Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - MELINTA THERAPEUTICS, INC. /NEW/ | d737502d8k.htm |
 Developing Well-
Differentiated Antibiotics
to Meet Medical Needs
Cempra Corporate
Presentation
Prabhavathi Fernandes, Ph.D.
President & CEO
June 2014
Exhibit 99.1 |
 Forward Looking Statement
2
This presentation contains forward-looking statements regarding future events. These
statements are just predictions and are subject to risks and uncertainties that could cause
the actual events or results to differ materially. These risks and uncertainties include,
among others: risks related to the costs, timing, regulatory review and results of our studies
and clinical trials; our need to obtain additional funding and our ability to obtain future
funding on acceptable terms; our anticipated capital expenditures and our estimates
regarding our capital requirements; our and our strategic partners’ ability to obtain FDA and
foreign regulatory approval of our product candidates; our dependence on the success of
solithromycin and TAKSTA; the possible impairment of, or inability to obtain, intellectual
property rights and the costs of obtaining such rights from third parties; the unpredictability
of the size of the markets for, and market acceptance of, any of our products, including
solithromycin and TAKSTA; our ability to produce and sell any approved products and the
price we are able to realize for those products; our ability to retain and hire necessary
employees and to staff our operations appropriately; our ability to compete in our industry;
innovation by our competitors; and our ability to stay abreast of and comply with new or
modified laws and regulations that currently apply or become applicable to our business.
Please refer to the documents that we file from time to time with the Securities and
Exchange Commission. |
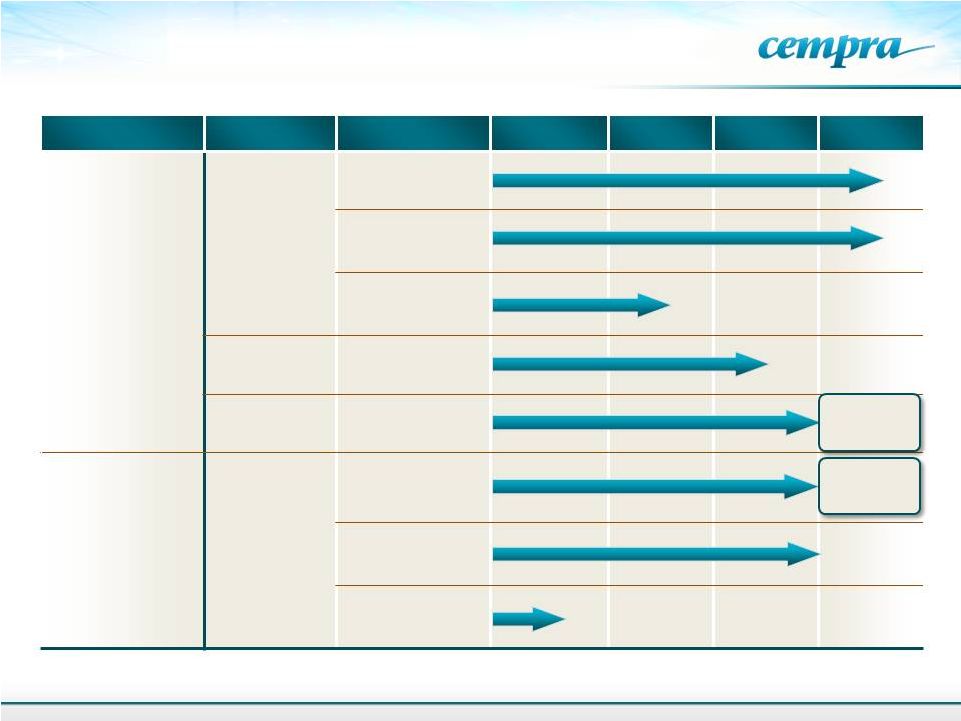 Product
Indication
Formulation
Preclinical
Phase 1
Phase 2
Phase 3
Solithromycin
(CEM-101)
Community
Acquired
Bacterial
Pneumonia
(CABP)
Oral
IV-to-Oral
Oral Pediatric
Biodefense
Animal Rule
Urethritis
Oral
TAKSTA
Fusidic Acid
Acute and
Chronic
Treatment of
Staph
(MRSA)
Oral –
Chronic
Prosthetic Joint
Infections
Oral –
ABSSSI
Oral Suspension/
Pediatric
