Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REPROS THERAPEUTICS INC. | v377691_8k.htm |

Developing clinical stage small molecule therapeutics to treat hormonal and reproductive system disorders

Repros Disclaimer Any statements made by the Company that are not historical facts contained in these slides (or in any oral accompanying discussion) are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to various risks, uncertainties and other factors that could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by such forward - looking statements. These statements often include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “project,” “potential,” “intend,” “belie ve, ” “plan,” “seek,” “could,” “can,” “should” or similar expressions. These statements are based on assumptions that the Company has made in light of the Company’s experience in the industry, as well as the Company’s perceptions of historical trends, current cond iti ons, expected future developments and other factors the Company believes are appropriate in these circumstances. Forward - looking statements include, but are not limited to, those relating to anticipated milestones for Androxal® and Proellex®, the conduct of planned clinical studies and the timing and nature of the results thereof, the markets for the Company’s products and the pot ent ial success of the Company in penetrating those markets and that the Company’s need for and use of financial resources. Such statements are based on current expectations that involve a number of known and unknown risks, uncertainties and other factor s that may cause actual events to be materially different from those expressed or implied by such forward - looking statements, including the ability to raise additional needed capital on a timely basis in order for it to continue to fund development of it s Androxal® and Proellex® programs, the ability to have success in the clinical development of its technologies, the reliabilit y o f interim results to predict final study outcomes, and such other risks as are identified in the Company's most recent Annual R epo rt on Form 10 - K and the subsequent quarterly report on Form 10 - Q and in the prospectus supplement and the accompanying prospectus included in the registration statement mentioned below. These documents are available on request from Repros or at www.sec.gov. Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a resul t of new information, future events or otherwise. In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry. We obtained t his information and these statistics from third - party sources, which we have supplemented where necessary with information from publicly available sources and our own internal estimates. Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information. While we believe that each of these studies and publications is reliable, we have not independently verified suc h d ata, and we make no any representation as to the accuracy of such information. Similarly, we believe our internal research is reli abl e, but it has not been verified by any independent sources.

Investment Highlights for 2014 Significant Value Creating Events for Androxal , An Oral Treatment for Low T Androxal Restores the Body’s ability to Produce Its Own Testosterone • Androxal ® 2014 Milestones – Complete all clinical studies – Complete head - to - head studies of Androxal versus Androgel 1.62 for label claims and demonstration of efficacy benefits for Androxal – Submit NDA • Proellex : “ An oral treatment for uterine fibroids and endometriosis” – Proellex 2014 Milestones • Position program to enter Phase 3 in 2015

Testosterone Market and Androxal Overview • US market for low testosterone exceeds $1.5 billion • Only approved non invasive therapies are hormone replacements • Repros believes 85% of hypogonadal men experience low T due to an endocrine disorder – These men have functional but un - stimulated testes – Hypothalamic - pituitary suppression due to estrogen • Androxal is the only oral medication in development that treats the underlying disorder for the majority of hypogonadal men

Who are the men using testosterone? European Male Aging Study Distribution and Selected Characteristics of Men Ages 40 - 79 ( Tajar et al) 0 10 20 30 40 50 60 70 80 90 100 Eugonadal Secondary Hypogonadal Primary Compensated Hypogonadism % of Subjects Age: 58.5 (10.7) BMI: 27.3 TT: 513.4 ng / dL LH: 5.2 U/L Age: 59.4 (10.4) BMI: 30.8 (4.8) TT: 250.9 ng / dL LH: 4.4 U/L Age: 70.0 (9.0) BMI: 29.0 (3.9) TT: 216 ng / dL LH: 18.0 U/L Age: 67.3 (9.9) BMI: 26.8 (3.6) TT: 527.8 LH: 14.1 U/L Data derived from over 3000 men Overweight BMI > 25 (6’ 190# male BMI=25.8) Obese BMI > 30 (6’ 230# male BMI =31.2) Obese men (BMI>30) 8.7X more likely to have low T than men of normal weight In 2010 there were ~90 million men in the US between the ages of 20 and 65 32% are obese

Day to Day Morning T Variability in Men with Secondary Hypogonadism Sampling of Placebo Subjects ZA - 003

