Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Aldeyra Therapeutics, Inc. | d782956d8k.htm |
| EX-99.2 - EX-99.2 - Aldeyra Therapeutics, Inc. | d782956dex992.htm |
 A Novel
Pharmaceutical Platform Focused on Trapping Aldehydes
September 2014
Exhibit 99.1 |
 Forward-Looking Statements
•
This
presentation
includes
statements
contains
forward-looking
statements
that
involve
risks
and
uncertainties.
In
some
cases,
you
can
identify
forward-looking
statements
by
terms
such
as
“may,”
“might,”
“will,”
“objective,”
“intend,”
“should,”
“could,”
“can,”
“would,”
“expect,”
“believe,”
“anticipate,”
“project,”
“target,”
“design,”
“estimate,”
“predict,”
“potential,”
“plan”
or the negative of these terms, and similar
expressions intended to identify forward-looking statements. These statements
reflect our current views with respect to future events and are
based
on
assumptions
and
subject
to
risks
and
uncertainties.
Given
these
uncertainties,
you
should
not
place
undue
reliance
on
these
forward-looking statements. Forward-looking statements include, but are
not limited to, statements about: our expectations regarding our expenses
and revenue, the sufficiency of our cash resources and needs for additional financing; our anticipated growth strategies; our
expectations regarding competition; the anticipated trends and challenges in our
business and the market in which we operate; the timing and
success
of
preclinical
studies
and
clinical
trials
conducted
by
us
and
our
development
partners;
the
ability
to
obtain
and
maintain
regulatory approval of our product candidates, and the labeling for any approved
products; the scope, progress, expansion, and costs of developing and
commercializing our product candidates; the size and growth of the potential markets for our product candidates and the
ability to serve those markets; the rate and degree of market acceptance of any of
our product candidates; our ability to establish and maintain
development
partnerships;
our
ability
to
attract
or
retain
key
personnel;
our
expectations
regarding
federal,
state
and
foreign
regulatory requirements; regulatory developments in the United States and foreign
countries; and our ability to obtain and maintain intellectual property
protection for our product candidates. •
Forward-looking statements involve known and unknown risks, uncertainties and
other factors which may cause our actual results, performance or
achievements to be materially different from any future results, performances or achievements expressed or implied by
the
forward-looking
statements.
Any
forward-looking
statement
made
by
us
in
this
presentation
speaks
only
as
of
the
date
on
which
it is
made. Except as required by law, we assume no obligation to update these
statements publicly, or to update the reasons actual results could differ
materially from those anticipated in these statements, even if new information becomes available in the future.
•
Although we believe that we have a reasonable basis for each forward-looking
statement contained in this presentation, we caution you that
forward-looking statements are not guarantees of future performance and that our actual results of operation, financial condition and
liquidity, and the development of the industry in which we operate may differ
materially from the forward-looking statements contained in this
presentation
as
a
result
of,
among
other
factors,
the
factors
referenced
in
our
final
prospectus
filed
under
Rule
424(b)(4)
with
the
Securities and Exchange Commission on May 2, 2014 and in our subsequent filings
with the Securities and Exchange Commission. In addition, even if our
results of operation, financial condition and liquidity, and the development of the industry in which we operate are
consistent with the forward-looking statements contained in this presentation,
they may not be predictive of results or developments in future
periods. •
You should read carefully our filings with the Securities and Exchange Commission,
including risk factors described therein, to better understand the risks
and uncertainties inherent in our business and underlying any forward-looking statements.
2 |
 Management and Directors
•
Todd Brady, M.D., Ph.D. –
President, CEO, and
Director
–
18 years of pharmaceutical business and
clinical development
–
Domain Associates, Phenome Sciences,
(acquired by Xanthus/Antisoma), Aderis
Pharmaceuticals (acquired by Schwarz/UCB)
•
Scott Young –
Chief Operating Officer
–
28 years of pharmaceutical clinical
development
–
Genzyme, Genetics Institute, Oxigene,
Repligen
•
Steve Tulipano, CPA –
Chief Financial Officer
–
27 years of financial experience
–
Biogen, Javelin Pharmaceuticals
3
Board of Directors
Boyd
Clarke
–
former
CEO
Aviron
(acquired by MedImmune)
Gary
Phillips,
M.D.
–
Chief
Strategy
Officer Mallinckrodt Pharmaceuticals
Ben Bronstein, M.D. –
former CEO
Peptimmune (acquired by Genzyme)
Neal
Walker,
D.O.
–
CEO
Aclaris
Therapeutics
Marty
Joyce
–
former
CFO
of
Serono
USA
Jesse
Treu,
Ph.D.
