Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Inspyr Therapeutics, Inc. | v382033_8k.htm |
EXHIBIT 99.01

Presentation at BIO International June 24, 2014 OTCQB : GNSZ

2 Safe Harbor Statement Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995 : Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera's anticipated growth strategies, the outcome of its clinical trials, future business development, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the Company's filings and future filings made with the United States Securities and Exchange Commission . Readers are advised that this information is intended for the use of investment professionals . Anyone interested in obtaining information on GenSpera should contact GenSpera directly . This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company's stock . This presentation is based upon information available to the public, as well as other information from sources which management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete . Opinions expressed herein are those of management as of the date of the presentation and are subject to change without notice .
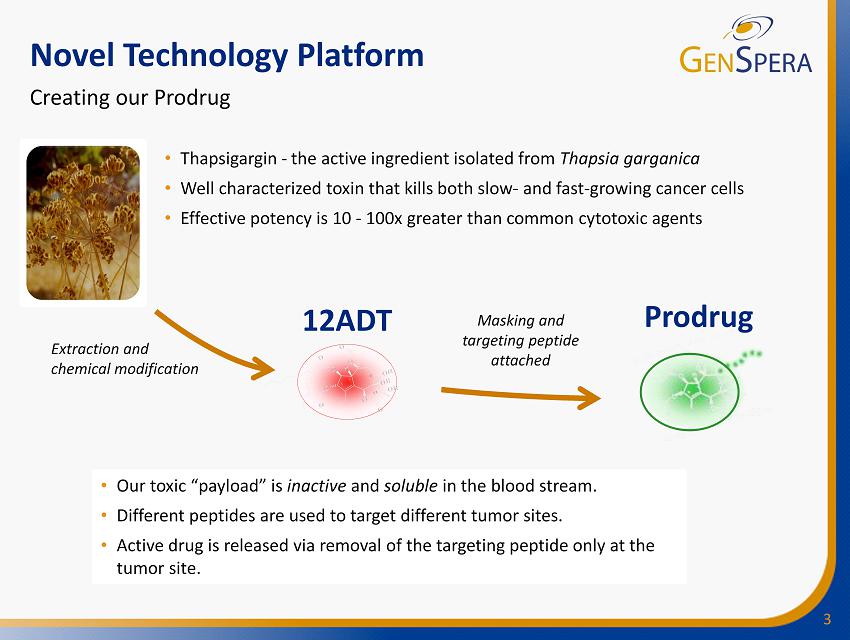
3 Novel Technology Platform 12ADT Prodrug • Thapsigargin - the active ingredient isolated from Thapsia garganica • Well characterized toxin that kills both slow - and fast - growing cancer cells • Effective potency is 10 - 100x greater than common cytotoxic agents Creating our Prodrug Extraction and chemical modification • Our toxic “payload” is inactive and soluble in the blood stream. • Different peptides are used to target different tumor sites. • Active drug is released via removal of the targeting peptide only at the tumor site. Masking and targeting peptide attached

4 RESEARCH PRE - CLINICAL PHASE 1 PHASE 2 Liver Cancer (HCC) GenSpera Clinical Programs Evaluating Multiple Oncology Indications TARGET SITE DRUG INDICATION Brain Cancer (GBM) Prostate cancer (PCa) Prostate cancer (PCa) Prostate cancer(PCa) Prostate cancer (PCa) G - 202 PSMA Blood vessels of most solid tumors Surface of all prostate cancer cells G - 114 PSA G - 301 hK2 G - 115 PSA DEVELOPMENT STAGE HUMAN CLINICAL TRIALS Over $20M deployed by GenSpera to advance its clinical programs Over $15M of grant funded research at Johns Hopkins PHASE 3 18 patients treated in Ph2 study at 5 sites 4 patients enrolled in Ph2 study at UCSD* Ph2 to begin Q2 2014 at UTHSC Houston* Efficacy in animal models Efficacy in animal models Pilot toxicology complete * Ph2 costs primarily supported by investigator funds Renal Cell Carcinoma (RCC) Ph2 to begin Q3 2014 at UTHSC Houston*

5 G - 202 Phase 2 Studies Targeting Large and Deadly Global Cancers • Most common liver cancer • 6th most prevalent type of cancer; 750,000 annual incidence worldwide • 3rd highest mortality • Most common type of primary brain malignancy; 10,000 new cases annually in US • Most frequently diagnosed cancer in men in the US; ~240,000 new cases each year Hepatocellular Carcinoma (HCC) Glioblastoma multiforme (GBM) Prostate Cancer (PCa) Incidence Market Potential Therapy snapshot Particularly high PSMA expression in tumor vasculature High PSMA expression in tumor • Only one approved drug (Nexavar®); provides ~2 months increase in median survival with significant side effects • Projected market over $1.5B by 2019 • Avastin® provides increase in median survival of ~ 4 months • Avastin® projected sales in GBM of $460M in 2017 • Viewed as stepping - stone to breast & lung cancer metastases to brain – 10 - fold larger market with little competition • Currently projected to be dominated by anti - hormonal treatments with significant side effects • Anticipated market of > $6.7 B by 2020
6 G - 202 Data in HCC Patients ( Ph I and Ph II) • All patients previously progressed on, or were intolerant of, Nexavar • Outstanding clinical safety profile • Well tolerated with easily managed and predictable side effects • No effect on bone marrow! • 80% of patients with stable disease (no tumor growth) at two months • 50 % of patients with stable disease for ≥ 4 months • Long - term disease stabilization (≥ 9 months) in 15% of patients • One patient remains on treatment > 22 months • End of bone pain in one patient with vertebral metastasis

7 Upcoming GenSpera Events • June 2014 – Open G - 202 Phase II clinical trial in prostate cancer • July 2014 – Present ongoing HCC trial data at APPLE 2014 Congress • 3Q 2014 – Initiate Phase II clinical trial in renal cell carcinoma • 1Q 2015 – Present interim data for ongoing glioblastoma trial

8 GenSpera Profile • GenSpera is a leader in developing prodrug therapeutics for the treatment of cancer • Unique, naturally derived, cancer killing drug has advantages in potency and significantly reduced side - effects • Experienced scientific and development team • Robust global IP position • G - 202, GenSpera’s lead candidate, is showing excellent clinical results • Highly differentiated from other anti - cancer approaches • Outstanding clinical safety profile • Data strongly suggestive of clinical activity in HCC • Will be evaluated in 4 different cancer indications by Q3 2014 • Multiple development opportunities based on our prodrug platform • Four drug candidates in the pipeline • Company is sufficiently financed through mid - 2015

9 Presentation at BIO International June 24, 2014 OTCQB : GNSZ
