Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - ALLERGAN INC | d758653dex991.htm |
| EX-99.3 - EX-99.3 - ALLERGAN INC | d758653dex993.htm |
| 8-K - FORM 8-K - ALLERGAN INC | d758653d8k.htm |
| EX-99.4 - EX-99.4 - ALLERGAN INC | d758653dex994.htm |
 July
21, 2014 Allergan
A Specialist in the Biopharmaceutical
& Medical Device Industries
Exhibit 99.2 |
 2
2
Forward-Looking Statements
®
& ™
Marks owned by Allergan, Inc.
JUVÉDERM
®
is
a
registered
trademark
of
Allergan
Industrie
SAS
All
other
products
are
registered
trademarks
of
their
respective
companies
This
presentation
contains
“forward-looking
statements,”
including
statements
regarding
product
acquisition
and
development,
regulatory
approvals, market potential, expected growth, operational efficiencies, a proposed
offer made by Valeant, and Allergan’s expected, estimated
or
anticipated
future
results,
including
Allergan’s
earnings
per
share
and
revenue
forecasts,
among
other
statements.
All
forward-looking statements herein are based on Allergan’s current
expectations of future events and represent Allergan’s judgment only as
of the date of this presentation. If underlying assumptions prove inaccurate or
unknown risks or uncertainties materialize, actual results could vary
materially from Allergan's expectations and projections. Therefore, you are cautioned not to rely on any of these forward-looking
statements and Allergan expressly disclaims any intent or obligation to update
these forward-looking statements except as required to do so by
law. Actual results may differ materially from Allergan’s current
expectations based on a number of factors affecting Allergan’s businesses,
including
changing
competitive,
market
and
regulatory
conditions;
the
timing
and
uncertainty
of
the
results
of
both
the
research
and
development and regulatory processes; domestic and foreign health care and cost
containment reforms, including government pricing, tax and reimbursement
policies; revisions to regulatory policies related to the approval of competitive generic products; technological advances
and
patents
obtained
by
competitors;
the
ability
to
obtain
and
maintain
adequate
protection
of
intellectual
property
rights;
the
performance
of new products, including obtaining government approval and consumer and physician
acceptance, the continuing acceptance of currently marketed products, and
consistency of treatment results among patients; the effectiveness of promotional and advertising campaigns; the
potential for negative publicity concerning any of Allergan’s products; the
timely and successful implementation of strategic initiatives, including
expansion of new or existing products into new markets; the results of any pending or future litigation, investigations or claims; the
uncertainty associated with the identification of, and successful consummation,
execution and integration of, external corporate development initiatives and
strategic partnering transactions; potential difficulties in manufacturing; and Allergan’s ability to obtain and
successfully maintain a sufficient supply of products to meet market demand in a
timely manner. In addition, matters generally affecting the U.S. and
international economies, including consumer confidence and debt levels, changes in interest and currency exchange rates,
political
uncertainty,
international
relations,
the
status
of
financial
markets
and
institutions,
impact
of
natural
disasters
or
geo-political
events
and the state of the economy worldwide, may materially affect Allergan’s
results. These and other risks and uncertainties affecting Allergan’s
businesses and operations may be found in Allergan’s most recently filed
Annual Report on Form 10-K and any subsequent Quarterly Reports on Form
10-Q, including under the heading “Risk Factors”. These
filings, as well as Allergan's other public filings with the U.S. Securities and
Exchange Commission (SEC), can be obtained without charge at
the
SEC's
web
site
at
www.sec.gov.
