Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Inspyr Therapeutics, Inc. | v388792_8k.htm |

Investor Presentation September 2014 OTCQB : GNSZ

2 Safe Harbor Statement Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995 : Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera's anticipated growth strategies, the outcome of its clinical trials, future business development, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the Company's filings and future filings made with the United States Securities and Exchange Commission . Readers are advised that this information is intended for the use of investment professionals . Anyone interested in obtaining information on GenSpera should contact GenSpera directly . This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company's stock . This presentation is based upon information available to the public, as well as other information from sources which management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete . Opinions expressed herein are those of management as of the date of the presentation and are subject to change without notice .

3 Company Overview • Technology platform combines a powerful, plant - derived cytotoxin (thapsigargin) with targeted release of drug candidates only within the tumor • Unique mechanism of action • Minimized side effects • Enhanced anti - tumor activity • Mipsagargin: Lead drug candidate • Encouraging Phase 2 results: hepatocellular carcinoma (liver cancer) • Phase 2: glioblastoma (brain cancer) underway • Phase 2: prostate cancer soon to enroll • Experienced scientific and development team • Robust global IP position

4 Craig A. Dionne, PhD Chairman & CEO • Over 25 years of experience in pharmaceutical industry SVP Drug Discovery at Cephalon, Inc. (acquired by Teva Pharmaceuticals ) EVP Prostate Cancer Foundation Peter E. Grebow, PhD Director • Over 37 years of experience in pharmaceutical industry EVP Drug Development Cephalon, Inc. Board member Optimer Pharmaceuticals, Q