Estrogen Exogenous Testosterone Override Suppresses LH and FSH

Head to Head Comparison of Two Doses of Androxal to Testim and Placebo, Study ZA - 203 An approved T gel significantly suppresses LH and FSH in a clinically relevant manner and suppresses sperm concentration to near severe oligospermia (median sperm concentration 6.2 million/mL) after only three months of treatment 0 2 4 6 8 10 12 14 12.5 mg 25 mg PL Testim FSH Treatment Effect of Treatment on Median FSH p versus Testim Before After p<0.00001 p=0.0004 0 10 20 30 40 50 60 70 80 90 100 12.5 mg 25 mg PL Testim [Sperm] in Millions/ml Treatment Effect of Treatment on Median Sperm Concentration p versus Testim Baseline EOS p=0.012 p=0.0021 p=0.0049 0 2 4 6 8 10 12 12.5 mg 25 mg PL Testim LH Treatment Effect of Treatment on Median LH p versus Testim Before After p=0.0004 0 50 100 150 200 250 300 350 400 450 500 12.5 mg 25 mg PL Testim TT in ng/dL Treatment Effect of Treatment on Median Serum TT p versus placebo Before After p<0.00001 p=0.0002 p<0.00001

• July 2002 IND for Androxal to Treat 2 ° Hypogonadism • Nov.’04 24hr Serial Testosterone to determine average and maximum concentration • Nov.’04(+1) Testosterone endpoint not acceptable because Androxal is not testosterone • 2007 Even though Androxal is non inferior to Androgel with numerous advantages, T still not an acceptable endpoint • Late 2007 FDA willing to entertain proof of concept head - to - head study of Androxal versus approved testosterone gel and impact on spermatogenesis • 2010 Endocrine Division accepts IND for to study Androxal’s glycemic effects in diabetic men • Nov. ‘10 Urology Division accepts co - primary of testosterone as an endpoint for studies of Androxal in the treatment of secondary hypogonadism along with spermatogenesis effects compared to testosterone control • June’12 SPA Obtained for Androxal using FDA suggested non inferiorty compared to placebo for changes in sperm concentration, comparisons to testosterone not recommended • Feb. ’14 FDA deems non inferiority compared to placebo as per SPA may be insufficient to assess risk benefit ratio • Apr. ’14 FDA recommends co primary spermatogenesis endpoints versus approved testosterone gel Evolving FDA Requirements for Endpoints

Head to Head Studies Comparing Androxal to Androgel 1.62 Endpoints Changed per Late April FDA Guidance • ZA - 304 & ZA - 305: Two identical studies not under SPA – 3 arm parallel design with 40 subjects per arm – 23 US clinical sites – Primary endpoint comparison of Androxal and Androgel with respect to 2 co - primary sperm endpoints • % of men with average sperm concentration <10 million/mL • % change from baseline – Numerous comparative secondary endpoints – 16 week duration for dosing plus 1 week follow - up – Anticipate topline results Oct. ‘14

ZA - 304 & ZA - 305 Efficacy Endpoints Endpoint Class Endpoint Goal Powering Primary % of subjects with sperm concentration <10X10 6 /mL Comparing Androxal to Androgel 1.62% Show Androxal superior to Androgel 10% vs 50% 98.6% % change from baseline comparing Androxal to Androgel 1.62% Show Androxal superior to Androgel - 5% vs - 65%, SD=85 80% power Secondary change from baseline for LH comparing Androxal to Androgel 1.62% Show Androxal superior to Androgel from perspective of no negative effect on pituitary secretions. One of two mechanisms of exogenous T suppression of pituitary and testicular function 3.6 vs - 2.4, SD=5 100% power change from baseline for FSH comparing Androxal to Androgel 1.62% Show Androxal superior to Androgel from perspective of no negative effect on pituitary secretions. One of two mechanisms of exogenous T suppression of pituitary and testicular function 3.7 vs - 4, SD=5 100% power Responder analysis assessing % of men within normal range for 24 - hr avg T and sperm count >15X10 6 /mL comparing Androxal to Androgel 1.62% Show Androxal superior to Androgel in normalizing testicular function 30% vs 70% 95.7% power Comparison of Androxal to placebo for 24 hr average T Androxal to be superior 400 vs 245, SD=160 99% Comparison of Androxal to Androgel 1.62% regarding morning T one week post end of active dosing period Show suppression of testicular function by Androgel as a result of suppressed pituitary function. Androxal expected to be superior to Androgel 440 vs 300, SD = 180 93% power Comparison of Androxal to Androgel 1.62% for effect on raising hematocrit by end of study Show Androxal is superior to Androgel regarding elevations of hematocrit. This is a key safety parameter and demonstrates less risk for Androxal in clinical practice 1.2 vs 2.6, SD=2.9 Power = 56.8, 80% power if Androxal is 1.06 instead

