Attached files
| file | filename |
|---|---|
| EX-1.1 - EX-1.1 - Karyopharm Therapeutics Inc. | a2220615zex-1_1.htm |
| EX-5.1 - EX-5.1 - Karyopharm Therapeutics Inc. | a2220615zex-5_1.htm |
| EX-23.1 - EX-23.1 - Karyopharm Therapeutics Inc. | a2220615zex-23_1.htm |
As filed with the Securities and Exchange Commission on June 25, 2014
Registration No. 333-196892
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1 to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
KARYOPHARM THERAPEUTICS INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
2834 (Primary Standard Industrial Classification Code Number) |
26-3931704 (I.R.S. Employer Identification No.) |
2 Mercer Road
Natick, MA 01760
(508) 975-4820
(Address, including zip code, and telephone number, including
area code, of registrant's principal executive offices)
Michael G. Kauffman, M.D., Ph.D.
Chief Executive Officer
Karyopharm Therapeutics Inc.
2 Mercer Road
Natick, MA 01760
(508) 975-4820
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
| Copies to: | ||||
Steven D. Singer, Esq. Joshua D. Fox, Esq. Wilmer Cutler Pickering Hale and Dorr LLP 60 State Street Boston, MA 02109 Telephone: (617) 526-6000 |
Christopher B. Primiano, Esq. Vice President, General Counsel Karyopharm Therapeutics Inc. 2 Mercer Road Natick, MA 01760 Telephone: (508) 975-4820 |
Patrick O'Brien, Esq. Ropes & Gray LLP Prudential Tower 800 Boylston Street Boston, MA 02199 Telephone: (617) 951-7000 |
||
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer ý (Do not check if a smaller reporting company) |
Smaller reporting company o |
CALCULATION OF REGISTRATION FEE
|
||||||||
| Class of Securities to be Registered |
Number of Shares to be Registered(1) |
Proposed Maximum Offering Price Per Share(2) |
Proposed Maximum Aggregate Offering Price |
Amount of Registration Fee(3) |
||||
|---|---|---|---|---|---|---|---|---|
Common Stock, par value $0.0001 per share |
2,530,000 | $43.20 | $109,296,000 | $14,077.32 | ||||
|
||||||||
- (1)
- Includes shares that the underwriters have the option to purchase.
- (2)
- Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933, as amended, and based on the average of the high and low sales price of the registrant's common stock as reported on the NASDAQ Global Select Market on June 18, 2014.
- (3)
- $14,812 previously paid on June 19, 2014.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We and the selling stockholders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion
Preliminary Prospectus dated June 25, 2014
PROSPECTUS
2,200,000 Shares

Common Stock
We are offering 2,000,000 shares of our common stock and the selling stockholders are offering 200,000 shares of our common stock. We will not receive any proceeds from the sale of shares by the selling stockholders.
Our common stock is listed on The NASDAQ Global Select Market under the symbol "KPTI." The last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014 was $41.37 per share.
We are an "emerging growth company" under federal securities laws and are subject to reduced public company disclosure standards. See "Prospectus Summary—Implications of Being an Emerging Growth Company."
Investing in the common stock involves risks that are described in the "Risk Factors" section beginning on page 15 of this prospectus.
| |
Per Share
|
Total
|
||
|---|---|---|---|---|
Public offering price |
$ | $ | ||
Underwriting discount(1) |
$ | $ | ||
Proceeds, before expenses, to us |
$ | $ | ||
Proceeds, before expenses, to selling stockholders |
$ | $ |
- (1)
- We refer you to "Underwriting" on page 36 for additional information regarding total underwriting compensation.
The underwriters may also exercise their option to purchase up to an additional 330,000 shares from us at the public offering price, less the underwriting discount, for 30 days after the date of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The shares will be ready for delivery on or about , 2014.
| BofA Merrill Lynch | Leerink Partners |
JMP Securities |
Wedbush PacGrow Life Sciences |
Oppenheimer & Co. |
The date of this prospectus is , 2014.
TABLE OF CONTENTS
You should rely only on the information contained in or incorporated by reference in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. We have not, the selling stockholders have not, and the underwriters have not, authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We and the selling stockholders are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained or incorporated by reference in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of shares of our common stock.
For investors outside the United States: We have not, the selling stockholders have not, and the underwriters have not, done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside the United States.
This summary highlights information contained elsewhere in this prospectus or incorporated by reference into this prospectus from our Annual Report on Form 10-K for the year ended December 31, 2013, our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 and our other filings with the Securities and Exchange Commission listed in the section of this prospectus entitled "Incorporation of Documents by Reference" and is qualified in its entirety by the more detailed information and consolidated financial statements included or incorporated by reference elsewhere in this prospectus. This summary does not contain all of the information that may be important to you. You should read and carefully consider the following summary together with the entire prospectus and the documents included herein by reference, including our consolidated financial statements and the notes thereto incorporated herein by reference and the matters discussed under "Risk Factors," "Selected Consolidated Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in each case appearing elsewhere in this prospectus or in our Annual Report on Form 10-K for the year ended December 31, 2013 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 incorporated by reference herein before deciding to invest in our common stock. Some of the statements in this prospectus constitute forward-looking statements that involve risks and uncertainties. See "Cautionary Note Regarding Forward-Looking Statements." Our actual results could differ materially from those anticipated in such forward-looking statements as a result of certain factors, including those discussed in "Risk Factors" and other sections of this prospectus and the documents incorporated herein by reference.
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to "Karyopharm" "the Company," "we," "us" and "our" refer to Karyopharm Therapeutics Inc. and, where appropriate, its consolidated subsidiaries.
Overview
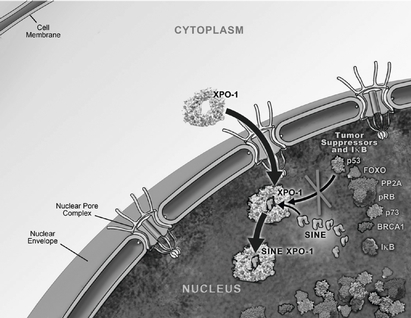
We are a clinical-stage pharmaceutical company founded in December 2008 by Dr. Sharon Shacham. We are focused on the discovery and development of novel first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases. Our scientific expertise is focused on the understanding of the regulation of intracellular transport between the nucleus and the cytoplasm. We have discovered and are developing wholly-owned, novel, small molecule, Selective Inhibitors of Nuclear Export, or SINE, compounds that inhibit the nuclear export protein XPO1. We have worldwide rights to these SINE compounds. Our lead drug candidate, Selinexor (KPT-330), is an XPO1 inhibitor being evaluated in multiple open-label Phase 1 and Phase 2 clinical trials in patients with heavily pretreated relapsed and/or refractory hematological and solid tumor malignancies. To date, we have administered Selinexor to over 330 patients across Phase 1 and Phase 2 clinical trials in hematologic and solid tumor indications. Evidence of anti-cancer activity has been observed in some patients and Selinexor has been sufficiently well-tolerated to allow many of these patients to remain on therapy for prolonged periods, including several who have remained on study for over 12 months. During 2014, we plan to initiate registration-directed clinical trials in three different hematological malignancy indications. We plan to initiate up to two additional registration-directed trials in hematological or solid tumor indications in late 2014 or early 2015. These registration-directed trials are designed to serve as the basis for an application seeking regulatory approval for Selinexor in such indications. To our knowledge, no other XPO1 inhibitors are in clinical development at the present time.
One of the ways in which the cell regulates the function of a particular protein is by controlling the protein's location within the cell, as a specific function may only occur within a particular location in the cell. In healthy cells, nuclear transport, both into and out of the nucleus, is a normal and regular occurrence that is tightly regulated and requires specific carrier proteins to occur. XPO1 mediates the export of approximately 220 different mammalian cargo proteins, including the vast majority of tumor
1
suppressor proteins. Moreover, XPO1 appears to be the only nuclear exporter for most of these tumor suppressor proteins. Cancer cells have increased levels of XPO1, causing the increased export of these tumor suppressor proteins from the nucleus. Since the tumor suppressor proteins need to be located in the nucleus to promote programmed cell death, or apoptosis, XPO1 overexpression in cancer cells counteracts the natural apoptotic process that protects the body from cancer. Due to XPO1 inhibition by our SINE compounds, the export of tumor suppressor proteins is prevented, thereby leading to their accumulation in the nucleus. This subsequently reinitiates and amplifies their natural apoptotic function in cancer cells with minimal effects on normal cells. The figure below depicts the process by which our SINE compounds inhibit the XPO1 nuclear export of tumor suppressor proteins.
Transient XPO1 Inhibition by SINE Compounds
We are currently conducting multiple open-label clinical trials of Selinexor, including three Phase 1 clinical trials, the first in patients with various advanced hematological malignancies, the second in patients with various advanced or metastatic solid tumor malignancies and the third, a food effect study, in patients who have metastatic, locally advanced or locally recurrent soft tissue or bone sarcomas. In these trials, we have observed evidence of anti-cancer activity of Selinexor across a spectrum of patients with advanced cancers who had received multiple previous treatments and, despite these treatments, had disease that was progressing at the time of enrollment in our clinical trials. Our hematological malignancy trial consists of six arms, in which Selinexor is administered as a monotherapy in Arms 1-5 and in combination in Arm 6. Arm 1 includes patients with certain chronic B-cell malignancies, including non-Hodgkin's lymphoma, or NHL, chronic lymphocytic leukemia, or CLL, multiple myeloma, or MM and Waldenström's Macroglobulinemia, or WM; Arm 2 includes patients with acute myeloid leukemia, or AML; Arm 3 includes patients with T-cell lymphomas; Arm 4 includes patients with chronic myeloid leukemia, or CML; Arm 5 includes patients with acute lymphocytic leukemia, or ALL; and Arm 6 includes patients on combination therapy who have MM and are taking low-dose dexamethasone (20mg) in combination with each biweekly dose of Selinexor.
Of the patients evaluated in our hematological malignancy trial as of May 13, 2014, we have observed complete responses or remissions, partial responses or remissions, minimal responses or stable disease in a number of these patients, all as determined in accordance with commonly accepted evaluation criteria for the specific indication. Partial or minimal responses or stable disease have been observed in 70% of patients with relapsed and/or refractory chronic B-cell malignancies. In patients
2
with relapsed and/or refractory acute myeloid leukemia, or AML, as of May 13, 2014, we have observed complete remissions, partial remissions, morphologic leukemia-free state or stable disease in 49% of patients, some for longer than three months. Among eight patients with multiple myeloma treated with Selinexor in combination with low-dose dexamethasone (20mg twice weekly), complete, partial or minor responses were observed in 75% of patients as of June 5, 2014. Of the patients in the solid tumor malignancy trial evaluated as of May 13, 2014, we have observed partial responses or stable disease in 49%, all as determined in accordance with Response Evaluation Criteria In Solid Tumors, or RECIST.
We have initiated Phase 2 studies of Selinexor in several indications, including prostate cancer, gynecologic malignancies (ovarian, cervical and uterine cancers) and glioblastoma and we expect initial data during 2015. In addition, we expect to initiate a Phase 2 clinical trial in squamous head, neck or lung cancers during 2014. We have also initiated a Phase 1/2 study of Selinexor in combination with decitabine (Dacogen) in patients with AML and additional combination studies are expected to begin in 2014 and 2015.
We have initiated one registration-directed clinical trial and, assuming positive results from our ongoing clinical trials and pending regulatory feedback, we plan to initiate registration-directed clinical trials of Selinexor in two additional hematological malignancy indications during 2014. We refer to these trials as registration-directed because they are designed to serve as the basis for an application seeking regulatory approval of Selinexor. On June 24, 2014, we announced the initiation of a registration-directed clinical trial for Selinexor in older patients with relapsed refractory AML. We expect to initiate registration-directed clinical trials in Richter's Syndrome (also called Richter's Transformation) during the middle of 2014 and in diffuse large B-cell lymphoma, or DLBCL, in the second half of 2014. Moreover, we plan to initiate up to two additional registration-directed trials in hematological or solid tumor indications, potentially including multiple myeloma, in late 2014 or early 2015. We plan to seek regulatory approvals of Selinexor in North America and Europe in each such indication with respect to which we receive positive results and positive regulatory feedback. We may seek such approvals in other geographies as well.
We believe that the XPO1-inhibiting SINE compounds that we have discovered and developed to date, including Selinexor, have the potential to provide a novel targeted therapy that enables tumor suppressor proteins to remain in the nucleus and promote apoptosis of cancer cells. Moreover, our SINE compounds spare normal cells, which, unlike cancer cells, do not have significant damage to their genetic material, and we believe this selectivity for cancer cells minimizes side effects. We believe that the oral administration of Selinexor and the lack of cumulative or major organ toxicities observed to date in patients treated with Selinexor in clinical trials create the potential for its broad use across many cancer types, including both hematological and solid tumor malignancies. We believe that no currently approved cancer treatments or current clinical-stage cancer drug candidates are selectively targeting the restoration and increase in the levels of multiple tumor suppressor proteins in the nucleus.
Another SINE compound, Verdinexor (KPT-335), which is closely related to Selinexor, is being developed for the treatment of pet dogs with lymphomas. The approval of drugs to treat animals is based on a New Animal Drug Application, or NADA, reviewed by the FDA's Center for Veterinary Medicine. Three of the four technical sections of the NADA for Verdinexor have been approved by the FDA, including Effectiveness, Safety and Environmental Impact. We anticipate obtaining a marketing collaborator to complete the final major technical section of the NADA, the Chemicals/Manufacturing/Controls section, which covers the commercial-scale manufacturing, or CMC. of Verdinexor. We believe that the approval of the effectiveness and safety sections of the NADA for Verdinexor for the treatment of dogs with lymphoma helps confirm the activity and tolerability of our novel SINE compounds for the treatment of cancers.