Holdings, Complexa . Bo Jesper Hansen, MD, PhD Director • Executive Chairman of Swedish Orphan Biovitrum AB Board member CMC AB, Orphazyme ApS, Newron , Onxeo SAS, Hyperion Therapeutics and Ablynx NV Scott Ogilvie Director • President and CEO AFIN International, Inc. Board member Research Solutions, Inc., and Neuralstem , Inc. Russell Richerson, PhD COO • Over 25 years of experience in biotechnology industry Previously COO Molecular Profiling Institute, VP Diagnostic R&D Prometheus, Abbott Labs Management, Directors, Advisors John T. Isaacs, PhD Chief Scientific Advisor • Professor at Johns Hopkins School of Medicine Co - Inventor of GenSpera’s technologies Samuel R. Denmeade, MD Chief Clinical Advisor • Professor at Johns Hopkins and Board Certified Medical Oncologist Co - Inventor of GenSpera’s technologies Søren Brøgger Christensen, PhD Scientific Advisor • Professor at University of Copenhagen. Expert in chemistry of thapsigargin Exploring further derivatives and manufacturing efficiencies for thapsigargin Experienced Scientific and Development Team
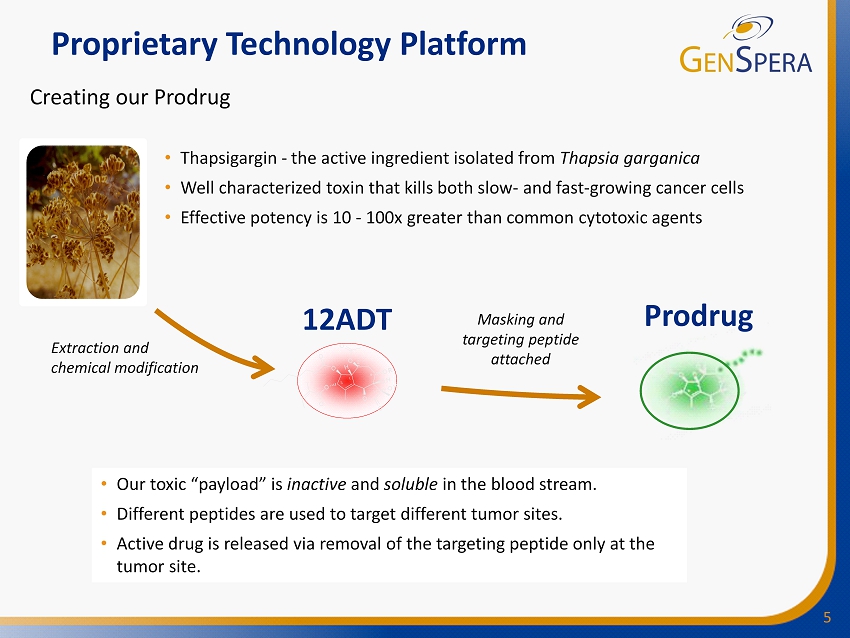
5 Proprietary Technology Platform 12ADT Prodrug • Thapsigargin - the active ingredient isolated from Thapsia garganica • Well characterized toxin that kills both slow - and fast - growing cancer cells • Effective potency is 10 - 100x greater than common cytotoxic agents Creating our Prodrug Extraction and chemical modification • Our toxic “payload” is inactive and soluble in the blood stream. • Different peptides are used to target different tumor sites. • Active drug is released via removal of the targeting peptide only at the tumor site. Masking and targeting peptide attached

6 Market Opportunity We believe mipsagargin may be effective in a wide range of malignant tumor types, which covers a multi - billion dollar industry opportunity Prostate Cancer: a. 240,000 new cases diagnosed in the U.S. each year; b. $6.7B by 2020 Liver Cancer: a. 3 rd largest cancer killer worldwide; b. $1.5B by 2019 Brain Cancer: a. 13,000 deaths/year in the U.S. alone; b. Approved drug, Avastin is expected to generate $460M in revenue by 2017 mipsagargin
