Attached files
| file | filename |
|---|---|
| EX-3.2 - EX-3.2 - ENDOSTIM, INC. | d718829dex32.htm |
| EX-3.1 - EX-3.1 - ENDOSTIM, INC. | d718829dex31.htm |
| EX-10.8 - EX-10.8 - ENDOSTIM, INC. | d718829dex108.htm |
| EX-10.4 - EX-10.4 - ENDOSTIM, INC. | d718829dex104.htm |
| EX-23.1 - EX-23.1 - ENDOSTIM, INC. | d718829dex231.htm |
| EX-10.1 - EX-10.1 - ENDOSTIM, INC. | d718829dex101.htm |
| EX-10.9 - EX-10.9 - ENDOSTIM, INC. | d718829dex109.htm |
| EX-10.7 - EX-10.7 - ENDOSTIM, INC. | d718829dex107.htm |
| EX-10.5 - EX-10.5 - ENDOSTIM, INC. | d718829dex105.htm |
| EX-10.16 - EX-10.16 - ENDOSTIM, INC. | d718829dex1016.htm |
| EX-10.19 - EX-10.19 - ENDOSTIM, INC. | d718829dex1019.htm |
| EX-10.14 - EX-10.14 - ENDOSTIM, INC. | d718829dex1014.htm |
| EX-10.13 - EX-10.13 - ENDOSTIM, INC. | d718829dex1013.htm |
| EX-10.18 - EX-10.18 - ENDOSTIM, INC. | d718829dex1018.htm |
| EX-10.15 - EX-10.15 - ENDOSTIM, INC. | d718829dex1015.htm |
| EX-10.10 - EX-10.10 - ENDOSTIM, INC. | d718829dex1010.htm |
| EX-10.17 - EX-10.17 - ENDOSTIM, INC. | d718829dex1017.htm |
| EX-4.2 - EX-4.2 - ENDOSTIM, INC. | d718829dex42.htm |
Table of Contents
As filed with the Securities and Exchange Commission on September 5, 2014.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
EndoStim, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 3845 | 27-0373496 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
4041 Forest Park Avenue, Suite 220
St. Louis, Missouri 63108
Tel: (314) 615-6345
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Bevil J. Hogg
President and Chief Executive Officer
EndoStim, Inc.
4041 Forest Park Avenue, Suite 220
St. Louis, Missouri 63108
Tel: (314) 615-6345
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| James L. Nouss, Jr. Robert J. Endicott Bryan Cave LLP One Metropolitan Square 211 North Broadway, Suite 3600 St. Louis, Missouri 63102 (314) 259-2000 |
Steven M. Skolnick Lowenstein Sandler LLP 1251 Avenue of the Americas New York, New York 10020 (646) 414-6947 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | x (Do not check if a smaller reporting company) | Smaller reporting company | ¨ |
CALCULATION OF REGISTRATION FEE
|
| ||||
| TITLE OF EACH CLASS OF SECURITIES TO BE REGISTERED |
PROPOSED MAXIMUM AGGREGATE OFFERING PRICE (1) |
AMOUNT OF REGISTRATION FEE (2) | ||
| Common Stock, $0.001 par value per share |
$40,250,000 | $5,184.20 | ||
|
| ||||
|
| ||||
| (1) | Estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended, and includes the offering price of shares of common stock that the underwriters have an option to purchase to cover over-allotments, if any. |
| (2) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS, SUBJECT TO COMPLETION, DATED SEPTEMBER 5, 2014
PROSPECTUS
Shares

Common Stock
This is the initial public offering of EndoStim, Inc. We are selling shares of our common stock. We anticipate that the initial public offering price of our shares of common stock will be between $ and $ per share.
After the pricing of the offering, we expect that the shares will trade on The NASDAQ Capital Market under the symbol “STIM.”
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 10 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012 and will be subject to reduced public company reporting requirements. See “Prospectus Summary — Implications of Being an Emerging Growth Company.”
| Per Share |
Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions (1) |
$ | $ | ||||||
| Proceeds to us, before expenses |
$ | $ | ||||||
| (1) | See “Underwriting” for additional disclosure regarding underwriting discounts, commissions and estimated offering expenses. |
We have granted a 30-day option to the representative of the underwriters to purchase up to additional shares of common stock solely to cover over-allotments, if any.
The underwriters expect to deliver our shares to purchasers in the offering on or about , 2014.
Wedbush PacGrow Life Sciences
| Craig-Hallum Capital Group | Roth Capital Partners |
The date of this prospectus is , 2014.
Table of Contents
| PAGE | ||||
| 1 | ||||
| 10 | ||||
| Special Note Regarding Forward-Looking Statements and Industry Data |
43 | |||
| 45 | ||||
| 46 | ||||
| 47 | ||||
| 49 | ||||
| 51 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
52 | |||
| 69 | ||||
| 100 | ||||
| 108 | ||||
| 120 | ||||
| 124 | ||||
| 128 | ||||
| 133 | ||||
| Material U.S. Federal Income Tax Consequences to Non-U.S. Holders |
135 | |||
| 138 | ||||
| 143 | ||||
| 143 | ||||
| 144 | ||||
| F-2 | ||||
You should rely only on the information contained in this prospectus and any free writing prospectus prepared by or on behalf of us or to which we have referred you. We have not, and the underwriters have not, authorized anyone to provide you with information that is different. We are offering to sell shares of our common stock, and seeking offers to buy shares of our common stock, only in jurisdictions where offers and sales are permitted. The information in this prospectus is complete and accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or any sale of shares of our common stock.
For investors outside the United States: neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of shares of our common stock and the distribution of this prospectus outside the United States.
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “EndoStim,” “the company,” “we,” “us,” “our” and similar references refer to EndoStim, Inc. and its subsidiary. We own various U.S. federal trademark registrations and applications, and unregistered trademarks and servicemarks, including EndoStim™ and our corporate logo. All other trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
i
Table of Contents
The following summary highlights information contained elsewhere in this prospectus and is qualified in its entirety by the more detailed information and financial statements included elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our common stock. Before you decide to invest in our common stock, you should read and carefully consider the following summary together with the entire prospectus, including our financial statements and the related notes thereto appearing elsewhere in this prospectus and the matters discussed in the sections in this prospectus entitled “Risk Factors,” “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Some of the statements in this prospectus constitute forward-looking statements that involve risks and uncertainties. See “Special Note Regarding Forward-Looking Statements and Industry Data.” Our actual results could differ materially from those anticipated in such forward-looking statements as a result of certain factors, including those discussed in the “Risk Factors” and other sections of this prospectus.
Overview
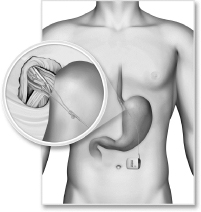
We are a medical device company focused on the development and commercialization of a novel neurostimulation system for the treatment of severe gastroesophageal reflux disease, or GERD. Severe GERD is a debilitating condition that significantly disrupts patients’ quality of life, work productivity and sleep. Based on several international third party studies, we believe the impact of severe GERD on quality of life can be worse than that of chronic back pain or urinary incontinence, two other chronic conditions that are successfully treated with neurostimulation. Moreover, the impact of severe GERD on work productivity has been shown to be significantly greater than that attributable to back pain. Our innovative approach targets the main cause of GERD — a dysfunctional lower esophageal sphincter, or LES, a muscle at the junction of the esophagus and stomach. The EndoStim neurostimulation system, which has a form and function similar to a pacemaker, delivers low energy electrical stimulation to the LES. In our clinical trials to date, this stimulation has been shown to improve the tone and function of the LES, thereby significantly reducing the pathological reflux symptoms of GERD. Our laparoscopically implantable neurostimulation system is minimally-invasive, reversible and preserves the anatomy of the esophagus and stomach.
GERD Therapy Gap
In patients with GERD, a dysfunctional LES allows gastric contents, including stomach acid and bile, to pass inappropriately from the stomach into the esophagus. As a result, patients often suffer from heartburn, regurgitation, chest pain, and difficulty swallowing, among other symptoms, which can severely impact their quality of life. In more serious cases, GERD can result in a precancerous condition called “Barrett’s esophagus” that can lead to esophageal cancer. Patients who suffer from daily GERD symptoms experience a sevenfold increase in the risk of esophageal cancer, for which the World Health Organization reported 456,000 new cases worldwide in 2012.
Today, GERD is most often treated with chronic medication, specifically proton pump inhibitors, or PPIs, such as Nexium and Prilosec, which suppress the production of acid in the stomach. However, PPIs do not address the main cause of GERD, a dysfunctional LES. Therefore, PPIs do not prevent the regurgitation of stomach contents into the esophagus. As a result, 38% of patients taking PPIs to treat GERD reported continuing symptoms that were not resolved by their medication, according to a 2010 study of over 1,000 patients by the American Gastroenterological Association. We expect to initially target a subset of these patients who experience severe symptoms while on medication, and whose GERD can be confirmed by an esophageal acid test. We estimate this subset to comprise approximately 4.6% of all GERD patients, or about 21 million patients worldwide.
The traditional alternative for patients with continuing symptoms on PPIs has been fundoplication, a surgical procedure where the top of the stomach is wrapped and secured around the lower esophagus to augment the LES
1
Table of Contents
in order to create a mechanical barrier to reflux. This is a highly invasive procedure which permanently changes the anatomy, can result in a number of side effects and is difficult to correct or reverse. As a result of these concerns, fewer than 1% of patients who are unsatisfied with PPIs are treated with fundoplication.
Several competing mechanical devices have been developed and evaluated in clinical trials in attempts to address this therapy gap. These include the LINX Reflux Management System (Torax Medical), EsophyX (EndoGastric Solutions) and Stretta (Mederi Therapeutics). Use of these devices, which typically employ mechanical constriction or thermal ablation of the anatomy to restrict the migration of stomach contents into the esophagus, have either shown low efficacy in suppressing esophageal acid or have led to unwanted side effects such as difficulty swallowing. We believe that our therapy offers superior clinical efficacy in controlling esophageal acid and also offers a better safety and side effect profile by preserving normal anatomy and swallow function.
Our Solution
Unlike fundoplication and other mechanical interventions, our treatment does not interfere with the natural LES relaxation which is necessary to allow for normal LES functions, such as swallowing and belching. We believe that our neurostimulation system, when compared with fundoplication and other available implantable devices, offers the following advantages:
| • | targets the main cause of GERD by restoring the natural functioning of a dysfunctional LES; |
| • | is effective in alleviating patient symptoms and reducing or eliminating daily PPI use; |
| • | is minimally-invasive and reversible; |
| • | has minimal side effects and adverse events based on available clinical data; |
| • | is CE marked for use with MRI procedures for head and limb imaging and is potentially compatible with 3T MRI systems for full body exposure; and |
| • | has the ability to be wirelessly customized at post procedure follow-ups to accommodate a patient’s individual lifestyle, responses to treatment, and personal preferences. |
Although our solution is novel for the treatment of GERD, neurostimulation has been used successfully over many years for the treatment of a wide variety of diseases, such as lower back pain, incontinence, various heart conditions and epilepsy.
Regulatory Approval and Commercialization
In 2012, we received CE Mark regulatory approval to commercialize our neurostimulation system in Europe and in other countries recognizing the CE Mark. We launched our system commercially in late 2013. More than 40 sites in Germany, Switzerland, Argentina and Colombia have obtained access to third-party reimbursement for the procedure. We currently use a direct sales force in key European countries and specialized distributors in other European countries, South America and Asia to commercialize our products.
In the United States, our device has not been approved for sale by the FDA. However, we have received approval from the FDA for our Investigational Device Exemption, or IDE, application, which allows us to conduct a pivotal clinical trial in the United States. The trial design is double-blind sham-controlled, which means that neither the investigator nor the patient knows whether or not the patient implanted with the device is actually receiving stimulation therapy. This guards against a “sham” or “placebo” effect where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment, or indirectly as a result of the investigator’s behavior. The clinical trial endpoints which the FDA will use to evaluate the effects of our neurostimulation treatment, are similar to the endpoints of our prior overseas clinical work. Most importantly, in our prior long-term clinical trials, we have consistently demonstrated the positive effect of our treatment on acid
2
Table of Contents
exposure to the esophagus. This is the outcome required by the FDA as the primary efficacy endpoint of our United States pivotal trial.
The FDA has recommended a minimum of 100 patients to be treated for one year; therefore, we plan to enroll a minimum of 110 patients to allow for dropouts. Based on our own prior open-label clinical results and published sham responses from other sham-controlled trials for GERD devices, we believe we can achieve a successful outcome with 100 completed patients. However, we have the ability to increase the total number of patients in the United States trial up to 200 if we believe it is necessary to successfully meet the primary endpoint. Provided our United States clinical trial is successful, we do not believe there would be any material hurdles to receiving FDA approval that would permit us to sell our neurostimulation system in the United States.
Clinical Trials
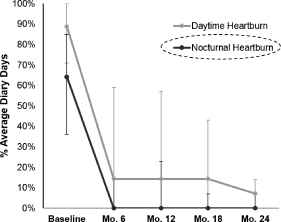
To date, over 130 patients have been implanted with our system, 65 of whom have been treated in open-label clinical trials. These clinical trials have shown promising outcomes with respect to the safety and clinical efficacy of our technology as measured by objective and subjective criteria described under “— Clinical Trials.” The results of these clinical trials have been published in peer-reviewed medical journals. The trials have been conducted in Europe, South America and Asia with a follow-up period of up to three years. A total of 39 patients in these trials have been treated for more than one year, of whom:
| • | 72% achieved successful control of objectively measured esophageal acid exposure, considered the “gold standard” within our industry in evaluating GERD therapies, at their last follow-up (where “success” is defined using FDA criteria of normalization of values or a decrease of more than 50% from a baseline); |
| • | 85% reported clinically significant improvement in symptoms, as evidenced by 50% or more improvement in GERD-HRQL score (a standard, internationally validated questionnaire-based measure of GERD-related quality of life), at their last follow-up; and |
| • | 97% were able to discontinue regular PPI use at their one-year evaluation (regular PPI use is defined as use of PPIs during 50% or more of diary days). |
For a more detailed description of these results, please refer to “Business — Clinical Trials — Summary of Findings” below. Although these trials have shown promising results, they were open-label studies performed at a limited number of clinical sites on a limited number of patients who were followed for a period of up to 36 months. Open-label trials are subject to various limitations that may exaggerate any therapeutic effect, including the “sham” or “placebo” effect described above under “— Regulatory Approval and Commercialization.” Moreover, these patients likely included a high number of severe sufferers, and their symptoms may have been bound to improve notwithstanding treatment with our neurostimulation system. Please refer to “Risk Factors — We have collected limited clinical data about the efficacy and safety of our neurostimulation system and may be unable to reproduce historical clinical results in large-scale and double-blind controlled clinical trials.” To partially mitigate the placebo effect in our open-label trials, we included the measurement of esophageal acid exposure described in the first bullet above, which provides a more objective measure of reflux.
Our Strategy
Our goal is to establish our neurostimulation system as the leading therapy for treatment of severe GERD. Currently, our target market is 21 million patients worldwide, a subset of the patients in the GERD “therapy gap.” The key components of our strategy include the following:
| • | we intend to drive sales growth primarily in countries where the marketing of our neurostimulation system is approved and where third-party reimbursement is available; |
3
Table of Contents
| • | we aim to expand regulatory approval and third-party reimbursement to additional countries worldwide; |
| • | we intend to seek FDA regulatory approval and third-party reimbursement to commercialize our system in the United States; and |
| • | we intend to broaden our customer base by developing new applications for, and enhancements to, our therapy. This is expected to include the development of an endoscopic approach to the delivery of our leads and electrodes, as well as the potential expansion of our therapy to other sphincter disorders. |
Our Risks and History of Losses
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. These risks are discussed more fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. These risks include, but are not limited to, the following:
| • | we have a history of losses and expect to continue losses in the future, and we may never generate enough revenue from the commercialization of our neurostimulation system to achieve or sustain profitability; |
| • | we will likely need to raise additional capital in the future to fund our operations, and we may not be able to do so on favorable terms or at all; |
| • | our neurostimulation system may never achieve broad market acceptance, and may not be commercially successful; |
| • | lack of third-party coverage and reimbursement for our neurostimulation system could delay or limit the adoption of our neurostimulation system; |
| • | while we have CE Mark approval, we do not have, and may never receive, approval to market our neurostimulation system in the United States; |
| • | our neurostimulation system has only been used in 130 patients worldwide and, while it has been shown to be safe, there is no certainty that this will continue to be the case; and |
| • | we may be unable to adequately protect our intellectual property, or our intellectual property may infringe the intellectual property rights of third parties, either of which could adversely affect our business. |
We have incurred net losses since our inception in 2009, including net losses of $7.5 million and $5.5 million before deduction for the accretion of redeemable preferred stock and undeclared dividends on Series A convertible preferred stock for the years ended December 31, 2013 and 2012, respectively, and $3.6 million for the six months ended June 30, 2014. As of June 30, 2014, our accumulated deficit was approximately $29.5 million. Due to the uncertainty of our ability to meet our current operating and capital expenses, our independent auditors included an explanatory paragraph regarding concerns about our ability to continue as a going concern in their report on our audited annual financial statements as of and for the year ended December 31, 2013.
Our Corporate Information
We were incorporated under the laws of the State of Delaware in 2009. Our principal executive offices are located at 4041 Forest Park Avenue, Suite 220, St. Louis, Missouri 63108, and our telephone number is (314) 615-6345. Our website address is http://www.endostim.com. Our website and the information contained on, or that can be accessed through, the website will not be deemed to be incorporated by reference in, and are not considered part of, this prospectus. You should not rely on any such information in making your decision whether to purchase our common stock.
4
Table of Contents
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
| • | a requirement to have only two years of audited financial statements and only two years of related management’s discussion and analysis; |
| • | an exemption from compliance with the auditor attestation requirement on the effectiveness of our internal controls over financial reporting; |
| • | an exemption from compliance with any requirement that the Public Company Accounting Oversight Board may adopt regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; |
| • | reduced disclosure about the company’s executive compensation arrangements; and |
| • | exemptions from the requirements to obtain a non-binding advisory vote on executive compensation or a stockholder approval of any golden parachute arrangements. |
We may take advantage of these provisions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenues, have more than $700 million in market value of our capital stock held by non-affiliates, or issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some, but not all, of the available benefits under the JOBS Act. We have taken advantage of some reduced reporting burdens in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to delay the adoption of some accounting standards until those standards would otherwise apply to private companies. We have elected to avail ourselves of delayed adoption of new or revised accounting standards and, therefore, our financial statements may not be comparable to the financial statements of other public companies.
5
Table of Contents
THE OFFERING SUMMARY
| Common stock to be offered by us |
shares |
| Common stock to be outstanding immediately following this offering |
shares |
| Over-allotment option |
We have granted the underwriters an option for 30 days from the date of this prospectus to purchase up to additional shares of common stock to cover over-allotments. |
| Use of proceeds |
We estimate that the net proceeds from this offering will be approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
| We currently expect to use the net proceeds from this offering for the following purposes: |
| • | approximately $ for commercialization activities; |
| • | approximately $ for clinical development activities, most importantly our United States pivotal trial to support a PMA filing with the FDA; and |
| • | any remaining proceeds for working capital and general corporate purposes. |
| See “Use of Proceeds” for a more complete description of the intended use of proceeds from this offering. |
| Risk factors |
You should read the “Risk Factors” section of this prospectus for a discussion of factors to carefully consider before deciding to invest in shares of our common stock. |
| Proposed NASDAQ Capital Market symbol |
“STIM” |
The number of shares of our common stock outstanding immediately following this offering set forth above is based on shares of our common stock outstanding as of June 30, 2014, and excludes the following:
| • | 1,335,946 shares of our common stock issuable upon the exercise of outstanding stock options as of June 30, 2014, at a weighted average price of $0.25 per share; |
| • | 889,453 shares of our common stock issuable upon the exercise of outstanding warrants as of June 30, 2014, of which warrants to purchase 550,000 shares have an exercise price of $0.01 per share, and warrants to purchase 339,453 shares have an exercise price of $0.50 per share; |
| • | shares of our common stock reserved for issuance pursuant to future awards under our 2014 Stock Incentive Plan, including 140,333 shares of our common stock reserved for issuance under our 2009 Stock Incentive Plan as of June 30, 2014, which will become available for issuance under our 2014 Stock Incentive Plan following the consummation of this offering. |
6
Table of Contents
Except as otherwise indicated, all information in this prospectus assumes:
| • | the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 11,719,975 shares of common stock, the conversion of which will occur immediately prior to the completion of this offering; |
| • | the automatic conversion of the principal and accrued interest on the approximately $6.4 million aggregate principal amount of convertible promissory notes issued in June 2014 and the approximately $1.1 million aggregate principal amount of convertible promissory notes issued in August 2014 into an aggregate of shares of common stock, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering; |
| • | the filing and effectiveness of our amended and restated certificate of incorporation in Delaware and the adoption of our amended and restated bylaws, each of which will occur immediately prior to the completion of this offering; and |
| • | no exercise by the underwriters of their option to purchase up to an additional shares of common stock from us in this offering. |
7
Table of Contents
SUMMARY FINANCIAL DATA
The following table summarizes our financial data. We have derived the following statements of operations data for the years ended December 31, 2013 and December 31, 2012 from our audited financial statements, included elsewhere in this prospectus. We have derived the statements of operations data for the six months ended June 30, 2014 and 2013 and the balance sheet data as of June 30, 2014 from our unaudited financial statements, included elsewhere in this prospectus. Our historical results are not necessarily indicative of results to be expected for any period in the future. The summary financial data presented below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes thereto, included elsewhere in this prospectus. The summary financial data in this section is not intended to replace our financial statements and the related notes thereto.
| Six months ended June 30, | Fiscal year ended December 31, | |||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| Consolidated statement of operations data: |
||||||||||||||||
| Revenue |
$ | 324,837 | $ | 106,466 | $ | 301,432 | $ | 33,350 | ||||||||
| Cost of revenue |
151,496 | 62,995 | 194,911 | 23,064 | ||||||||||||
| Gross margin |
173,341 | 43,471 | 106,521 | 10,286 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
1,256,966 | 1,337,758 | 3,195,979 | 2,613,429 | ||||||||||||
| Sales and marketing |
1,392,508 | 1,056,177 | 2,232,514 | 1,025,364 | ||||||||||||
| General and administrative |
1,125,466 | 1,055,172 | 2,211,222 | 1,910,423 | ||||||||||||
| Total operating expenses |
3,774,940 | 3,449,107 | 7,639,715 | 5,549,216 | ||||||||||||
| Loss from operations |
(3,601,599 | ) | (3,405,636 | ) | (7,533,194 | ) | (5,538,930 | ) | ||||||||
| Other (expense) income |
(20,184 | ) | 3,206 | 5,962 | 1,857 | |||||||||||
| Net loss |
(3,621,783 | ) | (3,402,430 | ) | (7,527,232 | ) | (5,537,073 | ) | ||||||||
| Accretion of redeemable preferred stock |
(1,247,583 | ) | (908,142 | ) | (1,884,822 | ) | (853,561 | ) | ||||||||
| Undeclared dividends on Series A convertible preferred stock |
(81,580 | ) | (23,562 | ) | (47,125 | ) | (71,446 | ) | ||||||||
| Net loss attributable to common stockholders |
$ | (4,950,946 | ) | $ | (4,334,134 | ) | $ | (9,459,179 | ) | $ | (6,462,080 | ) | ||||
| Basic and diluted net loss per common share attributable to common stockholders |
$ | (3.53 | ) | $ | (3.09 | ) | $ | (6.74 | ) | $ | (4.71 | ) | ||||
| Shares used in computing basic and diluted net loss per common share attributable to common shareholders |
1,403,721 | 1,403,721 | 1,403,721 | 1,371,863 | ||||||||||||
| As of June 30, 2014 | ||||||||
| Actual | As adjusted (1) | |||||||
| Consolidated balance sheet data: |
||||||||
| Cash and cash equivalents |
$ | 7,732,992 | $ | |||||
| Working capital |
6,659,531 | |||||||
| Total assets |
9,667,914 | |||||||
| Note payable |
4,345,281 | |||||||
| Embedded derivative |
1,651,084 | |||||||
| Deferred revenue |
277,179 | |||||||
| Total stockholders’ equity (deficit) |
(27,460,161 | ) | ||||||
| (1) | The “As adjusted” column reflects (i) the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 11,719,975 shares of common stock, the conversion of which will occur |
8
Table of Contents
| immediately prior to the completion of this offering, (ii) the automatic conversion of the principal and accrued interest on the approximately $6.4 million aggregate principal amount of convertible promissory notes issued in June 2014 and the approximately $1.1 million aggregate principal amount of convertible promissory notes issued in August 2014 into an aggregate of shares of common stock and (iii) the sale by us of $ of shares of our common stock in this offering after deducting the underwriting discount and estimated offering expenses payable by us, in each case assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering. |
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, working capital, total assets and total stockholders’ (deficit) equity by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) each of cash and cash equivalents, working capital, total assets and total stockholders’ (deficit) equity by approximately $ million, assuming that the assumed initial public offering price, which is the midpoint of the price range set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The “As adjusted” information discussed above is illustrative only and will adjust based on the actual initial public offering price and other terms of this offering determined at pricing.
9
Table of Contents
Before you invest in our common stock, you should understand the high degree of risk involved. You should carefully consider the following risks and other information in this prospectus, including our consolidated financial statements and related notes included elsewhere in this prospectus, before you decide to purchase shares of our common stock. The following risks may adversely impact our business and financial condition. As a result, the trading price of our common stock could decline and you could lose part or all of your investment.
Risks Related to Our Financial Condition and Capital Requirements
We have limited operating history, which may make it difficult to evaluate our current business and to forecast our future performance.
We have little operating history and are addressing an emerging market. As a result, our current and future business prospects are difficult to evaluate. All potential investors must consider our business prospects in light of the risks and difficulties we have encountered and will continue to encounter as a startup company in a rapidly evolving market. Some of these risks relate to our potential inability to:
| • | effectively manage our business and technology; |
| • | recruit and retain sales and marketing, scientific, technical and managerial personnel; |
| • | obtain required regulatory approvals and approvals from governmental and private insurers and medical expense reimbursement providers; |
| • | develop a safe and effective product; |
| • | recruit and retain sales personnel and appropriate distributor relationships; |
| • | successfully develop and protect our intellectual property portfolio; |
| • | successfully provide high levels of service as our business expands; and |
| • | successfully address other risks, as described in this prospectus or otherwise. |
If we do not address these risks successfully, it could have a material adverse effect on our business and financial condition.
We have a history of net losses. We expect to continue to incur net losses in the future and we may never generate sufficient revenue from the commercialization of our neurostimulation system to achieve or sustain profitability.
We have incurred net losses since our inception in 2009, including net losses of $7.5 million and $5.5 million before deduction for the accretion of redeemable preferred stock and undeclared dividends on Series A convertible preferred stock for the years ended December 31, 2013 and 2012, respectively, and $3.6 million for the six months ended June 30, 2014. As of June 30, 2014, our accumulated deficit was approximately $29.5 million. Although we have received CE Mark regulatory approval to commercialize our neurostimulation system in Europe and those countries worldwide that recognize the CE Mark, we expect to incur significant sales and marketing expenses prior to recording sufficient revenue to offset these expenses. In the United States, we expect to incur substantial additional losses as a result of the costs associated with our pivotal clinical trial, continued research and development, and, following the offering, costs associated with operating as a public company. Development of a new medical device, including conducting clinical trials and seeking regulatory approvals, is a long, expensive and uncertain process, and we expect that our expenses will increase as we seek regulatory approval for our neurostimulation system in the United States.
These losses have had, and will continue to have an adverse effect on our working capital, total assets and stockholders’ equity (deficit). Our ability to become and remain profitable will depend on our ability to generate significantly higher product sales outside the United States based on our CE Mark approval, which depends upon a number of factors, including successful sales, manufacturing, marketing and distribution of our products.
10
Table of Contents
Because of the numerous risks and uncertainties associated with our commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then sustain profitability would have a material adverse effect on our business and financial condition.
We will need to raise additional capital to fund our existing operations, commercialize our technology and expand our operations.
We believe that the net proceeds of this offering, together with our existing cash balances, will be sufficient to cover our cash needs for the next months. If our available cash balances, net proceeds from this offering, and anticipated cash flow from operations are lower than we anticipate, including due to changes in our business plan, a lower demand for our products or other risks described in this prospectus, we may seek to raise additional capital sooner than currently expected.
We may also consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities or for other reasons, including to:
| • | fund development of our products; |
| • | acquire, license or invest in technologies or intellectual property relating to our existing technology; |
| • | acquire or invest in complementary businesses or assets; and |
| • | finance capital expenditures and general and administrative expenses. |
Our present and future funding requirements will depend on many factors, including:
| • | success of our current commercialization efforts outside of the United States; |
| • | our revenue growth rate and ability to generate cash flows from product sales; |
| • | our research and product development activities, particularly those related to obtaining regulatory approval for our neurostimulation system in the United States; |
| • | effects of competing technological and market developments; |
| • | changes in regulatory oversight applicable to our products. |
The various alternatives for raising additional capital include short-term or long-term debt financings, equity offerings, collaborations or licensing arrangements and each one carries potential risks. If we raise funds by issuing equity securities, our stockholders will be further diluted. Any equity securities issued also could provide for rights, preferences or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, those debt securities would have rights, preferences and privileges senior to those of holders of our common stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations or our ability to issue additional equity securities or issue additional indebtedness. We may also be required under additional debt financing to grant security interests on our assets, including our intellectual property. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our neurostimulation system, or grant licenses on terms that are not favorable to us which could lower the economic value of those programs to us. The credit markets and the financial services industry have previously experienced unprecedented turmoil and upheaval characterized by the bankruptcy, failure, collapse, or sale of various financial institutions and an unprecedented level of intervention from the U.S. federal government. These events have generally made equity and debt financing more difficult to obtain. Accordingly, additional equity or debt financing might not be available on reasonable terms, if at all. If we cannot secure additional funding when needed, including due to changes in our business plan, a lower demand for our products or other risks described in this prospectus, we may have to delay, reduce the scope of, or eliminate one or more sales and marketing initiatives, clinical trials or other research and development programs all of which would have a materially adverse effect on our business.
11
Table of Contents
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to annual limitations on its ability to use its pre-change net operating loss carryforwards, or NOLs, or other tax attributes to offset future taxable income or reduce taxes. Our past issuances of stock and other changes in our stock ownership may have resulted in ownership changes within the meaning of Section 382 of the Code; accordingly, our pre-change NOLs may be subject to limitation under Section 382. State NOL carryforwards may be similarly limited. Furthermore, the closing of this offering, alone or together with transactions in our stock that have occurred in the past and may occur in the future, may trigger an ownership change pursuant to Section 382. Because of the cost and complexity involved in the analysis of a Section 382 ownership change and the fact that we do not have any taxable income to offset, we have not undertaken a study to assess whether an “ownership change” has occurred or whether there have been multiple ownership changes since we became a “loss corporation” as defined in Section 382. Shares issued in this offering or future changes in our stock ownership (either in combination with this offering or separately) could result in ownership changes under Section 382 of the Code further limiting our ability to utilize our NOLs. Furthermore, our ability to use NOLs of companies that we may acquire in the future may be subject to limitations. For these reasons, even if we attain profitability, we may not be able to use a material portion of our NOLs, and this would reduce our earnings and the valuation of our stock.
Our independent auditor’s report for the fiscal year ended December 31, 2013 includes an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern.
Due to the uncertainty of our ability to meet our current operating and capital expenses, in their report on our audited annual financial statements as of and for the year ended December 31, 2013, our independent auditors included an explanatory paragraph regarding concerns about our ability to continue as a going concern. Recurring losses from operations raise substantial doubt about our ability to continue as a going concern. If we are unable to continue as a going concern, we might have to liquidate our assets and the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements. In addition, the inclusion of an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern and our lack of cash resources may materially adversely affect our share price and our ability to raise new capital or to enter into critical contractual relations with third parties.
Risks Relating to Our Business
We operate in a very competitive business environment and if we are unable to compete successfully against existing GERD treatments, as well as future treatments, our sales and operating results may be negatively affected and we may not grow.
The health care industry is highly competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. Some of the competitive factors impacting success in our markets include the features and capabilities of product offerings, product safety, availability of compelling clinical data, recommendations of key opinion leaders and professional medical societies, competitive pricing, favorable reimbursement, strong customer service and continued product enhancements.
We are aware of several companies offering competitive medical device solutions for treating GERD and many of these companies already have FDA approval to sell their products in the United States. Such competitors include Torax Medical and EndoGastric Solutions. Other therapeutic devices for treating GERD are under development. In addition, some of our targeted patients may opt to undergo the Stretta Procedure, an outpatient endoscopic approach that delivers radio frequency ablation to the LES in an attempt to strengthen the LES. Our current and potential competitors may have significant competitive advantages over us, including:
| • | substantially greater financial, technical and other resources; |
| • | extensive intellectual property portfolios and greater resources for patent protection; |
12
Table of Contents
| • | current FDA and other regulatory clearances and approvals for their products; |
| • | greater experience in obtaining and maintaining FDA and other regulatory clearances and approvals for products and product enhancements; |
| • | greater experience in launching, marketing, distributing and selling products, including strong sales forces and established distribution networks; |
| • | established manufacturing operations and contract manufacturing relationships; |
| • | established relationships with healthcare providers and payors; |
| • | significantly longer operating history, more established reputations and widely recognized trademarks; |
| • | endoscopic rather than laparoscopic delivery of their therapeutic solution resulting in lower procedure cost and greater patient acceptance; and |
| • | more favorable pricing. |
We expect that the medical device market will continue to develop rapidly, and our future success will depend on our ability to develop and maintain a competitive position. Our competitors may succeed in developing products that render our neurostimulation system obsolete or noncompetitive. Additionally, developers of PPIs have developed and will continue to innovate and develop new pharmaceuticals that may satisfactorily address the symptoms of GERD. Further, we will face competition from existing surgical procedures, such as fundoplication.
We cannot be certain that our neurostimulation system will be able to achieve broad market acceptance, replace established treatments, or that physicians or the medical community will accept and utilize our products over competing devices, including those that are being developed. If physicians, surgeons and patients prefer the use of our competitors’ medical devices or current treatments, our ability to generate revenue will be significantly impaired which would have a material adverse effect on our business and financial condition.
Our current neurostimulation system may never find broad market acceptance and become commercially successful.
Our neurostimulation system is commercially available only in a limited number of countries and will not be available in other countries, including the United States, until clinical development is completed and regulatory approvals are obtained. These may never occur. The successful commercialization of our products will require significant, time-consuming and costly sales and marketing efforts. If the commercialization of our current neurostimulation system is unsuccessful or we are unable to market our neurostimulation system due to market developments, failure to obtain and maintain regulatory approvals, development of alternative treatments or otherwise, we will be required to expend significant additional resources on research and development to improve our neurostimulation system. A part of our business strategy is to expand the addressable patient market, and we may be unable to do so. The development of a new device will be subject to the risks of failure inherent in the creation of any innovative new medical technology. These risks include the possibilities that our device will not be effective or of acceptable quality, will fail to receive necessary regulatory clearances, will be uneconomical to manufacture or market or does not achieve broad market acceptance, and that third parties market a superior or equivalent product. Even if our product is effective, it may not be accepted by patients, who may find our neurostimulation system too harsh for an implanted device. The failure of our research and development activities to result in any commercially viable products would have a material adverse effect on our business and financial condition.
We have limited marketing and sales resources and personnel and have limited experience with the distributors on which we will depend to sell our products.
We have limited experience in sales, marketing and distribution of our products and are just beginning the process of developing a sales and marketing organization, including by establishment of a distributor network. Our lack of experience and/or limited resources could negatively impact our ability to enter into or maintain
13
Table of Contents
collaborative arrangements or other third party relationships which are important to the successful commercialization of our products and potential profitability. We may be unable to establish or maintain adequate sales and distribution capabilities.
In some countries we intend to market directly to customers through our own sales personnel. As a result, we will need to attract, train and retain sufficient personnel to maintain an effective sales and marketing force. Developing a sales force is expensive and time consuming, and our sales force will be competing with the experienced and well-funded marketing and sales organizations of our more established competitors. Also, we may not be able to recruit and retain skilled sales, marketing, service or support personnel with industry expertise. Even if we are successful in developing high-performing sales representatives with strong customer relationships, new or existing competitors may target our representatives in their recruitment efforts. If such representatives are recruited by our competitors, our sales could be materially adversely impacted.
In some countries we will rely on third party distributors to market and sell our products. In these markets, our profit margins will likely be lower than if we sold directly to customers ourselves. In addition, we would necessarily be relying on the skills and efforts of others for the successful marketing of our product.
If we are unable to establish and maintain effective sales and marketing capabilities, independently or with others, we may not be able to generate product revenue and may not become profitable. If we are unable to adequately address our customers’ needs, it could negatively impact sales and market acceptance of our products and we may not be able to generate significant revenue and may not become profitable.
We have collected limited clinical data about the efficacy and safety of our neurostimulation system and may be unable to reproduce historical clinical results in large-scale and double-blind controlled clinical trials.
Although the clinical trials performed to date using our neurostimulation system have shown promising results, these results were generated from open-label studies performed at a limited number of clinical sites on a limited number of patients over 36 months. Open-label trials are subject to various limitations that may exaggerate any therapeutic effect. Patients in open-label studies are aware that they are receiving treatment. Therefore, open-label trials may be subject to a “sham-effect” where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment. Patients selected for early clinical studies often include the most severe sufferers and their symptoms may have been bound to improve notwithstanding the new treatment. Finally, those assessing and reviewing the physiological outcomes of the clinical trials are aware which patients have received treatment and may interpret the information of the treated group more favorably given this knowledge. Our first clinical trial was completed in Chile. Patients in Chile are genetically similar to European and North American patients, but there may be unidentified genetic differences that may result in variable therapeutic response in patients in other countries. Our initial safety profile has been favorable, but safety could be dependent on operator skills. It is possible that we may experience a higher rate of adverse events in the future with wider application of our technology in real-world practice outside of clinical trials. Finally, GERD is a disease that is affected by patients’ diet and lifestyle and hence variation in these factors among different regions of the world may result in variation in observed therapeutic response to our therapy. The conclusive evidence of efficacy and magnitude of therapeutic effect of our therapy can only be established in a well-designed, randomized sham-controlled trial where patients are randomly selected to receive either electrical stimulation or no stimulation. We have never conducted such a clinical trial. If our planned pivotal trial fails to demonstrate sufficient efficacy of electrostimulation therapy over a non-active (sham) device implant, we may be unable to obtain regulatory approval and/or reimbursement coverage to successfully commercialize our neurostimulation system in the United States. Our commercialization efforts elsewhere in the world would also likely suffer due to reduced physician and patient acceptance of our neurostimulation system outside the United States based on studies conducted in the United States causing our financial results to be materially adversely effected.
14
Table of Contents
Any clinical trials that we may conduct may not begin on time, or at all, may not be completed on schedule, or at all, or may be more expensive that we expect, which could prevent or delay regulatory approval of our products or impair our financial position.
The commencement or completion of any clinical trials that we may conduct may be delayed or halted for numerous reasons, including, but not limited to, the following:
| • | the FDA or other regulatory authorities suspend or place on hold a clinical trial, or do not approve a clinical trial protocol or a clinical trial; |
| • | the data and safety monitoring committee or applicable hospital institutional ethics review board recommends that a trial be placed on hold or suspended; |
| • | fewer patients meet our clinical study criteria and our enrollment rate is lower than we expected; |
| • | patients do not return for follow-up as expected; |
| • | clinical trial sites decide not to participate or cease participation in a clinical trial; |
| • | patients experience adverse side effects or events related to our neurostimulation system or for unrelated reasons; |
| • | third-party clinical investigators do not perform our clinical trials on schedule or consistent with the clinical trial protocol and good clinical practices, or other third-party organizations do not perform data collection and analysis in a timely or accurate manner; |
| • | we fail regulatory inspections of our manufacturing facilities requiring us to undertake corrective action or suspend or terminate our clinical trials; |
| • | governmental regulations require additional testing not contemplated in our pivotal trial or implement administrative actions; |
| • | interim results of the clinical trial are inconclusive or negative; |
| • | pre-clinical or clinical data are interpreted by third parties in unanticipated ways; or |
| • | our trial design, although approved, is inadequate to demonstrate safety and/or efficacy. |
Patient enrollment in clinical trials and completion of patient follow-up in clinical trials depend on many factors, including the size of the patient population, the nature of the trial protocol, the proximity of patients to clinical sites and the eligibility criteria for the study and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures to assess the safety and effectiveness of our product candidates, or they may be persuaded to participate in contemporaneous trials of competitive products. Additionally, patients may be unwilling to participate in a trial of a surgical implant procedure that includes the potential for assignment to an arm that has a period of no therapy. Delays in patient enrollment or failure of patients to continue to participate in a study may cause an increase in costs and delays or result in the failure of the trial.
Our clinical trial costs will increase if we have material delays in those trials or if we need to perform more or larger trials than planned. Adverse events during a clinical trial could cause us to repeat a trial, terminate a trial or cancel an entire program. Should our clinical development plan be delayed, this could have a material adverse effect on our operations and financial condition.
15
Table of Contents
The safety of our neurostimulation system is supported by limited clinical data and our product may not be a safe and efficient alternative to existing treatments; unfavorable safety outcomes could lead to product recalls in markets where we are commercializing our products.
We have not yet obtained the endorsement of the relevant professional medical societies in each country in which we intend to commercialize our neurostimulation system and as such, physicians may be slow to incorporate the therapy into their respective treatment guidelines. Although initial clinical experience shows a favorable safety profile, future patient studies or a broader clinical experience may indicate that:
| • | patients may experience side effects during treatment with our neurostimulation system, which may be severe, such as: |
| • | prolonged difficulty in swallowing (dysphagia); |
| • | significant and prolonged discomfort or pain at the implant site; |
| • | system component dislodgement, migration or erosion into unintended areas leading to discomfort, pain, hemorrhage or unintended stimulation of other physiological structures, intestinal obstruction, or severe infections; or |
| • | discomfort from the neurostimulation system being implanted, especially with thinner patients; or |
| • | the neurostimulation system or any of its components could malfunction, causing a loss of therapeutic benefit or serious injuries to the patient, or may result in other unexpected or serious complications. |
Any of these side effects or complications could require surgical removal of the implant, and, in rare instances, could result in death, either from the complications themselves or from surgical implant or removal procedure. Additionally, the effective life of the neurostimulation system may be shorter than anticipated which would cause the neurostimulation system to be removed and replaced sooner than anticipated, requiring an unanticipated surgical procedure. Moreover, any such or other unexpected outcomes may also subject us to voluntary or mandatory product recalls, suspension or significant legal liability, or lead to withdrawal of any regulatory approvals that may be obtained. The FDA and other governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture or in the event that a product poses an unacceptable risk to health. Manufacturers may, under their own initiative, recall a product if any material deficiency is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of our neurostimulation system would divert managerial and financial resources, and could impair our ability to produce our neurostimulation system in a cost-effective and timely manner in order to meet our customers’ demands. Recall may be unsuccessful if we are unable to locate patients with an implanted neurostimulation system, potentially increasing our risk of liability. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits. Any of these outcomes could significantly reduce our ability to achieve expected sales and as a result could have a material adverse effect on our business and financial condition.
We face the risk of product liability claims that could be expensive, divert management’s attention and harm our reputation and business. We may not be able to obtain adequate product liability insurance.
Our business exposes us to a risk of product liability claims that is inherent in the testing, manufacturing and marketing of medical devices. The medical device industry has historically been subject to extensive litigation over product liability claims. We may be subject to product liability claims if our products cause, or appear to have caused, an injury. Claims may be made by consumers, governmental authorities, healthcare providers, third-party strategic collaborators or others selling our neurostimulation system.
We maintain product liability insurance which currently covers our neurostimulation system used in our clinical trials and those we commercially sell outside of the United States in an amount that we believe is sufficient for our line of business. However, our current product liability insurance may not continue to be available to us on
16
Table of Contents
acceptable terms, if at all, may not be expanded to cover new markets, and, if available, the coverage may not be adequate to protect us against any future product liability claims. If we are unable to obtain insurance at an acceptable cost and on acceptable terms for an adequate coverage amount, or otherwise to protect against potential product liability claims, we could be exposed to significant liabilities, which may harm our business. A product liability claim, recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse effect on our business, financial condition and results of operations. These liabilities could prevent or interfere with our product commercialization efforts. Defending a suit, regardless of merit, could be costly, could divert management attention and might result in adverse publicity, which could result in the withdrawal of, or inability to recruit, clinical trial volunteers or result in reduced acceptance of our products in the market.
In addition, we offer warranties for our products against defects in materials and workmanship. Significant product warranty claims would lead to unanticipated additional costs and a loss of reputation.
Some physicians and surgeons may have difficulty in procuring or maintaining liability insurance to protect them from liability when treating patients with our neurostimulation system. Medical malpractice carriers are withdrawing coverage in certain states or substantially increasing premiums. If this trend continues or worsens, physicians and surgeons may discontinue using our neurostimulation system or may choose to not purchase our neurostimulation system in the future due to the cost or inability to procure insurance coverage. This would limit the number of physicians using our electrostimulation therapy, reduce our sales and adversely impact our financial condition.
We currently rely on one company outside of the United States to manufacture our neurostimulation system. We may be unable to cost-effectively source our product to meet our quality standards, regulatory requirements and customer demand or we may experience disruptions in the sourcing process.
The manufacture of our neurostimulation system is a complex and costly operation involving a number of separate processes and components. We currently rely on a sole source in Uruguay to manufacture our neurostimulation system. Such reliance results in a number of risks including control over product quality, access to materials or components and the risks of doing business internationally, which may result in an interruption in supply of our products or an increase in our cost of goods. Vandalism, terrorism or a natural or other disaster, such as an earthquake, fire or flood, could damage or destroy the manufacturing equipment related to our neurostimulation system, and could cause substantial delays in our operations. The manufacturer could elect to stop making our product for any reason. If our manufacturer discontinues making our product or experiences delays, disruptions, capacity constraints or quality control problems in its manufacturing operations, becomes insolvent or otherwise fails to supply us with our products in sufficient quantities or on a timely basis, then shipments to us and our customers could be adversely impacted, which could restrict our growth and harm our competitive position and reputation.
The manufacturer was recently acquired by a larger medical device and component company, which may adversely affect our relationship with the manufacturer and may subject us to additional risks. We may now interact with different management and operating personnel. Also, our relationship may not be as important to the manufacturer since it is now part of a much larger company and our business will accordingly account for a smaller portion of the company’s total business. Additionally, the manufacturer may change its business priorities, and we may not be able to negotiate modifications or extensions of our current agreement on favorable pricing or other terms, or at all.
We cannot quickly replace our manufacturer or establish additional new suppliers for our products, particularly due to both the complex nature of the manufacturing process and the time and effort that would be required to establish appropriate quality control systems, and to obtain new regulatory approvals to use materials from alternative suppliers. There are a limited number of suppliers and third-party manufacturers that have the necessary expertise and capacity to manufacture our neurostimulation system and that operate under the FDA’s
17
Table of Contents
current Good Manufacturing Practices, or cGMP, and Quality System Regulation, or QSR, which is a prerequisite for commercialization of our product in the United States. As a result, if it were necessary to terminate our relationship with our existing manufacturer, or if the manufacturer terminates its relationship with any of its suppliers, it may be difficult for us to locate another manufacturer or for the manufacturer to locate other suppliers that could promptly fulfill our anticipated needs. In addition, if we are required to obtain an alternate manufacturer, we may be unable to negotiate an arrangement that is acceptable to us.
Further, because we place our orders based on forecasts of expected demand for our neurostimulation system, if we inaccurately forecast demand, we may be unable to obtain adequate quantities of neurostimulation system as needed by us to meet our customers’ delivery requirements or we may accumulate excess inventory. In determining the required quantities of our product, we must make significant judgments and estimates based on historical experience, inventory levels, current market trends and other related factors. An interruption in our clinical trials, sales and operations would occur if we encounter delays or difficulties in securing our neurostimulation systems from our manufacturer. We may also experience quality problems, substantial costs and unexpected delays in efforts to upgrade and expand our manufacturing, assembly and testing capabilities. Because of the inherent nature of estimates and the numerous uncertainties regarding the manufacturing process may fail to provide our customers with the neurostimulation systems they require, which could harm our business and results of operations.
We have limited manufacturing experience and may encounter difficulties in increasing production to provide an adequate supply to customers.
To date, our manufacturer has produced moderate quantities of our neurostimulation system for use in clinical studies and for initial commercial sales outside the United States. Increasing production to commercial quantities may require changes in the manufacturing processes. Neither we nor our current manufacturer have experience in manufacturing our neurostimulation system on a commercial scale. We do not know of any other contract manufacturers with experience in the production of 10,000 or more implantable neurostimulators per year. As a result, high-volume sourcing of our neurostimulation system may not be available or at prices which are acceptable to us, our customers, third-party payors or privately paying potential patients, and we may be unable to meet the expected future demand for our products.
We may encounter difficulties in increasing our supply due to limited manufacturing capacity and in manufacturing larger commercial quantities, including:
| • | maintaining quality control and assurance; |
| • | providing component and service availability; |
| • | maintaining adequate control policies and procedures; and |
| • | maintaining low cost of goods, either through direct purchase, import/export tariffs or currency exchange rates. |
Difficulties encountered in increasing our required manufacturing volumes or any shipment delays could harm perception of our business and have a material adverse effect on our business and financial condition.
We rely on third parties for various aspects of our business. Any delays or disruptions in the business of such third parties could have a material adverse effect on our business and financial condition.
In addition to our third-party manufacturer in business, we rely on various other suppliers for supplying our neurostimulation system, including individual components of our neurostimulation system, such as batteries. We also rely on third parties in the conduct of our clinical trials. Further, we rely on a single provider of warehousing services in Europe.
18
Table of Contents
Reliance on third parties entails many costs and risks to which we would not be subject if we conducted such operation ourselves, including costs associated with auditing and monitoring the third parties for regulatory and other compliance, the possibility of increases in pricing for our products, the possibility of breach of the applicable agreements by the third parties, third-party credit risk, and the possibility of termination or non-renewal of our agreements with the third parties. If any of these risks materialize, it could significantly increase our costs and negatively impact our ability to meet demand for our neurostimulation system. Further, since we rely on a single provider of warehousing services in Europe, vandalism, terrorism or a natural or other disaster, such as an earthquake, fire or flood, could damage or destroy a significant part of our inventory and could cause substantial delays in our operations.
If our third-party distributors will not perform according to our expectations, we could suffer from decreased sales or other adverse consequences, which could have a material adverse effect on our business and financial condition.
Third-party distributors are crucial for commercialization of our products outside of the United States, and we are subject to particular risks relating to these distributors. Distributors may sell products that compete with our products, and we may need to provide financial and other incentives to focus distributors on the sale of our products. We may rely on one or more key distributors for our neurostimulation system in some countries, and the loss of these distributors could reduce our revenue. Distributors may face financial difficulties, including bankruptcy, which could harm our collection of accounts receivable and financial results. Violations of the Foreign Corrupt Practices Act or similar laws by distributors or other third-party intermediaries could have a material adverse impact on our business. While we expect our distributors and sales representatives to provide services in accordance with our standards and in compliance with applicable laws and regulations, we do not control their efforts or oversee their daily activities. Our distributors and sales representatives may not always act in our best interests or in accordance with applicable laws and regulations. If we fail to manage risks related to our use of distributors it could have a material adverse effect on our business and financial condition.
Low current manufacturing volumes of our products results in high per unit costs, and if we are not able to reduce such costs through higher volumes and economies of scale, we may not become profitable.
The current unit costs for our neurostimulation system, based on limited manufacturing volumes, are high, and it will be necessary to achieve economies of scale to become profitable. Certain of our manufacturing components are expensive and pricing can only drop with economies of scale. Certain manufacturing processes are labor intensive, and achieving significant cost reductions will depend in part upon reducing the time required to complete these processes. If we are unable to achieve cost reductions in the manufacture of our neurostimulation system, our business may never achieve profitability.
The loss of any member of our senior management team or our inability to attract and retain highly skilled personnel could have a material adverse effect on our business.
Our success depends on the skills, experience and performance of key members of our senior management team, including Bevil Hogg, our President and Chief Executive Officer, Shai Policker, our Chief Operating Officer, and Paul Goode, Senior Vice President, Research and Development, as well as V. K. Sharma, our founder and Chief Medical Officer. The individual and collective efforts of our senior management team will be important as we continue to expand our commercial activities and develop additional products. The loss or incapacity of existing members of our senior management team could have a material adverse effect on our business and financial condition if we experience difficulties in hiring qualified successors. Our executive officers have employment agreements; however, the existence of an employment agreement does not guarantee the retention of the executive officer for any period of time. We do not maintain “key person” insurance on any of our employees.
Due to the specialized nature of the business and the small size of our company, we are highly dependent upon our ability to attract and retain qualified sales and marketing, scientific, technical and managerial personnel. The
19
Table of Contents
loss of the services of existing personnel, as well as the failure to recruit key sales, marketing, scientific, technical and managerial personnel in a timely manner would be detrimental to our development and could have a material adverse effect on our business and financial condition. Our anticipated growth and expansion into areas and activities requiring additional expertise, such as sales and marketing, may require the addition of new management personnel, both domestic and international. We also face competition from universities and public and private research institutions in recruiting and retaining highly qualified scientific personnel. All of our employees may terminate their employment at any time with short or no advance notice. We may have difficulties locating, recruiting or retaining qualified sales people. Recruiting and retention difficulties will limit our ability to support our research and development and sales programs and to build a commercially viable business.
Because our management structure is not centralized, the management of our business operations may be more expensive and more difficult.
As part of our strategy to attract the most qualified individuals, we do not require the members of our management team to relocate to a particular geographic area. Accordingly, the members of our management team are geographically dispersed. This decentralized structure might cause additional expenses in the conduct of our business. It might also delay communication between members of our management team, lower the quality of our management decisions or decrease our ability to take action quickly.
A variety of risks associated with international operations could materially adversely affect our business.
Our business strategy involves manufacturing our neurostimulation system exclusively outside of the United States and commercially launching our neurostimulation system in international markets governed by the CE Mark. As such, we will be subject to a number of international difficulties and risks, including:
| • | export restrictions, trade regulations foreign laws and controls relating to technology; |
| • | the availability and level of reimbursement within prevailing foreign healthcare payment systems; |
| • | currency fluctuations impacting both our cost of goods and the price at which we sell our productions when converted to U.S. dollars; |
| • | pricing pressure that we may experience internationally; |
| • | required compliance with existing and changing foreign regulatory requirements and laws; |
| • | laws and business practices favoring local companies; |
| • | longer payment cycles; |
| • | difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
| • | economic and political instability, including the risk of seizure of assets by a foreign government; |
| • | shipping delays; |
| • | potentially adverse tax consequences, tariffs and other trade barriers; |
| • | international terrorism and anti-American sentiment; |
| • | difficulties and costs of staffing and managing any foreign operations and risks from any alleged misclassification of our contractors and employees; and |
| • | difficulties in enforcing intellectual property rights. |
Assuming we receive FDA approval to market our products in the United States, the commercial launch of our neurostimulation system will require significant investment in sales and marketing in the months prior to approval, and therefore creating the need to repatriate cash from our international operations back to the United
20
Table of Contents
States. Laws in foreign countries and in the United States, including United States tax laws, may create, impede or increase our cost to repatriate capital or earnings from our international operations.
Our exposure to each of these risks may increase our costs, impair our ability to market and sell our products and require significant management attention. We cannot assure you that one or more of these factors will not harm our business. Additionally, we are subject to various anti-bribery laws including but not limited to the United States Foreign Corrupt Practices Act. Any violation of these laws by our distributors, our agents or by us could create a substantial liability for us and also cause a loss of reputation in the market. Contracts may be difficult to enforce, receivables may be difficult to collect and employment, distribution, and sales representative and other contracts may be difficult to terminate through a foreign country’s legal system. Our in ability to manage any of these risks may have a material adverse effect on our operations in any particular country and on our business as a whole.
In addition, the current manufacturer of our products is located in Uruguay. Although we may eventually move the production of our products to a manufacturing facility in the United States, until such time as this transfer occurs, any event causing a disruption of imports, including the imposition of import restrictions could have a material adverse effect on our business and financial condition.
