Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Cytosorbents Corp | v386965_8k.htm |
| EX-99.3 - PRESS RELEASE - Cytosorbents Corp | v386965_ex99-3.htm |
| EX-99.1 - TRANSCRIPT OF CONFERENCE CALL - Cytosorbents Corp | v386965_ex99-1.htm |
Exhibit 99.2

Cyto Sorbents Corporation OTCBB: CTSO An Emerging Leader in Critical Care Immunotherapy Q2 2014 Review – August 12, 2014

Safe Harbor Statement Statements in this presentation regarding CytoSorbents Corporation and its operating subsidiary CytoSorbents, Inc that are not historical facts are forward - looking statements and are subject to risks and uncertainties that could cause actual future events or results to differ materially from such statements . Any such forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . It is routine for our internal projections and expectations to change . Although these expectations may change, we are under no obligation to inform you if they do . Actual events or results may differ materially from those contained in the projections or forward - looking statements . The following factors, among others, could cause our actual results to differ materially from those described in a forward - looking statement : our history of losses ; potential fluctuations in our quarterly and annual results ; competition, inability to achieve regulatory approval for our device, technology systems beyond our control and technology - related defects that could affect the companies’ products or reputation ; risks related to adverse business conditions ; our dependence on key employees ; competition for qualified personnel ; the possible unavailability of financing as and if needed ; and risks related to protecting our intellectual property rights or potential infringement of the intellectual property rights of third parties . This list is intended to identify only certain of the principal factors that could cause actual results to differ from those discussed in the forward - looking statements . Readers are referred to a discussion of important risk factors detailed in the Company’s Form 10 - K filed with the Securities and Exchange Commission on March 31 , 2014 and other reports and documents filed from time to time by us, which are available online at www . sec . gov .

