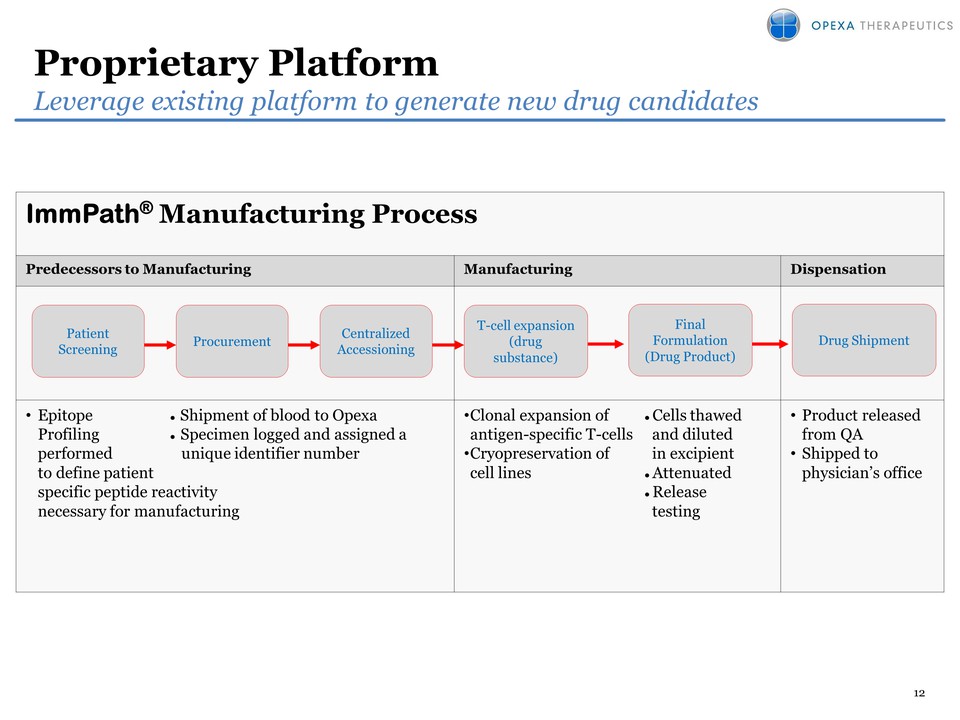
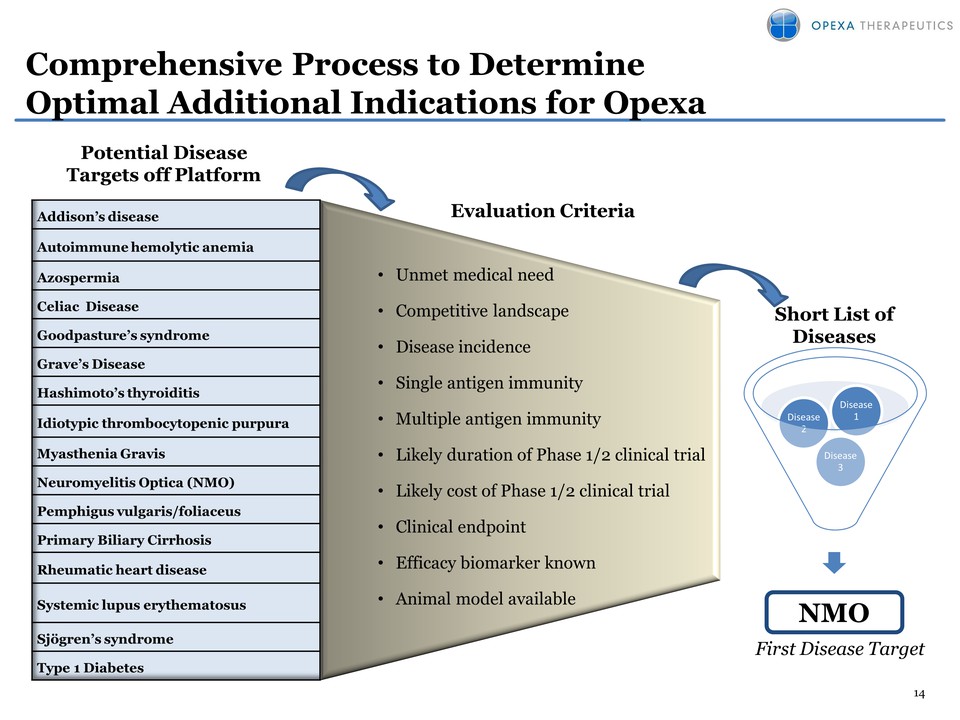
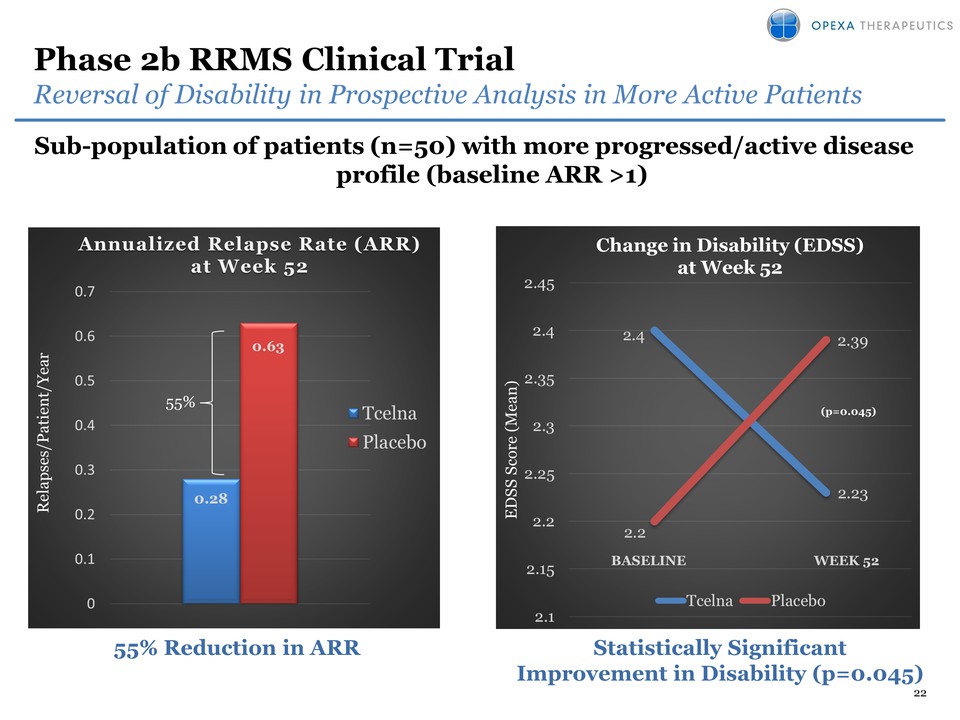
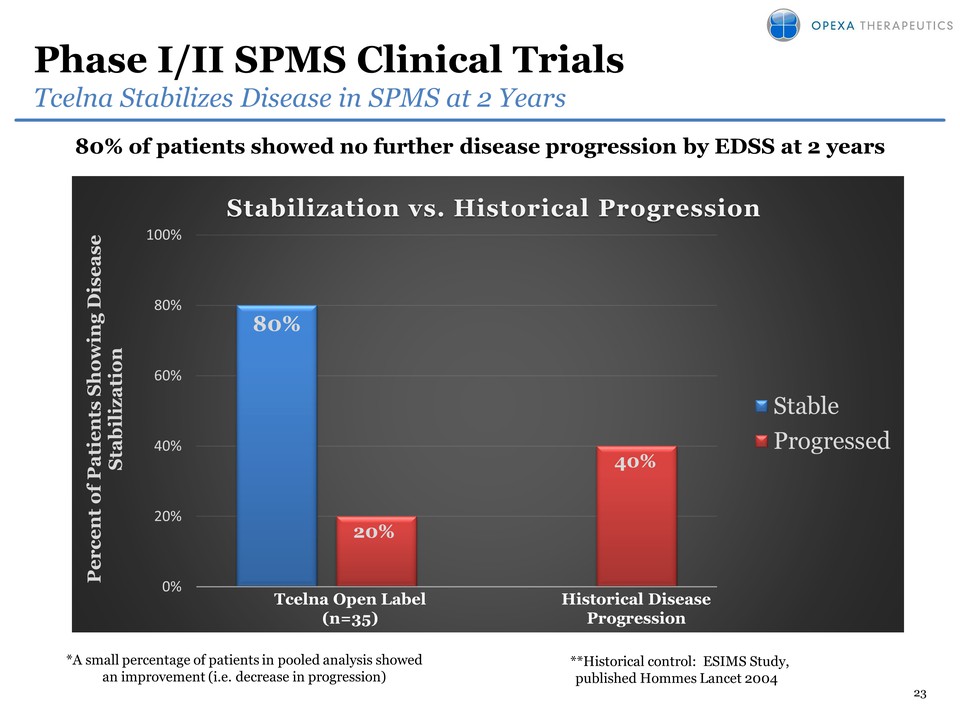
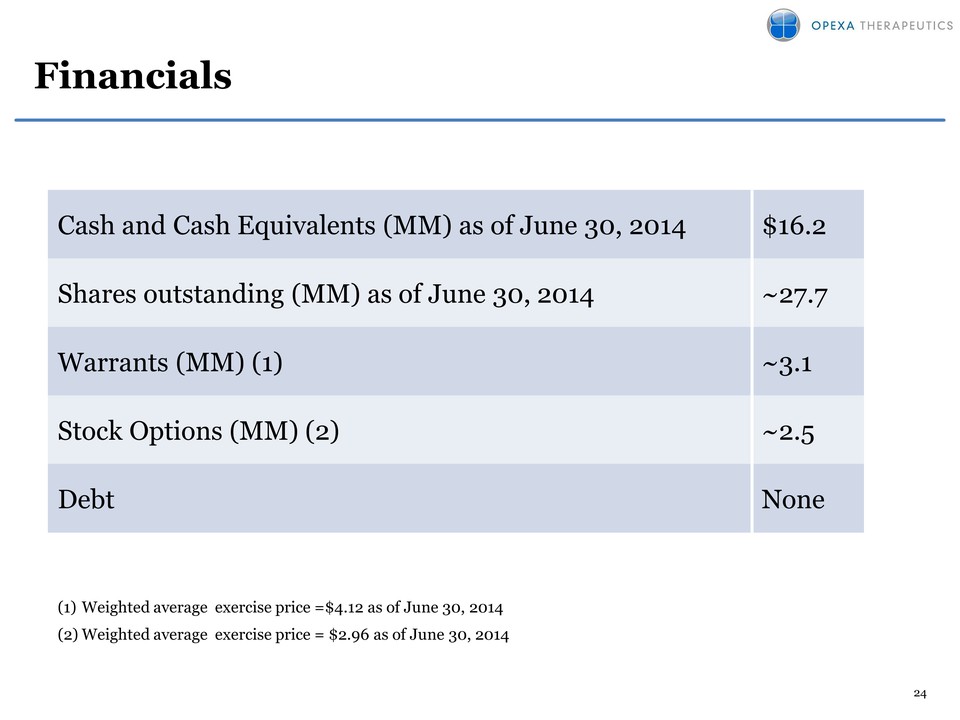
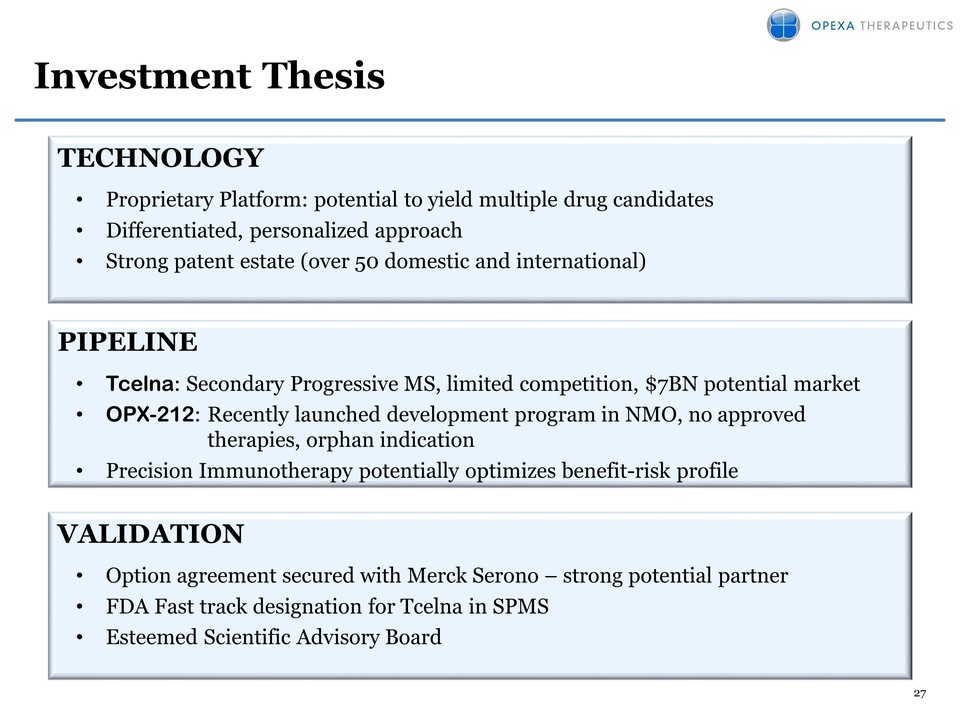
Attached files
| file | filename |
|---|---|
| 8-K - OPEXA THERAPEUTICS, INC. 8-K - Acer Therapeutics Inc. | a50937548.htm |
| EX-99.1 - EXHIBIT 99.1 - Acer Therapeutics Inc. | a50937548ex99_1.htm |
Exhibit 99.2

Opexa Therapeutics, Inc.
NASDAQ: OPXA Precision Immunotherapy September 2014 The Woodlands, TX
Precision Immunotherapy TM

2 Forward-Looking
Statements All statements in this presentation other than those of
historical fact,including statements regarding our preclinical and
clinical development plans for Tcelna® and OPX 212, our research and
other development programs, our ability to undertake certain activities
and accomplish certain goals, projected timelines for our research and
development activities and possible regulatory approvals, if any, our
expectations regarding the relative benefits of our product candidates