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
We anticipate growth in our business operations commensurate with the commercialization of our neurostimulation system outside of the United States. Since our inception in June 2009, we have increased our employees and independent contractors to 23 as of July 31, 2014, and we expect to continue hiring new employees and independent contractors to support our anticipated growth. This future growth could impose significant added responsibilities on members of our existing management and create strain on our organizational, administrative and operational infrastructure, including sales and marketing, quality control, and customer service. Our ability to manage our growth properly will require us to continue to improve our operational, financial, and management controls, as well as our reporting systems and procedures. Our status as a public company will require us to increase our investment in financial accounting and reporting. If our current infrastructure is unable to handle our growth, we may need to expand our infrastructure, to identify and recruit new staff and to implement new reporting systems. The time and resources required to implement such expansion and systems could adversely affect our operations. Our future financial performance and our ability to commercialize our neurostimulation system and to compete effectively will depend, in part, on our ability to manage this potential future growth effectively, without compromising quality.
Risks Related to Government Regulation and Third-Party Reimbursement
We may be unable to maintain or obtain governmental approvals to market our neurostimulation system outside the United States, including the European Union countries.
Any medical device placed on the European market must comply with the relevant legislation of the European Economic Community, or EEC, which requires manufacturers of medical products to obtain the right to affix the CE Mark to their products before selling them in the European Union, the European Economic Area and Switzerland. The CE Mark is an international symbol of adherence to quality assurance standards and compliance with applicable European medical device directives. In order to obtain the right to affix the CE Mark to products, a manufacturer must obtain certification that its processes meet specified quality and design standards. Compliance with the medical device directives, as certified by a recognized European Notified Body, permits the manufacturer to affix the CE Mark on its products and commercially distribute those products throughout the European Union, the European Economic Area and Switzerland. However, individual countries can lawfully request that a medical device be registered locally. Furthermore, states may have requirements in place in relation to the language of the device information, which would require additional compliance, review and approval. Although, we obtained CE Mark approval of our neurostimulation system on August 16, 2012, it is
21
Table of Contents
anticipated that minor revisions to our CE Mark certification will be required to address future improvements and changes to our products. Each of these revisions will be subject to review by the European Notified Body. Although we anticipate that such submissions will be approved in a reasonable period of time, if the approvals are not obtained in a timely matter, commercialization of certain new versions of our neurostimulation system may be delayed. In addition, the European Union is presently assessing an overhaul of its regulatory requirements that may make the CE Mark much more difficult to obtain or maintain.
To be able to market and sell our neurostimulation system in other countries, we must obtain regulatory approvals and comply with the regulations of those countries. These regulations, including the requirements for approvals and the time required for regulatory review, vary from country to country. Many non-European markets, including major markets in South America and Asia Pacific, have allowed for expedited regulatory review and approval based on an existing CE Mark. We intend to target countries with a regulatory approval process with similar requirements to CE Mark. However, obtaining and maintaining foreign regulatory approvals are complex and expensive and subject to delays, and management cannot be certain that we will receive and be able to maintain regulatory approvals in any foreign country in which we plan to market our neurostimulation system or in the time frame in which we expect.
There can be no assurance that any further regulatory approval will be obtained for any products developed by us in the future, including for modifications of the current neurostimulation system. Furthermore, regulatory approval may entail limitations on the indicated use(s) of a device. Because the products likely to result from our continued research and development programs might involve the application of new technologies or a new therapeutic approach, such products may be subject to substantial additional review by various government regulatory authorities and, as a result, regulatory approvals may be obtained more slowly, if at all, than for products based upon more conventional technologies. If we fail to obtain or maintain regulatory approvals in any country in which we plan to market our product, our ability to generate revenue will be harmed.
We have not received, and may never receive, approval from the FDA to market our neurostimulation system in the United States.
Before a new medical device can be marketed in the United States, it must first receive either premarket approval, or a PMA, or 510(k) clearance from the FDA, unless an exemption exists. A PMA submission, which is a higher standard than a 501(k) clearance, is used to demonstrate to the FDA that a new or modified device is safe and effective. The 510(k) is used to demonstrate that a device is “substantially equivalent” to a predicate device (one that has been cleared by the FDA). We are planning to seek premarket approval, a PMA for our product. The FDA approval process involves, among other things, successfully completing clinical trials and filing for and obtaining a PMA. The PMA process requires us to prove the safety and effectiveness of our neurostimulation system to the FDA’s satisfaction. This process, which includes preclinical studies and clinical trials, can take many years and requires the expenditure of substantial resources and may include post-marketing surveillance to establish the safety and efficacy of the neurostimulation system. Notwithstanding the effort and expense incurred, the process may never result in the FDA granting a PMA. Data obtained from preclinical studies and clinical trials are subject to varying interpretations that could delay, limit or prevent regulatory approval. Delays or rejections may also be encountered based upon changes in governmental policies for medical devices during the period of product development. Because our therapy represents a novel way to treat GERD, a non-life threatening condition, and because there is a large population of patients who might be eligible for treatment, it is possible that the FDA will review the application for approval of our neurostimulation system with greater scrutiny, which could cause that process to be lengthier and more involved than that for products without such characteristics. The FDA can delay, limit or deny approval of a PMA application for many reasons, including:
| • | our inability to demonstrate safety or effectiveness to the FDA’s satisfaction; |
| • | insufficient data from our preclinical studies and clinical trials to support approval; |
| • | failure of the facilities of our third-party manufacturer or suppliers to meet applicable requirements; |
| • | inadequate compliance with preclinical, clinical or other regulations; |
22
Table of Contents
| • | our failure to meet the FDA’s statistical requirements for approval; and |
| • | changes in the FDA’s approval policies, or the adoption of new regulations that require additional data or additional clinical studies. |
Modifications to products that are approved through a PMA application generally need FDA approval. Similarly, some modifications made to products cleared through a 510(k) may require a new 510(k). The FDA’s 510(k) clearance process usually takes from three to 12 months, but may last longer. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the application is submitted to the FDA until an approval is obtained. Our neurostimulation system is considered to be a class III device, which are considered to pose the greatest risk and the approval of which is governed by the strictest guidelines, and will require the submission and approval of a PMA in order for us to market if in the United States. We also may design new products in the future that could require the clearance of a 510(k).
Although we have received approval to proceed with clinical trials in the United States under the investigational device exemption, there can be no assurance that the current approval from the FDA to proceed with a double blind sham-controlled study will not be revoked, that the study will be successful, or that the FDA PMA approval will eventually be obtained and not revoked. Even if we obtain approval, the FDA or other regulatory authorities may require expensive or burdensome post-market testing or controls. Any delay in, or failure to receive or maintain, clearance or approval for our future products could prevent us from generating revenue from these products or achieving profitability. Additionally, the FDA and other regulatory authorities have broad enforcement powers. Regulatory enforcement or inquiries, or other increased scrutiny on us, could dissuade some surgeons from using our products and adversely affect our reputation and the perceived safety and efficacy of our products.
Our neurostimulation system and our operations are subject to extensive governmental regulation both in the United States and abroad, and our failure to comply with applicable requirements could cause our business to suffer.
Our neurostimulation system and our operations are subject to extensive regulation by the FDA and various other federal, state and foreign governmental authorities, such as the competent authorities of EEA countries. Government regulation of medical devices is meant to assure their safety and effectiveness, and includes regulation of, among other things:
| • | design, development and manufacturing; |
| • | testing, labeling, content and language of instructions for use and storage; |
| • | clinical trials; |
| • | product safety; |
| • | marketing, sales and distribution; |
| • | regulatory clearances and approvals including premarket clearance and approval; |
| • | conformity assessment procedures; |
| • | product traceability and record keeping procedures; |
| • | advertising and promotion; |
| • | product complaints, complaint reporting, recalls and field safety corrective actions; |
| • | post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; |
| • | post-market studies; and |
| • | product import and export. |
23
Table of Contents
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales.
Even after we have obtained the proper regulatory approval to market a product, the FDA has the power to require us to conduct postmarketing studies. For example, as a condition of approval, we may be required to conduct a post-approval study of our system. Failure to conduct required studies in a timely manner could result in the revocation of the PMA approval for the product that is subject to such a requirement and could also result in the recall or withdrawal of the product, which would prevent us from generating sales from that product in the United States.
To market and sell our products in other countries, we must seek and obtain regulatory approvals, certifications or registrations and comply with the laws and regulations of those countries. These laws and regulations, including the requirements for approvals, certifications or registrations and the time required for regulatory review, vary from country to country. Obtaining and maintaining foreign regulatory approvals, certifications or registrations are expensive, and we cannot be certain that we will receive regulatory approvals, certifications or registrations in any foreign country in which we plan to market our products. If we fail to obtain or maintain regulatory approvals, certifications or registrations in any foreign country in which we plan to market our products, our ability to generate revenue will be harmed.
Failure to comply with applicable laws and regulations could jeopardize our ability to sell our products and result in enforcement actions such as:
| • | warning letters; |
| • | fines; |
| • | injunctions; |
| • | civil penalties; |
| • | termination of distribution; |
| • | recalls or seizures of products; |
| • | delays in the introduction of products into the market; |
| • | total or partial suspension of production; |
| • | refusal of the FDA or other regulator to grant future clearances or approvals; |
| • | withdrawals or suspensions of current clearances or approvals, resulting in prohibitions on sales of our products; |
| • | withdrawal or suspension of the CE Certificates of Conformity granted by the Notified Body or delay in obtaining these certificates; and/or |
| • | in the most serious cases, criminal penalties. |
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, results of operations and financial condition.
Once marketed, modifications to our neurostimulation system, may require new PMAs or PMA supplements, or even require us to cease promoting or to recall the modified products until such approvals are obtained, and the FDA may not agree with our conclusions regarding whether new approvals are required.
Any modification to a device approved through the PMA pathway that impacts the safety or effectiveness of the device requires submission to the FDA and FDA approval of a PMA supplement or, in some cases, a new PMA application. In the future, we may submit PMA supplements for changes to our neurostimulation system. Certain
24
Table of Contents
changes to a device approved through the PMA pathway do not require submission and approval of a new PMA or PMA supplement and may only require notice to FDA in a PMA Annual Report. In the future, we may make changes to our neurostimulation system that we may determine do not require approval of a new PMA or PMA supplement. The FDA may not agree with our decisions not to seek approval of a new PMA or PMA supplement.
If the FDA disagrees with us and requires us to submit a new PMA or PMA supplement for modifications we have made to our neurostimulation system for which we did not seek approval of a PMA or PMA supplement, we may be required to cease promoting or to recall the modified product until we obtain approval. In addition, we could be subject to significant regulatory fines or other penalties. Furthermore, our products could be subject to recall if the FDA determines, for any reason, that our products are not safe or effective or that appropriate regulatory submissions were not made. Delays in receipt or failure to receive approvals, the loss of previously received approvals, or the failure to comply with any other existing or future regulatory requirements, could reduce our sales, profitability and future growth prospects.
If we or our suppliers fail to comply with ongoing FDA or other foreign regulatory authority requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain clearance or approval, and the manufacturing processes, reporting requirements, post-approval clinical data and promotional activities for such product, will be subject to continued regulatory review, oversight and periodic inspections by the FDA and other domestic and foreign regulatory bodies. In particular, we and our third-party suppliers are required to comply with the FDA’s Quality System Regulation, or QSR. These FDA regulations cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. Compliance with applicable regulatory requirements is subject to continual review and is monitored rigorously through periodic inspections by the FDA. If we, or our manufacturers, fail to adhere to QSR requirements in the United States, this could delay production of our products and lead to fines, difficulties in obtaining regulatory clearances, recalls, enforcement actions, including injunctive relief or consent decrees, or other consequences, which could, in turn, have a material adverse effect on our financial condition or results of operations.
In addition, the FDA assesses compliance with the QSR through periodic announced and unannounced inspections of manufacturing and other facilities. The failure by us or one of our suppliers to comply with applicable statutes and regulations administered by the FDA, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in any of the following enforcement actions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | unanticipated expenditures to address or defend such actions; |
| • | customer notifications or repair, replacement, refunds, recall, detention or seizure of our products; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusing or delaying our requests for 510(k) clearance or premarket approval of new products or modified products; |
| • | withdrawing 510(k) clearances or PMA approvals that have already been granted; |
| • | refusal to grant export approval for our products; or |
| • | criminal prosecution. |
Any of these sanctions could have a material adverse effect on our reputation, business, results of operations and financial condition. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
25
Table of Contents
If our products, or malfunction of our products, cause or contribute to a death or a serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA medical device reporting regulations, medical device manufacturers are required to report to the FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or one of our similar devices were to recur. If we fail to report these events to the FDA within the required timeframes, or at all, FDA could take enforcement action against us. Any such adverse event involving our products also could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
Our products may in the future be subject to product recalls. A recall of our products, either voluntarily or at the direction of the FDA or another governmental authority, including a third-country authority, or the discovery of serious safety issues with our products, could have a significant adverse impact on us.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In this case, the FDA, the authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. The FDA requires that certain classifications of recalls be reported to the FDA within 10 working days after the recall is initiated. A government-mandated or voluntary recall by us or one of our international distributors could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our reputation, results of operations and financial condition, which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be subject to liability claims, be required to bear other costs, or take other actions that may have a negative impact on our future sales and our ability to generate profits. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA or another third-country competent authority. We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the FDA or another third-country competent authority. If the FDA disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they were.
We are also required to follow detailed recordkeeping requirements for all firm-initiated medical device corrections and removals. In addition, in December of 2012, the FDA issued a draft guidance intended to assist the FDA and industry in distinguishing medical device recalls from product enhancements. Per the guidance, if any change or group of changes to a device that addresses a violation of the FDCA, that change would generally constitute a medical device recall and require submission of a recall report to the FDA.
If the third parties on which we rely to conduct our clinical trials and to assist us with pre-clinical development do not perform as contractually required or expected, we may not be able to obtain regulatory clearance, approval to commercialize our products.
We do not have the ability to independently conduct clinical trials for our neurostimulation system. We rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct our clinical trials and prepare our PMA submissions. If these third parties do not
26
Table of Contents
successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval or successfully commercialize our products on a timely basis, if at all, and our business, operating results and prospects may be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
The results of our clinical trials may not support our product candidate claims or may result in the discovery of adverse side effects.
Our ongoing research and development, pre-clinical testing and clinical trial activities involving our neurostimulation system, and any future products, are subject to extensive regulation and review by numerous governmental authorities both in the United States and abroad. We have obtained approved FDA approval to conduct clinical studies for our neurostimulation system. In the future we may conduct clinical trials to support approval of new products. Clinical studies must be conducted in compliance with FDA regulations or the FDA may take enforcement action. The data collected from these clinical studies may ultimately be used to support market clearance for these products. Even if our clinical trials are completed as planned, we cannot be certain that their results will support our product candidate claims or that the FDA will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our product candidates are safe and effective for the proposed indicated uses, which could cause us to abandon a product candidate and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our product submissions and, ultimately, our ability to commercialize our product candidates and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile.
U.S. legislative or FDA regulatory reforms may make it more difficult and costly for us to obtain regulatory approval of our product candidates and to manufacture, market and distribute our products after approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulatory approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of future products. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be.
For example, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. For example, in 2011, the FDA initiated a review of the premarket clearance process in response to internal and external concerns regarding the 510(k) program, announcing 25 action items designed to make the process more rigorous and transparent. In addition, as part of the Food and Drug Administration Safety and Innovation Act of 2012, or the FDASIA, Congress enacted several reforms entitled the Medical Device Regulatory Improvements and additional miscellaneous provisions which will further affect medical device regulation both pre- and post-approval. The FDA has implemented, and continues to implement, these reforms, which could impose additional regulatory requirements upon us and increase the costs of compliance.
27
Table of Contents
Lack of third-party coverage and reimbursement for our devices could delay or limit their adoption.
In both the United States and international markets, the use of medical devices is dependent in part on the availability of reimbursement from third-party payors, such as government and private insurance plans. Healthcare providers that use medical devices generally rely on third-party payors to pay for all or part of the costs and fees associated with the medical procedures being performed or to compensate them for their patient care services. Even though we have received initial reimbursement approval in Germany, where reimbursement for a limited number of our procedures is available to over 40 hospitals through a fast track method for the introduction of new technologies, in Switzerland, where initial coverage is available through existing code and in Colombia and Argentina, where approval for a small number of initial cases was obtained, there can be no assurance that our products will be considered cost-effective, that reimbursement will be available in other sites or in other countries, including the United States, if approved, or that reimbursement will be sufficient to allow sales of our neurostimulation system on a profitable basis. The coverage decisions of third-party payors are significantly influenced by the assessment of our neurostimulation system by health technology assessment bodies. Such assessments are outside our control and currently, no such body has conducted an evaluation of our neurostimulation system. There can be no assurance that such evaluations will be conducted or that they will have a favorable outcome.
If approved for use in the United States, we expect that our neurostimulation system will be purchased primarily by medical institutions, which will in turn bill various third-party payors for the health care services provided to patients at their facility, including the procedure to implant our neurostimulation system. The payors include the Centers for Medicare & Medicaid Services, or CMS, which administers the Medicare program and works in partnership with state governments to administer Medicaid, other government programs and private insurance plans. The process involved in applying for coverage and incremental reimbursement from CMS is lengthy and expensive. Further, Medicare coverage is based on our ability to demonstrate the treatment is “reasonable and necessary” for Medicare beneficiaries. Even if our neurostimulation system receives FDA and other regulatory clearance or approval, it may not be granted coverage and reimbursement by any payor, including by CMS. For some governmental programs, such as Medicaid, coverage and reimbursement differ from state to state and some state Medicaid programs may not pay adequate amounts for the procedure to implant our product, or any payment at all. Moreover, many private payors use coverage decisions and payment amounts determined by CMS as guidelines in setting their coverage and reimbursement policies and amounts. If CMS or other agencies limit coverage or decrease or limit reimbursement payments for doctors and hospitals, this may affect coverage and reimbursement determinations by many private payors.
If our medical neurostimulation system does not receive reimbursement, or receives reimbursement at a low rate from a third-party payor such as CMS, then a medical institution would have to absorb the cost of our neurostimulation system as part of the cost of the procedure. At this time, we do not know the extent to which medical institutions would consider insurers’ payment levels adequate to cover the cost of our neurostimulation system. Failure by hospitals and physicians to receive an amount that they consider to be adequate reimbursement for procedures in which our neurostimulation system is used could deter them from purchasing our neurostimulation system and limit our revenue growth. If medical institutions are unable to justify the costs of our neurostimulation system, they may refuse to purchase them, which would have a material adverse effect on our business and financial condition.
Adverse changes in reimbursement policies and procedures by payors may impact our ability to market and sell our neurostimulation system.
Healthcare costs have risen significantly over the past decade, and there have been and continue to be proposals by legislators, regulators and third-party payors to decrease costs. Third-party payors are increasingly challenging the prices charged for medical products and services and instituting cost containment measures to control or significantly influence the purchase of medical products and services.
28
Table of Contents
For example, in the United States, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively, PPACA, among other things, reduced and/or limited Medicare reimbursement to certain providers. The Budget Control Act of 2011, as amended by subsequent legislation, further reduces Medicare’s payments to providers by 2 percent through fiscal year 2024. These reductions may reduce providers’ revenues or profits, which could affect their ability to purchase new technologies. Furthermore, the healthcare industry in the United States has experienced a trend toward cost containment as government and private insurers seek to control healthcare costs by imposing lower payment rates and negotiating reduced contract rates with service providers. Legislation could be adopted in the future that limits payments for our products from governmental payors. In addition, commercial payors such as insurance companies, could adopt similar policies that limit reimbursement for medical device manufacturers’ products. Therefore, we cannot be certain that our product or the procedures or patient care performed using our product will be reimbursed at a cost-effective level. We face similar risks relating to adverse changes in reimbursement procedures and policies in other countries where we market or intend to market our neurostimulation system. Reimbursement and healthcare payment systems vary significantly among international markets. Our inability to obtain international reimbursement approval, or any adverse changes in the reimbursement policies of foreign payors, could negatively affect our ability to sell our neurostimulation system and have a material adverse effect on our business and financial condition.
If we or our sales personnel or distributors do not comply with the U.S. federal and state fraud and abuse laws, including anti-kickback laws for any products approved in the U.S., or with similar foreign laws where we market our products, we could face significant liability.
There are numerous United States federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback laws, false claims, and physician transparency laws. Our relationships with physicians and surgeons, hospitals and our independent distributors are subject to scrutiny under these laws. Violations of these laws are punishable by criminal and civil sanctions, including significant fines, damages and monetary penalties and in some instances, imprisonment and exclusion from participation in federal and state healthcare programs, including the Medicare, Medicaid and Veterans Administration health programs.
Healthcare fraud and abuse regulations are complex, and even minor irregularities can potentially give rise to claims that a statute or prohibition has been violated. The laws that may affect our ability to operate include:
| • | the federal healthcare program Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, paying, or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any good or service for which payment may be made under federal healthcare programs such as Medicare and Medicaid; |
| • | federal civil False Claims Act prohibits, among other things, knowingly presenting, or causing to be presented, claims for payment of government funds that are false or fraudulent or knowingly making, using or causing to be made or used a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology and Clinical Health Act of 2009, which, among other things, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | HIPAA also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services; |
29
Table of Contents
| • | the Federal Trade Commission Act and similar laws regulating advertisement and consumer protections; |
| • | the federal Foreign Corrupt Practices Act of 1997, which makes it illegal to offer or provide money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians. Some states, such as California, Massachusetts, Nevada, and Vermont mandate implementation of commercial compliance programs and/or impose restrictions on device manufacturer marketing practices and tracking and reporting of gifts, compensation and other remuneration to physicians. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from federal healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. To enforce compliance with the federal laws, the U.S. Department of Justice, or DOJ, has recently increased its scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Dealing with investigations can be time and resource consuming and can divert management’s attention from the business. In addition, settlements with the DOJ or other law enforcement agencies have forced healthcare providers to agree to additional onerous compliance and reporting requirements as part of a consent decree or corporate integrity agreement. Any violations of these laws, or any action against us for violation of these laws, even if we successfully defend against it, could have a material adverse effect on our reputation, business and financial condition.
Many foreign countries have enacted similar laws addressing fraud and abuse in the healthcare sector. The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance requirements in multiple jurisdictions increases the possibility that a healthcare company may run afoul of one or more of the requirements.
The implementation of the reporting and disclosure obligations of the Physician Payments Sunshine Act/Open Payments provisions of the Health Care Reform Law could adversely affect our business upon commercialization of our product in the U.S.
The federal Physician Payments Sunshine Act, being implemented as the Open Payments Program, requires certain applicable device manufacturers to engage in extensive tracking of payments and other transfers of value made to physicians and teaching hospitals, as well as physician ownership and investment interests, and public reporting of such data. Device manufacturers with products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program are required to have started tracking such payments or transfers of value on August 1, 2013, and must report such payments to the Centers for Medicare and Medicaid Services annually.
The final rule implementing the Physician Payments Sunshine Act is complex and broad in scope. If we receive PMA approval for our neurostimulation system, we will be considered an “applicable manufacturer” under the Physician Payments Sunshine Act and as such will be subject to reporting requirements. Accordingly, we will be required to collect and report detailed information regarding certain financial relationships we have with
30
Table of Contents
physicians, dentists and teaching hospitals. It is difficult to predict how the new requirements may impact existing relationships among manufacturers, distributors, physicians, dentists and teaching hospitals. The Physician Payments Sunshine Act preempts similar state reporting laws, although we may be required to continue to report under certain other provisions of such state laws. While we expect to have substantially compliant programs and controls in place to comply with the Physician Payments Sunshine Act requirements and similar state and foreign laws, our compliance with the Physician Payments Sunshine Act and similar state and foreign transparency laws imposes additional costs on us. Additionally, failure to comply with the Physician Payment Sunshine Act or similar state or foreign laws may subject the Company to monetary penalties.
We may become liable for significant damages or be restricted from selling our products if we engage in inappropriate promotion of our neurostimulation system.
The advertising and promotion of our neurostimulation system in Europe is subject to EEA Member States laws implementing Directive 90/385/EEC concerning Active Implantable Medical Devices, Directive 2006/114/EC concerning misleading and comparative advertising, and Directive 2005/29/EC on unfair commercial practices, as well as other EEA Member State legislation governing the advertising and promotion of medical devices. These laws may limit or restrict the advertising and promotion of our products to the general public and may impose limitations on our promotional activities with healthcare professionals. Our failure to comply with all these laws and requirements could have a material adverse effect on our business and financial condition.
If our neurostimulation system is approved for sale in the United States, our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of the “off-label” use of our neurostimulation system, including by using our neurostimulation system in a way not approved by the FDA. Healthcare providers may use our products off-label, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. However, if the FDA determines that our promotional materials, training or marketing efforts constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or marketing efforts or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties. Although we do not intend to engage in any activities that may be considered off-label promotion of our products, the FDA or another regulatory agency could disagree and conclude that we have engaged, directly or indirectly, in off-label promotion. In addition, the off-label use of our products may increase the risk of product liability claims. Product liability claims are expensive to defend and could result in substantial damage awards against us and harm our reputation.
Our financial performance may be adversely affected by medical device tax provisions in the healthcare reform laws.
PPACA imposes, among other things, an excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States, which began in 2013. Under these provisions, the Congressional Research Service predicts that the total cost to the medical device industry may be up to $20 billion over the next decade. The Internal Revenue Service issued final regulations implementing the tax in December 2012, which requires, among other things, bi-monthly payments and quarterly reporting. Once we market products, we will be subject to this excise tax on our sales of certain medical devices in the United States. We anticipate that primarily all of our sales of medical devices in the United States will be subject to this 2.3% excise tax.
31
Table of Contents
Intellectual Property Risks Related to Our Business
If we are unable to obtain or to adequately protect our intellectual property, others may be able to use our technology, which could adversely affect our ability to compete in the market.
We regard various features of our potential products and processes as proprietary and rely on a combination of patent and trade secret laws to protect our proprietary rights. We own intellectual property designed to protect our core technologies. The patent positions of medical development companies, including our patent position, are generally uncertain and involve complex legal and factual questions. We will be able to protect our intellectual property rights from unauthorized use by third parties only to the extent that technologies are covered by valid and enforceable patents or are effectively maintained as trade secrets. The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The process of seeking patent protection can be time consuming and expensive, and there can be no assurance that patents will be issued from currently pending or future applications or that we have filed patent applications in countries that become commercially significant markets or that any patents that may be issued will be sufficient in scope or strength to provide meaningful protection or any commercial advantage to us, including that we seek and obtain patent protection in the countries that are important for our operations. Further, there can be no assurance that others will not independently develop technologies that are substantially equivalent or superior to our technology, duplicate our products or design around any patents that may be issued to us.
Claims that our products infringe the patent rights of third parties could result in costly litigation or, in the event of an unfavorable outcome, could have an adverse impact on our ability to do business.
We cannot guarantee that our products, or the use of our products, do not infringe third-party patents.
Third parties might allege that we are infringing their patent rights or that we have misappropriated their trade secrets and we may be involved from time to time in litigation to determine the enforceability, scope and validity of any proprietary rights of ours or of third parties asserting infringement claims against us. We expect that developers of medical devices will be increasingly subject to infringement claims as the number of products and competitors in our industry grows and the functionality of products in different industry segments overlaps.
We may not be aware of all relevant third-party patents or patent applications. Furthermore, applications filed before November 29, 2000, and certain applications filed after that date that will not be filed outside the United States, remain confidential until patents issue. Patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing, which is referred to as the priority date. Therefore, patent applications covering our products or their use could have been filed by others without our knowledge. Third parties may have obtained, and may in the future obtain, patents under which such third parties may claim that the use of our products constitutes patent infringement. Furthermore, as we continue to commercialize our products in their current or future form, launch new products, and enter new markets, we expect that competitors may claim that our products infringe their intellectual property rights as part of business strategies designed to impede our successful commercialization and entry into new markets. We may receive letters from third parties inviting us to take licenses under, or alleging that we infringe, their patents.
If a third-party patent is successfully asserted against us, we could be barred from commercializing our current products and may owe a substantial damages award, all of which could have a material adverse impact on our cash position and business and financial condition. We could incur substantial costs and divert the attention of our management and technical personnel in defending ourselves against any of these claims. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a material adverse impact on our cash position and stock price. If we were to challenge the validity of any issued U.S. patent in court, we would need to overcome a statutory presumption of validity that attaches to every U.S. patent. This means that in order to prevail, we would have to present clear and convincing evidence as to the invalidity of the patent’s claims. There is no assurance that a court would find in our favor on questions of infringement or validity.
32
Table of Contents
In the event of a successful claim of infringement or misappropriation against us, we may be required to pay damages and obtain one or more licenses from third parties, or be prohibited from commercializing our current products. We may be unable to obtain these licenses at a reasonable cost, if at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. Moreover, we could encounter delays in product introductions while we attempt to develop alternative methods or products. Defense of any lawsuit or failure to obtain any of these licenses on favorable terms could prevent us from commercializing products, and the prohibition of sale of any of our products could materially affect our ability to commercialize our current or future products and have a material adverse effect on our business and financial condition.
Developments in patent law could have a material adverse effect on our business and financial condition.
From time to time, the U.S. Supreme Court, or the Supreme Court, other federal courts, the U.S. Congress or the U.S. Patent and Trademark Office, or the USPTO, may change the standards of patentability and any such changes could have a material adverse effect on our business and financial condition.
The Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged, and changes to the way patent applications are disputed during the examination process. These changes may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. The USPTO has developed new and untested regulations and procedures to govern the full implementation of the America Invents Act, and many of the substantive changes to patent law associated with the America Invents Act, and, in particular, the first-to-file provisions, became effective on March 16, 2013. Substantive changes to patent law associated with the America Invents Act may affect our ability to obtain patents, and if obtained, to enforce or defend them. Accordingly, it is not clear what, if any, impact the America Invents Act will ultimately have on the cost of prosecuting our patent applications, our ability to obtain patents based on our discoveries and our ability to enforce or defend any patents that may issue from our patent applications, all of which could have a material adverse effect on our business and financial condition.
We may be unable to protect or enforce our intellectual property effectively, which could harm our competitive position.
Obtaining and maintaining a strong patent position is important to our business. Some of our patent applications are in the early stages of prosecution and have not yet issued as patents. Patent law relating to the scope of claims in the technology fields in which we operate is complex and uncertain, so we cannot be assured that we will be able to obtain or maintain patent rights, or that the patent rights we may obtain will be valuable, provide an effective barrier to competitors or otherwise provide competitive advantages. Others have filed, and in the future are likely to file, patent applications that are similar or identical to ours or those of our licensors. To determine the priority of inventions, or demonstrate that we did not derive our invention from another, we may have to participate in interference or derivation proceedings in the USPTO or in court that could result in substantial costs in legal fees and could substantially affect the scope of our patent protection. We cannot be assured our patent applications will prevail over those filed by others. Also, our intellectual property rights may be subject to other challenges by third parties. Patents we obtain could be challenged in litigation or in administrative proceedings such as ex parte reexam, inter partes review, or post grant review in the United States or opposition proceedings in Europe or other jurisdictions.
Obtaining and maintaining a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity fees, various other governmental fees on patents and/or applications due in several stages over the lifetime of patents and/or applications, as well as the cost associated with complying with numerous procedural provisions during the patent application process. We may or may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure
33
Table of Contents
to make certain payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we choose to forgo patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer.
Legal actions to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or interferences against those that have infringed on our patents, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could have a material adverse effect on our business and financial condition.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position could be harmed, which could have a material adverse effect on our business.
In addition to patent protection, we also rely upon copyright and trade secret protection, as well as nondisclosure agreements and invention assignment agreements with our employees, consultants and third-parties, to protect our confidential and proprietary information. Some elements of our neurostimulation system are based on unpatented trade secrets and know-how that are not publicly disclosed. In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and legal recourse to which we are entitled against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, trade secrets may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed, which could have a material adverse effect on our business and financial condition.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to biotechnology. This could make it difficult for us to stop the infringement of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for the technology used in our products and the enforcement of intellectual property.
34
Table of Contents
Third parties may assert ownership or commercial rights to inventions we develop.
Third parties may in the future make claims challenging the inventorship or ownership of our intellectual property. We have written agreements with collaborators that provide for the ownership of intellectual property arising from our collaborations. These agreements provide that we must negotiate certain commercial rights with collaborators with respect to joint inventions or inventions made by our collaborators that arise from the results of the collaboration. In some instances, there may not be adequate written provisions to address clearly the resolution of intellectual property rights that may arise from a collaboration. If we cannot successfully negotiate sufficient ownership and commercial rights to the inventions that result from our use of a third-party collaborator’s materials where required, or if disputes otherwise arise with respect to the intellectual property developed with the use of a collaborator’s samples, we may be limited in our ability to capitalize on the market potential of these inventions. In addition, we may face claims by third parties that our agreements with employees, contractors, or consultants obligating them to assign intellectual property to us are ineffective, or in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes regarding intellectual property we have developed or will develop and interfere with our ability to capture the commercial value of such inventions. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property, or may lose our exclusive rights in that intellectual property. Either outcome could have a material adverse effect on our business and financial condition.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We employ individuals who were previously employed at universities or other diagnostic or life sciences companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs to us impacting our financial condition and be a distraction to management and other employees.
Third parties may build on our intellectual property and limit our ability to evolve the product.
Continued innovation and patenting is crucial to the success of any product-based company. The ability to do so depends on the resources assigned to research, development, and protection of intellectual property. Third parties who may have significantly more resources than us in these areas could innovate quicker and create significant intellectual property around next generation devices, treatment regimens, stimulation algorithms, delivery techniques, and stimulation protocols. This could limit our ability to sufficiently evolve our products to meet market needs, avoid obsolescence and fulfill customer demands, resulting in loss of market share.
Risks Related to this Offering and Ownership of our Common Stock
We do not know whether an active, liquid and orderly trading market will develop for our common stock or what the market price of our common stock will be; as a result, it may be difficult for you to sell your shares of our common stock.
Prior to this offering, no market for shares of our common stock existed and an active trading market for our shares may never develop or, if developed, may not be sustained following this offering. We will determine the initial public offering price for our common stock through negotiations with the underwriters, and the negotiated price may not be indicative of the market price of our common stock after this offering. The market value of our common stock may decrease significantly from the initial public offering price. The lack of an active market may
35
Table of Contents
impair your ability to sell your shares or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your shares. Furthermore, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic collaborations or acquire companies or products by using our shares of common stock as consideration.
The market price of our stock may be volatile, and you could lose all or part of your investment.
The trading price of our common stock following this offering is likely to be highly volatile and subject to wide fluctuations in response to various factors, some of which we cannot control. In addition to the factors discussed in this “Risk Factors” section and elsewhere in this prospectus, these factors include:
| • | the success of, or developments in, competitive products or technologies; |
| • | regulatory actions with respect to our products and our competitors; |
| • | the level of success of our commercialization strategy outside of the United States; |
| • | announcements related to developments in our pivotal U.S. clinical trial: |
| • | announcements by us or our competitors of significant acquisitions, strategic collaborations, joint ventures or capital commitments; |
| • | regulatory or legal developments in the United States and other countries; |
| • | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| • | recruitment or departure of key personnel; |
| • | expenses related to any of our development programs and our business in general; |
| • | actual or anticipated changes in financial estimates, development timelines or recommendations by securities analysts; |
| • | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| • | our ability or failure to raise additional capital in equity or debt transactions; |
| • | market acceptance of our neurostimulation system and developments in reimbursement; |
| • | the success of our ongoing development of our products, including post-commercialization improvements; |
| • | costs associated with our sales and marketing initiatives and manufacturing activities; |
| • | costs and timing of obtaining and maintaining FDA and other regulatory clearances and approvals for our neurostimulation system and potential additional products; |
| • | sales of our common stock by us, our insiders or our other stockholders; |
| • | changes in the structure of healthcare payment systems; |
| • | market conditions in the medical device sector; and |
| • | general economic, industry and market conditions. |
In addition, the stock market in general, and medical device companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of
36
Table of Contents
these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. The realization of any of the above risks or any of a broad range of other risks, including those described in this “Risk Factors” section, could have a dramatic and material adverse impact on the market price of our common stock.
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our common stock, the price of our common stock could decline.
The trading market for our common stock will rely in part on the research and reports that equity research analysts publish about us and our business. We do not control these analysts. The price of our common stock could decline if one or more equity analysts downgrade our common stock or if analysts issue other unfavorable commentary or cease publishing reports about us or our business.
If we fail to meet or delay in meeting clinical, regulatory, financial or other milestones, the price of our common stock could decline.
From time to time, we may estimate and publicly announce the timing anticipated for the accomplishment of various clinical, regulatory and other product development goals and financial targets, such as relating to sales, which we sometimes refer to as milestones. These milestones may include submissions for and receipt of clearances or approvals from regulatory authorities, other clinical and regulatory events or the launch of new products. These estimates are based on a variety of assumptions. The actual timing of these milestones may vary dramatically compared to our estimates, in some cases for reasons beyond our control. If we do not meet milestones as publicly announced, the commercialization of our products will be delayed and investors may lose confidence in our management team. As a result, they may sell their stock and our stock price may decline.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively, which could affect our results of operations and cause our stock price to decline.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section entitled “Use of Proceeds.” We intend to use the net proceeds from this offering to commercialize our technology in a number of countries that recognize the CE Mark and to pursue regulatory approval in the United States through the FDA premarket approval (PMA) process. As a result, you will be relying upon management’s judgment with only limited information about our specific intentions for the use of the balance of the net proceeds of this offering. You will not have the opportunity, as part of your investment decision, to assess whether we are using the proceeds appropriately. Our management might not apply our net proceeds in ways that ultimately increase the value of your investment. If we do not invest or apply the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our common stock may be volatile, and in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could have a material adverse effect on our business and financial condition.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
Prior to this offering, our executive officers, directors, holders of 5% or more of our capital stock and their respective affiliates beneficially owned approximately 59.7% of our voting stock and, upon completion of this offering, that same group will hold approximately % of our outstanding voting stock (assuming full exercise of
37
Table of Contents
the underwriters’ over-allotment option, no exercise of outstanding options and warrants and no purchases of shares in this offering by any of this group), in each case assuming the conversion of all outstanding shares of our convertible preferred stock into shares of our common stock immediately prior to the completion of this offering. After this offering, this group of stockholders will have the ability to exert significant influence over us through this ownership position. These stockholders may be able to exert significant influence over all matters requiring stockholder approval, including with respect to elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders. The interests of this group of stockholders may not always coincide with your interests or the interests of other stockholders and they may act in a manner that advances their best interests and not necessarily those of other stockholders.
Sales of a substantial number of shares of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have outstanding shares of common stock based on the number of shares outstanding as of June 30, 2014, assuming (i) the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 11,719,975 shares of common stock and (ii) the conversion of the principal and accrued interest on the approximately $6.4 million aggregate principal amount of convertible promissory notes issued in June 2014 and the approximately $1.1 million aggregate principal amount of convertible promissory notes issued August 2014 into an aggregate of shares of common stock, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering. This includes the shares that we sell in this offering, which may be resold in the public market immediately without restriction, unless purchased by our affiliates. Of the remaining shares, shares of our common stock are currently restricted as a result of securities laws or lock-up agreements but will be able to be sold after this offering as described in the “Shares Eligible for Future Sale” section of this prospectus. Moreover, after this offering, holders of a majority of our common stock (i) issuable upon conversion of our outstanding Series B, Series B-1 and Series C Convertible Preferred Stock, (ii) issuable upon the exercise of warrants issued to holders of our convertible promissory notes in June 2014 and in August 2014, and (iii) actually issued upon conversion of our outstanding convertible promissory notes, or a total of shares, will have rights, subject to certain conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We also intend to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the “Underwriting” section of this prospectus.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and will be able to avail ourselves of reduced disclosure requirements, which could make our common stock less attractive to investors and adversely affect the market price of our common stock.
We are an “emerging growth company” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various requirements including:
| • | the provisions of Section 404(b) of the Sarbanes-Oxley Act of 2002 requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; |
| • | the “say on pay” provisions (requiring a non-binding shareholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding shareholder vote to approve golden parachute arrangements for certain executive officers in connection |
38
Table of Contents
| with mergers and certain other business combinations) of the Dodd-Frank Act and some of the disclosure requirements of the Dodd-Frank Act relating to compensation of our executives; |
| • | the requirement to provide detailed compensation discussion and analysis in proxy statements and reports filed under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and instead provide a reduced level of disclosure concerning executive compensation; and |
| • | any rules that the Public Company Accounting Oversight Board may adopt requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements. |
We may take advantage of these exemptions until we are no longer an “emerging growth company.” We would cease to be an “emerging growth company” upon the earliest of: (i) the first fiscal year following the fifth anniversary of this offering; (ii) the first fiscal year after our annual gross revenues are $1 billion or more; (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt securities; or (iv) as of the end of any fiscal year in which the market value of our common stock held by non-affiliates exceeded $700 million as of the end of the second quarter of that fiscal year. Our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an “emerging growth company,” which may increase the risk that material weaknesses or significant deficiencies in our internal control over financial reporting go undetected. Likewise, so long as we qualify as an “emerging growth company,” we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would otherwise have been required to provide in filings we make with the Securities and Exchange Commission, or the SEC, which may make it more difficult for investors and securities analysts to evaluate our company.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to delay the adoption of some accounting standards until those standards would otherwise apply to private companies. We have elected to avail ourselves of this exemption and, therefore, we will not be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies, which may mean that our financial statements may not be comparable to companies that comply with all public company accounting standards.
We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile and may decline.
We will incur increased costs as a result of operating as a public company, and our management will devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. We will be subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Protection Act, as well as rules adopted, and to be adopted, by the SEC and the NASDAQ stock exchange. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, we expect these rules and regulations to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. The increased costs will increase our net loss. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the sufficient coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
39
Table of Contents
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, new SEC regulations and the NASDAQ stock exchange rules are creating uncertainty for public companies. We are presently evaluating and monitoring developments with respect to new and proposed rules and cannot predict or estimate the amount of the additional compliance costs we may incur or the timing of such costs. These new or changed laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and as a result, their application in practice may evolve over time as new guidance is provided by courts and regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. Maintaining appropriate standards of corporate governance and public disclosure will result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. In addition, if we fail to comply with new or changed laws, regulations and standards, regulatory authorities may initiate legal proceedings against us which could have a material adverse effect on our reputation, business and financial condition.
Our disclosure controls and procedures and internal control over financial reporting may not prevent or detect all errors or acts of fraud and we may be unable to accurately and timely report our financial results or file our periodic reports in a timely manner.
Upon completion of this offering, we will become subject to the periodic reporting requirements of the Exchange Act. We designed our disclosure controls and procedures to reasonably assure that information we must disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls.
In June 2014, we identified a “significant deficiency” in our internal control over financial reporting related to the design and operating effectiveness of financial reporting controls over the recording and disclosure of convertible redeemable preferred stock and the accretion of undeclared dividends on such stock. A deficiency in internal control over financial reporting exists when the design or operation of a control does not allow management or employees, in the normal course of performing their assigned functions, to prevent or detect misstatements on a timely basis. A significant deficiency is a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention by those responsible for the oversight of a company’s financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be prevented or detected on a timely basis.
Because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected. We are in the process of taking action to remediate the significant deficiency that we have identified by commencing the hiring process for additional full-time accounting staff. At this time, we cannot assure you that the measures we have taken will be effective in remediating the previously identified significant deficiency and mitigating or preventing significant deficiencies or material weaknesses in our internal control over financial reporting in the future. If we fail to effectively remediate deficiencies in our control environment or are unable to implement and maintain effective internal control over financial reporting to meet the demands that will be placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act, we may be unable to accurately report our financial results, or to report them within the timeframes required by law or exchange regulations.
40
Table of Contents
Additionally, we have engaged only a very limited number of accounting and finance personnel and we rely substantially on outside consultants. We will need to incur additional expenses to hire additional personnel with public company financial reporting expertise to build our financial management and reporting infrastructure, and further develop and document our accounting policies and financial reporting procedures. If we are unable to do so, we may not be able to accurately report our financial results or file our periodic reports in a timely manner, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would benefit our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws that will become effective immediately prior to the completion of this offering, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These provisions include:
| • | creating a classified board of directors whose members serve staggered three-year terms; |
| • | authorizing the issuance of “blank check” preferred stock, the terms of which we may establish and shares of which we may issue without stockholder approval; |
| • | prohibiting cumulative voting in the election of directors, which would otherwise allow for less than a majority of stockholders to elect director candidates; |
| • | prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; |
| • | eliminating the ability of stockholders to call a special meeting of stockholders; |
| • | requiring a supermajority vote to remove directors or to amend certain provisions of our certificate of incorporation; and |
| • | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management. Because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, or the DGCL, which may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under the DGCL, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Any provision of our amended and restated certificate of incorporation or amended and restated bylaws or Delaware law that has the effect of delaying or deterring a change of control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
We have never paid dividends on our capital stock and we do not anticipate paying any dividends in the foreseeable future. Consequently, any profits from an investment in our common stock will depend on whether the price of our common stock increases.
We have not paid dividends on any of our classes of capital stock to date and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
41
Table of Contents
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Our amended and restated certificate of incorporation and amended and restated bylaws provide that we will indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. In addition, as permitted by Section 145 of the Delaware General Corporation Law, our amended and restated bylaws to be effective immediately prior to the completion of this offering and our indemnification agreements that we have entered into with our directors and officers provide for the following:
| • | We will indemnify our directors and officers for serving us in those capacities or for serving other business enterprises at our request, to the fullest extent permitted by Delaware law. Delaware law provides that a corporation may indemnify such person if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the registrant and, with respect to any criminal proceeding, had no reasonable cause to believe such person’s conduct was unlawful. |
| • | We may, in our discretion, indemnify employees and agents in those circumstances where indemnification is permitted by applicable law. |
| • | We are required to advance expenses, as incurred, to our directors and officers in connection with defending a proceeding, except that such directors or officers shall undertake to repay such advances if it is ultimately determined that such person is not entitled to indemnification. |
| • | We will not be obligated pursuant to our amended and restated bylaws to indemnify a person with respect to proceedings initiated by that person against us or our other indemnitees, except with respect to proceedings authorized by our board of directors or brought to enforce a right to indemnification. |
| • | The rights conferred in our amended and restated bylaws are not exclusive, and we are authorized to enter into indemnification agreements with our directors, officers, employees and agents and to obtain insurance to indemnify such persons. |
| • | We may not retroactively amend our amended and restated bylaw provisions to reduce our indemnification obligations to directors, officers, employees and agents. |
If you purchase shares of common stock in this offering, you will experience immediate dilution in your investment. You will experience further dilution if we issue additional equity securities in future financing transactions.
Purchasers of common stock in this offering will pay a price per share that exceeds the net tangible book value per share of our common stock. Investors participating in this offering will incur immediate and substantial dilution. After giving effect to our receipt of approximately $ million of estimated net proceeds, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, from our sale of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, our pro forma as adjusted net tangible book value as of June 30, 2014, would have been $ million, or $ per share. This amount represents an immediate increase in net tangible book value of $ per share of our common stock to existing stockholders and an immediate dilution in net tangible book value of $ per share of our common stock to new investors purchasing shares of common stock in this offering. See the section entitled “Dilution” below for a more detailed illustration of the dilution you would incur if you purchase common stock in this offering.
If we issue additional common stock, or securities convertible into or exchangeable or exercisable for common stock, our stockholders, including investors who purchase shares of common stock in this offering, may experience additional dilution, and any such issuances may result in downward pressure on the price of our common stock. We also cannot assure you that we will be able to sell shares or other securities in any future offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders.
42
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS AND INDUSTRY DATA
This prospectus, including the sections entitled “Prospectus Summary,” “Risk Factors,” “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business,” contains forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “intends” or “continue,” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology.
Forward-looking statements include, but are not limited to, statements about:
| • | our plans to develop, commercialize and market our products and potential future products; |
| • | the rate and degree of market acceptance of our products and future products |
| • | our ability, or the ability of healthcare providers, to obtain reimbursement of our neurostimulation system or the implantation procedure; |
| • | our ability to continue relationships with our collaborators; |
| • | the effect of changes in reimbursement by payors; |
| • | the results of our efforts to advance and expand our portfolio of product candidates; |
| • | the results of clinical data and our ability to establish the safety and efficacy of our devices; |
| • | our use of the proceeds from the offering; |
| • | the success, cost and timing of our product development activities and clinical trials; |
| • | our ability to adequately source our products from third-party manufacturers and suppliers; |
| • | our ability to obtain additional financing necessary to implement our growth strategy; |
| • | the performance of our third-party suppliers and manufacturers; |
| • | the success of currently competitive products or those that may become available; |
| • | our estimates regarding expenses, future revenue, capital requirements and needs for additional capital; |
| • | economic, political and other risks associated with our international operations; |
| • | our ability to obtain and maintain regulatory approval for our neurostimulation system, and any related restrictions, limitations, and/or warnings in the label of the neurostimulation system; |
| • | costs of compliance and our failure to comply with new and existing governmental regulations in the U.S. and foreign countries, including, but not limited to, healthcare fraud, patient privacy and tax regulations; |
| • | our expectations regarding our ability to obtain and maintain intellectual property protection for our intellectual property; and |
| • | costs of litigation and the failure to successfully defend lawsuits and other claims against us. |
These statements are only current predictions and are subject to known and unknown risks, uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements. We discuss many of these risks in this prospectus in greater detail under the heading “Risk Factors” and elsewhere in this prospectus. You should not rely upon forward-looking statements as predictions of future events.
43
Table of Contents
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, after the date of this prospectus, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise.
We obtained the industry, market and competitive position data in this prospectus from our own internal estimates and research as well as from industry and general publications and research surveys and studies conducted by third parties. While we believe that each of these studies and publications is reliable, we have not independently verified market and industry data from third-party sources. While we believe our internal company research is reliable and the market definitions we use are appropriate, neither such research nor these definitions have been verified by any independent source.
44
Table of Contents
We estimate that our net proceeds from the sale of shares of our common stock in this offering will be approximately $ million, based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. If the representative of the underwriters exercises the over-allotment option in full, we estimate that our net proceeds will be approximately $ million based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
The principal purposes of this offering are to obtain additional capital to support our operations, establish a public market for our common stock and to facilitate our future access to the public capital markets. We currently expect to use the net proceeds from this offering for the following purposes:
| • | approximately $ for commercialization activities; |
| • | approximately $ for clinical development activities, most importantly our United States pivotal trial to support a PMA filing with the FDA; and |
| • | any remaining proceeds for working capital and general corporate purposes. |
Pending the application of the net proceeds as described above, we intend to invest the net proceeds of the offering in short-term, investment-grade, interest-bearing securities.
Our management will have broad discretion to allocate the net proceeds to us from this offering and investors will be relying on the judgment of our management regarding the application of the proceeds from this offering. We reserve the right to change the use of these proceeds as a result of certain contingencies such as competitive developments, the results of our commercialization efforts, acquisition and investment opportunities and other factors. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use the proceeds.
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase of 1.0 million shares in the number of shares offered by us, together with a concurrent $1.00 increase in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase the net proceeds to us from this offering by approximately $ million after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Conversely, a decrease of 1.0 million shares in the number of shares offered by us together with a concurrent $1.00 decrease in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would decrease the net proceeds to us from this offering by approximately $ million after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The as adjusted information discussed above is illustrative only and will adjust based on the actual initial public offering price and other terms of this offering determined at pricing.
45
Table of Contents
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business and do not intend to declare or pay any cash dividends in the foreseeable future. As a result, you will likely need to sell your shares of common stock to realize a return on your investment, and you may not be able to sell your shares at or above the price you paid for them. Payment of cash dividends, if any, in the future will be at the discretion of our board of directors and will depend on then-existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
46
Table of Contents
The following table sets forth our capitalization as of June 30, 2014, on:
| • | an actual basis; |
| • | a pro forma basis giving effect to the conversion of (i) all outstanding shares of our convertible preferred stock into an aggregate of 11,719,975 shares of common stock upon completion of this offering, and (ii) the automatic conversion of the principal and accrued interest on the approximately $6.4 million aggregate principal amount of convertible promissory notes issued in June 2014 and the approximately $1.1 million aggregate principal amount of convertible promissory notes issued in August 2014 into an aggregate of shares of common stock, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering; and |
| • | a pro forma as adjusted basis giving additional effect to the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, as if the sale of the shares in this offering had occurred on June 30, 2014. |
The information in this table is illustrative only and our capitalization following the completion of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this table in conjunction with the information contained in “Use of Proceeds,” “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” as well as the financial statements and the notes thereto included elsewhere in this prospectus.
| As of June 30, 2014 | ||||||||||||
| (in thousands, except share amounts) | Actual | Pro Forma | Pro Forma as Adjusted |
|||||||||
| Convertible promissory notes |
$ | 4,345 | $ | — | $ | — | ||||||
| Embedded derivative |
1,651 | — | — | |||||||||
| Series B convertible redeemable preferred stock, $0.001 par value; 3,999,951 shares authorized, 3,999,951 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted |
7,565 | — | — | |||||||||
| Series B-1 convertible redeemable preferred stock, $0.001 par value; 2,466,218 shares authorized, 2,466,218 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted |
6,515 | — | — | |||||||||
| Series C convertible redeemable preferred stock, $0.001 par value; 4,400,000 shares authorized, 3,754,713 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted |
14,999 | — | — | |||||||||
| Stockholders’ equity (deficit): |
||||||||||||
| Series A convertible preferred stock, par value $0.001; 1,499,093 shares authorized, 1,499,093 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted |
1 | — | — | |||||||||
| Common stock, $0.001 par value, 16,345,262 shares authorized, 2,203,721 shares issued and outstanding, actual; shares authorized, pro forma and pro forma as adjusted; shares issued and outstanding, pro forma; and shares issued and outstanding, pro forma as adjusted |
2 | |||||||||||
| Additional paid-in capital |
2,040 | |||||||||||
| Accumulated deficit |
(29,504 | ) | ||||||||||
| Total stockholders’ deficit |
(27,460 | ) | ||||||||||
| Total capitalization |
$ | 7,614 | $ | $ | ||||||||
47
Table of Contents
The number of shares of our common stock outstanding immediately following this offering set forth above excludes:
| • | 1,335,946 shares of our common stock issuable upon the exercise of outstanding stock options as of June 30, 2014, at a weighted average price of $0.25 per share; |
| • | 889,453 shares of our common stock issuable upon the exercise of outstanding warrants as of June 30, 2014, of which warrants to purchase 550,000 shares have an exercise price of $0.01 per share, and warrants to purchase 339,453 shares have an exercise price of $0.50 per share; and |
| • | shares of our common stock reserved for issuance pursuant to future awards under our 2014 Stock Incentive Plan, including 140,333 shares of our common stock reserved for issuance under our 2009 Stock Incentive Plan as of June 30, 2014, which will become available for issuance under our 2014 Stock Incentive Plan following the consummation of this offering. |
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ (deficit) equity and total capitalization by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ (deficit) equity and total capitalization by approximately $ million, assuming that the assumed initial public offering price, the midpoint of the price range set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will adjust based on the actual initial public offering price and other terms of this offering determined at pricing.
The pro forma adjustments reflected in the June 30, 2014 balance sheet assume the conversion of the redeemable convertible preferred shares, the convertible preferred shares then outstanding but excluding accreted dividends in arrears and the convertible promissory note and embedded derivative, to common shares upon an initial public offering of our common stock. The pro forma adjustments to reflect the conversion resulted in: i) a reduction to the redeemable preferred stock and convertible preferred stock amounts to zero, ii) the reversal of accretion for unpaid dividends accumulated in arrears as unpaid dividends in arrears do not convert into common stock upon an initial public offering, iii) a reduction to the convertible promissory note and embedded derivative to reflect the conversion of these items into additional paid in capital and iv) an increase to common stock and additional-paid-in-capital to reflect the conversion into common shares. Certain deferred financing costs related to the transactions were reduced from other long-term assets and a corresponding entry was made to reduce additional-paid-in-capital. Accrued interest related to the convertible note was reduced from other current liabilities and a corresponding entry was made to increase additional-paid-in capital.
48
Table of Contents
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the assumed initial public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering. Net tangible book value per share of our common stock is determined at any date by subtracting our total liabilities and convertible preferred stock from the amount of our total tangible assets (total assets less intangible assets) and dividing the difference by the number of shares of our common stock deemed to be outstanding at that date.
Our historical net tangible book value (deficit) as of June 30, 2014 was approximately $(27.8) million, or $(19.85) per share of common stock, based on 1,401,637 shares of common stock outstanding as of June 30, 2014 (which excludes 802,084 shares of unvested restricted stock subject to repurchase by us). The pro forma net tangible book value (deficit) as of June 30, 2014 is approximately $ million, or approximately $ per share of common stock. The pro forma net tangible book value (deficit) per share gives effect to (i) the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 11,719,975 shares of common stock, which will occur immediately prior to the completion of this offering, and (ii) the conversion of the principal and accrued interest on the approximately $6.4 million aggregate principal amount of convertible promissory notes issued in June 2014 and the approximately $1.1 million aggregate principal amount of convertible promissory notes issued in August 2014 into an aggregate of shares of common stock, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering.
