Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Savara Inc | d773164d8k.htm |
Exhibit 99.1

| NYSE MKT: MSTX Canaccord Genuity 34th Annual Growth Conference Brian M. Culley, CEO August 14, 2014 |

| 2 Forward-Looking Statements This presentation includes forward-looking statements about our business prospects, financial position, and development of MST-188 and AIR001 for therapeutic use in humans. Any statement that is not a statement of historical fact should be considered a forward-looking statement. Because forward-looking statements relate to the future, they are subject to inherent risks, uncertainties and changes in circumstances that are difficult to predict. Actual events or performance may differ materially from our expectations indicated by these forward- looking statements due to a number of factors, including, but not limited to, results of our pending and future clinical studies, the timeline for clinical and manufacturing activities and regulatory approval; our dependency on third parties to conduct our clinical studies and manufacture our clinical trial material; our ability to raise additional capital, as needed; our ability to establish and protect proprietary rights related to our product candidates; and other risks and uncertainties more fully described in our press releases and our filings with the SEC, including our annual report on Form 10-K filed with the SEC on March 26, 2014. We caution you not to place undue reliance on any of these forward-looking statements, which speak only as of the date of this presentation. We do not intend to update any forward-looking statement included in this presentation to reflect events or circumstances arising after the date of the presentation, except as may be required by law. |

| Publicly-traded biopharmaceutical company based in San Diego Developing MST-188 for diseases of membrane dysfunction Near-term focus on rare ("orphan") diseases Sickle Cell Disease (SCD) - phase 3 enrolling Acute Limb Ischemia (ALI) - phase 2 enrolling Longer-term growth into larger markets Heart Failure Stroke Recently acquired Aires Pharmaceuticals (AIR001) Phase 2 asset in Pulmonary Hypertension (PH) Complementary to MST-188 3 Corporate Overview |

| 4 Lead Program MST-188 |

| API Structure: CMC: Large polymer (8,500 Daltons) manufactured by chemical synthesis and proprietary purification process Administration: IV infusion 5 MST-188 Overview HO - (CH2CH2O)79- (CH2CHO)30- (CH2CH2O)79- H CH3 | 5 |

| No Affinity for Healthy Cell Membranes... But Adheres to Damaged Cell Membranes Hydrophobic core adheres to hydrophobic domains in circulation (e.g., damaged cell membranes) 6 MST-188 Mechanism of Action |
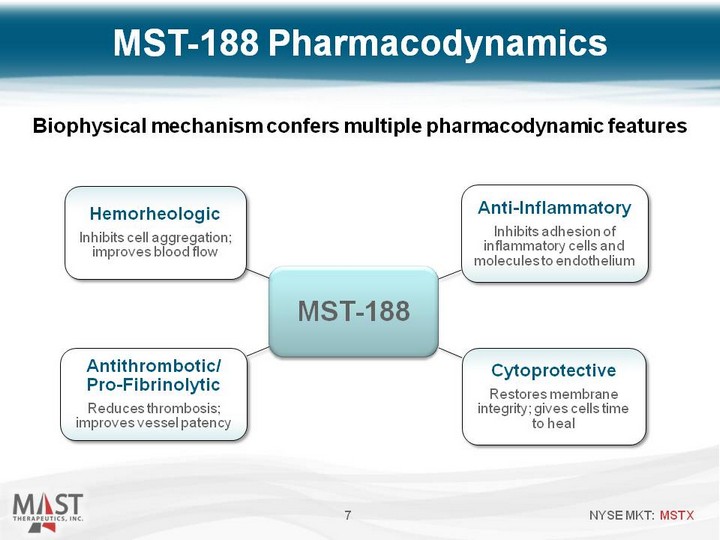
| 7 Biophysical mechanism confers multiple pharmacodynamic features MST-188 Pharmacodynamics |

| MST-188 Clinical Development 8 Preclinical Phase 1 Phase 2 Phase 3 2014 2014 2014 2014 2014 Sickle Cell Disease (orphan) Acute Limb Ischemia* (orphan) Acute Heart Failure Stroke* Enrolling Planned initiation: 1H 2015 Planned initiation: 2H 2014 *In combination with thrombolytics Enrolling |

