Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a14-11911_18k.htm |
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a14-11911_1ex99d1.htm |
Exhibit 99.2
|
|
1Q14 Financial Results Conference Call & Webcast May 5, 2014 |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus’ candidate drug products, cash runway, ongoing collaborations and the timing and reporting of results from clinical trials evaluating Amicus’ candidate drug products. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2013. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. |
|
|
Agenda Opening Remarks Summary of Phase 3 Monotherapy Study (Study 011) 12 and 24 Month Results Update on Next-Generation ERTs in Fabry, Pompe and MPS I Financial Results Q&A |
|
|
Migalastat Monotherapy: Study 011 12- and 24-Month Data - Key Findings Subjects who switched from placebo to migalastat after month 6 demonstrated a statistically significant reduction in kidney interstitial capillary GL-3 at month 12 (p=0.013*) Subjects who remained on migalastat for 12 months demonstrated a durable reduction in kidney interstitial capillary GL-3 Reduction in disease substrate also observed in plasma lyso-Gb3 in subjects who switched from placebo to migalastat (p<0.0001**). Subjects who remained on migalastat demonstrated a durable reduction in lyso-Gb3 Kidney function (estimated glomerular filtration rate (eGFR), iohexol mGFR) remained stable over 18-24 months Migalastat was generally safe and well-tolerated Of 41 subjects with GLP HEK amenable mutations who completed Study 011, 35 (85%) remain in voluntary extension study (Study 041) Migalastat Demonstrated Statistically Significant and Durable Substrate Reductions on 12-Month Pre-Specified Primary Analysis in Fabry Patients with Amenable Mutations *MMRM, **ANCOVA |
