Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Nuwellis, Inc. | a14-21020_18k.htm |
| EX-99.1 - EX-99.1 - Nuwellis, Inc. | a14-21020_1ex99d1.htm |
| EX-99.2 - EX-99.2 - Nuwellis, Inc. | a14-21020_1ex99d2.htm |
| EX-99.3 - EX-99.3 - Nuwellis, Inc. | a14-21020_1ex99d3.htm |
Exhibit 99.4
|
|
Progress of the Fully Implantable System Sep 16, 2014 www.sunshineheart.com |
|
|
Internal electro-hydraulic converter and TETS eliminate the percutaneous drive line and associated infection risks. Non-blood contacting Non-obligatory C-Pulse II Overview © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. Fully Implantable System |
|
|
Internal electro-hydraulic converter and TETS eliminate the percutaneous drive line and associated infection risks. Non-blood contacting Non-obligatory No percutaneous drive line © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. C-Pulse II Overview Fully Implantable System |
|
|
Internal electro-hydraulic converter and TETS eliminate the percutaneous drive line and associated infection risks. Non-blood contacting Non-obligatory No percutaneous drive line No implanted battery © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. C-Pulse II Overview Fully Implantable System |
|
|
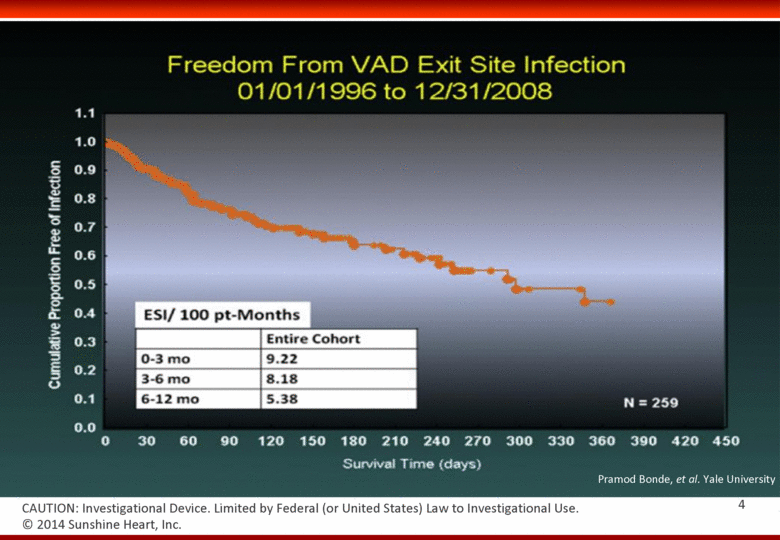