3 Conference Call Participants Dr . Phillip Chan, MD, PhD Chief Executive Officer and President Vincent Capponi, MS Chief Operating Officer Kathleen Bloch, MBA, CPA Chief Financial Officer Dr . Christian Steiner, MD Vice President of Sales and Marketing Christopher Cramer, MS, MBA Vice President of Business Development (Absent) Moderator: Amy Vogel – CytoSorbents Corporation

4 Cyto Sorbents is an Emerging Leader in the $20B Critical Care Immunotherapy Space Leading the Prevention or Treatment of Life - Threatening Inflammation in the ICU

5 Inflammation Plays a Major Role in Nearly Every Known Disease • Life threatening conditions like sepsis & trauma • Autoimmune diseases like rheumatoid arthritis, inflammatory bowel, psoriasis, and lupus • Heart disease, peripheral artery disease • Cancer, cancer cachexia, graft vs host disease • Neurodegenerative diseases such as Alzheimer’s, multiple sclerosis (MS), Parkinson’s • Many, many others others Uncontrolled inflammation wreaks havoc on the body and can be deadly
6 Severe Inflammation Drives Organ Failure Organ failure occurs when vital organs stop working, causing nearly half of all deaths in the ICU, but little can be done to treat or prevent it today

7 Cyto Sorb ® Removes the Fuel to the Fire • CytoSorb ® represents a powerful immunotherapy to control inflammation • Approved in the European Union as the only specifically approved extracorporeal cytokine filter • Clinically proven to reduce key cytokines in blood in critically - ill patients • Approved for use in any situation where cytokines are elevated • Safe: More than 3,000 human treatments, with no serious device related adverse events reported
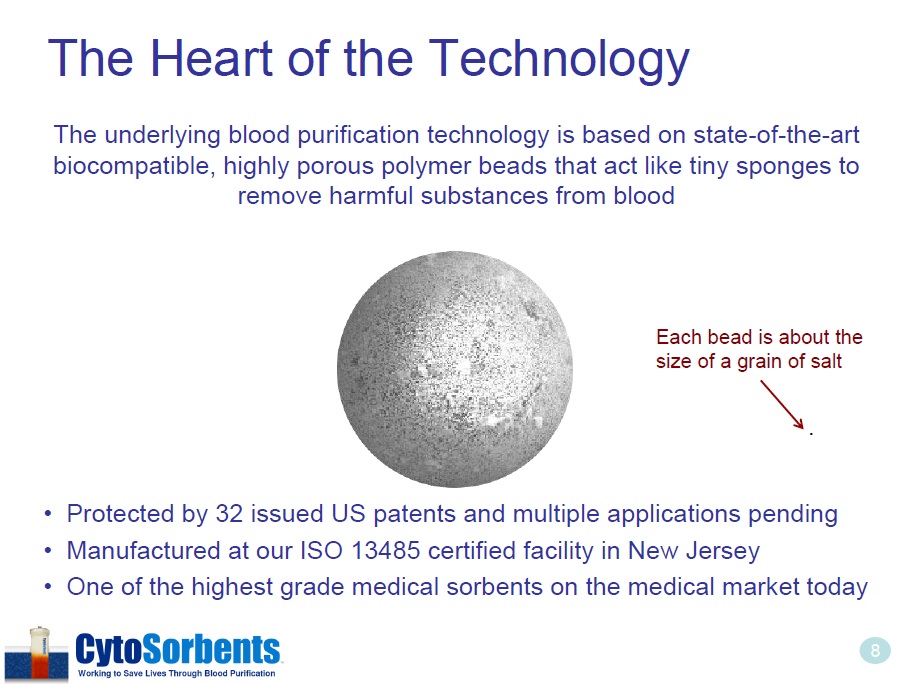
