Attached files
| file | filename |
|---|---|
| EX-1.1 - EX-1.1 - Intersect ENT, Inc. | d681748dex11.htm |
| EX-23.1 - EX-23.1 - Intersect ENT, Inc. | d681748dex231.htm |
Table of Contents
As filed with the Securities and Exchange Commission on July 18, 2014
Registration No. 333-196974
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 3
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Intersect ENT, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware | 3841 | 20-0280837 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
1555 Adams Drive
Menlo Park, California 94025
(650) 641-2100
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Lisa D. Earnhardt
President and Chief Executive Officer
Intersect ENT, Inc.
1555 Adams Drive
Menlo Park, California 94025
(650) 641-2100
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Matthew B. Hemington Brett D. White Seth J. Gottlieb Cooley LLP 3175 Hanover Street Palo Alto, California 94304 Telephone: (650) 843-5000 Facsimile: (650) 849-7400 |
B. Shayne Kennedy Thomas E. Mitchell Latham & Watkins LLP 650 Town Center Drive, 20th Floor Costa Mesa, California 92626 Telephone: (714) 540-1235 Facsimile: (714) 755-8290 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer x | Smaller reporting company ¨ | |||
| (Do not check if a smaller reporting company) |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to completion, dated July 18, 2014
Prospectus
5,000,000 Shares

Common Stock
This is the initial public offering of common stock of Intersect ENT, Inc. We are offering 5,000,000 shares of our common stock. We anticipate the initial public offering price will be between $11.00 and $13.00 per share.
Prior to this offering, there has been no public market for our common stock. We have applied for our common stock to be listed on The Nasdaq Global Market under the symbol “XENT.”
We are an “emerging growth company” as that term is defined under the federal securities laws of the United States and, as such, may elect to comply with certain reduced public company reporting requirements for this and future filings.
| Per Share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds, before expenses, to Intersect ENT, Inc. |
$ | $ | ||||||
| (1) | See “Underwriting” for additional disclosure regarding the compensation payable to the underwriters. |
We have granted to the underwriters an option to purchase up to 750,000 additional shares of common stock at the initial public offering price, less the underwriting discounts and commissions, for 30 days after the date of this prospectus.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 12 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares of common stock to investors on or about , 2014.
| J.P. Morgan | Piper Jaffray | |
| Leerink Partners | Wedbush PacGrow Life Sciences | |
The date of this prospectus is , 2014.
Table of Contents
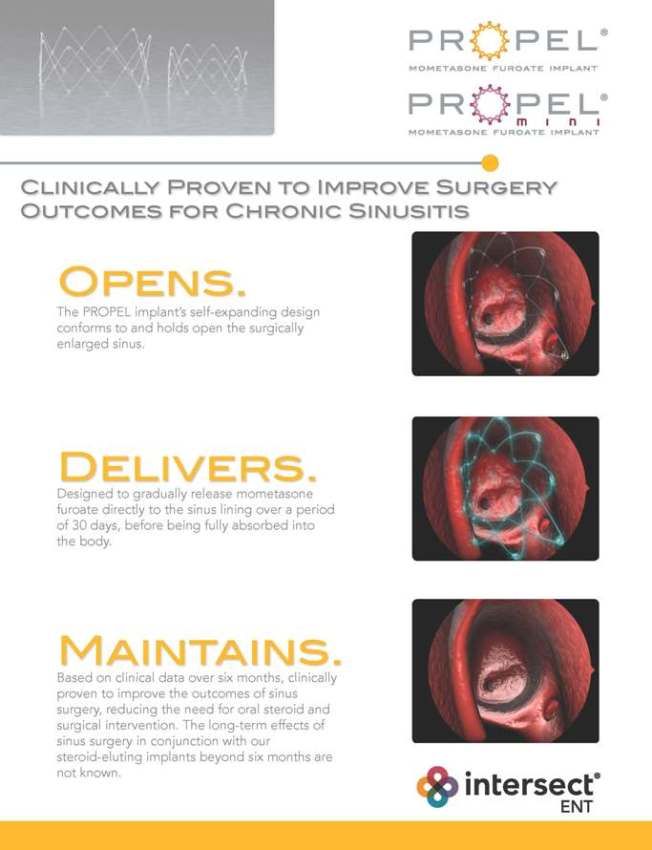
CLINICALLY PROVEN TO IMPROVE SURGERY OUTCOMES FOR CHRONIC SINUSITIS OPENS. The PROPEL implant’s self-expanding design conforms to and holds open the surgically enlarged sinus. DELIVERS. Designed to gradually release mometasone furoate directly to the sinus lining over a period of 30 days, before being fully absorbed into the body. MAINTAINS. Based on clinical data over six months, clinically proven to improve the outcomes of sinus surgery, reducing the need for oral steroid and surgical intervention. The long-term effects of sinus surgery in conjunction with our steroid-eluting implants beyond six months are not known.
Table of Contents
Neither we nor the underwriters have authorized anyone to provide you with information that is different from that contained in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We and the underwriters are offering to sell shares of common stock and are seeking offers to buy shares of common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date on the front of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
Until , 2014 (25 days after the commencement of this offering), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
TRADEMARKS
Intersect ENT, Inc. and our logo are our trademarks and are used in this prospectus. This prospectus also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, our trademarks and tradenames referred to in this prospectus appear without the ™ symbol, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames.
INVESTORS OUTSIDE THE UNITED STATES
Neither we nor any of the underwriters have taken any action that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons who have come into possession of this prospectus in a jurisdiction outside the United States are required to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
i
Table of Contents
This summary highlights information contained elsewhere in this prospectus. This summary is not complete and may not contain all the information you should consider before investing in our common stock. You should read this entire prospectus carefully, especially the risks of investing in our common stock discussed under the heading “Risk Factors,” and our financial statements and related notes included elsewhere in this prospectus before making an investment decision. Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “Intersect ENT,” “the company,” “we,” “us” and “our” refer to Intersect ENT, Inc.
Overview
We are a commercial stage drug-device company committed to improving the quality of life for patients with ear, nose and throat conditions. We have developed a drug-eluting bioabsorbable implant technology that enables targeted and sustained release of therapeutic agents. This targeted drug delivery technology is designed to allow ear, nose and throat, or ENT, physicians to improve patient care. Our initial products, PROPEL and PROPEL mini, are the first and only drug-eluting implants approved by the U.S. Food and Drug Administration, or FDA, for use in patients with chronic sinusitis. Inserted by a physician during ethmoid sinus surgery, the self-expanding implants are designed to conform to and hold open the surgically enlarged sinus, while gradually releasing an anti-inflammatory steroid over a period of 30 days, before being fully absorbed into the body. Use of our PROPEL implants is clinically proven to improve surgical outcomes by maintaining the open pathways created in surgery and reducing the need for oral steroids and additional surgical procedures. In addition, we are using our drug-eluting bioabsorbable implant technology to develop new, less-invasive and more cost-effective treatment options for the management of chronic sinusitis in the physician office setting to provide benefits for patients, physicians and payors. Any new products we develop, or changes that we make in the therapeutic agent used in PROPEL or PROPEL mini, will require FDA approval prior to commercialization in the United States.
Chronic sinusitis is one of the most prevalent chronic diseases in the United States and significantly impacts the quality of life of patients. According to the Centers for Disease Control and Prevention, or CDC, approximately 12% of the U.S. adult population, or 29 million people, are affected by chronic sinusitis, making it more prevalent than heart disease and asthma. Chronic sinusitis is an inflammatory condition in which the sinus lining becomes swollen and inflamed, leading to significant patient morbidity, including difficulty breathing, chronic headaches, recurrent infections, bodily pain, and loss of sense of smell and taste. These persistent symptoms can severely impact a patient’s day-to-day well-being, resulting in frequent doctor visits and lost work productivity and can lead to chronic fatigue and depression. Chronic sinusitis is managed by a combination of medical management and surgical intervention. The first line of therapy is medical management involving antibiotics, anti-inflammatory steroids and decongestants. Patients whose symptoms persist, despite medical management, are recommended to undergo functional endoscopic sinus surgery, or FESS. FESS is performed in the operating room to open the blocked sinus pathways by removing inflamed tissue and bone using surgical tools. Approximately 540,000 patients underwent sinus surgery for chronic sinusitis in 2013 in the United States. Although sinus surgery can be effective, a majority of patients experience recurrent symptoms which commonly necessitate additional treatment with medications and surgery.
Our two commercial products, PROPEL and PROPEL mini, are designed to improve the outcomes of sinus surgery by reducing postoperative inflammation and scarring from the underlying condition as well as the surgery. PROPEL and PROPEL mini were clinically proven in a meta-analysis of prospective, multicenter, randomized, controlled, double-blind clinical studies to improve surgical outcomes, including a 35% reduction in the need for postoperative oral steroid and surgical intervention.
In August 2011 we received Premarket Approval, or PMA, from the FDA for our first product, PROPEL, indicated for use in patients 18 years or older following ethmoid sinus surgery to maintain patency. Our second product, PROPEL mini, received PMA approval from the FDA in November 2012. PROPEL mini is designed for patients requiring less extensive surgery and those who have smaller anatomy, thus expanding the treatable patient population. In the first half of 2013, we began scaling our U.S. direct commercial presence. As of March 31, 2014, over 1,000 physicians in the United States have been trained and have incorporated our steroid-
1
Table of Contents
eluting implants into their practice. Based on the number of units shipped as of March 31, 2014, we estimate that physicians have treated over 25,000 patients with PROPEL and PROPEL mini. For the year ended December 31, 2013, we generated revenue of $17.9 million and had a net loss of $18.4 million. For the three months ended March 31, 2014, we generated revenue of $7.5 million and had a net loss of $4.4 million. As of March 31, 2014, we had an accumulated deficit of $82.7 million.
The Market
According to the CDC, approximately 12% of the U.S. adult population, or 29 million people, are affected by chronic sinusitis, making it more prevalent than heart disease and asthma. The CDC estimated that in 2005 the condition resulted in 12.6 million physician office and 1.2 million hospital out-patient department visits per year in the United States. Industry sources estimate that chronic sinusitis resulted in $8.6 billion in direct healthcare costs in 2007 in the United States.
Our target market consists of more than 3.5 million people with chronic sinusitis in the United States who are managed by ENT physicians and who we believe could benefit from products that incorporate our drug-eluting bioabsorbable implant technology. These patients are treated by approximately 7,500 ENT physicians who perform sinus surgery. Approximately 540,000 patients underwent sinus surgery for chronic sinusitis in 2013 in the United States. This includes patients who are under the age of 18 and surgeries on the frontal sinuses, both of which represent potential expanded future indications for PROPEL and PROPEL mini and would require FDA approval. Based on the number of sinus surgeries (including surgeries performed by the approximately 40% of surgeons that do not use packing materials in their surgeries), and assuming two devices per surgery and an average selling price of $695 per device, we estimate the annual total addressable market for PROPEL and PROPEL mini in the United States to be approximately $830 million. We further estimate the annual total addressable market for our products in clinical development in the United States that are specifically designed to address patients in the physician office setting to be approximately $4.0 billion. This estimate is based on our estimate that of the more than 3.5 million chronic sinusitis patients in the United States who are managed by ENT physicians, (i) approximately 34% have undergone prior FESS procedures and we assume that 64% of these patients would undergo approximately 1.5 procedures per year to treat recurring symptoms using two devices at an average selling price of $495 per device, and (ii) the remaining approximately 66% of all chronic sinusitis patients managed by ENT physicians would use a different device we have in clinical development assuming that these patients would undergo approximately one procedure per year using two devices at an average selling price of $595 per device.
While our current commercial focus is the U.S. market, we plan to initiate efforts that will allow for future expansion into international geographies. To that end, in July 2014, we received CE mark approval for PROPEL and PROPEL mini which enables market access in Europe. We estimate the annual total addressable market for PROPEL and PROPEL mini in these regions to be greater than $1.0 billion. This estimate is based on an estimated approximately 865,000 FESS procedures annually in the 16 countries in which we did research (including surgeries performed by the approximately 40% of surgeons that do not use packing materials in their surgeries), assuming that 85% involve ethmoid sinuses and 25% frontal sinuses and assuming using two devices at an average selling price of $695 per device. Therefore, we estimate the global annual total addressable market for our products and products in clinical development for chronic sinusitis patients to exceed $6.0 billion.
Current Treatments for Chronic Sinusitis and Their Limitations
The treatment of chronic sinusitis often entails a combination of medical management and surgical intervention to treat the underlying inflammation of the sinus lining, while addressing the secondary symptoms caused by obstruction of the natural drainage pathways.
Medical Management
The first line of therapy for chronic sinusitis is medical management, which typically includes prescribed antibiotics, anti-inflammatory steroids and decongestants. Steroids are prescribed in two forms, oral steroid pills and nasal steroid sprays, both of which have serious limitations. Oral steroid therapy is effective at reaching the sinus lining, but it does so by means of systemic exposure and therefore carries the risk of serious side effects,
2
Table of Contents
including glaucoma, bone loss, weight gain, psychosis and difficulty in controlling blood glucose levels in patients with diabetes. Nasal steroid sprays, commonly indicated for rhinitis, or inflammation of the nasal passage, are routinely prescribed for chronic sinusitis patients. While nasal steroid sprays avoid systemic exposure, an estimated 70% of the drug is swallowed and the remainder is directed to the nasal passages, instead of the sinuses, which limits efficacy. In a published study, the fraction of drug deposited in the sinuses from a nasal steroid spray was measured to be less than 2%. Poor patient compliance further limits the effectiveness of nasal steroid sprays. Although medical management can reduce symptoms, an estimated 20% or more of chronic sinusitis patients who receive medical therapy are unresponsive.
Sinus Surgery
In cases where patients are symptomatic despite medical management, a physician may recommend FESS. In the FESS procedure, the physician enlarges the inflamed and obstructed sinus pathways by removing inflamed tissue and bone in order to facilitate normal sinus drainage and aeration. While FESS is the standard of care for treating chronic sinusitis, it has several limitations:
| • | Limited effectiveness. Inflammation and scarring in the postoperative period are common and can compromise the surgical result by negatively impacting the ability of the sinuses to heal. This increases the need for continued medical management and additional surgical procedures. Within the first year after surgery, approximately 64% of patients experience recurrent symptoms. |
| • | Limited ability to address postoperative inflammation. While oral steroids prescribed postoperatively can be effective at addressing inflammation and scarring, the required doses are significant and can result in serious systemic side effects. We believe, as a result, only 20% of physicians prescribe them routinely after surgery. The absence of anti-inflammatory steroid therapy leaves the surgical wound susceptible to postoperative complications. |
| • | Pain and discomfort during postoperative period. During surgery, an ENT physician typically places sinus packing materials into the ethmoid sinuses to physically separate tissues in an attempt to prevent scarring and adhesions. The sinus packing materials are removed in the physician’s office postoperatively by pulling or suctioning them from the newly opened cavity, a painful and time-consuming process, often necessitating pain medication. Moreover, the use of sinus packing materials obstructs the newly opened sinuses, leading to patient symptoms of congestion and discomfort. Despite the use of packing materials, scarring and adhesions are common, necessitating painful removal of additional tissue during postoperative treatments. |
| • | Potential for revision surgery. Within the first year after FESS, approximately 10% of patients will return to the operating room to undergo a revision procedure, while additional patients will return for a revision procedure after one year. We believe the risk of potential revision surgery is a significant deterrent to some patients that would otherwise undergo FESS for chronic sinusitis. |
We believe that because of the limitations of medical management and lack of disease resolution after FESS, many chronic sinusitis patients remain undertreated. We estimate that only a third of patients recommended for sinus surgery proceed with the potentially beneficial procedure, which we believe is due to its limitations and high risk for additional medical management and surgical revision. While balloon sinuplasty has been introduced to open the frontal, maxillary and sphenoid sinuses, or dependent sinuses, in a less invasive manner, it cannot treat disease in the most commonly involved sinuses, the ethmoids, and does not address the underlying inflammation associated with chronic sinusitis. We believe that an opportunity exists to reach these undertreated patients by providing a more effective option to address their inflammatory disease, while improving the overall outcomes of FESS.
Our Solution
We have designed a drug-eluting implant that consists of bioabsorbable polymers that control drug release and provide structural support to adjacent tissues during the healing process, while the implant is gradually and fully absorbed into the body.
3
Table of Contents
Our PROPEL implants are designed to improve the outcomes of sinus surgery by holding open the sinus passageways, reducing postoperative inflammation and scarring. They are inserted by a physician under endoscopic visualization during sinus surgery in the ethmoids. Once inserted, the self-expanding implants conform to and hold open the surgically enlarged sinus, while gradually releasing an anti-inflammatory steroid, mometasone furoate, directly to the sinus lining over a period of 30 days. Mometasone furoate, which is available generically and has been approved by the FDA for use in PROPEL and PROPEL mini, is the active ingredient in NASONEX, a nasal spray used to treat allergic rhinitis and other indications. The implants fully absorb into the body after a period of four to six weeks or are removed at the discretion of a physician during a routine office visit. Once absorbed or removed, the implant no longer provides structural support. We believe the principal benefits of our steroid-eluting implants are:
| • | Improved surgical outcomes. Our PROPEL implants have been clinically proven to improve FESS results by reducing postoperative inflammation and scarring. In a meta-analysis of prospective, multicenter, randomized, controlled, double-blind clinical studies, our PROPEL implants provided a 46% relative reduction in inflammation and a 70% relative reduction in scarring compared to the control implant. The control implant was identical to the PROPEL implant in composition, size, structure and mode of insertion but lacked the mometasone furoate coating. |
| • | Targeted steroid therapy to address postoperative inflammation. Our PROPEL implants are the first and only drug-eluting bioabsorbable sinus implants. They deliver postoperatively an anti-inflammatory steroid directly to the sinus lining in a controlled fashion over a period of 30 days, which helps in the wound healing process. In a meta-analysis of prospective, multicenter, randomized, controlled, double-blind clinical studies, our PROPEL implants reduced the need for oral steroids by 40% compared to the control implant. |
| • | Reduced pain and discomfort during postoperative period. Our PROPEL implants improve postoperative care. Once inserted, the self-expanding implants are designed to conform to and hold open the surgically enlarged ethmoid sinuses until fully absorbed into the body, which improves wound healing without obstructing the sinuses and causing congestion. Our steroid-eluting implants are designed to obviate the need for sinus packing materials, which can be a significant source of postoperative pain and discomfort. Our PROPEL implants significantly reduce scarring and adhesions, which reduces the potential for pain in postoperative treatments. |
| • | Reduced surgical revisions. In clinical studies, our PROPEL implants demonstrated a significant reduction in need for postoperative surgical intervention. In a meta-analysis of prospective, multicenter, randomized, controlled, double-blind clinical studies, our PROPEL implants provided a 35% relative reduction in the need for postoperative oral steroid and surgical intervention compared to the control implant. We believe that patients who have been deterred by the high revision rates associated with FESS may now consider surgical intervention to treat their chronic sinusitis condition. |
We believe these benefits will help expand the size of the FESS market as referring physicians increase their prescription of surgical intervention for chronic sinusitis and more patients elect to undergo sinus surgery to resolve their condition. However, although we believe PROPEL and PROPEL mini have significant advantages over sinus packing materials and other treatment options, we do not have clinical results directly comparing the effectiveness of our PROPEL implants versus sinus packing materials. Additionally, PROPEL and PROPEL mini are expensive relative to packing materials and may not be reimbursed by third-party payors, which has led some ENT physicians to not use these products, or to use them sparingly. ENT physicians may be slow to adopt our PROPEL implants until further clinical evidence or physician experience exists. Until we are able to obtain widespread coverage of our products by third-party payors, our products may be viewed by some ENT physicians, hospitals or other healthcare providers as too expensive to employ in every FESS procedure.
4
Table of Contents
Our Strategy
Our goal is to be a leading drug-device company committed to advancing clinically proven therapeutic solutions that improve the quality of life for patients with ENT conditions. The key elements of our strategy include:
| • | Expand our sales and marketing organizations to support growth. We plan to continue to invest in the expansion of our sales and marketing organizations to provide ENT physicians in the United States with access to our steroid-eluting implants. |
| • | Promote awareness amongst ENT physicians, referring physicians and patients. We believe that many patients with chronic sinusitis are currently undertreated, and we intend to educate ENT physicians, allergists, primary care physicians or other referring medical professionals and patients to raise awareness of the disease and available treatment alternatives, including the advantages of using our steroid-eluting implants. |
| • | Advance our platform of innovative products for additional chronic sinusitis patients. We are developing additional products using our drug-eluting bioabsorbable implant technology that are specifically designed to treat and manage chronic sinusitis in the physician office setting during a routine visit. We are also working to expand indications for our PROPEL implants to allow for the treatment of the dependent sinuses. |
| • | Facilitate access to our products via third-party reimbursement. We believe that establishment of reimbursement codes specific to the use of drug-eluting implants for chronic sinusitis is an important factor in expanding access to our products, especially in the physician office setting. Therefore, our strategy includes efforts to engage physician societies and encourage third-party payors to establish coverage, coding and payment that will facilitate access to our drug-eluting implants as we expand our commercialization efforts in the United States. |
| • | Introduce our steroid-eluting implants in markets outside the United States. We plan to leverage our FDA PMA approved products and clinical data to access markets outside the United States, starting first with the development of clinical, regulatory and reimbursement strategies for countries in Europe and the Asia-Pacific region. |
Risks Related to Our Business
Our ability to successfully operate our business is subject to numerous risks, including those that are generally associated with operating in the medical device industry. Any of the factors set forth under the heading “Risk Factors” may limit our ability to successfully execute our business strategy. You should carefully consider all of the information set forth in this prospectus and, in particular, you should evaluate the specific factors set forth under the heading “Risk Factors” in deciding whether to invest in our common stock. Some of the principal risks relating to our business and our ability to execute our business strategy include:
| • | We have incurred significant operating losses since inception and may not be able to achieve profitability. |
| • | All of our revenue is generated from a limited number of products and we are completely dependent on the success of our PROPEL and PROPEL mini steroid-eluting implants, which have a limited commercial history. If these products fail to gain widespread market acceptance, our business will suffer. |
| • | The establishment of adequate coverage and reimbursement is important for sales of our products. Coverage and reimbursement policies for procedures using our steroid-eluting implants could affect the adoption of our products, particularly our product candidates for the physician office setting, and our future revenue. |
| • | Pricing pressure from our hospital and ambulatory surgery center customers due to limited coverage and reimbursement for our products may impact our ability to sell our products at prices necessary to support our current business strategies. |
| • | We may never obtain regulatory approval for future products or products outside the United States. |
| • | If we are unable to protect our intellectual property, or operate our business without infringing on the intellectual property rights of third parties, our business will be negatively affected. |
5
Table of Contents
Corporate Information
We were incorporated in Delaware in October 2003 as Sinexus, Inc. We changed our name to Intersect ENT, Inc. in November 2009. Our offices are located at 1555 Adams Drive, Menlo Park, California 94025. Our telephone number is (650) 641-2100. Our corporate website is at www.intersectent.com. The information contained on or that can be accessed through our website is not incorporated by reference into this prospectus, and you should not consider information on our website to be part of this prospectus or in deciding to purchase our common stock.
Preliminary Unaudited Second Quarter 2014 Financial Expectations
Set forth below are certain preliminary revenue, cost, expense and net loss expectations for the three and six months ended June 30, 2014. These preliminary results represent our estimates only based on currently available information and do not present all necessary information for an understanding of our financial condition as of June 30, 2014 or our results of operations for the three and six months ended June 30, 2014. As we complete our quarter-end financial close process and finalize our second quarter 2014 unaudited financial statements, we will be required to make significant judgments in a number of areas, including inventory, stock-based compensation and the liability for preferred stock warrants. This financial information has been prepared by and is the responsibility of our management. Our independent registered public accounting firm has not audited, reviewed or performed any procedures with respect to this preliminary financial data or the accounting treatment thereof and does not express an opinion or any other form of assurance with respect thereto. We expect to complete our unaudited financial statements for the quarter ended June 30, 2014 subsequent to the completion of this offering. It is possible that we or our independent registered public accounting firm may identify items that require us to make adjustments to the financial information set forth below and those changes could be material. Accordingly, undue reliance should not be placed on these preliminary estimates. These preliminary estimates are not necessarily indicative of any future period and should be read together with “Risk Factors,” “Special Note Regarding Forward-Looking Statements,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Selected Financial Data” and our financial statements and related notes included elsewhere in this prospectus.
We estimate that our total revenue for the three months ended June 30, 2014 was between approximately $8.4 million and $8.6 million as compared to $3.9 million for the three months ended June 30, 2013. We estimate that our total revenue for the six months ended June 30, 2014 was between approximately $15.9 million and $16.1 million as compared to $6.7 million for the six months ended June 30, 2013. The estimated increase in total revenue is primarily attributable to the growth of our revenue as we expanded our commercial sales force by increasing the number of our territory managers.
We estimate that our cost of sales was between approximately $2.3 million and $2.6 million for the three months ended June 30, 2014 as compared to cost of sales of $2.2 million for the three months ended June 30, 2013. We estimate that our cost of sales was between approximately $4.7 million and $5.0 million for the six months ended June 30, 2014 as compared to cost of sales of $4.2 million for the six months ended June 30, 2013. This estimated increase is primarily due to the increase in units sold.
We estimate that our operating expenses for the three months ended June 30, 2014 was between approximately $10.7 million and $11.2 million as compared to $6.5 million for the three months ended June 30, 2013. We estimate that our operating expenses for the six months ended June 30, 2014 was between approximately $19.9 million and $20.4 million as compared to $12.1 million for the six months ended June 30, 2013. This increase is primarily due to headcount increases associated with the commercial expansion of our sales force and related growth of our business and increased marketing and promotional expenses.
We estimate that our operating loss for the three months ended June 30, 2014 was between approximately $4.4 million and $5.4 million and between $8.5 million and $9.5 million for the six months ended June 30, 2014. We also estimate that our net loss for the three months ended June 30, 2014 was between approximately $4.4 million and $5.4 million and between $8.8 million and $9.8 million for the six months ended June 30, 2014. Consequently, we expect that our accumulated deficit increased to between approximately $87.1 million and $88.1 million as of June 30, 2014.
6
Table of Contents
Implications of Being an Emerging Growth Company
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and are eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. These include, but are not limited to, (1) reduced obligations with respect to the disclosure of selected financial data in registration statements filed with the Securities and Exchange Commission, or the SEC, including the registration statement on Form S-1 of which this prospectus is a part, (2) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, (3) an exception from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, and (4) exemptions from the requirements of holding a non-binding advisory vote on executive compensation and the requirement to obtain shareholder approval of any golden parachute payments not previously approved.
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenue, we have more than $700 million in market value of our stock held by non-affiliates or we issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of certain reduced reporting burdens in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
In addition, Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, or Securities Act, for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We intend to take advantage of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for private companies. Section 107 of the JOBS Act provides that we can elect to opt out of the extended transition period at any time, which election is irrevocable.
7
Table of Contents
The Offering
| Issuer |
Intersect ENT, Inc. |
| Common stock offered by us |
5,000,000 shares (or 5,750,000 shares if the underwriters exercise in full their option to purchase additional shares) |
| Common stock to be outstanding immediately after this offering |
22,501,808 shares (or 23,251,808 shares if the underwriters exercise in full their option to purchase additional shares) |
| Underwriters’ option to purchase additional shares |
750,000 shares |
| Use of proceeds |
We intend to use substantially all of the net proceeds from this offering for sales, marketing, research and development activities, clinical and regulatory initiatives, working capital and general corporate purposes. See “Use of Proceeds” on page 43 for a more complete description of the intended use of proceeds from this offering. |
| Risk factors |
See “Risk Factors” beginning on page 12 and other information included in this prospectus for a discussion of factors that you should consider carefully before deciding to invest in our common stock. |
| Proposed Nasdaq Global Market Symbol |
“XENT” |
The number of shares of common stock to be outstanding after this offering is based on 17,501,808 shares of common stock outstanding as of March 31, 2014, and excludes the following:
| • | 1,964,978 shares of common stock issuable upon the exercise of outstanding options as of March 31, 2014, having a weighted average exercise price of $1.34 per share; |
| • | 47,554 shares of common stock issuable upon the exercise and subsequent conversion of warrants to purchase our Series A convertible preferred stock at an exercise price of $3.68 per share; |
| • | Up to 20,313 shares of common stock issuable upon the exercise and subsequent conversion of a warrant to purchase our Series D convertible preferred stock at an exercise price of $6.89 per share, which, as of March 31, 2014, was exercisable for 5,803 shares; |
| • | 4,750,000 shares of common stock reserved for future issuance under our 2014 Equity Incentive Plan, as well as any automatic increases in the number of shares of common stock reserved for future issuance under this plan, which will become effective upon the execution of the underwriting agreement related to this offering; and |
| • | 496,092 shares of common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan, as well as any automatic increases in the number of shares of common stock reserved for future issuance under this plan, which will become effective upon the execution of the underwriting agreement related to this offering. |
Unless otherwise indicated, all information in this prospectus assumes:
| • | the automatic conversion of 15,703,875 shares of our convertible preferred stock outstanding as of March 31, 2014, into an aggregate of 15,703,875 shares of our common stock immediately prior to the closing of this offering; |
| • | the automatic conversion of all convertible preferred stock warrants outstanding as of March 31, 2014, into warrants to purchase up to an aggregate 67,867 shares of our common stock immediately prior to the closing of this offering; |
8
Table of Contents
| • | the filing of our amended and restated certificate of incorporation and the effectiveness of our amended and restated bylaws immediately prior to the closing of this offering; |
| • | no exercise of the underwriters’ option to purchase additional shares; and |
| • | a 1-for-4 reverse stock split of our common stock and preferred stock effected on July 11, 2014. |
9
Table of Contents
Summary Financial Data
The following tables set forth a summary of our historical financial data as of, and for the period ended on, the dates indicated. The statements of operations data for the years ended December 31, 2012 and 2013, are derived from our audited financial statements included elsewhere in this prospectus. The statements of operations data for the three months ended March 31, 2013 and 2014, and the balance sheet data as of March 31, 2014, are derived from our unaudited interim financial statements included elsewhere in this prospectus. We have prepared the unaudited interim financial statements on the same basis as the audited financial statements and have included, in our opinion, all adjustments, consisting only of normal recurring adjustments that we consider necessary for a fair presentation of the financial information set forth in those statements. You should read this data together with our audited financial statements and related notes appearing elsewhere in this prospectus and the information under the captions “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results are not necessarily indicative of our future results and are not necessarily indicative of results to be expected for the full year ending December 31, 2014, or any other period.
| Year
Ended December 31, |
Three Months Ended March 31, |
|||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (in thousands, except per share data) |
(unaudited) | |||||||||||||||
| Statements of Operations Data: |
||||||||||||||||
| Revenue |
$ | 5,863 | $ | 17,931 | $ | 2,744 | $ | 7,497 | ||||||||
| Cost of sales |
3,837 | 8,150 | 1,949 | 2,360 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
2,026 | 9,781 | 795 | 5,137 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Selling, general and administrative |
9,251 | 18,229 | 3,350 | 6,658 | ||||||||||||
| Research and development |
9,260 | 9,518 | 2,256 | 2,577 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
18,511 | 27,747 | 5,606 | 9,235 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(16,485 | ) | (17,966 | ) | (4,811 | ) | (4,098 | ) | ||||||||
| Other income (expense), net |
120 | (403 | ) | 58 | (311 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (16,365 | ) | $ | (18,369 | ) | $ | (4,753 | ) | $ | (4,409 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share, basic and diluted |
$ | (16.38 | ) | $ | (12.57 | ) | $ | (4.57 | ) | $ | (2.49 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average common shares used to compute net loss per share, basic and diluted |
999 | 1,461 | 1,039 | 1,773 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma net loss per share, basic and diluted (unaudited)(1) |
$ | (1.17 | ) | $ | (0.25 | ) | ||||||||||
|
|
|
|
|
|||||||||||||
| Weighted average common shares used to compute pro forma net loss per share, basic and diluted (unaudited)(1) |
15,655 | 17,474 | ||||||||||||||
|
|
|
|
|
|||||||||||||
10
Table of Contents
| As of March 31, 2014 | ||||||||||||
| (in thousands) |
Actual | Pro Forma(1) | Pro
Forma As Adjusted(2)(3) |
|||||||||
| (unaudited) | ||||||||||||
| Balance Sheet Data: |
||||||||||||
| Cash and cash equivalents |
$ | 7,478 | $ | 7,478 | $ | 60,278 | ||||||
| Working capital |
8,643 | 9,137 | 61,937 | |||||||||
| Total assets |
16,767 | 16,767 | 69,567 | |||||||||
| Preferred stock warrant liability |
494 | — | — | |||||||||
| Total debt |
1,280 | 1,280 | 1,280 | |||||||||
| Total liabilities |
6,783 | 6,289 | 6,289 | |||||||||
| Convertible preferred stock |
90,789 | — | — | |||||||||
| Accumulated deficit |
(82,668 | ) | (82,668 | ) | (82,668 | ) | ||||||
| Total stockholders’ (deficit) equity |
(80,805 | ) | 10,478 | 63,278 | ||||||||
| (1) | Reflects the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 15,703,875 shares of common stock immediately prior to the closing of this offering and the conversion of warrants to purchase up to 67,867 shares of convertible preferred stock into warrants to purchase up to 67,867 shares of common stock immediately prior to the closing of this offering. |
| (2) | Reflects the pro forma adjustments described in footnote (1) above and the sale by us of 5,000,000 shares of common stock in this offering at an assumed initial public offering price of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
| (3) | A $1.00 increase (decrease) in the assumed initial public offering price of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) each of pro forma as adjusted cash and cash equivalents, working capital, total assets and total stockholders’ equity by $4.7 million, assuming the number of shares we are offering, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) each of pro forma as adjusted cash and cash equivalents, working capital, total assets and total stockholders’ equity by approximately $11.2 million, assuming the assumed initial public offering price per share remains the same. The pro forma as adjusted information is illustrative only, and we will adjust this information based on the actual initial public offering price, number of shares offered and other terms of this offering determined at pricing. |
11
Table of Contents
An investment in our common stock involves a high degree of risk. You should carefully consider the following risk factors together with all of the other information contained in this prospectus, including our financial statements and the related notes, before deciding whether to invest in shares of our common stock. Each of these risks could harm our business, operating results, financial condition or growth prospects. As a result, the trading price of our common stock could decline and you may lose all or part of your investment.
Risks Related to Our Business
We have incurred significant operating losses since inception and may not be able to achieve profitability.
We have incurred net losses since our inception in 2003. For the years ended December 31, 2012 and 2013, and for the three months ended March 31, 2014, we had net losses of $16.4 million, $18.4 million and $4.4 million, respectively. As of March 31, 2014, we had an accumulated deficit of $82.7 million. To date, we have financed our operations primarily through private placements of our equity securities, certain debt-related financing arrangements and from sales of our approved products. We have devoted substantially all of our resources to research and development of our products, sales and marketing activities and clinical and regulatory initiatives to obtain approvals for our products. Our ability to generate sufficient revenue from our existing products or from any of our product candidates in development, and to transition to profitability and generate consistent positive cash flows is uncertain. Following this offering, we expect that our operating expenses will continue to increase as we continue to build our commercial infrastructure, develop, enhance and commercialize new products and incur additional operational and reporting costs associated with being a public company. As a result, we expect to continue to incur operating losses for the foreseeable future and may never achieve profitability.
All of our revenue is generated from our PROPEL and PROPEL mini steroid-eluting implants and we are completely dependent on the success of these products, which have a limited commercial history. If these products fail to gain widespread market acceptance, our business will suffer.
We started selling PROPEL in August 2011 and PROPEL mini in November 2012. We expect that sales of these products will account for substantially all of our revenue for the foreseeable future and therefore our ability to become profitable will depend upon the commercial success of these products. Because of their recent commercial introduction, PROPEL and PROPEL mini have limited product and brand recognition. We market these products primarily to ear, nose and throat, or ENT, physicians and believe they may be slow or fail to adopt our products for a variety of reasons, including, among others:
| • | lack of experience with our products; |
| • | lack of availability of adequate coverage and reimbursement for hospitals, ambulatory surgery centers and physicians; |
| • | lack of evidence supporting cost benefits or cost effectiveness of our products over existing alternatives; |
| • | lack of clinical data supporting patient benefits beyond six months; |
| • | perception that our products are unproven, investigational or experimental; |
| • | liability risks generally associated with the use of new products and procedures; and |
| • | training required to use new products. |
If we are unable to effectively demonstrate to ENT physicians the benefits of our products when used during sinus surgery and our products fail to achieve market acceptance, our future revenue will be adversely impacted. In addition, we believe recommendations and support of our products by influential ENT physicians are essential for market acceptance and adoption. If we do not receive support from these influential ENT physicians, ENT physicians in general may not use our products and our future revenue will be harmed.
Because of the numerous risks and uncertainties associated with our commercialization efforts, we are unable to predict the extent to which we will continue to generate revenue from our products or the timing for
12
Table of Contents
when or the extent to which we will become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on an ongoing basis.
Pricing pressure from our hospital and ambulatory surgery center customers due to limited coverage and reimbursement for our products may impact our ability to sell our products at prices necessary to support our current business strategies.
Hospital and other healthcare provider customers, including ambulatory surgery center customers, that purchase our products typically bill various third-party payors to cover all or a portion of the costs and fees associated with the sinus surgery procedures in which our products are used and bill patients for any deductibles or co-payments. Because there is often no separate reimbursement for supplies used in surgical procedures, the additional cost associated with the use of our steroid-eluting implants can impact the profit margin of the hospital or surgery center where the sinus surgery is performed. Some of our target customers may be unwilling to adopt our steroid-eluting implants in light of the additional associated cost. Further, any decline in the amount payors are willing to reimburse our customers for sinus surgery procedures could make it difficult for existing customers to continue using, or adopt, our steroid-eluting implants and could create additional pricing pressure for us. If we are forced to lower the price we charge for our products, our gross margins will decrease, which will adversely affect our ability to invest in and grow our business.
We are actively seeking new billing codes for our products and the procedure associated with their use, including codes that are established by the Centers for Medicare & Medicaid Services, or CMS, and the American Medical Association, or AMA. Our ability to obtain new billing codes will depend, in part, on support from the ENT community and physician acceptance of our technology. Although obtaining billing codes may result in payment amounts that better reflect the costs and resources of our products and related procedure, there is a possibility that they may not do so.
We cannot assure you that we will be successful in garnering the necessary support for new codes from the ENT community or from third-party payors, who are responsible for determining which billing codes are to be used for procedures performed on their insured population. Even if we are able to establish reimbursement codes for our products, we will continue to be subject to significant pricing pressure, which could harm our business, results of operations, financial condition and prospects.
Our future growth depends on physician awareness and adoption of our steroid-eluting implants.
We focus our sales, marketing and education efforts primarily on ENT physicians. We train physicians on the patient population that would benefit from our steroid-eluting implants. This patient population is based on those included in our clinical studies and includes, for example, patients with or without polyps as well as patients undergoing either primary or revision surgery. Some physicians may choose to utilize our products on a subset of their patients such as patients with severe polyp disease that they deem at higher risk for postoperative complications. If we are not able to effectively demonstrate to those physicians that our products are beneficial in a broad range of patients on which they operate, their adoption of our products will be limited.
We train our physician customers on the proper techniques in using our devices to achieve the intended outcome. The successful use of our steroid-eluting implants depends in large part on the physician’s adherence to the techniques that they are provided in training by our sales representatives. In the event that physicians do not adhere to these techniques or if they perceive that our products are too cumbersome for them to use, we may have difficulty facilitating adoption. Additionally, physicians may develop their own techniques for use of our products during insertion and during the period in which the drug is delivered and is bioabsorbed. For example, we are aware some physicians are removing our steroid-eluting implants before all of the drug has eluted into the surrounding tissue. While physicians were allowed to remove the implant at any time at their discretion in our clinical studies, early removal could lead to suboptimal outcomes. In addition, if physicians utilize our products in a manner that is inconsistent with how they were studied clinically, their outcomes may not be consistent with the outcomes achieved in our clinical studies, which may impact their perception of patient benefit and limit their adoption of our products.
In addition, the initial point of contact for many patients suffering from chronic sinusitis may be primary care physicians or other referring medical professionals who commonly treat patients experiencing sinus-related
13
Table of Contents
symptoms or complications. We believe that we must educate these primary care physicians and other referring medical professionals about our steroid-eluting implants in order to grow the market beyond the over 3.5 million patients with chronic sinusitis who are currently managed by ENT physicians. If we fail to do so, these primary care physicians and other referring medical professionals may not refer patients to an ENT physician who will perform sinus surgery and use our steroid-eluting implants. As a result, those patients may go untreated, attempt to manage their sinusitis through medical management alone or seek alternative surgical procedures. If we are not successful in educating primary care physicians and other referring medical professionals about our steroid-eluting implants, our ability to increase our revenue may be impaired.
We have limited experience marketing and selling our steroid-eluting implants, and if we are unable to expand, manage and maintain our direct sales and marketing organizations we may not be able to generate anticipated revenue.
We started selling our first approved product, PROPEL, on a limited basis in August 2011. We subsequently started selling PROPEL mini in November 2012, and in the first half of 2013 we began to expand and scale our direct sales and marketing organizations in the United States. As a result, we have limited experience marketing and selling our steroid-eluting implants. As of March 31, 2014, our direct sales organization, including marketing, customer service and reimbursement, consisted of 62 employees, having increased from 21 employees as of December 31, 2012. Our operating results are directly dependent upon the sales and marketing efforts of our employees. If our direct sales representatives fail to adequately promote, market and sell our products, our sales may suffer.
In order to generate our anticipated sales, we will need to expand the size and geographic scope of our direct sales organization. As a result, our future success will depend largely on our ability to continue to hire, train, retain and motivate skilled regional sales managers and direct sales representatives with significant technical knowledge of ENT. Because of the competition for their services, we cannot assure you we will be able to hire and retain additional direct sales representatives on favorable or commercially reasonable terms, if at all. Failure to hire or retain qualified sales representatives would prevent us from expanding our business and generating sales. Additionally, new hires require training and take time before they achieve full productivity. If we fail to train new hires adequately, new hires may not become as productive as may be necessary to maintain or increase our sales.
If we are unable to expand our sales and marketing capabilities, we may not be able to effectively commercialize our products, which would adversely affect our business, results of operations and financial condition.
Our clinical studies were designed to demonstrate the safety and efficacy of our steroid-eluting implants based on FDA requirements and may not be seen as compelling to physicians. Any subsequent clinical studies that are conducted and published may not be positive or consistent with our existing data, which would affect the rate of adoption of our products.
Our success depends on the medical community’s acceptance of our steroid-eluting implants as tools that are useful to ENT physicians treating patients with chronic sinusitis. We have sponsored three multi-center, prospective studies of over 200 patients to track outcomes of treatment with our steroid-eluting implants, which clinical data has resulted in the highest level of evidence generated for any product used in sinus surgery. The principal safety and efficacy information of our steroid-eluting implants is derived from the ADVANCE II study, a prospective, multicenter, randomized controlled, double-blind, pivotal study that was completed in September 2010. We also sponsored the ADVANCE study, a prospective, multicenter, single-cohort, open-label trial completed in December 2009 and the PROPEL Pilot Study, a prospective, multicenter, randomized, controlled, double-blind feasibility study completed in April 2009. While the results of these three studies collectively indicate a favorable safety and efficacy profile, the study designs and results may not be viewed as compelling to our physician customers. If physicians do not find our data compelling, they may choose not to use our products or limit their use. Our PROPEL Pilot study and ADVANCE II study incorporated an intra-patient control design comparing PROPEL to a non-drug-eluting control version of the implant in order to maintain blinding. Primary efficacy endpoints for these two studies were measured at 30 days after placement as we believe that proper healing in the immediate postoperative period is indicative of long-term outcomes. Additionally, it was important to allow for medical intervention after day 30 given one sinus side of each patient had the control device. Clinical
14
Table of Contents
efficacy demonstrated at this short-term endpoint does not guarantee long-term clinical benefits. Our ADVANCE study measured patient symptom improvements out to six months. The long-term effects of sinus surgery in conjunction with our steroid-eluting implants beyond six months are not known. Certain ENT physicians, hospitals and surgery centers may prefer to see longer term efficacy data than we have produced. We cannot assure you that any data that we or others generate will be consistent with that observed in these studies nor that results will be maintained beyond the time points studied. We also cannot assure you that any data that may be collected will be compelling to the medical community because the data may not be scientifically meaningful and may not demonstrate that sinus surgery using our steroid-eluting implants is an attractive procedure when compared against data from alternative treatments.
Each ENT physician’s individual experience with our steroid-eluting implants will vary, and we believe that physicians will compare actual long-term outcomes in their own practices using our steroid-eluting implants against sinus surgery used in conjunction with traditional sinus packing techniques. A long-term, adequately-controlled clinical study comparing sinus surgery performed in conjunction with our steroid-eluting implants against sinus surgery performed in conjunction with the variety of traditional sinus packing techniques incorporated by physicians would be expensive and time-consuming and we have not conducted, and are not currently planning to conduct, such a study. If the experience of physicians indicates that the use of our steroid-eluting implants in FESS is not as safe or efficacious as other treatment options or does not provide a lasting solution to patients with chronic sinusitis, adoption of our products may suffer and our business would be harmed.
We utilize third-party, single source suppliers for many of the components and materials used in our steroid-eluting implants, and the loss of any of these suppliers could harm our business.
The active pharmaceutical ingredient, or API, and a number of our critical components used in our steroid-eluting implants are supplied to us from single source suppliers. We rely on single source suppliers for some of our polymer materials, some extrusions and molded components, some off-the-shelf components and for finished goods testing. Our ability to supply our products commercially and to develop our product candidates depends, in part, on our ability to obtain these components in accordance with regulatory requirements and in sufficient quantities for commercialization and clinical testing. We have entered into manufacturing, supply or quality agreements with a number of our single source suppliers pursuant to which they supply the components we need. We are not certain that our single source suppliers will be able to meet our demand for their products, either because of the nature of our agreements with those suppliers, our limited experience with those suppliers or our relative importance as a customer to those suppliers. It may be difficult for us to assess their ability to timely meet our demand in the future based on past performance. While our suppliers have generally met our demand for their products on a timely basis in the past, they may subordinate our needs in the future to their other customers.
Establishing additional or replacement suppliers for the API or any of the components or processes used in our products, if required, may not be accomplished quickly. If we are able to find a replacement supplier, the replacement supplier would need to be qualified and may require additional regulatory authority approval, which could result in further delay. For example, the U.S. Food and Drug Administration, or FDA, could require additional supplemental data if we rely upon a new supplier for the API used in PROPEL and PROPEL mini. While we seek to maintain adequate inventory of the single source components and materials used in our products, any interruption or delay in the supply of components or materials, or our inability to obtain components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and cause them to cancel orders.
If our third-party suppliers fail to deliver the required commercial quantities of materials, on a timely basis and at commercially reasonable prices, and we are unable to find one or more replacement suppliers capable of production at a substantially equivalent cost in substantially equivalent volumes and quality, and on a timely basis, the continued commercialization of our products and the development of our product candidates would be impeded, delayed, limited or prevented, which could harm our business, results of operations, financial condition and prospects.
It is difficult to forecast future performance, which may cause our financial results to fluctuate unpredictably.
Our limited operating history and commercial experience make it difficult for us to predict future performance. As we gain additional commercial experience, a number of factors over which we have limited
15
Table of Contents
control may contribute to fluctuations in our financial results, such as seasonal variations in revenue. In the first quarter, our results can be impacted by adverse weather and by resetting of annual patient healthcare insurance plan deductibles, both of which may cause patients to delay elective procedures such as FESS. In the second quarter, demand may be impacted by the seasonal nature of allergies and the resultant onset of sinus-related symptoms. In the third quarter, the number of FESS procedures nationwide is historically lower than other quarters throughout the year, which we believe is attributable to the summer vacations of ENT physicians and their patients. In the fourth quarter, demand may be impacted by the onset of the cold and flu season and related symptoms, as well as the desire of patients to spend their remaining balances in flexible-spending accounts or because they have met their annual deductibles under their health insurance plans. Other factors that may impact our quarterly results include:
| • | ENT physician adoption of our steroid-eluting implants; |
| • | unanticipated pricing pressure; |
| • | the hiring, retention and continued productivity of our sales representatives; |
| • | our ability to expand the geographic reach of our sales and marketing efforts; |
| • | our ability to obtain regulatory clearance or approval for our products in development or for our current products outside the United States; |
| • | results of clinical research and trials on our existing products and products in development; |
| • | delays in receipt of anticipated purchase orders; |
| • | timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors; |
| • | delays in, or failure of, component and raw material deliveries by our suppliers; and |
| • | positive or negative coverage in the media or clinical publications of our steroid-eluting implants or products of our competitors or our industry. |
In the event our actual revenue and operating results do not meet our forecasts for a particular period, the market price of our common stock may decline substantially.
Our long-term growth depends on our ability to develop and commercialize additional ENT products.
It is important to our business that we continue to build a more complete product offering within the ENT market. We are using our drug-eluting bioabsorbable technology to develop new products for use in the physician office setting. Developing additional products is expensive and time-consuming and could divert management’s attention away from our current sinus surgery products and harm our business. Even if we are successful in developing additional products, including those currently in development for use in the physician office setting, the success of any new product offering or enhancement to an existing product will depend on several factors, including our ability to:
| • | properly identify and anticipate ENT physician and patient needs; |
| • | develop and introduce new products or product enhancements in a timely manner; |
| • | avoid infringing upon the intellectual property rights of third parties; |
| • | demonstrate, if required, the safety and efficacy of new products with data from preclinical studies and clinical trials; |
| • | obtain the necessary regulatory clearances or approvals for new products or product enhancements; |
| • | be fully FDA-compliant with marketing of new devices or modified products; |
| • | provide adequate training to potential users of our products; |
| • | receive adequate coverage and reimbursement for procedures performed with our products; and |
| • | develop an effective and FDA-compliant, dedicated sales and marketing team. |
16
Table of Contents
If we are unsuccessful in developing and commercializing additional products in other areas of ENT, our ability to increase our revenue may be impaired.
If clinical studies of our future products do not produce results necessary to support regulatory clearance or approval in the United States or, with respect to our current or future products, elsewhere, we will be unable to commercialize these products.
We will likely need to conduct additional clinical studies in the future to support new product approvals, or for the approval of the use of our products in some foreign countries. Clinical testing takes many years, is expensive and carries uncertain outcomes. The initiation and completion of any of these studies may be prevented, delayed, or halted for numerous reasons, including, but not limited to, the following:
| • | the FDA, institutional review boards or other regulatory authorities do not approve a clinical study protocol, force us to modify a previously approved protocol, or place a clinical study on hold; |
| • | patients do not enroll in, or enroll at a lower rate than we expect, or do not complete a clinical study; |
| • | patients or investigators do not comply with study protocols; |
| • | patients do not return for post-treatment follow-up at the expected rate; |
| • | patients experience unexpected adverse event or side effects for a variety of reasons that may or may not be related to our products; |
| • | sites participating in an ongoing clinical study withdraw, requiring us to engage new sites; |
| • | difficulties or delays associated with establishing additional clinical sites; |
| • | third-party clinical investigators decline to participate in our clinical studies, do not perform the clinical studies on the anticipated schedule, or are inconsistent with the investigator agreement, clinical study protocol, good clinical practices or other agency requirements; |
| • | third-party organizations do not perform data collection and analysis in a timely or accurate manner; |
| • | regulatory inspections of our clinical studies or manufacturing facilities require us to undertake corrective action or suspend or terminate our clinical studies; |
| • | changes in federal, state, or foreign governmental statutes, regulations or policies; |
| • | interim results are inconclusive or unfavorable as to immediate and long-term safety or efficacy; |
| • | the study design is inadequate to demonstrate safety and efficacy; or |
| • | the study does not meet the statistical endpoints. |
Clinical failure can occur at any stage of the testing. Our clinical studies may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical and non-clinical testing in addition to those we have planned. Our failure to adequately demonstrate the safety and efficacy of any of our devices would prevent receipt of regulatory clearance or approval and, ultimately, the commercialization of that device or indication for use. Even if our future products are approved in the United States, commercialization of our products in foreign countries would require approval by regulatory authorities in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials. Any of these occurrences may harm our business, results of operations, financial condition and prospects.
Consolidation in the healthcare industry could lead to demands for price concessions, which may impact our ability to sell our products at prices necessary to support our current business strategies.
Healthcare costs have risen significantly over the past decade, which has driven numerous cost reform initiatives by legislators, regulators and third-party payors. A typical FESS procedure is paid at a Medicare rate of approximately $10,000. Private insurer payment rates average 139 percent of Medicare rates nationally. Cost reform has elicited a consolidation trend in the healthcare industry to aggregate purchasing power, which may
17
Table of Contents
create more requests for pricing concessions in the future. Additionally, group purchasing organizations, independent delivery networks and large single accounts may continue to use their market power to consolidate purchasing decisions for hospitals and ambulatory surgery centers. We expect that market demand, government regulation, third-party coverage and reimbursement policies and societal pressures will continue to change the healthcare industry worldwide, resulting in further business consolidations and alliances among our customers, which may exert further downward pressure on the prices of our products and may adversely impact our business, results of operations, financial condition and prospects.
We compete or may compete in the future against other companies, some of which have longer operating histories, more established products and greater resources, which may prevent us from achieving significant market penetration or improved operating results.
Our industry is highly competitive, subject to change and significantly affected by new product introductions and other activities of industry participants. Many of the companies developing or marketing ENT products are publicly traded or are divisions of publicly-traded companies, including the Xomed division of Medtronic, the Gyrus ACMI division of Olympus, the Acclarent division of Johnson & Johnson, Stryker and ArthroCare. These companies enjoy several competitive advantages, including:
| • | greater financial and human capital resources; |
| • | significantly greater name recognition; |
| • | established relationships with ENT physicians, referring physicians, customers and third-party payors; |
| • | additional lines of products, and the ability to offer rebates or bundle products to offer greater discounts or incentives to gain a competitive advantage; and |
| • | established sales, marketing and worldwide distribution networks. |
Because of the size of the market opportunity for the treatment of chronic sinusitis, we believe potential competitors have historically dedicated and will continue to dedicate significant resources to aggressively promote their products or develop new products. New product developments that could compete more effectively with our products are possible because of the prevalence of chronic sinusitis and the extensive research efforts and technological progress that exist within the market. Large medical device companies with ENT divisions, such as Medtronic, also have capability in drug-eluting stents and smaller companies may develop competing products. Though we are not aware of any such products to date, these or other companies may develop drug-eluting products that could compete with our products.
Our commercially available products are designed to be used during sinus surgery. If another company successfully develops an approach for the treatment of chronic sinusitis that would not benefit from the use of our steroid-eluting implants, if another company develops a device to treat the inflammation and scarring associated with sinus surgery that is more efficacious than our steroid-eluting implants, or if a pharmaceutical company successfully develops a drug that addresses chronic sinusitis without the need for surgical intervention, sales of our products would be significantly and adversely affected.
We may be unable to manage our growth effectively.
Our past growth has provided, and our future growth may create, challenges to our organization. From December 31, 2012, to March 31, 2014, the number of our employees increased from 75 to 146. In the future, we expect to hire and train new personnel as we continue to grow and expand our operations. As a public company, we will need to further expand our scientific, sales and marketing, managerial, operational, financial and other resources to support our planned research, development and commercialization activities. This growth may place significant strain on our management, financial and operational resources. Successful growth is also dependent upon our ability to implement appropriate financial and management controls, systems and procedures and securing a sufficient amount of office space for additional personnel. If we fail to manage these challenges effectively, our business could be harmed.
18
Table of Contents
We face the risk of product liability claims that could be expensive, divert management’s attention and harm our reputation and business. We may not be able to maintain adequate product liability insurance.
