Attached files
| file | filename |
|---|---|
| EX-23.2 - CONSENT - Sunshine Biopharma, Inc | sbfm_ex232.htm |
| EX-5.2 - OPINION - Sunshine Biopharma, Inc | sbfm_ex52.htm |
| EX-23.3 - CONSENT - Sunshine Biopharma, Inc | sbfm_ex233.htm |
As Filed with the Securities and Exchange Commission on May 22, 2014
Reg. No. _________________
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C., 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
SUNSHINE BIOPHARMA, INC.
(Exact Name of Registrant as specified in its Charter)
|
Colorado
|
2836
|
20-5566275
|
||
|
(State or Other Jurisdiction of
|
(Primary Standard Industrial
|
(I.R.S. Employer Identification No.)
|
||
|
Incorporation or Organization)
|
Classification Code Number)
|
469 Jean-Talon West
3rd Floor
Montreal, Quebec, Canada H3N 1R4
(514) 764-9698
(Address and telephone number of principal executive offices)
Dr. Steve N. Slilaty, President
469 Jean-Talon West
3rd Floor
Montreal, Quebec, Canada H3N 1R4
Tel: (514) 764-9698
(Name, address, including zip code, and telephone number, including area code, of agent for service)
_________
Copies of communications to:
Andrew I. Telsey, Esq.
Andrew I., Telsey P.C.
12835 E Arapahoe Rd.
Tower 1 Penthouse #803
Centennial, CO 80112
Tel: (303) 768-9221 Fax: 303-768-9224
----------------------------------------------------------------------
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement.
----------------------------------------------------------------------
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. þ
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company.
|
Large accelerated filer
|
o
|
Accelerated filer
|
o
|
|
|
Non-accelerated filer
|
o
|
Smaller reporting company
|
þ
|
|
|
(Do not check if smaller reporting company)
|
CALCULATION OF REGISTRATION FEE
|
Title of Each
Class of Securities to
be Registered
|
Aggregate Proposed
Amount to be
Registered(1)
|
Proposed Maximum
Offering Price per
Share(2)
|
Proposed Maximum
Aggregate Offering
Price(2)
|
Amount of
Registration Fee
|
||||
|
Common Stock
|
13,400,000 shares
|
$0.15 per share
|
$2,010,000
|
$258.89
|
|
(1) Pursuant to Rule 416(a) under the Securities Act of 1933, this registration statement also covers any additional securities that may be offered or issued in connection with any stock split, stock dividend or similar transaction.
|
|
(2) Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(c) under the Securities Act of 1933. The price per share and aggregate offering price are based on the average of the high and low sales prices of the registrant’s common stock on May 20, 2014, as reported on the OTCQB.
|
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT, OR UNTIL THIS REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SECTION 8(a), MAY DETERMINE.
THE INFORMATION IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. WE MAY NOT OFFER OR SELL THESE SECURITIES UNTIL THE REGISTRATION STATEMENT FILED WITH THE SECURITIES AND EXCHANGE COMMISSION IS EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND IT IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY JURISDICTION WHERE THE OFFER OR SALE IS NOT PERMITTED.
SUBJECT TO COMPLETION, DATED MAY 22, 2014
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement is filed with the Securities and Exchange Commission and becomes effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the sale is not permitted.
|
PRELIMINARY PROSPECTUS
|
SUBJECT TO COMPLETION
|
MAY 22, 2014
|
PROSPECTUS
Sunshine Biopharma, Inc.
13,400,000 Shares of Common Stock
This prospectus relates to the offer and sale of up to 13,000,000 shares of our common stock by Dutchess Opportunity Fund, II, LP (“Dutchess”), which Dutchess has agreed to purchase from us pursuant to an investment agreement (“Investment Agreement”), dated as of April 23, 2014 between our company and Dutchess. In addition, this prospectus relates to an additional 400,000 shares of our common stock which we issued to Dutchess as an engagement fee Subject to the terms and conditions of the Investment Agreement, we have the right, but not the obligation, to “put,” or require Dutchess to purchase up to $2.5 million worth of our shares of common stock during a 36 month period commencing on the date of this prospectus. This arrangement is sometimes referred to as an “Equity Line.”
We will not receive any of the proceeds from Dutchess’ sale of these shares. However, we will receive proceeds from our initial sale of these shares to Dutchess pursuant to the Investment Agreement. We will sell these shares to Dutchess at a price equal to 90% of the lowest daily volume weighted average price (“VWAP”), of our common stock during the five (5) consecutive trading day period immediately preceding the date of delivery of the put notice. We have the right to withdraw all or any portion of any put before the closing, subject to certain limitations set forth in the Investment Agreement.
Dutchess may sell these shares from time to time in regular brokerage transactions, in transactions directly with market makers or in privately negotiated transactions. For additional information on the methods of sale that may be used by Dutchess, see the section entitled “Plan of Distribution” on page 20. We will bear the costs relating to the registration of these shares, but we will not pay any of the selling commissions, brokerage fees and related expenses.
Our common stock is currently quoted on the OTCQB under the symbol “SBFM.” Only a limited public market currently exists for our common stock. The closing price of our common stock on May 20, 2014 was $0.15 per share.
With the exception of Dutchess, which is an “underwriter” within the meaning of the Securities Act of 1933, no other underwriter or person has been engaged to facilitate the sale of shares of our common stock in this offering. The Securities and Exchange Commission may take the view that, under certain circumstances, any broker-dealer or agent that participates with the selling stockholder in the distribution of the shares may be deemed to be an “underwriter” within the meaning of the Securities Act. Commissions, discounts or concessions received by any such broker-dealer or agent may be deemed to be underwriting commissions under the Securities Act.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 3 of this prospectus to read about factors you should consider before investing in shares of our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
———————————
The date of this prospectus is ______, 2014
Table of Contents
|
PART I - INFORMATION REQUIRED IN PROSPECTUS
|
||
|
Prospectus Summary
|
1
|
|
|
Risk Factors
|
3
|
|
|
Risks Related To Our Operations
|
3
|
|
|
Risks Related To Our Common Stock
|
10
|
|
| Risks Related to this Offering | 11 | |
|
Special Note Regarding Forward-Looking Statements
|
12
|
|
|
Use of Proceeds
|
12
|
|
|
Determination of Offering Price
|
12
|
|
|
The Dutchess Equity Line Transaction
|
13
|
|
|
Selling Stockholder
|
15
|
|
|
Plan of Distribution
|
15
|
|
|
Description of Securities
|
16
|
|
|
Description of Our Business
|
17
|
|
|
Market for Common Equity and Related Stockholders Matters
|
21
|
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
23
|
|
|
Directors, Executive Officers, Promoters and Control Persons
|
25 | |
|
Security Ownership of Certain Beneficial Owners and Management
|
27
|
|
|
Related Party Transactions
|
27
|
|
|
Interest of Named Experts and Counsel
|
28
|
|
|
Disclosure of Commission Position on Indemnification for Securities Act Liabilities
|
28
|
|
|
Financial Statements
|
F-1
|
|
|
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS
|
||
|
Other Expenses of Issuance and Distribution
|
II-1
|
|
|
Indemnification of Directors and Officers
|
II-1
|
|
|
Recent Sales of Unregistered Securities
|
II-1
|
|
|
Exhibit Index
|
II-3
|
|
|
Undertakings
|
II-3
|
|
|
Signatures
|
II-4
|
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with additional or different information from that contained in this prospectus. You should assume that the information contained in this prospectus is accurate only as of any date on the front cover of this prospectus or the date of the document incorporated by reference, as applicable, regardless of the time of delivery of this prospectus or any sales under the Investment Agreement. Our business, financial condition, results of operations and prospects may have changed since those dates.
This summary highlights selected information contained elsewhere in this Prospectus. This summary does not contain all the information that you should consider before investing in the common stock of SMTP, Inc. (referred to herein as the “Company,” “Sunshine,” “we,” “our,” and “us”). You should carefully read the entire Prospectus, including Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements before making an investment decision.
About Us
We were incorporated in the State of Colorado on August 31, 2006 under the name “Mountain West Business Solutions, Inc.” Until October 2009 our business was to provide management consulting with regard to accounting, computer and general business issues for small and home-office based companies.
Effective October 15, 2009, we executed an agreement to acquire Sunshine Biopharma, Inc., a Colorado corporation, in exchange for the issuance of 21,962,000 shares of our Common Stock and 850,000 shares of Convertible Preferred Stock, each convertible into twenty (20) shares of our Common Stock (the “Agreement”). As a result of this transaction we changed our name to “Sunshine Biopharma, Inc.” On December 21, 2011, Advanomics Corporation, a privately held Canadian company (“Advanomics”), and our licensor, exercised its right to convert the 850,000 shares of Series “A” Preferred Stock it held in our Company into 17,000,000 shares of Common Stock.
We are currently a pharmaceutical company focused on the research, development and commercialization of drugs for the treatment of various forms of cancer. The preclinical studies for our lead compound, Adva-27a, a multi-purpose antitumor compound, were successfully completed in late 2011. We are now continuing our clinical development of Adva-27a by conducting the next sequence of steps comprised of Good Manufacturing Practice (“GMP”) manufacturing, Investigational New Drug (“IND”)-enabling studies, regulatory filing and Phase I clinical trials. We plan to conduct our Phase I clinical trials for Adva-27a at the Jewish General Hospital, Montreal, Canada, one of McGill University’s Hospital Centers. The planned indication will be pancreatic cancer in parallel to multidrug resistant breast cancer as Adva-27a has shown a positive effect on these two types of cancer for which there is currently little or no treatment options available. See “Description of Business - Clinical Trials” below.
We have licensed our technology on an exclusive basis from Advanomics, and we are planning to initiate our own research and development program as soon as practicable after our registration statement is deemed effective by the Securities and Exchange Commission and we have sufficient funds available to do so.
Offering Summary
|
Common stock offered by Dutchess, who is the Selling Stockholder
|
13,400,000 shares of common stock.
|
|
|
Common stock outstanding before the offering
|
65,775,728 shares of common stock as of May 21, 2014
|
|
|
Common stock outstanding after the offering after giving effect to the issuance of 13,400,000 shares to Dutchess pursuant to the Investment Agreement
|
78,775,728 shares of common stock.
|
|
|
Offering Price
|
To be determined by the prevailing market price for the shares at the time of sale or negotiated transactions.
|
|
|
Use of proceeds
|
We will not receive any of the proceeds from Dutchess’ sale of the shares of common stock covered by this prospectus. However, we may receive up to $2.5 million in proceeds from the sale of shares of common stock to Dutchess pursuant to the terms of the Investment Agreement. We anticipate that the net proceeds we receive under the Investment Agreement will be used for the clinical development of our cancer drug, Adva-27a more fully described below, including (i) GMP Manufacturing; (ii) IND-Enabling Studies; (iii) Regulatory Filings; (iv) Phase I Clinical Trials; and (v) working capital and other general corporate purposes. See “Description of Business” and “Use of Proceeds.”
|
|
|
OTCQB Trading Symbol
|
Our common stock is traded on the OTCQB under the symbol “SBFM.”
|
|
|
Risk Factors
|
The common stock offered hereby involves a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investment. See “Risk Factors” beginning on page 3.
|
1
SUMMARY FINANCIAL INFORMATION
THE FOLLOWING SUMMARY CONTAINS:
|
●
|
AUDITED FINANCIAL INFORMATION FOR THE YEARS ENDED DECEMBER 31, 2013 AND 2012 FROM BALANCE SHEETS AND STATEMENTS OF OPERATIONS DATA FROM OUR AUDITED FINANCIAL STATEMENTS; AND
|
|
●
|
UNAUDITED FINANCIAL INFORMATION FOR THE THREE MONTHS ENDED MARCH 31, 2014 FROM BALANCE SHEET AND STATEMENT OF OPERATIONS DATA FROM OUR UNAUDITED INTERIM FINANCIAL STATEMENTS.
|
THE INFORMATION CONTAINED IN THESE TABLES SHOULD BE READ IN CONJUNCTION WITH “MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS” HEREIN BELOW AND THE FINANCIAL STATEMENTS AND ACCOMPANYING NOTES INCLUDED HEREIN.
Our financial statements have been prepared in accordance with US GAAP. The accompanying unaudited financial information includes all adjustments considered necessary (consisting only of normal recurring adjustments) for a fair presentation. Results for the years ended December 31, 2013 and 2012 and the three months ended March 31, 2014 are not necessarily indicative of the results that may be expected for any future period.
Statement of Operations:
|
Year Ended December 31,
|
Three Months Ended March 31,
|
|||||||||||||||
|
2013
|
2012
|
2014
|
2013
|
|||||||||||||
|
Revenues
|
$ | — | $ | — | $ | — | $ | — | ||||||||
|
Total operating expenses
|
$ | 1,936,077 | $ | 706,680 | $ | 405,873 | $ | 922,891 | ||||||||
|
Income (Loss) from operations
|
$ | (1,936,077 | ) | $ | (706,680 | ) | $ | (405,873 | ) | $ | (922,891 | ) | ||||
|
Other income (expense)
|
(6,445 | ) | (1,272 | ) | (95,382 | ) | (5,330 | ) | ||||||||
|
Provision for income tax
|
$ | - | $ | — | $ | - | ||||||||||
|
Net income (loss)
|
$ | (2,491,483 | ) | $ | (707,952 | ) | $ | (501,255 | ) | $ | (1,477,172 | ) | ||||
|
Net income (loss) per share – (basic and fully diluted)
|
$ | (0.04 | ) | $ | (0.01 | ) | $ | (0.01 | ) | $ | (0.03 | ) | ||||
|
Weighted common shares outstanding
|
55,395,819 | 49,775,134 | 61,127,469 | 55,395,819 | ||||||||||||
Balance Sheet:
|
Year Ended
December 31,
2013
|
Year Ended
December 31,
2012
|
|||||||
|
Cash and cash equivalents
|
$
|
31,240
|
$
|
132,638
|
||||
|
Current assets
|
$
|
31,240
|
$
|
134,793
|
||||
|
Total assets
|
$
|
31.240
|
$
|
134,793
|
||||
|
Current liabilities
|
$
|
38,950
|
$
|
64,367
|
||||
|
Total liabilities
|
$
|
38,950
|
$
|
64,367
|
||||
|
Total stockholders’ equity
|
$
|
(7,710)
|
$
|
70,426
|
||||
2
RISK FACTORS
An investment in the securities offered involves a high degree of risk and represents a highly speculative investment. In addition to the other information contained in this prospectus, prospective investors should carefully consider the following risks before investing in our common stock. If any of the following risks actually occur, our business, operating results and financial condition could be materially adversely affected. As a result, the trading price of our common stock could decline, and you may lose all or part of your investment in our common stock. The risks discussed below also include forward-looking statements, and our actual results may differ substantially from those discussed in these forward-looking statements. See “Special Note Regarding Forward Looking Statements” in this prospectus.
Additional risks and uncertainties not currently known to us or that we presently deem to be immaterial may also materially and adversely affect our business, prospects, financial condition, results of operations and value of our stock. You should not purchase the securities offered unless you can afford the loss of your entire investment.
Risks Related to our Operations
We may not be able to continue as a going concern or fund our existing capital needs.
Our independent registered public accounting firm included an explanatory paragraph in their report included herein on our financial statements related to the uncertainty in our ability to continue as a going concern. The paragraph stated that we do not have sufficient cash on-hand or other funding available to meet our obligations and sustain our operations, which raises substantial doubt about our ability to continue as a going concern. Our cash and cash equivalents were sufficient to fund our existing development commitments, indebtedness and general operating expenses through December 31, 2013; however, we will not be generating any product-based revenues or realizing cash flows from operations in the near term, if at all, and may not have sufficient cash or other funding available to complete our anticipated business activities during 2014.
We have incurred losses in the past and expect to incur greater losses until we implement our business plan.
We are a development stage company and we have not yet begun generating revenues and we do not expect to begin generating revenues until the clinical trials for our sole product candidate is completed and is successful. In particular, our multi-purpose anti-tumor compound, Adva-27a, expects to be entering Phase I clinical trials for multidrug resistant breast cancer indication during 2014, provided that we are successful in obtaining the funding necessary to conduct these trials. We expected that we would begin these clinical trials during 2012, but were unable to secure sufficient funding to undertake this activity. While we believe that the funds received from Dutchess will be sufficient to allow us to complete our initial clinical trials there can be no assurances that we will have sufficient funds to complete the same, or that these trials will be successful. Further, there can be no assurance that the results obtained from laboratory or research studies will be replicated in human studies or that such human studies will not identify undesirable side effects. There can be no assurance that any of our therapeutic products will meet applicable health regulatory standards, obtain required regulatory approvals or clearances, be produced in commercial quantities at reasonable costs, be successfully marketed or be profitable enough that we will recoup the investment made in such product candidates.
We are a development stage company and may never attain product sales.
We have not received approval for any of our product candidates from the FDA. Any compounds that we discover or in-license will require extensive and costly development, preclinical testing and/or clinical trials prior to seeking regulatory approval for commercial sales. Our most advanced and only product candidate, Adva-27a, may never be approved for commercial sale. The time required to attain product sales and profitability is lengthy and highly uncertain, and we cannot assure you that we will be able to achieve or maintain product sales.
We expect our net operating losses to continue for at least several years, and we are unable to predict the extent of future losses or when we will become profitable, if ever. We have incurred significant net losses since our formation in 2009. We have incurred an accumulated deficit of $5,494,149 as of December 31, 2013 and $5,995,404 as of March 31, 2014. Our operating losses are due in large part to the significant research and development costs required to identify, validate and license potential product candidates, conduct preclinical studies and conduct clinical trials of our more advanced product candidates. To date, we have not generated any revenues and we do not anticipate generating any revenues in the near term, if ever. We expect to increase our operating expenses over the next several years as we plan to:
● Prepare and carry out for the development of Adva-27a;
● Expand our research and development activities;
● Increase our required corporate infrastructure and overhead.
3
As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with our research and product development efforts, we are unable to predict the extent of any future losses or when we will become profitable, if ever. Even if we do achieve profitability, we may not be able to sustain or increase profitability on an ongoing basis.
We have not conducted any significant business operations yet and have been unprofitable to date.
There is no prior operating history by which to evaluate the likelihood of our success or our contribution to our overall profitability. We may never complete clinical trials of our product and commence significant operations or, if we do complete these clinical trials there are no assurances that the results will be positive.
We may require additional funding to satisfy our future capital needs, and future financing strategies may adversely affect holders of our Common Stock.
Even after we complete our financing with Dutchess our operations may require significant additional funding in large part due to our research and development expenses, future preclinical and clinical testing costs, and the absence of any meaningful revenues in the near future. We do not know whether additional financing will be available to us on favorable terms or at all. If we cannot raise additional funds, we may be required to reduce our capital expenditures, scale back product development programs, reduce our workforce and license to others products or technologies that we may otherwise be able to commercialize.
To the extent we raise additional capital by issuing equity securities our stockholders could experience substantial dilution. Any additional equity securities we issue or issuances of debt we may enter into or undertake may have rights, preferences or privileges senior to those of existing holders of stock. To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us.
We have not recorded any revenues from the sale of therapeutic products, have accumulated significant losses since inception and expect to continue to incur losses in the future.
There can be no assurance that we will ever be able to achieve or sustain sufficient sales or other revenue growth in order to achieve profitability or positive cash flow. To become profitable we, either alone or with one or more partners, must develop, manufacture and successfully market therapeutic product candidates. There can be no assurance that we will be successful in achieving the sales levels required to achieve profitability. In addition, lower than anticipated revenues may negatively impact our cash flows, which could accelerate the need for additional capital.
The FDA may change its approval policies or requirements, or apply interpretations to its policies or requirements, in a manner that could delay or prevent commercialization of Adva-27a.