versus competitive therapies, our expectations regarding the possibility
of licensing or collaborating with third parties regarding our product
candidates or research, and our expectations regarding the therapeutic
and commercial potential of our product candidates, research,
technologies and intellectual property, are forward-looking statements.
The words “believe,” “may,” “will,” “estimate,” “continue,”
“anticipate,” “design,” “intend,” “expect,” “potential” and similar
expressions, as well as the negative version of these words and similar
expressions, are intended to identify forward-looking statements. Our
forward-looking statements do not constitute guarantees of future
performance, and are subject to a number of risks and uncertainties that
could cause actual results to differ materially and adversely from those
anticipated or implied in such statements. Our forward-looking
statements are based upon our current expectations and involve
assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from
those anticipated as a result of various risks and uncertainties which
include, without limitation, risks associated with the process of
discovering, developing and commercializing drugs that are safe and
effective for use as human therapeutics and risks inherent in the effort
to build a business around such drugs. Although we believe our
expectations are reasonable, we do not in any way guarantee future
results, level of activity, performance or achievements. In addition,
neither we nor any other person assumes responsibility for the accuracy
and completeness of any forward-looking statements. Our forward-looking
statements in this presentation speak only as of the date this
presentation is actually delivered by us in person. We assume no
obligation or undertaking to update or revise any statements to reflect
any changes in our expectations or any change in events, conditions or
circumstances on which any such statement is based. You should, however,
review additional disclosures we make that further describe risks and
uncertainties relevant to us in additional detail in our filings with
the Securities and Exchange Commission. You may get these documents for
free by visiting EDGAR on the SEC web site at http://www.sec.gov.
