Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a14-15551_18k.htm |
Exhibit 99.1
|
|
INNOVATIVE THERAPIES NOVEL PRODUCTS June 2014 ©2014 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
FORWARD LOOKING STATEMENT This presentation contains "forward-looking" statements that involve risks and uncertainties. These statements typically may be identified by the use of forward-looking words or phrases such as "may," "believe," "expect," "forecast," "intend," "anticipate," "predict," "should," "planned," "likely," "opportunity," "estimated," and "potential," the negative use of these words or other similar words. All forward-looking statements included in this presentation are based on our current expectations, and we assume no obligation to update any such forward-looking statements. The Private Securities Litigation Reform Act of 1995 provides a "safe harbor" for such forward-looking statements. In order to comply with the terms of the safe harbor, we note that a variety of factors could cause actual results and experiences to differ materially from the anticipated results or other expectations expressed in such forward-looking statements. The risks and uncertainties that may affect the operations, performance, development, and results of our business include but are not limited to: (1) our limited commercial experience with Qsymia® in the United States, or U.S.; (2) the timing of initiation and completion of the clinical studies required as part of the approval of Qsymia by the U.S. Food and Drug Administration, or FDA; (3) the response from the FDA to the data that we will submit relating to post-approval clinical studies; (4) the impact of the indicated uses and contraindications contained in the Qsymia label and the Risk Evaluation and Mitigation Strategy requirements; (5) our ability to continue to certify and add to the Qsymia retail pharmacy network and sell Qsymia through this network; (6) whether the Qsymia retail pharmacy network will simplify and reduce the prescribing burden for physicians, improve access and reduce waiting times for patients seeking to initiate therapy with Qsymia; (7) that we may be required to provide further analysis of previously submitted clinical trial data; (8) our assessment of the European Medicines Agency's Scientific Advice relating to our cardiovascular outcomes trial, or CVOT, and the resubmission of an application for the grant of a marketing authorization to the European Medicines Agency, or EMA, the timing of such resubmission, if any, the results of the CVOT, assessment by the EMA of the application for marketing authorization, and their agreement with the data from the CVOT; (9) our