|
|
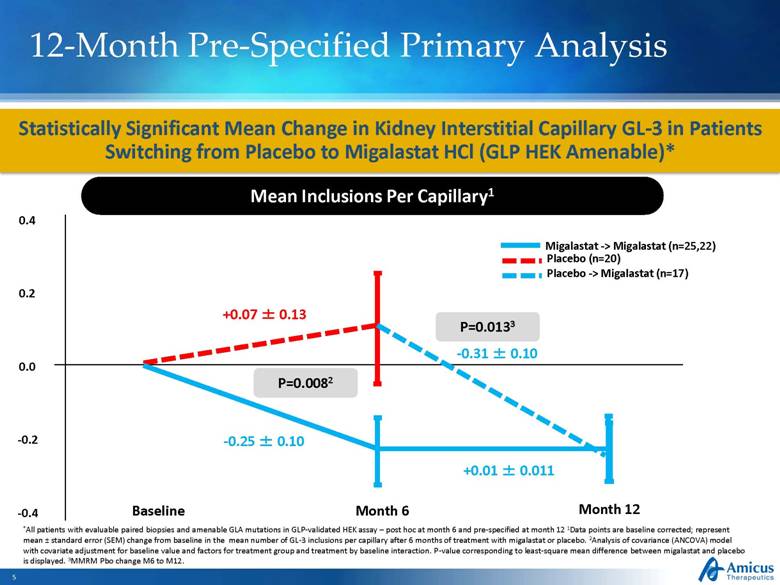
12-Month Pre-Specified Primary Analysis Statistically Significant Mean Change in Kidney Interstitial Capillary GL-3 in Patients Switching from Placebo to Migalastat HCl (GLP HEK Amenable)* Mean Inclusions Per Capillary1 Baseline Month 6 +0.07 ± 0.13 -0.25 ± 0.10 P=0.0082 *All patients with evaluable paired biopsies and amenable GLA mutations in GLP-validated HEK assay – post hoc at month 6 and pre-specified at month 12 1Data points are baseline corrected; represent mean ± standard error (SEM) change from baseline in the mean number of GL-3 inclusions per capillary after 6 months of treatment with migalastat or placebo. 2Analysis of covariance (ANCOVA) model with covariate adjustment for baseline value and factors for treatment group and treatment by baseline interaction. P-value corresponding to least-square mean difference between migalastat and placebo is displayed. 3MMRM Pbo change M6 to M12. Month 12 -0.31 ± 0.10 +0.01 ± 0.011 P=0.0133 0.4 0.0 -0.2 -0.4 0.2 Placebo -> Migalastat (n=17) Migalastat -> Migalastat (n=25,22) Placebo (n=20) |
|
|
Disease Substrate in Plasma (Plasma Lyso-GB3) Statistically Significant Reduction in Plasma Lyso-GB3 at Month 6 and Month 12 Following Treatment with Migalastat (GLP HEK Amenable)* 5.0 -5.0 -10.0 -15.0 10.0 Plasma Lyso-GB31 Baseline Month 6 +0.60 ± 2.4 -11.2±4.8 P=0.00332 -20.0 0.0 *Patients with amenable GLA mutations in GLP-validated HEK assay 1Baseline corrected. Error bars are SEM 2ANCOVA comparing migalstat to placebo in Stage 1 3ANCOVA comparing change from month 6 to month 12 in subjects switching from placebo to migalastat Month 12 +1.2 ±1.3 P<0.00013 Placebo -> Migalastat (n=13) Migalastat -> Migalastat (n=18,18) -15.5±6.2 Placebo (n=13) -25.0 |
|
|
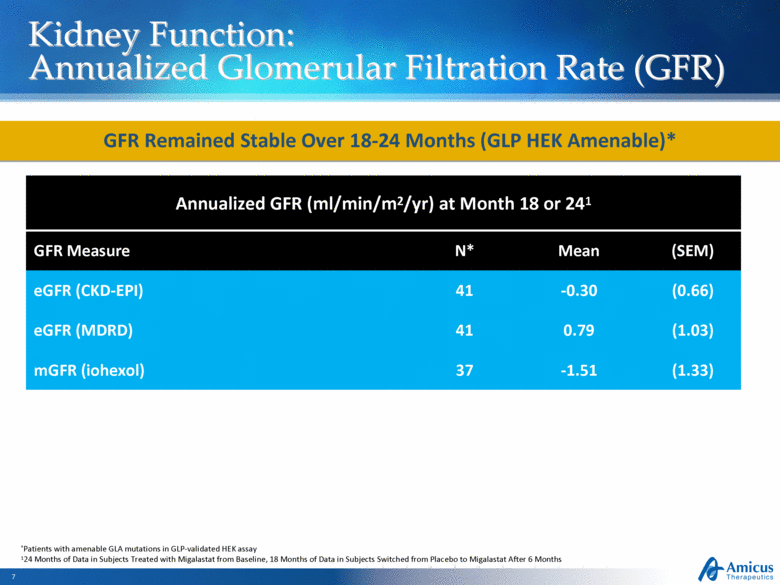
GFR Remained Stable Over 18-24 Months (GLP HEK Amenable)* Annualized GFR (ml/min/m2/yr) at Month 18 or 241 GFR Measure N* Mean (SEM) eGFR (CKD-EPI) 41 -0.30 (0.66) eGFR (MDRD) 41 0.79 (1.03) mGFR (iohexol) 37 -1.51 (1.33) Kidney Function: Annualized Glomerular Filtration Rate (GFR) *Patients with amenable GLA mutations in GLP-validated HEK assay 124 Months of Data in Subjects Treated with Migalastat from Baseline, 18 Months of Data in Subjects Switched from Placebo to Migalastat After 6 Months |
|
|
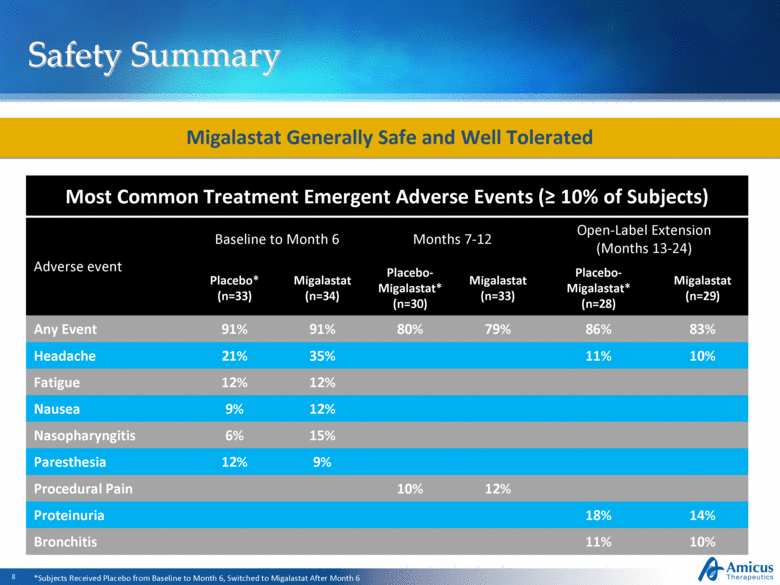
Safety Summary Most Common Treatment Emergent Adverse Events (> 10% of Subjects) Adverse event Baseline to Month 6 Months 7-12 Open-Label Extension (Months 13-24) Placebo* (n=33) Migalastat (n=34) Placebo-Migalastat* (n=30) Migalastat (n=33) Placebo-Migalastat* (n=28) Migalastat (n=29) Any Event 91% 91% 80% 79% 86% 83% Headache 21% 35% 11% 10% Fatigue 12% 12% Nausea 9% 12% Nasopharyngitis 6% 15% Paresthesia 12% 9% Procedural Pain 10% 12% Proteinuria 18% 14% Bronchitis 11% 10% Migalastat Generally Safe and Well Tolerated *Subjects Received Placebo from Baseline to Month 6, Switched to Migalastat After Month 6 |
