Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ALLERGAN INC | d750005d8k.htm |
| EX-99.1 - EX-99.1 - ALLERGAN INC | d750005dex991.htm |
| Exhibit 99.2
|
Exhibit 99.2
June 30, 2014 Allergan
Abicipar Pegol (Anti-VEGF DARPin®) & Bimatoprost
Sustained-Release Implant for Glaucoma
|
|
Forward-Looking Statements
This presentation contains “forward-looking statements,” including statements regarding product acquisition and development, regulatory approvals, market potential, expected growth, efficiencies, and Allergan’s expected, estimated or anticipated future results, including Allergan’s earnings per share and revenue forecasts, among other statements. All forward-looking statements herein are based on Allergan’s current expectations of future events and represent Allergan’s judgment only as of the date of this presentation. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from Allergan’s expectations and projections. Therefore, you are cautioned not to rely on any of these forward-looking statements and Allergan expressly disclaims any intent or obligation to update these forward-looking statements except as required to do so by law.
Actual results may differ materially from Allergan’s current expectations based on a number of factors affecting Allergan’s businesses, including changing competitive, market and regulatory conditions; the timing and uncertainty of the results of both the research and development and regulatory processes; domestic and foreign health care and cost containment reforms, including government pricing, tax and reimbursement policies; revisions to regulatory policies related to the approval of competitive generic products; technological advances and patents obtained by competitors; the ability to obtain and maintain adequate protection of intellectual property rights; the performance of new products, including obtaining government approval and consumer and physician acceptance, the continuing acceptance of currently marketed products, and consistency of treatment results among patients; the effectiveness of promotional and advertising campaigns; the potential for negative publicity concerning any of Allergan’s products; the timely and successful implementation of strategic initiatives, including expansion of new or existing products into new markets; the results of any pending or future litigation, investigations or claims; the uncertainty associated with the identification of, and successful consummation, execution and integration of, external corporate development initiatives and strategic partnering transactions; potential difficulties in manufacturing; and Allergan’s ability to obtain and successfully maintain a sufficient supply of products to meet market demand in a timely manner. In addition, matters generally affecting the U.S. and international economies, including consumer confidence and debt levels, changes in interest and currency exchange rates, political uncertainty, international relations, the status of financial markets and institutions, impact of natural disasters or geo-political events and the state of the economy worldwide, may materially affect Allergan’s results.
These and other risks and uncertainties affecting Allergan’s businesses and operations may be found in Allergan’s most recently filed
Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q, including under the heading “Risk Factors”. These filings, as well as Allergan’s other public filings with the U.S. Securities and Exchange Commission (SEC), can be obtained without charge at the SEC’s web site at www.sec.gov. These SEC filings are also available at Allergan’s web site at www.allergan.com along with copies of Allergan’s press releases and additional information about Allergan. For further information, you can contact the Allergan Investor
Relations Department by calling 714-246-4636.
© 2014 Allergan, Inc. All rights reserved.
® & ™ Marks owned by Allergan, Inc.
