UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
(Amendment No. 3)
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): July 31, 2014
Enumeral Biomedical Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 333-185891 | 99-0376434 | ||
| (State or Other Jurisdiction | (Commission File | (I.R.S. Employer | ||
| of Incorporation) | Number) | Identification Number) |
One Kendall Square,
Building 400, 4th Floor
Cambridge, MA 02139
(Address of principal executive offices, including zip code)
+1 (617) 674-1865
(Registrant’s telephone number, including area code)
Krizikova 22
Prague 8, 18600, Czech Republic
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| o | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| o | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| o | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| o | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
EXPLANATORY NOTE
This Form 8-K/A is filed by Enumeral Biomedical Holdings, Inc. (the “Company”) to update Items 2.01 and 9.01 to provide updated historical financial information as of June 30, 2014 and for the three and six months ended June 30, 2014. References to Items in Form 8-K which are not included herein refer to Items previously filed by the Company.
Table of Contents
| i |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Current Report on Form 8-K (this “Current Report”) contains forward-looking statements, including, without limitation, in the sections captioned “Description of Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and elsewhere. Any and all statements contained in this Current Report that are not statements of historical fact may be deemed forward-looking statements. Terms such as “may,” “might,” “would,” “should,” “could,” “project,” “estimate,” “pro-forma,” “predict,” “potential,” “strategy,” “anticipate,” “attempt,” “develop,” “plan,” “help,” “believe,” “continue,” “intend,” “expect,” “future,” and terms of similar import (including the negative of any of the foregoing) may be intended to identify forward-looking statements. However, not all forward-looking statements may contain one or more of these identifying terms. Forward-looking statements in this Current Report may include, without limitation, statements regarding (i) the plans and objectives of management for future operations, including plans or objectives relating to the development of commercially viable pharmaceuticals, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance, including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations included pursuant to the rules and regulations of the SEC, and (iv) the assumptions underlying or relating to any statement described in points (i), (ii) or (iii) above.
The forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation, our inability to obtain adequate financing, the significant length of time associated with drug development and related insufficient cash flows and resulting illiquidity, our inability to expand our business, significant government regulation of pharmaceuticals and the healthcare industry, lack of product diversification, volatility in the price of our raw materials, existing or increased competition, results of arbitration and litigation, stock volatility and illiquidity, and our failure to implement our business plans or strategies. A description of some of the risks and uncertainties that could cause our actual results to differ materially from those described by the forward-looking statements in this Current Report appears in the section captioned “Risk Factors” and elsewhere in this Current Report.
Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and uncertainties related to them and to the risk factors. We disclaim any obligation to update the forward-looking statements contained in this Current Report to reflect any new information or future events or circumstances or otherwise, except as required by law.
Readers should read this Current Report in conjunction with the discussion under the caption “Risk Factors,” our financial statements and the related notes thereto in this Current Report, and other documents which we may file from time to time with the Securities and Exchange Commission (the “SEC”).
We were incorporated as Cerulean Group, Inc. in Nevada on February 27, 2012. Prior to the Merger and Split-Off (each as defined below), we were a development stage company formed to develop and operate a website for self-travelers and backpackers.
As previously reported, on July 10, 2014, we (i) were converted from a Nevada corporation into a Delaware corporation under the name Enumeral Biomedical Holdings, Inc., and (ii) we increased our authorized capital stock from 75,000,000 shares of common stock, par value $0.001 per share, to 300,000,000 shares of common stock, par value $0.001 per share (the “Common Stock”), and 10,000,000 shares of “blank check” preferred stock, par value $0.001 per share.
Also as previously reported, on July 25, 2014, we completed a 4.62-for-1 forward split of our Common Stock in the form of a dividend, with the result that the 6,190,000 shares of Common Stock outstanding immediately prior to the stock split became 28,597,804 shares of Common Stock outstanding immediately thereafter. All share and per share numbers in this Current Report relating to our Common Stock have been adjusted to give effect to this stock split, unless otherwise stated.
On July 31, 2014, our wholly owned subsidiary, Enumeral Acquisition Corp., a corporation formed in the State of Delaware on July 21, 2014 (“Acquisition Sub”) merged (the “Merger”) with and into Enumeral Biomedical Corp., a corporation incorporated in the State of Delaware on December 11, 2009 (“Enumeral”). Enumeral was the surviving corporation in the Merger and became our wholly owned subsidiary. All of the outstanding Enumeral stock was converted into shares of our Common Stock, as described in more detail below.
In connection with the Merger and pursuant to the Split-Off Agreement (defined below), we transferred our pre-Merger assets and liabilities to our pre-Merger majority stockholder, in exchange for the surrender by her and cancellation of 23,100,000 shares of our Common Stock, leaving 5,497,804 shares outstanding before giving effect to the issuance of the Merger and the PPO (as defined below). See Item 2.01, “Split-Off”, below.
As a result of the Merger and Split-Off, we discontinued our pre-Merger business and acquired the business of Enumeral, and will continue the existing business operations of Enumeral as a publicly-traded company under the name Enumeral Biomedical Holdings, Inc.
Also on July 31, 2014, we closed a private placement offering (the “PPO”) of 21,549,510 Units of our securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of our Common Stock and a warrant to purchase one share of Common Stock at an exercise price of $2.00 per share and with a term of five years (the “PPO Warrants”). Additional information concerning the PPO and PPO Warrants is presented below under Item 2.01, “Merger and Related Transactions—the PPO” and “Description of Securities,” and Item 3.02, “Unregistered Sales of Equity Securities.”
In accordance with “reverse merger” accounting treatment, our historical financial statements as of period ends, and for periods ended, prior to the Merger will be replaced with the historical financial statements of Enumeral prior to the Merger in all future filings with the SEC.
Also on July 31, 2014, we changed our fiscal year from a fiscal year ending on October 31 of each year, which was used in our most recent filing with the SEC, to one ending on December 31 of each year, which is the fiscal year end of Enumeral.
As used in this Current Report henceforward, unless otherwise stated or the context clearly indicates otherwise, the terms the “Company,” the “Registrant,” “we,” “us,” and “our” refer to Enumeral Biomedical Holdings, Inc., incorporated in Delaware, after giving effect to the Merger, the Split-Off and the PPO.
This Current Report contains summaries of the material terms of various agreements executed in connection with the transactions described herein. The summaries of these agreements are subject to, and are qualified in their entirety by, reference to these agreements, which are filed as exhibits hereto and incorporated herein by reference.
| 2 |
This Current Report is being filed in connection with a series of transactions consummated by the Company and certain related events and actions taken by the Company.
This Current Report on Form 8-K/A responds to the following Items in Form 8-K:
Item 2.01. Completion of Acquisition or Disposition of Assets
Item 9.01. Financial Statements and Exhibits
Prior to the Merger, we were a “shell company” (as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)). As a result of the Merger, we have ceased to be a shell company. The information contained in this Current Report, together with the information contained in our Annual Report on Form 10-K for the fiscal year ended October 31, 2013, and our subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as filed with the SEC, constitute the current “Form 10 information” necessary to satisfy the conditions contained in Rule 144(i)(2) under the Securities Act of 1933, as amended (the “Securities Act”).
Item 2.01 Completion of Acquisition or Disposition of Assets
THE MERGER AND RELATED TRANSACTIONS
Merger Agreement
On July 31, 2014 (the “Closing Date”), the Company, Acquisition Sub and Enumeral entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), which closed on the same date. Pursuant to the terms of the Merger Agreement, Acquisition Sub merged with and into Enumeral, which was the surviving corporation and thus became our wholly-owned subsidiary.
Pursuant to the Merger, we acquired the business of Enumeral to discover and develop novel antibody therapeutics that help the human immune system attack diseased cells. (See “Description of Business” below.)
At the closing of the Merger (a) each share of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.102121 shares of our Common Stock, (b) each share of Enumeral’s Series A Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.598075 shares of our Common Stock, (c) each share of Enumeral’s Series A-1 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.790947 shares of our Common Stock, (d) each share of Enumeral’s Series A-2 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.997246 shares of our Common Stock, (e) each share of Enumeral’s Series B Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 2.927509 shares of our Common Stock, and (f) a convertible note was converted into 3,230,869 shares of our Common Stock. As a result, an aggregate of 22,700,649 shares of our Common Stock were issued to the holders of Enumeral’s stock (inclusive of 188,250 shares of common stock that is unvested and remains to a restricted stock award to each of Messrs. Rydzewski and Tinkelenberg as more fully described in “Executive Compensation – Employment Agreements”).
In addition, pursuant to the Merger Agreement:
| · | warrants to purchase 694,443 shares of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger were converted into warrants to purchase shares of our Common Stock at a conversion ratio of 1.102121 for one; |
| · | warrants to purchase 41,659 shares of Enumeral’s Series A Preferred Stock issued and outstanding immediately prior to the closing of the Merger were converted into warrants to purchase shares of our Common Stock at a conversion ratio of 1.598075 for one and |
| 3 |
| · | warrants to purchase 144,140 shares of Enumeral’s Series B Preferred Stock issued and outstanding immediately prior to the closing of the Merger were converted into warrants to purchase shares of our Common Stock at a conversion ratio of 2.927509 for one and |
| · | options to purchase 948,567 shares of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger were converted into options to purchase shares of our Common Stock at a conversion ratio of 1.102121 for one. |
As a result, warrants to purchase an aggregate of 1,253,899 shares of our Common Stock and options to purchase an aggregate of 1,045,419 shares of our Common Stock were issued in connection with the Merger. See “Description of Securities—Warrants” and “—Options” below for more information.
The Merger Agreement provided certain anti-dilution protection to the holders of the Company’s Common Stock immediately prior to the Merger (after giving effect to the Split-Off), in the event that the aggregate number of Units sold in the PPO after the final closing thereof were to exceed 15,000,000 Accordingly, based on the final amount of gross proceeds raised in the PPO, the Company issued 1,693,747 additional shares of Common Stock to the holders of the Company’s Common Stock immediately prior to the Merger.
The Merger Agreement contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions. Breaches of the representations and warranties will be subject to indemnification provisions. Each of the stockholders of Enumeral as of the date of the Merger initially received in the Merger 98% of the shares to which each such stockholder is entitled, with the remaining 2% of such shares being held in escrow for 18 months to satisfy post-closing claims for indemnification by the Company (“Indemnity Shares”). Any of the Indemnity Shares remaining in escrow at the end of such 18-month period shall be distributed to the pre-Merger stockholders of Enumeral on a pro rata basis. The Merger Agreement also contains a provision providing for a post-Merger share adjustment as a means for which claims for indemnity may be made by the pre-Merger stockholders of Enumeral. Pursuant to this provision, up to 500,000 additional shares (“R&W Shares”) of Common Stock may be issued to the pre-Merger stockholders of Enumeral, pro rata, during the 18-month period following the Merger for breaches of representations and warranties by the Company. The value of the Indemnity Shares and the R&W Shares issued pursuant to the foregoing adjustment mechanisms is fixed at $1.00 per share. The foregoing mechanisms are the exclusive remedies of the Company on one hand and the pre-Merger stockholders of Enumeral on the other hand for satisfying indemnification claims under the Merger Agreement.
The Merger will be treated as a recapitalization of the Company for financial accounting purposes. Enumeral will be considered the acquirer for accounting purposes, and our historical financial statements prior to the Merger will be replaced with the historical financial statements of Enumeral prior to the Merger in all future filings with the SEC.
The Merger is intended to be treated as a tax-free reorganization under Section 368 of the Internal Revenue Code of 1986, as amended.
The issuance of shares of our Common Stock to holders of Enumeral’s capital stock in connection with the Merger was not registered under the Securities Act, in reliance upon the exemption from registration provided by Section 4(a)(2) of the Securities Act, which exempts transactions by an issuer not involving any public offering, and Regulation D promulgated by the SEC under that section. These securities may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirement, and are subject to further contractual restrictions on transfer as described below.
We also agreed not to register under the Securities Act the resale of the shares of our Common Stock received in the Merger by our officers, directors and key employees and holders of 10% or more of our Common Stock for a period of one year following the closing of the Merger.
The Merger Agreement is filed as Exhibit 2.1 to this Current Report on Form 8-K. All descriptions of the Merger Agreement herein are qualified in their entirety by reference to the text thereof filed as an exhibit hereto, which is incorporated herein by reference.
| 4 |
Split-Off
Upon the closing of the Merger and under the terms of a split-off agreement and a general release agreement, the Company transferred all of its pre-Merger operating assets and liabilities to its wholly-owned special-purpose subsidiary, Cerulean Operating Corp., a Delaware corporation (“Split-Off Subsidiary”), formed on July 24, 2014. Thereafter, pursuant to the split-off agreement, the Company transferred all of the outstanding shares of capital stock of Split-Off Subsidiary to Olesya Didenko, the pre-Merger majority stockholder of the Company, and the former sole officer and director of the Company (the “Split-Off”), in consideration of and in exchange for (i) the surrender and cancellation of an aggregate of 23,100,000 shares of our Common Stock held by Olesya Didenko (which were cancelled and will resume the status of authorized but unissued shares of our Common Stock) and (ii) certain representations, covenants and indemnities. All descriptions of the split-off agreement and the general release agreement herein are qualified in their entirety by reference to the text thereof filed as Exhibits 10.1 and 10.2 hereto, which are incorporated herein by reference.
The PPO
Concurrently with the closing of the Merger and in contemplation of the Merger, we held a closing of our PPO in which we sold 21,549,510 Units of our securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of the our Common Stock and PPO Warrant to purchase one share of Common Stock at an exercise price of $2.00 per share and with a term of five years.
The investors in the Units (for so long as such investors hold shares of Common Stock) will have anti-dilution protection on those Units purchased in the PPO and not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning), such that if within two years after the final closing of the PPO the Company shall issue additional shares of Common Stock or Common Stock equivalents (subject to customary exceptions, including but not limited to (a) issuances of awards under the 2014 Plan (as defined below) and (b) other Exempt Securities (defined below)) for a consideration per share less than $1.00 (the “Lower Price”), each such investor will be entitled to receive from the Company additional Units in an amount such that, when added to the number of Units initially purchased by such investor, will equal the number of Units that such investor’s PPO subscription amount would have purchased at the Lower Price.
The PPO Warrants not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) have “weighted average” anti-dilution protection for the same period as the Units, subject to customary exceptions, including but not limited to issuances of awards under the 2014 Plan and other Exempt Securities.
“Exempt Securities” include: (i) shares of Common Stock issued or issuable upon conversion or exchange of any convertible securities or exercise of any options or warrants outstanding on the effective date of the Merger; (ii) shares of Common Stock issued or issuable upon exercise of the PPO Warrants or the Agent Warrants; (iii) shares of Common Stock issued in a registered public offering under the Securities Act; (iv) shares of Common Stock issued or issuable pursuant to the acquisition of another entity or business by the Company by merger, purchase of substantially all of the assets or other reorganization or pursuant to a joint venture or technology license agreement, but not including a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities; (v) shares of Common Stock issued or issuable to officers, directors and employees of, or consultants to, the Company pursuant to stock grants, option plans, purchase plans or other employee stock incentive programs or arrangements approved by the Board of Directors, or upon exercise of options or warrants granted to such parties pursuant to any such plan or arrangement; and (vi) securities issued to financial institutions, institutional investors or lessors in connection with credit arrangements, equipment financings, lease arrangements or similar transactions, in the aggregate not exceeding ten percent (10%) of the number of shares of Common Stock outstanding at any time, and in case of clauses (iii) through (vi) above, such issuance is approved by a majority of disinterested directors of the Company and includes no “death spiral” provision of any kind.
The aggregate gross proceeds of the PPO were $21,549,510 (before deducting placement agent fees and expenses and expenses of the offering estimated of approximately $2,938,000).
| 5 |
The PPO was exempt from registration under Section 4(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”), in reliance upon the exemption provided by Regulation D promulgated by the SEC thereunder. The PPO was sold to “accredited investors,” as defined in Regulation D, and was conducted on a “best efforts” basis.
The closing of the PPO and the closing of the Merger were conditioned upon each other.
In connection with the PPO, we agreed to pay our placement agents, EDI Financial, Inc. and Katalyst Securities LLC (the “Placement Agents”), a commission of equal to 10% of the gross proceeds raised from investors in the PPO). In addition, the Placement Agents collectively received warrants to purchase 10% of the number of shares of Common Stock included in the Units sold in the PPO, provided, however, that the Placement Agents are not entitled to any warrants on the sale of Units in excess of 20,000,000), with a term of five (5) years and an exercise price of $1.00 per share (the “Agent Warrants”). Any sub-agent of the Placement Agents or certain individuals identified by the Company that introduced investors to the PPO were entitled to share in the cash fees and warrants attributable to those investors as described above. We also agreed to pay to the Placement Agents a cash fee (a “Subsequent Offering Fee”) on the amount that any person or entity contacted by the Placement Agents, in connection with the Offering (each, an “Introduced Investor”), invests in the Company at any time prior to the date that is eighteen (18) months after the final closing of the PPO, whether or not such Introduced Investor invested in the PPO, provided, that such person was introduced to the Company by the Placement Agents prior to or during the PPO and was provided with a copy of the Private Placement Memorandum; and provided further, that an Introduced Investor shall not include (x) any investors who were investors in Enumeral prior to the Offering, (y) any employees, directors or officers of the Company or Enumeral, or (z) were introduced to the Company by employees, officers or directors of Enumeral (and who were not a client of the Placement Agents prior to the Offering), whether or not they participate in the placement. The Subsequent Offering Fee shall be equal to (i) the highest percentage of amount raised paid to any placement agent affiliated with the offering, or (ii) 10% if no placement agents are engaged as part of such offering.
As a result of the foregoing, the Placement Agents and their respective sub-agents were collectively paid an aggregate commission of $2,154,951 and were issued Agent Warrants to purchase an aggregate of 2,000,000 shares of our Common Stock. We were also required to reimburse the Placement Agents up to $30,000 of legal expenses incurred in connection with the PPO, in the aggregate.
We agreed to indemnify the Placement Agents and their respective sub-agents to the fullest extent permitted by law, against certain liabilities that may be incurred in connection with the PPO, including certain civil liabilities under the Securities Act, and, where such indemnification is not available, to contribute to the payments the Placement Agents and their respective sub-agents may be required to make in respect of such liabilities.
All descriptions of the PPO Warrants and the Agent Warrants herein are qualified in their entirety by reference to the text of the forms of such documents filed as Exhibits 10.5, 10.8 and 10.36 hereto, which are incorporated herein by reference.
| 6 |
Registration Rights
In connection with the PPO, we entered into a Registration Rights Agreement, pursuant to which we have agreed that promptly, but no later than 90 calendar days from the final closing of the PPO, the Company will file a registration statement with the SEC (the “Registration Statement”) covering (a) the shares of Common Stock issued in the PPO, (b) the shares of Common Stock issuable upon exercise of the PPO Warrants, (c) the shares of Common Stock underlying the Agent Warrants, (d) up to 50% of shares of Common Stock issued in the Merger in exchange for the preferred and common stock held by the former stockholders of Enumeral prior to the Merger (the “Enumeral Stockholders”) who are not parties to a lock-up agreement (provided that any registered Enumeral Stockholder that purchased Units in the PPO having a purchase price equal to at least 50% of the total amount invested by such holder in Enumeral stock prior to the PPO, the Registration Statement will include 100% of shares of Common Stock issued in the Merger to an Enumeral Stockholder in exchange for Enumeral’s preferred and common stock held by such person) and (e) the True-Up Shares, if any (clauses (a) through (e), collectively, the “Registrable Shares”). The Company agreed to use its commercially reasonable efforts to ensure that such Registration Statement is declared effective within 180 calendar days of filing with the SEC. If the Company is late in filing the Registration Statement or if the Registration Statement is not declared effective within 180 days of filing with the SEC, the Company will be required to pay the holders of Registrable Shares that have not been so registered, liquidated damages at a rate equal to 1.00% of the Offering Price per share for each full month that (i) the Company is late in filing the Registration Statement, (ii) the Registration Statement is late in being declared effective by the SEC or (iii) after the Registration Statement is declared effective, the Registration Statement ceases for any reason to remain continuously effective or the holders of the Registrable Shares are otherwise not permitted to utilize the prospectus therein to resell the Registrable Securities for a period of more than 30 consecutive trading days; provided, however, that in no event shall the aggregate of any such liquidated damages exceed 8% of the PPO offering price per share. No liquidated damages will accrue and accumulate with respect to (a) any Registrable Shares removed from the Registration Statement in response to a comment from the staff of the SEC limiting the number of shares of Common Stock which may be included in the Registration Statement (a “Cutback Comment”), or (b) after the shares may be resold under Rule 144 under the Securities Act or another exemption from registration under the Securities Act.
The Company is required to use its commercially reasonable efforts to keep the Registration Statement “evergreen” for two years from the date it is declared effective by the SEC or until Rule 144 is available to the holders of Registrable Shares who are not and have not been affiliates of the Company with respect to all of their registrable shares, whichever is earlier.
Prior to the Merger, the Company was a “shell company” as defined in Rule 12b-2 under the Exchange Act. Pursuant to Rule 144(i), securities issued by a current or former shell company that otherwise meet the holding period and other requirements of Rule 144 nevertheless cannot be sold in reliance on Rule 144 until twelve (12) months after the company (a) is no longer a shell company; and (b) has filed current “Form 10 information” (as defined in Rule 144(i)) with the SEC reflecting that it is no longer a shell company, and provided that at the time of a proposed sale pursuant to Rule 144, the company is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and has filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding twelve (12) months (or for such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports. As a result, the restrictive legends on certificates for our Common Stock and Warrants cannot be removed (a) except in connection with an actual sale meeting the foregoing requirements or (b) pursuant to an effective registration statement.
The holders of Registrable Shares (including any shares of Common Stock removed from the Registration Statement as a result of a Cutback Comment) and the holders of the Company’s common stock prior to the Merger (but not holders of the shares issued to the stockholders of Enumeral in consideration for the Merger) will have two “piggyback” registration rights for such shares with respect to any registration statement filed by the Company following the effectiveness of the Registration Statement that would permit the inclusion of such shares, subject to customary cut-backs on a pro rata basis if the underwriter or the Company determines that marketing factors require a limitation on the number of shares of stock or other securities to be underwritten.
We will pay all expenses in connection with any registration obligation provided in the Registration Rights Agreement, including, without limitation, all registration, filing, stock exchange fees, printing expenses, all fees and expenses of complying with applicable securities laws, and the fees and disbursements of our counsel and of our independent accountants. Each investor will be responsible for its own sales commissions, if any, transfer taxes and the expenses of any attorney or other advisor that such investor decides to employ.
We also have agreed not to file any other registration statements under the Securities Act, with customary exceptions, including shares issued in any acquisitions or covering any equity incentive plans, for a period of one year following the Merger.
All descriptions of the Registration Rights Agreement herein are qualified in their entirety by reference to the text of the form of such document filed as an Exhibit 10.9 hereto, which is incorporated herein by reference.
Composition of the Board; Voting Agreement
In connection with the Merger, the parties agreed that the Board of Directors of the Company will consist of seven members. The Board of Directors is initially composed of:
| 7 |
| (i) | six directors nominated by Enumeral, who shall be composed of |
| (a) | four of whom shall initially be John J. Rydzewski, Arthur H. Tinkelenberg, Allan Rothstein and Barry Buckland, |
| (b) | one of whom shall be a director nominated by Harris & Harris Group, Inc. (the “H&H” Director), who is reasonably acceptable to Enumeral and who shall initially be Daniel Wolfe; provided, that the right of H&H shall cease at such time as the number of shares of Common Stock owned directly by H&H is less than five percent of the total number of shares of Common Stock outstanding, and |
| (c) | one additional person to be designated by the directors specified in clauses (a) and (b) above (which director has not yet been designated), and |
| (ii) | one independent director nominated by Montrose Capital Limited and the Placement Agents, who is reasonably acceptable to the pre-Merger stockholders of Enumeral (which director has not yet been designated). |
In connection with the Merger, certain stockholders of the Company (holding in excess of 70.0% of the common stock), including all of the investors in the PPO, all of the pre-Merger stockholders of the Company and certain of the Enumeral Stockholders (including all of its officers and directors and certain of its principal stockholders), entered into a Voting Agreement in which they have agreed to vote their Company stock to maintain the composition of the Company’s Board of Directors as described above.
The Voting Agreement will terminate two years from the date of closing of the Merger. All descriptions of the Voting Agreement herein are qualified in their entirety by reference to the text thereof filed as an Exhibit 10.10 hereto, which is incorporated herein by reference.
2014 Equity Incentive Plan
Before the Merger, our Board of Directors adopted, and our stockholders approved, our 2014 Equity Incentive Plan (the “2014 Plan”), which provides for the issuance of incentive awards of up to 8,100,000 shares of our Common Stock. Incentive awards generally may be issued to officers, key employees, consultants and directors. In connection with the Merger, options to purchase an aggregate of 1,045,419 shares of our Common Stock were issued under the 2014 Plan in exchange for outstanding options that were previously issued to officers, key employees, consultants and directors of Enumeral. In addition, options to purchase 1,221,250 shares of our Common Stock were issued on the Closing Date, consisting of incentive stock options to purchase 1,105,000 shares of our Common Stock issued to our executive officers, and incentive stock options to purchase 116, 250 issued to other employees. Following the Merger, on August 4, 2014, nonqualified options to purchase 120,000 shares of our Common Stock were issued to the non-executive Directors of the Company. See “Market Price of and Dividends on Common Equity and Related Stockholder Matters—Securities Authorized for Issuance under Equity Compensation Plans” below for more information about the 2014 Plan and the outstanding stock options.
All descriptions of the 2014 Plan herein are qualified in their entirety by reference to the text thereof filed as an Exhibit 10.11 hereto, which is incorporated herein by reference.
Departure and Appointment of Directors and Officers
On the Closing Date, our Board of Directors was authorized to consist of, and currently consists of, five members, two of whom are independent. On the Closing Date, Olesya Didenko, our sole director before the Merger, resigned her position as a director, and Arthur H. Tinkelenberg, John J. Rydzewski, Daniel B. Wolfe, Allan Rothstein and Barry Buckland were appointed to the Board of Directors. Pursuant to the Voting Agreement, we expect to add two additional directors in the near future. See “—Composition of the Board; Voting Agreement” above.
| 8 |
Also on the Closing Date, Ms. Olesya Didenko, our President, Secretary and Treasurer before the Merger, resigned from these positions, and John J. Rydzewski was appointed as our Executive Chairman, Arthur H. Tinkelenberg was appointed as our Chief Executive Officer and President, Derek Brand was appointed as our Vice President of Business Development and Secretary, Anhco Nguyen was appointed as our Vice President of Research and Development and Kevin Sarney was appointed as our Vice President of Finance and Chief Accounting Officer.
See “—Security Ownership of Certain Beneficial Owners and Management – Directors and Executive Officers” below for information about our new directors and executive officers.
Lock-up Agreements and Other Restrictions
In connection with the Merger, each of our executive officers and directors named above, key employees and holders of five percent or more of our Common Stock after giving effect to the Merger and the PPO (“5% Stockholders” and together with our directors, officers and key employees, collectively, the “Restricted Holders”), holding in the aggregate 12,457,103 shares of our Common Stock (exclusive of shares they have the right to acquire upon exercise of options and warrants), entered into agreements (the “Lock-Up and No Shorting Agreements”), whereby they are restricted for a period of 18 months (in the case of directors and 5% Stockholders) or 24 months (in the case of officers and key employees) after the Merger from making certain sales or dispositions of our Common Stock held by them immediately after the Merger, except in certain limited circumstances (the “Lock-Up”) and subject to termination with the written approval of the lead underwriter of any underwritten public offering of the Company’s securities with gross proceeds of $40,000,000 or more or upon the Restricted Holder’s resignation from the board, resignation or termination as an employee of the Company or ownership of less than five percent of our Common Stock, as applicable.
Further, for the respective periods indicated above after the Merger, each Restricted Holder has agreed in the Lock-Up and No Shorting Agreements to be subject to restrictions on engaging in certain transactions, including effecting or agreeing to effect short sales, whether or not against the box, establishing any “put equivalent position” with respect to our Common Stock, borrowing or pre-borrowing any shares of our Common Stock, or granting other rights (including put or call options) with respect to our Common Stock or with respect to any security that includes, relates to or derives any significant part of its value from our Common Stock, or otherwise seeks to hedge his position in our Common Stock, but in any of the foregoing events, excluding the pledge of the Common Stock as collateral for any bona fide loan, provided that the lender agrees in writing that the Common Stock subject to the Lock-Up will continue to be subject to the restrictions on transfer set forth in Lock-Up and No Shorting Agreement.
The Company has agreed that, following the second anniversary of the closing of the Merger, and upon the request of Restricted Holders who collectively own at least 51% of the shares subject to Lock-Up, to promptly, but no later than 90 calendar days from the date of the notice, file a registration statement with the SEC covering up to 50% of the shares of Common Stock issued in the Merger to the Restricted Holders. The Company agreed to use its commercially reasonable efforts to ensure that such registration statement is declared effective within 180 calendar days of filing with the SEC. The Company is required to use its commercially reasonable efforts to keep the registration statement “evergreen” for nine months from the date it is declared effective by the SEC or until such time as none of the Restricted Holders are affiliates of the Company
All descriptions of the Lock-Up and No Shorting Agreements herein are qualified in their entirety by reference to the text of the form of such document filed as an Exhibit 10.3 hereto, which is incorporated herein by reference.
Pro Forma Ownership
Immediately after giving effect to (i) the Merger, (ii) the cancellation of 23,100,000 shares in the Split-Off, and (iii) the issuance of 21,549,510 shares at the closing of the PPO, there were 51,591,710 issued and outstanding shares of our Common Stock, as follows:
| · | the Enumeral Stockholders and a former holder of an Enumeral convertible note own 22,700,649 shares of our Common Stock (inclusive of 188,250 shares of common stock that is unvested and remains to a restricted stock award to each of Messrs. Rydzewski and Tinkelenberg as more fully described in “Executive Compensation – Employment Agreements”); |
| 9 |
| · | the stockholders of the Company prior to the Merger own 7,191,551 shares of our Common Stock (inclusive of 1,693,747 shares of common stock that were issued pursuant to the Merger Agreement’s anti-dilution provisions); |
| · | a sub-agent to one of the Placement Agents owns 150,000 shares of our Common Stock; and |
| · | investors in the PPO own 21,549,510 shares of our Common Stock. |
In addition,
| · | investors in the PPO hold PPO Warrants to purchase 21,549,510 shares of our Common Stock; |
| · | the Placement Agents and their respective sub-agents hold Agent Warrants to purchase 2,000,000 shares of our Common Stock; |
| · | warrants to purchase an aggregate of 1,253,899 shares of our Common Stock are held by certain former Enumeral warrant holders; and |
| · | the 2014 Plan authorizes issuance of up to 8,100,000 shares of our Common Stock and effective as of the Closing of the Merger options to purchase 2,266,669 shares of our Common Stock had been granted, consisting of options to purchase 1,045,419 shares of our Common Stock issued in exchange for Enumeral options in connection with the Merger, options to purchase 1,105,000 shares of our Common Stock issued to our officers on the Closing Date, and options to purchase 116,250 shares of our Common Stock issued to other employees. |
No other securities convertible into or exercisable or exchangeable for our Common Stock are outstanding.
Our common stock is quoted on the OTC Markets (OTCQB) under the symbol “ENUM,” which changed from “CEUL” on July 21, 2014.
Accounting Treatment; Change of Control
The Merger is being accounted for as a “reverse merger,” and Enumeral is deemed to be the acquirer in the reverse merger. Consequently, the assets and liabilities and the historical operations that will be reflected in the financial statements prior to the Merger will be those of Enumeral and will be recorded at the historical cost basis of Enumeral, and the consolidated financial statements after completion of the Merger will include the assets and liabilities of Enumeral, historical operations of Enumeral and operations of the Company and its subsidiaries from the closing date of the Merger. As a result of the issuance of the shares of our Common Stock pursuant to the Merger, a change in control of the Company occurred as of the date of consummation of the Merger. Except as described in this Current Report, no arrangements or understandings exist among present or former controlling stockholders with respect to the election of members of our Board of Directors and, to our knowledge, no other arrangements exist that might result in a change of control of the Company.
Following the Merger, we will continue to be a “smaller reporting company,” as defined under the Exchange Act, and an “emerging growth company” under the JOBS Act. We believe that as a result of the Merger we have ceased to be a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act).
Immediately following the Merger, the business of Enumeral became our business.
| 10 |
History
As described above, we were incorporated in Nevada as Cerulean Group, Inc. on February 27, 2012, and converted to a Delaware corporation on July 10, 2014. Our original business was to develop and operate a website for self-travelers and backpackers that would allow a person with a mobile device or computer and access to the Internet to build a trip. Prior to the Merger, our Board of Directors determined to discontinue operations in this area to seek a new business opportunity. As a result of the Merger, we have acquired the business of Enumeral. In connection with the Merger, we have also changed our name to Enumeral Biomedical Holdings, Inc. and changed our state of incorporation from Nevada to Delaware.
Our authorized capital stock currently consists of 300,000,000 shares of common stock, par value $0.001, and 10,000,000 shares of “blank check” preferred stock, par value $0.001. Our common stock is quoted on the OTC Markets (OTCQB) under the symbol “ENUM,” which changed from “CEUL” on July 21, 2014.
Our principal executive offices are located at One Kendall Square, Building 400, 4th Floor, Cambridge, MA 02139. Our telephone number is 1-617-674-1865. Our website address is www.enumeral.com.
Enumeral was incorporated on December 11, 2009 under the laws of the State of Delaware.
General
Enumeral is discovering and developing novel antibody therapeutics that help the immune system attack diseased cells (“immunomodulators”). We believe that we have a unique ability to extensively interrogate cells of the human immune system for drug candidate validation and that this ability gives us a distinct advantage in selecting potential best-in-class therapeutic candidates. We are building a pipeline of drug candidates for the treatment of cancer and inflammatory diseases and leveraging the breadth of our technology in order to drive near-term cash flows from out-licensing of our drug candidates and through strategic collaboration partnerships. Our near-term goals include performance of preclinical testing on drug candidates resulting from our internal programs to generate data to support our ability to obtain revenue from co-development partners. The Company has focused its research efforts on using its proprietary drug discovery platform utilizing, in part, technology licensed by Enumeral from M.I.T., Harvard University and other institutions to identify and elucidate antibodies and antigens that are relevant to diseases that affect millions of individuals and are underserved by current therapeutic alternatives. These diseases have included cancer, infectious, and inflammatory diseases. We believe that the breadth of our drug discovery platform offers us the potential to grow our business through revenue-generating collaborative research and development partnerships.
Our strategy is to generate superior antibodies that we will co-develop, either through collaborative partnerships, or out-licensing partnerships, with larger biotechnology or pharmaceutical companies. Our goal initially is to obtain such partners following completion of “ex vivo” human and animal preclinical studies, and prior to completion of full investigational new drug (“IND”)-enabling studies, or we may fund IND-enabling studies and seek collaboration partners at that point. The immediate commercial goal of our programs is to reach significant value inflection points in the next twelve to eighteen months in order to obtain from the sale or license of product discovery programs a combination of up-front payments; subsequent milestone payments as the candidate clears pre-clinical and clinical regulatory hurdles; and royalty payments upon future sales of the marketed drug.
The protein targets on immune cells on which we are focused on function as “immune checkpoint” components of regulatory processes within the body that help coordinate an appropriate immune response. Checkpoint proteins serve as “brakes” that the body uses to prevent runaway immune responses, which can be debilitating, or even deadly. However, checkpoint control mechanisms can hinder the anti-cancer immune response, or responses to other diseased cells. In addition, cancer cells can sabotage the function of these control mechanisms as a defense against an immune system attack. Thus, while checkpoints usually function to appropriately regulate immune responses, cancers can “co-opt” checkpoint processes to evade destruction by the immune system.
| 11 |
There is increasing evidence that autoimmune, inflammatory, and infectious disease processes may be under similar checkpoint controls in the body (see “Programmed death-1 pathway in cancer and autoimmunity”, Clinical Immunology (2014) 153, 145–152). Immunomodulators (or immunotherapies) are potential drugs (usually antibodies) that bind to checkpoint proteins and either enhance or block specific checkpoint processes. Such approaches to cancer therapy are designed to make cancers more susceptible to destruction by the body’s immune responses. Immunomodulators include Bristol-Meyers Squibb’s Yervoy (forecast to reach $1B to $1.5B in peak sales; see http://www.fiercebiotech.com/special-reports/fdas-top-10-blockbuster-decisions/ipilimumab-fdas-top-10-blockbuster-decisions) PD-1 antagonists from Merck (Pembrolizumab) and Bristol-Myers Squibb (Nivolumab), both currently in Phase 3 clinical trials.
The Company’s principal objective is to use its technology platform to enable and accelerate the discovery and development of therapeutic products, particularly antibody therapies. Such antibodies may originally be identified in human patients, or from more traditional sources such as target-focused animal immunization and screening.
We employ a proprietary and patented “microengraving” technology in the discovery and development of monoclonal antibodies (“mAbs”) and other novel biologics for use in the diagnosis and treatment of cancer, and infectious and inflammatory diseases. We seek to: (1) create valuable products through internal product discovery programs for novel therapeutics and biomarkers that can be licensed or sold; (2) create and co-own valuable products and generate revenues through collaborative partnerships with leading pharmaceutical companies that apply our microengraving technology in therapeutic and biomarker discovery; and (3) develop novel means to utilize our microengraving technology through foundation and government funded grants and contracts programs to further benefit our internal and collaborative discovery programs.
Our corporate objective is to apply our microengraving technology to the development of more effective therapeutics at a lower cost by enabling better understanding of which drug candidates might work in which patients. We believe that our discoveries will be valuable for the development of therapeutic products (including vaccines and drugs based on new monoclonal antibodies) as well as potential diagnostic products.
Our technology enables us to perform sensitive measurements in primary patient-derived biopsy tissue in order to mine the human immune system’s rich collection of cells for information that is difficult to obtain using other methods and that may guide the development of effective therapeutics and diagnostics. We believe that the efficiency and sensitivity of our platform technology increases the probability of finding rare cell types associated with disease or drug response, as well as rare and previously unknown antibodies that may have the characteristics essential to becoming safe and effective drugs.
In effect, our platform enables us to study at high resolution the responses of rare immune cells from a patient in a single chip, thereby potentially identifying drug candidates earlier in the drug discovery and development process. Enumeral’s vision is to employ its platform to better predict, using human tissues from patient cohorts, which antibody drug candidates are most likely to be effective in a targeted group of patients.
Background
Pharmaceutical Antibody and Biologics Market
The pharmaceutical industry is facing major challenges, which include increased pressure from sluggish prescription drug trends, intensifying generic competition, growing regulatory costs and rapidly escalating R&D costs that result in unproductive product pipelines, and an increasing rate of costly failures of drugs in Phase II and Phase III clinical trials. The significant imbalance between new product introductions and unprecedented patent losses of “blockbuster” (generally taken to mean a drug generating more than $1 billion of annual revenue) small molecule drugs has caused pharmaceutical companies to fundamentally re-orient their drug product portfolios to contain both large-molecule, protein and small-molecule, chemical therapeutics over the past decade-and-a-half.
The portfolio rebalancing has been so profound that it is now predicted that antibody products will dominate the list of top-selling products by 2018 (Evaluate Pharma, June 2013). As depicted in the following table, it is forecast that five of the top ten selling products in 2014 will be mAbs (Thomson Reuters). This compares to only one biotech product making it into the top 100 in 2000, Amgen’s erythropoietin agent, Epogen. Illustrating the importance of these products as industry growth drivers, EvaluatePharma, Ltd. projects in its World Preview 2016 Report that biotech drugs will account for 50% of the top 100 drugs in 2014.
| 12 |
Top Ten Products by Projected 2014 Revenues
| Rank | Product | Indication | Company | Worldwide Sales ($ billion) | Technology | |||||||
| 1 | Avastin | Cancer | Roche | $ | 8.9 | mAb | ||||||
| 2 | Humira | Arthritis | Abbott | $ | 8.5 | mAb | ||||||
| 3 | Enbrel | Arthritis | Pfizer/Amgen | $ | 8.0 | Recombinant (biologic) | ||||||
| 4 | Crestor | Cholesterol | AstraZeneca | $ | 7.7 | Small molecule chemistry | ||||||
| 5 | Remicade | Arthritis | Merck/JNJ | $ | 7.6 | mAb | ||||||
| 6 | Rituxan | Cancer | Roche | $ | 7.4 | mAb | ||||||
| 7 | Lantus | Diabetes | Sanofi | $ | 7.1 | Recombinant (biologic) | ||||||
| 8 | Advair | Asthma/COPD | GlaxoSmithKline | $ | 6.8 | Small molecule chemistry | ||||||
| 9 | Herceptin | Cancer | Roche | $ | 6.4 | mAb | ||||||
| 10 | Novolog | Diabetes | Novo Nordisk | $ | 5.7 | Recombinant (biologic) | ||||||
Source: Thomson Reuters
The Immune System and Monoclonal Antibodies
The immune system is a system of biological structures and processes within an organism that protects against diseases. In humans and other vertebrate species, the immune system can be classified into two subsystems, the innate immune system and the adaptive immune system. The innate immune system, also known as the non-specific immune system, comprises the cells and mechanisms that defend the host from infection by other organisms in a non-specific manner. The cells of the innate system recognize and respond to pathogens in a generic way, but, unlike the adaptive immune system, they do not confer long-lasting or protective immunity to the host. The adaptive immune system, also known as the acquired immune system, is composed of highly specialized, systemic cells and processes that eliminate or prevent pathogen growth, and, in certain cases, can protect against the inappropriate growth of cancer cells. Acquired immunity creates immunological memory after an initial response to a specific pathogen or disease antigen, leading to an enhanced response to subsequent encounters with that same pathogen or disease antigen. The cells of the acquired immune system are B and T lymphocytes (B cells and T cells). B cells respond to pathogens or disease antigens by producing large quantities of antibodies, which then neutralize foreign objects like bacteria and viruses, and disease antigen-specific cells. In response to pathogens or disease antigens, some T cells, called T helper cells, produce signaling molecules (“cytokines”) that direct the immune response, while other T cells, called cytotoxic T cells, produce toxic granules that contain powerful enzymes that induce the death of target cells. Following activation, B cells and T cells leave a lasting legacy of the disease they have encountered, in the form of memory cells.
An antibody is a specialized protein secreted by B cells that recognizes and binds to a specific target molecule, called an antigen, in an interaction similar to a ‘lock and key’. Antigens are often proteins from bacteria, viruses, and diseased cells. Antibodies circulate in the body until they find and attach to the antigen. Once attached, they can recruit other parts of the immune system to destroy the cells containing the antigen. Researchers have learned how to design antibodies that specifically target a certain antigen, such as one that is found on cancer cells. They can then make many copies of that antibody in the lab. These are known as monoclonal antibodies (“mAbs”).
| 13 |
Among pharmaceutical products, mAbs are one of the fastest growing product categories. Global sales are forecast to exceed $141 billion in 2017 (Reportbuyer.com), up from $44.6 billion in 2011, driven by growth in the more than 30 FDA-approved mAbs now on the market, and anticipated launch of several of the 200-plus mAbs currently in clinical studies and 600 more in preclinical development. Four of the top six selling drugs in 2014 are projected to be mAbs, and today seven have sales exceeding one billion-dollars. Four of the 15 fastest growing drugs (with 2012 revenues exceeding $500) are biologics that modulate a patient’s immune system. A detailed overview of the antibody therapeutic market and its dynamics can be found in “Therapeutic antibodies: Market considerations, disease targets and bioprocessing”, J.G. Elvin et al., International Journal of Pharmaceutics 440 (2013) 83– 98.
There are a number of reasons why antibodies have become a key therapeutic class for a broad range of diseases. Antibodies as drugs (versus traditional, orally-available drugs derived from small molecule chemistry) have enabled pharmaceutical companies to develop drugs against intractable target classes (e.g., secreted hormones and cytokines (proteins involved in cell-to-cell signaling), and cell surface sensing molecules that activate internal cellular responses) that have traditionally not been “druggable” via small molecule chemistry. The therapeutic potential of mAbs is derived from their high specificities for their antigen targets, which facilitates precise action. In addition, they often display long half-lives, enabling infrequent dosing.
Novel technologies have continued to be introduced that further improve through engineering or novel selection methods the potential of the antibody therapies, leading to improved risk-benefit ratios (“The safety and side effects of monoclonal antibodies”, T.T. Hansel et al., Nature Reviews Drug Discovery Vol. 9 (April 2010) 325-338. This potentially can lead to fewer side effects experienced with mAbs than with conventional, small-molecule drugs, and overall, is reflected in approval rates of around 20% compared with 5% for small molecule-based drugs.
However, antibody therapeutics have been associated with a range of adverse events related to the class and also to specific target-induced mechanisms of action. Both are desirable to eliminate, and increasing attention has been paid to this area, leading to interest in novel platforms that can mitigate such risks. (see “Monoclonal antibody successes in the clinic”, J.M. Reichert et al., Nature Biotechnology 23 (2005) 1073-1078, and “Development trends for human monoclonal antibody therapeutics”, A.L. Nelson et al., Nature Reviews Drug Discovery Vol. 9 (April 2010) 767 – 774).
Approved antibody therapies have so far been focused on the treatment of cancer, inflammatory and autoimmune disorders, and infectious disease. The two largest categories by worldwide pharmaceutical revenues are represented by the blockbuster oncology mAb products Avastin, Rituxan, Herceptin and Erbitux (a blockbuster not in the ‘Top Ten’) and the anti-TNF biologic drugs for the treatment of rheumatoid arthritis (“RA”), including Humira (antibody), Enbrel (fusion protein) and Remicade (antibody).
Continuing Challenges
Despite the exponential growth in the market for mAbs, considerable unmet medical needs remain. One area where the heretofore limited application of antibody therapeutics represents a particular opportunity is the development of antibodies targeting immune checkpoints — immunotherapies for cancer and other indications, where modulation of targets such as CTLA-4 (a protein receptor found on the surface of T cells that downregulates the immune system) and PD-1 (programmed cell death protein 1, which is expressed on the surface of T cells and other lymphocytes) has shown impressive results in clinical care (Yervoy, which targets CTLA-4, and was approved for Stage IV melanoma) and in ongoing clinical studies (PD-1, see http://www.fiercebiotech.com/story/asco-big-four-immuno-oncology-players-wager-13b-next-gen-cancer-drugs/2014-05-29). The so-called immunomodulatory therapies that utilize the power of the patient’s own immune system by targeting these proteins to fight cancer are predicted by analysts to enter widespread clinical use and comprise up to 50% of all cancer treatment within the next decade. A key bottleneck in the field is limited understanding of human patient immunology at the level of human cells to guide validation and development of best-in-class therapies that effectively target the right patients. Our human cell-based evaluation methods (developed under a National Cancer Institute (“NCI”) contract; see below, “—Grants and Contracts: National Cancer Institute”) coupled with our unique antibody screening capabilities should, in our opinion, provide us with a distinct competitive advantage in this field.
| 14 |
Advances in the identification of antibody drug candidates have progressed through multiple waves of technology with respect to species origin of the underlying protein sequences, starting with murine (mouse), to chimeric mouse-human, to humanized, to fully-human. Multiple platforms now exist, including camel- and rabbit-derived. Screening or panning technologies have also evolved, including human-engineered antibody protein fragment library approaches (e.g., phage display), cellular immortalization methods (e.g., hybridoma), and more recently, single cell technology approaches. As novel antibodies have entered drug development, unforeseeable negative attributes have been discovered, with the largest remaining challenge being fine-tuning precise targeting of antibodies to maximize therapeutic effect over induction of undesirable side effects. The latter can also be stated as poor understanding of the inherent target and antibody-induced variability among a targeted group of patients. Even with incremental improvements in widely employed discovery platforms, antibody drug discovery and development may continue to require significant time and resources and will yield little assurance of predictable outcomes once products are tested in human clinical trials (for discussion generally on these topics, see “The safety and side effects of monoclonal antibodies”, T.T. Hansel et al., Nature Reviews Drug Discovery Vol. 9 (April 2010) 325-338).