–
Domain
Associates
Todd
Brady
–
CEO
Aldeyra
Therapeutics |
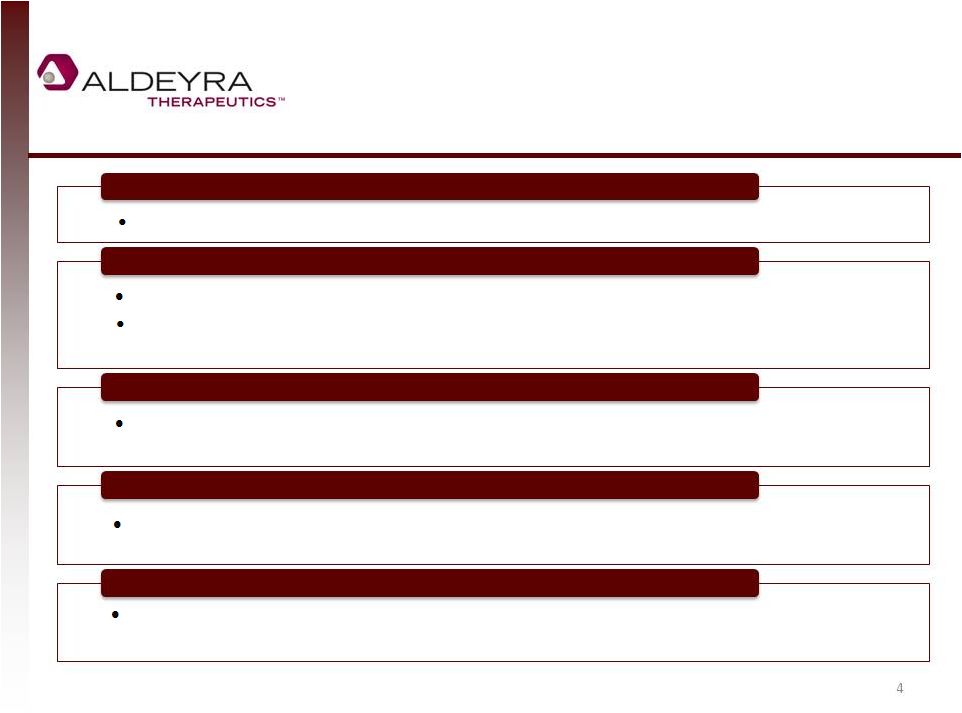 Investment
Highlights 4
Unique, Innovative Platform Technology to Trap Aldehydes
Orphan and mass-market diseases in which toxic aldehydes are implicated
Modest Funding Required for Multiple Clinical Events
Lead compound in two topical indications: one dermal and one ocular
Phase II/III results for Sjögren Larsson Syndrome (SLS) and Phase II results for
acute anterior uveitis in 2015
Large Markets with Significant Unmet Medical Need
Markets for orphan indications alone are substantial, and positive data may
suggest efficacy in a broad array of mass-market diseases
Strong Patent Portfolio of Compositions, Uses, and Formulations
Extend to late 2020s worldwide and to 2033 in US, assuming Hatch-Waxman
extension
Marquee Investors Validate Opportunity
Johnson & Johnson Development Corporation, Fidelity, and Domain Associates –
one of the oldest and largest healthcare venture capital funds worldwide
|
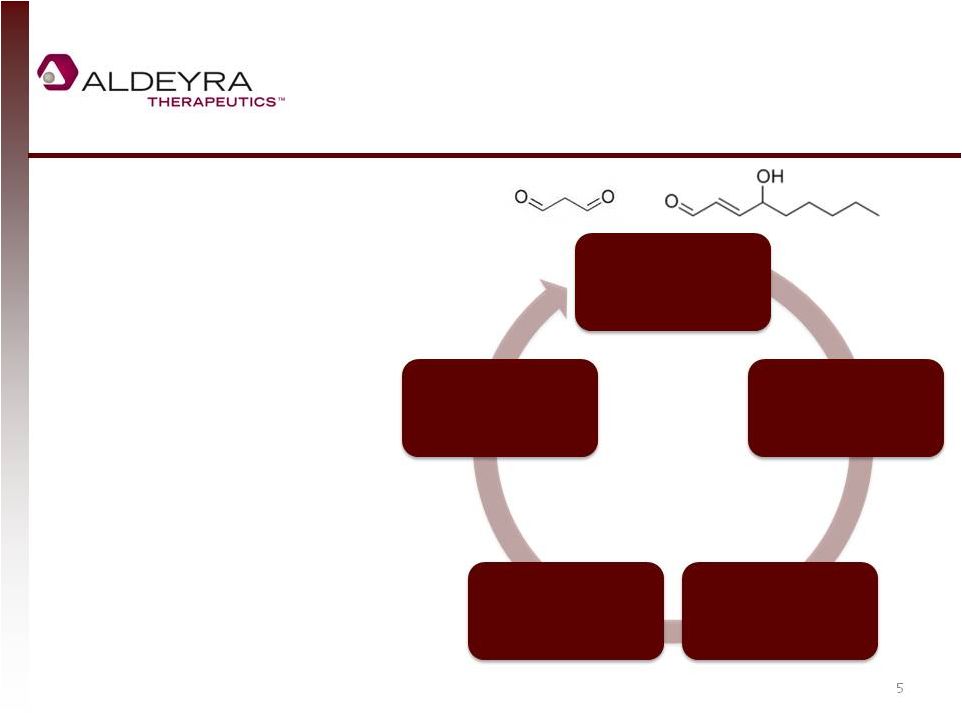 Aldehydes Are
Mediators of Disease
•