Pramod Bonde, et al. Yale University © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 4 |
|
|
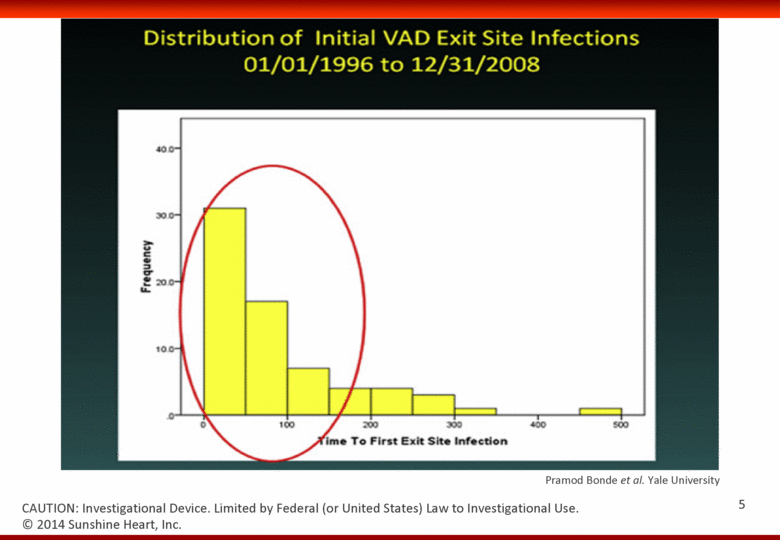
Pramod Bonde et al. Yale University © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 5 |
|
|
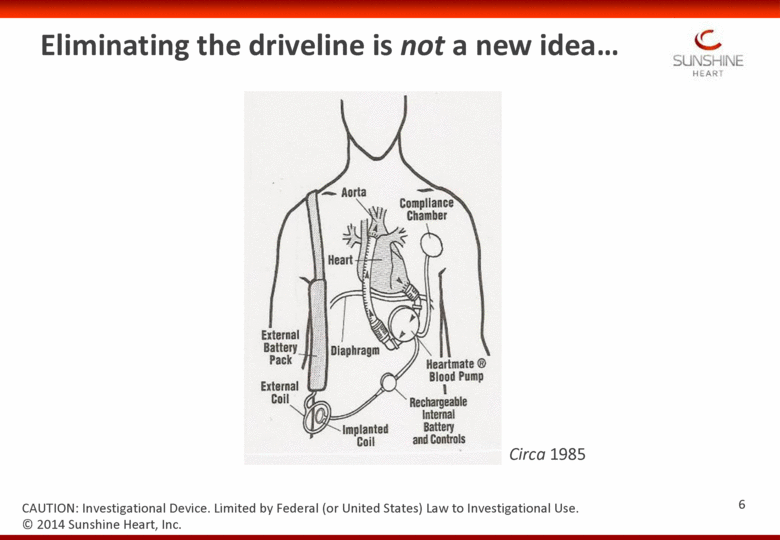
Eliminating the driveline is not a new idea Circa 1985 © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 6 |
|
|
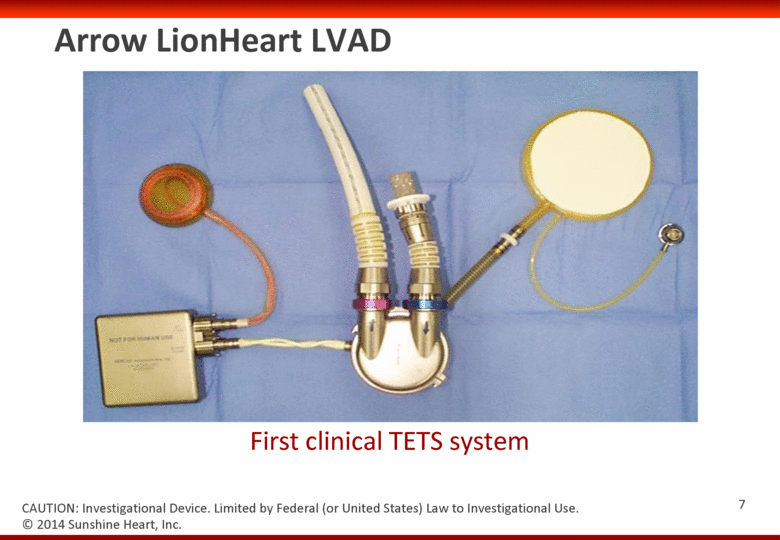
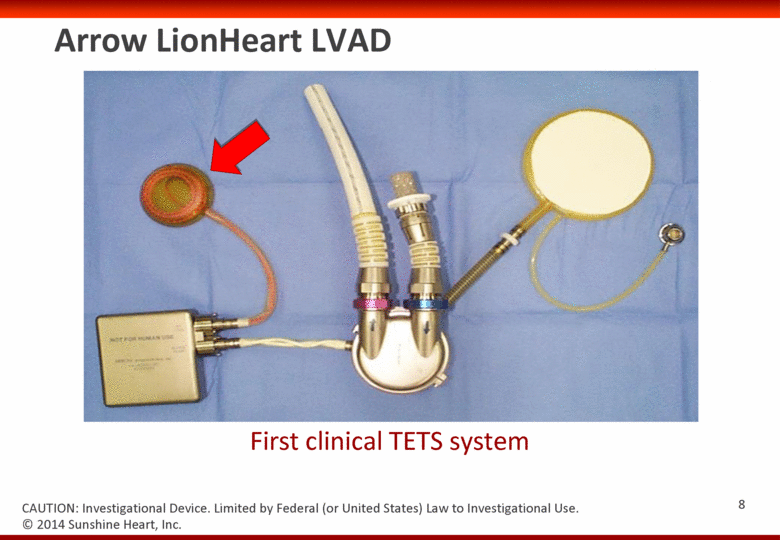
Arrow LionHeart LVAD First clinical TETS system © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 7 |
|
|
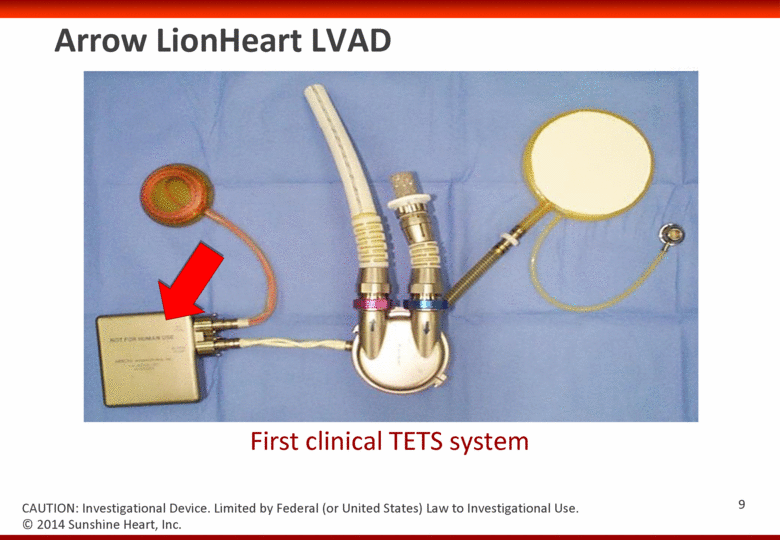
Arrow LionHeart LVAD First clinical TETS system © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 8 |
|
|
Arrow LionHeart LVAD First clinical TETS system © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 9 |
|
|
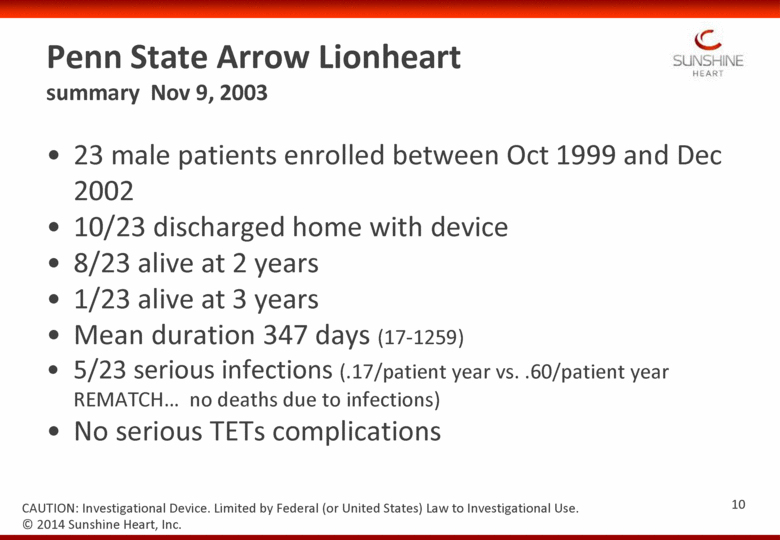
Penn State Arrow Lionheart summary Nov 9, 2003 23 male patients enrolled between Oct 1999 and Dec 2002 10/23 discharged home with device 8/23 alive at 2 years 1/23 alive at 3 years Mean duration 347 days (17-1259) 5/23 serious infections (.17/patient year vs. .60/patient year REMATCH no deaths due to infections) No serious TETs complications © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 10 |
|
|