Regulatory requirements may change in a manner that requires us to conduct additional clinical trials, which may delay or prevent commercialization of our only drug candidate, Adva-27a. We cannot provide any assurance that the FDA will not require us to repeat existing studies or conduct new or unforeseen experiments in order to demonstrate the safety and efficacy of Adva-27a before considering the approval of Adva-27a for the treatment of lung cancer or breast cancer indication. Further, FDA Advisory Panel meetings discussing such drug approvals may result in the heightened scrutiny of Adva-27a for the treatment of lung cancer, pancreatic cancer or breast cancer.
Our business would be materially harmed if we fail to obtain FDA approval of a New Drug Application (“NDA”) for Adva-27a.
We anticipate that our ability to generate any significant product revenues in the near future will depend solely on the successful development and commercialization of Adva-27a. The FDA may not approve in a timely manner, or at all, the NDA that we submit. If we are unable to submit an NDA for other product candidates, or if the NDA we submitted for Adva-27a is not approved by the FDA, we will be unable to commercialize any products in the United States and our business will be materially harmed. The FDA can and does reject NDAs, and often requires additional clinical trials, even when product candidates performed well or achieved favorable results in large-scale Phase III clinical trials. The FDA imposes substantial requirements on the introduction of pharmaceutical products through lengthy and detailed laboratory and clinical testing procedures, sampling activities and other costly and time-consuming procedures. Satisfaction of these requirements typically takes several years and may vary substantially based upon the type and complexity of the pharmaceutical product. Our product candidates are novel compounds or new chemical entities, which may further increase the period of time required for satisfactory testing procedures.
Data obtained from preclinical and clinical activities are susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. In addition, delays or rejections may be encountered based on changes in, or additions to, regulatory policies for drug approval during the period of product development and regulatory review. The effect of government regulation may be to delay or prevent the commencement of clinical trials or marketing of our product candidates for a considerable period of time, to impose costly procedures upon our activities and to provide an advantage to our competitors that have greater financial resources or are more experienced in regulatory affairs. The FDA may not approve our product candidates for clinical trials or marketing on a timely basis or at all. Delays in obtaining or failure to obtain such approvals would adversely affect the marketing of our product candidates and our liquidity and capital resources.
Drug products and their manufacturers are subject to continual regulatory review after the product receives FDA approval. Later discovery of previously unknown problems with a product or manufacturer may result in additional clinical testing requirements or restrictions on such product or manufacturer, including withdrawal of the product from the market. Failure to comply with applicable regulatory requirements can, among other things, result in fines, injunctions and civil penalties, suspensions or withdrawals of regulatory approvals, product recalls, operating restrictions or shutdown and criminal prosecution. We may lack sufficient resources and expertise to address these and other regulatory issues as they arise.
4
We may be sued or become a party to litigation, which could require significant management time and attention and result in significant legal expenses and may result in an unfavorable outcome which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
While we have no knowledge of any threatened material litigation matters, we may be subject to lawsuits from time to time arising in the ordinary course of our business. We may be forced to incur costs and expenses in connection with defending ourselves with respect to such litigation and the payment of any settlement or judgment in connection therewith if there is an unfavorable outcome. The expense of defending litigation may be significant. The amount of time to resolve lawsuits is unpredictable and defending ourselves may divert management’s attention from the day-to-day operations of our business, which could adversely affect our business, results of operations and cash flows. In addition, an unfavorable outcome in any such litigation could have a material adverse effect on our business, results of operations and cash flows.
Holders of our Common Stock may suffer significant dilution in the future.
In order to fully implement our business plan we will require additional capital, either debt or equity, or both. As a result, we expect to raise additional equity capital by selling shares of our Common Stock or other securities in the future to raise the funds necessary to allow us to implement our business plan. If we do so, investors will suffer significant dilution.
Our management and principal shareholders have the ability to significantly influence or control matters requiring a shareholder vote and other shareholders may not have the ability to influence corporate transactions.
Currently, Dr. Steve N. Slilaty owns or controls, either directly or indirectly, approximately 48.3% of our outstanding voting securities. As a result, he essentially has the ability to determine the outcome on all matters requiring approval of our shareholders, including the election of directors and approval of significant corporate transactions.
If we are unable to attract and retain qualified scientific, technical and key management personnel, or if our key executive, Dr. Steve N. Slilaty, discontinues his employment with us, it may delay our research and development efforts.
We rely on the services of Dr. Slilaty for strategic and operational management, as well as for scientific and/or medical expertise in the development of our products. The loss of Dr. Slilaty would result in a significant negative impact on our ability to implement our business plan. We have not entered into an employment agreement with any member of our management, including Dr. Slilaty. In addition, we do not maintain “key person” life insurance covering Dr. Slilaty or any other executive officer. The loss of Dr. Slilaty will also significantly delay or prevent the achievement of our business objectives.
Our business will expose us to potential product liability risks and there can be no assurance that we will be able to acquire and maintain sufficient insurance to provide adequate coverage against potential liabilities.
Our business will expose us to potential product liability risks that are inherent in the testing, manufacturing and marketing of pharmaceutical products. The use of our product candidates in clinical trials also exposes us to the possibility of product liability claims and possible adverse publicity. These risks will increase to the extent our product candidates receive regulatory approval and are commercialized. We do not currently have any product liability insurance, although we plan to obtain product liability insurance in connection with future clinical trials of our product candidates. There can be no assurance that we will be able to obtain or maintain any such insurance on acceptable terms. Moreover, our product liability insurance may not provide adequate coverage against potential liabilities. On occasion, juries have awarded large judgments in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us would decrease our cash reserves and could cause our stock price to fall significantly.
We face regulation and risks related to hazardous materials and environmental laws, violations of which may subject us to claims for damages or fines that could materially affect our business, cash flows, financial condition and results of operations.
Our research and development activities involve the use of controlled and/or hazardous materials and chemicals. The risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of an accident, we could be held liable for any damages or fines that result, and the liability could have a material adverse effect on our business, financial condition and results of operations. We are also subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products. If we fail to comply with these laws and regulations or with the conditions attached to our operating licenses, the licenses could be revoked, and we could be subjected to criminal sanctions and substantial liability or be required to suspend or modify our operations. In addition, we may have to incur significant costs to comply with future environmental laws and regulations. We do not currently have a pollution and remediation insurance policy.
We do not have any agreements with any collaborators or third party manufacturers to manufacture our products. If and when we do reach an agreement with these parties, they may not be able to manufacture our product candidates, which would prevent us from commercializing our product candidates.
If any of our product candidates is approved by the FDA or other regulatory agencies for commercial sale, we will need third parties to manufacture the product in larger quantities. If we are able to reach an agreement with any collaborator or third party manufacturer in the future, of which there can be no assurance, due to factors beyond our control these collaborators and/or third party manufacturers may not be able to increase their manufacturing capacity for any of our product candidates in a timely or economic manner, or at all. Significant scale-up of manufacturing may require additional validation studies, which the FDA must review and approve. If we are unable to increase the manufacturing capacity for a product candidate successfully, the regulatory approval or commercial launch of that product candidate may be delayed or there may be a shortage in the supply of the product candidate. Our product candidates require precise, high-quality manufacturing. The failure of collaborators or third party manufacturers to achieve and maintain these high manufacturing standards, including the incidence of manufacturing errors, could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously harm our business.
5
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market any products we may develop, we may be unable to generate revenues.
We do not currently have product sales and marketing capabilities. If we receive regulatory approval to commence commercial sales of any of our product candidates, we will have to establish a sales and marketing organization with appropriate technical expertise and distribution capabilities or make arrangements with third parties to perform these services in other jurisdictions. If we receive approval to commercialize Adva-27a for the treatment of breast cancer indication, we intend to engage additional pharmaceutical or health care companies with existing distribution systems and direct sales organizations to assist us in North America and abroad. We may not be able to negotiate favorable distribution partnering arrangements, if at all. To the extent we enter into co-promotion or other licensing arrangements, any revenues we receive will depend on the efforts of third parties and will not be under our control. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, our ability to generate product revenues, and become profitable, would be severely limited.
Our ability to generate any significant revenues in the near-term is dependent entirely on the successful commercialization and market acceptance of Adva-27a. Factors that may inhibit our efforts to commercialize Adva-27a or other product candidates without strategic partners or licensees include:
|
●
|
difficulty recruiting and retaining adequate numbers of effective sales and marketing personnel;
|
|
|
●
|
the inability of sales personnel to obtain access to, or persuade adequate numbers of, physicians to prescribe our products;
|
|
|
●
|
the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage against companies with broader product lines; and
|
|
|
●
|
unforeseen costs associated with creating an independent sales and marketing organization.
|
Even if we successfully develop and obtain approval for Adva-27a, our business will not be profitable if this product does not achieve and maintain market acceptance.
Even if our product candidate, Adva-27a, is approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of our approved product candidate by physicians, healthcare professionals, patients and third-party payors, and our resulting profitability and growth, will depend on a number of factors, including:
|
●
|
our ability to provide acceptable evidence of safety and efficacy;
|
|
|
●
|
relative convenience and ease of administration;
|
|
|
●
|
the prevalence and severity of any adverse side effects;
|
|
|
●
|
the availability of alternative treatments;
|
|
|
●
|
the details of FDA labeling requirements, including the scope of approved indications and any safety warnings;
|
|
|
●
|
pricing and cost effectiveness;
|
|
|
●
|
the effectiveness of our or our collaborators' sales and marketing strategy;
|
|
|
●
|
our ability to obtain sufficient third-party insurance coverage or reimbursement; and
|
|
|
●
|
our ability to have the product listed on insurance company formularies.
|
If our product candidate achieves market acceptance, we may not be able to maintain that market acceptance over time if new products or technologies are introduced that are received more favorably or are more cost effective. Complications may also arise, such as development of new know-how or new medical or therapeutic capabilities by other parties that render our product obsolete.
Because the results of preclinical studies for our preclinical product candidates are not necessarily predictive of future results, our product candidates may not have favorable results in later clinical trials or ultimately receive regulatory approval.
Our product candidate has not been tested in clinical trials. Positive results from preclinical studies are no assurance that later clinical trials will succeed. Preclinical trials are not designed to establish the clinical efficacy of our preclinical product candidate. We will be required to demonstrate through clinical trials that our product candidate is safe and effective for use before we can seek regulatory approvals for commercial sale. There is typically an extremely high rate of failure as product candidates proceed through clinical trials. If our product candidate fails to demonstrate sufficient safety and efficacy in any clinical trial, we would experience potentially significant delays in, or be required to abandon, development of that product candidate. This would adversely affect our ability to generate revenues and may damage our reputation in the industry and in the investment community.
6
The future clinical testing of our product candidates could be delayed, resulting in increased costs to us and a delay in our ability to generate revenues.
Our product candidate will require preclinical testing and extensive clinical trials prior to submitting a regulatory application for commercial sales. We do not know whether clinical trials will begin on time, if at all. Delays in the commencement of clinical testing could significantly increase our product development costs and delay product commercialization. In addition, many of the factors that may cause, or lead to, a delay in the commencement of clinical trials may also ultimately lead to denial of regulatory approval of a product candidate. Each of these results would adversely affect our ability to generate revenues.
The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
|
●
|
demonstrating sufficient safety to obtain regulatory approval to commence a clinical trial;
|
|
|
●
|
reaching agreement on acceptable terms with prospective research organizations and trial sites;
|
|
|
●
|
manufacturing sufficient quantities of a product candidate;
|
|
|
●
|
obtaining institutional review board approvals to conduct clinical trials at prospective sites; and
|
|
|
●
|
procuring adequate financing to fund the work.
|
In addition, the commencement of clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease, and the eligibility criteria for the clinical trial. If we are unable to enroll a sufficient number of evaluable patients, the clinical trials for our product candidates could be delayed until sufficient numbers are achieved.
We will face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively.
We are a development stage company with three employees. Most of our competitors, such as Bristol-Myers Squibb, Pfizer, TEVA, Amgen, and others, are large pharmaceutical companies with substantially greater financial, technical and human resources than we have. The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. The drug that we are attempting to develop will compete with existing therapies if we receive marketing approval. Because of their significant resources, our competitors may be able to use discovery technologies and techniques, or partnerships with collaborators, in order to develop competing products that are more effective or less costly than the product candidate we are developing. This may render our technology or product candidate obsolete and noncompetitive. Academic institutions, government agencies, and other public and private research organizations may seek patent protection with respect to potentially competitive products or technologies and may establish exclusive collaborative or licensing relationships with our competitors.
As a company, we do not have any experience in conducting clinical trials for our Adva-27a development program. Our competitors may succeed in obtaining FDA or other regulatory approvals for product candidates more rapidly than us. Companies that complete clinical trials, obtain required regulatory agency approvals and commence commercial sale of their drugs before we do may achieve a significant competitive advantage, including certain FDA marketing exclusivity rights that would delay or prevent our ability to market certain products. Any approved drugs resulting from our research and development efforts, or from our joint efforts with our existing or future collaborative partners, might not be able to compete successfully with our competitors' existing or future products.
Because our product candidate and our development and collaboration efforts depend on our intellectual property rights, adverse events affecting our intellectual property rights will harm our ability to commercialize products.
Our success will depend to a large degree on our own and our licensors' ability to obtain and defend patents for each party's respective technologies and the compounds and other products, if any, resulting from the application of such technologies. The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and technical questions. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date. Accordingly, we cannot predict the breadth of claims that will be allowed or maintained, after challenge, in our or other companies' patents.
7
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
|
●
|
we were the first to make the inventions covered by each of our pending patent applications;
|
|
|
●
|
we were the first to file patent applications for these inventions;
|
|
|
●
|
others will not independently develop similar or alternative technologies or duplicate any of our technologies;
|
|
|
●
|
any patents issued to us or our collaborators will provide a basis for commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties;
|
|
|
●
|
our pending patent applications will result in issued patents;
|
|
|
●
|
we will develop additional proprietary technologies that are patentable; or
|
|
|
●
|
the patents of others will not have a negative effect on our ability to do business.
|
If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborations, then our ability to receive patent protection or protect our proprietary information will be impaired. In addition, some of the technology we have licensed relies on patented inventions developed using U.S. and other governments’ resources. Under applicable law, the U.S. government has the right to require us to grant a nonexclusive, partially exclusive or exclusive license for such technology to a responsible applicant or applicants, upon terms that are reasonable under the circumstances, if the government determines that such action is necessary.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information and may not adequately protect our intellectual property.
We rely on trade secrets to protect our technology, particularly when we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. In order to protect our proprietary technology and processes, we rely in part on confidentiality and intellectual property assignment agreements with our employees, consultants, outside scientific collaborators and sponsored researchers and other advisors. These agreements may not effectively prevent disclosure of confidential information nor result in the effective assignment to us of intellectual property, and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information or other breaches of the agreements. In addition, others may independently discover our trade secrets and proprietary information, and in such case we could not assert any trade secret rights against such party. Enforcing a claim that a party illegally obtained and is using our trade secrets is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. Costly and time-consuming litigation could be necessary to seek to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
The implementation of our business plan will result in a period of rapid growth that will impose a significant burden on our current administrative and operational resources.
Our ability to effectively manage our growth will require us to substantially expand the capabilities of our administrative and operational resources by attracting, training, managing and retaining additional qualified personnel, including additional members of management, technicians and others. To successfully develop our products we will need to manage operating, producing, marketing and selling our products. There can be no assurances that we will be able to do so. Our failure to successfully manage our growth will have a negative impact on our anticipated results of operations.
The requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain executive management and qualified board members.
As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Act, and other applicable securities rules and regulations. Compliance with these rules and regulations increases our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an “emerging growth company,” as defined in the Jumpstart our Business Startups Act, or the JOBS Act. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to maintain and, if required, improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight may be required. As a result, management’s attention may be diverted from other business concerns which could adversely affect our business and operating results. We may need to hire more employees in the future or engage outside consultants which will increase our costs and expenses.
8
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and our business may be adversely affected.
However, for as long as we remain an “emerging growth company,” we may take advantage of certain exemptions from various reporting requirements that are applicable to public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.”
We would cease to be an “emerging growth company” upon the earliest of: (i) the first fiscal year following the fifth anniversary of our becoming a reporting company, (ii) the first fiscal year after our annual gross revenues are $1.0 billion or more, (iii) the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt securities, or (iv) as of the end of any fiscal year in which the market value of our Common Stock held by non-affiliates exceeded $700 million as of the end of the second quarter of that fiscal year.
We also expect that being a public company and these new rules and regulations will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our Board of Directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
As a result of disclosure of information in this Prospectus and in future filings required of a public company, our business and financial condition will become more visible, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business and operating results could be adversely affected, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and adversely affect our business and operating results.
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our Common Stock less attractive because we may rely on these exemptions. If some investors find our Common Stock less attractive as a result, there may be a less active trading market for our Common Stock and our stock price may be more volatile.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
9
Risks Related to our Common Stock
There is a limited trading market for our securities and there can be no assurance that such a market will develop in the future.
There is no assurance that a market will develop in the future or, if developed, that it will continue. In the absence of a public trading market, an investor may be unable to liquidate his investment in our Company.
We do not have significant financial reporting experience, which may lead to delays in filing required reports with the Securities and Exchange Commission and suspension of quotation of our securities on the OTCQM or a national exchange, which will make it more difficult for you to sell your securities.
The OTCQM and other national stock exchanges each limits quotations to securities of issuers that are current in their reports filed with the Securities and Exchange Commission. Because we do not have significant financial reporting experience, we may experience delays in filing required reporting with the Securities and Exchange Commission (the “SEC”). Because issuers whose securities are qualified for quotation on the OTCQM or any other national exchange are required to file these reports with the SEC in a timely manner, the failure to do so may result in a suspension of trading or delisting.
There are no automated systems for negotiating trades on the OTCQM and it is possible for the price of a stock to go up or down significantly during a lapse of time between placing a market order and its execution, which may affect your trades in our securities.
Because there are no automated systems for negotiating trades on the OTCBB, they are conducted via telephone. In times of heavy market volume, the limitations of this process may result in a significant increase in the time it takes to execute investor orders. Therefore, when investors place market orders, an order to buy or sell a specific number of shares at the current market price, it is possible for the price of a stock to go up or down significantly during the lapse of time between placing a market order and its execution.
Our stock will be considered a “penny stock” so long as it trades below $5.00 per share. This can adversely affect its liquidity.
Our Common Stock is currently considered a “penny stock” and will continue to be considered a penny stock so long as it trades below $5.00 per share and as such, trading in our Common Stock will be subject to the requirements of Rule 15g-9 under the Securities Exchange Act of 1934. Under this rule, broker/dealers who recommend low-priced securities to persons other than established customers and accredited investors must satisfy special sales practice requirements. The broker/dealer must make an individualized written suitability determination for the purchaser and receive the purchaser’s written consent prior to the transaction.
SEC regulations also require additional disclosure in connection with any trades involving a “penny stock,” including the delivery, prior to any penny stock transaction, of a disclosure schedule explaining the penny stock market and its associated risks. In addition, broker-dealers must disclose commissions payable to both the broker-dealer and the registered representative and current quotations for the securities they offer. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from recommending transactions in our securities, which could severely limit the liquidity of our securities and consequently adversely affect the market price for our securities. In addition, few broker or dealers are likely to undertake these compliance activities. Other risks associated with trading in penny stocks could also be price fluctuations and the lack of a liquid market.
We do not anticipate payment of dividends, and investors will be wholly dependent upon the market for the Common Stock to realize economic benefit from their investment.
As holders of our Common Stock, you will only be entitled to receive those dividends that are declared by our Board of Directors out of retained earnings. We do not expect to have retained earnings available for declaration of dividends in the foreseeable future. There is no assurance that such retained earnings will ever materialize to permit payment of dividends to you. Our Board of Directors will determine future dividend policy based upon our results of operations, financial condition, capital requirements, reserve needs and other circumstances.
10
Any adverse effect on the market price of our Common Stock could make it difficult for us to raise additional capital through sales of equity securities at a time and at a price that we deem appropriate.
Sales of substantial amounts of our Common Stock, or in anticipation that such sales could occur, may materially and adversely affect prevailing market prices for our Common Stock.