Cempra’s Antibiotic Portfolio
3
Phase 3
to be
initiated
FDA
Discussions
TM |
 Gary
Horwith, MD EVP Regulatory
David Moore, MBA
CCO
Mark Hahn, CPA
CFO
David Oldach, MD
SVP Clinical
Prabhavathi Fernandes, PhD
President & CEO
David Pereira, PhD
SVP Chemistry
Azactam
(aztreonam)
Biaxin
(clarithromycin)
Dificid
(fidaxomicin)
Levaquin
Topamax
Ultram
Nucynta
Proven Management Team
4
IPO and M&A
Athenix-Bayer CropScience
Charles
&
Colvard
(CTHR)
E&Y
S. aureus vaccine
Abelcet
Viread
GS-9190
Combinations against HCV
Injectable penicillins
Dobutamine HCl injection
Ranitidine injection |
 Broad
Hospital and Primary Care Indications
Due to resistance recurring, increasing need –
Global antibiotic sales over $40B
Safe
antibiotics
with
broad
use
potential
have
historically
been
blockbusters,
e.g.,
Biaxin
®
,
Z-Pak
®
,
Cipro
®
,
Levaquin
®
,
Amoxicillin
Broad use requires hospital and primary care sales and marketing
About
1
antibiotic
prescription
is
written
in
the
hospital
for
9
prescriptions
in
the
community
Retail sales can be carried by large company partners
More and more patients will need hospital admissions for IV therapy as out
patient treatment fails owing to resistance to generic antibiotics
Hospitalization is expensive and exposure to hospital acquired infections is
costly and not reimbursed to hospitals
Therefore there is a very important role for a new primary care antibiotic for
respiratory tract infections (RTIs) that is safe and effective
5 |
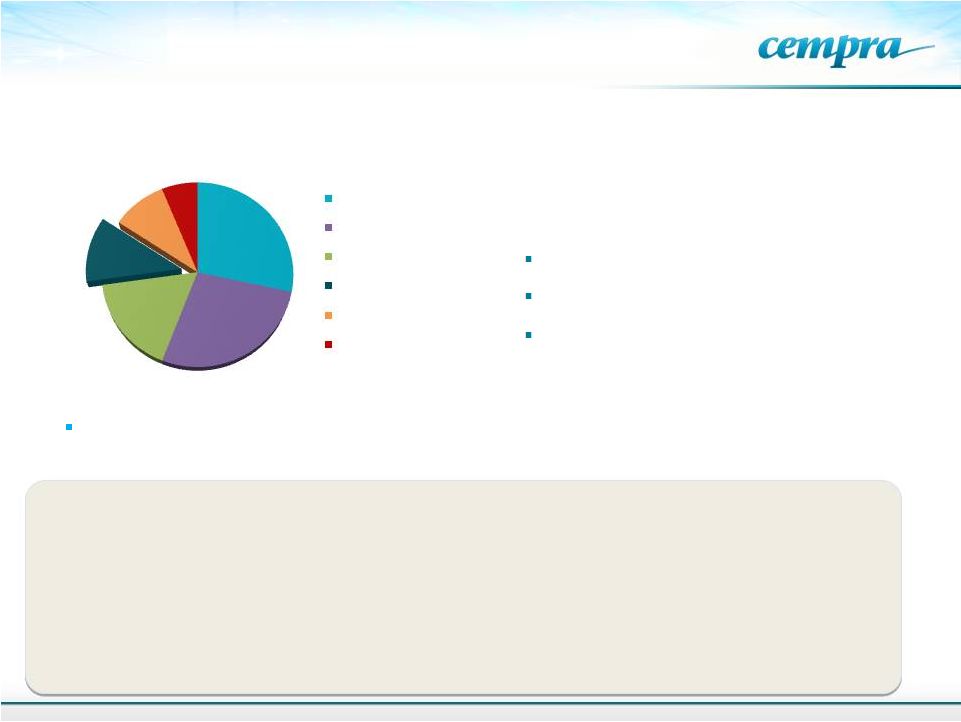 Large
Macrolide Market Opportunity 6
From Hamad, B. IMS Health: Nature Drug Discovery, 9:675-676, 2010.
Azithromycin, a macrolide, is the
most widely prescribed treatment
for CABP and other RTIs –
>60% of
market
Global Antibiotic Sales in 2009
Total $42B
Increasing
resistance
(40%
of
U.S.
pneumococci
a
;
96.4%
in
China
b
a
Jones,
RN.
DMID.
2013;75:107-109;
b
Kim,
SH.
AAC.
2012;56:1418-1426..
51 Million prescriptions were written in the US for azithromycin in the US. in
2013
Antimicrobial Resistance and Infection Control 2014: 3:16
Treatment failure of macrolide resistant pneumonia is attributed to
increased cost and heathcare utilization and hospital admission
Study by the Health Policy Institute, Univ. of California, Irvine.