These
SEC
filings
are
also
available
at
Allergan’s
web
site
at
www.allergan.com
along
with
copies
of Allergan’s press releases and additional information about Allergan. For
further information, you can contact the Allergan Investor Relations
Department by calling 714-246-4636. ©
2014 Allergan, Inc.
All rights reserved. |
 3
Allergan Continues to Execute &
Deliver
Increased Value to Stockholders
•
Continued strong performance and business momentum
•
Q2 and Q2 YTD sales growth of 16%
(1)
driven by strength across nearly all
product lines and geographies
•
Q2 and Q2 YTD EPS
(2)
growth in excess of 20%
•
Increase in both Sales & EPS
(2)
guidance for full year 2014
•
Execution of plan to drive further operational efficiencies & create a robust
platform for long-term sustainable growth
•
Streamline SG&A while continuing to focus on revenue growth
opportunities
•
Further enhance R&D productivity
•
Maintain robust double-digit revenue CAGR over long-term plan
•
Further enhanced long-term earnings outlook
•
Additional value from ongoing business development & capital return
(1)
Represents local currency sales growth
(2)
Non-GAAP Diluted Earnings per Share |
 4
4
Another Outstanding Quarter Highlights the Strength of
Allergan’s Business Model
Q2 2014 Guidance
(Issued May 7)
$1,725M -
$1,800M
$1.41 -
$1.44
16% -
18%
Actual Q2 2014
Results
$1,827M
$1.51
24%
Product Net Sales
Non-GAAP Diluted
EPS
Non-GAAP Diluted
YOY EPS Growth
Strong business momentum continued in 2nd quarter with record dollar sales
growth Depth
and
breadth
of
contribution
made
by
nearly
all
therapeutic
areas
and
geographies
Leverage of SG&A investments in a thoughtful & disciplined approach
|
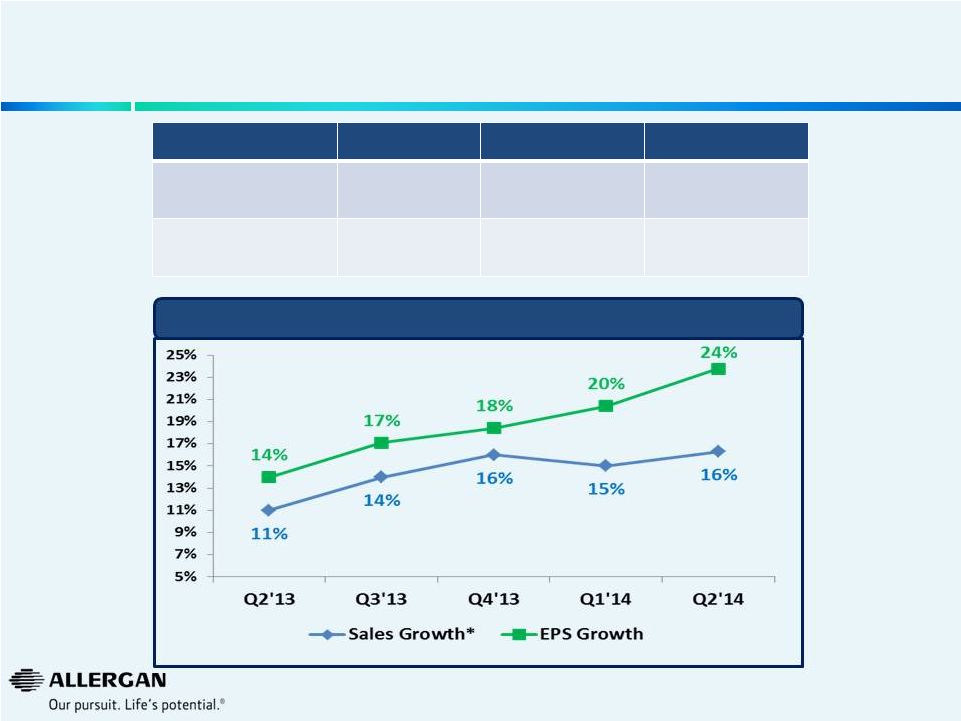 5
5
Strong Business Momentum Continues to Accelerate
Sales & EPS Growth
* Sales growth in local currency retrospectively adjusted to exclude
Obesity Intervention Business. **
2012
EPS
growth
includes
the
2012
Obesity
impact
of
$0.10
and
the
2012
R&D
Tax
Credit
impact
of
$0.06.
2013
EPS
Growth
–
Restating
2012
to exclude the 2012 Obesity impact of $0.10 and including the 2012 R&D Tax
Credit impact of $0.06. FY 2012
FY 2013
YTD Q2 2014
Sales Growth*
10%
12%
16%
EPS Growth**
15%
16%
22%
Quarterly Sales & EPS Performance |
 Allergan’s 2014 Full Year Earnings Outlook
Continues to Improve
FY 2014
Product Net Sales
Non-GAAP
Diluted EPS
Non-GAAP
Diluted EPS YOY
Growth
$6.8bn -
$7.0bn
$5.64 -
$5.73
18% -
20%
$6.7bn -
$7.0bn
$5.36 -
$5.48
12% -
15%
Feb. 5 Guidance
May 12 Guidance
July 21 Guidance
$6.9bn -
$7.1bn
$5.74 -
$5.80
20% -
22%
6 |
 7
Overview of Today’s Plan to Restructure
Our
Operations & Processes
~13% reduction in
workforce
Site closures
~1,500 employees &
~250 vacant positions
3
Annual Cost reductions*
~ $475M
Our ongoing effort to improve efficiency and productivity will further increase
stockholder value
* Includes $27M of Gross Margin enhancements |
 8
Driving Sustainable Operational Efficiencies
Without
Compromising Effectiveness