Toxic mediators of numerous
diseases
•
Modify cellular constituents,
lead to indigestible
aggregates, and are pro-
inflammatory
•
Dehydrogenases attempt to
eliminate free aldehydes
•
High levels are implicated in
autoimmune, inflammatory,
neurological, cardiovascular
and endocrinologic diseases
5
Oxidation,
Metabolism
Free Aldehydes
Lipid, DNA, Protein,
Carbohydrate
Modification
Cytokine Release
NF-KB Activation |
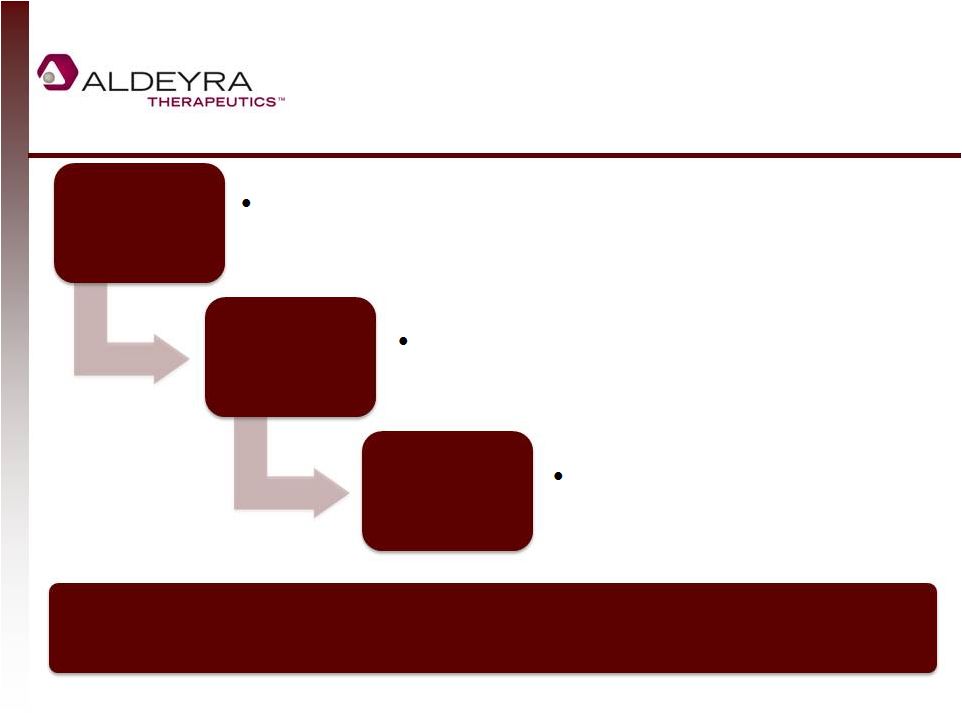 Aldehyde
Traps: A Novel Therapeutic Approach
6
Aldeyra’s
lead
aldehyde
trap,
NS2,
appears
to
have
minimal
pharmacology;
it
does
not
seem to affect receptors or proteins. No similar technology believed to be
available. Adduct
Transport
Aldehyde
Binding
Cellular
Disposal
Aldeyra’s compounds rapidly trap
free aldehydes
Trapped aldehydes are
transported to the lysosome
Drug and aldehydes are
metabolized within hours |
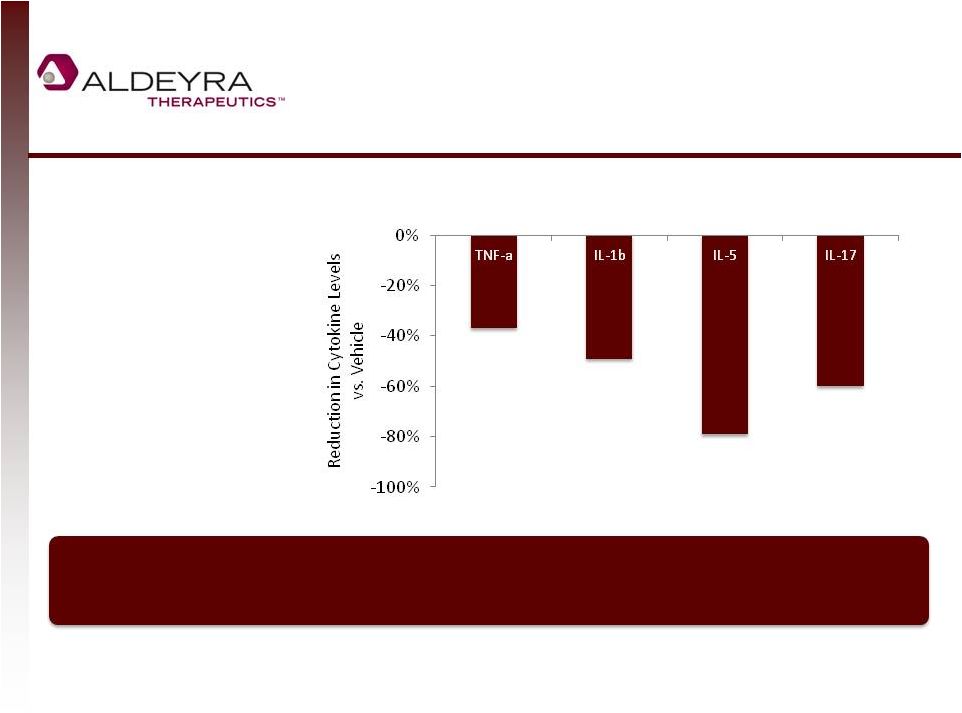 Trapping Aldehydes Generates a Broad
Anti-Inflammatory Response
7
Mice treated with NS2 or
vehicle 30 minutes prior to
endotoxin exposure;
cytokines measured two
hours after endotoxin