All other products are registered trademarks of their respective companies
|
|
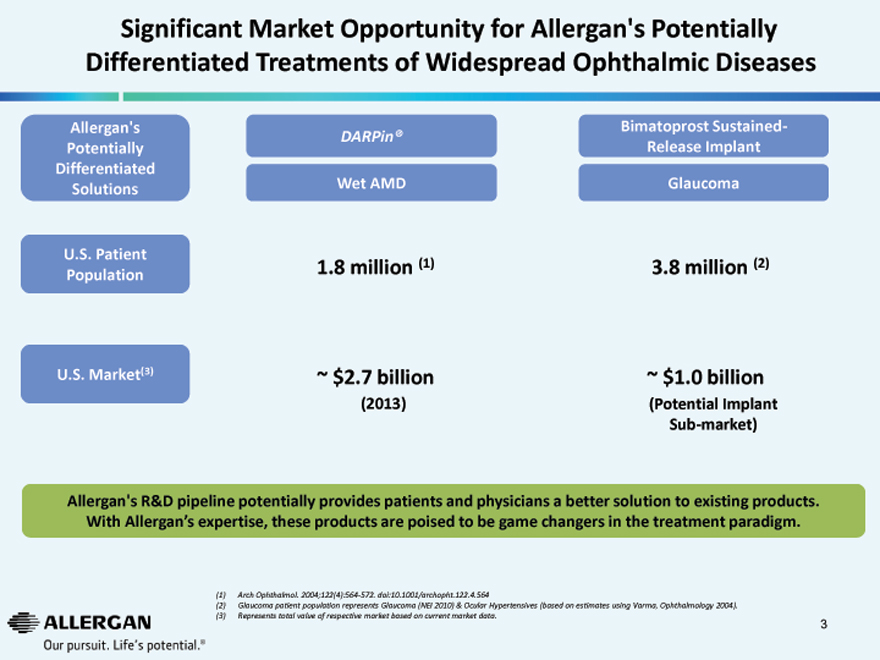
Significant Market Opportunity for Allergan’s Potentially Differentiated Treatments of Widespread Ophthalmic Diseases
Allergan’s Bimatoprost Sustained-
DARPin®
Potentially Release Implant
Differentiated
Solutions Wet AMD Glaucoma
U.S. Patient
Population 1.8 million (1) 3.8 million (2)
U.S. Market(3) ~ $2.7 billion ~ $1.0 billion
(2013) (Potential Implant
Sub-market)
Allergan’s R&D pipeline potentially provides patients and physicians a better solution to existing products.
With Allergan’s expertise, these products are poised to be game changers in the treatment paradigm.
| (1) |
|
Arch Ophthalmol. 2004;122(4):564-572. doi:10.1001/archopht.122.4.564 |
(2) Glaucoma patient population represents Glaucoma (NEI 2010) & Ocular Hypertensives (based on estimates using Varma, Ophthalmology 2004). (3) Represents total value of respective market based on current market data.
| 3 |
|
|
|
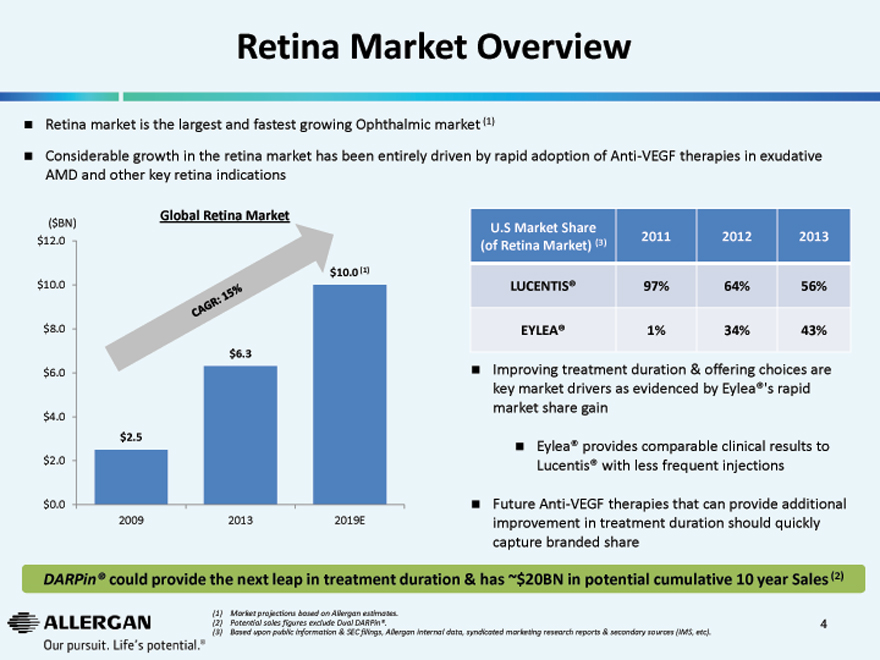
Retina Market Overview
Retina market is the largest and fastest growing Ophthalmic market (1)
Considerable growth in the retina market has been entirely driven by rapid adoption of Anti-VEGF therapies in exudative AMD and other key retina indications
($BN) Global Retina Market
$12.0
$10.0 (1)
$10.0
$8.0
$6.3
$6.0
$4.0
$2.5
$2.0
$0.0
2009 2013 2019E
U.S Market Share
(of Retina Market) (3) 2011 2012 2013
LUCENTIS® 97% 64% 56%
EYLEA® 1% 34% 43%
Improving treatment duration & offering choices are key market drivers as evidenced by Eylea®’s rapid market share gain
Eylea® provides comparable clinical results to Lucentis® with less frequent injections
Future Anti-VEGF therapies that can provide additional improvement in treatment duration should quickly capture branded share
DARPin® could provide the next leap in treatment duration & has ~$20BN in potential cumulative 10 year Sales (2)
| (1) |
|
Market projections based on Allergan estimates. |
| (2) |
|
Potential sales figures exclude Dual DARPin®. |
(3) Based upon public information & SEC filings, Allergan internal data, syndicated marketing research reports & secondary sources (IMS, etc).
