Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT OF GREGORY & ASSOCIATES, LLC. - Enochian Biosciences Inc | fs12014a6ex23i_dandritbio.htm |
As filed with the Securities and Exchange Commission on August 8, 2014
Registration No. 333 - 193965
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
PRE-EFFECTIVE AMENDMENT NO. 6
TO
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
DanDrit Biotech USA, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
||
|
Delaware
|
2834
|
45-2259340
|
||
|
(State or other jurisdiction of
incorporation or organization)
|
(Primary Standard Industrial
Classification Code Number)
|
(I.R.S. Employer
Identification Number)
|
DanDrit Biotech A/S
Fruebjergvej 3 Box 62
2100 Copenhagen, Denmark
+45 39179840 (Telephone Number)
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Dr. Eric Leire
Chief Executive Officer
c/o DanDrit Biotech USA, Inc.
P.O. Box 189
Randolph, VT 05060
212-727-7085
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
David N. Feldman, Esq.
Richardson & Patel, LLP
The Chrysler Building
405 Lexington Avenue, 49th Floor
New York, NY 10174
(212) 869-7000 (Telephone Number)
(917) 677-8165 (Facsimile Number)
|
Henry I. Rothman, Esq.
Joseph Walsh, Esq.
Troutman Sanders LLP
The Chrysler Building
405 Lexington Avenue
New York, NY 10174
(212) 704-6000 (Telephone Number)
(917) 704-6288 (Facsimile Number)
|
Approximate date of commencement of proposed sale to the public: Promptly after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: þ
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o
|
Accelerated filer o
|
|
|
Non-accelerated filer o (Do not check if a smaller reporting company)
|
Smaller reporting company þ
|
CALCULATION OF REGISTRATION FEE
|
Title of each class of securities to be registered
|
Proposed Maximum
Aggregate Offering
Price(1)
|
Amount of
Registration Fee
|
|||||
|
Common stock, par value $0.0001 per share (2)
|
$ |
12,000,000
|
$ |
1,545.60
|
|||
|
Common Stock par value $0.0001 per share (3)
|
$ |
200,000
|
$ |
25.76
|
|||
|
TOTAL
|
$ |
12,200,000
|
$ |
1,571.36
|
|||
|
(1)
|
Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) of the Securities Act of 1933, as amended (the “Securities Act”), for the public offering and Rule 457(a) of the offering by the security holder.
|
|
(2)
|
This registration statement covers under one prospectus, the registrant’s initial public offering of up to 2,400,000 shares of the registrant’s common stock, par value $0.0001 per share (the “Common Stock”) (based on an assumed offering price of $5.00 per share).
|
|
(3)
|
This registration statement also covers, under a separate prospectus, the resale (the “Resale”) of an aggregate of 40,000 shares of Common Stock owned by one (1) selling shareholder (the “Security Holder”) identified in the Resale Prospectus defined below. The Company will not receive any proceeds from the Resale.
|
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
EXPLANATORY NOTE
This registration statement contains two forms of prospectus, as set forth below.
|
|
●
|
Public Offering Prospectus. A prospectus to be used for the initial public offering by the registrant of 2,400,000 shares of common stock, (the “Public Offering Prospectus”) through the placement agents named on the cover page of the Public Offering Prospectus.
|
|
|
|
|
●
|
Resale Prospectus. A prospectus to be used in connection with the potential distribution by the Selling Security Holder of up to an aggregate of 40,000 shares of the registrant’s common stock (the “Resale Prospectus”).
|
The Public Offering Prospectus and the Resale Prospectus will be identical in all respects except for the following:
|
●
|
they contain different front covers;
|
|
|
●
|
they contain different tables of contents;
|
|
|
●
|
the summary of The Offering is deleted from the Resale Prospectus;
|
|
|
●
|
they contain different Use of Proceeds sections;
|
|
|
●
|
a Shares Registered for Distribution section is included in the Resale Prospectus;
|
|
|
●
|
they contain different Plan of Distribution sections;
|
|
|
●
|
the Legal Matters section in the Resale Prospectus deletes the reference to counsel for the placement agents; and
|
|
|
●
|
they contain different back covers.
|
The registrant has included in this registration statement, after the financial statements, a set of alternate pages to reflect the foregoing differences between the Resale Prospectus and the Public Offering Prospectus.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PROSPECTUS | Subject to Completion, Dated August 8, 2014 |

DANDRIT BIOTECH USA, INC.
Up to 2,400,000 Shares of Common Stock
We are offering up to $12,000,000 (2,400,000 shares) of our common stock at an assumed offering price of $5.00 per share in an initial public offering of our common stock. There is presently no public market for our common stock, however, we have applied for trading on the OTC Bulletin Board and the OTCQB.
We are an “emerging growth company” under the federal securities laws and will have the option to use reduced public company reporting requirements.
Investing in our common stock involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page 9 of this prospectus, and under similar headings in any amendments or supplements to this prospectus.
Sunrise Securities Corp. and The Benchmark Company, LLC (each a “Placement Agent” and, collectively, the “Placement Agents”) are the placement agents for our initial public offering. The Placement Agents are not purchasing or selling any shares of common stock nor is it required to sell any specific number or dollar amount of common stock but will use their best efforts to sell the common stock offered. There are no minimum purchase requirements that must be met before the offering terminates. We have not arranged to place the funds from investors in an escrow, trust or similar account. Once your subscription is received, you will not have the right to withdraw your funds. Once your subscription has been accepted by us, offering proceeds will be deposited into our operating account and used to conduct our business and operations in accordance with the section of this prospectus titled “Use of Proceeds”. This offering will terminate on , unless it is fully subscribed before that date or we decide to terminate the offering prior to that date. In either event, the offering may be closed without further notice to you.
|
Per Share
|
Total
|
|||||||
|
Public Offering Price
|
$
|
5.00
|
$
|
12,000,000
|
||||
|
Placement Agents' Commissions(1)
|
$
|
0.35
|
$
|
840,000
|
||||
|
Offering Proceeds before expenses (2)
|
$
|
4.65
|
$
|
11,160,000
|
||||
(1) For the purpose of estimating the Placement Agents’ commission, we have assumed that the Placement Agents will receive the maximum commission on all sales made in the offering. The Placement Agents will only receive commissions from proceeds raised from investors introduced to us by the Placement Agents. This figure does not include up to an aggregate amount of $75,000 for fees and expenses of counsel to the Placement Agents and an accountable expense reimbursement fee of up to 1% of the gross proceeds of this offering. See “Plan of Distribution” for more information on this offering and the arrangements we have with the Placement Agents.
(2) Does not include offering expenses that we will be required to pay. Because there is no minimum offering amount required as a condition to closing this offering, the actual public offering amount, the Placement Agents’ commissions, and proceeds to us, if any, are not presently determinable and may be substantially less than the total maximum offering set forth above. Once the offering price has been determined, it will remain fixed for the duration of the offering. See “Plan of Distribution” for more information on this offering and the arrangements we have with the Placement Agents.
The delivery of the shares of common stock will be made on or about , 2014.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| Sunrise Securities Corp. |  |
The date of this prospectus is , 2014.
|
Page
|
||
|
1
|
||
|
7
|
||
|
9
|
||
|
23
|
||
|
24
|
||
|
26
|
||
|
27
|
||
|
28
|
||
|
29
|
||
|
36
|
||
|
62
|
||
|
64
|
||
|
66
|
||
|
68
|
||
|
70
|
||
|
71
|
||
|
72
|
||
|
73
|
||
|
74
|
||
|
77
|
||
|
80
|
||
|
80
|
||
|
80
|
||
|
80
|
||
Dealer Prospectus Delivery Obligation
Until , 2014 (90 days after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as a placement agent and with respect to any unsold allotments or subscriptions.
ABOUT THIS PROSPECTUS
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with different or additional information. If anyone provides you with different or inconsistent information, you should not rely on it. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of securities described in this prospectus. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus or any prospectus supplement, as well as information we have previously filed with the Securities and Exchange Commission (the “SEC” or the “Commission”) and incorporated by reference herein, is accurate as of the date on the front of those documents only. Our business, financial condition, results of operations and prospects may have changed since those dates.
CURRENCY INFORMATION
The functional and reporting currency of DanDrit Biotech USA, Inc. is the U.S. Dollar. The functional currency of DanDrit Biotech A/S is the Danish Krone (“DKK” or “Danish Krone”) and our reporting currency is U.S. dollars ($) for the purpose of the financial statements and other financial data contained elsewhere in this prospectus. DanDrit Biotech A/S consolidated balance sheet accounts are translated into U.S. dollars at the period-end exchange rates (DKK 5.422, DKK 5.4127 and DKK 5.66 to $1 at March 31, 2014, December 31, 2013 and 2012, respectively) and all revenue and expenses reported for three months ended March 31, 2014 and the years ended December, 2013 and 2012 are translated into U.S. dollars at the average exchange rates prevailing during 2014, 2013 and 2012 (DKK 5.41735, DKK 5.54 and DKK 5.79 to $1, respectively).
FINANCIAL INFORMATION
As a result of the reverse acquisition resulting from the Share Exchange (as defined below), DanDrit Denmark (as defined below) is considered the accounting acquirer in the Share Exchange and the assets and liabilities and the historical operations that are reflected in our financial statements are those of DanDrit Denmark. Therefore, the historical financial data of DanDrit Denmark is deemed to be our historical financial data; provided, however, that all amounts have been restated to reflect the recapitalization of the exchange ratio applied as a result of the Share Exchange.
This summary may not contain all of the information that may be important to you. You should read the entire prospectus, including the financial statements and related notes, and the risk factors under the section titled “Risk Factors”. Unless otherwise indicated or the context otherwise requires, all references in this prospectus to “DanDrit,” “we,” “us,” “our” or the “Company” are to DanDrit Biotech USA, Inc., a Delaware corporation (“DanDrit USA”), together with its wholly-owned subsidiary DanDrit Biotech A/S, a Danish limited company, organized under the Danish Act on Limited Companies of the Kingdom of Denmark (“DanDrit Denmark,” or the “Subsidiary”).
Overview
We are a biotechnology company seeking to develop what we believe could be the world’s first vaccine approved for the treatment of colorectal cancer. For more than a decade we have developed and patented vaccines successfully used in initial clinical trials in Europe and Asia including: (i) MelCancerVac™ (MCV) for treatment of cancer (one phase I/II trial in Denmark and two phase II trials in Denmark and Singapore), (ii) Tolerogenic (producing immunologic tolerance) dendritic cell (TDC) (pre-clinical stage in Denmark) and (iii) Melvaccine (MV) a melanoma cell lysate used as stand-alone vaccine (pre-clinical state in Denmark). We plan to continue the clinical development program in the United States. Springing from academic roots in Denmark, DanDrit has built upon its scientific and medical skills to advance candidate therapies, targeted initially at non-small-cell-lung-cancer (NSCLC) and colorectal cancer (CRC). In 2001, MCV was developed as a result of the combined efforts and research of DanDrit researchers and employees. On September 22, 2008, the Singapore government granted to DanDrit Denmark a named-patient compassionate use program of MCV. DanDrit’s dendritic cell vaccine, MCV, was evaluated in three single-arm Phase II clinical trials in cancer where MCV demonstrated potential efficacy. However, these three clinical trials generated data reported in published papers which indicated that the data needed to be confirmed in a larger, comparative randomized clinical trial. As a result, DanDrit, with the assistance of experienced practitioners in colorectal cancer treatment, designed a randomized trial with 174 stage IV colorectal cancer patients. Neither the US Federal Drug Administration (FDA) nor any other comparable governmental agency has reviewed MCV. Therefore, any assessment of its safety or efficacy only reflects the opinion of the Company. Furthermore, it does not indicate that MCV will achieve favorable results in any later stage trials or that the FDA or comparable agency will ultimately determine that MCV is safe and effective for purposes of granting marketing approval.
Our Biotechnology
We plan to use a dendritic cell vaccine technology relatively similar to the technology behind Dendreon’s FDA approved Provenge™ cancer vaccine. However, we believe DanDrit’s next generation of dendritic cell vaccine may benefit from technological competitive advantages over other cancer vaccines including:
|
|
●
|
The vaccine will be generated within eight days from a patient’s peripheral blood. We will be able to generate the vaccine quickly because only 200 ml of blood is required. Leukapheresis, a medical technology in which the blood of a patient is passed through an apparatus (similar to a dialysis machine) that separates out one particular constituent and returns the remainder to the circulation which is used in Dendreon’s Provenge™ cancer vaccine, can be used but is not needed.
|
|
|
●
|
The vaccine will use an allogenic (using cells, tissues, or organs, sourced from a genetically non-identical member of the same species as the recipient (“Allogenic”) tumor lysate (a fluid containing the contents of lysed cells (lysis referring to the breaking down of a cell and a fluid containing the contents of lysed cells referred to as a “lysate”) as opposed to inconvenient autologous (from the patient) tumor lysate. A major limitation of autologous tumor cell vaccines is the low yield of autologous tumor cells that may compromise the number of immunizations given to patients (difficult to obtain enough cancer cells from the patient). A second inconvenience is the variability of GM-CSF (a protein that functions as a white blood cell growth factor) secretion among patients, which could be responsible for the different levels of responses observed. But above all, although autologous tumor cells may be a good source of tumor-associated antigens, present on some tumor cells and some normal cells (as opposed to tumor specific antigens only present on tumor cells) (TAA) for cancer vaccine development, limitations plus the significant time and expense required for the approval of each patient’s vaccine by the appropriate regulatory agencies severely limit the development of this type of immunization approach. DanDrit does not need a patient’s tumor cells to manufacture MCV. Therefore MCV is not classified as an autologous vaccine.
|
|
|
●
|
The vaccine will be polytopic (targets several cancer specific antigens). As a result, the risk of the tumor escaping is more limited and more T-cells can be activated than if the vaccine is targeting one antigen only. However, MCV has a focus on melanoma-associated antigen (“MAGE”)-A antigens that are only expressed by tumors (in many different types of cancer – not only melanoma) and absent in normal tissues.
|
|
|
●
|
Fast track production in two days is possible.
|
Our Proposed Clinical Trial
Parallel with the establishment of a cancer vaccine center in the European Union (“EU”), DanDrit intends to develop globally MCV in colorectal cancer, with opportunities to expand the scope of the treatment to other types of cancers after development in colorectal cancer. DanDrit proposes to focus its development program on a randomized multicenter Phase IIb/III clinical trial in stage IV colorectal cancer to be initiated in Italy. The proposed Proof of Concept (PoC) study with an adaptive seamless design plans to enroll 174 stage IV colorectal cancer patients after surgical resection of metastases and chemotherapy. The traditional drug clinical development is a sequence of independent trials or phases, where each phase has a different research objective, such as, determining the maximum toxicity point at which the drug can be administered (Phase I), assessing dosing requirements (Phase IIA) and determining efficacy at a prescribed dose (Phase IIB). Each phase of the drug development may also have a different group of randomized participants. A trial that is designed as an adaptive seamless clinical trial refers to a trial that combines the objectives of what are typically separate trials into a single uninterrupted trial with multiple objectives. Usually, the patient participants in this trial are constant and monitored through the course of the various phases and are not re-randomized except for new enrollments. Regulatory authorities in the United States and Europe have published guidance documents on the use and implementation of adaptive design trials. These documents include descriptions of adaptive trials and a requirement for prospectively written standard operating procedures and working processes for executing adaptive trials as well as a recommendation that sponsor companies engage with CROs that have the necessary experience in running such trials.
The proposed patients for the Phase IIb/III clinical trial have no evidence of disease but are not cured of cancer. Their Progression Free Survival (PFS), which refers to the length of time during and after treatment that a patient lives with the disease which does not get worse, is only 24 to 26 months. The objective of this multicenter Phase IIb/III clinical study is to lengthen the survival of these patients. Treatment will be double blinded (to the patients and physicians) against reference therapy. Patients will be included after surgical resection of their primary tumor and resectable metastases in liver and after appropriate peri-operative chemotherapy by stratification and random assignment to a non-vaccine control group or a vaccine group receiving five vaccinations with 14-day administration intervals followed by five vaccines with two-month intervals. Inclusion will take place one month after finishing the last round of peri-operative chemotherapy (FOLFOX or FOLFIRI) and after a negative tumor scan (head, thoracic and abdominal cavities) and normal carcinoembryonic antigen (CEA) prior to inclusion in the vaccine or the control groups. Patients will be screened for MAGE-A expression. The control group will receive five plus five injections with physiological saline. In the event of disease progression, as verified by tumor scan and biomarker levels during the vaccination schedule, vaccinations will be discontinued.
The initial Phase IIb/III trials are currently contemplated to be initiated in Italy. Following the initial closing of the offering described in the Registration Statement, we intend to file an Investigational Medicinal Product Dossier (IMPD) in Italy which is required to obtain a clinical trial authorization (CTA) to begin trials in any Europe state. The IMPD and CTA review and approval process is anticipated to take approximately two-three months, and we anticipate that patients will begin to be enrolled for the Phase IIb/III trials in Italy in October or November of 2014. DanDrit has not filed an investigational new drug (IND) application with the FDA in relation to the proposed trial but anticipates filing an IND application with the FDA by the end of 2014 to initiate the process to permit manufacturing capability of MCV in the U.S. and to include U.S. patients in the PhaseIIb/III trials. Once an IND application has been filed in the U.S., we believe that we will be able to expand the PhaseIIb/III trials initiated in Italy to the U.S. but we cannot estimate at this time when we will be able to begin enrolling U.S. patients in the trial. Although we were a sponsor for only one of the three MCV clinical trials completed to date, we have obtained the case report forms (CRF) with respect to two of the three trials and have requested the CRFs with respect to the third trial and therefore we intend to present the results obtained from the MCV clinical trials for all trials in which we have been able to obtain the related CRFs to the FDA in connection with our IND application, when filed. While we were not the sponsor or principal investigator for all of the trials, certain employees and directors of DanDrit were significantly involved in the design of the study and the analysis and interpretation of the data in all three studies and therefore believe that any weight applied to such trial in connection with our IND application will be focused on the results and data of the trials derived from the studies and disclosed in published papers or otherwise reflected in the CRFs rather than the level of our participation. In addition, we believe that the data received in connection with the Phase IIB/III trials contemplated to be initiated in Italy, will have the greatest weight applied in connection with its IND application anticipated to be filed with the FDA (see “Clinical Trials Data and Product Approvals).
Our Competitive Strengths
We believe the following strengths position us to increase our revenue and profitability:
|
●
|
Cutting Edge Technology. We believe, based on the current state of research, that immunotherapy is one of the waves of the future in cancer management.
|
|
|
●
|
Colorectal Therapy Potential. We believe the treatment of advanced colorectal cancer represents an opportunity to meet a well identified medical need for safe maintenance therapy. We believe the clinical data for MCV to date shows the potential for the vaccine to eventually become a standard of care for maintenance therapy. We believe, based on the available studies to date, that MCV has the potential to prolong periods of remission after response to chemotherapy. If MCV works as expected in advanced colorectal cancer, we believe it would likely prove beneficial in other tumors that over-express MAGE-A including lung, breast and esophageal cancers.
|
|
|
●
|
Regulatory Precedent. With Provenge™, its prostate cancer vaccine, Dendreon pioneered the regulatory pathway for MCV. Dendreon worked with the FDA to develop the protocols which could allow a cellular therapy such as MCV to be approved for clinical use. We believe that DanDrit is the next generation of dendritic cell vaccine with several improvements over its competition: stimulate a cellular immune response rather than just an antibody response, no need for leukapheresis to produce the vaccine, intradermal administration, convenience of an Allogenic vaccine (off-the-shelf cancer specific antigens), polytopic approach but with a focus on the MAGE-A antigen family and reliable cost-efficient manufacturing.
|
|
|
●
|
Use in Singapore. For the last five years, DanDrit and the Singapore National Cancer Center have provided MCV to colorectal cancer patients within an on-going compassionate use program in Singapore.
|
|
|
●
|
Strong IP Protection. The technology is patented with a long patent life. DanDrit owns 100% of the technology.
|
Our Strategy
Our strategy is focused on conducting a proof-of-concept clinical trial in advanced colorectal cancer. DanDrit intends to conduct a randomized multicenter Phase IIb/III clinical trial to determine the ability of MCV to prevent recidivism in stage IV colorectal patients with no evidence of disease after resection of metastasis and chemotherapy. This blinded comparative trial is planned to be completed within three years. We believe that positive clinical data will be the catalyst to unlock commercial revenues for DanDrit through either acquisition by pharmaceutical partner or licensing deals that would yield upfront and milestone payments as well as royalties or other strategic directions the Company may consider.
Furthermore, parallel to the previously described clinical trial, DanDrit may pursue a registration trial to support potential approval of MCV in China. This trial would be conducted under China’s State Food and Drug Administration (the “SFDA”) regulations with a Chinese oncology pharmaceutical partner. China has recently put in place a drug approval system.
DanDrit is headquartered in and runs operations from Denmark. However, DanDrit intends to establish a dendritic cell cancer vaccine good manufacturing practices (GMP) laboratory in the United States.
Industry Overview
We believe that major advances have been made the last three years in the field of immunotherapy. Molecular and cellular mechanisms controlling the immune system’s battle against cancer cells are now better understood.
However, cancer remains mostly treated by surgery, chemotherapy and radiotherapy. The current therapeutic approach is aggressive on the patient with significant side effects. Immunotherapy, however, can potentially solve these problems because the immune system, with its high level of specificity, can zero in on cancer cells that surgeons, drugs and radiations cannot reach.
For example, according to Dr. Adam Snook, “Our immune system is characterized by remarkable specificity, potency and memory – the ability of a single vaccine treatment to provide life-long-protection. No pharmacologic treatment for any indication can provide the same level of safety, efficacy, and long lasting effect than a vaccine can.”
In 2010, Dendreon published positive Phase III survival data for its immunotherapy, called Provenge™, in prostate cancer. During the last three years, we believe the field of cancer immunotherapy has been fast evolving. There has been recently positive clinical data (i.e. anti-programmed cell death protein-1) and approval of several immunotherapies for cancer. We believe that dendritic cell vaccines such as MCV are among the potential developments in the treatment of cancer.
CRC is the second largest cancer market in terms of numbers of patients diagnosed. In 2010, a total of around 1.58 million individuals were affected by CRC in the seven major markets, including the US, Japan, France, Germany, Italy, Spain and the UK. CRC was the leading cause of cancer prevalence among men and second among women in Europe. It was also observed that higher survival rates correlated with higher prevalence.
According to Decision Resources, the CRC market totaled $8.3 billion in 2011. The value of the CRC market is expected to decrease in the next ten years due to generic competition for a key cytotoxic agent, oxaliplatin (Sanofi’s Eloxatin/Eloxatine, Yakult Honsha’s Elplat), as well as the entry of biosimilar competitors for key targeted biological agents. In terms of number of patients, despite the risk being strongly associated with age, the effect of population aging may be limited by reduced risk of invasive disease due to screening at least in developed countries.
DanDrit develops MCV for the management of metastatic CRC (stage IV). Currently, about 20% of CRC patients are diagnosed with metastatic disease. Forecast improvements in the observed survival in the metastatic setting will increase the number of people living with metastatic CRC over the next 20 years, despite the number developing metastatic disease per year remaining relatively stable due to the combined effects of screening and forecast improvements in the management of metastatic recurrence.
Treatment of advanced CRC typically involves removal of sections of the colon (colectomy) or rerouting of the intestine by colostomy. Chemotherapy is also used to treat patients with stage IV colon cancer. Irinotecan, oxaliplatin, and 5-fluorouracil are the three most commonly used drugs. In addition, monoclonal antibodies, including cetuximab (Erbitux), panitumumab (Vectibix), and bevacizumab (Avastin) have been used alone or in combination with chemotherapy. CRC is considered cured in the absence of a recurrence within the first five years. Five year survival rates associated with CRC are as high as 90% in early stage disease, and 40–60% in late-stage disease. Stage I, II and III cancers are considered potentially curable. In most cases, stage IV cancer is not curable. Therefore, there is an unmet need for a safe maintenance therapy of stage IV CRC after surgery and chemotherapy.
Corporate History and Information
DanDrit was incorporated in Delaware on January 18, 2011 under the name “Putnam Hills Corp.” (“Putnam”) as a vehicle to pursue a business combination through the acquisition of, or merger with, an operating business. We filed a Registration Statement on Form 10 with the SEC on August 12, 2011.
On February 12, 2014, the Company signed and consummated the transactions contemplated by a Share Exchange Agreement (the "Share Exchange Agreement"), by and among DanDrit USA, DanDrit Denmark and N.E. Nielsen, as the representative of the shareholders of DanDrit Denmark, pursuant to which the Company will acquire 100% of the issued and outstanding equity securities in Dandrit Denmark in exchange for 6,000,000 of the issued and outstanding shares common stock par value $0.0001 per share of the Company. The initial share exchange was closed on February 12, 2014 pursuant to which holders of approximately 97% of the issued and outstanding capital stock of DanDrit Denmark (the “DanDrit Consenting Holders”) exchanged an aggregate of 3,879,624 equity interests of DanDrit Denmark for 5,814,947 shares of DanDrit USA (the “Share Exchange”) and as a result of which Putnam would become the parent of DanDrit Denmark. In accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark, DanDrit Denmark shareholders who have not consented to the Share Exchange (the “Non-Consenting Shareholders”) and therefore have not exchanged such DanDrit Denmark shareholder’s equity interests in DanDrit Denmark for shares of DanDrit USA, will be entitled to receive the 185,053 shares of common stock of DanDrit USA, reflected as issued and outstanding, that each such DanDrit Denmark shareholder would have been entitled to receive if such DanDrit Denmark shareholder had consented to the Share Exchange, up to an aggregate of 185,053 shares of common stock of DanDrit USA. As a result of the Share Exchange, the former shareholders of Dandrit Denmark became the controlling shareholders of the Company. The Share Exchange was accounted for as a reverse takeover/recapitalization effected by a share exchange, wherein Dandrit Denmark is considered the acquirer for accounting and financial reporting purposes. The capital, share price, and earnings per share amount in these consolidated financial statements for the period prior to the reverse merger were restated to reflect the recapitalization in accordance with the exchange ratio established in the merger.
Upon the closing of the Share Exchange, DanDrit USA and its majority shareholder immediately prior to the closing agreed to cancel up to 4,400,000 shares of our common stock. In addition, following the closing of the Share Exchange, DanDrit Biotech USA, Inc., a wholly owned subsidiary of the Company merged with and into the Company, thereby changing the Company’s name to “DanDrit Biotech USA, Inc.”
DanDrit USA owns approximately 97% of the outstanding equity interests of DanDrit Denmark. As a result of the Share Exchange, we changed our management and reconstituted our board of directors. As of the effective time of the Share Exchange, Samir Masri, the Chief Executive Officer, Chief Financial Officer, President, Secretary and sole director of Putnam resigned as the sole officer and director of Putnam and appointed NE Nielsen, Dr. Jacob Rosenberg, Dr. Eric Leire, Aldo Petersen and Robert E. Wolfe as directors of Putnam, and Dr. Eric Leire as Chief Executive Officer and President and Mr. Wolfe as Chief Financial Officer, Treasurer and Secretary.
Our principal executive offices are located at Fruebjergvej 3 Box 62, 2100 Copenhagen, Denmark, and our telephone number is +45 39179840. We maintain an Internet website at www.dandrit.com. The information contained in, or accessible from, our website is not a part of this prospectus.
Implications of being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012. An “emerging growth company” may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
Financial Disclosure. The financial disclosure in a registration statement filed by an “emerging growth company” pursuant to the Securities Act of 1933, as amended (the “Securities Act”), will differ from registration statements filed by other companies as follows:
|
●
|
audited financial statements are required for only two fiscal years;
|
|
|
●
|
selected financial data is required for only the fiscal years that were audited;
|
|
|
●
|
executive compensation only needs to be presented in the limited format now required for “smaller reporting companies”.
|
Because we are a smaller reporting company, we are already provided with the above exemptions under Regulation S-K promulgated under the Securities Act.
The JOBS Act also exempts us from any Public Company Accounting Oversight Board rules that, if adopted, would mandate auditor rotation or auditor discussion and analysis.
Internal Control Attestation. The JOBS Act provides an exemption to emerging growth companies from the audit of internal controls over financial reporting. We are also exempt from this requirement as a smaller reporting company.
Shareholder Advisory Votes. Section 102(a) of the JOBS Act exempts emerging growth companies from the requirements in Sections 14A(a) and (b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) to hold shareholder advisory votes approving executive compensation and golden parachute compensation paid in connection with an acquisition, merger, consolidation, or proposed sale or other disposition of all or substantially all the assets of an issuer.
Information about an Emerging Growth Company. Section 105(a) of the JOBS Act amended the Securities Act to provide an exception from the definition of the word “offer” for purposes of Sections 2(a)(10) and 5(c) of the Securities Act for research reports issued by a broker-dealer regarding an emerging growth company that is the subject of a proposed public equity offering.
The JOBS Act also prohibits the SEC and FINRA from adopting or maintaining any rule or regulation in connection with an initial public offering of an emerging growth company that restricts, based on “functional role”, which employees of a broker-dealer may arrange for communications between research analysts and prospective investors; prohibits research analysts from participating in communication with company management in the presence of non-research personnel such as investment banking or sales force personnel; or which prohibits the publication or distribution of a research report or making of a public appearance within any prescribed period of time either following the pricing date of the emerging growth company’s initial public offering or prior to the expiration of a company or shareholder lock-up agreement.
Election to Opt Out of Transition Period. Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standard.
The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. We have not elected to opt out of the transition period.
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act, which such fifth anniversary will occur in 2018. However, if certain events occur prior to the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.0 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period.
Because we have elected to take advantage of certain of the reduced disclosure obligations and may elect to take advantage of other reduced reporting requirements in future filings, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
THE OFFERING
|
Common stock offered by us
|
Up to 2,400,000 shares on a best efforts basis.
|
|
|
Common stock outstanding prior to the offering
|
8,040,000 shares, including 185,053 shares of common stock reserved for issuance to the Non-Consenting Shareholders of DanDrit Denmark and deemed issued and outstanding for accounting purposes.
|
|
|
Common stock to be outstanding after the offering
|
Up to 10,440,000 shares.
|
|
|
Use of proceeds
|
Based on an assumed offering price of $5.00 per share, after deducting the placement agents’ commissions and estimated offering expenses payable by us, we estimate that we will receive up to $10,785,464 in net proceeds from the sale of the shares of common stock in this offering. However, this is a best efforts offering, and there can be no assurance that the offering contemplated hereby will ultimately be consummated.
We intend to use the proceeds from this offering to invest approximately (i) $830,000 for the manufacturing of our products, (ii) $2,670,656 in SG&A/Administration, (iii) $526,689 in debt repayment to Sune Olsen Holding ApS, a shareholder, and (iv) $6,758,120 in our clinical trial. All remaining proceeds will be used for working capital and general corporate purposes.
If we are unable to raise gross proceeds equal to at least $12,000,000, we intend to first apply the proceeds towards $526,689 in outstanding debt to Sune Olsen Holding ApS, a shareholder, and then towards the development and marketing of our products and the engineering, development and testing of vaccines. However, to the extent that we are unable to raise a sufficient amount of proceeds in this offering, we may not be able to achieve all our business objectives in a timely manner.
In the event that we file a post-effective amendment to increase the offering amount pursuant to Rule 462(b) of the Securities Act, we plan to allocate the extra funds in strengthening our Phase II/III clinical trial with a larger sample size and in targeting patients with stage III colorectal cancer rather than metastatic (stage IV) colorectal cancer patients.
See “Use of Proceeds” for more information.
|
|
|
Potential purchases by affiliates
|
Certain of our affiliates may purchase shares of our common stock in this offering on the same terms as they are offered and sold to the public.
|
|
|
Risk factors
|
The shares of common stock offered hereby involve a high degree of risk. See “Risk Factors”.
|
|
|
Dividend policy
|
We currently intend to retain any future earnings to fund the development and growth of our business. Therefore, we do not currently anticipate paying cash dividends on our common stock.
|
|
|
Trading Symbol
|
There is not currently, and there has never been, any market for our common stock. In connection with this offering, we intend to arrange for a registered broker-dealer to apply to have our common stock quoted on the OTC Bulletin Board and on the OTCQB. We cannot guarantee that our application will be approved.
|
|
|
Lock-Up
|
All of the DanDrit Consenting Shareholders that were issued shares of common stock in the Share Exchange are subject to a lock-up agreement for a 180 day period beginning as of the filing date of the last amendment to the registration statement filed in connection with this Offering that is declared effective (the “Lock-Up Period”). The one selling shareholder (the “Security Holder”) identified in the Resale Prospectus will also be subject to a lock-up agreement restricting any sales of the Company’s common stock during the Lock-up Period. See “Plan of Distribution”. The Company will not receive any proceeds from the Resale of the shares of Common Stock by the Security Holder.
|
The following table sets forth selected historical statements of operations for the fiscal years ended December 31, 2013 and 2012 and for the three months ended March 31, 2014 and 2013; and balance sheet data as of December 31, 2013 and March 31, 2014. As a result of the reverse acquisition resulting from the Share Exchange, DanDrit Denmark is considered the accounting acquirer in the Share Exchange and the assets and liabilities and the historical operations that are reflected in our financial statements are those of DanDrit Denmark. Therefore, the historical financial data of DanDrit Denmark is deemed to be our historical financial data; provided, however, that all amounts have been restated to reflect the recapitalization of the exchange ratio applied as a result of the Share Exchange.
The balance sheet data as of December 31, 2013 and the statement of operations data for the fiscal years ended December 31, 2013 and 2012 have been derived from our audited financial statements for those years included elsewhere in this prospectus. The balance sheet data as of March 31, 2014 and the statement of operations data for the three months ended March 31, 2014 and 2013 have been derived from our unaudited condensed financial statements included elsewhere in this prospectus.
The following data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in this prospectus and with our financial statements and the related notes and other financial information included in this prospectus.
STATEMENTS OF OPERATIONS:
|
For the
Three Months
Ended
March 31,
2014
|
For the
Three
Months
Ended
March 31,
2013
|
For the Year
Ended
December 31,
2013
|
For the Year
Ended
December 31,
2012
|
|||||||||||||
| (Unaudited) | (Unaudited) | (Audited) | (Audited) | |||||||||||||
|
Net Sales
|
$ | - | $ | 31,558 | $ | 32,768 | $ | 62,806 | ||||||||
|
Cost of Goods Sold
|
17,739 | 15,360 | 109,299 | 64,385 | ||||||||||||
|
Gross Income (Loss)
|
(17,739 | ) | 16,198 | (76,531 | ) | (1,579 | ) | |||||||||
|
Operating Expenses:
|
||||||||||||||||
|
General and administrative expenses
|
326,428 | 175,016 | 1,233,683 | 1,036,005 | ||||||||||||
|
Depreciation and Amortization
|
6,794 | 8,600 | 38,297 | 56,600 | ||||||||||||
|
Consulting expenses
|
61,145 | 13,048 | 390,437 | 829,845 | ||||||||||||
|
Total Operating Expense
|
394,367 | 196,664 | 1,662,417 | 1,922,450 | ||||||||||||
|
Loss from Operations
|
(412,106 | ) | (180,466 | ) | (1,738,948 | ) | (1,924,029 | ) | ||||||||
|
Other Income (Expense)
|
||||||||||||||||
|
Interest (expense)
|
(13,999 | ) | (159,922 | ) | (652,703 | ) | (704,911 | ) | ||||||||
|
Gain (loss) on currency transactions
|
- | (100,327 | ) | 19,541 | 32,841 | |||||||||||
| Gain on forgiveness of debt | - | - | 49,016 | - | ||||||||||||
|
Gain on derivative liability
|
- | 41,643 | 175,732 | 153,430 | ||||||||||||
| Gain on sale of assets | - | - | 1 | 15,020 | ||||||||||||
|
Interest Income
|
51 | - | - | - | ||||||||||||
|
Total Other Income (Expense)
|
(13,948 | ) | (218,606 | ) | (408,413 | ) | (503,620 | ) | ||||||||
|
Loss Before Income Taxes
|
(426,054 | ) | (399,072 | ) | (2,147,361 | ) | (2,427,649 | ) | ||||||||
|
Income Tax Expense (Benefit)
|
- | - | - | - | ||||||||||||
|
Net Loss
|
$ | (426,054 | ) | $ | (399,072 | ) | $ | (2,147,361 | ) | $ | (2,427,649 | ) | ||||
BALANCE SHEETS:
|
(Unaudited)
March 31,
2014
|
December 31,
2013
|
|||||||
|
ASSETS
|
||||||||
|
CURRENT ASSETS:
|
||||||||
|
Cash
|
$
|
54,472
|
$
|
18,794
|
||||
|
Cash held in escrow
|
423,969
|
77,468
|
||||||
|
Other Receivables
|
69,712
|
25,456
|
||||||
|
Prepaid Expenses
|
11,621
|
19,774
|
||||||
|
Total Current Assets
|
559,774
|
141,492
|
||||||
|
PROPERTY AND EQUIPMENT, Net accumulated Depreciation
|
40,460
|
-
|
||||||
|
OTHER ASSETS
|
||||||||
|
Definite Life Intangible Assets
|
233,766
|
231,615
|
||||||
|
Deferred Stock Offering Costs
|
67,000
|
67,000
|
||||||
|
Deposits
|
10,466
|
10,360
|
||||||
|
Total Other Assets
|
311,232
|
308,975
|
||||||
|
TOTAL ASSETS
|
$
|
911,466
|
$
|
450,467
|
||||
|
LIABILITIES AND STOCKHOLDER'S DEFICIENCY
|
||||||||
|
CURRENT LIABILITIES:
|
||||||||
|
Notes Payable -Related Party, Current Portion
|
$
|
1,705,201
|
$
|
728,001
|
||||
|
Accounts Payable
|
568,175
|
548,501
|
||||||
|
Accrued Expenses
|
824,192
|
858,135
|
||||||
|
Total Current Liabilities
|
3,097,568
|
2,134,637
|
||||||
|
LONG TERM LIABILITIES
|
-
|
-
|
||||||
|
Notes Payable, Related Parties Less Current Portion
|
-
|
-
|
||||||
|
Total Long Term Liabilities
|
-
|
-
|
||||||
|
Total Liabilities
|
3,097,568
|
2,134,637
|
||||||
|
STOCKHOLDER'S DEFICIENCY:
|
||||||||
|
Preferred stock, $.0001 par value; 10,000,000 shares authorized; none issued and outstanding
|
-
|
-
|
||||||
|
Common stock, $.0001 par value 100,000,000 shares authorized, 8,040,000 and 6,000,000 issued and outstanding at March 31, 2014 and December 31, 2013, respectively (1)
|
804
|
600
|
||||||
|
Additional paid-in capital
|
17,788,110
|
17,867,546
|
||||||
|
Accumulated Deficit
|
(19,947,180
|
)
|
(19,521,126
|
)
|
||||
|
Other Comprehensive Income, net
|
(27,836
|
) |
(31,190
|
) | ||||
|
Total Stockholder’s (Deficit)
|
(2,186,102
|
) |
(1,684,170
|
) | ||||
|
TOTAL LIABILITIES AND STOCKHOLDER'S EQUITY (DEFICIT)
|
$
|
911,466
|
450,467
|
|||||
(1) As a result of the Share Exchange, the former shareholders of DanDrit Denmark became the controlling shareholders of the Company. The Share Exchange was accounted for as a reverse takeover/recapitalization effected by a share exchange, wherein DanDrit Denmark is considered the acquirer for accounting and financial reporting purposes. The capital, share price, and earnings per share amount in these consolidated financial statements for the period prior to the reverse merger were restated to reflect the recapitalization in accordance with the exchange ratio established in the merger.
You should carefully consider and evaluate all of the information in this prospectus, including the risk factors listed below. Risks and uncertainties in addition to those we describe below, that may not be presently known to us, or that may also harm our business and operations. If any of these risks occur, our business, results of operations and financial condition could be harmed, the price of our common stock could decline, and future events and circumstances could differ significantly from those anticipated in the forward-looking statements contained in this prospectus.
All references to DanDrit’s drugs and vaccine candidates in this section refer to DanDrit drugs and vaccine candidates that DanDrit developed in-house.
RISKS ASSOCIATED WITH DANDRIT’S BUSINESS AND INDUSTRY
DanDrit lacks an established operating history on which to evaluate its business and determine if it will be able to execute our business plan, and can give no assurance that operations will result in profits.
DanDrit was formed in Delaware in January 2011 as a vehicle to pursue a business combination through the acquisition of, or merger with, an operating business.
On February 12, 2014, the Company completed the Share Exchange as described herein, as a result of which it adopted the business and management of DanDrit Denmark.
DanDrit has a limited operating history that makes it difficult to evaluate its business. DanDrit has not begun sales of its products, and cannot say with certainty when it will begin to achieve profitability. No assurance can be made that DanDrit will ever derive meaningful revenues or become profitable.
DanDrit has incurred losses in prior periods and expect to incur losses in the future. DanDrit may never be profitable.
DanDrit’s independent registered public accounting firm has issued an unqualified opinion with an explanatory paragraph to the effect that there is substantial doubt about DanDrit’s ability to continue as a going concern. This unqualified opinion with an explanatory paragraph could have a material adverse effect on DanDrit’s business, financial condition, results of operations and cash flows.
DanDrit had net losses at March 31, 2014 and 2013 of $426,054 and $399,072, respectively and December 31, 2013 and 2012 of $2,147,361 and $2,427,649, respectively and an accumulated deficit at March 31, 2014 and December 31, 2013 of $19,947,180 and $19,521,126, respectively. DanDrit expects to continue to sustain losses for the foreseeable future.
As sales of DanDrit’s products have generated minimal operating revenues, DanDrit has relied on loans and on sales of its debt and equity securities to continue operations. If DanDrit is unable to raise funds through sales of its securities, there can be no assurance that DanDrit will be able to implement its business plan, generate sustainable revenue or ever achieve profitable operations. DanDrit expects to have operating losses until such time as it develops a substantial and stable revenue base. DanDrit cannot assure you that it can achieve or sustain profitability on a quarterly or annual basis in the future.
DanDrit may not be able to develop its vaccine candidates to yield satisfactory results and they may never be approved for use by regulatory authorities. If DanDrit is unable to successfully commercialize its vaccines, its prospects, financial position, results of operations and future opportunities will be materially adversely affected.
None of DanDrit’s vaccine candidates has completed full clinical development. Because DanDrit’s vaccine candidates generally belong to new classes of cell therapy, they will require extensive further development, testing and funding before we can seek regulatory approval for any of these vaccines.
DanDrit’s prospects in the short term, including DanDrit’s ability to generate revenue and make new strategic alliances, depend on DanDrit’s ability to develop, obtain regulatory approval for and commercialize its current vaccine candidates with satisfactory results. If DanDrit fails to develop its vaccine candidates, it will have a material adverse effect on DanDrit’s business, financial condition, results of operations and future growth opportunities.
There can be no assurance that DanDrit will succeed in implementing its Phase IIb/III clinical trials for advanced colorectal cancer so that the results of these clinical trials will support further preclinical or clinical studies, or that DanDrit will be able to develop new vaccine candidates or successfully commercialize any of those cancer vaccine candidates at a later time. If DanDrit does not do this, we cannot achieve our growth potential and this will have a material adverse effect on our prospects, financial position, results of operations and future opportunities.
Results of the early clinical trials do not insure future success.
The results of early clinical trials may not necessarily be indicative of future results. Achieving positive results in preclinical testing and early clinical trials does not constitute any assurance that in future clinical trials sufficient data can be obtained to document a vaccine candidate’s efficacy and safety. The safety and efficacy of a vaccine candidate in development must be supported by extensive data from preclinical studies and clinical trials.
A number of companies in the pharmaceutical industry and in the biopharmaceutical industry, including companies that have greater resources and more experience than DanDrit, have achieved significant negative results in clinical phase IIb and III trials, even after obtaining promising results in preclinical and early clinical studies. Results that are considered acceptable in early clinical studies may not be confirmed or may be interpreted differently in subsequent studies. DanDrit cannot predict whether the clinical phase IIb and III and other clinical trials that may be implemented will demonstrate sufficient efficacy and safety to obtain regulatory approval to market any of DanDrit’s vaccine candidates.
Negative or non-satisfactory results of clinical trials involving DanDrit’s vaccine candidates could lead to DanDrit or its collaborators having to perform additional nonclinical and/or clinical trials, which could result in higher costs and significantly delay the marketing authorization application for such vaccine candidates by the regulatory authority, or could lead to an application for a more narrowly defined use, or another indication for the vaccine candidate than originally expected. Such results could also lead to the complete elimination of a vaccine candidate. If any of these risks materialize, it could have a material adverse effect on DanDrit’s business, financial condition, results of operations and future growth opportunities.
We are a clinical-stage biopharmaceutical company which makes it difficult to assess our future viability.
We are a clinical-stage biopharmaceutical company. We have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical area. For example, to execute our business plan, we will need to successfully:
|
●
|
execute on product candidate development activities;
|
|
|
●
|
obtain required regulatory approvals for the development and commercialization of our product candidates;
|
|
|
●
|
maintain, leverage and expand our intellectual property portfolio;
|
|
|
●
|
gain market acceptance for our products;
|
|
|
●
|
develop and maintain any strategic relationships we elect to enter into; and;
|
|
|
●
|
manage our spending as costs and expenses increase due to preclinical development, clinical trials, regulatory approvals and commercialization.
|
If we are unsuccessful in accomplishing these objectives, we may not be able to develop product candidates, raise capital, expand our business or continue our operations.
DanDrit will be dependent on collaboration and licensing arrangement to develop and commercialize its products. These relationships may be unsuccessful and may not result in the development of vaccine candidates. In that case, our business, financial condition and growth opportunities will be materially adversely affected.
DanDrit expects to depend on collaboration and licensing agreements with third parties who we expected will provide additional personnel and other resources and funding required to develop and commercialize its products. Until these relationships are established, our plans for developing some of our vaccines may be uncertain. There can be no assurance that DanDrit will be able to enter into or maintain these agreements, that the results of these agreements will further the development of a vaccine, or that DanDrit will receive income from these agreements. Furthermore, collaborators that we anticipate may enter into agreements with us may change their priorities; make reallocation of resources; terminate the agreements; end or further delay the development of vaccine candidates; downgrade or change plans or strategies for regulatory approval or commercialization of the vaccine candidate; find it difficult to retain key employees; or be taken over by companies that are our competitors.
We expect that these collaboration and licensing agreements will entitle us to milestone payments and a percentage of sales related to the vaccine candidates that are commercialized. If a third party with which DanDrit has established a collaboration or licensing arrangement stops the development of a vaccine candidate, there can be no assurance that all rights in respect of the vaccine candidate will be reassigned to us. A transfer of these rights may be delayed for various reasons, which may result in the delay of all work performed for the vaccine candidate.
Since we are dependent on third parties to develop and commercialize our product candidates, any change in these anticipated relationships will have a material adverse effect on our business, financial condition, and future growth opportunities.
Regulatory requirements and regulations could have a material adverse effect on DanDrit.
DanDrit’s products are subject to extensive regulatory requirements, including public and/or regulatory limits set by the FDA and the European Medicines Agency (“EMA”). These laws and regulations, including those relating to reporting on safety, product safety and advertising and marketing of products cover all aspects of DanDrit’s business.
DanDrit and/or any future third party with which it has an effective collaboration or licensing agreement may be subject to changes in applicable governmental regulations and/or regulatory framework and be subject to additional or more onerous restrictions, which may make it necessary to make changes to personnel, facilities or procedures that could result in increased costs and adversely affect DanDrit’s business activities, including the development and commercialization of DanDrit’s vaccine candidates.
If DanDrit or its affiliates do not comply with applicable regulatory requirements or comply with significant legislative changes, DanDrit or its affiliates may be fined or risk having regulatory approvals suspended or withdrawn, risking recall or seizure of products, restrictions on activities and/or civil or criminal prosecution, which could have a material adverse effect on DanDrit’s business, financial condition, results of operations and future growth opportunities. Furthermore, we cannot guarantee that our vaccine candidates will be approved by the regulating agencies.