Organization Re-design
•
Refocusing our resources on the highest yielding initiatives
•
Right-sizing, adapting and simplifying our structure and processes
Selling, General and Administrative (SG&A)
•
Past strong investments have created critical mass that can now be leveraged
Research & Development (R&D)
•
Heavy concentration on programs already in clinic
From 1998 –
2014 Allergan Has Successfully Invested to Create Robust Top and Bottom Line Growth
•
Launched new markets, products and geographies
16% Sales CAGR
19% EPS CAGR
>2,000% Stock Price Appreciation
No compromise to our commercial
strategy and low impact to our long-term
revenue growth targets
Maintain strength in our R&D pipeline to
bring innovative therapies to patients
~$475M in annual operational efficiencies expected to be realized in 2015
(1)
(1)
(2)
(1)
Represents 1998 – 2014 CAGR. 2014 is midpoint of July 21, 2014 guidance. (2)
Calculation based on 1998 – July 18, 2014. |
 9
Principles Behind Our Enduring Organization
Customer
Centricity
•
DTC spend preserved at 2014 levels in all priority
brands
DTC spend maintained ~$200M
•
Sales force maintained in all key areas
~ 94% of sales force remains intact
Reductions mainly in breast & glaucoma
•
Maintain education and training focus
Innovation
and
Pipeline
•
No changes to Phase 2/3 clinical pharmaceutical
R&D programs
•
Outsource to achieve efficiencies in non-core
areas (e.g. data management, global monitoring)
Preserve study protocol design and
Phase 2B & Phase 3 trials in-house
Culture
and values
•
Success continues to be driven by action-
oriented culture with a premium placed on
generating results
•
Rewire, right-size and reduce complexity /
layers within the organization
•
Optimize processes and speed of
decision
making
Commercial
•
Focus resources on highest ROI areas
•
Preserve customer facing headcount
•
Reduce
complexity
and
layers
within
the
organization
R&D
Principles Underpinning Allergan’s Plan
Allergan will not compromise
our successful business model
Maintains a robust platform for long-term sustainable growth
|
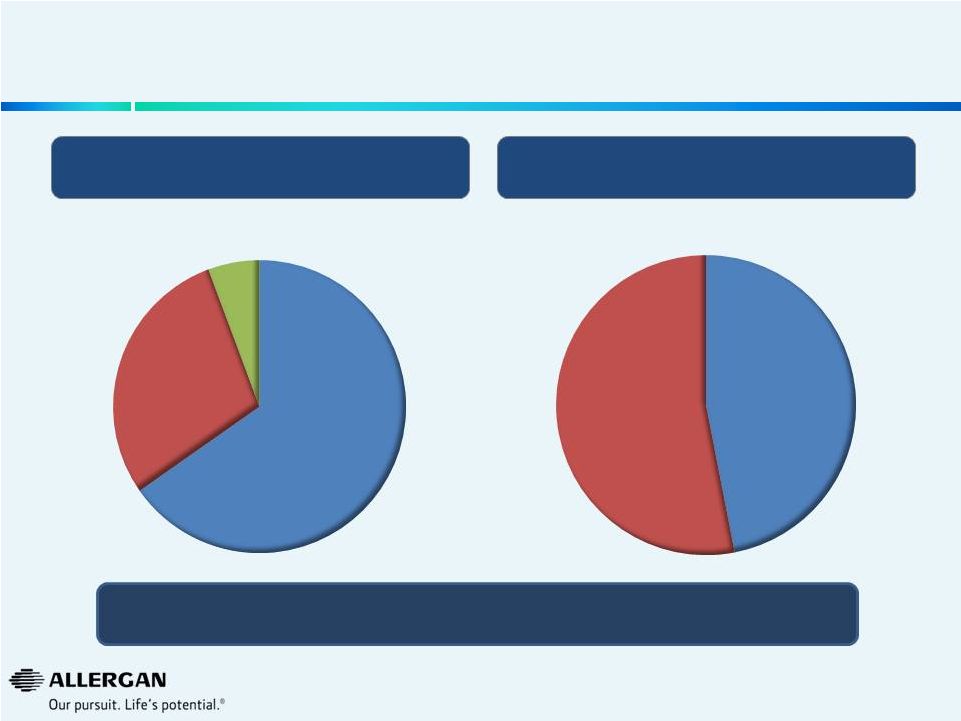 10
10
% of Operational Efficiencies from Headcount
Operational Efficiency Mix by P&L Line Item
~$475M Annual Cost Savings
To Be Realized in 2015
~
13%
Reduction
in
Allergan
Workforce
(1)
Implementation
expected
to
be
mostly
completed
in
2014
with
operational
efficiencies
expected to be fully realized in 2015 and beyond
Overview of Operational Efficiencies to be Realized
Gross Margin
6%
R&D
29%
SG&A
65%
Non
Headcount
53%
Headcount
47%
(1)
As part of the restructuring, Allergan will reduce its workforce by approximately 1,500 employees, or
approximately 13 percent of its current global headcount, and eliminate an additional
approximately 250 vacant positions
|
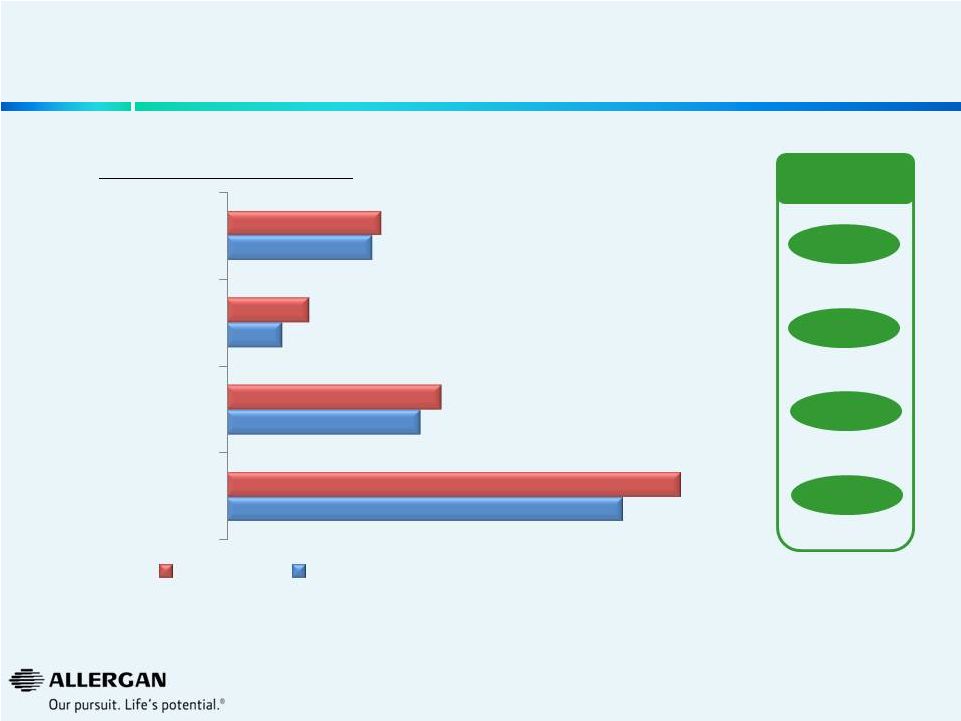 11
Operational Efficiencies Result in a ~13%
Reduction in Allergan
Workforce (1)
As of June 30, 2014. All headcount is rounded.