Our business exposes us to the risk of product liability claims that are inherent in the testing, manufacturing and marketing of medical devices and drug products. This risk exists even if a device or product is approved for commercial sale by the FDA and manufactured in facilities licensed and regulated by the FDA, such as the case with PROPEL and PROPEL mini, or an applicable foreign regulatory authority. Our products and product candidates are designed to affect important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with our products or our product candidates could result in patient injury or death. The medical device industry has historically been subject to extensive litigation over product liability claims, and we cannot offer any assurance that we will not face product liability suits. We may be subject to product liability claims if our steroid-eluting implants cause, or merely appear to have caused, patient injury or death. In addition, an injury that is caused by the activities of our suppliers, such as those who provide us with components and raw materials, may be the basis for a claim against us. Product liability claims may be brought against us by consumers, health care providers or others selling or otherwise coming into contact with our products or product candidates, among others. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities and reputational harm. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| • | costs of litigation; |
| • | distraction of management’s attention from our primary business; |
| • | the inability to commercialize our products or, if approved, our product candidates; |
| • | decreased demand for our products or, if approved, product candidates; |
| • | impairment of our business reputation; |
| • | product recall or withdrawal from the market; |
| • | withdrawal of clinical trial participants; |
| • | substantial monetary awards to patients or other claimants; or |
| • | loss of revenue. |
While we may attempt to manage our product liability exposure by proactively recalling or withdrawing from the market any defective products, any recall or market withdrawal of our products may delay the supply of those products to our customers and may impact our reputation. We can provide no assurance that we will be successful in initiating appropriate market recall or market withdrawal efforts that may be required in the future or that these efforts will have the intended effect of preventing product malfunctions and the accompanying product liability that may result. Such recalls and withdrawals may also be used by our competitors to harm our reputation for safety or be perceived by patients as a safety risk when considering the use of our products, either of which could have an adverse effect on our business.
In addition, although we have product liability and clinical study liability insurance that we believe is appropriate, this insurance is subject to deductibles and coverage limitations. Our current product liability insurance may not continue to be available to us on acceptable terms, if at all, and, if available, coverage may not be adequate to protect us against any future product liability claims. If we are unable to obtain insurance at an acceptable cost or on acceptable terms with adequate coverage or otherwise protect against potential product liability claims, we will be exposed to significant liabilities, which may harm our business. A product liability claim, recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse effect on our business, financial condition and results of operations.
19
Table of Contents
The misuse or off-label use of our products may harm our image in the marketplace, result in injuries that lead to product liability suits or result in costly investigations and sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
The products we currently market have been approved by the FDA for specific treatments. We train our marketing and direct sales force to not promote our products for uses outside of the FDA-approved indications for use, known as “off-label uses.” We cannot, however, prevent a physician from using our products off-label, when in the physician’s independent professional medical judgment he or she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use our products off-label. Furthermore, the use of our products for indications other than those approved by the FDA or any foreign regulatory body may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients.
Physicians may also misuse our products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. If our products are misused or used with improper technique, we may become subject to costly litigation by our customers or their patients. Product liability claims could divert management’s attention from our core business, be expensive to defend, and result in sizable damage awards against us that may not be covered by insurance. In addition, if the FDA or any foreign regulatory body determines that our promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs, and the curtailment of our operations. Any of these events could significantly harm our business and results of operations and cause our stock price to decline.
Our ability to maintain our competitive position depends on our ability to attract and retain highly qualified personnel.
We believe that our continued success depends to a significant extent upon the efforts and abilities of our key executives. All of our executive officers and other employees are at-will employees, and therefore may terminate employment with us at any time with no advance notice. The replacement of any of our key personnel likely would involve significant time and costs and may significantly delay or prevent the achievement of our business objectives and would harm our business.
Our future success also depends on our ability to continue to attract and retain our executive officers and other key employees. Many of our employees have become or will soon become vested in a substantial amount of stock or number of stock options. Our employees may be more likely to leave us if the shares they own or the shares underlying their vested options have significantly appreciated in value relative to the original purchase prices of the shares or the exercise prices of the options, or if the exercise prices of the options that they hold are significantly below the market price of our common stock. Further, our employees’ ability to exercise those options and sell their stock in a public market after the closing of this offering may result in a higher than normal turnover rate. We do not carry any “key person” insurance policies.
If our facilities or the facility of a supplier become inoperable, we will be unable to continue to research, develop, manufacture and commercialize our products and, as a result, our business will be harmed until we are able to secure a new facility.
We do not have redundant facilities. We perform substantially all of our research and development, manufacturing and commercialization activity and maintain all our raw material and finished goods inventory in a single location in Menlo Park, California. Menlo Park is situated on or near earthquake fault lines. Our facility and equipment would be costly to replace and could require substantial lead time to repair or replace. The facility may be harmed or rendered inoperable by natural or man-made disasters, including, but not limited to, earthquakes, flooding, fire and power outages, which may render it difficult or impossible for us to perform our research, development, manufacturing and commercialization activities for some period of time. The inability to perform those activities, combined with our limited inventory of reserve raw materials and finished product, may
20
Table of Contents
result in the inability to continue manufacturing our products during such periods and the loss of customers or harm to our reputation. Although we possess insurance for damage to our property and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and this insurance may not continue to be available to us on acceptable terms, or at all.
We expect to incur significant additional costs as a result of being a public company, which may adversely affect our operating results and financial condition.
We expect to incur costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act, as well as rules implemented by the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, or the Dodd-Frank Act, the Securities and Exchange Commission, or the SEC, and NASDAQ. These rules and regulations are expected to increase our accounting, legal and financial compliance costs and make some activities more time-consuming and costly. In addition, we will incur additional costs associated with our public company reporting requirements and we expect those costs to increase in the future. We also expect these rules and regulations to make it more expensive for us to maintain directors’ and officers’ liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees, or as executive officers. Increases in costs incurred as a result of becoming a publicly traded company may adversely affect our operating results and financial condition.
We estimate the additional annual cost that we will incur as a result of our public company reporting obligations is approximately $3.0 million. However, because these rules and regulations are often subject to varying interpretations, it is difficult to accurately estimate or predict the amount or timing of these additional costs. Further, the lack of specificity of many of the rules and regulations may result in an application in practice that may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
If we experience significant disruptions in our information technology systems, our business may be adversely affected.
We depend on our information technology systems for the efficient functioning of our business, including accounting, data storage, compliance, purchasing and inventory management. Our current systems provide virtual redundancy but are operated from one physical location in Menlo Park. However, we are in the process of upgrading the level of redundancy for our IT systems. We expect these upgrades to take one to two years to complete. While we will attempt to mitigate interruptions, we may experience difficulties in implementing some upgrades which could impact our business operations, or experience difficulties in operating our business during the upgrade, either of which could disrupt our operations, including our ability to timely ship and track product orders, project inventory requirements, manage our supply chain and otherwise adequately service our customers. In the event we experience significant disruptions as a result of the current implementation of our information technology systems, we may not be able to repair our systems in an efficient and timely manner. Accordingly, such events may disrupt or reduce the efficiency of our entire operation and have a material adverse effect on our results of operations and cash flows.
We are increasingly dependent on sophisticated information technology for our infrastructure. Our information systems require an ongoing commitment of significant resources to maintain, protect and enhance existing systems. Failure to maintain or protect our information systems and data integrity effectively could have a materially adverse effect on our business. For example, third parties may attempt to hack into our information systems and may obtain our proprietary information.
Fluctuations in insurance costs and availability could adversely affect our profitability or our risk management profile.
We hold a number of insurance policies, including product liability insurance, directors’ and officers’ liability insurance, general liability insurance, property insurance and workers’ compensation insurance. If the costs of maintaining adequate insurance coverage increase significantly in the future, our operating results could
21
Table of Contents
be materially adversely affected. Likewise, if any of our current insurance coverage should become unavailable to us or become economically impractical, we would be required to operate our business without indemnity from commercial insurance providers. If we operate our business without insurance, we could be responsible for paying claims or judgments against us that would have otherwise been covered by insurance, which could adversely affect our results of operations or financial condition.
Risks Relating to Regulatory Matters
The existence of adequate coverage and reimbursement is important for sales of our products. Inadequate coverage and reimbursement policies for procedures using our steroid-eluting implants could affect the adoption of our products and our future revenue.
Successful sales of our steroid-eluting implants depend on the availability of adequate coverage and reimbursement from third-party payors for either the products specifically, the procedures associated with the use of the products, or both. Providers that purchase our products generally rely on third-party payors to reimburse all or part of the costs and fees associated with the procedures performed with these medical devices or the devices themselves. Adequate coverage and reimbursement from third-party payors, including governmental payors, such as Medicare and Medicaid, therefore, is important for obtaining product acceptance and widespread adoption in the marketplace.
In the United States, coverage and reimbursement for medical devices vary among payors. In addition, payors continually review new technologies for possible coverage and can, without notice, deny coverage for these new products and procedures. We estimate that, as of March 31, 2014, private payors covering approximately 37% of U.S. covered lives currently have non-coverage policies with respect to PROPEL and PROPEL mini and they consider these products investigational or experimental. Some governmental and private third-party payors do not currently cover or reimburse our products because they have determined insufficient evidence of favorable clinical outcomes is available. Although they consider the steroid-eluting implants investigational or experimental at this time, these payors may in the future determine sufficient evidence has been developed to cover and reimburse our products and related procedures. We are actively working to reverse these non-coverage decisions but cannot provide assurance that we will be successful in these efforts. If we are not successful in reversing existing non-coverage policies, or if other third-party payors issue similar policies, this could have a material adverse effect on our business and operations. Further, third-party payors who currently cover and reimburse customers for procedures using our products may in the future choose to decrease current levels of reimbursement or eliminate reimbursement altogether, either of which will cause our business to suffer.
To contain costs of new technologies, governmental healthcare programs and third-party payors are increasingly scrutinizing new and even existing treatments by requiring extensive evidence of favorable clinical outcomes and cost effectiveness before extending or continuing coverage, respectively. Such evidence generally must be derived from well-designed independent studies and published in peer-reviewed journals. Payors also may be influenced by positive position statements on the value of this technology issued by medical specialty societies. For example, payors may be persuaded to extend coverage by positive clinical data demonstrating the long-term safety and efficacy of FESS performed with our steroid-eluting implants against FESS alone. A long-term clinical study randomizing FESS using our products against FESS alone would require an extremely large patient population to demonstrate these differences, and would be expensive and time-consuming. We have not conducted and are not currently planning to conduct such a study. Further, even positive study results do not guarantee adequate third-party payor coverage and reimbursement. Although the American Rhinologic Society has issued a positive position statement regarding the use of our steroid-eluting implants, if the society changes its position in an unfavorable manner, third-party payors may reverse existing favorable coverage and reimbursement policies or otherwise decline to adopt favorable policies for our products, either of which will cause our business to suffer.
Generally, third-party payors currently reimburse hospitals and ambulatory surgery centers and physicians for the FESS procedures during which our technology is implanted using existing Category I Current Procedure Terminology, or CPT, codes relating to the FESS procedures performed. These CPT codes do not currently distinguish between procedures performed with or without our steroid-eluting implants. The amount of reimbursement received by our customers from third-party payors is dependent generally on fee schedules
22
Table of Contents
established by these payors for the existing FESS CPT codes. For governmental payors, such as Medicare and Medicaid, the fee schedule amount is determined by statutory and regulatory formulas. For commercial payors, the reimbursement amount generally is dependent upon the specific contract terms between the provider and payor. We cannot provide assurance that government or private third-party payors will continue to reimburse for FESS with our products using the existing codes, nor can we provide assurance that the payment rates will be adequate. If providers and physicians are unable to obtain reimbursement for FESS with the use of our products at cost-effective levels, this could have a material adverse effect on our business and operations. Hospitals and ambulatory surgery centers are unlikely to purchase our products if they do not receive payment sufficient to cover the cost of our products and related procedures. In addition, in the event that the current coding and/or payment methodology for these procedures changes, this could have a material adverse effect on our business and business operations.
To secure separate payment for our products, whether for our currently marketed products or those under development, a unique billing code is required for either the implant, the procedure associated with use of our products, or both. Although a unique billing code currently exists for our marketed products it is not associated with payment by most payors and is not reportable to many payors, including Medicare. New billing codes, including CPT codes, may be developed which are both reportable and payable. In addition, new billing codes may be developed for the specific procedure performed to implant our products. As of now, it is not possible to assess the full impact of product or procedure-specific codes on our business or results of operations or the likelihood of securing such specific codes. If new product or procedure-specific codes are adopted, and the levels of reimbursement declines significantly below current levels, our business and results of operations would be harmed and our stock price would likely decline.
Healthcare cost containment pressures and legislative or administrative reforms resulting in restrictive reimbursement practices of third-party payors could decrease the demand for our products, the prices that customers are willing to pay and the number of procedures performed using our steroid-eluting implants, which could have an adverse effect on our business.
All third-party payors, whether governmental or commercial, whether inside the United States or outside, are developing increasingly sophisticated methods of controlling healthcare costs. These cost-control methods include prospective payment systems, bundled payment models, capitated arrangements, group purchasing, benefit redesign, pre-authorization processes, and requirements for second opinions prior to major surgery. These cost-control methods also potentially limit the amount that healthcare providers may be willing to pay for medical devices. Therefore, coverage or reimbursement for medical devices may decrease in the future.
Further, from time to time, typically on an annual basis, payment amounts are updated and revised by third-party payors. Because the cost of our products generally is recovered by the healthcare provider as part of the payment for performing a procedure and not separately reimbursed, these updates could directly impact the demand for our products. An example of payment updates is the Medicare program updates to physician payments, which is done on an annual basis using a prescribed statutory formula. In the past, when the application of the formula resulted in lower payment, Congress has passed interim legislation to prevent the reductions. Most recently, the Protecting Access to Medicare Act of 2014, signed into law in April 2014, provided for a 0.5% update from 2013 payment rates under the Medicare Physician Fee Schedule through 2014 and a 0% update from January 1 until April 1, 2015. If Congress fails to intervene to prevent the negative update factor in future years, the resulting decrease in payment may adversely affect our revenue and results of operations. In addition, the Medicare physician fee schedule has been adapted by some private payors into their plan-specific physician payment schedule. We cannot predict how pending and future healthcare legislation will impact our business, and any changes in coverage and reimbursement that further restricts coverage of our products or lowers reimbursement for procedures using our products could materially affect our business.
Reimbursement in international markets may require us to undertake country-specific reimbursement activities, including additional clinical studies, which could be time-consuming and expensive and may not yield acceptable reimbursement rates.
In international markets, market acceptance of our products will likely depend in large part on the availability of reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare
23
Table of Contents
payment systems in international markets vary significantly by country, and by region in some countries, and include both government-sponsored healthcare and private insurance. We may not obtain international reimbursement approvals in a timely manner, if at all. In addition, even if we do obtain international reimbursement approvals, the level of reimbursement may not be enough to commercially justify expansion of our business into the approving jurisdiction. To the extent we or our customers are unable to obtain reimbursement for our steroid-eluting implants in major international markets in which we seek to market and sell our products, our international revenue growth would be harmed, and our business and results of operations would be adversely affected.
Our products are subject to extensive regulation by the FDA, including the requirement to obtain premarket approval and the requirement to report adverse events. If we fail to obtain necessary FDA device or drug approvals for our products, or are subject to regulatory enforcement action as a result of our failure to properly report adverse events or otherwise comply with regulatory requirements, our commercial operations would be harmed.
Our steroid-eluting implants are subject to extensive regulation by the FDA and various other federal, state and foreign governmental authorities. The PMA and New Drug Application, or NDA, approval processes can be expensive and lengthy. Despite the time, effort and cost required to obtain approval, there can be no assurance that any product that we intend to commercialize in the future will be approved by the FDA in a timely fashion, if at all. For example, we do not have any prior experience in obtaining approval of an NDA, and this lack of experience may delay or adversely affect our ability to obtain approval for our steroid-eluting implant for refractory disease treated in the physician office setting, which will require NDA approval prior to commercialization in the United States.
Our marketed products are subject to Medical Device Reporting, or MDR, obligations, which require that we report to the FDA any incident in which our products may have caused or contributed to a death or serious injury, or in which our products malfunctioned and, if the malfunction were to recur, it could likely cause or contribute to a death or serious injury. We take a conservative approach in reporting MDRs and have had eight events occur postoperatively with PROPEL and PROPEL mini that we have reported to FDA as MDRs, six of which were for infections and two of which were for adhesions.
The FDA and state authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA or state agencies, which may include any of the following sanctions:
| • | adverse publicity, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | repair, replacement, recall or seizure of our products; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | delaying or refusing our requests for approval of new products, new intended uses or modifications to our existing products; |
| • | refusal to grant export approval for our products; |
| • | withdrawing PMAs that have already been granted; and |
| • | criminal prosecution. |
If any of these enforcement actions were to be taken by the government, our business could be harmed.
If we materially modify our FDA-approved products, we may need to seek and obtain new approvals, which, if not granted, would prevent us from selling our modified products.
A component of our strategy is to continue to modify and upgrade our steroid-eluting implants. Medical devices can be marketed only for the indications for which they are approved. We have received a number of PMA supplement approvals since the original approvals of PROPEL and PROPEL mini. We may not be able to obtain additional regulatory approvals for new products or for modifications to, or additional indications for, our existing product in a timely fashion, or at all. Delays in obtaining future approvals would adversely affect our
24
Table of Contents
ability to introduce new or enhanced products in a timely manner, which in turn would harm our revenue and potential future profitability.
We may fail to obtain foreign regulatory approvals to market our products in other countries.
We do not have any sales outside the United States. Sales of our steroid-eluting implants outside the United States are subject to foreign regulatory requirements that vary widely from country to country. In addition, the FDA regulates exports of medical devices from the United States. Complying with international regulatory requirements can be an expensive and time-consuming process and approval is not certain. The time required to obtain approvals, if required by other countries, may be longer than that required for FDA approvals, and requirements for such approvals may significantly differ from FDA requirements. In certain countries we may rely upon a third-party or third party distributors to obtain all required regulatory approvals, and these distributors may be unable to obtain or maintain such approvals. Our distributors in these countries may also incur significant costs in attempting to obtain and in maintaining foreign regulatory approvals or qualifications, which could increase the difficulty of attracting and retaining qualified distributors. If these distributors experience delays in receiving necessary qualifications, clearances or approvals to market our products outside the United States, or if they fail to receive those qualifications, clearances or approvals, we may be unable to market our products or enhancements in certain international markets effectively, or at all.
If we expand our operations outside the United States, we will need to expand our international marketing. International jurisdictions require separate regulatory approvals and compliance with numerous and varying regulatory requirements. The approval procedures vary among countries and may involve requirements for additional testing, and the time required to obtain approval may differ from country to country and from that required to obtain clearance or approval in the United States.
Approval in the United States does not ensure approval or certification by regulatory authorities in other countries or jurisdictions, and approval or certification by one foreign regulatory authority does not ensure approval or certification by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval or certification process may include all of the risks associated with obtaining FDA approval. In addition, some countries only approve or certify a product for a certain period of time, and we are required to re-approve or re-certify our products in a timely manner prior to the expiration of our prior approval or certification. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals or certifications and may not receive necessary approvals to commercialize our products in any market. If we fail to receive necessary approvals or certifications to commercialize our products in foreign jurisdictions on a timely basis, or at all, or if we fail to have our products re-approved or re-certified, our business, results of operations and financial condition could be adversely affected.
These and other factors may have a material adverse effect on our international operations or on our business, results of operations and financial condition generally.
If we or our suppliers fail to comply with ongoing FDA or foreign regulatory authority requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain approval, and the manufacturing processes, reporting requirements, post-approval clinical data and promotional activities for such product, will be subject to continued regulatory review, oversight and periodic inspections by the FDA and other domestic and foreign regulatory bodies. In particular, we and our third-party suppliers are required to comply with the FDA’s current good manufacturing practice. These FDA regulations cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. Compliance with applicable regulatory requirements is subject to continual review and is monitored rigorously through periodic inspections by the FDA. If we, or our manufacturers, fail to adhere to current good manufacturing practice requirements in the United States, this could delay production of our products and lead to fines, difficulties in obtaining regulatory approvals, recalls, enforcement actions, including injunctive relief or consent decrees, or other consequences, which could, in turn, have a material adverse effect on our financial condition or results of operations.
25
Table of Contents
In addition, the FDA audits compliance with the current good manufacturing practice through periodic announced and unannounced inspections of manufacturing and other facilities. The failure by us or one of our suppliers to comply with applicable statutes and regulations administered by the FDA, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in any of the following enforcement actions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | unanticipated expenditures to address or defend such actions; |
| • | customer notifications or repair, replacement, refunds, recall, detention or seizure of our products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | refusing or delaying our requests for regulatory approvals of new products or modified products; |
| • | withdrawing PMA approvals that have already been granted; |
| • | refusal to grant export approval for our products; or |
| • | criminal prosecution. |
Any of these sanctions could have a material adverse effect on our reputation, business, results of operations and financial condition. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
If we expand our operations outside the United States, our products and operations will be required to comply with standards set by foreign regulatory bodies, and those standards, types of evaluation and scope of review differ among foreign regulatory bodies. We intend to comply with the standards enforced by such foreign regulatory bodies as needed to commercialize our products. If we fail to comply with any of these standards adequately, a foreign regulatory body may take adverse actions similar to those within the power of the FDA. For example, if we seek to obtain CE marks permitting us to commercially distribute our products in Europe, we will be subject to a conformity assessment procedure under which a so-called Notified Body, an organization accredited by a member state of the European Economic Area, or EEA, will audit and examine our quality system for the manufacture, design, and release of our products and confirm adherence with applicable regulatory requirements. If we fail to obtain or maintain a CE mark in accordance with these requirements, we would be precluded from selling our products in the EEA. Any such action or circumstance may harm our reputation and business, and could have an adverse effect on our business, results of operations and financial condition.
Our products may in the future be subject to product recalls. A recall of our products, either voluntarily or at the direction of the FDA or another governmental authority, or the discovery of serious safety issues with our products, could have a significant adverse impact on us.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our products in their respective jurisdictions in the event of material deficiencies or defects in the design or manufacture of our products. We may, under our own initiative, recall a product if any material deficiency in our steroid-eluting implants is found. The FDA requires that recalls be reported to the FDA within 10 working days after the recall is initiated. A government-mandated or voluntary recall by us or one of our international distributors could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our reputation, results of operations and financial condition, which could impair our ability to produce our products in a cost- effective and timely manner in order to meet our customers’ demands. We may also be subject to liability claims, be required to bear other costs, or take other actions that may have a negative impact on our future sales and our ability to generate profits. Companies are required to maintain certain records of recalls, even if they are not
26
Table of Contents
reportable to the FDA. We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they were conducted.
If the third parties on which we rely to conduct our clinical trials and to assist us with pre-clinical development do not perform as contractually required or expected, we may not be able to obtain regulatory approval for or commercialize our products.
We often must rely on third parties, such as medical institutions, clinical investigators, contract research organizations and contract laboratories to conduct our clinical trials and provide data or prepare deliverables for our PMA or NDA submissions. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize, our products on a timely basis, if at all, and our business, operating results and prospects may be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
We may be subject to enforcement action if we engage in improper marketing or promotion of our products.
Our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of unapproved, or off-label, use. Physicians may use our products off-label, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. However, if the FDA determines that our promotional materials or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an off-label use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products could be impaired. Although our policy is to refrain from statements that could be considered off-label promotion of our products, the FDA or another regulatory agency could disagree and conclude that we have engaged in off-label promotion. In addition, the off-label use of our products may increase the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention, result in substantial damage awards against us, and harm our reputation.
If we fail to comply with U.S. federal and state healthcare regulatory laws, we could be subject to penalties, including, but not limited to, administrative, civil and criminal penalties, damages, fines, disgorgement, exclusion from participation in governmental healthcare programs, and the curtailment of our operations, any of which could adversely impact our reputation and business operations.
There are numerous U.S. federal and state healthcare regulatory laws, including, but not limited to, anti-kickback laws, false claims laws, privacy laws, and transparency laws. Our relationships with healthcare providers and entities, including but not limited to, physicians, hospitals, ambulatory surgery centers, group purchasing organizations and our international distributors are subject to scrutiny under these laws. Violations of these laws can subject us to penalties, including, but not limited to, administrative, civil and criminal penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in federal and state healthcare programs, including the Medicare, Medicaid and Veterans Administration health programs, and the curtailment of our operations. Healthcare fraud and abuse regulations are complex, and even minor irregularities can
27
Table of Contents
potentially give rise to claims that a statute or prohibition has been violated. The laws that may affect our ability to operate include, but are not limited to:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering, or paying remuneration, directly or indirectly, in cash or in kind, in exchange for or to induce either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or service for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid; |
| • | the federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent; knowingly making using, or causing to be made our used, a false record or statement to get a false or fraudulent claim paid or approved by the government; or knowingly making, using, or causing to be made or used, a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal criminal False Claims Act, which imposes criminal fines or imprisonment against individuals or entities who make or present a claim to the government knowing such claim to be false, fictitious or fraudulent; |
| • | the civil monetary penalties statute, which imposes penalties against any person or entity who, among other things, is determined to have presented or caused to be presented, a claim to a federal healthcare program that the person knows, or should know, is for an item or service that was not provided as claimed or is false or fraudulent; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, as amended, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose requirements on certain covered healthcare providers, health plans and healthcare clearinghouses as well as their business associates that perform services for them that involve individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization, including mandatory contractual terms as well as directly applicable privacy and security standards and requirements; |
| • | the Federal Trade Commission Act and similar laws regulating advertisement and consumer protections; |
| • | the federal Foreign Corrupt Practices Act of 1997, which prohibits corrupt payments, gifts or transfers of value to foreign officials; and |
| • | foreign or U.S. state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers. |
Further, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or, collectively, the Affordable Care Act, among other things, amends the intent requirements of the federal Anti-Kickback Statute and certain criminal statutes governing healthcare fraud. A person or entity can now be found guilty of violating the statute without actual knowledge of the statute or specific intent to violate it. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. Moreover, while we do not submit claims and our customers make the ultimate decision on how to submit claims, from time-to-time, we may provide reimbursement guidance to our customers. If a government authority were to conclude that we provided improper advice to our customers or encouraged the submission of false claims for reimbursement, we could face action against us by government authorities. Any violations of these laws, or any action against us for violation of these laws, even if we successfully defend against it, could result in a material adverse effect on our reputation, business, results of operations and financial condition.
28
Table of Contents
We have entered into consulting agreements with physicians, including some who influence the ordering of and use our products in procedures they perform. While we believe these transactions were structured to comply with all applicable laws, including state and federal anti-kickback laws, to the extent applicable, regulatory agencies may view these transactions as prohibited arrangements that must be restructured, or discontinued, or for which we could be subject to other significant penalties. We could be adversely affected if regulatory agencies interpret our financial relationships with ENT physicians who influence the ordering of and use our products to be in violation of applicable laws. This could subject us to the penalties described above.
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available under such laws, it is possible that some of our business activities, including our relationships with healthcare providers and entities, including, but not limited to, physicians, hospitals, ambulatory surgery centers, group purchasing organizations and our independent distributors and certain sales and marketing practices, including the provision of certain items and services to our customers, could be subject to challenge under one or more of such laws.
To enforce compliance with the healthcare regulatory laws, federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Responding to investigations can be time-and resource-consuming and can divert management’s attention from the business. Additionally, as a result of these investigations, healthcare providers and entities may have to agree to additional onerous compliance and reporting requirements as part of a consent decree or corporate integrity agreement. Any such investigation or settlement could increase our costs or otherwise have an adverse effect on our business.
In certain cases, federal and state authorities pursue actions for false claims on the basis that manufacturers and distributors are promoting off-label uses of their products. Pursuant to FDA regulations, we can only market our products for cleared or approved uses. Although physicians are permitted to use medical devices for indications other than those cleared or approved by the FDA in their professional medical judgment, we are prohibited from promoting products for off-label uses. We market our products and provide promotional materials and training programs to physicians regarding the use of our products. If it is determined that our business activities, including our marketing, promotional materials or training programs constitute promotion of unapproved uses, we could be subject to significant fines in addition to regulatory enforcement actions, including the issuance of a warning letter, injunction, seizure and criminal penalty.
In addition, there has been a recent trend of increased federal and state regulation of payments and transfers of value provided to healthcare professionals or entities. On February 8, 2013, the Centers for Medicare & Medicaid Services, or CMS, released its final rule implementing section 6002 of the Affordable Care Act known as the Physician Payment Sunshine Act that imposes new annual reporting requirements on device manufacturers for payments and other transfers of value provided by them, directly or indirectly, to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their family members. A manufacturer’s failure to submit timely, accurately and completely the required information for all payments, transfers of value or ownership or investment interests may result in civil monetary penalties of up to an aggregate of $150,000 per year, and up to an aggregate of $1.0 million per year for “knowing failures.” The period between August 1, 2013, and December 31, 2013 was the first reporting period, for which manufacturers were required to report aggregate payment data to CMS by March 31, 2014. Manufacturers also will be required to report to CMS detailed payment and transfers of value data and submit legal attestation to the accuracy of such data during Phase 2 of the program, which is expected to begin in May 2014 and extends for at least 30 days. Thereafter, manufacturers must submit reports by the 90th day of each subsequent calendar year. Due to the difficulty in complying with the Physician Payment Sunshine Act, we cannot assure you that we will successfully report all payments and transfers of value provided by us, and any failure to comply could result in significant fines and penalties. Some states, such as California and Connecticut, also mandate implementation of commercial compliance programs, and other states, such as Massachusetts and Vermont, impose restrictions on device manufacturer marketing practices and tracking and reporting of gifts, compensation and other remuneration to healthcare professionals and entities. The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance and reporting requirements in multiple jurisdictions increase the possibility that a healthcare company may fail to comply fully with one or more of these requirements.
29
Table of Contents
Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
Most of these laws apply to not only the actions taken by us, but also actions taken by our distributors. We have limited knowledge and control over the business practices of our distributors, and we may face regulatory action against us as a result of their actions which could have a material adverse effect on our reputation, business, results of operations and financial condition.
In addition, the scope and enforcement of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal or state regulatory authorities might challenge our current or future activities under these laws. Any such challenge could have a material adverse effect on our reputation, business, results of operations and financial condition. Any state or federal regulatory review of us, regardless of the outcome, would be costly and time-consuming. Additionally, we cannot predict the impact of any changes in these laws, whether or not retroactive.
Legislative or regulatory healthcare reforms may make it more difficult and costly for us to obtain regulatory approval of our products and to produce, market and distribute our products after approval is obtained.
FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of our products. Delays in receipt of, or failure to receive, regulatory approvals for our new products would have a material adverse effect on our business, results of operations and financial condition.
Federal and state governments in the United States have recently enacted legislation to overhaul the nation’s healthcare system. While the goal of healthcare reform is to expand coverage to more individuals, it also involves increased government price controls, additional regulatory mandates and other measures designed to constrain medical costs. The Affordable Care Act significantly impacts the medical device industry. Among other things, the Affordable Care Act:
| • | imposes an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States beginning in 2013; |
| • | establishes a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in comparative clinical effectiveness research in an effort to coordinate and develop such research; |
| • | implements payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models; and |
| • | creates an independent payment advisory board that will submit recommendations to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate. |
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. On August 2, 2011, President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and will stay in effect through 2024, unless additional congressional action is taken. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or the ATRA, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressure.
30
Table of Contents
Our financial performance may be adversely affected by medical device tax provisions in the healthcare reform laws.
The Affordable Care Act imposes, among other things, an excise tax of 2.3% on any entity that manufactures or imports specified medical devices offered for sale in the United States beginning in 2013. Under these provisions, the Congressional Research Service predicts that the total cost to the medical device industry may be up to $29 billion over the next decade. The Internal Revenue Service issued final regulations implementing the tax in December of 2012 which requires, among other things, bi-monthly payments and quarterly reporting. We anticipate that primarily all sales of our products in the United States will be subject to this 2.3% excise tax. During the year ended December 31, 2013, we recognized $0.3 million in tax expense associated with the medical device tax in the United States, which is included in selling, general and administrative expenses.
Our operations involve the use of hazardous and toxic materials, and we must comply with environmental laws and regulations, which can be expensive, and may affect our business and operating results.
We are subject to a variety of federal, state and local regulations relating to the use, handling, storage, disposal and human exposure to hazardous materials. Liability under environmental laws can be joint and several and without regard to comparative fault, and environmental laws could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations, which could harm our business. Although we believe that our activities conform in all material respects with environmental laws, there can be no assurance that violations of environmental and health and safety laws will not occur in the future as a result of human error, accident, equipment failure or other causes. The failure to comply with past, present or future laws could result in the imposition of fines, third-party property damage and personal injury claims, investigation and remediation costs, the suspension of production, or a cessation of operations. We also expect that our operations will be affected by other new environmental and health and safety laws on an ongoing basis. Although we cannot predict the ultimate impact of any such new laws, they will likely result in additional costs, and may require us to change how we manufacture our products, which could have a material adverse effect on our business.
Failure to comply with the United States Foreign Corrupt Practices Act, or the FCPA, and similar laws associated with any activities outside the United States could subject us to penalties and other adverse consequences.
We are subject to the FCPA and other anti-bribery legislation around the world. The FCPA generally prohibits covered entities and their intermediaries from engaging in bribery or making other prohibited payments, offers or promises to foreign officials for the purpose of obtaining or retaining business or other advantages. In addition, the FCPA imposes recordkeeping and internal controls requirements on publicly traded corporations and their foreign affiliates, which are intended to, among other things, prevent the diversion of corporate funds to the payment of bribes and other improper payments, and to prevent the establishment of “off books” slush funds from which such improper payments can be made. Although we do not currently have any operations outside the United States, in the future we may face significant risks if we fail to comply with the FCPA and other laws that prohibit improper payments, offers or promises of payment to foreign governments and their officials and political parties by us and other business entities for the purpose of obtaining or retaining business or other advantages. In many foreign countries, particularly in countries with developing economies, some of which represent significant markets for us, it may be a local custom that businesses operating in such countries engage in business practices that are prohibited by the FCPA or other laws and regulations. Although we have implemented a company policy requiring our employees and consultants to comply with the FCPA and similar laws, such policy may not be effective at preventing all potential FCPA or other violations. There can be no assurance that none of our employees and agents, or those companies to which we outsource certain of our business operations will not take actions that violate our policies or applicable laws, for which we may be ultimately held responsible. As a result of our focus on managing our growth, our development of infrastructure designed to identify FCPA matters and monitor compliance is at an early stage. Any violation of the FCPA and related policies could result in severe criminal or civil sanctions, which could have a material and adverse effect on our reputation, business, operating results and financial condition.
31
Table of Contents
Risks Relating to Intellectual Property Matters
Intellectual property rights may not provide adequate protection, which may permit third parties to compete against us more effectively.
Our success depends significantly on our ability to protect our proprietary rights to the technologies and inventions used in, or embodied by, our products. To protect our proprietary technology, we rely on patent protection, as well as a combination of copyright, trade secret and trademark laws, as well as nondisclosure, confidentiality and other contractual restrictions in our consulting and employment agreements. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage.
Patents
The process of applying for patent protection itself is time consuming and expensive and we cannot assure you that all of our patent applications will issue as patents or that, if issued, they will issue in a form that will be advantageous to us. The rights granted to us under our patents, including prospective rights sought in our pending patent applications, may not be meaningful or provide us with any commercial advantage and they could be opposed, contested or circumvented by our competitors or be declared invalid or unenforceable in judicial or administrative proceedings.
We own numerous issued patents and pending patent applications that relate to the sinus delivery of sustained release therapeutics, sinus delivery of implants, implant designs as well as individual components of our steroid-eluting implants. The API contained in our steroid-eluting implants is generic and is not the subject of independent patent protection. If any of our patents are challenged, invalidated or legally circumvented by third parties, and if we do not own other enforceable patents protecting our products, competitors could market products and use processes that are substantially similar to, or superior to, ours, and our business will suffer. In addition, the patents we own may not be of sufficient scope or strength to provide us with any meaningful protection or commercial advantage, and competitors may be able to design around our patents or develop products that provide outcomes comparable to ours without infringing on our intellectual property rights.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted, redefine prior art, may affect patent litigation, and switch the U.S. patent system from a “first-to-invent” system to a “first-to-file” system. Under a “first-to-file” system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor had made the invention earlier. The U.S. Patent and Trademark Office, or USPTO, recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first-to-file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition. In addition, patent reform legislation may pass in the future that could lead to additional uncertainties and increased costs surrounding the prosecution, enforcement, and defense of our patents and applications.
We may be subject to a third-party preissuance submission of prior art to the USPTO, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review, or other patent office proceedings or litigation, in the United States or elsewhere, challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights.
32
Table of Contents
Moreover, the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In addition, periodic maintenance fees on issued patents often must be paid to the USPTO and foreign patent agencies over the lifetime of the patent. While an unintentional lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain the patents and patent applications covering our products or procedures, we may not be able to stop a competitor from marketing products that are the same as or similar to our products, which would have a material adverse effect on our business.
Furthermore, we do not have patent rights in certain foreign countries in which a market may exist in the future, and the laws of many foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States. Thus, we may not be able to stop a competitor from marketing and selling in foreign countries products that are the same as or similar to our products.
Trademarks
We rely on our trademarks as one means to distinguish our products from the products of our competitors, and have registered or applied to register many of these trademarks. Our trademark applications may not be approved, however. Third parties may oppose our trademark applications, or otherwise challenge our use of the trademarks. In the event that our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition and could require us to devote resources to advertising and marketing new brands. Our competitors may infringe our trademarks and we may not have adequate resources to enforce our trademarks.
Trade Secrets and Know-How
We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by consultants, vendors, former employees or current employees, despite the existence generally of confidentiality agreements and other contractual restrictions. Monitoring unauthorized uses and disclosures of our intellectual property is difficult, and we do not know whether the steps we have taken to protect our intellectual property will be effective.
Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Competitors could purchase our steroid-eluting implants and attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property is not adequately protected so as to protect our market against competitors’ products and methods, our competitive position could be adversely affected, as could our business.
We may in the future be a party to patent and other intellectual property litigation and administrative proceedings that could be costly and could interfere with our ability to sell our steroid-eluting implants.
The medical device industry has been characterized by frequent and extensive intellectual property litigation. Additionally, the ENT market is extremely competitive. Our competitors or other patent holders may assert that our steroid-eluting implants and the methods employed in our steroid-eluting implants are covered by their patents. If our steroid-eluting implants or methods are found to infringe, we could be prevented from manufacturing or marketing our steroid-eluting implants. In the event that we become involved in such a dispute, we may incur significant costs and expenses and may need to devote resources to resolving any claims, which would reduce the cash we have available for operations and may be distracting to management. We do not know whether our competitors or potential competitors have applied for, will apply for, or will obtain patents that will prevent, limit or interfere with our ability to make, use, sell, import or export our steroid-eluting implants.
33
Table of Contents
Competing products may also be sold in other countries in which our patent coverage might not exist or be as strong. If we lose a foreign patent lawsuit, alleging our infringement of a competitor’s patents, we could be prevented from marketing our steroid-eluting implants in one or more foreign countries. We may also initiate litigation against third parties to protect our own intellectual property. Our intellectual property has not been tested in litigation. If we initiate litigation to protect our rights, we run the risk of having our patents invalidated, which would undermine our competitive position.
Litigation related to infringement and other intellectual property claims, with or without merit, is unpredictable, can be expensive and time-consuming and can divert management’s attention from our core business. If we lose this kind of litigation, a court could require us to pay substantial damages, treble damages and attorneys’ fees, and prohibit us from using technologies essential to our steroid-eluting implants, any of which would have a material adverse effect on our business, results of operations and financial condition. If relevant patents are upheld as valid and enforceable and we are found to infringe, we could be prevented from selling our steroid-eluting implants unless we can obtain licenses to use technology or ideas covered by such patents. We do not know whether any necessary licenses would be available to us on satisfactory terms, if at all. If we cannot obtain these licenses, we could be forced to design around those patents at additional cost or abandon our products altogether. As a result, our ability to grow our business and compete in the market may be harmed.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
Many of our employees were previously employed at other medical device companies, including our competitors or potential competitors, in some cases until recently. We could in the future be subject to claims that we or our employees have inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of these former employers or competitors. In addition, we have been and may in the future be subject to claims that we caused an employee to breach the terms of his or her non-competition or non-solicitation agreement. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and could be a distraction to management. If our defense to those claims fails, in addition to paying monetary damages, a court could prohibit us from using technologies or features that are essential to our products, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers. An inability to incorporate technologies or features that are important or essential to our products would have a material adverse effect on our business, and may prevent us from selling our products. In addition, we may lose valuable intellectual property rights or personnel. Any litigation or the threat thereof may adversely affect our ability to hire employees or contract with independent sales representatives. A loss of key personnel or their work product could hamper or prevent our ability to commercialize our products, which could have an adverse effect on our business, results of operations and financial condition.
Risks Relating to Our Capital Requirements and Finances
We may need substantial additional funding beyond the proceeds of this offering and may be unable to raise capital when needed, which would force us to delay, reduce, eliminate or abandon our commercialization efforts or product development programs.
Our ability to continue as a going concern may require us to obtain additional financing to fund our operations. We may need to raise substantial additional capital to:
| • | expand the commercialization of our products; |
| • | fund our operations and clinical studies; |
| • | continue our research and development activities; |
| • | defend, in litigation or otherwise, any claims that we infringe third-party patents or other intellectual property rights; |
| • | enforce our patent and other intellectual property rights; |
34
Table of Contents
| • | address legal or enforcement actions by the FDA or other governmental agencies and remediate underlying problems; |
| • | commercialize our new products in development, if any such products receive regulatory clearance or approval for commercial sale; and |
| • | acquire companies and in-license products or intellectual property. |
We believe that the net proceeds from this offering, together with our existing cash and cash equivalents, revenue, and available debt financing arrangements will be sufficient to meet our capital requirements and fund our operations until at least through 2016. However, we have based these estimates on assumptions that may prove to be wrong, and we could spend our available financial resources much faster than we currently expect. Any future funding requirements will depend on many factors, including:
| • | market acceptance of our products; |
| • | the scope, rate of progress and cost of our clinical studies; |
| • | the cost of our research and development activities; |
| • | the cost of filing and prosecuting patent applications and defending and enforcing our patent or other intellectual property rights; |
| • | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patents or other intellectual property rights; |
| • | the cost and timing of additional regulatory clearances or approvals; |
| • | the cost and timing of establishing additional sales, marketing and distribution capabilities; |
| • | costs associated with any product recall that may occur; |
| • | the effect of competing technological and market developments; |
| • | the extent to which we acquire or invest in products, technologies and businesses, although we currently have no commitments or agreements relating to any of these types of transactions; and |
| • | the costs of operating as a public company. |
If we raise additional funds by issuing equity securities, our stockholders may experience dilution. Any future debt financing into which we enter may impose upon us covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our stock, make certain investments and engage in certain merger, consolidation or asset sale transactions. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us. If we are unable to raise adequate funds, we may have to liquidate some or all of our assets, or delay, reduce the scope of or eliminate some or all of our development programs.
We cannot be certain that additional funding will be available on acceptable terms, if at all. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to reduce marketing, customer support or other resources devoted to our products or cease operations. Any of these factors could harm our operating results.
Our outstanding debt financing arrangements contain restrictive covenants that may limit our operating flexibility.
The agreements relating to our outstanding debt contain certain covenants limiting our ability to transfer or dispose of assets, merge with or acquire other companies, make investments, pay dividends, incur additional indebtedness and liens and conduct transactions with affiliates. We therefore may not be able to engage in any of the foregoing transactions until our current debt obligations are paid in full or we obtain the consent of the lenders. We cannot assure you that we will be able to generate sufficient cash flows or revenue to meet the
35
Table of Contents
financial covenants or pay the principal and interest on our debt. Furthermore, we cannot assure you that future working capital, borrowings or equity financing will be available to repay or refinance any such debt.
Our ability to use our net operating losses and research and development credit carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change,” generally defined as a greater than 50% change by value in its equity ownership over a three-year period, is subject to limitations on its ability to utilize its pre-change net operating losses, or NOLs, and its research and development credit carryforwards to offset future taxable income. Our existing NOLs and research and development credit carryforwards may be subject to limitations arising from previous ownership changes, and if we undergo an ownership change, our ability to utilize NOLs and research and development credit carryforwards could be further limited by Sections 382 and 383 of the Code. Future changes in our stock ownership, some of which might be beyond our control, could result in an ownership change under Section 382 of the Code. For these reasons, in the event we experience a change of control, we may not be able to utilize a material portion of the NOLs and research and development credit carryforwards, even if we attain profitability.
Risks Related to Our Common Stock and This Offering
There has been no prior public market for our common stock and an active trading market may not develop.
Prior to this offering, there has been no public market for our common stock. An active trading market may not develop following completion of this offering or, if developed, may not be sustained. The lack of an active market may impair the value of your shares and your ability to sell your shares at the time you wish to sell them. An inactive market may also impair our ability to both raise capital by selling shares and acquire other complementary products, technologies or businesses by using our shares as consideration.
We expect that the price of our common stock will fluctuate substantially and you may not be able to sell the shares you purchase in this offering at or above the offering price.
The initial public offering price for the shares of our common stock sold in this offering is determined by negotiation between the representatives of the underwriters and us. This price may not reflect the market price of our common stock following this offering. In addition, the market price of our common stock is likely to be highly volatile and may fluctuate substantially due to many factors, including:
| • | volume and timing of sales of our steroid-eluting implants; |
| • | the introduction of new products or product enhancements by us or others in our industry; |
| • | disputes or other developments with respect to our or others’ intellectual property rights; |
| • | our ability to develop, obtain regulatory clearance or approval for, and market new and enhanced products on a timely basis; |
| • | product liability claims or other litigation; |
| • | quarterly variations in our results of operations or those of others in our industry; |
| • | sales of large blocks of our common stock, including sales by our executive officers and directors; |
| • | media exposure of our steroid-eluting implants or products of others in our industry; |
| • | changes in governmental regulations or in the status of our regulatory approvals or applications; |
| • | changes in earnings estimates or recommendations by securities analysts; and |
| • | general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors. |
In recent years, the stock markets generally have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. Broad market
36
Table of Contents
and industry factors may significantly affect the market price of our common stock, regardless of our actual operating performance. These fluctuations may be even more pronounced in the trading market for our common stock shortly following this offering. If the market price of shares of our common stock after this offering does not ever exceed the initial public offering price, you may not realize any return on your investment in us and may lose some or all of your investment.
In addition, in the past, class action litigation has often been instituted against companies whose securities have experienced periods of volatility in market price. Securities litigation brought against us following volatility in our stock price, regardless of the merit or ultimate results of such litigation, could result in substantial costs, which would hurt our financial condition and operating results and divert management’s attention and resources from our business.
These and other factors may make the price of our stock volatile and subject to unexpected fluctuation.
Securities analysts may not publish favorable research or reports about our business or may publish no information at all, which could cause our stock price or trading volume to decline.
If a trading market for our common stock develops, the trading market will be influenced to some extent by the research and reports that industry or financial analysts publish about us and our business. We do not control these analysts. As a newly public company, we may be slow to attract research coverage and the analysts who publish information about our common stock will have had relatively little experience with our company or industry, which could affect their ability to accurately forecast our results and could make it more likely that we fail to meet their estimates. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us provide inaccurate or unfavorable research or issue an adverse opinion regarding our stock price, our stock price could decline. If one or more of these analysts cease coverage of our company or fail to publish reports covering us regularly, we could lose visibility in the market, which in turn could cause our stock price or trading volume to decline.
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to “emerging growth companies” will make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and we may take advantage of certain exemptions and relief from various reporting requirements that are applicable to other public companies that are not “emerging growth companies.” In particular, while we are an “emerging growth company” (1) we will not be required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act, (2) we will be exempt from any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotations or a supplement to the auditor’s report on financial statements, (3) we will be subject to reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and (4) we will not be required to hold nonbinding advisory votes on executive compensation or stockholder approval of any golden parachute payments not previously approved.
In addition, while we are an “emerging growth company” we will not be required to comply with any new financial accounting standard until such standard is generally applicable to private companies. As a result, our financial statements may not be comparable to companies that are not “emerging growth companies” or elect not to avail themselves of this provision.
We may remain an “emerging growth company” until as late as December 31, 2019, the fiscal year-end following the fifth anniversary of the completion of this initial public offering, though we may cease to be an “emerging growth company” earlier under certain circumstances, including (1) if the market value of our common stock that is held by nonaffiliates exceeds $700 million as of any June 30, in which case we would cease to be an “emerging growth company” as of the following December 31, or (2) if our gross revenue exceeds $1.0 billion in any fiscal year.
The exact implications of the JOBS Act are still subject to interpretations and guidance by the SEC and other regulatory agencies, and we cannot assure you that we will be able to take advantage of all of the benefits of the JOBS Act. In addition, investors may find our common stock less attractive if we rely on the exemptions and relief
37
Table of Contents
granted by the JOBS Act. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may decline or become more volatile.
If you purchase our common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
Investors purchasing common stock in this offering will pay a price per share that substantially exceeds the pro forma as adjusted net tangible book value per share. As a result, investors purchasing common stock in this offering will incur immediate dilution of $9.19 per share, based on an assumed initial public offering price of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, and our pro forma as adjusted net tangible book value per share as of March 31, 2014. For more information on the dilution you may suffer as a result of investing in this offering, see the section entitled “Dilution.”
This dilution is due to the substantially lower price paid by our investors who purchased shares prior to this offering as compared to the price offered to the public in this offering and the exercise of stock options granted to our employees. The exercise of any of these options would result in additional dilution. As a result of the dilution to investors purchasing shares in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell their shares, could result in a decrease in the market price of our common stock. Immediately after this offering, we will have outstanding 22,501,808 shares of common stock based on the number of shares outstanding as of March 31, 2014. This includes the shares that we are selling in this offering, which may be resold in the public market immediately without restriction, unless purchased by our affiliates. Of the remaining shares, 17,501,808 shares are currently restricted as a result of securities laws or lock-up agreements but will be able to be sold after the offering as described in the section of this prospectus entitled “Shares Eligible for Future Sale.” Moreover, immediately after this offering, holders of an aggregate of up to 15,771,742 shares of our common stock, including shares of our common stock issuable upon the exercise or, in certain cases, net exercise of outstanding warrants, will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders as described in the section of this prospectus entitled “Description of Capital Stock—Registration Rights.” We also intend to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the “Underwriting” section of this prospectus.
Our directors, officers and principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
After this offering, our officers, directors and principal stockholders each holding more than 5% of our common stock, collectively, will control approximately 65.9% of our outstanding common stock. As a result, these stockholders, if they act together, will be able to control the management and affairs of our company and most matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change of control and might adversely affect the market price of our common stock. This concentration of ownership may not be in the best interests of our other stockholders.
We have broad discretion in the use of proceeds of this offering for working capital and general corporate purposes.
The net proceeds of this offering will be allocated to sales, marketing, research and development activities, clinical and regulatory initiatives, working capital and general corporate purposes. We may also use a portion of the net proceeds of this offering to acquire complementary products, technologies or businesses. Within those
38
Table of Contents
categories, we have not determined the specific allocation of the net proceeds of this offering. Our management will have broad discretion over the use and investment of the net proceeds of this offering within those categories. Accordingly, investors in this offering have only limited information concerning management’s specific intentions and will need to rely upon the judgment of our management with respect to the use of proceeds.
If we experience material weaknesses in the future or otherwise fail to maintain an effective system of internal controls in the future, we may not be able to accurately report our financial condition or results of operations which may adversely affect investor confidence in us and, as a result, the value of our common stock.
As a result of becoming a public company, we will be required, under Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting beginning with our Annual Report on Form 10-K for the year ended December 31, 2015. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency or combination of deficiencies in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual and interim financial statements will not be detected or prevented on a timely basis.
We are further enhancing internal controls, processes and related documentation necessary to perform the evaluation needed to comply with Section 404. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are effective. The effectiveness of our controls and procedures may be limited by a variety of factors, including:
| • | faulty human judgment and simple errors, omissions or mistakes; |
| • | fraudulent action of an individual or collusion of two or more people; |
| • | inappropriate management override of procedures; and |
| • | the possibility that any enhancements to controls and procedures may still not be adequate to assure timely and accurate financial control. |
When we cease to be an “emerging growth company” under the federal securities laws, our auditors will be required to express an opinion on the effectiveness of our internal controls. If we are unable to confirm that our internal control over financial reporting is effective, or if our auditors are unable to express an opinion on the effectiveness of our internal controls, we could lose investor confidence in the accuracy and completeness of our financial reports, which could cause the price of our common stock to decline.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
Upon the closing of this offering, we will become subject to the periodic reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act. We designed our disclosure controls and procedures to provide reasonable assurance that information we must disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
39
Table of Contents
Provisions in our corporate charter documents and under Delaware law could make an acquisition of us more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and our amended and restated bylaws that will become effective upon the closing of this offering may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. Among others, these provisions include that:
| • | our board of directors has the right to expand the size of our board of directors and to elect directors to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors; |
| • | our stockholders may not act by written consent or call special stockholders’ meetings; as a result, a holder, or holders, controlling a majority of our capital stock would not be able to take certain actions other than at annual stockholders’ meetings or special stockholders’ meetings called by the board of directors, the chairman of the board, the chief executive officer or the president; |
| • | our certificate of incorporation prohibits cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
| • | the affirmative vote of holders of at least 66-2/3% of the voting power of all of the then outstanding shares of voting stock, voting as a single class, will be required (a) to amend certain provisions of our certificate of incorporation, including provisions relating to the size of the board, removal of directors, special meetings, actions by written consent and cumulative voting and (b) to amend or repeal our bylaws, although our bylaws may be amended by a simple majority vote of our board of directors; |
| • | stockholders must provide advance notice and additional disclosures in order to nominate individuals for election to the board of directors or to propose matters that can be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of our company; and |
| • | our board of directors may issue, without stockholder approval, shares of undesignated preferred stock; the ability to issue undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to acquire us. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
40
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements concerning our business, operations and financial performance and condition, as well as our plans, objectives and expectations for our business operations and financial performance and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These forward-looking statements include, but are not limited to, statements about:
| • | estimates of our future revenue, expenses, capital requirements and our needs for additional financing; |
| • | preliminary estimates of our revenue, cost, expense and net loss expectations for the three and six months ended June 30, 2014; |
| • | our ability to obtain additional financing in this or future offerings; |
| • | the implementation of our business model and strategic plans for our products, technologies and businesses; |
| • | our future clinical results; |
| • | competitive companies and technologies and our industry; |
| • | our ability to manage and grow our business by expanding our sales to existing customers or introducing our products to new customers; |
| • | our ability to establish and maintain intellectual property protection for our products or avoid claims of infringement; |
| • | extensive government regulation; |
| • | the timing or likelihood of regulatory filings and approvals; |
| • | our ability to hire and retain key personnel; |
| • | our financial performance; |
| • | the volatility of our share price; and |
| • | our expectations regarding use of proceeds from this offering. |
Forward-looking statements are based on management’s current expectations, estimates, forecasts, and projections about our business and the industry in which we operate, and management’s beliefs and assumptions and are not guarantees of future performance or development and involve known and unknown risks, uncertainties, and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this prospectus may turn out to be inaccurate. Furthermore, if the forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under “Risk Factors” and elsewhere in this prospectus. Potential investors are urged to consider these factors carefully in evaluating the forward-looking statements. These forward-looking statements speak only as of the date of this prospectus. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future. You should, however, review the factors and risks we describe in the reports we will file from time to time with the SEC after the date of this prospectus.