8 The Heart of the Technology The underlying blood purification technology is based on state - of - the - art biocompatible, highly porous polymer beads that act like tiny sponges to remove harmful substances from blood • Protected by 32 issued US patents and multiple applications pending • Manufactured at our ISO 13485 certified facility in New Jersey • One of the highest grade medical sorbents on the medical market today . Each bead is about the size of a grain of salt

9 Goal: To Prevent or Treat Organ Failure Sepsis ARDS Burn Injury Trauma Pancreatitis Influenza Surgical The Potential to Revolutionize Critical Care Medicine Improve Patient Outcome and Survival Decrease Costs Of ICU and Patient Care
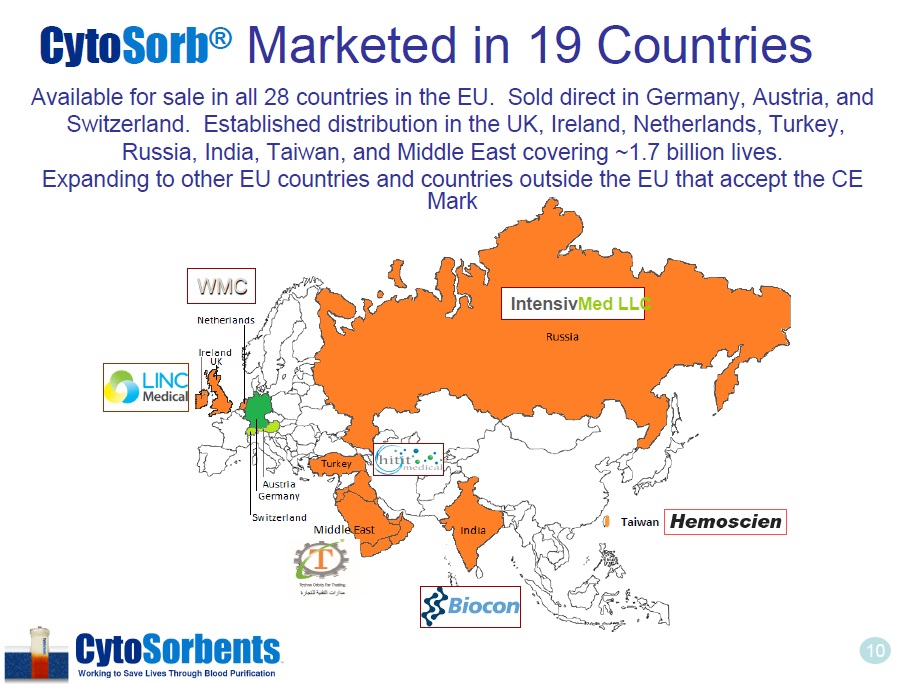
10 Available for sale in all 28 countries in the EU. Sold direct in Germany, Austria, and Switzerland. Established distribution in the UK, Ireland, Netherlands, Turkey, Russia, India, Taiwan, and Middle East covering ~1.7 billion lives. Expanding to other EU countries and countries outside the EU that accept the CE Mark WMC Intensiv Med LLC Taiwan Cyto Sorb ® Marketed in 19 Countries

11 • US Dept of Health and Human Services awarded $0.5M grant (2010) for therapies that can save lives and reduce costs under the QTDP Program • NIH grant awarded $7M five year (2006 - 2010) to University of Pittsburgh and Dr. John Kellum to research CytoSorb bead for treatment of sepsis • NIH/NHLBI awarded $0.2M Phase I SBIR to advance the HemoDefend purification technology intended to improve the quality and safety of blood transfusions (2013 - present) $15+ Million in US Government Support • DARPA awarded $3.8M five year (2012 - present) contract as part of “Dialysis - Like Therapeutics” program to treat sepsis by removing cytokines and pathogen - derived toxins • U.S. Army awarded $1.15M SBIR contracts for trauma and burn injury research (2011 - present) • U.S. Air Force is funding a 30 - patient human pilot study in trauma valued at $3M (2013 - present). FDA approved trial that has begun enrollment

12 Q2 2014 Operating and Financial Highlights
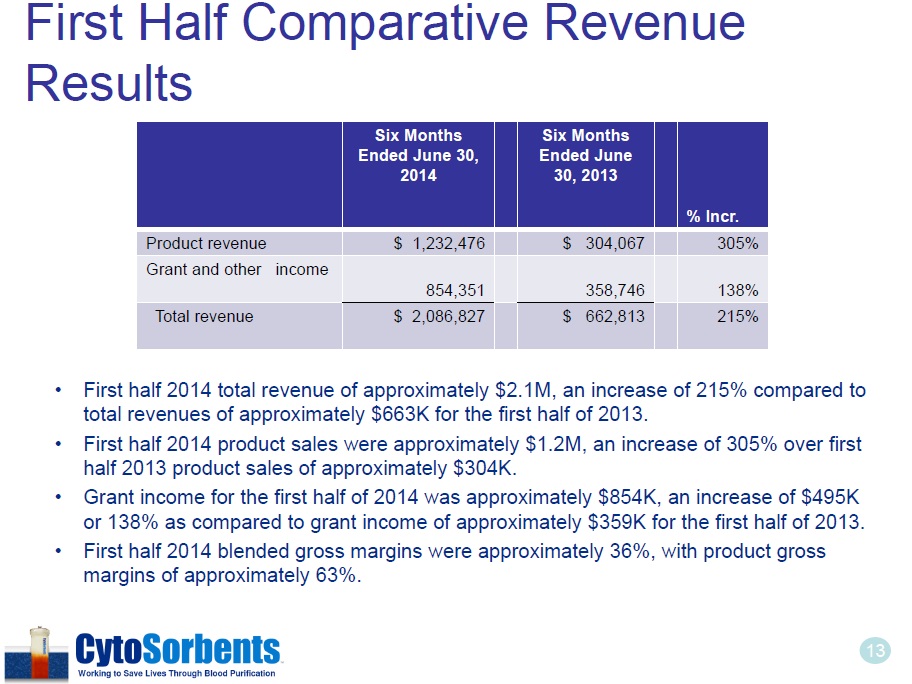
