Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a14-18616_18k.htm |
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a14-18616_1ex99d1.htm |
|
|
2Q14 Financial Results Conference Call & Webcast August 7, 2014 Exhibit 99.2 |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus’ candidate drug products, cash runway, ongoing collaborations and the timing and reporting of results from clinical trials evaluating Amicus’ candidate drug products. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2013. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. |
|
|
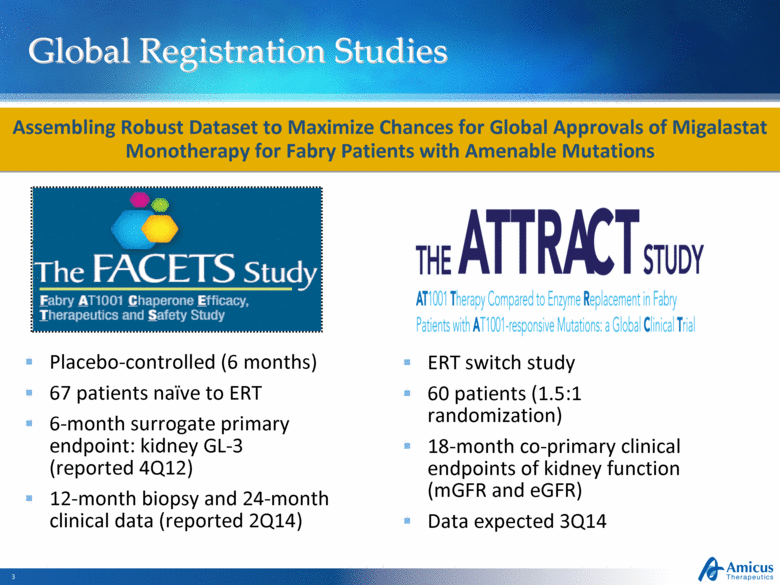
Global Registration Studies Placebo-controlled (6 months) 67 patients naïve to ERT 6-month surrogate primary endpoint: kidney GL-3 (reported 4Q12) 12-month biopsy and 24-month clinical data (reported 2Q14) ERT switch study 60 patients (1.5:1 randomization) 18-month co-primary clinical endpoints of kidney function (mGFR and eGFR) Data expected 3Q14 Assembling Robust Dataset to Maximize Chances for Global Approvals of Migalastat Monotherapy for Fabry Patients with Amenable Mutations |
|
|
Recap of Study 011 12- and 24-Month Data: Key Findings Subjects who switched from placebo to migalastat after month 6 demonstrated a statistically significant reduction in kidney interstitial capillary GL-3 at month 12 (p=0.013*) Subjects who remained on migalastat for 12 months demonstrated a durable reduction in kidney interstitial capillary GL-3 Reduction in disease substrate also observed in plasma lyso-Gb3 in subjects who switched from placebo to migalastat (p<0.0001**). Subjects who remained on migalastat demonstrated a durable reduction in lyso-Gb3 Kidney function (estimated glomerular filtration rate (eGFR), iohexol mGFR) remained stable over 18-24 months Migalastat was generally safe and well-tolerated Of 41 subjects with GLP HEK amenable mutations who completed Study 011, 35 (85%) remain in voluntary extension study (Study 041) 12- and 24-Month Results Released in April 2014 Demonstrated a Clear Efficacy Signal in Fabry Patients with Amenable Mutations *MMRM, **ANCOVA |