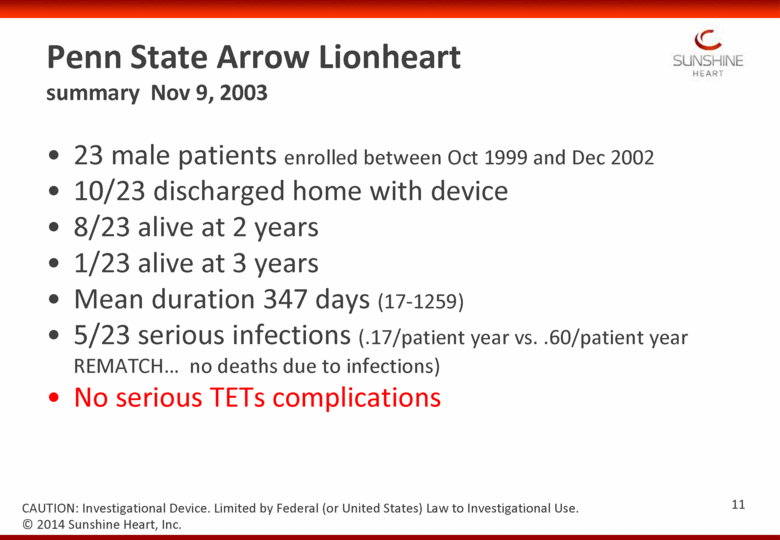
23 male patients enrolled between Oct 1999 and Dec 2002 10/23 discharged home with device 8/23 alive at 2 years 1/23 alive at 3 years Mean duration 347 days (17-1259) 5/23 serious infections (.17/patient year vs. .60/patient year REMATCH no deaths due to infections) No serious TETs complications Penn State Arrow Lionheart summary Nov 9, 2003 © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 11 |
|
|
Where will clinical implementation of TETS technology first find traction? © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 12 |
|
|
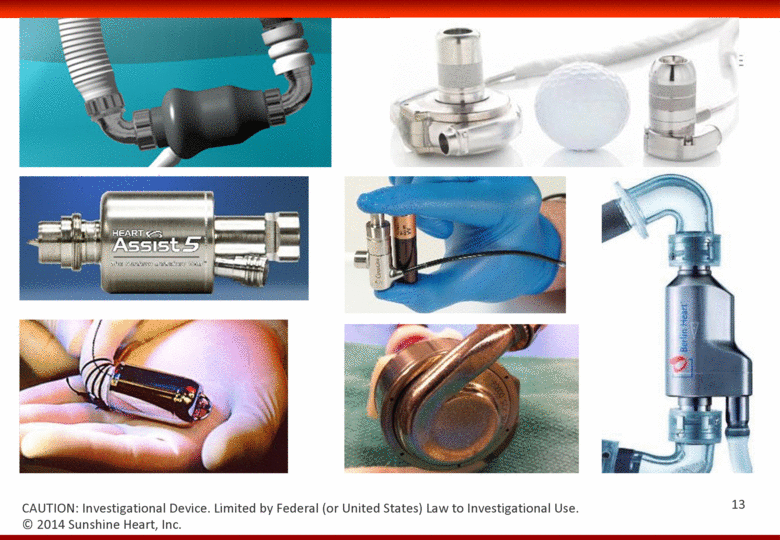
© 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 13 |
|
|
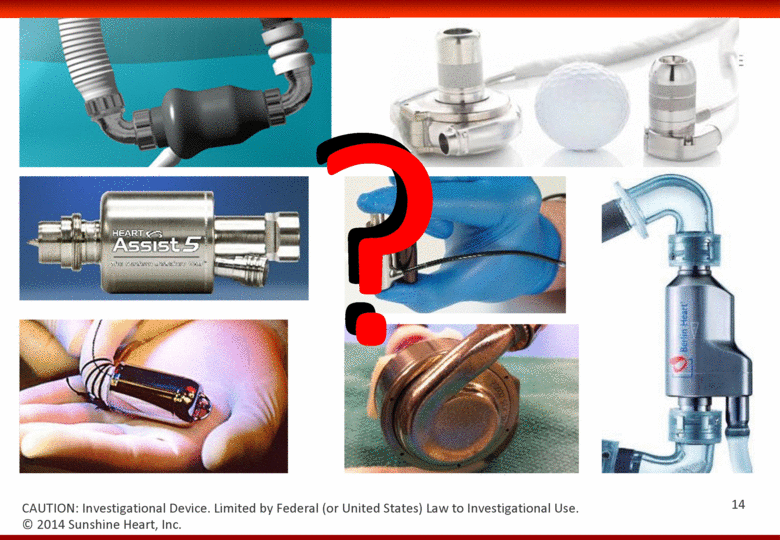
© 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. ? 14 |
|
|
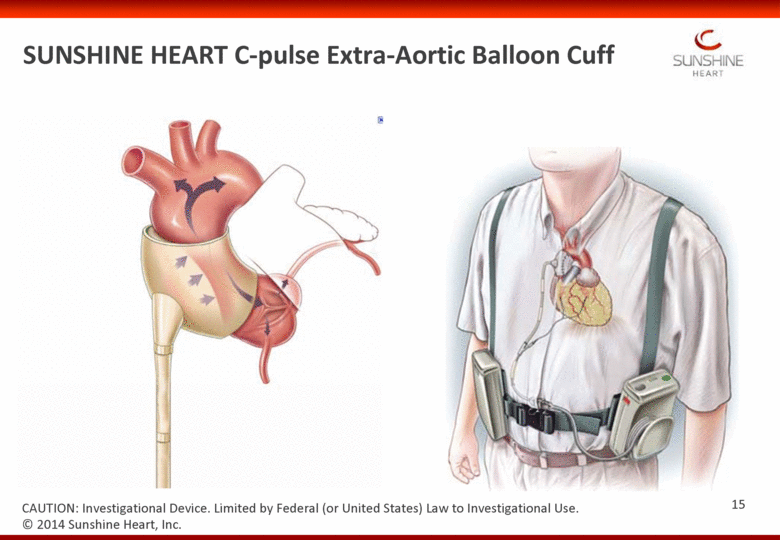
© 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. SUNSHINE HEART C-pulse Extra-Aortic Balloon Cuff 15 |
|
|
SUNSHINE HEART C-pulse Extra-Aortic Balloon Cuff © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. Non-obligatory No blood contact so 16 |
|
|
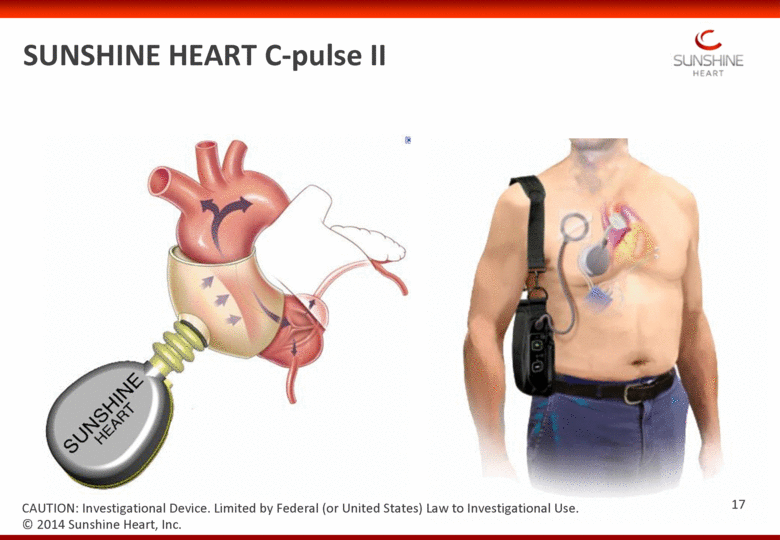
SUNSHINE HEART C-pulse II © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 17 |
|
|
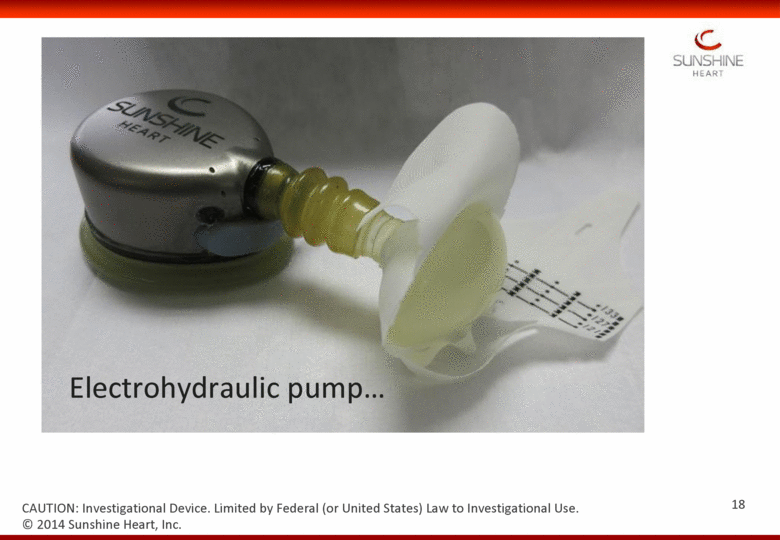