We are focused on building a leading oncology business. Karyopharm was founded by Sharon Shacham, Ph.D., M.B.A., our President and Chief Scientific Officer. We are led by Dr. Shacham and
3
Dr. Michael Kauffman, our Chief Executive Officer. Dr. Kauffman played a leadership role in the development and approval of Velcade® at Millennium Pharmaceuticals, and of Kyprolis® while serving as Chief Medical Officer at Proteolix and then Onyx Pharmaceuticals. Dr. Shacham has played a leadership role in the discovery and development of many novel drug candidates, which have been or are being tested in human clinical trials, prior to her founding of Karyopharm and while at Karyopharm.
In addition to cancer, we believe that our SINE compounds have the potential to provide therapeutic benefit in a number of additional indications, including autoimmune and inflammatory diseases, wound healing, HIV and influenza. We have discovered and are developing a pipeline of SINE compounds that have shown evidence of activity in preclinical models of inflammation, wound healing and viral infection. We may seek to enter into development, marketing and commercialization collaboration arrangements for our SINE compounds other than Selinexor in non-oncology indications globally.
The table below summarizes the current stages of development of our key human drug candidates and indications that we expect to initially focus on for each candidate. We expect to initiate the planned clinical trials of Selinexor described below assuming positive results from our ongoing clinical trials and pending regulatory feedback. We also expect a number of investigator-sponsored trials, or ISTs, to be initiated for Selinexor in a variety of cancer indications over the next year. These ISTs could consist of single agent or combination studies with other agents in both hematological and solid tumor malignancies.
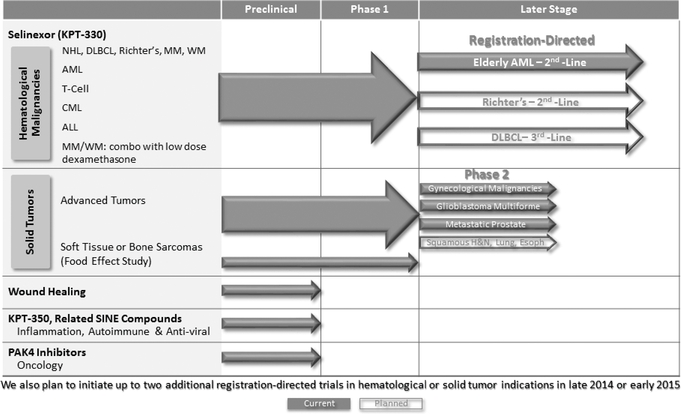
Recent Developments
We recently presented updated data from our ongoing Phase 1 clinical trials of Selinexor in patients with various advanced hematological malignancies and in patients with various advanced or metastatic solid tumor malignancies. All patients entering these trials have progressive disease upon enrollment that is relapsed after and/or refractory to all available classes of anti-cancer agents, typically
4
after multiple approved therapies and often after one or more experimental therapies. Anti-cancer activity has been observed with tumor reductions and durable disease control across many hematologic and solid tumor malignancies. Several patients have remained on single-agent oral Selinexor for over one year. Adverse events observed in our most recent patient data are generally mild, responsive to standard supportive care and consistent with those previously reported in patients in our Phase 1 clinical trials.
Response data presented herein are interim unaudited data based on reports by physicians at the clinical trial sites. Responses in the hematological trial are measured using commonly accepted evaluation criteria for the specific indication. Responses in the sold tumor trial are measured using RECIST. Responses include:
- •
- sCR – stringent complete response;
- •
- CR – complete response;
- •
- CR(i/p) – complete response without hematological recovery;
- •
- PR – partial response;
- •
- MLFS – morphological leukemia free state;
- •
- MR – minor response;
- •
- SD – stable disease;
- •
- PD – progressive disease;
- •
- WC – withdrew consent; or
- •
- NE – non-evaluable, meaning the patient's response could not be evaluated due to a number of potential factors, including when a patient withdraws consent or fails to comply with the therapeutic protocol for the trial.
Disease control rate, or DCR, refers to the percentage of responses that are SD or better (MR or better in the case of MM) and overall response rate, or ORR, refers to the percentage of responses that are PR or better.
Advanced Hematological Malignancies
We recently reported data from patients with non-Hodgkin's lymphoma, including diffuse large B-cell lymphoma and Richter's syndrome/transformation (Richter's), acute myeloid leukemia and multiple myeloma as part of our ongoing Phase 1 clinical trial of Selinexor in patients with various advanced hematological malignancies. The primary objectives of the Phase 1 trial are to determine the safety, tolerability and recommended Phase 2 dose of orally administered Selinexor. Patients were dosed 3 - 80mg/m2 of Selinexor orally over a four-week cycle, with lower doses initially given ten times per cycle and higher doses given twice weekly. Responses were evaluated every one to two cycles.
Non-Hodgkin's Lymphoma
Fifty-one heavily pretreated patients with relapsed and/or refractory NHL, including DLBCL and Richter's, and mean prior treatment regimens of 4.1 were enrolled in this arm of the Phase 1 clinical trial as of May 13, 2014. Of this group, 43 patients were evaluable, five patients had tumors deemed non-evaluable and three patients had tumors pending evaluation. Among the 43 evaluable patients, the DCR was 74% across all doses of Selinexor and the ORR was 28%. Responses were observed across all subtypes of NHL, independent of genetic abnormalities, with durable cancer control observed across several patients who remained on study for longer than nine months. Responses in 43 evaluable patients are shown below.
5
Best Responses in NHL Patients as of May 13, 2014
Cancer
|
N | DCR (%) | ORR(%) | CR (%) | PR (%) | SD (%) | PD (%) | WC (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DLBCL |
21 | 15 (70%) | 6 (29%) | 1 (5%) | 5 (25%) | 9 (40%) | 5 (25%) | 1 (5%) | ||||||||
Follicular |
7 | 6 (86%) | 1 (14%) | — | 1 (14%) | 5 (71%) | — | 1 (14%) | ||||||||
Mantle Cell |
3 | 2 (67%) | 1 (33%) | — | 1 (33%) | 1 (33%) | — | 1 (33%) | ||||||||
Transformed |
3 | 1 (33%) | 1 (33%) | — | 1 (33%) | — | 2 (67%) | — | ||||||||
T-Cell |
4 | 3 (75%) | 1 (25%) | 1 (25%) | — | 2 (50%) | — | 1 (25%) | ||||||||
Richter's Syndrome |
5 | 5 (100%) | 2 (40%) | — | 2 (40%) | 3 (60%) | — | — | ||||||||
| | | | | | | | | | | | | | | | | |
Total |
43 | 32 (74%) | 12 (28%) | 2 (5%) | 10 (23%) | 20 (47%) | 7 (16%) | 4 (9%) | ||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
The following chart indicates the maximum percentage shrinkage of tumors across various subtypes of NHL relative to baseline scans for the patients depicted.
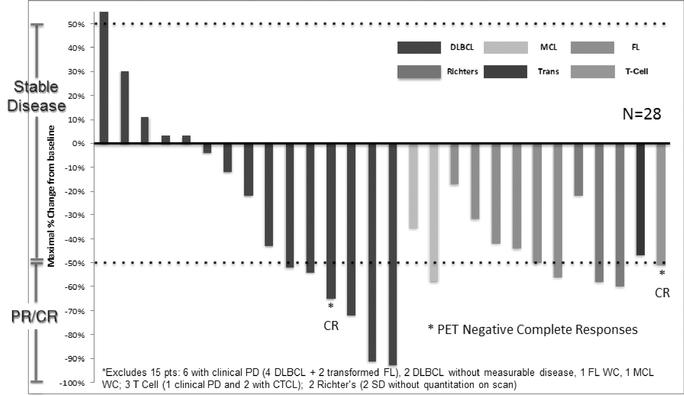
Among the 21 patients with heavily pretreated DLBCL, ORR and DCR were nearly identical across all subtypes of DLBCL. There are two major subtypes of DLBCL, namely Germinal Center B-Cell, or GCB and Activated B-Cell, or ABC, also called non-GCB. Many targeted therapies such as ibrutinib or lenalidomide show activity primarily against the ABC subtype. However, as detailed in the table below and consistent with the broadly applicable mechanism of action of Selinexor, Selinexor shows activity across both major subtypes.
Best Responses in Diffuse Large B-Cell Patients as of May 13, 2014
Type
|
N | DCR (%) | ORR (%) | CR (%) | PR (%) | SD (%) | PD (%) | WC (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
GCB |
11 | 8 (72%) | 3 (27%) | 1 (9%) | 2 (18%) | 5 (45%) | 2 (18%) | 1 (9%) | ||||||||
non-GCB |
4 | 3 (75%) | 1 (25%) | — | 1 (25%) | 2 (50%) | 1 (25%) | — | ||||||||
Unknown |
6 | 4 (67%) | 2 (33%) | — | 2 (33%) | 2 (33%) | 2 (33%) | — |
6
In addition, a minority of DLBCL patients have "double-hit" disease because these tumors over-express the two oncogenes MYC and BCL2 (or BCL6). Double-hit DLBCL is particularly difficult to treat due in part to its resistance to multi-agent immunochemotherapy and many targeted agents. Of four patients with double-hit DLBCL, there was one CR, two patients with SD (with 43% and 45% lymph node reductions, respectively), and one PD. We believe, together, these data and the consistent data across DLBCL subtypes indicate that Selinexor has the potential to treat a broad range of subtypes of DLBCL, largely independent of the cell of origin or oncogenic drivers.
Among all NHL patients, Grade 3/4 adverse events occurring in more than three patients included thrombocytopenia (20%), neutropenia (16%) and hyponatremia (6%). The most common Grade 1/2 adverse events were nausea (51%), anorexia (41%) and fatigue (36%). There have been no dose limiting toxicities among NHL patients as of May 13, 2014. The maximum tolerated dose was not reached, but based on biological activity and data from additional studies, the intended Phase 2 or Phase 3 trial dose of oral Selinexor for NHL is 60mg/m2 twice weekly.
Acute Myeloid Leukemia
Sixty-five heavily pretreated patients with progressive, relapsed and/or refractory AML, most with three or more prior treatment regimens, were enrolled in this arm of the Phase 1 clinical trial as of May 13, 2014. These patients were dosed 16.8 - 70mg/m2 in a four-week cycle, with lower doses initially given ten times per cycle and higher doses given twice weekly. Of these 65 patients, two patients had tumors pending evaluation, and among the 63 other patients, the complete response rate with or without full hematologic recovery was 11%, the ORR was 16% and the DCR was 49%; 16 (25%) of the 63 patients with AML were non-evaluable but were included in the AML response rate calculation. Responses were observed across multiple genetic subtypes of AML. Higher doses of Selinexor were associated with greater reductions in bone marrow blast counts, which were also observed across different AML subtypes. Responses in 63 patients are shown below. Two patients had tumors pending evaluation.
Best Responses in AML Patients as of May 13, 2014
N
|
DCR (%) | ORR (%) | CR (%) | CR(i/p) (%) | PR (%) | MLFS (%) | SD (%) | PD (%) | NE (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
63 |
31 (49%) | 10 (16%) | 5 (8%) | 2 (3%) | 1 (2%) | 2 (3%) | 21 (33%) | 16 (25%) | 16 (25%) |
Grade 3/4 adverse events occurring in more than three patients included fatigue (18%), thrombocytopenia (15%), neutropenia (11%), and nausea (8%). The most common Grade 1/2 adverse events were diarrhea (82%), anorexia (78%), nausea (74%), and fatigue (65%). There have been no dose limiting toxicities in AML patients as of May 13, 2014 and the maximum tolerated dose is ³70mg/m2 twice weekly. The intended Phase 2 or Phase 3 trial dose of oral Selinexor for AML is 55mg/m2 twice weekly.
Multiple Myeloma
As part of our Phase 1 clinical trial of Selinexor in patients with advanced hematological malignancies, patients with multiple myeloma were treated with either single-agent Selinexor or Selinexor in combination with "low-dose" (20mg) dexamethasone, all dosed twice weekly. Forty-four patients with multiple myeloma whose disease was relapsed and/or refractory to all available classes of approved therapies, with a mean of 5.7 prior therapies, and progressing on study entry have been enrolled in this trial as of June 5, 2014. Of these 44 patients, 34 received single-agent Selinexor therapy and eight patients were treated with Selinexor in combination with dexamethasone. Among the 34 patients receiving single-agent Selinexor therapy, including six patients who were non-evaluable, best responses include one PR (3%), five MRs (15%), 16 SDs (47%) and six PDs (18%). It should also be noted that some patients treated with single agent Selinexor receive very low doses of dexamethasone
7
(£12mg with each dose of selinexor) or another steroid as part of supportive care. These very low doses of steroids are not known to have any anti-myeloma activity, particularly in the patients enrolled in this study, whose disease is refractory to steroids and multiple other agents.
Eight patients were treated with 45mg/m2 of oral Selinexor and 20mg of dexamethasone, each dosed twice weekly. Two additional patients have been treated with combination therapy but had not yet completed the first treatment cycle as of June 5, 2014. This dose of dexamethasone is the standard low dose dexamethasone (40mg weekly or 20mg twice weekly) used with other anti-myeloma drugs including lenalidomide or pomalidomide. The patients enrolled in this study had received a median of 5.5 prior lines of therapy. All had received prior therapy with a proteasome inhibitor, such as Kyprolis or Velcade, and an immunomodulatory agent, such as pomalidomide, steroids (typically two or more times) while seven of the eight patients also received stem cell transplantations. As of June 5, 2014, the best responses among the eight patients were one stringent complete response (sCR) (12.5%), three PRs (37.5%), two MRs (25%), one PD (12.5%) and one NE (12.5%). The clinical benefit response rate (sCR+PR+MR) was 75% and the ORR was 50%. Five of the six responding patients remain on study as of June 5, 2014. Approximately 12 additional patients with multiple myeloma may be dosed with Selinexor in combination with dexamethasone in this ongoing Phase 1 study. Responses in these eight patients are described below.