The market price of our Common Stock may fluctuate significantly in the future.
The market price of our Common Stock may fluctuate in response to one or more of the following factors, many of which are beyond our control:
|
●
|
competitive pricing pressures;
|
|
|
●
|
our ability to produce and sell our products on a cost-effective and timely basis;
|
|
|
●
|
our inability to obtain working capital financing;
|
|
|
●
|
the introduction and announcement of one or more new alternatives to our products by our competitors;
|
|
|
●
|
changing conditions in the market;
|
|
|
●
|
changes in market valuations of similar companies;
|
|
|
●
|
stock market price and volume fluctuations generally;
|
|
|
●
|
regulatory developments;
|
|
|
●
|
fluctuations in our quarterly or annual operating results;
|
|
|
●
|
additions or departures of key personnel; and
|
|
|
●
|
future sales of our Common Stock or other securities.
|
The price at which you purchase shares of our Common Stock may not be indicative of the price that will prevail in the trading market. You may be unable to sell your shares of Common Stock at or above your purchase price, which may result in substantial losses to you and which may include the complete loss of your investment. In the past, securities class action litigation has often been brought against a company following periods of stock price volatility. We may be the target of similar litigation in the future. Securities litigation could result in substantial costs and divert management’s attention and our resources away from our business. Any of the risks described above could adversely affect our sales and profitability and also the price of our Common Stock.
We are registering the resale of 13,400,000 shares of common stock. Of these shares, 13,000,000 which may be issued to Dutchess under the Equity Line. The resale of such shares by Dutchess could depress the market price of our common stock and you may not be able to sell your investment for what you paid for it.
We are registering the resale of 13,400,000 shares of common stock under the registration statement of which this prospectus forms a part. We may issue up to 13,000,000 shares to Dutchess pursuant to the Equity Line. The sale of these shares into the public market by Dutchess could depress the market price of our common stock and you may not be able to sell your investment for what you paid for it.
Dutchess will pay less than the then-prevailing market price for our common stock under the Equity Line.
The common stock to be issued to Dutchess pursuant to the Investment Agreement will be purchased at a 10% discount to the lowest daily volume weighted average price, VWAP, of our common stock during the five consecutive trading day period preceding the date of delivery of a put notice by us to Dutchess, subject to certain exceptions. Dutchess has a financial incentive to sell our common stock upon receiving the shares to realize the profit equal to the difference between the discounted price and the market price. If Dutchess sells the shares, the price of our common stock could decrease.
Any draw downs under our Equity Line with Dutchess may result in dilution to our stockholders.
If we sell shares to Dutchess under the Equity Line, it will have a dilutive effect on the holdings of our current stockholders, and may result in downward pressure on the price of our common stock. If we draw down amounts under the Equity Line, we will issue shares to Dutchess at a 10% discount to the lowest daily volume weighted average price, VWAP, of our common stock during the five consecutive trading day period preceding the date of delivery of a put notice by us to Dutchess, subject to certain exceptions. If we draw down amounts under the Equity Line when our share price is decreasing, we will need to issue more shares to raise the same amount than if our stock price was higher. Issuances in the face of a declining share price will have an even greater dilutive effect than if our share price were stable or increasing, and may further decrease our share price.
Our Equity Line with Dutchess may not be available to us if we elect to make a draw down.
Our ability to put shares to Dutchess and obtain funds under the Equity Line is limited by the terms and conditions in the Investment Agreement, including restrictions on when we may exercise our put rights, restrictions on the amount we may put to Dutchess at any one time, which is determined in part by the trading volume of our common stock, a limitation on Dutchess’s obligation to purchase if such purchase would result in Dutchess beneficially owning more than 4.99% of our common stock or if the price of our common stock is lower than $0.10 per share. Accordingly, the Equity Line may not be available to satisfy all of our funding needs.
We cannot predict whether we will successfully effectuate our current business plan.
Each prospective purchaser is encouraged to carefully analyze the risks and merits of an investment in our Common Stock and should take into consideration when making such analysis, among others, the Risk Factors discussed above.
11
We have made statements in this Prospectus, including under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Description of Business” and elsewhere that constitute forward-looking statements. Forward-looking statements involve risks and uncertainties, such as statements about our plans, objectives, expectations, assumptions or future events. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “we believe,” “we intend,” “may,” “should,” “will,” “could” and similar expressions denoting uncertainty or an action that may, will or is expected to occur in the future. These statements involve estimates, assumptions, known and unknown risks, uncertainties and other factors that could cause actual results to differ materially from any future results, performances or achievements expressed or implied by the forward-looking statements.
Examples of forward-looking statements include:
|
●
|
the timing of the development of future products;
|
|
●
|
projections of costs, revenue, earnings, capital structure and other financial items;
|
|
●
|
statements of our plans and objectives;
|
|
●
|
statements regarding the capabilities of our business operations;
|
|
●
|
statements of expected future economic performance;
|
|
●
|
statements regarding competition in our market; and
|
|
●
|
assumptions underlying statements regarding us or our business.
|
The ultimate correctness of these forward-looking statements depends upon a number of known and unknown risks and events. We discuss our known material risks under the heading “Risk Factors” above. Many factors could cause our actual results to differ materially from the forward-looking statements. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
The forward-looking statements speak only as of the date on which they are made, and, except as required by law, we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events.
You should also assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business, financial condition, results of operations and prospects may have changed since that date.
We will not receive any proceeds from the resale of our common stock offered by Dutchess, the Selling Stockholder. We will, however, receive proceeds from the sale of our common stock to Dutchess pursuant to the Investment Agreement. We anticipate the proceeds from our exercise of the put option pursuant to the Investment Agreement will be used for the clinical development of our cancer drug, Adva-27amore fully described below, including (i) GMP Manufacturing; (ii) IND-Enabling Studies; (iii) Regulatory Filings; (iv) Phase I Clinical Trials; and (v) working capital and other general corporate purposes.
The offering price of the securities offered by Dutchess, the Selling Stockholder, will be determined by the prevailing market price for the shares at the time of sale or negotiated transactions.
12
Investment Agreement
We entered into the Investment Agreement with Dutchess on April 23, 2014. Pursuant to the Investment Agreement, Dutchess committed to purchase, subject to certain restrictions and conditions, up to that number of shares of the Company’s common stock having an aggregate purchase price of two million five hundred thousand dollars ($2,500,000), over a period of 36 months from the first trading day following the effectiveness of the registration statement of which this prospectus forms a part. We may terminate the Investment Agreement at any time, at our discretion, without any cost to us, upon notice to Dutchess.
We may draw funds from the Equity Line facility by selling shares of common stock to Dutchess from time to time, as and when we determine appropriate in accordance with the terms and conditions of the Investment Agreement, by our issuance of a put notice (the “Put Notice”) to Dutchess. The purchase price of the shares shall be ninety percent (90%) of the lowest daily volume weighted average price (VWAP) of our Common Stock during the five (5) consecutive trading day period immediately preceding the date of delivery of the applicable draw down notice. We refer to such five-day period as the “Pricing Period.”
Dutchess has no right to require any sales by us, but is obligated to make purchases from us as we direct in accordance with the Investment Agreement. There are no limitations on use of proceeds, financial or business covenants, restrictions on future funding, rights of first refusal, participation rights, penalties or liquidated damages in the Investment Agreement.
The maximum amount of each Put Notice is limited to maximum $100,000 and we may only issue a Put Notice ten (10) Trading Days after each prior Put Notice Date. During such time, we are not entitled to deliver another draw down notice. Further, if the price of our Common Stock falls below $0.10 per share, we are not entitled to deliver a Put Notice to Dutchess.
Certain conditions must be satisfied before we are entitled to put shares to Dutchess, including the following:
|
●
|
there must be an effective registration statement under the Securities Act to cover the resale of the shares by Dutchess;
|
|
●
|
our common stock cannot be suspended from trading or the Company shall not have been notified of any pending or threatened proceeding or other action to suspend the trading of the common stock;
|
|
●
|
we must have complied with our obligations and not otherwise be in default under the Investment Agreement and Registration Rights Agreement;
|
|
●
|
no injunction or other governmental action shall remain in force which prohibits the issuance of shares to Dutchess pursuant to the Equity Line; and
|
| ● | the share price of our common stock must be at or above $0.10 |
There is no guarantee that we will be able to meet the foregoing conditions or any other conditions under the Investment Agreement or that we will be able to draw down any portion of the amount available to us under the Equity Line.
The Investment Agreement further provides that we and Dutchess are each entitled to customary indemnification from the other for any losses or liabilities we or it suffers as a result of any breach by the other party of any provisions of the Investment Agreement or the Registration Rights Agreement, or as a result of any lawsuit brought by a third-party arising out of or resulting from the other party’s execution, delivery, performance or enforcement of the Investment Agreement or the Registration Rights Agreement.
The Investment Agreement also contains customary representations and warranties of each of the parties. The assertions embodied in those representations and warranties were made for purposes of the Investment Agreement and are subject to qualifications and limitations agreed to by the parties in connection with negotiating the terms of the Investment Agreement. In addition, certain representations and warranties were made as of a specific date, may be subject to a contractual standard of materiality different from what a stockholder or investor might view as material, or may have been used for purposes of allocating risk between the respective parties rather than establishing matters as facts.
Dutchess has also agreed pursuant to the Investment Agreement not to sell short any of our securities, either directly or indirectly through its affiliates, principals or advisors during the term of the Investment Agreement.
In connection with the preparation of the Investment Agreement and the Registration Rights Agreement, we paid Dutchess an engagement fee of 400,000 shares of our Common Stock, which is included in the 13,400,000 shares being registered in our registration statement.
13
Registration Rights Agreement
Pursuant to the terms of the Registration Rights Agreement, we are obligated to file one or more registration statements with the SEC to register the resale by Dutchess of shares of common stock issued or issuable under the Investment Agreement. We have filed with the SEC an initial registration statement of which this prospectus forms a part, in order to access the Equity Line, covering the resale of up to 13,400,000 shares of common stock. In addition, we are obligated to use all commercially reasonable efforts to have the registration statement remain effective by the SEC as provided for in the Investment Agreement.
The foregoing summary of the Investment Agreement with Dutchess does not purport to be complete and is qualified by reference to the Investment Agreement and the Registration Rights Agreement, copies of which have been filed as exhibits to the registration statement of which this prospectus is a part.
Effect of Performance of the Investment Agreement on Our Stockholders
All 13,400,000 shares of common stock that are registered in this offering which have been issued to Dutchess or which may be sold by us to Dutchess under the Investment Agreement are expected to be freely tradable. It is anticipated that shares registered in this offering will be sold over a period of up to 36 months from the date of this prospectus. The sale by Dutchess of a significant amount of shares registered in this offering at any given time could cause the market price of our common stock to decline and to be highly volatile. Dutchess may ultimately acquire all, some or none of the shares of common stock not issued but registered in this offering. After it has acquired such shares, it may sell all, some or none of such shares.
If and to the extent we issue common stock to Dutchess at a lower price per share, Dutchess will receive a higher number of shares, which equates to greater dilution to our other stockholders. The effect of this dilution may, in turn, cause the price of our common stock to decrease further because of the downward pressure on the stock price that would be caused by a large number of sales of our shares into the public market by Dutchess. Additionally, if certain of our existing stockholders disagree with our decision to sell shares to Dutchess at a time when our stock price is low, those stockholders may in response decide to sell additional shares of common stock, which could further decrease our stock price. Therefore, sales to Dutchess by us under the Investment Agreement may result in substantial dilution to the interests of other stockholders and a decrease in our stock price. However, we have the right to control the timing and amount of any sales of our shares to Dutchess and the Investment Agreement may be terminated by us at any time at our discretion without any cost to us.
In connection with entering into the Investment Agreement, we authorized the sale to Dutchess of up to $2,500,000 of our common stock. The number of shares ultimately offered for sale by Dutchess under this prospectus is dependent upon the number of shares purchased by Dutchess under the Investment Agreement. In the event we elect to issue more than the 13,400,000 shares offered hereby, we will be required to file a new registration statement and have it declared effective by the SEC. The following table sets forth the amount of proceeds we would receive from Dutchess from the sale of shares at varying purchase prices:
|
Assumed Average
Purchase Price
($)
|
Number of Registered
Shares to be Issued
if Full Purchase (1)(2)
|
Percentage of
Outstanding Shares
After Giving Effect
to the Issuance
to Dutchess (3)
|
Proceeds
from the Sale of
Registered Shares
to Dutchess Under the
Investment Agreement
|
|||
|
$0.09
|
13,000,000
|
16.5%
|
$1,170,000
|
|||
|
$0.10
|
13,000,000
|
16.5%
|
1,300,000
|
|||
|
$0.15(4)
|
13,000,000
|
16.5%
|
1,950,000
|
|||
|
$0.20
|
12,500,000
|
16%
|
2,500,000
|
|||
|
$0.25
|
10,000,000
|
13.2%
|
2,500,000
|
|||
|
$0.50
|
5,000,000
|
7%
|
2,500,000
|
|||
|
$1.00
|
2,500,000
|
3.7%
|
2,500,000
|
———————
|
(1)
|
Although the Investment Agreement provides that we may sell up to $2,500,000 of our common stock to Dutchess, we are only registering 13,400,000 shares, including 13,000,000 shares to be purchased thereunder, which may or may not cover all such shares purchased by them under the Investment Agreement, depending on the purchase price per share. As a result, we have included in this column only those shares which are registered in this offering.
|
|
(2)
|
The number of registered shares to be issued represents the number of shares to be purchased at the applicable price.
|
|
(3)
|
The denominator is based on 65,775,728 shares outstanding plus the corresponding number of shares set forth in the adjacent column. The numerator is based on the number of shares issuable under the Investment Agreement at the corresponding assumed purchase price set forth in the adjacent column.
|
|
(4)
|
The closing price of our common stock on May 20, 2014.
|
14
The shares of common stock being offered by Dutchess, the selling stockholder, are those to be issued to Dutchess under the Investment Agreement. We are registering the shares of common stock in order to permit Dutchess to offer the shares for resale from time to time. Dutchess is not a licensed broker-dealer or an affiliate of a licensed broker-dealer. Neither Dutchess nor any of its affiliates has held a position or office, or had any other material relationship, with us within the past three years.
We do not know when or in what amounts Dutchess may offer shares for sale. Dutchess may elect not to sell any or all of the shares offered by this prospectus. Because Dutchess may offer all or some of the shares, and because there are currently no agreements, arrangements or understandings with respect to the sale of any of the shares, we cannot estimate the number of the shares that will be held by Dutchess after completion of the offering. However, for purposes of this table, we have assumed that, after completion of the offering, all of the shares covered by this prospectus will be sold by Dutchess.
The following table presents information regarding Dutchess. The information concerning beneficial ownership has been taken from information provided to us by Dutchess. Beneficial ownership has been calculated in accordance with the rules of the SEC, which generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include shares of common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable within 60 days.
|
Selling Stockholder
|
Shares Beneficially
Owned Before
Offering
|
Percentage of
Outstanding Shares
Beneficially Owned
Before Offering
|
Shares to be Sold
in the Offering
Assuming the
Company Issues
Maximum No. of
Shares in the
Offering
|
Percentage of
Outstanding Shares
Beneficially Owned
After Offering
|
||||||||||||
|
Dutchess Opportunity Fund II, LP(1)
|
400,000 | 0.6% | 13,400,000 | (2) | 0.6 | % | ||||||||||
———————
|
(1)
|
The address of Dutchess is 50 Commonwealth Avenue, Suite 2, Boston, MA 02116. Dutchess is a Delaware limited partnership. Michael Novielli and Douglas H. Leighton are the managing members of Dutchess Capital Management, II, LLC, the general partner to Dutchess, which has the voting and investment power over the shares being offered under this prospectus.
|
|
(2)
|
Although the Investment Agreement provides what we may sell up to $2,500,000 of our common stock to Dutchess, we are only registering 13,400,000 shares issuable under the Investment Agreement pursuant to the registration statement of which this prospectus is a part. If we elect to issue more than the 13,400,000 shares offered by this prospectus, which we have the right but not the obligation to do, we must first register under the Securities Act any additional shares we may elect to sell to Dutchess before we can sell such additional shares.
|
The common stock offered by this prospectus is being offered by Dutchess, the selling stockholder. The common stock may be sold or distributed from time to time by the selling stockholder directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of the common stock offered by this prospectus may be effected in one or more of the following methods:
|
●
|
ordinary brokers’ transactions;
|
|
●
|
transactions involving cross or block trades;
|
|
●
|
through brokers, dealers, or underwriters who may act solely as agents;
|
|
●
|
“at the market” into an existing market for the common stock;
|
|
●
|
in other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents;
|
|
●
|
in privately negotiated transactions;
|
|
●
|
any combination of the foregoing; or
|
|
●
|
any other method permitted pursuant to applicable law.
|
The Selling Stockholder may also sell shares under Rule 144 under the Securities Act of 1933, if available, rather than under this prospectus.
15
In order to comply with the securities laws of certain states, if applicable, the shares may be sold only through registered or licensed brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the registration or qualification requirement is available and complied with.
Brokers, dealers, underwriters, or agents participating in the distribution of the shares as agents may receive compensation in the form of commissions, discounts, or concessions from the selling stockholder and/or purchasers of the common stock for whom the broker-dealers may act as agent. The compensation paid to a particular broker-dealer may be less than or in excess of customary commissions.
Dutchess is an “underwriter” within the meaning of the Securities Act.
Neither we nor Dutchess can presently estimate the amount of compensation that any agent will receive. We know of no existing arrangements between Dutchess, any other stockholder, broker, dealer, underwriter, or agent relating to the sale or distribution of the shares offered by this prospectus. At the time a particular offer of shares is made, a prospectus supplement, if required, will be distributed that will set forth the names of any agents, underwriters, or dealers and any compensation from the selling stockholder, and any other required information.
We will pay all of the expenses incident to the registration, offering, and sale of the shares to the public other than commissions or discounts of underwriters, broker-dealers, or agents. We have also agreed to indemnify Dutchess and related persons against specified liabilities, including liabilities under the Securities Act.
Dutchess and its affiliates have agreed not to engage in any direct or indirect short selling of our common stock during the term of the Investment Agreement.
While Dutchess is engaged in a distribution of the shares included in this prospectus, Dutchess is required to comply with Regulation M promulgated under the Exchange Act, and it is aware of its compliance obligations pursuant to Regulation M. With certain exceptions, Regulation M precludes the selling stockholder, any affiliated purchasers, and any broker-dealer or other person who participates in the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the subject of the distribution until the entire distribution is complete.
Regulation M also prohibits any bids or purchases made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability of the shares offered pursuant to this prospectus. This offering will terminate on the date that all shares offered by this prospectus have been sold by Dutchess or may be resold by Dutchess without restriction under Rule 144(b)(1)(i) under the Securities Act.
General
Our authorized capital stock consists of 205,000,000 shares, consisting of 200,000,000 shares of common stock, par value $.001 per share; and 5,000,000 shares of preferred stock (the "Preferred Stock"), par value $.10 per share. The following description of our capital stock is intended as a summary only and is qualified in its entirety by reference to our articles of incorporation, as amended and our bylaws, which have been filed previously with the SEC.
As of May 21, 2014, there were 65,775,728 shares of common stock outstanding held of record by 145 persons. No shares of preferred stock are currently outstanding.
Common Stock
Holders of our common stock are entitled to one vote for each share held of record on all matters to be voted on by stockholders. There is no cumulative voting with respect to the election of directors, with the result that directors will be elected by a plurality of the votes cast. Our stockholders have no conversion, preemptive or other subscription rights and there are no sinking fund or redemption provisions applicable to the common stock.
Holders of our common stock are entitled to receive dividends when, as and if declared by our board of directors out of funds legally available for this purpose. In the event of our liquidation, dissolution or winding up, holders of our common stock are entitled to receive on a proportional basis any assets remaining available for distribution after payment of our liabilities. All of the outstanding shares of our common stock are fully paid and non-assessable.