Investors participating in this offering will incur immediate and substantial dilution. After giving effect to our receipt of approximately $ million of estimated net proceeds, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, from our sale of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, our pro forma as adjusted net tangible book value (deficit) as of June 30, 2014, would have been $ million, or $ per share. This amount represents an immediate increase in net tangible book value of $ per share of our common stock to existing stockholders and an immediate dilution in net tangible book value of $ per share of our common stock to new investors purchasing shares of common stock in this offering.
The following table illustrates this dilution on a per share basis to new investors:
| Assumed initial public offering price per share |
$ | |||||||
| Historical net tangible book value (deficit) per share as of June 30, 2014 |
$ | (19.85 | ) | |||||
| Pro forma increase in net tangible book value (deficit) per share attributable to pro forma transactions described above |
$ | |||||||
| Pro forma net tangible book value (deficit) per share before this offering |
$ | |||||||
| Pro forma increase in net tangible book value (deficit) per share attributable to new investors |
$ | |||||||
| Pro forma as adjusted net tangible book value (deficit) per share after this offering |
$ | |||||||
| Dilution per share to new investors purchasing common stock in this offering |
$ | |||||||
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) our pro forma as adjusted net tangible book value (deficit) by $ million or by $ per share and the dilution to new investors in this offering by $ per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
We may also increase or decrease the number of shares we are offering. An increase of 1.0 million shares in the number of shares offered by us would increase our pro forma as adjusted net tangible book value (deficit) as of June 30, 2014 by approximately $ million or by $ per share and the dilution per share to new investors purchasing common stock in this offering by $ , assuming the number of shares offered by us, as set forth on
49
Table of Contents
the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Conversely, a decrease of 1.0 million shares in the number of shares offered by us would decrease our pro forma as adjusted net tangible book value (deficit) as of June 30, 2014 by approximately $ million or by $ per share and the dilution per share to new investors purchasing common stock in this offering by $ , assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriter exercises its over-allotment option in full, the pro forma as adjusted net tangible book value (deficit) per share after giving effect to this offering would be $ per share, which amount represents an immediate increase in pro forma net tangible book value (deficit) of $ per share of our common stock to existing stockholders and an immediate dilution in net tangible book value (deficit) of $ per share of our common stock to new investors purchasing shares of common stock in this offering.
The following table summarizes, as of June 30, 2014, after giving effect to the pro forma adjustments noted above, the differences between the number of shares purchased from us, the total consideration paid to us, and the average price per share paid to us by existing stockholders and by new investors purchasing shares in this offering, before deducting underwriting discounts and commissions and estimated offering expenses payable by us, at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus.
| Shares Purchased | Total Consideration | Average Price per Share | ||||||||
| Number | Percent | Amount | Percent | |||||||
| Existing stockholders |
||||||||||
| New investors |
||||||||||
| Total |
||||||||||
The number of shares of our common stock outstanding immediately following this offering is based on shares of our common stock outstanding as of June 30, 2014 (which excludes 802,084 shares of unvested restricted stock subject to repurchase by us), before giving effect to the pro forma transactions described above. This number also excludes:
| • | 1,335,946 shares of our common stock issuable upon the exercise of outstanding stock options as of June 30, 2014, at a weighted average price of $0.25 per share; |
| • | 889,453 shares of our common stock issuable upon the exercise of outstanding warrants as of June 30, 2014, of which warrants to purchase 550,000 shares have an exercise price of $0.01 per share, and warrants to purchase 339,453 shares, have an exercise price of $0.50 per share; and |
| • | shares of our common stock reserved for issuance pursuant to future awards under our 2014 Stock Incentive Plan, including 140,333 shares of our common stock reserved for issuance under our 2009 Stock Incentive Plan as of June 30, 2014, which will become available for issuance under our 2014 Stock Incentive Plan following the consummation of this offering. |
If all outstanding options and warrants to acquire our common stock had been exercised as of June 30, 2014, assuming the treasury stock method, our pro forma net tangible book value as of June 30, 2014 (calculated on the basis of the assumptions set forth above) would have been approximately $ million, or $ per share of our common stock, and the pro forma as adjusted net tangible book value would have been $ per share, representing dilution in our pro forma as adjusted net tangible book value per share to new investors.
To the extent that outstanding options and warrants are exercised, you will experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital by issuing equity securities or convertible debt, your ownership will be further diluted.
50
Table of Contents
You should read the following selected financial data together with the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of this prospectus and our consolidated financial statements and the accompanying notes appearing at the end of this prospectus. We have derived the statements of operations data for the years ended December 31, 2013 and 2012 and the balance sheet data as of December 31, 2013 and December 31, 2012 from our audited consolidated financial statements included elsewhere in this prospectus. We have derived the statements of operations data for the six months ended June 30, 2014 and 2013 and the balance sheet data as of June 30, 2014 from our unaudited financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of results to be expected for the full year or in any period in the future.
| Six months ended June 30, | Year ended December 31, | |||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| Consolidated statement of operations data: |
||||||||||||||||
| Revenue |
$ | 324,837 | $ | 106,466 | $ | 301,432 | $ | 33,350 | ||||||||
| Gross margin |
173,341 | 43,471 | 106,521 | 10,286 | ||||||||||||
| Net loss |
(3,621,783 | ) | (3,402,430 | ) | (7,527,232 | ) | (5,537,073 | ) | ||||||||
| Net loss attributable to common stockholders |
(4,950,946 | ) | (4,334,134 | ) | (9,459,179 | ) | (6,462,080 | ) | ||||||||
| Weighted average shares used in computing net loss attributable to common shareholders |
1,403,721 | 1,403,721 | 1,403,721 | 1,371,863 | ||||||||||||
| Net loss per share attributable to common shareholders: basic and diluted |
$ | (3.53 | ) | $ | (3.09 | ) | $ | (6.74 | ) | $ | (4.71 | ) | ||||
| As of June 30, | As of December 31, | |||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Consolidated balance sheet data: |
||||||||||||
| Cash and cash equivalents |
$ | 7,732,992 | $ | 5,389,003 | $ | 8,538,783 | ||||||
| Working capital |
6,659,531 | 4,701,592 | 7,606,550 | |||||||||
| Total assets |
9,667,914 | 6,351,838 | 8,999,609 | |||||||||
| Deferred Revenue |
$ | 277,179 | $ | 203,159 | $ | 81,464 | ||||||
| Total current liabilities |
2,052,098 | 1,576,941 | 1,310,034 | |||||||||
| Convertible promissory note |
4,345,281 | — | — | |||||||||
| Embedded derivative |
1,615,084 | — | — | |||||||||
| Convertible redeemable preferred stock |
29,079,612 | 27,832,029 | 21,400,387 | |||||||||
| Deficit accumulated during development stage |
(29,503,656 | ) | (24,634,290 | ) | (15,222,236 | ) | ||||||
| Total stockholders’ deficit |
(27,460,161 | ) | (23,057,132 | ) | (13,710,812 | ) | ||||||
51
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the consolidated financial statements and the notes to those statements included in this prospectus. This discussion and analysis may contain forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, such as those set forth under heading “Risk Factors,” and elsewhere in this prospectus.
This report includes various forward-looking statements that are subject to risks and uncertainties, many of which are beyond our control. Our actual results could differ materially from those anticipated in these forward looking statements as a result of various factors, including those set forth under “Risk Factors.” Forward-looking statements discuss matters that are not historical facts, and include, but are not limited to, discussions regarding our operating strategy, sales and marketing strategy, regulatory strategy, industry, economic conditions, financial condition, liquidity and capital resources and results of operations. Such statements include, but are not limited to, statements preceded by, followed by or that otherwise include the words “believes,” “expects,” “anticipates,” “intends,” “estimates,” “projects,” “can,” “could,” “may,” “will,” “would,” or similar expressions. You should not unduly rely on these forward-looking statements, which speak only as of the date on which they were made. They represent our expectations regarding the future but are not guarantees. We undertake no obligation to update publicly or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law.
Overview
We are a medical device company focused on the development and commercialization of a novel neurostimulation system for the treatment of severe gastroesophageal reflux disease, or GERD. Severe GERD is a debilitating condition that significantly disrupts patients’ quality of life, work productivity and sleep. Based on several international third party studies, we believe the impact of severe GERD on quality of life can be worse than that of chronic back pain or urinary incontinence, two other chronic conditions that are successfully treated with neurostimulation. Moreover, the impact of severe GERD on work productivity has been shown to be significantly greater than that attributable to back pain.
Our innovative approach targets the main cause of GERD — a dysfunctional lower esophageal sphincter, or LES, a muscle at the junction of the esophagus and stomach. The EndoStim neurostimulation system, which has a form and function similar to a pacemaker, delivers low energy electrical stimulation to the LES. In our clinical trials to date, this stimulation has been shown to improve the tone and function of the LES, thereby significantly reducing the pathological reflux symptoms of GERD. Our laparoscopically implantable neurostimulation system is minimally-invasive, reversible and preserves the anatomy of the esophagus and stomach.
GERD is the most common upper gastro-intestinal, or GI, disorder seen in clinical practice. It is estimated to affect 15-20% of the population in developed countries and 5% in developing nations. Today, GERD is most often treated with chronic medication, specifically proton pump inhibitors, or PPIs, such as Nexium and Prilosec, which suppress the production of acid in the stomach. However, PPIs do not address the main cause of GERD, a dysfunctional LES. Therefore, PPIs do not prevent the regurgitation of stomach contents into the esophagus. As a result, 38% of patients taking PPIs to treat GERD reported continuing symptoms that were not resolved by their medication, according to a 2010 study of over 1,000 patients by the American Gastroenterological Association. We expect to initially target a subset of these patients who experience severe symptoms while on medication, and whose GERD can be confirmed by an esophageal acid test. We estimate this subset to comprise approximately 4.6% of all GERD patients, or about 21 million patients worldwide.
52
Table of Contents
In 2012, we received CE Mark regulatory approval to commercialize our neurostimulation system in Europe and in other countries recognizing the CE Mark. We launched our system commercially in 2013. More than 40 sites in Germany, Switzerland, Argentina and Colombia have obtained access to third-party reimbursement for the procedure. We currently use a direct sales force in key European countries and specialized distributors in other European countries, South America and Asia.
In the United States, our device has not been approved for sale by the FDA. However, we have received approval from the FDA for our Investigational Device Exemption, or IDE, application, which allows us to conduct a pivotal clinical trial in the United States. The trial design is double-blind sham-controlled, which means that neither the investigator nor the patient knows whether or not the patient implanted with the device is actually receiving stimulation therapy. This guards against a “sham” or “placebo” effect where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment, or indirectly as a result of the investigator’s behavior. The clinical trial endpoints which the FDA will use to evaluate the effects of our neurostimulation treatment, are similar to the endpoints of our prior overseas clinical work. Most importantly, in our prior long-term clinical trials, we have consistently demonstrated the positive effect of our treatment on acid exposure to the esophagus. This is the outcome required by the FDA as the primary endpoint of our United States pivotal trial.
To date, over 130 patients have been implanted with our system, 65 of whom have been treated in open-label clinical trials. These clinical trials have shown promising outcomes with respect to the safety and clinical efficacy of our technology as measured by objective and subjective criteria described under “— Clinical Trials.” The results of these clinical trials have been published in peer-reviewed medical journals. The trials have been conducted in Europe, South America and Asia with a follow-up period of up to three years.
Our revenue is generated from sales of our implantable neurostimulator and our programmers. We are currently leveraging the CE Mark to commercialize our system in Europe, South America and Asia and all of our near term revenue will be generated outside of the United States. Since our inception through June 30, 2014, we have generated an aggregate of approximately $660,000 in revenue from sales of our products in 13 countries with a significant percentage of this revenue generated in Germany where reimbursement for a limited number of our procedures was available through a fast track method for the introduction of new technologies. Sites in Argentina and Colombia have obtained initial reimbursement approval for the procedure. We expect that the number of procedures and hospitals eligible for such reimbursement will increase. We intend to focus our sales efforts on those markets where reimbursement is available, including Colombia and Argentina where reimbursement for initial procedures was recently approved, and focus our marketing efforts to expand our hospital customer base and increase the flow of patients to those sites. We engage a direct sales force in key European countries, in particular Germany, and sell through specialized distributors or sales agents elsewhere, particularly in South America and Asia. We have a limited number of sales resources and intend to expand our sales force to market our products directly to hospitals in key countries and to train and manage our distributors in other areas of the world.
Our Implantable Pulse Generator (IPG) is developed and manufactured by a third party in Uruguay with extensive experience in developing and manufacturing active implantable devices. While we believe this manufacturer has sufficient capacity to accommodate our growth in the near future, as we increase our volume, we may move our manufacturing to a higher volume facility possibly within the United States.
Since our inception, our principal activities involved obtaining capital, business development, performing research and development activities, and funding prototype development. We have been classified as a development-stage company from our inception on June 3, 2009 through June 30, 2014.
For the years ended December 31, 2013 and 2012, our total revenues were approximately $301,000 and $33,000, respectively. We incurred net losses of $7.5 million and $5.5 million during the years ended December 31, 2013 and 2012, respectively prior to a deduction for the accretion of dividends related to our convertible redeemable
53
Table of Contents
preferred stock of $1.9 million and $0.9 million, and a deduction for undeclared dividends on Series A convertible preferred stock of $47,000 and $71,000, during the years ended December 31, 2013 and 2012, respectively. For the six months ended June 30, 2014 and 2013, our total revenues were approximately $325,000 and $106,000, respectively. We incurred net losses of $3.6 million and $3.4 million during the six months ended June 30, 2014 and 2013, respectively, prior to a deduction for the accretion of dividends related to our convertible redeemable preferred stock of $1.2 million and $0.9 million, and a deduction for undeclared dividends on Series A convertible preferred stock of $82,000 and $24,000, during the six months ended June 30, 2014 and 2013, respectively. As of June 30, 2014, we had cash and cash equivalents of $7.7 million and a deficit accumulated during development stage of $29.5 million. We are not profitable and expect to continue to incur significant losses for the foreseeable future. To date, we have funded our operations with proceeds from the sale of approximately $6.4 million of convertible promissory notes issued in June 2014, approximately $1.1 million of convertible promissory notes issued in August 2014, and $27.1 million of preferred stock, before deductions for offering expenses, including $1.5 million from the sale of our Series A convertible Preferred Stock (including the conversion of approximately $0.3 million in convertible notes), $6.0 million from the sale of our Series B convertible redeemable Preferred Stock, $5.5 million from the sale of our Series B-1 convertible redeemable Preferred Stock and $14.1 million from the sale of our Series C convertible redeemable Preferred Stock. Our net losses and negative cash flows have had and will continue to have an adverse effect on our stockholders’ deficit and working capital.
We expect to increase our sales and marketing expenses to expand the market for, and increase the adoption of, our products, train surgeons, seek hospital approvals and pursue third-party payor coverage and reimbursement. We expect to increase our research and development expenses as we pursue FDA approval for our products in the United States and to continue to make product improvements or introduce new technologies. We expect to increase our general and administrative expenses, including regulatory and quality, accounting and legal to support our growing organization and the cost of being a public company.
Critical Accounting Policies and Use of Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements which have been prepared in accordance with generally accepted accounting principles (GAAP). The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and related disclosure of contingent assets and liabilities, revenue and expenses at the date of the consolidated financial statements. We base our estimates on historical experience and on various other assumptions in accordance with GAAP that we believe to be reasonable under the circumstances. Actual results may differ from these estimates and such differences could be material to the financial position and results of operations.
Critical accounting policies and estimates are those that we consider the most important to the portrayal of our financial condition and results of operations because they require our most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. We believe the following accounting policies are critical to the judgments and estimates we use in preparing our financial statements.
Revenue Recognition
Our revenue is generated from the sale of our implantable neurostimulator and its associated programmer, which communicates wirelessly with the implanted neurostimulation system. We sell both directly to hospitals and through distributors. We recognize revenue when all four revenue recognition criteria have been met: persuasive evidence of an arrangement exists; delivery has occurred; the price is fixed or determinable; and collectability is reasonably assured. In general, our customers do not have any rights of return or exchange. Our revenue recognition policy generally results in revenue recognition at the time of delivery to the hospital or distributor for our programmers and upon the implantation of our neurostimulators.
54
Table of Contents
For certain transactions, we have multiple-element arrangements that include our neurostimulators and programmers. For multiple-element arrangements, revenue is allocated to each element based on its relative selling prices. Relative selling prices are based first on vendor specific objective evidence (“VSOE”), then on third-party evidence of selling price (“TPE”) when VSOE does not exist, and then on estimated selling price (“ESP”) when VSOE and TPE do not exist. We do not have VSOE for our neurostimulators and programmers because we recently began commercialization of our products and we do not yet have enough data on stand-alone transactions to establish VSOE. Because we have neither VSOE nor TPE for our products, the allocation of revenue has been based on our ESPs. The objective of ESP is to determine the price at which we would transact a sale if the product was sold on a stand-alone basis. We determine the ESPs for our products by considering multiple factors including, but not limited to, geography and market conditions. Amounts collected prior to satisfying the above revenue recognition criteria are reflected as deferred revenue.
Inventory Valuation
Our inventory generally consists of finished goods which are purchased from a third-party supplier located in Uruguay. We value our inventory at the lower of the actual cost of our inventory, as determined using the first-in, first-out (FIFO) method or its net realizable value. We periodically review our physical inventory for excess, obsolete or potentially impaired items, taking into account product development plans, product expiration and current inventory and reserve accordingly. As of June 30, 2014, we have not established a reserve because, based on our estimate of future use, we do not believe that we have excess, obsolete or impaired inventory. In the future, we may be required to take charges for excess and obsolete inventory that could have a significant impact on the value of our inventory and our operating results.
Stock Based Compensation and Common Stock Valuation
In 2009, our board of directors adopted our 2009 Stock Incentive Plan, which allows for the grant of incentive stock options and non-qualified stock options to employees, board members, and consultants. Options granted under the 2009 Stock Incentive Plan expire no later than 10 years from the date of grant. The exercise price of each incentive stock option must not be less than 100% of the fair value of the stock subject to the option on the date the option is granted. The vesting provisions of individual options may vary, but option grants generally vest ratably over a period of one to four years. Our compensation committee administers and grants equity awards under the 2009 Stock Incentive Plan.
We account for our stock-based compensation in accordance with ASC 718, Compensation — Stock Compensation, which establishes accounting for share-based awards, including stock options and restricted stock, exchanged for services and requires companies to expense the estimated fair value of these awards over the requisite service period, which is the vesting period. When we grant options to employees and directors, we record stock options at fair value as of the grant date and recognize the associated expense over the term of the service period.
We used the Black-Scholes pricing model to determine the fair value of stock options with the fair value of each option grant being estimated on the date of the grant. This model incorporates various assumptions including expected volatility, expected term, risk-free interest rates, and expected dividend yields. The assumptions used in the Black-Scholes option-pricing method for the six months ended June 30, 2014 and for the years ended December 31, 2013 and 2012 are set forth below:
| Six Months ended June 30, |
Year ended December 31, | |||||
| 2014 | 2013 | 2012 | ||||
| Risk-free interest rate |
1.53%-2.02% | 0.14%-0.76% | 0.26%-0.50% | |||
| Expected dividend yield |
0% | 0% | 0% | |||
| Expected term (in years) |
5.75-6.5 | 5-6 | 6 | |||
| Expected volatility |
60% | 60% | 60% | |||
55
Table of Contents
Risk-free interest rate. The risk-free interest rate is based on a Treasury instrument whose term is consistent with the expected life of the stock options.
Expected dividend yield. We have not paid, and do not anticipate paying or declaring, cash dividends on our common stock; therefore, the expected dividend yield is assumed to be zero.
Expected term. The expected term of options represents the period of time that options are expected to be outstanding. Because we have limited historic exercise behavior, we determine the expected term assumption using the simplified method, which is an average of the contractual term of the option and its ordinary vesting period.
Expected volatility. For purposes of using the Black-Scholes option-pricing model, and because our stock is not publicly traded, the volatility for options was determined based on an analysis of reported data for a peer group of companies. The expected volatility of options granted has been determined using an average of the historical volatility measures of this peer group. As a public company, we will also consider the historical volatility of our own stock.
The amount of compensation expense to be recorded in future periods may increase if we make additional grants of options or restricted shares or if the fair value of such grants on the date of measurement is based on a higher stock price or if we determine that actual forfeiture rates are less than anticipated. The amount of expense may also increase if we determine that the performance conditions related to 800,000 outstanding restricted share awards would be achieved. The amount of expense to be recorded in future periods may decrease if the actual forfeiture rates are greater than anticipated. Moreover, if we determine that other methods are more reasonable, or other methods for calculating these assumptions are prescribed by regulators, the fair value calculated for our stock options could change significantly. Higher volatility and longer expected lives would result in an increase to stock-based compensation expense determined at the date of grant. Stock-based compensation expense affects our research and development, sales and marketing and general and administrative expenses.
Common Stock Valuation
Due to the absence of an active market for our common stock, the fair value of our common stock for purposes of determining the exercise price for stock option grants was determined by our compensation committee, with the assistance and upon the recommendation of management, in good faith, based on a number of objective and subjective factors including:
| • | the prices at which we most recently sold our convertible preferred stock and our convertible redeemable preferred stock and the rights, preferences and privileges of the convertible preferred stock as compared to those of our common stock, including: |
| • | the redemption rights of certain classes of convertible redeemable preferred stock; |
| • | the liquidation preferences of all of the convertible preferred stock; |
| • | rights with respect to cumulative dividends of the convertible preferred stock; |
| • | anti-dilution protections for our convertible preferred stock; |
| • | certain voting rights of such stock as reflected in our charter; and |
| • | various other rights in other agreements among the stockholders, including registration rights, rights of first refusal and drag-along rights; |
| • | the progress made on our business plan; |
| • | our results of operations, our research and development program, the results of our clinical trials, regulatory status in countries we are targeting both outside and in the United States and our commercialization program; |
56
Table of Contents
| • | our financial position including the need for and availability of cash to fund our future operations considering the general market conditions for private company financings for development stage companies; and |
| • | the material risks related to our business. |
Valuation — 2012
February 17, 2012 through August 22, 2012. Our compensation committee granted 35,000 stock options on February 17, 2012, 5,000 stock options on May 3, 2012 and 69,000 stock options on August 22, 2012, all having an exercise price of $0.15 per share, which our compensation committee determined to be equal to the fair value of our common stock on the date of grant. In determining the exercise price, our compensation committee also considered:
| • | the sale in November 2011 of 822,071 shares of the first tranche of our Series B-1 convertible preferred stock at a purchase price of $2.25 per share for aggregate gross proceeds of about $1.85 million; |
| • | the rights, preferences and privileges of our convertible preferred stock, in particular the liquidation preferences, and the redemption rights of our convertible redeemable preferred stock; and |
| • | the regulatory status of our neurostimulation system. |
The committee determined the value considering the objective and subjective factors described above. As part of this determination, our compensation committee concluded on subsequent dates that no significant internal or external value-generating events had taken place since the February 17, 2012 valuation other than our completion of an additional closing of our Series B-1 convertible preferred stock financing in May 2012, which had been taken into consideration as part of the February 17, 2012 valuation, and our receipt of CE Mark approval of our neurostimulation system on August 16, 2012; however, our compensation committee did not accord significant weight to the CE Mark approval at that time because no commercialization activities had occurred.
November 6, 2012. Our compensation committee granted 30,000 stock options with an exercise price of $0.25 per share, which our compensation committee determined to be equal to the fair value of our common stock on the date of grant. The facts and circumstances considered by our compensation committee in making this determination included the factors described above. The resulting value, which represented the estimated fair value of our common stock as of November 6, 2012, was $0.25 per share. The increase in valuation between August 22, 2012 and November 6, 2012 resulted primarily from the commencement of early commercialization activities.
Valuation — 2013
February 20, 2013 through March 19, 2013. During this period, our compensation committee granted 295,000 stock options on February 20, 2013 and 54,000 stock options on March 19, 2013, all having an exercise price of $0.40 per share, which our compensation committee determined to be equal to the fair value of our common stock on the date of grant. In determining the exercise price, our compensation committee also considered the following:
| • | the initial closings in December 2012 of 2,435,682 shares of our Series C convertible redeemable preferred stock at a purchase price of $3.75 per share for aggregate gross proceeds of $9.1 million; |
| • | the rights, preferences and privileges of our convertible preferred stock, in particular the liquidation preferences, and the redemption rights of our convertible redeemable preferred stock; and |
| • | our progress on our early commercialization efforts; |
The increase in valuation between November 6, 2012 and February 20, 2013 resulted primarily from the successful completion of the Series C financing.
57
Table of Contents
May 31, 2013 through December 17, 2013 Valuations. Our compensation committee granted 112,500 stock options on May 31, 2013, 120,000 stock options on July 1, 2013, 25,000 stock options on August 21, 2013 and 30,000 stock options on December 17, 2013, all having an exercise price of $0.40 per share, which was determined to be equal to the fair value of our common stock on the date of grant. The committee determined the value considering the valuation as of February 20, 2013 and the other objective and subjective factors described above including early commercialization results and challenges. As part of this determination, our compensation committee concluded on such dates that no significant internal or external value-generating events had taken place since the February 20, 2013 valuation other than our completion of an additional closing of our Series C financing through May 2013, which had been taken into consideration as part of the February 20, 2013 valuation.
January 22, 2014 through February 19, 2014 Valuation. Our compensation committee granted 25,000 stock options on January 22, 2014 and 100,000 stock options on February 19, 2014, at an exercise price of $0.40 and $0.50 per share, respectively, which our compensation committee determined to be equal to the fair value of our common stock on the date of grant. Our compensation committee considered our current cash requirements as well as the general market conditions for financing development stage companies and our commercialization progress. The increase in valuation between December 17, 2013 and February 19, 2014 resulted primarily from an increase in commercialization activities.
Deferred Income Taxes
Deferred assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using the enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. We have established a valuation allowance against the entire amount of our deferred tax assets because we are not able to conclude, due to our history of operating losses, that it is more likely than not that we will be able to realize any portion of the deferred tax assets.
Convertible Redeemable Preferred Stock
We categorize our convertible redeemable preferred stock as liabilities or temporary equity within the consolidated balance sheets based on the terms of the preferred stock agreements. To the extent the redemption is mandatory, or would require us to settle in an undeterminable number of common shares that may not be solely within our control to issue, we record the securities as liabilities. We do not have any securities classified as liabilities for the years ended December 31, 2013 and 2012 or for the six months ended June 30, 2014. To the extent that the redemption is required as of a fixed or determinable date, upon the occurrence of certain contingent events not solely within our control or at the option of the holder, the preferred stock securities are recorded as temporary equity.
Convertible redeemable preferred stock is recorded at the initial carrying amount which is generally its fair value on the date of issuance within temporary equity. The convertible redeemable preferred shares are not currently redeemable but will become redeemable at the sixth anniversary from the issuance date at the option of the holders. We have accreted all declared or accrued and unpaid dividends over the period from the date of issuance to the sixth anniversary date using the interest method in subsequently measuring the redemption value. The amount of accrued unpaid dividends on convertible redeemable preferred stock within temporary equity is recorded as an increase in the deficit accumulated during development stage.
Convertible Promissory Notes
On June 25, 2014 and August 28, 2014, we issued secured convertible promissory notes which accrue interest at the rate of 10% per annum and mature on June 25, 2016, unless earlier converted. Under the terms of the notes, we agreed to issue warrants to purchase shares of our common stock. Amounts outstanding under the convertible promissory notes convert automatically into shares of our common stock at a 20% discount upon a qualified
58
Table of Contents
financing event of at least $10,000,000 as defined in the Note and Warrant Purchase Agreement. We determined that the conversion features were an embedded derivative instrument which was required to be bifurcated from the associated debt instrument and accounted for separately as a derivative instrument liability. The fair value of the 2014 Notes embedded derivative was calculated by using the “with and without” approach, whereby the value of the embedded derivatives was determined as the difference between the value of the related debt host instrument with the embedded features and the value of the debt host instrument without the embedded derivative features. The value of the debt host instrument with the embedded features was determined using a Monte Carlo simulation model. A Monte Carlo simulation uses statistical sampling to predict outcomes based on variable inputs. This valuation methodology uses Level 3 inputs and requires us to make assumptions related to the probabilities of various outcomes. The significant unobservable inputs used in our fair value measurement include the probability and price of conversion. Because our stock has not traded publicly, we estimated the future volatility based on the experience of other entities considered comparable. We estimated the fair value of the derivative using a Monte Carlo simulation model. A Monte Carlo simulation uses statistical sampling to predict outcomes based on variable inputs. This valuation methodology requires us to make assumptions related to the probabilities of various outcomes. We will revalue this derivative at the end of each reporting period and record any changes to the fair value as interest expense in the Statement of Operations. The warrants were determined to be free standing instruments and classified as equity. Debt issuance costs were allocated between the debt and the warrant using the relative fair value. Debt issuance costs related to the debt will be expensed using the effective interest method over the underlying term of the loan.
Recently Issued Accounting Pronouncements
Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are electing to delay such adoption of new or revised accounting standards, and as a result, we may not comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. As a result of this election, our financial statements may not be comparable to the financial statements of other public companies. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.”
The Financial Accounting Standards Board (the FASB) and the International Accounting Standards Board (the IASB) (collectively, the Boards) have jointly issued a new revenue recognition standard that will supersede virtually all revenue recognition guidance in US GAAP and IFRS. The new revenue standard creates a single source of revenue guidance for companies, regardless of industry and is more principles-based than the current revenue guidance. The new standard is effective for public entities for fiscal years beginning after December 15, 2016 and for interim periods therein. Early adoption is not permitted for public entities. The Company has not yet evaluated the transition approach or the impact of this standard on its results of operations or its financial condition.
Basis of Presentation
The following describes the line items set forth in our statements of operations.
Revenue
We generate revenue from the sale of our implantable neurostimulator and associated programmer, which communicates wirelessly with our implanted neurostimulation system. All of our revenue is generated from outside of the United States. In certain countries, primarily in Europe, we sell our products directly to hospitals. In many countries in which we do not have a presence, particularly in South America and Asia, we utilize a network of distributors which in turn, sell our products directly to hospitals, physicians or end customers. These distributors are generally providers of medical equipment used by surgeons, gastroenterologists or both.
59
Table of Contents
We anticipate our revenue will increase as we expand our sales and marketing infrastructure, increase awareness of our products and broaden third party reimbursement for our products. We expect to increase revenue by increasing adoption in the countries in which we are already commercializing and by expanding our geographic presence into other countries.
Cost of Revenue
We rely on a third-party supplier located in Uruguay to manufacture our products. Our cost of revenue primarily consists of costs of products purchased from our third-party supplier, accessories, shipping and warehousing costs, and overhead incurred in making our products available for sale or use.
We expect the average unit costs of our products to decline in future periods as a result of our ability to obtain volume discounts on purchases from our supplier. Although our contract with our current supplier is currently denominated in U.S. Dollars, we may also experience currency fluctuations related to other components of cost of revenue that could impact the cost of the products.
Our future gross margins will be impacted by numerous factors including the percentage of products sold through distributors as compared with direct customers, the countries in which our products are sold and our ability to realize manufacturing and distribution efficiencies as our production and sales volume increases.
Operating Expenses
Research and Development
Research and development expenses consist primarily of the costs associated with the development and clinical testing of our products. Research and development includes expenses related to third-party development costs for our implantable neurostimulator and associated programmer, personnel-related expenses, including salaries, benefits and travel, clinical trial expenses paid to hospitals and third-party consultants and related costs. We expense research and development expenses as they are incurred. We have made substantial investments in research and development since our inception and we expect that research and development expenses will continue to increase as we commence our U.S. and other clinical trial activities and continue to make improvements to our products.
Sales and Marketing
Our sales and marketing expenses primarily support our international commercialization strategy. Our sales and marketing expenses consist primarily of personnel-related expenses, including salaries, benefits and travel, and expenses for promotional and marketing activities, including scientific conferences and country specific promotional activities. We expect sales and marketing expenses to continue to increase to support our expanding commercialization activities.
General and Administrative
General and administrative expenses consist primarily of personnel-related expenses, including salaries and benefits for employees in our general management, regulatory, quality, finance and administrative functions. In addition, general and administrative expenses include professional services, such as corporate and intellectual property legal services and accounting and tax services. We expect legal, accounting and compliance costs to increase due to costs associated with our initial commercialization efforts and to being a public company, although we cannot be certain as to what these costs will be.
Other (Expense) Income
Other (expense) income consists primarily of interest expense related to our convertible promissory note.
60
Table of Contents
Result of Operations
Comparison of Six Months Ended June 30, 2014 and 2013
Revenue
| Six months ended June 30, |
Change | |||||||||||||||
| 2014 | 2013 | Dollars | % | |||||||||||||
| Revenue |
$ | 324,837 | $ | 106,466 | $ | 218,371 | 205 | % | ||||||||
The increase in revenue for the six months ended June 30, 2014 as compared to the six months ended June 30, 2013 was attributable to an increase in the number of active sites completing implantations of our neurostimulation system which resulted in more revenue. Additionally, the average revenue recognized per neurostimulation system increased because more neurostimulation systems were implanted in countries in which we sell directly to health care providers rather than through distributors. We realize a higher average selling price when we sell directly to a health care provider than we do when we sell to a distributor which in turn sells the product to a health care provider.
Cost of Revenue and Gross Margin
| Six months ended June 30, |
Change | |||||||||||||||
| 2014 | 2013 | Dollars | % | |||||||||||||
| Cost of revenue |
$ | 151,496 | $ | 62,995 | $ | 88,501 | 140 | % | ||||||||
| Gross margin |
$ | 173,341 | $ | 43,471 | $ | 129,870 | 299 | % | ||||||||
| Gross margin % |
53 | % | 41 | % | ||||||||||||
The increase in cost of revenue in absolute dollars for the six months ended June 30, 2014 as compared to the six months ended June 30, 2013 was attributable to the increase in sales volume as the number of active sites increased and more neurostimulation systems were implanted. The increase in gross margin as a percentage of revenue was attributable to higher average revenue realized per neurostimulation system implanted.
Research and Development Expense
| Six months ended June 30, |
Change | |||||||||||||||
| 2014 | 2013 | Dollars | % | |||||||||||||
| Research and development |
$ | 1,256,966 | $ | 1,337,758 | $ | (80,792 | ) | (6% | ) | |||||||
The decrease in research and development expense for the six months ended June 30, 2014 as compared to the six months ended June 30, 2013 was principally attributable to higher clinical trial activity during the 2013 period.
Sales and Marketing Expense
| Six months ended June 30, |
Change | |||||||||||||||
| 2014 | 2013 | Dollars | % | |||||||||||||
| Sales and marketing |
$ | 1,392,508 | $ | 1,056,177 | $ | 336,331 | 32 | % | ||||||||
The increase in sales and marketing expense for the six months ended June 30, 2014 as compared to the six months ended June 30, 2013 was principally attributable to an increase in personnel-related expenditures
61
Table of Contents
including compensation and travel to support the launch of our products in Europe and to a lesser extent to increase our marketing and promotional activities.
General and Administrative Expense
| Six months ended June 30, |
Change | |||||||||||||||
| 2014 | 2013 | Dollars | % | |||||||||||||
| General and administrative |
$ | 1,125,466 | $ | 1,055,172 | $ | 70,294 | 7 | % | ||||||||
The increase in general and administrative expense for the six months ended June 30, 2014 as compared to the six months ended June 30, 2013 was principally attributable to increased personnel related expenditures to support our increased commercialization activities and legal expenditures related to the maintenance of our intellectual property portfolio.
Accretion of redeemable preferred stock
| Six months ended June 30, |
Change | |||||||||||||||
| 2014 | 2013 | Dollars | % | |||||||||||||
| Accretion of redeemable preferred stock |
$ | 1,247,583 | $ | 908,142 | $ | 339,441 | 37 | % | ||||||||
The increase in the accretion of redeemable preferred stock for the six months ended June 30, 2014 as compared to the six months ended June 30, 2013 was attributable principally to the dividend associated with the Series C convertible redeemable preferred stock financing. We recorded an investment in our Series C convertible redeemable preferred stock of $9.1 million completed in December 2012 and $4.9 million during the six months ended June 30, 2013. During the six months ended June 30, 2014, we recorded dividends associated with the December 2012 investment and a full six months of the investments made during the six months ended June 30, 2013. In addition, our Series B and B-1 redeemable preferred stock also had an additional year of dividend accretion.
Other (expense) income
| Six months ended June 30, |
Change | |||||||||||||||
| 2014 | 2013 | Dollars | % | |||||||||||||
| Other (expense) income |
$ | (20,184 | ) | $ | 3,206 | $ | 23,390 | 730 | % | |||||||
The increase in other expense for the six months ended June 30, 2014 as compared to the six months ended June 30, 2013 was principally attributable to interest expense recorded for our convertible promissory notes.
Comparison of Twelve Months Ended December 31, 2013 and 2012
Revenue
| Twelve months ended December 31, |
Change 2013 v. 2012 |
|||||||||||||||
| 2013 | 2012 | Dollars | % | |||||||||||||
| Revenue |
$ | 301,432 | $ | 33,350 | $ | 268,082 | 804 | % | ||||||||
The increase in revenue for the year ended December 31, 2013 as compared to the year ended December 31, 2012 was attributable to a full year of commercial activities in 2013 as contrasted with approximately one
62
Table of Contents
calendar quarter of activities in 2012 as we received European regulatory approval to commence marketing our products in August 2012. As a result of these activities, we established more active sites in 2013 and we recognized more revenue as our customers implanted more neurostimulation systems.
Cost of Revenue and Gross Margin
| Twelve months ended December 31, |
Change | |||||||||||||||
| 2013 | 2012 | Dollars | % | |||||||||||||
| Cost of revenue |
$ | 194,911 | $ | 23,064 | $ | 171,847 | 745 | % | ||||||||
| Gross margin |
$ | 106,521 | $ | 10,286 | $ | 96,235 | 936 | % | ||||||||
| Gross margin % |
35 | % | 31 | % | ||||||||||||
The increase in cost of revenue in absolute dollars for the year ended December 31, 2013 as compared to the year ended December 31, 2012 was attributable to the increase in sales volume as our customers implanted more neurostimulation systems. As we are in the early stages of commercialization, the increase in gross margin percentage is not meaningful. We expect our gross margin to increase over time as our sales volume increases.
Research and Development Expense
| Twelve months ended December 31, |
Change | |||||||||||||||
| 2013 | 2012 | Dollars | % | |||||||||||||
| Research and development |
$ | 3,195,979 | $ | 2,613,429 | $ | 582,550 | 22 | % | ||||||||
The increase in research and development expense for the year ended December 31, 2013 as compared to the year ended December 31, 2012 was principally attributable to increased engineering expenditures related to product improvements and increased personnel costs associated with the management of our clinical trials offset by a reduction in direct clinical trial expenses as more patients were enrolled in clinical trials in 2012.
Sales and Marketing Expense
| Twelve months ended December 31, |
Change | |||||||||||||||
| 2013 | 2012 | Dollars | % | |||||||||||||
| Sales and marketing |
$ | 2,232,514 | $ | 1,025,364 | $ | 1,207,150 | 118 | % | ||||||||
The increase in sales and marketing expense for the year ended December 31, 2013 as compared to the year ended December 31, 2012 was principally attributable to an increase in personnel-related expenditures including salaries, benefits and taxes to support the launch of our products in Europe and to a lesser extent, in South America and Asia. We also increased our marketing research and general marketing activities and expanded our promotional activities into more countries.
General and Administrative Expense
| Twelve months ended December 31, |
Change | |||||||||||||||
| 2013 | 2012 | Dollars | % | |||||||||||||
| General and administrative |
$ | 2,211,222 | $ | 1,910,423 | $ | 300,799 | 16 | % | ||||||||
63
Table of Contents
The increase in general and administrative expense for the year ended December 31, 2013 as compared to the year ended December 31, 2012 was principally attributable to increased personnel related expenditures as we expanded our regulatory, quality and administrative activities in response to increased commercialization activities.
Accretion of redeemable preferred stock
| Twelve months ended December 31, |
Change | |||||||||||||||
| 2013 | 2012 | Dollars | % | |||||||||||||
| Accretion of redeemable preferred stock |
$ | 1,884,822 | $ | 853,561 | $ | 1,031,261 | 121 | % | ||||||||
The increase in the accretion of redeemable preferred stock for the year ended December 31, 2013 as compared to the year ended December 31, 2012 was attributable to the dividend associated with the Series C convertible redeemable preferred stock financing. In 2012, we recorded dividends associated with an investment of $9.1 million in our Series C convertible redeemable preferred stock completed in December 2012. In 2013, we recorded dividends associated with the December 2012 investment and with an additional $4.9 million investment in the first half of 2013. In addition, our Series B and B-1 redeemable preferred stock also had an additional year of dividend accretion.
Income Tax
Realization of deferred tax assets is dependent upon future earnings, the timing and amount of which are uncertain. Accordingly, net deferred tax assets have been fully offset by valuation allowances as of June 30, 2014 and December 31, 2013 and 2012 to reflect these uncertainties. As of December 31, 2013, we had federal net operating loss carryforwards of approximately $20.8 million which will expire at various dates between 2029 and 2033 if not utilized. We may not be able to utilize all of these loss carryforwards prior to their expiration. We have a wholly-owned subsidiary in the Netherlands. It is possible that this entity may generate income requiring the payment of taxes even though the parent company has a net operating loss.
Liquidity and Capital Resources
Since our inception in 2009, we have incurred significant losses, and as of June 30, 2014, we had a deficit accumulated during development stage of approximately $29.5 million. We have not yet achieved profitability, and anticipate that we will continue to incur losses. We expect that research and development, sales and marketing and general and administrative expenses will continue to grow, and, as a result, we will need to generate significant revenues to achieve profitability.
To date, we have funded our operations primarily with proceeds from the sale of shares of preferred stock which are convertible into shares of our common stock and from sale of convertible promissory notes that are automatically convertible into shares of common stock or preferred stock on terms set forth in the notes. Net proceeds from our sales of preferred stock and convertible notes total approximately $33.3 million to date, net of a deduction for offering expenses as follows:
| • | Series A Convertible Preferred Stock. In 2009, we issued 8% convertible promissory notes, which were converted into 275,000 shares of our Series A convertible preferred stock at the time of the Series A convertible preferred stock offering in 2010. We issued 1,224,093 of additional shares of Series A convertible preferred stock between September 2009 and March 2010. We realized approximately $1.4 million, net of deductions for offering costs on the sale of our Series A convertible preferred stock. |
| • | Series B Convertible Redeemable Preferred Stock. We issued 3,999,951 shares of our Series B convertible redeemable preferred stock between July 2010 and May 2011. We realized approximately $5.8 million, net of deductions for offering costs on the sale of our Series B convertible redeemable preferred stock. |
64
Table of Contents
| • | Series B-1 Convertible Redeemable Preferred Stock. We issued a total of 2,466,218 shares of our Series B-1 convertible redeemable preferred stock between November 2011 and May 2012 in three separate offerings, respectively. We realized approximately $5.5 million, net of deductions for offering costs on the sale of our Series B-1 convertible redeemable preferred stock. |
| • | Series C Convertible Redeemable Preferred Stock. We issued a total of 3,754,713 shares of Series C convertible redeemable preferred stock between December 2012 and May 2013. We realized approximately $13.1 million, net of deductions for offering costs on the sale of our Series C convertible redeemable preferred stock. |
| • | Convertible Promissory Notes. We issued $6,364,780 in aggregate principal amount of secured convertible promissory notes on June 25, 2014 and $1.1 million in aggregate principal amount on August 28, 2014. Interest on all amounts outstanding accrues at a rate of 10% per annum. The notes mature on June 25, 2016, unless earlier converted. The principal amount and all accrued but unpaid interest under these notes convert automatically upon the closing of this offering into shares of our common stock at a price per share equal to 80% of the public offering price (before underwriting discounts and commissions and other offering expenses). |
Our principal use of cash is to fund our operations, which includes the commercialization of our neurostimulator system in a number of countries that recognize the CE Mark, the pursuit of regulatory approval in the United States through the Federal Drug Administration (“FDA”) premarket approval (“PMA”) process, the continuation of efforts to improve our products, administrative support of our operations and other working capital requirements. We plan to enroll a minimum of 110 patients in our pivotal double-blind, sham-controlled clinical trial to pursue regulatory approval in the United States. We are scheduled to begin recruiting patients in 2015 for this trial, and expect to complete enrollment in 2016. We expect to submit our PMA application in 2017, which could result in FDA approval, subject to trial outcomes, in 2018. We currently anticipate that the cost of the trial will be approximately $7.5-8.5 million.
We believe that the net proceeds of this offering, together with our existing cash balances, will be sufficient to cover our cash needs for the next months. We may need to raise additional funds to support our operating activities, and such funding may not be available to us on acceptable terms, or at all. If we are unable to raise additional funds when needed, our operations and ability to execute our business strategy could be adversely affected. We may seek to raise additional funds through equity, equity-linked or debt financings. If we raise additional funds through the incurrence of indebtedness, such indebtedness would have rights that are senior to holders of our equity securities and could contain covenants that restrict our operations. Any additional equity financing may be dilutive to our stockholders.
As of June 30, 2014, we had approximately $7.7 million in cash. Approximately $315,000, or 4%, of our cash is held in an account in the Netherlands to support our international activities.
Summary of Cash Flows
| Six Months Ended June 30, |
Twelve Months Ended December 31, |
|||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| Cash used in operating activities |
$ | (3,057,671 | ) | $ | (3,824,912 | ) | $ | (7,696,600 | ) | $ | (5,343,483 | ) | ||||
| Cash used in investing activities |
— | — | — | (27,609 | ) | |||||||||||
| Cash provided by financing activities |
$ | 5,401,660 | 4,546,821 | 4,546,820 | 12,274,010 | |||||||||||
| Net increase in cash and cash equivalents |
$ | 2,343,989 | $ | 721,909 | $ | (3,149,780 | ) | $ | 6,902,918 | |||||||
65
Table of Contents
Operating activities
We used approximately $3.1 million and $3.8 million of cash in operating activities during the six months ended June 30, 2014 and 2013 respectively, principally to fund our operating losses. We incurred additional net losses of approximately $219,000 for the six months ended June 30, 2014 as compared to the six months ended June 30, 2013. In addition, we used approximately $386,000 less cash for working capital purposes during the 2014 period than during the 2013 period, principally due to the timing of payments associated with this offering.
We used approximately $7.7 million and $5.3 million of cash in operating activities during the years ended December 31, 2013 and 2012 respectively, principally to fund our operating loss. The increase in cash used in operating activities of approximately $2.4 million was primarily attributable to the increase in net loss of $2.0 million for the year ended December 31, 2013 as compared to the year ended December 31, 2012. In addition, we used approximately $0.2 million to fund working capital activities, principally related to sourcing our inventory during the 2013 period.
Investing activities
We did not purchase equipment during the six months ended June 30, 2014, or June 30, 2013.
During the year ended December 31, 2012, we used approximately $28,000 in cash to purchase equipment. We did not purchase equipment during the year ended December 31, 2013.
Financing activities
Historically we have funded our operations through the issuance of convertible preferred stock and convertible redeemable preferred stock.
Cash provided from financing activities increased from approximately $4.5 million during the six month period ended June 30, 2013 to $5.4 million during the six month period ended June 30, 2014. During the six months ended June 30, 2013, we issued 1,319,031 shares of Series C convertible redeemable preferred stock which generated net cash proceeds of approximately $4.5 million of cash. During the six months ended June 30, 2014, we issued $6,364,780 of convertible promissory notes. We used $949,000 of cash for financing costs principally related to our note and equity offerings.
Cash provided from financing activities decreased from approximately $12.3 million for the year ended December 31, 2012 to approximately $4.5 million for the year ended December 31, 2013. During the year ended December 31, 2013, we issued 1,319,031 shares of Series C convertible redeemable preferred stock which generated net cash proceeds of approximately $4.5 million of cash. During the year ended December 31, 2012, we issued (i) 1,644,147 shares of Series B-1 convertible preferred stock, which generated net cash proceeds of approximately $3.7 million of cash and (ii) 2,435,682 shares of Series C convertible preferred stock, which generated net cash proceeds of approximately $8.6 million of cash.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Contractual Obligations
We have agreements with the supplier of our products and with other contract service providers to assist in the performance of our research and development and commercial operations in the ordinary course of business. We can elect to discontinue the work under these agreements at any time. We could also enter into additional supplier agreements, or collaborative research contracts in the future, which may require upfront payments or long-term commitments of cash.
66
Table of Contents
As of June 30, 2014, we had an outstanding obligation to the supplier of our neurostimulation system in the amount of $793,000, net of deposits. We intend to take delivery of these products through 2015. If we cancel the purchase order or refuse to take delivery of the items, we would be liable for the materials purchased and work performed to date.
We lease our office space on a month to month basis.
On June 25, 2014, we issued $6.4 million in aggregate principal amount of secured convertible promissory notes. Interest on all amounts outstanding accrues at a rate of 10% per annum, and the notes mature on June 25, 2016, unless earlier converted. The total payments due on the convertible notes are $7.6 million, of which $0.6 million are due within one year of June 30, 2014 and $7.0 million are due 1-3 years from June 30, 2014. The principal amount and all accrued but unpaid interest under these notes convert automatically upon the closing of this offering into shares of our common stock at a price per share equal to 80% of the public offering price (before underwriting discounts and commissions and other offering expenses).
The following table summarizes our contractual obligations as of June 30, 2014:
| Payments Due by Period as of June 30, 2014 | ||||||||||||||||
| Total | 0-1 years | 1-3 years | More than 3 years | |||||||||||||
| Operating lease obligation (1) |
$ | 6,325 | $ | 3,300 | $ | 3,025 | $ | — | ||||||||
| Purchase obligations (2) |
792,554 | 792,554 | — | — | ||||||||||||
| Convertible promissory notes (3) |
7,639,480 | 636,400 | 7,003,080 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 8,468,359 | $ | 1,432,254 | $ | 7,006,105 | $ | — | ||||||||
| (1) | Consists of an operating lease for a printer at our corporate headquarters. |
| (2) | Purchase obligations consist of an outstanding obligation to the supplier of its products, net of deposits. We intend to take delivery of these products through 2015. |
| (3) | On June 25, 2015, we issued convertible promissory notes in the amount of $6,364,780. Interest is payable annually at the rate of 10% per annum on principal outstanding. |
On August 28, 2014, we issued an additional $1.1 million in aggregate principal amount of secured convertible promissory notes substantially on the same terms as the notes issued on June 25, 2014. The total payments due on the notes issued in August 2014 are $1.3 million, of which $0.1 are due within one year of June 30, 2014 and $1.2 are due 1-3 years from June 30, 2014.
Related Parties
For a description of our related party transactions, see “Certain Relationships and Related Party Transactions.”
Quantitative and Qualitative Disclosure About Market Risk
We are exposed to market risks in the ordinary course of our business. These market risks are principally limited to interest rate and foreign currency fluctuations.
Interest Rate Risk
Our cash consists primarily of funds in cash and money market accounts. The primary objective of our investment activities is to preserve principal and liquidity while maximizing income without significantly increasing risk. We do not enter into investments for trading or speculative purposes. Due to the short-term nature of our investment portfolio, we do not believe an immediate 10% increase in interest rates would have a material effect on the fair market value of our portfolio, and accordingly we do not expect our operating results or cash flows to be materially affected by a sudden change in market rates.
67
Table of Contents
Foreign Currency Exchange Risk
We operate in countries other than the United States, and, therefore, we are exposed to foreign currency risks. Historically, all of our revenue has been from outside of the United States and we will not generate sales within the United States until we obtain FDA approval to market our product. We generally bill our European customers in Euros and our other customers in U.S. dollars. During the six months ended June 30, 2014 and 2013, approximately 84% and 49% of our revenue, respectively, was billed in Euros. Certain operating expenses related to European sales are denominated in Euros, thereby limiting our transaction risk exposure. We believe that the risks to our operating income from foreign currency fluctuations are not significant.
68
Table of Contents
Overview
We are a medical device company focused on the development and commercialization of a novel neurostimulation system for the treatment of severe gastroesophageal reflux disease, or GERD. Severe GERD is a debilitating condition that significantly disrupts patients’ quality of life, work productivity and sleep. Based on several international third party studies, we believe the impact of severe GERD on quality of life can be worse than that of chronic back pain or urinary incontinence, two other chronic conditions that are successfully treated with neurostimulation. Moreover, the impact of severe GERD on work productivity has been shown to be significantly greater than that attributable to back pain.
Our innovative approach targets the main cause of GERD — a dysfunctional lower esophageal sphincter, or LES, a muscle at the junction of the esophagus and stomach. The EndoStim neurostimulation system, which has a form and function similar to a pacemaker, delivers low energy electrical stimulation to the LES. In our clinical trials to date, this stimulation has been shown to improve the tone and function of the LES, thereby significantly reducing the pathological reflux symptoms of GERD. Our laparoscopically implantable neurostimulation system is minimally-invasive, reversible and preserves the anatomy of the esophagus and stomach.
In patients with GERD, a dysfunctional LES allows gastric contents, including stomach acid and bile, to pass inappropriately from the stomach into the esophagus. As a result, patients often suffer from heartburn, regurgitation, chest pain, and difficulty swallowing, among other symptoms, which can severely impact their quality of life. In more serious cases, GERD can result in a precancerous condition called “Barrett’s esophagus” that can lead to esophageal cancer. Patients who suffer from daily GERD symptoms experience a sevenfold increase in the risk of esophageal cancer, for which the World Health Organization reported 456,000 new cases worldwide in 2012. GERD is the most common upper gastro-intestinal, or GI, disorder seen in clinical practice. It is estimated to affect 15-20% of the population in developed countries and 5% in developing nations. Today, GERD is most often treated with chronic medication, specifically proton pump inhibitors, or PPIs, such as Nexium and Prilosec, which suppress the production of acid in the stomach. However, PPIs do not address the main cause of GERD, a dysfunctional LES. Therefore, PPIs do not prevent the regurgitation of stomach contents into the esophagus. As a result, 38% of patients taking PPIs to treat GERD reported continuing symptoms that were not resolved by their medication, according to a 2010 study of over 1,000 patients by the American Gastroenterological Association. We expect to initially target a subset of these patients who experience severe symptoms while on medication, and whose GERD can be confirmed by an esophageal acid test. We estimate this subset to comprise approximately 4.6% of all GERD patients, or about 21 million patients worldwide.
The traditional alternative for patients with continuing symptoms on PPIs has been fundoplication, a surgical procedure where the top of the stomach is wrapped and secured around the lower esophagus to augment the LES in order to create a mechanical barrier to reflux. This is a highly invasive procedure which permanently changes the anatomy, can result in a number of side effects and is difficult to correct or reverse. As a result of these concerns, fewer than 1% of patients who are unsatisfied with PPIs are treated with fundoplication. Several competing mechanical devices have been developed and evaluated in clinical trials in attempts to address this GERD therapy gap. These include the LINX Reflux Management System (Torax Medical), EsophyX (EndoGastric Solutions) and Stretta (Mederi Therapeutics). Use of these devices, which typically employ mechanical constriction or thermal ablation of the anatomy to restrict the migration of stomach contents into the esophagus, have either shown low efficacy in suppressing esophageal acid or have led to unwanted side effects such as difficulty swallowing. We believe that our therapy offers superior clinical efficacy in controlling esophageal acid and also offers a better safety and side effect profile by preserving normal anatomy and swallow function.