Increasingly, regulators are looking for specific biomarker signatures that new drugs are both safe and effective, and in the areas of immunology and inflammation, are seeking efficacy and safety measurements at the level of the targeted immune cell subpopulations. In particular, we believe that the experience of Tegenero in 2006, and Raptiva and Tysabri in 2008 may lead regulators to require that drug developers prove that their products do not generate adverse, and potentially fatal, reactions in humans that were not observable in animal safety and toxicology studies (“Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies”, MAbs. 2010 May-Jun; 2(3):233–255). Such adverse events, including massive immune system-wide inflammation, known as “cytokine storms”, have occurred in human trials of novel antibody therapeutics (“Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release”, Br J Clin Pharmacol. 2013 Aug;76(2):299-315). Indeed, recent reports on Yervoy have demonstrated numerous immune-related adverse events in patients that did not correlate with response rate, and that were not observed in studies using monkeys (“Opportunistic Autoimmune Disorders Potentiated by Immune-Checkpoint Inhibitors Anti-CTLA-4 and Anti-PD-1”, Front Immunol. 2014 May 16;5:206; and “In vitro Characterization of the Anti-PD-1 Antibody Nivolumab, BMS-936558, and in vivo Toxicology in Non-Human Primates”, Cancer Immunol Res. 2014 May 28. pii: canimm.0040.2014).
So even with incremental improvements in these widely employed discovery platforms, antibody drug discovery and development may continue to require significant time and resources and will yield little assurance of predictable outcomes once products are tested in human clinical trials.
Our Strategy
Our objective is to discover and develop mAbs and other novel biologics for use in the diagnosis and treatment of cancer, and infectious and inflammatory diseases through our platform’s capability to identify antibodies based on their ability to modulate the activity of human immune cells directly in human biopsy samples from patients. If we are successful deploying our platform to measure patient responses to our antibody candidates, we believe we may substantially reduce the time-consuming testing processes typically employed to test novel antibodies, with the potential for both significantly lowered discovery and development costs and accelerated regulatory approval. Key elements of our strategy include:
| - | Employ our proprietary microengraving technology to discover monoclonal antibodies and other novel biologics. |
| - | Engage in internal product discovery programs to develop novel immunotherapies. |
| - | Establish collaborative partnerships with leading pharmaceutical companies to accelerate product development, regulatory approvals and commercialization and to leverage our technological resources. |
| - | Participate in foundation and government funded grants and contracts programs to further benefit our internal and collaborative discovery programs. |
| 15 |
Our Technology Platform
Currently, we have utilized our platform in two key areas: Therapeutic Discovery and Immune Profiling. In Therapeutic Discovery, our inputs are cellular libraries that are solely owned by Enumeral and that are derived from our own target-specific mouse immunization campaigns or cellular libraries from human patient donors sourced from commercial vendors or Enumeral’s own institutional review board-approved clinical operations activities. Antibody hits and leads are the output of our microengraving and data capture and our cell recovery and molecular biology activities. These hits and leads are further characterized for biological activity, confirmation of which may lead to drug candidates that can be brought to clinical trials. It is important to note that our platform enables rapid, low-cost generation of proprietary libraries, removing the need for in-licensing potentially expensive third-party libraries.
In Immune Profiling our inputs are tissue samples sourced from patients or commercial vendors, and the outputs are single cell functional profiles including assessment of the frequencies of functional immune cells. When such assays are carried out with cells in the presence of potential antibody candidates, as a way of measuring the potential modulation effects of the tested candidate, we believe such information will enable us to rationally select antibody therapeutic candidates with the best immune cell targeting properties – that is, those candidate that are observed, through application of our platform, to interact with and activate or modulate appropriate effector cells preferentially over potentially harmful inflammatory or other damaging cells.
Our microengraving technology integrates proprietary and standard laboratory processes with data acquisition and management software in order to link multiple parameters of cell function with high throughput and sensitivity. We have used the technology in partnerships with pharmaceutical company partners, and we believe we will generate additional partnerships based on internal validation data that we have generated.
The platform employs a proprietary chip that is manufactured by Enumeral, containing on its surface a soft molded dense array of spatially addressable subnanoliter microwells (the “Device”) that is affixed to a glass slide, and standard laboratory equipment such as manual pipetting devices, slide washers, microscopes and microarray readers. The device is currently manufactured from polydimethylsiloxane (PDMS) (a soft silicone-type organic polymer), but we believe other materials would work as well. As depicted in Figure 1 below, the platform facilitates:
| (i) | Cell culture and cell handling, working with a wide variety of cell types (T-Cells, B-Cells, PBMCs (peripheral blood mononuclear cells), mucosal, cerebral spinal fluid, bone marrow tissue types) sourced from humans and mice; |
| (ii) | Data capture about cell surface markers and numerous types of cell secretion (antibodies, cytokines) through our proprietary microengraving process utilizing microarrays and microscopy; and |
| (iii) | Recovery of individual cells in order to obtain single cell gene sequence information through reverse transcription polymerase chain reaction (“RT-PCR”), a method for amplifying and copying rare DNA molecules. |
| 16 |
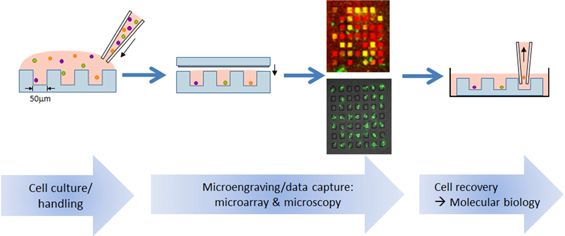
Figure 1: Platform Overview
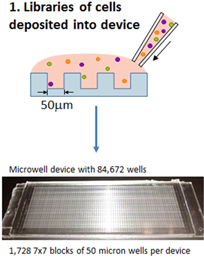
The first component of the platform process is a thin PDMS chip the size of a standard microscope slide that contains on its surface a molded dense array of spatially addressable subnanoliter microwells. It may be configured for arrays containing 85,000 or 250,000 microwells, depending on the experimental protocol. Utilizing custom-crafted molds, lab technicians are able to manufacture chips on-site in our laboratory.
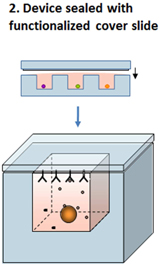
The second component of the process is a glass microscope slide (the “Slide”) that has been functionalized (i.e. coated with capture reagents) to enable it to create a “printed” microarray representation of secreted proteins from individual cells within each microwell following the Slide’s brief attachment to the top of the Device, in effect, sealing each individual subnanoliter microwell for a fixed period of time.
Sample preparation and loading onto the Device and subsequent microarray “printing” on the surface of the Slide follows routine laboratory procedures which can be performed and scaled rapidly. For screening of antibody-producing cells (B cells), a blood sample is fractionated, diluted and then spread across the Device via a simple pipetting step.
Tissue samples, for example biopsy specimens from the intestinal wall of human patients, can be disaggregated and similarly spread across the Device. Cells are loaded directly onto the Device without prior enrichment or other laboratory-based manipulations that invariably lead to loss of efficiency, viability, or both (e.g. Epstein-Barr Virus-based transformation), preserving the entire original population of cells for analysis.
As illustrated in the diagram of the microengraving process in Figure 2 below, after introduction of human cells (from blood, serum or tissue) to the Device, they are allowed to settle naturally from suspension (optimized at the number of cells/well that best meets experimental objectives) into microwells and weakly adhere to the bottom of the individual wells (Step 1). Device design facilitates cell dispersion across its surface, resulting in rapid loading and analysis by the user.
The microwell configuration defines a collection of independent and viable sub-nanoliter cultures of individual human cells (or mouse cells, as the experimental protocol dictates). The small volumes used to confine each cell enables rapid detection of material secreted by the living cells. Because an individual cell within a well represents a significant portion of the volume of the well in which it settles, commonly employed artificial signal amplification procedures that can bias research output, e.g. cell proliferation, prove unnecessary.
Detectable secreted material include antibodies, cytokines and other pharmacologically relevant substances. The extremely small scale ensures that adequate quantities of secreted materials are available for analysis after very short incubation periods (10–60 minutes).
| 17 |
The Device is then placed in contact with a Slide and the cell secretions occurring in each microwell during the incubation period are captured via a ‘printing’ step where each location on the now printed Slide is spatially registered to a corresponding microwell in the Device (Step 2).
To accomplish this transfer/printing, the Device is temporarily attached and sealed to the Slide which has already been functionalized (with user-defined capture reagents) to bind the secreted material from each cell onto the surface of the Slide. Slides can be functionalized with multiple capture reagents, enabling multiplexed assays to be performed.
After Device and Slide are subsequently separated (Step 3), the Slide surface retains a microarray (essentially, a recording) of secreted material where each spot in the microarray corresponds to the secretions/metabolic activity of an individual cell, and the Device retains the individual viable cells within the microwells.
The Slide’s surface is then studied (“interrogated”) in a manner identical to other microarrays using fluorescent-labeled detection reagents and off-the-shelf slide scanners to reveal and characterize, for example, antibodies and their properties, such as potential affinity and specificity. These data are similar to those obtained by more laborious assays for function (such as ELISpot, Enzyme-Linked ImmunoSpot assay, a widely-used method for monitoring responses of immune cells that relies on populations rather than single cells), phenotype (such as FACS, Fluorescence-activated cell sorting, whereby cells are labeled with fluorescent dyes and suspended in a stream of fluid and passed by a laser-based detection apparatus), and genotype (RT-PCR). However, unlike those assays, we believe our platform permits more efficient assessment of all of these functions within the context of the single cell microwells and retrieval of single viable cells in a planar format, enabling higher sensitivity and detection of rare events.
While analysis of the Slide’s microarray proceeds, the cells are retained in their Device microwells and remain viable during the printing process and analysis of phenotype. Assay methods for determining cell function and phenotype (ELISpot, FACs, et. al.) that are common to other approaches usually result in the death of the cells under study. Thus, with our platform, individual cell secretions can be analyzed while retaining viable cells for further analysis.
The cells that correspond to the antibodies of interest can later be retrieved from individual wells by manual or automatic micromanipulation for further analysis, including cell-cell assays, gene-based analysis and gene recovery via RT-PCR. In addition, further sequential rounds of micro-engraving onto additional multiplexed capture Slides can be performed, greatly expanding the analytic capability of our platform.
| 18 |
Screening
 |
 |
 | ||
| Simple pipetting step achieves desired cell density, e.g. single cell occupancy | Secreted proteins bind to capture antibodies on cover slide | Spatially-registered custom protein microarray allows identification of cells for recovery |
Analysis & Recovery
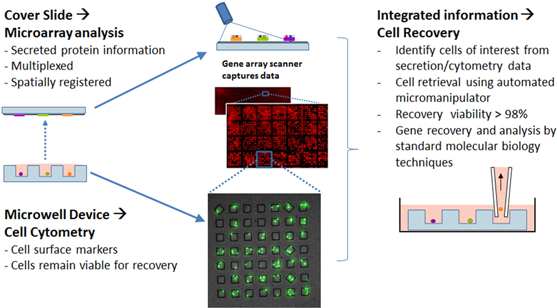
Figure 2: Schematic Diagram of our Microengraving process – Screening, Analysis and Recovery.
In Figure 3 below, the relationship between the Device (in which cells have been distributed and settled) and the cover Slide (which registers secretions from the deposited cells) is illustrated.
The cover Slide is diagrammed on the right as having been “functionalized”, meaning it is coated with a “capture reagent”. As a result of this functionalization process, proteins (including antibodies) that the in-well cells secrete will now bind on the surface of the Slide.
| 19 |
When the Slide is read by our automated slide reader, we observe the antibodies secreted by the cells as colored features produced by fluorescent dyes as displayed in an actual Slide image as shown on the lower left. The spatial registration of the Slide and the Device enable one-to-one identification of image and cell. Software is provided by third-party vendors and developed by Enumeral to implement automated data collection and registration.

Figure 3: Reading of antibody secretion events from single cells.
In Figure 4 below, the cover Slide on the left depicts several antibodies attaching themselves to the capture agent that coats the cover Slide, as shown previously. But now, the target (Antigen, or Ag) is diagramed as attaching or “binding” to certain of the antibodies that had been captured by the specific reagents which had functionalized the cover Slide.
To enable us to “see” where the target has bound to antibodies, a fluorescent reagent that produces a specific color is introduced and it signals those locations where the target is present in the middle right Slide image (shown as green in this case). The spatial registration of the Slide and the Device enable one-to-one identification of image and cell.
When the two florescent channels are read on the Slide as a merge of antibody (Ig+) and antigen (Ag+) by the slide reader, we now know which of our individual cells– alive and safely retained in the Device – are capable of producing antibodies to the target of interest.
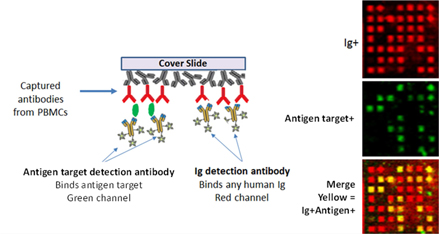
| Figure 4: Monoclonal antibodies (“mAbs”) secreted from single cells screened for binding to an Antigen target. |
| 20 |
After identifying cells that generate antibodies of interest, we retrieve the individual cells and perform RT-PCR in order to obtain DNA sequence information corresponding to the potential antibodies.
Enumeral’s single cell RT-PCR recovery rates for matched heavy and light DNA sequences using our established protocols are typically greater than 50%, with 80-90% for antibody heavy chain and light chain genes individually, compared to the industry standard of around 50% for heavy chains, and significantly less for antibody chain genes (see “Cloning and expression of murine Ig genes from single B cells”, T. Tiller et al., Journal of Immunological Methods 350 (2009) 183–193). Subsequent to this verification, DNA samples are sent to a commercial vendor for DNA sequencing. When we obtain gene sequences, following in-house bioinformatics analysis, we select and then express sufficient quantity of antibodies to confirm their target-binding properties and perform other target-specific assays to establish whether or not they demonstrate desired functional properties.
In practice, we have thus far employed our technology for retrieval of antibody secreting cells of interest and to obtain gene sequence information for antibodies binding to a number of disease-relevant protein targets.
Application of Our Technology Platform to a Broad Range of Cellular Analyses
While the antibody recovery and characterization at the single-cell level is the critical technical capability enabling us to generate diverse and proprietary antibody libraries for our own and partnered programs, the ability to measure multiple functional determinants during the same process, including multiple secretion measurements and single cell surface marker expression, and other profiling advances are integral to our discovery, corporate collaboration and grant proposal efforts.
Single-cell functional profiling also makes possible (i) simultaneous and unbiased analysis of an extensive repertoire of cellular expression from the same population of cells, including antibodies, cytokines, other proteins and genetic sequencing and (ii) advanced profiling options such as quantitation of secretion rates and on-device cell:cell assays.
To illustrate the microengraving process involved in a broader range of cellular analyses, the single print process previously shown in Figure 1 is expanded below to depict the creation of multiple microengraving prints in order to detect multiple protein secretions from the same array of cells in Figure 5 below. Such assays have been developed and employed in one of our ongoing partnership programs.
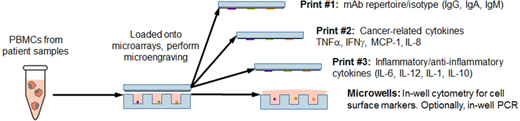
| Figure 5: Assay design, parallel assessment of B- and T-cell repertoire from human PBMCs |
Since microengraving technology has use independent of cell type, the applicability of single cell interrogation in our system is generally limited only by the availability of binding reagents, the availability of commercial reagents such as labels used in assays, and ex-vivo cell viability (e.g. B-cells are relatively fragile while T-cells can persist as long as 10-14 days).
In the case of antibodies, the cells distributed across the Device can be exposed to (“probed with”) an antibody that is a potential drug candidate, to determine at the single cell level potential effects of the antibody upon cellular functional activity. In the case of cell:cell assays (discussed below), target (diseased, e.g., virally infected) cells can be loaded onto the Device and treated with a discovered antibody candidate, followed by administration of CD8+ human T-cells - one of the key immune cell types thought to be involved in attacking diseased cells. Outcomes (e.g. target cell killed) can be observed on a well-by-well basis; when combined with a cytokine release assay by use of our printed microarrays (the Slide), function can be linked to expression phenotype. The expected output of such an experiment might be the identification of an antibody that promotes or enhances the ability of CD8+ T-cells to kill the target cells, potentially defining a new class of drugs that might be effective in treating cancer or other diseases.
| 21 |
For example, we have shown the ability to detect secreted antibodies and secreted cytokines in parallel, using the same single cell population, using multi-print assays with appropriate combinations of capture and detection agents on the functionalized cover slides as shown in Figure 6. On the left, Print #1 measures antibody secretion and identifies the isotype (IgA, IgG, and IgM) of the secreted mAbs; on the right, the cytokines TNFα, IL2, IFNγ, and IL17 were measured. Representative microarray images are shown for each, with the distribution of isotypes and cytokines presented below.
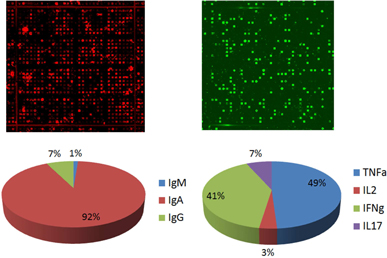 |
| Figure 6: Antibody secretion and isotype identification (left) and cytokine secretion (right) from human mucosal biopsy tissue. Data shown on the left is percentage of total number of wells positive for antibody secretion (of all isotypes). Data shown on the right is percentage of total number of wells positive for cytokine secretion (of any of the four cytokines measured). |
In addition to being able to multiplex secretion information, secretion and surface expression data are mapped to individual cells, allowing identification of rare secretion phenotypes and determination of cell lineage. An example of our ability to measure both cell surface marker expression and multiple cytokine secretion is shown below, identifying T-cell subsets (CD4+, CD127+, and CD25+) and their respective secretion of cytokines IFNγ, TNF-α, IL10, and IL17. This is particularly advantageous in applications such as prediction of “Cytokine Storm”, using PBMCs dosed ex-vivo with drug candidates of interest. This ability to multiplex secretion data and determine cell lineages is one of the central advantages of the technology, and is important across all of our business activities.
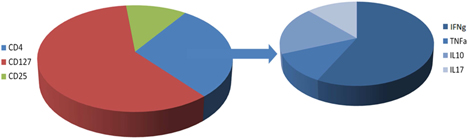 |
| Figure 7: Linking surface marker expression with secretion. The distribution of surface expression markers is shown on the left and secretion profiles of the CD4+ compartment shown on the right. Data shown on the left is percentage of total number of wells positive for any of three cell surface markers showing the respective frequency for cell surface markers (CD4, CD127, CD25). Data shown on the right provides the breakdown of how many of the CD4+ wells contained cells secreting the noted cytokines as a percentage of the total number of CD4+ wells secreting cytokines. |
| 22 |
As a further illustration of the platform’s ability provide multiple indicia of function of a set of cells, the identification of T-cells that are able to kill virus infected cells has been demonstrated and published based on research performed in our scientific founder’s laboratory at MIT (see “A high-throughput single-cell analysis of human CD8+ T cell functions reveals discordance for cytokine secretion and cytolysis”, N. Varadarajan et al., Journal of Clinical Investigation, doi:10.1172/JCI58653).
Figure 8 below, shows the schematic for the experiment on the left, with CD8+ T-cells and target (HIV) cells loaded onto the device. The outputs of the experiment are shown on the right, where secretion information (IFNγ and IL2) is shown in addition to information as to whether the T-cells in the wells were able to kill the resident target cell.
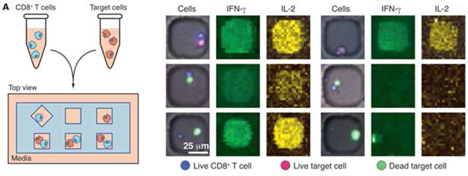 |
| Figure 8: Measurement of T-cell effector function (published as “A high-throughput single-cell analysis of human CD8+ T cell functions reveals discordance for cytokine secretion and cytolysis”, in Journal of Clinical Investigation, 2011, doi:10.1172/JCI58653) |
We believe this type of assay could become a more integral part of discovery and development efforts in an effort to understand the biology of discovered therapies prior to the investment in costly clinical trials, and understanding patient- and cell-specific mechanisms early in the development process is a key area where we believe we can add value to partners beyond discovery of new antibodies, potentially helping bring therapies to the right subsets of patients where they will be most effective.
Internal Discovery Programs - Immunomodulatory Antibodies to Treat Cancer and Other Diseases
Enumeral’s Technology Platform in Drug Discovery
The Company is currently engaged in internal programs to discover and develop antibodies. The aim of our internal discovery programs is to generate superior antibodies that we will co-develop, either through collaborative partnerships, or through out-licensing partnerships, with larger biotechnology or pharmaceutical companies. Our goal initially is to obtain such partners following completion of “ex vivo” human and animal preclinical studies, and prior to completion of full IND-enabling studies, or we may fund IND-enabling studies and seek collaboration partners at that point. The immediate commercial goal of our programs is to reach significant value inflection points in the next twelve to eighteen months in order to obtain from the sale or license of product discovery programs a combination of up-front payments; subsequent milestone payments as the candidate clears pre-clinical and clinical regulatory hurdles; and royalty payments upon future sales of the marketed drug.
| 23 |
We believe that the key advantages of our approach include our ability to perform drug candidate validation at each of these targets, in the following ways: we can understand at the single cell level, within biopsy samples containing very few immune cells, whether or not these proteins are expressed, and the variety of cell types upon which they are displayed – all of which could impact whether or not a given product candidate would produce a beneficial effect, or, a toxic effect, within a given patient, and, a given disease state. These single cell functional profiling capabilities provide for a deeper understanding of the variety of human responses, and thus, may provide a rational basis to guide product design and development. Indeed, recent reports indicate that the phase 3 candidates targeting PD-1 exhibit different side effect profiles in patients, suggesting that they may have fundamentally different mechanisms of action (see for example, “Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma”, N Engl J Med. 2013 Jul 11;369(2):134-44, and “Safety, activity, and immune correlates of anti-PD-1 antibody in cancer”, N Engl J Med. 2012 Jun 28;366(26):2443-54). Such differences and lack of understanding may hinder further development and label expansion. Enumeral’s capabilities may provide significant opportunities to rationally develop PD-1 targeted therapies using its ex vivo human cell targeting assays.
In our view, our technology platform can improve the prospects of antibody discovery programs by enabling quantitative screening of antibody-secreting cells in a population of cells without introducing bias from prior sorting according to cell surface markers or other low-yield transformation and handling steps. It is also agnostic as to the species of cell sample origin – for example, human, mouse or rabbit. Our internal programs seek to leverage both sources – proprietary immunized mouse-based libraries as well as proprietary human patient donor-derived libraries.
When applied to cells derived from patient samples, we believe that our technology increases the probability of finding rare antibodies that have the characteristics that are essential to becoming safe and effective drugs while also providing for an assessment of antibody affinity, specificity and potential tolerability much earlier in the antibody discovery and development process than has previously been achieved. Further, quantifying individual human immune response directly in blood or biopsy samples should enable the development of immune-based diagnostic products for the classification of responders and non-responders to therapeutics.
We believe our technology makes possible screening against complex targets, such as whole virus or cells, while simultaneously providing for counter-screening against undesirable targets. It can be employed with individual single cells from any sample of isolated tissue, including PBMCs, bone marrow, precious samples of Cerebral Spinal Fluid (“CSF”) or biopsy material from tumors or mucosal tissues lining intestinal, oral, nasal and pulmonary tracts. Most of these tissue types are inaccessible from other technologies. Measuring single-cell secreted products can also be used to improve product quality of production cell lines.
Native human antibodies offer many compelling advantages over antibodies derived from non-human systems. As an example, native antibodies that specifically bind tumor antigens frequently arise in cancer patients. These antibodies result from the interaction between cancer cells and the host immune system and could be adapted for use as therapeutics or diagnostic reagents. In addition, the study of cloned antibodies may reveal the complexity of an effective humoral immune response while producing antibodies for the prevention and treatment of infectious diseases by passive immunization.
The systematic examination of the antibodies that accompany auto-immune diseases may provide clues to the pathogenesis of these diseases as well as guide the creation of novel immuno-modulatory drugs. Unfortunately, because of the difficulty in rapidly and reliably immortalizing human B-cells and the scarcity of human B cells expressing antibodies of the desired antigen specificity and affinity, the potential utility of native human mAbs has not been realized. Our platform is not limited by such requirements for immortalization.
Current Drug Discovery Programs.
The Company is currently engaged in internal programs to discover and develop antibodies targeting three checkpoint protein targets for the treatment of cancer and other diseases. Included among our programs are PD-1, OX40 and Lag-3, three checkpoint proteins expressed on the surface of the body’s immune cells.
Therapeutics targeting these proteins may have application in treating multiple diseases in addition to cancer, including inflammatory, autoimmune, and infectious diseases, and we believe application of our platform will enable us to determine rational development strategies across these diseases using our ex vivo human preclinical studies.
| 24 |
PD-1 and OX40, along with, CTLA-4 (the target of Yervoy) and Lag-3, are a group of functionally-related proteins expressed on various immune cells, including T cells, that together function as immune checkpoints (discussed above; for detailed discussion see “Advances in targeting cell surface signaling molecules for immune modulation”, S. Yao et al., Nature Reviews Drug Discovery, Vol. 12 (February 2013) 130-146). A full understanding of the myriad cell types, disease situations, and variability with respect to human populations, where these proteins are expressed, and exactly how and when they function, is not presently known – breaking this “code” is the opportunity that we believe Enumeral’s platform technology advantages could provide, and may present a significant business opportunity for us.
Antibody Screening. We have developed assays to identify animal- and human-derived native antibodies that bind to PD-1, and OX40, and Lag-3. Initial screens performed by a scientist have identified within three months the first potential anti-OX40, anti-PD-1, and anti-Lag-3 native human antibodies from cells obtained from patients. Further characterization of these antibodies is ongoing. Importantly, the existence of such antibodies in a variety of autoimmune patient donors may suggest that modulation of these targets could be relevant for treatment of these diseases, or for the development of biomarkers and patient stratification strategies for accelerated clinical trials.
Our internal program objectives include application of our technology and assays to stratify patients with various cancers and other disease that might benefit from immunomodulatory treatment. Disease-specific assays can be rapidly developed for each type of patient population. Key to creating best-in-class programs will be the development of stratification tools based on our platform that will enable truly predictive decision making during development, which we believe will lead to faster, less costly and, ultimately, more successful clinical development.
Multiple immune correlates have been described in the literature for immunomodulator target function that we can rapidly verify in human micro-biopsy and apply to our own product development programs, including expression and function of various receptors on T cells from within the tumor microenvironment. Such assays will be applied in ex vivo “human preclinical” trials using biopsies from the intent-to-treat population to select lead candidates that exhibit the best immunomodulatory profiles, including appropriate activation of immune cells that target the tumor cells. We will also apply our assays for measurement of the potential for more general unwanted immune-related effects, such as inappropriate cytokine release or lymphocyte activation, especially immune-suppressive or tumor-promoting lymphocytes, to aid in selection of best-in-class lead candidates.
Screening for anti-PD-1 antibodies from immunized rodents commenced at the end of the fourth quarter of 2013 and has led within three months to the identification of novel antibodies that bind to PD-1. Significantly, multiple diverse antibody sequences were identified in our screening, two of which contain similar sequences to two different phase 3 product candidates, Pembrolizumab (Merck) and Nivolumab (Bristol-Myers Squibb), indicating the potential of our platform to rapidly identify commercially-relevant antibody candidates.
Our commercial goal of monetizing a significant value inflection point requires that we have active lead candidates against our targets, along with ex vivo “human preclinical” response profiling data, and completion of initial animal studies. We plan to identify partners and CROs (contract research organizations) to undertake further required preclinical disease model and IND-enabling safety and toxicity studies as well as formulation and CMC package development. We currently expect to have such preclinical and ex vivo human data packages on our first program by early 2015, and our second and third programs, within the second half of 2015.
Other Programs and Capabilities:
Antibodies and Vaccine Response for Influenza. Our scientists have developed screening assays utilizing our platform technology to identify responses to the influenza virus hemagglutinin (“HA”) molecule, the primary determinant of seasonal flu vaccines. As part of these efforts, we have also shown the ability to potentially discover rare, broadly reactive antibodies from vaccinated humans that could form the cornerstone of future work to discover and develop the best immunogen candidates. These antibodies would serve as templates for guiding the design of novel immunogens, and may themselves be potential therapeutic candidates for treatment of influenza infection in at-risk patient groups in the acute care setting (hospitalized, immunocompromised). Enumeral has built a proprietary collection of samples from human donors, collected just prior to and following vaccination.
| 25 |
Vaccine immunogen candidates would be obtained through our proprietary antibody-directed multiplex screening capabilities, using multiple different broadly neutralizing antibodies firstly recovered from our proprietary collection, and assays that we have designed and validated. Work in our R&D labs has demonstrated the necessary capabilities through the identification of 450+ anti-viral antibodies from a human donor during a single day of screening. In addition, broadly cross-reactive antibodies with multiple viral antigen specificities have been identified from human donors.
Our program objectives would include identifying immunogen candidates with the capability of eliciting the broadest number and types of antibodies reactive to viral antigens, in addition to maximal stimulation of immune memory compartments thought to be associated with long-lasting and broad protection. We believe our screening work could lead to first-in-class immunogen candidates for preclinical testing using already-validated assays. In addition, we would leverage our platform to perform animal model in vivo rank-ordering of immunogen candidates for those that elicit the broadest antibody response with minimal stimulation of potential side effects.
Our influenza screening and profiling capabilities are currently the subject of ongoing collaboration discussions with a pharmaceutical company partner, and further plans for progressing a program internally are subject to the outcome of these negotiations.
Antibodies Targeting Inflammatory Mediators. We have discovered novel and selective antibodies binding to secreted protein mediators of inflammation. Such antibodies may represent drug candidates for the treatment of osteoarthritis and diabetic macular edema, and other inflammatory conditions. The protein target is a short, “self” peptide target, representing a particularly challenging target for antibody-directed drug developers employing traditional methods. We are initiating second and third screens using additional antibody-secreting cell libraries as well as samples from our human autoimmune donor samples, in an effort to identify native human antibodies that might indicate features critical for a successful drug candidate, and to build the broadest possible intellectual property estate around this high-value target. This program is currently the focus of partnership discussions with an existing pharmaceutical partner. Through these discussions, we will determine whether additional internal resources, if any, will be devoted to this program.
Our goal of achieving a significant value inflection point requires our mapping of the broadest possible antibody repertoire “space” and completing initial animal model testing of lead candidates along with companion ex vivo “human preclinical” assays to verify candidate safety and efficacy. We would then identify partners and CROs to undertake further required preclinical disease model and IND-enabling safety and toxicity studies as well as formulation and CMC package development.
Antibodies for Paramyxovirus Infections. Paramyxoviruses are an important class of viruses which are associated with respiratory ailments, and common childhood diseases such as measles and mumps. Our scientists have developed screening capabilities for libraries of antibody-secreting cells from both immunized mice and exposed human donors in order to find novel antibodies targeting neutralizing sites on the viral fusion glycoprotein of Respiratory Syncytial Virus (“RSV”). The goals of this program were to establish platform capabilities within the Company’s facilities following technology transfer from the laboratories of our founder at M.I.T., and to initiate and develop our clinical operations infrastructure for obtaining human patient samples. Data and capabilities generated with these efforts have supported further technology development and business development marketing activities.
While our paramyxovirus programs are not currently ongoing due to a shift in our strategic focus, our program assets and capabilities may be of interest to potential vaccine and infectious disease therapeutic companies, and could form the basis of a future collaboration.
Collaborative Partnerships
Collaboration Strategy.
We seek to enter into collaborative partnerships in order to engage in product discovery and development where we can benefit from the potential leverage provided by large companies, including capital, research and development capabilities, manufacturing, clinical and regulatory expertise, and sales and marketing. Many of the functions necessary for new medical products to be developed are not currently part of Enumeral’s infrastructure, and as such, a key part of our strategy is to identify collaborative partners to help move products forward toward commercialization.
| 26 |
Our collaborative partnerships have initially taken the form of proof-of-concept projects that have provided for modest cash payments (in the hundreds of thousands of dollars per project), and one has provided commercialization rights including shared (equal) ownership in any Enumeral-discovered antibodies, and rights to certain therapeutic and diagnostic outcomes. These collaborations have validated our technology in a commercial setting as we advance a partner’s research objective. The primary purpose of engaging in proof-of-concept projects has been to leverage specific scientific outcomes into larger and potentially profitable collaborations that employ our technology across the wider spectrum of our respective collaborative partner’s discovery and development activities. We believe that large-scale discovery collaborations will provide revenues that will reduce future operating losses and equity requirements, and we do not currently anticipate entering into further proof-of-concept collaborations.
The large scale collaborations that we seek to enter into are intended to provide to partners the same advantages of our technology that we anticipate realizing in our internal discovery programs – significantly lower drug discovery and development costs and/or reduced time required for their therapeutic candidates to move to the next stage toward successful product launch. In return, we seek to capture a meaningful portion of the value that our collaborations create for our partners by obtaining some combination of technology access fees, full time equivalent (FTE)-based research funding and the right to sell or license to our partner (or third party) our ownership position in jointly owned therapeutic discoveries. In turn, a sale or license of our ownership interest could result in the Company obtaining a combination of up-front payments, subsequent milestone payments as the candidate clears pre-clinical and clinical regulatory hurdles, and royalty payments upon future sales of the marketed drug.
Discussions with partners regarding potential large scale collaborations may prove inconclusive for a variety of reasons, including changes in research or other corporate priorities among any of our current or prospective partners. Partnership agreements that we may ultimately enter into may terminate without benefit to us if the underlying products are not fully developed or if the contractual research aims are not met. If we fail to meet our obligations under these agreements, they could terminate and we might need to enter into relationships with other collaborators and to spend additional time, money, and other valuable resources in the process. We cannot predict whether our collaborators will continue their development efforts or, if they do, whether their efforts will achieve success. Many of our collaborators face the same kinds of risks and uncertainties in their business that we face.
Current Collaborations.
Presented below are summary descriptions of five proof-of-concept collaborations with four leading pharmaceutical companies including descriptions of data that we have developed that we believe demonstrates compelling value of our technology. Work with Collaboration Partner #1 (Sanofi US Services, Inc.) is nearing completion. It has prompted our partner to review additional targets in their discovery pipeline that could potentially benefit from the use of our technology platform. Our work with Collaboration Partner #2 (Crucell Holland B.V.), involving two separate proof-of-concept projects, has been completed. Our partner requested that we propose the basis on which we would further collaborate and these discussions are ongoing. Our work with Collaboration Partner #3 (Anthrogenesis Corporation d/b/a Celgene Cellular Therapeutics) is nearing completion, and we anticipate that discussions of a long-term strategic collaboration with this partner will follow submission of our final report. Our work with Collaboration Partner #4 (Novartis Pharma AG) is also nearing completion, and it has already resulted in an invitation to discuss additional ways we can collaborate.
In the next twelve months we are focused on entering into one or more large-scale corporate collaborations engaged in joint product discovery and development programs that can provide up-front payments, follow-on fees and research funding which will reduce the Company’s overall operating losses and need for additional equity capital. And we would seek to retain commercialization rights to certain therapeutic and diagnostic applications of the discoveries resulting from large-scale corporate collaborations.
| 27 |
There are several factors that potentially benefit our ability to secure larger-scale collaborations. The data generated in our internal product discovery programs is of a type that partners have unsuccessfully sought to obtain from other technologies. Platform technology skills in therapeutic discovery and immune profiling activities perfected by our scientists on internal discovery programs enables them to more effectively construct attractive research to prospective corporate collaboration partners. We are optimistic that we shall achieve our objective of entering into large-scale joint therapeutic discovery and development corporate collaborations that will enable us to follow the precedent of other companies that have successfully leveraged corporate partnerships to create value for their shareholders.
Collaboration Partner #1 (Sanofi US Services, Inc.) – Monoclonal Antibody Discovery
In our work with this partner, we have used our technology to screen for monoclonal antibodies that bind to a validated target that is thought to play a role in multiple inflammatory conditions. Antibody screening from single antibody-secreting cells is one of the more straightforward applications of our microengraving technology. As a condition to proceeding with this collaboration partner, we have agreed to not engage with a third party in a program related to antibody discovery against this target for a period of seven years from end of the term of the collaboration. Enumeral believes that the target in question is unique to this program and will not have an adverse impact on its other collaborations and future potential collaborations.
The cellular libraries for this project originated from mice immunized by the partner, specifically mouse B cells (splenocytes) and plasma cells (bone marrow), the latter representing an immune compartment not routinely utilized for generating target-specific antibody diversity.
Our completed screens have repeatedly identified screening hits against the antibody target of interest using both fresh and frozen cells, including bone marrow-derived antibody secreting cells, with greater efficiency than that used by our partner.
Inasmuch as bone marrow is a potentially untapped reservoir of antigen specific antibody secreting cells, we believe that bone marrow represents a more diverse source of antibodies than what can be found elsewhere in the circulation – our ability to screen for antibodies from bone marrow displays the potential for our platform as a “discovery engine” for identifying antibodies against intractable targets.
Our partner informed us that they intend to evaluate other projects that could potentially benefit from the use of our technology platform.
Collaboration Partner #2 (Crucell Holland B.V.) - Vaccine Research
Our work with this partner consisted of two contractually separate, but scientifically related, proof-of-concept projects. Both programs were designed to showcase the efficiency of our platform at identifying interactions between important targets and disease-protective antibodies sourced from human patients.
These proof-of-concept projects have concluded. At our partner’s request discussions of further collaboration efforts have begun, including initial negotiations of draft economic and business terms. Importantly, this partner has determined that it may seek to license our platform for multiple applications, including antibody discovery and immune profiling, as well as vaccine research.
Collaboration Partner #3 (Anthrogenesis Corporation d/b/a Celgene Cellular Therapeutics) – Immune Profiling
Our work with this partner focuses on measuring complex immune functional signatures from specific subpopulations of cells from patient biopsy samples. These information sets include protein secretion and cell-surface markers in parallel and the ability to retrieve cells of interest for further study (similar to the experiments we have described above in our NCI-sponsored research program).
The major capability of our technology that is valuable to this partner is the analysis of samples where cell counts are very limited; this is particularly well suited to tissues only accessible via biopsy or other invasive means. This is important because it allows measurement of cellular function at the site of disease, rather than reliance on a circulating marker (which may be at a very low concentration or, potentially, absent in some patients).
| 28 |
Our work will potentially allow for the determination of the immune signatures reflective of a successful response to novel therapies, and there are clinical-stage novel immunomodulators in this company’s research pipeline that are of specific interest. Such an assay would in effect be a “potency assay” for such novel products, and may also enable stratification of patients based on good responses versus poor responses. In particular, we are focused on analyzing rare subsets of cells which can’t be measured with more traditional approaches, but whose activity might be critically indicative of product success or failure.
Whereas conventional alternatives such as fluorescence-activated cell sorting (FACS), have limited resolving power on these rare cells, we believe that our technology is able to identify and analyze this fraction with comparative ease because of our platform’s ability to look directly at these rare subpopulations and resolve both secretion and cell surface markers at the single cell level.
A key point resulting from this program is that our capability to analyze sample sizes of a few thousand cells – typically the case with mucosal tissue, biopsies, fine needle aspirates, and cerebrospinal fluid (CSF) – is a differentiated competitive capability, providing insight into tissue properties and cellular function that was heretofore inaccessible.
The research program has been completed and preparation of a final report is in progress. Success on this project will represent further commercial validation of the platform’s immune profiling and biomarker discovery capabilities. We believe that successful completion of this project represents a gateway to developing the basis for a broader collaboration with this partner.
Collaboration Partner #4 (Novartis Pharma AG) – Immune Profiling
Our work with this partner is focused on using our platform’s immune profiling capabilities to measure the biological effects of administered therapies in ex-vivo “human preclinical” patient cohorts as a predictor of potential adverse events in later clinical development. Such capabilities, we believe, are an important application of our platform, and is planned to impact all of our internal programs – rank ordering candidates not only for their potential on-target mechanistic efficacy, but also, their potential for inappropriate activation of potentially damaging or inflammatory cell types, such as macrophages.
Such inappropriate immune cell activation is one of the major risks inherent in developing immunotherapies, as it is possible for the immune system to “fire all at once”. Many immune cells, while normally quiescent, when inappropriately activated can unleash toxic payloads in the form of tissue degrading enzymes, inflammatory activators, and other secreted protein mediators that activate cell death pathways. The effects of such inappropriate activation are illustrated by the 2006 clinical safety study of the Tegenero antibody TGN1412. When administered to six healthy volunteers at what was believed to be a “sub-clinical” dose (500 times lower than the safe dose in monkeys), all six volunteers were hospitalized in intensive care units with life threatening conditions involving multiple organ failure.
One of the reasons that our platform is useful in this application is that the “predictive” studies are often performed on animal models which may be poorly predictive of responses in humans, such as was the case with the Tegenero antibody. While this fact has been noted in the field for some time, this was described further in a publication in the journal Science (Cameron et al, 2013) regarding off-target effects resulting from drugs against MAGE-A3, which was the target of a cancer immunotherapy developed by Glaxosmithkline in a Phase III study in 2013. Though there are currently no reliably predictive animal models in this area, the use of human cells derived from patient cohorts and the delineation of which cells are causing any adverse reactions, we believe, will be a key aspect of our internal development programs achieving a higher likelihood of success.
Work with this partner is nearing completion and it has already resulted in an invitation to discuss additional ways we can collaborate.
| 29 |
Future Collaborations
We have proposed larger collaborations with pharmaceutical companies. If successfully negotiated, we shall meet our goal of leveraging proof-of-concept results for corporate partners into larger collaborations.
In all of these proposals we are looking for our technology to play a pivotal enabling role in accelerating the discovery, development and/or regulatory approval of a partner’s product program and when it is possible to obtain valuable co-ownership rights for joint discoveries. Areas we are exploring with these prospective partners include:
| (i) | Antibody discovery (screening) programs based on a partner’s libraries and biological materials; |
| (ii) | Functional immunophenotyping for patient stratification: to accelerate clinical-stage drug programs or repurpose marketed or clinical-stage drug programs; |
| (iii) | Ex vivo testing of late preclinical monoclonal antibodies against immunology and inflammation targets for potential safety issues in a population of intent-to-treat patients, while retaining companion diagnostic rights; and |
| (iv) | Technology development focused on expanding the use of the platform to identify drug and diagnostic candidates against novel cell biology targets. |
Where the scope of a program allows, we seek to retain rights based in areas that are considered non-core to the partner. As an example, on a program where we were performing immunophenotyping, we would seek to retain the rights to screen those patient samples for internal discovery programs. As a note, the ability to retain these rights is bolstered by our ability to obtain patient samples, rather than relying on partners to supply them for us.
Grants and Contracts
To improve the commercial potential of our internal discovery programs and our corporate collaborations, we will also seek to develop novel applications of our technology through fully-funded foundation and government grant and contract programs, such as through the National Institutes of Health’s SBIR program. If awarded, these foundation and government grants and contracts will provide non-dilutive capital dedicated to the advancement of our technology and R&D capabilities through recovery of both direct and overhead costs and a small profit margin. Another important aspect of the grants and contracts is that the assets that might be created through these efforts will be, in most cases, fully owned by the Company, subject to the retention of the rights for use by the U.S. federal government, and can either be out-licensed or serve as proof-of concept data to support larger corporate collaborations. The programs also offer a forum for communicating our technology to the larger scientific community which, in turn may enable us to build relationships with leading scientists and academic research institutions that can provide the basis for future internal discovery programs and collaborations.
National Cancer Institute (NCI)
We have completed a Phase I research contract with NCI, a unit of the National Institutes of Health (“NIH”), to analyze B- and T-cells (at the single cell level) in parallel with cell surface markers in both diseased and healthy intestinal tissue samples from patients with colorectal cancer. This work established our capabilities in measuring samples of with limited numbers of cells, such as punch biopsies, as this is a limiting factor with many technologies in use today. Our final report was accepted in November 2013.
This completed work has funded the development of assays and capabilities by our scientific team that will be highly useful in our internal programs – specifically in profiling human gut tissues, which will be highly relevant in cancer immunotherapy development and, potentially, infectious and inflammatory diseases as well. These data can be used to drive additional partnerships, as analysis of biopsies and other small samples represent an advantage of our technology over current alternatives. At the invitation of NCI, we have since submitted an application for a “Phase II” contract, which was not accepted.
| 30 |
Intellectual Property
We have a license agreement (“License”) with MIT, Harvard University (“Harvard”), the Whitehead Institute for Biomedical Research, and the Massachusetts General Hospital, that provides us with exclusive worldwide rights for certain key patented intellectual property (IP) underlying the core technology platform developed by our Scientific Founder. The license provides the Company with rights in all fields of use, enabling broad flexibility in developing the technology for use in its internal and partnered drug discovery and development programs, as well as providing for the flexibility to commercialize, non-core biotechnology applications such as diagnostics and bioprocess as part of its overall financing strategy. The license is subject to the completion by the Company of certain due diligence milestones.
Our licensed patent portfolio currently comprises 6 patent families, with three issued or allowed and eight pending U.S. applications, including four continuations of the foundational issued patent. We are pursuing the prosecution of various pending claims in multiple international jurisdictions and intend to file patents on or keep trade secret improvements to our core platform technology.
We recently received a notice of allowance from the U.S. Patent Office for one of our continuations of the issued U.S. patent covering methods for antibody screening as well as our patent application covering our novel device. This latter patent represents the first of the Company’s licensed estate that will issue providing broad device claims. The allowed continuation further enhances our overall IP position as it covers methods of using our device and methods of performing our core microengraving technology, as well as applications of these methods. Together, we believe these allowed patents will provide us with a significant barrier to entry commercially and a strong patent position, both defensively and offensively.
We anticipate filing additional patents covering novel antibodies and biomarkers that we discover. On an opportunistic basis, the Company will seek to in-license “picket fence” patents to protect its core processes and platform, as well as patents covering novel product candidates that may be synergistic with its current programs and approach. The Company has filed an Opposition in the European patent office against a recently granted European patent controlled by a company with a microwell array platform. Should the Opposition succeed, the Company believes such a result would place it in a strategically advantageous position with respect to the business of the third party which holds the patent subject to the Opposition. Should the Opposition fail, the Company believes it shall not have a material adverse impact to its business. We expect to learn the Office’s preliminary opinion in the matter within the next six to eighteen months.
Our technology may also provide certain advantages in formulating novel intellectual property around biological discoveries. The Company’s platform, with its ability to relatively quickly and easily generate multiple antibodies with desired properties against a target antigen, is particularly advantageous in light of two recent patent law developments.
First, according to a decision of the United States Court of Appeals for the Federal Circuit in 2011, in Centocor Ortho Biotech Inc. v. Abbott Laboratories, when one is claiming antibodies with specific properties, where generating such antibodies is not conventional and routine, using well-developed, mature technology, the patent application must describe antibody(ies) that fall within the scope of the claim in order for the written description requirement for patentability to be satisfied. We believe that the speed with which we can generate antibodies with the desired properties should facilitate having such representative antibodies described in the patent application. Second, the conversion of the US patent system to a “first to file” system under the America Invents Act places even greater emphasis than in the past on getting an early filing date. Our technology facilitates having quickly the necessary information to file a patent application that satisfies the written description requirement, so as to allow an early filing.
Competition
We compete with companies engaged in antibody discovery and development and other companies focused on responder and profiling. We believe that the competing screening and response profiling technologies that these competitors employ can be classified as fitting into one of three categories: enzyme-linked immunosorbent assay (ELISA)-style supernatant approaches; flow cytometry-based approaches; and phage display (limited only to antibody screening). There may be other approaches that we are not aware of.
| 31 |
Each of these alternative methods offers some of the benefits of our microengraving technology, but, in practice, we believe each has drawbacks compared to our technology, and that most of which we are aware are not useful if starting cell numbers are low, or if the objective is to understand comprehensive response, such as from a patient-derived biopsy sample.
Antibody Discovery. Antibody discovery requires immunization of a host animal with a pre-determined, often recombinant, protein antigen (target). This first step engenders an immune response whereby the host animal generates large numbers of antibody-secreting cells. Two key problems follow from this: the resulting antibodies are not human-derived sequences, nor have they been optimized via the secondary pathways of the human immune system (that is, they are not “host evolved” in a way that might be optimal for a therapeutic intended to treat humans), and perhaps more importantly, screening for and identifying rare antibodies of interest remains a key issue.
Various solutions in the industry have emerged, including “humanization”, “fully-human” mice, and phage display (microbial cell system-based display of antibody sets generated through molecular cloning techniques). Whichever source of antibody-secreting (or displaying) cells is used, the efficiency in each step of the various processes that leads to detection of an antibody of interest is poor, and the associated processes are time-consuming. For cells derived from animal hosts, immortalized versions of the antibody-secreting cells must be generated, either via ‘hybridoma’ techniques or EBV transformation. Yields are poor, and follow-on steps for detection of antibodies introduce further loss and bias as highly-secreting clones tend to be over-represented.