exposure
**
p<0.01
***
p<0.001
**
**
***
**
In an endotoxin model of cytokine generation in mice, NS2 administration significantly
reduced levels of a broad array of pro-inflammatory cytokines.
|
 8
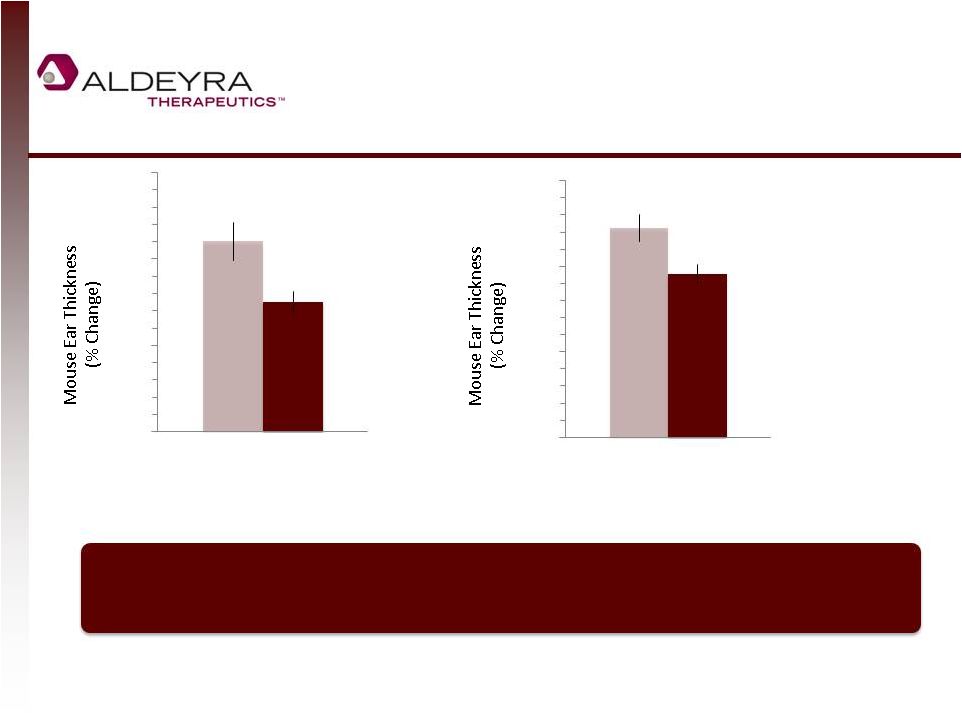
NS2 Decreases Dermal
Inflammation in Animal Models
**
Vehicle
NS2
Vehicle
NS2
*
*
p<0.05
**
p<0.01
Murine Model of Contact Dermatitis (PMA)
6.5 hours after NS2 Administration
Murine Model of Allergic Dermatitis (Oxazolone)
24.5 hours after NS2 Administration
Single dose of NS2 has early and potent anti-inflammatory effect that reduces
swelling in two different models of skin inflammation
150
50
0
100
150
50
0
100 |
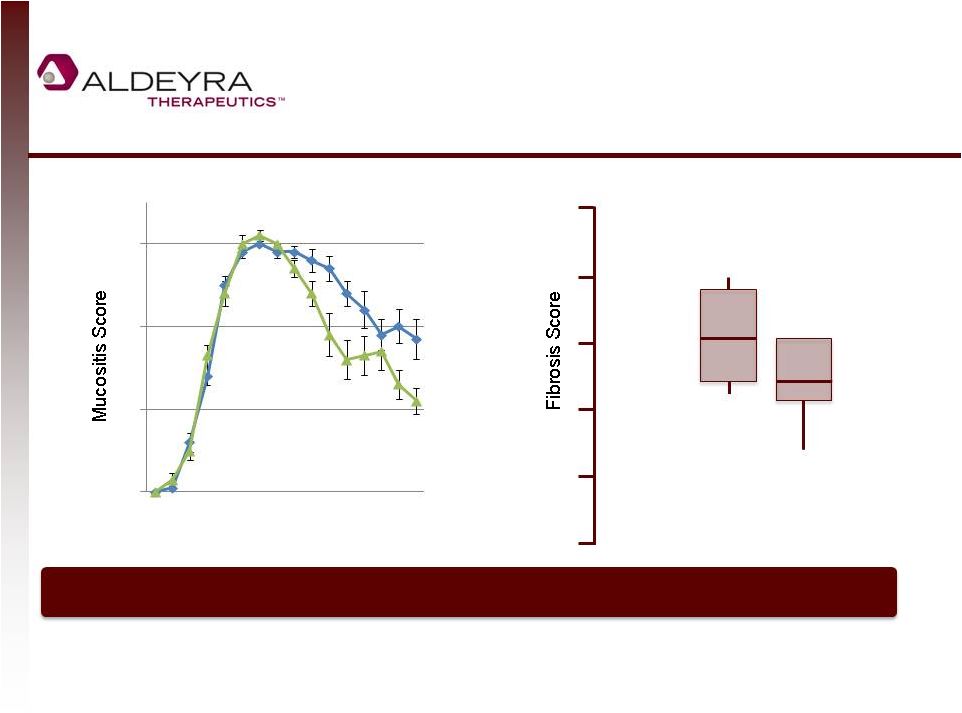 NS2
Speeds Healing and Reduces Scarring of Lesions in Animal Models
9
Vehicle
NS2
p=0.01
Day
6
21 36
p=0.1
NS
2
Vehicle
None
Minimal
Mild
Marked
Moderate
Severe
Hamster cheek pouch radiation-induced oral mucositis
NS2 speeds lesion healing and reduces scarring in a model of skin and eye
disease 2
3
1
0 |
 10
NS2 Protects a Key Lipid Relevant to
Skin and Eye Disease in Cell Systems
Aldehyde-
