Attached files
| file | filename |
|---|---|
| 8-K - 8-K - EAGLE PHARMACEUTICALS, INC. | a14-14843_18k.htm |
Exhibit 99.1
|
|
Jeffries 2014 Global Healthcare Conference - June 5, 2014 Scott Tarriff, CEO David Riggs, CFO NASDAQ: EGRX |
|
|
Forward Looking Statements 2 This presentation contains certain forward-looking information about Eagle Pharmaceuticals that is intended to be covered by the safe harbor for "forward-looking statements" provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. Words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “strong,” “up coming,” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding our ability to successfully develop and commercialize our therapeutic products; our ability to expand our long-term business opportunities; financial projections and estimates and their underlying assumptions; and future performance. All such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the Company’s control, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include, but are not limited to: whether any of our product candidates will advance further in the clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether our product candidates will be successfully marketed if approved; whether our discovery and development efforts relating to improved formulations and delivery methods for existing FDA-approved products will be successful and/or our ability to select and acquire or in-license product candidates; our ability to achieve the results contemplated by our collaboration arrangements; the strength and enforceability of our intellectual property rights; competition from biotechnology and pharmaceutical companies; our ability to raise additional capital to fund our operations on terms acceptable to us; general economic conditions; and the other risk factors included in our final prospectus filed with the Securities and Exchange Commission on February 13, 2014 relating to our Registration Statement on Form S-1 (File No. 333-192984) for our IPO. Our audience is cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. |
|
|
Eagle Pharmaceuticals Nasdaq: EGRX Specialty pharmaceutical company Differentiated forms of branded injectables Validated strategy Two PDUFA dates in July 2014 7/6 Bendamustine RTD Near-term high-margin 7/22 Ryanodex commercial opportunities Long life cycles = significant value Strong IP around differentiating features Orphan drug designations Well capitalized to execute Over $50M in cash |
|
|
Validated Business Model In-market product: Argatroban Improved formulation of branded drug Ready-to-use; no dilution Significant reduction in drug waste Entered market three years before first generic can enter Undifferentiated ANDAs must await patent expiry (6/30/14) Our partners net sales & margins over last 12 months 1 Source: IMS Net Sales $ 24.6M COGS 4.9 Gross Profit $ 19.7M 79% margin |
|
|