Electrohydraulic pump © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 18 |
|
|
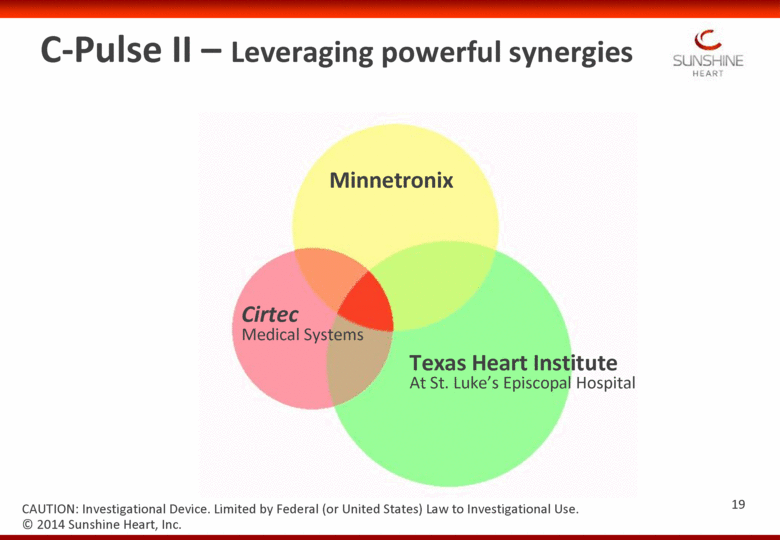
C-Pulse II – Leveraging powerful synergies Minnetronix Cirtec Medical Systems Texas Heart Institute At St. Luke’s Episcopal Hospital © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 19 |
|
|
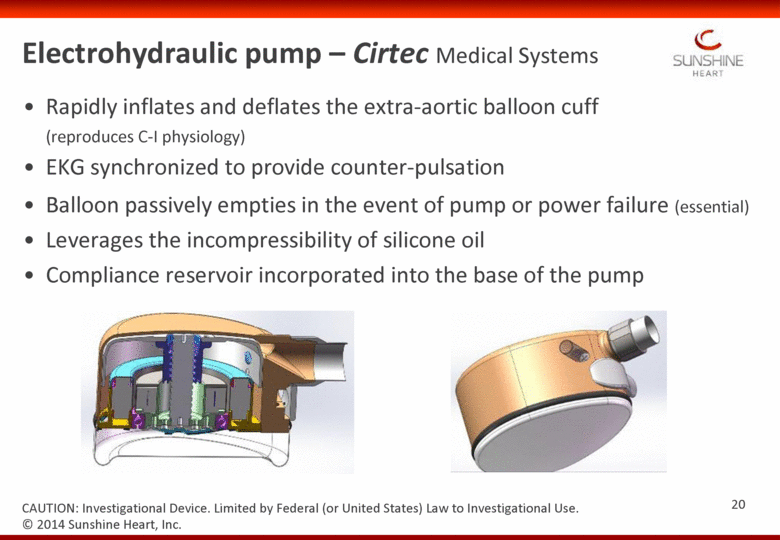
Electrohydraulic pump – Cirtec Medical Systems Rapidly inflates and deflates the extra-aortic balloon cuff (reproduces C-I physiology) EKG synchronized to provide counter-pulsation Balloon passively empties in the event of pump or power failure (essential) Leverages the incompressibility of silicone oil Compliance reservoir incorporated into the base of the pump © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 20 |
|
|
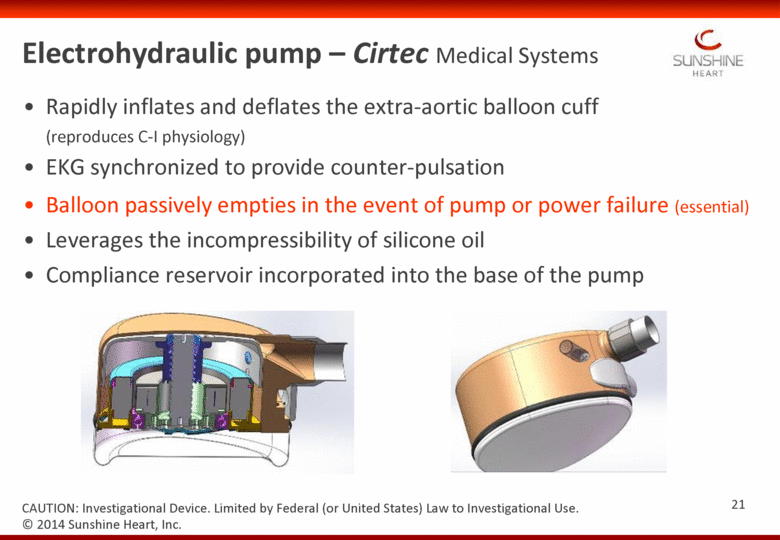
Rapidly inflates and deflates the extra-aortic balloon cuff (reproduces C-I physiology) EKG synchronized to provide counter-pulsation Balloon passively empties in the event of pump or power failure (essential) Leverages the incompressibility of silicone oil Compliance reservoir incorporated into the base of the pump © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. Electrohydraulic pump – Cirtec Medical Systems 21 |
|
|
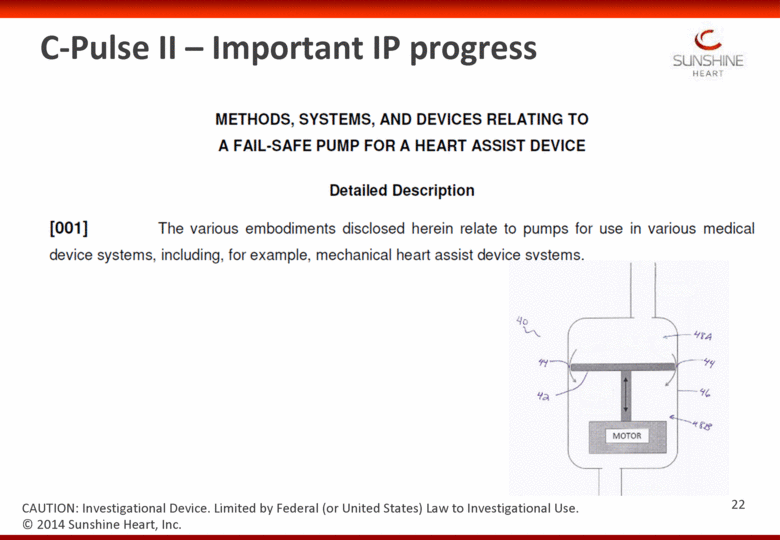
