Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Theravance Biopharma, Inc. | a14-20614_18k.htm |
| EX-99.1 - EX-99.1 - Theravance Biopharma, Inc. | a14-20614_1ex99d1.htm |
Exhibit 99.2
|
|
TD-4208 LAMA Phase 2b Study 0117 Results September 8, 2014 |
|
|
Safe Harbor Statement This presentation contains certain "forward-looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives and future events. Theravance Biopharma intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. The words “anticipate”, “expect”, “goal,” “intend”, “objective,” “opportunity,” “plan”, “potential”, “target” and similar expressions are intended to identify such forward-looking statements. Examples of such statements include statements relating to: the strategies, plans and objectives of Theravance Biopharma, the status and timing of clinical studies, data analysis and communication of results, the potential benefits and mechanisms of action of product candidates, the enabling capabilities of Theravance Biopharma's approach to drug discovery and Theravance Biopharma's proprietary insights, expectations for product candidates through development and commercialization, and the timing of seeking regulatory approval of product candidates. These statements are based on the current estimates and assumptions of the management of Theravance Biopharma as of the date of this presentation and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance Biopharma to be materially different from those reflected in the forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to: the disruption of operations during the transition period following the spin-off of Theravance Biopharma from Theravance, Inc., including the diversion of management's and employees' attention from the business, adverse impacts upon the progress of discovery and development efforts, disruption of relationships with collaborators and increased employee turnover, delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective, dependence on third parties to conduct clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products and risks associated with establishing distribution capabilities for telavancin with appropriate technical expertise and supporting infrastructure. Other risks affecting Theravance Biopharma are described under the heading "Risk Factors" contained in Theravance Biopharma's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 14, 2014. In addition to the risks described above and in Theravance Biopharma's other filings with the SEC, other unknown or unpredictable factors also could affect Theravance Biopharma's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance Biopharma assumes no obligation to update its forward-looking statements on account of new information, future events or otherwise, except as required by law. |
|
|
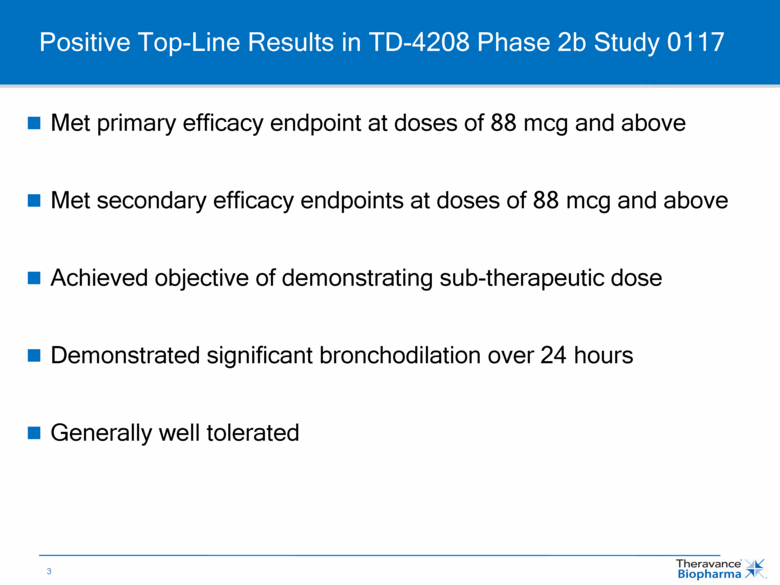
Positive Top-Line Results in TD-4208 Phase 2b Study 0117 Met primary efficacy endpoint at doses of 88 mcg and above Met secondary efficacy endpoints at doses of 88 mcg and above Achieved objective of demonstrating sub-therapeutic dose Demonstrated significant bronchodilation over 24 hours Generally well tolerated |