3 Opexa Key Investment
Highlights • Personalized T-cell immunotherapy platform – Autologous
cell therapy – Potential to address multiple therapeutic areas – Strong
Patent Estate: 50 patents issued on T-cell platform (domestic and
international) • Lead Indication: Multiple Sclerosis – Ongoing Phase 2b
trial in Secondary Progressive Multiple Sclerosis (SPMS) – Potential
SPMS market in North America alone could exceed $7 Billion – Fast Track
designation from the U. S. FDA for the treatment of SPMS – Option
Agreement with Merck Serono, a strong potential commercial partner •
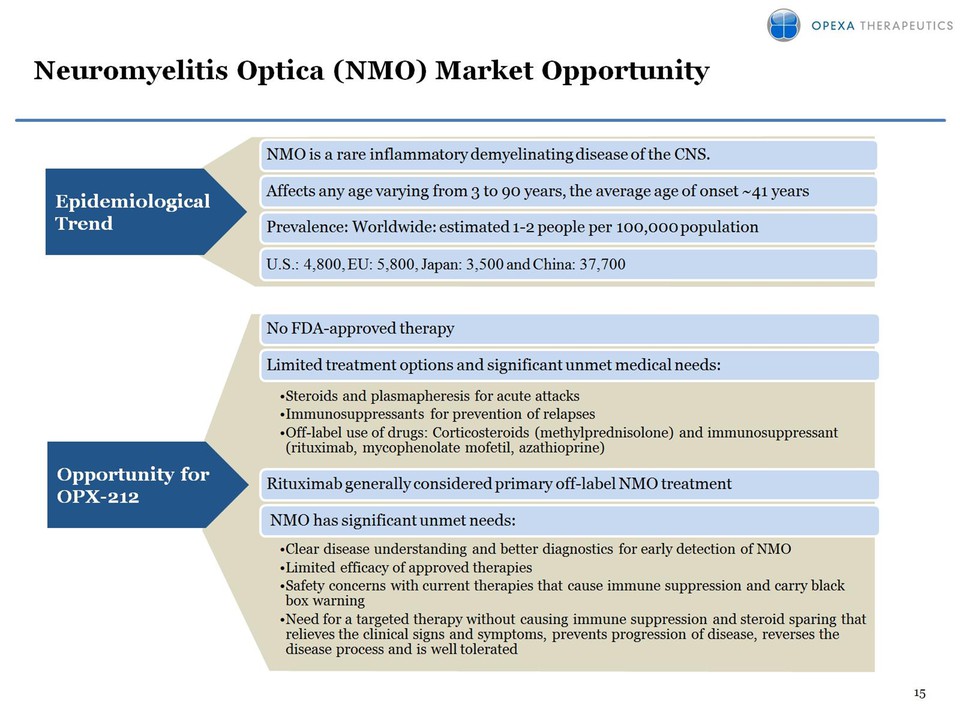
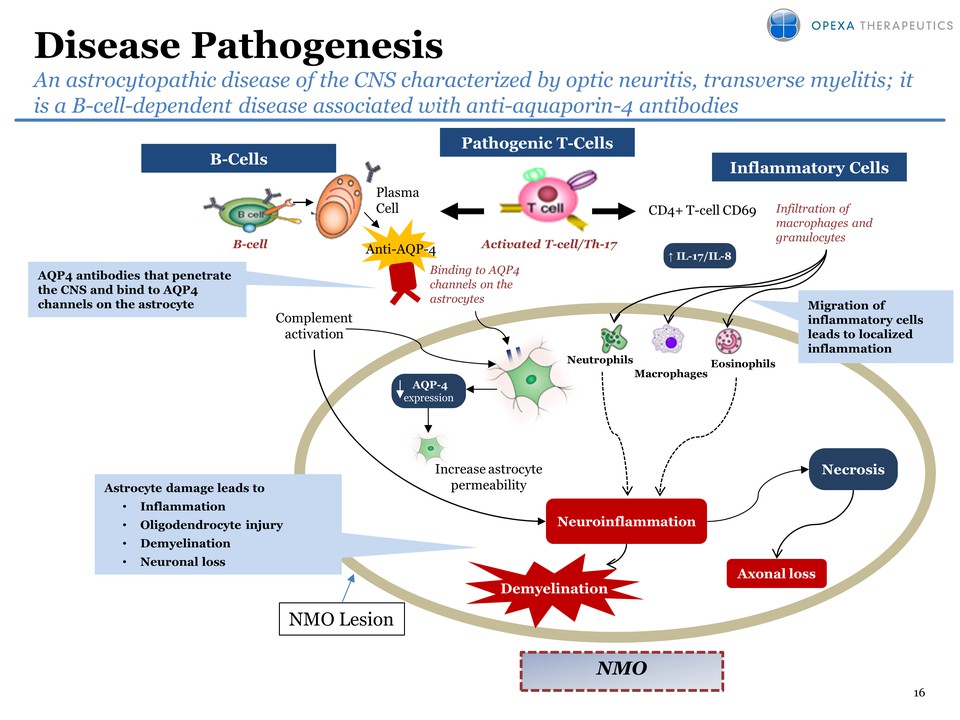
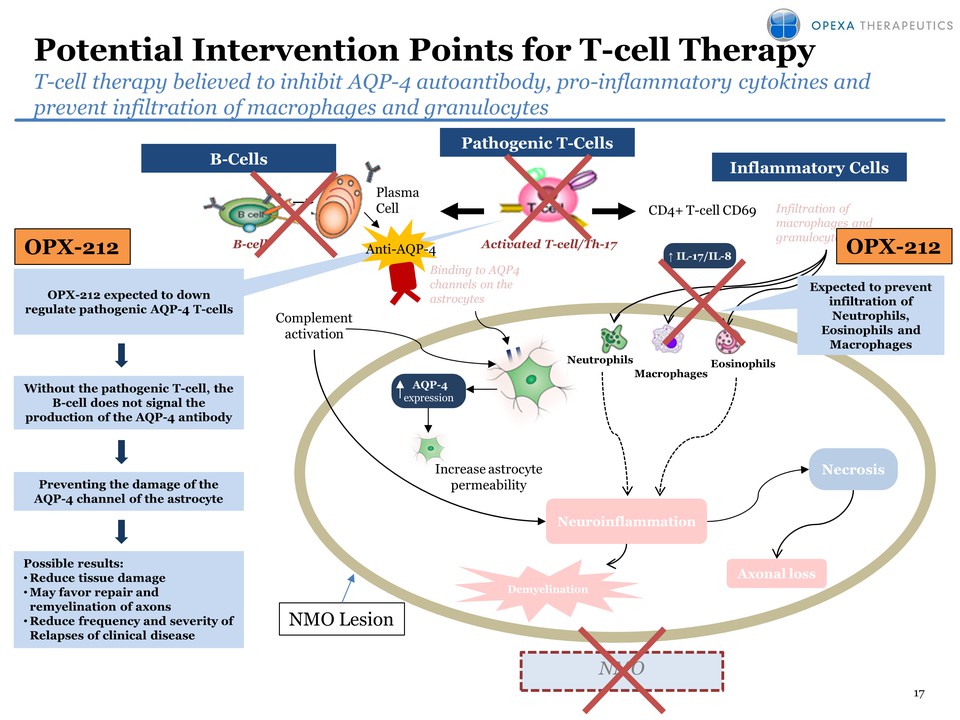
Pipeline (platform) expansion: OPX-212 for Neuromyelitis Optica (NMO) –
No FDA approved drugs for the treatment of NMO – Identified target
antigen: Aquaporin-4 – NMO is classified as an orphan disease
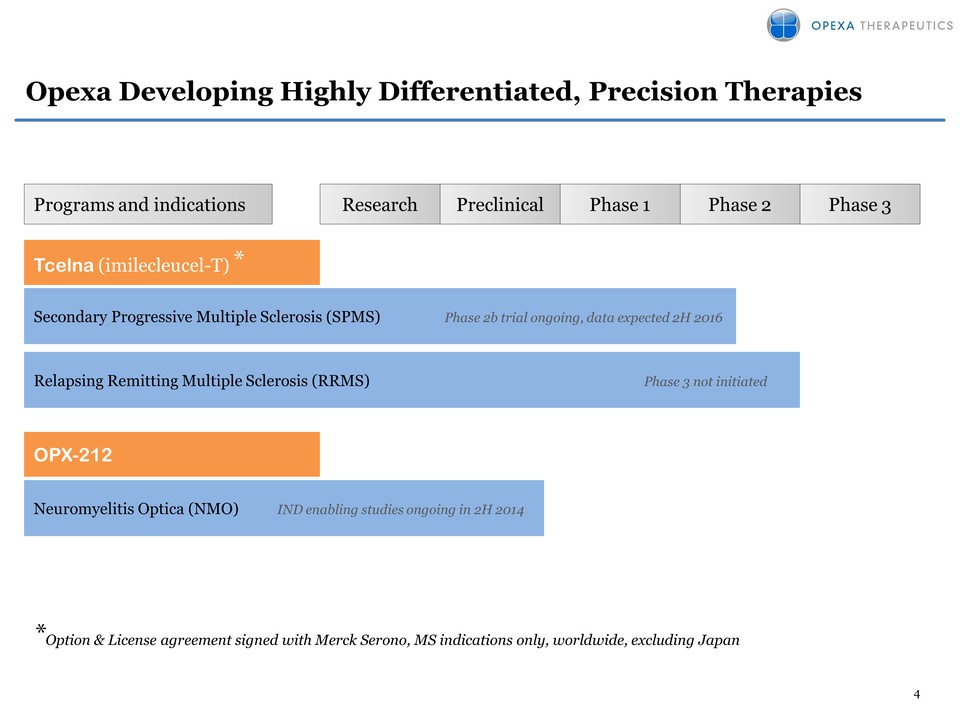
4 Opexa Developing Highly
