Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Matinas BioPharma Holdings, Inc. | v376464_8k.htm |

Corporate Presentation

Forward Looking Statement This presentation contains "forward - looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995, including those relating to the Company’s product development, clinical and regulatory timelines, market opportunity, cash flow and other statements that are predictive in nature, that depend upon or refer to future events or conditions. All statements other than statements of historical fact are statements that could be forward - looking statements. Forward - looking statements include words such as “expects,” “anticipates,” “intends,” “plans,“ “could”, “believes,” “estimates” and similar expressions. These statements involve known and unknown risks, uncertainties and other factors which may cause actual results to be materially different from any future results expressed or implied by the forward - looking statements. Forward - looking statements are subject to a number of risks and uncertainties, including, but not limited to, our ability to successfully complete research and further development and commercialization of MAT9001; our ability to obtain additional capital to meet our liquidity needs on acceptable terms, or at all, including the additional capital which will be necessary to complete the clinical trials for MAT9001; the uncertainties inherent in clinical testing; the timing, cost and uncertainty of obtaining regulatory approvals; our ability to protect the Company's intellectual property; the loss of any executive officers or key personnel or consultants; competition; changes in the regulatory landscape or the imposition of regulations that affect the Company's products; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10 - K, 10 - Q and 8 - K. Prospective investors are cautioned not to place undue reliance on such forward - looking statements, which speak only as of the date of this presentation. Matinas does not undertake any obligation to release publicly any revisions to such forward - looking statement to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. Matinas BioPharma’s lead product candidate MAT9001 is in a development stage and is not available for sale or use. 2

Team out of Reliant Pharmaceuticals ( Lovaza ® ) with strong development & commercialization track record Unique and differentiating expertise in lipidomics and lipid chemistry Historical lack of innovation/investment in lipid - based therapies Initial focus on lower risk hypertriglyceridemia opportunity in a large and growing metabolic/CV disease arena 3

HIGH triglycerides Pancreatitis Cardiovascular Disease Type 2 Diabetes Fatty Liver Disease HIGH risk: 4

HIGH number of patients with high triglycerides 5 ~4M ~65M (TG≥150mg/dl) (TG≥500mg/dl)

2012 2013 Lack of Good Prescription Options 6 ~2.5M Rx/ yr lost US NON - LDL - LOWERING PRESCRIPTIONS – 2012 & 2013 Total Prescriptions TRx Source: IMS 35.0 32.5

The good news: Research shows strong evidence that Omega - 3s lower triglyceride levels 7

Not all Omega - 3s are the same: Not all Rx Omega - 3s are the same: 8 COMMON OMEGA - 3S EPA DHA is associated with an increase in LDL cholesterol UNIQUE OMEGA - 3S DHA ALA ETA HPA LOVAZA (EPA and DHA) EPANOVA (EPA and DHA) VASCEPA (Pure EPA) High DHA Low DHA DPA SDA