41
Table of Contents
MARKET, INDUSTRY AND OTHER DATA
This prospectus contains estimates, projections and other information concerning our industry, our business, and the markets for our products and product candidates, including data regarding the estimated size of those markets, their projected growth rates, the perceptions and preferences of patients and physicians regarding certain therapies and other prescription, prescriber and patient data, as well as data regarding market research, estimates and forecasts prepared by our management. We obtained the industry, market and other data throughout this prospectus from our own internal estimates and research, as well as from industry publications and research, surveys and studies conducted by third parties.
Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. In some cases, we do not expressly refer to the sources from which this data is derived.
42
Table of Contents
We estimate that the net proceeds from this initial public offering of 5,000,000 shares of common stock will be approximately $52.8 million, or $61.2 million if the underwriters exercise their option to purchase additional shares in full, assuming an initial public offering price of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting the underwriting discounts and commissions and estimated offering expenses.
A $1.00 increase (decrease) in the assumed initial public offering price of $12.00 per share would increase (decrease) the net proceeds from this offering by approximately $4.7 million, assuming that the number of shares we are offering, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1,000,000 shares in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering by approximately $11.2 million, assuming that the assumed initial public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
We expect to use approximately $7.0 million of the net proceeds from this offering to fund the Phase 3 clinical trial of our Steroid-Eluting Implant for Refactory Disease in Office and pre-clinical development our Steroid-Eluting Implant for Primary Disease Management in Office products in development and approximately $3.3 million for research and development expenses related to these products. We expect to use the remainder of the net proceeds from this offering for sales, marketing, working capital and general corporate purposes.
We may also use a portion of our net proceeds to acquire and invest in complementary products, technologies or businesses; however, we currently have no agreements or commitments to complete any such transaction. The amounts and timing of our expenditures will depend upon numerous factors, including:
| • | rate of adoption of our products; |
| • | the expenses we incur in selling and marketing PROPEL and PROPEL mini; |
| • | the scope of research and development efforts; |
| • | the timing and success of preclinical studies or clinical trials we may commence in the future; and |
| • | the timing of regulatory submissions. |
Accordingly, our management will have broad discretion over the use of the net proceeds from this offering. Pending the use of the proceeds from this offering, we intend to invest the net proceeds in short-term, interest-bearing, investment-grade securities, certificates of deposit or government securities.
We have never declared or paid any cash dividends on our capital stock, and we do not currently intend to pay any cash dividends on our capital stock in the foreseeable future. In addition, our credit facility with Silicon Valley Bank restricts our ability to pay dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support operations and to finance the growth and development of our business. Any future determination to pay dividends will be made at the discretion of our board of directors subject to applicable laws, and will depend upon, among other factors, our results of operations, financial condition, contractual restrictions and capital requirements. Our future ability to pay cash dividends on our capital stock may also be limited by the terms of any future debt or preferred securities or future credit facility.
43
Table of Contents
The following table sets forth our equipment loan, convertible preferred stock warrant liability and capitalization as of March 31, 2014:
| • | on an actual basis; |
| • | on a pro forma basis to reflect: (a) the conversion of all outstanding shares of convertible preferred stock into an aggregate of 15,703,875 shares of common stock immediately prior to the closing of this offering; (b) the automatic conversion of warrants to purchase up to 67,867 shares of our convertible preferred stock into warrants to purchase up to 67,867 shares of common stock immediately prior to the closing of this offering; and (c) the effectiveness of our amended and restated certificate of incorporation; and |
| • | on a pro forma as adjusted basis to give further effect to the issuance and sale of 5,000,000 shares of common stock in this offering at an assumed initial public offering price of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting the underwriting discounts and commissions and estimated offering expenses. |
You should read this information together with our financial statements and related notes appearing elsewhere in this prospectus and the information set forth under the headings “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| As of March 31, 2014 | ||||||||||||
| Actual | Pro Forma(1) |
Pro Forma As Adjusted(1) |
||||||||||
| (in thousands, except for share and per share amounts) |
(unaudited) | |||||||||||
| Equipment loans |
$ | 1,280 | $ | 1,280 | $ | 1,280 | ||||||
| Convertible preferred stock warrant liability |
494 | — | — | |||||||||
| Convertible preferred stock, $0.001 par value, 15,907,322 shares authorized, 15,703,875 shares issued and outstanding, actual; no shares issued and outstanding pro forma and pro forma as adjusted |
90,789 | — | — | |||||||||
| Stockholders’ (deficit) equity: |
||||||||||||
| Preferred stock, $0.001 par value, no shares authorized, issued and outstanding, actual; 10,000,000 shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted |
— | — | — | |||||||||
| Common stock, $0.001 par value; |
||||||||||||
| 150,000,000 shares authorized, 1,797,933 shares issued and outstanding, actual; 150,000,000 shares authorized, 17,501,808 shares issued and outstanding, pro forma and 22,501,808 shares issued and outstanding, pro forma as adjusted |
2 | 18 | 23 | |||||||||
| Additional paid-in capital |
2,065 | 93,332 | 146,127 | |||||||||
| Notes receivable from related party |
(204 | ) | (204 | ) | (204 | ) | ||||||
| Accumulated deficit |
(82,668 | ) | (82,668 | ) | (82,668 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ (deficit) equity |
(80,805 | ) | 10,478 | 63,278 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | 10,478 | $ | 10,478 | 63,278 | |||||||
|
|
|
|
|
|
|
|||||||
| (1) | Each $1.00 increase (decrease) in the assumed initial price to the public of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) each of additional paid-in capital, total stockholders’ equity and total capitalization by approximately $4.7 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1,000,000 shares in the number of shares offered by us would increase (decrease) each of additional paid-in capital, total stockholders’ equity and total capitalization by approximately $11.2 million, assuming that the |
44
Table of Contents
| assumed initial price to the public remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will adjust based on the actual initial price to the public and other terms of this offering determined at pricing. |
The number of shares of our common stock reflected in the discussion and tables above is based on 17,501,808 shares of our common stock outstanding as of March 31, 2014, including convertible preferred stock on an as-converted basis, and excludes the following:
| • | 1,964,978 shares of common stock issuable upon the exercise of outstanding options as of March 31, 2014, having a weighted average exercise price of $1.34 per share; |
| • | 47,554 shares of common stock issuable upon the exercise and subsequent conversion of warrants to purchase our Series A convertible preferred stock at an exercise price of $3.68 per share; |
| • | Up to 20,313 shares of common stock issuable upon the exercise and subsequent conversion of a warrant to purchase our Series D convertible preferred stock at an exercise price of $6.89 per share, which, as of March 31, 2014, was exercisable for 5,803 shares; |
| • | 4,750,000 shares of common stock reserved for future issuance under our 2014 Equity Incentive Plan, as well as any automatic increases in the number of shares of common stock reserved for future issuance under this plan, which will become effective upon the execution of the underwriting agreement related to this offering; and |
| • | 496,092 shares of common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan, as well as any automatic increases in the number of shares of common stock reserved for future issuance under this plan, which will become effective upon the execution of the underwriting agreement related to this offering. |
To the extent that any outstanding options or warrants are exercised, new options are issued under our stock-based compensation plans or we issue additional shares of common stock in the future, there will be further dilution to investors participating in this offering.
45
Table of Contents
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the assumed initial public offering price per share and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Historical net tangible book value (deficit) per share represents our total tangible assets less our liabilities and convertible preferred stock that is not included in equity divided by the total number of shares outstanding. As of March 31, 2014, our historical net tangible book value (deficit) was approximately $(80.8) million, or $(44.94) per share. Our pro forma net tangible book value as of March 31, 2014, was approximately $10.5 million, or $0.60 per share after giving effect to the conversion of all of our outstanding convertible preferred stock into 15,703,875 shares of common stock upon the consummation of this offering.
After giving further effect to receipt of the net proceeds from our sale of 5,000,000 shares of common stock at an assumed initial public offering price of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses, our pro forma as adjusted net tangible book value as of March 31, 2014, would have been approximately $63.3 million, or $2.81 per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $2.21 per share to our existing stockholders and an immediate dilution of $9.19 per share to investors purchasing common stock in this offering.
The following table illustrates this dilution to new investors on a per share basis:
| Assumed initial public offering price per share |
$ | 12.00 | ||||||
| Historical net tangible book value (deficit) per share as of March 31, 2014 |
$ | (44.94 | ) | |||||
| Pro forma increase in net tangible book value (deficit) per share attributable to the conversion of our convertible preferred stock |
45.54 | |||||||
|
|
|
|||||||
| Pro forma net tangible book value per share as of March 31, 2014 |
0.60 | |||||||
| Increase in net tangible book value per share attributable to new investors purchasing shares in this offering |
2.21 | |||||||
|
|
|
|||||||
| Pro forma as adjusted net tangible book value per share after this offering |
2.81 | |||||||
|
|
|
|||||||
| Dilution per share to new investors participating in this offering |
$ | 9.19 | ||||||
|
|
|
If the underwriters’ option to purchase additional shares in this offering is exercised in full, the pro forma as adjusted net tangible book value would be $3.08 per share, and the dilution to new investors participating in this offering would be $8.92 per share.
Each $1.00 increase (decrease) in the assumed initial public offering price of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted net tangible book value, by $0.21 per share and the dilution per share to new investors by $0.79 per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses.
We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 shares in the number of shares we are offering would increase (decrease) our pro forma as adjusted net tangible book value by approximately $11.2 million, or $0.36 per share, and decrease (increase) the pro forma dilution per share to investors in this offering by $0.36 per share, assuming that the assumed initial public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses. The pro forma information discussed above is illustrative only and will change based on the actual initial public offering price, number of shares and other terms of this offering determined at pricing.
46
Table of Contents
The table below summarizes, as of March 31, 2014, on the pro forma as adjusted basis described above, the number of shares of our common stock, the total consideration, and the average price per share (1) paid to us by our existing stockholders and (2) to be paid by new investors participating in this offering at an assumed initial public offering price of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, before deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| (in thousands, except share, per share and percentages) | Shares Purchased | Total Consideration | Average Price Per Share |
|||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||||
| Existing stockholders |
17,501,808 | 77.8 | % | $ | 92,479,490 | 60.7 | % | $ | 5.28 | |||||||||||
| New investors |
5,000,000 | 22.2 | 60,000,000 | 39.3 | $ | 12.00 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
22,501,808 | 100.0 | % | $ | 152,479,490 | 100.0 | % | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
In addition, if the underwriters’ option to purchase additional shares is exercised in full, the number of shares held by existing stockholders will be reduced to 75.3% of the total number of shares of common stock to be outstanding upon completion of this offering, and the number of shares of common stock held by new investors participating in this offering will be further increased to 24.7% of the total number of shares of common stock to be outstanding upon completion of the offering.
Each $1.00 increase (decrease) in the assumed initial public offering price of $12.00 per share would increase (decrease) total consideration paid by new investors by $5.0 million assuming the number of shares we are offering, as set forth on the cover page of this prospectus, remains the same, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares offered by us would increase (decrease) total consideration paid by new investors by $12.0 million, assuming that the assumed initial price to the public remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The number of shares of our common stock reflected in the discussion and tables above is based on 17,501,808 shares of our common stock outstanding as of March 31, 2014, and excludes:
| • | 1,964,978 shares of common stock issuable upon the exercise of outstanding options as of March 31, 2014, having a weighted average exercise price of $1.34 per share; |
| • | 47,554 shares of common stock issuable upon the exercise and subsequent conversion of warrants to purchase our Series A convertible preferred stock at an exercise price of $3.68 per share; |
| • | Up to 20,313 shares of common stock issuable upon the exercise and subsequent conversion of a warrant to purchase our Series D convertible preferred stock at an exercise price of $6.89 per share, which, as of March 31, 2014, was exercisable for 5,803 shares; |
| • | 4,750,000 shares of common stock reserved for future issuance under our 2014 Equity Incentive Plan, as well as any automatic increases in the number of shares of common stock reserved for future issuance under this plan, which will become effective upon the execution of the underwriting agreement related to this offering; and |
| • | 496,092 shares of common stock reserved for future issuance under our 2014 Employee Stock Purchase Plan, as well as any automatic increases in the number of shares of common stock reserved for future issuance under this plan, which will become effective upon the execution of the underwriting agreement related to this offering. |
Effective as of the date of the effectiveness of the registration statement of which this prospectus forms a part, an aggregate of 4,750,000 shares of our common stock will be reserved for issuance under the 2014 Equity Incentive Plan and 496,092 shares of our common stock will be reserved for issuance under the 2014 Employee Stock Purchase Plan. Furthermore, we may choose to raise additional capital through the sale of equity or convertible debt securities due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that any of our options are exercised, new options are issued under our equity incentive plans or we issue additional shares of common stock or other equity or convertible debt securities in the future, there will be further dilution to investors participating in this offering.
47
Table of Contents
You should read the following selected financial data together with our audited and unaudited financial statements, the related notes appearing at the end of this prospectus and the information under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” The selected financial data included in this section are not intended to replace the financial statements and the related notes included elsewhere in this prospectus.
We derived the selected statements of operations data for the years ended December 31, 2012 and 2013, and the balance sheet data as of December 31, 2012 and 2013, from our audited financial statements appearing elsewhere in this prospectus. The statements of operations data for the three months ended March 31, 2013 and 2014, and the balance sheet data as of March 31, 2014, are derived from our unaudited interim financial statements included elsewhere in this prospectus. We have prepared the unaudited interim financial statements on the same basis as the audited financial statements and have included, in our opinion, all adjustments, consisting only of normal recurring adjustments that we consider necessary for a fair presentation of the financial information set forth in those statements. You should read this data together with our audited financial statements and related notes appearing elsewhere in this prospectus and the information under the captions “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results are not necessarily indicative of our future results and our interim results are not necessarily indicative of results to be expected for the full year ending December 31, 2014, or any other period.
| Year Ended December 31, |
Three Months Ended March 31, |
|||||||||||||||
| (in thousands, except per share data) |
2012 | 2013 | 2013 | 2014 | ||||||||||||
| (unaudited) | ||||||||||||||||
| Statements of Operations Data: |
||||||||||||||||
| Revenue |
$ | 5,863 | $ | 17,931 | $ | 2,744 | $ | 7,497 | ||||||||
| Cost of sales |
3,837 | 8,150 | 1,949 | 2,360 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
2,026 | 9,781 | 795 | 5,137 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Selling, general and administrative |
9,251 | 18,229 | 3,350 | 6,658 | ||||||||||||
| Research and development |
9,260 | 9,518 | 2,256 | 2,577 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
18,511 | 27,747 | 5,606 | 9,235 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(16,485 | ) | (17,966 | ) | (4,811 | ) | (4,098 | ) | ||||||||
| Other income (expense), net |
120 | (403 | ) | 58 | (311 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (16,365 | ) | $ | (18,369 | ) | $ | (4,753 | ) | $ | (4,409 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share, basic and diluted |
$ | (16.38 | ) | $ | (12.57 | ) | $ | (4.57 | ) | $ | (2.49 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average common shares used to compute net loss per share, basic and diluted |
999 | 1,461 | 1,039 | 1,773 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma net loss per share, basic and diluted (unaudited)(1) |
$ | (1.17 | ) | $ | (0.25 | ) | ||||||||||
|
|
|
|
|
|||||||||||||
| Weighted average common shares used to compute pro forma net loss per share, basic and diluted (unaudited)(1) |
15,655 | 17,474 | ||||||||||||||
|
|
|
|
|
|||||||||||||
48
Table of Contents
| As of December 31, |
As of March 31, 2014 |
|||||||||||
| 2012 | 2013 | |||||||||||
| (in thousands) |
(unaudited) | |||||||||||
| Balance Sheet Data: |
||||||||||||
| Cash and cash equivalents |
$ | 2,060 | $ | 12,294 | $ | 7,478 | ||||||
| Working capital |
1,152 | 13,118 | 8,643 | |||||||||
| Total assets |
7,227 | 21,035 | 16,767 | |||||||||
| Preferred stock warrant liability |
93 | 237 | 494 | |||||||||
| Total debt |
2,000 | 1,452 | 1,280 | |||||||||
| Total liabilities |
5,627 | 6,892 | 6,783 | |||||||||
| Convertible preferred stock |
60,320 | 90,760 | 90,789 | |||||||||
| Accumulated deficit |
(59,890 | ) | (78,259 | ) | (82,668 | ) | ||||||
| Total stockholders’ deficit |
(58,720 | ) | (76,617 | ) | (80,805 | ) | ||||||
| (1) | Reflects the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 15,703,875 shares of common stock immediately prior to the closing of this offering and the conversion of warrants to purchase up to 67,867 shares of convertible preferred stock into warrants to purchase up to 67,867 shares of common stock immediately prior to the closing of this offering. |
49
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations together with the section entitled “Selected Financial Data,” should be read in conjunction with our financial statements and the related notes to those statements included elsewhere in this prospectus. In addition to historical financial information, the following discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results and timing of selected events may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those discussed under “Risk Factors” and elsewhere in this prospectus.
Overview
We are a commercial stage drug-device company committed to improving the quality of life for patients with ear, nose and throat conditions. We have developed a drug-eluting bioabsorbable implant technology that enables targeted and sustained release of therapeutic agents. This targeted drug delivery technology is designed to allow ear, nose and throat, or ENT, physicians to improve patient care. Our initial products, PROPEL and PROPEL mini, are the first and only drug-eluting implants approved by the U.S. Food and Drug Administration, or FDA, for use in patients with chronic sinusitis. Inserted by a physician during ethmoid sinus surgery, the self-expanding implants are designed to conform to and hold open the surgically enlarged sinus, while gradually releasing an anti-inflammatory steroid over a period of 30 days, before being fully absorbed into the body. Use of our PROPEL implants is clinically proven to improve surgical outcomes by maintaining the open pathways created in surgery and reducing the need for oral steroids and additional surgical procedures. In addition, we are using our drug-eluting bioabsorbable implant technology to develop new, less-invasive and more cost-effective treatment options for the management of chronic sinusitis in the physician office setting to provide benefits for patients, physicians and payors. Any new products we develop, or changes that we make in the therapeutic agent used in PROPEL or PROPEL mini, will require FDA approval prior to commercialization in the United States.
Our two commercial products, PROPEL and PROPEL mini, are designed to improve the outcomes of sinus surgery by reducing postoperative inflammation and scarring, both from the underlying condition as well as the surgery. Prior to obtaining FDA approval, we devoted substantially all of our resources to the design and clinical development of our steroid-eluting implants. Following approval, we commenced a limited commercial launch of PROPEL in the United States in September 2011, with four territory managers. In November 2012, we received FDA approval and commenced a limited commercial launch of PROPEL mini. In the first half of 2013 we began scaling our U.S. direct commercial presence. In addition to PROPEL and PROPEL mini, we are developing additional products using our drug-eluting bioabsorbable implant technology that are specifically designed to treat chronic sinusitis in the physician office setting during a routine visit.
Our direct sales force engages in sales efforts and promotional activities focused on ENT physicians. We increased the number of employees in our sales, marketing and reimbursement organizations from 21 as of December 31, 2012, to 62 as of March 31, 2014, and we expect to continue to expand this infrastructure to further penetrate the chronic sinusitis market. As of March 31, 2014, over 1,000 ENT physicians in the United States have been trained and have incorporated our steroid-eluting implants into their practice. Although our sales and marketing efforts are directed at ENT physicians because they are the primary users of our technology, we consider the hospitals and ambulatory surgery centers where the procedure is performed to be our customers, as they typically are responsible for making the decisions to purchase our products. No single customer accounted for more than 6% of our revenue during 2012, 2013, and 2014. We manufacture our steroid-eluting implants at our 32,500 square foot facility in Menlo Park, California with components supplied by external suppliers. On March 31, 2014, our manufacturing organization included 53 people and we expect our existing facility to meet expected demand for the foreseeable future.
During the year ended December 31, 2013, our revenue was $17.9 million, and during the three months ended March 31, 2014, our revenue was $7.5 million. During the year ended December 31, 2013, our net loss was $18.4 million, and during the three months ended March 31, 2014, our net loss was $4.4 million. We have not been profitable since inception and as of March 31, 2014, our accumulated deficit was $82.7 million. Since
50
Table of Contents
inception, we have financed our operations primarily through private placements of our convertible preferred securities and, to a lesser extent, certain debt financing arrangements.
Components of Our Results of Operations
Revenue
All of our revenue is currently derived from sales of PROPEL and PROPEL mini in the United States. We expect our revenue to increase as we expand our sales, marketing and reimbursement infrastructure and increase awareness of our products. We also expect our revenue to fluctuate from quarter to quarter due to a variety of factors. In the first quarter, our results can be impacted by adverse weather and by resetting of annual patient healthcare insurance plan deductibles, both of which may cause patients to delay elective procedures such as functional endoscopic sinus surgery, or FESS. In the second quarter, demand may be impacted by the seasonal nature of allergies and the resultant onset of sinus-related symptoms. In the third quarter, the number of FESS procedures nationwide is historically lower than other quarters throughout the year, which we believe is attributable to the summer vacations of ENT physicians and their patients. In the fourth quarter, demand may be impacted by the onset of the cold and flu season and related symptoms, as well as the desire of patients to spend their remaining balances in flexible-spending accounts or because they have met their annual deductibles under their health insurance plans.
Our currently approved products are commonly treated as general supplies utilized in sinus surgery and are paid for as part of the FESS procedure. We believe that establishment of reimbursement codes specific to the use of drug-eluting implants for chronic sinusitis is an important factor in expanding access to our products, especially in the physician office setting.
Cost of Sales and Gross Profit
PROPEL and PROPEL mini are manufactured at our facility in Menlo Park, California. Cost of sales consists primarily of manufacturing overhead costs, material costs and direct labor. A significant portion of our cost of sales currently consists of manufacturing overhead costs. These overhead costs include the cost of quality assurance, material procurement, inventory control, facilities, equipment and operations supervision and management. We expect overhead costs as a percentage of revenue to become less significant as our production volume increases. Cost of sales also includes depreciation expense for production equipment and certain direct costs such as shipping costs. We expect cost of sales to increase in absolute dollars primarily as, and to the extent, our revenue grows.
We calculate gross margin as gross profit divided by revenue. Our gross margin has been and will continue to be affected by a variety of factors, primarily production volumes, manufacturing costs and product yields, and to a lesser extent the implementation of cost-reduction strategies. We expect our gross margin to increase over the long term as our production volume increases and as we spread the fixed portion of our manufacturing overhead costs over a larger number of units produced, thereby significantly reducing our per unit manufacturing costs. However, our gross margin will likely fluctuate from quarter to quarter.
Selling, General and Administrative Expenses
Selling, general and administrative, or SG&A, expenses consist primarily of compensation for personnel, including stock-based compensation, related to selling, marketing, business development, finance, information technology, and human resource functions. Other SG&A expenses include commissions, training, travel expenses, promotional activities, conferences, trade shows, professional services fees, audit fees, insurance costs and general corporate expenses including allocated facilities-related expenses. We expect SG&A expenses to continue to increase in absolute dollars for the foreseeable future as we expand our commercial infrastructure to both drive and support the anticipated growth in revenue and incur additional legal, accounting, insurance and other professional service fees associated with being a public company. However, for the foreseeable future we expect SG&A expenses to continue to decrease as a percentage of revenue.
Research and Development Expenses
Research and development, or R&D, expenses consist primarily of engineering, product development, clinical and regulatory affairs, consulting services, materials, depreciation and other costs associated with
51
Table of Contents
products and technologies in development. These expenses include employee and non-employee compensation, including stock-based compensation, supplies, materials, quality assurance expenses allocated to R&D programs, consulting, related travel expenses and facilities expenses. Clinical expenses include clinical trial design, clinical site reimbursement, data management and travel expenses, and the cost of manufacturing products for clinical trials. We expect R&D expenses to increase in absolute dollars for the foreseeable future as we continue to develop, enhance and commercialize new products and technologies. However, we expect R&D expenses as a percentage of revenue to vary over time depending on the level and timing of initiating new product development efforts as well as our clinical development activities.
Results of Operations
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (in thousands, except percentages) | (unaudited) | |||||||||||||||
| Revenue |
$ | 5,863 | $ | 17,931 | $ | 2,744 | $ | 7,497 | ||||||||
| Cost of sales |
3,837 | 8,150 | 1,949 | 2,360 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
2,026 | 9,781 | 795 | 5,137 | ||||||||||||
| Gross margin |
35 | % | 55 | % | 29 | % | 69 | % | ||||||||
| Operating expenses: |
||||||||||||||||
| Selling, general and administrative |
9,251 | 18,229 | 3,350 | 6,658 | ||||||||||||
| Research and development |
9,260 | 9,518 | 2,256 | 2,577 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
18,511 | 27,747 | 5,606 | 9,235 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(16,485 | ) | (17,966 | ) | (4,811 | ) | (4,098 | ) | ||||||||
| Other income (expense), net |
120 | (403 | ) | 58 | (311 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (16,365 | ) | $ | (18,369 | ) | $ | (4,753 | ) | $ | (4,409 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
Comparison of Three Months Ended March 31, 2013 and 2014
Revenue
Revenue increased $4.8 million, or 173%, to $7.5 million during the three months ended March 31, 2014, compared to $2.7 million during the three months ended March 31, 2013. The growth in revenue was attributable to an increase in unit sales of PROPEL and PROPEL mini from 3,900 units, to 10,800 units, or 177%. Our average selling price per unit was consistent for both periods. The increase in units was driven by an expansion of our sales, marketing and reimbursement organizations. In addition, PROPEL mini had minimal sales in the three months ended March 31, 2013, as compared to the three months ended March 31, 2014, as we continued its commercial introduction.
Cost of Sales and Gross Margin
Cost of sales increased $0.5 million, or 21%, to $2.4 million during the three months ended March 31, 2014, compared to $1.9 million during the three months ended March 31, 2013. The increase in cost of sales was primarily attributable to the growth in the number of PROPEL and PROPEL mini units sold. In addition, cost of sales incurred during the three months ended March 31, 2013, included $0.7 million for expenses associated with establishing and qualifying our new manufacturing facility in Menlo Park, California. After qualification of the Menlo Park facility in June 2013, we closed our facility in Palo Alto, California.
Gross margin for the three months ended March 31, 2014, increased to 69%, compared to 29% for the three months ended March 31, 2013. The increase in gross margin was primarily due to the growth in unit sales which allowed us to spread the fixed portion of our manufacturing overhead costs over more production units, and the impact of the completion of qualification of our Menlo Park facility in June 2013. The fixed portion of our manufacturing overhead allows our costs of sales to grow at a slower rate than our revenue. As a result, we expect gross margin to continue to increase.
52
Table of Contents
Selling, General and Administrative Expenses
SG&A expenses increased $3.3 million, or 99%, to $6.7 million during the three months ended March 31, 2014, compared to $3.4 million during the three months ended March 31, 2013. The increase in SG&A expenses was primarily due to the build out of our infrastructure to support the commercialization of PROPEL and PROPEL mini. The primary driver of this increase was employee-related expenses of our sales, marketing and reimbursement organizations which increased $2.9 million as we increased headcount to 62 as of March 31, 2014, compared to 26 at March 31, 2013. In addition, other SG&A expenses increased $0.4 million primarily due to an increase in headcount.
Research and Development Expenses
R&D expenses increased $0.3 million, or 14%, to $2.6 million during the three months ended March 31, 2014, compared to $2.3 million during the three months ended March 31, 2013. The increase in R&D expenses was primarily due to an increase in clinical trial costs for the evaluation of our steroid-eluting implant for refractory disease for use in the physician office setting.
Other Income (Expense), Net
Other income (expense), net, decreased $0.4 million, or 636%, to an expense of $0.3 million during the three months ended March 31, 2014, compared to an income of $0.1 million during the three months ended March 31, 2013. The decrease in other income (expense), net was attributable to a fair value adjustment of the convertible preferred stock warrants, which are accounted for as liabilities and marked-to-market at each reporting period, and interest expense related to our debt financing arrangements.
Comparison of Years Ended December 31, 2012 and 2013
Revenue
Revenue increased $12.0 million, or 206%, to $17.9 million during the year ended December 31, 2013, compared to $5.9 million during the year ended December 31, 2012. The growth in revenue was attributable to an increase in unit sales of PROPEL and PROPEL mini from 8,400 units to 25,600 units, or 205%. Our average selling price per unit was consistent for both periods. The increase in units was driven by the increased commercialization of PROPEL and the introduction of PROPEL mini in late 2012, supported by an expansion of our sales, marketing and reimbursement organizations.
Cost of Sales and Gross Margin
Cost of sales increased $4.4 million, or 112%, to $8.2 million during the year ended December 31, 2013, compared to $3.8 million during the year ended December 31, 2012. The increase in cost of sales was primarily attributable to the growth in the number of PROPEL and PROPEL mini units sold, partially offset by a decrease of $0.6 million for expenses associated with establishing and qualifying our new manufacturing facility in Menlo Park, California. After qualification of the Menlo Park facility in June 2013, we closed our facility in Palo Alto, California.
Gross margin for the year ended December 31, 2013, increased to 55%, compared to 35% for the year ended December 31, 2012. The increase in gross margin was primarily due to the growth in unit sales which allowed us to spread the fixed portion of our manufacturing overhead costs over more production units, and the impact of the completion of the qualification of our Menlo Park facility in June 2013. The fixed portion of our cost of sales allows our cost of sales to grow at a slower rate than our revenue.
Selling, General and Administrative Expenses
SG&A expenses increased $8.9 million, or 97%, to $18.2 million during the year ended December 31, 2013, compared to $9.3 million during the year ended December 31, 2012. The increase in SG&A expenses was primarily due to the build out of our infrastructure to support the commercialization of PROPEL and PROPEL mini. The primary driver of this increase was employee-related expenses of our sales, marketing and
53
Table of Contents
reimbursement organizations, which increased $8.3 million as we increased headcount to 55 as of December 31, 2013, compared to 21 at December 31, 2012. In addition, other SG&A expenses increased $0.7 million primarily due to the newly enacted medical device tax.
Research and Development Expenses
R&D expenses increased $0.2 million, or 3%, to $9.5 million during the year ended December 31, 2013, compared to $9.3 million during the year ended December 31, 2012. The increase in R&D expenses was primarily due to an increase of $1.9 million in clinical trial costs for the evaluation of our steroid-eluting implant for refractory disease for use in the physician office setting. This was partially offset by a decrease of $1.0 million in development costs as that product transitioned into clinical trials and a decrease of $0.6 million in quality assurance costs allocated to R&D programs.
Other Income (Expense), Net
Other income (expense), net, decreased $0.5 million to an expense of $0.4 million during the year ended December 31, 2013, compared to an income of $0.1 million during the year ended December 31, 2012. The decrease in other income (expense), net was primarily attributable to a fair value adjustment of the preferred stock financing option in connection with our Series D convertible preferred stock financing, the fair value adjustment of the preferred stock warrants, which are accounted for as liabilities and marked-to-market at each reporting period, and interest expense related to our debt financing arrangements.
Quarterly Results of Operations Data
The following tables set forth our unaudited quarterly statements of operations and comprehensive loss data for each quarter in the year ended December 31, 2013, and the quarter ended March 31, 2014. We have prepared the quarterly data on a consistent basis as our audited financial statements included in this prospectus. This information should be read in conjunction with the audited financial statements and related notes for the year ended December 31, 2013, included elsewhere in this prospectus. The results of historical periods are not necessarily indicative of the results of our operations for the year ending December 31, 2014, or any future period.
| Three Months Ended | ||||||||||||||||||||
| March 31, 2013 |
June 30, 2013 |
September 30, 2013 |
December 31, 2013 |
March 31, 2014 |
||||||||||||||||
| (in thousands, except percentages) | (unaudited) | |||||||||||||||||||
| Revenue |
$ | 2,744 | $ | 3,936 | $ | 4,279 | $ | 6,972 | $ | 7,497 | ||||||||||
| Cost of sales(1) |
1,949 | 2,202 | 1,438 | 2,561 | 2,360 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
795 | 1,734 | 2,841 | 4,411 | 5,137 | |||||||||||||||
| Gross margin(1) |
29 | % | 44 | % | 66 | % | 63 | % | 69 | % | ||||||||||
| Operating expenses: |
||||||||||||||||||||
| Selling, general and administrative |
3,350 | 4,087 | 4,700 | 6,092 | 6,658 | |||||||||||||||
| Research and development |
2,256 | 2,421 | 2,077 | 2,764 | 2,577 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
5,606 | 6,508 | 6,777 | 8,856 | 9,235 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(4,811 | ) | (4,774 | ) | (3,936 | ) | (4,445 | ) | (4,098 | ) | ||||||||||
| Other income (expense), net(2) |
58 | (111 | ) | (163 | ) | (187 | ) | (311 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (4,753 | ) | $ | (4,885 | ) | $ | (4,099 | ) | $ | (4,632 | ) | $ | (4,409 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | The three months ended March 31, 2013, included $0.7 million for establishing and qualifying our new manufacturing facility. The three months ended June 30, 2013, included a charge of $0.5 million related to a packaging issue and $0.1 million for establishing and qualifying our new manufacturing facility. |
| (2) | The three months ended June 30 and September 30, 2013, each included a $0.1 million fair value expense adjustment for the preferred stock financing option. The three months ended December 31, 2013, and March 31, 2014, included a $0.1 million and $0.3 million fair value expense adjustment for the preferred stock warrants classified as liabilities, respectively. |
54
Table of Contents
Seasonality
Our business is affected by seasonality. In the first quarter, our results can be impacted by adverse weather and by resetting of annual patient healthcare insurance plan deductibles, both of which may cause patients to delay elective procedures such as FESS. In the second quarter, demand may be impacted by the seasonal nature of allergies and the resultant onset of sinus-related symptoms. In the third quarter, the number of FESS procedures nationwide is historically lower than other quarters throughout the year, which we believe is attributable to the summer vacations of ENT physicians and their patients. In the fourth quarter, demand may be impacted by the onset of the cold and flu season and related symptoms, as well as the desire of patients to spend their remaining balances in flexible-spending accounts or because they have met their annual deductibles under their health insurance plans.
Liquidity and Capital Resources
Overview
As of March 31, 2014, we had cash and cash equivalents of $7.5 million and an accumulated deficit of $82.7 million, compared to cash and cash equivalents of $12.3 million and an accumulated deficit of $78.3 million as of December 31, 2013. We currently believe that our existing cash and cash equivalents, revenue and available debt financing arrangements will be sufficient to meet our capital requirements and fund our operations until at least December 31, 2014. Our primary sources of capital have been from private placements of convertible preferred securities and debt financing agreements. To date, we have raised $91.4 million from private placements of convertible preferred securities from our investors. In August 2013, we entered into a loan and security agreement with Silicon Valley Bank for up to $12.0 million of debt financing consisting of an $8.0 million growth capital facility and a $4.0 million revolving accounts receivable line of credit. As of March 31, 2014, we had no outstanding amounts under the loan and security agreement. We entered into equipment loans totaling $2.1 million, of which $1.3 million is outstanding as of March 31, 2014. There are no remaining amounts available under these loans.
Cash Flows
| Year Ended December 31, |
Three Months Ended March 31, |
|||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (in thousands) | (unaudited) | |||||||||||||||
| Net cash (used in) provided by: |
||||||||||||||||
| Operating activities |
$ | (16,148 | ) | $ | (19,101 | ) | $ | (6,118 | ) | $ | (4,546 | ) | ||||
| Investing activities |
5,956 | (467 | ) | (52 | ) | (136 | ) | |||||||||
| Financing activities |
2,302 | 29,802 | 20,674 | (134 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net (decrease) increase in cash and cash equivalents |
$ | (7,890 | ) | $ | 10,234 | $ | 14,504 | $ | (4,816 | ) | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
Net cash Used in Operating Activities
During the three months ended March 31, 2014, net cash used in operating activities was $4.5 million, consisting primarily of a net loss of $4.4 million and an increase in net operating assets of $0.8 million, partially offset by non-cash charges of $0.7 million. The cash used in operations was primarily due to the ongoing commercialization of PROPEL and PROPEL mini. To support the commercialization of these products, we continued to expand our sales, marketing and reimbursement organizations and manufacturing supply chain. The increase in net operating assets was primarily due to increases in accounts receivable and inventory to support the growth of our operations and a decrease in accrued compensation as annual bonuses were paid, partially offset by increases in accounts payable and other liabilities as we expanded our operations. The non-cash charges primarily consisted of the change in fair value of convertible preferred stock warrants accounted for as liabilities and stock-based compensation as the underlying value of our company increased with the expansion of our business.
During the three months ended March 31, 2013, net cash used in operating activities was $6.1 million, consisting primarily of a net loss of $4.8 million and an increase in net operating assets of $1.6 million, partially
55
Table of Contents
offset by non-cash charges of $0.3 million. The increase in net operating assets was primarily due to increases in accounts receivable and inventory as we expanded our sales, marketing and reimbursement organizations and manufacturing supply chain and a decrease in accrued compensation as annual bonuses were paid. The non-cash charges primarily consisted of depreciation and amortization and the forgiveness of a related party loan.
Net cash used in operating activities for 2013 was $19.1 million, consisting primarily of a net loss of $18.4 million and an increase in net operating assets of $2.1 million, partially offset by non-cash charges of $1.4 million. The cash used in operations was primarily due to the expansion our sales, marketing and reimbursement organizations to support the ongoing commercialization of PROPEL and PROPEL mini resulting in increases in accounts receivable and inventory, offset by an increase in accrued compensation due to the growth in our sales, marketing and reimbursement organizations. Non-cash charges consisted primarily of depreciation from the investment in our Menlo Park manufacturing facility and stock-based compensation. As of December 31, 2013, 87% of accounts receivable was less than 60 days old.
Net cash used in operating activities for 2012 was $16.1 million, consisting primarily of a net loss of $16.4 million and an increase in net operating assets of $0.4 million, partially offset by non-cash charges of $0.6 million. The increase in net operating assets was primarily due to increases in accounts receivable and inventory as we accelerated our commercial launch of PROPEL and introduced PROPEL mini, partially offset by an increase in accrued compensation related to expansion of our sales, marketing and reimbursement organizations. Non-cash charges consisted primarily of depreciation and stock-based compensation.
Net Cash (Used in) Provided by Investing Activities
During each of the three months ended March 31, 2013 and 2014, net cash used in investing activities was $0.1 million, consisting of purchases of property and equipment.
Net cash used in investing activities in 2013 was $0.5 million consisting of purchases of property and equipment. Net cash provided by investing activities in 2012 was $6.0 million in 2012, consisting primarily of net proceeds from short-term investment of $7.3 million, partially offset by purchases of property and equipment of $1.4 million.
Net Cash (Used in) Provided by Financing Activities
During the three months ended March 31, 2014, net cash used in financing activities was $0.1 million, consisting of repayments related to the equipment financing arrangements. During the three months ended March 31, 2013, net cash provided by financing activities was $20.7 million, consisting primarily of net proceeds from the issuance of Series D convertible preferred stock.
Net cash provided by financing activities in 2013 was $29.8 million, consisting primarily of net proceeds from the issuance of our Series D convertible preferred stock of $30.1 million. Net cash provided by financing activities in 2012 was $2.3 million, primarily from proceeds of a $2.0 million equipment loan.
Liquidity
In August 2013, we entered into a loan and security agreement, or the Loan Agreement, with Silicon Valley Bank, or SVB, for a total commitment of $12.0 million. The commitment consists of an $8.0 million growth capital facility and a $4.0 million revolving accounts receivable line of credit.
The $8.0 million growth capital facility consists of two $4.0 million tranches. The first $4.0 million tranche is available until October 31, 2014, and the second $4.0 million tranche will be available until March 31, 2015. Payment on both tranches will be interest only until March 31, 2015, at which time the outstanding balance will convert to a 30-month fully amortizing loan. The interest rate will be fixed at the time of advance and will be the greater of 3.65% or the three-year U.S. Treasury note plus 3.0%. At the end of the amortization period, we will make a final payment in the amount of 3.9% of the advanced amounts. There is a prepayment penalty of 2.0% of the prepaid principal during the first year, 1.0% during the second year and no prepayment penalty during the third year.
The $4.0 million revolving accounts receivable line of credit will expire in August 2016. Advances on this line of credit will be made for up to 80% of eligible accounts receivables. An annual loan fee of 0.75% is owed at the beginning of August in each of the three years. The interest rate is determined monthly and is the greater of 4.25% or SVB Prime Rate plus 0.25%.
56
Table of Contents
As of March 31, 2014, we have not received advances under the Loan Agreement.
We currently believe that our existing cash and cash equivalents and available debt financing arrangements will be sufficient to meet our capital requirements and fund our operations until at least December 31, 2014. If these sources are insufficient to satisfy our liquidity requirements, we may seek to sell additional equity or debt securities or obtain an additional credit facility. If we raise additional funds by issuing equity securities, our stockholders would experience dilution. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders. Additional financing may not be available at all, or in amounts or on terms unacceptable to us. If we are unable to obtain additional financing, we may be required to delay the development, commercialization and marketing of our products.
Off-Balance Sheet Arrangements
As of December 31, 2013, and March 31, 2014, we were not a party to any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources.
Contractual Obligations
The following table sets out, as of December 31, 2013, our contractual obligations due by period.
| Payments Due by Period | ||||||||||||||||||||
| Less Than 1 Year |
1-3 Years |
3-5 Years |
More Than 5 Years |
Total | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating lease obligations |
$ | 928 | $ | 390 | $ | — | $ | — | $ | 1,318 | ||||||||||
| Purchase commitments |
407 | 54 | — | — | 461 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| $ | 1,335 | $ | 444 | $ | — | $ | — | $ | 1,779 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Our contractual obligations as of March 31, 2014, have not significantly changed from December 31, 2013.
Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk
The risk associated with fluctuating interest rates is primarily limited to our cash equivalents which are carried at quoted market prices. We do not currently use or plan to use financial derivatives in our investment portfolio.
In September 2012, we entered into an equipment loan with an aggregate principal amount of $2.0 million, all of which was drawn down in December 2012. Payments will be made in monthly installments over a 36-month period with a fixed interest rate of 5.1%. As of March 31, 2014, the amount outstanding under this equipment loan was $1.2 million.
Credit Risk
As of December 31, 2012 and 2013, and March 31, 2014, our cash and cash equivalents were maintained with one financial institution in the United States, and our current deposits are likely in excess of insured limits. We have reviewed the financial statements of this institution and believe it has sufficient assets and liquidity to conduct its operations in the ordinary course of business with little or no credit risk to us.
Our accounts receivable primarily relate to revenue from the sale of PROPEL and PROPEL mini to hospitals and ambulatory surgery centers in the United States. No single customer represented more than 5% of our accounts receivable as of December 31, 2013, and March 31, 2014.
Foreign Currency Risk
Our business is conducted in U.S. dollars. Any transactions that may be conducted in foreign currencies are not expected to have a material effect on our results of operations, financial position or cash flows.
Related Parties
For a description of our related party transactions, see “Certain Relationships and Related Party Transactions.”
57
Table of Contents
Critical Accounting Policies and Estimates
Management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions for the reported amounts of assets, liabilities, revenue, expenses and related disclosures. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions and any such differences may be material.
While our significant accounting policies are more fully described in Note 2 of our financial statements included in this prospectus, we believe the following discussion addresses our most critical accounting policies, which are those that are most important to the portrayal of our financial condition and results of operations and require our most difficult, subjective and complex judgments.
Revenue Recognition
We derive revenue from the sale of PROPEL and PROPEL mini to hospitals and ambulatory surgery centers in the United States. We recognize revenue when the following revenue recognition criteria are met:
| • | Persuasive evidence of an arrangement exists. We consider this criterion satisfied when we have an agreement or contract with the customer in place. |
| • | Delivery has occurred or services rendered. We principally determine this criterion to be satisfied when we ship the product to the customer. |
| • | The fee is fixed or determinable and collectibility is reasonably assured. We determine the satisfaction of these criteria based on our judgment regarding the nature of the fee charged for products, contractual agreements entered into, and the collectibility of those fees under any contract or agreement. |
We must make significant assumptions regarding the future collectibility of amounts receivable from customers to determine whether revenue recognition criteria have been met. If collectibility is not assured at the time of shipment, we defer revenue until such criteria have been met. Our standard terms and conditions of sale do not allow for product returns, and we generally do not allow product returns, except in the case of damaged goods, and we have not experienced any significant returns of our products. We expense shipping and handling costs as incurred and include them in the cost of sales. In those cases where we bill shipping and handling costs to customers, we classify the amounts billed as a component of revenue.
Common Stock Valuation and Stock-based Compensation
We maintain an equity incentive plan to provide long-term incentive for employees, consultants, and members of the board of directors. The plan allows for the issuance of non-statutory and incentive stock options to employees and non-statutory stock options to consultants and non-employee directors.
We are required to determine the fair value of equity incentive awards and recognize compensation expense for all equity incentive awards made to employees and directors, including employee stock options. We recognize this expense over the requisite service period. In addition, we recognize stock-based compensation expense in the statements of operations and comprehensive loss based on awards expected to vest and, therefore, the amount of expense has been reduced for estimated forfeitures. We use the straight-line method for expense attribution, except for awards issued with performance-based conditions which require an accelerated attribution method over the requisite performance and service periods.
Under the applicable accounting guidance for equity incentive awards granted to non-employees, the measurement date at which the fair value of the equity incentive awards is measured is equal to the earlier of (1) the date at which a commitment for performance by the counterparty to earn the equity instrument is reached or (2) the date at which the counterparty’s performance is complete. We recognize stock-based compensation expense for the fair value of the vested portion of the equity incentive awards in the statements of operations and comprehensive loss. We remeasure the fair value of options granted to consultants and non-employee directors as the options vest.
58
Table of Contents
The valuation model we used for calculating the fair value of awards for stock-based compensation expense is the Black-Scholes option-pricing model, or the Black-Scholes model. The Black-Scholes model requires us to make assumptions and judgments about the variables used in the calculation, including the expected term weighted average period of time that the options granted are expected to be outstanding, the volatility of common stock, an assumed risk-free interest rate and an estimated forfeiture rate. We use the “simplified method” to determine the expected term of the stock option. Volatility is based on an average of the historical volatilities of the common stock of publicly-traded companies with characteristics similar to us. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods corresponding with the expected term of the option. Potential forfeitures of awards are estimated based on our historical forfeiture experience and an analysis of similar companies. The estimate of forfeitures will be adjusted over the service period to the extent that actual forfeitures differ, or are expected to differ, from prior estimates.
The following table summarizes the assumptions we used to determine the fair value of stock options:
| Year Ended December 31, 2013 |
Three Months Ended March 31, 2014 |
|||||||
| (unaudited) | ||||||||
| Expected life (years) |
6.0 | 6.0 | ||||||
| Expected volatility |
67 | % | 60 | % | ||||
| Risk-free interest rate |
1.6 | % | 1.8 | % | ||||
| Dividend yield |
0.0 | % | 0.0 | % | ||||
Fair Value of Common Stock. As discussed below, the fair value of the shares of our common stock underlying the stock options has historically been determined by our board of directors. Because there has been no public market for our common stock, our board of directors has determined the fair value of our common stock at the time of grant of the option by considering a number of objective and subjective factors, including valuations of comparable companies, sales of our convertible preferred stock, our operating and financial performance and the general and industry-specific economic outlook.
Expected Term. The expected term of stock options represents the weighted average period that the stock options are expected to remain outstanding. We estimated the expected term based on the simplified method, which is the average of the weighted average vesting period and contractual term of the option.
Expected Volatility. Since there has been no public market for our common stock and lack of company specific historical volatility, we have determined the share price volatility for options granted based on an analysis of the volatility of a peer group of publicly traded companies. In evaluating similarity, we consider factors such as industry, stage of life cycle and size.
Risk-free Interest Rate. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of the grant for zero-coupon U.S. Treasury notes with remaining terms similar to the expected term of the options.
Dividend Rate. We assumed the expected dividend to be zero as we have never paid dividends and have no current plans to do so.
Expected Forfeiture Rate. We are required to estimate forfeitures at the time of grant, and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. We use historical data to estimate pre-vesting option forfeitures and record share-based compensation expense only for those awards that are expected to vest. To the extent actual forfeitures differ from the estimates, we record the difference as a cumulative adjustment in the period that the estimates are revised.
Service period. We amortize all stock-based compensation over the requisite service period of the awards, which is generally the same as the vesting period of the awards. We amortize the fair value cost on a straight-line basis over the expected service periods. We estimate when and if performance-based grants will be earned. If we consider the award to be probable, we recognize expense over the estimated service period, which would be the estimated period of performance. If we do not consider the awards probable of achievement, we recognize no amount of stock-based compensation.
If factors change and we employ different assumptions, stock-based compensation expense may differ significantly from what we have recorded in the past. If there are any modifications or cancellations of the
59
Table of Contents
underlying unvested securities, we may be required to accelerate, increase or cancel any remaining unearned stock-based compensation expense. We currently estimate when and if performance-based grants will be earned. If we do not consider the awards probable of achievement, we recognize no amount of stock-based compensation. If we consider the award to be probable, we record expense over the estimated service period. To the extent that our assumptions are incorrect, the amount of stock-based compensation recorded will change.
Our intent has been to grant all options with an exercise price not less than the fair value of our common stock underlying those options on the date of grant. We have determined the estimated fair value of our common stock at each valuation date in accordance with the guidelines outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation. Our board of directors, with the assistance of management, developed these valuations using significant judgment and taking into account numerous factors, including developments at our company, market conditions and contemporaneous independent third-party valuations.
For all option grant dates through March 31, 2014, our board determined the enterprise value based on the Market Approach, Income Approach, Option Pricing Method, or OPM, and Probability Weighted Expected Return Method, or PWERM, or a weighted combination of the OPM and PWERM methods. Under the Market Approach we estimate the value based upon analysis of similar companies. We then apply these derived multiples or values to our financial metrics to estimate our market value. The Income Approach, or Discounted Cash Flow Method, estimates value based on the expectation of future net cash flows, which are then discounted back to the present using a rate of return derived from companies of similar type and risk profile. The allocation of these enterprise values to each part of our capital structure, including our common stock, was done based on OPM. OPM treats the rights of the holders of preferred and common stock as equivalent to call options on any value of the enterprise above certain break points of value based upon the liquidation preferences of the holders of preferred stock, as well as their rights to participation and conversion. Thus, the estimated value of the common stock can be determined by estimating the value of its portion of each of these call option rights. OPM derives the implied equity value of a company from a recent transaction involving the company’s own securities issued on an arms-length basis. Under the PWERM the value is estimated based upon analysis of future values for the enterprise under varying scenarios, probabilities are ascribed to these scenarios based on expected future outcomes. Following the closing of this offering, the fair value of our common stock will be determined based on the closing price of our common stock on The Nasdaq Global Market.
In January 2014, we decided to pursue an initial public offering, or an IPO. As a result, in connection with the preparation of our financial statements included in this prospectus, and in preparing for our proposed IPO, we reexamined the estimated fair value of our common stock associated with our stock option grants since our prior common stock valuation report as of October 31, 2013, for financial reporting purposes.
Our board of directors granted options to purchase common stock on December 3, 2013, primarily to employees who were recently hired since we last issued option grants, with each option having an exercise price of $0.33 per share. In establishing this exercise price, our board of directors considered input from management, including the previously issued common stock valuation report which was performed as of October 31, 2013, as well as various objective and subjective factors including:
| • | the continued lack of liquidity of our common stock as a private company; |
| • | the second tranche of our Series D convertible preferred stock in October 2013; |
| • | the rights, preferences and privileges of our convertible preferred stock as compared to those of our common stock, including the liquidation preferences of our convertible preferred stock; |
| • | the impact of significant ongoing expenses associated with research and development, ongoing clinical trials and commercialization; and |
| • | the low likelihood of achieving a liquidity event for holders of our common stock, such as an IPO or sale of our company, given the prevailing market conditions. |
60
Table of Contents
At the time of the grants, our board of directors determined that, at the grant date, the collective effect of these events and circumstances did not indicate a significant change in the fair value of our common stock. Based on these factors, our board of directors determined that the fair value of our common stock on the grant date listed above was $1.32 per share.
In preparation for filing a registration statement with the SEC in connection with this offering, we evaluated whether or not in retrospect the value of the common stock on December 3, 2013, was appropriate for accounting purposes. We determined that in retrospect, the valuation of the common stock as determined by the valuation report issued as of December 31, 2013, provided a closer approximation of the fair value of the common stock for accounting purposes. For the calculation of stock-based compensation expense solely for financial reporting purposes, we have used a fair value of $4.24 per share for the December 3, 2013, stock option awards.
The following table illustrates our stock option grant information from January 1, 2013, through March 31, 2014, including the estimated fair value of our common stock on the date of grant:
| Grant Date |
Shares Subject to Options Granted |
Exercise Price |
Reassessed Estimated Fair Market Value |
Grant Date Aggregate Intrinsic Value |
||||||||||||
| April 23, 2013 |
888,498 | $ | 1.20 | $ | 1.20 | — | ||||||||||
| June 11, 2013 |
54,750 | 1.20 | 1.20 | — | ||||||||||||
| September 5, 2013 |
90,000 | 1.20 | 1.20 | — | ||||||||||||
| December 3, 2013 |
94,375 | 1.32 | 4.24 | $ | 275,575 | |||||||||||
| March 5, 2014 |
103,560 | 6.20 | 6.20 | — | ||||||||||||
The intrinsic value of all outstanding vested and unvested options as of March 31, 2014, was $20.9 million based on the assumed initial public offering price of $12.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, and based on 1,964,978 shares of common stock issuable upon the exercise of options outstanding as of March 31, 2014, with a weighted average exercise price of $1.34 per share.
Convertible Preferred Stock Warrant Liability
For a warrant classified as a derivative liability, we record the fair value of that warrant on the balance sheet at the inception of such classification and adjust to fair value at each financial reporting date. We record the changes in the fair value of the warrants in the statement of operations as a component of interest and other income or expense as appropriate. We will continue to adjust the carrying value of the convertible preferred stock warrant liability for changes in the fair value of the warrants until the earlier of: the exercise of the warrants, at which time we will reclassify the liability to temporary equity; the conversion of the underlying preferred stock into common stock, at which time we will reclassify the liability to stockholders’ deficit; or the expiration of the warrant, at which time we would reverse the entire amount and reflect it in the statement of operations. Our assumptions with regard to the warrant valuation are based on estimates of the valuation of the underlying preferred stock, volatility, interest rate and such estimates could vary significantly.
Convertible Preferred Stock Financing Option
For a freestanding option to purchase a future round of financing, we determine the fair value of that option based on the underlying estimated future fair value of the convertible preferred stock, the timing of when such obligation will be satisfied, the discount factor applied based on the timing of the satisfaction of the obligation and the probability that the obligation will be satisfied. We record the initial fair value of the obligation on the balance sheets at the inception of such arrangement and adjust it to fair value at each financial reporting date. We record the change in the fair value of the convertible preferred stock financing option in the statement of operations as a component of interest and other income or expense as appropriate. We adjust the carrying values of the convertible preferred stock financing option liability for changes in the fair value of the option and will continue to do so until the earlier of when all of the options have been exercised or expired. The assumptions used in determining the fair value of the obligation require significant judgment.
61
Table of Contents
JOBS Act Accounting Election
As an emerging growth company under the Jumpstart Our Business Startups Act of 2012, we are eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. We have elected to take advantage of the extended transition period for adopting new or revised accounting standards that have different effective dates for public and private companies until such time as those standards apply to private companies. There have been no new accounting pronouncements or changes to accounting pronouncements that are of significance or potential significance to us.