As long as the relevant regulatory authorities have not considered and approved applications for DanDrit’s vaccine candidates (New Drug Application (NDA) or equivalent) DanDrit and its affiliates cannot commercialize DanDrit’s vaccine candidates. Production and marketing of DanDrit’s products and DanDrit’s ongoing research and development activities are subject to rules set by numerous public authorities throughout the world. The regulatory authorities of each country can set their own requirements and may refuse to approve a product or may require additional data before approving a product, even if the product is approved by another regulating agency. Approvals may include restrictions on the marketing or use of products, which could adversely affect the amount of DanDrit’s revenue from the sale of those products.
We are conducting, and may in the future conduct, clinical trials for MCV or any future product candidates in sites outside the United States and the FDA may not accept data from trials conducted in such locations.
We have conducted, and may in the future choose to conduct, one or more of our clinical trials outside of the United States. Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of this data is subject to certain conditions imposed by the FDA. For example, the clinical trial must be well designed and conducted and performed by qualified investigators in accordance with ethical principles. The study population must also adequately represent the U.S. population, and the data must be applicable to the U.S. population and U.S. medical practice in ways that the FDA deems clinically meaningful. Generally, the patient population for any clinical studies conducted outside of the United States must be representative of the population for whom we intend to label the product in the United States. In addition, while these clinical trials are subject to the applicable local laws, FDA acceptance of the data will be dependent upon its determination that the studies also complied with all applicable U.S. laws and regulations. There can be no assurance the FDA will accept data from trials conducted outside of the United States. If the FDA does not accept the data from our clinical trial conducted outside the United States, it would likely result in the need for additional trials within the United States, which would be costly and time-consuming and delay or permanently halt our development of MCV or any future vaccine candidates.
In connection with the anticipated filing of our IND application with the FDA, we plan to submit trial results for trials we did not sponsor, which the FDA may refuse to consider.
DanDrit was only a sponsor of one of the clinical trials completed to date for MCV and while DanDrit Denmark employees and certain affiliates were closely involved in the design of the studies and the analysis and interpretation of the resulting data of all three studies. As a result, DanDrit intends to present all applicable data with respect to the current trials that is available to it, regardless of DanDrit’s specific role in any one of the trials. There are no assurances that the FDA will accept any data for any clinical trial in which it was not a sponsor or principal investigator that DanDrit submits in support of its IND application.
There can be no assurance that regulators will complete their review process in a timely manner, or that DanDrit vaccine candidates will obtain regulatory approval.
If DanDrit or any third party with which we have an effective collaboration or licensing agreement experience difficulties or delays in obtaining regulatory approvals, the development and commercialization of our vaccine candidates may be significantly delayed or even discontinued. Such difficulties or delays could result in significantly increased development costs and/or a delay or elimination of payments to us from our collaborators. This would have a material adverse effect on our business, financial condition, results of operations and future growth opportunities.
DanDrit will be dependent on external suppliers of certain services and technologies.
DanDrit will be dependent on a number of external parties such as contract laboratories and clinical research organizations, and in some cases our collaborators to:
|
●
|
Implement preclinical studies (pharmacology, toxicology testing and safety pharmacological evaluations).
|
|
|
●
|
Provide DanDrit with vaccine materials and support DanDrit’s activities related to preclinical and clinical studies.
|
|
|
●
|
Implement, inspect and/or monitor some or all aspects of the preclinical or clinical studies with DanDrit’s product candidates.
|
|
|
●
|
Ensure compliance with regulatory requirements such as Good Clinical Practice (“GCP”), Good Manufacturing Practice (“GMP”) and Good Laboratory Practices (“GLP”).
|
|
|
●
|
Deliver IT services.
|
|
|
●
|
Produce vaccine drugs and vaccines in accordance with GMP. The third parties DanDrit depends on may not be available when needed, or might not, if available, comply with all statutory and contractual requirements, and / or otherwise provide their services in a timely manner or in an acceptable manner.
|
DanDrit is dependent on its ability to recruit and retain qualified scientific and management personnel.
Recruiting and retaining qualified scientific and management personnel for the planning and execution of research and development; preparation of applications for intellectual property rights and regulatory approvals; and negotiating and maintaining cooperation with existing and new partners is essential for DanDrit.
While DanDrit has not thus far experienced any difficulty in recruiting and retaining qualified scientific and management personnel, DanDrit may in the future require additional expertise and manpower in areas such as preclinical trials, management of clinical trials, regulatory affairs, marketing, business development and management of partnerships. There can be no assurance that DanDrit will continue to be able to attract and retain such persons in light of demand for experienced employees from numerous pharmaceutical companies, chemical companies, specialized biopharmaceutical companies, universities and other research institutions. DanDrit’s employment contracts contain no limitation on competition that would prevent DanDrit’s current employees from being employed by DanDrit’s competitors or partners, if they choose to leave DanDrit. Inability to obtain or develop such expertise, or hire the employees they need, on reasonable terms, could have a material adverse effect on DanDrit’s business, financial condition, results of operations and future growth opportunities.
If our employees commit fraud or other misconduct, including noncompliance with regulatory standards, our business may experience serious adverse consequences.
DanDrit is exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations, to provide accurate information to the FDA, to comply with manufacturing standards we have established, to comply with federal and state health-care fraud and abuse laws and regulations, to report financial information or data accurately or to disclose unauthorized activities to us.
In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We have adopted a Code of Business Conduct and Ethics but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
DanDrit’s products may not achieve market acceptance. This would have a material adverse effect on our business, financial condition, results of operations and future growth opportunities.
The drugs DanDrit or its collaborators may develop, may not gain market acceptance among physicians, patients, third-party payors and the medical community, even if they are approved for marketing. The degree of market acceptance of the products approved for sale depends on a number of factors, including:
|
●
|
The ability of DanDrit or its collaborators to demonstrate the clinical efficacy, safety and benefits of the products.
|
|
|
●
|
The ability of DanDrit or its collaborators to demonstrate that the product has advantages over existing therapies or new alternative treatments.
|
|
|
●
|
The frequency and severity of any adverse effects arising from the use of the products.
|
|
|
●
|
The price of the products.
|
|
|
●
|
The subsidies DanDrit receives.
|
|
|
●
|
Efficacy within the therapeutic range for the illnesses the products are directed towards.
|
|
|
●
|
Patient comfort and user administration.
|
|
|
●
|
Requirements for marking.
|
|
|
●
|
The level of support for marketing and distribution.
|
We have no control over most of these factors. Furthermore, it may be difficult for us, to the extent that competitors are able to commercialize competing products before our vaccine candidates obtain regulatory approval, to develop a market for a vaccine because doctors, patients or third-party payors may have become accustomed to using a competing, existing product or for other reasons, even though our drug may be more effective or has other advantages.
If any of the vaccines we develop fail to achieve market acceptance in the future, we may not be able to generate significant revenue, which would have a material adverse effect on our business, financial condition, results of operations and future growth opportunities.
The use of DanDrit’s drugs or vaccine candidates may lead to unforeseen side effects. If any of our drugs or vaccine candidates is deemed to be unsafe, our business, financial condition, results of operations and future growth opportunities could be materially adversely affected.
All drugs are associated with a risk of allergic, immunologic genes or hyper-sensitivities. We test for allergic and immunological genes actions in preclinical and clinical studies, but if any of our products cause other allergic or immunological reactions than those considered acceptable by patients, doctors or regulatory authorities, we or our collaborators may be required to conduct additional clinical trials that will cause delays and increase costs for the development of a product, or development may have to be terminated or suspended on the grounds that participants will be exposed to unacceptable health risks.
Even in cases where pre-clinical or clinical studies have been successful, or received regulatory approval, a product can later prove to be unsafe. The incidence of adverse events may make it necessary for us and for our collaborators to carry out further investigations and studies. If a product is determined to be unsafe, we and our collaborators can be fined or risk having regulatory approvals suspended or withdrawn, be required to cease selling activities relating to the product, be required to recall the product, be subject to seizure of products, or be exposed to civil or criminal prosecution. Any of these results could have a material adverse effect on our business, financial condition, results of operations and future growth opportunities.
Third party reimbursement and reform measures on health care could have a material adverse effect on the commercial success of DanDrit’s vaccine candidates.
Market acceptance of DanDrit’s vaccine candidates depends in part on the extent to which the public and private health insurance and other third-party payors will subsidize DanDrit’s drugs.
Governments, insurance companies and health organizations are increasingly seeking to reduce healthcare costs by limiting coverage, price and reimbursement levels of new vaccine products as well as in some cases rejecting coverage. Reimbursement practices vary significantly from country to country, and some countries require that products undergo a lengthy review by the authorities before they meet the public support requirements.
In the United States, in Canada and in many other countries, pricing and/or profitability of some or all prescription pharmaceuticals and biopharmaceuticals are subject to varying degrees of government control. Healthcare reform and controls on healthcare spending may limit the price we charge for any products and the amounts thereof that we can sell. In particular, in the United States, the federal government and private insurers have changed and have considered ways to change, the manner in which healthcare services are provided. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, PPACA, became law in the United States. PPACA substantially changes the way healthcare is financed by both governmental and private insurers and significantly affects the healthcare industry. The provisions of PPACA of importance to our product candidates include the following:
|
●
|
an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs;
|
|
|
●
|
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program, to 23.1% and 13.0% of the average manufacturer price for most branded and generic drugs, respectively;
|
|
|
●
|
expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance;
|
|
●
|
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for a manufacturer’s outpatient drugs to be covered under Medicare Part D;
|
|
|
●
|
extension of a manufacturer’s Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations;
|
|
|
●
|
expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the federal poverty level beginning in 2014, thereby potentially increasing a manufacturer’s Medicaid rebate liability;
|
|
|
●
|
expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
|
|
●
|
new requirements under the federal Open Payments program and its implementing regulations;
|
|
|
●
|
a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; and
|
|
|
●
|
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
|
In addition, other legislative changes have been proposed and adopted since PPACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect on April 1, 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our customers and accordingly, our financial operations.
We anticipate that PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and additional downward pressure on the reimbursement we may receive for any approved product. Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, the Middle Class Tax Relief and Job Creation Act of 2012 requires the Centers for Medicare & Medicaid Services, or CMS, to reduce the Medicare clinical laboratory fee schedule by 2% in 2013, which in turn will serve as a base for 2014 and subsequent years. CMS also recently proposed to re-examine payment amounts for tests reimbursed under the Medicare clinical laboratory fee schedule due to changes in technology and, in addition, proposed to bundle the Medicare payments for certain laboratory tests ordered while a patient received services in a hospital outpatient setting. Such changes went into effect January 1, 2014. Levels of reimbursement may be impacted by current and future legislation, regulation or reimbursement policies of third-party payors in a manner that may harm the demand and reimbursement available for our products, which in turn, could harm our future product pricing and sales. Any reduction in reimbursement from Medicare and other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
There may be delays or difficulties in the recruitment and monitoring of patients in clinical trials. Any such delays could have a material adverse effect on DanDrit’s business, financial condition, results of operations and future growth opportunities.
All clinical development of new vaccine candidates depends on the recruitment of volunteer suitable patients for clinical trials. While DanDrit has not experience difficulties in recruiting patients to date, the ability to recruit patients depends on certain factors, including the prevalence of the disease in the population and it may be more difficult to find a sufficient number of patients to participate in clinical trials for drugs being developed for a disease that is common among the general population. Even if a disease is frequent among the population, there may be a number of other companies developing drugs that target the same disease who may eventually have more success in recruiting among the total group of potential patients for their clinical studies. In addition, trials in which patients may receive a placebo are typically more difficult to populate. DanDrit’s next clinical trial will include a comparative placebo arm. While DanDrit currently plans to address these potential difficulties, potentially through 2:1 randomization that increases a patient’s potential to receive active treatment vs. a placebo, if we or our collaborators find it difficult to recruit a sufficient number of patients to participate in clinical trials for one of its vaccine candidates DanDrit and/or its collaborators may have to postpone or discontinue ongoing clinical trials. Delays may also result in increased costs for clinical studies and may affect the implementation of studies required for a vaccine candidate’s approval. Delay or complete termination of a clinical trial program could have a material adverse effect on DanDrit’s business, financial condition, results of operations and future growth opportunities.
DanDrit may not be able to make, or cause others to conduct, animal testing in the future. This could have a material adverse effect on our research and development work.
Research into dendritic cell vaccines does not generally involve animals. But, certain aspects of DanDrit’s biotechnology research and development may be carried out on animals. Changes to laws and regulations, recognized clinical procedures, or experimental protocols may have a negative impact on this research and development. Pressure from society, which may lead to restrictions on the use of animals or result in actions against DanDrit, its affiliates or its clinical research organizations from groups of people or individuals who are against animal testing may also have a material adverse effect on research and development work.
DanDrit faces extensive competition. If our vaccine candidates cannot compete successfully, our business, financial condition, results of operations and future growth opportunities could be materially, adversely affected.
There is extensive competition in the biopharmaceutical industry and the technology is developing rapidly. DanDrit is developing a vaccine for the treatment of advanced colorectal cancer, where competing products may be introduced. If these newly developed products are more efficient, cheaper, more patient-friendly, safer, or better placed than DanDrit’s vaccine candidates, or if DanDrit’s competitors develop drugs that reduce or eliminate the need for DanDrit’s vaccine candidates, such competition could reduce or eliminate DanDrit’s commercial opportunities. Many of DanDrit’s competitors have substantially greater financial, technical and human resources than DanDrit and significantly more experience than DanDrit with preclinical and clinical research and development and in obtaining regulatory approval of pharmaceutical products.
DanDrit’s drugs may face competition as a result of many factors, including the route of administration (e.g. oral administration vs. injection), the availability and cost of production, efficiency of DanDrit’s partners’ marketing and sales efforts as well as the price of DanDrit’s products. DanDrit has limited or no previous experience in these areas. DanDrit’s inability to compete effectively would have a material adverse effect on DanDrit’s business, financial condition, results of operations and future growth opportunities.
DanDrit is likely to be exposed to product liability claims. If product liability lawsuits are successfully brought against us, our insurance may be inadequate. If a judgment were to exceed our insurance coverage, our business could be materially, adversely affected.
DanDrit will be exposed, by virtue of the nature of its business, to the risk of potential product liability claims, which is a natural part of the clinical development, manufacture and marketing of drugs. Even in cases where DanDrit has granted licenses to third parties to manufacture and sell its products, there can be no assurance that DanDrit will not be included in any product liability claims relating to these medicines, or claims by third parties, including DanDrit’s partners, for indemnity or other compensation from DanDrit in connection with any such claims.
We plan to obtain product liability insurance coverage once our clinical trials commence. However, our insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If and when we obtain marketing approval for our product candidates, we intend to expand our insurance coverage to include the sale of commercial products; however, we may be unable to obtain this product liability insurance on commercially reasonable terms. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be substantial. A successful product liability claim or series of claims brought against us could cause our share price to decline (assuming a trading market in our common stock is established) and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
We will need substantial additional funds to support our operations, and such funding may not be available to us on acceptable terms, or at all, which would force us to terminate, delay, reduce or suspend our operations, research and development programs and other commercialization efforts.
The completion of the development and the potential commercialization of MCV and any future product candidates, should they receive approval, will require substantial funds. As of December 31, 2013 and March 31, 2014, we had approximately $96,262 and $478,441 in cash and cash equivalents, respectively. We project that cash will be derived from revenues received from eight patients each month beginning November 1, 2014 in connection with the MyTomorrows program and committed financing secured by Paseco ApS for DKK 2,000,000 (the “Paseco Note”) or approximately $369,920, if necessary. This note accrues annual interest at 5% per annum and is due and payable on February 1, 2015. Our projected revenues and cash from financing activities for the next 12 months are approximately $980,000 and $1,300,000, respectively, and our projected expenses are approximately $1,700,000. On April 29, 2014, DanDrit Denmark and Paseco amended the Paseco Note to extend the term to February 1, 2015, with a further extension permitted at the Company’s option for an additional year with an increase in the interest rate to 7.00% per annum. We believe that the anticipated revenues and financing described above will supply us with sufficient cash and cash equivalents to sustain our operations for the next 12 months based on our existing business plan. Our future financing requirements will depend on many factors, some of which are beyond our control, including the following:
|
●
|
the rate of progress and cost of our clinical studies;
|
|
|
●
|
the timing of, and costs involved in, seeking and obtaining approvals from the FDA and other regulatory authorities;
|
|
●
|
the cost of preparing to manufacture MCV on a larger scale;
|
|
|
●
|
the costs of commercialization activities if MCV or any future product candidate is approved, including product sales, marketing, manufacturing and distribution;
|
|
|
●
|
the degree and rate of market acceptance of any products launched by us or future partners;
|
|
|
●
|
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
|
|
|
●
|
our ability to enter into additional collaboration, licensing, commercialization or other arrangements and the terms and timing of such arrangements;
|
|
|
●
|
the emergence of competing technologies or other adverse market developments; and
|
|
|
●
|
the costs of attracting, hiring and retaining qualified personnel.
|
Other than the Paseco Note, we do not have any committed external source of funds or other support for our operational or development efforts. Until we can generate a sufficient amount of product revenue to finance our cash requirements, which we may never do, we expect to finance future cash needs through a combination of public or private equity offerings, debt financings, collaborations, strategic alliances, licensing arrangements and other marketing and distribution arrangements. Additional financing may not be available to us when we need it or it may not be available on favorable terms. If we raise additional capital through marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish certain valuable rights to MCV or potential future product candidates, technologies, future revenue streams or research programs, or grant licenses on terms that may not be favorable to us. If we raise additional capital through public or private equity offerings, the ownership interest of our existing stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect our stockholders’ rights.
If we raise additional capital through debt financing, we may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we are unable to obtain adequate financing when needed, we may have to delay, reduce the scope of, or suspend one or more of our clinical studies or research and development programs or our commercialization efforts.
Raising capital in the future could cause dilution to our existing shareholders, and may restrict our operations or require us to relinquish rights.
In the future, we may seek additional capital through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a shareholder. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates, or grant licenses on terms that are not favorable to us.
We depend on intellectual property and the failure to protect our intellectual property could adversely affect our future growth and success. This would have a material adverse effect on our business, financial condition and results of operations.
We rely on patent, trademark and copyright law, trade secret protection, and confidentiality and other agreements with employees, customers, collaborators and others to protect our intellectual property. However, some of our intellectual property is not covered by any patent or patent application, and, despite precautions, it may be possible for third parties to obtain and use our intellectual property without authorization.
We do not know whether any patents will be issued from pending or future patent applications or whether the scope of the issued patents is sufficiently broad to protect our technologies or processes. The patent position of biotechnology companies is generally uncertain because it involves complex legal and factual considerations. The standards applied by the United States Patent and Trademark Office and foreign patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in biotechnology and pharmaceutical patents. Consequently, patents may not issue from our pending patent applications. As such, we do not know the degree of future protection that we will have on our proprietary products and technology.
We endeavor to aggressively protect our technologies through patents covering compositions of matter, drug targets and aspects of mechanism of action, drug product formulation, methods of use and methods of manufacture, and trade secrets. We have filed patent applications and in-licensed others with respect to our technology both domestically and internationally and anticipate filing multiple patent applications, in the future. While we believe that we will be able to secure adequate and enforceable patent protection for our products and technologies, there is no guarantee that patent protection can be obtained, and even if it is obtained that such patent protection will ultimately be deemed valid, sufficiently enforceable, sufficient to preclude competition or not infringe upon the rights of other parties. Furthermore, the laws of some foreign countries may not protect intellectual property rights to the same extent as do the laws of the United States and Denmark.
The patents protecting our proprietary technologies expire after a period of time. Currently, our patents have expiration dates ranging from 2021 through 2024. Although we have attempted to incorporate technology from our core patents into specific patented product applications, product designs and packaging to extend the lives of our patents, this approach may not be successful in protecting our proprietary technology. If we are not successful in protecting our proprietary technology, it could have a material adverse effect on our business, financial condition and results of operations.
We may not be successful in protecting our proprietary rights. Any infringement upon our intellectual property rights could have an adverse effect on our ability to develop our products and sell them commercially.
Issued patents covering one or more of our product candidates could be found invalid or unenforceable if challenged in court. If that were to happen, our business would be adversely impacted.
If we were to initiate legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the U.S. Patent and Trademark Office, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on one or more of our product candidates or certain aspects of our platform technology. Such a loss of patent protection could have a material adverse impact on our business.
We may infringe on the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing our products.
Our commercial success depends significantly on our ability to operate without infringing the patents and other intellectual property rights of third parties. For example, there could be issued patents of which we are not aware that our products infringe. There also could be patents that we believe we do not infringe, but that we may ultimately be found to have infringed. Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our products infringe. For example, pending applications may exist that provide support or can be amended to provide support for a claim that results in an issued patent that our product infringes.
Third parties may assert that we are employing their proprietary technology without authorization. If a court held that any third-party patents are valid, enforceable and cover our products or their use, the holders of any of these patents may be able to block our ability to commercialize our products unless we obtained a license under the applicable patents, or until the patents expire. We may not be able to enter into licensing arrangements or make other arrangements at a reasonable cost or on reasonable terms. Any inability to secure licenses or alternative technology could result in delays in the introduction of our products or lead to prohibition of the manufacture or sale of products by us.
Unfavorable outcomes in intellectual property litigation could limit our research and development activities and/or our ability to commercialize certain products.
If third parties successfully assert intellectual property rights against us, we might be barred from using certain aspects of our technology, or barred from developing and commercializing certain products. Prohibitions against using certain technologies, or prohibitions against commercializing certain products, could be imposed by a court or by a settlement agreement between us and a plaintiff. In addition, if we are unsuccessful in defending against allegations of patent infringement or misappropriation of trade secrets, we may be forced to pay substantial damage awards to the plaintiff. There is inevitable uncertainty in any litigation, including intellectual property litigation. There can be no assurance that we would prevail in any intellectual property litigation, even if the case against us is weak or flawed. If litigation leads to an outcome unfavorable to us, we may be required to obtain a license from the patent owner, in order to continue our research and development programs or to market our product(s). It is possible that the necessary license will not be available to us on commercially acceptable terms, or at all. This could limit our research and development activities, our ability to commercialize certain products, or both.
Most of our competitors are larger than we are and have substantially greater resources. They are, therefore, likely to be able to sustain the costs of complex patent litigation longer than we could. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our internal research programs, in-license needed technology, or enter into strategic partnerships that would help us bring our product candidates to market.
In addition, any future patent litigation, interference or other administrative proceedings will result in additional expense and distraction of our personnel. An adverse outcome in such litigation or proceedings may expose us or any future collaborators to loss of our proprietary position, expose us to significant liabilities, or require us to seek licenses that may not be available on commercially acceptable terms, if at all.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity. Therefore, obtaining and enforcing pharmaceutical patents is costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. The United States Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. PTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Confidentiality agreements with employees and third parties may not prevent unauthorized disclosure of trade secrets and other proprietary information.
In addition to patents, we rely on other methods to protect our trade secrets, technical know-how, and proprietary information. In the course of our research and development activities and our business activities, we often rely on confidentiality agreements to protect our proprietary information. Such confidentiality agreements are used, for example, when we talk to vendors of laboratory or clinical development services or potential collaborators. We take steps to protect our proprietary information, and our confidentiality agreements are carefully drafted to protect our proprietary interests. Nevertheless, there can be no guarantee that an employee or an outside party will not make an unauthorized disclosure of our proprietary confidential information. This might happen intentionally or inadvertently. It is possible that a competitor will make use of such information in spite of any legal action we might take against persons making such unauthorized disclosures.
Trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, or outside collaborators might intentionally or inadvertently disclose our trade secret information to competitors. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States sometimes are less willing than U.S. courts to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
Foreign currency fluctuations could adversely impact financial performance.
Our reporting currency is the United States dollar. Because of our activities in Denmark, the United Kingdom and continental Europe, we are exposed to fluctuations in foreign currency rates. We may manage the risk to such exposure by entering into foreign currency futures and option contracts. Foreign currency fluctuations may have a significant effect on our operations in the future.
Assuming that our vaccine candidates receive regulatory approval and we begin sales of these products, our results may fluctuate due to certain regulatory, marketing and competitive factors over which we have little or no control.
Assuming that our vaccine candidates receive regulatory approval and we begin the sale of these products, the factors listed below, some of which we cannot control, may cause our revenue and results of operations to fluctuate significantly:
|
●
|
Actions taken by regulatory bodies relating to the verification, registration or health effects of our products.
|
|
|
●
|
The extent to which existing and newly developed products obtain market acceptance.
|
|
|
●
|
The timing and size of customer purchases.
|
|
|
●
|
Customer concerns about the stability of our business, which could cause them to seek alternatives to our solutions and products; and
|
|
|
●
|
Increases in raw material costs.
|
We will incur significant costs as a result of operating as a public company, and our management may be required to devote substantial time to compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses which we estimate to be in excess of $300,000 annually. In addition, the Sarbanes-Oxley Act, as well as rules subsequently implemented by the SEC, have imposed various requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls as well as mandating certain corporate governance practices. Our management and other personnel will devote a substantial amount of time and financial resources to these compliance initiatives.
If we fail to staff our accounting and finance function adequately, or maintain internal control systems adequate to meet the demands that are placed upon us as a public company, we may be unable to report our financial results accurately or in a timely manner and our business and stock price, assuming that a market for our stock develops, may suffer. The costs of being a public company, as well as diversion of management’s time and attention, may have a material adverse effect on our future business, financial condition and results of operations.
A significant portion of our assets and the majority of our officers and directors are located outside of the United States and therefore it may be difficult for an investor to enforce within the United States any judgments obtained against us or such officers and directors.
A significant portion of our assets are located outside of the United States. In addition, the majority of our officers and directors are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons’ assets are located outside the United States. As a result, it may be difficult for an investor to effect service of process or enforce within the United States any judgments obtained against us or such officers or directors, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof. In addition, there is uncertainty as to whether the courts of other jurisdictions would recognize or enforce judgments of United States courts obtained against us or our directors and officers predicated upon the civil liability provisions of the securities laws of the United States or any state thereof, or be competent to hear original actions brought in other jurisdictions against us, or such officers and directors predicated upon the securities laws of the United States or any state thereof.
RISKS RELATED TO OWNERSHIP OF OUR COMMON STOCK
There is currently no trading market for our common stock and we cannot guarantee you that a trading market will develop. You may be unable to sell your shares of our common stock if you need to liquidate your investment.
While our class of common stock is registered under the Exchange Act and we are a full SEC “reporting company,” our individual shares of common stock are not currently registered under the securities laws of any state or other jurisdiction, and none of such shares currently may publicly trade through exemptions from such registration. Accordingly there is no public trading market for our common stock. Further, we cannot guarantee you that a public trading market will develop in the foreseeable future. Currently, outstanding shares of our common stock cannot be offered, sold, pledged or otherwise transferred unless registered pursuant to, or exempt from registration under, the Securities Act and any other applicable federal or state securities laws or regulations. Compliance with the criteria for securing exemptions under federal securities laws and the securities laws of the various states is extremely complex. If you purchase shares of our common stock, there may be no market in which to sell them if you need to liquidate your investment in the future or the transfer or sale you wish to make may not comply with federal or state securities laws and would, therefore, be prohibited.
If a public market for our common stock develops, it may be volatile. This may affect the ability of our investors to sell their shares as well as the price at which they sell their shares.
If a market for our common stock develops, the market price for shares of our common stock may be significantly affected by factors such as variations in quarterly and yearly operating results, general trends in the biotechnology industry, and changes in state or federal regulations affecting us and our industry.
Furthermore, in recent years the stock market has experienced extreme price and volume fluctuations that are unrelated or disproportionate to the operating performance of the affected companies. Such broad market fluctuations may adversely affect the market price of our common stock, if a market for it develops.
As an “emerging growth company” under the JOBS Act, we are permitted to rely on exemptions from certain disclosure requirements.
We qualify as an “emerging growth company” under the JOBS Act. As a result, we are permitted to and may rely on exemptions from certain disclosure requirements. For so long as we are an emerging growth company, we will not be required to:
|
●
|
have an auditor report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act;
|
|
|
●
|
comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis);
|
|
|
●
|
submit certain executive compensation matters to shareholder advisory votes, such as “say-on-pay”, “say-on-frequency” and “say-on-golden parachute;” and
|
|
|
●
|
disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive’s compensation to median employee compensation.
|
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are not choosing to “opt out” of this provision. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable. As a result of our election, not to “opt out” of Section 107, DanDrit’s financial statements may not be comparable to companies that comply with public company effective dates.
We will remain an “emerging growth company” until the last day of our fiscal year following the fifth anniversary of the date of our first sale of common equity securities pursuant to an effective registration under the Securities Act, or until the earliest of (i) the last day of the first fiscal year in which our total annual gross revenues exceed $1 billion, (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, which would occur if the market value of our ordinary shares that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period.
Until such time, however, we cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
We have never paid dividends on our common stock.
We have never paid dividends on our common stock and do not presently intend to pay any dividends in the foreseeable future. We anticipate that any funds available for payment of dividends will be re-invested into the Company to further its business strategy. Because we do not anticipate paying dividends in the future, the only opportunity for our stockholders to realize the creation of value in our common stock will likely be through a sale of those shares.
The offering is being made on a best efforts basis with no minimum amount of shares required to be sold for the offering to proceed. If we are unable to raise the funds we need to fully implement our business plan, investors who participate in the offering may lose their entire investment.
In order to fully implement our business plan, we must raise $12,000,000 from this offering. However, our offering is being made on a best efforts basis with no minimum amount of shares required to be sold for the offering to proceed. If we raise only a nominal amount of proceeds we would likely be unable to implement our business plan, we may have to suspend or cease operations and investors who participate in this offering may lose their entire investments.
We arbitrarily determined the price of the common stock we are offering.
We determined the offering price of the common stock we are offering. The offering price does not bear any direct relationship to the value of our physical assets, the book value of our common stock, or any other generally accepted criteria of valuation. The price and other terms were based on a number of factors including, without limitation, estimates of our business potential and earnings prospects and the consideration of such potential earnings in relation to market valuations of comparable companies. The offering price is not an indication of the actual value of our common stock at the time of this offering.
Furthermore, if a market for our common stock develops, the price of our common stock following this offering may be highly volatile as the securities of emerging businesses often are. Factors such as our financial results and the introduction of new products by us or by our competitors, and various factors affecting the biotechnology industry generally, may have a significant impact on the market price of our securities. Additionally, in recent years, the stock market has experienced a high level of price and volume volatility and market prices for the securities of many companies, particularly of small and emerging growth companies, the common stock of which trade in the over-the-counter market, have experienced wide price fluctuations which have not necessarily been related to the operating performance of these companies.
The Company has not received any commitments to purchase the common stock under the Public Offering Prospectus. There is no minimum offering amount.
The Company will use its best efforts to sell all of the common stock offered under the Public Offering Prospectus. These sales may be made either directly by the Company, through its officers and directors, or with the assistance of the Placement Agents. No underwriter has been engaged in connection with the offering or performed any due diligence activities which would otherwise confirm the accuracy of our disclosures in the registration statement of which this prospectus is a part and our price of the offered common stock. None of the Company, the Placement Agents, or any other person, has made any commitment to purchase any of the shares offered hereby. Consequently, there can be no assurance that any of the shares will be sold. There is no minimum offering amount required for us to accept subscriptions for our Common Stock. To the extent that the net proceeds raised by the Company are substantially less than the maximum offering amount, the Company's opportunities would be severely diminished. In the event that an alternate source of financing is not obtained in a timely manner, those investors who participate in this offering risk the loss of their entire investments. In the event that we file a post-effective amendment to increase the offering amount pursuant to Rule 462(b) of the Securities Act, we plan to allocate the extra funds in strengthening our Phase II/III clinical trial with a larger sample size and in targeting patients with stage III colorectal cancer rather than metastatic (stage IV) colorectal cancer patients. See "Use of Proceeds," "Management’s Discussion and Analysis of Financial Condition and Results of Operations" and "Plan of Distribution."
We have the right to issue shares of preferred stock. If we were to issue preferred stock, it is likely to have rights, preferences and privileges that may adversely affect the common stock.
We are authorized to issue 10,000,000 shares of “blank check” preferred stock, with such rights, preferences and privileges as may be determined from time-to-time by our board of directors. No preferred stock is currently issued and outstanding. Our board of directors is empowered, without stockholder approval, to issue preferred stock in one or more series, and to fix for any series the dividend rights, dissolution or liquidation preferences, redemption prices, conversion rights, voting rights, and other rights, preferences and privileges for the preferred stock. No shares of preferred stock are presently issued and outstanding and we have no immediate plans to issue shares of preferred stock. The issuance of shares of preferred stock, depending on the rights, preferences and privileges attributable to the preferred stock, could adversely reduce the voting rights and powers of the common stock and the portion of the Company’s assets allocated for distribution to common stockholders in a liquidation event, and could also result in dilution in the book value per share of the common stock being offered. The preferred stock could also be utilized, under certain circumstances, as a method for raising additional capital or discouraging, delaying or preventing a change in control of the Company, to the detriment of the investors in the common stock being offered. We cannot assure you that the Company will not, under certain circumstances, issue shares of its preferred stock.
We may use the net proceeds from this offering in ways which differ from our estimates and with which you may not agree.
The discussion titled “Use of Proceeds”, which appears elsewhere in this prospectus, sets forth the way in which we expect to use the net proceeds of this offering. The discussion represents our estimates based upon our current plans, assumptions regarding industry and general economic conditions, and our anticipated future revenues and expenditures. The amounts and timing of our actual expenditures will depend on numerous factors, including market conditions, cash generated by our operations, business developments and the rate of our growth. We may find it necessary or advisable to use portions of the proceeds from this offering for purposes other than those stated in the Use of Proceeds discussion. Circumstances that may give rise to a change in the use of proceeds and the alternate purposes for which the proceeds may be used are also discussed in the section of this prospectus titled “Use of Proceeds”. You will not have an opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use our proceeds. As a result, you and other shareholders may not agree with the decisions made by our management relating to the way in which the proceeds from this offering are used. See the discussion titled “Use of Proceeds” for additional information.
You will experience immediate dilution in the book value per share of the common stock you purchase.
Because the price per share of our common stock being offered is substantially higher than the book value per share of our common stock, you will experience substantial dilution in the net tangible book value of the common stock you purchase in this offering. Based on an assumed offering price of $5.00 per share, if you purchase shares of common stock in this offering, you will experience immediate and substantial dilution of $3.87 per share in the net tangible book value of the common stock at March 31, 2014. See the section titled “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
Penny stock regulations may impose certain restrictions on the marketability of our securities.
The SEC has adopted regulations which generally define “penny stock” to be any equity security that has a market price (as defined) less than $5 per share, is not listed on a national exchange and fails to meet other specific criteria, subject to certain exceptions. We anticipate that our common stock, when trading commences, will be subject to these regulations which impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000, excluding the net value of the person’s primary residence, or annual income exceeding $200,000, or $300,000 together with the investor’s spouse). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of such securities and have received the purchaser’s written consent to the transaction prior to the purchase. Additionally, for any transaction involving a “penny stock”, unless exempt, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the SEC relating to the “penny stock” market. The broker-dealer must also disclose the commission payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the “penny stock” held in the account and information on the limited market in “penny stocks”. Consequently, if and when our common stock becomes publicly traded, the “penny stock” rules may restrict the ability of broker-dealers to sell our securities and may negatively affect the ability of purchasers of our shares of common stock to sell such securities.
A significant portion of our total outstanding shares of common stock is restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market, including shares issuable upon the effectiveness of this registration statement or upon the expiration of any statutory holding period under Rule 144, or existing lock-up agreements could occur in the future. These sales, or the perception in the market that the holders of a large number of shares of common stock intend to sell shares, could reduce the market price of our common stock. Our directors, executive officers and certain shareholders, including those shareholders that were issued common stock in the Share Exchange, and the selling shareholders described herein are subject to a lock-up agreement pursuant to which these persons have agreed, subject to specified exceptions, not to sell, transfer, dispose of, contract to sell, sell any option or contract to purchase, or otherwise transfer or dispose of, directly or indirectly, without the written consent of Sunrise Securities Corp., one of the Placement Agents, any shares of our common stock or any securities convertible into or exercisable or exchangeable for shares of our common stock for a period of 180 days beginning as of the filing date of the last pre-effective amendment to this prospectus. Once these lock-up provisions expire, and provided that one year has passed following the date the company filed Form 10 information with the SEC, may be sold in the public market pursuant to Rule 144, which could cause the market price of our common stock to drop significantly.
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. You can find many (but not all) of these statements by looking for words such as “approximates,” “believes,” “hopes,” “expects,” “anticipates,” “estimates,” “projects,” “intends,” “plans,” “would,” “should,” “could,” “may” or other similar expressions in this prospectus. These statements may be found in the sections of this prospectus titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business”, as well as in this prospectus generally. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from our historical experience and our present expectations or projections. Factors that could cause actual results to differ from those discussed in the forward-looking statements include, but are not limited to:
|
●
|
future financial and operating results, including projections of revenues, income, expenditures, cash balances and other financial items;
|
|
|
●
|
our capital requirements and the need for additional financing;
|
|
|
●
|
our ability to protect our intellectual property rights and secure the right to use other intellectual property that we deem to be essential to the conduct of our business;
|
|
|
●
|
our ability to execute our growth, expansion and acquisition strategies;
|
|
|
●
|
current and future economic and political conditions;
|
|
|
●
|
overall industry and market performance;
|
|
|
●
|
competition;
|
|
|
●
|
management’s goals and plans for future operations; and
|
|
|
●
|
other assumptions described in this prospectus underlying or relating to any forward-looking statements.
|
We describe material risks, uncertainties and assumptions that could affect our business, including our financial condition and results of operations, under “Risk Factors.” We base our forward-looking statements on our management’s beliefs and assumptions based on information available to our management at the time the statements are made. We caution you that actual outcomes and results may differ materially from what is expressed, implied or forecast by our forward-looking statements. Accordingly, you should be careful about relying on any forward-looking statements. Reference is made in particular to forward-looking statements regarding growth strategies, financial results, product development, competitive strengths, intellectual property rights, litigation, mergers and acquisitions, market acceptance or continued acceptance of our products, accounting estimates, financing activities, ongoing contractual obligations and sales efforts. Except as required under the federal securities laws and the rules and regulations of the SEC, we do not have any intention or obligation to update publicly any forward-looking statements after the distribution of this prospectus, whether as a result of new information, future events, changes in assumptions, or otherwise.
Assuming an initial public offering price of $5.00 per share, after deducting commissions and estimated offering expenses payable by us, we estimate that we will receive net proceeds up to $10,785,464 from the sale of 2,400,000 shares of our common stock (100% of the offering), $8,024,464 from the sale of 1,800,000 shares of our common stock (75% of the offering), $5,265,464 from the sale of 1,200,000 shares of our common stock (50% of the offering) and $2,505,464 from the sale of 600,000 shares of our common stock (25% of the offering).
We intend to use the proceeds from this offering to conduct the Phase IIb/III clinical trial with MCV and pay $526,689 in outstanding debt received from Sune Holding ApS, a shareholder of DanDrit, which accrues interest at the rate of 5% per annum and is due upon the earlier of 14 days following the closing of this offering or February 1, 2015 (the “Sune Loan”). All remaining proceeds will be used for working capital and general corporate purposes. In the event that we receive maximum proceeds under the offering, we anticipate that we will be able to (i) complete the first step of our Phase II/III clinical trial in metastatic colorectal cancer, (ii) refine the design of the second step (Phase III trial) and (iii) initiate a collaboration agreement with a pharmaceutical partner. If we receive 75% of the maximum proceeds of the offering, we anticipate that we will be able to (i) provide comparative randomized confirmation of efficacy on a large scale that may trigger a partnership with a pharmaceutical company and (ii) pursue the Patient Name Use program with our partner MyTomorrows. If we receive 50% of the maximum proceeds of the offering, we anticipate that we will be able to (i) provide comparative randomized confirmation of efficacy on a smaller scale with a lower hazard ratio (HR) (a ratio used in time to event analysis) that may still trigger a less attractive partnership with a pharmaceutical company and (ii) pursue the Patient Name Use program with our partner MyTomorrows. HR is the hazard of an event in the studied arm divided by the hazard of the same event in the control arm. In our context the event is survival. An HR of 1 means that survival will be the same in both arms. An HR of 0.5 means that at a determined time, half as many patients in the vaccine group will have survived proportionally to the placebo control group. If we receive 25% of the maximum proceeds of the offering, we anticipate that we will be able to conduct a small non-comparative trial that allow us to pursue the patient Name Use Program with our partner MyTomorrows.
If we are unable to raise net proceeds equal to at least $10,700,000, we intend to first apply any proceeds raised towards repayment of the Sune Loan and the development and marketing of our products and the engineering, development and testing of vaccines. However, to the extent that we are unable to raise a sufficient amount of proceeds in this offering, we may not be able to achieve all our business objectives in a timely manner.
In the event that we file a post-effective amendment to increase the offering amount pursuant to Rule 462(b) of the Securities Act, we plan to allocate the extra funds in strengthening our Phase II/III clinical trial with a larger sample size and in targeting patients with stage III colorectal cancer rather than metastatic (stage IV) colorectal cancer patients.
Pending any ultimate use of any portion of the proceeds from this offering, we intend to invest the proceeds in a variety of capital preservation investments, including short-term, interest-bearing instruments such as United States government securities and municipal bonds.
The anticipated use of proceeds for the offering funds based on assumed amounts of 100%, 75%, 50% and 25% of total offering is summarized below.
|
Use of the Net Proceeds from the Sale of Shares at 100% of the offering:
|
||||||||||||||||
|
USD
|
Year 1
|
Year 2
|
Year 3
|
Total
|
||||||||||||
|
Manufacturing
|
230,000
|
285,000
|
315,000
|
830,000
|
||||||||||||
|
General & Administrative expenses
|
975,000
|
978,000
|
717,656
|
2,670,656
|
||||||||||||
|
Clinical trial costs
|
1,861,464
|
2,430,000
|
2,466,656
|
6,758,120
|
||||||||||||
|
Debt Repayment
|
526,689
|
526,689
|
||||||||||||||
|
Total
|
3,593,153
|
3,693,000
|
3,499,311
|
10,785,464
|
||||||||||||
|
Use of the Net Proceeds from the Sale of Shares at 75% of the offering:
|
||||||||||||||||
|
USD
|
Year 1
|
Year 2
|
Year 3
|
Total
|
||||||||||||
|
Manufacturing
|
156,400
|
193,800
|
214,200
|
564,400
|
||||||||||||
|
General & Administrative expenses
|
860,000
|
863,000
|
602,656
|
2,325,656
|
||||||||||||
|
Clinical trial costs
|
1,296,715
|
1,685,675
|
1,626,330
|
4,608,720
|
||||||||||||
|
Debt Repayment
|
526,689
|
526,689
|
||||||||||||||
|
Total
|
2,839,804
|
2,742,475
|
2,4443,185
|
8,025,464
|
||||||||||||
|
Use of the Net Proceeds from the Sale of Shares at 50% of the offering:
|
||||||||||||||||
|
USD
|
Year 1
|
Year 2
|
Year 3
|
Total
|
||||||||||||
|
Manufacturing
|
92,000
|
108,300
|
119,700
|
320,000
|
||||||||||||
|
General & Administrative expenses
|
740,000
|
743,000
|
482,656
|
1,965,656
|
||||||||||||
|
Clinical trial costs
|
755,664
|
923,400
|
774,056
|
2,453,120
|
||||||||||||
|
Debt Repayment
|
526,689
|
526,689
|
||||||||||||||
|
Total
|
2,114,353
|
1,774,700
|
1,376,411
|
5,265,464
|
||||||||||||
|
Use of the Net Proceeds from the Sale of Shares at 25% of the offering:
|
||||||||||||||||
|
USD
|
Year 1
|
Year 2
|
Year 3
|
Total
|
||||||||||||
|
Manufacturing
|
34,500
|
0
|
0
|
34,500
|
||||||||||||
|
General & Administrative expenses
|
700,000
|
439,656
|
442,656
|
1,582,311
|
||||||||||||
|
Clinical trial costs
|
278,700
|
82,264
|
0
|
360,964
|
||||||||||||
|
Debt Repayment
|
526,689
|
526,689
|
||||||||||||||
|
Total
|
1,539,889
|
521,920
|
442,656
|
2,504,464
|
||||||||||||
The amounts and timing of our actual expenditures will depend on numerous factors, including market conditions, results from our research and development efforts, business developments and opportunities and the rate of our growth, sales and marketing activities and competition. Accordingly, our management will have broad discretion in the application of the net proceeds, and investors will be relying on the judgment of our management regarding the application of the proceeds from this offering. We may find it necessary or advisable to use portions of the proceeds from this offering for other purposes. Circumstances that may give rise to a change in the use of proceeds and the alternate purposes for which the proceeds may be used include:
|
●
|
the existence of unforeseen or other opportunities or the need to take advantage of changes in the timing of our existing activities;
|
|
|
●
|
the need or desire on our part to accelerate, increase, reduce, change or eliminate one or more existing initiatives due to, among other things, changing market conditions and competitive developments or interim results of research and development efforts;
|
|
●
|
results from our business development and marketing efforts, including opportunities that may materialize;
|
|
|
●
|
the effect of federal, state, and local regulation on us and on our identified industries;
|
|
|
●
|
our ability to attract development funding or to license or sell our vaccine candidates;
|
|
|
●
|
the presentation of strategic opportunities of which we are not currently aware (including acquisitions, joint ventures, licensing and other similar transactions); and/or
|
|
|
●
|
the filing of a post-effective amendment pursuant to Rule 462(b) of the Securities Act in order to raise additional funds to strengthen our Phase II/III clinical trial with a larger sample size and target patients with stage III colorectal cancer rather than metastatic (stage IV) colorectal cancer patients.
|
From time to time, we evaluate these and other factors and we anticipate continuing to make such evaluations to determine if the existing allocation of resources, including the proceeds of this offering, is being optimized.
We do not intend to declare or pay dividends on our common stock in the foreseeable future. Instead, we generally intend to invest any future earnings in our business. Subject to Delaware law, our board of directors will determine the payment of future dividends on our common stock, if any, and the amount of any dividends in light of:
|
●
|
any contractual restrictions limiting our ability to pay dividends that may be applicable at such time;
|
|
|
●
|
our earnings and cash flow;
|
|
|
●
|
our capital requirements;
|
|
|
●
|
our financial condition; and
|
|
|
●
|
other factors our board of directors deems relevant.
|
The table below sets forth our cash and cash equivalents and capitalization, each as of March 31, 2014 on an actual basis.
You should consider this table in conjunction with our financial statements and the notes thereto included elsewhere in this prospectus.
|
As of March 31,
2014
|
||||
| Unaudited | ||||
|
Cash and cash held in escrow
|
$ | 559,774 | ||
|
Notes payable - related party, current portion
|
1,705,201 | |||
|
Accounts payable and accrued liabilities
|
1,392,367 | |||
|
Total Debt obligations and payables
|
3,097,568 | |||
|
STOCKHOLDERS’ DEFICIT:
|
||||
|
Common stock; par value $0.0001, 100,000,000 shares authorized, 8,040,000, 9,054,947 and 10,254,947 shares issued and outstanding respectively (1)
|
804
|
|||
|
Additional Paid-in Capital
|
17,788,110 | |||
|
Other Comprehensive Income, net
|
(27,836 | ) | ||
|
Accumulated Deficit
|
(19,947,180 | ) | ||
|
Total Stockholders’ (Deficit)
|
(2,186,102 | ) | ||
|
Total Capitalization
|
$ | 911,466 | ||
(1) Reflects a recapitalization of the Company’s historical financial statements resulting from the closing of the Share Exchange and an aggregate of 8,040,000 shares of common stock issued and outstanding following the Share Exchange (including 185,053 shares of common stock issuable to the DanDrit Denmark Non-Consenting Shareholders).