Patients with Relapsed/Refractory MM Treated with Twice Weekly
Oral Selinexor 45mg/m2 + Dexamethasone 20mg
| MM Type | Number of Prior therapies |
Maximal Change |
Response | Study Days |
|||||
|---|---|---|---|---|---|---|---|---|---|
| IgG-k | 7 | -73% | PR | 122+ | |||||
| FLC-l | 5 | * | NE | 15 | |||||
| FLC-k | 3 | -53% | PR | 45 | |||||
| FLC-k | 5 | -98% | sCR | 107+ | |||||
| IgG-k | 7 | -81% | PR | 81+ | |||||
| IgG-k | 4 | * | PD | 38 | |||||
| IgA-k | 6 | -48% | MR | 51+ | |||||
| IgG-k | 7 | -32% | MR | 46+ |
- *
- Patients did not undergo post-cycle 1 scan.
Adverse events in patients receiving single-agent Selinexor were generally low-grade, consistent with events observed in patients with other hematological malignancies and responsive to standard supportive care. Compared with Selinexor given alone, fewer adverse events in patients receiving Selinexor in combination with dexamethasone were reported, particularly levels of nausea, consistent with dexamethasone's reduction in Selinexor's main side effects of nausea, anorexia, and fatigue.
In addition to patients with multiple myeloma achieving durable responses and disease control on Selinexor single-agent therapy, Selinexor with low dose dexamethasone showed activity with rapid M-protein reductions and good tolerability, even in patients with disease refractory to pomalidomide and/or carfilzomib. We believe that Selinexor may have synergistic potential with other therapies.
Advanced or Metastatic Solid Tumor Malignancies
We recently reported data from our ongoing Phase 1 clinical trial of Selinexor in patients with advanced or metastatic solid tumor malignancies. All patients entered the study with advanced or
8
metastatic solid tumor cancers relapsed or refractory after multiple previous treatments and objectively progressing on study entry. The primary objectives of the Phase 1 dose escalation trial are to determine the safety, tolerability and recommended Phase 2 dose of orally administered Selinexor. These patients were dosed 3 - 85mg/m2 of oral Selinexor over a four-week cycle, with lower doses initially given ten times per cycle and higher doses given twice weekly. Response evaluation was done every two cycles in accordance with RECIST.
As of May 13, 2014, 129 patients were enrolled in this Phase 1 clinical trial. Enrolled patients had received a mean of 3.7 prior therapies. Of these patients, 106 were evaluable and 23 patients were non-evaluable or pending evaluation as of May 13, 2014. Of these evaluable patients, the DCR was 49%. PRs were observed in four patients, one each with colorectal cancer (KRAS mutant), melanoma (BRAFwt), ovarian adenocarcinoma and cervical cancer. SD was noted in 47 patients, with 17 patients (16%) experiencing SD for six months or longer. Eight of nine evaluable patients with hormone and chemotherapy refractory prostate cancer achieved stable disease and have remained on study for between 70 and 317 days. Among 14 evaluable patients with head and neck cancer, nine achieved stable disease with eight on study for 75 to over 401 days. Responses in 106 evaluable patients are shown below.
Best Responses in Solid Tumor Patients as of May 13, 2014
Cancer Type
|
N | DCR (%) |
PR (%) | SD (%) | PD (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
Colorectal |
39 | 14 (36%) | 1 (3%) | 13 (33%) | 25 (64%) | |||||
Head & Neck |
14 | 9 (64%) | — | 9 (64%) | 5 (36%) | |||||
Prostate |
8 | 7 (88%) | — | 7 (88%) | 1 (12%) | |||||
Cervical |
5 | 4 (80%) | 1 (20%) | 3 (60%) | 1 (20%) | |||||
Ovarian |
5 | 3 (60%) | 1 (20%) | 2 (40%) | 2 (40%) | |||||
GBM |
5 | — | — | — | 5 (100%) | |||||
Melanoma |
3 | 2 (67%) | 1 (33%) | 1 (33%) | 1 (33%) | |||||
Sarcoma |
8 | 7 (88%) | — | 7 (88%) | 1 (12%) | |||||
Other |
19 | 6 (32%) | — | 6 (32%) | 13 (68%) | |||||
| | | | | | | | | | | |
Total |
106 | 52 (49%) | 4 (4%) | 48 (45%) | 54 (51%) | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Side effects were generally low grade and typically gastrointestinal in nature, or fatigue. These common side effects decreased over time, in part due to prophylactic use of standard supportive care. Two DLTs were observed in solid tumor patients receiving 85 mg/m2 dosed twice weekly. Grade 3/4 adverse events occurring in six or more patients in the first cycle included fatigue hyponatremia (8%), fatigue (7%), and thrombocytopenia (7%). The most common Grade 1/2 adverse events in the first cycle were nausea (62%), fatigue (52%), anorexia (48%) and vomiting (35%). One was Grade 3 asymptomatic hyponatremia and the other was acute cerebellar syndrome that reversed over several weeks with markedly improving ataxia and dysarthria. No additional central nervous system toxicities were observed in any other patients receiving Selinexor to date. Major organ dysfunction or clinically significant cumulative toxicities have not been observed. The intended Phase 2 or Phase 3 trial dose of oral Selinexor in solid tumors is 65mg/m2 twice weekly.
Registration-Directed Trial Design
On June 24, 2014, we announced the initiation of our Phase 2 study of Selinexor in patients 60 years of age or older with relapsed or refractory acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy and/or transplantation. We have titled this trial the Selinexor in Older Patients with Relapsed/Refractory AML, or SOPRA, study. We also expect to initiate registration-
9
directed clinical trials for Selinexor in Richter's Syndrome during the middle of 2014 and in DLBCL in the second half of 2014.
In SOPRA, 150 patients with AML which has relapsed after, or was refractory to, first line therapy will be randomized in a 2:1 fashion to Selinexor provided orally twice per week at a dose of 55mg/m2 versus one of four physician choices. Physician choices include best supportive care, or BSC, alone, or BSC plus either azacytidine (Vidaza), decitabine (Dacogen), or low dose cytosine arabinoside (LD-AraC). Overall survival is the primary endpoint. SOPRA was designed based on data from the ongoing Phase 1 study of Selinexor in patients with advanced hematologic malignancies, including AML. SOPRA is expected to take approximately two years to complete.
Our registration-directed trial in Richter's Syndrome is designed as a single-arm trial in patients with CLL who experienced a transformation to Richter's Syndrome and subsequently relapsed after chemotherapy. The primary endpoint of this trial is ORR and the trial is expected to enroll approximately 50 patients who will be treated with Selinexor given at a dose of 60mg/m2, administered orally two times per week.
Our registration-directed trial in DLBCL is designed as a single-arm trial in patients that are relapsed and/or refractory to two lines of chemotherapy. The primary endpoint of this trial is ORR and the trial is expected to enroll approximately 150 patients who will be treated with Selinexor given at a dose of 60mg/m2 in combination with low dose dexamethasone (12mg), each administered orally two times per week.
Our Strategy
As a clinical-stage pharmaceutical company focused on the discovery and development of orally available, novel first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases, the critical components of our business strategy are to:
- •
- Develop and seek regulatory approval of Selinexor, our novel lead drug candidate, in North America and Europe.
- •
- Maximize the commercial value of Selinexor.
- •
- Maintain our competitive advantage and scientific expertise in the field of nuclear transport.
- •
- Develop novel drug candidates by leveraging our proprietary drug discovery and optimization platform and our understanding
of nuclear transport.
- •
- Collaborate with key opinion leaders to conduct investigator-sponsored trials of Selinexor.
- •
- Maximize the value of our other SINE compounds in non-oncology indications and veterinary oncology through collaborations.
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the "Risk Factors" section of this prospectus immediately following this prospectus summary and in our Annual Report on Form 10-K for the year ended December 31, 2013 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 incorporated by reference herein. These risks include the following:
- •
- We depend heavily on the success of Selinexor, our lead drug candidate, which is currently in Phase 1 and Phase 2 clinical trials, and we cannot be certain that we will receive regulatory approval for Selinexor or will successfully commercialize Selinexor even if we receive such regulatory approval.
10
- •
- Our approach to the discovery and development of drug candidates that target Exportin 1, or XPO1, is unproven, and
we do not know whether we will be able to develop any drugs of commercial value.
- •
- If clinical trials of our drug candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory
authorities or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization
of our drug candidates; to date, both adverse and serious adverse events have been experienced by patients in our clinical trials of Selinexor, including several which have been determined to relate
to Selinexor.
- •
- We cannot be certain that, even if successful, our registration-directed trials will be sufficient to allow us to file
for, or receive approval for, Selinexor.
- •
- We have incurred significant losses since inception. We expect to incur losses for the foreseeable future and may never
achieve or maintain profitability. As of March 31, 2014, we had an accumulated deficit of $76.3 million.
- •
- Our short operating history may make it difficult for you to evaluate the success of our business to date and to assess
our future viability.
- •
- We will need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay,
reduce or eliminate our research and drug development programs or commercialization efforts.
- •
- We expect to depend on third parties for the development, marketing and/or commercialization of our drug candidates. If
those collaborations are not successful, we may not be able to capitalize on the market potential of these drug candidates.
- •
- We have applied for, but not yet received, patent protection for our key drug candidates and if we are unable to obtain and maintain patent protection for our drug candidates and other discoveries, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize drugs and other discoveries similar or identical to ours, and our ability to successfully commercialize our drug candidates and other discoveries may be adversely affected.
Our Corporate Information
We were incorporated under the laws of the State of Delaware in December 2008. Our executive offices are located at 2 Mercer Road, Natick, MA 01760, and our telephone number is (508) 975-4820. Our website address is www.karyopharm.com. The information contained in, or accessible through, our website does not constitute part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference. The trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners.
Implications of Being an Emerging Growth Company
As a company with less than $1 billion in revenue during our last fiscal year, we qualify as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
- •
- reduced disclosure about our executive compensation arrangements;
- •
- exemption from holding the non-binding advisory votes on executive compensation, including golden parachute arrangements; and
11
- •
- exemption from the auditor attestation requirement in the assessment of our internal controls over financial reporting.
Generally, we may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1 billion in annual revenue, we have more than $700 million in market value of our stock held by non-affiliates or we issue more than $1 billion of non-convertible debt over a three-year period. We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of certain reduced reporting burdens in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
12
Common stock offered by us |
2,000,000 shares | |
Common stock offered by the selling stockholders |
200,000 shares |
|
Common stock to be outstanding after this offering |
31,762,499 shares |
|
Option to purchase additional shares |
The underwriters have an option for a period of 30 days to purchase up to 330,000 additional shares of our common stock. |
|
Use of proceeds |
We intend to use the net proceeds from this offering as follows: approximately $65.0 million to fund the continued clinical development of our lead drug candidate, Selinexor (KPT-330), including by initiating and conducting planned registration-directed clinical trials of Selinexor in three hematological malignancy indications, up to two additional registration-directed clinical trials of Selinexor in hematological or solid tumor indications, Phase 2 clinical trials of Selinexor in solid tumor indications and additional clinical trials of Selinexor in additional cancer indications as a single agent as well as in combination with other therapies; approximately $4.0 million to continue the preclinical development of our drug candidates for anti-inflammatory, viral and wound-healing indications; approximately $8.0 million for discovery, research, preclinical development and clinical trials of additional drug candidates; and the balance for working capital and other general corporate purposes. |
|
|
We will not receive any of the proceeds from any sale of shares by the selling stockholders. |
|
|
See "Use of Proceeds" for more information. |
|
NASDAQ Global Select Market symbol |
"KPTI" |
The number of shares of our common stock to be outstanding after this offering is based on 29,762,499 shares of our common stock issued and outstanding as of March 31, 2014, including 143,620 shares of unvested restricted stock subject to repurchase by us, and excludes:
- •
- 2,505,749 shares of our common stock issuable upon exercise of stock options outstanding as of March 31, 2014 at a
weighted-average exercise price of $9.21 per share; and
- •
- 1,722,111 and 242,424 additional shares of our common stock available for future issuance, as of March 31, 2014, under our 2013 stock incentive plan and our 2013 employee stock purchase plan, respectively.
Unless otherwise indicated, this prospectus reflects and assumes the following:
- •
- no exercise of the outstanding options described above;
- •
- no exercise by the underwriters of their option to purchase up to 330,000 additional shares of our common stock;
and
- •
- no purchases of shares of our common stock by our existing stockholders in this offering.