62
Table of Contents
Overview
We are a commercial stage drug-device company committed to improving the quality of life for patients with ear, nose and throat conditions. We have developed a drug-eluting bioabsorbable implant technology that enables targeted and sustained release of therapeutic agents. This targeted drug delivery technology is designed to allow ear, nose and throat, or ENT, physicians to improve patient care. Our initial products, PROPEL and PROPEL mini, are the first and only drug-eluting implants approved by the U.S. Food and Drug Administration, or FDA, for use in patients with chronic sinusitis. Inserted by a physician during ethmoid sinus surgery, the self-expanding implants are designed to conform to and hold open the surgically enlarged sinus while gradually releasing an anti-inflammatory steroid over a period of 30 days, before being fully absorbed into the body. Use of our PROPEL implants is clinically proven to improve surgical outcomes by maintaining the open pathways created in surgery and reducing the need for oral steroids and additional surgical procedures. In addition, we are using our drug-eluting bioabsorbable implant technology to develop new, less-invasive, more cost-effective treatment options for the management of chronic sinusitis in the physician office setting to provide benefits for patients, physicians and payors. Any new products we develop, or changes that we make in the therapeutic agent used in PROPEL or PROPEL mini, will require FDA approval prior to commercialization in the United States.
Chronic sinusitis is one of the most prevalent chronic diseases in the United States and significantly impacts the quality of life of patients. According to the Centers for Disease Control and Prevention, or CDC, approximately 12% of the U.S. adult population, or 29 million people, are affected by chronic sinusitis, making it more prevalent than heart disease and asthma. Chronic sinusitis is an inflammatory condition in which the sinus lining becomes swollen and inflamed, leading to significant patient morbidity, including difficulty breathing, chronic headaches, recurrent infections, bodily pain, and loss of sense of smell and taste. These persistent symptoms can severely impact a patient’s day-to-day well-being, resulting in frequent doctor visits and lost work productivity and can lead to chronic fatigue and depression. Chronic sinusitis is managed by a combination of medical management and surgical intervention. The first line of therapy is medical management involving antibiotics, anti-inflammatory steroids and decongestants. Despite limited efficacy of the use of antibiotics in this patient population and the consequence of increasing bacterial resistance, there is pervasive overuse of these drugs, which could lead to patient resistance and has resulted in sinusitis being identified as a major target in national efforts to reduce unnecessary medical intervention. Sinusitis is the most common reason for adult outpatient antibiotic use in the United States, comprising 11% of all antibiotic prescriptions. Patients whose symptoms persist despite medical management, are recommended to undergo functional endoscopic sinus surgery, or FESS. FESS is performed in the operating room to open the blocked sinus pathways by removing inflamed tissue and bone using surgical tools. Although sinus surgery can be effective, a majority of patients experience recurrent symptoms which commonly necessitate additional treatment with medications and surgery.
Our two commercial products, PROPEL and PROPEL mini, are designed to improve the outcomes of sinus surgery by reducing postoperative inflammation and scarring from the underlying condition as well as the surgery. PROPEL and PROPEL mini were clinically proven in a meta-analysis of prospective, multicenter, randomized, controlled, double-blind clinical studies to improve surgical outcomes, including a 35% reduction in the need for postoperative oral steroid and surgical intervention.
Our target market consists of more than 3.5 million people with chronic sinusitis in the United States who are managed by ENT physicians and who we believe could benefit from products that incorporate our drug-eluting bioabsorbable implant technology. Approximately 540,000 patients underwent sinus surgery for chronic sinusitis in 2013 in the United States. This includes patients who are under the age of 18 and surgeries on the frontal sinuses, both of which represent potential expanded future indications for PROPEL and PROPEL mini and would require FDA approval. Based on the number of sinus surgeries (including surgeries performed by the approximately 40% of surgeons that do not use packing materials in their surgeries), and assuming two devices per surgery and an average selling price of $695 per device, we estimate the annual total addressable market for PROPEL and PROPEL mini in the United States to be approximately $830 million. We further estimate the annual total addressable market for our products in clinical development in the United States that are specifically designed to address patients in the physician office setting to be approximately $4.0 billion. This estimate is based on our estimate that of the more than 3.5 million chronic sinusitis patients in the United States who are
63
Table of Contents
managed by ENT physicians, (i) approximately 34% have undergone prior FESS procedures and we assume that 64% of these patients would undergo approximately 1.5 procedures per year to treat recurring symptoms using two devices at an average selling price of $495 per device, and (ii) the remaining approximately 66% of all chronic sinusitis patients managed by ENT physicians would use a different device we have in clinical development assuming that these patients would undergo approximately one procedure per year using two devices at an average selling price of $595 per device. While our current commercial focus is the U.S. market, we plan to initiate efforts that will allow for future expansion into international geographies. We estimate the annual total addressable market for PROPEL and PROPEL mini in these regions to be greater than $1.0 billion. This estimate is based on an estimated approximately 865,000 FESS procedures annually in the 16 countries in which we did research (including surgeries performed by the approximately 40% of surgeons that do not use packing materials in their surgeries), assuming that 85% involve ethmoid sinuses and 25% frontal sinuses and assuming using two devices at an average selling price of $695 per device. Therefore, we estimate the global annual total addressable market for our products and products in clinical development for chronic sinusitis patients to exceed $6.0 billion.
In August 2011 we received Premarket Approval, or PMA, from the FDA for our first product, PROPEL, indicated for use in patients 18 years or older following ethmoid sinus surgery to maintain patency. Our second product, PROPEL mini, received PMA approval from the FDA in November 2012.
PROPEL mini is designed for patients requiring less extensive surgery and those who have smaller anatomy, thus expanding the treatable patient population. In the first half of 2013, we began scaling our U.S. direct commercial presence. As of March 31, 2014, over 1,000 physicians in the United States have been trained and have incorporated our steroid-eluting implants into their practice. Based on the number of units shipped as of March 31, 2014, we estimate that physicians have treated over 25,000 patients with PROPEL and PROPEL mini. For the year ended December 31, 2013, we generated revenue of $17.9 million and had a net loss of $18.4 million. For the three months ended March 31, 2014, we generated revenue of $7.5 million and had a net loss of $4.4 million. As of March 31, 2014, we had an accumulated deficit of $82.7 million.
We expanded our sales, marketing and reimbursement organizations from 21 people as of December 31, 2012, to 62 people as of March 31, 2014, and have plans to increase sales by further expanding these organizations. We believe this expansion will allow us to further penetrate and expand the market by demonstrating the benefits of our steroid-eluting implants to our physician customers. We expect this would, in turn, persuade more patients to undergo sinus surgery to treat their condition as they are convinced of the ability of our products to improve recovery and surgical outcomes, allowing us to further penetrate and expand the market. In addition, we are using our drug-eluting bioabsorbable implant technology to develop new products for use in the physician office setting, expanding the patient population that can benefit.
64
Table of Contents
Overview of Sinusitis
The sinuses are a system of connected air-filled cavities located within the bones around the nose and eyes that allow for natural ventilation and drainage of mucus. There are four sinus cavities: ethmoid, frontal, maxillary and sphenoid. One of each type of sinus lies on either side of the face. The sinuses are lined with soft, pink tissue called mucosa, which serves to constantly cleanse the sinuses of impurities such as dust, dirt, allergens, pollutants and bacteria. To clear these inhaled pathogens, the sinus lining secretes mucus which is then cleared away by small, hair-like structures called cilia, which act in coordination to sweep the mucus through the sinus pathways and out through the back of the throat.
The ethmoid sinuses, which lie between the eyes, are a series of small cells with multiple, often interconnected openings in a honeycomb-like formation. These sinuses serve as the central aeration and drainage pathway for all other sinuses. The frontal, maxillary and sphenoid sinuses are known as dependent sinuses, as they each consist of one large cell that drains through an opening, or ostium, into the ethmoid sinus.
Chronic sinusitis is an inflammatory condition in which the sinus lining becomes swollen and inflamed, leading to significant patient morbidity including difficulty breathing, chronic headaches, recurrent infections, bodily pain, and loss of sense of smell and taste. These persistent symptoms can severely impact a patient’s day-to-day well-being, resulting in frequent doctor visits and can lead to chronic fatigue and depression. The condition significantly reduces work productivity from absenteeism and reduced on-the-job effectiveness, especially meaningful given the average chronic sinusitis patient age of approximately 37 years.
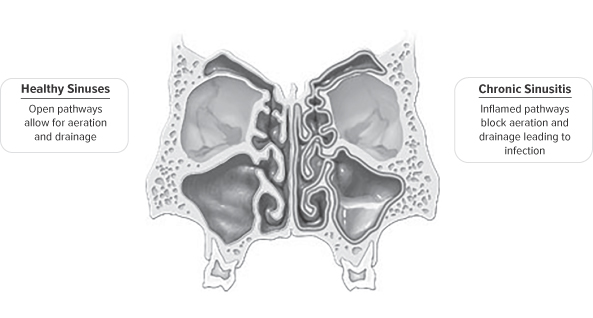
65
Table of Contents
The debilitating patient symptoms and quality of life impairments attributed to chronic sinusitis create a significant healthcare burden to patients, insurers and employers. According to the CDC, approximately 12% of the U.S. adult population, or 29 million people, are affected by the disease, making it more prevalent than heart disease and asthma. The CDC estimated that in 2005 the condition resulted in 12.6 million physician office and 1.2 million hospital out-patient department visits per year in the United States. It is estimated that chronic sinusitis resulted in $8.6 billion in direct healthcare costs in 2007 in the United States. The indirect costs of chronic sinusitis are also significant, with one study attributing a 38% loss in work productivity due to the illness. In addition, a study of over 200,000 managed care patients found that chronic sinusitis patients made 43% more physician office visits and 25% more urgent care visits, ranking chronic sinusitis among the top 10 most costly conditions to U.S. employers.
Our target market consists of more than 3.5 million people with chronic sinusitis in the United States who are managed by ENT physicians and who we believe could benefit from products that incorporate our drug-eluting bioabsorbable implant technology. While approximately 1.6 million chronic sinusitis patients are offered surgery as a treatment option, approximately one third or 540,000 patients will proceed to surgery. This includes patients who are under the age of 18 and surgeries on the frontal sinuses, both of which are indications that require FDA approval before PROPEL and PROPEL mini may be indicated for use in these procedures. Of the 540,000 patients, we estimate that 85% underwent surgeries for the ethmoid sinuses for which PROPEL and PROPEL mini have been approved for use in adults, and 25% underwent surgeries on the frontal sinuses. We believe that less than 5% of all the procedures were performed on patients under 18 years old. Patients suffering from chronic sinusitis are treated by approximately 7,500 ENT physicians who perform sinus surgery. Based on the number of sinus surgeries, and assuming two devices per surgery and using our estimated average selling price of PROPEL and PROPEL mini of $695 per device, we estimate the annual total addressable market for PROPEL and PROPEL mini in the United States, including for future use in frontal sinus surgeries and on patients under 18 years old, to be approximately $830 million. Penetration of this addressable market would require adoption of PROPEL and PROPEL mini by the 60% of ENT physicians who place sinus packing material into the ethmoid sinuses during surgery and the remaining ENT physicians who do not place packing materials in the ethmoid sinuses or frontal sinuses during surgery. We further estimate the annual total addressable market for our products in clinical development in the United States that are specifically designed to address patients in the physician office setting to be approximately $4.0 billion. This estimate is based on an estimated 3.5 million sinusitis patients at all stages of the disease who are managed by ENT physicians in the United States, for which we estimate (i) approximately 34% have undergone FESS procedures and we assume that 64% of these patients would undergo approximately 1.5 procedures per year to treat recurring symptoms using two devices at an average selling price of $495 per device, and (ii) the remaining approximately 66% of all chronic sinusitis patients managed by ENT physicians would use a different device we have in clinical development assuming that these patients would undergo approximately one procedure per year using two devices at an average selling price of $595 per device.
While our current commercial focus is the U.S. market, we plan to initiate efforts that will allow for future expansion into international geographies. To that end, in July 2014, we received CE mark approval for PROPEL and PROPEL mini which enables market access in Europe. Based on the number of sinus surgeries in Europe and the Asia-Pacific region and our estimated average selling price per implant, and assuming two implants per procedure, we estimate the annual total addressable market for PROPEL and PROPEL mini in these regions to be greater than $1.0 billion. This estimate is based on an estimated approximately 865,000 FESS procedures annually in the 16 countries in which we did research (including surgeries performed by the approximately 40% of surgeons that do not use packing materials in their surgeries), assuming that 85% involve ethmoid sinuses and 25% frontal sinuses and assuming using two devices at an average selling price of $695 per device. Therefore, we estimate the global annual total addressable market for our products and products in clinical development for chronic sinusitis patients to exceed $6.0 billion.
66
Table of Contents
Current Treatments for Chronic Sinusitis and Their Limitations
The treatment of chronic sinusitis often entails a combination of medical management and surgical intervention to treat the underlying inflammation of the sinus lining, while addressing the secondary symptoms caused by obstruction of the natural drainage pathways.
Medical Management
The first line of therapy for chronic sinusitis is medical management, which typically includes prescribed antibiotics, anti-inflammatory steroids and decongestants. Despite limited efficacy of use of antibiotics in this patient population and the consequence of increasing bacterial resistance, there is pervasive overuse of these drugs, which could lead to patient resistance and has resulted in sinusitis being identified as a major target in national efforts to reduce unnecessary medical intervention. Sinusitis is the most common reason for adult outpatient antibiotic use in the United States, comprising 11% of all antibiotic prescriptions. In addition, physicians often prescribe decongestants and other drugs to target mucus accumulation.
Steroids are prescribed in two forms, oral steroid pills and nasal steroid sprays, both of which have serious limitations. Oral steroid therapy is effective at reaching the sinus lining, but it does so by means of systemic exposure and therefore carries the risk of serious side effects, including glaucoma, bone loss, weight gain, psychosis and difficulty in controlling blood glucose levels in patients with diabetes. Nasal steroid sprays, commonly indicated for rhinitis, or inflammation of the nasal passage, are routinely prescribed for chronic sinusitis patients. While nasal steroid sprays avoid systemic exposure and thus lack such serious side effects, an estimated 70% of the drug is swallowed and the remainder is directed to the nasal passages, instead of the sinuses, which limits efficacy. In a published study, the fraction of drug deposited in the sinuses from a nasal steroid spray was measured to be less than 2%. Poor patient compliance further limits the effectiveness of nasal steroid sprays. Although medical management can reduce symptoms, an estimated 20% or more of chronic sinusitis patients who receive medical therapy are unresponsive.
Sinus Surgery
In cases where patients are symptomatic despite medical management, a physician may recommend FESS. In the FESS procedure, the physician enlarges the inflamed and obstructed sinus pathways by removing inflamed tissue and bone in order to facilitate normal sinus drainage and aeration. First introduced in the United States in the 1980s, FESS is considered the standard of care for surgical intervention to treat chronic sinusitis. During the procedure, the honeycomb-like cells of the ethmoid sinuses are removed, resulting in one large open cavity. Given their essential role in sinus function, the ethmoid sinuses are opened in over 85% of FESS procedures. The dependent sinuses each drain into the ethmoid sinuses through ostia, which may be enlarged by either surgically removing tissue or via balloon sinuplasty.
FESS is typically performed under general anesthesia in an operating room. During the procedure, a physician inserts an endoscope into the nasal cavity to provide visualization of the patient’s anatomy. Surgical instruments, powered cutting tools and balloon sinuplasty devices are used to remove or dilate obstructive tissue and bone. Following the surgical intervention, physicians often pack the newly opened ethmoid sinuses with gauze or other obstructive sinus packing materials to hold the sinus cavities open. A follow-up office visit is required several days after the procedure to remove the sinus packing materials and an additional one to two follow-up visits typically occur over the first four to six weeks following surgery to monitor for and treat complications. A typical FESS procedure is paid at a Medicare rate of approximately $10,000. Private insurer payment rates average 139 percent of Medicare rates nationally.
Since the introduction of FESS, several new technologies, such as image-guided surgical navigation systems, powered surgical tools and balloon sinuplasty devices have expanded the addressable patient population who can benefit from FESS and allowed for treatment of patients in the physician office setting. For instance, balloon sinuplasty devices were developed to be used in combination with traditional surgical instruments during FESS to treat the dependent sinuses. Balloon sinuplasty is now also used during a procedure in the physician office setting to treat mild chronic sinusitis patients who may not be willing to undergo or are not candidates for sinus surgery under general anesthesia in the operating room setting.
67
Table of Contents
While FESS is the standard of care for treating chronic sinusitis, it has several limitations:
| • | Limited effectiveness. Inflammation and scarring in the postoperative period are common and can compromise the surgical result by negatively impacting the ability of the sinuses to heal. This increases the need for continued medical management and additional surgical procedures. Within the first year after surgery, approximately 64% of patients experience recurrent symptoms. |
| • | Limited ability to address postoperative inflammation. While oral steroids prescribed postoperatively can be effective at addressing inflammation and scarring, the required doses are significant and can result in serious systemic side effects, including glaucoma, bone loss, weight gain, and psychosis. Further, they have restricted use in diabetic individuals, patients with glaucoma and certain psychological disorders. We believe, as a result, only 20% of physicians prescribe them routinely after surgery. The absence of anti-inflammatory steroid therapy leaves the surgical wound susceptible to postoperative complications. |
| • | Pain and discomfort during postoperative period. During surgery, an ENT physician typically places sinus packing materials into the ethmoid sinuses to physically separate tissues in an attempt to prevent scarring and adhesions. Following surgery, physicians see patients two to three times in order to monitor for and, if necessary, to treat complications as well as remove these sinus packing materials. The sinus packing materials are removed by pulling or suctioning them from the newly opened cavity, a painful and time-consuming process, often necessitating pain medication. Moreover, the use of sinus packing materials obstructs the newly opened sinuses, leading to patient symptoms of congestion and discomfort. Despite the use of packing materials, scarring and adhesions are common, necessitating painful removal of additional tissue during postoperative treatments. |
| • | Potential for revision surgery. Within the first year after FESS, approximately 10% of patients will return to the operating room to undergo a revision procedure, while additional patients will return for a revision procedure after one year. We believe the risk of potential revision surgery is a significant deterrent to some patients that would otherwise undergo FESS for chronic sinusitis. |
We believe that because of the limitations of medical management and lack of disease resolution after FESS, many chronic sinusitis patients remain undertreated. We estimate that only a third of patients recommended for sinus surgery proceed with the potentially beneficial procedure, which we believe is due to its limitations and high risk for additional medical management and surgical revision. While balloon sinuplasty has been introduced to open frontal, maxillary and spenoid sinuses, or dependent sinuses, in a less invasive manner, it cannot treat disease in the most commonly involved sinuses, the ethmoids, and does not address the underlying inflammation associated with chronic sinusitis. We believe that an opportunity exists to reach these undertreated patients by providing a more effective option to address their inflammatory disease, while improving the overall outcomes of FESS.
Our Solution
We have designed a drug-eluting implant that consists of bioabsorbable polymers that control drug release and provide structural support to adjacent tissues during the healing process, while the implant is gradually and fully absorbed into the body. We believe the development, manufacturing, and regulatory approval for products incorporating this technology requires capabilities in polymer science, drug delivery, analytical testing and combination products. These competencies allow our technical team to tailor drug formulation, polymer design, drug release duration, implant radial strength and degradation period to meet different clinical needs. This expertise has been utilized in the development of PROPEL and PROPEL mini and is currently being leveraged in the development of our pipeline products. Any new products we develop, or changes that we make in the therapeutic agent used in our PROPEL or PROPEL mini, will require FDA approval prior to commercialization in the United States.
Our PROPEL implants are designed to improve the outcomes of sinus surgery by holding open the sinus passageways, reducing postoperative inflammation and scarring. They are inserted by a physician under endoscopic visualization during sinus surgery in the ethmoids. Once inserted, the self-expanding implants conform to and hold open the surgically enlarged sinus, while gradually releasing an anti-inflammatory steroid,
68
Table of Contents
mometasone furoate, directly to the sinus lining over a period of 30 days. Mometasone furoate, which is available generically and has been approved by the FDA for use in PROPEL and PROPEL mini, is the active ingredient in NASONEX, a nasal spray used to treat allergic rhinitis and other indications. The implants fully absorb into the body over a period of four to six weeks or are removed at the discretion of a physician during a routine office visit. Once absorbed or removed, the implant no longer provides structural support. The graphic below illustrates the operation of PROPEL and PROPEL mini in the sinuses:
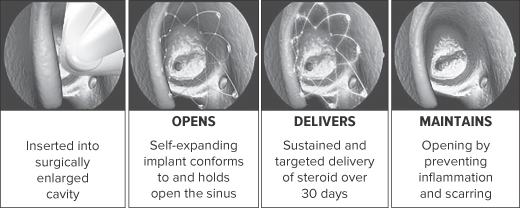
We believe the principal benefits of our steroid-eluting implants are:
| • | Improved surgical outcomes. Our PROPEL implants have been clinically proven to improve FESS results by reducing postoperative inflammation and scarring. In a meta-analysis of prospective, multicenter, randomized, controlled, double-blind clinical studies, our PROPEL implants provided a 46% relative reduction in inflammation and a 70% relative reduction in scarring compared to the control implant. The control implant was identical to the PROPEL implant in composition, size, structure and mode of insertion but lacked the mometasone furoate coating. Our PROPEL implants also demonstrated a significant reduction in postoperative complications, including severe inflammation, such as polyps, and scarring, which are both common reasons for FESS failure. |
| • | Targeted steroid therapy to address postoperative inflammation. Our PROPEL implants are the first and only drug-eluting bioabsorbable sinus implants. They deliver postoperatively an anti-inflammatory steroid directly to the sinus lining in a controlled fashion over a period of 30 days, which helps in the wound healing process. In a meta-analysis of prospective, multicenter, randomized, controlled, double-blind clinical studies, our PROPEL implants reduced the need for oral steroids by 40% compared to the control implant. |
| • | Reduced pain and discomfort during postoperative period. Our PROPEL implants improve postoperative care. Once inserted, the self-expanding implants are designed to conform to and hold open the surgically enlarged ethmoid sinuses until fully absorbed into the body, which improves wound healing without obstructing the sinuses and causing congestion. Our steroid-eluting implants are designed to obviate the need for sinus packing materials, which can be a significant source of postoperative pain and discomfort. Our PROPEL implants significantly reduce scarring and adhesions, which reduces the potential for pain in postoperative treatments. |
| • | Reduced surgical revisions. In clinical studies, our PROPEL implants demonstrated a significant reduction in need for postoperative surgical intervention. In a meta-analysis of prospective, multicenter, randomized, controlled, double-blind clinical studies, our PROPEL implants provided a 35% relative reduction in the need for postoperative oral steroid and surgical intervention compared to the control implant. We believe that patients who have been deterred by the high revision rates associated with FESS may now consider surgical intervention to treat their chronic sinusitis condition. |
We believe these benefits will help expand the size of the FESS market as referring physicians increase their prescription of surgical intervention for chronic sinusitis and more patients elect to undergo sinus surgery to treat their condition.
69
Table of Contents
Our Strategy
Our goal is to be a leading drug-device company committed to advancing clinically proven therapeutic solutions that improve the quality of life for patients with ENT conditions. The key elements of our strategy include:
| • | Expand our sales and marketing organizations to support growth. In the territories in which we focus, our sales team has been able to achieve meaningful adoption and utilization since our first product launch in September 2011. After refining our commercial model, we began to scale our sales and marketing organizations in the first half of 2013 and as of March 31, 2014 are selling in 35 territories. We plan to continue to invest in the expansion of this scalable infrastructure to provide ENT physicians in the United States with access to our steroid-eluting implants. We believe that investment in our sales and marketing organizations will drive continued adoption of our products to establish our steroid-eluting implants as the standard of care. |
| • | Promote awareness amongst ENT physicians, referring physicians and patients. We believe that many patients with chronic sinusitis are currently undertreated, and we intend to educate ENT physicians, allergists, primary care physicians or other referring medical professionals and patients to raise awareness of the disease and available treatment alternatives, including the advantages of using our steroid-eluting implants. We intend to continue promoting this awareness through conducting advertising campaigns, training and educating physicians, publishing additional clinical data demonstrating the benefits of our devices including improved patient outcomes and exhibiting at tradeshows. We have various ongoing marketing initiatives focused on improving referring patterns to our trained physicians. In addition, we plan to launch direct-to-patient marketing programs that we believe will further increase product awareness. |
| • | Advance our platform of innovative products for additional chronic sinusitis patients. We believe improving treatment options for chronic sinusitis patients across the continuum of care holds tremendous market opportunity, and has significant potential to reduce healthcare costs. We are developing additional products using our drug-eluting bioabsorbable implant technology that are specifically designed to treat and manage chronic sinusitis in the physician office setting during a routine visit. This technology is currently under clinical evaluation in the United States. We are also working to expand indications for our PROPEL implants to allow for the treatment of the dependent sinuses. |
| • | Facilitate access to our products via third-party reimbursement. Our currently approved products are commonly treated as general supplies utilized in sinus surgery and if covered by third-party payors, are paid for as part of the FESS procedure. We believe that establishment of reimbursement codes specific to the use of drug-eluting implants for chronic sinusitis is an important factor in expanding access to our products, especially in the physician office setting. Therefore, our strategy includes efforts to engage physician societies and encourage third-party payors to establish coverage, coding and payment that will facilitate access to our drug-eluting implants as we expand our commercialization efforts in the United States. |
| • | Introduce our steroid-eluting implants in markets outside the United States. While our current commercial focus is the U.S. market, we plan to initiate efforts that will allow for future expansion into international geographies. To that end, in July 2014, we received CE mark approval for PROPEL and PROPEL mini which enables market access in Europe. Based on the number of sinus surgeries in Europe and the Asia-Pacific region, we estimate the annual total addressable market for PROPEL and PROPEL mini in these regions to be greater than $1.0 billion. We plan to leverage our FDA PMA approved products and clinical data to access these markets, starting first with the development of clinical, regulatory and reimbursement strategies for countries in Europe and the Asia-Pacific region. |
Our Products
Our Technology Platform
Our drug-eluting bioabsorbable implant technology consists of a polymer-based implant that is coated with a drug and polymer matrix. In fabricating the implant, we use polymers that are bioabsorbable and, over time,
70
Table of Contents
gradually and fully absorb into the body. The polymers chosen are materials with established safety profiles and have been used in medical devices for over 30 years. Individual polymer properties can further enhance biocompatibility in the sinus anatomy. For example, the polyethylene glycol used in the drug coating for PROPEL and PROPEL mini has been proven to be an anti-inflammatory, protein-resistant barrier.
Our innovative design process enables us to develop the mechanical and drug delivery features independently, lending to our customization capability. Our highly specialized bioabsorbable polymer engineering capability, allows us to design the implant with various physical characteristics depending on the size, radial strength and other attributes that are appropriate to enable treatment of the underlying disease in its location. Our implants are designed to be self-expanding, which facilitates insertion when compressed, and expand to conform to the surrounding anatomy after insertion. The ability to control radial strength is important in enabling us to address different diseases at different states. For example, in some instances an implant may be used to maintain an already open passageway. In other situations an implant with significantly greater strength may mechanically dilate a diseased passageway.
Our expertise in drug delivery allows us to effectively pair appropriate polymer delivery matrices with desired therapeutic agents. This allows us to select the therapeutic agent based on its clinical effectiveness and tailor the platform accordingly. In the case of PROPEL, we considered the wide range of off-patent corticosteroids, chose the one best suited for treatment of sinus inflammation, then customized the polymer delivery. Once a drug is selected, our specialized capability in drug formulation allow us to control the speed at which the drug elutes, allowing us to design implants from which the drug is released over a matter of weeks to even longer durations. As a result, we have the flexibility to select the type of drug to be used on the implant and then engineer the implant to control the amount, timing and delivery of the drug.
PROPEL Implant and Delivery System
Our steroid-eluting implants are the first and only FDA-approved drug-eluting implants for chronic sinusitis sufferers. They are indicated for use in patients 18 years or older following ethmoid sinus surgery to maintain patency.
The PROPEL implant is formed from a bioabsorbable polymer fiber, poly-(L-lactide-co-glycolide), or PLG, a material that has been used in medical devices for 30 years. The implant is coated with a drug-eluting coating containing 370 micrograms of mometasone furoate, a corticosteroid with anti-inflammatory properties, in a polymer matrix containing PLG and polyethylene glycol, or PEG. Because of its bioabsorbable nature, the implant will be fully and gradually absorbed into the body over a period of four to six weeks, or it may be removed prior to such time at the discretion of the physician. The implant is designed to accommodate the size and variability of the surgically enlarged ethmoid sinus anatomy and features a nominal expanded implant length of 23 millimeters. PROPEL is delivered using a one-handed ergonomically designed delivery system that provides access to the ethmoid sinuses using endoscopic visualization. The delivery system is comprised of a sheath with a curved distal tip, inside which the implant is housed for deployment. A funnel tool is included in the product kit and is attached to the distal tip of the delivery system to assist in the loading of the implant into the delivery system.
71
Table of Contents
We designed the steroid drug release of PROPEL for a duration of 30 days to match the postoperative healing cycle characterized in published medical literature. We selected mometasone furoate as the anti-inflammatory agent among numerous evaluated compounds based on three important characteristics: absorbability, binding affinity and low systemic bioavailability. The compound preferentially absorbs into the sinus lining instead of the surrounding mucous fluid. The drug has the highest glucocorticoid receptor binding affinity, making it highly potent in preventing inflammation once within tissue. Glucocorticoid receptors are the molecules in the surface membranes of cells throughout the body to which corticosteroids chemically bind. Additionally, the compound has low systemic bioavailability, meaning that it has negligible systemic safety side effects.

PROPEL and PROPEL mini steroid-eluting implants |
PROPEL and PROPEL mini implant delivery systems |
PROPEL Mini Implant and Delivery System
The PROPEL mini implant, is a smaller version of PROPEL and is manufactured from the same bioabsorbable polymer fiber and with the same drug-eluting coating and other design characteristics. The nominal expanded implant length of the PROPEL mini is 16 millimeters. The profile of the delivery system is smaller, facilitating treatment of smaller anatomy. The distal tip is curved to allow for treatment of additional sinuses as future indications are obtained. A crimper, loading tool and funnel are provided in the product kit to assist in the crimping and loading of the implant into the delivery system.
Product Pipeline and Research and Development
We continue to expand our product pipeline by applying our proprietary drug-eluting bioabsorbable implant technology to additional chronic sinusitis-targeted applications, which we believe will expand the patient population that can benefit from our steroid-eluting technology.
Steroid-Eluting Implant for Refractory Disease in Office
Our steroid-eluting implants are clinically proven to improve surgical results for patients undergoing FESS, reducing the need for postoperative surgical and steroid intervention. However, given the underlying chronic inflammation associated with this condition, recurrent obstruction of the sinus cavity can occur over time following sinus surgery, especially in patients afflicted with polyps, a sign of severe inflammation. Improving care of such chronic patients holds meaningful opportunity to significantly reduce healthcare costs by preventing revision surgery. We have developed an alternative treatment option for patients who are candidates for revision surgery that can be placed in the physician office setting during a routine visit. This implant is based on the same drug-eluting bioabsorbable implant technology as PROPEL, but is designed to have greater radial strength in order to dilate an obstructed, polyp-filled sinus cavity, and deliver drug for an extended period of time. This implant is currently under clinical evaluation in the United States and, unlike PROPEL, which is regulated as a medical device, the implant is subject to regulation as a drug product and will require the submission to the FDA of a New Drug Application, or NDA. We intend to pursue the NDA for this implant under Section 505(b)(2) of the FDCA. Section 505(b)(2) would enable us to rely, in part, on the FDA’s findings of safety and efficacy for an existing product, or published literature, in support of our NDA, which could expedite the development program of this implant by potentially decreasing the amount of clinical data that we would need to generate in order to obtain FDA approval.
72
Table of Contents
Steroid-Eluting Implant for Primary Disease Management in Office
We are also developing another steroid-eluting implant designed to fit the openings of the dependent sinuses following ostial dilation using balloon sinuplasty. This implant will allow treatment of patients, who have not yet had surgery, in the physician office setting. We believe this product will further expand the addressable patient population to those that are either indicated for sinus surgery and choose not to proceed or those with less severe disease that would otherwise be treated with medical management. A single center, non-randomized clinical study to assess the feasibility of this implant was recently initiated in the United States.
Other Products
Beyond chronic sinusitis, we plan to apply our drug-eluting bioabsorbable implant technology to a variety of other ENT conditions, such as conditions affecting the ear and throat. We believe there are many opportunities to replace medical management and improve current surgical interventions with targeted drug-eluting implants. We are in various stages of development for these potential new products.
In addition to building our product offering through internal product development efforts, we may selectively license or acquire complementary products and technologies, leveraging our experience at bringing new products to market and our commercial channel.
Research and Development
As of March 31, 2014, we had 26 employees in our research and development department, including eight employees responsible for clinical development, six for quality assurance and three for regulatory affairs. The primary focus of this group is to develop new steroid-eluting implants that leverage our drug-eluting bioabsorbable implant technology. Research and development expenses for 2012 and 2013 were $9.3 million and $9.5 million, respectively.
Clinical Results and Studies
PROPEL
The safety and efficacy of PROPEL has been studied in three prospective, multicenter clinical trials conducted in the United States enrolling a total of 205 patients. The principal safety and efficacy information is derived from the ADVANCE II randomized clinical trial and is supported by the ADVANCE clinical trial and an initial pilot study. A meta-analysis that pooled data from the ADVANCE II study and the initial pilot study provides further evidence of efficacy. In all three studies, implants were placed following ethmoid sinus surgery, or ethmoidectomy, which entails removal of the honeycomb-like partitions between the ethmoid sinuses in order to create larger sinus cavities. Based on the number of units shipped between the initial FDA approval in August 2011, and March 31, 2014, we estimate that physicians have treated over 25,000 patients with our steroid-eluting implants.
All three studies were designed to measure PROPEL’s ability to improve the outcomes of sinus surgery. This included measuring the need for postoperative interventions such as adhesion lysis, which is a procedure to separate scar tissue and adhesions within the sinus cavity, and the need to prescribe oral steroids to treat inflammation. The studies also measured the impact of PROPEL on other postoperative complications, such as occurrence of polyposis and middle turbinate lateralization. Prevention of these complications contributes to the long-term success of sinus surgery. Polyposis represents a higher level inflammatory disease. The middle turbinate is a bony structure in the middle of the sinus, responsible for filtration, heating, and humidification of nasal air flow. Middle turbinate lateralization is an undesired complication where this structure curves towards the outer, or lateral, wall of the nose resulting in blockage of the ethmoid sinus passage.
The ADVANCE II Study
We sponsored the ADVANCE II study, a prospective, multicenter, randomized, controlled, double-blind, pivotal study that enrolled 105 adult patients undergoing FESS with bilateral ethmoidectomy for treatment of chronic sinusitis refractory to medical management at 11 study centers in the United States between December 2009 and July 2010. The study utilized an intra-patient control design to minimize variability and allow for blinded assessment of the safety and efficacy of the drug-eluting implant compared to a non-drug-eluting control version of the implant. Patients received a drug-eluting implant on one randomized side and an identical non-drug-eluting
73
Table of Contents
control version of the implant on the contralateral side. During the initial 30-day follow-up, no oral or topical steroid sprays were permitted. The primary efficacy endpoint was the reduction in need for postoperative interventions at day 30, determined by grading of video-endoscopies from day 30 by a panel of three independent blinded sinus surgeons. Postoperative interventions were a composite endpoint defined as either surgical intervention required to separate an adhesion and/or oral steroid intervention to resolve recurrent ethmoid sinus inflammation. Additional efficacy endpoints were determined by endoscopic grading done by clinical investigators at the study centers.
Ocular safety was characterized by measurement of intraocular pressure, or IOP, at baseline through day 90 and lens examination for cataract formation or progression performed at baseline and day 90. The primary safety endpoint was defined as the absence of clinically significant IOP elevation through day 90.
Implants were successfully deployed in all 210 sinuses. Compared to the control implant, the drug-eluting implant provided a 29% relative reduction in postoperative interventions, a 52% relative reduction in adhesion lysis, and a 29% relative reduction in oral steroid intervention. The relative reduction in frank polyposis was 45%. The primary ocular safety endpoints were met as there were no clinically significant IOP increases and no clinically significant changes from baseline in lens opacities.
| Principal Efficacy Results at Day 30 as graded by independent, blinded panel of physicians |
||||||||||||||||||||
| Outcome Measure |
Evaluable Patients* |
Treatment Sides (n=105) No. (%) |
Control Sides (n=105) No. (%) |
Relative Reduction (%) |
P Value | |||||||||||||||
| Postoperative intervention |
96 | 32 (33.3) | 45 (46.9) | -29 | 0.0280 | |||||||||||||||
| Adhesion lysis |
100 | 14 (14.0) | 29 (29.0) | -52 | 0.0053 | |||||||||||||||
| Oral steroid intervention |
86 | 20 (23.3) | 28 (32.6) | -29 | 0.0881 | |||||||||||||||
| Frank polyposis |
85 | 16 (18.8) | 29 (34.1) | -45 | 0.0023 | |||||||||||||||
| * | Evaluable subjects were those with gradable sinuses on both sides |
Meta-Analysis
Two prospective, multicenter, randomized, controlled, double-blind studies enrolled a total of 143 patients utilizing an intra-patient control design. The results of these two trials, the ADVANCE II study and the initial Pilot study, were then pooled for a meta-analysis, which represents the first and only Level 1a evidence for any devices used in sinus surgery today. Level 1a evidence is the highest level of evidence according to the criteria of the Centre for Evidence-Based Medicine at the University of Oxford. For evidence Level 1a, a meta-analysis of multiple randomized controlled trials is required.
Compared to the control implant, the drug-eluting implant provided a 35% relative reduction in postoperative interventions, a 51% relative reduction in adhesion lysis and a 40% relative reduction in oral steroid intervention. The relative reduction in frank polyposis was 46%. Additional efficacy endpoints of significant, or severe, adhesions and middle turbinate lateralization, determined by clinical investigators at the study centers, were reduced by 70% (p=0.0013) and 75% (p=0.0225), respectively.
| Principal Efficacy Results at Day 30 as graded by independent, blinded panel of physicians |
||||||||||||||||||||
| Outcome Measure |
Evaluable Patients* |
Treatment Sides (n=143) No. (%) |
Control Sides (n=143) No. (%) |
Relative Reduction (%) |
P Value | |||||||||||||||
| Postoperative intervention |
128 | 42 (32.8) | 65 (50.8) | -35 | 0.0008 | |||||||||||||||
| Adhesion lysis |
134 | 19 (14.2) | 39 (29.1) | -51 | 0.0016 | |||||||||||||||
| Oral steroid intervention |
113 | 25 (22.1) | 42 (37.2) | -40 | 0.0023 | |||||||||||||||
| Frank polyposis |
111 | 22 (19.8) | 41 (36.9) | -46 | <0.0001 | |||||||||||||||
| * | Evaluable subjects were those with gradable sinuses on both sides |
The ADVANCE Study
We sponsored the ADVANCE study, a prospective, multicenter, single-cohort, open-label trial that enrolled 50 adult patients undergoing primary or revision FESS with either unilateral or bilateral ethmoidectomy for treatment of chronic sinusitis refractory to medical management at seven study centers in the United States between March 2009 and December 2009. At the end of the FESS procedure, drug-eluting implants were placed
74
Table of Contents
in the patient’s ethmoid sinus cavities. During the initial 30-day follow-up, no oral or topical steroid sprays were permitted. Follow-up assessments included endoscopic examination and scoring through 60 days, with patient symptom scoring done through six months. Patient symptom scoring was assessed using validated disease-specific symptom-scoring instruments, namely the Sinonasal Outcomes Test-22, or SNOT-22, a questionnaire that includes 22 symptoms related to sinusitis, and the Rhinosinusitis Disability Index, or RSDI, which is a validated measure of health-related quality of life in rhinitis or rhinosinusitis that contains 30 items that measure symptoms such as facial pain, congestion and sense of smell.
Local sinus safety was assessed by monitoring for device-related adverse events. Ocular safety was characterized by measurement of IOP at baseline through day 30 and lens examination for cataract formation or progression, performed at baseline and day 30.
Implants were successfully placed in all 90 sinuses. Mean inflammation scores were minimal at all time points. At one month, the prevalence of polypoid edema was nine of 90 sinuses (10.0%), frank polyposis occurred in two sinuses (2.2%), significant adhesion occurred in one sinus (1.1%), and middle turbinate lateralization occurred in four sinuses (4.4%). There were no clinically significant changes from baseline in lens opacities or IOP. The mean changes from baseline to day 60 and six months in total RSDI scores were -36.2 and -29.7, respectively, reflecting statistically significant improvement. The mean changes from baseline to day 60 and six months for the SNOT-22 scores were -1.9 and -1.7, respectively, reflecting statistically significant improvement.
PROPEL Pilot Study
We sponsored an initial pilot study, a prospective, multicenter, randomized, controlled, double-blind feasibility study that enrolled 50 adult patients undergoing FESS with bilateral ethmoidectomy for treatment of chronic sinusitis refractory to medical management at four study centers in the United States between March 2008 and February 2009. A total of 43 patients received the PROPEL and control implants. The other seven patients were the first patients enrolled in the study and received an earlier implant version that was subsequently discontinued. The study utilized an intra-patient control design to minimize variability and allow for blinded assessment of the efficacy of the drug-eluting implant compared to an identical non-drug-eluting control version of the implant. Thirty-eight patients received the drug-eluting implant on one randomized side and an identical non-drug-eluting control implant on the contralateral side. Five patients received bilateral drug-eluting implants to assess systemic safety. During the initial 30-day follow-up, no oral or topical steroid sprays were permitted.
Implants were successfully deployed in all 86 sinuses. Compared to the control implant, the drug-eluting implant provided a statistically significant reduction in ethmoid sinus inflammation from day 21 to 45. The drug-eluting implant reduced the frequency of middle turbinate lateralization, significant adhesion lysis, and frank polyposis through day 30, compared to the control implant. There was no evidence of systemic drug exposure. The steroid was not quantifiable systemically at any time point and there was no evidence of adrenal cortical suppression.
| Principal Efficacy Results at Day 30 as graded by independent, blinded panel of physicians |
||||||||||||||||||||
| Outcome Measure |
Evaluable Patients* |
Treatment Sides (n=38) No. (%) |
Control Sides (n=38) No. (%) |
Relative Reduction (%) |
P Value | |||||||||||||||
| Postoperative intervention |
31 | 10 (32.3) | 20 (64.5) | -50 | 0.0063 | |||||||||||||||
| Adhesion lysis |
34 | 5 (14.7) | 10 (29.4) | -50 | ns | |||||||||||||||
| Oral steroid intervention |
27 | 5 (18.5) | 14 (51.9) | -64 | 0.0039 | |||||||||||||||
| Frank polyposis |
26 | 6 (23.1) | 12 (46.2) | -50 | 0.0312 | |||||||||||||||
| * | Evaluable subjects were those with gradable sinuses on both sides. |
Future Products
Steroid-Eluting Implant for Refractory Disease in Office
We are developing a steroid-eluting implant for refractory disease for the treatment of patients in the physician office setting. This product candidate has a primary mode of action of drug, and for this reason will require NDA approval from the FDA rather than PMA approval. We are sponsoring four studies of this product candidate, a pilot study, a pharmacokinetic study, RESOLVE and RESOLVE II in order to support an NDA application with the FDA.
75
Table of Contents
Pilot Study. We sponsored an initial pilot study which was a prospective, multicenter feasibility study that enrolled 12 adult patients who had prior FESS, but experienced recurrent polyposis refractory to medical management at four study sites in the United States in November 2011. Implants were delivered under topical or anesthesia in a physician’s office. Reductions in mean bilateral polyp grade as well as patient reported symptoms were demonstrated over 30 days and were sustained through six months. At six months follow-up, 64% of patients were no longer revision FESS candidates. No serious adverse events occurred.
This study provided initial clinical evidence of the feasibility, safety, and efficacy of in-office drug-eluting implant placement in chronic sinusitis patients with recurrent polyposis after FESS. Based on these study results, we planned the RESOLVE study program.
Pharmacokinetic Study. A single-center pharmacokinetic study was conducted in 2013. The protocol required five patients to undergo serial blood sampling for one month following bilateral implant placement. The study demonstrated that there is negligible systemic exposure from the drug.
The RESOLVE Study Program. RESOLVE and RESOLVE II are randomized, controlled clinical trials. RESOLVE is a 100-patient, prospective, multicenter, randomized, controlled, double-blind, clinical study that follows patients through six months. As we had previously agreed with the FDA, the intent of RESOLVE was to direct the final study design and sample size required for the subsequent, larger RESOLVE II, Phase 3 study. Enrollment for the RESOLVE study was completed in November 2013 with primary outcome measurements at three months. The primary safety endpoints were met and no serious adverse events occurred. The study included endpoints designed to assess improvements in both patient-reported symptoms (sensation of nasal obstruction/congestion) and objective endoscopic outcomes determined by clinicians (sino-nasal polyp burden and the overall volume of sinus obstruction). The improvement from baseline in nasal obstruction/congestion score (co-primary endpoint) in the treatment group was clinically meaningful and was twice as large as that of the control group, although this finding did not achieve statistical significance due in part to higher than anticipated variability in the measure. In patients with greater polyp burden at baseline, representing approximately 75% of the study population, the improvement in nasal obstruction/congestion achieved statistical significance. Endoscopically, statistically significant reductions in nasal polyp burden and the overall volume of sinus obstruction were observed. We expect all analyses for the RESOLVE study, including the endoscopic grading by an independent panel (co-primary endpoint) and the 6-month data, to be available in September 2014. We believe RESOLVE provides important information with regard to sample size and other design elements and we plan to utilize the RESOLVE study results in determining the design of the Phase 3, RESOLVE II study.
Steroid-Eluting Implant for Primary Disease Management in Office
We are also developing another steroid-eluting implant designed to fit the openings of the dependent sinuses following ostial dilation using balloon sinuplasty. This implant will allow treatment of patients, who have not yet had surgery, in the physician office setting. A single center, non-randomized clinical study to assess the feasibility of this implant was recently initiated in the United States.
Sales and Marketing and Physician Training
Sales and Marketing
We market and sell our steroid-eluting implants through our direct sales and marketing organizations in the United States. As of March 31, 2014, our sales, marketing and reimbursement organizations consisted of 62 employees including 52 employees in sales, nine in marketing and customer service and one in reimbursement.
Of the approximately 10,000 ENT physicians in the United States, our target customer market consists of the approximately 7,500 ENT physicians who perform sinus surgery. We believe this makes for a well-defined physician customer base that is accessible by a direct sales organization. We are focused on developing strong relationships with our customers and educating physicians on the proper use and applications of our products and in identifying the appropriate patient candidates who could benefit from our products. While we sell directly to hospitals, the ENT physician typically drives the purchasing decision. In addition, we sell products to ambulatory surgery centers. We plan to increase the size of our commercial organization to expand the current customer account base and increase utilization of our steroid-eluting implants.
76
Table of Contents
Through education, training and research programs, we are supporting physicians to provide the best care for sinus surgery patients. Our physician education program, clinical studies and medical conferences are key means for generating product awareness, adoption and utilization.
Physician referrals and peer-to-peer education and training are important components of our strategy. We have marketing programs targeted at appropriately educating medical professionals who in many instances represent the primary point of contact with the patient. We plan to implement marketing initiatives targeted at increasing patient awareness of our products through direct-to-patient marketing programs. These efforts are currently focused on informing patients of local physicians trained in the use of our steroid-eluting implants through a physician locator application on our website.
Physician Training
We devote resources to training and educating ENT physicians and their support teams on the proper use of our steroid-eluting implants. We strongly believe proper physician preparation and training optimize the proper use of our products and improve surgical outcomes. We believe one of the most effective ways to introduce and build market demand for our products is by training ENT physicians.
We have designed our products to be ergonomically intuitive to an ENT physician so that their function and use is based on common sinus surgery techniques. Our training process consists of a presentation by one of our sales representatives at the physician’s office to demonstrate the use of our products, without requiring physician attendance at a formal training course. Although we are not required to do so, we may have one of our field personnel present during the initial cases performed by a newly-trained ENT physician. Our efficient in-servicing process makes for a cost-effective method of training new physicians and staff.
As of March 31, 2014, we had trained over 1,000 ENT physicians in the proper use of our products. Physician users of our steroid-eluting implants frequently assist in our training efforts as we believe these physicians are instrumental in demonstrating the benefits of our products through peer-to-peer education. These educational events, conducted across the country, typically consist of instructional presentations, clinical study reviews and filmed case studies.
Competition
Our industry is highly competitive, subject to change and significantly affected by new product introductions and other activities of industry participants. Many of the companies developing or marketing ENT products are publicly traded or are divisions of publicly-traded companies, and these companies enjoy several competitive advantages, including:
| • | greater financial and human capital resources; |
| • | significantly greater name recognition; |
| • | established relationships with ENT physicians, referring physicians, customers and third-party payors; |
| • | additional lines of products, and the ability to offer rebates or bundle products to offer greater discounts or incentives to gain a competitive advantage; and |
| • | established sales, marketing and worldwide distribution networks. |
Because of the size of the market opportunity for the treatment of chronic sinusitis, potential competitors have historically dedicated and will continue to dedicate significant resources to aggressively promote their products or develop new products. New product developments that could compete with us more effectively are possible because of the prevalence of chronic sinusitis and the extensive research efforts and technological progress that exist within the market. Large medical device companies with ENT divisions, such as Medtronic, also have capability in drug-eluting stents. Though we are not aware of any such products to date, these or other companies may develop drug-eluting products that could compete with our products.
Our commercially available products are designed to be used following sinus surgery. If another company successfully develops a surgical approach to the treatment of sinusitis that would not benefit from the use of our
77
Table of Contents
steroid-eluting implants, if another company develops a device to treat the inflammation and scarring associated with sinus surgery that is more efficacious than our steroid-eluting implants or if a pharmaceutical company successfully develops a drug that addresses chronic sinusitis without the need for surgical intervention, sales of our products would be significantly and adversely affected.
The alternative to delivering steroids to the sinuses post-operatively with PROPEL is the prescription of oral steroids. While oral steroids prescribed postoperatively can be effective at addressing inflammation and scarring, the required doses are significant and can result in serious systemic side effects, including glaucoma, bone loss, weight gain, and psychosis. Further, oral steroids have restricted use in diabetic individuals, patients with glaucoma and some psychological disorders. We believe, as a result, only 20% of physicians prescribe them routinely after surgery. Additionally, there are commercially available packing materials on the market that provide a spacing function and are less expensive than PROPEL. During surgery, approximately 60% of ENT physicians place sinus packing materials, either absorbable or non-absorbable, into the ethmoid sinuses to physically separate tissues in an attempt to prevent scarring and adhesions. Sinus packing materials are not placed in the frontal sinuses. Following surgery, the sinus packing materials are removed by pulling or suctioning them from the newly opened cavity, a painful and time-consuming process, often necessitating pain medication. Despite the use of packing materials, scarring and adhesions are common, necessitating painful removal of additional tissue during postoperative treatments. Some physicians choose to soak these packing materials with steroid in liquid form in an effort to deliver steroid to the sinus. This practice is off-label and is not supported by clinical data. However, although we believe PROPEL and PROPEL mini have significant advantages over sinus packing materials and other treatment options, they are expensive relative to packing materials and may not be reimbursed by third-party payors. As a result, ENT physicians may choose to use oral steroid delivery or packing materials or a combination of the two, which are less expensive, in lieu of PROPEL or PROPEL mini.
We believe that our continued ability to compete favorably depends on:
| • | successfully expanding our commercial operations; |
| • | continuing to innovate and maintain scientifically-advanced technology; |
| • | developing technologies for applications in the sinuses and other areas of ENT; |
| • | attracting and retaining skilled personnel; |
| • | obtaining patents or other intellectual property protection for our products; and |
| • | conducting clinical studies and obtaining and maintaining regulatory approvals. |
Intellectual Property
As of March 31, 2014, we owned 41 issued patents globally, of which 24 were issued U.S. patents. As of March 31, 2014, we owned 41 patent applications pending globally, of which 13 were patent applications pending in the United States. Subject to payment of required maintenance fees, annuities and other charges, our issued U.S. patents have expiration dates between 2017 and 2030, with one of our issued U.S. patents expiring before 2020, 13 expiring between 2021 and 2025, and the remaining 10 expiring after 2025.
As of March 31, 2014, our trademark portfolio contained 20 trademark registrations, four of which were U.S. trademark registrations, as well as three pending foreign trademark applications.
We also rely upon trade secrets, know-how, continuing technological innovation, and may rely upon licensing opportunities in the future, to develop and maintain our competitive position. We protect our proprietary rights through a variety of methods, including confidentiality agreements and proprietary information agreements with suppliers, employees, consultants and others who may have access to proprietary information.
Manufacturing and Supply
We manufacture our steroid-eluting implants at our facility in Menlo Park, California with components supplied by external suppliers. We perform inspections of these components before use in our manufacturing operations. Using these components, we assemble, inspect, test and package our implants, and send them to a
78
Table of Contents
third-party sterilization vendor. After sterilization, we perform inspections of the finished implants internally and via third party laboratories to determine compliance with our specifications, release the lot to inventory and then ship the finished product to customers.
The active pharmaceutical ingredient, or API, and a number of our critical components used in our implants are supplied to us from single source suppliers. We rely on single source suppliers for some of our polymer materials, some extrusions and molded components, some off-the-shelf components and for finished goods testing. Our ability to commercially supply our products and to develop our product candidates depends, in part, on our ability to obtain successfully the API and polymer materials used in these products in accordance with regulatory requirements and in sufficient quantities for commercialization and clinical testing. We have entered into manufacturing, supply or quality agreements with a number of our single source suppliers pursuant to which they supply the API and components we need. We generally acquire our single source components pursuant to purchase orders placed in the ordinary course of business. However, we are not certain that our single source suppliers will be able to meet our demand for their products, whether because of the nature of our agreements with those suppliers, our limited experience with those suppliers or our relative importance as a customer to those suppliers. It may be difficult for us to assess their ability to timely meet our demand in the future based on past performance. To date, we have not experienced any significant supply constraints or delays in procuring components and materials and while our suppliers have generally met our demand for their products on a timely basis in the past, they may subordinate our needs in the future to the needs of their other customers.
We are currently improving our manufacturing capabilities and increasing capacity as we increase the extent of our commercialization efforts. We believe our manufacturing operations are in compliance with regulations mandated by the FDA. We are an FDA-registered medical device manufacturer and we were granted PMA approval for PROPEL in August 2011. Our manufacturing facilities and processes are subject to periodic inspections and audits by various federal, state and foreign regulatory agencies. For example, our facilities were inspected by the FDA in April and May 2011, December 2012 and March 2013. The State of California regulatory authority audited our manufacturing facility in connection with granting a California Device Manufacturing License to us in August 2009. Our facility was last inspected in October 2013 by the European Notified Body in Ireland, National Standards Authority of Ireland, and no major nonconformance reports were issued as a result of that inspection.
