Attached files
| file | filename |
|---|---|
| EX-31.1 - EX-31.1 - ARIAD PHARMACEUTICALS INC | d658676dex311.htm |
| EX-31.2 - EX-31.2 - ARIAD PHARMACEUTICALS INC | d658676dex312.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K/A
(Amendment No. 1)
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2013
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 001-36172
ARIAD Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 22-3106987 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
26 Landsdowne Street, Cambridge, Massachusetts 02139-4234
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (617) 494-0400
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Name of each exchange on which registered | |
| Common Stock, $.001 par value Preferred Stock Purchase Rights |
The NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | x | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant’s common stock held by nonaffiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the common stock was last sold, as of the last business day of the registrant’s most recently completed second fiscal quarter was approximately $3.1 billion.
As of April 28, 2014, the registrant had 186,798,293 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE: None
Table of Contents
This Amendment No. 1 on Form 10-K/A (this “Form 10-K/A”) amends the Annual Report on Form 10-K of ARIAD Pharmaceuticals, Inc. (the “Company”) for the fiscal year ended December 31, 2013, as originally filed with the Securities and Exchange Commission (the “SEC”) on March 3, 2014 (the “Original Filing”). This Form 10-K/A amends the Original Filing to include the information required by Part III of the Original Filing because the Company has not and will not file a definitive proxy statement within 120 days after the end of its 2013 fiscal year. In addition, this Form 10-K/A amends Item 15 of Part IV of the Original Filing to include new certifications by our principal executive officer and principal financial officer under Section 302 of the Sarbanes-Oxley Act of 2002, as required by Rule 12b-15 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
Except for the foregoing, we have not modified or updated disclosures presented in the Original Filing in this Form 10-K/A. Accordingly, this Form 10-K/A does not modify or update the disclosures in the Original Filing to reflect subsequent events, results or developments or facts that have become known to us after the date of the Original Filing. Information not affected by this amendment remains unchanged and reflects the disclosures made at the time the Original Filing was filed. Therefore, this Form 10-K/A should be read in conjunction with any documents incorporated by reference therein and our filings made with the SEC subsequent to the Original Filing.
This Form 10-K/A contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements are based on management’s expectations and are subject to certain factors, risks and uncertainties that may cause actual results, outcome of events, timing and performance to differ materially from those expressed or implied by such forward-looking statements. Forward-looking statements should be evaluated together with the many uncertainties that affect our business, particularly those mentioned in the section entitled “Certain Factors That May Affect Future Results of Operations” and in the Risk Factors in Item 1A of our Original Filing and in our periodic reports on Form 10-Q and Form 8-K. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to us or to any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
Unless the content requires otherwise, references to “ARIAD,” “company,” “we,” “our,” and “us,” in this Form 10-K/A refer to ARIAD Pharmaceuticals, Inc. and our subsidiaries.
1
Table of Contents
| 1 | ||||||||
| 1 | ||||||||
| Item 10: |
3 | |||||||
| Item 11: |
13 | |||||||
| Item 12: |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
45 | ||||||
| Item 13: |
Certain Relationships and Related Transactions, and Director Independence |
47 | ||||||
| Item 14: |
49 | |||||||
| Item 15: |
51 | |||||||
| 52 | ||||||||
2
Table of Contents
| ITEM 10: | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Board of Directors
The Board currently consists of nine members classified into three classes. Listed below are our nine directors by class. At each annual meeting of stockholders, the term for one class of directors expires, and directors are elected for a full term of three years to succeed the directors of such class.