69
Table of Contents
To date, over 130 patients have been implanted with our system, 65 of whom have been treated in open-label clinical trials. These clinical trials have shown promising outcomes with respect to the safety and clinical efficacy of our technology as measured by objective and subjective criteria described under “— Clinical Trials.” The results of these clinical trials have been published in peer-reviewed medical journals. The trials have been conducted in Europe, South America and Asia with a follow-up period of up to three years. A total of 39 patients in these trials have been treated for more than one year, of whom:
| • | 72% achieved successful control of objectively measured esophageal acid exposure, considered the “gold standard” within our industry in evaluating GERD therapies, at their last follow-up (where “success” is defined using the FDA criteria of normalization of values or a decrease of more than 50% from a baseline); |
| • | 85% reported clinically significant improvement in symptoms, as evidenced by 50% or more improvement in GERD-HRQL score (a standard, internationally validated questionnaire-based measure of GERD-related quality of life), at their last follow-up; and |
| • | 97% were able to discontinue regular PPI use at their one-year evaluation (regular PPI use is defined as use of PPIs during 50% or more of diary days). |
For a more detailed description of these results, please refer to “— Clinical Trials — Summary of Findings” below. Although these trials have shown promising results, they were open-label studies performed at a limited number of clinical sites on a limited number of patients who were followed for a period of up to 36 months. Open-label trials are subject to various limitations that may exaggerate any therapeutic effect, including the “sham” or “placebo” effect described below under “— Regulatory Status.” Moreover, these patients likely included a high number of severe sufferers, and their symptoms may have been bound to improve notwithstanding treatment with our neurostimulation system. Please refer to “Risk Factors — We have collected limited clinical data about the efficacy and safety of our neurostimulation system and may be unable to reproduce historical clinical results in large-scale and double-blind controlled clinical trials.” To partially mitigate the placebo effect in our open-label trials, we included the measurement of esophageal acid exposure described in the first bullet above, which provides a more objective measure of reflux.
|
|
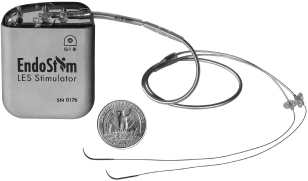
|
EndoStim System and Implant
Unlike fundoplication and other mechanical interventions, our treatment does not interfere with the natural LES relaxation which is necessary to allow for normal LES functions, such as swallowing and belching. We believe that our neurostimulation system, when compared with fundoplication and other available implantable devices, offers the following advantages:
| • | targets the main cause of GERD by restoring the natural functioning of a dysfunctional LES; |
70
Table of Contents
| • | is effective in alleviating patient symptoms and reducing or eliminating daily PPI use; |
| • | is minimally-invasive and reversible; |
| • | has minimal side effects and adverse events based on available clinical data; |
| • | is CE marked for use with MRI procedures for head and limb imaging and is potentially compatible with 3T MRI systems for full body exposure; and |
| • | has the ability to be wirelessly customized, to accommodate a patient’s individual lifestyle, responses to treatment, and personal preferences, at post procedure follow-ups. |
Neurostimulation to Treat Chronic Lifestyle Diseases
Although our solution is novel for the treatment of GERD, neurostimulation has been used successfully over many years for the treatment of a wide variety of other chronic diseases, such as lower back pain, incontinence, various heart conditions and epilepsy. The total worldwide annual revenues for neurostimulation devices is estimated to be about $5 billion and is expected to grow at a 11.6% compound annual growth rate through 2017. Of particular interest to us is the market for two chronic lifestyle conditions, chronic back pain and urinary incontinence, whose worldwide prevalence is comparable to GERD and whose quality of life impact on patients has been shown to be comparable to or lower than that of severe GERD, as indicated below:
| • | Prevalence: For each of these diseases, a separate study measured the worldwide prevalence of all forms of each such disease, including severe indications. The prevalence was estimated at 10% for chronic back pain, 8% for urinary incontinence and 10% for GERD. |
| • | Quality of life impairment: Several international studies have measured the subjective average quality of life of patients suffering from each of these diseases. A separate study covered each disease and used the same standardized questionnaire, the EQ-5D. Using this questionnaire, subjects rated their perceived level of health related to each of five health states (anxiety/depression, mobility, self-care, pain/discomfort, and usual activities), resulting in a composite, algorithmically-validated index score. Comparing the average index score reported in each study to the average score of 0.78 for a general male population aged 55 to 75, the results showed: |
| • | a 25% impairment for chronic back pain in patients with long-lasting back pain; |
| • | a 26% impairment for urinary incontinence measured in patients with stress incontinence (defined as the loss of urine without intent during physical activity, such as coughing, sneezing, laughing, or exercise); and |
| • | a 36% impairment for patients with severe GERD, and a 14% impairment for patients with moderate GERD. |
A portion of patients suffering from chronic back pain and urinary incontinence are treated with neurostimulation. The combined annualized revenues for neurostimulation treatment of these diseases is estimated to total almost $3 billion, with an estimated $2.5 billion from the treatment of chronic back pain with spinal cord stimulation and $400 million from the treatment of urinary incontinence with sacral nerve stimulation. We believe this illustrates the important role that can be played by neurostimulation in treating patients suffering from conditions impacting their quality of life who are inadequately treated on medication but reluctant to pursue a more invasive surgical alternative.
Regulatory Status
In 2012, we received CE Mark regulatory approval to commercialize our neurostimulation system in Europe and in other countries recognizing the CE Mark. We launched our system commercially in late 2013. More than 40 sites in Germany, Switzerland, Argentina and Colombia have obtained access to third-party reimbursement for
71
Table of Contents
the procedure. We currently use a direct sales force in key European countries and specialized distributors in other European countries, South America and Asia to commercialize our products.
In the United States, our device has not been approved for sale by the FDA. However, we have received approval from the FDA for our Investigational Device Exemption, or IDE, application, which allows us to conduct a pivotal clinical trial in the United States. The trial design is double-blind sham-controlled, which means that neither the investigator nor the patient knows whether or not the patient implanted with the device is actually receiving stimulation therapy. This guards against a “sham” or “placebo” effect where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment, or indirectly as a result of the investigator’s behavior. The clinical trial endpoints which the FDA will use to evaluate the effects of our neurostimulation treatment, are similar to the endpoints of our prior overseas clinical work. Most importantly, in our prior long-term clinical trials, we have consistently demonstrated the positive effect of our treatment on acid exposure to the esophagus. This is the outcome required by the FDA as the primary efficacy endpoint of our United States pivotal trial.
The FDA has recommended a minimum of 100 patients to be treated for one year; therefore, we plan to enroll a minimum of 110 patients to allow for dropouts. Based on our own prior open-label clinical results and published sham responses from other sham-controlled trials for GERD devices, we believe we can achieve a successful outcome with 100 completed patients. However, we have the ability to increase the total number of patients in the United States trial up to 200 if we believe it is necessary to successfully meet the primary endpoint. Provided our United States clinical trial is successful, we do not believe there would be any material hurdles to receiving FDA approval that would permit us to sell our neurostimulation system in the United States.
Our Strategy
Our goal is to establish our neurostimulation system as the leading therapy for treatment of severe GERD. Currently, we are focusing on a segment of patients that fall within the GERD “therapy gap.” The key components of our strategy include the following:
We intend to drive sales growth primarily in countries where the marketing of our neurostimulation system is approved and where third-party reimbursement is available.
In markets where our neurostimulation system is approved and reimbursement is available, we will continue to build our direct sales force and our distributor network and invest in a focused marketing program to expand our hospital customer base as well as increase the flow of GERD patients to these sites. Consequently, we will initially focus our efforts in Germany and other countries where our neurostimulation system has received preliminary reimbursement authorization from the government.
We aim to expand regulatory approval and third-party reimbursement to additional countries worldwide.
Additionally, we intend to expand and achieve permanent third-party reimbursement approvals in Germany, Switzerland, Colombia and Argentina, and seek reimbursement approvals from private health insurers and governments in other countries. We expect to receive reimbursement approvals, over time, in an expanding number of countries worldwide.
We intend to seek FDA regulatory approval and third-party reimbursement to commercialize our system in the United States.
We intend to seek FDA regulatory approval to market our neurostimulation system pursuant to the PMA process and have received approval from the FDA for our IDE application, which allows us to proceed with a pivotal clinical trial in the United States for this purpose. We will also seek approvals for third-party reimbursement in the United States. We believe that we will be in a strong position to obtain such approvals, because of the
72
Table of Contents
availability of an existing CPT code, the high level of clinical evidence that we expect to receive from our pivotal clinical trial, and potential endorsements from medical societies. However, we believe we can reach profitability based solely on revenues from outside the United States.
We intend to broaden our customer base by developing new applications for, and enhancements to, our therapy.
We are pursuing ways to broaden our customer base:
| • | We intend to offer the neurostimulation system to patients who respond to PPI therapy but who have concerns with the long-term risks of taking daily PPIs, or who are unsatisfied with the quality of life impact of daily medication dependence. We are already pursuing this strategy in European countries where the neurostimulation system has been approved for use with such patients. |
| • | Our neurostimulation system has been implanted in a single patient who also received a sleeve gastrectomy. The latter procedure is being performed on a growing number of patients worldwide to treat obesity, and is known to lead to or exacerbate GERD. |
| • | We are researching ways to deliver the neurostimulation system leads endoscopically through the mouth and throat, rather than laparoscopically. This could result in even less invasive procedures that we believe could be performed by qualified gastroenterologists rather than by surgeons alone. |
| • | Potential expansion of our therapy to other sphincter disorders, such as fecal incontinence. |
Gastroesophageal Reflux Disease
Overview
GERD is typically caused by a dysfunctional LES, the sphincter muscle at the junction of the esophagus and stomach. The dysfunctional LES allows gastric contents, including stomach acid and bile, to pass inappropriately from the stomach into the esophagus. As a result, patients often suffer from heartburn, regurgitation, chest pain, and difficulty swallowing, among other symptoms, which can severely impact their quality of life. In more serious cases, GERD can result in a precancerous condition called “Barrett’s esophagus” that can lead to esophageal cancer, whose prevalence is growing faster than any other cancer in the Western world. Patients who suffer from daily GERD symptoms experience a sevenfold increase in the risk of esophageal cancer, for which the World Health Organization reported 456,000 new cases worldwide in 2012.
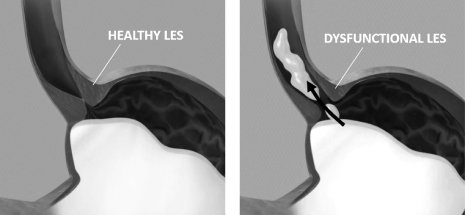
Healthy and dysfunctional LES
73
Table of Contents
Disease severity
Severe GERD is a debilitating condition that significantly disrupts patients’ quality of life, work productivity and sleep. As illustrated by the following graphs, the impact of severe GERD on quality of life can be worse than that of chronic back pain or urinary incontinence, two other chronic conditions that are successfully treated with neurostimulation. Moreover, the impact of severe GERD on work productivity has been shown to be significantly greater than that attributable to back pain. Further detail on each graph appears below.
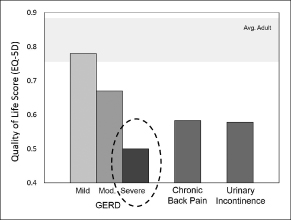
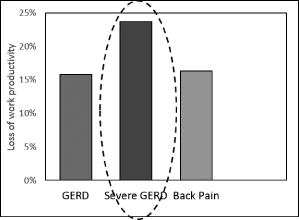
Impact of Severe GERD on Quality of Life and Work Productivity
Quality of Life Comparison. As described above under “—Neurostimulation to Treat Chronic Diseases,” several international studies measured the subjective average quality of life for patients suffering from each of chronic back pain, urinary incontinence and various levels of GERD (from mild to severe), using the same standardized questionnaire, the EQ-5D. Using this questionnaire, subjects rated their perceived level of health related to each of five health states, resulting in a composite index score. The above-left graph compares the average index score reported in studies of approximately 200 subjects with long-lasting back pain, approximately 200 subjects with stress incontinence, and approximately 1,000 subjects with GERD, with a lower number representing a greater impairment in perceived quality of life. As noted above, in measuring impairment from baseline, the index score was compared to the average score of 0.78 for a general male population aged 55 to 75.
Work Productivity Comparison. In the above-right graph, we compare loss of work productivity from back pain, GERD and severe GERD. Loss of work productivity is measured using a standard questionnaire, the Work Productivity and Activity Impairment Questionnaire. This measure combines both the time an individual is absent from work as well as the impairment while working (called presenteeism), with the result given as a percentage drop in the potential work productivity of the individual. One study including over 1,000 employees at a major U.S. service industry employer reported the work productivity impact of certain health conditions, including GERD and back pain, on employees who suffered from these conditions. This study did not include urinary incontinence. A published analysis of five other studies showed that those with disruptive GERD had 2.4-times and 1.5-times higher mean rates of absenteeism and presenteeism, respectively. Based on these studies, we estimate that the impact of severe GERD on work productivity loss is approximately 1.5-times greater than average, or moderate, GERD.
Therapy Gap
GERD is the most common upper gastro-intestinal, or GI, disorder seen in clinical practice. It is estimated to affect 15-20% of the population in the United States and other Western countries, 5% in Asia and in developing nations. Medication therapy, specifically PPIs, has been widely accepted as the solution of choice for most patients suffering from symptoms of GERD. PPI medications target acid production in the stomach, and are
74
Table of Contents
effective in suppressing the acid component of reflux. However, PPIs do not address the dysfunction of the LES, which is the main cause of reflux. Many patients using maximum daily doses of PPIs continue to suffer from regurgitation of weakly acidic or non-acidic gastric contents. It is very common for these patients to experience GERD symptoms at night, or nocturnal GERD. Accordingly, 38% of patients taking PPIs to treat GERD reported continuing symptoms that were not resolved by their medication.
| Traditionally, the alternative for patients who undergo medical PPI therapy, but for whom the maximum dosage is insufficient to control GERD, has been fundoplication, a highly invasive surgery that is not easily reversible. During this procedure, the top of the stomach is wrapped and secured around the lower esophagus to augment the LES in order to create a mechanical barrier to reflux, as illustrated in the accompanying drawing. While fundoplication has a high short-term success rate, the procedure can result in a number of side effects including difficulty swallowing, or dysphagia, as well as diarrhea and gas bloat. Fundoplication is also expensive, costing up to $30,000 per procedure, and up to |
Fundoplication | |
| two-thirds of patients continue to require PPI medication over the long-term after the procedure. Favorable outcomes are highly dependent on surgical expertise and experience. Less than 1% of patients experiencing breakthrough symptoms on medication currently opt to undergo fundoplication due to the surgery’s limited long-term efficacy and known risk of side effects. | ||
Recently, less-invasive alternative treatments have been developed. The LINX Reflux Management System, which is a series of titanium beads with magnetic cores forming a collar around the lower esophagus, received FDA approval in 2012.
In addition, other approaches deliver radiofrequency energy to the LES (Stretta therapy) or involve minimally-invasive surgery to the LES (Medigus MUSETM or EsophyXTM). All of these alternatives seek to mechanically restrict the migration of stomach contents into the esophagus. Use of these devices, which typically employ mechanical constriction or thermal ablation of the anatomy to restrict the migration of stomach contents into the esophagus, have either shown low efficacy in suppressing esophageal acid or have led to unwanted side effects such as difficulty swallowing. Accordingly, we believe that the need still exists for treatment with improved clinical effectiveness, which for these therapies we define as efficacy in controlling esophageal acid exposure and their safety and side-effect profile.
Patients suffering from GERD symptoms for whom PPI therapy is not adequate and for whom fundoplication is neither attractive nor appropriate fall within a “therapy gap.” We expect patients who experience continuing symptoms despite PPI medication, or approximately 38% of GERD patients on PPI, to continue to seek alternative treatments to manage their condition. However, not all patients will fall into the initial primary target for our neurostimulation therapy. We expect to initially target a subset of these patients who experience severe symptoms while on medication, and thus would be most interested in undergoing a laparoscopic procedure to address their condition, and whose GERD can be confirmed by an esophageal acid test. As illustrated by the following chart, we estimate this subset to comprise approximately 4.6% of all GERD patients, or about
75
Table of Contents
21 million patients worldwide and approximately 3 million patients in the United States. We intend to seek, though have not obtained, approval from the FDA to market our neurostimulation system in the United States.
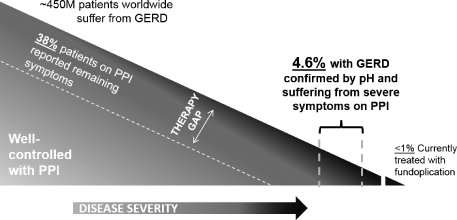
GERD therapy gap
Our Solution
EndoStim’s Approach
Our neurostimulation system offers a minimally invasive, anatomy preserving and reversible treatment intended to safely and efficaciously improve and/or normalize LES function. We believe that this solution will appeal to both patients and treating physicians as it provides a superior treatment alternative to patients in the GERD “therapy gap” compared with fundoplication and other available implantable devices by offering reflux control while minimizing unwanted side effects, as also described in more detail below under “— Measurement of Treatment Success and Our Neurostimulation System in Comparison.” Additionally, the treatment can be customized, typically eliminates the need for daily medications and in many patients avoids the side effects associated with current surgical and device-based interventions. Although our solution is novel for the treatment of GERD, neurostimulation is used successfully for the treatment of a wide variety of diseases, such as lower back pain, incontinence, various heart conditions and epilepsy.
Potential Mechanisms of Action
Low LES pressure, or hypotensive LES, and transient LES relaxations (tLESRs) are the two major causes of GERD. Multiple animal studies and human trials of LES stimulations have consistently shown a significant improvement in LES pressure with electrical stimulation. In these studies, the effect of LES stimulation on LES pressure enhancement lasted beyond the duration of the stimulation session. This suggests that the rise in LES pressure most likely is mediated through the release of neuropeptides (e.g. Tachykinins) in the LES. In long-term human trials, LES stimulation also resulted in an increase in the length of the high pressure zone (HPZ) of the LES resulting in an enhanced LES barrier function. This may also contribute to the control of GERD.
Our preliminary data on tLESR indicates that LES stimulation may have a therapeutically positive effect on tLESR events. The review of esophageal pH data demonstrates a significant reduction in the “short-reflux” events which are typically mediated by tLESRs, and additional data using prolonged high-resolution impedance manometry (HRIM) is being collected to confirm the effect.
76
Table of Contents
Our Neurostimulation System
The EndoStim LES Stimulator is designed to treat patients with GERD by delivering electrical stimulation to the LES. The system consists of the following components:
| • | EndoStim Implantable Pulse Generator, or IPG |
| • | EndoStim Implantable Bipolar Lead |
| • | EndoStim Programmer, an external device used by physicians to interrogate and program the IPG |
The IPG is an internally-powered device which delivers electrical stimulation signals to the LES for approximately 30 minutes, 6-12 times a day, using our proprietary algorithms. Our first generation neurostimulation system battery is expected to have a life of approximately 10 years under typical use. The IPG is programmable; its various parameters and algorithms can be set by the attending medical/technical personnel to suit each patient’s needs via wireless communication from the EndoStim Programmer. The IPG is connected to an EndoStim implantable bipolar lead which is used to deliver the electrical neurostimulation therapy to the LES.
Implantation of the Neurostimulation System
Our neurostimulation system is implanted using standard laparoscopic techniques in an approximately one-hour outpatient or inpatient procedure that is typically performed using a short-acting general anesthetic. We expect that a vast majority of the procedures in the United States following FDA approval will be performed on an outpatient basis. The EndoStim lead is implanted in the LES muscle present at the junction of the esophagus and stomach. The two electrodes are implanted in a proprietary configuration in the sphincter muscle, away from the vagal nerve, and secured on the esophageal wall with sutures and titanium clips. Our procedure follows a longstanding and well established gastrointestinal lead implantation methodology that has been proven effective in preventing lead dislodgement, erosion or migration. The proximal end of the lead is then brought out through the abdominal wall using one of the trocar sites into the subcutaneous space. The IPG is connected to the lead and placed under the skin in the abdomen. In most cases, the patient can be released from the hospital or clinic on the same day.
This technique of minimal dissection around the LES maintains the normal anatomy, minimizing any damage to the area of the implant, thus potentially eliminating long-term side effects typically seen with other anti-reflux procedures and making the procedure reversible if need be.
Measurement of Treatment Success and Our Neurostimulation System in Comparison
We believe that an optimal solution for severe GERD successfully meets the following criteria:
| • | pH normalization and improvement. The acid exposure is the primary standard for measuring treatment success, and although other standards, such as GERD-HRQL scores or elimination of need for daily PPI medication, are used, acid exposure is considered the “gold standard” within our industry, including the medical community and practitioners, for the evaluation of the efficacy of GERD treatments. The standard pH test evaluates the amount of time the esophagus is exposed to acid during a 24-hour period. Results below 4-5%, depending on the measurement device, are considered normal. According to the FDA, a 50% drop in exposure time is also considered a therapeutic success even when the exposure levels are not normalized. The measurements of pH level give an objective picture of the effectiveness of a device so long as the testing is done while the patient does not receive PPIs or other anti-acid medication. In the absence of such medications, the measured acid level in the esophagus gives an accurate indicator of actual reflux events, because the stomach contents are naturally acidic. If no acid is detected, it is because the contents of the stomach have not passed into the esophagus. |
Based on objective measures of pH normalization and improvement in clinical trials, we believe that our neurostimulation system has generated positive results compared to the alternatives.
77
Table of Contents
| • | Alleviation of symptoms. Symptoms are typically measured based on patients’ own evaluation. In clinical trials these symptoms are quantified using validated questionnaires such as the GERD-HRQL (“The Gastroesophageal Reflux Disease-Health Related Quality of Life”). GERD-HRQL is a patient questionnaire containing 11 questions that measures symptomatic improvement of both medical and surgical treatment for GERD. It includes 10 questions on various aspects of the disease impact such as heartburn, difficulty and pain swallowing and medication use and also one question on overall satisfaction. Each of the first 10 questions is answered on a scale of 0 to 5 (5 being the most bothersome) and the sum of these answers provides a composite score between 0 to 50 where a score of “0” represents no symptoms. An acceptable measure of successful treatment by the FDA is a 50% drop in the composite GERD-HRQL score. The GERD-HRQL has been validated in several languages and is a widely used tool for evaluating new GERD treatments. |
In our clinical trials, 82% of the patients who received our neurostimulation therapy for at least one year reported significant improvement in their symptoms, including GERD-HRQL score improvements.
| • | Invasiveness and reversibility. The treatment should itself not carry the risk of serious adverse consequences, whether due to invasiveness or otherwise. Treatments that are mostly reversible are preferred to non-reversible treatments. |
Our current laparoscopic procedure is state of the art and is considered to be less invasive than other laparoscopic procedures required to perform certain other GERD therapies. We believe that our procedure is also reversible.
| • | Side effects. The treatment should have as few side effects as possible, such as dysphagia, diarrhea or gas bloat. |
Patients treated with our therapy have reported very few side effects. Notably, dysphagia is a rare side effect of our therapy, observed in two patients out of 38, both of whom also had a hiatal hernia repair procedure.
There have been no device or therapy related serious adverse events with our commercially approved products. In our clinical trials, 3% of the patients experienced serious adverse events related to our device or procedure. Of those, two cases of lead erosions were reported with a first generation 5mm lead which is not part of our current commercial system.
| • | Need for continued PPI use. Following treatment, the patient should not need to continue PPI use at prior levels. |
Based on our clinical trial results to date, we expect that fewer patients will need to continue PPI use with our treatment compared to the other alternatives.
| • | Compatibility. The device should be compatible with potentially necessary diagnostic devices or treatments, most significantly with MRI evaluation. |
Currently, our neurostimulation system is CE marked for use with 1.5T MRI for head and limb imaging. We believe, based on preliminary testing, that our neurostimulation system is compatible with 3T MRI systems for full body exposure. We intend to seek regulatory approval for this feature, which would allow current and future patients to undergo full-body MRI scans following the implantation of our neurostimulation system.
Based on clinical experience thus far, we believe our therapy has the potential to become a market leader in minimally-invasive medical devices for the treatment of severe GERD both in safety and efficacy measures, as shown in the table below comparing all major treatment outcomes uniformly reported for all devices. Although the information included in the table is based on a literature review and the sources examined may contain minor
78
Table of Contents
differences in the definition of measured outcomes, we believe it nevertheless provides an accurate comparison of alternative treatments.
| 12 month follow-up |
EndoStim (n=38) |
LINX (n=98) |
EsophyX (n=79) |
Medigus (n=72, 6 mos.) |
Stretta (n=94) | |||||
| Mechanism of Action | Neurostimulation | Mechanical | Mechanical | Mechanical | Thermal | |||||
| % pH Normalized or Improved ³50% (FDA standard for PMA success) |
67% | 64% | 37%5 | 33% | 36% (n=98) | |||||
| % avg. pH improvement from baseline at 6 mo. | ||||||||||
| Daily/Regular PPI Use |
3% | 9% | 15% | 35% (any PPI) | 30% | |||||
| Reversibility |
Yes | Possible | Possible | Possible | No | |||||
| Anatomical Alteration |
Minimal | Moderate | Moderate | Moderate | Minimal | |||||
| Non-invasive Adjustment Post-op |
Yes | No | No | No | No | |||||
| Serious Adverse Events |
3%1 | 6%4 | 4% | 14% | 1% | |||||
| MRI Compatibility (>0.7T) |
Yes2 | No | Yes | Yes | Yes | |||||
| Dysphagia |
5%3 | 68% | 4% | 0% | 1% | |||||
| Median GERD-HRQL |
2.5 | 2 | 7 | 6 | 9 | |||||
| (1) | Includes all patients in EndoStim studies (CS004, CS005, Registry; n=95). No device or therapy related serious adverse events with our commercially approved system |
| (2) | Head and extremity MRI CE approved. Full body MRI compatibility testing ongoing |
| (3) | No dysphagia reported in patients implanted with our system who did not undergo hiatal repair procedure |
| (4) | LINX® serious adverse events were all related to the device and/or procedure and two-thirds necessitated device removal |
| (5) | pH normalized or improved >30% |
Clinical Trials
Our clinical development program is intended to generate compelling outcomes data to:
| • | support regulatory approvals; |
| • | optimize therapy; |
| • | create market awareness; |
| • | support reimbursement; |
| • | broaden our target patient population; and |
| • | generally increase acceptance and adoption of our technology. |
To date, as measured by objective and subjective criteria described below, we have demonstrated the safety and clinical efficacy of our neurostimulation system in short-term and long-term open-label clinical trials conducted in Europe, South America and Asia, as well as in commercial use, with more than 100 patients treated so far. These clinical trials resulted in the European CE Mark approval in August 2012. We are currently pursuing a trial in the United States for the PMA process. We have received approval from the FDA pursuant to the investigational device exemption, or IDE, to proceed with a sham-controlled trial. The FDA has recommended a minimum of 100 patients treated for one year; therefore, we plan to enroll a minimum of 110 patients to allow for dropouts. We have the ability to increase the number of patients in the trial up to 200 if we believe it is necessary to successfully meet the primary endpoint for the trial.
The FDA trial endpoints are based on the similar clinical outcomes as our prior overseas clinical work. Most importantly, in our long-term clinical trials, we have consistently measured acid exposure of the esophagus, the “gold standard” for purposes of FDA approval. We have not conducted a sham-controlled trial which would provide the highest (Tier 1) level of evidence to conclusively establish that improvement in GERD is due to our
79
Table of Contents
therapy and not a sham response. However, clinical trials with other devices have not found any significant sham response when measuring esophageal acid exposure, which is an objectively measured outcome.
The following provides an overview of the studies sponsored by us from the initial “proof of concept” study to the ongoing trials.
EndoStim-Sponsored Studies — Short-Term
The initial studies relating to our neurostimulation system focused on measuring the effect of neurostimulation on the pressure in the LES, which is a standard acute measure of LES functionality. Pressure is measured using pressure sensors placed on a catheter that is inserted into the patient’s esophagus. Such acute measure provided us valuable initial data to proceed with further trials.
| • | Endoscopic proof of concept. LES stimulation using endoscopically implanted temporary leads in GERD patients was performed at the Asian Institute of Gastroenterology in Hyderabad, India. The study included five GERD patients whose lower esophageal sphincter pressure was consistently enhanced following stimulation. |
| • | Surgical (laparoscopic) proof of concept. LES stimulation using laparoscopically implanted temporary leads in ten GERD patients was performed at Clinica Indisa in Santiago, Chile. The study confirmed the results of the previous endoscopic study. This study also tested various stimulation signals, electrode configurations and treatment algorithms and formed the basis of EndoStim LES stimulation treatment. |
The results of these two short term studies are demonstrated by the following graphic:
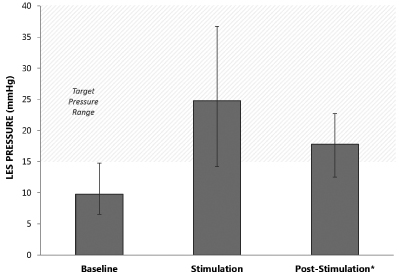
*Post-stimulation LES pressure measured 30-60 minutes after cessation of stimulation
Figure 1: LES Pressure in Short-Term Trials
Figure 1 shows the effect of LES stimulation on LES pressure. LES pressure is a measure for LES barrier function (blocking acid flow from the stomach to the esophagus) and is a common diagnostic in standard clinical use. LES pressure is typically measured in millimeters (mm) of Mercury (Hg). Baseline average patient LES pressure was about 10 mm Hg. The LES pressure was increased following stimulation to a target pressure of >15 mm Hg. This enhanced pressure was maintained at a higher level than baseline at 30-60 minutes post stimulation. These results show a sustained enhancement in the barrier function of the LES after a session of electrical stimulation.
80
Table of Contents
EndoStim-Sponsored Trials — Long-Term (open-label)
In our long-term trials, we have evaluated the impact of our treatment on all key GERD treatment outcomes, including the objectively measured pH exposure and subjective measures such as GERD-HRQL.
Our initial long-term trials consists of the following:
| • | CS004. Our first long-term trial was conducted in Santiago, Chile. The one-year results in 23 patients served as the main clinical evidence for CE Mark approval. The results, as described in more detail below, show highly statistically significant and clinically meaningful improvements in all key GERD treatment outcomes, including the objectively measured “gold standard” within the medical industry and practitioners, the 24 hour acid exposure. No serious adverse events relating to the electrical stimulation therapy or the EndoStim neurostimulation system were reported. |
| • | CS005. The CS005 trial is currently ongoing at 10 leading GERD centers worldwide. The study has enlisted both international and local key opinion leaders in GERD as principal investigators in Europe (Netherlands, UK), Asia Pacific (India, New Zealand, Hong Kong) and Latin America (Chile, Colombia, Mexico). As of June 30, 2014, the study had reached its target enrollment of 41 patients. To date, clinical results in the CS005 trial confirm the results seen in the CS004 trial. In addition, the trial is evaluating two additional GERD-related issues, sleep quality and work productivity, which have significant impact on GERD patients’ lives. Interim results indicate that EndoStim therapy significantly improves sleep quality and reduces overall work impairment in patients with GERD. No serious adverse events relating to the electrical stimulation therapy were reported. Two cases of lead erosion were reported with a first generation 5mm lead which is not part of our current commercial system. Additionally, a single case of suspected lead migration has been recorded, probably due to excessive post-operative retching most likely related to anesthesia. The lead was replaced before there were any adverse effects. |
Long-Term Clinical Results
We believe that our long-term clinical studies have shown exemplary results, using clinical trial methodologies and end points similar to those recognized by the FDA. These results are captured in Figures 2-4 below, followed by a summary of findings. Figures 2-4 provide information for all patients enrolled in trials CS004 and CS005. The measures used in Figure 2 and Figure 3 are explained above under “— Measurement of Treatment Success and Our Neurostimulation System in Comparison.” Note that these were open-label trials, which means that efficacy results could have been influenced by a placebo effect. However, we believe that the objective measure
81
Table of Contents
of esophageal acid exposure is less sensitive to a placebo effect than subjective measures such as subjectively reported patient symptoms.
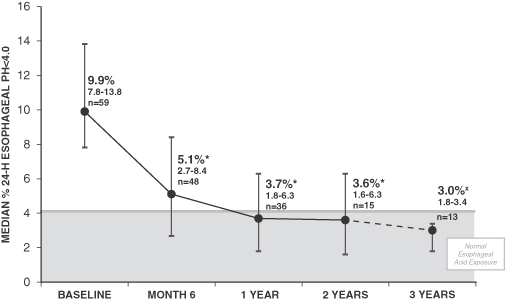
*Statistically significant and sustained improvement in esophageal acid exposure vs. baseline all time points (p<0.001). Data: Medians, IQR. Includes all patients in CS004 and CS005 receiving continuous (per protocol) EndoStim® therapy and having pH test performed at that time point.
xPreliminary data, unmonitored
Figure 2: Median % 24-hour Esophageal Acid Exposure
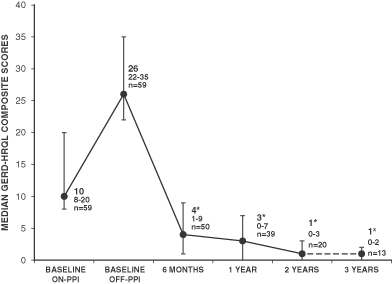
*p<0.01 vs. Baseline Off and On PPI for all comparisons. Includes all patients in CS004 and CS005 receiving continuous (per protocol) EndoStim® therapy. Data: medians, IQR.
xPreliminary data, unmonitored
82
Table of Contents
Figure 3: Median Composite GERD-HRQL Scores
| Heartburn |
Regurgitation | |||
|
|
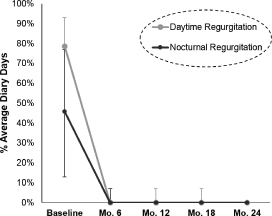
|
|||
n=53 at baseline, 48 at Month 6, 37 at Month 12, 26 at Month 18, 17 at Month 24. Includes all patients in CS004 and CS005 receiving continuous (per protocol) EndoStim® therapy. Data: medians, IQR. The measure “ % Average Diary Days” measures the average percent of days out of a 14-day daily diary period that patients experienced symptoms of heartburn and/or regurgitation, during the day and/or at night.
Figure 4: Improvement in Daytime and Nocturnal Symptoms
We performed a sub-study where the neurostimulation therapy was turned off for at least three months after more than one year of successful treatment in four patients. The patients were told prior to the commencement of the sub-study that their therapy could (but may not be) turned off as part of the study, thus resulting in a blinded design. An increase in acid exposure in all four patients was shown after the turn-off, and a decrease after the therapy was turned back on. The results of the sub-study are not statistically relevant due to the small sample size. We believe, however, that the outcomes of the sub-study provide some anecdotal support to the claim that
83
Table of Contents
our neurostimulation therapy contributes to an improvement in esophageal acid exposure, and the outcome is not due to a sham effect. The results of the sub-study are shown in Figure 5 below.
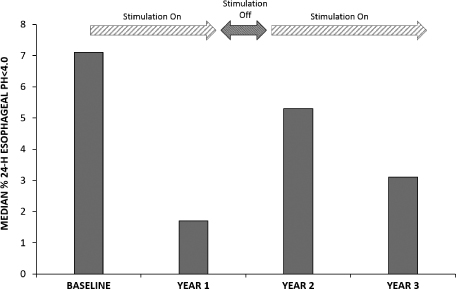
Figure 5: Device Turn-Off Sub-Study: Median % 24-hour Esophageal Acid Exposure
n=4 from Baseline through year 2; n=3 at year 3
Summary of Findings
The following summary of findings is based on outcomes of all patients in CS004 and CS005 trials receiving continuous (per protocol) therapy with 24-month follow-up data available. All outcomes were measured at 24 months, except for LES pressure, which was measured at 12 months.
| • | Esophageal Acid Exposure. Median esophageal acid exposure (esophageal pH less than 4.0) decreased from 9.9% (off-PPI) at baseline to 3.6% during a 24-hour period (see Figure 2 above). The esophageal acid exposure was either normalized (pH less than 4.0 for less than 4% of the time) or improved by decreasing more than 50%, compared to the baseline, in 73% of the patients. |
| • | Patient Symptoms. |
| • | GERD-HRQL. Median composite GERD-HRQL scores improved from baseline scores of 26.0 (off-PPI) and 10.0 (on PPI) to 0.5 (see Figure 3 above). |
| • | PPI Usage. 93% of patients reported being off regular use of PPI (more than half of the diary-days without PPI use) and 81% reported no PPI use at 24 months. |
| • | Sleep Quality. Percent of patients reporting any impact of heartburn on sleep dropped from 68% (on PPI alone) and 95% (off-PPI) at baseline to 9% (using our neurostimulation system). |
| • | Dysphagia. Percent of patients reporting bothersome difficulty in swallowing dropped from 14% (on PPI) and 59% (off-PPI) to 0% (using our neurostimulation system). |
| • | LES Pressure. Sustained increase in LES end expiratory pressure without any change in LES relaxation was observed compared to baseline as measured using high resolution manometry. |
84
Table of Contents
Additional Quality of Life Outcomes
In our CS005 international multicenter trial, we have collected additional data regarding the impact of GERD and EndoStim therapy on patients’ work productivity.
Figure 6 illustrates the preliminary results of the impact of EndoStim therapy on work productivity for patients who have reached 6 months and 12 months in the study.
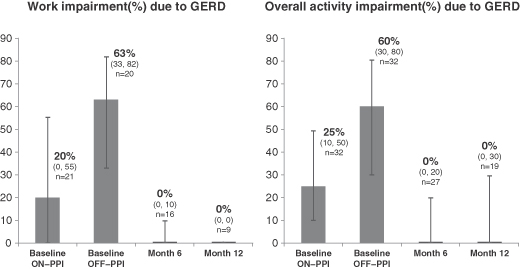
Figure 6: Improvement in Work Productivity
Figure 6 shows the improvement (reduction) in the effect of GERD symptoms on patients’ work and activity impairment while on EndoStim therapy, measured by the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP) questionnaire. Patients reported a median of no work impairment or overall activity impairment due to GERD while on EndoStim therapy compared to a median of >60% impairment off-PPI and >20% impairment in both outcomes on PPI at baseline.
Investigator-Initiated Trials
We are cooperating with non-affiliated physicians and institutions interested in pursuing independent investigator-initiated research with limited support and involvement from us. Studies that are currently being planned include:
| • | a multi-center randomized sham control trial in three centers in France, Belgium and Italy, the goal of which is to provide an early indication of the benefit of our neurostimulation therapy in a sham trial; |
| • | studies in Maastricht, Netherlands, and Buenos Aires, Argentina, to assess the efficacy of our system in patients with post-sleeve gastrectomy GERD; and |
| • | an open-label study in Hong Kong, to assess the efficacy of our system in the Asian population. |
Ongoing and Future Trials
The following clinical activities are ongoing or are planned to support the objectives of our clinical development program:
Randomized double-blind sham controlled trial for FDA approval
85
Table of Contents
We have received approval from the FDA for our Investigational Device Exemption, or IDE, application, which allows us to conduct a pivotal double-blind, sham-controlled clinical trial to pursue regulatory approval in the United States. The trial will include a very similar patient population as our prior open-label trials. The study end points are also based on the same clinical outcomes as our prior trials, and include esophageal pH, GERD-HRQL, PPI use, and quality of life. The FDA has recommended a minimum of 100 patients to be treated for one year for PMA approval. We plan to enroll a minimum of 110 patients to allow for dropouts. Based on our own open-label clinical results and published sham responses from other sham-controlled trials of GERD devices, we believe we can achieve a successful outcome with 100 completed patients. However, we have the ability to increase the total number of patients in the trial up to 200 if we believe it is necessary to successfully meet the primary endpoint. We are scheduled to begin recruiting patients in 2015 for this trial, and expect to complete enrollment in 2016. We expect to submit our PMA in 2017, which could result in FDA approval, subject to trial outcomes, in 2018.
In this pivotal trial, our neurostimulation system will be implanted in all patients, who will then be randomly assigned to two groups. For a six month period, one group will receive neurostimulation and the other group will not. After six months, all patients will receive neurostimulation for a total treatment duration of at least twelve months. If successful, this will provide us with “Tier 1” evidence demonstrating the efficacy of our therapy, the highest level of clinical evidence considered by third-party payors. The final results of this trial are expected to be used both for gaining regulatory approval and to obtain third-party reimbursement in the United States.
Registry Trial
We intend to enroll up to 350 patients at 40 commercial sites located in countries with current regulatory approval into a registry trial. The goal of this trial is to collect real-world standard of care data to evaluate patient outcomes and support reimbursement activities. As of June 30, 2014, 37 patients are enrolled at five centers.
Our LES Neurostimulation System
Pulse Generator and Leads
The IPG is an internally-powered device that delivers electrical stimulation therapy to the LES. We have developed two generations of the IPG.
First Generation Neurostimulation system
Our first generation neurostimulation system is equipped with a battery with approximately a 10-year life, but has larger overall dimensions than the second generation neurostimulation system described below.
| First Generation EndoStim LES Stimulator (IPG and Leads shown).
Currently available in certain markets outside of the United States
(Not actual size) |
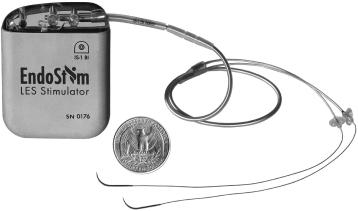
|
86
Table of Contents
Second Generation Neurostimulation system
Our second generation neurostimulation system is about 40% smaller in volume than our first generation neurostimulation system, and is anticipated to have a battery life of approximately 7 years. Because of its thin profile, it is better suited for implantation in slim or smaller patients. This neurostimulation system is expected to be available during the second half of 2014. We intend to develop further generations of the neurostimulation system, potentially offering even smaller dimensions and a battery recharge capability.
| Second Generation EndoStim LES Stimulator (IPG & Leads Shown)
Expected to be available in 2014 in certain markets outside of the United States
(Not actual size) |
|
Lead Wires
The lead electrodes are implanted in the LES through a simple laparoscopic procedure. The electrodes receive low-energy electrical pulses from the IPG, and deliver pre-programmed therapy to the LES without the need for patient intervention.
Programmer
The programmer is a device that is used for optimizing our neurostimulation therapy wirelessly. At the time of optimization, the programmer is connected to a computer that permits medical personnel to:
| • | enable or disable therapy; |
| • | read and modify simulation parameters (such as number of sessions, timing of sessions, sleep mode, pulse parameters); and |
| • | read and log IPG and lead status (such as battery life, impedance, therapy delivery, device events). |
Research and Development
Our research and development efforts are currently focused on supporting our ongoing clinical trials, including preparing for the PMA submission. However, we intend to undertake research and development efforts to investigate potential new applications of our neurostimulation system for the treatment GERD sub-populations, to address other sphincter disorders, and to enhance both the system and its delivery. These efforts include the following:
| • | PPI responders seeking alternative therapy. There are growing concerns about the long-term safety of PPIs, resulting in multiple FDA warnings. Therefore, we may have the opportunity to capture a segment of the “PPI Responder” market — patients who respond adequately to PPI therapy but have concerns with long-term risk of taking daily PPIs, or are unsatisfied with the quality of life impact of daily medication dependence. Considering that PPIs represent a $24 billion market worldwide, which is |
87
Table of Contents
| the third largest class of drugs prescribed in the U.S., we believe expansion of our target patient base to include “PPI Responder” market could significantly enhance our commercialization opportunities. The American Gastroenterology Association’s Center for GI Innovation and Technology (AGA-CGIT) recently recommended to the FDA to expand our PMA trial patient population to include these PPI responders seeking alternative therapy for various safety and quality of life reasons. The FDA has also indicated that it is open to considering the proposed patient population expansion in the future through a separate clinical trial. |
| • | Pediatric GERD. Severe GERD in infant and children is associated with failure to thrive resulting in significant morbidity and rarely mortality due to pulmonary complications. Fundoplication, despite poor long-term results, side effects and associated mortality, continues to be one of the most prevalent pediatric surgical procedures. We estimate that over 3,000 such procedures are performed annually in the United States. We believe that our second generation, smaller IPG should help make our approach viable for pediatric use. |
| • | Sleeve gastrectomy. In 2006, about 200,000 bariatric procedures were performed in the United States. The sleeve gastrectomy procedure is growing in popularity to treat obesity, now comprising over 36% of bariatric procedures performed in the United States. Sleeve gastrectomies are known to lead to GERD or exacerbate pre-existing GERD. Up to 20% of patients who have undergone sleeve procedures develop severe reflux post-surgery or exacerbate symptoms of an existing GERD condition. Due to the resection of most of the stomach, including the gastric fundus, such patients are unable to undergo fundoplication to treat GERD. Additionally, we estimate that almost 20% of obese patients are excluded from the sleeve gastrectomy procedure because of pre-existing GERD. We are aware that a physician has used our neurostimulation system to treat GERD in a sleeve gastrectomy patient, with excellent short-term results. Several additional sites are considering implanting our neurostimulation system in sleeve gastrectomy patients with some procedures expected in 2014. |
| • | Patients with proximal GERD and Laryngo-Pharyngeal Reflux, or LPR. A significant patient population not included in EndoStim’s initial target market suffers from LPR, including throat symptoms due to acid hitting the back of the throat, or proximal reflux. These patients have poor response to PPIs and are reluctant to undergo a more invasive fundoplication procedure. Our clinical trial data showed a complete elimination of proximal reflux in seven patients originally presenting with abnormal proximal acid exposure following our neurostimulation therapy. |
| • | Patients with ineffective esophageal motility. Ineffective esophageal motility, or IEM, is found in 20-60% of patients undergoing esophageal manometry, a test of esophageal function. It is the most prevalent finding in patients with GERD, observed in up to 32% of cases, and it is associated with the degree of reflux and the severity of symptoms. Patients with severe IEM are reluctant to undergo fundoplication. Our therapy does not affect esophageal body motility and does not cause dysphagia, hence it is not expected to have a negative effect on these patients. Our neurostimulation system has demonstrated early success in treating such patients and if the results continue to hold in larger patient populations, our system may become the treatment of choice in this subgroup of patients. |
| • | Other sphincter disorders. We believe, based on published animal and human data, that electrical stimulation can potentially play an important role in treating fecal/bowel incontinence. By using the same direct neuromuscular stimulation technique that we have developed to treat GERD, we believe we can extend our therapy to a large group of patients who suffer from fecal/bowel incontinence due to a dysfunctional rectal sphincter. Fecal incontinence is a condition that tends to be under-reported and responds poorly to surgical techniques. Prevalence of weekly symptoms is estimated to affect 2.7% of adults in the United States. Medtronic has recently received regulatory approval in the United States for the use of their sacral nerve stimulator to treat bowel incontinence and we believe that our direct neurostimulation approach may achieve equal or better results. |
| • | Development of an endoscopic delivery solution. Our neurostimulation system is currently designed for laparoscopic lead implantation by a surgeon. We are currently exploring ways to deliver the leads |
88
Table of Contents
| endoscopically rather than laparoscopically, as this would result in an even less invasive procedure that could be performed by qualified gastroenterologists. In the endoscopic delivery method, we expect to combine two conventional gastro-intestinal techniques: PEG, or feeding tube delivery procedure, and POEM, or endoscopic achalasia procedure. We intend to perform the first-in-man clinical trial for this technique in 2015. |
We have spent a significant portion of our capital resources on research and development. Our research and development expenses were $3.2 million in 2013 and $2.6 million in 2012 and approximately $1.3 million for the six months ended June 30, 2014. We expect our research and development expenditures to be significant as we continue to commit resources to developing our neurostimulation system and expanding future indications for our therapy.
Commercialization Strategy
We are currently focusing our commercialization efforts in countries which have favorable third-party reimbursement policies.
Sales and Marketing
We are currently leveraging our CE Mark to commercialize our neurostimulation system outside the United States. Our initial commercial activities are focused on select countries with early access to third party reimbursement and clinical adoption by local physician champions. Our strategy is to develop the following three elements which we believe are critical for successful commercial adoption of our therapy:
| • | training surgeons who can perform the procedure and are interested in offering it to their reflux patients; |
| • | achieving third party reimbursement that can support the procedure and system cost for a large patient population; and |
| • | generating a continuous flow of our target patients to surgical sites through either patient self-referrals or physician referrals, as described below under “—Marketing to Increase Patient Flow to Surgical Sites.” |
While we are initially focused on markets where reimbursement is available, we have entered into contracts with qualified distributors in a total of 12 countries including several in the European Union (EU) and others ranging from Colombia and Argentina to Hong Kong and New Zealand, many of which do not currently have reimbursement available for EndoStim procedures, in preparation for future growth.
Surgical Training
We are primarily directing our efforts towards surgeons specializing in esophageal procedures. The development of these surgical sites into EndoStim implanting sites is done by our own direct product specialists and in collaboration with local distributors. So far, over 20 surgeons around the world successfully completed our training program and implanted our product, about half as part of a clinical trial and half as part of our commercial training program.
Marketing to Increase Patient Flow to Surgical Sites
Patients reach surgical sites typically through one of two pathways: (1) self-referral, where a patient independently requests such a consultation and referral to the surgeon’s clinic, and (2) physician referrals, typically by a general practitioner or a specialist such as a gastroenterologist or ear-nose-and-throat expert. We use several marketing tools to enhance the flow of our target patients through both of these referral pathways.
89
Table of Contents
Our direct-to-patient marketing programs to increase patient self-referrals include:
| • | traditional media and co-marketing with hospitals or surgeons, such as local news, office and hospital based collateral, patient seminars and other public relations; and |
| • | digital, country-specific campaigns, including patient websites, landing pages, geo-targeted search engine campaigns and social media. |
Our efforts to develop referral networks and to educate physicians about our therapy include:
| • | user group meetings and scientific symposia at international conferences; |
| • | support to key opinion leaders with publication, podium presentation and proctorship opportunities; and |
| • | outreach to referring gastroenterologists and general practitioners through medical journals and local conferences. |
We intend to continue and expand these marketing activities based on our ongoing assessment and experience with their relative effectiveness.
Third-Party Reimbursement
Our marketing efforts and resources are mainly focused on countries with established reimbursement potential or a quicker pathway to third-party reimbursement.
Reimbursement in Germany, Switzerland, Argentina and Colombia
In 2013, we received preliminary reimbursement authorization from the government in Germany, pursuant to the “NUB” (Neue Untersuchungs- und Behandlungsmethoden) process, a fast-track method for the introduction of new and innovative technologies. This process can only be filed by hospitals and is limited to new technologies that have just been introduced into Germany. The approval of the NUB is limited to the hospital which filed the application, lasts for one year, and can be renewed on an annual basis. The NUB approval relating to our product allows an increasing number of hospitals to access reimbursement, currently at a level of approximately $14,000 per case. In 2013, preliminary approval was granted for nine hospitals and this was expanded to 40 hospitals in 2014. In Switzerland, we were able to use an existing reimbursement code, with the first procedure performed in 2013. In similar fashion, we expect to receive reimbursement approvals, over time, for an expanding number of countries in Europe ranging from Sweden to Austria and The Netherlands. In 2014, we received the first reimbursement approvals for procedures conducted in Colombia and Argentina.
Third-Party Reimbursement in the United States
We will actively seek third-party reimbursement in the United States concurrently with the seeking of regulatory approval from the FDA. We believe that the following factors will put us in a strong position to receive third-party reimbursement in the United States post-approval.
| • | The availability of an existing CPT insurance code covering the procedure. We believe, based on assessment by a certified coding expert that an existing CPT code (64590) for gastric stimulation will apply to our procedure and neurostimulation system. The current payment (based on the Medicare reimbursement level in 2014) under such CPT code is $17,232. Additionally, there may be an additional payment for lead insertion, which could increase the total payment for the procedure. |
| • | The availability of scientifically rigorous sham-controlled clinical data. Our planned FDA pivotal study is expected to generate rigorous sham-controlled data on the efficacy of our technology in treating GERD and, if successful, will provide Tier I clinical evidence for commercial approval. |
90
Table of Contents
| • | Endorsement by key medical societies. We have advanced our clinical development program in the United States in collaboration with an independent panel of experts convened by the Center for GI Innovation and Technology (CGIT), a joint body of American Gastroenterology Association and Society of American Gastrointestinal and Endoscopic Surgeons. The panel supports our study design and, as discussed below, has made a recommendation to the FDA that we should consider expanding our patient eligibility criteria. We believe this is a good indication of the society’s potential willingness to recommend EndoStim for reimbursement in the United States after a successful PMA trial. |
Competition
The annual direct cost of treating the approximately 63 million GERD patients in the United States is estimated to be about $10 billion, of which approximately 5% is spent on surgical procedures. This rapidly changing market, comprised of pharmaceutical products, medical devices and potential other treatments for GERD, is significantly affected by new product introductions and other market activities of industry participants. We believe the principal competitive factors in this market include:
| • | safety and improved patient outcomes; |
| • | absence of side effects; |
| • | access to and acceptance by leading physicians and professional medical and surgical societies; |
| • | product quality and reliability and risks relating to its use; |
| • | cost-effectiveness and comparative effectiveness; |
| • | training and support; |
| • | physician relationships; and |
| • | sales and marketing support. |
We have numerous competitors and potential competitors in the medical device and pharmaceutical industries. Patients and physicians may opt for more established existing therapies to treat GERD, including PPI treatment or fundoplication. PPIs are currently being offered by several large pharmaceutical manufacturers, each of whom has significantly greater financial, clinical, manufacturing, marketing, distribution and technical resources and experience than we have.
Over the last decade a number of medical devices and treatments have been introduced to address the GERD “therapy gap” and treat GERD less invasively than fundoplication, including the following options that are currently commercialized:
| • | LINX® Reflux Management System (Torax Medical) — an implantable magnetic mechanical collar around the LES. |
| • | Stretta (Mederi Therapeutics) — catheter to deliver radiofrequency energy to the LES to stimulate collagen deposition (scarring) in the LES. |
| • | Medigus MUSETM — device to endoscopically replicate a partial fundoplication. |
| • | EsophyXTM Transoral Incisionless Fundoplication (EndoGastric Solutions) — device to endoscopically replicate a partial fundoplication (no longer commercialized outside the United States). |
In addition, new companies have been, and are likely to continue to be, formed to pursue opportunities in our market.
We believe our therapy, which offers a minimally invasive, anatomy preserving and reversible treatment intended to safely and efficaciously improve and/or normalize LES function while avoiding the side effects
91
Table of Contents
associated with current surgical and device-based interventions, offers a superior treatment alternative to those of our competitors.
Manufacturing and Procurement
In March 2013, we entered into a Development and Supply Agreement with Centro de Construcción (CCC) in Uruguay. CCC is a long-standing contract supplier of neurostimulators and pacemakers to companies in the United States. It has manufactured and marketed over a dozen models of cardiac pacemakers since the 1970s. Under the agreement, CCC manufactures and continues to develop the neurostimulation system for us, including the programmer and implantable lead. The agreement also grants us the rights to all test results, designs, inventions and intellectual property developed as a result of the agreement. Over the first three years of the agreement, CCC must be able to supply minimum quantities of the IPGs and implantable leads, and we must purchase minimum quantities of IPGs and implantable leads. The agreement may be terminated by either party with 90 days’ written notice, provided that such termination shall not relieve either party of its payment and supply obligations accrued prior to the date of termination.
Intellectual Property
We own intellectual property designed to protect our core technologies and we continue to strengthen our patent portfolio through new filings. We initially acquired patents and other intellectual property from Dr. Virender K. Sharma through a Patent Assignment Agreement and an Assignment Agreement concurrently executed on September 2, 2009 and subsequently amended in January 2010. Pursuant to these agreements, Dr. Sharma transferred six categories of rights to us:
| • | All right, title, and interest to the inventions disclosed or claimed in U.S. Patent Nos. 6,901,295 and 7,738,961 and any domestic or foreign patents related thereto. |
| • | All right, title, and interest to discoveries, intellectual property and know-how developed by Dr. Sharma, as of September 2, 2009, in the field of (i) the treatment or therapy of gastroesophageal reflux or gastroesophageal reflux diseases and all esophageal and extraesophageal conditions caused by gastroesophageal reflux or gastroesophageal reflux disease and/or obesity using electrostimulation in the stomach alone or in combination with the esophagus and (ii) the treatment or therapy of any condition or indication using electrostimulation in the esophagus, which we refer to as the “Field.” |
| • | All right, title, and interest to U.S. Patent No. 8,543,210 and all domestic and foreign patents related thereto, with our practice of the inventions embodied in the patent No. 8,543,210 being limited to the Field. Pursuant to an exclusive license agreement dated January 11, 2010, Dr. Sharma has the exclusive right to practice the inventions embodied in this patent outside the Field. |
| • | All right, title, and interest to discoveries, intellectual property and know-how developed by Dr. Sharma in the field of the treatment or therapy of gastroesophageal reflux disease and/or obesity using electro-stimulation of the esophagus and/or stomach through to September 2, 2019. |
| • | A first right of refusal to purchase rights to the inventions covered by U.S. Patent Application No. 12/030,222 and all patents related thereto. |
| • | A first right of refusal to purchase rights to all other discoveries, intellectual property and know-how developed by Dr. Sharma outside the Field. |
Under these agreements, we are solely responsible for prosecuting and maintaining the patents in all jurisdictions we select based on our marketing strategy.