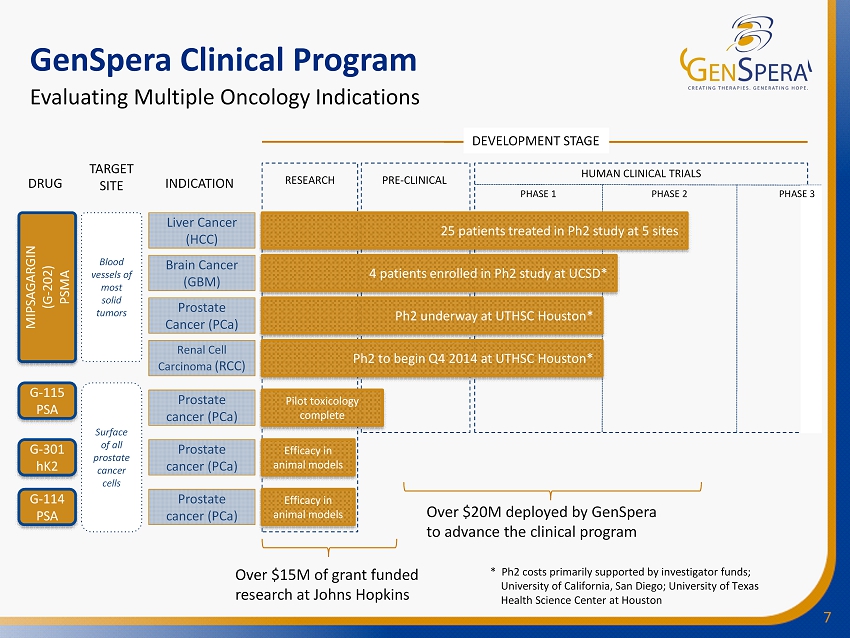
7 RESEARCH PRE - CLINICAL PHASE 1 PHASE 2 Liver Cancer (HCC) GenSpera Clinical Program Evaluating Multiple Oncology Indications TARGET SITE DRUG INDICATION Brain Cancer (GBM) Prostate Cancer (PCa) Prostate cancer (PCa) Prostate cancer (PCa) Prostate cancer (PCa) MIPSAGARGIN (G - 202) PSMA Blood vessels of most solid tumors Surface of all prostate cancer cells G - 114 PSA G - 301 hK2 G - 115 PSA DEVELOPMENT STAGE HUMAN CLINICAL TRIALS Over $20M deployed by GenSpera to advance the clinical program Over $15M of grant funded research at Johns Hopkins PHASE 3 25 patients treated in Ph2 study at 5 sites 4 patients enrolled in Ph2 study at UCSD* Ph2 underway at UTHSC Houston* Efficacy in animal models Efficacy in animal models Pilot toxicology complete * Ph2 costs primarily supported by investigator funds; University of California, San Diego; University of Texas Health Science Center at Houston Renal Cell Carcinoma (RCC) Ph2 to begin Q4 2014 at UTHSC Houston*
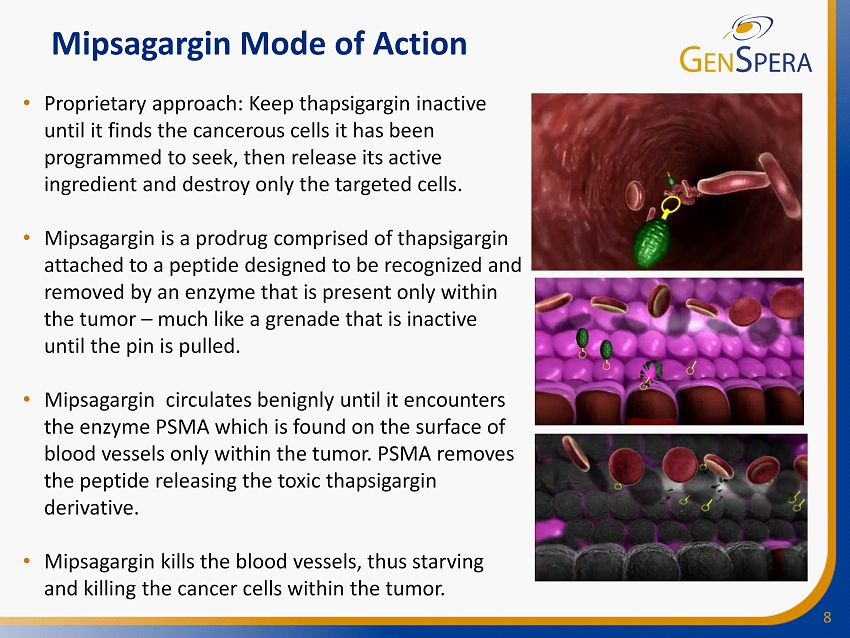
8 Mipsagargin Mode of Action • Proprietary approach: Keep thapsigargin inactive until it finds the cancerous cells it has been programmed to seek, then release its active ingredient and destroy only the targeted cells. • Mipsagargin is a prodrug comprised of thapsigargin attached to a peptide designed to be recognized and removed by an enzyme that is present only within the tumor – much like a grenade that is inactive until the pin is pulled. • Mipsagargin circulates benignly until it encounters the enzyme PSMA which is found on the surface of blood vessels only within the tumor. PSMA removes the peptide releasing the toxic thapsigargin derivative. • Mipsagargin kills the blood vessels, thus starving and killing the cancer cells within the tumor.