ability to successfully seek approval for Qsymia in other territories outside the U.S. and European Union, or EU; (10) whether healthcare providers, payors and public policy makers will recognize the significance of the American Medical Association officially recognizing obesity as a disease, or the new American Association of Clinical Endocrinologists guidelines; (11) our ability to successfully commercialize Qsymia including risks and uncertainties related to expansion to retail distribution, the broadening of payor reimbursement, the expansion of Qsymia's primary care presence, and the outcomes of our discussions with pharmaceutical companies and our strategic and franchise-specific pathways for Qsymia; (12) our ability to focus our promotional efforts on health-care providers and on patient education that, along with increased access to Qsymia and ongoing improvements in reimbursement, will result in the accelerated adoption of Qsymia; (13) our ability to eliminate expenses that are not essential to expanding the use of Qsymia and fully realize the anticipated benefits from a cost reduction plan, including the timing thereof; (14) the impact of lower annual net cost savings than currently expected; (15) the impact of the cost reduction plan on our business and unanticipated charges not currently contemplated that may occur as a result of the cost reduction plan; (16) our ability to ensure that the entire supply chain for Qsymia efficiently and consistently delivers Qsymia to our customers; (17) risks and uncertainties related to the timing, strategy, tactics and success of the launches and commercialization of STENDRA™ (avanafil) or SPEDRA™ (avanafil) by our sublicensees in the United States, Canada, the EU, Australia, New Zealand, Africa, the Middle East, Turkey, and the Commonwealth of Independent States, including Russia; (18) our ability to successfully complete on acceptable terms, and on a timely basis, avanafil partnering discussions for other territories under our license with Mitsubishi Tanabe Pharma Corporation in which we do not have a commercial collaboration; (19) the timing of the qualification and subsequent approval by regulatory authorities of Sanofi Chimie and Sanofi Winthrop Industrie as a qualified supplier of STENDRA/SPEDRA, Sanofi Chimie's ability to undertake worldwide manufacturing of the avanafil active pharmaceutical ingredient and Sanofi Winthrop Industrie's ability to undertake worldwide manufacturing of the tablets for avanafil; (20) whether the FDA will approve the amendment for the new prescribing information we have submitted, and/or the European Commission, following an opinion by the EMA, will