|
|
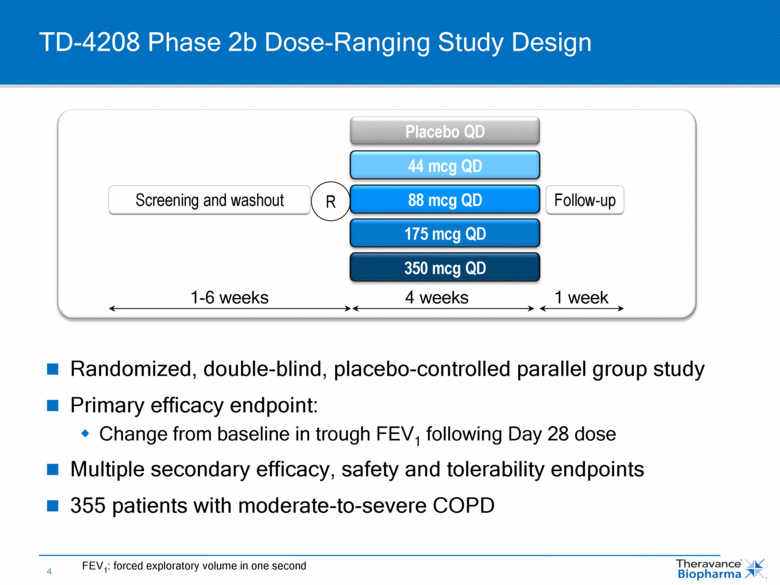
Randomized, double-blind, placebo-controlled parallel group study Primary efficacy endpoint: Change from baseline in trough FEV1 following Day 28 dose Multiple secondary efficacy, safety and tolerability endpoints 355 patients with moderate-to-severe COPD TD-4208 Phase 2b Dose-Ranging Study Design R Placebo QD 44 mcg QD 175 mcg QD 88 mcg QD 350 mcg QD Screening and washout Follow-up 1-6 weeks 4 weeks 1 week FEV1: forced exploratory volume in one second |
|
|
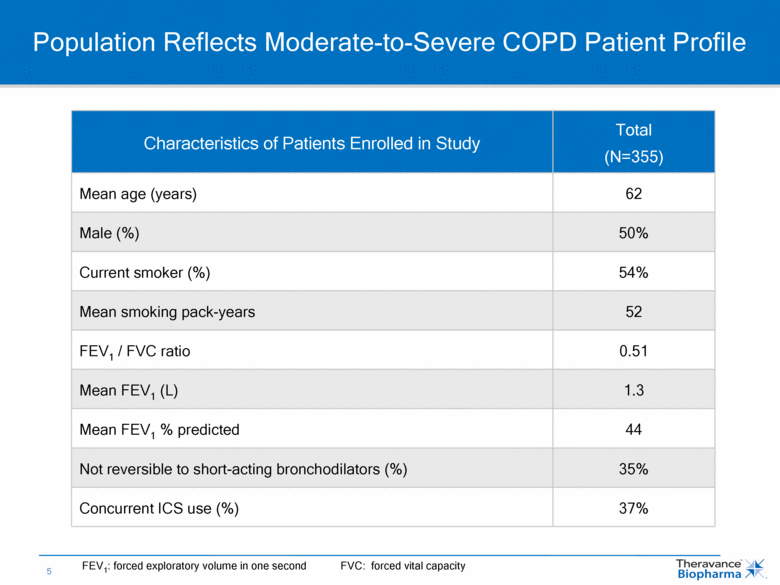
Population Reflects Moderate-to-Severe COPD Patient Profile Characteristics of Patients Enrolled in Study Total (N=355) Mean age (years) 62 Male (%) 50% Current smoker (%) 54% Mean smoking pack-years 52 FEV1 / FVC ratio 0.51 Mean FEV1 (L) 1.3 Mean FEV1 % predicted 44 Not reversible to short-acting bronchodilators (%) 35% Concurrent ICS use (%) 37% FEV1: forced exploratory volume in one second FVC: forced vital capacity |
|
|
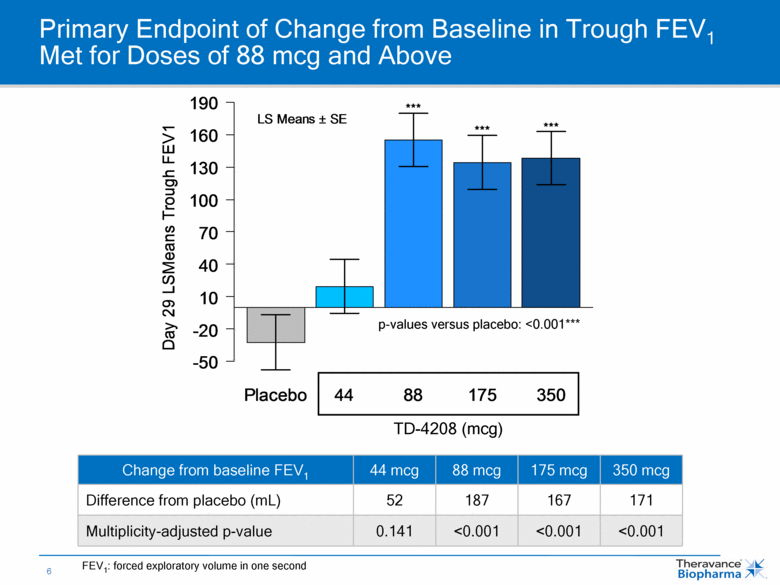
Primary Endpoint of Change from Baseline in Trough FEV1 Met for Doses of 88 mcg and Above Change from baseline FEV1 44 mcg 88 mcg 175 mcg 350 mcg Difference from placebo (mL) 52 187 167 171 Multiplicity-adjusted p-value 0.141 <0.001 <0.001 <0.001 TD-4208 (mcg) p-values versus placebo: <0.001*** FEV1: forced exploratory volume in one second Placebo 44 88 175 350 Day 29 LSMeans Trough FEV1 -50 -20 10 40 70 100 130 160 190 *** *** *** LS Means ± SE |
|
|
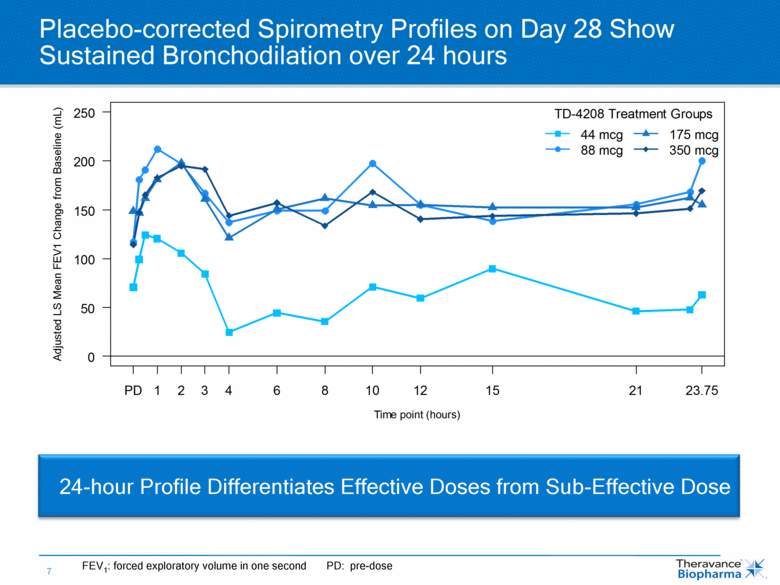