Preferred Stock
Our Articles of Incorporation provides that our board of directors may, by resolution, establish one or more classes or series of preferred stock having the number of shares and relative voting rights, designations, dividend rates, liquidation, and other rights, preferences, and limitations as may be fixed by them without further stockholder approval. The holders of our preferred stock may be entitled to preferences over common stockholders with respect to dividends, liquidation, dissolution, or our winding up in such amounts as are established by the resolutions of our board of directors approving the issuance of such shares.
16
The issuance of our preferred stock may have the effect of delaying, deferring or preventing a change in control of us without further action by the holders and may adversely affect voting and other rights of holders of our common stock. In addition, issuance of preferred stock, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, could make it more difficult for a third party to acquire a majority of the outstanding shares of voting stock. As of the date of this prospectus, no shares of our preferred stock have been issued and we have no plans to issue any shares of preferred stock.
We have no outstanding options or warrants to purchase any of our securities.
Registration Rights
We have entered into a registration rights agreement with Dutchess. Please see “The Dutchess Equity Line Transaction” on page 18 of this prospectus.
Transfer Agent
The transfer agent for our common stock is Corporate Stock Transfer, Inc. of Denver, Colorado. Their address is 3200 Cherry Creek South Drive, Suite 430, Denver, Colorado, 80209. Their phone number is (303) 282-4800.
DESCRIPTION OF BUSINESS
History
We were incorporated in the State of Colorado on August 31, 2006 under the name “Mountain West Business Solutions, Inc.” Until October 2009 our business was to provide management consulting with regard to accounting, computer and general business issues for small and home-office based companies.
Effective October 15, 2009, we executed an agreement to acquire Sunshine Biopharma, Inc., a Colorado corporation, in exchange for the issuance of 21,962,000 shares of our Common Stock and 850,000 shares of Convertible Preferred Stock, each convertible into twenty (20) shares of our Common Stock (the “Agreement”). As a result of this transaction we changed our name to “Sunshine Biopharma, Inc.” On December 21, 2011, Advanomics Corporation, a privately held Canadian company (“Advanomics”), and our licensor, exercised its right to convert the 850,000 shares of Series “A” Preferred Stock it held in our Company into 17,000,000 shares of Common Stock.
Description of Current Business
We are currently a pharmaceutical company focused on the research, development and commercialization of drugs for the treatment of various forms of cancer. The preclinical studies for our lead compound, Adva-27a, a multi-purpose antitumor compound, were successfully completed in late 2011. We are now continuing our clinical development of Adva-27a by conducting the next sequence of steps comprised of Good Manufacturing Practice (“GMP”) manufacturing, Investigational New Drug (“IND”)-enabling studies, regulatory filing and Phase I clinical trials. We plan to conduct our Phase I clinical trials for Adva-27a at the Jewish General Hospital, Montreal, Canada, one of McGill University’s Hospital Centers. The planned indication will be pancreatic cancer in parallel to multidrug resistant breast cancer as Adva-27a has shown a positive effect on both of these cancer types for which there is currently little or no treatment options available. See “Clinical Trials” below.
We have licensed our technology on an exclusive basis from Advanomics Corporation, and we are planning to initiate our own research and development program as soon as practicable once financing is in place. There are no assurances that we will obtain the financing necessary to allow us to implement this aspect of our business plan, or to enter clinical trials. See “Management's Discussion and Analysis of Financial Condition-Liquidity and Capital Resources.”
Carbon-Difluoride Platform Technology
Many therapeutically important compounds contain diester bonds that link different parts of the molecule together. Diester bonds are naturally unstable often leading to suboptimal performance when the molecule is administered to patients. Diester bonds have specific three-dimensional, as well as electrostatic properties that cannot be easily mimicked by other bonds. Bonds that do not mimic the diester bond correctly invariably render the compound inactive. In collaboration with Institut National des Sciences Appliquées de Rouen in France (“INSA”), Advanomics Corporation has developed a way to replace the diester bond with a Carbon-Difluoride bond which acts as a diester isostere. An isostere is a different chemical structure that mimics the properties of the original. In the body, Carbon-Difluoride compounds are resistant to metabolic degradation but recognized similarly to the diester compounds (see Figure 1).
17
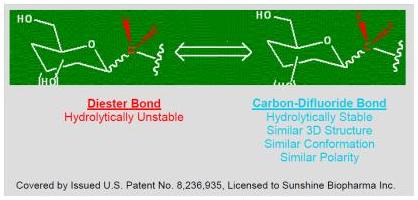
Figure 1
While no assurances can be provided, we are planning to expand our product line through acquisitions and/or in-licensing as well as in-house research and development.
Our Lead Compound (Adva-27a)
Our initial drug candidate is Adva-27a, a GEM-difluorinated C-glycoside derivative of Podophyllotoxin, targeted for various forms of cancer. If we are successful in our current financing efforts, Adva-27a is expected to enter Phase I clinical trials for pancreatic cancer and multidrug resistant breast cancer in mid to late 2015 (see “Clinical Development Path” and “Clinical Trials” below). Etoposide, which is also a derivative of Podophyllotoxin, is currently on the market and is used to treat various types of cancer including leukemia, lymphoma, testicular cancer, lung cancer, brain cancer, prostate cancer, bladder cancer, colon cancer, ovarian cancer, liver cancer and several other forms of cancer. Like Etoposide, Adva-27a is a Topoisomerase II inhibitor; however, unlike Etoposide and other anti-tumor drugs currently in use, Adva-27a is able to destroy multidrug resistant cancer cells. Adva-27a is a new chemical entity and has been shown to have distinct and more desirable biological properties compared to Etoposide. Most notably, Adva-27a is very effective against multidrug resistant breast cancer cells while Etoposide has no activity against this aggressive form of cancer (see Figure 2). In other side-by-side studies against Etoposide as a reference, Adva-27a showed markedly improved cell killing activity in various other cancer types, particularly prostate, colon and lung cancer (see Table 1). Our preclinical studies to date have shown that:
| ● | Adva-27a is effective at killing different types of multidrug resistant cancer cells, including: | |
| - |
Breast Cancer Cells (MCF-7/MDR)
|
|
| - |
Small Cell Lung Cancer Cells (H69AR)
|
|
| - |
Uterine Cancer (MES-SA/Dx5)
|
|
| - |
Pancreatic Cancer (Panc-1)
|
|
| ● |
Adva-27a is unaffected by P-Glycoprotein, the enzyme responsible for making cancer cells resistant to anti-tumor drugs.
|
|
| ● |
Adva-27a has excellent clearance time (half-life = 54 minutes) as indicated by human microsomes stability studies and pharmacokinetics data in rats.
|
|
| ● |
Adva-27a clearance is independent of Cytochrome P450, a mechanism that is less likely to produce toxic intermediates.
|
|
| ● |
Adva-27a is an excellent inhibitor of Topoisomerase II with an IC50 of only 13.7 micromolar (this number has recently been reduce to 1.44 micromolar as a result of resolving the two isomeric forms of Adva-27a).
|
|
| ● |
Adva-27a has shown excellent pharmacokinetics profile as indicated by studies done in rats.
|
|
| ● |
Adva-27a does not inhibit tubulin assembly.
|
|
These and other preclinical data have recently been published in ANTICANCER RESEARCH, a peer-reviewed International Journal of Cancer Research and Treatment. The manuscript entitled “Adva-27a, a Novel Podophyllotoxin Derivative Found to Be Effective Against Multidrug Resistant Human Cancer Cells” appeared in print in the October 2012 issue of the journal [ANTICANCER RESEARCH 32: 4423-4432 (2012)]. A copy of the full manuscript as it appeared in the journal is available on our website atwww.sunshinebiopharma.com.
18
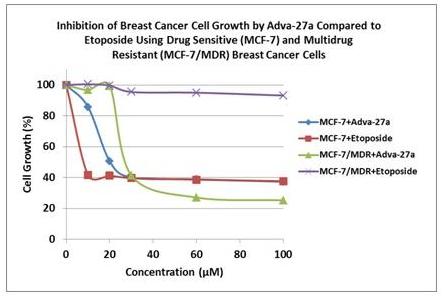
Figure 2

Table 1
Clinical Development Path
The early stage preclinical studies for our lead compound, Adva-27a, were successfully completed in late 2011 and the results have recently been published [ANTICANCER RESEARCH 32: 4423-4432 (2012)]. We are now continuing our clinical development program of Adva-27a by conducting the next sequence of steps comprised of the following:
|
●
|
GMP Manufacturing of 1 kilogram for use in IND-Enabling Studies and Phase I Clinical Trials
|
|
●
|
IND-Enabling Studies
|
|
●
|
Regulatory Filing (Fast-Track Status Anticipated)
|
|
●
|
Phase I Clinical Trials (Multidrug Resistant Breast Cancer Indication)
|
Clinical Trials
Adva-27a’s initial indication will be Pancreatic cancer and multidrug resistant breast cancer for which there are currently little or no treatment options. In June 2011 we concluded an agreement with McGill University’s Jewish General Hospital in Montreal, Canada to conduct Phase I clinical trials for this indication. All aspects of the planned clinical trials in Canada will employ U.S. Food and Drug Administration (“FDA”) standards at all levels. We anticipate that the clinical trials will be completed by mid2016, at which time we, together with our licensor, expect to file for limited marketing approval with the regulatory authorities in Canada and the FDA in the U.S. See “Marketing,” below.
Marketing
According to the American Cancer Society, nearly 1.5 million new cases of cancer are diagnosed in the U.S. each year. Given the terminal and limited treatment options available for the multidrug resistant breast cancer indication we are planning to study, we anticipate being granted limited marketing approval (“compassionate-use”) for our Adva-27a following receipt of funding and a successful Phase I clinical trial. There are no assurances that either will occur. Such limited approval will allow us to make the drug available to various hospitals and health care centers for experimental therapy and/or “compassionate-use”, thereby generating some revenues in the near-term.
19
We believe that upon successful completion of Phase I Clinical Trials we may receive one or more offers from large pharmaceutical companies to buyout or license our drug. However, there are no assurances that our Phase I Trials will be successful, or if successful, that any pharmaceutical companies will make an acceptable offer to us. In the event we do not consummate such a transaction, we will require significant capital in order to manufacture and market our new drug.
Intellectual Property
We are the exclusive licensee for the U.S. territory of Advanomics Corporation’s Adva-27a which is covered by international patent applications filed on April 27, 2007 (PCT/FR2007/000697). These patent applications, which are now issued in Europe and the United States (US 8,236,935) and are still pending elsewhere around the world, were originally owned by Institut National des Sciences Appliquées de Rouen (France) and have recently been purchased by Advanomics Corporation. On January 14, 2013, Advanomics Corporation filed a new patent application covering Adva-27a manufacturing processes as well as new Adva-27a derivatives and compositions.
Our Lead Anti-Cancer Compound, Adva-27a, in 3D
Government Regulations
Our existing and proposed business operations are subject to extensive and frequently changing federal, state, provincial and local laws and regulations. We will be subject to significant regulations in the U.S. in order to obtain the approval of the FDA to offer our product on the market. The approximate procedure for obtaining FDA approval involves an initial filing of an IND application following which the FDA would give the go ahead with Phase I clinical (human) trials. Following completion of Phase I, the results are filed with the FDA and a request is made to proceed to Phase II. Similarly, following completion of Phase II the data are filed with the FDA and a request is made to proceed to Phase III. Following completion of Phase III, a request is made for marketing approval. Depending on various issues and considerations, the FDA could provide limited marketing approval on a humanitarian basis if the drug treats terminally ill patients with limited treatment options available. As of the date of this Prospectus we have not made any filings with the FDA or other regulatory bodies in other jurisdictions. We have however had extensive discussions with clinicians at the McGill University’s Jewish General Hospital in Montreal where we plan to undertake our Phase I study for multidrug resistant breast cancer they believe that Health Canada is likely to grant us a so-called fast-track process on the basis of the terminal nature of the cancer which we will be treating. There are no assurances this will occur.
Employees
As of the date of this Prospectus we have three (3) employees, our management. We anticipate that if we receive financing we will hire additional employees in the areas of accounting, regulatory affairs, marketing and laboratory personnel.
20
Competition
We will be competing with publicly and privately held companies engaged in developing cancer therapies. There are numerous other entities engaged in this business that have greater resources, both financial and otherwise, than the resources presently available to us. Nearly all major pharmaceutical companies including Amgen, Roche, Pfizer, Bristol-Myers Squibb and Novartis, to name just a few, have on-going anti-cancer drug development programs and some of the drug they may develop could be in direct competition with our drug. Also, a number of small companies are also working in the area of cancer and could develop drugs that may be in competition with ours. However, none of these competitor companies can use molecules similar to ours as they would be infringing our patents.
Trademarks-Tradenames
We are the exclusive licensee for the U.S. territory of Advanomics’ Adva-27a which is covered by international patent applications filed on April 27, 2007 (PCT/FR2007/000697). These patent applications, which are now issued in Europe and the United States (US 8,236,935) and which are still pending elsewhere around the world, were originally owned by Institut National des Sciences Appliquées de Rouen (France) and have recently been purchased by Advanomics.
DESCRIPTION OF PROPERTY
Our principal place of business is located at 469 Jean-Talon West, 3rd Floor, Montreal, Quebec, Canada, H3N 1R4, where we relocated in June, 2012. Previously, our principal place of business was located at 2015 Peel Street, 5th Floor, Montreal, Quebec, Canada, H3A 1T8. This is also the location of our licensor, Advanomics Corporation, who is providing this space to us on a rent free basis as of the date of this Prospectus. If and when we are able to secure financing we expect that we will lease our own office and laboratory space. Our current space consists of approximately 1,000 square feet of executive office space. We anticipate that this will be sufficient for our needs until financing is in place, of which there is no assurance.
LEGAL PROCEEDINGS
To the best of our management’s knowledge and belief, there are no material claims of any merit that have been brought against us nor have there been any claims threatened.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDERS MATTERS
Trading of our Common Stock commenced on the OTCBB in September 2007 under the symbol “MWBN.” Effective November 30, 2009, the trading symbol for our Common Stock was changed to “SBFM” as a result of our name change discussed above.
The table below sets forth the reported high and low bid prices for the periods indicated. The bid prices shown reflect quotations between dealers, without adjustment for markups, markdowns or commissions, and may not represent actual transactions in our Common Stock.
|
Quarter Ended
|
High
|
Low
|
||||||
|
March 31, 2013
|
$ | 0.44 | $ | 0.24 | ||||
|
June 30, 2013
|
$ | 0.28 | $ | 0.19 | ||||
|
September 31, 2013
|
$ | 0.23 | $ | 0.16 | ||||
|
December 31, 2013
|
$ | 0.21 | $ | 0.13 | ||||
|
March 31, 2012
|
$ | 0.25 | $ | 0.13 | ||||
|
June 30, 2012
|
$ | 0.28 | $ | 0.19 | ||||
|
September 31, 2012
|
$ | 0.60 | $ | 0.18 | ||||
|
December 31, 2012
|
$ | 0.42 | $ | 0.19 |
As of May 20, 2014, the closing bid price of our Common Stock was $0.15.
Trading volume in our Common Stock is very limited. As a result, the trading price of our Common Stock is subject to significant fluctuations. See “Risk Factors.”
21
The Securities Enforcement and Penny Stock Reform Act of 1990
The Securities and Exchange Commission has also adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges or quoted on the Nasdaq system, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system).
As of the date of this Prospectus, our Common Stock is defined as a “penny stock” under the Securities and Exchange Act. It is anticipated that our Common Stock will remain a penny stock for the foreseeable future. The classification of penny stock makes it more difficult for a broker-dealer to sell the stock into a secondary market, which makes it more difficult for a purchaser to liquidate his/her investment. Any broker-dealer engaged by the purchaser for the purpose of selling his or her shares in us will be subject to Rules 15g-1 through 15g-10 of the Securities and Exchange Act. Rather than creating a need to comply with those rules, some broker-dealers will refuse to attempt to sell penny stock.
The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document prepared by the Commission, which:
|
●
|
contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading;
|
|
|
●
|
contains a description of the broker's or dealer's duties to the customer and of the rights and remedies available to the customer with respect to a violation to such duties or other requirements of the Securities Act of 1934, as amended;
|
|
|
●
|
contains a brief, clear, narrative description of a dealer market, including "bid" and "ask" prices for penny stocks and the significance of the spread between the bid and ask price;
|
|
|
●
|
contains a toll-free telephone number for inquiries on disciplinary actions;
|
|
|
●
|
defines significant terms in the disclosure document or in the conduct of trading penny stocks; and
|
|
|
●
|
contains such other information and is in such form (including language, type, size and format) as the Securities and Exchange Commission shall require by rule or regulation;
|
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, to the customer:
|
●
|
the bid and offer quotations for the penny stock;
|
|
|
●
|
the compensation of the broker-dealer and its salesperson in the transaction;
|
|
|
●
|
the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and
|
|
|
●
|
monthly account statements showing the market value of each penny stock held in the customer's account.
|
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules; the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written acknowledgment of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitability statement. These disclosure requirements will have the effect of reducing the trading activity in the secondary market for our stock because it will be subject to these penny stock rules. Therefore, stockholders may have difficulty selling their securities.
Holders
We had 145 holders of record of our Common Stock as of the date of this prospectus, not including those persons who hold their shares in “street name.”
Dividends
We have not paid any dividends since our incorporation and do not anticipate the payment of dividends in the foreseeable future. At present, our policy is to retain earnings, if any, to develop and market our products. The payment of dividends in the future will depend upon, among other factors, our earnings, capital requirements, and operating financial conditions.
22
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our audited financial statements and notes thereto included herein. In connection with, and because we desire to take advantage of, the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, we caution readers regarding certain forward looking statements in the following discussion and elsewhere in this Prospectus and in any other statement made by, or on our behalf, whether or not in future filings with the Securities and Exchange Commission. Forward looking statements are statements not based on historical information and which relate to future operations, strategies, financial results or other developments. Forward looking statements are necessarily based upon estimates and assumptions that are inherently subject to significant business, economic and competitive uncertainties and contingencies, many of which are beyond our control and many of which, with respect to future business decisions, are subject to change. These uncertainties and contingencies can affect actual results and could cause actual results to differ materially from those expressed in any forward looking statements made by, or on our behalf. We disclaim any obligation to update forward looking statements.
Overview and History
We were incorporated in the State of Colorado on August 31, 2006 under the name “Mountain West Business Solutions, Inc.” During our fiscal year ended July 31, 2009 our business was to provide management consulting with regard to accounting, computer and general business issues for small and home-office based companies. Effective October 15, 2009, we executed an agreement to acquire Sunshine Biopharma, Inc., a Colorado corporation (“SBI”), in exchange for the issuance of 21,962,000 shares of our Common Stock and 850,000 shares of Convertible Preferred Stock, each convertible into twenty (20) shares of our Common Stock (the “Agreement”). As a result of this transaction our officers and directors resigned their positions with us and were replaced by our current management. As a result of this transaction we have changed our name to “Sunshine Biopharma, Inc.”
Our principal place of business is located at 469 Jean-Talon West, 3rd Floor, Montreal, Quebec, Canada H3N 1R4. Our phone number is (514) 764-9698 and our website address is www.sunshinebiopharma.com.
We have not been subject to any bankruptcy, receivership or similar proceeding.
Results Of Operations
Comparison of Results of Operations for the three months ended March 31, 2014 and 2013
For the three months ended March 31, 2014 and 2013 we did not generate any revenues.
