Attached files
| file | filename |
|---|---|
| EX-3.1 - EX-3.1 - Rhythm Holding Company, LLC | a2220945zex-3_1.htm |
| EX-10.7 - EX-10.7 - Rhythm Holding Company, LLC | a2220945zex-10_7.htm |
| EX-10.12 - EX-10.12 - Rhythm Holding Company, LLC | a2220945zex-10_12.htm |
| EX-10.11 - EX-10.11 - Rhythm Holding Company, LLC | a2220945zex-10_11.htm |
| EX-3.2 - EX-3.2 - Rhythm Holding Company, LLC | a2220945zex-3_2.htm |
| EX-10.8 - EX-10.8 - Rhythm Holding Company, LLC | a2220945zex-10_8.htm |
| EX-10.9 - EX-10.9 - Rhythm Holding Company, LLC | a2220945zex-10_9.htm |
| EX-21.1 - EX-21.1 - Rhythm Holding Company, LLC | a2220945zex-21_1.htm |
| EX-23.1 - EX-23.1 - Rhythm Holding Company, LLC | a2220945zex-23_1.htm |
| EX-10.10 - EX-10.10 - Rhythm Holding Company, LLC | a2220945zex-10_10.htm |
| EX-10.13 - EX-10.13 - Rhythm Holding Company, LLC | a2220945zex-10_13.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
RHYTHM HOLDING COMPANY, LLC INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
As filed with the Securities and Exchange Commission on August 27, 2014
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
RHYTHM HOLDING COMPANY, LLC
(to be converted into Rhythm Pharmaceuticals, Inc.)
(Exact name of Registrant as specified in its charter)
| Delaware (State or Other Jurisdiction of Incorporation or Organization) |
2834 (Primary Standard Industrial Classification Code Number) |
46-2170017 (I.R.S. Employer Identification Number) |
855 Boylston Street
11th Floor
Boston, MA 02116
(857) 264-4280
(Address, including zip code, and telephone number, including area code, of Registrant's principal executive offices)
Keith M. Gottesdiener, M.D.
Chief Executive Officer
Rhythm Holding Company, LLC
855 Boylston Street
11th Floor
Boston, MA 02116
(857) 264-4280
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| Please send copies of all communications to: | ||
Julio E. Vega Laurie A. Cerveny Bingham McCutchen LLP One Federal Street Boston, MA 02110 (617) 951-8000 |
Steven D. Singer Lisa Firenze Wilmer Cutler Pickering Hale and Dorr LLP 60 State Street Boston, MA 02109 (617) 526-6000 |
|
Approximate date of commencement of the proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer ý (Do not check if a smaller reporting company) |
Smaller reporting company o |
CALCULATION OF REGISTRATION FEE
|
||||
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee(3) |
||
|---|---|---|---|---|
Common Stock, $0.01 par value per share |
$86,250,000 | $11,109.00 | ||
|
||||
- (1)
- Includes
additional shares of common stock that the underwriters have the option to purchase.
- (2)
- Estimated
solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended.
- (3)
- Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Rhythm Holding Company, LLC, or the LLC entity, the registrant whose name appears on the cover of this registration statement, is a Delaware limited liability company. Prior to the effectiveness of this registration statement, the LLC entity will convert into a Delaware corporation and change its name from Rhythm Holding Company, LLC to Rhythm Pharmaceuticals, Inc. We refer to this conversion throughout the prospectus included in this registration statement as the "Conversion." Shares of the common stock of Rhythm Pharmaceuticals, Inc. are being offered by the prospectus included in this registration statement.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED AUGUST 27, 2014
PRELIMINARY PROSPECTUS

Shares
Rhythm Pharmaceuticals, Inc.
Common Stock
$ per share
This is the initial public offering of our common stock. We are selling shares of our common stock. We currently expect the initial public offering price to be between $ and $ per share of common stock.
We have granted the underwriters an option to purchase up to additional shares of common stock to cover over-allotments.
We intend to apply to have the common stock listed on The NASDAQ Global Market under the symbol "RYTM."
We are an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012, and therefore have elected to comply with certain reduced public company reporting requirements.
Investing in our common stock involves risks. See "Risk Factors" beginning on page 11.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| |
Per Share | Total | ||
|---|---|---|---|---|
| Public Offering Price | $ | $ | ||
| Underwriting Discount(1) | $ | $ | ||
| Proceeds to Rhythm Pharmaceuticals, Inc. (before expenses) | $ | $ |
- (1)
- We refer you to "Underwriting" beginning on page 158 for additional information regarding underwriting compensation.
The underwriters expect to deliver the shares to purchasers on or about , 2014 through the book-entry facilities of The Depository Trust Company.
Citigroup |
Cowen and Company |
Canaccord Genuity |
Oppenheimer & Co. | Cantor Fitzgerald & Co. |
, 2014
We are responsible for the information contained in this prospectus. We have not authorized anyone to provide you with different information, and we take no responsibility for any other information others may give you. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than its date.
Through and including , 2014 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
For investors outside the United States: Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus outside of the United States.
ii
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should read the entire prospectus carefully, including the sections entitled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and the related notes.
Prior to the effectiveness of the registration statement of which this prospectus forms a part, we will convert into a Delaware corporation, change our name to Rhythm Pharmaceuticals, Inc. and engage in other related transactions. See "Conversion." Except where the context otherwise requires or where otherwise indicated, the terms "Rhythm," "we," "us," "our," "our company," "the company," and "our business" refer, prior to the Conversion discussed below, to Rhythm Holding Company, LLC (or, as applicable, its predecessor company) and its consolidated subsidiaries and, after the Conversion, to Rhythm Pharmaceuticals, Inc. and its consolidated subsidiaries.
We are a biopharmaceutical company focused on the development and commercialization of peptide therapeutics for the treatment of gastrointestinal, or GI, diseases, and genetic deficiencies that result in metabolic disorders. Our lead product candidate, relamorelin, is a potent, best-in-class, Phase 2 ghrelin agonist for the treatment of diabetic gastroparesis, a GI complication of diabetes, and other GI functional disorders. We are also developing a second product candidate, RM-493, which is a potent, first-in-class, Phase 2 ready melanocortin-4, or MC4, receptor agonist for the treatment of obesity caused by genetic deficiencies in the MC4 pathway. We believe our product candidates, which are both administered by subcutaneous injection, and for which we have exclusive worldwide rights, have the potential to treat these diseases for which there are currently limited therapeutic options. We believe that ghrelin and MC4 are compelling targets because of their critical role in regulating GI function and metabolism, and that peptide therapeutics are well-suited for activating these targets.
Relamorelin targets the receptor for ghrelin, which is a naturally occurring hormone that plays a critical role in GI motility, or the movement of food through the GI tract, digestion and the absorption of nutrients. Prior drugs targeted the same GI motility disorders, primarily through either the serotonin or dopamine receptors, but had significant safety issues. First generation ghrelin agonists were small molecules with limited potency and efficacy. In contrast, the relamorelin peptide retains the specificity and functionality of the naturally occurring ghrelin peptide, and is designed to increase GI motility, with markedly greater potency than both naturally occurring ghrelin and the first generation small molecule agonists of ghrelin.
RM-493 targets the receptor for MC4, which is a key pathway that regulates energy, homeostasis, and food intake. The first generation of MC4 agonists were predominantly small molecules that failed in clinical trials due to safety issues, particularly increases in blood pressure, and had limited efficacy. In contrast, the RM-493 peptide retains the specificity and functionality of the naturally occurring hormone that activates MC4, and our initial Phase 1 and Phase 2 clinical trials have shown promising evidence of weight loss without adversely increasing blood pressure.
1
The following chart depicts key information regarding our product candidates, their indications, current states of development, and anticipated upcoming milestones:
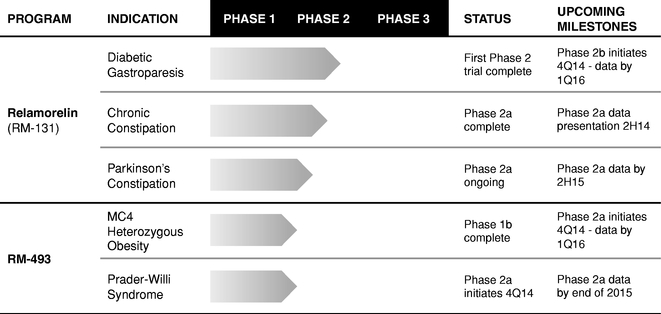
Relamorelin: A Best-In-Class Phase 2 Ghrelin Agonist
Relamorelin is a potent, best-in-class, Phase 2 ghrelin agonist which stimulates GI motility, and aids digestion and the absorption of nutrients. We have completed a Phase 2 clinical trial of relamorelin for the treatment of diabetic gastroparesis. Based on consultation with regulatory authorities, we expect to initiate a Phase 2b clinical trial for the treatment of diabetic gastroparesis by the end of 2014. The U.S. Food and Drug Administration, or FDA, has granted Fast Track designation to relamorelin for the treatment of diabetic gastroparesis. Fast Track designation is designed to facilitate and expedite the review of drugs for serious conditions with high unmet need, in order to get new drugs to patients earlier. We intend to manage our relamorelin clinical development program such that, if our clinical trials are successful, we can file a new drug application, or NDA, by 2018. We are also conducting a Phase 2a clinical trial for chronic refractory constipation in Parkinson's disease patients and a Phase 2a benchmark clinical trial in patients with chronic constipation.
Diabetic gastroparesis is a disorder in which there is a substantial delay in stomach emptying along with characteristic symptoms of vomiting, nausea, abdominal pain, early satiety and bloating. Moderate to severe diabetic gastroparesis results in significant debility and hospitalizations, and can interfere with nutrition and the absorption of medications. An estimated 2.3 million type 1 and type 2 diabetic patients in the United States have moderate or severe gastroparesis symptoms. Available therapies to treat this disorder are limited and exhibit significant side effects. No new therapies have been approved in the United States for the treatment of gastroparesis in more than 30 years.
To date, as part of our clinical program, we have treated approximately 260 subjects and patients with relamorelin. In our Phase 2 clinical trial, relamorelin had statistically significant effects on the most important diabetic gastroparesis symptoms, such as vomiting, as well as accelerated stomach emptying, and was well-tolerated. The results of these clinical trials support our belief that relamorelin has the potential to be a safe and effective treatment for diabetic gastroparesis.
2
RM-493: A First-In-Class Phase 2 Ready MC4 Agonist
RM-493 is a potent, first-in-class, Phase 2 ready MC4 agonist, which modulates a key pathway in humans that regulates energy homeostasis and food intake. MC4's critical role in weight regulation was validated with the discovery that a heterozygous mutation of the MC4 receptor gene, a mutation in just one of the two copies of the MC4 receptor gene, results in early onset obesity and severe obesity. We expect to initiate two Phase 2a clinical trials in patients with genetic deficiencies in the MC4 pathway by the end of 2014, one for the treatment of obesity in patients with a single mutation of the MC4 receptor gene, and one in patients with Prader Willi Syndrome, or PWS.
Genetic defects in the MC4 gene are the most common genetic cause of obesity. A 2005 epidemiological study performed in Europe reported a prevalence of 1-2% of genetic defects in the MC4 gene in the obese population with a body mass index, or BMI, of greater than 30 kg/m2, and studies performed in both Europe and the United States, in 2000 and 2003, respectively, reported a prevalence of up to 4% of these genetic defects in more severely obese populations with a BMI of greater than 35 kg/m2. These prevalence rates suggest that there are approximately one million people in the United States with obesity caused by a mutation of the MC4 gene. These patients have a higher risk than the general population for early onset obesity and severe obesity and their complications such as diabetes. There are currently no approved drugs in the United States that have been studied in obesity due to MC4 deficiency.
PWS, another form of genetic MC4 deficiency, is an orphan disease with a prevalence ranging from approximately one in 8,000 to one in 25,000 patients in the United States. A hallmark of PWS is severe hyperphagia, an overriding physiological drive to eat, leading to severe obesity and other complications. For PWS patients, obesity is the greatest threat to their health, and these patients are likely to die prematurely as a result of choking, stomach rupture, or from complications caused by morbid obesity. Currently, there is no approved treatment for the obesity and hyperphagia associated with PWS. Based on recent scientific studies, we believe that deficiencies in the MC4 pathway are a cause of the obesity and associated symptoms of PWS, such as hyperphagia, and that an MC4 agonist can directly impact these symptoms.
To date, as part of our clinical program, we have treated approximately 190 subjects and patients with RM-493. We have completed a Phase 1b proof-of-concept clinical trial with RM-493 in obese patients, including one cohort of patients with mutations in one of the MC4 receptor genes. This clinical trial demonstrated promising weight loss in these patients with a four-week treatment.
Based on the promising clinical results to date of relamorelin in diabetic gastroparesis, and the initial, favorable clinical profile of RM-493 for the treatment of obesity, including obesity caused by certain genetic deficiencies in the MC4 pathway, we believe these product candidates are well positioned to significantly improve the treatment of these indications. The key components of our strategy include:
- •
- Pursue development and regulatory approval for our lead product, relamorelin, for the treatment
of diabetic gastroparesis. We have completed a successful Phase 2 clinical trial of relamorelin for diabetic gastroparesis and plan to initiate a Phase 2b
clinical trial for this indication by the end of 2014. We plan to enroll and manage our Phase 2b and Phase 3 clinical trials such that, if the clinical trials are successful, we can file
an NDA by 2018.
- •
- Develop and seek regulatory approval for relamorelin in expansion indications in GI functional disorders. We intend to target expansion indications for both upper and lower GI functional disorders where the unmet need is high, including idiopathic gastroparesis, or gastroparesis without a specific cause, and refractory chronic constipation in Parkinson's disease patients.
3
- •
- Establish commercial and marketing capabilities in the United States
for relamorelin. We intend to establish our own commercial and marketing organization in the United States and to selectively establish
partnerships in markets outside the United States. We intend to target physicians who treat a high concentration of diabetic gastroparesis patients with a specialist sales force that focuses on
gastroenterologists and primary care physicians who are high prescribers of treatments for diabetic gastroparesis.
- •
- Develop RM-493 for obesity caused by genetic deficiencies in the MC4
pathway. We expect to initiate two Phase 2a clinical trials in patients with genetic deficiencies in the MC4 pathway by the end
of 2014, one for the treatment of obesity in patients with a single mutation of the MC4 receptor gene, and one in patients with PWS. Based on FDA consultations to date, we believe we can seek an
indication for obesity caused by genetic deficiencies in the MC4 pathway. If we are successful, we believe the time to receive regulatory approval for this product candidate may be reduced. We are
taking a personalized medicine approach to developing RM-493 for use in restoring MC4 function in patients who have genetic deficiencies in the MC4 pathway.
- •
- Maximize the therapeutic potential of RM-493 in additional obesity indications. We will consider entering into relationships with strategic partners that enable the expansion of the ongoing clinical development of RM-493, while retaining significant value for us.
Assuming we receive regulatory approval for diabetic gastroparesis, we believe that regulatory approval for these indications will follow thereafter.
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the "Risk Factors" section beginning on page 11 of this prospectus. These risks include the following:
- •
- We are a development stage biopharmaceutical company with a limited operating history and have not generated any revenue
from product sales. We have incurred significant operating losses since our inception, anticipate that we will incur continued losses for the foreseeable future, and may never achieve profitability.
As of June 30, 2014, we had an accumulated deficit of $65.4 million.
- •
- Even if this offering is successful, we will need to raise additional funding, which may not be available on acceptable
terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
- •
- Positive results from early clinical trials of relamorelin and RM-493 may not be predictive of the results of later
clinical trials of relamorelin and RM-493. If we cannot generate positive results in our later clinical trials of relamorelin and RM-493, we may be unable to successfully develop, obtain regulatory
approval for, and commercialize relamorelin and RM-493.
- •
- Failures or delays in the commencement or completion of our planned clinical trials of relamorelin and RM-493 could
result in increased costs to us and could delay, prevent or limit our ability to generate revenue and continue our business.
- •
- Changes in regulatory requirements, FDA guidance or unanticipated events during our clinical trials of relamorelin and RM-493 may occur, which may result in changes to clinical trial protocols or additional clinical trial requirements, which could result in increased costs to us and could delay our development timeline. Additionally, it may be necessary to validate different or additional instruments for measuring subjective symptoms, and to show that relamorelin and RM-493 have a clinically meaningful impact on those endpoints in order to obtain regulatory approval.
4
- •
- Even if we complete the necessary clinical trials, the regulatory and marketing approval process is expensive, time
consuming and uncertain and may prevent us from obtaining approvals for the commercialization of some or all of our product candidates. We depend almost entirely on the success of two product
candidates, relamorelin and RM-493, which are still in Phase 2 clinical development. We cannot be certain that we will be able to obtain regulatory approval for, or successfully commercialize,
relamorelin and RM-493. If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize our product candidates, and our ability
to generate revenue will be materially impaired.
- •
- If we experience delays or difficulties in the enrollment of patients in clinical trials, our receipt of necessary
regulatory approvals could be delayed or prevented.
- •
- Our product candidates may cause undesirable side effects that could delay or prevent their regulatory approval, limit
the commercial profile of an approved labeling, or result in significant negative consequences following marketing approval, if any.
- •
- Even if approved, reimbursement policies could limit our ability to sell relamorelin and RM-493.
- •
- Competing products and technologies could emerge adversely affecting our opportunity to generate revenue from the sale of
relamorelin and RM-493.
- •
- If we are unable to obtain and maintain patent protection for our products and technology, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be impaired.
Rhythm Holding Company, LLC, or the LLC entity, was formed on March 21, 2013 as a holding company in connection with a corporate restructuring, or the Prior Restructuring, by Rhythm Pharmaceuticals, Inc., a Delaware corporation that was organized in November 2008. Rhythm Pharmaceuticals, Inc. changed its name to Rhythm Health, Inc., or Rhythm Health, on July 7, 2014. Rhythm Health and Rhythm Metabolic, Inc., or Rhythm Metabolic, a Delaware corporation organized on March 21, 2013, became the wholly owned subsidiaries of the LLC entity as a result of certain exchange of equity transactions in connection with the Prior Restructuring. As part of the Prior Restructuring, Rhythm Health contributed all of its patent rights and other intellectual property rights with respect to RM-493 and Rhythm Health's related MC4 program to Rhythm Metabolic. As a result of the Prior Restructuring, our two product candidates and their related programs have been separated and each is held by a different legal entity, and the LLC entity became the holding company of both of these legal entities. Our wholly owned subsidiary, Rhythm Health, holds all of our patent rights and other intellectual property rights with respect to relamorelin and our related ghrelin agonist program, and our wholly owned subsidiary, Rhythm Metabolic, holds all of our patent rights and other intellectual property rights with respect to RM-493 and our related MC4 agonist program. See "Prior Restructuring" for additional information.
Prior to the effectiveness of the registration statement of which this prospectus forms a part, the LLC entity will convert from a Delaware limited liability company into a Delaware corporation to be named Rhythm Pharmaceuticals, Inc. See "Conversion" and "Description of Capital Stock" for additional information, including a description of the terms of our capital stock following the Conversion and the terms of our amended and restated certificate of incorporation and amended and restated bylaws that will be in effect upon the closing of this offering.
Our principal executive offices are located at 855 Boylston Street, 11th Floor, Boston, MA 02116, and our telephone number is (857) 264-4280. Our corporate website address is www.rhythmtx.com.
5
Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
This prospectus contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies' trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Implications of Being an Emerging Growth Company
We are an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of this offering, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period. We refer to the Jumpstart Our Business Startups Act of 2012 in this prospectus as the "JOBS Act," and references in this prospectus to "emerging growth company" shall have the meaning ascribed to it in the JOBS Act.
An emerging growth company may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
- •
- the ability to present only two years of audited financial statements and only two years of related Management's
Discussion and Analysis of Financial Condition and Results of Operations in this prospectus;
- •
- an exemption from the requirements to comply with the auditor attestation requirements of Section 404 of the
Sarbanes-Oxley Act of 2002, as amended;
- •
- reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and
registration statements; and
- •
- exemptions from the requirement to hold a nonbinding advisory vote on executive compensation and to obtain stockholder approval of any golden parachute payments not previously approved.
We may use these provisions until such time as we cease to be an emerging growth company.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus forms a part and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different from the information that you might receive from other public reporting companies in which you hold equity interests.
The JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. We have irrevocably elected not to avail ourselves of this exemption and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
6
Common stock we are offering |
shares | |
Common stock outstanding after giving effect to this offering |
shares |
|
Underwriters' option to purchase additional shares |
shares |
|
Use of proceeds |
We estimate that our net proceeds from this offering will be approximately $ million at an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds from this offering, together with our existing cash resources, as follows: |
|
|
• approximately $ million to fund the manufacturing and development of relamorelin in connection with our Phase 2 clinical trials and planned Phase 3 clinical trials for the treatment of diabetic gastroparesis through at least the initiation of Phase 3 clinical trials; |
|
|
• approximately $ million to fund the manufacturing and development of RM-493 through completion of our Phase 2a clinical trials for the treatment of MC4 heterozygous obesity and Prader Willi Syndrome; and |
|
|
• the remainder for working capital purposes, including general operating expenses. |
|
Risk factors |
See "Risk Factors" beginning on page 11 and the other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. |
|
NASDAQ Global Market Symbol |
"RYTM" |
The number of shares of our common stock to be outstanding after this offering assumes the Conversion takes place prior to the effectiveness of the registration statement of which this prospectus forms a part (see "Conversion"), and is based on shares of our common stock outstanding as of , 2014, including shares of unvested restricted common stock, and also assumes:
- •
- the conversion of all of our outstanding series A preferred stock and series B preferred stock into
shares of our common stock upon the completion of this offering;
- •
- no exercise by the underwriters of their option to purchase up to an additional shares of our common
stock to cover over-allotments; and
- •
- the filing of our amended and restated certificate of incorporation and the adoption of our amended and restated bylaws upon the closing of this offering.
7
In this prospectus, unless otherwise indicated, the number of shares of common stock outstanding and the other information based thereon does not reflect:
- •
- 9,790,000 shares of common stock issuable upon the exercise of outstanding warrants as of , 2014, at an
exercise price of $0.001 per share, which will automatically expire upon the closing of this offering if not previously exercised; and
- •
- shares of common stock reserved for future issuance under our 2014 equity incentive plan.
8
SUMMARY CONSOLIDATED FINANCIAL DATA
The following summary consolidated financial data for the years ended December 31, 2012 and 2013 are derived from our audited consolidated financial statements included elsewhere in this prospectus. The summary consolidated financial data as of June 30, 2014 and for the six months ended June 30, 2013 and 2014 have been derived from our unaudited consolidated financial statements included elsewhere in this prospectus. These unaudited consolidated financial statements have been prepared on a basis consistent with our audited consolidated financial statements and, in our opinion, contain all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of such financial data. You should read this data together with our audited consolidated financial statements and related notes included elsewhere in this prospectus and the information under the captions "Selected Consolidated Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations." Our historical results are not necessarily indicative of our future results, and our operating results for the six-month period ended June 30, 2014 are not necessarily indicative of the results that may be expected for the fiscal year ending December 31, 2014 or any other interim periods or any future year or period.
9
| |
Years Ended December 31, | Six Months Ended June 30, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2013 | 2014 | |||||||||
| |
|
|
(unaudited) |
||||||||||
| |
(in thousands, except per share data) |
||||||||||||
Statement of Operations and Comprehensive Loss Data: |
|||||||||||||
Operating Expenses: |
|||||||||||||
Research and development |
$ | 15,678 | $ | 18,015 | $ | 9,079 | $ | 5,470 | |||||
General and administrative |
2,460 | 3,433 | 1,753 | 1,503 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses |
18,138 | 21,448 | 10,832 | 6,973 | |||||||||
| | | | | | | | | | | | | | |
Loss from operations |
(18,138 | ) | (21,448 | ) | (10,832 | ) | (6,973 | ) | |||||
Other (expense) income, net |
8 | 17 | 11 | 5 | |||||||||
| | | | | | | | | | | | | | |
Net loss |
$ | (18,130 | ) | $ | (21,431 | ) | $ | (10,821 | ) | $ | (6,968 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Other comprehensive income (loss) |
7 | (1 | ) | 3 | (1 | ) | |||||||
| | | | | | | | | | | | | | |
Comprehensive loss |
$ | (18,123 | ) | $ | (21,432 | ) | $ | (10,818 | ) | $ | (6,969 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net loss applicable to common holders |
$ | (21,498 | ) | $ | (27,229 | ) | $ | (13,454 | ) | $ | (10,128 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net loss per common unit, basic and diluted(1) |
$ | (7.46 | ) | $ | (3.65 | ) | $ | (2.25 | ) | $ | (0.99 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Weighted average common units outstanding, basic and diluted |
2,883,087 | 7,467,781 | 5,979,829 | 10,198,713 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma net loss per common share applicable to common stockholders, basic and diluted(1) |
$ | (0.15 | ) | $ | (0.04 | ) | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma weighted average common shares outstanding, basic and diluted(2) |
139,901,617 | 156,198,713 | |||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| |
As of June 30, 2014 |
|
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Pro Forma As Adjusted(3) |
||||||||
| |
Actual | Pro Forma(2) | ||||||||
| |
(unaudited in thousands) |
|||||||||
Balance Sheet Data: |
||||||||||
Cash and cash equivalents |
$ | 8,864 | $ | 8,864 | $ | |||||
Working capital |
8,210 | 8,210 | ||||||||
Total assets |
10,687 | 10,687 | ||||||||
Convertible preferred units |
72,586 | — | ||||||||
Accumulated deficit |
(65,406 | ) | (65,406 | ) | ||||||
Total stockholders' and members' equity (deficit) |
$ | (64,338 | ) | $ | 8,248 | $ | ||||
- (1)
- See
Notes 2 and 10 within the notes to our financial statements appearing elsewhere in this prospectus for a description of the methods used to
calculate basic and diluted net loss per share and pro forma basic and diluted net loss per share.
- (2)
- Pro
forma to reflect the Conversion to a corporation and the conversion of all outstanding shares of series A preferred stock and series B preferred stock
into shares of common stock.
- (3)
- Pro forma as adjusted to further reflect the sale of shares of our common stock in this offering, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
10
Investing in our common stock involves a high degree of risk. You should carefully consider the following risks and uncertainties, together with all other information in this prospectus, including our consolidated financial statements and related notes, before investing in our common stock. Any of the risk factors we describe below could adversely affect our business, financial condition or results of operations. The market price of our common stock could decline if one or more of these risks or uncertainties actually occur, causing you to lose all or part of the money you paid to buy our common stock. Additional risks that we currently do not know about or that we currently believe to be immaterial may also impair our business. Certain statements below are forward-looking statements. See "Cautionary Note Regarding Forward-Looking Statements" in this prospectus.
Risks Related to Our Financial Position and Need for Capital
We are a development stage biopharmaceutical company with a limited operating history and have not generated any revenue from product sales. We have incurred significant operating losses since our inception, anticipate that we will incur continued losses for the foreseeable future, and may never achieve profitability.
We are a development stage company with a limited operating history on which to base your investment decision. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We were incorporated in November 2008. Our operations to date have been limited primarily to organizing and staffing our company and conducting research and development activities for our lead product candidate, relamorelin, and our second product candidate, RM-493. We have never generated any revenue from product sales. We have not obtained regulatory approvals for any of our product candidates.
Since our inception, we have focused substantially all of our efforts and financial resources on research and development of relamorelin and RM-493, which are currently in Phase 2 clinical development. We have funded our operations to date primarily through proceeds from sales of preferred stock and have incurred losses in each year since our inception. Our comprehensive losses were $12.3 million for the year ended December 31, 2011, $18.1 million for the year ended December 31, 2012 and $21.4 million for the year ended December 31, 2013. As of June 30, 2014, we had an accumulated deficit of $65.4 million. Substantially all of our operating losses resulted from costs incurred in connection with our development program and from general and administrative costs associated with our operations. We expect to incur increasing levels of operating losses over the next several years and for the foreseeable future. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders' deficit and working capital. We expect our research and development expenses to significantly increase in connection with our additional clinical trials of relamorelin and RM-493 and development of any other product candidates we may choose to pursue. In addition, if we obtain marketing approval for relamorelin and/or RM-493, we will incur significant sales, marketing and outsourced manufacturing expenses. Once we are a public company, we will incur additional costs associated with operating as a public company. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with developing pharmaceutical products, we are unable to predict the extent of any future losses or when we will become profitable, if at all. Even if we do become profitable, we may not be able to sustain or increase our profitability on a quarterly or annual basis.
Our ability to become profitable depends upon our ability to generate revenue. To date, we have not generated any revenue from our product candidates, and we do not know when, or if, we will generate any revenue. We do not expect to generate significant revenue unless and until we obtain
11
marketing approval for, and begin to sell, relamorelin and/or RM-493. Our ability to generate revenue depends on a number of factors, including, but not limited to, our ability to:
- •
- initiate and successfully complete later-stage clinical trials that meet their clinical endpoints;
- •
- initiate and successfully complete all safety studies required to obtain U.S. and foreign marketing approval for
relamorelin as a treatment for diabetic gastroparesis, a gastrointestinal complication of diabetes, and other gastrointestinal, or GI, functional disorders and/or RM-493 as a treatment for obesity
caused by genetic deficiencies in the melanocortin-4, or MC4, pathway;
- •
- successfully manufacture or contract with others to manufacture our product candidates;
- •
- commercialize relamorelin and RM-493, if approved, by building a sales force or entering into collaborations with third
parties; and
- •
- achieve market acceptance of relamorelin and RM-493 in the medical community and with third-party payors.
Absent our entering into a collaboration or partnership agreement, we expect to incur significant sales and marketing costs as we prepare to commercialize relamorelin. Even if relamorelin is approved for commercial sale, and we incur these costs, relamorelin may not be a commercially successful drug. We may not achieve profitability soon after generating product sales, if ever. If we are unable to generate product revenue, we will not become profitable and may be unable to continue operations without continued funding. In addition, any income we earn from sales of relamorelin may be used in our continued development of RM-493. Although the expected time frame for commercialization of RM-493 is longer than that for relamorelin, the commercialization of RM-493 is subject to the same risks as relamorelin.
Even if this offering is successful, we will need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
We are currently advancing our relamorelin and RM-493 product candidates through clinical development. Developing peptide therapeutic products is expensive, and we expect our research and development expenses to increase substantially in connection with our ongoing activities, particularly as we advance our product candidates in clinical trials. We intend to use the proceeds of this offering primarily for the clinical development and regulatory approval of relamorelin. Depending on the status of regulatory approval and, if approved, commercialization of relamorelin, as well as the progress we make in selling relamorelin, we will likely require additional capital to fund the continued development of RM-493 and our operating needs thereafter. We may also need to raise additional funds if we choose to pursue additional indications and/or geographies for relamorelin and RM-493 or otherwise expand more rapidly than we presently anticipate.
As of June 30, 2014, our cash, cash equivalents and marketable securities were $9.3 million. We estimate that the net proceeds from this offering will be approximately $ million, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and offering expenses payable by us. We expect that the net proceeds from this offering, together with our existing cash and cash equivalents will be sufficient to fund our operating expenses through at least the first half of 2016. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements, or a combination of these approaches. We will also require additional capital to obtain regulatory approval for, and to
12
commercialize, our product candidates. Raising funds in the current economic environment may present additional challenges. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, and may cause the market price of our shares to decline. The sale of additional equity or convertible securities would dilute all of our stockholders. The incurrence of indebtedness would result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights, and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or other third parties at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our product candidates or technologies or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any product candidate or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially adversely affect our business, financial condition and results of operations.
Our short operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
We are an early stage company. We were incorporated in November 2008 and commenced active operations in February 2010. Our operations to date have been limited to organizing and staffing our company, acquiring rights to intellectual property, business planning, raising capital, developing our technology, identifying potential product candidates, undertaking preclinical studies and, beginning in November 2010, conducting clinical trials. We have not yet demonstrated our ability to successfully complete a pivotal Phase 3 clinical trial, obtain marketing approvals, manufacture at commercial scale, or arrange for a third party to do so on our behalf, or conduct sales, marketing and distribution activities necessary for successful product commercialization. Consequently, any predictions made about our future success or viability may not be as accurate as they could be if we had a longer operating history.
In addition, as a new business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition at some point from a company with a research and development focus to a company capable of supporting commercial activities and we may not be successful in such a transition.
We expect our financial condition and operating results to continue to fluctuate significantly from quarter-to-quarter and year-to-year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any quarterly or annual periods as indications of future operating performance.
13
Risks Related to the Development of Our Product Candidates
Positive results from early clinical trials of relamorelin and RM-493 may not be predictive of the results of later clinical trials of relamorelin and RM-493. If we cannot generate positive results in our later clinical trials of relamorelin and RM-493, we may be unable to successfully develop, obtain regulatory approval for, and commercialize relamorelin and RM-493.
Positive results from any of our Phase 1 and Phase 2 clinical trials of relamorelin and RM-493 may not be predictive of the results of later clinical trials. This may be partly due to the possibility that the design of our Phase 2b and Phase 3 clinical trials of relamorelin as a treatment for diabetic gastroparesis may differ in several respects from our recently completed Phase 2 clinical trial for diabetic gastroparesis. For example, in later-stage clinical trials, we will likely be subject to more rigorous statistical analyses, such as intent to treat analyses. In addition, the effect of relamorelin on the four subjective symptoms of gastroparesis, nausea, abdominal pain, bloating, and early satiety, was only significant in a large subset of patients with vomiting at baseline. That subgroup was not pre-specified, and post-hoc analyses of subgroups may not be predictive of later trials. Placebo effects are also very common in clinical trials of GI disorders and may also be observed in obesity trials. Large placebo effects can make it harder, or even impossible, to demonstrate significant improvement on trial endpoints when the product candidate is compared to placebo. For both programs, the duration of effect of our products has only been studied for durations less than the expected duration of pivotal Phase 3 clinical trials. It is possible that the effects seen in shorter term clinical trials will not be replicated at later time points or in larger clinical trials. There may be other reasons why our early clinical trials are not predictive of later clinical trials. For RM-493, we may also need to develop a "companion diagnostic" genetic test to help identify patients with genetic deficiencies in the MC4 pathway, a process that can also be lengthy and cause additional delays in completing our clinical trials.
Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in early-stage development, and we cannot be certain that we will not face similar setbacks. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain U.S. Food and Drug Administration, or FDA, approval. If we fail to produce positive results in our planned Phase 2 or Phase 3 clinical trials of relamorelin and RM-493, the development timeline and regulatory approval and commercialization prospects for our product candidates, and, correspondingly, our business and financial prospects, would be materially adversely affected.
Failures or delays in the commencement or completion of our planned clinical trials of relamorelin and RM-493 could result in increased costs to us and could delay, prevent or limit our ability to generate revenue and continue our business.
We plan to commence our Phase 2b clinical trial in relamorelin and our Phase 2a clinical trials in RM-493 by the end of 2014. Successful initiation and completion of such clinical trials is a prerequisite to submitting a new drug application, or NDA, to the FDA and, consequently, the ultimate approval and commercial marketing of relamorelin and RM-493. We do not know whether any of these Phase 2 or Phase 3 clinical trials will begin or be completed on schedule, if at all, as the commencement and completion of clinical trials can be delayed or prevented for a number of reasons, including, among others:
- •
- the FDA may deny permission to proceed with our planned Phase 2 or Phase 3 clinical trials or any other
clinical trials we may initiate, or may place a clinical trial on hold;
- •
- delays in filing or receiving approvals or additional investigational new drug, or IND, applications that may be
required;
- •
- negative results from our ongoing and planned preclinical studies;
14
- •
- delays in commencing additional necessary preclinical studies, including carcinogenicity studies;
- •
- delays in reaching or failing to reach agreement on acceptable terms with prospective contract research organizations, or
CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
- •
- inadequate quantity or quality of a product candidate or other materials necessary to conduct clinical trials, including
delays in the manufacturing of sufficient supply of finished drug product;
- •
- difficulties obtaining Institutional Review Board, or IRB, approval to conduct a clinical trial at a prospective site or
sites;
- •
- challenges in recruiting and enrolling patients to participate in clinical trials, including the size and nature of the
patient population, the proximity of patients to clinical trial sites, eligibility criteria for the clinical trial, the nature of the clinical trial protocol, the availability of approved effective
treatments for the relevant disease and competition from other clinical trial programs for similar indications;
- •
- severe or unexpected drug-related side effects experienced by patients in a clinical trial, including side effects
previously identified in our completed clinical trials;
- •
- delays in validating the Patient Reported Outcome, or PRO, questionnaire for measuring subjective symptoms which is
likely to be a key endpoint in relamorelin clinical trials in diabetic gastroparesis or other clinical trials;
- •
- delays in validating any measures of hunger and related endpoints that may be utilized in a clinical trial for Prader
Willi Syndrome, or PWS;
- •
- delays in validating and, if necessary, obtaining approval for any needed genetic tests, for example, to identify
patients with MC4 deficiencies;
- •
- delays in identifying and recruiting patients with genetic causes of obesity;
- •
- the FDA may disagree with our clinical trial design, which may cause delays in initiating our clinical trials;
- •
- reports from preclinical or clinical testing of other diabetic gastroparesis, GI functional or weight loss therapies that
raise safety or efficacy concerns; and
- •
- difficulties retaining patients who have enrolled in a clinical trial but may be prone to withdraw due to rigors of the clinical trial, lack of efficacy, side effects, personal issues or loss of interest.
Clinical trials may also be delayed or terminated as a result of ambiguous or negative interim results. In addition, a clinical trial may be suspended or terminated by us, the FDA, the IRBs at the sites where the IRBs are overseeing a clinical trial, a data and safety monitoring board overseeing the clinical trial at issue, or other regulatory authorities due to a number of factors, including, among others:
- •
- failure to conduct the clinical trial in accordance with regulatory requirements or our clinical trial protocols;
- •
- inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities that reveals
deficiencies or violations that require us to undertake corrective action, including the imposition of a clinical hold;
- •
- unforeseen safety issues, adverse side effects or lack of effectiveness;
- •
- changes in government regulations or administrative actions;
- •
- problems with clinical trial supply materials; and
- •
- lack of adequate funding to continue the clinical trial.
15
Changes in regulatory requirements, FDA guidance or unanticipated events during our clinical trials of relamorelin and RM-493 may occur, which may result in changes to clinical trial protocols or additional clinical trial requirements, which could result in increased costs to us and could delay our development timeline. Additionally, it may be necessary to validate different or additional instruments for measuring subjective symptoms, and to show that relamorelin and RM-493 have a clinically meaningful impact on those endpoints in order to obtain regulatory approval.
Changes in regulatory requirements, FDA guidance or unanticipated events during our clinical trials may force us to amend clinical trial protocols or the FDA may impose additional clinical trial requirements. For instance, the FDA issued draft guidance on developing products for weight management in February 2007, but this guidance may be revised in the near future. In March 2012, the FDA's Endocrinologic and Metabolic Drugs Advisory Committee met to discuss possible changes to how the FDA evaluates the cardiovascular safety of weight-management drugs, and the FDA may require additional studies to support registration. In addition, the FDA is considering broader applicability of requirements for cardiovascular outcomes trials, or CVOTs, that exclude some degree of risk of cardiovascular risk pre-approval, including for obesity products. The FDA has also stated it is developing a guidance document for the development of products to treat gastroparesis. Any of these activities could result in additional clinical requirements for relamorelin or RM-493, increases in our costs, or delay of approval of relamorelin or RM-493.
Amendments to our clinical trial protocols would require resubmission to the FDA and IRBs for review and approval, which may adversely impact the cost, timing or successful completion of a clinical trial. If we experience delays completing, or if we terminate, any of our clinical trials, or if we are required to conduct additional clinical trials, the commercial prospects for relamorelin and RM-493 may be harmed and our ability to generate product revenue will be delayed.
In addition, prior to commencing our Phase 3 clinical trials for relamorelin as a treatment for diabetic gastroparesis and other GI functional disorders, we will need to validate and seek FDA concurrence with the PRO questionnaire for measuring subjective endpoints such as nausea, abdominal pain, bloating, and early satiety. If we experience delays in validation or do not receive approval based on the content validity, reliability, other validity, and ability to detect a change in study groups then we may not be able to proceed with the Phase 3 clinical trials of relamorelin.
If we experience delays or difficulties in the enrollment of patients in clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for relamorelin, RM-493 or other product candidates that we may develop if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. In addition, some of our competitors have ongoing clinical trials for product candidates that treat the same indications as relamorelin and RM-493, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors' product candidates.
Patient enrollment is affected by other factors including:
- •
- the severity of the disease under investigation;
- •
- the eligibility criteria for the clinical trial in question;
- •
- the perceived risks and benefits of the product candidate under study;
- •
- the success of efforts to facilitate timely enrollment in clinical trials;
- •
- the patient referral practices of physicians;
16
- •
- the ability to monitor patients adequately during and after treatment; and
- •
- the proximity and availability of clinical trial sites for prospective patients.
Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays, could require us to abandon one or more clinical trials altogether and could delay or prevent our receipt of necessary regulatory approvals. Enrollment delays in our clinical trials may result in increased development costs for our product candidates, which would cause the value of our company to decline and limit our ability to obtain additional financing.
Our product candidates may cause undesirable side effects that could delay or prevent their regulatory approval, limit the commercial profile of an approved labeling, or result in significant negative consequences following marketing approval, if any.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive labeling or the delay or denial of regulatory approval by the FDA or other regulatory authorities.
Ghrelin agonists may potentially cause impaired glucose tolerance due to secretion of growth hormone, and may potentially increase hunger and possibly to lead to increased weight. In addition, short-lived changes to other hormones, such as cortisol levels, have also been noted, though the clinical significance is unclear. Some changes in liver function tests were also noted in one Phase 1 clinical trial. While these increases were not replicated in the Phase 2 clinical trial in diabetic gastroparesis in larger numbers of diabetic patients, a risk for liver abnormalities may be seen in larger clinical trials. Injection site reactions may also occur with any subcutaneous injected product. Ghrelin may also have other potential mechanism-based and non-mechanism-based side effects.
Setbacks in late-stage clinical trials may be caused by, among other things, preclinical findings and safety observations in clinical trials, including previously unreported adverse events. In particular, long-term animal toxicity studies have not been completed for either of our product candidates, and while shorter studies have shown potentially large margins of safety, it is unclear if the margins will be smaller with longer studies or if new findings will appear that might have a significant impact on the clinical trials or approval by the FDA.
RM-493 is an MC4 agonist. Potential side effects of MC4 agonism, which have been noted either with RM-493 or with other MC4 agonists in clinical trials and preclinical studies, include:
- •
- adverse effects on cardiovascular parameters, such as increases in heart rate and blood pressure;
- •
- erections and similar effects in women, such as clitoral swelling and hypersensitivity, and sexual arousal;
- •
- nausea and vomiting; and
- •
- effects on mood, depression, anxiety and other psychiatric manifestations.
Injection site reactions have been seen in subcutaneous injections with RM-493. In addition, RM-493 has likely off-target effects on the closely related MC-1 receptor, the receptor that mediates tanning in response to sun exposure. Other MC-1 mediated effects include darkening of skin blemishes, such as freckles and moles. These effects have generally been reversible in clinical trials, but it is still unknown if they will be reversible with long-term exposure. The MC-1 mediated effects may also carry risks. The long-term impact of MC-1 activation has not been tested in clinical trials, and could potentially include increases in skin cancer, excess biopsy procedures, and cosmetic blemishes.
The safety data we have disclosed to date represents our interpretation of the data at the time of disclosure and they are subject to our further review and analysis. There have been no serious adverse events, or SAEs, attributed to relamorelin in our clinical trials. The only SAE possibly attributed to RM-493 in our clinical trials was one report of atypical chest pain, with no evidence of any serious
17
respiratory or cardiac cause on careful examination. In addition, our interpretation of the safety data from our clinical trials is contingent upon the review and ultimate approval of the FDA. The FDA may not agree with our methods of analysis or our interpretation of the results.
Further, if relamorelin or RM-493 receives marketing approval and we or others identify undesirable side effects caused by the product (or any other similar product) before or after the approval, a number of potentially significant negative consequences could result, including:
- •
- regulatory authorities may withdraw or limit their approval of the product;
- •
- regulatory authorities may require the addition of labeling statements, such as a "boxed" warning or a contraindication;
- •
- the FDA and other regulatory authorities may require the addition of Risk Evaluation and Mitigation Strategy, or REMS, or
post-market safety studies;
- •
- we may be required to change the way the product is distributed or administered, conduct additional clinical trials or
change the labeling of the product;
- •
- we may decide to remove the products from the marketplace;
- •
- we could be sued and held liable for injury caused to individuals exposed to or taking our product candidates; and
- •
- our reputation may suffer.
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product candidate and could substantially increase the costs of commercializing our product candidates and significantly impact our ability to successfully commercialize our product candidates and generate revenues.
Prior drugs and product candidates focused on the same indications as those targeted by relamorelin and RM-493 have achieved only limited efficacy and have posed serious safety concerns.
Relamorelin is a peptide that targets the receptor for ghrelin, a naturally occurring hormone that facilitates GI motility, or the movement of food through the GI tract, for the treatment of diabetic gastroparesis. Prior drugs targeted the same GI motility disorders, primarily through either the serotonin or dopamine receptors but had significant safety issues. Additionally, the first generation ghrelin agonists were small molecules that had limited potency and efficacy. There is no guarantee that relamorelin will achieve sufficient levels of efficacy and safety to qualify for regulatory approval. Similarly, first generation MC4 agonists were predominantly small molecules that failed in clinical trials due to safety issues, particularly increases in blood pressure, and had limited efficacy. There is no guarantee that RM-493 will achieve greater success than prior treatments in gaining regulatory approval.
We may not be able to translate the current formulations of our product candidates for methods of delivery that would be acceptable to the FDA or commercially successful.
Both relamorelin and RM-493 are currently administered by subcutaneous injection using small insulin-type needles, which is generally less well-received by patients than other methods of administration, such as oral administration. Considerable additional resources and efforts may be necessary in order to translate the current formulations of relamorelin and RM-493 into forms that will be acceptable to the FDA and/or to patients. While we plan to develop new and useful formulations of both relamorelin and RM-493, we cannot estimate the probability of success, nor the resources and time needed to succeed. If we are unable to develop new formulations, our products may not achieve significant market acceptance and our business, financial condition and results of operations may be materially harmed.
18
Our approach to treating one type of genetic form of obesity, heterozygous MC4 deficiency, requires identification and recruitment of patients with specific MC4 receptor genetic deficiencies. Development of such genetic testing will require substantial financial resources and regulatory approval, and may raise ethical, legal and social issues related to the use of genetic information, and genetic testing may cause less demand for our products. It is also possible that the FDA will require that we show that RM-493 is particularly useful for these patients, to demonstrate the need and usefulness of genetic pre-selection.
We intend to focus our development of RM-493 as a treatment for obesity caused by certain genetic deficiencies in the MC4 pathway. For some genetic deficiencies in this pathway, for example PWS, no specific genetic testing is expected to be required prior to initiation of therapy with RM-493. For others, for example, in the heterozygous MC4 patient population, this approach requires genetic testing, identification, and recruitment of patients, which may require substantial financial resources as well as regulatory approvals for these genetic tests. The development of these tests to accompany a clinical program has been difficult, and may delay our current timelines. In addition, our estimates of the prevalence and incidence of these genetic deficiencies are based primarily on rates reported in scientific publications and it is uncertain if these rates will be confirmed as subjects are screened for clinical trials. If these rates are lower than reported, recruitment and screening would be more costly and require additional time.
In order to assist in identifying this subset of patients, a companion diagnostic, which is a test or measurement that evaluates the presence of biomarkers in a patient, could be used. We anticipate that the development of a companion diagnostic concurrently with our product candidates will help us more accurately identify the patients who belong to the target subset. Use of a companion diagnostic in this way during clinical trials will result in product labeling, if the product candidate is approved, that limits use to only those patients who express the biomarker measured by the companion diagnostic. As a result, the companion diagnostic must be approved by the FDA on or before the date of approval of our product candidate. We may need to rely on third party collaborators to successfully develop and commercialize a companion diagnostic.
Companion diagnostics are subject to regulation by the FDA and comparable foreign regulatory authorities as medical devices and require separate regulatory clearance or approval prior to their commercialization. We may be dependent on the sustained cooperation and effort of any third-party collaborators with whom we may partner in the future to develop and obtain approval for these companion diagnostics. We and our potential future collaborators may encounter difficulties in developing and obtaining approval for these companion diagnostics, including issues relating to the selectivity and/or specificity of the diagnostic, analytical validation, reproducibility, or clinical validation. Any delay or failure by us or our potential future collaborators to develop or obtain regulatory approval of any companion diagnostics could delay or prevent approval of our related product candidates.
Additionally, we may be required to show that RM-493 has special benefits in genetic populations. In some of the patients with deficiencies in the MC4 pathway, such as those with PWS, we believe that any meaningful benefit from RM-493 treatment will be enough to support registration and approval. For others, such as the heterozygous MC4 patient population, we may need to show that this genetic subset will derive selective and meaningful benefit from RM-493 as a treatment for obesity. This may mean that we need to provide more than just the usual demonstration of safety and efficacy with RM-493 to support the value of identifying the MC4 heterozygous population for the treatment with RM-493. For example, we may need to show that RM-493 works better in the heterozygote than the wild-type, or genetically normal, patient population. Alternatively, we may need to demonstrate that RM-493 provides effective treatment for patients who fail to achieve weight loss on an already approved anti-obesity medication in the heterozygous population but not in the general population, thereby showing an advantage to the administration of RM-493 as well as identifying a population of MC4 heterozygotes.
19
The development of our product candidates may require substantial financial resources and may ultimately be unsuccessful.
In addition to the development of relamorelin, we are pursuing the development of RM-493 and may pursue the development of our other early-stage development programs. Other than relamorelin and RM-493, no other potential product candidates have commenced any clinical trials, and there are a number of FDA requirements that we must satisfy before we can commence clinical trials. Satisfaction of these requirements will entail substantial time, effort and financial resources. We may never satisfy these requirements. Any time, effort and financial resources we expend on RM-493 and our other early-stage development programs may adversely affect our ability to continue development and commercialization of relamorelin, and we may never complete our clinical trials of RM-493 or commence clinical trials of our other development programs despite expending significant resources in pursuit of their development. If we do commence clinical trials for our other potential product candidates, such product candidates may never be approved by the FDA.
We may not be successful in our efforts to identify or discover additional product candidates.
While we are not currently intending to identify or discover additional product candidates, it is possible that this plan will change over time. If so, the success of our business may depend primarily on our ability to identify, develop and commercialize these products. Although relamorelin and RM-493 are currently in clinical development, our research programs may fail to identify other potential product candidates for clinical development for a number of reasons. Our research methodology may be unsuccessful in identifying potential product candidates or our potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval.
If any of these events occur, we may be forced to abandon our development efforts for a program or programs, which would have a material adverse effect on our business and could potentially cause us to cease operations. Research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
Risks Related to the Commercialization of Our Product Candidates
Even if approved, reimbursement policies could limit our ability to sell relamorelin and RM-493.
Market acceptance and sales of relamorelin and RM-493 will depend on reimbursement policies and may be affected by healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels for those medications. Cost containment is a primary concern in the U.S. healthcare industry and elsewhere. Government authorities and these third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. We cannot be sure that reimbursement will be available for relamorelin or RM-493 and, if reimbursement is available, the level of such reimbursement. Reimbursement may impact the demand for, or the price of, relamorelin or RM-493. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize relamorelin or RM-493.
In some foreign countries, particularly in Canada and European countries, the pricing of prescription pharmaceuticals is subject to strict governmental control. In these countries, pricing negotiations with governmental authorities can take six to 12 months or longer after the receipt of regulatory approval and product launch. To obtain favorable reimbursement for the indications sought or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of relamorelin and RM-493 with other available therapies. If reimbursement for
20
relamorelin or RM-493 is unavailable in any country in which we seek reimbursement, if it is limited in scope or amount, if it is conditioned upon our completion of additional clinical trials, or if pricing is set at unsatisfactory levels, our operating results could be materially adversely affected.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell relamorelin and RM-493, if approved, we may not be able to generate any revenue.
We do not currently have infrastructure in place for the sale, marketing or distribution of pharmaceutical products. In order to market relamorelin and RM-493, if approved by the FDA or any other regulatory body, we must build our sales, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, or if we are unable to do so on commercially reasonable terms, our business, results of operations, financial condition and prospects would be materially adversely affected.
Even if we receive marketing approval for relamorelin and RM-493 in the United States, we may never receive regulatory approval to market relamorelin and RM-493 outside of the United States.
We intend to pursue marketing approval for relamorelin and RM-493 in the European Union and in other countries worldwide. In order to market any product outside of the United States, we must establish and comply with the numerous and varying safety, efficacy and other regulatory requirements of other countries. Approval procedures vary among countries and can involve additional product candidate testing and additional administrative review periods. The time required to obtain approvals in other countries might differ from that required to obtain FDA approval. The marketing approval processes in other countries may implicate all of the risks detailed above regarding FDA approval in the United States as well as other risks. In particular, in many countries outside of the United States, products must receive pricing and reimbursement approval before the product can be commercialized. Obtaining this approval can result in substantial delays in bringing products to market in such countries. Marketing approval in one country does not ensure marketing approval in another, but a failure or delay in obtaining marketing approval in one country may have a negative effect on the regulatory process in others. Failure to obtain marketing approval in other countries or any delay or other setback in obtaining our potential market share could have a material adverse impact on our business, results of operations and prospects.
Even if we receive marketing approval for relamorelin and RM-493, our product candidates may not achieve broad market acceptance, which would limit the revenue that we generate from their sale.
The commercial success of relamorelin and RM-493, if approved by the FDA or other applicable regulatory authorities, will depend upon the awareness and acceptance of relamorelin and RM-493 within the medical community, including physicians, patients and healthcare payors. Market acceptance of relamorelin and RM-493, if approved, will depend on a number of factors, including, among others:
- •
- relamorelin's ability to treat diabetic gastroparesis and other GI functional disorders and RM-493's ability to treat
obesity caused by certain genetic deficiencies in the MC4 pathway and, if required by any applicable regulatory authority in connection with the approval for these indications, to provide patients
with incremental health benefits, as compared with other available treatments, therapies, devices or surgeries;
- •
- the relative convenience and ease of subcutaneous injections as the necessary method of administration of relamorelin and
RM-493;
- •
- the prevalence and severity of any adverse side effects associated with relamorelin and RM-493;
21
- •
- limitations or warnings contained in the labeling approved, as well as the existence of a REMS, for relamorelin and
RM-493 by the FDA;
- •
- availability of alternative treatments, including a number of competitive GI functional, obesity and diabetes therapies
already approved or expected to be commercially launched in the near future;
- •
- the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
- •
- the strength of marketing and distribution support and timing of market introduction of competitive products;
- •
- publicity concerning our products or competing products and treatments;
- •
- pricing and cost effectiveness;
- •
- the effectiveness of our sales and marketing strategies;
- •
- our ability to increase awareness of relamorelin and RM-493 through marketing efforts;
- •
- our ability to obtain sufficient third-party coverage or reimbursement; and
- •
- the willingness of patients to pay out-of-pocket in the absence of third-party coverage.
If relamorelin or RM-493 is approved but does not achieve an adequate level of acceptance by patients, physicians and third-party payors, we may not generate sufficient revenue from our product candidates to become or remain profitable. Before granting reimbursement approval, healthcare payors may require us to demonstrate that, in addition to treating gastroparesis and other GI functional disorders and obesity caused by certain genetic deficiencies in the MC4 pathway, relamorelin and RM-493 also provide incremental health benefits to patients. Our efforts to educate the medical community and third-party payors about the benefits of relamorelin and RM-493 may require significant resources and may never be successful.
Competing products and technologies could emerge, adversely affecting our opportunity to generate revenue from the sale of relamorelin and RM-493.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. We have competitors in a number of jurisdictions, many of which have substantially greater name recognition, commercial infrastructures and financial, technical and personnel resources than we have. Established competitors may invest heavily to quickly discover and develop compounds that could make relamorelin and RM-493 obsolete or uneconomical. Any new product that competes with an approved product may need to demonstrate compelling advantages in efficacy, convenience, tolerability and safety to be commercially successful. Other competitive factors, including generic competition, could force us to lower prices or could result in reduced sales. In addition, new products developed by others could emerge as competitors to relamorelin and RM-493. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
Although there is a significant unmet medical need for drugs that improve motility, in GI functional disorders the development pipeline for prokinetic, or pro-motility, drugs is limited and overall there are few treatments approved or in late-stage clinical development for diabetic gastroparesis:
- •
- Reglan (metoclopramide), a mixed dopamine and serotonin receptor modulator, is the only drug indicated for the treatment of diabetic gastroparesis in the United States but is only approved for short-term use due to intolerance and acute side effects, predominantly an involuntary
22
- •
- EVK-001 is an intranasal formulation of metoclopramide under development by Evoke Pharma, Inc. Evoke has initiated a
Phase 3 clinical trial for the treatment of female patients with symptoms of diabetic gastroparesis. A recently completed Phase 2b clinical trial did not achieve statistical significance
in improving symptoms of diabetic gastroparesis for the total study population, while a post hoc analysis demonstrated efficacy in females.
- •
- GlaxoSmithKline plc is evaluating a motilin agonist, currently in Phase 2 clinical trials, for the
treatment of ICU gastric stasis and diabetic gastroparesis. Earlier programs with motilin agonists developed by Abbott Laboratories and Chugai Pharmaceutical Co. were discontinued because of
lack of efficacy.
- •
- Velusetrag, currently in late-stage clinical development by Theravance, Inc. and Alfa Wasserman S.p.A., is
a serotonin 5-HT4 receptor agonist. Velusetrag has completed Phase 2 clinical trials for chronic constipation and is currently undergoing limited Phase 2 clinical evaluation for diabetic
gastroparesis.