We believe that our technology can circumvent these limitations by screening every single antibody-secreting cell in a collection directly – whether from humans or from immunized animals.
Following immunization (or creation of phage library), antibodies of interest must be detected and recovered. Competing antibody screening and detection technologies can be classified as fitting into one of three categories: ELISA-style supernatant approaches; flow cytometry-based approaches; and phage display. These alternative methods offer some of the benefits of our microengraving technology, but in practice, each may also have drawbacks as compared to our technology.
Typically, only one key capability – multi-parameter characterization in a single assay, cell secretion measurement, detection of cell surface markers or gene sequence analysis – can be performed at the expense of others.
ELISA-style Supernatant Assays. Assays are performed in standard microplate formats (96, 384 or 1,536 well plates) measure cell secretions and have inherently lower sensitivity because they measure secretions of cell populations, not individual cells and individual B cell growth and antibody secretion rates vary widely. Effective sensitivity is further lowered if replicate sampling is undertaken to achieve multi-parameter characterization. Typically, the input cells are derived from immortalization steps (hybridoma or EBV transformation), so the screen begins with significant signal loss prior to initiation of the assay.
Flow Cytometry. Flow Cytometry incorporates both multi-parameter characterization and the ability to study single cells, though it faces several challenges not associated with our technology. First, B-Cells have variable amounts of surface Ig, effectively reducing detection sensitivity. Second, single cell flow sorting requires downstream cloning/expression of large numbers of irrelevant clones to find those of interest. That is, since flow-based approaches does not directly measure secreted Ig, inference is used to determine whether a cell might be capable of secreting an antibody, while the set of interest to the investigator, would be the subset that is both actually secreted by the cells and that binds to a relevant target. The cloning process must overcome the reduced cell viability associated with flow sorting.
Phage Display. Phage Display has multiple assay development challenges. First, library generation is considerably more cumbersome than direct screening of B cells. Second, the optimal native and Heavy and Light chain pairings are typically greatly diluted by non-native pairings as this approach is essentially ‘blind’ to native antibodies. Third, the highest affinity antibodies are difficult to recover from any panning process without a labor intensive process (lysing the page or cell to recover the DNA and re-cloning into a new library). Fourth, phage display is a single parameter screen; cell surface display combined with flow cytometry introduces the same drawbacks as direct use of flow.
| 32 |
Many of the original innovator companies that developed the indirect mAb discovery techniques such as bacterial phage display and humanized mice (Medarex, Abgenix, and Cambridge Antibody Technology) have been purchased by major pharmaceutical companies.
Other Systems. A number of “next generation” humanized organism (mice and yeast) companies have been formed to take advantage of genome-level improvements in their respective systems. Companies such as Adimab, Ablexis, and Kymab have improved representation of human genes, but still lack access to full human population-based antibody repertoire screening, as we believe is enabled by the Company’s platform technology. It should be noted that there may still remain differences of biology between a human, and a “fully human” mouse, in terms of the evolution of an immune response that produces antibodies.
There are companies that have developed technologies for the discovery of antibodies directly from screening human B cells on the same premise as us but, we believe, with far less efficient technologies that are incapable of comprehensive population-level analysis. We believe that they appear to combine ELISA-style technologies applied in high density wells created by microlithography.
These companies include Vivalis (SC World), Trellis Bioscience, Theraclone Sciences, Single Cell Technology, Natimab Therapeutics. The following summarizes the key similarities and differences of which we are aware compared to our technology:
| · | Vivalis (SC World) – Prior to its acquisition of SC World, Vivalis relied on approaches employed by Trellis and Theraclone (see below). With this recent acquisition, Vivalis now uses a microwell array similar to our platform for spatial segregation of cells, but all detection is done in-well using an ELISA-style assay (i.e. they are not able to practice protein microengraving), which limits multiplexing capability and signal amplification via accumulation on the two-dimensional device surface. The SC World approach does not appear to allow for detection of multiple events from the same cell and is not capable of dynamic measurements of secretion phenotypes, only endpoint measurements. |
| · | Trellis Biosciences – Their CellSpot™ system employs a 96- and 384-well plate formatted-platform for discovering fully human monoclonal antibodies from patient-derived B cells; EBV transformation/hybridoma formation and proliferation, and multiple weeks of culturing; micro-colonies are required amplification step so outcome is biased against gene products (antibodies) that might slow cell growth; insufficient for repertoire mapping, lacks single cell capability nor assessment of comprehensive population-level gene usage, cell lineage assessment, or other functional parameters of non-proliferative antibody cells from a given patient sample. |
| · | Theraclone Sciences – Their I-STAR™ Technology employs similar approach to that of the Trellis and, though it relies on feeder cell layers and mitogenic activation instead of EBV transformation, but in our assessment it has similar limitations as Trellis’s technology. |
| · | Single Cell Technology – uses a microwell array formatted-system for capturing secreted products on planar surface from single cells but, to our knowledge, their system is not likely, in our opinion, to be as robust as binding is detected in-well rather than on a slide following separation and secondary labeling and analysis, and as such, will only provide endpoint measurements rather than dynamic measurements. |
| · | NatiMab Therapeutics S.r.l. – combines EBV transformation of IgG producing B cells from immune donors and a phage display methodology that allows for the display of the antibody variable region. |
| 33 |
| · | HiFi Bio and Raindance – these two companies employ droplet-based encapsulation of cells with highthroughput laser-based sorting. In theory, this is an improvement over FACS-based methods that seek to infer secretion; however, owing to the speed of analysis (retention time) which may limit sensitivity, it is possible that secretion events at the single cell level will be challenging to detect. Verification of live cells and multiplexed quantitative outputs may also be challenging to obtain. These platforms apparently do not support both phenotyping (cell surface markers) and functional output (secretion). As a method for identifying rare cells within a background of irrelevant cells, these systems may face similar limitation to FACS, we believe. |
The approaches that rely on EBV transformation introduce signal loss immediately as the hybridoma formation process has poor efficiency (at best, perhaps 30%-70%). Additionally, due to the fragility of B Cells most of these companies’ processes begin with B cell enrichment either via negative selection or some other method, introducing further signal loss as potential antibody producing cells are lost via the enrichment methods. Generally, any extra handling of B cells introduces bias due to loss of cell viability.
Our technology sidesteps the whole issue of fragile B cells by depositing and containing the Cells in picoliter volume-sized wells, enabling the concentrations of secreted antibodies to rapidly exceed current detection limits and obviate that need for cell proliferation to effect signal amplification.
In this way the antibodies secreted can be picked up very quickly by a capture antibody on the cover Slide; within 20 minutes sufficient antibody can be captured to be usefully analyzed by standard microarray equipment. To our knowledge, the other single cell microwell array format approaches lack our microengraved cover Slide feature which enables our speed, dynamic endpoint or multiplexing capabilities, or near one-for-one interrogation of all cells within a given patient sample.
For example, in the space of a day, a Company scientist can prepare a sample, load the cells into the microwell array device containing ~85,000 wells, capture secreted antibodies on a cell by cell basis from each of the B cells using the microengraving technology.
Having detected an antibody of interest, the corresponding secreting cell can be retrieved from the Device while the B cell is still viable. Unlike these competing technologies, the Company’s scientists can match cell-by-cell specific lineage characteristics and additionally interrogate other cell types in the sample, such as T cells, for their functional status.
Responder Profiling. Understanding human disease as a distribution of population-based variations has emerged in the last two decades as one of the most important advances impacting drug discovery, development, diagnostics, clinical decision making and patient-specific drug utilization.
Rarely are clinical conditions determined by a binary factor, but when they are, much can be learned about the causative physiology of the disease in question, and pharmacologic strategies for treatment. For example, genetic deletion of the receptor for Low Density Lipoprotein, while not a lethal event in humans, gives rise to pronounced elevation of blood cholesterol (in particular, “bad” cholesterol, LDL) and early atherosclerosis and with dramatic elevation of risk of heart disease and heart attack at an early age.
Similarly, humans who are deficient for the chemokine receptors CCR5 or CXCR4 while otherwise healthy are resistant to developing AIDS when they become infected with the causative virus, HIV. Pharmaceutical companies have developed strategies for lowering LDL and blocking the CCR5 protein as strategies for treating the referenced diseases, as a result of these fundamental insights.
A logical extension of these discoveries is implementing in routine clinical care tests to determine whether a patient is likely to benefit from a particular drug, either based on the presence of the specific drug target or a combination of other biomarkers that might be indicative of beneficial response, including both disease remediation and minimal side effect burden.
| 34 |
Most famously, Roche’s Herceptin monoclonal antibody drug that targets the Her2 receptor is only prescribed for patients who test positive for the presence of the Her2 protein in their tumor biopsy specimens. Most diseases and conditions are determined by more than one factor however, making simple binary tests likely to be more the exception than the rule. Indeed, Genomic Health’s OncotypeDx test for guiding chemotherapy selection for the treatment of breast cancer relies on measuring gene expression of multiple genes within tumor biopsies.
Pioneering genomics companies such as Millenium Pharmaceuticals endeavored to understand genetic variation at the level of the genome as a way of discovering such “biomarkers” that could be useful in the clinical diagnostic setting. For many aspects of physiology, genetic variation determines probability of a condition developing rather than actual active disease state or response to a therapy, and as such is insufficient for use in most routine clinical settings. One exception that is noteworthy is human genotypic variation of the drug-metabolizing cytochrome P450 enzymes that break down the anti-platelet drug clopidogrel (Plavix), thereby accounting for potential side effects of this class of drugs.
For most disease and conditions, the specific levels of certain proteins or the activities of certain cell types in the blood are likely to be more indicative of a patient’s health and a physician’s likely course of action in treatment than the patient’s genotype. For example, cardiologist routinely measure brain natriuretic peptide (“BNP”) in the blood, a peptide produced at low levels by healthy hearts, but at elevated levels in over-worked hearts. Elevated BNP is a sign of heart failure, and treatment can be monitored by following BNP levels. Oncologists routinely assess lymphocyte subsets in patient suspected of having leukemia or lymphoma, but are currently limited by adequate tools for quantitative assessment of lineage markers and cell function, which could help determine which therapies might prove most beneficial for a given patient.
We believe that our platform is uniquely enabling for rapid assessment of single cell lineage and function, including cytokine and antibody repertoire, within a population of cells from a patient blood or biopsy sample. Through application of our platform we believe that we can develop “cell function biomarkers”, which would enable rapid assessment of disease state and best treatment course. Further, as the immune system and the activities of subsets of immune cells are very sensitive bell-weathers of acute and chronic changes in a person’s health status, we believe that its platform will have applicability in biomarker discovery in a broad range of immunologic conditions, including infectious disease, inflammatory and autoimmune disease, and cancer.
There are some companies competing within this field that we are aware of. For example, Nodality is a company developing a single cell platform for biomarker discovery. It refers to its platform as “Single Cell Network Profiling”, which measures intracellular signal transduction phenotypes in single cells from patient samples following stimulation of the cells. This technology appears to have utility for discovery of novel pathways and drug targets and may therefore prove useful for understanding an individual patient’s likely response to certain classes of drugs, in particular kinase inhibitors.
We believe that in our technology may prove to be more efficient than Nodality’s platform as a biomarker discovery tool. Furthermore, because Nodality’s platform measures intracellular signal transduction, it is possible that researchers may find it complimentary to our platform in that it may provide a better understanding of the biologic and genetic mechanisms underlying functional responses that lead to therapeutic advances discovered by our technology.
Isoplexis is a recently formed company deploying a device comprised of soft materials similar to ours, and a similar capture slide arrangement. However, their technology contains cells within an array of 8,000 larger microwell (almost 1 microliter volume) of rectangular shape, such that a pre-functionalized cover slide can be applied with a highly-multiplexed “barcode” arrangement of capture reagents for immune cell profiling. Their system may not support microscopy or cell retrieval.
Employees
As of August 19, 2014, the Company employed five business executives and nine scientists, and one operations assistant. None of our employees is represented by a collective bargaining agreement, nor have we experienced work stoppages. We believe that our relations with our employees are good.
| 35 |
The Company’s offices and research labs currently consist of approximately 4,782 square feet of space at One Kendall Square, Building 400, 4th Floor, in Cambridge, Massachusetts, at a current annual rent of $229,536, which will increase to $253,446 over the remaining term of the lease. The lease on this space expires at the end of November 2015. We retain an option to renew the lease for one additional period of three years at the then fair market value. We maintain a small corporate office at 1370 Broadway, New York, New York, at a current annual rate of $21,833. Our lease expires on May 30, 2016, and we may extend in one year increments.
AN INVESTMENT IN OUR SECURITIES IS HIGHLY SPECULATIVE AND INVOLVES A HIGH DEGREE OF RISK. WE FACE A VARIETY OF RISKS THAT MAY AFFECT OUR OPERATIONS OR FINANCIAL RESULTS AND MANY OF THOSE RISKS ARE DRIVEN BY FACTORS THAT WE CANNOT CONTROL OR PREDICT. BEFORE INVESTING IN THE SECURITIES YOU SHOULD CAREFULLY CONSIDER THE FOLLOWING RISKS, TOGETHER WITH THE FINANCIAL AND OTHER INFORMATION CONTAINED IN THIS REPORT. IF ANY OF THE FOLLOWING RISKS ACTUALLY OCCURS, OUR BUSINESS, PROSPECTS, FINANCIAL CONDITION AND RESULTS OF OPERATIONS COULD BE MATERIALLY ADVERSELY AFFECTED. IN THAT CASE, THE TRADING PRICE OF OUR COMMON STOCK WOULD LIKELY DECLINE AND YOU MAY LOSE ALL OR A PART OF YOUR INVESTMENT. ONLY THOSE INVESTORS WHO CAN BEAR THE RISK OF LOSS OF THEIR ENTIRE INVESTMENT SHOULD CONSIDER AN INVESTMENT IN OUR SECURITIES.
THIS REPORT CONTAINS CERTAIN STATEMENTS RELATING TO FUTURE EVENTS OR THE FUTURE FINANCIAL PERFORMANCE OF OUR COMPANY. PROSPECTIVE INVESTORS ARE CAUTIONED THAT SUCH STATEMENTS ARE ONLY PREDICTIONS AND INVOLVE RISKS AND UNCERTAINTIES, AND THAT ACTUAL EVENTS OR RESULTS MAY DIFFER MATERIALLY. IN EVALUATING SUCH STATEMENTS, PROSPECTIVE INVESTORS SHOULD SPECIFICALLY CONSIDER THE VARIOUS FACTORS IDENTIFIED IN THIS REPORT, INCLUDING THE MATTERS SET FORTH BELOW, WHICH COULD CAUSE ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE INDICATED BY SUCH FORWARD-LOOKING STATEMENTS.
If any of the following or other risks materialize, the Company’s business, financial condition, and results of operations could be materially adversely affected which, in turn, could adversely impact the value of our Common Stock. In such a case, investors in our Common Stock could lose all or part of their investment.
Prospective investors should consider carefully whether an investment in the Company is suitable for them in light of the information contained in this Current Report and the financial resources available to them. The risks described below do not purport to be all the risks to which the Company could be exposed. This section is a summary of certain risks and is not set out in any particular order of priority. They are the risks that we presently believe are material to the operations of the Company. Additional risks of which we are not presently aware or which we presently deem immaterial may also impair the Company’s business, financial condition or results of operations.
Risks Related to our Business and the Industry in Which We Operate
We may face uncertainties relating to technological approaches.
To date, Enumeral has not developed or commercialized any products based on its technological approaches and utilizing its proprietary drug discovery platform. There can be no assurance that these approaches will enable the Company to successfully discover mAbs and other novel biologics for use in the diagnosis and treatment of cancer, infectious and inflammatory diseases.
| 36 |
The development of mAbs and other novel biologics for use in the diagnosis and treatment of cancer, infectious and inflammatory diseases also will be subject to the risks of failure inherent in the development of products based on new technologies. These risks include the possibilities that any products based on these technologies will be found to be ineffective or toxic, or otherwise fail to receive necessary regulatory approvals; the products, if safe and effective, will be difficult to manufacture on a large scale or will be uneconomical to market; proprietary rights of third parties will preclude the Company or its collaborative partners from marketing products; or third parties will market superior or equivalent products. As a result, there can be no assurance that the Company’s research and development activities will result in any commercially viable products.
Biotechnology and pharmaceutical technologies have undergone and are expected to continue to undergo rapid and significant change. The Company’s future success will depend in large part on its ability to maintain a competitive position with respect to these technologies. Rapid technological developments by the Company or others may result in products or processes becoming obsolete before the Company recovers any expenses it incurs in connection with the development of such products.
We may not achieve profitability.
Enumeral has incurred operating losses in each year since its inception and expects to have negative cash flow from operations through at least the end of 2016. Enumeral experienced net losses of approximately, $1.7 million in 2011, $3.2 million in 2012 and $3.8 million in 2013. Our ability to become profitable will depend on our ability to increase our revenues, which is subject to a number of factors including our ability to successfully penetrate and expand the research and discovery market through collaborations with third parties, our ability to successfully market our core technology and develop effective research, the success of our collaborative programs, our ability to compete effectively against current and future competitors, availability of public funding through grants and other federal funds, the time required to reach commercial revenue and profitability, if at all, and global economic and political conditions.
The Company’s ability to become profitable also depends on its expense levels, which are influenced by a number of factors, including the resources it devotes to developing and supporting its projects and potential products, the continued progress of its research and development of potential products, its ability to improve its research and development efficiencies, license fees or royalties it may be required to pay, and the potential need to acquire licenses to new technology or intellectual property, the availability of intellectual property for licensing or acquisition, or to use its technology in new markets, which could require the Company to pay unanticipated license fees and royalties in connection with these licenses.
Our expansion efforts may prove more expensive than we currently anticipate, and we may not succeed in increasing our revenues to offset higher expenses. These expenses, among other things, may cause our net income and working capital to decrease. If the Company fails to grow its revenue and manage its expenses, it may never achieve profitability, which would adversely and materially affect its ability to provide a return to its investors.
We will require additional capital to support business growth, and such capital might not be available.
We will continue to expend substantial resources for research and development, including costs associated with developing our technology and conducting internal research and development to support our technology development, marketing and business development efforts. Our future capital requirements will depend on a number of factors, including, by way of example:
| · | the size and complexity of research and development programs; |
| · | the scope and results of technology testing and development, if any; |
| · | the retention of existing and establishment of further partnerships, if any; |
| · | continued scientific progress in our research and development programs; |
| · | competing technological and market developments; |
| · | the time and expense of filing and prosecuting patent applications and enforcing patent claims; and |
| · | the cost of conducting commercialization activities and arrangements and in-licensing products, if it proves necessary to do so. |
| 37 |
The Company intends to continue to make investments to support business growth and may require additional funds to respond to business challenges, which include the need to develop new products or enhance existing products, conduct clinical trials through its collaborations, if needed, enhance its operating infrastructure and acquire complementary business and technologies. Equity and debt financing, however, might not be available when needed or, if available, might not be available on terms satisfactory to us. In addition, to the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in dilution to the Company’s shareholders. In addition, these securities may be sold at a discount from the market price of the Company’s Common Stock. If the Company is unable to obtain adequate financing or financing on terms satisfactory to it, its ability to continue to support its business growth and to respond to business challenges could be significantly impaired and there would be a material adverse effect on its business and financial condition.
If we cannot successfully commercialize our discovery platform, our business would be harmed.
Our technology represents a new approach to discovering novel therapeutic product candidates. We are in the research stage for the generation of product candidates employing our antibody technology. To date, neither we nor our partners have identified any product candidates employing our human antibody technology that can be further developed with a view to submitting such product to the FDA or other regulatory body for approval to enter clinical trials. Product candidates discovered based upon the application of our technology may not advance beyond the early stages of product development or demonstrate clinical safety and effectiveness.
Our technology may not generate antibodies or other information useful to product discovery in an efficient and timely manner, if at all. If our technology fails to generate antibody product candidates, or if we or our partners do not succeed in the development of products employing our antibody technology, those product candidates may not be approved or commercialized and our business, financial condition and results of operations may be materially harmed. Furthermore, if we are unable to successfully gain market acceptance of our discovery platform with third parties, we will be unable to generate significant revenues, which will prevent us from achieving profitability and being a sustainable business.
We rely on relationships with collaboration partners and other third parties for development of potential products, supply and will rely on collaboration partners and other third parties for the marketing and sale of any products, if developed, and such collaborative partners or other third parties could fail to perform sufficiently.
We believe that our success in penetrating our target markets depends on our ability to develop and maintain collaborative relationships with other companies. Relying on collaborative relationships is risky to our future success because, among other things:
| · | our collaborative partners may not devote sufficient resources to the success of our collaboration; |
| · | our collaborative partners may not obtain regulatory approvals necessary to continue the collaborations in a timely manner; |
| · | our collaborative partners may be acquired by another company and decide to terminate our collaborative partnership or become insolvent; |
| · | our collaborative partners may develop or license technologies or components competitive with our products; |
| · | components developed by collaborators could fail to meet specifications, possibly causing us to lose potential projects and subjecting us to liability; |
| · | disagreements with collaborators could result in the termination of the relationship or litigation; |
| · | collaborators may not have sufficient capital resources; |
| · | collaborators may pursue tests or other products that will not generate significant volume for us, but may consume significant research and development and manufacturing resources; |
| · | our existing collaborations may preclude us from entering into additional future arrangements; or |
| · | we may not be able to negotiate future collaborative arrangements, or renewals of existing collaborative agreements, on acceptable terms. |
Because these and other factors may be beyond our control, the development or commercialization of our products may delayed or otherwise adversely affected.
| 38 |
If we or any of our collaborative partners terminate a collaborative arrangement, we may be required to devote additional resources to product development and commercialization or we may need to cancel some development programs, which could adversely affect our product pipeline and business.
Successful development of therapeutic and diagnostic candidates and products is uncertain.
Our development of future product candidates is subject to the risks of failure inherent in the development of new pharmaceutical products and products based on new technologies. These risks include:
| · | delays in product research and development, clinical testing or manufacturing; |
| · | unplanned expenditures in product development, clinical testing or manufacturing; |
| · | failure in clinical trials or failure to receive regulatory approvals; |
| · | emergence of superior or equivalent products; |
| · | inability to manufacture on our own, or through others, product candidates on a commercial scale; |
| · | inability to market products due to third-party proprietary rights; |
| · | election by our partners not to pursue product development; |
| · | failure by our partners to develop products successfully; and |
| · | failure to achieve market acceptance. |
Because of these risks, our research and development efforts or those of our partners may not result in any commercially viable products. If a significant portion of these development efforts is not successfully completed, required regulatory approvals are not obtained or any approved products are not commercially successful, our business, financial condition and results of operations may be materially harmed.
Because we and our partners have not identified product candidates to bring to clinical trials, our revenue and profit potential are unproven and our limited operating history makes it difficult for an investor to evaluate our business and prospects. Our technology may not result in any meaningful benefits to our current or potential partners.
Our ability to access the drug discovery research and development market is uncertain.
A significant amount of our revenues will depend on long term collaborations with third party biotechnology and pharmaceutical companies for the development and commercialization of new therapeutics and diagnostics. Many factors may affect our ability to access this market, including:
| · | timely development of a therapeutic candidates to bring to clinical trials through our collaborations for a condition or a disease, which will generate significant sales; |
| · | the ability of our collaboration partners to obtain medical insurance reimbursement for such any therapeutic or diagnostic product; |
| · | the results of clinical trials through our collaborations needed to support any required regulatory approvals; |
| · | our ability to obtain requisite United States Food and Drug Administration (FDA) and comparable foreign agencies or other regulatory clearances or approvals required for products developed and on a timely basis; |
| · | demand for the products we are able to develop and introduce; |
| · | our ability to convince our potential customers of the advantages and economic value of our discovery platform over competing technologies and products; and |
| · | the breadth of our product and service menu relative to competitors; |
In particular, we believe that the success of our business will depend in large part on our ability to commercialize our products either through our collaboration partners or alone for the clinical market. We believe that successfully building our business to produce therapeutic and diagnostic products is critical to our long-term goals and success. We have limited experience as a company in utilizing our platform in the therapeutic or diagnostic market and, as a result, we have limited ability to forecast future demand for our products in this market. Failure to successfully penetrate the research and discovery market to exploit our discovery platform will have a material and adverse effect on the company and its sustainability.
| 39 |
The regulatory approval process is expensive, time-consuming, and uncertain and may prevent us from obtaining required approvals for the commercialization of some of our products.
Although we do not have to seek approval from the United States Food and Drug Administration (“FDA”) for our technology platform or its use in connection with the research market, in the clinical market, any therapeutic and diagnostic products are regulated by the FDA and comparable agencies of other countries. In particular, FDA regulations govern activities such as product development, product testing, product labeling, product storage, premarket clearance or approval, manufacturing, advertising, promotion, product sales, reporting of certain product failures and distribution.
Any therapeutic or diagnostic products, depending on their intended use, will likely require regulatory approval from the FDA or comparable foreign agencies. The FDA approval process can be costly, lengthy and uncertain. Clinical trials are generally required to support the submissions for approval to market, especially in the therapeutic field. Clinical trials are expensive and time-consuming. In addition, the commencement or completion of any clinical trials may be delayed or halted for any number of reasons, including adverse medical events, efficacy, changes in intended use, changes in medical practice and issues with evaluator Institutional Review Boards.
Failure to comply with the applicable requirements can result in, among other things, warning letters, administrative or judicially imposed sanctions such as injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, refusal to grant premarket clearance or premarket approval for devices, withdrawal of marketing clearances or approvals, or criminal prosecution.
With regard to any therapeutics or diagnostic products for which we or our collaborators or licensees may seek clearance or approval from the FDA, any failure or material delay to obtain such clearance or approval could harm our business. In addition, it is possible that the current regulatory framework could change or additional regulations could arise at any stage during our product development or marketing, which may adversely affect our ability to obtain or maintain approval of our products and could harm our business.
Prior to marketing, any new therapeutic developed by the Company and its collaborative partners must undergo an extensive regulatory approval process in the United States and other countries. This regulatory process, which includes preclinical studies and clinical trials, and may include post-marketing surveillance, of each compound to establish its safety and efficacy, can take many years and require the expenditure of substantial resources. Data obtained from preclinical studies and clinical trials are susceptible to varying interpretations that could delay, limit or prevent regulatory approval. The rate of completion of clinical trials is dependent upon, among other factors, the enrollment of patients. Patient accrual is a function of many factors, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the study and the existence of competitive clinical trials.
Delays in planned patient enrollment in clinical trials may result in increased costs, program delays or both, which could have a material adverse effect on the Company. Delays or rejections may also be encountered based upon changes in the FDA policies for drug approval during the period of product development and FDA regulatory review of each submitted new drug application (“NDA”) in the case of new pharmaceutical agents, or biologics license application (“BLA”) in the case of biologics. Similar delays also may be encountered in the regulatory approval of any diagnostic product and in obtaining regulatory approval in foreign countries. There can be no assurance that regulatory approval will be obtained for any drugs or diagnostic products developed by the Company or its collaborative partners. Furthermore, regulatory approval may entail limitations on the indicated use of a drug. Because certain of the products likely to result from the Company’s disease research programs involve the application of new technologies and may be based upon a new therapeutic approach, such products may be subject to substantial additional review by various government regulatory authorities and, as a result, regulatory approvals may be obtained more slowly than for products using more conventional technologies.
| 40 |
Even if regulatory approval is obtained, a marketed product and its manufacturer are subject to continuing review. Discovery of previously unknown problems with a product many have adverse effects on the Company’s business, financial condition and results of operations, including withdrawal of the product from the market. Violations of regulatory requirements at any state, including preclinical studies and clinical trials, the approval process or post-approval, may result in various adverse consequences to the Company, including the FDA’s delay in approval or refusal to approve a product, withdrawal of an approved product from the market or the imposition of criminal penalties against the manufacturer and NDA or BLA holder. The Company has not submitted an investigational new drug application (“IND”) for any product candidate, and no product candidate has been approved for commercialization in the United States or elsewhere. No assurance can be given that the Company or any of its strategic partners will be able to identify a product candidate to submit for approval, conduct clinical testing or obtain the necessary approvals from the FDA or other regulatory authorities for any products. Failure to obtain required governmental approvals will delay or preclude the Company’s collaborative partners from marketing drugs or diagnostic products developed by the Company or limit the commercial use of such products and could have a material adverse effect on the Company’s business, financial condition and results of operations.
We rely on licenses of key technology from third parties and may require additional licenses for many of our new product candidates.
We rely on third-party licenses for our core platform, and we could lose any of our third-party licenses for a number of reasons, including, for example, early termination of such agreements due to breaches or alleged breaches by us to the agreement. Our licenses typically subject us to various commercialization, sublicensing and other material obligations or milestones, with which if we fail to comply, we could lose certain rights under such license, such as a field of use for an application or the right to exclusivity in a specified market, or in some cases, the license could be terminated. If we are unable to enter into a new agreement for such licensed technologies, either on terms that are acceptable to us or at all, we may be unable to use our core platform for a broad range of activities or access some geographic or industry markets. In particular, our core platform relies upon a patent license from Massachusetts Institute of Technology granting to us, among other rights, the right to use certain intellectual property central to our core platform. The continued use of this license is subject to completion by the Company of certain due diligence milestones. In the event that the Company fails to achieve these milestones, the license could be limited or terminated, which would have a substantial material adverse effect on our business.
We also may need to introduce new technologies, products and services in order to market to a broader customer base and grow our revenues, and many new technologies, products and services could require us to obtain additional licenses and pay additional license fees and royalties. Our ability to develop, and offer new technologies, products and services, and our strategic plans and growth, could be impaired if we are unable to obtain these licenses or if these licenses are terminated or expire and cannot be renewed.
In addition, any of our licensors could lose patent protection for a number of reasons, including the invalidity or unenforceability of the licensed patent. We typically do not receive significant indemnification under such arrangements from a licensor against third party claims of intellectual property infringement.
If our competitors and potential competitors develop superior products and technologies, our competitive position and results of operations would suffer.
We face intense competition from a number of companies that offer products and services in our target markets. Information concerning potential competition is set forth in the section entitled “Description of Business – Competition.”
If our discovery platform does not perform as expected, or the reliability of the technology is questioned, we could experience lost revenue, delayed or reduced market acceptance of our products, increased costs and damage to our reputation.
Our success depends on the market’s confidence that we can provide a platform that will produce reliable and credible results. Our reputation and the public image of our products or technologies may be impaired if our products fail to perform as expected; or our products are perceived as difficult to use.
| 41 |
We may be subject to third-party claims that we require additional licenses for our products and we could face costly litigation, which could cause us to pay substantial damages and limit our ability to sell some or all of our products.
Our industry is characterized by a large number of patents, claims of which appear to overlap in many cases. As a result, there is a significant amount of uncertainty regarding the extent of patent protection and infringement. Companies may have pending patent applications (which are can be confidential for up to the first eighteen months following filing) that cover technologies we incorporate in our products. Our products are based on complex, rapidly developing technologies. These products could be developed without knowledge of previously filed patent applications that mature into patents that cover some aspect of these technologies. In addition, our belief that our products do not infringe the technology covered by valid and enforceable patents could be successfully challenged by third parties. As a result, we may be subjected to substantial damages for past infringement or be required to modify our products or stop selling them if it is ultimately determined that our products infringe a third party’s proprietary rights. Due to these factors, there remains a constant risk of intellectual property litigation affecting our business. To avoid or settle legal claims, it may be necessary or desirable in the future to obtain licenses relating to one or more products or relating to current or future technologies, and we cannot be assured that we will be able to obtain these licenses or other rights on commercially reasonable terms.
The cost of litigation and the amount of management time associated with infringement cases is significant. Should an infringement case be filed against the Company, there can be no assurance that these matters would be resolved favorably; that we would continue to be able to research, develop or sell the products in question or other products as a result; or that any legal costs associated with defending such claims or any monetary or other damages assessed against us would not have a material adverse effect on the company. Even a successful outcome may take years to achieve and the costs associated with such litigation, in terms of dollars spent and diversion of management time and resources, could seriously harm the our business. Moreover, if a third party claims an intellectual property right to technology that we use, we may be forced to discontinue the use of our discovery platform as it is currently used, an important research and development program, product or product line, alter our discovery platform, products and processes, pay license fees, pay damages for past infringement or cease certain activities. In addition, if claims are made against intellectual property that we currently license, we will not have any meaningful input in resolution of such disputes. Under these circumstances, we may attempt to obtain a license to such intellectual property, but we may not be able to do so on commercially reasonable terms, or at all.
If we fail to maintain and protect our intellectual property rights, our competitors could use our technology to develop competing products and our business will suffer.
Our competitive success will be affected in part by our continued ability to obtain and maintain patent protection for our inventions, technologies and discoveries, including our intellectual property that includes technologies that we license. Our ability to do so will depend on, among other things, complex legal and factual questions. We have licensed our technology under patents of Harvard, Massachusetts General Hospital, the Whitehead Institute and MIT. We cannot assure you that our patents and licenses will successfully preclude others from using our technology. Our pending patent applications may lack priority over others’ applications or may not result in the issuance of patents. Even if issued, our patents may not be sufficiently broad to provide protection against competitors with similar technologies and may be challenged, invalidated or circumvented.
In addition to patents, we rely on a combination of trade secrets, copyright and trademark laws, nondisclosure agreements, licenses and other contractual provisions and technical measures to maintain and develop our competitive position with respect to intellectual property. Nevertheless, these measures may not be adequate to safeguard the technology underlying our products. For example, employees, consultants and others who participate in the development of our products may breach their agreements with us regarding our intellectual property and we may not have adequate remedies for the breach. We also may not be able to effectively protect our intellectual property rights in some foreign countries, as many countries do not offer the same level of legal protection for intellectual property as the United States. Furthermore, for a variety of reasons, we may decide not to file for patent, copyright or trademark protection outside of the United States. Our trade secrets could become known through other unforeseen means.
| 42 |
Notwithstanding our efforts to protect our intellectual property, our competitors may independently develop similar or alternative technologies or products that are competitive with, equal or superior to our technology. Our competitors may also develop similar products without infringing on any of our owned or licensed intellectual property rights or design around our proprietary technologies. Furthermore, any efforts to enforce our intellectual property rights could result in disputes and legal proceedings that could be costly and divert attention from our business.
We may need to initiate lawsuits to protect or enforce our patents, which would be expensive and, if we lose, may cause us to lose some, if not all, of our intellectual property rights, and thereby impair our ability to compete.
We rely on patents to protect a large part of our intellectual property. To protect or enforce our patent rights, we may initiate patent litigation against third parties, such as infringement suits or interference proceedings. These lawsuits could be expensive, take significant time and divert management’s attention from other business concerns. They would also put our patents at risk of being invalidated or interpreted narrowly, and our patent applications at risk of not issuing. We may also provoke these third parties to assert claims against us. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and, consequently, patent positions in our industry are generally uncertain. We cannot assure you that we would prevail in any of these suits or that the damages or other remedies awarded, if any, would be commercially valuable. During the course of these suits, there may be public announcements of the results of hearings, motions and other interim proceedings or developments in the litigation. Any public announcements related to these suits could cause our stock price to decline.
If we fail to retain key members of our staff our ability to conduct and expand our business would be impaired. We are highly dependent on the principal members of our management and scientific staff.
The loss of services of any of these persons could seriously harm our product development and commercialization efforts. In addition, we will require additional skilled personnel in areas such as biologists, biochemists, microbiologists, statisticians, software developers and business development. Attracting, retaining and training personnel with the requisite skills remains challenging, and, as general economic conditions improve, it is becoming increasingly competitive, particularly in the Greater Cambridge area of Massachusetts where our main office and laboratory is located. If at any point we are unable to hire, train and retain a sufficient number of qualified employees to match our growth, our ability to conduct and expand our business could be seriously reduced.
If we become subject to claims relating to improper handling, storage or disposal of hazardous materials, we could incur significant cost and time to comply.
Our research and development processes involve the controlled storage, use and disposal of hazardous materials, including biological hazardous materials, chemicals and various radioactive materials. We are subject to federal, state and local regulations governing the use, manufacture, storage, handling and disposal of materials and waste products. We also may be subject to the laws regarding the use, manufacture, storage, handling and disposal of materials and waste products in countries outside the United States. We may incur significant costs complying with both existing and future environmental laws and regulations. We are unable to predict whether any agency will adopt any regulations that would have a material adverse effect on our operations. The risk of accidental contamination or injury from hazardous materials cannot be eliminated completely. In the event of an accident, we could be held liable for any damages that result, and any liability could exceed the limits or fall outside the coverage of our insurance.
If a catastrophe strikes our research facilities, we may be unable to complete our projects for a substantial amount of time and we would experience lost revenue.
Our research and development facilities are located in Cambridge, Massachusetts. Although we have commercial insurance, our facilities and some pieces of equipment and biological samples are difficult to replace and could require substantial replacement lead-time. Various types of disasters, including earthquakes, fires, floods and acts of terrorism, may affect our research and development facilities. In the event our existing research and development facilities or equipment is affected by man-made or natural disasters, we may be unable to research and develop potential products, or meet partner demands. If our research and development operations were curtailed or ceased, it would seriously harm our business. Although we have access to back-up power generators, in the event of a prolonged or even short-term power failure, we may lose biological samples stored in our freezers or growing in our incubators that could impact our ability to complete projects for ourselves or partners.
| 43 |
We may face product liability claims related to the use or misuse of products employing our antibody technology.
The administration of drugs to humans, in clinical trials or after commercialization, may expose us to product liability claims. Consumers, healthcare producers or persons selling products based on our technology may be able to bring claims against us based on the use of our products in clinical trials and the sale of products based on our technology. Product liability claims may be expensive to defend and may result in large judgments against us.
Pharmaceutical pricing, reimbursement and related matters are uncertain.
Our business, financial condition and results of operation may be materially and adversely affected by the continuing efforts of the government and third party payors to contain or reduce the costs of health care through various means. For example, in certain foreign markets pricing and profitability of prescription pharmaceuticals are subject to government control. In the United States, we expect that there will continue to be a number of federal and state proposals to implement similar government control. In addition, increasing emphasis on managed care in the United States will continue to put pressure on the pricing of pharmaceutical products and diagnostic tests. Cost control initiatives could decrease the price that we or any of our collaborative partners receives for any products in the future and have a material adverse effect on our business, financial condition and results of operations. Further, to the extent that cost control initiatives have a material adverse effect on our collaborative partners, our ability to commercialize its products and to realize royalties may be adversely affected.
The ability of the Company and its strategic partners to commercial pharmaceutical or diagnostic products may depend in part on the extent to which reimbursement for the products will be available from government and health administration authorities, private health insurers and other third party payors. Significant uncertainty exists as to the reimbursement status of newly approved health care products. Third party payors, including Medicare, increasingly are challenging the prices charged for medical products and services. Government and other third party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for new therapeutic products and by refusing in some cases to provide coverage for uses of approved products for disease indications for which the FDA has not granted labeling approval. There can be no assurance that any third party insurance coverage will be available to patients for any products discovered and developed by the Company or its strategic partners. If adequate coverage and reimbursement levels are not provided by government and other third party payors for the Company’s products, the market acceptance of these products may be reduced, which may have a material adverse effect on the Company’s business, financial condition and results of operations.
Investment Risks
You could lose all of your investment.
An investment in our securities is speculative and involves a high degree of risk. Potential investors should be aware that the value of an investment in the Company may go down as well as up. In addition, there can be no certainty that the market value of an investment in the Company will fully reflect its underlying value. You could lose your entire investment.
| 44 |
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders and the purchasers of our Units offered hereby. The Company will be authorized to issue an aggregate of 300,000,000 shares of Common Stock and 10,000,000 shares of “blank check” preferred stock. We may issue additional shares of our Common Stock or other securities that are convertible into or exercisable for our Common Stock in connection with hiring or retaining employees, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our Common Stock may create downward pressure on the trading price of the Common Stock. We will need to raise additional capital in the near future to meet our working capital needs, and there can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with these capital raising efforts, including at a price (or exercise prices) below the price you paid for your stock.
The ability of our Board of Directors to issue additional stock may prevent or make more difficult certain transactions, including a sale or merger of the Company.
Our Board of Directors will be authorized to issue up to 10,000,000 shares of preferred stock with powers, rights and preferences designated by it. See “Preferred Stock” in the section of this Current Report titled “Description of Securities.” Shares of voting or convertible preferred stock could be issued, or rights to purchase such shares could be issued, to create voting impediments or to frustrate persons seeking to effect a takeover or otherwise gain control of the Company. The ability of the Board of Directors to issue such additional shares of preferred stock, with rights and preferences it deems advisable, could discourage an attempt by a party to acquire control of the Company by tender offer or other means. Such issuances could therefore deprive stockholders of benefits that could result from such an attempt, such as the realization of a premium over the market price for their shares in a tender offer or the temporary increase in market price that such an attempt could cause. Moreover, the issuance of such additional shares of preferred stock to persons friendly to the Board of Directors could make it more difficult to remove incumbent officers and directors from office even if such change were to be favorable to stockholders generally.
There currently is no active public market for our Common Stock and there can be no assurance that an active public market will ever develop. Failure to develop or maintain a trading market could negatively affect the value of our Common Stock and make it difficult or impossible for you to sell your shares.
There is currently no active public market for shares of our Common Stock and one may never develop. Our Common Stock is quoted on the OTC Markets. The OTC Markets is a thinly traded market and lacks the liquidity of certain other public markets with which some investors may have more experience. We may not ever be able to satisfy the listing requirements for our Common Stock to be listed on a national securities exchange, which is often a more widely-traded and liquid market. Some, but not all, of the factors which may delay or prevent the listing of our Common Stock on a more widely-traded and liquid market include the following: our stockholders’ equity may be insufficient; the market value of our outstanding securities may be too low; our net income from operations may be too low; our Common Stock may not be sufficiently widely held; we may not be able to secure market makers for our Common Stock; and we may fail to meet the rules and requirements mandated by the several exchanges and markets to have our Common Stock listed. Should we fail to satisfy the initial listing standards of the national exchanges, or our Common Stock is otherwise rejected for listing, and remains listed on the OTC Markets or is suspended from the OTC Markets, the trading price of our Common Stock could suffer and the trading market for our Common Stock may be less liquid and our Common Stock price may be subject to increased volatility, making it difficult or impossible to sell shares of our Common Stock.
Our Common Stock is subject to the “penny stock” rules of the SEC and the trading market in the securities is limited, which makes transactions in the stock cumbersome and may reduce the value of an investment in the stock.
Rule 15g-9 under the Exchange Act establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require: (a) that a broker or dealer approve a person’s account for transactions in penny stocks; and (b) the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must: (a) obtain financial information and investment experience objectives of the person and (b) make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
| 45 |
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form: (a) sets forth the basis on which the broker or dealer made the suitability determination; and (b) confirms that the broker or dealer received a signed, written agreement from the investor prior to the transaction. Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our Common Stock and cause a decline in the market value of our Common Stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker or dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Our stock may be traded infrequently and in low volumes, so you may be unable to sell your shares at or near the quoted bid prices if you need to sell your shares.
Until our Common Stock is listed on a national securities exchange such as the New York Stock Exchange or the Nasdaq Stock Market, we expect our Common Stock to remain eligible for quotation on the OTC Markets, or on another over-the-counter quotation system, or in the “pink sheets.” In those venues, however, the shares of our Common Stock may trade infrequently and in low volumes, meaning that the number of persons interested in purchasing our common shares at or near bid prices at any given time may be relatively small or non-existent. An investor may find it difficult to obtain accurate quotations as to the market value of our Common Stock or to sell his or her shares at or near bid prices or at all. In addition, if we fail to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our Common Stock, which may further affect the liquidity of our Common Stock. This would also make it more difficult for us to raise capital.
We do not anticipate paying dividends on our Common Stock, and investors may lose the entire amount of their investment.
Cash dividends have never been declared or paid on our Common Stock, and we do not anticipate such a declaration or payment for the foreseeable future. We expect to use future earnings, if any, to fund business growth. Therefore, stockholders will not receive any funds absent a sale of their shares of Common Stock. If we do not pay dividends, our Common Stock may be less valuable because a return on your investment will only occur if our stock price appreciates. We cannot assure stockholders of a positive return on their investment when they sell their shares, nor can we assure that stockholders will not lose the entire amount of their investment.
Being a public company is expensive and administratively burdensome.
As a public reporting company, we are subject to the information and reporting requirements of the Securities Act, the Exchange Act and other federal securities laws, rules and regulations related thereto, including compliance with the Sarbanes-Oxley Act. Complying with these laws and regulations requires the time and attention of our Board of Directors and management, and increases our expenses. Among other things, we are required to:
| · | maintain and evaluate a system of internal controls over financial reporting in compliance with the requirements of Section 404 of the Sarbanes-Oxley Act and the related rules and regulations of the SEC and the Public Company Accounting Oversight Board; |
| · | maintain policies relating to disclosure controls and procedures; |
| · | prepare and distribute periodic reports in compliance with our obligations under federal securities laws; |
| · | institute a more comprehensive compliance function, including with respect to corporate governance; and |
| · | involve, to a greater degree, our outside legal counsel and accountants in the above activities. |
| 46 |
The costs of preparing and filing annual and quarterly reports, proxy statements and other information with the SEC and furnishing audited reports to stockholders are expensive and much greater than that of a privately-held company, and compliance with these rules and regulations may require us to hire additional financial reporting, internal controls and other finance personnel, and will involve a material increase in regulatory, legal and accounting expenses and the attention of management. There can be no assurance that we will be able to comply with the applicable regulations in a timely manner, if at all. In addition, being a public company makes it more expensive for us to obtain director and officer liability insurance. In the future, we may be required to accept reduced coverage or incur substantially higher costs to obtain this coverage. These factors could also make it more difficult for us to attract and retain qualified executives and members of our Board of Directors, particularly directors willing to serve on an audit committee which we expect to establish.
As an emerging growth company, we intend to follow certain permitted corporate governance practices instead of the otherwise applicable SEC and stock exchange requirements, which may result in less protection than is accorded to investors in a non-emerging growth company.
As an emerging growth company, we will be permitted, and intend to follow, certain permitted corporate governance practices instead of those otherwise required by the SEC. Following our emerging growth company governance practices as opposed to the requirements that would otherwise apply to a company listed on a stock exchange may provide less protection to you than what is accorded to investors under a stock exchange’s listing requirements applicable to non-emerging growth company issuers.
As an emerging growth company, we may delay adoption of new or revised accounting standards, which may make our stock less attractive and our trading price more volatile.
Pursuant to the JOBS Act, as an emerging growth company, we have elected to take advantage of an extended transition period for any new or revised accounting standards that may be issued by the Financial Accounting Standards Board (FASB) or the SEC, which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can delay adoption of the standard until it applies to private companies. This may make a comparison of our financial statements with any other public company that is either not an emerging growth company or is an emerging growth company that has opted out of using the extended transition period difficult, as different or revised standards may be used. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile and could decline.
Any failure to maintain effective internal control over our financial reporting could materially adversely affect us.
Section 404 of the Sarbanes-Oxley Act of 2002 requires us to include in our annual reports on Form 10-K an assessment by management of the effectiveness of our internal control over financial reporting. In addition, at such time, if any, as we are no longer a “smaller reporting company,” our independent registered public accounting firm will have to attest to and report on management’s assessment of the effectiveness of such internal control over financial reporting.
While we intend to diligently and thoroughly document, review, test and improve our internal control over financial reporting in order to ensure compliance with Section 404, management may not be able to conclude that our internal control over financial reporting is effective. Furthermore, even if management were to reach such a conclusion, if our independent registered public accounting firm is not satisfied with the adequacy of our internal control over financial reporting, or if the independent auditors interpret the requirements, rules or regulations differently than we do, then they may decline to attest to management’s assessment or may issue a report that is qualified. Any of these events could result in a loss of investor confidence in the reliability of our financial statements, which in turn could negatively affect the price of our Common Stock.
| 47 |
In particular, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management and (if required in future) our independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting, as required by Section 404. Our compliance with Section 404 may require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we will need to retain the services of additional accounting and financial staff or consultants with appropriate public company experience and technical accounting knowledge to satisfy the ongoing requirements of Section 404. We intend to review the effectiveness of our internal controls and procedures and make any changes management determines appropriate, including to achieve compliance with Section 404 by the date on which we are required to so comply.
Provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable.