Differentiated, Precision Therapies OPX-212 Preclinical Phase 1 Phase 2
Phase 3 Programs and indications Neuromyelitis Optica (NMO) IND enabling
studies ongoing in 2H 2014 Tcelna (imilecleucel-T) * Relapsing Remitting
Multiple Sclerosis (RRMS) Phase 3 not initiated *Option & License
agreement signed with Merck Serono, MS indications only, worldwide,
excluding Japan ResearchSecondary Progressive Multiple Sclerosis (SPMS)
Phase 2b trial ongoing, data expected 2H 2016

5 Recent and Upcoming
Expected Milestones 1H 2014: Completed Enrollment in Phase 2b SPMS trial
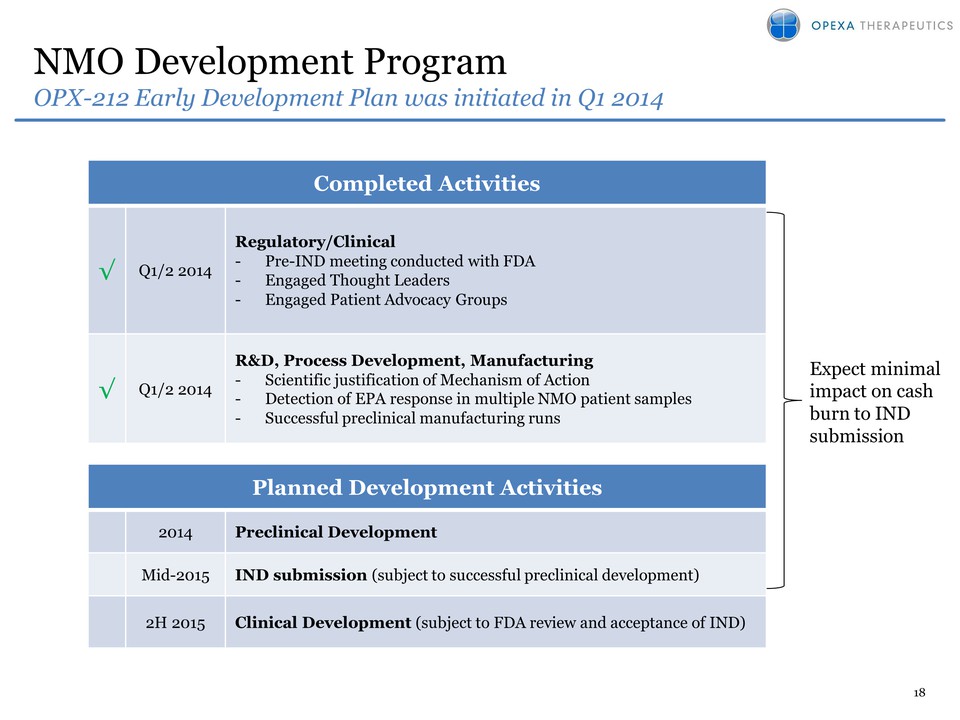
1H 2014: Initiated Early Development Plan for second indication (NMO)•
2H 2014: Advance Preclinical Development in NMO• Mid- 2015: Submit IND
for OPX-212 in NMO to FDA• 2H 2015: Initiation of Clinical Development
in NMO (assuming IND accepted)• 2H 2015: Apply for Orphan Designation
and Fast Track for OPX-212 in NMO• 2H 2016: Top line results for Abili-T