Late Stage Portfolio Targeting Proven Markets Bendamustine RTD Bendamustine RTD Low-volume, shorter infusion time Ryanodex (dantrolene): Malignant Hyperthermia Ryanodex: Exertional Heat Stroke RTU bivalirudin Pemetrexed Argatroban 7/6/14 PDUFA date 7/22/14 PDUFA date 1 Source: publicly filed reports with the SEC, independent market research and management’s estimates Preclinical Clinical NDA filing Aproval In-market Market Opp1 Orphan Drug $99M $709M+ $709M+ $20M $150M $550M $1.21B |
|
|
Bendamustine RTD Improved formulation of Treanda1 for CLL2 & NHL3 Ready-to-dilute concentrated liquid of cytotoxic API Same volume diluent, same infusion time 7/6/14 PDUFA date – expecting tentative approval Commercial launch early 2015 through early 2016 Patent litigation, Orphan Drug, FDA Approval Potential blockbuster based on improved product attributes Longevity post-launch: 1 issued patent & 4 filed Expiry expected 2031-33 Patent litigation – ahead of ANDA litigation 1 Treanda® (bendamustine HCl) (Cephalon, Inc., a wholly-owned subsidiary of Teva Pharmaceutical Industries Ltd.) 2 CLL: chronic lymphocytic leukemia 3 NHL: indolent B-cell non-Hodgkin lymphoma |
|
|
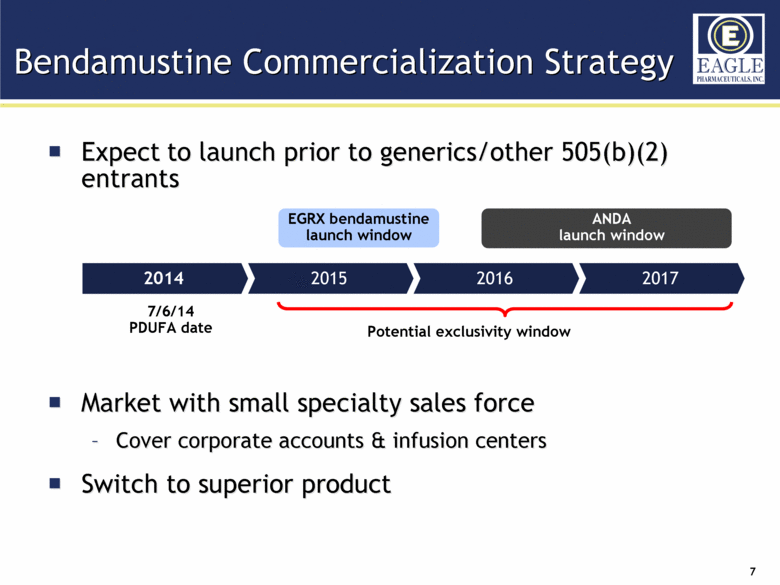
Expect to launch prior to generics/other 505(b)(2) entrants Market with small specialty sales force Cover corporate accounts & infusion centers Switch to superior product Bendamustine Commercialization Strategy 2014 2017 2015 2016 EGRX bendamustine launch window Potential exclusivity window ANDA launch window 7/6/14 PDUFA date |
|
|
Eagle Shorter Infusion Time Highlights Patient Benefit Less chair time 30 or 60 minutes reduced to 10 minutes Less volume and the issues related to 500ml vs. 50ml Nurse Less nursing time required Medical/Patient 90% reduction in NaCl – renal suppressed population Economic Benefit Additional patient treated in the cancer clinic due to shorter infusion time Anticipated to sell slightly below innovator |
|
|
Lower-volume Shorter Infusion Time Bendamustine RTD Same product as bendamustine RTD Potential to convert market based on improved product attributes Orphan drug designation, if granted, may afford 7 years exclusivity on approval One issued patent to June 2031 |
|
|
Lower-volume Shorter Infusion Time Bendamustine RTD Randomized, blinded clinical trial Last patient expected to receive first dose shortly Plan to submit NDA to FDA by YE 2014 – requesting priority review |
|
|
No change to SOC for MH treatment in over 30 years Often fatal if untreated Current product requires 720 ml (12 vials) Eagle optimized formulation reduces to 5ml (1 vial) Breakthrough change Expected to take over market 3 patents issued + 1 filed Orphan drug designation for MH PDUFA date 7/22/14 (priority review) Potential launch summer 2014 EU regulatory submission to be filed by mid-2015 Ryanodex (dantrolene) for Malignant Hyperthermia (MH) 1 Retrospective study conducted by the Malignant Hyperthermia Association of the United States (MHAUS) |
|
|
Ryanodex US Commercial Landscape Branded dantrolene sales for MH ~$20M in 2013 Ryanodex advantages support premium pricing Faster administration Reduced volume Significant reduction of Mannitol (diuretic) All US hospitals required to stock dantrolene MHAUS1 recommends a minimum of 36 vials Market unlikely to revert to old 720ml formulation Eagle to commercialize (without partnering) Requires only small contract sales force to launch & call on stakeholders 1 Joint Commission 2 MHAUS: Malignant Hyperthermia Association of the United States |