approve the new prescribing information we intend to submit, to include the recently announced clinical study results showing avanafil is effective for sexual activity within 15 minutes in men with erectile dysfunction; (21) the ability of our partners to maintain regulatory approvals to manufacture and adequately supply our products to meet demand; (22) our ability to accurately forecast Qsymia demand; (23) our ability to increase Qsymia sales in 2014 through growth in certified retail pharmacies, expansion of reimbursement coverage and the use of a more focused selling message; (24) the number of Qsymia prescriptions dispensed through the mail order system and through certified retail pharmacies; (25) the impact of promotional programs for Qsymia on our net product revenue and net income (loss) in future periods; (26) our history of losses and variable quarterly results; (27) substantial competition; (28) risks related to the failure to protect our intellectual property and litigation in which we may become involved; (29) uncertainties of government or third-party payor reimbursement; (30) our reliance on sole-source suppliers; (31) our reliance on third parties and our collaborative partners; (32) our failure to continue to develop innovative investigational drug candidates and drugs; (33) risks related to the failure to obtain FDA or foreign authority clearances or approvals and noncompliance with FDA or foreign authority regulations; (34) our ability to demonstrate through clinical testing the quality, safety, and efficacy of our investigational drug candidates; (35) the timing of initiation and completion of clinical trials and submissions to foreign authorities; (36) the results of post-marketing studies are not favorable; (37) compliance with post-marketing regulatory standards, post-marketing obligations or pharmacovigilance rules is not maintained; (38) the volatility and liquidity of the financial markets; (39) our liquidity and capital resources; (40) our expected future revenues, operations and expenditures; (41) potential change in our business strategy to enhance long-term stockholder value; (42) the impact, if any, of the expansion of our Board of Directors to include predominantly new members, the recent appointment of a new Chief Executive Officer and an interim Chief Financial Officer, the resignation of our President, the decision of our Chief Financial Officer to exercise his right to terminate his employment for Good Reason (as defined in his Amended and Restated Change of Control and Severance Agreement with the Company, effective as of July 1, 2013) and the assumption of the Chief Commercial Officer's duties