Phase 2b SPMS trial• 2H 2016: Completion and unblinding of Immune
Monitoring data

TCELNA® (imilecleucel-T) MULTIPLE SCLEROSIS 6
7 Tcelna®Lead Program
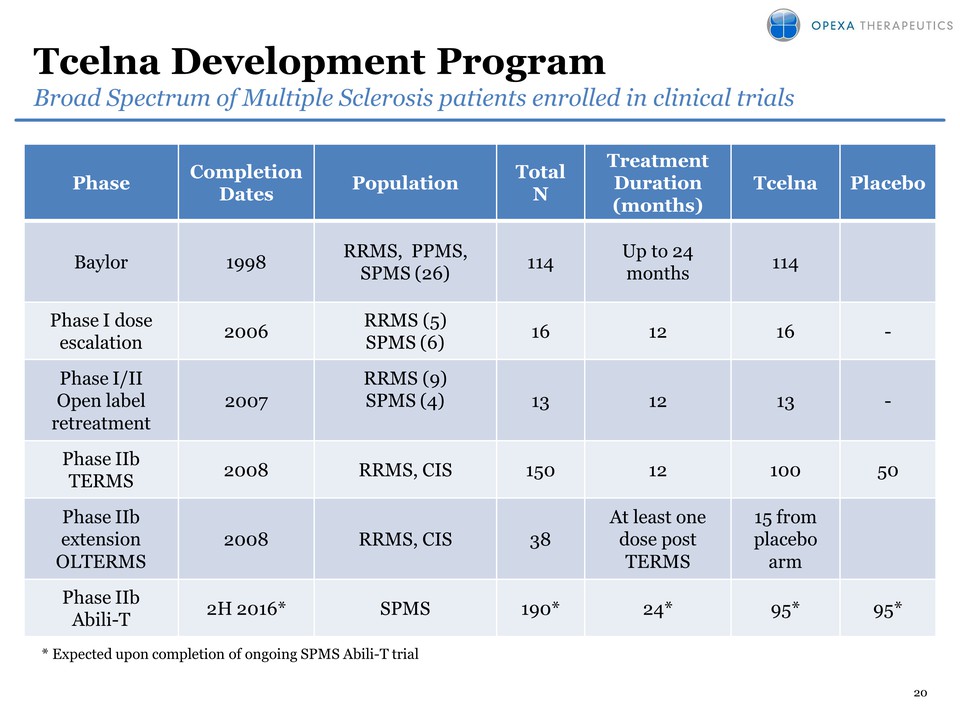
Targeting Secondary Progressive MS patientsFast Track Designation by FDA
• Phase 2b clinical trial ongoing• Trial is fully enrolled: 190 patients
with SPMS• Top line data expected in 2H 2016• Design– Double-blind, 1:1
randomized, placebo-controlled– 35 clinical sites in USA and Canada– Two
annual courses of personalized therapy– Efficacy Endpoints:
Primary-Whole Brain Atrophy, Secondary-Disease Progression• Immune
Monitoring being conducted in parallel– Comprehensive biomarker analysis
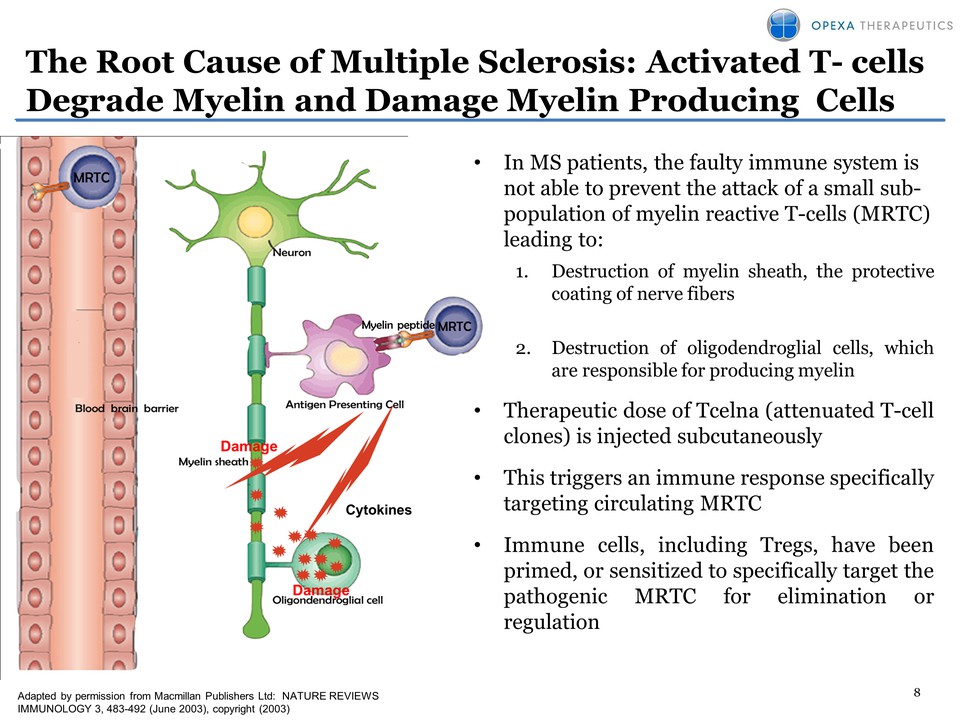
8 The Root Cause of
Multiple Sclerosis: Activated T- cells Degrade Myelin and Damage Myelin
Producing CellsAdapted by permission from Macmillan Publishers Ltd:
NATURE REVIEWS IMMUNOLOGY 3, 483-492 (June 2003), copyright (2003)