ZA - 305 Baseline Morning Testosterone (fully enrolled, n=127) Mean Age: 47 Mean BMI: 33.4 Baseline Morning Testosterone (ng/ dL ) n 127 Mean 216.2 Median 214.0 Standard Deviation 54.1 (Minimum, Maximum) (79.0, 299.0)

ZA - 305 Baseline Semen Concentration 0 2 4 6 8 10 12 14 16 18 20 22 24 15 25 35 45 55 65 75 85 95 100 150 200 250 More Number of Subjects with Baseline Semen Concentration Baseline Semen Concentration Histogram Mean Semen Concentration 78.0 million/mL Median Semen Concentration 61.3 million/mL

Experience with Percentage Change in Semen Concentration Androxal (ZA-301) Androxal (ZA-302) Androxal 12.5 mg (ZA-203) Androxal 25 mg (ZA-203) Testim (ZA-203) Median -3.3 -1.3 -19.5 -15.9 -88.2 -100.0 -90.0 -80.0 -70.0 -60.0 -50.0 -40.0 -30.0 -20.0 -10.0 0.0 Median Percentage Change from Baseline in Semen Concentration

Median Semen Concentrations in ZA - 203 Baseline 3 Months 1 Week Post Drug 4 Weeks Post Drug 12.5 mg 106.8 60.3 72.5 67.0 25 mg 66.0 47.0 98.8 64.0 Placebo 44.0 77.0 70.4 73.4 Testim 60.0 6.4 2.2 19.3 0.0 20.0 40.0 60.0 80.0 100.0 120.0 Sperm Concentration (million/mL)
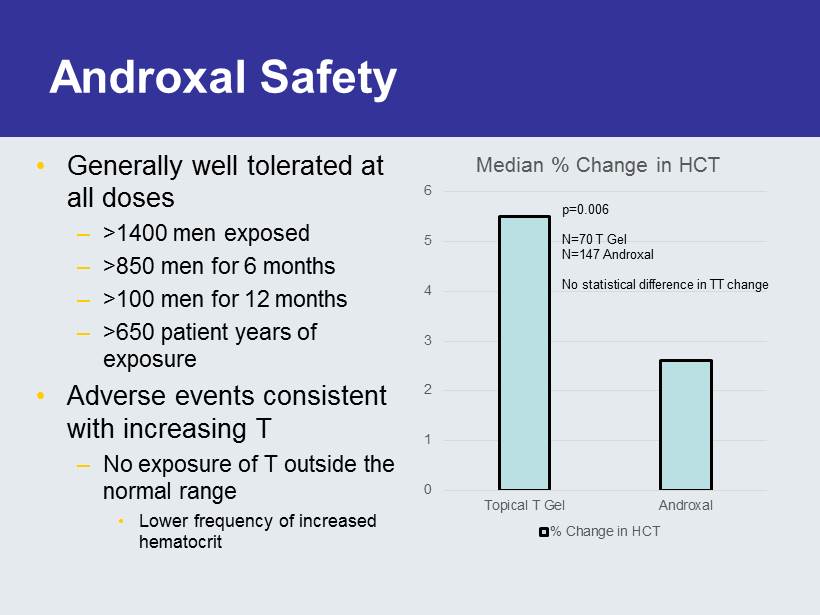
Androxal Safety • Generally well tolerated at all doses – >1400 men exposed – >850 men for 6 months – >100 men for 12 months – >650 patient years of exposure • Adverse events consistent with increasing T – No exposure of T outside the normal range • Lower frequency of increased hematocrit 0 1 2 3 4 5 6 Topical T Gel Androxal Median % Change in HCT % Change in HCT p=0.006 N=70 T Gel N=147 Androxal No statistical difference in TT change
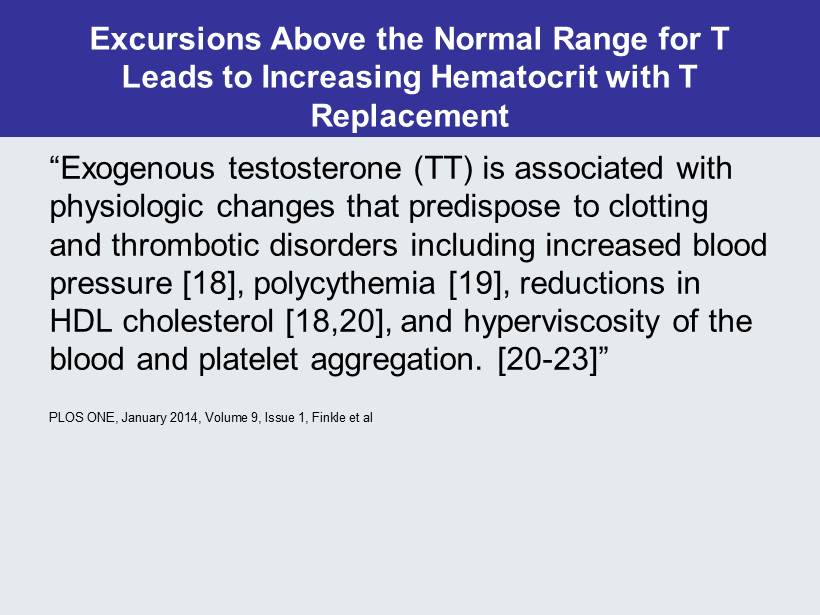
Excursions Above the Normal Range for T Leads to Increasing Hematocrit with T Replacement “Exogenous testosterone (TT) is associated with physiologic changes that predispose to clotting and thrombotic disorders including increased blood pressure [18], polycythemia [19], reductions in HDL cholesterol [18,20], and hyperviscosity of the blood and platelet aggregation. [20 - 23 ]” PLOS ONE, January 2014, Volume 9, Issue 1, Finkle et al