$11.9B
$11.5B
$7.1B
$4.8B
$4B
$2.6B
Excellent tissue/intracellular distribution
and anti-inflammatory activity
Good safety
Cephalosporins
beta-lactams
Fluoroquinolones
Macrolides
Other antibacterials
Tetracyclines /
aminoglycosides
Broad spectrum of activity
2013
IMS
New
Prescription
Audit)
( |
 What
is Solithromycin? Basic characteristics
A
4
generation
macrolide
antibiotic,
the
first
fluoroketolide
–
2
Phase
3
trials
Active against macrolide resistant pathogens
Inhibits
bacterial
protein
synthesis
–
binds
three
regions
of
the
bacterial
ribosome,
overcoming
resistance to azithromycin (Zithromax) which binds to one site only
Extended spectrum of activity
Best
orally
bioavailable
macrolide,
best
distribution
into
cells
and
tissues
Chemically stable
Strong anti-inflammatory properties
Clinical
New
oral
and
IV
antibiotic
in
development
in
CABP
–
Well
tolerated
to
date
IV switch to oral provides flexibility for dosing and a pharmacoeconomic advantage
TQT negative-
advantage over older macrolides
Demonstrated
efficacy
and
safety
in
Phase
2
CABP
trial
–
132
patients
Oral
capsules,
intravenous
formulation
and
pediatric
suspension
in
development
–
first
in
>20
years
An
antibiotic
for
all
age
groups,
hospital
as
well
as
outpatient
use
7
th |
 Solithromycin Highlights
8
Clinical Trials
Phase 3 Oral, CABP study enrolling; Enrollment
expected to complete Q4 2014, data
expected – Q1 2015
Phase 3 intravenous-oral step down study; enrolling
Phase 2 in gonorrhea completed with 100% cure
in culture proven cases. Phase 3 planned to start 2H 2014
Phase 1 in pediatrics, enrolling
Strategic
Partnerships
BARDA
HHS:
$58MM
contract
–
Development
of
Soli
for
pediatric
use
and against bioterror pathogens
Toyama Chemical (FujiFilm): Global development program –
exclusive license for Japan. $10MM upfront; up to $60MM in
milestones; tiered royalties based on sales
Regulatory & IP
Qualified Infectious Disease Products (QIDP) granted by FDA
designations; oral and IV formulations for CABP
Provides
priority
review
–
8
months
Only QIDP drug in CABP
QIDP for gonorrhea
NCE patent to 2025, additional patents and PTEs |
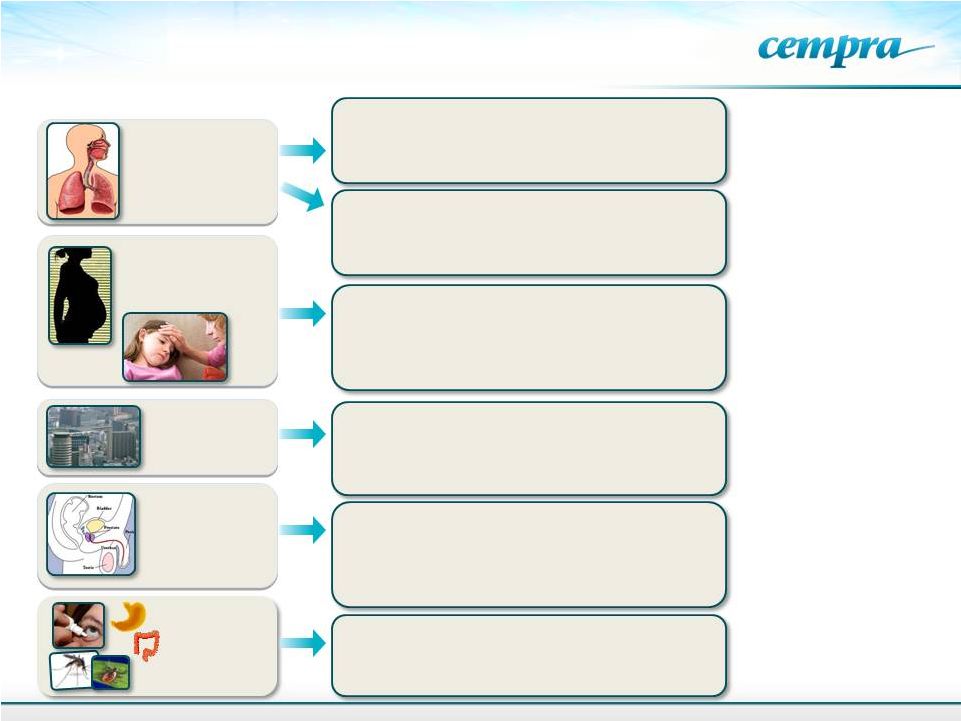 Solithromycin’s Broad Use Potential
HAP, Simple RTI’s, Pharyngitis, Sinusitis, Bronchitis,
Acute Exacerbation of Chronic Bronchitis (AECB)
Respiratory Tract Infections (RTI)
Antibacterial and Anti-inflammatory
COPD, Cystic fibrosis, Panbronchiolitis, NASH
Special
Populations
BARDA funded
Pediatrics and Pregnancy
No pediatric drug with broad potential
in development
Infections
in
pregnancy
–
neonatal
sepsis
Infections
In
Utero
–
Premature,
Cerebral
palsy,
Autism
Biodefense
BARDA funded
Multiple Unidentified Pathogens
Anthrax, Tularemia
Sexually
Transmitted
Diseases
Genital Infections
Major
public
health
crisis
–
multi
drug
resistance,
no
oral
therapy. Most common reportable infectious diseases
Primary cause of infertility in white women
Infertility, PID
GI & Others
Ophthalmic
Helicobacter gastritis, Campylobacteria, tick and insect
borne diseases, diarrhea, and ophthalmic drops
CABP
Primary
Indication
Other Infections
9 |
 10
Why is a New Antibiotic Needed for CABP?