- •
- TC-6499, a small molecule that activates the alpha3beta4 and other neuronal nicotinic receptors, is in a Phase 1/2 exploratory clinical trial by Targacept, Inc. for gastroparesis. TC-6499 has completed a Phase 1 clinical trial suggesting that it may increase gastric motility.
movement disorder, known as tardive dyskinesia, the symptoms of which mimic those of Parkinson's disease. In 2009, the FDA required that a black box warning be added to the metoclopramide label because of the risk of tardive dyskinesia with long-term use, and recommended that its use be limited to 12 weeks. The European Medicines Agency is even more stringent, and recommends metoclopramide for use of no more than five days, and not for chronic conditions such as gastroparesis.
Although drugs approved for general obesity can be used in obese patients with MC4 deficiencies, there are currently no approved treatments in the United States specifically for these indications—either for obesity caused by heterozygous mutations of the MC4 gene or for PWS. The available treatments for general obesity have significant shortcomings, and have not been evaluated for safety or efficacy in the treatment of obesity caused by MC4 deficiencies. Bariatric surgery is the most successful treatment for obesity, and the only treatment resulting in an average of more than 15% documented weight loss over 10 years. This treatment has dramatic positive effects on most, but not on all, cardiovascular disease risk factors over a 10-year period. It has favorable effects on established diabetes and prevents the development of new cases of diabetes. The diabetes-preventive effect of bariatric surgery is particularly strong among patients with impaired fasting glucose at baseline. The incidence rates of cardiovascular disease events and cancer, as well as overall mortality, are reduced by bariatric surgery. Quality of life is also markedly improved. However, the number of bariatric surgery procedures in the U.S. has not increased for several years, despite greatly expanded guideline recommendations and new supportive clinical data. The implications are that there are substantial patient concerns about the morbidity and mortality associated with this surgery.
We face potential product liability exposure, and, if claims are brought against us, we may incur substantial liability.
The use of relamorelin or RM-493 in clinical trials and the sale of relamorelin or RM-493, if approved, exposes us to the risk of product liability claims. Product liability claims might be brought against us by patients, healthcare providers or others selling or otherwise coming into contact with relamorelin or RM-493. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, including as a result of interactions with alcohol or other drugs, negligence, strict liability and a breach of warranties. Claims could also be asserted under
23
state consumer protection laws. If we become subject to product liability claims and cannot successfully defend ourselves against them, we could incur substantial liabilities. In addition, regardless of merit or eventual outcome, product liability claims may result in, among other things:
- •
- withdrawal of patients from our clinical trials;
- •
- substantial monetary awards to patients or other claimants;
- •
- decreased demand for relamorelin, RM-493 or any future product candidates following marketing approval, if obtained;
- •
- damage to our reputation and exposure to adverse publicity;
- •
- litigation costs;
- •
- distraction of management's attention from our primary business;
- •
- loss of revenue; and
- •
- the inability to successfully commercialize relamorelin, RM-493 or any future product candidates, if approved.
We maintain product liability insurance coverage for our clinical trials with a $5.0 million annual aggregate coverage limit. Our insurance coverage may be insufficient to reimburse us for any expenses or losses we may suffer. Moreover, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses, including if insurance coverage becomes increasingly expensive. If and when we obtain marketing approval for relamorelin or RM-493, we intend to expand our insurance coverage to include the sale of commercial products. However, we may not be able to obtain this product liability insurance on commercially reasonable terms. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be substantial, particularly in light of the size of our business and financial resources. A product liability claim or series of claims brought against us could cause our stock price to decline and, if we are unsuccessful in defending such a claim or claims and the resulting judgments exceed our insurance coverage, our financial condition, business and prospects could be materially adversely affected.
Risks Related to Our Dependence on Third Parties
We rely, and expect that we will continue to rely, on third parties to conduct any future clinical trials for relamorelin and RM-493. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize relamorelin and RM-493 and our business could be substantially harmed.
We enter into agreements with third-party CROs to provide monitors for and to manage data for our ongoing clinical trials. We rely heavily on these parties for the execution of clinical trials for relamorelin and RM-493 and control only certain aspects of their activities. As a result, we have less direct control over the conduct, timing and completion of these clinical trials and the management of data developed through the clinical trials than would be the case if we were relying entirely upon our own staff. Communicating with outside parties can also be challenging, potentially leading to mistakes as well as difficulties in coordinating activities. Outside parties may:
- •
- have staffing difficulties;
- •
- fail to comply with contractual obligations;
- •
- experience regulatory compliance issues;
- •
- undergo changes in priorities or become financially distressed; or
24
- •
- form relationships with other entities, some of which may be our competitors.
These factors may materially adversely affect the willingness or ability of third parties to conduct our clinical trials and may subject us to unexpected cost increases that are beyond our control. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on CROs does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with current Good Clinical Practices, or cGCPs, which are guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities for any products in clinical development. The FDA enforces these regulations and cGCP guidelines through periodic inspections of clinical trial sponsors, principal investigators and trial sites. If we or our CROs fail to comply with applicable cGCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, the FDA will determine that any of our clinical trials comply with cGCPs. In addition, our clinical trials must be conducted with product produced under current Good Manufacturing Practices, or cGMPs, regulations and will require a large number of test subjects. Our failure or the failure of our CROs to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process and could also subject us to enforcement action up to and including civil and criminal penalties.
If any of our relationships with these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain are compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, any such clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize relamorelin and RM-493. As a result, our financial results and the commercial prospects for relamorelin and RM-493 in the subject indication would be harmed, our costs could increase and our ability to generate revenue could be delayed.
We rely completely on third-party suppliers to manufacture our clinical drug supplies for relamorelin or RM-493, and we intend to rely on third parties to produce commercial supplies of relamorelin or RM-493 and preclinical, clinical and commercial supplies of any future product candidate.
We do not currently have, nor do we plan to acquire, the infrastructure or capability to internally manufacture our clinical drug supply of relamorelin or RM-493, or any future product candidates, for use in the conduct of our preclinical studies and clinical trials, and we lack the internal resources and the capability to manufacture any product candidates on a clinical or commercial scale. The facilities used by our contract manufacturers to manufacture the active pharmaceutical ingredient and final drug product must pass inspection by the FDA and other comparable foreign regulatory agencies pursuant to inspections that would be conducted after we submit our NDAs or relevant foreign regulatory submission to the applicable regulatory agency. In addition, our clinical trials must be conducted with products produced under current cGMP regulations. Our failure or the failure of our CROs to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process and could also subject us to enforcement action, including civil and criminal penalties.
We currently contract with a third party for the manufacture of our product candidates and intend to continue to do so in the future. Both of our subsidiaries have entered into a process development and manufacturing services agreement with Peptisyntha SA under which Peptisyntha will provide certain process development and manufacturing services in connection with the manufacture of both relamorelin and RM-493. Under both agreements, we pay Peptisyntha for services in accordance with the terms of mutually agreed upon work orders, which we and Peptisyntha may enter into from time to
25
time. The agreements also provide that, subject to certain conditions, for a period following each product launch date, we will source from Peptisyntha a portion of our requirements for that product being sourced from non-affiliate third parties. We may need to engage additional third-party suppliers to manufacture our clinical drug supplies. We cannot be certain that we can engage third-party suppliers on terms as favorable as those that are currently in place.
We do not control the manufacturing process of, and are completely dependent on, our contract manufacturers to comply with cGMPs for manufacture of both active drug substances and finished drug products. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or applicable foreign regulatory agencies, they will not be able maintain a satisfactory manufacturing history. In addition, although we have no direct control over our contract manufacturers' ability to maintain adequate quality control, quality assurance and qualified personnel, we are ultimately responsible for ensuring that our drug substances and finished product are manufactured in accordance with cGMPs. Furthermore, all of our contract manufacturers are engaged with other companies to supply and/or manufacture materials or products for such companies, which exposes our manufacturers to regulatory risks for the production of such materials and products. As a result, failure to satisfy the regulatory requirements for the production of those materials and products may affect the regulatory clearance of our contract manufacturers' facilities generally. If the FDA or an applicable foreign regulatory agency does not approve these facilities for the manufacture of our product candidates or if it withdraws its approval in the future, we may need to find alternative manufacturing facilities, which would adversely impact our ability to develop, obtain regulatory approval for or market our product candidates.
We are currently in the process of manufacturing finished drug product for use in our upcoming clinical trials. We believe we currently have sufficient drug substance to complete our planned clinical trials. Finished drug product, diluent and placebo are expected to be available by the end of 2014. Aside from our planned Phase 2b clinical trial in patients with diabetic gastroparesis, we will not be able to commence any additional clinical trials without the production of additional finished drug product. The manufacturing process is under active development and these projections could change based on delays encountered with manufacturing activities, equipment scheduling and material lead times. Any such delays in the manufacturing of finished drug product could delay our planned clinical trials of relamorelin and RM-493, which could delay, prevent or limit our ability to generate revenue and continue our business.
We do not have long-term supply agreements in place with our contractors, and each batch of relamorelin and RM-493 is individually contracted under a quality and supply agreement. If we engage new contractors, such contractors must be approved by the FDA and other applicable foreign regulatory agencies. We will need to submit information to the FDA and other regulatory authorities describing the manufacturing changes. If manufacturing changes occur post-approval, the FDA will have to approve these changes. We plan to continue to rely upon contract manufacturers and, potentially, collaboration partners to manufacture commercial quantities of relamorelin and RM-493, if approved. Our current scale of manufacturing appears adequate to support all of our needs for clinical trial supplies for relamorelin, though further optimization may be required for RM-493. If relamorelin and RM-493 are approved, we will need to identify contract manufacturers or partners to produce relamorelin and RM-493 on a larger scale.
26
Risks Related to Our Intellectual Property Rights
If we are unable to adequately protect our proprietary technology or maintain issued patents that are sufficient to protect relamorelin and RM-493, others could compete against us more directly, which would have a material adverse impact on our business, results of operations, financial condition and prospects.
Our commercial success will depend in part on our success in obtaining and maintaining issued patents and other intellectual property rights in the United States and elsewhere and protecting our proprietary technology. If we do not adequately protect our intellectual property and proprietary technology, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
We cannot provide any assurances that any of our patents have, or that any of our pending patent applications that mature into issued patents will include, claims with a scope sufficient to protect relamorelin and RM-493 or our other product candidates. Other parties have developed technologies that may be related or competitive to our approach, and may have filed or may file patent applications and may have received or may receive patents that may overlap or conflict with our patent applications, either by claiming the same methods or formulations or by claiming subject matter that could dominate our patent position. The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, the issuance, scope, validity and enforceability of any patent claims that we may obtain cannot be predicted with certainty.
Although an issued patent is presumed valid and enforceable, its issuance is not conclusive as to its validity or its enforceability and such patent may not provide us with adequate proprietary protection or competitive advantages against competitors with similar products. Patents, if issued, may be challenged, deemed unenforceable, invalidated, or circumvented. U.S. patents and patent applications may also be subject to interference proceedings, ex parte reexamination, or inter partes review proceedings, post-grant review proceedings, supplemental examination and challenges in district court. Patents may be subjected to opposition or comparable proceedings lodged in various foreign, both national and regional, patent offices. These proceedings could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, such proceedings may be costly. Thus, any patents that we may own or exclusively license may not provide any protection against competitors. Furthermore, an adverse decision in an interference proceeding can result in a third party receiving the patent right sought by us, which in turn could affect our ability to develop, market or otherwise commercialize relamorelin and RM-493.
Competitors may also be able to design around our patents. Other parties may develop and obtain patent protection for more effective technologies, designs or methods. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in these countries. If these developments were to occur, they could have a material adverse effect on our sales.
In addition, proceedings to enforce or defend our patents could put our patents at risk of being invalidated, held unenforceable, or interpreted narrowly. Such proceedings could also provoke third parties to assert claims against us, including that some or all of the claims in one or more of our patents are invalid or otherwise unenforceable. If any of our patents covering relamorelin or RM-493 are invalidated or found unenforceable, our financial position and results of operations would be materially and adversely impacted. In addition, if a court found that valid, enforceable patents held by third parties covered relamorelin or RM-493, our financial position and results of operations would also be materially and adversely impacted.
27
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
- •
- any of our patents, or any of our pending patent applications, if issued, will include claims having a scope sufficient
to protect relamorelin and RM-493 or any other products or product candidates;
- •
- any of our pending patent applications will issue as patents;
- •
- we will be able to successfully commercialize relamorelin or RM-493, if approved, before our relevant patents expire;
- •
- we were the first to make the inventions covered by each of our patents and pending patent applications;
- •
- we were the first to file patent applications for these inventions;
- •
- others will not develop similar or alternative technologies that do not infringe our patents;
- •
- any of our patents will be found to ultimately be valid and enforceable;
- •
- any patents issued to us will provide a basis for an exclusive market for our commercially viable products, will provide
us with any competitive advantages or will not be challenged by third parties;
- •
- we will develop additional proprietary technologies or product candidates that are separately patentable; or
- •
- our commercial activities or products will not infringe upon the patents of others.
We rely upon unpatented trade secrets, unpatented know-how and continuing technological innovation to develop and maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with our employees, consultants, collaborators and vendors. We also have agreements with our employees and selected consultants that obligate them to assign their inventions to us. It is possible that technology relevant to our business will be independently developed by a person who is not a party to such an agreement. We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or trade secrets by consultants, collaborators, vendors, former employees and current employees. Furthermore, if the parties to our confidentiality agreements breach or violate the terms of these agreements, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets through such breaches or violations. Further, our trade secrets could otherwise become known or be independently discovered by our competitors.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming and divert the attention of our management and key personnel from our business operations. Even if we prevail in any lawsuits that we initiate, the damages or other remedies awarded may not be commercially meaningful. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid, is unenforceable and/or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Interference proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party
28
does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock.
We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing relamorelin or RM-493, if approved.
Our success will depend in part on our ability to operate without infringing the intellectual property and proprietary rights of third parties. We cannot assure you that our business, products and methods do not or will not infringe the patents or other intellectual property rights of third parties. For example, numerous third party U.S. and non-U.S. patents and pending applications exist that cover ghrelin analogs, melanocortin receptor analogs and methods of using these analogs. We are aware of one U.S. patent that broadly claims the method of use we are developing for relamorelin. While we believe that these broad claims are invalid, we cannot assure you that the patent holder will not attempt to assert these claims against us. If these claims were asserted against us and were found to be valid and enforceable, we may be forced to stop or delay developing, manufacturing, selling or otherwise commercializing relamorelin, or to obtain a license which may not be available on commercially reasonable terms or at all.
The pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may allege that relamorelin or RM-493 or the use of our technologies infringes patent claims or other intellectual property rights held by them or that we are employing their proprietary technology without authorization. For example, we received a letter in early 2013 from a third party bringing to our attention several patents and patent applications, both U.S. and non-U.S. We believe that these third-party patents do not impact our ability to operate without infringing same, but cannot assure you that the holder of these third-party patents will not attempt to assert these patents against us.
Patent and other types of intellectual property litigation can involve complex factual and legal questions, and their outcome is uncertain. Any claim relating to intellectual property infringement that is successfully asserted against us may require us to pay substantial damages, including treble damages and attorney's fees if we are found to be willfully infringing another party's patents, for past use of the asserted intellectual property and royalties and other consideration going forward if we are forced to take a license. In addition, if any such claim were successfully asserted against us and we could not obtain such a license, we may be forced to stop or delay developing, manufacturing, selling or otherwise commercializing relamorelin or RM-493.
If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court, or redesign our products. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion.
29
In addition, in order to avoid infringing the intellectual property rights of third parties and any resulting intellectual property litigation or claims, we could be forced to do one or more of the following, which may not be possible and, even if possible, could be costly and time-consuming:
- •
- cease development and commercialization of relamorelin or RM-493;
- •
- pay substantial damages for past use of the asserted intellectual property;
- •
- obtain a license from the holder of the asserted intellectual property, which license may not be available on reasonable
terms, if at all; and
- •
- in the case of trademark claims, rename our relamorelin or RM-493 product candidates.
Any of these risks coming to fruition could have a material adverse effect on our business, results of operations, financial condition and prospects.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may also be subject to claims that former employees, collaborators or other third parties have an ownership interest in our patents or other intellectual property. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, such intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The U.S. Patent and Trademark Office, or U.S. PTO, and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court.
If we or one of our licensing partners threatened or initiated legal proceedings against a third party to enforce a patent covering our product candidate, the defendant could claim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include alleged failures to meet any one of several statutory requirements, including novelty, non-obviousness and enablement. Grounds for unenforceability assertions include allegations that someone connected with prosecution of the patent withheld material information from the U.S. PTO, or made a misleading statement, during patent prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, inter partes review, post grant review and equivalent proceedings in foreign jurisdictions, for example, opposition proceedings. Such proceedings could result in revocation or amendment of our patents in such a way that they no longer cover our product candidates or competitive products. The outcome following legal assertions of invalidity and/or unenforceability is unpredictable. With respect to validity, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a
30
defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection would have a material adverse impact on our business.
We do not seek to protect our intellectual property rights in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection.
Filing, prosecuting and defending patents on product candidates in all countries and jurisdictions throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States could be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
We are dependent on licensed intellectual property. If we were to lose our rights to licensed intellectual property, we may not be able to continue developing or commercializing relamorelin, RM-493 or our other product candidates, if approved.
We have licensed our rights to relamorelin and RM-493 from Ipsen Pharma SAS, or Ipsen. Our licenses with Ipsen impose various obligations on us, and each provides Ipsen the right to terminate the license thereunder in the event of our material breach of the license agreement, our failure to initiate or complete development of a licensed product or our commencement of an action seeking to have an Ipsen licensed patent right declared invalid. Termination of our licenses from Ipsen would result in our loss of the right to use the licensed intellectual property, which would materially adversely affect our ability to develop and commercialize relamorelin or RM-493, as applicable, as well as harm our competitive business position and our business prospects.
We may enter into additional licenses to third-party intellectual property that are necessary or useful to our business. Future licensors may also allege that we have breached our license agreement and may accordingly seek to terminate our license with them. In addition, future licensors may have the right to terminate our license at will. Any termination could result in our loss of the right to use the licensed intellectual property, which could materially adversely affect our ability to develop and
31
commercialize a product candidate or product, if approved, as well as harm our competitive business position and our business prospects.
We have not yet registered trademarks for a commercial trade name for relamorelin or RM-493 and failure to secure such registrations could adversely affect our business.
We have not yet registered trademarks for a commercial trade name for relamorelin or RM-493. Any future trademark applications may be rejected during trademark registration proceedings. Although we would be given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the U.S. PTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. Moreover, any name we propose to use with our product candidates in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. If the FDA objects to any of our proposed proprietary product names, we may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA.
If we do not obtain additional protection under the Hatch-Waxman Amendments and similar foreign legislation by extending the patent terms and obtaining product exclusivity for relamorelin or RM-493, our business may be materially harmed.
Depending upon the timing, duration and specifics of FDA marketing approval for relamorelin or RM-493, one or more of the U.S. patents we license may be eligible for limited patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent term restoration of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, we may not be granted an extension because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or restoration or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our ability to generate revenues could be materially adversely affected.
While we believe that our product candidates contain active ingredients that would be treated by the FDA as new chemical entities, or new drug products, and, therefore, if approved, should be afforded five years of marketing exclusivity, the FDA may disagree with that conclusion and may approve generic products within a period that is less than five years. Manufacturers may seek to launch these generic products following the expiration of the applicable marketing exclusivity period, even if we still have patent protection for our product. Competition that our products may face from generic versions of our products could materially and adversely impact our future revenue, profitability and cash flows and substantially limit our ability to obtain a return on the investments we have made in those product candidates.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
The United States has recently enacted and is currently implementing the America Invents Act of 2011, wide-ranging patent reform legislation. Further, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain
32
circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain future patents, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the U.S. PTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents or future patents.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Our employees have been previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we are not aware of any claims currently pending against us, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of the former employers of our employees. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail in defending such claims, in addition to paying money claims, we may lose valuable intellectual property rights or personnel. A loss of key personnel or their work product could hamper or prevent our ability to commercialize relamorelin and RM-493, which would materially adversely affect our commercial development efforts.
Risks Related to Regulatory Approval and Marketing of Our Product Candidates and Other Legal Compliance Matters
Even if we complete the necessary clinical trials, the regulatory and marketing approval process is expensive, time consuming and uncertain and may prevent us from obtaining approvals for the commercialization of some or all of our product candidates. We depend almost entirely on the success of two product candidates, relamorelin and RM-493, which are still in Phase 2 clinical development. We cannot be certain that we will be able to obtain regulatory approval for, or successfully commercialize, relamorelin and RM-493. If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired.
We currently have only two product candidates, relamorelin and RM-493, in clinical development, and our business depends almost entirely on their successful clinical development, regulatory approval and commercialization. We currently have no drug products for sale and may never be able to develop marketable drug products. Relamorelin, which is currently in Phase 2 clinical development as a treatment for diabetic gastroparesis and other GI functional disorders, and RM-493, which is currently in Phase 2a clinical development as a treatment for genetic deficiencies in the MC4 pathway, will each require substantial additional clinical development, testing and regulatory approval before we are permitted to commence their commercialization. The clinical trials of our product candidates are, and the manufacturing and marketing of our product candidates will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where we intend to test and, if approved, market any product candidate. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must demonstrate through non-clinical testing and clinical trials that the product candidate is safe and effective for use in each target indication. This process can take many years and approval, if any, may be conditional on post-marketing studies and surveillance, and will require the expenditure of substantial resources beyond the proceeds we raise in this offering. Of the large number of drugs in development in the United States, only a small percentage will successfully complete the FDA regulatory approval process and will be commercialized. In addition, we have not discussed all of our proposed development programs with the FDA and the FDA may determine that a specific indication, such as constipation in patients with Parkinson's disease, is inappropriate for drug approval and require us to seek approval in a broader population to obtain a constipation claim. Accordingly, even if we are able to obtain the
33
requisite financing to continue to fund our development and clinical trials, we cannot assure you that relamorelin, RM-493 or any of our other product candidates will be successfully developed or commercialized.
We are not permitted to market relamorelin or RM-493 in the United States until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries. Relamorelin has already completed a Phase 2 clinical trial for the treatment of diabetic gastroparesis in which the drug had significant effects on both stomach emptying and vomiting symptoms. We expect that the FDA will require us to conduct at least two Phase 3 clinical trials in order to submit an NDA for relamorelin as a treatment for diabetic gastroparesis, and other Phase 3 trials to submit an NDA for other GI functional disorders. The FDA or other regulatory authorities may also require that we conduct additional pivotal trials. Pursuant to the FDA's February 2007 draft guidance on the development of weight management drugs, in order to reasonably estimate the safety of a weight-management drug, Phase 3 clinical trials must randomize approximately 3,000 subjects to active doses of the product and 1,500 subjects to placebo for one year of treatment. Our focus is not to develop RM-493 for the general category of weight management drugs, but we expect to seek an indication for obesity caused by genetic deficiencies in the MC4 pathway. If RM-493 produces significant treatment effects in these patients, coupled with an acceptable safety profile, our overall clinical program may be less time consuming and require fewer patients than a program for a broader obesity indication. The FDA has advised us, however, that such a program would require development of a systematic and clearly defined approach to classifying patients' various mutations and selecting patients with mutations of unquestionable clinical relevance. In addition we would be required to demonstrate the predictive utility of MC4 genotype related to RM-493-induced weight loss. For example, we may need to show that RM-493 works better in the heterozygote than the wild-type, or genetically normal, population. Alternatively, we may need to demonstrate that RM-493 provides effective treatment for patients who fail to achieve weight loss on an already approved anti-obesity medication in the heterozygous population but not in the general population, thereby showing an advantage to the administration of RM-493 as well as identifying a population of MC4 heterozygotes. Either of these scenarios would require that we study both our intended patient population as well as the general obesity population and could prove costly and time consuming. In addition, the FDA may not agree with our plans, may not support the size and length of our proposed clinical development program, and may not allow us to gain an indication for these genetic causes of obesity. In addition, the FDA may impose additional requirements beyond the February 2007 draft guidance in order to support approval. For example, the FDA has required other sponsors to conduct large CVOTs to rule out excess cardiovascular risk. Overall, it is not yet possible to determine the size, duration, endpoints, and definition of success for our approach. We have not yet commenced any of these clinical trials.
Our plan is to expand our internal clinical development operations and capabilities so that we can enroll and manage our Phase 2b and Phase 3 clinical trials, if any, of relamorelin for the treatment of diabetic gastroparesis such that, if the clinical trials are successful, we can file an NDA in the United States by 2018. Based on initial discussions with the FDA, we anticipate that the pivotal Phase 3 clinical trials required for regulatory approval in the United States of relamorelin for the treatment of diabetic gastroparesis will assess efficacy based on 12 weeks of treatment. However, we have not finalized the design, timing and size of our Phase 3 trials with the FDA and therefore cannot assure you that they will start on time. In addition, obtaining approval of an NDA is a complex, lengthy, expensive and uncertain process, and the FDA may delay, limit or deny approval of relamorelin and RM-493 for many reasons, including, among others:
- •
- the FDA may disagree with our clinical trial design and our interpretation of data from clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design for our clinical trials;
34
- •
- we may not be able to demonstrate that relamorelin is safe and effective in treating diabetic gastroparesis and GI
functional disorders and that RM-493 is safe and effective in treating obesity caused by certain genetic deficiencies in the MC4 pathway, to the satisfaction of the FDA;
- •
- the results of our clinical trials may not be interpretable or meet the level of statistical or clinical significance
required by the FDA for marketing approval. For example, the potential unblinding of RM-493 studies due to easily identifiable adverse events may raise the concern that potential bias has affected the
clinical trial results;
- •
- the FDA may disagree with the number, design, size, conduct or implementation of our clinical trials;
- •
- the FDA may require that we conduct additional clinical trials;
- •
- the FDA or the applicable foreign regulatory agency may identify deficiencies in our chemistry, manufacturing or controls
of relamorelin or RM-493;
- •
- the CROs that we retain to conduct our clinical trials may take actions outside of our control that materially adversely
impact our clinical trials;
- •
- the FDA may find the data from preclinical studies and clinical trials insufficient to demonstrate that relamorelin or
RM-493's clinical and other benefits outweigh its safety risks;
- •
- the FDA may disagree with our interpretation of data from our preclinical studies and clinical trials;
- •
- the FDA may not accept data generated at our clinical trial sites;
- •
- if our NDA, if and when submitted, is reviewed by an advisory committee, the FDA may have difficulties scheduling an
advisory committee meeting in a timely manner or the advisory committee may recommend against approval of our application or may recommend that the FDA require, as a condition of approval, additional
preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions;
- •
- the FDA or the applicable foreign regulatory agency may deem our manufacturing processes or facilities inadequate to
preserve the identity, strength, quality, purity, or potency of our products; or
- •
- the FDA may change its approval policies or adopt new regulations and guidance.
Any of these factors, many of which are beyond our control, could jeopardize our ability to obtain regulatory approval for and successfully market relamorelin and RM-493. Moreover, because our business is almost entirely dependent upon these product candidates, any such setback in our pursuit of regulatory approval would have a material adverse effect on our business and prospects.
Our Fast Track designation may not lead to a faster development or regulatory review or approval process and, moreover, does not assure FDA approval of our product.
The FDA has granted Fast Track designation to relamorelin for the treatment of diabetic gastroparesis. However, even with this designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures and there is no assurance that relamorelin will be approved by the FDA. The FDA may withdraw Fast Track designation if the FDA believes that the designation is no longer supported by data from our clinical development program. Fast Track designation does not guarantee that a sponsor will qualify for or be able to take advantage of any FDA expedited review procedures.
35
Our failure to obtain marketing approval in foreign jurisdictions would prevent our product candidates from being marketed abroad, and any approval we are granted for our product candidates in the United States would not assure approval of such product candidates in foreign jurisdictions.
In order to market and sell relamorelin, RM-493 and any other product candidate that we may develop in the European Union and many other jurisdictions, we or our third-party collaborators must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be sold in that country. We or these third parties may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market.
Even if we obtain marketing approval for relamorelin and RM-493, the terms of approvals and ongoing regulation of our products may limit how we manufacture and market our products and compliance with such requirements may involve substantial resources, which could materially impair our ability to generate revenue.
Even if we receive marketing approval for relamorelin and RM-493, regulatory authorities may impose significant restrictions on relamorelin and RM-493's indicated uses or marketing or impose ongoing requirements for potentially costly post-approval studies. Relamorelin and RM-493 will also be subject to ongoing FDA requirements governing the labeling, packaging, storage and promotion of these products and recordkeeping and submission of safety and other post-market information. The FDA has significant post-market authority, including, for example, the authority to require labeling changes based on new safety information and to require post-market studies or clinical trials to evaluate serious safety risks related to the use of a drug. The FDA also has the authority to require, as part of an NDA or post-approval, the submission of a REMS. Any REMS required by the FDA may lead to increased costs to assure compliance with new post-approval regulatory requirements and potential requirements or restrictions on the sale of approved products, all of which could lead to lower sales volume and revenue.
Manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMPs and other regulations. If we or a regulatory agency discover problems with relamorelin or RM-493, such as adverse events of unanticipated severity or frequency, or problems with the facility where relamorelin or RM-493 is manufactured, a regulatory agency may impose restrictions on relamorelin or RM-493, the manufacturer or us, including requiring withdrawal of relamorelin or RM-493 from the market or suspension of manufacturing. If we, relamorelin or RM-493 or the manufacturing facilities for relamorelin or RM-493 fail to comply with applicable regulatory requirements, a regulatory agency may, among other things:
- •
- issue warning letters or untitled letters;
- •
- seek an injunction or impose civil or criminal penalties or monetary fines;
- •
- suspend or withdraw marketing approval;
- •
- suspend any ongoing clinical trials;
- •
- refuse to approve pending applications or supplements to applications submitted by us;
36
- •
- suspend or impose restrictions on operations, including costly new manufacturing requirements; or
- •
- seize or detain products, refuse to permit the import or export of products, or request that we initiate a product recall.
Accordingly, assuming we receive marketing approval for one or more of our product candidates, we and our contract manufacturers will continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance and quality control. If we are not able to comply with post-approval regulatory requirements, we could have the marketing approvals for our products withdrawn by regulatory authorities and our ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Thus, the cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
We are subject to healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and others will play a primary role in the recommendation and prescription of relamorelin and RM-493, if approved. Our future arrangements with third-party payors will expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute relamorelin and RM-493, if we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include the following:
- •
- The federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully
soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or
recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid.
- •
- The federal False Claims Act imposes criminal and civil penalties, including those from civil whistleblower or qui tam
actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to
avoid, decrease, or conceal an obligation to pay money to the federal government.
- •
- The federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology
for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory
contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information.
- •
- The federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material
fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services.
- •
- The federal transparency requirements, sometimes referred to as the "Sunshine Act," under the Patient Protection and
Affordable Care Act, require manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to physician payments and
other transfers of value and physician ownership and investment interests.
- •
- Analogous state laws and regulations, such as state anti-kickback and false claims laws and transparency laws, may apply to sales or marketing arrangements and claims involving healthcare
37
items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures and drug pricing.
Ensuring that our future business arrangements with third parties comply with applicable healthcare laws and regulations could be costly. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations, including anticipated activities to be conducted by our sales team, were found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines and exclusion from government funded healthcare programs, such as Medicare and Medicaid, any of which could substantially disrupt our operations. If any of the physicians or other providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Any product candidate for which we obtain marketing approval will be subject to strict enforcement of post-marketing requirements and we could be subject to substantial penalties, including withdrawal of our product from the market, if we fail to comply with all regulatory requirements.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include, but are not limited to, restrictions governing promotion of an approved product, submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, and requirements regarding the distribution of samples to physicians and recordkeeping. The FDA and other federal and state agencies, including the Department of Justice, closely regulate compliance with all requirements governing prescription drug products, including requirements pertaining to marketing and promotion of drugs in accordance with the provisions of the approved labeling and manufacturing of products in accordance with cGMP requirements. Violations of such requirements may lead to investigations alleging violations of the Federal Food, Drug, and Cosmetic Act and other statutes, including the False Claims Act and other federal and state health care fraud and abuse laws as well as state consumer protection laws.
For example, the FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, such as relamorelin and RM-493, if approved. In particular, a product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product's approved labeling. If we receive marketing approval for relamorelin as a treatment for diabetic gastroparesis and other GI functional disorders and RM-493 as a treatment for obesity caused by certain genetic deficiencies in the MC4 pathway, physicians may nevertheless prescribe relamorelin and RM-493 to their patients in a manner that is inconsistent with the approved labeling. If we are found to have promoted such off-label uses, we may become subject to significant liability. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. If we cannot successfully manage the promotion of relamorelin and RM-493, if approved, we could become subject to significant liability, which would materially adversely affect our business and financial condition.
38
Our employees may engage in misconduct or other improper activities, including violating applicable regulatory standards and requirements or engaging in insider trading, which could significantly harm our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with the regulations of the FDA and applicable non-U.S. regulators, provide accurate information to the FDA and applicable non-U.S. regulators, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of, including trading on, information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We have adopted a code of conduct, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may be ineffective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
Our future growth depends, in part, on our ability to penetrate foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future profitability will depend, in part, on our ability to commercialize relamorelin and RM-493 in foreign markets for which we intend to rely on collaboration with third parties. If we commercialize relamorelin and RM-493 in foreign markets, we would be subject to additional risks and uncertainties, including:
- •
- our customers' ability to obtain reimbursement for relamorelin and RM-493 in foreign markets;
- •
- our inability to directly control commercial activities because we are relying on third parties;
- •
- the burden of complying with complex and changing foreign regulatory, tax, accounting and legal requirements;
- •
- different medical practices and customs in foreign countries affecting acceptance in the marketplace;
- •
- import or export licensing requirements;
- •
- longer accounts receivable collection times;
- •
- longer lead times for shipping;
- •
- language barriers for technical training;
- •
- reduced protection of intellectual property rights in some foreign countries;
- •
- foreign currency exchange rate fluctuations; and
- •
- the interpretation of contractual provisions governed by foreign laws in the event of a contract dispute.
Foreign sales of relamorelin and RM-493 could also be adversely affected by the imposition of governmental controls, political and economic instability, trade restrictions and changes in tariffs.
39
Laws and regulations governing any international operations we may have in the future may preclude us from developing, manufacturing and selling certain product candidates and products outside of the United States and require us to develop and implement costly compliance programs.
If we expand our operations outside of the United States, we must dedicate additional resources to comply with numerous laws and regulations in each jurisdiction in which we plan to operate. The Foreign Corrupt Practices Act of 1977, or the FCPA, prohibits any U.S. individual or business from paying, offering, authorizing payment or offering anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of such third party in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the company, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the FCPA presents particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials. Certain payments to hospitals in connection with clinical trials and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions.
Various laws, regulations and executive orders also restrict the use and dissemination outside of the United States, or the sharing with certain non-U.S. nationals, of information classified for national security purposes, as well as certain products and technical data relating to those products. If we expand our presence outside of the United States, it will require us to dedicate additional resources to comply with these laws, and these laws may preclude us from developing, manufacturing or selling certain product candidates and products outside of the United States, which could limit our growth potential and increase our development costs.
The failure to comply with laws governing international business practices may result in substantial civil and criminal penalties and suspension or debarment from government contracting. The Securities and Exchange Commission also may suspend or bar issuers from trading securities on U.S. exchanges for violations of the FCPA's accounting provisions.
Risks Related to Employee Matters and Managing Growth
Our future success depends on our ability to retain our key employees and consultants, and to attract, retain and motivate qualified personnel.
We are highly dependent on Keith M. Gottesdiener, M.D., our Chief Executive Officer, Bart Henderson, our President, Lex H.T. Van der Ploeg, Ph.D., our Chief Scientific Officer, and Elizabeth Stoner, M.D., our Chief Medical Officer. We have entered into employment agreements with Dr. Gottesdiener, Mr. Henderson, and Dr. Van der Ploeg, but they may terminate their employment with us at any time. Dr. Stoner is currently a consultant and, while we have entered into a consulting arrangement with her, she may terminate her relationship with us at any time. Although we do not have any reason to believe that we will lose the services of any of our key employees or consultants in the foreseeable future, the loss of their services might impede the achievement of our research, development and commercialization objectives. We also do not have any key-man life insurance on any of our key employees. We rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us and may not be subject to our standard non-compete agreements. Recruiting and retaining qualified scientific personnel and sales and
40
marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. Failure to succeed in clinical trials may make it more challenging to recruit and retain qualified scientific personnel.
We will need to develop and expand our company, and we may encounter difficulties in managing this development and expansion, which could disrupt our operations.
As of June 30, 2014, we had three full-time employees and one part-time employee, and in connection with becoming a public company, we expect to increase our number of employees and the scope of our operations. To manage our anticipated development and expansion, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Also, our management may need to divert a disproportionate amount of its attention away from their day-to-day activities and devote a substantial amount of time to managing these development activities. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. This may result in weaknesses in our infrastructure, and give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. The physical expansion of our operations may lead to significant costs and may divert financial resources from other projects, such as the development of relamorelin and RM-493. If our management is unable to effectively manage our expected development and expansion, our expenses may increase more than expected, our ability to generate or increase our revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize relamorelin and RM-493, if approved, and compete effectively will depend, in part, on our ability to effectively manage the future development and expansion of our company.
In order to satisfy our obligations as a public company, we will need to hire additional qualified accounting and financial personnel with appropriate public company experience.
As a newly public company, we will need to establish and maintain effective disclosure and financial controls and make changes in our corporate governance practices. We will need to hire additional accounting and financial personnel with appropriate public company experience and technical accounting knowledge, and it may be difficult to recruit and maintain such personnel. Even if we are able to hire appropriate personnel, our existing operating expenses and operations will be impacted by the direct costs of their employment and the indirect consequences related to the diversion of management resources from product development efforts.
Unfavorable global economic conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. The recent global financial crisis caused extreme volatility and disruptions in the capital and credit markets. A severe or prolonged economic downturn, such as the recent global financial crisis, could result in a variety of risks to our business, including weakened demand for our product candidates and our ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could also strain our suppliers, possibly resulting in supply disruption, or cause our customers to delay making payments for our services. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
41
Our internal computer systems, or those of our third-party CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our relamorelin and RM-493 development programs.
Despite the implementation of security measures, our internal computer systems and those of our third-party CROs and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we have not experienced any such system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our programs. For example, the loss of clinical trial data for relamorelin and RM-493 could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications or other data or applications relating to our technology or product candidates, or inappropriate disclosure of confidential or proprietary information, we could incur liabilities and the further development of relamorelin and RM-493 could be delayed.
We may acquire businesses or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions.
We may acquire additional businesses or products, form strategic alliances or create joint ventures with third parties that we believe will complement or augment our existing business. If we acquire businesses with promising markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing, manufacturing and marketing any new products resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business. We cannot assure you that, following any such acquisition, we will achieve the expected synergies to justify the transaction.
Risks Related to Our Common Stock and This Offering
Our executive officers, directors, principal stockholders and their affiliates will continue to exercise significant control over our company after this offering, which will limit your ability to influence corporate matters and could delay or prevent a change in corporate control.
Upon the completion of this offering, the existing holdings of our executive officers, directors, principal stockholders and their affiliates, including Pfizer Inc., investment funds affiliated with MPM Capital, investment funds affiliated with Third Rock Ventures, and investment funds affiliated with New Enterprise Associates will represent beneficial ownership, in the aggregate, of approximately % of our outstanding common stock, assuming no exercise of the underwriters' option to acquire additional common stock in this offering and assuming we issue the number of shares of common stock as set forth on the cover page of this prospectus. As a result, these stockholders, if they act together, will be able to influence our management and affairs and control the outcome of matters submitted to our stockholders for approval, including the election of directors and any sale, merger, consolidation, or sale of all or substantially all of our assets. These stockholders acquired their shares of common stock for substantially less than the price of the shares of common stock being acquired in this offering, and these stockholders may have interests, with respect to their common stock, that are different from those of investors in this offering and the concentration of voting power among these stockholders may have an adverse effect on the price of our common stock. In addition, this concentration of ownership might adversely affect the market price of our common stock by:
- •
- delaying, deferring or preventing a change of control of us;
- •
- impeding a merger, consolidation, takeover or other business combination involving us; or
42
- •
- discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us.
See "Principal Stockholders" in this prospectus for more information regarding the ownership of our outstanding common stock by our executive officers, directors, principal stockholders and their affiliates.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, even one that may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Upon completion of the Conversion, we will be a Delaware corporation. Provisions in our amended and restated certificate of incorporation and amended and restated bylaws may delay or prevent an acquisition of us or a change in our management. In addition, because we will be incorporated in Delaware, we will be governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us. Although we believe these provisions collectively will provide for an opportunity to obtain greater value for stockholders by requiring potential acquirers to negotiate with our board of directors, they would apply even if an offer rejected by our board were considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
If you purchase our common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
You will suffer immediate and substantial dilution in the net tangible book value of the common stock you purchase in this offering. Assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, purchasers of common stock in this offering will experience immediate dilution of $ per share in net tangible book value of the common stock. In addition, investors purchasing common stock in this offering will contribute % of the total amount invested by stockholders since inception but will only own % of the shares of common stock outstanding. See "Dilution" for a more detailed description of the dilution to new investors in the offering.
An active trading market for our common stock may not develop, and you may not be able to resell your shares at or above the initial public offering price.
Prior to this offering, there has been no public market for shares of our common stock. Although we anticipate our common stock will be approved for listing on The NASDAQ Global Market, an active trading market for our shares may never develop or be sustained following this offering. The initial public offering price of our common stock will be determined through negotiations between us and the underwriters. This initial public offering price may not be indicative of the market price of our common stock after this offering. In the absence of an active trading market for our common stock, investors may not be able to sell their common stock at or above the initial public offering price or at the time that they would like to sell.
If securities or industry analysts do not publish research or reports or publish unfavorable research or reports about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. We do not currently have and may never obtain research coverage by securities and industry analysts. If no
43
securities or industry analysts commence coverage of our company, the trading price for our stock would be negatively impacted. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our stock, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to regularly publish reports on us, interest in our stock could decrease, which could cause our stock price or trading volume to decline.
Market volatility may affect our stock price and the value of your investment.
Following this offering, the market price for our common stock is likely to be volatile, in part because our common stock has not been previously traded publicly. In addition, the market price of our common stock may fluctuate significantly in response to a number of factors, most of which we cannot control, including, among others:
- •
- plans for, progress of, or results from preclinical studies and clinical trials of relamorelin and RM-493;
- •
- the failure of the FDA to approve relamorelin or RM-493;
- •
- announcements of new products, technologies, commercial relationships, acquisitions or other events by us or our
competitors;
- •
- the success or failure of other GI functional or weight loss therapies;
- •
- regulatory or legal developments in the United States and other countries;
- •
- failure of relamorelin or RM-493, if approved, to achieve commercial success;
- •
- fluctuations in stock market prices and trading volumes of similar companies;
- •
- general market conditions and overall fluctuations in U.S. equity markets;
- •
- variations in our quarterly operating results;
- •
- changes in our financial guidance or securities analysts' estimates of our financial performance;
- •
- changes in accounting principles;
- •
- our ability to raise additional capital and the terms on which we can raise it;
- •
- sales of large blocks of our common stock, including sales by our executive officers, directors and significant
stockholders;
- •
- additions or departures of key personnel;
- •
- discussion of us or our stock price by the press and by online investor communities; and
- •
- other risks and uncertainties described in these risk factors.
Our quarterly operating results may fluctuate significantly.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
- •
- variations in the level of expenses related to our development programs;
- •
- addition or termination of clinical trials;
- •
- any intellectual property infringement lawsuit in which we may become involved;
- •
- regulatory developments affecting our product candidates;
- •
- our execution of any collaborative, licensing or similar arrangements, and the timing of payments we may make or receive under these arrangements;
44
- •
- the achievement and timing of milestone payments under our existing collaboration and license agreements; and
- •
- if relamorelin or RM-493 receives regulatory approval, the level of underlying demand for that product and wholesalers' buying patterns.
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially.
We will have broad discretion in how we use the proceeds of this offering. We may not use these proceeds effectively, which could affect our results of operations and cause our stock price to decline.
We will have considerable discretion in the application of the net proceeds of this offering. We intend to use the net proceeds from this offering to advance the clinical development of relamorelin as a treatment for diabetic gastroparesis and other GI functional disorders and RM-493 as a treatment for obesity caused by certain genetic deficiencies in the MC4 pathway and to fund working capital and other general corporate purposes, which may include funding for the hiring of additional personnel, capital expenditures and the costs of operating as a public company. As a result, investors will be relying upon management's judgment with only limited information about our specific intentions for the use of the balance of the net proceeds of this offering. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
Our ability to use certain net operating loss carryovers and other tax attributes may be limited.
Our corporate subsidiaries have incurred substantial losses during our history and we do not expect to become profitable in the near future and may never achieve profitability. Under the Internal Revenue Code of 1986, as amended, or the Code, a corporation is generally allowed a deduction for net operating losses carried over from a prior taxable year. Under that provision, we can carry forward certain taxable losses of our subsidiaries to offset future taxable income, if any, until such losses are used or expire. The same is true of other unused tax attributes, such as research tax credits. As of December 31, 2013, we had approximately $47.0 million of unused carryforwards of net operating losses, or NOLs, and approximately $2.1 million of unused carryforwards of tax credits.
If a corporation undergoes an "ownership change," generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, Sections 382 and 383 of the Code, limit the corporation's ability to use carryovers of its pre-change NOLs, credits and certain other tax attributes to reduce its tax liability for periods after the ownership change. Our issuance of common stock pursuant to this offering may result in a limitation under Sections 382 and 383, either separately or in combination with certain prior or subsequent shifts in the ownership of our common stock. As a result, our ability to use carryovers of pre-change NOLs and credits to reduce our future U.S. federal income tax liability may be subject to limitations. This could result in increased U.S. federal income tax liability for us if we generate taxable income in a future period. Limitations on the use of NOLs and other tax attributes could also increase our state tax liability.
The use of our tax attributes will also be limited to the extent that we do not generate positive taxable income in future tax periods. We do not expect to generate positive taxable income in the near future and we may never achieve tax profitability.
45
Sales of a substantial number of shares of our common stock in the public market could cause our stock price to fall.
If those stockholders who hold shares of common stock immediately following the Conversion sell, or indicate an intention to sell, substantial amounts of our common stock in the public market after the lock-up and other legal restrictions on resale discussed in this prospectus lapse, the market price of our common stock could decline and our ability to raise adequate capital through the sale of additional equity securities could be impaired. Upon completion of this offering, there will be shares of our common stock outstanding, assuming no exercise of the underwriters' over-allotment option. Of these shares, as of the date of this prospectus, approximately shares of our common stock, plus any shares sold upon exercise of the underwriters' over-allotment option, will be freely tradable, without restriction, in the public market immediately following this offering (except for shares purchased by affiliates), and the remaining shares may be sold upon expiration of the lock-up agreements pertaining to this offering 180 days after the date of this offering (subject in some cases to volume limitations). The representatives of the underwriters, however, may, in their sole discretion, permit our officers, directors and other stockholders who are subject to these lock-up agreements to sell shares prior to the expiration of the lock-up agreements.
After the lock-up agreements expire, based upon the number of shares of common stock, on an as-converted basis, outstanding as of , 2014, up to an additional shares of common stock will be eligible for sale in the public market, of which shares are held by directors, executive officers and other affiliates and will be subject to Rule 144 under the Securities Act of 1933, as amended, or the Securities Act.
In addition, as of , 2014, shares of common stock that are reserved for future issuance under our equity incentive plans will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements and Rule 144 and Rule 701 under the Securities Act. If these additional shares of common stock are sold, or if it is perceived that they will be sold, in the public market, the market price of our common stock could decline.
After this offering, the holders of approximately 147,710,000 shares of our common stock will be entitled to rights with respect to the registration of their shares under the Securities Act, subject to the lock-up agreements described above, including requiring us to file registration statements covering the shares of our common stock they hold or to include their shares in registration statements that we may file for ourselves or other stockholders. See "Description of Capital Stock—Registration Rights" in this prospectus for more information regarding these registration rights. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates. Any sales of securities by these stockholders could have a material adverse effect on the market price of our common stock.
We also intend to register all the shares of common stock that we may issue under our equity incentive plans. Effective upon the effectiveness of the registration statement of which this prospectus is a part, an aggregate of shares of our common stock will be reserved for future issuance under these plans. Once we register these shares, which we plan to do shortly after the completion of this offering, they can be freely sold in the public market upon issuance, subject to the lock-up agreements referred to above. If a large number of these shares are sold in the public market, the sales could reduce the trading price of our common stock. See "Shares Eligible for Future Sale" for a more detailed description of sales that may occur in the future.
We are an "emerging growth company," and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are an "emerging growth company" as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other
46
public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. If we choose not to comply with the auditor attestation requirements of Section 404, our auditors will not be required to attest to the effectiveness of our internal control over financial reporting. As a result, investors may become less comfortable with the effectiveness of our internal controls and the risk that material weaknesses or other deficiencies in our internal controls go undetected or may increase. If we choose to provide reduced disclosures in our periodic reports and proxy statements while we are an emerging growth company, investors would have access to less information and analysis about our executive compensation, which may make it difficult for investors to evaluate our executive compensation practices. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an "emerging growth company." We will remain an "emerging growth company" until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of this offering, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporation governance policies.
As a public company, and particularly after we are no longer an emerging growth company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The NASDAQ Global Market and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified members of our board of directors.
We are evaluating these rules and regulations, and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
For as long as we remain an emerging growth company, we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not applicable to emerging growth companies as described in the preceding risk factor.
Pursuant to Section 404, we will be required to furnish a report by our management on our internal control over financial reporting. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued
47
by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses in our internal control over financial reporting, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
We do not intend to pay dividends on our common stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid any cash dividend on our common stock and do not currently intend to do so in the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. Therefore, the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which you purchased them.
48
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements, which reflect our current views with respect to, among other things, our operations and financial performance. You can identify these forward-looking statements by the use of words such as "outlook," "believes," "expects," "potential," "continues," "may," "will," "should," "seeks," "approximately," "predicts," "intends," "plans," "estimates," "anticipates" or the negative version of these words or other comparable words. Such forward-looking statements are subject to various risks and uncertainties. Accordingly, there are or will be important factors that could cause actual outcomes or results to differ materially from those indicated in these statements. We believe these factors include but are not limited to those described under "Risk Factors" and include, among other things:
- •
- the success, cost and timing of our product development activities and clinical trials;
- •
- our ability to obtain and maintain regulatory approval for relamorelin, RM-493, and any of our future product candidates,
and any related restrictions, limitations, and/or warnings in the label of an approved product candidate;
- •
- our ability to obtain funding for our operations;
- •
- the commercialization of our product candidates, if approved;
- •
- our plans to research, develop and commercialize our product candidates;
- •
- our ability to attract collaborators with development, regulatory and commercialization expertise;
- •
- our expectations regarding our ability to obtain and maintain intellectual property protection for our product
candidates.
- •
- future agreements with third parties in connection with the commercialization of relamorelin, RM-493 or any of our future
product candidates;
- •
- the size and growth potential of the markets for our product candidates, and our ability to serve those markets;
- •
- the rate and degree of market acceptance of our product candidates, as well as the reimbursement coverage for our product
candidates;
- •
- regulatory developments in the United States and foreign countries;
- •
- the performance of our third-party suppliers and manufacturers;
- •
- the success of competing therapies that are or may become available;
- •
- our ability to attract and retain key scientific or management personnel;
- •
- the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional
financing;
- •
- our expectations regarding the period during which we qualify as an emerging growth company under the JOBS Act; and
- •
- our use of the proceeds from this offering.
These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in this prospectus. We undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise.
49
MARKET, INDUSTRY AND OTHER DATA
This prospectus includes market, industry and other data and forecasts that we have derived from independent consultant reports, publicly available information, various industry publications, other published industry sources and our internal data and estimates. Independent consultant reports, industry publications and other published industry sources generally indicate that the information contained therein was obtained from sources believed to be reliable.
Our internal data and estimates are based upon information obtained from trade and business organizations and other contacts in the markets in which we operate and our management's understanding of industry conditions.
50
We estimate that the net proceeds to us from the sale of the shares of our common stock offered by us will be approximately $ million, based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting underwriting discounts and commissions. If the underwriters' option to purchase additional shares in this offering is exercised in full, we estimate that our net proceeds will be approximately $ million, after deducting underwriting discounts and commissions.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the net proceeds to us from this offering by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions. Similarly, each increase (decrease) of one million shares in the number of shares of common stock offered by us would increase (decrease) the net proceeds to us from this offering by approximately $ million, assuming the assumed initial public offering price remains the same and after deducting underwriting discounts and commissions.
As of June 30, 2014, we had cash, cash equivalents and marketable securities of $9.3 million. We intend to use the net proceeds from this offering, together with our existing cash resources, as follows:
- •
- approximately $ million to fund the manufacturing and development of relamorelin in connection with
our Phase 2 clinical trials and planned Phase 3 clinical trials for the treatment of diabetic gastroparesis through at least the initiation of Phase 3 clinical trials;
- •
- approximately $ million to fund the manufacturing and development of RM-493 through completion of
our Phase 2a clinical trials for the treatment of MC4 heterozygous obesity and Prader Willi Syndrome; and
- •
- the remainder for working capital purposes, including general operating expenses.
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the closing of this offering, or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of the net proceeds will vary depending on numerous factors, including the progress of our clinical trials and other development efforts for relamorelin and other factors described in "Risk Factors" beginning on page 10, as well as the amount of cash we use in our operations. As a result, our management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds of this offering. In addition, we might decide to postpone or not pursue clinical trials or preclinical activities if the net proceeds from this offering and the other sources of cash are less than expected.
Pending application of the net proceeds, we intend to invest the net proceeds from this offering in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
51
We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any dividends on our common stock in the foreseeable future. Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant.
52
Rhythm Holding Company, LLC, or the LLC entity, was formed on March 21, 2013 as a holding company in connection with a corporate restructuring, or the Prior Restructuring, by Rhythm Pharmaceuticals, Inc., a Delaware corporation that was organized in November 2008. Rhythm Pharmaceuticals, Inc. changed its name to Rhythm Health, Inc., or Rhythm Health, on July 7, 2014. Rhythm Health and Rhythm Metabolic, Inc., or Rhythm Metabolic, a Delaware corporation organized on March 21, 2013, became the wholly owned subsidiaries of the LLC entity as a result of the exchange of equity transactions described further below in connection with the Prior Restructuring. As part of the Prior Restructuring, Rhythm Health contributed all of its patent rights and other intellectual property rights with respect to RM-493 and Rhythm Health's related MC4 program to Rhythm Metabolic. As a result of the Prior Restructuring, our two product candidates and their related programs were separated and each is held by a different legal entity, and the LLC entity became the holding company for both of these legal entities. Our wholly owned subsidiary, Rhythm Health, holds all of our patent rights and other intellectual property rights with respect to relamorelin and our related ghrelin agonist program, and our wholly owned subsidiary, Rhythm Metabolic, holds all of our patent rights and other intellectual property rights with respect to RM-493 and our related MC4 agonist program.
The Prior Restructuring involved the following transactions:
- •
- each outstanding share of series A preferred stock of Rhythm Health was exchanged for one series A
preferred unit of the LLC entity;
- •
- each outstanding share of series B preferred stock of Rhythm Health was exchanged for one series B
preferred unit of the LLC entity;
- •
- each outstanding share of common stock of Rhythm Health was exchanged for one common unit of the LLC entity, and if such
outstanding share of common stock was restricted stock subject to vesting at the time of such exchange, then such common unit was issued by the LLC entity subject to continued vesting to the same
extent as such outstanding share of restricted stock was still subject to vesting;
- •
- each outstanding stock option to purchase a share of common stock of Rhythm Health was cancelled and the LLC entity
issued to the holder of such cancelled stock option one common unit of the LLC entity, and if such outstanding stock option was subject to vesting at the effective time of its cancellation, then such
common unit was issued by the LLC entity subject to continued vesting to the same extent as such outstanding stock option was still subject to vesting at the effective time of its cancellation;
- •
- each outstanding warrant to purchase common stock was adjusted to become a warrant to purchase an equivalent number of
common units; and
- •
- Rhythm Health distributed to the LLC entity all outstanding shares of capital stock of Rhythm Metabolic, which was originally organized as a wholly owned subsidiary of Rhythm Health, resulting in Rhythm Metabolic becoming a direct wholly owned subsidiary of the LLC entity just like Rhythm Health.
53
The charts below show our organizational structure upon the consummation of the Prior Restructuring and immediately prior to and immediately following the conversion of the LLC entity to a corporation, as described below under "Conversion."
| Pre-Conversion | Post-Conversion | |
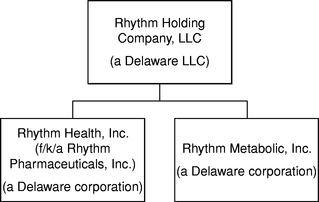
|
|
Prior to the effectiveness of the registration statement of which this prospectus forms a part, we will engage in the following transactions, which we refer to collectively as the Conversion:
- •
- we will convert from a Delaware limited liability company to a Delaware corporation by filing a certificate of conversion
with the Secretary of State of the State of Delaware; and
- •
- we will change our name from Rhythm Holding Company, LLC to Rhythm Pharmaceuticals, Inc.
As part of the Conversion:
- •
- each outstanding series A preferred unit will be converted into one share of our series A preferred stock;
- •
- each outstanding series B preferred unit will be converted into one share of our series B preferred stock;
- •
- each outstanding common unit will be converted into one share of common stock, and if such outstanding common unit was a
restricted common unit subject to vesting at the time of the Conversion then the share of common stock into which such outstanding common unit will convert will be subject to continued vesting to the
same extent as such outstanding common unit was subject to vesting; and
- •
- each outstanding warrant to purchase common units will be adjusted to become a warrant to purchase an equivalent number of shares of common stock.
After converting to a corporation and changing our name to Rhythm Pharmaceuticals, Inc., we will initially be governed by a certificate of incorporation to be filed with the Delaware Secretary of State and amended and restated bylaws. On the effective date of the Conversion, the members of the board of managers of Rhythm Holding Company, LLC will become the members of the board of directors of Rhythm Pharmaceuticals, Inc. and the officers of Rhythm Holding Company, LLC will become the officers of Rhythm Pharmaceuticals, Inc.