13 First Half Comparative Revenue Results • First half 2014 total revenue of approximately $2.1M, an increase of 215% compared to total revenues of approximately $663K for the first half of 2013. • First half 2014 product sales were approximately $1.2M, an increase of 305% over first half 2013 product sales of approximately $304K. • Grant income for the first half of 2014 was approximately $854K, an increase of $495K or 138% as compared to grant income of approximately $359K for the first half of 2013. • First half 2014 blended gross margins were approximately 36%, with product gross margins of approximately 63%. Six Months Ended June 30, 2014 Six Months Ended June 30, 2013 % Incr . Product revenue $ 1,232 ,476 $ 304,067 305% Grant and other income 854,351 358,746 138% Total revenue $ 2,086,827 $ 662,813 215%
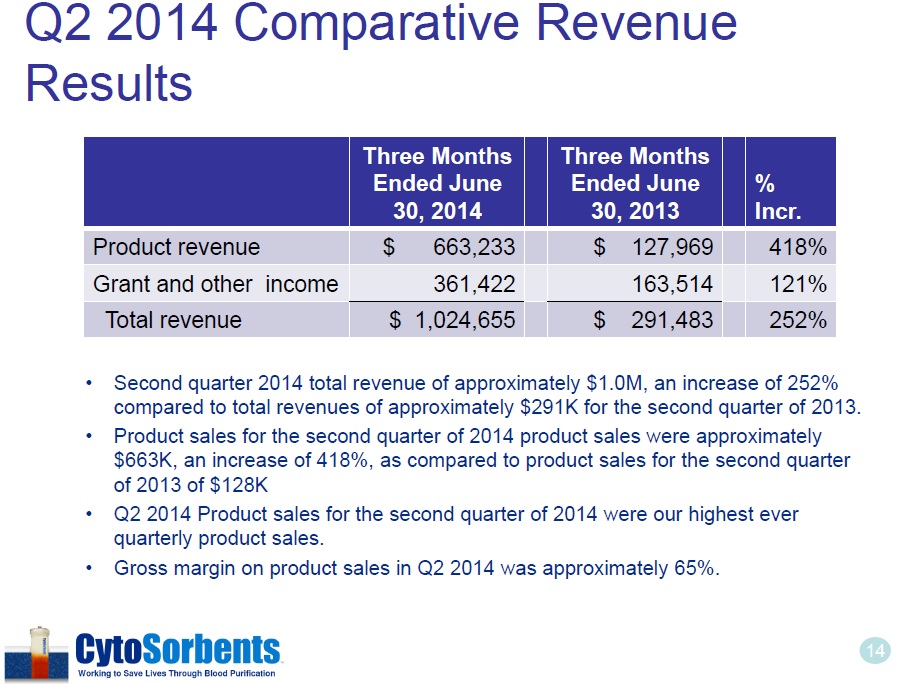
14 Q2 2014 Comparative Revenue Results • Second quarter 2014 total revenue of approximately $1.0M, an increase of 252% compared to total revenues of approximately $291K for the second quarter of 2013. • Product sales for the second quarter of 2014 product sales were approximately $663K, an increase of 418%, as compared to product sales for the second quarter of 2013 of $128K • Q2 2014 Product sales for the second quarter of 2014 were our highest ever quarterly product sales. • Gross margin on product sales in Q2 2014 was approximately 65%. Three Months Ended June 30, 2014 Three Months Ended June 30, 2013 % Incr . Product revenue $ 663,233 $ 127,969 418% Grant and other income 361,422 163,514 121% Total revenue $ 1,024,655 $ 291,483 252%
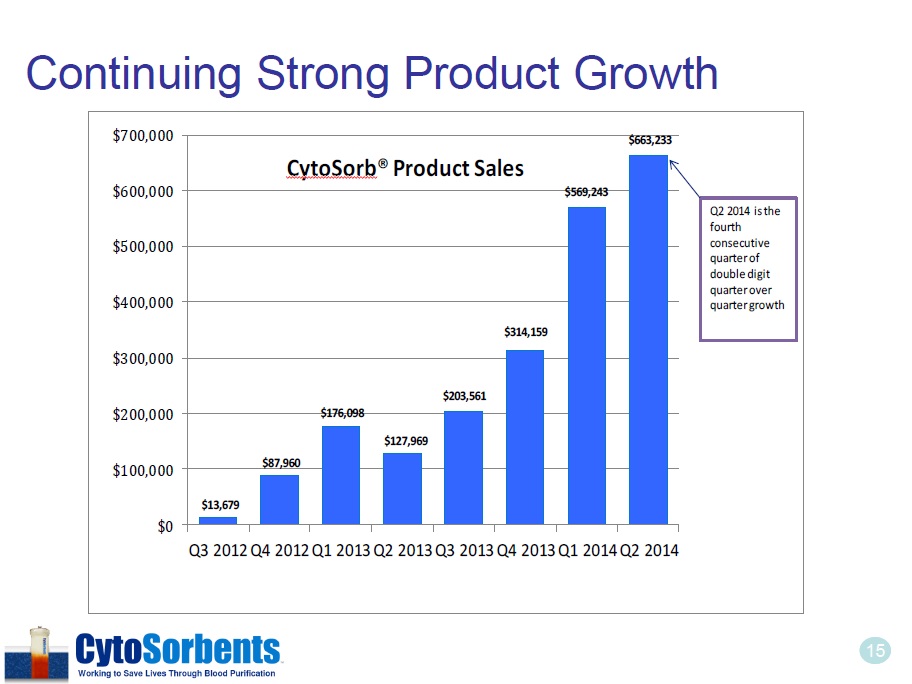
15 Continuing Strong Product Growth $13,679 $87,960 $176,098 $127,969 $203,561 $314,159 $569,243 $663,233 $0 $100,000 $200,000 $300,000 $400,000 $500,000 $600,000 $700,000 Q3 2012 Q4 2012 Q1 2013 Q2 2013 Q3 2013 Q4 2013 Q1 2014 Q2 2014 CytoSorb ® Product Sales Q2 2014 is the fourth consecutive quarter of double digit quarter over quarter growth 15
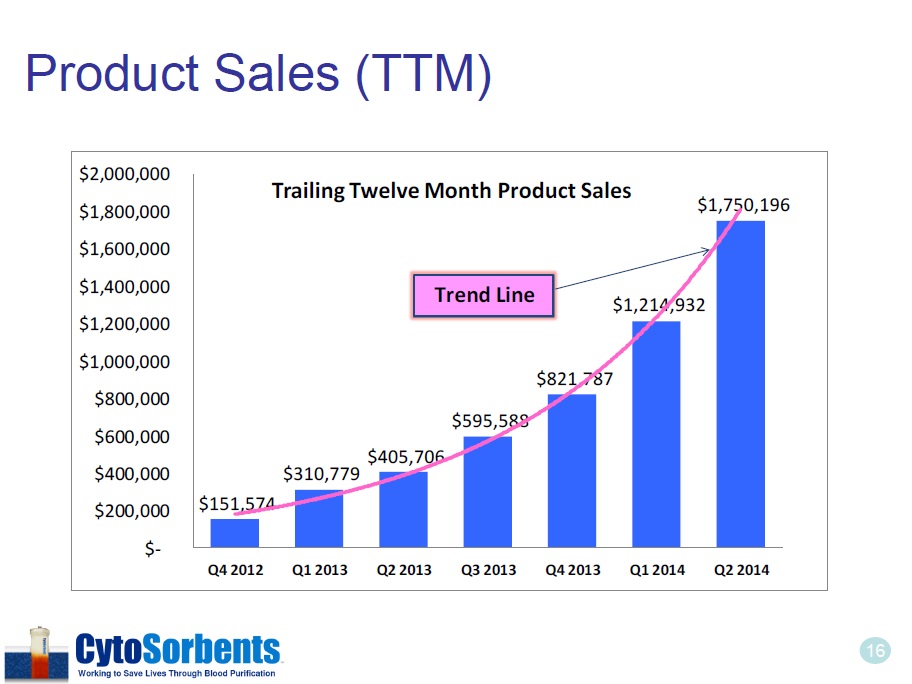