Provisions in our certificate of incorporation and by-laws, as amended and restated prior to the closing of this offering, may have the effect of delaying or preventing a change of control or changes in our management. These provisions may include the following:
| · | authorize the issuance of “blank check” preferred stock that could be issued by our board of directors to defend against a takeover attempt; |
| · | establish a classified board of directors, as a result of which the successors to the directors whose terms have expired will be elected to serve from the time of election and qualification until the third annual meeting following their election; |
| · | require that directors only be removed from office for cause and only upon a supermajority stockholder vote; |
| · | provide that vacancies on the board of directors, including newly created directorships, may be filled only by a majority vote of directors then in office rather than by stockholders; |
| · | prevent stockholders from calling special meetings; and |
| · | prohibit stockholder action by written consent, requiring all actions to be taken at a meeting of the stockholders. |
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in a broad range of business combinations with any “interested” stockholder for a period of three years following the date on which the stockholder becomes an “interested” stockholder.
Also, in connection with the Merger, certain stockholders of the Company (holding in excess of 70% of the common stock), including all of the investors in the PPO, all of the pre-Merger stockholders of the Company and certain of the Enumeral Stockholders including all of its officers directors and principal stockholders entered into a Voting Agreement in which they have agreed to vote their Company stock to maintain the composition of the Company’s Board of Directors. The Voting Agreement will terminate two years from the date of closing of the Merger.
***
The risks above do not necessarily comprise all of those associated with an investment in the Company. This Current Report contains forward looking statements that involve unknown risks, uncertainties and other factors that may cause the actual results, financial condition, performance or achievements of the Company to be materially different from any future results, performance or achievements expressed or implied by such forward looking statements. Factors that might cause such a difference include, but are not limited to, those set out above.
| 48 |
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following management’s discussion and analysis should be read in conjunction with the historical financial statements and the related notes thereto contained in this report. The management’s discussion and analysis contains forward-looking statements, such as statements of our plans, objectives, expectations and intentions. Any statements that are not statements of historical fact are forward-looking statements. When used, the words “believe,” “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect” and the like, and/or future tense or conditional constructions (“will,” “may,” “could,” “should,” etc.), or similar expressions, identify certain of these forward-looking statements. These forward-looking statements are subject to risks and uncertainties, including those under “Risk Factors” in this Current Report, that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. The Company’s actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of several factors. The Company does not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this report, except as required by law.
As a result of the Merger and the change in business and operations of the Company, a discussion of the past financial results of the Company is not pertinent, and under applicable accounting principles the historical financial results of Enumeral, the accounting acquirer, prior to the Merger are considered the historical financial results of the Company.
The following discussion highlights Enumeral’s results of operations and the principal factors that have affected our financial condition as well as our liquidity and capital resources for the periods described, and provides information that management believes is relevant for an assessment and understanding of the statements of financial condition and results of operations presented herein. The following discussion and analysis are based on Enumeral’s audited and unaudited financial statements contained in this Current Report, which we have prepared in accordance with United States generally accepted accounting principles. You should read the discussion and analysis together with such financial statements and the related notes thereto.
Basis of Presentation
The audited financial statements of Enumeral for the fiscal years ended December 31, 2013 and 2012, and the unaudited condensed financial statements of Enumeral for the three months and six months ended June 30, 2014, include a summary of our significant accounting policies and should be read in conjunction with the discussion below. In the opinion of management, all material adjustments necessary to present fairly the results of operations for such periods have been included in these audited financial statements. All such adjustments are of a normal recurring nature.
Overview
Enumeral was incorporated on December 11, 2009 and has devoted substantially all of its resources to the discovery of monoclonal antibodies and other novel biologics for use in the diagnosis and treatment of cancer, infectious and inflammatory diseases. To date, all of the Company’s revenue has resulted from payments from strategic partners and the Company has not received any revenue from the sale of products or services. As of June 30, 2014, the Company had total stockholders’ deficiency of $10,509,846, including an accumulated deficit of $11,908,142.
The Company has entered into several strategic alliances, which have provided the Company with research funding. Revenue recognized under these collaborations through June 30, 2014 has aggregated $565,867.
Internal product discovery programs that achieve significant value inflection points in the next twelve to eighteen months may generate cash flow to the Company from asset sales or licensing transactions. Revenues from corporate collaborations also serve to minimize future operating losses and equity requirements.
However, Enumeral’s actual capital requirements may vary significantly and will depend on many factors, including progress of our proprietary programs, and the number and breadth of these programs; retention of existing and establishment of additional corporate collaborations and licensing arrangements; achievement of specific research objectives under our corporate collaboration arrangements; and the progress of the development efforts of the Company’s collaboration partners.
| 49 |
To date, our proof-of-concept corporate collaborations have provided modest revenues, and one has provided commercialization rights including shared (equal) ownership in any Enumeral-discovered antibodies, and rights to certain therapeutic and diagnostic outcomes. Our efforts to secure corporate collaborations in the future are focused on entering into one or more full-scale joint product discovery and development corporate collaborations that can provide significant up-front payments, up-front fees, FTE-based research funding, milestone payments from Company and partner achievements, and license fees, and we would seek to retain commercialization rights to certain therapeutic and diagnostic applications of the discoveries resulting from large-scale corporate collaborations.
Even if the Company is successful in entering into large corporate collaborations, we may incur increasing expenses and additional losses for at least the next several years due to expanding internal product discovery programs; significant fluctuations in both timing and amounts of payments under corporate collaborations and licensing arrangements; expenses related to commercializing the Company’s rights retained in its corporate collaborations; competing technological and market developments; and the costs involved in obtaining and enforcing patent claims and other intellectual property rights.
Consequently, Enumeral may require significant additional financing in the future, which it may seek to raise through public or private equity offerings, debt financings or additional corporate collaborations. No assurance can be given that additional financing, corporate collaborations and licensing arrangements will be available when needed or that, if available, such financing will be obtained on terms favorable to the Company or its stockholders. (See “Risk Factors.”)
Results of Operations
Six months ended June 30, 2014 as compared to six months ended June 30, 2013
| For The | For The | |||||||
| Six Months | Six Months | |||||||
| Ended | Ended | |||||||
| June 30, 2014 | June 30, 2013 | |||||||
| Collaboration and license revenues | $ | 50,714 | $ | 67,167 | ||||
| Grant revenues | - | 133,037 | ||||||
| Total revenues | 50,714 | 200,204 | ||||||
| Cost of revenue and expenses | ||||||||
| Research and development | 1,476,517 | 1,166,403 | ||||||
| General and administrative | 846,700 | 703,285 | ||||||
| Total cost of revenue and expenses | 2,323,217 | 1,869,688 | ||||||
| Loss from operations | (2,272,503 | ) | (1,669,484 | ) | ||||
| Other income (expense) | ||||||||
| Interest expense | (128,081 | ) | (58,932 | ) | ||||
| Change in fair value of derivatives | 3,679 | 168 | ||||||
| Total other income (expense), net | (124,402 | ) | (58,764 | ) | ||||
| Net loss before income taxes | (2,396,905 | ) | (1,728,248 | ) | ||||
| Provisions for income taxes | - | - | ||||||
| Net loss | $ | (2,396,905 | ) | $ | (1,728,248 | ) | ||
Revenue decreased 74.7% to $50,714 in the six months ended June 30, 2014 from $200,204 in the six months ended June 30, 2013. The decreases in revenue are primarily attributable to the completion of multiple collaboration projects by the end of 2013, as well as a decrease in grant revenue following completion of the NCI project in 2013.
| 50 |
Research and development expenses increased 26.6% to $1,476,517 in the six months ended June 30, 2014 from $1,166,403 in the six months ended June 30, 2013. The increases was primarily attributable to increased payroll and personnel expenses of $55,520 from $435,548 to $491,068, as the Company hired additional research and development personnel, patent expenses of $193,343 from $63,905 to $257,248, and an increase in consulting expenses of $63,926 from $63,905 to $127,831. The Company expects research and development expenses to continue to increase as personnel and research and development facilities are expanded to accommodate the Company’s proprietary internal programs. Such expenses will also increase to the extent that the Company enters into additional strategic alliances with third parties.
General and administrative expenses increased 20.4% to $846,700 in the six months ended June 30, 2014 from $703,285 in the six months ended June 30, 2013. The increase was primarily attributable increases in professional service fees of $116,566 from $183,390 to $299,956, increased travel and other expenses of $35,184 from $87,676 to $122,860, an increase in non-cash stock compensation expense of $102,929 from $100,808 to $203,737, offset by a decrease in payroll and personnel expenses expense of $149,380 from $303,880 to $154,500.
Net interest expense increased 111.7% to $128,081 in the six months ended June 30, 2014 from $58,932 in the six months ended June 30, 2013. This increase is largely attributable to the debt discounts recorded for the beneficial conversion feature and the value ascribed to the warrants issued in connection with the convertible notes, which are accreted to interest expense over the term of the loan using the effective interest rate for the six months ended June 30, 2014. No such instruments were outstanding during the six months ended June 30, 2013. For more information regarding the convertible promissory notes issued during the six months ended June 30, 2014.
Net loss increased 38.7% to $2,396,905 in the six months ended June 30, 2014 from $1,728,248 in the six months ended June 30, 2013. This increase was due to increased research and development expenses and general and administrative expenses, along with decreased revenues.
As of June 30, 2014, the Company has accumulated losses of $11,908,142 since inception and, therefore, has not paid any federal taxes. Realization of deferred tax assets is dependent on future earnings, if any, the timing and amount of which are uncertain. Accordingly, valuation allowances in amounts equal to the deferred tax assets have been established to reflect these uncertainties. Utilization of the deferred tax asset, consisting of net operating loss and research and development credit carryforwards may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986 due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating loss and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively.
| 51 |
Three months ended June 30, 2014 as compared to three months ended June 30, 2013
| For The | For The | |||||||
| Three Months | Three Months | |||||||
| Ended | Ended | |||||||
| June 30, 2014 | June 30, 2013 | |||||||
| Collaboration and license revenues | $ | - | $ | - | ||||
| Grant revenues | - | 66,609 | ||||||
| Total revenues | - | 66,609 | ||||||
| Cost of revenue and expenses | ||||||||
| Research and development | 791,105 | 609,014 | ||||||
| General and administrative | 342,623 | 362,571 | ||||||
| Total cost of revenue and expenses | 1,133,728 | 971,585 | ||||||
| Loss from operations | (1,133,728 | ) | (904,976 | ) | ||||
| Other income (expense) | ||||||||
| Interest expense | (75,720 | ) | (28,020 | ) | ||||
| Change in fair value of derivatives | 3,019 | (96 | ) | |||||
| Total other income (expense), net | (72,701 | ) | (28,116 | ) | ||||
| Net loss before income taxes | (1,206,429 | ) | (933,092 | ) | ||||
| Provisions for income taxes | - | - | ||||||
| Net loss | $ | (1,206,429 | ) | $ | (933,092 | ) | ||
Revenue decreased 100.0% to 0 in the three months ended June 30, 2014 from $66,609 in the three months ended June 30, 2013. The decreases in revenue are primarily attributable to the completion of multiple collaboration projects by the end of 2013 as well as a decrease in grant revenue following completion of the NCI project in 2013.
Research and development expenses decreased 29.9% to $791,105 in the three months ended June 30, 2014 from $609,014 in the three months ended June 30, 2013. The increase was primarily attributable to increases in payroll and personnel expenses of $30,631 from $206,819 to $237,450, as the Company hired additional research and development personnel, patent expenses of $132,121 from $41,251 to $173,372, offset by a decrease in laboratory costs of $19,747 from $146,357 to $126,610. The Company expects research and development expenses to continue to increase as personnel and research and development facilities are expanded to accommodate the Company’s existing proprietary programs. Such expenses will also increase to the extent that the Company enters into additional strategic alliances with third parties.
General and administrative expenses decreased 5.5% to $342,623 in the three months ended June 30, 2014 from $362,571 in the three months ended June 30, 2013. This decrease was primarily attributable to decreases in payroll and personnel expenses of $116,497 from $136,471 to $19,974, offset by increases in professional services fees of $47,540 from $44,393 to $48,999, travel and other expenses of $4,606 from $44,393 to $48,999 and an increase in noncash stock compensation expense of $35,716 from $38,070 to $73,786.
Net interest expense increased 158.6% to $75,720 in the three months ended June 30, 2014 from $28,020 in the three months ended June 30, 2013. This increase is largely attributable to the debt discounts recorded for the beneficial conversion feature and the value ascribed to the warrants issued in connection with the convertible notes, which are accreted to interest expense over the term of the loan using the effective interest rate for the three months ended June 30, 2014. No such instruments were outstanding during the three months ended June 30, 2013.
Net loss increased 29.3% to $1,206,429 in the three months ended June 30, 2014 from $933,092 in the three months ended June 30, 2013. This increase was due to increased research and development expenses and general and administrative expenses, along with decreased revenues.
| 52 |
Year ended December 31, 2013 as compared to year ended December 31, 2012
| Year Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| Collaboration and license revenues | $ | 266,039 | $ | 49,281 | ||||
| Grant revenues | 182,997 | 16,836 | ||||||
| Total revenues | 449,036 | 66,117 | ||||||
| Cost of revenue and expenses | ||||||||
| Research and development | 2,369,414 | 1,831,144 | ||||||
| General and administrative | 1,832,088 | 1,377,158 | ||||||
| Total cost of revenue and expenses | 4,201,502 | 3,208,302 | ||||||
| Loss from operations | (3,752,466 | ) | (3,142,185 | ) | ||||
| Other income (expense) | ||||||||
| Interest expense | (109,064 | ) | (106,768 | ) | ||||
| Change in fair value of derivatives | (3,090 | ) | (4,897 | ) | ||||
| Other income (expense) | 31 | (284 | ) | |||||
| Total other income (expense), net | (112,123 | ) | (111,949 | ) | ||||
| Net loss before income taxes | (3,864,589 | ) | (3,254,134 | ) | ||||
| Provisions for income taxes | - | - | ||||||
| Net loss | $ | (3,864,589 | ) | $ | (3,254,134 | ) | ||
Revenue increased 579.2% to $449,036 in calendar year 2013 from $66,117 in calendar year 2012. The increase in 2013 was due to increases in revenue from collaborations– from $49,281 in 2012 to $266,039 in 2013 – and in grant revenues – from $16,836 in 2012 to $182,997 in 2013. Increases in revenue from collaboration are primarily attributable to the Company’s initiation of new collaborations in 2013 with Novartis. Increases in grant revenue reflect continuation of the Company’s work on its program for NCI, which began in October 2012 and for which the majority of the work – and subsequent achievement of milestones to recognize revenue – was performed in 2013.
Research and development expenses increased 29.4% to $2,369,414 in calendar year 2013 from $1,831,144 in calendar year 2012. The increase was primarily attributable to increased payroll and personnel expenses of $106,733 from $999,372 to $1,106,105, as the Company hired additional research and development personnel, increased laboratory expenses of $140,289 from $335,635 to $475,924, and increased equipment depreciation and facilities expenses of $93,004 from $187,721 to $280,725, and $120,047 from $218,845 to $338,892, respectively, in connection with the expansion of the Company’s research efforts. The Company expects research and development expenses to continue to increase as personnel and research and development facilities are expanded to accommodate the Company’s existing strategic alliances. Such expenses will also increase to the extent that the Company enters into additional strategic alliances with third parties.
General and administrative expenses increased 33.0% to $1,832,088 in calendar year 2013 from $1,377,158 in calendar year 2012. The increase was primarily attributable an increase in in legal fees of $297,518 from $171,098 to $468,616, and partially offset by decreases in recruiting fees of $68,438 from $84,438 to $16,000, and other professional fees of $19,207 from $19,357 to $150. It is anticipated that general and administrative expenses will increase in 2014 and later years as the Company continues to grow.
Net interest expense increased 2.2% to $109,064 in calendar year 2013 from $106,768 in calendar year 2012. This increase was due to increased interest expense incurred on the loan from Square 1 Bank (see “Liquidity and Capital Resources” below) partially offset by increased interest income earned on higher balances of cash and investment securities.
| 53 |
Net loss increased 18.8% to $3,864,589 in calendar year 2013 from $3,254,134 in calendar year 2012. This increase was due to increased research and development expenses and general and administrative expenses partially offset by increased revenues.
As of December 31, 2013, the Company has accumulated losses of $9,511,237 since inception and, therefore, has not paid any federal taxes. Realization of deferred tax assets is dependent on future earnings, if any, the timing and amount of which are uncertain. Accordingly, valuation allowances in amounts equal to the deferred tax assets have been established to reflect these uncertainties. Accordingly, valuation allowances in amounts equal to the deferred tax assets have been established to reflect these uncertainties. Utilization of the deferred tax asset, consisting of net operating loss and research and development credit carryforwards may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986 due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating loss and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively.
Liquidity and Capital Resources
Enumeral has financed its operations since inception primarily through private placement of preferred stock, venture debt, and revenues from corporate collaborations and the contract with NCI. Enumeral raised gross proceeds of $10.4 million in four financing rounds, consisting of: (i) a $3 million Series A Preferred Stock financing completed in early 2011; (ii) a $2.6 million Series A-1 Preferred financing completed in mid-2012; (iii) a $2.6 million Series A-2 financing completed in mid-2013; and (iv) a $1.6 million Series B Preferred financing in April 2014. Each share of Enumeral preferred stock issued in the above financing rounds were converted into our Common Stock in the Merger.
To fund operations during 2014 prior to the completion of the Series B financing, we completed a $750,000 bridge note financing that, in connection with the Merger, was converted into shares of Common Stock. Please refer to “Unregistered Sales of Equity Securities - Sales of Unregistered Securities” below for a further description of the bridge note and its conversion.
In addition, in December 2011, we entered into a venture debt financing with Square 1 Bank for $1.79 million which was advanced during 2011 and 2012. As of July 31, 2014 approximately $1.1 million has been repaid under its terms. Interest accrues at an annual rate of 7.00%. The remaining amounts are due and payable in equal monthly installments of principal plus accrued interest. The maturity date is July 5, 2015, at which time a success fee of $15,000 will be due to Square 1 Bank. The financing, including the success fee, was repaid in full in August 2014.
To date, revenue from contracts with our collaboration partners and the NCI totaled $565,867, of which $449,036 was realized in calendar 2013 and $50,714 was realized in the first quarter of 2014.
As of June 30, 2014 and December 31, 2013, Enumeral had approximately $603,612 and $263,910 in cash, cash equivalents and short-term investments, respectively.
The financial statements included in this Current Report have been prepared in conformity with generally accepted accounting principles that contemplate our continuance as a going concern. The Company has generated losses from operation since its inception. The continuation of the Company as a going concern is dependent upon the Company obtaining adequate capital to fund operating losses until it becomes profitable, if ever. The financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
| 54 |
Following the PPO, the Company has $18.6 million in cash and cash equivalents and believes that its existing cash and investment securities and anticipated cash flow from strategic alliances will be sufficient to support the Company’s operations for at least the next 12 months. The Company’s actual future capital requirements, however, will depend on many factors, including progress of its research and development programs, the number and breadth of these programs, achievement of milestones under strategic alliance arrangements, the ability of Enumeral to establish and maintain additional strategic alliance and licensing arrangements, and the progress of the development efforts of Enumeral’s strategic partners. These factors also include the level of Enumeral’s activities relating to commercialization rights it has retained in its strategic alliance arrangements, competing technological and market developments, the costs involved in obtaining and enforcing patent claims and other intellectual property rights and the costs and timing of regulatory approvals.
The Company expects that it will require significant additional financing in the future, which it may seek to raise through public or private equity offerings, debt financings or additional strategic alliance and licensing arrangements. No assurance can be given that additional financing or strategic alliance and licensing arrangements will be available when needed or that, if available, such financing will be obtained on terms favorable to Enumeral or its stockholders. It is possible the Company may never reach profitability. See “Risk Factors—Risks Related to our Business and the Industry in Which We Operate—We will require additional capital to support business growth, and such capital might not be available.”
Critical Accounting Policies, Estimates, and Judgments
Recently Adopted Accounting Standards
On June 10, 2014, the FASB issued Accounting Standards Update (ASU) No. 2014-10, Development Stage Entities (Topic 915) – Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation, which eliminates the concept of a development stage entity (DSE) in its entirety from current accounting guidance. Amendments to the consolidation guidance may result in more DSEs being considered variable interest entities (VIEs). The new guidance applies to all entities that previously met the definition of a DSE. This ASU is effective for public business entities for annual reporting periods beginning after December 15, 2015, and interim periods therein. Early adoption of the new standard is permitted. Management has elected to early adopt this ASU, as permitted and, accordingly, has not included the inception-to-date disclosures and other previously required disclosures for development stage entities.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (U.S. GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reported period. Significant estimates used in preparing the Company’s financial statements include the estimation of stock based compensation, stock warrants liability, accrued expenses, revenue recognition and income taxes. Actual results could differ from those estimates.
Fair Value of Financial Instruments
Fair values of financial instruments included in current assets and current liabilities are estimated to approximate their book values, due to the short maturity of such instruments. All debt is based on current rates at which the Company could borrow funds with similar remaining maturities and approximates fair value. The Company’s assets and liabilities that are measured at fair value on a recurring basis are measured in accordance with ASC 820 under the Financial Accounting Standards Board (“FASB”), which establishes a three-level valuation hierarchy for measuring fair value and expands financial statement disclosures about fair value measurements. The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability as of the measurement date.
Property and Equipment
Property and equipment are recorded at cost. Expenditures for maintenance and repairs are charged to expense as incurred, whereas major betterments are capitalized as additions to property and equipment. Depreciation is provided using the straight-line method over the estimated useful lives of the assets as follows:
| Lab equipment | 5 years |
| Computer equipment and software | 3 years |
| Furniture | 3 years |
| Leasehold improvements | Shorter of useful life or life of the lease |
| 55 |
Revenue Recognition
Collaboration and license revenue
Non-refundable license fees are recognized as revenue when the Company has a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and the Company has no further performance obligations under the license agreement. Multiple element arrangements, such as license and development arrangements are analyzed to determine whether the deliverables, which often include license and performance obligations such as research and steering committee services, can be separated or whether they must be accounted for as a single unit of accounting in accordance with GAAP.
The Company recognizes up-front license payments as revenue upon delivery of the license only if the license has stand-alone value and the fair value of the undelivered performance obligations, typically including research and/or steering committee services, can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations would then be accounted for separately as performed. If the license is considered to either (i) not have standalone or (ii) have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement would then be accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
Whenever the Company determines that an arrangement should be accounted for as a single unit of accounting, it must determine the period over which the performance obligations will be performed and revenue will be recognized. Revenue will be recognized using either a relative performance or straight-line method. The Company recognizes revenue using the relative performance method provided that the Company can reasonably estimate the level of effort required to complete its performance obligations under an arrangement and such performance obligations are provided on a best-efforts basis. Direct labor hours or full-time equivalents are typically used as a measure of performance. Revenue recognized under the relative performance method would be determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of substantive milestones, by the ratio of level of effort incurred to date to estimated total level of effort required to complete the Company’s performance obligations under the arrangement. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the relative performance method, as of each reporting period.
If the Company cannot reasonably estimate the level of effort required to complete its performance obligations under an arrangement, the performance obligations are provided on best-efforts basis and the Company can reasonably estimate when the performance obligation ceases or the remaining obligations become inconsequential and perfunctory. At that time, the total payments under the arrangement, excluding royalties and payments contingent upon achievement of substantive milestones, would be recognized as revenue on a straight-line basis over a period the Company expects to complete its performance obligations.
Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date.
Grant Revenue
The Company recognizes nonrefundable grant revenue that is earned in connection with its Research Agreement with the National Cancer Institute (the “NIH”), which was executed in September 2012. Grant revenue consists of a portion of the funds received to date by the NIH, which allow the Company to conduct research on colon cancer tissues. Revenue is recognized as the related research services are performed in accordance with the terms of the agreement.
Research and Development Expenses
Research and development expenditures are charged to the statement of operations as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, clinical supply costs, contract services, depreciation and amortization expense and other related costs. Costs associated with acquired technology, in the form of upfront fees or milestone payments, are charged to research and development expense as incurred. Legal fees incurred in connection with patent applications along with fees associated with the license to the Company’s core technology are expensed as research and development expense.
| 56 |
Accounting for Preferred Stock Warrants
Warrants for the purchase of redeemable Convertible Preferred Series A Stock are carried at fair value and reported as a derivative liability on the accompanying balance sheets. Changes in the fair value of warrants for the purchase of convertible preferred stock are included in other income in the statements of operations.
Stock-Based Compensation
The Company has elected to use the Black-Scholes option pricing model to determine the grant date fair value of share-based awards. The Company recognizes the compensation cost of employee share-based awards on a straight-line basis over the employee’s requisite service period of each award, which is generally the vesting period.
The fair value of the restricted stock awards granted to employees is based upon the fair value of the common stock on the date of grant. Expense is recognized over the vesting period.
Expected volatility for the Company’s Common Stock was determined based on the historical volatility of comparable publically traded companies in a similar industry. The risk-free interest rate is based on the yield of U.S. Treasury securities consistent with the expected term of the option. No dividend yield was assumed as the Company does not pay dividends on its Common Stock. The expected term of the options granted represents the period of time that options granted are expected to be outstanding and is calculated using the simplified method.
The fair value of the common stock has typically been determined by the Board of Directors (the “Board”) at each date of grant based upon a variety of factors, including the Company’s financial position and historical financial performance, the status of developments within the Company’s research and development activities, the composition and ability of the current climate in the market place, the illiquid nature of the common stock, the effect of the rights and preferences of the preferred shareholders, and the prospects of a liquidity event, among others.
The Company engaged a third party to develop an estimate of the fair value of a share the Company’s common stock on a fully-diluted, minority, non-marketable basis as of December 31, 2013 and 2012. The estimates used in the valuation at December 31, 2013 have not materially changed as of June 30, 2014 and the same estimate of the fair value of common shares was used at June 30, 2014.
Based upon unaudited historical, pro-forma and/or forecast financial and operational information, which Enumeral management represented as accurately reflecting the company’s operating results and financial position, the third party utilized both a Market Approach (using various financial statement metrics of similar enterprises’ equity securities to estimate the fair value of the Company’s equity securities) and an Income Approach (which bases value on expectations of future income and cash flows) in their analyses.
The fair value of a single share of Common Stock was determined using the Option Pricing Method, which treats Common and Preferred Stock as call options on the aggregate enterprise value and using traditional models, including Black-Scholes or binomial models, to calculate share values.
Recent Authoritative Guidance
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers: Topic 606. This Update affects any entity that either enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets, unless those contracts are within the scope of other standards. The guidance in this Update supersedes the revenue recognition requirements in Topic 605, Revenue Recognition and most industry-specific guidance. The core principle of the guidance is that an entity should recognize revenue to illustrate the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The new guidance also includes a cohesive set of disclosure requirements that will provide users of financial statements with comprehensive information about the nature, amount, timing, and uncertainty of revenue and cash flows arising from a reporting organization’s contracts with customers. This ASU is effective retrospectively for fiscal years, and interim periods within those years beginning after December 15, 2016 for public companies and 2017 for non-public entities. Management is evaluating the effect, if any, on the Company’s financial position and results of operations
| 57 |
In June 2014, the FASB issued Accounting Standards Update (ASU) No. 2014-12, Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. The amendments in the ASU require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in Topic 718, Compensation – Stock Compensation, as it relates to awards with performance conditions that affect vesting to account for such awards. The performance target should not be reflected in estimating the grant-date fair value of the award. Compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation cost should be recognized prospectively over the remaining requisite service period. The total amount of compensation cost recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved.
Off-Balance Sheet Arrangements
The Company did not engage in any “off-balance sheet arrangements” (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) as of June 30, 2014.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. In accordance with Securities and Exchange Commission rules, shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days of the date of the table below are deemed beneficially owned by the holders of such options and warrants and are deemed outstanding for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage of ownership of any other person. Subject to community property laws, where applicable, the persons or entities named in the tables below have sole voting and investment power with respect to all shares of our Common Stock indicated as beneficially owned by them.
The following table sets forth information with respect to the beneficial ownership of our Common Stock as of August 21, 2014 (the “Determination Date”) following the consummation of the Merger, the Split-Off and the PPO, by (i) each stockholder known by us to be the beneficial owner of more than 5% of our Common Stock (our only classes of voting securities), (ii) each of our directors and executive officers, and (iii) all of our directors and executive officers as a group. To the best of our knowledge, except as otherwise indicated, each of the persons named in the table has sole voting and investment power with respect to the shares of our Common Stock beneficially owned by such person, except to the extent such power may be shared with a spouse. To our knowledge, none of the shares listed below are held under a voting trust or similar agreement, except as noted. Other than the Merger, to our knowledge, there is no arrangement, including any pledge by any person of securities of the Company or any of its parents, the operation of which may at a subsequent date result in a change in control of the Company.
| 58 |
| Title of class | Name and address of beneficial owner (1) | Amount and nature of beneficial ownership | Percent
of class (2) | |||||||
| Common Stock | Harris & Harris Group, Inc. (3) 1450 Broadway, 24th Floor New York, NY 10018 | 9,763,988 | 18.3 | % | ||||||
| Common Stock | Daniel B. Wolfe c/o Harris & Harris Group, Inc. (3) 1450 Broadway, 24th Floor New York, NY 10018 | 9,763,988 | 18.3 | % | ||||||
| Common Stock | Mark Tompkins (4) | 6,155,179 | 11.7 | % | ||||||
| Common Stock | Allan Rothstein (5) | 2,054,881 | 4.0 | % | ||||||
| Common Stock | John J. Rydzewski (6) | 1,461,755 | 2.8 | % | ||||||
| Common Stock | Arthur H. Tinkelenberg (7) | 1,452,555 | 2.8 | % | ||||||
| Common Stock | Derek Brand (8) | 128,273 | * | |||||||
| Common Stock | Anhco Nguyen (9) | 90,255 | * | |||||||
| Common Stock | Barry Buckland (10) | 290,753 | * | |||||||
| Common Stock | Kevin Sarney (11) | — | — | |||||||
| Common Stock | All of our directors and executive officers as a group (8 persons) (12) | 15,242,460 | 27.7 | % | ||||||
| * | Less than 1% |
| (1) | Unless otherwise set forth above, the address for each of the persons or entities listed above is c/o Enumeral Biomedical Holdings, Inc., One Kendall Square, Building 400, 4th Floor, Cambridge, MA 02139. |
| (2) | Applicable percentage ownership is based on 51,591,710 shares of Common Stock outstanding as of the Determination Date, together with securities exercisable or convertible into shares of Common Stock within 60 days after the Determination Date, for each shareholder. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. |
| (3) | Consists of 7,966,368 shares of common stock owned directly by Harris & Harris Group, Inc. (“Harris”), 255,120 shares that Harris has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date and 1,500,000 shares that Harris has the right to acquire upon the exercise of PPO Warrants that are exercisable within 60 days of the Determination Date, and 23,333 shares of common stock that Daniel B. Wolfe, a director of the Company, has the right to acquire upon the exercise of options that is exercisable within 60 days of the Determination Date provided however that Mr. Wolfe has assigned the economic benefit of the option to Harris. 7,966,368 shares of common stock and warrants to purchase 1,755,120 shares of common stock owned by Harris is pledged as security for a margin account. Mr. Wolfe, along with Douglas W. Jamison, Alexei Andreev and Misti Ushio, are managing directors of Harris and as such may be deemed to have shared voting and dispositive power over the share of common Stock beneficially owned by Harris. However, each of such persons disclaims beneficial ownership over such shares of Common Stock except as to their pecuniary interest therein of 330,961, 379,194, 308,611 and 295,472 shares, respectively, as of the filing of Harris’ most recent proxy statement on May 1, 2014. |
| (4) | Consists of 4,955,179 shares of common stock owned directly by Mr. Tompkins and 1,200,000 shares that Mr. Tompkins has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date, based on information provided by the transfer agent as of July 24, 2014. Mr. Tompkins does not serve as an officer or director of the Company. |
| (5) | Consists of 1,709,912 shares of common stock owned directly by Mr. Rothstein, 320,427 shares that Mr. Rothstein has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date and 42,042 shares that Mr. Rothstein has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. |
| (6) | Consists of 1,264,637 shares of common stock owned directly by Mr. Rydzewski, 188,752 shares that Mr. Rydzewski has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date and 4,167 shares that Mr. Rydzewski has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. Does not include shares that Mr, Rydzewski has the right to acquire upon exercise of options subject to performance based criteria, which criteria has not yet been achieved. |
| 59 |
| (7) | Consists of 1,272,018 shares of common stock owned directly by Dr. Tinkelenberg and 172,204 shares, 172,204 shares that Dr. Tinkelenberg has the right to acquire upon the exercise of warrants that are exercisable within 60 days of the Determination Date and 4,167 shares that Dr. Tinkelenberg has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. Does not include shares that Dr. Tinkelenberg has the right to acquire upon exercise of options subject to performance based criteria, which criteria has not yet been achieved. |
| (8) | Consists of 124,268 shares that Mr. Brand has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. Terms of certain options are described in the Exhibits. Does not include shares that Mr. Brand has the right to acquire upon exercise of options subject to performance based criteria, which criteria has not yet been achieved. |
| (9) | Consists of 87,763 shares that Mr. Nguyen has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. Terms of certain options are described in the Exhibits. Does not include shares that Mr. Nguyen has the right to acquire upon exercise of options subject to performance based criteria, which criteria has not yet been achieved |
| (10) | Consists of 244,168 shares of common stock owned by Mr. Buckland and 62,248 shares that Mr. Buckland has the right to acquire upon the exercise of options that are exercisable within 60 days of the Determination Date. |
| (11) | Mr. Sarney started with the Company on July 31, 2014. As of the date hereof, Mr. Sarney did not beneficially own any shares. |
| (12) | Shares are subject to the Voting Agreement as described above under the captions “The Merger and Related Transactions—Composition of the Board; Voting Agreement” |
Directors and Executive Officers
Below are the names of and certain information regarding the Company’s current executive officers and directors who were appointed effective as of the closing of the Merger:
| Name | Age | Position | Date Named to Board of Directors/as Executive Officer |
Director
Class (if applicable) | ||||
| John J. Rydzewski, | 61 | Chairman of the Board | July 31, 2014 | I | ||||
| Arthur H. Tinkelenberg, Ph.D. | 47 | President, Chief Executive Officer and a Director | July 31, 2014 | I | ||||
| Anhco Nguyen, Ph.D. | 41 | Vice President of Research and Development | July 31, 2014 | - | ||||
| Derek Brand | 37 | Vice President of Business Development | July 31, 2014 | - | ||||
| Kevin Sarney | 49 | Vice President of Finance and Chief Accounting Officer | July 31, 2014 | - | ||||
| Daniel B. Wolfe | 37 | Director | July 31, 2014 | III | ||||
| Allan Rothstein | 56 | Director | July 31, 2014 | II | ||||
| Barry Buckland | 66 | Director | July 31, 2014 | II |
Directors are elected to serve until the next annual meeting of stockholders and until their successors are elected and qualified. Directors are elected by a plurality of the votes cast at the annual meeting of stockholders and hold office until the expiration of the term for which he or she was elected and until a successor has been elected and qualified.
| 60 |
The Board of Directors is divided into three classes, designated Class I, Class II and Class III. Each class consists, as nearly as may be possible, of one-third of the total number of directors constituting the entire Board of Directors. Class I directors have been elected initially for a one-year term which will expire in 2015, Class II directors initially for a two-year term which will expire in 2016 and Class III directors initially for a three-year term which will expire in 2017. At each succeeding annual meeting of stockholders beginning in 2015, successors to the class of directors whose term expires at that annual meeting will be elected for a three-year term. If the number of directors is changed, any increase or decrease will be apportioned among the classes so as to maintain the number of directors in each class as nearly equal as possible, and any additional director of any class will hold office for a term that will coincide with the remaining term of that class, but in no case will a decrease in the number of directors shorten the term of any incumbent director.
A majority of the authorized number of directors constitutes a quorum of the Board of Directors for the transaction of business. The directors must be present at the meeting to constitute a quorum. However, any action required or permitted to be taken by the Board of Directors may be taken without a meeting if all members of the Board of Directors individually or collectively consent in writing to the action.
Executive officers are appointed by the Board of Directors and serve at its pleasure.
The principal occupation and business experience during the past five years for our executive officers and directors is as follows:
John J. Rydzewski – Executive Chairman of the Board of Directors and Co-Founder
Mr. Rydzewski has been Executive Chairman of the Board of the Company since the Merger and prior thereto served as Executive Chairman of Enumeral since October 2011. Mr. Rydzewski is a Class I Director and his term on the Board will expire in 2015. Mr. Rydzewski has significant life sciences industry experience as an institutional investor, investment banker and corporate director. Before joining Enumeral, he was managing director of Christofferson, Robb & Co., an investment firm where he monetized life-sciences intellectual property covering large and small molecule therapeutics from July 2006 to September 2011. His banking career included partnership responsibilities at Benedetto, Gartland & Co., an investment bank serving corporate and institutional clients, and officer positions in the merchant banking and healthcare specialty finance units of Kidder, Peabody & Co. Incorporated and Dean Witter Reynolds Inc. Prior to banking, he was a manager in the Bankruptcy Services Group of Price Waterhouse & Co. and he held positions in industry. He continues to serve as a director of several healthcare companies, including Vision-Sciences, Inc. and he is Co-Chairman of the Board of Advisors of the RAND Corporation’s health policy research unit. He received an M.B.A. and a B.S. in Economics with Honors from The Wharton School of the University of Pennsylvania.
Arthur H. Tinkelenberg – President, Chief Executive Officer, Director and Co-Founder
Dr. Tinkelenberg has been President and Chief Executive Officer and a Director of the Company since the Merger and prior thereto was President, Chief Executive Officer and a director of Enumeral since 2010. He is a Class I Director and his term on the Board will expire in 2015. Dr. Tinkelenberg has broad experience in the business and finance of healthcare enterprises and basic life sciences research. Prior to joining Enumeral, he was a partner in Ascent Biomedical Ventures, a life sciences venture capital firm focused on early stage biomedical technologies, where he was responsible for originating and managing numerous biotechnology and medical device company investments from 2003 through 2009. Prior to venture capital, he served in the healthcare corporate finance and mergers and acquisitions group of Robertson Stephens from January 2001 through July 2002. Prior to industry, he conducted research in lipid metabolism, genomics, biochemistry, and cell biology as a Research Scientist at Columbia University from 1996 through 2000. He is a published author and inventor on a number of patents from projects initiated by him during his tenure in academic research. He received his Ph.D. in molecular genetics from The Rockefeller University and his B.A. in Biology from Grinnell College.
Anhco Nguyen - Vice President of Research and Development
Dr. Nguyen has been Vice President of Research and Development since the Merger and prior thereto, was Vice President of Research and Development of Enumeral since January 2012. Dr. Nguyen leads our research and development team and is responsible for both our proprietary discovery and corporate partnership programs. He participates with the Company’s business development team in structuring new research collaboration initiatives. He brings to this role significant industry experience in the development of therapeutic antibodies for treatment of cancer and multiple sclerosis having held positions of increasing responsibility, most notably at Forma Therapeutics where he served as a staff scientist from June 2009 to January 2012 and Genzyme Corp. where he served as a staff scientist from June 2006 to May 2009. He completed postdoctoral training at MIT’s Cancer Center and was the recipient of a Presidential Postdoctoral Fellowship, which he completed at the Novartis Institute for Biomedical Research. He received his Ph.D. Immunology from Washington University and his A.B. Biology, Harvard College. He has co-authored numerous articles in the fields of cancer and immunology.
| 61 |
Derek Brand - Vice President of Business Development
Mr. Brand has been Vice President of Business Development since the Merger and prior thereto, was a director and Vice President of Business Development and Director of Finance of Enumeral since 2010. Mr. Brand is responsible for initiating and executing technology and business collaborations with biopharmaceutical companies as well as our grant submission efforts. He is establishing our base of clinical operations and partnerships with leading academic research centers that provide research materials essential to our proprietary drug discovery programs and that potentially contribute to our receiving more favorable terms when negotiating corporate collaborations. He manages our financial reporting requirements and develops related budgets and financial projections. He has broad-based experience creating and implementing growth strategies for biomedical startup companies, having served in both business roles and scientific capacities. In previous roles in his career, he has secured corporate funding from research grants and corporate collaborations and he directed key elements of clinical trial design, implementation and analysis as a research scientist. He has been involved in multiple startup companies and also had the opportunity to lead a successful business turnaround for an analytical technology developed by General Electric for the pharmaceutical industry. He received his MBA from Babson College and his B.A. in Biology from Hamilton College.
Kevin Sarney – Vice President of Finance and Chief Accounting Officer
Kevin G. Sarney has been Vice President of Finance and Chief Accounting Officer since the Merger. From September 2013 to July 2014 , Mr. Sarney was Vice President, Finance and Administration for Avaxia Biologics, Incorporated, a clinical-stage biopharmaceutical company developing gut-targeted therapeutics. Prior to joining Avaxia, he worked as a consultant from May 2009 to August 2013 at various biotechnology and medical device companies in interim VP of Finance and Controller roles. From March 2005 to April 2009, Mr. Sarney was the Corporate Controller and Principal Accounting Officer of Nitromed, Inc., a publicly traded cardiovascular-focused pharmaceutical company. Mr. Sarney earned a B.S. in business management from the University of Hartford, an M.B.A. from Boston University and an M.A. in accounting from Suffolk University. Mr. Sarney is a certified public accountant in the Commonwealth of Massachusetts.
Daniel B. Wolfe - Director
Dr. Wolfe has been a member of the Board of Directors of the Company since the Merger and prior thereto, served as member of the Board of Directors of Enumeral since 2009. He is a Class III Director and his term on the Board will expire in 2017. Dr. Wolfe serves on the Board of the Company and Enumeral pursuant to a Voting Agreement with Harris & Harris Group, Inc. Dr. Wolfe has been President and Chief Operating Officer of Harris & Harris Group, Inc., a publicly traded venture capital firm that invests in transformative companies enabled by disruptive science, since January 2009 and a Managing Director of Harris & Harris Group, Inc. since January 2008. Prior to that, he served as Harris & Harris Group, Inc.’s Chief Financial Officer from January 2008 to December 31, 2012 and Treasurer from May 2008 to December 31, 2012. Since January 2009, Dr. Wolfe has served as President and Chief Operating Officer and from January 2009 to December 31, 2012, as Chief Financial Officer of H&H Ventures Management, Inc., a wholly-owned subsidiary of Harris & Harris Group, Inc., and from October 2008 to May 2012, he served as a director. At the request of Harris & Harris Group, Inc., Dr. Wolfe served as Chief Executive Officer of Evolved Nanomaterial Sciences from July 1, 2008 to September 28, 2009. Evolved Nanomaterial Sciences filed for bankruptcy protection under Chapter 7 of the U.S. Bankruptcy Code on September 30, 2009. Dr. Wolfe also serves on the boards of SiOnyx, Inc. and SynGlyco, Inc. He completed his Ph.D. and A.M. in Chemistry at Harvard University, and graduated from Rice University with a B.A. in Chemistry.
| 62 |
Allan P. Rothstein - Director
Mr. Rothstein has been a member of the Board of Directors of the Company since the Merger and prior thereto served as member of the Board of Directors of Enumeral since September 20, 2013. He is a Class II Director and his term on the Board will expire in 2016. Mr. Rothstein has been a principal of Healthcare Recovery Services LLC, servicing financial aspects and enhancing revenue cycles of hospitals and other healthcare service enterprises, since December 2011. Mr. Rothstein has owned and managed Hedge Capital Partners LLC, an investment vehicle for venture capital, portfolio management and asset management, since April 2004 and is the owner of Shizoom LLC, a business funding company formed in March 2013. Mr. Rothstein was also head of trading at Centurion Partners from September 2010 to August 2011. He actively invests in emerging technology companies. He was founder and Chairman of NanoDynamics Inc. from 2002 through 2007. He served as a member of the National Nanotechnology Business Alliance and the New York Nanotechnology Business Alliance advisory boards and the Dean’s Council of the Graduate School of SUNY Stonybrook.
He has over 30 years’ experience in the financial industry, having started his career at Shearson Loeb Rhoades where he handled arbitrage lines for domestic and international customers. In 1982, he became a member of the New York Futures Exchange. In 1990 he joined Fahnestock & Company (presently, Oppenheimer & Company) as a NASDAQ market maker. In his role as Senior Vice President and co-head of NASDAQ Trading, Mr. Rothstein was responsible for trading in over 500 markets, oversight of all NASDAQ market making and trading systems construction and integration. Mr. Rothstein graduated as a Benjamin Franklin Scholar from The University of Pennsylvania, magna cum laude in 1980.
Barry Buckland – Director, Co-Founder and Co-Chairman of the Scientific Advisory Board
Dr. Buckland has been a member of the Board of Directors of the Company since the Merger and prior thereto, served as member of the Board of Directors of Enumeral since December 2009. He is a Class II Director and his term on the Board will expire in 2016. Dr. Buckland’s career in the pharmaceutical industry includes his leadership of Merck’s world class bioprocess Research and Development Group which saw the market launch of major biological products, including Gardasil, Rotateq, and Zostavax. Dr. Buckland has been the Chief Executive Officer of BiologicB LLC, a consulting firm working with biotechnology and vaccine products, since May 2009. He is the recipient of numerous awards, among them the Donald Medal, UK Institute of Chemical Engineering in 2002, Prix Galien award as a member of the team that received the Vaccine Award for Gardasil in 2007, the Marvin Johnson award by ACS for lifetime contribution to Biotechnology in 2008, and the PhRMA Discoverer of the Year for development of the Merck HPV vaccine (along with Eliav Barr and Kathrin Jansen) in 2009. He has chaired multiple International Conferences related to Bioprocess Research and Development and Vaccine Technology and is an author on over 70 papers. He has been a Visiting Professor at University College London since 1995, and earned his Ph.D. in Biochemical Engineering at University College London in 1974.
Scientific Advisors and Consultants
The Company retains scientific and clinical professionals on an as-needed, consulting basis. The Company also consults Scientific Advisors who have significant experience that is directly relevant to our discovery and collaboration efforts. The following is a list of the Company’s Scientific Advisors:
J. Christopher Love, Ph.D., Scientific Founder and Co-Chairman of the Scientific Advisory Board.
Dr. Love is an Associate Professor in the Department of Chemical Engineering at the Massachusetts Institute of Technology, and an associate member at the Eli and Edythe L. Broad Institute. Dr. Love began work on a number of the unconventional methods to fabricate micro- and nanoscale structures using both soft lithography and self-assembly which are foundational to our technology platform while in the laboratory of George Whitesides at Harvard.
Dr. Love extended his research into immunology and pathology when he was a research fellow at Harvard Medical School and then as an Instructor at the Immune Disease Institute (formerly CBRI). Drawing on his expertise in surface chemistry and microtechnologies, Dr. Love studied both the dynamics of phagosomal maturation in dendritic cells and the activation of naïve B cells by live cell imaging and microfluids. Dr. Love earned his Ph.D. in physical chemistry at Harvard and B.S. in Chemistry from University of Virginia
| 63 |
Florian P. Schödel, M.D.
Dr. Schödel, formerly of Merck Research Labs, has over 20 years of experience leading teams in the development of preventative and therapeutic vaccines and biologics, and is considered a leading authority in biologics and vaccine development. While at Merck, Dr. Schödel played key roles in the development and implementation of several leading vaccines including the shingles vaccine ZOSTAVAX® and the rotavirus vaccine RotaTeq®, as well as the natural product based cholesterol-reducing medicine ZOCOR®.
Prior to joining Merck, Dr. Schödel performed important research focused on developing vaccines against such diseases as hepatitis B, malaria and typhoid at the Max-Planck Institute for Biochemistry, Walter Reed Army Institute of Research and Inserm.
Dr. Schödel completed post doctoral studies at Ludwig-Maximilians-Universität, München, 1991, Habilitation for Hygiene and Medical Microbiology (academic degree, second thesis: Dr. med. habil, and prerequisite for professorship in Germany). Dr. Schödel completed Medical School, Ludwig-Maximilians-Universität, München, 1986, Thesis, Dr. med. (summa cum laude) and Medical School, Technische Universität, München, 1983, Graduation and Approbation, (M.D. License).
Wenyan Shen, Ph.D.
Dr. Shen is Vice President, Global Head of Biologics Development at TEVA Pharmaceuticals. Dr. Shen is a member of TEVA Pharmaceutical’s executive team and is responsible for setting the company’s strategy for BioSimilars, BioBetters and innovative biologics. In this capacity, Mr. Shen oversees research, process development and CMC up to phase II clinical materials. Dr. Shen previously led research and process development teams at Merck and Amgen.
As a senior executive in charge of research and process development teams in major biotech and pharmaceutical companies, Dr. Shen is well-recognized for his leadership role in the discovery of new technologies (20 patents or applications and more than 20 peer reviewed papers) with important industrial applications.