We have further acquired an intellectual property portfolio from Dr. Edy Soffer of the University of Southern California, a gastrointestinal motility researcher with experience in gastrointestinal electrical stimulation and LES stimulation animal work. We acquired Dr. Soffer’s intellectual property pursuant to a Patent Assignment
92
Table of Contents
Agreement and an Assignment Agreement concurrently executed on December 15, 2009. Under the agreements, we received all right, title and interest to Dr. Soffer’s patent application concerning electrical stimulation of the lower esophageal sphincter, or the LES Application, in exchange for an assignment fee and two contingent assignment fees, the conditions of which were met. Under the agreements, we are solely responsible for prosecuting the LES Application, maintaining any patents issuing therefrom, and prosecuting and maintaining any patent applications or patents related thereto. Additionally, pursuant to a Patent Option Agreement executed December 15, 2009, we acquired an option to purchase Dr. Soffer’s patent concerning a device for the automatic detection of eating and drinking, or the AED Application, in exchange for an option fee. As of today, we have not elected to exercise our option rights. Until we exercise our option rights, Dr. Soffer and/or his co-inventors are entirely responsible for prosecuting and maintaining the AED Application under the agreement and have the right to terminate prosecution or abandon the AED Application.
In the United States, we have obtained additional patent coverage over functional features that represent some of the operational advantages of our neurostimulation system. For example, we have patented devices and treatment methods that, in combination with other functions, stimulate a patient’s LES in a manner that maintains the therapeutic effect after stimulation ceases. Our patent for this device and methods expires on February 23, 2028. We have also patented devices and treatment methods that, together with other elements, normalize the functionality of a patient’s LES, while not inhibiting a patient’s ability to swallow. Our patent for this device and method expires on June 4, 2031. With these features claimed, we are also pursuing patents directed to devices and methods which incorporate novel features related to energy usage, diagnostics, form factor, surgical techniques, stimulation triggers based on a patient’s supine position, and specific stimulation parameters. We have made patent filings in the United States, in the European Union, Australia, New Zealand and China.
We have also evaluated and accumulated third-party intellectual property that offers valuable, though optional, feature sets and device implementations. We view this intellectual property as being useful for possible future generation devices as well as potentially for defensive positioning for alternative implementations that could be developed by competing entities.
Regulation of Our Business
Medical Device Products
Pursuant to its authority under the Federal Food, Drug and Cosmetic Act, or FDCA, FDA has jurisdiction over medical devices. Our product is a medical device subject to extensive regulation by the FDA and other U.S. federal and state regulatory bodies and comparable authorities in other countries. To ensure that medical products distributed domestically and internationally are safe and effective for their intended use, the FDA and comparable authorities in other countries have imposed regulations that govern, among other things, the following activities that we or our partners perform and will continue to perform:
| • | product design and development; |
| • | product testing; |
| • | product manufacturing; |
| • | product labeling; |
| • | product storage; |
| • | premarket clearance, approval or comparable foreign actions; |
| • | pre-clinical testing in animals and in the laboratory; |
| • | clinical investigations in humans; |
| • | advertising and promotion; |
93
Table of Contents
| • | record keeping and document retention procedures; |
| • | product marketing, sales, distribution and recalls; and |
| • | post-market surveillance reporting, including reporting of death, serious injuries, device malfunctions or other adverse events. |
United States FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device distributed commercially in the United States requires either prior 510(k) clearance or premarket approval, or PMA, from the FDA. The FDA classifies medical devices into one of three classes. Class I devices are subject to only general controls, such as establishment registration and device listing, labeling, medical device reporting, and prohibitions against adulteration and misbranding. Class II medical devices generally require prior 510(k) clearance before they may be commercially marketed in the United States. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device, are placed in Class III, generally requiring submission of a PMA supported by clinical trial data. Our neurostimulation system is considered to be a Class III product and thus will require submission and approval of a PMA. In the future, we may develop new products that are considered to be Class II and require the clearance of a 510(k).
510(k) Clearance Pathway
To obtain 510(k) clearance, a premarket notification must be submitted to FDA demonstrating that the proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of premarket approval applications. FDA’s 510(k) clearance pathway usually takes from three to twelve months, but it can take significantly longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, require premarket approval. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k), or a premarket approval, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket approval is obtained. If the FDA requires a 510(k) holder to seek 510(k) clearance or premarket approval for any modifications to a previously cleared product, the 510(k) holder also may be required to cease marketing or recall the modified device until this clearance or approval is obtained.
Premarket Approval Pathway
A PMA must be supported by extensive data, including but not limited to data obtained from technical, preclinical and clinical studies and relating to manufacturing and labeling to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device.
After a PMA submission is sufficiently complete, the FDA will accept the application and begin an in-depth review, which generally takes between one and three years, but may take significantly longer. During this review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will conduct a pre-approval inspection of the manufacturing facility to ensure compliance with Quality System Regulation, or QSR. New PMA applications or PMA
94
Table of Contents
supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application and may not require as extensive clinical data or the convening of an advisory panel.
Clinical Trials
Clinical trials are almost always required to support a PMA. To perform a clinical trial in the United States for a significant risk device, FDA requires the device sponsor to file an Investigational Device Exemption, or IDE, application with the FDA and obtain IDE approval prior to commencing the human clinical trial. An IDE amendment or supplement must also be submitted before initiating a significant change to the clinical protocol or device under an existing IDE. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, and any available data on human clinical experience, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound.
The IDE must be approved in advance by the FDA for a specific number of patients. Clinical trials conducted in the United States for significant risk devices may begin once the IDE application is approved by the FDA and the appropriate institutional review boards, or IRBs, overseeing the welfare of the research subjects and responsible for that particular clinical trial. Under its regulations, the FDA responds to an IDE or an IDE amendment within 30 days. The FDA may approve the IDE or amendment, grant an approval with certain conditions, or identify deficiencies and request additional information. It is common for the FDA to require additional information before approving an IDE or amendment for a new trial, and thus final FDA approval on a submission may require more than the initial 30 days. The FDA may also require that a small-scale feasibility study be conducted before a pivotal trial may commence. In a feasibility trial, the FDA limits the number of patients, sites and investigators that may participate. Feasibility trials are typically structured to obtain information on safety and to help determine how large a pivotal trial should be to obtain statistically significant results.
Clinical trials are subject to extensive recordkeeping and reporting requirements. Our clinical trials must be conducted under the oversight of an IRB for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. We are also required to obtain the patients’ informed consent in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the IRB may suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and effectiveness of the device or may otherwise not be sufficient to obtain FDA approval to market the product in the United States. Similarly, in Europe the clinical study must be approved by a local ethics committee and in some cases, including studies with high-risk devices, by the ministry of health in the applicable country.
Pervasive and Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. These include:
| • | product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
| • | Quality System Regulation, or QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
| • | labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication; |
95
Table of Contents
| • | approval of product modifications that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our approved devices; |
| • | approval of product modifications that affect the safety or effectiveness of one of our approved devices; |
| • | medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; |
| • | post-approval restrictions or conditions, including post-approval study commitments; |
| • | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
| • | the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
| • | regulations pertaining to voluntary recalls; and |
| • | notices of corrections or removals. |
We will be required to register with FDA as a medical device manufacturer within 30 days of commercial distribution of our neurostimulation system and must obtain all necessary state permits or licenses to operate our business. As a manufacturer, we are subject to announced and unannounced inspections by FDA to determine our compliance with quality system regulation and other regulations, and these inspections may include the manufacturing facilities of our suppliers. We have not yet been inspected by the FDA. We believe that we are in substantial compliance with quality system regulation and other regulations. Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or state authorities, which may include any of the following sanctions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | unanticipated expenditures to address or defend such actions; |
| • | customer notifications for repair, replacement, refunds; |
| • | recall, detention or seizure of our products; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusing or delaying our requests for premarket approval of new products or modified products; |
| • | operating restrictions; |
| • | withdrawing PMA approvals that have already been granted; |
| • | refusal to grant export approval for our products; or |
| • | criminal prosecution. |
Further, we are subject to various federal and state laws concerning health care fraud and abuse, including false claims and anti-kickback laws. Violations of these laws can result in criminal and/or civil punishment, including fines, penalties, damages, imprisonment and, in the United States, exclusion from participation in federal health care programs. The scope of these laws and related regulations is broad and their interpretation is evolving. In addition, these laws and their interpretations are subject to change. Increased funding for enforcement of these laws and regulations has resulted in greater scrutiny of marketing practices in our industry and resulted in numerous investigations by various government authorities. If a governmental authority were to determine that we do not comply with these laws and regulations, then we and our officers and employees could be subject to criminal and civil sanctions, including exclusion from participation in federal health care programs.
96
Table of Contents
Advertising and promotion of medical devices are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Promotional activities for FDA-regulated products have been the subject of significant enforcement actions brought under healthcare reimbursement laws, “fraud and abuse” laws (such as those prohibiting kickbacks and false claims, discussed below), and consumer protection statutes, among other theories. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims.
Fraud and Abuse and False Claims
We are subject to various federal and state laws governing our relationship with healthcare providers and pertaining to healthcare fraud and abuse, including anti-kickback laws. In particular, the federal healthcare program Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, paying, or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any health care items or services for which payment may be made in whole or part under federal healthcare programs, such as Medicare and Medicaid. Penalties for violations include criminal, civil and administrative penalties, damages, fines, imprisonment and exclusion from participation in Medicare, Medicaid and other federal healthcare programs. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. There are a number of statutory exceptions as well as regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny.
The Federal civil False Claims Act prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowing and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government. Violation of the False Claims Act can result in significant civil and administrative penalties, up to treble damages and exclusion from participation in federal health care programs like Medicare and Medicaid. The False Claims Act also allows a private individual or entity to sue on behalf of the government. Medical device manufacturers and other health care companies have been investigated by the U.S. Department of Justice and have reached substantial financial settlements with the federal government under the civil False Claims Act for a variety of alleged improper marketing activities, including providing free product, providing consulting fees, grants, free travel and other benefits to physicians to induce them to prescribe the company’s products, and for causing false claims to be submitted as a result of the marketing of their products for unapproved, and thus non-reimbursable, uses. Resolution of such investigations has often included manufacturers entering into corporate integrity agreements with the Office of Inspector General for the U.S. Department of Health and Human Services that require, among other things, substantial reporting and remedial actions.
Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the payer.
The federal Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the U.S. Securities and Exchange Commission. Violations of United States or foreign laws or regulations could result in the imposition of substantial fines, interruptions of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits,
97
Table of Contents
and other legal or equitable sanctions. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence.
We engage in a variety of activities that are potentially regulated under these laws, including, for example, consulting arrangements with cardiothoracic surgeons, grants for training and other education, grants for research, and other interactions with doctors. Failure to comply with applicable legal requirements could potentially result in substantial penalties to us and significant adverse effect on our ability to operate. Even though we structure our programs with the intent of compliance with such laws, there can be no certainty that we would not need to defend against enforcement or litigation, in light of the fact that there is significant enforcement interest in medical device manufacturers in the United States, and some of the applicable laws are quite broad in scope.
We may also be subject to various federal and state marketing laws, such as the federal Physician Payments Sunshine Act, which generally require certain types of expenditures in the United States and the particular states to be tracked and reported. The federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires certain pharmaceutical and medical device manufacturers to engage in extensive tracking of payments or transfers of value to physicians and teaching hospitals, maintenance of a payments database, and public reporting of the payment data. Device manufacturers with products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program are required to track and report such payments. Moreover, several states have enacted legislation requiring pharmaceutical and medical device companies to establish marketing compliance programs or even prohibit providing meals to prescribers or other marketing related activities. Compliance with such requirements may require investment in infrastructure to ensure that tracking and reporting is performed properly. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated.
We believe that our marketing practices are not in violation of the laws mentioned above or their state equivalents, but we cannot assure you that individuals or enforcement authorities will not attempt to take action against us and, if such action were successful, we could be required to pay significant fines and penalties and change our marketing practices. Such enforcement could have a significant adverse effect on our ability to operate.
International
International sales of medical devices are subject to foreign governmental regulations, which vary substantially from country to country. The time required to obtain certification or approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ.
The European Union, or EU, which consists of 25 member countries, has adopted numerous directives and has promulgated voluntary standards regulating the design, manufacture, labeling, clinical trials and adverse event reporting for medical devices. Devices that comply with the requirements of relevant directives will be entitled to bear CE conformity marking, and, accordingly, can be commercially distributed throughout the member states of the European Union and other countries that comply with or mirror these directives. The method for assessing conformity varies depending upon the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a notified body, which is an independent and neutral institution appointed by a country to conduct the conformity assessment. This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s device. Such an assessment in one country within the EU is required for a manufacturer to commercially distribute the product throughout the EU. In addition, compliance with voluntary harmonizing standards ISO 9001 and ISO 13845 issued by the International Organization for Standards establishes the presumption of conformity with the essential requirements for a CE marking. Our facilities were certified by DEKRA, an accredited certification body, to ISO 13485 in 2013 to aid in registration in other countries. We have the authorization to affix the CE Mark to our lead product and to commercialize the neurostimulation system in the European Union for the
98
Table of Contents
treatment of patients with chronic GERD with symptom duration of six months or longer. We received the CE Mark certification for our neurostimulation system in August 2012. In order to maintain CE Mark certification, we are subject to both announced and unannounced inspections by the notified body. Significant changes to the device or manufacturer’s quality system are also subject to approval by the notified body. Reporting of certain adverse events and product recalls to the national health authorities is required for compliance with the directive. Member states may also require registration of devices and translation of labeling into national languages.
We have received approval for clinical investigation by the appropriate government agencies in Mexico, Colombia, and Singapore. The clinical investigation is conducted under approval of the ethics committee and is subject to reporting requirements of the government authorities. We have received approval for commercial distribution of our neurostimulation systems in Argentina, Australia, Colombia, and Peru. The approved indications in Argentina, Colombia, and Peru are the same as those in the EU. Approval of the neurostimulation system in Australia is limited to the treatment of patients with chronic GERD with symptom duration of 6 months or longer that has been shown to be refractory to pharmaceutical treatment.
Employees
As of June 30, 2014, we had 13 full-time employees, with four in research and development and clinical functions, five in commercial operations, and four in general and administrative, regulatory and quality and business development functions. As of June 30, 2014, we had two part-time employees, with one in a clinical function and one in a general and administrative function. In addition, we have engaged a network of independent contractors. None of our employees is represented by a labor union with respect to his or her employment with us.
Facilities
We lease approximately 2,020 square feet of space on a month to month basis in St. Louis, Missouri, where we have our executive offices.
Legal Proceedings; Product Liability
From time to time, we may be subject to legal proceedings and claims in the ordinary course of business. Currently, we are not a party to any legal proceedings or subject to any claims. The results of any future litigation cannot be predicted with certainty, and regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
99
Table of Contents
Directors and Executive Officers
The following table sets forth the name, age (as of July 1, 2014) and position of each of our directors and executive officers.
| Name |
Age |
Position | ||||
| Bevil Hogg |
66 | President and Chief Executive Officer, Director | ||||
| Shai Policker |
45 | Chief Operating Officer | ||||
| Paul Goode, Ph.D. |
47 | Senior Vice President, Research and Development | ||||
| Peggy S. Stohr |
49 | Chief Financial Officer | ||||
| Douglas D. French (1)(2)(3) |
60 | Chairman of the Board | ||||
| Virender K. Sharma, M.D. (4) |
45 | Director, Chief Medical Officer | ||||
| Ralph G. Dacey, Jr., M.D. (2)(3) |
65 | Director | ||||
| Fred C. Goad (1) |
73 | Director | ||||
| Jeffrey M. McDonnell (1)(3) |
51 | Director | ||||
| Raul E. Perez, M.D. (2) |
64 | Director | ||||
| (1) | Member of the Audit Committee |
| (2) | Member of the Compensation Committee |
| (3) | Member of the Nominating and Corporate Governance Committee |
| (4) | Dr. Sharma provides consulting services as our Chief Medical Officer but does not function as an executive officer in such capacity. |
The following includes a brief biography for each of our executive officers and directors, with each director biography including information regarding the experiences, qualifications, attributes or skills that caused our board of directors to determine that each member of our board of directors should serve as a director as of the date of this prospectus. There are no family relationships among any of our executive officers or directors.
Executive Officers
Bevil J. Hogg. Mr. Hogg is one of our founders and has served as our President and Chief Executive Officer since June 2009. From June 1997, to December 31, 2008, Mr. Hogg served as Chief Executive Officer of Stereotaxis, Inc., a publicly traded medical device company. From 1994 through 1996 Mr. Hogg served as President and Chief Executive officer of Everest & Jennings International Ltd., a publicly traded manufacturer of wheelchairs and other hospital, home care and nursing home products. Earlier in his career, Mr. Hogg co-founded Trek Bicycle Corporation, a technologically advanced U.S. bicycle corporation. Mr. Hogg received a Diplôme Superior d’Études Françaises from the Sorbonne (University of Paris, France). We believe that Mr. Hogg’s extensive knowledge of our company and our business from service as our President and Chief Executive Officer, as well as his prior service as president and chief executive officer at two publicly traded healthcare related companies, makes him well-suited to serve as a member of our board of directors.
Shai Policker, Chief Operating Officer. Mr. Policker has served as our Chief Operating Officer since February 2014. From January 2011 to January 2014, Mr. Policker served as our Senior Vice President of Operations, and from 2009 to January 2011 we engaged him as a consultant. From 2007 to 2009, Mr. Policker was Vice President of Business Development for MetaCure Inc., an implantable stimulator company for the treatment of type 2 diabetes. From August 2000 to August 2005, Mr. Policker served as the Vice President for Research at Impulse Dynamics Inc., an implantable stimulator company for the treatment of congestive heart failure. Mr. Policker holds an undergraduate degree in electrical engineering from the Technion, Israel Institute of Technology and a M.B.A. from Columbia University.
Paul Goode, Ph.D., Senior Vice President, Research and Development. Dr. Goode has served as our Senior Vice President, Research and Development since June 2010. From January 2006 to May 2010, Dr. Goode was Vice President of Research and Development for MetaCure Inc., an implantable stimulator company for the
100
Table of Contents
treatment of type 2 diabetes. From 2004 to 2006, Dr. Goode served as Director of Engineering for Impulse Dynamics Inc., an implantable stimulator company for the treatment of congestive heart failure. From 2001 to 2004, Dr. Goode was Director of Engineering for DexCom, a glucose sensor company with both implantable and external sensing products. Previously, Dr. Goode served in several engineering and scientific positions at MiniMed (now part of Medtronic Diabetes), Guidant Corporation (now Boston Scientific) and Intermedics (now Boston Scientific). Dr. Goode received his B.S., M.S., and Ph.D. degrees in electrical engineering from North Carolina State University.
Peggy S. Stohr, Chief Financial Officer. Ms. Stohr has served as our Chief Financial Officer since September 2010. Since October 2009, Ms. Stohr has served as the Chief Financial Officer for several development stage organizations, including Pulse Therapeutics (medical device, stroke) from September 2011, Graematter, Inc. (healthcare information technology) from 2011 to July 2014 and Verto Medical Solutions (performance headphones) from October 2009 to May 2011. Following the completion of this offering, Ms. Stohr will become a full-time employee of us. From 1999 to 2009, she held various positions at Stereotaxis, Inc., most recently as Vice President — Administration and Controller. Previously, Ms. Stohr served in progressive financial and administrative management positions for two Boston-area software companies, as an auditor with Coopers & Lybrand in Boston, MA. Ms. Stohr is a certified public accountant. Ms. Stohr earned an M.B.A. from Washington University, St. Louis, an M.S. (Tax) from Bentley College in Waltham, Massachusetts and a B.S.B.A. in Accounting from the University of Dayton.
Non-Employee Directors
Douglas D. French, Chairman of the Board. Mr. French has served as a member of our Board of Directors since July 2010 and as the Chairman of the Board since May 2014. Since 2007, Mr. French has served as a founder and Managing Director of Santé Ventures, an investor in early stage life science and healthcare companies. From 2001 to 2004, Mr. French served as the President and Chief Executive Officer of the Ascension Health system and creator of the Ascension Health Ventures corporate fund. Previously, Mr. French served as a Healthcare Venture Partner with Austin Ventures. Mr. French currently serves on the board of Herman Miller and is a former board member of Ascension Health, Catholic Healthcare West, EMAGEON and Old National Bancorp. Mr. French holds a B.S. Degree from Trevecca Nazarene College in Nashville, Tennessee and a Masters in Hospital and Health Services Administration from Xavier University in Cincinnati, Ohio. We believe that the experience of Mr. French in the venture capital industry, including with other successful companies in the life sciences and healthcare industry, including his service as a director of a publicly traded companies, makes him well suited to serve as a member of our board of directors.
Ralph G. Dacey, Jr., M.D., FACS, FRCSI (Hon), Director. Dr. Dacey has served as member of our Board of Directors since our inception in 2009. Since 1989, Dr. Dacey has been a professor at the Department of Neurological Surgery at Washington University in St. Louis and currently serves as the Henry G. and Edith R. Schwartz Professor and Chairman of the Department of Neurological Surgery. Dr. Dacey previously served as Chairman of the American Board of Neurological Surgery and President of the Congress of Neurological Surgeons, the American Academy of Neurological Surgery and most recently, the Society of Neurological Surgeons. Dr. Dacey received a B.A. from Harvard University, an M.D. from the University of Virginia. Dr. Dacey completed residency at the University of Virginia in both internal medicine and neurological surgery and is board certified in Internal Medicine and Neurological Surgery. We believe that Dr. Dacey’s deep professional knowledge in the medical field and his contribution to the conduct of our clinical program make him well-suited to serve as a member of our board of directors.
Fred C. Goad, Director. Mr. Goad has served as a member of our Board of Directors since 2010. Since August 2001, Mr. Goad has served as a co-founder and partner of Voyent Partners, LLC, a private investment firm focused on early stage investments across a variety of sectors including healthcare. From 1985 to June 1996, Mr. Goad served as President and Chief Executive Officer of ENVOY Corporation, a provider of electronic data interchange services to participants in the health care market. From June 1996 to March 1999, Mr. Goad served as Co-Chief Executive Officer and Chairman of ENVOY Corporation. From March 1999 to May 2000, Mr. Goad
101
Table of Contents
served as a member of the board of directors of Quintiles Transnational Corporation and as Senior Advisor to the Office of the President of the transaction services division of the Quintiles Transnational Corporation. From June 2000 to March 2001, Mr. Goad served as Co-Chief Executive Officer of the transaction services division of WebMD Corporation. Mr. Goad currently serves on the boards of directors Luminex Corporation, a manufacturer of innovative biological testing technologies with applications throughout the diagnostic and life science industries, Informatics Corporation of America, an early-stage healthcare informatics company creating electronic medical records for regional health information organizations, large healthcare systems, hospitals and multisite practices, and Sy.Med Development, a provider of enrollment and credentialing software to healthcare organizations. Mr. Goad previously served on the boards of directors of Performance Food Group, a food service distributor and Emageon Inc., a provider of enterprise-level information technology solutions for the clinical analysis and management of digital medical images within health care provider organizations. Mr. Goad holds a B.S. from the University of Virginia. We believe that Mr. Goad’s experience in the venture capital industry, including in leadership positions with other successful companies in the life sciences and healthcare industry, and his prior service as a director of other publicly traded companies makes him well suited to serve as a member of our board of directors.
Jeffrey M. McDonnell, Director. Mr. McDonnell has served as a member of our Board of Directors since 2010. Mr. McDonnell has served as an officer of J&J Management Services, Inc., a private management company since 2000. Mr. McDonnell currently serves on the boards of KETC, the St. Louis metro region public television station, The Center for Emerging Technologies, a non-profit technology incubator, and Love Savings Holding Company, a savings and loan holding company. Mr. McDonnell serves on the investment and oversight committees for the venture capital firms Oakwood Medical and Rivervest. Mr. McDonnell holds a B.A. in economics from Princeton University, an M.B.A. from the University of Michigan and a certification as a Chartered Financial Analyst (CFA). We believe that Mr. McDonnell’s experience in senior leadership positions at various organizations, including venture capital firms, and his financial and accounting expertise makes him well-suited to serve as a member of our board of directors.
Raul E. Perez, M.D., Director. Dr. Perez is one of our founders and served as Chairman of our Board of Directors from 2009 until May 2014. Since 1997, Dr. Perez has served as the President of Oakwood Medical Investors, a life science venture capital fund. Dr. Perez holds an undergraduate degree from St. Louis University and an M.D. from University UAG in Mexico and the University of Maryland, Baltimore (at the Maryland General Hospital). Dr. Perez completed residency in obstetrics and gynecology at St. John’s Mercy Medical Center in St. Louis, Missouri. Dr. Perez practiced medicine until 2000 as a board certified OB/GYN. Dr. Perez has also served as a director of Miravant Medical Technologies (formerly known as PDT Inc.), a publicly-traded biomedical company and as advisory director to Computer Motion Inc., a publicly-traded medical device company, which merged with Intuitive Surgical, Inc. We believe that the medical background of Dr. Perez, his experience in managing venture investments in the life sciences field, and extensive knowledge of our business as one of the founders makes him well-suited to serve as a member of our board of directors.
Virender K. Sharma, M.D., FASGE, FACG, AGAF, Chief Medical Officer, Director. Dr. Sharma is one of our founders and has served as our Chief Medical Officer, on a part-time basis, since June 2011. From June 2007 to January 2010, Dr. Sharma was a Professor of Medicine, Division of Gastroenterology and Hepatology, Director of the Esophageal Clinic and Vice-Chair for Gastroenterology Research at the Mayo Clinic in Arizona. Currently Dr. Sharma maintains an active, full-time private clinical practice, serving as the Director of the Arizona Center of Digestive Health. Dr. Sharma holds an MB BS from the All India Institute of Medical Science, New Delhi, India. Dr. Sharma completed his internship in internal medicine at the State University of New York Health Sciences Center, Brooklyn, his residency at State University of New York Health Sciences Center, Syracuse, New York, and his subspecialty training in gastroenterology at the University of South Carolina, Columbia, South Carolina. We believe that Dr. Sharma’s expertise in the field of gastroenterology and clinical research and his extensive knowledge of our business and technology as one of the founders and the inventor of significant intellectual property makes him well-suited to serve as a member of our board of directors.
102
Table of Contents
Composition of the Board of Directors
Our bylaws provide that the size of our board of directors will be determined from time to time by resolution of our board of directors. Our board of directors currently consists of seven directors, five of whom qualify as independent directors under the rules and regulations of the Securities and Exchange Commission, or SEC, and NASDAQ Stock Market, LLC, or NASDAQ.
Election of Directors
Immediately prior to the completion of this offering, our amended and restated certificate of incorporation will provide for a classified board of directors consisting of three classes of directors. We will have three directors in Class I and two directors in each of Class II and Class III, each serving a staggered three-year term. At each annual meeting of stockholders, our stockholders will elect successors to directors whose terms then expire to serve from the time of election and qualification until the third annual meeting following election. After the completion of this offering, our directors will be divided among the three classes as follows:
| • | Class I directors will be , and their terms will expire at the annual meeting of stockholders to be held in 2015; |
| • | Class II directors will be , and their terms will expire at the annual meeting of stockholders to be held in 2016; and |
| • | Class III directors will be , and their terms will expire at the annual meeting of stockholders to be held in 2017. |
The classification of our board of directors may have the effect of delaying or preventing changes in control of our company. We expect that additional directorships resulting from an increase in the number of directors, if any, will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors.
Voting Arrangements
We have entered into a stockholders’ agreement with certain of our stockholders who hold, collectively, the majority of our common stock, on an as-converted basis. Pursuant to the stockholders’ agreement, all stockholders that are party to the agreement have agreed to vote to all of their shares in such manner as may be necessary to elect and maintain in office as members of our Board of Directors the following individuals: (i) two designees of Santé Health Ventures I, LP, or its affiliates, (ii) one designee of the holders of a majority of the then-outstanding shares of Series A Preferred Stock, (iii) two designees of the holders of a majority of the then-outstanding shares of Common Stock and (iv) two designees of a majority of the common and preferred stockholders, voting together as a single class, provided that one such designee of all stockholders is always our chief executive officer. The stockholders’ agreement will terminate upon the earlier of (a) the completion of this offering, (b) a change in control of our company, (c) when the agreement is terminated in writing executed by each of (i) the Company, (ii) the holders of 60% of shares of all series of preferred stock, voting together on an as-converted basis, and (iii) the holders of a majority of the common stock, or (d) the dissolution or winding out of the Company.
Independence of the Board of Directors and Board Committees
Rule 5605 of the NASDAQ Marketplace Rules, or the NASDAQ Listing Rules, requires that independent directors compose a majority of a listed company’s board of directors within one year of listing. In addition, the NASDAQ Listing Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation, and nominating and corporate governance committees be independent and that audit committee members also satisfy independence criteria set forth in Rule 10A-3 under the Exchange Act of 1934, as amended, or the Exchange Act. Under NASDAQ Listing Rule 5605(a)(2), a director will only qualify as an “independent director” if, in the opinion of our board of directors, that person does not have a relationship that would interfere
103
Table of Contents
with the exercise of independent judgment in carrying out the responsibilities of a director. In order to be considered independent for purposes of Rule 10A-3 under the Exchange Act, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: (1) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (2) be an affiliated person of the listed company or any of its subsidiaries. In addition to satisfying general independence requirements under the NASDAQ Listing Rules, members of the compensation committee must also satisfy additional independence requirements set forth in NASDAQ Listing Rule 5605(d)(2). In order to be considered independent for purposes of NASDAQ Listing Rule 5605(d)(2), a member of a compensation committee of a listed company may not, other than in his or her capacity as a member of the compensation committee, the board of directors, or any other board committee, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries. Additionally, the board of directors of the listed company must consider whether the compensation committee member is an affiliated person of the listed company or any of its subsidiaries and, if so, must determine whether such affiliation would impair the director’s judgment as a member of the compensation committee.
In 2014, our board of directors undertook a review of the composition of our board of directors and its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family and other relationships, including those relationships described under “Certain Relationships and Related Party Transactions,” our board of directors determined that none of Dr. Perez, Dr. Dacey, and Messrs. French, Goad and McDonnell, representing five of our seven directors, has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under Rule 5605(a)(2) of the NASDAQ Listing Rules. Mr. Hogg is not considered independent because he currently serves as our Chief Executive Officer. Dr. Sharma is not considered independent because he currently serves as our Chief Medical Officer. Our board of directors also determined that each member of the audit, compensation, and nominating and corporate governance committees satisfy the independence standards for such committees established by the SEC and the NASDAQ Listing Rules, as applicable. In making these determinations on the independence of our directors, our board of directors considered the relationships that each such non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining independence, including the beneficial ownership of our capital stock by each non-employee director.
Leadership Structure of the Board
Our amended and restated bylaws and corporate governance guidelines, which will become effective immediately prior to the consummation of this offering, will provide our board of directors with flexibility to combine or separate the positions of Chairman of the board of directors and Chief Executive Officer and/or the implementation of a lead director in accordance with its determination that utilizing one or the other structure would be in the best interests of our company. Mr. French currently serves as the Chairman of our board of directors. In that role, Mr. French presides over the executive sessions of the board of directors in which Mr. Hogg does not participate and serves as a liaison to Mr. Hogg and management on behalf of the board of directors.
Our board of directors has concluded that our current leadership structure is appropriate at this time. However, our board of directors will continue to periodically review our leadership structure and may make such changes in the future as it deems appropriate.
Role of Board in Risk Oversight Process
Risk assessment and oversight are an integral part of our governance and management processes. Our board of directors encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. Management discusses strategic and operational risks at regular
104
Table of Contents
management meetings, and conducts specific strategic planning and review sessions during the year that include a focused discussion and analysis of the risks facing us. Throughout the year, senior management reviews these risks with the board of directors at regular board meetings as part of management presentations that focus on particular business functions, operations or strategies, and presents the steps taken by management to mitigate or eliminate such risks.
Our board of directors does not have a standing risk management committee, but rather administers this oversight function directly through our board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure and our audit committee is responsible for overseeing our major financial risk exposures and the steps our management has taken to monitor and control these exposures. The audit committee also monitors compliance with legal and regulatory requirements. Our nominating and governance committee monitors the effectiveness of our corporate governance guidelines and considers and approves or disapproves any related-persons transactions. Our compensation committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
Board Committees
Audit Committee
Our audit committee oversees our corporate accounting and financial reporting process. Among other matters, the audit committee:
| • | appoints our independent registered public accounting firm; |
| • | evaluates the independent registered public accounting firm’s qualifications, independence and performance; |
| • | determines the engagement of the independent registered public accounting firm; |
| • | reviews and approves the scope of the annual audit and the audit fee; |
| • | discusses with management and the independent registered public accounting firm the results of the annual audit and the review of our quarterly financial statements; |
| • | approves the retention of the independent registered public accounting firm to perform any proposed permissible non-audit services; |
| • | monitors the rotation of partners of the independent registered public accounting firm on our engagement team as required by law; |
| • | is responsible for reviewing our financial statements and our management’s discussion and analysis of financial condition and results of operations to be included in our annual and quarterly reports to be filed with the SEC; |
| • | reviews our critical accounting policies and estimates; and |
| • | annually reviews the audit committee charter and the committee’s performance. |
The current members of our audit committee are Messrs. McDonnell (Chair), French and Goad. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and NASDAQ. Our board of directors has determined that Mr. McDonnell is an audit committee financial expert as defined under the applicable rules of the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of NASDAQ. Under the rules of the NASDAQ, members of the audit committee must also meet heightened independence standards. However, a minority of the members of the audit committee may be exempt from the heightened audit committee independence standards for one year from the date of effectiveness of the registration statement of which this prospectus forms a part. Our board of directors
105
Table of Contents
has determined that each of the members of the audit committee are independent under the applicable rules of NASDAQ. The audit committee operates under a written charter that satisfies the applicable standards of the SEC and NASDAQ.
Compensation Committee
Our compensation committee reviews and recommends policies relating to compensation and benefits of our officers and employees. The compensation committee reviews and recommends corporate goals and objectives relevant to compensation of our Chief Executive Officer and other executive officers, evaluates the performance of these officers in light of those goals and objectives and recommends to our board of directors the compensation of these officers based on such evaluations. The compensation committee also recommends to our board of directors the issuance of stock options and other awards under our stock plans. The current members of our compensation committee are Messrs. French (Chair) and Perez and Dr. Dacey. Each of the members of our compensation committee is independent under the applicable rules and regulations of The NASDAQ Capital Market, is a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act and is an “outside director” as that term is defined in Section 162(m) of the U.S. Internal Revenue Code of 1986, as amended, or Section 162(m). The compensation committee operates under a written charter that satisfies the applicable standards of the SEC and NASDAQ.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee is responsible for making recommendations to our board of directors regarding candidates for directorships and the size and composition of our board of directors. In addition, the nominating and corporate governance committee is responsible for overseeing our corporate governance policies and reporting and making recommendations to our board of directors concerning governance matters. The current members of our nominating and corporate governance committee are Dr. Dacey (Chair) and Messrs. French and McDonnell. Each of the members of our nominating and corporate governance committee is an independent director under the applicable rules and regulations of NASDAQ relating to nominating and corporate governance committee independence. The nominating and corporate governance committee operates under a written charter that satisfies the applicable standards of the SEC and NASDAQ.
Board Diversity
Upon consummation of this offering, our nominating and corporate governance committee will be responsible for reviewing with the board of directors, on an annual basis, the appropriate characteristics, skills and experience required for the board of directors as a whole and its individual members. In evaluating the suitability of individual candidates (both new candidates and current members), the nominating and corporate governance committee, in recommending candidates for election, and the board of directors, in approving (and, in the case of vacancies, appointing) such candidates, will take into account many factors, including the following:
| • | personal and professional integrity; |
| • | ethics and values; |
| • | experience in corporate management, such as serving as an officer or former officer of a publicly held company; |
| • | experience in the industries in which we compete; |
| • | experience as a board member or executive officer of another publicly held company; |
| • | diversity of expertise and experience in substantive matters pertaining to our business relative to other board members; |
| • | conflicts of interest; and |
| • | practical and mature business judgment. |
106
Table of Contents
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee has ever been an officer or employee of the company. None of our executive officers serves, or has served during the last three years, as a member of the board of directors, compensation committee or other board committee performing equivalent functions of any entity that has one or more executive officers serving as one of our directors or on our compensation committee.
107
Table of Contents
EXECUTIVE AND DIRECTOR COMPENSATION
Executive Compensation
The following is a discussion and analysis of compensation arrangements of our named executive officers, or NEOs. This discussion contains forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt may differ materially from currently planned programs as summarized in this discussion. As an “emerging growth company” as defined in the JOBS Act, we are not required to include a Compensation Discussion and Analysis section and have elected to comply with the scaled disclosure requirements applicable to emerging growth companies.
Our compensation committee, who is appointed by our board of directors, is responsible for establishing, implementing and monitoring our compensation philosophy and objectives. We seek to ensure that the total compensation paid to our executive officers is reasonable and competitive. Compensation of our executives is structured around the achievement of individual performance and near-term corporate targets as well as long-term business objectives.
Summary Compensation Table
The following table sets forth information for the year ended December 31, 2013, regarding compensation awarded to or earned by our named executive officers.
| Name and Principal Position |
Year | Salary ($) |
Option Awards ($) (1) |
Non-Equity Incentive Plan Compensation ($) |
Total ($) | |||||||||||||||
| Bevil Hogg |
2013 | 304,337 | 22,000 | 81,250 | 407,587 | |||||||||||||||
| President and Chief Executive Officer |
||||||||||||||||||||
| Shai Policker |
2013 | 240,666 | 9,900 | 20,313 | 270,879 | |||||||||||||||
| Chief Operating Officer (2) |
||||||||||||||||||||
| Paul Goode |
2013 | 213,570 | 4,400 | 20,313 | 238,283 | |||||||||||||||
| Senior VP of Research and Development |
||||||||||||||||||||
| (1) | Amounts listed in this column represent the aggregate fair value of the awards computed as of the grant date of each award in accordance with Financial Accounting Standards Board Accounting Standards Codification No. 718, Compensation-Stock Compensation, or FASB ASC Topic 718, rather than amounts paid to or realized by the named individual. See the notes to our consolidated financial statements for a discussion of assumptions made in determining the grant date fair value and compensation expense of our stock options. These amounts do not necessarily correspond to the actual value that the named executive officers may realize upon exercise. |
| (2) | During 2013, Mr. Policker served as Senior Vice President of Operations. |
Narrative Disclosure to Summary Compensation Table
In 2010, we entered into employment agreements with our named executive officers. These agreements provide for a minimum level of compensation that is payable to each executive. The compensation is reviewed by our compensation committee annually. In the past, the compensation committee has granted limited annual increases in base salary, although salary increases may be larger in connection with internal promotions or increase of responsibilities. We expect to continue this procedure for determining executive compensation following the closing of this offering. However, we expect that the compensation committee will evaluate the need for revisions to our executive compensation program to ensure that our program is competitive with the companies with which we compete for executive talent and that it is appropriate for a public company.
108
Table of Contents
The main components of our executive compensation program are base salary, incentive bonuses under our management bonus plan and equity incentive awards. Our executives are also eligible to receive benefits granted to our other salaried employees.
Base Salary
We use base salaries to recognize the experience, skills, knowledge and responsibilities required of all of our employees, including our named executive officers. Our employment agreements provide for an annual salary for each of our named executive officers, which may be increased at the discretion of the compensation committee.
Incentive Bonus
Pursuant to the employment agreements, each of the named executive officers is eligible to receive an incentive bonus. The performance of each of the named executive officers are evaluated quarterly, based on achievement of goals and objectives set by the compensation committee or the Board in advance of each quarterly period. In 2013, the quarterly performance objectives included financial goals, such as revenue or expenses, and operational goals, such as commercial, clinical or regulatory approval milestones. The incentive bonuses are payable quarterly to Mr. Hogg and annually to Messrs. Policker and Goode.
Equity Incentives
We do not have a formal policy with respect to the grant of equity incentive awards to our named executive officers. The employment agreements provided for initial equity grants to our named executive officers. Any further grants, including those disclosed in the 2013 summary compensation table above, are made at the discretion at the compensation committee. The compensation committee has granted, and expects to grant, such equity awards in order to align the interests of our executives and our stockholders. All stock options granted in 2013 were granted pursuant to our 2009 Stock Incentive Plan. Following the closing of the offering, our employees and executives will be eligible to receive equity incentive awards pursuant to our 2014 Stock Incentive Plan.
109
Table of Contents
Outstanding Equity Awards at Fiscal Year-End
The following table provides information regarding equity awards held by the named executive officers that were outstanding as of December 31, 2013.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||
| Name |
Grant Date | Number of Securities Underlying Unexercised Options Exercisable (#) |
Number of Securities Underlying Unexercised Options Unexercisable (#) |
Option Exercise Price ($) |
Option Expiration Date |
Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested (#) |
Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested ($) |
|||||||||||||||||||||
| Bevil Hogg |
5/25/2010 | — | — | — | — | 400,000 | (8) | 100,000 | ||||||||||||||||||||
| 11/16/2011 | 10,416 | 9,584 | (1) | 0.150 | 11/16/2021 | — | — | |||||||||||||||||||||
| 7/1/2013 | — | 100,000 | (2)(9) | 0.400 | 7/1/2023 | — | — | |||||||||||||||||||||
| Shai Policker |
12/19/2009 | 15,000 | — | 0.050 | 12/19/2019 | — | — | |||||||||||||||||||||
| 6/17/2010 | 26,250 | 3,750 | (3) | 0.075 | 6/17/2020 | — | — | |||||||||||||||||||||
| 6/17/2010 | 26,250 | 3,750 | (4) | 0.075 | 6/17/2020 | — | — | |||||||||||||||||||||
| 5/3/2011 | 15,000 | 5,000 | (5) | 0.150 | 5/3/2021 | — | — | |||||||||||||||||||||
| 11/16/2011 | 10,416 | 9,584 | (1) | 0.150 | 11/16/2021 | — | — | |||||||||||||||||||||
| 3/19/2013 | — | 45,000 | (6) | 0.400 | 3/19/2023 | — | — | |||||||||||||||||||||
| Paul Goode |
6/17/2010 | 50,000 | — | 0.075 | 6/17/2020 | — | — | |||||||||||||||||||||
| 5/3/2011 | 15,000 | 5,000 | (5) | 0.150 | 5/3/2021 | — | — | |||||||||||||||||||||
| 11/16/2011 | 10,416 | 9,584 | (1) | 0.150 | 11/16/2021 | — | — | |||||||||||||||||||||
| 2/20/2013 | — | 20,000 | (7) | 0.400 | 2/20/2023 | — | — | |||||||||||||||||||||
| (1) | 25% of the shares subject to the option vested on the one year anniversary of the November 16, 2011 vesting commencement date and 1/48th of the shares subject to the option vest in equal monthly installments thereafter over the next three years. |
| (2) | 25% of the shares subject to the option vest the one year anniversary of the July 1, 2013 vesting commencement date and 1/48th of the shares subject to the option vest in equal monthly installments thereafter over the next three years. |
| (3) | 1/48th of the shares subject to the option vest in equal monthly installments over four years. |
| (4) | 1/48th of the shares subject to the option vest in equal monthly installments over four years, and began vesting upon achievement of a milestone. |
| (5) | 1/36th of the shares subject to the option vest in equal monthly installments over three years, with additional performance-based restrictions. |
| (6) | 25% of the shares subject to the option vested on February 20, 2014 and 1/48th of the shares subject to the option vest in equal monthly installments thereafter over the next three years. |
| (7) | 25% of the shares subject to the option vested on the one year anniversary of the February 20, 2013 vesting commencement date and 1/48th of the shares subject to the option vest in equal monthly installments thereafter over the next three years. |
| (8) | Restricted stock awards are subject to vesting upon achievement of certain performance criteria, which relate to the internal rate of return that may be achieved by the holders of Series B Preferred Stock. The performance criteria may be amended at the discretion of the compensation committee. |
| (9) | In the second quarter of 2014, Mr. Hogg voluntarily forfeited the options granted on July 1, 2013. |
Restricted Stock Agreements
In May 2010, in connection with our Series B preferred stock financing, we issued 400,000 shares of our restricted common stock to each of Mr. Hogg and Dr. Perez pursuant restricted stock agreements. The agreements provide that the shares are subject to vesting upon achievement of certain performance criteria, which
110
Table of Contents
relate to the internal rate of return that may be achieved by the holders of Series B preferred stock. The performance criteria may be further amended at the discretion of the compensation committee. If the performance criteria are not satisfied upon certain liquidity events described in the agreements, we may repurchase all or a portion of the shares at a nominal purchase price. A “liquidity event” includes a sale of 50% of the voting power of the company’s stock, a merger or consolidation in which our stockholders do not own 50% of the voting power of the surviving entity, a sale or license of substantially all of our assets, an initial public offering of our common stock, including this offering, and the sale or exclusive license of a significant portion of our assets followed by a distribution to our stockholders of a substantial portion of the proceeds of such sale or license in cash or other liquid assets.
Agreements with Our Named Executive Officers
Each of our named executive officers has an employment agreement with us, providing for certain benefits, including severance benefits upon the termination of employment.
Agreement with Mr. Hogg
On May 25th, 2010 we entered into an At-Will Employment Agreement with Mr. Hogg setting forth the terms of his employment. Pursuant to the agreement, Mr. Hogg shall serve as our President, Chief Executive Officer and as a member of the Board.
Pursuant to the agreement, Mr. Hogg is entitled to an annual base salary, currently set at $304,337, and a quarterly bonus opportunity, currently at $25,000. Our Compensation Committee has approved an increase in Mr. Hogg’s base salary contingent on the completion of a funding event, which includes this offering. The increased salary of $308,902 would become effective as of January 1, 2014. The payment of the quarterly bonus is determined by our board of directors based upon our achievement of goals and objectives for the relevant quarter. The Compensation Committee has approved an increase in Mr. Hogg’s total bonus opportunity for 2014 to $150,000, contingent on the completion of a funding event, which includes this offering. For Mr. Hogg’s salary and bonus information for 2013, see “— Summary Compensation Table.” Pursuant to the agreement, Mr. Hogg received in 2010 an initial grant of 400,000 shares of our restricted common stock which are subject to our stockholders agreement and a restricted stock agreement executed at the same time as the employment agreement (for more information, see “— Restricted Stock Agreements”). Mr. Hogg is eligible to receive future equity awards at the Board’s discretion. For awards outstanding as of December 31, 2013, see “— Outstanding Equity Awards at Fiscal Year-End.” Mr. Hogg also receives 4 weeks of paid vacation per year, office space, secretarial services and reimbursement for reasonable business expenses, including travel expenses.
Pursuant to his employment agreement with us, if we terminate Mr. Hogg’s employment without “cause,” as defined in the agreement, Mr. Hogg is entitled to receive a salary continuance in an amount dependent on whether we engage in a “liquidity event,” which includes (i) the sale of 50% or more of the combined voting power of our outstanding securities; (ii) our merger or consolidation with any other entity resulting in our securities outstanding before the merger or consolidation representing less than 50% of the combined voting power of the securities of the surviving entity; (iii) the sale or license of all or substantially all of our assets; (iv) the completion of this offering; or (v) the sale or exclusive license of a significant portion of our assets to any person or group followed by a distribution in the form of liquid assets to our stockholders of a substantial portion of the proceeds from such sale or license. Currently, the severance payment equals Mr. Hogg’s base salary on the date of termination for the lesser of (w) the period from the date of Mr. Hogg’s termination of employment until Mr. Hogg commences employment with a new employer or (x) 12 months. If we engage in a liquidity event, including completion of this offering, the severance payment equals Mr. Hogg’s base salary on the date of termination for the lesser of (y) the period from the date of Mr. Hogg’s termination of employment until Mr. Hogg commences employment with a new employer or (z) 18 months.
For purposes of Mr. Hogg’s agreement, “cause” means gross misconduct or gross negligence that materially impacts us, such as breach of fiduciary duty, dishonesty, theft or commission of a crime involving moral turpitude.
111
Table of Contents
Agreement with Mr. Goode
On June 1, 2010 we entered into an Employment Agreement with Mr. Goode setting forth the terms of his employment. Pursuant to the agreement, Mr. Goode shall serve as our Vice President of Research and Development.
Pursuant to the agreement, Mr. Goode is entitled to an annual base salary, currently set at $213,570, and an annual bonus opportunity, currently at $25,000, whether in cash or our stock, upon completion of certain mutually agreed upon milestones or other criteria. Our Compensation Committee has approved an increase in Mr. Goode’s base salary contingent on the completion of a funding event, which includes this offering. The increased salary of $216,774 would become effective as of January 1, 2014. The Compensation Committee has approved an increase in Mr. Goode’s total bonus opportunity for 2014 at $35,000, contingent on the completion of a funding event, which includes this offering. Mr. Goode’s total cash compensation is eligible for annual review. For Mr. Goode’s salary and bonus information for 2013, see “— Summary Compensation Table.”
Pursuant to the agreement, Mr. Goode received options to purchase 50,000 shares of our common stock that vested in monthly installments on the condition that Mr. Goode remained employed with us. Mr. Goode is eligible to receive future equity awards at the Board’s discretion. For awards outstanding, including status of vesting as of December 31, 2013, see “— Outstanding Equity Awards at Fiscal Year-End.” Mr. Goode is further eligible for any additional benefits available to our employees, including group life, medical, health, dental and/or disability insurance or other benefits. Mr. Goode also receives 15 days of paid vacation per year under the agreement.
If we terminate Mr. Goode’s employment without “cause,” Mr. Goode is eligible to receive payments equal to (i) the portion of his then-current salary accrued to the date of termination but unpaid as of the termination date and (ii) his salary rate then in effect for 180 days. “Cause” is defined as (A) material breach of the agreement by the executive, (B) commission by the executive of a willful act of fraud or dishonesty involving us, or the commission of, or being charged with, any crime constituting a felony or involving theft, fraud or moral turpitude or (C) engagement by the executive in willful or reckless misconduct or gross negligence having an adverse effect on us.
Agreement with Mr. Policker
On June 1, 2010 we entered into an Employment Agreement with Mr. Policker setting forth the terms of his employment. Pursuant to the agreement, Mr. Policker served as our Vice President of Operations. Mr. Policker has since transitioned to the role of Chief Operating Officer.
Pursuant to the agreement, Mr. Policker is entitled to an annual base salary, currently set at $265,000, and an annual bonus opportunity, currently at $50,000, whether in cash or our stock, upon completion of certain mutually agreed upon milestones or other criteria. Mr. Policker’s total cash compensation is eligible for annual review. For Mr. Policker’s salary and bonus information for 2013, see “— Summary Compensation Table.”
Pursuant to the agreement, Mr. Policker received options to purchase 60,000 shares of our common stock upon completion of our Series B financing, of which 30,000 vest in 48 equal monthly installments beginning on the execution of the employment agreement and 30,000 vest in 48 equal monthly installments beginning on the achievement of a milestone. Mr. Policker is eligible to receive future equity awards at the Board’s discretion. For awards outstanding, including status of vesting as of December 31, 2013, see “— Outstanding Equity Awards at Fiscal Year-End.” Mr. Policker is further eligible for any additional benefits available to our employees, including group life, medical, health, dental and/or disability insurance or other benefits. Mr. Policker also receives 15 days of paid vacation per year under the agreement.
If we terminate Mr. Policker’s employment without “cause,” he is eligible to receive payments equal to (i) the portion of his then-current salary accrued to the date of termination but unpaid as of the termination date and (ii) his salary rate then in effect for 120 days. “Cause” is defined in the same way as in Mr. Goode’s agreement.
112
Table of Contents
Other Benefits
Our named executive officers are eligible to participate in all of our employee benefit plans, such as medical, dental, group life, short and long-term disability in each case on the same basis as other employees, subject to applicable laws. We also provide vacation and other paid holidays to all employees, including our named executive officers.
We believe these benefits are important to attracting and retaining experienced executives. Like many private companies, we do not currently provide perquisites to our executive officers, given our attention to the cost-benefit tradeoff of such benefits, and the board of directors’ knowledge of the benefit offerings at other private companies.
Tax and Accounting Considerations
Section 162(m) of the Code generally disallows a tax deduction for compensation in excess of $1.0 million paid to our chief executive officer and our three other most highly paid executive officers, other than our principal financial officer. Qualifying performance-based compensation is not subject to the deduction limitation if specified requirements are met. We may structure the performance-based portion of our executive compensation, when feasible, to comply with exemptions in Section 162(m) so that the compensation remains tax deductible to us. However, our board of directors may, in its judgment, authorize compensation arrangements and payments that do not comply with the exemptions in Section 162(m).
The compensation committee also takes into account whether components of our compensation program may be subject to the penalty tax associated with Section 409A of the Code, and aims to structure the elements of compensation to be compliant with or exempt from Section 409A to avoid such potential adverse tax consequences.
Equity Compensation Plans and Other Benefit Plans
2014 Stock Incentive Plan
Prior to the completion of this offering, our board of directors will adopt, and we expect our stockholders to approve, our 2014 Stock Incentive Plan, which we refer to as the 2014 Plan, for the purpose of attracting and retaining non-employee directors, executive officers and other key employees and service providers, including officers, employees and service providers of our subsidiaries, and to stimulate their efforts toward our continued success, long-term growth and profitability. The 2014 Plan provides for the grant of stock options, stock appreciation rights, restricted stock, unrestricted stock, stock units, dividend equivalent rights, other equity-based awards and cash bonus awards. We have reserved shares of common stock (which includes 140,333 shares available for issuance under our 2009 Plan as of June 30, 2014) for issuance pursuant to the 2014 Plan, subject to certain adjustments and automatic annual increases as set forth in the plan. Any shares of common stock underlying awards outstanding under the 2009 Plan as of the closing of this offering, which thereafter terminate by expiration, forfeiture, cancellation or otherwise without the issuance of such shares, shall be added to, and become available for issuance under, the 2014 Plan. This summary is qualified in its entirety by the detailed provisions of the 2014 Plan, which is filed as an exhibit to the registration statement of which this prospectus is a part.
Administration of the 2014 Plan. Our compensation committee will administer the 2014 Plan and determine all terms of awards under the plan. Each member of our compensation committee that administers the plan will be both a “non-employee director” within the meaning of Rule 16b-3 of the Exchange Act, and an “outside director” within the meaning of Section 162(m) of the Code. Our compensation committee will also interpret the provisions of the plan. During any period of time in which we do not have a compensation committee, or otherwise at the election of our board of directors, our board of directors or another committee appointed by our board of directors will administer the plan. References below to the compensation committee include a reference to the board of directors or another committee appointed by the board of directors for those periods in which the board of directors or such other committee appointed by the board of directors is acting.
113
Table of Contents
Eligibility. All of our employees and the employees of our subsidiaries are eligible to receive awards under the 2014 Plan. In addition, our non-employee directors and consultants and advisors who perform services for us and our subsidiaries may receive awards under the 2014 Plan, other than incentive stock options.
Share Authorization. As stated above, we have reserved shares of common stock for issuance under the 2014 Plan, which includes all shares of common stock that remain available for issuance under the 2009 Plan as of the completion of this offering. In connection with stock splits, dividends, recapitalizations and certain other events, our board will make proportionate adjustments that it deems appropriate in the aggregate number of shares of common stock that we may issue under the 2014 Plan and the terms of outstanding awards. If any shares of stock covered by an award granted under the 2014 Plan or the 2009 Plan are not purchased or are forfeited or expire, or if an award otherwise terminates without delivery of any shares of stock subject thereto, or is settled in cash in lieu of shares of stock, then such number of shares with respect to such award shall be available for making awards under the 2014 Plan. If shares of stock subject to an award are surrendered in connection with the purchase of shares of stock upon exercise of an option, deducted from payment of an award in connection with tax withholding obligations or purchased by the Company with proceeds from option exercises, then such shares shall not be available for making awards under the 2014 Plan. The number of shares reserved for issuance or transfer pursuant to awards under the 2014 Plan will be increased annually, on the first day of each fiscal year beginning in 2015 and ending in 2024, equal to the least of (A) shares, (B) percent ( %) of the shares of common stock outstanding (on an as-converted basis) on the last day of the immediately preceding fiscal year, and (C) such smaller number of shares of stock as determined by our board of directors.
During any time that the transition period under Section 162(m) of the Code has expired or does not apply, the maximum number of shares of common stock subject to options or stock appreciation rights that we can issue under the 2014 Plan to any person is in any single calendar year. The maximum number of shares of common stock that we can issue under the 2014 Plan to any person other than pursuant to an option or stock appreciation right is in any single calendar year. The maximum amount that any one person may earn as an annual incentive award or other cash award in any calendar year is $ and the maximum amount that any one person may earn as a performance award or other cash award in respect of a performance period is $ .