Damaged
Lipid
Control
Aldehyde
NS2+Aldehyde
Human Skin Cells
Aldehyde-
Damaged
Lipid
Normal
Cells
SLS
Mutants
SLS Mutants +
NS2
p<0.01
p<0.01
NS2 prevents aldehyde-mediated damage of lipid that is critical to dermal moisture
barrier and ocular tear integrity |
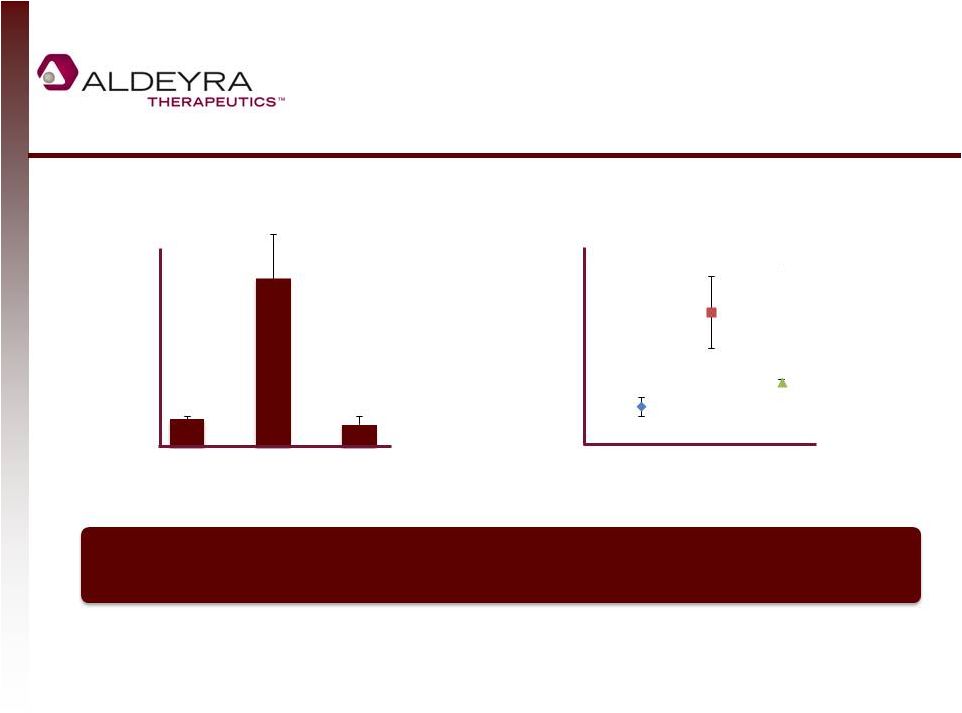
 NS2 Traps Aldehydes Generated by Dry
Conditions in Human Tissue
11
Malondialdehyde concentration in human
tissues after 72 hours of NS2
Dry Tissue + NS2
Eye Drop
Normal Tissue
Dry Tissue
p < 0.01
0
5
10
15
20
25
30
0
2
4
6
8
10
12
Dry Tissue + NS2
Dermatologic
Normal Tissue
Dry Tissue
p < 0.05
Human Ocular Tissue
Human Skin Tissue
Potential to reduce aldehyde-mediated damage in diseases characterized by dry tissue
(Including Sjögren-Larsson Syndrome and Ocular Rosacea with Meibomian Gland
Dysfunction) |
 NS2 Summary of
Efficacy: Multiple Mechanisms of Action
12
NS2
Lipid Protection
Anti-fibrotic
Lesion Healing
Anti-
inflammatory
Decreased
Aldehyde
Load
The same biological mechanisms may apply to many orphan and prevalent diseases.
|
 Positive NS2 Eye
Drop Phase I Results
o