13
SUMMARY CONSOLIDATED FINANCIAL DATA
The following table presents our summary consolidated financial data. We have derived the following summary of our statement of operations data for the years ended December 31, 2013, 2012 and 2011 from our audited consolidated financial statements incorporated by reference in this prospectus from our Annual Report on Form 10-K for the year ended December 31, 2013. We have derived the following summary of our statement of operations data for the three months ended March 31, 2014 and 2013 from our unaudited consolidated financial statements incorporated by reference in this prospectus from our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2014. We have derived the summary of our balance sheet data as of March 31, 2014 from our unaudited consolidated financial statements incorporated by reference herein. Our historical results are not necessarily indicative of the results that may be expected in the future. The summary of our consolidated financial data set forth below should be read together with our consolidated financial statements and the related notes to those statements, as well as "Management's Discussion and Analysis of Financial Condition and Results of Operations," all included elsewhere or incorporated by reference in this prospectus. For more details on how you can obtain the documents incorporated by reference in this prospectus, see "Where You Can Find More Information" and "Incorporation of Documents by Reference" appearing elsewhere in this prospectus.
| |
|
|
|
Three Months Ended, March 31, |
Period from December 22, 2008 (Inception) to March 31, 2014 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | ||||||||||||||||||
| |
2013 | 2012 | 2011 | 2014 | 2013 | ||||||||||||||
| |
(in thousands, except share and per share data) |
||||||||||||||||||
Consolidated Statement of Operations Data: |
|||||||||||||||||||
Contract and grant revenue |
$ | 387 | $ | 634 | $ | 152 | $ | 171 | $ | 233 | $ | 1,437 | |||||||
| | | | | | | | | | | | | | | | | | | | |
Operating expenses: |
|||||||||||||||||||
Research and development |
28,452 | 14,095 | 8,623 | 10,979 | 4,965 | 63,814 | |||||||||||||
General and administrative |
5,885 | 2,429 | 1,840 | 2,904 | 879 | 13,721 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses |
34,337 | 16,524 | 10,463 | 13,883 | 5,844 | 77,535 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Loss from operations |
(33,950 | ) | (15,890 | ) | (10,311 | ) | (13,712 | ) | (5,611 | ) | (76,098 | ) | |||||||
Interest income (expense), net |
3 | 2 | — | 18 | — | (165 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net loss |
$ | (33,947 | ) | $ | (15,888 | ) | $ | (10,311 | ) | $ | (13,694 | ) | $ | (5,611 | ) | $ | (76,263 | ) | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Net loss per share applicable to common stockholders—basic and diluted |
$ | (5.59 | ) | $ | (8.95 | ) | $ | (10.27 | ) | $ | (0.46 | ) | $ | (2.52 | ) | $ | (24.70 | ) | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Weighted-average number of common shares used in net loss per share applicable to common stockholders—basic and diluted |
6,067,679 | 1,775,323 | 1,004,144 | 29,606,683 | 2,225,596 | 3,087,622 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| |
As of March 31, 2014 | ||||||
|---|---|---|---|---|---|---|---|
| |
Actual | As Adjusted(1) |
|||||
| |
(in thousands) |
||||||
Consolidated Balance Sheet Data: |
|||||||
Cash and cash equivalents |
$ | 144,893 | 221,919 | ||||
Working capital |
142,551 | 219,577 | |||||
Total assets |
149,285 | 226,311 | |||||
Total stockholders' equity |
144,100 | 221,126 | |||||
- (1)
- The as adjusted balance sheet data give effect to our issuance and sale of 2,000,000 shares of common stock in this offering at an assumed public offering price of $41.37 per share, which is the last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
14
Investing in our common stock involves a high degree of risk. Before investing in our common stock, you should consider carefully the risks described below, together with the other information contained in this prospectus and incorporated by reference in this prospectus, including the risk factors incorporated by reference from our Annual Report on Form 10-K for the year ended December 31, 2013, our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 and other filings we make with the SEC. We believe the risks described below and incorporated by reference herein are the risks that are material to us as of the date of this prospectus. If any of the following risks or the risks incorporated by reference herein occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment.
Risks Related To This Offering
Our executive officers, directors and principal stockholders maintain the ability to control all matters submitted to stockholders for approval.
As of June 1, 2014, our executive officers, directors and a small number of stockholders own more than a majority of our outstanding common stock. As a result, if these stockholders were to choose to act together, they would be able to control all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other stockholders may desire.
If you purchase shares of common stock in this offering, you will suffer immediate dilution of your investment.
The price of our common stock in this offering will be substantially higher than the net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. To the extent shares are issued under outstanding options, you will incur further dilution. Based on an assumed public offering price of $41.37 per share, which is the last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014, you will experience immediate dilution of $34.41 per share, representing the difference between our as adjusted net tangible book value per share after giving effect to this offering and the assumed public offering price per share.
An active trading market for our common stock may not be sustained following this offering.
Although our common stock is listed on The NASDAQ Global Select Market, an active trading market for our shares may not be sustained following this offering. If an active market for our common stock does not continue, it may be difficult for you to sell shares you purchase in this offering without depressing the market price for the shares, or at all. An inactive trading market for our common stock may also impair our ability to raise capital to continue to fund our operations by selling shares and may impair our ability to acquire other companies or technologies by using our shares as consideration.
We have broad discretion in the use of our cash and cash equivalents, including the net proceeds we receive in this offering, and may not use them effectively.
Our management has broad discretion to use our cash and cash equivalents, including the net proceeds we receive in this offering, to fund our operations and could spend these funds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by
15
our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our drug candidates. Pending their use to fund our operations, we may invest our cash and cash equivalents, including the net proceeds from this offering, in a manner that does not produce income or that loses value.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, of our common stock will be the sole source of gain for our stockholders.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be the sole source of gain for our stockholders for the foreseeable future.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have outstanding 31,762,499 shares of common stock based on 29,762,499 shares outstanding as of March 31, 2014. This assumes no exercise by the underwriters of their option to purchase additional shares and includes the shares that we and the selling stockholders are selling in this offering, which may be resold in the public market immediately without restriction, unless purchased by our affiliates. Of the remaining shares, a majority are currently restricted as a result of securities laws, including under Rule 144 or under lock-up agreements but will be able to be sold after the offering as described in the "Shares Eligible for Future Sale" section of this prospectus. Moreover, following this offering, based on information we have available to us, we believe holders of an aggregate of 17,819,662 shares of our common stock will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We have also registered shares of common stock that we have issued upon the exercise of outstanding stock options, and that we may in the future issue, under our equity compensation plans. These can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the "Underwriting" section of this prospectus.
16
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference into it contain forward-looking statements that involve substantial risks and uncertainties. These statements include all matters that are not related to present facts or current conditions or that are not historical facts, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward- looking statements.
The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "predict," "project," "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
- •
- the timing, progress and results of current and future preclinical studies and clinical trials, and our research and
development programs;
- •
- our plans to develop and commercialize our drug candidates;
- •
- the timing or likelihood of regulatory filings and approvals;
- •
- the implementation of our business model and strategic plans for our business, drug candidates and technology;
- •
- our commercialization, marketing and manufacturing capabilities and strategy;
- •
- the rate and degree of market acceptance and clinical utility of our drugs;
- •
- our competitive position;
- •
- our intellectual property position;
- •
- developments and projections relating to our competitors and our industry;
- •
- our ability to establish collaborations or obtain additional funding;
- •
- our expectations regarding the time during which we will be an emerging growth company under the JOBS Act;
- •
- our expectations related to the use of proceeds from this offering; and
- •
- our estimates regarding expenses, future revenue, capital requirements and needs for additional financing.
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this prospectus, particularly in the "Risk Factors" section and the other documents incorporated by reference herein, that could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we may make.
You should read this prospectus, the documents that we incorporate by reference or otherwise reference herein and the documents that we have filed as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from what we expect. The forward-looking statements contained in this prospectus and in the documents incorporated herein by reference are made as of the date of this prospectus and we do not assume any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
17
We estimate that the net proceeds to us from this offering will be approximately $77.0 million, based on an assumed public offering price of $41.37 per share, which is the last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their option to purchase additional shares of our common stock in full, we estimate that our net proceeds will be approximately $89.9 million after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We will not receive any of the proceeds from any sale of shares in this offering by the selling stockholders.
As of March 31, 2014, we had cash and cash equivalents of $144.9 million. We currently intend to use the net proceeds from this offering as follows:
- •
- approximately $65.0 million to fund the continued clinical development of our lead drug candidate, Selinexor
(KPT-330), including: approximately $52.0 million for initiating and conducting planned registration-directed clinical trials of Selinexor in three hematological malignancy indications and up
to two additional registration-directed clinical trials of Selinexor in hematological or solid tumor indications; approximately $13.0 million for initiating and conducting clinical trials of
Selinexor in additional clinical trials of Selinexor in additional cancer indications as a single agent as well as in combination with other therapies;
- •
- approximately $4.0 million to continue our preclinical development of our drug candidates for anti-inflammatory,
viral and wound-healing indications;
- •
- approximately $8.0 million for discovery, research, preclinical development and clinical trials of additional drug
candidates; and
- •
- the balance for working capital and other general corporate purposes.
This expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development, feedback from regulatory authorities, the status of and results from clinical trials, as well as any collaborations that we may enter into with third parties for our drug candidates, and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
Pending use of the proceeds as described above, we intend to invest the proceeds in a variety of capital preservation investments, including short-term, interest-bearing, investment-grade instruments and U.S. government securities.
18
Our common stock began trading on The NASDAQ Global Select Market under the symbol "KPTI" on November 6, 2013. Prior to that time, there was no public market for our common stock. The following table sets forth the high and low sale prices per share of our common stock, as reported on The NASDAQ Global Select Market, for the periods indicated.
| |
High | Low | |||||
|---|---|---|---|---|---|---|---|
Year Ended December 31, 2013 |
|||||||
Fourth quarter (from November 6, 2013) |
$ | 25.69 | $ | 15.50 | |||
Year Ending December 31, 2014 |
|||||||
First quarter |
$ | 47.87 | $ | 21.13 | |||
Second quarter (through June 24, 2014) |
$ | 47.98 | $ | 23.86 | |||
On June 24, 2014, the last reported sale price of our common stock on The NASDAQ Global Select Market was $41.37 per share. As of the date of this prospectus, we had approximately 25 holders of record of our common stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
We have not declared or paid any cash dividends on our capital stock since our inception. We intend to retain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future.
We obtained the industry, market and competitive position data in this prospectus from our own internal estimates and research as well as from industry and general publications and research, surveys and studies conducted by third parties. Industry and general publications and research, surveys and studies conducted by third parties generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information.
19
The following table sets forth our cash and cash equivalents and capitalization as of March 31, 2014, as follows:
- •
- on an actual basis; and
- •
- on an as adjusted basis to give effect to our issuance and sale of 2,000,000 shares of common stock in this offering at an assumed public offering price of $41.37 per share, which was the last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
You should read this information in conjunction with our consolidated financial statements and the related notes and the "Management's Discussion and Analysis of Financial Condition and Results of Operations" section, the "Selected Financial Data" section and other financial information in our Annual Report on Form 10-K for the year ended December 31, 2013 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014, which are incorporated by reference in this prospectus.
| |
As of March 31, 2014 | ||||||
|---|---|---|---|---|---|---|---|
| |
Actual | As Adjusted | |||||
| |
(in thousands, except share and per share data) |
||||||
Cash and cash equivalents |
$ | 144,893 | $ | 221,919 | |||
| | | | | | | | |
Undesignated preferred stock, $0.0001 par value: 5,000,000 shares authorized and no shares issued or outstanding |
— | — | |||||
Common stock, par value $0.0001 per share; 100,000,000 shares authorized, 29,618,879 shares issued and outstanding, actual; and 31,618,879 shares issued and outstanding, as adjusted |
3 | 3 | |||||
Additional paid-in capital |
220,360 | 297,386 | |||||
Deficit accumulated during the development stage |
(76,263 | ) | (76,263 | ) | |||
| | | | | | | | |
Total stockholders' equity |
144,100 | 221,126 | |||||
| | | | | | | | |
Total capitalization |
$ | 144,100 | $ | 221,126 | |||
| | | | | | | | |
| | | | | | | | |
The table above does not include:
- •
- 143,620 shares of unvested restricted stock subject to repurchase by us;
- •
- 2,505,749 shares of our common stock issuable upon exercise of stock options outstanding as of March 31, 2014 at a
weighted-average exercise price of $9.21 per share;
- •
- 1,722,111 and 242,424 additional shares of our common stock available for future issuance, as of March 31, 2014,
under our 2013 stock incentive plan and our 2013 employee stock purchase plan, respectively; and
- •
- 330,000 shares of our common stock available for purchase by the underwriters pursuant to their option.
20
PRINCIPAL AND SELLING STOCKHOLDERS
The following table sets forth information with respect to the beneficial ownership of our common stock, as of May 31, 2014, by:
- •
- each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock;
- •
- each of our directors;
- •
- each of our named executive officers;
- •
- all of our executive officers and directors as a group; and
- •
- each of the selling stockholders.
The column entitled "Shares Beneficially Owned Prior to Offering—Percentage" is based on a total of 29,786,403 shares of our common stock outstanding as of May 31, 2014. The column entitled "Shares Beneficially Owned After Offering—Percentage" is based on shares of our common stock to be outstanding after this offering, including 2,000,000 shares of our common stock that we are selling in this offering, and 200,000 shares of our common stock that the selling stockholders are selling in this offering, each at an assumed public offering price of $41.37 per share, which was the last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014, but not including any additional shares issuable upon exercise of outstanding options or the underwriters' option to purchase additional shares.
The number of shares beneficially owned by each stockholder is determined under rules issued by the Securities and Exchange Commission and includes voting or investment power with respect to securities. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that person, shares of common stock subject to options or other rights held by such person that are currently exercisable or will become exercisable within 60 days of May 31, 2014, are considered outstanding, although such shares subject to options or other rights are not considered outstanding for purposes of computing the percentage ownership of any other person. Unless otherwise indicated, the address of all listed stockholders is c/o Karyopharm Therapeutics Inc., 2 Mercer Road, Natick, Massachusetts 01760. Each of the stockholders listed has sole voting and investment power with respect to the shares beneficially owned by the stockholder unless noted otherwise, subject to community property laws where applicable.
21
The table below does not reflect any shares of our common stock that our directors, executive officers, 5% stockholders or their affiliated entities may purchase in this offering.
| |
Shares Beneficially Owned Prior to Offering |
|
Shares Beneficially Owned After Offering |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Shares Offered | |||||||||||||||
Name of Beneficial Owner
|
Number | Percentage | Number | Percentage | ||||||||||||
5% Stockholders |
||||||||||||||||
Chione Ltd.(1) |
10,258,079 | 34.44% | — | 10,258,079 | 32.30 | % | ||||||||||
Entities Affiliated with Franklin Resources, Inc.(2) |
3,542,004 | 11.89% | — | 3,542,004 | 11.15 | % | ||||||||||
Plio Limited(3) |
3,193,473 | 10.72% | — | 3,193,473 | 10.05 | % | ||||||||||
Entities Affiliated with Foresite Capital(4) |
2,378,615 | 7.99% | — | 2,378,615 | 7.49 | % | ||||||||||
Entities Affiliated with FMR LLC(5) |
2,346,000 | 7.88% | — | 2,346,000 | 7.39 | % | ||||||||||
Entities Affiliated with Delphi Ventures(6) |
2,217,552 | 7.44% | — | 2,217,552 | 6.98 | % | ||||||||||
Named Executive Officers and Directors |
||||||||||||||||
Michael G. Kauffman, M.D., Ph.D.(7) |
1,836,821 | 6.17% | 100,000 | (15) | 1,636,821 | 5.15 | % | |||||||||
Sharon Shacham, Ph.D., M.B.A.(8) |
1,836,821 | 6.17% | 100,000 | (16) | 1,636,821 | 5.15 | % | |||||||||
Paul Brannelly |
66,136 | * | — | 66,136 | * | |||||||||||
Deepa R. Pakianathan, Ph.D.(9) |
2,217,552 | 7.44% | — | 2,217,552 | 6.98 | % | ||||||||||
Mansoor Raza Mirza, M.D.(10) |
69,478 | * | — | 69,478 | * | |||||||||||
Alan T. Barber(11) |
10,952 | * | — | 10,952 | * | |||||||||||
Barry E. Greene(12) |
5,301 | * | — | 5,301 | * | |||||||||||
Kenneth E. Weg(13) |
2,683 | * | — | 2,683 | * | |||||||||||
Garen Bohlin |
0 | * | — | 0 | * | |||||||||||
All executive officers and directors as a group (9 persons)(14) |
4,208,923 | 14.13% | 200,000 | 4,008,923 | 12.62 | % | ||||||||||
All selling stockholders |
1,836,821 | 6.17% | 200,000 | 1,636,821 | 5.15 | % | ||||||||||
- *
- Less
than 1%.