Current IDSA/ATS recommendation to give broad spectrum coverage:
In clinical practice a definitive microbial diagnosis of CABP is not feasible
leading to empiric therapy to provide broad spectrum coverage
Requires intravenous
cephalosporin
(e.g., ceftriaxone)
and hospitalization
Azithromycin added to treat
Legionella
and
Mycoplasma.
Pneumoccocci can be resistant
Mortality rates
in hospitalized
CABP patients
is 23%
–
30-day rate
a
a
Freeman, MK. CABP: A Primer for
Pharmacists: US Pharmacist. July 1, 2013.
IV
and
Oral
available
–
and
have
broad
spectrum
activity,
however, they are not in favor for treating CABP because:
Treatment failures from resistant strain selection
Kill
bowel
flora
–
associated
with
C.difficile
colitis
Adverse tendonitis, Achilles tendon rupture, hepatotoxicity and
peripheral neuritis, retinal detachment
Not approved for use in pediatrics
Or
1)
A ß-lactam plus
a macrolide
2)
A fluoroquinolone
(e.g., Levaquin,
Avelox) |
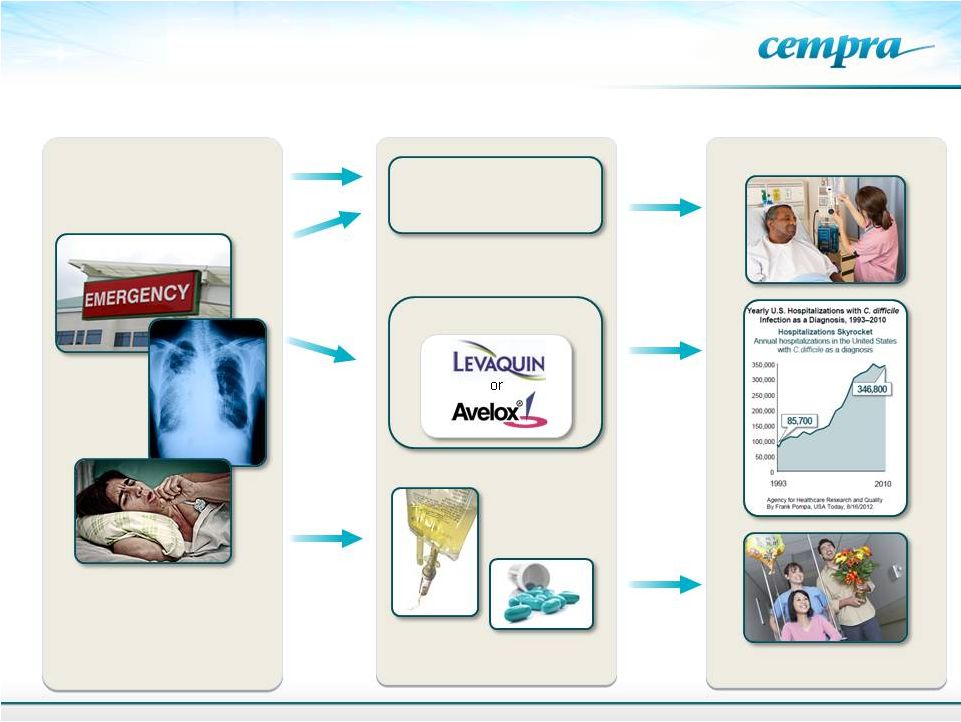 A
Fluoroquinolone Current and Future Treatment of CABP
11
Hospital / IV 7–10 Days
or
Indication
Treatment
Implication
Ceftriaxone plus
Azithromycin
Current
Treatment
Options
Future
Options
With Soli
IV-Oral Step-down Therapy
Fewer Hospital Days
Use Leads
to Higher
C. diff
Incidence |
 Problem of Hospital
Acquired Infections 12
NEJM 2014. 1198-1208, 2014
Magill, SS. And CDC and Emory Authors
2013 National Prevention Target –
30%
reduction
in
C.
difficile
(
Srinivasan,
CID
2012;
55426-31
-
Not on target
Of
all
health-care
associated
infections-
C.
difficile
caused
by
fluoroquinolone
and
broad
spectrum
antibiotics
was
ranked
#1 |
 13
Large VA study -
14 million unique persons, between Sept. 1999, and April 2012.