|
|
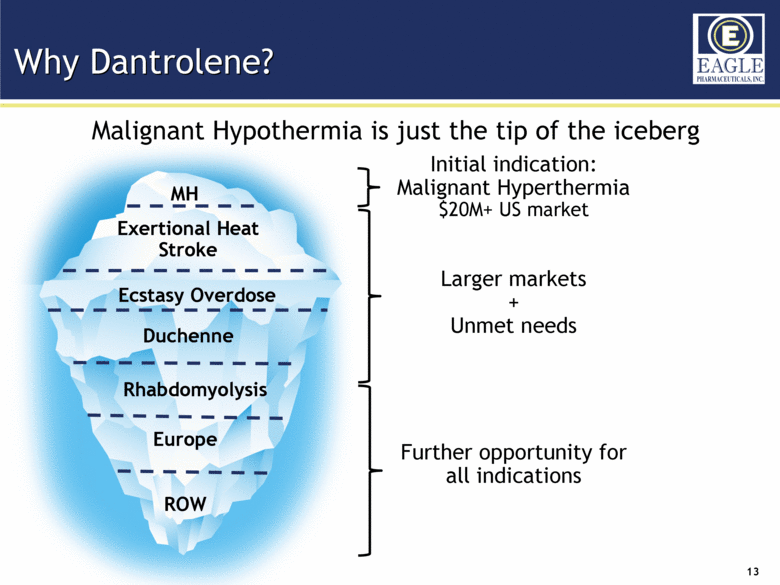
Why Dantrolene? MH Exertional Heat Stroke Ecstasy Overdose Duchenne Europe ROW Initial indication: Malignant Hyperthermia $20M+ US market Larger markets + Unmet needs Further opportunity for all indications Malignant Hypothermia is just the tip of the iceberg Rhabdomyolysis |
|
|
Ryanodex Label Expansion Opportunity Exertional Heat Stroke (EHS) Est. 23,000-38,000 cases/year1 A leading cause of student athlete death (US) & non-combat military deaths Similarities to MH Potential for Ryanodex to be first to market for EHS Orphan drug designation for EHS Potential for 7-10 year life cycle w/o competition Market estimated at $150M Working with the U.S. military Pilot study planned to start 2H 2014 in Saudi Arabia 1 Estimate based on 2010 population projections |
|
|
Bivalirudin: $550M – Approximate Brand Sales Enhanced RTU version of the anticoagulant Angiomax Completed three registration batches for NDA submission 2 patents issued; third patent filed 1Q:14 NDA submission targeted for Q2:15 US launch planned as early as Q1:16 |
|
|
Pemetrexed: $1.21B Opportunity Liquid version of Alimta, a powder cytotoxic API No reconstitution & dilution Less exposure to cytotoxic vapors for healthcare staff Expect to launch with generics (2017-21) |
|
|
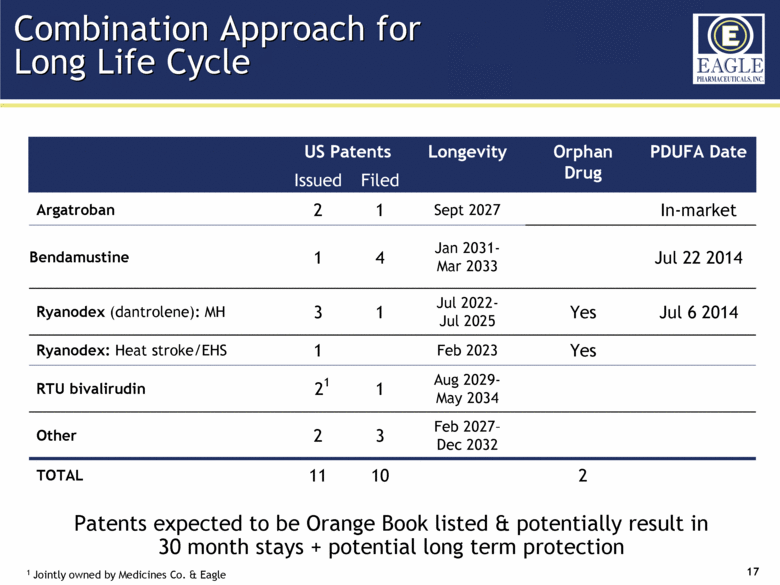
US Patents Longevity Orphan Drug PDUFA Date Issued Filed Argatroban 2 1 Sept 2027 In-market Bendamustine 1 4 Jan 2031- Mar 2033 Jul 22 2014 Ryanodex (dantrolene): MH 3 1 Jul 2022- Jul 2025 Yes Jul 6 2014 Ryanodex: Heat stroke/EHS 1 Feb 2023 Yes RTU bivalirudin 21 1 Aug 2029- May 2034 Other 2 3 Feb 2027– Dec 2032 TOTAL 11 10 2 Combination Approach for Long Life Cycle 1 Jointly owned by Medicines Co. & Eagle Patents expected to be Orange Book listed & potentially result in 30 month stays + potential long term protection |
|
|
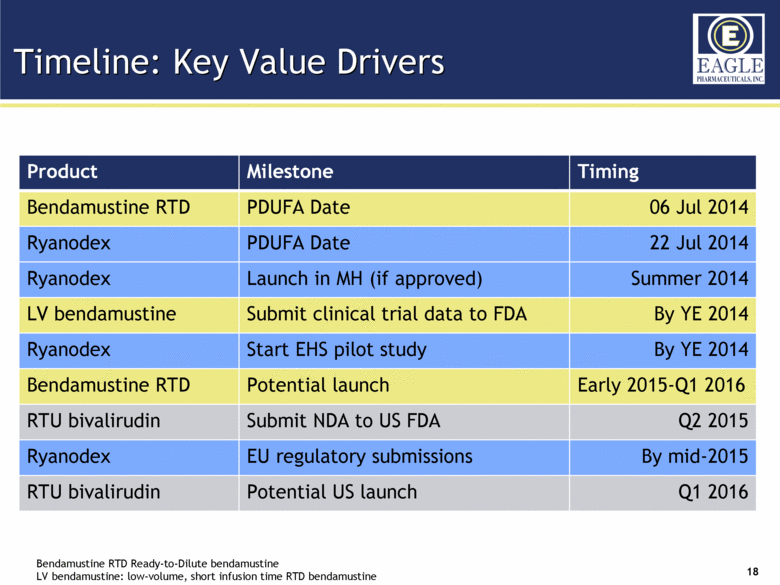
Timeline: Key Value Drivers Bendamustine RTD Ready-to-Dilute bendamustine LV bendamustine: low-volume, short infusion time RTD bendamustine Product Milestone Timing Bendamustine RTD PDUFA Date 06 Jul 2014 Ryanodex PDUFA Date 22 Jul 2014 Ryanodex Launch in MH (if approved) Summer 2014 LV bendamustine Submit clinical trial data to FDA By YE 2014 Ryanodex Start EHS pilot study By YE 2014 Bendamustine RTD Potential launch Early 2015-Q1 2016 RTU bivalirudin Submit NDA to US FDA Q2 2015 Ryanodex EU regulatory submissions By mid-2015 RTU bivalirudin Potential US launch Q1 2016 |
|
|
Investment Considerations Over $3.4 billion opportunity in injectable products space Two PDUFA dates next month (bendamustine RTD & Ryanodex) Near term product launch opportunities with high margins 2 Orphan Drugs: Ryanodex EHS & MH Strong IP portfolio to protect market position: 11 patents issued Long life cycle creates significant value opportunity Strong balance sheet with over $50M in cash |
|
|
June 2014 |