ZA - 204 24 Hr Assessment Androgel 1% 0 500 1000 1500 2000 2500 3000 0 5 10 15 20 Total Testosterone Hours 3 of 13 Subjects in Androgel Arm Exhibited T>1040 2012 2011 2052

24 Hour T & LH 25 mg Androxal Subject Study ZA - 204 Subject 2 - 003 Age: 55, BMI: 32 Baseline 0 time: 8:09 AM Week 6 0 time: 7:25 AM

ZA - 300 Study • 499 Subjects randomized – Baseline Morning T < 300 – 23.5% Type II Diabetes – 30.6% taking c holesterol medication – 33.3% previous T exposure • 6 months of treatment • 2 months of follow - up post treatment • 393 Subjects (78.8%) completed study – 23 (4.6%) Withdrew due to an Adverse Event
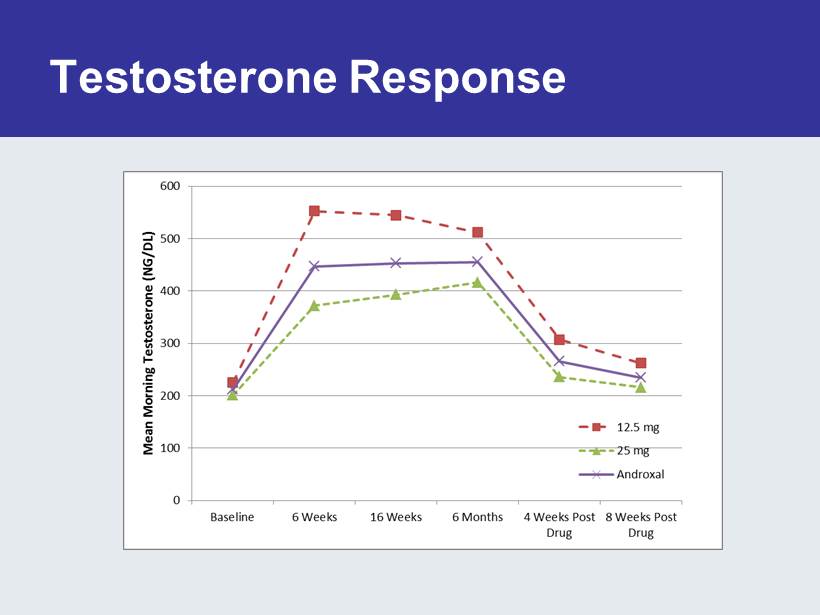
Testosterone Response