Veterans who received azithromycin 48% higher death rate and a 77% higher risk
of cardiac arrhythmias in the first 5 days of treatment when compared to
amoxicillin.
Those who received levofloxacin had a 149% and 143% higher risk of death and
cardiac arrhythmia than amoxicillin–treated patients in the first 5 day with
higher rates as the duration of treatment increased.
Reports
of
Cardiac
Arrhythmias
-
Azithromycin
and
Levofloxacin are No Longer Preferred Options
Ann.Fam Med 12:121-127, 2014
Gowtham A. Rao, M.D.., Ph.D., MPH, et al. |
 Community
Acquired
Bacterial
Pneumonia
(CABP)
–
#1
cause
of
death
from an infection
The 6th most common cause of death from all causes in the U.S. and a leading
cause of death worldwide
5 to10 million CABP cases annually, 1.1 million patients hospitalized per year
More common in older adults (
>65 years) and young children (CABP infection rate ranges
from 74–92 (
<2yrs) and 33–52 (3–6 yrs) per 1,000 children
a, b
)
Appropriate empiric therapy is critical to positive outcomes
Multiple
pathogens
can
be
involved
-
Pneumococcus
–
the
most
frequent
cause
CABP –
Most Frequent Infectious Disease in
the U.S.
14
Pneumococcal infections cause more deaths per year in
U.S. than breast or prostate cancer.
Xu, et al. Deaths: Final Data for 2007. Natl Vital Stat Rep.
2010;58:1-51. Respiratory disease incidence is increasing;
growing numbers of COPD and asthma patients.
Drug Discovery News, May 2012.
a
Freeman, MK. CABP: A Primer for Pharmacists.:US Pharmacist. July1, 2013.
b
Ostapchuk,
M.
et.al.
American
Family
Physician.
2004;70:899-908
.
a |
 Solithromycin –
Spectrum of Activity
that Addresses CABP Pathogens
Solithromycin has class leading potency and spectrum in vitro against CABP
pathogens
15
Interacts
with
bacterial
ribosome
at
three
sites
–
Resistance
rare
and
it
could
only
occur if mutations occur at three distinct sites
Gram
Positive
Negative
Positive
Atypical
Atypical
Atypical
Streptococcus
pneumoniae
Haemophilus
influenzae
Staphylococcus
aureus
Legionella
pneumophila
Mycoplasma
pneumoniae
Chlamydophila
pneumoniae
Organisms
Solithromycin
Azithromycin
Cephalosporin
Fluoroquinolone |
 Why We Need a New
Macrolide 16
Susceptibility data for solithromycin and comparators against 927 S.
pneumoniae
from RTI Samples Collected in 2012-2013.
Morrissey, I. ECCMID 2014. Abstr. P1584.
Antibiotic
Region
Percentage
MIC
(µg/mL)
S
R
90%
Solithromycin
Europe (418)
100
0
0.06
NA (380)
100
0
0.25
Asia (129)
100
0
0.5
Azithromycin
Europe (418)
71.3
28.0
> 1
NA (380)
56.3
42.6
> 1
Asia (129)
28.7
70.5
> 1 |
 Solithromycin: Phase 3 CABP Studies
Combined safety and efficacy data from two CABP Phase 3
studies is expected to be sufficient for capsules and
intravenous NDA
Phase 3 study 1: SOLITAIRE Oral study currently enrolling
Initiated Dec 2012, enrollment expected to complete 4Q 2014
800mg
LD/400mg
–
total
5
days
vs.