- (1)
- The
address for Chione Ltd. is Simou Menardou 8, Ria Court 8, Office 101, 6015 Larnaca, Cyprus. The board of directors of Chione, comprised of Marcin
Czernik, Andreas Hadjimichael and Amalia Hadjimichael, and its sole stockholder, Wiaczeslaw Smolokowski, may be deemed to share voting and investment power with respect to the shares held by such
entity and each of them disclaims beneficial ownership of such shares except to the extent of any pecuniary interest therein. The shares held by Chione Ltd. do not include the shares held by
Plio Limited.
- (2)
- The address for Franklin Resources Inc. ("FRI") and affiliates is One Franklin Parkway, San Mateo, CA 94403-1906. Franklin Advisers, Inc. ("Franklin Advisers") directly holds 3,449,809 shares prior to this offering. Franklin Templeton Portfolio Advisors, Inc. ("FTPA") directly holds 47,232 shares prior to this offering. Fiduciary Trust Company International ("FTCI") directly holds 11,200 shares prior to this offering. Franklin Templeton Investments (Asia) Ltd. ("Franklin Asia") directly holds 863 shares prior to this offering. Each of Franklin Advisers, FTPA, FTCI and Franklin Asia (collectively, the "Franklin Funds") are investment companies or other managed accounts that are investment management clients of investment managers that are direct and indirect subsidiaries of FRI. Charles B. Johnson and Rupert H. Johnson, Jr. (the "FRI Principal Shareholders") each own in excess of 10% of the outstanding common stock of FRI and are the principal stockholders of FRI. FRI and the FRI Principal Shareholders may be deemed to be the beneficial owners of securities held by persons and entities for whom or for which FRI subsidiaries provide investment management services. For information regarding FRI, the Franklin Funds, and the FRI Principal Shareholders we have relied on the Schedule 13G filed on June 10, 2014.
22
- (3)
- The
address for Plio Limited is Simou Menardou 8, Ria Court 8, Office 101, 6015 Larnaca, Cyprus. The board of directors of Plio, comprised of Marcin
Czernik, Andreas Hadjimichael and Amalia Hadjimichael, and its sole stockholder, Gregory Jankilevitsch, may be deemed to share voting and investment power with respect to the shares held by such
entity and each of them disclaims beneficial ownership of such shares except to the extent of any pecuniary interest therein. The shares held by Plio Limited do not include the shares held by
Chione Ltd.
- (4)
- The
address for Foresite Capital and affiliates is One Montgomery Street, Suite 2500, San Francisco, California 94104. This consists of
(i) 1,585,743 shares of common stock held by Foresite Capital Fund I, L.P. (ii) 688,705 shares of common stock held by Foresite Capital IV-B, LLC and (iii) 104,167 shares
of common stock held by Foresite Capital IV-C, LLC. James B. Tananbaum is the managing member of Foresite Capital Management I, LLC, the general partner of Foresite Capital
Fund I, L.P., is the managing member of Foresite Capital IV-B Management, LLC, the managing member of Foresite Capital IV-B, LLC, and is the managing member of Foresite Capital IV-C Management,
LLC, the managing member of Foresite Capital IV-C, LLC, and as such, James B. Tananbaum may be deemed to have sole voting and investment power over the securities held by Foresite Capital
Fund I, L.P., Foresite Capital IV-B, LLC and Foresite Capital IV-C, LLC. James B. Tananbaum disclaims beneficial ownership of these securities except to the extent of his pecuniary interest
therein. For information regarding Foresite Capital and its affiliates, we have relied on the Schedule 13G filed by Foresite Capital Fund I, L.P. with the SEC on
November 15, 2013.
- (5)
- The
address for Fidelity SelectCo, LLC ("SelectCo") is 1225 17th Street, Suite 1100, Denver, Colorado 80202 and the address for Fidelity
Select Biotechnology Portfolio ("Portfolio") is 245 Summer Street, Boston, Massachusetts 02210. SelectCo, a wholly-owned subsidiary of FMR LLC and an investment adviser registered under
Section 203 of the Investment Advisers Act of 1940, may be deemed to be the beneficial owner of 2,346,000 shares of common stock as a result of acting as investment adviser to various
investment companies registered under Section 8 of the Investment Company Act of 1940, including Portfolio, which holds 2,055,682 shares of common stock. Additional shares of common stock are owned by
other investment companies. Members of the family of Edward C. Johnson 3d, Chairman of FMR LLC, are the predominant owners, directly or through trusts, of Series B voting
common shares of FMR LLC, representing 49% of the voting power of FMR LLC. For information regarding FMR LLC and its affiliates, we have relied on the Schedule 13G filed by FMR LLC with
the SEC on February 14, 2014.
- (6)
- The
address for Delphi Ventures and affiliates is 3000 Sand Hill Road, #1-135, Menlo Park, California 94025. Delphi Ventures VIII, L.P. ("Delphi
VIII") directly holds 2,196,109 shares. Delphi BioInvestments VIII, L.P. ("DBI VIII") directly holds 21,443 shares. Delphi Management Partners VIII, L.L.C. ("DMP VIII") is the general partner
of Delphi VIII and DBI VIII (together, the "Delphi VIII Funds") and may be deemed to have sole voting and dispositive power over the shares held by the Delphi VIII Funds. DMP VIII and each of James J.
Bochnowski, David L. Douglass, Douglas A. Roeder and Deepa R. Pakianathan, Ph.D., the Managing Members of DMP VIII who may be deemed to share voting and dispositive power over the reported securities,
disclaim beneficial ownership of the reported securities held by Delphi VIII Funds except to the extent of any pecuniary interest therein.
- (7)
- Consists of (a) 44,759 shares of common stock underlying options held by Michael Kauffman that are exercisable as of May 31, 2014 or will become exercisable within 60 days after such date, (b) 697,596 shares of common stock held by Dr. Kauffman, (c) 175,503 shares of common stock underlying options held by Sharon Shacham, who is the spouse of Dr. Kauffman, that are exercisable as of May 31, 2014 or will become exercisable within 60 days after such date and (d) 918,963 shares of common stock held by Dr. Shacham.
23
- (8)
- Consists
of (a) 175,503 shares of common stock underlying options held by Dr. Shacham that are exercisable as of May 31, 2014 or will
become exercisable within 60 days after such date, (b) 918,963 shares of common stock held by Dr. Shacham, (c) 44,759 shares of common stock underlying options held by
Dr. Kauffman, who is Dr. Shacham's spouse, that are exercisable as of May 31, 2014 or will become exercisable within 60 days after such date and (d) 697,596 shares
of common stock held by Dr. Kauffman.
- (9)
- Delphi
Ventures VIII, L.P. ("Delphi VIII") directly holds 2,196,109 shares. Delphi BioInvestments VIII, L.P. ("DBI VIII") directly holds
21,443 shares. Delphi Management Partners VIII, L.L.C. ("DMP VIII") is the general partner of Delphi VIII and DBI VIII (together, the "Delphi VIII Funds") and may be deemed to have sole voting and
dispositive power over the shares held by the Delphi VIII Funds. DMP VIII and each of James J. Bochnowski, David L. Douglass, Douglas A. Roeder and Deepa R. Pakianathan, Ph.D., the Managing Members of
DMP VIII who may be deemed to share voting and dispositive power over the reported securities, disclaim beneficial ownership of the reported securities held by Delphi VIII Funds except to the extent
of any pecuniary interest therein.
- (10)
- Consists
of (a) 46,751 shares of common stock underlying options that are exercisable as of May 31, 2014 or will become exercisable within
60 days after such date of which 41,954 are held by Dr. Mirza personally and 4,797 are held by his consulting firm, and (b) 22,727 shares of common stock.
- (11)
- Consists
of 10,952 shares of common stock underlying options that are exercisable as of May 31, 2014 or will become exercisable within
60 days after such date.
- (12)
- Consists
of 5,301 shares of common stock underlying options that are exercisable as of May 31, 2014 or will become exercisable within 60 days
after such date.
- (13)
- Consists
of 2,683 shares of common stock that are exercisable as of May 31, 2014 or will become exercisable within 60 days after such date.
- (14)
- Includes
285,949 shares of common stock underlying options that are exercisable as of May 31, 2014 or will become exercisable within 60 days
after such date.
- (15)
- This
number does not include 100,000 shares being offered by Sharon Shacham, the spouse of Dr. Kauffman, of which Dr. Kauffman was a beneficial owner prior
to this offering.
- (16)
- This number does not include 100,000 shares being offered by Michael Kauffman, the spouse of Dr. Shacham, of which Dr. Shacham was a beneficial owner prior to this offering.
24
General
Our authorized capital stock consists of 100,000,000 shares of common stock, par value $0.0001 per share, and 5,000,000 shares of preferred stock, par value $0.0001 per share. The following description of our capital stock and provisions of our certificate of incorporation and by-laws are summaries and are qualified by reference to our certificate of incorporation and by-laws. Copies of these documents have been filed with the Securities and Exchange Commission as exhibits to our registration statement, of which this prospectus forms a part.
Common Stock
As of March 31, 2014, there were 29,762,499 shares of our common stock outstanding and held of record by approximately 25 stockholders.
Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have cumulative voting rights. An election of directors by our stockholders shall be determined by a plurality of the votes cast by the stockholders entitled to vote on the election. Holders of common stock are entitled to receive proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights of any series of preferred stock that we may designate and issue in the future.
In the event of our liquidation or dissolution, the holders of common stock are entitled to receive proportionately our net assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of common stock have no preemptive, subscription, redemption or conversion rights. Our outstanding shares of common stock are, and the shares offered by us in this offering will be, when issued and paid for, validly issued, fully paid and nonassessable. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our certificate of incorporation, our board of directors is authorized to direct us to issue shares of preferred stock in one or more series without stockholder approval. Our board of directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The purpose of authorizing our board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a majority of our outstanding voting stock. As of March 31, 2014, there were no shares of preferred stock outstanding, and we have no present plans to issue any shares of preferred stock.
Options
As of March 31, 2014, options to purchase 2,505,749 shares of our common stock at a weighted average exercise price of $9.21 per share were outstanding.
25
Registration Rights
We have entered into a third amended and restated investors' rights agreement, dated July 26, 2013, which we refer to as the investor rights agreement, with the former holders of shares of our preferred stock. Holders of a total of 17,819,662 shares of our common stock as of March 31, 2014 have the right to require us to register these shares under the Securities Act, and to participate in future registrations of securities by us, under the circumstances described below. After registration pursuant to these rights, these shares will become freely tradable without restriction under the Securities Act except that affiliates will need to comply with resale restrictions under Rule 144 of the Securities Act. If not otherwise exercised, the rights described below will expire five years after the closing of our initial public offering.
Demand Registration Rights
Subject to specified limitations set forth in the investor rights agreement, at any time, the holders of 50% or more of the then outstanding shares having rights under the investor rights agreement, which we refer to as registrable shares, may at any time demand in writing that we register all or a portion of the registrable shares under the Securities Act if the total amount of registrable shares registered have an anticipated aggregate offering price of at least $5,000,000. We are not obligated to file a registration statement pursuant to this provision on more than two occasions.
In addition, subject to specified limitations set forth in the investor rights agreement, at any time after we become eligible to file a registration statement on Form S-3, holders of registrable shares outstanding may demand that we register on Form S-3 the registrable shares held by them so long as the total amount of registrable shares being registered has an aggregate offering price of at least $1,000,000. We are not obligated to file a registration statement pursuant to this provision on more than two occasions in any 12-month period.
Incidental Registration Rights
If we propose to file a registration statement to register any of our securities under the Securities Act, either for our own account or for the account of any of our stockholders, other than pursuant to the demand registration rights described above and other than pursuant to a Form S-4 or Form S-8, the holders of our registrable shares are entitled to notice of registration and, subject to specified limitations set forth in the investor rights agreement, we will be required to use best efforts to register the registrable shares then held by them that they request that we register.
In the event that any registration in which the holders of registrable shares participate pursuant to our investor rights agreement is an underwritten public offering, we agree to enter into an underwriting agreement containing customary representations and warranties and covenants.
In the event that any registration in which the holders of registrable shares participate pursuant to our investor rights agreement is an underwritten public offering, we will use our best efforts to include the requested registrable shares to be included, but may be limited by market conditions.
Expenses
Pursuant to the investor rights agreement, we are required to pay all registration, qualification and filing fees, printing and accounting expenses, fees and expenses of one counsel to represent the selling stockholders and other reasonable direct costs for the selling stockholders (such selling stockholder expenses not to exceed $50,000), but excluding underwriting discounts, selling commissions and the fees and expenses of selling stockholders' own counsel (other than the counsel selected to represent all selling stockholders). We are not required to pay registration expenses if the registration request under the investor rights agreement is withdrawn at the request of holders of a majority of the
26
registrable shares, unless such holders agree to forfeit their right to one demand registration, or the withdrawal is due to discovery of a materially adverse change in our business.