48 healthy volunteers
o
Double-blinded and placebo controlled
o
Two treatment stages for two drug concentrations:
-
Single day 0.25% & 0.5% bid &
qid
-
Seven day 0.25% & 0.5% qid
o
Eye drops were well tolerated in all treatment groups
o
No plasma exposure detected by LC-MS/MS (<5 ng/ml)
13
NS2 is Phase II-ready as an eye drop |
 Acute
Anterior Uveitis: A Rare Inflammatory Ocular Disease
14
Uveitis
Acute anterior
ocular
inflammation
Pain,
photophobia,
loss of vision
Estimated
25,000 US
patients/year
Aldehydes are inflammatory mediators of ocular diseases, and can
lead to
degradation of tear quality |
 Anticipated
Clinical Trial Designs for Ocular Disease
Formulation
Control
Total Patients
Treatment Time
Endpoints
15
Acute Anterior
Uveitis
Eye Drop
Active 1:1:1
45 Patients
8 weeks
Cell Counts, Symptoms |
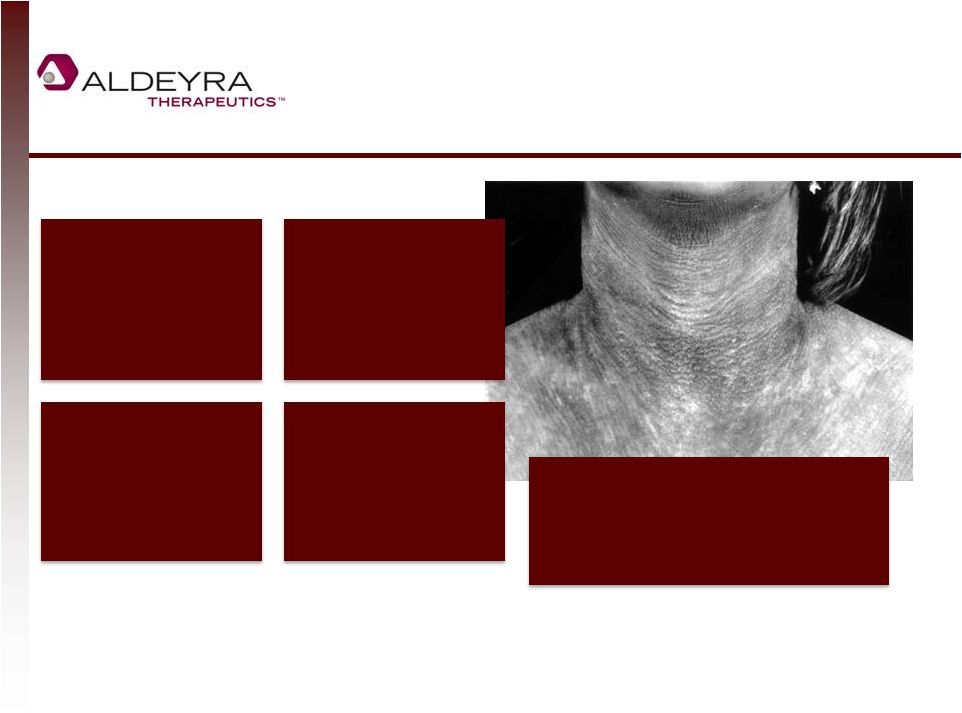 Sjögren-Larsson Syndrome (SLS):
Orphan Disease with No Therapy
16
(1) Extrapolating from a Swedish estimate, it is generally assumed that there are approximately 1,000
SLS patients in the United States and a greater number of SLS patients in Europe.