| 4 |
|
|
|
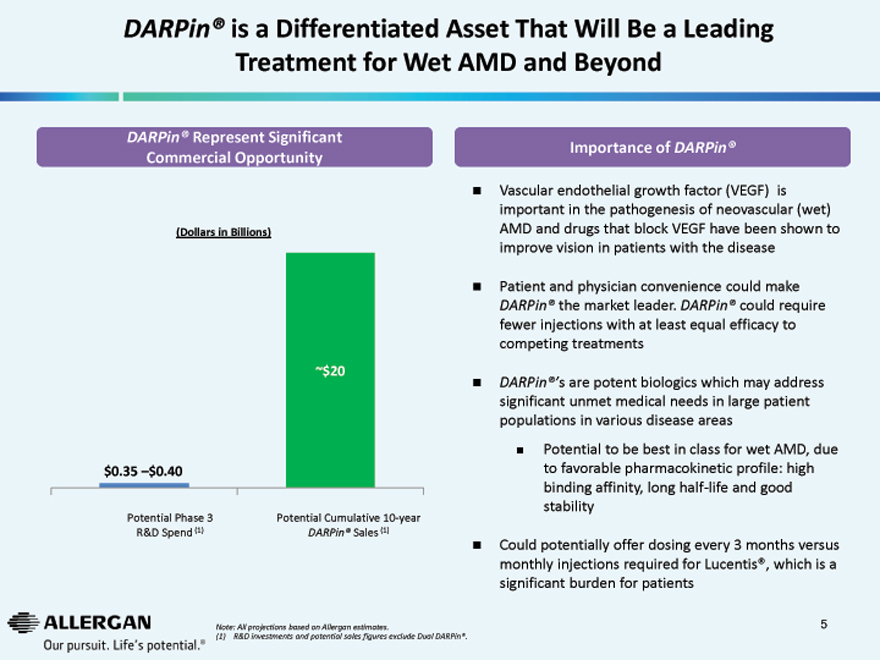
DARPin® is a Differentiated Asset That Will Be a Leading Treatment for Wet AMD and Beyond
DARPin® Represent Significant
Commercial Opportunity
(Dollars in Billions)
~$20
$0.35 –$0.40
Potential Phase 3 Potential Cumulative 10-year
R&D Spend (1) DARPin® Sales (1)
Importance of DARPin®
Vascular endothelial growth factor (VEGF) is
important in the pathogenesis of neovascular (wet)
AMD and drugs that block VEGF have been shown to
improve vision in patients with the disease
Patient and physician convenience could make
DARPin® the market leader. DARPin® could require
fewer injections with at least equal efficacy to
competing treatments
DARPin®’s are potent biologics which may address
significant unmet medical needs in large patient
populations in various disease areas
Potential to be best in class for wet AMD, due
to favorable pharmacokinetic profile: high
binding affinity, long half-life and good
stability
Could potentially offer dosing every 3 months versus
monthly injections required for Lucentis®, which is a
significant burden for patients
Note: All projections based on Allergan estimates.