MAT9001 A next generation prescription - only omega - 3 fatty acid medication 9

MAT9001 – Unique engineered Omega - 3 composition • Severe Hypertriglyceridemia (≥500mg/dL) • Highest potency • Unique Mechanism of Action • Trace amounts of DHA 10 EPA SPECIFICALLY DESIGNED TO TREAT DYSLIPIDEMIA DPA
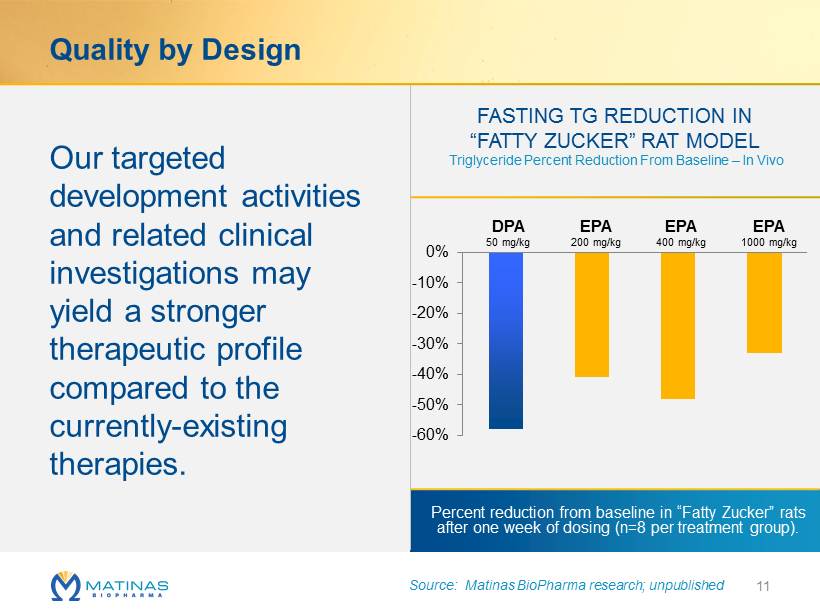
Quality by Design Our targeted development activities and related clinical investigations may yield a stronger therapeutic profile compared to the currently - existing therapies. 11 FASTING TG REDUCTION IN “FATTY ZUCKER” RAT MODEL Triglyceride Percent Reduction From Baseline – In Vivo -60% -50% -40% -30% -20% -10% 0% 50 200 400 1000 EPA 200 mg/kg EPA 400 mg/kg EPA 1000 mg/kg DPA 50 mg/kg Percent reduction from baseline in “Fatty Zucker ” rats after one week of dosing ( n =8 per treatment group). Source: Matinas BioPharma research; unpublished
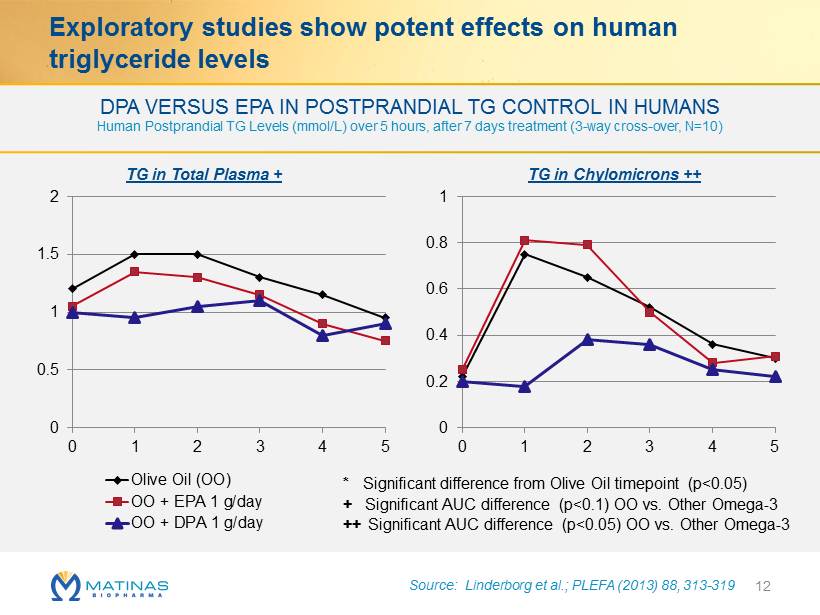