Placebo-corrected Spirometry Profiles on Day 28 Show Sustained Bronchodilation over 24 hours 24-hour Profile Differentiates Effective Doses from Sub-Effective Dose FEV1: forced exploratory volume in one second PD: pre-dose 0 50 100 150 200 250 PD 1 2 3 4 6 8 10 12 15 21 23.75 Time point (hours) Adjusted LS Mean FEV1 Change from Baseline (mL) TD-4208 Treatment Groups 44 mcg 88 mcg 175 mcg 350 mcg |
|
|
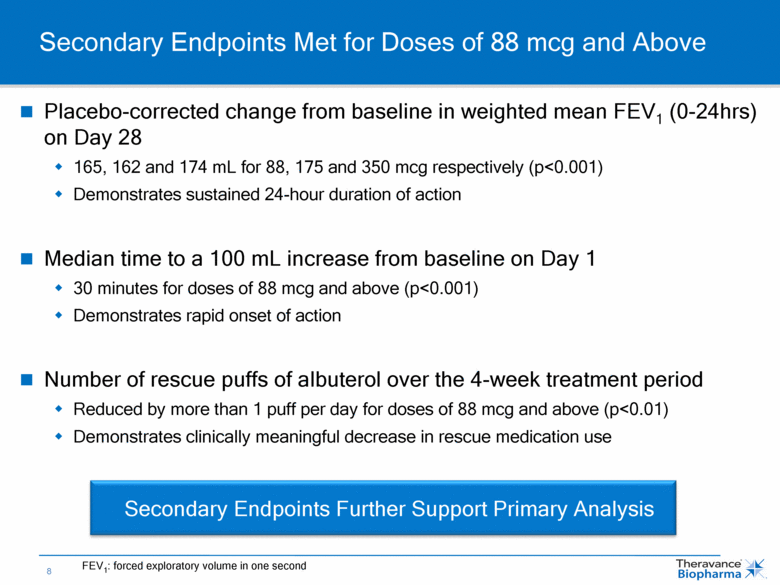
Placebo-corrected change from baseline in weighted mean FEV1 (0-24hrs) on Day 28 165, 162 and 174 mL for 88, 175 and 350 mcg respectively (p<0.001) Demonstrates sustained 24-hour duration of action Median time to a 100 mL increase from baseline on Day 1 30 minutes for doses of 88 mcg and above (p<0.001) Demonstrates rapid onset of action Number of rescue puffs of albuterol over the 4-week treatment period Reduced by more than 1 puff per day for doses of 88 mcg and above (p<0.01) Demonstrates clinically meaningful decrease in rescue medication use Secondary Endpoints Met for Doses of 88 mcg and Above Secondary Endpoints Further Support Primary Analysis FEV1: forced exploratory volume in one second |
|
|
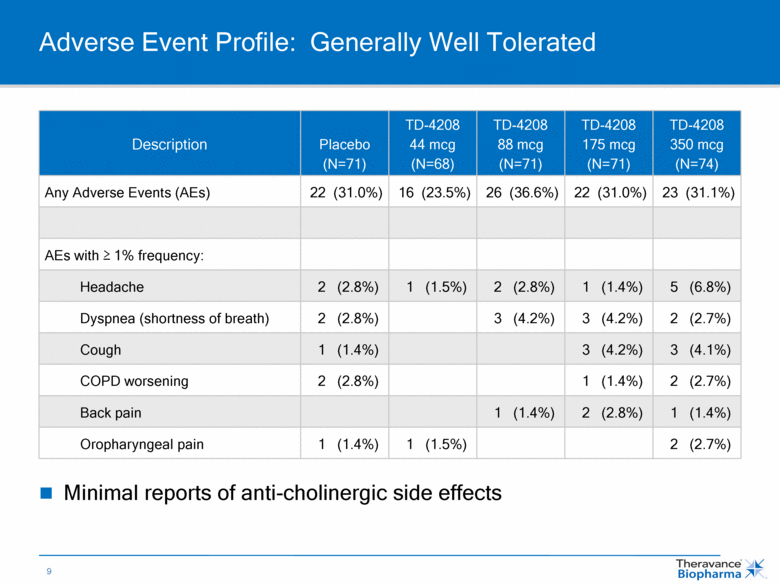
Adverse Event Profile: Generally Well Tolerated Description Placebo (N=71) TD-4208 44 mcg (N=68) TD-4208 88 mcg (N=71) TD-4208 175 mcg (N=71) TD-4208 350 mcg (N=74) Any Adverse Events (AEs) 22 (31.0%) 16 (23.5%) 26 (36.6%) 22 (31.0%) 23 (31.1%) AEs with > 1% frequency: Headache 2 (2.8%) 1 (1.5%) 2 (2.8%) 1 (1.4%) 5 (6.8%) Dyspnea (shortness of breath) 2 (2.8%) 3 (4.2%) 3 (4.2%) 2 (2.7%) Cough 1 (1.4%) 3 (4.2%) 3 (4.1%) COPD worsening 2 (2.8%) 1 (1.4%) 2 (2.7%) Back pain 1 (1.4%) 2 (2.8%) 1 (1.4%) Oropharyngeal pain 1 (1.4%) 1 (1.5%) 2 (2.7%) Minimal reports of anti-cholinergic side effects |