54
Upon the closing of this offering, all outstanding shares of our series A preferred stock and series B preferred stock will be automatically converted into shares of our common stock pursuant to the terms of our certificate of incorporation and we will file an amended and restated certificate of incorporation to reflect the conversion of our series A preferred stock and our series B preferred stock. The material portions of our amended and restated certificate of incorporation and amended and restated bylaws are described in "Description of Capital Stock."
For the convenience of the reader, except as the context otherwise requires, all information included in this prospectus is presented giving effect to the Conversion.
55
The following table sets forth our cash, cash equivalents and marketable securities and capitalization as of June 30, 2014, as follows:
- •
- on an actual basis;
- •
- on a pro forma basis after giving effect to the Conversion and the conversion of all outstanding shares of our
series A preferred stock and series B preferred stock into 146,000,000 shares of our common stock; and
- •
- on a pro forma as adjusted basis to give further effect to our issuance and sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of the offering determined at pricing. You should read this information in conjunction with our consolidated financial statements and the related notes appearing at the end of this prospectus and the "Management's Discussion and Analysis of Financial Condition and Results of Operations" section and other financial information contained in this prospectus. You should also read this table together with the information contained in this prospectus, including "Conversion," "Use of Proceeds," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the historical financial statements and related notes included elsewhere in this prospectus.
56
| |
As of June 30, 2014 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Actual | Pro Forma | Pro Forma As Adjusted |
|||||||
| |
(unaudited) |
|||||||||
| |
(in thousands) |
|||||||||
Cash and cash equivalents |
$ | 8,864 | $ | 8,864 | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Convertible preferred units: |
||||||||||
Series A convertible preferred units; 61,000,000 units authorized, issued and outstanding actual; no units authorized, issued or outstanding pro forma or pro forma as adjusted (aggregate liquidation preferences of $40,254 at June 30, 2014) |
$ | 30,307 | — | |||||||
Series B convertible preferred units; 85,000,000 units authorized, issued and outstanding actual; no units authorized, issued or outstanding pro forma or pro forma as adjusted (aggregate liquidation preferences of $48,365 at June 30, 2014) |
42,279 |
— |
||||||||
Stockholders' and members' equity (deficit): |
||||||||||
Common units, 179,539,000 units authorized, 15,369,832 units issued and 10,938,609 units outstanding at June 30, 2014; no units authorized, issued or outstanding pro forma or pro forma as adjusted |
53 | — | ||||||||
Common stock, $0.001 par value; no shares authorized, issued or outstanding actual; shares authorized and 156,938,609 shares issued pro forma; shares authorized and shares issued pro forma as adjusted |
— | 157 | ||||||||
Additional paid-in capital |
1,006 | 73,488 | ||||||||
Accumulated other comprehensive income |
9 | 9 | ||||||||
Accumulated deficit |
(65,406 | ) | (65,406 | ) | ||||||
| | | | | | | | | | | |
Total stockholders' and members' equity (deficit) |
(64,338 | ) | 8,248 | |||||||
Total capitalization |
$ | (64,338 | ) | $ | 8,248 | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
The information above is illustrative only and our capitalization following the completion of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the estimated price range shown on the cover page of this prospectus, would increase (decrease) the amount of cash and cash equivalents, additional paid-in capital, total stockholders' equity (deficit) and total capitalization on a pro forma as adjusted basis by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of one million shares offered by us would increase (decrease) cash and cash equivalents, total stockholders' equity (deficit) and total capitalization on a pro forma as adjusted basis by approximately $ million, assuming the assumed initial public offering price remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. A share increase in the number of shares offered by us together with a concomitant $1.00 increase in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase each of cash and cash equivalents and total stockholders' (deficit) equity by approximately $ million after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Conversely, a share decrease in the number of shares offered by us together with a concomitant $1.00 decrease in the assumed initial public offering
57
price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would decrease each of cash and cash equivalents and total stockholders' (deficit) equity by approximately $ million after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
The actual, pro forma and pro forma as adjusted information set forth in the table excludes:
- •
- 9,790,000 shares of common stock issuable upon the exercise of outstanding warrants at an exercise price of $0.001 per
share, which outstanding warrants will automatically expire upon the closing of this offering if not previously exercised; and
- •
- shares of common stock reserved for future issuance under our 2014 equity incentive plan.
58
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per share and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Our historical net tangible book value as of June 30, 2014 was approximately $(64.3) million, or $(5.88) per common unit. Our historical net tangible book value is the amount of our total tangible assets less our total liabilities, including the liquidation preference of our convertible preferred units. Net historical tangible book value per common unit is our historical net tangible book value divided by the number of common units outstanding as of June 30, 2014.
Our pro forma net tangible book value as of June 30, 2014 after giving effect to the Conversion was $8.3 million, or $0.05 per share of common stock.
The following table illustrates this dilution on a per share basis:
Assumed initial public offering price per share |
$ | ||||||
Historical net tangible book value per share as of June 30, 2014 |
$ | ||||||
Pro forma net tangible book value per share as of June 30, 2014 |
$ | ||||||
Increase per share attributable to new investors |
$ | ||||||
| | | | | | | | |
Pro forma as adjusted net tangible book value per share at June 30, 2014 after giving effect to the offering |
$ | ||||||
| | | | | | | | |
Dilution per share to new investors |
$ | ||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted net tangible book value per share after this offering by approximately $ per share and the dilution per share to investors participating in this offering by approximately $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering costs payable by us.
The following table summarizes, on a pro forma basis as of June 30, 2014, the total number of shares purchased from us, the total consideration paid, or to be paid, and the average price per share paid, or to be paid, by existing stockholders and by new investors in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. As the table below shows, new investors purchasing shares in this offering will pay an average price per share substantially higher than our existing stockholders paid.
| |
Shares Purchased | Total Consideration | |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Average Price Per Share |
|||||||||||||||
| |
Number | Percentage | Amount | Percentage | ||||||||||||
| |
|
|
(in thousands) |
|
|
|||||||||||
Existing stockholders |
% | $ | % | $ | ||||||||||||
New investors |
% | % | ||||||||||||||
| | | | | | | | | | | | | | | | | |
Total |
% | $ | % | $ | ||||||||||||
59
Except as otherwise indicated, the discussion and tables above assume no exercise of the underwriters' option to purchase additional shares of our common stock in this offering. If the underwriters' option to purchase additional shares is exercised in full:
- •
- the percentage of outstanding common stock held by existing stockholders will be reduced to % of the total
number of shares of common stock to be outstanding upon completion of this offering; and
- •
- the number of shares of common stock held by investors participating in this offering will be increased to shares, or % of the total number of shares of common stock to be outstanding upon completion of this offering.
Effective immediately upon closing of this offering, an aggregate of shares of our common stock will be reserved for issuance under our 2014 equity incentive plan. Furthermore, we may choose to raise additional capital through the sale of equity or convertible debt securities due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that any new options are issued under our equity incentive plans or we issue additional shares of common stock or other equity or convertible debt securities in the future, there will be further dilution to investors participating in this offering.
60
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated statements of operations data for the years ended December 31, 2012 and 2013 and the consolidated balance sheet data as of December 31, 2012 and 2013 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The selected consolidated statements of operations data for the six months ended June 30, 2013 and 2014 and the balance sheet data as of June 30, 2014 have been derived from our unaudited consolidated financial statements included elsewhere in this prospectus. In our opinion, these unaudited consolidated financial statements have been prepared on a basis consistent with our audited consolidated financial statements and contain all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of such financial data. You should read this data together with our audited consolidated financial statements and related notes appearing elsewhere in this prospectus and the information under the caption "Management's Discussion and Analysis of Financial Condition and Results of Operations." Our historical results are not necessarily indicative of our future results, and our operating results for the six-month period ended June 30, 2014 are not necessarily indicative of the results that may be expected for the fiscal year ending December 31, 2014 or any other interim periods or any future year or period.
| |
Years Ended December 31, | Six Months Ended June 30, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2013 | 2014 | |||||||||
| |
|
|
(unaudited) |
||||||||||
| |
(in thousands, except share and per share data) |
||||||||||||
Statement of Operations and Comprehensive Loss Data: |
|||||||||||||
Operating Expenses: |
|||||||||||||
Research and development |
$ | 15,678 | $ | 18,015 | $ | 9,079 | $ | 5,470 | |||||
General and administrative |
2,460 | 3,433 | 1,753 | 1,503 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses |
18,138 | 21,448 | 10,832 | 6,973 | |||||||||
| | | | | | | | | | | | | | |
Loss from operations |
(18,138 | ) | (21,448 | ) | (10,832 | ) | (6,973 | ) | |||||
Other (expense) income, net |
8 | 17 | 11 | 5 | |||||||||
| | | | | | | | | | | | | | |
Net loss |
$ | (18,130 | ) | $ | (21,431 | ) | $ | (10,821 | ) | $ | (6,968 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Other comprehensive income (loss) |
7 | (1 | ) | 3 | (1 | ) | |||||||
| | | | | | | | | | | | | | |
Comprehensive loss |
$ | (18,123 | ) | $ | (21,432 | ) | $ | (10,818 | ) | $ | (6,969 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net loss applicable to common holders |
$ | (21,498 | ) | $ | (27,229 | ) | $ | (13,454 | ) | $ | (10,128 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net loss per common unit, basic and diluted(1) |
$ | (7.46 | ) | $ | (3.65 | ) | $ | (2.25 | ) | $ | (0.99 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Weighted average common units outstanding, basic and diluted |
2,883,087 | 7,467,781 | 5,979,829 | 10,198,713 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma net loss per common share applicable to common stockholders, basic and diluted(1) |
$ | (0.15 | ) | $ | (0.04 | ) | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma weighted average common shares outstanding, basic and diluted(2) |
139,901,617 | 156,198,713 | |||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- (1)
- See
Notes 2 and 10 within the notes to our financial statements appearing elsewhere in this prospectus for a description of the methods used to
calculate basic and diluted net loss per share and pro forma basic and diluted net loss per share.
- (2)
- Pro forma to reflect the Conversion to a corporation and the conversion of all outstanding shares of series A preferred stock and series B preferred stock into shares of common stock.
61
| |
December 31, | June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2014 | |||||||
| |
|
|
(unaudited) |
|||||||
Balance Sheet Data: |
||||||||||
Cash and cash equivalents |
$ | 11,766 | $ | 7,364 | $ | 8,864 | ||||
Working capital |
16,353 | 15,079 | 8,210 | |||||||
Total assets |
18,922 | 18,186 | 10,687 | |||||||
Convertible preferred stock/units |
52,704 | 72,586 | 72,586 | |||||||
Accumulated deficit |
(37,007 | ) | (58,438 | ) | (65,406 | ) | ||||
Total stockholders' and members' deficit |
$ | (36,284 | ) | $ | (57,460 | ) | $ | (64,338 | ) | |
62
MANAGEMENT'S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included elsewhere in this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those set forth in the "Risk Factors" section of this prospectus, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. Unless otherwise indicated, the numbers in the text below are expressed in thousands.
Overview
We are a biopharmaceutical company focused on the development and commercialization of peptide therapeutics for the treatment of gastrointestinal, or GI, diseases and genetic deficiencies that result in metabolic disorders. Our lead product candidate, relamorelin, is a potent, best-in-class, Phase 2 ghrelin agonist for the treatment of diabetic gastroparesis, a GI complication of diabetes, and other GI functional disorders. We are also developing a second product candidate, RM-493, which is a potent, first-in-class, Phase 2 ready melanocortin-4, or MC4, receptor agonist for the treatment of obesity caused by genetic deficiencies in the MC4 pathway. We believe our product candidates, for which we have exclusive worldwide rights, have the potential to treat these diseases for which there currently are limited therapeutic options.
We have completed a Phase 2 clinical trial of relamorelin for the treatment of diabetic gastroparesis. Based on consultation with regulatory authorities, we expect to initiate a Phase 2b clinical trial for the treatment of diabetic gastroparesis by the end of 2014. The U.S. Food and Drug Administration, or the FDA, has granted Fast Track designation to relamorelin for the treatment of diabetic gastroparesis. Fast Track designation is designed to facilitate and expedite the review of drugs for serious conditions with high unmet need, in order to get drugs to patients earlier. We intend to manage our relamorelin clinical development program such that, if our clinical trials are successful, we can file a new drug application, or NDA, by 2018. We are also conducting a Phase 2a clinical trial of relamorelin for the treatment of refractory chronic constipation in Parkinson's disease patients and a Phase 2a benchmark clinical trial of relamorelin in patients with chronic constipation. RM-493 is in clinical development for the treatment of obesity caused by genetic deficiencies in the MC4 pathway. We expect to initiate two Phase 2a clinical trials in patients with genetic deficiencies in the MC4 pathway by the end of 2014, one for the treatment of obesity in patients with a single mutation of the MC4 receptor gene, and one in patients with Prader Willi Syndrome, or PWS.
We have operated as a virtual company that leverages skilled experts, consultants, contract research organizations, or CROs, and contractors to manage our clinical operations under the leadership and direction of our management. We expect to expand our infrastructure to manage our clinical, finance and commercial operations with a higher proportion of full-time employees.
Our operations to date have been limited primarily to organizing and staffing our company and conducting research and development activities for our lead product candidate, relamorelin, and our second product candidate, RM-493. To date, we have not generated any product revenue and have financed our operations primarily through the private placement of our equity securities to venture capital investors in exchange for proceeds of approximately $73.0 million. We will not generate revenue from product sales unless and until we or a partner successfully complete development and obtain regulatory approval for one or more of our product candidates, which we expect will take a number of years and is subject to significant uncertainty. We expect to fund our operations through the sale of equity, debt financings or other sources, including potential collaborations with pharmaceutical
63
companies. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms, or at all. If we fail to raise capital or enter into such other arrangements as, and when, needed, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates. See "Risk Factors" for additional information.
As of June 30, 2014, we had an accumulated deficit of $65.4 million. Our net losses were $18.1 million and $21.4 million for the years ended December 31, 2012 and 2013, respectively, and $10.8 million and $7.0 million, for the six months ended June 30, 2013 and 2014, respectively. We expect to continue to incur significant expenses and increasing operating losses over the foreseeable future. We expect our expenses will increase substantially in connection with our ongoing activities, as we:
- •
- continue to conduct clinical trials for our product candidates;
- •
- manufacture our product candidates for clinical trials;
- •
- seek regulatory approval for our product candidates;
- •
- build a marketing and commercialization infrastructure; and
- •
- operate as a public company.
As of June 30, 2014, our existing cash, cash equivalents and marketable securities were $9.3 million. We expect that the net proceeds of this offering, together with our existing cash, cash equivalents and marketable securities, will enable us to fund our operating expenses through at least the first half of 2016.
The Prior Restructuring
Rhythm Holding Company, LLC, or the LLC entity, was formed on March 21, 2013 as a holding company in connection with a corporate restructuring, or the Prior Restructuring, by Rhythm Pharmaceuticals, Inc., a Delaware corporation that was organized in November 2008. Rhythm Pharmaceuticals, Inc. changed its name to Rhythm Health, Inc., or Rhythm Health, on July 7, 2014. Rhythm Health and Rhythm Metabolic, Inc., or Rhythm Metabolic, a Delaware corporation organized on March 21, 2013, became the wholly owned subsidiaries of the LLC entity as a result of certain exchange of equity transactions in connection with the Prior Restructuring. Pursuant to the Prior Restructuring, each outstanding share of series A preferred stock, series B preferred stock and common stock of Rhythm Health was exchanged for one series A preferred unit, series B preferred unit and common unit, respectively of the LLC entity. Each outstanding warrant to purchase common stock of Rhythm Health was converted into a warrant to purchase an equivalent number of common units of the LLC entity. In addition, each outstanding stock option to purchase a share of common stock of Rhythm Health was cancelled and the LLC entity issued to the holder of such cancelled stock option one common unit of the LLC entity. If such outstanding stock option was subject to vesting at the effective time of its cancellation, then such common unit was issued by the LLC entity subject to continued vesting to the same extent as such outstanding stock option was still subject to vesting at the effective time of its cancellation. See "The Prior Restructuring" for additional information.
The Conversion
Prior to the effectiveness of the registration statement of which this prospectus forms a part, the LLC entity will convert from a Delaware limited liability company to a Delaware corporation named Rhythm Pharmaceuticals, Inc. by filing a certificate of conversion with the Secretary of State of the State of Delaware. As part of the Conversion:
- •
- each outstanding common unit of the LLC entity will be converted into one share of common stock of Rhythm Pharmaceuticals, Inc.;
64
- •
- each outstanding series A preferred unit of the LLC entity will be converted into one share of series A
preferred stock of Rhythm Pharmaceuticals, Inc.;
- •
- each outstanding series B preferred unit of the LLC entity will be converted into one share of series B
preferred stock of Rhythm Pharmaceuticals, Inc.;
- •
- each outstanding vested restricted common unit of the LLC entity will be converted into one share of common stock of
Rhythm Pharmaceuticals, Inc.;
- •
- each outstanding unvested restricted common unit of the LLC entity will be converted into one share of restricted common
stock of Rhythm Pharmaceuticals, Inc., subject to vesting on the same terms as prior to the Conversion; and
- •
- each outstanding warrant to purchase common units of the LLC entity will be converted into a warrant to purchase an equivalent number of shares of common stock of Rhythm Pharmaceuticals, Inc.
Upon the closing of this offering, all outstanding shares of our series A preferred stock and series B preferred stock will be automatically converted into shares of our common stock pursuant to the terms of our certificate of incorporation and we will file an amended and restated certificate of incorporation to reflect the conversion of our series A preferred stock and our series B preferred stock. The material portions of our amended and restated certificate of incorporation and amended and restated bylaws are described in "Description of Capital Stock."
Financial operations overview
Revenue
To date, we have not generated any revenue from product sales and do not expect to generate any revenue from the sale of any of our product candidates for at least several years. We cannot predict if, when, or to what extent we will generate revenues from the commercialization and sale of our product candidates. We may never succeed in achieving regulatory approval for any of our product candidates.
Research and development expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our drug discovery efforts, and the development of our product candidates, which include:
- •
- expenses incurred under agreements with third parties, including CROs that conduct research and development and
preclinical activities on our behalf, and the cost of consultants and contract manufacturing organizations, or CMOs, that manufacture drug products for use in our preclinical studies and clinical
trials;
- •
- employee-related expenses, including salaries, benefits, and stock-based compensation expense;
- •
- the cost of lab supplies and acquiring, developing, and manufacturing preclinical study materials; and
- •
- facilities, depreciation, and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance, and other operating costs.
We expense research and development costs to operations as incurred. Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses and capitalized. The capitalized amounts are expensed as the related goods are delivered or the services are performed.
65
The following table summarizes our current research and development programs for relamorelin and RM-493.
| |
Years Ended December 31, |
Six Months Ended June 30, |
Period from Inception through |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Research and Development Summary
|
2012 | 2013 | 2013 | 2014 | June 30, 2014 | |||||||||||
| |
(in thousands) |
|
||||||||||||||
Relamorelin Program |
$ | 7,558 | $ | 7,459 | $ | 3,820 | $ | 1,390 | $ | 22,403 | ||||||
RM-493 Program |
8,120 | 10,556 | 5,259 | 4,080 | 32,963 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total Research and Development |
$ | 15,678 | $ | 18,015 | $ | 9,079 | $ | 5,470 | $ | 55,366 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
We are unable to predict the duration and costs of the current or future clinical trials of our product candidates. The duration, costs, and timing of clinical trials and development of our product candidates will depend on a variety of factors, including:
- •
- the scope, rate of progress, and expense of our ongoing, as well as any additional, clinical trials and other research
and development activities;
- •
- the rate of enrollment in clinical trials;
- •
- the safety and efficacy demonstrated by our product candidates in future clinical trials;
- •
- changes in regulatory requirements;
- •
- changes in clinical trial design; and
- •
- the timing and receipt of any regulatory approvals.
A change in the outcome of any of these variables with respect to the development of any of our product candidates would significantly change the costs and timing associated with the development of that product candidate.
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect research and development costs to increase significantly for the foreseeable future as our product candidate development programs progress. However, we do not believe that it is possible at this time to accurately project total program-specific expenses to commercialization and there can be no guarantee that we can meet the funding needs associated with these expenses.
General and administrative expenses
General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation for personnel. Other significant costs include rent, legal fees relating to patent and corporate matters and fees for accounting and consulting services.
We anticipate that our general and administrative expenses will increase in the future to support continued and expanding development efforts, potential commercialization of our product candidates and increased costs of operating as a public company. These increases will likely include increased costs related to the hiring of additional personnel and fees to outside consultants, lawyers and accountants, among other expenses. Additionally, we anticipate increased costs associated with being a public company including expenses related to maintaining compliance with exchange listing and Securities and Exchange Commission requirements, insurance, and investor relations costs.
We have operated as a virtual company that leverages skilled experts, consultants, CROs, and contractors to manage our clinical operations, under the leadership and direction of our management. As of June 30, 2014, we had four employees, two of whom hold Ph.D. or M.D. degrees. Of these employees, two were engaged in development activities and two were engaged in support
66
administration, including business development and finance. We will expand our infrastructure to manage our clinical, finance and commercial operations with additional full-time employees.
Critical accounting policies and estimates
Our discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which we have prepared in accordance with United States generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those described in greater detail below. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our consolidated financial statements included elsewhere in this prospectus, we believe that the following accounting policies are the most critical to aid in fully understanding and evaluating our financial condition and results of operations.
Accrued research and development expenses
As part of the process of preparing our consolidated financial statements, we are required to estimate the value associated with goods and services received in the period in connection with research and development activities. This process involves reviewing quotations and contracts, identifying services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost, or alternatively, the deferral of amounts paid for goods or services to be incurred in the future. The majority of our service providers invoice us monthly in arrears for services performed or when contractual milestones are met. We make estimates of our accrued expenses or prepaid expense as of each balance sheet date in our consolidated financial statements based on facts and circumstances known to us at the time those financial statements are prepared. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. The significant estimates in our accrued research and development expenses include fees paid to CROs and CMOs in connection with research and development activities.
We accrue our expenses related to CROs and CMOs based on our estimates of the services received and efforts expended pursuant to quotes and contracts with CROs and CMOs that conduct research and development and manufacturing on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. The allocation of CRO upfront expenses for both clinical trials and preclinical studies generally tracks actual work activity. However there may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees delivered over a period of time, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust accrued or prepaid expense accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimates of the status and timing of services performed differ from the actual status and timing of services performed, it could result in us reporting amounts that are too high or too low in any particular period. To date, there have been no material differences between our estimates of such expenses and the amounts actually incurred.
67
Income taxes
We account for uncertain tax positions in accordance with the provisions of Accounting Standards Codification, or ASC, Topic 740, Accounting for Income Taxes. When uncertain tax positions exist, we recognize the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances. As of June 30, 2014, we do not have any uncertain tax positions.
Income taxes are recorded in accordance with ASC 740, Accounting for Income Taxes, which provides for deferred taxes using an asset and liability approach. We recognize deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. We determine our deferred tax assets and liabilities based on differences between financial reporting and tax bases of assets and liabilities, which are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances are provided, if based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
Interest and penalties on uncertain tax positions are recorded in the provision (benefit) for income taxes in the statements of operations. During the years ended December 31, 2012 and 2013 and the six months ended June 30, 2013 and 2014, we had no amounts accrued for interest and penalties related to uncertain tax positions.
The LLC entity has elected to be taxed as a partnership for U.S. federal income tax purposes. Accordingly, all income and deductions of the LLC entity are reported on the members' individual income tax returns and no income taxes are recorded by the LLC entity. The LLC entity does not have any operations.
As of December 31, 2013, we had net operating loss, or NOL, carryforwards to reduce federal and state incomes taxes of approximately $36.0 million and $11.0 million, respectively. If not utilized, these carryforwards begin to expire in 2030. At December 31, 2013, we also had available research and development tax credits for federal and state income tax purposes of approximately $1.9 million and $0.2 million respectively. The federal and state credits begin to expire in 2030 and 2026, respectively.
Utilization of the NOL and tax credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations that have occurred previously or that could occur in the future, as provided by Section 382 of the Internal Revenue Code of 1986 as amended, or Section 382, as well as similar state provisions and other provisions of the Internal Revenue Code. Ownership changes may limit the amount of NOLs and tax credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Section 382, results from transactions that increase the ownership of 5.0% shareholders in the stock of a corporation by more than 50% in the aggregate over a three-year period.
Stock-based compensation
Pursuant to the Prior Restructuring, which became effective on March 21, 2013, all stock options that were exercisable for shares of common stock of Rhythm Health were cancelled and the LLC entity issued to each holder of such cancelled stock options a number of our common units equal to the number of shares of common stock underlying such holder's cancelled stock option. If any stock option that was cancelled pursuant to the Prior Restructuring was subject to vesting at the time of its cancellation, the LLC entity entered into a written agreement with the holder of such stock option at the effective time of its cancellation pursuant to which any common units issued by the LLC entity with respect to such cancelled stock option would continue to be subject to vesting to the same extent as such cancelled stock option was subject to vesting at the effective time of its cancellation. Under such written agreement, if the holder of any common units subject to vesting, which we refer to as restricted common units, ceases to be an employee or consultant of ours and our subsidiaries, the holder will forfeit all of
68
such restricted common units. As part of the Conversion, each outstanding vested restricted common unit of the LLC entity will be converted into one share of common stock of Rhythm Pharmaceuticals, Inc., and each outstanding unvested restricted common unit of the LLC entity will be converted into one share of restricted common stock of Rhythm Pharmaceuticals, Inc., subject to vesting on the same terms as prior to the Conversion.
As a general rule, we apply the fair value recognition provisions of Financial Accounting Standards Board, or FASB, ASC Topic 718, Compensation—Stock Compensation, or ASC 718, to account for stock-based compensation. We recognize stock-based compensation expense related to stock options granted by Rhythm Health before the Prior Restructuring and to any restricted common units granted by the LLC entity from and after the Prior Restructuring to employees and consultants for compensatory purposes, based on the estimated fair value of each stock option or restricted common unit on the date of grant, net of estimated forfeitures. The grant date fair value of awards subject to service-based vesting, net of estimated forfeitures, is recognized on a straight-line basis over the requisite service period, which is generally the vesting period of the respective awards. In accordance with the ASC 718, stock-based compensation expense related to stock options and restricted common units that are subject to both performance- and service-based vesting conditions is recognized using an accelerated recognition model. Unless the context otherwise requires, any reference to our stock options, common stock or preferred stock in this description of our stock-based compensation is a reference to stock options granted by Rhythm Health before the Prior Restructuring, the common stock of Rhythm Health and the preferred stock of Rhythm Health, respectively, in each case as Rhythm Health was constituted before the Prior Restructuring, and any reference to common units, restricted common units or preferred units in this description of our stock-based compensation is a reference to common units, restricted common units or preferred units of the LLC entity, respectively, as we are constituted after the Prior Restructuring and prior to the Conversion. Following the Conversion, we expect to issue stock options and other stock-based compensation pursuant to our 2014 equity incentive plan as described in "Executive and Director Compensation—Employee Benefit and Stock Plans—2014 Equity Incentive Plan."
We use the Black-Scholes option pricing model to estimate the fair value of stock option using various assumptions that require management to apply judgment and make estimates, including:
- •
- the expected term of the award, which we calculate using the simplified method, as prescribed by the Securities and
Exchange Commission Staff Accounting Bulletin No. 107, Share-Based Payment, as we have insufficient historical information regarding our stock
options to provide a basis for an estimate;
- •
- the expected volatility of the underlying common stock or of our common units, which we estimate based on the historical
volatility of a representative group of publicly traded biopharmaceutical companies with similar characteristics to us, including development candidates in earlier stages of drug development and areas
of therapeutic focus;
- •
- the risk-free interest rate, which we based on the yield curve of U.S. Treasury securities with periods commensurate with
the expected term of the stock options or restricted common units being valued;
- •
- the expected dividend yield, which we estimate to be zero based on the fact that we have never paid cash dividends and
have no present intention to pay cash dividends; and
- •
- the fair value of a share of our common stock or a common unit on the date of grant of the applicable award.
If factors change and different assumptions are used, our stock-based compensation expense could be materially different in the future.
The fair value of a restricted common unit is estimated based on its fair value on the measurement date as if the restricted common unit was fully vested at that date, which is equivalent to the fair value of a common unit. Refer to our later discussion on the determination of the fair value of a common unit.
69
The weighted-average assumptions used to estimate the fair value of stock options using the Black-Scholes option pricing model were as follows:
| |
Years Ended December 31, | |||
|---|---|---|---|---|
| |
2012 | 2013 | ||
Expected volatility |
80.0% | 80% - 65% | ||
Risk-free interest rate |
0.82% - 1.11% | 1.14% | ||
Expected option term (in years) |
6.08 | 6.08 | ||
Expected dividend yield |
0.0% | 0.0% | ||
The weighted-average assumptions used to estimate the fair value of common units for the three months ended March 31, 2014 using the Probability Weighted Expected Return Method, or the PWERM, were as follows: volatility of 59.0%, risk-free interest rate of 0.45% and 2.0 years to liquidity event.
In addition to the assumptions used in our Black-Scholes option-pricing model, the amount of compensation expense we recognize in our consolidated statements of operations includes an estimate of stock option or restricted common unit forfeitures. Under ASC 718, we are required to estimate the level of forfeitures expected to occur and record compensation expense only for those awards that we ultimately expect will vest. Due to the lack of historical forfeiture activity, we estimate our forfeiture rate based on data from our representative group of companies. Changes in the estimated forfeiture rate can have a significant impact on our stock-based compensation expense as the cumulative effect of adjusting the rate is recognized in the period the forfeiture estimate is changed. For example, if a revised forfeiture rate is higher than the previously estimated forfeiture rate, an adjustment is made that will result in a decrease to the stock-based compensation expense recognized in the consolidated financial statements. If a revised forfeiture rate is lower than the previously estimated forfeiture rate, an adjustment is made that will result in an increase to the stock-based compensation expense recognized in our consolidated financial statements. To date our actual forfeitures have not been material.
At June 30, 2014, the total unrecognized compensation expense related to unvested restricted common unit awards, including estimated forfeitures, was $0.27 million, which we expect to recognize over a weighted-average period of approximately one year. We also have unrecognized stock-based compensation expense of $0.05 million related to restricted common units with milestone-based vesting criteria that is not considered probable of achievement as of June 30, 2014; therefore we have not recognized any portion of this expense on these awards.
Determination of the fair value of common stock or common units
We have operated as a private company with no active public market for our common stock or our common units. Therefore, our board of directors estimated the fair value of our common stock or common units at various dates, with input from management, considering our most recently available third-party valuations of common stock or common units and its assessment of additional objective and subjective factors that it believed were relevant and which may have changed from the date of the most recent valuation through the date of the applicable grant or award. Once our shares begin trading in an open market there will no longer be a need for such estimates as the market will determine the fair value.
We determined the estimated per share fair value of our common stock or common units at various dates using contemporaneous valuations performed in accordance with the guidance outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation, or the Practice Aid, for financial reporting purposes.
70
In conducting the contemporaneous valuations, we considered all objective and subjective factors that we believed to be relevant for each valuation conducted, including our best estimate of our business condition, prospects and operating performance at each valuation date. Within the contemporaneous valuations performed, a range of factors, assumptions and methodologies were used. The significant factors included:
- •
- the lack of an active public market for our common and preferred stock or for our common and preferred units;
- •
- the prices at which we sold our preferred stock or preferred units to outside investors in arm's length transactions, and
the rights, preferences and privileges of those preferred stock or preferred units relative to our common stock or common units;
- •
- our results of operations, financial position and the status of our research and preclinical development efforts,
including our relamorelin and RM-493 programs;
- •
- the material risks related to our business;
- •
- the market performance of publicly traded companies in the life sciences and biotechnology sectors, and recently
completed mergers and acquisitions of companies comparable to us;
- •
- the likelihood of achieving a liquidity event for the holders of our common stock or our common units, such as an initial
public offering, or IPO, or sale of the company given prevailing market conditions; and
- •
- any recent contemporaneous valuations of our common stock or our common units prepared in accordance with methodologies outlined in the Practice Aid.
Valuation methodologies
Our valuations of common stock and common units were prepared utilizing the option-pricing method, or OPM, the PWERM, and a hybrid of the PWERM and OPM, which we refer to as the hybrid method:
- •
- Option Pricing Method. Under the OPM, common and
preferred stock or units are valued by creating a series of call options with exercise prices based on the liquidation preferences and conversion terms of each equity class. Under this method, a share
of common stock or a common unit only has value if the funds available for distribution to stockholders exceed the value of the liquidation preferences at the time of a liquidity event, such as a
strategic sale or merger. Under the OPM, the value of one security, such as preferred stock or preferred units, is used to determine the value of the equity and the corresponding value of the common
stock or the common units, respectively. We used the OPM to estimate the value of our common stock relative to the pricing of our series B preferred stock, and to estimate the value of our
common units relative to the pricing of our series B preferred units.
- •
- Probability-Weighted Expected Return Method. The PWERM is a scenario-based analysis that estimates the value per share based on the probability-weighted present value of expected future investment returns, considering each of the possible outcomes available to us, as well as the economic and control rights of each equity class. In the PWERM used by us, three types of future-event scenarios were considered: an IPO, a sale and dissolution.
71
- •
- Hybrid Method. The hybrid method is a PWERM where the
equity value in one of the scenarios is calculated using an OPM. In the hybrid method used by us, three types of future-event scenarios were considered: an IPO, a sale and an unspecified liquidity
event scenario. We used the OPM to allocate equity value for the unspecified liquidity event.
- •
- The enterprise value for the IPO and sale scenarios was determined using a market approach. Under the market approach, we
estimated enterprise value using the guideline public company method. The guideline public company method includes comparisons to publicly traded companies in the relevant industry that recently
completed IPOs. For the sale scenarios, we estimated a premium to the IPO value. The enterprise values are discounted back to the valuation date at an appropriate risk-adjusted discount rate.
- •
- The enterprise value for the unspecified liquidity event was determined using the OPM backsolve approach.
Retrospective Valuations
In April 2014, our board of directors, based on market conditions and the clinical advancement of our relamorelin and RM-493 clinical programs, authorized the management team to assess the feasibility of an IPO in the second half of 2014. In early May 2014, we selected underwriters and held an organizational meeting. In connection with the preparation of the consolidated financial statements for the year ended December 31, 2013 and in preparing for this offering, we re-valued, solely for financial reporting purposes, our common units as of March 21, 2013. We also performed additional retrospective valuations of our common stock or common units, as applicable, as of January 30, 2013, June 5, 2013 and September 12, 2013 to coincide with the issuance of certain of the equity grants. Finally, we performed a retrospective valuation of our common units as of December 31, 2013 and a contemporaneous valuation as of March 21, 2014. We believe that the preparation of the retrospective valuations was necessary due to the fact that the timeframe and probability for a potential IPO had accelerated significantly since the time of our initial contemporaneous valuations, and that such acceleration would have a significant impact on the fair value of our common stock. We concluded that retrospective valuations for grant dates prior to January 30, 2013 were not required due to the lack of clarity and risk related to our early stage research programs and determined it would not be reasonable to assign a probability to a future IPO.
Retrospective valuations of common stock or common units, as applicable, as of January 30, 2013 and March 21, 2013. We performed the retrospective valuations as of January 30, 2013 and March 21, 2013 to coincide with certain equity grants on January 30, 2013, and the Prior Restructuring on March 21, 2013. In conducting the valuations as of January 30, 2013 and March 21, 2013, we estimated the value of our common stock or common units, as applicable, based on the price at which we sold shares of our series B convertible preferred stock. We concluded that the price paid for the series B convertible preferred stock was representative of fair value since our November 2012 series B preferred stock financing included a significant investment from a new unaffiliated lead investor. We utilized the OPM to estimate the enterprise value as of January 30, 2013 and March 21, 2013 that was implied by the arm's length series B convertible preferred stock financing.
Retrospective valuations of common units as of June 5, 2013, September 12, 2013 and December 31, 2013. We performed the retrospective valuations as of June 5, 2013 and September 12, 2013 to coincide with the issuance of certain of the equity grants. Although no equity grants were made on December 31, 2013, given the possibility of an IPO in the second half of 2014, we also conducted a retrospective valuation as of December 31, 2013. Given the prospect for an IPO in 2014, we used the hybrid method.
72
Contemporaneous valuation of common units as of March 31, 2014
We performed a contemporaneous valuation of our common units on March 31, 2014 to coincide with our preparation for this offering. Pursuant to the Practice Aid, the PWERM is appropriate when the time to a liquidity event is short, making the range of possible future outcomes relatively easy to predict. Given the proximity to a potential IPO transaction, we chose to use the PWERM. We have not performed any subsequent contemporaneous valuations since March 31, 2014.
Results of Operations
Comparison of years ended December 31, 2012 and 2013
The following table summarizes our results of operations for the years ended December 31, 2012 and 2013, together with the changes in those items in dollars and as a percentage:
| |
Years Ended December 31, | Change | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | $ | % | |||||||||
| |
(in thousands) |
||||||||||||
Statement of Operations Data: |
|||||||||||||
Operating Expenses: |
|||||||||||||
Research and development |
$ | 15,678 | $ | 18,015 | $ | 2,337 | 15 | % | |||||
General and administrative |
2,460 | 3,433 | 973 | 40 | % | ||||||||
| | | | | | | | | | | | | | |
Total operating expenses |
18,138 | 21,448 | 3,310 | 18 | % | ||||||||
| | | | | | | | | | | | | | |
Loss from operations |
(18,138 | ) | (21,448 | ) | (3,310 | ) | (18 | )% | |||||
Other (expense) income, net |
8 | 17 | 9 | 113 | % | ||||||||
| | | | | | | | | | | | | | |
Net loss |
$ | (18,130 | ) | $ | (21,431 | ) | $ | (3,301 | ) | (18 | )% | ||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Other comprehensive (loss) income |
7 | (1 | ) | (8 | ) | (114 | )% | ||||||
| | | | | | | | | | | | | | |
Comprehensive loss |
$ | (18,123 | ) | $ | (21,432 | ) | $ | (3,309 | ) | (18 | )% | ||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Research and development expense. Research and development expense increased by $2,337 to $18,015 in 2013 from $15,678 in 2012, an increase of 15%. The increase was primarily due to an increase in external services in 2013 related to the initiation of new Phase 2 clinical trials for both relamorelin and RM-493.
General and administrative expense. General and administrative expense increased by $973 to $3,433 in 2013 from $2,460 in 2012, an increase of 40%. The increase in general and administrative expenses was primarily attributable to external legal patent expenses.
Interest income. The increase in interest income was nominal and primarily related to an increase in investment balances during the twelve-month period ended December 31, 2013 versus the investments on hand during the twelve-month period ended December 31, 2012.
73
Comparison of six months ended June 30, 2013 and 2014
The following table summarizes our results of operations for the six months ended June 30, 2013 and 2014, together with the changes in those items in dollars and as a percentage:
| |
Six Months Ended June 30, |
Change | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | $ | % | |||||||||
| |
(in thousands) |
||||||||||||
Statement of Operations Data: |
|||||||||||||
Operating Expenses: |
|||||||||||||
Research and development |
$ | 9,079 | $ | 5,470 | $ | (3,609 | ) | (40 | )% | ||||
General and administrative |
1,753 | 1,503 | (250 | ) | (14 | )% | |||||||
| | | | | | | | | | | | | | |
Total operating expenses |
10,832 | 6,973 | (3,859 | ) | (36 | )% | |||||||
| | | | | | | | | | | | | | |
Loss from operations |
(10,832 | ) | (6,973 | ) | 3,859 | 36 | % | ||||||
Other (expense) income, net |
11 | 5 | (6 | ) | (55 | )% | |||||||
| | | | | | | | | | | | | | |
Net loss |
$ | (10,821 | ) | $ | (6,968 | ) | $ | 3,853 | 36 | % | |||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Other comprehensive (loss) income |
3 | (1 | ) | (4 | ) | (133 | )% | ||||||
| | | | | | | | | | | | | | |
Comprehensive loss |
$ | (10,818 | ) | $ | (6,969 | ) | $ | 3,849 | 36 | % | |||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Research and development expense. Research and development expense decreased by $3,609 to $5,470 for the six months ended June 30, 2014 from $9,079 for the six months ended June 30, 2013, a decrease of 40%. The decrease in research and development expenses was primarily attributable to a decrease in contract research costs as a result of the completion in 2013 of our relamorelin Phase 2 clinical trial. For the remainder of 2014, we expect our research and development expenses to remain relatively steady as we complete certain Phase 2 trials, initiate new trials for relamorelin and RM-493, and hire additional personnel in the clinical operations department.
General and administrative expense. General and administrative expense decreased by $250 to $1,503 for the six months ended June 30, 2014 from $1,753 for the six months ended June 30, 2013, a decrease of 14%. The decrease in general and administrative expenses was primarily attributable to reduction in patent expenses due to fewer patent filings in 2014 in comparison to the same period in 2013. For the remainder of 2014 we expect a slight increase in general and administrative expenses associated with hiring in the finance department.
Interest income. The decrease in interest income was nominal and is primarily attributable to a decrease in our investments outstanding for the six months ended June 30, 2014 compared to the six-month period ended June 30, 2013.
Liquidity and Capital Resources
Since our inception and through June 30, 2014, we have raised an aggregate of approximately $73,000 from the issuance of preferred stock. We also have been awarded a $1,350 grant from the Michael J. Fox Foundation to help fund our relamorelin Phase 2a clinical trial. As of June 30, 2014, we had $9,333 in cash, cash equivalents and marketable securities.
74
Cash flows
The following table provides information regarding our cash flows for the years ended December 31, 2012 and 2013, and the six months ended June 30, 2013 and 2014:
| |
Years Ended December 31, |
|
Six Months Ended June 30, |
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | Change | 2013 | 2014 | Change | |||||||||||||
| |
|
|
|
(unaudited) |
(unaudited) |
|
|||||||||||||
| |
(in thousands) |
|
|||||||||||||||||
Net cash provided by (used in): |
|||||||||||||||||||
Operating activities |
$ | (17,830 | ) | $ | (19,156 | ) | $ | (1,326 | ) | $ | (9,496 | ) | $ | (8,189 | ) | $ | 1,307 | ||
Investing activities |
(5,270 | ) | (5,128 | ) | 142 | (5,839 | ) | 9,929 | $ | 15,768 | |||||||||
Financing activities |
22,440 | 19,882 | (2,558 | ) | 8,939 | (240 | ) | $ | (9,179 | ) | |||||||||
| | | | | | | | | | | | | | | | | | | | |
Net decrease in cash and cash equivalents |
$ | (660 | ) | $ | (4,402 | ) | $ | (3,742 | ) | $ | (6,396 | ) | $ | 1,500 | $ | 7,896 | |||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Net cash used in operating activities
The use of cash in all periods resulted primarily from our net losses adjusted for non-cash charges and changes in components of working capital.
Net cash used in operating activities was $17,830 for the year ended December 31, 2012, and consisted primarily of a net loss of $18,130 adjusted for non-cash items, including depreciation and stock-based compensation. The significant items in the change in operating assets and liabilities includes an increase in accounts payable, accrued expenses and other current liabilities of $1,459 and an increase of approximately $1,258 in prepaid expenses and other current assets.
Net cash used in operating activities was $19,156 for the year ended December 31, 2013, and consisted primarily of a net loss of $21,431 adjusted for non-cash items, including depreciation and stock-based compensation. The significant items in the change in operating assets and liabilities includes an increase in accounts payable, accrued expenses and other current liabilities of $558 offset by a decrease of approximately $1,442 in prepaid expenses and other current assets.
Net cash used in operating activities was $9,496 for the six-month period ended June 30, 2013, and consisted primarily of a net loss of $10,821 adjusted for non-cash items including depreciation and stock-based compensation. The significant items in the change in operating assets and liabilities includes a decrease in accounts payable, accrued and other current liabilities expenses of $471 and a decrease of approximately $677 in prepaid expenses and other current assets.
Net cash used in operating activities was $8,189 for the six-month period ended June 30, 2014, and consisted primarily of a net loss of $6,968 adjusted for non-cash items including depreciation and stock-based compensation. The significant items in the change in operating assets and liabilities includes a decrease in accounts payable, accrued expenses and other current liabilities of $619 and an increase of approximately $700 in prepaid expenses and other current assets.
Net cash (used in) provided by investing activities
Net cash used in investing activities was $5,270 for the year ended December 31, 2012, and consisted primarily of purchases of marketable securities of $8,799 offset by maturities of marketable securities of approximately $3,529.
Net cash used in investing activities was $5,128 for the year ended December 31, 2013, and consisted primarily of purchases of marketable securities of $28,741 offset by maturities of marketable securities of approximately $23,613.
Net cash used in investing activities was $5,839 for the six months ended June 30, 2013, and consisted primarily of purchases of marketable securities of $15,447 offset by maturities of marketable securities of approximately $9,608.
75
Net cash provided by investing activities was $9,929 for the six months ended June 30, 2014, and consisted primarily of purchases of marketable securities of $3,404 offset by maturities of marketable securities of approximately $13,333.
Net cash provided by financing activities
Net cash provided by financing activities was $22,440 for the year ended December 31, 2012 and consisted primarily of the proceeds from the issuance of 45 million shares of our series B preferred stock.
Net cash provided by financing activities was $19,882 for the year ended December 31, 2013 and consisted of the proceeds from the issuance of 40 million shares of our series B preferred stock.
Net cash provided by financing activities was $8,939 for the six months ended June 30, 2013 and consisted of the proceeds from the issuance of 18 million shares of our series B preferred stock.
Net cash used in financing activities was $240 for the six months ended June 30, 2014, and consisted of issuance costs related to our planned initial public offering.
Funding requirements
We expect our expenses to increase in connection with our ongoing activities, particularly as we continue the clinical development of relamorelin and RM-493 and seek marketing approval for either of our product candidates. In addition, if we obtain marketing approval for either or both of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution to the extent that such sales, marketing and distribution are not the responsibility of potential collaborators. We also expect to incur additional costs associated with operating as a public company upon the closing of this offering.
We expect that the net proceeds from this offering, together with our existing cash, cash equivalents and marketable securities, will enable us to fund our operating expenses at least through the first half of 2016. We may need to obtain substantial additional funding in connection with our research and development activities and any continuing operations thereafter. If we are unable to raise capital when needed or on favorable terms, we would be forced to delay, reduce or eliminate our research and development programs or future commercialization efforts.
Our future capital requirements will depend on many factors, including:
- •
- the scope, progress, results and costs of clinical trials for our relamorelin and RM-493 programs;
- •
- the costs, timing and outcome of regulatory review of our relamorelin and RM-493 programs;
- •
- the obligations owed to Ipsen Pharma SAS, or Ipsen, pursuant to our license agreement;
- •
- the extent to which we acquire or in-license other product candidates and technologies;
- •
- the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property
rights and defending intellectual property-related claims; and
- •
- our ability to establish and maintain additional collaborations on favorable terms, if at all.
Developing our relamorelin and RM-493 programs is a time-consuming, expensive and uncertain process that may take years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of our product candidates that we do not expect to be commercially available for several years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all.
76
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. We do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise funds through additional collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our relamorelin and RM-493 programs on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market our relamorelin and RM-493 programs that we would otherwise prefer to develop and market ourselves.
Contractual obligations
The following table summarizes our significant contractual obligations as of payment due date by period at December 31, 2013:
| |
Total | 1 year | 2 to 3 years | 3 to 5 years | More than 5 years |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(in thousands) |
|
||||||||||||||
Operating lease obligations |
$ | 223 | $ | 138 | $ | 85 | $ | — | $ | — | ||||||
| | | | | | | | | | | | | | | | | |
Total |
$ | 223 | $ | 138 | $ | 85 | $ | — | $ | — | ||||||
| | | | | | | | | | | | | | | | | |
We enter into agreements in the normal course of business with CROs and CMOs for clinical trials and clinical supply manufacturing and with vendors for clinical research studies and other services and products for operating purposes. Excluded from the payments in the table are contractual obligations where the contracts are cancelable at any time by us, generally upon 30 days' prior written notice to the vendor.
Other than the specific payments noted in the table of contractual obligations and as described in footnote 1 above, milestone and royalty payments associated with our license agreements with Ipsen have not been included in the above table of contractual obligations as we cannot reasonably estimate if or when they will occur. Under the terms of each Ipsen license agreement, assuming that relamorelin or RM-493, as applicable, is successfully developed, receives regulatory approval and is commercialized, Ipsen may receive aggregate payments of up to $40.0 million upon the achievement of certain development and commercial milestones under such license agreement and royalties on future product sales. Substantially all of such aggregate payments under each Ipsen license agreement are for milestones that may be achieved no earlier than first commercial sale of relamorelin or RM-493, as applicable.
Off-balance sheet arrangements
We did not have, during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under applicable Securities and Exchange Commission rules.
Quantitative and qualitative disclosures about market risk
We are exposed to market risk related to changes in interest rates. As of December 31, 2013 and June 30, 2014, we had cash, cash equivalents and marketable securities of $17,762 and $9,333, respectively, consisting primarily of investments in U.S. government sponsored enterprise securities. Our
77
marketable securities are accounted for as available-for-sale securities and consisted of investments in U.S. government sponsored enterprise securities. As these securities were available to fund current operations, they are classified as current assets. Marketable securities are stated at fair value, with unrealized gains and losses included, until realized, as a component of other comprehensive income, which is a separate component of stockholders' equity. Realized gains and losses are determined on the specific-identification method and are included in investment income. There were nominal realized gains for the years ended December 31, 2012 and 2013, respectively. Other-than-temporary impairments of investments are recognized in the statements of operations if we have the intent to sell the security, or if it is more likely than not that we will be required to sell the security before recovery of the amortized cost basis. Even if we do not expect to sell a security, we evaluate expected cash flows to be received to determine if a credit loss has occurred. In the event of a credit loss, only the amount associated with the credit loss is recognized in the statements of operations. The amount of loss relating to other factors is recorded in other comprehensive income (loss). There have been no impairment losses recognized in any period presented. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments are in short-term marketable securities. Our marketable securities are subject to interest rate risk and could fall in value if market interest rates increase. A hypothetical change of 1% in U.S. interest rates would not be material to the carrying value of our investments.
We are not exposed to market risk related to change in foreign currency exchange rates.
JOBS Act
In April 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an "emerging growth company," or EGC, can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. Thus, an EGC can delay the adoption of certain newly implemented accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements under the JOBS Act. Subject to certain conditions, as an EGC, we intend to rely on certain of these exemptions, including without limitation, (i) providing an auditor's attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an EGC until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of this offering, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
78
Overview
We are a biopharmaceutical company focused on the development and commercialization of peptide therapeutics for the treatment of gastrointestinal, or GI, diseases, and genetic deficiencies that result in metabolic disorders. Our lead product candidate, relamorelin, is a potent, best-in-class, Phase 2 ghrelin agonist for the treatment of diabetic gastroparesis, a GI complication of diabetes, and other GI functional disorders. We are also developing a second product candidate, RM-493, which is a potent, first-in-class, Phase 2 ready melanocortin-4, or MC4, receptor agonist for the treatment of obesity caused by genetic deficiencies in the MC4 pathway. We believe our product candidates, which are both administered by subcutaneous injection, and for which we have exclusive worldwide rights, have the potential to treat these diseases for which there are currently limited therapeutic options. We believe that ghrelin and MC4 are compelling targets because of their critical role in regulating GI function and metabolism, and that peptide therapeutics are well-suited for activating these targets.
Relamorelin targets the receptor for ghrelin, which is a naturally occurring hormone that plays a critical role in GI motility, or the movement of food through the GI tract, digestion and the absorption of nutrients. Prior drugs targeted the same GI motility disorders, primarily through either the serotonin or dopamine receptors, but had significant safety issues. First generation ghrelin agonists were small molecules with limited potency and efficacy. In contrast, the relamorelin peptide retains the specificity and functionality of the naturally occurring ghrelin peptide, and is designed to increase GI motility, with markedly greater potency than both naturally occurring ghrelin and the first generation small molecule agonists of ghrelin.
RM-493 targets the receptor for MC4, which is a key pathway that regulates energy, homeostasis, and food intake. The first generation of MC4 agonists were predominantly small molecules that failed in clinical trials due to safety issues, particularly increases in blood pressure, and had limited efficacy. In contrast, the RM-493 peptide retains the specificity and functionality of the naturally occurring hormone that activates MC4, and our initial Phase 1 and Phase 2 clinical trials have shown promising evidence of weight loss without adversely increasing blood pressure.
Relamorelin: A Best-In-Class Phase 2 Ghrelin Agonist
Relamorelin is a potent, best-in-class, Phase 2 ghrelin agonist which stimulates GI motility, and aids digestion and the absorption of nutrients. We have completed a Phase 2 clinical trial of relamorelin for the treatment of diabetic gastroparesis. Based on consultation with regulatory authorities, we expect to initiate a Phase 2b clinical trial for the treatment of diabetic gastroparesis by the end of 2014. The U.S. Food and Drug Administration, or FDA, has granted Fast Track designation to relamorelin for the treatment of diabetic gastroparesis. Fast Track designation is designed to facilitate and expedite the review of drugs for serious conditions with high unmet need, in order to get drugs to patients earlier. We intend to manage our relamorelin clinical development program such that, if our clinical trials are successful, we can file a new drug application, or NDA, by 2018. We are also conducting a Phase 2a clinical trial for refractory chronic constipation in Parkinson's disease patients and a Phase 2a benchmark clinical trial in patients with chronic constipation. In September 2013, we were awarded a $1.35 million grant from the Michael J. Fox Foundation to help fund a Phase 2 clinical trial of relamorelin.
Diabetic gastroparesis is a disorder in which there is a substantial delay in stomach emptying along with characteristic symptoms of vomiting, nausea, abdominal pain, early satiety and bloating. Moderate to severe diabetic gastroparesis results in significant debility and hospitalizations, and can interfere with nutrition and the absorption of medications. An estimated 2.3 million type 1 and type 2 diabetic patients in the United States have moderate or severe gastroparesis symptoms. Available therapies to
79
treat this disorder are limited and exhibit significant side effects. No new therapies have been approved in the United States for the treatment of gastroparesis in more than 30 years.
To date, as part of our clinical program, we have treated approximately 260 subjects and patients with relamorelin. In our Phase 2 clinical trial, relamorelin had statistically significant effects on the most important diabetic gastroparesis symptoms, such as vomiting, as well as accelerated stomach emptying, and was well-tolerated. The results of these clinical trials support our belief that relamorelin has the potential to be a safe and effective treatment for diabetic gastroparesis.
We submitted an Investigational New Drug application, or IND, with the FDA for the indication of diabetic gastroparesis on October 20, 2010. In addition, we submitted a second IND to support an ongoing pilot study in anorexia nervosa on June 26, 2012. These INDs remain open and have not been subject to any clinical hold.
RM-493: A First-In-Class Phase 2 Ready MC4 Agonist
RM-493 is a potent, first-in-class, Phase 2 ready MC4 agonist, which modulates a key pathway in humans that regulates energy homeostasis and food intake. MC4's critical role in weight regulation was validated with the discovery that a heterozygous mutation of the MC4 receptor gene, a mutation in just one of the two copies of the MC4 receptor gene, results in early onset obesity and severe obesity. We expect to initiate two Phase 2a clinical trials in patients with genetic deficiencies in the MC4 pathway by the end of 2014, one for the treatment of obesity in patients with a single mutation of the MC4 receptor gene, and one in patients with Prader Willi Syndrome, or PWS.
Genetic defects in the MC4 gene are the most common genetic cause of obesity. A 2005 epidemiological study performed in Europe reported a prevalence of 1-2% of genetic defects in the MC4 gene in the obese population with a body mass index, or BMI, of greater than 30 kg/m2, and studies performed in both Europe and the United States, in 2000 and 2003, respectively, reported a prevalence of up to 4% of these genetic defects in more severely obese populations with a BMI of greater than 35 kg/m2. These prevalence rates suggest that there are approximately one million people in the United States with obesity caused by a mutation of the MC4 gene. These patients have a higher risk than the general population for early onset obesity and severe obesity and their complications such as diabetes. There are currently no approved drugs in the United States that have been studied in obesity due to MC4 deficiency.
PWS, another form of genetic MC4 deficiency, is an orphan disease with a prevalence ranging from approximately one in 8,000 to one of 25,000 patients in the United States. A hallmark of PWS is severe hyperphagia, an overriding physiological drive to eat, leading to severe obesity and other complications. For PWS patients, obesity is the greatest threat to their health, and these patients are likely to die prematurely as a result of choking, stomach rupture, or from complications caused by morbid obesity. Currently, there is no approved treatment for the obesity and hyperphagia associated with PWS. Based on recent scientific studies, we believe that deficiencies in the MC4 pathway are a cause of the obesity and associated symptoms of PWS, such as hyperphagia, and that an MC4 agonist can directly impact these symptoms.
To date, as part of our clinical program, we have treated approximately 190 subjects and patients with RM-493. We have completed a Phase 1b proof-of-concept clinical trial with RM-493 in obese patients, including one cohort of patients with mutations in one of the MC4 receptor genes. This clinical trial demonstrated promising weight loss in these patients with a four-week treatment.
We submitted an IND with the FDA for the indication of obesity on October 12, 2011. This IND remains open and has not been subject to any clinical hold.
80
Our Strategy
Based on the promising clinical results to date of relamorelin in diabetic gastroparesis, and the initial, favorable clinical profile of RM-493 for the treatment of obesity, including obesity caused by certain genetic deficiencies in the MC4 pathway, we believe these product candidates are well positioned to significantly improve the treatment of these indications. The key components of our strategy include:
- •
- Pursue development and regulatory approval for our lead product, relamorelin, for the treatment
of diabetic gastroparesis. We have completed a successful Phase 2 clinical trial of relamorelin for diabetic gastroparesis and plan to initiate a Phase 2b
clinical trial for this indication by the end of 2014. We plan to enroll and manage our Phase 2b and Phase 3 clinical trials such that, if the clinical trials are successful, we can file
an NDA by 2018.
