Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a14-19392_18k.htm |
| EX-99.2 - EX-99.2 - AMICUS THERAPEUTICS, INC. | a14-19392_1ex99d2.htm |
|
|
Phase 3 Fabry Monotherapy Study (Study 012) Results Conference Call August 20, 2014 Exhibit 99.1 |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus’ candidate drug products, cash runway, ongoing collaborations and the timing and reporting of results from clinical trials Amicus’ candidate drug products. Words such as, evaluating Amicus but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2013. All forward-looking statements are qualified in their entirety by this cautionary statement, 2 and Amicus undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. |
|
|
Top Study 012-Line 18-Month Data: Key Findings Migalastat Successfully Met Co-Primary Endpoints of Comparability to ERT on Key Measures of Kidney Function Migalastat had a comparable effect to ERT on patients’ kidney function as measured by the change in both eGFR* and mGFR* with 100% overlap in the 95% confidence intervals for both measures Levels of plasma lyso-Gb3, an important biomarker of disease, remained low and stable in patients with amenable mutations who switched from ERT to migalastat Migalastat was generally safe and well-tolerated Of 46 subjects with GLP HEK-amenable mutations who completed Study 012, 46 (96%) elected to continue with the 12-month treatment extension and 45 remain on migalastat today as their only treatment for Fabry disease 3 * Estimated Glomerular Filtration Rate, ** Measured Glomerular Filtration Rate |
|
|
Phase 3 ATTRACT Study (Study 012) Migalastat 150 mg Every- Other-Day (QOD) 1.5:1 Randomization Stratified by Sex and *Proteinuria Open-Label Open-Label Migalastat 150 mg QOD Screening 2 Months MONTH MONTH ERT 18 30 BASELINE Optional Extension 12 Months Open-Label Treatment Period 18 Months Open-Label mGFR: BL 6-mo 12-mo 18-mo 30-mo 4 *Proteinuria Stratification: High (> 0.1 g/24h) Low (< 0.1 g/24h) |
|
|
Study 012 Statistical Analysis Plan Co-Primary Outcome Measures Assessing Comparability Between Migalastat and ERT on Key Measures of Kidney Function . Comparability between migalastat and ERT in eGFR and mGFR assessed by: – Overlap of 95% CI >50% – Means within 2.2 mL/min/1.73 m2/yr . Statistical model: – Primary analysis group, sex, age, efficacy using ANCOVA model accounting for treatment baseline GFR, and baseline proteinuria 5 |
|
|
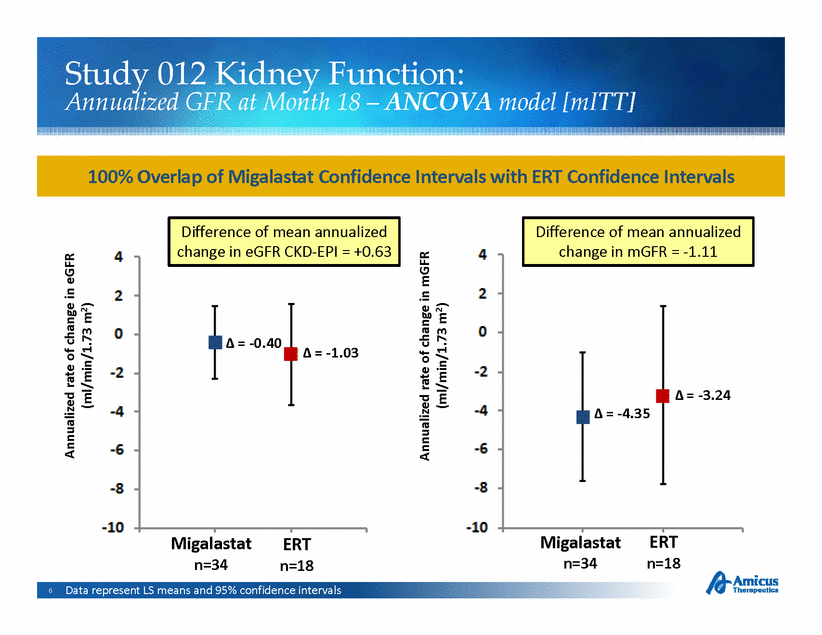
Study 012 Kidney Function: Annualized GFR at Month 18 – ANCOVA model [ mITT ] 100% Overlap of Migalastat Confidence Intervals with ERT Confidence Intervals mGFR Difference of mean annualized change in mGFR = -1.11 Difference of mean annualized change in eGFR CKD-EPI = +0.63 GFR of change in m /1.73 m2) of change in e /1.73 m2) . Migalastat n=34 ERT n=18 Migalastat n=34 ERT n=18 Data represent LS means and 95% confidence intervals |
|
|