Options. The 2014 Plan authorizes our compensation committee to grant incentive stock options (under Section 422 of the Code) and options that do not qualify as incentive stock options (or non-qualified stock options). All of the shares of stock available for issuance under the 2014 Plan at the time of this offering shall be available for issuance pursuant to incentive stock options. The compensation committee will determine the exercise price of each option, provided that the price will be equal to at least the fair market value of the shares of common stock on the date on which the option is granted. If we were to grant incentive stock options to any 10% stockholder, the exercise price may not be less than 110% of the fair market value of our shares of common stock on the date of grant.
The term of an option cannot exceed 10 years from the date of grant. If we were to grant incentive stock options to any 10% stockholder, the term cannot exceed five years from the date of grant. The compensation committee determines at what time or times each option may be exercised and the period of time, if any, after retirement, death, disability or termination of employment during which options may be exercised. Options may be made exercisable in installments. The compensation committee may accelerate the exercisability of options. Except in connection with a corporate transaction, the exercise price of an option may not be amended or modified after the grant of the option, and an option may not be surrendered in consideration of or exchanged for a grant of a new option having an exercise price below that of the option which was surrendered or exchanged without stockholder approval.
The aggregate fair market value, determined at the time of grant, of our common stock with respect to incentive stock options that are exercisable for the first time by an optionee during any calendar year under all of our stock plans may not exceed $100,000. We will generally treat options or portions thereof that exceed such limit as non-qualified stock options.
114
Table of Contents
Stock Awards. The 2014 Plan also provides for the grant of stock awards (which includes restricted stock). A stock award is an award of shares of common stock that may be subject to restrictions on transferability and other restrictions as our compensation committee determines in its sole discretion on the date of grant. The restrictions, if any, may lapse over a specified period of time or through the satisfaction of conditions, in installments or otherwise, as our compensation committee may determine. A participant who receives a restricted stock award will have all of the rights of a stockholder as to those shares, including the right to vote and the right to receive dividends or distributions on the shares, except that the board of directors may require any dividends to be reinvested in shares, which may be subject to the same vesting conditions applicable to the restricted stock. During the period, if any, when stock awards are non-transferable or forfeitable, a participant is prohibited from selling, transferring, assigning, pledging or otherwise encumbering or disposing of his or her award shares.
Stock Appreciation Rights. The 2014 Plan authorizes our compensation committee to grant stock appreciation rights that provide the recipient with the right to receive, upon exercise of the stock appreciation right, cash, shares of common stock or a combination of the two. The amount that the recipient will receive upon exercise of the stock appreciation right generally will equal the excess of the fair market value of our common stock on the date of exercise over the shares’ fair market value on the date of grant. Stock appreciation rights will become exercisable in accordance with terms determined by our compensation committee. Stock appreciation rights may be granted in tandem with an option grant or independently from an option grant. The term of a stock appreciation right cannot exceed 10 years from the date of grant.
Stock Units. The 2014 Plan also authorizes our compensation committee to grant stock units. Stock units represent the participant’s right to receive a compensation amount, based on the value of the shares of common stock, if vesting criteria established by the compensation committee are met. If the vesting criteria are met, we will pay stock units in cash, shares of common stock or a combination thereof.
Bonuses. We may base cash performance bonuses payable under the 2014 Plan on the attainment of performance goals that the compensation committee establishes that relate to one or more performance criteria described in the plan. Cash performance bonuses, for which there is no minimum payout, must be based upon objectively determinable bonus formulas established in accordance with the 2014 Plan, as determined by the compensation committee.
Dividend Equivalents. Our compensation committee may grant dividend equivalents in connection with the grant of any equity-based award other than options and stock appreciation rights. Dividend equivalents may be paid currently or may be deemed to be reinvested in additional shares of stock, which may thereafter accrue additional equivalents, and may be payable in cash, shares of common stock or a combination of the two. Our compensation committee will determine the terms of any dividend equivalents.
Performance Awards. Section 162(m) of the Code limits publicly held companies to an annual deduction for U.S. federal income tax purposes of $1.0 million for compensation paid to each of their principal executive officer and their three highest compensated executive officers (other than the chief executive officer or the chief financial officer) determined at the end of each year, referred to as covered employees. However, performance-based compensation and compensation meeting certain initial public offering transition rule requirements is excluded from this limitation. The 2014 Plan is designed to permit the compensation committee to grant awards that qualify as performance-based for purposes of satisfying the conditions of Section 162(m) once the transition rule is no longer applicable, but it is not required under the 2014 Plan that awards qualify for this exception. To help assure that the compensation attributable to performance-based awards will so qualify, our compensation committee can structure such awards so that stock or cash will be issued or paid pursuant to such award only after the achievement of certain pre-established performance goals during a designated performance period.
We may select performance goals from one or more of the following: (1) net earnings or net income; (2) operating earnings; (3) pretax earnings; (4) earnings per share of stock; (5) stock price, including growth measures and total stockholder return; (6) earnings before interest and taxes; (7) earnings before interest, taxes,
115
Table of Contents
depreciation and/or amortization; (8) sales or revenue growth, whether in general, by type of product or service, or by type of customer; (9) gross or operating margins; (10) return measures, including return on assets, capital, investment, equity, sales or revenue; (11) cash flow, including operating cash flow, free cash flow, cash flow return on equity and cash flow return on investment; (12) productivity ratios; (13) expense targets; (14) market share; (15) financial ratios as provided in credit agreements of our company; (16) working capital targets; (17) completion of acquisitions of business or companies; (18) completion of divestitures and asset sales; (19) revenues under management; (20) funds from operations; (21) successful implementation of clinical trials, including components thereof; (22) submitting regulatory filings; (23) obtaining regulatory approvals; (24) entering into contractual arrangements; (25) meeting contractual requirements; (26) achieving contractual milestones; (27) entering into collaborations; (28) receipt of grant funding; (29) regulatory body approval for commercialization of a product; (30) implementation or completion of critical projects; (31) product development; (32) licensing; (33) laboratory testing capacity, including laboratory testing volume; (34) facility development; (35) government relations; (36) production volume levels; and (37) any combination of any of the foregoing business criteria.
We may base performance goals on a company-wide basis, with respect to one or more business units, divisions, affiliates, or business segments, and in either absolute terms or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. We may not adjust upward any awards that we intend to qualify as performance-based compensation. The plan administrator retains the discretion to adjust performance-based awards downward, either on a formula or discretionary basis, or any combination as the compensation committee determines. Performance goals may differ from participant to participant and from award to award.
Other Equity-Based Awards. Our compensation committee may grant other types of equity-based awards under the 2014 Plan. Other equity-based awards are payable in cash, shares of common stock or other equity, or a combination thereof, and may be restricted or unrestricted, as determined by our compensation committee. The terms and conditions that apply to other equity-based awards are determined by the compensation committee.
Change in Control. If we experience a change in control, our compensation committee may determine whether (i) the successor corporation may assume or substitute for each outstanding award, (ii) the vesting of such awards held by current service providers may be accelerated in full, and/or (iii) all outstanding awards are to be cancelled as of the effective date of the consummation of the transaction in exchange for the payment of a cash amount that would have been attained upon exercise or vesting of such awards. In the case of performance shares and performance units, our compensation committee shall determine what adjustments, accelerations or amendments, if any, shall be applied to such awards, either at the time or grant or prior to the change in control.
Amendment; Termination. Our board of directors may amend or terminate the 2014 Plan at any time; provided that no amendment may adversely impair the benefits of participants with outstanding awards. Our stockholders must approve any amendment if such approval is required under applicable law or rules of a stock exchange on which the common stock is then listed. Unless terminated sooner by our board of directors or extended with stockholder approval, the 2014 Plan will terminate on the tenth anniversary of the completion of this offering.
2009 Stock Incentive Plan
General. In 2009, our board of directors and our stockholders adopted our 2009 Stock Incentive Plan, which has been subsequently amended, most recently in 2013. Our board of directors has determined not to grant any additional awards under the 2009 Plan after the completion of this offering. However, the 2009 Plan will continue to govern the terms and conditions of the outstanding awards granted under the 2009 Plan.
Share Reserve. As of June 30, 2014, a total of 1,600,000 shares of our common stock had been authorized for issuance under the 2009 Plan. As of June 30, 2014, options to purchase a total of 1,335,946 shares of our common stock were issued and outstanding, a total of 123,721 shares of our common stock had been issued upon
116
Table of Contents
the exercise of options or pursuant to other awards granted under the 2009 Plan, of which 2,084 shares of our common stock were issued upon the early exercise of stock options and subject to repurchase and 140,333 shares remained available for future grant. We have agreed to issue options to purchase 12,000 shares of our common stock to a consultant. Such remaining share balance will become available for issuance under the 2014 Plan upon completion of this offering.
Types of Awards. Our 2009 Plan provides for the grant of incentive stock options and nonqualified stock options to our employees, directors and consultants. Our board of directors has the authority to determine the terms and conditions of the awards granted under the 2009 Plan.
Options granted under the 2009 Plan may be early exercised prior to vesting under the terms of our stock option agreement. Under this agreement and upon termination of services, unvested exercised shares are subject to repurchase by the Company at the lesser of the exercise price or then fair market value.
Our 2009 Plan does not allow for the transfer of awards other than by will or the laws of descent or distribution and only the recipient of an award may exercise such awards during his or her lifetime, unless our board of directors provides for additional transfer terms as permitted by Rule 701 of the Securities Act. Furthermore, awards may only be granted on the condition that stock purchased under such awards will be held solely for investment purposes, and not with a view to resell or distribute. Our board of directors may make provisions to release this condition upon the registration of the stock subject to the 2009 Plan with the SEC, or upon the happening of any other contingency warranting the release of the condition.
Corporate Transaction. Our 2009 Plan provides that in the event of our merger with another corporation, more than 50% of the total voting power of our capital stock is acquired, or the sale of all or substantially all of our assets, our board of directors may determine whether (i) the successor corporation may assume or substitute for each outstanding award, (ii) the vesting of such awards held by current service providers may be accelerated in full, and/or (iii) all outstanding awards are to be cancelled as of the effective date of the consummation of the transaction in exchange for the payment of a cash amount that would have been attained upon exercise of such awards.
Non-Employee Director Compensation
Cash and Equity Compensation
Our board of directors is contemplating the adoption of a formal non-employee director compensation policy following the completion of the offering, but has not decided on the material terms of such policy at this time. We currently do not pay cash retainers to our directors and do not intend to pay cash retainers in the near future, but such policy may include annual cash retainers and potentially additional annual cash retainers to committee chairs and potentially the Chairman of the Board.
The non-employee director compensation policy is expected to provide for grants of options to purchase shares of our common stock or other stock awards pursuant to the terms and conditions of our 2014 Plan, as described above.
117
Table of Contents
Director Compensation Table
The following table sets forth information concerning compensation accrued or paid to our non-employee directors for the year ended December 31, 2013, for their service on our board of directors. Hr. Hogg, our Chief Executive Officer, receives no additional compensation for his services as a director and is not included in the table below.
| Director Name |
Fees Earned or Paid in Cash ($) |
Option Awards ($) (1) |
All Other Compensation ($) |
Total ($) |
||||||||||||
| Douglas D. French |
— | 3,300 | — | 3,300 | ||||||||||||
| Ralph G. Dacey, Jr. |
— | 3,300 | — | 3,300 | ||||||||||||
| Fred C. Goad |
— | 3,300 | — | 3,300 | ||||||||||||
| Jeffrey M. McDonnell |
— | 3,300 | — | 3,300 | ||||||||||||
| Virender K. Sharma |
— | 3,300 | 68,000 | (2) | 71,300 | |||||||||||
| Raul E. Perez |
— | 3,300 | — | 3,300 | ||||||||||||
| (1) | Amounts reflect the grant date fair value of option awards granted in 2013 in accordance with ASC 718. For information regarding assumptions underlying the value of equity awards, see Note 1 to our financial statements and the discussion under “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Critical Accounting Policies — Stock-Based Compensation,” included elsewhere in this prospectus. These amounts do not necessarily correspond to the actual value that the named directors may recognize. |
| (2) | Amount represents the amount paid to Dr. Sharma as a Chief Medical Officer pursuant to a consulting agreement for his service in such capacity. |
The following table provides information regarding equity awards held by each director, other than Mr. Hogg, as of December 31, 2013:
| Name |
Options Outstanding (#) |
Restricted Stock Outstanding (#) |
||||||
| Douglas D. French |
15,000 | — | ||||||
| Ralph G. Dacey, Jr. |
85,000 | — | ||||||
| Fred C. Goad |
60,000 | — | ||||||
| Jeffrey M. McDonnell |
60,000 | — | ||||||
| Virender K. Sharma |
30,000 | (1) | — | |||||
| Raul E. Perez |
30,000 | 400,000 | (2) | |||||
| (1) | The table does not reflect shares of common stock that Dr. Sharma acquired upon the exercise of stock options prior to vesting thereof. 6,250 of such shares remained unvested and subject to repurchase by us as of December 31, 2013. |
| (2) | Restricted stock awards are subject to vesting upon achievement of certain performance criteria, which relate to the internal rate of return that may be achieved by the holders of Series B Preferred Stock. The performance criteria may be amended at the discretion of the compensation committee. |
Limitation of Liability and Indemnification Agreements
Our amended and restated certificate of incorporation and amended and restated bylaws, each to become effective immediately prior to the completion of this offering, provide that we will limit the liability of our directors, and may indemnify our directors and officers, to the maximum extent permitted by the Delaware General Corporation Law, or DGCL. The DGCL provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
| • | breach of their duty of loyalty to the corporation or its stockholders; |
118
Table of Contents
| • | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payment of dividends or redemption of shares; or |
| • | transaction from which the directors derived an improper personal benefit. |
These limitations of liability do not apply to liabilities arising under federal securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission.
We intend to enter into separate indemnification agreements with our directors and officers in addition to the indemnification provided for in our amended and restated bylaws. These indemnification agreements are expected to provide, among other things, that we will indemnify our directors and officers for certain expenses, including damages, judgments, fines, penalties, settlements and costs and attorneys’ fees and disbursements, incurred by a director or officer in any claim, action or proceeding arising in his or her capacity as a director or officer of our company or in connection with service at our request for another corporation or entity. The indemnification agreements also provide for procedures that will apply in the event that a director or officer makes a claim for indemnification.
We also maintain a directors’ and officers’ insurance policy pursuant to which our directors and officers are insured against liability for actions taken in their capacities as directors and officers. We believe that these indemnification provisions and insurance are useful to attract and retain qualified directors and officers.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
119
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
We describe below transactions and series of similar transactions, since January 1, 2011, to which we were a party or will be a party, in which:
| • | the amounts involved exceeded or will exceed $120,000; and |
| • | any of our directors, executive officers, holders of more than 5% of our capital stock or any member of their immediate family had or will have a direct or indirect material interest, other than compensation arrangements with directors and executive officers, which are described where required under “Executive and Director Compensation — Narrative Disclosure to Summary Compensation Table,” “Executive and Director Compensation — Agreements with Our Named Executive Officers” and “Executive and Director Compensation — Non-Employee Director Compensation.” |
We refer to these transactions as related person transactions.
Preferred Stock Financings
Issuance of Series B Convertible Preferred Stock
Between July 13, 2010 and May 24, 2011, we issued and sold 3,999,951 shares of our Series B convertible preferred stock at a price per share of $1.50, for gross proceeds of $6.0 million. The table below sets forth the number of shares of Series B convertible preferred stock purchased by officers, directors or beneficial owners of 5% or more of our capital stock and their affiliated entities in such financing. Each share of Series B convertible preferred stock in the table below will be converted into one share of our common stock upon the completion of this offering.
| Participants |
Series B Convertible Preferred Stock (#) |
Aggregate Purchase Price of Series B Convertible Preferred Stock ($) |
||||||
| Virender K. Sharma |
34,363 | 51,545 | ||||||
| Santé Health Ventures I, LP (1) |
2,666,667 | 4,000,001 | ||||||
| Prolog Capital III, LP |
333,333 | 500,000 | ||||||
| 1998 Co-Investing, LLC (2) |
91,637 | 137,456 | ||||||
| (1) | Douglas D. French, our Chairman of the Board, is a founder and managing director of Santé Health Ventures I, LP. |
| (2) | Jeffrey McDonnell, one of our directors, is the manager of 1998 Co-Investing, LLC. |
Issuance of Series B — 1 Convertible Preferred Stock
Between November 22, 2011 and May 18, 2012, we issued and sold 2,466,218 shares of our Series B-1 convertible preferred stock at a price per share of $2.25, for gross proceeds of $5.5 million. The table below sets forth the number of shares of Series B-1 convertible preferred stock purchased by officers, directors or beneficial owners of 5% or more of our capital stock and their affiliated entities in such financing. Each share of Series B-1 convertible preferred stock in the table below will be converted into one share of our common stock upon the completion of this offering.
| Participants |
Series B–1 Convertible Preferred Stock (#) |
Aggregate Purchase Price of Series B–1 Convertible Preferred Stock ($) |
||||||
| Virender K. Sharma |
22,221 | 49,997 | ||||||
| Fred C. Goad |
111,111 | 250,000 | ||||||
| Santé Health Ventures I, LP (1) |
1,555,555 | 3,499,999 | ||||||
| Prolog Capital III, L.P. |
324,000 | 729,000 | ||||||
| 1998 Co-Investing, LLC (2) |
144,444 | 324,999 | ||||||
120
Table of Contents
| (1) | Douglas D. French, our Chairman of the Board, is a founder and managing director of Santé Health Ventures I, LP. |
| (2) | Jeffrey McDonnell, one of our directors, is the manager of 1998 Co-Investing, LLC. |
Issuance of Series C Convertible Preferred Stock
Between December 5, 2012 and May 15, 2013 we issued and sold 3,754,713 shares of our Series C convertible preferred stock at a price per share of $3.75, for gross proceeds of $14.1 million. The table below sets forth the number of shares of Series C convertible preferred stock purchased by officers, directors or beneficial owners of 5% or more of our capital stock and their affiliated entities in such financing. Each share of Series C convertible preferred stock in the table below will be converted into one share of our common stock upon the completion of this offering.
| Participants |
Series C Convertible Preferred Stock (#) |
Aggregate Purchase Price of Series C Convertible Preferred Stock ($) |
||||||
| Ralph G. Dacey |
11,000 | 41,250 | ||||||
| Fred C. Goad |
319,999 | 1,199,996 | ||||||
| Santé Health Ventures I, LP (1) |
666,667 | 2,500,001 | ||||||
| Prolog Capital III, L.P. |
133,333 | 499,999 | ||||||
| 1998 Co-Investing LLC (2) |
160,000 | 600,000 | ||||||
| (1) | Douglas D. French, our Chairman of the Board, is a founder and managing director of Santé Health Ventures I, LP. |
| (2) | Jeffrey McDonnell, one of our directors, is the manager of 1998 Co-Investing, LLC. |
Convertible Note Financing
On June 25, 2014, we entered into a Note and Warrant Purchase Agreement, or the 2014 Note Agreement, with various accredited investors. A number of additional accredited investors joined in that agreement on August 28, 2014. We refer to the accredited investors party to the 2014 Note Agreement collectively as the 2014 Note Investors.
Pursuant to the 2014 Note Agreement, we issued an aggregate of $7.5 million in secured convertible promissory notes, or the 2014 Notes, to the 2014 Note Investors. Interest on all amounts outstanding accrues at a rate of 10% per annum, and the notes mature on June 25, 2016, unless earlier converted. Under the 2014 Note Agreement, we agreed to issue warrants to purchase up to 399,998 shares of our common stock at an exercise price of $0.50 per share, or the 2014 Warrants, to the 2014 Note Investors.
Amounts outstanding under the 2014 Notes convert automatically on the first to occur of the following:
| • | In the event we consummate an underwritten public offering pursuant to which we receive at least $10,000,000 from the sale of our equity securities in such offering, or a Qualified Initial Public Offering, then all outstanding principal and accrued but unpaid interest under the 2014 Notes will automatically convert into fully paid and nonassessable shares of the equity securities issued in the Qualified Initial Public Offering at a price per share equal to 80% of the price to the public (before underwriting discounts and commissions and other offering expenses) in the Qualified Initial Public Offering; and |
| • | In the event we complete a transaction or series of transactions pursuant to which we issue and sell shares of a new series of our preferred stock for aggregate gross proceeds of at least $10,000,000 with the principal purpose of raising capital, or a Qualified Financing, then all outstanding principal and accrued but unpaid interest under the 2014 Notes will automatically convert into fully paid and nonassessable shares of the class of our stock issued in such Qualified Financing at a price per share equal to 80% of the lowest price paid by investors in the Qualified Financing. |
121
Table of Contents
This offering will constitute a Qualified Initial Public Offering and the convertible notes will convert into shares of our common stock upon completion of this offering. The 2014 Warrants are expected to remain outstanding upon completion of this offering. The 2014 Warrants will expire on June 25, 2019. The 2014 Notes are secured by all of our current or after-acquired assets and may be subordinated to any of our senior indebtedness and to any liens securing such senior indebtedness. The liens will be terminated upon completion of this offering, as the loans underlying the 2014 Notes will be satisfied.
The table below sets forth the aggregate amount of securities purchased by officers, directors or beneficial owners of 5% or more of our capital stock and their affiliated entities in such financing.
| Participants |
Aggregate Principal Amount of 2014 Notes ($) |
Shares of Common Stock Underlying 2014 Warrants |
||||||
| Ralph G. Dacey |
32,858 | 1,752 | ||||||
| Fred C. Goad |
300,000 | 15,999 | ||||||
| Virender K. Sharma |
35,220 | 1,878 | ||||||
| Santé Health Ventures I, LP (1) |
1,000,000 | 53,333 | ||||||
| 1998 Co-Investing, LLC (2) |
500,000 | 26,667 | ||||||
| (1) | Douglas D. French, our Chairman of the Board, is a founder and managing director of Santé Health Ventures I, LP. |
| (2) | Jeffrey McDonnell, one of our directors, is the manager of 1998 Co-Investing, LLC. |
Investor Rights Agreement
We are party to the Third Amended and Restated Investors’ Rights Agreement, dated June 25, 2014, or the Investor Rights Agreement, with certain holders of our convertible preferred stock, 2014 Notes and 2014 Warrants. The Investor Rights Agreement provides that the holders of the majority of the common stock (i) issuable upon conversion of our outstanding Series B, Series B-1 and Series C Convertible Preferred Stock, (ii) issuable upon the exercise of the 2014 Warrants, and (iii) actually issued upon conversion of the 2014 Notes have the right to demand that we file a registration statement or request that their shares of common stock be covered by a registration statement that we are otherwise filing. In addition to the registration rights, the Investor Rights Agreement provides for certain information rights and rights of first refusal. The provisions of the investor rights agreement will terminate upon the earlier of such date (i) on or after the closing of our first registered public offering of Common Stock, on which all shares of registrable securities held or entitled to be held upon conversion by a holder may immediately be sold under Rule 144 during any ninety (90) day period, and (ii) six years after the closing of this offering, other than certain rights related to information and inspection rights and certain covenants which will terminate upon the completion of this offering. The registration rights are described in more detail under “Description of Capital Stock — Registration Rights.”
Other Transactions
We have entered into various employment-related agreements with our executive officers that, among other things, provide for compensatory benefits. For a description of these agreements, see the sections entitled “Executive and Director Compensation — Narrative Disclosure to Summary Compensation Table” and “Executive and Director Compensation — Agreements with Our Named Executive Officers.”
We intend to enter into indemnification agreements with each of our current directors and officers. See “Executive and Director Compensation — Limitation of Liability and Indemnification Agreements.”
Policies and Procedures for Related Person Transactions
All related person transactions will be reviewed and approved by our nominating and corporate governance committee (or any other committee of the board consisting of independent directors) or our board of directors.
122
Table of Contents
This review covers any material transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant, and a related person had or will have a direct or indirect material interest, including, purchases of goods or services by or from the related party or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. A “related person” is any person who is or was one of our executive officers, directors or director nominees or is a beneficial owner of more than 5% of our common stock, or their immediate family members or any entity owned or controlled by any of the foregoing persons.
All of the transactions described above were entered into prior to the adoption of this policy and were approved by our board of directors.
123
Table of Contents
The following table sets forth certain information known to us regarding the beneficial ownership of our common stock as of June 30, 2014, as adjusted to reflect the conversion of all outstanding shares of our convertible preferred stock, by:
| • | each of our named executive officers; |
| • | each of our directors; |
| • | all of our executive officers and directors as a group; and |
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of any class of our voting securities. |
We have based our calculation of beneficial ownership prior to this offering on 13,923,696 shares of common stock outstanding on June 30, 2014, assuming the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 11,719,975 shares of our common stock immediately prior to the closing of this offering. We have based our calculation of beneficial ownership after this offering on shares of our common stock outstanding immediately following the completion of this offering, which gives effect to the issuance of shares of common stock in this offering and the conversion of (i) all outstanding shares of our convertible preferred stock into an aggregate of 11,719,975 shares of our common stock, and (ii) the principal and accrued interest on the approximately $6.4 million aggregate principal amount of convertible promissory notes issued in June 2014 and the approximately $1.1 million aggregate principal amount of convertible promissory notes issued in August 2014 into an aggregate of shares of common stock, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering. Ownership information provided below assumes no exercise of the underwriters’ over-allotment option.
124
Table of Contents
Information with respect to beneficial ownership has been furnished to us by each director, executive officer or stockholder who beneficially owns more than 5% of any class of our voting securities, as the case may be. Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he or she possesses sole or shared voting or investment power of that security, including the right to acquire beneficial ownership of that security within 60 days, including through outstanding options and warrants that are currently exercisable within 60 days of June 30, 2014. Options to purchase shares of our common stock that are exercisable within 60 days of June 30, 2014 are deemed to be beneficially owned by the persons possessing those rights for the purpose of computing percentage ownership of that person, but are not treated as outstanding for the purpose of computing any other person’s ownership percentage. Except as indicated in the footnotes below, each of the beneficial owners named in the table below has, and upon completion of this offering will have, to our knowledge, sole voting and investment power with respect to all shares of common stock listed as beneficially owned by him or her, except for shares owned jointly with that person’s spouse. Unless otherwise indicated, the address for each of the stockholders in the table below is EndoStim, Inc., 4041 Forest Park Avenue, Suite 220, St. Louis, Missouri 63108.
| Name and Address of Beneficial Owner |
Shares of Common Stock Beneficially Owned |
Percentage of Shares Beneficially Owned |
||||||||||||||
| Before Offering | After Offering (14) | Before Offering | After Offering | |||||||||||||
| Named Executive Officers and Directors: |
||||||||||||||||
| Bevil J. Hogg (1) |
664,122 | 664,122 | 4.7 | % | % | |||||||||||
| Douglas D. French (2) |
4,957,222 | 35.4 | % | % | ||||||||||||
| Ralph Dacey (3) |
147,752 | 1.1 | % | % | ||||||||||||
| Fred Goad (4) |
532,109 | 3.8 | % | % | ||||||||||||
| Jeffrey McDonnell (5) |
884,237 | 6.2 | % | % | ||||||||||||
| Virender K. Sharma (6) |
736,584 | 736,584 | 5.3 | % | % | |||||||||||
| Raul Perez (7) |
730,744 | 730,744 | 5.2 | % | % | |||||||||||
| Paul Goode (8) |
90,694 | 90,694 | * | % | ||||||||||||
| Shai Policker (9) |
126,402 | * | % | |||||||||||||
| All executive officers and directors as a group (10 persons) (10) |
8,912,678 | 59.7 | % | % | ||||||||||||
| 5% Stockholders: |
||||||||||||||||
| Santé Health Ventures I, LP (11) |
4,942,222 | 35.4 | % | % | ||||||||||||
| Prolog Capital III, L.P. (12) |
790,666 | 790,666 | 5.7 | % | % | |||||||||||
| 1998 Co-Investing, LLC (13) |
824,237 | 5.8 | % | % | ||||||||||||
| * | Represents beneficial ownership of less than one percent. |
| (1) | The number of shares beneficially owned before and after this offering consists of (a) 600,372 shares of common stock, (b) 50,000 shares of common stock issuable upon the exercise of a warrant, and (c) 13,750 shares of common stock issuable upon the exercise of stock options exercisable within 60 days after June 30, 2014. The shares of common stock include 400,000 shares of restricted stock subject to vesting upon achievement of certain performance criteria, which relate to the internal rate of return that may be achieved by the holders of Series B Preferred Stock. The performance criteria may be amended at the discretion of the compensation committee. |
| (2) | The number of shares beneficially owned before and after this offering consists of (a) the shares described in note (11) below, and (b) 15,000 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of June 30, 2014. |
| (3) | The number of shares beneficially owned before this offering consists of (a) 61,000 shares of common stock held by a trust controlled by Mr. Dacey, (b) 85,000 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of June 30, 2014, and (c) 1,752 shares of common stock issuable upon the exercise of 2014 Warrants. The number of shares beneficially owned after this offering consists of the number of shares beneficially owned before this offering plus the number of shares issuable upon the automatic conversion of the principal and accrued interest on $32,858 principal amount of convertible promissory notes issued in June 2014 into an aggregate of shares of common stock. |
125
Table of Contents
| (4) | The number of shares beneficially owned before this offering consists of (a) 297,777 shares of common stock, (b) 53,333 shares of common stock held by the Franye Goad Johnson Dynasty Trust, (c) 40,000 shares of common stock held by the Franye Goad Johnson 1999 Generation Skipping Trust, (d) 40,000 shares of common stock held by the Franye Goad Johnson 1998 Children’s Trust, (e) 85,000 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of June 30, 2014, (f) 15,999 shares of common stock issuable upon the exercise of 2014 Warrants. The number of shares beneficially owned after this offering consists of the number of shares beneficially owned before this offering plus the number of shares issuable upon the automatic conversion of the principal and accrued interest on the $300,000 principal amount of convertible promissory notes issued in June 2014 into an aggregate of shares of common stock. |
| (5) | The number of shares beneficially owned before and after this offering consists of (a) the shares described in note (13) below, and (b) 60,000 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of June 30, 2014. |
| (6) | The number of shares beneficially owned before and after this offering consists of (a) 100,000 shares of common stock, (b) 306,584 shares of common stock held by Sharma Family Revocable Trust, (c) 300,000 shares of common stock held by the Sharma Family Irrevocable Trust, and (d) 30,000 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of June 30, 2014. |
| (7) | The number of shares beneficially owned before and after this offering consists of (a) 600,744 shares of common stock, (b) 100,000 shares of common stock issuable upon exercise of a warrant held by the Mary Ellen Perez Revocable Trust, and (c) 30,000 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of June 30, 2014. The shares of common stock include 400,000 shares of restricted stock subject to vesting upon achievement of certain performance criteria, which relate to the internal rate of return that may be achieved by the holders of Series B Preferred Stock. The performance criteria may be amended at the discretion of the compensation committee. |
| (8) | The number of shares beneficially owned before and after this offering consists of shares of common stock issuable upon the exercise of stock options exercisable within 60 days of June 30, 2014. |
| (9) | The number of shares beneficially owned before this offering consists of (a) 125,069 shares of common stock issuable upon the exercise of stock options exercisable within 60 days of June 30, 2014 and (b) 1,333 shares of common stock issuable upon the exercise of 2014 Warrants. The number of shares beneficially owned after this offering consists of the number of shares beneficially owned before this offering plus the number of shares issuable upon the automatic conversion of the principal and accrued interest on the $25,000 principal amount of convertible promissory notes issued in June 2014 into an aggregate of shares of common stock. |
| (10) | The number of shares beneficially owned before this offering consists of (a) 350,000 shares of common stock issuable upon the exercise of warrants, (b) 552,325 shares of common stock issuable upon the exercise of options each exercisable within 60 days after June 30, 2014 and (c) 99,084 shares of common stock issuable upon the exercise of 2014 Warrants. The number of shares beneficially owned after this offering consists of the number of shares beneficially owned before this offering plus the number of shares issuable upon the automatic conversion of the principal and accrued interest on the $1,857,858 principal amount of convertible promissory notes issued in June 2014 into an aggregate of shares of common stock. |
| (11) | The number of shares beneficially owned before this offering includes 53,333 shares of common stock issuable upon the exercise of 2014 Warrants. The number of shares beneficially owned after this offering consists of the number of shares beneficially owned before this offering plus the number of shares issuable upon the automatic conversion of the principal and accrued interest on the $1,000,000 principal amount of convertible promissory notes issued in June 2014 into an aggregate of shares of common stock. Douglas French, our Chairman of the Board, shares voting and investment power with respect to the shares held by, and is a founder and managing director of, Santé Health Ventures I, LP. SHV Management Services, LLC, the general partner of SHV Management Services, LP, which is in turn the general partner of Santé Health Ventures I, LP, has voting and investment power over these securities. Douglas D. French, Kevin M. Lalande and Joe H. Cunningham, M.D. are the sole managers of SHV Management Services, LLC. Messrs. French and Lalande and Dr. Cunningham each disclaim beneficial ownership of such securities except to the extent of any pecuniary interest therein. |
126
Table of Contents
| (12) | Prolog Ventures III, LLC, the general partner of Prolog Capital III, L.P., has voting and investment power over these securities. Greg R. Johnson, Ilya B. Nykin and Brian L. Clevinger are the sole managing directors of Prolog Ventures III, LLC. Messrs. Johnson and Clevinger and Ms. Nykin each disclaim beneficial ownership of such securities except to the extent of any pecuniary interest therein. |
| (13) | The number of shares beneficially owned before this offering consists of (a) 597,570 shares of common stock, (b) 200,000 shares of common stock issuable upon the exercise of a warrant exercisable within 60 days of June 30, 2014, and (c) 26,667 shares of common stock issuable upon the exercise of 2014 Warrants. The number of shares beneficially owned after this offering consists of the number of shares beneficially owned before this offering plus the number of shares issuable upon the automatic conversion of the principal and accrued interest on the $500,000 principal amount of convertible promissory notes issued in June 2014 into an aggregate of shares of common stock. Jeffrey McDonnell, one of our directors, is the sole manager of 1998 Co-Investing, LLC and has sole voting and investment power with respect to shares held by 1998 Co-Investing, LLC. |
| (14) | The number of shares beneficially owned after this offering includes the number of shares issuable upon the automatic conversion of the principal and accrued interest on the convertible promissory notes issued in June 2014 into shares of common stock, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering. |
127
Table of Contents
Upon the completion of this offering, our amended and restated certificate of incorporation will authorize us to issue up to shares of common stock, par value $0.001 per share, and shares of convertible preferred stock, par value $0.001 per share. As of June 30, 2014, there were outstanding:
| • | 2,203,721 shares of our common stock held by 146 stockholders; |
| • | 11,719,975 shares of our common stock issuable upon the automatic conversion of all outstanding shares of our convertible preferred stock, the conversion of which will occur immediately prior to the completion of this offering; |
| • | shares of our common stock issuable upon the automatic conversion of the principal and accrued interest on the approximately $6.4 million aggregate principal amount of convertible promissory notes issued in June 2014 and the approximately $1.1 million aggregate principal amount of convertible promissory notes issued in August 2014, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering; |
| • | 1,335,946 shares of our common stock issuable upon the exercise of outstanding stock options as of June 30, 2014, at a weighted average price of $0.25 per share; and |
| • | 889,453 shares of our common stock issuable upon the exercise of outstanding warrants as of June 30, 2014, of which warrants to purchase 550,000 shares have an exercise price of $0.01 per share, and warrants to purchase 339,453 shares each with an exercise price of $0.50 per share. |
The following description of our capital stock is not complete and is subject to and qualified in its entirety by our amended and restated certificate of incorporation and amended and restated bylaws and by the provisions of applicable Delaware law. Copies of these documents are filed with the SEC as exhibits to our registration statement, of which this prospectus forms a part. The descriptions of our common stock, convertible preferred stock and warrants reflect changes to our capital structure that will occur immediately in connection with the completion of this offering.
Common Stock
Voting Rights
Each holder of common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders.
Dividends
Subject to preferences that may apply to any outstanding convertible preferred stock, holders of our common stock are entitled to receive ratably any dividends that our board of directors may declare out of funds legally available for that purpose.
Liquidation
In the event of our liquidation, dissolution or winding up, the holders of our common stock are entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preference of any outstanding convertible preferred stock.
Rights and Preferences
Holders of our common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of our convertible preferred stock that we may designate in the future.
128
Table of Contents
Fully Paid and Nonassessable
All outstanding shares of our common stock are fully paid and non-assessable, and the shares of common stock to be issued upon completion of this offering will be fully paid and non-assessable.
Preferred Stock
Upon the completion of this offering, all outstanding shares of our convertible preferred stock will convert into shares of common stock. Upon completion of this offering, our board of directors will have the authority, without further action by our stockholders, to issue up to shares of convertible preferred stock in one or more series and to fix the number, rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, and sinking fund terms, and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of common stock. The issuance of our convertible preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of convertible preferred stock could have the effect of delaying, deferring or preventing a change of control or other corporate action. We have no current plan to issue any shares of convertible preferred stock.
Convertible Notes
In June 2014 and in August 2014, in connection with the 2014 Note Agreement, we issued the convertible notes for an aggregate principal amount of $7.5 million. In connection with the completion of this offering, all amounts outstanding under these notes will automatically convert into shares of our common stock, assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering.
Warrants
In connection with a bridge loan financing in July 2009, we issued warrants to accredited investors to purchase 550,000 shares of our common stock at an exercise price of $0.01 per share. The warrants will expire starting on July 13, 2019.
In connection with the 2014 Note Agreement, on June 25, 2014 and August 28, 2014, we issued the 2014 Warrants exercisable for an aggregate of 339,453 and 60,545 shares of our common stock, respectively, at an exercise price of $0.50 per share. The 2014 Warrants are expected to remain outstanding upon completion of this offering. The 2014 Warrants will expire on June 25, 2019.
Registration Rights
Holders of a majority of our common stock (i) issuable upon conversion of our outstanding Series B, Series B-1 and Series C Convertible Preferred Stock, (ii) issuable upon the exercise of our 2014 Warrants and (iii) actually issued upon conversion of the 2014 Notes have the right to demand that we file a registration statement or request that we cover their shares by a registration statement that we otherwise file, as described below. As of June 30, 2014, 10,560,335 shares of our common stock were issuable upon conversion of such preferred stock and upon exercise of the 2014 Warrants and no shares were issued upon conversion of the June 2014 Notes. Assuming that following the completion of this offering an additional shares of common stock will be issued upon the conversion of the principal and accrued interest on the approximately $7.5 million aggregate principal amount of 2014 Notes (assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and a conversion date of , 2014, which is the expected closing date of this offering), the holders of the majority of shares of our common stock will have such registration rights.
129
Table of Contents
Demand Registration Rights
At any time 180 days after the completion of this offering, the holders of a majority of the shares having demand registration rights may request that we register all or a portion of their shares of common stock for sale under the Securities Act. We will effect the registration as requested, unless, in the good faith judgment of our board of directors, such registration would be materially detrimental to the company and its stockholders and should be delayed. In addition, when we are eligible for the use of Form S-3, or any successor form, holders of at least twenty-five percent of the shares having demand registration rights may make requests that we register all or a portion of their common stock for sale under the Securities Act on Form S-3, or any successor form.
Incidental Registration Rights
In addition, if at any time after this offering we register any shares of our common stock, the holders of all shares having piggyback registration rights are entitled to notice of the registration and to include all or a portion of their shares of common stock in the registration.
Other Provisions
In the event that any registration in which the holders of registrable shares participate is an underwritten public offering, the number of registrable shares to be included may, in specified circumstances, be limited due to market conditions.
We will pay all registration expenses, other than underwriting discounts and selling commissions, related to any demand, piggyback and Form S-3 registration. The registration rights agreement contains customary cross-indemnification provisions. The demand, piggyback and Form S-3 registration rights described above will expire, with respect to any particular stockholder, three years after the completion of this offering.
Anti-Takeover Provisions
Our Certificate of Incorporation and Bylaws
Our amended and restated certificate of incorporation and amended and restated bylaws that will become effective upon completion of this offering will include a number of provisions that may deter or impede hostile takeovers or changes of control or management. These provisions include:
| • | Issuance of undesignated convertible preferred stock. Our board of directors has the authority, without further action by the stockholders, to issue up to shares of undesignated convertible preferred stock with rights and preferences, including voting rights, designated from time to time by the board of directors. The existence of authorized but unissued shares of convertible preferred stock enables our board of directors to make it more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. |
| • | Classified board. Our amended and restated certificate of incorporation will provide for a classified board of directors consisting of three classes of directors, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. This provision may have the effect of delaying a change in control of the board of directors. |
| • | Board of directors vacancies. Our amended and restated certificate of incorporation and amended and restated bylaws will authorize only our board of directors to fill vacant directorships. In addition, the number of directors constituting our board of directors may be set only by resolution adopted by a majority vote of our entire board of directors. These provisions prevent a stockholder from increasing the size of our board of directors and gaining control of our board of directors by filling the resulting vacancies with its own nominees. |
130
Table of Contents
| • | Stockholder action; special meetings of stockholders. Our amended and restated certificate of incorporation will provide that our stockholders may not take action by written consent, but may only take action at annual or special meetings of our stockholders. Stockholders will not be permitted to cumulate their votes for the election of directors. Our amended and restated certificate of incorporation will further provide that special meetings of our stockholders may be called only by the chairman of our board of directors or by a majority of our board of directors. |
| • | Advance notice requirements for stockholder proposals and director nominations. Our amended and restated bylaws will provide advance notice procedures for stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for election as directors at our annual meeting of stockholders. Our bylaws also specify certain requirements as to the form and content of a stockholder’s notice. These provisions may make it more difficult for our stockholders to bring matters before our annual meeting of stockholders or to nominate directors at our annual meeting of stockholders. |
| • | Supermajority voting. Our amended and restated certificate of incorporation will provide that our directors may be removed for cause only with the approval of holders of % of the shares of our common stock. Additionally, certain provisions of our certificate of incorporation will only be able to be amended only with the affirmative vote of holders of at least % of the common stock. These supermajority voting requirements may inhibit the ability of a potential acquirer to effect removal of a director or effect amendments to our organizational documents to facilitate an unsolicited takeover attempt. |
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of us. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. However, these provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they may also reduce fluctuations in the market price of our shares that could result from actual or rumored takeover attempts.
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the DGCL, which prohibits a Delaware corporation from engaging in a business combination with any interested stockholder for a period of three years following the date the person became an interested stockholder, with the following exceptions:
| • | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested holder; |
| • | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (a) by persons who are directors and also officers and (b) pursuant to employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines business combination to include the following:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
131
Table of Contents
| • | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| • | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| • | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the entity’s or person’s affiliates and associates, beneficially owns, or is an affiliate of the corporation and within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Choice of Forum
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a breach of fiduciary duty owed by any director, officer or employee to us or our stockholders, any action asserting a claim against us arising pursuant to the DGCL or any action asserting a claim against us that is governed by the internal affairs doctrine. However, several lawsuits involving other companies have been brought challenging the validity of choice of forum provisions in certificates of incorporation, and it is possible that a court could rule that this provision is inapplicable or unenforceable.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is . The transfer agent and registrar’s address is .
132
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, no public market for our common stock existed, and a liquid trading market for our common stock may not develop or be sustained after this offering. Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options and warrants, or the anticipation of such sales, could adversely affect prevailing market prices of our common stock from time to time and could impair our future ability to raise equity capital in the future. Furthermore, because only a limited number of shares of our common stock will be available for sale shortly after this offering due to certain contractual and legal restrictions on resale described below, sales of substantial amounts of our common stock in the public market after such restrictions lapse, or the anticipation of such sales, could adversely affect the prevailing market price of our common stock and our ability to raise equity capital in the future.
Based on the number of shares of common stock outstanding as of June 30, 2014, upon completion of this offering, shares of our common stock will be outstanding. The number of shares outstanding upon completion of this offering assumes no exercise of outstanding options or warrants.
All of the shares sold in this offering will be freely tradable unless purchased by our affiliates. The remaining shares of common stock outstanding after this offering will be restricted as a result of securities laws or lock-up agreements as described below. Following the expiration of the lock-up period, all shares will be eligible for resale, subject to compliance with Rule 144 or Rule 701 of the Securities Act of 1933, as amended, or the Securities Act, to the extent these shares have been released from any repurchase option that we may hold.
We may issue shares of common stock from time to time as consideration for future acquisitions, investments or other corporate purposes. In the event that any such acquisition, investment or other transaction is significant, the number of shares of common stock that we may issue may in turn be significant. We may also grant registration rights covering those shares of common stock issued in connection with any such acquisition and investment.
In addition, shares of common stock that are either subject to outstanding options or warrants or reserved for future issuance under our equity incentive plans will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements and Rule 144 and Rule 701 of the Securities Act.
Rule 144
In general, under Rule 144 of the Securities Act, as in effect on the date of this prospectus, beginning 90 days after the date of this prospectus, any person who is not our affiliate at any time during the preceding three months, and who has beneficially owned their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares of our common stock provided current public information about us is available, and, after owning such shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares of our common stock without restriction.
Beginning 90 days after the date of this prospectus, a person who is our affiliate or who was our affiliate at any time during the preceding three months, and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell within any three-month period a number of shares that does not exceed the greater of:
| • | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares, or shares if the underwriters exercise their over-allotment option in full, immediately following this offering, based on the number of shares of our common stock outstanding upon completion of this offering; or |
133
Table of Contents
| • | the average weekly trading volume of our common stock on NASDAQ during the four calendar weeks preceding the filing of a Notice of Proposed Sale of Securities pursuant to Rule 144 with respect to the sale. |
Sales under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Upon expiration of the 180-day lock-up period described below, shares of our common stock will be eligible for sale under Rule 144. We cannot estimate the number of shares of our common stock that our existing stockholders will elect to sell under Rule 144.
Rule 701
In general, under Rule 701 of the Securities Act, any of an issuer’s employees, directors, officers, consultants or advisors who purchases shares from the issuer in connection with a compensatory stock or option plan or other written agreement before the effective date of a registration statement under the Securities Act, is entitled to sell such shares 90 days after such effective date in reliance on Rule 144. An affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirement, and non-affiliates of the issuer can resell shares in reliance on Rule 144 without having to comply with the current public information and holding period requirements.
Lock-up Agreements
We, along with our directors and executive officers and substantially all of our other stockholders have agreed with the underwriters that, for a period of 180 days following the date of this prospectus, we or they will not offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or otherwise dispose of any shares of our common stock (including any shares issued in this offering or other issuer-directed shares), or any options or warrants to purchase any shares of our common stock, or any securities convertible into, exchangeable for or that represent the right to receive shares of our common stock, whether now owned or later acquired, owned directly or with respect to which we or they have beneficial ownership within the rules and regulations of the SEC, subject to specified exceptions. The underwriters may, in their sole discretion, at any time without prior notice, release all or any portion of the shares from the restrictions in any such agreement.
Equity Incentive Plans
We intend to file one or more registration statements on Form S-8 under the Securities Act to register all shares of common stock subject to outstanding stock options and common stock issuable under our equity incentive plans. We expect to file the registration statement covering such shares shortly after the date of this prospectus, permitting the resale of such shares by non-affiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance with the resale provisions of Rule 144. For more information on our equity incentive plans, see “Executive and Director Compensation — Equity Compensation Plans and Other Benefit Plans.”
Registration Rights
Holders of a majority of our common stock (i) issuable upon conversion of our outstanding Series B, Series B-1 and Series C Convertible Preferred Stock, (ii) issuable upon the exercise of our 2014 Warrants and (iii) actually issued upon conversion of the 2014 Notes have the right to demand that we file a registration statement or request that we cover their shares by a registration statement that we otherwise file. For more information, see “Description of Capital Stock — Registration Rights.” Except for shares purchased by affiliates, registration of their shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration statement, subject to the expiration of the lock-up period and to the extent these shares have been released from any repurchase option that we may hold.
134
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following summary describes the material U.S. federal income and estate tax consequences of the acquisition, ownership and disposition of our common stock acquired in this offering by non-U.S. holders (as defined below). This discussion does not address all aspects of U.S. federal income and estate taxes and does not deal with foreign, state and local consequences that may be relevant to non-U.S. holders in light of their particular circumstances, nor does it address U.S. federal tax consequences other than income and estate taxes. Special rules different from those described below may apply to certain non-U.S. holders.
This is a summary of certain material U.S. federal income tax and estate tax consequences relating to the purchase, ownership and disposition of our common stock applicable to non-U.S. holders, as defined below. This summary is based on the Internal Revenue Code of 1986, or the Code, as amended to the date hereof, Treasury regulations promulgated thereunder, administrative pronouncements and judicial decisions, changes to any of which subsequent to the date of the registration statement may affect the tax consequences described herein. We undertake no obligation to update this tax summary in the future. This summary applies only to non-U.S. holders that will hold our common stock as capital assets within the meaning of Section 1221 of the Code. It does not purport to be a complete analysis of all the potential tax consequences that may be material to a non-U.S. holder based on his or her particular tax situation. This summary does not address tax consequences applicable to non-U.S. holders that may be subject to special tax rules, such as banks, tax-exempt organizations, pension funds, insurance companies or dealers in securities or foreign currencies, or persons that have a functional currency other than the U.S. dollar. This summary does not address the tax treatment of partnerships or persons who hold their interests through a partnership or another pass-through entity. This summary does not consider the effect of any applicable state, local, foreign or other tax laws.
When we refer to a non-U.S. holder, we mean a beneficial owner of common stock that for U.S. federal income tax purposes is:
| • | a nonresident alien individual (other than certain former citizens and residents of the U.S. subject to tax as expatriates); |
| • | a foreign corporation or other entity taxable as a corporation for U.S. federal income tax purposes; or |
| • | a foreign estate or trust. |
We urge you to consult your tax advisor about the U.S. federal tax consequences of purchasing, holding, and disposing of our common stock in your particular circumstances, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Taxation of Dividends and Dispositions
Dividends on Common Stock. In general, if distributions are made with respect to our common stock, such distributions will be treated as dividends to the extent of our current and accumulated earnings and profits as determined under the Code. Any portion of a distribution that exceeds our current and accumulated earnings and profits will first be applied in reduction of the non-U.S. holder’s basis in the common stock, and to the extent such portion exceeds the non-U.S. holder’s basis, the excess will be treated as gain from the disposition of the common stock, the tax treatment of which is discussed below under “Dispositions of Common Stock”.
Generally, dividends paid to a non-U.S. holder will be subject to the U.S. withholding tax at a 30% rate, subject to the two following exceptions.
| • | Dividends effectively connected with a trade or business of a non-U.S. holder within the U.S. generally will not be subject to withholding if the non-U.S. holder complies with applicable IRS certification requirements and generally will be subject to U.S. federal income tax on a net income basis at regular graduated rates. In the case of a non-U.S. holder that is a corporation, such effectively connected |
135
Table of Contents
| income also may be subject to the branch profits tax, which generally is imposed on a foreign corporation on the deemed repatriation from the U.S. of effectively connected earnings and profits at a 30% rate (or such lower rate as may be prescribed by an applicable tax treaty). |
| • | The withholding tax might not apply, or might apply at a reduced rate, under the terms of an applicable tax treaty. Under the Treasury regulations, to obtain a reduced rate of withholding under a tax treaty, a non-U.S. holder generally will be required to satisfy applicable certification and other requirements. |
Dispositions of Common Stock. Generally, a non-U.S. holder will not be subject to U.S. federal income tax with respect to gain recognized upon the disposition of such holder’s shares of common stock unless:
| • | the non-U.S. holder is an individual who is present in the U.S. for 183 days or more in the taxable year of disposition and certain other conditions are met; |
| • | such gain is effectively connected with the conduct by a non-U.S. holder of a trade or business within the U.S. and, if certain tax treaties apply, is attributable to a U.S. permanent establishment maintained by the non-U.S. holder; or |
| • | we are or have been a “U.S. real property holding corporation” for federal income tax purposes and, assuming that the common stock is deemed to be “regularly traded on an established securities market,” the non-U.S. holder held, directly or indirectly at any time during the five-year period ending on the date of disposition or such shorter period that such shares were held, more than five percent of our common stock. |
We believe we are not currently, and do not anticipate becoming, a “U.S. real property holding corporation” for U.S. federal income tax purposes.
Special rules may apply to certain non-U.S. holders, such as “controlled foreign corporations”, “passive foreign investment companies”, “foreign personal holding companies” and corporations that accumulate earnings to avoid U.S. federal income tax, that are subject to special treatment under the Code. Such entities should consult their own tax advisors to determine the U.S. federal, state, local, foreign and other tax consequences that may be relevant to them.
Federal Estate Tax
Common stock owned or treated as owned by an individual non-U.S. holder at the time of death generally will be included in such holder’s gross estate for U.S. federal estate tax purposes, unless an applicable estate tax treaty provides otherwise.
Information Reporting and Backup Withholding
Information Reporting. The payment of a dividend to a non-U.S. holder is generally not subject to information reporting on IRS Form 1099 if applicable certification requirements are satisfied. The payment of proceeds from the sale of common stock by a broker to a non-U.S. holder is generally not subject to information reporting, if the broker or payor does not have actual knowledge or reason to know that the payee is a U.S. person and:
| • | the beneficial owner of the common stock certifies its non-U.S. status under penalties of perjury, or otherwise establishes an exemption; or |
| • | the sale of the common stock is effected outside the U.S. by a foreign office of a broker, unless the broker is: |
| • | a U.S. person; |
| • | a foreign person that derives 50% or more of its gross income for certain periods from activities that are effectively connected with the conduct of a trade or business in the U.S.; |
136
Table of Contents
| • | a controlled foreign corporation for U.S. federal income tax purposes; or |
| • | a foreign partnership more than 50% of the capital or profits of which is owned by one or more U.S. persons or which engages in a U.S. trade or business. |
In addition to the foregoing, we must report annually to the IRS and to each non-U.S. holder on IRS Form 1042-S the entire amount of any distribution irrespective of any estimate of the portion of the distribution that represents a taxable dividend. This information may also be made available to the tax authorities in the country in which the non-U.S. holder resides under the provisions of an applicable income tax treaty.
Backup Withholding. Backup withholding is only required on payments that are subject to the information reporting requirements, discussed above, and if other requirements are satisfied. Even if the payment of proceeds from the sale of common stock is subject to the information reporting requirements, the payment of sale proceeds from a sale outside the U.S. will not be subject to backup withholding unless the payor has actual knowledge the payee is a U.S. person. Backup withholding does not apply when any other provision of the Code requires withholding. As withholding is generally required on dividends paid to non-U.S. holders, as discussed above, backup withholding is not also imposed. Thus, backup withholding may be required on payments subject to information reporting, and not otherwise subject to withholding.
Backup withholding is not an additional tax. Any amount withheld from a payment to a non-U.S. holder under these rules will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is furnished timely to the IRS.
The U.S. federal income tax and estate tax summary set forth above is included for general information only and may not be applicable depending upon your particular situation. You should consult your own tax advisors with respect to the tax consequences to you of the purchase, ownership and disposition of the common stock, including the tax consequences under state, local, foreign and other tax laws and the possible effects of changes in federal or other tax laws.
Foreign Accounts
The Foreign Account Tax Compliance Act (FATCA) generally imposes a U.S. federal withholding tax of 30% on dividends on and the gross proceeds of a disposition of our common stock, paid to a “foreign financial institution” (as specially defined under these rules), unless such institution enters into an agreement with the U.S. government, among other things, to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding the U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or otherwise establishes an exemption. A U.S. federal withholding tax of 30% also applies to dividends and the gross proceeds of a disposition of our common stock paid to a “passive non-financial foreign entity” (as specifically defined for purposes of these rules) unless such entity provides the withholding agent with a certification identifying certain substantial direct and indirect U.S. owners of the entity, certifies that there are none or otherwise establishes an exemption. The withholding provisions under FATCA will generally apply to dividends on our common stock paid on or after July 1, 2014 and with respect to the gross proceeds of a sale or other disposition of our common stock on or after January 1, 2017. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Prospective investors are encouraged to consult with their own tax advisors regarding the possible implications of this legislation on their investment in our common stock.
137
Table of Contents
Wedbush Securities Inc. is acting as representative of each of the underwriters named below. Subject to the terms and conditions set forth in an underwriting agreement among us and the underwriters, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the number of shares of common stock set forth opposite its name below.
| Underwriters | Number of Shares | |||
| Wedbush Securities Inc. |
— | |||
| Craig-Hallum Capital Group LLC |
— | |||
| Roth Capital Partners, LLC |
— | |||
| Total |
— | |||
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the shares sold under the underwriting agreement if any of these shares are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
The following table shows the per share and total underwriting discount to be paid to the underwriters by us at the public offering price listed on the cover page of this prospectus, less underwriting discount. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| No Exercise | Full Exercise | |||||||
| Per Share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
The representative has advised us that the underwriters propose to offer directly to the public the shares purchased pursuant to the underwriting agreement at to certain dealers at that price less a concession not in excess of $ per share of common stock. After the offering, the representative may change the offering price and other selling terms.