- •
- Develop and seek regulatory approval for relamorelin in expansion indications in GI functional
disorders. We intend to target expansion indications for both upper and lower GI functional disorders where the unmet need is high, including idiopathic gastroparesis, or
gastroparesis without a specific cause, and refractory chronic constipation in Parkinson's disease patients. Assuming we receive regulatory approval for diabetic gastroparesis, we believe that
regulatory approval for these indications will follow thereafter.
- •
- Establish commercial and marketing capabilities in the United States
for relamorelin. We intend to establish our own commercial and marketing organization in the United States and to selectively establish
partnerships in markets outside the United States. We intend to target physicians who treat a high concentration of diabetic gastroparesis patients with a specialist sales force that focuses on
gastroenterologists and primary care physicians who are high prescribers of treatments for diabetic gastroparesis.
- •
- Develop RM-493 for obesity caused by genetic deficiencies in the MC4
pathway. We expect to initiate two Phase 2a clinical trials in patients with genetic deficiencies in the MC4 pathway by the end
of 2014, one for the treatment of obesity in patients with a single mutation of the MC4 receptor gene, and one in patients with PWS. We are taking a personalized medicine approach to developing RM-493
for use in restoring MC4 function in patients who have genetic deficiencies in the MC4 pathway. Based on FDA consultations to date, we believe we can seek an indication for obesity caused by genetic
deficiencies in the MC4 pathway. If we are successful, we believe the time to receive regulatory approval for this product candidate for the treatment of obesity caused by certain types of genetic
deficiencies in the MC4 pathway may be reduced. In some of the patients with deficiencies in the MC4 pathway, such as those with PWS, this is based on our belief that the number of patients required
for pivotal trials may be small based on previous indications approved in PWS patients. For others, such as the heterozygous MC4 patient population, the time to receive regulatory approval is more
uncertain, as we will need to show that this genetic subset will derive selective and meaningful benefit from RM-493 as a treatment for obesity.
- •
- Maximize the therapeutic potential of RM-493 in additional obesity indications. We will consider entering into relationships with strategic partners that enable the expansion of the ongoing clinical development of RM-493, while retaining significant value for us.
81
Our Product Pipeline
The following chart depicts key information regarding our product candidates, their indications, current states of development, and anticipated upcoming milestones:

Relamorelin: A Best-in-Class Phase 2 Ghrelin Agonist
Overview of the GI Functional Disorder Market
The health of the GI system has a major effect on an individual's daily activities and quality of life. A retrospective review published by the National Institute of Diabetes and Digestive and Kidney Diseases estimated that in 2004 there were more than 97 million ambulatory care visits by patients with a diagnosis of a GI disorder in the United States alone. The annual cost of these GI disorders in 2004, not including digestive cancers and viral diseases, was estimated to be greater than $114.0 billion in direct and indirect expenditures, including hospital, physician and nursing services as well as over-the-counter and prescription drugs. In 2008, Research and Markets in Business Insights estimated worldwide sales of GI drugs to be worth $49.9 billion, with approximately 38% of this market represented by sales in the United States totaling $18.8 billion. Four major GI market segments can be distinguished:
- •
- upper GI functional disorders resulting from impaired motility, including diabetic and idiopathic gastroparesis,
gastroesophageal reflux disease, or GERD, and functional dyspepsia;
- •
- lower GI functional disorders resulting from impaired motility affecting the small and large intestines, such as
constipation and irritable bowel syndrome, or IBS;
- •
- inflammatory GI disorders such as Crohn's disease and ulcerative colitis; and
- •
- disorders of organs involved in the digestive process, such as the gallbladder, liver and pancreas.
Historically, GI product development efforts have focused on indications with the largest patient populations, such as GERD, constipation, peptic ulcers and IBS. As a result, limited innovation has occurred in other segments of the GI market, in particular upper GI functional disorders, even though these disorders affect several million patients worldwide. Consequently, due to the limited safe and effective treatments available for upper GI functional disorders resulting from impaired motility, we believe there is a substantial market opportunity for us to address these significant unmet medical
82
needs. Therefore, we are focused on the subset of GI disorders associated with underlying defects in GI motility that affect both the upper and the lower GI tract.
Diabetic Gastroparesis
Our initial focus is on diabetic gastroparesis because there is a substantial unmet medical need, prevalence is high, and few therapeutic options exist. The only approved drug for gastroparesis has significant limitations and was approved more than 30 years ago. In addition, we believe successful clinical trials in diabetic gastroparesis will be the foundation for expansion into other upper GI functional indications resulting from impaired GI motility.
Diabetic gastroparesis is a debilitating GI disorder in which there is substantial delay in stomach emptying along with associated symptoms of vomiting, nausea, abdominal pain, early satiety and bloating. Patients often limit their food and liquid intake leading to poor nutrition and dehydration, which can ultimately require hospitalization. If untreated, diabetic gastroparesis causes significant acute and chronic medical problems, including additional diabetic complications resulting from poor glucose control.
Diabetes is the most commonly identified cause of gastroparesis. The underlying mechanism of diabetic gastroparesis is unknown although it is thought to be related in part to neuropathic changes in the major nerves that supply the GI tract. These nerves control the movement of food through the digestive tract and, when damaged, forward movement of food through the GI tract is delayed and/or vomiting may occur. The prevalence of diabetes in the United States is rapidly rising with the Centers for Disease Control estimating that one in 10 adults currently suffer from the disease. Sedentary lifestyles, poor dietary habits and a consequent rising prevalence of obesity are expected to cause this number to grow substantially. At present, an estimated 2.3 million type 1 and type 2 diabetic patients in the United States have moderate or severe gastroparesis symptoms and are seeking treatment for these symptoms.
Idiopathic Gastroparesis
Idiopathic gastroparesis afflicts a comparable number of non-diabetics who have the same symptoms of vomiting, nausea, abdominal pain, early satiety and bloating, along with delayed stomach emptying. Idiopathic gastroparesis, or gastroparesis without a specific cause such as diabetes, can be a manifestation of many systemic illnesses, neuromuscular dysfunction, a complication of select surgical procedures, or due to unknown causes. In a 1998 study, 29% of gastroparesis cases were associated with diabetes, 13% developed as a complication of surgery and 36% were due to unknown causes. According to the American Motility Society Task Force on Gastroparesis, 4% of the United States population experiences symptomatic manifestations of gastroparesis.
Limitations of Gastroparesis Treatment
Both diabetic and idiopathic gastroparesis have a significant impact on patients' lives, as well as significant economic impact. The number of gastroparesis-related hospitalizations has been increasing in the United States, suggesting an increasing prevalence of gastroparesis. Based on the most recent available data, which is from 2004, the economic impact of gastroparesis-related hospitalizations is significant and increasing. Vomiting is the symptom requiring the most medical intervention and is the leading cause of both emergency room visits and hospitalization for gastroparesis patients, followed by abdominal pain.
While there is a high prevalence of gastroparesis, there is a lack of safe and effective therapies approved for the treatment of this disorder. Reglan (metoclopramide), a mixed dopamine and serotonin receptor modulator, is the only drug indicated for the treatment of diabetic gastroparesis in the United States. Metoclopramide is only somewhat effective for short-term use. However, some patients
83
discontinue therapy due to intolerance and acute side effects, predominantly an involuntary movement disorder, known as tardive dyskinesia, the symptoms of which mimic Parkinson's disease. Even with this limitation, we believe metoclopramide continues to be prescribed for a substantial number of patients. In 2009, the FDA required that a black box warning be added to the metoclopramide label because of the risk of tardive dyskinesia with long-term use, and recommended that its use be limited to 12 weeks. The European Medicines Agency is even more stringent, and recommends metoclopramide for use no more than five days, and not for chronic conditions such as gastroparesis.
Other drugs that are used for diabetic gastroparesis but not approved for this indication in the United States are domperidone, a dopamine receptor modulator, and erythromycin, both of which stimulate stomach emptying. In addition, anti-emetics are used to attempt to manage vomiting and nausea. The efficacy of domperidone as a pro-motility agent is not fully established, and studies have demonstrated that domperidone may cause heart rhythm abnormalities. Erythromycin is a macrolide antibiotic that also is a motilin receptor agonist and is used primarily for short-term treatment of gastroparesis. Erythromycin has significant potential to interact with other medications and has also been associated with cardiac arrhythmias and death.
Propulsid (cisapride) was a widely prescribed prokinetic drug approved for GERD and used broadly for upper GI indications. It was frequently prescribed for gastroparesis symptoms though not approved for that indication. Sales of Propulsid exceeded $1 billion before being withdrawn due to cardiovascular safety issues.
Refractory Functional Dyspepsia and Refractory GERD
Functional dyspepsia is characterized by symptoms that are similar to gastroparesis, such as stomach pain or discomfort, early satiety, bloating, fullness, nausea, and vomiting. Functional dyspepsia occurs in approximately 10% of the general population, and approximately 25-35% of patients with functional dyspepsia have impaired stomach emptying. Up to one third of adult patients with GERD also have delayed stomach emptying and are refractory to drugs that treat acid reflux. Relamorelin is a potential therapy for both functional dyspepsia and refractory GERD, since these patients also suffer from impaired GI motility. However, we have not yet initiated clinical trials of relamorelin in patients with functional dyspepsia and refractory GERD.
There are currently no safe and effective GI motility, or prokinetic, drugs approved in the United States that address impaired motility in GI functional disorders. The need is particularly acute in refractory functional dyspepsia and refractory GERD, where few treatment options exist for chronic therapy.
Lower GI Functional Disorders
Twelve to 19% of the U.S. population seeks medical care annually for chronic constipation and IBS, and approximately 38% of this group is dissatisfied with traditional treatment options due to lack of efficacy. These conditions are two of the most common GI functional disorders, with significant health consequences and symptoms including constipation, abdominal pain, nausea, bloating, and decreased appetite.
Our initial relamorelin lower GI clinical trials focus on refractory constipation, including refractory constipation in patients with Parkinson's disease. Approximately one million people in the United States are living with Parkinson's disease. Constipation is common among patients with Parkinson's disease, with studies reporting that more than 50% of patients suffer from moderate to severe constipation, many of whom are refractory to existing therapies. In addition, GI functional disorders in Parkinson's disease patients can affect the upper GI tract, resulting in gastroparesis symptoms. GI functional disorders in Parkinson's disease patients also may undermine the GI absorption of L-DOPA, interfering with this drug's efficacy in managing the symptoms of Parkinson's disease.
84
Current treatments for lower GI functional disorders are predominantly over-the-counter medications such as laxatives, stool softeners or fiber supplementation, as well as changes to diet. The newest prescription therapies for chronic constipation and IBS are Amitiza (lubiprostone) and Linzess (linaclotide). There remains a large number of patients who are refractory to these treatments, resulting in a significant unmet medical need for effective therapies. Zelnorm (tegaserod) was a GI motility drug approved for IBS in the United States, where sales exceeded $500 million, but it was eventually withdrawn due to cardiovascular safety issues.
Relamorelin: Clinical Development
We have completed a Phase 2 clinical trial of relamorelin, which is also known as RM-131, in diabetic gastroparesis. We currently plan to initiate a Phase 2b clinical trial in diabetic gastroparesis by the end of 2014. The data from the completed Phase 2 clinical trial in diabetic gastroparesis showed statistically significant improvements in vomiting symptoms, and in a focused subgroup, relamorelin also showed statistically significant improvements in the other symptoms of gastroparesis. Our clinical trials showed relamorelin to be generally well-tolerated.
Relamorelin has demonstrated an ability to accelerate both stomach emptying, which is important for the diabetic gastroparesis indication, and small bowel and colonic motility, which is important for lower GI indications, supporting the theory that relamorelin has effects on GI motility across the whole GI tract. The FDA has granted Fast Track designation to relamorelin for the treatment of diabetic gastroparesis.
In addition, we are currently conducting a Phase 2a clinical trial of relamorelin for refractory chronic constipation in patients with Parkinson's disease.
85
The following chart depicts the completed and ongoing relamorelin Phase 2 clinical trials:
Relamorelin: Completed and Ongoing Phase 2 Clinical Trials
Population
|
Diabetic Gastroparesis |
Chronic Constipation |
Refractory Constipation in Parkinson's Disease |
|||
|---|---|---|---|---|---|---|
Clinical trial phase |
Phase 2 | Phase 2a | Phase 2a | |||
Status |
Phase |
Initial biomarker data complete |
Ongoing |
|||
Treatment groups |
Placebo, relamorelin |
Placebo, relamorelin |
Placebo, relamorelin |
|||
Dose |
10 mcg once daily |
100 mcg once daily |
100 mcg once daily |
|||
Number of patients(3) |
204(2) |
48 |
56 |
|||
Duration of relamorelin treatment |
4 weeks |
2 weeks |
2 weeks |
|||
Endpoints |
Stomach emptying; |
Lower GI motility; |
Constipation |
- (1)
- One
Phase 2 clinical trial (RM-131-004) is complete. We currently plan to initiate a Phase 2b clinical trial in diabetic gastroparesis by the
end of 2014.
- (2)
- A
very small cohort of additional patients and dosing regimens was used adaptively to provide initial variability estimates to design the main part of the
clinical trial.
- (3)
- Approximate
number of patients.
- (4)
- Using a Rhythm-developed Patient Reported Outcome, or PRO, instrument, that has not yet been validated by the FDA.
mcg=microgram
Relamorelin: Clinical Development in Diabetic Gastroparesis
Primary and Secondary Clinical Trial Endpoints
We measured stomach emptying in Phase 1 and Phase 2 clinical trials as an important biomarker for effects on upper GI motility, and we plan to continue evaluating stomach emptying in our Phase 2b and Phase 3 clinical trials. However, we anticipate that diabetic gastroparesis symptoms are likely to be the primary and key secondary endpoints for our Phase 2b and Phase 3 clinical trials, and that stomach emptying data will only be a supportive endpoint.
We measured stomach emptying primarily using the Gastric Emptying Breath Test, or GEBT, a simple, non-invasive, non-radioactive breath test designed to simply measure stomach emptying of solids in humans. While we have primarily used the GEBT as a clinical endpoint to measure rates of stomach emptying, the GEBT is being developed by AB Diagnostics to identify patients with delayed stomach emptying (gastroparesis), though it is currently not approved by the FDA. If the GEBT is not approved, it is possible that the FDA may not allow clinical data measured by the GEBT (stomach emptying) to be reflected in the product labeling. However, if approved, we believe the increased use
86
of the GEBT in the healthcare industry will simplify the ability of physicians to diagnose diabetic gastroparesis.
We measured diabetic gastroparesis symptoms, which include vomiting, nausea, abdominal pain, early satiety and bloating, using a PRO. A composite measure combining four of the subjective symptoms, including nausea, abdominal pain, early satiety, and bloating (excluding vomiting), may also be analyzed. This PRO has been developed, and is in the process of being validated, by us in collaboration with the FDA and in a manner consistent with FDA guidance. However, since the PRO is likely to be a key endpoint in relamorelin clinical trials in diabetic gastroparesis, validating the PRO questionnaire for measuring gastroparesis symptoms is a critical step in relying on symptom endpoint data to support regulatory approval.
Phase 2b and Phase 3 Clinical Development Programs
We expect to initiate our Phase 2b clinical trial of relamorelin by the end of 2014, and we expect it will include approximately 200-400 moderate to severe diabetic gastroparesis patients receiving 12 weeks of treatment. Based on initial discussions with the FDA, we anticipate that the pivotal Phase 3 clinical trials required for regulatory approval in the United States of relamorelin for the treatment of diabetic gastroparesis will assess three months of treatment. We expect that for both our Phase 2b clinical trial and our Phase 3 clinical trial, key endpoints will be improvement in diabetic gastroparesis symptoms as assessed with a PRO. We intend to also measure stomach emptying as a supportive endpoint. Our plan is to manage our relamorelin clinical development program such that, if our clinical trials and chronic toxicology and carcogenicity studies are successful, we anticipate filing an NDA by 2018.
Phase 2 Clinical Trial
We designed our Phase 2 clinical trial to evaluate the effect of relamorelin in patients with moderate to severe diabetic gastroparesis on (i) the primary endpoint of GI motility by measuring stomach emptying using the GEBT, (ii) the secondary endpoints of the symptoms of diabetic gastroparesis, assessed with a PRO, and (iii) safety. The clinical trial assigned patients to treatment with either placebo or relamorelin administered either 10 mcg once daily or twice daily for a four-week period. The clinical trial's primary endpoint was the effect of relamorelin on stomach emptying.
Analysis of the data indicates that relamorelin administered twice daily for four weeks in patients with moderate to severe diabetic gastroparesis:
- •
- resulted in statistically significant acceleration of stomach emptying;
- •
- resulted in statistically significant improvements in vomiting symptoms;
- •
- in a subgroup of approximately 60% of patients who had vomiting symptoms at the time the clinical trial started, had
statistically significant improvements in a composite of the other symptoms of diabetic gastroparesis—nausea, abdominal pain, bloating, and early satiety, in addition to improving stomach
emptying and vomiting; and
- •
- was generally well-tolerated, with little evidence of safety concerns. Overall, the most frequent adverse experiences included constipation, urinary tract infection, changes in diabetic status, headache and dizziness, though incidence was low and generally similar between placebo and treatment groups.
Phase 2 Diabetic Gastroparesis Clinical Trial Design
This clinical trial was randomized and double-blind, and each patient was evaluated over a one-week baseline period, then assigned to one of three subcutaneous treatments for four weeks:
87
(i) placebo, or a sugar solution, (ii) 10 mcg of relamorelin once daily, or (iii) 10 mcg of relamorelin twice daily (with all patients receiving two injections per day). Patients had a mean age of approximately 55 years, were 10-15% type 1 diabetics and 86-90% type 2 diabetics, and the duration of their diabetes ranged from nine to 28 years. Patients also displayed signs and symptoms of moderate to severe diabetic gastroparesis. Stomach emptying was measured at baseline and day 28, and daily PRO-recorded patient symptoms included vomiting frequency and severity. Safety was carefully evaluated, including adverse events. Sixty-seven to 69 patients were randomized to each treatment group.
Phase 2 Diabetic Gastroparesis Clinical Trial Results
Relamorelin, administered twice daily for four weeks in patients with moderate to severe diabetic gastroparesis, showed statistically significant improvement in stomach emptying and symptoms of diabetic gastroparesis:
- •
- Met the primary endpoint: relamorelin showed statistically significant acceleration in stomach emptying in patients with
diabetic gastroparesis by 15.4 minutes versus placebo (p=0.03).
- •
- Met the following key secondary endpoints: relamorelin resulted in clinically important, statistically significant
improvements in vomiting. Weekly vomiting episodes were reduced by 63% versus placebo (p=0.033) and ratings of vomiting severity were reduced by 58% versus placebo (p=0.005).
- •
- For the subjective diabetic gastroparesis symptoms (nausea, abdominal pain, bloating, and early satiety), there was a
strong placebo effect in the overall clinical trial group; relamorelin improved symptoms versus placebo but did not reach statistical significance.
- •
- A retrospective analysis was conducted in the large subgroup of patients who had vomiting symptoms at the time the
clinical trial started, which was approximately 60% of patients. We focused on this subgroup because vomiting is usually considered the most serious and bothersome symptom in diabetic gastroparesis,
and is often the symptom that brings patients into treatment and/or for hospitalization. In this subgroup of patients, relamorelin showed statistically significant improvements on the signs and
symptoms of diabetic gastroparesis:
- •
- Met the primary clinical trial endpoint in this subgroup: relamorelin showed statistically significant acceleration in
stomach emptying by 25.0 minutes versus placebo (p=0.02).
- •
- Met key secondary endpoints in this subgroup: relamorelin resulted in clinically important, statistically significant
improvements in vomiting. Weekly vomiting episodes were reduced by 63% versus placebo (p=0.041) and ratings of vomiting severity were reduced by 59% versus placebo (p=0.006).
- •
- Met other key secondary endpoints in this subgroup: relamorelin showed improvements in the other subjective symptoms of diabetic gastroparesis—nausea, abdominal pain, bloating, and early satiety. A composite score of these four subjective symptoms showed statistically significant differences from placebo (p=0.043).
For almost all endpoints, relamorelin administered once daily showed less efficacy than twice daily treatment, and did not show statistical significance versus placebo.
88
Relamorelin: Effects on Individual Diabetic Gastroparesis Symptoms in Patients with
Vomiting Symptoms at the Start of the Clinical Trial(1)
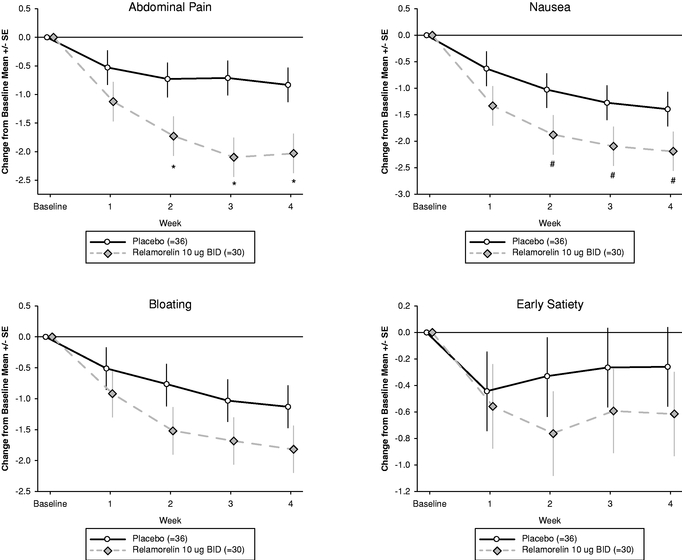
- (1)
- BID =
twice daily
- *
- p-value
<0.05 active versus placebo. A p-value is a statistical calculation that relates to the probability that a difference between groups
happened by chance. Generally, the FDA considers a p-value less than 0.05 a statistically significant result sufficient to demonstrate efficacy in pivotal Phase 3 clinical trials. A p-value
0.05-1.0 may, in earlier stage trials, suggest clinical activity supportive of efficacy and worthy of further exploration in Phase 3 clinical trials.
- #
- 0.05< p-value <0.10
89
Relamorelin: Effects on a Composite Analysis of Diabetic Gastroparesis Symptoms in Patients with
Vomiting Symptoms at the Start of the Clinical Trial(1)(2)
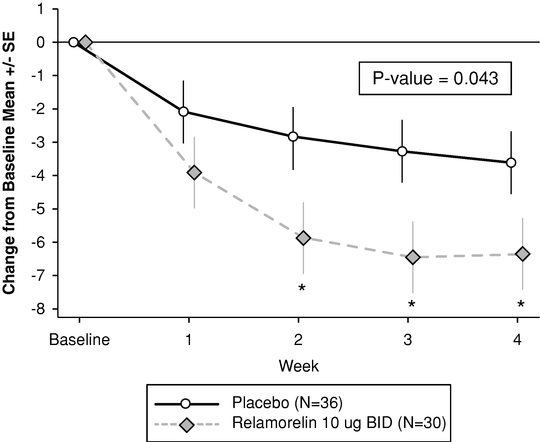
- (1)
- BID =
twice daily
- (2)
- Composite
analysis of four subjective symptoms (nausea, abdominal pain, early satiety, bloating).
- *
- p-value <0.05 active versus placebo
Phase 2 Diabetic Gastroparesis Clinical Trial Safety Data
There was little evidence of safety concerns for relamorelin in this clinical trial, and it was well-tolerated. Of the 204 patients in the three treatment groups, 179, or 87.7%, completed the clinical trial. Discontinuations due to adverse events after initiation of treatment in the clinical trial, or TEAEs, were rare, and occurred in two, five, and one patients in the placebo, 10 mcg once daily and 10 mcg twice daily treatment groups, respectively. Treatment emergent serious adverse experiences were also rare, and occurred in two, one and four patients in the placebo, 10mcg once daily and 10mcg twice daily treatment groups, respectively, and none were considered drug-related. Overall, TEAEs were evenly distributed between the placebo and treatment groups. There were 30 (43.5%), 32 (47.8%,) and 25 (36.8%) TEAEs in the placebo, 10 mcg once daily and 10 mcg twice daily treatment groups, respectively. The most frequent adverse events included constipation, urinary tract infection, changes in diabetic status, headache and dizziness, which were of low incidence and generally similar between placebo and treatment groups. Laboratory test data (including fasting glucose), vital signs (including weight), electrocardiograms, physical exam and injection site evaluations showed little, if any evidence of adverse changes with treatment.
90
Phase 1 Clinical Development in Diabetic Gastroparesis
We completed three Phase 1 clinical trials in both healthy volunteers and in patients with type 1 and type 2 diabetes suffering from diabetic gastroparesis. Our Phase 1 clinical trials in 71 healthy volunteers demonstrated the positive effects of relamorelin on stomach emptying, with good safety and tolerability.
In the single ascending dose clinical trial conducted in healthy volunteers, single doses from 3 to 2400 mcg were studied. In the multiple ascending dose clinical trial conducted in 40 healthy volunteers, 32 of whom received relamorelin, multiple doses from 10 to 300 mcg per day were studied. In that study, relamorelin reduced the time of stomach emptying by 35-50%. To confirm the positive effects of relamorelin on stomach emptying, we conducted a two-part, Phase 1b, single dose crossover clinical trial in 20 type 1 and type 2 diabetic patients suffering from diabetic gastroparesis. In that clinical trial, a single dose 100 mcg of relamorelin substantially improved stomach emptying, accelerating stomach emptying by 54% in type 1 diabetics and 66% in type 2 diabetics versus placebo (p=ns and p=0.011, respectively). In addition, the type 1 diabetic patients had statistically significant improvement in diabetic gastroparesis symptoms (p<0.041).
Preclinical Development in Diabetic Gastroparesis
We have extensively profiled relamorelin in preclinical studies, which demonstrated strong potency for accelerating stomach emptying when compared to naturally occurring ghrelin and first generation ghrelin agonists. For example, relamorelin was more than 1000-fold more potent in restoring gastric emptying in a rodent model of delayed gastric emptying when compared directly to TZP-102, a small molecule ghrelin agonist developed by Tranzyme for the treatment of diabetic gastroparesis. In addition, we have shown in preclinical models that relamorelin has strong anti-inflammatory effects. We completed one and three month toxicology studies, with doses and exposures between 100-760 fold greater than the Phase 2 clinical trial doses and exposures, without evidence of clinically relevant toxicological findings. We plan to initiate carcogenicity studies, one of which is expected to be two years in length.
Relamorelin Formulation Development
Our previously conducted and ongoing clinical trials, and our planned Phase 2b clinical trial, use a subcutaneous injection with an insulin gauge needle. We are developing two new formulations of relamorelin. We are developing a 28-day pen injector for administering relamorelin as a subcutaneous injection that is convenient for patients. We anticipate that the initial NDA filing of relamorelin for diabetic gastroparesis will use this formulation. We also are in preclinical development of a patch formulation of relamorelin that uses a third-party proprietary microneedle technology. We believe the patch formulation could lead to market expansion opportunities by providing a more convenient treatment alternative to injection and may extend patent protection. We will supply both the pen injector and patch formulations prefilled with relamorelin. We expect that both the pen injector and patch formulations will be classified as drug-device combination products. Both formulations will require FDA approval before marketing and we expect the device constituents will be reviewed as part of each NDA. Some safety and/or efficacy testing of the device alone may be necessary.
Relamorelin: Clinical Development in Lower GI Disorders
Phase 2 Clinical Trials in Lower GI Disorders
Relamorelin is in Phase 2 development for lower GI functional disorders. Our first Phase 2a clinical trial is designed to evaluate the effects of relamorelin on constipation symptoms and on biomarkers of lower GI motility in patients with chronic constipation. Our second Phase 2a clinical trial, which is called the MOVE-PD Study, is designed to evaluate the effects of relamorelin on constipation symptoms in Parkinson's disease patients with refractory constipation.
91
Phase 2a Clinical Trial in Chronic Constipation
In our first lower GI Phase 2a clinical trial of relamorelin, administered by subcutaneous injection at a dose of 100 mcg once daily for two weeks, we assessed the effect of relamorelin compared to placebo on GI and colonic motility (primary objective) as well as on symptoms of chronic constipation. We enrolled 48 patients in the clinical trial, with a pre-specified interim analysis of the GI motility biomarkers on the first 24 patients who completed the clinical trial. This allows us to benchmark relamorelin against other constipation therapies that have been studied previously in similar clinical trials. All 48 patients are included in the clinical symptom analysis using an intention-to-treat approach.
This Phase 2a clinical trial also demonstrated an improvement in both GI motility and clinical symptoms. The initial biomarker results on GI motility were presented in May 2014 at Digestive Diseases Week 2014, the leading GI scientific meeting. The biomarker data indicates that relamorelin administered once daily for two weeks in patients with chronic constipation resulted in statistically significant acceleration of lower GI motility, supporting utility in the treatment of chronic constipation. This clinical trial also demonstrated improvement in other biomarkers of GI motility, stomach emptying and small bowel motility, confirming relamorelin's pro-motility effect on both upper and lower GI function. In addition, relamorelin improved clinical symptoms of constipation. Specific highlights of the clinical trial include:
- •
- Relamorelin showed statistically significant acceleration in colonic motility at 32 and 48 hours after ingestion
of a meal (p=0.029 and p=0.012, respectively), but not at 24 hours.
- •
- Relamorelin also showed statistically significant acceleration in stomach emptying (p=0.032) and a strong trend toward
significance in small bowel motility (p=0.051).
- •
- Relamorelin also showed statistically significant improvements in the clinical symptoms of spontaneous bowel movements (p=0.002), ease of stool passage (straining; p=0.042), and time to first bowel movement (p=0.004), but not in stool consistency (Bristol Stool Scale; p=0.184). We are awaiting results on additional clinical symptoms.
Phase 2 Clinical Trial in Parkinson's Disease Patients with Refractory Constipation
In our second lower GI Phase 2a clinical trial, the MOVE-PD Study, we are evaluating the effects of relamorelin on symptoms of refractory constipation in Parkinson's disease patients. The majority of Parkinson's disease patients suffer from moderate to severe chronic constipation, which can be the initial hallmark of the disease, and may appear before motor symptoms become apparent. We are studying patients who suffer moderate to severe constipation and are refractory to previous therapies.
In our MOVE-PD Study, we are assessing the effect of relamorelin, administered by subcutaneous injection at a dose of 100 mcg once daily for two weeks, on the symptoms of constipation. We are conducting the MOVE-PD Study in partnership with the Parkinson's Study Group, one of the largest academic Parkinson's disease clinical consortia. This clinical trial is supported with a $1.35 million grant from the Michael J. Fox Foundation, which we believe underscores the priority within the Parkinson's disease community concerning better treatments for these GI complications. In consideration for the grant, we must endeavor to complete the study according to a mutually agreed timeline as well as submit certain required communications and deliverables. Failure to perform these obligations may result in the suspension or termination of payments under the grant. In the event that relamorelin generates commercial sales in the indication of chronic constipation in Parkinson's disease above a certain threshold, we are obligated to pay a limited royalty to the Michael J. Fox Foundation up to the amount of the grant. We anticipate that enrollment will be complete, and data will be available for the MOVE-PD study, in the second half of 2015.
92
Phase 1 Clinical Trial Results Supporting Lower GI Indications
Early biomarker data from our Phase 1 multiple ascending dose clinical trial of relamorelin in healthy volunteers also support the effects of relamorelin on lower GI motility. In this clinical trial, patients swallowed an ingestible wireless medical sensor, which allowed us to measure the time for the sensor to pass through the digestive tract. In this Phase 1 clinical trial, our exploratory analysis showed a strong trend that relamorelin reduced colonic motility time by up to 54%—from approximately 42 hours in placebo subjects to approximately 18 hours in those receiving relamorelin. This was achieved with little, if any, evidence of associated symptoms, such as diarrhea, abdominal pain or abdominal cramping. We presented these results at the American College of Gastroenterology 2012 Annual Scientific Meeting.
RM-493: A First-in-Class Phase 2 Ready MC4 Agonist
Our second product candidate, RM-493, is entering into Phase 2 clinical development for the treatment of obesity caused by genetic deficiencies in the MC4 pathway. RM-493 is a potent, first-in-class, Phase 2 ready MC4 agonist peptide administered by subcutaneous injection. MC4 modulates a key pathway in humans that regulates energy, homeostasis and food intake. The critical role of MC4 in weight regulation was validated with the discovery that a mutation of the MC4 receptor gene resulted in early onset obesity and severe obesity. The first generation of MC4 agonists were small molecules that failed primarily due to safety issues, particularly increases in blood pressure. In contrast, RM-493 is a peptide that retains the specificity and functionality of the naturally occurring hormone that activates MC4, and has not been shown to adversely affect blood pressure in our Phase 1 and Phase 2 clinical trials.
Market Overview
We are preparing to initiate two Phase 2a clinical trials by the end of 2014, each for the treatment of obesity caused by a specific genetic deficiency in the MC4 pathway. The first clinical trial is a personalized medicine approach for treating obesity in people with a mutation of the MC4 receptor gene to assess weight loss and safety. We have completed a Phase 1b proof-of-concept clinical trial of RM-493 in this patient population that demonstrated weight loss with four-week treatment. Our second RM-493 Phase 2a clinical trial is in PWS patients; recent scientific evidence supports a role for genetic defects in the MC4 pathway in PWS.
Obesity Caused by Genetic Deficiencies in the MC4 Pathway
Mutation of the MC4 Receptor Gene
MC4 deficiency due to heterozygous mutations in the MC4 gene is the most common genetic cause of obesity. RM-493 may restore MC4 function by increasing activity in the one normal copy of the MC4 gene (see figure below). A 2005 epidemiological study performed in Europe reported a prevalence of 1-2% of genetic defects in the MC4 gene in the obese population with a body mass index, or BMI, of greater than 30 kg/m2, and studies performed in both Europe and the United States, in 2000 and 2003, respectively, reported a prevalence of up to 4% of these genetic defects in more severely obese populations with a BMI of greater than 35 kg/m2. These prevalence rates suggest that there are approximately one million people in the United States with obesity caused by a mutation of the MC4 gene. These patients have a higher risk than the general population for early onset obesity and severe obesity and their complications such as diabetes. Furthermore, MC4 deficiency may offset the beneficial effects of diet and exercise for sustained weight loss, limiting treatment options for these individuals.
93
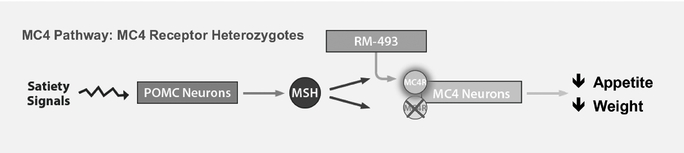
Prader Willi Syndrome, or PWS
PWS, another form of genetic MC4 deficiency, is an orphan disease with a prevalence ranging from approximately one in 8,000 to one in 25,000 patients in the United States. PWS patients exhibit intellectual disability and delayed growth. A hallmark of the disease is severe hyperphagia, which leads to severe obesity and other complications.
For PWS patients, obesity is the greatest threat to their health and these patients often die at a young age from obesity-related complications. Hyperphagia has a significant negative impact on the patients' quality of life as well as causes obesity and a range of associated co-morbidities. Normal satiety, or the feeling of fullness after eating, does not exist in a person with PWS. The physiological drive to eat is so powerful and overwhelming that most PWS patients will go to great lengths to eat large quantities of food, even if it is spoiled, indigestible, or unpalatable to others.
Hyperphagia impairs the PWS patients' ability to live independently, requiring costly and constant supervision to prevent overeating. Without supervision, PWS patients are likely to die prematurely as a result of choking, stomach rupture or tissue necrosis, or from complications caused by morbid obesity, such as right heart failure and respiratory failure. While a small number of PWS patients are cared for in costly group homes, the majority of PWS patients are cared for in their homes and their families undertake substantial effort to create physical barriers to eating. These efforts result in extremely stressful environments as caregivers often place locks and alarms on cabinets and refrigerators that contain food to impede PWS patients' efforts to obtain food at all times. The typical annual cost of treating a PWS patient is approximately $100,000, excluding the often significant costs of drug therapies related to other medical and psychological conditions, and the costs of any lost time from work experienced by their families due to responsibilities related to the care of a PWS patient.
Currently, there is no approved treatment for PWS, and most research to date has targeted the treatment of specific symptoms. For many individuals affected by the disorder, the elimination of some of the most difficult aspects of the syndrome, such as hyperphagia and obesity, would represent a significant improvement in quality of life and provide the potential opportunity for patients to live independently.
The genetics of PWS are complex, involving several genes on chromosome 15 that are not properly expressed. Recent discoveries highlight a defect in one of these, the MAGEL2 gene, as one of the dominant contributors to PWS pathology. Defects in MAGEL2 in humans result in autism spectrum disorders, reduced intellectual ability and most aspects of behavior and metabolism associated with PWS. Unraveling the function of the MAGEL2 gene in MAGEL2 deficient mice revealed functionally defective pro-opiomelanocortin, or POMC, neurons. These neurons normally promote satiety by activating MC4. This inherent defect in POMC neurons can be bypassed and MAGEL2 deficient mice are responsive to therapeutic activation of the MC4 receptor, resulting in control of appetite (see figure below). These findings provide the rationale for the treatment of PWS patients with an MC4 agonist.
94
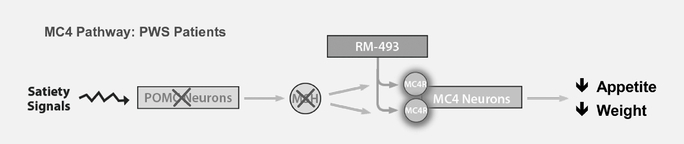
Limitations of Current Therapies
First generation MC4 agonists were predominantly small molecules that were neither effective nor safe, and no therapies that target MC4 have been approved for any indication. Although drugs approved for general obesity can be used in obese patients with MC4 deficiencies, there are currently no approved treatments in the United States specifically for these indications—either for obesity caused by heterozygous mutations of the MC4 gene or for PWS. In addition, the available treatments for general obesity have significant shortcomings.
In general obesity, there is a clear need for effective anti-obesity drugs that are a viable alternative to surgery. Drugs that can deliver sustained, clinically-relevant weight loss with good safety have the potential to improve healthcare outcomes dramatically. However, treatment options are limited with few approved drugs available.
Orlistat (tetrahydrolipstatin), Adipex-P (phentermine), Qsymia (phentermine/topiramate), and Belviq (lorcaserin) are pharmaceutical products that have been approved for the treatment of obesity in the United States. Orlistat is marketed in the United States by Roche Holdings AG under the brand name Xenical and is generally prescribed for short-term use. Orlistat is associated with GI side effects, the nature of which can be socially constraining. These include flatulence and fecal incontinence and urgency. Orlistat was also launched in 2007 by GlaxoSmithKline plc in over-the-counter form at half the prescribed dose under the brand name allí. Phentermine is also generally prescribed for short-term use. Phentermine is a Schedule IV controlled substance and, according to the FDA-approved product information, has an amphetamine-like profile, an increased risk for abuse potential, and may be associated with adverse cardiovascular or central nervous system effects. Vivus, Inc. commercially launched its combination phentermine/topiramate product in the United States under the name Qsymia in September 2012. Qsymia treatment has resulted in approximately 7% placebo-subtracted weight loss over a period of one year. In June 2012, Arena Pharmaceuticals, Inc., or Arena, obtained FDA approval for its product, lorcaserin, which was launched in the United States in June 2013 under the name Belviq. Belviq treatment has demonstrated only modest weight loss. Both Qsymia and Belviq have an increased risk for abuse potential and, therefore, both are designated as Schedule IV controlled substances. The FDA also required a Risk Evaluation and Mitigation Strategy, or REMS, for Qsymia to inform prescribers and women of reproductive potential about the increased risk of congenital malformation in infants exposed to Qsymia in early pregnancy.
RM-493: Phase 2 Clinical Development
We are initiating Phase 2a clinical trials of RM-493 for the treatment of obesity caused by genetic deficiencies in the MC4 pathway. Our RM-493 clinical program targets segments of the obese population that are genetically defined by defects in the MC4 pathway, as a personalized medicine and focused approach to restore function and improve weight regulation in these patients. Based on FDA consultations to date, we believe we can seek an indication for obesity caused by genetic deficiencies in the MC4. If we are successful, we believe the time to receive regulatory approval for this product candidate may be reduced.
95
We plan to initiate two Phase 2a clinical trials, one in each of two genetically targeted populations: (1) those with MC4 deficiency caused by heterozygous mutations of the MC4 gene; and (2) those with PWS. Proof-of-concept for patients with heterozygous mutations of the MC4 gene has been achieved in one cohort in a four-week, Phase 1b clinical trial. We plan to initiate these clinical trials by the end of 2014.
We intend to use the results of our Phase 2a clinical trials of RM-493 in these indications potentially as the foundation for planning pivotal registration clinical trials. It is our intention to work with the FDA on a focused clinical program for each indication.
To date, most of our RM-493 clinical trials have been in the general obese population. We are currently conducting a Phase 2a clinical trial in general obese patients, with interim 12-week weight data now available. In preceding Phase 1 clinical trials of two to four weeks, patients treated with RM-493 achieved weight loss without adversely increasing blood pressure.
RM-493: Phase 2 Clinical Program in MC4 Deficiency Obesity and General Obesity
| |
MC4 Gene Heterozygous Mutation Obesity |
Prader Willi Syndrome (PWS) |
General Obesity | |||
|---|---|---|---|---|---|---|
Clinical trial phase |
Phase 2a | Phase 2a | Phase 2a | |||
Status |
Initiates 4Q2014 |
Initiates 4Q2014 |
Ongoing |
|||
Patient population |
MC4 gene heterozygous mutation |
PWS |
General obesity |
|||
Treatment groups |
RM-493 (SQ Injection QD), |
RM-493 (SQ Injection QD), |
RM-493 (SQ Injection QD), |
|||
Number of patients(1) |
30 - 50 |
36 |
100 |
|||
Patient demographics |
BMI 30 - 60 kg/m2 in MC4 deficient |
PWS Adults/Adolescents |
BMI 30 - 40 kg/m2 |
|||
Duration of treatment |
12 weeks |
8 - 10 weeks |
12 weeks |
|||
Location |
United States, Canada, Europe |
United States |
United States |
- (1)
- Approximate number of patients.
SQ = subcutaneous
QD = once daily
RM-493: Clinical Development Program in Genetically Defined Segments of Obesity
Phase 1 and Phase 2a Clinical Development in Patients with Heterozygous MC4 Gene Mutations
We plan to initiate a Phase 2a clinical trial in patients who are obese and have a heterozygous genetic mutation of the MC4 gene resulting in full or partial loss of MC4 function. We anticipate that approximately 30-50 patients, identified by genetic screening, will be chosen at random to receive either placebo or RM-493 by once daily subcutaneous injection for approximately 12 weeks of treatment. The primary endpoint for this clinical trial is expected to be percent weight loss, with additional endpoints including metabolic parameters that are likely to be improved by substantial weight loss. We plan to
96
initiate this clinical trial by the end of 2014, and to conduct this clinical trial in the United States, Canada, and Europe.
We established proof-of-concept for efficacy of RM-493 in patients with an MC4 heterozygous genetic mutation in one cohort of patients in our Phase 1 clinical trial. This clinical trial was a double-blind, placebo-controlled, randomized Phase 1b clinical trial designed to evaluate the effect of RM-493 on weight loss and safety in obese patients, including the one cohort of patients with a heterozygous mutation of the MC4 gene. The initial cohort of eight patients treated for four weeks with RM-493 or placebo showed approximately 2.6kg more weight loss in the RM-493 treatment group than in the placebo group. Other parameters supporting weight loss were also positively affected by RM-493. We believe that these results support the hypothesis that RM-493 can be effective in weight loss in MC4 deficient patients, and support the initiation of our Phase 2a clinical trial.
The following chart depicts preliminary data relating to our RM-493 Phase 1b clinical trial in heterozygous patients:
RM-493 Phase 1b MC4 Heterozygous Patients: Placebo Subtracted Differences(1)(2)
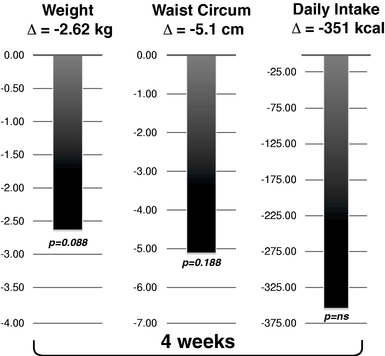
- (1)
- Over
four weeks of treatment with RM-493 0.01 mpk/day by continuous subcutaneous infusion.
- (2)
- Preliminary data.
Phase 2 Clinical Development in PWS Patients
We plan to initiate a Phase 2a, proof-of-concept, double-blind, placebo-controlled, randomized clinical trial in approximately 36 PWS patients for up to eight weeks of active treatment administered once daily by subcutaneous injection, to assess the effects of RM-493 on weight reduction, and PWS-specific hyperphagia-related behaviors, as well as determine its safety profile. Based on the data from this Phase 2a clinical trial, we believe we may proceed into a Phase 3 clinical trial that could lead to an indication for the treatment of PWS patients. We plan to initiate our Phase 2a clinical trial by the end of 2014.
97
Clinical Development in General Obesity Patients
Our initial RM-493 clinical trials have focused on the general obese population, though we do not have plans to develop and commercialize RM-493 for this population while we focus on genetic segments of obesity where the medical need is greater. The general obese population is defined as having a BMI of greater than or equal to 30 kg/m2. In our initial clinical trials, we delivered RM-493 with continuous subcutaneous infusion using an insulin pump. More recently, our administration has been converted to a once daily injectable formulation.
- •
- Phase 2a Clinical Trial Results with Continuous
Infusion. We conducted our first Phase 2a clinical trial of RM-493 using continuous subcutaneous infusion. This was a 12-week,
Phase 2a proof-of-concept clinical trial in general obese patients using the subcutaneous continuous infusion formulation delivered by an insulin pump. We treated approximately 74 obese
patients with either placebo or RM-493 at a dose of 1 mg over 24 hours with no serious adverse events or other safety indications from laboratory tests, electrocardiograms, or vital signs noted
in the RM-493 treatment group. Evaluation of the pharmacokinetics, or blood levels, of RM-493 from this clinical trial demonstrated that the subcutaneous continuous infusion method of drug
administration was not optimal. A large number of patients did not meet the target pharmacokinetic exposures of RM-493 that our Phase 1b clinical trials suggested would have to be achieved in
order for RM-493 to show efficacy. This clinical trial did not demonstrate statistically significant weight loss compared to the placebo. We believe patients in this clinical trial lacked adequate
exposure to RM-493, and concluded that all future efficacy clinical trials in obese patients should be conducted using the subcutaneous injection method. This belief is based on a prior Phase 1
pharmacokinetic study, which used the subcutaneous injection formulation and demonstrated higher pharmacokinetic exposures in obese patients.
- •
- Ongoing Phase 2a Clinical Trial. We are currently conducting a Phase 2a clinical trial in general obese patients, with 12-week weight data now available from an interim analysis. This clinical trial is a Phase 2a, 12-week treatment clinical trial, in which we are administering in approximately 100 obese patients either (i) RM-493 at a daily dose of 1.5-2 mg by subcutaneous injection or (ii) a matching placebo by subcutaneous injection. We designed this Phase 2a clinical trial to bridge between the earlier clinical trials that used a continuous infusion by insulin pump and all future clinical trials that use the new formulation for once daily subcutaneous injection. Patients in this clinical trial entered in staggered cohorts, with decreasing training and supervision of patient dosing. In the interim data, there was significant placebo-subtracted percent weight loss for these cohorts that ranged from -2.47 to -4.67% (p-values ranging from 0.005 to <0.001) between active and placebo group at 12-weeks. Despite the new formulation, pharmacokinetic exposures continued to be suboptimal in this population. Rhythm remains blinded to most other results until the database is finalized.
Phase 1 Clinical Development in the General Obese Population
We have completed a Phase 1 single-ascending dose, or SAD, clinical trial of RM-493, as well as five cohorts in the Phase 1 multiple-ascending dose, or MAD, clinical trial of RM-493. Both clinical trials were in healthy obese volunteers, and included a double-blind, placebo-controlled randomized escalating dose design. Subjects received treatment in these Phase 1 clinical trials for one day at doses up to 0.1 mg/kg/day, which is a total daily dose of approximately 10 mg/day, and for up to 28 days at doses up to 0.015 mg/kg/day, which is a total daily dose of approximately 1.5 mg/day.
In the SAD clinical trial, our extensive monitoring of heart rate and blood pressure did not demonstrate any notable change with RM-493 treatment compared with placebo. Similarly, in the MAD clinical trial, there was no evidence of any notable changes in cardiovascular parameters compared to placebo when assessed by 24-hour ambulatory blood pressure monitoring. We determined
98
that the terminal half-life of RM-493 is approximately nine hours, making it suitable for once daily dosing.
Four cohorts of the Phase 1b MAD clinical trial that included doses of greater than 0.01 mg/kg/day, which is approximately 1 mg/day, for two to four weeks, demonstrated placebo-subtracted weight differences, or the difference in the amount of weight gained or lost in the active treatment group as compared to the placebo treatment group. Most panels showed statistically significant, placebo-subtracted weight reduction that ranged from 0.6 to 1.4 kg/week, with a mean of approximately 0.9 kg/week, over the two to four weeks of treatment in Phase 1b.
RM-493: Phase 1b General Obesity Patients: Placebo Subtracted Differences(1)
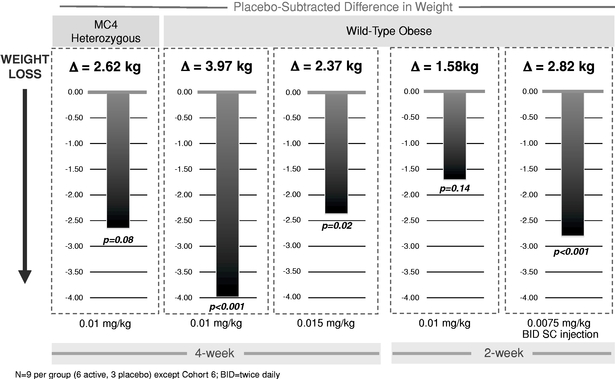
- (1)
- Over
two to four weeks of treatment with RM-493 by continuous subcutaneous infusion. Placebo subtracted differences are the FDA's primary weight loss
analysis approach, assessing the weight difference between active and placebo treatment groups for changes from baseline for weight. In one of these panels, both the placebo group and the active
treatment group gained weight, which we believe was due to the ad lib access to food allowed in this study. The placebo-subtracted weight difference in
this panel was primarily due to the placebo group experiencing significantly more weight gain than the small amount of weight gained by the active treatment group.
- (2)
- Preliminary data.
pbo =
Placebo
D = change from placebo
Phase 1 Energy Expenditure Clinical Trial
In collaboration with the National Institute of Diabetes, Digestive and Kidney Diseases, we investigated RM-493 in a Phase 1 clinical trial to determine the effects on energy expenditure, a mechanism for weight loss, in addition to the well-known effects of MC4 agonists on appetite and food intake. Twelve obese adults were randomized to receive RM-493 or placebo by continuous subcutaneous infusion over 72 hours, followed immediately by crossover to the other treatment.
99
RM-493 showed statistically significant increases in resting energy expenditure by 6.4%, supporting a role for RM-493 in weight regulation.
Phase 2a POMC Homozygous Patients Proof of Concept Clinical Trial
In collaboration with Charite Medical University (Berlin, Germany), we are conducting a Phase 2a proof of concept clinical trial in patients who have homozygous pro-opiomelanocortin, or POMC, deficiency. The very rare phenotype of POMC deficient patients consists of severe early-onset obesity and other hormonal pathology. The obesity develops due to the lack of the Melanocyte-Stimulating-Hormone, or MSH, the hormone that normally activates the MC-4 receptor in the MC4 pathway. This lack of MSH leads to an improper regulation of energy expenditure and satiety. In this open label Phase 2a proof of concept clinical trial, up to ten POMC deficient patients will receive open label doses of RM-493 by subcutaneous injection once daily for up to 12 weeks. This clinical trial is expected to initiate in the fall of 2014, with preliminary results available in the first half of 2015. This clinical trial may provide proof of concept for PWS, where a defect in the POMC neurons is also hypothesized.
Safety and Tolerability
Clinical data with other MC4 therapies suggests that MC4-mediated effects may include changes in blood pressure and heart rate, increased erections in males and other changes in libido and sexual function in females, nausea and vomiting, and increases in yawning and stretching. It is noteworthy that the pattern of effects appears different among other MC4 therapies, underscoring the complex receptor physiology of the MC4 receptor. Careful monitoring for these potential adverse events is included in all RM-493 clinical trials.
RM-493 generally was well-tolerated in our Phase 1 and Phase 2a clinical trials. Overall, except as outlined below, the number and patterns of adverse events was generally low, the intensity was generally mild, and infrequently related to clinical trial discontinuation. There has been only a single serious adverse experience possibly attributed to RM-493 in our clinical trials: one patient was hospitalized for unusual chest pain, but no evidence of any serious respiratory or cardiac cause was found after careful evaluation. There were no treatment-related changes in physical examination, except as noted below, and few, if any, clinically relevant changes in electrocardiograms, laboratory data, injection site reactions and/or anti-drug antibodies.
To demonstrate that RM-493 had the potential to provide a safer cardiovascular profile, we extensively validated RM-493 in obese primate preclinical studies, with special attention to cardiovascular effect. The results of these studies supported testing in clinical trials. In the clinical trials, we monitored blood pressure and heart rate extensively, primarily by 24-hour ambulatory blood pressure monitoring, or ABPM. In most clinical trials, there were multiple 24-hour monitoring periods, both on a pre-treatment and post-treatment basis. Careful review of the 24-hour ABPM data shows little, if any, evidence of changes in heart rate and/or blood pressure even at the highest doses tested in Phase 1 and Phase 2a clinical trials, preliminarily supporting an important differentiation of RM-493 from previous MC4 therapies. While the preliminary data is encouraging, there will be continued concerns about cardiovascular risk until addressed in larger and longer clinical trials, and there is no guarantee that RM-493 will achieve greater success than prior treatments in gaining regulatory approval.
There was a small increase in penile erections in male patients, as well as signs of sexual arousal in a small number of female patients. These symptoms were infrequent, generally mild, not painful, and were short-lived. Most often these symptoms were reported in the first week of treatment. There was a small indication for nausea and vomiting, which usually was reported as mild, early in treatment, and short-lived. A small number of patients had dose reductions and/or discontinued treatment due to nausea and vomiting.
100
We also noted darkening of skin and skin lesions, like moles and freckles, in many patients who received RM-493. This was likely caused by activation of the closely related MC1 receptor, the receptor that mediates skin darkening in response to sun exposure. This was noted generally after one to two weeks of treatment, most often plateaued by two to four weeks of treatment, and like for sun-related tanning, generally returned to baseline after cessation of exposure.
Preclinical Development
We demonstrated activity of RM-493 in reducing body weight and restoring insulin sensitivity in nonclinical models of obesity in rodents. We demonstrated activity in obese non-human primates, where approximately 13% weight loss was demonstrated with eight weeks of treatment, without evidence of cardiovascular toxicity. We also studied obese primates in crossover studies to confirm the lack of cardiovascular toxicity by RM-493 in obese primates. These preclinical studies confirmed the cardiovascular effects of previous MC4 therapies that had produced cardiovascular toxicity in humans. In contrast, RM-493 was without cardiovascular effects.
We completed one and three month toxicology studies, with doses and exposures that are more than 500-fold greater than those at the anticipated clinical doses without evidence of clinically relevant toxicological findings. We also plan to conduct chronic toxicity studies, including carcogenicity studies, one of which is expected to be two years in length.
In addition to developing the once-daily injectable formulation of RM-493 that we plan to use in all future clinical trials, we have developed compelling preclinical data with a once weekly slow release formulation, and we plan to develop this latter formulation as a product line extension.
Competition
Our industry is highly competitive and subject to rapid and significant technological change. While we believe that our development experience and scientific knowledge provide us with competitive advantages, we may face competition from large pharmaceutical and biotechnology companies, smaller pharmaceutical and biotechnology companies, including specialty pharmaceutical companies and generic drug companies, academic institutions, government agencies, research institutions, and others.
Many of our competitors may have significantly greater financial, technical and human resources than we have. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Our commercial opportunity could be reduced or eliminated if our competitors develop or market products or other technologies that are more effective, safer or less costly, or obtain regulatory approval more rapidly, than any that we expect to commercialize.
Relamorelin: Competition
While there is a high prevalence of diabetic gastroparesis, there is a lack of safe and effective therapies approved for the treatment of this disorder. Reglan (metoclopramide) is the only drug indicated for the treatment of diabetic gastroparesis in the United States and is only approved for short-term use due to intolerance and acute side effects, predominantly an involuntary movement disorder, known as tardive dyskinesia, the symptoms of which mimic Parkinson's disease. Other drugs that are used for diabetic gastroparesis, but not approved for this indication in the United States, are domperidone (a dopamine receptor modulator, not FDA-approved for any indication in the United States) and erythromycin, both of which stimulate stomach emptying. Anti-emetics are also used for vomiting and nausea.
Two prokinetic drugs were used widely in the United States for both upper and lower GI functional disorders before being withdrawn from the market. Propulsid (cisapride) was approved for GERD and was used broadly for upper GI indications, with sales that exceeded $1 billion before being
101
withdrawn for cardiovascular safety issues. Zelnorm (tegaserod) was marketed for IBS in the United States, where sales rapidly exceeded $500 million before its withdrawal for cardiovascular safety issues.
Although there is significant unmet medical need for drugs that improve motility in GI functional disorders, the development pipeline for prokinetic drugs is limited and overall there are few treatments approved or in late-stage clinical development for diabetic gastroparesis:
- •
- Reglan (metoclopramide), a mixed dopamine and serotonin receptor
modulator, is the only drug indicated for the treatment of diabetic gastroparesis in the United States and is approved for short-term use only due to intolerance and acute side effects, predominantly
an involuntary movement disorder, known as tardive dyskinesia, the symptoms of which mimic Parkinson's disease. In 2009, the FDA required that a black box warning be added to the metoclopramide label
because of the risk of tardive dyskinesia with long-term use, and recommended that its use be limited to 12 weeks. The European Medicines Agency is even more stringent, and recommends
metoclopramide for use no more than five days, and not for chronic conditions such as gastroparesis.