ZA - 300 Additional Findings after 6 months of treatment • Androxal improves symptoms of metabolic syndrome – Androxal reduces total cholesterol and improves HDL/LDL ratio – Androxal reduces fasting plasma glucose levels – Androxal shifts A1c into normal range in a statistically significant manner • Men previously exposed to T treatment do not respond as well

Influence of Prior Testosterone

ZA - 300: Safety • 499 subjects treated with Androxal – For up to 26 weeks • No new safety issues identified – No deaths – No significant cardiovascular findings – No significant eye findings • Venous Thromboembolism – One case (02 - 029) with deep vein thrombosis (DVT) and pulmonary emboli (PE), multiple risk factors – Another case (17 - 007) of DVT in prior T subject entering study with high hematocrit, resolved with treatment continued – Caused by high hematocrit – A known complication of testosterone elevation

Two Cases of VTE from ZA - 300 39 44 49 54 59 0 50 100 150 200 250 300 350 400 450 Screening Baseline Week 6 Week 16 Morning T Hematocrit 39 44 49 54 59 0 100 200 300 400 500 600 700 Screening Baseline Week 6 Week 16 Week 26 Morning T Hematocrit Subject 02 - 029, 12.5 - 25 mg dose, African American, 49 years old, BMI: 39, Height 6’5” Subject 17 - 007, 12.5mg dose, Middle Eastern, 54 years old, BMI: 27.4, Height 5’7” T use immediately before enrolling, LH =0.1 VTE at week 6 after long air flight VTE at week 18 after long trip Subject discontinued

Treatment Emergent Cardiac Events ZA - 300 EVENT 12.5 mg (N=207)* n (%) 25 mg (N=283)* n (%) CARDIAC DISORDERS 2 (0.97) 2 (0.71) Atrial Flutter 0 (0) 1 (0.35) Bradycardia 2 (0.97) 0 (0) Palpitations 0 (0) 1 (0.35) * Some men withdrew from the study before the first visit after baseline and did not return to the clinic such that any AE’s c oul d be determined
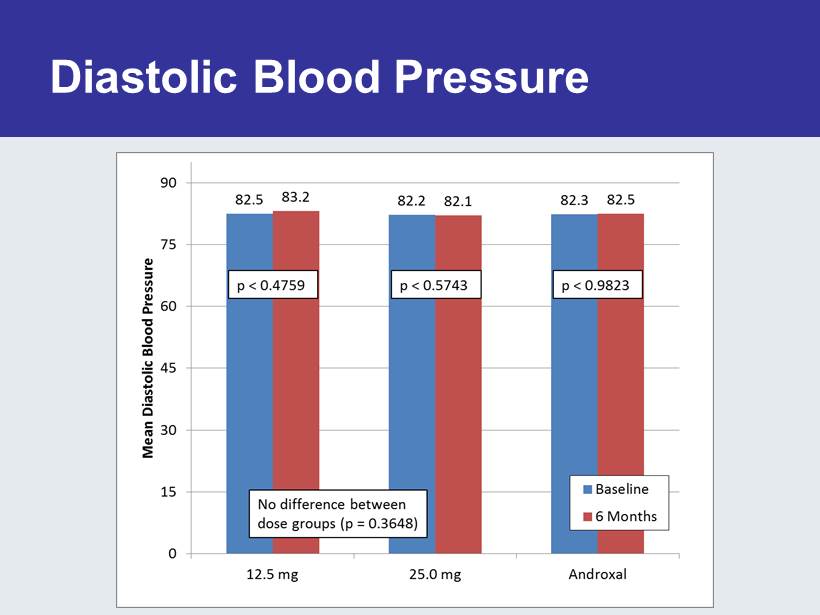
Diastolic Blood Pressure