(2)
As
part
of
the
restructuring,
Allergan
will
reduce
its
workforce
by
approximately
1,500
employees,
or
approximately
13
percent of its current global headcount, and eliminate an additional approximately
250 vacant positions Headcount (#’s are approximate) -6%
-33%
-10%
-13%
(1)
(2)
% Reduction
10,200
5,000
1,450
3,750
11,700
5,550
2,150
4,000
Total Company
All Other Functions
R&D
Sales Force
Post Operational Efficiencies Plan
Current |
 12
$180M
$182M
$26M
$22M
$65M
Focus resources on highest
value opportunities
Streamline organization structure
Simplify processes and interfaces
Optimize site footprint
Enhance strategic sourcing
of goods and services
I
II
III
IV
V
$475M
Total Operational Efficiencies
Five Performance Improvement Levers
Drive
Further Operational Efficiencies |
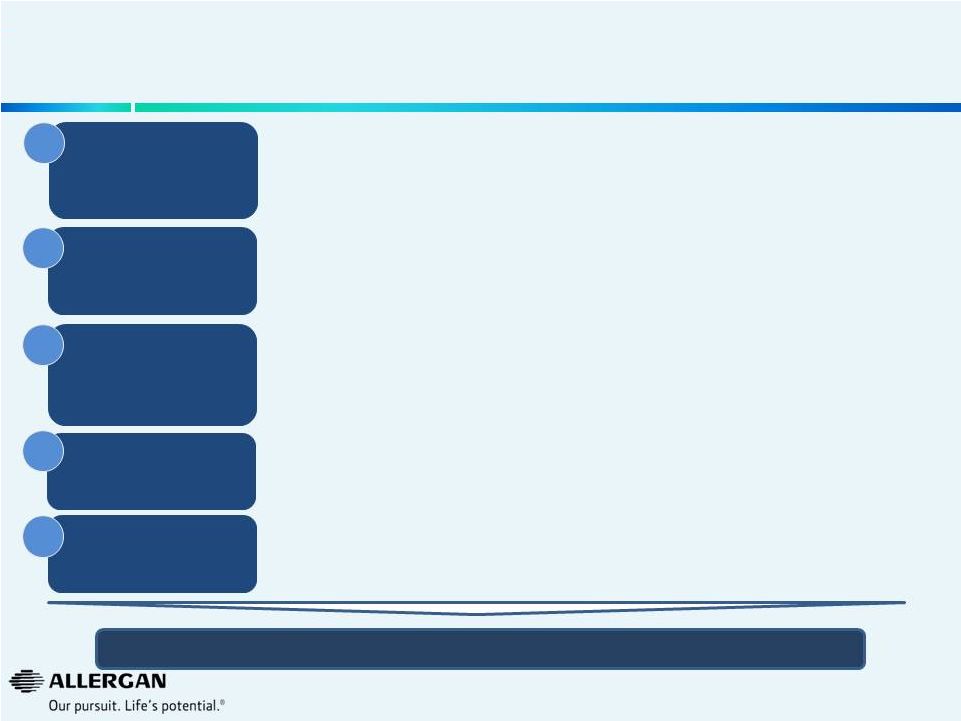 13
13
Streamlining SG&A While Continuing to
Focus on Revenue Growth Opportunities
Focus resources on
highest value
opportunities
•
Invest
in
key
growth
drivers
(Retina,
Facial
Aesthetics,
RESTASIS
®
,
BOTOX
®
Chronic
Migraine
& Urology)
•
Rationalize spend in lower growth franchises (Breast & Glaucoma IOP
Drops) •
Rebalance
resources
in
favor
of
customer
facing
headcount
and
drive
commercial
excellence
Streamline
Organization
Structure
•
Optimize regional marketing structures and commercial support operations
•
Rationalize
global
functions
(e.g.