The expenses of the offering, not including the underwriting discount, are estimated at $ and are payable by us.
Option to Purchase Additional Shares
We have granted an option to the underwriters, exercisable for 30 days after the date of this prospectus, to purchase up to additional shares at the public offering price, less the underwriting discount. If the underwriters exercise this option, each will be obligated, subject to conditions contained in the underwriting agreement, to purchase a number of additional shares proportionate to that underwriter’s initial amount reflected in the above table.
138
Table of Contents
Lock-Up Agreements
We, all of our directors and executive officers, and holders of substantially all of our outstanding stock have agreed that, for a period of 180 days, or the lock-up period, after the date of this prospectus subject to certain limited exceptions described below, we and they will not directly or indirectly, without the prior written consent of Wedbush Securities Inc., (1) offer for sale, sell, pledge, or otherwise dispose of (or enter into any transaction or device that is designed to, or could be expected to, result in the disposition by any person at any time in the future of) any shares of common stock (including, without limitation, shares of common stock that may be deemed to be beneficially owned by us or them in accordance with the rules and regulations of the SEC and shares of common stock that may be issued upon exercise of any options or warrants) or securities convertible into or exercisable or exchangeable for common stock, (2) enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of common stock, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of common stock or other securities, in cash or otherwise, (3) make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable for common stock or any of our other securities, or (4) publicly disclose the intention to do any of the foregoing.
These lock-up restrictions will not apply to: (1) bona fide gifts, sales or other dispositions made exclusively by the holder to the holder’s family, partners, members, stockholders or affiliates (as applicable), and transfers or other dispositions by will, other testamentary documents or intestate succession, provided , that such transferee agrees to be bound by the terms of the lock-up agreement, the parties agree to not make any filing or public announcement regarding such transfer or disposition prior to the expiration of the lock-up period and the holder notifies Wedbush Securities Inc. at least two business days prior to the proposed transfer or disposition; (2) the exercise of warrants or stock options granted pursuant to the Company’s stock option/incentive plans or otherwise, or the conversion of securities, in each case outstanding on the date of this prospectus, provided that the restrictions shall apply to the shares of common stock issued upon such exercise or conversion; (3) the establishment of any trading plan established pursuant to Rule 10b5-1 under the Exchange Act, provided that no sales or securities convertible into common stock shall be made pursuant to such plan prior to the expiration of the lock-up period, and we do not, and are not required to, report the establishment of such plan in any public report or filing with the SEC under the Exchange Act prior to the expiration of the lock-up period; (4) any forfeiture, sale or other transfer to us in connection with the termination of the holder’s employment with or services to the company; (5) the transfer of shares to the company to satisfy withholding taxes for any equity award granted prior to the date of this prospectus; (6) any distribution of shares to members, partners or stockholders of the undersigned; provided, that the restrictions shall apply to the shares of common stock distributed; and (7) transactions relating to shares of common stock or other securities acquired in open market transactions after the completion of the offering.
Wedbush Securities Inc. may release the common stock and other securities subject to the lock-up agreements described above in whole or in part at any time. When determining whether or not to release common stock and other securities from lock-up agreements, Wedbush Securities Inc. will consider, among other factors, the holder’s reasons for requesting the release, the number of shares of common stock and other securities for which the release is being requested and market conditions at the time. If Wedbush Securities, Inc. grants a release to any stockholder, it is required to grant the same type of release to all directors, officers and greater than 1% stockholders.
At least three business days before the effectiveness of any release or waiver of any of the restrictions described above with respect to an officer or director of us, Wedbush Securities Inc. will notify us of the impending release or waiver and we have agreed to announce the impending release or waiver by press release through a major news service at least two business days before the effective date of the release or waiver.
139
Table of Contents
Offering Price Determination
Prior to this offering, there has been no public market for our common stock. The initial public offering price was negotiated between the representative and us. In determining the initial public offering price of our common stock, the representative considered:
| • | the history and prospects for the industry in which we compete; |
| • | our financial information; |
| • | the ability of our management and our business potential and earning prospects; |
| • | the prevailing securities markets at the time of this offering; and |
| • | the recent market prices of, and the demand for, publicly traded shares of generally comparable companies. |
An active trading market for the shares may not develop. It is also possible that after the offering the shares will not trade in the public market at or above the initial public offering price.
The underwriters do not expect to sell more than 5% of the shares in the aggregate to accounts over which they exercise discretionary authority.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
Stabilization, Short Positions and Penalty Bids
The representative may engage in stabilizing transactions, short sales and purchases to cover positions created by short sales, and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, in accordance with Regulation M under the Exchange Act:
| • | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. |
| • | A short position involves a sale by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase in the offering, which creates the syndicate short position. This short position may be either a covered short position or a naked short position. In a covered short position, the number of shares involved in the sales made by the underwriters in excess of the number of shares they are obligated to purchase is not greater than the number of shares that they may purchase by exercising their option to purchase additional shares. In a naked short position, the number of shares involved is greater than the number of shares in their option to purchase additional shares. The underwriters may close out any short position by either exercising their option to purchase additional shares and/or purchasing shares in the open market. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through their option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. |
| • | Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. |
| • | Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
140
Table of Contents
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of the common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on the NASDAQ Capital Market or otherwise and, if commenced, may be discontinued at any time.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor any of the underwriters make any representation that the representative will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Listing on The NASDAQ Capital Market
We expect the shares to be approved for listing on the NASDAQ Capital Market under the symbol “STIM.”
Electronic Distribution
In connection with the offering, certain of the underwriters or securities dealers may distribute prospectuses by electronic means, such as e-mail.
Other Relationships
Some of the underwriters and their affiliates have provided in the past to us and our affiliates and may provide from time to time in the future certain financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, the underwriters and their affiliates may effect transactions for their own account or the accounts of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
141
Table of Contents
NOTICE TO INVESTORS
Notice to Investors in the United Kingdom
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of any securities which are the subject of the offering contemplated by this prospectus supplement and the related prospectus may not be made in that Relevant Member State except that an offer to the public in that Relevant Member State of any such securities may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
(a) to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts;
(c) by the underwriter to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive); or
(d) in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of these securities shall result in a requirement for the publication by the issuer or the underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer to the public” in relation to any of the securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any such securities to be offered so as to enable an investor to decide to purchase any such securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
Each underwriter has represented, warranted and agreed that:
(a) it has only communicated or caused to be communicated and will only communicate or cause to be communicated any invitation or inducement to engage in investment activity (within the meaning of section 21 of the Financial Services and Markets Act 2000 (the FSMA)) received by it in connection with the issue or sale of any of the securities in circumstances in which section 21(1) of the FSMA does not apply to the issuer; and
(b) it has complied with and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the securities in, from or otherwise involving the United Kingdom.
European Economic Area
In particular, this document does not constitute an approved prospectus in accordance with European Commission’s Regulation on Prospectuses no. 809/2004 and no such prospectus is to be prepared and approved in connection with this offering. Accordingly, in relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (being the Directive of the European Parliament and of the Council 2003/71/EC and including any relevant implementing measure in each Relevant Member State) (each, a Relevant Member State), with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State (the Relevant Implementation Date) an offer of securities to the public may not be made in that Relevant Member State prior to the publication of a prospectus in relation to such
142
Table of Contents
securities which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that it may, with effect from and including the Relevant Implementation Date, make an offer of securities to the public in that Relevant Member State at any time:
| • | to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| • | to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000; and (3) an annual net turnover of more than €50,000,000, as shown in the last annual or consolidated accounts; or |
| • | in any other circumstances which do not require the publication by the Issuer of a prospectus pursuant to Article 3 of the Prospectus Directive. |
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any of the securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State. For these purposes the shares offered hereby are “securities.”
The validity of the shares of our common stock to be issued in this offering will be passed upon for us by our counsel, Bryan Cave LLP, St. Louis, Missouri. As of the date of this prospectus, James L. Nouss of Bryan Cave, LLP beneficially owned less than one percent of the outstanding shares of our common stock and is our corporate secretary. Certain legal matters relating to this offering will be passed upon for the underwriters by Lowenstein Sandler LLP, New York, New York.
The consolidated financial statements of EndoStim, Inc. at December 31, 2013 and 2012, and for each of the two years in the period ended December 31, 2013, and for the period from June 3, 2009 (inception) to December 31, 2013, appearing in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon (which contains an explanatory paragraph describing conditions that raise substantial doubt about the Company’s ability to continue as a going concern as described in Note 1 to the consolidated financial statements) appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
143
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of our common stock offered by this prospectus. This prospectus, which constitutes part of that registration statement, does not contain all of the information set forth in the registration statement or the accompanying exhibits and schedules. Some items included in the registration statement are omitted from this prospectus in accordance with the rules and regulations of the SEC. For further information with respect to us and the common stock offered in this prospectus, we refer you to the registration statement and the accompanying exhibits and schedules. Statements contained in this prospectus regarding the contents of any contract, agreement or any other document are summaries of the material terms of these contracts, agreements or other documents. With respect to each of these contracts, agreements or other documents filed as an exhibit to the registration statement, reference is made to such exhibit for a more complete description of the matter involved.
A copy of the registration statement and the accompanying exhibits and schedules and any other document we file may be inspected without charge and copied at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. The public may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the SEC’s website is http://www.sec.gov.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act, and we will file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at http://www.endostim.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports, proxy statements and other information filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
144
Table of Contents
| CONSOLIDATED FINANCIAL STATEMENTS |
||
| EndoStim, Inc. (A Development Stage Company) Years Ended December 31, 2013 and 2012, and Period From June 3, 2009 (Inception) to December 31, 2013 With Report of Independent Registered Public Accounting Firm |
||
F-1
Table of Contents
(A Development Stage Company)
Consolidated Financial Statements
Years Ended December 31, 2013 and 2012, and
Period From June 3, 2009 (Inception) to December 31, 2013
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 | ||||
F-2
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of EndoStim, Inc.
We have audited the accompanying consolidated balance sheets of EndoStim, Inc. (a development stage company) (the Company) as of December 31, 2013 and 2012, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the two years in the period ended December 31, 2013, and for the period from June 3, 2009 (inception) to December 31, 2013. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of EndoStim, Inc. at December 31, 2013 and 2012, and the consolidated results of its operations and its cash flows for each of the two years in the period ended December 31, 2013 and for the period from June 3, 2009 (inception) to December 31, 2013, in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has recurring losses from operations and working capital requirements in excess of committed capital that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Ernst & Young LLP
St. Louis, Missouri
May 8, 2014
F-3
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
| December 31 | ||||||||
| 2013 | 2012 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 5,389,003 | $ | 8,538,783 | ||||
| Accounts receivable |
83,980 | 13,200 | ||||||
| Inventory |
342,416 | 63,290 | ||||||
| Deferred cost of revenue |
103,425 | 49,980 | ||||||
| Prepaid expenses and other current assets |
359,709 | 251,331 | ||||||
|
|
|
|||||||
| Total current assets |
6,278,533 | 8,916,584 | ||||||
| Property and equipment, net |
11,653 | 20,595 | ||||||
| Other long-term assets |
61,652 | 62,430 | ||||||
|
|
|
|||||||
| Total assets |
$ | 6,351,838 | $ | 8,999,609 | ||||
|
|
|
|||||||
| Liabilities and stockholders’ equity (deficit) |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 500,691 | $ | 625,203 | ||||
| Accrued expenses |
833,669 | 601,259 | ||||||
| Deferred revenue |
203,159 | 81,464 | ||||||
| Other current liabilities |
39,422 | 2,108 | ||||||
|
|
|
|||||||
| Total liabilities |
1,576,941 | 1,310,034 | ||||||
| Commitment and contingencies |
– | – | ||||||
| Series B convertible redeemable preferred stock, $0.001 par value; 3,999,951 and 4,000,000 shares authorized at December 31, 2013 and 2012; 3,999,951 shares issued and outstanding at December 31, 2013 and 2012 | 7,248,707 | 6,903,239 | ||||||
| Series B-1 convertible redeemable preferred stock, $0.001 par value; 2,466,218 and 2,666,666 shares authorized at December 31, 2013 and 2012; 2,466,218 shares issued and outstanding at December 31, 2013 and 2012 | 6,253,890 | 5,852,363 | ||||||
| Series C convertible redeemable preferred stock, $0.001 par value; 4,400,000 and 3,200,000 shares authorized at December 31, 2013 and 2012; 3,754,713 and 2,435,682 shares issued and outstanding at December 31, 2013 and 2012 | 14,329,432 | 8,644,785 | ||||||
| Stockholders’ equity (deficit): |
||||||||
| Series A convertible preferred stock, $0.001 par value; 1,499,093 shares authorized; 1,499,093 shares issued and outstanding at December 31, 2013 and 2012 | 1,499 | 1,499 | ||||||
| Common stock, $0.001 par value; 16,345,262 shares authorized; 2,203,721 shares issued and outstanding at December 31, 2013 and 2012 | 2,204 | 2,204 | ||||||
| Additional paid-in capital |
1,573,455 | 1,507,721 | ||||||
| Deficit accumulated during development stage |
(24,634,290) | (15,222,236) | ||||||
|
|
|
|||||||
| Total stockholders’ equity (deficit) |
(23,057,132) | (13,710,812) | ||||||
|
|
|
|||||||
| Total liabilities and stockholders’ equity (deficit) |
$ | 6,351,838 | $ | 8,999,609 | ||||
|
|
|
|||||||
See accompanying notes.
F-4
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Consolidated Statements of Operations
| Year Ended December 31, |
Period From June 3, 2009 (Inception) to December 31, 2013 |
|||||||||||
| 2013 | 2012 | |||||||||||
| Revenue |
$ | 301,432 | $ | 33,350 | $ | 334,782 | ||||||
| Cost of revenue |
194,911 | 23,064 | 217,975 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross margin |
106,521 | 10,286 | 116,807 | |||||||||
| Operating expenses: |
||||||||||||
| Research and development |
3,195,979 | 2,613,429 | 11,624,233 | |||||||||
| Sales and marketing |
2,232,514 | 1,025,364 | 3,257,878 | |||||||||
| General and administrative |
2,211,222 | 1,910,423 | 6,715,512 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
7,639,715 | 5,549,216 | 21,597,623 | |||||||||
| Loss from operations |
(7,533,194) | (5,538,930) | (21,480,816) | |||||||||
| Other income: |
||||||||||||
| Grant income |
– | – | 244,478 | |||||||||
| Interest income |
5,962 | 1,857 | 17,091 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other income |
5,962 | 1,857 | 261,569 | |||||||||
| Income tax |
– | – | – | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(7,527,232) | (5,537,073) | (21,219,247) | |||||||||
| Accretion of redeemable preferred stock |
(1,884,822) | (853,561) | (3,415,043) | |||||||||
| Undeclared dividends on Series A convertible preferred stock |
(47,125) | (71,446) | (357,336) | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common stockholders |
$ | (9,459,179) | $ | (6,462,080) | $ | (24,991,626) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss per common share attributable to common stockholders: |
||||||||||||
| Basic and diluted |
$ | (6.74) | $ | (4.71) | ||||||||
| Weighted-average shares used in computing net loss per common share attributable to common stockholders: |
||||||||||||
| Basic and diluted |
1,403,721 | 1,371,863 | ||||||||||
| Pro forma net loss, per common share (see Note 11) |
$ | (0.59 | ) | |||||||||
See accompanying notes.
F-5
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Consolidated Statements of Stockholders’ Equity (Deficit)
| Convertible Preferred Stock Series A |
Common Stock | Additional Paid-In Capital |
Accounts Receivable Sale of Stock |
Deficit Accumulated During Development Stage |
Total Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||
| Initial capital contribution |
– | $ | – | 805,000 | $ | 805 | $ | 24,450 | $ | (25,000) | $ | – | $ | 255 | ||||||||||||||||||
| Issuance of Series A preferred stock at $1.00 per share from the conversion of a bridge note into Series A preferred stock | 275,000 | 275 | – | – | 274,725 | – | – | 275,000 | ||||||||||||||||||||||||
| Issuance of Series A preferred stock at $1.00 per share, net of issuance costs of $75,747 | 476,593 | 476 | – | – | 400,370 | – | – | 400,846 | ||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (501,448) | (501,448) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2009 |
751,593 | 751 | 805,000 | 805 | 699,545 | (25,000) | (501,448) | 174,653 | ||||||||||||||||||||||||
| Issuance of Series A shares at $1.00 per share |
747,500 | 748 | – | – | 746,752 | – | – | 747,500 | ||||||||||||||||||||||||
| Exercise of stock options |
– | – | 28,125 | 28 | 3,878 | – | – | 3,906 | ||||||||||||||||||||||||
| Grant of restricted shares |
– | – | 1,275,000 | 1,275 | (750) | – | – | 525 | ||||||||||||||||||||||||
| Payments on accounts receivable |
– | – | – | – | – | 25,000 | – | 25,000 | ||||||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | – | – | – | – | – | (170,658) | (170,658) | ||||||||||||||||||||||||
| Share-based compensation |
– | – | – | – | 9,152 | – | 9,152 | |||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (3,062,380) | (3,062,380) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2010 |
1,499,093 | 1,499 | 2,108,125 | 2,108 | 1,458,577 | – | (3,734,486) | (2,272,302) | ||||||||||||||||||||||||
| Exercise of stock options |
– | – | 50,000 | 50 | 3,700 | – | – | 3,750 | ||||||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | – | – | – | – | – | (506,002) | (506,002) | ||||||||||||||||||||||||
| Share-based compensation |
– | – | – | – | 18,049 | – | 18,049 | |||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (4,591,114) | (4,591,114) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2011 |
1,499,093 | 1,499 | 2,158,125 | 2,158 | 1,480,326 | – | (8,831,602) | (7,347,619) | ||||||||||||||||||||||||
| Exercise of stock options |
– | – | 45,596 | 46 | 2,702 | – | – | 2,748 | ||||||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | – | – | – | – | – | (853,561) | (853,561) | ||||||||||||||||||||||||
| Share-based compensation |
– | – | – | – | 24,693 | – | – | 24,693 | ||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (5,537,073) | (5,537,073) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2012 |
1,499,093 | 1,499 | 2,203,721 | 2,204 | 1,507,721 | – | (15,222,236) | (13,710,812) | ||||||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | – | – | – | – | – | (1,884,822) | (1,884,822) | ||||||||||||||||||||||||
| Share-based compensation |
– | – | – | – | 65,734 | – | – | 65,734 | ||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (7,527,232) | (7,527,232) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2013 |
1,499,093 | $ | 1,499 | 2,203,721 | $ | 2,204 | $ | 1,573,455 | $ | – | $ | (24,634,290) | $ | (23,057,132) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
See accompanying notes.
F-6
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Consolidated Statements of Cash Flows
| Year Ended December 31 |
Period From June 3, 2009 (Inception) to December 31, 2013 |
|||||||||||
| 2013 | 2012 | |||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (7,527,232) | $ | (5,537,073) | $ | (21,219,247) | ||||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation |
8,942 | 7,014 | 15,956 | |||||||||
| Share-based compensation |
65,734 | 24,693 | 117,628 | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(70,780) | (13,200) | (83,980) | |||||||||
| Inventory |
(279,126) | (63,290) | (342,416) | |||||||||
| Deferred cost of revenue |
(53,445) | (49,980) | (103,425) | |||||||||
| Prepaid expenses and other current assets |
(108,378) | (161,753) | (359,709) | |||||||||
| Other long-term assets |
778 | (62,430) | (61,652) | |||||||||
| Accounts payable |
(124,512) | 86,719 | 500,691 | |||||||||
| Accrued expenses |
232,410 | 342,245 | 833,669 | |||||||||
| Deferred revenue |
121,695 | 81,464 | 203,159 | |||||||||
| Other current liabilities |
37,314 | 2,108 | 39,422 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(7,696,600) | (5,343,483) | (20,459,904) | |||||||||
| Investing activities |
||||||||||||
| Purchase of equipment |
– | (27,609) | (27,609) | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
– | (27,609) | (27,609) | |||||||||
| Financing activities |
||||||||||||
| Issuance of convertible note |
– | – | 275,000 | |||||||||
| Proceeds from issuance of preferred and common stock |
4,946,366 | 12,833,138 | 26,878,964 | |||||||||
| Issuance costs for preferred and common stock |
(399,546) | (561,876) | (1,287,852) | |||||||||
| Proceeds from exercise of stock options |
– | 2,748 | 10,404 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
4,546,820 | 12,274,010 | 25,876,516 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net (decrease) increase in cash and cash equivalents |
(3,149,780) | 6,902,918 | 5,389,003 | |||||||||
| Cash and cash equivalents, beginning of period |
8,538,783 | 1,635,865 | – | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 5,389,003 | $ | 8,538,783 | $ | 5,389,003 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental schedule of non-cash financing activity: |
||||||||||||
| Accretion of redeemable preferred stock |
$ | (1,884,822) | $ | (853,561) | $ | (3,415,043) | ||||||
See accompanying notes.
F-7
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
Years Ended December 31, 2013 and 2012, and
Period from June 3, 2009 (Inception) to
December 31, 2013
1. Description of Business
EndoStim, Inc. (EndoStim or the Company) is a medical device company developing a novel treatment for severe gastroesophageal reflux disease, or GERD. EndoStim’s proprietary technology uses electrical stimulation of the lower esophageal sphincter (LES) to normalize esophageal functions and has been shown in clinical trials to improve esophageal pH and patient symptoms. In 2012, EndoStim obtained the right to apply the CE Mark to its device and has begun to commercialize its products in Europe and in other countries worldwide that recognize the CE Mark. Founded in 2009, EndoStim is a development-stage company headquartered in St. Louis, Missouri, U.S.A., with offices in The Hague, The Netherlands.
Since its inception, the Company’s principal activities involved obtaining capital, business development, performing research and development activities and funding prototype development. During 2012, the Company commenced international commercialization operations. The Company was classified as a development-stage company from its inception on June 3, 2009 through December 31, 2013.
Since its inception, the Company has generated significant losses, given that the Company is a development-stage organization that has recently begun commercialization of its products. As of December 31, 2013, the Company incurred cumulative net losses of $21,219,247. EndoStim expects to incur additional losses as it continues the commercialization of its product, conducts additional research and development activities, and funds additional sales and marketing initiatives throughout 2014. Historically, its cash needs for operating expenses have been principally met by proceeds from the issuance of preferred stock. The Company will be required to raise capital or pursue other financing strategies to continue operations. Although the Company anticipates that it will be able to achieve adequate liquidity for the remainder of fiscal year 2014 by raising additional investments from current or new investors and/or securing a credit facility, there is no assurance that the Company will be able to obtain such investment or financing on favorable terms, if at all. In the event the Company fails to raise additional financing, the Company would be required to delay seeking further regulatory approval for its products, delay commercialization of its products internationally, or reduce the sales, marketing, customer support or other resources devoted to its products and product development, any of which could have a material adverse effect on its business, financial condition and results of operations. In addition, the Company could be required to cease operations if the Company fails to raise additional financing. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
2. Summary of Significant Accounting Policies
Principles of consolidation
The financial statements of the Company have been prepared in accordance with principles generally accepted in the United States (GAAP). The consolidated financial statements include the
F-8
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
accounts of EndoStim, Inc. and its wholly owned subsidiary, EndoStim, BV located in The Netherlands which has de minimus operations and net assets. All intercompany accounts and transactions have been eliminated.
Accounting Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates, and such differences could be material to the financial position and results of operations.
Concentration of Risk and Other Uncertainties
The Company sells its products to distributors and directly to hospitals in various countries outside of the United States and grants credit to certain of these customers. The Company commenced international commercialization efforts in the fourth quarter of 2012. There are two customers, Ev. Krankenhaus Castop-Rauxel and Hospimedics S.A., that contributed 11.3% and 17.5% of revenue, respectively, for the 12 months ended December 31, 2013. There are three customers, Ardmore Healthcare Ltd., Tronda Electronics Inc., and Equipos Medicos Zepeda y Cia. Ltda that contributed 27.3%, 13.7% and 49.9% of revenue, respectively, for the 12 months ended December 31, 2012.
The Company relies upon a single third-party supplier located in Uruguay to produce its finished products for sale.
The Company currently maintains the majority of its cash and cash equivalents with two high-credit-quality financial institutions in the United States and deposits in excess of $250,000 are not Federal Deposit Insurance Corporation insured. Cash equivalents are accounted for at cost, which approximates fair value. The Company believes that it is not exposed to significant credit risk due to the financial position of the depository institutions in which these deposits are held and of the money market funds in which investments are made. Additionally, the Company’s investment policy is designed to preserve principal and provide liquidity.
Cash and Cash Equivalents
For the purposes of the consolidated statement of cash flows, the Company considers all short-term deposits purchased with original maturities of three months or less and money market funds to be cash equivalents.
Accounts Receivable and Allowance for Uncollectible Accounts
During the ordinary course of its business, the Company grants unsecured credit to its customers, principally hospitals. Accounts receivable are carried at cost less an allowance for doubtful accounts. On a regular basis, the Company evaluates accounts receivable and estimates an allowance for doubtful accounts, as needed, based on various factors such as customers’ current credit conditions,
F-9
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
length of time past due and the general economic conditions. As of December 31, 2013 and 2012, the Company has not established a reserve because the Company believes that the amounts outstanding are fully collectible.
Inventory
Inventory principally consists of finished goods that are purchased from a third-party supplier. Inventory is valued at the lower of the actual cost of the inventory, as determined using the first-in, first-out (FIFO) method or its net realizable value. Actual cost of inventory includes purchased cost and freight costs. The Company periodically reviews its physical inventory for excess, obsolete or potentially impaired items, taking into account product development plans, product expiration and current inventory levels. As of December 31, 2013 and 2012, the Company has not established a reserve because, based on the Company’s estimate of future use, the Company does not believe that it has excess, obsolete or impaired inventory.
Property and Equipment, net
Property and equipment, net consists of research and development equipment that has alternative future use to the Company and is stated at cost. Depreciation is calculated using the straight-line method over the estimated useful life of the assets, which is three years.
Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, accounts receivable, and accounts payable.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value hierarchy that prioritizes the use of inputs used in valuation techniques is as follows:
Level 1—quoted prices in active markets for identical assets and liabilities;
Level 2—observable inputs other than quoted prices in active markets, such as quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data;
Level 3—unobservable inputs reflecting management’s assumptions, consistent with reasonably available assumptions made by other market participants. These valuations require significant judgment.
The carrying value of cash and cash equivalents, accounts receivable, and accounts payable approximates fair values based on the short-term nature of these financial instruments.
F-10
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Revenue
The Company’s revenue consists of product revenue resulting from the sale of its programmable Implantable Pulse Generators (IPG) and programmers, which communicate wirelessly with the IPGs. All product sales originate from the United States. The Company recognizes revenue when all four revenue recognition criteria have been met: persuasive evidence of an arrangement exists; delivery has occurred or service has been rendered; the price is fixed or determinable; and collectability is reasonably assured. The Company sells its products to distributors with substantial independent operations under sales arrangements whose terms do not allow for rights of return or price protection on unsold products held by them. The Company’s revenue recognition policy generally results in revenue recognition at delivery to the hospital or distributor for programmers and upon implant into patient for IPGs. The Company defers revenue on the sale of IPGs until implant into the patient as the Company may grant concessions allowing for the resterilization or exchange of IPGs upon expiration. Amounts collected prior to satisfying the above revenue recognition criteria are reflected as deferred revenue, and the corresponding cost of revenue is reflected as deferred cost of revenue in the consolidated balance sheets. For certain transactions, the Company has multiple-element arrangements that include the IPGs and programmer. For multiple-element arrangements, revenue is allocated to each element based on its relative selling price. Relative selling prices are based first on vendor-specific objective evidence (VSOE), then on third-party evidence of selling price (TPE) when VSOE does not exist, and then on estimated selling price (ESP) when VSOE and TPE do not exist. The Company does not have VSOE for the IPG and programmer. The Company recently began commercialization of its products in many different countries, each of which is expected to have a different fair value. The Company does not yet have enough data on stand-alone transactions to establish VSOE in any of the markets in which it is commercializing its products. Because the Company has neither VSOE nor TPE for its products, the allocation of revenue has been based on the Company’s ESPs. The objective of ESP is to determine the price at which the Company would transact a sale if the product was sold on a stand-alone basis. The Company determines ESP for its products by considering multiple factors, including, but not limited to, geography and market conditions, reimbursement applications specific to a particular country and price lists provided to customers. When billed to the customer, shipping and handling costs are included in revenue and cost of revenue, and were immaterial for the periods presented. Costs of revenue include direct product costs and overhead costs, shipping costs and estimated warranty costs.
Product Warranty Provisions
The Company provides a warranty for products against defects in material or workmanship for up to two years following delivery for programmers or implant for IPGs. The Company’s warranty obligations at December 31, 2013 and 2012 were immaterial.
Research and Development Costs
Research and development costs are expensed in the period incurred. These costs include, but are not limited, to employee-related expenses such as salaries, benefits and travel, product development expenditures and clinical trial expenditures.
Patent Costs
Costs related to filing, pursuing and defending patents are expensed as incurred, as recoverability of such expenditures is uncertain.
F-11
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Advertising Costs
The Company expenses advertising costs as incurred. The Company incurred approximately $37,400 in advertising costs during the year ended December 31, 2013. There were no advertising costs for the year ended December 31, 2012.
Deferred Income Taxes
Deferred income tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using the enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. The Company has established a valuation allowance against the entire amount of its deferred tax assets because the Company is unable to conclude, due to its history of operating losses, that it is more likely than not that the Company will be able to realize any portion of the deferred tax assets.
Accounting for Stock-Based Compensation
The Company accounts for its stock-based compensation in accordance with Accounting Standards Codification (ASC) 718, Compensation—Stock Compensation, which establishes accounting for share-based awards, including stock options and restricted stock, exchanged for services and requires companies to expense the estimated fair value of these awards over the requisite service period.
The Company utilized the Black-Scholes valuation model to determine the fair value of share-based payments at the grant date using a risk-free interest rate based on the Treasury yield on the date of the grant and expected volatility based on an analysis of reported data for a peer group of companies. The resulting compensation expense is recognized on a straight-line basis over the requisite service period, generally one to four years. Compensation expense is recognized only for those awards expected to vest, with forfeitures estimated based on future expectations. The estimate of forfeitures will be adjusted over the requisite service period to the extent that actual forfeitures differ, or are expected to differ, from such estimates. Changes in estimated forfeitures will be recognized through a cumulative catch-up adjustment in the period of change and will also impact the amount of stock compensation expense to be recognized in future periods. Restricted common shares granted are valued at the fair market value at the date of grant. The Company amortizes the amount to expense over the service period on a straight-line basis for those shares with graded vesting. If the shares are subject to performance objectives, the resulting compensation expense is amortized over the anticipated vesting period when achievement of the performance objectives is probable and is subject to adjustment based on the actual achievement of objectives.
Fair Value of Common Stock
The Board of Directors considers objective and subjective factors to determine the fair value of the Company’s common stock at each meeting at which awards were approved. These factors include, but are not limited to: (i) third-party valuations of the common stock; (ii) the prices, rights, preferences and privileges of the Company’s convertible preferred stock relative to those of the common stock;
F-12
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
(iii) the lack of marketability of the Company’s common stock; (iv) the Company’s actual operating and financial results, current business conditions and projections including future cash needs; and (v) the likelihood of achieving a liquidity event, such as an initial public offering or sale of the Company, given prevailing market conditions.
Basic and Diluted Net Loss per Share of Common Stock
Basic net loss per share of common stock is computed by dividing net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period. Net loss attributable to common stockholders includes deductions for the accretion of convertible redeemable preferred stock and undeclared dividends on Series A convertible preferred stock which accumulate dividends. Diluted net loss per share of common stock is computed by dividing the net loss attributable to common stockholders by the sum of the weighted-average number of common shares outstanding during the period plus the potential dilutive effects of convertible preferred stock outstanding during the period calculated in accordance with the if-converted method, as well as outstanding options and warrants, if any, to the extent their inclusion is not anti-dilutive. Because the impact of these items is anti-dilutive during periods presented, there was no difference between basic and diluted net loss per share of common stock attributable to common stockholders for the years ended December 31, 2013 and 2012.
Reclassifications
The Company reclassified certain prior period amounts in its Consolidated Financial Statements to be consistent with the current period presentation. Except for the reclassification of the Series B, B-1 and C convertible redeemable preferred stock from stockholders’ equity (deficit) to temporary or mezzanine equity (a classification between liabilities and stockholders’ equity (deficit) pursuant to Securities and Exchange Commissoin (SEC) Accounting Series Release No. 268, Presentation in Financial Statements of ‘Redeemable Preferred Stocks’), the effect of these reclassifications was not material.
Subsequent Events
Events and transactions were evaluated for potential recognition or disclosure through May 8, 2014, which is the date the consolidated financial statements were issued. There were no subsequent events that require recognition or disclosure.
3. Property and Equipment, net
Property and equipment consists of the following:
| December 31, 2013 | December 31, 2012 | |||||||
| Research and development equipment |
$ 27,609 | $ 27,609 | ||||||
| Less: accumulated depreciation |
(15,956) | (7,014) | ||||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ 11,653 | $ 20,595 | ||||||
|
|
|
|
|
|||||
F-13
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
4. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of the following:
| December 31, 2013 | December 31, 2012 | |||||||
| Deposits on inventory purchases |
$ 256,903 | $ 190,755 | ||||||
| Prepaid insurance and other prepaid expenses |
58,647 | 56,063 | ||||||
| Other current assets |
44,159 | 4,513 | ||||||
|
|
|
|
|
|||||
| Total prepaid expenses and other current assets |
$ 359,709 | $ 251,331 | ||||||
|
|
|
|
|
|||||
5. Accrued Expenses
Accrued expenses consist of the following:
| December 31, 2013 | December 31, 2012 | |||||||
| Accrued salaries, bonuses and benefits |
$ 247,719 | $ 152,117 | ||||||
| Accrued research and development costs |
510,600 | 231,550 | ||||||
| Accrued legal and professional fees |
52,850 | 193,800 | ||||||
| Accrued other |
22,500 | 23,792 | ||||||
|
|
|
|
|
|||||
| Total accrued expenses |
$ 833,669 | $ 601,259 | ||||||
|
|
|
|
|
|||||
6. Related-Party Transactions
During the years ended December 31, 2013 and 2012, the Company recorded operating expenses within research and development of $68,000 and $88,000, respectively, related to payments made to a member of the Board of Directors as compensation for consulting services unrelated to his services as a director. As of December 31, 2013 and 2012, $20,500 and $18,500 was included in accounts payable or accrued expenses, respectively.
During the years ended December 31, 2013 and 2012, the Company recorded operating expenses within general and administrative of $84,551 and $85,499, respectively, related to its principal legal firm, in which a partner is an investor and principal counsel to the Company. During the years ended December 31, 2013 and 2012, the Company recorded $82,654 and $156,007, respectively, related to its principal legal firm in which a partner is the Secretary of the Company and an investor and principal counsel to the Company, which were recorded as a reduction to the proceeds from the respective preferred offerings. As of December 31, 2013 and 2012, $32,002 and $191,711 was included in accounts payable or accrued expenses, respectively.
The Company’s placement agent for its Series C financing offering also serves as a general partner of an investor in the Company. This investor owns 333,333 shares of the Company’s Series B
F-14
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
convertible redeemable preferred stock, 222,222 shares of the Company’s Series B-1 convertible redeemable preferred stock and 133,333 shares of the Company’s Series C convertible redeemable preferred stock. During the years ended December 31, 2013 and 2012, the Company made payments of $286,532 and $353,123, respectively, for commissions due as placement agent and for reimbursement of expenses, which were recorded as a reduction to the proceeds from the offering.
7. Commitments and Contingencies
Purchase Obligations
As of December 31, 2013, the Company had an outstanding purchase commitment to the supplier of its product in the amount of $999,803, net of deposits. The Company intends to take delivery of these products through 2015. Should the Company cancel the purchase order or refuse to take delivery of the items, the Company would be liable for any materials purchased and work performed at the date of cancellation.
Operating Leases
The Company leases its office space and certain equipment under operating leases that expire through May 2016. At December 31, 2013, the minimum aggregate payment due under these leases is summarized as follows:
| Year |
Payment | |||
| 2014 |
$ 16,971 | |||
| 2015 |
3,300 | |||
| 2016 |
1,375 | |||
|
|
|
|||
| Total |
$ 21,646 | |||
|
|
|
|||
The Company leases its office space under a two-year operating lease through April 30, 2014. This lease was subsequently renewed in May 2014 on a month-to-month basis. For the years ended December 31, 2013 and 2012, rent expense was $36,456 and $20,936, respectively. Amounts are due monthly under this lease in the amount of $3,418.
The Company leases a printer under a three-year operating lease. For the year ended December 31, 2013, the Company expensed $1,926 under this lease. Amounts are due monthly under this lease in the amount of $275 through May 2016.
8. Convertible Redeemable Preferred Stock
The Company assesses the terms of its redeemable preferred stock as liabilities or temporary equity within the consolidated balance sheets based on the terms of the preferred stock agreements. To the extent the redemption is mandatory or would require the Company to settle in an undeterminable number of common shares that may not be solely within the control of the Company to issue, the Company would record the securities as liabilities. The Company does not have any redeemable preferred stock classified as liabilities for the years ended December 31, 2013 and 2012.
F-15
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
The Series B, B-1 and C convertible redeemable preferred stock was recorded at the initial carrying amount, which is generally its fair value on the date of issuance within temporary equity. The redeemable preferred shares are not currently redeemable but probable of becoming redeemable at the sixth anniversary from the issuance date at the option of the holders. The Company has accreted all accrued and unpaid dividends, commencing from the date of issuance using the interest method and accordingly, the convertible redeemable preferred stock is recorded at its redemption value. The amount of accrued unpaid dividends on convertible redeemable preferred stock within temporary equity is reflected as an increase to accumulated deficit.
The following is a summary of the Company’s convertible redeemable preferred stock from inception to December 31, 2013.
| Preferred Stock | Preferred Stock | Preferred Stock | ||||||||||||||||||||||||||
| Series B | Series B-1 | Series C | ||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Total | ||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Balance at December 31, 2009 |
– | $ | – | – | $ | – | – | $ | – | $ | – | |||||||||||||||||
| Issuance of Series B shares at $1.50 per share, net of offering costs of $142,638 |
2,413,728 | 3,477,954 | – | – | – | – | 3,477,954 | |||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | 170,658 | – | – | – | – | 170,658 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Balance at December 31, 2010 |
2,413,728 | 3,648,612 | – | – | – | – | 3,648,612 | |||||||||||||||||||||
| Issuance of Series B shares at $1.50 per share, net of offering costs of $22,065 |
1,586,223 | 2,357,270 | – | – | – | – | 2,357,270 | |||||||||||||||||||||
| Issuance of Series B-1 shares at $2.25 per share, net of offering costs of $85,980 |
– | – | 822,071 | 1,763,680 | – | – | 1,763,680 | |||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | 481,340 | – | 24,662 | – | – | 506,002 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Balance at December 31, 2011 |
3,999,951 | 6,487,222 | 822,071 | 1,788,342 | – | – | 8,275,564 | |||||||||||||||||||||
| Issuance of Series B-1 shares at $2.25 per share, net of offering costs of $5,643 |
– | – | 1,644,147 | 3,693,688 | – | – | 3,693,688 | |||||||||||||||||||||
| Issuance of Series C shares at $3.75 per share, net of offering costs of $556,233 |
– | – | – | – | 2,435,682 | 8,577,574 | 8,577,574 | |||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | 416,017 | – | 370,333 | – | 67,211 | 853,561 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Balance at December 31, 2012 |
3,999,951 | 6,903,239 | 2,466,218 | 5,852,363 | 2,435,682 | 8,644,785 | 21,400,388 | |||||||||||||||||||||
| Issuance of Series C shares at $3.75 per share, net of offering costs of $399,546 |
– | – | – | – | 1,319,031 | 4,546,820 | 4,546,819 | |||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | 345,468 | – | 401,527 | – | 1,137,827 | 1,884,822 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Balance at December 31, 2013 |
3,999,951 | $ | 7,248,707 | 2,466,218 | $ | 6,253,890 | 3,754,713 | $ | 14,329,432 | $ | 27,832,029 | |||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
F-16
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Each share of Series B, B-1 and C convertible redeemable preferred stock is convertible at any time at the option of the holder into a number of common shares as determined by dividing the original issue price by the then-applicable conversion price, which is one share of common stock as of December 31, 2013 and 2012. In addition, each share of convertible redeemable preferred stock will be automatically converted into shares of common stock, at the then-effective conversion rate, (a) upon completion by the Company of an initial public offering (IPO) and i) the IPO per share price is at least $7.75 (as adjusted for any stock dividends, combinations, splits or recapitalizations and ii) the net cash IPO proceeds to the Company (net of issuance costs) are at least $35,000,000, or (b) at such time that the holders of at least 60% of the outstanding preferred shares, voting together as a single class, vote affirmatively to convert such shares into common stock. The initial conversion rate is 1:1 subject to adjustment for stock splits and combinations; stock dividends and distributions; and reclassifications, reorganizations, mergers, or consolidations. In addition, subject to certain exceptions each of the convertible redeemable preferred stock conversion rates is subject to adjustment in accordance with the weighted-average anti-dilution provisions in the Company’s Amended and Restated Certificate of Incorporation, if the Company issues or sells shares of preferred or common stock below the preferred stock conversion rate then in effect for each series.
If declared by the Board of Directors, convertible redeemable preferred stockholders are entitled to cumulative dividends at the rate of 8% per share per annum on each outstanding share of Series B, B-1 and C preferred stock, as adjusted for stock splits and recapitalizations, payable in preference to common stock dividends. Series C dividends rank equally with dividend rights on the Series B-1 in proportion to the original issue prices of the series plus accrued dividends, and the Series C and Series B-1 dividends are payable in preference to the dividend rights of holders of the Company’s Series B. Series B dividends are payable in preference to the dividend rights of holders of the Company’s Series A preferred stock and common stock. No dividends have been declared or paid by the Company. Cumulative dividends in arrears are $3,192,483 and $1,463,489 at December 31, 2013 and 2012.
Holders of Series B, B-1 and C preferred stock are entitled to the number of votes equal to the number of shares of common stock into which such series are convertible and are entitled to vote with the common stock on all matters submitted to the Company’s stockholders for a vote. Additionally, the holders of at least 60% of the outstanding preferred shares, voting together as a single class, must approve certain other actions by the Company. Certain holders of the Series B, B-1 and C have a right of first refusal with respect to participation in additional equity financings the Company undertakes, subject to defined exceptions.
The holders of each of the Series B, B-1 and C convertible redeemable preferred stock will have the right to require the Company to purchase its shares for cash commencing any time after the sixth anniversary of the original issue date of the respective preferred stock (or (i) with respect to the Series C, upon any earlier date that the holders of the Series B or Series B-1 have elected to be redeemed or (ii) with respect to the Series B-1, upon any earlier date that the holders of the Series B have elected to be redeemed). The redemption price is an amount equal to the original issue price plus all declared or accumulated and unpaid dividends in arrears. If the holders of one or more series have made redemption elections that are otherwise payable concurrently with the other series, the redemption amounts will be payable among the holders of Series C and Series B-1 entitled to
F-17
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
redemption ratably in proportion to the full amounts to which they would otherwise be respectively entitled, and in preference to any redemption amount payable to the holders of the Series B. As the redemption is as of a fixed and determinable date but at the option of the holder, the redemption is not solely within the control of the Company. Therefore, the Company has recorded the convertible redeemable preferred stock within mezzanine equity at the amount of the initial proceeds, net of issuance costs, which was deemed to approximate fair value, adjusted for the accretion of cumulative undeclared dividends earned at 8% using the interest method at 8% per annum using the interest rate method.
In addition, subject to certain exceptions, the conversion rate for each series of convertible redeemable preferred stock is subject to adjustment in accordance with the weighted average anti-dilution provisions in the Company’s Amended and Restated Certificate of Incorporation if the Company issues or sells shares or other securities convertible into common stock, below the applicable preferred stock conversion rate then in effect for each such series.
9. Stockholders’ Equity
Common Stock
The Company has outstanding shares of common stock that are subject to the Company’s right to repurchase at the original issuance price upon the occurrence of certain events as defined in the agreements.
The Company has reserved shares of common stock for the conversion of preferred stock, the exercise of warrants, and the issuance of options granted and outstanding under the Company’s stock option plan as follows:
| December 31, 2013 | December 31, 2012 | |||||||
| Convertible preferred stock |
12,365,262 | 11,365,759 | ||||||
| Warrants |
550,000 | 550,000 | ||||||
| Stock option plan |
1,344,800 | 800,280 | ||||||
|
|
|
|
|
|||||
|
|
|
|
|
|||||
| Total shares |
14,260,062 | 12,716,039 | ||||||
|
|
|
|
|
|||||
Series A Convertible Preferred Stock
In 2009, the Company issued 8% Convertible Promissory Notes, which was converted into 275,000 shares of its Series A convertible preferred stock at the time of the Series A convertible preferred stock offering. The Series A convertible preferred stock is convertible into shares of the Company’s common stock, par value $0.001 per share, at the original issue price of $1.00 per share of common stock.
Each share of Series A is convertible at any time at the option of the holder into a number of common shares as determined by dividing the original issue price by the then applicable conversion
F-18
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
price, which is one share of common stock as of December 31, 2013 and 2012. In addition, each share of Series A will be automatically converted into shares of common stock, at the then-effective conversion rate, (a) upon completion by the Company of an initial public offering (IPO) and i) the IPO per share price is at least $7.75 (as adjusted for any stock dividends, combinations, splits or recapitalizations and ii) the net cash proceeds to the Company (net of issuance costs) from the IPO are at least $35,000,000, or (b) at such time that the holders of at least 60% of the outstanding preferred shares, voting together as a single class, vote affirmatively to convert such shares into common stock. The initial conversion rate is 1:1 subject to adjustment for stock splits and combinations; stock dividends and distributions; and reclassifications, reorganizations, mergers, or consolidations. In addition, subject to certain exceptions, the conversion rate for the Series A is subject to adjustment in accordance with the weighted average anti-dilution provisions in the Company’s Amended and Restated Certificate of Incorporation if the Company issues or sells shares or other securities convertible into common stock below the applicable preferred stock conversion rate then in effect for the Series A.
If declared by the Board of Directors, Series A stockholders are entitled to cumulative dividends at the rate of 8% per share per annum on each outstanding share, as adjusted for stock splits and recapitalizations, payable in preference to common stock dividends. Series A dividends are payable in preference to the dividend rights of holders of the Company’s common stock but are subordinated to the dividend rights of holders of Series B, B-1 and C. No dividends have been declared or paid by the Company. Cumulative dividends in arrears are $357,336 and $310,211 at December 31, 2013 and 2012, respectively.
Holders of Series A are entitled to the number of votes equal to the number of shares of common stock into which such Series A are convertible and are entitled to vote with the common stock on all matters submitted to the Company’s stockholders for a vote. Additionally, the holders of at least 60% of the outstanding preferred shares, voting together as a single class, must approve certain other actions by the Company.
Warrants
In 2009, the Company issued 8% Convertible Promissory Notes, and the holders of the 8% Convertible Promissory Notes also received warrants to purchase 550,000 shares of common stock at $0.01 per share and are exercisable through July 2019. The warrants were determined to be freestanding instruments upon issuance and only provide for physical share settlement. The warrants are classified as equity instruments at fair value as of the issuance date of July 1, 2009; however, the fair value ($16,500) of these warrants was determined to be immaterial and therefore no amounts were recorded at the date of issuance. The warrants are outstanding at December 31, 2013.
Share-Based Compensation
In 2009, the Board of Directors adopted a stock incentive plan (the 2009 Stock Incentive Plan), which, as amended, authorizes for issuance a total of 1,600,000 shares of common stock. The 2009 Stock Incentive Plan allows for the grant of incentive stock options and non-qualified stock options to employees, board members and consultants. Options granted under the 2009 Stock Incentive Plan
F-19
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
expire no later than 10 years from the date of grant. The exercise price of each incentive stock option shall not be less than 100% of the fair value of the stock subject to the option on the date the option is granted. The vesting provisions of individual options may vary, but option grants generally vest ratably over a period of one to four years.
The Board of Directors approved performance-based restricted common share awards. Performance-based restricted shares vest upon the achievement of performance objectives, which are based on the achievement of specified returns at the time of a liquidation event, which is defined in the agreement and includes an initial public offering, consummation of the sale or exclusive license of a significant portion of the Company’s assets, or the stockholders of the Company consummate a merger or consolidation of the Company with any other entity. There is no maximum term associated with such grants. As of December 31, 2013 and 2012, the Company had 800,000 performance-based restricted shares outstanding. During the year ended December 31, 2013, the Company determined that it was not probable that the performance conditions related to its outstanding restricted share awards would be achieved and accordingly has not recorded share-based compensation expense related to these awards.
As of December 31, 2013 and 2012, 761,989 and 607,487 options were vested and outstanding under the 2009 Stock Incentive Plan, respectively, at a weighted-average exercise price of $0.15 and $0.09, respectively.
F-20
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
A summary of the options outstanding is as follows:
| Number of Shares |
Range of Exercise Prices |
Weighted- Average Exercise Price per Share |
Weighted- Average Grant Date Fair Value |
|||||||||||||
| Outstanding, December 31, 2009 |
150,000 | $ 0.05 | $ 0.05 | $ 0.03 | ||||||||||||
| Granted |
370,000 | 0.075–0.15 | 0.09 | 0.05 | ||||||||||||
| Exercised |
(28,125) | 0.05–0.15 | 0.14 | 0.08 | ||||||||||||
| Forfeited |
- | - | - | - | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding, December 31, 2010 |
491,875 | 0.05–0.15 | 0.075 | 0.04 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Granted |
325,000 | 0.15 | 0.15 | 0.08 | ||||||||||||
| Exercised |
(50,000) | 0.075 | 0.075 | 0.04 | ||||||||||||
| Forfeited |
- | - | - | - | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding, December 31, 2011 |
766,875 | 0.05–0.015 | 0.11 | 0.06 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Granted |
139,000 | 0.15–0.25 | 0.17 | 0.23 | ||||||||||||
| Exercised |
(45,596) | 0.05–0.075 | 0.06 | 0.03 | ||||||||||||
| Forfeited |
(60,000) | 0.075–0.15 | 0.14 | 0.11 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding, December 31, 2012 |
800,279 | 0.05–0.25 | 0.12 | 0.09 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Granted |
636,500 | 0.40 | 0.40 | 0.22 | ||||||||||||
| Exercised |
- | - | - | - | ||||||||||||
| Forfeited |
(91,980) | 0.15–0.40 | 0.31 | 0.19 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding, December 31, 2013 |
1,344,799 | $ 0.05–0.40 | $ 0.24 | $ 0.14 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
As of December 31, 2013, the weighted-average remaining contractual term of the options outstanding was 8.1 years. Of the 1,344,799 options outstanding as of December 31, 2013, 761,989 were exercisable with a weighted average exercise price of $0.15 per share and a weighted-average remaining contractual term of 7.3 years.
A summary of the stock options outstanding by range of exercise price is as follows:
| Year ended December 31, 2013 | ||||||||||||||||||||
| Range of Exercise Prices |
Options Outstanding |
Weighted Average Remaining Life |
Weighted Average Exercise Price |
Number of Options Currently Exercisable |
Weighted- Average Exercise Price per Vested Share |
|||||||||||||||
| $0.05–0.075 |
336,279 | 6.3 years | $ 0.07 | 324,404 | $ 0.07 | |||||||||||||||
| $0.15–0.25 |
429,520 | 7.8 years | $ 0.16 | 329,171 | $ 0.16 | |||||||||||||||
| $0.40 |
579,000 | 9.3 years | $ 0.40 | 108,414 | $ 0.40 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| 1,344,799 | 8.1 years | $ 0.24 | 761,989 | $ 0.15 | ||||||||||||||||
F-21
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
The Company used the Black-Scholes pricing model to determine the fair value of stock options with the fair value of each option grant being estimated on the date of the grant. This model incorporates various assumptions including expected volatility, expected term, risk-free interest rates, and expected dividend yields. The following assumptions were used for each respective period to calculate stock-based compensation for each stock option grant:
| Year Ended December 31, | ||||
| 2013 | 2012 | |||
| Risk–free rate |
0.14%-0.76% | 0.26%-0.50% | ||
| Expected dividend yield |
0% | 0% | ||
| Expected term (in years) |
5-6 | 6 | ||
| Expected volatility |
60% | 60% | ||
For purposes of using the Black-Scholes option-pricing model, and because the Company’s stock is not publicly traded, the volatility for options was determined based on an analysis of reported data for a peer group of companies. The expected volatility of options granted has been determined using an average of the historical volatility measures of this peer group. The expected life of options granted has been determined using the shortcut method prescribed in Staff Accounting Bulletin No. 107, Share-Based Payment. The risk-free interest rate is based on a Treasury instrument whose term is consistent with the expected life of the stock options. The Company has not paid, and does not anticipate paying or declaring, cash dividends on its shares of common stock; therefore, the expected dividend yield is assumed to be zero.
The total fair value of shares vested during the years ended December 31, 2013 and 2012, was $46,329 and $17,942, respectively. For the years ended December 31, 2013 and 2012, the Company recognized $65,734 and $24,693 respectively, of compensation expense related to stock option plans, classified as follows in the Consolidated Statements of Operations:
| Year Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| Research and development |
$40,321 | $16,556 | ||||||
| Sales and marketing |
2,781 | 58 | ||||||
| General and administrative |
22,632 | 8,079 | ||||||
| Total fair value of shares vested |
$65,734 | $24,693 | ||||||
At December 31, 2013 and 2012, there was $111,203 and $176,936, respectively, of total unrecognized compensation cost related to non-vested, share-based compensation arrangements. The cost is expected to be recognized over a weighted-average period of 3.5 years.
The intrinsic value of options is calculated as the difference between the exercise price of the underlying awards and the quoted price of the Company’s common stock for the options that were in-the-money at December 31, 2013. The intrinsic value of options outstanding at December 31, 2013 was $485,630. The intrinsic value of the fully vested options at December 31, 2013 was $341,632. The
F-22
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
aggregate intrinsic value of options exercised under the Company’s stock option plan was $0 and $4,092 for the year ended December 31, 2013 and 2012, respectively.
10. Income Taxes
The provision for income taxes consists of the following:
| Year Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| Deferred: |
||||||||
| Federal taxes |
$ 2,537,732 | $ 1,866,401 | ||||||
| State and local taxes, net of federal tax benefit |
207,904 | 152,222 | ||||||
|
|
|
|
|
|||||
| 2,745,636 | 2,018,623 | |||||||
| Valuation allowance |
(2,745,636) | (2,018,623) | ||||||
|
|
|
|
|
|||||
|
|
|
|
|
|||||
| Income tax |
$ - | $ - | ||||||
|
|
|
|
|
|||||
The provision for income taxes varies from the amount determined by applying the U.S. federal statutory rate to the loss before income taxes as a result of the following:
| Year Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| U.S. statutory income tax rate |
34.0% | 34.0% | ||||||
| State and local taxes, net of federal tax benefit |
2.8% | 2.8% | ||||||
| Permanent difference between book and tax |
(7.3)% | (4.9)% | ||||||
| Valuation allowances |
(29.5)% | (31.9)% | ||||||
|
|
|
|
|
|||||
| Effective income tax rate |
0.0% | 0.0% | ||||||
|
|
|
|
|
|||||
In assessing the realizability of deferred tax assets, the Company considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Based upon the level of historical taxable losses, and projections for future periods over which the deferred tax assets are deductible, the Company determined that a 100% valuation allowance of deferred tax assets was appropriate.
F-23
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
The components of the deferred tax asset are as follows:
| Year Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| Depreciation and amortization |
$ | 37,235 | $ | 39,991 | ||||
| Deferred compensation |
45,817 | 22,413 | ||||||
| State and federal net operating loss carryforwards |
7,654,610 | 4,927,934 | ||||||
|
|
|
|
|
|||||
| Deferred tax assets |
7,737,662 | 4,990,338 | ||||||
| Valuation allowance |
(7,737,662) | (4,990,338) | ||||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | - | $ | - | ||||
|
|
|
|
|
|||||
As of December 31, 2013 and 2012, the Company’s primary deferred tax assets consist of its net operating loss carryforwards. As of both December 31, 2013 and 2012, a valuation allowance was recorded against the entire deferred tax asset. As of December 31, 2013, the Company had $20,808,795 of net operating loss carryforwards. Unless otherwise utilized, net operating loss carryforwards will expire at various dates beginning in 2029 through 2033.