Dr. Shen received his Ph.D. in Molecular Biology from the University of Toronto and completed his post-doctoral training at The Whitehead Institute, MIT. He earned his B.Sc. in Biochemistry from the East China University of Science and Technology in Shanghai, China.
Director Independence
We are not currently subject to listing requirements of any national securities exchange or inter-dealer quotation system which has requirements that a majority of the board of directors be “independent” and, as a result, we are not at this time required to have our Board of Directors comprised of a majority of “independent directors.” Nevertheless, our Board has determined that Messrs. Buckland and Wolfe are independent directors under the applicable standards of the SEC and NYSE MKT stock market.
Family Relationships
There are no family relationships among our Directors or executive officers.
Involvement in Certain Legal Proceedings
Except as described in the “Directors and Executive Officers” section above, none of our directors or executive officers has been involved in any of the following events during the past ten years:
| 64 |
| · | any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| · | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
| · | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his or her involvement in any type of business, securities or banking activities; or |
| · | being found by a court of competent jurisdiction (in a civil action), the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
Board Committees
On August 4, 2014, the Company established an audit committee and compensation committee of the Board of Directors, each of which is composed of Messrs. Buckland, Rothstein and Wolfe. Our Board of Directors may designate from among its members an executive committee and one or more other committees in the future. We do not have a nominating committee or a nominating committee charter. Further, we do not have a policy with regard to the consideration of any director candidates recommended by security holders. To date, other than as described above, no security holders have made any such recommendations. Other than the audit committee and compensation committee, the entire Board of Directors performs all functions that would otherwise be performed by committees. If we are able to grow our business and increase our operations, we intend to expand the size of our board and allocate responsibilities accordingly.
Audit Committee Financial Expert
On August 4, 2014, the Company established an audit committee composed of Messrs. Buckland, Rothstein and Wolfe with Mr. Wolfe serving as chair. The audit committee oversees our audits and auditing procedures. Neither the Board of Directors or the audit committee has at this time determined whether any director is an “audit committee financial expert” within the meaning of Item 407(d)(5) for SEC regulation S-K. Based on his experience as CFO of Harris & Harris Group, we believe that Daniel Wolfe would qualify as an audit committee financial expert.
Compensation Committee Interlocks and Insider Participation
On August 4, 2014, the Company established a compensation committee composed of Messrs. Buckland, Rothstein and Wolfe with Mr. Rothstein serving as chair. No executive officer of the Company has served as a director or member of the compensation committee (or other committee serving an equivalent function) of any other entity.
Code of Ethics
The Company currently has not adopted a written code of ethics.
The following table sets forth information concerning the total compensation paid or accrued by the Company and Enumeral during the last two fiscal years indicated to (i) all individuals that served as our or Enumeral’s principal executive officer or acted in a similar capacity for the Company or Enumeral at any time during the most recent fiscal year indicated; (ii) the two most highly compensated executive officers who were serving as executive officers of the Company or Enumeral at the end of the most recent fiscal year indicated; and (iii) up to two additional individuals for whom disclosure would have been provided pursuant to clause (ii) above but for the fact that the individual was not serving as an executive officer of the Company or Enumeral at the end of the most recent fiscal year indicated.
| 65 |
Summary Compensation Table
| Name & Principal Position | Fiscal Year ended October 31, | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Non-Qualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||||||||
| Olesya Didenko | 2013 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| President, Treasurer and Secretary (1) | 2012 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Fiscal
Year ended December 31, | ||||||||||||||||||||||||||||||||||||
| Arthur H. Tinkelenberg | 2013 | 250,000 | — | 87,480 | — | — | — | — | 337,480 | |||||||||||||||||||||||||||
| President and CEO (2) | 2012 | 250,000 | — | — | — | — | — | — | 250,000 | |||||||||||||||||||||||||||
| John J. Rydzewski | 2013 | 200,000 | — | 87,480 | — | — | — | — | 287,480 | |||||||||||||||||||||||||||
| Chairman (2) | 2012 | 200,000 | — | — | — | — | — | — | 200,000 | |||||||||||||||||||||||||||
| Derek Brand (2) | 2013 | 157,758 | 13,500 | — | 22,923 | — | — | — | 194,181 | |||||||||||||||||||||||||||
| 2012 | 141,258 | 12,000 | — | 21,600 | — | — | — | 174,858 | ||||||||||||||||||||||||||||
| (1) | On July 31, 2014, Olesya Didenko resigned as sole officer and director of the Company. |
| (2) | Reflects compensation received from Enumeral. |
We have no plans in place and have never maintained any plans that provide for the payment of retirement benefits or benefits that will be paid primarily following retirement including, but not limited to, tax qualified deferred benefit plans, supplemental executive retirement plans, tax-qualified deferred contribution plans and nonqualified deferred contribution plans.
Except as indicated below, we have no contracts, agreements, plans or arrangements, whether written or unwritten, that provide for payments to the named executive officers listed above.
Outstanding Equity Awards at Fiscal Year-End
We have one compensation plan approved by our stockholders, the 2014 Plan. As of the end of our last completed fiscal year, we had not granted any awards under the 2014 Plan. As of December 31, 2013, Enumeral had one equity compensation plan, its 2009 Equity Incentive Plan (the “2009 Plan”), which provided for awards of stock options, restricted stock and restricted stock units. The table below provides information with respect to the outstanding equity awards of Messrs. Tinkelenberg, Rydzewski and Brand as of December 31, 2013 without giving effect to the Merger and represent awards in shares of Enumeral common stock. In connection with the Merger, the 2009 Plan was terminated and options to purchase 948,567 shares of Enumeral common stock were converted into options to purchase 1,045,419 shares of our Common Stock. See “Description of Securities—Options” below for more information.
| 66 |
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name | Number of Securities Under- lying Unexer- cised Options Exercis- able (#) | Number of Securities Under- lying Unexer- cised Options Unexer- cisable (#) | Equity Incentive Plan Awards: Number of Securities Under- lying Unexer- cised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number
of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) | |||||||||||||||||||||||||||
| Arthur H. Tinkelenberg | - | - | - | - | - | 229,500 | (a) | $ | 229,500 | - | - | |||||||||||||||||||||||||
| John J. Rydzewski | - | - | - | - | - | 229,500 | (a) | $ | 229,500 | - | - | |||||||||||||||||||||||||
| Derek Brand | 33,625 | (b) | 14,375 | - | $ | 0.23000 | 11/30/2021 | |||||||||||||||||||||||||||||
| 21,225 | (c) | 63,675 | - | $ | 0.27000 | 10/26/2022 | ||||||||||||||||||||||||||||||
| 2,500 | (d) | 17,500 | 60,000 | $ | 0.27000 | 7/25/2023 | ||||||||||||||||||||||||||||||
Footnotes –
| (a) | Consists of 324,000 shares of Enumeral common stock subject to a restricted stock award granted as of January 2, 2013. Of which 13,500 shares were immediately vested upon grant and the balance vests in 46 equal monthly installments beginning on January 26, 2013. |
| (b) | Consists of incentive stock options granted November 30, 2011 and exercisable for 48,000 shares of Enumeral common stock. 3,000 shares vested as of the grant date; the remaining 45,000 shares vest as follows: 30,000 shares will vest according to a time-based schedule as follows (the “time-based shares”): twenty-five percent (25%) of the time-based shares will vest on the first anniversary of the Vesting Commencement Date (“VCD”), the remaining unvested portion of the time-based shares will vest over the three-year period following the first anniversary of the VCD, with vesting of such unvested portion to occur on a monthly basis on the last day of each month (1/36th per month) during such three-year period. 15,000 shares will vest according to the satisfactory achievement of performance-based objectives. These objectives have been achieved. |
| (c) | Consists of incentive stock options granted October 26, 2012 and exercisable for 84,900 shares of Enumeral common stock. 56,600 shares shall vest monthly over four years in 48 equal monthly installments, beginning on November 26, 2012; 28,300 shares shall vest upon satisfactory achievement of performance-based objectives. These objectives have been achieved. |
| (d) | Consists of incentive stock options granted July 25, 2013 and exercisable for 80,000 shares of Enumeral common stock. 20,000 shares shall vest monthly over four years in 48 equal monthly installments, beginning on July 25, 2013; 60,000 shares shall vest upon satisfactory achievement of performance-based objectives. These objectives have not been met. |
Employment Agreements
John J. Rydzewski
On July 21, 2014, Enumeral and Mr. Rydzewski amended and restated his existing employment agreement, dated as of October 1, 2011. His amended and restated employment agreement provides, following the closing of the Merger and PPO, for (i) a term of employment of two years from July 21, 2014 that automatically renews for additional two-year terms unless terminated by Enumeral or Mr. Rydzewski, (ii) a base salary of $262,500, (iii) entitlement to participate in bonus plans which are approved by the Board for Enumeral employees generally, (iv) a stock option award to purchase 100,000 shares of Enumeral’s or, if applicable, the Company’s common stock subject to the vesting conditions set forth in the employment agreement and with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors, (v) an incentive stock option award to purchase 200,000 shares of Enumeral’s or, if applicable, the Company’s common stock subject to vesting conditions set forth in the employment agreement and with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors, and (vi) a warrant to purchase 47,058 shares of Enumeral’s Series B Convertible Preferred Stock subject to vesting conditions set forth in the employment agreement and exercisable at $2.125 per share.
| 67 |
Pursuant to the October 1, 2011 employment agreement, Mr. Rydzewski received (a) a restricted stock grant of 100,000 shares and (b) a stock option award to purchase 50,000 shares of Enumeral’s common stock, in each case subject to the vesting conditions set forth in the employment agreement (and which were fully vested prior to the closing of the Merger) and with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors. Mr. Rydzewski exercised his option to purchase 50,000 options on July 25, 2012. Additionally, on October 26, 2012, Mr. Rydzewski received a restricted stock grant of 324,000 shares, of which 13,500 shares were immediately vested upon grant and the balance vests in 46 equal monthly installments beginning on January 26, 2013. As of the date of the Merger, 188,250 shares of subject to this restricted stock award remained unvested.
Contemporaneous with the closing of the Merger and PPO, the warrant to purchase 47,058 shares of Enumeral’s Series B Convertible Preferred Stock vested in its entirety and was converted into a warrant to purchase the Company’s Common Stock as described herein.
On July 31, 2014, the Company agreed to assume the obligations of Enumeral under the amended and restated employment agreement.
Arthur H. Tinkelenberg
On July 21, 2014, Enumeral and Dr. Tinkelenberg amended and restated his existing employment agreement, dated as of July 1, 2010. His amended and restated employment agreement provides, following the closing of the Merger and PPO, for (i) a term of employment of two years from July 21, 2014 that automatically renews for additional two-year terms unless terminated by Enumeral or Dr. Tinkelenberg, (ii) a base salary of $262,500, (iii) entitlement to participate in bonus plans which are approved by the Board for Enumeral employees generally, (iv) a stock option award to purchase 100,000 shares of Enumeral’s or, if applicable, the Company’s common stock subject to the vesting conditions set forth in the employment agreement and with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors, (v) an incentive stock option award to purchase 200,000 shares of Enumeral’s or, if applicable, the Company’s common stock subject to vesting conditions set forth in the employment agreement and with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors, and (vi) a warrant to purchase 58,823 shares of Enumeral’s Series B Convertible Preferred Stock subject to vesting conditions set forth in the employment agreement and exercisable at $2.125 per share.
Pursuant to the July 1, 2010 employment agreement, Dr. Tinkelenberg received (a) a restricted stock grant of 393,750 shares and (b) a stock option award to purchase 51,576 shares of Enumeral’s common stock, in each case subject to the vesting conditions set forth in the employment agreement (and which were fully vested prior to the closing of the Merger) and with an exercise price equal to the fair market value of Enumeral’s common stock as determined by Enumeral’s board of directors. Dr. Tinkelenberg exercised his 51,576 options on July 27, 2012. Additionally, on October 26, 2012, Dr. Tinkelenberg received a restricted stock grant of 324,000 shares, of which 13,500 shares were immediately vested upon grant and the balance vests in 46 equal monthly installments beginning on January 26, 2013. As of the date of the Merger, 188,250 shares of subject to this restricted stock award remained unvested.
Contemporaneous with the closing of the Merger and PPO, the warrant to purchase 58,823 shares of Enumeral’s Series B Convertible Preferred Stock vested in its entirety and was converted into a warrant to purchase the Company’s Common Stock as described herein.
On July 31, 2014, the Company agreed to assume the obligations of Enumeral under the amended and restated employment agreement.
| 68 |
Derek Brand
On May 19, 2011, Enumeral and Mr. Brand entered an employment agreement. His employment agreement provides for (i) “at will” employment where either Enumeral or Mr. Brand may terminate Mr. Brand’s employment with or without cause, and with or without notice, at any time, (ii) a base salary of $120,000 which may be increased to $125,000 upon Mr. Brand’s performance of certain objectives set by the President and approved by Enumeral’s board of director, (iii) eligibility to receive a bonus of 10% of Mr. Brand’s base salary upon Mr. Brand’s performance of certain objectives set by the President and approved by Enumeral’s board of director, (iv) a stock option grant to purchase 48,000 shares of Enumeral’s common stock subject to the vesting conditions set forth in the employment agreement.
Contemporaneous with the closing of the Merger and PPO, the stock option award for 48,000 vested in its entirety and converted as described herein.
On July 31, 2014, the Company agreed to assume the obligations of Enumeral under the employment agreement.
Kevin Sarney
Pursuant to his Offer Letter, dated July 25, 2014, Kevin Sarney serves as Vice President of Finance and Chief Accounting Officer of the Company. The Base Salary of the Mr. Sarney shall be $200,000. At the discretion of the Board of Directors, Mr. Sarney shall be entitled to a target bonus of 25% of his base salary based on achievement of target milestone parameters. Mr. Sarney shall be entitled to receive options to purchase 350,000 shares of Common Stock with an exercise price at the then fair market value of the Common Stock as determined by the Board of Directors. The options shall be subject to a vesting with 300,000 vesting over 4 years with the first 25% vesting on the one year anniversary of his employment and the remaining shares vesting monthly over the next three years. Options to purchase the remaining 50,000 shares shall vest subject to vesting conditions set forth in the offer letter.
This summary descriptions of the amended and restated employment agreements and arrangements described above are qualified in their entirety by reference to the agreements filed as Exhibits 10.16, 10.17, 10.18 and 10.44 to this Current Report.
Potential Payments upon Termination or Change in Control
Employment Agreements of Mr. Rydzewski and Dr. Tinkelenberg
If Mr. Rydzewski’s or Dr. Tinkelenberg’s employment was terminated for cause, death, disability or resigns for any reason (other than resignation for Good Reason), their employment agreements provide that such executive would have received:
| · | any accrued but unpaid salary through the date of termination plus any accrued vacation |
| · | any earned but unpaid bonus for the most recently completed year |
| · | reimbursement of any unreimbursed expenses and |
| · | immediate termination and cancellation of all unvested stock options. |
If Mr. Rydzewski’s or Dr. Tinkelenberg’s employment is terminated by the Company other than for cause or by Mr. Rydzewski or Dr. Tinkelenberg for good reason where a Change in Control has not occurred, the executive will receive:
| · | base salary for the greater of twelve months or the remaining time until two years from the date of the closing of the Merger and PPO, |
| 69 |
| · | all earned and declared but unpaid bonuses which are due and payable, including a pro rata portion of the current years’ potential bonus as determined in good faith by the Compensation Committee of the Board of Directors, as applicable, |
| · | twelve months of continued coverage under the Company’s health plans and participation in the Company’s disability and/or life insurance program, |
| · | continued vesting of any non-vested stock options and restricted stock granted pursuant to the employment agreement for one year after the executive’s termination of employment pursuant to the vesting schedule set forth therein, provided that if the executive breaches his employment agreement, all of the executive’s unvested options and shares of restricted stock will be immediately forfeited, and |
| · | payment for all accrued but unused vacation. |
If Mr. Rydzewski’s or Dr. Tinkelenberg’s employment is terminated or Mr. Rydzewski or Dr. Tinkelenberg resigned for Good Reason where a Change in Control has occurred, the executive will receive:
| · | one year of base salary, payable immediately in one lump sum, |
| · | all bonuses which are due and payable, including the full amount of the current years’ target bonus as if the current target milestones were achieved, |
| · | twelve months of continued coverage under the Company’s health plans and participation in the Company’s disability and/or life insurance program, |
| · | all non-vested stock options or restricted stock shall best immediately and become exercisable or non-forfeitable and shall be exchanged for cash or freely tradable stock of the acquiring company, and |
| · | payment for all accrued but unused vacation. |
“Cause” is defined in the executives’ employment agreements as:
| · | the executive’s conviction of any felony involving dishonesty, breach of trust or misappropriation or entry of a plea of nolo contendere thereto, |
| · | commission of an act of fraud upon, breaching the duty of loyalty to, the Company, |
| · | conviction for willful violation of any law governing operation of the Company or any of its subsidiaries that is punishable by six months or more imprisonment, |
| · | substantial and continuing refusal of the executive, after seven (7) days’ written notice thereof, to perform his or her job duties and responsibilities which failure is committed in bad faith, |
| · | the executive’s breach of his employment agreement that continues for more than seven days after written notice has been given to the executive, and |
| · | deliberate and willful disregard for the Company’s rules or policies that results in material and substantial loss, damage or injury to the Company. |
“Good Reason” is defined in the executives’ employment agreements as:
| · | a reduction in the executive’s then-current base salary or target bonus percentage, |
| 70 |
| · | any failure to offer to the executive at least the same level of benefits offered to similarly situated employees, |
| · | a significant diminution in the executive’s managerial authority, duties and responsibilities following a change in control of the Company relocation of the executive’s primary business location to a location outside of the New York metropolitan area, |
| · | failure to pay to the executive any portion of his current base salary, bonus or benefits within 20 days of when such compensation is due, and |
| · | the Company’s failure to obtain a reasonably satisfactory agreement from any successor to the Company to assume and agree to perform the executive’s employment agreement. |
“Change in control” is defined in the executives’ employment agreements as:
| · | merger or consolidation of the Company with another entity, where, immediately after the transaction, |
| o | the Company’s stockholders immediately prior to the merger or consolidation beneficially own less than 50% of the voting shares of the surviving entity, or |
| o | persons who constitute the Company’s board of directors prior to the transaction cease to constitute at least a majority of the board of directors of the surviving entity |
| · | sale, lease or other transfer of all or substantially all of the Company’s assets or the exclusive license of all or substantially all of its patents, provisional patent applications and patent applications for substantially all uses to a third party where, immediately after the transaction, |
| · | any person becomes the beneficial owner of the Company’s securities representing at least 50% of the combined voting power of the Company’s then outstanding securities having the right to vote in an election of the Company’s board of directors. |
Employment Agreement of Derek Brand
If Mr. Brand’s employment was terminated for cause on December 31, 2013, death or disability, his employment agreements provide that the Executive would have received:
| · | any unpaid base salary through the date of termination plus any accrued vacation, |
| · | any earned but unpaid bonus for 2013, |
| · | reimbursement of any unreimbursed expenses, and |
| · | immediate termination and cancellation of all unvested stock options. |
If Mr. Brand’s employment is terminated by the Company other than for cause or by Mr. Brand for good reason, the executive will receive:
| · | three months of base salary, |
| · | six months of continued coverage under the Company’s health plans and participation in the Company’s disability and/or life insurance program, |
| · | if there has not been a change in control of the Company, continued vesting of the option granted pursuant to the employment agreement for one year after the executive’s termination of employment pursuant to the vesting schedule set forth therein, provided that if the executive breaches his employment agreement, all of the executive’s unvested portion of the option will be immediately forfeited, and |
| 71 |
| · | if there has been a change in control of the Company, immediate vesting of the executive’s outstanding stock option awards granted pursuant to the executive’s employment agreement. |
“Cause” is defined in the executives’ employment agreements as:
| · | the executive’s conviction of any felony involving dishonesty, breach of trust or misappropriation or entry of a plea of nolo contendere thereto, |
| · | commission of an act of fraud upon, breaching the duty of loyalty to, the Company, |
| · | conviction for willful violation of any law governing operation of the Company or any of its subsidiaries that is punishable by six months or more imprisonment, |
| · | substantial and continuing refusal of the executive, after written notice thereof, to reasonably attempt to perform his or her job duties and responsibilities which failure is committed in bad faith or is not in the best interests of the Company, |
| · | the executive’s breach of his employment agreement that continues for more than seven days after written notice has been given to the executive, and |
| · | deliberate and willful disregard for the Company’s rules or policies that results in material and substantial loss, damage or injury to the Company. |
“Good reason” is defined in the executives’ employment agreements as:
| · | a reduction in the executive’s then-current base salary or bonus percentage, |
| · | any failure to offer to the executive at least the same level of benefits offered to similarly situated employees, |
| · | a requirement that the executive engage in materially greater or different activities than set forth in his employment agreement, |
| · | failure to pay to the executive any portion of his current base salary, bonus or benefits within 20 days of when such compensation is due, or |
| · | the Company’s material breach of its obligation under his employment agreement. |
“Change in control” is defined in the executives’ employment agreements as:
| · | merger or consolidation of the Company with another entity, where, immediately after the transaction, |
| o | the Company’s stockholders immediately prior to the merger or consolidation beneficially own less than 50% of the voting shares of the surviving entity or |
| o | persons who constitute the Company’s board of directors prior to the transaction cease to constitute at least a majority of the board of directors of the surviving entity |
| · | sale, lease or other transfer of all or substantially all of the Company’s assets or the exclusive license and sublicense of all or substantially all of its patents, provisional patent applications and patent applications for substantially all uses to a third party where, immediately after the transaction |
| 72 |
| o | the Company’s stockholders immediately prior to the transaction beneficially own less than 50% of the voting shares of such third party or |
| o | Persons who constitute the Company’s board of directors prior to such transaction cease to constitute at least a majority of directors of such third party and |
| · | any person becomes the beneficial owner of the Company securities representing at least 50% of the combined voting power of the Company’s then outstanding securities having the right to vote in an election of the Company’s board of directors. |
The Company’s 2014 Equity Incentive Plan
Please refer to “Market Price of and Dividends on Common Equity and Related Stockholder Matters—Securities Authorized for Issuance under Equity Compensation Plans—The Company’s 2014 Equity Incentive Plan—Change in Control” for discussion regarding changes in control under the 2014 Plan.
Director Compensation
Non-employee directors’ compensation generally is determined and awarded by the Board of Directors. The Board of Directors is responsible for, among other things, reviewing, evaluating and designing a director compensation package of a reasonable total value, typically based on comparisons with similar firms, and aligned with long-term interests of the stockholders of the Company, and reviewing director compensation levels and practices and considering, from time to time, changes in such compensation levels and practices. These matters also include making equity awards to non-employee directors from time to time under the Company’s equity-based plans. As part of these responsibilities, the Board of Directors may request that management of the Company provide it with recommendations on non-employee director compensation and/or common director compensation practices, although the Board of Directors retains its ultimate authority to take compensatory actions.
Prior to the Merger, the Company did not pay its non-employee directors an annual retainer. On August 4, 2014, the Board of Directors approved a Director Compensation package for non-executive directors. Newly elected non-executive Board members will receive an option grant of 60,000 shares that will vest on a monthly basis over three years from date of grant and a one-time option grant of 20,000 shares that will be fully vested upon their election to the Board. The Chairman of the Board, if such Chairman is not receiving other compensation from the Company for employment, will receive either cash compensation or shares in addition to the base grant in an amount/number agreed to with the Board of Directors of the Company. Non-Executive directors shall also be entitled an annual retainer at the rate of $20,000 per annum. If such Directors serve on the Audit Committee, then the Audit Committee member shall be entitled to compensation at the rate of $3,000 per annum with the Chair being entitled to an additional compensation at the rate of $6,000 per annum. Any Director serving on the Compensation Committee shall be entitled to compensation at the rate of $2,000 per annum with the Chair being entitled to additional compensation at the rate of $4,000 per annum. A director who serves in the position of Lead Independent Director or Lead Non-Management Director shall be entitled to compensation at the rate of $6,000 per annum. All such fees shall be paid quarterly in advance provided that the Director is still serving as a director when such payment is due. Individual Directors may elect to take, in lieu of cash compensation for the annual retainer as a Director (but not any committee related payment), restricted stock or options. The election shall be made at least 30 days prior to the date when compensation shall be due for the annual period. The restricted stock or options shall be subject to one year vesting and ¼ shall vest on each fiscal quarter provided director continues to serve as a director.
On August 4, 2014, pursuant to the adoption of the Director Compensation package discussed above, the Company granted each of Messrs. Buckland, Rothstein and Wolfe an option to purchase 20,000 shares of common stock with an exercise price of $1.00 per share which are fully vested. Additionally, on August 4, 2014, the Company granted Mr. Wolfe an option to purchase 60,000 shares of common stock with an exercise price of $1.00 per share which shall vest monthly over a three-year period commencing on September 1, 2014.
| 73 |
In connection with the Merger, John Rydzewski was elected Chairman of the Board of Directors and, pursuant to the adoption of his employment agreement, was granted an option to purchase 300,000 shares of the Company’s common stock. Also in connection with the Merger, Arthur Tinkelenberg was elected Director and, pursuant to the adoption of his employment agreement, was granted an option to purchase 300,000 shares of the Company’s common stock. Each of the option awards were made under our 2014 Plan. Additional information regarding the option awards may be found in “—Employment Agreements” and “Description of Securities – Options.”
The following table sets forth compensation actually paid to or earned or accrued by Enumeral non-executive directors during 2013:
| Name | Fees earned or paid in cash ($) | Stock awards ($) | Option awards ($) | Non-equity incentive plan compensation ($) | Nonqualified deferred compensation earnings ($) | All other compensation ($) | Total ($) | |||||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | |||||||||||||||||||||
| Barry Buckland (1) | 53,125 | — | — | — | — | — | 53,125 | |||||||||||||||||||||
| Allan Rothstein (2) | — | 135,000 | — | — | — | — | 135,000 | |||||||||||||||||||||
| Michael B. Weiss (1) | 9,500 | — | — | — | — | — | 9,500 | |||||||||||||||||||||
| (1) | Represents fees earned per consulting agreements for service on Scientific Advisory Board and/or Business Advisory Board. |
| (2) | Represents grant of Enumeral common stock for consulting services rendered to Enumeral. See “Certain Relationships and Related Transactions” for further information. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
SEC rules require us to disclose any transaction or currently proposed transaction in which the Company is a participant and in which any related person has or will have a direct or indirect material interest involving the lesser of $120,000.00 or one percent (1%) of the average of the Company’s total assets as of the end of last two completed fiscal years. A related person is any executive officer, director, nominee for director, or holder of 5% or more of the Company’s common stock, or an immediate family member of any of those persons.
The descriptions set forth above under the captions “The Merger and Related Transactions—Merger Agreement,” “—Split-Off,” “—the PPO,” “—Registration Rights,” “—Composition of the Board; Voting Agreement,” “—2014 Equity Incentive Plan,” “—Lock-up Agreements and Other Restrictions” and “Executive Compensation—Employment Agreements” and “—Director Compensation” and below under “Description of Securities—Options” are incorporated herein by reference.
As more fully described in “Item 3.02. Unregistered Sales of Equity Securities,” we completed the following offerings in which a person who was a related party at the time of the transaction participated (share and per share numbers below are without giving effect to the conversion of all Enumeral shares into shares of our Common Stock in the Merger):
| (a) | During April and June, 2011, Enumeral completed a private placement of 2,766,926 shares of Series A Preferred Stock. John J. Rydzewski, the Executive Chairman of Enumeral, Arthur H. Tinkelenberg, the Chief Executive of Enumeral, Barry Buckland, a director of Enumeral, and Harris & Harris Group, Inc., a holder of 5% or more of Enumeral’s securities, purchased 43,104, 65,000, 21,552 and 646,551, respectively, of the securities in this offering, |
| (b) | During April 2011, Harris & Harris Group, Inc. (holder or 5% or more of Enumeral’s securities) received 310,487 shares of Series A Convertible Preferred Stock converted from principal of the Bridge Note that it acquired in December 2009 and from 8.00% interest earned on the Bridge Note for a total cost of $276,833. |
| 74 |
| (c) | During June and September, 2012, Enumeral completed a private placement of 2,047,207 shares of Series A-1 Preferred Stock. John J. Rydzewski, the Executive Chairman of Enumeral, Arthur H. Tinkelenberg, the Chief Executive of Enumeral, Barry Buckland, a director of Enumeral, and Harris & Harris Group, Inc. (holder or 5% or more of Enumeral’s securities) purchased 76,924, 57,693, 19,231, and 576,923, respectively, of the securities in this offering. |
| (d) | During April and September, 2013, Enumeral completed a private placement of 1,833,798 shares of Series A-2 Preferred Stock. John J. Rydzewski, the Executive Chairman of Enumeral, Arthur H. Tinkelenberg, the Chief Executive of Enumeral, Allan Rothstein, who became a director of Enumeral in September 2013, Jay and Tammy Levine, Mr. Rothstein’s brother-in-law and sister, and Harris & Harris Group, Inc. (holder or 5% or more of Enumeral’s securities) purchased 34,483, 34,483, 100,000, 100,000, and 724,138, respectively, of the securities in this offering. |
| (e) | During February and March, 2014, Enumeral completed a private placement of $750,000 principal amount of 12% Convertible Promissory Notes and warrants to purchase 694,443 shares of Enumeral Common Stock. John J. Rydzewski, the Executive Chairman of Enumeral, Emanuele Ostuni, a director of Enumeral at the time, Allan Rothstein, a director of Enumeral, Michael Weiss, a director of Enumeral at the time, Harris & Harris Group, Inc. (holder or 5% or more of Enumeral’s securities) purchased $50,000 principal amount of the Bridge Notes and warrants to purchase 46,296 shares of common stock, $10,000 principal amount of the Bridge Notes and warrants to purchase 9,259 shares of common stock, $265,000 principal amount of the Bridge Notes and warrants to purchase 245,370 shares of common stock, $50,000 principal amount of the Bridge Notes and warrants to purchase 46,296 shares of common stock, and $250,000 principal amount of the Bridge Notes and warrants to purchase 231,481 shares of common stock, respectively, of the securities in this offering. |
| (f) | During April 2014, Enumeral completed a private placement of 948,823 shares of Series B Preferred Stock. Harris & Harris Group, Inc. (holder or 5% or more of Enumeral’s securities) purchased 470,588 of the securities in this offering. |
In September 2013, Enumeral entered into a consulting agreement with Allan Rothstein, a director of the Company, and his father, Norman Rothstein, to consult with Enumeral’s chief executive officer and senior members of management on various financing-related matters. Messrs. Rothstein each received 500,000 shares of Enumeral common stock valued at $0.27 per share as payment for their services provided to Enumeral. These grants vested in 1/3 increments in September 2013, on December 14, 2013 and on March 10, 2014. The consulting agreement was terminated on July 30, 2014.
In 2011, pursuant to a license agreement with Massachusetts Institute of Technology (“MIT”) Enumeral licensed certain intellectual property in exchange for the payment of upfront license fees and a commitment to pay annual license fees, patent costs, milestone payments, and, upon product commercialization, royalties sales of products covered by the licenses or income from corporate partners. Dr. Love, a co-founder of the Company and Co-Chairman of our Scientific Advisory Board is an Associate Professor in the Department of Chemical Engineering at MIT and was the benefially owner of more than holder of 5% or more of Enumeral’s common stock , at the time Enumeral entered into the license agreement.
MARKET PRICE OF AND
DIVIDENDS ON COMMON EQUITY
AND RELATED STOCKHOLDER MATTERS
Our Common Stock is quoted on the OTC Markets (OTCQB) under the symbol “ENUM”, which changed from “CEUL” on July 21, 2014.
| 75 |
Prior to the Merger, there was very limited trading in the Common Stock and the last trade was reported on May 2, 2014 at $0.15 per share. Since the Merger through August 21, 2014, the high and low bid price for the Common Stock was $2.25 and $1.20 respectively.
Prior to the Merger, we had 28,597,804 shares of Common Stock outstanding held by 11 stockholders of record. Immediately after the Split-Off, the Merger and the PPO, we had 51,591,710 shares of Common Stock outstanding held by 316 stockholders of record.
Dividend Policy
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon financial condition, results of operations, capital requirements and such other factors as the Board of Directors deems relevant.
Securities Authorized for Issuance under Equity Compensation Plans
Enumeral’s 2009 Equity Incentive Plan
Prior to the Merger, Enumeral had one equity compensation plan, the 2009 Plan. In connection with the Merger, options to purchase 948,567 shares of Enumeral common stock were converted into options to purchase 1,045,419 shares of our Common Stock under our 2014 Equity Incentive Plan. See “Description of Securities—Options” below for more information. In addition, Enumeral had granted restricted stock awards under the 2009 Plan for 324,000 shares of common stock to each of Messrs. Rydzewski and Tinkelenberg, which were converted into shares of our Common Stock in connection with the Merger. As of the Merger, 188,250 of such shares issued to each of them remain unvested.
The following table provides information with respect to the 2009 Plan as of December 31, 2013 and does not give effect to the Merger.
| Equity Compensation Plan Information | ||||||||||||
| (a) | (b) | (c) | ||||||||||
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| Equity compensation plans approved by security holders | 948,567 | $ | 0.24 | 421,096 | ||||||||
| Equity compensation plans not approved by security holders |
N/A |
N/A |
N/A | |||||||||
| Total | 948,567 | $ | 0.24 | 421,096 | ||||||||
| (1) | A total of 2,119,239 shares of Enumeral’s common stock were authorized for issuance pursuant to awards under the 2009 Plan as of December 31, 2013 (which was increased to 3,200,437 in April 2014). A total of 648,000 shares of restricted stock and 1,115,143 stock options have been awarded to participants in the 2009 Plan as of December 31, 2013. |
| 76 |
The 2009 Plan was administered by Enumeral’s Board of Directors. Subject to the terms of the 2009 Plan, Board had complete authority and discretion to determine the terms of awards under the 2009 Plan. The 2009 Plan was terminated in connection with the Merger.
The Company’s 2014 Equity Incentive Plan
The Company had no equity compensation plans as of the end of fiscal year 2013.
On July 31, 2014, our Board of Directors of the Company adopted, and on July 31, 2014, our stockholders approved, the 2014 Equity Incentive Plan, which reserves a total of 8,100,000 shares of our Common Stock for incentive awards. Incentive awards generally may be issued to officers, key employees, consultants and directors. In connection with the Merger, options to purchase an aggregate of 1,045,419 shares of our Common Stock were issued under the 2014 Plan in exchange for outstanding options that were previously issued to officers, key employees, consultants and directors of Enumeral.
Adjustment for Awards and Payouts
Unless determined otherwise by the compensation committee or the Board of Directors in the absence of such a committee, unless a participant has received a benefit of ownership such as dividend or voting rights with respect to the incentive award, the following transactions will restore, on a one-for-one basis, the number of shares available for issuance under the Plan:
| 1. | A payout of a stock appreciation right (“SAR”) or a tandem SAR in cash; |
| 2. | A cancellation, termination, expiration, forfeiture or lapse for any reason (with the exception of the termination of a tandem SAR upon exercise of the related options, or the termination of a related option upon exercise of the corresponding tandem SAR) of any award payable in shares; |
| 3. | Shares tendered in payment of the exercise price of an option; |
| 4. | Shares withheld for payment of federal, state or local taxes; |
| 5. | Shares repurchased by the Company with proceeds collected in connection with the exercise of outstanding options; and |
| 6. | The net shares issued in connection with the exercise of SARs (as opposed to the full number of shares underlying the exercised portion of the SAR). |
In addition, the number of shares of our Common Stock subject to the 2014 Plan, any number of shares subject to any numerical limit in the 2014 Plan, and the number of shares and terms of any incentive award are expected to be adjusted in the event of any change in our outstanding our Common Stock by reason of any stock dividend, spin-off, split-up, stock split, reverse stock split, recapitalization, reclassification, merger, consolidation, liquidation, business combination or exchange of shares or similar transaction.
Administration
The compensation committee of the Board of Directors, or the Board of Directors in the absence of such a committee, will administer the 2014 Plan. Subject to the terms of the 2014 Plan, the compensation committee of the Board of Directors, or the Board of Directors in the absence of such a committee, has complete authority and discretion to determine the terms of awards under the 2014 Plan.
| 77 |
Grants
The 2014 Plan authorizes the grant by the compensation committee to participants of nonqualified stock options, incentive stock options, restricted stock awards, restricted stock units, performance units and performance shares (which may be designed to comply with Section 162(m) of the Internal Revenue Code (as amended, the “Code”)) and SARs, as described below:
| · | Options granted entitle the grantee, upon exercise, to purchase a specified number of shares from us at a specified exercise price per share. The exercise price for shares of our Common Stock covered by an option cannot be less than the fair market value of our Common Stock on the date of grant. In addition, in the case of an incentive stock option granted to an employee who, at the time the incentive stock option is granted, owns stock representing more than 10% of the voting power of all classes of stock of the Company or any parent or subsidiary, the per share exercise price will be no less than 110% of the fair market value of our Common Stock on the date of grant. |
| · | Restricted stock awards and restricted stock units may be awarded on terms and conditions established by the compensation committee, or the Board of Directors in the absence of such a committee, which may include time-based and performance based-conditions for restricted stock awards and the lapse of restrictions on the achievement of one or more performance goals for restricted stock units. |
| · | A performance share award and/or a performance unit award may be granted to participants. Each performance unit will have an initial value that is established by the compensation committee, or the Board of Directors in the absence of such a committee, at the time of grant. Each performance share will have an initial value equal to the fair market value of one share of Common Stock on the date of grant. Such awards may be earned based upon satisfaction of certain specified performance criteria, subject to such other terms and conditions as the compensation committee, or the Board of Directors in the absence of such a committee, deems appropriate. |
| · | SARs entitle the participant to receive a distribution in an amount not to exceed the number of shares of our Common Stock subject to the portion of the SAR exercised multiplied by the difference between the market price of a share of our Common Stock on the date of exercise of the SAR and the market price of a share of our Common Stock on the date of grant of the SAR. An Option and a SAR may be granted “in tandem” with each other. An option and a SAR are considered to be in tandem with each other because the exercise of the option aspect of the tandem unit automatically cancels the right to exercise the SAR aspect of the tandem unit, and vice versa. The option may be an incentive stock option or a nonqualified stock option. |
Change in Control
Generally upon the occurrence of a Change in Control:
| 1. | any and all options and SARs granted shall become fully-vested and immediately exercisable; |
| 2. | any periods of restriction and any restrictions imposed on restricted stock or RSUs which are not intended to qualify for the performance-based exception shall lapse; and |
| 3. | any award intended to qualify for the performance-based exception under Section 162(m) of the Code shall be earned in accordance with the applicable award agreement. |
For purposes of the 2014 Plan, a “Change in Control” shall be deemed to have occurred as of the first day that any one or more of the following conditions shall have been satisfied:
| · | the “Beneficial Ownership” of securities as defined in Rule 13d-3 under the Exchange Act representing more than thirty-three percent (33%) of the combined voting power of the Company is acquired by any “person” as defined in Section 3(a)(9) of the Exchange Act (other than the Company, any trustee or other fiduciary holding securities under an employee benefit plan of the Company, or any corporation owned, directly or indirectly, by the stockholders of the Company in substantially the same proportions as their ownership of stock of the Company); or |
| 78 |
| · | the consummation of a definitive agreement to merge or consolidate the Company with or into another corporation or to sell or otherwise dispose of all or substantially all of its assets, or adopt a plan of liquidation; or |
| · | during any period of three consecutive years, individuals who at the beginning of such period were members of the Board of Directors cease for any reason to constitute at least a majority thereof (unless the election, or the nomination for election by the Company’s stockholders, of each new director was approved by a vote of at least a majority of the directors then still in office who were directors at the beginning of such period or whose election or nomination was previously so approved). |
Notwithstanding the foregoing, with respect to any incentive award subject to Code Section 409A, a “change in control” of the Company is defined in a manner to insure compliance with Code Section 409A.
Duration, Amendment, and Termination
The Board of Directors upon recommendation of the compensation committee, has the power to amend, suspend or terminate the 2014 Plan without stockholder approval or ratification at any time or from time to time. No change may be made that increases the total number of shares of our Common Stock reserved for issuance pursuant to incentive awards, materially increase, the benefits accruing to participants or materially modify the requirements for participation in the 2014 Plan, unless such change is authorized by our stockholders. Unless sooner terminated, the 2014 Plan will terminate ten years after it is adopted.
As of the date hereof, options to purchase an aggregate of 1,911,479 shares of our Common Stock have been issued under the 2014 Plan. See “Description of Securities—Options” below for more information.
All descriptions of the 2014 Plan herein and the option agreements herein are qualified in their entirety by reference to the text thereof filed as an Exhibits 10.11, 10.12, 10.13, 10.14 and 10.15 hereto, which is incorporated herein by reference.
We have authorized capital stock consisting of 300,000,000 shares of Common Stock and 10,000,000 shares of preferred stock. As of the date of this Current Report, we had 51,591,710 shares of Common Stock issued and outstanding, and no shares of preferred stock issued and outstanding. Prior to the Merger, the Company was incorporated in Nevada, but has recently been reincorporated in Delaware in connection with the Merger.
Common Stock
The holders of outstanding shares of common stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends of such times and in such amounts as Board of Directors from time to time may determine. Holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. On the Closing Date, we implemented a staggered board consisting of three classes of directors, with Class I having an initial term expiring in 2015, Class II having an initial term expiring in 2016 and Class III having an initial term expiring in 2017. There is no cumulative voting of the election of directors then standing for election. The common stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon liquidation, dissolution or winding up of our company, the assets legally available for distribution to stockholders are distributable ratably among the holders of the common stock after payment of liquidation preferences, if any, on any outstanding payment of other claims of creditors.
| 79 |
This classification of our board of directors may prevent stockholders from changing the membership of the entire board of directors in a relatively short period of time. At least two annual meetings, instead of one, generally will be required to change the majority of directors. The classified board provisions could have the effect of prolonging the time required for a stockholder with significant voting power to gain majority representation on our board of directors. Where majority or supermajority board of directors approval is necessary for a transaction, such as in the case of an interested stockholder business combination, the inability immediately to gain majority representation on the board of directors could discourage takeovers and tender offers. See “The Merger and Related Transactions—Composition of the Board; Voting Agreement.”
Preferred Stock
Shares of preferred stock may be issued from time to time in one or more series, each of which will have such distinctive designation or title as shall be determined by our Board of Directors prior to the issuance of any shares thereof. Preferred stock will have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof, as shall be stated in such resolution or resolutions providing for the issue of such class or series of preferred stock as may be adopted from time to time by the Board of Directors prior to the issuance of any shares thereof. The number of authorized shares of preferred stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then outstanding shares of our capital stock entitled to vote generally in the election of the directors, voting together as a single class, without a separate vote of the holders of the preferred stock, or any series thereof, unless a vote of any such holders is required pursuant to any preferred stock designation.
While we do not currently have any plans for the issuance of additional preferred stock, the issuance of such preferred stock could adversely affect the rights of the holders of common stock and, therefore, reduce the value of the Common Stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of the Common Stock until the Board of Directors determines the specific rights of the holders of the preferred stock; however, these effects may include:
| · | Restricting dividends on the Common Stock; |
| · | Diluting the voting power of the Common Stock; |
| · | Impairing the liquidation rights of the Common Stock; or |
| · | Delaying or preventing a change in control of the Company without further action by the stockholders. |
Other than in connection with our classified board structure (as explained above) and shares of preferred stock (as explained above), which preferred stock is not currently designated nor contemplated by us, we do not believe that any provision of our charter or By-Laws would delay, defer or prevent a change in control.
Options
Options to purchase an aggregate of 1,911,479 shares of our Common Stock have been issued under the 2014 Plan, as follows:
| · | A combination of Incentive Stock Options (ISOs) and Non-Qualified Stock Options (NSOs) to purchase 948,567 shares of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger (“Pre-Merger Options”) were converted into options to purchase 1,045,419 shares of our Common Stock, with a weighted average exercise price of $0.3432 per share. These option grants retain their character as ISOs or NSOs and have a term that expires on the same date that the Pre-Merger Options would have expired. These option grants also have the same vesting schedule as the Pre-Merger Options, generally vesting over a period of four years or less with monthly vesting throughout the term. In some cases, the options provide vesting or accelerated vesting upon the occurrence of either performance milestones or a change in control of the Company, or both. |
| 80 |
| · | Options to purchase 1,105,000 shares of our Common Stock were granted to in the aggregate to our executive officers, Messrs. Rydzewski, Tinkelenberg, Brand, Nguyen and Sarney, in connection with the Merger. These option grants have an exercise price of $1.00 per share and have a term of ten years. |
| · | The grant to each of Messrs. Rydzewski and Tinkelenberg will vest as follows: (a) 100,000 shares will vest over four years in equal monthly installments and (b) 200,000 shares upon the earlier of (1) upon the price of the Company’s common stock being above $3, $4, $5, $6, $7, $8, $9 and $10 per share (each a “Price Based Vesting Milestone”) on a volume-weighted daily basis (adjusted for any stock-splits, recapitalizations or dividends) for at least 60 trading days (“Measuring Period”), with such vesting to occur evenly across all stock prices provided that if a higher Price Based Vesting Milestone is maintained for the Measuring Period, then the lower Priced Based Vesting Milestones shall also be deemed to have been achieved; or (2) entering into one or more collaborations or joint ventures with third party biotechnology, pharmaceutical or other companies in the life sciences or healthcare industry sector under which the Company would receive gross proceeds of at least $20 million, with twenty five percent (25%) of the shares covered by this portion of the option vesting upon each receipt by the Company of $5 million increments of gross proceeds to the Company (“Collaboration Proceeds”). The above vesting is additive, not exclusionary, and is based on the original amount of the grant, of New Option B without reduction for earlier vesting, meaning that, for example, if the Company achieves the Price Based Vesting Milestone for the Measuring Period and then the Company receives $5 million in Collaboration Proceeds, the Executive would be entitled to vest on 25,000 shares or 12.5% of the original grant amount of New Option B plus 50,000 shares or 25% of the original grant amount of New Option B. |
| · | The grant to Mr. Brand will vest as follows: (a) 15,000 shares will vest over four years in equal monthly installments; (b) 12,500 shares will vest upon the development, submission, and acceptance by President of the Company of commercial operating plan for identified cell therapy; (c) 12,500 will vest upon the execution of any agreement for supply of human patient tissues from one or more sites for an indication specified by President of the Company and (d) 25,000 will vest upon the receipt of term sheet for additional partnership with a pharmaceutical or biotechnology company acceptable to President with upfront payments or milestones within the first year of the partnership above an agreed amount. |
| · | The grant to Mr. Nguyen will vest as follows: (a) 15,000 shares will vest over four years in equal monthly installments; (b) 12,500 shares will vest upon the development, submission, and acceptance by President of R&D operating plan for an identified cell therapy; (c) 12,500 shares will vest upon the filing of patent application for certain identified antibodies; (d) 12,500 will vest upon the filing of patent application on a certain method identified by the Company; (e) 12,500 will vest upon the generation of comparative data using of certain identified targets using the Company’s proprietary method, acceptable to President; and (f) 25,000 will vest upon the attainment of first proof-of-concept data in human cells from 5 patients using Enumeral proprietary antibody, acceptable to President. |
| · | The grant to Mr. Sarney will vest as follows: (a) 300,000 vesting over 4 years with the first 25% vesting on the one year anniversary of his employment and (b) 50,000 shares shall vest upon the earlier of (1) pursuant to the Price Based Vesting Milestone on a volume-weighted daily basis for at least 60 trading days or (2) pursuant to the achievement of the Collaboration Proceeds. |
| · | Options to purchase 116,250 shares of our Common Stock were granted to our other officers and employees in connection with the Merger. These option grants have an exercise price of $1.00 per share, will be exercisable over four years with a monthly vesting schedule and have a term of ten years. |
This summary description of the options described above is qualified in their entirety by reference to the forms of such options filed as Exhibits 10.11, 10.37, 10.38, 10.39, 10.40 and 10.41 to this Current Report.
| 81 |
Warrants
As of the date hereof:
| · | The PPO Warrants entitle their holders to purchase 21,549,510 shares of Common Stock, with a term of five years and an exercise price of $2.00 per share. |
| · | The Agent Warrants entitle their holders to purchase 2,000,000 shares of Common Stock, with a term of five years and an exercise price of $1.00 per share. |
| · | Other warrants entitle their holders to purchase 1,253,899 shares of Common Stock, with terms ranging from 7 to 10 years and a weighted average exercise price ranging of $0.431 per share. |
The PPO Warrants, the Agent Warrants and another warrant entitling its holder to purchase 70,915 shares of Common Stock contain “weighted average” anti-dilution protection in the event that we issue Common Stock or securities convertible into or exercisable for shares of Common Stock at a price lower than the subject warrant’s exercise price, subject to certain customary exceptions, as well as customary provisions for adjustment in the event of stock splits, subdivision or combination, mergers, etc.
See Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Registration Rights” for a description of the registration rights granted to (among others) the holders of the PPO Warrants and the Agent Warrants, which description is incorporated herein by reference.
This summary descriptions of the warrants described above is qualified in their entirety by reference to the forms of such warrants filed as Exhibits 10.5 and 10.8 to this Current Report.
Convertible Notes
Prior to the Merger, Enumeral had outstanding $750,000 principal amount of 12% Convertible Promissory Notes due July 1, 2015 (the “Notes”). Upon closing of the Merger, the principal and interest on the Notes were converted into 3,230,869 shares of Company Common Stock.
Other Convertible Securities
As of the date hereof, other than the securities described above, the Company does not have any outstanding convertible securities.
Transfer Agent
The transfer agent for our Common Stock is Island Stock Transfer. The transfer agent’s address is 15500 Roosevelt Boulevard, Suite 301, Clearwater, Florida 33760 and its telephone number is +1-727-289-0010.
Restrictions on Transfer of Securities
The shares of Common Stock contained in the Units and underlying the PPO Warrants are subject to restrictions on transfer and have not been registered under the Securities Act or any state securities laws. Such securities must be held indefinitely unless:
| · | there is in effect a registration statement under the Securities Act covering the proposed disposition or transfer and such disposition or transfer is made in accordance with such registration statement; or |
| · | the shares are sold pursuant to an exemption from the registration requirements of the Securities Act and applicable state securities laws, and our counsel, or an outside counsel reasonably satisfactory to us, provides a legal opinion that such disposition is exempt from registration under the Securities Act and applicable state securities laws. |
| 82 |
The shares of Common Stock contained in the Units and issuable upon exercise of the PPO Warrants will bear a legend setting forth these restrictions on transfer and any legends required by state securities laws.
CHANGES IN AND DISAGREEMENTS
WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE
Information concerning the Company’s dismissal of Anton & Chia, LLP as its independent registered public accounting firm and its retention of Friedman LLP as its new independent registered public accounting firm is presented below under “Item 4.01. Changes in Registrant’s Certifying Accountant.” During the Company’s last two fiscal years, the Company had no disagreements with Anton & Chia, LLP on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure.
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
We are currently not aware of any pending legal proceedings to which we are a party or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by any governmental authority.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
The Delaware General Corporation Law (the “DGCL”) and our Amended and Restated Certificate of Incorporation (the “Certificate”) allow us to indemnify our officers and directors from certain liabilities and our Certificate states that every director, to the fullest extent permitted by the DGCL, will not be held personally liable for a breach of fiduciary duty as a director, except for liability (i) for any breach of the director’s duty of loyalty to the Corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174 of the DGCL or (iv) for any transaction from which the director derived any improper personal benefit. If the DGCL is amended to authorize further eliminating or limiting the personal liability of directors, then the liability of a director of the Corporation shall be eliminated or limited to the fullest extent permitted by the DGCL as so amended.
Our Certificate and our Bylaws provide that we will indemnify, to the fullest extent permitted by applicable law, any person (an “Indemnified Person”) who was or is made or is threatened to be made a party or is otherwise involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative (a “Proceeding”), by reason of the fact that such person is or was a director or an officer of the Corporation or, while a director or an officer of the Corporation, is or was serving at the request of the Corporation as a director, officer, employee or agent of another corporation or of a partnership, joint venture, limited liability company, trust, enterprise or nonprofit entity, including service with respect to employee benefit plans, against all liability and loss (including liabilities arising under the Securities Act) suffered and expenses (including attorneys’ fees) reasonably incurred by such Indemnified Person in such Proceeding. Our Certificate and Bylaws also provide for the advancement of expenses (including attorney’s fees) incurred in defending any such Proceeding in advance of its final disposition.
Our Bylaws provide that if an indemnification claim is not paid in full by the Corporation within 60 days after a written claim has been received by the Corporation, except in the case of a claim for an advancement of expenses, in which case the applicable period shall be 20 days, the Indemnified Person may bring suit against the Corporation to recover the unpaid amount of the claim to the fullest extent permitted by law. If successful in whole or in part in any such suit, or in a suit brought by the Corporation to recover an advancement of expenses pursuant to the terms of an undertaking, the Indemnified Person shall be entitled to be paid also the expense of prosecuting or defending such suit.
| 83 |
In addition to the indemnification in our Certificate and Bylaws, we have entered into indemnification agreements with each member of our board of directors and each of our executive officers. These agreements provide for the indemnification of our directors and officers for certain expenses and liabilities (including liabilities arising under the Securities Act) incurred in connection with any action, suit, proceeding or alternative dispute resolution mechanism, or hearing, inquiry or investigation that may lead to the foregoing, to which they are a party, or are threatened to be made a party, by reason of the fact that they are or were a director, officer, employee, agent or fiduciary of our company, or any of our subsidiaries, by reason of any action or inaction by them while serving as an officer, director, agent or fiduciary, or by reason of the fact that they were serving at our request as a director, officer, employee, agent or fiduciary of another entity. In the case of an action or proceeding by or in the right of our company or any of our subsidiaries, no indemnification will be provided for any claim where a court determines that the indemnified party is prohibited from receiving indemnification. We believe that these charter and bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
Our Bylaws provide that we may maintain insurance, at our expense, to protect us and any director, officer, employee or agent of the Company or another corporation, partnership, joint venture, trust or other enterprise against any expense, liability or loss, whether or not we would have the power to indemnify such person against such expense, liability or loss under the DGCL. We have an insurance policy covering our directors and officers with respect to certain liabilities, including liabilities arising under the Securities Act, which might be incurred by them in such capacities and against which they cannot be indemnified by us.
The limitation of liability and indemnification provisions may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. Moreover, a stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
As discussed above, our Certificate, Bylaws, and indemnification agreement provide for indemnification of our officers or directors against liability under the Securities Act. Insofar as indemnification for liabilities arising under the Securities Act may be required or permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, the Company has been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
This summary descriptions of the indemnifications described above is qualified in their entirety by reference to our Certificate, Bylaws and form of indemnification agreement the forms of such warrants filed as Exhibits 3.1, 3.2 and 10.45.
| Item 9.01 | Financial Statements and Exhibits. |
| (a) | Financial statements of business acquired. |
In accordance with Item 9.01(a), Enumeral’s audited financial statements as of, and for the fiscal years ended, December 31, 2013 and 2012, and Enumeral’s unaudited condensed financial statements as of, and for the three and six months ended, June 30, 2014, and the accompanying notes, are included in this Current Report beginning on Page F-1.
| (b) | Pro forma financial information. |
In accordance with Item 9.01(b), unaudited pro forma condensed combined financial statements for the fiscal years ended December 31, 2013 and 2012, and unaudited condensed financial statements as of, and for the six months ended, June 30, 2014, and the accompanying notes, are included in this Current Report beginning on Page F-46.
| 84 |
| (d) | Exhibits |
In reviewing the agreements included or incorporated by reference as exhibits to this Current Report on Form 8-K, please remember that they are included to provide you with information regarding their terms and are not intended to provide any other factual or disclosure information about the Company or the other parties to the agreements. The agreements may contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties have been made solely for the benefit of the parties to the applicable agreement and:
| · | should not in all instances be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; |
| · | have been qualified by disclosures that were made to the other party in connection with the negotiation of the applicable agreement, which disclosures are not necessarily reflected in the agreement; |
| · | may apply standards of materiality in a way that is different from what may be viewed as material to you or other investors; and |
| · | were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement and are subject to more recent developments. |
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date they were made or at any other time. Additional information about the Company may be found elsewhere in this Current Report on Form 8-K and the Company’s other public filings, which are available without charge through the SEC’s website at http://www.sec.gov.
| Exhibit Number |
Description | |
| 2.1* | Agreement and Plan of Merger and Reorganization, dated as of July 31, 2014, by and among the Registrant, Acquisition Sub and Enumeral Biomedical Corp. | |
| 3.1* | Amended and Restated Certificate of Incorporation of the Registrant | |
| 3.2* | Amended and Restated By-Laws of the Registrant | |
| 3.3* | Certificate of Merger of Enumeral Biomedical Corp., with and into Acquisition Sub, filed July 31, 2014 | |
| 10.1* | Split-Off Agreement, dated as of July 31, 2014, by and among the Registrant, Cerulean Operating Corp. and Olesya Didenko | |
| 10.2* | General Release Agreement, dated as of July 31, 2014, by and among the Registrant, Cerulean Operating Corp. and Olesya Didenko | |
| 10.3* | Form of Lock-Up Agreement between the Registrant and the officers, directors and shareholders party thereto | |
| 10.4* | Form of Subscription Agreement between the Registrant and the investors party thereto | |
| 10.5* | Form of PPO Warrant for Common Stock of the Registrant | |
| 10.6* | Placement Agency Agreement, dated July 3, 2014, between the Registrant and EDI Financial, Inc., as amended by Amendment No. 1 dated as of July 21, 2014 |
| 85 |
| Exhibit Number |
Description | |
| 10.7* | Placement Agency Agreement, dated July 25, 2014, between the Registrant and Katalyst Securities LLC | |
| 10.8* | Form of Agent Warrant for Common Stock of the Registrant | |
| 10.9* | Form of Registration Rights Agreement dated July 31, 2014, among the Registrant and the other parties thereto | |
| 10.10* | Voting Agreement dated July 31, 2014, among the Registrant and the other parties thereto | |
| 10.11*† | The Registrant’s 2014 Equity Incentive Plan | |
| 10.12*† | Form of Non-Qualified Stock Option Agreement under 2014 Equity Incentive Plan | |
| 10.13*† | Form of Stock Appreciation Right Grant Agreement under 2014 Equity Incentive Plan | |
| 10.14*† | Form of Restricted Stock Agreement under 2014 Equity Incentive Plan | |
| 10.15*† | Form of Incentive Stock Option Agreement under 2014 Equity Incentive Plan | |
| 10.16*† | Amended and Restated Employment Agreement, dated as of July 21, 2014 between Enumeral Biomedical Corp. and Arthur Tinkelenberg, as assumed by the Registrant | |
| 10.17*† | Amended and Restated Employment Agreement, dated as of July 21, 2014 between Enumeral Biomedical Corp. and John Rydzewski, as assumed by the Registrant | |
| 10.18*† | Employment Agreement, dated as of May 19, 2011 between Enumeral Biomedical Corp. and Derek Brand, as assumed by the Registrant | |
| 10.19* | Real Estate Lease Agreement, dated as of July 16, 2012, between Enumeral Biomedical Corp. and RB Kendall Fee, LLC | |
| 10.20* | Loan and Security Agreement, dated as of December 5, 2011, between Enumeral Biomedical Corp. and Square 1 Bank, as amended | |
| 10.21*‡ | Form of Warrant, issued by Enumeral Biomedical Corp. to Square 1 Bank | |
| 10.22* | Exclusive Patent License Agreement, dated as of April 15, 2011, between Enumeral Biomedical Corp. and the Massachusetts Institute of Technology (confidential portions have been omitted and filed separately with the Commission) | |
| 10.23* | First Amendment to Exclusive Patent License Agreement, effective as of March 8, 2013, between Enumeral Biomedical Corp. and the Massachusetts Institute of Technology (confidential portions have been omitted and filed separately with the Commission) | |
| 10.24* | Second Amendment to Exclusive Patent License Agreement, effective as of July 16, 2014, between Enumeral Biomedical Corp. and the Massachusetts Institute of Technology | |
| 10.25* | Contract Research Agreement, dated as of February 28, 2012, between Enumeral Biomedical Corp. and Crucell Holland B.V. (confidential portions have been omitted and filed separately with the Commission) |
| 86 |
| Exhibit Number |
Description | |
| 10.26* | 1st Amendment to Contract Research Agreement, dated as of March 22, 2013, between Enumeral Biomedical Corp. and Crucell Holland B.V. (confidential portions have been omitted and filed separately with the Commission) | |
| 10.27* | Master Service Provider Agreement, dated as of March 14, 2012, between Enumeral Biomedical Corp. and Anthrogenesis Corporation D/B/A Celgene Cellular Therapeutics (confidential portions have been omitted and filed separately with the Commission) | |
| 10.28* | Change Order, dated as of December 16, 2013, between Enumeral Biomedical Corp. and Celgene Cellular Therapeutics (confidential portions have been omitted and filed separately with the Commission) | |
| 10.29* | Research Agreement, dated as of June 18, 2012, between Enumeral Biomedical Corp. and sanofi-aventis U.S. Inc. (confidential portions have been omitted and filed separately with the Commission) | |
| 10.30* | Services Agreement, dated as of October 8, 2013, between Enumeral Biomedical Corp. and Novartis Pharma AG (confidential portions have been omitted and filed separately with the Commission) | |
| 10.31* | Agreement, dated as of March 21, 2013, between Enumeral Biomedical Corp. and Fikst (confidential portions have been omitted and filed separately with the Commission) | |
| 10.32* | Award/Contract, dated as of September 17, 2012, between Enumeral Biomedical Corp. and National Cancer Institute (confidential portions have been omitted and filed separately with the Commission) | |
| 10.33*‡ | Form of Warrant issued by Enumeral Biomedical Corp. to the investors in the Bridge Notes offering | |
| 10.34*‡ | Warrant dated April 15, 2014 issued by Enumeral Biomedical Corp. to John J. Rydzewski. | |
| 10.35*‡ | Warrant dated April 15, 2014 issued by Enumeral Biomedical Corp. to Arthur Tinkelenberg. | |
| 10.36*‡ | Warrant dated April 25, 2014 issued by Enumeral Biomedical Corp. to BlackBook Capital LLC | |
| 10.37* | Option Agreement dated July 31, 2014 between Registrant and John J. Rydzewski | |
| 10.38* | Option Agreement dated July 31, 2014 between Registrant and Arthur Tinkelenberg | |
| 10.39* | Option Agreement dated July 31, 2014 between Registrant and Derek Brand | |
| 10.40* | Option Agreement dated July 31, 2014 between Registrant and Anhco Nguyen | |
| 10.41* | Option Agreement dated July 31, 2014 between Registrant and Kevin Sarney |
| 87 |
| Exhibit Number |
Description | |
| 10.42*‡ | Option Agreement dated July 25, 2013 between Enumeral Biomedical Corp. and Derek Brand | |
| 10.43*‡ | Option Agreement dated July 25, 2013 between Enumeral Biomedical Corp. and Anhco Nguyen | |
| 10.44*‡ | Offer Letter dated July 25, 2014, between Enumeral Biomedical Corp. and Kevin Sarney | |
| 10.45* | Form of Indemnification Agreement between the Registrant and each of its directors and officers | |
|
16.1* |
Letter, dated August 11, 2014, from Anton & Chia, LLP to the Securities and Exchange Commission | |
| 99.1* | Press Release of the Registrant, dated July 31, 2014 |
| * | Previously filed. |
| † | Management contract or compensatory plan or arrangement |
| ‡ | Represents an option or warrant issues by Enumeral Biomedical Corp. and as such the number of shares issuable upon exercise and the exercise have not been revised to reflect the conversion to the right to receive common stock of the Registrant |
| 88 |
Enumeral Biomedical Corp.
FINANCIAL STATEMENTS
Table of Contents
| Page Number | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| Audited Financial Statements for the Years Ended December 31, 2013 and 2012 | |
| Balance Sheet | F-3 |
| Statement of Operations | F-4 |
| Statement of Members’ Equity | F-5 |
| Statements of Cash Flows | F-6 |
| Notes to Financial Statements | F-7 |
| Condensed Financial Statements for the three and six months ended June 30, 2014 (unaudited) | |
| Condensed Balance Sheets | F-25 |
| Condensed Statements of Operations | F-26 |
| Condensed Statement of Members Equity (Deficiency) | F-27 |
| Condensed Statements of Cash Flows | F-28 |
| Notes to Condensed Financial Statements | F-29 |
| Pro Forma Financial Statements (Unaudited) | |
| Pro Forma Combined Balance Sheet as of June 30, 2014 | F-47 |
| Pro Forma Combined Statements of Operations for the six months ended June 30, 2014 | F-48 |
| Pro Forma Combined Statements of Operations for the year ended December 31, 2013 | F-49 |
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board
of Directors and Stockholders
of Enumeral Biomedical Corp.
We have audited the accompanying balance sheets of Enumeral Biomedical Corp. (the “Company”) as of December 31, 2013 and 2012, and the related statements of operations, stockholders’ deficiency, and cash flows for the years then ended. Enumeral Biomedical Corp.’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Enumeral Biomedical Corp. as of December 31, 2013 and 2012, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has deficiencies in working capital and stockholders’ equity and incurred recurring losses from operations and negative cash flows from operations, which raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ FRIEDMAN LLP
New York, NY
July 10, 2014
| F-2 |
ENUMERAL BIOMEDICAL CORPORATION
BALANCE SHEETS
| As of December 31, | ||||||||
| 2013 | 2012 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 263,910 | $ | 1,747,534 | ||||
| Accounts receivable | 125,000 | 53,689 | ||||||
| Prepaid expenses and other current assets | 142,741 | 83,779 | ||||||
| Total current assets | 531,651 | 1,885,002 | ||||||
| Property and equipment, net | 747,888 | 965,565 | ||||||
| Other assets: | ||||||||
| Restricted cash | 32,666 | 32,635 | ||||||
| Other assets | 6,100 | 6,000 | ||||||
| $ | 1,318,305 | $ | 2,889,202 | |||||
| LIABILITIES AND STOCKHOLDERS' DEFICIENCY | ||||||||
| Current liabilities | ||||||||
| Accounts payable ($109,740 and $75,166 due to related parties, respectively) | $ | 342,595 | $ | 358,468 | ||||
| Accrued expenses and other current liabilities ($50,942 and $48,000 due to related parties, respectively) | 206,035 | 137,735 | ||||||
| Deferred rent | 15,101 | 15,101 | ||||||
| Deferred revenue | 25,714 | 73,827 | ||||||
| Current portion of long-term debt | 716,000 | 716,000 | ||||||
| Total current liabilities | 1,305,445 | 1,301,131 | ||||||
| Deferred rent, net of current portion | 37,267 | 48,851 | ||||||
| Long-term debt, net | 339,348 | 1,048,735 | ||||||
| Preferred stock warrant liability | 41,466 | 38,376 | ||||||
| Total liabilities | $ | 1,723,526 | $ | 2,437,093 | ||||
| Commitments and Contingencies | ||||||||
| Series A convertible preferred stock, $0.0001 par value, 2,879,000 shares authorized; 2,766,926 shares issued and outstanding at December 31 2013 and 2012 (liquidation preference of $3,209,634 at December 31, 2013) | 3,046,537 | 3,046,537 | ||||||
| Series A-1 convertible preferred stock, $0.0001 par value, 3,461,538 authorized; 2,047,207 shares issued and outstanding at December 31 2013 and 2012 (liquidation preference of $2,661,369 at December 31, 2013) | 2,608,190 | 2,608,190 | ||||||
| Series A-2 convertible preferred stock, $0.0001 par value, 3,448,275 shares authorized; 1,833,798 shares issued and outstanding at December 31, 2013 (liquidation preference of $2,659,007 at December 31, 2013) | 2,540,975 | - | ||||||
| Total convertible preferred stock | 8,195,702 | 5,654,727 | ||||||
| Stockholders' deficiency | ||||||||
| Common stock, $0.0001 par value; 15,000,000 shares authorized: 4,482,948 and 2,467,460 shares issued and outstanding at December 31, 2013 and 2012, respectively | 448 | 247 | ||||||
| Additional paid-in capital | 909,866 | 443,783 | ||||||
| Accumulated deficiency | (9,511,237 | ) | (5,646,648 | ) | ||||
| Total stockholders’ deficiency | (8,600,923 | ) | (5,202,618 | ) | ||||
| Total liabilities and stockholders’ deficiency | $ | 1,318,305 | $ | 2,889,202 | ||||
The accompanying notes are an integral part of the financial statements
| F-3 |
| ENUMERAL BIOMEDICAL CORP. | |||
| STATEMENTS OF OPERATIONS |
| Year Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| Collaboration and license revenues | $ | 266,039 | $ | 49,281 | ||||
| Grant revenues | 182,997 | 16,836 | ||||||
| Total revenues | 449,036 | 66,117 | ||||||
| Cost of revenue and expenses | ||||||||
| Research and development | 2,369,414 | 1,831,144 | ||||||
| General and administrative | 1,832,088 | 1,377,158 | ||||||
| Total cost of revenue and expenses | 4,201,502 | 3,208,302 | ||||||
| Loss from operations | (3,752,466 | ) | (3,142,185 | ) | ||||
| Other income (expense) | ||||||||
| Interest expense | (109,064 | ) | (106,768 | ) | ||||
| Change in fair market value of derivatives | (3,090 | ) | (4,897 | ) | ||||
| Other income (expense) | 31 | (284 | ) | |||||
| Total other income (expense), net | (112,123 | ) | (111,949 | ) | ||||
| Net loss before income taxes | (3,864,589 | ) | (3,254,134 | ) | ||||
| Provisions for income taxes | - | - | ||||||
| Net loss | $ | (3,864,589 | ) | $ | (3,254,134 | ) | ||
The accompanying notes are an integral part of the financial statements
| F-4 |
STATEMENTS OF STOCKHOLDERS' DEFICIENCY
| Common Stock | ||||||||||||||||||||||||
| Shares | Amount | Additional Paid-In Capital | Accumulated Deficiency | Treasury Stock | Total Stockholders' Deficiency | |||||||||||||||||||
| Balance at January 1, 2012 | 3,705,356 | $ | 370 | $ | 295,084 | $ | (2,392,514 | ) | $ | (135 | ) | $ | (2,097,195 | ) | ||||||||||
| Stock-based compensation expense | - | - | 131,567 | - | - | 131,567 | ||||||||||||||||||
| Retirement of treasury stock | (1,358,465 | ) | (135 | ) | - | - | 135 | - | ||||||||||||||||
| Exercise of common stock options | 101,576 | 10 | 12,006 | - | - | 12,016 | ||||||||||||||||||
| Issuance of common stock - license agreement | 18,993 | 2 | 5,126 | - | - | 5,128 | ||||||||||||||||||
| Net loss | - | - | - | (3,254,134 | ) | (3,254,134 | ) | |||||||||||||||||
| Balance at December 31, 2012 | 2,467,460 | $ | 247 | $ | 443,783 | $ | (5,646,648 | ) | $ | - | $ | (5,202,618 | ) | |||||||||||
| Stock-based compensation expense | - | - | 366,897 | - | - | 366,897 | ||||||||||||||||||
| Issuance of common stock | 234,648 | 23 | 63,331 | - | - | 63,354 | ||||||||||||||||||
| Issuance of restricted stock | 1,648,000 | 165 | - | - | - | 165 | ||||||||||||||||||
| Issuance of common stock - license agreement | 132,840 | 13 | 35,855 | - | - | 35,868 | ||||||||||||||||||
| Net loss | - | - | - | (3,864,589 | ) | - | (3,864,589 | ) | ||||||||||||||||
| Balance at December 31, 2013 | 4,482,948 | $ | 448 | $ | 909,866 | $ | (9,511,237 | ) | $ | - | $ | (8,600,923 | ) | |||||||||||
The accompanying notes are an integral part of the financial statements
| F-5 |
STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| Operating activities | ||||||||
| Net loss | $ | (3,864,589 | ) | $ | (3,254,134 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 280,725 | 187,721 | ||||||
| Stock-based compensation | 367,062 | 131,567 | ||||||
| Change in fair value of derivatives | 3,090 | 4,897 | ||||||
| Accretion of debt discount | 6,613 | 6,677 | ||||||
| Issuance of common stock pursuant to license agreement | 35,868 | 5,128 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (71,311 | ) | (53,689 | ) | ||||
| Prepaid expenses and other assets | (59,061 | ) | (62,873 | ) | ||||
| Accounts payable | (15,872 | ) | 295,630 | |||||
| Accrued expenses and other current liabilities | 68,299 | (53,383 | ) | |||||
| Deferred rent | (11,584 | ) | 63,952 | |||||
| Deferred revenue | (48,113 | ) | 73,827 | |||||
| Net cash used in operating activities | (3,308,873 | ) | (2,654,680 | ) | ||||
| Investing activities | ||||||||
| Purchases of property and equipment | (63,049 | ) | (539,376 | ) | ||||
| Change in restricted cash | (31 | ) | (26,177 | ) | ||||
| Net cash used in investing activities | (63,080 | ) | (565,553 | ) | ||||
| Financing activities | ||||||||
| Proceeds from issuance of Series A-1 convertible preferred stock, net of issuance costs | - | 2,608,190 | ||||||
| Proceeds from issuance of Series A-2 convertible preferred stock, net of issuance costs | 2,540,975 | - | ||||||
| Proceeds from issuance of common stock | 63,354 | - | ||||||
| Proceeds from exercise of common stock options | 12,016 | |||||||
| Payments on ST & LT debt | (716,000 | ) | - | |||||
| Proceeds from long-term debt | - | 640,000 | ||||||
| Net cash provided by financing activities | 1,888,329 | 3,260,206 | ||||||
| Net (decrease) increase in cash and cash equivalents | (1,483,624 | ) | 39,973 | |||||
| Cash and cash equivalents, beginning of year | 1,747,534 | 1,707,561 | ||||||
| Cash and cash equivalents, end of year | $ | 263,910 | $ | 1,747,534 | ||||
| Cash paid for interest | $ | 107,265 | $ | 94,840 | ||||
The accompanying notes are an integral part of the financial statements
| F-6 |
NOTES TO FINANCIAL STATEMENTS
1 - NATURE OF BUSINESS
Enumeral Biomedical Corporation (“Enumeral” or “the Company”) was founded in 2009 in the state of Delaware as Enumeral Technologies, Inc. The name was later changed to Enumeral Biomedical Corporation. Enumeral is discovering and developing novel antibody therapeutics that help the immune system attack diseased cells (“immunomodulators”). The Company believes it has a unique ability to extensively interrogate cells of the human immune system for drug candidate validation, and that this ability could give the Company a distinct advantage in selecting potential best-in-class candidates. The Company is building a pipeline of drug candidates for the treatment of cancer, infectious, and inflammatory diseases and leveraging the breadth of the Company’s technology in order to drive near-term cash flows from out-licensing of the Company’s drug candidates and through strategic collaboration partnerships. The Company’s near-term goals include performance of preclinical testing on drug candidates resulting from internal programs to generate data to support the Company’s ability to obtain revenue from co-development partners. The Company has focused its research efforts on using its proprietary drug discovery platform utilizing, in part, technology licensed by Enumeral from M.I.T., Harvard University and other institutions to identify and elucidate antibodies and antigens that are responsible for diseases that affect millions of individuals and are underserved by current therapeutic alternatives. These diseases have included cancer, infectious, and inflammatory diseases. The Company believes that the breadth of the drug discovery platform offers the potential to grow through revenue-generating collaborative research and development partnerships.
2 - RISK AND UNCERTAINTIES
The Company is subject to a number of risks common to emerging companies in the life sciences industry. Principal among these risks are the uncertainties of the drug development process, technological innovations, dependence on key individuals, development of the same or similar technological innovations by the Company’s competitors, protection of proprietary technology, compliance with government regulations and approval requirements, uncertainty of market acceptance of products, product liability, and the need to obtain financing necessary to fund future operations.
Enumeral has funded operations since inception through private placement of equity securities, venture debt, and revenues from corporate collaborations and a contract with the National Cancer Institute (NCI). The Company received gross proceeds through completing a $ 3.1 million Series A convertible preferred stock financing during 2011, a $ 2.7 million Series A-1 convertible preferred stock financing during 2012 and a $2.7 million Series A-2 convertible preferred stock financing during 2013.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company has a working capital deficiency of $773,794 and an accumulated deficiency of $9,511,237 as of December 31, 2013. Additionally, the Company has incurred negative cash flows from operations and net losses to date. The Company is in the process of completing corporate collaborations to gain market acceptance of its technology and increase revenues. These factors raise a substantial doubt about the Company's ability to continue as a going concern. Without realization of additional capital, it would be unlikely for the Company to continue as a going concern. The financial statements do not include any adjustments that might result from this uncertainty
In view of these matters, continuation as a going concern is dependent upon the continued operations of the Company, which in turn is dependent upon the Company's ability to meet its financial requirements, raise additional capital, and the success of its future operations.
| F-7 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
2 - RISK AND UNCERTAINTIES (Continued)
In February 2014, the Company raised $750,000 by issuing convertible promissory notes with a maturity date of July, 2015. In connection with the issuance of the convertible promissory notes, the Company issued warrants with respect to 694,444 underlying shares exercisable into common stock at $0.27 per share with a term of 10 years. In April, 2014 the Company issued 948,823 shares of Series B Convertible Preferred Stock at an issue price of $2.125 per share for proceeds of $1,742,220, net of issuance costs of $274,027. The Company is planning to complete a reverse merger with a public shell company and a private equity offering prior to September 15, 2014 whereby the Company will raise a minimum of $10 million and a maximum of $25 million, including a $10 million over-allotment, if available, all prior to the deduction of expenses related to the transaction. The new capital will be used to fund ongoing operations of the successor company.
Management believes that it will be successful in obtaining sufficient financing to execute its operating plan. However, no assurances can be provided that the Company will secure additional financing or achieve and sustain a profitable level of operations. To the extent that the Company is unsuccessful in its plans, the Company may find it necessary to contemplate the sale of its assets and curtail operations.
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Recently Adopted Accounting Standards
On June 10, 2014, the FASB issued Accounting Standards Update (ASU) No. 2014-10, Development Stage Entities (Topic 915) – Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation, which eliminates the concept of a development stage entity (DSE) in its entirety from current accounting guidance. Amendments to the consolidation guidance may result in more DSEs being considered variable interest entities (VIEs). The new guidance applies to all entities that previously met the definition of a DSE. This ASU is effective for public business entities for annual reporting periods beginning after December 15, 2015, and interim periods therein. Early adoption of the new standard is permitted. Management has elected to early adopt this ASU, as permitted and, accordingly, has not included the inception-to-date disclosures and other previously required disclosures for development stage entities.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (U.S. GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reported period. Significant estimates used in preparing the Company’s financial statements include the estimation of stock based compensation, stock warrants liability, accrued expenses, revenue recognition and income taxes. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash equivalents represent high liquid investments with remaining maturities of ninety days or less at the date of purchase. Cash equivalents are stated at cost, which approximates fair value.
Concentration of Credit Risk
The Company has no significant off-balance sheet concentrations of credit risk as foreign currency exchange contracts, option contracts or other hedging arrangements. Financial instruments that subject the Company to credit risk consists primarily of cash and cash equivalents. The Company generally invests its cash in money market funds, U.S. Treasury securities and U.S. Agency securities that are subject to minimal credit and market risk. Management has established guidelines relative to credit ratings and maturities intended to safeguard principal balances and maintain liquidity. At times, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation.
| F-8 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Fair Value of Financial Instruments
Fair values of financial instruments included in current assets and current liabilities are estimated to approximate their book values, due to the short maturity of such instruments. All debt is based on current rates at which the Company could borrow funds with similar remaining maturities and approximates fair value. The Company’s assets and liabilities that are measured at fair value on a recurring basis are measured in accordance with ASC 820 under the Financial Accounting Standards Board (“FASB”), which establishes a three-level valuation hierarchy for measuring fair value and expands financial statement disclosures about fair value measurements.
The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability as of the measurement date. The three levels are defined as follows:
Level 1 Inputs to the valuation methodology are quoted prices (unadjusted) for identical assets in active markets.
Level 2 Inputs to the valuation methodology included quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument.
Level 3 Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The Company’s cash equivalents carried at fair value are primarily comprised of investments in a U.S. Treasury and federal agency backed money market fund. The valuation of the Company’s warrants liability is discussed below and in Note 5. The following table presents information about the Company’s financial assets and liabilities measured at a fair value on a recurring basis as of December 31, 2013 and 2012:
| December 31, 2013 | Quoted Prices in Active Markets (Level 1) | Observable Inputs (Level 2) | Unobservable Inputs (Level 3) | |||||||||||||
| Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 263,910 | $ | 263,910 | $ | - | $ | - | ||||||||
| Liabilities | ||||||||||||||||
| Preferred stock warrants | $ | 41,466 | $ | - | $ | - | $ | 41,466 | ||||||||
| December 31, 2012 | Quoted Prices in Active Markets (Level 1) | Observable Inputs (Level 2) | Unobservable Inputs (Level 3) | |||||||||||||
| Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 1,747,534 | $ | 1,747,534 | $ | - | $ | - | ||||||||
| Liabilities | ||||||||||||||||
| Preferred stock warrants | $ | 38,376 | $ | - | $ | - | $ | 38,376 | ||||||||
| F-9 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Fair Value Measurements (continued)
The following table provides a roll forward of the fair value of the Company’s preferred stock warrant liabilities, using Level 3 inputs:
| Balance at January 1, 2012 | $ | 27,348 | ||
| Issuance of preferred stock warrant | 6,131 | |||
| Change in fair value | 4,897 | |||
| Balance at December 31, 2012 | $ | 38,376 | ||
| Change in fair value | 3,090 | |||
| Balance at December 31, 2013 | $ | 41,466 |
Accounts Receivable and Allowance for Doubtful Accounts
Trade receivables are recorded at the invoiced amount. The Company maintains allowances for doubtful accounts, if needed, for estimated losses resulting from the inability of customers to make required payments. This allowance is based on specific customer account reviews and historical collections experience. There was no allowance for doubtful accounts as of December 31, 2013 or 2012.
Property and Equipment
Property and equipment are recorded at cost. Expenditures for maintenance and repairs are charged to expense as incurred, whereas major betterments are capitalized as additions to property and equipment. Depreciation is provided using the straight-line method over the estimated useful lives of the assets as follows:
| Lab equipment | 5 years |
| Computer equipment and software | 3 years |
| Furniture | 3 years |
| Leasehold improvements | Shorter of useful life or life of the lease |
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment annually or whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. When such events occur, the Company compares the carrying amounts of the assets to their undiscounted expected future cash flows. If this comparison indicated that there is impairment, the amount of the impairment is calculated as the difference between the carrying value and fair value. There have been no impairments recognized during the years ended December 31, 2013 or 2012.
Revenue Recognition
Collaboration and license revenue
Non-refundable license fees are recognized as revenue when the Company has a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and the Company has no further performance obligations under the license agreement. Multiple element arrangements, such as license and development arrangements are analyzed to determine whether the deliverables, which often include license and performance obligations such as research and steering committee services, can be separated or whether they must be accounted for as a single unit of accounting in accordance with GAAP. The Company recognizes up-front license payments as revenue upon delivery of the license only if the license has stand-alone value and the fair value of the undelivered performance obligations, typically including research and/or steering committee services, can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations would then be accounted for separately as performed. If the license is considered to either (i) not have stand-alone or (ii) have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement would then be accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
| F-10 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Revenue Recognition (continued)
Whenever the Company determines that an arrangement should be accounted for as a single unit of accounting, it must determine the period over which the performance obligations will be performed and revenue will be recognized. Revenue will be recognized using either a relative performance or straight-line method. The Company recognizes revenue using the relative performance method provided that the Company can reasonably estimate the level of effort required to complete its performance obligations under an arrangement and such performance obligations are provided on a best-efforts basis. Direct labor hours or full-time equivalents are typically used as a measure of performance. Revenue recognized under the relative performance method would be determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of substantive milestones, by the ratio of level of effort incurred to date to estimated total level of effort required to complete the Company’s performance obligations under the arrangement. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the relative performance method, as of each reporting period.
Collaboration and license revenue (continued)
If the Company cannot reasonably estimate the level of effort required to complete its performance obligations under an arrangement, the performance obligations are provided on best-efforts basis and the Company can reasonably estimate when the performance obligation ceases or the remaining obligations become inconsequential and perfunctory. At that time, the total payments under the arrangement, excluding royalties and payments contingent upon achievement of substantive milestones, would be recognized as revenue on a straight-line basis over a period the Company expects to complete its performance obligations.
Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date.
In March 2012, the Company and Celgene Corporation (“Celgene”) entered into an agreement whereby the Company would perform services using their technology to perform research related to an inflammatory disease. Under the terms of the agreement, Celgene paid an upfront research support payment of $40,000. The Company completed two of the remaining deliverables and received additional payments in 2013. The final deliverable, submission of a final report, is anticipated to occur in the second half of 2014.
In June 2012, the Company and Sanofi-Aventis (“Sanofi”) entered into a research agreement, where Sanofi funded a research project to be performed by the Company for the purpose of evaluating the Company’s technology and capabilities, to obtain useful antibodies to a biologically active peptide, and to determine if the respective parties wish to consider further business arrangements and transactions with the other party. Under the Agreement, the Company is responsible for screening monoclonal antibodies that meet specific and challenging antigen binding characteristic requirements using its single cell microengraving technology (“technology”) and to compare such antibodies to monoclonal antibodies obtained by Sanofi in parallel using a modified traditional ELISA screening approach. The Company is responsible for obtaining antibody sequences and providing Sanofi a final report of the research performed. Under the terms of the agreement, Sanofi paid an upfront research support payment of $50,000. The Company received $50,000 in March 2014 after completion of a second deliverable, and jointly owns all of the inventions disclosed from the collaboration.
| F-11 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Revenue Recognition (continued)
Collaboration and license revenue (continued)
In February 2012, the Company and Crucell Holland (“Crucell”) entered into a research agreement, where Crucell funded a research project to be performed by the Company for the purpose of evaluating the Company technology and capabilities and to identify truncated hemagglutinin clones (mini-HA) that react with monoclonal anti-HA antibodies and display favorable aqueous solubility along with B-cell repertoire analysis of patient samples for native broadly HA-specific antibodies. Under the terms of the agreement, Crucell paid $50,000 after the delivery of the first interim library screening report. Terms of the agreement were amended in August 2013, aftewhich the Company received an additional $12,500. Upon Crucell communicating to the Company that it had established “proof of concept”, Crucell canceled the agreement in December 2013 and requested the Company engage in discussions of further collaboration. These discussions began in January 2014 and are ongoing.
In October 2013, the Company and Novartis Pharma (“Novartis”) entered into a research agreement, where Novartis engaged the Company to evaluate the applicability of the technology for the ex-vivo assessment of T-cell profiles for human samples after exposure to therapeutic proteins for immunogenicity screening. The Company received an upfront research support payment of $50,000 upon signing of the agreement. The final deliverable, submission of a final report, is anticipated to occur in the second half of 2014.
The Company recognized $266,039 and $49,281 of collaboration and license revenue during the years ended December 31, 2013 and 2012, respectively. The difference between the total consideration received to date and the revenue recognized is recorded as deferred collaboration revenue and totals $25,714 and $40,705 at December 31, 2013 and 2012, respectively.
Grant Revenue
The Company recognizes nonrefundable grant revenue that is earned in connection with its Research Agreement with the National Cancer Institute (the “NIH”), which was executed in September 2012. Grant revenue consists of a portion of the funds received to date by the NIH, which allow the Company to conduct research on colon cancer tissues. Revenue is recognized as the related research services are performed in accordance with the terms of the agreement. An upfront payment of $49,958 is recognized on a straight line basis over the period the Company was expected to complete its performance obligations. The Company recognized $182,997 and $16,836 of grant revenue during the years ended December 31, 2013 and 2012. The difference between the total consideration received to date and the revenue recognized is recorded as deferred grant revenue and totals $0 and $33,122 at December 31, 2013 and 2012, respectively.
Research and Development Expenses
Research and development expenditures are charged to the statement of operations as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, clinical supply costs, contract services, depreciation and amortization expense and other related costs. Costs associated with acquired technology, in the form of upfront fees or milestone payments, are charged to research and development expense as incurred. Legal fees incurred in connection with patent applications along with fees associated with the license to the Company’s core technology are expensed as research and development expense.
| F-12 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Accounting for Preferred Stock Warrants
Warrants for the purchase of redeemable convertible preferred stock are carried at fair value and reported as a derivative liability on the accompanying balance sheets.
Changes in the fair value of warrants for the purchase of convertible preferred stock are included in other income in the statements of operations. For the years ended December 31, 2013 and 2012, the Company recorded expenses of $3,090 and $4,897, respectively as a result of the change in the fair value of the warrants. The Company used the Black-Scholes option-pricing model to estimate the fair values of the issued and outstanding warrants as of December 31, 2013 with the following weighted average assumptions: risk-free interest rate of 1.75%, remaining term of 5.02 years, volatility rate of 48% and no expected dividends. As of December 31, 2012, the following weighted average assumptions were used: risk-free interest rate of 1.18%, remaining term of 6.02 years, a volatility rate of 48% and no expected dividends.
Stock-Based Compensation
The Company has elected to use the Black-Scholes option pricing model to determine the grant date fair value of share-based awards. The Company recognizes the compensation cost of employee share-based awards on a straight-line basis over the employee’s requisite service period of each award, which is generally the vesting period.
The fair value of the restricted stock awards granted to employees is based upon the fair value of the common stock on the date of grant. Expense is recognized over the vesting period.
The Company has recorded stock-based compensation expense of $367,062 and $131,567 for the years ended December 31, 2013 and 2012, respectively. The Company has an aggregate of $272,403 of unrecognized stock-based compensation cost as of December 31, 2013 to be amortized over a weighted average period of 1.4 years.
The fair value of each option award is estimated on the date of grant using the Black-Scholes option pricing model that uses the weighted average assumptions noted in the following table:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Stock Price | $ | 0.27 | $ | 0.27 | ||||
| Risk-free interest rate | 1.74 | % | 0.70 | % | ||||
| Expected dividend yield | - | - | ||||||
| Expected term – employees | 6 years | 6 years | ||||||
| Expected term – nonemployees | 10 years | 10 years | ||||||
| Volatility | 53 | % | 50 | % | ||||
| Determined grant date fair value per option | $ | 0.19 | $ | 0.19 | ||||
Expected volatility for the Company’s common stock was determined based on the historical volatility of comparable publically traded companies in a similar industry. The risk-free interest rate is based on the yield of U.S. Treasury securities consistent with the expected term of the option. No dividend yield was assumed as the Company does not pay dividends on its common stock. The expected term of the options granted represents the period of time that options granted are expected to be outstanding and is calculated using the simplified method.
The fair value of the common stock has typically been determined by the Board of Directors (the “Board”) at each date of grant based upon a variety of factors, including the Company’s financial position and historical financial performance, the status of developments within the Company’s research and development activities, the composition and ability of the current climate in the market place, the illiquid nature of the common stock, the effect of the rights and preferences of the preferred shareholders, and the prospects of a liquidity event, among others.
| F-13 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Stock-Based Compensation (continued)
The Company engaged a third party to develop an estimate of the fair value of a share of the Company’s common stock on a fully-diluted, minority, non-marketable basis as of December 31, 2013 and 2012.
Based upon unaudited historical, pro-forma and/or forecast financial and operational information, which Enumeral management represented as accurately reflecting the company’s operating results and financial position, the third party utilized both a Market Approach (using various financial statement metrics of similar enterprises’ equity securities to estimate the fair value of the Company’s equity securities) and an Income Approach (which bases value on expectations of future income and cash flows) in their analyses.
The fair value of a single share of common stock was determined using the Option Pricing Method, which treats Common and Preferred Stock as call options on the aggregate enterprise value and using traditional models, including Black-Scholes or binomial models, to calculate share values.
Income Taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, and operating loss and tax credit carryforwards. The Company’s financial statements contain certain deferred tax assets, which have arisen primarily as a result of operating losses, as well as other temporary differences between financial and tax accounting. The Company would establish a valuation allowance if the likelihood of realization of the deferred tax assets is reduced based on an evaluation of objective verifiable evidence. Significant management judgment is required in determining the Company’s provision for income taxes, the Company’s deferred tax assets and liabilities and any valuation allowance recorded against those net deferred assets. The Company evaluates the weight of all available evidence to determine whether it is more likely than not that some portion or all of the net deferred income tax assets will not be realized.
The Company accounts for uncertain tax positions in accordance with GAAP and has no uncertain tax liabilities at December 31, 2013 or 2012. The guidance requires the Company to determine whether it is more likely than not that a tax position will be sustained upon examination by the appropriate taxing authority. If a tax position meets the more likely than not recognition criteria, the guidance requires the tax position be measured at the largest amount of benefit greater than 50% likely of being realized upon ultimate settlement.
New Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers: Topic 606. This Update affects any entity that either enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets, unless those contracts are within the scope of other standards. The guidance in this Update supersedes the revenue recognition requirements in Topic 605, Revenue Recognition and most industry-specific guidance. The core principle of the guidance is that an entity should recognize revenue to illustrate the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The new guidance also includes a cohesive set of disclosure requirements that will provide users of financial statements with comprehensive information about the nature, amount, timing, and uncertainty of revenue and cash flows arising from a reporting organization’s contracts with customers. This ASU is effective retrospectively for fiscal years, and interim periods within those years beginning after December 15, 2016 for public companies and 2017 for non-public entities. Management is evaluating the effect, if any, on the Company’s financial position and results of operations.
| F-14 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
New Accounting Pronouncements (Continued)
In June 2014, the FASB issued Accounting Standards Update (ASU) No. 2014-12, Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. The amendments in the ASU require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in Topic 718, Compensation – Stock Compensation, as it relates to awards with performance conditions that affect vesting to account for such awards. The performance target should not be reflected in estimating the grant-date fair value of the award. Compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation cost should be recognized prospectively over the remaining requisite service period. The total amount of compensation cost recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved.
4 - PROPERTY AND EQUIPMENT
Property and equipment, net consist of the following:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Laboratory equipment | $ | 1,082,438 | $ | 1,035,280 | ||||
| Computer/office equipment and software | 87,563 | 71,672 | ||||||
| Furniture, fixtures and office equipment | 18,772 | 18,772 | ||||||
| Leasehold improvements | 112,507 | 112,507 | ||||||
| 1,301,280 | 1,238,231 | |||||||
| Less - Accumulated depreciation and amortization | (553,392 | ) | (272,666 | ) | ||||
| $ | 747,888 | $ | 965,565 | |||||
Depreciation and amortization expense for the years December 31, 2013 and 2012 were $280,725, and $187,721 respectively.
5 - DEBT AND WARRANTS
In December 2011, the Company entered into a $1.79 million venture debt financing with Square 1 Bank. The Term Loans require the Company to comply with certain covenants on an annual basis. The Company is required to meet a maximum cash burn or liquidity ratio as indicated in the term loan agreement. The Company was in compliance with its covenants as of December 31, 2013 and 2012.
The financing and security agreement is comprised of three separate loans:
“Term Loan A” is a $500,000 loan initiated at the signing of the loan agreement. “Term Loan B” is a second $500,000 loan, which was extended to the Company upon raising cash proceeds of at least $2,000,000. The Company achieved this milestone with the completion of its Series A-1 convertible preferred stock issuance in 2012. The Term Loans bear interest at a variable annual rate equal to the greater of: (A) 3.75% above the Prime Rate then in effect; or (B) 7.00%. The annual rate was capped not to exceed 8.5% during the first 18 months and will not exceed 9.50% thereafter. The Term A and B loans are collateralized by substantially all of the assets of the Company.
| F-15 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
5 – DEBT AND WARRANTS (Continued)
“Equipment Loan” consisted of a credit extension of up to $790,000. The Equipment Loan bears interest under the same as Term Loan A and B and is collateralized by the equipment purchased.
The initial drawdown occurred in December 2011, consisting of the entirety of Term Loan A of $500,000 and $650,000 of the Equipment loan for a total of $1,150,000. The Company exercised an additional $106,875 of its Equipment Loan in April 2012. In October 2012, the Company drew down the remaining $33,125 from its Equipment Loan, along with the entirety of Loan B of $500,000.
Monthly principal payments on Term Loan A and B and the Equipment Loan of $59,667 commenced on January 5, 2013 and continue until maturity on June 5, 2015.
Future principal payments under debt obligations at December 31, 2013 are as follows:
| Year Ending December 31, | ||||
| 2014 | $ | 716,000 | ||
| 2015 | 358,000 | |||
| $ | 1,074,000 | |||
In connection with the December 5, 2011 financing and security agreement, the Company issued to the lender warrants to purchase an aggregate of 33,944 shares of Series A convertible preferred stock at an exercise price of $1.16 per share, exercisable for seven years. The estimated fair value of the warrants at the time of issuance was determined to be $26,348 using the Black-Sholes pricing model and the following assumptions: expected term of seven years, 47% volatility, and a risk-free rate of 1.49%. As part of a June 12, 2012 amendment to the loan and security agreement, the Company issued warrants to Square 1 Bank to purchase an aggregate of 7,715 shares of Series A convertible preferred stock at an exercise price of $1.16 per share, exercisable for seven years. The estimated fair value of these warrants at the time of issuance was determined to be $6,131 using the Black-Sholes pricing model and the following assumptions: expected term of seven years, 48% volatility, and a risk-free rate of 1.12%. The warrants are classified as a derivative liability in the accompanying balance sheets and measured at fair value on a recurring basis.