16 Product Sales (TTM) $151,574 $310,779 $405,706 $595,588 $821,787 $1,214,932 $1,750,196 $- $200,000 $400,000 $600,000 $800,000 $1,000,000 $1,200,000 $1,400,000 $1,600,000 $1,800,000 $2,000,000 Q4 2012 Q1 2013 Q2 2013 Q3 2013 Q4 2013 Q1 2014 Q2 2014 Trailing Twelve Month Product Sales Trend Line

17 The Path to Up - listing Completed: x Met with NYSE and NASDAQ x Evaluation of listing requirements x Transitioned to a new SEC counsel to provide guidance during up - listing process x Adopted Code of Business Conduct and Ethics x Updating our Insider Trading Policy x Improvements to our system of internal control In Process: • Fully Independent Audit Committee • Selecting third party providers to assist with documentation and testing of our system of internal controls • Increasing exposure to institutional investors • Simplify capital structure • Simultaneous reverse stock split with up - listing
18 U.S. Cardiac Surgery Pivotal Trial
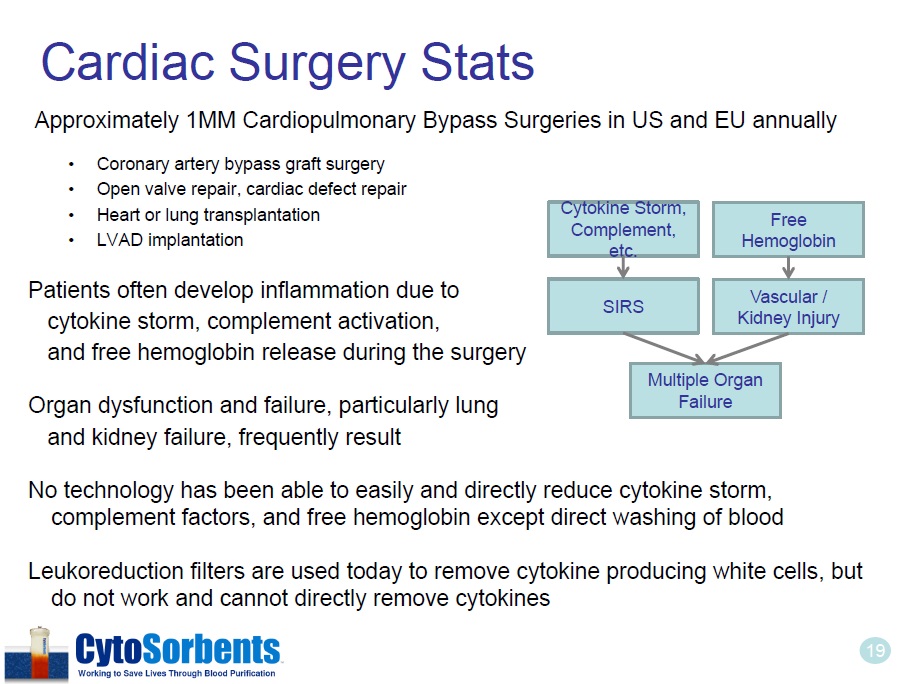
19 Cardiac Surgery Stats Approximately 1MM Cardiopulmonary Bypass Surgeries in US and EU annually • Coronary artery bypass graft surgery • Open valve repair, cardiac defect repair • Heart or lung transplantation • LVAD implantation Patients often develop inflammation due to cytokine storm, complement activation, and free hemoglobin release during the surgery Organ dysfunction and failure, particularly lung and kidney failure, frequently result No technology has been able to easily and directly reduce cytokine storm, complement factors, and free hemoglobin except direct washing of blood Leukoreduction filters are used today to remove cytokine producing white cells, but do not work and cannot directly remove cytokines Cytokine Storm, Complement, etc. SIRS Multiple Organ Failure Free Hemoglobin Vascular / Kidney Injury
20 Intra - operative versus Post - Operative