General and administrative expenses during the three month period ended March 31, 2014 were $405,873, compared to general and administrative expense of $922,891 incurred during the three month period ended March 31, 2013, a decrease of $517,018. This decrease is attributable to the fact that during the aforesaid period in 2014, we incurred $170,000 in financial consulting fees but incurred $622,610 in financial consulting fees during the similar period in 2013. We also incurred $96,000 in research and development costs in the three months ended March 31, 2014, compared to $23,400 during the similar period in 2013. Legal Fees increased during the three months ended March 31, 2014 to $45,372, compared to $19,034 during the three months ended march 31, 2013, primarily as a result of our attempts to re-domicile into Canada. We have subsequently elected not to proceed with this reincorporation primarily as a result of our successful efforts to secure funding. See “Liquidity and Capital Resources,” below. We also incurred $83,333 in license fees payable to Advanomics Corporation during the three months ended March 31, 2014, compared to license fees of $250,000 paid during the three months ended March 31, 2013. Most of our other expenses remained relatively constant during 2014 compared to 2013. We also incurred $95,382 in interest expense during the three months ended March 31, 2014, compared to $5,330 in interest expense during the similar period in 2013. Finally, in 2013 we also incurred $548,281 in costs associated with a beneficial conversion feature to our then outstanding convertible debentures. No such costs were incurred in 2014.
As a result, we incurred a net loss of 501,255 (approximately $0.01 per share) for the three month period ended March 31, 2014, compared to a net loss of $1,477,172 (approximately $0.03 per share) during the three month period ended March 31, 2013.
Comparison of Results of Operations for the fiscal years ended December 31, 2013 and 2012
Total expenses, including general and administrative expenses and research and development expenses for our fiscal year ended December 31, 2013 were $2,491.483, compared to $707,952 during our fiscal year ended December 31, 2012, an increase of $1,783,531. During our fiscal year ended December 31, 2013, our principal expenses included $1,186,610 in financial consulting fees, compared to $316,375 during 2012, an increase of $870,235 as a result of intensified efforts to secure funding for the next phase or our Adva-27a drug development program. Other principal expenses included $475,000 in licensing fees compared to $250,000 incurred during 2012, $137,400 in research and development costs in 2013 compared to $1,829 in 2012 and increased accounting fees of approximately $5,000 in 2013 compared to 2012. Legal fees decreased approximately $14,000 in 2013 compared to 2012.
In addition, we incurred a loss of $548,951 which arose from the conversion of outstanding convertible notes. On March 30, 2013 we issued 2,590,426 shares of our Common Stock upon the conversion of Convertible Notes payable on or before March 31, 2013 (“Convertible Notes”) valued at $621,703 representing principal of $513,000 and interest of $5,086. These Convertible Notes contained a beneficial conversion feature convertible at our option and were convertible at a fixed conversion price of $0.20. The market price on the issuance of these Convertible Notes varied from a low of $0.21 per share and a high of $0.46 per share with an average of $0.36 per share. Consequently, the Convertible Notes were considered to have a beneficial conversion feature and under ASC 470-20-25-10 the beneficial conversion feature was calculated to be $548,951 in total based on the issuance date and the share price on that date. This amount was booked to interest expense and Additional Paid in Capital for the period as all of the Convertible Notes have been converted by quarter end.
23
As a result, we incurred a net loss of $2,491,483 (approximately $0.04 per share) for the fiscal year ended December 31, 2013, compared to a net loss of $707,952 during our fiscal year ended December 31, 2012 (approximately $0.01 per share).
Because we have not generated any revenues, following is our Plan of Operation.
Plan of Operation
As of the date of this prospectus we are a pharmaceutical company focused on the research, development and commercialization of drugs for the treatment of various forms of cancer. Our lead compound, Adva-27, a multi-purpose anti-tumor compound, is expected to enter Phase I clinical trials in 2014. We have licensed our technology on an exclusive basis from Advanomics Corporation, a privately held Canadian company (“Advanomics”), and we are planning to initiate our own research and development program as soon as practicable, once financing is in place. There are no assurances that we will obtain the financing necessary to allow us to implement this aspect of our business plan, or to enter clinical trials. More details about our Plan of Operations are provided above under “Business.”
Liquidity and Capital Resources
As of March 31, 2014, we had cash or cash equivalents of $70,460.
Net cash used in operating activities was $90,780 during the three month period ended March 31, 2014, compared to $339,721 for the three month period ended March 31, 2013. The decrease is due to a decrease in issuance of stock for services during 2014. However, stock issued for payment of expenses and for interest on note payables increased during 2014. We anticipate that overhead costs in current operations will increase in the future once our research and development activities discussed above increase.
Cash flows from financing activities were $130,000 for the three month periods ended March 31, 2014, compared to $463,000 during the three months ended March 31, 2013. Cash flows used by investing activities were $0 for the three month periods ended March 31, 2014 and 2013.
In June 2012, we conducted a private placement of our Common Stock for the purposes of supporting our working capital whereby we sold 250,000 shares at a price of $0.20 per share and received proceeds of approximately $50,000 therefrom.
Between July and October 2012, we conducted a private placement of our Common Stock to fund our drug development program whereby we sold 1,410,000 shares of our Common Stock at a price of $0.25 per share and received proceeds of approximately $352,500 therefrom.
In December 2012, we commenced a private offering of Convertible Notes. We issued nine Convertible Notes to six accredited investors (as that term is defined under the Securities Act of 1933, as amended) in the aggregate amount of $513,000. These notes accrued interest at the rate of 6% per annum and were convertible at our option into shares of our Common Stock at $0.20 per share on or before March 31, 2013. We elected to convert these notes with interest accrued thereon and issued an aggregate of 2,590,426 shares of Common Stock to these investors. The Convertible Notes were considered to have a beneficial conversion feature and under ASC 470-20-25-10 the beneficial conversion feature was calculated to be $548,951 in total based on the issuance date and the share price on that date. This amount was booked to interest expense and Additional Paid in Capital for the period as all of the Convertible Notes were converted by March 31, 2013.
During the three months ended March 31, 2014 we conducted a private placement of our Common Stock for the purposes of supporting our working capital whereby we sold 266,667 shares at a price of $0.20 per share and received proceeds of approximately $53,333 therefrom.
On March 27, 2014, we issued a Convertible Note to one accredited investor (as that term is defined under the Securities Act of 1933, as amended) in the aggregate amount of $100,000 plus 500,000 Common shares (paid) and $20,000 (unpaid) for origination fee. This Convertible Note accrues interest at the rate of 10% per annum and is convertible at the option of the Holder into shares of our Common Stock at $0.20 per share on or before September 27, 2014. Since the Note was issued at a premium no value is apportioned to the conversion feature when recording the issue per ASC 470-20-05. The debt and its interest are reported as if it were a nonconvertible debt. Upon conversion the issued stock may be valued at either the book value or the market value of the note.
We are not generating revenue from our operations, and our ability to implement our business plan for the future will depend on the future availability of financing. Such financing will be required to enable us to further develop our drug research and development capabilities and continue operations. We intend to raise funds through private placements of our Common Stock and through short-term borrowing. We estimate that we will require approximately $5 million in debt and/or equity capital to fully implement our business plan in the future and there are no assurances that we will be able to raise this capital. While we have engaged in discussions with various investment banking firms and venture capitalists to provide us these funds, as of the date of this prospectus we have not reached any agreement with any party that has agreed to provide us with the capital necessary to effectuate our business plan. Our inability to obtain sufficient funds from external sources when needed will have a material adverse effect on our plan of operation, results of operations and financial condition.
Our cost to continue operations as they are now conducted is nominal, but these are expected to increase once we commence Phase I clinical trials. We do not have sufficient funds to cover the anticipated increase in these expenses. We need to raise additional funds in order to continue our existing operations, to initiate research and development activities, and to finance our plans to expand our operations for the next year. If we are successful in raising additional funds, our research and development efforts will continue and expand.
24
Subsequent Event
On April 23, 2014, we entered into an Investment Agreement (the “Investment Agreement”) with Dutchess Opportunity Fund, II, LP (“Dutchess”), for the sale of up to $2.5 million of shares of our Common stock over a three-year commitment period. Under the terms of the Investment Agreement, we may, from time to time and in our sole discretion, issue shares of our Common stock to Dutchess at a price equal to ninety percent (90%) of the lowest daily volume weighted average price during a Trading Day of our Common Stock during the five (5) consecutive Trading Days immediately preceding the Put Notice Date, up to $2.5 million. In connection with the Investment Agreement, we also issued to Dutchess an engagement fee in the form of 400,000 “restricted” shares of our Common Stock.
The amount of each tranche under the Investment Agreement is limited to maximum $100,000 and we may only issue a Put Notice (as defined under the Investment Agreement) ten (10) Trading Days after each prior Put Notice Date. We are not obligated to utilize any of the $2.5 million available under the Investment Agreement and there are no minimum commitments or minimum use penalties.
The Investment Agreement does not impose any restrictions on our operating activities. During the term of the Investment Agreement, Dutchess is prohibited from engaging in any short selling or hedging transactions, either directly or indirectly, related to our Common stock.
Inflation
Although our operations are influenced by general economic conditions, we do not believe that inflation had a material effect on our results of operations during the three month period ended March 31, 2014.
Critical Accounting Policies and Estimates
Critical Accounting Estimates
The discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. The following represents a summary of our critical accounting policies, defined as those policies that we believe are the most important to the portrayal of our financial condition and results of operations and that require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effects of matters that are inherently uncertain.
Leases – We follow the guidance in SFAS No. 13 “Accounting for Leases,” as amended, which requires us to evaluate the lease agreements we enter into to determine whether they represent operating or capital leases at the inception of the lease.
Recently Adopted Accounting Standards – As of November 1, 2011, we adopted new guidance on the testing of goodwill impairment that allows the option to assess qualitative factors to determine whether performing the two step goodwill impairment assessment is necessary. Under the option, the calculation of the reporting unit's fair value is not required to be performed unless as a result of the qualitative assessment, it is more likely than not that the fair value of thereporting unit is less than the unit's carrying amount. The adoption of this guidance impacts testing steps only, and therefore adoption did not have an impact on our consolidated financial statements. As of November 1, 2011, we adopted new guidance regarding disclosures about fair value measurements. The guidance requires new disclosures related to activity in Level 3 fair value measurements. This guidance requires purchases, sales, issuances, and settlements to be presented separately in the roll-forward of activity in Level 3 fair value measurements. We have complied with the disclosure requirements of the new guidance within Note 10, Fair value measurements. There were various other accounting standards and interpretations issued during 2010 and 2011, none of which are expected to have a material impact on our consolidated financial position, operations or cash flows.
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Following is a list of our officers and directors:
|
Name
|
Age
|
Position(s)
|
||
|
Dr. Steve N. Slilaty
|
62
|
President, Chief Executive Officer, and Chairman
|
||
|
Michele Di Turi
|
37
|
Chief Operating Officer and Director
|
||
|
Camille Sebaaly
|
55
|
Chief Financial Officer, Secretary and Director
|
25
Our directors serve as directors until our next Annual Meeting of Stockholders and the election and qualification of the director’s respective successor or until the director’s earlier death, removal or resignation.
Following is biographical information of our current management:
Dr. Steve N. Slilaty was appointed as our CEO, President and Chairman of our Board of Directors on October 15, 2009. In addition, since February 2002, Dr. Slilaty has been President and Chief Scientific Officer of Advanomics Corporation, Montreal, Canada, a privately held company engaged in the research, development and commercialization of drugs for the treatment of various forms of cancer. Advanomics Corporation is the third in a line of biotechnology companies that Dr. Slilaty founded and managed through their early and mid-stages of development. The first, Quantum Biotechnologies Inc. later known as Qbiogene Inc., was founded in 1991 and grew to over $60 million in annual sales. Today, Qbiogene is a member of a family of companies owned by MP Biomedicals, one of the largest international suppliers of biotechnology reagents with a catalogue containing over 55,000 products. The second company which Dr. Slilaty founded, Genomics One Corporation, now known as Alert B&C Corporation, conducted an initial public offering (IPO) of its capital stock in 1999 and, on the basis of its ownership of Dr. Slilaty’s patented TrueBlue® Technology, Genomics One became one of the handful of participants in the Human Genome Project. Formerly a research team leader of the Biotechnology Research Institute, a division of the National Research Council of Canada, Dr. Slilaty also served as a consultant in a management and advisory capacity for a major Canadian biotechnology company between 1995 and 1997 during which time the company completed one of the largest biotechnology IPO‘s in Canada raising over $34 million. Dr. Slilaty received his Ph.D degree from the University of Arizona in 1983 and a Bachelor of Science degree from Cornell University in 1976. In addition, Dr. Slilaty holds a position as Adjunct Professor at Université du Québec in the Department of Microbiology and Biotechnology. He devotes approximately 50% of his time to our business affairs.
Michele Di Turi was appointed as our Chief Operating Officer and a Director of our Company on October 15, 2009. Since November 2008, Mr. Di Turi has been President of Sunshine Bio Investments, Inc., a privately held Canadian corporation engaged in the sale of nonregulated biotechnology and medical products. In addition, since March 2013 Mr. Di Turi has been Chief Executive Officer, President and a director of Kisses From Italy, Inc., which is currently a privately held “start-up” company engaged in the development of Italian fast food restaurants in the US. Prior, from February 2003 through November 2008, he was employed by Mazda President, Inc., Montreal, Canada, as a sales representative and director of customer service. He devotes only such time as necessary to our business affairs.
Camille Sebaaly was appointed as our Chief Financial Officer, Secretary and a Director of our Company on October 15, 2009. Since 2001, Mr. Sebaaly has been self-employed as a business consultant, primarily in the biotechnology and biopharmaceutical sectors, as well as in the hydrogen generation and energy savings fields. He was a co-founder of Advanomics Corporation with Dr. Slilaty. He received a Bachelor of Science degree in electrical and computer engineering from the State University of New York at Buffalo in 1987. He devotes approximately 50% of his time to our business affairs.
There are no family relationships between any of our former or current officers and directors.
Code of Ethics
Our board of directors has not adopted a code of ethics but plans to do so in the near future.
Committees of the Board of Directors
There are no committees of the Board of Directors but it is anticipated that we will establish an audit committee, nominating committee and governance committee once independent directors are appointed, which is expected to occur in the near future.
Executive Compensation
We have not and do not expect to pay salaries to any of our executive officers or directors until such time as we are able to secure adequate funding for our operations.
Employment Agreements
None of our executive officers is party to an employment agreement with us.
Stock Plan
We have not adopted any stock option or other employee plans as of the date of this Prospectus. We may adopt such plans in the future.
26
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding the ownership of Common Stock as of the date of this prospectus, by (i) each person known to us to own more than 5% of our outstanding Common Stock as of the date of this Prospectus, (ii) each of our directors, (iii) each of our executive officers, and (iv) all of our directors and executive officers as a group. Unless otherwise indicated, all shares are owned directly and the indicated person has sole voting and investment power.
|
Title of
Class
|
Name and Address
Of Beneficial Owner
|
Amount and Nature
Of Beneficial Ownership
|
Percent
Of Class
|
|||||||
|
Common
|
Dr. Steve N. Slilaty(1)
|
31,752,067 | (2) | 48.3 | % | |||||
| 579 rue Lajeunesse | ||||||||||
| Laval, Quebec | ||||||||||
| Canada H7X 3K4 | ||||||||||
|
Common
|
Michele Di Turi(1)
|
234,373 | * | |||||||
| 3100 Boulevard Des Gouverneurs | ||||||||||
| Laval, Quebec | ||||||||||
| Canada H7E 5J3 | ||||||||||
|
Common
|
Camille Sebaaly(1)
|
234,373 | * | |||||||
| 14464 Gouin W, #B | ||||||||||
| Montreal, Quebec | ||||||||||
| Canada H9H 1B1 | ||||||||||
|
Common
|
All Officers and Directors
|
32,220,813 | 49 | % | ||||||
| As a Group (3 persons) | ||||||||||
|
|
_________________________
|
|
*
|
Less than 1%
|
|
(1)
|
Officer and Director of our Company.
|
|
(2)
|
Includes 31,517,694 shares held in the name of Advanomics Corporation. Dr. Slilaty is an officer, director and principal shareholder of Advanomics Corporation and as a result, controls the disposition of these shares.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Related Party Transactions
We licensed our technology on an exclusive basis (“Exclusive License Agreement”) from Advanomics Corporation (“Advanomics”), a privately held Canadian company. Dr. Steve N. Slilaty, our Chief Executive Officer and a Director, is an Officer, Director and principal shareholder of Advanomics. In 2009 we issued an aggregate of 17,109,194 shares of our Common Stock valued at $73,000 and 850,000 shares of Series “A” Convertible Preferred Stock valued at $73,000 in exchange for this license, and had an option to purchase 2,000,000 shares of Advanomics Common Stock at $5 (U.S.) per share within 1 year of September 30, 2009, as well as a second option to purchase an additional 1,000,000 shares of Advanomics’ Common Stock at an exercise price of $10.00 (U.S.) per share also for a 1 year term. We advanced further funds pursuant to this contract of $300,000. The total transaction costs to date of $446,000 have been written off as impaired.
Pursuant to a notice of conversion received from Advanomics on December 21, 2011, we issued 17,000,000 shares of our Common Stock in exchange for the 850,000 shares of Preferred Stock held by Advanomics. On December 21, 2011, we executed an amendment to the Exclusive License Agreement which waived a condition of termination and revised the consideration payable to Advanomics. The original Exclusive License Agreement required us to exercise an option to purchase shares in Advanomics for aggregate consideration of $9,700,000.00 ($5.00 per share). This obligation was waived and replaced with an annual licensing fee of $360,000.00 and reimbursement of research and development expenses incurred by Advanomics in connection with the Licensed Material as defined in the original Exclusive License Agreement.
We have moved our principal place of business to 469 Jean-Talon West, 3rd Floor, Montreal, Quebec, Canada, H3N 1R4. This is also the location of our licensor, Advanomics Corporation, who is providing this space to us on a rent free basis as of the date of this Prospectus. Dr. Steve N. Slilaty, our Chief Executive Officer and a Director, is an Officer, Director and principal shareholder of Advanomics. If and when we are able to secure financing we expect that we will lease our own office and laboratory space.
There are no other related party transactions that are required to be disclosed pursuant to Regulation S-K promulgated under the Securities Act of 1933, as amended.
27
Director Independence
None of our current directors are deemed “independent” pursuant to SEC rules. We anticipate appointing independent directors in the foreseeable future.
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis, or had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in the registrant or any of its parents or subsidiaries. Nor was any such person connected with the registrant or any of its parents or subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
The validity of the shares of our common stock offered under this prospectus is being passed upon for us by Andrew I. Telsey, P.C., Centennial, Colorado. Andrew Telsey, sole shareholder of the firm, owns 1,401,838 shares of our common stock.
The financial statements as of and for the years ended December 31, 2013 and December 31, 2012 included in this prospectus and the registration statement have been audited by B F Borgers CPA PC, to the extent and for the periods set forth in their report appearing elsewhere herein and in the registration statement, and are included in reliance upon such report given upon the authority of said firm as experts in auditing and accounting.
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is therefore unenforceable.
AVAILABLE INFORMATION
We filed with the Securities and Exchange Commission a registration statement under the Securities Act of 1933 for the shares of common stock in this offering. This prospectus does not contain all of the information in the registration statement and the exhibits and schedule that were filed with the registration statement. For further information with respect to us and our common stock, we refer you to the registration statement and the exhibits and schedule that were filed with the registration statement. Statements contained in this prospectus about the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and we refer you to the full text of the contract or other document filed as an exhibit to the registration statement. A copy of the registration statement and the exhibits and schedules that were filed with the registration statement may be inspected without charge at the Public Reference Room maintained by the Securities and Exchange Commission at 100 F Street, N.E. Washington, DC 20549, and copies of all or any part of the registration statement may be obtained from the Securities and Exchange Commission upon payment of the prescribed fee. Information regarding the operation of the Public Reference Room may be obtained by calling the Securities and Exchange Commission at 1-800-SEC-0330. The Securities and Exchange Commission maintains a website that contains reports, proxy and information statements, and other information regarding registrants that file electronically with the SEC. The address of the website is www.sec.gov.