C-Pulse II – Important IP progress © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 22 |
|
|
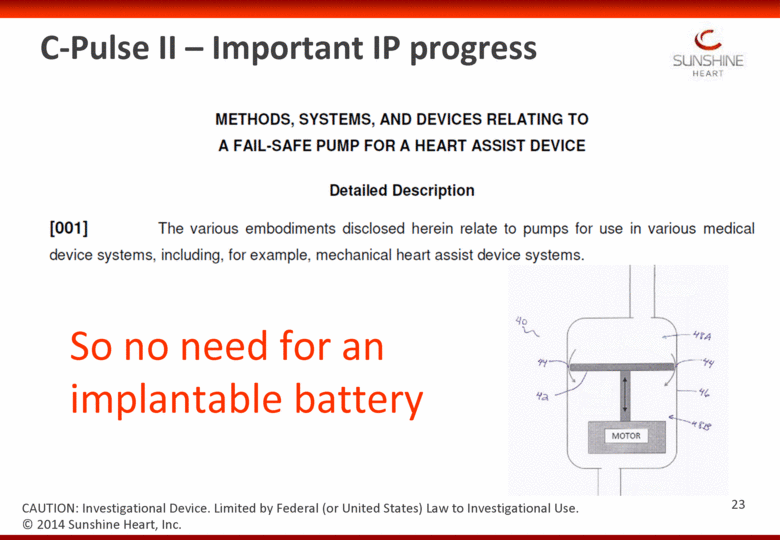
C-Pulse II – Important IP progress So no need for an implantable battery © 2014 Sunshine Heart, Inc. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 23 |
|
|
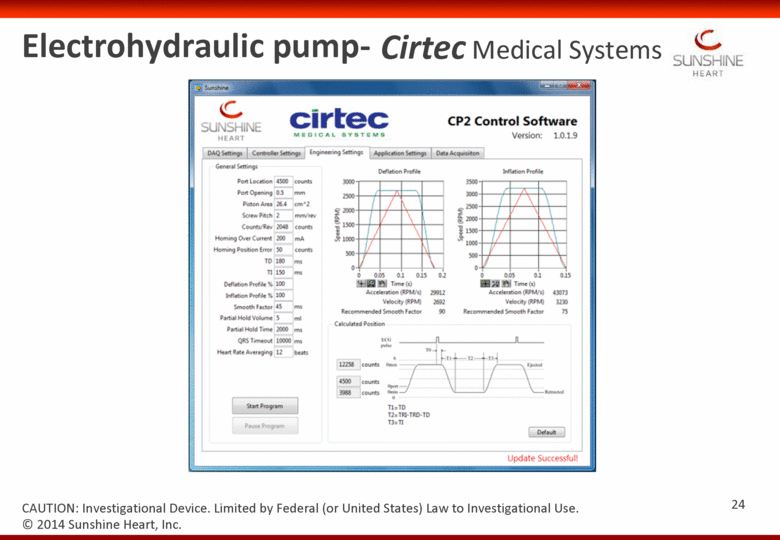
Cirtec Medical Systems Electrohydraulic pump- CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 24 © 2014 Sunshine Heart, Inc. |
|
|
How are we going to power it? CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 25 © 2014 Sunshine Heart, Inc. |
|
|
Trans-cutaneous Energy Transfer System (TETS) DC battery pack outside the body DC current is put through an oscillator to make AC AC current energizes external coil (1o) to generate an oscillating magnetic field Oscillating magnetic field goes through the skin Oscillating magnetic field is picked up by a tuned internal coil (2o) resulting in induction of AC current AC current rectified into DC used to run the internal device CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 26 © 2014 Sunshine Heart, Inc. |
|
|
Standard transformer CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 27 © 2014 Sunshine Heart, Inc. |
|
|
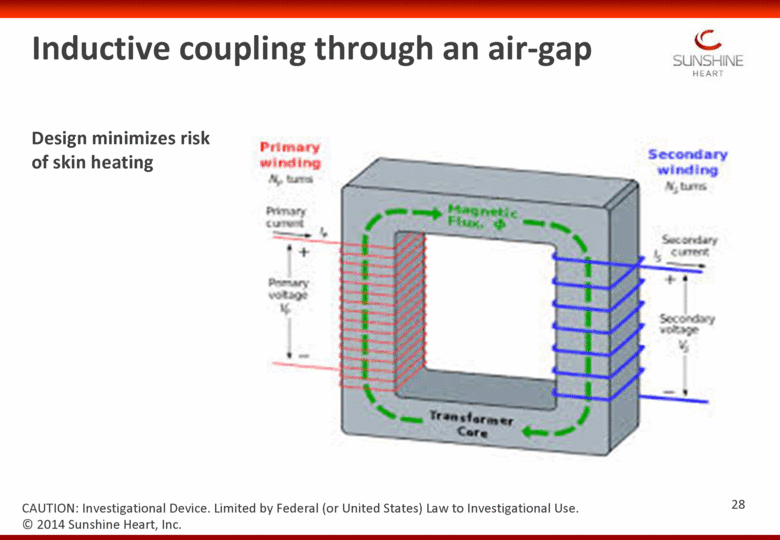