21 U.S. Cardiac Surgery Pivotal Trial 2 1 1) Inflammatory Biomarker Reduction • Randomized controlled multi - center trial <150 patients • Enrich for cardiac surgery patients at high risk for inflammation and hemolysis and treat DURING surgery with CytoSorb in a bypass circuit • Primary endpoint: Reduction of inflammatory biomarkers • Regulatory path: De novo 510(k) 2) Intra - Operative Usage of CytoSorb to Prevent Post - operative Complications • Randomized controlled multi - center trial • Enrich for cardiac surgery patients at high risk for inflammation and hemolysis and treat DURING surgery with CytoSorb in a bypass circuit • Primary endpoint: Reduction in incidence of organ dysfunction • Regulatory path: PMA 3) Post - Operative Usage of CytoSorb to Treat Post - operative SIRS • Multi - center randomized controlled trial, PMA path • CytoSorb is used AFTER surgery to treat patients who develop SIRS • Primary endpoint: Relative reduction in incidence of organ dysfunction • Regulatory path: PMA Three potential paths to U.S. regulatory approval for CytoSorb in cardiac surgery, pending discussions with FDA. Expect to submit IDE application in Q4 2014
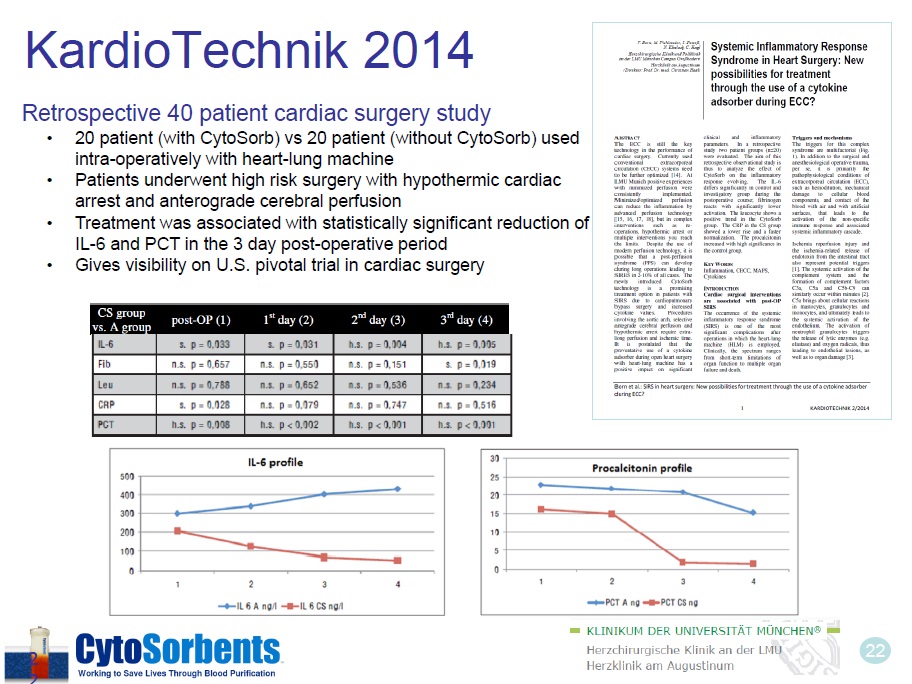
22 KardioTechnik 2014 Retrospective 40 patient cardiac surgery study • 20 patient (with CytoSorb) vs 20 patient (without CytoSorb) used intra - operatively with heart - lung machine • Patients underwent high risk surgery with hypothermic cardiac arrest and anterograde cerebral perfusion • Treatment was associated with statistically significant reduction of IL - 6 and PCT in the 3 day post - operative period • Gives visibility on U.S. pivotal trial in cardiac surgery 2 2
23 Ebola Virus • Ebola is one of the most deadly viruses known with a very high mortality rate of 50 - 90% depending upon the strain, and very contagious (bodily fluids) • Following an incubation phase of 2 - 21 days, has an abrupt onset of symptoms including high fever, chills, weakness and body pain, followed by more severe symptoms including diarrhea, cough, headache, and bleeding with vomiting of blood or blood in stool • Patients typically die with time to death (from onset of symptoms) of 6 - 16 days. Culminates in a cytokine release syndrome • Key to why Ebola is