Purchasers of the shares of our common stock offered by this prospectus will suffer immediate and substantial dilution in the net tangible book value per share of our common stock. Our net tangible book value (unaudited) as of March 31, 2014, was a deficit of approximately $(2,486,868), or $(0.32) per share. Net tangible book value per share is calculated by subtracting our total liabilities from our total tangible assets, which is total assets less intangible assets, and dividing this amount by the number of shares of common stock outstanding as of March 31, 2014.
Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers in this offering and the as adjusted net tangible book value per share of our common stock immediately after this offering.
The shares of common stock issuable upon the exercise of our outstanding warrants and the exercise price in respect thereof are subject to adjustment in certain circumstances.
To the extent that any options or warrants are exercised, new options are issued, any new stock option or stock incentive plans are adopted or we otherwise issue additional shares of common stock in the future, there will be further dilution to investors in this offering.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking statements reflecting our current expectations that involve risks and uncertainties. See “Disclosure Regarding Forward-Looking Statements” for a discussion of the uncertainties, risks and assumptions associated with these statements. Actual results and the timing of events could differ materially from those discussed in our forward-looking statements as a result of many factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We are a biotechnology company committed to developing what we believe could be the world’s first vaccine against colorectal cancer. For more than a decade, we have developed and patented compounds successfully used in initial clinical trials in Europe and Asia including: (i) MelCancerVac™ (MCV) for treatment of cancer (one phase I/II trial in Denmark and two phase II trials in Denmark and Singapore), (ii) Tolerogenic dendritic cell (TDC) (pre-clinical stage in Denmark) and (iii) Melvaccine (MV) a melanoma cell lysate used as stand-alone vaccine (pre-clinical state in Denmark). We expect to continue our clinical development program in the United States, Europe and Asia. Springing from academic roots in the Danish Cancer Society, we have built upon our scientific and medical skills to advance a number of candidate therapies, targeted initially at non-small-cell-lung-cancer (NSCLC) and colorectal-cancer (CRC). In 2001, MCV was developed as a result of the combined efforts and research of DanDrit researchers and employees. On September 22, 2008, MCV was authorized by the Singapore government for named patient compassionate use for CRC. Three single-arm Phase II clinical trials in cancer have been conducted where DanDrit’s dendritic cell vaccine, MCV demonstrated potential efficacy. The three clinical trials generated data indicating prospects in a larger and different clinical setting. More specifically, this efficacy data needed to be confirmed in a comparative randomized trial with advanced colorectal cancer patients. Neither the FDA nor any other comparable governmental agency has yet to review MCV. Therefore, any assessment of its safety or efficacy only reflects the opinion of the Company. Furthermore, it does not indicate that MCV will achieve favorable results in any later stage trials or that the FDA or comparable agency will ultimately determine that MCV is safe and effective for purposes of granting marketing approval.
As a result, DanDrit Denmark, with the assistance of key opinion leaders in colorectal cancer treatment, has designed a randomized trial with 174 stage IV colorectal cancer patients after surgical resection and chemotherapy. Using an adaptive design clinical study that includes a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data (usually interim data) from subjects in the study (an “Adaptive Design Clinical Study”), we significantly reduced the cost and duration of a Phase IIb/III study and we believe we can complete the study within three years. Regulatory authorities in the United States and Europe have both published guidance documents on the use and implementation of adaptive design trials. These documents both include description of adaptive trials and include a requirement for prospectively written standard operating procedures and working processes for executing adaptive trials and a recommendation that sponsor companies engage with CROs that have the necessary experience in running such trials.
Share Exchange
DanDrit was incorporated in Delaware on January 18, 2011 under the name “Putnam Hills Corp.” as a vehicle to pursue a business combination through the acquisition of, or merger with, an operating business. We filed a Registration Statement on Form 10 with the SEC on August 12, 2011.
On February 12, 2014, the Company signed and consummated the transactions contemplated by the Share Exchange Agreement, by and among DanDrit USA, DanDrit Denmark and N.E. Nielsen, as the representative of the shareholders of DanDrit Denmark, pursuant to which the Company will acquire 100% of the issued and outstanding equity securities in DanDrit Denmark in exchange for 6,000,000 of the issued and outstanding shares common stock par value $0.0001 per share of the Company. The Share Exchange was closed on February 12, 2014. In accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark, the Non-Consenting Shareholders are entitled to receive the 185,053 shares of common stock of DanDrit USA that each such DanDrit Denmark shareholder would have been entitled to receive if such DanDrit Denmark shareholder had consented to the Share Exchange, up to an aggregate of 185,053 shares of common stock of DanDrit USA, reflected as issued and outstanding. As a result of the Share Exchange, the former shareholders of DanDrit Denmark became the controlling shareholders of the Company. The Share Exchange was accounted for as a reverse takeover/recapitalization effected by a share exchange, wherein DanDrit Denmark is considered the acquirer for accounting and financial reporting purposes. The capital, share price, and earnings per share amount in these consolidated financial statements for the period prior to the reverse merger were restated to reflect the recapitalization in accordance with the exchange ratio established in the merger.
Upon the closing of the Share Exchange, DanDrit USA and the its majority shareholder immediately prior to the closing agreed to cancel up to 4,400,000 share of common stock. In addition, following the closing of the Share Exchange, the wholly owned subsidiary of the company formed solely for the purposes of changing the company’s name, DanDrit Biotech USA, Inc., merged with and into the company and the company adopted the name of its wholly owned subsidiary “DanDrit Biotech USA, Inc.”
DanDrit USA will own approximately 100% of the outstanding equity interests of DanDrit Denmark. As a result of the Share Exchange, we changed our management and reconstituted our board of directors. As of the effective time of the Share Exchange, Samir Masri, the Chief Executive Officer, Chief Financial Officer, President, Secretary and sole director of Putnam resigned as the sole officer and director of Putnam and appointed NE Nielsen, Dr. Jacob Rosenberg, Dr. Eric Leire, Aldo Petersen and Robert E. Wolfe as directors of Putnam, and Dr. Eric Leire as Chief Executive Officer and President and Mr. Wolfe as Chief Financial Officer, Treasurer and Secretary.
Recent Developments
Negotiation with Etablissement Francais du Sang (EFS) regarding the GeniusVac Technology
DanDrit has recently entered into a negotiation with the Etablissement Francais du Sang (EFS) regarding access to their GeniusVac Technology. The GeniusVac technology is an Allogenic irradiated plasmacytoid dendritic cell line. This technology may allow DanDrit to develop a 100% off-the-shelf cancer vaccine. DanDrit has conducted and completed due diligence under CDA. DanDrit USA and EFS are now working on a feasibility proof-of-concept test before establishing further collaboration.
Results of Operations
Three months ended March 31, 2014 compared to the three months ended March 31, 2013
The following table sets forth our revenues, expenses and net income for the three months ended March 31, 2014 and 2013. The financial information below is derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus.
|
For The
Three Months
Ended
March 31, 2014
|
For The
Three Months
Ended
March 31, 2013
|
|||||||
|
Revenues
|
$
|
-
|
$
|
31,558
|
||||
|
Cost of Goods Sold
|
17,739
|
15,360
|
||||||
|
Gross Income(Loss)
|
(17,739
|
) |
16,198
|
|||||
|
Operating Expenses
|
||||||||
|
General and Administrative Expenses
|
326,428
|
175,016
|
||||||
|
Depreciation and Amortization
|
6,794
|
8,600
|
||||||
|
Consulting Expenses
|
61,145
|
13,048
|
||||||
|
Total Operating Expense
|
394,367
|
196,664
|
||||||
|
(LOSS) FROM OPERATIONS
|
(412,106
|
)
|
(180,466
|
) | ||||
|
Other Income (Expense)
|
||||||||
|
Interest (expense)
|
(13,999
|
) |
(159,922
|
)
|
||||
|
Gain (loss) on Currency Transactions
|
-
|
(100,327
|
) | |||||
|
Gain on Derivative Liability
|
-
|
41,643
|
||||||
|
Interest Income
|
51
|
-
|
||||||
|
Total Other Income (Expense)
|
(13,948
|
)
|
(218,606
|
) | ||||
|
(Loss) Before Income Taxes
|
(426,054
|
) |
(399,072
|
) | ||||
|
Income Tax Expense (Benefit)
|
-
|
-
|
||||||
|
NET (LOSS)
|
$
|
(426,054
|
) |
$
|
(399,072
|
) | ||
|
BASIC AND DILUTED LOSS PER SHARE
|
$
|
(0.06
|
) |
(0.08
|
) | |||
|
WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING - BASIC AND DILUTED
|
7,065,333
|
5,318,151
|
||||||
Year ended December 31, 2013 compared to the year ended December 31, 2012
The following table sets forth our revenues, expenses and net income for the years ended December 31, 2013 and 2012. The financial information below is derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus.
|
For the Year Ended
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
Net Sales
|
$
|
32,768
|
$
|
62,806
|
||||
|
Cost of Goods Sold
|
102,299
|
64,385
|
||||||
|
Gross Loss
|
(76,531
|
)
|
(1,579
|
) | ||||
|
Operating Expenses:
|
||||||||
|
General and administrative expenses
|
1,233,683
|
1,036,005
|
||||||
|
Depreciation and Amortization
|
38,297
|
56,600
|
||||||
|
Consulting expenses
|
390,437
|
829,845
|
||||||
|
Total Operating Expense
|
1,662,417
|
1,922,450
|
||||||
|
Loss from Operations
|
(1,738,948
|
)
|
(1,924,029
|
)
|
||||
|
Other Income (Expense)
|
||||||||
|
Interest (expense)
|
(652,703
|
) |
(704,911
|
)
|
||||
|
Gain on forgiveness of debt
|
49,016
|
-
|
||||||
|
Gain (loss) on currency transactions
|
19,541
|
32,841
|
||||||
|
Gain on derivative liability
|
175,732
|
153,430
|
||||||
|
Gain on sale of fixed assets
|
1
|
15,020
|
||||||
|
-
|
||||||||
|
Total Other Income (Expense)
|
(408,413
|
)
|
(503,620
|
)
|
||||
|
Loss Before Income Taxes
|
(2,147,361
|
)
|
(2,427,649
|
)
|
||||
|
Income Tax Expense (Benefit)
|
-
|
|||||||
|
Net Loss
|
(2,147,361
|
)
|
(2,427,649
|
)
|
||||
|
BASIC AND DILUTED LOSS PER SHARE
|
$ |
(0.40
|
) | $ |
(0.46
|
) | ||
|
WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING - BASIC AND DILUTED
|
5,332,721
|
5,318,151
|
||||||
31
Comparison of the three months ended March 31, 2014 and March 31, 2013
Revenues
Revenues from operations for the three months ended March 31, 2014 and March 31, 2013 were $0 and $31,558, respectively. The revenues for the three month period ending March 31, 2013 were attributable to the sale of lysate to the Singapore NCC compassionate use program by DanDrit Biotech A/S.
Cost of Goods Sold
Our cost of goods sold was $17,739 and $15,360 during the three months ended March 31, 2014 and 2013, respectively.
Gross Loss
Gross loss for the three months ended March 31, 2014 was $17,739 compared to gross income of $16,198 for same period in 2013. The increase in gross loss was due to lower sales and higher cost of goods sold for the three months ended March 31, 2014.
Expenses
Our operating expense for the three months ended March 31, 2014 totaled $394,367, representing an increase of $197,703, or approximately 101%, compared to $196,664 for the three months ended March 31, 2013. The largest contributors to the increase in operating expenses were fees associated with raising funds through an equity offering.
General and administrative expenses for the three months ended March 31, 2014 and 2013 were $326,428 and $175,016, respectively. The differences in expenses were primarily due to a total of $156,903 in legal fees for the three months ended March 31, 2014, representing an increase of $151,412, or approximately 87%, compared to $29,042 for the three months ended March 31, 2013; and audit expenses of $36,793, representing an increase of $15,917, or approximately 55%, compared to $20,876 for the three months ended March 31, 2013.
Depreciation and amortization expenses for the three months ended March 31, 2014 and 2013 were $6,794 and $8,600, respectively.
Consulting expenses for the three months ended March 31, 2014 and 2013 were $61,145 and $13,048, respectively. The difference in consulting expenses is attributable to the Company engaging consultants to assist in connection with planning and executing the Company’s going public strategy in the United States.
Other income (expense) net for the three month period ending March 31, 2014 and March 31, 2013 were $13,948 and $218,606, respectively. Other expense is associated with interest on related party loans, losses on currency transaction and gains on the derivative related to the convertible bond.
Net Loss
Net loss for the three months ended March 31, 2014 was $(426,054) comprised of legal, accounting, audit and other professional service fees incurred in relation to the preparation and filing of the Company’s periodic reports and general and administrative expenses, compared to a net loss of $(399,072) for the three months ended March 31, 2013 comprised of general and administrative expenses and interest expense, representing an increase of $26,982, or 7%. Net loss for the three month period ending March 31, 2014 and March 31, 2013 attributed to common stockholders was $(426,054) and $(399,072), respectively, or $(0.06) and $(0.08) per share, respectively. In 2014, the losses increased due to the Company’s efforts to secure financings through debt and equity offerings.
Comparison of the years ended December 31, 2013 and December 31, 2012
Revenues
Our revenues for the year ended December 31, 2013 were $32,768 compared to revenues of $62,806 for the year ended December 31, 2012, representing a decrease in revenues of $30,038, or 47.8%. Net sales to the Singapore compassionate use program decreased during 2013 as DanDrit Denmark was transferring manufacturing technologies to a new supplier. The transfer of manufacturing technology was completed and DanDrit Denmark should be able to supply the Singapore compassionate use program with larger quantities of cGMP lysate.
Cost of Goods Sold
Our cost of goods sold increased by $44,914, or 69.8%, during the year ended December 31, 2013, to $109,299, from $64,385 in cost of goods sold for the year ended December 31, 2012. The increase in cost of goods sold was due to the additional costs of transferring manufacturing technologies of CGMP lysate to a new supplier during 2013.
Gross Loss
Gross loss for the year ended December 31, 2013 was $76,531 compared to a loss of $1,579 for same period in 2012, representing an increase in the loss of $74,952, or 4,746.8%. The increase in gross loss was due to lower sales and higher cost of goods sold for the year ended December 31, 2013.
Expenses
Our operating expense for the year ended December 31, 2013 totaled $1,662,417, representing a decrease of $260,033, or 14%, compared to $1,922,450 for the year ended December 31, 2012.
General and administrative expenses for the year ended December 31, 2013 were $1,233,683 compared to $1,036,005 for the year ended December 31, 2012, representing an increase of $197,678, or 19.1%. This increase was due primarily to costs associated with an increase in salary and employee cost of $294,545. General and administrative expenses include office rental, website management and insurance and salary.
Depreciation and amortization expenses for the year ended December 31, 2013 and 2012 were $38,297 and $56,600, respectively.
Consulting expenses for the year ended December 31, 2013 were $390,437 compared to $829,845 for the year ended December 31, 2012, a decrease of $439,408, or 53%. The differences are largely attributable to the expenses of going public in the United States during 2013, as described above and the expenses of going public in Denmark in 2012. Of the $390,437 in expenses for the year ended December 31, 2013, $334,545 was related to expenses incurred in connection with developing and planning a US going public strategy including the preparation of this registration statement and converting our financial statements into US GAAP.
32
Net Loss
Net loss attributable to DanDrit Denmark for the year ended December 31, 2013 was $2,147,361compared to a net loss of $2,427,649 for the year ended December 31, 2012, representing a decrease of $280,288, or 11.5%. The decrease was primarily attributable to a decrease in total operating expenses of $185,081 from $1,924,029 for the year ended December 31, 2012 to $1,738,948 for the year ended December 31, 2013, as well as a decrease of $52,208 in interest expense.
Liquidity and Capital Resources
We have historically satisfied our capital and liquidity requirements through funding from our largest shareholders and the issuance of convertible notes, which over time have been converted into shares of our common stock. On December 16, 2013, DanDrit Denmark issued 681,849 common shares upon the conversion of $5,050,492 in loan and convertible bonds payable and related accrued interest and derivative liabilities. As of March 31, 2014, we had notes payable to related parties and related accrued interest totaling $1,705,201 consisting of (1) a $187,932 loan payable at the rate of 5% per annum and is payable upon the earlier of 14 days following the closing of the offering or February 1, 2015 (“Loan 1”), (2) a $526,689 loan payable at the rate of 5% per annum due upon the earlier of 14 days following the closing of this offering or February 1, 2015 (“Loan 2”), (3) a $888,880 loan payable to an existing shareholder which accrues interest at the rate of 5% per annum and is due February 1, 2015 and can be extended at the Company’s option for an additional year with an increase in the interest rate to 7.00% (“Loan 3”) and (4) $101,700 in other loans payable on demand. On April 29, 2014, Loan 1, Loan 2 and Loan 3 were amended whereby the terms of the 2014 loans are payable on February 1, 2015 and can be extended at the Company’s option for an additional year with an increase in the interest rate to 7.00%. The Company plans to repay Loan 1 from operations. The Company has an additional funding commitment of approximately $368,868 until February 2015 for the Company to draw upon to support operations. The Company plans to repay Loan 1 as described in the “Use of Proceeds” section herein.
As of March 31, 2014 the Company had cash of $54,472, cash held in escrow of $423,969 and an accumulated deficit of $(2,186,102) as compared to March 31, 2013, when the Company had $18,794 in cash and cash equivalents, cash held in escrow of $77,468 and an accumulated deficit of $(1,684,170). The change in cash is primarily due to the additional financings obtained by the Company’s subsidiary. The decrease in the working capital is primarily related to the acquisition of DanDrit Biotech A/S on February 12, 2014. At December 31, 2013, we had cash of $18,794, cash held in escrow of $77,468 and working capital deficit of $(1,993,145).
The following is a summary of the Company's cash flows provided by (used in) operating, investing, and financing activities:
|
Three Months
Ended
March 31,
2014
|
Three Months
Ended
March 31,
2013
|
|||||||
|
Net Cash (Used by) Operating Activities
|
$
|
(437,746
|
)
|
$
|
(1,123,899
|
) | ||
|
Net Cash (Used by) Investing Activities
|
(395,906
|
) |
-
|
|||||
|
Net Cash Provided by Financing Activities
|
$
|
865,976
|
$
|
987,911
|
||||
|
Loss on Currency Translation
|
3,354
|
139,279
|
||||||
|
Net Increase (Decrease) in Cash and Cash Equivalents
|
$
|
35,678
|
$
|
3,291
|
||||
The Company is dependent upon the receipt of capital investment or other financing to fund its ongoing operations. The Company has secured an additional funding commitment of $368,868 through February 2015 from a related party. In addition, the Company is dependent upon certain related parties to provide continued funding and capital resources. If continued funding and capital resources are unavailable or unavailable at reasonable terms, the Company may not be able to implement its plan of operations.
We believe that our cash flow together with currently available funds from our existing lines of credit and other potential sources of funds, such as loans from shareholders, will be sufficient to fund our anticipated working capital needs and capital spending requirements for the next 12 months. The Company projects that revenues and expenses for the next 12 months will be $983,108 and $1,404,187, respectively. The Company secured financing of $461,877 and $424,927 on February 15, 2014 and March 18, 2014 as amended on April 29, 2014, respectively. The Company has secured an additional funding commitment until February 2015 of 2,000,000 DKK or approximately $368,868, determined by using the current currency conversion rate. However, if the additional funding commitment is not funded or if we were to incur any unanticipated expenditures or the positive trend of our operating cash flow does not continue or our shareholders determine not to invest in the Company either in connection with the sale of debt or equity securities, such circumstances could put a substantial burden on our cash resources.
We may also need additional funds for possible future strategic acquisitions of businesses, products or technologies complementary to our business. If additional funds are required, we may raise such funds from time to time through public or private sales of equity or debt securities. Financing may not be available on acceptable terms, or at all, and our failure to raise capital when needed could materially adversely impact our growth plans and our financial condition and results of operations.
Our auditors have prepared the financial statements included with this prospectus in conformity with generally accepted accounting principles of the United States of America, which contemplate continuation of the Company as a going concern. However, since the Company has incurred significant losses and has not yet been successful in establishing profitable operations, our auditors have expressed doubts about the ability of the Company to continue as a going concern. In this regard, management plans to mitigate this doubt by raising additional funds through debt and/or equity offerings and by substantially increasing sales once approval for the Company’s product is obtained. There is no assurance that the Company will be successful in achieving profitable operations. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
33
Cash Flows
Cash used in operating activities for the year ended December 31, 2013 was $2,130,628, representing an increase of $1,197,616, compared to cash used in operating activities of $933,012 for the year ended December 31, 2012. This increase was primarily due to $1,161,004 in cash provided by increases in accounts payable and accrued expense for the year ended December 31, 2012 compared to $408,825 is cash used by accounts payable and accrued expense for the year ended December 31, 2013. During 2013, the Company obtained additional loans, which were converted to common stock to pay down approximately $1,038,000 in accounts payable and accrued expenses. Cash used by operating activities for the three months ended March 31, 2014 was $437,746, representing a decrease of $686,153, or approximately 61%, compared to cash loss from operating activities of $1,123,899 for the three months ended March 31, 2013. The net cash used by operating activities was primarily due to fund raising efforts of the Company and that from the operations of DanDrit Biotech A/S.
There were no major changes in the total assets as of December 31, 2013 compared to December 31, 2012. Total liabilities decreased by approximately $2,596,058 as of December 31, 2013 compared to December 31, 2012. The decrease was mainly due to the conversion of promissory notes and bonds to related parties to shares of common stock. Total assets as of March 31, 2014 were $911,466 compared to $450,467 as of March 31, 2013. Total liabilities increased to $3,097,568 as of March 31, 2014 compared to $2,134,637 as of March 31, 2013. The increases to total assets and total liabilities were mainly due to consummation of the Share Exchange.
Cash used in investing activities was $105,015 for the year ended December 31, 2013, as compared to cash used in investing activities of $84,643 for the year ended December 31, 2012. Cash used for investing activities increased during the year ended December 31, 2013 primarily due to an increase in the amount of $77,468 of cash held in escrow. While our purchase of intangible assets declined by $72,115, we have invested, and we anticipate that we will continue to invest, in additional production equipment in order to meet the continuing increase in the demand for our products.
Cash provided by financing activities was $2,469,526 for the year ended December 31, 2013, as compared to cash provided by financing activities of $856,754 for the year ended December 31, 2012. The increase of approximately $1,612,772 in cash provided by financing activities in the year ended December 31, 2013, compared to the year ended December 31, 2012, was due to cash received in connection with the issuance of related party notes net of $218,136 in payments on related party notes and $67,000 paid in offering costs.
Other than as discussed above, we know of no trends, events or uncertainties that are reasonably likely to impact our future liquidity.
Off Balance Sheet Arrangements
As of March 31, 2014, we had no off-balance sheet arrangements. We are not aware of any material transactions which are not disclosed in our financial statements.
Significant Accounting Policies and Critical Accounting Estimates
The methods, estimates, and judgments that we use in applying our accounting policies have a significant impact on the results that we report in our financial statements. Some of our accounting policies require us to make difficult and subjective judgments, often as a result of the need to make estimates regarding matters that are inherently uncertain. In addition, Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are not choosing to “opt out” of this provision. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable. As a result of our election, not to “opt out” of Section 107, DanDrit’s financial statements may not be comparable to companies that comply with public company effective dates.
Property and Equipment — Property and equipment are stated at cost. Expenditures for major renewals and betterments that extend the useful lives of property and equipment are capitalized, upon being placed in service. Expenditures for maintenance and repairs are charged to expense as incurred. Depreciation is computed for financial statement purposes on a straight-line basis over the estimated useful lives of the assets which range from four to six years.
Intangible Assets — Definite life intangible assets include patents. The Company accounts for definite life intangible assets in accordance with Financial Accounting Standards Board, (“FASB”) Accounting Standards Codification, (“ASC”) Topic 350, “Goodwill and Other Intangible Assets” and amortized the patents on a straight line basis over the estimated useful life of twenty years. Costs incurred in relation to patent applications are capitalized costs and amortized over the estimated useful life of the patent. If it is determined that a patent will not be issued, the related remaining patent application costs are charged to expense.
Revenue Recognition and Sales — The Company’s sales of its MelCancerVac colorectal cancer treatment have been limited to a compassionate use basis in Singapore after stage IIA trials and the vaccine is not currently approved for sale for any other use or location. The Company accounts for revenue recognition in accordance with Securities and Exchange Commission Staff Accounting Bulletin No. 101, “Revenue Recognition in Financial Statements” (SAB 101), FASB ASC 605 Revenue Recognition. The Company recognizes revenue when rights and risk of ownership have passed to the customer, when there is persuasive evidence of an arrangement, product has been shipped or delivered to the customer, the price and terms are finalized, and collection of the resulting receivable is reasonably assured. Products are primarily shipped FOB shipping point at which time title passes to the customer.
Value Added Tax - In Denmark, Value Added Tax (“VAT”) of 25% of the invoice amount is collected in respect of the sales of goods on behalf of tax authorities. The VAT collected is not revenue of the Company; instead, the amount is recorded as a liability on the balance sheet until such VAT is paid to the authorities. VAT of 25% is also paid to Danish and EU vendors on invoices. These amounts are refundable from the respective governmental authority and recorded as other receivables in the accompanying financial statements.
Accounting Estimates — The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimated.
Recent Enacted Accounting Standards
For a description of accounting changes and recent accounting standards, including the expected dates of adoption and estimated effects, if any, on our consolidated financial statements, see “Note 1: Recently Enacted Accounting Standards” in the financial statements included elsewhere in this prospectus.
Quantitative and Qualitative Disclosures About Market Risk
We are not required to provide quantitative and qualitative disclosures about market risk because we are a smaller reporting company.
Overview
We are a biotechnology company seeking to develop what we believe could be the world’s first vaccine against colorectal cancer. We have developed and patented compounds used in initial clinical trials in Europe and Asia including: (i) MelCancerVac™ (MCV) for treatment of cancer (one phase I/II trial in Denmark and two phase II trials in Denmark and Singapore), (ii) Tolerogenic dendritic cell (TDC) (pre-clinical stage in Denmark) and (iii) Melvaccine (MV) a melanoma cell lysate used as stand-alone vaccine (pre-clinical state in Denmark). We expect to continue these trials in the United States. Springing from academic roots in Denmark, DanDrit has built upon its scientific and medical skills to advance a number of candidate therapies, targeted initially at non-small-cell-lung-cancer (NSCLC) and colorectal-cancer (CRC). On September 22, 2008, MCV was authorized by the Singapore government for a named patient compassionate use for CRC. MCV, demonstrated potential efficacy in three single-arm Phase II clinical trials in cancer,. The three clinical trials generated data indicating prospects in a larger and different clinical setting. More specifically, this efficacy data needed to be confirmed in a comparative randomized trial. As a result, DanDrit Denmark, with the assistance of experienced practitioners in colorectal cancer treatment, has designed a randomized trial with 174 stage IV colorectal cancer patients. Neither the FDA nor any other comparable governmental agency has reviewed MCV. Therefore, any assessment of its safety or efficacy only reflects the opinion of the Company. Furthermore, it does not indicate that MCV will achieve favorable results in any later stage trials or that the FDA or comparable agency will ultimately determine that MCV is safe and effective for purposes of granting marketing approval.
Our Biotechnology
We plan to use a dendritic cell vaccine technology relatively similar to the technology behind Dendreon’s FDA approved Provenge™ cancer vaccine. However, we believe DanDrit’s next generation of dendritic cell vaccine may benefit from technological competitive advantages over other cancer vaccines including:
|
●
|
The vaccine will be generated within eight days from a patient’s peripheral blood. We will be able to generate the vaccine quickly because only 200 ml of blood is required to be drawn. Leukapheresis (a medical technology in which the blood of a patient is passed through an apparatus -dialysis machine- that separates out one particular constituent and returns the remainder to the circulation which is used in Denderon’s Provenge™ cancer vaccine) is not needed.
|
|
|
●
|
The vaccine will use an allogenic (using cells, tissues, or organs, sourced from a genetically non-identical member of the same species as the recipient (“Allogenic”) tumor lysate (a fluid containing the contents of lysed cells, cells broken down by viral, enzymic or osmotic mechanisms that compromise their integrity “lysed cells”) as opposed to inconvenient autologous (from the patient) tumor lysate. A major limitation of autologous tumor cell vaccines is the low yield of autologous tumor cells that may compromise the number of immunizations given to patients (difficult to obtain enough cancer cells from the patient). A second inconvenience is the variability of GM-CSF (a protein that functions as a white blood cell growth factor) secretion among patients, which could be responsible for the different levels of responses observed. But above all, although autologous tumor cells may be a good source of TAA for cancer vaccine development, limitations plus the significant time and expense required for the approval of each patient’s vaccine by the appropriate regulatory agencies severely limits the development of this type of immunization approach. DanDrit does not need a patient’s tumor cells to manufacture MCV. Therefore MCV is not classified as an autologous vaccine.
|
|
|
●
|
The vaccine will be polytopic (targets several cancer specific antigens, or antibody generator is any substance which provokes an adaptive immune response “antigens”). As a result, the risk of the tumor escaping is more limited and more T-cells can be activated than if the vaccine is targeting one antigen only. However, MCV has a focus on melanoma-associated antigen (“MAGE”)-A antigens that are only expressed by tumors and absent in normal tissues.
|
|
|
●
|
Fast track production in two days is possible.
|
Our Proposed Clinical Trial
Parallel with the establishment of a cancer vaccine center in the EU, DanDrit intends to develop globally MCV in colorectal cancer, with opportunities to expand the scope of the treatment to other types of cancer. DanDrit, as a sponsor, will focus on a randomized adaptive seamless Phase IIb/III clinical trial in stage IV colorectal cancer. The proposed Proof of Concept (PoC) study (a study conducted to provide the first evidence that a candidate drug such as MCV might be effective for a disease) with an adaptive design plans to enroll 174 stage IV colorectal cancer patients after resection of metastases and therefore no evidence of disease. Regulatory authorities in the United States and Europe have published guidance documents on the use and implementation of adaptive design trials. These documents include descriptions of adaptive trials and a requirement for prospectively written standard operating procedures and working processes for executing adaptive trials, as well as a recommendation that sponsor companies engage with CROs that have the necessary experience in running such trials.
The clinical study is designed as a randomized, placebo controlled, multicenter, adaptive seamless Phase IIb/III clinical study. Treatment is double blinded (to the patients and physicians). Patients will be included after resection of their primary tumor and resectable metastases and after appropriate peri-or post-operative chemotherapy by stratification and random assignment to a non-vaccine control group or a vaccine group receiving five vaccinations with 14-day administration intervals followed by ten vaccines with two-month intervals. Inclusion will take place one month after finishing the last round of peri- or post-operative chemotherapy (FOLFOX or FOLFIRI) and after a negative tumor scan (head, thoracic and abdominal cavities) and normal CarcinoEmbryonic Antigen (CEA) prior to inclusion in the vaccine or the control groups. Patients will be screened for MAGE-A expression. The control group will receive five plus ten injections with physiological saline. In the event of disease progression, as verified by tumor scan during the vaccination schedule, vaccinations will be discontinued.
The initial Phase IIb/III trials are currently contemplated to be initiated in Italy. Following the initial closing of the offering, we intend to file an Investigational Medicinal Product Dossier (IMPD) in Italy which is required to obtain a clinical trial authorization (CTA) to begin trials in any Europe state. The IMPD and CTA review and approval process is anticipated to take approximately two-three months, and we anticipate that patients will begin to be enrolled for the Phase IIb/III trials in Italy in October or November of 2014. DanDrit has not filed an investigational new drug (IND) application with the FDA in relation to the proposed trial but anticipates filing an IND application with the FDA by the end of 2014 to initiate the process to permit manufacturing capability of MCV in the U.S. and to include U.S. patients in the Phase IIb/III trials. Once an IND application has been filed in the U.S., we believe that we will be able to expand the PhaseIIb/III trials initiated in Italy to the U.S., however, we cannot estimate at this time when we will be able to begin enrolling U.S. patients in the trial. Although we were a sponsor for only one of the three MCV clinical trials completed to date, we have obtained the case report forms (CRF) with respect to two of the three trials and have requested the CRFs with respect to the third trial and therefore we intend to present the results obtained from the MCV clinical trials for all trials in which we have been able to obtain the related CRFs to the FDA in connection with our IND application, when filed. While we were not the sponsor or principal investigator for all of the trials, certain employees and directors of DanDrit were significantly involved in the design of the study and the analysis and interpretation of the data in all three studies and therefore believe that any weight applied to such trial in connection with our IND application will be focused on the results and data of the trials derived from the studies and disclosed in published papers or otherwise reflected in the CRFs rather than the level of our participation. In addition, we believe that the data received in connection with the Phase IIB/III trials contemplated to be initiated in Italy, will have the greatest weight applied in connection with its IND application anticipated to be filed with the FDA (see “Clinical Trials Data and Product Approvals).
Corporate History and Information
DanDrit was incorporated in Delaware on January 18, 2011 under the name “Putnam Hills Corp.” (“Putnam”) as a vehicle to pursue a business combination through the acquisition of, or merger with, an operating business. We filed a Registration Statement on Form 10 with the U.S. Securities and Exchange Commission (the “SEC”) on August 12, 2011.
On February 12, 2014, the Company signed the Share Exchange Agreement, by and among DanDrit USA, DanDrit Denmark and N.E. Nielsen, as the representative of the shareholders of DanDrit Denmark, pursuant to which the Company will acquire 100% of the issued and outstanding equity securities in DanDrit Denmark in exchange for 6,000,000 of the issued and outstanding shares common stock par value $0.0001 per share of the Company. The Share Exchange was closed on February 12, 2014 pursuant to which the DanDrit Consenting Holders exchanged an aggregate of 3,879,624 equity interests of DanDrit Denmark for 5,814,947 shares of DanDrit USA. In accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark, the Non-Consenting Shareholders will be entitled to receive the 185,053 shares of common stock of DanDrit USA, reflected as issued and outstanding, that each such DanDrit Denmark shareholder would have been entitled to receive if such DanDrit Denmark shareholder had consented to the Share Exchange, up to an aggregate of 185,053 shares of common stock of DanDrit USA. As a result of the Share Exchange, the former shareholders of DanDrit Denmark became the controlling shareholders of the Company. The Share Exchange was accounted for as a reverse takeover/recapitalization effected by a share exchange, wherein DanDrit Denmark is considered the acquirer for accounting and financial reporting purposes. The capital, share price, and earnings per share amount in these consolidated financial statements for the period prior to the reverse merger were restated to reflect the recapitalization in accordance with the exchange ratio established in the merger.
Upon the closing of the Share Exchange, DanDrit USA and the its majority shareholder immediately prior to the closing agreed to cancel up to 4,400,000 shares of our common stock. In addition, following the closing of the Share Exchange, DanDrit Biotech USA, Inc., the wholly owned subsidiary of the Company, merged with and into the Company, thereby changing and the Company’s name to “DanDrit Biotech USA, Inc.”
DanDrit USA will own 100% of the outstanding equity interests of DanDrit Denmark. As a result of the Share Exchange, we changed our management and reconstituted our board of directors. As of the effective time of the Share Exchange, Samir Masri, the Chief Executive Officer, Chief Financial Officer, President, Secretary and sole director of Putnam resigned as the sole officer and director of the company and appointed NE Nielsen, Dr. Jacob Rosenberg, Dr. Eric Leire, Aldo Petersen and Robert E. Wolfe as directors of Putnam, and Dr. Eric Leire as Chief Executive Officer and President and Mr. Wolfe as Chief Financial Officer, Treasurer and Secretary.
Our principal executive offices are located Fruebjergvej 3 Box 62, 2100 Copenhagen, Denmark, and our telephone number is +45 39179840. We maintain an Internet website at www.dandrit.com. The information contained in, or accessible from, our website is not a part of this prospectus.
Products
DanDrit plans to assess its lead compound, MelCancerVac™ (MCV), a cellular therapy, in a comparative Phase IIb/III clinical trial in advanced colorectal cancer. DanDrit uses a dendritic cell technology similar to the Dendreon’s FDA approved Provenge™ cancer vaccine.
DanDrit’s MCV demonstrated potential efficacy in three separate Phase IIa clinical trials in colorectal and non-small cell lung cancer. Even if MCV can be used for various cancers, DanDrit has decided to initiate MCV’s clinical development with advanced colorectal cancer. We believe that a maintenance therapy for advanced colorectal cancer represents a genuine commercial opportunity for MCV. A clear and unmet medical need for a safe maintenance therapy offers the opportunity to confirm the potential efficacy of MCV in a favorable setting. Neither the FDA nor or any other comparable governmental agency has reviewed MCV. Therefore, any assessment of its safety or efficacy only reflects the opinion of the Company. Furthermore, it does not indicate that MCV will achieve favorable results in any later stage trials or that the FDA or comparable agency will ultimately determine that MCV is safe and effective for purposes of granting marketing approval.
DanDrit plans to conduct a randomized multicenter (anticipated to be located in Europe), company sponsored clinical trial to determine the safety and efficacy of MCV as adjuvant therapy in advanced colorectal cancer. The study will determine the ability of MCV to prevent recidivism in stage IV colorectal patients with no evidence of disease after surgical resection of liver metastasis and chemotherapy. Using an Adaptive Design Clinical Study, which allows modification made to trail and/or statistical procedures of ongoing clinical trials based on accrued data, the double blinded randomized Proof-of-Concept study (a study conducted in order to confirm feasibility) will evaluate MCV with standard of care against standard of care alone in 174 colorectal cancer patients using as primary endpoints Progression Free Survival at 18 months and Overall Survival. We anticipated that the blinded comparative trial can be completed within three years. We are confident that positive upcoming clinical data will be the catalyst to unlock commercial revenues for DanDrit through either acquisition by a pharmaceutical partner or licensing deals that would yield upfront and milestone payments as well as royalties.
DanDrit has learned how to manufacture dendritic cells, immune cells forming part of the mammalian immune system with the main function of processing antigen material and presenting it on the surface to other cells of the immune system, functioning as antigen-presenting cells, in vitro from monocyte (a type of white blood cell) precursor cells taken from patients eligible for DanDrit’s therapies. The preparation of tumor lysate containing selections of cancer-specific non-self antigens, allows DanDrit to sensitize patients’ dendritic cells. The use of the patient’s own monocyte cells from peripheral blood (autologous cell therapy) overcomes the issues associated with non-self allergic reactions to immune therapies.
37
DanDrit’s intellectual property is protected with patents and trademarks. DanDrit’s candidate vaccines are based on the MCV platform that is protected by a family of issued or submitted patents. DanDrit’s lead product has completed Phase II clinical trials in Denmark and Singapore. DanDrit’s Singapore Phase II data was compelling enough that the Singapore authorities allowed the use of MCV for CRC on a humanitarian named patient basis. Outside the United States, named patient programs provide controlled, pre-approval access to drugs in response to requests by physicians on behalf of specific, or “named”, patients before those medicines are licensed in the patient’s home country. Governments worldwide, such as Singapore’s government, have created provisions for granting access to drugs prior to approval for patients who have exhausted all alternative treatment options and do not match clinical trial entry criteria. Often grouped under the labels of compassionate use, expanded access, or named patient supply, these programs are governed by rules which vary by country defining access criteria, data collection, promotion, and control of drug distribution. Through these programs, patients are able to access drugs in late-stage clinical trials or approved in other countries for a genuine, unmet medical need, before those drugs have been licensed in the patient’s home country. In September 2008, DanDrit Denmark and the National Cancer Centre of Singapore (NCC) entered a collaboration agreement regarding a clinical named patient program conducted in Singapore at NCC with the dendritic cell vaccine MCV. NCC has established a GMP approved laboratory in which the manufacturing of MCV takes place. NCC has received approval from the relevant governmental authorities for the import of lysate necessary for production of MCV. The clinical and research and development activities of the named patient program relate to the Company’s product, MCV. The purpose for the Singapore named patient program is to provide patients with advanced colorectal cancer or other forms of cancer(s) with the presence of MAGE antigen expression an alternative treatment for the vaccination with MCV, where there is no further indication for surgery or treatment with chemotherapy. Patients are recruited on named patient basis according to the patient inclusion and exclusion criteria stated in the phase IIa study protocol. However, there may be some exceptional cases where treatment will be made based on a doctor’s discretion regarding the patient’s quality of life. An estimated total of 50 patients have been recruited for the Singapore named patient program and 8 patients are currently still active in the named patient program. To date we have not received a detailed report regarding the final outcomes for patients participating in the Singapore trials.
The colorectal cancer patients who are eligible for the humanitarian program in Singapore must present a profile similar to the one of the patients who were recruited in the phase IIa clinical trial. Also, patients are monitored according to the previous phase II study protocol.
To date, clinical trials of MCV have been targeted to patients in terminal stages of cancer with non-resectable bulky tumors who failed to respond to surgery and chemotherapy. Several patients showed extended overall survival with good quality of life. Several patients showed stable disease with no progression of tumors. There was evidence of tumor regression in some patients (see “Clinical Trials Data and Product Approvals”).
These achievements have been built on a carefully executed R&D program that generated practical solutions to scientific and medical challenges. Through this development program, DanDrit gained advanced understanding of the role of dendritic cells in immunoregulation and cancer.
Non-core applications of dendritic cell technologies mastered by DanDrit have applications in infectious diseases and auto-immune diseases such as diabetes (seventh leading cause of death in the US). These other applications represent opportunities for out-licensing and cooperation. Where other companies have non-core technologies of relevance to DanDrit’s core business in dendritic cell cancer therapy, we will pursue a policy of cooperation and in-licensing.
DENDRITIC CELLS, THE THERAPEUTIC PLATFORM
Summary
Early academic work at the Danish Cancer Society was spun-out into DanDrit Denmark. None of the personnel at the Danish Cancer Society, or any other third-party, retains any rights to the intellectual property underlying the Company’s business, technology or product candidates, including MCV. The fundamental scientific postulate of DanDrit is the fact that key cells in the immune system can be sensitized to cancer cells that carry foreign (or non-self) antigens. These key antigen-presenting cells are the dendritic cells. Dendritic cells encounter and recognize foreign antigens. Dendritic cells can assimilate and process the cells expressing these antigens. The key components of these antigens (known as epitopes and several epitopes are known as polytopes) are subsequently presented on the cell surface of the dendritic cell. Dendritic cells travel to lymph nodes and other lymphatic tissues where the epitopes are presented to other immune cells, including cell-killing T lymphocytes. T lymphocytes sensitized by dendritic cells can then recognize and kill tumor cells carrying tumor-specific antigens recognized by the dendritic cells. The main aim is to kill tumor cells without killing normal body tissues.
Dendritic cell interacting with T-lymphocyte
The above photograph (courtesy Science Photo Library) illustrates a dendritic cell (blue/green) communicating with a T-lymphocyte (gold). From DanDrit’s point of interest, this might represent a dendritic cell instructing a T-lymphocyte to kill tumor cells.
DanDrit presents itself as a “Cancer Vaccine” company and its lead product, MelCancerVac® (MCV), a polytopic vaccine, targets colorectal cancer in the first instance. In addition, DanDrit has developed several technologies relevant to dendritic cell production, including:
| ● |
Generation of fast track dendritic cells
|
|
| ● |
Processing and presentation of protein antigen
|
|
| ● |
Characterization of DanDrit dendritic cells
|
|
| ● |
Analysis of lysate uptake by DanDrit dendritic cells
|
|
| ● |
MicroRNA profiling of DanDrit dendritic cells
|
|
| ● |
Effect of Resiquimod (a drug that acts as an immune response modifier, and has antiviral and anti-tumoral activity) on production of Interleukin 12 (Il-12), a secreted protein factor that is naturally produced by dendritic cells in response to antigenic stimulation and Interleukin 10 (IL-10), a protein that inhibits the synthesis of a number of other signaling proteins.
|
| ● |
Generation of tolerogenic dendritic cells
|
|
| ● |
Development of Il-12 based potency assay
|
DanDrit’s vaccine candidates are based on the MCV platform and are protected by a family of issued and submitted patents. DanDrit’s lead product has completed Phase II clinical trials in Denmark and Singapore. DanDrit’s Singapore Phase II data were compelling enough for the Singapore authorities to make MCV available on a limited humanitarian named patient basis.
To date, clinical trials of MCV have been targeted to patients in terminal stages of disease who failed to respond to surgical resection and chemotherapies. Some patients showed extended overall survival with good quality of life. Many patients showed stable disease with no progression of tumor. There was evidence of tumor regression in some patients. (see “Clinical Trials Data and Product Approvals”).
Scientific and medical research is adding to DanDrit’s clinical and pre-clinical development pipeline in cancer. Some of this research in dendritic cells could have implications that reach beyond DanDrit’s cancer vaccine vision.
Dendritic Cells and the immune response
Dendritic cells were first recognized by Paul Langerhans in the late 19th century. For this reason such cells in the skin may still be referred to as Langerhans cells. The term “dendritic cell” was first used by Ralph Steinman and Zanvil Cohn in 1973. Steinman received the 2007 Lasker Award for this work and the 2011 Medicine Nobel Prize.
Like macrophages, cells whose role is to phagocytose, or engulf and then digest, cellular debris and pathogens, either as stationary or as mobile cells, dendritic cells are involved in the processing of antigens and their presentation to the cells that directly carry out the immune response through antibody generation (B lymphocytes) or cell killing activity (T- lymphocytes). Like macrophages, dendritic cells are mobile and once stimulated by an antigen, activated macrophages and dendritic cells move from their host tissue (usually skin or epithelial tissue such as gut, mucous membranes, lung etc.) to lymphatic tissues where they encounter and stimulate cells that mediate the immune response.
Unsurprisingly, macrophages and dendritic cells are closely related. Both are derived from circulating blood cells known as monocytes, a type of white blood cell which constitutes roughly 10% of all white blood cells. Monocytes, macrophages and immature dendritic cells are all phagocytic cells, that is, they engulf and process foreign antigens. On activation by the uptake of antigen, dendritic cells mature and become mobile. The mobile mature dendritic cells are capable of stimulating T-lymphocytes through the expression of T-cell stimulatory antigens on their cell surfaces.
It is possible to force monocytes to differentiate in vitro into immature dendritic cells. This is the basis of DanDrit’s proprietary dendritic cell production process. As in nature, DanDrit’s process involves a subtle communication between monocytes and cytokines (small proteins that important in the communication process that governs basic cellular activities and coordinates cell actions). Dendritic cells produced by DanDrit are functionally, morphologically and biochemically very similar – if not identical – to natural dendritic cells.
Figure 2 Principle of Dendritic Cell cancer vaccines
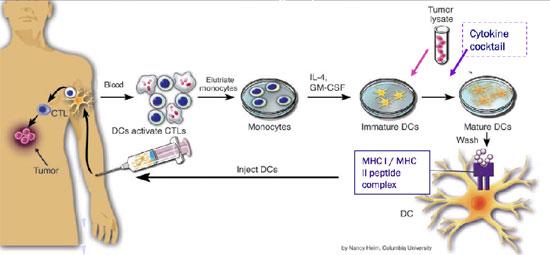
DanDrit’s platform technology is based on isolating patient monocytes and transforming them into immature dendritic cells in vitro. This is achieved by exposing monocytes to cytokines (interleukin 4, IL-4; and granulocyte macrophage colony stimulating factor, GM-CSF). Still in vitro these immature dendritic cells are activated by exposure to a cancer cell line lysate. This cancer cell lysate contains many “non-self” antigens of the cancer/testis family. Although coded by the human genome, these antigens are not normally expressed in tissues other than cancer or testis (note that testis and immune system are isolated from each other). Once sensitized in vitro, the immature dendritic cells are matured by exposure to a DanDrit proprietary cytokine cocktail. The now mature dendritic cells can be re-injected to the patient via a simple 0.2 ml intra-dermal injection and they will find their way to the lymphatic tissues. There, they will stimulate multiple cell killing (T) lymphocytes which will become sensitized to the cancer-specific antigens present in the lysate.