9 Mipsagargin Phase 2 Trial in Liver Cancer • Advanced Hepatocellular Carcinoma (HCC) patients who have progressed or are intolerant of Nexavar® • Multiple clinical sites in US • Drug administered as a one - hour intravenous infusion in saline on Days 1, 2 and 3 of 28 - day cycle • Minimal and manageable side effects (fatigue, nausea, rash, reversible creatinine increase ) • No apparent effect on bone marrow
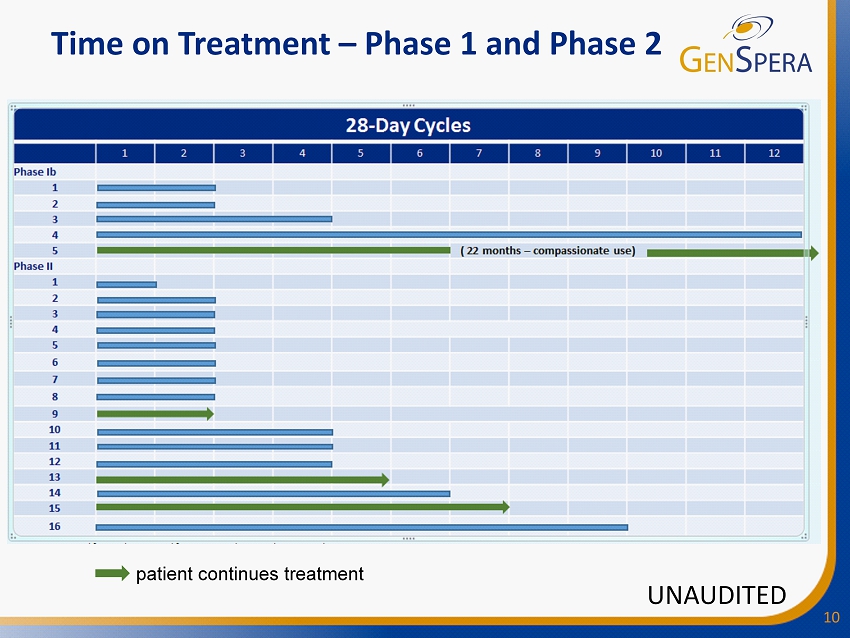
10 Time on Treatment – Phase 1 and Phase 2 patient continues treatment UNAUDITED
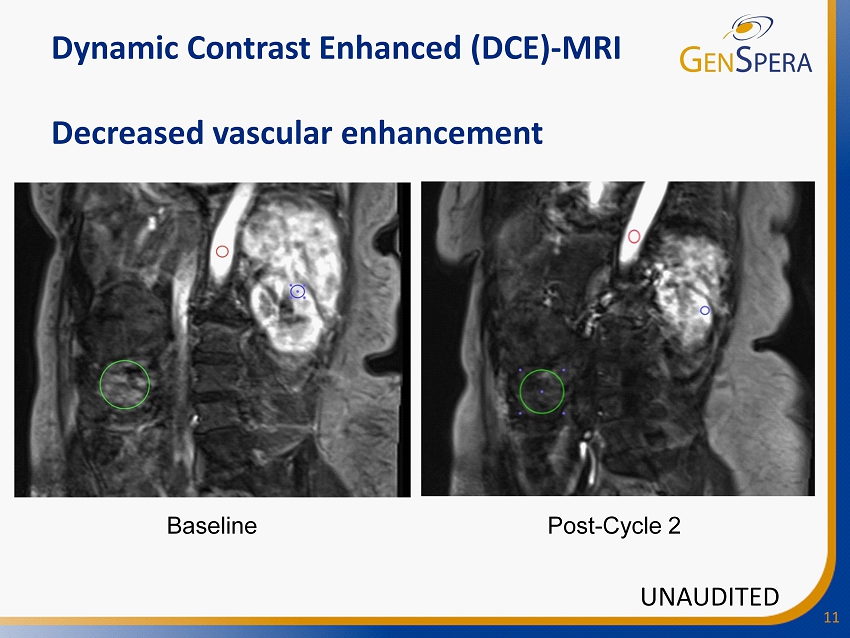
11 Dynamic Contrast Enhanced (DCE) - MRI Decreased vascular enhancement Baseline Post - Cycle 2 UNAUDITED
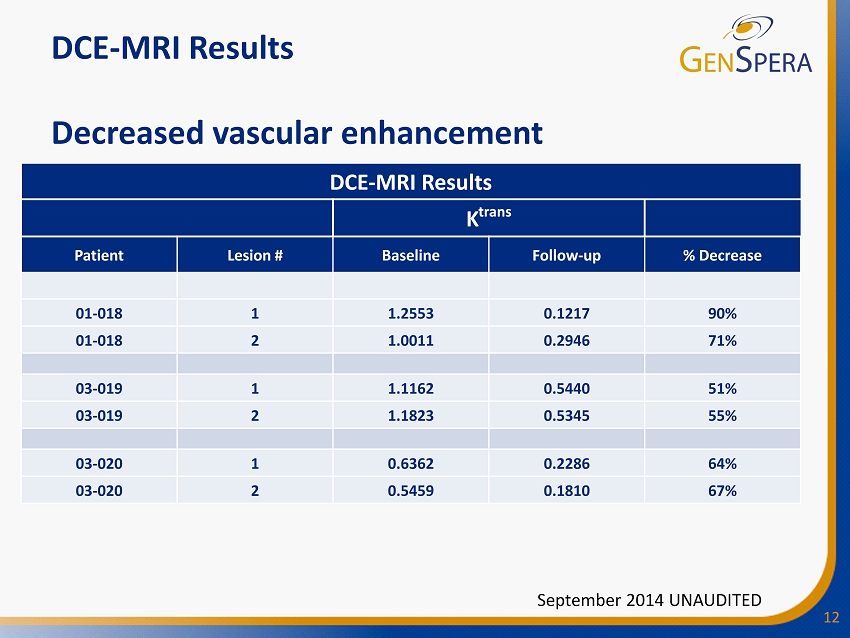
12 September 2014 UNAUDITED DCE - MRI Results K trans Patient Lesion # Baseline Follow - up % Decrease 01 - 018 1 1.2553 0.1217 90% 01 - 018 2 1.0011 0.2946 71% 03 - 019 1 1.1162 0.5440 51% 03 - 019 2 1.1823 0.5345 55% 03 - 020 1 0.6362 0.2286 64% 03 - 020 2 0.5459 0.1810 67% DCE - MRI Results Decreased vascular enhancement
13 Clinical Observations – Phase 1 & Phase 2 • Mipsagargin administration results in Disease Control Rate (CR + PR + SD) at two months of 80% • One patient with vertebral metastases has significant reduction in bone pain • 50% of patients with Stable Disease for ≥ 4 months – Primary Endpoint • Long - term disease stabilization ≥ 9 months in 4 patients (20%) • DCE - MRI data (three patients to date) demonstrate dramatic effect on tumor vasculature UNAUDITED
14 Distinctive Mipsagargin Characteristics Distinctive characteristics compared to other chemotherapeutic agents being tested in liver cancer patients: 1. Unique molecular mechanism of action. 2. Prolonged disease stabilization in a significant percentage of patients – 20% with stable disease > 9 months. 3. Side - effect profile – Minimal and manageable side effects (fatigue, nausea, rash, reversible creatinine increase). No apparent effect on bone marrow. 4. Proof of Concept – Imaging studies demonstrate dramatic effects on tumor blood flow.