so deadly is its ability to evade the immune response, resulting in advanced infection that culminates in cytokine storm, deadly inflammation, and multiple organ failure • Initial suppression of the anti - viral cytokine immune response allows rampant viral replication • Release of soluble viral glycoproteins (sGP) that interfere with white blood cell activation and act as a decoy so that antibodies cannot neutralize the virus • CytoSorb may have benefit by reducing cytokine storm and sGP, buying time for the immune system to kill the virus

24 Could Cyto Sorb ® Help? The 2014 Ebola epidemic in West Africa, called an “international emergency” by the WHO, continues to grow and has already infected nearly 1,900 people, and claimed more than 1,000 lives. Epidemic is expected to continue throughout the year. Our Strategy for outreach: • WHO • FDA • CDC • Government agencies such as USAMRIID (US Army Medical Research Institute of Infectious Diseases) • Non - profit organizations • Hospitals treating Ebola patients inside/outside of West Africa • Europe • U.S. 2 4

25 Critical Care, High Risk Surgery Our Bead Technology Enables a Diverse and Valuable Pipeline Blood Collection & Transfusion CT Imaging, Interventional Radiology Contrast Sorb Drug Overdose, Chemo Removal Drug Sorb Beta Sorb Improving Dialysis Under Development CE Mark Approved
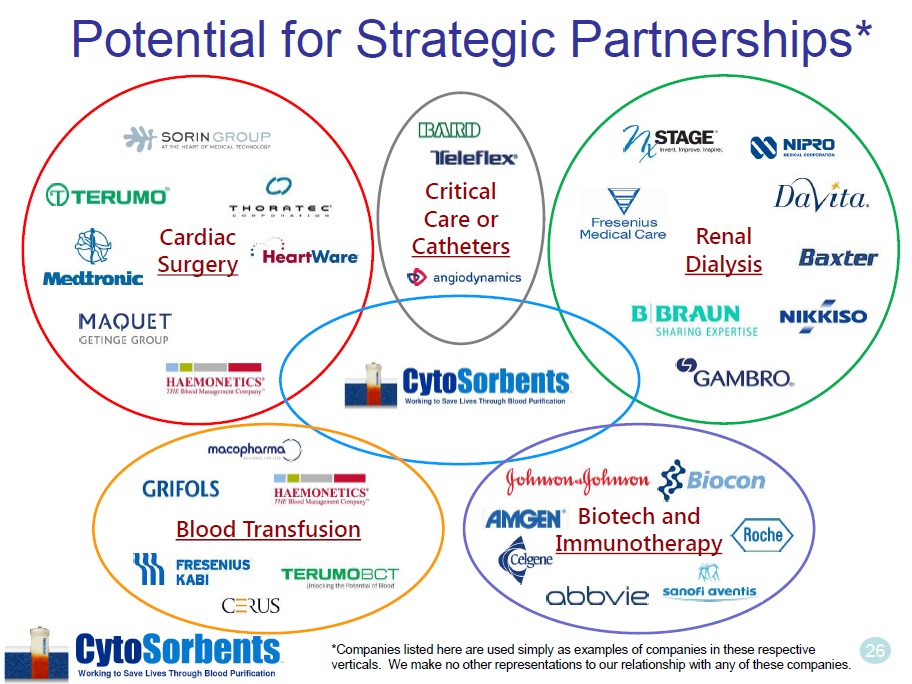
26 Potential for Strategic Partnerships* Cardiac Surgery Renal Dialysis Blood Transfusion Biotech and Immunotherapy Critical Care or Catheters *Companies listed here are used simply as examples of companies in these respective verticals. We make no other representations to our relationship with any of these companies .
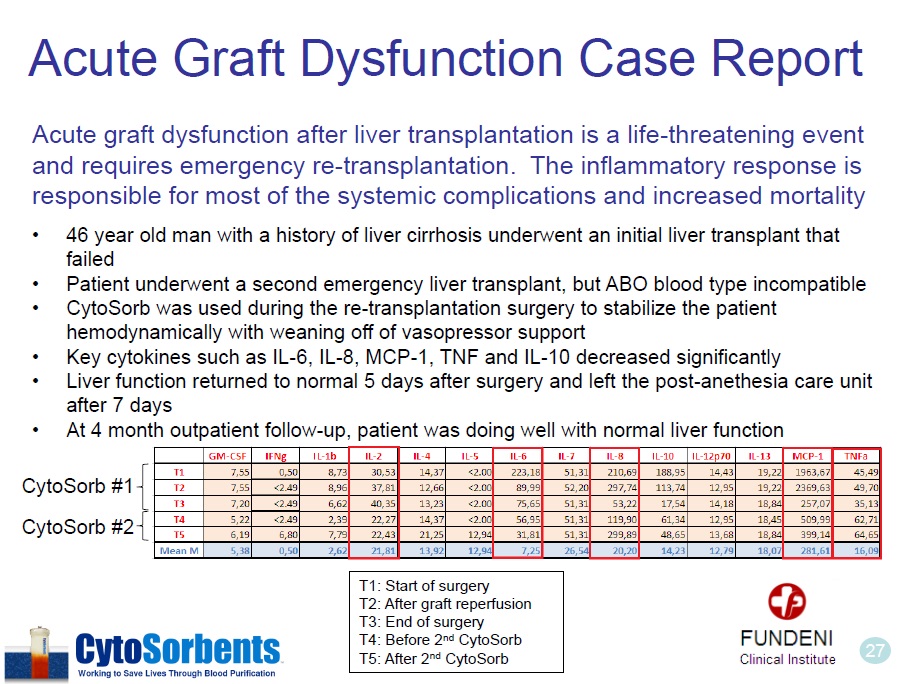