Study 012 Kidney Function: Annualized Change in GFR at Month 18 – ANCOVA model [mITT] Migalastat Successfully Met Both Co-Primary Endpoints of Comparability to ERT on Key Measures of Kidney Function ANCOVA 95% CI overlap Difference of means Migalastat group (mean ± SEM) ERT group (mean ± SEM) Difference [mITT] >50%? within 2.2? n = 34 n = 18 in means eGFR (CKD-EPI) . . -0.40 ± 0.93 -1.03 ± 1.29 +0.63 mGFR (iohexol) . . -4.35 ± 1.64 -3.24 ± 2.27 -1.11 7 Terms in the model included treatment, sex, age, baseline mGFR, baseline proteinuria; LS means are presented |
|
|
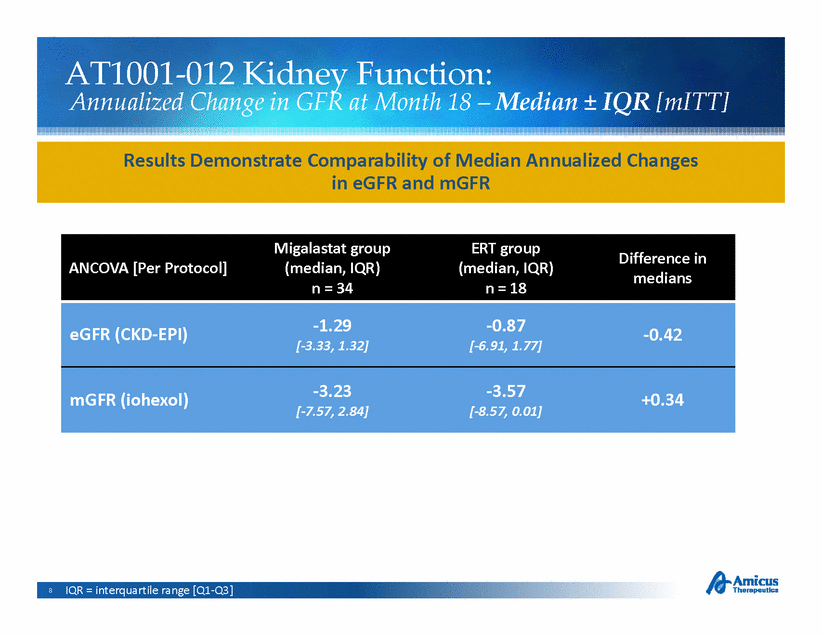
AT1001-Function: 012 Kidney Annualized Change in GFR at Month 18 – Median ± IQR [mITT] Results Demonstrate Comparability of Median Annualized Changes in eGFR and mGFR ANCOVA [Per Protocol] Migalastat group (median, IQR) ERT group (median, IQR) Difference in medians n = 34 n = 18 eGFR (CKD-EPI) -1.29 [-3.33, 1.32] -0.87 [-6.91, 1.77] -0.42 mGFR (iohexol) -3.23 [-7.57, 2.84] -3.57 [-8.57, 0.01] +0.34 8 IQR = interquartile range [Q1-Q3] |
|
|
Disease Substrate in Plasma (Plasma Lyso-Gb3) No Change in Plasma Lyso-Gb3 over 18 months Following Switch From ERT to Migalastat in Subjects with Amenable Mutations . In subjects with amenable mutations the plasma lyso-Gb3 levels were comparable for migalastat and ERT. In two male subjects with non-amenable mutations plasma lyso-Gb3 increased 9 Data points represent the mean, Error bars are SD; Based on subjects with available samples for this analysis following switch from ERT as compared to two (1M, 1F) who remained on ERT |
|
|
Safety Summary Common AEs (>10%) Migalastat Was Generally Safe and Well-Tolerated Migalastat ERT N subjects 36 21 n subjects with TEAEs (%) 34 (94%) 20 (95%) Nasopharyngitis 33% 33% Headache 25% 24% Dizziness 17% 10% Influenza 14% 19% Abdominal Pain 14% 10% Diarrhea 14% 10% Nausea 14% 10% Back Pain 11% 14% Upper Respiratory Tract Infection 11% 5% Urinary Tract Infection 11% 5% Cough 8% 24% Vomiting 8% 14% Sinusitis 8% 14% Arthralgia 8% 10% Bronchitis 6% 14% Edema Peripheral 6% 10% Vertigo 3% 10% Dry Mouth 3% 10% 10 Gastritis 3% 10% Pain In Extremity 3% 10% Dyspnea 3% 10% Procedural Pain - 10% |
|
|
Migalastat Monotherapy Experience 97 Patients Today Take Migalastat HCl as Only Therapy for Fabry Disease1 Total Fabry patients who have taken migalastat: Total subjects on migalastat today: Total patient years of therapy: 143 97 377 (drug no drug-related SAEs) Maximum years on therapy: 8.6 years Average retention rate into next study*: 96% 11 1 All patients are receiving investigational drug, migalastat HCl, as part of ongoing clinical trials *Retention defined as # of patients who complete a study and chose to enter extension, e.g., 011 12-mo into 12-mo extension or 011 into 041 As of Aug 4 2014 |
|
|
Global Regulatory Strategy Pursuing Fastest Path to Approval for Migalastat • Totality of clinical data • 8+ years of data in extension studies • Complete data set from Phase 3 studies (011 and 012) • Centralized Procedure underway • EMA Pre Pre-Submission Meeting planned for 4Q 14 • Clear regulatory pathway • Non Non-inferiority to ERT (Study 012) 12 |