The Platform Technology, MelCancerVac®
MelCancerVac® (MCV) is a cellular immunotherapy for treatment of cancer. MCV has been studied in two cancers: Non-Small Cell Lung cancer (NSCLC) and colorectal cancer (CRC). The use of MCV is extended to other tumors in the mid-term such as the two types of esophageal cancers: esophageal squamous cell carcinoma (EC) and esophageal adenocarcinoma (EAC).
DanDrit’s platform technology comprises two arms:
|
●
|
autologous dendritic cells obtained by the activation of patient-derived monocytes; and
|
|
|
●
|
proprietary lysate from melanoma-derived cell line expressing a range of cancer/testis antigens, notably the MAGE-A family
|
The melanoma lysate component of MCV is manufactured from a melanoma cell line established by DanDrit scientists. This cell line was isolated from a melanoma tumor that expressed antigens found in a wide range of tumors but not in normal tissues (other than the testis). These antigens belong to a family of cancer/testis antigens (including mostly MAGE-A antigens) found in many tumors.
Furthermore, by exposing DanDrit’s proprietary melanoma tumor cell to 5-aza-deoxycytadine (5-aza-CdR/Decitabine), which is an inhibitor of DNA methylation, DanDrit has shown that derived tumor lysates (MCV5AZA) express a far wider range of tumor-specific antigens.
Antigen characterization
For a patient to respond favorably to MCV, it is necessary that the antigens presented by the patient’s tumor show a significant match with the antigens in the lysate. The level of expression of antigens in each batch of lysate is determined by a procedure known as Reverse Transcriptase Quantitative Polymerase Chain Reaction or “RT-QPCR”. Clearly all patient cells will present many thousands of antigens, as will the lysate. MCV’s lysate component is isolated from a melanoma cell line that expresses a great many cancer/testis antigens at significant level. This broad spectrum of cancer/testis antigens is what makes MCV a good cancer vaccine. Figure 3 (below) shows how RT-QPCR can analyze levels of antigen expression as measured by messenger RNA.
Figure 3 Comparison of tumor antigen expression in MCV with two patient biopsies
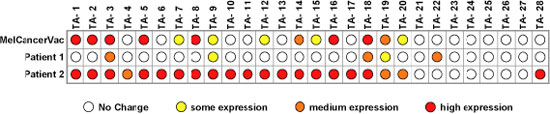
In this example, TA-1 to TA-28 are 28 known tumor antigens (antigens that are only expressed by cancer cells and not by normal cells). We can see that 14 of these antigens are present in MCV. Twenty-one cancer-specific antigens are expressed by the tumor in patient two, which indicates a good chance of promoting a cancer killing response. In patient one there is not a strong overlap of MCV antigens and the five patient’s tumor antigens. The chances of promoting a strong immune response are less but still significant (TA-3, TA-9, TA-18, and TA-19 are shared).
By analyzing patient’s tumors by RT-QPCR, it is possible to select patients that have the best chance of success with MCV. However, other uncharacterized antigens may also be present that might promote a response.
Clinical Trials Data and product approvals
Overall clinical results
No dendritic cell-based vaccination has to date demonstrated life-threatening side effects. The only potential adverse events associated with dendritic cell vaccines to date are a flu-like symptom with fevers (up to 39-40 degrees Celsius), chills, and headaches in some patients. The occurrence of these adverse events did not require additional treatment or hospitalization. Some patients may also develop a vitiligo, a skin condition in which there is a loss of brown color (pigment) from areas of skin, resulting in irregular white patches that feel like normal skin, when melanocyte differentiation antigens are used as targets in immunotherapy. However, this has not occurred, to DanDrit’s knowledge, with MCV in clinical trials that have been conducted to date.
MCV is produced according to the principles of Good Manufacturing Practice (GMP) in facilities approved by the Danish Medicine Agency and EU regulation for the production of medicines from patient blood in aseptic conditions. No products of animal origin are used during vaccine preparation. Quality control is performed for each individual batch of the vaccine as well as for the lysate used in the loading of dendritic cells.
MCV was originally developed in 2001 as a result of the combined research efforts of DanDrit researchers and employees and has been tested in clinical trials for the treatment of colorectal cancer (CRC) and non-small-cell lung cancer (NSCLC).
CRC Clinical Trials
|
●
|
Phase I/II at Gentofte Hospital, Denmark (investigator-sponsored trial)– Completed, November 2004 – April 2006
|
|
|
●
|
Phase II at the National Cancer Centre, Singapore (investigator-sponsored trial) – Completed, November 2005 – March 2007
|
NSCLC Clinical Trials
|
●
|
Phase II at Herlev Hospital, Denmark(Dandrit-sponsored trial) – Completed, January 2006 – September 2009
|
ColoRectal Cancer (CRC) in Denmark
The clinical trial using MCV at the University Hospital of Copenhagen, Gentofte, in Denmark was an investigator sponsored trial. The principal investigator and sponsor of the trial was Dr. Anders Fischer, a recognized specialist in surgical oncology in Demark and the department head of the Dept. of Surgical Gastroenterology at Copenhagen University Hospital in Gentofte, who received a grant to fund the trials provided by the Aase & Einar Danielsen Foundation. Dr. Jacob Rosenberg, a professor and surgeon, working in the Dept. of Surgical Gastroenterology at Copenhagen University and Dr. Mogen Claesson, a director of DanDrit designed the trial and proposed it to Dr. Fischer to act as sponsor and principal investigator. Enrollment of CRC patients started in October 2004 and the study ended in September 2006. Certain DanDrit staff, including Dr. Rosenberg and Dr. Claesson, as well as two other employees of DanDrit Denmark at the time of the study closely collaborated with the researchers at Gentofte Hospital responsible for the day to day work of the study with respect to the study design, analysis and interpretation of data obtained in the Denmark CRC Trial. In addition, the patents and proprietary knowledge of DanDrit and its employees were utilized in connection with the analysis and interpretation of the data that resulted from the study. The results and findings of the trial were published in established scientific journals (Phase I study: J Exp Clin Cancer Res. 2006 Jun;25(2):201-6., Phase II study, clinical data: Oncol Rep. 2008 Dec;20(6):1305-11., Immunological data: Acta Oncol. 2009;48(8):1157-64.), which were co-authored by Dr. Rosenberg, Dr. Claesson and the two other DanDrit staff researchers.
The data described in this prospectus with respect to the Copenhagen CRC trial have been obtained from the published papers issued in connection with the study. Twenty patients with advanced colorectal cancer (Dukes D - not curable by resection and no further conventional therapy options available) were included in the study (six patients in phase I and 14 in phase II).
The purpose of this open phase I/II study was to study the tolerability and effect of MCV given as intradermal injections to patients with metastasizing colorectal cancer, where there was no indication for surgery or chemotherapy. The first part was a phase I study to investigate whether treatment with MCV is in any way toxic. No toxicity was observed and the study continued into phase II to study the effect and tolerability of MCV. At the completion of the study stable disease was observed in twenty percent of the enrolled patients. This data was achieved with DanDrit’s early MCV vaccine, which has since been replaced by an improved MCV. The MCV was improved subsequent to the completion of the clinical trials described in this prospectus, but included the addition of aza-cytidine to the DDM-1 culture to de-methylize the genome in order to optimize tumor specific antigen expression. The benefit was marginal and did not justify switching to a different product during the trials. As a result, all trials we will present to the FDA and EMEA will use the same cell line and the same manufacturing process.
Inclusion criteria:
|
●
|
Age 25-75
|
|
|
●
|
No chemo or radiotherapy within six weeks prior to inclusion
|
|
|
●
|
Expected survival > four month
|
|
●
|
Performance status two according to the performing status of WHO
|
|
|
●
|
Adequate hepatic and renal function
|
|
|
●
|
Adequate hematopoietic and coagulation capacity
|
|
|
●
|
Normal EKG or non-clinical significant abnormal EKG
|
|
|
●
|
Preserved pulmonary function
|
Exclusion criteria for the trial:
|
●
|
Uncontrolled serious infection
|
|
|
●
|
Systemic corticosteroid treatment or other immune suppressive treatment in the last two months
|
|
|
●
|
Participation in other clinical trials over the former six weeks
|
|
|
●
|
For women, pregnancy or lactation
|
Study design: dendritic cells were generated from autologous peripheral blood mononuclear cells (PBMC). In order to increase the level of circulating leukocytes, patients exercised five minutes on a treadmill before 200 ml of blood was drawn. Patients were scheduled for ten vaccinations consisting of 3-5x106 dendritic cells. Vaccinations were given bi-weekly intra-dermally on the proximal thigh with two injections each thigh. Adverse events were monitored and classified according to the National Cancer Institute’s Common Toxicity Criteria (NCI’s CTC). Evaluation of responses was made according to the Response Evaluation Criteria in Solid Tumors (“RECIST”) criteria and patients were CT scanned before entering the study, after five vaccinations and after ten vaccinations. Quality of life was monitored by questionnaires bi-weekly. The study was performed at the Department of Surgical Gastroenterology at Gentofte University Hospital, Copenhagen, Denmark according to ICH Guidelines for Good Clinical Practice (European Directive on GCP 2001/20/EC).
RECIST is a set of published rules that define when tumors in cancer patients improve ("respond"), stay the same ("stabilize"), or worsen ("progress") during treatments. The criteria were published in February 2000 by an international collaboration including the European Organization for Research and Treatment of Cancer (“EORTC”), National Cancer Institute of the United States, and the National Cancer Institute of Canada Clinical Trials Group. Today, the majority of clinical trials evaluating cancer treatments for objective response in solid tumors are using RECIST.
The aim of the phase II CRC study in Denmark was to evaluate the effect of treating patients with advanced colorectal cancer with a cancer vaccine based on dendritic cells pulsed with an allogenic tumor cell lysate. Twenty patients with advanced colorectal cancer were consecutively enrolled, with 17 completed the full study. Dendritic cells (DC) were generated from autologous peripheral blood mononuclear cells and pulsed with allogenic tumor cell lysate containing high levels of cancer-testis antigens. Vaccines were biweekly administered intra-dermally with a total of 10 vaccines per patient. CT scans were performed and responses were graded according to the RECIST criteria. Quality of life was monitored with the SF-36 questionnaire. Four patients of the 17 were graded with stable disease, two of whom remained stable throughout the entire study period. Analysis of changes in the patients’ quality of life revealed stability in the sub-groups: “physical function” (p=0.872), “physical role limitation” (p=0.965), “bodily pain” (p= 0.079), “social function” (p=0.649), “emotional role limitation” (p=0.252) and “mental health” (p=0.626). The median survival from inclusion was 5.3 months (range 0.2 - 29.2 months) with one patient still being alive almost 30 months after inclusion in the trial. Toxicity and adverse events were graded according to the National Cancer Institute’s common Toxicity Criteria. At the first evaluating CT scan, four patients were categorized with stable disease and at the second evaluating CT scan two of these patients still had stable disease and one of them received additional monthly vaccines because of the remaining stability in the disease. DanDrit determined that treatment with this DC-based cancer vaccine was safe and non-toxic. Stable disease was found in 24% (4/17) of the patients participating in the full study. The quality of life remained stable for most categories stable throughout the study period. Stable disease is defined as a tumor that is neither growing nor shrinking. Stable disease also means that no new tumors have developed and that the cancer has not spread to any new regions of the body (the cancer is not getting better or worse) and quality of life, measured using a global health score, was at the baseline with no or minimal variation. Variations in the patients' self-reported quality of life during the study period, assessed by the SF-36 questionnaire, were estimated using Freidman's statistical analysis. There were no significant variation in the patients' ‘physical function’ (p=0.872), ‘physical role limitation’ (p=0.965), ‘bodily pain’ (p=0.079), ‘social function’ (p=0.649), ‘emotional role limitation’ (p=0.252) and ‘mental health’ (p=0.626). There was a significant variation concerning ‘general health perception’ (p=0.006) and ‘vitality’ (p=0.011).
Primary endpoints of the study were tumor response according to RECIST criteria and quality of life (Burgdorf SK, Fischer A, Myschetzky PS, Munksgaard SB, Zocca MB, Claesson MH, Rosenberg J. Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. Oncol Rep. 2008 Dec;20(6):1305-11. PubMed PMID:19020707) and secondary endpoints for the study were responses measured by immunological parameters (Burgdorf SK, Claesson MH, Nielsen HJ, Rosenberg J. Changes in cytokine and biomarker blood levels in patients with colorectal cancer during dendritic cell-based vaccination. Acta Oncol. 2009;48(8):1157-64. doi:10.3109/02841860903099964. PubMed PMID: 19863224).
As a measure of quality of life for the colorectal cancer trial in Denmark, DanDrit used the SF-36 Global Health Score questionnaire to evaluate the patients' quality of life throughout the study period. At the time of the trial, this questionnaire from the Medical Outcome Study (MOS), conducted by the RAND Corporation, was both recommended and validated. All patients in the trial independently filled in the questionnaire every two weeks. The SF-36 Global Health Score questionnaire consists of eight scaled scores, which are the weighted sums of the questions in their section. Each scale is directly transformed into a 0-100 scale on the assumption that each question carries equal weight. The lower the score is the more disability is reported by the patient. Higher scores reflect less disability i.e. a score of zero is equivalent to maximum disability and a score of 100 is equivalent to no disability. The eight different aspects of quality of life reflecting different aspects of the patient’s self-reported quality of life are:
|
●
|
vitality
|
|
| ● |
physical functioning
|
|
| ● |
bodily pain
|
|
| ● |
general health perceptions
|
|
|
●
|
physical role functioning
|
|
|
●
|
emotional role functioning
|
|
|
●
|
social role functioning
|
|
| ● |
mental health
|
The fact that patients' SF-36 Global Health Score was high signifies that when entering the study the patients’ quality of life was comparable to the healthy background population. The fact that it remained stable signifies that there were no significant changes in the patients’ quality of life during treatment. This correlates with the fact that the treatment was well tolerated by all patients and that investigators did not observe severe adverse effects from the treatment.
A more in depth analysis of the components of the patients’ quality of life revealed a stability in certain parameters that measure quality of life. The “p” refers to the p-value. In a statistical test, the p-value is the probability of getting the same value for a model built around two hypotheses, one is the "neutral" hypothesis, and the other is the hypothesis under testing. In the Friedman analysis that was used for QOL testing in this study, a p-value below 0.05 means that the values varied throughout the observation period (but says nothing about increase or decrease). A p-value above 0.05 means that the QOL values were stable throughout the observation period.
The graph below indicates the specific p-values of “general health perception” and “vitality” throughout the study:
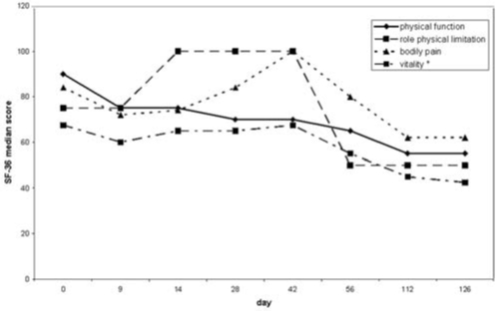
The Company does not believe that any significant information can be inferred from the variation observed, even if statistically significant, with respect to these two parameters as it would have been anticipated that these extremely sick patients with a progressive disease would have resulted in variations in QOL over the course of the study. For this same reason, it is, however, more significant that 6 of the 8 parameters showed stability as it can be inferred that the treatment had a positive impact on the QOL of patients.
Non-small cell lung cancer (NSCLC) in Denmark
DanDrit sponsored and funded this MCV clinical trial conducted at Herlev Hospital, University of Copenhagen, in Denmark by Quintiles A/S (“Quintiles”) and ACRO Nordic A/S (“ACRO”) as contract research organizations (CROs). The title of the study is: “Vaccination with Autologous Dendritic Cells Pulsed with Allogeneic Tumor Lysate (MelCancerVac) for the Treatment of Patients with Advanced or Metastatic Non-Small Cell Lung Cancer”. The principal investigator was Dr. Anders Mellemgaard, the head of the Department of Lung Medicine at Herlev Hospital. Dr. Claesson and three other DanDrit employees worked and collaborated with the researchers at the Herley Hospital with respect to study design and the analysis and interpretation of data obtained from the NSCLC Trial. The results of the trial were published in a recognized scientific journal (World Journal of Vaccines, 2013, 3, 68-76) in a paper that was co-authored by Dr. Claesson and the three other DanDrit employees that collaborated with the NSCLC Trial researchers.
The NSCLC trial was designed as an open-label, phase II clinical study. Enrolled patients had disseminated, inoperable NSCLC after chemotherapy; the patients did not want further chemotherapy: and no other systemic treatments could be offered to them.
The primary objective was to measure the antigen specific immunological reaction between vaccine antigens and the patients’ immune system in vivo and in vitro. The secondary objectives were to estimate the patients’ survival time, the tumor response according to RECIST criteria, and the patients’ quality of life during the study period. Primary endpoint was tumor response, assessed by clinical benefit rate, the percentage of patients with advanced or metastatic cancer who have achieved complete response, partial response and stable disease to a therapeutic intervention in clinical trials of anticancer agents (CBR), however the study also evaluated PFS and overall survival (OS) as secondary endpoints. Complete response (CR) is a figure representing the percentage of patients whose cancer disappears after treatment. Partial response (PR) is a figure representing the percentage of patients whose cancer shrinks after treatment. PR describes a tumor that has decreased in size by at least 30%. The term stable disease (SD) describes a tumor that is neither growing nor shrinking. SD also means that no new tumors have developed, and that the cancer has not spread to any new regions of the body (the cancer is not getting better or worse). The median overall survival was 7.4 months (95% confidence interval (CI), used to indicate the reliability of an estimate, 4.5-17.5 months). Two patients were still alive at the time of analysis. An exploratory analysis showed that patients with PR and SD had significantly better survival (median, 18.1 months) compared to those with progressive disease (median, 6.2 months; P = .007). Although the median time to tumor progression was short at 2.4 months (95% CI, 1.9-4.1 months), five patients experienced a prolonged PFS of more than 6 months; and two of them (reviewed below) continued to be progression-free at time of analysis (PFS >27 and >37 months).
The first patient was included in January 2007. A total of 28 patients were included in the trial. Treatments prior to DC vaccinations, tumor histology, smoking status, number of vaccinations, age and gender were recorded. The median age was 58.5 years (46-74 years). All patients received systemic anti-cancer treatment prior to inclusion. At the time of inclusion, 15 patients were in performance status (PS) 0 and seven patients were in PS 1. Fifteen months after termination of the trial, 4 patients (patient number 1, 2, 12 and 13) were still alive. These four patients who remained in stable disease after more than 10 vaccinations had different histology subtypes: one broncho-alveolar carcinoma, one squamous cell carcinoma and two adenocarcinoma. In this Phase IIa trial a 43% CBR (the percentage of patients with advanced or metastatic cancer who have achieved CR, PR and SD to a therapeutic intervention in clinical trials of anticancer agents) was observed, with six patients showing stable disease. Five of these patients were immunologically responding to the vaccine (ELISPOT –IFN Gamma positive) while eight of nine patients with no clinical response had no IFN gamma response. Sixteen patients received at minimum six vaccines and were evaluated by CT scans. Of those, nine patients showed progression on the 1st evaluation CT scan three months after initiation of treatment, and seven patients had stable disease, representing a 43% CBR. For these 7 patients remaining in stable disease (SD) for a variable period of time, the overall survival curve showed a plateau after two years.
In this NSCLC trial, quality of life was measured by self-administered questionnaire using EORTC Quality of Life Questionnaire (QLQ)-C30 version 3 and QLQ-LC13. The QLQ-C30 is composed of both multi-item scales and single-item measures. These include five functional scales, three symptom scales, a global health status/quality of life scale, and six single items. Each of the multi-item scales includes a different set of items - no item occurs in more than one scale. All of the scales and single-item measures range in score from 0 to 100. A high scale score represents a higher response level. Thus, a high score for a functional scale represents a high/healthy level of functioning, a high score for the global health status/quality of life represents a high quality of life, but a high score for a symptom scale/item represents a high level of symptomatology/problems. Version 3.0 is currently the standard version of the QLQ-C30, and should be used for all new studies. An essential component of the EORTC QLQ development strategy involves the use of cancer-specific supplementary questionnaire modules which, when employed in conjunction with the QLQ-C30, can provide more detailed information relevant to evaluating the quality of life in specific patient populations. The additional QLQ-LC13 questionnaire is specifically designed for lung cancer patients. The QLQ-LC13 includes questions assessing lung cancer-associated symptoms (cough, hemoptysis, dyspnea and site specific pain), treatment-related side effects (sore mouth, dysphagia, peripheral neuropathy and alopecia) and pain medication. The questionnaire was filled by the patients at baseline, and by the time of the 5th, 6th, 7th, 8th, 9th and 10th vaccinations. The data from the quality of life questionnaires was collected and coded according to EORTC. An overall evaluation of general quality of life-score for the global question of “How do you rate your overall quality of life during the past week” remained stable throughout the study period. More specific factors such as anxiety and lung specific symptoms also remained unchanged during the study-period.
The NSCLC trial in Denmark evaluated the clinical and immunological effects of dendritic cell (DC) vaccination in patients with NSCLC. Autologous DCs were pulsed with a MAGE containing allogenic melanoma cell lysate (MCV). Twenty-two patients initiated the vaccination program including a total of ten vaccinations. Seven patients remained in SD three months after the first vaccination. After 10 vaccinations, six months after vaccine initiation, four patients still showed SD and continued vaccinations on a monthly basis. These four patients received a total of 12, 16, 26 and 35 vaccinations, respectively. Five patients showed unexpectedly prolonged survival. The treatment was well tolerated and only minor adverse events were reported. Quality of life did not change during the study period. In four out of seven patients with SD, vaccine-specific T cells were detected by interferon gamma (IFNγ) (a small protein that plays a role in immunity against infections and for tumor control mostly by activating microphages) EliSpot assays, whereas only one patient with progressive disease (PD) showed vaccine-specific responses. This DC-based vaccine trial has indicated a correlation between vaccine-specific immunity and sustained SD. The finding of a significant correlation between prolonged disease stabilization and vaccine-specific cellular responses may support the latter notion and support the hypothesis that immune responses may play a role in disease control even long time after the actual treatment. This is in sharp contrast to the rapid effect of anti-cancer treatments such as chemotherapy and radiotherapy. Furthermore, the trial demonstrated an unexpectedly prolonged survival in some patients, which may indicate delayed effect of DC vaccination after completion of the treatment. In addition, the investigators reported that this kind of vaccine treatment was feasible and the logistics were manageable in this patient group.
In conclusion, 7 out of 22 NSCLC patients vaccinated with autologous DC pulsed with an allogenic Clinical Trial Authorization (CTA) containing tumor cell lysate had prolonged disease stabilization. In the course of DC vaccination vaccine-specific IFNγ responses were detected in peripheral blood of four of patients with SD and one patient with progressive disease. However, from this study it is not possible to conclude whether the vaccine treatment and the subsequent IFNγ responses are involved in the clinical cause of these patients. To elucidate the full efficacy of vaccine treatment of patients with NSCLC, the investigators recommended that a randomized trial should be conducted.
Colorectal Cancer (CRC) in Singapore
A single arm phase II clinical study was also sponsored and funded by the Singapore National Cancer Centre (NCC) to investigate the efficacy of intradermal vaccination with MCV in patients with advanced colorectal cancer. The principal investigator of the Singapore CRC Trial was Dr. Han Chong Toh, a recognized specialist in medical oncology in Singapore. While Dandrit Denmark was not sponsor of the trial, it assisted in the design and proposal of the trial to the SNCC and Dr. Toh. Dr. Claesson and two other DanDrit employees collaborated with the researchers at the Singapore National Cancer Center, with respect to the study design and the analysis and interpretation of data obtained from the Singapore CRC Trial. The results and findings of the Singapore CRC trial were published in recognized scientific journals (Clinical results: Clin. Cancer Res. 2009 Dec 15;15(24):7726-7736., Immunological data: Vaccine. 2009 Dec 11;28(2):542-7.) that was co-authored by Dr. Claesson and the two other employees of DanDrit Denmark that collaborated with the researchers at the NCC in Singapore.
The study used DanDrit’s patented procedure for generating dendritic cells. All included patients had tumors which antigenically correlated with the vaccine, i.e. were MAGE-A positive. The purpose of the study was to investigate the objective efficacy and specific immunologic response of the MCV vaccination. The first patient was enrolled in June 2005, and by June 2007 a total of 20 patients had been treated and evaluated.
The vaccine was given to advanced colorectal cancer patients pre-treated with chemotherapy, where there was no further indication for surgery or treatment with chemotherapy.
Treatment with MCV did not appear to adversely affect the patient’s quality of life, measured based on a global health score of 68.3 prior to treatment with minimal variation through the course of the treatment. The health-related quality of life assessment quantifies how the individual's well-being may be affected over time by a disease, such as cancer. Health-related quality of life is assessed using patient questionnaires. These questionnaires are multidimensional and cover physical, social, emotional, cognitive, work- or role-related and spiritual aspects, as well as a wide variety of cancer related symptoms, therapy induced side effects, and the financial impact of cancer. The questionnaire from the Eastern Cooperative Oncology Group (ECOG) is most commonly used to evaluate the impact of cancer on sufferers. MCV induced objective responses in seven of 20 patients (six responses were stable disease and one response was partial regression of tumor mass). Significant immunological and clinical correlation was observed. Results from the trial were presented orally at the AACR meeting in Singapore in November 2007.
The CRC trial in Singapore evaluated the efficacy and toxicity of MCV in advanced colorectal cancer patients expressing at least one of six MAGE-A antigens. Dendritic cells were cultured from peripheral blood mononuclear cells (PBMCs) and pulsed with allogenic lysate and matured using cytokines to achieve high CD83 and CCR7 expressing dendritic cells. Each patient received up to 10 intradermal vaccinations (3-5 x 106 cells/dose) at biweekly intervals. Twenty patients received a total of 161 vaccinations. Treatment was well-tolerated with minimal adverse events. Quality of life measurement using global health score was high at baseline and did not change during the duration of the trial. In this study, statistical testing was done with repeated t-tests comparing baseline with each time point. or similar. A “t-test” is a statistical analysis used to determine whether there is a statistical difference between averages or means of a group with a small sample size. The “baseline” is a starting point from which a comparison can be made and is typically established prior to the beginning of a study as a point of comparison for monitoring and evaluating data at various point in a study. The term “comparing baseline with each time point” refers to the comparison of data at a defined point in time against the originally established “baseline”.
Since the p-values did not change during the duration of the trial, we believe that there were no statistical differences regarding quality of life in this study in any of the parameters at any time points.
The colorectal cancer patients who are eligible for the humanitarian program in Singapore must present a profile similar to the one of the patients who were recruited in the phase IIa clinical trial. However, there have been some exceptional cases where treatment has been based on a doctor’s discretion on the patient’s quality of life. Also, patients are monitored according to the previous phase II study protocol. To date we have not received a detailed report regarding the final outcomes for patients participating in the Singapore trials.
MAGE-A-expressing metastatic colorectal cancer patients with prior progressive disease treated with MCV achieved a competitive Clinical Benefit Rate of 40%. While patients with single metastatic sites in either lung or nodal regions tended to have more durable responses (see patients 1, 2 and 9 in table below), Stable Disease was also attained in patients with bulky multiple metastases (see patient 6 in table below). Five patients notably remained progression-free for over six months and two patients with significant tumor burden (see patients 1 and 9 in table below) were still progression-free for over 27 and 37 months respectively. We recognize that adopting the primary endpoint of Clinical Benefit Rate using RECIST criteria has limitations. This study protocol was designed in 2005 where objective response rate (ORR) and Clinical Benefit Rate evaluation as primary endpoints in Phase II cancer vaccine trials were not uncommon. Nevertheless, the investigators did evaluate Progression Free Survival and Overall Survival as secondary endpoints, which may better reflect true vaccine efficacy.
A meta-analysis of 32 cancer vaccine clinical studies in patients with advanced colorectal cancer reported a Clinical Benefit Rate in 11.2% of patients and an overall response rate (Complete Response and Partial Response) of 0.9%. The defined clinical benefit rate (Complete Response, Partial Response, Stable Disease) was observed in 17% (12/70) of colorectal cancer patients who received Dendritic Cell vaccines.
Patients’ Characteristics
|
ID
|
Age
(years) |
Sex
|
PS
|
Site of disease
|
No. of
Chemo-regimens |
Disease at Accrual
|
No. of vaccinations
|
BOR
|
Time to Tumor response (months)
|
Duration of response (months)
|
TTP
(months) |
Survival Time (months)
|
||||||||||||||
| 1 | 72 | F | 1 |
LN
|
1 |
PD
|
10 |
SD
|
2.7 |
> 25.0
|
* |
> 27.7
|
* | 39.7 | † | |||||||||||
| 2 | 67 | F | 1 |
Lung
|
0 |
PD
|
10 |
SD
|
2.9 | 4.2 | 7.1 | 35.6 | ||||||||||||||
| 3 | 53 | F | 2 |
Lung, LN, Pelvic, Bone
|
4 |
PD
|
10 |
SD
|
1.7 | 5.2 | 6.9 | 6.9 | ||||||||||||||
| 4 | 43 | F | 1 |
Lung, Adrenal, LN
|
4 |
PD
|
3 |
PD
|
- | - | 2.6 | 5.9 | ||||||||||||||
| 5 | 54 | M | 1 |
Liver, Lung, Ascites, LN
|
3 |
PD
|
3 |
ND‡
|
- | - |
> 3.8
|
‡ | 3.8 | ‡ | ||||||||||||
| 6 | 76 | M | 0 |
Liver, Peritoneum, Pelvic, Lung, LN, Serosa
|
3 |
PD
|
10 |
SD
|
1.8 | 2.4 | 4.1 | 7.6 | ||||||||||||||
| 7 | 33 | F | 1 |
Bone
|
2 |
PD
|
9 |
PD
|
- | - | 2.0 | 6.5 | ||||||||||||||
| 8 | 75 | F | 0 |
Lung
|
0 |
PD
|
10 |
PD
|
- | - | 1.9 | 13.1 | ||||||||||||||
| 9 | 62 | F | 1 |
LN, Lung and Pelvic
|
3 |
PD
|
10 |
PR
|
2.5 |
> 35.4
|
* |
> 37.9
|
* | 37.9 | † | |||||||||||
| 10 | 73 | M | 0 |
Liver, LN
|
2 |
PD
|
10 |
PD
|
- | - | 2.1 | 19.6 | ||||||||||||||
| 11 | 64 | M | 0 |
Liver
|
1 |
PD
|
10 |
PD
|
- | - | 2.1 | 6.4 | ||||||||||||||
| 12 | 57 | M | 1 |
Lung, Liver, LN
|
2 |
PD
|
10 |
PD
|
- | - | 2.3 | 7.5 | ||||||||||||||
| 13 | 65 | F | 1 |
LN, Pleural, Lung, Liver
|
5 |
PD
|
5 |
PD
|
- | - | 1.6 | 2.9 | ||||||||||||||
| 14 | 49 | M | 1 |
Lung, Liver, Peritoneum
|
5 |
PD
|
8 |
SD
|
2.3 | 1.2 | 3.5 | 7.2 | ||||||||||||||
| 15 | 72 | M | 0 |
LN, Pleural, Liver, Lung
|
2 |
PD
|
4 |
PD
|
- | - | 1.8 | 3.2 | ||||||||||||||
| 16 | 77 | M | 1 |
Liver, Bone, Lung
|
4 |
PD
|
10 |
SD
|
1.6 | 1.9 | 3.5 | 13.0 | ||||||||||||||
| 17 | 75 | F | 0 |
Lung, Liver
|
1 |
PD
|
10 |
PD
|
- | - | 1.9 | 17.5 | ||||||||||||||
| 18 | 54 | F | 0 |
LN, Lung
|
1 |
PD
|
10 |
SD
|
1.8 | 4.9 | 6.7 | 23.2 | ||||||||||||||
| 19 | 75 | M | 0 |
Lung, Liver,
|
2 |
PD
|
3 |
PD
|
- | - | 2.0 | 2.9 | ||||||||||||||
| 20 | 41 | F | 1 |
Lung, Skin, LN, Bone
|
5 |
PD
|
6 |
PD
|
- | - | 1.9 | 4.5 |
* Indicates that the patient has not progressed at the time of analysis.
† Patients who are alive at the time of analysis have their survival time censored at the time of last follow up.
‡ Patient withdrawn due to poor performance status; survival time was censored at last date in the study.
Abbreviations: PS, performance status according to Eastern Cooperative Oncology Group; CT, chemotherapy; RT, radiotherapy; BOR, best overall response; TTP, time to tumor progression; LN, lymph node; F, female; M, male; SD, stable disease of at least 4 weeks; PD, progressive disease; ND, CT scan not done; PR, partial response.
|
ECOG PERFORMANCE STATUS
|
||
|
Grade
|
ECOG
|
|
|
0
|
Fully active, able to carry on all pre-disease performance without restriction
|
|
|
1
|
Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work
|
|
|
2
|
Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours
|
|
|
3
|
Capable of only limited self-care, confined to bed or chair more than 50% of waking hours
|
|
|
4
|
Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair
|
|
|
5
|
Dead
|
|
As of 2006, there were a total of eight clinical DC vaccination studies in patients with metastatic colon cancer, all with peptide-pulsed DC. To our knowledge, this study which adopted an allogenic tumor lysate-based DC vaccine achieves the highest Clinical Benefit Rate in advanced colorectal cancer patients compared to these previous Dendritic Cell vaccination clinical trials. The clinical activity of this present Dendritic Cell-based vaccine might reflect its polytopic nature, its allogenic adjuvant-like components, the quality of the Dendritic Cell preparation (i.e. high uniform expression of CD83, CD86, HLA class II, and CCR7), the intradermal route of vaccine injection securing optimal lymph drainage to regional lymph nodes, the presence of MAGE expression in both patients and vaccine and the increased frequency of delivery (ten injections).
Quality of life measurement using global health score was high at baseline and did not vary much across time. In this study, statistical testing was done with repeated t-tests comparing baseline with each time point., Since the p. values did not vary across time, we believe that there were no statistical differences regarding quality of life in this study in any of the parameters at any time points.
Treatment with MCV did not appear to adversely affect the patient’s quality of life, measured based on a global health score of 68.3 prior to treatment with minimal variation through the course of the treatment. The health-related quality of life assessment quantifies how the individual's well-being may be affected over time by a disease, such as cancer. Health-related quality of life is assessed using patient questionnaires. These questionnaires are multidimensional and cover physical, social, emotional, cognitive, work- or role-related and spiritual aspects, as well as a wide variety of cancer related symptoms, therapy induced side effects, and the financial impact of cancer. The questionnaire from the Eastern Cooperative Oncology Group (ECOG) is most commonly used to evaluate the impact of cancer on sufferers. MCV induced objective responses in seven of 20 patients (six responses were stable disease and one response was partial regression of tumor mass). Significant immunological and clinical correlation was observed.
Compassionate Use/Named Patient Approval
Further to the data emerging from the Singapore CRC trial, the Singapore government requested and approved (22 September 2008) that named patients be offered MCV therapy at cost. This first compassionate use approval marked a significant milestone for the progress and acceptability of the MCV therapeutic model. This compassionate program could be used as a model to initiate sales of MCV in other countries of the ASEA such as Thailand or Malaysia. Outside the United States, named patient programs provide controlled, pre-approval access to drugs in response to requests by physicians on behalf of specific, or “named”, patients before those medicines are licensed in the patient’s home country. Governments worldwide, such as Singapore’s government, have created provisions for granting access to drugs prior to approval for patients who have exhausted all alternative treatment options and do not match clinical trial entry criteria. Often grouped under the labels of compassionate use, expanded access, or named patient supply, these programs are governed by rules which vary by country defining access criteria, data collection, promotion, and control of drug distribution. Through these programs, patients are able to access drugs in late-stage clinical trials or approved in other countries for a genuine, unmet medical need, before those drugs have been licensed in the patient’s home country. In September 2008, DanDrit Denmark and the National Cancer Centre of Singapore (NCC) entered a collaboration agreement regarding a clinical named patient program conducted in Singapore at NCC with the dendritic cell vaccine MCV. NCC has established a GMP approved laboratory in which the manufacturing of MCV takes place. NCC has received approval from the relevant governmental authorities for the import of lysate necessary for production of MCV. The clinical and research and development activities of the named patient program relate to the Company’s product, MCV. The purpose for the Singapore named patient program is to provide patients with advanced colorectal cancer or other forms of cancer(s) with the presence of MAGE antigen expression an alternative treatment for the vaccination with MCV, where there is no further indication for surgery or treatment with chemotherapy. Patients are recruited on named patient basis according to the patient inclusion and exclusion criteria stated in the phase IIa study protocol. However, there may be some exceptional cases where treatment will be made based on a doctor’s discretion regarding the patient’s quality of life. Approximately 50 patients have been recruited for the Singapore named patient program and 8 patients are still active in the named patient program.
Future: 100% Off-the-Shelve Vaccines
Autologous (from the patient) dendritic cells cancer vaccines are tailor made for each individual patient. This personalized medicine approach is appealing to the patients but may present several drawbacks to a pharmaceutical company. Creating a new, unique vaccine for each patient may be perceived as complex, time consuming, and expensive. Therefore, DanDrit is pursuing two programs to offer in addition to its personalized vaccine 100% off-the-shelve cancer vaccines: MCV2 and MelVaxin™. These two programs presented below capitalize on the knowledge and the expertise gained with DanDrit’s proprietary lysate used for MCV.
48
Allogenic DC based vaccine: MCV2
First, DanDrit is developing MCV2 a 100% off-the-shelf dendritic cell vaccine through collaboration with the Etablissement Francais du Sang (EFS) / GeniusVac (France) pursuant to a Confidential Disclosure Agreement entered into in March 2013, with a Materials Transfer and Feasibility Study Agreement currently under negotiation. Through the proposed agreement, DanDrit would provide EFS with a certain quantity of its proprietary DDM-1.7 lysate, for the limited purpose of EFS using its GEN-T plasmacytoid DC line technology to conduct a feasibility study in collaboration with DanDrit, to demonstrate the potential efficacy of EFS’ technology and evaluate prospects of further cooperation between the parties. The GEN-T cell line is generated by EFS’ R&D laboratory in Grenoble. This cell line, loaded with tumor-derived antigenic peptides (HLA-A*0201 restriction) is being used as a cellular vaccine, to treat cancer patients. The DDM-1.7 lysate is produced by DanDrit to load autologous dendritic cells used to treat cancer patients. In this context, the main objective of the project is to determine if DDM1.7 lysate and GEN-T cell line can be combined to develop a new cancer vaccine. The main point is to validate that the GEN-T cell line, once loaded with the DDM1.7 lysate is able to present the DDM1.7-derived Ag to specific T lymphocytes. MCV2 is a cell-based immunotherapeutic product consisting of an irradiated plasmacytoid dendritic cell line presenting DanDrit’s proven lysate. DanDrit sourced the allogenic dendritic cells from EFS /GeniusVac. EFS/GeniusVac produces an allogenic plasmacytoid dendritic cells (pDCs) line that has demonstrated the induction of multi-specific and highly functional cytotoxic cell responses directed against tumor targets both In vitro and in vivo. These irradiated antigen-presenting pDCs have a strong power to induce specific antitumor response by cytotoxic DC8+ T-cells. The safety and efficacy allogenic pDC platform has been proven. Stimulation of PBMC from HLA-A*0201+ donors by HLA-A*0201 matched allogenic pDCs pulsed with tumor-derived peptides triggered high levels of antigen-specific and functional cytotoxic T cell responses (up to 98% tetramer+ CD8 T cells (a group of white blood cells known as lymphocytes and play a central role in cell-mediated immunity). The pDC vaccine demonstrated anti-tumor therapeutic in vivo efficacy as shown by the inhibition of tumor growth in a humanized mouse model. It also elicited functional tumor-specific T cells ex-vivo from PBMC and TIL of stage I-IV melanoma patients. Responses against MelA, GP100, tyrosinase and MAGE-3 antigens reached tetramer levels up to 62%, 24%, 85% and 43% respectively. pDC vaccine-primed T cells specifically killed patients’ own autologous melanoma tumor cells. This semi-allogenic pDC vaccine was more effective than conventional myeloid DC- based vaccines.
This Allogenic approach offers multiple advantages:
|
●
|
The vaccine could then be mass-produced in a unique manufacturing facility
|
 |
|
| ● |
Cost effective process
|
||
| ● |
All manufacturing process can then be out-sourced (DanDrit does not need to support its own GMP manufacturing facility)
|
||
| ● |
The MCV2 vaccine could be more likely to have higher potential efficacy than MCV. The allogenic DCs are further regarded as MHC-incompatible foreign invaders. Then, they induce an inflammatory reaction that further promotes the recruitment and activation of endogenous DCs at the vaccination site. This hypothesis has now been verified in rat and mouse cancer models in which tumor growth was significantly reduced by therapeutic vaccinations with tumor-loaded allogenic DCs.
|
|
●
|
MCV2 is still using the clinically proven lysate used in MCV as cancer specific antigen
|
|
|
●
|
“One- To- Many”: the same drug product could be used to treat several patients (consistent with current pharma business model).
|
|
|
●
|
Fully standardizable product
|
|
|
●
|
Guarantee of homogeneity of the clinical trials
|
Melvaxin™
A second platform product, MelVaxin™ is also evaluated. MelVaxin™ is similar to the lysate component of MCV. DanDrit proposed injecting MelVaxin™ into the skin to promote natural dendritic cell responses that will attack the tumor expressing cancer/testis antigens. It is necessary to inject MelVaxin™ with an immuno-stimulator such as GM-CSF, BCG or novel adjuvants (such as 3M’s TLR7 and TLR8 agonists). A preclinical program has been planned in minipigs. These animals have immune response profiles, particularly of skin injection, that are very close to human. This program, currently on hold, can be reinitiated when staff is available to manage this program. This takes second place to the MCV2 program and illustrates DanDrit’s professional commitment to advancing lead clinical products.
Other Future Products
Other cancers
DanDrit has already made progress with clinical trials of NSCLC and CRC. DanDrit is now focusing its clinical development on advanced colorectal cancer, but DanDrit may if opportunity arises extend its range of cancer targets to answer the desperate need for effective new therapies. As an illustration, esophageal cancers may be one of these opportunities. The two types of esophageal cancers the esophageal squamous cell carcinoma (EC) and the esophageal adenocarcinoma (EAC) expressed MAGE -A. Worldwide, EC is the most frequent malignant esophageal cancer accounting for at least 10,000 deaths per year.
But in Western countries, EAC is the most rapidly increasing cancer compared with other malignancies. Surgical resection is currently the only potential cure with or without neo-adjuvant or adjuvant chemo-and/or radiotherapy, the five year survival rate is less than 20%. At first presentation, approximately 50-60% of patients with esophageal cancer are not eligible for surgery and have very poor outcome.
Tolerogenic Dendritic Cells
Some dendritic cells seem to instruct cell-killing T cell clones to abandon their mission by self-destructing through an apoptotic pathway. This may offer the possibility of eliminating those T cells responsible for the manifestation of auto-immune disease. In MCV dendritic cells are derived in such a way that the resulting dendritic cells promote an immune reaction. However, dendritic cells may also be derived in such a way that they are tolerogenic, they promote immune tolerance. Promoting immune tolerance can be used to treat autoimmune diseases such as early stage type I diabetes (where insulin secreting cells are still present) or even to help prevent rejection of tissue transplantation. In this way the tolerogenic dendritic cells are used to turn off an undesirable immune reaction. DanDrit has established methods to derive tolerogenic dendritic cells from peripheral blood monocytes, similar to the approach used to generate immunogenic dendritic cells in MCV. Tolerogenic dendritic cells are easily distinguished by their function in vitro. DanDrit has filed patents to cover the generation of tolerogenic dendritic cells.
Non-Core Products – Out-licensing
Non-core patents are being developed for application in dendritic cell related applications that are not cancer-related. Revenues from licensing such non-core products will support core product and core technology development.
The principal non-core intellectual property relates to tolerogenic dendritic cells, their production and application in auto-immune diseases to include type 1 diabetes. DanDrit’s fast track production methods for dendritic cells might be out-licensed for non-competitive applications in areas other than cancer.
Fast-track production of Dendritic Cells
The generation of mature immunogenic dendritic cells from peripheral blood monocytes requires eight days of growth in culture. The efficiency of producing MCV could be improved if the time required to generate dendritic cells could be significantly reduced. DanDrit has tested many protocols for generating dendritic cells quickly. Two promising methods have emerged from intensive research activities to generate dendritic cells in either two days or five days. The fast track methods for generating dendritic cells produce immunogenic dendritic cells that are comparable to cells generated using DanDrit’s standard technique. These new fast track methods are covered by DanDrit’s existing dendritic cell technology patent.
This fast-track production technology could be of commercial interest for other companies working in non-competitive areas of dendritic cell technology.
MicroRNAs for dendritic cell quality control
DanDrit patented a method using microRNAs to characterize dendritic cells and establish a basis for quality control. To date there are few dendritic-cell specific antigens and those existing are covered by patents. DanDrit has patented its microRNA approach developed with Bioneer (note that patents are 100% owned by DanDrit).
Proposed Clinical Trial
The proposed PoC study with an adaptive design plans to enroll 174 stage IV colorectal cancer patients after surgical resection of metastases and chemotherapy. Regulatory authorities in the United States and Europe have published guidance documents on the use and implementation of adaptive design trials. These documents include descriptions of adaptive trials and a requirement for prospectively written standard operating procedures and working processes for executing adaptive trials, as well as a recommendation that sponsor companies engage with CROs that have the necessary experience in running such trials.
The proposed patients in the trial will therefore have no evidence of disease. The clinical study is designed as a randomized, placebo controlled, multicenter, Phase IIb/III clinical study. Treatment is double blinded (to the patients and physicians). Patients will be included after resection of their primary tumor and resectable metastases in liver and after appropriate peri- or post-operative chemotherapy by stratification and random assignment to a non-vaccine control group or a vaccine group receiving five vaccinations with 14-day administration intervals followed by ten vaccines with two-month intervals. Inclusion is planned to take place one month after finishing the last round of peri- or post-operative chemotherapy (FOLFOX or FOLFIRI) and after a negative tumor scan (head, thoracic and abdominal cavities) and normal CEA prior to inclusion in the vaccine or the control groups. Patients will be screened for MAGE-A expression. The control group will receive five plus ten injections with physiological saline. In the event of disease progression, as verified by tumor scan during the vaccination schedule, vaccinations will be discontinued. The table below summarizes the key features of the proposed PoC clinical study.
Traditionally drug development has consisted of a sequence of independent trials organized in different phases. Full development typically involved (1) a learning phase II trial and (2) one or two confirmatory pivotal phase III trial(s). The new seamless phase II/III designs are aimed at interweaving the two phases of full development by combining them into one single, uninterrupted study conducted in two steps. Adaptive seamless clinical trial designs have proved to be effective in several clinical research areas, such as the development of Velcade intended for multiple myeloma and non-Hodgkin's lymphoma or a long-acting glucagon-like peptide-1 analog (dulaglutide) in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Adaptive seamless phase II/III designs enable a clinical trial to be conducted in steps with the sample size calculation selected on the basis of data observed in the first step to continue along to the second step. The main statistical challenge in such a design is ensuring control of the type I error rate. Most methodology for such trials is based on the same endpoint being used for interim and final analyses. However, in some settings like our clinical trial, the primary endpoint, overall survival, can be observed only after long-term follow-up. In this case the design includes a shorter term endpoint data, in our case, progression free survival at 18 months. If short-term data are available for some patients for whom the primary endpoint is not available, basing treatment selection on these data may lead to increase of the type I error rate (false positive).