| Class | Name | Position with ARIAD | Age* | Director Since | ||||
| 1 |
Alexander J. Denner, Ph.D. | Director | 44 | 2014 | ||||
| Athanase Lavidas, Ph.D. | Director | 66 | 2003 | |||||
| Massimo Radaelli, Ph.D. | Director | 56 | 2008 | |||||
| 2 |
Jay R. LaMarche | Director | 67 | 1992 | ||||
| Norbert G. Riedel, Ph.D. | Director | 56 | 2011 | |||||
| Robert M. Whelan, Jr. | Director | 62 | 2010 | |||||
| 3 |
Harvey J. Berger, M.D. | Chairman of the Board of Directors, Chief Executive Officer and President | 64 | 1991 | ||||
| Sarah J. Schlesinger, M.D. | Director | 54 | 2013 | |||||
| Wayne Wilson | Lead Director | 65 | 2008 |
| * | Ages are provided as of June 25, 2014, which is the date of our 2014 annual meeting of stockholders. |
Certain biographical information is set forth below for our directors. Additionally, information about the specific experience, qualifications, attributes or skills that led to our Board of Directors’ conclusion that each person listed below should serve as a director is also set forth below.
Class 1 Directors (Term to Expire in 2016)
Alexander J. Denner, Ph.D. founded Sarissa Capital, a registered investment advisor, in 2012. Sarissa Capital focuses on improving the strategies of companies to better provide stockholder value. From 2006 to November 2011, Dr. Denner served as a Senior Managing Director of Carl C. Icahn’s investment activities. Prior to that, he served as a portfolio manager at Viking Global Investors, a private investment fund, and Morgan Stanley Investment Management, a global asset management firm, for the Health Sciences Trust Fund and the Biotechnology Fund. Dr. Denner is presently a director of Biogen Idec Inc. and VIVUS, Inc. During the past five years, Dr. Denner had also served as a director of the following life sciences companies: Amylin Pharmaceuticals, Inc., Enzon Pharmaceuticals, Inc. and Mast Therapeutics, Inc. Prior to that time, he served as a director of ImClone Systems Incorporated, where he also served as Chairman of the Executive Committee.
Dr. Denner has been a member of our Board since February 2014. He is a member of the Nominating and Corporate Governance Committee. Dr. Denner brings to the Board a strong background overseeing the operations and research and development of biopharmaceutical companies and evaluating corporate governance matters. He also has extensive experience as an investor, particularly with respect to healthcare companies, and has broad healthcare-industry knowledge.
Dr. Denner received his S.B. degree from the Massachusetts Institute of Technology and his M.S., M.Phil. and Ph.D. degrees from Yale University.
Athanase Lavidas, Ph.D. has been the Chairman and Chief Executive Officer of the Lavipharm Group, a pharmaceutical, cosmetics and consumer health-products company headquartered in Greece, since 1976. Dr. Lavidas is also Chairman of the Greece-U.S. Business Council and Chairman of SEV Business Council for International Activities, the international arm of the Hellenic Federation of Industries and Enterprises (SEV).
3
Table of Contents
Dr. Lavidas has been a member of our Board since September 2003 and served as our lead director from November 2008 until January 2014. He is chair of the Nominating and Corporate Governance Committee and a member of the Compensation Committee. Dr. Lavidas brings to the Board over thirty years of international pharmaceutical industry experience in strategic development and operational management. Dr. Lavidas has expertise in the research, development and commercialization of innovative pharmaceutical and cosmetic products, as well as global pharmaceutical and biotechnology collaborations.
Dr. Lavidas received his B.S. and M.S. in chemistry from the University of Munich, his M.B.A. from the Institut Superieur de Marketing et de Management in Paris and his Ph.D. degree in pharmaceutical chemistry from the University of Athens.
Massimo Radaelli, Ph.D. is the President and Chief Executive Officer of Noventia Pharma, a specialty pharmaceutical company focused on orphan drugs for the treatment of rare diseases, in particular for the central nervous system and respiratory system. Prior to joining Noventia in May 2009, Dr. Radaelli was President and Chief Executive Officer of Dompé International SA, the international pharmaceutical company of the Dompé Group. He joined Dompé in 1996 as director of corporate business development. Dr. Radaelli is also Executive Chairman of Bioakos Pharma Laboratories, a specialty pharmaceuticals company concentrated in the fields of gynecology, dermatology, ear, nose and throat and pediatrics and a director of Arriani International, SA, the international subsidiary of Arriani Pharmaceuticals, a pharmaceutical company in southeastern Europe. Since January 2014, Dr. Radaelli has served as a director of NovaBay Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company. He also serves as a director of Innotex Sa, a privately held specialty pharmaceuticals and cosmetics business, and IDRI, a non-profit organization focused on neglected diseases.