We file periodic reports under the Securities Exchange Act of 1934, including annual, quarterly and special reports, and other information with the Securities and Exchange Commission. These periodic reports and other information are available for inspection and copying at the regional offices, public reference facilities and website of the Securities and Exchange Commission referred to above.
We make available free of charge on or through our internet website www.sunshinebiopharma.com our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission.
CAUTIONARY STATEMENT CONCERNING FORWARD-LOOKING STATEMENTS
Information included or incorporated by reference in this Prospectus may include “forward-looking statements”. This information may involve known and unknown risks, uncertainties and other factors which could cause actual results, financial performance, operating performance or achievements expressed or implied by such forward-looking statements not to occur or be realized. Such forward-looking statements generally are based upon our best estimates of future results, performance or achievement and based upon current conditions and the most recent results of operations. Forward-looking statements may be identified by the use of forward-looking terminology such as “believes,” “could,” “possibly,” “probably,” “anticipates,” “estimates,” “projects,” “expects,” “may,” “will,” or “should” or the negative thereof or other variations thereon or comparable terminology. This Prospectus contains forward-looking statements, including statements regarding, among other things, our projected sales and profitability, our growth strategies, anticipated trends in our industry and our future plans. These statements may be found under “Description of Business”, as well as in this Prospectus generally. Our actual results or events may differ materially from the results discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and elsewhere in this Prospectus. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Moreover, we do not assume responsibility for the accuracy or completeness of the forward-looking statements after the date of this Prospectus.
28
|
Page
|
|
|
Financial Statements for the Fiscal Years Ended December 31, 2013 and 2012
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Balance Sheets
|
F-3
|
|
Statements of Operations
|
F-4
|
|
Statements of Shareholder’s Equity
|
F-5
|
|
Statements of Cash Flows
|
F-6
|
|
Notes to Financial Statements
|
F-7
|
|
Financial Statements for the Three Months Ended March 31, 2013 and 2012
|
|
|
Condensed Balance Sheets (unaudited)
|
F-16
|
|
Condensed Statements of Operations (unaudited)
|
F-17
|
|
Condensed Statements of Cash Flows (unaudited)
|
F-18
|
|
Notes to Financial Statements (unaudited)
|
F-19
|
Sunshine Biopharma, Inc.
Balance Sheet
(A Development Stage Company)
|
Unaudited
|
Audited
|
|||||||
|
March 31,
|
December 31,
|
|||||||
|
2014
|
2013
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
70,460
|
$
|
31,240
|
||||
|
Prepaid expenses
|
1,345
|
-
|
||||||
|
Total Current Assets
|
71,805
|
31,240
|
||||||
|
TOTAL ASSETS
|
$
|
71,805
|
$
|
31,240
|
||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
Current Liabilities:
|
||||||||
|
Current portion of note payable
|
132,500
|
12,500
|
||||||
|
Accounts payable
|
49,514
|
23,809
|
||||||
|
Interest payable
|
3,023
|
2,641
|
||||||
|
TOTAL LIABILITIES
|
185,037
|
38,950
|
||||||
|
SHAREHOLDERS' EQUITY
|
||||||||
|
Preferred stock, $0.10 par value per share;
|
||||||||
|
Authorized 5,000,000 Shares; Issued
|
||||||||
|
and outstanding -0- shares.
|
-
|
-
|
||||||
|
Common Stock, $0.001 per share;
|
||||||||
|
Authorized 200,000,000 Shares; Issued
|
||||||||
|
and outstanding 62,675728 and 60,299,061 at
|
||||||||
|
March 31, 2014 and December 31, 2013 respectively
|
62,675
|
60,299
|
||||||
|
Capital paid in excess of par value
|
5,819,496
|
5,426,140
|
||||||
|
Accumulated other comprehensive (Loss)
|
-
|
-
|
||||||
|
(Deficit) accumulated during the development stage
|
(5,995,404
|
)
|
(5,494,149
|
)
|
||||
|
TOTAL SHAREHOLDERS' EQUITY
|
(113,232
|
)
|
(7,710
|
)
|
||||
|
TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY
|
$
|
71,805
|
$
|
31,240
|
||||
See Accompanying Notes To These Financial Statements.
F-1
Sunshine Biopharma, Inc.
Unaudited Statement Of Operations
(A Development Stage Company)
|
Unaudited
|
Unaudited
|
Unaudited
|
||||||||||
|
3 Months
|
3 Months
|
August 17,
|
||||||||||
|
Ended
|
Ended
|
2009 (inception)
|
||||||||||
|
March 31,
|
March 31,
|
through March 31,
|
||||||||||
|
2014
|
2013
|
2014
|
||||||||||
|
Revenue:
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
|
General & Administrative Expenses
|
||||||||||||
|
Research and Development
|
96,000
|
23,400
|
377,879
|
|||||||||
|
Accounting
|
5,180
|
3,780
|
78,455
|
|||||||||
|
Consulting
|
170,000
|
622,610
|
1,835,342
|
|||||||||
|
Legal
|
45,372
|
19,034
|
376,748
|
|||||||||
|
Licenses
|
83,333
|
250,000
|
1,258,333
|
|||||||||
|
Office
|
4,905
|
4,067
|
50,680
|
|||||||||
|
Merger Cost
|
-
|
-
|
155,150
|
|||||||||
|
Public Relations
|
-
|
-
|
241,768
|
|||||||||
|
Stock Transfer Fee
|
1,083
|
-
|
23,013
|
|||||||||
|
Writedown of intangible assets
|
-
|
-
|
945,976
|
|||||||||
|
Total G & A
|
405,873
|
922,891
|
5,343,344
|
|||||||||
|
(Loss) from operations
|
(405,873
|
)
|
(922,891
|
)
|
(5,343,344
|
)
|
||||||
|
Other (expense):
|
||||||||||||
|
Interest expense
|
(95,382
|
)
|
(5,330
|
)
|
(103,109
|
)
|
||||||
|
Beneficial conversion feature
|
-
|
(548,951
|
)
|
(548,951
|
)
|
|||||||
|
Total Other (Expense)
|
(95,382
|
)
|
(554,281
|
)
|
(652,060
|
)
|
||||||
|
Net (loss)
|
$
|
(501,255
|
)
|
$
|
(1,477,172
|
)
|
$
|
(5,995,404
|
)
|
|||
|
Basic (Loss) per common share
|
$
|
(0.01
|
)
|
$
|
(0.03
|
)
|
||||||
|
Weighted Average Common Shares Outstanding
|
61,127,469
|
55,395,819
|
||||||||||
See Accompanying Notes To These Financial Statements.
F-2
Sunshine Biopharma, Inc.
Unaudited Statement Of Cash Flows
(A Development Stage Company)
|
Unaudited
|
Unaudited
|
Unaudited
|
||||||||||
|
3 Months
|
3 Months
|
August 17,
|
||||||||||
|
Ended
|
Ended
|
2009 (inception)
|
||||||||||
|
March 31,
|
March 31,
|
through March 31,
|
||||||||||
|
2014
|
2013
|
2014
|
||||||||||
|
Cash Flows From Operating Activities:
|
||||||||||||
|
Net (Loss)
|
$
|
(501,255
|
)
|
$
|
(1,477,172
|
)
|
$
|
(5,995,404
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in
|
||||||||||||
|
operating activities:
|
||||||||||||
|
Stock issued for licenses, services, and other assets
|
267,400
|
576,310
|
2,941,102
|
|||||||||
|
Stock issued for payment interest on notes payable
|
75,000
|
5,086
|
80,086
|
|||||||||
|
Stock issued for payment of expenses
|
43,333
|
143,333
|
||||||||||
|
Beneficial conversion feature on note conversion
|
-
|
548,951
|
548,951
|
|||||||||
|
(Increase) Decrease in prepaid expenses
|
(1,345
|
)
|
(883
|
)
|
(1,345
|
)
|
||||||
|
Increase (Decrease) in Accounts Payable
|
25,705
|
7,743
|
49,514
|
|||||||||
|
Increase in interest payable
|
382
|
244
|
3,023
|
|||||||||
|
Net Cash Flows (used) in operations
|
(90,780
|
)
|
(339,721
|
)
|
(2,230,740
|
)
|
||||||
|
Cash Flows From Investing Activities:
|
||||||||||||
|
Net Cash Flows (used) in Investing activities
|
-
|
-
|
-
|
|||||||||
|
Cash Flows From Financing Activities:
|
||||||||||||
|
Proceed from note payable
|
60,000
|
463,000
|
585,500
|
|||||||||
|
Note payable used to pay expenses
|
60,000
|
-
|
60,000
|
|||||||||
|
Sale of common stock
|
10,000
|
-
|
1,655,700
|
|||||||||
|
Net Cash Flows provided by financing activities
|
130,000
|
463,000
|
2,301,200
|
|||||||||
|
Net Increase (Decrease) In Cash and cash equivalents
|
39,220
|
123,279
|
70,460
|
|||||||||
|
Cash and cash equivalents at beginning of period
|
31,240
|
132,638
|
-
|
|||||||||
|
Cash and cash equivalents at end of period
|
$
|
70,460
|
$
|
255,917
|
$
|
70,460
|
||||||
|
Supplementary Disclosure Of Cash Flow Information:
|
||||||||||||
|
Stock issued for services, licenses and other assets
|
$
|
266,000
|
$
|
576,310
|
$
|
2,930,702
|
||||||
|
Stock issued for note conversions
|
$
|
-
|
$
|
513,000
|
$
|
542,645
|
||||||
|
Stock issued for net deficit of MWBS
|
$
|
-
|
$
|
-
|
$
|
(29,465
|
)
|
|||||
|
Stock issued for interest
|
$
|
95,000
|
$
|
-
|
$
|
95,000
|
||||||
|
Stock issued for payment of expenses
|
$
|
43,333
|
$
|
-
|
$
|
143,333
|
||||||
|
Loan proceeds used to pay expenses
|
$
|
40,000
|
$
|
-
|
$
|
40,000
|
||||||
|
Cash paid for interest
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
|
Cash paid for income taxes
|
||||||||||||
See Accompanying Notes To These Financial Statements.
F-3
Sunshine Biopharma, Inc.
Notes To Unaudited Financial Statements
For The Three Month Interim Period Ended March 31, 2014
Note 1 – Unaudited Financial Information
The unaudited financial information included for the three month interim period ended March 31, 2014 was taken from the books and records without audit. However, such information reflects all adjustments, consisting only of normal recurring adjustments, which in the opinion of management are necessary to reflect properly the results of the interim periods presented. The results of operations for the three month interim period ended March 31, 2014 are not necessarily indicative of the results expected for the fiscal year ending December 31, 2014.
Note 2 – Notes Payable
The Company had outstanding loans of $12,500 accruing interest at a rate of 12% and $100,000 accruing interest at 10%. At March 31, 2014 and December 31, 2013 accrued interest was $3,023 and $2,641, respectively.
Note 3 – Issuance of Common Stock
During the three months ended March 31, 2014 the Company issued 2,376,667 shares of $0.001 par value Common Stock as follows:
In January 2014 the Company issued 200,000 shares of $0.001 par value Common Stock for cash of $40,000 or $0.20 per share and was paid directly to an affiliated company for licensing rights.
In January 2014 the Company issued 600,000 shares of $0.001 par value Common Stock for R&D services valued at $96,000 or $0.16 per share.
In February 2014 the Company issued 66,667 shares of $0.001 par value Common Stock for cash of $13,333 or $0.20 per share and $3,333 was paid directly to an affiliated company for licensing rights.
In March 2014 the Company issued 10,000 shares of $0.001 par value Common Stock for services valued at $1,400 or $0.14 per share.
In March 2014 the Company issued 1,000,000 shares of $0.001 par value Common Stock for services valued at $170,000 or $0.17 per share.
F-4
Sunshine Biopharma, Inc.
Notes To Unaudited Financial Statements
For The Three Month Interim Period Ended March 31, 2014
Note 3 – Issuance of Common Stock (Continued)
On March 27, 2014 the Company issued 500,000 shares of $0.001 par value Common Stock for origination fee valued at $75,000 or $0.15 per share as part of a convertible note payable for $100,000.
Note 4 – Convertible Notes
March 27, 2014 the Company issued a Convertible Note to one accredited investor (as that term is defined under the Securities Act of 1933, as amended) in the aggregate amount of $100,000 plus 500,000 Common shares (paid) and $20,000 (unpaid) for origination fee. This Convertible Note accrues interest at the rate of 10% per annum and is convertible at the option of the Holder into shares of the Company’s Common Stock at $0.20 per share on or before September 27, 2014. Since the Note was issued at a premium no value is apportioned to the conversion feature when recording the issue per ASC 470-20-05. The debt and its interest are reported as if it were a nonconvertible debt. Upon Conversion, the stock may be valued at either the book value or the market value of the bonds.
Note 4 – Financial Statements
For a complete set of footnotes, reference is made to the Company’s Report on Form 10-K for the year ended December 31, 2013 as filed with the Securities and Exchange Commission and the audited financial statements included therein.
F-5
|
Page No.
|
||||
|
Independent Accountant’s Audit Report
|
F-1
|
|||
|
Consolidated Balance Sheet
|
F-2
|
|||
|
Consolidated Statement of Operations
|
F-3
|
|||
|
Consolidated Statement of Cash Flow
|
F-4
|
|||
|
Statement of Shareholders’ Equity
|
F-5-F-6
|
|||
|
Notes to the Consolidated Financial Statements
|
F-7-F-14
|
|||
Sunshine Biopharma, Inc.
(A Development Stage Company)
CONSOLIDATED FINANCIAL STATEMENTS
With Independent Accountant’s Audit Report
At December 31, 2013 and 2012
And the period August 17, 2009 (inception) through December 31, 2013
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Sunshine Biopharma, Inc.:
We have audited the accompanying consolidated balance sheets of Sunshine Biopharma, Inc.(a development stage company) as of December 31, 2013 and 2012 and the related statements of operations, stockholders’ equity (deficit) and cash flows for the years then ended, and for the period from August 17, 2009 (inception) through December 31, 2013. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Sunshine Biopharma, Inc., as of December 31, 2013 and 2012, and the results of its operations and its cash flows for the years then ended, and for the period from August 17, 2009 (inception) through December 31, 2013 in conformity with generally accepted accounting principles in the United States of America.
The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the Company's internal control over financial reporting. Accordingly, we express no such opinion.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company’s significant operating losses raise substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ B F Borgers CPA PC
B F Borgers CPA PC
Denver, CO
March 17, 2014
F-1
Sunshine Biopharma, Inc.
Balance Sheet
(A Development Stage Company)
|
Audited
|
Audited
|
|||||||
|
December 31,
|
December 31,
|
|||||||
|
2013
|
2012
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
31,240
|
$
|
132,638
|
||||
|
Prepaid expenses
|
-
|
2,155
|
||||||
|
Total Current Assets
|
31,240
|
134,793
|
||||||
|
TOTAL ASSETS
|
$
|
31,240
|
$
|
134,793
|
||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
Current Liabilities:
|
||||||||
|
Current portion of note payable
|
12,500
|
62,500
|
||||||
|
Accounts payable
|
23,809
|
595
|
||||||
|
Interest payable
|
2,641
|
1,272
|
||||||
|
TOTAL LIABILITIES
|
38,950
|
64,367
|
||||||
|
SHAREHOLDERS' EQUITY
|
||||||||
|
Preferred stock, $0.10 par value per share;
|
||||||||
|
Authorized 5,000,000 Shares; Issued
|
||||||||
|
and outstanding -0- shares.
|
-
|
-
|
||||||
|
Common Stock, $0.001 per share;
|
||||||||
|
Authorized 200,000,000 Shares; Issued
|
||||||||
|
and outstanding 60,299,061 and 51,416,092 at
|
||||||||
|
December 31, 2013 and December 31, 2012 respectively
|
60,299
|
51,416
|
||||||
|
Capital paid in excess of par value
|
5,426,140
|
3,021,676
|
||||||
|
Accumulated other comprehensive (Loss)
|
-
|
-
|
||||||
|
(Deficit) accumulated during the development stage
|
(5,494,149
|
)
|
(3,002,666
|
)
|
||||
|
TOTAL SHAREHOLDERS' EQUITY
|
(7,710
|
)
|
70,426
|
|||||
|
TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY
|
$
|
31,240
|
$
|
134,793
|
||||
See Accompanying Notes To These Financial Statements.
F-2
Sunshine Biopharma, Inc.
Unaudited Statement Of Operations
(A Development Stage Company)
|
Unaudited
|
||||||||||||
|
August 17,
|
||||||||||||
|
2009 (inception)
|
||||||||||||
|
December 31,
|
December 31,
|
through December 31,
|
||||||||||
|
2013
|
2012
|
2013
|
||||||||||
|
Revenue:
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
|
General & Administrative Expenses
|
||||||||||||
|
Research and Development
|
137,400
|
1,829
|
281,879
|
|||||||||
|
Accounting
|
23,640
|
18,190
|
73,275
|
|||||||||
|
Consulting
|
1,186,610
|
316,375
|
1,665,342
|
|||||||||
|
Legal
|
88,381
|
101,907
|
331,376
|
|||||||||
|
Licenses
|
475,000
|
250,000
|
1,175,000
|
|||||||||
|
Office
|
18,963
|
15,089
|
45,775
|
|||||||||
|
Merger Cost
|
-
|
-
|
155,150
|
|||||||||
|
Public Relations
|
-
|
-
|
241,768
|
|||||||||
|
Stock Transfer Fee
|
6,083
|
3,290
|
21,930
|
|||||||||
|
Writedown of intangible assets
|
-
|
-
|
945,976
|
|||||||||
|
Total G & A
|
1,936,077
|
706,680
|
4,937,471
|
|||||||||
|
(Loss) from operations
|
(1,936,077
|
)
|
(706,680
|
)
|
(4,937,471
|
)
|
||||||
|
Other (expense):
|
||||||||||||
|
Interest expense
|
(6,455
|
)
|
(1,272
|
)
|
(7,727
|
)
|
||||||
|
Beneficial conversion feature
|
(548,951
|
)
|
-
|
(548,951
|
)
|
|||||||
|
Total Other (Expense)
|
(555,406
|
)
|
(1,272
|
)
|
(556,678
|
)
|
||||||
|
Net (loss)
|
$
|
(2,491,483
|
)
|
$
|
(707,952
|
)
|
$
|
(5,494,149
|
)
|
|||
|
Basic (Loss) per common share
|
$
|
(0.04
|
)
|
$
|
(0.01
|
)
|
||||||
|
Weighted Average Common Shares Outstanding
|
55,395,819
|
49,775,134
|
||||||||||
See Accompanying Notes To These Financial Statements.
F-3
Sunshine Biopharma, Inc.