| 9 Sickle Cell Disease |

| A chronic, genetic disorder and rare (orphan) disease Affects 90,000 to 100,000 people in the U.S. Characterized by severe deformation (i.e., "sickling") of red blood cells Hallmark of disease is a "vaso-occlusive crisis" Exceedingly painful condition Leading cause of hospitalization Significant unmet need No approved agents to shorten duration or severity of crisis Standard of care (hydration and analgesics) unchanged for >10 years Vaso-occlusion is associated with early death Obstructed blood flow -> hypoxia -> tissue death -> organ failure Average age at death; 42 years (males), 48 years (females) 10 Overview of Sickle Cell Disease |

| The Complex Pathophysiology of Sickle Cell Disease Requires Multiple Sites of Intervention sRBC Adherence to Endothelium VASO-OCCLUSION ORGAN DAMAGE/FAILURE ANEMIA PAIN INTRAVASCULAR Hemolysis NITRIC OXIDE DEPLETION VASOCONSTRUCTION INFLAMMATION ACTIVATION OF COAGULATION TISSUE ISCHEMIA LOCAL HYPOXIA STRESS LARGE VESSEL DAMAGE Microvascular DAMAGE WBC adherence to RBCs Activated WBCs WBC adherence to endothelium Activated platelets Release of Inflammatory Cytokines Endothelial Cell Activation Free Radical Formation Loss of antithrombotic action Loss of anti- inflammatory action Release of Inflammatory Cytokines Endothelial Cell Retraction/ Exposed Subendothelial Matrix Release of Inflammatory Cytokines Free Radical Formation Stimulation of nociceptors Increased Epinephrine Levels Activated platelets Endothelial Cell Retraction/ Exposed Subendothelial Matrix Increased Platelet Count Activated WBCs Increased WBC Count Release of Inflammatory Cytokines Externalization of phosphatidylserine Polymerization of HbS Membrane Damage Red Cell Dehydration Increased Reticulocyte Counts Plasma Free Hb Irreversibly Sickled Cells Red Cell Arginase Release |

| 12 MST-188: Reduces adhesion of cells to endothelium (anti-inflammatory) Reduces RBC aggregation, improves RBC deformability, lowers viscosity, and restores flow (rheologic) Vaso-Occlusion: Adhesion of poorly-deformable, "sticky" cells to endothelium Physical entrapment of rigid, sickled cells and vessel obstruction Role of MST-188 in Sickle Cell Disease 12 Ischemia: RBCs cannot traverse occlusion to deliver oxygen to tissue, resulting in ischemia, hypoxia and infarction |