and responsibilities by the Chief Executive Officer; and (43) other factors that are described from time to time in our periodic filings with the U.S. Securities and Exchange Commission, or the SEC, or the Commission, including our Annual Report on Form 10-K for the year ended December 31, 2013, as amended by the Form 10-K/A filed on April 30, 2014. ©2014 VIVUS Inc. All rights reserved | www.vivus.com 1 |
|
|
VIVUS PRIORITIES - 2014 Drive Qsymia Demand Increase Third Party Coverage for Qsymia Enhance Direct-to-Patient Programs Establish a Qsymia Commercial Alliance Maintain Positive Momentum for STENDRA/SPEDRA Alliances ©2014 VIVUS Inc. All rights reserved | www.vivus.com 2 |
|
|
Obesity Market & Qsymia 3 ©2014 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
HTN: 20.0 M HTN2/ Dyslipidemia: 11.4 M HTN/Diabetes/ Dyslipidemia: 21.9 M Diabetes/ Dyslipidemia: 5.0 M Dyslipidemia: 35.0 M HTN/ Diabetes: 2.8 M Diabetes: 1.4 M U.S. Obese and Overweight w/co-morbidity population1 Over 110 million US adults are obese or overweight with a weight-related comorbidity Approximately 40% are currently on drug therapy for at least one comorbidity3, while only ~2% are on drug therapy for obesity SOURCE: NHANES (2010); VIVUS, Inc. 1 Chart simplified for representation purposes; does not include overweight without comorbidity population; 2 HTN = hypertension; 3 Qsymia patient research OVER 110 MILLION US ADULTS ARE OBESE OR OVERWEIGHT WITH A COMORBIDITY 4 Obese without comorbidities: 13.2 M |
|
|
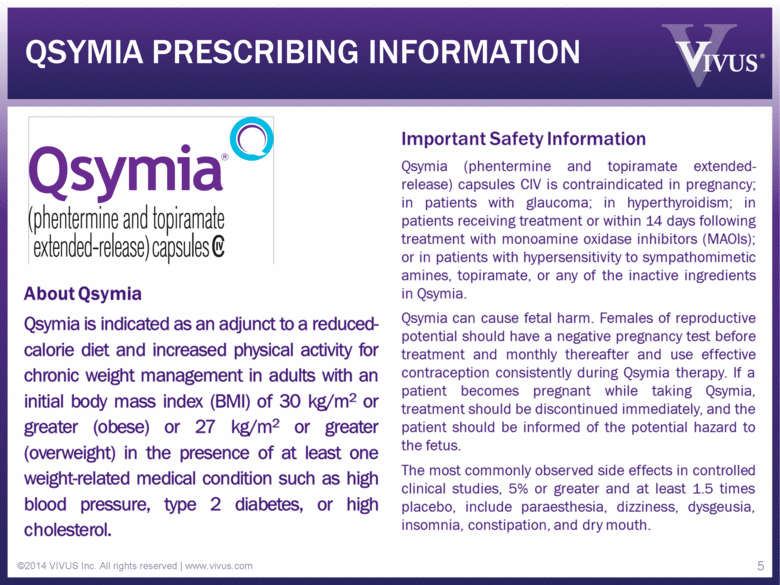
QSYMIA PRESCRIBING INFORMATION ©2014 VIVUS Inc. All rights reserved | www.vivus.com 5 |
|
|
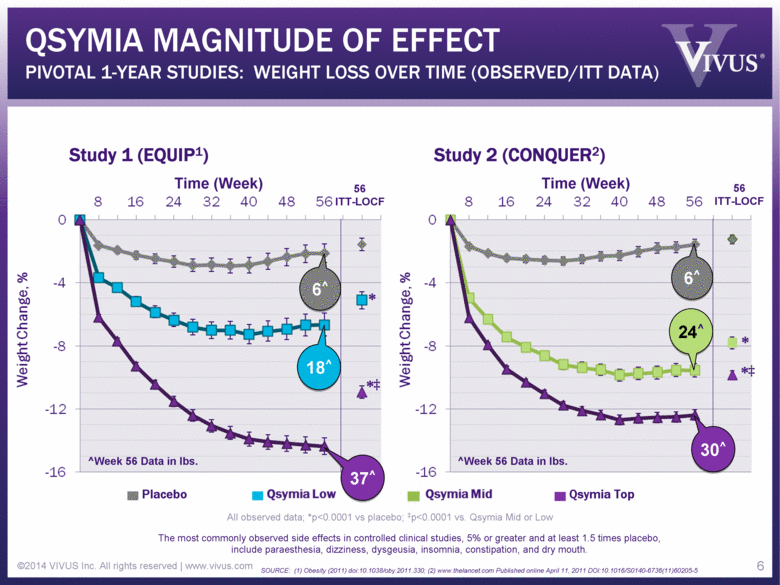
QSYMIA MAGNITUDE OF EFFECT PIVOTAL 1-YEAR STUDIES: WEIGHT LOSS OVER TIME (OBSERVED/ITT DATA) ©2014 VIVUS Inc. All rights reserved | www.vivus.com 6 Study 1 (EQUIP1) Study 2 (CONQUER2) Time (Week) Time (Week) 56 ITT-LOCF 56 ITT-LOCF All observed data; *p<0.0001 vs placebo; ‡p<0.0001 vs. Qsymia Mid or Low The most commonly observed side effects in controlled clinical studies, 5% or greater and at least 1.5 times placebo, include paraesthesia, dizziness, dysgeusia, insomnia, constipation, and dry mouth. SOURCE: (1) Obesity (2011) doi:10.1038/oby.2011.330; (2) www.thelancet.com Published online April 11, 2011 DOI:10.1016/S0140-6736(11)60205-5 Placebo Qsymia Top * *‡ * *‡ 6 6^ 24^ 30^ 37^ 18^ 6^ ^Week 56 Data in lbs. ^Week 56 Data in lbs. |
|
|
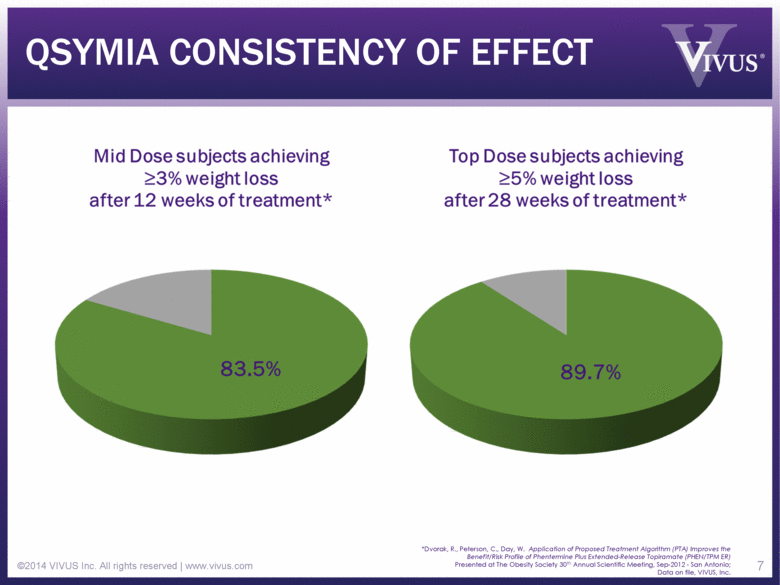
QSYMIA CONSISTENCY OF EFFECT ©2014 VIVUS Inc. All rights reserved | www.vivus.com 7 *Dvorak, R., Peterson, C., Day, W. Application of Proposed Treatment Algorithm (PTA) Improves the Benefit/Risk Profile of Phentermine Plus Extended-Release Topiramate (PHEN/TPM ER) Presented at The Obesity Society 30th Annual Scientific Meeting, Sep-2012 - San Antonio; Data on file, VIVUS, Inc. |