Phase IIb/III Overview
|
Purpose
|
To determine the safety and efficacy of our investigational vaccine in colorectal cancer and to determine its ability to prevent recidive in stage IV colorectal patients with no evidence of disease (after surgical resection of metastase and chemotherapy)
|
|
Study Type
|
Interventional
|
|
Study design
|
|
|
Endpoint (primary)
|
Efficacy : Progression Free Survival at 18 months
|
|
Endpoint (secondary)
|
Overall Survival; Carcino-Embryonic Antigen (CEA); Quality of Life
|
|
Intervention Model
|
Parallel assignment 174 patients
|
|
Masking
|
Double blind
|
|
Allocation
|
Randomized
|
|
Power
|
80%
|
|
Adaptive Design
|
Purpose: seamless Phase II/III clinical trial
|
|
Treatment
|
Five vaccines bi-weekly (intra-dermal administration) followed by ten vaccines every two months
|
|
Location
|
Europe, and USA
|
|
Expected Duration
|
Three years
|
|
Eligibility
|
Stage IV colorectal cancer patients
After resection of metastase and no evidence of disease (CT scan and CEA back to normal)
● Stratification for risk factor
Vaccine therapy given after FOLFOX or FOLFIRI (after completion of course of chemotherapy)
Screen for MAGE-A expression
|
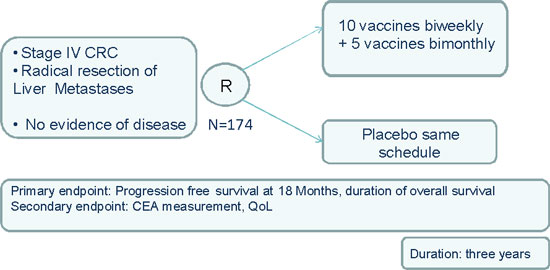
Critical Success Factors
The points below are a specific, focused list of critical factors and challenges that need to be considered for the project, during the critical start-up phase and throughout the project life cycle. In addition to the sections noted below, during the course of the study, DanDrit will be pro-active in discussing the Critical Factors with the investigators.
Oncology studies by their nature have a degree of complexity not always encountered in other therapeutic areas. We believe success of the CRC study will be related to these Critical Success Factors. Our approach to each critical factor is detailed below. DanDrit identified the following key factors for success:
|
●
|
Patient accrual and site selection
|
|
|
●
|
Assessment of patient response
|
|
|
●
|
Study design and collection of patient data
|
|
|
●
|
Vaccine supply
|
|
|
●
|
Patient safety
|
|
|
●
|
Multinational regulatory requirements
|
|
|
●
|
CRO previous experience
|
|
|
●
|
Adaptive trial design experience
|
Patient accrual and site selection
The proposed study is anticipated to enroll 174 patients at approximately 22 sites in Europe. Although the number of potential stage IV patients is significant, only a portion of these, approximately 10-20%, will have complete resections of their primary tumors and metastatic disease with no detectable residual disease (i.e. clear resection margins). This coupled with a competitive oncology vaccine research environment will put pressure on accrual milestones.
The selected patient population will be easier to work with than the patients in Phase IIa. It is reasonable to expect the response rate to be greater for MCV, or for any immunotherapy, in a patient population with No evident Disease (NED). Consequently in this Phase IIb/III trial, patients will have to be NED, raising the likelihood that the immune system can generate a response against cancer as it re-occurs. We believe this may ultimately lead to better data from the Phase IIb/III trial. Careful selection of study sites using evidence based feasibility research, discussion with colorectal key opinion leaders (KOLs), contact with investigators at key sites and our past clinical experience in this indication and with cancer vaccines will be required. While there are currently no definitive agreements in place, the Company and its management are currently in negotiations with respect to various study sites to complete these trials, including the Dana Farber Harvard University in the US, the Institut Gustave Roussy (IGR) in France, and the GISCAD Foundation for Research on Cancer (GISCAD) in Italy (Pr. Sobrero), all of which are recognized as among the world’s premier cancer research and treatment facilities and leaders in immunotherapy research. In addition, the Company is also considering the option to conduct the trial in Italy exclusively in collaboration with GISCAD, which conducted a clinical trial evaluating FOLFOX-4 3 months vs. 6 Months and Bevacizumab as adjuvant therapy for patients with Stage II/III CRC. The network of Italian hospitals enrolled 3,800 patients in the recent Thiazolidinediones or Sulfonylureas and Cardiovascular Accidents (TOSCA) trial.
Accrual rates are estimates and can be further refined. Inadequate enrollment is one of the biggest drivers of wasted cost and time in clinical trials. Therefore, DanDrit has taken a very conservative position regarding site selection and patient enrollment.
Assessment of patient response
In general, in oncology vaccine studies, the relationship between clinical response, survival (and other measures of efficacy) and immune response may be unclear. Changes in patients’ immunological profiles during vaccination protocols, their response to the vaccine components as measured by delayed-type hypersensitivity (DTH), used as the primary measure of the ability to immunize a patient to a tumor cell or specific tumor antigen; the enzyme-linked immunosorbent spot (ELISPOT), a common method for monitoring immune responses In humans; cluster of differentiation (CD) antigen profiles, protocol used for the identification and investigation of cell surface molecules; and other strategies to attempt to correlate treatment outcome with the results of vaccination are variable. The paper describing the Phase II study in CRC patients by Toh et al indicates that a plasma protein expression profile has been identified for responding patients. Continued evaluation of immunological profiles of the patients and the collection of these data and correlation with outcomes may be desirable but for this POC study will not be necessary.
In a guidance document by the FDA, “Clinical Considerations for Therapeutic Cancer Vaccines” (September 2009), the agency recognizes that immunological approaches to tumor control may require significant time to develop, and that careful clinical assessment of patients must be performed as well as the use of methods that rely on radiological measurement of tumor size (e.g. RECIST). The guidance indicates that for cancer vaccines, patients may be observed to develop indications of progressive disease based on radiological measurement, but that these indications may also be transient and that tumor regression is still a possibility as the immunological response develops. Methods to incorporate such an approach will help avoid premature termination of study treatment for some patients.
Tumor burden has also been a confounding problem for oncology vaccine development because of tumor-induced immune-suppression in some patients and because of progression prior to immune response. These issues may be obviated in this study of no-evident-disease subjects.
Patient safety
DanDrit believes that MCV appears to be safe and well tolerated in studies to date. Adverse events related to the vaccine appear to be Grade 1-2 and consist of mostly superficial toxicities as describe above. Patients in the proposed study will have recovered from previous treatments and will be apparently disease free: thus, at this time, only general safety precautions and observations related to the patient population are recommended.
Injection site reactions and other toxicities expected in the class of DC vaccines will be included in site training. Some volume of Severe Adverse Events can be anticipated in a population of advanced CRC patients. Discrimination of events related to vaccine against a background of underlying disease and prior chemo or and/or radiotherapy will be necessary.
The CRO’s pharmacovigilance scientists will prepare a Safety Management Plan to specifically outline the procedures to be followed and will train the site personnel to obtain, collect, verify, transmit and coordinate a timely and efficient manner.
In addition to reviewing assignment of causality, a DSMB may assist in the (proposed) interim assessment for efficacy and its constitution should be considered.
Regulatory
DanDrit will seek scientific advice from the European Medicines Agency EMEA (ATMP) in connection with the Phase IIb/III trials in Italy and will request a pre-Phase II/III meeting with the FDA. The CRO Regulatory Affairs group or GISCAD will provide preparation and assistance for the Scientific Advice process in EMEA including the following activities:
|
●
|
Regulatory review of pertinent data
|
|
|
●
|
Discussions/kick off meeting with DanDrit contact(s), for background, pertinent issues, proposed questions, strategic discussion etc.
|
|
|
●
|
Prepare a briefing package for Scientific Advice includes QC (one review round with DanDrit) using the existing information in the Investigational Medicinal Product Dossier(IMPD)/Investigator Brochure(IB) as the basis for the package
|
|
|
●
|
Set up and attend meeting with EU regulatory agency and conduct all associated administrative tasks (letters, post meeting minutes, etc.)
|
Upon receipt of funding in connection with the initial closing of this offering, we anticipate seeking a clinical trial authorization to commence a Phase IIb/III trial in Italy which we anticipate will run over a three year period. DanDrit also has the option to evolve the Phase IIb to a Phase III trial through the use of an adaptive design. Ordinarily a drug requires two Phase III trials before it can apply for FDA approval. Consequently the first patient for Phase IIb could be considered commencement of ‘pivotal’ trials for MCV. Also, DanDrit intends to move to a pivotal trial in China with a Chinese partner. Currently, the China Food and Drug Administration offers a low-cost clinical development pathway for cancer drugs developed, manufactured and commercialized in China. A separate local CRO will be recruited for this Chinese trial. DanDrit intends to file an investigational new drug (IND) application with the FDA to initiate the process to permit manufacturing capability of MCV in the U.S. and to include U.S. patients in the PhaseIIb/III trials initiated in Italy. Once an IND application has been filed in the U.S., we believe that we will be able to expand the PhaseIIb/III trials initiated in Europe to the U.S., however we cannot estimate at this time when we will be able to begin enrolling U.S. patients in the trial.
Our Competitive Strengths
We believe our following strengths position us to increase our revenue and profitability:
|
●
|
Cutting Edge Technology. Immunotherapy is one of the waves of the future in cancer treatment.
|
|
|
●
|
Colorectal Market Potential. Colorectal cancer is a large market with a well identified unmet medical need for safe maintenance therapy. The clinical data for MCV to date gives the potential for the vaccine to eventually become the standard of care for maintenance therapy. MCV has the potential to alter the treatment paradigm by prolonging periods of remission after response to chemotherapy. If MCV works as expected in colorectal cancer, we believe it would likely prove beneficial in other tumors that over-express MAGE-A including lung, breast and esophageal cancer.
|
|
|
●
|
Regulatory Precedent. Dendreon with Provenge™, its prostate cancer vaccine, pioneered the regulatory pathway for MCV. Dendreon worked with the FDA to develop the protocols allowing a cellular therapy such as MCV to be approved for clinical use. DanDrit could be the next generation of dendritic cell vaccine with several improvements over its competition: stimulate a cellular immune response rather than just an antibody response, no need for leukapheresis to produce the vaccine, intradermal administration, convenience of an allogenic vaccine, polytopic approach but with a focus on the MAGE-A antigen family and reliable manufacturing.
|
|
|
●
|
Successful Use in Singapore. For the last five years, the Singapore National Cancer Center have provided MCV to colorectal cancer patients within an on-going compassionate use program in Singapore. Based on that experience, DanDrit is building a potential collaboration with a Chinese oncology pharma partner that may speed up large scale commercialization of MCV.
|
|
|
●
|
Strong IP Protection. The technology is patented with a long patent life. DanDrit owns 100% of the technology, without intellectual property issues.
|
Our Strategy
Our strategy is focused on conducting a proof-of-concept clinical trial in advanced colorectal cancer that may trigger partnership deal that should bring a significant return on investment (based on analysis of past acquisitions of peer cancer vaccine companies).
DanDrit intends to conduct a randomized multicenter clinical trial to determine the ability of MCV to prevent recidivism in stage IV colorectal patients with no evidence of disease after surgical resection of metastasis and chemotherapy. The same need for a safe effective maintenance therapy exists for stage III colorectal cancer patients with no evidence of disease after surgical resection.
This blinded comparative trial is planned to be completed within three years. DanDrit’s management is confident that upcoming clinical data will be the catalyst to unlock commercial revenues for DanDrit through either acquisition by pharmaceutical partner or licensing deals that would yield upfront and milestone payments as well as royalties.
We are also considering a registration trial to support potential approval of MCV in China. This trial would be conducted under China Food and Drug Administration regulations with a Chinese oncology pharmaceutical partner, such as the TASLY Group or 3S Bio. Contacts with 3S Bio and the TASLY Group have already been initiated. China has recently put in place a drug approval system that includes a low-cost first clinical approval pathway especially for Chinese biotechnology companies. The approval for local biotechnology players is advantageous, since costs for a pivotal clinical trial in China are estimated at one tenth of EU or U.S. costs. Therefore, we plan to collaborate with a Chinese company such as the TASLY Group to develop, manufacture and sell MCV in China. Several factors are also making a partnership with a Chinese pharmaceutical company attractive:
|
●
|
For registration, the clinical trial can only be performed in sites approved by the China Food and Drug Administration. By November 2010, there were 112 oncology sites in Mainland China.
|
|
|
●
|
Screening for MAGE-A could be attractive to the China Food and Drug Administration, but tumor samples could not be shipped outside of China for genomic testing. Therefore a partner who can perform MAGE-A screening in China is valuable.
|
In addition, the China Food and Drug Administration relies more than other agencies on risk benefit assessment. Risk benefit assessment in China remains the “heart” of determining the value of products and is a more favorable assessment approach to MCV as the vaccine is, thus far, well tolerated with what DanDrit believes to be a strong safety profile (due to dendritic cell technology).
Furthermore, due to high unmet medical need, the approval for cancer drugs is also more favorable than in other regions of the world. Because cancer is the first cause of mortality in China, the approval process for oncology drugs benefits from easier rules than those that govern drugs targeting other diseases. The State Food and Drug Administration (the predecessor of China Food and Drug Administration) granted 114 CTA approvals for oncology global/regional trials from 2005 to 2010. Generally, in order to approve a cancer drug in China:
|
●
|
Usually only one pivotal study is required
|
|
|
●
|
With only 100 to 800 patients (most likely 300 patients)
|
|
|
●
|
An open-label study design is accepted (without placebo control)
|
|
|
●
|
The statistical consideration are also attractive as relatively low statistical significance (P value 0.03~0.05) is required
|
|
|
●
|
Overall additional flexibility exists for oncology drugs, driven by the benefit/risk ratio
|
Furthermore, a special review and approval procedure applies to oncology drugs. The review and approval procedure could shorten the review time and can enhance communication with the China Food and Drug Administration. By the end of 2010, 28 drugs obtained approval, and more than half were oncology drugs (ten chemical drugs, and five biologics).
We believe that it is important to take advantage of this development opportunity quickly as the paradigm for oncology drug development is changing rapidly in China:
|
●
|
There is an unprecedented number of anti-cancer therapies in development and the standard of care changes quickly
|
|
|
●
|
The complexity of information concerning tumor genetics and signaling pathways is growing and will bring greater opportunities for personalized medicine
|
Industry
Cancer deaths remain constant, partly because people are living longer. DanDrit’s lead products for NSCLC and CRC address about 40% of all cancer deaths. Other important cancers include Breast (8% of deaths), Prostate (6% of deaths) and Pancreas (6% of deaths). Together these top 5 cancers are responsible for 60% of all cancer deaths.
Cancers Diagnosed (deaths in parentheses) each year
|
Region
|
Population
1000,000s |
All Cancer
1000s |
NSCLC
1000s |
CRC
1000s
|
Breast
1000s
|
Prostate
1000s
|
Pancreas
1000s
|
|
USA
|
300
|
1400 (560)
|
190 (125)
|
150 (50)
|
185 (40)
|
185 (30)
|
37 (30)
|
|
EU
|
500
|
2300 (900)
|
315 (205)
|
250 (85)
|
305 (67)
|
305 (50)
|
60 (50)
|
|
Combined
|
800
|
3700 (1300)
|
505 (330)
|
400 (135)
|
490 (107)
|
490 (80)
|
97 (80)
|
With the 12,667,500 estimated number of new cancer cases in 2008, cancer remains a large market opportunity. Cancer is still the main cause of death in developed countries – accounting for ~33% of death and remains an area of huge unmet medical need. The cancer market has a high growth potential for the coming years with an expected 7% annual growth rate for the years 2011- 2018. The American Cancer Society figured out 1,638,910 new cancer cases in the US for 2012 with 577,190 associated deaths.
In Europe the number of new cancer cases for 2012 was estimated at 3.45 million with a 1.75 million deaths. The cancer market is the fastest growing pharmaceutical market with $83 billion expected growth of the cancer drug market by 2020.
The per-treatment price of chemotherapy for CRC is approximately $30,000. We expect that, if our vaccine is approved for use in CRC patients, the cost per-treatment will be approximately equal to the per-treatment cost of chemotherapy.
Due to its safety profile, MCV should fit easily into the treatment paradigm of most cancers. The initial label of adjuvant therapy for stage IV colorectal cancer with no evidence of disease after surgical resection of metastases could be a door opener for the larger colorectal cancer market. DanDrit’s pharmaceutical partner should be able to grow the label to the larger adjuvant for stage III colorectal cancer market.
Colorectal Cancer
The figure below presents the market opportunity for MCV in advanced colorectal treatment. The global colorectal cancer market peak opportunity for MCV can be valued at US$4.6 billion using quite conservative assumptions.
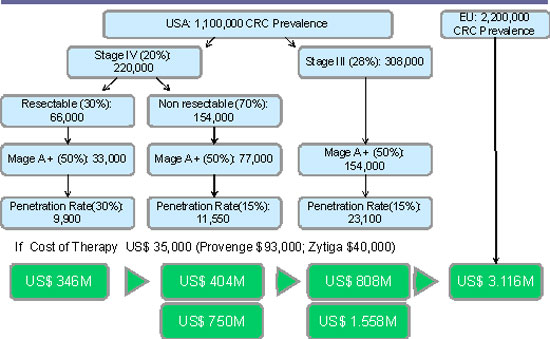
Despite numerous therapeutic advances, colorectal cancer continues to be associated with one of the worst survival rates of all cancers. Metastatic liver disease is found in 10% to 25% of patients having surgery for primary colorectal cancer instead of liver metastasis are detected in 40-50% of patients with diagnosed colon cancer. Then, standard of care “treatment” for colorectal cancer patients after resection surgery and chemotherapy is only observation. When surgical resections of liver metastases are possible, as in 20% of the affected patients, five years survival may approach 35%. According to the most recent papers, the median PFS in patients receiving combined surgery and chemotherapy with No Evidence of Disease is 24-26 months. The same need for a safe effective maintenance therapy exists for stage III colorectal cancer patients with no evidence of disease after surgical resection and chemotherapy.
We believe that it is of great importance for colorectal cancer patients receiving surgery alone or surgery combined with peri- or post-operative chemotherapy, that new and more effective therapies are developed and offered in the post-treatment period. The aim of the proposed trial is to study whether our lead vaccine can increase the progression-free survival for these patients.
Licensing Potential and Cooperation Agreements
The following discussion represents opportunities that we believe can expand the use of our technology.
Alliance with Chinese Company
In addition of the size of their national market, Chinese biotech firms currently benefit from a low cost first clinical development path. The Chinese approval process is favorable for local biotechnology companies. With a Chinese partner, we plan to conduct a Phase III trial in China for lower costs than in the U.S. and at a faster pace. A successful Phase III trial could result in large scale commercialization in China and Southeast Asia.
Furthermore, the domestic market in China for cancer therapies is expected to grow due to a large aging population, expanded insurance coverage, higher government healthcare spending, rising disposable incomes and the high incidence of cancer among the population. In spite of recent price cuts, we believe that the market for cancer therapies in China represents a long-term opportunity based on the factors set forth above. In 2010, oncology agents (17.1%) ranked second in sales, at 17.1%, only after anti-infective agents (23.1%) and before cardiovascular drugs (13.4%). This market segment is expected to continue to grow at a CAGR of over 20% from 2009 to 2014.
Alliance Strategy
In addition to its lead compound MCV, DanDrit has built a pipeline of dendritic cell based cancer therapies, currently addressing 40% of all cancer-related deaths. MCV can be indicated to cancers over-expressing MAGE-A. Cancers over-expressing MAGE-A include among others, lung cancer, colorectal cancer, breast cancer, and esophageal cancers. The 2010 CDC cancer incidence and mortality statistics report listed 575,000 deaths by cancer in the US in 2010. In the same 2010 CDC report, mortality of lung cancer (160,000 deaths), colorectal cancer (52,000 deaths), Breast cancer (41,000 deaths) and melanoma (9,500 deaths) summed up to 262,500 deaths. DanDrit intends to work with strategic partners to strengthen the in-house pipeline.
We control key technologies with relevance outside our core business area and these we may out-license or co-develop with suitable partners.
MyTomorrows
In December 2013, DanDrit entered an agreement with MyTomorrows (“MT”), a Dutch company, regarding a Patient Name Use Program (PNU) for MCV. This program will allow DanDrit to sell MCV for one year of treatment (10 vaccines) to cancer patients through MT. MT offers a worldwide online platform providing access to non-registered medicines for patients with life threatening diseases.
MT is a turnkey solution and will be in charge of regulatory, recruitment, logistics, and pharmacovigilance. DanDrit’s potential liabilities are limited to quality control of cGMP manufacturing of MCV. DanDrit expects several benefits from this agreement. First, in 2014, DanDrit anticipates short term revenue generation as MT will transfer payment as soon as a patient orders MCV. DanDrit also anticipates that this program may contribute to lowering the cost of manufacturing of the clinical lot through economy of scale. This program may also generate real life data for MCV.
Manufacturing
In 2011 and 2012, DanDrit has out-sourced the GMP manufacturing of its lysate. We believe that proving that our technology transfer was possible was a key step in finding and working with a future pharmaceuticals partner. DanDrit evaluated several possible EU-based contract manufacturing organizations (CMOs) and selected PX Therapeutics SA, a CMO based in Grenoble, France. The collaboration with PX Therapeutics SA in France demonstrated that GMP production of lysate could not only be transferred but that the production could be scaled up. We consider that the potential economy of scale that can be expected in the cost of lysate production could become a competitive advantage versus other cancer vaccine companies using recombinant production of cancer-specific antigens (i.e. Mage-A3 from GSK). Also, the collaboration with the French CMO is based on a pure fee-for-services basis and can be discontinued at any time without notice.
In addition, DanDrit will spend a small part of the net proceeds on improving the manufacturing of the MCV vaccine. DanDrit intends to establish a closed fully automatized manufacturing process. We learned from the Dendreon’s experience that an efficient manufacturing process should be in place before approval for commercialization. Cost saving should be expected from a fully automatized vaccine production. We also assume that a fully efficient manufacturing process may increase the value of a deal with a pharmaceutical partner.
Cell Banking
The melanoma cell lines used by DanDrit in the production of our lysate (MCL) are stored at ultra-low temperature in liquid nitrogen at Symbion Science Park, Copenhagen, Denmark. Both master- and working cell banks are stored this way and the contents of the cell banks (both master and working) are recorded in log books. Nitrogen levels are maintained by the staff of DanDrit Biotech at least once a week and any activity in regards to storage (shipment of cells, nitrogen levels etc.) are documented in the appropriate log book.
Furthermore, for security reasons, samples of the master cell banks are also stored at specialized cell storage facilities in England. In addition, samples of one working cell bank from the DDM1.7 cell line are stored at PX Therapeutics in France for production purposes.
Sales, Marketing and Distribution
The business model of DanDrit is to focus on early development of dendritic cell based vaccine. We have significantly reduced the fixed costs linked with our operation and do not intend to build an expensive marketing, sales and distribution organization. We will rely on pharmaceutical partners with demonstrated relevant experience in commercialization of cancer products to market, sell and distribute MCV. Therefore, we have already identified and establish a communication line with several potential future pharmaceutical partners. At completion of the comparative clinical trial, we plan to enter into a collaboration agreement with a pharmaceutical partner regarding the regulatory approval, marketing, sales and distribution of MCV.
Intellectual Property
As a company primarily focused on pharmaceutical research, we expect that our most valuable assets are our intellectual property. This includes U.S. and foreign patents, patent applications, common-law trademarks, trade secrets and know-how. We are pursuing an aggressive intellectual property strategy.
DanDrit intends to aggressively defend its patents through legal process if necessary. Where appropriate, DanDrit may in-license intellectual property that may add to the strength and defense of our core business. DanDrit’s intellectual property comprises patents, trademarks, copyright and secret know-how.
DanDrit’s core business is cancer therapy. Where DanDrit’s patents and secret know-how are applicable to non-core business areas we will consider out-licensing for relevant non-core applications.
DanDrit filed its first Patent Cooperation Treaty (PCT) patent application on November 29, 2002 with priority claimed from 2001 with the Danish application, shortly after our formation.
DanDrit may continue to patent its innovations, such as novel dendritic cell production systems or dendritic cell quality control. To support potential income streams DanDrit may patent non-core applications of its dendritic cell technologies so as to secure future revenue streams from out-licensing activity.
Patents
|
●
|
Pharmaceutical composition for inducing an immune response in a human or animal (2001 Denmark (DK), 2002 PCT)
|
|
|
This patent was first filed in November 2002. The patent covers and describes the usage of an allogenic melanoma cell lysate (MCL)-pulsed autologous DC vaccine expressing at least one of six MAGE-A antigens overexpressed by the cell line being the source of the lysate. The patent covers the antigen composition used in the generation of MelCancerVac and the claims for producing MelCancerVac. In this patent the antigens are specified to mainly belong to the cancer testis family. The family of antigens is expressed in a wide variety of cancer forms. In the International Preliminary Report on Patentability (IPRP) all claims were determined to be novel and inventive. The patent expiry date is November 29, 2022. This patent has been granted in: Europe, the USA, China, Australia, Singapore, Japan, Russia, Hong Kong. This patent is pending in: Israel and Norway. This patent is owned by the Company and was not licensed from third parties. The patent protection means that the cancer specific antigen-rich lysate obtained from our cell line cannot be commercially made, used, distributed or sold without DanDrit's consent. These patent rights can be usually enforced in a court, which, in most systems, holds the authority to stop patent infringement.
|
||
|
●
|
Protocol for generating dendritic cells (2005 DK, 2008 PCT)
|
|
|
This patent covers the generation of dendritic cells based on a blood sample of 200 ml. The patent differs from other DC generating patents by the utilization of reduced temperature and a single blood sample. DCs exposed to tumor antigens followed by treatment with T(h)1-polarizing differentiation signals have paved the way for the development of DC-based cancer vaccines. Critical parameters for generation of optimal functional clinical grade DCs are a very competitive area. DanDrit has developed a method that covers the generation of immature dendritic cells under reduced temperature settings which by further activation has been shown to give a high yield of homogeneous and fully matured DCs. This patent was filed on December 7, 2006. In the International Preliminary Report on Patentability (IPRP) a large majority of claims were found to be novel and inventive. The patent expiry date is 2032. This patent was granted in 2012 in China, Eurasia, Russia, Europe, Israel, Mexico, Malaysia, New Zealand. This patent is owned by DanDrit and was not licensed from third parties. The patent protection means that the method that DanDrit use to generate dendritic cells cannot be commercially used, distributed or sold without DanDrit's consent. These patent rights can be usually enforced in a court, which, in most systems, holds the authority to stop patent infringement.
|
||
|
●
|
Method for generating tolerogenic dendritic cells employing decreased temperature (2007)
|
|
|
DanDrit has expanded the method of development of mature dendritic cells to also include the generation of regulatory DCs. In addition to DCs used for cancer immunotherapy, DanDrit has developed an additional arm of DCs, namely regulatory/tolerogenic DCs to be used for treatment of various autoimmune diseases such as Type 1 diabetes and Multiple Sclerosis. This patent was filed on November 13, 2008. Patent pending: worldwide. 1st Office Action received in Europe August 25, 2010. This patent is owned by the Company and was not licensed from third parties. The patent protection means that the method that DanDrit use to produce tolerogenic dendritic cells cannot be commercially used, distributed or sold without DanDrit's consent. These patent rights can be usually enforced in a court, which, in most systems, holds the authority to stop patent infringement.
|
||
|
●
|
Micro RNAs as markers of the functional state of a dendritic cell
|
|
|
This patent covers and demonstrates that functionally different DCs carry unique microRNA signatures. By examining a handful of microRNA profiles one can analyze the function of DC vaccines. This is a valuable addition to other vaccine quality control measures that are currently used in studies that involve DCs. Critical parameters for assessment of the optimal functional state of DCs and prediction of the vaccine potency of activated DCs have in the past been based on measurements of differentiation surface markers like HLA-DR, CD80, CD83, CD86, and CCR7 and the level of secreted cytokines like interleukin-12p70. However, the level of these markers does not provide a complete picture of the DC phenotype and may be insufficient for prediction of clinical outcome for DC-based therapy. We have identified additional biomarkers by investigating the differential expression of microRNAs (miRNAs) in mature DCs relative to immature DCs. The patent was filed on November 14, 2008. In the International Preliminary Report on Patentability, a large majority of claims were found to be novel and inventive. Patent pending: Europe and USA. 1st Office Action received in Europe on August 18, 2010. Follow up action on election restriction received in the USA on October 21, 2010. This patent is owned by the Company and was not licensed from third parties. The patent protection means that the method that DanDrit use to test and release its dendritic cells cannot be commercially used, distributed or sold without DanDrit's consent. These patent rights can be usually enforced in a court, which, in most systems, holds the authority to stop patent infringement.
|
All of the above patents are protected by relevant international extensions.
Trademarks
A policy of product trademarking and branding has been adopted by DanDrit. Trademarks have been obtained for
MelCancerVac™
MelVaxin™
DanDrit™
Commercial Secrets
In addition to intellectual property protected by patents and copyright, DanDrit has commercial secrets relating to its products, production processes, know-how and future strategies. Where it is expedient to share such secret information this shall be done under the legal protection of a confidentiality (or secrecy) agreement. Such agreements shall bind the signing parties, and especially the recipient of DanDrit’s secret information, unless:
|
●
|
at the time of disclosure was already known to the recipient as evidenced by written record pre-dating such disclosure;
|
|
|
●
|
at the time of disclosure is generally available to the public or subsequently becomes available to the public other than by an act of omission on the part of the recipient; or
|
|
|
●
|
shall be made available to the recipient (on a non-confidential basis) by a third party having the lawful right to do so.
|
Government Regulation
Orphan Drug status for MCV
The United States and Europe may designate drugs for relatively small patient populations as orphan drugs. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process, but does make the product eligible for orphan drug exclusivity, reduced filing fees and specific tax credits. Generally, if a company receives the first marketing approval for a product with an orphan drug designation in the clinical indication for which it has such designation, the product is entitled to orphan drug exclusivity. Orphan drug exclusivity means that the FDA will not approve another application to market the same drug for the same indication, except in limited circumstances, for a period of seven years in the United States. This exclusivity, however, could block the approval of our proposed product candidates if a competitor obtains marketing approval before us. We plan to apply for orphan drug status for MCV to treat stage IV CRC with NED after surgical resection and chemotherapy if we meet the eligibility criteria. However, note that, even if we obtain orphan drug exclusivity MCV, we may not be able to maintain the status. For example, if a competitive product is shown to be clinically superior to our product, any orphan drug exclusivity we have will not block the approval of such competitive product.
Fast Track designation for development of MCV
We intend to request fast Track designation for MCV. If a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the drug sponsor may apply for FDA Fast Track designation for a particular indication. Marketing applications filed by sponsors of products in Fast Track development may qualify for priority review under the policies and procedures offered by the FDA, but the Fast Track designation does not assure any such qualification or ultimate marketing approval by the FDA. Receipt of Fast Track designation may not result in a faster development process, review or approval compared to drugs considered for approval under conventional FDA procedures. In addition, the FDA may withdraw any Fast Track designation at any time. We may seek Fast Track designation for our vaccine product candidates or any other product candidates, but the FDA may not grant this status to any of our proposed product candidates.
Approval for Commercialization
MCV and any future product candidates that we will be developing will require approval of the FDA before they can be marketed in the U.S. Although our focus at this time is primarily on the U.S. market, in the future similar approvals will need to be obtained from foreign regulatory agencies before we can market our current and proposed product candidates in other countries.
The process for filing and obtaining FDA approval to market therapeutic products is both time-consuming and costly, with no certainty of a successful outcome. The historical failure rate for companies seeking to obtain FDA approval of therapeutic products is high and, with the exception of Dendreon Corp.’s dendritic cell vaccine for the treatment of prostate cancer, no cancer stem cell or dendritic cell-based cancer vaccine has to date been approved by the FDA. This process includes conducting extensive pre-clinical research and clinical testing, which may take longer and cost more than we initially anticipate due to numerous factors, including without limitation, difficulty in securing appropriate centers to conduct trials, difficulty in enrolling patients in conformity with required protocols in a timely manner, unexpected adverse reactions by patients in the trials to our proposed product candidates and changes in the FDA’s requirements for our testing during the course of that testing.
The time required to obtain FDA and other approvals is unpredictable but often can exceed five years following the commencement of clinical trials, depending upon the complexity of the product and other factors. Any analysis we perform of data from preclinical and clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We may also encounter unexpected delays or increased costs due to a variety of reasons, including new government regulations from future legislation or administrative actions or from changes in FDA policy during the period of product development, clinical trials and FDA regulatory review.
Any delay or failure in our clinical trial program and in obtaining required approvals would have a material adverse effect on our ability to generate revenues from the particular product. Furthermore, any regulatory approval to market a product may be subject to limitations on the indicated uses for which we may market the product. These limitations may limit the size of the market for the product.
Environmental matters
We are subject to a broad range of federal, state, local and foreign environmental laws and regulations which govern, among other things, air emissions, wastewater discharges and the handling, storage disposal and release of wastes and hazardous substances. It is our policy to comply with applicable environmental requirements at all of our facilities. We are also subject to laws, such as the Comprehensive Environmental Response, Compensation and Liability Act (“CERCLA”), that may impose liability retroactively and without fault for releases or threatened releases of hazardous substances at on-site or off-site locations. We are subject to similar requirements in Denmark and other European countries.
Research and Development
We currently have one full-time employee working on maintaining our research and development. For the years ended December 31, 2013 and 2012 and the three months ended March 31, 2014 and 2013 we did not spend any money for research and development.
Competition
Several companies are trying to capitalize on the growing interest for immunotherapy in the treatment of cancer.
Two Directly Competing Companies
The figure below outlines the competitive landscape for MCV. Note that colorectal cancer, while providing a large market opportunity (it is the second most killer cancer after lung cancer), offers a more robust competitive landscape than other cancers. In the colorectal cancer space DanDrit faces two main competitors:
|
●
|
Bavarian Nordic (“BN”): CV-301, BN’s second compound, is in clinical development with advanced colorectal cancer patients
|
|
|
●
|
Immatics: its second compound is in clinical development in early stage colorectal cancer patients
|

Bavarian Nordic (BAVA.CO) and CV-301

With its lead vaccine Prostvac™ from Therion Biologics and NCI, Bavarian Nordic (BN) also acquired Panvac™ vaccine. This PANVAC™ vaccine failed to prove efficacy in patients with advanced pancreatic cancer who failed gemcitabine. BN is currently focusing its efforts on Prostvac™ for treatment of prostate cancer, currently in Phase III. However, in 2012, BN re-initiated the clinical development of Panvac™ (re-named CV-301).
CV-301 is a cancer immunotherapy product candidate incorporating two antigens, CEA and MUC-1, in a viral vector. CV-301 is an off-the-shelf immunotherapy product candidate for the treatment of multiple cancers. It originates from the same poxvirus technology platform as PROSTVAC™. Both PROSTVAC™ and CV-301 are prime-boost vaccines sequentially combining two different poxviruses (vaccinia and fowlpox).
CV-301 had been studied in different cancers in clinical trials led by the National Cancer Institute. One study was a randomized Phase II trial in patients with metastatic breast cancer. The study enrolled 48 patients to receive CV-301 in combination with docetaxel or docetaxel alone. The primary study endpoint was PFS, while secondary endpoints included overall survival and immunologic correlative studies. A preliminary analysis of the study showed PFS of 6.6 months in the CV-301 group versus 3.8 months among those receiving docetaxel alone. Final study data are pending results from five patients that remained on study at the time of the analysis. Because of its size the study was not designed to reach statistical significance.
More directly relevant to DanDrit was the colorectal Phase II study of CV-301 conducted by Morse at Duke University. The objective of the trial was to determine whether one of two vaccines based on dendritic cells and poxvectors encoding CEA and MUC1 would lengthen survival in patients with resected metastases of colorectal cancer. The studied patients were, disease-free after CRC metastasectomy and perioperative chemotherapy (n = 74). They were randomized to injections of autologous DCs modified with PANVAC (DC/PANVAC) or PANVAC with per injection GM-CSF (granulocyte-macrophage colony-stimulating factor). Endpoints were recurrence-free survival overall survival, and rate of CEA-specific immune responses. Clinical outcome was compared with that of an unvaccinated, contemporary group of patients who had undergone CRC metastasectomy, received similar perioperative therapy, and would have otherwise been eligible for the study.
The recurrence-free survival at two years was similar (47% and 55% for DC/PANVAC and PANVAC/GM-CSF, respectively). At a median follow-up of 35.7 months, there were two of 37 deaths in the DC/PANVAC arm and five of 37 deaths in the PANVAC/GM-CSF arm. The rate and magnitude of T-cell responses against CEA was statistically similar between study arms.
As a group, vaccinated patients had superior survival compared with the contemporary unvaccinated group. Both DC and pox-vector vaccines had similar activity. Survival was longer for vaccinated patients than for a contemporary unvaccinated group.
In 2013, Bavarian Nordic expanded its license with the National Cancer Institute (NCI) for CV-301 to include colon cancer. The original collaboration agreement executed in 2011, involved multiple cancers including breast, lung, ovarian and other cancers.
Immatics Biotechnologies

The second direct competitor is Immatics (previously known as Biomira), a German biotech company who currently focuses its clinical efforts on a Phase III in Renal Cell Carinoma for its lead vaccine. However, Immatic also develops a vaccine in colorectal cancer (enter Phase I in 2012). Note that Immatic’s technology is peptide-based rather than a dendritic cell approach and that Immatics is targeting its vaccine toward early stage colorectal cancer rather than resected advanced colorectal cancer like Bavarian and DanDrit. This private German company only discovers and develops tumor-associated peptides for the immunotherapy of cancer. Immatics reports that they raised €53.8million in a Series C financing round to finance a Phase III pivotal trial of their lead product IMA901 which in data reported in June at ASCO demonstrated the potential to confer an overall survival benefit in patients with advanced renal cell carcinoma.
Other cancer vaccine companies
The global cancer vaccines market was worth $3,483.0m in 2010, after increasing at a compound annual growth rate (CAGR) of 63.7% during 2006--2010. During 2010--2018, the market is expected to record a CAGR of 12.7%, to reach $9,077.9m by 2018.
The following companies (by alphabetical order) are part of the competitive landscape but not direct competitors. They are presented in this plan to illustrate the growing interest in cancer vaccines.
|
●
|
Agenus (www.agenus.com)
|
|
|
●
|
Argos Therapeutics (www.argostherapeutics.com)
|
|
|
●
|
BioVest (www.biovest.com)
|
|
|
●
|
Celldex (www.celldextherapeutics.com)
|
|
|
●
|
ImmunoCellular Therapeutics Ltd (www.imuc.com)
|
|
|
●
|
North West Biotherapeutics (www.nwbio.com)
|
|
|
●
|
Prima Biomed (www.primabiomed.com.au)
|
|
|
●
|
TVax Biomedical (www.tvax.com)
|
Employees
As of August 8, 2014, we had three employees, two of which are full time employees.
Properties
Our corporate headquarters are located in Symbion Science Park, Fruebjergvej 3, 2100 Copenhagen, Denmark and our U.S. mailing address is P.O. Box 189, Randolph, VT 05060. We lease approximately 1,108 square feet at our Symbion location which is used for work and storage of cells and biological material in freezers. The lease is for a term of three years until March 2016. We also currently occupy approximately 1,620 square feet at Bredgade 75, 3rd Floor, 1263 Copenhagen K, Denmark, which is used for office space. The Company’s lease can be terminated by either the Company or the landlord with a three months’ notice of termination.
Legal Proceedings
From time to time, we may be involved in litigation relating to claims arising out of our operations in the normal course of business. We are not currently a party to in any legal proceeding that we believe would have a material adverse effect on our business, financial condition or operating results.
Set forth below is information concerning our directors, senior executive officers and other key employees.
On the effective date of the Share Exchange the following individuals were named to the Board and executive management of the Company.
|
Name
|
|
Age
|
|
Titles
|
|
Dr. Eric Leire
|
|
56
|
|
Chief Executive Officer, President and Director (Principal Executive Officer)
|
|
Robert E. Wolfe
|
|
51
|
|
Chief Financial Officer, Treasurer, Secretary and Director (Principal Financial and Accounting Officer)
|
|
NE Nielsen
|
|
65
|
|
Chairman of the Board
|
|
Dr. Jacob Rosenberg
|
|
49
|
|
Director
|
|
Aldo Michael Noes Petersen
|
|
52
|
|
Director
|
Executive officers are appointed by and serve at the pleasure of the Board of Directors. A brief biography of each director and executive officer follows:
Dr. Eric Patterson Leire, MD, MBA. Dr. Eric Leire has served as the Chief Executive Officer, President and a director of DanDrit since April 2011. Dr. Leire also serves as Chief Executive Officer of DKTI A/S, a listed Danish investment company since September 2012. Prior to these roles, Dr. Leire was a partner at BioFund Venture Capital, a Finnish biotech venture fund, from August 2006 through September 2010 and a partner at Medwell Capital Corp., a Canadian venture fund, from April 2010 through May 2011. Dr. Leire has worked globally for many international pharmaceutical organizations, including Schering-Plough, Pfizer, Inc., Boots Pharmaceuticals Company PLC, Harvard AIDS Institute and bioStrategies Group. Dr. Leire also served as the CEO of US biotech companies APT Therapeutics and Paringenix and currently serves on the board of directors of Novicol Canada, DKTI A/S and DanDrit Corp. Ltd. Dr. Leire received his medical degree from the University of Medicine of Grenoble in 1980 and his MBA from ISA-HEC and the Kellogg School of Management at Northwestern University in 1991. The Board believes that Dr. Leire’s significant global experience in the pharmaceutical and biotechnology industries will be a significant asset to DanDrit as it carries out its business plan and for those reasons determined that he should serve on the Board of Directors.
Robert E. Wolfe. Mr. Wolfe has served as the Chief Financial Officer, Treasurer and Secretary since January 1, 2014. Mr. Wolfe also serves as Chairman and CEO of IProcess Manager Inc. from April 2010, and as a director of Iso-Ware A/S from February 2006. In addition, Mr. Wolfe has served as Chairman, CEO and CFO of Advanced Oxygen Technologies, Inc., a publicly traded company, since December 1997, which owns Anton Nielsen Vojens ApS, a Danish commercial real estate company. Mr. Wolfe has served as President, CEO and director of Crossfield, Inc., Crossfield Investments, LLC, Drumbeg Ltd, Baldwin Construction Inc. and Ludlow Leasing, Inc. from May 1989 to the present. The Board believes that Mr. Wolfe’s experience with U.S. public companies and Danish entities qualifies Mr. Wolfe to serve as a director of the Company.
NE Nielsen. Mr. Nielsen is a lawyer and partner at Lett Law Firm in Denmark. His practice areas are capital market conditions, securities law, boards of directors, managerial, finance and acquisitions. He currently serves as the chairman and a board member of numerous companies, including as Chairman of DanDrit Biotech A/S since June 2013, director of the board of Pele Holding A/S since May 1982, director of the board of Charles Christensen A/S since 1983 and as Chairman since May 2010, Chairman of Charles Gulve Engros A/S since June 2002, Chairman of InterMail A/S since January 1995, Chairman of Gammelrand Holding A/S since December 2009, Chairman of Gammelrand Skærvefabrik A/S since May 1995, director of the board of Ejendomsaktieselskabet Matr. 43 Ei Avedøre since August 2000 and as Chairman since February 2009, Chairman of Gammelrand Beton A/S since April 2001, director of the board of P.O.A. Ejendomme A/S since July 1994 and Chairman since April 2007, and Chairman of Konveloutfabrikken Danmakrs Fond and Brøndbyerns I.F. Fodbold A/S since June 2013. Within the last five years Mr. Nielsen has served as a board member or chairman in the following companies: Amagerbanken Aktieselskab under konkurs from December 1999 to November 2010, Ambu A/S from February 1999 to December 2012, Carepoint Haslev/Ringsted under konkurs from June 2009 to August 2009, Cimber Sterling A/S under konkurs from September 2000 to September 2010, Cimber Sterling Group A/S under konkurs from September 2005 to September 2010, Danica-Elektronik ApS from March 1993 to September 2012, GPV Industri A/S under konkurs from December 1986 to June 2011, GPV International A/S from April 2009 to June 2009, Henrik Olsen Automobiler A/S from April 2010 to May 2010, Kirk & Thorsen Invest A/S from February 2013 to April 2013, Olsen Biler Administraton A/S from April 2007 to May 2010, Olsen Biler Ringsted-Haslev A/S from April 2007 to May 2010, Satair A/S from November 1994 to October 2011, Satair Service A/S from May 1995 to May 2011, Torm A/S from September 2000 to January 2013 and Weibel Scientific A/S from January 1986 to September 2012. Mr. Nielsen’s significant global experience as a member of the board of directors or chairman of various entities lead the Board to believe that Mr. Nielsen is qualified to serve as a director of the Company.
Dr. Jacob Rosenberg. Dr. Jacob Rosenberg currently serves as a director of DanDrit Denmark, a position he has held since 2012. Prior to this role, Dr. Rosenberg served as Chairman of DanDrit Denmark’s board from 2003 to 2009 and Chairman of T-cellic A/S from 2007 to 2008. Dr. Rosenberg is also currently Chairman of the board of DKTI A/S. Dr. Rosenberg was appointed as a Professor of Surgery at the University of Copenhagen in 2003, where he also received his M.D. in 1991. He also has a D.Sc. from the University of Copenhagen. During the years 1997-2003 he received 6 honorary research prizes. Professor Rosenberg has overseen DanDrit’s Copenhagen based clinical trials. The Board believes that Dr. Rosenberg is one of the leading experts in research in cancer and dendritic cells and, as a result, that he has a thorough understanding of our company and our technology. Because of his research background, the Board believes that Dr. Rosenberg is uniquely qualified to serve as a director.
Aldo Petersen. Aldo Petersen has been chairman of LiqTech International, Inc. since August 2011. He has been the Chief Executive Officer of APE Invest A/S, a private Danish investment company, since 2006 when he sold Telepartner A/S, a formerly NASDAQ-listed company that he founded in 1986. Prior to Telepartner, he started and sold one of Denmark’s first hedge funds, Dansk Fromue Invest. Mr. Petersen was a major investor in Greentech Energy Systems A/S, a renewable energy company that builds wind farms in Denmark, Germany, Poland and Italy. He is a private investor in wind farms in Germany and France, and was also a major investor in Football Club Copenhagen (listed on the Copenhagen Stock Exchange). Mr. Petersen has a B.A. degree in Economics from Copenhagen Business School. The Board believes that Mr. Petersen’s experience as a businessman and his knowledge of the capital markets qualifies him to be a director.
Our certificate of incorporation provides for the annual election of directors. At each annual meeting of stockholders, our directors will be elected to serve until their respective successors have been elected and qualified.
Family Relationships
None of the directors or executive officers has a family relationship as defined in Item 401 of Regulation S-K.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has, during the past 10 years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K.
Director Independence
Our Board of Directors has determined that Messrs. Nielsen, Rosenberg and Petersen are independent as that term is defined in the listing standards of the NYSE MKT. In making these determinations, our Board of Directors has concluded that none of our independent directors has an employment, business, family or other relationship which, in the opinion of our Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Our other directors, Dr. Leire and Mr. Wolfe, are not considered independent under these rules because each serves as an executive officer.
Insider Participation Concerning Executive Compensation
DanDrit’s Board of Directors has historically made all determinations regarding executive officer compensation, including compensation decisions during the years ended December 31, 2012 and December 31, 2013.
Nominating Committee
We have not adopted any procedures by which security holders may recommend nominees to our Board of Directors.
The Board of Directors acts as the audit committee. The Company does not have a qualified financial expert at this time because it has inadequate financial resources at this time to hire such a qualified candidate. The Company intends to continue to search for a qualified individual for hire.
Code of Ethics and Business Conduct
On July 12, 2012, the Company adopted a formal code of ethics statement for senior officers and directors (the “Code of Ethics”) that is designed to deter wrongdoing and to promote ethical conduct and full, fair, accurate, timely and understandable reports that the Company files or submits to the SEC and others. A form of the Code of Ethics was filed as Exhibit 14.1 to the Company’s Annual Report on Form 10-K filed with the SEC on July 17, 2012 and is incorporated herein by reference. Requests for copies of the Code of Ethics should be sent in writing to DanDrit Biotech USA, Inc., PO Box 189, Randolph, VT 05060.