Cytokines Damage Damage• In MS patients, the faulty immune system is not
able to prevent the attack of a small subpopulation of myelin reactive
T-cells (MRTC) leading to: 1. Destruction of myelin sheath, the
protectivecoating of nerve fibers 2. Destruction of oligodendroglial
cells, which are responsible for producing myelin• Therapeutic dose of
Tcelna (attenuated T-cell clones) is injected subcutaneously • This
triggers an immune response specifically targeting circulating MRTC •
Immune cells, including Tregs, have been primed, or sensitized to
specifically target the pathogenic MRTC for elimination or regulation
Myelin peptide
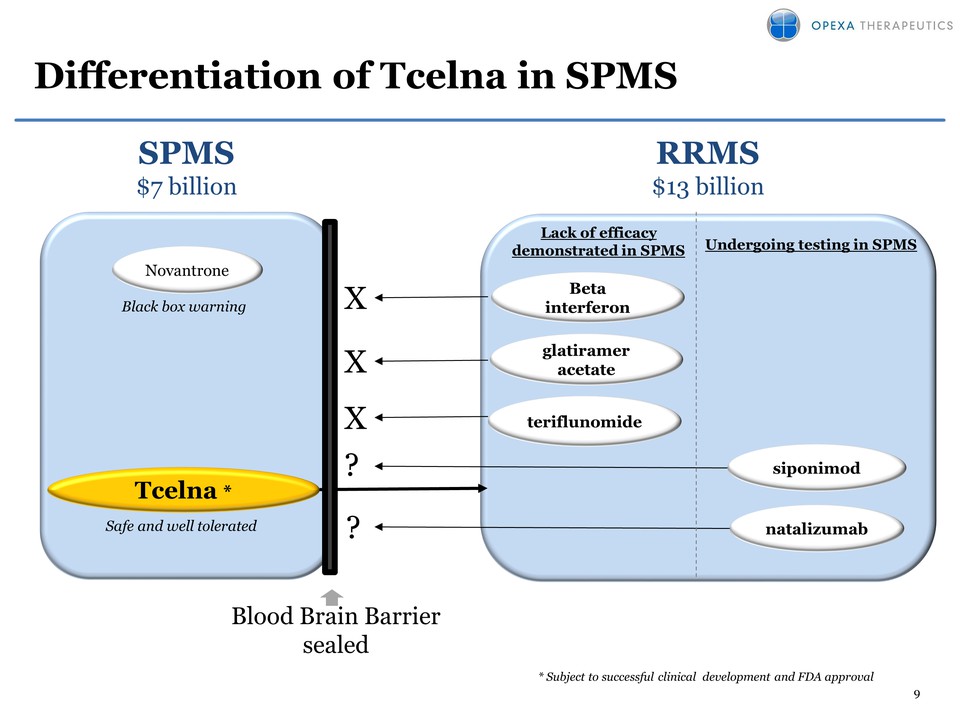
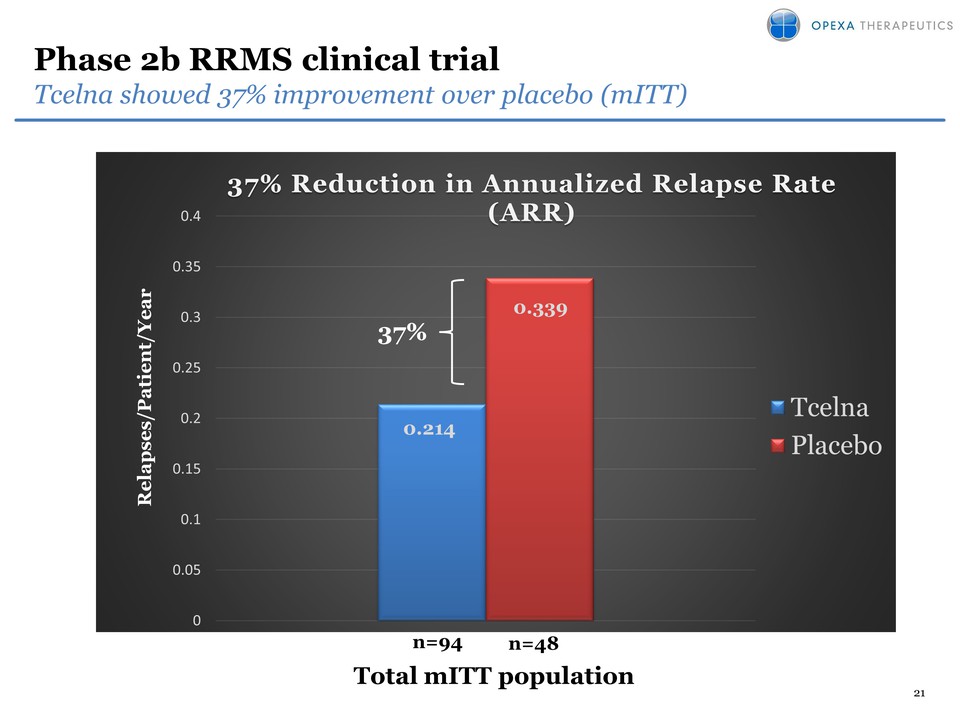
9 Differentiation of Tcelna
in SPMS SPMS $7 billion? RRMS $13 billion ?* glatiramer acetate
Novantrone Blood Brain Barrier sealed natalizumab
Betainterferon teriflunomide X Safe and well tolerated siponimod *
Subject to successful clinical development and FDA approval X Lack of
efficacy demonstrated in SPMS Undergoing testing in SPMS X Black box
warning

10 Merck Serono Agreement
signed 2013; strong potential partner Option and License Agreement for
worldwide rights to all Multiple Sclerosis indications, excluding Japan
• If Merck Serono exercises option: – Merck Serono to fund Phase 3,
pre-commercial and commercial activities – Merck Serono obtains rights
to develop Tcelna for all MS indications – Worldwide rights excluding
Japan • Opexa received $5 million upfront option fee at signing • Opexa
has potential to receive additional $220 million in milestone payments,
and • Royalties ranging from 8% to 15% of annual net sales with step-ups
occurring if net sales exceed $500 million, $1 B & $2 B • Opexa
maintains key rights – Development and commercialization rights to
Tcelna in Japan – Certain manufacturing rights – Co-development funding
option in exchange for increased royalties – Rights to all other disease
indications