Unaudited Statement Of Cash Flows
(A Development Stage Company)
|
August 17,
|
||||||||||||
|
2009 (inception)
|
||||||||||||
|
December 31,
|
December 31,
|
through December 31,
|
||||||||||
|
2013
|
2012
|
2013
|
||||||||||
|
Cash Flows From Operating Activities:
|
||||||||||||
|
Net (Loss)
|
$
|
(2,491,483
|
)
|
$
|
(707,952
|
)
|
$
|
(5,494,149
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in
|
||||||||||||
|
operating activities:
|
||||||||||||
|
Stock issued for licenses, services, and other assets
|
1,246,310
|
272,875
|
2,673,702
|
|||||||||
|
Stock issued for payment of expenses
|
100,000
|
100,000
|
||||||||||
|
Stock issued for payment interest on notes payable
|
5,086
|
-
|
5,086
|
|||||||||
|
Beneficial conversion feature on note conversion
|
548,951
|
-
|
548,951
|
|||||||||
|
(Increase) Decrease in prepaid expenses
|
2,155
|
43,590
|
-
|
|||||||||
|
Increase (Decrease) in Accounts Payable
|
23,214
|
(2,839
|
)
|
23,809
|
||||||||
|
Increase in interest payable
|
1,369
|
1,272
|
2,641
|
|||||||||
|
Net Cash Flows (used) in operations
|
(564,398
|
)
|
(393,054
|
)
|
(2,139,960
|
)
|
||||||
|
Cash Flows From Investing Activities:
|
||||||||||||
|
Net Cash Flows (used) in Investing activities
|
-
|
-
|
-
|
|||||||||
|
Cash Flows From Financing Activities:
|
||||||||||||
|
Proceed from note payable
|
463,000
|
62,500
|
525,500
|
|||||||||
|
Sale of common stock
|
-
|
402,500
|
1,645,700
|
|||||||||
|
Net Cash Flows provided by financing activities
|
463,000
|
465,000
|
2,171,200
|
|||||||||
|
Net Increase (Decrease) In Cash and cash equivalents
|
(101,398
|
)
|
71,946
|
31,240
|
||||||||
|
Cash and cash equivalents at beginning of period
|
132,638
|
60,692
|
-
|
|||||||||
|
Cash and cash equivalents at end of period
|
$
|
31,240
|
$
|
132,638
|
$
|
31,240
|
||||||
|
Supplementary Disclosure Of Cash Flow Information:
|
||||||||||||
|
Stock issued for services, licenses and other assets
|
$
|
1,237,310
|
$
|
266,875
|
$
|
2,664,702
|
||||||
|
Stock issued for payment of expenses
|
$
|
100,000
|
$
|
-
|
$
|
100,000
|
||||||
|
Stock issued for note conversions
|
$
|
513,000
|
$
|
-
|
$
|
542,465
|
||||||
|
Stock issued for net deficit of MWBS
|
$
|
-
|
$
|
-
|
$
|
(29,465
|
)
|
|||||
|
Cash paid for interest
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
|
Cash paid for income taxes
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
See Accompanying Notes To These Financial Statements.
F-4
Sunshine Biopharma, Inc.
(A Development Stage Company)
Statement of Shareholders' Equity
|
Deficit accumulated
|
||||||||||||||||||||||||||||||||||||
|
Number Of
|
Capital Paid
|
Number Of
|
Stock
|
During the
|
||||||||||||||||||||||||||||||||
|
Common
|
Common
|
in Excess
|
Preferred
|
Preferred
|
Subscription
|
Comprehensive
|
development
|
|||||||||||||||||||||||||||||
|
Shares Issued
|
Stock
|
of Par Value
|
Shares Issued
|
Stock
|
Receivable
|
Income
|
stage
|
Total
|
||||||||||||||||||||||||||||
|
Balance at August 17, 2009 (Inception)
|
-
|
$
|
-
|
$
|
-
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||||||||||||||
|
August 17, 2009 issued 703,118
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for services valued at or $.004 per share
|
703,118
|
703
|
2,297
|
3,000
|
||||||||||||||||||||||||||||||||
|
August 19, 2009 issued 218,388
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for services valued at or $.004 per share
|
218,388
|
218
|
714
|
932
|
||||||||||||||||||||||||||||||||
|
August 20, 2009 issued 17,109,194
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock and
|
||||||||||||||||||||||||||||||||||||
|
730,000 share of par value $0.10 preferred stock
|
||||||||||||||||||||||||||||||||||||
|
for license agreement Advanomics:
|
||||||||||||||||||||||||||||||||||||
|
Common valued at or $.004 per share
|
||||||||||||||||||||||||||||||||||||
|
and Preferred valued at or $.086 per share
|
17,109,194
|
17,109
|
55,891
|
850,000
|
73,000
|
146,000
|
||||||||||||||||||||||||||||||
|
September 24, 2009 :
|
||||||||||||||||||||||||||||||||||||
|
Private Placement-The Company undertook to sell
|
||||||||||||||||||||||||||||||||||||
|
2,220,552 shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for cash of $649,000 or $.2922 per share.
|
||||||||||||||||||||||||||||||||||||
|
Company bought 1,150,693 share of par value $.001
|
||||||||||||||||||||||||||||||||||||
|
stock for cash of $336,312 or $.2922 per share;
|
||||||||||||||||||||||||||||||||||||
|
the remaining 1,069,859 shares were collected for
|
||||||||||||||||||||||||||||||||||||
|
cash of $312,688 in October 2009.
|
1,150,693
|
1,151
|
335,161
|
336,312
|
||||||||||||||||||||||||||||||||
|
September 24, 2009 Common stock subscription
|
||||||||||||||||||||||||||||||||||||
|
(see notation above) for 1,069,074 shares of par value
|
||||||||||||||||||||||||||||||||||||
|
$.001 common stock valued at $.2922 per share
|
(312,688
|
)
|
312,688
|
-
|
||||||||||||||||||||||||||||||||
|
September 30, 2009 issued 1,710,748
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for asset purchase from Sunshine Bio Investment
|
||||||||||||||||||||||||||||||||||||
|
valued at or $.2922 per share
|
1,710,748
|
1,711
|
498,289
|
-
|
500,000
|
|||||||||||||||||||||||||||||||
|
Net (Loss)
|
(650,130
|
)
|
(650,130
|
)
|
||||||||||||||||||||||||||||||||
|
Balance at September 30, 2009
|
20,892,141
|
20,892
|
892,352
|
850,000
|
73,000
|
(312,688
|
)
|
312,688
|
(650,130
|
)
|
336,114
|
|||||||||||||||||||||||||
F-5
|
October 31, 2009 issuance of common stock
|
||||||||||||||||||||||||||||||||||||
|
subscription, upon receipt of cash 1,069,859 shs of par
|
||||||||||||||||||||||||||||||||||||
|
value $.001 common stock valued at $.2922 per share
|
1,069,859
|
1,070
|
311,618
|
312,688
|
(312,688
|
)
|
312,688
|
|||||||||||||||||||||||||||||
|
October 31, 2009 Outstanding stock of MWBS
|
||||||||||||||||||||||||||||||||||||
|
counted as issued for MWBS net deficit
|
888,000
|
888
|
(30,353
|
)
|
(29,465
|
)
|
||||||||||||||||||||||||||||||
|
Subtotal-at October 31, 2009 reverse merger date
|
||||||||||||||||||||||||||||||||||||
|
for accounting purposes
|
22,850,000
|
22,850
|
1,173,617
|
850,000
|
73,000
|
-
|
-
|
(650,130
|
)
|
619,337
|
||||||||||||||||||||||||||
|
November 16, 2009 Note conversions, several,
|
||||||||||||||||||||||||||||||||||||
|
Principle of $26,500 and interest of $2,965
|
6,810,000
|
6,810
|
22,655
|
29,465
|
||||||||||||||||||||||||||||||||
|
Fractional Shares
|
7
|
-
|
||||||||||||||||||||||||||||||||||
|
Net (Loss)
|
(551,000
|
)
|
(551,000
|
)
|
||||||||||||||||||||||||||||||||
|
Balance at December 31, 2009
|
29,660,007
|
29,660
|
1,196,272
|
850,000
|
73,000
|
-
|
-
|
(1,201,130
|
)
|
97,802
|
||||||||||||||||||||||||||
|
June 2, 2010 issued 1,675,000
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for services valued at or $.94 per share
|
1,675,000
|
1,675
|
1,572,825
|
1,574,500
|
||||||||||||||||||||||||||||||||
|
September 30, 2010 reversed issuance of 1,625,000
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for services valued at or $.94 per share
|
(1,625,000
|
)
|
(1,625
|
)
|
(1,525,875
|
)
|
(1,527,500
|
)
|
||||||||||||||||||||||||||||
|
September 30, 2010 issued 166,667
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for cash at or $.60 per share
|
166,667
|
167
|
99,833
|
100,000
|
||||||||||||||||||||||||||||||||
|
October 1, 2010 issued 217,000
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for services valued at or $.60 per share
|
217,000
|
217
|
129,983
|
130,200
|
||||||||||||||||||||||||||||||||
|
October 29, 2010 issued 100,000
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for services valued at or $.60 per share
|
100,000
|
100
|
59,900
|
60,000
|
||||||||||||||||||||||||||||||||
|
October 31, 2010 issued 419,334
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for cash at or $.60 per share
|
419,334
|
419
|
251,181
|
251,600
|
||||||||||||||||||||||||||||||||
|
November 30, 2010 issued 78,334
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for cash at or $.60 per share
|
78,334
|
78
|
46,922
|
47,000
|
||||||||||||||||||||||||||||||||
|
Net (Loss)
|
(537,382
|
)
|
(537,382
|
)
|
||||||||||||||||||||||||||||||||
|
Balance at December 30, 2010
|
30,691,342
|
$
|
30,691
|
$
|
1,831,040
|
850,000
|
$
|
73,000
|
$
|
-
|
$
|
-
|
$
|
(1,738,512
|
)
|
$
|
196,220
|
|||||||||||||||||||
F-6
|
March 29, 2011 issued 20,000
|
||||||||||||||||||||||||||||||||||||
|
shares of par value $.001 common stock
|
||||||||||||||||||||||||||||||||||||
|
for services valued at $ 12,000 or $.60 per share
|
20,000
|
20
|
11,980
|
12,000
|
||||||||||||||||||||||||||||||||
|
September 1, 2011 issued 326,00 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $.001 common stock in a
|
||||||||||||||||||||||||||||||||||||
|
private offering for cash at $0.60 per share
|
326,000
|
326
|
195,274
|
195,600
|
||||||||||||||||||||||||||||||||
|
November 3, 2011 issued 400,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
for services valued at $ 200,000 or $.50 per share
|
400,000
|
400
|
199,600
|
200,000
|
||||||||||||||||||||||||||||||||
|
December 16, 2011 issued 291,500 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
for services valued at $ 55,385 or $.19 per share
|
291,500
|
292
|
55,094
|
55,385
|
||||||||||||||||||||||||||||||||
|
December 21, 2011 converted 850,000 shares of
|
||||||||||||||||||||||||||||||||||||
|
preferred stock into 17,000,000 shares of par value
|
||||||||||||||||||||||||||||||||||||
|
$.001 common stock
|
17,000,000
|
17,000
|
56,000
|
(850,000
|
)
|
(73,000
|
)
|
-
|
||||||||||||||||||||||||||||
|
Net (Loss)
|
-
|
(556,202
|
)
|
(556,202
|
)
|
|||||||||||||||||||||||||||||||
|
Balance at December 31, 2011
|
48,728,842
|
$
|
48,729
|
$
|
2,348,988
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
(2,294,714
|
)
|
$
|
103,003
|
F-7
|
June 28, 2012 issued 250,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock in a
|
||||||||||||||||||||||||||||||||||||
|
private offering for cash at $.20 per share
|
||||||||||||||||||||||||||||||||||||
|
or $50,000
|
250,000
|
250
|
49,750
|
50,000
|
||||||||||||||||||||||||||||||||
|
June 28, 2012 issued 230,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 69,000 or $0.30 per share
|
230,000
|
230
|
68,770
|
69,000
|
||||||||||||||||||||||||||||||||
|
July 2012 issued 840,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock in a
|
||||||||||||||||||||||||||||||||||||
|
private offering for cash at $.25 per share
|
||||||||||||||||||||||||||||||||||||
|
or $50,000
|
840,000
|
840
|
209,160
|
210,000
|
||||||||||||||||||||||||||||||||
|
July 25, 2012 issued 44,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 15,400 or $0.35 per share
|
44,000
|
44
|
15,356
|
15,400
|
||||||||||||||||||||||||||||||||
|
August 2012 issued 570,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock in a
|
||||||||||||||||||||||||||||||||||||
|
private offering for cash at $.25 per share
|
||||||||||||||||||||||||||||||||||||
|
or $142,500
|
570,000
|
570
|
141,929
|
142,500
|
||||||||||||||||||||||||||||||||
|
August 17, 2012 issued 128,250 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 38,475 or $0.30 per share
|
128,250
|
127
|
38,348
|
38,475
|
||||||||||||||||||||||||||||||||
|
August 31, 2012 issued 600,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 144,000 or $0.24 per share
|
600,000
|
600
|
143,400
|
144,000
|
||||||||||||||||||||||||||||||||
|
August 31, 2012 issued 600,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 144,000 or $0.24 per share
|
25,000
|
25
|
5,975
|
6,000
|
||||||||||||||||||||||||||||||||
|
Net (Loss)
|
-
|
(707,952
|
)
|
(707,952
|
)
|
|||||||||||||||||||||||||||||||
|
Balance at December 31, 2012
|
51,416,092
|
$
|
51,416
|
$
|
3,021,676
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
(3,002,666
|
)
|
$
|
70,426
|
F-8
|
January 11, 2013 issued 350,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 136,500 or $0.39 per share
|
350,000
|
350
|
136,150
|
136,500
|
||||||||||||||||||||||||||||||||
|
March 28, 2013 issued 918,500 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 220,440 or $0.24 per share
|
918,500
|
919
|
219,522
|
444,896
|
665,336
|
|||||||||||||||||||||||||||||||
|
March 30, 2013 issued 259,043 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 219,370 or $0.24 per share
|
914,043
|
914
|
218,456
|
219,370
|
||||||||||||||||||||||||||||||||
|
March 30, 2013 issued 2,590,428 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for conversion
|
||||||||||||||||||||||||||||||||||||
|
of debt in the amount of $513,000 and interest
|
||||||||||||||||||||||||||||||||||||
|
of $5,086 or $0.24 per share
|
2,590,426
|
2,590
|
515,496
|
518,086
|
||||||||||||||||||||||||||||||||
|
Beneficial conversion feature
|
548,951
|
548,951
|
||||||||||||||||||||||||||||||||||
|
May 14, 2013 issued 250,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 60,000 or $0.24 per share
|
250,000
|
250
|
59,750
|
60,000
|
F-9
|
August 1, 2013 issued 150,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 30,000 or $0.20 per share
|
150,000
|
150
|
29,850
|
30,000
|
||||||||||||||||||||||||||||||||
|
August 23, 2013 issued 250,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 50,000 or $0.20 per share
|
250,000
|
250
|
49,750
|
50,000
|
||||||||||||||||||||||||||||||||
|
October 4, 2013 issued 60,0000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 15,000 or $0.25 per share
|
60,000
|
60
|
14,940
|
15,000
|
||||||||||||||||||||||||||||||||
|
November 4, 2013 issued 500,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for cash
|
||||||||||||||||||||||||||||||||||||
|
of $95,000 or $0.19 per share
|
500,000
|
500
|
94,500
|
95,000
|
||||||||||||||||||||||||||||||||
|
November 20, 2013 issued 425,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for cash
|
||||||||||||||||||||||||||||||||||||
|
of $85,000 or $0.20 per share
|
425,000
|
425
|
84,575
|
85,000
|
||||||||||||||||||||||||||||||||
|
November 20, 2013 issued 600,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 114,000 or $0.19 per share
|
600,000
|
600
|
113,400
|
114,000
|
||||||||||||||||||||||||||||||||
|
December 2, 2013 issued 75,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for cash
|
||||||||||||||||||||||||||||||||||||
|
of $15,000 or $0.20 per share
|
75,000
|
75
|
14,925
|
15,000
|
||||||||||||||||||||||||||||||||
|
December 27, 2013 issued 1,800,000 shares
|
||||||||||||||||||||||||||||||||||||
|
of par value $0.001 common stock for services
|
||||||||||||||||||||||||||||||||||||
|
valued at $ 306,000 or $0.17 per share
|
1,800,000
|
1,800
|
304,200
|
306,000
|
||||||||||||||||||||||||||||||||
|
Net (Loss)
|
-
|
-
|
-
|
(2,491,483
|
)
|
(2,491,483
|
)
|
|||||||||||||||||||||||||||||
|
Balance at December 31, 2013
|
60,299,061
|
$
|
60,299
|
$
|
5,426,140
|
-
|
$
|
444,896
|
$
|
-
|
$
|
-
|
$
|
(5,494,149
|
)
|
$
|
437,186
|
See Accompanying Notes To These Financial Statements.
F-10
Sunshine Biopharma, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012 and
The period August 17, 2009 (inception) through December 31, 2013
Note 1 - Organization and Summary of Significant Accounting Policies
ORGANIZATION
Mountain West Business Solutions, Inc. (“MWBS”) was incorporated August 31, 2006 in the State of Colorado. Sunshine Etopo, Inc. (formerly Sunshine Biopharma, Inc.) was incorporated in the State of Colorado on August 17, 2009. Effective October 15, 2009 MWBS was acquired by Sunshine Etopo, Inc. in a transaction classified as a reverse acquisition. MWBS concurrently changed its name to Sunshine Biopharma, Inc. The financial statements represent the activity of Sunshine Etopo, Inc. from August 17, 2009 (inception) through October 15, 2009, and the consolidated activity of Sunshine Etopo, Inc. and Sunshine Biopharma Inc. from October 15, 2009 forward. Sunshine Etopo, Inc. and Sunshine Biopharma, Inc. are hereinafter referred to collectively as the "Company". The Company was formed for the purposes of conducting research, development and commercialization of drugs for the treatment of various forms of cancer. The Company may also engage in any other business that is permitted by law, as designated by the Board of Directors of the Company.
PRINCIPLES OF CONSOLIDATION
The accompanying consolidated financial statements include the accounts of the Company and its wholly owned subsidiary. All intercompany accounts and transactions have been eliminated in consolidation.
USE OF ESTIMATES
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
STATEMENT OF CASH FLOWS
For purposes of the statement of cash flows, the Company considered demand deposits and highly liquid-debt instruments purchased with maturity of three months or less to be cash equivalents. Cash paid for interest during the years ended December 31, 2013 and 2012 was $0. Cash paid for income taxes during the years ended December 31, 2013 and 2012 was $0.
F-11
Sunshine Biopharma, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
December 31, 2012 and 2011 and
The period August 17, 2009 (inception) through December 31, 2013
Note 1 - Organization and Summary of Significant Accounting Policies (Continued)
BASIC EARNINGS PER SHARE
The net income (loss) per share is computed by dividing the net income (loss) by the weighted average number of shares of common stock outstanding. Warrants, stock options, and common stock issuable upon the conversion of the Company's preferred stock (if any), are not included in the computation if the effect would be anti-dilutive and would increase the earnings or decrease loss per share.
REVENUE RECOGNITION
The Company is a development stage pharmaceutical company focused on the research, development and commercialization of drugs for the treatment of various forms of cancer. The Company does not expect to generate revenues until clinical trials of its proposed products are completed. Once completed, revenues would be recognized as its technology is sold or its products become marketable.
FINANCIAL INSTRUMENTS
The carrying value of the Company’s financial instruments as reported in the accompanying balance sheet approximates fair value.
STOCK BASED COMPENSATION
The Company accounts for employee and non-employee stock awards under ASC 718, whereby equity instruments issued to employees for services are recorded based on the fair value of the instrument issued and those issued to non-employees are recorded based on the fair value of the consideration received or the fair value of the equity instrument, whichever is more reliably measurable.
DATE OF MANAGEMENT’S REVIEW
Subsequent events have been evaluated through the date of the issuance of these financial statements.
F-12
Sunshine Biopharma, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012 and
The period August 17, 2009 (inception) through December 31, 2013
OFFICER COMPENSATION
Through the period ended December 31, 2013 the officers and directors of the Company have not received any cash, stock, or other forms of compensation.
LEGAL FEES
During the years ended December 31, 2013 and 2012 legal fees were incurred as a result of services provided to the Company to assist with its regulatory requirements with the Securities and Exchange Commission.
Note 2 – Basis of Presentation
In the course of its life the Company has had limited operations, and has a working capital deficit. This raises substantial doubt about the Company’s ability to continue as a going concern.
The Company believes it can raise capital through equity sales and borrowing to fund its operations. Management believes this will contribute toward its subsequent profitability. The accompanying financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
Note 3 – Capital Stock
The Company’s authorized capital is comprised of 200,000,000 shares of $0.001 par value Common Stock and 5,000,000 shares of $0.10 par value Preferred Stock, to have such rights and preferences as the directors of the Company have or may assign from time to time. Out of the authorized Preferred Stock, the Company has designated 850,000 shares as Series A Preferred Stock (“Series A”). The Series A is convertible at any time after issuance into 20 shares of the Company's Common Stock with no further consideration, has full voting rights at 20 votes per share, and has superior liquidation rights to the common stock. Through December 31, 2013 and December 31, 2012, the Company has issued and outstanding a total of 60,299,061 and 51,416,092 shares of Common Stock and 0 and 0 shares of Series A Preferred Stock, respectively.