The investor rights agreement contains customary cross-indemnification provisions, pursuant to which we are obligated to indemnify the selling stockholders in the event of material misstatements or omissions in the registration statement attributable to us, and they are obligated to indemnify us for material misstatements or omissions in the registration statement attributable to them.
Anti-Takeover Provisions
We are subject to Section 203 of the Delaware General Corporation Law. Subject to certain exceptions, Section 203 prevents a publicly held Delaware corporation from engaging in a "business combination" with any "interested stockholder" for three years following the date that the person became an interested stockholder, unless the interested stockholder attained such status with the approval of our board of directors or unless the business combination is approved in a prescribed manner or the interested stockholder acquired at least 85% of our outstanding voting stock in the transaction in which it became an interested stockholder. A "business combination" includes, among other things, a merger or consolidation involving us and the "interested stockholder" and the sale of more than 10% of our assets. In general, an "interested stockholder" is any entity or person beneficially owning 15% or more of our outstanding voting stock and any entity or person affiliated with or controlling or controlled by such entity or person.
Staggered Board; Removal of Directors
Our certificate of incorporation and our by-laws divide our board of directors into three classes with staggered three-year terms. In addition, such certificate of incorporation and by-laws provide that, subject to the rights of holders of any series of preferred stock, a director may be removed only for cause and only by the affirmative vote of the holders of at least 75% of the votes that all our stockholders would be entitled to cast in an annual election of directors. Under our certificate of incorporation and by-laws, any vacancy on our board of directors, including a vacancy resulting from an enlargement of our board of directors, may be filled only by vote of a majority of our directors then in office. Furthermore, our certificate of incorporation provides that the authorized number of directors may be changed only by the resolutions of our board of directors.
The classification of our board of directors and the limitations on the removal of directors and filling of vacancies could make it more difficult for a third party to acquire, or discourage a third party from seeking to acquire, control of our company.
Super-Majority Voting
The Delaware General Corporation Law provides generally that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation's certificate of incorporation or by-laws, unless a corporation's certificate of incorporation or by-laws, as the case may be, requires a greater percentage. Our by-laws may be amended or repealed by a majority vote of our board of directors or the affirmative vote of the holders of at least 75% of the votes that all our stockholders would be entitled to cast in any annual election of directors. In addition, the affirmative vote of the holders of at least 75% of the votes that all our stockholders would be entitled to cast in any annual election of directors is required to amend or repeal, or to adopt any provisions inconsistent with, any of the provisions of our certificate of incorporation described under "Staggered Board; Removal of Directors" above.
27
Stockholder Action; Special Meeting of Stockholders; Advance Notice Requirements for Stockholder Proposals and Director Nominations
Our certificate of incorporation and our by-laws provide that any action required or permitted to be taken by our stockholders at a duly called annual meeting or special meeting of stockholders may only be taken if it is properly brought before such meeting and may not be taken by written action in lieu of a meeting. Our certificate of incorporation and our by-laws also provide that, except as otherwise required by law, special meetings of our stockholders can only be called by our board of directors, chairman of the board or chief executive officer. In addition, our by-laws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of stockholders, including proposed nominations of candidates for election to our board of directors. Stockholders at an annual meeting may only consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of our board of directors, or by a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has delivered timely written notice in proper form to our secretary of the stockholder's intention to bring such business before the meeting. These provisions could have the effect of delaying until the next stockholder meeting stockholder actions that are favored by the holders of a majority of our outstanding voting stock. These provisions also could discourage a third party from making a tender offer for our common stock, because even if it acquired a majority of our outstanding voting stock, it would be able to take action as a stockholder, such as electing new directors or approving a merger, only at a duly called stockholders meeting and not by written consent.
Authorized But Unissued Shares
The authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval, subject to any limitations imposed by the listing standards of The NASDAQ Global Select Market. These additional shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could make more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A.
NASDAQ Global Select Market
Our common stock is listed on The NASDAQ Global Select Market under the symbol "KPTI."
28
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of common stock in the public market, or the perception that such sales may occur, could adversely affect the market price of our common stock. Our common stock is listed on The NASDAQ Global Select Market under the symbol "KPTI."
Upon the closing of this offering, we will have outstanding an aggregate of 31,762,499 shares of common stock, based on the number of shares of our common stock outstanding as of March 31, 2014, assuming the issuance of 2,000,000 shares of common stock by us in this offering and assuming no exercise by the underwriters of their option to purchase additional shares and no exercise of options outstanding as of March 31, 2014. Of these shares, all shares sold in this offering, including both the shares sold by us and the shares sold by the selling stockholders, will be freely tradable without restriction or further registration under the Securities Act, except for any shares purchased by our "affiliates," as that term is defined in Rule 144 under the Securities Act, whose sales would be subject to the Rule 144 resale restrictions described below, other than the holding period requirement.
The remaining 27,762,499 shares of common stock will be "restricted securities," as that term is defined in Rule 144 under the Securities Act, of which 4,008,923 shares of common stock will further be subject to restrictions on transfer under the lock-up agreements described below. The lock-up agreements entered into in connection with this offering restrict transfer for a period from the date of execution of the lock-up agreement to 90 days from the date of the underwriting agreement described under "Underwriting". Following the expiration of these restrictions, these shares will become eligible for public sale if they are registered under the Securities Act or if they qualify for an exemption from registration under Rules 144 or 701 under the Securities Act, which are summarized below.
In addition, of the 2,505,749 shares of our common stock that were subject to stock options outstanding as of March 31, 2014, options to purchase 359,420 shares of common stock were vested as of March 31, 2014 and, upon exercise, these shares will be eligible for sale, subject to the lock-up agreements described in this section and Rules 144 and 701 under the Securities Act.
Lock-Up Agreements
We and each of our directors and executive officers and the selling stockholders, who collectively beneficially own 4,208,923 shares of our common stock as of March 31, 2013, without the prior written consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated on behalf of the underwriters, will not, subject to limited exceptions, during the period from the date of execution of the lock-up agreement to 90 days from the effectiveness of the registration statement of which this prospectus forms a part, directly or indirectly:
- •
- offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell,
grant any option, right or warrant for the sale of, or otherwise dispose of or transfer any shares of our common stock or any securities convertible into or exchangeable or exercisable for our common
stock, except for the shares made available by our selling stockholders as part of this prospectus;
- •
- exercise any right with respect to the registration of any shares of our common stock or any security convertible into or
exercisable or exchangeable for our common stock, or with respect to the filing of any registration statement in connection therewith under the Securities Act; or
- •
- enter into any swap or any other agreement or any transaction that transfers, in whole or in part, the economic consequence of ownership of our common stock,
whether any transaction described above is to be settled by delivery of our common stock or such other securities, in cash or otherwise.
29
The Company announced on June 14, 2014 that each of Drs. Kauffman and Shacham entered into a pre-arranged stock trading plan (a "10b5-1 Plan") pursuant to Rule 10b5-1 of the Securities Exchange Act of 1934, as amended. Pursuant to an agreement with the underwriters, each of Drs. Kauffman and Shacham have agreed to terminate their respective 10b5-1 Plan effective as of, and conditioned upon, the execution of the underwriting agreement related to this offering.
The lock-up restrictions are described in more detail in the section of this prospectus entitled "Underwriting."
Rule 144
Affiliate Resales of Restricted Securities
In general, a person who is an affiliate of ours, or who was an affiliate at any time during the 90 days before a sale, who has beneficially owned shares of our common stock for at least six months would be entitled to sell in "broker's transactions" or certain "riskless principal transactions" or to market makers, a number of shares within any three-month period that does not exceed the greater of:
- •
- 1% of the number of shares of our common stock then outstanding, which will equal approximately 317,625 shares immediately
after this offering; or
- •
- the average weekly trading volume in our common stock on The NASDAQ Global Select Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale.
Affiliate resales under Rule 144 are also subject to the availability of current public information about us. In addition, if the number of shares being sold under Rule 144 by an affiliate during any three-month period exceeds 5,000 shares or has an aggregate sale price in excess of $50,000, the seller must file a notice on Form 144 with the Securities and Exchange Commission and NASDAQ concurrently with either the placing of a sale order with the broker or the execution directly with a market maker.
Non-Affiliate Resales of Restricted Securities
In general, a person who is not an affiliate of ours at the time of sale, and has not been an affiliate at any time during the 90 days preceding a sale, and who has beneficially owned shares of our common stock for at least six months but less than a year, is entitled to sell such shares subject only to the availability of current public information about us. If such person has held our shares for at least one year, such person can resell under Rule 144(b)(1) without regard to any Rule 144 restrictions, including the current public information requirement.
Non-affiliate resales are not subject to the manner of sale, volume limitation or notice filing provisions of Rule 144.
Rule 701
In general, under Rule 701, any of an issuer's employees, directors, officers, consultants or advisors who purchases shares from the issuer in connection with a qualified compensatory stock or option plan or other written agreement before our initial public offering is entitled to sell such shares in reliance on Rule 144. An affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirement, and non-affiliates of the issuer can resell shares in reliance on Rule 144 without having to comply with the current public information and holding period requirements.
The Securities and Exchange Commission has indicated that Rule 701 will apply to typical stock options granted by an issuer before it becomes subject to the reporting requirements of the
30
Securities Exchange Act of 1934, as amended, or the Exchange Act, along with the shares acquired upon exercise of such options, including exercises after an issuer becomes subject to the reporting requirements of the Exchange Act.
Equity Plans
We have filed a registration statement on Form S-8 under the Securities Act to register all shares of common stock subject to outstanding stock options and common stock issuable under our stock plans, permitting the resale of such shares by non-affiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance with the resale provisions of Rule 144.
Registration Rights
As of March 31, 2014, the holders of 17,819,662 shares of our common stock or their transferees are entitled to various rights with respect to the registration of these shares under the Securities Act. Registration of these shares under the Securities Act would result in these shares becoming fully tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, subject to the terms of the lock-up agreement to which the holder is subject and except for shares purchased by affiliates. See "Description of Capital Stock—Registration Rights" for additional information.
31
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
OF COMMON STOCK
The following is a general discussion of material U.S. federal income and estate tax considerations relating to the purchase, ownership and disposition of shares of our common stock by a non-U.S. holder. For purposes of this discussion, a non-U.S. holder means a beneficial owner of shares of our common stock that is not for U.S. federal income tax purposes:
- •
- an individual who is a citizen or resident of the United States;
- •
- a corporation, or other entity treated as a corporation for U.S. federal income tax purposes, created or organized in or
under the laws of the United States or of any state or political subdivision of the United States;
- •
- an estate the income of which is subject to U.S. federal income taxation regardless of its source; or
- •
- a trust if (1) a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust or (2) the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
This discussion does not address the tax treatment of partnerships or other entities that are pass-through entities for U.S. federal income tax purposes or persons that hold their common stock through partnerships or other pass-through entities. A partner in a partnership or other pass-through entity that will hold our common stock should consult his, her or its own tax advisor regarding the tax consequences of acquiring, holding and disposing of our common stock through a partnership or other pass-through entity, as applicable.
This discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code, existing and proposed U.S. Treasury Regulations promulgated or proposed thereunder and current administrative and judicial interpretations thereof, all as in effect as of the date of this prospectus and all of which are subject to change or to differing interpretation, possibly with retroactive effect. Any change could alter the tax consequences to non-U.S. holders described in this prospectus. There can be no assurance that the Internal Revenue Service, or the IRS, will not challenge one or more of the tax consequences described in this prospectus. We assume in this discussion that each non-U.S. holder holds shares of our common stock as a capital asset (generally, property held for investment).
This discussion does not address all aspects of U.S. federal income and estate taxation that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder's individual circumstances nor does it address any aspects of U.S. state, local or non-U.S. taxes, the alternative minimum tax, the Medicare tax on net investment income or, except as explicitly addressed herein, U.S. federal taxes other than income and estate taxes. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax considerations that may be applicable to particular non-U.S. holders, such as:
- •
- insurance companies;
- •
- tax-exempt organizations;
- •
- financial institutions;
- •
- brokers or dealers in securities;
- •
- regulated investment companies;
- •
- pension plans;
32
- •
- controlled foreign corporations;
- •
- passive foreign investment companies;
- •
- persons that have a "functional currency" other than the U.S. dollar;
- •
- persons that acquire shares of our common stock as compensation for services;
- •
- owners that hold shares of our common stock as part of a straddle, hedge, conversion transaction, synthetic security or
other integrated investment; and
- •
- certain U.S. expatriates.
This discussion is for general information only and is not tax advice. Accordingly, all prospective non-U.S. holders of our common stock should consult their own tax advisors regarding the U.S. federal, state, local and non-U.S. income and other tax considerations of acquiring, holding and disposing of shares of our common stock.
Distributions on Common Stock
Distributions on our common stock generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles and will be subject to withholding as described in the paragraphs below. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder's investment, up to such holder's tax basis in its shares of our common stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below under the heading "Gain on Sale, Exchange or Other Taxable Disposition of Shares of Our Common Stock." Any such distributions will also be subject to the discussion below under the section titled "Withholding and Information Reporting Requirements—FATCA."
Dividends paid to a non-U.S. holder generally will be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder's country of residence.
Dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States, and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by the non-U.S. holder within the United States, are generally exempt from the 30% withholding tax if the non-U.S. holder satisfies applicable certification and disclosure requirements. To obtain this exemption, a non-U.S. holder must generally satisfy certain disclosure and certification requirements, including providing us with a properly executed IRS Form W-8ECI. However, such U.S. effectively connected income, net of specified deductions and credits, is taxed at the same graduated U.S. federal income tax rates applicable to U.S. persons (as defined in the Code). Any U.S. effectively connected income received by a non-U.S. holder that is treated as a corporation for U.S. federal income tax purposes may also, under certain circumstances, be subject to an additional "branch profits tax" at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder's country of residence.