Avelox
400
mg
7
days
Top line results expected in Q1 2015
Phase 3 study 2: SOLITAIRE Intravenous to step down oral
Initiated 4Q 2013. Enrolling
400 mg IV followed by oral 800 mg LD/400 mg for up to 7 days
vs. Avelox 400 mg IV to oral 7 days
Both Phase 3 studies are global studies
Plan to enroll ~800 patients
Comparator
Avelox
(moxifloxacin)
–
A
fluoroquinolone
that
is
used
worldwide at same dose
17 |
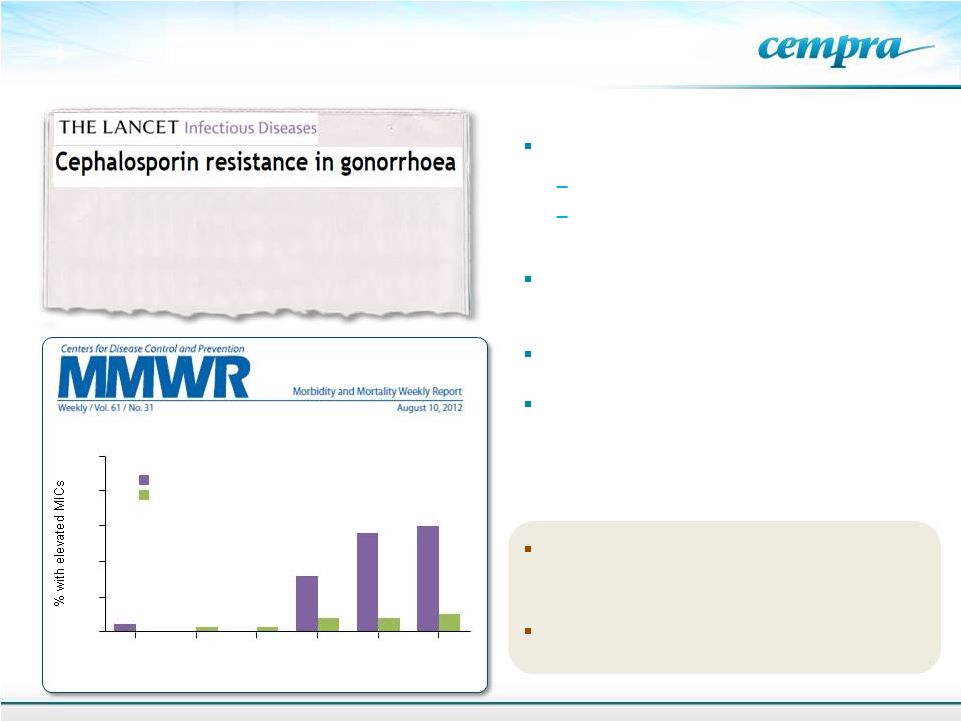 Gonococcal Surveillance Project, US. 2006-2011.
Expanding Solithromycin Use
Gonorrhea Studies
Current treatment
–
IM ceftriaxone
No oral drug to treat partners
The second most common
communicable disease
QIDP granted for solithromycin for
gonorrhea treatment
CDC and WHO list as urgent need
Soli Phase 2 results: culture negative
cure in 100% (all body sites) of
evaluable patients
Phase 3 planned to be initiated 2H 2014
–
Shared costs of study by Australian
public health
A single Phase 3 study is expected to be
sufficient for FDA approval
Oral Cephalosporins No Longer a Recommended
Treatment for Gonococcal Infections
2006
2007*
2008*
2009
2010
2011
†
0
0.5
1.0
1.5
2.0
2.5
Cefixime
Ceftriaxone
Year
18
Lancet ID 13: 728-730, 2013
…….A new drug on the horizon is the
fluoroketolide solithromycin, to which N
gonorrhoeae
is
very
sensitive
in
vitro,
and
this
agent is currently in pneumonia trials……
The second most common
communicable disease |
 Pediatrics Studies
–
Suspension
formulation Developed (BARDA funded)
19
First NCE since azithromycin to be developed as a suspension formulation for
pediatric
Oral drugs are even more important in pediatrics. About 80M prescriptions are
written for pediatrics in the US alone
When children get MDR infections, most commonly respiratory infections, what do
we have left? Amoxicillin 1972, Azithromycin 1988
Solithromycin is being developed for pediatric use because of its oral
bioavailability, safety and tolerability profile, stability in suspension
Otitis
media
is
the
most
common
reason
for
pediatric
antibiotic
prescription
2013 IMS New Prescription Audit
Solithromycin is effective in the chinchilla model of experimental otitis media
which has been found to consistently correlate with subsequent clinical trials
in children |
 TAKSTA™
(Fusidic Acid)
20
An
Oral
Antibiotic
for
MRSA
Infections
Being
Developed
for
Chronic
Use
in
Prosthetic
Joint
Infections
in
the
U.S. |
 TAKSTA
Cempra’s proprietary fusidic acid dosing regimen
Fusidic acid approved in Western Europe, Australia, and other countries
40
years
of
established
safety
and
efficacy
profile
in
acute
and
chronic
use
in
staph
infections
ex-U.S.