0 0.5 1 1.5 2 0 1 2 3 4 5 Olive Oil (OO) OO + EPA 1 g/day OO + DPA 1 g/day 0 0.2 0.4 0.6 0.8 1 0 1 2 3 4 5 DPA VERSUS EPA IN POSTPRANDIAL TG CONTROL IN HUMANS Human Postprandial TG Levels ( mmol /L) over 5 hours, after 7 days treatment (3 - way cross - over, N=10) Exploratory studies show potent effects on human triglyceride levels 12 * Significant difference from Olive Oil timepoint (p<0.05) + Significant AUC difference (p<0.1) OO vs. Other Omega - 3 ++ Significant AUC difference (p<0.05) OO vs. Other Omega - 3 Source: Linderborg et al.; PLEFA (2013) 88, 313 - 319 TG in Chylomicrons ++ TG in Total Plasma +
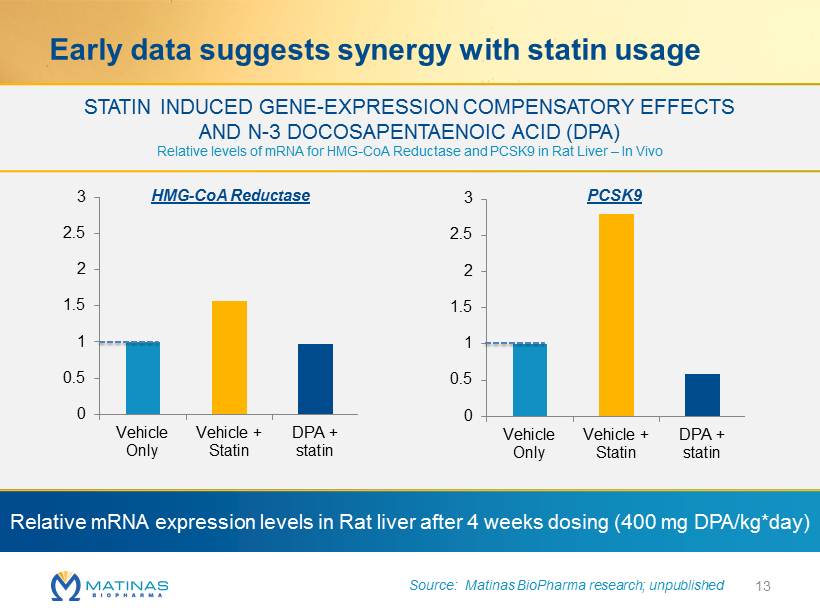
Early data suggests synergy with statin usage 13 STATIN INDUCED GENE - EXPRESSION COMPENSATORY EFFECTS AND N - 3 DOCOSAPENTAENOIC ACID (DPA) Relative levels of mRNA for HMG - CoA Reductase and PCSK9 in Rat Liver – In Vivo 0 0.5 1 1.5 2 2.5 3 Vehicle Only Vehicle + Statin DPA + statin 0 0.5 1 1.5 2 2.5 3 Vehicle Only Vehicle + Statin DPA + statin Relative mRNA expression levels in Rat liver after 4 weeks dosing (400 mg DPA/kg*day) Source: Matinas BioPharma research; unpublished PCSK9 HMG - CoA Reductase