| (1) |
|
R&D investments and potential sales figures exclude Dual DARPin®. |
| 5 |
|
|
|
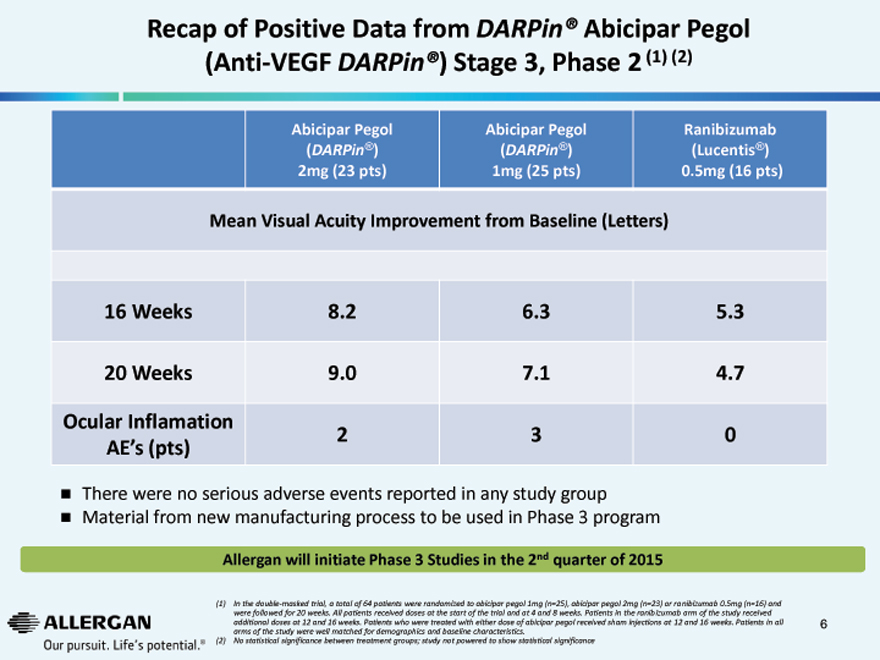
Recap of Positive Data from DARPin® Abicipar Pegol (Anti-VEGF DARPin®) Stage 3, Phase 2 (1) (2)
Abicipar Pegol Abicipar Pegol Ranibizumab
(DARPin®) (DARPin®) (Lucentis®)
2mg (23 pts) 1mg (25 pts) 0.5mg (16 pts)
Mean Visual Acuity Improvement from Baseline (Letters)
16 Weeks 8.2 6.3 5.3
20 Weeks 9.0 7.1 4.7
Ocular Inflamation
| 2 |
|
3 0 |
AE’s (pts)
There were no serious adverse events reported in any study group Material from new manufacturing process to be used in Phase 3 program
Allergan will initiate Phase 3 Studies in the 2nd quarter of 2015
(1) In the double-masked trial, a total of 64 patients were randomized to abicipar pegol 1mg (n=25), abicipar pegol 2mg (n=23) or ranibizumab 0.5mg (n=16) and were followed for 20 weeks. All patients received doses at the start of the trial and at 4 and 8 weeks. Patients in the ranibizumab arm of the study received additional doses at 12 and 16 weeks. Patients who were treated with either dose of abicipar pegol received sham injections at 12 and 16 weeks. Patients in all arms of the study were well matched for demographics and baseline characteristics.
| (2) |
|
No statistical significance between treatment groups; study not powered to show statistical significance |
| 6 |
|
|
|
Glaucoma Market Overview
Global Glaucoma Pharma Market (1)
($BN)
$7.4
$7.2
$7.2
$7.0
$6.8
$6.8
$6.6
$6.4
$6.2 $6.1
$6.0
$5.8
$5.6
$5.4
2014 2018 2022
Glaucoma is a global and growing epidemic
Impacts 3.8 million people in the U.S.(2)
Leading cause of irreversible blindness globally ? Loss of vision decreases quality of life and daily functioning ? Significant indirect societal cost ~ $1.5Bn per annum in the U.S.(3)
Market projections based on Allergan estimates
Glaucoma patient population represents Glaucoma (NEI 2010) & Ocular Hypertensives (based on estimates using Varma, Ophthalmology 2004).