Orphan disease caused
by mutation in Fatty
Aldehyde
Dehydrogenase, leading
to high levels of toxic
aldehydes
Symptoms include
severe skin thickening
(ichthyosis), retinal
disease, and
neurological disorders
Diagnosed at birth, but
no approved therapy
that addresses disease;
patients survive into
50s
Estimated 0.4
births/100,000 = about
1000 patients in US and
a greater number in
Europe (1)
Therapeutic aldehyde trap
would be analogous to an
enzyme replacement therapy |
 Anticipated
Clinical Trial Designs For Dermatologic Disease
Formulation
Control
Total Patients
Treatment Time
Endpoints
17
Sjögren-Larsson
Syndrome
Dermal
Topical
Placebo
1:1
Visual Rating
12 Patients
8 weeks |
 Unmet Medical Need
for Our Clinical Indications
18
Market demand is substantial for a novel therapy that is safe and effective in the
indications that we intend to develop
There is no FDA-approved therapy for Sjögren-
Larsson Syndrome
Therapies for acute anterior uveitis are
associated with significant side effects |
 Orphan Topical:
Attractive Pricing, Large Market
19
Total US SLS market: ~$200M
Payer research
confirms similar or
higher pricing for a
topical SLS therapy
Estimated
>$200,000
per year
Twice per day
treatment
25% of body
surface area
treated
Orphan
Topical Pricing
for Cutaneous
Lymphoma |
 Intellectual Property
Portfolio: Composition of Matter into the 2030s
20
*Pending in Brazil, India
Formulation
Composition
Method
Topical
dosing
Uses for aldehyde traps in
diseases
NS2 to 2033 in US, assuming Hatch-
Waxman extension; Issued or allowed
worldwide* |
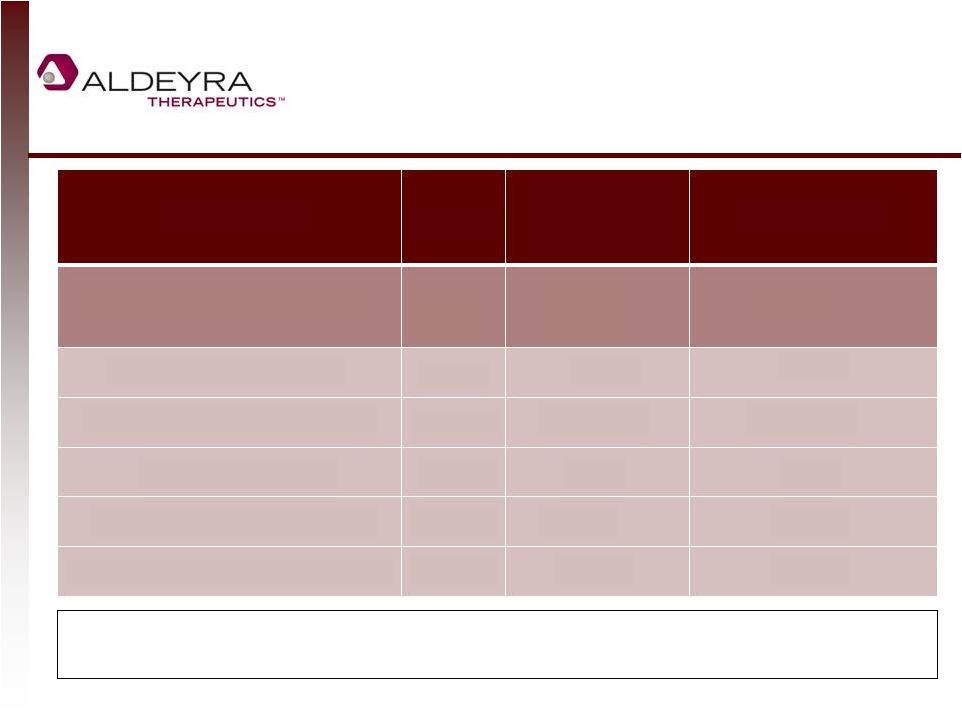 Orphan Disease
Company Valuation Comparables
21
Orphan disease-focused biotechnology companies are highly valued, but Aldeyra has
potential to expand to prevalent diseases as well.