|
|
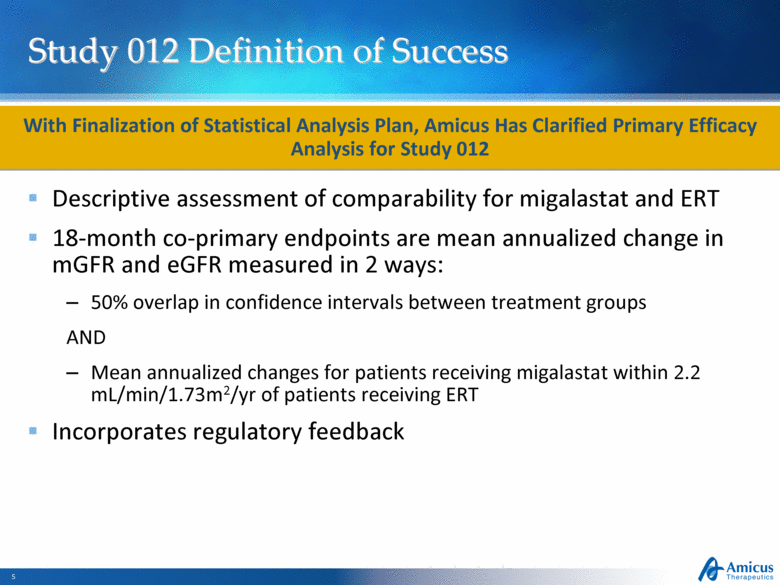
Study 012 Definition of Success Descriptive assessment of comparability for migalastat and ERT 18-month co-primary endpoints are mean annualized change in mGFR and eGFR measured in 2 ways: 50% overlap in confidence intervals between treatment groups AND Mean annualized changes for patients receiving migalastat within 2.2 mL/min/1.73m2/yr of patients receiving ERT Incorporates regulatory feedback With Finalization of Statistical Analysis Plan, Amicus Has Clarified Primary Efficacy Analysis for Study 012 |
|
|
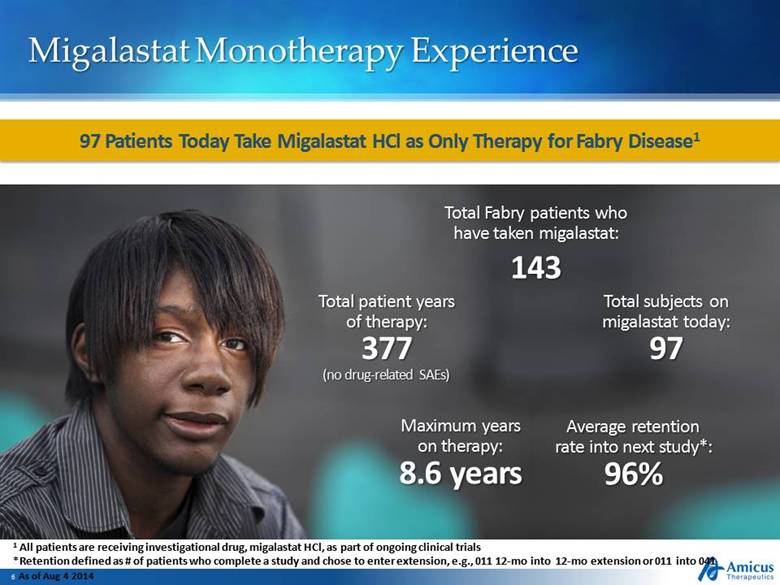
Migalastat Monotherapy Experience 97 Patients Today Take Migalastat HCl as Only Therapy for Fabry Disease1 1 All patients are receiving investigational drug, migalastat HCl, as part of ongoing clinical trials *Retention defined as # of patients who complete a study and chose to enter extension, e.g., 011 12-mo into 12-mo extension or 011 into 041 As of Aug 4 2014 Total subjects on migalastat today: 97 Total patient years of therapy: 377 (no drug-related SAEs) Maximum years on therapy: 8.6 years Average retention rate into next study*: 96% Total Fabry patients who have taken migalastat: 143 |
|
|
Migalastat Monotherapy: Global Regulatory Strategy Totality of clinical data 8+ years of data in extension studies Complete data from Phase 3 Studies (011 and 012) Clear regulatory pathway Non-inferiority to ERT (Study 012) Data from Study 011 (Reported) and Study 012 (Expected 3Q14) to Support Global Approvals of Migalastat Monotherapy for Patients with Amenable Mutations |
|
|
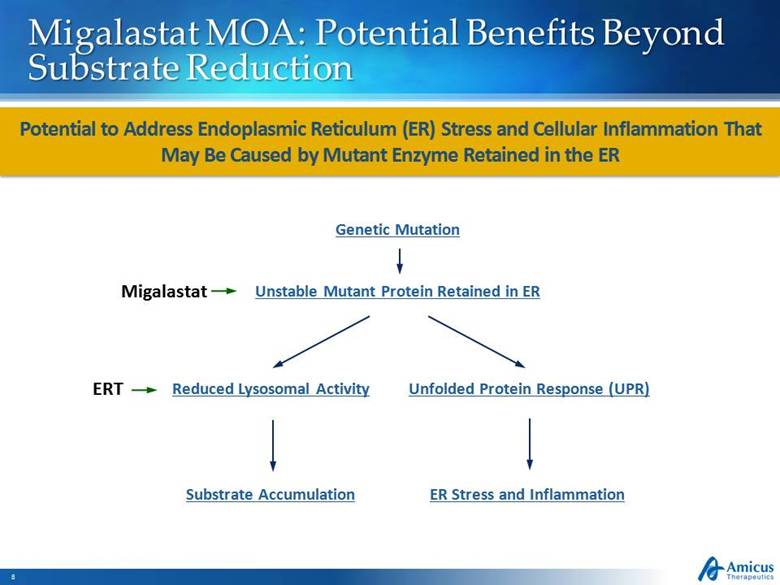