Market
Research
&
Medical
Affairs)
•
Reduce management layers and increase span of control (company-wide)
Simplify
Processes &
Interfaces
•
Clarify roles of global, regional and country organizations to speed
decision-making •
Enhance and expand use of regional shared service centers for high volume
transactional
services (e.g. Austin, TX, Westport, Ireland)
•
Reduce number of product promotional cycles and refocus marketing material
creation Enhance strategic
sourcing of goods
and services
•
Improve procurement of vendor services (e.g. advertising agencies and media
buying, market research)
2015 SG&A Operational Efficiencies: ~$310M
Optimize site footprint
•
Close Carlsbad, CA facility acquired in SkinMedica acquisition
•
Streamline commercial regional headquarter locations (Europe, Asia Pacific,
Latin America & Canada)
$145M
$112M
$6M
$38M
$9M |
 14
14
R&D Project Rationalization & Increased Efficiencies
Rationalize Projects
$52m
Increase Efficiency
$86m
Outsource non-core functions
Consolidate global development sites
Right-size organization to
align with future R&D budgets
Consolidate R&D vendors
Refocus discovery on core areas of
expertise (e.g. Eye care & Biologics)
Cancel 4 current early stage
discovery projects
Cancel 4 pre-clinical projects with
projected approvals dates post 2020
Reduce investment in Breast franchise
innovation –
no impact pre 2020 |
 15
15
•
Focus discovery resources in core areas of expertise (e.g. Eye Care and
Biologics) •
Focus
development
activities
on
high
value
projects
(e.g.
Retina,
Dry
Eye,
Fillers
and
new
BOTOX
®
indications)
•
Continue
to
implement
“quick
kill”
concept
•
Close Santa Barbara, CA and Medford, MA sites and relocate core activities to
other sites •
Consolidate development sites to enable scale and operational efficiency and
effectiveness (Europe, Asia Pacific)
•
Improve procurement of vendor services (e.g. Contract Research Organizations
(CRO’s)) Total 2015 R&D Operational Efficiencies: ~$138M
Further Enhancing R&D Productivity
•
Outsource select non-core functions (e.g. data management) to ensure
flexibility and scalability •
Consolidate duplicative activities within R&D (e.g. create centralized vendor
management group) •
Right-size organization for revised R&D spend
Focus resources on
highest value
opportunities
Streamline
Organization
Structure
Simplify
Processes &
Interfaces
Enhance strategic
sourcing of goods
and services
Optimize site footprint
•
Create single management structure across key R&D functions (e.g. project
management, clinical operations)
•
Integrate R&D IT into Corporate IT
$35M
$65M
$8M
$22M
$8M |
 16
16
1 cpd for new indication
ph1
Maintain Rich, Highly Diversified Clinical R&D Pipeline
Post
Approval
Pre-Clinical
Phase I
Phase II –
POC
Phase II –
Confirmatory
Phase III
Registration
Discontinued
Allergan remains committed to an industry leading R&D engine
Limited number of pre-clinical projects have been
cancelled
(all with projected approval dates after 2020)
1857 Migraine
Derma-
tology
•
1 rosacea programs ph1
•
2 acne programs ph1
•
One early preclinical acne
program
•
One early pre
-clinical derm
program for a new indication
•
Bim Hair Growth
•
BTX Masseter
•
Medytox Aesthetic
•
BTX CFL Asia
•
BTX Forehead Lines
•
LATISSE
®
Brow
•
Oxymetazoline
Rosacea
•
ACZONE
X
•
BTX CFL
•
LATISSE
®
US
•
LATISSE
®
Japan
Urology
•
BTX PE
•
SER-120
•
BTX OAB
•
BTX NDO
Neurology
& Pain
•
AGN
-
ph1
•
BTX X with potential for higher
dose and/or longer duration
ph1
•
Senrebotase (TEM)
Pain
•
BTX Depression
•
BTX OA Pain
•
BTX New
Indication
•
Medytox
Therapeutic
•
BTX
Spasticity Adult
LL, Adult UL, Ped LL,
Ped UL
•
AGN-1763 BTX
LL Spasticity
•
“
SEMPRANA
®
”
Headache
•
BTX
Headache
Retina
•
Dual anti
-PDGF/VEGF
DARPin
®
•
1 retina
program
ph1
•
2 early preclinical retina
programs
•
DARPin
®
AMD
•
DARPin
®
DME
•
Brimo
DDS Dry
AMD
•
OZURDEX
®
RVO
China
•
OZURDEX
®
DME (Europe)
•
OZURDEX
®
RVO
•
OZURDEX
®
DME
3B
Anterior
Segment
•
1 NCE
for
Dry
Eye
ph1
•
1 cpd for new
indication
•
•
NCE Dry Eye
•
Androgen Front of
Eye
•
RESTASIS
®
X
•
LASTACAFT
®
Japan
•
RESTASIS
®
EU
•
1 cpd
for front of eye
indication
Glaucoma
•
NCE Glaucoma ph2
•
Combo glaucoma Japan
ph3
•
Bimatoprost
SR
•
LUMIGAN
®
UD
•
LUMIGAN
®
0.01%
EU
•
GANFORT™
UD
•
GANFORT™
China
®
ph2
AMD ph2 |
 17
17
R&D Outsourcing Will Ensure Flexibility and Scalability
Strategic partnering with global
CROs for lower overhead & wages
Increased Outsourcing Across R&D for
More Nimble & Focused Development
PharmSci
Clin Ops
Biostatistics
Safety
In-house
Outsourced
Core functions retained
in-house
Allows flexibility and scale for
future R&D spend
Data Mgmt
Global
Monitoring
Global
Medical
Writing
Monitoring
Writing
Medical |
 18
18
Total 2015 Gross Margin Operational Efficiencies: $27M*
Further Improvement in Gross Margin Efficiencies
•
Create a Global Supply Chain organization
•
Network optimization
•
Global Procurement
•
Re-design organization at each site and centre
Focus resources on
highest value
opportunities
Streamline
Organization
Structure
Simplify
Processes &
Interfaces
Enhance strategic
sourcing of goods
and services
Optimize site footprint
•
Manufacturing excellence program
•
Optimize activity at select sites
*~$50M by 2016 primarily due to inventory rollout |
 19
Driving Sustainable Operational Efficiencies
Without
Compromising Effectiveness
No compromise to our commercial
strategy and low impact to our long-
term revenue growth targets
Maintain strength in our R&D
pipeline to bring innovative
therapies to patients
~$475M in annual operational efficiencies expected to be realized in 2015
Allergan 2014 -
2019
Double-Digit Sales CAGR
despite a ~50 b.p. reduction*
>20% EPS CAGR
2016 EPS -
~$10.00
Additional free cash flow of ~$18bn** and significant borrowing capacity to drive
strategic options and financial flexibility
* There
was
a
~50
basis
point
reduction
in
the
Sales
CAGR
(2014E
–
2019E)
between
guidance
provided
on
May 12, 2014 vs. the guidance provided on July 21, 2014.