|
|
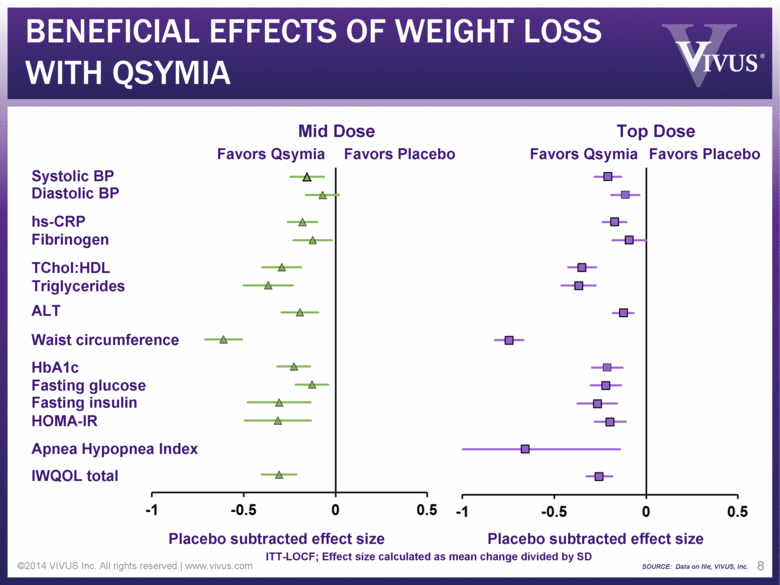
BENEFICIAL EFFECTS OF WEIGHT LOSS WITH QSYMIA ©2014 VIVUS Inc. All rights reserved | www.vivus.com 8 Diastolic BP Systolic BP Fibrinogen Triglycerides TChol:HDL IWQOL total Fasting glucose HOMA-IR Fasting insulin HbA1c hs-CRP ALT Apnea Hypopnea Index Placebo subtracted effect size Placebo subtracted effect size Favors Qsymia Favors Placebo Favors Qsymia Favors Placebo Waist circumference ITT-LOCF; Effect size calculated as mean change divided by SD SOURCE: Data on file, VIVUS, Inc. Mid Dose Top Dose |
|
|
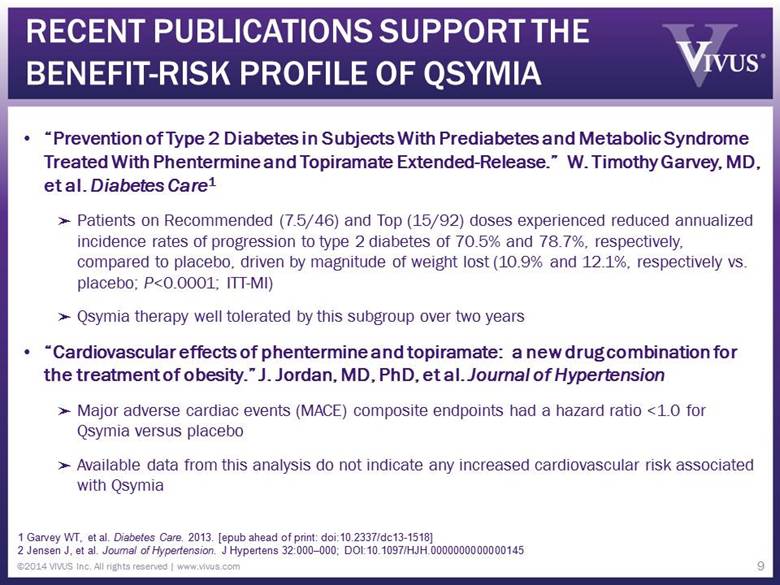
RECENT PUBLICATIONS SUPPORT THE BENEFIT-RISK PROFILE OF QSYMIA “Prevention of Type 2 Diabetes in Subjects With Prediabetes and Metabolic Syndrome Treated With Phentermine and Topiramate Extended-Release.” W. Timothy Garvey, MD, et al. Diabetes Care1 Patients on Recommended (7.5/46) and Top (15/92) doses experienced reduced annualized incidence rates of progression to type 2 diabetes of 70.5% and 78.7%, respectively, compared to placebo, driven by magnitude of weight lost (10.9% and 12.1%, respectively vs. placebo; P<0.0001; ITT-MI) Qsymia therapy well tolerated by this subgroup over two years “Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity.” J. Jordan, MD, PhD, et al. Journal of Hypertension Major adverse cardiac events (MACE) composite endpoints had a hazard ratio <1.0 for Qsymia versus placebo Available data from this analysis do not indicate any increased cardiovascular risk associated with Qsymia 9 1 Garvey WT, et al. Diabetes Care. 2013. [epub ahead of print: doi:10.2337/dc13-1518] 2 Jensen J, et al. Journal of Hypertension. J Hypertens 32:000–000; DOI:10.1097/HJH.0000000000000145 ©2014 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
Obesity Treatment Evolution 10 ©2014 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
AACE TREATMENT ALGORITHM: MEDICAL THERAPY IS RECOMMENDED 11 Obesity management and FDA-approved anti-obesity medications now recommended for: Prediabetes Diabetes Dyslipidemia Hypertension 1st ever inclusion of drug therapy in treatment algorithm Primary Care Physicians refer to AACE algorithm ©2014 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