| MST-188 Placebo Before Infusion (Crisis Baseline) Velocity (mm/sec) 0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2-Hours After Loading Infusion 7-Hours After Loading Infusion Source: J. Investig. Med. 2004;52(6):402-6 (p = 0.00003) 13 MST-188 improved microvascular blood flow in SCD patients in crisis 13 MST-188 Improves Blood Flow Red cell velocity (mm/s) measured by video microscopy in nine sickle cell patients with vaso-occlusive crisis. |
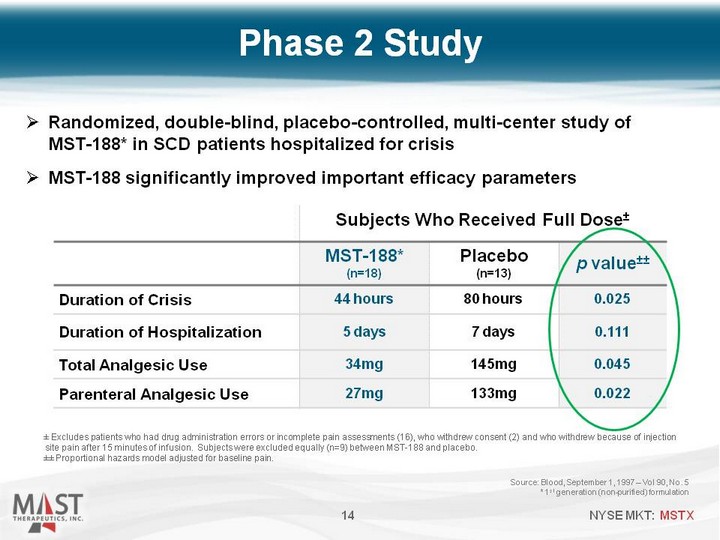
| Phase 2 Study Source: Blood, September 1, 1997 - Vol 90, No. 5 * 1st generation (non-purified) formulation 14 Subjects Who Received Full Dose+- Subjects Who Received Full Dose+- Subjects Who Received Full Dose+- MST-188* (n=18) Placebo (n=13) p value+-+- Duration of Crisis 44 hours 80 hours 0.025 Duration of Hospitalization 5 days 7 days 0.111 Total Analgesic Use 34mg 145mg 0.045 Parenteral Analgesic Use 27mg 133mg 0.022 +- Excludes patients who had drug administration errors or incomplete pain assessments (16), who withdrew consent (2) and who withdrew because of injection site pain after 15 minutes of infusion. Subjects were excluded equally (n=9) between MST-188 and placebo. +-+- Proportional hazards model adjusted for baseline pain. Randomized, double-blind, placebo-controlled, multi-center study of MST-188* in SCD patients hospitalized for crisis MST-188 significantly improved important efficacy parameters |
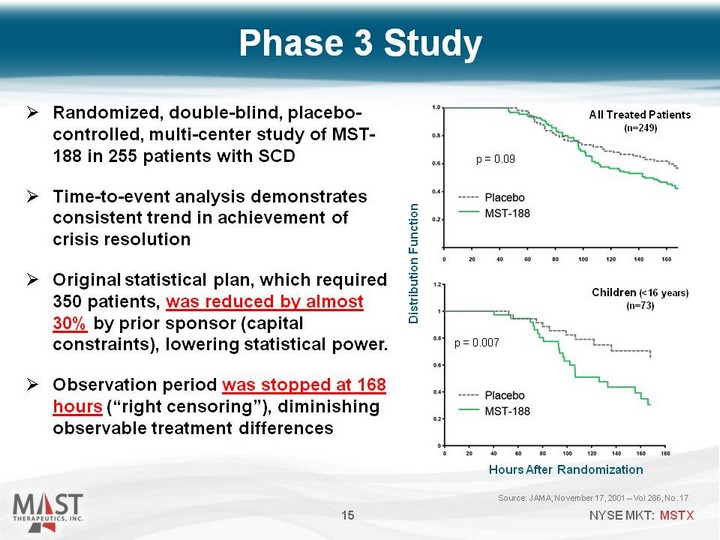
| Source: JAMA, November 17, 2001 - Vol 286, No. 17 15 Randomized, double-blind, placebo- controlled, multi-center study of MST- 188 in 255 patients with SCD Time-to-event analysis demonstrates consistent trend in achievement of crisis resolution Original statistical plan, which required 350 patients, was reduced by almost 30% by prior sponsor (capital constraints), lowering statistical power. Observation period was stopped at 168 hours ("right censoring"), diminishing observable treatment differences Phase 3 Study 15 p = 0.09 All Treated Patients (n=249) Hours After Randomization Distribution Function Children (<16 years) (n=73) p = 0.007 |
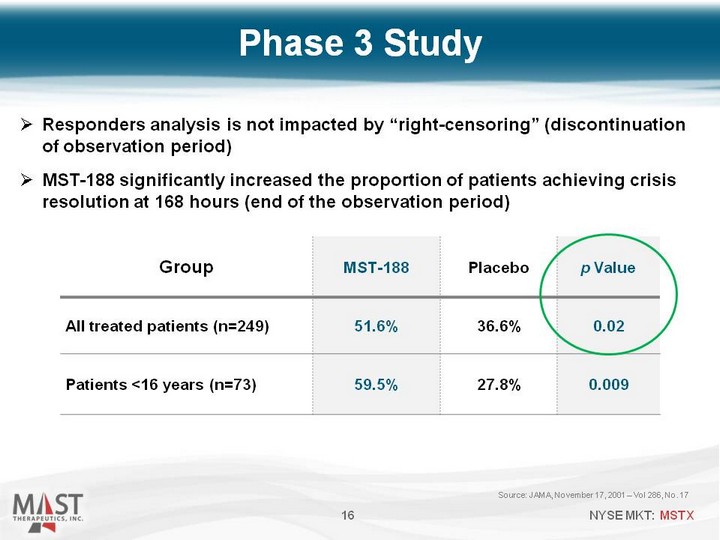
| Source: JAMA, November 17, 2001 - Vol 286, No. 17 16 Group MST-188 Placebo p Value All treated patients (n=249) 51.6% 36.6% 0.02 Patients <16 years (n=73) 59.5% 27.8% 0.009 Phase 3 Study Responders analysis is not impacted by "right-censoring" (discontinuation of observation period) MST-188 significantly increased the proportion of patients achieving crisis resolution at 168 hours (end of the observation period) |