|
|
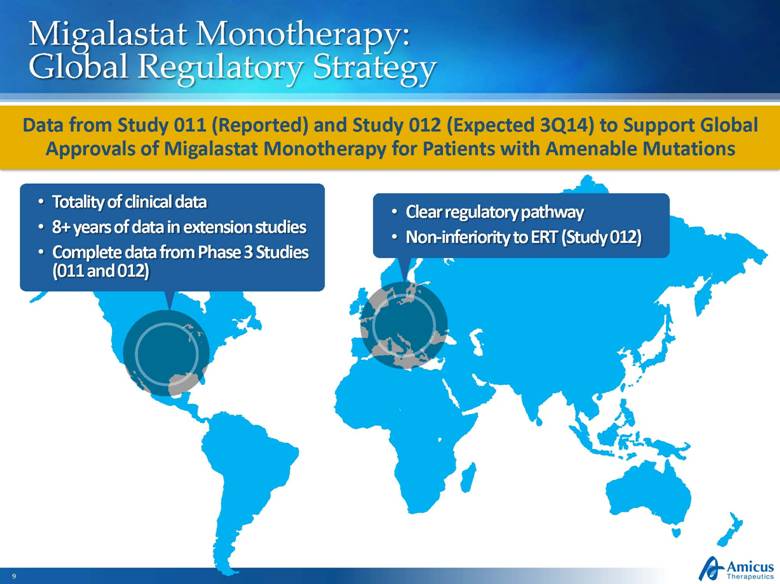
Migalastat Monotherapy: Global Regulatory Strategy Totality of clinical data 8+ years of data in extension studies Complete data from Phase 3 Studies (011 and 012) Clear regulatory pathway Non-inferiority to ERT (Study 012) Data from Study 011 (Reported) and Study 012 (Expected 3Q14) to Support Global Approvals of Migalastat Monotherapy for Patients with Amenable Mutations |
|
|
3-in-3 Strategy: Pathway to Clinic Milestones Fabry Next-Generation ERT 1H14 Phase 1 study initiation of IV migalastat in healthy volunteers 2H14 Phase 1/2 study initiation Executing Strategy to Advance 3 Next-Generation ERTs into Clinic in Next 3 Years with Lead Programs in Fabry, Pompe and MPS I Milestones Pompe Next-Generation ERT 1Q14 Initial preclinical proof-of-concept presented at LDN WORLD Ongoing Longer-term preclinical proof-of-concept studies to optimize product for clinic with better tissue uptake and enzyme stability Ongoing Manufacturing scale-up activities 2015 Phase 1/2 study initiation |
|
|
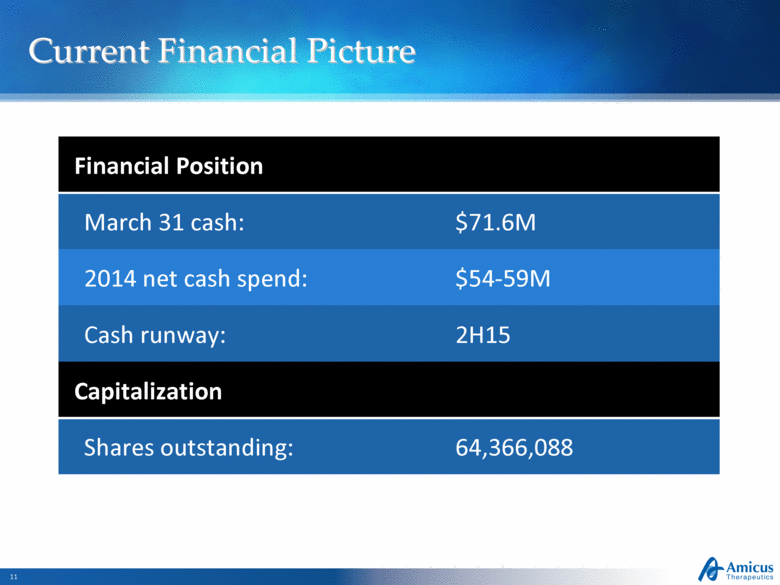
Current Financial Picture Slide 11 Financial Position March 31 cash: $71.6M 2014 net cash spend: $54-59M Cash runway: 2H15 Capitalization Shares outstanding: 64,366,088 |
|
|
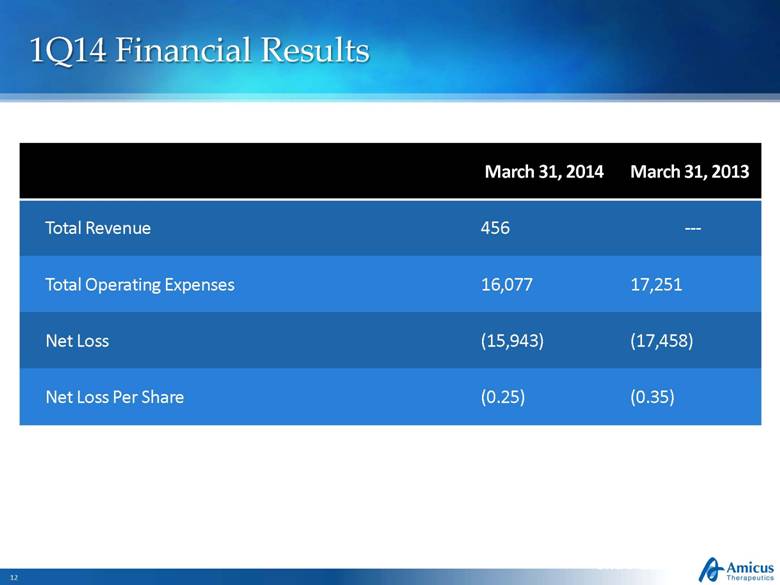
1Q14 Financial Results Slide 12 March 31, 2014 March 31, 2013 Total Revenue 456 --- Total Operating Expenses 16,077 17,251 Net Loss (15,943) (17,458) Net Loss Per Share (0.25) (0.35) |
|
|
Questions & Answers |
|
|
1Q14 Financial Results Conference Call & Webcast May 5, 2014 |