Utilization of the net operating losses are subject to annual limitations due to ownership change limitations that have occurred or that could occur in the future, as required by Section 382 of the Internal Revenue Code of 1986, as amended (the Code), as well as similar state and foreign provisions. These ownership changes may limit the amount of net operating loss carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an “ownership change” as defined by Section 382 of the Code results from a transaction or series of transactions over a three-year period resulting in an ownership change of more than 50 percentage points of the outstanding stock of a company by certain stockholders. The Company has not performed an analysis as to whether any of its credits are limited by Section 382.
The Company files income tax returns in the U.S. federal jurisdiction and various state and local jurisdictions. As the Company has a federal net operating loss carryforward from the year ended December 31, 2009 forward, all tax years from 2009 forward are subject to examination. As states have varying carryforward periods, the states are generally subject to examination for the previous 10 years or less. The Company also files income tax returns for its wholly owned subsidiary in The Netherlands. The Company has no operating results in The Netherlands for the periods presented, and accordingly had no foreign tax obligations.
No provision for uncertain tax positions was considered necessary at December 31, 2013 or 2012, respectively.
F-24
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
11. Net Loss per Common Share Attributable to Common Stockholders and Pro Forma Net Loss Per Share
The following table sets forth the number of common shares that were excluded from the computation of diluted earnings per share attributable to common stockholders because their inclusion would have been anti-dilutive as follows:
| December 31, 2013 |
December 31, 2012 |
|||||||
| Shares issued |
||||||||
| Unvested Restricted common awards |
800,000 | 800,000 | ||||||
| Shares issuable upon vesting/ exercise of: |
||||||||
| Options to purchase common stock |
1,344,799 | 800,279 | ||||||
| Warrants |
550,000 | 550,000 | ||||||
|
|
|
|
|
|||||
| 2,694,799 | 2,150,279 | |||||||
|
|
|
|
|
|||||
Pro forma net loss per common share calculation:
| December 31, 2013 |
||||
| Numerator: |
||||
| Net loss |
$ | (7,527,232) | ||
|
|
|
|||
| Denominator: |
||||
| Weighted average shares outstanding as presented |
1,403,721 | |||
| Weighted average shares issued upon conversion of convertible redeemable preferred stock |
9,868,339 | |||
| Weighted average shares issued upon conversion of Series A convertible preferred stock |
1,499,093 | |||
|
|
|
|||
| Pro forma weighted average shares outstanding |
12,771,153 | |||
|
|
|
|||
| Pro forma net loss per common share |
$ | (0.59) | ||
12. Segment Information
The Company considers all of its revenues to be part of a single operating segment. All of the Company’s revenues in the years ended December 31, 2013 and 2012, were to international customers. All of the Company’s long-lived assets are located in the United States.
During the 12 months ended December 31, 2013, the Company’s first full year of commercial activity, the Company’s revenue in Germany and Colombia was approximately 32% and 18% of total revenue for the period, respectively.
F-25
Table of Contents
| UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS |
||
| EndoStim, Inc. (A Development Stage Company) Six Months Ended June 30, 2014 and 2013, and Period From June 3, 2009 (Inception) to June 30, 2014 |
||
F-26
Table of Contents
Unaudited Consolidated Financial Statements
Six Months Ended June 30, 2014 and 2013, and
Period From June 3, 2009 (Inception) to June 30, 2014
Contents
| F-28 | ||
| F-29 | ||
| Unaudited Consolidated Statements of Stockholders’ Equity (Deficit) |
F-30 | |
| F-31 | ||
| F-32 | ||
F-27
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
| June 30, 2014 |
December 31, 2013 |
Pro Forma June 30, 2014 |
||||||||||
| (unaudited) | (unaudited) | |||||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
$ | 7,732,992 | $ | 5,389,003 | $ | 7,732,992 | ||||||
| Accounts receivable |
106,902 | 83,980 | 106,902 | |||||||||
| Inventory |
404,213 | 342,416 | 404,213 | |||||||||
| Deferred cost of revenue |
143,386 | 103,425 | 143,386 | |||||||||
| Prepaid expenses and other current assets |
324,136 | 359,709 | 324,136 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
8,711,629 | 6,278,533 | 8,711,629 | |||||||||
| Property and equipment, net |
7,181 | 11,653 | 7,181 | |||||||||
| Other long-term assets |
949,104 | 61,652 | 121,973 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
$ | 9,667,914 | $ | 6,351,838 | $ | 8,840,783 | ||||||
|
|
|
|
|
|
|
|||||||
| Liabilities and stockholders’ equity (deficit) |
||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 829,971 | $ | 500,691 | $ | 829,971 | ||||||
| Accrued expenses |
932,030 | 833,669 | 932,030 | |||||||||
| Deferred revenue |
277,179 | 203,159 | 277,179 | |||||||||
| Other current liabilities |
12,918 | 39,422 | 4,199 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
2,052,098 | 1,576,941 | 2,043,379 | |||||||||
| Convertible promissory note, net of debt discount |
4,345,281 | – | – | |||||||||
| Embedded derivative |
1,651,084 | – | – | |||||||||
| Commitment and contingencies |
– | – | – | |||||||||
| Series B convertible redeemable preferred stock, $0.001 par value; 3,999,951 shares authorized at June 30, 2014 and December 31, 2013; 3,999,951 shares issued and outstanding at June 30, 2014 and December 31, 2013; no shares authorized, issued or outstanding, pro forma | 7,565,175 | 7,248,707 | – | |||||||||
| Series B-1 convertible redeemable preferred stock, $0.001 par value; 2,466,218 shares authorized at June 30, 2014 and December 31, 2013; 2,466,218 shares issued and outstanding at June 30, 2014 and December 31, 2013; no shares authorized, issued or outstanding, pro forma | 6,515,272 | 6,253,890 | – | |||||||||
| Series C convertible redeemable preferred stock, $0.001 par value; 4,400,000 authorized at June 30, 2014 and December 31, 2013; 3,754,713 shares issued and outstanding at June 30, 2014 and December 31, 2013; no shares authorized, issued or outstanding, pro forma | 14,999,165 | 14,329,432 | – | |||||||||
| Stockholders’ equity (deficit): |
||||||||||||
| Series A convertible preferred stock, $0.001 par value; 1,499,093 shares authorized at June 30, 2014 and December 31, 2013; 1,499,093 shares issued and outstanding at June 30, 2014 and December 31, 2013, no shares authorized, issued or outstanding pro forma | 1,499 | 1,499 | – | |||||||||
| Common stock, $0.001 par value; 16,345,262 shares authorized; 2,203,721 shares issued and outstanding at June 30, 2014 and December 31, 2013; 13,923,696 shares outstanding, pro forma | 2,204 | 2,204 | 13,924 | |||||||||
| Additional paid-in capital |
2,039,792 | 1,573,455 | 31,624,510 | |||||||||
| Deficit accumulated during development stage |
(29,503,656) | (24,634,290) | (24,841,030) | |||||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity (deficit) |
(27,460,161) | (23,057,132) | 6,797,404 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities and stockholders’ equity (deficit) |
$ | 9,667,914 | $ | 6,351,838 | $ | 8,840,783 | ||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes.
F-28
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Consolidated Statements of Operations
(Unaudited)
| Six Months Ended June 30, |
Period From June 3, 2009 (Inception) to June 30, 2014 |
|||||||||||
| 2014 | 2013 | |||||||||||
| Revenue | $ | 324,837 | $ | 106,466 | $ | 659,619 | ||||||
| Cost of revenue |
151,496 | 62,995 | 369,471 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross margin | 173,341 | 43,471 | 290,148 | |||||||||
| Operating expenses: | ||||||||||||
| Research and development |
1,256,966 | 1,337,758 | 12,881,199 | |||||||||
| Sales and marketing |
1,392,508 | 1,056,177 | 4,650,386 | |||||||||
| General and administrative |
1,125,466 | 1,055,172 | 7,840,978 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses | 3,774,940 | 3,449,107 | 25,372,563 | |||||||||
| Loss from operations | (3,601,599) | (3,405,636) | (25,082,415) | |||||||||
| Other (expense) income: | ||||||||||||
| Grant income |
– | – | 244,478 | |||||||||
| Interest income |
1,113 | 3,206 | 18,204 | |||||||||
| Interest expense |
(21,297) | – | (21,297) | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other (expense) income |
(20,184 | ) | 3,206 | 241,385 | ||||||||
| Income tax | – | – | – | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss | (3,621,783) | (3,402,430) | (24,841,030) | |||||||||
| Accretion of redeemable preferred stock | (1,247,583) | (908,142) | (4,662,626) | |||||||||
| Undeclared dividends on Series A convertible preferred stock | (81,580) | (23,562) | (438,916) | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common stockholders | $ | (4,950,946) | $ | (4,334,134) | $ | (29,942,572) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss per common share attributable to common stockholders: | ||||||||||||
| Basic and diluted |
$ | (3.53) | $ | (3.09) | ||||||||
| Weighted-average shares used in computing net loss per common share attributable to common stockholders: | ||||||||||||
| Basic and diluted |
1,403,721 | 1,403,721 | ||||||||||
|
|
|
|
|
|||||||||
| Pro forma net loss per common share | ||||||||||||
| (See Note 12) | $ | (0.27) | ||||||||||
See accompanying notes.
F-29
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Consolidated Statements of Stockholders’ Equity (Deficit)
(Unaudited)
| Convertible Preferred Stock Series A |
Common Stock | Additional Paid-In Capital |
Accounts Receivable Sale of Stock |
Deficit Accumulated During Development Stage |
Total Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||
| Initial capital contribution |
– | $ | – | 805,000 | $ | 805 | $ | 24,450 | $ | (25,000) | $ | – | $ | 255 | ||||||||||||||||||
| Issuance of Series A preferred stock at $1.00 per share from the conversion of a bridge note into Series A preferred stock |
275,000 | 275 | – | – | 274,725 | – | – | 275,000 | ||||||||||||||||||||||||
| Issuance of Series A preferred stock at $1.00 per share, net of issuance costs of $75,747 |
476,593 | 476 | – | – | 400,370 | – | – | 400,846 | ||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (501,448 | ) | (501,448) | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2009 |
751,593 | 751 | 805,000 | 805 | 699,545 | (25,000 | ) | (501,448 | ) | 174,653 | ||||||||||||||||||||||
| Issuance of Series A shares at $1.00 per share |
747,500 | 748 | – | – | 746,752 | – | – | 747,500 | ||||||||||||||||||||||||
| Exercise of stock options |
– | – | 28,125 | 28 | 3,878 | – | – | 3,906 | ||||||||||||||||||||||||
| Grant of restricted shares |
– | – | 1,275,000 | 1,275 | (750 | ) | – | – | 525 | |||||||||||||||||||||||
| Payments on accounts receivable |
– | – | – | – | – | 25,000 | – | 25,000 | ||||||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | – | – | – | – | – | (170,658) | (170,658) | ||||||||||||||||||||||||
| Share-based compensation |
– | – | – | – | 9,152 | – | – | 9,152 | ||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (3,062,380) | (3,062,380) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2010 |
1,499,093 | 1,499 | 2,108,125 | 2,108 | 1,458,577 | – | (3,734,486) | (2,272,302) | ||||||||||||||||||||||||
| Exercise of stock options |
– | – | 50,000 | 50 | 3,700 | – | – | 3,750 | ||||||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | – | – | – | – | – | (506,002) | (506,002) | ||||||||||||||||||||||||
| Share-based compensation |
– | – | – | – | 18,049 | – | – | 18,049 | ||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (4,591,114) | (4,591,114) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2011 |
1,499,093 | 1,499 | 2,158,125 | 2,158 | 1,480,326 | – | (8,831,602) | (7,347,619) | ||||||||||||||||||||||||
| Exercise of stock options |
– | – | 45,596 | 46 | 2,702 | – | – | 2,748 | ||||||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | – | – | – | – | – | (853,561) | (853,561) | ||||||||||||||||||||||||
| Share-based compensation |
– | – | – | – | 24,693 | – | – | 24,693 | ||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (5,537,073) | (5,537,073) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2012 |
1,499,093 | 1,499 | 2,203,721 | 2,204 | 1,507,721 | – | (15,222,236) | (13,710,812) | ||||||||||||||||||||||||
| Accretion of redeemable preferred stock |
– | – | – | – | – | – | (1,884,822) | (1,884,822) | ||||||||||||||||||||||||
| Share-based compensation |
– | – | – | – | 65,734 | – | – | 65,734 | ||||||||||||||||||||||||
| Net loss |
– | – | – | – | – | – | (7,527,232) | (7,527,232) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2013 |
1,499,093 | $ | 1,499 | 2,203,721 | $ | 2,204 | $ | 1,573,455 | $ | – | $ | (24,634,290) | $ | (23,057,132) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Accretion of redeemable preferred stock |
- | - | - | - | - | - | (1,247,583) | (1,247,583) | ||||||||||||||||||||||||
| Share-based compensation |
- | - | - | - | 100,277 | - | - | 100,277 | ||||||||||||||||||||||||
| Issuance of warrant, net of offering costs |
- | - | - | - | 366,060 | - | - | 366,060 | ||||||||||||||||||||||||
| Net loss |
- | - | - | - | - | - | (3,621,783) | (3,621,783) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at June 30, 2014 |
1,499,093 | $ | 1,499 | 2,203,721 | $ | 2,204 | $ | 2,039,792 | $ | – | $ | (29,503,656) | $ | (27,460,161) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
See accompanying notes.
F-30
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Consolidated Statements of Cash Flows
(Unaudited)
| Six Months Ended June 30, |
Period From June 30, 2014 |
|||||||||||
| 2014 | 2013 | |||||||||||
|
|
|
|
|
|||||||||
| Operating activities |
||||||||||||
| Net loss |
$ (3,621,783) | $ (3,402,430) | $ | (24,841,030) | ||||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation |
4,472 | 4,471 | 20,428 | |||||||||
| Share-based compensation |
100,277 | 27,197 | 217,905 | |||||||||
| Interest accretion |
11,661 | – | 11,661 | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(22,922) | (51,613) | (106,902) | |||||||||
| Inventory |
(61,797) | (114,100) | (404,213) | |||||||||
| Deferred cost of revenue |
(39,961) | (84,130) | (143,386) | |||||||||
| Prepaid expenses and other current assets |
35,573 | (48,041) | (324,136) | |||||||||
| Other long-term assets |
61,652 | – | - | |||||||||
| Accounts payable |
329,280 | (269,657) | 829,971 | |||||||||
| Accrued expenses |
98,361 | (11,280) | 932,030 | |||||||||
| Deferred revenue |
74,020 | 120,654 | 277,179 | |||||||||
| Other current liabilities |
(26,504) | 4,017 | 12,918 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(3,057,671) | (3,824,912) | (23,517,575) | |||||||||
| Investing activities |
||||||||||||
| Purchase of equipment |
– | – | (27,609) | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
– | – | (27,609) | |||||||||
| Financing activities |
||||||||||||
| Proceeds from the issuance of convertible notes |
6,364,780 | – | 6,639,780 | |||||||||
| Proceeds from issuance of preferred and common stock | – | 4,946,367 | 26,878,964 | |||||||||
| Issuance costs for preferred and common stock and note payable | (14,016) | (399,546) | (1,301,868) | |||||||||
| Deferred financing costs |
(949,104) | – | (949,104) | |||||||||
| Proceeds from exercise of stock options |
– | – | 10,404 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
5,401,660 | 4,546,821 | 31,278,176 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase in cash and cash equivalents |
2,343,989 | 721,909 | 7,732,992 | |||||||||
| Cash and cash equivalents, beginning of period |
5,389,003 | 8,538,783 | – | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 7,732,992 | $ | 9,260,692 | $ | 7,732,992 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental schedule of non-cash financing activity: | ||||||||||||
| Accretion of redeemable preferred stock |
$ | (1,247,583) | $ | (908,142) | $ | (4,662,626) | ||||||
See accompanying notes.
F-31
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
(Unaudited)
1. Description of Business
EndoStim, Inc. (EndoStim or the Company) is a medical device company developing a novel treatment for severe gastroesophageal reflux disease, or GERD. EndoStim’s proprietary technology uses electrical stimulation of the lower esophageal sphincter (LES) to normalize esophageal functions and has been shown in clinical trials to improve esophageal pH and patient symptoms. In 2012, EndoStim obtained the right to apply the CE Mark to its device and has begun to commercialize its products in Europe and in other countries worldwide that recognize the CE Mark. Founded in 2009, EndoStim is a development-stage company headquartered in St. Louis, Missouri, U.S.A., with offices in The Hague, The Netherlands.
Since its inception, the Company’s principal activities involved obtaining capital, business development, performing research and development activities and funding prototype development. During 2012, the Company commenced international commercialization operations. The Company was classified as a development-stage company from its inception on June 3, 2009 through June 30, 2014.
Since its inception, the Company has generated significant losses, given that the Company is a development-stage organization that has recently begun commercialization of its products. As of June 30, 2014, the Company has incurred cumulative net losses attributable to common stockholders of $29,942,572. EndoStim expects to incur additional losses as it continues the commercialization of its product, conducts additional research and development activities, and funds additional sales and marketing initiatives throughout 2014. Historically, its cash needs for operating expenses have been met principally by proceeds from the issuance of preferred stock. The Company will be required to raise capital or pursue other financing strategies to continue operations. On June 25, 2014, the Company issued $6,364,780 in secured convertible promissory notes as described in Note 2. The Company believes that together with its existing cash, such amounts will be sufficient to fund its operations through 2014. Although the Company anticipates that it will be able to achieve adequate liquidity to fund future operations by raising additional investments from current or new investors and/or securing a credit facility, there is no assurance that the Company will be able to obtain such investment or financing on favorable terms, if at all. In the event the Company fails to raise additional financing, the Company would be required to delay seeking further regulatory approval for its products, delay commercialization of its products internationally, or reduce the sales, marketing, customer support or other resources devoted to its products and product development, any of which could have a material adverse effect on its business, financial condition and results of operations. In addition, the Company could be required to cease operations if the Company fails to raise additional financing. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
F-32
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited financial statements of EndoStim, Inc. have been prepared in accordance with generally accepted accounting principles for interim financial information and with the instructions to Form 10-Q. Accordingly, they do not include all the disclosures required by U.S. generally accepted accounting principles for complete financial statements. In the opinion of management, they include all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of the results for the interim periods presented. Operating results for the six months ended June 30, 2014 are not necessarily indicative of the results that may be expected for the year ended December 31, 2014 or for future operating periods.
These interim financial statements and related notes should be read in conjunction with the annual financial statements and notes.
Principles of Consolidation
The financial statements of the Company have been prepared in accordance with principles generally accepted in the United States (GAAP). The consolidated financial statements include the accounts of EndoStim, Inc. and its wholly owned subsidiary, EndoStim, BV located in The Netherlands which has de minimus operations and net assets. All intercompany accounts and transactions have been eliminated.
Accounting Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates, and such differences could be material to the financial position and results of operations.
Concentration of Risk and Other Uncertainties
The Company sells its products to distributors and directly to hospitals in various countries outside of the United States and grants credit to certain of these customers. The Company commenced international commercialization efforts in the fourth quarter of 2012.
F-33
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
During the six months ended June 30, 2014 and 2013, certain customers of the Company provided more than 10% of revenue for the respective period as follows:
| Six Months Ended June 30, |
||||||||
| 2014 | 2013 | |||||||
| Marienkrankenhaus Soest GmbH |
15 | % | 16 | % | ||||
| Klinikum Memmingen |
21 | % | * | |||||
| Hospimedics S.A. |
* | 28 | % | |||||
| Synmed Medicinteknik AB |
* | 15 | % | |||||
| EV Hospital Castrop-Rauxel |
15 | % | 11 | % | ||||
* less than 10%
The Company relies upon a single third-party supplier located in Uruguay to produce its finished products for sale.
The Company currently maintains the majority of its cash and cash equivalents with two high-credit-quality financial institutions in the United States and deposits in excess of $250,000 are not Federal Deposit Insurance Corporation insured. The Company maintains a bank account in the Netherlands to support its European operations. Cash equivalents are accounted for at cost, which approximates fair value. The Company believes that it is not exposed to significant credit risk due to the financial position of the depository institutions in which these deposits are held and of the money market funds in which investments are made. Additionally, the Company’s investment policy is designed to preserve principal and provide liquidity.
Cash and Cash Equivalents
For the purposes of the consolidated statement of cash flows, the Company considers all short-term deposits purchased with original maturities of three months or less and money market funds to be cash equivalents.
Accounts Receivable and Allowance for Uncollectible Accounts
During the ordinary course of its business, the Company grants unsecured credit to its customers, principally hospitals. Accounts receivable are carried at cost less an allowance for doubtful accounts. On a regular basis, the Company evaluates accounts receivable and estimates an allowance for doubtful accounts, as needed, based on various factors such as customers’ current credit conditions, length of time past due and the general economic conditions. As of June 30, 2014 and December 31, 2013, the Company has not established a reserve because the Company believes that the amounts outstanding are fully collectible.
F-34
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Inventory
Inventory principally consists of finished goods that are purchased from a third-party supplier. Inventory is valued at the lower of the actual cost of the inventory, as determined using the first-in, first-out (FIFO) method or its net realizable value. Actual cost of inventory includes purchased cost and freight costs. The Company periodically reviews its physical inventory for excess, obsolete or potentially impaired items, taking into account product development plans, product expiration and current inventory levels. As of June 30, 2014 and December 31, 2013, the Company has not established a reserve because, based on the Company’s estimate of future use, the Company does not believe that it has excess, obsolete or impaired inventory.
Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value hierarchy that prioritizes the use of inputs used in valuation techniques is as follows:
Level 1—quoted prices in active markets for identical assets and liabilities;
Level 2—observable inputs other than quoted prices in active markets, such as quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data;
Level 3—unobservable inputs reflecting management’s assumptions, consistent with reasonably available assumptions made by other market participants. These valuations require significant judgment.
Refer to Note 8 for a discussion of the Company’s fair value measurements.
Revenue
The Company’s revenue consists of product revenue resulting from the sale of its programmable Implantable Pulse Generators (IPG) and programmers, which communicate wirelessly with the IPGs. All product sales originate from the United States. The Company recognizes revenue when all four revenue recognition criteria have been met: persuasive evidence of an arrangement exists; delivery has occurred or service has been rendered; the price is fixed or determinable; and collectability is reasonably assured. The Company sells its products to distributors with substantial independent operations under sales arrangements whose terms do not allow for rights of return or price protection on unsold products held by them. The Company’s revenue recognition policy generally results in revenue recognition at delivery to the hospital or distributor for programmers and upon implant into patient for IPGs. The Company defers revenue on the sale of IPGs until implant into the patient as the Company may grant concessions allowing for the resterilization or exchange of IPGs upon expiration. Amounts collected prior to satisfying the above revenue recognition criteria are reflected as deferred revenue, and the corresponding cost of revenue is reflected as deferred cost of revenue in the consolidated balance sheets. For certain transactions, the Company has multiple-element
F-35
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
arrangements that include the IPGs and programmer. For multiple-element arrangements, revenue is allocated to each element based on its relative selling price. Relative selling prices are based first on vendor-specific objective evidence (VSOE), then on third-party evidence of selling price (TPE) when VSOE does not exist, and then on estimated selling price (ESP) when VSOE and TPE do not exist. The Company does not have VSOE for the IPG and programmer. The Company recently began commercialization of its products in many different countries, each of which is expected to have a different fair value, and does not yet have enough data on stand-alone transactions to establish VSOE in any of the markets in which it is commercializing its products. Because the Company has neither VSOE in any of the markets in which it is commercializing nor TPE for its products, the allocation of revenue has been based on the Company’s ESPs. The objective of ESP is to determine the price at which the Company would transact a sale if the product was sold on a stand-alone basis. The Company determines ESP for its products by considering multiple factors, including, but not limited to, geography and market conditions, reimbursement applications specific to a particular country and price lists provided to customers. When billed to the customer, shipping and handling costs are included in revenue and cost of revenue, and are immaterial for the periods presented. Costs of revenue include direct product costs and overhead costs, shipping costs and estimated warranty costs.
Accounting for Stock-Based Compensation
The Company accounts for its stock-based compensation in accordance with Accounting Standards Codification (ASC) 718, Compensation—Stock Compensation, which establishes accounting for share-based awards, including stock options and restricted stock, exchanged for services and requires companies to expense the estimated fair value of these awards over the requisite service period.
Basic and Diluted Net Loss per Share of Common Stock
Basic net loss per share of common stock is computed by dividing net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period. Net loss attributable to common stockholders includes deductions for the accretion of convertible redeemable preferred stock and undeclared dividends on Series A convertible preferred stock which accumulate dividends. Diluted net loss per share of common stock is computed by dividing the net loss attributable to common stockholders by the sum of the weighted-average number of common shares outstanding during the period plus the potential dilutive effects of convertible preferred stock outstanding during the period calculated in accordance with the if-converted method, as well as outstanding options and warrants, if any, to the extent their inclusion is not anti-dilutive. The Company excluded the effect of the shares issuable upon conversion of the 2014 Notes because it is not possible to determine the number of shares into which the 2014 Notes will convert until the date of conversion. Because the impact of these items is anti-dilutive during periods presented, there was no difference between basic and diluted net loss per share of common stock attributable to common stockholders for the six months ended June 30, 2014 and 2013.
Subsequent Events
Events and transactions were evaluated for potential recognition or disclosure through September 5, 2014, which is the date the consolidated financial statements were issued.
F-36
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
On August 28, 2014, the Company issued $1,135,220 in aggregate principal amount of secured convertible promissory notes substantially on the same terms as the notes issued on June 25, 2014.
Pro Forma Disclosures of the Effect of Convertible Redeemable Preferred Stock and Convertible Promissory Note
The pro forma adjustments reflected in the June 30, 2014 balance sheet assume the conversion of the redeemable convertible preferred shares, the convertible preferred shares then outstanding but excluding accreted dividends in arrears and the convertible promissory note and embedded derivative, to common shares upon an initial public offering of the Company’s common stock. The pro forma adjustments to reflect the conversion resulted in: i) a reduction to the redeemable preferred stock and convertible preferred stock amounts to zero, ii) the reversal of accretion for unpaid dividends accumulated in arrears as unpaid dividends in arrears do not convert into common stock upon an initial public offering, iii) a reduction to the convertible promissory note and embedded derivative to reflect the conversion of these items into additional paid in capital and iv) an increase to common stock and additional-paid-in-capital to reflect the conversion into common shares. Because the Company cannot determine the number of shares into which the convertible promissory note will convert until the date of conversion, common stock was not adjusted and the entire amount was reflected within additional paid in capital. Certain deferred financing costs related to the transactions were reduced from other long-term assets and a corresponding entry was made to reduce additional-paid-in-capital. Accrued interest related to the convertible note was reduced from other current liabilities and a corresponding entry was made to increase additional-paid-in capital.
Recently Issued Accounting Pronouncements
The Financial Accounting Standards Board (the FASB) and the International Accounting Standards Board (the IASB) (collectively, the Boards) have jointly issued a new revenue recognition standard that will supersede virtually all revenue recognition guidance in US GAAP and IFRS. The new revenue standard creates a single source of revenue guidance for companies, regardless of industry and is more principles-based than the current revenue guidance. The new standard is effective for public entities for fiscal years beginning after December 15, 2016 and for interim periods therein. Early adoption is not permitted for public entities. The Company has not yet evaluated the transition approach or the impact of this standard on its results of operations or its financial condition.
3. Property and Equipment, net
Property and equipment consists of the following:
| June 30, 2014 | December 31, 2013 | |||||||
| Research and development equipment |
$ 27,609 | $ 27,609 | ||||||
| Less: accumulated depreciation |
(20,428) | (15,956) | ||||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ 7,181 | $ 11,653 | ||||||
|
|
|
|
|
|||||
F-37
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
4. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of the following:
| June 30, 2014 | December 31, 2013 | |||||||
| Deposits, principally on inventory purchases |
$ 228,733 | $ 256,903 | ||||||
| Prepaid insurance and other prepaid expenses |
72,871 | 58,647 | ||||||
| Other current assets |
22,532 | 44,159 | ||||||
|
|
|
|
|
|||||
| Total prepaid expenses and other current assets |
$ 324,136 | $ 359,709 | ||||||
|
|
|
|
|
|||||
5. Other Long-term Assets
Other long-term assets consist of the following:
| June 30, 2014 | December 31, 2013 | |||||||
| Deferred financing |
$ 949,104 | $ - | ||||||
| Other long-term assets |
- | 61,652 | ||||||
|
|
|
|
|
|||||
| Total long-term assets |
$ 949,104 | $ 61,652 | ||||||
|
|
|
|
|
|||||
6. Accrued Expenses
Accrued expenses consist of the following:
| June 30, 2014 | December 31, 2013 | |||||||
| Accrued salaries, bonuses and benefits |
$ 282,148 | $ 247,719 | ||||||
| Accrued research and development costs |
428,352 | 510,600 | ||||||
| Accrued legal and professional fees |
144,100 | 52,850 | ||||||
| Accrued other |
77,430 | 22,500 | ||||||
|
|
|
|
|
|||||
| Total accrued expenses |
$ 932,030 | $ 833,669 | ||||||
|
|
|
|
|
|||||
7. Long-term debt
On June 25, 2014, the Company entered into a Note and Warrant Purchase Agreement (the “2014 Note Agreement”) pursuant to which the Company issued $6,364,780 in secured convertible promissory notes (the “2014 Notes”). Interest on all amounts outstanding accrues at a rate of 10% per annum with interest after the first year payable in cash. The 2014 Notes mature on June 25, 2016, unless earlier converted. The 2014 Notes are secured by all of the Company’s current or after-acquired assets and may be subordinated to any senior indebtedness and to any liens securing such senior indebtedness. Amounts outstanding under the 2014 Notes convert automatically on the first to occur of the following:
| — | In the event the Company consummates an underwritten public offering pursuant to which the Company receives at least $10,000,000 from the sale of its equity securities |
F-38
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
| in such offering, or a qualified initial public offering as outlined in the 2014 Note Agreement, all outstanding principal and accrued but unpaid interest under the 2014 Notes will automatically convert into fully paid and nonassessable shares of the equity securities issued in the qualified initial public offering at a price per share equal to 80% of the price to the public (before underwriting discounts and commissions and other offering expenses); or |
| — | In the event the Company completes a transaction or series of transactions pursuant to which the Company issues and sells shares of a new series of its preferred stock for aggregate gross proceeds of at least $10,000,000 with the principal purpose of raising capital, or a qualified financing as outlined in the 2014 Note Agreement, all outstanding principal and accrued but unpaid interest under the 2014 Notes will automatically convert into fully paid and nonassessable shares of the class of the Company’s stock issued in such qualified financing at a price per share equal to 80% of the lowest price paid by investors. |
The Company determined that the conversion features represented an embedded derivative instrument because the features were not clearly and closely related to the 2014 Notes. Consequently, the features were bifurcated from the associated debt instrument and recorded as a derivative instrument liability at fair value. As of June 30, 2014, the Company recorded $1,651,084 for the embedded derivative within an Embedded Derivatives line item in long-term liabilities on the Consolidated Balance Sheet.
Under the 2014 Note Agreement, the Company agreed to issue warrants to purchase up to 339,453 shares of the Company’s common stock at an exercise price of $0.50 per share to the investors in the 2014 Notes. These warrants were determined to be free standing instruments and classified as equity. The proceeds from the issuance of the 2014 Notes were allocated to the 2014 Notes and warrants based on their relative fair values: $5,984,704 was allocated to the 2014 Notes (inclusive of the embedded features noted above) and $380,076 was allocated to the warrants. The fair value of the warrants was determined using the Monte Carlo simulation described below. Debt issuance costs were allocated between the debt and the warrant using the relative fair value method. Debt issuance costs related to the 2014 Notes will be expensed using the effective interest method over the underlying term of the 2014 Notes.
Upon issuance of the 2014 Notes on June 25, 2014, the Company allocated the proceeds and deferred financing costs as follows:
|
Allocation of Proceeds |
Deferred Financing Costs |
|||||||
| Convertible 2014 Notes |
$ 4,333,620 | $ 159,808 | ||||||
| Embedded derivative |
1,651,084 | - | ||||||
| Warrant |
380,076 | 14,016 | ||||||
|
|
|
|
|
|||||
| Total shares |
$ 6,364,780 | $ 173,824 | ||||||
|
|
|
|
|
|||||
F-39
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
The 2014 Notes will be accreted from the initial recorded value to the face value of $6,364,780 with a charge to interest expense, using the effective interest method over the term of the 2014 Notes. The accretion of the debt discount and amortization of the offering costs resulted in additional interest expense of $12,578 during the six months ended June 30, 2014. The embedded derivative liability will be re-measured at fair value at the end of each reporting period or when settled and any changes will be recognized as interest expense in the Statement of Operations.
Long-term debt consists of the following:
| June 30, 2014 | ||||||
| Convertible 2014 Notes |
$ 4,333,620 | |||||
| Accretion of debt discount |
11,661 | |||||
|
|
|
|||||
| Total long-term debt |
$ 4,345,281 | |||||
|
|
|
|||||
|
|
|
| ||||
8. Fair Value Measurements
Recurring fair value measurements
The Company measures certain financial assets and liabilities at fair value on a recurring basis, consistent with the fair value hierarchy provisions described above in Note 2. At June 30, 2014, the Company’s embedded derivative associated with its 2014 Notes was valued using “Level 3” inputs and was determined to be $1,651,084.
The fair value of the 2014 Notes embedded derivative was calculated by using the “with and without” approach; whereby, the value of the embedded derivative was determined as the difference between the value of the related debt host instrument with the embedded features and the value of the debt host instrument without the embedded derivative features. The value of the debt host instrument with the embedded features was determined using a Monte Carlo simulation model. A Monte Carlo simulation uses statistical sampling to predict outcomes based on variable inputs. This valuation methodology uses Level 3 inputs and requires the Company to make assumptions related to the probabilities of various outcomes. The significant unobservable inputs used in the Company’s fair value measurement include the probability and price of conversion. Because the Company’s stock has not traded publicly, the Company estimated the future volatility based on the experience of other entities considered comparable. The Company considered whether the instrument would be held to maturity or whether it would convert into equity in a qualified financing as defined in the agreement and assumed that it was 90% likely that the instrument would be converted and 10% likely that it would be held to maturity. The Company also considered the effect of its current capitalization structure. Further considerations included the timing of any such transaction, the range of projected stock prices and expected volatility of 70%, interest rate of 12% and a discount for lack of maturity of 10%.
There were no transfers between Level 1, 2 or 3 during the six months ended June 30, 2014 and 2013. There were no changes in the fair value of the embedded derivative during the six months ended June 30, 2014 as the Company determined that the fair value of the embedded derivative had not changed between the date the 2014 Notes were issued, June 25, 2014, and June 30, 2014.
F-40
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
Fair value of the other financial instruments
The fair values of the Company’s other financial instruments, including cash and cash equivalents, accounts receivable, and accounts payable approximates fair value based on the short-term nature of these financial instruments.
The fair value of the Company’s convertible promissory note (excluding valuation of the embedded derivative discussed above) as of June 30, 2014 was approximately $6,100,000 compared to the carrying value of $4,345,281. The Company measures the fair value of its convertible promissory notes based on the estimated borrowing rate of 12% to discount the cash flows to their present value (Level 2 valuation).
9. Commitments and Contingencies
As of June 30, 2014, the Company had an outstanding purchase commitment to the supplier of its product in the amount of $792,554, net of deposits. The Company intends to take delivery of these products through 2015. Should the Company cancel the purchase order or refuse to take delivery of the items, the Company would be liable for any materials purchased and work performed at the date of cancellation.
The Company leases its office space under a two-year operating lease through April 30, 2014 which was subsequently renewed in May 2014 on a month-to-month basis. For the six months ended June 30, 2014 and 2013, rent expense was $20,507 and $15,950, respectively. Amounts are due monthly under this lease in the amount of $3,418.
The Company leases a printer under a three-year operating lease. For the six months ended June 30, 2014 and 2013, the Company expensed $1,651 and $564 under this lease. Amounts are due monthly under this lease in the amount of $275 through May 2016.
10. Convertible Redeemable Preferred Stock
The Company assesses the terms of its redeemable preferred stock as liabilities or temporary equity within the consolidated balance sheets based on the terms of the preferred stock agreements. To the extent the redemption is mandatory or would require the Company to settle in an undeterminable number of common shares that may not be solely within the control of the Company to issue, the Company would record the securities as liabilities. The Company does not have any redeemable preferred stock classified as liabilities for the periods ended June 30, 2014 and December 31, 2013.
The Series B, B-1 and C convertible redeemable preferred stock was recorded at its initial carrying amount, which is generally its fair value on the date of issuance within temporary equity. The redeemable preferred shares are not currently redeemable but probable of becoming redeemable at the sixth anniversary from the issuance date at the option of the holders. The Company has accreted all accrued and unpaid dividends commencing from the date of issuance at a rate of 8% per annum using the effective interest method and accordingly, the convertible redeemable preferred stock is recorded at its redemption value. The amount of accrued unpaid dividends on redeemable preferred stock within temporary equity is reflected as an increase to accumulated deficit.
F-41
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
The following is a summary of the Company’s convertible redeemable preferred stock activity for the six months ended June 30, 2014.
| Preferred Stock | Preferred Stock | Preferred Stock | ||||||||||||||||||||||||||
| Series B | Series B-1 | Series C | ||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Total | ||||||||||||||||||||||
| Balance at December 31, |
3,999,951 | $ | 7,248,707 | 2,466,218 | $ | 6,253,890 | 3,754,713 | $ | 14,329,432 | $ | 27,832,029 | |||||||||||||||||
| Accretion of redeemable preferred stock |
– | 316,468 | – | 261,382 | – | 669,733 | 1,247,583 | |||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
| Balance at June 30, 2014 |
3,999,951 | $ | 7,565,175 | 2,466,218 | $ | 6,515,272 | 3,754,713 | $ | 14,999,165 | $ | 29,079,612 | |||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
11. Stockholders’ Equity
Common Stock
The Company has outstanding shares of common stock that are subject to the Company’s right to repurchase at the original issuance price upon the occurrence of certain events as defined in the agreements.
The Company has reserved shares of common stock for the conversion of preferred stock, the exercise of warrants, and the issuance of options granted and outstanding under the Company’s stock option plan as follows:
| June 30, 2014 | December 31, 2013 | |||||||
| Convertible preferred stock |
12,365,262 | 12,365,262 | ||||||
| Warrants |
889,453 | 550,000 | ||||||
| Stock option plan |
1,335,946 | 1,344,799 | ||||||
|
|
|
|
|
|||||
| Total shares |
14,590,661 | 14,260,061 | ||||||
|
|
|
|
|
|||||
Warrants
In 2009, the Company issued 8% Convertible Promissory Notes and the holders of the 8% Convertible Promissory Notes also received warrants to purchase 550,000 shares of common stock at $0.01 per share which are exercisable through July 2019. The warrants were determined to be freestanding instruments upon issuance and only provide for physical share settlement. The warrants are classified as equity instruments at fair value as of the issuance date of July 1, 2009; however, the fair value ($16,500) of these warrants was determined to be immaterial and therefore no amounts were recorded at the date of issuance. The warrants are outstanding at June 30, 2014.
The holders of the 2014 Notes received warrants to purchase 339,453 shares of common stock at $0.50 per shares which are exercisable through June 25, 2019. The warrants were determined to be
F-42
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
freestanding instruments upon issuance and only provide for physical share settlement. The warrants have a fair value of $380,076 and were classified in equity on the date of issuance. All of the warrants are outstanding at June 30, 2014.
Share-Based Compensation
In 2009, the Board of Directors adopted a stock incentive plan (the 2009 Stock Incentive Plan) which, as amended, authorizes for issuance a total of 1,600,000 shares of common stock. The 2009 Stock Incentive Plan allows for the grant of incentive stock options and non-qualified stock options to employees, board members and consultants. Options granted under the 2009 Stock Incentive Plan expire no later than 10 years from the date of grant. The exercise price of each incentive stock option shall not be less than 100% of the fair value of the stock subject to the option on the date the option is granted. The vesting provisions of individual options may vary, but option grants generally vest ratably over a period of one to four years.
The Board of Directors approved performance-based restricted common share awards. Performance-based restricted shares vest upon the achievement of performance objectives, which are based on the achievement of specified returns at the time of a liquidation event, which is defined in the agreement and includes an initial public offering, consummation of the sale or exclusive license of a significant portion of the Company’s assets, or the stockholders of the Company consummate a merger or consolidation of the Company with any other entity. There is no maximum term associated with such grants. As of June 30, 2014 and 2013, the Company had 800,000 performance-based restricted shares outstanding. During the period ended June 30, 2014, the Company determined that it was not probable that the performance conditions related to its outstanding restricted share awards would be achieved and accordingly has not recorded share-based compensation expense related to these awards.
As of June 30, 2014 and December 31, 2013, 942,751 and 761,989 options were vested and outstanding under the 2009 Stock Incentive Plan, respectively, at a weighted-average exercise price of $0.19 and $0.15, respectively.
A summary of the options activity for the six month period ended June 30, 2014 is as follows:
| Number of Shares |
Range of Exercise Prices |
Weighted- Average Exercise Price per Share |
| |||||||||||
| Outstanding, December 31, 2013 |
1,344,799 | $ 0.05–0.40 | $ 0.24 | |||||||||||
|
|
|
|
|
|
| |||||||||
| Granted |
125,000 | 0.40-0.50 | 0.48 | |||||||||||
| Exercised |
- | - | - | |||||||||||
| Forfeited |
(133,853) | 0.15–0.40 | 0.38 | |||||||||||
|
|
|
|
|
|
| |||||||||
| Outstanding, June 30, 2014 |
1,335,946 | $ 0.05–0.50 | $ 0.25 | |||||||||||
|
|
|
|
|
|
| |||||||||
F-43
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
For the six months ended June 30, 2014 and 2013, the Company recognized $100,277 and $27,197 respectively, of compensation expense related to stock option plans.
At June 30, 2014 and December 31, 2013, there was $215,752 and $111,203 respectively, of total unrecognized compensation cost related to non-vested, share-based compensation arrangements.
12. Net Loss per Common Share Attributable to Common Stockholders and Pro Forma Net Loss Per Share
The following table sets forth the number of common shares that were excluded from the computation of diluted earnings per share attributable to common stockholders because their inclusion would have been anti-dilutive as follows:
| June 30, 2014 | June 30, 2013 | |||||||
| Shares issued |
||||||||
| Unvested restricted common awards |
800,000 | 800,000 | ||||||
| Shares issuable upon vesting/ exercise of: |
||||||||
| Options to purchase common stock |
1,335,946 | 1,225,634 | ||||||
| Warrants |
889,453 | 550,000 | ||||||
|
|
|
|
|
|||||
| 3,025,399 | 2,575,634 | |||||||
|
|
|
|
|
|||||
The Company has excluded shares issuable upon the conversion of the 2014 Notes because the Company cannot determine the number of shares into which the 2014 Note will convert until the date of conversion.
F-44
Table of Contents
EndoStim, Inc.
(A Development Stage Company)
The following table sets for the pro forma net loss per common share calculation as follows:
| Six months ended |
||||
| Numerator: |
||||
| Net loss |
$(3,621,783) | |||
| Add back interest expense on 2014 Notes |
21,297 | |||
|
|
|
|||
| Net loss before interest on 2014 Notes |
$(3,600,486) | |||
|
|
|
|||
| Denominator: |
||||
| Weighted average shares outstanding as presented |
1,403,721 | |||
| Weighted average shares issued upon conversion of convertible redeemable preferred stock |
10,220,882 | |||
| Weighted average shares issued upon conversion of Series A convertible preferred stock |
1,499,093 | |||
| Shares issuable on conversion of 2014 Notes |
[ ] | |||
|
|
|
|||
| Pro forma weighted average shares outstanding |
13,123,696 | |||
|
|
|
|||
| Pro forma net loss per common share |
$ | (0.27) | ||
The Company cannot determine the number of shares into which the 2014 Notes will convert until the date of conversion and consequently, such shares were excluded from the pro forma weighted average shares outstanding calculation.
F-45
Table of Contents
Shares

Common Stock
PROSPECTUS
Wedbush PacGrow Life Sciences
| Craig-Hallum Capital Group | Roth Capital Partners |
, 2014
Through and including , 2014 (the 25th day after the date of this offering), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable in connection with the registration of the common stock hereunder. All amounts are estimates, except the SEC registration fee, the FINRA filing fee and The NASDAQ Capital Market listing fee.
| AMOUNT | ||||
| SEC registration fee |
$ | 5,184.20 | ||
| FINRA filing fee |
6,037.50 | |||
| NASDAQ Capital Market listing fee |
* | |||
| Accountants’ fees and expenses |
* | |||
| Legal fees and expenses |
* | |||
| Blue Sky fees and expenses |
* | |||
| Transfer Agent’s fees and expenses |
* | |||
| Printing and engraving expenses |
* | |||
| Miscellaneous |
* | |||
| Total |
$ | * | ||
| * | To be filed by amendment |
Item 14. Indemnification of Directors and Officers.
Section 102(b)(7) of the Delaware General Corporation Law, or DGCL, provides that a Delaware corporation, in its certificate of incorporation, may limit the personal liability of a director to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
| • | transaction from which the director derived an improper personal benefit; |
| • | act or omission not in good faith or that involved intentional misconduct or a knowing violation of law; |
| • | unlawful payment of dividends or redemption of shares; or |
| • | breach of the director’s duty of loyalty to the corporation or its stockholders. |
Section 145(a) of the DGCL provides, in general, that a Delaware corporation may indemnify any person who was or is a party, or is threatened to be made a party, to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) because that person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or other enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, so long as the person acted in good faith and in a manner he or she reasonably believed was in or not opposed to the corporation’s best interests, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
Section 145(b) of the DGCL provides, in general, that a Delaware corporation may indemnify any person who was or is a party, or is threatened to be made a party, to any threatened, pending or completed action or suit by or in the right of the corporation to obtain a judgment in its favor because the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or other enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action, so long as the person acted in good faith and in a manner the person reasonably believed was in or
II-1
Table of Contents
not opposed to the corporation’s best interests, except that no indemnification shall be permitted without judicial approval if a court has determined that the person is to be liable to the corporation with respect to such claim. Section 145(c) of the DGCL provides that, if a present or former director or officer has been successful in defense of any action referred to in Sections 145(a) and (b) of the DGCL, the corporation must indemnify such officer or director against the expenses (including attorneys’ fees) he or she actually and reasonably incurred in connection with such action.
Section 145(g) of the DGCL provides, in general, that a corporation may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or other enterprise against any liability asserted against and incurred by such person, in any such capacity, or arising out of his or her status as such, whether or not the corporation could indemnify the person against such liability under Section 145 of the DGCL.
Our amended and restated certificate of incorporation and our amended and restated bylaws, each of which will be effective upon the completion of this offering, provide for the indemnification of our directors and officers to the fullest extent permitted under the DGCL.
We have entered into separate indemnification agreements with our directors and officers in addition to the indemnification provided for in our amended and restated bylaws. These indemnification agreements provide, among other things, that we will indemnify our directors and officers for certain expenses, including damages, judgments, fines, penalties, settlements and costs and attorneys’ fees and disbursements, incurred by a director or officer in any claim, action or proceeding arising in his or her capacity as a director or officer of our company or in connection with service at our request for another corporation or entity. The indemnification agreements also provide for procedures that will apply in the event that a director or officer makes a claim for indemnification.
We also maintain a directors’ and officers’ insurance policy pursuant to which our directors and officers are insured against liability for actions taken in their capacities as directors and officers.
We have entered into an underwriting agreement, which provides for indemnification by the underwriters of us, our officers and directors, for certain liabilities, including liabilities arising under the Securities Act of 1933, as amended, or the Securities Act.
See also the undertakings set out in response to Item 17 herein.
Item 15. Recent Sales of Unregistered Securities.
The following lists set forth information regarding all securities sold or granted by us within the past three years that were not registered under the Securities Act, and the consideration, if any, received by us for such securities:
| (1) | Between July 13, 2010 and May 24, 2011, we issued and sold to accredited investors an aggregate of 3,999,951 shares of our Series B convertible preferred stock in exchange for cash at a price per share of $1.50 for gross proceeds of $6.0 million. Each share of Series B convertible preferred stock will convert into one share of our common stock upon completion of this offering. |
| (2) | Between November 22, 2011 and May 18, 2012, we issued and sold to accredited investors an aggregate of 2,466,218 shares of our Series B-1 convertible preferred stock in exchange for cash at a price per share of $2.25 for gross proceeds of $5.5 million. Each share of Series B–1 convertible preferred stock will convert into one share of our common stock upon completion of this offering. |
| (3) | Between December 5, 2012 and May 15, 2013 we issued and sold to accredited investors an aggregate of 3,754,713 shares of our Series C convertible preferred stock in exchange for cash at a price per share of $3.75, for gross proceeds of $14.1 million. Each share of Series C convertible preferred stock will convert into one share of our common stock upon completion of this offering. |
II-2
Table of Contents
| (4) | On June 25, 2014, we issued convertible promissory notes to accredited investors for an aggregate principal amount of $6,364,780. Such notes are automatically convertible into shares of common stock or preferred stock on terms set forth in the notes. |
| (5) | On June 25, 2014, in connection with the convertible note offering, we granted to accredited investors warrants to purchase 339,453 shares of our common stock at an exercise price of $0.50 per share, which are expected to remain outstanding upon completion of this offering. |
| (6) | On August 28, 2014, we issued convertible promissory notes to accredited investors for an aggregate principal amount of $1,135,220. Such notes are automatically convertible into shares of common stock or preferred stock on terms set forth in the notes. |
| (7) | On August 28, 2014, in connection with the convertible note offering, we granted warrants to accredited investors to purchase 60,545 shares of our common stock at an exercise price of $0.50 per share, which are expected to remain outstanding upon completion of this offering. |
| (8) | Since January 1, 2011, we have granted stock options to purchase an aggregate of 1,225,500 shares of our common stock with an average exercise price of $0.32 per share to our employees and directors pursuant to the 2009 Plan. |
| (9) | Since January 1, 2011 we have issued an aggregate of 95,596 shares of our common stock at prices ranging from $0.05 to $0.15 per share to certain of our employees, directors and consultants pursuant to the exercise of stock options under the 2009 Plan. |
We deemed the offers, sales and issuances of the securities described in paragraphs (1) through (9) above to be exempt from registration under the Securities Act, in reliance on Section 4(a)(2) of the Securities Act, including for the securities described in paragraphs (1) through (7), Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving a public offering. All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options described in paragraph (8) above and the issuance of shares discussed in paragraph (9) above, except to the extent described above as exempt pursuant to Section 4(a)(2) of the Securities Act, to be exempt from registration under the Securities Act in reliance on Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All of the foregoing securities are deemed restricted securities for purposes of the Securities Act. The certificates representing the securities issued in the transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
See the Index to Exhibits attached to this registration statement, which is incorporated by reference herein.
(b) Financial Statement Schedules
No financial statement schedules are provided, because the information called for is not required or is shown either in the financial statements or the notes thereto.
II-3
Table of Contents
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | The registrant will provide to the underwriters at the closing as specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser. |
| (2) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (3) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of St. Louis, in the State of Missouri, on this 5th day of September, 2014.
| ENDOSTIM, INC. | ||
| By: |
/s/ Bevil J. Hogg | |
| Bevil J. Hogg | ||
| Chief Executive Officer | ||
Each person whose individual signature appears below hereby authorizes and appoints Bevil J. Hogg and Margaret S. Stohr and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file any and all amendments to this registration statement, including any and all post-effective amendments and amendments thereto, and any subsequent registration statement relating to the same offering as this registration statement that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
| Signature |
Title |
Date | ||
| /s/ Bevil J. Hogg Bevil J. Hogg |
Director, President and Chief Executive Officer (Principal Executive Officer) |
September 5, 2014 | ||
| /s/ Peggy S. Stohr Peggy S. Stohr |
Chief Financial Officer (Principal Financial and Accounting Officer) |
September 5, 2014 | ||
| /s/ Douglas D. French Douglas D. French |
Chairman of the Board | September 5, 2014 | ||
| /s/ Raul E. Perez Raul E. Perez |
Director | September 5, 2014 | ||
| /s/ Ralph G. Dacey, Jr. Ralph G. Dacey, Jr. |
Director | September 5, 2014 | ||
| /s/ Fred C. Goad Fred C. Goad |
Director | September 5, 2014 | ||
| /s/ Jeffrey M. McDonnell Jeffrey M. McDonnell |
Director | September 5, 2014 | ||
| /s/ Virender K. Sharma Virender K. Sharma |
Director | September 5, 2014 | ||
Table of Contents
INDEX TO EXHIBITS
| EXHIBIT |
EXHIBIT DESCRIPTION | |
| 1.1* | Form of Underwriting Agreement. | |
| 3.1 | Amended and Restated Certificate of Incorporation of the company, as amended, as currently in effect. | |
| 3.2 | Bylaws of the company, as currently in effect. | |
| 3.3* | Amended and Restated Certificate of Incorporation of the company, to be in effect immediately prior to the completion of this offering. | |
| 3.4* | Amended and Restated Bylaws of the company, to be in effect immediately prior to the completion of this offering. | |
| 4.1* | Specimen Common Stock Certificate of the company. | |
| 4.2 | Form of Warrant to purchase Common Stock issued on July 13, 2009. | |
| 5.1* | Opinion of Bryan Cave LLP. | |
| 10.1 | Third Amended and Restated Investors’ Rights Agreement dated as of June 25, 2014, as amended, by and among the company and the investors named therein. | |
| 10.2*+ | 2014 Stock Incentive Plan. | |
| 10.3*+ | Form of Option Agreement under 2014 Stock Incentive Plan. | |
| 10.4+ | 2009 Stock Incentive Plan, as amended. | |
| 10.5+ | Form of Option Agreement under 2009 Stock Incentive Plan. | |
| 10.6*+ | Form of Indemnification Agreement by and between the company and each of its directors and officers. | |
| 10.7+ | At-Will Employment Agreement dated May 25, 2010, by and between EndoStim, Inc. and Bevil J. Hogg, as amended. | |
| 10.8+ | Employment Agreement dated June 1, 2010, by and between EndoStim, Inc. and Paul Goode. | |
| 10.9+ | Employment Agreement dated June 1, 2010, by and between EndoStim, Inc. and Shai Policker. | |
| 10.10+ | Consulting and Non-Competition Agreement dated May 21, 2010, by and between EndoStim, Inc. and Virender K. Sharma, as amended. | |
| 10.11*+ | Restricted Stock Agreement dated May 25, 2010, by and between EndoStim, Inc. and Bevil J. Hogg, as amended. | |
| 10.12*+ | Restricted Stock Agreement dated May 25, 2010, by and between EndoStim, Inc. and Raul E. Perez. | |
| 10.13^ | Development and Supply Agreement dated March 31, 2013, by and between EndoStim, Inc. and C.C.C. Del Uruguay, S.A. | |
| 10.14 | Patent Assignment Agreement dated September 2, 2009, by and among Virender Sharma, Sarah M. Sharma, EndoStim, Inc., a Delaware corporation and Endostim, Inc., an Arizona corporation. | |
| 10.15 | Amendment No. 1 to Patent Assignment Agreement dated January 11, 2010, by and among Virender Sharma, Sarah M. Sharma, EndoStim, Inc., a Delaware corporation and EndoStim, Inc., an Arizona corporation. | |
| 10.16 | Exclusive License Agreement dated January 11, 2010, by and between Virender Sharma and EndoStim, Inc. | |
Table of Contents
| EXHIBIT |
EXHIBIT DESCRIPTION | |
| 10.17 | Patent Assignment Agreement dated December 15, 2009, by and among Edy E. Soffer, Jeffrey Conklin and EndoStim, Inc. | |
| 10.18 | Patent Option Agreement dated December 15, 2009, by and among Edy E. Soffer, Jeffrey Conklin, Claudia Sanmiguel and EndoStim, Inc. | |
| 10.19 | Form of Convertible Promissory Notes issued on June 25, 2014 and August 28, 2014. | |
| 23.1 | Consent of Ernst & Young, LLP. | |
| 23.2* | Consent of Bryan Cave LLP (included in Exhibit 5.1). | |
| 24.1 | Power of Attorney (included on the signature page to this registration statement). | |
| * | To be filed by amendment |
| + | Management contract or compensatory plan |
| ^ | Confidential treatment has been sought regarding the agreement |