|
|
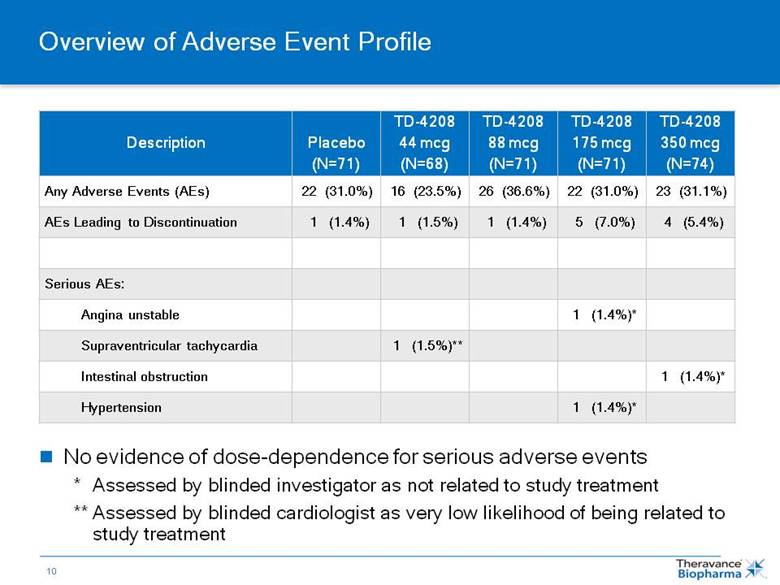
Overview of Adverse Event Profile Description Placebo (N=71) TD-4208 44 mcg (N=68) TD-4208 88 mcg (N=71) TD-4208 175 mcg (N=71) TD-4208 350 mcg (N=74) Any Adverse Events (AEs) 22 (31.0%) 16 (23.5%) 26 (36.6%) 22 (31.0%) 23 (31.1%) AEs Leading to Discontinuation 1 (1.4%) 1 (1.5%) 1 (1.4%) 5 (7.0%) 4 (5.4%) Serious AEs: Angina unstable 1 (1.4%)* Supraventricular tachycardia 1 (1.5%)** Intestinal obstruction 1 (1.4%)* Hypertension 1 (1.4%)* No evidence of dose-dependence for serious adverse events ** Assessed by blinded investigator as not related to study treatment ** Assessed by blinded cardiologist as very low likelihood of being related to study treatment |
|
|
No clinically significant abnormalities in any laboratory parameters No clinically significant changes in vital signs No clinically significant change in ECG heart rate or QTcF interval No Clinically Significant Findings in Other Safety Measures |
|
|
TD-4208 Phase 2b Study Summary Met primary and secondary efficacy endpoints at doses of 88 mcg and above Achieved objective of demonstrating sub-therapeutic dose of 44 mcg TD-4208 generally well tolerated TD-4208 demonstrated significant bronchodilation over 24 hours Results demonstrate TD-4208 potential to be best-in-class single agent product for COPD patients on nebulized therapy |