We have manufacturing, supply or quality agreements with a number of our single source suppliers:
| • | In April 2014, we entered into an agreement with Hovione Inter Ltd., or Hovione, pursuant to which we are required to purchase 80% of our API from Hovione, in quantities to be specified in 12-month forecasts provided by us and updated on a quarterly basis. This agreement extends until April 2019. Either we or Hovione may terminate the agreement prior to that date for uncured material breach by or insolvency of the other party. We may also terminate the agreement in the event Hovione loses any required FDA approval rendering it unable to fulfill its contractual obligations, or if Hovione is engaged in felonious or fraudulent activities. Either we or Hovione may terminate the Agreement in the event regulatory agencies require changes to the product specifications that materially affect Hovione’s cost of production, and we are unable to reach an agreement with Hovione regarding an equitable pricing adjustment. |
| • | In January 2014, we entered into an agreement with AIM Plastics, Inc., or AIM, pursuant to which AIM provides us with injection molded components in quantities to be specified in rolling quarterly forecasts provided by us. The agreement extends for an initial period of one year from its effective date, with automatic one-year renewal periods applying thereafter unless we or AIM provide written notice of non-renewal at least 30 days prior to the end of the then-current term. Either we or AIM may terminate the agreement for an uncured material breach by the other party. We may also terminate the agreement upon 12 months written notice in the event of a change in control of AIM. |
| • | In November 2013, we entered into an agreement with Stephen Gould Corporation, or SGC, pursuant to which SGC supplies us with packaging components in accordance with a rolling quarterly forecast provided by us. The agreement extends for an initial period of two years from the effective date, with automatic one-year renewal periods applying thereafter unless we or SGC provide written notice of |
79
Table of Contents
| intent not to renew at least 30 days prior to the end of the then-current term. Either we or SGC may terminate the agreement for uncured material breach by the other party. We may also terminate the agreement at will upon 90 days’ prior written notice to SGC, or upon 12 months’ prior written notice in the event of a change in control of SGC. |
| • | In December 2013, we entered into an agreement with Exova Group Limited, pursuant to which Exova supplies us with analytical testing services for our finished product. The agreement extends until December 2015. |
| • | In April 2014, we entered into an agreement with Polymer Solutions Incorporated, or PSI, pursuant to which PSI supplies us with analytical testing services for our API and polymer materials. The agreement has an indefinite term, but may be terminated by us at any time upon 30 days’ notice to PSI or immediately upon written notice in the event of an uncured material breach by PSI. |
Each of these suppliers manufactures the components they produce for us or tests our components and devices to our specifications. We expect to be able to negotiate new agreements with these vendors in advance of the expiration of the current agreements. We intend to maintain sufficient supplies of the API and components from these single source suppliers in the event that our agreements with one or more of these suppliers were to terminate to enable us to continue to manufacture our implants for a sufficient amount of time necessary to obtain another source of API or components.
Government Regulation
United States Regulation of Medical Devices and Drugs
Our products and product candidates are drug-eluting bioabsorbable implants that are regulated as combination products by the FDA. FDA’s Office of Combination Products designates a primary mode of action for such drug-device combination products, with the respective primary Center within FDA leading the regulatory review for the product, in consultation with the secondary designated Center. FDA determined that the primary mode of action for PROPEL and PROPEL mini was that of a medical device, so these products have been approved and are regulated in the United States as medical devices. Our product candidate for the physician office setting, however, was designated as having a drug primary mode of action, and thus our RESOLVE trials are and will be conducted under an Investigational New Drug, or IND, application, and if successful, this product candidate will need to be approved under the NDA pathway.
The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant or sponsor to a variety of administrative or judicial sanctions, including refusal by FDA to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters and other types of letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, or civil or criminal investigations and penalties brought by FDA and the Department of Justice, or DOJ, or other governmental entities.
Medical Devices
Our PROPEL implants are regulated in the United States as Class III medical devices by the FDA under the Federal Food, Drug and Cosmetic Act, or FDCA. The FDA classifies medical devices into one of three classes based upon controls the FDA considers necessary to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls such as labeling, adherence to good manufacturing practices and maintenance of product complaint records, but are usually exempt from premarket notification requirements. Class II devices are subject to the same general controls and also are subject to special controls such as performance standards, and FDA guidelines, and may also require clinical testing prior to approval. Class III devices are subject to the highest level of controls and require rigorous clinical testing prior to their approval and generally require a PMA, or a PMA supplement approval prior to their sale.
80
Table of Contents
Manufacturers must file an Investigational Device Exemption, or IDE, application if human clinical studies of a device are required and if the investigational use of the device represents a potential for significant risk to the patient. The IDE application must be supported by data, typically including the results of animal and engineering testing of the device. If the IDE application is approved by the FDA, human clinical studies may begin at a specific number of investigational sites with a maximum number of patients, as approved by the FDA. The clinical studies must be conducted under the review of an independent institutional review board to ensure the protection of the patients’ rights.
Generally, upon completion of these human clinical studies, a manufacturer seeks approval of a Class III medical device from the FDA by submitting a PMA application. A PMA application must be supported by extensive data, including the results of the clinical studies, as well as testing and literature to establish the safety and effectiveness of the device. PMA approval may be conditioned upon the conduct of certain post-approval studies, such as long term follow-up studies.
FDA regulations require us to register as a medical device manufacturer with the FDA. Additionally, the California Department of Health Services, or CDHS, requires us to register as a medical device manufacturer within the state. Because of this, the FDA and the CDHS inspect us on a routine basis for compliance with the current good manufacturing practice. These regulations require that we manufacture our products and maintain related documentation in a prescribed manner with respect to manufacturing, testing and control activities, and product release for distribution. We have undergone and expect to continue to undergo regular current good manufacturing practice inspections in connection with the manufacture of our products at our facility. Further, the FDA requires us to comply with various FDA regulations regarding labeling. Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or state authorities, which may include any of the following sanctions:
| • | warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | customer notifications, repair, replacement, refunds, recall or seizure of our products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | delay in processing marketing applications for new products or modifications to existing products; |
| • | mandatory product recalls; |
| • | withdrawing approvals that have already been granted; and |
| • | criminal prosecution. |
The Medical Device Reporting laws and regulations require us to provide information to the FDA on deaths or serious injuries alleged to have been associated with the use of our devices, as well as product malfunctions that likely would cause or contribute to death or serious injury if the malfunction were to recur. In addition, the FDA prohibits an approved device from being marketed for “off-label” use. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability, including substantial monetary penalties and criminal prosecution.
Drugs
The clinical testing, manufacturing, labeling, storage, distribution, record keeping, advertising, promotion, import, export and marketing, among other things, of our product candidates that are subject to FDA’s drug authority are governed by extensive regulation by governmental authorities in the United States and other countries. The FDA, under the FDCA, regulates pharmaceutical products in the United States. The steps required before a drug may be approved for marketing in the United States generally include:
| • | preclinical laboratory tests and animal tests conducted under Good Laboratory Practices, or GLP; |
| • | the submission to the FDA of an IND application for human clinical testing, which must become effective before human clinical trials commence; |
81
Table of Contents
| • | adequate and well-controlled human clinical trials to establish the safety and efficacy of the product and conducted in accordance with Good Clinical Practices, or GCP; |
| • | the submission to the FDA of an NDA; |
| • | FDA acceptance, review and approval of the NDA; and |
| • | satisfactory completion of an FDA inspection of the manufacturing facilities at which the product is made to assess compliance with current Good Manufacturing Practices, or cGMPs. |
The testing and approval process requires substantial time, effort and financial resources, and the receipt and timing of any approval is uncertain. The FDA may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
Preclinical studies include laboratory evaluations of the product candidate, as well as animal studies to assess the potential safety and efficacy of the product candidate. The results of the preclinical studies, together with manufacturing information and analytical data, are submitted to the FDA as part of the IND, which must become effective before clinical trials may be commenced. The IND will become effective automatically 30 days after receipt by the FDA, unless the FDA raises concerns or questions about the conduct of the trials as outlined in the IND prior to that time. In this case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can proceed.
Each clinical trial must be reviewed and approved by an independent institutional review board, or IRB. The IRB will consider, among other things, ethical factors, the safety of human subjects and the possible liability of the institution.
Clinical trials are typically conducted in three sequential phases prior to approval, but the phases may overlap. These phases generally include the following:
Phase 1. Phase 1 clinical trials represent the initial introduction of a product candidate into human subjects, frequently healthy volunteers. In Phase 1, the product candidate is usually tested for safety, including adverse effects, dosage tolerance, absorption, distribution, metabolism, excretion and pharmacodynamics.
Phase 2. Phase 2 clinical trials usually involve studies in a limited patient population to (1) evaluate the efficacy of the product candidate for specific indications, (2) determine dosage tolerance and optimal dosage and (3) identify possible adverse effects and safety risks.
Phase 3. If a product candidate is found to be potentially effective and to have an acceptable safety profile in Phase 2 studies, the clinical trial program will be expanded to Phase 3 clinical trials to further demonstrate clinical efficacy, optimal dosage and safety within an expanded patient population at geographically dispersed clinical study sites.
Phase 4 clinical trials are conducted after approval to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of drugs approved under accelerated approval regulations, or when otherwise requested by the FDA in the form of post-market requirements or commitments. Failure to promptly conduct any required Phase 4 clinical trials could result in withdrawal of approval.
The results of preclinical studies and clinical trials, together with detailed information on the manufacture, composition and quality of the product, are submitted to the FDA in the form of an NDA, requesting approval to market the product. The application must be accompanied by a significant user fee payment. The FDA has substantial discretion in the approval process and may refuse to accept any application or decide that the data is insufficient for approval and require additional preclinical, clinical or other studies.
Review of Application. Once the NDA has been accepted for filing, which occurs, if at all, 60 days after submission, the FDA sets a Prescription Drug User Fee Act date that informs the applicant of the specific date by which the FDA intends to complete its review. This is typically 10 months from the date of submission. The review process is often extended by FDA requests for additional information or clarification. The FDA reviews NDAs to determine, among other things, whether the proposed product is safe and effective for its intended use,
82
Table of Contents
and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. Before approving an NDA, the FDA may inspect the facilities at which the product is manufactured and will not approve the product unless the manufacturing facility complies with cGMPs and may also inspect clinical trial sites for integrity of data supporting safety and efficacy. During the approval process, the FDA also will determine whether a risk evaluation and mitigation strategy, or REMS, is necessary to assure the safe use of the product. If the FDA concludes a REMS is needed, the sponsor of the application must submit a proposed REMS; the FDA will not approve the application without an approved REMS, if required. A REMS can substantially increase the costs of obtaining approval. The FDA may also convene an advisory committee of external experts to provide input on certain review issues relating to risk, benefit and interpretation of clinical trial data. The FDA may delay approval of an NDA if applicable regulatory criteria are not satisfied or the FDA requires additional testing or information. The FDA may require post-marketing testing and surveillance to monitor safety or efficacy of a product. FDA will issue either an approval of the NDA or a Complete Response Letter, detailing the deficiencies and information required in order for reconsideration of the application.
Post-Approval Requirements. Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, some manufacturing and supplier changes are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for the establishments at which such products are manufactured, as well as new application fees for certain supplemental applications.
The FDA may impose a number of post-approval requirements as a condition of approval of an PMA or NDA. For example, the FDA may require post-marketing testing, including phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In addition, entities involved in the manufacture and distribution of approved devices or drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with QSR and cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from QSR or cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain QSR and cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| • | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| • | fines, warning letters or holds on post-approval clinical trials; |
| • | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of products; or |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the
83
Table of Contents
approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
The Hatch-Waxman Amendments. We are pursuing development of our steroid-eluting implant for refractory disease treated in office as a 505(b)(2) NDA. As an alternative path to FDA approval for modifications to formulations or uses of products previously approved by the FDA, an applicant may submit an NDA under 505(b)(2) of the FDCA. 505(b)(2) was enacted as part of the Hatch-Waxman Amendments and permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by, or for, the applicant and for which the applicant has not obtained a right of reference. If the 505(b)(2) applicant can establish that reliance on FDA’s previous findings of safety and effectiveness is scientifically appropriate, it may eliminate the need to conduct certain preclinical or clinical studies of the new product. The FDA may also require companies to perform additional studies or measurements, including clinical trials, to support the change from the approved branded reference drug. The FDA may then approve the new product candidate for all, or some, of the label indications for which the branded reference drug has been approved, as well as for any new indication sought by the 505(b)(2) applicant.
Orange Book Listing. In seeking approval for a drug through an NDA, including an Abbreviated New Drug Application, or ANDA, or a 505(b)(2) NDA, applicants are required to list with the FDA certain patents whose claims cover the applicant’s product. Upon approval of an NDA, each of the patents listed in the application for the drug is then published in the Orange Book. Any applicant who files an ANDA seeking approval of a generic equivalent version of a drug listed in the Orange Book or a 505(b)(2) NDA referencing a drug listed in the Orange Book must certify to the FDA that: (1) no patent information on the drug product that is the subject of the application has been submitted to the FDA, a Paragraph I Certification; (2) such patent has expired, Paragraph II Certification; (3) the date on which such patent expires, a Paragraph III Certification; or (4) such patent is invalid or will not be infringed upon by the manufacture, use or sale of the drug product for which the application is submitted, a Paragraph IV Certification. The applicant may also elect to submit a “section viii” statement certifying that its proposed label does not contain, or carves out, any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. An applicant submitting a Paragraph IV Certification must provide notice to each owner of the patent that is the subject of the certification and to the holder of the approved NDA to which the ANDA or 505(b)(2) application refers. If the reference NDA holder and patent owners assert a patent challenge directed to one of the Orange Book listed patents within 45 days of the receipt of the Paragraph IV Certification notice, the FDA is prohibited from approving the application until the earlier of 30 months from the receipt of the Paragraph IV Certification, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the applicant. We are currently evaluating whether we will file a Paragraph II Certification, Paragraph III Certification, Paragraph IV Certification, or section viii statement for our steroid-eluting implant for refractory disease treated in office.
The ANDA or 505(b)(2) application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the branded reference drug has expired as described in further detail below.
Non-Patent Exclusivity. In addition to patent exclusivity, the holder of the NDA for the listed drug may be entitled to a period of non-patent exclusivity, during which the FDA cannot approve an ANDA or 505(b)(2) application that relies on the listed drug. For example, a pharmaceutical manufacturer may obtain five years of non-patent exclusivity upon NDA approval of a new chemical entity, or NCE, which is a drug that contains an active moiety that has not been approved by FDA in any other NDA. An “active moiety” is defined as the molecule or ion responsible for the drug substance’s physiological or pharmacologic action. During the five year exclusivity period, FDA cannot accept for filing any ANDA seeking approval of a generic version of that drug or any 505(b)(2) NDA for the same active moiety and that relies on the FDA’s findings regarding that drug, except that FDA may accept an application for filing after four years if the follow-on applicant makes a paragraph IV certification.
A drug, including one approved under 505(b)(2), may obtain a three-year period of exclusivity for a particular condition of approval, or change to a marketed product, such as a new formulation for a previously approved product, if one or more new clinical studies, other than bioavailability or bioequivalence studies, was essential to the approval of the application and was conducted or sponsored by the applicant. Should this occur,
84
Table of Contents
the FDA would be precluded from approving any ANDA or 505(b)(2) application for the protected modification until after that three-year exclusivity period has run. However, unlike NCE exclusivity, the FDA can accept an application and begin the review process during the exclusivity period.
Foreign Regulation
In order to market any product outside of the United States, we would need to comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we would need to obtain the necessary approvals by the comparable foreign regulatory authorities before we can commence clinical trials or marketing of the product in foreign countries and jurisdictions. Although many of the issues discussed above with respect to the United States apply similarly in the context of the European Union and other geographies, the approval process varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Federal and State Fraud and Abuse, Data Privacy and Security and Transparency Laws
In addition to FDA restrictions on marketing and promotion of drugs and devices, other federal and state laws restrict our business practices. These laws include, without limitation, anti-kickback and false claims laws, data privacy and security laws, as well as transparency laws regarding payments or other items of value provided to healthcare providers.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any good, facility, item or service reimbursable, in whole or in part, under Medicare, Medicaid or other federal healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated.
Additionally, the intent standard under the Anti-Kickback Statute was amended by the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, collectively the Affordable Care Act, to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the Affordable Care Act codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The federal civil False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval to the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. The civil False Claims Act also applies to false submissions that cause the government to be paid less than the amount to which it is entitled, such as a rebate. Intent to deceive is
85
Table of Contents
not required to establish liability under the civil False Claims Act. Several pharmaceutical, device and other healthcare companies have been prosecuted under these laws for, among other things, allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of products for unapproved, and thus non-covered, uses.
The government may further prosecute conduct constituting a false claim under the criminal False Claims Act. The criminal False Claims Act prohibits the making or presenting of a claim to the government knowing such claim to be false, fictitious, or fraudulent and, unlike the civil False Claims Act, requires proof of intent to submit a false claim.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the Anti-Kickback Statute, the Affordable Care Act amended the intent standard for certain healthcare fraud under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
Also, many states have similar fraud and abuse statutes or regulations that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs.
Under HIPAA, the Department of Health and Human Services, or HHS, has issued regulations to protect the privacy and security of protected health information used or disclosed by covered entities including health care providers, such as us. HIPAA also regulates standardization of data content, codes and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include civil and criminal penalties. We have developed and implemented processes designed to comply with these regulations, specifically in the training process for our field sales team. The requirements under these regulations may change periodically and could have an effect on our business operations if compliance becomes substantially more costly than under current requirements. Additionally, a breach of unsecured protected health information, such as by employee error or an attack by an outsider, could have an adverse effect on our business in terms of potential penalties and corrective action required. In addition to federal privacy regulations, there are a number of state laws governing confidentiality and security of health information that are applicable to our business. New laws governing privacy may be adopted in the future as well. We have taken steps to comply with health information privacy requirements to which we are aware that we are subject. However, we can provide no assurance that we are or will remain in compliance with diverse privacy requirements in all of the jurisdictions in which we do business. Failure to comply with privacy requirements could result in civil or criminal penalties, which could have a materially adverse effect on our business.
Additionally, there has been a recent trend of increased federal and state regulation of payments and transfers of value provided to healthcare professionals or entities. On February 8, 2013, the Centers for Medicare & Medicaid Services, or CMS, released its final rule implementing section 6002 of the Affordable Care Act known as the Physician Payment Sunshine Act that imposes new annual reporting requirements on device manufacturers for payments and other transfers of value provided by them, directly or indirectly, to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their family members. A manufacturer’s failure to submit timely, accurately and completely the required information for all payments, transfers of value or ownership or investment interests may result in civil monetary penalties of up to an aggregate of $150,000 per year, and up to an aggregate of $1 million per year for “knowing failures.” The period between August 1, 2013, and December 31, 2013, was the first reporting period, for which manufacturers were required to report aggregate payment data to CMS by March 31, 2014. Manufacturers also will be required
86
Table of Contents
to report to CMS detailed payment and transfers of value data and submit legal attestation to the completeness and accuracy of such data during Phase 2 of the program, which is expected to begin in May 2014 and extends for at least 30 days. Thereafter, manufacturers must submit reports by the 90th day of each subsequent calendar year. Any failure to comply could result in significant fines and penalties. Certain states, such as California and Connecticut, also mandate implementation of commercial compliance programs, and other states, such as Massachusetts and Vermont, impose restrictions on device manufacturer marketing practices and require tracking and reporting of gifts, compensation and other remuneration to healthcare professionals and entities. The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance or reporting requirements in multiple jurisdictions increase the possibility that a healthcare company may fail to comply fully with one or more of these requirements.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities, including certain sales and marketing practices and the provision of certain items and services to our customers, could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to penalties, including potentially significant criminal and civil and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. To the extent that any of our products are sold in a foreign country, we may be subject to similar foreign laws, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws and implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals.
Coverage and Reimbursement
Our currently approved products are commonly treated as general supplies utilized in sinus surgery and if covered by third-party payors, are paid for as part of the FESS procedure. We believe that establishment of reimbursement codes specific to the use of drug-eluting implants for chronic sinusitis is an important factor in expanding access to our products, especially in the physician office setting. Therefore, our strategy includes efforts to engage physician societies and encourage third-party payors to establish coverage, coding and payment that will facilitate access to our drug-eluting implants as we expand our commercialization efforts in the United States. Our success in these efforts depends in part on the extent to which governmental authorities, private health insurers and other third-party payors provide coverage for and establish adequate reimbursement levels for the procedures during which our products are used. Failure by physicians, hospitals, ambulatory surgery centers and other users of our products to obtain sufficient coverage and reimbursement from healthcare payors for procedures in which our products are used, or adverse changes in government and private third-party payors’ policies would have a material adverse effect on our business, financial condition, results of operations and future growth prospects.
In the United States, third-party payors continue to implement initiatives that restrict the use of certain technologies to those that meet certain clinical evidentiary requirements. We are aware of a number of third-party payors in the United States, including Aetna and some Blue Cross Blue Shield plans, that consider our products to be experimental or investigational. These payors have developed policies that deny coverage and separate payment for our products, but based on our experience to date, these payors generally reimburse for the surgical procedures in which our products are used, as long as the patient meets the established medical necessity criteria for surgery. In addition, some payors are moving toward a managed care system and control their health care costs by limiting authorizations for surgical procedures, including elective procedures using our devices. Failure to obtain favorable payor policies could have a material adverse effect on our business and operations.
In addition to uncertainties surrounding coverage policies, there are periodic changes to reimbursement. Third-party payors regularly update reimbursement amounts and also from time to time revise the methodologies used to determine reimbursement amounts. This includes annual updates to payments to physicians, hospitals and ambulatory surgery centers for procedures during which our products are used. Because the cost of our products generally is recovered by the healthcare provider as part of the payment for performing a procedure and not
87
Table of Contents
separately reimbursed, these updates could directly impact the demand for our products. An example of payment updates is the Medicare program’s updates to hospital and physician payments, which are done on an annual basis using a prescribed statutory formula. In the past, when the application of the formula resulted in lower payment, Congress has passed interim legislation to prevent the reductions. Most recently, the Protecting Access to Medicare Act of 2014, signed into law in April 2014, provided for a 0.5% update from 2013 payment rates under the Medicare Physician Fee Schedule through 2014 and a 0% update from January 1 until April 1, 2015. If Congress fails to intervene to prevent the negative update factor in future years, the resulting decrease in payment may adversely affect our revenues and results of operations.
Any changes in coverage and reimbursement that further restricts coverage of our products or lowers reimbursement for procedures using our devices could materially affect our business.
Healthcare Reform
Current and future legislative proposals to further reform healthcare or reduce healthcare costs may result in lower reimbursement for our products, or for the procedures associated with the use of our products, or limit coverage of our products. The cost containment measures that payors and providers are instituting and the effect of any healthcare reform initiative implemented in the future could significantly reduce our revenues from the sale of our products. Alternatively, the shift away from fee-for-service agreements to capitated payment models supports the value of our products, as they have been proven to reduce longitudinal resource utilization, which can be cost saving-for both payors and providers.
The recent implementation of the Affordable Care Act is an example that has the potential to substantially change healthcare financing and delivery by both governmental and private insurers, and significantly impact the pharmaceutical and medical device industries.
The Affordable Care Act imposed, among other things, a new federal excise tax on the sale of certain medical devices, established an annual, nondeductible fee on any entity that manufactures or imports certain specified branded prescription drugs and biologic agents, revised the methodology by which rebates owed by manufacturers to the state and federal government for covered outpatient drugs under the Medicaid Drug Rebate Program are calculated, increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program, extended the Medicaid Drug Rebate program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations, and provided incentives to programs that increase the federal government’s comparative effectiveness research. In addition, the Affordable Care Act implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. On August 2, 2011, President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013, and will stay in effect through 2024 unless congressional action is taken. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products or product candidates or additional pricing pressure.
The Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act, or the FCPA, prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official,
88
Table of Contents
political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations. Activities that violate the FCPA, even if they occur wholly outside the United States, can result in criminal and civil fines, imprisonment, disgorgement, oversight, and debarment from government contracts.
Research and Development
Our research and development expenses totaled $9.3 million and $9.5 million in the years ended December 31, 2012 and 2013, respectively, and $2.3 million and $2.6 million in the three months ended March 31, 2013 and 2014, respectively. For more information, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Employees
As of March 31, 2014, we had 146 employees, of whom 47 are in manufacturing, 26 are in research and development and 62 are in sales, marketing and reimbursement.
Properties
We occupy approximately 32,500 square feet of leased office and laboratory space located in Menlo Park, California. The term of our lease expires in June 2015. We believe that our facilities are sufficient to meet our current needs and that suitable additional space will be available as and when needed on acceptable terms.
Legal Proceedings
We are not currently a party to any material legal proceedings. We may at times be involved in litigation and other legal claims in the ordinary course of business. When appropriate in our estimation, we may record reserves in our financial statements for pending litigation and other claims.
89
Table of Contents
Executive Officers, Key Employees and Directors
The following table sets forth information regarding our executive officers, key employees and directors, as of June 4, 2014:
| Name |
Age | Position(s) | ||||
| Executive Officers and Key Employees |
||||||
| Lisa D. Earnhardt |
44 | President, Chief Executive Officer and Director | ||||
| Jeryl L. Hilleman |
56 | Chief Financial Officer | ||||
| Richard E. Kaufman |
52 | Senior Vice President and Chief Operating Officer | ||||
| Robert H. Binney, Jr. |
44 | Vice President, Sales | ||||
| James Stambaugh |
44 | Vice President, Clinical and Reimbursement | ||||
| Susan P. Stimson |
41 | Vice President, Marketing | ||||
| Amy Wolbeck |
42 | Vice President, Regulatory Affairs and Quality | ||||
| Non-Employee Directors |
||||||
| Rick D. Anderson(1)(3) |
53 | Director | ||||
| Casper L. de Clercq(1) |
50 | Director | ||||
| Mark Fletcher |
57 | Director | ||||
| Cynthia L. Lucchese(1)(3) |
54 | Director | ||||
| Dana G. Mead, Jr.(2) |
55 | Director | ||||
| Frederic H. Moll, MD(3) |
62 | Director | ||||
| Casey M. Tansey(2) |
56 | Director | ||||
| (1) | Member of the audit committee. |
| (2) | Member of the compensation committee. |
| (3) | Member of the nominating and corporate governance committee. |
Executive Officers and Key Employees
Lisa D. Earnhardt has served as our President and Chief Executive Officer and as a member of our board of directors since March 2008. Prior to joining us, Ms. Earnhardt served as President of Boston Scientific’s Cardiac Surgery division (formerly known as Guidant Corporation, or Guidant) from June 2006 to January 2008 until its sale to Getinge Group. From August 1996 to April 2006, Ms. Earnhardt worked at Guidant in a variety of sales and marketing leadership positions. Ms. Earnhardt served on the board of directors of Kensey Nash, a publicly traded company from 2011 until it was acquired by Royal DSM NA in 2012, where she served on the board’s nominating and governance and audit committees. Ms. Earnhardt serves as the Chair of the Kellogg Alumni Council. Ms. Earnhardt holds an M.B.A. from Northwestern’s Kellogg School of Management and a B.S. in Industrial Engineering from Stanford University. We believe Ms. Earnhardt’s experience in the industry, her role as our President and Chief Executive Officer and her knowledge of our company enables her to make valuable contributions to our board of directors.
Jeryl L. Hilleman has served as our Chief Financial Officer since June 2014. From September 2013 to May 2014, Ms. Hilleman served as Chief Financial Officer and Secretary of Ocera Therapeutics, Inc., a biopharmaceutical company, where she was responsible for managing Ocera’s financial and accounting operations. From 2012 to 2013, Ms. Hilleman provided independent financial and strategic consulting for biotech and cleantech companies. From 2008 to 2012, she served as Chief Financial Officer of Amyris, Inc., a multi-national, renewable products company based in California and Brazil, where she was responsible for managing Amyris’s financial and accounting operations. Prior to Amyris, Ms. Hilleman served as Chief Financial Officer of Symyx Technologies, a public company selling instruments, software and research services to pharmaceutical and chemical companies, and led the finance function at two public biotechnology companies, Geron Corporation and Cytel Corporation. Ms. Hilleman is also a member of the board of directors and chair of the audit committee of XenoPort, Inc. Ms. Hilleman holds an A.B. from Brown University and an M.B.A. from the Wharton Graduate School of Business, University of Pennsylvania.
90
Table of Contents
Richard E. Kaufman has served as our Senior Vice President of R&D and Operations and Chief Operating Officer since January 2007. Prior to joining us, Mr. Kaufman was the Vice President and General Manager of Abbott Bioabsorbable Vascular Solutions. Mr. Kaufman held several R&D and Operations leadership positions at Guidant from 1998 through 2006. Previously Mr. Kaufman worked at McGhan Medical from 1993 to 1998 developing products for the plastic surgery field and at IMPRA from 1990 to 1993 developing products for orthopedics and vascular grafts. Mr. Kaufman holds a B.S. in Biomedical Engineering from Arizona State University.
Robert H. Binney, Jr. has served as our Vice President of Sales since July 2011. From June 2007 to July 2011, Mr. Binney served as Director of Sales for AccessClosure, Inc., a privately-held medical device company, where he was responsible for eastern United States sales. From June 2004 to June 2007, Mr. Binney served as Peripheral Sales Representative at Boston Scientific’s Peripheral Division. Mr. Binney holds a B.A. in Communications from the University of North Carolina at Chapel Hill.
James Stambaugh has served as our Vice President of Clinical and Reimbursement since October 2006. Prior to joining us, Mr. Stambaugh served as Director of Clinical Operations at Guidant’s Vascular Intervention division, from March 2005 to October 2006. Prior to serving as Director of Clinical Operations Mr. Stambaugh held various positions at Guidant within the pre-clinical, clinical and regulatory environment. Mr. Stambaugh holds a B.S. in Chemistry from the University of California at Davis.
Susan P. Stimson has served as our Vice President of Marketing since March 2010 and served as our Director of Marketing Development from April 2007 to March 2010. Prior to joining us, Ms. Stimson served as Director of Marketing and Clinical Research at Mitralign, Inc., from October 2003 to December 2006. From February 1997 to September 2003, Ms. Stimson worked within multiple areas and divisions of Guidant. Ms. Stimson holds a B.S. in Biomedical Engineering from Marquette University, Milwaukee, Wisconsin.
Amy Wolbeck has served as our Vice President, Regulatory Affairs and Quality since January 2011 and served as our Director of Regulatory Affairs from January 2009 to January 2011. Prior to joining us, Ms. Wolbeck was the Director of Regulatory Affairs and Quality Assurance at Sonoma Orthopedic Products. Ms. Wolbeck served in various roles at Medtronic from 1998-2007, including Director of Regulatory Affairs within Medtronic’s Cardiovascular Division and Director of Quality for Medtronic Vascular. Ms. Wolbeck holds a B.S. in Mechanical Engineering from the University of Massachusetts at Amherst.
Non-Employee Directors
Rick D. Anderson has served as a member of our Board of Directors since April 2012. Mr. Anderson has served as a Managing Director of PTV Healthcare Capital, a venture capital firm since January 2008. From September 2008 to August 2011, Mr. Anderson served as a member of the board of directors of InSite Vision, a publicly traded company, where he was the chair of the board’s nominating and governance committee and a member of the audit committee. From April 2008 to June 2012, Mr. Anderson served as a member of the board of directors of Cameron Health until its acquisition by Boston Scientific. From July 2009 to August 2013, Mr. Anderson served as Chairman of the board of directors for IDEV Technologies, until its acquisition by Abbott. Currently, Mr. Anderson also serves as a member of the board of directors for several private companies. Mr. Anderson holds a B.B.A. in Marketing from Mississippi State University. He served five years in the U.S. Army where he obtained the rank of captain. Mr. Anderson is a member of the Economic Advisory Board of Mississippi State University and a consultant member of the Society for Cardiovascular Angiography and Interventions. He participates in the AdvaMed Venture Capital Advisory Council and the Dartmouth Device Development Symposium Executive Committee, and he is a Director for the Heart Rhythm Society Consulting Services and a member of the Accelerated Innovation Program Working Group for the National Heart Lung and Blood Institute. We believe Mr. Anderson’s experience in the industry and his knowledge of our company enables him to make valuable contributions to our board of directors.
Casper de Clercq has served as a member of our Board of Directors since February 2013. Mr. de Clercq has been a partner at Norwest Venture Partners, a venture capital firm, since January 2011. Prior to joining Norwest Venture Partners, Mr. de Clercq was a Partner at U.S. Venture Partners, a venture capital firm, from August 2004 to January 2011. He currently serves on the boards of directors of several privately held companies and was
91
Table of Contents
previously on the board of directors of Basis Science, Inc. prior to its acquisition by Intel, and Ilypsa, Inc. prior to its acquisition by Amgen. Mr. de Clercq holds a B.A. in biochemistry from Dartmouth College, an M.S. in biological science from Stanford University, and a M.B.A. from Stanford University Graduate School of Business. We believe Mr. de Clercq’s experience in the industry and his knowledge of our company enables him to make valuable contributions to our board of directors.
Mark Fletcher has served as a member of our Board of Directors since November 2010. Mr. Fletcher has served as the Senior Vice-President of Medtronic, Inc. and the President of its Surgical Technologies Division, consisting of four business segments including ENT, Neurosurgery, Advanced Energy and Global Services since May 2011. Prior to this position, Mr. Fletcher served as the President of Medtronic’s ENT Division from 1999 to 2011. Mr. Fletcher also serves on the board of Inspire Medical, an early-stage ENT technology company. Mr. Fletcher holds a B.S. in Finance from St. Mary’s University. We believe Mr. Fletcher’s experience in the industry and his knowledge of our company enables him to make valuable contributions to our board of directors.
Cynthia L. Lucchese was appointed as a member of our board of directors in July 2014. From August 2009 to October, 2012, Ms. Lucchese served as a member of the board of directors of Brightpoint Inc., a publicly traded company, where she was the chair of the board’s audit committee and a member of the nominating and governance committee. From February 2008 to March 2014, Ms. Lucchese served as Senior Vice President and Chief Financial Officer of Hillenbrand, Inc., a manufacturing company. From 2005 to 2007, she served as Senior Vice President and Chief Financial Officer for Thoratec Corporation, a medical device company. From 1994 to 2005 she worked for Guidant Corporation, now a part of Boston Scientific Corporation, in a variety of finance roles, including Vice President, Treasurer and Chief Accounting Officer. Ms. Lucchese serves on the Dean’s Council of the Indiana University Kelley School of Business, and the Board of the Indiana Sport Corporation as treasurer. Ms. Lucchese is a Certified Public Accountant and holds a B.S. with distinction in Accounting and an MBA in Finance from the Indiana University Kelley School of Business. We believe Ms. Lucchese’s experience in the industry with publicly traded companies enables her to make valuable contributions to our board of directors.
Dana G. Mead, Jr. has served as a member of our Board of Directors since June 2007. Mr. Mead has been a Partner at Kleiner Perkins Caufield & Byers since May 2005. Mr. Mead currently serves on the boards of several privately held Kleiner Perkins Caufield & Byers portfolio companies. From 1992 to 2005, Mr. Mead worked for Guidant where he held numerous positions including, most recently, President of Guidant Vascular Intervention. Mr. Mead received his B.A. from Lafayette College and holds an M.B.A. from the University of Southern California. We believe Mr. Mead’s experience with medical device companies and role in the venture capital industry enables him to make valuable contributions to our board of directors.
Frederic H. Moll, M.D. has served as a member of our Board of Directors since March 2006. From September 2002 until May 2012, Dr. Moll served as a member of the Board of Directors of Hansen Medical, Inc., including its Executive Chairman of the Board from June 2010 until December 2011. Dr. Moll also served as the Chief Executive Officer of Hansen Medical, Inc. from September 2002 until June 2010 and its President from March 2009 until June 2010. Dr. Moll has served as the Chairman of the Board of Auris Surgical Robotics, Inc. since June 2011 and as its Chief Executive Officer since August 2012. In November 1995, Dr. Moll co-founded Intuitive Surgical, Inc., a medical device company, and served as its first Chief Executive Officer and later, its Vice President and Medical Director until September 2003. In 1989, Dr. Moll co-founded Origin Medsystems, Inc., a medical device company, which later became an operating company within Guidant Corporation following its acquisition by Eli Lilly in 1992. Dr. Moll served as Medical Director of Guidant’s surgical device division until November 1995. Dr. Moll also serves on the board of directors of MAKO Surgical Corp. and Restoration Robotics. Dr. Moll holds a B.A. from the University of California, Berkeley, an M.S. from Stanford University and an M.D. from the University of Washington School of Medicine. We believe Dr. Moll’s experience as a physician, chief executive officer of medical technology companies, familiarity with serving on the boards of public companies and his knowledge of our company enables him to make valuable contributions to our board of directors.
Casey M. Tansey has served as a member of our Board of Directors since March 2006. Mr. Tansey has served as a General Partner of U.S. Venture Partners since April 2005. From September 2001 to December 2004, Mr. Tansey served as President and Chief Executive Officer of Epicor Medical, Inc., until its acquisition by
92
Table of Contents
St. Jude Medical. From June 1999 to May 2001, Mr. Tansey served as President, CEO and Director of Heartport Inc., a publicly traded company that was acquired by Johnson & Johnson. Mr. Tansey joined Heartport as Vice President of Sales and Marketing in December 1995. Mr. Tansey currently serves on the Board of Directors for several privately held U.S. Venture Partners portfolio companies. Mr. Tansey holds a B.S. and M.B.A. from the College of Notre Dame. We believe Mr. Tansey’s experience in the industry and his knowledge of our company enables him to make valuable contributions to our board of directors.
Family Relationships
There are no family relationships among any of our directors or executive officers.
Board Composition
The primary responsibilities of our board of directors are to provide oversight, strategic guidance, counseling and direction to our management. Our board of directors meets on a regular basis and additionally as required. Our board of directors currently consists of seven directors. The members of our board of directors were elected in compliance with the provisions of our amended and restated certificate of incorporation and a voting agreement among certain of our stockholders. The voting agreement will terminate upon the closing of this offering and none of our stockholders will have any special rights regarding the election or designation of members of our board of directors. Our amended and restated certificate of incorporation to become effective upon the completion of this offering, or the amended and restated certificate of incorporation, will permit our board of directors to establish by resolution the authorized number of directors. Each director serves until the expiration of the term for which such director was elected or appointed, or until such director’s earlier death, resignation or removal. At each annual meeting of stockholders, directors whose terms then expire will be elected to serve from the time of election and qualification until the next annual meeting following election. Our amended and restated certificate of incorporation provides that the authorized number of directors may be changed only by resolution of the board of directors.
Director Independence
Upon the completion of this offering, we anticipate that our common stock will be listed on The Nasdaq Global Market. Under the listing requirements and rules of The Nasdaq Global Market, independent directors must compose a majority of a listed company’s board of directors within 12 months after its initial public offering. In addition, the rules of The Nasdaq Global Market require that, subject to specified exceptions and phase in periods following its initial public offering, each member of a listed company’s audit and compensation, nominating and governance committee be independent. Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Under the rules of The Nasdaq Global Market, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
To be considered to be independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of our audit committee, our board of directors, or any other board committee: (1) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (2) be an affiliated person of the listed company or any of its subsidiaries.
Our board of directors has undertaken a review of its composition, the composition of its committees, and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment, and affiliations, including family relationships, our board of directors has determined that all of our board of directors except Lisa D. Earnhardt do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the applicable rules and regulations of the SEC, and the listing requirements and rules of The Nasdaq Global Market. In making this determination, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director. Our board
93
Table of Contents
of directors also determined that Casper L. de Clercq and Rick D. Anderson and Cynthia L. Lucchese, who compose our audit committee, upon the completion of this offering, satisfy the independence standards for the audit committee established by applicable Securities and Exchange Commission, or SEC, rules and the listing standards of The Nasdaq Global Market and Rule 10A-3 of the Exchange Act. Our board of directors has determined that Messrs. Mead and Tansey who compose our compensation committee, upon the completion of this offering, are “outside directors” as that term is defined in Section 162(m) of the Internal Revenue Code of 1986, as amended, or Section 162(m). Each member of the nominating and corporate governance committee is independent within the meaning of the applicable NASDAQ listing standards, is a non-employee director and is free from any relationship that would interfere with the exercise of his or her independent judgment.
Board Committees
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee. Our board of directors may establish other committees to facilitate the management of our business. The composition and functions of each committee are described below. Members serve on these committees until their resignation or until otherwise determined by our board of directors.
Audit Committee
Our audit committee consists of Casper L. de Clercq, Rick D. Anderson and Cynthia L. Lucchese. The chair of our audit committee is Ms. Lucchese, who our board of directors has determined is an “audit committee financial expert” as that term is defined under the SEC rules implementing Section 407 of the Sarbanes-Oxley Act of 2002, and possesses financial sophistication, as defined under the listing standards of The Nasdaq Global Market. Our board of directors has also determined that each member of our audit committee can read and understand fundamental financial statements in accordance with applicable requirements. In arriving at these determinations, the board of directors has examined each audit committee member’s scope of experience and the nature of their experience in the corporate finance sector.
The primary purpose of the audit committee is to discharge the responsibilities of our board of directors with respect to our accounting, financial and other reporting and internal control practices and to oversee our independent registered public accounting firm. Specific responsibilities of our audit committee include:
| • | selecting a qualified firm to serve as the independent registered public accounting firm to audit our financial statements; |
| • | helping to ensure the independence and performance of the independent registered public accounting firm; |
| • | discussing the scope and results of the audit with the independent registered public accounting firm and reviewing, with management and the independent accountants, our interim and year-end operating results; |
| • | developing procedures for employees to submit concerns anonymously about questionable accounting or audit matters; |
| • | reviewing our financial statements and critical accounting policies and estimates; |
| • | reviewing the adequacy and effectiveness of our internal controls; |
| • | reviewing our policies on risk assessment and risk management; |
| • | reviewing related-party transactions; |
| • | obtaining and reviewing a report by the independent registered public accounting firm, at least annually, that describes our internal quality-control procedures, any material issues with such procedures and any steps taken to deal with such issues when required by applicable law; and |
| • | approving (or, as permitted, pre-approving) all audit and all permissible non-audit services, other than de minimis non-audit services, to be performed by the independent registered public accounting firm. |
94
Table of Contents
Compensation Committee
Our compensation committee consists of Dana G. Mead, Jr. and Casey M. Tansey. The chair of our compensation committee is Dana G. Mead, Jr.
The primary purpose of our compensation committee is to discharge the responsibilities of our board of directors to oversee our compensation policies, plans and programs and to review and determine the compensation to be paid to our executive officers, directors and other senior management, as appropriate. Specific responsibilities of our compensation committee include:
| • | reviewing and approving, or recommending that our board of directors approve, the compensation of our executive officers; |
| • | reviewing and recommending to our board of directors the compensation of our directors; |
| • | reviewing and approving, or recommending that our board of directors approve, the terms of compensatory arrangements with our executive officers; |
| • | administering our stock and equity incentive plans; |
| • | selecting independent compensation consultants and assessing whether there are any conflicts of interest with any of the committees compensation advisers; |
| • | reviewing and approving, or recommending that our board of directors approve, incentive compensation and equity plans, severance agreements, change-of-control protections and any other compensatory arrangements for our executive officers and other senior management, as appropriate; and |
| • | reviewing and establishing general policies relating to compensation and benefits of our employees and reviewing our overall compensation philosophy. |
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee consists of Rick D. Anderson, Frederic H. Moll and Cynthia L. Lucchese. The chair of our nominating and corporate governance committee is Rick D. Anderson. Specific responsibilities of our nominating and corporate governance committee include:
| • | identifying, evaluating and selecting, or recommending that our board of directors approve, nominees for election to our board of directors; |
| • | evaluating the performance of our board of directors and of individual directors; |
| • | considering and making recommendations to our board of directors regarding the composition of the committees of the board of directors; |
| • | reviewing developments in corporate governance practices; |
| • | evaluating the adequacy of our corporate governance practices and reporting; |
| • | reviewing management succession plans; |
| • | developing and making recommendations to our board of directors regarding corporate governance guidelines and matters; and |
| • | overseeing an annual evaluation of the board of directors’ performance. |
Role of the Board in Risk Oversight
The audit committee of the board of directors is primarily responsible for overseeing our risk management processes on behalf of the board of directors. Going forward, we expect that the audit committee will receive reports from management on at least a quarterly basis regarding our assessment of risks. In addition, the audit committee reports regularly to the board of directors, which also considers our risk profile. The audit committee and the board of directors focus on the most significant risks we face and our general risk management strategies. While the board of directors oversees our risk management, management is responsible for day-to-day risk management processes. Our
95
Table of Contents
board of directors expects management to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively implement risk management strategies adopted by the audit committee and the board of directors. We believe this division of responsibilities is the most effective approach for addressing the risks we face and that our board of directors leadership structure, which also emphasizes the independence of the board of directors in its oversight of its business and affairs, supports this approach.
Code of Business Conduct and Ethics
Effective upon the closing of this offering, we have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Following the closing of this offering, the code of business conduct and ethics will be available on our website at www.intersectent.com. We intend to disclose any amendments to the code, or any waivers of its requirements, on our website to the extent required by the applicable rules and exchange requirements. The inclusion of our website address in this prospectus does not incorporate by reference the information on or accessible through our website into this prospectus.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee has ever been an officer or employee of the company. None of our executive officers serve, or have served during the last fiscal year, as a member of the board of directors, compensation committee or other board committee performing equivalent functions of any entity that has one or more executive officers serving as one of our directors or on our compensation committee.
Director Compensation
We have a policy of reimbursing our directors for their reasonable out-of-pocket expenses in connection with attending board of directors and committee meetings. From time to time, we have granted stock options to certain of our non-employee directors as compensation for their services. None of our non-employee directors were granted a stock option during the year ended December 31, 2013. Dr. Moll and Ms. Lucchese are our only non-employee directors who hold options to purchase our common stock. As of December 31, 2013, Dr. Moll held options to purchase 25,000 shares of our common stock. In July 2014, our board of directors granted Ms. Lucchese an option to purchase 25,000 shares of our common stock, vesting monthly over four years.
96
Table of Contents
Compensation
Summary Compensation Table
The following summary compensation table shows information regarding the compensation awarded to, earned by or paid to our named executive officers during the year ended December 31, 2013.
| Name and Principal Position |
Year | Salary | Stock Options(1) |
Non-Equity Incentive Plan Compensation |
All Other Compensation |
Total | ||||||||||||||||||
| Lisa D. Earnhardt |
2013 | $ | 334,750 | $ | 210,688 | $ | 109,195 | (2) | $ | 343 | (4) | $ | 654,976 | |||||||||||
| President and Chief Executive Officer |
||||||||||||||||||||||||
| Richard E. Kaufman |
2013 | 293,150 | 20,795 | 68,304 | (2) | 100,000 | (5) | 482,249 | ||||||||||||||||
| Senior Vice President and Chief Operating Officer |
||||||||||||||||||||||||
| Robert H. Binney, Jr. |
2013 | 154,500 | 51,076 | 154,583 | (3) | 14,400 | (6) | 374,559 | ||||||||||||||||
| Vice President, Sales |
||||||||||||||||||||||||
| (1) | The amounts included in the Stock Options column represent the grant date fair value of stock options granted, calculated in accordance with ASC Topic 718. For a discussion of the assumptions made in the valuations reflected in this column, see Note 10 of the Financial Statements included elsewhere in this prospectus. |
| (2) | Represents payments pursuant to our corporate bonus plan. At the beginning of each year, the compensation committee approves specific company performance milestones. Bonuses are determined at year-end by the compensation committee based upon the level of achievement of the milestones. Approve bonuses are paid in March of the following year. |
| (3) | Consists of (a) $33,333 paid pursuant to our management business objectives bonus plan and (b) $121,250 pursuant to our sales commission plan. |
| (4) | Represents life insurance premiums paid by us. |
| (5) | Represents the portion of a loan to Mr. Kaufman forgiven by us. |
| (6) | Represents a car allowance paid by us. |
Outstanding Equity Awards as of December 31, 2013
The following table shows stock options outstanding for each of our named executive officers as of December 31, 2013.
| Name |
Vesting Commencement Date |
Number of Securities Underlying Unexercised Options(1) |
Option Exercise Price |
Option Expiration Date |
||||||||||||||||
| Exercisable | Unexercisable | |||||||||||||||||||
| Lisa D. Earnhardt |
04/23/13 | 89,375 | (2) | — | $ | 1.20 | 04/22/23 | |||||||||||||
| 11/30/13 | 144,374 | (3) | — | $ | 1.20 | 04/22/23 | ||||||||||||||
| Richard E. Kaufman |
04/23/13 | 14,250 | (4) | — | $ | 1.20 | 04/22/23 | |||||||||||||
| 11/30/13 | 14,250 | (3) | — | $ | 1.20 | 04/22/23 | ||||||||||||||
| Robert H. Binney, Jr. |
07/18/11 | 87,500 | (5) | — | $ | 1.08 | 09/21/21 | |||||||||||||
| 04/23/13 | 35,000 | (4) | — | $ | 1.20 | 04/22/23 | ||||||||||||||
| 11/30/13 | 35,000 | (3) | — | $ | 1.20 | 04/22/23 | ||||||||||||||
| (1) | The outstanding stock options are exercisable prior to vesting. |
| (2) | None of the shares subject to this option were vested as of December 31, 2013, and the remainder vest in equal increments on monthly basis from November 23, 2014, through April 23, 2017. |
| (3) | Approximately 2.0% of the shares subject to this option were vested as of December 31, 2013, and the remainder vest in equal increments on a monthly basis thereafter through November 30, 2017. |
| (4) | Approximately 16.6% of the shares subject to this option were vested as of December 31, 2013, and the remainder vest in equal increments on a monthly basis thereafter through April 23, 2017. |
97
Table of Contents
| (5) | Approximately 60.4% of the shares subject to this option were vested as of December 31, 2013, and the remainder vest in equal increments on a monthly basis thereafter through July 18, 2015. |
Each of the options reflected in the table above were granted with an exercise price equal to the fair value of our common stock on the date of grant, and may be exercised prior to vesting. Stock issued upon exercise of unvested options will be subject to repurchase by the company until vested.
Employee Benefits
We provide standard employee benefits to our full- and part-time employees, including our named executive officers, in the United States (in the case of part-time, those that work 30 or more hours per week), including health, disability and life insurance and a 401(k) plan as a means of attracting and retaining our executives and employees.
Tax Considerations
Our Board has considered the potential future effects of Section 162(m) of the Internal Revenue Code on the compensation paid to our named executive officers. Section 162(m) disallows a tax deduction for any publicly held corporation for individual compensation exceeding $1.0 million in any taxable year for our chief executive officer and each of the other named executive officers (other than our chief financial officer), unless compensation is performance-based. As we only recently became publicly-traded, our Board has not previously taken the deductibility limit imposed by Section 162(m) into consideration in setting compensation.
Pension Benefits
We do not maintain any defined benefit pension plans.
Non-qualified Deferred Compensation
We do not maintain any non-qualified deferred compensation plans.
Limitation on Liability and Indemnification
Upon the closing of this offering, our amended and restated certificate of incorporation will contain provisions that limit the liability of our current and former directors for monetary damages to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for any breach of fiduciary duties as directors, except liability for:
| • | any breach of the director’s duty of loyalty to the corporation or its stockholders; |
| • | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions; or |
| • | any transaction from which the director derived an improper personal benefit. |
Such limitation of liability does not apply to liabilities arising under federal securities laws and does not affect the availability of equitable remedies, such as injunctive relief or rescission. Our amended and restated certificate of incorporation and our amended and restated bylaws will provide that we are required to indemnify our directors to the fullest extent permitted by Delaware law. Our amended and restated bylaws will also provide that, upon satisfaction of certain conditions, we shall advance expenses incurred by a director in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law. Our amended and restated certificate of incorporation and amended and restated bylaws will also provide our board of directors with discretion to indemnify our officers and employees when determined appropriate by the board. We have entered and expect to continue to enter into agreements to indemnify our directors and executive officers. With certain exceptions, these agreements provide for indemnification for related expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any
98
Table of Contents
action or proceeding. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain customary directors’ and officers’ liability insurance.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought and we are not aware of any threatened litigation that may result in claims for indemnification.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, or the Securities Act, may be permitted for directors, executive officers or persons controlling us, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Offer Letters and Bonus Plan
We extended offer letters to each of our named executive officers in connection with their employment. The letters generally provide for at-will employment and set forth the named executive officer’s initial base salary, initial equity grant amount and eligibility for employee benefits. In addition, each of our named executive officers has executed a form of our standard confidential information and invention assignment agreement. The key terms of the offer letters extended to our named executive officers that continue to be in effect are described below.
Lisa D. Earnhardt
In January 2008, we extended an offer letter to Ms. Earnhardt to be our President and Chief Executive Officer. The letter was subsequently amended in July 2013. Pursuant to her offer letter, as amended, if, within 12 months following a change of control, Ms. Earnhardt’s employment is terminated without “cause” or she resigns for “good reason,” all unvested shares subject to her outstanding options shall accelerate in full. In addition, in the event of Ms. Earnhardt’s death, permanent disability, resignation for “good reason” or termination without “cause,” (1) all unvested shares subject to her outstanding options shall accelerate in full, (2) she shall receive a lump sum payment equal to her annual target bonus and (3) she shall receive 12 months of her base salary, to be paid monthly.
Pursuant to our corporate bonus plan, Ms. Earnhardt is eligible to receive a bonus of up to 35% of her annual base salary upon our achievement of milestones established by the compensation committee for the particular year.
Robert H. Binney, Jr.
In June 2011, we extended an offer letter to Mr. Binney to be our Vice President of Sales. The letter was subsequently amended in November 2013. Pursuant to his offer letter, as amended, upon the occurrence of a change of control, all outstanding stock options held by Mr. Binney shall be accelerated such that 50% of the unvested shares shall be fully vested. In addition, if, within 12 months following a change of control, Mr. Binney’s employment is terminated without “cause” or he resigns for “good reason,” and provided such termination is in connection with the change of control and constitutes a “separation from service,” (1) all unvested shares subject to his outstanding options shall accelerate in full, (2) he shall receive a prorated, lump sum payment equal to his annual target bonus and (3) he shall receive six months of his base salary, to be paid monthly.
As a member of our sales team, Mr. Binney is a participant in (1) our sales commission plan, which provides for quarterly commissions based on the sales team’s achievement of its quotas and (2) our management business objectives bonus plan, which provides for a quarterly bonus upon the achievement of management targets established by the compensation committee for the particular year.
99
Table of Contents
Richard E. Kaufman
In December 2006, we extended an offer letter to Mr. Kaufman to be our Vice President and Chief Operating Officer. The letter was subsequently amended in November 2013. Pursuant to his offer letter, as amended, upon the occurrence of a change of control, all outstanding stock options held by Mr. Kaufman shall be accelerated such that 50% of the unvested shares shall be fully vested. In addition, if, within 12 months following a change of control, Mr. Kaufman’s employment is terminated without “cause” or he resigns for “good reason,” and provided such termination is in connection with such change of control and constitutes a “separation from service,” (1) all unvested shares subject to his outstanding options shall accelerate in full, (2) he shall receive a prorated, lump sum payment equal to his annual target bonus and (3) he shall receive six months of his base salary, to be paid monthly.
Pursuant to our corporate bonus plan, Mr. Kaufman is eligible to receive a bonus of up to 25% of his annual base salary, upon the achievement of certain milestones established by the compensation committee for the particular year.
As set forth in these offer letters: (1) “cause” includes commissions of crimes or other material acts of dishonesty, engagement in any activity the executive officer knows could materially harm our business or reputation, material failure to adhere to our corporate codes, policies or procedures, material violation of any statutory, contractual, or common law duty or obligation to us or material breach of the executive officer’s confidentiality agreement with us, or the failure to substantially perform assigned duties or responsibilities after notice and opportunity to cure; and (2) “good reason” includes a relocation of the office where the executive officer is assigned to a location of more than 35 miles away (50 miles in the case of Ms. Earnhardt), a material decrease in base salary (except for salary decreases generally applicable to our other executive employees), or a material reduction in the scope of duties or responsibilities, in each case without the executive officer’s written consent; provided, however, that, in the case of Mr. Binney and Mr. Kaufmann, to resign for Good Reason the executive officer must provide notice to us and give us 30 days to cure the event giving rise to Good Reason, and if not cured then must resign within 90 days of the expiration of the cure period.
Stock Plans
The principal features of our equity incentive plans and our 401(k) plan are summarized below. These summaries are qualified in their entirety by reference to the actual text of the plans, which, other than the 401(k) plan, are filed as exhibits to the registration statement of which this prospectus is a part.