Data as of 8/26/14
(1) INDs anticipated by end of 2014, pending FDA review, among other contingencies.
(1)
Company
Stage
Diseases in
Phase II or III
Clinical Trials
Valuation
Aldeyra Therapeutics (ALDX)
Phase II
2
$23M
Bluebird Bio (BLUE)
Phase II
2
$1.1B
Sarepta Therapeutics (SRPT)
Phase II
1
$950M
Ultragenyx (RARE)
Phase II
2
$1.7B
Synageva BioPharma (GEVA)
Phase III
1
$2.6B
Intercept Pharmaceuticals (ICPT)
Phase III
4
$6.4B |
 Post-IPO Updates
•
Closed IPO on May 7, 2014 raising gross proceeds of $12M
•
Proceeds from IPO to be used to complete two clinical trials and
expected to provide working capital through 2015
•
2014 Society for Investigational Dermatology poster on novel
treatment for dry skin and eye diseases selected for Late
Breaking and Industry Review sessions
•
Clinical development timelines remain on track
•
Team expansion completed thru 2015
22 |
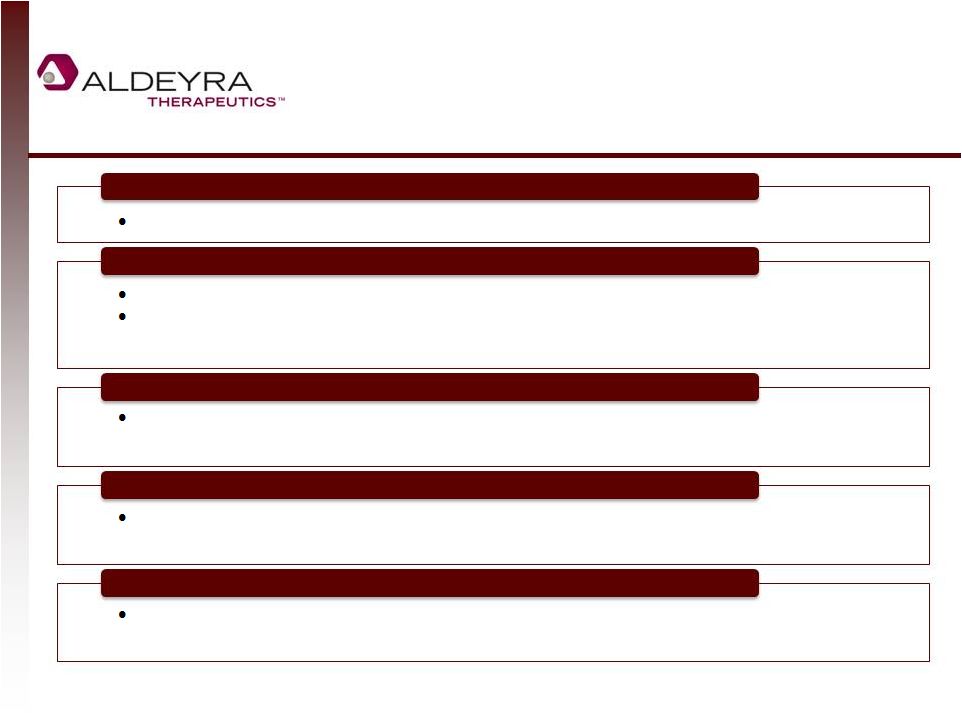 Investment
Highlights 23
Marquee Investors Validate Opportunity
Strong Patent Portfolio of Compositions, Uses, and Formulations
Large Markets with Significant Unmet Medical Need
Modest Funding Required for Multiple Clinical Events
Unique, Innovative Platform Technology to Trap Aldehydes
Orphan and mass-market diseases in which toxic aldehydes are implicated
Lead compound in two topical indications: one dermal and one ocular
Phase II/III results for Sjögren Larsson Syndrome (SLS) and Phase II results for
acute anterior uveitis in 2015
Markets for orphan indications alone are substantial, and positive data may
suggest efficacy in a broad array of mass-market diseases
Extend to late 2020s worldwide and to 2033 in US, assuming Hatch-Waxman
extension
Johnson & Johnson Development Corporation, Fidelity, and Domain Associates –
one of the oldest and largest healthcare venture capital funds worldwide
|