As of December 31, 2013, warrants to purchase 41,659 shares of Series A convertible preferred stock at a price of $1.16 per share are outstanding and expire on December 5, 2018 and June 12, 2019.
6 - COMMITMENTS
Operating Leases
In May, 2011, the Company entered into a 24 month operating lease for office and research space consisting of 1,746 square feet in Cambridge, Massachusetts. The lease was terminated in December 2012.
In October 2012, the company entered into a 37 month operating lease for office and research space consisting of 4,782 square feet in Cambridge, Massachusetts. The term of the lease expires on November 30, 2015. The Company maintains a security deposit relating to the facility, recorded as restricted cash on the accompanying balance sheet.
Total rent expense was $292,390 and $218,605, for the years ended December 31, 2013 and 2012 respectively.
| F-16 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
6 – COMMITMENTS (Continued)
Future operating lease commitments are as follows:
| Year Ending December 31, | ||||
| 2014 | $ | 234,318 | ||
| 2015 | 228,919 | |||
| $ | 463,237 | |||
Employment Agreements
The Company has employment agreements with members of management which contain minimum annual salaries and severance benefits if terminated prior to the term of the agreements.
7 - LICENSE AGREEMENT AND RELATED-PARTY TRANSACTIONS
License Agreement
In 2011, the Company licensed certain intellectual property from the Massachusetts Institute of Technology (“MIT”), a related party, in exchange for the payment of upfront license fees and a commitment to pay annual license fees, patent costs, milestone payments, and, upon product commercialization, royalties sales of products covered by the licenses or income from corporate partners.
All amounts paid related to the license fees have been expensed as research and development by the Company as incurred. The Company incurred $20,000 and $15,000 in the years ended December 31, 2013 and 2012, respectively. In addition to potential future royalty and milestone payments that the Company may have to pay MIT per the terms of their license agreement, the Company is obligated to pay an annual fee of $25,000 in 2014, $30,000 in 2015, $40,000 in 2016, and $50,000 every year thereafter unless the license agreement is terminated. To date, no milestone payments have been made. No royalty payments have been payable as the Company has not commercialized any products as set forth in the agreement. The Company reimburses the costs to MIT and Harvard University for the continued prosecution of the licensed patent estate. For the years ended December 31, 2013 and 2013, the Company paid $207,220 and $168,849 for MIT and $27,812 and $18,786 for Harvard, respectively.
Upon execution of this agreement, the Company issued MIT and affiliates 66,303 shares of common stock, and recognized $15,250 as research and development expense. As of the effective date of the agreement through the date upon which $7,500,000 of funding for the Company’s capital stock has been achieved, the Company is required to issue MIT and affiliates an aggregate amount of shares equaling 3% of the Company’s issued and outstanding common stock on a fully diluted basis.
During the year ended December 31, 2011, the Company issued an additional 94,307 shares of common stock under the license agreement and recognized $21,691 as research and development expense. During the years ended December 31, 2013 and 2012, the Company issued to MIT and affiliates 132,840 and 18,993 shares of common stock, respectively under the license agreement and recognized $35,867 and $5,128 as research and development expense, respectively. In April of 2013, the Company satisfied the $7,500,000 funding requirement through the Series A-2 financing which fulfilled the provision in the license agreement that required the Company to issue shares to MIT and affiliates.
| F-17 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
7- LICENSE AGREEMENT AND RELATED-PARTY TRANSACTIONS (Continued)
License Agreement (continued)
The license agreement also contains a provision whereby after the $7,500,000 funding requirement is met, the Company may have to issue additional shares to MIT and affiliates if the Company issues common stock at a price per share that is less than the fair market value per share of the common stock issued to MIT and affiliates. The Company could be required to issue additional common shares to MIT and affiliates based upon a weighted average formula set forth in the License Agreement. The Company has not issued any additional common shares under this provision of the License agreement to date.
Consulting Agreements
A Company co-founder/stockholder is employed by MIT. On April 19, 2011, the Company entered into a consulting agreement with this Company co-founder/stockholder. The agreement has a three year term. During the years ended December 31, 2013 and 2012, the Company paid for consulting services $25,000 and $18,750, respectively.
On April 19, 2011, the Company entered into a consulting agreement with a director of the Company. Pursuant to the original agreement, the consultant will be compensated through the issuance of 159,045 restricted common shares that vest one quarter on the execution of the contract, one quarter on April 19, 2012 and the remainder 2.08% vesting monthly through April 1, 2014. The term of the consulting agreement is three years. On February 11, 2013, the consulting agreement was amended so that the consultant would receive $75,000 per year for a period of one year. On August 14, 2013, the consulting agreement was amended so that the consultant would receive $37,500 per year for a period of one year. During the years ended December 31, 2013 and 2012, the Company recognized $18,750 and $53,125 of expense related to the consulting agreement, respectively.
On September 20, 2013, the Company entered into a consulting agreement with Allan Rothstein, and Norman Rothstein (collectively the “consultants”). Pursuant to the consulting agreement, Allan Rothstein was elected as a member of the Board of Directors of the Company. The consultants were compensated through the issuance of 1,000,000 shares of restricted common stock of the Company with one third vesting upon the execution of the consulting agreement, one third vesting on December 10, 2013 and one third vesting on March 10, 2014. The consulting agreement has a term of two years. During the year ended December 31, 2013, the Company recognized $201,236 of expense related to the consulting agreement.
8 - COMMON AND PREFERRED STOCK
Common Stock
During year ended December 31, 2012, the Company retired 1,358,465 shares of common stock held in treasury.
On October 16, 2013, the Company issued 234,648 shares of common stock to members of management in exchange for consideration of $63,354.
Convertible Preferred Stock
In June and September 2012, the Company issued 2,047,207 shares of Series A-1 Convertible Preferred Stock (“Series A-1”) at an issuance price of $1.30 per share for proceeds of $2,608,190, net of issuance costs of $53,179.
In May and September 2013, the Company issued 1,833,798 shares of Series A-2 Convertible Preferred Stock (“Series A-2”) at an issuance price of $1.45 per share for proceeds of $2,540,975, net of issuance costs of $118,032.
Rights, Preferences, and Privileges
The rights, preferences, and privileges of Series A, Series A-1 and Series A-2 (collectively, “Preferred Stock”) are as follows:
| F-18 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
8 - COMMON AND PREFERRED STOCK (Continued)
Conversion
Each share of Preferred Stock is convertible, at the option of the holder, at any time after issuance into such number of fully paid and non-assessable shares of common stock as determined by dividing the Original Issue Price by the Preferred Stock conversion price in effect at the time of conversion. The Preferred Stock conversion price is subject to adjustment in the event of certain dilutive events.
Voting Rights
The holder if each share of Preferred Stock shall have the right to one vote for each share of common stock into which the Preferred Stock can then be converted, and such holder will have full voting rights and powers equal to those of the holders of common stock and be entitled to vote on all matters. The holders of record of the shares of Series A have the right to elect three members of the Board of Directors.
Liquidation
In the event of any liquidation, dissolution, or winding up of the Company, either voluntary or involuntary, as defined, the holders of the Series A, Series A-1 and Series A-2 shall be entitled to receive cash in the amount of $1.16, $1.30 and $1.45 per share, respectively, subject to appropriate adjustment for any stock splits, stock dividends, combination, or any other similar recapitalization affecting such shares, plus any dividends declared but unpaid. The Series A-2 holders have preference over the holders of Series A, Series A-1 and common stock. Insufficient assets to pay any class of holders in order of preference and in the full amount to which they are entitled are distributed on a pro rata basis. After the payment of all preferential amounts, any remaining assets available for distribution will be distributed ratably among the holders of common stock and holders of Series A, Series A-1 and Series A-2 on an as-if converted to common stock basis.
9 - STOCK OPTIONS AND RESTRICTED STOCK
Stock Options
In December 2009, the Company adopted the 2009 Stock Incentive Plan. The 2009 Stock Incentive Plan provides for the granting of incentive and non-statutory stock options, restricted stock, restricted stock units and other equity awards to employees, officers, directors, consultants and advisors of the Company. Provisions such as vesting, repurchase, and exercise conditions and limitations are determined by the Board of Directors on the grant date. Under the terms of the Company’s 2009 Stock Incentive Plan, options expire 10 years after the date of grant. The Company initially reserved 2,500,000 shares of common stock under the 2009 Stock Incentive Plan for issuance to eligible employees, nonemployees and consultants. In April 2011, the Company decreased number of shares in its 2009 Stock Incentive Plan to 971,204, in June 2012 increased shares 1,407,427 and in September 2012 increased shares to 1,623,556. This number is subject to adjustment in the event of a stock split, reverse stock split, stock dividend, or other change in the Company’s capitalization.
Generally, shares that are expired, terminated, surrendered or cancelled without having been fully exercised will be available for future awards. In addition, shares of common stock that are tendered to the Company by a participant to exercise an award are added to the number of shares of common stock available for the grant of awards.
During the years ended December 31, 2013 and 2012 the Company granted 130,000 and 419,900 stock options, respectively, to certain employees and directors. The vesting of these awards is time-based and the restrictions typically lapse 25% after 12 months and monthly thereafter for the next 36 months for employees and annually over three years for directors.
During the years ended December 31, 2013 and 2012 the Company granted 0 and 50,000 stock options, respectively, to certain nonemployees in exchange for certain consulting services.
| F-19 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
9 - STOCK OPTIONS AND RESTRICTED STOCK (Continued)
Stock Options (continued)
Vesting on these awards is time-based and the vesting period is generally over three years. Stock option expense for employees was $19,472 and $11,661 for the years ended December 31, 2013 and 2012, respectively, while non-employee stock option expense was $6,542 and $6,775, respectively, for the years ended December 31, 2013 and December 31, 2012.
A summary stock option activity for the years ended December 31, 2013 and 2012 are as follows:
| Number of Options | Weighted Average Exercise Price Per Share | |||||||
| January 1, 2012, Outstanding | 359,743 | $ | 0.15 | |||||
| Granted | 469,900 | 0.26 | ||||||
| Exercised | (101,576 | ) | 0.12 | |||||
| Forfeited | (15,000 | ) | 0.27 | |||||
| December 31, 2012, Outstanding | 713,067 | $ | 0.23 | |||||
| Granted | 130,000 | 0.27 | ||||||
| Exercised | — | - | ||||||
| Forfeited | (50,000 | ) | 0.27 | |||||
| December 31, 2013, Outstanding | 793,067 | $ | 0.24 | |||||
| Exercisable | 395,056 | $ | 0.20 | |||||
| F-20 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
9 - STOCK OPTIONS AND RESTRICTED STOCK (Continued)
Stock Options (continued)
Summary information regarding the options outstanding and exercisable at December 31, 2013 is as follows:
| Outstanding | Exercisable | |||||||||||||||||||||
| Exercise Prices | Number Outstanding (in shares) | Weighted Average Remaining Contractual Life (in years) | Weighted Average Exercise Price | Number Exercisable (in shares) | Weighted Average Exercise Price | |||||||||||||||||
| $ | 0.01 | 72,500 | 6.76 | $ | 0.01 | 72,500 | $ | 0.01 | ||||||||||||||
| $ | 0.23 | 205,667 | 7.10 | $ | 0.23 | 148,552 | $ | 0.23 | ||||||||||||||
| $ | 0.27 | 514,900 | 8.95 | $ | 0.27 | 174,004 | $ | 0.27 | ||||||||||||||
| 793,067 | 395,056 | |||||||||||||||||||||
The intrinsic value of options exercisable at December 31, 2013 was $24,792. The aggregate intrinsic value was calculated as the difference between the exercise price of the stock options and the fair value of the underlying common stock as of the balance sheet date.
Restricted Stock
In February 2011, the Company issued a total of 393,750 shares of the Company’s common stock to an executive of the Company as consideration for services. The fair value of these shares was $3,938 at the issuance date. The shares are subject to repurchase restrictions that lapse over a 41-month period, with 160,787 shares vesting in 2011 and the remaining shares vesting in equal monthly installments thereafter over the vesting period.
In connection with the issuance of Series A Preferred Stock for consideration during April of 2011, the Company placed vesting restrictions on 1,565,295 common shares held by three founders and a director of the Company. The total fair value of the shares at the date of issuance was $360,018. The shares vested 25% upon issuance, with 25% vesting on the first year anniversary date and the balance vesting over evenly over 24 months.
In 2011, the Company issued a total of 100,000 shares of the Company’s common stock to an executive of the Company as consideration for services. The fair value of these shares was $23,000. The shares are subject to repurchase restrictions that lapse over a 4-year period, with one-third of the shares vesting in four equal quarterly installments over the first year, and two-thirds of the shares vesting in equal monthly installments thereafter over the vesting period.
In 2013, the Company issued a total of 1,648,000 shares of the Company’s common stock to two executives of the Company and consultants (see note 7) as consideration for services. The fair value of these shares was $444,960. The restricted stock awards issued to executives are subject to repurchase restrictions that lapse over a 4-year period, with the shares vesting over 48 months.
Restricted stock expense was $341,048 and $113,131 for the year ended December 31, 2013 and December 31, 2012, respectively.
| F-21 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
9 - STOCK OPTIONS AND RESTRICTED STOCK (Continued)
Restricted stock (Continued)
A summary of restricted stock activity for the years ended December 31, 2013 and 2012 is as follows:
| Number of shares | Weighted Average Grant Date Fair Value | |||||||
| Balance of unvested restricted stock at January 1, 2012 | 1,498,601 | $ | 0.20 | |||||
| Vested | (775,118 | ) | (0.20 | ) | ||||
| Balance of unvested restricted stock at December 31, 2012 | 723,483 | 0.19 | ||||||
| Issuance of restricted stock | 1,648,000 | 0.27 | ||||||
| Vested | (1,361,569 | ) | (0.24 | ) | ||||
| Balance of unvested restricted stock at December 31, 2013 | 1,009,914 | $ | 0.25 | |||||
10 - INCOME TAX
There is no provision for income taxes because the Company has historically incurred operating losses and maintains a full valuation allowance against its gross deferred tax assets. The reported amount of income tax expense differs from the amount that would result from applying domestic federal statutory tax rates to pretax losses primarily because of changes in the valuation allowance.
Significant components of the Company’s net deferred tax asset at December 31, 2013 and 2012 are as follows:
| December 31, | ||||||||
| 2013 | 2012 | |||||||
| Deferred tax assets: | ||||||||
| Net operating losses | $ | 1,956,406 | $ | 981,191 | ||||
| Accrued expenses | 167,880 | 166,872 | ||||||
| Tax credit carryforwards | 120,700 | 73,700 | ||||||
| Depreciation and amortization | (137,528 | ) | (36,395 | ) | ||||
| Other | 5,842 | 5,842 | ||||||
| Capitalized R&D | 1,371,494 | 870,919 | ||||||
| Total gross deferred tax assets | 3,484,794 | 2,062,129 | ||||||
| Valuation allowance | (3,484,794 | ) | (2,062,129 | ) | ||||
| Total deferred tax assets | $ | - | $ | - | ||||
As of December 31, 2013 the Company had federal and state net operating loss carryforwards of $5,094,191 and $4,250,631, respectively which begin to expire in 2032 and 2018, respectively. As of December 31, 2013, the Company had federal and state research and development tax credits carryforwards of $21,736 and $88,382, respectively, available to reduce future income taxes payable which begin to expire in 2031 and 2026, respectively.
Management of the Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets, which are comprised principally of net operating loss carryforwards and research and development credits. Under the applicable accounting standards, management has considered the Company’s history of losses and concluded that it is more likely than not that the Company will not recognize the benefits of federal and state deferred tax assets. Accordingly, a full valuation allowance of $3,484,794 and $2,062,129 has been established at December 31, 2013 and 2012, respectively. The valuation allowance increased $1,422,665 and $1,226,162 during 2013 and 2012, respectively.
| F-22 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
10 - INCOME TAX (Continued)
For tax positions meeting the more likely than not threshold, the tax amount recognized in the financial statements is reduced by the largest benefit that has greater than fifty percent likelihood of being realized upon the ultimate settlement with the relevant tax authority. The Company determined that the adoption of this authoritative guidance did not have a material effect on the consolidated financial statements.
The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. In the normal course of business the Company is subject to examination by federal and state jurisdictions, where applicable. There are currently no pending tax examinations. The Federal and State tax returns for the years ending 2009 to present are currently open. Earlier years may be examined to the extent that credit or loss-carry-forwards are used in it. The Company’s policy is to record interest and penalties related to income taxes as part of the tax provision.
Utilization of the net operating loss and research and development credit carryforwards may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986 due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating loss and research and development credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively.
11 - CONCENTRATIONS
During the year ended December 31, 2013, the Company recorded sales of $182,997 (41%), $122,821 (27%), $62,500 (14%) and $56,058 (12%) in excess of 10% of the Company’s total sales. At December 31, 2013, accounts receivable consisted of amounts due from three customers which represented approximately 40%, 40% and 20% of the outstanding accounts receivable balance.
During the year ended December 31, 2012, the Company recorded sales of $43,943 (66%), and $16,836 (25%) in excess of 10% of the Company’s total sales. At December 31, 2012, amounts due from one customer represented approximately 93% of the outstanding accounts receivable balance.
12 - SUBSEQUENT EVENTS
In February 2014, the Company raised $750,000 by issuing convertible promissory notes at 12% interest per annum. The maturity date is July 2015. The notes can be converted into shares of common stock at a conversion price of $0.27 per share. In connection with the issuance of the convertible promissory notes, the Company issued warrants with respect to 694,444 underlying shares exercisable into common stock at $0.27 per share with a term of 10 years.
On April 8, 2014, the Company amended the articles of incorporation to increase the number of authorized shares of common stock from 15,000,000 to 24,000,000 and to increase the number of authorized shares of preferred stock from 9,788,813 to 12,000,000 and designated a new class of Series B Preferred Stock.
In April 2014, the Company issued options to two executive officers to purchase 105,881 underlying shares of convertible preferred series B shares in connection with the Company’s Series B financing. These options were issued in relation to these executives taking a salary reduction prior to the Series B round of financing.
In April, 2014 the Company issued 948,823 shares of Series B Convertible Preferred Stock at an issue price of $2.125 per share for proceeds of $1,597,860, net of issuance costs of $418,390. The Series B Preferred Stock ranks pari passu in all respects to the Company’s Series A-2, A-1 and Series A Preferred Stock. In connection with the offering, the Company compensated the placement agent through the payment of $81,000 and the issuance of a warrant with respect to 38,259 Series B shares exercisable at $2.125 per share for a term of 5 years.
| F-23 |
ENUMERAL BIOMEDICAL CORP.
NOTES TO FINANCIAL STATEMENTS
12 - SUBSEQUENT EVENTS (Continued)
In April 2014 the Company amended the 2009 Equity Incentive Plan to increase the number of shares authorized to 3,200,437.
In February 2014, the Company revised the terms of their Loan and Security Agreement with Square 1 Bank, whereby the Company must complete an equity financing for gross cash proceeds of $2.0 million by March 31, 2014 and $4.0 million by June 30, 2014. Additionally, beginning on the date that the Company completes an equity financing for gross cash proceeds of $2.0 million, a monthly minimum unrestricted cash balance is required based upon three times the trailing three month cash burn.
In June 2014, the Company revised the terms of their Loan and Security Agreement with Square 1 Bank, whereby the Company extended their deadline to complete an equity financing for gross cash proceeds of $4.0 million to August 1, 2014. Additionally, the Company amended their minimum cash requirement beginning June 26, 2014 through August 1, 2014, whereas, the Company is required to maintain a minimum of $300,000 in unrestricted cash.
| F-24 |
Enumeral Biomedical Corporation
Condensed Balance Sheets
(Unaudited)
| June 30, | December 31, | |||||||
| 2014 | 2013 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 603,612 | $ | 263,910 | ||||
| Accounts receivable | - | 125,000 | ||||||
| Prepaid expenses and other current assets | 304,514 | 142,741 | ||||||
| Total current assets | 908,126 | 531,651 | ||||||
| Property and equipment, net | 624,527 | 747,888 | ||||||
| Other assets: | ||||||||
| Restricted cash | 32,666 | 32,666 | ||||||
| Other assets | 10,100 | 6,100 | ||||||
| Total assets | $ | 1,575,419 | $ | 1,318,305 | ||||
| LIABIITIES AND STOCKHOLDERS' DEFICIENCY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable ($224,508 and $109,740 due to related parties, respectively) | $ | 547,194 | $ | 342,595 | ||||
| Accrued expenses and other current liabilities ($12,500 and $50,942 due to related parties, respectively) | 435,011 | 206,035 | ||||||
| Deferred rent | 15,101 | 15,101 | ||||||
| Deferred revenue | - | 25,714 | ||||||
| Current portion of long-term debt | 700,654 | 716,000 | ||||||
| Total current liabilities | 1,697,960 | 1,305,445 | ||||||
| Deferred rent, net of current portion | 29,682 | 37,267 | ||||||
| Convertible promissory note, net of discount | 526,274 | - | ||||||
| Long-term debt, net of current portion and discount | - | 339,348 | ||||||
| Preferred stock warrant liability | 37,787 | 41,466 | ||||||
| Total liabilities | 2,291,703 | 1,723,526 | ||||||
| Commitments and contigencies | ||||||||
| Series A convertible preferred stock, $0.0001 par value; 2,879,000 shares authorized; 2,766,926 shares issued and outstanding at June 30, 2014 and December 31, 2013 (liquidation preference of $3,209,634 at June 30, 2014 and December 31, 2013) | 3,046,537 | 3,046,537 | ||||||
| Series A-1 convertible preferred stock, $0.0001 par value; 3,461,538 shares authorized; 2,047,207 shares issued and outstanding at June 30, 2014 and December 31, 2013 (liquidation preference of $2,661,369 at June 30, 2014 and December 31, 2013) | 2,608,190 | 2,608,190 | ||||||
| Series A-2 convertible preferred stock, $0.0001 par value; 3,448,275 shares authorized; 1,833,798 shares issued and outstanding at June 30, 2014 and December 31, 2013 (liquidation preference of $2,659,007 at June 30, 2014 and December 31, 2013) | 2,540,975 | 2,540,975 | ||||||
| Series B convertible preferred stock, $0.0001 par value; 2,211,187 shares authorized; 948,823 shares issued and outstanding at June 30, 2014 and none at December 31, 2013 (liquidation preference of $2,016,250 at June 30, 2014) | 1,597,860 | - | ||||||
| Total convertible preferred stock | 9,793,562 | 8,195,702 | ||||||
| Stockholders' deficiency | ||||||||
| Common stock, $.0001 par value; 24,000,000 shares authorized: 4,482,948 shares issued and outstanding at June 30, 2014 and December 31, 2013, respectively | 448 | 448 | ||||||
| Additional paid-in-capital | 1,397,848 | 909,866 | ||||||
| Accumulated deficiency | (11,908,142 | ) | (9,511,237 | ) | ||||
| Total stockholders’ deficiency | (10,509,846 | ) | (8,600,923 | ) | ||||
| Total liabilities, convertible preferred stock and stockholders’ deficiency | $ | 1,575,419 | $ | 1,318,305 | ||||
The accompanying notes are an integral part of the financial statements.
| F-25 |
Enumeral Biomedical Corporation
Condensed Statements of Operations
(Unaudited)
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| 2014 | 2013 | 2014 | 2013 | |||||||||||||
| Revenue: | ||||||||||||||||
| Collaboration and license revenues | $ | - | $ | - | $ | 50,714 | $ | 67,167 | ||||||||
| Grant revenue | - | 66,609 | - | 133,037 | ||||||||||||
| - | 66,609 | 50,714 | 200,204 | |||||||||||||
| Cost of revenue and expenses: | ||||||||||||||||
| Research and development | 791,105 | 609,014 | 1,476,517 | 1,166,403 | ||||||||||||
| General and administrative | 342,623 | 362,571 | 846,700 | 703,285 | ||||||||||||
| Total cost of revenue and expenses | 1,133,728 | 971,585 | 2,323,217 | 1,869,688 | ||||||||||||
| Loss from operations | (1,133,728 | ) | (904,976 | ) | (2,272,503 | ) | (1,669,484 | ) | ||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | (75,720 | ) | (28,020 | ) | (128,081 | ) | (58,932 | ) | ||||||||
| Change in fair value of derivatives | 3,019 | (96 | ) | 3,679 | 168 | |||||||||||
| Total other income (expense), net | (72,701 | ) | (28,116 | ) | (124,402 | ) | (58,764 | ) | ||||||||
| Net loss before income taxes | (1,206,429 | ) | (933,092 | ) | (2,396,905 | ) | (1,728,248 | ) | ||||||||
| Provision for income taxes | - | - | - | - | ||||||||||||
| Net loss | $ | (1,206,429 | ) | $ | (933,092 | ) | $ | (2,396,905 | ) | $ | (1,728,248 | ) | ||||
The accompanying notes are an integral part of the financial statements
| F-26 |
Enumeral Biomedical Corporation
Condensed Statements of Stockholders’ Deficiency
(Unaudited)
| Common Stock | ||||||||||||||||||||
| Shares | Amount | Additional Paid- In Capital | Accumulated Deficiency | Total Stockholders' Deficiency | ||||||||||||||||
| Balance at December 31, 2013 | 4,482,948 | $ | 448 | $ | 909,866 | $ | (9,511,237 | ) | $ | (8,600,923 | ) | |||||||||
| Stock-based compensation expense | - | - | 206,424 | - | 206,424 | |||||||||||||||
| Issuance of common stock warrants in connection with convertible promissory notes | - | - | 140,779 | - | 140,779 | |||||||||||||||
| Beneficial conversion feature associated with convertible promissory note | - | - | 140,779 | - | 140,779 | |||||||||||||||
| Net loss | - | - | - | (2,396,905 | ) | (2,396,905 | ) | |||||||||||||
| Balance at June 30, 2014 | 4,482,948 | $ | 448 | $ | 1,397,848 | $ | (11,908,142 | ) | $ | (10,509,846 | ) | |||||||||
The accompanying notes are an integral part of the financial statements
| F-27 |
Enumeral Biomedical Corporation
Condensed Statements of Cash Flows
(Unaudited)
| Six Months Ended June 30, | ||||||||
| 2014 | 2013 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (2,396,905 | ) | $ | (1,728,248 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activites: | ||||||||
| Depreciation and Amortization | 141,529 | 137,395 | ||||||
| Stock-based compensation | 206,424 | 103,584 | ||||||
| Change in fair value of derivatives | (3,679 | ) | (168 | ) | ||||
| Accretion of debt discount | 61,138 | 3,306 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivables | 125,000 | 28,689 | ||||||
| Prepaid expenses and other assets | (165,773 | ) | 18,396 | |||||
| Accounts payable | 204,599 | (240,707 | ) | |||||
| Accrued expenses and other current liabilities | 228,976 | 39,313 | ||||||
| Deferred rent | (7,585 | ) | (5,194 | ) | ||||
| Deferred revenue | (25,714 | ) | (31,741 | ) | ||||
| Net cash used in operating activities | (1,631,990 | ) | (1,675,375 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchases of property and equipment | (18,168 | ) | (51,221 | ) | ||||
| Net cash used in investing activities | (18,168 | ) | (51,221 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from issuance of Series A-2 convertible preferred stock, net of issuance costs | - | 1,480,005 | ||||||
| Proceeds from issuance of Series B convertible preferred stock, net of issuance costs | 1,597,860 | - | ||||||
| Proceeds from issuance of convertible promisory note | 750,000 | - | ||||||
| Payments on ST & LT debt | (358,000 | ) | (358,000 | ) | ||||
| Net cash provided by financing activities | 1,989,860 | 1,122,005 | ||||||
| Net Increase (decrease) in cash and cash equivalents | 339,702 | (604,591 | ) | |||||
| Cash and cash equivalents, beginning of period | 263,910 | 1,747,534 | ||||||
| Cash and cash equivalents, end of period | $ | 603,612 | $ | 1,142,943 | ||||
| Supplemental cash flow disclosures: | ||||||||
| Cash paid for interest | $ | 30,466 | $ | 55,654 | ||||
The accompanying notes are an integral part of the financial statements
| F-28 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
1 - NATURE OF BUSINESS
Enumeral Biomedical Corporation (“Enumeral” or “the Company”) was founded in 2009 in the state of Delaware as Enumeral Technologies, Inc. The name was later changed to Enumeral Biomedical Corporation. Enumeral is discovering and developing novel antibody therapeutics that help the immune system attack diseased cells (“immunomodulators”). The Company believes it has a unique ability to extensively interrogate cells of the human immune system for drug candidate validation, and that this ability could give the Company a distinct advantage in selecting potential best-in-class candidates. The Company is building a pipeline of drug candidates for the treatment of cancer, infectious, and inflammatory diseases and leveraging the breadth of the Company’s technology in order to drive near-term cash flows from out-licensing of the Company’s drug candidates and through strategic collaboration partnerships. The Company’s near-term goals include performance of preclinical testing on drug candidates resulting from internal programs to generate data to support the Company’s ability to obtain revenue from co-development partners. The Company has focused its research efforts on using its proprietary drug discovery platform utilizing, in part, technology licensed by Enumeral from M.I.T., Harvard University and other institutions to identify and elucidate antibodies and antigens that are responsible for diseases that affect millions of individuals and are underserved by current therapeutic alternatives. These diseases have included cancer, infectious, and inflammatory diseases. The Company believes that the breadth of the drug discovery platform offers the potential to grow through revenue-generating collaborative research and development partnerships.
2 - RISK AND UNCERTAINTIES
The Company is subject to a number of risks common to emerging companies in the life sciences industry. Principal among these risks are the uncertainties of the drug development process, technological innovations, dependence on key individuals, development of the same or similar technological innovations by the Company’s competitors, protection of proprietary technology, compliance with government regulations and approval requirements, uncertainty of market acceptance of products, product liability, and the need to obtain financing necessary to fund future operations.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company has a working capital deficiency of $789,834 and accumulated deficit of $11,908,142 as of June 30, 2014. Additionally, the Company has incurred negative cash flows from operations and net losses to date. The Company is in the process of completing corporate collaborations to gain market acceptance of its technology and increase revenues. The Company completed a reverse merger on July 31, 2014 whereby the Company raised $21.5 million, prior to the deduction of expenses related to the transaction.
In view of these matters, continuation as a going concern is dependent upon the continued operations of the Company, which in turn is dependent upon the Company's ability to meet its financial requirements, raise additional capital, and the success of its future operations.
Management believes that it will be successful in obtaining sufficient financing to execute its operating plan. However, no assurances can be provided that the Company will secure additional financing or achieve and sustain a profitable level of operations. To the extent that the Company is unsuccessful in its plans, the Company may find it necessary to contemplate the sale of its assets and curtail operations.
| F-29 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Recently Adopted Accounting Standards
On June 10, 2014, the FASB issued Accounting Standards Update (ASU) No. 2014-10, Development Stage Entities (Topic 915) – Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation, which eliminates the concept of a development stage entity (DSE) in its entirety from current accounting guidance. Amendments to the consolidation guidance may result in more DSEs being considered variable interest entities (VIEs). The new guidance applies to all entities that previously met the definition of a DSE. This ASU is effective for public business entities for annual reporting periods beginning after December 15, 2015, and interim periods therein. Early adoption of the new standard is permitted. Management has elected to early adopt this ASU, as permitted and, accordingly, has not included the inception-to-date disclosures and other previously required disclosures for development stage entities.
Basis of Presentation
The accompanying unaudited financial statements were prepared using generally accepted accounting principles for interim financial information and the instructions to Form 10-Q and Regulation S-X. Accordingly, these financial statements do not include all information or notes required by generally accepted accounting principles for annual financial statements and should be read in conjunction with the 2013 Financial Statements included herein.
The preparation of financial statements in conformity with these accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements; and the reported amounts of expenses during the reported period. Ultimate results could differ from the estimates of management.
In the opinion of management, the unaudited financial statements included herein contain all adjustments necessary to present fairly the Company's financial position and the results of its operations and cash flows for the interim periods presented. Such adjustments are of a normal recurring nature. The results of operations for the three and six months ended June 30, 2014 may not be indicative of results for the full year.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (U.S. GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reported period. Significant estimates used in preparing the Company’s financial statements include the estimation of stock based compensation, stock warrants liability, accrued expenses, revenue recognition and income taxes. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash equivalents represent high liquid investments with remaining maturities of ninety days or less at the date of purchase. Cash equivalents are stated at cost, which approximates fair value.
Concentration of Credit Risk
The Company has no significant off-balance sheet concentrations of credit risk such as foreign currency exchange contracts, option contracts or other hedging arrangements. Financial instruments that subject the Company to credit risk consists primarily of cash and cash equivalents. The Company generally invests its cash in money market funds, U.S. Treasury securities and U.S. Agency securities that are subject to minimal credit and market risk. Management has established guidelines relative to credit ratings and maturities intended to safeguard principal balances and maintain liquidity. At times, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation.
| F-30 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Fair Value of Financial Instruments
Fair values of financial instruments included in current assets and current liabilities are estimated to approximate their book values, due to the short maturity of such instruments. All debt is based on current rates at which the Company could borrow funds with similar remaining maturities and approximates fair value. The Company’s assets and liabilities that are measured at fair value on a recurring basis are measured in accordance with ASC 820 under the Financial Accounting Standards Board (“FASB”), which establishes a three-level valuation hierarchy for measuring fair value and expands financial statement disclosures about fair value measurements.
The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability as of the measurement date. The three levels are defined as follows:
Level 1 Inputs to the valuation methodology are quoted prices (unadjusted) for identical assets in active markets.
Level 2 Inputs to the valuation methodology included quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument.
Level 3 Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The Company’s cash equivalents carried at fair value are primarily comprised of investments in a U.S. Treasury and federal agency backed money market fund. The valuation of the Company’s preferred warrants liability is discussed below and in Note 5. The following table presents information about the Company’s financial assets and liabilities measured at a fair value on a recurring basis as of June 30, 2014 and December 31, 2013:
| June 30, 2014 | Quoted Prices in Active Markets (Level 1) | Observable Inputs (Level 2) | Unobservable Inputs (Level 3) | |||||||||||||
| Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 603,612 | $ | 603,612 | $ | - | $ | - | ||||||||
| Liabilities | ||||||||||||||||
| Preferred stock warrants | $ | 37,787 | $ | - | $ | - | $ | 37,787 | ||||||||
| F-31 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Fair Value Measurements (continued)
| December 31, 2013 | Quoted Prices in Active Markets (Level 1) | Observable Inputs (Level 2) | Unobservable Inputs (Level 3) | |||||||||||||
| Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 263,910 | $ | 263,910 | $ | - | $ | - | ||||||||
| Liabilities | ||||||||||||||||
| Preferred stock warrants | $ | 41,466 | $ | - | $ | - | $ | 41,466 | ||||||||
The following table provides a roll forward of the fair value of the Company’s preferred stock warrant liabilities, using Level 3 inputs:
| Balance at December 31, 2013 | $ | 41,466 | ||
| Change in fair value | (3,679 | ) | ||
| Balance at June 30, 2014 | $ | 37,787 |
Accounts Receivable and Allowance for Doubtful Accounts
Trade receivables are recorded at the invoiced amount. The Company maintains allowances for doubtful accounts, if needed, for estimated losses resulting from the inability of customers to make required payments. This allowance is based on specific customer account reviews and historical collections experience. There was no allowance for doubtful accounts as of June 30, 2014 or December 31, 2013.
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets included $237,636 of costs associated with the Company’s reverse merger and private placement offering with Enumeral Biomedical Holdings, Inc. (f.k.a Cerulean Group, Inc.) that occurred in July 2014 – See Subsequent Events.
Property and Equipment
Property and equipment are recorded at cost. Expenditures for maintenance and repairs are charged to expense as incurred, whereas major betterments are capitalized as additions to property and equipment. Depreciation is provided using the straight-line method over the estimated useful lives of the assets as follows:
| Lab equipment | 5 years |
| Computer equipment and software | 3 years |
| Furniture | 3 years |
| Leasehold improvements | Shorter of useful life or life of the lease |
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment annually or whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. When such events occur, the Company compares the carrying amounts of the assets to their undiscounted expected future cash flows. If this comparison indicated that there is impairment, the amount of the impairment is calculated as the difference between the carrying value and fair value. There have been no impairments recognized during the six months ended June 30, 2014 or 2013, respectively.
| F-32 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Revenue Recognition
Collaboration and license revenue
Non-refundable license fees are recognized as revenue when the Company has a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and the Company has no further performance obligations under the license agreement. Multiple element arrangements, such as license and development arrangements are analyzed to determine whether the deliverables, which often include license and performance obligations such as research and steering committee services, can be separated or whether they must be accounted for as a single unit of accounting in accordance with GAAP. The Company recognizes up-front license payments as revenue upon delivery of the license only if the license has stand-alone value and the fair value of the undelivered performance obligations, typically including research and/or steering committee services, can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations would then be accounted for separately as performed. If the license is considered to either (i) not have stand-alone or (ii) have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement would then be accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
Whenever the Company determines that an arrangement should be accounted for as a single unit of accounting, it must determine the period over which the performance obligations will be performed and revenue will be recognized. Revenue will be recognized using either a relative performance or straight-line method. The Company recognizes revenue using the relative performance method provided that the Company can reasonably estimate the level of effort required to complete its performance obligations under an arrangement and such performance obligations are provided on a best-efforts basis. Direct labor hours or full-time equivalents are typically used as a measure of performance. Revenue recognized under the relative performance method would be determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of substantive milestones, by the ratio of level of effort incurred to date to estimated total level of effort required to complete the Company’s performance obligations under the arrangement. Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the relative performance method, as of each reporting period.
If the Company cannot reasonably estimate the level of effort required to complete its performance obligations under an arrangement, the performance obligations are provided on best-efforts basis and the Company can reasonably estimate when the performance obligation ceases or the remaining obligations become inconsequential and perfunctory. At that time, the total payments under the arrangement, excluding royalties and payments contingent upon achievement of substantive milestones, would be recognized as revenue on a straight-line basis over a period the Company expects to complete its performance obligations.
Revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date.
The difference between the total consideration received to date and the revenue recognized is recorded as deferred collaboration revenue and totals $0 and $25,714 at June 30, 2014 and December 31, 2013, respectively.
| F-33 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Grant Revenue
The Company recognizes nonrefundable grant revenue that is earned in connection with its Research Agreement with the National Cancer Institute (the “NIH”), which was executed in September 2012. Grant revenue consists of a portion of the funds received to date by the NIH, which allow the Company to conduct research on colon cancer tissues. Revenue is recognized as the related research services are performed in accordance with the terms of the agreement. The difference between the total consideration received to date and the revenue recognized is recorded as deferred grant revenue and totals $0 at both June 30, 2014 and December 31, 2013.
Research and Development Expenses
Research and development expenditures are charged to the statement of operations as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, clinical supply costs, contract services, depreciation and amortization expense and other related costs. Costs associated with acquired technology, in the form of upfront fees or milestone payments, are charged to research and development expense as incurred. Legal fees incurred in connection with patent applications along with fees associated with the license to the Company’s core technology are expensed as research and development expense.
Accounting for Warrants
Preferred Stock
Warrants for the purchase of redeemable Convertible Preferred Series A Stock are carried at fair value and reported as a derivative liability on the accompanying balance sheets.
Changes in the fair value of warrants for the purchase of Convertible Preferred Series A Stock are included in other income in the statements of operations. For the six months ended June 30, 2014 and 2013, the Company recorded income of $3,679 and $168, respectively, as a result of the change in the fair value of the preferred Series A warrants. The Company recorded income of $3,019 for the three months ended June 30, 2014 and expense of $96 for the three months ended June 30, 2013, as a result of the change in the fair value of the preferred Series A warrants. The Company used the Black-Scholes option-pricing model to estimate the fair values of the issued and outstanding preferred warrants as of June 30, 2014 with the following weighted average assumptions: risk-free interest rate of 1.62%, remaining term of 4.96 years, volatility rate of 72.9% and no expected dividends. As of June 30, 2013, the following weighted average assumptions were used: risk-free interest rate of 1.41%, remaining term of 5.96 years, a volatility rate of 74.5% and no expected dividends.
In connection with the Company’s issuance of Convertible Preferred Series B Stock, the Company issued 144,140 shares of warrants for the purchase of Convertible Preferred Series B Stock - See Footnote 8 Common and Preferred Stock.
| F-34 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Accounting for Warrants (continued)
Common Stock
Warrants exercisable into shares of common stock at a price per share of $0.27 issued in connection with the convertible promissory note in February, 2014 were recorded based upon the relative fair value of the convertible promissory notes and the warrants at issuance and are reported as equity on the accompanying balance sheets.
Stock-Based Compensation
The Company has elected to use the Black-Scholes option pricing model to determine the grant date fair value of share-based awards. The Company recognizes the compensation cost of employee share-based awards on a straight-line basis over the employee’s requisite service period of each award, which is generally the vesting period.
The fair value of the restricted stock awards granted to employees is based upon the fair value of the common stock on the date of grant. Expense is recognized over the vesting period.
The Company has recorded stock-based compensation expense of $206,424 and $79,983 for six months ended June 30, 2014 and 2013, respectively, and $75,130 and $39,362 for three months ended June 30, 2014 and 2013, respectively. The Company has an aggregate of $224,674 of unrecognized stock-based compensation cost as of June 30, 2014 to be amortized over a weighted average period of 0.83 years.
The fair value of each option award is estimated on the date of grant using the Black-Scholes option pricing model that uses the weighted average assumptions noted in the following table:
| June 30, | December 31, | |||||||
| 2014 | 2013 | |||||||
| Stock Price | $ | 0.27 | $ | 0.27 | ||||
| Risk-free interest rate | 2.73 | 1.74 | % | |||||
| Expected dividend yield | - | - | ||||||
| Expected term – employees | 6 years | 6 years | ||||||
| Expected term – nonemployees | 10 years | 10 years | ||||||
| Volatility | 68 | % | 53 | % | ||||
| Determined grant date fair value per option | $ | 0.20 | $ | 0.19 | ||||
Expected volatility for the Company’s common stock was determined based on the historical volatility of comparable publically traded companies in a similar industry. The risk-free interest rate is based on the yield of U.S. Treasury securities consistent with the expected term of the option. No dividend yield was assumed as the Company does not pay dividends on its common stock. The expected term of the options granted represents the period of time that options granted are expected to be outstanding and is calculated using the simplified method.
The fair value of the common stock has typically been determined by the Board of Directors (the “Board”) at each date of grant based upon a variety of factors, including the Company’s financial position and historical financial performance, the status of developments within the Company’s research and development activities, the composition and ability of the current climate in the market place, the illiquid nature of the common stock, the effect of the rights and preferences of the preferred shareholders, and the prospects of a liquidity event, among others.
| F-35 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Stock-Based Compensation (continued)
The Company engaged a third party to develop an estimate of the fair value of a share of the Company’s common stock on a fully-diluted, minority, non-marketable basis as of December 31, 2013. The estimates used in the valuation at December 31, 2013 have not materially changed as of June 30, 2014 and the same estimate of the fair value of common shares was used at June 30, 2014.
Based upon unaudited historical, pro-forma and/or forecast financial and operational information, which Enumeral management represented as accurately reflecting the company’s operating results and financial position, the third party utilized both a Market Approach (using various financial statement metrics of similar enterprises’ equity securities to estimate the fair value of the Company’s equity securities) and an Income Approach (which bases value on expectations of future income and cash flows) in their analyses.
The fair value of a single share of common stock was determined using the Option Pricing Method, which treats Common and Preferred Stock as call options on the aggregate enterprise value and using traditional models, including Black-Scholes or binomial models, to calculate share values.
Income Taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, and operating loss and tax credit carryforwards. The Company’s financial statements contain certain deferred tax assets, which have arisen primarily as a result of operating losses, as well as other temporary differences between financial and tax accounting. The Company would establish a valuation allowance if the likelihood of realization of the deferred tax assets is reduced based on an evaluation of objective verifiable evidence. Significant management judgment is required in determining the Company’s provision for income taxes, the Company’s deferred tax assets and liabilities and any valuation allowance recorded against those net deferred assets. The Company evaluates the weight of all available evidence to determine whether it is more likely than not that some portion or all of the net deferred income tax assets will not be realized.
The Company accounts for uncertain tax positions in accordance with GAAP and has no uncertain tax liabilities at June 30, 2014 or December 31, 2013. The guidance requires the Company to determine whether it is more likely than not that a tax position will be sustained upon examination by the appropriate taxing authority. If a tax position meets the more likely than not recognition criteria, the guidance requires the tax position be measured at the largest amount of benefit greater than 50% likely of being realized upon ultimate settlement.
| F-36 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
New Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers: Topic 606. This Update affects any entity that either enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets, unless those contracts are within the scope of other standards. The guidance in this Update supersedes the revenue recognition requirements in Topic 605, Revenue Recognition and most industry-specific guidance. The core principle of the guidance is that an entity should recognize revenue to illustrate the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The new guidance also includes a cohesive set of disclosure requirements that will provide users of financial statements with comprehensive information about the nature, amount, timing, and uncertainty of revenue and cash flows arising from a reporting organization’s contracts with customers. This ASU is effective retrospectively for fiscal years, and interim periods within those years beginning after December 15, 2016 for public companies and 2017 for non-public entities. Management is evaluating the effect, if any, on the Company’s financial position and results of operations.
In June 2014, the FASB issued Accounting Standards Update (ASU) No. 2014-12, Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. The amendments in the ASU require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in Topic 718, Compensation – Stock Compensation, as it relates to awards with performance conditions that affect vesting to account for such awards. The performance target should not be reflected in estimating the grant-date fair value of the award. Compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If the performance target becomes probable of being achieved before the end of the requisite service period, the remaining unrecognized compensation cost should be recognized prospectively over the remaining requisite service period. The total amount of compensation cost recognized during and after the requisite service period should reflect the number of awards that are expected to vest and should be adjusted to reflect those awards that ultimately vest. The requisite service period ends when the employee can cease rendering service and still be eligible to vest in the award if the performance target is achieved.
| F-37 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
4 - PROPERTY AND EQUIPMENT
Property and equipment, net consist of the following:
| June 30, | December 31, | |||||||
| 2014 | 2013 | |||||||
| Laboratory equipment | $ | 1,097,494 | $ | 1,082,438 | ||||
| Computer/office equipment and software | 90,675 | 87,563 | ||||||
| Furniture, fixtures and office equipment | 18,772 | 18,772 | ||||||
| Leasehold improvements | 112,507 | 112,507 | ||||||
| 1,319,448 | 1,301,280 | |||||||
| Less - Accumulated depreciation and amortization | (694,921 | ) | (553,392 | ) | ||||
| $ | 624,527 | $ | 747,888 | |||||
Depreciation and amortization expense for the six months ended June 30, 2014 and 2013 were $141,529, and $137,395 respectively, and, for the three months ended June 30, 2014 and 2013 were $70,766, and $70,005 respectively.
5 – DEBT AND WARRANTS
Square 1 Financing and Security Agreement
In December 2011, the Company entered into a $1.79 million venture debt financing with Square 1 Bank. The Term Loans require the Company to comply with certain covenants on an annual basis. The Company is required to meet a maximum cash burn or liquidity ratio as indicated in the term loan agreement. In February 2014, the Company revised the terms of their Loan and Security Agreement with Square 1 Bank, whereby the Company must complete an equity financing for gross cash proceeds of $2.0 million by March 31, 2014 and $4.0 million by June 30, 2014. Additionally, beginning on the date that the Company completes an equity financing for gross cash proceeds of $2.0 million, a monthly minimum unrestricted cash balance is required based upon three times the trailing three month cash burn. In June 2014, the Company revised the terms of their Loan and Security Agreement with Square 1 Bank, whereby the Company extended their deadline to complete an equity financing for gross cash proceeds of $4.0 million to August 1, 2014. The Company was in compliance with its covenants as of June 30, 2014 and December 31, 2013.
Additionally, the Company amended their minimum cash requirement beginning June 26, 2014 through August 1, 2014, whereas, the Company is required to maintain a minimum of $300,000 in unrestricted cash.