Migalastat MOA: Potential Benefits Beyond Substrate Reduction Potential to Address Endoplasmic Reticulum (ER) Stress and Cellular Inflammation That May Be Caused by Mutant Enzyme Retained in the ER ER Stress and Inflammation Unstable Mutant Protein Retained in ER Substrate Accumulation Reduced Lysosomal Activity Unfolded Protein Response (UPR) Genetic Mutation Migalastat ERT |
|
|
Migalastat Restores Trafficking of Mutant Enzyme Migalastat Restores Ability of Mutant -Gal A Retained in ER to Traffic to Lysosomes and Reduces a Marker of ER Stress in a Fabry Mouse Model Wild-Type Untreated R301Q Untreated R301Q Migalastat-Treated -Gal in lysosomes -Gal retained in ER -Gal in lysosomes Migalastat leads to reduced levels of mutant -Gal in ER in fibroblast cells Migalastat also reduces levels of ER stress marker BiP in white blood cells |
|
|
Role of UPR and ER Stress in Nephropathy and Potential Role of Migalastat There is growing evidence that UPR and ER stress contribute to nephropathy ER stress markers are increased in kidney biopsies from patients with nephropathy Abnormal protein accumulation and ER stress in podocytes and tubular cells can lead to severe proteinuria and tubular apoptosis By chaperoning mutant -Gal out of the ER, migalastat may reduce UPR and ER stress, potentially addressing aspects of kidney disease not currently addressed by ERT Further Research Into the Role of UPR and ER stress in Fabry and the Potential for Migalastat to Address this Pathology Is Ongoing Sources: Cybulsky 2013; Inagi 2014 |
|
|
Pompe Disease Overview Deficiency of lysosomal enzyme GAA Age of onset ranges from infancy to adulthood Glycogen accumulation in muscle tissue Incidence 1:28,0001 Current ERT suboptimal Elevated Glycogen in Muscle Severe, progressive, fatal neuromuscular disease 1Evidence Report – Newborn Screening for Pompe Disease – June 2013 – HRSA.gov |
|
|