| Randomized, Double-Blind, Placebo-Controlled, Multicenter 388 patients Standard of care +/- MST-188 Primary Efficacy Assessment Duration of crisis Secondary Efficacy Assessments Re-hospitalization for crisis within 14 days Occurrence of acute chest syndrome Power 90% power to detect a 16-hour difference (p=0.05) 85% power to detect a 24-hour difference (p=0.01) 17 Evaluation of Purified 188 In Crisis (EPIC): Pivotal Phase 3 Study Design |

| Enrollment on-track at 14 months (announced Aug 2014) >45 U.S. sites open Multiple ex-U.S. sites open Most Advanced New Drug in SCD Potential to be first approved drug to treat an on-going vaso-occlusive crisis Substantial head start versus other new drugs in development for SCD Positive Factors for Regulatory Decision-Making Significant unmet need Fast Track designation Orphan Drug designation Healthcare disparity 18 EPIC Success Factors |

| 19 Arterial Disease (MST-188 In Combination with Thrombolytics) Acute Limb Ischemia Stroke |
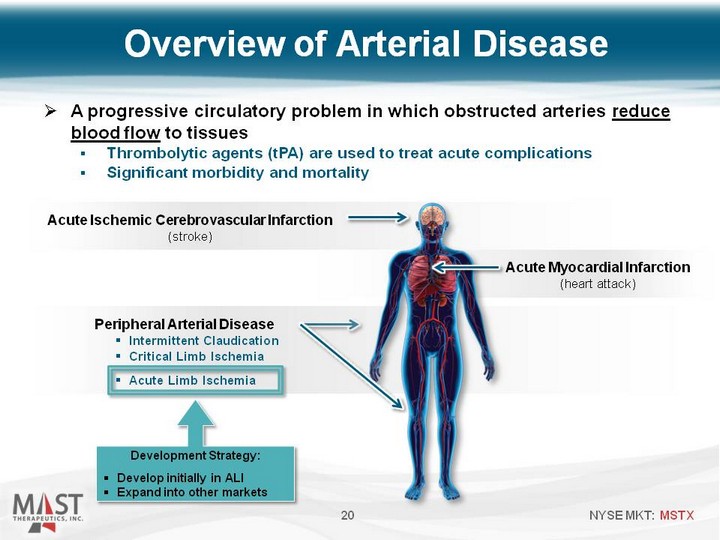
| A progressive circulatory problem in which obstructed arteries reduce blood flow to tissues Thrombolytic agents (tPA) are used to treat acute complications Significant morbidity and mortality 20 Acute Ischemic Cerebrovascular Infarction (stroke) Acute Myocardial Infarction (heart attack) Peripheral Arterial Disease Intermittent Claudication Critical Limb Ischemia Acute Limb Ischemia Development Strategy: Develop initially in ALI Expand into other markets Overview of Arterial Disease |