- •
- EVK-001 is an intranasal formulation of metoclopramide under development by Evoke Pharma, Inc. Evoke has initiated
a Phase 3 clinical trial for the treatment of female patients with symptoms of diabetic gastroparesis. A recently completed Phase 2b clinical trial did not achieve statistical
significance in improving symptoms of diabetic gastroparesis for the total study popluation, while a post hoc analysis demonstrated efficacy in females.
- •
- GlaxoSmithKline plc is evaluating a motilin agonist, currently in Phase 2 clinical trials, for the
treatment of ICU gastric stasis and diabetic gastroparesis. Earlier programs with motilin agonists developed by Abbott Laboratories and Chugai Pharmaceutical Co. were discontinued because of
lack of efficacy.
- •
- Velusetrag, currently in late-stage clinical development by Theravance, Inc. and Alfa Wasserman S.p.A., is
a serotonin 5-HT4 receptor agonist like Zelnorm. Velusetrag has completed Phase 2 clinical trials for chronic constipation and is currently undergoing limited Phase 2 clinical evaluation
for diabetic gastroparesis.
- •
- TC-6499, a small molecule that activates the alpha3beta4 and other neuronal nicotinic receptors, is in a Phase 1/2 exploratory clinical trial by Targacept, Inc. for gastroparesis. TC-6499 has completed a Phase 1 clinical trial suggesting that it may increase gastric motility.
RM-493: MC4 Competition
Although drugs approved for general obesity can be used in obese patients with MC4 deficiencies, there are currently no approved treatments in the United States specifically for these indications—either for obesity caused by heterozygous mutations of the MC4 gene or for PWS. The available treatments for general obesity have not been evaluated for safety or efficacy for the treatment of obesity caused by MC4 deficiencies, and have significant shortcomings.
Bariatric surgery is the most successful treatment for general obesity, and the only treatment resulting in an average of more than 15% documented weight loss sustained over a 10 year period. However, the number of bariatric surgery procedures in the United States has not increased substantially in recent years, despite new supportive clinical data and new guideline recommendations that expanded the range of patients eligible for these procedures. The implications of this trend likely indicate that there are substantial patient concerns about the morbidity and mortality associated with this surgery. In addition, bariatric surgery is contraindicated in the treatment of PWS due to poor outcomes and complications.
102
In general obesity, there is a clear need for effective anti-obesity drugs that are a viable alternative to surgery. Drugs that can deliver sustained, clinically-relevant weight loss with good safety have the potential to improve healthcare outcomes dramatically.
Orlistat (tetrahydrolipstatin), Qsymia (phentermine/topiramate) and Belviq (lorcaserin) are three recently approved and currently marketed pharmaceutical products in the United States for the treatment of obesity, along with several older agents, indicated for short-term administration, Adipex-P (phentermine), Obezine (phendimetrazine), Didrex (benzphetamine) and Tenuate (diethylpropion). Orlistat is marketed in the United States by Roche Holdings AG under the brand name Xenical and over-the-counter in the United States at half the prescribed dose by GlaxoSmithKline plc under the brand name Alli. In June 2013, Arena launched its lorcaserin product, which is marketed in the United States under the name Belviq and in September 2012, Vivus, Inc. commercially launched its combination product, phentermine/topiramate, under the trade name Qsymia.
Despite the large market opportunity for anti-obesity agents, there are relatively few competitive products in late-stage clinical development. Other companies pursuing pharmaceutical treatments for obesity include Neurosearch A/S, Novo Nordisk A/S, Takeda Pharmaceutical Company Limited, and Orexigen Therapeutics, Inc. resubmitted its NDA for Contrave in December 2013. In addition, Essentials, Inc. is developing DCCR for PWS, and Zafgen, Inc. is developing beloranib for obesity including for PWS.
For more information on the market for relamorelin and RM-493, our competitors and the products that may compete with our product candidates, see "Overview of the GI Functional Disorder Market" and "Market Overview," respectively.
Licensing Agreements
In February 2010, we entered into a license agreement with Ipsen pursuant to which Ipsen granted to us an exclusive, sublicenseable, worldwide license to certain patents and other intellectual property rights to research, develop, and commercialize compounds that were discovered or researched by Ipsen in the course of conducting its ghrelin program or MC4 program or that otherwise were covered by the licensed patents. Our rights under the license included the right to research, develop and commercialize relamorelin and RM-493. Pursuant to the license, Ipsen also granted to us a non-exclusive, sublicenseable, worldwide license to certain patents and other intellectual property rights that were licensed by Ipsen from a third party or that Ipsen may develop in the future to research, develop, and commercialize any of the compounds exclusively licensed by Ipsen to us pursuant to the license.
On March 21, 2013, we completed the Prior Restructuring pursuant to which, among other things, we amended the existing license with Ipsen so that the rights licensed by us from Ipsen with respect to each of our ghrelin program and our MC4 program would be held separately by each of our two wholly owned subsidiaries. Rhythm Health holds the rights related to the ghrelin program, including the rights to develop and commercialize relamorelin, and Rhythm Metabolic holds the rights to the MC4 program, including the rights to develop and commercialize RM-493.
Under the terms of each Ipsen license agreement, Ipsen will receive payments of up to $40.0 million upon the achievement of certain development and commercial milestones in connection with the development, regulatory approval and commercialization of applicable licensed products, and royalties on future sales of the licensed products. Substantially all of such aggregate payments under each Ipsen license agreement are for milestones that may be achieved no earlier than first commercial sale of the applicable licensed product. Royalties in the mid-single digits on future sales of the applicable licensed products will be due under each Ipsen license agreement on a licensed product-by-licensed product and country-by-country basis until the later of the date when sales of a licensed product in a particular country are no longer covered by patent rights licensed pursuant to such Ipsen license agreement and the 10th anniversary of the date of the first commercial sale of the
103
applicable licensed product in the applicable country. In connection with the original license agreement between Ipsen and Rhythm Health, in March 2010, and after giving pro forma effect to the Prior Restructuring and the Conversion, an affiliate of Ipsen acquired 410,000 shares of our common stock and two warrants exercisable for a total of 9,790,000 shares of our common stock, which warrants expire upon consummation of this offering. The term of each Ipsen license agreement continues until the expiration of the applicable royalty term on a country-by-country and product-by- product basis. Upon expiration of the term of each agreement, the licensed rights granted to us under that agreement, to the extent they remain in effect at the time of expiration, will thereafter become irrevocable, perpetual and fully paid-up licenses that survive the expiration of the term. We have a right to terminate each license agreement at any time during the term for any reason on 180 days' written notice to Ipsen. Ipsen has a right to terminate each agreement prior to expiration of their respective terms for our material breach of the agreement, our failure to initiate or complete development of a licensed product or our bringing an action seeking to have an Ipsen license patent right declared invalid. Upon any early termination of each license agreement not due to Ipsen's material breach, all licensed rights granted under the terminated license agreement will terminate.
Commercial Operations
For relamorelin, we intend to establish our own commercial and marketing organization in the United States and to selectively establish partnerships in markets outside the United States. We intend to target physicians who treat a high concentration of diabetic gastroparesis patients with a specialist sales force that targets gastroenterologists and primary care physicians who are high prescribers of treatments for diabetic gastroparesis. We expect that the sales force will be supported by sales management, internal sales support, an internal marketing group and distribution support. Additionally, we expect that the sales and marketing teams will manage relationships with key accounts such as managed care organizations, group-purchasing organizations, hospital systems, physician group networks, and government accounts. To develop the appropriate commercial infrastructure, we expect to invest significant amounts of financial and management resources, some of which will be committed prior to any confirmation that relamorelin will be approved. We could invest resources and then later learn that a particular product candidate will not be approved.
For RM-493, we will consider entering into relationships with strategic partners that enable the expansion of the ongoing clinical development of RM-493, while retaining significant value for us. For PWS and other MC4 genetic deficiencies, we may either commercialize RM-493 on our own or by establishing alliances with pharmaceutical companies, depending on development costs and our available resources. These pharmaceutical company partnerships could focus on specific patient populations and their caregivers, on regional development, or on distribution and sales.
Patents and Proprietary Rights
We have in-licensed two patent portfolios from Ipsen, one each for our ghrelin and melanocortin programs. Each portfolio includes multiple patent families, and all of these in-licensed patent families are being prosecuted or maintained by Ipsen in consultation with us. We have also filed patent applications in two families which are exclusively owned and maintained by Rhythm that relate to the ghrelin and melanocortin programs.
Our ghrelin portfolio, which includes patents and applications directed to relamorelin, consists of 12 patent families currently being prosecuted or maintained, which include applications and patents directed to compositions of matter, formulations, methods of treatment and methods of preparing relamorelin. As of June 23, 2014, the portfolio licensed for the ghrelin program consists of 10 issued U.S. patents and 56 issued non-U.S. patents across nine of the 12 families. Furthermore, we are actively pursuing seven U.S. patent applications, two of which are provisional applications, one Patent Cooperation Treaty, or PCT, application, and 40 non-U.S. patents in 14 jurisdictions.
104
In the patent family directed to the composition of matter for relamorelin, we have two issued U.S. patents and 18 issued non-U.S. patents, including Australia, Canada, Europe, Hong Kong, India, Japan, Mexico, Russia, Singapore and Taiwan. The standard 20-year term for patents in this family would expire in 2023, but the U.S. patents will expire in 2024 and 2025 due to patent term adjustments. Patent term extensions for delays in marketing approval may also extend the terms of patents in this family. In addition, in the patent family directed to the use of relamorelin for stimulating gastrointestinal motility, we have issued one patent in each of the United States, Japan, and Russia. The standard 20-year term for patents in this family would expire in 2026, but the U.S. patent will expire in 2027 due to a patent term adjustment. Patent term extensions for delays in marketing approval may also extend the terms of patents in this family.
Our MC-4 portfolio, which includes RM-493, consists of 12 patent families currently being prosecuted or maintained, which include applications and patents directed to compositions of matter, formulations and methods of treatment using RM-493. As of June 23, 2014, the portfolio licensed for the MC-4 program consists of two issued U.S. patents and 26 issued non-U.S. patents across four of the 12 families. We are actively pursuing eight U.S. patent applications, three of which are provisional applications, three PCT applications and 42 non-U.S. applications in 14 jurisdictions.
In the patent family directed to the composition of matter for RM-493, we have one issued U.S. patent and 16 issued non-U.S. patents, including Australia, Canada, Europe, Hong Kong, Israel, Japan, Korea, New Zealand, Russia and Singapore. The standard 20-year term for patents in this family would expire in 2026, but the U.S. patent will expire in 2027 due to a patent term adjustment. Patent term extensions for delays in marketing approval may also extend the terms of patents in this family.
Intellectual Property Protection Strategy
We currently seek, and intend to continue seeking, patent protection whenever commercially reasonable for any patentable aspects of our existing products or product candidates and related technology or any new products or product candidates we acquire in the future. Where our intellectual property is not protected by patents, we may seek to protect it through other means, including maintenance of trade secrets and careful protection of our proprietary information. Our licenses from Ipsen for each of the ghrelin and melanocortin programs, respectively, require Ipsen, subject to certain exceptions and upon consultation with us, to prosecute and maintain its patent rights as they relate to the licensed compounds and methods. If Ipsen decides to cease prosecution or maintenance of any of the licensed patent rights, we have the option to take over prosecution and maintenance of those patents and Ipsen will assign to us all of its rights in such patents. For those patent rights that we own exclusively, we control all prosecution and maintenance activities.
The patent positions of biopharmaceutical companies are generally uncertain and involve complex legal, scientific and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Consequently, we do not know whether any of the product candidates we in-license will be protectable or remain protected by enforceable patents. We cannot predict whether the patent applications we are currently pursuing will issue as patents in any particular jurisdiction, and furthermore, we cannot determine whether the claims of any issued patents will provide sufficient proprietary protection to protect us from competitors, or will be challenged, circumvented or invalidated by third parties. Because patent applications in the United States and certain other jurisdictions are maintained in secrecy for 18 months, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. This potential issue is exacerbated by the fact that, prior to March 16, 2013, in the United States, the first to make the claimed invention may be entitled to the patent. On March 16, 2013, the United States transitioned to a "first to file" system in which the first inventor to file a patent application may be entitled to the patent. Therefore, we may have to participate in interference
105
proceedings declared by the U.S. PTO or a foreign patent office to determine priority of invention. Moreover, we may have to participate in other proceedings declared by the U.S. PTO or a foreign patent office, such as post-grant proceedings and oppositions, that challenge the validity of a granted patent. Such proceedings could result in substantial cost, even if the eventual outcome is favorable to us.
Although we currently have issued patents directed to a number of different attributes of our products, and pending applications on others, there can be no assurance that any issued patents would be held valid by a court of competent jurisdiction. An adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using specific compounds or technology. To the extent prudent, we intend to bring litigation against third parties that we believe are infringing our patents.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent's term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. PTO in granting a patent, or may be shortened if a patent is terminally disclaimed over another patent with an earlier expiration date.
As mentioned above, in the United States, the patent term of a patent that covers an FDA-approved drug may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. In the future, if and when our pharmaceutical products receive FDA approval, we expect to apply for patent term extensions on patents covering those products. We intend to seek patent term adjustments and extensions to any of our issued patents in any jurisdiction where these are available, however there is no guarantee that the applicable authorities, including the FDA in the United States, will agree with our assessment of whether such extensions should be granted, and even if granted, the length of such adjustments or extensions.
To protect our rights to any of our issued patents and proprietary information, we may need to litigate against infringing third parties, or avail ourselves of the courts or participate in hearings to determine the scope and validity of those patents or other proprietary rights. These types of proceedings are often costly and could be very time-consuming to us, and we cannot be certain that the deciding authorities will rule in our favor. An unfavorable decision could result in the invalidation or a limitation in the scope of our patents or forfeiture of the rights associated with our patents or pending patent applications. Any such decision could result in our key technologies not being protectable, allowing third parties to use our technology without being required to pay us licensing fees or may compel us to license needed technologies from third parties to avoid infringing third-party patent and proprietary rights. Such a decision could even result in the invalidation or a limitation in the scope of our patents or could cause us to lose our rights under existing issued patents or not to have rights granted under our pending patent applications.
In addition, we intend to seek orphan drug status in jurisdictions in which it is available. An orphan drug designation may be granted where a drug is developed specifically to treat a rare or uncommon medical treatment. If a product which has an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, meaning that the applicable regulatory authority may not approve any other applications to market the same drug for the same indication, except in certain very limited circumstances, for a period of seven years in the United States and 10 years in the European Union. Orphan drug designation does not prevent competitors from developing or marketing different drugs for an indication.
106
We also rely on trade secret protection for our confidential and proprietary information. Although we take steps to protect our proprietary information and trade secrets, including through contractual means with our employees and consultants, no assurance can be given that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technology, or that we can meaningfully protect our trade secrets. It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual's relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual will be our exclusive property. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for our trade secrets in the event of unauthorized use or disclosure of such information.
Manufacturing
We currently contract with a third party for the manufacture of our product candidates and intend to continue to do so in the future. Each of our subsidiaries has entered into a process development and manufacturing services agreement with Peptisyntha SA under which Peptisyntha will provide certain process development and manufacturing services in connection with the manufacture of both relamorelin and RM-493. Under both agreements, we pay Peptisyntha for services in accordance with the terms of mutually agreed upon work orders, which we and Peptisyntha may enter into from time to time. The agreements also provide that, subject to certain conditions, for a period following each product launch date, we will source from Peptisyntha a portion of our requirements for that product being sourced from non-affiliate third parties. Under both agreements, each party is subject to customary indemnification provisions.
The agreement related to relamorelin will continue, unless earlier terminated pursuant to its terms, until the later of (i) six years from the July 2, 2010 effective date, or (ii) the completion of all services under all work plans executed in accordance with the terms of the agreement prior to the sixth anniversary of its effective date. The agreement related to RM-493 will continue, unless earlier terminated pursuant to its terms, until the later of (i) six years from the July 17, 2013 effective date, or (ii) the completion of all services under all work plans executed in accordance with the terms of the agreement prior to the sixth anniversary of its effective date. Each agreement may be extended by us continuously for additional two-year periods upon written notice to Peptisyntha. We also may terminate each of the agreements or any work order thereunder upon at least 30 days' prior written notice to Peptisyntha.
We currently have no plans to build our own clinical or commercial scale manufacturing capabilities. To meet our projected needs for clinical supplies to support our activities through regulatory approval and commercial manufacturing, the contract manufacturing organizations, or CMOs with whom we currently work will need to increase scale of production or we expect that we will need to secure alternate suppliers. We have not currently identified alternate suppliers in the event the current CMOs we utilize are unable to scale production. Because we rely on these CMOs, we have personnel with pharmaceutical development and manufacturing experience who are responsible for maintaining our CMO relationships.
107
Regulatory Matters
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the U.S. Food and Drug Administration, or the FDA. The Federal Food, Drug, and Cosmetic Act and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications, or NDAs, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution.
Pharmaceutical product development for a new product or certain changes to an approved product in the U.S. typically involves preclinical laboratory and animal tests, the submission to the FDA of an investigational new drug, or IND, application, which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity, and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation, and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the IND to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with good clinical practice, or GCP, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators, and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to IRB for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB's requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence on effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a
108
particular indication, dosage tolerance, and optimum dosage, and to identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In most cases the FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 trial with other confirmatory evidence may be sufficient in rare instances where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all preclinical, clinical, and other testing and a compilation of data relating to the product's pharmacology, chemistry, manufacture, and controls. The cost of preparing and submitting an NDA is substantial. The submission of most NDAs is additionally subject to a substantial application user fee, currently exceeding $2,169,000, and the manufacturer and/or sponsor under an approved new drug application are also subject to annual product and establishment user fees, currently exceeding $104,000 per product and $554,000 per establishment. These fees are typically increased annually.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency's threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of new drug applications. Most such applications for standard review drug products are reviewed within 10-12 months; most applications for priority review drugs are reviewed in six to eight months. Priority review can be applied to drugs that the FDA determines offer major advances in treatment, or provide a treatment where no adequate therapy exists. For biologics, priority review is further limited only for drugs intended to treat a serious or life-threatening disease relative to the currently approved products. The review process for both standard and priority review may be extended by the FDA for three additional months to consider certain late-submitted information, or information intended to clarify information already provided in the submission.
The FDA may also refer applications for drug products, or drug products that present difficult questions of safety or efficacy, to an advisory committee—typically a panel that includes clinicians and other experts—for review, evaluation, and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with current Good Manufacturing Practices, or cGMPs, regulations is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA's satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included.
109
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a REMS to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug's safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, REMS and surveillance to monitor the effects of an approved product, or the FDA may place conditions on an approval that could restrict the distribution or use of the product. In addition, quality-control, drug manufacture, packaging, and labeling procedures must continue to conform to current cGMPs after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money, and effort in the areas of production and quality-control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition—generally a disease or condition that affects fewer than 200,000 individuals in the U.S. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. The first NDA applicant to receive FDA approval for a particular active ingredient to treat a particular disease with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the U.S. for that product, for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not
110
prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee.
Fast Track Designation and Accelerated Approval
The FDA is required to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the condition. Under the fast track program, the sponsor of a new drug candidate may request that the FDA designate the drug candidate for a specific indication as a fast track drug concurrent with, or after, the filing of the IND for the drug candidate. The FDA must determine if the drug candidate qualifies for fast track designation within 60 days of receipt of the sponsor's request.
Under the fast track program and the FDA's accelerated approval regulations, the FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A drug candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the drug from the market on an expedited basis. All promotional materials for drug candidates approved under accelerated regulations are subject to prior review by the FDA.
In addition to other benefits such as the ability to use surrogate endpoints and engage in more frequent interactions with the FDA, the FDA may initiate review of sections of a fast track drug's NDA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA's time period goal for reviewing an application does not begin until the last section of the NDA is submitted. Additionally, the fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Pediatric Information
Under the Pediatric Research Equity Act, or PREA, NDAs or supplements to NDAs must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may grant full or partial waivers, or deferrals, for submission of data. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for which orphan designation has been granted.
The Best Pharmaceuticals for Children Act, or BPCA, provides NDA holders a six-month extension of any exclusivity—patent or non-patent—for a drug if certain conditions are met. Conditions for exclusivity include the FDA's determination that information relating to the use of a new drug in the pediatric population may produce health benefits in that population, the FDA making a written
111
request for pediatric studies, and the applicant agreeing to perform, and reporting on, the requested studies within the statutory timeframe. Applications under the BPCA are treated as priority applications, with all of the benefits that designation confers.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA-regulated products, including drugs, are required to register and disclose certain clinical trial information. Information related to the product, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed until the new product or new indication being studied has been approved. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
The Hatch-Waxman Amendments
Orange Book Listing
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant's product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application, or ANDA. An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing to be therapeutically equivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct, or submit results of, pre-clinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way are commonly referred to as "generic equivalents" to the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA's Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The ANDA applicant may also elect to submit a section viii statement certifying that its proposed ANDA label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. If the applicant does not challenge the listed patents, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
A certification that the new product will not infringe the already approved product's listed patents, or that such patents are invalid, is called a Paragraph IV certification. If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA applicant.
The ANDA application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the referenced product has expired.
112
Exclusivity
Upon NDA approval of a new chemical entity or NCE, which is a drug that contains no active moiety that has been approved by the FDA in any other NDA, that drug receives five years of marketing exclusivity during which the FDA cannot receive any ANDA seeking approval of a generic version of that drug. Certain changes to a drug, such as the addition of a new indication to the package insert, are associated with a three-year period of exclusivity during which the FDA cannot approval an ANDA for a generic drug that includes the change.
An ANDA may be submitted one year before NCE exclusivity expires if a Paragraph IV certification is filed. If there is no listed patent in the Orange Book, there may not be a Paragraph IV certification, and, thus, no ANDA may be filed before the expiration of the exclusivity period.
Patent Term Extension
After NDA approval, owners of relevant drug patents may apply for up to a five year patent extension. The allowable patent term extension is calculated as half of the drug's testing phase—the time between IND application and NDA submission—and all of the review phase—the time between NDA submission and approval up to a maximum of five years. The time can be shortened if the FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the extension may not exceed 14 years.
For patents that might expire during the application phase, the patent owner may request an interim patent extension. An interim patent extension increases the patent term by one year and may be renewed up to four times. For each interim patent extension granted, the post-approval patent extension is reduced by one year. The director of the U.S. PTO must determine that approval of the drug covered by the patent for which a patent extension is being sought is likely. Interim patent extensions are not available for a drug for which an NDA has not been submitted.
Anti-Kickback, False Claims Laws
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce; or in return for; purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. The Patient Protection and Affordable Care Act, or PPACA, amended the intent element of the federal statute so that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers, and formulary managers on the other. Violations of the anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties, and exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. This includes claims made to programs where the federal government reimburses, such as Medicaid, as well as programs where the federal government is a direct purchaser, such as when it purchases off the Federal Supply Schedule. Recently, several
113
pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. Additionally, PPACA amended the federal healthcare program anti-kickback statute such that a violation of that statute can serve as a basis for liability under the federal false claims law. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
Other Federal and State Regulatory Requirements
The Centers for Medicare & Medicaid Services has issued a final rule that requires manufacturers of prescription drugs to collect and report information on payments or transfers of value to physicians and teaching hospitals, as well as investment interests held by physicians and their immediate family members. Manufacturers were required to begin collecting information on August 1, 2013, with the first reports due March 31, 2014. The reported data will be posted in searchable form on a public website beginning September 30, 2014. Failure to submit required information may result in civil monetary penalties.
In addition, several states now require prescription drug companies to report expenses relating to the marketing and promotion of drug products and to report gifts and payments to individual physicians in these states. Other states prohibit various other marketing-related activities. Still other states require the posting of information relating to clinical studies and their outcomes. In addition, California, Connecticut, Nevada, and Massachusetts require pharmaceutical companies to implement compliance programs and/or marketing codes. Several additional states are considering similar proposals. Compliance with these laws is difficult and time consuming, and companies that do not comply with these state laws face civil penalties.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our product candidates to the extent we choose to sell any products outside of the United States. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. As in the United States, post-approval regulatory requirements, such as those regarding product manufacture, marketing, or distribution would apply to any product that is approved outside the United States.
Employees
We have operated as a virtual company that leverages skilled experts, consultants, contract research organizations, and contractors to manage our clinical operations, under the leadership and direction of our management. We will expand our infrastructure to manage our clinical, finance and commercial operations with additional full-time employees.
As of June 30, 2014, we had four employees, two of whom hold Ph.D. or M.D. degrees. Of these employees, two were engaged in development activities and two were engaged in support administration, including business development and finance. None of our employees are represented by
114
labor unions or covered by collective bargaining agreements. We consider our relationship with our employees to be good.
Facilities
Our offices are located at a 2,930 square foot facility in Boston, Massachusetts used primarily for corporate functions. Our lease for this space expires in July 2015. We believe that our existing facility is sufficient for our needs for the foreseeable future.
Legal Proceedings
We are not currently a party to any material legal proceedings.
115
Executive Officers and Directors
The following table sets forth the names, ages and positions of the directors and executive officers of Rhythm each of whom is elected annually and serves until his or her re-election, or earlier resignation or removal:
Name
|
Age | Position(s) | ||
|---|---|---|---|---|
Keith M. Gottesdiener, M.D. |
60 | Chief Executive Officer and Chairman of the Board of Directors | ||
Bart Henderson |
55 | President & Founder | ||
Elizabeth Stoner, M.D. |
64 | Chief Development Officer & Founder | ||
Lex H.T. Van der Ploeg, Ph.D. |
59 | Chief Scientific Officer | ||
Christian de la Tour |
54 | Director | ||
Todd Foley |
42 | Director | ||
Ed Mathers |
54 | Director | ||
Neil Exter |
56 | Director |
Keith M. Gottesdiener, M.D. | Chief Executive Officer and Chairman of the Board of Directors
Dr. Gottesdiener has been our Chief Executive Officer and a member of our board of directors since October 2011. He joined Rhythm after 16 years at Merck Research Laboratories. Dr. Gottesdiener joined Merck early clinical development in 1995, helping to transition compounds from the bench to the bedside and through to proof of concept. He held positions of increasing responsibility, eventually leading Merck's early clinical development across all therapeutic areas from 2001 through early 2006. From 2006-2011, he was a leader of Merck's late clinical development organization, first overseeing the development of Merck's infectious diseases and vaccine products through pivotal trials, registration, and life cycle management, including Gardasil™ (HPV Vaccine), Rotateq™ (rotavirus vaccine), Zostavax™ (zoster vaccine) and Isentress™ (HIV integrase inhibitor), among others. In 2008, Dr. Gottesdiener was appointed Late Stage Therapeutic Group Leader, and in that role led Merck's late-stage clinical development efforts (from Phase 2 thru patent expiry) across all therapeutic areas. After Merck's merger with Schering Plough in 2009, he continued as co-head of late development. Dr. Gottesdiener received his B.A. from Harvard College and his M.D. from the University of Pennsylvania. He completed his residency and fellowship at the Brigham and Women's Hospital-Beth Israel Medical Center-Dana Farber Cancer Institute Children's Hospital programs. After his fellowship, Dr. Gottesdiener did postdoctoral research in the laboratory of Dr. Jack Strominger at Dana Farber Cancer Institute working on the molecular immunology of the T-cell receptor. In 1986, he joined the faculty as an assistant professor at Columbia University, started an independent research laboratory with NIH RO-1 funding, focusing on gene transcription, and was Associate Clinical Professor of Medicine at the time he left to join Merck in 1995. We believe that Dr. Gottesdiener's detailed knowledge of our company, his extensive experience in the pharmaceutical industry as a senior executive, and his research work for both medical and academic institutions provide him with the qualifications to serve as chairman of our board of directors.
Bart Henderson, MBA | President & Founder
Mr. Henderson has been our President since February 2010 and is a Founder of Rhythm. Previously, he was Entrepreneur-in-Residence at MPM Capital and Chief Business Officer of Radius, where he was a founding employee and led the acquisition of four pipeline programs. Mr. Henderson founded sales and marketing at Vertex when the company launched its first product, broadened its product pipeline, signed corporate partnerships valued at $1.2 billion, and raised $715 million in financing. He founded business development at Microbia (now Ironwood Pharmaceuticals Inc.), which
116
signed its first corporate partnerships and significantly expanded its pipeline during his tenure. In addition, Mr. Henderson held marketing management positions at Amgen and Merck. He holds an MBA from Dartmouth's Tuck School of Business and a B.A. from Amherst College.
Elizabeth Stoner, M.D. | Chief Development Officer & Founder
Dr. Stoner is a Founder of Rhythm and has been our Chief Development Officer since 2010. Dr. Stoner has been a Managing Director at MPM Capital since October 2007. Prior to joining MPM Capital, Dr. Stoner served in various roles, most recently as Senior Vice President of Global Clinical Development Operations at Merck Research Laboratories, or MRL, since 1985. Dr. Stoner currently serves as a director of Radius Health, Inc. and Momenta Pharmaceuticals Inc., and she served as a director of Metabasis Therapeutics, Inc. from 2009 to 2010. Dr. Stoner received an M.D. from Albert Einstein College of Medicine, an M.S. in Chemistry from the State University of New York at Stony Brook and a B.S. in Chemistry from Ottawa University, Kansas.
Lex H.T. Van der Ploeg, Ph.D. | Chief Scientific Officer
Dr. Van der Ploeg has been our Chief Scientific Officer since October 2011. He has more than 25 years of drug development experience focused on obesity, metabolic disorders, oncology, and neurodegenerative diseases. Before joining Rhythm, he was Senior Vice President of Integrative Medicine and Translational Science at Abraxis Bioscience and Head of R&D at Abraxis Health; both companies were acquired by Celgene Corporation. Prior to that, he held R&D leadership roles at MRL directing drug development programs in metabolism, oncology, and neurodegenerative diseases as Vice President, Basic Research and Site Head, MRL Boston; Site Head, MRL San Diego; and Head, Obesity Research for Merck Rahway and Banyu, Japan. Previously, Dr. Van der Ploeg was an associate professor in the Department of Genetics and Development at Columbia University. He has received numerous awards and grants for his research and has published more than 200 peer-reviewed research papers. Dr. Van der Ploeg is a named inventor on more than 50 patents and patent applications. He received an M.S. in Biochemistry from the University of Amsterdam and a Ph.D. in Biochemistry/Enzymology/Genetics from the University of Amsterdam/Netherlands Cancer Institute.
Christian de la Tour
Mr. de la Tour has served as a member of our board of directors since March 2013. Since 1983 when Mr. de la Tour joined Ipsen, he has gained experience in various commercial and corporate business development functions. Since 2009, Mr. de la Tour has served as Chairman and Director of Ipsen Ltd. and Ipsen Biopharm Ltd. and since 2011 he has served as Vice President in charge of Ipsen Corporate Alliance Management (Ipsen Biopharm Ltd). Mr. de la Tour is a founding member and served as Vice President of Ipsen's Corporate Business Development department, which he led from 1998 to 2009. His efforts were instrumental in Ipsen's emergence as a leader in global pharmaceutical partnering. Mr. de la Tour holds a degree from Institut Superieur de Gestion business school in Paris. We believe that Mr. de la Tour's extensive experience in the pharmaceutical industry as a senior executive at Ipsen provides him with the qualifications to serve as a director of our company.
Todd Foley
Mr. Foley has served as a member of our board of directors since July 2014. Mr. Foley is a managing director with MPM Capital, a venture capital firm, which he joined in 1999. Prior to joining MPM, Mr. Foley worked in business development at Genentech and in management consulting with Arthur D. Little. Mr. Foley currently serves as a member of the board of directors of Aires Pharmaceuticals, Celladon Corporation, Chiasma, Inc., Iconic Therapeutics, Inc., OSS Inc., Proteon Therapeutics Inc., Selexys Pharmaceuticals Corporation, Valeritas, Inc., and Zalicus, Inc. Mr. Foley received a B.S. in chemistry from the Massachusetts Institute of Technology and an MBA from Harvard
117
Business School. We believe that Mr. Foley's broad experience in the life sciences industry as a venture capitalist, as well as his service on the boards of directors of numerous companies provide him with the qualifications to serve as a director of our company.
Ed Mathers
Mr. Mathers has served as a member of our board of directors since March 2013. He has been a Partner at New Enterprise Associates, or NEA, a venture capital firm, since August 2008. Mr. Mathers currently serves on the boards of directors of Envisia Therapeutics Inc., Intarcia Therapeutics, Inc., Liquidia Technologies, Inc., Lumos Pharma, Inc., Lumena Pharmaceuticals, Inc., Mirna Therapeutics, Inc., Ra Pharmaceuticals, Inc., and Satori Pharmaceuticals Incorporated, all of which are biotechnology companies. In addition, Mr. Mathers is a member of the Biotechnology Industry Organization board, the Southeast BIO board and the North Carolina State Physical and Mathematical Sciences Foundation board. Prior to joining NEA, Mr. Mathers served in various corporate development roles at MedImmune, Inc., a biotechnology company that was acquired by AstraZeneca PLC in 2007, culminating in the position of Executive Vice President, Corporate Development and Venture. In this role, he also led the company's venture capital subsidiary, MedImmune Ventures, Inc., from 2002 to 2008. Mr. Mathers was a director of MedImmune, LLC, from 2007 to 2008. From 2000 to 2002, Mr. Mathers was Vice President, Marketing and Corporate Licensing and Acquisitions at Inhale Therapeutic Systems, Inc., a biopharmaceutical company, which is now known as Nektar Therapeutics, Inc. Previously, for 15 years, Mr. Mathers was at Glaxo Wellcome, Inc., where he held sales and marketing positions of increasing responsibility. Mr. Mathers received a B.S. in chemistry from North Carolina State University. We believe that Mr. Mather's extensive experience in the life sciences industry as a venture capitalist and senior executive, as well as his service on the boards of directors of numerous companies provide him with the qualifications to serve as a director of our company.
Neil Exter
Mr. Exter has served as a member of our board of directors since April 2014. He is a partner at Third Rock Ventures, where he plays an integral role in the formation, development, business strategy, and business development efforts of portfolio companies. He has more than 20 years of business development and strategic experience, facilitating the successful development and implementation of operations and collaborations across the spectrum of newly emerging and established biotech companies. Prior to joining Third Rock Ventures, Mr. Exter was CBO of Alantos Pharmaceuticals and led the sale of that company to Amgen. Previously, he served as Vice President of Business Development for Millennium Pharmaceuticals. Mr. Exter is a board member of CytomX Therapeutics, Cibiem, Lotus Tissue Repair, Coridea NC1, Coridea NC2, Element Science, and Seventh Sense. He is a member of the Research Committee of Children's Hospital Boston, the investment committee of the Innovation Research Fund at Partners Healthcare, the Board of Directors of the New England Venture Capital Association, the Advisory Council of the Electrical and Computer Engineering Department at Cornell University, and the Board of Visitors of Columbia College. He holds an MBA as a Baker Scholar from Harvard Business School, an M.S. from Stanford University, and a B.S. from Cornell University. We believe that Mr. Exter's extensive experience in the life sciences industry as a venture capitalist and senior executive, as well as his service on the boards of directors of numerous companies provide him with the qualifications to serve as a director of our company.
Composition of the Board of Directors after this Offering
Our amended and restated bylaws will provide that our board of directors will consist of such number of directors as our board of directors may determine from time to time. Our board of directors currently consists of five directors. Immediately upon the consummation of this offering, our board of
118
directors will consist of (i) one director designated by MPM Capital, who is currently Todd Foley, and who will be continuing as a director following the offering, (ii) one director designated by NEA, who is currently Ed Mathers, and who will be continuing as a director following the offering, (iii) one director designated by Third Rock Ventures, who is currently Neil Exter, and who will be continuing as a director following the offering, (iv) one additional independent director, and (v) our chief executive officer. Our board of directors has determined that are independent for the purpose of serving on our board of directors under the independence standards promulgated by NASDAQ.
Board leadership structure
Our board of directors is currently led by a combined chairman of the board and chief executive officer. Our board of directors believes that this leadership structure is the most effective for us at this time. Because our chief executive officer is closest to the many facets of our business, our board of directors believes that the chief executive officer is in the best position to lead our board of directors most effectively and, accordingly, to serve in the critical role of chairman of the board. In addition, as the chief executive officer is directly involved in managing the company, having a chairman who also serves as chief executive officer facilitates timely communication with the board on critical business matters. Furthermore, we believe that this combined leadership structure is appropriate for our company because (i) our chairman and chief executive officer conveys a singular, cohesive message to our stockholders, employees, industry partners and the investment community and (ii) this structure eliminates any ambiguity as to who is accountable for the company's performance. Our directors and management team engage frequently and directly in the flow of information and ideas and we believe our combined leadership structure facilitates the quality, quantity and timeliness of the information flow and communication. Our board of directors believes that there is a well-functioning and effective balance between strong company leadership and oversight by active, independent directors.
Lead independent director
Our board of directors has appointed to serve as our lead independent director. As lead independent director, presides over periodic meetings of our independent directors, serves as a liaison between our chairman of the board and the independent directors and performs such additional duties as our board of directors may otherwise determine and delegate.
Board Committees
Following the Conversion and prior to the consummation of this offering, our board of directors will establish the following committees: an audit committee, a compensation committee and a governance and nominating committee. The initial composition and responsibilities of each committee are described below. Members will serve on these committees until their resignation or until otherwise determined by our board of directors.
Audit Committee
Our audit committee will provide oversight of our accounting and financial reporting process, the audit of our financial statements and our internal control function. Among other matters, the audit committee will be responsible for the following: assisting the board of directors in oversight of the independent auditors' qualifications, independence and performance; the engagement, retention and compensation of the independent auditors; reviewing the scope of the annual audit; reviewing and discussing with management and the independent auditors the results of the annual audit and the review of our quarterly consolidated financial statements, including the disclosures in our annual and quarterly reports filed with the SEC; reviewing our risk assessment and risk management processes; establishing procedures for receiving, retaining and investigating complaints received by us regarding
119
accounting, internal accounting controls or audit matters; and approving audit and permissible non-audit services provided by our independent auditor.
The initial members of our audit committee will be , who will be the chair of the committee, and . All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and NASDAQ. Our board of directors has determined that is an audit committee financial expert as defined under the applicable rules of the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of NASDAQ. All of the members of our audit committee are independent directors as defined under the applicable rules and regulations of the SEC and NASDAQ.
Compensation Committee
Our compensation committee will adopt and administer the compensation policies, plans and benefit programs for our executive officers and all other members of our executive team. Our compensation committee will also be responsible for making recommendations regarding non-employee director compensation to the full board of directors. In addition, among other things, our compensation committee will evaluate annually, in consultation with the board of directors, the performance of our chief executive officer, review and approve corporate goals and objectives relevant to compensation of our chief executive officer and other executives and evaluate the performance of these executives in light of those goals and objectives. Our compensation committee will also adopt and administer our equity compensation plans. The initial members of our compensation committee will be , who will be the chair of the committee, and . All of the members of our compensation committee are independent under the applicable rules and regulations of the SEC and NASDAQ, and qualify as outside directors under Section 162(m) of the Internal Revenue Code of 1986, or the Code.
Governance and Nominating Committee
Our governance and nominating committee will be responsible for, among other things, making recommendations regarding corporate governance, the composition of our board of directors, the identification, evaluation and nomination of director candidates and the structure and composition of committees of our board of directors. In addition, our governance and nominating committee will oversee our corporate governance guidelines, approve our committee charters, oversee compliance with our code of business conduct and ethics, contribute to succession planning, review policies and procedures with respect to our related party transactions policy and oversee the board self-evaluation process. The initial members of our governance and nominating committee will be , who will be the chair of the committee, and . All of the members of our governance and nominating committee are independent under the applicable rules and regulations of NASDAQ.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee is or has at any time during the past year been one of our officers or employees. None of our executive officers currently serves or in the past year has served as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
Role of the board in risk oversight
The audit committee of the board of directors is primarily responsible for overseeing our risk management processes on behalf of the board of directors. Going forward, we expect that the audit committee will receive reports from management at least quarterly regarding our assessment of risks. In addition, the audit committee reports regularly to the board of directors, which also considers our risk
120
profile. The audit committee and the board of directors focus on the most significant risks we face and our general risk management strategies. While the board of directors oversees our risk management, management is responsible for day-to-day risk management processes. Our board of directors expects management to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively implement risk management strategies adopted by the audit committee and the board of directors. We believe this division of responsibilities is the most effective approach for addressing the risks we face and that the leadership structure of our board of directors, which also emphasizes the independence of the board of directors in its oversight of its business and affairs, supports this approach.
Scientific Advisory Board
Our management team is supported by a scientific advisory board composed of leading academic and industry scientists. Our scientific advisory board consists of:
| John Amatruda, M.D. | Dr. Amatruda has broad clinical development expertise in metabolic disease. Most recently, he was Senior Vice President and Franchise Head for Diabetes and Obesity at Merck & Co., Inc. | |
Michael Camilleri, M.D. |
Dr. Camilleri is a Professor of Medicine and Physiology in the Mayo Clinic College of Medicine. He is a leading expert in gastroenterology, with a research focus on enteric neurosciences and the physiology, pathophysiology, and treatment of diseases that affect gastrointestinal motility, including gastroparesis, diabetes, obesity, and irritable bowel syndrome. |
|
William Chin, M.D. |
Dr. Chin is Executive Dean for Research at Harvard Medical School, following a 10-year career at Eli Lilly and Company, where he was most recently Senior Vice President for Discovery Research and Clinical Investigation. |
|
Lee Kaplan, M.D., Ph.D. Chairman of Scientific Advisory Board |
Dr. Kaplan is Director of the Obesity, Metabolism, & Nutrition Institute and was Founding Director of the Weight Center at the Massachusetts General Hospital. He is an Associate Professor of Medicine at Harvard Medical School. |
Code of Business Conduct and Ethics
We will adopt a code of business conduct and ethics that will apply to all of our employees, including our executive officers, and directors, and those employees responsible for financial reporting. The code of business conduct and ethics will be available on our website. We expect that, to the extent required by law, any amendments to the code, or any waivers of its requirements, will be disclosed on our website.
121
EXECUTIVE AND DIRECTOR COMPENSATION
This section discusses the material elements of compensation for our named executive officers and the most important factors relevant to an analysis of these policies. It provides qualitative information regarding the manner and context in which compensation is awarded to and earned by our executive officers named in the "Summary Compensation Table" below, or our named executive officers, and is intended to place in perspective the data presented in the following tables and the corresponding narrative. Our named executive officers are Keith M. Gottesdiener, M.D., Bart Henderson and Lex H.T. Van der Ploeg, Ph.D. Our other executive officer, Elizabeth Stoner, M.D., is a consultant and is not a named executive officer of the company.
In preparing to become a public company, we have begun a thorough review of all elements of the compensation of our executives, including our compensation philosophy and the function and design of our equity incentive programs. We have begun and expect to continue to evaluate the existing executive compensation program to ensure our program is competitive with the companies with which we compete for executive talent and is appropriate for a public company.
Summary Compensation Table
The following table presents compensation awarded in 2013 to our principal executive officer and our two other most highly compensated persons serving as executive officers as of December 31, 2013 or paid to or accrued for those executive officers for services rendered during 2013.
Name & Principal Position
|
Year | Salary ($) |
Equity Awards ($)(1) |
Non-Equity Incentive Plan Compensation ($)(2) |
All Other Compensation ($) |
Total ($) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Keith M. Gottesdiener, M.D. |
2013 | $ | 437,750 | $ | 107,700 | $ | 109,438 | — | $ | 654,888 | ||||||||
Chairman and Chief Executive Officer |
||||||||||||||||||
Bart Henderson |
2013 |
$ |
311,446 |
$ |
43,200 |
$ |
75,594 |
— |
$ |
430,240 |
||||||||
President |
||||||||||||||||||
Lex H.T. Van der Ploeg, Ph.D.(3) |
2013 |
$ |
167,375 |
$ |
11,520 |
$ |
20,085 |
— |
$ |
198,980 |
||||||||
Chief Scientific Officer |
||||||||||||||||||
- (1)
- We
performed a retrospective valuation of our common stock as of January 30, 2013 and determined the fair value of the common stock to be $0.06 per
share. For further discussion of the retrospective valuation see "Management's Discussion and Analysis of Financial Conduction and Results of Operations—Stock Based Compensation."
- (2)
- Amounts
represent incentive payments earned in 2013, and paid during 2014, based on achievement of performance goals and other factors determined by our
board of directors. Our 2013 company objectives related primarily to development and strategic achievements.
- (3)
- Reflects Dr. Van der Ploeg's part-time employment with the Company.
Executive Compensation
Overview
Our executive compensation program is based on a pay-for-performance philosophy. We designed our executive compensation program to achieve the following primary objectives: provide compensation
122
and benefit levels that will attract, retain, motivate and reward a highly talented executive team within the context of responsible cost management; establish a direct link between our individual/team performance and results and our executives' compensation; and align the interests and objectives of our executives with those of our stockholders/unitholders by linking executive equity awards to stockholder/unitholder value creation. Compensation for our executive officers is composed primarily of the following three main components: base salary, annual cash incentives and long-term equity incentives.
Base Salary
Base salaries are determined for each named executive officer by our board of directors, which gives consideration to each officer's experience, expertise and performance, as well as market compensation levels for similar positions.
Name
|
2013 Base Salary ($) |
2014 Base Salary ($) |
|||||
|---|---|---|---|---|---|---|---|
Keith M. Gottesdiener, M.D. |
437,750 | 450,882 | |||||
Bart Henderson |
311,446 | 320,789 | |||||
Lex H.T. Van der Ploeg, Ph.D. |
167,375 | 172,396 | |||||
The 2013 base salary for each named executive officer became effective on January 1, 2013. The 2014 base salary for each named executive officer became effective on January 1, 2014.
Annual Performance-Based Incentive Opportunity
In addition to base salaries, our named executive officers are eligible to receive annual performance-based cash incentives, which are designed to motivate our executives to achieve defined annual corporate goals and to reward our executives for their contributions towards achievement of these goals. The annual performance-based incentive each named executive officer is eligible to receive is generally based on the extent to which we achieve the corporate goals that our board of directors establishes at the beginning of each year. After the end of each year, our board of directors reviews our performance against each corporate goal and determines the extent to which we achieved each of our corporate goals.
Our board of directors generally considers each named executive officer's individual contributions towards reaching our annual corporate goals but does not typically establish specific individual goals for our named executive officers. Pursuant to the terms of their respective agreements governing their employment relationship with us (described below under "—Agreements with our Named Executive Officers."), Dr. Gottesdiener is eligible to receive a target bonus of up to 50% of his base salary, Mr. Henderson is eligible to receive a target bonus of up to 50% of his base salary, and Dr. Van der Ploeg is eligible to receive a target bonus of up to 20% of his base salary. However, there is no minimum bonus percentage or amount established for the named executive officers and, as a result, the bonus amounts vary from year to year based on corporate and individual performance.
In January 2014, our compensation committee reviewed our 2013 corporate goals and determined that on an overall basis, we had made significant progress towards achieving all of those goals. In recognition of this achievement and the efforts of each executive, our compensation committee recommended awarding each of our named executive officers eligible for performance bonuses a portion of their target bonus opportunity for 2013, and our board of directors adopted such recommendation. For 2013, Dr. Gottesdiener received 50% of his target bonus percentage, for a net bonus of 25% of his base salary, Mr. Henderson received 50% of his target bonus percentage, for a net bonus of 25% of his base salary, and Dr. Van der Ploeg received 60% of his target bonus percentage, for a net bonus of 12% of his base salary.
123
Pension Benefits
Our named executive officers did not participate in, or otherwise receive any benefits under, any pension or retirement plan sponsored by the Company during 2013, other than pursuant to the 401(k) plan described under "—401(k) Plan."
Nonqualified Deferred Compensation
Our named executive officers did not participate in, or earn any benefits under, a nonqualified deferred compensation plan during 2013.
Agreements with our Named Executive Officers
Below are descriptions of our current employment and consulting agreements with our named executive officers. In connection with the Conversion, we plan to enter into new letter agreements with each of our executive officers.
Agreement with Dr. Gottesdiener. We entered into a letter agreement with Dr. Gottesdiener in October 2011 that governs the current terms of his employment with us. Pursuant to the agreement, Dr. Gottesdiener was appointed to our board of directors, is entitled to an annual base salary of at least $425,000, is eligible to receive an annual target performance bonus of up to 40% of his base salary (subsequently increased to 50%) based on objectives mutually agreed upon between him and our board of directors, and was granted time-vested stock options to purchase an aggregate of 4,144,200 shares of Rhythm Health common stock, and was entitled to earn options to purchase up to 830,000 shares of Rhythm Health common stock, which were subsequently earned and awarded. Dr. Gottesdiener is additionally entitled to certain severance benefits pursuant to his agreement, the terms of which are described below under "—Potential Payments Upon Termination or Change of Control."
Agreement with Mr. Henderson. We entered into an employment agreement with Mr. Henderson in February 2010 that governs the current terms of his employment with us. Pursuant to the agreement, Mr. Henderson is entitled to an annual base salary of at least $295,000, is eligible to receive an annual target performance bonus of up to 50% of his base salary, as determined by our board of directors, was granted 650,000 shares of Rhythm Health common stock, and was granted stock options to purchase an aggregate of 1,500,000 shares of Rhythm Health common stock. Mr. Henderson is additionally entitled to certain severance benefits pursuant to his agreement, the terms of which are described below under "—Potential Payments Upon Termination or Change of Control."
Agreement with Dr. Van der Ploeg. We entered into a letter agreement with Dr. Van der Ploeg in October 2011 that governs the current terms of his part-time employment with us, under which he is required to devote approximately 50% of his full working time to our business. Pursuant to the agreement, Dr. Van der Ploeg is entitled to an annual base salary of at least $162,500, is eligible to receive an annual target performance bonus of up to 20% of his base salary, as determined by our board of directors, and was granted stock options to purchase an aggregate of 775,000 shares of Rhythm Health common stock, 360,000 of which were subject to the prior achievement by Rhythm Health of subsequent closings. Dr. Van der Ploeg is additionally entitled to certain severance benefits pursuant to his agreement, the terms of which are described below under "—Potential Payments Upon Termination or Change of Control."
124
Potential Payments Upon Termination or Change of Control
Regardless of the manner in which a named executive officer's employment terminates, the named executive officer is entitled to receive amounts earned during his term of employment, including salary and unused vacation pay. In addition, each of our named executive officers is eligible to receive certain benefits pursuant to his or her employment or letter agreements with us as described below.
Under the terms of Dr. Gottesdiener's letter agreement, upon termination without "cause," as defined below, or for "good reason," as defined below, Dr. Gottesdiener is entitled to receive a severance payment in an aggregate amount equal to 12 months of his base salary then in effect, paid in 12 equal monthly installments.
In addition, under the terms of his letter agreement, upon termination Dr. Gottesdiener may exercise each option then outstanding and exercisable, but only until the earlier to occur of (i) the expiration of the term of such option, or (ii) the expiration of the limited period of time set forth in the applicable plan and/or stock option agreement following the termination date; provided, however, that upon termination "without cause" or for "good reason" at any time following an exclusive license or licenses of all of our development programs, and subject to the prior occurrence of specified events, the vesting and exercisability of all his options will be accelerated in full and he shall have one year from the termination date to exercise each option, but not later than the expiration date of the option.
Under the terms of Dr. Van der Ploeg's letter agreement, upon termination he may exercise each option then outstanding and exercisable, but only until the earlier to occur of (i) the expiration of the term of such option, or (ii) the expiration of the limited period of time set forth in the applicable plan and/or stock option agreement following the termination date. Under the terms of Dr. Van der Ploeg's letter agreements, upon termination "without cause" or for "good reason," and subject to the prior occurrence of specified events, the vesting and exercisability of such executive's options to purchase shares of common stock will be accelerated in full; provided that if he remains our consultant following any termination of his employment with us, he shall have 90 days from the termination date of his consulting relationship to exercise each option, but not later than the expiration date of the option.
For purposes of Dr. Gottesdiener and Dr. Van der Ploeg's letter agreements, "cause" generally means the occurrence of any of the following events, conditions or actions: (i) a material breach of the employment agreement by the executive, including the non-disclosure obligations; (ii) the executive's material failure to adhere to our company policies; (iii) the executive's appropriation of one of our business opportunities; (iv) the executive's misappropriation of our funds or property; (v) a conviction, guilty plea, or plea of no contest to a felony, or any crime involving fraud, dishonesty, or misappropriation by the executive; (vi) willful misconduct or failure by the executive to perform duties requested by our board of directors; (vii) bad faith or gross negligence by the executive in the performance of his duties; or (viii) commission of an act of fraud, embezzlement, breach of any fiduciary duty owed to us or our stockholders or any other act of material dishonesty by the executive.
For purposes of Dr. Gottesdiener and Dr. Van der Ploeg's letter agreements, "good reason" generally means the occurrence of any of the following events, conditions or actions: (i) a diminution in the scope of the executive's authorities or duties; (ii) removal of the executive from his role; (iii) a significant reduction in the executive's base salary other than a reduction of less than 5% that applies to all executives and employees; and (iv) a breach by us of the terms set forth in the employment agreement.
In addition, pursuant to the terms of Dr. Gottesdiener and Dr. Van der Ploeg's letter agreements, in the event of a "change of control," the vesting and exercisability of the executive's options to purchase shares of common stock will be accelerated in full. A change of control is defined as the closing of any sale of our company, the direct or indirect acquisition, in a single transaction or a series of related transactions, by any person or group of beneficial ownership of previously outstanding capital
125
securities of our company, unless such transactions are excluded from the scope of the definition by consent in writing.
Under the terms of Mr. Henderson's employment agreement, upon termination without "cause," as defined below, Mr. Henderson is entitled to receive a severance payment of his base salary then in effect through the end of the earlier of (a) six months or (b) the date on which he commences new full-time employment, and the vesting and exercisability of Mr. Henderson's options to purchase shares of common stock will be accelerated in full.
For purposes of Mr. Henderson's employment agreement, "cause" generally means the occurrence of any of the following events, conditions or actions: (i) arbitrary, unreasonable or intentional failure by such executive to follow instructions of our board of directors or to perform his duties; (ii) gross negligence or intentional misconduct by such executive in the performance of his duties; (iii) any behavior by such executive that is materially injurious to us, financially or otherwise; (iv) commission of an act constituting fraud, embezzlement, breach of any fiduciary duty owed to us or our stock holders, or any other act of material dishonesty by such executive; (v) a conviction, guilty plea, or plea of no contest to a felony, or any crime involving moral turpitude by such executive; or (vi) material breach of the employment agreement or the non-disclosure agreement entered into by such executive.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth the outstanding equity awards held by each of our named executive officers as of December 31, 2013.
Name
|
Number of securities that have vested (*) |
Number of securities that have not vested (*) |
Total market value of securities that have not vested |
Grant date | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Keith M. Gottesdiener, M.D. |
1,790,083 | 1,514,117 | (1) | $ | 348,247 | 10/13/2011 | |||||||
Keith M. Gottesdiener, M.D. |
455,078 | 384,922 | (1) | $ | 88,532 | 10/13/2011 | |||||||
Keith M. Gottesdiener, M.D. |
364,246 | 555,754 | (2) | $ | 127,823 | 12/05/2012 | |||||||
Keith M. Gottesdiener, M.D. |
224,830 | 190,170 | (3) | $ | 43,739 | 12/05/2012 | |||||||
Keith M. Gottesdiener, M.D. |
364,237 | 555,763 | (4) | $ | 127,825 | 01/30/2013 | |||||||
Keith M. Gottesdiener, M.D. |
— | 460,000 | (5) | $ | 105,800 | 01/30/2013 | |||||||
Keith M. Gottesdiener, M.D. |
224,830 | 190,170 | (6) | $ | 43,739 | 01/30/2013 | |||||||
Bart Henderson |
250,000 | (7) | — | $ | — | 06/08/2010 | |||||||
Bart Henderson |
400,000 | (8) | — | $ | — | 07/30/2010 | |||||||
Bart Henderson |
190,042 | 289,958 | (2) | $ | 66,690 | 12/05/2012 | |||||||
Bart Henderson |
190,037 | 289,963 | (4) | $ | 66,692 | 01/30/2013 | |||||||
Bart Henderson |
— | 240,000 | (5) | $ | 55,200 | 01/30/2013 | |||||||
Lex H.T. Van der Ploeg, Ph.D. |
164,307 | 250,693 | (9) | $ | 57,722 | 12/06/2011 | |||||||
Lex H.T. Van der Ploeg, Ph.D. |
81,164 | 123,836 | (9) | $ | 28,482 | 12/06/2011 | |||||||
Lex H.T. Van der Ploeg, Ph.D. |
61,368 | 93,632 | (9) | $ | 21,535 | 06/05/2012 | |||||||
Lex H.T. Van der Ploeg, Ph.D. |
50,678 | 77,322 | (2) | $ | 17,784 | 12/05/2012 | |||||||
Lex H.T. Van der Ploeg, Ph.D. |
50,677 | 77,324 | (4) | $ | 17,784 | 01/30/2013 | |||||||
Lex H.T. Van der Ploeg, Ph.D. |
— | 64,000 | (5) | $ | 14,720 | 01/30/2013 | |||||||
- (*)
- The
number of securities represent restricted common units issued pursuant to our Operating Agreement.
- (**)
- For
purposes of this table, the fair market value of our common units as of December 31, 2013 was based on qualitative and quantitative factors and
the results of our retrospective valuation analysis. See "Management's Discussion and Analysis of Financial Condition and Results of Operations." The fair value of our common units for financial
reporting purposes as of December 31, 2013 was $0.24 per share.
- (1)
- Pursuant to the terms of the applicable award agreement, 25% of the securities vested on October 17, 2012, and an additional 2.084% of the remaining securities vest on the first day of each month commencing on November 1, 2012. All of the securities become fully vested on October 1, 2015.
126
- (2)
- Pursuant
to the terms of the applicable award agreement, 12.5% of the securities vested on December 5, 2012, and an additional 2.084% of the
remaining securities vest after the twenty-fifth day of each month commencing on December 25, 2012. All of the securities become fully vested on May 25, 2016.