Select Milestones in Pompe Drug Development 16 Years After First Pompe Cell Line, Significant Unmet Medical Needs Remain 1998 2001 2005 2006 2007 2008 2012 2013 2014 Amicus initial Phase 2 POC for CHART in Pompe (chaperone + Myozyme/Lumizyme) Myozyme Phase 3 data in infants Callidus initial PoC for rhGAA cell line with improved carbohydrate structure Amicus acquires Callidus Preclinical POC and scale up for ATB200 cell line at Amicus Myozyme approved Novazyme initial PoC for rhGAA cell line - acquired by Genzyme Myozyme Phase 3 data in late-onset patients First rhGAA cell line developed First infant Pompe patient has immune system ablated |
|
|
Three Challenges with Pompe ERT Activity/ Stability Tolerability / Immunogenicity Uptake/ Targeting Infusion-associated reactions in ~50% of late-onset patients3 High antibody titers shown to affect treatment outcomes4,5 Rapid denaturation of ERT in pH of blood1 Low M6P receptor uptake into skeletal muscle2 Majority of rhGAA is not delivered to lysosomes2 1Khanna et al., PLoS ONE, 2012; 2Zhu et al., Amer. Soc. Gene Therapy, 2009 June; 3Banati et al., Muscle Nerve, 2011 Dec. ; 4Banugaria et al., Gen. Med., 2011 Aug.; 5de Vries et al., Mol Genet Metab., 2010 Dec. |
|
|
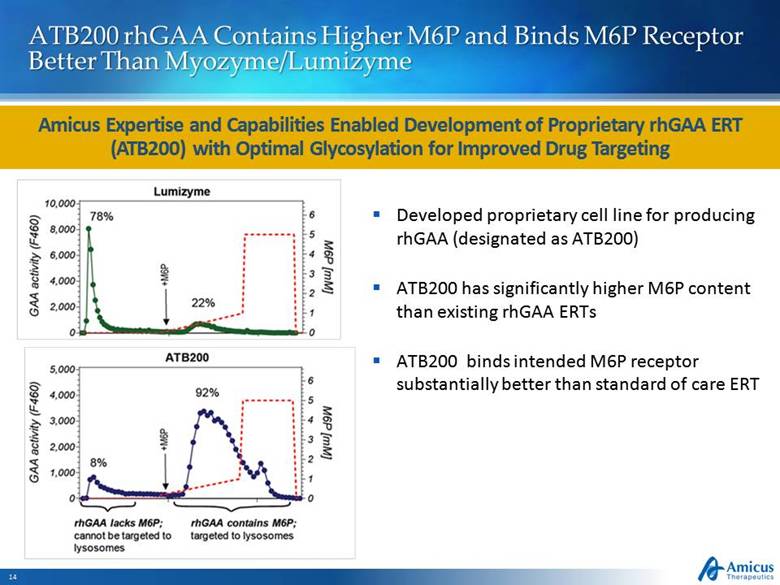
ATB200 rhGAA Contains Higher M6P and Binds M6P Receptor Better Than Myozyme/Lumizyme Amicus Expertise and Capabilities Enabled Development of Proprietary rhGAA ERT (ATB200) with Optimal Glycosylation for Improved Drug Targeting Developed proprietary cell line for producing rhGAA (designated as ATB200) ATB200 has significantly higher M6P content than existing rhGAA ERTs ATB200 binds intended M6P receptor substantially better than standard of care ERT |
|
|
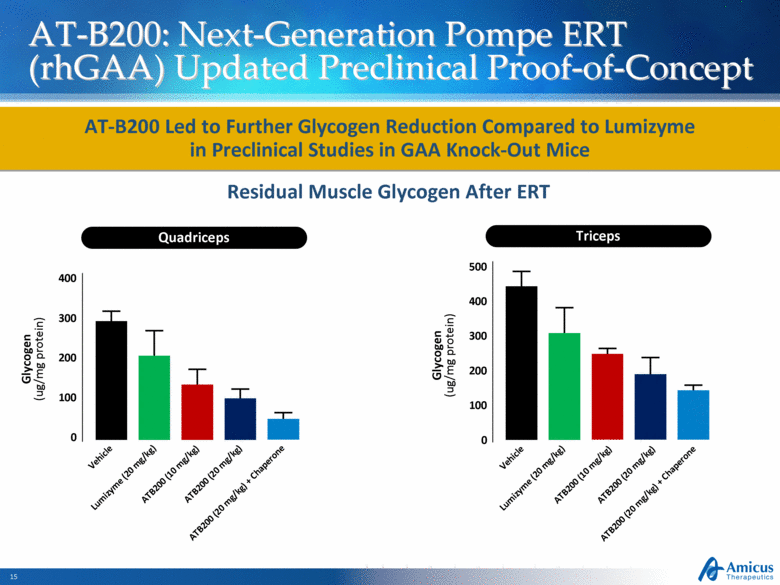
AT-B200: Next-Generation Pompe ERT (rhGAA) Updated Preclinical Proof-of-Concept Residual Muscle Glycogen After ERT AT-B200 Led to Further Glycogen Reduction Compared to Lumizyme in Preclinical Studies in GAA Knock-Out Mice Quadriceps Triceps ATB200 (20 mg/kg) ATB200 (10 mg/kg) ATB200 (20 mg/kg) + Chaperone ATB200 (20 mg/kg) ATB200 (10 mg/kg) ATB200 (20 mg/kg) + Chaperone Glycogen (ug/mg protein) Vehicle Lumizyme (20 mg/kg) 300 200 100 0 400 Vehicle Lumizyme (20 mg/kg) Glycogen (ug/mg protein) 300 200 100 0 400 500 |