15 Worldwide Patent Strategy • Current Infusion Formulation • Patent coverage in U.S. to 2023 • Data exclusivity outside of U.S. • Future Injectable Nanoemulsion Formulation • Composition of matter PCT filed August 2013 • Worldwide Patent exclusivity expected through 2033 • Injectable form is easier than infusion for patients and treatment centers
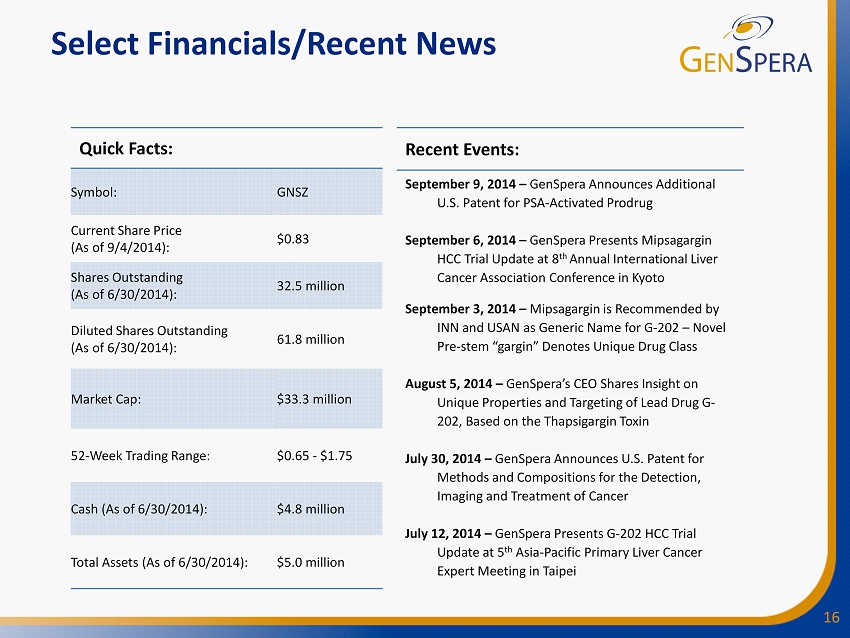
16 Select Financials/Recent News Quick Facts: Symbol: GNSZ Current Share Price (As of 9/4/2014): $0.83 Shares Outstanding (As of 6/30/2014): 32.5 million Diluted Shares Outstanding (As of 6/30/2014): 61.8 million Market Cap: $33.3 million 52 - Week Trading Range : $0.65 - $1.75 Cash (As of 6/30/2014 ) : $4.8 million Total Assets (As of 6/30/2014): $5.0 million Recent Events: September 9, 2014 – GenSpera Announces Additional U.S . Patent for PSA - Activated Prodrug September 6, 2014 – GenSpera Presents Mipsagargin HCC Trial Update at 8 th Annual International Liver Cancer Association Conference in Kyoto September 3, 2014 – Mipsagargin is R ecommended by INN and USAN as Generic Name for G - 202 – Novel Pre - stem “gargin” D enotes Unique Drug Class August 5, 2014 – GenSpera’s CEO Shares Insight on Unique Properties and Targeting of Lead Drug G - 202, Based on the Thapsigargin Toxin July 30, 2014 – GenSpera Announces U.S. Patent for Methods and Compositions for the Detection, Imaging and Treatment of Cancer July 12, 2014 – GenSpera Presents G - 202 HCC Trial Update at 5 th Asia - Pacific Primary Liver Cancer Expert Meeting in Taipei

17 Investment Highlights • Intellectual property created through ~$35M in funding and 15 years of research at Johns Hopkins Medical Center and other renowned centers • Distinctive characteristics • Mechanism of action • Excellent side - effect profile • Signs of clinical efficacy • Proof of concept via imaging • Lead candidate, mipsagargin • Liver cancer: Phase 2 enrollment nearly complete • Brain cancer: Four patients treated in Phase 2 • Prostate cancer: Beginning enrollment • Billion dollar market opportunity • Liver Cancer: 3 rd largest cancer killer worldwide & estimated to total $1.5B by 2019 • Brain Cancer: 13,000 deaths/year in U.S. alone • Prostate Cancer: $6.7B by 2020

18 Contact Us PRESENTATION BY: GenSpera (OTC QB: GNSZ) 2511 N. Loop 1604 W, Suite 204 San Antonio, TX 78258 Craig Dionne, PhD President, CEO and Chairman (210) 479 - 8112 info@genspera.com Investor Relations Capital Markets Group, LLC Alan Sheinwald (914) 669 - 0222 alan@capmarketsgroup.com