A non-U.S. holder of shares of our common stock who claims the benefit of an applicable income tax treaty between the United States and such holder's country of residence generally will be required to provide a properly executed IRS Form W-8BEN or W-BEN-E (or applicable successor form) and satisfy applicable certification and other requirements. Non-U.S holders are urged to consult their own tax advisors regarding their entitlement to benefits under a relevant income tax treaty.
33
A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty or that is otherwise not subject to U.S. withholding tax may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS.
Gain on Sale, Exchange or Other Taxable Disposition of Shares of Our Common Stock
Subject to the discussion below under the section titled "Withholding and Information Reporting Requirements—FATCA," a non-U.S. holder generally will not be subject to U.S. federal income tax on gain realized on a sale, exchange or other taxable disposition of shares of our common stock unless:
- •
- the gain is effectively connected with the non-U.S. holder's conduct of a trade or business in the United States, and, if
an applicable income tax treaty so provides, the gain is attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the United States; in these cases, the non-U.S.
holder generally will be taxed on a net income basis at the regular graduated rates and in the manner applicable to United States Persons (as defined in the Code), and, if the non-U.S. holder is a
non-U.S. corporation, an additional branch profits tax at a rate of 30%, or a lower rate as may be specified by an applicable income tax treaty, may also apply;
- •
- the non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more in the
taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax
treaty between the United States and such holder's country of residence) on the net gain derived from the disposition, which may be offset by certain U.S. source capital losses of the non-U.S. holder,
if any; or
- •
- we are or have been, at any time during the five-year period preceding such disposition (or the non-U.S. holder's holding period, if shorter) a "U.S. real property holding corporation" unless our common stock is regularly traded on an established securities market and the non-U.S. holder held no more than 5% of our outstanding common stock, directly or indirectly, during the shorter of the 5-year period ending on the date of the disposition or the period that the non-U.S. holder held our common stock. Generally, a corporation is a "U.S. real property holding corporation" if the fair market value of its "U.S. real property interests" (within the meaning of the Code) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we believe that we are not currently, and we do not anticipate becoming, a "U.S. real property holding corporation" for U.S. federal income tax purposes. No assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described above.
Information Reporting and Backup Withholding Tax
We must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions on shares of our common stock paid to such holder and the tax withheld, if any, with respect to such distributions. These information reporting requirements apply even if withholding is not required. Non-U.S. holders may have to comply with specific certification procedures to establish that the holder is not a United States person (as defined in the Code) or is otherwise subject to an exemption in order to avoid backup withholding at the applicable rate with respect to dividends paid with respect to shares of our common stock. Dividends paid to non-U.S. holders subject to the U.S. federal withholding tax, as described above in "Dividends," generally will be exempt from U.S. backup withholding.
34
Information reporting and backup withholding generally will apply to the payment of the proceeds of a disposition of shares of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or non-U.S., unless the holder certifies that it is not a U.S. person (as defined in the Code) and satisfies certain other requirements, or otherwise establishes an exemption. For information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker and dispositions otherwise effected through a non-U.S. office generally will not be subject to information reporting. Generally, backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected through a non-U.S. office of a broker. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder can be refunded or credited against the non-U.S. holder's U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
Withholding and Information Reporting Requirements—FATCA
The Foreign Account Tax Compliance Act, or FATCA, generally imposes a U.S. federal withholding tax at a rate of 30% on payments of dividends on, or gross proceeds from the sale or other disposition of, our common stock paid to a foreign entity unless (i) if the foreign entity is a "foreign financial institution," such foreign entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the foreign entity is not a "foreign financial institution," such foreign entity identifies certain of its U.S. investors, if any, or (iii) the foreign entity is otherwise exempt under FATCA. Under applicable U.S. Treasury regulations, withholding under FATCA will only apply (1) to payments of dividends on our common stock made after June 30, 2014, and (2) to payments of gross proceeds from a sale or other disposition of our common stock made after December 31, 2016. Under certain circumstances, a non-U.S. holder may be eligible for refunds or credits of the tax. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult their own tax advisors regarding the possible implications of this legislation on their investment in our common stock and the entities through which they hold our common stock, including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of the 30% withholding tax under FATCA.
U.S. Federal Estate Tax
Shares of our common stock owned or treated as owned at the time of death by an individual who is not a citizen or resident of the United States (as specially defined for U.S. federal estate tax purposes) are considered U.S. situs assets and will be included in the individual's gross estate for U.S. federal estate tax purposes and, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax or other treaty provides otherwise.
The preceding discussion of material U.S. federal tax considerations is for general information only. It is not tax advice. Prospective investors should consult their own tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing of shares of our common stock, including the consequences of any proposed changes in applicable laws.
35
Merrill Lynch, Pierce, Fenner & Smith Incorporated and Leerink Partners LLC are acting as representatives of each of the underwriters named below. Subject to the terms and conditions set forth in an underwriting agreement among us, the selling stockholders and the underwriters, we and the selling stockholders have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us and the selling stockholders, the number of shares of common stock set forth opposite its name below.
| Underwriter |
Number of Shares |
|||
|---|---|---|---|---|
Merrill Lynch, Pierce, Fenner & Smith |
||||
Incorporated |
||||
Leerink Partners LLC |
||||
JMP Securities LLC |
||||
Wedbush Securities Inc |
||||
Oppenheimer & Co. Inc. |
||||
| | | | | |
Total |
2,200,000 | |||
| | | | | |
| | | | | |
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the shares sold under the underwriting agreement if any of these shares are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated.
We and the selling stockholders have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officers' certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
The representatives have advised us and the selling stockholders that the underwriters propose initially to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After the initial offering, the public offering price, concession or any other term of the offering may be changed.
The following table shows the public offering price, underwriting discount and proceeds before expenses to us and to the selling stockholders. We will not receive any of the proceeds from the sale of the common stock by the selling stockholders. The information assumes either no exercise or full exercise by the underwriters of their option to purchase additional shares of our common stock.
| |
Per Share | Without Option | With Option | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Public offering price |
$ | $ | $ | |||||||
Underwriting discount |
$ | $ | $ | |||||||
Proceeds, before expenses, to us |
$ | $ | $ | |||||||
Proceeds, before expenses, to the selling stockholders |
$ | $ | — | |||||||
36
The expenses of the offering to us, not including the underwriting discount, are estimated at $750,000. We have agreed to reimburse the underwriters for all expenses related to the clearing of this offering with the Financial Industry Regulatory Authority and the qualification of our common stock under state securities laws (in an amount not to exceed in the aggregate $35,000).
Option to Purchase Additional Shares
We have granted an option to the underwriters, exercisable for 30 days after the date of this prospectus, to purchase up to 330,000 additional shares at the public offering price, less the underwriting discount. If the underwriters exercise this option, each will be obligated, subject to conditions contained in the underwriting agreement, to purchase a number of additional shares proportionate to that underwriter's initial amount reflected in the above table. The selling stockholders have not granted such an option to the underwriters.
No Sales of Similar Securities
We, our executive officers and directors and the selling stockholders have agreed not to sell or transfer any common stock or securities convertible into or exchangeable or exercisable for common stock, for 90 days after the date of this prospectus without first obtaining the written consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated. Specifically, we and these other persons have agreed, with certain limited exceptions, not to directly or indirectly
- •
- offer, pledge, sell or contract to sell any common stock;
- •
- sell any option or contract to purchase any common stock;
- •
- purchase any option or contract to sell any common stock;
- •
- grant any option, right or warrant for the sale of any common stock;
- •
- otherwise dispose of or transfer any common stock;
- •
- request or demand that we file a registration statement related to the common stock; or
- •
- enter into any swap or other agreement or any transaction that transfers, in whole or in part, the economic consequence of ownership of any common stock, whether any such swap, agreement or transaction is to be settled by delivery of shares or other securities, in cash or otherwise.
This lock-up provision applies to common stock and to securities convertible into or exchangeable or exercisable for common stock. It also applies to common stock owned now or acquired later by the person executing the agreement or for which the person executing the agreement later acquires the power of disposition.
NASDAQ Global Select Market Listing
Our common stock is listed on the NASDAQ Global Select Market under the symbol "KPTI."
Price Stabilization, Short Positions
Until the distribution of the shares is completed, SEC rules may limit underwriters and selling group members from bidding for and purchasing our common stock. However, the representatives may engage in transactions that stabilize the price of the common stock, such as bids or purchases to peg, fix or maintain that price.
In connection with the offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchases on the open market to cover
37
positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering. "Covered" short sales are sales made in an amount not greater than the underwriters' option described above. The underwriters may close out any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase shares granted to them. "Naked" short sales are sales in excess of such option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of common stock made by the underwriters in the open market prior to the completion of the offering.
Similar to other purchase transactions, the underwriters' purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. The underwriters may conduct these transactions on the NASDAQ Global Select Market, in the over-the-counter market or otherwise.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor any of the underwriters make any representation that the representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Passive Market Making
In connection with this offering, underwriters and selling group members may engage in passive market making transactions in the common stock on the NASDAQ Global Select Market in accordance with Rule 103 of Regulation M under the Exchange Act during a period before the commencement of offers or sales of common stock and extending through the completion of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker's bid, that bid must then be lowered when specified purchase limits are exceeded. Passive market making may cause the price of our common stock to be higher than the price that otherwise would exist in the open market in the absence of those transactions. The underwriters and dealers are not required to engage in passive market making and may end passive market making activities at any time.
Electronic Distribution
In connection with the offering, certain of the underwriters or securities dealers may distribute prospectuses by electronic means, such as e-mail.
Other Relationships
Some of the underwriters and their affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They have received, or may in the future receive, customary fees and commissions for these transactions.
38
In addition, in the ordinary course of their business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
A managing director of Leerink Partners LLC, one of the underwriters in this offering, has a 0.1% interest in Foresite Capital Fund I, L.P., who holds 1,585,743 shares of our common stock as of March 31, 2014.
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area (each, a "Relevant Member State"), no offer of shares may be made to the public in that Relevant Member State other than:
- A.
- to
any legal entity which is a qualified investor as defined in the Prospectus Directive;
- B.
- to
fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons
(other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives; or
- C.
- in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of shares shall require the Company or the representatives to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
Each person in a Relevant Member State who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed that it is a "qualified investor" within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive. In the case of any shares being offered to a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representatives has been obtained to each such proposed offer or resale.
The Company, the representatives and their affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgements and agreements.
This prospectus has been prepared on the basis that any offer of shares in any Relevant Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of shares. Accordingly any person making or intending to make an offer in that Relevant Member State of shares which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for the Company or any of the underwriters to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither the Company nor the underwriters have authorized, nor do they authorize, the making of any offer of shares in circumstances in which an obligation arises for the Company or the underwriters to publish a prospectus for such offer.
39
For the purpose of the above provisions, the expression "an offer to the public" in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in the Relevant Member State by any measure implementing the Prospectus Directive in the Relevant Member State and the expression "Prospectus Directive" means Directive 2003/71/EC (including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member States) and includes any relevant implementing measure in the Relevant Member State and the expression "2010 PD Amending Directive" means Directive 2010/73/EU.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are "qualified investors" (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the "Order") and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as "relevant persons"). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange ("SIX") or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company or the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes ("CISA"). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in the Dubai International Financial Centre
This prospectus relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority ("DFSA"). This prospectus is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth herein and has no responsibility for the prospectus. The shares to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the shares offered should conduct their own due diligence on the shares. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
40
Notice to Prospective Investors in Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission ("ASIC"), in relation to the offering. This prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001 (the "Corporations Act"), and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the shares may only be made to persons (the "Exempt Investors") who are "sophisticated investors" (within the meaning of section 708(8) of the Corporations Act), "professional investors" (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act.
The shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring shares must observe such Australian on-sale restrictions.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice to Prospective Investors in Hong Kong
The shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to "professional investors" as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a "prospectus" as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to Prospective Investors in Japan
The shares have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) and, accordingly, will not be offered or sold, directly or indirectly, in Japan, or for the benefit of any Japanese Person or to others for re-offering or resale, directly or indirectly, in Japan or to any Japanese Person, except in compliance with all applicable laws, regulations and ministerial guidelines promulgated by relevant Japanese governmental or regulatory authorities in effect at the relevant time. For the purposes of this paragraph, "Japanese Person" shall mean any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
41
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the "SFA"), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
- (a)
- a
corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the
entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
- (b)
- a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor,
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except:
- (a)
- to
an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in
Section 275(1A) or Section 276(4)(i)(B) of the SFA;
- (b)
- where
no consideration is or will be given for the transfer;
- (c)
- where
the transfer is by operation of law;
- (d)
- as
specified in Section 276(7) of the SFA; or
- (e)
- as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore.
42
The validity of the shares of common stock offered hereby will be passed upon for us by Wilmer Cutler Pickering Hale and Dorr LLP, Boston, Massachusetts. Ropes & Gray LLP, Boston, Massachusetts, has acted as counsel for the underwriters in connection with certain legal matters related to this offering.
The consolidated financial statements incorporated in this Prospectus by reference to the Annual Report on Form 10-K for the year ended December 31, 2013 have been audited by McGladrey LLP, an independent registered public accounting firm, as stated in their report incorporated by reference herein, and have been so incorporated in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information about us and the common stock offered hereby, we refer you to the registration statement and the exhibits and schedules filed therewith. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement or incorporated herein by reference are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement or incorporated herein by reference. We are required to file periodic reports, proxy statements, and other information with the SEC pursuant to the Exchange Act. You may read and copy this information at the SEC's public preference room, which is located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements and other information about registrants, like us, that file electronically with the SEC. The address of that site is www.sec.gov.
43
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to "incorporate by reference" information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus.
We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC.
- •
- our Annual Report on Form 10-K for the fiscal year ended December 31, 2013 filed on March 21, 2014;
- •
- our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 filed on May 8, 2014;
- •
- our Current Reports on Form 8-K filed on each of April 1, 2014, April 17, 2014, and June 13,
2014;
- •
- our Proxy Statement for our 2014 annual meeting of stockholders filed on April 28, 2014 (solely to the extent
incorporated by reference into part III of our Annual Report on Form 10-K); and
- •
- the description of our common stock contained in our Registration Statement on Form 8-A filed on November 1, 2013, including any amendment or report filed for the purpose of updating such description.