|
|
Program Updates Fabry Next-Generation ERT Phase 1 migalastat IV PK study successfully completed Manufacturing of co-formulated JR-051 drug supply complete Phase 1/2 study in Fabry patients will begin pending outcome of ongoing BD discussion on future sources of Fabry enzyme Parkinson’s Early-stage Biogen collaboration to conclude in September Amicus retains WW rights to most advanced Parkinson’s compound (AT3375) |
|
|
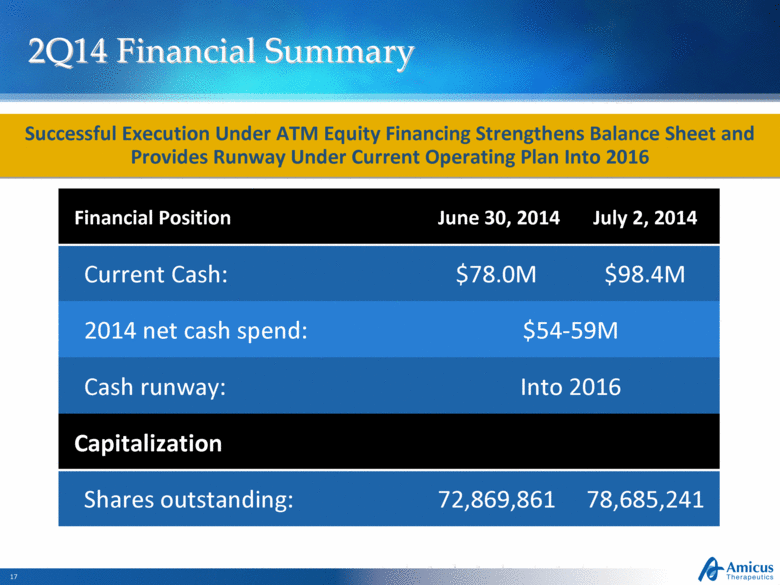
2Q14 Financial Summary Financial Position June 30, 2014 July 2, 2014 Current Cash: $78.0M $98.4M 2014 net cash spend: $54-59M Cash runway: Into 2016 Capitalization Shares outstanding: 72,869,861 78,685,241 Successful Execution Under ATM Equity Financing Strengthens Balance Sheet and Provides Runway Under Current Operating Plan Into 2016 |
|
|
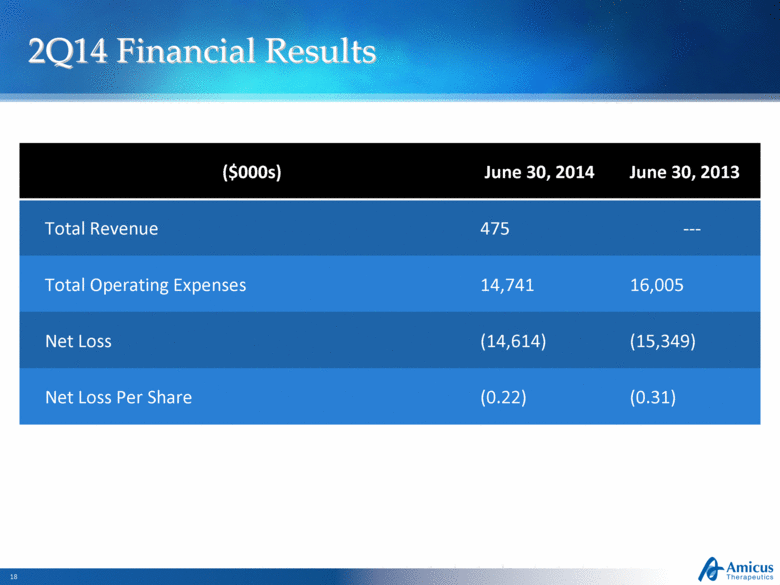
2Q14 Financial Results Slide 18 ($000s) June 30, 2014 June 30, 2013 Total Revenue 475 --- Total Operating Expenses 14,741 16,005 Net Loss (14,614) (15,349) Net Loss Per Share (0.22) (0.31) |
|
|
Continuity of Leadership at Amicus Chairman and CEO John Crowley to take temporary leave of absence (~32 Weeks) to serve active military duty Crowley, a commissioned officer in the United States Navy Reserve, will begin a temporary leave of absence in late September in support of Operation Enduring Freedom (Afghanistan) Crowley will remain CEO and Chairman and expects to continue to advise Amicus on major strategic and business issues during this time Bradley Campbell, Chief Operating Officer, will oversee day to day operations and chair executive leadership team in Mr. Crowley’s absence Mr. Crowley expected to return full-time from active duty service in 2Q15 |
|
|
Questions & Answers |
|
|
2Q14 Financial Results Conference Call & Webcast August 7, 2014 |