Quigley and Broman. Br J Ophthalmol. 2006.
| 7 |
|
|
|
Bimatoprost Sustained-Release Implant is a Significant Global Opportunity
Bimatoprost Sustained-Release Implant is a Significant Innovation
A sustained-release, Disruptive, first-in-class
prostamide-loaded, technology
bioerodible implant Data from Phase 2 clinical trials
Injected into the anterior suggests that bimatoprost
chamber sustained-release implant efficacy
Can be performed in the is comparable to daily topical
office bimatoprost with duration of 4-6
Ensures patient compliance months
Noncompliance is an Issue with
Current Treatments
Inability to correctly and
reliably instill eye drops (1)(2)
Adverse effects associated
with taking eye drops (3)
The number of medication
and the complexity of the
dosing schedule (4)(5)(6)(7)
Understanding of glaucoma
including the consequences
and its treatment (1)(2)(8)
Medication cost (9)
Patient forgetfulness (2)(5)(8)
Of Patients are
~ 20% Not Well Managed
On Topical Drops (10)
If approved, bimatoprost sustained-release implant for glaucoma has potential to change the treatment paradigm
| (1) |
|
Wu and Yin. Chinese J Ophthalmology. 2010. (6) Olthoff et al. Ophthalmology. 2005. |
| (2) |
|
Stryker et al. J Glaucoma. 2010. (7) Stewart et al. J Ocul Pharmacol Ther. 2004. |
| (3) |
|
Zimmerman et al. J Ocular Pharmacol Ther. 2009. |
| (4) |
|
Robin and Covert. Ophthalmology. 2005. (8) Taylor et al. J Ocul Pharmacol Ther. 2002. |
| (9) |
|
Schwartz and Quigley. Surv Ophthalmol. 2008. |
(5) Patel and Spaeth. Ophthalmic Surg. 1995. (10) Truven Health MarketScan® Research Databases, August 2013 (Data on file with Allergan) & Anwar Z et al.
Current Opinions Ophthalmology 2013.
| 8 |
|
|
|
Important Information
Allergan, its directors and certain of its officers and employees are participants in solicitations of Allergan stockholders. Information regarding the names of Allergan’s directors and executive officers and their respective interests in Allergan by security holdings or otherwise is set forth in Allergan’s proxy statement for its 2014 annual meeting of stockholders, filed with the SEC on March 26, 2014, as supplemented by the proxy information filed with the SEC on April 22, 2014. Additional information can be found in Allergan’s Annual Report on Form 10-K for the year ended December 31, 2013, filed with the SEC on February 25, 2014 and its Quarterly Report on Form 10-Q for the quarter ended March 31, 2014, filed with the SEC on May 7, 2014. To the extent holdings of Allergan’s securities have changed since the amounts printed in the proxy statement for the 2014 annual meeting of stockholders, such changes have been reflected on Initial Statements of Beneficial Ownership on Form 3 or Statements of Change in Ownership on Form 4 filed with the
SEC. These documents are available free of charge at the SEC’s website at www.sec.gov.
STOCKHOLDERS ARE ENCOURAGED TO READ ANY ALLERGAN SOLICITATION STATEMENT (INCLUDING ANY SUPPLEMENTS THERETO) AND ANY OTHER RELEVANT DOCUMENTS THAT ALLERGAN MAY FILE WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION.
Stockholders will be able to obtain, free of charge, copies of any solicitation statement and any other documents filed by Allergan with the SEC at the SEC’s website at www.sec.gov. In addition, copies will also be available at no charge at the Investors section of Allergan’s website at www.allergan.com.
|
|
June 30, 2014 Allergan
Abicipar Pegol (Anti-VEGF DARPin®) & Bimatoprost
Sustained-Release Implant for Glaucoma