GREATER RECOGNITION OF OBESITY AS A DISEASE, PHARMACOTHERAPY AS A TREATMENT OPTION Obesity is now recognized as a disease by the leading medical association in the U.S. AMA recognition is important support for policy makers, healthcare providers and payors Additional recent publications support long-term use of drug treatment “Medications approved for long-term obesity treatment, when used as an adjunct to lifestyle intervention, lead to greater mean weight loss and an increased likelihood of achieving clinically meaningful 1-year weight loss relative to placebo. By discontinuing medication in patients who do not respond with weight loss of at least 5%, clinicians can decrease their patients’ exposure to the risks and costs of drug treatment when there is little prospect of long-term benefit.” JAMA. 2014;311(1):74-86. doi:10.1001/jama.2013.281361. ©2014 VIVUS Inc. All rights reserved | www.vivus.com 12 Clinical practice guidelines, developed in conjunction with National Heart, Lung and Blood Institute, by American Heart Association American College of Cardiology The Obesity Society Guidelines urge HCPs to take an active role in helping patients achieve & maintain healthy body weight Include obesity medication as adjunct to lifestyle intervention to help appropriate patients (BMI>30 or BMI>27 with at least one obesity-associated co-morbid condition) achieve weight loss goal Jensen MD, Ryan DH, Apovian CM, Loria CM, Ard JD, Millen BE, Comuzzie AG, Nonas CA, Donato KA, Pi-Sunyer FX, Hu FB, Stevens J, Hubbard VS, Stevens VJ, Jakicic JM, Wadden TA, Kushner RF, Wolfe BM, Yanovski SZ, 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults, Journal of the American College of Cardiology (2013), doi: 10.1016/j.jacc.2013.11.004. |
|
|
Qsymia Commercialization 13 ©2014 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
HEALTHCARE PROVIDER TARGETING Re-prioritized targets based on prescribing behavior and other factors Analyzed and redefined territories Reconfigured and redeployed sales resources against highest value targets ©2014 VIVUS Inc. All rights reserved | www.vivus.com 14 |
|
|
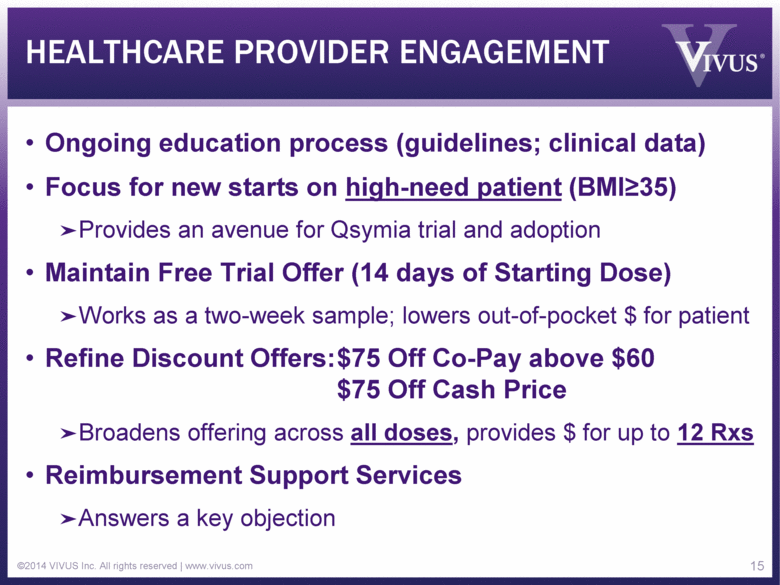
HEALTHCARE PROVIDER ENGAGEMENT Ongoing education process (guidelines; clinical data) Focus for new starts on high-need patient (BMI>35) Provides an avenue for Qsymia trial and adoption Maintain Free Trial Offer (14 days of Starting Dose) Works as a two-week sample; lowers out-of-pocket $ for patient Refine Discount Offers: $75 Off Co-Pay above $60 $75 Off Cash Price Broadens offering across all doses, provides $ for up to 12 Rxs Reimbursement Support Services Answers a key objection ©2014 VIVUS Inc. All rights reserved | www.vivus.com 15 |
|
|
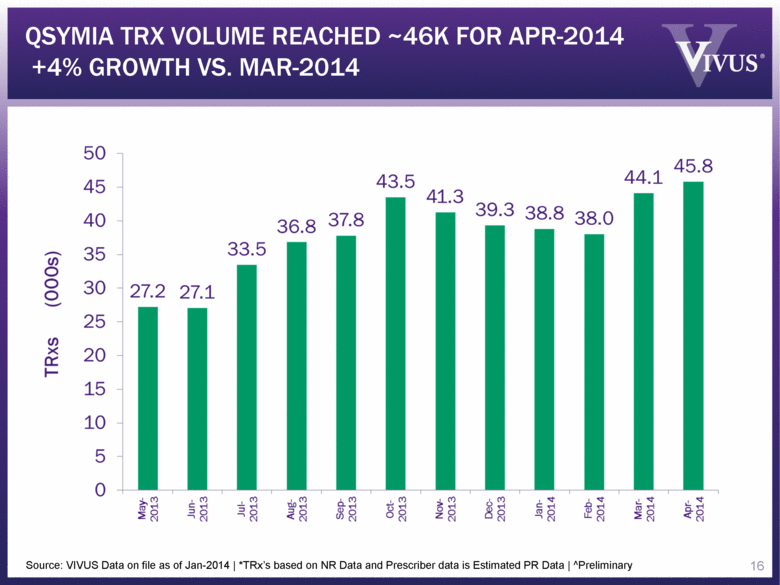
Source: VIVUS Data on file as of Jan-2014 | *TRx’s based on NR Data and Prescriber data is Estimated PR Data | ^Preliminary QSYMIA TRX VOLUME REACHED ~46K FOR APR-2014 +4% GROWTH VS. MAR-2014 16 |
|
|
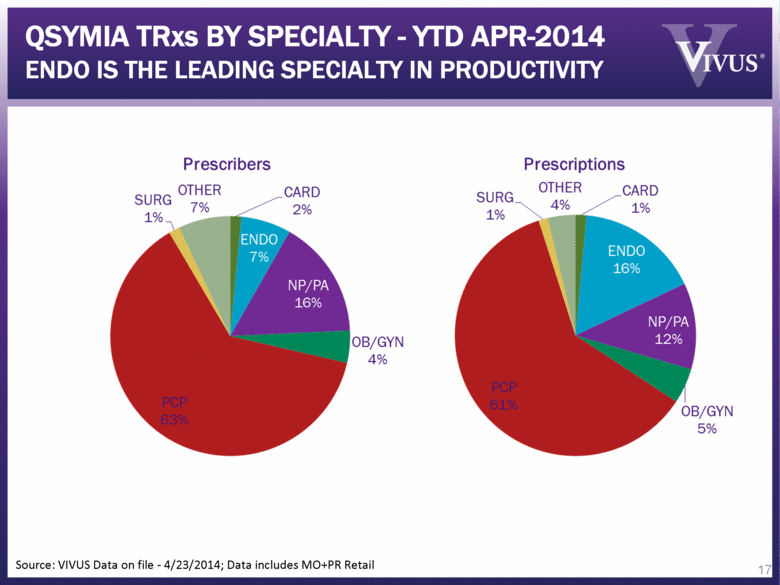