2014 Equity Incentive Plan
Our board of directors adopted our 2014 Equity Incentive Plan, or the 2014 Plan, in July, 2014, and our stockholders subsequently approved the 2014 Plan in July, 2014. The 2014 Plan will become effective on the date the registration statement of which this prospectus forms a part is declared effective by the SEC. Once the 2014 Plan becomes effective, no further grants will be made under our 2013 Equity Incentive Plan, which is described below.
Our 2014 Plan provides for the grant of incentive stock options, or ISOs, within the meaning of Section 422 of the Code, to our employees and our parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options, or NSOs, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance-based stock awards, and other forms of equity compensation to our employees, directors and consultants. Addition, our 2014 Plan provides for the grant of performance cash awards to our employees, directors and consultants.
Authorized Shares. The maximum number of shares of our common stock that may be issued under our 2014 Plan is 4,750,000. The number of shares of our common stock reserved for issuance under our 2014 Plan will automatically increase on January 1 of each year, beginning on January 1, 2015, and continuing through and including January 1, 2024, by 3% of the total number of shares of our capital stock outstanding on December 31 of the preceding calendar year, or a lesser number of shares determined by our board of directors. The maximum number of shares that may be issued upon the exercise of ISOs under our 2014 Plan is 10,000,000.
100
Table of Contents
Shares issued under our 2014 Plan will be authorized but unissued or reacquired shares of our common stock. Shares subject to stock awards granted under our 2014 Plan that expire or terminate without being exercised in full, or that are paid out in cash rather than in shares, will not reduce the number of shares available for issuance under our 2014 Plan. Additionally, shares issued pursuant to stock awards under our 2014 Plan that we repurchase or that are forfeited, as well as shares used to pay the exercise price of a stock award or to satisfy the tax withholding obligations related to a stock award, will become available for future grant under our 2014 Plan.
Plan Administration. Our board of directors, or a duly authorized committee of our board of directors, will administer our 2014 Plan. Our board of directors may also delegate to one or more of our officers the authority to (1) designate employees (other than officers) to receive specified stock awards, and (2) determine the number of shares subject to such stock awards. Subject to the terms of our 2014 Plan, the board of directors has the authority to determine the terms of awards, including recipients, the exercise, purchase or strike price of stock awards, if any, the number of shares subject to each stock award, the fair market value of a share of our common stock, the vesting schedule applicable to the awards, together with any vesting acceleration, and the form of consideration, if any, payable upon exercise or settlement of the award and the terms of the award agreements for use under our 2014 Plan.
Our board of directors has the power to modify outstanding awards under our 2014 Plan. Our board of directors has the authority to reprice any outstanding option or stock appreciation right, cancel any outstanding stock award in exchange for new stock awards, cash or other consideration, or take any other action that is treated as a repricing under generally accepted accounting principles, with the consent of any adversely affected participant.
Section 162(m) Limits. At such time as necessary for compliance with Section 162(m) of the Code, no participant may be granted stock awards covering more than 1,000,000 shares of our common stock under our 2014 Plan during any calendar year pursuant to stock options, stock appreciation rights and other stock awards whose value is determined by reference to an increase over an exercise price or strike price of at least 100% of the fair market value of our common stock on the date of grant. Additionally, no participant may be granted in a calendar year a performance stock award covering more than 1,000,000 shares of our common stock or a performance cash award having a maximum value in excess of $1,000,000 under our 2014 Plan. These limitations are designed to allow us to grant compensation that will not be subject to the $1,000,000 annual limitation on the income tax deductibility of compensation paid per covered executive officer imposed by Section 162(m) of the Code.
Performance Awards. Our 2014 Plan permits the grant of performance-based stock and cash awards that may qualify as performance-based compensation that is not subject to the $1,000,000 limitation on the income tax deductibility of compensation paid per covered executive officer imposed by Section 162(m) of the Code. Our compensation committee can structure such awards so that the stock or cash will be issued or paid pursuant to such award only following the achievement of certain pre-established performance goals during a designated performance period.
Our compensation committee may establish performance goals by selecting from one or more of the following performance criteria: (1) earnings (including earnings per share and net earnings); (2) earnings before interest, taxes and depreciation; (3) earnings before interest, taxes, depreciation and amortization; (4) earnings before interest, taxes, depreciation, amortization and legal settlements; (5) earnings before interest, taxes, depreciation, amortization, legal settlements and other income (expense); (6) earnings before interest, taxes, depreciation, amortization, legal settlements, other income (expense) and stock-based compensation; (7) earnings before interest, taxes, depreciation, amortization, legal settlements, other income (expense), stock-based compensation and changes in deferred revenue; (8) total stockholder return; (9) return on equity or average stockholder’s equity; (10) return on assets, investment, or capital employed; (11) stock price; (12) margin (including gross margin); (13) income (before or after taxes); (14) operating income; (15) operating income after taxes; (16) pre-tax profit; (17) operating cash flow; (18) sales or revenue targets; (19) increases in revenue or product revenue; (20) expenses and cost reduction goals; (21) improvement in or attainment of working capital levels; (22) economic value added (or an equivalent metric); (23) market share; (24) cash flow; (25) cash flow per share; (26) share price performance; (27) debt reduction; (28) implementation or completion of projects or processes; (29) user satisfaction; (30) stockholders’ equity; (31) capital expenditures; (32) debt levels; (33) operating profit or net operating profit; (34) workforce diversity; (35) growth of net income or operating
101
Table of Contents
income; (36) billings; (37) bookings; (38) the number of users, including but not limited to unique users; (39) employee retention; and (40) to the extent that an Award is not intended to comply with Section 162(m) of the Code, other measures of performance selected by the Board.
Our compensation committee may establish performance goals on a company-wide basis, with respect to one or more business units, divisions, affiliates, or business segments, and in either absolute terms or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. Unless specified otherwise (1) in the award agreement at the time the award is granted or (2) in such other document setting forth the performance goals at the time the goals are established, our compensation committee will appropriately make adjustments in the method of calculating the attainment of the performance goals as follows: (a) to exclude restructuring or other nonrecurring charges; (b) to exclude exchange rate effects; (c) to exclude the effects of changes to generally accepted accounting principles; (d) to exclude the effects of any statutory adjustments to corporate tax rates; and (e) to exclude the effects of any “extraordinary items” as determined under generally accepted accounting principles; (f) to exclude the dilutive effects of acquisitions or joint ventures; (g) to assume that any business divested achieved performance objectives at targeted levels during the balance of a Performance Period following such divestiture; (h) to exclude the effect of any change in the outstanding shares of our common stock by reason of any stock dividend or split, stock repurchase, reorganization, recapitalization, merger, consolidation, spin-off, combination or exchange of shares or other similar corporate change, or any distributions to common stockholders other than regular cash dividends; (i) to exclude the effects of stock based compensation and the award of bonuses under our bonus plans; (j) to exclude costs incurred in connection with potential acquisitions or divestitures that are required to be expensed under generally accepted accounting principles; (k) to exclude the goodwill and intangible asset impairment charges that are required to be recorded under generally accepted accounting principles; and (l) to exclude the effect of any other unusual, non-recurring gain or loss or other extraordinary item.
Corporate Transactions. Our 2014 Plan provides that in the event of certain specified significant corporate transactions, as defined under our 2014 Plan, each outstanding award will be treated as the administrator determines. The administrator may (1) arrange for the assumption, continuation or substitution of a stock award by a successor corporation; (2) arrange for the assignment of any reacquisition or repurchase rights held by us to a successor corporation; (3) accelerate the vesting, in whole or in part, of the stock award and provide for its termination prior to the transaction; (4) arrange for the lapse, in whole or in part, of any reacquisition or repurchase rights held by us; or (5) cancel or arrange for the cancellation of the stock award prior to the transaction in exchange for a cash payment, if any, determined by the board. The plan administrator is not obligated to treat all stock awards or portions of stock awards, even those that are of the same type, in the same manner.
Transferability. A participant may not transfer stock awards under our 2014 Plan other than by will, the laws of descent and distribution or as otherwise provided under our 2014 Plan.
Plan Amendment or Termination. Our board of directors has the authority to amend, suspend, or terminate our 2014 Plan, provided that such action does not materially impair the existing rights of any participant without such participant’s written consent. No ISOs may be granted after the tenth anniversary of the date our board of directors adopted our 2014 Plan.
2014 Employee Stock Purchase Plan
Our board of directors adopted our 2014 Employee Stock Purchase Plan, or the ESPP, in July 2014, and our stockholders subsequently approved the ESPP in July, 2014. The ESPP will become effective immediately upon the execution and delivery of the underwriting agreement related to this offering. The purpose of the ESPP is to secure the services of new employees, to retain the services of existing employees and to provide incentives for such individuals to exert maximum efforts toward our success and that of our affiliates. The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Code. The maximum aggregate number of shares of our common stock that may be issued under our ESPP is shares.
Authorized Shares. The maximum aggregate number of shares of our common stock that may be issued under our ESPP is 496,092 shares. Additionally, the number of shares of our common stock reserved for issuance under our ESPP will increase automatically each year, beginning on January 1, 2015, and continuing through and including
102
Table of Contents
January 1, 2024, by the lesser of (1) 1% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year; or (2) such lesser number as determined by our board of directors. Shares subject to purchase rights granted under our ESPP that terminate without having been exercised in full will not reduce the number of shares available for issuance under our ESPP.
Administration. Our board of directors, or a duly authorized committee thereof, will administer our ESPP. Our board of directors has delegated concurrent authority to administer our ESPP to our compensation committee under the terms of the compensation committee’s charter. The ESPP is implemented through a series of offerings under which eligible employees are granted purchase rights to purchase shares of our common stock on specified dates during such offerings. Under the ESPP, we may specify offerings with durations of not more than 27 months, and may specify shorter purchase periods within each offering. Each offering will have one or more purchase dates on which shares of our common stock will be purchased for employees participating in the offering. An offering under the ESPP may be terminated under certain circumstances.
Payroll Deductions. Generally, all regular employees, including executive officers, employed by us or by any of our designated affiliates, may participate in the ESPP and may contribute, normally through payroll deductions, up to 15% of their earnings (as defined in the ESPP) for the purchase of our common stock under the ESPP. Unless otherwise determined by our board of directors, common stock will be purchased for the accounts of employees participating in the ESPP at a price per share equal to the lower of (a) 85% of the fair market value of a share of our common stock on the first date of an offering or (b) 85% of the fair market value of a share of our common stock on the date of purchase. For the initial offering, which we expect will commence upon the execution and delivery of the underwriting agreement relating to this offering, the fair market value on the first day of the initial offering will be the price at which shares are first sold to the public.
Limitations. Our employees, including executive officers, or any of our designated affiliates may have to satisfy one or more of the following service requirements before participating in our ESPP, as determined by the administrator: (1) customary employment with us or one of our affiliates for more than 20 hours per week and more than five months per calendar year, or (2) continuous employment with us or one of our affiliates for a minimum period of time, not to exceed two years, prior to the first date of an offering. An employee may not be granted rights to purchase stock under our ESPP if such employee (1) immediately after the grant would own stock possessing 5% or more of the total combined voting power or value of our common stock, or (2) holds rights to purchase stock under our ESPP that would accrue at a rate that exceeds $25,000 worth of our stock for each calendar year that the rights remain outstanding.
Changes to Capital Structure. In the event that there occurs a change in our capital structure through such actions as a stock split, merger, consolidation, reorganization, recapitalization, reincorporation, stock dividend, dividend in property other than cash, large nonrecurring cash dividend, liquidating dividend, combination of shares, exchange of shares, change in corporate structure or similar transaction, the board of directors will make appropriate adjustments to (1) the number of shares reserved under the ESPP, (2) the maximum number of shares by which the share reserve may increase automatically each year, (3) the number of shares and purchase price of all outstanding purchase rights and (4) the number of shares that are subject to purchase limits under ongoing offerings.
Corporate Transactions. In the event of certain significant corporate transactions, as defined in the ESPP, any then-outstanding rights to purchase our stock under the ESPP may be assumed, continued or substituted for by any surviving or acquiring entity (or its parent company). If the surviving or acquiring entity (or its parent company) elects not to assume, continue or substitute for such purchase rights, then the participants’ accumulated payroll contributions will be used to purchase shares of our common stock within 10 business days prior to such corporate transaction, and such purchase rights will terminate immediately.
ESPP Amendments, Termination. Our board of directors has the authority to amend or terminate our ESPP, provided that except in certain circumstances such amendment or termination may not materially impair any outstanding purchase rights without the holder’s consent. We will obtain stockholder approval of any amendment to our ESPP as required by applicable law or listing requirements.
103
Table of Contents
2013 Equity Incentive Plan
Our board of directors adopted and our stockholders approved our 2013 Equity Incentive Plan, or the 2013 Plan, in September 2013. The 2013 Plan provides for the discretionary grant of ISOs, nonstatutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, and other stock awards to our employees, directors and consultants or our affiliates. ISOs may be granted only to our employees or employees of our affiliates.
The 2013 Plan will be terminated on the date the 2014 Plan becomes effective. However, any outstanding options granted under the 2014 Plan will remain outstanding, subject to the terms of our 2013 Plan and stock option agreements, until such outstanding options are exercised or until they terminate or expire by their terms.
Authorized Shares. The maximum number of shares of our common stock that may be issued under our 2013 Plan is 596,817, plus any shares subject to stock options or other stock awards granted under the 2003 Equity Incentive Plan, described below, that would have otherwise returned to our 2003 Plan (such as upon the expiration or termination of a stock award prior to vesting). The maximum number of shares that may be issued upon the exercise of ISOs under our 2013 Plan is two (2) times the 2013 Plan share reserve.
Plan Administration. Our board of directors or a duly authorized committee of our board of directors administers our 2013 Plan and the stock awards granted under it. Our board of directors may also delegate to one or more of our officers the authority to (1) designate officers and employees to receive specified stock awards and (2) determine the number of shares subject to such stock awards. Subject to the terms of our 2013 Plan, the board of directors has the authority to determine and amend the terms of awards, including recipients, the exercise, purchase or strike price of stock awards, if any, the number of shares subject to each stock award, the fair market value of a share of our common stock, the vesting schedule applicable to the awards, together with any vesting acceleration, and the form of consideration, if any, payable upon exercise or settlement of the award and the terms of the award agreements for use under our 2013 Plan.
Our board of directors has the power to modify outstanding awards under our 2013 Plan. Our board of directors has the authority to reprice any outstanding option or stock appreciation right, cancel any outstanding stock award in exchange for new stock awards, cash or other consideration, or take any other action that is treated as a repricing under generally accepted accounting principles, with the consent of any adversely affected participant.
Corporate Transactions. Our 2013 Plan provides that in the event of certain specified significant corporate transactions, as defined under our 2013 Plan, each outstanding award will be treated as the administrator determines. The administrator may (1) arrange for the assumption, continuation or substitution of a stock award by a successor corporation, or the acquiring corporation’s parent company; (2) arrange for the assignment of any reacquisition or repurchase rights held by us to a successor corporation, or the acquiring corporation’s parent company; (3) accelerate the vesting, in whole or in part, of the stock award and provide for its termination prior to the transaction; (4) arrange for the lapse, in whole or in part, of any reacquisition or repurchase rights held by us; (5) cancel or arrange for the cancellation of the stock award prior to the transaction in exchange for a cash payment, if any, determined by the board; or (6) make a payment in such form as determined by the board of directors equal to the excess of the value of the property that would have been received and the exercise price. The plan administrator is not obligated to treat all stock awards or portions of stock awards, even those that are of the same type, in the same manner.
Transferability. A participant may not transfer stock awards under our 2013 Plan other than by will, the laws of descent and distribution, or as otherwise provided under our 2013 Plan.
Plan Amendment or Termination. Our board of directors has the authority to amend, suspend or terminate our 2013 Plan, provided that such action is approved by our stockholders to the extent stockholder approval is necessary and that such action does not impair the existing rights of any participant without such participant’s written consent. As described above, our 2013 Plan will be terminated upon the effective date of this offering and no future stock awards will be granted thereunder.
104
Table of Contents
2003 Equity Incentive Plan
Our board of directors adopted and our stockholders approved our 2003 Equity Incentive, or the 2003 Plan, in October 2003. The 2003 Plan was last amended in February 2013. The 2003 Plan provides for the discretionary grant of ISOs, nonstatutory stock options, stock bonuses, and rights to acquire restricted stock to our employees, directors and consultants or our affiliates. ISOs may be granted only to our employees or employees of our affiliates.
The 2003 Plan was terminated on the date the 2013 Plan became effective. However, any outstanding options granted under the 2003 Plan remain outstanding, subject to the terms of our 2003 Plan and stock option agreements, until such outstanding options are exercised or until they terminate or expire by their terms.
Authorized Shares. The maximum number of shares of our common stock that may be issued under our 2003 Plan is 3,774,005.
Plan Administration. Our board of directors or a duly authorized committee of our board of directors administers our 2003 Plan and the stock awards granted under it. Our board of directors has the power to modify outstanding awards under our 2003 Plan, with the consent of any adversely affected participant.
Corporate Transactions. Our 2003 Plan provides that in the event of certain specified significant corporate transactions, as defined under our 2003 Plan, the surviving or acquiring corporation may assume, continue or substitute similar stock awards for outstanding stock awards under the 2003 Plan and any reacquisition or repurchase rights held by us may be assigned to the surviving or acquiring corporation. In the event that outstanding stock awards are not so assumed, continued or substituted, the vesting and, if applicable, exercisability of any such stock awards held by participants whose continuous service has not terminated prior to the effective time of the corporate transaction will be accelerated in full to a date prior to the effective time of such corporate transaction, and such stock awards will terminate if not exercised (if applicable) at or prior to the effective time of such corporation transaction, and any reacquisition or repurchase rights held by us will lapse, contingent upon the effectiveness of such corporate transaction. With respect to any other outstanding stock awards that are not so assumed, continued or substituted, the vesting and, if applicable, exercisability of any such stock awards will not be accelerated, unless otherwise provided in a written agreement between us and the participant, and all such stock awards will terminate if not exercised (if applicable) prior to the effective time of the corporate transaction.
Transferability. A participant may not transfer stock awards under our 2003 Plan other than by will, the laws of descent and distribution, or as otherwise provided under our 2003 Plan.
Plan Amendment or Termination. Our board of directors has the authority to amend, suspend or terminate our 2003 Plan, provided that such action does not impair the existing rights of any participant without such participant’s written consent. As described above, our 2003 Plan terminated upon the effective date of the 2013 Plan.
105
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a description of transactions since January 1, 2011, to which we have been a party, in which the amount involved exceeds $120,000, and in which any of our directors, executive officers or beneficial holders of more than 5% of our common stock, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest.
Each agreement described below is filed as an exhibit to the registration statement of which this prospectus forms a part, and the following descriptions are qualified by reference to such agreements.
Compensation arrangements for our directors and named executive officers are described in this prospectus under the section entitled “Executive Compensation.”
Sale of Series C Convertible Preferred Stock
In August 2011, we completed the closing of the sale of an aggregate of 2,655,698 shares of our Series C convertible preferred stock at a purchase price of $5.84 per share for an aggregate purchase price of $15,509,288. Our Series C convertible preferred stock will convert on a one-for-one basis into common stock immediately prior to the closing of this offering. The following table summarizes purchases of shares of our Series C convertible preferred stock by our executive officers, directors and holders of more than 5% of our capital stock.
| Name |
Series C Convertible Preferred Stock |
Aggregate Purchase Price |
||||||
| U.S. Venture Partners IX, L.P.(1) |
646,108 | $ | 3,773,272 | |||||
| KPCB Holdings, Inc., as nominee(2) |
646,108 | 3,773,272 | ||||||
| PTV Sciences II, L.P.(3) |
599,315 | 3,500,000 | ||||||
| Medtronic, Inc.(4) |
505,137 | 2,950,000 | ||||||
| Entities affiliated with DAG Ventures(5) |
135,363 | 790,516 | ||||||
| Frederic H. Moll(6) |
42,808 | 249,999 | ||||||
| (1) | Mr. Tansey, a member of our board of directors, is affiliated with U.S. Venture Partners IX, L.P. |
| (2) | Mr. Mead, a member of our board of directors, is affiliated with KPCB Holdings, Inc., as nominee. |
| (3) | Mr. Anderson, a member of our board of directors, is affiliated with PTV Sciences II, L.P. |
| (4) | Mr. Fletcher, a member of our board of directors, is affiliated with Medtronic, Inc. |
| (5) | Includes (a) 123,613 shares held by DAG Ventures III-QP, (b) 11,627 shares held by DAG Ventures III, L.P. and (c) 121 shares held by DAG Ventures GP Fund III, LLC |
| (6) | Dr. Moll is a member of our board of directors. |
106
Table of Contents
Sale of Series D Convertible Preferred Stock
In February and March 2013, we completed the initial closings of the sale of an aggregate of 3,026,765 shares of our Series D convertible preferred stock at a purchase price of $6.89 per share for an aggregate purchase price of $20,860,554, or the initial closings. In October 2013, we completed the second closing of the sale of an aggregate of 1,369,008 shares of our Series D convertible preferred stock at a purchase price of $6.89 per share for an aggregate purchase price of $9,435,213, or the second closing. Our Series D convertible preferred stock will convert on a one-for-one basis into common stock immediately prior to the closing of this offering. The following table summarizes purchases of shares of our Series D convertible preferred stock by our executive officers, directors and holders of more than 5% of our capital stock in each of the initial closings and the second closing.
| Name |
Initial Closings Shares of Series D Convertible Preferred Stock |
Initial Closings Aggregate Purchase Price |
Second Closing Shares of Series D Convertible Preferred Stock |
Second Closing Aggregate Purchase Price |
||||||||||||
| Norwest Venture Partners XI, LP(1) |
1,538,015 | $ | 10,599,999 | 710,969 | $ | 4,900,000 | ||||||||||
| U.S. Venture Partners IX, L.P.(2) |
509,375 | 3,510,613 | 236,679 | 1,631,195 | ||||||||||||
| PTV Sciences II, L.P.(3) |
276,047 | 1,902,518 | 128,264 | 883,999 | ||||||||||||
| KPCB Holdings, Inc., as nominee(4) |
217,943 | 1,502,068 | 101,267 | 697,930 | ||||||||||||
| Medtronic, Inc.(5) |
128,360 | 884,661 | 59,642 | 411,054 | ||||||||||||
| Entities affiliated with DAG Ventures(6) |
85,961 | 592,445 | 39,942 | 275,280 | ||||||||||||
| Frederic H. Moll(7) |
17,558 | 121,012 | 8,158 | 56,228 | ||||||||||||
| Monika A. De Martini(8) |
16,299 | 112,338 | — | — | ||||||||||||
| Robert H. Binney, Jr.(9) |
4,352 | 29,999 | — | — | ||||||||||||
| Richard E. Kaufman(10) |
16,299 | 112,338 | — | — | ||||||||||||
| Amy Wolbeck(11) |
2,901 | 19,999 | — | — | ||||||||||||
| (1) | Mr. de Clercq, a member of our board of directors, is affiliated with Norwest Venture Partners XI, LP. |
| (2) | Mr. Tansey, a member of our board of directors, is affiliated with U.S. Venture Partners IX, L.P. |
| (3) | Mr. Anderson, a member of our board of directors, is affiliated with PTV Sciences II, L.P. |
| (4) | Mr. Mead, a member of our board of directors, is affiliated with KPCB Holdings, Inc., as nominee. |
| (5) | Mr. Fletcher, a member of our board of directors, is affiliated with Medtronic, Inc. |
| (6) | Includes (a) 114,975 shares held by DAG Ventures III-QP, (b) 10,815 shares held by DAG Ventures III, L.P. and (c) 113 shares held by DAG Ventures GP Fund III, LLC. |
| (7) | Dr. Moll is a member of our board of directors. |
| (8) | Shares held in the David P. and Monika A. De Martini Living Trust dated 11/16/2004. Ms. De Martini is our former Chief Financial Officer. |
| (9) | Mr. Binney is our Vice President, Sales. |
| (10) | Mr. Kaufman is our Senior Vice President and Chief Operating Officer. |
| (11) | Ms. Wolbeck is our Vice President, Regulatory Affairs and Quality. |
Employee Loans
In May 2013, we loaned Lisa Earnhardt, our President and Chief Executive Officer, $499,854 in connection with her exercise of options to purchase 586,575 shares of our common stock, or the Exercise Shares. The loan was evidenced by full recourse promissory notes, accrued interest on the outstanding principal amount at the rate of 1% per annum and was secured by a pledge of the Exercise Shares. The promissory notes provided that the loan would be forgiven at a rate of one-fourth of the original principal amount and all accrued and unpaid interest on each anniversary of the loan. The entire principal amount and all accrued and unpaid interest of the loan was forgiven in full in June 2014.
In February 2007, we loaned Richard E. Kaufman, our Senior Vice President and Chief Operating Officer, $286,050. In February 2011, our board of directors approved the forgiveness of Mr. Kaufman’s loan over a
107
Table of Contents
period of three years, provided that he remained employed with us and in good standing. The loan to Mr. Kaufman was forgiven in full in January 2014.
Indemnification Agreements
Our amended and restated certificate of incorporation, which will be effective upon the closing of this offering, will contain provisions limiting the liability of directors, and our amended and restated bylaws will provide that we will indemnify each of our directors to the fullest extent permitted under Delaware law. Our amended and restated certificate of incorporation and amended and restated bylaws will also provide our board of directors with discretion to indemnify our officers and employees when determined appropriate by the board. In addition, we have entered and expect to continue to enter into agreements to indemnify our directors and executive officers. For more information regarding these agreements, see the section of this prospectus entitled “Executive Compensation—Limitation on Liability and Indemnification.”
Investor Rights Agreement
We are party to an investor rights agreement that provides holders of our convertible preferred stock, including certain holders of 5% of our capital stock and entities affiliated with certain of our directors, with certain registration rights, including the right to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing. The investor rights agreement also provides for a right of first refusal in favor of certain holders of our stock with regard to certain issuances of our capital stock. The rights of first refusal will not apply to, and will terminate upon, closing of this offering. For a more detailed description of these registration rights, see the section of this prospectus entitled “Description of Capital Stock—Registration Rights.”
Voting Agreement
We are party to a voting agreement under which certain holders of our capital stock, including certain holders of 5% of our capital stock and entities affiliated with certain of our directors, have agreed to vote in a certain way on certain matters, including with respect to the election of directors. Pursuant to the voting agreement, each of U.S. Venture Partners IX, L.P., Kleiner Perkins Caufield & Byers, PTV Sciences II, L.P., Medtronic, Inc. and Norwest Venture Partners XI, LP had the right to designate one member of our board of directors. Casey M. Tansey, Dana G. Mead, Jr., Rick D. Anderson, Mark Fletcher and Casper L. de Clercq were designated by U.S. Venture Partners IX, L.P., Kleiner Perkins Caufield & Byers, PTV Sciences II, L.P., Medtronic, Inc. and Norwest Venture Partners XI, LP, respectively, under the voting agreement. The voting agreement and rights regarding the election or designation of members of our board of directors will terminate upon the closing of this offering and none of our stockholders will have any special rights regarding the election or designation of members of our board of directors.
Right of First Refusal and Co-Sale Agreement
We are party to a right of first refusal and co-sale agreement with holders of our convertible preferred stock and our founders, including certain holders of 5% of our capital stock and entities affiliated with certain of our directors, pursuant to which the holders of convertible preferred stock have a right of first refusal and co-sale in respect of certain sales of securities by our founders. The right of first refusal and co-sale agreement will terminate upon the closing of this offering.
Employment Arrangements
We have extended offer letters to our executive officers in connection with their employment as described in greater detail in the section of this prospectus titled “Executive Compensation—Offer Letters and Bonus Plan.”
Policies and Procedures for Related Party Transactions
Our board of directors has adopted a written related-person transaction policy setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy covers, with
108
Table of Contents
certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar or related transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person.
In addition, under our code of conduct, our employees and directors have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest to our legal department, or, if the employee is an executive officer, to our board of directors.
In considering related-person transactions, our audit committee (or other independent body of our board of directors) will take into account the relevant available facts and circumstances including, but not limited to, the risks, costs and benefits to us, the terms of the transaction, the availability of other sources for comparable services or products and, if applicable the impact on a director’s independence in the event that the related person is a director, immediate family member of a director or an entity with which a director is affiliated.
109
Table of Contents
The following table presents information as to the beneficial ownership of our common stock as of March 31, 2014, for:
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock; |
| • | each named executive officer; |
| • | each of our directors; and |
| • | all executive officers and directors as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Unless otherwise indicated below, to our knowledge, the persons and entities named in the table have sole voting and sole investment power with respect to all shares beneficially owned, subject to community property laws where applicable. Common stock subject to options that are currently exercisable or exercisable within 60 days of March 31, 2014, are deemed to be outstanding and to be beneficially owned by the person holding the options for the purpose of computing the percentage ownership of that person, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
Percentage ownership of our common stock “prior to this offering” in the table is based on 17,501,808 shares of common stock issued and outstanding as of March 31, 2014, adjusted for the automatic conversion of all outstanding shares of convertible preferred stock into shares of common stock upon the completion of this offering. Percentage ownership of our common stock “after this offering” in the table is based on 22,501,808 shares of common stock issued and outstanding on March 31, 2014, which gives effect to the issuance of 5,000,000 shares of common stock in this offering and assumes no exercise of the underwriters’ option to purchase additional shares. Unless otherwise indicated, the address of each of the individuals and entities named below is c/o Intersect ENT, Inc., 1555 Adams Drive, Menlo Park, California 94025.
| Percentage of Shares Beneficially Owned |
||||||||||||||||||||
| Name and Address of Shares Beneficially Owned |
Shares Beneficially Owned(1) |
Shares Exercisable Within 60 days |
Total
Shares Beneficially Owned |
Before the Offering |
After the Offering |
|||||||||||||||
| 5% Stockholders: |
||||||||||||||||||||
| U.S. Venture Partners IX, L.P.(2) 2735 Sand Hill Road Menlo Park, CA 94025 |
3,975,433 | — | 3,975,433 | 22.7 | % | 17.7 | % | |||||||||||||
| KPCB Holdings, Inc., as nominee(3) 2750 Sand Hill Road Menlo Park, CA 94025 |
3,548,589 | — | 3,548,589 | 20.3 | % | 15.8 | % | |||||||||||||
| PTV Sciences II, L.P.(4) 3600 N. Capital of Texas Highway Building B, Suite 180 Austin, TX 78746 |
2,576,966 | — | 2,576,966 | 14.7 | % | 11.5 | % | |||||||||||||
| Entities affiliated with Norwest Venture Partners(5) 525 University Ave, Suite 800 Palo Alto, CA 94301 |
2,248,984 | — | 2,248,984 | 12.9 | % | 10.0 | % | |||||||||||||
| Medtronic, Inc.(6) 710 Medtronic Parkway Mail Stop: L100 Minneapolis, MN 55432 |
1,198,276 | — | 1,198,276 | 6.8 | % | 5.3 | % | |||||||||||||
110
Table of Contents
| Outstanding Shares Beneficially Owned(1) |
Shares Exercisable Within 60 days |
Total
Shares Beneficially Owned |
Percentage of Shares Beneficially Owned |
|||||||||||||||||
| Name and Address of Shares Beneficially Owned |
Before the Offering |
After the Offering |
||||||||||||||||||
| Directors and Named Executive Officers: |
||||||||||||||||||||
| Rick D. Anderson(4) |
2,576,966 | — | 2,576,966 | 14.7 | % | 11.5 | % | |||||||||||||
| Casper L. de Clercq(5) |
2,248,984 | — | 2,248,984 | 12.8 | % | 10.0 | % | |||||||||||||
| Mark Fletcher(6) |
1,198,276 | — | 1,198,276 | 6.8 | % | 5.3 | % | |||||||||||||
| Cynthia L. Lucchese |
— | — | — | * | * | |||||||||||||||
| Dana G. Mead, Jr.(3) |
3,548,589 | — | 3,548,589 | 20.3 | % | 15.8 | % | |||||||||||||
| Frederic H. Moll, MD |
276,178 | 37,083 | 313,261 | 1.8 | % | 1.4 | % | |||||||||||||
| Casey M. Tansey(2) |
3,975,433 | — | 3,975,433 | 22.7 | % | 17.7 | % | |||||||||||||
| Lisa D. Earnhardt(7) |
751,050 | 233,749 | 984,799 | 5.6 | % | 4.3 | % | |||||||||||||
| Richard E. Kaufman(8) |
224,189 | 28,500 | 252,689 | 1.4 | % | 1.1 | % | |||||||||||||
| Robert H. Binney, Jr. |
4,352 | 157,500 | 161,852 | * | * | % | ||||||||||||||
| All directors and named executive officers as a group (13 persons)(9) |
14,834,732 | 941,803 | 15,776,535 | 85.5 | % | 67.3 | % | |||||||||||||
| * | Represents beneficial ownership of less than one percent of the outstanding common stock, adjusted for the automatic conversion of all outstanding shares of convertible preferred stock into 15,703,875 shares of common stock upon the completion of this offering. |
| (1) | Represents shares of common stock held by such individuals. Includes shares held in the beneficial owner’s name or jointly with others, or in the name of a bank, nominee or trustee for the beneficial owner’s account. |
| (2) | Presidio Management Group IX, L.L.C., or PMG IX, is the general partner of U.S. Venture Partners IX, L.P., or USVP IX, and may be deemed to have sole voting and dispositive power over the shares held by USVP IX. Casey M. Tansey, one of our directors, and each of Irwin Federman, Steven M. Krausz, David E. Liddle, Paul A. Matteucci, Jonathan D. Root, and Philip M. Young are managing members of PMG IX, and may be deemed to share voting and dispositive power over the shares held by USVP IX. Such persons and entities disclaim beneficial ownership of shares held by USVP IX, except to the extent of any proportionate pecuniary interest therein. |
| (3) | Shares beneficially owned consist of (a) 3,112,232 shares held by Kleiner Perkins Caufield & Byers XI-A, L.P., or KPCB XI-A, (b) 72,272 shares held by Kleiner Perkins Caufield & Byers XI-B, L.P., or KPCB XI-B, and (c) 364,085 shares held by individuals and entities associated with Kleiner Perkins Caufield & Byers. All shares are held for convenience in the name of “KPCB Holdings, Inc. as nominee,” for the accounts of such individuals and entities who each exercise their own voting and dispositive power over such shares. The general partner of KPCB XI-A and KPCB XI-B is KPCB XI Associates, LLC. Brook H. Byers, L. John Doerr, Raymond J. Lane and Theodore E. Schlein, the managers of KPCB XI Associates, exercise shared voting and dispositive power over the shares directly held by KPCB XI-A and KPCB XI-B. |
| (4) | Pinto Technology Ventures GP II, L.P., or PTV GP II, is the general partner of PTV Sciences II, L.P., or PTVS II. Pinto TV GP Company LLC, or TV GP, is the general partner of PTV GP II. Each of Matthew Crawford and Rick Anderson, one of our directors, is a manager of TV GP and may be deemed to have beneficial ownership of the shares held by PTVS II. Such persons and entities disclaim beneficial ownership of shares held by PTVS II, except to the extent of any proportionate pecuniary interest therein. |
| (5) | Shares beneficially owned reflect a transfer subsequent to March 31, 2014 and consist of (a) 1,124,492 shares held by Norwest Venture Partners XI, L.P., or NVP XI, and (b) 1,124,492 shares held by Norwest Venture Partners XII, L.P., or NVP XII. Genesis VC Partners XI, LLC, or Genesis XI, is the general partner of NVP XI and may be deemed to have sole voting and dispositive power over the shares held by NVP XI. Genesis VC Partners XII, LLC, or Genesis XII, is the general partner of NVP XII and may be deemed to have sole voting and dispositive power over the shares held by NVP XII. NVP Associates, LLC, and each of Promod Haque, Jeffrey Crowe and Matthew Howard, the managing members of Genesis XI and Genesis XII may be deemed to share voting and dispositive power over the shares held by NVP XI and NVP XII. Such persons and entities disclaim beneficial ownership of shares held by NVP XI and NVP XII, except to the extent of any proportionate pecuniary interest therein. |
| (6) | Shares beneficially owned consist of shares held by Medtronic, Inc. Mr. Fletcher, President of Surgical Technologies of Medtronic, Inc. may be deemed to share voting and investment power with respect to the shares held by Medtronic. Mr. Fletcher disclaims beneficial ownership of these shares except to the extent of his pecuniary interest therein. |
| (7) | Shares beneficially owned reflect a stock transfer subsequent to March 31, 2014 and consist of (a) 724,868 shares of common stock held directly by Ms. Earnhardt of which 148,724 shares may be repurchased by us at the original exercise price within 60 days of March 31, 2014, and (b) 26,182 shares of common stock held by Ms. Earnhardt as custodian for her son. |
111
Table of Contents
| (8) | Shares beneficially owned consist of (a) 207,890 shares of common stock of which 24,557 shares may be repurchased by us at the original exercise price within 60 days of March 31, 2014, and (b) 16,299 shares of convertible preferred stock. |
| (9) | Consists of (a) 1,286,484 shares held by the current directors and executive officers, of which 173,281 shares may be repurchased by us at the original exercise price within 60 days of March 31, 2014, and (b) 13,548,248 shares held by entities affiliated with certain of our directors. |
112
Table of Contents
Capital Structure
The following description of our capital stock and certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws are summaries and are qualified by reference to the amended and restated certificate of incorporation and the amended and restated bylaws that will be in effect upon the closing of this offering. Copies of these documents will be filed with the SEC as exhibits to our registration statement, of which this prospectus forms a part. The descriptions of our common stock and convertible preferred stock reflect changes to our capital structure that will occur upon the closing of this offering.
General
Upon the closing of this offering, our authorized capital stock will consist of 160,000,000 shares, all with a par value of $0.001 per share, of which:
| • | 150,000,000 shares are designated as common stock; and |
| • | 10,000,000 shares are designated as preferred stock. |
Common stock
As of March 31, 2014, after giving effect to the conversion of all outstanding shares of convertible preferred stock into 15,703,875 shares of common stock immediately prior to the closing of this offering, we had outstanding 17,501,808 shares of common stock held of record by 90 stockholders.
Voting Rights. Each holder of our common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of stockholders, except as otherwise expressly provided in our amended and restated certificate of incorporation or required by applicable law. Cumulative voting for the election of directors is not provided for in our amended and restated certificate of incorporation, which means that the holders of a majority of our shares of common stock can elect all of the directors then standing for election.
Dividends and Distributions. Subject to preferences that may apply to any shares of convertible preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends out of funds legally available at the times and in the amounts that our board of directors may determine.
Liquidation Rights. Upon our liquidation, dissolution or winding-up, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our common stock and any participating convertible preferred stock outstanding at that time after payment of liquidation preferences, if any, on any outstanding shares of convertible preferred stock and payment of other claims of creditors.
The rights, preferences, and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of holders of shares of any series of convertible preferred stock that we may designate and issue in the future.
Preemptive or Similar Rights. Our common stock is not entitled to preemptive rights and is not subject to conversion, redemption or sinking fund provisions.
Convertible Preferred Stock
As of March 31, 2014, there were 15,703,875 shares of our convertible preferred stock outstanding. Immediately prior to the closing of this offering, all outstanding shares of our convertible preferred stock will convert into 15,703,875 shares of our common stock.
Upon the closing of this offering, our board of directors may, without further action by our stockholders, fix the rights, preferences, privileges and restrictions of up to an aggregate of 10,000,000 shares of preferred stock in one or more series and authorize their issuance. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of such series, any or all of which may be greater than
113
Table of Contents
the rights of our common stock. The issuance of our preferred stock could adversely affect the voting power of holders of our common stock and the likelihood that these holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change of control or other corporate action. Upon the closing of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Warrants
As of March 31, 2014, we had warrants to purchase an aggregate of 47,554 shares of our Series A convertible preferred stock outstanding with an exercise price of $3.68 per share. Unless earlier exercised, these warrants will expire in November 2017. Upon the closing of this offering, these warrants will become exercisable for 47,554 shares of our common stock with an exercise price of $3.68 per share.
As of March 31, 2014, we had a warrant to purchase an aggregate of up to 20,313 shares of our Series D convertible preferred stock outstanding with an exercise price of $6.89 per share. As of March 31, 2014, the warrant was exercisable for 5,803 shares of our Series D convertible preferred stock and may become exercisable for additional shares if we make draw downs under our credit facility. Unless earlier exercised, these warrants will expire in August 2023. Upon the closing of this offering, these warrants will become exercisable for up to 20,313 shares of our common stock with an exercise price of $6.89 per share.
Registration Rights
We are party to an investor rights agreement that provides that holders of our convertible preferred stock and certain holders of common stock that received the common stock upon conversion of convertible preferred stock have certain registration rights. This investor rights agreement was entered into in March 2006 and has been amended or amended and restated from time to time in connection with our convertible preferred stock financings. The registration of shares of our common stock pursuant to the exercise of registration rights described below would enable the holders who have these rights to sell these shares without restriction under the Securities Act when the applicable registration statement is declared effective. We will pay the registration expenses, other than underwriting discounts and commissions, of the shares registered pursuant to the demand, piggyback and Form S-3 registrations described below.
Generally, in an underwritten offering, the managing underwriter, if any, has the right, subject to specified conditions and limitations, to limit the number of shares the registration rights holders participating in any offering may include in any particular registration. The demand, piggyback and Form S-3 registration rights described below will expire on the earlier of (1) the date that is five years after the closing of this offering or (2) with respect to each stockholder following the closing of this offering, at such time as such stockholder can sell all of its shares pursuant to Rule 144 of the Securities Act during any three month period.
Demand registration rights. The holders of an aggregate of 15,771,742 shares of our common stock (including shares previously issued upon conversion of convertible preferred stock, shares issuable upon conversion of outstanding convertible preferred stock and shares issuable upon conversion of shares of convertible preferred stock issuable upon the exercise or, in certain cases, net exercise of outstanding warrants) are entitled to certain demand registration rights. At any time beginning six months after the closing of this offering, the holders of not less than 30% of these shares may, on not more than two occasions, request that we file a registration statement having an aggregate offering price to the public of not less than $10,000,000 to register at least 20% of their shares.
Piggyback registration rights. In connection with this offering, the holders of an aggregate of 15,771,742 shares of our common stock previously issued upon conversion of convertible preferred stock and common stock issuable upon conversion of outstanding convertible preferred stock and shares of convertible preferred stock issuable upon the exercise or, in certain cases, net exercise of outstanding warrants, were entitled to, and the necessary percentage of holders waived, rights to include their shares of registrable securities in this offering. In the event that we propose to register any of our securities under the Securities Act, either for our own account or for the account of other security holders, the holders of these shares will be entitled to certain “piggyback” registration rights allowing them to include their shares in such registration, subject to certain marketing and
114
Table of Contents
other limitations. As a result, whenever we propose to file a registration statement under the Securities Act other than with respect to a demand registration or a registration statement on Form S-3, S-4 or S-8, the holders of these shares are entitled to notice of the registration and have the right, subject to limitations that the underwriters may impose on the number of shares included in the registration, to include their shares in the registration.
Form S-3 registration rights. The holders of an aggregate of 15,771,742 shares of our common stock previously issued upon conversion of convertible preferred stock and common stock issuable upon conversion of outstanding convertible preferred stock and shares of convertible preferred stock issuable upon the exercise or, in certain cases, net exercise of outstanding warrants will be entitled to certain Form S-3 registration rights. Such holders may make a request that we register their shares on Form S-3 if we are qualified to file a registration statement on Form S-3.
Anti-Takeover Provisions
Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
Because our stockholders do not have cumulative voting rights, our stockholders holding a majority of the voting power of our shares of common stock outstanding will be able to elect all of our directors. Our amended and restated certificate of incorporation and amended and restated bylaws to be effective upon the closing of this offering will provide that all stockholder actions must be effected at a duly called meeting of stockholders and not by consent in writing. A special meeting of stockholders may be called only by a majority of our whole board of directors, the chair of our board of directors, or our chief executive officer.
Our amended and restated certificate of incorporation will further provide that, immediately after this offering, the affirmative vote of holders of at least sixty-six and two-thirds percent (66-2/3%) of the voting power of all of the then outstanding shares of voting stock, voting as a single class, will be required to amend certain provisions of our certificate of incorporation, including provisions relating to the size of the board, removal of directors, special meetings, actions by written consent and cumulative voting. The affirmative vote of holders of at least sixty-six and two-thirds percent (66-2/3%) of the voting power of all of the then outstanding shares of voting stock, voting as a single class, will be required to amend or repeal our bylaws, although our bylaws may be amended by a simple majority vote of our board of directors.
The foregoing provisions will make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of our company by replacing our board of directors. Since our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change the control of our company.
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of our company. These provisions are also designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage certain tactics that may be used in proxy rights. However, these provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of deterring hostile takeovers or delaying changes in control of our company or our management. As a consequence, these provisions also may inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts.
Our amended and restated certificate of incorporation provides that stockholder litigation alleging certain claims against us or our board of directors may only be brought in the courts located within the State of Delaware.
115
Table of Contents
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
| • | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| • | upon closing of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned by (1) persons who are directors and also officers and (2) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines business combination to include the following:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| • | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| • | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
| • | the receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Limitations on Liability and Indemnification
See the section of this prospectus titled “Executive Compensation—Limitation on Liability and Indemnification.”
Listing
We have applied to have our common stock listed on The Nasdaq Global Market under the trading symbol “XENT.”
Transfer Agent and Registrar
Upon the closing of this offering, the transfer agent and registrar for our common stock will be Computershare Trust Company, N.A.
116
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of our common stock in the public market, or the availability of such shares for sale in the public market, could adversely affect market prices prevailing from time to time. As described below, only a limited number of shares will be available for sale shortly after this offering due to contractual and legal restrictions on resale. Nevertheless, sales of our common stock in the public market after such restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price at such time and our ability to raise equity capital in the future.
Based on the number of shares outstanding as of March 31, 2014, and after giving effect to the conversion of our outstanding convertible preferred stock into an aggregate of 15,703,875 shares of common stock upon the completion of this offering, 22,501,808 shares of common stock will be outstanding, or 23,251,808 shares of common stock if the underwriters exercise their option to purchase additional shares in full. All of the 5,000,000 shares sold in this offering will be freely tradable unless purchased by our affiliates, as that term is defined in Rule 144 under the Securities Act. The remaining outstanding shares of our common stock will be “restricted securities” as that term is defined under Rule 144 of the Securities Act.
As a result of the lock-up agreements and the provisions of Rules 144 and 701 under the Securities Act, the shares of common stock that will be deemed restricted securities after this offering will be available for sale in the public market as follows:
| • | no shares will be available for sale until 180 days after the date of this prospectus, subject to certain limited exceptions provided for in the lock-up agreements; and |
| • | 17,501,808 shares will be eligible for sale beginning more than 180 days after the date of this prospectus, subject, in the case of shares held by our affiliates, to the volume limitations under Rule 144. |
Rule 144
In general, under Rule 144, beginning 90 days after the date of this prospectus, a person who has beneficially owned restricted shares for at least six months would be entitled to sell those securities provided that (1) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, a sale and (2) we have been subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale and are current in filing our periodic reports. Persons who have beneficially owned restricted shares of common stock for at least six months but who are our affiliates at the time of, or any time during the 90 days preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed 1% of the number of our common stock then outstanding, which will equal approximately 225,018 shares immediately after this offering, based on the number of shares of common stock outstanding as of March 31, 2014. Such sales by affiliates must also comply with the manner of sale and notice provisions of Rule 144 and to the availability of current public information about us.
Rule 701
Rule 701 under the Securities Act, as in effect on the date of this prospectus, permits resales of shares in reliance upon Rule 144 but without compliance with certain restrictions of Rule 144, including the holding period requirement. Most of our employees, executive officers or directors who purchased shares under a written compensatory plan or contract may be entitled to rely on the resale provisions of Rule 701, but all holders of Rule 701 shares are required to wait until 90 days after the date of this prospectus before selling their shares. However, substantially all Rule 701 shares are subject to lock-up agreements as described below and will become eligible for sale upon the expiration of the restrictions set forth in those agreements.
Lock-Up Agreements
We, our executive officers and directors and all of our stockholders have agreed, with certain limited exceptions, that for a period of 180 days from the date of this prospectus, we and they will not, without the prior
117
Table of Contents
written consent of J.P. Morgan Securities LLC and Piper Jaffray & Co., dispose of or hedge any shares or any securities convertible into or exchangeable for our common stock. J.P. Morgan Securities LLC and Piper Jaffray & Co. in their sole discretion may release any of the securities subject to these lock-up agreements at any time. These agreements are described in the section of this prospectus captioned “Underwriting.”
J.P. Morgan Securities LLC and Piper Jaffray & Co. have advised us that they have no present intent or arrangement to release any common stock subject to a lock-up, and will consider the release of any lock-up on a case-by-case basis. Upon a request to release any shares of common stock subject to a lock-up, J.P. Morgan Securities LLC and Piper Jaffray & Co. would consider the particular circumstances surrounding the request, including, but not limited to, the length of time before the lock-up expires, the number of shares of common stock requested to be released, the reasons for the request, the possible impact on the market for our common stock and whether the holder of our common stock requesting the release is an officer, director or other affiliate of ours.
118
Table of Contents
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSEQUENCES
TO NON-U.S. HOLDERS
The following is a discussion of the material U.S. federal income tax considerations applicable to non-U.S. holders (as defined below) with respect to their ownership and disposition of shares of our common stock issued pursuant to this offering, but does not purport to be a complete analysis of all potential tax effects. All prospective non-U.S. holders of our common stock should consult their own tax advisors with respect to the U.S. federal income tax consequences of the purchase, ownership and disposition of our common stock, as well as any consequences arising under the laws of any other taxing jurisdiction, including any state, local and non-U.S. tax consequences and any U.S. federal non-income tax consequences. In general, a non-U.S. holder means a beneficial owner of our common stock (other than a partnership or an entity or arrangement treated as a partnership for U.S. federal income tax purposes) that is not, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation, or an entity treated as a corporation for U.S. federal income tax purposes, created or organized in the United States or under the laws of the United States or of any state thereof or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| • | a trust if (1) a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons have the authority to control all of the trust’s substantial decisions or (2) the trust has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person. |
This discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code, existing U.S. Treasury Regulations promulgated thereunder, published administrative rulings and judicial decisions, all as in effect as of the date of this prospectus. These laws are subject to change and to differing interpretation, possibly with retroactive effect. Any change or differing interpretation could alter the tax consequences to non-U.S. holders described in this prospectus.
This discussion is limited to non-U.S. holders that hold shares of our common stock as a capital asset within the meaning of Section 1221 of the Code (generally, for investment). This discussion does not address all aspects of U.S. federal income taxation that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder’s individual circumstances, nor does it address any aspects of U.S. estate or gift tax, or any state, local or non-U.S. taxes. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax rules applicable to particular non-U.S. holders, such as holders that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below), corporations that accumulate earnings to avoid U.S. federal income tax, tax-exempt organizations, banks, financial institutions, insurance companies, brokers, dealers or traders in securities, commodities or currencies, tax-qualified retirement plans, holders subject to the alternative minimum tax or Medicare contribution tax, holders who hold or receive our common stock pursuant to the exercise of employee stock options or otherwise as compensation, holders holding our common stock as part of a hedge, straddle or other risk reduction strategy, conversion transaction or other integrated investment, holders deemed to sell our common stock under the constructive sale provisions of the Code, controlled foreign corporations, passive foreign investment companies, corporations that accumulate earnings to avoid U.S. federal income tax, and U.S. expatriates and certain former citizens or long-term residents of the United States.
In addition, this discussion does not address the tax treatment of partnerships (or entities or arrangements that are treated as partnerships for U.S. federal income tax purposes) or persons that hold their common stock through such partnerships or such entities or arrangements. If a partnership, including any entity or arrangement treated as a partnership for U.S. federal income tax purposes, holds shares of our common stock, the U.S. federal income tax treatment of a partner in such partnership will generally depend upon the status of the partner, the activities of the partnership and certain determinations made at the partner level. Such partners and partnerships should consult their own tax advisors regarding the tax consequences of the purchase, ownership and disposition of our common stock.
119
Table of Contents
There can be no assurance that the Internal Revenue Service, which we refer to as the IRS, will not challenge one or more of the tax consequences described herein, and we have not obtained, nor do we intend to obtain, a ruling with respect to the U.S. federal income tax consequences with respect to the matters discussed below.
Distributions on Our Common Stock
Distributions, if any, on our common stock generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder’s investment, up to such holder’s adjusted tax basis in the common stock. Any remaining excess will be treated as capital gain from the sale or exchange of such common stock, subject to the tax treatment described below in “Gain on Sale, Exchange or Other Disposition of Our Common Stock.”
Subject to the discussion below regarding backup withholding and foreign accounts, dividends paid to a non-U.S. holder will generally be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. A non-U.S. holder of our common stock who claims the benefit of an applicable income tax treaty generally will be required to provide a properly executed IRS Form W-8BEN (or successor form) and satisfy relevant certification and other requirements. Non-U.S. holders are urged to consult their tax advisors regarding their entitlement to benefits under a relevant income tax treaty.
Dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by the non-U.S. holder within the United States, are generally exempt from the 30% withholding tax if the non-U.S. holder satisfies applicable certification and disclosure requirements. To claim the exemption, the non-U.S. holder must furnish to us or our paying agent a valid IRS Form W-8ECI (or applicable successor form), certifying that the dividends are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States. However, such U.S. effectively connected income, net of specified deductions and credits, is taxed at the same graduated U.S. federal income tax rates applicable to U.S. persons (as defined in the Code), unless a specific treaty exemption applies. Any U.S. effectively connected income received by a non-U.S. holder that is a corporation may also, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
Gain on Sale, Exchange or Other Disposition of Our Common Stock
Subject to the discussion below regarding backup withholding and foreign accounts, in general, a non-U.S. holder will not be subject to any U.S. federal income tax on any gain realized upon such holder’s sale, exchange or other disposition of shares of our common stock unless:
| • | the gain is effectively connected with a U.S. trade or business of the non-U.S. holder and, if an applicable income tax treaty so provides, is attributable to a permanent establishment or a fixed base maintained in the United States by such non-U.S. holder, in which case the non-U.S. holder generally will be taxed at the graduated U.S. federal income tax rates applicable to U.S. persons (as defined in the Code) and, if the non-U.S. holder is a foreign corporation, the branch profits tax described above in “Distributions on Our Common Stock” may also apply; |
| • | the non-U.S. holder is a nonresident alien individual who is present in the United States for 183 days or more in the taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty) on the net gain derived from the disposition, which may be offset by U.S. source capital losses of the non-U.S. holder, if any (even though the individual is not considered a resident of |
120
Table of Contents
| the United States) provided the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses; or |
| • | our common stock constitutes a U.S. real property interest because we are, or have been, at any time during the five-year period preceding such disposition (or the non-U.S. holder’s holding period, if shorter) a “U.S. real property holding corporation.” Even if we are or become a U.S. real property holding corporation, provided that our common stock is regularly traded on an established securities market, our common stock will be treated as a U.S. real property interest only with respect to a non-U.S. holder that holds more than 5% of our outstanding common stock, directly or indirectly, actually or constructively, during the shorter of the 5-year period ending on the date of the disposition or the period that the non-U.S. holder held our common stock. In such case, such non-U.S. holder generally will be taxed on its net gain derived from the disposition at the graduated U.S. federal income tax rates applicable to U.S. persons (as defined in the Code). Generally, a corporation is a U.S. real property holding corporation only if the fair market value of its U.S. real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we do not believe that we are, or have been, a U.S. real property holding corporation, or that we are likely to become one in the future. No assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described above. |
Backup Withholding and Information Reporting
We must report annually to the IRS and to each non-U.S. holder the gross amount of the dividends on our common stock paid to such holder and the tax withheld, if any, with respect to such dividends. Non-U.S. holders will have to comply with specific certification procedures to establish that the holder is not a U.S. person (as defined in the Code) in order to avoid backup withholding at the applicable rate with respect to dividends on our common stock. U.S. backup withholding generally will not apply to a Non-U.S. holder who provides a properly executed IRS Form W-8BEN or otherwise establishes an exemption.