27 Acute Graft Dysfunction Case Report Acute graft dysfunction after liver transplantation is a life - threatening event and requires emergency re - transplantation. The inflammatory response is responsible for most of the systemic complications and increased mortality • 46 year old man with a history of liver cirrhosis underwent an initial liver transplant that failed • Patient underwent a second emergency liver transplant, but ABO blood type incompatible • CytoSorb was used during the re - transplantation surgery to stabilize the patient hemodynamically with weaning off of vasopressor support • Key cytokines such as IL - 6, IL - 8, MCP - 1, TNF and IL - 10 decreased significantly • Liver function returned to normal 5 days after surgery and left the post - anethesia care unit after 7 days • At 4 month outpatient follow - up, patient was doing well with normal liver function T1: Start of surgery T2: After graft reperfusion T3: End of surgery T4: Before 2 nd CytoSorb T5: After 2 nd CytoSorb CytoSorb #1 CytoSorb #2
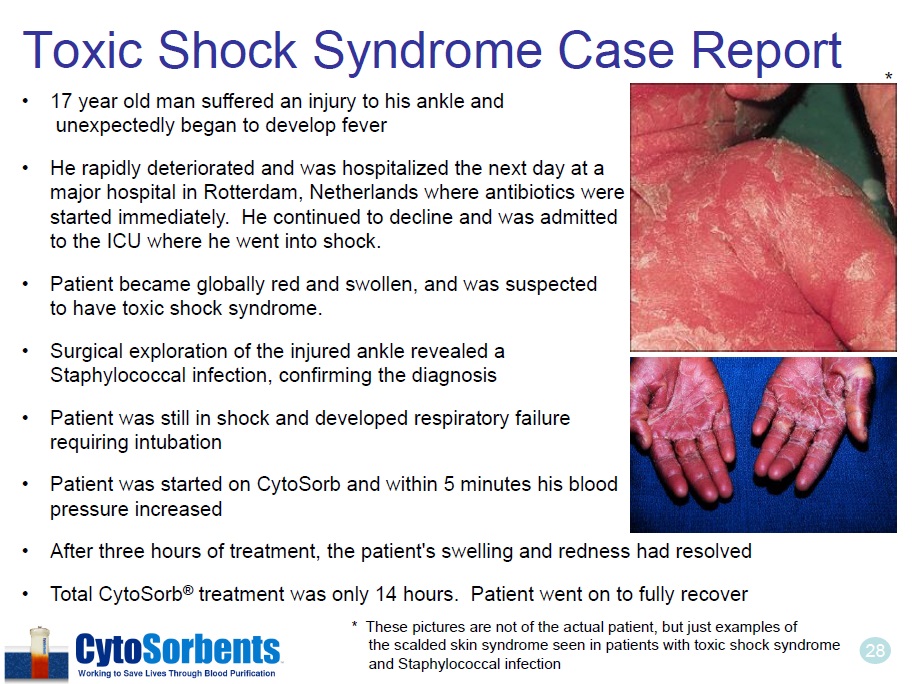
28 Toxic Shock Syndrome Case Report • 17 year old man suffered an injury to his ankle and unexpectedly began to develop fever • He rapidly deteriorated and was hospitalized the next day at a major hospital in Rotterdam, Netherlands where antibiotics were started immediately. He continued to decline and was admitted to the ICU where he went into shock. • Patient became globally red and swollen, and was suspected to have toxic shock syndrome. • Surgical exploration of the injured ankle revealed a Staphylococcal infection, confirming the diagnosis • Patient was still in shock and developed respiratory failure requiring intubation • Patient was started on CytoSorb and within 5 minutes his blood pressure increased • After three hours of treatment, the patient’s swelling and redness had resolved • Total CytoSorb ® treatment was only 14 hours. Patient went on to fully recover * * These pictures are not of the actual patient, but just examples of the scalded skin syndrome seen in patients with toxic shock syndrome and Staphylococcal infection

29 Phillip P. Chan, MD, PhD - CEO 7 Deer Park Drive, Suite K Monmouth Junction, NJ 08852 pchan@cytosorbents.com Cyto Sorbents Corporation OTCBB: CTSO The Rise of An Emerging Critical Care Immunotherapy Company Q&A Session