- (3)
- Pursuant
to the terms of the applicable award agreement, 29.168% of the securities vested on December 5, 2012, and an additional 2.084% of the
remaining securities vest on the first day of each month commencing on January 1, 2013. All of the securities become fully vested on October 1, 2015.
- (4)
- Pursuant
to the terms of the applicable award agreement, 16.667% of the securities vested on January 30, 2013, and an additional 2.084% of the
remaining securities vest after the twenty-fifth day of each month commencing on February 25, 2013. All of the securities become fully vested on May 25, 2016.
- (5)
- Pursuant
to the terms of the applicable award agreement, prior to a Liquidation Event (as such term is defined in the applicable award agreement) the number
of vested Compensatory Units shall be determined by the Board. In the event of a Liquidation Event that is a Sale of the Company (as such term is defined in the applicable award agreement), all of the
securities shall immediately vest.
- (6)
- Pursuant
to the terms of the applicable award agreement, 31.252% of the securities vested on January 30, 2013, and an additional 2.084% of the
remaining securities vest on the first day of each month commencing on February 1, 2013. All of the securities become fully vested on October 1, 2015.
- (7)
- Pursuant
to the terms of the applicable award agreement, the securities became fully vested on the date of the grant, which was June 8, 2010.
- (8)
- Pursuant
to the terms of the applicable award agreement, the securities became fully vested on the date of the grant, which was July 30, 2010.
- (9)
- Pursuant to the terms of the applicable award agreement, 12.5% of the securities vested on November 1, 2012, and the remaining securities vest in thirty-six equal monthly installments on the first day of each month thereafter. All of the securities become fully vested on May 25, 2016.
Employee Benefit and Stock Plans
Prior to 2014—Restricted Common Units
Pursuant to the 2013 Rhythm Holding Company, LLC Operating Agreement, as amended by the First Amendment to Operating Agreement, Rhythm Holding Company, LLC, or the Operating Agreement, was authorized to grant restricted common units, referred to in the Operating Agreement as compensatory units, to employees, directors, consultants, and other members as compensation for services. The restricted common units are subject to vesting terms approved by the board of managers and set forth in a written restricted common unit vesting agreement entered into by Rhythm Holding Company, LLC and the service provider receiving restricted common units. Pursuant to these restricted common unit agreements, vesting may accelerate upon the occurrence of various events, including termination, with or without a change of control, and in certain cases, upon a change in control. As part of the Conversion, each outstanding vested restricted common unit will be converted into one share of common stock and each outstanding unvested restricted common unit will be converted into one share of restricted common stock, subject to vesting on the same terms as prior to the Conversion.
2014 Equity Incentive Plan
We expect our board of directors to adopt and our stockholders to approve the 2014 equity incentive plan which will become effective immediately prior to the completion of this offering. The following summary of the material terms of the 2014 equity incentive plan does not purport to be complete and is qualified by reference to the full text of the 2014 equity incentive plan, which we will file as an exhibit to our registration statement of which this prospectus is a part.
The 2014 equity incentive plan provides for the grant of incentive stock options and nonstatutory stock options, stock appreciation rights, restricted stock and restricted stock unit awards, performance
127
units, stock grants and any of the foregoing intended to constitute qualified performance-based awards under Section 162(m) of the Code, which we collectively refer to as "awards" in connection with the 2014 equity incentive plan. Our directors, officers and other employees, as well as others performing consulting or advisory services for us, are eligible for grants under the 2014 equity incentive plan. The purpose of the 2014 equity incentive plan is to provide incentives that will attract, retain and motivate highly competent officers, directors, employees and consultants to promote the success of our business and align employees' interests with stockholders' interests.
Administration
Under its terms, the 2014 equity incentive plan is administered by the compensation committee of our board of directors which is made up of independent outside non-employee directors for purposes of applicable securities and tax laws. The board of directors itself may also exercise any of the powers and responsibilities under the 2014 equity incentive plan. The compensation committee may delegate to an executive officer or officers the authority to grant certain awards under the 2014 equity incentive plan subject to applicable law and to guidelines specified by the compensation committee. Subject to the terms of the 2014 equity incentive plan, the compensation committee will select the recipients of awards and determine, among other things, the:
- •
- number of shares of common stock covered by awards and the dates upon which such awards become exercisable or any
restrictions to which they are subject lapse, as applicable;
- •
- type of award and the exercise or purchase price and method of payment for each such award;
- •
- vesting period for awards, risks of forfeiture and any potential acceleration of vesting or lapses in risks of
forfeiture; and
- •
- duration of awards.
All decisions, determinations and interpretations made in good faith by the compensation committee with respect to the 2014 equity incentive plan and the terms and conditions of or operation of any award are final and binding on all participants, beneficiaries, heirs, assigns or other persons holding or claiming rights under the 2014 equity incentive plan or any award.
Available Shares
The aggregate number of shares of our common stock which may be issued under the 2014 equity incentive plan or with respect to which awards may be granted may not exceed shares, which may be either authorized and unissued shares of our common stock or shares of common stock held in or acquired for our treasury. In general, if awards under the 2014 equity incentive plan are for any reason cancelled, or expire or terminate unexercised, the number of shares covered by such awards will again be available for the grant of awards under the 2014 equity incentive plan. The number of shares authorized under the 2014 equity incentive plan will be increased each January 1, commencing on January 1, 2015, by an amount equal to 4% of outstanding shares of stock as of the end of the immediately preceding fiscal year. Notwithstanding the foregoing, our board of directors may act prior to January 1 of a given year to provide that there will be no such January 1 increase in the number of shares of stock authorized under the 2014 equity incentive plan for such year or that the increase in the number of shares of stock authorized under the 2014 equity incentive plan for such year will be a lesser number than would otherwise occur pursuant to the preceding sentence. Notwithstanding the preceding sentences, in no event shall the number of shares available for issuance pursuant to incentive options exceed shares of stock.
128
Eligibility for Participation
Members of our board of directors, as well as employees of, and consultants to, us or any of our subsidiaries and affiliates are eligible to receive awards under the 2014 equity incentive plan. The selection of participants is within the sole discretion of the compensation committee.
Individual Limitations
The maximum number of shares of common stock that may be subject to options or stock appreciation rights or any combination thereof granted to any one person during any single calendar year shall be . The maximum number of shares of common stock that may be subject to all other awards granted to any one person during any single calendar year that are intended to be qualified performance-based awards shall be . The maximum value of awards denominated in cash granted to any one person during any single calendar year and that are intended to be qualified performance-based awards shall be . Each of the foregoing limitations shall be doubled with respect to awards granted to an individual during the first calendar year in which he or she commences employment.
Incentive Stock Options
Incentive stock options are options that are intended to qualify as incentive stock options under Section 422 of the Code, and will be granted pursuant to incentive stock option agreements. The compensation committee will determine the exercise price for an incentive stock option, which may not be less than 100% of the fair market value of the stock underlying the option determined on the date of grant. In addition, incentive options granted to employees who own, or are deemed to own, more than 10% of our voting stock, must have an exercise price not less than 110% of the fair market value of the stock underlying the option determined on the date of grant.
Nonstatutory Stock Options
Nonstatutory stock options are not intended to qualify as incentive stock options under Section 422 of the Code and will be granted pursuant to nonstatutory stock option agreements. The compensation committee will determine the exercise price for a nonstatutory stock option.
Stock Appreciation Rights
A stock appreciation right, or a SAR, entitles a participant to receive a payment equal in value to the difference between the fair market value of a share of stock on the date of exercise of the SAR over a specified exercise price of the SAR. SARs may be granted in tandem with a stock option, such that the recipient has the opportunity to exercise either the stock option or the SAR, but not both. The exercise price (above which any appreciation is measured) will not be less than 100% of the fair market value of the common stock on the date of grant of the SAR or, in the case of an SAR granted in tandem with a stock option, the exercise price will be the same as the exercise price of the related stock option. The compensation committee may pay that amount in cash, in shares of our common stock, or a combination of cash and shares of our common stock. The terms, methods of exercise, methods of settlement, form of consideration payable in settlement, and any other terms and conditions of any SAR will be determined by the compensation committee at the time of the grant of the award and will be reflected in the award agreement.
Restricted Stock and Restricted Stock Units
A restricted stock award or restricted stock unit award is the grant of shares of our common stock either currently (in the case of restricted stock) or at a future date (in the case of restricted stock units) at a price determined by the compensation committee (including zero), based on satisfaction of
129
certain vesting conditions, including continuing employment or other service, or achievement of performance goals. During the vesting period, participants holding shares of restricted stock shall, except as otherwise provided in an individual award agreement, have full voting and dividend rights with respect to such shares but any stock dividends or other distributions payable in shares of stock or other securities of ours will be subject to the same vesting conditions that apply to the shares of restricted stock in respect of which the dividend was made. The receipt of cash dividends may also be deferred or required to be invested in additional shares of restricted stock. Participants holding restricted stock units may be entitled to receive payments equivalent to any dividends declared with respect to the common stock referenced in the grant of the restricted stock units, but only following the close of the applicable restriction period and then only if the employment or other service and/or performance goals have been met. The restrictions will lapse in accordance with a schedule or other conditions determined by the compensation committee.
Performance Units
A performance unit award is a contingent right to receive the value of a specified number of shares of our common stock over an initial value for such number of shares (which may be zero) established by the compensation committee at the time of grant if certain performance goals or other business objectives are met with the specified performance period. The value of performance units will depend on the degree to which the specified performance goals are achieved. The compensation committee may, in its discretion, pay earned performance units in cash, or stock, or a combination of both cash and stock.
The compensation committee has discretion to select the length of any applicable restriction or performance period, the kind and/or level of the applicable performance goal, and whether the performance goal is to apply to us, one of our subsidiaries or any division or business unit, or to the recipient.
Stock Grants
A stock grant is an award of shares of common stock without restriction. Stock grants may only be made in limited circumstances, such as in lieu of other earned compensation. Stock grants are made without any forfeiture conditions.
Qualified Performance-Based Awards
Qualified performance-based awards include performance criteria intended to satisfy Section 162(m) of the Code. Section 162(m) of the Code limits our federal income tax deduction for compensation to each of our CEO and our three most highly compensated employees other than the CEO and CFO to $1 million dollars, but excludes from that limit "performance-based compensation." Any form of award permitted under the 2014 equity incentive plan other than a stock grant may be granted as a qualified performance-based award, but, in the case of awards other than stock options and SARs, will be subject to satisfaction of pre-established, objective performance goals. Qualified performance-based awards include any of the foregoing awards that are granted subject to vesting and/or payment based on the attainment of specified performance goals. Performance criteria upon which performance goals are established by the plan administrator may include but are not limited to: (i) net earnings (either before or after one or more of (A) interest, (B) taxes, (C) depreciation and (D) amortization); (ii) gross or net sales or revenue; (iii) net income (either before or after taxes); (iv) adjusted net income; (v) operating earnings or profit; (vi) cash flow (including, but not limited to, operating cash flow and free cash flow); (vii) return on assets; (viii) return on capital; (ix) return on stockholders' equity; (x) total stockholder return; (xi) return on sales; (xii) gross or net profit or operating margin; (xiii) costs; (xiv) expenses; (xv) working capital; (xvi) earnings per share; (xvii) adjusted earnings per share; (xviii) price per share; (xix) regulatory body approval for
130
commercialization of a product; (xx) implementation, completion or attainment of objectives relating to research, development, regulatory, commercial, or strategic milestones or developments; (xxi) market share; (xxii) economic value; (xxiii) revenue; (xxiv) revenue growth; and (xxv) operational and organizational metrics.
Transferability
Awards, other than stock grants, granted under the 2014 equity incentive plan are generally nontransferable (other than by will or the laws of descent and distribution), except that the compensation committee may, at the time of grant or thereafter, provide for the transferability of nonstatutory stock options or restricted stock to certain family members.
Adjustment for Corporate Actions
In the event of any change in the outstanding shares of common stock as a result of a reorganization, recapitalization, reclassification, stock dividend, stock split, reverse stock split or other similar distribution with respect to the shares of common stock, an appropriate and proportionate adjustment will be made in (i) the maximum numbers and kinds of shares subject to the 2014 equity incentive plan, (ii) the numbers and kinds of shares or other securities subject to then outstanding awards, (iii) the exercise price for each share or unit of any other securities subject to then outstanding stock options or SARs (without change in the aggregate purchase price as to which such stock options or SARs remain exercisable), and (iv) the repurchase price of each share of restricted stock then subject to a risk of forfeiture in the form of a company repurchase right. Any such adjustment in awards will be determined and made by the compensation committee in its sole discretion.
Transactions
In the event of a transaction, including (i) any merger or consolidation of our company with or into another entity as a result of which our stock is converted into or exchanged for the right to receive cash, securities or other property or is cancelled, (ii) any sale or exchange of all of our common stock for cash, securities, or other property, (iii) any sale, transfer or other disposition of all or substantially all of our assets to one or more other persons in a single transaction or series of related transactions, or (iv) any liquidation or dissolution of our company, the compensation committee may, with respect to all or any outstanding stock options and SARS, (1) provide that such awards will be assumed, or substantially equivalent rights shall be provided in substitution therefor by the acquiring or succeeding entity (or an affiliate thereof), (2) upon written notice to the recipient, provide that the recipient's unexercised awards will terminate immediately prior to the consummation of such transaction unless exercised within a specified period following the date of such written notice, (3) provide that outstanding awards shall become exercisable in whole or in part prior to or upon the transaction, (4) provide for cash payments, net of applicable tax withholdings, to be made to the recipients, (5) provide that, in connection with our liquidation or dissolution, awards shall convert into the right to receive liquidation proceeds net of the exercise price of the awards and any applicable tax withholdings, or (6) any combination of the foregoing. With respect to outstanding awards other than stock options or SARs, that are not terminated prior to or upon the transaction, upon the occurrence of a transaction other than our liquidation or dissolution which is not part of another form of transaction, our repurchase and other rights under each such award will transfer to our successor and inure to the benefit of our successor, and shall, unless the compensation committee determines otherwise, apply to the cash, securities or other property which the stock was converted into or exchanged for pursuant to such transaction in the same manner and to the same extent as they applied to the award.
131
Change of Control
In the event of a change of control, to the extent that the surviving entity declines to continue, convert, assume or replace outstanding awards, then all such awards shall become fully vested and exercisable and any restrictions applicable to any such awards shall lapse in connection with the transaction. Upon or in anticipation of a change of control, the plan administrator may cause any outstanding awards to terminate at a specified time in the future and give the participant the right to exercise such awards during a period of time determined by the plan administrator. Individual award agreements may provide for additional accelerated vesting and payment provisions.
A change of control is defined as the occurrence of any of the following: (1) a transaction, as described above, unless securities possessing more than 50% of the total combined voting power of the resulting entity or acquiror's outstanding securities (or the securities of any parent thereof) are held by a person or persons who held securities possessing more than 50% of the total combined voting power of our outstanding securities immediately prior to the transaction; (2) any person or group of persons, excluding our company and certain other related entities or any of its affiliates, directly or indirectly acquires, including but not limited to by means of a merger or consolidation, beneficial ownership of securities possessing more than 50% of the total combined voting power of our outstanding securities, unless pursuant to a tender or exchange offer made directly to our stockholders that our board of directors recommends such stockholders accept; or (3) over a period of no more than 36 consecutive months there is a change in the composition of our board such that a majority of the board members ceases to be composed of individuals who either (i) have been board members continuously since the beginning of that period, or (ii) have been elected or nominated for election as board members during such period by at least a majority of the remaining board members who have been board members continuously since the beginning of that period; or (4) a majority of the board of directors votes in favor of a decision that a change of control has occurred.
Amendment and Termination
Our board of directors may at any time amend any or all of the provisions of the 2014 equity incentive plan, or suspend or terminate it entirely, retroactively or otherwise. However, except as set forth in the 2014 equity incentive plan, we must obtain stockholder approval to increase the number of shares available under the 2014 equity incentive plan, or to change the description of persons eligible for awards, or as otherwise required by law or applicable stock exchange rules. Unless otherwise required by law or specifically provided in the 2014 equity incentive plan, the rights of a participant under awards granted prior to any amendment, suspension or termination may not be adversely affected without the consent of the participant. Unless the 2014 equity incentive plan is earlier terminated by our board of directors, the 2014 equity incentive plan terminates immediately prior to the tenth anniversary of the earlier of the adoption of the 2014 equity incentive plan by our board of directors and approval of the 2014 equity incentive plan by our stockholders.
Allocation of Awards; Plan Benefits.
It is not presently possible to determine the dollar value of award payments that may be made or the number of options, shares of restricted stock, restricted stock units, or other awards that may be granted under the 2014 equity incentive plan in the future, or the individuals who may be selected to receive such awards because awards under the 2014 equity incentive plan are granted at the discretion of the compensation committee.
2014 Employee Stock Purchase Plan
We expect our board of directors to adopt and our stockholders to approve the 2014 employee stock purchase plan, or the ESPP, which will become effective immediately prior to the completion of
132
this offering. The following summary of the material terms of the ESPP does not purport to be complete and is qualified by reference to the full text of the ESPP, which we will file as an exhibit to our registration statement of which this prospectus is a part.
The ESPP provides an incentive to, and encourages stock ownership by, all of our eligible employees and those of our participating subsidiaries so that they may share in our growth by acquiring or increasing their share ownership in the Company. It is intended that the ESPP constitute an "employee stock purchase plan" within the meaning of Section 423 of the Code. Under the ESPP, eligible employees may purchase shares of our common stock through payroll deductions.
Administration
The ESPP is administered by the compensation committee of our board of directors. The board of directors itself may exercise any of the powers and responsibilities under the ESPP. The compensation committee may delegate its duties in order to facilitate the purchase and transfer of shares of our common stock and for the day-to-day administration of the ESPP. The compensation committee, has the discretion, subject to the provisions of the ESPP, to make or to select the manner of making all determinations with respect to options granted under the ESPP. Further, the compensation committee has complete authority to interpret the ESPP, to prescribe, amend and rescind rules and regulations relating to it, and to make all other determinations necessary or advisable for the administration of the ESPP. All decisions, determinations and interpretations made in good faith by the compensation committee with respect to the ESPP are final and binding on all persons having or claiming any interest in the ESPP or any option granted under the ESPP.
Shares Subject to the Plan
The shares issued or to be issued under the ESPP are authorized but unissued shares of our common stock. The ESPP authorizes the issuance of up to shares of common stock. The number of shares authorized under the ESPP will be increased each January 1, commencing on January 1, 2015 and ending on (and including) January 1, 2024, by an amount equal to the lesser of (i) 1% of outstanding shares as of the end of the immediately preceding fiscal year and (ii) . Notwithstanding the foregoing, our board of directors may act prior to January 1 of a given year to provide that there will be no such January 1 increase in the number of shares authorized under the ESPP for such year, or that the increase in the number of shares authorized under the ESPP for such year will be a lesser number than would otherwise occur pursuant to the preceding sentence.
Terms of Participation
The ESPP will be implemented through a series of purchase periods called "plan periods." The initial plan period shall commence on such date following the closing of our initial public offering as the compensation committee may determine in its sole discretion and continue until April 30, 2015. After the initial plan period, there will be two consecutive six-month plan periods, during each twelve month period thereafter, with the first six-month plan period beginning on May 1 and ending on the immediately following October 31, and the second six-month plan period beginning on November 1 and ending on the immediately following April 30. An eligible employee will be granted an option at the beginning of the plan period, and can accumulate money to pay the exercise price for the option by electing to have payroll deductions taken from each payroll during a plan period of an amount, in whole percentages, between 1% and 15% of his or her compensation, but will not exceed $25,000 on an annual basis. At the end of each plan period, unless the participating employee has withdrawn from the ESPP, the option will be exercised by applying the employee's accumulated payroll deductions to the purchase of Common Stock. The exercise price paid by the employee will be the lower of 85% of
133
the fair market value of our common stock at (i) the commencement of the plan period and (ii) the end of the plan period.
Withdrawal
An employee may withdraw from participation in an offering up to two weeks prior to the plan period termination date and permanently draw out the balance accumulated in his or her account. In such case, the employee's option for the plan period he or she is withdrawing from will be automatically terminated. A participant's withdrawal from a plan period will not have any effect upon his or her eligibility to participate in a succeeding plan period or in any similar plan which we may adopt. If a participant's employment ends prior to a plan period termination date for any reason, including retirement or death, the contributions credited to his or her account will be returned to him or her or, in the case of his or her death, to his or her designated beneficiaries, and his or her option will be automatically terminated.
Eligibility
Our employees and those of a participating subsidiary are eligible to participate in the ESPP if we employ them for at least 20 hours per week and more than five months per year. However, no employee shall be granted an option under the ESPP if, immediately after the grant, the employee would own stock, including any outstanding options to purchase stock, equaling 5% or more of the total voting power or value of all classes of our stock. In addition, the ESPP provides that no employee may be granted an option if the option would permit the employee to purchase stock under all of our employee stock purchase plans in an amount that exceeds $25,000 of the fair market value of such stock for each calendar year in which the option is outstanding.
Adjustment for Corporate Actions
In the event of any change in the outstanding shares of common stock as a result of a reorganization, recapitalization, reclassification, stock dividend, stock split, reverse stock split, or other similar distribution with respect to the shares of common stock, an appropriate and proportionate adjustment will be made in (i) the maximum numbers and kinds of shares subject to the ESPP, (ii) the numbers and kinds of shares or other securities subject to the then outstanding options, and (iii) the exercise price for each share or other unit of any other securities subject to then outstanding options.
Corporate Transactions
In the event of our dissolution or liquidation, the plan period then in progress will terminate unless otherwise provided by the compensation committee. In the event of another significant corporate transaction such as a merger or consolidation of us with and into another person or entity or the sale or transfer of all or substantially all of our assets, each right to purchase stock under the ESPP may be assumed, or an equivalent right substituted by, the successor corporation or a parent or subsidiary of the successor corporation. In the event that the successor corporation refuses to assume each purchase right or to substitute an equivalent right, any ongoing offering period will be shortened so that employees' rights to purchase stock under the ESPP are exercised prior to the transaction, unless the employee has withdrawn.
Amendment and Termination
Our board of directors has the power to amend or terminate the ESPP and to change or terminate plan periods as long as any such action does not adversely affect any outstanding rights to purchase stock; provided, however, that the board of directors may amend or terminate the ESPP or a plan period even if it would adversely affect outstanding options in order to avoid our incurring adverse
134
accounting charges or if the board of directors determines that termination of the ESPP and/or plan period is in our best interest and the best interest of our stockholders. The ESPP will continue in effect until the tenth anniversary of the closing of the offering described in this prospectus, unless earlier terminated by the board of directors.
Amount of Benefits
The dollar value of benefits that will be received by any employee or group of employees in the ESPP is not determinable due to the voluntary nature of the ESPP and the variables involved in the calculation of any such benefits (including our stock price).
401(k) Plan
We maintain a tax-qualified retirement plan that provides eligible U.S. employees with an opportunity to save for retirement on a tax advantaged basis. Eligible employees are able to defer eligible compensation up to certain Code limits, which are updated annually. We have the ability to make matching and discretionary contributions to the 401(k) plan but have not done so to date. Employee contributions are allocated to each participant's individual account and are then invested in selected investment alternatives according to the participants' directions. Employees are immediately and fully vested in their own contributions. The 401(k) plan is intended to be qualified under Section 401(a) of the Code, with the related trust intended to be tax exempt under Section 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan are deductible by us when made, and contributions and earnings on those amounts are not taxable to the employees until withdrawn or distributed from the 401(k) plan.
Non-Employee Director Compensation
No compensation was paid to or earned by our non-employee directors during our 2013 fiscal year. Following the closing of this offering, we intend to consider and adopt a compensation policy for non-employee directors.
Limitation on Liability and Indemnification Matters
Section 145 of the Delaware General Corporation Law authorizes a corporation's board of directors to grant, and authorizes a court to award, indemnity to officers, directors, and other corporate agents.
As permitted by Delaware law, our amended and restated certificate of incorporation provides that, to the fullest extent permitted by Delaware law, no director will be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director. Pursuant to Delaware law, such protection would be not available for liability:
- •
- for any breach of a duty of loyalty to us or our stockholders;
- •
- for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law;
- •
- for any transaction from which the director derived an improper benefit; or
- •
- for an act or omission for which the liability of a director is expressly provided by an applicable statute, including unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law.
Our amended and restated certificate of incorporation also provides that if Delaware law is amended after the approval by our stockholders of the amended and restated certificate of incorporation to authorize corporate action further eliminating or limiting the personal liability of
135
directors, then the liability of our directors will be eliminated or limited to the fullest extent permitted by Delaware law.
Our amended and restated bylaws further provide that we must indemnify our directors and officers to the fullest extent permitted by Delaware law. Our amended and restated bylaws also authorize us to indemnify any of our employees or agents and permit us to secure insurance on behalf of any officer, director, employee or agent for any liability arising out of his or her action in that capacity, whether or not Delaware law would otherwise permit indemnification.
In addition, our amended and restated bylaws provide that we are required to advance expenses to our directors and officers as incurred in connection with legal proceedings against them for which they may be indemnified and that the rights conferred in the amended and restated bylaws are not exclusive.
We intend to enter into indemnification agreements with each of our directors and executive officers. These agreements, among other things, would require us to indemnify each director and officer to the fullest extent permitted by Delaware law, the amended and restated certificate of incorporation and amended and restated bylaws, for expenses such as, among other things, attorneys' fees, judgments, fines, and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action by or in our right, arising out of the person's services as our director or executive officer or as the director or executive officer of any subsidiary of ours or any other company or enterprise to which the person provides services at our request. We also maintain directors' and officers' liability insurance.
The Securities and Exchange Commission has taken the position that personal liability of directors for violation of the federal securities laws cannot be limited and that indemnification by us for any such violation is unenforceable. The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors and officers for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder's investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions.
Rule 10b5-1 sales plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, pursuant to which, if adopted, they would contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from them. The director or officer may amend a Rule 10b5-1 plan in some circumstances and may terminate a plan at any time. Our directors and executive officers also may buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material nonpublic information subject to compliance with the terms of our insider trading policy. Prior to 180 days after the date of this offering (subject to early termination), the sale of any shares under such plan would be subject to the lock-up agreement that the director or officer has entered into with the underwriters.
136
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
We describe below transactions and series of similar transactions, during our last three fiscal years, to which we were a party or will be a party, in which:
- •
- the amounts involved exceeded or will exceed $120,000; and
- •
- any of the directors, executive officers or holders of more than 5% of our voting equity, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest.
Compensation arrangements for our directors and named executive officers are described elsewhere in this prospectus.
Financings
Series A Preferred Stock Financings
In February 2010, we entered into a series A preferred stock purchase agreement, which we amended in November 2010, pursuant to which we issued, in a series of closings, an aggregate of 61.0 million shares of our series A preferred stock, at a purchase price of $0.50 per share, for aggregate consideration of approximately $30.5 million.
The following table summarizes the participation in the series A preferred stock financing by our directors, executive officers, holders of more than 5% of our voting securities, or any member of the immediate family of the foregoing persons:
Name
|
Shares of Series A Preferred Stock |
Date(s) Purchased |
|||
|---|---|---|---|---|---|
MPM BioVentures V, L.P.(1) |
20,333,334 | March 9, 2010 | |||
|
August 10, 2010 | ||||
|
November 29, 2011 | ||||
New Enterprise Associates 13, L.P. |
20,293,333 |
March 9, 2010 |
|||
|
August 10, 2010 | ||||
|
November 29, 2011 | ||||
NEA Ventures 2009, Limited Partnership |
40,000 |
March 9, 2010 |
|||
Third Rock Ventures, L.P. |
20,333,333 |
August 10, 2010 |
|||
|
November 29, 2011 | ||||
- (1)
- In 2012, shares were redistributed and split between MPM BioVentures V, L.P. (19,572,961 shares) and MPM Asset Management Investors BV5 LLC (760,373 shares).
Series B Preferred Stock Financings
In May 2012, we entered into a series B preferred stock purchase agreement, which we amended in November 2012, pursuant to which we issued, in a series of closings, an aggregate of 63.0 million shares of our series B preferred stock, at a purchase price of $0.50 per share, for aggregate consideration of approximately $31.5 million.
137
The following table summarizes the participation in the series B preferred stock financing by our directors, executive officers, holders of more than 5% of our voting securities, or any member of the immediate family of the foregoing persons:
Name
|
Shares of Series B Preferred Stock |
Date(s) Purchased |
|||
|---|---|---|---|---|---|
MPM BioVentures V, L.P. |
8,811,042 | May 25, 2012 | |||
|
September 24, 2012 | ||||
MPM Asset Management Investors BV5 LLC |
342,292 |
May 25, 2012 |
|||
|
September 24, 2012 | ||||
New Enterprise Associates 13, L.P. |
22,533,333 |
May 25, 2012 |
|||
|
September 24, 2012 | ||||
|
February 8, 2013 | ||||
Third Rock Ventures, L.P. |
22,533,333 |
May 25, 2012 |
|||
|
September 24, 2012 | ||||
|
February 8, 2013 | ||||
Sutrepa SAS |
780,000 |
September 24, 2012 |
|||
Pfizer Inc. |
8,000,000 |
November 20, 2012 |
|||
|
February 8, 2013 | ||||
Series B Preferred Unit Financings
As a part of the Prior Restructuring on March 21, 2013, we entered into a series B preferred unit purchase agreement pursuant to which we issued, in a closing in July 2013, an aggregate of 22.0 million series B preferred units, at a purchase price of $0.50 per unit, for aggregate consideration of approximately $11.0 million.
The following table summarizes the participation in the series B preferred unit financing by any of our directors, executive officers, holders of more than 5% of our voting securities, or any member of the immediate family of the foregoing persons:
Name
|
Shares of Series B Preferred Units |
Date(s) Purchased |
|||||
|---|---|---|---|---|---|---|---|
New Enterprise Associates 13, L.P. |
7,000,000 | July 16, 2013 | |||||
Third Rock Ventures, L.P. |
7,000,000 | July 16, 2013 | |||||
Pfizer, Inc. |
8,000,000 | July 16, 2013 | |||||
Operating Agreement
Prior to the Conversion, Rhythm Holding Company, LLC was governed by its Operating Agreement, as amended by that certain First Amendment to Operating Agreement. We intend to consummate the Conversion immediately prior to the effectiveness of the registration statement of which this prospectus forms a part. The Operating Agreement will terminate upon the Conversion. See "Conversion" for more information.
Conversion
Prior to the effectiveness of the registration statement of which this prospectus forms a part, we will convert into a Delaware corporation and change our name to Rhythm Pharmaceuticals, Inc. and engage in other related transactions. In this prospectus, we refer to these transactions collectively as the Conversion. See "Conversion" and "Description of Capital Stock" for additional information, including a description of the terms of our common stock following the Conversion and the terms of our
138
amended and restated certificate of incorporation and amended and restated bylaws as will be in effect upon the closing of this offering.
Investor Agreements
In connection with our sale and issuance of series B preferred units in July 2013, we entered into investors' rights and right of first refusal and co-sale agreements containing information rights, rights of first refusal and registration rights, among other things, with certain holders of our preferred stock and certain holders of our common stock, including all of the holders of more than 5% of our capital stock or entities affiliated with such holders. The right of first refusal and co-sale agreement terminates upon the closing of our initial public offering. In addition, all obligations under the investors' rights agreement, other than certain registration rights more fully described below in "Description of Capital Stock—Registration Rights," terminate immediately prior to the consummation of our initial public offering.
Indemnification Agreements
Upon the completion of this offering, we intend to enter into indemnification agreements with each of our directors and executive officers. These agreements, among other things, will require us to indemnify each director and officer to the fullest extent permitted by Delaware law, our amended and restated certificate of incorporation and amended and restated bylaws, for expenses such as, among other things, attorneys' fees, judgments, fines, and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action by or in our right, arising out of the person's services as our director or executive officer or as the director or executive officer of any subsidiary of ours or any other company or enterprise to which the person provides services at our request. We also maintain directors' and officers' liability insurance. See "Executive and Director Compensation—Limitation on Liability and Indemnification Matters."
Employment Arrangements
We currently have written employment or consulting agreements with our executive officers. For information about our employment agreements with our named executive officers, refer to "Executive and Director Compensation—Agreements with our Named Executive Officers."
Consulting Arrangements
Pursuant to an original Service Agreement, as amended, between us, MPM Asset Management LLC, or MPM, and Dr. Stoner, Dr. Stoner provides us with certain consulting services. Dr. Stoner is an employee of MPM and we pay a consulting fee to MPM for Dr. Stoner's services. In 2012 and 2013, this fee was approximately $195,000 and $285,000, respectively.
Pursuant to an original Service Agreement between us, Third Rock Ventures and Louis Tartaglia, a former director and officer, Mr. Tartaglia provided us with certain consulting services. Mr. Tartaglia was a former partner of Third Rock Ventures and we paid Third Rock Ventures a consulting fee of approximately $290,000 in 2011 for Mr. Tartaglia's services.
Related Party Transactions Policy
Our board of directors has adopted a policy, effective upon the closing of this offering, that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock and any members of the immediate family of any of the foregoing persons are not permitted to enter into a related person transaction with us without the prior review and approval of our governance and nominating committee. Any request for us to enter into a transaction with an executive officer, director, nominee for election as a director, beneficial owner of more than 5% of any class of our common stock or any member of the immediate family of any of the foregoing persons in which the amount involved exceeds $120,000 and such person would have a direct
139
or indirect material interest must first be presented to our governance and nominating committee for review, consideration and approval. In approving or rejecting any such proposal, our governance and nominating committee is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related person's interest in the transaction. We did not have a formal review and approval policy for related party transactions at the time of any of the transactions described above. However, all of the transactions described above under "Certain Relationships And Related Party Transactions" were entered into after presentation, consideration and approval by our board of directors.
140
The following table sets forth information regarding the beneficial ownership of our common stock as of June 23, 2014, after giving effect to the Conversion and the conversion of our series A preferred shares and series B preferred shares into shares of common stock, by:
- •
- each of our directors and named executive officers;
- •
- all of our directors and executive officers as a group; and
- •
- each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock.
Beneficial ownership is determined according to the rules of the Securities and Exchange Commission and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, convertible securities that are currently exercisable or exercisable within 60 days after June 23, 2014. Shares of our common stock issuable pursuant to warrants are deemed outstanding for computing the percentage of the person holding such warrants and the percentage of any group of which the person is a member but are not deemed outstanding for computing the percentage of any other person. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons named in the table below have sole voting and investment power with respect to all shares of common stock shown that they beneficially own, subject to community property laws where applicable. The information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Section 13(d) and 13(g) of the Securities Act.
Our calculation of the percentage of beneficial ownership prior to this offering is based on 161,360,874 shares of common stock outstanding as of June 23, 2014, assuming the completion of the Conversion and the conversion of our series A preferred stock and series B preferred stock into common stock, as if these conversions had occurred as of June 23, 2014. Our calculation of the percentage of beneficial ownership after this offering is based on shares of common stock outstanding immediately after the closing of this offering (assuming no exercise of the underwriters' over-allotment option to purchase additional shares of our common stock).
141
Except as otherwise noted below, the address for persons listed in the table is c/o Rhythm Holding Company, LLC, 855 Boylston Street, First Floor, Boston, MA 02116. Beneficial ownership representing less than 1% is denoted with an asterisk(*).
| |
Prior to the Offering | After the Offering | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name and Address of Beneficial Owner
|
Number of shares of common stock |
Percent of class |
Number of shares of common stock |
Percent of class |
|||||||||
5% Stockholders: |
|||||||||||||
New Enterprise Associates 13, L.P.(1) |
50,366,666 | 31.21 | % | % | |||||||||
1954 Greenspring Drive, Suite 600 |
|||||||||||||
Third Rock Ventures, L.P.(2) |
50,166,666 |
31.09 |
% |
% |
|||||||||
29 Newbury Street |
|||||||||||||
MPM BioVentures V, L.P.(3) |
29,986,668 |
18.58 |
% |
% |
|||||||||
200 Clarendon Street, 54th Floor |
|||||||||||||
Pfizer Inc. |
16,000,000 |
9.92 |
% |
% |
|||||||||
235 East 42nd Street |
|||||||||||||
Directors and Named Executive Officers: |
|||||||||||||
Keith M. Gottesdiener, M.D.(4) |
7,274,200 | 4.51 | % | % | |||||||||
Bart Henderson(5) |
3,350,000 | 2.08 | % | % | |||||||||
Lex H.T. Van der Ploeg, Ph.D.(6) |
1,095,000 | * | % | ||||||||||
Christian de la Tour(7) |
— | * | % | ||||||||||
Todd Foley(8) |
— | * | % | ||||||||||
Ed Mathers(9) |
— | * | % | ||||||||||
Neil Exter(10) |
— | * | % | ||||||||||
All executive officers and directors as a group (8 persons) |
12,769,200 | 7.91 | % | % | |||||||||
- (1)
- Consists of (i) 500,000 shares of common stock held by New Enterprise Associates 13, L.P. ("NEA 13"); (ii) 40,000 shares of common stock issuable upon conversion of series A preferred stock held by NEA Ventures 2009, Limited Partnership ("NEA Ventures"); (iii) 20,293,333 shares of common stock issuable upon conversion of series A preferred stock held by NEA 13; and (iv) 29,533,333 shares of common stock issuable upon conversion of series B preferred stock held by NEA 13. The shares directly held by NEA 13 are indirectly held by NEA Partners 13, L.P. ("NEA Partners 13"), the sole general partner of NEA 13, NEA 13 GP, LTD ("NEA 13 LTD"), the sole general partner of NEA Partners 13 and each of the individual Directors of NEA 13 LTD. The individual directors (collectively, the "Directors") of NEA 13 LTD are M. James Barrett, Peter J. Barris, Forest Baskett, Ryan D. Drant, Patrick J. Kerins, Krishna Kolluri, David M. Mott, Scott D. Sandell, Ravi Viswanathan and Harry R. Weller. NEA 13, NEA Partners 13, NEA 13 LTD and the Directors share voting and dispositive power with regard to the shares held by NEA 13. Karen P. Welsh, the general partner of NEA Ventures, holds voting and dispositive power over the shares held by NEA Ventures. All indirect holders of the above referenced shares disclaim beneficial ownership of applicable shares except to the extent of their pecuniary interest therein.
142
- (2)
- All
shares are held directly by Third Rock Ventures, L.P. ("TRV LP"). Each of Third Rock Ventures GP, LP ("TRV GP"), the
general partner of TRV LP, and Third Rock Ventures GP, LLC ("TRV LLC"), the general partner of TRV GP, and Mark Levin, Kevin Starr and Robert Tepper, the managers of
TRV LLC, may be deemed to have voting and dispositive power over the shares held by TRV LP. Each of TRV GP, TRV LLC, Mark Levin, Kevin Starr and Robert Tepper disclaims
beneficial ownership of all shares held by TRV LP except to the extent of their pecuniary interest therein.
- (3)
- Includes
(a) 481,302 shares of common stock held by MPM BioVentures V, L.P. ("Bioventures LP"), (b) 18,698 shares of common
stock held by MPM Asset Management Investors BV5 LLC ("Asset Management LLC"), (c) 19,572,961 shares of common stock issuable upon conversion of series A preferred stock
held by Bioventures LP, (d) 760,373 shares of common stock issuable upon conversion of series A preferred stock held by Asset Management LLC, (e) 8,811,042 shares of
common stock issuable upon conversion of series B preferred stock held by Bioventures LP and (f) 342,292 shares of common stock issuable upon conversion of series B
preferred stock held by Asset Management LLC. MPM BioVentures V GP LLC is the General Partner of Bioventures LP. MPM BioVentures V LLC is the Managing Member of MPM
BioVentures V GP LLC and the Manager of Asset Management LLC. The Members of MPM BioVentures V LLC share voting and investment power of the shares. Luke Evnin, Todd Foley,
Ansbert Gadicke, Vaughn Kailian, James Scopa and John Vander Vort are the Members of MPM BioVentures V LLC and have shared power to vote, hold and dispose of the shares beneficially held by
such entity. Each disclaims beneficial ownership of the shares except to the extent of his respective pecuniary interest therein.
- (4)
- Dr. Gottesdiener
has beneficial ownership of 7,274,200 shares of restricted common stock.
- (5)
- Mr. Henderson
holds 1,500,000 shares of common stock. Mr. Henderson also has beneficial ownership of 1,850,000 shares of restricted common
stock.
- (6)
- Dr. Van
Der Ploeg has beneficial ownership of 1,095,000 shares of restricted common stock.
- (7)
- Mr. de
la Tour is the Vice President in charge of Ipsen Corporate Alliance Management (Ipsen Biopharm Ltd) and Chairman of Ipsen UK companies,
which is affiliated with our stockholder, Sutrepa SAS. Mr. de la Tour disclaims beneficial ownership of the shares held by such entities.
- (8)
- Mr. Foley
is a managing director of MPM Capital, an affiliate of MPM BioVentures V, L.P. and MPM Asset Management Investors BV5 LLC.
Mr. Foley disclaims beneficial ownership of the shares held by such entities except to the extent of his pecuniary interest therein.
- (9)
- Mr. Mathers
is a partner at New Enterprise Associates, an affiliate of New Enterprise Associates 13, L.P. and NEA Ventures 2009,
Limited Partnership. Mr. Mathers disclaims beneficial ownership of the shares held by such entities except to the extent of his pecuniary interest therein.
- (10)
- Mr. Exter is a partner at Third Rock Ventures, an affiliate of Third Rock Ventures, L.P. Mr. Exter does not hold voting or dispositive power over the shares held by TRV LP.
143
The following description of our capital stock and provisions of our amended and restated certificate of incorporation and amended and restated bylaws as they will be in effect upon the consummation of this offering are summaries and are qualified in their entirety by reference to our amended and restated certificate of incorporation and amended and restated bylaws that will be in effect upon the closing of this offering, the forms of which are filed as exhibits to the registration statement of which this prospectus forms a part, and by applicable law. The description of our common stock reflects the completion of the Conversion which will occur prior to the effectiveness of the Registration Statement of which this prospectus forms a part. See "Conversion" for more information concerning the Conversion.
Upon consummation of this offering, our authorized capital stock will consist of shares of common stock, par value $0.01 per share and shares of preferred stock, par value $0.01 per share. Unless our board of directors determines otherwise, we will issue all shares of our capital stock in uncertificated form. Assuming the completion of the Conversion and the conversion of all outstanding shares of our series A preferred stock and series B preferred stock into shares of our common stock and (2) the issuance by us of shares of common stock in this offering, there will be shares of common stock and no shares of preferred stock outstanding upon closing of this offering.
Common Stock
Holders of shares of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. The holders of a plurality of the shares of our common stock entitled to vote in any election of directors can elect all of the directors standing for election.
Holders of shares of our common stock are entitled to receive dividends when and if declared by our board of directors out of funds legally available therefor, subject to any statutory or contractual restrictions on the payment of dividends and to any restrictions on the payment of dividends imposed by the terms of any outstanding preferred stock.
Upon our dissolution or liquidation or the sale of all or substantially all of our assets, after payment in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any, the holders of shares of our common stock will be entitled to receive pro rata our remaining assets available for distribution.
Holders of shares of our common stock do not have preemptive, subscription, redemption or conversion rights.
Preferred Stock
After giving effect to the Conversion, each of the issued and outstanding series A preferred units of Rhythm Holding Company, LLC will be converted into one issued and outstanding share of our series A preferred stock and each of the issued and outstanding series B preferred units of Rhythm Holding Company, LLC will be converted into one issued and outstanding share of our series B preferred stock. Upon consummation of this offering, each share of our series A preferred stock and series B preferred stock will be converted into one share of our common stock
Our amended and restated certificate of incorporation authorizes our board of directors to establish one or more series of preferred stock (including convertible preferred stock). Unless required by law or by any stock exchange, the authorized shares of preferred stock will be available for issuance without further action by our stockholders. Our board of directors is able to determine, with respect to any series of preferred stock, the terms and rights of that series, including:
- •
- the designation of the series;
144
- •
- the number of shares of the series, which our board may, except where otherwise provided in the preferred stock
designation, increase or decrease, but not below the number of shares then outstanding;
- •
- the voting rights, if any, of the holders of the series;
- •
- whether dividends, if any, will be cumulative or non-cumulative and the dividend rate of the series;
- •
- the dates at which dividends, if any, will be payable;
- •
- the rights of priority and amounts payable, if any, on shares of the series in the event of any voluntary or involuntary
liquidation, dissolution or winding-up of the affairs of our company;
- •
- the redemption rights and price or prices, if any, for shares of the series;
- •
- the terms of any purchase, retirement or sinking fund, if any, provided for shares of the series;
- •
- the terms, if any, upon which the shares of the series will be convertible into or exchangeable for shares of any other
class, classes or series, or other securities, whether or not issued by our company or any other entity;
- •
- restrictions, if any, upon issuance of indebtedness of our company so long as any shares of the series are outstanding;
and
- •
- restrictions, if any, on the issuance of shares of the same series or of any other class or series.
We could issue a series of preferred stock that could, depending on the terms of the series, impede or discourage an acquisition attempt or other transaction that some, or a majority, of our stockholders might believe to be in their best interests or in which our stockholders might receive a premium for their shares of common stock over the market price of the shares of common stock.
Warrants
As of , 2014, 9,790,000 shares of our common stock were issuable upon exercise of outstanding warrants to purchase common stock with an exercise price of $0.001 per share. These warrants provide for the adjustment of the number of shares issuable upon the exercise of the warrant in the event of stock splits, recapitalizations, reclassifications and consolidations. Upon the closing of this offering, these warrants will automatically expire if not previously exercised.
Restricted Common Units
As of , 2014, we have issued restricted common units pursuant to our Operating Agreement to employees, directors, consultants, and other members as compensation for services. These restricted common units are subject to vesting terms approved by the board of managers and set forth in written restricted common unit vesting agreements. As part of the Conversion, each outstanding vested restricted common unit will be converted into one share of common stock and each outstanding unvested restricted common unit will be converted into one share of restricted common stock, subject to vesting on the same terms as prior to the conversion.
Options
Upon completion of the offering, we will have no options to purchase our common stock outstanding. See "Executive and Director Compensation—Employee Benefit and Stock Plans" for a discussion of the terms of our 2014 equity incentive plan.
145
Authorized but Unissued Capital Stock
The Delaware General Corporation Law does not require stockholder approval for any issuance of authorized shares. However, the listing requirements of NASDAQ, which would apply so long as our common stock remains listed on NASDAQ, require stockholder approval of certain issuances equal to or exceeding 20% of the then outstanding voting power or then outstanding number of shares of common stock. These additional shares may be used for a variety of corporate purposes, including future public offerings, to raise additional capital or to facilitate acquisitions.
One of the effects of the existence of unissued and unreserved common stock or preferred stock may be to enable our board of directors to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of our company by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive our stockholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices.
Anti-Takeover Effects of Provisions of Delaware Law and Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
Undesignated Preferred Stock
The ability to authorize undesignated preferred stock will make it possible for our board of directors to issue preferred stock with super voting, special approval, dividend or other rights or preferences on a discriminatory basis that could impede the success of any attempt to acquire us or otherwise effect a change in control of us. These and other provisions may have the effect of deferring, delaying or discouraging hostile takeovers, or changes in control or management of our company.
Requirements for Advance Notification of Stockholder Meetings, Nominations and Proposals
Our amended and restated certificate of incorporation provides that special meetings of the stockholders may be called only by or at the direction of our board of directors, two or more of our directors, the chairman of our board, our chief executive officer or one or more holders of at least a minimum percentage of the voting power of the outstanding shares of our capital stock. This minimum will initially be 25% and will automatically increase to 51% on the first date on which the holders of outstanding shares of our common stock hold more than 51% of the voting power of all outstanding shares of our capital stock. Our amended and restated bylaws prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions may have the effect of deferring, delaying or discouraging hostile takeovers, or changes in control or management of our company.
Our amended and restated bylaws establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors or a committee of the board of directors. In order for any matter to be "properly brought" before a meeting, a stockholder will have to comply with advance notice requirements and provide us with certain information. Additionally, vacancies and newly created directorships may be filled only by a vote of a majority of the directors then in office, even though less than a quorum, and not by the stockholders. Our amended and restated bylaws will allow the presiding officer at a meeting of the stockholders to adopt rules and regulations for the conduct of meetings which may have the effect of precluding the conduct of certain business at a meeting if the rules and regulations are not followed. These provisions may also defer, delay or discourage a potential acquirer from conducting a solicitation of proxies to elect the acquirer's own slate of directors or otherwise attempting to obtain control of our company.
146
Our amended and restated certificate of incorporation provides that the board of directors is expressly authorized to adopt, amend, or repeal our amended and restated bylaws.
No Cumulative Voting
The Delaware General Corporation Law provides that stockholders are not entitled to cumulate votes in the election of directors unless our amended and restated certificate of incorporation provides otherwise. Our amended and restated certificate of incorporation does not expressly provide for cumulative voting.
Removal of Directors
In accordance with the terms of our amended and restated certificate of incorporation and amended and restated bylaws that will become effective upon the closing of this offering, our board of directors will be divided into three staggered classes of directors of the same or nearly the same number and each will be assigned to one of the three classes. At each annual meeting of the stockholders, a class of directors will be elected for a three-year term to succeed the directors of the same class whose terms are then expiring. The initial term of office of the directors of Class I shall expire as of the first annual meeting of the Company's stockholders following the closing of this offering; the initial term of office of the directors of Class II shall expire as of the second annual meeting of the Company's stockholders following the closing of this offering; and the initial term of office of the directors of Class III shall expire as of the third annual meeting of the Company's stockholders following the closing of this offering.
- •
- Our Class I directors will
be
;
- •
- Our Class II directors will
be and ; and
- •
- Our Class III directors will be and .
Our amended and restated certificate of incorporation and amended and restated bylaws provide that the number of our directors shall be fixed from time to time by a resolution of the majority of our board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class shall consist of one third of the board of directors.
The division of our board of directors into three classes with staggered three-year terms may delay or prevent stockholder efforts to effect a change of our management or a change in control.
Our amended and restated certificate of incorporation and amended and restated bylaws that will become effective upon the closing of this offering also provide that our directors may be removed only for cause by the affirmative vote of the holders of at least 75% of the outstanding shares of capital stock of the Company entitled to vote in the election of directors or class of directors, voting together as a single class, at a meeting of the stockholders called for that purpose. This requirement of a supermajority vote to remove directors could enable a minority of our stockholders to prevent a change in the composition of our board. Any vacancy on our board of directors, including a vacancy resulting from an enlargement of our board of directors, may be filled only by the vote of a majority of the remaining directors then in office, although less than a quorum, or by the sole remaining director.
Amendments to Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
The Delaware General Corporation Law provides that, unless a corporation's certificate of incorporation provides otherwise, the affirmative vote of holders of shares constituting a majority of the votes of all shares entitled to vote may approve amendments to the certificate of incorporation.
147
Our amended and rested certificate of incorporation and amended and restated bylaws will provide that the affirmative vote of holders of at least 75% of the outstanding shares of capital stock, voting together as a single class, and entitled to vote in the election of directors will be required to amend, alter, change, or repeal the amended and restated certificate of incorporation and the amended and restated bylaws. This requirement of a supermajority vote to approve amendments to our amended and restated certificate of incorporation and amended and restated bylaws could enable a minority of our stockholders to exercise veto power over such amendments.
Stockholder Action by Written Consent
Pursuant to Section 228 of the Delaware General Corporation Law, any action required to be taken at any annual or special meeting of the stockholders may be taken without a meeting, without prior notice and without a vote if a consent or consents in writing, setting forth the action so taken, is signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares of our stock entitled to vote thereon were present and voted, unless the corporation's certificate of incorporation provides otherwise. Our amended and restated certificate of incorporation will prohibit the taking of any action of our stockholders by written consent without a meeting.
Delaware Anti-Takeover Statute
We have not opted out of, and therefore are subject to, Section 203 of the Delaware General Corporation Law. Section 203 provides that, subject to certain exceptions specified in the law, a publicly-held Delaware corporation shall not engage in certain "business combinations" with any "interested stockholder" for a three-year period after the date of the transaction in which the person became an interested stockholder unless:
- •
- prior to the date of the transaction, the board of directors of the corporation approved either the business combination
or the transaction that resulted in the stockholder becoming an interested stockholder;
- •
- upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the stockholder
owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (1) shares
owned by persons who are directors and also officers and (2) shares owned under employee stock plans in which employee participants do not have the right to determine confidentially whether
shares held subject to the plan will be tendered in a tender or exchange offer; or
- •
- on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock which is not owned by the interested stockholder.
Generally, a business combination includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. An interested stockholder is a person who, together with affiliates and associates, owns or, within three years prior to the determination of interested stockholder status, did own 15% or more of a corporation's outstanding voting stock. Since Section 203 will apply to us, we expect that it would have an anti-takeover effect with respect to transactions our board of directors does not approve in advance. In such event, we would also anticipate that Section 203 could discourage attempts that might result in a premium over the market price for the shares of common stock held by stockholders.
148
Under certain circumstances, Section 203 makes it more difficult for a person who would be an "interested stockholder" to effect various business combinations with a corporation for a three-year period. The provisions of Section 203 may encourage companies interested in acquiring our company to negotiate in advance with our board of directors because the stockholder approval requirement would be avoided if our board of directors approves either the business combination or the transaction that results in the stockholder becoming an interested stockholder. These provisions also may make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Registration Rights
Following the closing of this offering, the holders of 147,710,000 shares of our common stock, or their transferees, will be entitled to the registration rights set forth below with respect to registration of the resale of such shares under the Securities Act pursuant to the investors' rights agreement, by and among us and certain of our stockholders.
Demand Registration Rights
At any time after 180 days after the effective public offering date set forth on the cover page of this prospectus, upon the written request of at least a majority of the holders of the registrable securities then outstanding that we file a registration statement under the Securities Act covering the registration of registrable securities owned by such holder(s) having an anticipated aggregate offering price, net of selling expenses, of at least $15.0 million, we will be obligated to notify all holders of registrable securities of such request. As soon as practicable thereafter, and in any event within 60 days after the date such request is given, we will be required to register the sale on a registration statement on Form S-1 of all registrable securities that holders may request to be registered, subject to specified exceptions, conditions and limitations. We may postpone the filing of a registration statement for up to 120 days once in any 12-month period if in the good faith judgment of our board of directors such registration would be detrimental to us, and we are not required to effect the filing of a registration statement during the period starting with the date that is 60 days prior to our good faith estimate of the date of filing of a registration statement initiated by us and ending on a date 180 days after the effective date of a registration statement initiated by us. The underwriters of any underwritten offering will have the right to limit the number of shares having registration rights to be included in the registration statement.
"Piggyback" Registration Rights
If we register any securities for public sale, holders of registration rights will have the right to include their shares in the registration statement. The underwriters of any underwritten offering will have the right to limit the number of registrable securities to be included in the registration statement, but such number may not be below 30% of the total number of shares included in such registration statement. The holders of registration rights have waived any and all rights to have their shares included in this offering.
Form S-3 Registration Rights
If we are eligible to file a registration statement on Form S-3, holders of at least 30% of our registrable securities then outstanding have the right to request that we file a registration statement on Form S-3, so long as the aggregate price to the public of the securities to be sold under the registration statement on Form S-3 is at least $5.0 million or consists of all the remaining registrable securities, and subject to specified exceptions, conditions and limitations.
149
Expenses of Registration
Pursuant to the investors' rights agreement, we are generally required to bear all registration expenses, including the fees and expenses of one counsel representing the selling holders, incurred in connection with the demand, piggyback and Form S-3 registrations described above. We are not required to bear selling expenses, which include all underwriting discounts, selling commissions, stock transfer taxes applicable to the sale of registrable securities, and fees and disbursements of any additional counsel for any selling holder. We are not required to pay registration expenses if the registration request under the investors' rights agreement is withdrawn at the request of the holders of a majority of the registrable securities unless (i) the holders of a majority of the registrable securities agree to forfeit their right to one registration under the investors' rights agreement or (ii) the withdrawal is due to the discovery of a material adverse change in our business.
Termination of Registration Rights
The demand, piggyback and Form S-3 registration rights discussed above will terminate as to a given holder of registrable securities upon the earlier of (i) five years following the closing of this offering or (ii) when all units held by the holders can be sold under SEC Rule 144 within a 90 day period.
Limitations of Liability and Indemnification
See "Executive and Director Compensation—Limitation on Liability and Indemnification Matters."
Market Listing
We will apply to list our common stock on The NASDAQ Global Market under the symbol "RYTM."
Transfer Agent and Registrar
Upon the completion of this offering, the transfer agent and registrar for our common stock will be Computershare.
150
MATERIAL UNITED STATES FEDERAL INCOME AND ESTATE TAX CONSEQUENCES TO
NON-U.S. HOLDERS OF OUR COMMON STOCK
The following is a general discussion of the material U.S. federal income and estate tax consequences of the acquisition, ownership, and disposition of our common stock to a non-U.S. holder that purchases shares of our common stock for cash in this offering. For purposes of this discussion, a "non-U.S. holder" means a beneficial owner of our common stock that is not, for U.S. federal income tax purposes:
- •
- an individual who is a citizen or resident alien of the United States;
- •
- a corporation or any other organization taxable as a corporation for U.S. federal income tax purposes, created or
organized in the United States or under the laws of the United States or of any state thereof or the District of Columbia;
- •
- an estate, the income of which is subject to U.S. federal income tax regardless of its source; or
- •
- a trust if (i) the trust is subject to the primary supervision of a U.S. court and all substantial decisions of the trust are controlled by one or more U.S. persons or (ii) the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
This discussion does not address the tax treatment of partnerships or other entities that are pass-through entities for U.S. federal income tax purposes or persons that hold their common stock through partnerships or other pass-through entities. The tax treatment of a partner in a partnership or other pass-through entity that will hold our common stock generally will depend upon the status of the partner and the activities of the partner and the partnership. Such a partner should consult his, her, or its own tax advisor regarding the tax consequences of the acquisition, ownership and disposition of our common stock through a partner or other pass-through entity, as applicable.