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
You may request, orally or in writing, a copy of any or all of the documents incorporated herein by reference. These documents will be provided to you at no cost, by contacting: Investor Relations, Karyopharm Therapeutics Inc., 2 Mercer Road, Natick, MA 01760, (508) 975-4820. In addition, copies of any or all of the documents incorporated herein by reference may be accessed at our website at www.karyopharm.com. The information contained in, or accessible through, our website does not constitute part of this prospectus.
44
2,200,000 Shares

Common Stock
PROSPECTUS
BofA Merrill Lynch
Leerink Partners
JMP Securities
Wedbush PacGrow Life Sciences
Oppenheimer & Co.
, 2014
Part II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table indicates the expenses to be incurred in connection with the offering described in this registration statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimated except the Securities and Exchange Commission registration fee and the Financial Industry Regulatory Authority, Inc. ("FINRA") filing fee.
| |
Amount | |||
|---|---|---|---|---|
Securities and Exchange Commission registration fee |
$ | 14,812 | ||
FINRA filing fee |
$ | 17,750 | ||
Accountants' fees and expenses |
150,000 | |||
Legal fees and expenses |
400,000 | |||
Transfer Agent's fees and expenses |
20,000 | |||
Printing and engraving expenses |
125,000 | |||
Miscellaneous |
22,438 | |||
| | | | | |
Total expenses |
750,000 | |||
| | | | | |
| | | | | |
Item 14. Indemnification of Directors and Officers.
Section 102 of the General Corporation Law of the State of Delaware permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificate of incorporation provides that no director of the Registrant shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the General Corporation Law of the State of Delaware prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the General Corporation Law of the State of Delaware provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys' fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he was or is or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
Our certificate of incorporation provides that we will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding whether civil, criminal, administrative or investigative (other than an action by or in the
II-1
right of us) by reason of the fact that he or she is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (including any employee benefit plan) (all such persons being referred to as an "Indemnitee"), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys' fees), liabilities, losses, judgments, fines (including excise taxes and penalties arising under the Employee Retirement Income Security Act of 1974), and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful. Our certificate of incorporation provides that we will indemnify any Indemnitee who was or is a party to any threatened, pending, or completed action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (including any employee benefit plan), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys' fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys' fees) actually and reasonably incurred in connection therewith. Expenses must be advanced to an Indemnitee under certain circumstances.
We have entered into indemnification agreements with each of our directors. These indemnification agreements may require us, among other things, to indemnify our directors for some expenses, including attorneys' fees, judgments, fines and settlement amounts incurred by a director in any action or proceeding arising out of his or her service as one of our directors, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request.
We maintain a liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
In any underwriting agreement we enter into in connection with the sale of common stock being registered hereby, the underwriters will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us within the meaning of the Securities Act of 1933, as amended (the "Securities Act"), against certain liabilities.
II-2
Item 15. Recent Sales of Unregistered Securities.
Set forth below is information regarding shares of capital stock issued by us within the past three years. Also included is the consideration received by us for such shares and information relating to the section of the Securities Act, or rule of the Securities and Exchange Commission, under which exemption from registration was claimed.
- (a)
- Issuances of Preferred Stock
- •
- In February 2012, we entered into a stock purchase agreement for the purpose of issuing and selling up to an aggregate of
1,538,461 shares of our series A-4 preferred stock, at a purchase price per share of $1.30, for an aggregate purchase price of $1,999,999. We issued these shares in August 2013.
- •
- In October 2011, we entered into a stock purchase agreement for the purpose of issuing and selling up to an aggregate of
(i) 7,000,000 shares of our series A-2 preferred stock and (ii) 3,000,000 shares of our series A-3 preferred stock. Between October 2011 and February 2013, we became
obligated to issue 6,100,000 shares of our series A-2 preferred stock at a purchase price per share of $1.15, for an aggregate purchase price of $7,015,000, and 1,764,706 shares of our
series A-3 preferred stock at a purchase price per share of $1.70, for an aggregate purchase price of $3,000,000. We issued these shares in August 2013.
- •
- In July 2013, we issued and sold an aggregate of 8,636,362 shares of our series B-1 preferred stock at a price per
share of $2.20 for an aggregate purchase price of $18,999,996.
- •
- Between April and September 2013, we issued and sold an aggregate of 24,100,000 shares of our series B preferred
stock at a price per share of $2.00 for an aggregate purchase price of $48,200,000.
- •
- Between October 2010 and January 2013, we issued and sold an aggregate of 20,937,500 shares of our series A preferred stock, at a purchase price per share of $1.00, for an aggregate purchase price of $20,937,500.
No underwriters were involved in the foregoing sales of securities. The securities described in this section (a) of Item 15 were issued to third parties pursuant to Regulation D promulgated under the Securities Act or pursuant to Regulation S promulgated under the Securities Act, in each case related to transactions by an issuer not involving any public offering. All purchasers of shares of preferred stock described above represented to us in connection with their purchase that they were accredited investors, and were acquiring the shares for their own account for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
- (b)
- Issuances of Common Stock
- •
- In July 2013, we issued an aggregate of 12,121 shares of our common stock to the holders of our special participation
stock, in connection with the election of the holders of a majority of such shares of special participation stock to convert all outstanding shares of special participation stock into common stock.
- •
- In December 2011, we issued an aggregate of 45,454 shares of our common stock to two of our directors, each of whom was also providing consulting services to us at that time, at a purchase price per share of $0.26, for an aggregate purchase price of $12,000.
II-3
- •
- In March 2011, we issued an aggregate of 162,684 shares of our common stock to a service provider in consideration for services provided to us under a master services agreement with such service provider.
The common stock described in this section (b) of Item 15 was issued pursuant to (i) with respect to the shares issued in July 2013, the exemption from the registration requirements of the Securities Act provided by Section 3(a)(9) of the Securities Act, (ii) with respect to the shares issued in December 2011, qualified written compensatory plans or arrangements with our employees, directors, officers, consultants and advisors, in reliance on the exemption from registration provided by Rule 701 promulgated under the Securities Act, or (iii) with respect to the shares issued in March 2011, in reliance on the exemption from registration provided by Section 4(2) under the Securities Act, relative to transactions by an issuer not involving any public offering. All recipients either received adequate information about us or had access, through employment or other relationships, to such information.
- (c)
- Stock Option Grants
Since January 1, 2011 and through March 31, 2014, we have issued to certain employees, directors and consultants options to purchase an aggregate of 2,908,677 shares of common stock, of which options issued between January 1, 2011 and March 31, 2014, as of March 31, 2014, options to purchase 460,880 shares of common stock had been exercised, options to purchase 113,836 shares had been forfeited and options to purchase 2,505,749 shares of common stock remained outstanding at a weighted-average exercise price of $9.21 per share.
The issuance of stock options and the common stock issuable upon the exercise of such options as described in this section (c) of Item 15 were issued pursuant to written compensatory plans or arrangements with our employees, directors and consultants, in reliance on the exemption from the registration requirements of the Securities Act provided by Rule 701 promulgated under the Securities Act, or pursuant to Section 4(2) under the Securities Act. All recipients either received adequate information about us or had access, through employment or other relationships, to such information.
Item 16. Exhibits and Financial Statement Schedules.
- (a)
- Exhibits.
The exhibits to this registration statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
The undersigned registrant hereby undertakes to provide to the underwriters, at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-4
The undersigned hereby undertakes that:
- (1)
- For
purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration
statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be
deemed to be part of this registration statement as of the time it was declared effective.
- (2)
- For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Pursuant to the requirements of the Securities Act, the registrant has duly caused this Amendment No. 1 to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the Town of Natick, Commonwealth of Massachusetts, on this 25th day of June, 2014.
| KARYOPHARM THERAPEUTICS INC. | ||||
By: |
/s/ MICHAEL G. KAUFFMAN Michael G. Kauffman, M.D., Ph.D. Chief Executive Officer |
|||
II-6
SIGNATURES AND POWER OF ATTORNEY
Pursuant to the requirements of the Securities Act of 1933, Amendment No. 1 to the Registration Statement has been signed by the following persons in the capacities held on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ MICHAEL G. KAUFFMAN Michael G. Kauffman, M.D., Ph.D. |
Chief Executive Officer and Director (principal executive officer) | June 25, 2014 | ||
/s/ PAUL BRANNELLY Paul Brannelly |
Senior Vice President, Finance and Administration, and Treasurer (principal financial and accounting officer) |
June 25, 2014 |
||
* Garen G. Bohlin |
Director |
June 25, 2014 |
||
* Barry E. Greene |
Director |
June 25, 2014 |
||
* Deepa R. Pakianathan, Ph.D. |
Director |
June 25, 2014 |
||
* Mansoor Raza Mirza, M.D. |
Director |
June 25, 2014 |
||
* Kenneth E. Weg |
Director |
June 25, 2014 |
*By: |
/s/ PAUL BRANNELLY |
|
II-7
| Exhibit Number |
Description of Exhibit | ||
|---|---|---|---|
| Underwriting Agreement | |||
| 1.1 | Underwriting Agreement | ||
| Articles of Incorporation and Bylaws | |||
| 3.1 | Restated Certificate of Incorporation of the Registrant(1) | ||
| 3.2 | Amended and Restated By-Laws of the Registrant(1) | ||
| Instruments Defining the Rights of Security Holders, Including Indentures | |||
| 4.1 | Specimen Stock Certificate evidencing the shares of common stock(2) | ||
| 4.2 | Third Amended and Restated Investors' Rights Agreement dated as of July 26, 2013(3) | ||
| Opinion re Legality | |||
| 5.1 | Opinion of Wilmer Cutler Pickering Hale and Dorr LLP | ||
| Material Contracts—Management Contracts and Compensatory Plans | |||
| 10.1 | 2010 Stock Incentive Plan(3) | ||
| 10.2 | Forms of Non-Qualified Stock Option Agreement under 2010 Stock Incentive Plan(3) | ||
| 10.3 | 2013 Stock Incentive Plan(2) | ||
| 10.4 | Form of Incentive Stock Option Agreement under 2013 Stock Incentive Plan(2) | ||
| 10.5 | Form of Nonstatutory Stock Option Agreement under 2013 Stock Incentive Plan(2) | ||
| 10.6 | 2013 Employee Stock Purchase Plan(2) | ||
| 10.7 | Employment Agreement dated December 6, 2010 between the Registrant and Michael Kauffman, M.D., Ph.D.(2) | ||
| 10.8 | Employment Agreement dated October 19, 2010, as amended on December 6, 2010, between the Registrant and Sharon Shacham, Ph.D., M.B.A.(2) | ||
| 10.9 | Employment Agreement dated May 29, 2013 between the Registrant and Paul Brannelly(2) | ||
| 10.10 | Consulting Agreement, dated as of October 28, 2010, between the Registrant and Alan T. Barber(3) | ||
| 10.11 | Consulting Agreement, dated as of September 1, 2012, between the Registrant and Mirza Consulting(3) | ||
| 10.12 | Form of Indemnification Agreement between the Registrant and each of its Directors(3) | ||
| 10.13 | Offer Letter from the Registrant to Christopher Primiano dated March 3, 2014(4) | ||
| Material Contracts—Leases | |||
| 10.14 | Lease, dated as of December 10, 2010, as amended on January 10, 2012 and July 29, 2013, between the Registrant and Nivek Investments I, LLC(3) | ||
II-8
| Exhibit Number |
Description of Exhibit | ||
|---|---|---|---|
| Material Contracts—Research Agreements | |||
| 10.15† | Research Agreement, dated as of July 18, 2011, between the Registrant and the Multiple Myeloma Research Foundation, Inc.(3) | ||
| 10.16 | Office Lease Agreement between NS Wells Acquisition LLC and Karyopharm Therapeutics Inc., dated March 27, 2014(5) | ||
| Additional Exhibits | |||
| 21.1 | Subsidiaries of the Registrant(4) | ||
| 23.1 | Consent of McGladrey LLP | ||
| 23.2 | Consent of Wilmer Cutler Pickering Hale and Dorr LLP (included in Exhibit 5.1) | ||
| 24.1 | * | Power of Attorney (included on signature page) | |
| 101.INS XBRL | ** | Instance Document(4) | |
| 101.SCH XBRL | ** | Schema Document(4) | |
| 101.CAL XBRL | ** | Calculation Linkbase Document(4) | |
| 101.LAB XBRL | ** | Labels Linkbase Document(4) | |
| 101.PRE XBRL | ** | Presentation Linkbase Document(4) | |
| 101.DEF XBRL | ** | Definition Linkbase Document(4) | |
- *
- Previously
Filed
- †
- Confidential
treatment has been granted as to portions of the exhibit.
- **
- Pursuant
to Rule 406T of Regulation S-T, these interactive data files are furnished and not filed or part of a registration statement or
prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933 or Section 18 of the Securities Exchange Act of 1934 and otherwise are not subject to liability under these
sections.
- (1)
- Incorporated
by reference to the Registrant's Current Report on Form 8-K (File No. 001-36167) filed with the Securities and Exchange
Commission on November 18, 2013.
- (2)
- Incorporated
by reference to the Registrant's Amendment No. 1 to Registration Statement on Form S-1 (File No. 333-191584) filed with
the Securities and Exchange Commission on October 28, 2013.
- (3)
- Incorporated
by reference to the Registrant's Registration Statement on Form S-1 (File No. 333-191584) filed with the Securities and Exchange
Commission on October 4, 2013.
- (4)
- Incorporated
by reference to the Registrant's Annual Report on Form 10-K (File No. 001-36167) filed with the Securities and Exchange
Commission on March 21, 2014.
- (5)
- Incorporated by reference to the Registrant's Current Report on Form 8-K (File No. 001-36167) filed with the Securities and Exchange Commission on April 1, 2014.
II-9