On August 17, 2009, the Company issued 703,118 shares of $0.001 par value Common Stock for services valued at $3,000 or $0.004 per share.
F-13
Sunshine Biopharma, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012 and
The period August 17, 2009 (inception) through December 31, 2013
Note 3 – Capital Stock (Continued)
On August 19, 2009, the Company issued 218,388 shares of $0.001 par value Common Stock for services valued at $932 or $0.004 per share.
On August 20, 2009, the Company issued 17,109,194 shares of $0.001 par value Common Stock for licenses valued at $73,000 or $0.004 per share.
On August 20, 2009, the Company issued 850,000 shares of $0.10 par value of Series “A” Convertible Preferred Stock for licenses valued at $73,000, or $0.086 per share.
In September and October, 2009, the Company issued 2,220,552 shares of $0.001 par value Common Stock for cash of $649,000 or $0.2922 per share as part of a private offering.
On September 30, 2009, the Company issued 1,710,748 shares of $0.001 par value Common Stock for assets valued at $500,000 or $0.2922 per share.
On October 31, 2009, the outstanding stock of Mountain West Business Solutions was counted as issued 888,000 shares of $0.001 par value Common Stock for Mountain West Business Solutions deficit of $ 29,465.
On November 16, 2009, the Company note holders converted their notes to 6,810,000 shares of $0.001 par value Common Stock for principal of $26,500 and interest of $2,965.
On June 2, 2010, the Company issued 1,675,000 shares of $0.001 par value Common Stock for services valued at $1,574,500 or $0.94 per share.
On September 30, 2010, the Company reversed issuance of 1,625,000 shares of $0.001 par value Common Stock for services valued at $1,527,500 or $0.94 per share.
On September 30, 2010, the Company issued 166,667 shares of $0.001 par value Common Stock for cash at $100,000 or $0.60 per share.
On October 1, 2010, the Company issued 217,000 shares of $0.001 par value Common Stock for services valued at $130,000 or $0.60 per share.
F-14
Sunshine Biopharma, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012 and
The period August 17, 2009 (inception) through December 31, 2013
Note 3 – Capital Stock (Continued)
On October 29, 2010, the Company issued 100,000 shares of $0.001 par value Common Stock for services valued at $60,000 or $0.60 per share.
On October 31, 2010, the Company issued 419,334 shares of $0.001 par value Common Stock for cash at $251,600 or $0.60 per share.
On November 30, 2010, the Company issued 78,334 shares of $0.001 par value Common Stock for cash at $47,000 or $0.60 per share.
On March 29, 2011, the Company issued 20,000 shares of $0.001 par value Common Stock for services valued at $12,000 or $0.60 per share.
On September 1, 2011, the Company issued 326,000 shares of $0.001 par value Common Stock for cash at $195,600 or $0.60 per share.
On November 3, 2011, the Company issued 400,000 shares of $0.001 par value Common Stock for services valued at $200,000 or $0.50 per share.
On September 16, 2011, the Company issued 291,500 shares of $0.001 par value Common Stock for services valued at $55,385 or $0.19 per share.
On December 21, 2011, the Company issued 17,000,000 shares of $0.001 par value Common Stock in exchange for the 850,000 shares of outstanding Series A Convertible Preferred Stock. At December 31, 2011 there is no Preferred Stock outstanding.
In June of 2012 the Company issued 230,000 shares of $0.001 par value restricted common stock for services valued at $69,000 or $0.30 per share.
In July 2012 the Company issued 44,000 shares of $0.001 par value restricted common stock for services valued at $15,400 or $0.35 per share.
In August 2012 the Company issued 128,250 shares of $0.001 par value restricted common stock for services valued at $38,475 or $0.30 per share.
F-15
Sunshine Biopharma, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012 and
The period August 17, 2009 (inception) through December 31, 2013
Note 3 – Capital Stock (Continued)
In August 2012 the Company issued 600,000 shares of $0.001 par value restricted common stock for services valued at $144,000 or $0.24 per share.
In October 2012 the Company issued 25,000 shares of $0.001 par value restricted common stock for services valued at $6,000 or $0.24 per share.
In January 2013 the Company issued 350,000 shares of $0.001 par value restricted common stock for services valued at $136,500 or $0.39 per share.
In March 2013 the Company issued 4,422,969 shares of $0.001 par value restricted common stock for services valued at $444,896 or $0.24 per share.
In May 2013 the Company issued 250,000 shares of $0.001 par value restricted common stock for services valued at $60,000 or $0.24 per share.
In August 2013 the Company issued 400,000 shares of $0.001 par value restricted common stock for services valued at $80,000 or $0.20 per share.
In October 2013 the Company issued 60,000 shares of $0.001 par value restricted common stock for services valued at $15,000 or $0.25 per share.
In November 2013 the Company issued 500,000 shares of $0.001 par value restricted common stock for cash of $95,000 or $0.19 per share that was used to pay expenses.
In November 2013 the Company issued 425,000 shares of $0.001 par value restricted common stock for cash of $85,000 or $0.20 per share that was used to pay expenses.
In November 2013 the Company issued 600,000 shares of $0.001 par value restricted common stock for R&D services valued at $114,000 or $0.20 per share.
In December 2013 the Company issued 75,000 shares of $0.001 par value restricted common stock for services valued at $15,000 or $0.20 per share.
F-16
Sunshine Biopharma, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012 and
The period August 17, 2009 (inception) through December 31, 2013
Note 3 – Capital Stock (Continued)
In December 2013 the Company issued 1,800,000 shares of $0.001 par value restricted common stock for services valued at $306,000 or $0.17 per share.
The Company has declared no dividends through December 31, 2013.
Note 4 – Income Taxes
Deferred income taxes arise from the temporary differences between financial statement and income tax recognition of net operating losses. These loss carryovers are limited under the Internal Revenue Code should a significant change in ownership occur. The Company accounts for income taxes pursuant to ASC 740. At December 31, 2013 and December 31, 2012, the Company had approximately $5,494,149 and $3,002,666, respectively, in unused federal net operating loss carryforwards, which begin to expire principally in the year 2029. A deferred tax asset at each date of approximately $1,098,830 and $600,533 resulting from the loss carryforwards has been offset by a 100% valuation allowance. The change in the valuation allowance for the period ended December 31, 2013 and December 31, 2012 was approximately $498,297 and $141,590.
The Company’s income tax filings are subject to audit by various taxing authorities. The Company’s open audit periods are 2010, 2011, and 2012, although, the statute of limitations for the 2010 tax year will expire effective March 15, 2014. In evaluating the Company’s provisions and accruals, future taxable income, and reversal of temporary differences, interpretations and tax planning strategies are considered. The Company believes its estimates are appropriate based on current facts and circumstances.
F-17
Sunshine Biopharma, Inc.
(A Development Stage Company)
Notes to Consolidated Financial Statements
December 31, 2013 and 2012 and
The period August 17, 2009 (inception) through December 31, 2013
Note 5 – Related Transactions
The Company has licensed its technology on an exclusive basis from Advanomics Corporation, a privately held Canadian company. Dr. Steve N. Slilaty, the Company’s Chief Executive Officer and a Director, is an Officer, Director and principal shareholder of Advanomics. In 2009 the Company issued an aggregate of 17,109,194 shares of its Common Stock valued at $73,000 and 850,000 shares of Series “A” Convertible Preferred Stock valued at $73,000 in exchange for this license, and has an option to purchase 2,000,000 shares of Advanomics common stock at $5 (US) per share within 1 year of September 30, 2009 as well as a second option to purchase an additional 1,000,000 shares of Licensor’s Common Stock at an exercise price of $10.00 (US) per share also for a 1 year term. The Company advanced further funds pursuant to this contract of $300,000. The total transaction costs to date of $446,000 have been written off as impaired.
In September 2009, the Company acquired certain assets from Sunshine Bio Investments Inc. Michele Di Turi, the Company’s Chief Operating Officer and Director, is President and Director of Sunshine Bio Investments Inc. The Company issued 1,710,748 shares of Common Stock valued at $500,000 in consideration for these assets. Goodwill was recognized on the transaction of $499,976 which has been written off.
Note 6 – Reverse Acquisition
On October 15, 2009 MWBS entered into an acquisition agreement (the "Agreement") with Sunshine Etopo, Inc., acquiring 100% of the outstanding common stock of Sunshine Etopo, Inc. through the issuance of 21,962,000 shares of its common stock with no readily available market price. The transaction was accounted for as a reverse acquisition as the shareholders of Sunshine Etopo, Inc. retained the majority of the outstanding common stock of MWBS after the share exchange. Effective with the Agreement, the Company's stockholders' equity was retroactively recapitalized as that of Sunshine Etopo, Inc., while 100% of the assets and liabilities of MWBS valued at $(29,465), consisting of notes payable and accrued interest of $29,465, were recorded as being acquired in the reverse acquisition for its 888,000 outstanding common shares on the acquisition date. (Immediately prior to the acquisition MWBS had 9,388,000 outstanding common shares. 8,500,000 of these shares were surrendered by the holders for cancellation). Subsequent to the October 15, 2009 recapitalization, MWBS and Sunshine Etopo, Inc. remain separate legal entities (with MWBS as the parent of Sunshine Etopo, Inc.). The accompanying consolidated financial statements exclude the financial position, results of operations and cash flows of MWBS prior to the October 15, 2009 acquisition. MWBS concurrent with the transaction changed its name to Sunshine Biopharma, Inc.
F-18
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth all costs and expenses, payable by us in connection with the sale of the common stock being registered. All amounts shown are estimates except for the SEC registration fee.
|
Amount to
|
||||
|
be Paid
|
||||
|
SEC registration fee
|
$ | 254 | ||
|
Edgar expenses
|
$ | 1,500 | ||
|
Legal fees and expenses
|
$ | 15,000 | ||
|
Accounting fees and expenses
|
$ | 3,500 | ||
|
Transfer agent and registrar fees and expenses
|
$ | 2,000 | ||
|
Miscellaneous expenses
|
$ | 3,500 | ||
|
Total
|
$ | 25,754 | ||
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Under the Colorado Statutes and our Articles of Incorporation, our directors and officers will have no personal liability to us or our shareholders for monetary damages incurred as the result of the breach or alleged breach by a director or officer of his “duty of care.” This provision does not apply to the directors’: (i) acts or omissions that involve intentional misconduct, fraud or a knowing and culpable violation of law, or (ii) approval of an unlawful dividend, distribution, stock repurchase or redemption. This provision would generally absolve directors of personal liability for negligence in the performance of his duties, including gross negligence.
The effect of this provision in our Articles of Incorporation is to eliminate the rights of our Company and our shareholders (through shareholder’s derivative suits on behalf of our Company) to recover monetary damages against a director for breach of his fiduciary duty of care as a director (including breaches resulting from negligent or grossly negligent behavior) except in the situations described in clauses (i) and (ii) above. This provision does not limit nor eliminate the rights of our Company or any shareholder to seek non-monetary relief such as an injunction or rescission in the event of a breach of a director’s duty of care. Section 7-109-102 of the Colorado Business Corporation Act provides corporations the right to indemnify their directors, officers, employees and agents in accordance with applicable law.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers, or persons controlling the Company pursuant to the foregoing provisions, the Company has been informed that, in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is therefore unenforceable.
Our Company has issued the following securities during the past three (3) years without registering the securities under the Securities Act:
|
●
|
On September 1, 2011, the Company issued 326,000 shares of $0.001 par value Common Stock for cash at $195,600 or $0.60 per share.
|
|
●
|
On November 3, 2011, the Company issued 400,000 shares of $0.001 par value Common Stock for services valued at $200,000 or $0.50 per share.
|
|
●
|
On September 16, 2011, the Company issued 291,500 shares of $0.001 par value Common Stock for services valued at $55,385 or $0.19 per share.
|
II-1
|
●
|
On December 21, 2011, the Company issued 17,000,000 shares of $0.001 par value Common Stock in exchange for the 850,000 shares of outstanding Series A Convertible Preferred Stock. At December 31, 2011 there is no Preferred Stock outstanding.
|
|
●
|
In June of 2012 the Company issued 230,000 shares of $0.001 par value restricted common stock for services valued at $69,000 or $0.30 per share.
|
|
●
|
In July 2012 the Company issued 44,000 shares of $0.001 par value restricted common stock for services valued at $15,400 or $0.35 per share.
|
|
●
|
In August 2012 the Company issued 128,250 shares of $0.001 par value restricted common stock for services valued at $38,475 or $0.30 per share.
|
|
●
|
In August 2012 the Company issued 600,000 shares of $0.001 par value restricted common stock for services valued at $144,000 or $0.24 per share.
|
|
●
|
In October 2012 the Company issued 25,000 shares of $0.001 par value restricted common stock for services valued at $6,000 or $0.24 per share.
|
|
●
|
In January 2013 the Company issued 350,000 shares of $0.001 par value restricted common stock for services valued at $136,500 or $0.39 per share.
|
|
●
|
In March 2013 the Company issued 4,422,969 shares of $0.001 par value restricted common stock for services valued at $444,896 or $0.24 per share.
|
|
●
|
In May 2013 the Company issued 250,000 shares of $0.001 par value restricted common stock for services valued at $60,000 or $0.24 per share.
|
|
●
|
In August 2013 the Company issued 400,000 shares of $0.001 par value restricted common stock for services valued at $80,000 or $0.20 per share.
|
|
●
|
In October 2013 the Company issued 60,000 shares of $0.001 par value restricted common stock for services valued at $15,000 or $0.25 per share.
|
|
●
|
In November 2013 the Company issued 500,000 shares of $0.001 par value restricted common stock for cash of $95,000 or $0.19 per share that was used to pay expenses.
|
|
●
|
In November 2013 the Company issued 425,000 shares of $0.001 par value restricted common stock for cash of $85,000 or $0.20 per share that was used to pay expenses.
|
|
●
|
In November 2013 the Company issued 600,000 shares of $0.001 par value restricted common stock for R&D services valued at $114,000 or $0.20 per share.
|
|
●
|
In December 2013 the Company issued 75,000 shares of $0.001 par value restricted common stock for services valued at $15,000 or $0.20 per share.
|
|
●
|
In December 2013 the Company issued 1,800,000 shares of $0.001 par value restricted common stock for services valued at $306,000 or $0.17 per share.
|
|
●
|
In January 2014 the Company issued 200,000 shares of $0.001 par value Common Stock for cash of $40,000 or $0.20 per share and was paid directly to an affiliated company for licensing rights.
|
|
●
|
In January 2014 the Company issued 600,000 shares of $0.001 par value Common Stock for R&D services valued at $96,000 or $0.16 per share.
|
|
●
|
In February 2014 the Company issued 66,667 shares of $0.001 par value Common Stock for cash of $13,333 or $0.20 per share and $3,333 was paid directly to an affiliated company for licensing rights.
|
|
●
|
In March 2014 the Company issued 10,000 shares of $0.001 par value Common Stock for services valued at $1,400 or $0.14 per share.
|
|
●
|
In March 2014 the Company issued 1,000,000 shares of $0.001 par value Common Stock for services valued at $170,000 or $0.17 per share.
|
|
●
|
On March 27, 2014 the Company issued 500,000 shares of $0.001 par value Common Stock for origination fee valued at $75,000 or $0.15 per share as part of a convertible note payable for $100,000.
|
|
●
|
On April 23, 2014, the Company issued 400,000 shares of its $0.001 par value Common Stock as an origination fee to the Selling Shareholder, which shares were valued at $60,000, or $0.15 per share.
|
|
●
|
On May 15, 2014, the Company issued 500,000 shares of its $0.001 par value Common Stock for services valued at $75,000, or $0.15 per share.
|
No underwriters were utilized and no commissions or fees were paid with respect to any of the above transactions. These persons were the only offerees in connection with these transactions. We relied on Regulation D and Section 4(2) of the Securities Act to issue these securities since the transactions did not involve any public offering.
II-2
ITEM 16. EXHIBIT INDEX.
|
No.
|
Description
|
Filed With
|
Date
|
|||
|
3.1
|
Articles of Incorporation
|
Form SB-2 Registration Statement
|
October 19, 2007
|
|||
|
3.2
|
Bylaws
|
Form SB-2 Registration Statement
|
October 19, 2007
|
|||
|
3.3
|
Articles of Amendment (Name Change)
|
Form 8-K Dated November 2, 2009
|
November 6, 2009
|
|||
|
3.5
|
Articles of Amendment (Increase Authorized)
|
Form 10-Q For Quarter Ended June 30, 2010
|
August 4, 2010
|
|||
|
Opinion of Andrew I. Telsey, P.C. re: legality
|
Filed herewith
|
|||||
|
10.1
|
Share Exchange Agreement with Sunshine Biopharma, Inc.
|
Form 8-K Dated October 15, 2009
|
October 20, 2009
|
|||
|
10.2
|
License Agreement with Advanomics, Inc.
|
Form 8-K/A1 Dated October 15, 2009
|
January 19, 2010
|
|||
|
10.3
|
Amendment No. 1 to License Agreement with Advanomics, Inc.
|
Form 8-K/A1 Dated October 15, 2009
|
January 19, 2010
|
|||
|
10.4
|
Research Agreement with The Research Foundation of the State University of New York
|
Form 8-K Dated January 20, 2011
|
January 20, 2011
|
|
No.
|
Description
|
Filed With
|
Date
|
|||
|
10.5
|
Research Agreement with Jewish General Hospital
|
Form 8-K Dated June 14, 2011
|
June 17, 2011
|
|||
|
10.6
|
Amendmentt No. 2 to License Agreement with Advanomics, Inc.
|
Form 8-K Dated December 21, 2011
|
December 27, 2011
|
|||
|
10.7
|
Investment Agreement with Dutchess Investment Group II
|
Form 8-K dated April 23, 2014
|
April 28, 2014
|
|||
|
10.8
|
Registration Rights Agreement with Dutchess Investment Group II
|
Form 8-K dated April 23, 2014
|
April 28, 2014
|
|||
|
16.1
|
Letter from Ronald R. Chadwick
|
Form 8-K dated January 30, 2012
|
February 7, 2012
|
|||
|
16.2
|
Letter from Borgers and Culter CPA’s PLLC
|
Form 8-K dated February 25, 2013
|
February 25, 2013
|
|||
|
21.1
|
List of Subsidiaries
|
Form 10-K For Fiscal Year Ended July 31, 2009
|
October 30, 2009
|
|||
|
Consent of Ben Borgers CPA PC
|
Filed herewith
|
|||||
|
Consent of Andrew I. Telsey, P.C.
|
Filed herewith
|
ITEM 22. UNDERTAKINGS.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2)That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3)To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4)That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Montreal, Province of Ontario on May 22, 2014.
|
SUNSHINE BIOPHARMA, INC.
|
|||
|
By:
|
/s/ Dr. Steve N. Slilaty
|
||
|
Dr. Steve N. Slilaty, President, Principal Executive Officer
|
|||
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
By:
|
/s/ Dr. Steve N. Slilaty
|
|
|
Dr. Steve N. Slilaty
|
||
|
Principal Executive Officer
|
||
|
President and Director
|
||
|
Date: May 22, 2014
|
||
|
By:
|
/s/ Camille Sebaaly
|
|
|
Camille Sebaaly
|
||
|
Principal Accounting Officer,
|
||
|
Principal Financial Officer,
|
||
|
Director
|
||
|
Date: May 22, 2014
|
||
|
By:
|
/s/ Michele Di Turi
|
|
|
Michele Di Turi
|
||
|
Director
|
||
|
Date: May 22, 2014
|
II-4