| ‹#› |

| 22 Parameter MST-188* Control Difference p Value N=114 Myocardial Infarct Size (median) 16% 26% 38% reduction 0.031 Myocardial Salvage (median) 13% 4% 125% increase 0.033 Ejection Fraction (median) 52% 46% 13% improvement 0.020 Incidence of Reinfarction 1% 13% 92% reduction 0.016 MST-188 Showed Synergy with Thrombolytics in Heart Attack Source: Circulation 1996; 94: 298-307 *1st generation (non-purified) formulation |

| 23 Clinical Proof-of-Concept Study Biomarkers Clinical outcomes Study Design Randomized, double-blind, and active-controlled Rutherford Class 2A/2B and catheter-directed thrombolysis t-PA +/- low or high dose MST-188 60 subjects (20 per arm) Timing Study initiated Q1 2014 Completion of enrollment anticipated Q4 2015 ALI data can be supportive of clinical development in stroke Phase 2 Study in ALI |

| 24 Heart Failure (Acute Decompensation) |
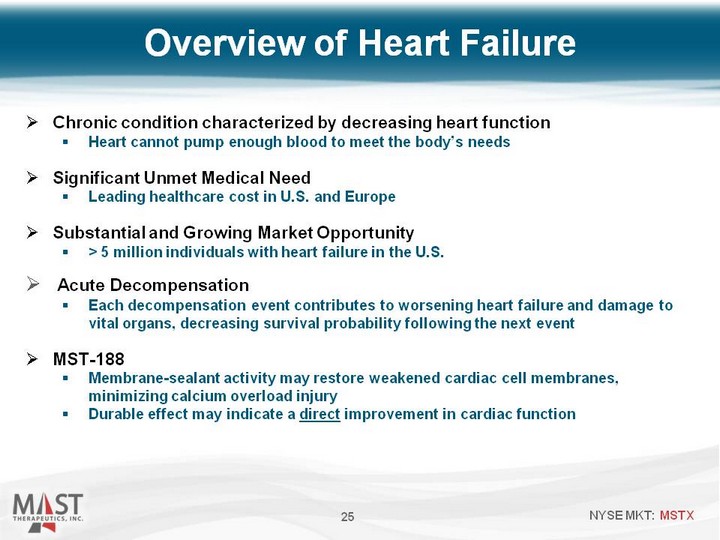
| Overview of Heart Failure 25 Chronic condition characterized by decreasing heart function Heart cannot pump enough blood to meet the body's needs Significant Unmet Medical Need Leading healthcare cost in U.S. and Europe Substantial and Growing Market Opportunity > 5 million individuals with heart failure in the U.S. Acute Decompensation Each decompensation event contributes to worsening heart failure and damage to vital organs, decreasing survival probability following the next event MST-188 Membrane-sealant activity may restore weakened cardiac cell membranes, minimizing calcium overload injury Durable effect may indicate a direct improvement in cardiac function |

| Proof-of-Concept in Heart Failure (nonclinical model of chronic heart failure) 26 Single 2h infusion resulted in improvements in hemodynamic parameters (LVEF, CO) and biomarkers (troponin, NT-proBNP) Troponin associated with 180-day all-cause mortality Elevated NT-proBNP associated with poor prognosis Potentially novel mechanism, compatible with existing treatments Planning to initiate Phase 2 in acute decompensated HF in 1H 2015 Source: data on-file (CHART) * = p<0.05 vs. Control * * * (CHART) * = p<0.05 vs. Control * * * * * |