Source: VIVUS Data on file - 4/23/2014; Data includes MO+PR Retail 17 QSYMIA TRxs BY SPECIALTY - YTD APR-2014 ENDO IS THE LEADING SPECIALTY IN PRODUCTIVITY |
|
|
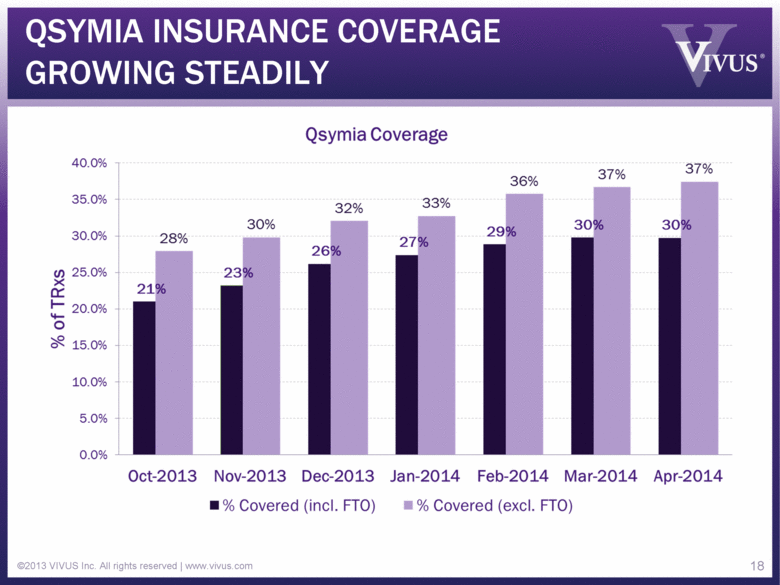
QSYMIA INSURANCE COVERAGE GROWING STEADILY ©2013 VIVUS Inc. All rights reserved | www.vivus.com 18 |
|
|
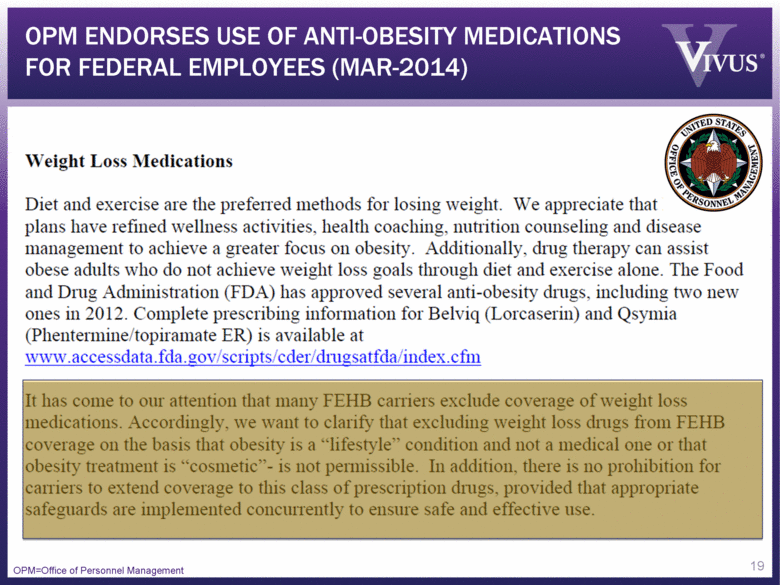
OPM ENDORSES USE OF ANTI-OBESITY MEDICATIONS FOR FEDERAL EMPLOYEES (MAR-2014) OPM=Office of Personnel Management 19 |
|
|
SHARI BELAFONTE, OBESITY SPOKESPERSON Inspired to Lose starring Shari Belafonte VIVUS-sponsored program to encourage patient-physician dialog about weight Satellite Media Tour 45.9 million impressions* to date *aired/published and pending stories ~225 TV broadcasts www.inspiredlosing.com ©2013 VIVUS Inc. All rights reserved | www.vivus.com 20 |
|
|
Qsiva | CVOT | Qsymia IP 21 ©2014 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
QSYMIA EUROPEAN STRATEGY Goal Satisfy FDA post-approval requirements with a single CVOT (AQCLAIM/OB-401) that may also support EU registration Strategy Create interim analysis CVOT so that ~two years of data may be used to support an application while trial continues Tactics Dialog with Scientific Advice Working Party (SAWP) - COMPLETED Revised OB-401 protocol – UNDER REVIEW AT FDA 10 years of exclusivity via CHMP central process ©2014 VIVUS Inc. All rights reserved | www.vivus.com 22 |
|
|
AQCLAIM STUDY (CVOT) A Qsymia® CardiovascuLAr morbIdity and Mortality (AQCLAIM) Study in Subjects with Documented Cardiovascular Disease Primary Efficacy Endpoint: Time to 1st occurrence of nonfatal MI, nonfatal stroke or CV death Number of Subjects: ~11,000 –16,000 Number of Sites: ~450 (North America, Europe, RoW) Analyzed and incorporated feedback from CHMP & FDA Budget: $180MM - $220MM ©2014 VIVUS Inc. All rights reserved | www.vivus.com 23 |
|
|
NEW PATENTS EXTEND QSYMIA COVERAGE TO 2029 Two additional VIVUS patents with claims covering QSYMIA issued 4Q2013, extending coverage by nine years U.S. Patent No. 8,580,298 26 claims directed to unit dosage forms or packaged pharmaceutical preparations U.S. Patent No. 8,580,299 Nine method-of-use claims for effecting weight loss over a two-year period Continuation applications on file VIVUS now has six patents covering Qsymia listed in the Orange Book; coverage extends to 2029 Pending in EP, AU, BR, CA, MX, IN, IL, CN, CL, JP, TR, KR, ZA ©2014 VIVUS Inc. All rights reserved | www.vivus.com 24 |
|
|
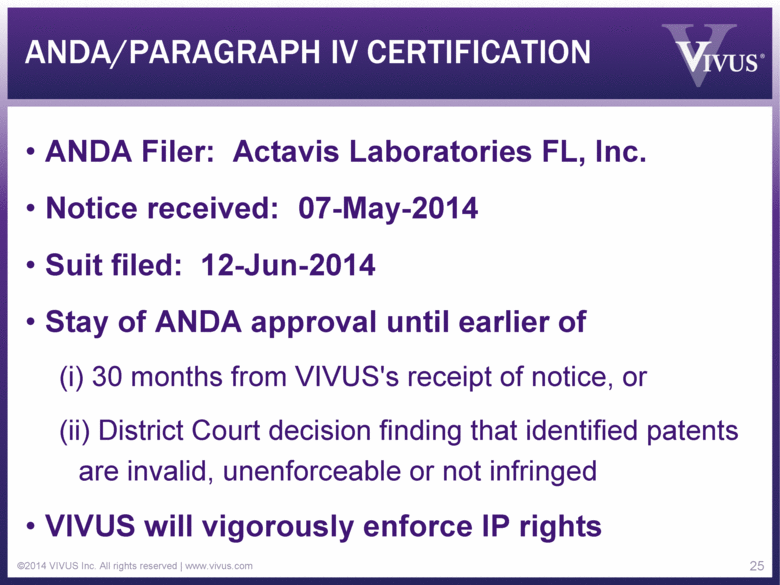