This discussion is based upon the provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code, the U.S. Treasury regulations promulgated thereunder, judicial decisions, and published rulings and administrative procedures of the Internal Revenue Service, which we refer to as the IRS, all as in effect as of the date hereof. These authorities are subject to change and to differing interpretations, possibly with retroactive effect, which could result in U.S. federal income tax consequences different from those summarized below. No ruling has been or will be sought from the IRS with respect to the matters summarized below, and there can be no assurance that the IRS will not take a contrary position regarding the U.S. federal income tax consequences of the acquisition, ownership, or disposition of our common stock, or that any such contrary position would not be sustained by a court.
This discussion is not a complete analysis of all of the potential U.S. federal income tax consequences relating to the acquisition, ownership, and disposition of our common stock by non-U.S. holders, nor does it address any U.S. federal gift tax consequences, any tax consequences arising under any state, local, or non-U.S. tax laws, any consequences under the unearned income Medicare contribution tax pursuant to the Health Care and Education Reconciliation Act of 2010, the alternative minimum tax or any consequences under other U.S. federal tax laws. In addition, this discussion does not address tax consequences resulting from a non-U.S. holder's particular circumstances or to non-U.S. holders that may be subject to special tax rules, including, without limitation:
- •
- non-U.S. governments or entities they control;
- •
- "controlled foreign corporations" and their shareholders;
- •
- "passive foreign investment companies" and their shareholders;
- •
- corporations that accumulate earnings to avoid U.S. federal income tax;
151
- •
- former citizens or residents of the United States;
- •
- banks, insurance companies or other financial institutions;
- •
- tax-exempt pension funds or other tax-exempt organizations;
- •
- tax-qualified retirement plans;
- •
- traders, brokers, or dealers in securities, commodities, or currencies;
- •
- persons that have a functional currency other than the U.S. dollar;
- •
- persons who hold our common stock as a position in a hedging transaction, "straddle," "conversion transaction" or other
risk reduction transaction;
- •
- persons who do not hold our common stock as a capital asset within the meaning of Section 1221 of the Code
(generally, for investment purposes);
- •
- persons who receive our common stock in the Conversion or who hold or receive our common stock pursuant to the exercise
of any employee stock option or otherwise as compensation; or
- •
- persons deemed to sell our common stock under the constructive sale provisions of the Code.
Prospective investors should consult their tax advisors regarding the particular U.S. federal income tax consequences to them of acquiring, owning, and disposing of our common stock, as well as any tax consequences arising under any state, local, or foreign tax laws and any other U.S. federal tax laws. Prospective investors should also consult their tax advisors regarding the potential impact of any applicable income tax treaty between the United States and such non-U.S. holder's country of residence and of the rules described below under the heading "Foreign Account Tax Compliance Act."
Distributions on Common Stock
As described in the section entitled "Dividend Policy," we currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any dividends on our common stock in the foreseeable future. The disclosure in this section addresses the consequences should our board of directors, in the future, determine to make a distribution of cash or property with respect to our common stock (other than certain distributions of stock which may be made free of tax), or to effect a redemption that is treated for tax purposes as a distribution. Any such distribution will generally constitute a dividend for U.S. federal tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent such a distribution exceeds both our current and our accumulated earnings and profits, such excess will be allocated ratably among the shares of common stock with respect to which the distribution is made, will constitute a return of capital, and will first be applied against and reduce the non-U.S. holder's adjusted tax basis in those shares of common stock, but not below zero. Distributions in excess of our current and accumulated earnings and profits and in excess of a non-U.S. holder's tax basis in that non-U.S. holder's shares of common stock then will be treated as gain from the sale of that common stock, subject to the tax treatment described below under "Gain on Disposition of Common Stock." A non-U.S. holder's adjusted tax basis in a share of common stock is generally the purchase price of the share, reduced by the amount of any distributions constituting a return of capital with respect to that share.
Any dividend paid to a non-U.S. holder of our common stock generally will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividend, or such lower rate as may be specified by an applicable income tax treaty between the United States and such non-U.S. holder's country of residence. If a non-U.S. holder is eligible for benefits under an income tax treaty and wishes to claim a reduced rate of withholding, the non-U.S. holder generally will be required to provide us or our paying agent with a properly completed IRS Form W-8BEN, Form W-8BEN-E, or other applicable
152
form, certifying under penalties of perjury the non-U.S. holder's qualification for the reduced rate. This certification must be provided to us or our paying agent prior to the payment of the dividend and may be required to be updated periodically. Special certification requirements apply to non-U.S. holders that hold common stock through certain foreign intermediaries. Non-U.S. holders that do not timely provide the required certifications, but that qualify for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. If we are not able to determine whether or not a distribution will exceed current and accumulated earnings and profits at the time the distribution is made, we may withhold tax on the entire amount of any distribution at the same rate as we would withhold on a dividend. However, a non-U.S. holder may obtain a refund of amounts that we withhold to the extent attributable to the portion of the distribution in excess of our current and accumulated earnings and profits.
If a non-U.S. holder holds our common stock in connection with the conduct of a trade or business in the U.S., and dividends paid on the common stock are effectively connected with the non-U.S. holder's U.S. trade or business (and, if required by an applicable income tax treaty between the United States and such non-U.S. holder's country of residence, are attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the U.S., as defined under the applicable treaty), the non-U.S. holder will be exempt from U.S. federal withholding tax on the dividends. To claim the exemption, the non-U.S. holder must furnish a properly executed IRS Form W-8ECI (or other applicable form) prior to the payment of the dividends. Any dividends paid on our common stock that are effectively connected with a non-U.S. holder's U.S. trade or business (and satisfy any other applicable treaty requirements) generally will be subject to U.S. federal income tax on a net income basis at the regular graduated U.S. federal income tax rates generally applicable to U.S. persons (as defined in the Code) or at such lower rate as may be specified by an applicable income tax treaty between the United States and such non-U.S. holder's country of residence. A non-U.S. holder that is treated as a corporation for U.S. federal income tax purposes also may be subject to an additional branch profits tax equal to 30% (or such lower rate as is specified by an applicable income tax treaty between the United States and such non-U.S. holder's country of residence) of a portion of its earnings and profits for the taxable year that are effectively connected with a U.S. trade or business, as adjusted for certain items.
Gain on Disposition of Common Stock
Subject to the discussion below regarding backup withholding and foreign accounts, a non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized upon the sale, exchange, or other taxable disposition of our common stock unless:
- •
- the gain is effectively connected with the non-U.S. holder's conduct of a U.S. trade or business (and, if required by an
applicable income tax treaty between the United States and such non-U.S. holder's country of residence, the gain is attributable to a permanent establishment or fixed base maintained by the non-U.S.
holder in the U.S.), in which case the non-U.S. holder will generally be required to pay tax on the net gain derived from the sale, exchange, or other taxable disposition (net of certain deductions or
credits) under regular graduated U.S. federal income tax rates generally applicable to U.S. persons or at such lower rate as may be specified by an applicable income tax treaty between the United
States and such non-U.S. holder's country of residence, and in the case of a non-U.S. holder that is treated as a corporation for U.S. federal income tax purposes, such non-U.S. holder may be subject
to a branch profits tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such non-U.S. holder's country of residence;
- •
- the non-U.S. holder is an individual who is present in the U.S. for a period or periods aggregating 183 days or more during the taxable year in which the sale, exchange, or other taxable disposition occurs and certain other conditions are met, in which case the non-U.S.
153
- •
- our common stock is a "United States real property interest" by reason of our status as a "United States real property holding corporation," or USRPHC, for U.S. federal income tax purposes during the five-year period preceding such sale, exchange or other taxable disposition (or the non-U.S. holder's holding period, if shorter). Generally, a corporation is a USRPHC only if the fair market value of its U.S. real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business.
holder will be subject to U.S. federal income tax at a flat 30% rate (or such lower rate as is specified by an applicable income tax treaty between the United States and such non-U.S. holder's country of residence) on the net gain derived from the sale, exchange, or other taxable disposition, which gain may be offset by U.S. source capital losses (even though the non-U.S. holder is not considered a resident of the U.S.) provided that the non-U.S. holder has timely filed U.S. federal income tax returns reporting those losses; or
We believe we are not now and we do not anticipate becoming a USRPHC. However, there can be no assurance that we are not now a USRPHC or will not become one in the future. Even if we are or become a USRPHC, for so long as our common stock is "regularly traded," as defined by applicable U.S. Treasury regulations, on an established securities market, sales of our common stock generally will not be subject to tax for non-U.S. holders that have held less than 5% of our common stock, actually or constructively, during the five-year period preceding such non-U.S. holder's sale, exchange or other taxable disposition of our common stock (or the non-U.S. holder's holding period, if shorter). If we are determined to be a USRPHC and the foregoing exception does not apply, then a purchaser may withhold 10% of the proceeds payable to a non-U.S. holder from a sale of our common stock and the non-U.S. holder generally will be taxed on its net gain derived from the disposition at the graduated U.S. federal income tax rates applicable to U.S. persons.
U.S. Federal Estate Tax
Shares of our common stock that are owned or treated as owned at the time of death by an individual who is not a citizen or resident of the United States, as specifically defined for U.S. federal estate tax purposes, are considered U.S. situs assets and will be included in the individual's gross estate for U.S. federal estate tax purposes. Such shares, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax or other treaty between the United States and such non-U.S. holder's country of residence provides otherwise.
Information Reporting and Backup Withholding
Generally, we must report annually to the IRS and to each non-U.S. holder the gross amount of dividends and other distributions on our common stock paid to the non-U.S. holder and the amount of tax withheld, if any, with respect to those distributions. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in the non-U.S. holder's country of residence or incorporation.
A non-U.S. holder may be subject to backup withholding with respect to dividends paid on shares of our common stock, unless, generally, the non-U.S. holder certifies under penalties of perjury (usually on IRS Form W-8BEN or W-8BEN-E) that the non-U.S. holder is not a U.S. person or otherwise establishes an exemption. The backup withholding rate is 28%. Dividends that are paid to non-U.S. holders subject to the withholding of U.S. federal income tax, as described above under the heading "Distributions on Common Stock" generally will be exempt from U.S. backup withholding.
154
Additional rules relating to information reporting requirements and backup withholding with respect to payments of the proceeds from the disposition of shares of our common stock are as follows:
- •
- If the proceeds are paid to or through the U.S. office of a broker, the proceeds generally will be subject to backup
withholding and information reporting, unless the non-U.S. holder certifies under penalties of perjury (usually on IRS Form W-8BEN or W-8BEN-E) that the non-U.S. holder is not a U.S. person and
satisfies certain other requirements or otherwise establishes an exemption.
- •
- If the proceeds are paid to or through a non-U.S. office of a broker that is not a U.S. person and is not a foreign
person with certain specified U.S. connections, which we refer to below as a "U.S.-related person," information reporting and backup withholding generally will not apply.
- •
- If the proceeds are paid to or through a non-U.S. office of a broker that is a U.S. person or a U.S.-related person, the proceeds generally will be subject to information reporting (but not to backup withholding), unless the non-U.S. holder certifies under penalties of perjury (usually on IRS Form W-8BEN or W-8BEN-E) that the non-U.S. holder is not a U.S. person.
Backup withholding is not a tax. Any amounts withheld from a non-U.S. holder under the backup withholding rules may be allowed as a refund or a credit against the non-U.S. holder's U.S. federal income tax liability, if any, provided that the non-U.S. holder timely furnishes the required information to the IRS. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Foreign Account Tax Compliance Act
Legislation enacted in 2010, commonly referred to as the Foreign Account Tax Compliance Act, or FATCA, generally imposes withholding tax on certain types of payments made to "foreign financial institutions" and other non-U.S. entities after June 30, 2014 (or, in certain cases, after later dates) unless those institutions and entities meet additional certification, information reporting and other requirements. FATCA generally imposes a 30% withholding tax on dividends on, or gross proceeds from the sale or other disposition of, our common stock paid to a foreign financial institution unless the foreign financial institution enters into an agreement with the U.S. Treasury to, among other things, (i) undertake to identify accounts held by certain U.S. persons (including certain equity and debt holders of such institution) or by U.S.-owned foreign entities, (ii) annually report certain information about such accounts, and (iii) withhold 30% on payments to account holders whose actions prevent it from complying with these reporting and other requirements. In addition, subject to certain exceptions, the legislation imposes a 30% withholding tax on the same types of payments to a foreign entity that is not a foreign financial institution unless the entity certifies that it does not have any substantial U.S. owners (which generally include any U.S. persons who directly or indirectly own more than 10% of the entity) or furnishes identifying information regarding each such substantial U.S. owner. These withholding taxes will be imposed on dividends paid on our common stock after June 30, 2014 (or, in certain cases, after later dates), and on gross proceeds from sales or other dispositions of our common stock after December 31, 2016. Withholding under FATCA generally will not be reduced or limited by bilateral income tax treaties. However, a non-U.S. holder may be exempt from FATCA withholding under an applicable intergovernmental agreement between the U.S. and a foreign government relating to the implementation of FATCA, provided that the non-U.S. holder and the foreign government comply with the terms of the agreement. Non-U.S. holders should consult their own tax advisors regarding the possible implications of FATCA on their investment in our common stock and the entities through which they hold our common stock, including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of the 30% withholding tax under FATCA.
155
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for shares of our common stock. We cannot predict the effect, if any, that future sales of shares of common stock, or the availability for future sale of shares of common stock, will have on the market price of shares of our common stock prevailing from time to time. The sale of substantial amounts of shares of our common stock in the public market, or the perception that such sales could occur, could harm the prevailing market price of shares of our common stock.
Currently, no shares of our common stock are outstanding. Upon completion of the Conversion and the conversion of our series A preferred stock and series B preferred stock to common stock prior to the closing of this offering, 161,360,874 shares of our common stock will be outstanding, and there will be 21 holders of our common stock.
Upon completion of this offering, we will have a total of shares of our common stock outstanding (or shares of common stock if the underwriters exercise in full their option to purchase additional shares of common stock). All of these shares of common stock will have been sold in this offering and will be freely tradable without restriction or further registration under the Securities Act by persons other than our "affiliates." Under the Securities Act, an "affiliate" of an issuer is a person who directly or indirectly controls, is controlled by or is under common control with that issuer. In addition, shares of common stock may be granted under our 2014 equity incentive plan. See "Executive and Director Compensation—Employee Benefit and Stock Plans—2014 Equity Incentive Plan."
Our amended and restated certificate of incorporation authorizes us to issue additional shares of common stock and options, rights, warrants and appreciation rights relating to common stock for the consideration and on the terms and conditions established by our board of directors in its sole discretion. In accordance with the Delaware General Corporation Law and the provisions of our amended and restated certificate of incorporation, we may also issue preferred stock that has designations, preferences, rights, powers and duties that are different from, and may be senior to, those applicable to shares of common stock. See "Description of Capital Stock."
Lock-Up Agreements
We, along with our directors, executive officers and all of our other stockholders have agreed with the underwriters that for a period of 180 days (the restricted period), after the date of this prospectus, subject to specified exceptions, we or they will not offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock. All 161,360,874 shares are subject to a lock-up Agreement. Upon expiration of the "restricted" period, certain of our stockholders will have the right to require us to register their shares under the Securities Act of 1933, as amended, or the Securities Act. See "—Registration Rights" below and "Description of Capital Stock—Registration Rights."
After this offering, certain of our employees, including our executive officers and/or directors, may enter into written trading plans that are intended to comply with Rule 10b5-1 under the Securities Exchange Act of 1934, as amended. Sales under these trading plans would not be permitted until the expiration of the lock-up agreements relating to the offering described above.
156
Registration Rights
Upon closing of this offering, the holders of 147,710,000 shares of our common stock will be entitled to rights with respect to the registration of their shares under the Securities Act, subject to the lock-up agreements described under "—Lock-Up Agreements" above. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates, immediately upon the effectiveness of the registration statement of which this prospectus is a part. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock. See "Description of Capital Stock—Registration Rights."
Rule 144
In general, under Rule 144 a person (or persons whose shares are aggregated) who may be deemed our affiliate is entitled to sell within any three-month period a number of restricted securities that does not exceed the greater of 1% of the then outstanding shares of common stock and the average weekly trading volume during the four calendar weeks preceding each such sale, provided that at least six months has elapsed since such shares of common stock were acquired from us or any affiliate of ours and certain manner of sale, notice requirements and requirements as to availability of current public information about us are satisfied. Any person who is deemed to be our affiliate must also comply with such provisions of Rule 144 (other than the six-month holding period requirement) in order to sell shares of common stock which are not restricted securities (such as shares of common stock acquired by affiliates through purchases in the open market following this offering). Upon completion of this offering, 143,289,200 shares of our common stock will be "restricted securities" as such term is defined in Rule 144. A person who is not our affiliate, and who has not been our affiliate at any time during the 90 days preceding any sale, is entitled to sell shares of common stock (i) subject only to the requirements as to availability of current public information about us, provided that a period of at least six months has elapsed since the shares of common stock were acquired from us or any affiliate of ours, and (ii) without regard to the requirements as to availability of current public information about us or any other requirement of Rule 144, provided that at least one year has elapsed since the shares of common stock were acquired from us or any affiliate of ours.
Stock Options and Restricted Stock
As soon as practicable after the completion of this offering, we intend to file a Form S-8 registration statement under the Securities Act to register shares of our common stock subject to options outstanding or reserved for issuance under our 2014 equity incentive plan. This registration statement will become effective immediately upon filing, and shares covered by this registration statement will thereupon be eligible for sale in the public markets, subject to vesting restrictions, the lock-up agreements described above and Rule 144 limitations applicable to affiliates. For a more complete discussion of our stock plans, see "Executive and Director Compensation—Employee Benefit and Stock Plans."
157
Citigroup Global Markets Inc. and Cowen and Company, LLC are acting as joint book-running managers of the offering and as representatives of the underwriters named below. Subject to the terms and conditions stated in the underwriting agreement dated the date of this prospectus, each underwriter named below has severally agreed to purchase, and we have agreed to sell to that underwriter, the number of shares of our common stock set forth opposite the underwriter's name.
Underwriter
|
Number of Shares |
|||
|---|---|---|---|---|
Citigroup Global Markets Inc. |
||||
Cowen and Company, LLC |
||||
Canaccord Genuity Inc. |
||||
Oppenheimer & Co. Inc. |
||||
Cantor Fitzgerald & Co. |
||||
| | | | | |
Total |
||||
| | | | | |
| | | | | |
The underwriting agreement provides that the obligations of the underwriters to purchase the shares of our common stock included in this offering are subject to approval of legal matters by counsel and to other conditions. The underwriters are obligated to purchase all of the shares of our common stock (other than those covered by the over-allotment option described below) if they purchase any of the shares.
Shares of our common stock sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any shares of our common stock sold by the underwriters to securities dealers may be sold at a discount from the initial public offering price not to exceed $ per share. If all the shares of our common stock are not sold at the initial offering price, the underwriters may change the offering price and the other selling terms. The representatives have advised us that the underwriters do not intend to make sales to discretionary accounts.
If the underwriters sell more shares of our common stock than the total number set forth in the table above, we have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to additional shares of our common stock at the public offering price less the underwriting discount. The underwriters may exercise the option solely for the purpose of covering over-allotments, if any, in connection with this offering. To the extent the option is exercised, each underwriter must purchase a number of additional shares of our common stock approximately proportionate to that underwriter's initial purchase commitment. Any shares of our common stock issued or sold under the option will be issued and sold on the same terms and conditions as the other shares of our common stock that are the subject of this offering.
We, our officers and directors, our stockholders and our option holders have agreed that, subject to specified limited exceptions, for a period of 180 days from the date of this prospectus, we and they will not, without the prior written consent of Citigroup Global Markets Inc. and Cowen and Company, LLC, dispose of or hedge any shares of our common stock or any securities convertible into or exchangeable for our common stock. Citigroup Global Markets Inc. and Cowen and Company, LLC in their sole discretion may release any of the securities subject to these lock-up agreements at any time, which, in the case of officers and directors, shall be with notice.
Prior to this offering, there has been no public market for our shares. Consequently, the initial public offering price for the shares of our common stock will be determined by negotiations between us and the representatives. Among the factors considered in determining the initial public offering price will be our results of operations, our current financial condition, our future prospects, our markets, the
158
economic conditions in and future prospects for the industry in which we compete, our management, and currently prevailing general conditions in the equity securities markets, including current market valuations of publicly traded companies considered comparable to our company. We cannot assure you, however, that the price at which the shares of our common stock will sell in the public market after this offering will not be lower than the initial public offering price or that an active trading market in our shares of common stock will develop and continue after this offering.
We have applied to have our shares of common stock listed on the Nasdaq Global Market under the symbol "RYTM."
The following table shows the underwriting discounts and commissions that we are to pay to the underwriters in connection with this offering. These amounts are shown assuming both no exercise and full exercise of the underwriters' over-allotment option.
| |
Paid by Rhythm | ||||||
|---|---|---|---|---|---|---|---|
| |
No Exercise | Full Exercise | |||||
Per share |
$ | $ | |||||
Total |
$ | $ | |||||
We estimate that our portion of the total expenses of this offering will be $ . We have agreed to reimburse the underwriters for certain of their expenses as set forth in the underwriting agreement.
In connection with the offering, the underwriters may purchase and sell shares of common stock in the open market. Purchases and sales in the open market may include short sales, purchases to cover short positions, which may include purchases pursuant to the over-allotment option, and stabilizing purchases.
- •
- Short sales involve secondary market sales by the underwriters of a greater number of shares of our common stock than
they are required to purchase in the offering.
- •
- "Covered" short sales are sales of shares of our common stock in an amount up to the number of shares of our common stock
represented by the underwriters' over-allotment option.
- •
- "Naked" short sales are sales of shares of our common stock in an amount in excess of the number of shares of our common
stock represented by the underwriters' over-allotment option.
- •
- Covering transactions involve purchases of shares of our common stock either pursuant to the underwriters' over-allotment
option or in the open market in order to cover short positions.
- •
- To close a naked short position, the underwriters must purchase shares of our common stock in the open market. A naked
short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of our common stock in the open market after pricing that
could adversely affect investors who purchase in the offering.
- •
- To close a covered short position, the underwriters must purchase shares of our common stock in the open market or must
exercise the over-allotment option. In determining the source of shares of our common stock to close the covered short position, the underwriters will consider, among other things, the price of shares
of our common stock available for purchase in the open market as compared to the price at which they may purchase shares of our common stock through the over-allotment option.
- •
- Stabilizing transactions involve bids to purchase shares of our common stock so long as the stabilizing bids do not exceed a specified maximum.
159
Purchases to cover short positions and stabilizing purchases, as well as other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in the market price of the shares. They may also cause the price of the shares of our common stock to be higher than the price that would otherwise exist in the open market in the absence of these transactions. The underwriters may conduct these transactions on the Nasdaq Global Market, in the over-the-counter market or otherwise. If the underwriters commence any of these transactions, they may discontinue them at any time.
Conflicts of Interest
The underwriters are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, principal investment, hedging, financing and brokerage activities. The underwriters and their respective affiliates may, from time to time, engage in transactions with and perform services for us in the ordinary course of their business for which they may receive customary fees and reimbursement of expenses. In the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (which may include bank loans or credit default swaps) for their own account and for the accounts of their customers and may at any time hold long and short positions in such securities and instruments. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long or short positions in such securities and instruments. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make because of any of those liabilities.
Notice to Prospective Investors in the European Economic Area
In relation to each member state of the European Economic Area that has implemented the Prospectus Directive (each, a relevant member state), with effect from and including the date on which the Prospectus Directive is implemented in that relevant member state (the relevant implementation date), an offer of shares of our common stock described in this prospectus may not be made to the public in that relevant member state other than:
- •
- to any legal entity which is a qualified investor as defined in the Prospectus Directive;
- •
- to fewer than 100 or, if the relevant member state has implemented the relevant provision of the 2010 PD Amending
Directive, 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of
the relevant Dealer or Dealers nominated by us for any such offer; or
- •
- in any other circumstances falling within Article 3(2) of the Prospectus Directive,
provided that no such offer of shares of our common stock shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive.
For purposes of this provision, the expression an "offer of securities to the public" in any relevant member state means the communication in any form and by any means of sufficient information on the
160
terms of the offer and the shares of our common stock to be offered so as to enable an investor to decide to purchase or subscribe for the shares of our common stock, as the expression may be varied in that member state by any measure implementing the Prospectus Directive in that member state, and the expression "Prospectus Directive" means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the relevant member state) and includes any relevant implementing measure in the relevant member state. The expression 2010 PD Amending Directive means Directive 2010/73/EU.
The sellers of the shares of our common stock have not authorized and do not authorize the making of any offer of shares of our common stock through any financial intermediary on their behalf, other than offers made by the underwriters with a view to the final placement of the shares of our common stock as contemplated in this prospectus. Accordingly, no purchaser of the shares of our common stock, other than the underwriters, is authorized to make any further offer of the shares of our common stock on behalf of the sellers or the underwriters.
Notice to Prospective Investors in the United Kingdom
This prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, or the Order, or (ii) high net worth entities, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (each such person being referred to as a relevant person). This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom that is not a relevant person should not act or rely on this document or any of its contents.
Notice to Prospective Investors in Australia
No prospectus or other disclosure document (as defined in the Corporations Act 2001 (Cth) of Australia, or the Corporations Act) in relation to the common stock has been or will be lodged with the Australian Securities & Investments Commission, or ASIC. This document has not been lodged with ASIC and is only directed to certain categories of exempt persons. Accordingly, if you receive this document in Australia:
- •
- you confirm and warrant that you are either:
- •
- a "sophisticated investor" under section 708(8)(a) or (b) of the Corporations Act;
- •
- a "sophisticated investor" under section 708(8)(c) or (d) of the Corporations Act and that you have
provided an accountant's certificate to us which complies with the requirements of section 708(8)(c)(i) or (ii) of the Corporations Act and related regulations before the offer has been
made;
- •
- a person associated with the company under section 708(12) of the Corporations Act; or
- •
- a "professional investor" within the meaning of section 708(11)(a) or (b) of the Corporations Act, and to
the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Corporations Act any offer made to you under
this document is void and incapable of acceptance; and
- •
- you warrant and agree that you will not offer any of the common stock for resale in Australia within 12 months of that common stock being issued unless any such resale offer is exempt from the requirement to issue a disclosure document under section 708 of the Corporations Act.
161
Notice to Prospective Investors in France
Neither this prospectus nor any other offering material relating to the shares of our common stock described in this prospectus has been submitted to the clearance procedures of the Autorité des Marchés Financiers or of the competent authority of another member state of the European Economic Area and notified to the Autorité des Marchés Financiers. The shares of our common stock have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France. Neither this prospectus nor any other offering material relating to the shares of our common stock has been or will be:
- •
- released, issued, distributed or caused to be released, issued or distributed to the public in France; or
- •
- used in connection with any offer for subscription or sale of the shares of our common stock to the public in France.
Such offers, sales and distributions will be made in France only:
- •
- to qualified investors (investisseurs qualifiés) and/or to
a restricted circle of investors (cercle restreint d'investisseurs), in each case investing for their own account, all as defined in, and in accordance
with articles L.411-2, D.411-1, D.411-2, D.734-1, D.744-1, D.754-1 and D.764-1 of the French Code monétaire et financier;
- •
- to investment services providers authorized to engage in portfolio management on behalf of third parties; or
- •
- in a transaction that, in accordance with article L.411-2-II-1°-or-2°-or 3° of the French Code monétaire et financier and article 211-2 of the General Regulations (Règlement Général) of the Autorité des Marchés Financiers, does not constitute a public offer (appel public à l'épargne).
The shares of our common stock may be resold directly or indirectly, only in compliance with articles L.411-1, L.411-2, L.412-1 and L.621-8 through L.621-8-3 of the French Code monétaire et financier.
Notice to Prospective Investors in Hong Kong
The shares of our common stock may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong), or (ii) to "professional investors" within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a "prospectus" within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong) and no advertisement, invitation or document relating to the shares of our common stock may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares of our common stock which are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Notice to Prospective Investors in Japan
The shares of our common stock offered in this prospectus have not been and will not be registered under the Financial Instruments and Exchange Law of Japan. The shares of our common stock have not been offered or sold and will not be offered or sold, directly or indirectly, in Japan or to or for the account of any resident of Japan (including any corporation or other entity organized under
162
the laws of Japan), except (i) pursuant to an exemption from the registration requirements of the Financial Instruments and Exchange Law and (ii) in compliance with any other applicable requirements of Japanese law.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares of our common stock may not be circulated or distributed, nor may the shares of our common stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to compliance with conditions set forth in the SFA.
Where the shares of our common stock are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
- •
- a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of
which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
- •
- a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor,
shares, debentures and units of shares of our common stock and debentures of that corporation or the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares of our common stock pursuant to an offer made under Section 275 of the SFA except:
- •
- to an institutional investor (for corporations, under Section 274 of the SFA) or to a relevant person defined in
Section 275(2) of the SFA, or to any person pursuant to an offer that is made on terms that such shares, debentures and units of shares of our common stock and debentures of that corporation or
such rights and interest in that trust are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction, whether such amount is to be paid for
in cash or by exchange of securities or other assets, and further for corporations, in accordance with the conditions specified in Section 275 of the SFA;
- •
- where no consideration is or will be given for the transfer; or
- •
- where the transfer is by operation of law.
Notice to Prospective Investors in Switzerland
The shares of our common stock may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or SIX, or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares of our common stock or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
163
Neither this document nor any other offering or marketing material relating to the offering, our company, or the shares of our common stock have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of shares of common stock has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares of our common stock.
Notice to Prospective Investors in the Dubai International Financial Centre
This prospectus relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority, or DFSA. This prospectus is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with the Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth herein and has no responsibility for the prospectus. The shares of our common stock to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the shares of our common stock offered should conduct their own due diligence on the shares. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
164
The validity of the shares of common stock offered hereby will be passed upon for us by Bingham McCutchen LLP, Boston, Massachusetts. Certain legal matters in connection with this offering will be passed upon for the underwriters by Wilmer Cutler Pickering Hale and Dorr LLP, New York, New York.
The consolidated financial statements of Rhythm Holding Company, LLC and its subsidiaries, as of December 31, 2012 and 2013, and for the years then ended, included in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 (including exhibits, schedules, and amendments) under the Securities Act with respect to the shares of common stock offered by this prospectus. This prospectus does not contain all the information set forth in the registration statement. For further information about us and the shares of common stock to be sold in this offering, you should refer to the registration statement. Statements contained in this prospectus relating to the contents of any contract, agreement or other document are not necessarily complete and are qualified in all respects by the complete text of the applicable contract, agreement or other document, a copy of which has been filed as an exhibit to the registration statement. Whenever this prospectus refers to any contract, agreement, or other document, you should refer to the exhibits that are a part of the registration statement for a copy of the contract, agreement, or document.
You may read and copy all or any portion of the registration statement or any other information we file at the SEC's public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You can request copies of these documents, upon payment of a duplicating fee, by writing to the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the operation of the public reference rooms. Our SEC filings, including the registration statement, are also available to you on the SEC's Website (http://www.sec.gov).
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act. Under the Exchange Act, we will file annual, quarterly and current reports, as well as proxy statements and other information with the SEC. These periodic reports, proxy statements, and other information will be available for inspection and copying at the SEC's Public Reference Room and the website of the SEC referred to above.
165
RHYTHM HOLDING COMPANY, LLC
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The
Board of Managers and Members of
Rhythm Holding Company, LLC
We have audited the accompanying consolidated balance sheets of Rhythm Holding Company, LLC and subsidiaries (the Company) as of December 31, 2012 and 2013, and the related consolidated statements of operations and comprehensive loss, convertible preferred stock, convertible preferred units, stockholders' and members' equity (deficit), and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Rhythm Holding Company, LLC and subsidiaries at December 31, 2012 and 2013, and the results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Boston,
Massachusetts
July 14, 2014
F-2
RHYTHM HOLDING COMPANY, LLC
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share and unit and per unit data)
| |
December 31, | |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
June 30, 2014 |
Pro Forma June 30, 2014 |
|||||||||||
| |
2012 | 2013 | |||||||||||
| |
|
|
(unaudited) |
||||||||||
Assets |
|||||||||||||
Current assets: |
|||||||||||||
Cash and cash equivalents |
$ | 11,766 | $ | 7,364 | $ | 8,864 | $ | 8,864 | |||||
Marketable securities |
5,270 | 10,398 | 469 | 469 | |||||||||
Prepaid research and development expenses |
1,370 | 264 | 43 | 43 | |||||||||
Prepaid expenses and other current assets |
449 | 113 | 1,273 | 1,273 | |||||||||
| | | | | | | | | | | | | | |
Total current assets |
18,855 | 18,139 | 10,649 | 10,649 | |||||||||
Property and equipment, net |
44 | 24 | 15 | 15 | |||||||||
Restricted cash |
23 | 23 | 23 | 23 | |||||||||
| | | | | | | | | | | | | | |
Total assets |
$ | 18,922 | $ | 18,186 | $ | 10,687 | $ | 10,687 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Liabilities, convertible preferred stock, convertible preferred units, stockholders' and members' equity (deficit) |
|||||||||||||
Current liabilities: |
|||||||||||||
Accounts payable |
$ | 2,245 | $ | 1,465 | $ | 523 | $ | 523 | |||||
Accrued expenses and other current liabilities |
257 | 1,595 | 1,916 | 1,916 | |||||||||
| | | | | | | | | | | | | | |
Total current liabilities |
2,502 | 3,060 | 2,439 | 2,439 | |||||||||
Commitments and contingencies (Notes 6 and 11) |
|||||||||||||
Convertible preferred stock: |
|||||||||||||
Series A convertible preferred stock; $0.001 par value per share: 61,000,000 shares authorized, issued and outstanding at December 31, 2012; no shares authorized, issued or outstanding at December 31, 2013, June 30, 2014 and June 30, 2014 (pro forma) |
30,307 | — | — | — | |||||||||
Series B convertible preferred stock; $0.001 par value per share: 69,000,000 shares authorized and 45,000,000 shares issued and outstanding at December 31, 2012; no shares authorized, issued or outstanding at December 31, 2013, June 30, 2014 and June 30, 2014 (pro forma) |
22,397 |
— |
— |
— |
|||||||||
Convertible preferred units: |
|||||||||||||
Series A convertible preferred units; 61,000,000 units authorized, issued and outstanding at December 31, 2013 and June 30, 2014; no units issued or outstanding at December 31, 2012 or June 30, 2014 (pro forma) (aggregate liquidation preferences $38,828 at December 31, 2013 and $40,254 at June 30, 2014) |
— | 30,307 | 30,307 | — | |||||||||
Series B convertible preferred units; 85,000,000 units authorized, issued and outstanding at December 31, 2013 and June 30, 2014; no units authorized, issued or outstanding at December 31, 2012 or June 30, 2014 (pro forma) (aggregate liquidation preferences of $46,632 at December 31, 2013 and $48,365 at June 30, 2014) |
— |
42,279 |
42,279 |
— |
|||||||||
Stockholders' and members' equity (deficit): |
|||||||||||||
Common stock, $0.001 par value: 179,584,000 shares authorized; 3,485,000 shares issued and outstanding at December 31, 2012 ; no shares authorized, issued or outstanding at December 31, 2013 or June 30, 2014; 156,938,609 shares issued and outstanding at June 30, 2014 (pro forma) |
3 | — | — | 157 | |||||||||
Common units, 179,539,000 units authorized; 15,650,986 and 15,371,709 units issued and 9,675,334 and 10,938,609 units outstanding at December 31, 2013 and June 30, 2014, respectively; no units authorized, issued or outstanding December 31, 2012 and June 30, 2014 (pro forma) |
— | 53 | 53 | — | |||||||||
Additional paid-in capital |
713 | 917 | 1,006 | 73,488 | |||||||||
Accumulated other comprehensive income |
7 | 8 | 9 | 9 | |||||||||
Accumulated deficit |
(37,007 | ) | (58,438 | ) | (65,406 | ) | (65,406 | ) | |||||
| | | | | | | | | | | | | | |
Total stockholders' and members' equity (deficit) |
(36,284 | ) | (57,460 | ) | (64,338 | ) | 8,248 | ||||||
| | | | | | | | | | | | | | |
Liabilities, convertible preferred stock, convertible preferred units, stockholders' and member's equity (deficit) |
$ | 18,922 | $ | 18,186 | $ | 10,687 | $ | 10,687 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements
F-3
RHYTHM HOLDING COMPANY, LLC
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except share and per share and unit and per
unit data)
| |
Years Ended December 31, | Six Months Ended June 30, 2014 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2013 | 2014 | |||||||||
| |
|
|
(unaudited) |
||||||||||
Operating expenses: |
|||||||||||||
Research and development |
$ | 15,678 | $ | 18,015 | $ | 9,079 | $ | 5,470 | |||||
General and administrative |
2,460 | 3,433 | 1,753 | 1,503 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses |
18,138 | 21,448 | 10,832 | 6,973 | |||||||||
| | | | | | | | | | | | | | |
Loss from operations |
(18,138 | ) | (21,448 | ) | (10,832 | ) | (6,973 | ) | |||||
Other income (expense): |
|||||||||||||
Other income, net |
8 | 17 | 11 | 5 | |||||||||
| | | | | | | | | | | | | | |
Net loss |
$ | (18,130 | ) | $ | (21,431 | ) | $ | (10,821 | ) | $ | (6,968 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Change in unrealized gain on marketable securities |
7 | (1 | ) | 3 | (1 | ) | |||||||
| | | | | | | | | | | | | | |
Comprehensive loss |
$ | (18,123 | ) | $ | (21,432 | ) | $ | (10,818 | ) | $ | (6,969 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net loss applicable to common holders |
$ | (21,498 | ) | $ | (27,229 | ) | $ | (13,454 | ) | $ | (10,128 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net loss per common unit, basic and diluted |
$ | (7.46 | ) | $ | (3.65 | ) | $ | (2.25 | ) | $ | (0.99 | ) | |
Weighted average common units outstanding, basic and diluted |
2,883,087 |
7,467,781 |
5,979,829 |
10,198,713 |
|||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma net loss per common share applicable to common stockholders, basic and diluted (unaudited) |
$ | (0.15 | ) | $ | (0.04 | ) | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma weighted average common shares outstanding, basic and diluted (unaudited) |
139,901,617 | 156,198,713 | |||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-4
RHYTHYM HOLDING COMPANY, LLC
STATEMENTS OF CONVERTIBLE PREFERRED STOCK, CONVERTIBLE PREFERRED UNITS, STOCKHOLDERS' AND MEMBERS' EQUITY (DEFICIT)
(in thousands, except share and per share and unit and per unit data)
| |
Series A Convertible Preferred Stock |
Series B Convertible Preferred Stock |
Series A Convertible Preferred Units |
Series B Convertible Preferred Units |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common Stock | Common Units | |
Accumulated Other Comprehensive Income |
|
Total Stockholders' and Members' Equity (Deficit) |
|||||||||||||||||||||||||||||||||||||||||||
| |
Additional Paid-In Capital |
Accumulated Deficit |
|||||||||||||||||||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Units | Amount | Units | Amount | Shares | Amount | Units | Amount | |||||||||||||||||||||||||||||||||||||
Balance at December 31, 2011 |
61,000,000 | $ | 30,307 | — | $ | — | — | $ | — | — | $ | — | 2,635,000 | $ | 2 | — | $ | — | $ | 596 | $ | — | $ | (18,877 | ) | $ | (18,279 | ) | |||||||||||||||||||||
Issuance of Series B convertible preferred stock, net of issuance costs of $103 |
— | — | 45,000,000 | 22,397 | — | — | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
Issuance of common stock upon exercise of stock options |
— | — | — | — | — | — | — | — | 850,000 | 1 | — | — | 42 | — | — | 43 | |||||||||||||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | — | — | — | — | — | — | 75 | — | — | 75 | |||||||||||||||||||||||||||||||||
Change in unrealized gain on marketable scurities |
— | — | — | — | — | — | — | — | — | — | — | — | — | 7 | — | 7 | |||||||||||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | (18,130 | ) | (18,130 | ) | |||||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2012 |
61,000,000 | 30,307 | 45,000,000 | 22,397 | — | — | — | — | 3,485,000 | 3 | — | — | 713 | 7 | (37,007 | ) | (36,284 | ) | |||||||||||||||||||||||||||||||
Issuance of Series B convertible preferred stock, net of issuance costs of $62 |
— | — | 18,000,000 | 8,938 | — | — | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
Exchange of convertible preferred A and B stock and common stock for convertible preferred A and B units and common units all on a one-to-one basis as a result of the corporate reorganization |
(61,000,000 | ) | (30,307 | ) | (63,000,000 | ) | (31,335 | ) | 61,000,000 | 30,307 | 63,000,000 | 31,335 | (3,485,000 | ) | (3 | ) | 3,485,000 | 53 | (50 | ) | — | — | — | ||||||||||||||||||||||||||
Exchange of vested stock options for vested restricted common units on a one-to-one basis as a result of the corporate reorganization |
— | — | — | — | — | — | — | — | — | — | 4,178,463 | — | — | — | — | — | |||||||||||||||||||||||||||||||||
Issuance of Series B convertible preferred units, net of issuance costs of $56 |
— | — | — | — | — | — | 22,000,000 | 10,944 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
Stock based compensation expense |
— | — | — | — | — | — | — | — | — | — | — | — | 254 | — | — | 254 | |||||||||||||||||||||||||||||||||
Vesting of restricted common units |
— | — | — | — | — | — | — | — | — | — | 2,011,871 | — | — | — | — | — | |||||||||||||||||||||||||||||||||
Change in unrealized gain on marketable scurities |
— | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | 1 | |||||||||||||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | — | — | — | — | — | — | (21,431 | ) | (21,431 | ) | |||||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2013 |
— | — | — | — | 61,000,000 | 30,307 | 85,000,000 | 42,279 | — | — | 9,675,334 | 53 | 917 | 8 | (58,438 | ) | (57,460 | ) | |||||||||||||||||||||||||||||||
Vesting of restricted common units (unaudited) |
1,263,275 | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
Stock-based compensation expense(unaudited) |
— | — | — | — | — | — | — | — | — | — | — | — | 89 | — | 89 | ||||||||||||||||||||||||||||||||||
Change in unrealized gain on marketable scurities(unaudited) |
— | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | 1 | |||||||||||||||||||||||||||||||||
Net loss(unaudited) |
— | — | — | — | — | — | — | — | — | — | — | — | — | (6,968 | ) | (6,968 | ) | ||||||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at June 30, 2014(unaudited) |
— | — | — | — | 61,000,000 | 30,307 | 85,000,000 | 42,279 | — | — | 10,938,609 | 53 | 1,006 | 9 | (65,406 | ) | (64,338 | ) | |||||||||||||||||||||||||||||||
Conversion of convertible preferred units and common units into common stock on a one-to-one basis |
(61,000,000 | ) | (30,307 | ) | (85,000,000 | ) | (42,279 | ) | 156,938,609 | 157 | (10,938,609 | ) | (53 | ) | 72,482 | 72,586 | |||||||||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Pro forma balance at June 30, 2014 (unaudited) |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | 156,938,609 | $ | 157 | — | $ | — | $ | 73,488 | $ | 9 | $ | (65,406 | ) | $ | 8,248 | ||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements
F-5
RHYTHM HOLDING COMPANY, LLC
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands, except share and per share and unit and per unit data)
| |
Years Ended December 31, | Six Months Ended June 30, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2013 | 2014 | |||||||||
| |
|
|
(unaudited) |
||||||||||
Operating activities |
|||||||||||||
Net loss |
$ | (18,130 | ) | $ | (21,431 | ) | $ | (10,821 | ) | $ | (6,968 | ) | |
Adjustments to reconcile net loss to cash used in operating activities: |
|||||||||||||
Depreciation expense |
17 | 20 | 9 | 9 | |||||||||
Change in unrealized gain on marketable securities |
7 | 1 | (3 | ) | 1 | ||||||||
Stock-based compensation |
75 | 254 | 171 | 88 | |||||||||
Changes in operating assets and liabilities: |
|||||||||||||
Prepaid expenses and other current assets |
(1,258 | ) | 1,442 | 677 | (700 | ) | |||||||
Accounts payable |
1,576 | (780 | ) | 63 | (940 | ) | |||||||
Accrued expenses and other current liabilities |
(117 | ) | 1,338 | 408 | 321 | ||||||||
| | | | | | | | | | | | | | |
Net cash used in operating activities |
(17,830 | ) | (19,156 | ) | (9,496 | ) | (8,189 | ) | |||||
Investing activities |
|||||||||||||
Purchases of marketable securities |
(8,799 | ) | (28,741 | ) | (15,447 | ) | (3,404 | ) | |||||
Maturities of marketable securities |
3,529 | 23,613 | 9,608 | 13,333 | |||||||||
| | | | | | | | | | | | | | |
Net cash provided by (used in) investing activities |
(5,270 | ) | (5,128 | ) | (5,839 | ) | 9,929 | ||||||
Financing activities |
|||||||||||||
Proceeds from issuance of series B preferred stock/units, net of issuance costs |
22,397 | 19,882 | 8,939 | — | |||||||||
Proceeds from issuance of common stock |
43 | — | — | — | |||||||||
Issuance costs related to planned issuance of common stock |
— | — | — | (240 | ) | ||||||||
| | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities |
22,440 | 19,882 | 8,939 | (240 | ) | ||||||||
| | | | | | | | | | | | | | |
Net decrease in cash and cash equivalents |
(660 | ) | (4,402 | ) | (6,396 | ) | 1,500 | ||||||
Cash and cash equivalents at beginning of period |
12,426 | 11,766 | 11,766 | 7,364 | |||||||||
| | | | | | | | | | | | | | |
Cash and cash equivalents at end of period |
$ | 11,766 | $ | 7,364 | $ | 5,370 | $ | 8,864 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements
F-6
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
1. Nature of Business
Rhythm Holding Company, LLC (the "LLC entity") is a biopharmaceutical company focused on the development and commercialization of peptide therapeutics for the treatment of gastrointestinal, or GI, diseases and genetic deficiencies which result in metabolic disorders. The LLC entity's lead product candidate, relamorelin, is a ghrelin agonist in Phase 2 development for the treatment of diabetic gastroparesis, a gastrointestinal complication of diabetes, and other GI functional disorders. The LLC entity is also developing a second product candidate, RM-493, which is a Phase 2 ready melanocortin-4, or MC4, receptor agonist for the treatment of obesity caused by genetic deficiencies in the MC4 pathway.
Corporate Restructuring
Rhythm Pharmaceuticals, Inc. ("RPI") was incorporated in Delaware in November 2008. On March 21, 2013 RPI underwent a corporate restructuring (the "Prior Restructuring"), pursuant to which the two product candidates were separated into different legal entities, both wholly owned by the LLC Entity. As part of the Prior Restructuring, the LLC entity was established as a holding company in 2013. RPI, focused on the ghrelin program, became a wholly-owned subsidiary of the LLC entity through an exchange of equity. RPI's MC4 program was contributed to Rhythm Metabolic, Inc. ("RMI"), a newly formed company incorporated in Delaware on March 21, 2013 and wholly owned by the LLC entity. The license between RMI and Ipsen (see Note 11) was amended to narrow the rights granted under that license to only the MC4 program. The Prior Restructuring did not result in a change in control; therefore all assets and liabilities continue to be accounted for at their historical cost.
In the Prior Restructuring, all outstanding shares of series A and series B convertible preferred stock, common stock, and warrants to purchase common stock of RPI were converted into an equal number of series A and series B convertible preferred units, common units, and warrants to purchase common units, respectively, of the LLC entity. All 12,313,700 common stock options of RPI outstanding as of March 21, 2013 were exchanged for an equal number of restricted common units of the LLC entity. Vesting of the restricted common units continued on the same schedule as per the respective original option agreements. While the stock options carried exercise prices, restricted common units do not. See Notes 7 and 8 for a description for the rights and privileges of the series A and series B convertible preferred units, common units, restricted common units and warrants to purchase common units. The Prior Restructuring resulted in no economic change to the holders of the series A and series B convertible preferred units, common units, restricted common units and warrants to purchase common units.
Since inception, the LLC entity has devoted substantially all of the LLC entity's efforts to clinical and preclinical development, business planning, acquiring operating assets, seeking intellectual property protection for its technology and product candidates and fundraising.
For periods prior to the Prior Restructuring on March 21, 2013, these notes and disclosures refer to RPI and subsequent to the Prior Restructuring, these notes and disclosures refer to the LLC entity.
F-7
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
1. Nature of Business (Continued)
Liquidity
The LLC entity has incurred losses since inception and negative cash flows from operating activities. As of December 31, 2013 and June 30, 2014 the LLC entity had an accumulated deficit of $58,438 and $65,406, respectively. The LLC entity anticipates that it will continue to incur significant operating losses for the next several years as it continues to develop relamorelin and RM-493. In the future the LLC entity will be dependent on obtaining funding from third parties, such as proceeds from the issuance of debt, sale of equity, and funded research and development programs, to maintain the LLC entity's operations and meet the LLC entity's obligations. There is no guarantee that additional equity or other financings will be available to the LLC entity on acceptable terms, or at all. If the LLC entity fails to obtain additional funding when needed, the LLC entity would be forced to scale back, or terminate its operations or seek to merge with or be acquired by another company. Management believes that the LLC entity's existing cash resources will be sufficient to fund the LLC entity's operating plan through early 2015.
2. Summary of Significant Accounting Policies
Basis of Presentation
The LLC entity's financial statements have been prepared in conformity with accounting principles generally accepted in the United States ("GAAP"). Any reference in these notes to applicable guidance is meant to refer to the authoritative United States generally accepted accounting principles as found in the Accounting Standards Codification ("ASC") and Accounting Standards Update ("ASU") of the Financial Accounting Standards Board ("FASB"). The LLC entity's financial statements include all majority owned subsidiaries, reported as a single operating segment, for which the LLC entity maintains controlling interests. Intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. The LLC entity bases its estimates on historical experience and other market-specific or other relevant assumptions that it believes to be reasonable under the circumstances. This process may result in actual results differing materially from those estimated amounts used in the preparation of the financial statements if these results differ from historical experience, or other assumptions do not turn out to be substantially accurate, even if such assumptions are reasonable when made. Significant estimates relied upon in preparing these financial statements include the fair value of convertible preferred stock and convertible preferred units, accrued expenses, the determination of the fair value of stock and unit awards issued, stock-based compensation expense, and the valuation allowance on the LLC entity's deferred tax assets.
F-8
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
2. Summary of Significant Accounting Policies (Continued)
The LLC entity utilizes significant estimates and assumptions in determining the fair value of its equity awards. The LLC entity utilized various valuation methodologies in accordance with the framework of the 2004 American Institute of Certified Public Accountants Technical Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation, to estimate the fair value of its equity awards. Each valuation methodology includes estimates and assumptions that require the LLC entity's judgment. These estimates and assumptions include a number of objective and subjective factors, including external market conditions, the prices at which the LLC entity sold convertible preferred stock or convertible preferred units, the superior rights and preferences of securities senior to the LLC entity's common stock or common units at the time and the likelihood of achieving a liquidity event, such as an initial public offering or sale. Significant changes to the key assumptions used in the valuations could result in different fair values of common stock or common units at each valuation date, as applicable.
Unaudited Interim Presentation
The accompanying interim consolidated balance sheet as of June 30, 2014, the statements of operations and comprehensive loss and cash flows for the six months ended June 30, 2013 and 2014 and the statement of convertible preferred units and members' equity (deficit) for the six months ended June 30, 2014 and the related footnote disclosures are unaudited. These unaudited interim consolidated financial statements have been prepared in accordance with GAAP. In management's opinion, the unaudited interim consolidated financial statements have been prepared on the same basis as the audited consolidated financial statements and include all adjustments, which include only normal recurring adjustments necessary for the fair presentation of the interim consolidated financial statements. The results for the six months ended June 30, 2014 are not necessarily indicative of the results expected for the full fiscal year.
Unaudited Pro Forma Presentation
In 2014, the LLC entity's board of managers (the "Board") authorized management of the LLC entity to pursue the filing of a registration statement with the Securities and Exchange Commission ("SEC") for the LLC entity to sell shares of its common stock to the public (the "IPO"). Upon effectiveness of the registration statement, the LLC entity intends to convert to a corporation. Upon the conversion to a corporation, all outstanding convertible preferred units and common units of the LLC entity will automatically convert to preferred stock and common stock, respectively, and at the closing of the IPO, all preferred stock will convert to common stock. The unaudited pro forma information in the accompanying consolidated financial statements as of, and for the six months ended June 30, 2014 reflects the conversion of all outstanding preferred units and common units of the LLC entity to common stock as of the balance sheet date. Shares of common stock issued in such IPO and any net proceeds therefrom are excluded from such pro forma information. The unaudited pro forma loss per share applicable to common stockholders for the year ended December 31, 2013 and the six months ended June 30, 2014 were computed using the weighted average pro forma number of shares of common stock outstanding, including the pro forma effect of the conversion of all outstanding
F-9
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
2. Summary of Significant Accounting Policies (Continued)
preferred units and common units into common stock, as if such conversion had occurred at the beginning of the respective periods, or the date of issuance, if later.
Off-Balance Sheet Risk and Concentrations of Credit Risk
The LLC entity has no off-balance sheet risk, such as foreign exchange contracts, option contracts, or other foreign hedging arrangements. The primary objectives for the LLC entity's investment portfolio are the preservation of capital and the maintenance of liquidity. The LLC entity's investment policy includes guidelines on the quality of the institutions and financial instruments and defines allowable investments that the LLC entity believes minimizes the exposure to concentration of credit risk.
Segment Information
Operating segments are defined as components of an entity about which separate discrete information is available for evaluation by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. The LLC entity views its operations and manages its business in one operating segment operating exclusively in the United States.
Cash and Cash Equivalents
The LLC entity considers all highly liquid investments with original or remaining maturity from the date of purchase of three months or less to be cash equivalents. Cash and cash equivalents include bank demand deposits and money market funds that invest primarily in U.S. government treasuries. Cash equivalents are carried at cost, which approximates their fair market value.
Marketable Securities
The LLC entity held $5,270, $10,398 and $469, in marketable securities at December 31, 2012 and 2013 and at June 30, 2014, respectively. The LLC entity's marketable securities were accounted for as available-for-sale securities and consisted of investments in U.S. Treasury and U.S. Government Sponsored Enterprise securities. As these securities were available to fund current operations, they are classified as current assets. Marketable securities are stated at fair value, with unrealized gains and losses included, until realized, as a component of accumulated other comprehensive income (loss), which is a separate component of stockholders'equity/members' equity. Realized gains and losses are determined on the specific-identification method and are included in investment income. The net unrealized gains and losses for the years ended December 31, 2012 and 2013, and the six months ended June 30, 2013 and 2014, were immaterial. Other-than-temporary impairments of investments are recognized in the consolidated statements of operations if the LLC entity has the intent to sell the security, or if it is more likely than not that the LLC entity will be required to sell the security before recovery of the amortized cost basis. Even if the LLC entity does not expect to sell a security, the LLC entity evaluates expected cash flows to be received to determine if a credit loss has occurred. In the event of a credit loss, only the amount associated with the credit loss is recognized in the statements of
F-10
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
2. Summary of Significant Accounting Policies (Continued)
operations. The amount of loss relating to other factors is recorded in other comprehensive income (loss). There have been no impairment losses recognized in any period presented.
Principles of Consolidation
The LLC entity's consolidated financial statements include the Company's accounts and the accounts of the wholly owned subsidiaries. All intercompany transactions have been eliminated in consolidation. The consolidated financial statements have been prepared in conformity with generally accepted accounting principles or GAAP.
Fair Value of Financial Instruments
The valuation of assets and liabilities are subject to fair value measurements using a three tiered approach and fair value measurement is classified and disclosed by the LLC entity in one of the following three categories:
Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
Level 2: Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability; or
Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e. supported by little or no market activity).
As of December 31, 2013, the carrying amounts of the LLC entity's financial instruments, which include cash equivalents and marketable securities, approximate their fair values.
The LLC entity determined the fair value of all cash equivalents and marketable securities at December 31, 2013 and 2012 using Level 1 inputs. The LLC entity did not have any nonfinancial assets or liabilities that should be recognized or disclosed at fair value on a recurring basis at December 31, 2013 or 2012.
Deferred Issuance Costs
Deferred issuance costs, which primarily consist of direct incremental legal and accounting fees relating to the potential IPO, are capitalized. The deferred issuance costs will be offset against IPO proceeds upon the consummation of the offering. In the event the offering is terminated, deferred issuance costs will be expensed. No amounts were capitalized as of December 31, 2012 and 2013 and $1,010 was capitalized at June 30, 2014, and is included in prepaid expenses and other current assets.
F-11
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
2. Summary of Significant Accounting Policies (Continued)
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist primarily of costs incurred in advance of services being received including services related to clinical trial programs of $263, in addition to $1,010 of deferred issuance costs at June 30, 2014.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Property and equipment are depreciated and amortized using the straight-line method over the estimated useful lives of the assets. Maintenance and repairs are expensed as incurred. The following estimated useful lives are used to depreciate the LLC entity's assets:
| |
Estimated Useful Life | |
|---|---|---|
| Computer equipment and software | 3 years | |
| Lab equipment | 5 years | |
| Furniture and fixtures | 5 years | |
| Leasehold improvements | Lesser of initial lease term or useful life |
The LLC entity reviews long-lived assets when events or changes in circumstances indicate the carrying value of the assets may not be recoverable. Recoverability is measured by comparison of the assets' book value to the future net undiscounted cash flows the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the book value of the assets exceed their fair value, which is measured based on the projected discounted future net cash flows arising from the assets. No impairment losses have been recorded through June 30, 2014.
Research and Development Expenses
Costs incurred in the research and development of the LLC entity's products are expensed to operations as incurred. Research and development expenses consist of costs incurred in performing research and development activities, including salaries and benefits, facilities cost, overhead costs, contract services and other outside costs. The value of goods and services received from contract research organizations or contract manufacturing organizations in the reporting period are estimated based on the level of services performed and progress in the period for which the LLC entity has not yet received an invoice from the supplier.
Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses, and expensed as the related goods are delivered or the services are performed.