Unique structure, no known cross resistance with any other antibiotic
Orally bioavailable
Targeted
against
gram-positive
pathogens,
including
MRSA
–
pathogens
that
causes
bone
and joint infections requiring long-term treatment
Exclusivity
NCE in the U.S.: Hatch Waxman exclusivity, 5 years obtained by Cempra, exclusive
supply agreement
of
drug
substance
–
a
fermentation
product
GAIN
Act
adds
an
additional
5
years
of
data exclusivity –
A minimum of 10 years of data exclusivity
or 12 years if Orphan
Loading dose patent Protects U.S. Market Exclusivity to 2029 + patent term
extension Orphan
Drug
Designation
for
PJI
granted
–
October
2013
What is TAKSTA™?
21 |
 Current and Future Treatment of
Prosthetic Joint Infections
22
IV Vancomycin and Oral
Antibiotics –
4–6 Weeks
Surgery
Spacers In
Surgery New
Prosthesis Inserted
Cempra’s
Trial
Current U.S.
Treatments
Surgery
Sterile Joint
Potential for Cost Savings, Surgery
Decrease, Patient Comfort and
Pharmacoeconomic Advantages Gain
Physician, Payor Acceptance
Cempra Aims to Bring the Lower Cost, More Effective
European PJI Treatment Paradigm to the U.S.
Debride
and Treat
Two-stage
Revision
Debride, IV
Vancomycin
for 1–7 Days
Oral Fusidic
Acid Plus Oral
Rifampin for
6–12 Months
Orthopedic
Surgery |
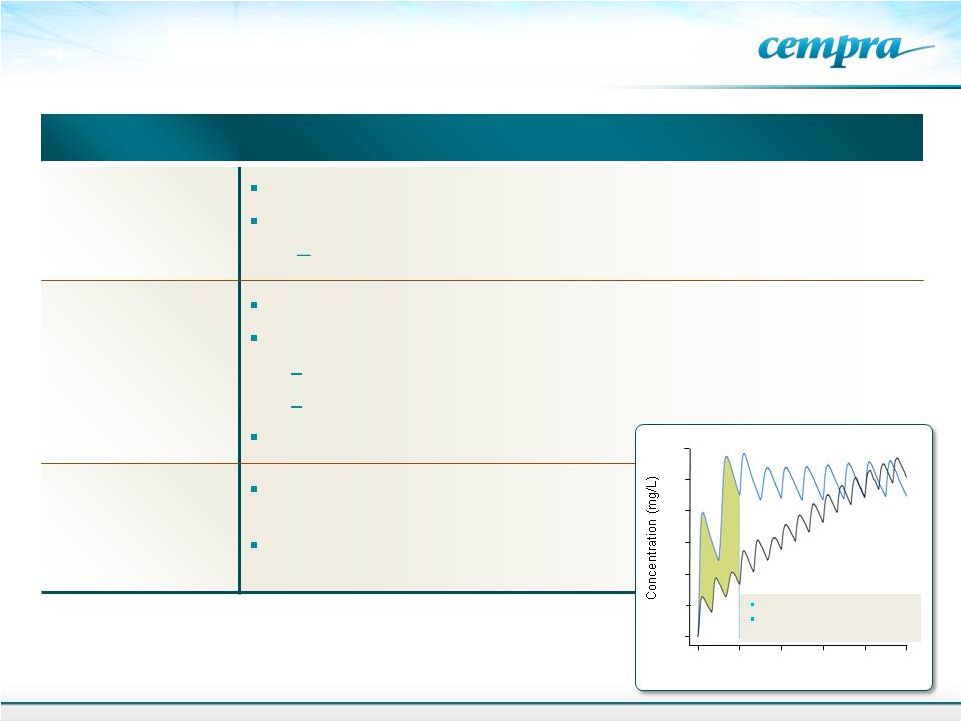 Taksta Update
23
0
20
40
60
80
100
120
European dosing
Cempra’s loading
dose
0
24
48
72
96
120
Time (hrs)
EU Dose 500 mg dose
Cempra dose 1200 mg Q12h
Day followed by 600 mg Q12h
Clinical Trials
Phase 2 PJI study data reported, study stopped
New strategy for the pivotal study is being designed
No PJI guidance
-
Discussion with FDA planned
Regulatory
Orphan Drug Designation for PJI granted by FDA (October 2013)
Orphan Drug designation benefits
7 year exclusivity
Tax credit for 50% of clinical trial cost and PDUFA fees exempted
GAIN
pathogen
MRSA-Potential
QIDP
Intellectual
Property
Loading dose patent protection
into 2029 and PTEs
Well
tolerated
in
ABSSSI
Phase
2
study;
no resistance seen |
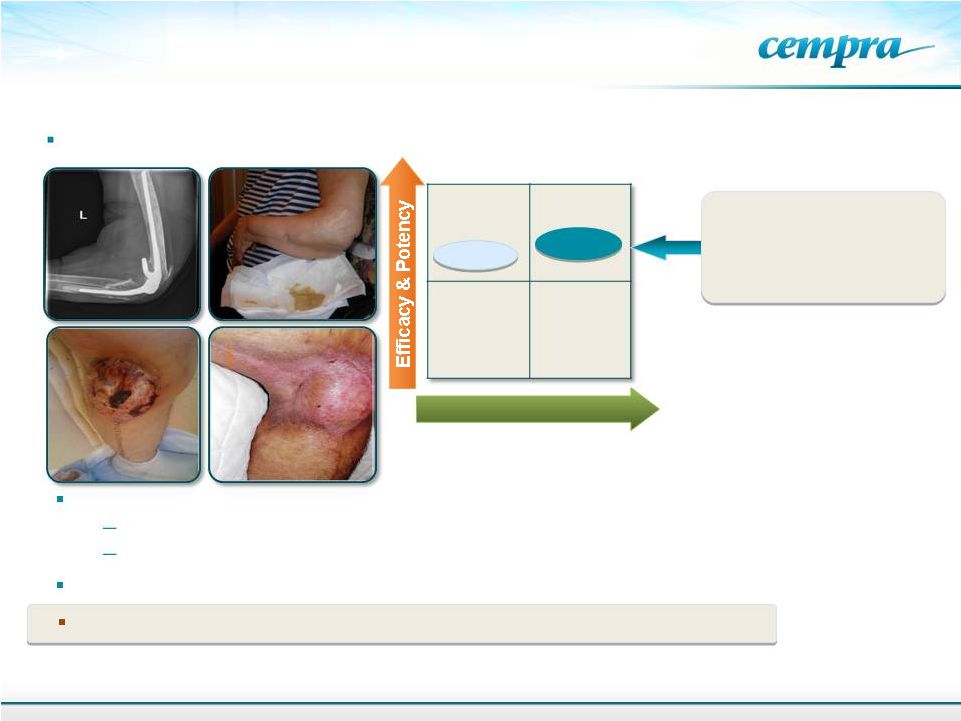 Two
compassionate use cases of bone/prosthesis infections in North America
Safety
Linezolid
TAKSTA
Prosthetic surgery facts
20,000
hip
and
knee
PJIs
in
2007
a
Total
joint
and
hardware
procedures
–
3,286,000/year
b,c