The financing and security agreement is comprised of three separate loans:
“Term Loan A” is a $500,000 loan initiated at the signing of the loan agreement. “Term Loan B” is a second $500,000 loan, which was extended to the Company upon raising cash proceeds of at least $2,000,000. The Company achieved this milestone with the completion of its Series A-1 convertible preferred stock issuance in 2012. The Term Loans bear interest at a variable annual rate equal to the greater of: (A) 3.75% above the Prime Rate then in effect; or (B) 7.00%. The annual rate was capped not to exceed 8.5% during the first 18 months and will not exceed 9.50% thereafter. The Term A and B loans are collateralized by substantially all of the assets of the Company.
“Equipment Loan” consisted of a credit extension of up to $790,000. The Equipment Loan bears interest under the same as Term Loan A and B and is collateralized by the equipment purchased. The initial drawdown occurred in December 2011, consisting of the entirety of Term Loan A of $500,000 and $650,000 of the Equipment loan for a total of $1,150,000. The Company exercised an additional $106,875 of its Equipment Loan in April 2012. In October 2012, the Company drew down the remaining $33,125 from its Equipment Loan, along with the entirety of Loan B of $500,000.
| F-38 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
5 – DEBT AND WARRANTS (Continued)
Square 1 Financing and Security Agreement (continued)
Monthly principal payments on Term Loan A and B and the Equipment Loan of $59,667 commenced on January 5, 2013 and continue until maturity on June 5, 2015.
In August 2014 the Company fully repaid this loan.
In connection with the December 5, 2011 financing and security agreement, the Company issued to the lender warrants to purchase an aggregate of 33,944 shares of Series A convertible preferred stock at an exercise price of $1.16 per share, exercisable for seven years. The estimated fair value of the warrants at the time of issuance was determined to be $26,348 using the Black-Sholes pricing model and the following assumptions: expected term of seven years, 47% volatility, and a risk-free rate of 1.49%. As part of a June 12, 2012 amendment to the Loan and Security Agreement, the Company issued warrants to Square 1 Bank to purchase an aggregate of 7,715 shares of Series A convertible preferred stock at an exercise price of $1.16 per share, exercisable for seven years. The estimated fair value of these warrants at the time of issuance was determined to be $6,131 using the Black-Sholes pricing model and the following assumptions: expected term of seven years, 48% volatility, and a risk-free rate of 1.12%. The warrants are classified as a derivative liability in the accompanying balance sheets and measured at fair value on a recurring basis.
As of June 30, 2014, warrants to purchase 41,659 shares of Series A convertible preferred stock at a price of $1.16 per share are outstanding and expire on December 5, 2018 and June 12, 2019.
Convertible Promissory Notes
In February 2014, the Company raised $750,000 by issuing convertible promissory notes at 12% interest per annum. The maturity date is July 2015. The notes can be converted into shares of common stock at a conversion price of $0.27 per share. The convertible promissory notes have various repayment terms and conversion terms discussed below:
Liquidation Event:
If prior to maturity or conversion, the Company consummates a liquidation event as defined, at the election of the holder, 1) the Company shall pay the holder and amount equal to the sum of three times the total principal amount then outstanding under this note, plus all accrued and unpaid interest due, 2) the Note Balance amount shall be converted into shares of the Company’s Series A-2 Preferred Stock at the closing of the liquidation event at the Series A-2 Original Issue Price, 3) the note balance shall be converted into shares of the Company’s common stock at the closing of the liquidation event at a price per share of $0.27.
Voluntary Conversion:
The Holder has the option at any time prior to the Maturity date or qualified financing to convert the principal balance and accrued interest into either 1) if there has been a non-qualifying financing of the Company, shares of the financing securities sold in such non-qualified financing shares of common stock at a price per share of $0.27.
| F-39 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
5 – DEBT AND WARRANTS (Continued)
Mandatory Conversion:
In the event that, prior to the Maturity date, the Company closes a qualified financing, then at the closing of the qualified financing, the holder shall convert the note balance amount into either 1) shares of the financing at the financing price 2) shares of common stock at $0.27 per share.
In connection with the issuance of the convertible promissory notes, the Company issued warrants with respect to 694,442 underlying shares exercisable into common stock at $0.27 per share with a term of 10 years.
The Company allocated proceeds to the convertible notes and the warrants based upon the relative fair value on the issuance date which resulted in a $140,779 debt discount on the convertible promissory notes and $140,778 allocated to the warrants, recorded in equity. The allocation of proceeds to the warrants gave rise to a beneficial conversion feature which was recorded at the intrinsic value calculated as the difference between the adjusted conversion price of approximately $0.22 and the estimated fair value of the Company’s common stock of $0.27
In July 2014, the Company completed a reverse merger and subsequent private placement. As a result of the Merger, all issued and outstanding common and preferred shares of the Company were exchanged for common shares of Enumeral Biomedical Holdings, Inc. Additionally, the in connection with the private placement offering, the mandatory conversion feature of the convertible promissory notes as triggered and all outstanding principal and accrued interest under the convertible promissory notes was converted into common shares of the Company.
Future principal payments under debt obligations at June 30, 2014 are as follows:
| Year Ending December 31, | ||||
| 2014 | $ | 358,000 | ||
| 2015 | 1,108,000 | |||
| $ | 1,466,000 | |||
6 - COMMITMENTS
Operating Leases
In October 2012, the company entered into a 37 month operating lease for office and research space consisting of 4,782 square feet in Cambridge, Massachusetts. The term of the lease expires on November 30, 2015. The Company maintains a security deposit relating to the facility, recorded as restricted cash on the accompanying balance sheet.
Total rent expense was $153,067 and $141,234 for the six months ended June 30, 2014 and 2013, respectively, and $76,622 and $72,969 for the three months ended June 30, 2014 and 2013, respectively.
Future operating lease commitments as of June 30, 2014 are as follows:
| Year Ending December 31, | ||||
| 2014 | $ | 119,550 | ||
| 2015 | 228,919 | |||
| $ | 348,469 | |||
Employment Agreements
The Company has employment agreements with members of management which contain minimum annual salaries and severance benefits if terminated prior to the term of the agreements.
| F-40 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
7 – LICENSE AGREEMENT AND RELATED-PARTY TRANSACTIONS
License Agreement
In 2011, the Company licensed certain intellectual property from the Massachusetts Institute of Technology (“MIT”), a related party, in exchange for the payment of upfront license fees and a commitment to pay annual license fees, patent costs, milestone payments, and, upon product commercialization, royalties sales of products covered by the licenses or income from corporate partners.
All amounts paid related to the license fees have been expensed as research and development by the Company as incurred. The Company incurred $12,500 and $9,918 in the six months ended June 30, 2014 and 2013, respectively, and $6,250 and $4,996 in the three months ended June 30, 2014 and 2013, respectively.
In addition to potential future royalty and milestone payments that the Company may have to pay MIT per the terms of their license agreement, the Company is obligated to pay an annual fee of $25,000 in 2014, $30,000 in 2015, $40,000 in 2016, and $50,000 every year thereafter unless the license agreement is terminated. To date, no milestone payments have been made. No royalty payments have been payable as the Company has not commercialized any products as set forth in the agreement. The Company reimburses the costs to MIT and Harvard University for the continued prosecution of the licensed patent estate. For the six months ended June 30, 2014 and 2013, the Company paid $123,890 and $149,170 for MIT and $100,781 and $17,008 for Harvard, respectively. For the three months ended June 30, 2014 and 2013, the Company paid $99,092 and $52,538 for MIT and $100,781 and $17,008 for Harvard, respectively.
As of the effective date of the agreement through the date upon which $7,500,000 of funding for the Company’s capital stock has been achieved, the Company is required to issue MIT and affiliates an aggregate amount of shares equaling 3% of the Company’s issued and outstanding common stock on a fully diluted basis.
In April of 2013, the Company satisfied the $7,500,000 funding requirement through the Series A-2 financing which fulfilled the provision in the license agreement that required the Company to issue shares to MIT and affiliates.
The license agreement also contains a provision whereby after the $7,500,000 funding requirement is met, the Company may have to issue additional shares to MIT and affiliates if the Company issues common stock at a price per share that is less than the fair market value per share of the common stock issued to MIT and affiliates. The Company could be required to issue additional common shares to MIT and affiliates based upon a weighted average formula set forth in the License Agreement. The Company has not issued any additional common shares under this provision of the License agreement to date.
Consulting Agreements
A Company co-founder/stockholder is employed by MIT. On April 19, 2011, the Company entered into a consulting agreement with this Company co-founder/stockholder. The agreement has a three year term. During six months ended June 30, 2014 and 2013, the Company recognized consulting services of $12,500 and $12,500, respectively, and during the three months ended June 30, 2014 and 2013, the Company recognized consulting services of $6,250 and $6,250, respectively.
| F-41 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
7 – LICENSE AGREEMENT AND RELATED-PARTY TRANSACTIONS (Continued)
Consulting Agreements (continued)
On April 19, 2011, the Company entered into a consulting agreement with a director of the Company. Pursuant to the original agreement, the consultant will be compensated through the issuance of 159,045 restricted common shares that vest one quarter on the execution of the contract, one quarter on April 19, 2012 and the remainder 2.08% vesting monthly through April 1, 2014. The term of the consulting agreement is three years. On February 11, 2013, the consulting agreement was amended so that the consultant would receive $75,000 per year for a period of one year. On August 14, 2013, the consulting agreement was amended so that the consultant would receive $37,500 per year for a period of one year. During the six months ended June 30, 2014 and 2013, the Company recognized $18,750 and $37,500 of expense related to the consulting agreement, respectively, and during the three months ended June 30, 2014 and 2013, the Company recognized $9,375 and $18,750 of expense related to the consulting agreement, respectively.
On September 20, 2013, the Company entered into a consulting agreement with Allan Rothstein, and Norman Rothstein (collectively the “consultants”). Pursuant to the consulting agreement, Allan Rothstein was elected as a member of the Board of Directors of the Company. The consultants were compensated through the issuance of 1,000,000 shares of restricted common stock of the Company with one third vesting upon the execution of the consulting agreement, one third vesting on December 10, 2013 and one third vesting on March 10, 2014. The consulting agreement has a term of two years. During the three and six months ended June 30, 2014, the Company recognized $0 and $68,764 of expense related to the consulting agreement, respectively.
8 – COMMON AND PREFERED STOCK
Common Stock
On April 8, 2014, the Company amended the articles of incorporation to increase the number of authorized shares of common stock from 15,000,000 to 24,000,000 and to increase the number of authorized shares of preferred stock from 9,788,813 to 12,000,000 and designated a new class of Series B Preferred Stock.
Convertible Preferred Stock
In April 2014, the Company issued 948,823 shares of Series B Convertible Preferred Stock at an issue price of $2.125 per share for proceeds of $1,597,860 net of issuance costs of $418,390. The Series B Preferred Stock ranks pari passu in all respects to the Company’s Series A-2, A-1 and Series A Preferred Stock. In connection with the offering, the Company compensated the placement agent through the payment of $81,000 and the issuance of a warrant with respect to 38,259 Series B shares exercisable at $2.125 per share for a term of five years. These costs were included in the total issuance costs. The estimated fair value of the warrants at the time of issuance was determined to be $49,854 using the Black-Sholes pricing model and the following assumptions: expected term of five years, 74.6% volatility, and a risk-free rate of 1.72%.
In April 2014, the Company issued options to two executive officers to purchase 105,881 underlying shares of Convertible Preferred Series B shares in connection with the Company’s Series B financing. These options were issued in relation to these executives taking a salary reduction prior to the Series B round of financing. The estimated fair value of the warrants at the time of issuance was determined to be $137,770 using the Black-Sholes pricing model and the following assumptions: expected term of five years, exercise price of $2.125 per share, 74.6% volatility, and a risk-free rate of 1.63%. This warrant vests over six months. During the six months ended June 30, 2014 the Company recorded $57,404 as stock based compensation expense.
| F-42 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
9 - STOCK OPTIONS AND RESTRICTED STOCK
Stock Options
In December 2009, the Company adopted the 2009 Stock Incentive Plan. In April 2014 the Company amended the 2009 Stock Incentive Plan to increase the number of shares authorized to 3,200,437. As of June 30, 2014 there are 2,407,370 shares available for issuance to eligible employees, nonemployees and consultants. This number is subject to adjustment in the event of a stock split, reverse stock split, stock dividend, or other change in the Company’s capitalization.
Generally, shares that are expired, terminated, surrendered or cancelled without having been fully exercised will be available for future awards. In addition, shares of common stock that are tendered to the Company by a participant to exercise an award are added to the number of shares of common stock available for the grant of awards.
During the six months ended June 30, 2014, no stock options were granted to employees or directors. The vesting of employee and director awards is time-based and the restrictions typically lapse 25% after 12 months and monthly thereafter for the next 36 months for employees and annually over three years for directors.
During the six months ended June 30, 2014, no stock options were granted to nonemployees in exchange for services. Vesting on non-employee awards is time-based and the vesting period is generally over three years.
Stock option expense for employees was $9,448 and $8,653 for the six months ended June 30, 2014 and 2013, respectively, while non-employee stock option expense was $2,231 and $1,423, respectively, for the six months ended June 30, 2014 and 2013. Stock option expense for employees was $4,700 and $4,220 for the three months ended June 30, 2014 and 2013, respectively, while non-employee stock option expense was $606 and $188, respectively, for the three months ended June 30, 2014 and 2013.
A summary stock option activity for the six months ended June 30, 2014 is as follows:
| Number of Options | Weighted Average Exercise Price Per Share | |||||||
| December 31, 2013, Outstanding | 793,067 | $ | 0.24 | |||||
| Granted | - | - | ||||||
| Exercised | - | - | ||||||
| Forfeited | - | - | ||||||
| June 30, 2014, Outstanding | 793,067 | $ | 0.24 | |||||
| Exercisable | 446,102 | $ | 0.21 | |||||
Summary information regarding the options outstanding and exercisable at June 30, 2014 is as follows:
| Outstanding | Exercisable | |||||||||||||||||||||
| Exercise Prices | Number Outstanding (in shares) | Weighted Average Remaining Contractual Life (in years) | Weighted Average Exercise Price | Number Exercisable (in shares) | Weighted Average Exercise Price | |||||||||||||||||
| $ | 0.01 | 72,500 | 6.26 | $ | 0.01 | 72,500 | $ | 0.01 | ||||||||||||||
| $ | 0.23 | 205,667 | 7.41 | $ | 0.23 | 170,698 | $ | 0.23 | ||||||||||||||
| $ | 0.27 | 514,900 | 8.50 | $ | 0.27 | 202,904 | $ | 0.27 | ||||||||||||||
| 793,067 | 446,102 | |||||||||||||||||||||
| F-43 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
9 - STOCK OPTIONS AND RESTRICTED STOCK (Continued)
Stock Options (continued)
The intrinsic value of options exercisable at June 30, 2014 was $25,678. The aggregate intrinsic value was calculated as the difference between the exercise price of the stock options and the fair value of the underlying common stock as of the balance sheet date.
Restricted Stock
Restricted stock expense was $137,342 and $69,924 for the six months ended June 30, 2014 and 2013, respectively, and $12,420 and $34,945 for the three months ended June 30, 2014 and 2013, respectively.
A summary of restricted stock activity for the six months ended June 30, 2014 is as follows:
| Number of shares | Weighted Average Grant Date Fair Value | |||||||
| Balance of unvested restricted stock at December 31, 2013 | 1,009,914 | 0.25 | ||||||
| Issuance of restricted stock | - | - | ||||||
| Vested | (602,064 | ) | (0.24 | ) | ||||
| Balance of unvested restricted stock at June 30, 2014 | 407,850 | 0.27 | ||||||
10 - CONCENTRATIONS
During the six months ended June 30, 2014, the Company recorded sales of $50,714 to one customer. During the six months ended June 30, 2013, the Company recorded sales to two customers of $133,037 (66%), $60,544 (30%) in excess of 10% of the Company’s total sales. At June 30, 2013, accounts receivable consisted of amounts due from one customer which represented approximately 100% of the outstanding accounts receivable balance.
11 - SUBSEQUENT EVENTS
In July 2014, the Company completed a reverse merger and subsequent private placement offering with Enumeral Biomedical Holdings, Inc. (f.k.a Cerulean Group, Inc.).
On July 31, 2014, a wholly owned subsidiary of Enumeral Biomedical Holdings, Inc, Enumeral Acquisition Corp., a corporation formed in the State of Delaware on, July 16th 2014 (“Acquisition Sub”) merged (the “Merger”) with and into Enumeral Biomedical Corp. Enumeral was the surviving corporation in the Merger and became a wholly owned subsidiary of Enumeral Biomedical Holdings Inc. In connection with the Merger and pursuant to the Split-Off Agreement, the pre-Merger assets and liabilities of Enumeral Biomedial Holdings Inc. were transferred to the pre-Merger majority stockholder, in exchange for the surrender by her and cancellation of 23,100,000 shares of Common Stock in Enumeral Biomedical Holdings.
As a result of the Merger, all issued and outstanding common and preferred shares of the Company were exchanged for common shares of Enumeral Biomedical Holdings, Inc. Additionally, the in connection with the private placement offering described below, the mandatory conversion feature of the convertible promissory notes as triggered and all outstanding principal and accrued interest under the convertible promissory notes was converted into common shares of the Company.
| F-44 |
ENUMERAL BIOMEDICAL CORPORATION
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
11 - SUBSEQUENT EVENTS (Continued)
As a result of the Merger and Split-Off, Enumeral Biomedical Holdings, Inc. discontinued its pre-Merger business and acquired the business of Enumeral, and will continue the existing business operations of Enumeral as a publicly-traded company under the name Enumeral Biomedical Holdings, Inc. In accordance with “reverse merger” accounting treatment, historical financial statements for Enumeral Biomedical Holdings Inc. as of period ends, and for periods ended, prior to the Merger will be replaced with the historical financial statements of Enumeral prior to the Merger in all future filings with the SEC.
Also on July 31, 2014, Enumeral Biomedical Holdings closed a private placement offering (the “PPO”) of 21,549,510 Units of securities, at a purchase price of $1.00 per Unit, each Unit consisting of one share of Common Stock and a warrant to purchase one share of Common Stock at an exercise price of $2.00 per share and with a term of five years (the “PPO Warrants”).
The PPO was conducted on a “best efforts” basis. Enumeral Biomedical Corp agreed to pay the placement agents in the offering, registered broker-dealers, a cash commission of 10% of the gross funds raised from investors in the PPO. In addition, the placement agents received warrants exercisable for a period of five (5) years to purchase a number of shares of Common Stock equal to 10% of the number of shares of common stock with a per share exercise price of $1.00 (the “Agent Warrants); provided, however, that the placement agents are not entitled to any warrants on the sale of Units in excess of 20,000,000. Any sub-agent of the Placement Agents that introduced investors to the PPO was entitled to share in the cash fees and warrants attributable to those investors as described above. The Company was also required to reimburse the placement agents $30,000 in the aggregate for legal expenses incurred by the placement agents’ counsels in connection with the PPO, as dictated by the private placement agreements.
As a result of the foregoing, the placement agents were paid an aggregate commission of $2,154.951 and were issued Agent Warrants to purchase 2,000,000 shares of the Company’s common stock. The Agent Warrants have a term of five (5) years and an exercise price of $1.00 per share. The Agent Warrants not subsequently transferred or sold (other than transfers to trusts or affiliates of such investors for the purpose of estate planning) have “weighted average” anti-dilution protection for the same period as the Units, subject to customary exceptions. The Company incurred approximately $500,000 of expenses in connection with the offering outside of the placement agent commissions and issued the subagent to one of the Placement Agents 150,000 shares of common stock. The net proceeds received from the PPO of approximately $18,600,000 was reflected as an increase to cash. The value ascribed to the Agent Warrants has been included as an expense netted against the common stock and a corresponding increase to the common stock with zero net impact on the balance sheet.
The Merger Agreement provided certain anti-dilution protection to the holders of the Company’s Common Stock immediately prior to the Merger (after giving effect to the Split-Off), in the event that the aggregate number of Units sold in the PPO after the final closing thereof were to exceed 15,000,000 Accordingly, based on the final amount of gross proceeds raised in the PPO, the Company issued 1,693,747 additional shares of Common Stock to the holders of the Company’s Common Stock immediately prior to the Merger.
Options to purchase 1,221,250 shares of the Company’s Common Stock were issued on the Closing Date, consisting of incentive stock options to purchase 1,105,000 shares of the Company’s Common Stock that was issued to the Company’s executive officers, and incentive stock options to purchase 116,250 shares were issued to Company employees.
On July 31, 2014, The Board of Directors of the Company adopted, and the Company’s stockholders approved, the 2014 Equity Incentive Plan, which reserves a total of 8,100,000 shares of the Company’s Common Stock for incentive awards. Incentive awards generally may be issued to officers, key employees, consultants and directors. In connection with the Merger, options to purchase an aggregate of 1,045,419 shares of the Company’s Common Stock were issued under the 2014 Plan in exchange for outstanding options that were previously issued to officers, key employees, consultants and directors of Enumeral.
| F-45 |
Enumeral Biomedical Holdings, Inc. (f.k.a. Cerulean Corp) and
Enumeral Biomedical Corp.
Proforma Combined Financial Statements
(Unaudited)
The following unaudited proforma financial information has been prepared to illustrate the effect of Enumeral Biomedical Corp’s reverse acquisition of Enumeral Biomedical Holdings, Inc. The unaudited proforma information has been prepared treating the transaction as a reverse merger whereby Enumeral Biomedical Corp. is the acquirer for accounting purposes.
The following unaudited proforma combined balance sheet assumes the related transaction described in the notes hereto had occurred on the date for the period presented. The unaudited proforma combined balance sheet as of June 30, 2014 is based upon the reviewed balance sheet of Enumeral Biomedical Corp. and the historical balance sheet of Enumeral Biomedical Holdings, Inc. as of April 30, 2014 as filed in its Quarterly Report on Form 10-Q filed June 16, 2014.
The following unaudited proforma combined statement of operations for the six months ended June 30, 2014 assumes the related transaction described in the notes hereto had occurred at the beginning of the calendar quarter presented. The statement is based on the reviewed statement of operations of Enumeral Biomedical Corp. for the quarter ended June 30 2014. The reviewed statement of operations of Enumeral Biomedical Holdings, Inc. for the quarter ended April 30, 2014 is based on its statement of operations as filed in its Quarterly Report on Form 10-Q filed June 16, 2014.
The following unaudited proforma combined statement of operations for the year ended December 31, 2013 assumes the related transaction described in the notes hereto had occurred at the beginning of the calendar year presented. The statement is based on the audited statement of operations of Enumeral Biomedical Corp. for the year ended December 31 2013. The audited statement of operations of Enumeral Biomedical Holdings, Inc. for the fiscal year ended October 31, 2013 is based on its statement of operations as filed in its Annual Report on Form 10-K filed January 29, 2014.
The proforma combined statements of operations give effect to the merger as if it occurred at the beginning of each period presented. These unaudited proforma combined financial statements are prepared by management for informational purposes and are not necessarily indicative of future results or of actual results that would have been achieved had the acquisition been consummated as of the dates presented, and should not be taken as representative of future consolidated results or operations of financial position of the Company.
| F-46 |
Enumeral Biomedical Holdings, Inc. (f.k.a. Cerulean Group, Inc.)
and
Enumeral Biomedical Corporation
Proforma Combined Balance Sheets
As of June 30, 2014
(unaudited)
| Enumeral Biomedical Corporation at June 30, 2014 | Enumeral Biomedical Holdings, Inc. at April 30, 2014 | Adjustments | Notes | Pro Forma | ||||||||||||||
| ASSETS | ||||||||||||||||||
| Current assets: | ||||||||||||||||||
| Cash and cash equivalents | $ | 603,612 | $ | 244 | (244 | ) | (2) | |||||||||||
| 18,611,510 | (4) | 19,215,122 | ||||||||||||||||
| Accounts receivable | - | - | - | |||||||||||||||
| Prepaid expenses and other current assets | 304,514 | 1,826 | (1,826 | ) | (2) | 304,514 | ||||||||||||
| Total current assets | 908,126 | 2,070 | 18,609,440 | 19,519,636 | ||||||||||||||
| Property and equipment, net | 624,527 | - | - | 624,527 | ||||||||||||||
| Other assets: | ||||||||||||||||||
| Restricted cash | 32,666 | - | - | 32,666 | ||||||||||||||
| Other assets | 10,100 | - | - | 10,100 | ||||||||||||||
| Total assets | $ | 1,575,419 | $ | 2,070 | 18,609,440 | $ | 20,186,929 | |||||||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | ||||||||||||||||||
| Current liabilities: | ||||||||||||||||||
| Accounts payable | $ | 547,194 | $ | 3,820 | (3,820 | ) | (2) | $ | 547,194 | |||||||||
| Accrued expenses and other current liabilities | 435,011 | - | (41,506 | ) | (3) | 393,505 | ||||||||||||
| Deferred rent | 15,101 | - | - | 15,101 | ||||||||||||||
| Current portion of long-term debt | 700,654 | - | - | 700,654 | ||||||||||||||
| Loan from shareholder | - | 16,217 | (16,217 | ) | (2) | - | ||||||||||||
| Total current liabilities | 1,697,960 | 20,037 | (61,543 | ) | 1,656,454 | |||||||||||||
| Deferred rent, net of current portion | 29,682 | - | - | 29,682 | ||||||||||||||
| Convertible promissory note, net of discount | 526,274 | - | (526,274 | ) | (3) | - | ||||||||||||
| Long-term debt, net of current portion and discount | - | - | - | - | ||||||||||||||
| Preferred stock warrant liability | 37,787 | - | - | 37,787 | ||||||||||||||
| PPO Warrant Liability | - | - | 10,731,899 | (4) | 10,731,899 | |||||||||||||
| Total liabilities | 2,291,703 | 20,037 | 10,144,082 | 12,455,822 | ||||||||||||||
| Series A convertible preferred stock | 3,046,537 | - | (3,046,537 | ) | (2) | - | ||||||||||||
| Series A-1 convertible preferred stock | 2,608,190 | - | (2,608,190 | ) | (3) | - | ||||||||||||
| Series A-2 convertible preferred stock | 2,540,975 | - | (2,540,975 | ) | (3) | - | ||||||||||||
| Series B convertible preferred stock | 1,597,860 | - | (1,597,860 | ) | ||||||||||||||
| - | - | |||||||||||||||||
| Total convertible preferred stock | 9,793,562 | - | (9,793,562 | ) | - | |||||||||||||
| Stockholders’ deficiency | ||||||||||||||||||
| Common stock | 448 | 6,190 | (692 | ) | (2) | |||||||||||||
| 22,253 | (3) | |||||||||||||||||
| 21,700 | (4) | |||||||||||||||||
| 1,694 | (6) | 51,592 | ||||||||||||||||
| Additional paid-in capital | 1,397,848 | 22,610 | (22,610 | ) | (2) | |||||||||||||
| 10,450,952 | (3) | |||||||||||||||||
| 7,857,912 | (4) | |||||||||||||||||
| 1,692,053 | (6) | 21,398,765 | ||||||||||||||||
| Accumulated deficiency | (11,908,142 | ) | (46,767 | ) | 41,269 | (2) | ||||||||||||
| (111,863 | ) | (3) | ||||||||||||||||
| (1,693,747 | ) | (5) | (13,719,250 | ) | ||||||||||||||
| Total stockholders’ deficiency | (10,509,846 | ) | (17,967 | ) | 18,258,920 | 7,731,107 | ||||||||||||
| Total liabilities and stockholders’ deficiency | $ | 1,575,419 | $ | 2,070 | 18,609,440 | $ | 20,186,929 | |||||||||||
| F-47 |
Enumeral Biomedical Holdings, Inc. (f.k.a. Cerulean Group, Inc.)
and
Enumeral Biomedical Corporation
Proforma Combined Statement of Operations
For the Six Months Ended June 30, 2014 and the Six Months Ended April 30, 2014
(unaudited)
| Enumeral Biomedical Corporation Six Months Ended June 30, 2014 | Enumeral Biomedical Holdings, Inc. Six Months Ended April 30, 2014 | Adjustments | Notes | Pro Forma | ||||||||||||||
| Revenue: | ||||||||||||||||||
| Collaboration and license revenues | $ | 50,714 | $ | - | $ | - | $ | 50,714 | ||||||||||
| Grant revenue | - | - | - | - | ||||||||||||||
| Total revenue | 50,714 | - | - | 50,714 | ||||||||||||||
| Cost of revenue and expenses: | ||||||||||||||||||
| Research and development | 1,476,517 | - | - | 1,476,517 | ||||||||||||||
| General and administrative | 846,700 | 27,078 | (27,078 | ) | (2) | 846,700 | ||||||||||||
| Total cost of revenue and expenses | 2,323,217 | 27,078 | (27,078 | ) | 2,323,217 | |||||||||||||
| Loss from operations | (2,272,503 | ) | (27,078 | ) | 27,078 | (2,272,503 | ) | |||||||||||
| Other income (expense): | ||||||||||||||||||
| Interest expense | (128,081 | ) | - | (130,784 | ) | (3) | (258,865 | ) | ||||||||||
| Additional Shareholder Comp | - | - | (1,693,747 | ) | (5) | (1,693,747 | ) | |||||||||||
| Change in fair market value of derivatives | 3,679 | - | - | 3,679 | ||||||||||||||
| Total other income (expense), net | (124,402 | ) | - | (1,824,531 | ) | (1,948,933 | ) | |||||||||||
| Net Loss before income taxes | (2,396,905 | ) | (27,078 | ) | (1,797,453 | ) | (4,221,436 | ) | ||||||||||
| Provision for income taxes | - | - | - | - | ||||||||||||||
| Net Loss | $ | (2,396,905 | ) | $ | (27,078 | ) | $ | (1,797,453 | ) | $ | (4,221,436 | ) | ||||||
| PER SHARE INFORMATION - BASIC AND FULLY DILUTED (Note 6): | ||||||||||||||||||
| Weighted average shares outstanding | 41,480,145 | |||||||||||||||||
| Net loss per share, basic and fully diluted | $ | (0.10 | ) | |||||||||||||||
| F-48 |
Enumeral Biomedical Holdings, Inc. (f.k.a. Cerulean Group, Inc.)
and
Enumeral Biomedical Corporation
Proforma Condensed Combined Statement of Operations
For the Year Ended December 31, 2013
(unaudited)
| Enumeral Biomedical Corporation Year Ended December 31, 2013 | Enumeral Biomedical Holdings, Inc. Year Ended October 31, 2013 | Adjustments | Notes | Pro Forma | ||||||||||||||
| Revenue: | ||||||||||||||||||
| Collaboration and license revenues | $ | 266,039 | $ | - | $ | - | $ | 266,039 | ||||||||||
| Grant revenue | 182,997 | - | - | 182,997 | ||||||||||||||
| Total revenue | 449,036 | - | - | 449,036 | ||||||||||||||
| Cost of revenue and expenses: | ||||||||||||||||||
| Research and development | 2,369,414 | - | - | 2,369,414 | ||||||||||||||
| General and administrative | 1,832,088 | 19,406 | (19,406 | ) | (2) | 1,832,088 | ||||||||||||
| Total cost of revenue and expenses | 4,201,502 | 19,406 | (19,406 | ) | 4,201,502 | |||||||||||||
| Loss from operations | (3,752,466 | ) | (19,406 | ) | 19,406 | (3,752,466 | ) | |||||||||||
| Other income (expense): | ||||||||||||||||||
| Interest expense | (109,064 | ) | - | - | (109,064 | ) | ||||||||||||
| Change in fair market value of derivatives | (3,090 | ) | - | - | (3,090 | ) | ||||||||||||
| Other income (expense) | 31 | 31 | ||||||||||||||||
| Total other income (expense), net | (112,123 | ) | - | - | (112,123 | ) | ||||||||||||
| Net Loss before income taxes | (3,864,589 | ) | (19,406 | ) | 19,406 | (3,864,589 | ) | |||||||||||
| Provision for income taxes | - | - | - | - | ||||||||||||||
| Net Loss | $ | (3,864,589 | ) | $ | (19,406 | ) | $ | 19,406 | $ | (3,864,589 | ) | |||||||
| PER SHARE INFORMATION - BASIC AND FULLY DILUTED (Note 6): | ||||||||||||||||||
| Weighted average shares outstanding | 34,891,042 | |||||||||||||||||
| Net loss per share, basic and fully diluted | $ | (0.11 | ) | |||||||||||||||
| F-49 |
Note 1. Financial Periods for Financial Statements
Enumeral Biomedical Holdings, Inc. (f.k.a. Cerulean Group, Inc.), a Delaware corporation (“Parent”) had a fiscal year ending October 31st during the periods presented. The most recent financial information available for Parent is for the six months ended April 30, 2014. There has been minimal operating activity in Parent after April 30, 2014. As a result, the information presented for Parent as of April 30, 2014 is deemed to be current for these proforma combined financial statements. Enumeral Biomedical Corp, a Delaware corporation (the “Company”) reports on a calendar year basis and is utilizing financial statements as of June 30, 2014 for these proforma combined financial statements. As of July 10th, 2014, Parent redomiciled from Nevada to Delaware and changed its name to Enumeral Biomedical Holdings, Inc.
Note 2. Recapitalization of Enumeral Biomedical Holdings, Inc.
On July 25, 2014, Parent enacted a forward stock split of 4.62 shares to each share of Common Stock, resulting in the 6,190,000 shares issued and outstanding adjusting to 28,597,804 shares issued and outstanding.
The Company is reflecting the change in par value of the common stock of Enumeral Biomedical Holdings Inc., immediately prior to the Merger, from $0.001 to $0.0001 for the surviving entity and the Company pre-merger.
Note 3. Reincorporation; Reverse Merger Transaction
On July 31, 2014, Parent merged into Enumeral Biomedical Corp, which was the surviving corporation in the merger. Prior to the reincorporation merger, on July 10th 2014 (i) Parent converted from a Nevada corporation into a Delaware corporation under the name Enumeral Biomedical Holdings, Inc., and (ii) it increased its authorized capital stock from 75,000,000 shares of common stock, par value $0.001 per share, to 300,000,000 shares of common stock, par value $0.001 per share (the “Common Stock”), and 10,000,000 shares of “blank check” preferred stock, par value $0.001 per share.
In February 2014, the Company raised $750,000 by issuing convertible promissory notes. The Merger met the criteria of a qualified financing, generating more than $4,000,000 in proceeds as defined in the Company’s agreement with the note holders, and thus the principal balance and all accrued interest under the note was converted into shares of common stock at $0.27 per share. In connection with the issuance of the convertible promissory notes, the Company issued warrants with respect to 694,442 underlying shares exercisable into common stock at $0.27 per share with a term of 10 years. The Notes had accrued interest of $40,520 at the date of the transaction and a debt discount for the warrants and a beneficial conversion feature as of June 30, 2014 of approximately $111,000, each. Upon conversion the debt discount associated with the beneficial conversion feature of $130,784 was written off to interest expense and reflected in an adjustment to the accumulated deficiency on the balance sheet. The remaining debt balance and accrued interest were converted to commons shares of Enumeral.
All of the outstanding Company stock was converted into shares of Parent common stock as follows: (a) each share of Enumeral’s common stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.102121 shares of our Common Stock for a total of 4,940,744 shares post-Merger, (b) each share of Enumeral’s Series A Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.598075 shares of our Common Stock for a total of 4,421,744 shares post-Merger, (c) each share of Enumeral’s Series A-1 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.790947 shares of our Common Stock for a total of 3,666,428 shares post-Merger, (d) each share of Enumeral’s Series A-2 Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 1.997594 shares of our Common Stock for a total of 3,663,177 shares post-Merger, (e) each share of Enumeral’s Series B Preferred Stock issued and outstanding immediately prior to the closing of the Merger was converted into 2.927509 shares of our Common Stock for a total of 2,777,687 shares post merger and (f) a convertible note was converted into 3,230,869 shares of our Common Stock. As a result, an aggregate of 22,700,649 shares of Common Stock were issued to the holders of Enumeral’s stock (inclusive of 188,250 shares of common stock that is unvested and remains to a restricted stock award to each of Messrs. Rydzewski and Tinkelenberg as more fully described in “Executive Compensation – Employment Agreements”).
| F-50 |
As a result of the Merger, Parent discontinued its pre-Merger business and acquired the business of the Company, and will continue the existing business operations of the Company as a publicly-traded company.
For financial reporting purposes, the transaction will be accounted for as a “reverse merger” rather than a business combination, because the sellers of the Company effectively control the combined companies immediately following the transaction. As such, the Company is deemed to be the accounting acquirer in the transaction and, consequently, the transaction is being treated as a reverse acquisition by the Company. Accordingly, the assets and liabilities and the historical operations that will be reflected in Parent’s ongoing financial statements will be those of the Company and will be recorded at the historical cost basis of the Company. All of Parent’s assets and liabilities were split off (see below) as part of the transaction and results of operations will be those of the Company after consummation of the transaction. Parent’s historic capital accounts and retained earnings will be retroactively adjusted to reflect the equivalent number of shares issued by it in the transaction while the Company’s historical retained earnings will be carried forward.
The historical financial statements of Parent before the transaction will be replaced with the historical financial statements of the Company before the transaction in all future filings with the Securities and Exchange Commission, or SEC. The Merger is intended to be treated as a tax-free exchange under Section 368(a) of the Internal Revenue Code of 1986, as amended.
In connection with the Merger, the Company transferred all of its pre-Merger operating assets and liabilities to its wholly-owned special-purpose subsidiary, Cerulean Operating Corp., a Delaware corporation (“Split-Off Subsidiary”), formed on July 24, 2014. Thereafter, pursuant to the split-off agreement, the Company transferred all of the outstanding shares of capital stock of Split-Off Subsidiary to Olesya Didenko, the pre-Merger majority stockholder of the Company, and the former sole officer and director of the Company (the “Split-Off”), in consideration of and in exchange for (i) the surrender and cancellation of an aggregate of 23,100,000 shares of Common Stock held by Olesya Didenko (which were cancelled and will resume the status of authorized but unissued shares of our Common Stock) and (ii) certain representations, covenants and indemnities.
The Split-Off Agreement resulted in the reduction of all assets, liabilities and retained earnings of Parent in the proforma financial statements and an adjustment to the Company’s common stock value of $692 to give effect to the 23,100,000 shares canceled and the change in par value from $0.001 to $0.0001.
Note 4. Financing Transaction
Concurrently with the closing of the Merger and in contemplation of the Merger, we held a closing of its private placement offering (“PPO”) in which 21,549,510 Units were sold, at a purchase price of $1.00 per Unit, each Unit consisting of one share of the our Common Stock and PPO Warrant to purchase one share of Common Stock at an exercise price of $2.00 per share and with a term of five years. The closing of this PPO and the closing of the Merger were conditioned upon each other.
The PPO was conducted on a “best efforts” basis. Enumeral Biomedical Corp agreed to pay the placement agents in the offering, registered broker-dealers, a cash commission of 10% of the gross funds raised from investors in the PPO. In addition, the placement agents received warrants exercisable for a period of five (5) years to purchase a number of shares of Common Stock equal to 10% of the number of shares of common stock with a per share exercise price of $1.00 (the “Agent Warrants); provided, however, that the placement agents are not entitled to any warrants on the sale of Units in excess of 20,000,000. Any sub-agent of the Placement Agents that introduced investors to the PPO was entitled to share in the cash fees and warrants attributable to those investors as described above. The Company was also required to reimburse the placement agents up to an aggregate of $30,000 for legal expenses incurred by them in connection with the PPO, as dictated by the private placement agreements.
As a result of the foregoing, the placement agents were paid an aggregate commission of $2,154,951 and were issued Agent Warrants to purchase 2,000,000 shares of the Company’s common stock. The Company incurred approximately $500,000 of expenses in connection with the offering outside of the placement agent commissions and issued the subagent to one of the Placement Agents 150,000 shares of common stock. The net proceeds received from the PPO of approximately $18,600,000 was reflected as an increase to cash.
| F-51 |
In connection with the warrants issued to the placement agent and through the PPO, the Company recorded a derivative liability upon effective date of the merger. Due to the warrants having certain anti-dilution protection against subsequent share issuance for a lower exercise price, the Company determine that fair value of the warrant would be treated as a liability and recorded in the balances sheet upon the merger becoming effective. The fair value was determined through the Black Scholes Model calculation as of the date of effectiveness.
Note 5. Issuance of Common Shares
The Merger Agreement provided certain anti-dilution protection to the holders of the Company’s Common Stock immediately prior to the Merger (after giving effect to the Split-Off), in the event that the aggregate number of Units sold in the PPO after the final closing thereof were to exceed 15,000,000. Accordingly, based on the final amount of gross proceeds raised in the PPO, the Company issued 1,693,747 additional shares of Common Stock to the holders of the Company’s Common Stock immediately prior to the Merger.
Note 6. Earnings Per Share
The proforma weighted average shares outstanding gives effect to the issuance of 21,699,510 shares of common stock in connection with the Merger and the Split-Off as if it occurred at the beginning of the periods presented and the 5,497,804 shares outstanding in Parent post-merger and Split-Off.
The effect of any potentially dilutive instruments including warrants and options were anti-dilutive. Therefore, dilutive earnings per share is equivalent to basic earnings per share.
| F-52 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ENUMERAL BIOMEDICAL HOLDINGS, INC. | ||
| Dated: August 28, 2014 | By: | /s/ Arthur H. Tinkelenberg |
| Name: Arthur H. Tinkelenberg | ||
| Title: Chief Executive Officer | ||
Exhibit Listing
| Exhibit Number |
Description | |
| 2.1* | Agreement and Plan of Merger and Reorganization, dated as of July 31, 2014, by and among the Registrant, Acquisition Sub and Enumeral Biomedical Corp. | |
| 3.1* | Amended and Restated Certificate of Incorporation of the Registrant | |
| 3.2* | Amended and Restated By-Laws of the Registrant | |
| 3.3* | Certificate of Merger of Enumeral Biomedical Corp., with and into Acquisition Sub, filed July 31, 2014 | |
| 10.1* | Split-Off Agreement, dated as of July 31, 2014, by and among the Registrant, Cerulean Operating Corp. and Olesya Didenko | |
| 10.2* | General Release Agreement, dated as of July 31, 2014, by and among the Registrant, Cerulean Operating Corp. and Olesya Didenko | |
| 10.3* | Form of Lock-Up Agreement between the Registrant and the officers, directors and shareholders party thereto | |
| 10.4* | Form of Subscription Agreement between the Registrant and the investors party thereto | |
| 10.5* | Form of PPO Warrant for Common Stock of the Registrant | |
| 10.6* | Placement Agency Agreement, dated July 3, 2014, between the Registrant and EDI Financial, Inc., as amended by Amendment No. 1 dated as of July 21, 2014 | |
| 10.7* | Placement Agency Agreement, dated July 25, 2014, between the Registrant and Katalyst Securities LLC | |
| 10.8* | Form of Agent Warrant for Common Stock of the Registrant | |
| 10.9* | Form of Registration Rights Agreement dated July 31, 2014, among the Registrant and the other parties thereto | |
| 10.10* | Voting Agreement dated July 31, 2014, among the Registrant and the other parties thereto | |
| 10.11*† | The Registrant’s 2014 Equity Incentive Plan | |
| 10.12*† | Form of Non-Qualified Stock Option Agreement under 2014 Equity Incentive Plan | |
| 10.13*† | Form of Stock Appreciation Right Grant Agreement under 2014 Equity Incentive Plan | |
| 10.14*† | Form of Restricted Stock Agreement under 2014 Equity Incentive Plan | |
| 10.15*† | Form of Incentive Stock Option Agreement under 2014 Equity Incentive Plan | |
| 10.16*† | Amended and Restated Employment Agreement, dated as of July 21, 2014 between Enumeral Biomedical Corp. and Arthur Tinkelenberg, as assumed by the Registrant |
| F-53 |
| Exhibit Number |
Description | |
| 10.17*† | Amended and Restated Employment Agreement, dated as of July 21, 2014 between Enumeral Biomedical Corp. and John Rydzewski, as assumed by the Registrant | |
| 10.18*† | Employment Agreement, dated as of May 19, 2011 between Enumeral Biomedical Corp. and Derek Brand, as assumed by the Registrant | |
| 10.19* | Real Estate Lease Agreement, dated as of July 16, 2012, between Enumeral Biomedical Corp. and RB Kendall Fee, LLC | |
| 10.20* | Loan and Security Agreement, dated as of December 5, 2011, between Enumeral Biomedical Corp. and Square 1 Bank, as amended | |
| 10.21*‡ | Form of Warrant, issued by Enumeral Biomedical Corp. to Square 1 Bank | |
| 10.22* | Exclusive Patent License Agreement, dated as of April 15, 2011, between Enumeral Biomedical Corp. and the Massachusetts Institute of Technology (confidential portions have been omitted and filed separately with the Commission) | |
| 10.23* | First Amendment to Exclusive Patent License Agreement, effective as of March 8, 2013, between Enumeral Biomedical Corp. and the Massachusetts Institute of Technology (confidential portions have been omitted and filed separately with the Commission) | |
| 10.24* | Second Amendment to Exclusive Patent License Agreement, effective as of July 16, 2014, between Enumeral Biomedical Corp. and the Massachusetts Institute of Technology | |
| 10.25* | Contract Research Agreement, dated as of February 28, 2012, between Enumeral Biomedical Corp. and Crucell Holland B.V. (confidential portions have been omitted and filed separately with the Commission) | |
| 10.26* | 1st Amendment to Contract Research Agreement, dated as of March 22, 2013, between Enumeral Biomedical Corp. and Crucell Holland B.V. (confidential portions have been omitted and filed separately with the Commission) | |
| 10.27* | Master Service Provider Agreement, dated as of March 14, 2012, between Enumeral Biomedical Corp. and Anthrogenesis Corporation D/B/A Celgene Cellular Therapeutics (confidential portions have been omitted and filed separately with the Commission) | |
| 10.28* | Change Order, dated as of December 16, 2013, between Enumeral Biomedical Corp. and Celgene Cellular Therapeutics (confidential portions have been omitted and filed separately with the Commission) | |
| 10.29* | Research Agreement, dated as of June 18, 2012, between Enumeral Biomedical Corp. and sanofi-aventis U.S. Inc. (confidential portions have been omitted and filed separately with the Commission) | |
| 10.30* | Services Agreement, dated as of October 8, 2013, between Enumeral Biomedical Corp. and Novartis Pharma AG (confidential portions have been omitted and filed separately with the Commission) |
| 2 |
| Exhibit Number |
Description | |
| 10.31* | Agreement, dated as of March 21, 2013, between Enumeral Biomedical Corp. and Fikst (confidential portions have been omitted and filed separately with the Commission) | |
| 10.32* | Award/Contract, dated as of September 17, 2012, between Enumeral Biomedical Corp. and National Cancer Institute (confidential portions have been omitted and filed separately with the Commission) | |
| 10.33*‡ | Form of Warrant issued by Enumeral Biomedical Corp. to the investors in the Bridge Notes offering | |
| 10.34*‡ | Warrant dated April 15, 2014 issued by Enumeral Biomedical Corp. to John J. Rydzewski. | |
| 10.35*‡ | Warrant dated April 15, 2014 issued by Enumeral Biomedical Corp. to Arthur Tinkelenberg. | |
| 10.36*‡ | Warrant dated April 25, 2014 issued by Enumeral Biomedical Corp. to BlackBook Capital LLC | |
| 10.37* | Option Agreement dated July 31, 2014 between Registrant and John J. Rydzewski | |
| 10.38* | Option Agreement dated July 31, 2014 between Registrant and Arthur Tinkelenberg | |
| 10.39* | Option Agreement dated July 31, 2014 between Registrant and Derek Brand | |
| 10.40* | Option Agreement dated July 31, 2014 between Registrant and Anhco Nguyen | |
| 10.41* | Option Agreement dated July 31, 2014 between Registrant and Kevin Sarney | |
| 10.42*‡ | Option Agreement dated July 25, 2013 between Enumeral Biomedical Corp. and Derek Brand | |
| 10.43*‡ | Option Agreement dated July 25, 2013 between Enumeral Biomedical Corp. and Anhco Nguyen | |
| 10.44*‡ | Offer Letter dated July 25, 2014, between Enumeral Biomedical Corp. and Kevin Sarney | |
| 10.45* | Form of Indemnification Agreement between the Registrant and each of its directors and officers | |
| 16.1* | Letter, dated August 11, 2014, from Anton & Chia, LLP to the Securities and Exchange Commission | |
| 99.1* | Press Release of the Registrant, dated July 31, 2014 |
* Previously filed.
† Management contract or compensatory plan or arrangement
‡ Represents an option or warrant issues by Enumeral Biomedical Corp. and as such the number of shares issuable upon exercise and the exercise have not been revised to reflect the conversion to the right to receive common stock of the Registrant
| 3 |