** 2014 -
2019 |
 20
20
Allergan’s Promising Outlook on Long-Term Organic Growth
Driven by New Product Innovation and Operational Excellence
2013A
2014E
Guidance
2019E
2015E
Guidance
Revenue
$6.2bn
EPS
$4.77
2016E
Guidance
2017E
2018E
* As a percentage of sales.
Additional stockholder value generated from strong business momentum & further
operational efficiencies
Revenue
Growth
Double Digit
EPS
~$10.00
Revenue
$6.9 -
$7.1bn
EPS
$5.74 -
$5.80
EPS Growth
20% -
22%
Revenue
Growth
Double Digit
EPS
$8.20 -
$8.40 |
 21
Strategic Options Available to Further Increase
Stockholder Value
•
Value from acquisitions
•
Value from capital return
•
Stock repurchases
•
“Special”
dividend
Additional free cash flow of ~$18bn
generated over strategic planning period |
 Allergan’s Standalone Performance Continues To Create
Significant Near-Term and Long-Term Value for Stockholders
(3)
Low Estimate
-
Former
High Estimate
-
Former
Low Estimate
-
Current
High Estimate
-
Current
Pre-Operational
Efficiencies
(2)
Post-Operational
Efficiencies
(1)
22.5x NTM P/E Multiple
25.0x NTM P/E Multiple
Future Value Per Share Allergan
Low Estimate based on 22.5x NTM P/E
High Estimate based on 25.0x NTM P/E
$371
$316
$390
$351
150.00
200.00
250.00
300.00
350.00
$400.00
Current
12/31/2014
12/31/2015
12/31/2016
12/31/2017
12/31/2018
Note: Allergan multiple range (as a standalone company) is based on Wall Street Research as of
07/15/14. (1)
2014 based on July 21, 2014 EPS guidance of $5.74- $5.80, 2015 and 2016 based on guidance of
$8.20-$8.40 and $10.00, respectively, as stated on July 21, 2014. 2017 – 2019 is
implied using 2014-2019 CAGR of >20%.
(2)
2014 based on May 12, 2014 EPS guidance of $5.64 -$5.73, 2015 based on guidance of 20% -
25% YOY EPS growth as stated on May 12, 2014 and 2016 – 2019 assumes 20% YOY EPS growth. (3)
Current Allergan share price of $165.82 as of 07/17/14. (3)
2014 based on midpoint of EPS guidance issued on Feb. 5, 2014 of $5.36 - $5.48, 2015 assumes 22.5%
growth (midpoint of 20% - 25% growth). (4)
2014 based on midpoint of May 12, 2014 EPS guidance of $5.64 -$5.73, 2015 calculated using
midpoint of 20% - 25% YOY EPS growth as stated on May 12, 2014 and 2016 – 2019 assumes
20% YOY EPS growth. (5)
2014 based on midpoint of July 21, 2014 EPS guidance of $5.74- $5.80, 2015 based on
midpoint of $8.20-$8.40 as stated on July 21, 2014, 2016 based on $10.00 and 2017-
2019 is implied using 2014-2019 CAGR of >20%.