Potential use in osteomyelitis, septic arthritis, and diabetic foot
TAKSTA for Chronic Use in Prosthetic
Joint Device (PJI) Infections
24
a
N Engl J Med 2009;361:787-794.
b
Life Science Intelligence market research report. U.S. Markets for Large
Replacement Technologies in 2012. March, 2012. c
Life Science Intelligence market research report. U.S. Markets for Small Joint
Implants and Hardware for the Extremities. January, 2012. Cempra’s Phase
2 PJI study closed- strategizing for pivotal study
Useful for Oral Chronic
Use in All Patient
Populations |
 Achieved and Projected Milestones
1Q 14: QIDP designation by FDA for solithromycin for
gonorrhea 1Q 14: Negative TQT results for
solithromycin 1Q 14: Pediatric trial initiated
for solithromycin 2H 14: Meet with FDA to define
pivotal study for Taksta 2H 14:
Initiate Phase 3 gonorrhea study
1Q 15: Data expected SOLITAIRE Oral Phase 3 CABP
25 |
 Take
Away Notes Solithromycin is being developed as adult oral, intravenous and
pediatric formulations. If Phase 3 data shows safety and efficacy, it
could allow broad use of solithromycin like older macrolides
Last antibiotic with oral, intravenous and pediatric suspension is azithromycin
51 million prescriptions were written last year in the US for azithromycin
A new macrolide with activity against resistant strains and improved safety is
needed Solithromycin –
4
th
generation macrolide antibiotic, the first fluoroketolide
Unlike azithromycin, monotherapy for CABP
Well tolerated to date. Negative in TQT study
IV switch to oral provides flexibility for dosing and a pharmacoeconomic advantage
Late-stage clinical development -Enrollment in the oral Phase 3 trial
expected to complete in 2014
26 |
 Cash & Equivalents (3/31/14)
$79.6MM
Long-Term Debt (3/31/14)
$12.5MM
Shares Outstanding (6/2/14)
33.2MM
Capitalization
27 |
 Pricing Models: U.S. & EU Countries
A 4
th
generation macrolide
Branding –
Product Recognition
Clinical trial name is SOLITAIRE
Mono-therapy
Commercialization Activities
28
Solithromycin Value
Proposition;
Activity, Safety, different
dosing formulations
Market-specific Evidence
Requirements for Access
and Reimbursement
Marketing
&
Sales
U.S.:
Co-promote with Partner
EU: Partner
Japan: Toyama/Fujifilm
Pharmacoeconomic
Advantages |