| 27 AIR001 (sodium nitrite) inhalation solution |
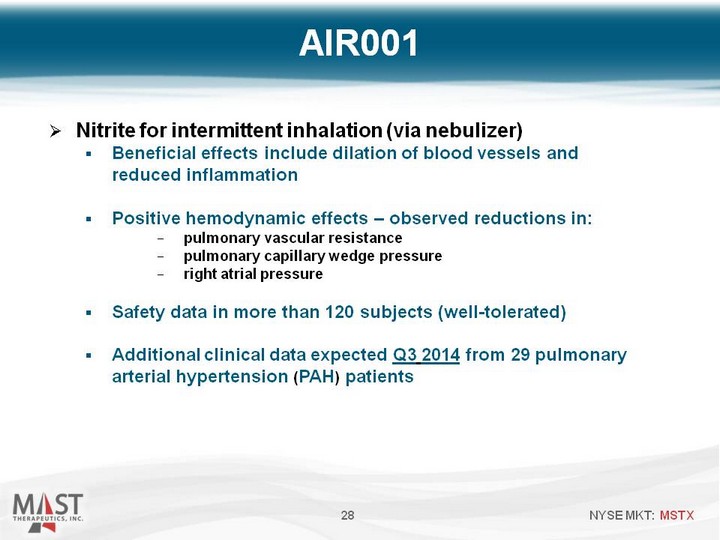
| Nitrite for intermittent inhalation (via nebulizer) Beneficial effects include dilation of blood vessels and reduced inflammation Positive hemodynamic effects - observed reductions in: pulmonary vascular resistance pulmonary capillary wedge pressure right atrial pressure Safety data in more than 120 subjects (well-tolerated) Additional clinical data expected Q3 2014 from 29 pulmonary arterial hypertension (PAH) patients 28 AIR001 |
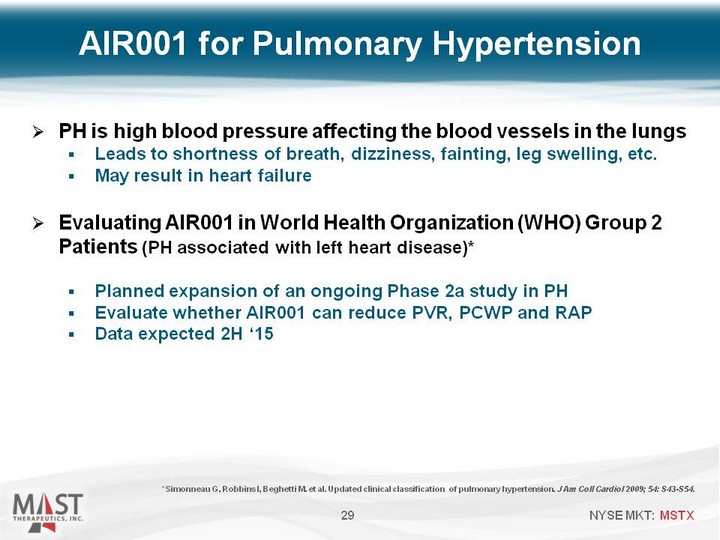
| PH is high blood pressure affecting the blood vessels in the lungs Leads to shortness of breath, dizziness, fainting, leg swelling, etc. May result in heart failure Evaluating AIR001 in World Health Organization (WHO) Group 2 Patients (PH associated with left heart disease)* Planned expansion of an ongoing Phase 2a study in PH Evaluate whether AIR001 can reduce PVR, PCWP and RAP Data expected 2H '15 29 AIR001 for Pulmonary Hypertension * Simonneau G, Robbins I, Beghetti M. et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54: S43-S54. |

| 30 Upcoming News & Events |

| Cash/investments at 6/30/14: $46.4 million Market capitalization: ~$75 million* Shares outstanding: ~121 million* Average daily volume (3 mo): ~1.2 million* No debt 31 * As of August 11, 2014 MSTX Financial Overview |

| A Leader in Areas of Significant Unmet Need Sickle Cell Disease: Most advanced (Phase 3) new drug in development Acute Limb Ischemia: Initiated Phase 2 study in Q1 2014 Heart Failure: potential new mechanism with durable effects Recently acquired Aires Pharmaceuticals (AIR001) New Phase 2 opportunities in pulmonary hypertension and heart failure Cash and marketable securities $46.4 million at 6/30/14 No debt 32 Mast Investment Summary |