Inductive coupling through an air-gap CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. Design minimizes risk of skin heating 28 © 2014 Sunshine Heart, Inc. |
|
|
July 10 1856 – January 7 1943 Nikola Tesla CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 29 © 2014 Sunshine Heart, Inc. |
|
|
Newest systems are: Smaller size so easier to implant More energy efficient so improved battery life More tolerant of geometric misalignment Minnetronix Leaders in Transcutaneous Energy Transfer Systems (TETS) CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 30 © 2014 Sunshine Heart, Inc. |
|
|
Minnetronix Leaders in Transcutaneous Energy Transfer Systems (TETS) CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 31 © 2014 Sunshine Heart, Inc. |
|
|
Improvement in TETS component geometry and function Minnetronix CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 32 © 2014 Sunshine Heart, Inc. |
|
|
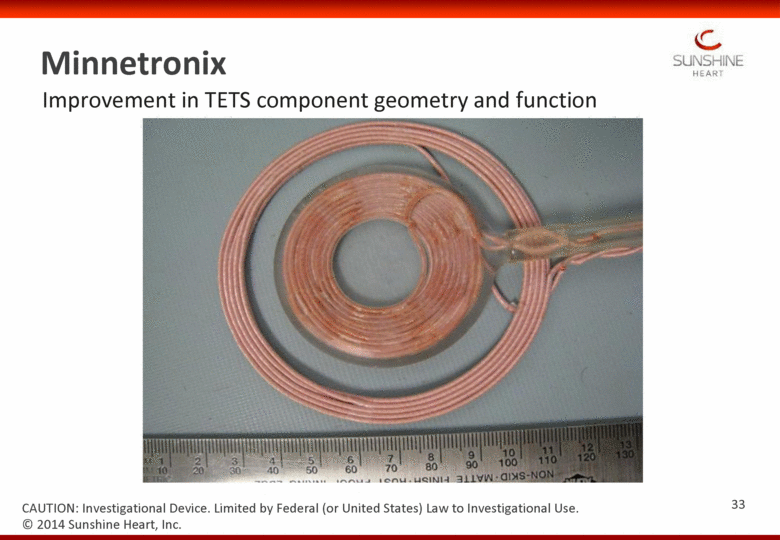
Improvement in TETS component geometry and function Minnetronix CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 33 © 2014 Sunshine Heart, Inc. |
|
|
How are we going to power it? Where are we going to test it? CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 34 © 2014 Sunshine Heart, Inc. |
|
|
CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. TEXAS HEART INSTITUTE at St. Luke’s Episcopal Hospital © 2014 Sunshine Heart, Inc. 35 |
|
|
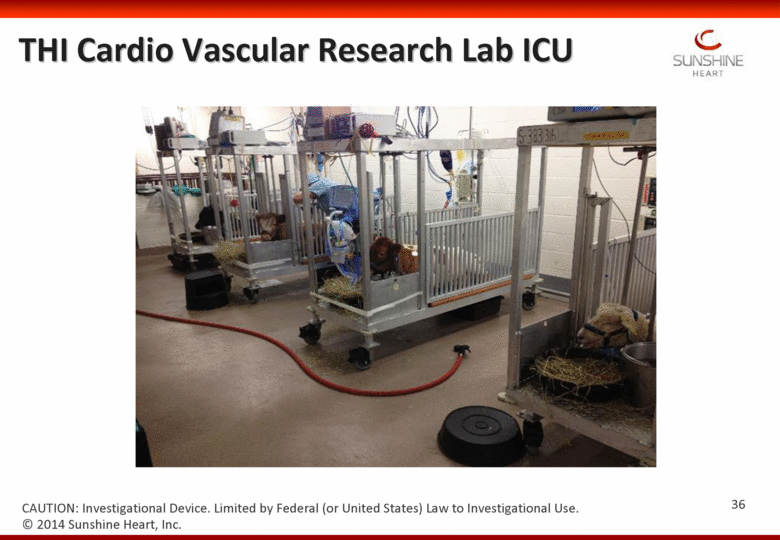
THI Cardio Vascular Research Lab ICU 36 CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. |
|
|
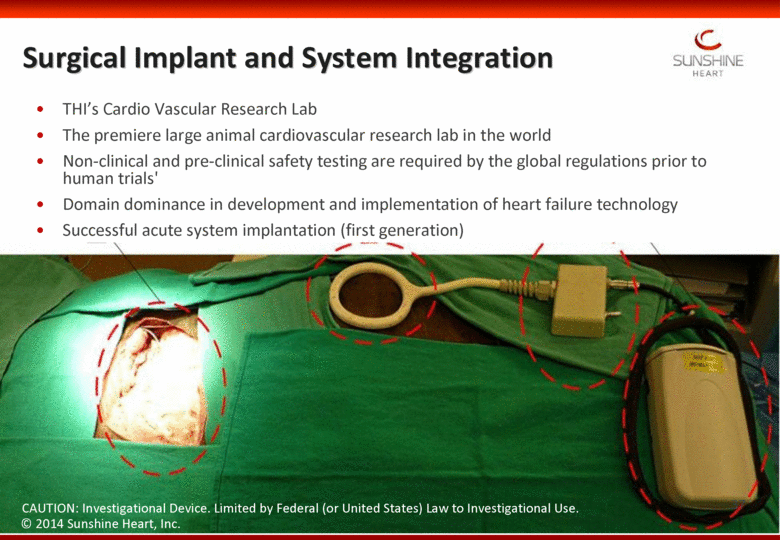
Surgical Implant and System Integration THI’s Cardio Vascular Research Lab The premiere large animal cardiovascular research lab in the world Non-clinical and pre-clinical safety testing are required by the global regulations prior to human trials' Domain dominance in development and implementation of heart failure technology Successful acute system implantation (first generation) CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. 37 © 2014 Sunshine Heart, Inc. |
|
|
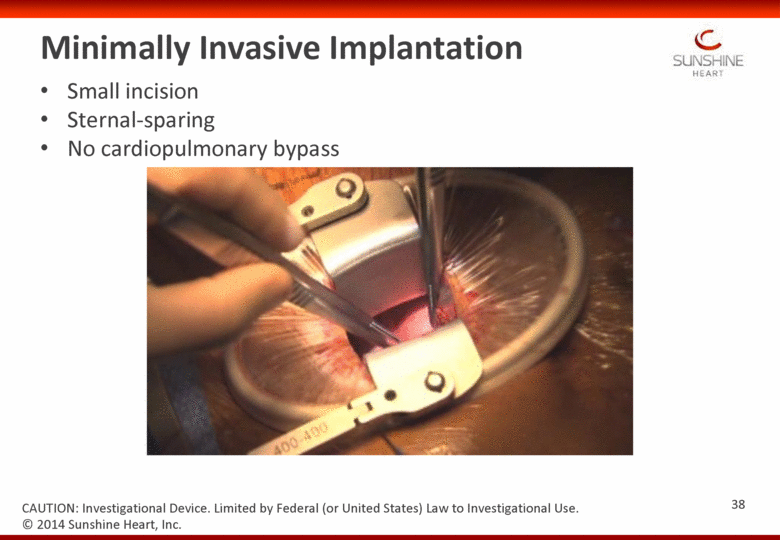
Minimally Invasive Implantation Small incision Sternal-sparing No cardiopulmonary bypass 38 CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. |
|
|
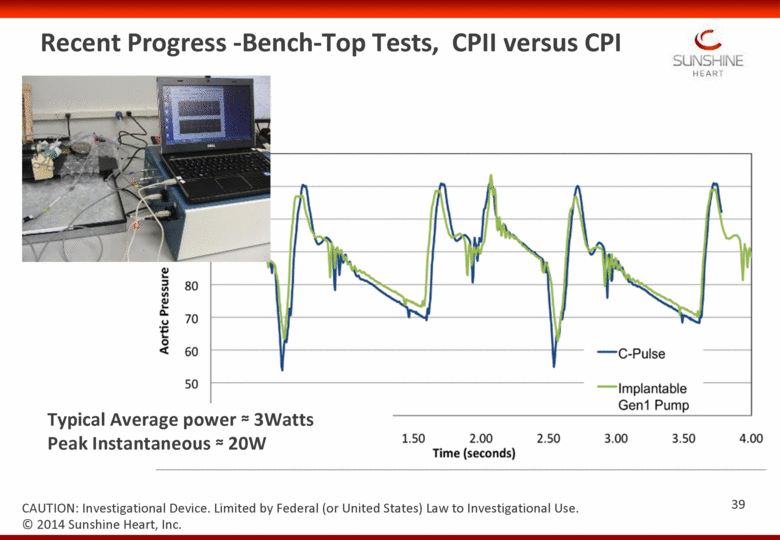
Recent Progress -Bench-Top Tests, CPII versus CPI Typical Average power 3Watts Peak Instantaneous 20W 39 CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. |
|
|
40 CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. |
|
|
41 CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. |