Summary Compensation Table
The following table sets forth certain information with respect to compensation for the years ended December 31, 2013 and 2012 earned by or paid to our chief executive officer and our one other executive officer in 2013 whose total compensation exceeded $100,000 (the “named executive officers”).
Summary Compensation Table
|
Name and
Principal |
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($) |
Nonequity
Incentive Plan
Compensation
|
Nonqualified Deferred Compensation
Earnings
|
Other |
Total
($)(1) |
||||||||||||||||||
|
Dr. Eric Leire,
|
2013
|
308,471
|
-
|
-
|
-
|
-
|
-
|
-
|
308,471
|
||||||||||||||||||
|
CEO and Director
|
2012
|
171,938
|
-
|
-
|
-
|
-
|
-
|
-
|
171,938
|
||||||||||||||||||
|
Dina Rosenberg Asmussen,
|
2013
|
165,746
|
165,746
|
||||||||||||||||||||||||
|
Former CFO
|
2012
|
162,357
|
-
|
-
|
-
|
-
|
-
|
-
|
162,357
|
||||||||||||||||||
(1) All values are reported on an as-converted basis from Danish Krone (DKK) to U.S. dollars ($) based on the currency exchange rate of $1.00 = DKK 5.43, as of December 31, 2013. We do not make any representation that the Danish Krone amounts could have been, or could be, converted into U.S. dollars at such rate on December 31, 2013, or at any other rate.
Employment Arrangements
Agreements with Named Executive Officers
We have employment agreements with Dr. Leire and Mr. Wolfe and a consultancy agreement with Mrs. Asmussen.
Leire Employment Agreement
Effective March 1, 2012, DanDrit Denmark entered into an Employment Agreement with Dr. Eric Leire (the “Leire Employment Agreement”), to serve as its Managing Director. Pursuant to the terms and conditions of the Employment Agreement, Dr. Leire will be employed by DanDrit Denmark for an indefinite term unless earlier terminated pursuant to the terms therein. The Leire Employment Agreement provides that the Dr. Leire will receive a salary of 2,100,000 DKK ($381,125) gross per year, to be paid monthly on the last day of each month and subject to annual review and increases by our Board, as it deems appropriate.
In addition to his salary, Dr. Leire will be entitled to receive: (i) a company car at a value up of DKK 5,100 ($926) per month (monthly lease value) and DanDrit Denmark shall defray all expenses in connection with the running of the car; (ii) coverage of all expenses relating to Dr. Leire’s mobile phone, home computer, Internet connection as well as his home phone; (iii) coverage of all the expenses relating to Dr. Leire’s subscription to a fitness club; (iv) a bonus of up to DKK 400,000 ($72,595) per year if Dr. Leire reaches certain conditions as specified in the Employment Agreement; and (v) shall be covered by DanDrit Denmark’s pension scheme.
DanDrit Denmark may terminate the employment with 12 months’ notice to the end of a month. If DanDrit Denmark terminates Dr. Leire’s employment, he shall be entitled to be released from his duty to work (in Danish “fritstillet”) during the notice period. Dr. Leire may terminate the employment at six months’ notice to the end of a month. In case of material breach, the non-defaulting party can terminate the Leire Employment Agreement without notice and can claim damages in accordance with the general Danish law of damages. If Dr. Leire suspends payments, or insolvency proceedings are commenced against his estate, DanDrit Denmark can terminate the employment without notice. The employment shall cease without notice to the end of the month in which Dr. Leire attains the age of 70. The Leire Employment Agreement contains non-competition and non-solicitation clauses.
Wolfe CFO Service Agreement
Effective January 1, 2014, DanDrit Denmark entered into a CFO Service Agreement with Robert Wolfe to serve as its Chief Financial Officer and Chief Financial Officer of DanDrit USA following the closing of the Share Exchange. Pursuant to the terms and conditions of the CFO Service Agreement, Mr. Wolfe will be employed by DanDrit Denmark for an indefinite term unless earlier terminated pursuant to the terms therein. The CFO Service Agreement provides that Mr. Wolfe will receive a salary of $78,000 gross per year, to be paid monthly on the last day of each month and subject to annual review and increases by our Board, as it deems appropriate and a one-time sign on fee of $6,000.
In addition to his salary, Mr. Wolfe will be entitled to receive reimbursement of all reasonable costs relating to his work for the Company, including travel, accommodation and meal expenses in connection with work outside the agreed premises are to be paid by the Company according to vouchers submitted.
DanDrit Denmark may terminate the employment with three months’ notice to the end of a month during the first six months of the term of the CFO Service Agreement. Thereafter six months’ notice is required for a termination. Mr. Wolfe may terminate the employment at three months’ notice to the end of a month. In case of material breach, the non-defaulting party can terminate the CFO Service Agreement without notice and can claim damages in accordance with the general Danish law of damages. If Mr. Wolfe suspends payments, or insolvency proceedings are commenced against his estate, DanDrit Denmark can terminate the employment without notice. The employment shall cease without notice to the end of the month in which Mr. Wolfe attains the age of 70. The CFO Service Agreement contains a non-competition clause.
The foregoing description of the terms and conditions of the employment agreements provides only a brief summary and is qualified in its entirety by reference to the full text of the employment agreements filed as exhibits to this registration statement on Form S-1.
Outstanding Equity Awards
As of December 31, 2013, there were no outstanding equity awards to our named executive officers.
Compensation of Directors
For the fiscal year ended December 31, 2013, we did not compensate our directors for their services other than to reimburse them for out-of-pocket expenses incurred in connection with their attendance at meetings of the Board of Directors. In order to attract and retain qualified independent directors, we may in the future adopt a compensation plan for non-employee directors that includes cash as well as equity-based compensation.
The following table sets forth, as of July 1, 2014, certain information regarding the beneficial ownership of the shares in DanDrit USA, of (i) our executive officers, (ii) our directors, (iii) our executive officers and directors as a group, (iv) each person known to us who is known to be the beneficial owner of more than 5% of the shares in DanDrit USA and (v) the persons who own more than 5% of the shares of DanDrit USA as a group. In accordance with the rules of the SEC, “beneficial ownership” includes voting or investment power with respect to securities. To our knowledge, except as indicated in the footnotes to this table and pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock beneficially owned by them. Unless indicated otherwise, the address for the beneficial holders is c/o DanDrit Biotech USA, Inc., P.O. Box 189, Randolph, VT 05060.
|
Shares in
|
% of shares in
|
|||||||
|
DanDrit
|
DanDrit
|
|||||||
|
Biotech
|
Biotech
|
|||||||
|
Name of Beneficial Owner
|
USA, Inc. (1)
|
USA, Inc. (1)
|
||||||
|
Directors/Officers:
|
||||||||
|
Eric Jean Marie Leire(2)
|
8,615 | 0.11 | % | |||||
|
Robert E. Wolfe
|
- | - | ||||||
|
NE Nielsen(3)
|
- | - | ||||||
|
Dr. Jacob Rosenberg(4)
|
31,476 | 0.39 | % | |||||
|
Aldo Petersen
|
- | - | ||||||
|
Directors/Officers Total:
|
40,091 | 0.50 | % | |||||
|
5% Shareholders:
|
||||||||
|
Sune Olsen Holdings ApS(5)
|
777,588 | 9.67 | % | |||||
|
Media-Invest Danmark A/S(6)
|
793,923 | 9.87 | % | |||||
|
Bele Invest ApS(7)
|
486,677 | 6.05 | % | |||||
|
Jonas Petterson (7)
|
486,677 | 6.05 | % | |||||
|
Sune Olsen (5)
|
1,157,500 | 14.40 | % | |||||
|
Thomas Ulletved Rasmussen (6)
|
793,923 | 9.87 | % | |||||
|
DKTI A/S (8)
|
555,869 | 6.91 | % | |||||
|
NLBDIT 2010 Services, LLC (9)
|
600,000 | 7.46 | % | |||||
|
5% Shareholders Total:
|
5,652,157 | 70.30 | % | |||||
|
Total:
|
5,732,339 | 71.30 | % | |||||
|
(1)
|
Based on 8,040,000 shares issued as of March 31, 2014, following the Share Exchange including 185,053 shares of common stock reserved for issuance to the non-consenting shareholders of DanDrit Denmark and deemed issued and outstanding for accounting purposes.
|
|
(2)
|
The holder has an address of Hambros Alle 12, 2900 Hellerup, Denmark.
|
|
(3)
|
The holder has an address of Lett Law Firm, Raadhuspladsen 4, DK-1550 Copenhagen, Denmark.
|
|
(4)
|
Shares are owned by Jaro Holding ApS, a Danish entity with an address of C.F. Richs Vej 44, 2000 Frederiksberg Denmark. The voting and disposition of the shares owned by the company are controlled by Dr. Rosenberg.
|
|
(5)
|
Shares are owned by Sune Olsen Holding ApS, Biotech Invest ApS and Sardinian Solar Park ApS, all Danish entities with an address of Jagtvej 169 B 4, 2100 Copenhagen, Denmark. The voting and disposition of the shares owned by the companies are controlled by Mr. Olsen.
|
|
(6)
|
Shares are owned by Media-Invest Danmark ApS (“Media-Invest”), a Danish entity with an address of Ostergade 61 4, 1100 Copenhagen, Denmark. The voting and disposition of the shares owned by Media-Invest are controlled by Mr. Rasmussen.
|
|
(7)
|
Shares are owned by Bele Invest ApS (“Bele Invest”), a Danish entity with an address of Vermehrensvej 7, 2930 Klampenborg Denmark. The voting and disposition of the shares owned by Bele Invest are controlled by Mr. Petterson.
|
|
(8)
|
DKTI A/S is a Danish public limited liability company with an address of Frederiksgade 21 1, 1265 Copenhagen, Denmark. DKTI was, until September 19, 2013, listed at the stock exchange OMX Nasdaq Copenhagen. DKTI has 189 shareholders. Dr. Eric Leire as CEO of DKTI A/S has voting and dispositive power over the shares owned by DKTI A/S.
|
|
(9)
|
NLBDIT 2010 Services, LLC (“NLBDIT”) has an address of c/o Sunrise Securities Corp., 600 Lexington Avenue, 23rd Floor, New York, NY 10022. The voting and disposition of the shares owned by NLBDIT are controlled by Nathan Low, principal of Sunrise Securities Corp., one of the Placement Agents.
|
We know of no arrangements, including pledges, by or among any of the forgoing persons, the operation of which could result in a change of control of DanDrit USA.
Summarized below are the principal terms of the agreements that govern our indebtedness and is subject to, and is qualified in its entirety by, such agreements, which are filed as exhibits to the registration statement to which this prospectus forms a part.
For the period January 18, 2011 (Inception) to March 31, 2013, professional fees of $38,225 were paid on behalf of the Company by Sunrise Financial Group Inc. (“SFG”). The President of SFG was the Company’s former President and sole stockholder. As of March 31, 2013, the outstanding balance of $38,235 for professional fees paid by SFG and amounts advanced to the Company are reported as loan payable - related party. These funds were advanced interest free and are unsecured. There is no written or oral agreement in effect with respect to the SFG Advances, provided, that the Company intends to attempt to reimburse the SFG Advances at the time of the closing of a business combination; however, there is no assurance that the Company will reimburse SFG.
On May 26, 2011, the Company issued an aggregate of 5,000,000 shares of its common stock to NLBDIT 2010 Services, LLC (“NLBDIT Services”) for an aggregate purchase price of $25,000 pursuant to the terms and conditions set forth in that certain common stock purchase agreement (the “NLBDIT Services CSPA”). The Low Trust owns 100% of the outstanding membership interests of NLBDIT Services and may be deemed to beneficially own the shares of Common Stock held of record by NLBDIT Services. Nathan Low is the family trustee of the Low Trust and has voting and dispositive control over any securities owned of record or beneficially by the Low Trust. Therefore, Mr. Low may be deemed to beneficially own the shares of Common Stock held of record by NLBDIT Services and beneficially by the Low Trust. The Company issued these shares of Common Stock under the exemption from registration provided by Section 4(2) of the Securities Act. The NLBDIT Services CSPA was filed as an exhibit to the Company’s Registration Statement on Form 10 filed with the SEC on August 12, 2011 and incorporated herein by this reference.
On June 3, 2011, the Company issued a note (the “NLBDIT Enterprises Note”) in favor of NLBDIT 2010 Enterprises, LLC (“NLBDIT Enterprises”) pursuant to which the Company agreed to repay NLBDIT Enterprises the sum of any and all amounts that NLBDIT Enterprises may advance to the Company (the “Principal Amount”) on or before the date that the Company consummates a business combination with a private company or reverse takeover transaction or other transaction after which the Company would cease to be a shell company (as defined in Rule 12b-2 under the Exchange Act). NLBDIT Enterprises is wholly owned by the Low Trust. Nathan Low, a principal of our sole shareholder, is the family trustee of the Low Trust and a principal of NLBDIT Enterprises. Interest accrues on the outstanding Principal Amount of the NLBDIT Enterprises Note on the basis of a 360-day year from June 3, 2011 until paid in full at the rate of six percent (6%) per annum. The lender has agreed to forego all accrued and unpaid interest through August 20, 2012. At December 31, 2013, $1,887 of accrued interest was payable.
In connection with the Share Exchange, Putnam and DanDrit agreed that upon the closing of a financing of at least $12 million in gross proceeds raised by Putnam following the Share Exchange (the “Offering”), DanDrit would repay up to an aggregate of $70,000 (the “Loan Amount”) in existing Putnam indebtedness including the professional fees and the NLBDIT Enterprises Note. In the event less than $12 million in gross proceeds is raised in connection with the Offering, the principal amount of the loan amount shall be converted into a note payable upon the one year anniversary of the earlier of the closing of the Offering or the termination of the Offering. Any amounts payable by Putnam, up to approximately $8,000, from the date of the letter of intent related to the Offering until the closing of the Share Exchange, for the purpose of maintaining the periodic and other filings required to be filed with the SEC in accordance with Putnam’s reporting requirements pursuant to the Exchange Act, including but not limited to, legal and accounting fees and expenses, shall be paid directly by DanDrit.
On December 1, 2011, DanDrit Denmark borrowed $1,500,000 and issued 6% convertible bonds. The bonds may not be converted during the four weeks following the publication of the annual report. The bond may not be repaid until the bonds expiration on November 30, 2014. The bonds shall not accrue interest after expiration. The bonds and related accrued interest are convertible into common share of the Company at an initial rate of $9.58 per common share. On December 16, 2013, the bond, related accrued interest and derivative liability were converted into 174,578 shares of DanDrit Denmark common stock.
During the years ended December 31, 2012 and 2011, Advance Biotech Invest, (“Advance Biotech”) an entity controlled and owned by Sune Olsen Holding ApS, a shareholder of the Company (“Sune Olsen Holding”), loaned DanDrit Denmark DKK 338,719 ($59,854) and DKK 143,750 ($25,019). The notes accrued interest at 6% and DanDrit Denmark recorded interest expense of DKK 20,469 ($3,617) and DKK 2,689 ($468) during the years ended December 31, 2012 and 2011, respectively. On December 16, 2013 the notes and related accrued interest were converted into shares of DanDrit Denmark and subsequently converted into shares of DanDrit USA common stock in connection with the Share Exchange.
On January 18, 2013, February 15, 2013 and March 1, 2013 Advance Biotech loaned DanDrit Denmark an additional DKK 1,000,000 and DKK 187,724 and DKK 80,000 (approximately $178,661, $33,685 and $14,075, respectively). The notes accrued interest at 6%. DanDrit Denmark recorded interest expense of DKK 86,047 ($15,617) during the year ended December 31, 2013. On December 16, 2013 the notes and related accrued interest were converted into shares of DanDrit Denmark and subsequently converted into shares of DanDrit USA common stock in connection with the Share Exchange.
On April 14, 2013 Sune Olsen Holding acquired DKK 4,375,932 (approximately $773,000) in liabilities owed by DanDrit Denmark for past due rent in exchange for a note which accrued interest at 5%, approximately DKK 139,670 ($25,349) during the year ended December 31, 2013. On December 16, 2013 the note and related accrued interest were converted into shares of DanDrit Denmark and subsequently converted into shares of DanDrit USA common stock in connection with the Share Exchange.
On June 20, 2013 Sune Olsen Holding paid DKK 1,500,000, ($265,000) in accrued legal fees of DanDrit Denmark in exchange for a note in aggregate principal amount of DKK 1,500,000 ($265,000). The note accrued interest at5%, approximately DKK 20,959 ($3,804) during the year ended December 31, 2013. On December 16, 2013 the note and related accrued interest were converted into shares of DanDrit Denmark and subsequently converted into shares of DanDrit USA common stock in connection with the Share Exchange.
On July 26, 2013 and August 15, 2013 Sune Olsen Holding loaned DanDrit Denmark an additional DKK 1,000,000, ($177,239) and DKK 750,000 ($133,343). The notes accrued interest at 5%, approximately DKK 15,575 ($2,827) during the year ended December 31, 2013. On December 16, 2013 the notes and related accrued interest were converted into shares of DanDrit Denmark and subsequently converted into shares of DanDrit USA common stock in connection with the Share Exchange.
On December 16, 2013, the notes payable, assumed expenses and liabilities and related accrued interest due to Sune Olsen Holdings totaling DKK 7,802,137, ($1,433,004) were converted into an aggregate of 223,245 shares of DanDrit Denmark common stock.
On November 11, 2013, November 20, 2013 and December 2, 2013, DanDrit Denmark received loans under an unsecured loan facility from Sune Olsen Holding ApS, a shareholder, with a goal of ensuring financing until new equity has been brought in, in aggregate principal amounts of DKK 1,500,000 ($276,651), DKK 405,000 ($74,696) and DKK 900,000 ($165,990), respectively. The loans are due May 1, 2014 and accrue interest at 5% per year DKK 50,706 ($9,352) at March 31, 2014. During March 2014, DanDrit Denmark extended maturity date of the loans with Sune Olsen Holdings ApS from May 1, 2014 to 14 days after the completion of the contemplated stock offering of DanDrit USA or February 1, 2015.
DanDrit Biotech A/S has received a loan from Sune Olsen ensuring financing until new equity has been brought in. The loan in the amount of $184,434 (DKK 1,000,000) was issued on December 20, 2013. The loan is due May 1, 2014 and accrues interest at 5% per year. During March 2014, the Company extended maturity date of the loan from May 1, 2014 to 14 days after the completion of the contemplated stock offering of DanDrit USA or February 1, 2015.
DanDrit Biotech A/S has received a loan on February 15, 2014 and March 18, 2014, and amended April 29, 2014 for DKK 2,500,000 ($461,877) and DKK 2,300,000 ($424,927), respectively, from Paseco ApS, a shareholder of DanDrit Biotech USA, Inc. The loans are payable on February 1, 2015, and accrue interest at 5% per annum. The Company may extend the term of the loan for 1 year by giving Paseco written notice by December 31, 2014. Should the Company extend the notes the interest rate will increase to 7.00% per annum.
DanDrit Biotech A/S has received an additional loan commitment on May 2, 2014 for 2,000,000 DKK ($368,868) from Paseco ApS, an entity owned by a shareholder of the DanDrit Biotech USA, Inc., payable by February 1, 2015. The Company has an option to extend the loan for one year by giving notice to Paseco by December 31, 2014 whereby the interest rate would increase to 7.00% per annum.
General
The following is a summary description of our capital stock and is subject to, and is qualified in its entirety by, the provisions of our certificate of incorporation and bylaws, which are filed as exhibits to the registration statement to which this prospectus forms a part.
Common Stock
Our Certificate of Incorporation authorizes the issuance of up to 100,000,000 shares of common stock, par value $.0001 per share. As of the date of this prospectus, there were 64 holders of record of our common stock. Holders of our common stock are entitled to such dividends as may be declared by our board of directors out of funds legally available for such purpose, subject to any preferential dividend rights of any then outstanding preferred stock. The shares of common stock are neither redeemable nor convertible. Holders of common stock have no preemptive or subscription rights to purchase any of our securities.
All outstanding shares of common stock are of the same class and have equal rights and attributes. Each holder of our common stock is entitled to one vote for each such share outstanding in the holder’s name on all matters submitted to a vote of stockholders of the Company. Directors shall be elected by a plurality of the votes of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors. Any other action shall be authorized by a majority of the votes cast except where the Delaware General Corporation Law (the “DGCL”) prescribes a different percentage of votes and/or a different exercise of voting power and except as may be otherwise prescribed by the provisions of the Company’s certificate of incorporation and bylaws. All holders are entitled to share equally in dividends, if any, as may be declared from time to time by the board of directors out of funds legally available. The holders of common stock do not have cumulative or preemptive rights.
In the event of our liquidation, dissolution or winding up, the holders of our common stock are entitled to receive pro rata our assets, which are legally available for distribution, after payments of all debts and other liabilities and subject to the prior rights of any holders of preferred stock then outstanding. All of the outstanding shares of our common stock are fully paid and non-assessable. The shares of common stock offered by this prospectus will also be fully paid and non-assessable.
Preferred Stock
Our Certificate of Incorporation authorizes the issuance of up to 10,000,000 shares of preferred stock, par value $.0001 per share with designations, voting and other rights and preferences as may be determined from time to time by the Board of Directors of the Company. As of the date of this prospectus, no shares of preferred stock are outstanding.
The issuance of preferred stock with certain voting, conversion and/or redemption rights could adversely affect the rights of holders of our common stock, including with respect to voting, dividends and liquidation. Preferred stock could also be issued quickly with terms calculated to delay, defer or prevent a change in control of our company or to make removal of management more difficult. Additionally, the issuance of preferred stock may decrease the market price of our common stock.
Dividend Policy
The Company has not declared or paid any cash dividends on its common stock and does not intend to declare or pay any cash dividend in the foreseeable future. The payment of dividends, if any, is within the discretion of the Board of Directors and will depend on the Company’s earnings, if any, its capital requirements and financial condition and such other factors as the Board of Directors may consider.
Securities Authorized for Issuance under Equity Compensation Plans
The Board of Directors has adopted the DanDrit Biotech USA, Inc. 2014 Equity Incentive Plan. We have reserved 1,206,000 shares of our common stock for issuance in accordance with the terms of the plan. As of the date of this prospectus, no awards have been made from the plan.
Section 145 of the DGCL provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses including attorneys’ fees, judgments, fines and amounts paid in settlement in connection with various actions, suits or proceedings, whether civil, criminal, administrative or investigative other than an action by or in the right of the corporation (a derivative action), if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, if they had no reasonable cause to believe their conduct was unlawful. A similar standard is applicable in the case of derivative actions, except that indemnification only extends to expenses including attorneys’ fees incurred in connection with the defense or settlement of such actions, and the statute requires court approval before there can be any indemnification where the person seeking indemnification has been found liable to the corporation. The statute provides that it is not exclusive of other indemnification that may be granted by a corporation’s certificate of incorporation, bylaws, agreement, a vote of stockholders or disinterested directors or otherwise.
DanDrit’s Certificate of Incorporation provides that it will indemnify and hold harmless, to the fullest extent permitted by Section 145 of the DGCL, as amended from time to time, each person that such section grants us the power to indemnify.
The DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability for:
|
●
|
any breach of the director’s duty of loyalty to the corporation or its stockholders;
|
|
|
●
|
acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law;
|
|
|
●
|
payments of unlawful dividends or unlawful stock repurchases or redemptions; or
|
|
|
●
|
any transaction from which the director derived an improper personal benefit.
|
DanDrit’s Certificate of Incorporation provides that, to the fullest extent permitted by applicable law, none of our directors will be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director. Any repeal or modification of this provision will be prospective only and will not adversely affect any limitation, right or protection of a director of our company existing at the time of such repeal or modification.
Delaware law and DanDrit’s Certificate of Incorporation and Bylaws may permit indemnification for liabilities under the Securities Act of 1933, as amended (“Securities Act”) or the Securities Exchange Act of 1934, as amended (“Exchange Act”). Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling DanDrit pursuant to the foregoing provisions, DanDrit has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Transfer Agent and Registrar
The transfer agent and registrar for the common stock is Continental Stock Transfer & Trust Company 17 Battery Place, 8th Floor, New York, New York 10004.
There is not currently, and there has never been, any market for any of our common stock. Our securities are not eligible for trading on any national securities exchange or any over-the-counter markets, including the OTC Bulletin Board or the quotation systems of the OTC Markets, and we cannot assure you that they will become eligible. In connection with this offering, we intend to arrange for a registered broker-dealer to apply to have our common stock quoted on the OTC Bulletin Board and on the OTCQB but we cannot assure you that our application will be approved.
Holders of Common Stock
As of March 31, 2014, we had 8,040,000 shares of common stock outstanding held of record by 64 persons, which includes 185,053 shares of common stock reserved for issuance to the Non-Consenting Shareholders of DanDrit Denmark also deemed issued and outstanding for accounting purposes. Our common stock is more fully described in the section of this prospectus entitled “Description of Securities.” For a description of the shares of our common stock that may be sold pursuant to Rule 144, please see the section of this prospectus titled “Shares Eligible for Future Sale”.
Securities Authorized for Issuance under Equity Compensation Plans
On February 12, 2014, the sole director and the majority stockholder of Putnam adopted the DanDrit Biotech USA, Inc. 2014 Equity Incentive Plan (the “Plan”) that governs equity awards to employees, directors and consultants of the Company, effective upon the closing of the Share Exchange. There are 1,206,000 shares of common stock reserved for issuance under the Plan.
The Plan has a term of ten years. The types of awards permitted under the Plan include qualified incentive stock options and non-qualified stock options, and restricted stock. Each option will be exercisable at such times and subject to such terms and conditions as the Board may specify. Stock options will generally vest over four years and expire no later than ten years from the date of grant. As of the date of this prospectus the Company has not issued any awards from the Plan.
A substantial number of shares of our common stock could be sold in the public market after the lapse of the contractual and legal restrictions described below. The sale of a substantial amount of our common stock in the public market could adversely affect the prevailing market price of our common stock.
As of March 31, 2014, we had an aggregate of 8,040,000 shares of our common stock outstanding, including 185,053 shares of common stock reserved for issuance to the non-consenting shareholders of DanDrit Denmark and deemed issued and outstanding for accounting purposes and 1,206,000 shares of common stock reserved for issuance under the DanDrit Biotech USA, Inc. 2014 Equity Incentive Plan. On February 12, 2014, pursuant to which holders of approximately 97% of the issued and outstanding capital stock of DanDrit Denmark (the “DanDrit Consenting Holders”) exchanged an aggregate of 3,879,624 equity interests of DanDrit Denmark for 5,814,947 shares of DanDrit USA (the “Share Exchange”) and as a result of which Putnam would become the parent of DanDrit Denmark. In accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark, DanDrit Denmark shareholders who have not consented to the Share Exchange (the “Non-Consenting Shareholders”) and therefore have not exchanged such DanDrit Denmark shareholder’s equity interests in DanDrit Denmark for shares of DanDrit USA, will be entitled to receive the 185,053 shares of common stock of DanDrit USA that each such DanDrit Denmark shareholder would have been entitled to receive if such DanDrit Denmark shareholder had consented to the Share Exchange, up to an aggregate of 185,053 shares of common stock of DanDrit USA. As a result of the Share Exchange, the former shareholders of DanDrit Denmark became the controlling shareholders of the Company. The Share Exchange was accounted for as a reverse takeover/recapitalization effected by a share exchange, wherein DanDrit Denmark is considered the acquirer for accounting and financial reporting purposes. The capital, share price, and earnings per share amount in these consolidated financial statements for the period prior to the reverse merger were restated to reflect the recapitalization in accordance with the exchange ratio established in the merger.
The shares of our common stock issued in the Share Exchange and the related transactions, as well as other outstanding shares of our common stock, our warrants, and the shares of our common stock issuable upon exercise of our warrants are, and will be, “restricted securities” as that term is defined in Rule 144 under the Securities Act. These restricted securities may be sold in the public market if they are registered or if the sale qualifies for an exemption from registration under Rule 144 promulgated under the Securities Act, as summarized below; and with respect to those shares of common stock issued pursuant to the exemption from registration provided by Regulation S under the Securities Act, in accordance with Regulation S.
Lock-Up Agreements
Our executive officers, directors and certain stockholders have agreed to a 180-day lock-up with respect to 7,854,947 shares of our outstanding common stock other than certain permitted transfers described in the form of Lock-Up Agreement. Holders of the common stock subject to the Lock-Up Agreement shall also be permitted to exercise options or receive stock through equity awards, and waive any rights to request or demand registration of the common stock.
Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies
Rule 144 is not available for resale of securities issued by any shell companies (other than business combination-related shell companies) or any issuer that has been at any time previously a shell company. The SEC has provided an exception to this prohibition, however, if the following conditions are met:
|
●
|
the issuer of the securities that was formerly a shell company has ceased to be a shell company;
|
|
|
●
|
the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act;
|
|
|
●
|
the issuer of the securities has filed all Exchange Act reports and materials required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports; and
|
|
|
●
|
at least one year has elapsed from the time that the issuer filed current Form 10 type information with the SEC reflecting its status as an entity that is not a shell company.
|
As a result, none of our stockholders is currently able to sell shares of our common stock in reliance on Rule 144. Assuming we continue to meet the requirements set forth above, Rule 144 will become available to our stockholders one year after the date we file the information required in SEC Form 10. Our stockholders may currently sell their shares of our common stock only pursuant to a registration statement that has been declared effective under the Securities Act or pursuant to another exemption from registration.
Policies and Procedures for Related Party Transactions
We do not have any special committee, policy or procedure related to the review, approval or ratification of transactions with related persons that are required to be disclosed pursuant to Item 404(a) of Regulation S-K, other than as required by the DGCL.
SEC regulations define the related party transactions that require disclosure to include any transaction, arrangement or relationship in which the amount involved exceeds the lesser of $120,000 or one percent of the average of the Company’s total assets at year end for the last two completed fiscal years in which we were or are to be a participant and in which a related person had or will have a direct or indirect material interest. A related person is: (i) an executive officer, director or director nominee of the Company, (ii) a beneficial owner of more than 5% of our common stock, (iii) an immediate family member of an executive officer, director or director nominee or beneficial owner of more than 5% of our common stock, or (iv) any entity that is owned or controlled by any of the foregoing persons or in which any of the foregoing persons has a substantial ownership interest or control.
For the period from January 18, 2011 (inception), through the date of this prospectus (the “Reporting Period”), described below are certain transactions or series of transactions between us and certain related persons.
Transactions with related persons.
During the Reporting Period, professional fees of $38,225 were paid on behalf of the Company by Sunrise Financial Group Inc. (“SFG”). The President of SFG was the Company’s former President and sole stockholder. As of March 31, 2013, the outstanding balance of $38,235 for professional fees paid by SFG and amounts advanced to the Company are reported as loan payable - related party. These funds were advanced interest free and are unsecured. There is no written or oral agreement in effect with respect to the SFG advances, provided, that the Company intends to attempt to reimburse the SFG advances at the time of the closing of a business combination; however, there is no assurance that the Company will reimburse SFG.
On May 26, 2011, the Company issued an aggregate of 5,000,000 shares of Common Stock to NLBDIT 2010 Services, LLC (“NLBDIT Services”) for an aggregate purchase price of $25,000 pursuant to the terms and conditions set forth in that certain common stock purchase agreement (the “NLBDIT Services CSPA”). Following the purchase, NLBDIT 2010 Services, LLC became the holder of more than 5% of our common stock. The Low Trust owns 100% of the outstanding membership interests of NLBDIT Services and may be deemed to beneficially own the shares of Common Stock held of record by NLBDIT Services. Nathan Low, a principal of our majority shareholder prior to the Share Exchange, is the family trustee of the Low Trust and has voting and dispositive control over any securities owned of record or beneficially by the Low Trust.
On June 3, 2011, the Company issued a note (the “NLBDIT Enterprises Note”) in favor of NLBDIT 2010 Enterprises, LLC (“NLBDIT Enterprises”) pursuant to which the Company agreed to repay NLBDIT Enterprises the sum of any and all amounts that NLBDIT Enterprises may advance to the Company (the “Principal Amount”) on or before the date that the Company consummates a business combination with a private company or reverse takeover transaction or other transaction after which the Company would cease to be a shell company (as defined in Rule 12b-2 under the Exchange Act). NLBDIT Enterprises is wholly owned by the Low Trust. Nathan Low, a principal of our sole shareholder, is the family trustee of the Low Trust and a principal of NLBDIT Enterprises. Interest accrues on the outstanding Principal Amount of the NLBDIT Enterprises Note on the basis of a 360-day year from June 3, 2011 until paid in full at the rate of six percent (6%) per annum. The lender has agreed to forego all accrued and unpaid interest through August 20, 2012. At December 31, 2013, $1,887 of accrued interest was payable.
The Company engaged Samir Masri CPA Firm P.C. to provide accounting services to the Company. Samir Masri, the Company’s former Chief Executive Officer, Chief Financial Officer, President, Secretary and director, is the founder and President of Samir Masri CPA Firm P.C. The Company agreed to pay Samir Masri CPA Firm P.C. for services rendered in connection with the preparation of the financial statements required to be filed in the Company’s registration statement on Form 10 and subsequent periodic reports in an aggregate amount equal to $10,000 per fiscal year until the date that the Company consummates a merger or similar transaction with an operating business.
On February 12, 2014, in connection with the closing of the Share Exchange, the Company and NLBDIT entered into a Share Cancellation Agreement pursuant to which NLBDIT agreed to the cancellation of an aggregate of 4,400,000 issued and outstanding shares of common stock of the Company owned by NLBDIT prior to the Share Exchange, effective upon the closing of the Share Exchange.
During the years ended December 31, 2012 and 2011, DanDrit Denmark received loans (the “2012 Loans”) of DKK 338,719 ($59,854) and DKK 143,750 ($25,019) from Advance Biotech Invest, (“Advance Biotech”) an entity controlled and owned by Sune Olsen Holding ApS, a shareholder of the Company (“Sune Olsen Holding”). The 2012 Loans accrued interest at 6% and DanDrit Denmark recorded interest expense of DKK 20,469 ($3,617) and DKK 2,689 ($468) during the years ended December 31, 2012 and 2011, respectively.
Sune Olsen Holding ApS
On January 18, 2013, February 15, 2013 and March 1, 2013, Advance Biotech loaned DanDrit Denmark an additional DKK 1,000,000 and DKK 187,724 and DKK 80,000, respectively (approximately $178,661, $33,685 and $14,075, respectively) (the “Spring 2013 Loans”). The Spring 2013 Loans accrued interest at 6%. DanDrit Denmark recorded interest expense of DKK 86,047 ($15,617) during the year ended December 31, 2013 for the Spring 2013 Loans. On December 16, 2013, the Spring 2013 Loans were converted into 52,618 shares of common stock of the Company.
On April 14, 2013, Sune Olsen Holding paid debt to Symbion on behalf of DanDrit Denmark (the “Symbion Debt”) of DKK 4,171,771 in payables equivalent to approximately $762,950. The Symbion Debt accrued interest at 5% from April 12, 2013 until repaid and is payable on demand. DanDrit Denmark recorded interest expense of DKK 139,670 ($25,349) during the year ended December 31, 2013 for the Symbion Debt. On December 16, 2013, the Symbion Debt and accrued interest was converted into 86,204 shares of common stock of the Company.
On June 20, 1013, Sune Olsen Holding paid debt to Lett Law Firm on behalf of DanDrit Denmark (the “Lett Debt”) of DKK 1,500,000 in payables equivalent to $274,300. The Lett Debt accrued interest at 5% per year and is payable on demand. DanDrit Denmark recorded interest expense of DKK 20,959 ($3,804) during the year ended December 31, 2013 for the Lett Debt. On December 16, 2013, the Lett Debt and accrued interest was converted into 29,036 shares of common stock of the Company.
On July 26, 2013 and August 15, 2013 Sune Olsen Holding loaned DanDrit Denmark DKK 1,000,000, ($177,239) and DKK 750,000 ($133,343) (the “Summer 2013 Loans” and together with the 2012 Loans and the Spring 2013 Loans, collectively, the “Sune Olsen Debt”). The Summer 2013 Loans accrued interest at 5%, or approximately DKK 15,575 ($2,827) during the year ended December 31, 2013, to be repaid in full by the end of the calendar year 2014. The Summer 2013 Loans may be terminated by Sune Olsen Holding with three months’ prior written notice. On December 16, 2013, the Summer Loans and accrued interest was converted into 33,705 shares of common stock of the Company.
During the year ended December 31, 2013 the total debt due to Sune Olsen Holding under the Sune Olsen Debt, including accrued interest, was DKK 9,641,065 (approximately USD 1,770,758). On the December 16, 2013, the full amount of the Sune Olsen Debt was converted into 275,863 shares of common stock of the Company.
Subsequent to the conversion of the Sune Olsen Debt, DanDrit Denmark received a loan facility (the “Subsequent Sune Olsen Holding Loan”) from Sune Olsen Holding to ensure financing until new equity is brought in. Under the Sune Olsen Holding Loan DanDrit Denmark has received the following amounts: on November 11, DKK 1,500,000 ($276,651), on November 20, 2013 DKK 405,000 ($74,696), on December 2, 2013 DKK 900,000 ($165,990), in total DKK 2,805,000 ($517,337). The Subsequent Sune Olsen Holding Loan is to be repaid the latest of 14 days after completion of the contemplated public offering of the Company or February 1, 2015 and each carry an interest of 5% per year, or a total of approximately DKK 50,706 ($9,352) as of March 31, 2014.
DanDrit Denmark has received an additional loan (the “New Sune Olsen Loan”) from Sune Olsen, managing member of Sune Olsen Holding, to ensure financing until new equity is brought in. The New Sune Olsen Loan in the amount of DKK 1,000,000 ($184,434) was issued on December 20, 2013. The New Sune Olsen Loan is to be repaid May 1, 2014 and carries an interest of 5% per year, or approximately DKK18,966 ($3498) as of March 31, 2014. During March 2014, DanDrit Denmark extended the maturity date of the New Sune Olsen Loan from May 1, 2014 to the latest of 14 days after the completion of the contemplated stock offering of the Company or February 1, 2015.
DKTI A/S
During 2012 DanDrit Denmark entered into a loan facility (“DKTI Loan 1”) of DKK 5,000,000 (approximately $880,000) accruing interest at 6% per annum. DKTI Loan 1 is secured by of DanDrit Denmark’s intellectual property rights, including its patents and its patent applications. During the year ended December 31, 2012, DanDrit Denmark borrowed an additional DKK 4,431,862 ($783,139) plus DKK 71,563 ($12,646) in interest (“DKTI Loan 2”). During the year ended December 31, 2013, DanDrit Denmark borrowed an additional DKK 310,000 (approximately $55,000) plus DKK 230,377 (approximately $42,000) in interest (“DKTI Loan 3” and together with DKTI Loan 1 and DKTI Loan 2, the “DKTI Loans”). On December 16, 2013, the DKTI Loans, including accrued interest, were converted into 144,321 shares of common stock of the Company.
As of October 31, 2013, DanDrit Denmark had a $1,500,000 convertible bond issued to DKTI A/S. The principal amount including accrued interest was $1,672,455 as of October 31, 2013. On December 16, 2013 the convertible bond, including accrued interest, was converted into 261,665 shares of common stock of the Company.
Paseco ApS
On February 15, 2014 and March 18, 2014, DanDrit Denmark received loans (the “2014 Loans”) of DKK 2,500,000 and 2,300,000, respectively, equivalent to approximately $460,549 and $423,705, respectively (based on the currency exchange rate of $1.00 = DKK 5.4283). The 2014 Loans, evidenced by that certain Loan Agreement by and between DanDrit Denmark and Paseco ApS, a shareholder of DanDrit USA (“Paseco”) dated March 21, 2014, carry an interest of 5% per year and are payable on February 1, 2015. On April 29, 2014, DanDrit Denmark and Paseco entered into an amendment whereby the terms of the 2014 Loans can be extended at the Company’s option for an additional year with an increase in the interest rate to 7.00%. As of March 31, 2014, the outstanding balance on the 2014 Loans, including accrued interest, was $880,880. During the three months ended March 31, 2014 the Company record related party interest on the 2014 Loans of DKK 19,507 ($3,598).
DanDrit Denmark received a loan commitment on May 2, 2014, for 2,000,000 DKK ($368,868) from Paseco, payable by February 1, 2015. The Company has an option to extend the loan for one year by giving notice to Paseco by December 31, 2014, whereby the interest rate would increase to 7.00% per annum.
Lease Agreements
On April 1, 2013, the Company entered into an operating lease agreement with Ordnung ApS, a company controlled by a shareholder, to lease office space. The lease calls for monthly payments of 1,150 DKK (approximately $200) and can be terminated by the Company or landlord with three month notice.
On July 8, 2013, the Company entered into an operating lease agreement for lab space with Symbion A/S. The lease calls for months payments of 6,000 DKK (approximately$1,000) and expires on March 31, 2016 but may be terminated by the Company with three month notice.
On March 27, 2014, the Company entered into an operating sublease agreement for office space with Paseco. The lease calls for months payments of DKK 10,000 increasing to DKK 20,000 as of July 1, 2014 (approximately $1,842 and 3,684, respectively). The lease can be terminated by either the Company or the landlord by giving three month notice.
LETT Advokatpartnerselskab
As of March 31, 2014 a total of DKK 1,468,500 (approximately $289,284) was accrued and due and payable to the Lett Law Firm for legal services provided for DanDrit Denmark.
Except as otherwise indicated herein, there have been no other related party transactions, or any other transactions or relationships required to be disclosed pursuant to Item 404 and Item 407(a) of Regulation S-K.
Employment Agreements
Leire Employment Agreement
Effective March 1, 2012, DanDrit Denmark entered into an Employment Agreement with Dr. Eric Leire (the “Leire Employment Agreement”), to serve as its Managing Director. Pursuant to the terms and conditions of the Employment Agreement, Dr. Leire will be employed by DanDrit Denmark for an indefinite term unless earlier terminated pursuant to the terms therein. The Leire Employment Agreement provides that the Dr. Leire will receive a salary of 2,100,000 DKK ($381,125) gross per year, to be paid monthly on the last day of each month and subject to annual review and increases by our Board, as it deems appropriate.
In addition to his salary, Dr. Leire will be entitled to receive: (i) a company car at a value up of DKK 5,100 ($926) per month (monthly lease value) and DanDrit Denmark shall defray all expenses in connection with the running of the car; (ii) coverage of all expenses relating to Dr. Leire’s mobile phone, home computer, Internet connection as well as his home phone; (iii) coverage of all the expenses relating to Dr. Leire’s subscription to a fitness club; (iv) a bonus of up to DKK 400,000 ($72,595) per year if Dr. Leire reaches certain conditions as specified in the Employment Agreement; and (v) shall be covered by DanDrit Denmark’s pension scheme.
DanDrit Denmark may terminate the employment with 12 months’ notice to the end of a month. If DanDrit Denmark terminates Dr. Leire’s employment, he shall be entitled to be released from his duty to work (in Danish “fritstillet”) during the notice period. Dr. Leire may terminate the employment at 6 months’ notice to the end of a month. In case of material breach, the non-defaulting party can terminate the Leire Employment Agreement without notice and can claim damages in accordance with the general Danish law of damages. If Dr. Leire suspends payments, or insolvency proceedings are commenced against his estate, DanDrit Denmark can terminate the employment without notice. The employment shall cease without notice to the end of the month in which Dr. Leire attains the age of 70. The Leire Employment Agreement contains non-competition and non-solicitation clauses.
Wolfe Executive Service Agreement
Effective January 1, 2014, DanDrit Denmark entered into a CFO Service Agreement with Robert Wolfe, to serve as its Chief Financial Officer and Chief Financial Officer of DanDrit USA following the closing of the Share Exchange. Pursuant to the terms and conditions of the CFO Service Agreement, Mr. Wolfe will be employed by DanDrit Denmark for an indefinite term unless earlier terminated pursuant to the terms therein. The CFO Service Agreement provides that Mr. Wolfe will receive a salary of $78,000 gross per year, to be paid monthly on the last day of each month and subject to annual review and increases by our Board, as it deems appropriate, and a one-time sign on fee of $6,000.
In addition to his salary, Mr. Wolfe will be entitled to receive reimbursement of all reasonable costs relating to his work for the Company, including travel, accommodation and meals expenses in connection with work outside the agreed premises, are to be paid by the Company according to vouchers submitted.
DanDrit Denmark may terminate the employment with three (3) months’ notice to the end of a month during the first six (6) months of the term of the Executive Service Agreement. Thereafter six (6) months’ notice is required for a termination. Mr. Wolfe may terminate the employment at three (3) months’ notice to the end of a month. In case of material breach, the non-defaulting party can terminate the CFO Service Agreement without notice and can claim damages in accordance with the general Danish law of damages. If Mr. Wolfe suspends payments, or insolvency proceedings are commenced against his estate, DanDrit Denmark can terminate the employment without notice. The employment shall cease without notice to the end of the month in which Mr. Wolfe attains the age of 70. The CFO Service Agreement contains a non-competition clause.
Distribution
The shares will be offered and sold on a best efforts basis through Sunrise Securities Corp. and The Benchmark Company, LLC (each a “Placement Agent” and, collectively, the “Placement Agents”), broker-dealers who are members of the Financial Industry Regulatory Authority (“FINRA”) and through other participating broker-dealers who are members of FINRA.
The maximum offering amount is $12,000,000. The broker-dealers are not obligated to obtain any subscriptions, and there is no assurance that any shares will be sold.
Investors can purchase common stock in this offering by completing a Subscription Agreement, a copy of which is filed as Exhibit 99.1 to the registration statement of which this prospectus is a part, and sending it together with payment in full to the escrow agent. Subscriptions will be effective only on acceptance by DanDrit and the right is reserved to reject any subscription in whole or in part. Subscribers must be provided a copy of this Prospectus. DanDrit and/or the Placement Agents will send each investor a written confirmation of the acceptance of the investor’s subscription for shares.
This offering will terminate on , unless it is fully subscribed before that date or we decide to terminate the offering prior to that date. In either event, the offering may be closed without further notice to you.
Certain of our affiliates may purchase shares of our common stock in this offering on the same terms as they are offered and sold to the public.
Compensation
The Placement Agents’ commissions will be equal to 7% of the gross proceeds received in this offering raised from investors introduced to the Company by the Placement Agent. After commissions, we shall receive the following for the shares sold in the Offering. The following table illustrates the net proceeds that we will receive from this offering, after payment of the Placement Agents’ commissions but before the payment of expenses.
|
Per Share
|
Total
|
|||||||
|
Public offering price
|
$
|
5.00
|
$
|
12,000,000
|
||||
|
Commissions
|
0.35
|
840,000
|
||||||
|
Proceeds, before expenses, to us
|
4.65
|
11,160,000
|
||||||
We estimate that the total expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the Placement Agents’ commissions, will be approximately $254,536, all of which are payable by us.
In addition to the commissions discussed above, the Placement Agents have received, or will receive, reimbursement of accountable expenses of up to $120,000 or 1% of the gross proceeds received in this offering. We have also agreed to reimburse the Placement Agents for reasonable fees and expenses of counsel up to an aggregate amount of $75,000 in connection with this offering.
Troutman Sanders LLP, counsel to the Placement Agents, acquired 40,000 shares of our common stock on February 12, 2014 upon closing of the Share Exchange, which shares have been deemed compensation by FINRA and are, therefore, subject to a 180-day lock-up pursuant to FINRA Rule 5110(g)(1). Pursuant to FINRA Rule 5110(g)(1), such shares shall not be sold during this offering, or sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date of the effectiveness or commencement of sales of this offering, subject to certain exceptions set forth in FINRA Rule 5110(g)(2).
Determination of Offering Price
There is no established public market for the shares of common stock that we are offering. The offering price has been arbitrarily determined and does not bear any relationship to our assets, results of operations, or book value, or to any other generally accepted criteria of valuation. The fixed price of $5.00 at which our common stock is being offered pursuant to this prospectus was determined based on, without limitation, the estimates of the business potential and earnings prospects of DanDrit and the consideration of such potential earnings in relation to market valuations of comparable companies.