SHORT TRACK TO PHASE III Solid Safety Record with Omega - 3 Products MAT9001 Development Plan 2014 2015 2016 2017 API manufacturing, Animal Study and IND Comparative PK Phase III in patients with Severe HTG (TG≥ 500mg/dL) PK/Drug Interaction Studies Additional Phase III? File NDA & FDA Review 14

Management by Design Roelof Rongen – President and CEO George Bobotas , PhD – Chief Scientific Officer Abdel Fawzy , PhD – EVP Pharmaceutical & Supply Chain Development Gary Gaglione , CPA – VP Finance, Acting CFO Jerome Jabbour , JD – Chief Business Officer & General Counsel Acquired by GlaxoSmithKline for $1.65B in 2007 15 $16M raised to date

Board of Directors with Strong Pharma Experience Herbert Conrad, Chairman – Former President, Hoffmann LaRoche Pharmaceuticals – Co - Founder/Director: Reliant Pharmaceuticals – Chairman: Pharmasset , GenVec , Sapphire, Bone Care – Director: Celldex , Reliant, Dura, Sicor , Savient Stefano Ferrari, Director – Murami Pharma , Bioseutica/KD - Pharma (leading manufacturer of omega - 3 concentrates) – Prospa , Societa Prodotti Antibiotici (developed first omega - 3 based medication) James Scibetta , Director – Current CFO Pacira , Bioenvision/Genzyme , Merrimack – Director: Labopharm , Nephros Adam Stern, Director – Aegis Capital Corp. / CEO, SternAegis Ventures – Director: Organovo , InVivo Therapeutics, Prolor Biotech, LabStyle Roelof Rongen , Director – Reliant, Abbott/BASF Pharma , e - FAT, EPAX/ Trygg Pharma , The Wilkerson Group, Arthur D. Little 16
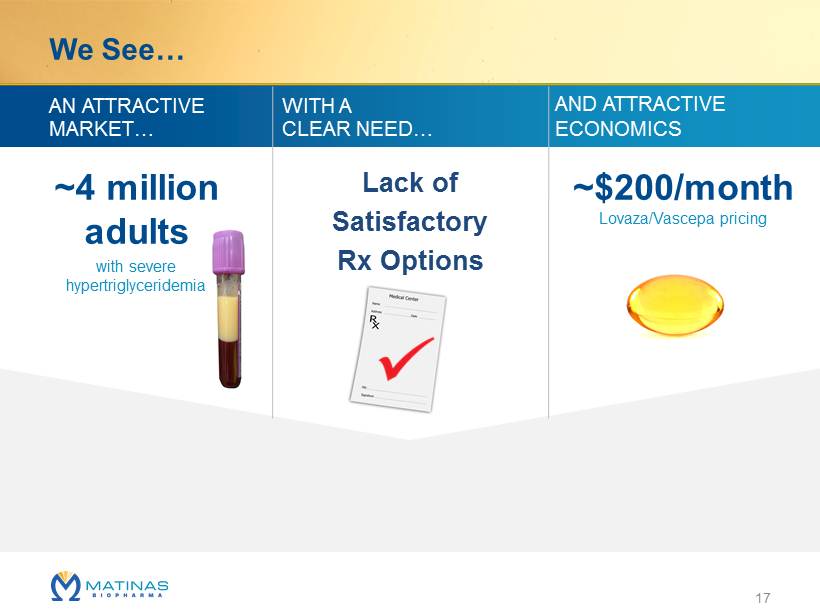
We See… ~4 million adults with severe hypertriglyceridemia ~$200/month Lovaza/Vascepa pricing AN ATTRACTIVE MARKET… AND ATTRACTIVE ECONOMICS WITH A CLEAR NEED… 17 Lack of Satisfactory Rx Options
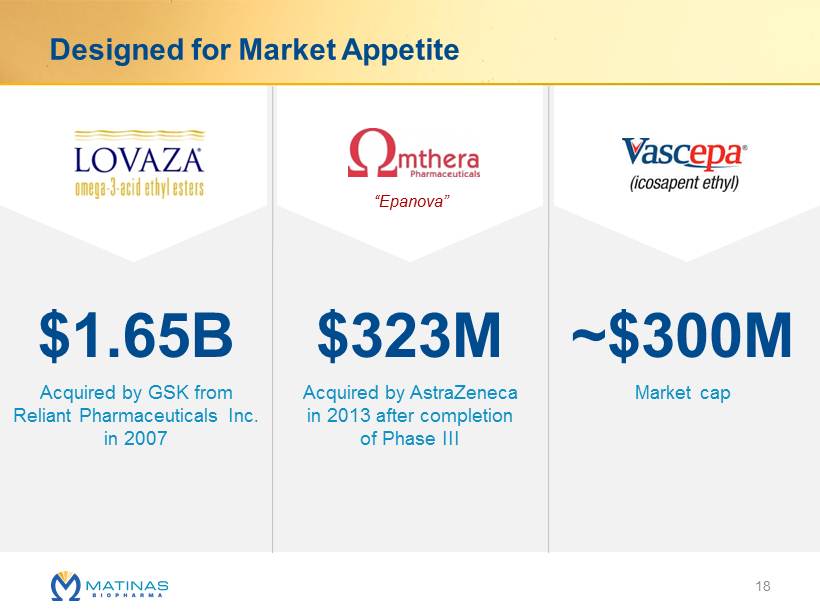
Designed for Market Appetite $1.65B $323M ~$300M Acquired by GSK from Reliant Pharmaceuticals Inc. in 2007 Market cap Acquired by AstraZeneca in 2013 after completion of Phase III “ Epanova ” 18

Also in our Omega - 3 pipeline MAT8800 – Proprietary Omega - 3 Discovery Program Treating Fatty Liver Disease NAFLD NASH • Common: 30% of U.S. population • A leading cause of cirrhosis • NO APPROVED TREATMENT OPTION MAT9001 Dyslipidemia & cardiovascular indications • Heart disease is the #1 killer 19

20 + HIGH Need HIGH Impact HIGH Value =

Thank You