Information reporting and backup withholding will generally apply to the proceeds of a disposition of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder may be allowed as a credit against the non-U.S. holder’s U.S. federal income tax liability, if any, and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Accounts
The Code generally imposes a U.S. federal withholding tax of 30% on dividends and the gross proceeds of a disposition of our common stock paid to a “foreign financial institution” or a “non-financial foreign entity” (as specifically defined for this purpose), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the foreign financial institution is subject to the diligence and reporting
121
Table of Contents
requirements, such institution must enter into an agreement with the U.S. government to, among other things, withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which may include certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing these withholding and reporting requirements may be subject to different rules. This U.S. federal withholding tax of 30% also applies to dividends and the gross proceeds of a disposition of our common stock paid to a non-financial foreign entity, unless such entity provides the withholding agent with either a certification that it does not have any substantial direct or indirect U.S. owners or provides information regarding substantial direct and indirect U.S. owners of the entity. The withholding tax described above will not apply if the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from the rules. The withholding provisions described above will generally apply to dividends on our common stock paid on or after July 1, 2014, and with respect to gross proceeds of a sale or other disposition of our common stock on or after January 1, 2017. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. Non-U.S. holders are encouraged to consult with their own tax advisors regarding the possible implications of the legislation on their investment in our common stock.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAW, AS WELL AS TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL, NON-U.S. OR U.S. FEDERAL NON-INCOME TAX LAWS.
122
Table of Contents
We are offering the shares of common stock described in this prospectus through a number of underwriters. J.P. Morgan Securities LLC and Piper Jaffray & Co. are acting as joint bookrunning managers of the offering and as representatives of the underwriters. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of shares of common stock listed next to its name in the following table:
| Name |
Number of Shares |
|||
| J.P. Morgan Securities LLC |
||||
| Piper Jaffray & Co. |
||||
| Leerink Partners LLC |
||||
| Wedbush Securities Inc. |
||||
|
|
|
|||
| Total |
5,000,000 | |||
|
|
|
|||
The underwriters are committed to purchase all the common stock offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The underwriters propose to offer the common stock directly to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. Any such dealers may resell shares to certain other brokers or dealers at a discount of up to $ per share from the initial public offering price. After the initial public offering of the shares, the offering price and other selling terms may be changed by the underwriters. Sales of shares made outside of the United States may be made by affiliates of the underwriters.
The underwriters have an option to purchase up to 750,000 additional shares of common stock from us to cover sales of shares by the underwriters which exceed the number of shares specified in the table above. The underwriters have 30 days from the date of this prospectus to exercise this option to purchase additional shares. If any shares are purchased with this option to purchase additional shares, the underwriters will purchase shares in approximately the same proportion as shown in the table above. If any additional shares of common stock are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to us per share of common stock. The underwriting fee is $ per share. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Without exercise of option to purchase additional shares exercise |
With
full exercise of option to purchase additional shares exercise |
|||||||
| Per Share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $3,000,000. We have also agreed to reimburse the underwriters for certain of their expenses in an amount up to $35,000.
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to
123
Table of Contents
allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We have agreed that we will not (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, or file with the Securities and Exchange Commission a registration statement under the Securities Act of 1933, or the Securities Act, relating to, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, or (2) enter into any swap or other agreement that transfers all or a portion of the economic consequences associated with the ownership of any shares of common stock or any such other securities (regardless of whether any of these transactions are to be settled by the delivery of shares of common stock or such other securities, in cash or otherwise), in each case without the prior written consent of J.P. Morgan Securities LLC and Piper Jaffray & Co. for a period of 180 days after the date of this prospectus.
Our directors and executive officers and all holders of our common stock and securities exercisable for or convertible into our common stock outstanding immediately prior to this offering have entered into lock-up agreements with the underwriters prior to the commencement of this offering pursuant to which each of these persons or entities, with limited exceptions, for a period of 180 days after the date of this prospectus, may not, without the prior written consent of J.P. Morgan Securities LLC and Piper Jaffray & Co., (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock (including, without limitation, common stock or such other securities which may be deemed to be beneficially owned by such directors, executive officers, managers and members in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant) or publicly disclose the intention to make any offer, sale, pledge or disposition, or (2) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock or such other securities, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of common stock or such other securities, in cash or otherwise, or (3) make any demand for or exercise any right with respect to the registration of any shares of our common stock or any security convertible into or exercisable or exchangeable for our common stock.
In the case of our directors, executive officers and holders of our common stock, subject to certain qualifications, the foregoing restrictions shall not apply to:
| • | transfers of shares of our common stock or any securities convertible into or exercisable or exchangeable for common stock (1) to any relationship by blood, marriage, domestic partnership or adoption, no more remote than a first cousin, or a trust formed for the exclusive benefit of the security holder or an immediate family member in a transaction not involving a distribution for value, (2) by bona fide gift, will or intestacy, (3) that occurs by operation of law, such as rules of descent and distribution, or pursuant to a qualified domestic order or in connection with a divorce settlement, (4) if the security holder is a corporation, partnership or other business entity, to an Affiliate (as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, or the Exchange Act), in a transaction not involving a distribution for value, or (5) if the security holder is a trust, to a trustor or beneficiary of the trust or (6) to the security holder’s estate; |
| • | distributions of shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock to members, partners or stockholders of the security holder; |
| • | shares of our common stock or securities convertible into or exercisable or exchangeable for our common stock acquired in open market transactions after the completion of this offering; |
| • | entering into a written plan pursuant to Rule 10b5-1 under the Exchange Act; or |
| • | the cash exercise of options to purchase shares of our common stock and the “net” or “cashless” exercise of options to purchase shares of our common stock and the transfer of our common stock to us upon the exercise of any options to cover tax withholding obligations in connection with such exercise. |
124
Table of Contents
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act.
We have applied to have our common stock approved for listing on The Nasdaq Global Market under the symbol “XENT.”
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common stock in the open market for the purpose of preventing or retarding a decline in the market price of the common stock while this offering is in progress. These stabilizing transactions may include making short sales of the common stock, which involves the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase additional shares, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares through the option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These activities may have the effect of raising or maintaining the market price of the common stock or preventing or retarding a decline in the market price of the common stock, and, as a result, the price of the common stock may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on The Nasdaq Global Market, in the over-the-counter market or otherwise.
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be determined by negotiations between us and the representatives of the underwriters. In determining the initial public offering price, we and the representatives of the underwriters expect to consider a number of factors including:
| • | the information set forth in this prospectus and otherwise available to the representatives; |
| • | our prospects and the history and prospects for the industry in which we compete; |
| • | an assessment of our management; |
| • | our prospects for future earnings; |
| • | the general condition of the securities markets at the time of this offering; |
| • | the recent market prices of, and demand for, publicly traded common stock of generally comparable companies; and |
| • | other factors deemed relevant by the underwriters and us. |
Neither we nor the underwriters can assure investors that an active trading market will develop for our common stock, or that the shares will trade in the public market at or above the initial public offering price.
125
Table of Contents
Relationships with Underwriters
The underwriters and their respective affiliates are full-service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and their affiliates have not, during the 180-day period preceding the date of the initial filing of the Registration Statement on Form S-1 of which this prospectus forms a part, but may, in the future, provide from time to time certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they may receive customary fees and commissions. Except as disclosed in this prospectus, we have no present arrangements with any of the underwriters for any further services. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
Selling Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
This document is only being distributed to and is only directed at (1) persons who are outside the United Kingdom or (2) to investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (3) high net worth entities, and other persons to whom it may lawfully be communicated, falling with Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), from and including the date on which the European Union Prospectus Directive (the “EU Prospectus Directive”) was implemented in that Relevant Member State (the “Relevant Implementation Date”) an offer of securities described in this prospectus may not be made to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the EU Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of securities described in this prospectus may be made to the public in that Relevant Member State at any time:
| • | to any legal entity which is a qualified investor as defined under the EU Prospectus Directive; |
| • | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural or legal persons (other than qualified investors as defined in the EU Prospectus Directive); or |
| • | in any other circumstances falling within Article 3(2) of the EU Prospectus Directive, provided that no such offer of securities described in this prospectus shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the EU Prospectus Directive. |
126
Table of Contents
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the EU Prospectus Directive in that Member State. The expression “EU Prospectus Directive” means Directive 2003/71/EC (and any amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Notice to Prospective Investors in the United Kingdom
Each underwriter has represented and agreed that:
| • | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the FSMA) received by it in connection with the issue or sale of the shares in circumstances in which Section 21(1) of the FSMA does not apply to the Issuer; and |
| • | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares in, from or otherwise involving the United Kingdom. |
Notice to Prospective Investors in Australia
No prospectus or other disclosure document (as defined in the Corporations Act 2001 (Cth) of Australia, or Corporations Act) in relation to the common stock has been or will be lodged with the Australian Securities & Investments Commission, or ASIC. This document has not been lodged with ASIC and is only directed to certain categories of exempt persons. Accordingly, if you receive this document in Australia:
| • | you confirm and warrant that you are either: |
| a. | a “sophisticated investor” under section 708(8)(a) or (b) of the Corporations Act; |
| b. | a “sophisticated investor” under section 708(8)(c) or (d) of the Corporations Act and that you have provided an accountant’s certificate to us which complies with the requirements of section 708(8)(c)(i) or (ii) of the Corporations Act and related regulations before the offer has been made; |
| c. | a person associated with the company under section 708(12) of the Corporations Act; or |
| d. | a “professional investor” within the meaning of section 708(11)(a) or (b) of the Corporations Act, and to the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Corporations Act any offer made to you under this document is void and incapable of acceptance; and |
| • | you warrant and agree that you will not offer any of the common stock for resale in Australia within 12 months of that common stock being issued unless any such resale offer is exempt from the requirement to issue a disclosure document under section 708 of the Corporations Act. |
Notice to Prospective Investors in France
We and the underwriters have not offered or sold and will not offer or sell, directly or indirectly, shares to the public in France, and have not distributed or caused to be distributed and will not distribute or cause to be distributed to the public in France, this prospectus or any other offering material relating to the shares. Offers, sales and distributions that have been and will be made in France have been and will be made only to (a) providers of the investment service of portfolio management for the account of third parties, and (b) qualified investors (investisseurs qualifi´es), other than individuals, all as defined in, and in accordance with, Articles L. 411-1, L. 411-2, and D. 411-1 of the French Code mon´etaire et financier.
Shares may be resold directly or indirectly only in compliance with Article L. 411-1, L. 411-2, L. 412-1 and L. 621-8 to L. 621-8-3 of the French Code mon´etaire et financier.
127
Table of Contents
Neither this prospectus prepared in connection with the shares nor any other offering material relating to the shares has been submitted to the clearance procedures of the Autorit´e des march´es financiers or notified to the Autorit´e des march´es financiers by the competent authority of another member state of the European Economic Area.
Notice to Prospective Investors in Germany
The shares offered by this prospectus have not been and will not be offered to the public within the meaning of the German Securities Prospectus Act (Wertpapierprospektgesetz). No securities prospectus pursuant to the German Securities Prospectus Act has been or will be published or circulated in Germany or filed with the German Federal Financial Supervisory Authority (Bundesanstalt f¨ur Finanzdienstleistungsaufsicht). This prospectus does not constitute an offer to the public in Germany, and it does not serve for public distribution of the shares in Germany. Neither this prospectus, nor any other document issued in connection with this offering, may be issued or distributed to any person in Germany except under circumstances that do not constitute an offer to the public under the German Securities Prospectus Act. Prospective Investors should consult with their legal or tax advisor before investing into the shares.
Notice to Prospective Investors in Ireland
This prospectus and any other material in relation to the shares described herein is only being distributed in Ireland:
| • | in circumstances which do not require the publication of a prospectus pursuant to Article 3(2) of Directive 2003/71/EC as amended by Directive 2010/73/EC; |
| • | in compliance with the provisions of the Irish Companies Acts 1963-2009; and |
| • | in compliance with the provisions of the European Communities (Markets in Financial Instruments) Regulations 2007 (S.I. No. 60 of 2007) (as amended), and in accordance with any codes or rules of conduct and any conditions or requirements, or any other enactment, imposed or approved by the Central Bank of Ireland with respect to anything done by them in relation to the shares. |
Notice to Prospective Investors in Italy
The offering of the shares has not been registered pursuant to Italian securities legislation and, accordingly, no shares may be offered, sold or delivered, nor may copies of the prospectus or of any other document relating to the shares be distributed in the Republic of Italy, except:
| 1. | to qualified investors (investitori qualificati), as defined pursuant to Article 100 of Legislative Decree No. 58 of 24 February 1998, as amended (the Financial Services Act) and Article 34-ter, first paragraph, letter b) of CONSOB Regulation No. 11971 of 14 May 1999, as amended from time to time (Regulation No. 11971); or |
| 2. | in other circumstances which are exempted from the rules on public offerings pursuant to Article 100 of the Financial Services Act and Article 34-ter of Regulation No. 11971. |
Any offer, sale or delivery of the shares or distribution of copies of the prospectus or any other document relating to the shares in the Republic of Italy under (1) or (2) above must be:
| a. | made by an investment firm, bank or financial intermediary permitted to conduct such activities in the Republic of Italy in accordance with the Financial Services Act, CONSOB Regulation No. 16190 of 29 October 2007 (as amended from time to time) and Legislative Decree No. 385 of 1 September 1993, as amended (the Banking Act); and |
| b. | in compliance with Article 129 of the Banking Act, as amended, and the implementing guidelines of the Bank of Italy, as amended from time to time, pursuant to which the Bank of Italy may request information on the issue or the offer of shares in the Republic of Italy; and |
128
Table of Contents
| c. | in compliance with any other applicable laws and regulations or requirement imposed by CONSOB or other Italian authority. Please note that in accordance with Article 100-bis of the Financial Services Act, where no exemption from the rules on public offerings applies under (1) and (2) above, the subsequent distribution of the shares on the secondary market in Italy must be made in compliance with the public offer and the prospectus requirement rules provided under the Financial Services Act and Regulation No. 11971. Failure to comply with such rules may result in the sale of such shares being declared null and void and in the liability of the intermediary transferring the shares for any damages suffered by the investors. |
Notice to Prospective Investors in the Netherlands
The shares will not be offered or sold, directly or indirectly, in the Netherlands, other than:
| • | with a minimum denomination of €50,000 or the equivalent in another currency per investor; |
| • | for a minimum consideration of €50,000 or the equivalent in another currency per investor; |
| • | to fewer than 100 individuals or legal entities other than ‘Qualified Investors’ (see below); or |
| • | solely to Qualified Investors, all within the meaning of Article 4 of the Financial Supervision Act Exemption Regulation (Vrijstellingsregeling Wet op het financieel toezicht) and Article 1:12 and Article 5:3 of the Financial Supervision Act (Wet op het financieel toezicht, FSA). |
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or the SIX, or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this prospectus nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland. Neither this prospectus nor any other offering or marketing material relating to the offering, the Company, the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or the CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in Hong Kong
The shares may not be offered or sold by means of any document other than (1) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (2) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (3) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or
129
Table of Contents
invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (1) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (2) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (3) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
Notice to Prospective Investors in Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (the Financial Instruments and Exchange Law) and each underwriter has agreed that it will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
130
Table of Contents
The validity of the issuance of our common stock offered in this prospectus will be passed upon for us by Cooley LLP, Palo Alto, California. Latham & Watkins LLP, Costa Mesa, California, is acting as counsel for the underwriters in connection with this offering.
The financial statements of Intersect ENT, Inc. at December 31, 2012 and 2013, and for each of the two years in the period ended December 31, 2013, appearing in this prospectus and registration statement have been audited by Ernst & Young LLP, an independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information with respect to Intersect ENT, Inc. and the shares of common stock offered hereby, reference is made to the registration statement and the exhibits and schedules filed therewith. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. A copy of the registration statement and the exhibits and schedules filed therewith may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street N.E., Room 1580, Washington, D.C. 20549, and copies of all or any part of the registration statement may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address is www.sec.gov.
We maintain a website at www.intersectent.com. The reference to our website address does not constitute incorporation by reference of the information contained on our website, and you should not consider the contents of our website in making an investment decision with respect to our common stock.
You may also request a copy of these filings, at no cost to you, by writing or telephoning us at the following address:
Intersect ENT, Inc.
1555 Adams Drive
Menlo Park, CA 94025
Attention: Chief Financial Officer
(650) 641-2100
131
Table of Contents
INTERSECT ENT, INC.
Years ended December 31, 2012 and 2013 (audited) and
Three month periods ended March 31, 2013 and 2014 (unaudited)
| Page | ||||
| F-2 | ||||
| Financial Statements: |
||||
| F-3 | ||||
| F-4 | ||||
| Statements of Convertible Preferred Stock and Stockholders’ (Deficit) Equity |
F-5 | |||
| F-6 | ||||
| F-7 | ||||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
Intersect ENT, Inc.
We have audited the accompanying balance sheets of Intersect ENT, Inc. as of December 31, 2012, and 2013, and the related statements of operations and comprehensive loss, convertible preferred stock and stockholders’ deficit and cash flows for each of the two years in the period ended December 31, 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Intersect ENT, Inc. at December 31, 2012 and 2013, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2013, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young
Redwood City, California
May 1, 2014, except for Note 17, as to which the date is July 11, 2014
F-2
Table of Contents
INTERSECT ENT, INC.
(in thousands, except per share data)
| December 31, | March
31, 2014 |
March
31, 2014 Pro Forma Stockholders’ Equity |
||||||||||||||
| 2012 | 2013 | |||||||||||||||
| (unaudited) | ||||||||||||||||
| Assets | ||||||||||||||||
| Current assets |
||||||||||||||||
| Cash and cash equivalents |
$ | 2,060 | $ | 12,294 | $ | 7,478 | ||||||||||
| Accounts receivable, net |
1,299 | 4,200 | 4,382 | |||||||||||||
| Inventory |
1,375 | 2,197 | 2,463 | |||||||||||||
| Restricted cash |
62 | 62 | — | |||||||||||||
| Prepaid expenses and other current assets |
452 | 449 | 507 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
5,248 | 19,202 | 14,830 | |||||||||||||
| Property and equipment, net |
1,755 | 1,707 | 1,716 | |||||||||||||
| Note receivable from related party |
100 | — | — | |||||||||||||
| Other non-current assets |
124 | 126 | 221 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 7,227 | $ | 21,035 | $ | 16,767 | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Liabilities, Convertible Preferred Stock and Stockholders’ (Deficit) Equity | ||||||||||||||||
| Current liabilities |
||||||||||||||||
| Accounts payable |
$ | 1,074 | $ | 1,451 | $ | 1,647 | ||||||||||
| Accrued compensation |
1,904 | 2,955 | 2,409 | |||||||||||||
| Equipment loans – current portion |
636 | 696 | 705 | |||||||||||||
| Preferred stock warrant liability |
93 | 237 | 494 | $ | — | |||||||||||
| Other current liabilities |
389 | 745 | 932 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
4,096 | 6,084 | 6,187 | |||||||||||||
| Equipment loans – non-current portion |
1,364 | 756 | 575 | |||||||||||||
| Other non-current liabilities |
167 | 52 | 21 | |||||||||||||
| Commitments and contingencies (see note 11) |
||||||||||||||||
| Convertible preferred stock issuable in series, $0.001 par value; |
||||||||||||||||
| 15,907 shares authorized at December 31, 2013 and March 31, 2014, actual, none pro forma; 11,283, 15,701 and 15,704 issued and outstanding at December 31, 2012 and 2013 and March 31, 2014, respectively, actual, none pro forma; aggregate liquidation preference: $91,433 and $91,441 at December 31, 2013 and March 31, 2014, respectively, actual, none pro forma |
60,320 | 90,760 | 90,789 | — | ||||||||||||
| Stockholders’ (deficit) equity |
||||||||||||||||
| Preferred stock, $0.001 par value; no shares authorized, issued and outstanding, actual; 10,000 shares authorized; no shares issued or outstanding pro forma |
— | |||||||||||||||
| Common stock, $0.001 par value; |
||||||||||||||||
| 150,000 authorized at December 31, 2013 and March 31, 2014, actual; 1,021, 1,761 and 1,798 issued and outstanding at December 31, 2012 and 2013 and March 31, 2014, respectively, actual; 150,000 shares authorized, 17,502 issued and outstanding pro forma |
1 | 2 | 2 | 18 | ||||||||||||
| Additional paid-in capital |
1,169 | 1,859 | 2,065 | 93,332 | ||||||||||||
| Notes receivable from related party |
— | (219 | ) | (204 | ) | |||||||||||
| Accumulated deficit |
(59,890 | ) | (78,259 | ) | (82,668 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total stockholders’ (deficit) equity |
(58,720 | ) | (76,617 | ) | (80,805 | ) | 10,478 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities, convertible preferred stock and stockholders’ (deficit) equity |
$ | 7,227 | $ | 21,035 | $ | 16,767 | $ | 16,767 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
See accompanying notes to financial statements.
F-3
Table of Contents
INTERSECT ENT, INC.
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except per share data)
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Revenue |
$ | 5,863 | $ | 17,931 | $ | 2,744 | $ | 7,497 | ||||||||
| Cost of sales |
3,837 | 8,150 | 1,949 | 2,360 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
2,026 | 9,781 | 795 | 5,137 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Selling, general and administrative |
9,251 | 18,229 | 3,350 | 6,658 | ||||||||||||
| Research and development |
9,260 | 9,518 | 2,256 | 2,577 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
18,511 | 27,747 | 5,606 | 9,235 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(16,485 | ) | (17,966 | ) | (4,811 | ) | (4,098 | ) | ||||||||
| Other income (expense), net: |
||||||||||||||||
| Interest and other income |
156 | 86 | 82 | 1 | ||||||||||||
| Interest and other expense |
(36 | ) | (489 | ) | (24 | ) | (312 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense), net |
120 | (403 | ) | 58 | (311 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (16,365 | ) | $ | (18,369 | ) | $ | (4,753 | ) | $ | (4,409 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other comprehensive income: |
||||||||||||||||
| Unrealized gain on short-term investments |
7 | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss |
$ | (16,358 | ) | $ | (18,369 | ) | $ | (4,753 | ) | $ | (4,409 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share, basic and diluted |
$ | (16.38 | ) | $ | (12.57 | ) | $ | (4.57 | ) | $ | (2.49 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average common shares used to compute net loss per share, basic and diluted |
999 | 1,461 | 1,039 | 1,773 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Pro forma net loss per share, basic and diluted (unaudited) |
$ | (1.17 | ) | $ | (0.25 | ) | ||||||||||
|
|
|
|
|
|||||||||||||
| Weighted average common shares used to compute pro forma net loss per share, basic and diluted (unaudited) |
15,655 | 17,474 | ||||||||||||||
|
|
|
|
|
|||||||||||||
See accompanying notes to financial statements.
F-4
Table of Contents
INTERSECT ENT, INC.
STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ (DEFICIT) EQUITY
(in thousands)
| Convertible Preferred Stock |
Common Stock | Additional Paid-in Capital |
Note Receivable From Related Party |
Accumulated Other Comprehensive Loss |
Accumulated Deficit |
Total Stockholders’ (Deficit) Equity |
||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||
| Balance at December 31, 2011 |
11,226 | $ | 60,107 | 907 | $ | 1 | $ | 928 | $ | — | $ | (7 | ) | $ | (43,525 | ) | $ | (42,603 | ) | |||||||||||||||||||
| Issuance of Series A convertible preferred stock upon exercise of warrants |
57 | 213 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||
| Issuance common stock upon exercise of stock options |
— | — | 115 | — | 89 | — | — | — | 89 | |||||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 152 | — | — | — | 152 | |||||||||||||||||||||||||||||
| Unrealized gain on short-term investments |
— | — | — | — | — | — | 7 | — | 7 | |||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (16,365 | ) | (16,365 | ) | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2012 |
11,283 | 60,320 | 1,022 | 1 | 1,169 | — | — | (59,890 | ) | (58,720 | ) | |||||||||||||||||||||||||||
| Issuance of Series A convertible preferred stock upon exercise of warrants |
22 | 82 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||
| Issuance of Series D convertible preferred stock, net of issuance cost and preferred stock financing option |
4,396 | 30,358 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||
| Issuance common stock upon exercise of stock options |
— | — | 740 | 1 | 364 | — | — | — | 365 | |||||||||||||||||||||||||||||
| Notes receivable from related party |
— | — | — | — | — | (275 | ) | — | — | (275 | ) | |||||||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | 326 | 56 | — | — | 382 | |||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | (18,369 | ) | (18,369 | ) | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at December 31, 2013 |
15,701 | 90,760 | 1,762 | 2 | 1,859 | (219 | ) | — | (78,259 | ) | (76,617 | ) | ||||||||||||||||||||||||||
| Issuance of Series A convertible preferred stock upon exercise of warrants (unaudited) |
3 | 29 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||
| Issuance common stock upon exercise of stock options (unaudited) |
— | — | 36 | — | 39 | — | — | — | 39 | |||||||||||||||||||||||||||||
| Stock-based compensation expense (unaudited) |
— | — | — | — | 167 | 15 | — | — | 182 | |||||||||||||||||||||||||||||
| Net loss (unaudited) |
— | — | — | — | — | — | — | (4,409 | ) | (4,409 | ) | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at March 31, 2014 (unaudited) |
15,704 | $ | 90,789 | 1,798 | $ | 2 | $ | 2,065 | $ | (204 | ) | $ | — | $ | (82,668 | ) | $ | (80,805 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Pro forma: |
||||||||||||||||||||||||||||||||||||||
| Conversion of convertible preferred stock to common stock (unaudited) |
(15,704 | ) | (90,789 | ) | 15,704 | 16 | 90,773 | — | — | — | 90,789 | |||||||||||||||||||||||||||
| Conversion of convertible preferred stock warrants to common stock warrants (unaudited) |
— | — | — | — | 494 | — | — | — | 494 | |||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance at March 31, 2014 (unaudited) |
— | $ | — | 17,502 | $ | 18 | $ | 93,332 | $ | (204 | ) | $ | — | $ | (82,668 | ) | $ | 10,478 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
See accompanying notes to financial statements.
F-5
Table of Contents
INTERSECT ENT, INC.
(in thousands)
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Cash flows from operating activities: |
||||||||||||||||
| Net loss |
$ | (16,365 | ) | $ | (18,369 | ) | $ | (4,753 | ) | $ | (4,409 | ) | ||||
| Adjustments to reconcile net loss to cash used in operating activities: |
||||||||||||||||
| Depreciation and amortization |
351 | 505 | 124 | 135 | ||||||||||||
| Amortization of net investment premium paid |
83 | — | — | — | ||||||||||||
| Stock-based compensation expense |
152 | 382 | 38 | 182 | ||||||||||||
| Change in fair value of convertible preferred stock warrants |
(128 | ) | 134 | — | 286 | |||||||||||
| Change in fair value of convertible preferred stock financing option |
— | 212 | — | — | ||||||||||||
| Forgiveness of notes receivable from related party |
116 | 100 | 100 | 100 | ||||||||||||
| Loss on disposal of fixed assets |
33 | 26 | — | — | ||||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||||
| Accounts receivable, net |
(1,011 | ) | (2,901 | ) | (398 | ) | (182 | ) | ||||||||
| Inventory |
(1,004 | ) | (822 | ) | (341 | ) | (266 | ) | ||||||||
| Prepaid expenses and other current assets |
(241 | ) | 3 | 15 | (96 | ) | ||||||||||
| Other non-current assets |
(90 | ) | (2 | ) | — | (95 | ) | |||||||||
| Accounts payable |
331 | 360 | (234 | ) | 189 | |||||||||||
| Accrued compensation |
1,271 | 1,051 | (741 | ) | (546 | ) | ||||||||||
| Other liabilities |
354 | 220 | 72 | 156 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash used in operating activities |
(16,148 | ) | (19,101 | ) | (6,118 | ) | (4,546 | ) | ||||||||
| Cash flows from investing activities: |
||||||||||||||||
| Purchases of short-term investments |
(1,324 | ) | — | — | — | |||||||||||
| Sales of short-term investments |
8,622 | — | — | — | ||||||||||||
| Purchases of property and equipment |
(1,413 | ) | (467 | ) | (52 | ) | (136 | ) | ||||||||
| Proceeds from disposal of fixed assets |
4 | — | — | — | ||||||||||||
| Change in restricted cash and deposits |
67 | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash provided by (used in) investing activities |
5,956 | (467 | ) | (52 | ) | (136 | ) | |||||||||
| Cash flows from financing activities: |
||||||||||||||||
| Proceeds from issuance of common stock |
89 | 122 | 36 | 36 | ||||||||||||
| Proceeds from equipment loan |
2,000 | — | — | — | ||||||||||||
| Proceeds from capital lease financing |
— | 106 | — | — | ||||||||||||
| Repayments related to equipment loans |
— | (636 | ) | (160 | ) | (163 | ) | |||||||||
| Repayments related to capital lease financing |
— | (18 | ) | — | (7 | ) | ||||||||||
| Proceeds from the exercise of Series A convertible preferred warrants |
213 | 82 | 82 | — | ||||||||||||
| Proceeds from issuance of Series D convertible preferred stock net of issuance costs |
— | 30,146 | 20,716 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash provided by (used in) financing activities |
2,302 | 29,802 | 20,674 | (134 | ) | |||||||||||
| Net (decrease) increase in cash and cash equivalents |
(7,890 | ) | 10,234 | 14,504 | (4,816 | ) | ||||||||||
| Cash and cash equivalents |
||||||||||||||||
| Beginning of the period |
9,950 | 2,060 | 2,060 | 12,294 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| End of the period |
$ | 2,060 | $ | 12,294 | $ | 16,564 | $ | 7,478 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Supplemental cash flow information |
||||||||||||||||
| Interest paid |
$ | — | $ | 93 | $ | 20 | $ | 20 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Noncash investing and financing activities |
||||||||||||||||
| Warrants issued for growth capital facility |
$ | — | $ | 10 | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Property and equipment included in accounts payable |
$ | — | $ | 17 | $ | — | $ | 7 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Notes issued to related party for the exercise of stock options |
$ | — | $ | 500 | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
See accompanying notes to financial statements.
F-6
Table of Contents
INTERSECT ENT, INC.
| 1. | Organization |
Description of Business
Intersect ENT, Inc. (the “Company”) was incorporated as Sinexus, Inc. in the state of Delaware in October 2003 (inception) and its facilities are located in Menlo Park, California. By written consent of the stockholders, the Company changed its name from Sinexus, Inc. to Intersect ENT, Inc., effective November 2009. The Company is a commercial stage drug-device company committed to improving the quality of life for patients with ear, nose and throat conditions. The Company’s sole commercial products are the PROPEL and PROPEL mini drug-eluting implants for patients undergoing sinus surgery to treat chronic sinusitis. The Company received approval from the U.S. Food and Drug Administration (“FDA”) for PROPEL, in August 2011 and for PROPEL mini, in November 2012. In the first half of 2013, the Company began scaling its U.S. direct commercial presence and currently markets its products only in the United States.
Liquidity and Business Risks
As of March 31, 2014, the Company had cash and cash equivalents of $7.5 million, and an accumulated deficit of $82.7 million. The Company has financed its operations with a combination of debt and equity financing arrangements. As of March 31, 2014, the Company expects its cash and cash equivalents, revenue and available debt financing arrangements will be sufficient to fund its operations through at least December 31, 2014. However, the Company expects to continue to incur additional substantial losses in 2014 as a result of its commercialization and research and development activities. If these sources are insufficient to satisfy our liquidity requirements, the Company may seek to sell additional equity or debt securities or obtain an additional credit facility. Debt financing, if available, may involve covenants restricting the Company’s operations or its ability to incur additional debt. Any debt financing or additional equity that the Company raises may contain terms that are not favorable to it or its stockholders. Additional financing may not be available at all, or in amounts or on terms unacceptable to the Company. If the Company is unable to obtain additional financing, it may be required to delay the development, commercialization and marketing of its products.
| 2. | Summary of Significant Accounting Policies |
Basis of Preparation
The financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) and pursuant to the rules and regulations of the United States Securities and Exchange Commission (“SEC”).
Certain amounts in the financial statements have been reclassified to conform to the current year presentation.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts and disclosures reported in the financial statements. Management uses significant judgment when making estimates related to its common stock valuation and related stock-based compensation, the valuations of the convertible preferred stock warrant liability, convertible preferred stock financing option, clinical trial accruals, as well as certain accrued liabilities. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ from those estimates.
Unaudited Interim Financial Statements
The accompanying balance sheet as of March 31, 2014, the statements of operations and comprehensive loss and cash flows for the three months ended March 31, 2013 and 2014, and the statements of convertible preferred
F-7
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 2. | Summary of Significant Accounting Policies (Continued) |
stock and stockholders’ deficit as of March 31, 2014, are unaudited. The financial data and other information disclosed in these notes to the financial statements related to March 31, 2014, and the three months ended March 31, 2013 and 2014, are also unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to state fairly the Company’s financial position as of March 31, 2014, and the results of its operations and cash flows for the three months ended March 31, 2013 and 2014. The results for the three months ended March 31, 2014, are not necessarily indicative of results to be expected for the year ending December 31, 2014, or for any other interim period or for any future year.
Unaudited Pro Forma Stockholders’ Equity
Pro forma basic and diluted net loss per share has been computed to give effect to: (1) the assumed conversion of the 15,701,481 and 15,703,912 shares of convertible preferred stock outstanding as of December 31, 2013 and March 31, 2014, respectively, into the same number of common stock in connection with the Company’s proposed initial public offering, or IPO; (2) the conversion of all warrants exercisable for convertible preferred stock outstanding as of December 31, 2013 into warrants exercisable for shares of common stock, resulting in the reclassification of the related convertible preferred stock warrant liabilities to additional paid-in capital, which when added to the existing 1,761,167 and 1,797,933 shares of common stock outstanding as of December 31, 2013 and March 31, 2014, respectively, results in a total of 17,462,647 and 17,501,808 shares of common stock outstanding, respectively; and (3) the effectiveness of the Company’s amended and restated certificate of incorporation. The pro forma net loss per share does not include the shares expected to be sold and related proceeds to be received from the IPO. For purposes of pro forma basic and diluted net loss per share, all shares of convertible preferred stock have been treated as though they had been converted to common stock in all periods in which such shares were outstanding.
Cash and Cash Equivalents
The Company considers all highly liquid securities, readily convertible to cash, that mature within three months or less from the original date of purchase to be cash equivalents.
Cash equivalents are considered available-for-sale marketable securities and are recorded at fair value, based on quoted market prices. Unrealized gains and losses are recorded in other comprehensive income (loss) and included as a separate component of stockholders’ deficit. As of December 31, 2012 and 2013, and March 31, 2014, the Company’s cash and cash equivalents were entirely composed of bank deposits, cash, and investments in money market funds.
Fair Value of Financial Instruments
Available-for-sale marketable securities are recorded at fair value in the Company’s balance sheets and are categorized based upon the level of judgment associated with the inputs used to measure their fair value. ASC Subtopic 820-10 defines a three-level valuation hierarchy for disclosure of fair value measurements.
F-8
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 2. | Summary of Significant Accounting Policies (Continued) |
The Company measures certain financial assets and liabilities at fair value on a recurring basis, including cash equivalents, the convertible preferred stock warrant liability and the convertible preferred stock financing option. Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability. A three-tier fair value hierarchy is established as a basis for considering such assumptions and for inputs used in the valuation methodologies in measuring fair value:
| Level 1 | Observable inputs such as quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| Level 2 | Include other inputs that are based upon quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active and model-based valuation techniques for which all significant inputs are observable in the market or can be derived from observable market data. Where applicable, these models project future cash flows and discount the future amounts to a present value using market-based observable inputs including interest rate curves, foreign exchange rates, and credit ratings. | |
| Level 3 | Unobservable inputs that are supported by little or no market activities, which would require the Company to develop its own assumptions. |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
As of December 31, 2012 and 2013, and March 31, 2014, cash equivalents were all categorized as Level 1 and primarily consisted of money market funds, and convertible preferred stock warrant liabilities and convertible preferred stock financing option were categorized as Level 3.
There were no transfers in and out of Level 1 and Level 2 fair value measurements during the years ended December 31, 2012 and 2013, and the three months ended March 31, 2014.
Convertible Preferred Stock Warrants
The following table sets forth a summary of the changes in the estimated fair value of the Company’s convertible preferred stock warrants, which represents financial instruments with valuations classified as Level 3. When a determination is made to classify a financial instrument within Level 3, the determination is based upon the significance of the unobservable inputs to the overall fair value measurement. However, Level 3 financial instruments typically include, in addition to the unobservable inputs, observable inputs (that is, components that are actively quoted and can be validated to external sources). Accordingly, the expense in the table below includes changes in fair value due in part to observable factors that are part of the Level 3 methodology (in thousands):
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Beginning of the period |
$ | 221 | $ | 93 | $ | 93 | $ | 237 | ||||||||
| Issued |
— | 10 | — | — | ||||||||||||
| Exercised |
— | — | — | (29 | ) | |||||||||||
| Expired |
— | — | — | — | ||||||||||||
| Change in fair value |
(128 | ) | 134 | — | 286 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| End of the period |
$ | 93 | $ | 237 | $ | 93 | $ | 494 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-9
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 2. | Summary of Significant Accounting Policies (Continued) |
The fair value of the convertible preferred stock warrants was determined using the option pricing method or the probability weighted expected return method using the following assumptions:
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Expected life (years) |
3.0 | 2.0 | 3.0 | 2.0 | ||||||||||||
| Expected volatility |
58 | % | 45 | % | 58 | % | 46 | % | ||||||||
| Risk-free interest rate |
0.4 | % | 0.4 | % | 0.4 | % | 0.5 | % | ||||||||
| Dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | 0.0 | % | ||||||||
Series D Convertible Preferred Stock Financing Option
The Series D convertible preferred stock contained a provision that obligated the investors to purchase additional shares (“convertible preferred stock financing option”) at the same price as the initial closing upon notification by the Company that it had achieved an annualized revenue rate of at least $16.0 million over a trailing three month period. This convertible preferred stock financing option to purchase Series D convertible preferred stock in the future tranche was considered to be a freestanding financial instrument for accounting purposes. Therefore, in February 2013, the Company recorded a financing liability of $0.9 million representing the fair value of this convertible preferred stock financing option at the time of issuance. In October 2013, shortly after achieving the annualized revenue rate, the Company issued the additional 1,369,009 shares of Series D convertible preferred stock to the investors for net proceeds of $9.4 million. Since the convertible preferred stock financing option expired in October 2013 as a result of the issuance of Series D convertible preferred stock, the carrying and fair value of the convertible preferred stock financing option of $1.1 million was reclassified from liability to Series D convertible preferred stock. The Company recorded a charge related to the change in fair value in 2013 of $0.2 million related to this financing option liability.
The following table sets forth a summary of the changes in the estimated fair value of the Company’s convertible preferred stock financing option, which represents financial instruments with valuations classified as Level 3 (in thousands):
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Beginning of the period |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Issued |
— | 885 | 885 | — | ||||||||||||
| Exercised |
— | (1,097 | ) | — | — | |||||||||||
| Expired |
— | — | — | — | ||||||||||||
| Change in fair value |
— | 212 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| End of the period |
$ | — | $ | — | $ | 885 | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The fair value of the convertible preferred stock financing liability was determined using the present value methodology with the following assumptions which are categorized as Level 3:
| February 2013 |
October 2013 |
|||||||
| Total consideration per share |
$ | 1.72 | $ | 1.72 | ||||
| Additional investment per share |
1.72 | 1.72 | ||||||
| Discount rate |
20.0 | % | 20.0 | % | ||||
| Probability of achievement |
95.0 | % | 95.0 | % | ||||
| Years until milestone achieved |
0.5 | 0.7 | ||||||
F-10
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 2. | Summary of Significant Accounting Policies (Continued) |
Concentration of Credit Risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of cash equivalents and accounts receivable. The Company believes that the credit risk in its accounts receivable is mitigated by its credit evaluation process, relatively short collection terms and dispersion of its customer base. The Company generally does not require collateral and losses on accounts receivable have historically been within management’s expectations.
The Company’s investment policy limits investments to certain types of debt securities issued by the U.S. government, its agencies, and institutions with investment-grade credit ratings, as well as corporate debt or commercial paper issued by the highest quality financial and non-financial companies, and places restrictions on maturities and concentration by type and issuer. The Company is exposed to credit risk in the event of a default by the financial institutions holding its cash and cash equivalents and issuers of investments to the extent recorded on the balance sheets. However, as of December 31, 2012 and 2013, and March 31, 2014, the Company has limited its credit risk associated with cash and cash equivalents by placing its investments with banks it believes are highly creditworthy and with highly rated money market funds.
Allowance for Doubtful Accounts
The Company will provide for uncollectible accounts receivable by recording an allowance for doubtful accounts for accounts receivable deemed uncollectible. The Company evaluates the collectibility of its accounts receivable based on known collection risks and historical experience. In circumstances where the Company is aware of a specific customer’s inability to meet its financial obligations to the Company (e.g., bankruptcy filings, substantial downgrading of credit ratings), the Company records a specific allowance for bad debts against amounts due to reduce the carrying amount of accounts receivable to the amount it reasonably believes will be collected.
If circumstances change, such as higher-than-expected defaults or an unexpected material adverse change in a major customer’s ability to meet its financial obligations, the Company’s estimates of the recoverability of the amounts due could be reduced by a significant amount. Based on the high creditworthiness of the customers that the Company sells to, the Company has experienced no significant collection issues. Therefore, the Company does not have an allowance for doubtful accounts established as of December 31, 2012 and 2013, and March 31, 2014.
Inventory
Inventory is valued at the lower of cost or market, computed on a first-in, first out basis, or net realizable value. The Company estimates the recoverability of its inventory by reference to internal estimates of future demands and product life cycles, including expiration of inventory prior to sale.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Depreciation is determined using the straight-line method over the estimated useful lives of the respective assets, generally three to five years. Leasehold improvements are amortized on a straight-line basis over the shorter of their estimated useful lives or the term of the lease. Amortization of assets under capital leases is included in depreciation and amortization expense. Maintenance and repairs are charged to operations as incurred.
Impairment of Long-lived Assets
Long-lived assets consists primarily of property and equipment and are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If circumstances require that a long-lived asset be tested for possible impairment, the Company compares the undiscounted cash flows expected to be generated by the asset to the carrying amount of the asset. If the carrying amount of the long-lived asset is not recoverable on an undiscounted cash flow basis, an impairment is
F-11
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 2. | Summary of Significant Accounting Policies (Continued) |
recognized to the extent that the carrying amount exceeds its fair value. The Company determines fair value using the income approach based on the present value of expected future cash flows or other appropriate measures of estimated fair value. The Company’s cash flow assumptions consider historical and forecasted revenue and operating costs and other relevant factors. Since inception, the Company has not recorded impairment charges on long-lived assets.
Convertible Preferred Stock Warrant Liability
For a warrant classified as a derivative liability, the fair value of that warrant is recorded on the balance sheet at the inception of such classification and adjusted to fair value at each financial reporting date. The changes in the fair value of the warrants are recorded in the statement of operations as a component of interest and other income or expense as appropriate. The Company will continue to adjust the carrying value of the convertible preferred stock warrant liability for changes in the fair value of the warrants until the earlier of: the exercise of the warrants, at which time the liability will be reclassified to temporary equity; the conversion of the underlying convertible preferred stock into common stock, at which time the liability will be reclassified to stockholders’ deficit; or the expiration of the warrant, at which time the entire amount would be reversed and reflected in the statement of operations.
Convertible Preferred Stock Financing Option
For a freestanding option to purchase in a future round of financing, the fair value of that option is recorded on the balance sheet at the inception of such classification and adjusted to fair value at each financial reporting date. The change in the fair value of the convertible preferred stock financing option is recorded in the statement of operations as a component of interest and other income or expense as appropriate. The Company adjusts the carrying value of the convertible preferred stock financing option liability for changes in the fair value of the option and continues to do so until the earlier of when the convertible preferred stock financing option has been exercised or expired.
Revenue Recognition
Revenue is derived from the sale of PROPEL and PROPEL mini to hospitals and ambulatory surgery centers in the United States. Revenue is recognized when the following revenue recognition criteria are met:
| • | Persuasive evidence of an arrangement exists. The Company considers this criterion satisfied when it has an agreement or contract with the customer in place. |
| • | Delivery has occurred or services rendered. The Company principally determines this criterion to be satisfied when it ships the product to the customer. |
| • | The fee is fixed or determinable and collectibility is reasonably assured. Determination of the satisfaction of these criteria is based on the Company’s judgment regarding the nature of the fee charged for products, contractual agreements entered into, and the collectibility of those fees under any contract or agreement. |
The Company must make significant assumptions regarding the future collectibility of amounts receivable from customers to determine whether revenue recognition criteria have been met. If collectibility is not assured at the time of shipment, the Company will defer revenue until such criteria have been met. The Company’s standard terms and conditions of sale do not allow for product returns and it generally does not allow product returns, except in the case of damaged goods, and it has not experienced any significant returns of its products. The Company expenses shipping and handling costs as incurred and includes them in the cost of sales. In those cases where the Company bills shipping and handling costs to customers, it will classify the amounts billed as a component of revenue.
F-12
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 2. | Summary of Significant Accounting Policies (Continued) |
Cost of Sales
Cost of sales consists primarily of manufacturing overhead costs, material costs and direct labor. A significant portion of the Company’s cost of sales currently consists of manufacturing overhead costs. These overhead costs include the cost of quality assurance, material procurement, inventory control, facilities, equipment and operations supervision and management. Cost of sales also includes depreciation expense for production equipment and certain direct costs such as shipping costs.
Research and Development
Research and development expenses consist primarily of engineering, product development, clinical and regulatory affairs, consulting services, materials, depreciation and other costs associated with products and technologies in development. These expenses include employee and non-employee compensation, including stock-based compensation, supplies, materials, consulting, related travel expenses and facility costs. Clinical expenses include clinical trial design, clinical site reimbursement, data management and travel expenses, and the cost of manufacturing products for clinical trials.
Common Stock Valuation and Stock-based Compensation
The Company maintains a payment equity incentive plan to provide long-term incentives for employees, consultants and members of the board of directors. The plan allows for the issuance of non-statutory and incentive stock options to employees and non-statutory stock options to consultants and non-employee directors.
The Company is required to determine the fair value of equity incentive awards and recognize compensation expense for all equity incentive awards made to employees and directors, including employee stock options. Such expense is recognized over the requisite service period. In addition, stock-based compensation expense recognized in the statements of operations and comprehensive loss is based on awards expected to vest and therefore the amount of expense has been reduced for estimated forfeitures. The Company uses the straight-line method for expense attribution, except for awards issued with performance-based conditions which require an accelerated attribution method over the requisite performance and service periods.
Under the applicable accounting guidance for equity incentive awards issued to non-employees, the date at which the fair value of such awards is measured is equal to the earlier of: 1) the date at which a commitment for performance by the counterparty to earn the equity instrument is reached; or 2) the date at which the counterparty’s performance is complete. The Company recognizes stock-based compensation expense for the fair value of the vested portion of the non-employee awards in the statements of operations and comprehensive loss. The fair value of options granted to non-employees is remeasured as the options vest.
The valuation model used for calculating the fair value of awards for stock-based compensation expense is the Black-Scholes option-pricing model (the “Black-Scholes model”). The Black-Scholes model requires the Company to make assumptions and judgments about the variables used in the calculation, including the expected term (weighted average period of time that the options granted are expected to be outstanding), the volatility of common stock, an assumed risk-free interest rate and an estimated forfeiture rate. The Company uses the “simplified method” to determine the expected term of the stock option. Volatility is based on an average of the historical volatilities of the common stock of publicly-traded companies with characteristics similar to those of the Company. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods corresponding with the expected term of the option. Potential forfeitures of awards are estimated based on the Company’s historical forfeiture experience and an analysis of similar companies. The estimate of forfeitures will be adjusted over the service period to the extent that actual forfeitures differ, or are expected to differ, from prior estimates.
F-13
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 2. | Summary of Significant Accounting Policies (Continued) |
Deferred Rent
Rent expense is recognized on a straight-line basis over the non-cancelable term of the Company’s operating lease and, accordingly, the Company records the difference between cash rent payments and the recognition of rent expense as a deferred rent liability. The Company also records lessor-funded lease incentives, such as reimbursable leasehold improvements, as a deferred rent liability, which is amortized as a reduction of rent expense over the non-cancelable term of its operating lease.
Advertising Expenses
The Company expenses the costs of advertising, including promotional expenses, as incurred. Advertising expenses were $23,000 and $46,000 during the years ended December 31, 2012 and 2013, respectively and $11,000 and $19,000 during the three months ended March 31, 2013 and 2014, respectively.
Income Taxes
The Company accounts for income taxes under the liability method. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates that will be in effect when the differences are expected to reverse. Valuation allowances against deferred tax assets are established when necessary to reduce deferred tax assets to the amounts expected to be realized. Currently, the Company has recorded a full valuation allowance against its deferred tax assets and there is no provision for income taxes, as the Company has incurred operating losses to date. The Company’s policy is to record interest and penalties expense related to uncertain tax positions as “other expense” in the statement of operations.
Net Loss and Unaudited Pro Forma Net Loss per Share
Basic net loss per share is computed by dividing the net loss by the weighted average number of shares of common stock outstanding during the period. Diluted net loss per share is computed by dividing the net loss by the weighted average number of shares of common stock and dilutive potential shares of common stock outstanding during the period. Because the Company has reported a net loss for all periods presented, diluted net loss per share is the same as basic net loss per share for those periods as all potentially dilutive shares consisting of convertible preferred stock, stock options and warrants were antidilutive in those periods.
The Company allocates no loss to participating securities because they have no contractual obligation to share in the losses of the Company. The shares of the Company’s convertible preferred stock participate in any dividends declared by the Company and are therefore considered to be participating securities.
Net loss per share was determined as follows (in thousands, except per share amounts):
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Net loss |
$ | (16,365 | ) | $ | (18,369 | ) | $ | (4,753 | ) | $ | (4,409 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average common stock outstanding |
999 | 1,461 | 1,039 | 1,773 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share, basic and diluted |
$ | (16.38 | ) | $ | (12.57 | ) | $ | (4.57 | ) | $ | (2.49 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
F-14
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 2. | Summary of Significant Accounting Policies (Continued) |
The following potentially dilutive securities outstanding have been excluded from the computations of diluted weighted average shares outstanding because such securities have an antidilutive impact due to losses reported, in common stock equivalent shares (in thousands):
| December 31, | March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Convertible preferred stock outstanding |
11,283 | 15,704 | 14,332 | 15,704 | ||||||||||||
| Convertible preferred stock warrants |
74 | 57 | 51 | 53 | ||||||||||||
| Common stock options |
1,594 | 1,928 | 1,538 | 1,965 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| 12,951 | 17,689 | 15,921 | 17,722 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The unaudited pro forma basic and diluted net loss per share for the year ended December 31, 2013, and the three months ended March 31, 2014, have been computed using the weighted average number of shares of common stock outstanding after giving pro forma effect to the assumed conversion of all shares of convertible preferred stock upon an IPO by treating all shares of convertible preferred stock as if they had been converted to common stock in all periods in which such shares were actually outstanding.
The following table sets forth the computation of the Company’s unaudited pro forma basic and diluted net loss per share during the year ended December 31, 2013, and three months ended March 31, 2014 (in thousands, except for per share amounts):
| Year Ended December 31, 2013 |
Three Months Ended March 31, 2014 |
|||||||
| (unaudited) | (unaudited) | |||||||
| Net loss |
$ | (18,369 | ) | $ | (4,409 | ) | ||
|
|
|
|
|
|||||
| Pro forma net loss |
$ | (18,369 | ) | $ | (4,409 | ) | ||
|
|
|
|
|
|||||
| Weighted average common stock outstanding |
1,461 | 1,773 | ||||||
| Pro forma weighted average convertible preferred stock outstanding |
14,194 | 15,701 | ||||||
|
|
|
|
|
|||||
| Pro forma weighted average common shares used to compute net loss per share, basic and diluted |
15,655 | 17,474 | ||||||
|
|
|
|
|
|||||
| Pro forma net loss per share, basic and diluted |
$ | (1.17 | ) | $ | (0.25 | ) | ||
|
|
|
|
|
|||||
Comprehensive Loss
Comprehensive loss consists of net loss and changes in unrealized gains and losses on available-for-sale marketable securities. Accumulated other comprehensive income (loss) is presented in the accompanying balance sheets, when applicable.
Segment, Geographical and Customer Concentration
The Company operates in one segment. All of the Company’s assets and revenue are based in the U.S. and no single customer accounted for more than 6% of its revenue during the years ended December 31, 2012 and 2013 and the three months ended March 31, 2013 and 2014.
JOBS Act Accounting Election
As an emerging growth company under the Jumpstart Our Business Startups Act (“JOBS Act”), unlike many other public companies, the Company is eligible to take advantage of certain exemptions from various
F-15
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 2. | Summary of Significant Accounting Policies (Continued) |
reporting requirements that are applicable to other public companies that are not emerging growth companies. The Company has elected to take advantage of the extended transition period for adopting new or revised accounting standards that have different effective dates for public and private companies until such time as those standards apply to private companies. There have been no new accounting pronouncements or changes to accounting pronouncements that are of significance or potential significance to the Company.
| 3. | Composition of Certain Financial Statement Items |
Inventory (in thousands):
| December 31, | March
31, 2014 |
|||||||||||
| 2012 | 2013 | |||||||||||
| (unaudited) | ||||||||||||
| Raw materials |
$ | 334 | $ | 472 | $ | 667 | ||||||
| Work-in-process |
111 | 241 | 187 | |||||||||
| Finished goods |
930 | 1,484 | 1,609 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,375 | $ | 2,197 | $ | 2,463 | |||||||
|
|
|
|
|
|
|
|||||||
Property and Equipment (in thousands):
| December 31, | March
31, 2014 |
|||||||||||
| 2012 | 2013 | |||||||||||
| (unaudited) | ||||||||||||
| Computer equipment and software |
$ | 337 | $ | 398 | $ | 446 | ||||||
| Furniture and office equipment |
135 | 252 | 255 | |||||||||
| Laboratory equipment |
2,176 | 2,262 | 2,350 | |||||||||
| Leasehold improvements |
228 | 124 | 128 | |||||||||
|
|
|
|
|
|
|
|||||||
| 2,876 | 3,036 | 3,179 | ||||||||||
| Less: accumulated depreciation and amortization |
(1,121 | ) | (1,329 | ) | (1,463 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,755 | $ | 1,707 | $ | 1,716 | |||||||
|
|
|
|
|
|
|
|||||||
Depreciation and leasehold improvements and capital lease amortization expenses were $0.4 million and $0.5 million during the years ended December 31, 2012 and 2013, respectively, and $0.1 million during each of the three months ended March 31, 2013 and 2014. Amortization for the office equipment purchased on a capital lease entered into in April 2013 was $11,000 and $5,000 during the year ended December 31, 2013 and the three months ended March 31, 2014, respectively.
Prepaid Expenses and Other Current and Non-current Assets (in thousands):
| December 31, | March
31, 2014 |
|||||||||||
| 2012 | 2013 | |||||||||||
| (unaudited) | ||||||||||||
| Prepaid expenses |
$ | 204 | $ | 349 | $ | 507 | ||||||
| Note receivable from related party |
100 | 100 | — | |||||||||
| Other |
148 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Prepaid expenses and other current assets |
$ | 452 | $ | 449 | $ | 507 | ||||||
|
|
|
|
|
|
|
|||||||
F-16
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 3. | Composition of Certain Financial Statement Items (Continued) |
As of December 31, 2012 and 2013, and March 31, 2014, other non-current assets consist of two-thirds of the security deposit on the Company’s operating lease for its headquarters in Menlo Park, California of $0.1 million.