Lock-Up Agreements
We and our officers, directors, and certain existing stockholders, including the Security Holder identified in the Resale Prospectus, have agreed, subject to certain exceptions, not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any shares of our common stock or other securities convertible into or exercisable or exchangeable for shares of our common stock after the effective date of the registration statement of which this prospectus is a part without the prior written consent of Sunrise Securities Corp. The lock-up period is for 180 days beginning as of the filing date of the last amendment to the registration statement of which this prospective is a part that is declared effective. Notwithstanding the foregoing, the lock-up period shall not apply, and the parties may transfer the shares in the transactions described in clauses (i) through (vi) below as follows:
|
(i)
|
as a bona fide gift or gifts; or
|
|
(ii)
|
to any trust for the direct or indirect benefit of the undersigned or the immediate family of the transferring party; or
|
|
(iii)
|
to the transferring party and/or any member of the immediate family of the transferring party from or by a grantor retained (or like-kind) annuity trust which exists as of the date hereof and was established for the direct or indirect benefit of the transferring party and/or any member of the immediate family of the transferring party pursuant to the terms of such trust;
|
|
(iv)
|
if the transferring party is a corporation, partnership or other business entity (A) to another corporation, partnership or other business entity that is an affiliate (as defined under Rule 12b-2 of the Exchange Act) of the transferring party or (B) any distribution or dividend to equity holders of the transferring party as part of a distribution or dividend by the transferring party (including upon the liquidation and dissolution of the transferring party pursuant to a plan of liquidation approved by the transferring party 's equity holders), or if the transferring party is a trust, to a grantor or beneficiary of the trust;
|
|
(v)
|
in the event of a default under a pledge which exists as of the date hereof as security for a margin or loan account pursuant to the terms of such account;
|
|
(vi)
|
pursuant to any 10b5-l trading plans in effect as of the date of the Offering; or
|
|
|
(vii)
|
with the prior written consent of Sunrise Securities Corp., as representative of the Placement Agents.
|
For purposes of this lock-up agreement, “immediate family” shall mean any relationship by blood, marriage or adoption, not more remote than first cousin.
Furthermore, notwithstanding the foregoing, during the lock-up period, the transferring party may sell shares of common stock of DanDrit on the open market following the consummation of the offering if and only if (i) such sales are not required to be reported in any public report or filing with the Securities Exchange Commission, or otherwise and (ii) the transferring party does not otherwise voluntarily effect any public filing or report regarding such sales.
Indemnification
We have agreed to indemnify the Placement Agents against liabilities relating to the offering arising under the Securities Act, liabilities arising from breaches of some or all of the representations and warranties contained in the Placement Agent Agreement, and to contribute to payments that the Placement Agents may be required to make for these liabilities.
Relationships
Nathan Low is the family trustee of the Low Trust, the beneficial owner of NLBDIT Services, our majority shareholder prior to the Share Exchange, and has voting and dispositive control over any securities owned of record or beneficially by the Low Trust, subject to the agreement of the independent trustee. Mr. Low is also the founder and President of Sunrise Securities Corp., one of the Placement Agents.
See “Related Party Transactions” in this prospectus, and “Item 15. Recent Sales of Unregistered Securities” in the registration statement of which this prospectus is a part, for a description of certain transactions involving Sunrise Securities Corp., one of the Placement Agents, or its affiliates and our Company.
Each Placement Agent or its affiliates from time to time and may in the future provide investment banking, financial advisory and other related services to us and our affiliates for which they have received and may continue to receive customary fees and commissions.
Foreign Sales
Notice to Investors in the United Kingdom
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of any securities which are the subject of the offering contemplated by this prospectus may not be made in that Relevant Member State except that an offer to the public in that Relevant Member State of any such securities may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
|
(a)
|
to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
|
|
(b)
|
to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts;
|
|
(c)
|
by the Placement Agents to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive); or
|
|
(d)
|
in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of shares shall result in a requirement for the publication by the issuer or the Placement Agents of a prospectus pursuant to Article 3 of the Prospectus Directive.
|
For the purposes of this provision, the expression an “offer to the public” in relation to any security in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide to purchase such securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
Each Placement Agent has represented, warranted and agreed that:
|
(a)
|
it has only communicated or caused to be communicated and will only communicate or cause to be communicated any invitation or inducement to engage in investment activity (within the meaning of section 21 of the Financial Services and Markets Act 2000 (the FSMA)) received by it in connection with the issue or sale of any of our securities in circumstances in which section 21(1) of the FSMA does not apply to the issuer; and
|
|
(b)
|
it has complied with and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to our securities in, from or otherwise involving the United Kingdom.
|
European Economic Area
In particular, this document does not constitute an approved prospectus in accordance with European Commission’s Regulation on Prospectuses no. 809/2004 and no such prospectus is to be prepared and approved in connection with this offering. Accordingly, in relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (being the Directive of the European Parliament and of the Council 2003/71/EC and including any relevant implementing measure in each Relevant Member State) (each, a Relevant Member State), with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State (the Relevant Implementation Date) an offer of securities to the public may not be made in that Relevant Member State prior to the publication of a prospectus in relation to such securities which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that any placement agent may, with effect from and including the Relative Implementation Date, make an offer of securities to the public in that Relevant Member State at any time:
|
●
|
to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
|
|
●
|
to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than 43,000,000 euros; and (3) an annual net turnover of more than 50,000,000 euros, as shown in the last annual or consolidated accounts; or
|
|
●
|
in any other circumstances which do not require the publication by the issuer of a prospectus pursuant to Article 3 of the Prospectus Directive.
|
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any common shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe such securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State. For these purposes the shares of common stock are “securities.”
Validity of the securities offered by this prospectus will be passed upon for us by Richardson & Patel, LLP, New York, New York. Certain legal matters related to the offering will be passed upon for the Placement Agents by Troutman Sanders LLP, New York, New York.
Putnam historically retained Raich Ende Malter & Co. LLP (“Raich”) as its principal accountant. In connection with the closing of the Share Exchange, we terminated Raich and retained Gregory & Associates, LLC (“Gregory”) as our principal accountant. Our Board of Directors approved the change.
Raich’s reports on the financial statements for the years ended March 31, 2013 and 2012 included in the Form 10-K for the year ended March 31, 2013 as filed with the SEC did not contain an adverse opinion or a disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles, except that their reports included disclosure of uncertainty regarding Putnam’s ability to continue as a going concern.
From inception through February 12, 2014, Putnam had no disagreements with Raich on matters of accounting principles or practices, financial statement disclosure, or auditing scope or procedure. Putnam had not consulted with Gregory on any matter prior to the Share Exchange.
We have authorized Raich to respond fully to the inquiries of Gregory concerning any matters discussed above. We have provided Raich with a copy of the above statements. We have requested that Raich furnish us with a letter addressed to the SEC stating whether Raich agrees with the above statements and, if not, stating the respects in which it does not agree. A copy of such letter from Raich is filed as an exhibit to the registration statement of which this prospectus forms a part.
The audited financial statements of DanDrit Biotech USA, Inc. as of December 31, 2013 and December 31, 2012 included in this prospectus have been audited by Gregory & Associates, LLC, who is an independent registered public accounting firm, as set forth in their report appearing elsewhere herein, and are included herein in reliance upon such report given upon the authority of said firm as experts in auditing and accounting.
We file annual, quarterly and special reports and other information with the SEC. These filings contain important information that does not appear in this prospectus. For further information about us, you may read and copy any reports, statements and other information filed by us at the SEC’s Public Reference Room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549-0102. You may obtain further information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Our SEC filings are also available on the SEC Internet site at http://www.sec.gov, which contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
DANDRIT BIOTECH USA, INC. AND SUBSIDIARIES
INDEX TO FINANCIAL STATEMENTS
Consolidated Financial Statements
|
Page
|
||
|
F-1
|
||
|
F-2
|
||
|
F-3
|
||
|
F-4
|
||
|
F-5
|
||
|
F-6
|
||
|
F-7
|
||
|
F-8
|
81
(FORMERLY PUTNAM HILLS CORP.)
(A Development Stage Company)
Index to Unaudited Financial Statements
|
Page
|
||
|
F-1
|
||
|
F-2
|
||
|
F-3
|
||
|
F-4
|
||
|
F-5
|
||
|
F-6
|
||
|
F-7
|
||
|
F-8
|

(801) 277-2763 Phone • (801) 277-6509 Fax
Board of Directors
DANDRIT BIOTECH USA, INC.
(Restated DanDrit Biotech A/S)
Jagtvej 169 A
2100 Copenhagen, Denmark
We have audited the accompanying balance sheets of DanDrit Biotech USA, Inc. (the restated financial statements of DanDrit Biotech A/S) as of December 31, 2013 and 2012, and the related statements of operations, other comprehensive income, stockholders’ deficit and cash flows for the years ended December 31, 2013 and 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, and audit of its internal controls over financial reporting for the year ended December 31, 2013 and 2012. Our audit included consideration of internal controls over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal controls over financial reporting for the year ended December 31, 2013 and 2012. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, based on our audit, the financial statements audited by us present fairly, in all material respects, the financial position of DanDrit Biotech USA, Inc. (the restated financial statements of DanDrit Biotech A/S) as of December 31, 2013 and 2012 and the results of their operations and their cash flows for the years ended December 31, 2013, and 2012, in conformity with generally accepted accounting principles in the United States of America.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has not yet established profitable operations and has incurred significant losses since its inception. These factors raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regards to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
The financial statements for the three months ended March 31, 2014 and 2013 are unaudited and we have not preformed any audit procedures since the report dated below.
/s/ Gregory & Associates, LLC
Gregory & Associates, LLC
June 13, 2014
Salt Lake City, Utah
(FORMERLY PUTNAM HILLS CORP.)
CONSOLIDATED BALANCE SHEET
|
(Unaudited)
March 31,
2014
|
December 31,
2013
|
December 31,
2012
|
||||||||||
|
ASSETS
|
||||||||||||
|
CURRENT ASSETS:
|
||||||||||||
|
Cash
|
$ | 54,472 | $ | 18,794 | 4,381 | |||||||
|
Cash held in escrow
|
423,969 | 77,468 | - | |||||||||
|
Other Receivables
|
69,712 | 25,456 | 81,802 | |||||||||
|
Prepaid Expenses
|
11,621 | 19,774 | 19,747 | |||||||||
|
Total Current Assets
|
559,774 | 141,492 | 105,930 | |||||||||
|
PROPERTY AND EQUIPMENT, Net accumulated Depreciation
|
40,460 | - | 2,706 | |||||||||
|
OTHER ASSETS
|
||||||||||||
|
Definite Life Intangible Assets
|
233,766 | 231,615 | 239,658 | |||||||||
|
Deferred Stock Offering Costs
|
67,000 | 67,000 | - | |||||||||
|
Deposits
|
10,466 | 10,360 | 14,570 | |||||||||
|
Total Other Assets
|
311,232 | 308,975 | 254,228 | |||||||||
|
TOTAL ASSETS
|
$ | 911,466 | $ | 450,467 | 362,864 | |||||||
|
LIABILITIES AND STOCKHOLDER'S DEFICIENCY
|
||||||||||||
|
CURRENT LIABILITIES:
|
||||||||||||
|
Notes Payable -Related Party, Current Portion
|
$ | 1,705,201 | $ | 728,001 | 106,349 | |||||||
|
Accounts Payable
|
568,175 | 548,501 | 551,175 | |||||||||
|
Accrued Expenses
|
824,192 | 858,135 | 1,429,098 | |||||||||
|
Total Current Liabilities
|
3,097,568 | 2,134,637 | 2,086,622 | |||||||||
|
LONG TERM LIABILITIES
|
- | - | - | |||||||||
|
Notes Payable, Related Parties Less Current Portion
|
- | - | 795,785 | |||||||||
|
Bonds Payable – Related Parties, net of $0, $0 and 502,465 discount
|
- | - | 997,535 | |||||||||
|
Derivative Liability
|
- | - | 850,753 | |||||||||
|
Total Long Term Liabilities
|
- | - | ||||||||||
|
Total Liabilities
|
3,097,568 | 2,134,637 | 4,730,695 | |||||||||
|
STOCKHOLDER'S DEFICIENCY:
|
||||||||||||
|
Preferred stock, $.0001 par value; 10,000,000 shares authorized; none issued and outstanding
|
- | - | - | |||||||||
|
Common stock, par value $0.0001, 100,000,000 shares authorized, 8,040,000, 6,000,000 and 5,318,151 issued and outstanding at March 31, 2014 and December 31, 2013 and 2012, respectively
|
804 | 600 | 532 | |||||||||
|
Additional paid-in capital
|
17,788,110 | 17,867,546 | 12,817,122 | |||||||||
|
Accumulated Deficit
|
(19,947,180 | ) | (19,521,126 | ) | (17,373,765 | ) | ||||||
|
Other Comprehensive Income, net
|
(27,836 | ) | (31,190 | ) | 188,280 | |||||||
|
Total Stockholder’s (Deficit)
|
(2,186,102 | ) | (1,684,170 | ) | (4,367,831 | ) | ||||||
|
TOTAL LIABILITIES AND STOCKHOLDER'S EQUITY
|
$ | 911,466 | 450,467 | 362,864 | ||||||||
See accompanying notes to the financial statements.
(FORMERLY PUTNAM HILLS CORP.)
CONSOLIDATED STATEMENT OF OPERATIONS
|
(Unaudited)
For The
Three Months
Ended
March 31,
2014
|
(Unaudited)
For The
Three Months
Ended
March 31,
2013
|
For the
Year Ended
December 31,
2013
|
For the
Year Ended
December 31,
2012
|
|||||||||||||
|
Revenues
|
$
|
-
|
$
|
31,558
|
32,768
|
62,806
|
||||||||||
|
Cost of Goods Sold
|
17,739
|
15,360
|
109,299
|
64,385
|
||||||||||||
|
Gross Income(Loss)
|
(17,739
|
)
|
16,198
|
(76,531
|
)
|
(1,579
|
)
|
|||||||||
|
Operating Expenses
|
||||||||||||||||
|
General and Administrative Expenses
|
326,428
|
175,016
|
1,233,683
|
1,036,005
|
||||||||||||
|
Depreciation and Amortization
|
6,794
|
8,600
|
38,297
|
56,600
|
||||||||||||
|
Consulting Expenses
|
61,145
|
13,048
|
390,437
|
829,845
|
||||||||||||
|
Total Operating Expense
|
394,367
|
196,664
|
1,662,417
|
1,922,450
|
||||||||||||
|
(LOSS) FROM OPERATIONS
|
(412,106
|
)
|
(180,466
|
)
|
(1,738,948
|
)
|
(1,924,029
|
) | ||||||||
|
Other Income (Expense)
|
||||||||||||||||
|
Interest (expense)
|
(13,999
|
)
|
(159,922
|
)
|
(652,703
|
)
|
(704,911
|
)
|
||||||||
|
Gain (loss) on Currency Transactions
|
-
|
(100,327
|
)
|
19,541
|
32,841
|
|||||||||||
|
Gain on forgiveness of debt
|
- |
|
-
|
49,016
|
-
|
|||||||||||
|
Gain on Derivative Liability
|
-
|
41,643
|
175,732
|
153,430
|
||||||||||||
|
Gain on Sale of Assets
|
-
|
-
|
1
|
15,020
|
||||||||||||
|
Interest Income
|
51
|
-
|
-
|
-
|
||||||||||||
|
Total Other Income (Expense)
|
(13,948
|
)
|
(218,606
|
)
|
(408,413
|
)
|
(503,620
|
)
|
||||||||
|
(Loss) Before Income Taxes
|
(426,054
|
)
|
(399,072
|
)
|
(2,147,361
|
)
|
(2,427,649
|
)
|
||||||||
|
Income Tax Expense (Benefit)
|
-
|
-
|
-
|
-
|
||||||||||||
|
NET (LOSS)
|
$
|
(426,054
|
)
|
$
|
(399,072
|
)
|
(2,147,361
|
)
|
2,427,649
|
)
|
||||||
|
BASIC AND DILUTED LOSS PER SHARE
|
$
|
(0.06
|
)
|
(0.08
|
)
|
(0.40
|
)
|
(0.46
|
)
|
|||||||
|
WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING - BASIC AND DILUTED
|
7,065,333
|
5,318,151
|
5,332,721
|
5,318,151
|
||||||||||||
See accompanying notes to the financial statements.
(FORMERLY PUTNAM HILLS CORP.)
STATEMENTS OF OTHER COMPREHENSIVE LOSS
|
(Unaudited)
|
||||||||||||||||
|
|
For the Three Months Ended
|
For the Years Ended
December 31
|
||||||||||||||
|
|
March 31
|
|||||||||||||||
|
|
2014
|
2013
|
2013
|
2012
|
||||||||||||
|
|
||||||||||||||||
|
Net Loss
|
(426,054 | ) | (399,072 | ) | $ | (2,147,361 | ) | $ | (2,427,649 | ) | ||||||
|
Currency Translation, Net of Taxes
|
(3,354 | ) | (139,052 | ) | (219,470 | ) | 85,702 | |||||||||
|
|
||||||||||||||||
|
Other Comprehensive Loss
|
$ | (429,408 | ) | $ | (538,124 | ) | $ | (2,366,831 | ) | $ | (2,341,947 | ) | ||||
See accompanying notes to the financial statements.
(FORMERLY PUTNAM HILLS CORP.)
CONSOLIDATED STATEMENT OF STOCKHOLDER'S DEFICIENCY
(Unaudited)
|
Other
|
||||||||||||||||||||||||||||
|
Additional
|
Compre-
|
|||||||||||||||||||||||||||
|
Preferred Stock
|
Common Stock
|
Paid-in
|
Accumulated
|
hensive
|
||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Deficit
|
Income
|
||||||||||||||||||||||
|
BALANCE, December 31, 2011
|
-
|
-
|
5,318,151
|
$
|
532
|
$
|
12,817,122
|
$
|
(14,946,116
|
)
|
273,981
|
|||||||||||||||||
|
Equity Adjustment for Foreign Currency Translation
|
-
|
-
|
-
|
-
|
-
|
-
|
(85,701
|
)
|
||||||||||||||||||||
|
Net Loss for the Year Ended December 31, 2012
|
-
|
-
|
-
|
-
|
-
|
(2,427,649
|
)
|
|||||||||||||||||||||
|
BALANCE, December 31, 2012
|
-
|
$
|
-
|
5,318,151
|
$
|
532
|
$
|
12,817,122
|
$
|
(17,373,765
|
)
|
$
|
188,280
|
|||||||||||||||
|
Common shares issued upon conversion of bond payable - related party and derivative liability at $9.00 per shares, December 2013
|
-
|
-
|
261,665
|
26
|
2,353,322
|
-
|
-
|
|||||||||||||||||||||
|
Common shares issued in payment of notes payable - related party at $6.42 per shares, December 2013
|
-
|
-
|
144,321
|
14
|
926,372
|
-
|
-
|
|||||||||||||||||||||
|
Common shares issued in payment of notes payable - related party at $6.42 per shares, December 2013
|
-
|
-
|
275,863
|
28
|
1,770,730
|
-
|
-
|
|||||||||||||||||||||
|
Equity Adjustment for Foreign Currency Translation
|
-
|
-
|
-
|
-
|
-
|
-
|
(219,470
|
)
|
||||||||||||||||||||
|
Net Loss for the Year Ended December 31, 2013
|
-
|
-
|
-
|
-
|
-
|
(2,147,361
|
)
|
-
|
||||||||||||||||||||
|
BALANCE, December 31, 2013
|
-
|
-
|
6,000,000
|
$
|
600
|
$
|
17,867,546
|
$
|
(19,521,126
|
)
|
$
|
(31,190
|
)
|
|||||||||||||||
|
To record the recapitalization of Subsidiary in connection with the February 12, 2014 Share Exchange Agreement wherein the DanDrit Biotech USA Inc. (“Parent”) issued 6,000,000 common shares to acquire a 100% interest in DanDrit Biotech A/S (“Subsidiary”) DanDrit Biotech USA Inc., (Formerly Putnam Hills Corp),
|
-
|
-
|
2,040,000
|
204
|
(79,436
|
)
|
-
|
-
|
||||||||||||||||||||
|
Equity Adjustment for Foreign Currency Translation
|
-
|
-
|
-
|
-
|
-
|
-
|
3,354
|
|||||||||||||||||||||
|
Net Loss for the Three Months Ended March 31, 2014
|
-
|
-
|
-
|
-
|
-
|
(426,054
|
)
|
-
|
||||||||||||||||||||
|
BALANCE, March 31, 2014
|
-
|
-
|
8,040,000
|
$
|
804
|
$
|
17,788,110
|
$
|
(19,947,180
|
)
|
$
|
(27,836
|
)
|
|||||||||||||||
See accompanying notes to the financial statements.
(FORMERLY PUTNAM HILLS CORP.)
CONSOLIDATED STATEMENT OF CASH FLOWS
(Unaudited)
|
(Unaudited)
|
(Unaudited)
|
|||||||||||||||
|
|
For The
Three Months
Ended
March 31,
2014
|
For The
Three Months
Ended
March 31,
2013
|
For the
Year Ended
December 31,
2013
|
For the
Year Ended
December 31,
2012
|
||||||||||||
|
|
||||||||||||||||
|
NET (LOSS)
|
$ | (426,054 | ) | $ | (399,072 | ) | $ | (2,147,361 | ) | $ | 2,427,649 | |||||
|
|
||||||||||||||||
|
ADJUSTMENT TO RECONCILE NET LOSS TO NET CASH USED IN OPERATING ACTIVITIES:
|
||||||||||||||||
|
Depreciation and Amortization
|
6,794 | 15,860 | 38,297 | 56,600 | ||||||||||||
|
(Gain)/Loss on sale of equipment
|
- | - | - | (15,020 | ) | |||||||||||
|
(Gain)/Loss on sale of subsidiary
|
- | - | (1 | ) | - | |||||||||||
|
Accretion of Discount on Bond Payable
|
- | 123,557 | 502,465 | 461,279 | ||||||||||||
|
(Gain)/Loss on Derivative Liability
|
- | (41,643 | ) | (175,732 | ) | (142,579 | ) | |||||||||
|
CHANGES IN ASSETS AND LIABILITIES:
|
||||||||||||||||
|
(Increase)Decrease in Other Receivables
|
(44,256 | ) | 43,915 | 56,346 | (17,705 | ) | ||||||||||
|
(Increase)Decrease in Prepaid Expenses/Deposits
|
8,047 | (8,962 | ) | 4,183 | (8,943 | ) | ||||||||||
|
Increase(Decrease) in Accounts Payable
|
19,674 | 463,472 | (2,674 | ) | 450,924 | |||||||||||
|
Increase(Decrease) in Accrued Expenses
|
(1,951 | ) | (1,321,026 | ) | (406,151 | ) | 710,080 | |||||||||
|
Total Adjustments
|
(11,692 | ) | (724,827 | ) | 16,733 | 1,494,636 | ||||||||||
|
|
||||||||||||||||
|
NET CASH USED IN OPERATING ACTIVITIES
|
(437,746 | ) | (1,123,899 | ) | (2,130,628 | ) |
(933,013
|
) | ||||||||
|
|
||||||||||||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||||||||||
|
Proceeds from sale of subsidiary
|
- | - | 1 | - | ||||||||||||
|
Proceeds from sale of equipment
|
- | - | - | 15,020 | ||||||||||||
|
Purchase of fixed assets
|
(41,498 | ) | - | - | - | |||||||||||
|
Net (Increase) in Cash held in Escrow
|
(346,501 | ) | - | (77,468 | ) | - | ||||||||||
|
Purchase of Intangible Assets
|
(7,907 | ) | - | (27,548 | ) | (99,663 | ) | |||||||||
|
NET CASH USED BY INVESTING ACTIVITIES
|
(395,906 | ) | - | (105,015 | ) | (84,643 | ) | |||||||||
|
|
||||||||||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||||||||||
|
Proceeds from Notes Payable – Related Party
|
865,976 | 987,911 | 2,754,662 | 856,754 | ||||||||||||
|
Payment of Stock Offering Costs
|
- | - | (67,000 | ) | - | |||||||||||
|
Payments on Notes Payable – Related Party
|
- | - | (218,136 | ) | - | |||||||||||
|
NET CASH PROVIDED BY(USED BY) FINANCING ACTIVITIES
|
865,976 | 987,911 | 2,469,526 | 856,754 | ||||||||||||
|
|
||||||||||||||||
|
Gain (Loss) on Currency Translation
|
3,354 | 139,279 | (219,470 | ) | (85,701 | ) | ||||||||||
|
|
||||||||||||||||
|
NET INCREASE (DECREASE) IN CASH
|
35,678 | 3,291 | 14,413 | (246,603 | ) | |||||||||||
|
|
||||||||||||||||
|
CASH, BEGINNING OF PERIOD
|
18,794 | 4,381 | 4,381 | 250,984 | ||||||||||||
|
|
||||||||||||||||
|
CASH, END OF PERIOD
|
$ | 54,472 | $ | 7,672 | $ | 18,794 | $ | 4,381 | ||||||||
|
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION
|
||||||||||||||||
|
Cash paid during the periods for:
|
||||||||||||||||
|
Interest
|
- | - | $ | 12,632 | - | |||||||||||
|
Income Taxes
|
- | - | - | - | ||||||||||||
|
SUPPLEMENTAL DISCLOSURES OF NON-CASH INVESTING AND FINANCING ACTIVITIES
|
||||||||||||||||
|
Accretion of Discount on Bond Payable
|
$ | - | $ | 123,557 | $ | 502,465 | $ | 461,279 | ||||||||
|
Change in Fair Market Value of Derivative Liability
|
$ | - | $ | (41,643 | ) | $ | (175,732 | ) | $ | 142,579 | ) | |||||
See accompanying notes to the financial statements.
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business and Basis of Presentation – DanDrit Biotech USA, Inc. (“DanDrit USA”, the “Company”, “we”, “us”, “our”) (formerly Putnam Hills Corp) was originally incorporated in the state of Delaware on January 18, 2011 as a vehicle to pursue a business combination through the acquisition of, or merger with, an operating business.
DanDrit BioTech A/S, a Danish Corporation was incorporated on April 1, 2001 (“DanDrit Denmark”). The Company engages in the research and development, manufacturing and clinical trials of pharmaceutical and biological products for the human treatment of cancer using the dendritic cell technology.
Reverse Acquisition - On February 12, 2014, the Company signed and consummated the transactions contemplated by a Share Exchange Agreement (the "Share Exchange Agreement"), by and among DanDrit USA (formerly known as Putnam Hills Corp.), DanDrit Denmark and N.E. Nielsen, as the representative of the shareholders of DanDrit Denmark, pursuant to which the Company agreed to acquire 100% of the issued and outstanding equity securities in Dandrit Denmark in exchange for 6,000,000 shares of common stock, par value $0.0001 per share of the Company (the “Share Exchange”). The Share Exchange closed on February 12, 2014 pursuant to which holders of approximately 97% of the issued and outstanding capital stock of DanDrit Denmark (the “DanDrit Consenting Holders”) exchanged an aggregate of 3,879,624 equity interests of DanDrit Denmark for 5,814,947 shares of DanDrit USA. As a result of the closing of the Share Exchange the Company became the parent of DanDrit Denmark. In accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark, DanDrit Denmark shareholders who have not consented to the Share Exchange (the “Non-Consenting Shareholders”) and therefore have not exchanged such DanDrit Denmark shareholder’s equity interests in DanDrit Denmark for shares of DanDrit USA, are entitled to receive up to an aggregate of 185,053 shares of common stock of DanDrit USA in exchange for the DanDrit Denmark equity interests by such Non-Consenting Shareholders. As a result of the Share Exchange, the former shareholders of Dandrit Denmark became the controlling shareholders of the Company.
Restatement - The Share Exchange was accounted for as a reverse takeover/recapitalization effected by a share exchange, wherein Dandrit Denmark is considered the acquirer for accounting and financial reporting purposes. The capital, share price, and earnings per share amount in these consolidated financial statements for the period prior to the reverse merger were restated to reflect the recapitalization in accordance with the exchange ratio established in the merger.
Upon the closing of the Share Exchange, DanDrit USA and the its majority shareholder immediately prior to the closing agreed to cancel up to 4,400,000 shares of our common stock. In addition, following the closing of the Share Exchange, DanDrit Biotech USA, Inc., a wholly owned subsidiary of the Company merged with and into the Company, thereby changing the Company’s name to “DanDrit Biotech USA, Inc.”
Consolidation - For the three months ended March 31, 2014, the consolidated financial statements include the accounts and operations of the DanDrit Denmark and the accounts and operations of DanDrit USA, Inc from February the date of acquisition of February 12, 2014 through March 31, 2014. For the years ended December 31, 2013 and 2012 and the three months ended March 31, 2013, the consolidated financial statements include the accounts and operations of the DanDrit Denmark with the capital, share price, and earnings per share amount in these consolidated financial statements restated to reflect the recapitalization in accordance with the exchange ratio established in the merger. All material inter-company transactions and accounts have been eliminated in the consolidation.
On December 16, 2013, the DanDrit Denmark sold, for $1.00, the wholly-owned dormant subsidiary DanDrit Corporation PTE. LTD. a Singapore limited liability company incorporated on July 1, 2008. As this Singapore entity was a dormant subsidiary the financial statements include the $1 proceeds and gain on sale of the former subsidiary.
Functional Currency / Foreign currency translation — The functional currency of DanDrit USA, Inc. is the U.S. Dollar. The functional currency of DanDrit Biotech A/S is the Danish Kroner (“DKK”). The Company’s reporting currency is the U.S. Dollar for the purpose of these financial statements. The Company’s balance sheet accounts are translated into U.S. dollars at the period-end exchange rates and all revenue and expenses are translated into U.S. dollars at the average exchange rates prevailing during the years 2013 and 2012 and the period ending March 31, 2014. Translation gains and losses are deferred and accumulated as a component of other comprehensive income in stockholders’ equity. Transaction gains and losses that arise from exchange rate fluctuations from transactions denominated in a currency other than the functional currency are included in the statement of operations as incurred.
Cash and Cash Equivalents — The Company considers all highly liquid debt instruments purchased with a maturity of three months or less to be cash equivalents. The Company had no balances held in financial institutions in the United States in excess of federally insured amounts at March 31, 2014 and December 31, 2013 and 2012.
Property and Equipment — Property and equipment are stated at cost. Expenditures for major renewals and betterments that extend the useful lives of property and equipment are capitalized, upon being placed in service. Expenditures for maintenance and repairs are charged to expense as incurred. Depreciation is computed for financial statement purposes on a straight-line basis over the estimated useful lives of the assets which range from four to six years (See Note 3).
Intangible Assets — The Company accounts for definite life intangible assets, including patents, in accordance with Financial Accounting Standards Board, (“FASB”) Accounting Standards Codification, (“ASC”) Topic 350, “Goodwill and Other Intangible Assets” and amortized the patents on a straight line basis over the estimated useful life of twenty years. Costs incurred in relation to patent applications are capitalized cost and amortized over the estimated useful life of the patent. If it is determined that a patent will not be issued, the related remaining patent application costs are charged to expense.
DANDRIT BIOTECH USA, INC.
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Impairment of Long-Lived Assets - Long-lived assets, such as property, plant, and equipment and patents are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life.
Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of would be separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell, and would no longer be depreciated. The depreciable basis of assets that are impaired and continue in use is their respective fair values.
Revenue Recognition and Sales — The Company’s sales of its MCV colorectal cancer vaccine have been limited to a compassionate use basis in Singapore after stage IIA trials and is not approved for current sale for any other use or location. The Company's accounts for revenue recognition in accordance with the Securities and Exchange Commission Staff Accounting Bulletin No. 101, “Revenue Recognition in Financial Statements” (SAB 101), and FASB ASC 605 Revenue Recognition. The Company recognizes revenue when rights and risk of ownership have passed to the customer, when there is persuasive evidence of an arrangement, product has been shipped or delivered to the customer, the price and terms are finalized, and collections of resulting receivable is reasonably assured. Products are primarily shipped FOB shipping point at which time title passes to the customer.
Value Added Tax - In Denmark, Value Added Tax (“VAT”) of 25% of the invoice amount is collected in respect of the sales of goods on behalf of tax authorities. The VAT collected is not revenue of the Company; instead, the amount is recorded as a liability on the balance sheet until such VAT is paid to the authorities. VAT of 25% is also paid to Danish and EU vendors on invoices these amounts are refundable from the respective governmental authority and recorded as other receivables in the accompanying financial statements.
Research and Development Cost — The Company expenses research and development costs for the development of new products as incurred and is included in operating expense. There was no research and development costs for the three month period ended March 31, 2014 and 2013 and years ended December 31, 2013 and 2012.
Income Taxes — The Company accounts for income taxes in accordance with FASB ASC Topic 740 Accounting for Income Taxes. This statement requires an asset and liability approach for accounting for income taxes.
Loss Per Share — The Company calculates earnings /(loss) per share in accordance with FASB ASC 260 Earnings Per Share. Basic earnings per common share (EPS) are based on the weighted average number of common shares outstanding during each period. Diluted earnings per common share are based on shares outstanding (computed as under basic EPS) and potentially dilutive common shares. Potential common shares included in the diluted earnings per share calculation include in-the-money stock options that have been granted but have not been exercised.
Derivatives - We generally do not use derivative financial instruments to hedge exposures to cash-flow, market or foreign-currency risks. However, we have entered into certain other financial instruments and contracts, such as debt financing arrangements with features that are either (i) not afforded equity classification, (ii) embody risks not clearly and closely related to host contracts, or (iii) may be net-cash settled by the counterparty. These instruments are required to be carried as derivative liabilities, at fair value.
DANDRIT BIOTECH USA, INC.
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
We estimate fair values of all derivative instruments, such as embedded conversion features utilizing Level 3 inputs (defined below in Note 1: Fair Value of Financial Instruments). We use the Black-Scholes option valuation technique because it embodies all of the requisite assumptions (including trading volatility, estimated terms and risk free rates) necessary to fair value these instruments. Estimating fair values of derivative financial instruments requires the development of significant and subjective inputs that may, and are likely to, change over the duration of the instrument with related changes in internal and external market factors. In addition, option-based techniques are highly volatile and sensitive to changes in our market price of our common stock, which have historically had high volatility. Since derivative financial instruments are initially and subsequently carried at fair value, our income will reflect the volatility in these estimate and assumption changes.
We report our derivative liabilities at fair value.
Fair Value of Financial Instruments — The Company accounts for fair value measurements for financial assets and financial liabilities in accordance with FASB ASC Topic 820, “Fair Value Measurements”. The authoritative guidance, which, among other things, defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is defined as the exit price, representing the amount that would either be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
|
●
|
Level 1. Observable inputs such as quoted prices in active markets for identical assets or liabilities;
|
|
●
|
Level 2. Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and
|
|
●
|
Level 3. Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
|
Unless otherwise disclosed, the fair value of the Company’s financial instruments including cash, accounts receivable, prepaid expenses, investments, accounts payable, accrued expenses, capital lease obligations and notes payable approximates their recorded values due to their short-term maturities.
Accounting Estimates — The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Actual results could differ from those estimated.
Recent Accounting Pronouncements — Recent accounting pronouncements issued by the FASB did not or are not believed by management to have a material impact on the Company’s present or future financial statements.
Reclassification - The financial statements for the periods ended March 31, 2013 and December 31, 2013 and 2012 have been reclassified to conform to the headings and classifications used in the March 31, 2014 financial statements.
DANDRIT BIOTECH USA, INC.
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 — GOING CONCERN
The accompanying financial statements have been prepared in conformity with generally accepted accounting principles of the United States of America, which contemplate continuation of the Company as a going concern. However, the Company has incurred significant losses and has not yet been successful in establishing profitable operations. These factors raise substantial doubt about the ability of the Company to continue as a going concern. In this regard, management plans to mitigate this doubt by raising additional funds through debt and/or equity offerings and by substantially increasing sales once approval for the Company’s product is obtained. There is no assurance that the Company will be successful in achieving profitable operations. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
NOTE 3 — PROPERTY AND EQUIPMENT
Property and equipment consisted of the following at March 31, 2014 and December 31, 2013 and 2012:
|
Useful
Life
|
March 31,
2014
|
December 31,
2013
|
December 31,
2012
|
|||||||||||||
|
Lab equipment and instruments
|
4-6 | $ | 202,632 | $ | 194,143 | $ | 194,143 | |||||||||
|
Computer equipment
|
4-6 | 110,898 | 66,493 | 66,493 | ||||||||||||
| 313,530 | 260,636 | 260,636 | ||||||||||||||
|
Less Accumulated Depreciation
|
(273,070 | ) | (260,636 | ) | (257,930 | ) | ||||||||||
|
Net Property and Equipment
|
$ | 40,460 | $ | - | 2,706 | |||||||||||
Depreciation expense amounted to $1,038 and $788 for the three month period ended March 31, 2014 and 2013, respectively. Depreciation expense amounted to $2,706 and $4,477, for the year ended December 31, 2013 and 2012, respectively. The Company’s property and equipment is held as collateral on the notes payable related party.
NOTE 4 — DEFINITE-LIFE INTANGIBLE ASSETS
At March 31, 2014 and December 31, 2013 and 2012, definite-life intangible assets, net of accumulated amortization, consist of patents on the Company’s products and processes of $233,766, $231,615, and $239,658 respectively. The patents are recorded at cost and amortized over twenty years from the date of application. Amortization expense for the three months ended March 31, 2014 and 2013 was $5,756 and $6,414, respectively. Amortization expense for the years ended December 31, 2013 and 2012 was $38,297 and $56,600, respectively. Expected future amortization expense for the years ended are as follows:
|
Year ending December 31,
|
||||
|
2014
|
$
|
17,283
|
||
|
2015
|
20,106
|
|||
|
2016
|
20,106
|
|||
|
2017
|
20,106
|
|||
|
2018
|
20,106
|
|||
|
Thereafter
|
136,059
|
|||
|
$
|
233,766
|
|||
DANDRIT BIOTECH USA, INC.
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 — NOTES PAYABLE – RELATED PARTY
Notes payable to related parties consists of the following as of March 31, 2014 and December 31, 2013 and 2012:
|
March 31,
2014 |
December 31,
2013 |
December 31,
2012 |
||||||||||
|
5% Note Payable Paseco ApS
|
$ | 888,880 | $ | - | - | |||||||
|
6% Note Payable DKTI A/S
|
- | - | 795,785 | |||||||||
|
6% Note Payable - Sune Olsen Holding ApS
|
- | - | 85,731 | |||||||||
|
Non-Interest Bearing Loan Payable Sunrise Financial Group Inc.
|
38,235 | - | - | |||||||||
|
Note Payable ML Group
|
21,520 | 21,557 | 20,618 | |||||||||
|
6% Promissory Note payable to NLBDIT 2010 Enterprises, LLC
|
41,945 | - | - | |||||||||
|
5% Note Payable - Sune Olsen Holding ApS
|
526,689 | 521,390 | - | |||||||||
|
5% Note Payable - Sune Olsen
|
187,932 | 185,054 | - | |||||||||
|
Total Notes Payable – Related Party
|
1,705,201 | 728,001 | 902,134 | |||||||||
|
Less Current Maturities
|
(1,705,201 | ) | (728,001 | ) | (106,349 | ) | ||||||
|
Note Payables – Related Party Long Term
|
$ | - | $ | - | 795,785 | |||||||
The following represents the future maturities of long-term debt as of March 31, 2014:
|
Year ending December 31,
|
||||
|
2014
|
1,705,201
|
|||
|
2015
|
- | |||
|
2016
|
-
|
|||
|
2017
|
-
|
|||
|
2018
|
-
|
|||
|
Thereafter
|
-
|
|||
|
-
|
||||
On February 15, 2014 and March 18, 2014, DanDrit Denmark received DKK 2,500,000 ($461,084) and DKK 2,300,000 ($424,198) loans (the “2014 Loans”), respectively, from Paseco ApS, a shareholder of the Company. The 2014 Loans are payable 14 days after the completion of the Company’s contemplated public offering or February 1, 2015, and accrue interest at 5% per annum. On April 29, 2014, DanDrit Denmark and Paseco entered into an amendment to extend the terms of the 2014 Loans to February 1, 2015, or at the Company’s option for an additional year with an increase in the interest rate to 7.00%. As of March 31, 2014, the outstanding balance on the 2014 Loans including accrued interest was $880,880. During the three months ended March 31, 2014 the Company record related party interest on the 2014 Loans of DKK 19,507 ($3,598).
DanDrit Denmark received an unsecured loan facility from Sune Olsen Holding ApS (“Sune Olsen Holding”), a shareholder, with a goal of ensuring financing until new equity has been brought in, pursuant to which DanDrit Denmark has received the following amounts: On November 11, 2013 DKK 1,500,000 ($276,651), on November 20, 2013 DKK 405,000 ($74,696), and on December 2, 2013 DKK 900,000 ($165,990). The loans are due on May 1, 2014 and accrue interest at a rate of 5% per year. There was DKK 50,706 ($9,352) of interest accrued at March 31, 2014. During March 2014, the maturity date of the loans with Sune Olsen Holding was extended from May 1, 2014 to the latest of 14 days after the completion of the contemplated stock offering of the Company or February 1, 2015.
DanDrit Denmark received an additional unsecured loan from Sune Olsen, managing member of Sune Olsen Holding, with a goal of ensuring financing until new equity has been brought in. The loan in the amount of DKK 1,000,000 ($184,434) was issued on December 20, 2013. The loan is due May 1, 2014 and accrues interest of 5% per year. There was DKK18,966 ($3,498) of interest accrued at March 31, 2014. During March 2014, the maturity date of the DKK 1,000,000 loans with Sune Olsen was extended from May 1, 2014 to the later of 14 days after the completion of the contemplated stock offering of the Company or February 1, 2015.
During March 2014, DanDrit Denmark received a 2,000,000 DKK ($368,868) letter of support from Paseco ApS an entity owned by a shareholder of the Company, to finance operations until February 1, 2015 to be issued with terms substantially similar to those contained in the 2014 Loans. The Company has an option to extend the loan for one year by giving notice to Paseco by December 31, 2014 whereby the interest rate would increase to 7.00% per annum.
During 2012, DKTI A/S, a shareholder of DanDrit Denmark, which is controlled by officers and directors of the Company agreed to loan the Company up to DKK 5,000,000 (approximately $880,000) accruing interest at 6% per annum. The loan is secured by all the Company’s intellectual property rights, including its patents and its patent applications. During the year ended December 31, 2012 the Company borrowed DKK 4,431,862 ($783,139) plus DKK 71,563 ($12,646) in interest. During the year ended December 31, 2013, the Company borrowed an additional DKK 310,000 (approximately $55,000) on the loan and accrued interest of DKK 230,377 (approximately $42,000). The notes with related accrued interest were converted into 96,288 common shares of DanDrit Denmark on December 16, 2013 which were exchanged for 144,321 shares of common stock of the Company upon the closing of the Share Exchange.
During the years ended December 31, 2012 and 2011 Sune Olsen Holding loaned DanDrit Denmark DKK 338,719 ($59,854) and DKK 143,750 ($25,019), respectively. The Company added the accrued interest at 6% and the Company recorded interest expense of DKK 20,469 ($3,617) and DKK 2,689 ($468) during the years end December 31, 2012 and 2011 respectively. The loans are payable upon three month written notice of the shareholder. On December 16, 2013, the notes with related accrued interest were converted into 35,106 shares of DanDrit Denmark which were exchanged for 52,618 shares of common stock of the Company upon the closing of the Share Exchange.
DANDRIT BIOTECH USA INC
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 — NOTES PAYABLE – RELATED PARTY (Continued)
On January 18, 2013, February 15, 2013 and March 1, 2013 Advance Biotech loaned DanDrit Denmark an additional DKK 1,000,000 and DKK 187,724 and DKK 80,000 (approximately $178,661, $33,685 and $14,075, respectively). The notes accrued interest at 6%. DanDrit Denmark recorded interest expense of DKK 86,047 ($15,617) during the year ended December 31, 2013. On December 16, 2013 the notes and related accrued interest were converted into shares of DanDrit Denmark and subsequently converted into shares of DanDrit USA common stock in connection with the Share Exchange.
On April 14, 2013, Sune Olsen Holding, a shareholder of the Company, assumed DKK 4,375,932 (approximately$773,000) in liabilities owed by DanDrit Denmark for past due rent from a vendor in exchange for a note payable. The note accrued interest at 5% and is payable on demand. On December 31, 2013, the note with related interest of DKK 139,670 ($25,349) were converted into 86,204 shares of DanDrit Denmark. Such shares of common stock were exchanged for 129,206 shares of common stock of the Company upon the closing of the Share Exchange.
On June 20, 2013, Sune Olsen Holding, a shareholder of the Company, paid DKK 1,500,000, ($265,000) in accrued legal fees owed by DanDrit Denmark in exchange for a DKK1,500,000 ($265,000) 5% note payable to Sune Olsen Holding. On December 16, 2013, the note with related accrued interest of DKK 20,959 ($3,804) was converted into 29,036 shares of DanDrit Denmark common stock. Such shares of common stock were exchanged for 31,414 shares of common stock of the Company upon the closing of the Share Exchange.
On July 26, 2013 and August 15, 2013, Sune Olsen Holding, a shareholder of the Company, loaned DanDrit Denmark an additional DKK 1,000,000 ($177,239) and DKK 750,000 ($133,343), respectively. The notes accrue interest at 5% per annum and are payable upon three month written notice of the shareholder. The notes with related accrued interest of DKK 15,575 ($2,827) were converted into 33,705 shares of DanDrit Biotech A/S common stock on December 16, 2013. Such shares of common stock were exchanged for 50,518 shares of common stock of the Company upon the closing of the Share Exchange.
On April 30, 2013 Stratega ApS, a shareholder of the Company, loaned the Company DKK 1,000,000 ($175,359). The note accrues interest at 1% per month and is payable on September 1, 2013. As of September 1, 2013 the loan was outstanding, and thereby incurred a penalty of DKK 50,000 ($8,863). The outstanding loan accrued interest at 2.5% per month beginning September 2, 2013. DKTI Invest AS has secured the loan by pledging 25,000 common shares of DKTI Invest AS. DKTI Invest AS pledged the collateral on behalf of the Company, and the Company granted DKTI Invest AS worldwide use of the Company’s DDM master cell bank (“Use Agreement”) more specifically of the Company’s working cell bank DDM 1-7203-01, manufactured in 2008, for research, manufacturing and commercial purposes. The note was repaid in November 2013, the security was released and the Use Agreement cancelled.
As of March 31, 2014, the outstanding balance of $38,235 for professional fees paid by a shareholder and amounts advanced to the Company are reported as loan payable - related party. The $38,235 loans payable were acquired in the reverse acquisition. The amounts are unsecured, non-interest bearing and have no stipulated repayment terms.
A 6% Promissory Note payable (the “Note”) to NLBDIT 2010 Enterprises, LLC, an entity controlled by a shareholder of the Company, in aggregate principal amount of up to $80,000, plus any accrued unpaid interest, was acquired by DanDrit Denmark in the Share Exchange. The note is payable upon or prior to the closing of at least $12 million in gross proceeds raise by the Company following the closing of the Share Exchange. In the event less than $12 million is raised in an offering of the Company, the Note shall be payable upon the one year anniversary of the earlier of the closing of the offering or the termination of the offering. As of March 31, 2014, the outstanding balance on the Note, including accrued interest, was $41,945. During the three months ended March 31, 2014 the Company record related party interest on the Note of $927.
NOTE 6 — CONVERTIBLE BOND PAYABLE – RELATED PARTY
On December 1, 2011, DanDrit Denmark borrowed $1,500,000 from DKTI A/S and issued 6% convertible bonds. The bonds may not be converted during the four weeks following the publication of the annual report. The bond may not be repaid until the bonds’ expiration on November 30, 2014. The bonds shall not accrue interest after expiration. The bonds and related accrued interest are convertible into common shares of the Company at an initial rate of $9.58 per common share.