Androxal Exhibits Unique Profile with Numerous Advantages vs Approved Hormone Replacement • The Androxal Advantages – Oral – Not controlled substance, cannot be abused – Less CV risk than T’s??? • No supernormal levels of T achieved – No transference risk – Restores normal function (no loss of testicular function) – Does not develop dependency – Avoids withdrawal symptoms – With lifestyle change can reverse disorder and result in no need for therapy

Commercial Goals for Androxal • Low T market already at primary care, 75% of written scripts • Specialists readily reached by Repros initiative – Conservative Quintiles assessment full year 1 sales = $120 million using small sales force (n=41) – Cash - flow positive during the first year of launch – Androxal is a high margin product • Androxal asset unencumbered • Repros goal to maximize shareholder return

Proellex for the Treatment of Uterine Fibroids and Endometriosis • Over 30 million women of reproductive age in the US afflicted with symptomatic uterine fibroids or endometriosis • Over 300,000 hysterectomies performed every year in the US to treat these two disorders • No acceptable chronic therapeutic options available today
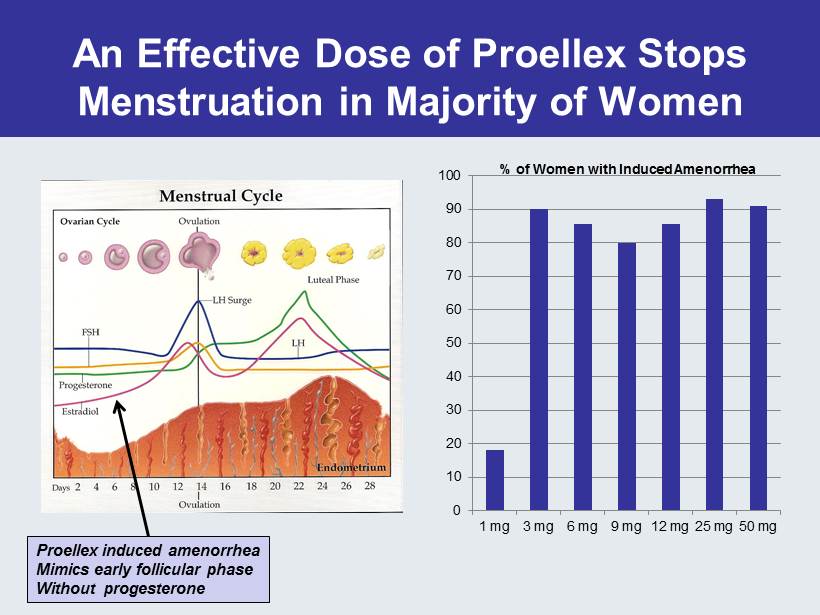
An Effective Dose of Proellex Stops Menstruation in Majority of Women 0 10 20 30 40 50 60 70 80 90 100 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg % of Women with Induced Amenorrhea Proellex induced amenorrhea Mimics early follicular phase Without progesterone
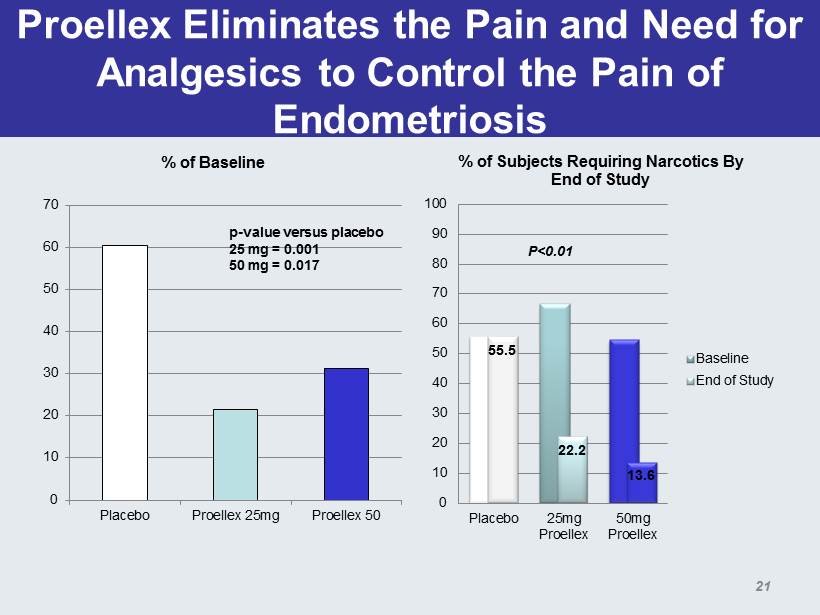
Proellex Eliminates the Pain and Need for Analgesics to Control the Pain of Endometriosis 0 10 20 30 40 50 60 70 Placebo Proellex 25mg Proellex 50 % of Baseline p - value versus placebo 25 mg = 0.001 50 mg = 0.017 55.5 22.2 13.6 0 10 20 30 40 50 60 70 80 90 100 Placebo 25mg Proellex 50mg Proellex % of Subjects Requiring Narcotics By End of Study Baseline End of Study P<0.01 21