ANDA/PARAGRAPH IV CERTIFICATION ANDA Filer: Actavis Laboratories FL, Inc. Notice received: 07-May-2014 Suit filed: 12-Jun-2014 Stay of ANDA approval until earlier of (i) 30 months from VIVUS's receipt of notice, or (ii) District Court decision finding that identified patents are invalid, unenforceable or not infringed VIVUS will vigorously enforce IP rights ©2014 VIVUS Inc. All rights reserved | www.vivus.com 25 |
|
|
STENDRA PRESCRIBING INFORMATION About STENDRA™ STENDRA is a prescription medicine used to treat erectile dysfunction (ED). STENDRA (avanafil) is licensed from Mitsubishi Tanabe Pharma Corporation. VIVUS has development and commercial rights to STENDRA for the treatment of sexual dysfunction worldwide with the exception of certain Asian Pacific Rim countries. Important Safety Information Do not take STENDRA if you take nitrates, often prescribed for chest pain, as this may cause a sudden, unsafe drop in blood pressure. Tell your healthcare provider about all the medicines you take and discuss your general health status to ensure that you are healthy enough to engage in sexual activity. If you experience chest pain, nausea, or any other discomforts during sex, seek immediate medical help. In the rare event of an erection lasting more than 4 hours, seek immediate medical help to avoid long-term injury. STENDRA in combination with other treatments for erectile dysfunction is not recommended. STENDRA does not protect against sexually transmitted diseases, including HIV. The most common side effects of STENDRA are headache, flushing, runny nose and congestion. ©2014 VIVUS Inc. All rights reserved | www.vivus.com 26 |
|
|
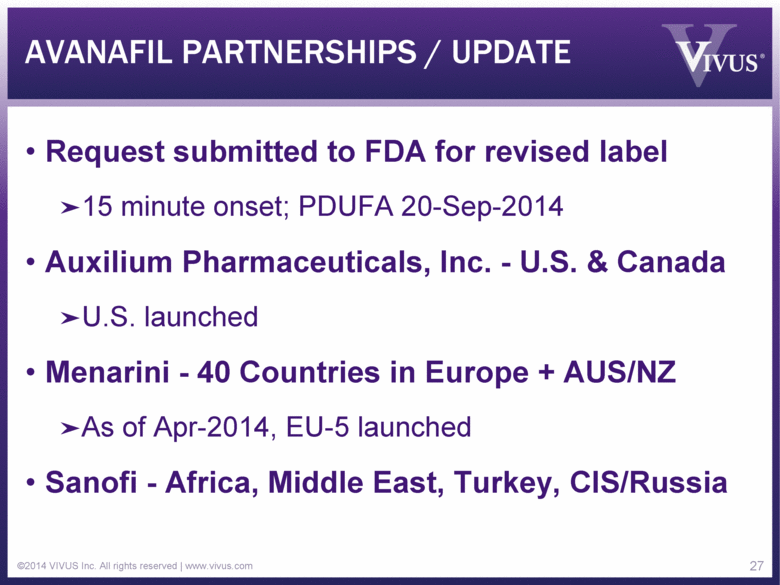
AVANAFIL PARTNERSHIPS / UPDATE Request submitted to FDA for revised label 15 minute onset; PDUFA 20-Sep-2014 Auxilium Pharmaceuticals, Inc. - U.S. & Canada U.S. launched Menarini - 40 Countries in Europe + AUS/NZ As of Apr-2014, EU-5 launched Sanofi - Africa, Middle East, Turkey, CIS/Russia ©2014 VIVUS Inc. All rights reserved | www.vivus.com 27 |
|
|
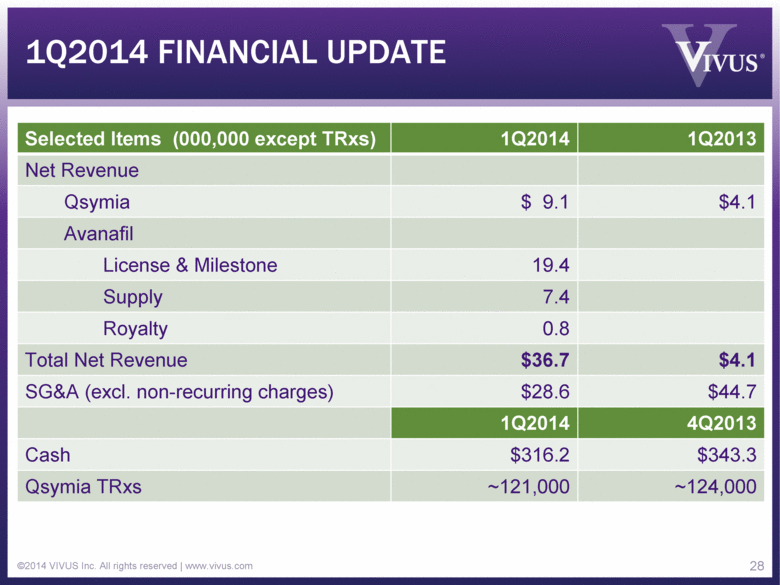
1Q2014 FINANCIAL UPDATE ©2014 VIVUS Inc. All rights reserved | www.vivus.com 28 Selected Items (000,000 except TRxs) 1Q2014 1Q2013 Net Revenue Qsymia $ 9.1 $4.1 Avanafil License & Milestone 19.4 Supply 7.4 Royalty 0.8 Total Net Revenue $36.7 $4.1 SG&A (excl. non-recurring charges) $28.6 $44.7 1Q2014 4Q2013 Cash $316.2 $343.3 Qsymia TRxs ~121,000 ~124,000 |
|
|
VIVUS PRIORITIES - 2014 Drive Qsymia Demand Increase Third Party Coverage for Qsymia Enhance Direct-to-Patient Programs Establish a Qsymia Commercial Alliance Maintain Positive Momentum for STENDRA/SPEDRA Alliances ©2014 VIVUS Inc. All rights reserved | www.vivus.com 29 |
|
|
INNOVATIVE THERAPIES NOVEL PRODUCTS June 2014 ©2014 VIVUS Inc. All rights reserved | www.vivus.com |