DANDRIT BIOTECH USA INC
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 — CONVERTIBLE BOND PAYABLE – RELATED PARTY (Continued)
The conversion /adjustment features had an estimated fair value of $1,003,557 using the Black-Scholes pricing Model using the assumptions below and bifurcated and properly classified as derivative instruments required to be recorded at fair value (Note 7). The proceeds from the bond have been allocate to the note and conversion / adjustment feature of the convertible bond and recorded at a discount which was amortized to interest expense through conversion. During the years ended December 31, 2013 and 2012, the Company recorded interest expense of $502,465 and $461,279, respectively, for the accretion of the discount on the note.
As of December 31, 2012, there was $1,500,000 outstanding on the convertible bond payable with related accrued interest of $97,497. As of December 31, 2012 the remaining discounts on the bond was $502,465, respectively.
On December 16, 2013, the $1,500,000 convertible bond accrued interest of $179,612 and the $673,736 derivative liability were converted into 174,578 shares of DanDrit Denmark common stock. Such shares of common stock were exchanged for 261,665 shares of common stock of the Company upon the closing of the Share Exchange.
The assumptions used to determine the initial fair value of the conversion feature of the convertible bond were expected volatility of 65%, expected life of two years to eleven months, risk – free interest rates of .41%, and no dividend yield.
NOTE 7 – DERIVATIVE LIABILITIES
The Company does not use derivative financial instruments to hedge exposures to cash-flow, market or foreign-currency risks. However, the Company has entered into certain other financial instruments and contracts, such as debt financing arrangements with features that are either (i) not afforded equity classification, (ii) embody risks not clearly and closely related to host contracts, or (iii) may be net-cash settled by the counterparty. These instruments are required to be carried as derivative liabilities, at fair value.
The fair value of the shares to be issued upon conversion of the bond was recorded as a derivative liability, with the change in the fair value recorded as a gain or loss in the accompanying statement of operations. During the three months March 31, 2014 and 2013 the Company recorded a gain of $0 and $51,456 respectively. On December 16, 2013, the $1,500,000 convertible bond, accrued interest of $179,612 and the $673,736 derivative liability were converted into 174,578 shares of DanDrit Denmark common stock or 261,665 shares of common stock of the Company upon the closing of the Share Exchange.
The following table discloses the fair value of the Company’s derivative liabilities and their location in the balance sheets as of December 31, 2013 and 2012. The Company held no asset derivatives at either reporting date.
|
Liability Derivatives
|
||||||||||
|
December 31, 2013
|
December 31, 2012
|
|||||||||
|
Balance Sheet
Location
|
|
Fair
Value
|
Balance Sheet
Location
|
|
Fair
Value
|
|||||
|
Derivatives not designated as hedging instruments
|
|
|
||||||||
|
Conversion feature on Convertible Bond
|
Derivative Liabilities
|
|
$
|
-
|
|
Derivative Liabilities
|
|
$
|
850,753
|
|
|
|
|
|||||||||
|
Total derivatives not designated as hedging instruments
|
|
$
|
-
|
|
|
$
|
850,753
|
|
||
The following table summarizes liabilities measured at fair value on a recurring basis for the periods presented:
|
December 31, 2013
|
December 31, 2012
|
|||||||||||||||||||||||||||||||
|
Fair Value Measurements Using:
|
Level 1
|
Level 2
|
Level 3
|
Total
|
Level 1
|
Level 2
|
Level 3
|
Total
|
||||||||||||||||||||||||
|
Liabilities
|
|
|
|
|
|
|||||||||||||||||||||||||||
|
Derivative Liabilities
|
- | - | - | - | - | - | $ | 850,753 | $ | 850,753 | ||||||||||||||||||||||
DANDRIT BIOTECH USA, INC.
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 — LEASES
Operating Leases — The Company leases laboratory and production space under operating lease agreements which can be cancelled with 3 month notice. The lease calls for monthly payments of DKK 6,000 (approximately $1,107 at March 31, 2014).
Lease expense charged to operations was $7,255 and $31,775, for the three months ended March 31, 2014 and 2013, respectively. Lease expense charged to operations was $65,702 and $76,969, for the year ended December 31, 2013, and 2012, respectively.
On March 27, 2014 the Company entered into an operating lease agreement for office space from a related party. The lease calls for monthly payments of DKK 10,000 (approximately $1,844), increasing to DKK 20,000 (approximately $3,689) on July 1, 2014. The lease can be terminated by either the Company or the landlord by giving the other 3 months’ notice.
NOTE 9 — INCOME TAXES
The Company accounts for income taxes in accordance with FASB ASC Topic 740, Accounting for Income Taxes; which requires the Company to provide a net deferred tax asset or liability equal to the expected future tax benefit or expense of temporary reporting differences between book and tax accounting and any available operating loss or tax credit carry forwards. The amount of and ultimate realization of the benefits from the deferred tax assets for income tax purposes is dependent, in part, upon the tax laws in effect, the Company’s future earnings, and other future events, the effects of which cannot be determined.
As of March 31, 2014 and December 31, 2013 the Company had net operating loss carry-forwards of approximately $10,200,000 and $9,800,000 for Danish tax purposes which do not expire and Company had net operating loss carry-forwards of approximately $120,000 for U.S. Federal Tax purposes which expire through 2033, a portion of which shall be limited due to the change in control of the Parent.
The Company files U.S. and Danish income tax returns, and they are generally no longer subject to tax examinations for years prior to 2010 and 2007, respectively.
The temporary differences, tax credits and carry forwards gave rise to the following deferred tax asset (liabilities) at March 31, 2014 and December 31, 2013 and 2012:
|
March 31,
2014
|
December 31,
2013
|
December 31,
2012
|
||||||||||
|
Excess of Tax over book depreciation Fixed assets
|
$ | 87,578 | $ | 87,578 | $ | 108,980 | ||||||
|
Excess of Tax over book depreciation Patents
|
114,028 | 114,028 | 8,076 | |||||||||
|
Net Operating Loss Carry forward
|
2,254,812 | 1,642,598 | 1,213,617 | |||||||||
|
Valuation Allowance
|
(2,456,418 | ) | (1,844,204 | ) | (1330,673 | ) | ||||||
|
Total Deferred Tax Asset (Liabilities)
|
$ | - | $ | - | - | |||||||
In accordance with prevailing accounting guidance, the Company is required to recognize and disclose any income tax uncertainties. The guidance provides a two-step approach to recognize and disclose any income tax uncertainties. The guidance provides a two-step approach to recognizing and measuring tax benefits and liabilities when realization of the tax position is uncertain. The first step is to determine whether the tax position meet the more-likely-than-not condition for recognition and the second step is to determine the amount to be recognized based on the cumulative probability that exceeds 50%. The amount of and ultimate realization of the benefits from the deferred tax assets for income tax purposes is dependent, in part, upon the tax laws in effect, the Company’s future earnings, and other future events, the effects of which can be difficult to determine and can only be estimated. Management estimates that it is more likely than not that the Company will not generate adequate net profits to use the deferred tax assets; and consequently, a valuation allowance was recorded for all deferred tax assets.
DANDRIT BIOTECH USA INC
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 — INCOME TAXES (Continued)
A reconciliation of income tax expense at the federal statutory rate to income tax expense at the company’s effective rate is as follows at March 31, 2014 and 2013 and December 31, 2013 and 2012:
|
March 31,
2014
|
March 31,
2013
|
December 31,
2013
|
December 31,
2012
|
|||||||||||||
|
Computed Tax at Expected Statutory Rate
|
$ | (144,858 | ) | $ | (135,684 | ) | $ | (752,882 | ) | $ | (825,401 | ) | ||||
|
Non-US Income Taxed at Different Rates
|
44,858 | 38,059 | 199,292 | 218,488 | ||||||||||||
|
Non-Deductible expenses
|
- | 27,850 | 283,381 | 96,328 | ||||||||||||
|
Valuation allowance
|
100,000 | 69,775 | 270,209 | 510,585 | ||||||||||||
|
Income Tax Expense
|
$ | - | $ | - | - | - | ||||||||||
The components of income tax expense (benefit) from continuing operations for the three months ended March 31, 2014 and 2013 and December 31, 2013 and 2012 consisted of the following
|
Current Tax Expense
|
March 31,
2014
|
March 31,
2012
|
December 31,
2013
|
December 31,
2012
|
||||||||||||
|
Danish Income Tax
|
$ | - | $ | - | $ | - | $ | - | ||||||||
|
Total Current Tax Expense
|
- | - | - | - | ||||||||||||
|
Deferred Income Tax Expense (Benefit)
|
||||||||||||||||
|
Excess of Tax over Book Depreciation Fixed Assets
|
- | - | 26,364 | 10,710 | ||||||||||||
|
Excess of Tax over Book Depreciation Patents
|
- | - | (105,585 | ) | (53,961 | ) | ||||||||||
|
Net Operating Loss Carry forwards
|
(100,000 | ) | (69,775 | ) | (2,319,956 | ) | (1,993,700 | ) | ||||||||
|
Change in the Valuation allowance
|
100,0000 | 69,775 | 2,399,177 | 2,036,951 | ||||||||||||
|
Total Deferred Tax Expense
|
$ | - | $ | - | $ | - | $ | - | ||||||||
Deferred income tax expense / (benefit) results primarily from the reversal of temporary timing differences between tax and financial statement income.
NOTE 10 — LOSS PER SHARE
The following data shows the amounts used in computing loss per share and the effect on income and the weighted average number of shares of potential dilutive common stock for the three month period ended March 31, 2014 and 2013 and December 31, 2013 and 2012:
|
For the Three Months
Ended
March 31,
|
For the Years
Ended
December 31,
|
|||||||||||||||
|
2014
|
2013
|
2013
|
2012
|
|||||||||||||
|
Net (Loss)
|
(426,054 | ) | (399,072 | ) | (2,147,361 | ) | (2,427,649 | ) | ||||||||
|
Weighted average number of shares of common stock used in basic earnings per share
|
7,065,333 | 5,318,151 | 5,332,721 | 5,318,151 | ||||||||||||
|
Effect of dilutive securities, stock options and warrants
|
- | - | - | - | ||||||||||||
|
Weighted average number of shares of common stock and potential dilutive common shares outstanding used in dilutive earnings per share
|
7,065,333 | 5,318,151 | 5,332,721 | 5,318,151 | ||||||||||||
For the three months ended March 31, 2014 and year ended December 31, 2013, the Company had no common stock equivalents. For the three months ended March 31, 2013 and for the year ended December 31, 2012 the Company had a convertible bond wherein the holder could convert the bond and underlying accrued interest into a minimum of 234,683 shares of common stock which were not included in the loss per share computation because their effect would be anti-dilutive.
DANDRIT BIOTECH USA, INC.
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11 — STOCKHOLDERS’ EQUITY
Common Stock — The Company has 100,000,000 authorized shares of common stock $0.0001. As of March 31, 2014 there were 8,040,000 shares issued and outstanding, including 185,053 shares of common stock reserved for issuance to the non-consenting shareholders of DanDrit Denmark and deemed issued and outstanding for accounting purposes.
Share Exchange Agreement/Reverse Acquisition - On February 12, 2014, the Company signed and consummated the transactions contemplated by a Share Exchange Agreement (the "Share Exchange Agreement"), by and among DanDrit USA (formerly known as Putnam Hills Corp.), DanDrit Denmark and N.E. Nielsen, as the representative of the shareholders of DanDrit Denmark, pursuant to which the Company agreed to acquire 100% of the issued and outstanding equity securities in Dandrit Denmark in exchange for 6,000,000 shares of common stock, par value $0.0001 per share of the Company (the “Share Exchange”). The Share Exchange closed on February 12, 2014 pursuant to which holders of approximately 97% of the issued and outstanding capital stock of DanDrit Denmark (the “DanDrit Consenting Holders”) exchanged an aggregate of 3,879,624 equity interests of DanDrit Denmark for 5,814,947 shares of DanDrit USA. As a result of the closing of the Share Exchange the Company became the parent of DanDrit Denmark. In accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark, DanDrit Denmark shareholders who have not consented to the Share Exchange (the “Non-Consenting Shareholders”) and therefore have not exchanged such DanDrit Denmark shareholder’s equity interests in DanDrit Denmark for shares of DanDrit USA, are entitled to receive up to an aggregate of 185,053 shares of common stock of DanDrit USA in exchange for the DanDrit Denmark equity interests owned by such Non-Consenting Shareholders. As a result of the Share Exchange, the former shareholders of Dandrit Denmark became the controlling shareholders of the Company. The Share Exchange was accounted for as a reverse takeover/recapitalization effected by a share exchange, wherein Dandrit Denmark is considered the acquirer for accounting and financial reporting purposes. The capital, share price, and earnings per share amount in these consolidated financial statements for the period prior to the reverse merger were restated to reflect the recapitalization in accordance with the exchange ratio established in the merger.
On February 11, 2014 1,400,000 and 40,000 common shares of the Company were issued for consulting and legal services valued at $5 per share or $7,000,000 and $200,000, respectively. These shares were issued by the Company prior to the reverse acquisition of DanDrit Denmark and the $7,200,000 was closed out to additional paid in capital in connection with the recapitalization of the Subsidiary.
On February 12, 2014, the Company’s majority shareholder cancelled 4,400,000 shares of common stock.
Upon the closing of the Share Exchange, the Company and the its majority shareholder immediately prior to the closing agreed to cancel up to 4,400,000 shares of our common stock. In addition, following the closing of the Share Exchange, DanDrit Biotech USA, Inc., a wholly owned subsidiary of the Company merged with and into the Company, thereby changing the Company’s name to “DanDrit Biotech USA, Inc.”
On December 16, 2013, DanDrit Denmark issued 174,578 shares of its common stock which were exchanged for 261,665 shares of common stock of the Company upon the closing of the Share Exchange in payment of a $1,500,000 convertible bond, $179,612 of accrued interest and the remaining $673,736 of derivative liability associated with the conversion feature of the bond.
On December 16, 2013, DanDrit Denmark issued 96,288 shares of its common stock which were exchanged for 144,321 shares of common stock of the Company upon the closing of the Share Exchange in payment of $926,386 of notes payable and related accrued interest payable to DKTI.
On December 16, 2013, DanDrit Denmark issued 184,051 shares of its common stock which were exchanged for 275,863 shares of common stock of the Company upon the closing of the Share Exchange in payment of $1,770,757 of notes payable and related accrued interest payable to Sune Olsen Holdings ApS and Advance Biotech Invest AS.
Voting- Holders of the Company’s common stock are entitled to one vote for each share held of record on each matter submitted to a vote of stockholders, including the election of directors, and do not have any right to cumulate votes in the election of directors.
Dividends- Holders of the Company’s common stock are entitled to receive ratably such dividends as our Board of Directors from time to time may declare out of funds legally available.
Liquidation Rights- In the event of any liquidation, dissolution or winding-up of affairs of the Company, after payment of all of our debts and liabilities, the holders of the Company’s common stock will be entitled to share ratably in the distribution of any of our remaining assets.
Other Matters- Holders of the Company’s common stock have no conversion, preemptive or other subscription rights, and there are no redemption rights or sinking fund provisions with respect to the common stock. All of the issued and outstanding shares of common stock on the date of this report are validly issued, fully paid and non-assessable.
DANDRIT BIOTECH USA INC
(FORMERLY PUTNAM HILLS CORP.)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12 — COMMITMENTS AND CONTINGENCIES
Shares held for non-consenting shareholders – In connection with the Share Exchange agreement certain shareholders of Dandrit Denmark had not been identified or did not consent to the exchange of shares. In accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark, the Non-Consenting Shareholders that did not exchange the DanDrit Denmark equity interests owned by such Non-Consenting Shareholders for shares of the Company, will be entitled to receive up to 185,053 shares of common stock of the Company that each such Non-Consenting Shareholder would have been entitled to receive if such shareholder had consented to the Share Exchange. The 185,053 shares have been reflected as issued and outstanding in the accompanying financial statements.
Patient Name Use Program - On December 16, 2013, DanDrit Biotech A/S entered into an agreement with MyTomorrows (MT), a Dutch company, regarding a Patient Name Use Program (PNU) for the Company’s MelCancerVac (MCV). This program will allow DanDrit Biotech A/S to sell MCV for a year of treatment (10 vaccines) to cancer patients through MT. MT offers a worldwide online platform providing access to non-registered medicines for patients with life threatening diseases. MT is a turnkey solution and will be in charge of regulatory, recruitment, logistics, and pharmacovigilance. The Company will pay MT a royalty on a country to country basis for 20 years on MCV sales sold under the agreement. Either party may terminate the agreement with 180 day written notice.
Food and Drug Administration (FDA) - The FDA has extensive regulatory authority over biopharmaceutical products (drugs and biological products), manufacturing protocols and procedures and the facilities in which they will be manufactured. Any new bioproduct intended for use in humans is subject to rigorous testing requirements imposed by the FDA with respect to product efficacy and safety, possible toxicity and side effects. FDA approval for the use of new bioproducts (which can never be assured) requires several rounds of extensive preclinical testing and clinical investigations conducted by the sponsoring pharmaceutical company prior to sale and use of the product. At each stage, the approvals granted by the FDA include the manufacturing process utilized to produce the product. Accordingly, the Company’s cell systems used for the production of therapeutic or biotherapeutic products are subject to significant regulation by the FDA under the Federal Food, Drug and Cosmetic Act, as amended.
Product liability -The contract production services for therapeutic products offered exposes an inherent risk of liability as biotherapeutic substances manufactured, at the request and to the specifications of customers, could foreseeably cause adverse effects. The Company seeks to obtain agreements from contract production customers indemnifying and defending the Company from any potential liability arising from such risk. There can be no assurance, however, that the Company will be successful in obtaining such agreements in the future or that such indemnification agreements will adequately protect the Company against potential claims relating to such contract production services. The Company may also be exposed to potential product liability claims by users of its products. A successful partial or completely uninsured claim against the Company could have a material adverse effect on the Company’s operations.
Employment Agreements -The Company and its Subsidiary have employment agreements with officers of the Company.
Contingencies - The Company is from time to time involved in routine legal and administrative proceedings and claims of various types. While any proceedings or claim contains an element of uncertainty, management does not expect a material impact on our results of operations or financial position.
During 2013, the Danish law firm Horten made a claim of DKK 184,144 ($33,421), including accrued interest, against DanDrit Biotech A/S related to services performed for a former shareholder who was selling his shares. DanDrit Biotech A/S did not engage Horten nor did the Company request the services of Horten. Horten submitted the invoices only after Horten went into bankruptcy. Management intends to vigorously defend against any claims made by Horton.
NOTE 13 — DISPOSITION
On December 16, 2013, DanDrit Denmark sold its dormant Singapore subsidiary, DanDrit Corporation PTE, LTD, for $1 and the accompanying financial statements of the Company include the $1 proceeds and gain on sale of the former subsidiary.
NOTE 14 — SUBSEQUENT EVENT
On April 29, 2014, the terms of the loan with Paseco ApS were amended to provide that the Company may extend the term of the loan for 1 year by giving Paseco written notice by December 31, 2014. Per the terms of the amendment, should the Company extend the notes, the interest rate will increase to 7.00% per annum.
DanDrit Denmark received a loan commitment on May 2, 2014 for 2,000,000 DKK ($36,868) from Paseco ApS, a shareholder of the Company, payable by February 1, 2015. The Company has an option to extend the loan for one year by giving notice to Paseco by December 31, 2014 whereby the interest rate would increase to 7.00% per annum.

Up to 2,400,000 Shares
DanDrit Biotech USA, Inc.
Common Stock
PROSPECTUS DATED ,
[Alternate Page for Resale Prospectus]
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
PROSPECTUS Subject to Completion, Dated August 8, 2014

DANDRIT BIOTECH USA, INC.
Up to 40,000 Shares of Common Stock
This registration statement of DanDrit Biotech USA, Inc. (the “Company”) and the prospectus contained herein register the resale (the “Resale”) of an aggregate of 40,000 shares of our common stock (the “Resale Shares”) owned by one (1) selling security holder (the “Security Holder”) described more fully herein.
The Security Holder is offering the Resale Shares of our common stock, par value $0.0001 per share (the “Common Stock”) pursuant to this prospectus, and the registration statement of which it is a part. The Security Holder will receive all of the proceeds from the sale of the Resale Shares and we will receive none of those proceeds. The Security Holder is offering our Common Stock at an initial price of $5.00 until and unless our shares are traded on a national securities exchange or quoted on the OTCBB or OTCQB markets (which we can provide no assurance will be accomplished) and thereafter at prevailing market prices or privately negotiated prices.
The Security Holder and intermediaries through whom the Resale Shares are sold may be deemed “underwriters” within the meaning of the Securities Act of 1933, as amended (the “Securities Act”), with respect to the Resale Shares offered hereby, and any profits realized or commissions received may be deemed underwriting compensation.
Prior to the Resale, which shall be commenced upon the expiration of the lock-up period as described in the Plan of Distribution, we have an offering (the “Public Offering”) of our Common Stock that will have a dilutive effect on any purchaser of Common Stock under this prospectus and the registration statement of which it is a part. We are conducting an offering pursuant to another prospectus (the “Public Offering Prospectus”), which registers the sale by the Company of up to 2,400,000 shares (the “Public Offering Shares”) of our Common Stock, which will be sold by Sunrise Securities Corp. and The Benchmark Company, LLC (each a “Placement Agent” and, collectively, the “Placement Agents”). The Placement Agents are not required to sell any specific number or dollar amount of securities but will use their best efforts to sell the Public Offering Shares. If we sell all of the Public Offering Shares we are offering, we will pay to the Placement Agents up to $840,000, or 7%, of the gross proceeds received in the Public Offering; provided, however, that the Placement Agents will only receive commissions based on the gross proceeds raised from investors introduced to the Company by the Placement Agents. In addition, we will pay accountable expenses of up to $120,000 or 1% of the gross proceeds received in the Public Offering. We have also agreed to reimburse the Placement Agent for reasonable fees and expenses of counsel up to an aggregate amount of $75,000 in connection with the Public Offering. The sales of the Public Offering Shares are not covered by this prospectus. As such, there are a total of 3,182,252 shares of our Common Stock registered for sale or resale under this and the other prospectus referred to above, although there is no guarantee that all of the shares will be sold or resold.
We are an “emerging growth company” under the federal securities laws and will have the option to use reduced public company reporting requirements. Investing in our common stock involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page 7 of this prospectus, and under similar headings in any amendments or supplements to this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2014.
[Alternate Page for Resale Prospectus]
TABLE OF CONTENTS
|
Page
|
|
|
1
|
|
|
7
|
|
|
9
|
|
|
23
|
|
|
24
|
|
|
26
|
|
|
27
|
|
|
28
|
|
|
29
|
|
|
36
|
|
|
62
|
|
|
64
|
|
|
66
|
|
|
68
|
|
|
70
|
|
|
71
|
|
|
72
|
|
|
73
|
|
|
74
|
|
| 76 | |
|
77
|
|
|
80
|
|
|
80
|
|
|
80
|
|
|
80
|
Dealer Prospectus Delivery Obligation
Until , 2014 (90 days after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as a placement agent and with respect to any unsold allotments or subscriptions.
[Alternate Page for Resale Prospectus]
USE OF PROCEEDS
There will not be any proceeds from the sale of the Resale Shares by the Security Holder. All proceeds from the sale of the Resale Shares will be paid directly to the selling Security Holder.
SHARES REGISTERED FOR RESALE
As part of this prospectus, we are registering 40,000 shares of Common Stock for resale, which are subject to a 180 day lock-up for sales into the public market. The shares described in the following table consist of shares of Common Stock that were issued in connection with the Share Exchange. A discussion of the material terms of this transaction are included in the subsection of this prospectus titled “Share Exchange” under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” The Company, by agreement, granted the Security Holder the right to have the Security Holder’s Common Stock registered in the event that the Company filed an initial registration statement for the sale of its securities to the public.
Concurrently with the filing of this Resale Prospectus, we are filing the Public Offering Prospectus for an initial public offering of our Common Stock. As a result, we became obligated to register the Security Holder’s shares for resale under the same registration statement.
The Security Holder does not have, nor within the past three years has the Security Holder had, any position, office or other material relationship with us or any of our predecessors or affiliates other than as described in the section of this prospectus titled “Related Party Transactions” and as a result of the ownership of our securities.
The following table provides certain information with respect to the Security Holder’s ownership of our securities as of July 1, 2014, the total number of securities the Security Holder may distribute under this prospectus from time to time, and the number of securities the Security Holder will own thereafter assuming no other acquisitions or dispositions of our securities. The Security Holder may distribute all, some or none of their securities, thus we have no way of determining the number of securities the Security Holder will hold after the Resale. Therefore, we have prepared the table below on the assumption that the Security Holder will sell all shares covered by this prospectus.
The Security Holder may dividend or distribute their shares from time to time to their shareholders or affiliates. The Security Holder may also transfer shares they own by gift, and upon any such transfer the donee would have the same right of resale as the Security Holder.
See our discussion titled “Plan of Distribution” for further information regarding the Security Holder’s method of distribution of these shares.
Security Holder Table
|
Name
|
Securities
Beneficially
Owned Prior
to
Offering (1)
|
Securities Being
Offered
|
Securities
Beneficially
Owned After
Offering (2)
|
% Beneficial
Ownership
After
Offering
|
|||||||
|
Troutman Sanders LLP (3)
|
40,000
|
40,000
|
0
|
0
|
%
|
||||||
|
Total:
|
40,000
|
40,000
|
0
|
0
|
%
|
||||||
|
(1)
|
The Security Holder listed in the table below acquired the securities being offered in connection with the Share Exchange. Percentages stated in the above table are based on a total of 8,040,000 shares of Common Stock outstanding as of March 31, 2014, including 185,053 shares of common stock reserved for issuance to the non-consenting shareholders of DanDrit Denmark and deemed issued and outstanding for accounting purposes.
|
|
(2)
|
Assumes that all of the shares offered hereby are resold and that shares owned before the offering but not offered hereby are not sold.
|
|
(3)
|
The address of this security holder is 405 Lexington Ave., New York, NY 10174. Troutman Sanders LLP is counsel to the Placement Agents in the Offering.
|
PLAN OF DISTRIBUTION
Lock-Up Agreements
The Security Holder, counsel to the Placement Agents in connection with the Public Offering, acquired the Resale Shares on February 12, 2014 upon the closing of the Share Exchange, which shares have been deemed compensation by FINRA and are, therefore, subject to a 180-day lockup pursuant to FINRA Rule 5110(g)(1). Pursuant to FINRA Rule 5110(g)(1), the Resale Shares shall not be sold during the Public Offering, or sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of the Public Offering, subject to certain exceptions set forth in FINRA Rule 5110(g)(2).
We and our officers, directors, and certain existing stockholders, have agreed, subject to certain exceptions, not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any shares of our common stock or other securities convertible into or exercisable or exchangeable for shares of our common stock after the effective date of the registration statement of which this prospectus is a part without the prior written consent of Sunrise Securities Corp. The lock-up period is for 180 days beginning as of the filing date of the last amendment to the registration statement of which this prospective is a part that is declared effective. Notwithstanding the foregoing, the lock-up period shall not apply, and the parties may transfer the shares in the transactions described in clauses (i) through (vi) below as follows:
|
(i)
|
as a bona fide gift or gifts;
|
|
|
(ii)
|
to any trust for the direct or indirect benefit of the undersigned or the immediate family of the transferring party;
|
|
|
(iii)
|
to the transferring party and/or any member of the immediate family of the transferring party from or by a grantor retained (or like-kind) annuity trust which exists as of the date hereof and was established for the direct or indirect benefit of the transferring party and/or any member of the immediate family of the transferring party pursuant to the terms of such trust
|
|
|
(iv)
|
if the transferring party is a corporation, partnership or other business entity (A) to another corporation, partnership or other business entity that is an affiliate (as defined under Rule 12b-2 of the Exchange Act) of the transferring party or (B) any distribution or dividend to equity holders of the transferring party as part of a distribution or dividend by the transferring party (including upon the liquidation and dissolution of the transferring party pursuant to a plan of liquidation approved by the transferring party 's equity holders), or if the transferring party is a trust, to a grantor or beneficiary of the trust;
|
|
|
(v)
|
in the event of a default under a pledge which exists as of the date hereof as security for a margin or loan account pursuant to the terms of such account;
|
|
|
(vi)
|
pursuant to any 10b5-l trading plans in effect as of the date of the Offering; or
|
|
|
(vii)
|
with the prior written consent of Sunrise Securities Corp., one of the Placement Agents.
|
For purposes of this lock-up agreement, “immediate family” shall mean any relationship by blood, marriage or adoption, not more remote than first cousin.
Furthermore, notwithstanding the foregoing, during the lock-up period, the transferring party may sell shares of common stock of DanDrit on the open market following the consummation of the offering if and only if (i) such sales are not required to be reported in any public report or filing with the Securities Exchange Commission, or otherwise and (ii) the transferring party does not otherwise voluntarily effect any public filing or report regarding such sales.
Resale
Following the expiration of the lock-up period, the Security Holder and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of Common Stock on the Over-the-Counter Bulletin Board, Nasdaq Capital Market or any other stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. These individuals may use any one or more of the following methods when selling shares:
|
●
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
●
|
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
|
●
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
●
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
●
|
privately negotiated transactions;
|
|
|
●
|
settlement of short sales entered into after the effective date of the registration statement of which this prospectus is a part;
|
|
|
●
|
broker-dealers may agree with the selling security holder to sell a specified number of such shares at a stipulated price per share;
|
|
|
●
|
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
|
|
|
●
|
a combination of any such methods of sale; or
|
|
|
●
|
any other method permitted pursuant to applicable law.
|
Each Security Holder may distribute the shares of which it is the owner by means of a dividend or other form of distribution, including in connection with a declaration of a dividend or distribution, reorganization, combination, consolidation and dissolution.
The Security Holder may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by any selling Security Holder may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling Security Holder (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated, but the maximum amount of compensation to be received by any participating FINRA member may not exceed 8%. We are required to pay certain fees and expenses incurred by us incident to the registration of the Resale Shares.
Since the Security Holder may be deemed to be an “underwriter” within the meaning of the Securities Act, the Security Holder will be subject to the prospectus delivery requirements of the Securities Act including Rule 172 thereunder. There is no underwriter or single coordinating broker acting in connection with the proposed sale of the Resale Shares by the Security Holder.
We agreed to keep this prospectus and the registration statement which this prospectus forms a part effective until the earlier to occur of (i) such time as Rule 144 or another similar exemption under the Securities Act is available for the sale of all the Resale Shares, to the extent the Security Holder has distributed the Resale Shares to its shareholders, by its shareholders, without volume or manner of sale restrictions during a three month period without registration or (ii) all of the Resale Shares have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The Resale Shares will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the Resale Shares may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the Resale Shares may not simultaneously engage in market making activities with respect to the Common Stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Security Holder will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of shares of the Common Stock by any person. We will make copies of this prospectus available to the Security Holder and have informed the Security Holder of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
[Alternate Page for Resale Prospectus]
Validity of the securities offered by this prospectus will be passed upon for us by Richardson & Patel, LLP, New York, New York.
Putnam historically retained Raich Ende Malter & Co. LLP (“Raich”) as its principal accountant. In connection with the closing of the Share Exchange, we terminated Raich and retained Gregory & Associates, LLC (“Gregory”) as our principal accountant. Our Board of Directors approved the change.
Raich’s reports on the financial statements for the years ended March 31, 2013 and 2012 included in the Form 10-K for the year ended March 31, 2013 as filed with the SEC did not contain an adverse opinion or a disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope, or accounting principles, except that their reports included disclosure of uncertainty regarding Putnam’s ability to continue as a going concern.
From inception through February 14, 2014, Putnam had no disagreements with Raich on matters of accounting principles or practices, financial statement disclosure, or auditing scope or procedure. Putnam had not consulted with Gregory on any matter prior to the Share Exchange.
We have authorized Raich to respond fully to the inquiries of Gregory concerning any matters discussed above. We have provided Raich with a copy of the above statements. We have requested that Raich furnish us with a letter addressed to the SEC stating whether Raich agrees with the above statements and, if not, stating the respects in which it does not agree. A copy of such letter from Raich is filed as an exhibit to the registration statement of which this prospectus forms a part.
The audited financial statements of DanDrit Denmark as of December 31, 2013 and December 31, 2012 included in this prospectus have been audited by Gregory & Associates, LLC, who is an independent registered public accounting firm, as set forth in their report appearing elsewhere herein, and are included herein in reliance upon such report given upon the authority of said firm as experts in auditing and accounting.
We file annual, quarterly and special reports and other information with the SEC. These filings contain important information that does not appear in this prospectus. For further information about us, you may read and copy any reports, statements and other information filed by us at the SEC’s Public Reference Room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549-0102. You may obtain further information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Our SEC filings are also available on the SEC Internet site at http://www.sec.gov, which contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
[Alternate Page for Resale Prospectus]

Up to Shares
DanDrit Biotech USA, Inc.
Common Stock
PROSPECTUS DATED ,
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. Other Expenses of Issuance and Distribution |
The following table sets forth the various costs and expenses payable by us in connection with the sale of the securities being registered. All such costs and expenses shall be borne by us. Except for the SEC registration fee, all the amounts shown are estimates.
|
Amount
to be Paid
|
||||
|
SEC registration fee
|
$
|
1,571.36
|
||
|
FINRA filing fee
|
3,600.09
|
|||
|
Legal fees and expenses
|
175,000
|
|||
|
Accounting fees and expenses
|
60,000
|
|||
|
Printing and miscellaneous expenses
|
14,364.55
|
|||
|
Total
|
$
|
254,536
|
||
| Item 14. Indemnification of Directors and Officers |
Section 145 of the Delaware General Corporation Law (the “DGCL”) provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses including attorneys’ fees, judgments, fines and amounts paid in settlement in connection with various actions, suits or proceedings, whether civil, criminal, administrative or investigative other than an action by or in the right of the corporation, a derivative action, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, if they had no reasonable cause to believe their conduct was unlawful. A similar standard is applicable in the case of derivative actions, except that indemnification only extends to expenses including attorneys’ fees incurred in connection with the defense or settlement of such actions, and the statute requires court approval before there can be any indemnification where the person seeking indemnification has been found liable to the corporation. The statute provides that it is not exclusive of other indemnification that may be granted by a corporation’s certificate of incorporation, bylaws, agreement, a vote of stockholders or disinterested directors or otherwise.
DanDrit’s Certificate of Incorporation provides that it will indemnify and hold harmless, to the fullest extent permitted by Section 145 of the DGCL, as amended from time to time, each person that such section grants us the power to indemnify.
The DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability for:
|
●
|
any breach of the director’s duty of loyalty to the corporation or its stockholders;
|
|
|
●
|
acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law;
|
|
|
●
|
payments of unlawful dividends or unlawful stock repurchases or redemptions; or
|
|
|
●
|
any transaction from which the director derived an improper personal benefit.
|
DanDrit’s Certificate of Incorporation provides that, to the fullest extent permitted by applicable law, none of our directors will be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director. Any repeal or modification of this provision will be prospective only and will not adversely affect any limitation, right or protection of a director of our company existing at the time of such repeal or modification.
| Item 15. Recent Sales of Unregistered Securities |
On May 26, 2011, the Company issued an aggregate of 5,000,000 shares of Common Stock to NLBDIT 2010 Services, LLC (“NLBDIT Services”) for an aggregate purchase price of $25,000 pursuant to the terms and conditions set forth in that certain common stock purchase agreement (the “NLBDIT Services CSPA”). The Low Trust owns 100% of the outstanding membership interests of NLBDIT Services and may be deemed to beneficially own the shares of Common Stock held of record by NLBDIT Services. Nathan Low, a principal of our majority shareholder prior to the Share Exchange, is the family trustee of the Low Trust and has voting and dispositive control over any securities owned of record or beneficially by the Low Trust. Therefore, Mr. Low may be deemed to beneficially own the shares of Common Stock held of record by NLBDIT Services and beneficially by the Low Trust. The Company issued these shares of Common Stock under the exemption from registration provided by Section 4(a)(2) of the Securities Act.
On December 1, 2011 DanDrit Denmark borrowed $1,500,000 and issued 6% convertible bonds. The bonds may not be converted during the four weeks following the publication of the annual report. The bond may not be repaid until the bonds expiration on November 30, 2014. The bonds shall not accrue interest after expiration. The bonds and related accrued interest are convertible into common share of the Company at an initial rate of $9.58 per common share.
Service Issuances
On February 11, 2014 the Company issued a total of 40,000 shares of its common stock to its legal counsel in consideration for legal services in connection with the filing of the registration statement of which this prospectus is a part.
On February 11, 2014 the Company entered into that certain Consulting Agreement with Paseco ApS (the “Consultant”), pursuant to which the Company issued 1,400,000 shares of the Company’s common stock to certain designees of the Consultant in consideration of advisory and consulting services provided by the Consultant in connection with the Share Exchange and the filing of the registration statement of which this prospectus is a part.
Share Exchange
On February 12, 2014, the Company signed and consummated the transactions contemplated by a Share Exchange Agreement (the "Share Exchange Agreement"), by and among DanDrit USA (formerly known as Putnam Hills Corp.),
DanDrit Denmark and N.E. Nielsen, as the representative of the shareholders of DanDrit Denmark, pursuant to which the Company agreed to acquire 100% of the issued and outstanding equity securities in Dandrit Denmark in exchange for 6,000,000 shares of common stock, par value $0.0001 per share of the Company (the “Share Exchange”). The Share Exchange closed on February 12, 2014 pursuant to which holders of approximately 97% of the issued and outstanding capital stock of DanDrit Denmark (the “DanDrit Consenting Holders”) exchanged an aggregate of 3,879,624 equity interests of DanDrit Denmark for 5,814,947 shares of DanDrit USA. As a result of the closing of the Share Exchange the Company became the parent of DanDrit Denmark. In accordance with Section 70 of the Danish Companies Act and the Articles of Association of DanDrit Denmark, DanDrit Denmark shareholders who have not consented to the Share Exchange (the “Non-Consenting Shareholders”) and therefore have not exchanged such DanDrit Denmark shareholder’s equity interests in DanDrit Denmark for shares of DanDrit USA, are entitled to receive up to an aggregate of 185,053 shares of common stock of DanDrit USA in exchange for the DanDrit Denmark equity interests owned by such Non-Consenting Shareholders. As a result of the Share Exchange, the former shareholders of Dandrit Denmark became the controlling shareholders of the Company. The Share Exchange was accounted for as a reverse takeover/recapitalization effected by a share exchange, wherein Dandrit Denmark is considered the acquirer for accounting and financial reporting purposes. The capital, share price, and earnings per share amount in these consolidated financial statements for the period prior to the reverse merger were restated to reflect the recapitalization in accordance with the exchange ratio established in the merger.
Upon the closing of the Share Exchange, the Company and the its majority shareholder immediately prior to the closing agreed to cancel up to 4,400,000 share of common stock. In addition, following the closing of the Share Exchange, the wholly owned subsidiary of the company formed solely for the purposes of changing the company’s name, Dandrit Biotech USA, Inc., merged with and into the company and the company adopted the name of its wholly owned subsidiary “DanDrit Biotech USA, Inc.”
As a result of the Share Exchange, we changed our management and reconstituted our board of directors. As of the effective time of the Share Exchange, Samir Masri, the Chief Executive Officer, Chief Financial Officer, President, Secretary and sole director of Putnam resigned as the sole officer and director of the company and appointed NE Nielsen, Dr. Jacob Rosenberg, Dr. Eric Leire, Aldo Petersen and Robert E. Wolfe as directors of Putnam, and Dr. Eric Leire as Chief Executive Officer and President and Mr. Wolfe as Chief Financial Officer, Treasurer and Secretary.
We believe that the above issuances of securities were exempt from registration under the Securities Act of 1933, as amended (the “Act”), pursuant to Section 4(2) of the Act or Regulation S promulgated under the Act in that the securities were offered and sold to accredited investors or foreign persons and the Company did not engage in any general advertisement, general solicitation or directed selling efforts in connection with the offering of the securities.
| Item 16. Exhibits |
A list of exhibits filed with this registration statement on Form S-1 is set forth on the Exhibit Index and is incorporated in this Item 16 by reference.
Item 17. Undertakings
(1) Insofar as indemnification for liabilities arising under the Securities Act may be permitted as to directors, officers and controlling persons of the registrant pursuant to the provisions described in Item 14, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes:
(2) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increases or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(3) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(4) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(5) That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(6) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: (i) any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; (ii) any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; (iii) the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and (iv) any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(7) To provide to the underwriter at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
(8) That, for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(9) That, for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Gentofte, Denmark, on the 8th day of August, 2014.
| DANDRIT BIOTECH USA, INC. | ||||
|
By:
|
/s/ Dr. Eric Leire
|
By:
|
/s/ Robert E. Wolfe
|
|
|
Dr. Eric Leire
|
Robert E. Wolfe
|
|||
|
Chief Executive Officer and President
|
Chief Financial Officer, Treasurer and Secretary
|
|||
|
(Principal Executive Officer)
|
(Principal Financial and Accounting Officer)
|
|||
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
||
|
/s/ Dr. Eric Leire
|
Chief Executive Officer and Director
|
August 8, 2014
|
||
|
Dr. Eric Leire
|
(Principal Executive Officer)
|
|||
|
/s/ Robert E. Wolfe
|
Chief Financial Officer, Treasurer, Secretary and Director
|
August 8, 2014
|
||
|
Robert E. Wolfe
|
(Principal Accounting Officer)
|
|||
|
/s/ NE Nielsen*
|
Chairman of the Board
|
August 8, 2014
|
||
|
NE Nielsen
|
||||
|
/s/ Dr. Jacob Rosenberg*
|
Director
|
August 8, 2014
|
||
|
Dr. Jacob Rosenberg
|
||||
|
/s/ Aldo Michael Noes Petersen*
|
Director
|
August 8, 2014
|
||
|
Aldo Michael Noes Petersen
|
||||
*Pursuant to a Power of Attorney
|
Exhibit Index
|
|
Exhibit
No.
|
Description
|
|
|
1.1
|
Form of Placement Agent Agreement. (1)
|
|
|
|
||
|
2.1
|
Agreement and Plan of Share Exchange dated February 12, 2014. (4)
|
|
|
2.2
|
Share Cancellation Agreement dated February 12, 2014. (4)
|
|
|
3.1
|
Certificate of Incorporation. (9)
|
|
|
3.2
|
Bylaws. (5)
|
|
|
3.3
|
Articles of Association of DanDrit Denmark, as amended, dated February 26, 2004. (4)
|
|
|
3.4
|
Certificate of Ownership and Merger, dated February 12, 2014. (1)
|
|
|
4.1
|
Form of Common Stock Certificate. (3)
|
|
|
4.2
|
Form of Lock-Up Agreement. (7)
|
|
|
5.1
|
Form of Opinion of Richardson and Patel, LLP. (2)
|
|
|
10.1
|
Intellectual Property Assignment by and between DanDrit Denmark and Alexei Kirkin dated June 5, 2002. (4)
|
|
|
10.2
|
Collaboration Agreement by and between DanDrit Denmark and National Cancer Centre of Singapore Pte Ltd dated November 11, 2008. (4)
|
|
|
10.3
|
Master Services Agreement by and between DanDrit Denmark and Aptiv Solutions (UK) Ltd dated October 11, 2011. (4)
|
|
|
10.4
|
Employment Agreement by and between DanDrit Denmark and Dr. Eric Leire dated February 5, 2012. (4)
|
|
|
10.5
|
Box Packing and Moving Agreement by and between DanDrit Denmark and Bryde & Sonner A/S dated May 23, 2012. (4)
|
|
|
10.6
|
Debt Instrument by and between DanDrit Denmark and Sune Olsen Holding ApS dated March 31, 2013. (4)
|
|
|
10.7
|
Lease Agreement by and between Symbion A/S and DanDrit Denmark dated July 8, 2013. (4)
|
|
|
10.8
|
Lease Agreement by and between Ordnung ApS and DanDrit Denmark. (4)
|
|
|
10.9
|
Debt Instrument by and between DanDrit Denmark and Sune Olsen Holding ApS dated January 17, 2014. (4)
|
|
|
10.10
|
Consultancy Agreement by and between DanDrit Denmark and Dina Rosenberg dated February 4, 2014. (7)
|
|
|
10.11
|
Consulting Agreement by and between DanDrit Denmark and Paseco ApS dated February 11, 2014. (5)
|
|
|
10.12
|
DanDrit Biotech USA, Inc. 2014 Equity Incentive Plan. (7)
|
|
|
10.13
|
Loan Agreement by and between DanDrit Denmark and Paseco ApS dated March 21, 2014. (7)
|
|
|
10.14
|
Lease Agreement by and between Paseco ApS and DanDrit Denmark dated March 27, 2014. (7)
|
|
10.15
|
Confidential Disclosure Agreement by and between Etablissement Francais Du Sang and DanDrit Biotech A/S, dated March 8, 2013. (3)
|
|
|
10.16
|
Loan Agreement by and between DanDrit Denmark and Paseco ApS dated April 29, 2014. (8)
|
|
|
10.17
|
Letter of Support of Paseco ApS related to committed 2M DKK in additional financing, dated May 2, 2014. (8)
|
|
|
10.18
|
Early Access Agreement by and between DanDrit Denmark and Impatients, N.V. dated December 20, 2013. (4)
|
|
|
10.19
|
CFO Service Agreement by and between DanDrit Denmark and Mr. Robert Wolfe dated February 10, 2014. (2)
|
|
|
14.1
|
Code of Ethics. (6)
|
|
|
16.1
|
Letter from Raich Ende Malter & Co. LLP, dated February 14, 2014, to the SEC. (4)
|
|
|
21.1
|
List of Subsidiaries. (4)
|
|
|
23.1
|
Consent of Gregory & Associates, LLC.*
|
|
|
23.2
|
Consent of Richardson & Patel LLP (included in Exhibit 5.1). (2)
|
|
|
24.1
|
Powers of Attorney of the Directors and Officers of the Registrant (included in signature pages). (4)
|
|
|
99.1
|
Form of Subscription Agreement.(1)
|
|
|
101.1INS
|
XBRL Instant Document(2)
|
|
|
101.1SCH
|
XBRL Taxonomy Extension Schema Document(2)
|
|
|
101.1CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document(2)
|
|
|
101.1DEF
|
XBRL Taxonomy Extension Definition Linkbase Document(2)
|
|
|
101.1LAB
|
XBRL Taxonomy Extension Label Linkbase Document(2)
|
|
|
101.1PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document(2)
|
|
*
|
Filed herewith.
|
|
(1)
|
Filed as an Exhibit to the Company’s Form S-1/A filed with the SEC on July 3, 2014 and incorporated herein by reference.
|
|
(2)
|
Filed as an exhibit to the Company’s Form S-1/A filed with the SEC on June 23, 2014 and incorporated herein by reference.
|
|
(3)
|
Filed as an exhibit to the Company’s Form S-1/A filed with the SEC on May 16, 2014 and incorporated herein by reference.
|
|
(4)
|
Filed as an exhibit to the Company’s Form S-1 filed with the SEC on February 14, 2014 and incorporated herein by reference.
|
|
(5)
|
Filed as an exhibit to the Company’s Form 10 filed with the SEC on August 12, 2011 and incorporated herein by reference.
|
|
(6)
|
Filed as an exhibit to the Company’s Annual Report on Form 10-K filed with the SEC on July 17, 2012 and incorporated herein by reference.
|
|
(7)
|
Filed as an exhibit to the Company’s Form S-1/A filed with the SEC on March 31, 2014 and incorporated herein by reference.
|
|
(8)
|
Filed as an exhibit to the Company’s Form 10-Q for the quarter ended March 31, 2014 filed with the SEC on May 14, 2014 and incorporated herein by reference.
|
|
(9)
|
Filed as an exhibit to the Company’s Form 10 filed with the SEC on August 12, 2011 and incorporated herein by reference.
|
II-6