22 |
 Value Drivers Available to Standalone Allergan to
Further Increase Stockholder Value
Potential
Future
Standalone
Stock Price
Value from Momentum in the Business
Value from Capital Return
Value from Pipeline Assets
Value from Ongoing Business Development
Current Stock
Price
Value Drivers Available to Allergan:
Value from Operational Efficiencies
23 |
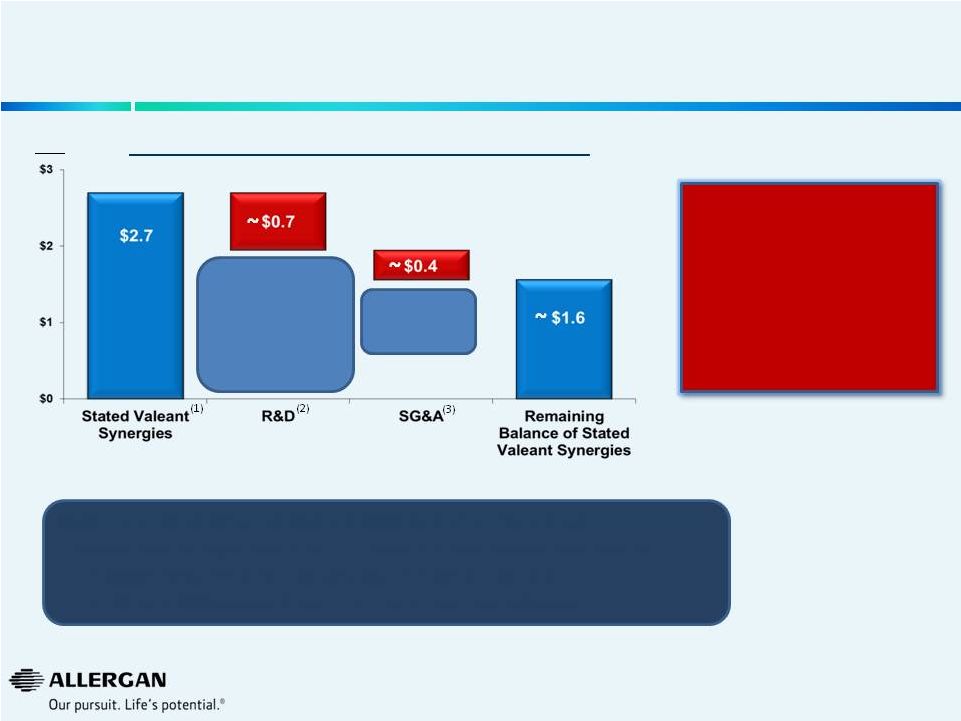 Allergan Actions Have Increased Standalone Value &
Significantly Reduced Synergies Available to Valeant
Reduction To Proposed Valeant Synergies*
Valeant’s purported $2.7bn of R&D and SG&A cuts are unrealistic
given: •
Valeant’s stated objectives in continuing certain R&D projects
post-closing •
the SG&A investments required to sustain Allergan’s products
•
the R&D and SG&A operational efficiencies announced by Allergan
Additional spend for
R&D programs that
Valeant claims to
continue post-closing
and Operational
Efficiencies
announced by
Allergan
Operational
Efficiencies
announced by
Allergan
$bn
Combination of higher
standalone value for Allergan
and Pro-forma impact of
Valeant’s reduced synergies
($1.1bn), has transferred
value from Valeant to
Allergan Stockholders
24
* Illustrative for 2015
(1)
Valeant SEC filings
(2)
Programs
Valeant
claims
to
continue
after
potential
post
closing
of
transaction.
Includes
all
Phase
3
pharmaceutical
programs
(including
DARPin®
&
Bimatoprost
Sustained
Release
Implant),
BOTOX®
Phase
2
&
3
programs,
Medytox
®,
Late-Stage Filler programs, IIT’s
(3)
Based on operational efficiencies announced on May 12, 2104 and July 21, 2014
|
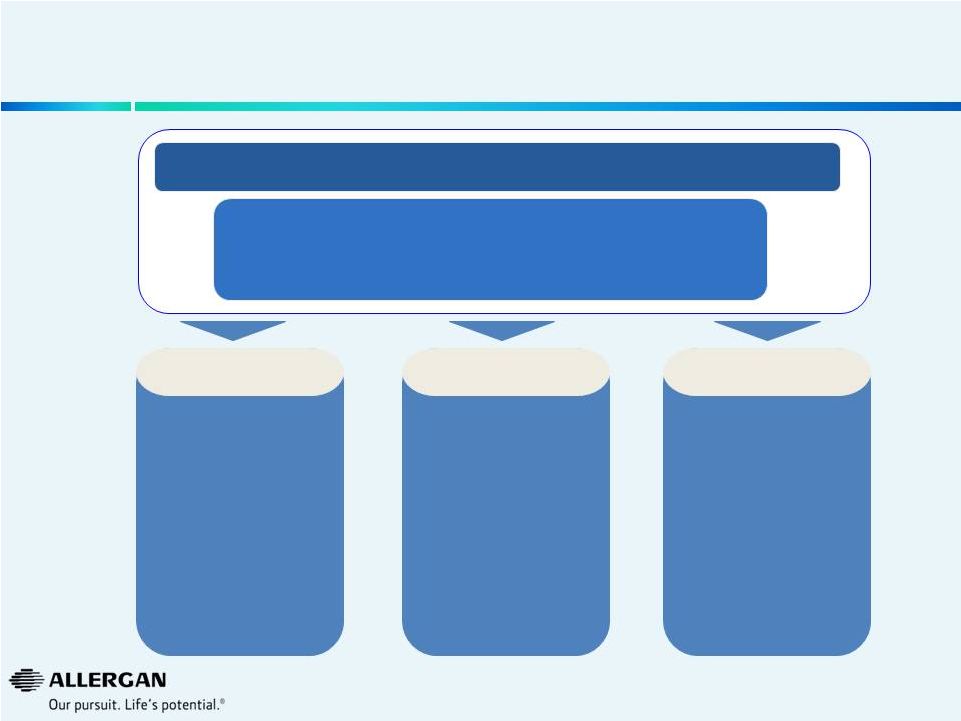 Allergan Has an Enduring Vision and a Goal to Create an
Even Stronger Company
Revenue Goal: ~$12bn by 2019
•
Double
digit
sales
growth
(2014E
–
2019E)
•
>20%
EPS
CAGR
(2014E
–
2019E)
•
Franchise leadership: #1 or #2 in every category
Our earnings growth is
founded on quality net
sales growth
In short, our profit
comes from serving the
needs of physicians and
their patients
Customer-centric
We have a unique
employee culture that
drives our success
Action orientated and
flexible with a premium
placed on generating
results
Culture & Values
We have an enduring
model for the future
founded on an R&D
pipeline that is informed
by our intimate
knowledge of our
customers and the
franchises in which we
choose to operate
Innovation
25 |
 26
26
Allergan’s promising outlook on long-term growth driven by new product
innovation and operational excellence:
—
Additional free cash flow of ~$18bn to drive strategic options and financial
flexibility —
5-year double digit revenue growth and >20% EPS CAGR
Investor
community
has
realized
an
increase
in
Allergan
value
as
our
management
team
has
and will continue to enhance business performance and outlook
To further enhance stockholder value, Allergan remains focused on ongoing value
driving opportunities
Allergan
management
team
best
equipped
to
deliver
significant
value
for
stockholders
–
our
track record speaks for itself
Conclusions
Allergan management and Board of Directors are committed to delivering
the highest value for stockholders |
 27
Reconciliation of Selected Non-GAAP Financial Measures
“GAAP” refers to financial information presented in accordance with generally accepted
accounting principles in the United States.
In this presentation, Allergan included historical non-GAAP financial measures, as defined in
Regulation G promulgated by the Securities and Exchange Commission, with respect to estimates