Key Symptom Driving Women to Seek Therapy for Uterine Fibroids Excessive Menstrual Bleeding and Bulk Symptoms Early Development Results 0 20 40 60 80 100 120 140 160 BL Mo 1 Mo 2 Mo 3 PBAC Score Pictorial Blood Loss Assessment Placebo Proellex 12.5 mg P<0.0001 0 5 10 15 20 25 30 35 40 45 Placebo Proellex 12.5 Normal Symptom Severity Menorrhagia=PBAC>80 US Phase 2b (n=127)
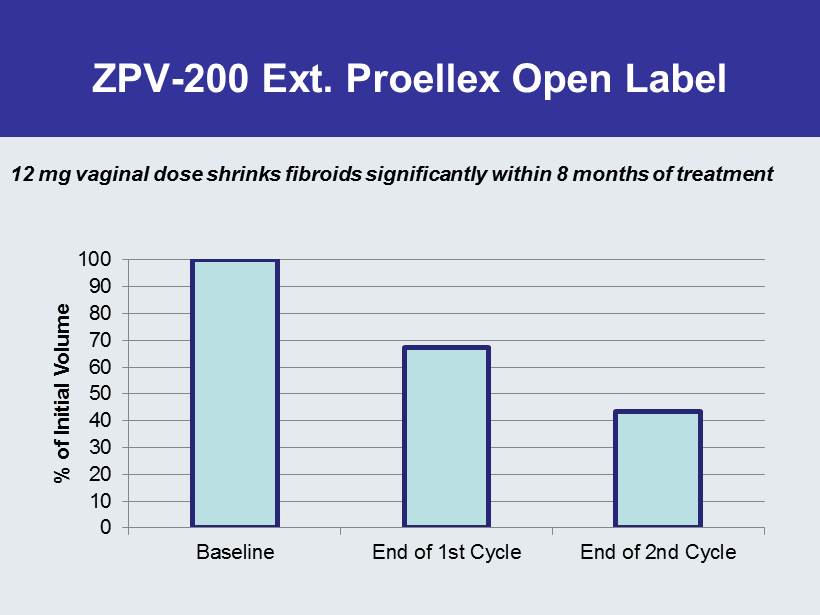
ZPV - 200 Ext. Proellex Open Label 0 10 20 30 40 50 60 70 80 90 100 Baseline End of 1st Cycle End of 2nd Cycle % of Initial Volume 12 mg vaginal dose shrinks fibroids significantly within 8 months of treatment

Proellex Program Status & Goals • All Pre - clinical Studies Complete • 60 Subject Phase 2 Low Dose Oral Endometriosis Study Enrolling – Adding foreign sites – Expect full enrollment in the second half of 2014 • Phase 2 Low Dose Oral Fibroid Study Submitted to FDA – Full hold lifted to partial hold to allow Phase 1 and 2 studies • Phase 2b Vaginal Fibroid Study to be submitted to FDA in Q - 2, 2014 – No clinical hold issues • Enter Phase 3 • Uterine Fibroids: 2015 • Endometriosis: Late 2015

Financial Summary • Cash and equivalents (as of 3/31/14, unaudited) $68 M • Cash runway: Through 2016* – Anticipate Androxal approved and launched • Current shares outstanding: 23.1 M shares – Warrants Outstanding – Series A – 877,137 (purchased in unit deal @ $2.45); Series B – 809,805 @ $2.49 exercise price. *Excludes marketing and commercialization expenses for Androxal