F-12
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
2. Summary of Significant Accounting Policies (Continued)
Income Taxes
The LLC entity has elected to be treated under the Partnership provisions of the Internal Revenue Code. Accordingly, all income and deductions of the LLC entity are reported on the members' individual income tax returns and no income taxes are recorded by the LLC entity. The LLC entity does not have any operations.
RPI continues to be taxed as a C corporation for federal income tax purposes and RMI is also taxed as a C corporation. These entities will each file separate corporate income tax returns. Income taxes for these entities are recorded in accordance with FASB ASC Topic 740, Income Taxes ("ASC 740"), which provides for deferred taxes using an asset and liability approach. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Tax benefits are recognized when it is more likely than not that a tax position will be sustained during an audit. Deferred tax assets are reduced by a valuation allowance if current evidence indicates that it is considered more likely than not that these benefits will not be realized.
Stock-Based Compensation
The LLC entity accounts for stock-based compensation awards in accordance with FASB ASC Topic 718, Compensation—Stock Compensation ("ASC 718"). ASC 718 requires all stock-based compensation to employees, including grants of employee stock options and restricted stock and modifications to existing stock options, to be recognized in the statement of operations and comprehensive loss based on their fair values.
Compensation expense related to awards to employees is recognized on a straight-line basis, based on the grant date fair value, over the requisite service period of the award, which is generally the vesting term. Awards to non-employees are adjusted through stock-based compensation expense as the awards vest to reflect the current fair value of such awards, and are expensed using the accelerated method.
Net Loss Per Unit
Prior to the Prior Restructuring on March 21, 2013, RPI issued convertible preferred stock, common stock, warrants to purchase common stock and options to purchase common stock. Subsequent to the Prior Restructuring all RPI instruments were cancelled and reissued on a one-to-one basis as convertible preferred units, common units, warrants to purchase common units and restricted common units, respectively, of the LLC entity, respectively. Net loss per common unit is calculated using the two-class method, which is an earnings allocation formula that determines net loss per share for the holders of the LLC entity's common units and participating securities. Prior to the Prior Restructuring the convertible preferred stock and, subsequent to the Prior Restructuring, the convertible preferred units and restricted common units, contain participation rights in any dividend to be paid to the holders of common units. Therefore, the convertible preferred stock, convertible
F-13
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
2. Summary of Significant Accounting Policies (Continued)
preferred units and restricted common units are deemed to be participating securities. Net loss is allocated to common and participating securities on an as-if-converted basis as if all of the earnings for the period had been distributed. The participating securities do not include a contractual obligation to share in losses and therefore are not included in the calculation of net loss per share or unit in the periods that have a net loss.
Diluted net loss per unit is computed using the more dilutive of (a) the two-class method, or (b) the treasury stock method. The weighted-average number of common units included in the computation of diluted net loss gives effect to all potentially dilutive common equivalent units, including outstanding stock options, restricted common units and outstanding warrants. Common equivalent units are excluded from the computation of diluted net loss per share if their effect is antidilutive.
Basic and diluted earnings per unit is calculated as follows:
| |
Year Ended December 31, | Six Months Ended June 30, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2013 | 2014 | |||||||||
| |
|
|
(unaudited) |
(unaudited) |
|||||||||
Numerator: |
|||||||||||||
Net loss |
$ | (18,130 | ) | $ | (21,431 | ) | $ | (10,821 | ) | $ | (6,968 | ) | |
Cumulative dividends on convertible preferred units |
(3,368 | ) | (5,798 | ) | (2,633 | ) | (3,160 | ) | |||||
| | | | | | | | | | | | | | |
Loss attributable to common units—basic and diluted |
$ | (21,498 | ) | $ | (27,229 | ) | $ | (13,454 | ) | $ | (10,128 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Denominator: |
|||||||||||||
Weighted-average number of common units—basic and diluted |
2,883,087 | 7,467,781 | 5,979,829 | 10,198,713 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Loss per common unit—basic and diluted |
$ | (7.46 | ) | $ | (3.65 | ) | $ | (2.25 | ) | $ | (0.99 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
F-14
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
2. Summary of Significant Accounting Policies (Continued)
The following potentially dilutive securities, prior to the use of the treasury stock method, have been excluded from the computation of diluted weighted-average shares outstanding, as they would be anti-dilutive:
| |
Year Ended December 31, | Six Months Ended June 30, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2013 | 2014 | |||||||||
| |
|
|
(unaudited) |
||||||||||
Convertible preferred stock or units |
106,000,000 | 146,000,000 | 124,000,000 | 146,000,000 | |||||||||
Options to purchase common stock |
9,162,700 | — | — | — | |||||||||
Unvested restricted common units |
— | 5,975,650 | 7,213,101 | 4,431,224 | |||||||||
Warrants to purchase common units |
9,790,000 | 9,790,000 | 9,790,000 | 9,790,000 | |||||||||
Pro forma earnings per share is computed as follows:
| |
Year Ended December 31, 2013 |
Six Months Ended June 30, 2014 |
|||||
|---|---|---|---|---|---|---|---|
| |
(unaudited) |
||||||
Numerator: |
|||||||
Net loss attributable to common units—basic and diluted |
$ | (27,229 | ) | $ | (10,128 | ) | |
Add: Cumulative dividends on convertible preferred units |
5,798 | 3,160 | |||||
| | | | | | | | |
Net loss attributable to common stockholders—basic and diluted |
$ | (21,431 | ) | $ | (6,968 | ) | |
| | | | | | | | |
| | | | | | | | |
Denominator: |
|||||||
Weighted average common units outstanding—basic and diluted |
7,467,781 | 10,198,713 | |||||
Add: Adjustment to reflect assumed conversion of convertible preferred stock to common stock |
132,433,836 | 146,000,000 | |||||
| | | | | | | | |
Pro forma weighted-average shares outstanding |
139,901,617 | 156,198,713 | |||||
| | | | | | | | |
| | | | | | | | |
Pro forma net loss per share—basic and diluted |
$ | (0.15 | ) | $ | (0.04 | ) | |
| | | | | | | | |
| | | | | | | | |
Patent Costs
Costs to secure and defend patents are expensed as incurred and are classified as general and administrative expenses. Patent costs were $122 and $623 for the years ended December 31, 2012 and 2013, and $402 and $119 for the six month periods ended June 30, 2013 and 2014, respectively.
Comprehensive Income (Loss)
Comprehensive income (loss) is defined as the change in equity of a business entity during a period from transactions, other events, and circumstances from non-owner sources. Comprehensive income (loss) consists of net income (loss) and other comprehensive income (loss), which includes
F-15
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
2. Summary of Significant Accounting Policies (Continued)
certain changes in equity that are excluded from net income (loss). The LLC entity's comprehensive loss consists of net loss and unrealized gains (losses) on marketable securities.
Application of new or revised accounting standards
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies and adopted by the LLC entity as of the specified effective date. Unless otherwise discussed, the LLC entity believes that the impact of recently issued standards that are not yet effective will not have a material impact on its financial position or results of operations upon adoption.
On April 5, 2012, the Jump-Start Our Business Startups Act (the "JOBS Act") was signed into law. The JOBS Act contains provisions that, among other things, reduce certain reporting requirements for an "emerging growth company." As an emerging growth company, the LLC entity has elected to not take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards, and as a result, will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies.
In June 2014, the FASB issued ASU No. 2014-10, Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation which guidance removes all incremental financial reporting requirements from GAAP for development stage entities, including the removal of Topic 915 from the FASB Accounting Standards Codification. The amendments in this ASU eliminated certain disclosure requirements to (1) present inception-to-date information in the statements of income, cash flows, and shareholder equity, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. The ASU also clarifies that disclosures about risks and uncertainties required by Topic 275 also apply to entities that have not commenced planned principal operations. Further, the ASU eliminates the exception to the sufficiency-of-equity-at-risk criterion in paragraph 810-10-15-16 for development stage entities will require all reporting entities that have an interest in a development stage entity to apply consistent guidance for transactions that are economically the same or similar.
Upon adoption, entities will no longer present or disclose any information required by Topic 915. The LLC entity has elected to early-adopt the amendments described in this ASU. The amendments primarily relate to disclosure matters and therefore have no impact on LLC entity's financial statements, other than the omission of previously required disclosures including inception-to-date financial information. With respect to the exception to the sufficiency-of-equity-at-risk criterion in paragraph 810-10-15-16, this exception had no impact on the LLC entity's determination to consolidate entities for which the LLC entity has a variable interest.
F-16
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
2. Summary of Significant Accounting Policies (Continued)
In July 2013, the FASB issued Accounting Standards Update No. 2013-11, Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exist ("ASU 2013-11"). ASU 2013-11 amends the presentation requirements of ASC 740 Income Taxes and requires an unrecognized tax benefit to be presented in the financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, similar tax loss, or a tax credit carryforward. To the extent the tax benefit is not available at the reporting date under the governing tax law or if the entity does not intend to use the deferred tax asset for such purpose, the unrecognized tax benefit should be presented as a liability and not combined with deferred tax assets. ASU 2013-11 is effective for annual periods, and interim periods within those years, beginning after December 15, 2013, which is the LLC entity's fiscal 2014. The amendments are to be applied to all unrecognized tax benefits that exist as of the effective date and may be applied retrospectively to each prior reporting period presented. The LLC entity is currently evaluating the impact of the adoption of ASU 2013-11 on its financial statements. The LLC entity adopted this standard in the quarter ended March 31, 2014 without impact on its financial statements, as its current practice is consistent with this standard.
In February 2013, the FASB issued ASU No. 2013-04, Liabilities (Topic 405): Obligations Resulting from Joint and Several Liability Arrangements for Which the Total Amount of the Obligation is Fixed at the Reporting Date ("ASU 2013-04"). This guidance changes how an entity measures obligations resulting from joint and several liability arrangements by requiring that when measuring the obligation, an entity will include the amount the entity agreed to pay for the arrangement between the entity and other entities that are also obligated to the liability, as well as any additional amount the entity expects to pay on behalf of the other entities. ASU 2013-04 also requires additional disclosures surrounding such obligations. ASU 2013-04 is effective for interim and annual reporting periods beginning after December 15, 2013 and is required to be applied retrospectively. The LLC entity has adopted this guidance in the first quarter of 2014 without any material impact on the LLC entity's financial position or results of operations.
Subsequent Events
The LLC entity considers events or transactions that occur after the balance sheet date but prior to the issuance of the financial statements to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure.
3. Marketable Securities
As referenced in Note 2, accounting principles provide guidance for using fair value to measure assets and liabilities based on a hierarchy of inputs and require management to make judgments and consider factors specific to the asset or liability.
The LLC entity's financial instruments consist of cash and cash equivalents, marketable securities, accounts payable and accrued liabilities. The carrying amount of cash and cash equivalents, marketable
F-17
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
3. Marketable Securities (Continued)
securities, accounts payable, and accrued liabilities approximate their fair values because of the short-term nature of the instruments.
The following tables summarize the cash equivalents and marketable securities measured at fair value on a recurring basis as of December 31, 2012, December 31, 2013, and June 30, 2014:
| |
Fair Value Measurement at December 31, 2012 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quoted Prices in Active Markets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||
Assets: |
|||||||||||||
Cash and money market funds |
$ | 7,403 | $ | — | $ | — | $ | 7,403 | |||||
Government-sponsored enterprise securities |
4,363 | — | — | 4,363 | |||||||||
| | | | | | | | | | | | | | |
Total cash equivalents |
11,766 | — | — | 11,766 | |||||||||
Marketable securities |
|||||||||||||
Government-sponsored enterprise securities |
5,270 | — | — | 5,270 | |||||||||
| | | | | | | | | | | | | | |
Total |
$ | 17,036 | $ | — | $ | — | $ | 17,036 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| |
Fair Value Measurement at December 31, 2013 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quoted Prices in Active Markets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||
Assets: |
|||||||||||||
Cash and money market funds |
$ | 6,764 | $ | — | $ | — | $ | 6,764 | |||||
Government-sponsored enterprise securities |
600 | 600 | |||||||||||
| | | | | | | | | | | | | | |
Total cash equivalents |
7,364 | — | — | 7,364 | |||||||||
Marketable securities |
— |
||||||||||||
Government-sponsored enterprise securities |
10,398 | — | — | 10,398 | |||||||||
| | | | | | | | | | | | | | |
Total |
$ | 17,762 | $ | — | $ | — | $ | 17,762 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
F-18
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
3. Marketable Securities (Continued)
| |
Fair Value Measurement at June 30, 2014 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quoted Prices in Active Markets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||
Assets: |
|||||||||||||
Cash and money market funds |
$ | 6,513 | $ | — | $ | — | $ | 6,513 | |||||
Government-sponsored enterprise securities |
2,351 | — | — | 2,351 | |||||||||
| | | | | | | | | | | | | | |
Total cash equivalents |
8,864 | — | — | 8,864 | |||||||||
Marketable securities |
|||||||||||||
US treasury securities |
$ | — | — | — | $ | — | |||||||
Government-sponsored enterprise securities |
469 | — | — | 469 | |||||||||
| | | | | | | | | | | | | | |
Total marketable securities |
469 | — | — | 469 | |||||||||
| | | | | | | | | | | | | | |
Total |
$ | 9,333 | $ | — | $ | — | $ | 9,333 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The fair value measurements of the LLC entity's cash held in money market funds and marketable securities at December 31, 2012 and 2013 and June 30, 2014 is summarized in the table below:
| |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
December 31, 2012 |
|||||||||||||
Cash and cash equivalents: |
|||||||||||||
Cash and money market funds |
$ | 7,397 | $ | 6 | $ | — | $ | 7,403 | |||||
Government-sponsored enterprise securities |
4,363 | — | — | 4,363 | |||||||||
| | | | | | | | | | | | | | |
Total cash and cash equivalents |
11,760 | 6 | — | 11,766 | |||||||||
| | | | | | | | | | | | | | |
Marketable securities: |
|||||||||||||
Government-sponsored enterprise securities |
5,269 | 1 | 5,270 | ||||||||||
| | | | | | | | | | | | | | |
Total marketable securities |
5,269 | 1 | — | 5,270 | |||||||||
| | | | | | | | | | | | | | |
Total cash, cash equivalents, and marketable securities |
$ | 17,029 | $ | 7 | $ | — | $ | 17,036 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
F-19
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
3. Marketable Securities (Continued)
| |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
December 31, 2013 |
|||||||||||||
Cash and cash equivalents: |
|||||||||||||
Cash and money market funds |
$ | 6,755 | $ | 9 | $ | — | $ | 6,764 | |||||
U.S. Treasury securities |
— | — | — | — | |||||||||
Government-sponsored enterprise securities |
600 | — | — | 600 | |||||||||
| | | | | | | | | | | | | | |
Total cash and cash equivalents |
7,355 | 9 | — | 7,364 | |||||||||
| | | | | | | | | | | | | | |
Marketable Securities: |
|||||||||||||
Government-sponsored enterprise securities |
10,399 | — | 1 | 10,398 | |||||||||
| | | | | | | | | | | | | | |
Total marketable securities |
10,399 | — | 1 | 10,398 | |||||||||
| | | | | | | | | | | | | | |
Total cash, cash equivalents, and marketable securities |
$ | 17,754 | $ | 9 | $ | — | $ | 17,762 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
June 30, 2014 |
|||||||||||||
Cash and cash equivalents: |
|||||||||||||
Cash and money market funds |
$ | 6,505 | $ | 8 | $ | — | $ | 6,513 | |||||
U.S. Treasury securities |
— | — | — | — | |||||||||
Government-sponsored enterprise securities |
2,351 | — | — | $ | 2,351 | ||||||||
| | | | | | | | | | | | | | |
Total cash and cash equivalents |
8,856 | 8 | — | 8,864 | |||||||||
| | | | | | | | | | | | | | |
Marketable Securities: |
|||||||||||||
U.S. Treasury Securities |
— | — | — | — | |||||||||
Government-sponsored enterprise securities |
468 | 1 | — | $ | 469 | ||||||||
| | | | | | | | | | | | | | |
Total marketable securities |
468 | 1 | — | 469 | |||||||||
| | | | | | | | | | | | | | |
Total cash, cash equivalents, and marketable securities |
$ | 9,324 | $ | 9 | $ | — | $ | 9,333 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
F-20
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
4. Property and Equipment
Property and equipment and related accumulated depreciation and amortization are as follows:
| |
December 31, | June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2014 | |||||||
Lab equipment |
$ | 12 | $ | 12 | $ | 12 | ||||
Computer equipment |
17 | 17 | 17 | |||||||
Furniture and office equipment |
34 | 34 | 34 | |||||||
Leasehold improvements |
7 | 7 | 7 | |||||||
| | | | | | | | | | | |
|
70 | 70 | 70 | |||||||
Less accumulated depreciation. |
(26 | ) | (46 | ) | (55 | ) | ||||
| | | | | | | | | | | |
|
$ | 44 | $ | 24 | $ | 15 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Depreciation expense for the years ended December 31, 2012 and 2013 was $17 and $20, and for the six months ended June 30, 2013 and 2014 was $9 and $9, respectively.
5. Accrued Expenses
Accrued expenses consisted of the following:
| |
December 31, | June 30, | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2014 | |||||||
Payroll and payroll related |
$ | 2 | $ | 258 | $ | 6 | ||||
Research and development costs |
136 | 1,256 | 926 | |||||||
Professional fees |
96 | 69 | 974 | |||||||
Other |
23 | 12 | 10 | |||||||
| | | | | | | | | | | |
Accrued expenses |
$ | 257 | $ | 1,595 | $ | 1,916 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
6. Commitments
Operating Leases
The LLC entity leases office space in Boston, Massachusetts under the terms of a lease initially ending in July 2014. In April 2014 the lease was amended to extend the lease to July 31, 2015. The lease is not renewable. Rent expense for the years ended December 31, 2012 and 2013 and for the six months ended June 30, 2013 and 2014 was approximately $127, $134, $66 and $75, respectively.
F-21
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
6. Commitments (Continued)
At December 31, 2013, future minimum commitments due under all operating leases, including the April 2014 lease extension, are as follows:
2014 |
$ | 138 | ||
2015 |
85 | |||
| | | | | |
Total minimum lease payments |
$ | 223 | ||
| | | | | |
| | | | | |
7. Convertible Preferred Stock and Convertible Preferred Units
In March and August 2010, RPI issued 36,000,000 shares of series A convertible preferred stock at a price of $0.50 per share, resulting in net proceeds of $17,820. In November 2011, RPI issued 25,000,000 shares of series A convertible preferred stock at a price of $0.50 per share, resulting in net proceeds of $12,487. An amendment to the series A convertible preferred stock purchase agreement dated July 30, 2010, provided for the issuance by RPI of up to 19,000,000 additional shares of series A convertible preferred stock.
In May, September and November 2012, RPI issued 45,000,000 shares of series B convertible preferred stock at $0.50 per share, resulting in net proceeds of $22,397. Upon the initial closing under the series B convertible preferred stock purchase agreement in May 2012, the ability for RPI to issue additional shares of series A convertible preferred stock under the July 30, 2010 amended series A convertible preferred stock purchase agreement was terminated.
The series B convertible preferred stock purchase agreement, as amended and restated November 20, 2012, provided for the issuance by RPI of up to 40,000,000 additional shares of series B convertible preferred stock at $0.50 per share upon the achievement of certain clinical development milestones, as defined therein (the date of any such issuances referred as the "Second Subsequent Closing" and the "Third Subsequent Closing", as applicable).
Pursuant to the Second Subsequent Closing in February 2013, RPI issued an additional 18,000,000 shares of series B convertible preferred stock at $0.50 per share, resulting in net proceeds of $8,938.
On March 21, 2013 as a result of the Prior Restructuring, (i) each outstanding share of series A convertible preferred stock was exchanged for one series A convertible preferred unit of the LLC entity, (ii) each outstanding share of series B convertible preferred stock was exchanged for one series B convertible preferred unit of the LLC entity, and (iii) each outstanding share of common stock was exchanged for one common unit of the LLC entity.
The LLC entity determined the exchange of the series A and series B convertible preferred stock upon the Prior Restructuring was a modification of the preferred stock. As a result the series A and series B convertible preferred units were recorded at their historical carrying values, on the date of the Prior Restructuring.
F-22
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
7. Convertible Preferred Stock and Convertible Preferred Units (Continued)
In July 2013, at the Third Subsequent Closing, the LLC entity issued 22.0 million series B preferred units at $0.50 per unit, resulting in net proceeds of $10,944.
The LLC entity has evaluated the investor right and/or obligation to purchase additional preferred stock (or units) upon the achievement of certain milestones and determined such right and/or obligation to be an embedded financial instrument that does not require bifurcation pursuant to the provisions of ASC 480, Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity, and ASC 815, Accounting for Derivative Instruments and Hedging Activities.
The holders of the series A and series B convertible preferred units have the following rights:
Conversion
Each convertible preferred unit of the LLC entity automatically converts into one common unit at the election of the holders of a majority of the convertible preferred units. In addition, each convertible preferred unit held by a member of the LLC entity that defaults in its obligation to purchase additional series B convertible preferred units automatically converts into one common unit upon the date of closing of the issuance under which such member defaulted. Pursuant to the LLC operating agreement, prior to the consummation of our IPO if approved by the LLC entity's board and holders of a majority of the convertible preferred units, the LLC entity will convert to a corporation, and the convertible preferred units will then convert to common stock on a one-to-one basis. Any such conversion ratio is subject to adjustment for certain dilutive events, including, but not limited to, stock splits, dividends and, with respect to the convertible preferred units, certain issuances of units at below the purchase price of the convertible preferred units.
The LLC entity has evaluated the series A and series B convertible preferred units and determined that they should be considered an "equity host" and not a "debt host." The evaluation is necessary to determine if any embedded features require bifurcation and separate accounting as a derivative liability. The LLC entity's analysis was based on a consideration of the economic characteristics and risks and more specifically, evaluated all the stated and implied substantive terms and features including (i) whether the preferred unit includes redemption features, (ii) how and when any redemption features could be exercised, (iii) whether the preferred unit holders were entitled to dividends, (iv) the voting rights of the preferred units and (v) the existence and nature of any conversion rights. As a result of the LLC entity's determination that the convertible preferred unit is an "equity host," the embedded conversion option is not considered a derivative liability.
Dividends
Prior to the Prior Restructuring, the holders of series B convertible preferred stock were entitled to receive cumulative dividends at the rate per annum of 8.0% of the original issue price of the series B convertible preferred stock (subject to appropriate adjustment in the event of any stock dividend, stock split, combination or other similar recapitalization affecting such shares), prior and in preference to the payment of any dividends on the series A convertible preferred stock or the common
F-23
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
7. Convertible Preferred Stock and Convertible Preferred Units (Continued)
stock (except in certain specific circumstances) when, as, and if declared by the board of directors of RPI. Holders of series A convertible preferred stock were entitled to receive cumulative dividends at the rate per annum of 8.0% of the original issue price of the series A convertible preferred stock (subject to appropriate adjustment in the event of any stock dividend, stock split, combination or other similar recapitalization affecting such shares), prior and in preference to the payment of any dividends on the common stock when, as and if declared by the board. No dividends were declared or paid by RPI through March 21, 2013.
Following the Prior Restructuring, if and when the board of managers of the LLC entity decides to distribute available cash to its members, the holders of all preferred units are entitled to a "preferred return" meaning, a cumulative non-compounding annual return of 8.0% on each such holder's unreturned capital contribution with respect to such preferred unit. The preferred return accrues daily until the earlier of (i) the date on which the full amount of such holder's capital contribution has been returned and (ii) the date on which such preferred unit is otherwise no longer outstanding. The series B convertible preferred units are entitled to such preferred return in preference to the Series A convertible preferred units and the common units, and the series A convertible preferred units are entitled to such preferred return in preference to the common units. No such preferred return is payable in connection with a conversion of a convertible preferred unit.
Voting
The holders of convertible preferred units and common units of the LLC entity are each entitled to one vote per unit. The holders of the convertible preferred units must consent to certain material changes in the LLC entity.
Liquidation
Prior to the Prior Restructuring, in the event of any voluntary or involuntary liquidation, dissolution or winding-up of RPI (a "Liquidation"), the holders of shares of series B convertible preferred stock were entitled to receive, prior to and in preference to the holders of series A convertible preferred stock and common stock, $0.50 per share, plus any cumulative, unpaid dividends on such shares. After the payment of all preferential amounts to the holders of series B convertible preferred stock, in the event of any Liquidation, the holders of shares of series A convertible preferred stock were entitled to receive, prior to and in preference to the holders of common stock, $0.50 per share, plus any cumulative, unpaid dividends on such shares. After the payment of all preferential amounts to the holders of convertible preferred stock, the remaining assets of RPI would be distributed among the holders of the shares of convertible preferred stock and common stock, pro rata based on the number of shares of common stock held by each, assuming conversion of the convertible preferred stock into common stock. Should the assets of RPI have been insufficient to pay the full amount due to the holders of the series B convertible preferred stock or series A convertible preferred stock, as the case may be, the holders of the particular class of shares to which the shortage applies, would share ratably in proportion to the amount they would otherwise be entitled, in the remaining distributable
F-24
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
7. Convertible Preferred Stock and Convertible Preferred Units (Continued)
amount. Liquidation would be deemed to occur if RPI entered into a merger or consolidation with another company that represents a change of control. Following the Prior Restructuring, in the event of any voluntary or involuntary liquidation, dissolution or winding-up (a "Liquidation"), each holder of a series B convertible preferred unit is entitled, prior and in preference to the holders of series A convertible preferred units and common units, to their preferred return and such holder's unreturned capital contribution. In event of any Liquidation, each holder of a series A convertible preferred unit is entitled, prior and in preference to the holders of common units, to their preferred return and such holder's unreturned capital contribution. After the payment of all preferential amounts to the holders of convertible preferred units, should the remaining assets of the LLC entity be insufficient to pay the full amount due to the holders of the series B convertible preferred units or series A convertible preferred units, as the case may be, the holders of the particular class of units to which the shortage applies, would share ratably in proportion to the amount they would otherwise be entitled, in the remaining distributable amount. A change in control is a deemed liquidated event.
8. Common Stock and Common Units
In March 2010, RPI issued 2,260,000 shares of common stock at a purchase price of $0.001 per share. In August 2010, RPI issued 300,000 shares of common stock also at a purchase price of $0.001 per share. In November 2011, RPI issued 75,000 shares of common stock at a price of $0.06 per share. Each of such foregoing issuances was determined by the board of directors of RPI to be at the fair value at the date of the grant.
Prior to the Prior Restructuring, holders of common stock were entitled to one vote per share and to receive dividends, when and if declared by the board of directors of RPI. The voting, dividend, and liquidation rights of the holders of the common stock were subject to, and qualified by, the rights of the holders of the convertible preferred stock.
Following the Prior Restructuring, each holder of a common unit is entitled to the same rights and privileges as the holders of common stock prior to the Prior Restructuring. The Prior Restructuring resulted in no economic change to the holders of the common units.
F-25
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
8. Common Stock and Common Units (Continued)
The LLC entity reserved for future issuance the following number of common units for the conversion of preferred units, and exercise of warrants:
| |
As of | ||||||
|---|---|---|---|---|---|---|---|
| |
December 31, 2013 | June 30, 2014 | |||||
Series A Convertible Preferred Unit |
61,000,000 | 61,000,000 | |||||
Series B Convertible Preferred Unit |
85,000,000 | 85,000,000 | |||||
Common Unit Warrants |
9,790,000 | 9,790,000 | |||||
| | | | | | | | |
|
155,790,000 | 155,790,000 | |||||
| | | | | | | | |
| | | | | | | | |
Additionally, as of December 31, 2013 and June 30, 2014 the LLC entity has 5,975,650 and 4,431,244 unvested restricted common units which will be included as common units outstanding as they vest.
9. Common Stock and Unit Warrants
In March 2010, RPI issued a fully vested warrant to purchase 4,690,000 shares of common stock to Ipsen in connection with a license agreement (see Note 11) at an exercise price of $0.001 per share. The warrant was determined to have a fair value of $230 as determined using the Black-Scholes model and was recorded as a charge to research and development expense on the date of issuance and within stockholders' equity. The warrant, if unexercised, expires on the earlier of March 9, 2015 or the first sale of securities to the public in an offering pursuant to an effective registration statement under the Securities Act of 1933, as amended (the "Securities Act").
In addition, in March 2010, RPI issued an unvested warrant to purchase 5,100,000 shares of common stock to Ipsen in connection with the above-mentioned license agreement at an exercise price of $0.001. The warrant vested in November 2011 upon the achievement of a regulatory milestone related to the product candidate that is the subject of the license agreement. As a result, the fair value of the warrant of $302, as determined using the Black-Scholes model, was recorded as a charge to research and development expense when the milestone was achieved. The warrant, if unexercised, expires on the earlier of March 9, 2015 or the first sale of securities to the public in an offering pursuant to an effective registration statement under the Securities Act.
As a result of the Prior Restructuring, each warrant to purchase a share of common stock of RPI was converted to a warrant to purchase one common unit of the LLC entity. The restructuring resulted in no economic change to the holders warrants to purchase common units.
10. 2010 Equity Incentive Plan and Restricted Common Units
The 2010 Equity Incentive Plan, as amended (the "Plan"), provides for the grant of incentive and non-qualified stock options and restricted stock grants to employees, consultants, advisors and directors, as determined by the board of directors.
F-26
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
10. 2010 Equity Incentive Plan and Restricted Common Units (Continued)
As a result of the Prior Restructuring, all outstanding option grants under the Plan were cancelled. Each holder of a stock option that was cancelled was issued a restricted common unit in its place on a one-for-one basis. Restricted common unit vesting agreements were contracted between the LLC entity and the restricted common unit holder granting the holder the same vesting terms as originally granted in the particular option agreement. Any unvested portion of the stock option at the Prior Restructuring would continue to vest under those original time frames and conditions. Strike prices were eliminated as they are not applicable to common unit instruments, and all equity incentive grants after the Prior Restructuring were of restricted common units.
The holder of a restricted common unit is entitled to one vote per unit. After the payment of all preferential amounts to the holders of the convertible preferred units the holder of a restricted common unit is entitled to their pro rata share of the remaining consideration, if any, based on the number of restricted common units held by the holder.
Stock Options and Compensatory Units
A summary of stock option activity under the 2010 Stock Plan for the year ended December 31, 2013, is as follows:
| |
Number of Shares |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Term (in years) |
Aggregate Intrinsic value (in thousands) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Outstanding as of December 31, 2012 |
9,162,700 | $ | 0.05 | 8.82 | $ | — | |||||||
Granted |
3,151,000 | 0.04 | |||||||||||
Exercised |
— | — | |||||||||||
Cancelled |
(12,313,700 | ) | 0.05 | ||||||||||
| | | | | | | | | | | | | | |
Outstanding as of December 31, 2013 |
— | $ | — | — | $ | — | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Common Stock Options
Prior to the Prior Restructuring on March 21, 2013, RPI issued common stock options pursuant to the Plan. RPI estimated the fair value of its common stock-based awards to employees and consultants using the Black-Scholes option pricing model, which requires the input of subjective assumptions, including (a) the expected stock price volatility, (b) the expected term of the award, (c) the risk-free interest rate and (d) expected dividends. Due to the lack of a public market for the trading of its common stock and a lack of company-specific historical and implied volatility data, RPI based its estimate of expected volatility on the historical volatility of a representative group of companies that are publicly traded. RPI selected a representative group of companies with comparable characteristics to it, including risk profiles, position within the industry, and with historical share price information sufficient to meet the expected term of the stock-based awards. RPI computed the historical volatility
F-27
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
10. 2010 Equity Incentive Plan and Restricted Common Units (Continued)
of this selected group using the daily closing prices for the selected companies' shares during the equivalent period of the calculated expected term of the stock-based awards. RPI estimated the expected life of its employee stock options whereby, the expected life equals the arithmetic average of the vesting term and the original contractual term of the option due to its lack of sufficient historical data. The risk-free interest rates for periods within the expected term of the option are based on the U.S. Treasury securities with a maturity date commensurate with the expected term of the associated award. The LLC entity has never paid, and does not expect to pay, dividends in the foreseeable future; therefore, the expected dividend yield is assumed to be zero.
Restricted Common Units
Upon the Prior Restructuring, all 12,313,700 common stock options of RPI outstanding as of March 21, 2013 were exchanged on a one-to-one basis for 12,313,700 restricted common units of the LLC entity. Vesting continued on the same schedule as originally granted per the respective option agreement. At the time of the exchange, the LLC entity determined the fair value of a restricted common unit to be $0.06 per unit, equivalent to the fair value of a common unit, based on the methods described in Note 2. The fair value of stock options immediately prior to the Prior Restructuring was determined using a Black-Scholes option pricing model, based on the methods described in Note 2 and in this Note 10, and ranged in value from $0.03 to $0.04. The exchange was accounted for as a modification in accordance with ASC 718, with the incremental fair value determined to be $255, of which $99 was recognized immediately upon the Prior Restructuring for the portion related to the vested awards, and the remaining $156 will be recognized over the remaining service period of the restricted common units, net of estimated forfeitures.
All restricted common units granted subsequent to the Prior Restructuring were valued at the fair value of the LLC entity's common unit on the date of grant and will be expensed over their respective service period. Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term "forfeitures" is distinct from "cancellations" and represents only the unvested portion of the surrendered unit. Ultimately, the actual expense recognized over the vesting period will only be for those options that vest.
F-28
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
10. 2010 Equity Incentive Plan and Restricted Common Units (Continued)
A summary of the LLC entity's restricted common unit activity for the year ended December 31, 2013 and the six months ended June 30, 2014, is as follows:
| |
Number of Units |
Weighted- Average Grant- Date Fair Value Per Unit |
|||||
|---|---|---|---|---|---|---|---|
Outstanding unvested as of December 31, 2012 |
— | $ | — | ||||
Exchange of unvested stock options for unvested restricted common units |
8,135,237 | 0.06 | |||||
Granted |
105,000 | 0.06 | |||||
Vested |
(2,011,871 | ) | 0.06 | ||||
Cancelled |
(252,716 | ) | 0.06 | ||||
| | | | | | | | |
Outstanding unvested as of December 31, 2013 |
5,975,650 | 0.06 | |||||
Granted |
— | — | |||||
Vested |
(1,263,275 | ) | 0.06 | ||||
Cancelled |
(281,151 | ) | 0.06 | ||||
| | | | | | | | |
Outstanding unvested as of June 30, 2014 |
4,431,224 | $ | 0.06 | ||||
| | | | | | | | |
| | | | | | | | |
The LLC entity recorded total stock-based compensation expense for stock options and restricted common units granted to employees, directors and non-employees from the Plan of $75, $254, $171 and $88 during the years ended December 31, 2012 and 2013 and the six months ended June 30, 2013 and 2014, respectively. As of December 31, 2013 and June 30, 2014, unrecognized compensation cost of $352 and $271, respectively, related to non-vested employee stock-based compensation arrangements is expected to be recognized over weighted-average periods of 1.1 and 1.0 years, respectively.
The LLC entity recorded stock-based compensation expense for stock options and restricted common units in the statements of operations and comprehensive loss as follows:
| |
Year Ended December 31, |
Six Months Ended June 30, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | 2013 | 2014 | |||||||||
| |
|
|
(unaudited) |
||||||||||
Research and development |
$ | 27 | $ | 74 | $ | 51 | $ | 34 | |||||
General and administrative |
48 | 180 | 120 | 54 | |||||||||
| | | | | | | | | | | | | | |
Total |
$ | 75 | $ | 254 | $ | 171 | $ | 88 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
F-29
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
10. 2010 Equity Incentive Plan and Restricted Common Units (Continued)
The weighted-average assumptions used to estimate the fair value of stock options using the Black-Scholes option pricing model were as follows:
| |
Year Ended December 31, | |||
|---|---|---|---|---|
| |
2012 | 2013 | ||
Expected volatility |
80.0% | 80% - 65% | ||
Risk-free interest rate |
0.82% - 1.11% | 1.14% | ||
Expected option term (in years) |
6.08 | 6.08 | ||
Expected dividend yield |
0.0% | 0.0% | ||
Restricted Common Unit Grants to Non-Employees
During the year ended December 31, 2013, and subsequent to the Prior Restructuring, the LLC entity granted restricted common units to non-employee consultants. The LLC entity valued these restricted common units based on their fair value on the date of grant, determined to be the fair value of a common unit.
The unvested restricted common units held by consultants have been and will be remeasured using the LLC entity's estimate of fair value at each reporting period through the remaining vesting period. Stock-based compensation expense of $5, $2, $0 and $17 was recorded for the years ended December 31, 2012 and 2013 and the six months ended June 30, 2013 and 2014, respectively, relating to non-employee and option awards.
11. Significant Agreements
License Agreements
RPI entered into a license agreement on February 26, 2010 with Ipsen Pharma, S.A.S. (Ipsen) that granted the LLC entity full worldwide right for two programs that include the clinical candidates RM-493, which is in Phase 1 clinical trials and relamorelin which is in Phase 2 clinical trials. In connection with the license agreement, RPI issued two warrants to purchase up to 9,790,000 shares of common stock at an exercise price of $0.001. As a result of the Prior Restructuring, Ipsen's warrants to purchase common stock were converted to warrants to purchase an equal number of common units (see Note 8). Also, as a result of the Prior Restructuring, the Ipsen license was converted to separate license agreements for each of the RM-493 and relamorelin programs, respectively. Under the terms of each Ipsen license agreement, assuming that relamorelin or RM-493, as applicable, is successfully developed, receives regulatory approval and is commercialized, Ipsen may receive aggregate payments of up to U.S. $40,000 upon the achievement of certain development and commercial milestones under such license agreements and royalties on future product sales in the mid-single digits. Substantially all of such aggregate payments of up to $40,000 under each Ipsen license agreement are for milestones that may be achieved no earlier than first commercial sale of relamorelin or RM-493, as applicable.
In connection with the original license agreement between Ipsen and RPI, in March 2010, after giving effect to the Prior Restructuring, an affiliate of Ipsen acquired 410,000 common units of the LLC entity.
F-30
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
11. Significant Agreements (Continued)
Michael J. Fox Foundation
On September 10, 2013 the LLC entity was awarded a $1,350 grant from the Michael J. Fox Foundation to help fund a Phase 2 clinical trial of relamorelin. In consideration for the grant the LLC entity must endeavor to complete the study according to a mutually agreed to timeline as well as submit certain required communications and deliverables. Failure to perform these obligations may result in the suspension or termination of payments under the grant. In the event that the relamorelin generates commercial sales in the indication of chronic constipation in Parkinson's disease above a certain threshold, the LLC entity is obligated to pay a limited royalty to the Michael J. Fox Foundation up to the amount of the grant.
During the year ended December 31, 2013 and the six months ended June 30, 2014, the LLC entity had received $100 and $100, respectively from the Michael J. Fox Foundation. These amounts have been classified as a reduction of research and development expenses, and have been recognized as the related research activities have been performed and expenses incurred.
12. Related-Party Transactions
Employees of certain holders of series A and series B convertible preferred units have been retained as consultants supporting development activities of the LLC entity for which the holders are paid cash compensation pursuant to consulting arrangements. Compensation payments related to these consultants totaled $178, $241, $162 and $97 for the years ended December 31, 2012 and 2013 and for the six month periods ended June 30, 2013 and 2014, respectively.
The LLC entity also engaged Ipsen to perform development services. The LLC entity paid $39, $26, $0 and $0 for the years ended December 31, 2012 and 2013, and for the six months ended June 30, 2013 and 2014, respectively, for services performed.
13. Income Taxes
As noted previously, the LLC entity has elected to be treated under the partnership provisions of the Internal Revenue Code and is therefore not a taxable entity. However, its two wholly-owned subsidiaries, RPI and RMI, are taxable corporations. The folowing discussion of income taxes represents the combined tax attributes of RPI and RMI. The LLC entity accounts for income taxes under ASC 740. Deferred income tax assets and liabilities are determined based upon differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
For the years ended December 31, 2012 and 2013, the LLC entity did not have a current or deferred income tax expense or benefit as the LLC is a flow-through entity not subject to tax at the entity level. Additionally, RPI and RMI have incurred losses since inception.
F-31
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
13. Income Taxes (Continued)
As of December 31, 2013, RPI and RMI had federal and state net operating loss carryforwards of approximately $36,000 and $11,000, respectively which are available to reduce future taxable income. The net operating loss carryforwards expire at various times beginning in 2030 for federal and state purposes. RPI also had federal and state tax credits of approximately $1,922 and $226, respectively, which may be used to offset future tax liabilities. These tax credit carryforwards will begin to expire at various times beginning in 2030 for federal purposes and 2026 for state purposes.
The Prior Restructuring transaction generated a taxable gain to RPI in the amount of $9,900 which was fully offset by utilization of current year losses in 2013.
The net operating loss and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. Net operating loss and tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant stockholders over a three-year period in excess of 50%, as defined under Sections 382 and 383 of the Internal Revenue Code, respectively, as well as similar state provisions and other provisions within the Internal Revenue Code. This could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value of the LLC entity immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years.
The LLC entity has not recorded any reserves for uncertain tax positions as of December 31, 2012 or 2013. The LLC entity has not, as yet, conducted a study of research and development credit carryforwards. This study may result in an adjustment to the LLC entity's research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position. A full valuation allowance has been provided against the LLC entity's research and development credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the valuation allowance. Thus, there would be no impact to the balance sheets or statements of operations and comprehensive loss if an adjustment were required.
Interest and penalty charges, if any, related to unrecognized tax benefits will be classified as income tax expense in the accompanying statements of operations and comprehensive loss. As of December 31, 2013, the LLC entity had no accrued interest or penalties related to uncertain tax positions.
Since the LLC entity is in a loss carryforward position, the LLC entity is generally subject to examination by the U.S. federal, state and local income tax authorities for all tax years in which a loss carryforward is available. The LLC entity is not currently under examination by the Internal Revenue Service or any other jurisdictions for any tax years.
F-32
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
13. Income Taxes (Continued)
The principal components of the LLC entity's deferred tax assets are as follows:
| |
As of December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Deferred tax assets: |
|||||||
Net operating loss carryforwards |
$ | 14,185 | $ | 18,635 | |||
Research and development credits |
760 | 2,071 | |||||
Capitalized start up costs |
60 | 55 | |||||
Capitalized license fee |
183 | 169 | |||||
Other |
39 | 35 | |||||
| | | | | | | | |
Total gross deferred tax assets |
15,227 | 20,965 | |||||
Deferred tax liability |
— | — | |||||
Valuation allowance |
(15,227 | ) | (20,965 | ) | |||
| | | | | | | | |
Net deferred tax assets |
$ | — | $ | — | |||
| | | | | | | | |
| | | | | | | | |
ASC 740 requires a valuation allowance to reduce the deferred tax assets reported if, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. After consideration of all the evidence, both positive and negative, the LLC entity has recorded a full valuation allowance against its deferred tax assets at December 31, 2012 and 2013 because the LLC entity's management has determined that is it more likely than not that these assets will not be realized. The increase in the valuation allowance of $7,163 in 2012 and $5,738 in 2013 primarily relates to the net loss incurred by the LLC entity during each period.
A reconciliation of the income tax expense at the federal statutory tax rate to the LLC entity's effective income tax rate follows:
| |
Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Statutory tax rate |
34.00 | % | 34.00 | % | |||
State taxes, net of federal benefit |
5.28 | % | 2.65 | % | |||
Research and development credit |
0.44 | % | 3.59 | % | |||
Gain on contribution |
0.00 | % | (15.71 | )% | |||
Other |
(0.21 | )% | 2.24 | % | |||
Change in valuation allowance |
(39.51 | )% | (26.77 | )% | |||
| | | | | | | | |
Effective tax rate |
0.00 | % | 0.00 | % | |||
| | | | | | | | |
| | | | | | | | |
F-33
Rhythm Holding Company, LLC
Notes to Consolidated Financial Statements (Continued)
(including data applicable to unaudited periods)
(Information as of June 30, 2014 and for the six months ended
June 30, 2013 and 2014 is unaudited)
(In thousands, except share and per share information)
14. Contingencies
Legal Matters
The LLC entity, from time to time, may be party to litigation arising in the ordinary course of its business. The LLC entity is not currently party to any litigation.
15. Subsequent Events
In April 2014, the corporate office operating lease term was amended to extend the termination date from July 31, 2014 to July 31 2015. The amendment does not include an option for further extension by either party beyond July 31, 2015. The monthly rent was increased from $11 per month to $12 during each month of the lease extension period.
F-34
Shares
Rhythm Pharmaceuticals, Inc.
Common Stock

PRELIMINARY PROSPECTUS
, 2014
Citigroup |
Cowen and Company |
Canaccord Genuity |
Oppenheimer & Co. | Cantor Fitzgerald & Co. |
Until , 2014 (25 days after the date of this prospectus), all dealers that effect buy, sell or trade shares of our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriter and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the fees and expenses in connection with the issuance and distribution of the securities being registered (excluding the underwriting discount). Except for the Securities and Exchange Commission registration fee and the FINRA filing fee, all amounts are estimates.
| |
Amount Paid or to be Paid |
|||
|---|---|---|---|---|
SEC registration fee |
$ | 11,109.00 | ||
FINRA filing fee |
* | |||
NASDAQ listing fee |
* | |||
Legal fees and expenses |
* | |||
Accounting fees and expenses |
* | |||
Printing expenses |
* | |||
Transfer and registrar fee |
* | |||
Miscellaneous |
* | |||
| | | | | |
Total |
$ | * | ||
| | | | | |
| | | | | |
- *
- To be provided by amendment.
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law authorizes a corporation's board of directors to grant, and authorizes a court to award, indemnity to officers, directors, and other corporate agents.
As permitted by Delaware law, our amended and restated certificate of incorporation provides that, to the fullest extent permitted by Delaware law, no director will be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director. Pursuant to Delaware law such protection would be not available for liability:
- •
- for any breach of a duty of loyalty to us or our stockholders;
- •
- for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law;
- •
- for any transaction from which the director derived an improper benefit; or
- •
- for an act or omission for which the liability of a director is expressly provided by an applicable statute, including unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law.
Our amended and restated certificate of incorporation also provides that if Delaware law is amended after the approval by our stockholders of the amended and restated certificate of incorporation to authorize corporate action further eliminating or limiting the personal liability of directors, then the liability of our directors will be eliminated or limited to the fullest extent permitted by Delaware law.
Our amended and restated bylaws further provide that we must indemnify our directors and officers to the fullest extent permitted by Delaware law. Our amended and restated bylaws also authorize us to indemnify any of our employees or agents and permit us to secure insurance on behalf
II-1
of any officer, director, employee or agent for any liability arising out of his or her action in that capacity, whether or not Delaware law would otherwise permit indemnification.
In addition, our amended and restated bylaws also provide that we are required to advance expenses to our directors and officers as incurred in connection with legal proceedings against them for which they may be indemnified and that the rights conferred in the amended and restated bylaws are not exclusive.
We intend to enter into indemnification agreements with each of our directors and executive officers. These agreements, among other things, would require us to indemnify each director and officer to the fullest extent permitted by Delaware law, the amended and restated certificate of incorporation and amended and restated bylaws, for expenses such as, among other things, attorneys' fees, judgments, fines, and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action by or in our right, arising out of the person's services as our director or executive officer or as the director or executive officer of any subsidiary of ours or any other company or enterprise to which the person provides services at our request. We also maintain directors' and officers' liability insurance.
The SEC has taken the position that personal liability of directors for violation of the federal securities laws cannot be limited and that indemnification by us for any such violation is unenforceable. The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors and officers for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder's investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions.
Item 15. Recent Sales of Unregistered Securities
Set forth below is information regarding securities we have issued within the past three years that were not registered under the Securities Act:
(1) Issuances of Capital Stock
On May 25, 2012, we issued and sold to investors an aggregate of 19,000,000 shares of our series B preferred stock, at a purchase price of $0.50 per share, for aggregate consideration of approximately $9,5000,000, which was paid for in cash.
On September 24, 2012, we issued and sold to investors an aggregate of 22,000,000 shares of our series B preferred stock, at a purchase price of $0.50 per share, for aggregate consideration of approximately $11,000,000, which was paid for in cash.
On November 20, 2012, we issued and sold to an investor an aggregate of 4,000,000 shares of our series B preferred stock, at a purchase price of $0.50 per share, for aggregate consideration of approximately $2,000,000, which was paid for in cash.
On February 8, 2013, we issued and sold to investors an aggregate of 18,000,000 shares of our series B preferred stock, at a purchase price of $0.50 per share, for aggregate consideration of approximately $9,000,000, which was paid for in cash.
On July 16, 2013, we issued and sold to investors an aggregate of 22,000,000 shares of our series B preferred stock, at a purchase price of $0.50 per share, for aggregate consideration of approximately $11,000,000, which was paid for in cash.
II-2
(2) Stock Option Grants
From March 9, 2010 to March 21, 2013, under the 2010 equity incentive plan of Rhythm Health, as amended, an aggregate of 17,824,000 shares of our common stock were authorized to be granted to our employees, directors, and consultants. These options to purchase shares of common stock were cancelled in exchange for an equal number of restricted common units as part of the Prior Restructuring on March 21, 2013.
(3) Restricted Common Unit Grants
From March 21, 2013 to July 7, 2014, we granted 12,403,700 restricted common units under our operating agreement, as amended, to employees, directors and consultants.
No underwriters were involved in the foregoing issuances of securities. The offers, sales and issuances of the securities described above were deemed to be exempt from registration under the Securities Act in reliance upon Rule 701 or Section 4(a)(2) of the Securities Act. The offers, sales and issuances of the securities that were deemed to be exempt in reliance on Rule 701 were transactions under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The offers, sales and issuances of the securities that were deemed to be exempt in reliance upon Section 4(a)(2) were each transactions not involving any public offering, and all recipients of these securities were accredited investors within the meaning of Rule 501 of Regulation D of the Securities Act who were acquiring the applicable securities for investment and not distribution and had represented that they could bear the risks of the investment. Each of the recipients of securities in these transactions had adequate access, through employment, business or other relationships, to information about us.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
| Number | Description | ||
|---|---|---|---|
| 1.1 | * | Form of Underwriting Agreement | |
3.1 |
2013 Rhythm Holding Company, LLC Operating Agreement, as currently in effect |
||
3.2 |
First Amendment to 2013 Rhythm Holding Company, LLC Operating Agreement, as currently in effect |
||
3.3 |
* |
Form of Certificate of Incorporation to become effective upon the Conversion |
|
3.4 |
* |
Form of Amended and Restated Certificate of Incorporation to become effective upon the closing of this offering |
|
3.5 |
* |
Form of Amended and Restated Bylaws to become effective upon the closing of this offering |
|
4.1 |
* |
Form of Common Stock Certificate of the Registrant |
|
4.2 |
* |
Investors' Rights Agreement, dated as of March 21, 2013, by and among Rhythm Holding Company, LLC and the investors set forth therein. |
|
5.1 |
* |
Opinion of Bingham McCutchen LLP |
|
10.1 |
*† |
Form of Indemnification Agreement by and between the Registrant and its directors and officers |
|
10.2 |
*† |
Rhythm Pharmaceuticals, Inc. 2014 Equity Incentive Plan and Forms of Option Agreement, Notice of Exercise |
|
II-3
| Number | Description | ||
|---|---|---|---|
| 10.3 | *† | Employment Agreement, dated February 26, 2010, by and between Rhythm Pharmaceuticals, Inc. and Bart Henderson. | |
10.4 |
*† |
Offer Letter, dated October 13, 2011, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and Keith Gottesdiener. |
|
10.5 |
*† |
Offer Letter, dated October 27, 2011, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and Lex Van der Ploeg. |
|
10.7 |
‡ |
Amended and Restated License Agreement, dated March 21, 2013, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and Ipsen Pharma S.A.S. |
|
10.8 |
‡ |
License Agreement, dated March 21, 2013, by and between Rhythm Metabolic, Inc. and Ipsen Pharma S.A.S. |
|
10.9 |
‡ |
Development and Manufacturing Services Agreement, dated as of July 2, 2010, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and Peptisyntha Inc. (n/k/a Peptisyntha SA), as amended on June 28, 2013. |
|
10.10 |
‡ |
Development and Manufacturing Services Agreement, dated July 17, 2013, by and between Rhythm Metabolic and Peptisyntha Inc. (n/k/a Peptisyntha SA). |
|
10.11 |
‡ |
Agreement regarding Therapeutic Pipeline Program 2013, dated August 13, 2013, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and The Michael J. Fox Foundation for Parkinson's Research |
|
10.12 |
Assignment and Assumption, dated as of April 25, 2011, by and among Echo Bridge Capital Management, LLC and North Hampton Partners Corporation (collectively, as Assignor), and Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.), as Assignee. |
||
10.13 |
First Amendment to Lease, dated March 24, 2014, by and between Gateway Longwood, Inc., as Landlord, and Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.), as Tenant. |
||
10.14 |
* |
Rhythm Pharmaceuticals, Inc. 2014 Employee Stock Purchase Plan. |
|
21.1 |
List of subsidiaries |
||
23.1 |
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm |
||
23.2 |
* |
Consent of Bingham McCutchen LLP. Reference is made to Exhibit 5.1. |
|
24.1 |
Power of Attorney. Reference is made to the signature page hereto. |
||
- *
- To
be filed by amendment
- †
- Indicates
management contract or compensatory plan
- ‡
- Indicates confidential treatment has been requested with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.
(b) Financial Statement Schedules
All schedules have been omitted because they are not required or because the required information is given in the financial statements or notes to those statements.
II-4
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
- (1)
- For
purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration
statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be
deemed to be part of this registration statement as of the time it was declared effective.
- (2)
- For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Boston, Commonwealth of Massachusetts on August 27, 2014.
| RHYTHM HOLDING COMPANY, LLC | ||||
By: |
/s/ KEITH M. GOTTESDIENER Keith M. Gottesdiener Chief Executive Officer |
|||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Keith M. Gottesdiener and Bart Henderson his true and lawful attorney-in-fact and agent with full power of substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to sign any registration statement for the same offering covered by the registration statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents or any of them, or his or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Name
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ KEITH M. GOTTESDIENER Keith M. Gottesdiener |
Chief Executive Officer and Director (Principal Executive Officer) | August 27, 2014 | ||
/s/ BART HENDERSON Bart Henderson |
President (Principal Financial and Accounting Officer) |
August 27, 2014 |
||
/s/ CHRISTIAN DE LA TOUR Christian de la Tour |
Director |
August 27, 2014 |
||
/s/ TODD FOLEY Todd Foley |
Director |
August 27, 2014 |
II-6
Name
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ ED MATHERS Ed Mathers |
Director | August 27, 2014 | ||
/s/ NEIL EXTER Neil Exter |
Director |
August 27, 2014 |
II-7
| Number | Description | ||
|---|---|---|---|
| 1.1 | * | Form of Underwriting Agreement | |
| 3.1 | 2013 Rhythm Holding Company, LLC Operating Agreement, as currently in effect | ||
| 3.2 | First Amendment to 2013 Rhythm Holding Company, LLC Operating Agreement, as currently in effect | ||
| 3.3 | * | Form of Certificate of Incorporation to become effective upon the Conversion | |
| 3.4 | * | Form of Amended and Restated Certificate of Incorporation to become effective upon the closing of this offering | |
| 3.5 | * | Form of Amended and Restated Bylaws to become effective upon the closing of this offering | |
| 4.1 | * | Form of Common Stock Certificate of the Registrant | |
| 4.2 | * | Investors' Rights Agreement, dated as of March 21, 2013, by and among Rhythm Holding Company, LLC and the investors set forth therein. | |
| 5.1 | * | Opinion of Bingham McCutchen LLP | |
| 10.1 | *† | Form of Indemnification Agreement by and between the Registrant and its directors and officers | |
| 10.2 | *† | Rhythm Pharmaceuticals, Inc. 2014 Equity Incentive Plan and Forms of Option Agreement, Notice of Exercise, | |
| 10.3 | *† | Employment Agreement, dated February 26, 2010, by and between Rhythm Pharmaceuticals, Inc. and Bart Henderson. | |
| 10.4 | *† | Offer Letter, dated October 13, 2011, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and Keith Gottesdiener. | |
| 10.5 | *† | Offer Letter, dated October 27, 2011, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and Lex Van der Ploeg. | |
| 10.7 | ‡ | Amended and Restated License Agreement, dated March 21, 2013, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and Ipsen Pharma S.A.S. | |
| 10.8 | ‡ | License Agreement, dated March 21, 2013, by and between Rhythm Metabolic, Inc. and Ipsen Pharma S.A.S. | |
| 10.9 | ‡ | Development and Manufacturing Services Agreement, dated as of July 2, 2010, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and Peptisyntha Inc. (n/k/a Peptisyntha SA), as amended on June 28, 2013. | |
| 10.10 | ‡ | Development and Manufacturing Services Agreement, dated July 17, 2013, by and between Rhythm Metabolic and Peptisyntha Inc. (n/k/a Peptisyntha SA). | |
| 10.11 | ‡ | Agreement regarding Therapeutic Pipeline Program 2013, dated August 13, 2013, by and between Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.) and The Michael J. Fox Foundation for Parkinson's Research | |
| 10.12 | Assignment and Assumption, dated as of April 25, 2011, by and among Echo Bridge Capital Management, LLC and North Hampton Partners Corporation (collectively, as Assignor), and Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.), as Assignee. | ||
II-8
| Number | Description | ||
|---|---|---|---|
| 10.13 | First Amendment to Lease, dated March 24, 2014, by and between Gateway Longwood, Inc., as Landlord, and Rhythm Pharmaceuticals, Inc. (n/k/a Rhythm Health, Inc.), as Tenant. | ||
| 10.14 | * | Rhythm Pharmaceuticals, Inc. 2014 Employee Stock Purchase Plan. | |
| 21.1 | List of subsidiaries | ||
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | ||
| 23.2 | * | Consent of Bingham McCutchen LLP. Reference is made to Exhibit 5.1. | |
| 24.1 | Power of Attorney. Reference is made to the signature page hereto. | ||
- *
- To
be filed by amendment
- †
- Indicates
management contract or compensation plan
- ‡
- Indicates confidential treatment has been requested with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and Exchange Commission pursuant to Rule 406 of the Securities Act of 1933, as amended.-
II-9