for the year ended December 31, 2013, and the corresponding periods for 1999 through 2012. The
information for 2012 and 2011 has been retrospectively adjusted to reflect the obesity
intervention unit, which was sold on December 2, 2013, as discontinued operations.
Allergan believes that its presentation of historical non-GAAP financial measures provides
useful supplementary information to investors. The presentation of historical non-GAAP financial
measures is not meant to be considered in isolation from or as a substitute for results prepared in
accordance with GAAP.
In this presentation, Allergan reported certain financial measures including “Adjusted
Sales”, “Adjusted SG&A”, “Adjusted R&D”, “Adjusted
EPS”, “Pro forma Growth” and “Sales Growth at constant exchange rates” as
adjusted for Non-GAAP items. Allergan uses these financial measures to enhance the investor’s
overall understanding of the financial performance and prospects for the future of
Allergan’s core business activities. Specifically, Allergan believes that a report
of these financial measures provides consistency in Allergan’s financial reporting and
facilitates the comparison of results of core business operations between its current, past and
future periods. Adjusted Sales, Adjusted SG&A, Adjusted R&D, Adjusted EPS, Pro forma Growth and Sales
Growth are the primary indicators management uses for planning and forecasting in future
periods. Allergan also uses Adjusted Sales, Adjusted R&D and Adjusted EPS for
evaluating management performance for compensation purposes.
A reconciliation of non-GAAP items may be found under the heading “Non-GAAP Financial
Reconciliation” in the investor
relations
section
of
the
www.Allergan.com
website. |
 28
28
Important Information
Allergan, its directors and certain of its officers and employees are participants in solicitations of
Allergan stockholders. Information regarding the names of Allergan's directors and executive
officers and their respective interests in Allergan by security holdings or otherwise is set
forth in Allergan's proxy statement for its 2014 annual meeting of stockholders, filed with the
SEC on March 26, 2014, as supplemented by the proxy information filed with the SEC on April 22,
2014. Additional information can be found in Allergan's Annual Report on Form 10-K for the
year ended December 31, 2013, filed with the SEC on February 25, 2014 and its Quarterly Report
on Form 10-Q for the quarter ended March 31, 2014, filed with the SEC on May 7, 2014. To the extent
holdings of Allergan's securities have changed since the amounts printed in the proxy statement for
the 2014 annual meeting of stockholders, such changes have been reflected on Initial Statements
of Beneficial Ownership on Form 3 or Statements of Change in Ownership on Form 4 filed with the
SEC. These documents are STOCKHOLDERS ARE ENCOURAGED TO READ ANY ALLERGAN SOLICITATION STATEMENT (INCLUDING
ANY SUPPLEMENTS THERETO) AND ANY OTHER RELEVANT DOCUMENTS THAT ALLERGAN MAY FILE
WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY BECAUSE THEY WILL CONTAIN IMPORTANT
INFORMATION. Stockholders will be able to obtain, free of charge, copies of any solicitation statement
and any
www.sec.gov.
www.allergan.com.
available
free
of
charge
at
the
SEC’s
website
at
other
documents
filed
by
Allergan
with
the
SEC
at
the
SEC's
website
at
www.sec.gov.
In
addition,
copies
will
also
be
available
at
no
charge
at
the
Investors
section
of
Allergan's
website
at |
 July
21, 2014 Allergan
A Specialist in the Biopharmaceutical
& Medical Device Industries
29 |