Other Current and Non-Current Liabilities (in thousands):
| December 31, | March
31, 2014 |
|||||||||||
| 2012 | 2013 | |||||||||||
| (unaudited) | ||||||||||||
| Manufacturing expenses |
$ | 206 | $ | 267 | $ | 451 | ||||||
| Deferred rent |
50 | 115 | 121 | |||||||||
| Sales and use tax |
32 | 104 | 165 | |||||||||
| Facilities expenses |
25 | 104 | 113 | |||||||||
| Other |
76 | 155 | 82 | |||||||||
|
|
|
|
|
|
|
|||||||
| Other current liabilities |
$ | 389 | $ | 745 | $ | 932 | ||||||
|
|
|
|
|
|
|
|||||||
Other non-current liabilities consist of deferred rent as of December 31, 2012 and 2013, and March 31, 2014.
Interest and Other Income (in thousands):
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Interest income |
$ | 27 | $ | 7 | $ | 3 | $ | 1 | ||||||||
| Decrease in fair value of convertible preferred stock warrant |
128 | — | — | — | ||||||||||||
| Other |
1 | 79 | 79 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 156 | $ | 86 | $ | 82 | $ | 1 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Interest and Other Expense (in thousands):
| Year Ended December 31, | Three Months Ended March 31, |
|||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Interest expense |
$ | 4 | $ | 117 | $ | 24 | $ | 26 | ||||||||
| Loss on disposal of assets |
32 | 26 | — | — | ||||||||||||
| Increase in fair value of convertible preferred stock warrant |
— | 134 | — | 286 | ||||||||||||
| Increase in fair value of convertible preferred stock financing option |
— | 212 | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 36 | $ | 489 | $ | 24 | $ | 312 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| 4. | Notes Receivable from Related Parties |
In April 2013, options held by the President and Chief Executive Officer, Ms. Earnhardt, a related party, were modified to permit exercise with promissory notes of up to $0.5 million. The promissory notes were issued in May 2013 for $0.5 million. Under the terms of the notes, one quarter of the principal and interest will be forgiven on each anniversary date of the note as long as Ms. Earnhardt remains the Company’s Chief Executive Officer. In addition, the entire principal and interest of the notes will be forgiven on the earlier of an initial public offering or the closing of a liquidation event (as defined in the articles of incorporation) in each case where total
F-17
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 4. | Notes Receivable from Related Parties (Continued) |
proceeds payable to the Company or its stockholders is greater than $200.0 million. The Company has the option to accelerate the maturity date if, at the Company’s reasonable discretion, such acceleration may be necessary due to any applicable law, rule or regulation, including, without limitation, the Sarbanes-Oxley Act of 2002. The full recourse promissory notes are secured by a pledge of the exercised shares.
In January 2007, the Company loaned amounts to its Chief Operation Officer, Mr. Kaufman, a related party, and another employee in connection with the commencement of their employment with the Company. The loans were evidenced by promissory notes for $0.3 million from Mr. Kaufman and $0.1 million from the other employee. These notes bore interest at the applicable federal rate and interest was determined in accordance with Section 7872 of the Internal Revenue Code and has been reflected as income to the employee.
Pursuant to the terms of the promissory notes, provided the individuals remained continuous, full-time employees of the Company, repayment of the notes was to begin after the third year of employment when 50% of the employees’ annual bonus would have been applied to the loan balance until the loan was paid in full. The notes mature and become immediately due and payable within 30 days following the date on which the employee ceases, voluntarily or involuntarily, to be employed by the Company. In August 2009, management agreed to forgive the $0.1 million loan, provided the individual remained employed by the Company, at the rate of one-third annually in February 2010, 2011 and 2012. This note was fully forgiven as of December 31, 2012. For Mr. Kaufman’s loan, no payment was received in the year ended December 31, 2011, since the Company did not pay an annual bonus in that year. In February 2011, the Company agreed to forgive Mr. Kaufman’s loan over three years, provided Mr. Kaufman remained employed by the Company, with $0.1 million forgiven in January 2012, $0.1 million in January 2013 and $0.1 million in January 2014. The notes are secured by any stock and options the employees hold in the Company. The note receivable balance outstanding at December 31, 2012 and 2013 was $0.2 million and $0.1 million, respectively. Mr. Kaufman’s note was fully forgiven as of March 31, 2014.
| 5. | Loan and Security Agreement |
In August 2013, the Company entered into a loan and security agreement (“Loan Agreement”) with Silicon Valley Bank (“SVB”) for a total commitment of $12.0 million. The commitment consists of an $8.0 million growth capital facility and a $4.0 million revolving accounts receivable line of credit.
The $8.0 million growth capital facility consists of two $4.0 million tranches. The first $4.0 million tranche was available immediately upon execution of the Loan Agreement and will continue to be available until October 31, 2014. The second $4.0 million tranche was contingent upon the Company achieving a trailing three-month revenue of at least $7.0 million. The Company achieved that condition as of January 31, 2014, and therefore the second tranche will be available until March 31, 2015. Payment under both tranches will be interest-only until March 31, 2015, at which time the outstanding balance will convert to a 30-month fully amortizing loan. The interest rate will be fixed at the time of advance and will be the greater of 3.65% or the three-year U.S. Treasury note plus 3%. At the end of the amortization period, the Company will make a final payment of 3.9% of the advanced amounts. There is a prepayment penalty of 2% of the prepaid principal during the first year, 1% during the second year and no prepayment penalty during the third year.
In addition, the Company issued a warrant to SVB to purchase up to 20,313 shares of the Company’s Series D convertible preferred stock for an aggregate exercise price of 0.5% of the total growth capital facility commitment, or $40,000. As of March 31, 2014, this warrant was exercisable for 5,803 shares of the Company’s Series D convertible preferred stock at an exercise price of $6.89 per share as of December 31, 2013, and March 31, 2014. Following any draw-downs under the growth capital facility, the warrant will become exercisable for additional shares of Series D convertible preferred stock equal to 1.25% of the advanced amounts under tranche one and two divided by $6.89. The initial value of the warrants issued was $10,000 and has been recognized as convertible preferred stock warrant liability and interest expense.
F-18
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 5. | Loan and Security Agreement (Continued) |
The $4.0 million revolving accounts receivable line of credit will expire in three years. Advances will be made for up to 80% of eligible accounts receivable. An annual loan fee of 0.75% will be paid at the beginning of each of the three years. The interest will be the greater of 4.25% or SVB Prime Rate plus 0.25%.
As of March 31, 2014, the Company had not received any advances under the Loan Agreement.
| 6. | Equipment Loans |
In September 2012, the Company entered into an equipment loan with an aggregate principal amount of $2.0 million, all of which was drawn down in December 2012. Payments will be made in monthly installments over a 36-month period with an annual interest rate of 5.1%. A total of $4,000 and $0.1 million was recorded as interest expense during the years ended December 31, 2012 and 2013, respectively, and $24,000 and $16,000 during the three months ended March 31, 2013 and 2014, respectively. The amounts outstanding under this loan at December 31, 2012 and 2013, and March 31, 2014, were $2.0 million, $1.4 million and $1.2 million, respectively.
In April 2013, the Company entered into an equipment loan for a principal amount of $0.1 million. Payments will be made in monthly installments over a 38-month period with an interest rate of 14.88%. A total of $11,000 was recorded as interest expense during the year ended December 31, 2013, and $3,000 during the three months ended March 31, 2014. The amounts outstanding under this loan at December 31, 2013, and March 31, 2014, were $87,000 and $80,000, respectively.
At December 31, 2013 and March 31, 2014, future minimum payments under these equipment loans are as follows (in thousands):
| Year Ending December 31, |
December 31, 2013 |
March 31, 2014 |
||||||
| (unaudited) | ||||||||
| 2014 (remaining) |
$ | 761 | $ | 570 | ||||
| 2015 |
761 | 761 | ||||||
| 2016 |
21 | 21 | ||||||
| Thereafter |
— | — | ||||||
|
|
|
|
|
|||||
| Total minimum payments |
1,543 | 1,352 | ||||||
| Less: amount representing interest |
(91 | ) | (72 | ) | ||||
|
|
|
|
|
|||||
| Present value of future payments |
1,452 | 1,280 | ||||||
| Less: current portion |
(696 | ) | (705 | ) | ||||
|
|
|
|
|
|||||
| Non-current portion |
$ | 756 | $ | 575 | ||||
|
|
|
|
|
|||||
| 7. | Convertible Preferred Stock Warrant Liability |
As of December 31, 2012 and 2013, and March 31, 2014, the following warrants to purchase shares of convertible preferred stock were outstanding and exercisable (in thousands, except per share data):
| In Connection |
Series |
Exercise Price |
Shares Outstanding at | Initial Value |
Fair Value at | |||||||||||||||||||||||||||||||||
| Dates |
December 31, | March
31, 2014 |
December 31, | March
31, 2014 |
||||||||||||||||||||||||||||||||||
| Issuance |
Expiration |
2012 | 2013 | 2012 | 2013 | |||||||||||||||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||||||||||||||||
| Jan to Mar 2006 |
Jan to Mar 2013 | Convertible notes | A | $ | 3.68 | 22,417 | — | — | $ | 2 | $ | — | $ | — | $ | — | ||||||||||||||||||||||
| Mar 2007 |
Mar 2014 | Equipment loan | A | 3.68 | 4,076 | 4,076 | — | 4 | 7 | 17 | — | |||||||||||||||||||||||||||
| Nov 2007 |
Nov 2017 | Venture loan | A | 3.68 | 47,554 | 47,554 | 47,554 | 63 | 86 | 200 | 447 | |||||||||||||||||||||||||||
| Aug 2013 |
Aug 2023 | Loan Agreement | D | 6.89 | — | 5,803 | 5,803 | 10 | — | 20 | 47 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
| 74,047 | 57,433 | 53,357 | $ | 93 | $ | 237 | $ | 494 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||
F-19
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 7. | Convertible Preferred Stock Warrant Liability (Continued) |
During the years ended December 31, 2012 and 2013, warrants to purchase 57,744 and 22,418 shares, respectively, of Series A convertible preferred stock were exercised at a price of $3.68 per share. During the year ended December 31, 2012, warrants to purchase 13,587 shares of Series A convertible preferred stock expired unexercised. During the three months ended March 31, 2013, warrants to purchase 22,418 shares of Series A convertible preferred stock were exercised at a price of $3.68 per share. During the three months ended March 31, 2014, warrants to purchase 4,076 shares of Series A convertible preferred stock were exercised though a cashless exercise provision. Net shares of 2,431 were issued and the remaining 1,645 shares were withheld for the exercise price.
In connection with the execution of the Loan Agreement, warrants were issued to purchase the Company’s Series D convertible preferred stock. The warrants may currently be exercised for 5,803 shares of Series D convertible preferred stock at an exercise price of $6.89 per share. Alternatively, the bank has the option to exercise the warrants to purchase shares of the class of equity sold to investors in a future round of equity financing with proceeds of at least $5.0 million. The exercise price would be the price paid by the lead investor in the new financing and the number of shares exercisable would be based on an aggregate exercise price of $40,000, representing 0.5% of the total growth capital facility. This represents a warrant for 5,803 shares at an exercise price of $6.89 per share as of December 31, 2013, and March 31, 2014. The warrants expire in August 2023 or upon the occurrence of an acquisition of the Company in exchange for cash or marketable securities. Following any draw-downs under the growth capital facility, the warrant will become exercisable for additional shares of Series D convertible preferred stock equal to 1.25% of the advanced amounts under tranche one and two divided by $6.89.
| 8. | Convertible Preferred Stock |
A summary of the Company’s convertible preferred stock is as follows:
| December 31, 2012 | December 31, 2013 | March 31, 2014 | ||||||||||||||||||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||||||||||||||||
| Series |
Shares Authorized |
Shares Issued and Outstanding |
Carrying Value (in thousands) |
Shares Authorized |
Shares Issued and Outstanding |
Carrying Value (in thousands) |
Shares Authorized |
Shares Issued and Outstanding |
Carrying Value (in thousands) |
|||||||||||||||||||||||||||
| A |
2,809,846 | 2,722,201 | $ | 9,905 | 2,796,259 | 2,744,629 | $ | 9,987 | 2,796,259 | 2,747,060 | $ | 10,016 | ||||||||||||||||||||||||
| B |
3,249,662 | 3,249,663 | 19,894 | 3,249,662 | 3,249,663 | 19,894 | 3,249,662 | 3,249,663 | 19,894 | |||||||||||||||||||||||||||
| C |
5,687,500 | 5,311,402 | 30,521 | 5,311,401 | 5,311,402 | 30,521 | 5,311,401 | 5,311,402 | 30,521 | |||||||||||||||||||||||||||
| D |
— | — | — | 4,550,000 | 4,395,788 | 30,358 | 4,550,000 | 4,395,788 | 30,358 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| 11,747,008 | 11,283,253 | $ | 60,320 | 15,907,322 | 15,701,444 | $ | 90,760 | 15,907,322 | 15,703,875 | $ | 90,789 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
A summary of the Company’s convertible preferred stock terms is as follows:
| Series |
Liquidation Preference Per Share |
8% Dividend Per Share |
||||||
| A |
$ | 3.68 | $ | 0.2944 | ||||
| B |
6.16 | 0.4928 | ||||||
| C |
5.84 | 0.4672 | ||||||
| D |
6.89 | 0.5514 | ||||||
The Company recorded its convertible preferred stock at fair value on the dates of issuance, net of issuance costs. A redemption event will only occur upon the liquidation or winding up of the Company, a greater than 50% change in control, or sale of substantially all of the assets of the Company. As the redemption event is outside the control of the Company, all shares of convertible preferred stock have been presented outside of permanent equity. Further, the Company has also elected not to adjust the carrying values of the convertible preferred stock to the redemption value of such shares, since it is uncertain whether or when a redemption event
F-20
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 8. | Convertible Preferred Stock (Continued) |
will occur. Subsequent adjustments to increase the carrying value to the redemption values will be made when it becomes probable that such redemption will occur. As of December 31, 2012 and 2013, and March 31, 2013, it was not probable that such redemption would occur.
Series D Convertible Preferred Stock
In February 2013, the Company completed the initial closing of the Series D convertible preferred stock financings. The total net cash proceeds from this initial closing totaled $18.2 million and 2,656,638 shares of Series D convertible preferred stock were issued. In March 2013, the Company completed an additional closing of the Series D convertible preferred stock financings. The total net cash proceeds from this additional closing totaled $2.5 million and 370,140 shares of Series D convertible preferred stock were issued. The March 2013 closing included 72,548 shares purchased by certain employees of the Company for $0.5 million.
The Series D convertible preferred stock financing contained a provision that obligated the investors to purchase additional shares (“convertible preferred stock financing option”) at the same price as the initial closing upon notification by the Company that it had achieved an annualized revenue rate of at least $16.0 million over a trailing three-month period. This convertible preferred stock financing option to purchase Series D convertible preferred stock in the future tranche was considered to be a freestanding financial instrument for accounting purposes. Therefore, in February 2013, the Company recorded a financing liability of $0.9 million representing the fair value of this convertible preferred stock financing option at the time of issuance. In October 2013, shortly after achieving the annualized revenue rate, the Company issued the additional 1,369,070 shares of Series D convertible preferred stock to the investors for net proceeds of $9.4 million. Since the convertible preferred stock financing option expired in October 2013 as a result of the issuance of Series D convertible preferred stock, the carrying and fair value of the convertible preferred stock financing option of $1.1 million was reclassified from liability to Series D convertible preferred stock. The Company recorded a charge related to the change in fair value in October 2013 of $0.2 million related to this financing option liability.
Dividends
The holders of Series A, B, C and D convertible preferred stock are entitled to receive dividends (as determined on a per annum basis and on an as-converted basis), from any assets legally available, prior and in preference to any declaration or payment of any dividend to the common stockholders. No dividends other than those payable solely in common stock shall be paid on any common stock, Series A or Series B convertible preferred stock until they are paid on each outstanding share of Series C and D convertible preferred stock. Such dividends are payable when and if declared by the Board of Directors and are not cumulative. No dividends have been declared through December 31, 2013.
Liquidation
In the event of any liquidation, dissolution, or winding up of the Company, whether voluntary or involuntary (“Liquidation Event”), the holders of Series C and D convertible preferred stock are entitled to receive their liquidation preferences, prior to and in preference of any distribution of any assets of the Company to the holders of common stock, Series A or Series B convertible preferred stock, as adjusted for any stock splits, combinations, reorganizations, or similar transactions, plus any declared and unpaid dividends. If, upon the occurrence of such an event, the proceeds thus distributed among the holders of Series C and D convertible preferred stock shall be insufficient to permit the payment to such holders of the full preferential amounts, then the entire amount legally available for distribution shall be distributed among the holders of Series C and D convertible preferred stock in proportion to the full preferential amount that each such holder is otherwise entitled to receive had such proceeds been available.
After payment in full of the Series C and D convertible preferred stock liquidation preference, Series A and B convertible preferred stock liquidation preferences will be paid, as adjusted for any stock splits, combinations,
F-21
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 8. | Convertible Preferred Stock (Continued) |
reorganizations, or similar transactions. If, upon the occurrence of such an event, the proceeds thus distributed among the holders of Series A and B convertible preferred stock shall be insufficient to permit the payment to such holders of the full preferential amounts, then the entire amount legally available for distribution (after payment in full of the Series C and D convertible preferred stock liquidation preference) shall be distributed among the holders of Series A and B convertible preferred stock in proportion to the full preferential amount that each such holder is otherwise entitled to receive had such proceeds been available.
After liquidation preference payments have been made to the holders of the convertible preferred stock as described above, any remaining assets and funds of the Company are to be distributed ratably among the holders of common stock and Series A, B, C and D convertible preferred stock, assuming conversion of all shares of convertible preferred stock.
Prior to the Liquidation Event, the holders of all series of preferred stock may convert their preferred stock to common stock at the then-applicable conversion rate. Distribution of the Company’s assets to the new and existing common stockholders would then be made pro rata according to the number of shares held by each investor.
If convertible preferred stock is not converted to common stock prior to the Liquidation Event, the holders of Series C and D convertible preferred stock are entitled to receive their liquidation preferences, prior to and in preference of distribution of any assets of the Company to the holders of common stock, Series A and Series B convertible preferred stock, by reason of their ownership (as adjusted for any stock splits, combinations, reorganizations, or similar transactions), plus any declared but unpaid dividends on such shares. The holders of Series A and B convertible preferred stock are entitled to receive their liquidation preferences, prior to and in preference of distribution of any assets of the Company to the holders of common stock, by reason of their ownership (as adjusted for any stock splits, combinations, reorganizations, or similar transactions), plus any declared but unpaid dividends on such shares.
Voting
Except as otherwise required by law, the holders of common and convertible preferred stock vote together as a single class. The holders of the convertible preferred stock are entitled to the number of votes equal to the number of shares of common stock into which the convertible preferred stock could be converted on the record date for the vote, or upon the written consent of the stockholders.
Redemption
The convertible preferred shares are not redeemable.
Conversion
Each share of Series A, B, C and D convertible preferred stock, at the option of the holder and at any time after the date of issuance, is convertible into shares of common stock, subject to certain anti-dilution adjustments, in accordance with the conversion formula provided in the Company’s Articles of Incorporation (currently 1-for-1). In the event of a stock split, the conversion price in effect immediately prior to such split will be proportionately decreased. Conversion is automatic at the conversion rate: (1) immediately upon the closing of a firm commitment, underwritten initial public offering pursuant to an effective registration statement under the Securities Act of 1933, as amended, in which the public offering price reflects a pre-money valuation of at least $200.0 million and with net proceeds raised of at least $40.0 million or (2) at the election of the holders of at least 70% of the then outstanding shares of such series of preferred stock, the election of the holders of at least 65% of the Series C convertible preferred stock and the election of the holders of at least 52% of the Series D convertible preferred stock, voting as a separate class. The convertible preferred stock warrants become exercisable for common stock.
F-22
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 9. | Stockholder’s Equity |
Founders’ Restricted Stock Agreements
The Company and the founders entered into restricted stock agreements in October 2003 for 142,500 shares of common stock, in June 2005 for 50,000 shares of common stock and in October 2005 for 57,500 shares of common stock.
The 2003 restricted stock was issued for cash consideration of $0.001 per share. The 2005 restricted stock was issued for cash consideration of $0.02 per share. The restricted stock issued was subject to a Company repurchase option at the original exercise price in the event of termination of a founder’s services with the Company. All 250,000 shares related to founders’ stock agreements are issued and outstanding and are included within common stock at December 31, 2012 and 2013, and March 31, 2014. None of these shares are subject to repurchase as of December 31, 2012 and 2013, and March 31, 2014.
2013 Equity Incentive Plan
Under the 2013 Equity Incentive Plan (the “2013 Plan”) approved by the Company’s Board of Directors in September 2013, 2,216,396 shares of common stock have been reserved for the issuance of incentive stock options (“ISOs”), non-statutory stock options (“NSOs”), stock bonuses and rights to acquire restricted stock to employees, officers, directors and consultants of the Company as of December 31, 2012. ISOs and NSOs may be granted with exercise prices at no less than 100% and 85%, respectively, of the fair value of the common stock on the date of grant. Options granted to a 10% stockholder shall be at no less than 110% of the fair value and ISO stock option grants to such 10% stockholders expire five years from the date of grant. ISOs granted under the 2013 Plan generally vest 25% after the completion of 12 months of service and the balance vests in equal monthly installments over the next 36 months of service and expire 10 years from the grant date, unless subject to provisions regarding 10% stockholders. NSOs vest per the specific agreement and expire 10 years from the date of grant. There were no options issued with 100% of the shares vested as of the date of grant during the years ended December 31, 2012 and 2013, and the three months ended March 31, 2013 and 2014. New shares are issued upon exercise of options under the stock plan.
The 2013 Plan is the successor to the 2003 Equity Incentive Plan (“2003 Plan”) which expired in September 2013. Options available for grant under the 2003 Plan of 1,702,916 were incorporated into the 2013 Plan. Options outstanding under the 2003 Plan will be returned to the 2013 Plan upon forfeiture.
A summary of the Company’s stock option activity and related information is as follows (options in thousands):
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||||||||||
| 2012 | 2013 | 2014 | ||||||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||||
| Options | Price | Options | Price | Options | Price | |||||||||||||||||||
| Outstanding, beginning of period |
1,425 | $ | 0.80 | 1,594 | $ | 0.88 | 1,928 | $ | 1.08 | |||||||||||||||
| Granted—at fair market value |
296 | 1.16 | 1,033 | 1.20 | 104 | 6.20 | ||||||||||||||||||
| Granted—below fair market value |
— | — | 94 | 1.32 | — | — | ||||||||||||||||||
| Exercised |
(115 | ) | 0.76 | (740 | ) | 0.84 | (37 | ) | .96 | |||||||||||||||
| Forfeited |
(12 | ) | 0.88 | (53 | ) | 1.08 | (30 | ) | 1.08 | |||||||||||||||
| Expired |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Outstanding, end of period |
1,594 | 0.88 | 1,928 | 1.08 | 1,965 | 1.34 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Vested and expected to vest |
1,461 | 0.84 | 1,752 | 1.08 | 1,791 | 1.34 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Exercisable |
1,206 | 0.80 | 1,314 | 1.00 | 1,337 | 1.08 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
F-23
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 9. | Stockholder’s Equity (Continued) |
The following is a further breakdown of the options outstanding at December 31, 2013 and March 31, 2014 (options in thousands):
| December 31, 2013 |
||||||||||||||||||||
| Range of Prices |
Options Outstanding |
Weighted Average Remaining Contractual Life (years) |
Weighted Average Exercise Price |
Options Exercisable |
Weighted Average Exercise Price |
|||||||||||||||
| $0.02-$0.48 |
129 | 3.1 | $ | 0.48 | 129 | $ | 0.48 | |||||||||||||
| 0.72-0.76 |
261 | 7.0 | 0.72 | 232 | 0.72 | |||||||||||||||
| 1.00-1.08 |
327 | 7.2 | 1.04 | 253 | 1.04 | |||||||||||||||
| 1.20-1.32 |
1,211 | 9.3 | 1.20 | 700 | 1.20 | |||||||||||||||
|
|
|
|
|
|
||||||||||||||||
| $0.02-$1.32 |
1,928 | 8.2 | 1.08 | 1,314 | 1.01 | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
| March 31, 2014 |
||||||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Range of Prices |
Options Outstanding |
Weighted Average Remaining Contractual Life (years) |
Weighted Average Exercise Price |
Options Exercisable |
Weighted Average Exercise Price |
|||||||||||||||
| $0.02-$0.48 |
125 | 2.9 | $ | 0.48 | 125 | $ | 0.48 | |||||||||||||
| 0.72-0.76 |
244 | 6.7 | 0.72 | 225 | 0.72 | |||||||||||||||
| 1.00-1.08 |
323 | 7.0 | 1.04 | 263 | 1.04 | |||||||||||||||
| 1.20-1.32 |
1,170 | 9.1 | 1.20 | 713 | 1.20 | |||||||||||||||
| 6.20-6.20 |
103 | 9.9 | 6.20 | 11 | 6.20 | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
| $0.02-$6.20 |
1,965 | 8.1 | 1.34 | 1,337 | 1.06 | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
As of December 31, 2013 and March 31, 2014, the aggregate pre-tax intrinsic value of options outstanding and exercisable was $4.2 million and $13.5 million, respectively, and options outstanding were $6.1 million and $19.2 million, respectively. The aggregate pre-tax intrinsic value of options exercised was $37,000 and $0.3 million during the years ended December 31, 2012 and 2013, respectively, and $23,000 and $0.2 million during the three months ended March 31, 2013 and 2014, respectively. The aggregate pre-tax intrinsic value was calculated as the difference between the exercise prices of the underlying options and the estimated fair value of the common stock on the date of exercise. The total cash received upon the exercise of options was $0.1 million and $0.6 million during the years ended December 31, 2012 and 2013, respectively, and $36,000 during each of the three months ended March 31, 2013 and 2014.
Early Exercise of Stock Options
Stock options granted under the Plan may provide option holders the right to elect to exercise unvested options in exchange for restricted common stock. During the year ended December 31, 2013, employees exercised options with 69,037 unvested shares. As of December 31, 2013 and March 31, 2014, 53,796 and 47,470 shares, respectively, remained subject to a repurchase right held by the Company at the original issuance price in the event the optionees’ service is voluntarily or involuntarily terminated. As of December 31, 2013 and March 31, 2014, the related liability was $31,000 and $28,000, respectively. There were no shares subject to repurchase at December 31, 2012.
F-24
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 9. | Stockholder’s Equity (Continued) |
Reserved Shares
The Company is required to reserve and keep available out of its authorized but unissued shares of common stock a number of shares sufficient to effect the conversion of all outstanding shares of convertible preferred stock (and preferred stock warrants), plus options granted and available for grant under the incentive stock plan.
The number of such shares of common stock reserved for the conversion, exercise and issuance of the following options and shares outstanding as of December 31, 2013 and March 31, 2014, are as follows (in thousands):
| December 31, 2013 |
March 31, 2014 |
|||||||
| (unaudited) | ||||||||
| Convertible preferred stock: |
||||||||
| Series A: |
||||||||
| Outstanding |
2,745 | 2,747 | ||||||
| Warrants issued in connection with: |
||||||||
| Equipment loans |
4 | — | ||||||
| Venture loan financing |
48 | 48 | ||||||
| Series B outstanding |
3,250 | 3,250 | ||||||
| Series C outstanding |
5,311 | 5,311 | ||||||
| Series D: |
||||||||
| Outstanding |
4,396 | 4,396 | ||||||
| Warrants issued in connection with the Loan Agreement |
6 | 6 | ||||||
| 2003 Equity Incentive Plan, options outstanding |
1,747 | 1,683 | ||||||
| 2013 Equity Incentive Plan: |
||||||||
| Options outstanding |
181 | 282 | ||||||
| Options available for future grant |
260 | 187 | ||||||
|
|
|
|
|
|||||
| 17,948 | 17,910 | |||||||
|
|
|
|
|
|||||
| 10. | Stock-Based Compensation Expense |
Total stock-based compensation recognized, before taxes, during the years ended December 31, 2012 and 2013 and the three months ended March 31, 2013 and 2014, are as follows (in thousands):
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Cost of sales |
$ | 10 | $ | 21 | $ | 3 | $ | 9 | ||||||||
| Sales and marketing |
36 | 94 | 11 | 45 | ||||||||||||
| General and administrative |
67 | 179 | 14 | 99 | ||||||||||||
| Research and development |
39 | 88 | 10 | 29 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total stock-based compensation |
$ | 152 | $ | 382 | $ | 38 | $ | 182 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The amount of unearned stock-based compensation currently estimated to be expensed from now through the year 2017 related to unvested employee stock-based payment awards as of December 31, 2013 and March 31, 2014 is $1.5 million and $1.4 million, respectively. The weighted average period over which the unearned stock-based compensation is expected to be recognized as of December 31, 2013 and March 31, 2014, is 3.2 years and 3.1 years, respectively. If there are any modifications or cancellations of the underlying unvested securities, the Company may be required to accelerate, increase or cancel any remaining unearned stock-based compensation expense. Future stock-based compensation expense and unearned stock-based compensation will increase to the extent that the Company grants additional share-based payments.
F-25
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 10. | Stock-Based Compensation Expense (Continued) |
The Company estimates the fair value of stock-based compensation on the date of grant using the Black-Scholes option-pricing model. The Black-Scholes model determines the fair value of stock-based payment awards based on the fair market value of the Company’s common stock on the date of grant and is affected by assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, the fair market value of the Company’s common stock, volatility over the expected term of the awards and actual and projected employee stock option exercise behaviors. The Company has opted to use the “simplified method” for estimating the expected term of options, whereby the expected term equals the arithmetic average of the vesting term and the original contractual term of the option. Due to the Company’s limited operating history and a lack of company specific historical and implied volatility data, the Company has based its estimate of expected volatility on the historical volatility of a group of similar companies that are publicly traded. When selecting these public companies on which it has based its expected stock price volatility, the Company generally selected companies with comparable characteristics to it, including enterprise value, stages of clinical development, risk profiles, position within the industry and with historical share price information sufficient to meet the expected life of the stock-based awards. The historical volatility data was computed using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the share-based payments. The Company will continue to analyze the historical stock price volatility and expected term assumptions as more historical data for the Company’s common stock becomes available. The risk-free rate assumption is based on the U.S. Treasury instruments with maturities similar to the expected term of the Company’s stock options. The expected dividend assumption is based on the Company’s history of not paying dividends and its expectation that it will not declare dividends for the foreseeable future.
As stock-based compensation expense recognized in the financial statements is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from estimates. Forfeitures are estimated based on estimated future employee turnover and historical experience.
The fair value of the options granted to employees or directors during the years ended December 31, 2012 and 2013, and the three months ended March 31, 2013 and 2014, was estimated as of the grant date using the Black-Scholes model assuming the weighted average assumptions listed in the following table:
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013(1) | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Expected life (years) |
6.0 | 6.0 | — | 6.0 | ||||||||||||
| Expected volatility |
66 | % | 67 | % | — | 60 | % | |||||||||
| Risk-free interest rate |
0.9 | % | 1.6 | % | — | 1.8 | % | |||||||||
| Dividend yield |
0.0 | % | 0.0 | % | — | 0.0 | % | |||||||||
| Expected forfeitures |
7 | % | 5 | % | — | 5 | % | |||||||||
| Weighted average fair value |
$ | 0.68 | $ | 0.96 | — | $ | 3.44 | |||||||||
| (1) | No options were granted during the period |
Option Modification
In April 2013, options held by the President and Chief Executive Officer, Ms. Earnhardt, a related party, were modified to permit exercise with promissory notes of up to $0.5 million. Under the terms of the notes, one quarter of the principal and interest will be forgiven on each anniversary date of the note as long as Ms. Earnhardt remains the Company’s Chief Executive Officer. In addition, the entire principal and interest of the notes will be forgiven on the earlier of an initial public offering or the closing of a liquidation event (as defined in the articles of incorporation), in each case where total proceeds payable to the Company or its stockholders is greater than $200.0 million. The Company has the option to accelerate the maturity date if, at the
F-26
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 10. | Stock-Based Compensation Expense (Continued) |
Company’s reasonable discretion, such acceleration may be necessary due to any applicable law, rule or regulation, including, without limitation, the Sarbanes-Oxley Act of 2002. The economic effect of the modification was equivalent to converting options to purchase 0.6 million shares to a grant of restricted stock with four-year vesting and a contingent vesting acceleration provision. The incremental cost of the modification was $0.3 million, of which $50,000 and $13,000 was recognized in the year ended December 31, 2013, and three months ended March 31, 2014, respectively.
Performance-Based Options
Options issued during the year ended December 31, 2011, included grants to certain employees totaling 289,253 shares that contained vesting conditions contingent on the achievement of certain milestones. Assuming continued service by the employee, the vesting would begin on the date both milestones were achieved and vest monthly thereafter over the following four years. At December 31, 2011, the Company determined that it was probable that both milestones would be achieved and therefore began recording stock-based compensation expense related to these options in the year ended December 31, 2011. The milestones were achieved by the year ended December 31, 2013, and therefore began vesting accordingly. Stock-based compensation expense totaling $29,000 and $30,000 was recorded during the years ended December 31, 2012 and 2013, respectively, and $8,000 and $5,000 during the three months ended March 31, 2013 and 2014, respectively.
Options issued during the year ended December 31, 2013, included grants to certain employees totaling 363,000 shares that contained vesting conditions contingent on the achievement of certain milestones. Assuming continued service by the employee, the vesting would begin on the date both milestones were achieved and vest monthly over the following four years. As of December 31, 2013, the Company determined it was probable that both milestones would be achieved and therefore began recording stock-based compensation expense related to these options in the year ended December 31, 2013. The milestones were achieved by the year ended December 31, 2013, and therefore began vesting accordingly. Stock-based compensation expense totaling $0.1 million and $27,000 were recorded during the year ended December 31, 2013, and the three months ended March 31, 2014, respectively.
Options Issued to Consultants
During the years ended December 31, 2012 and 2013, non-statutory options were issued to non-employees to purchase 15,000 and 5,000 shares of common stock, respectively, for current and future services at weighted-average exercise prices of $0.96 and $1.20 per share, respectively. There were no options were issued to non-employees during the three months ended March 31, 2013 and 2014. The options were valued using the Black-Scholes model based on the following weighted average assumptions:
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Expected life (years) |
10.0 | 9.0 | 9.0 | 8.0 | ||||||||||||
| Expected volatility |
66 | % | 66 | % | 66 | % | 65 | % | ||||||||
| Risk-free interest rate |
1.8 | % | 2.4 | % | 2.0 | % | 2.8 | % | ||||||||
| Dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | 0.0 | % | ||||||||
The fair value of these options is expensed over the vesting period, which ranges from five months to three years. Stock-based compensation expense was $7,000 and $15,000 during the years ended December 31, 2012 and 2013, respectively, and $2,000 and $17,000 during the three months ended March 31, 2013 and 2014, respectively, related to consultants was charged to expense in the statements of operations and comprehensive loss.
F-27
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 11. | Commitments and Contingencies |
Building Lease
As of December 31, 2013 and March 31, 2014, the Company has one leased facility under an operating lease agreement. Rental payments on operating leases are charged to expense on a straight-line basis over the period of the lease. The Company entered into this 36-month lease in March 2012, effective June 1, 2012, for a facility with larger production and office space. The lease agreement requires the Company to pay executory costs such as real estate taxes, insurance and repairs, and includes a renewal provision allowing the Company to extend this lease for an additional three years at 95% of the then-current fair market rental rate.
Future minimum annual operating lease payments are as follows (in thousands):
| Year Ending December 31, |
December 31, 2013 |
March 31, 2014 |
||||||
| (unaudited) | ||||||||
| 2014 (remaining) |
$ | 928 | $ | 699 | ||||
| 2015 |
390 | 390 | ||||||
| Thereafter |
— | — | ||||||
|
|
|
|
|
|||||
| 1,318 | 1,089 | |||||||
|
|
|
|
|
|||||
As of December 31, 2012 and 2013, and March 31, 2014, two-thirds of the Company’s security deposit is recorded in other non-current assets and the remaining one-third is restricted cash.
Rent expense was $0.6 million and $1.4 million during the years ended December 31, 2012 and 2013, respectively, and $0.3 million during each of the three months ended March 31, 2013 and 2014, respectively.
Purchase Commitments
The Company had commitments to suppliers for purchases totaling $0.5 million and $0.7 million as of December 31, 2013 and March 31, 2014, respectively.
| 12. | Employee Retirement Plan |
In January 2007, the Company established a qualified retirement plan under section 401(k) of the Internal Revenue Code (“IRC”) under which participants may contribute up to 100% of their eligible compensation, subject to maximum deferral limits specified by the IRC. The Company may make a discretionary profit sharing contribution to each eligible employee, subject to limits specified by the IRC, on an annual basis, provided the employee is employed with the Company on the last day of the plan year which is December 31. In addition, the Company may also make a matching contribution of up to 3% of an employee’s eligible compensation on a quarterly basis. The Company’s contributions will vest 25% per year over four years. As of December 31, 2013 and March 31, 2014, the Company has not made any profit sharing or matching contributions.
| 13. | Income Taxes |
The Company has a history of losses and therefore has made no provision for income taxes. All losses result from domestic operations.
F-28
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 13. | Income Taxes (Continued) |
The amount computed by applying the federal statutory rate to income before taxes reconciles to the provision for income taxes is as follows (in thousands):
| Year Ended December 31, | ||||||||
| 2012 | 2013 | |||||||
| Tax at federal statutory rate |
$ | (5,564 | ) | $ | (6,245 | ) | ||
| State, net of federal benefit |
(1,131 | ) | (826 | ) | ||||
| Permanent items |
59 | 368 | ||||||
| R&D tax credit |
— | (584 | ) | |||||
| Other |
(3 | ) | (2 | ) | ||||
| Change in valuation allowance |
6,639 | 7,289 | ||||||
|
|
|
|
|
|||||
| $ | — | $ | — | |||||
|
|
|
|
|
|||||
Significant components of net deferred tax assets are as follows (in thousands):
| December 31, | ||||||||
| 2012 | 2013 | |||||||
| Deferred tax assets: |
||||||||
| Net operating losses |
$ | 21,719 | $ | 28,236 | ||||
| R&D tax credit |
1,642 | 2,394 | ||||||
| Accruals and other |
1,259 | 1,279 | ||||||
|
|
|
|
|
|||||
| 24,620 | 31,909 | |||||||
| Valuation allowance |
(24,620 | ) | (31,909 | ) | ||||
|
|
|
|
|
|||||
| $ | — | $ | — | |||||
|
|
|
|
|
|||||
The Company had deferred tax assets of $24.6 million and $31.9 million as of December 31, 2012 and 2013, respectively. Deferred income taxes reflect the tax effects of net operating loss and tax credit carryforwards and the net temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
Realization of the deferred tax assets is dependent upon the generation of future taxable income, if any, the amount and timing of which are uncertain. Based on available objective evidence, management believes it is more likely than not that the deferred tax assets are not recognizable and will not be recognizable until the Company has sufficient taxable income. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by $6.6 million and $7.3 million during the years ended December 31, 2012 and 2013, respectively.
As of December 31, 2013, the Company’s federal net operating loss carryforwards of $71.6 million will expire at various dates beginning in 2026, if not utilized. Federal research and development tax credits of $1.9 million will begin to expire in 2026. In addition, net operating loss carryforwards for state income tax purposes of $49.2 million will begin to expire in 2016 and state research and development tax credits of $1.7 million do not expire.
Utilization of the net operating loss carryforwards may be subject to an annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986 and similar state provisions. The annual limitation may result in the expiration of the net operating loss before utilization.
The Company had unrecognized tax benefit of $0.5 million and $0.7 million as of and December 31, 2012 and 2013, respectively, all of which is offset by a full valuation allowance. These unrecognized tax benefits, if recognized, would not affect the effective tax rate. There was no interest or penalties accrued at the adoption date and at December 31, 2013.
F-29
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 13. | Income Taxes (Continued) |
A reconciliation of the change in the unrecognized tax benefit during the year is as follows (in thousands):
| December 31, | ||||||||
| 2012 | 2013 | |||||||
| Beginning of year |
$ | 442 | $ | 505 | ||||
| Additions for tax positions related to: |
||||||||
| Current year |
63 | 210 | ||||||
| Prior years |
— | — | ||||||
|
|
|
|
|
|||||
| End of year |
$ | 505 | $ | 715 | ||||
|
|
|
|
|
|||||
The Company does not expect a significant change to its unrecognized tax benefits over the next twelve months. The unrecognized tax benefits may increase or change during the next twelve months for items that arise in the ordinary course of business.
The Company files income tax returns in the U.S. federal and various state jurisdictions. Tax years beginning in 2003 through 2013, remain open to examination by the major taxing authorities to which the Company is subject to. The Company’s policy is to record interest related to uncertain tax positions as interest and any penalties as other expense in its statement of operations. As of the date of adoption of ASC Topic No. 740 and through December 31, 2013, the Company did not have any interest or penalties associated with unrecognized tax benefits.
| 14. | Related Parties |
In April 2013, options held by the President and Chief Executive Officer, Ms. Earnhardt, a related party, were modified to permit exercise with promissory notes of up to $0.5 million. The promissory notes were issued in May 2013 for $0.5 million. Under the terms of the notes, one quarter of the principal and interest will be forgiven on each anniversary date of the note as long as Ms. Earnhardt remains the Company’s Chief Executive Officer. In addition, the entire principal and interest of the notes will be forgiven on the earlier of an initial public offering or the closing of a liquidation event (as defined in the articles of incorporation), where the proceeds to the Company or its stockholders is greater than $200.0 million. The Company has the option to accelerate the maturity date if, at the Company’s reasonable discretion, such acceleration may be necessary due to any applicable law, rule or regulation, including, without limitation, the Sarbanes-Oxley Act of 2002. The promissory notes are secured by the shares received upon exercise with the promissory notes.
In January 2007, the Company loaned amounts to its Chief Operation Officer, Mr. Kaufman, a related party, in connection with the commencement of his employment with the Company. The loans were evidenced by promissory notes for $0.3 million. This note bore interest at the applicable federal rate and interest was determined in accordance with Section 7872 of the Internal Revenue Code and has been reflected as income to Mr. Kaufman.
Pursuant to the terms of the promissory note, provided Mr. Kaufman remained a continuous, full-time employee of the Company, repayment of the notes was to begin after the third year of employment when 50% of his annual bonus would have been applied to the loan balance until the loan was paid in full. The note matures and becomes immediately due and payable within 30 days following the date on which Mr. Kaufman ceases, voluntarily or involuntarily, to be employed by the Company. No payment was received in the year ended December 31, 2011, since the Company did not pay an annual bonus in that year. In February 2011, the Company agreed to forgive Mr. Kaufman’s loan over three years, provided Mr. Kaufman remained employed by the Company, with $0.1 million forgiven in January 2012, $0.1 million in January 2013 and $0.1 million in January 2014. The note is secured by any stock and options Mr. Kaufman holds in the Company. The note receivable balance outstanding at December 31, 2012 and 2013 was $0.2 million and $0.1 million, respectively. Mr. Kaufman’s note was fully forgiven as of March 31, 2014.
F-30
Table of Contents
INTERSECT ENT, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 14. | Related Parties (Continued) |
In March 2009, the Company purchased three sinus irrigation tool patents from Medilyfe Inc., a Canadian corporation. A member of the Company’s Medical Advisory Board holds an executive-level position with Medilyfe Inc. The agreement calls for a $40,000 milestone payment upon execution of the agreement and an additional $35,000 upon the first anniversary of the agreement’s effective date in addition to the issuance of a warrant to purchase 47,350 shares of the Company’s common stock at $1.00 per share.
The warrant vested fully upon grant and will expire April 7, 2019. The value of the warrant was $30,000 determined using the Black-Scholes model. The cost of the patents is included in research and development expense, since substantial research and development efforts will be required prior to commercialization and there is no alternative future use of the technology rights. A $35,000 cash payment was made in 2012, since one additional patent claim was allowed by the U.S. Patent Office. The Company recorded $35,000 in research and development expense related to this agreement for the year ended December 31, 2012. An additional $80,000 cash payment will be due upon the first achievement of net sales of products incorporating this technology exceeding $1.0 million in a given calendar year. Such additional payment was not owed as of December 31, 2013, since this technology is unrelated to the Company’s current activities.
| 15. | Subsequent Events |
For the audited financial statements, management has reviewed and evaluated material subsequent events from the balance sheet date of December 31, 2013, through the financial statements’ issue date of May 1, 2014. No subsequent events have been identified for disclosure.
| 16. | Subsequent Events (unaudited) |
For the interim condensed financial statements as of March 31, 2014, and for the three months then ended, the Company has evaluated events through June 23, 2014, which is the date the financial statements were available to be issued. No subsequent events have been identified for disclosure other than as noted below. The promissory notes of $0.5 million issued to the Company’s Chief Executive Officer in May 2013 for the exercise of stock options were fully forgiven by the Company in June 2014. In connection with the forgiveness of the note in June 2014, the unamortized modification cost relating to the vested options of $0.2 million was immediately recognized. The remaining unamortized modification cost of $0.1 million will be amortized over the remaining vesting period.
| 17. | Subsequent Events |
On July 11, 2014, the Board of Directors and stockholders approved, and the Company filed, an amended and restated certificate of incorporation effecting a 1-for-4 reverse stock split of common stock and all convertible preferred stock. The par value of the common and convertible preferred stock was not adjusted as a result of the reverse stock split. All issued and outstanding common stock, convertible preferred stock, warrants for preferred stock, and per share amounts contained in the financial statements have been retroactively adjusted to reflect this reverse stock split for all periods presented.
F-31
Table of Contents

intersect®
ENT
ADVANCING CLINICALLY PROVEN
THERAPEUTIC SOLUTIONS
FOR PATIENTS WITH CHRONIC SINUSITIS
Table of Contents
5,000,000 Shares

Common Stock
PROSPECTUS
| J.P. Morgan | Piper Jaffray | |||
| Leerink Partners | Wedbush PacGrow Life Sciences | |||
, 2014
Table of Contents
PART II
Information Not Required in Prospectus
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable by the registrant in connection with the sale of common stock being registered. All amounts are estimates except for the Securities and Exchange Commission, or SEC, registration fee, the FINRA filing fee and The Nasdaq Global Market listing fee.
| Item |
Amount to be paid |
|||
| SEC registration fee |
9,628 | |||
| FINRA filing fee |
12,500 | |||
| The Nasdaq Global Market Listing fee |
125,000 | |||
| Printing and engraving expenses |
250,000 | |||
| Legal fees and expenses |
1,300,000 | |||
| Accounting fees and expenses |
1,200,000 | |||
| Transfer Agent fees and expenses |
2,500 | |||
| Miscellaneous expenses |
100,372 | |||
|
|
|
|||
| Total |
$ | 3,000,000 | ||
|
|
|
|||
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act of 1933, as amended, or the Securities Act. Our amended and restated certificate of incorporation to be in effect upon the completion of this offering provides for indemnification of our directors, officers, employees and other agents to the maximum extent permitted the Delaware General Corporation Law, and our amended and restated bylaws to be in effect upon the completion of this offering provide for indemnification of our directors, officers, employees and other agents to the maximum extent permitted by the Delaware General Corporation Law. In addition, we have entered into indemnification agreements with our directors, officers and some employees containing provisions that may be in some respects broader than the specific indemnification provisions contained in the Delaware General Corporation Law. The indemnification agreements may require us, among other things, to indemnify our directors against certain liabilities that may arise by reason of their status or service as directors, officers and employees and to advance their expenses incurred as a result of any proceeding against them as to which they could be indemnified.
We maintain a directors’ and officers’ insurance policy.
The underwriting agreement to be entered into in connection with this offering will provide that the underwriters will indemnify us, our directors and certain of our officers against liabilities resulting from information furnished by or on behalf of the underwriters specifically for use in the Registration Statement.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. Please read “Item 17. Undertakings” for more information on the SEC’s position regarding such indemnification provisions.
II-1
Table of Contents
Item 15. Recent Sales of Unregistered Securities.
Since January 1, 2011, we have made sales of the following unregistered securities (does not give retroactive effect to the 1-for-4 reverse stock split that occurred on July 11, 2014):
| • | We granted stock options under our 2003 Equity Incentive Plan and 2013 Equity Incentive Plan to purchase an aggregate of 10,932,448 shares of our common stock at a weighted average exercise price of $0.67 to a total of 186 employees, directors and consultants. Of these, stock options to purchase an aggregate of 558,295 shares have been cancelled without being exercised, 2,356,029 have been exercised and 8,018,124 remain outstanding. |
| • | We issued and sold an aggregate of 4,048,373 shares of our common stock to employees, directors and consultants at a weighted average exercise price of $0.29 upon the exercise of stock options granted under our 2003 Equity Incentive Plan and 2013 Equity Incentive Plan. Of these, none have been repurchased and all shares remain outstanding. |
| • | In August 2011, we issued an aggregate of 10,622,800 shares of our Series C convertible preferred stock to 17 accredited investors at a per share price of $1.46, for aggregate consideration of approximately $15,509,288. |
| • | In February 2013, we issued an aggregate of 10,626,550 shares of our Series D convertible preferred stock to 8 accredited investors at a per share price of $1.72, for aggregate consideration of approximately $18,309,546. |
| • | In March 2013, we issued an aggregate of 1,480,562 shares of our Series D convertible preferred stock to 27 accredited investors at a per share price of $1.72, for aggregate consideration of approximately $2,551,008. |
| • | In October 2013, we issued an aggregate of 5,476,038 shares of our Series D convertible preferred stock to 18 accredited investors at a per share price of $1.72, for aggregate consideration of approximately $9,435,214. |
| • | In March 2014, we issued an aggregate of 9,725 shares of our Series A convertible preferred stock to 1 accredited investor at a per share price of $0.92 upon the net exercise of preferred stock warrants, for aggregate consideration of approximately $8,947. |
Unless otherwise stated, the sales of the above securities were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were placed upon the stock certificates issued in these transactions.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits. See the Exhibit Index attached to this registration statement, which is incorporated by reference herein.
(b) Financial Statement Schedules. Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the Financial Statements or notes thereto.
Item 17. Undertakings.
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification by the Registrant for liabilities arising under the Securities Act may be permitted to directors, officers, and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise,
II-2
Table of Contents
the Registration has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer, or controlling person of the Registrant in the successful defense of any action, suit, or proceeding) is
asserted by such director, officer, or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The Registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For purposes of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-3
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Amendment No. 3 to the Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in Menlo Park, California, on July 18, 2014.
| Intersect ENT, Inc. | ||||
| Date: July 18, 2014 | By: | /S/ LISA D. EARNHARDT | ||
| Lisa D. Earnhardt President and Chief Executive Officer | ||||
| Date: July 18, 2014 | By: | /S/ JERYL L. HILLEMAN | ||
| Jeryl L. Hilleman Chief Financial Officer | ||||
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Name |
Title |
Date | ||
| /S/ LISA D. EARNHARDT Lisa D. Earnhardt |
President and Chief Executive Officer (Principal Executive Officer) and Director |
July 18, 2014 | ||
| /S/ JERYL L. HILLEMAN Jeryl L. Hilleman |
Chief Financial Officer (Principal Financial and Accounting Officer) |
July 18, 2014 | ||
| * Rick D. Anderson |
Director | July 18, 2014 | ||
| * Casper L. de Clercq |
Director | July 18, 2014 | ||
| * Mark Fletcher |
Director | July 18, 2014 | ||
|
Cynthia L. Lucchese |
Director | |||
| * Dana G. Mead, Jr. |
Director | July 18, 2014 | ||
| * Frederic H. Moll |
Director | July 18, 2014 | ||
| * Casey M. Tansey |
Director | July 18, 2014 | ||
| * Pursuant to Power of Attorney | ||
| By: | /S/ JERYL L. HILLEMAN | |
| Jeryl L. Hilleman Attorney-in-Fact | ||
II-4
Table of Contents
EXHIBIT INDEX
| Exhibit |
Description | |
| 1.1 | Form of Underwriting Agreement. | |
| 3.1† | Amended and Restated Certificate of Incorporation as currently in effect. | |
| 3.2† | Form of Amended and Restated Certificate of Incorporation to become effective upon closing of this offering. | |
| 3.3† | Bylaws, as currently in effect and Certificate of Amendment of Bylaws, dated September 5, 2013. | |
| 3.4† | Form of Amended and Restated Bylaws to become effective upon closing of this offering. | |
| 4.1† | Form of Common Stock Certificate of the Registrant. | |
| 5.1† | Opinion of Cooley LLP. | |
| 10.1† | Form of Indemnity Agreement between the Registrant and its directors and officers. | |
| 10.2† | 2003 Equity Incentive Plan, as amended, and Form of Stock Option Grant Notice, Option Agreement and Form of Notice of Exercise. | |
| 10.3† | 2013 Equity Incentive Plan and Form of Stock Option Grant Notice, Option Agreement and Form of Notice of Exercise. | |
| 10.4† | 2014 Equity Incentive Plan and Form of Stock Option Grant Notice, Option Agreement and Form of Notice of Exercise. | |
| 10.5† | 2014 Employee Stock Purchase Plan. | |
| 10.6† | Third Amended and Restated Investor Rights Agreement, dated as of February 15, 2013, by and among the registrant and certain of its stockholders. | |
| 10.7† | Lease by and between the registrant and Menlo Business Park, LLC, dated as of March 2, 2012. | |
| 10.8† | Offer Letter by and between the registrant and Lisa Earnhardt, dated as of January 28, 2008, as amended. | |
| 10.9† | Offer Letter by and between the registrant and Robert Binney, dated as of June 23, 2011, as amended. | |
| 10.10† | Offer Letter by and between the registrant and Jeryl L. Hilleman, dated as of May 15, 2014. | |
| 10.11† | Offer Letter by and between the registrant and Richard Kaufman, dated as of December 6, 2006, as amended. | |
| 10.12† | Offer Letter by and between the registrant and James Stambaugh, dated as of September 15, 2006, as amended. | |
| 10.13† | Offer Letter by and between the registrant and Susan Stimson, dated as of January 30, 2007, as amended. | |
| 10.14† | Offer Letter by and between the registrant and Amy Conuel Wolbeck, dated as of November 24, 2008, as amended. | |
| 10.15† | Loan and Security Agreement by and between the registrant and Silicon Valley Bank, dated as of August 30, 2013. | |
| 10.16†# | Supply Agreement by and between the registrant and HOVIONE Inter Ltd, dated as of April 14, 2014. | |
| 10.17†# | Supply Agreement by and between the registrant and AIM Plastics Inc., dated as of Supply January 28, 2014. | |
Table of Contents
| Exhibit |
Description | |
| 10.18†# | Supply Agreement by and between the registrant and Stephen Gould Corporation, dated as of November 14, 2013. | |
| 10.19†# | Analytical Testing Partnership Program 2014-2015 by and between the registrant and Exova, dated as of December 6, 2013. | |
| 10.20†# | Master Services Agreement by and between the registrant and Polymer Solutions Corporation, dated as of April 9, 2014. | |
| 23.1 | Consent of Independent Registered Public Accounting Firm. | |
| 23.2† | Consent of Cooley LLP. Reference is made to Exhibit 5.1. | |
| 24.1† | Power of Attorney (see signature page hereto). | |
| † | Previously filed. |
| # | Confidential Treatment Requested. |