|
|
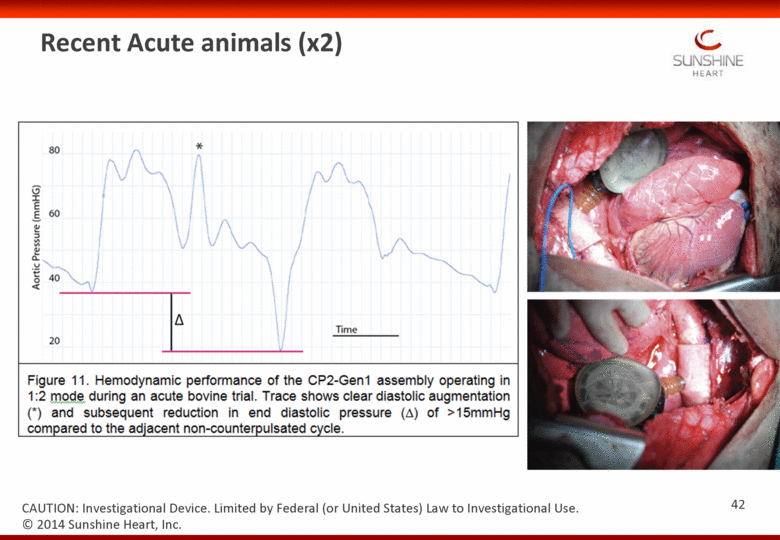
Recent Acute animals (x2) 42 CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. |
|
|
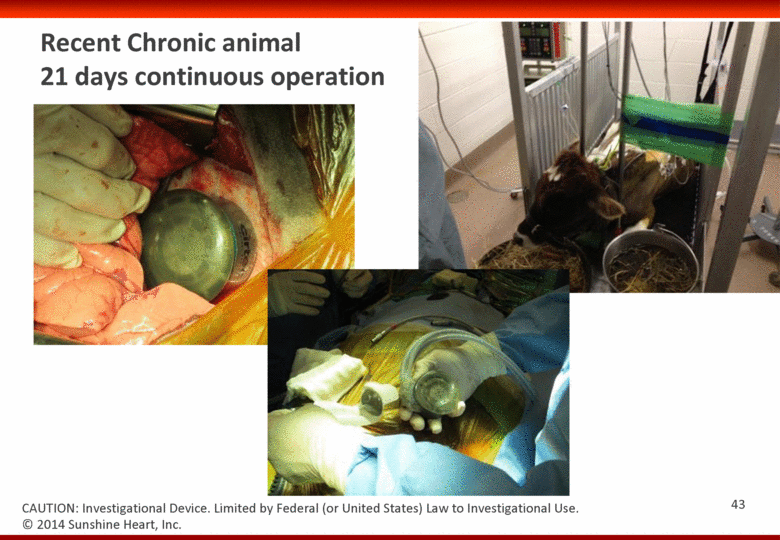
Recent Chronic animal 21 days continuous operation 43 CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. |
|
|
44 CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. |
|
|
CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. 45 |
|
|
The SUNSHINE HEART C-Pulse II has the potential to be the first completely self-contained therapy for heart failure since the bi-ventricular pacer Lack of blood contact and non-obligatory feature make it the most likely candidate to leverage TETS in a mechanical circulatory assist device Pump innovation has facilitated development of a novel technology, avoiding the safety and regulatory risks of an implantable battery The system is well suited for implantation off-pump through a small sternal sparing incision, making it well suited for patients earlier in the course of heart failure Early pre-clinical testing suggests the design is performing as intended and within established safety parameters In summary 46 CAUTION: Investigational Device. Limited by Federal (or United States) Law to Investigational Use. © 2014 Sunshine Heart, Inc. |