Dr. Radaelli is a member of the Italian Society of Pharmacology and has been awarded the highest ranking honor of the Italian Republic by the President and Prime Minister of Italy for merit acquired in the fields of science and biopharma and for his commitment to patients with rare diseases and unmet medical needs.
Dr. Radaelli has been a member of our Board since October 2008. He brings over twenty-five years of industry experience to our Board, including senior leadership positions with major European pharmaceutical companies. He is a member of the Audit Committee and Science and Medicine Committee. Dr. Radaelli brings to the Board significant strategic and operational industry experience, including expertise in pharmaceutical business development, strategic planning, alliance management, and product development and commercialization.
Dr. Radaelli received a University Degree in pharmaceutical sciences and a Ph.D. in clinical pharmacology from the University of Milan and an Executive Master of Business from Bocconi University of Milan.
Class 2 Directors (Term to Expire in 2014)
Jay R. LaMarche is a retired financial executive who brings to our Board over forty years of financial and senior operating experience. He has served us for over twenty years as a director and in executive leadership positions including Chief Financial Officer and Treasurer from January 1992 to November 2000. Mr. LaMarche was our Executive Vice President from March 1997 to November 2000 and Senior Vice President, Finance from January 1992 to February 1997. Prior to joining ARIAD, he was Chief Financial Officer and a director of ChemDesign Corporation, a fine chemicals manufacturer. Previously, Mr. LaMarche was an audit partner with Deloitte Haskins & Sells, a public accounting firm. Mr. LaMarche also served as an officer in the United States Navy.
Mr. LaMarche has been a member of our Board since January 1992. He is a member of the Audit Committee and the Nominating and Corporate Governance Committee. Mr. LaMarche provides the Board with an extensive knowledge of our operations, as well as expertise in financial and accounting issues, particularly as they relate to the pharmaceutical and biotechnology industry. Mr. LaMarche’s management experience and financial background serve him well in providing guidance concerning our operations and business strategy.
Mr. LaMarche received his B.B.A. degree in public accountancy from the University of Notre Dame.
Norbert G. Riedel, Ph.D. is the President and Chief Executive Officer of Naurex Inc., a clinical-stage biopharmaceutical company developing therapies for difficult-to-treat depression as well as orphan and other challenging diseases of the central nervous system. Prior to joining Naurex Inc. in January 2014, he was Corporate Vice President and Chief Scientific Officer of Baxter International Inc., a diversified healthcare company from
4
Table of Contents
March 2001 until January 2013. Between January 2013 and January 2014, Dr. Riedel served on our Board of Directors and the boards of directors of the other companies noted below. Before assuming this role, from 1998 to 2001, Dr. Riedel served as President and General Manager of the recombinant therapeutic proteins business unit and Vice President of Research and Development at Baxter’s bioscience business. Prior to joining Baxter, from 1996 to 1998, he was head of worldwide biotechnology and worldwide core research functions at Hoechst-Marion Roussel, now Sanofi-Aventis, a global pharmaceutical company. Previously, he held a series of scientific management positions at Hoechst-Marion Roussel and Hoechst AG.
Dr. Riedel has been a member of the board of directors of Jazz Pharmaceuticals since May 2013 and was a member of the Supervisory Board of MediGene AG, a biotechnology company from 2003 to 2013. He is a member of the Board of Directors of the Illinois Biotechnology Industry Organization and also serves on the Advisory Board of Northwestern University’s Kellogg School of Management Center for Biotechnology, and the McCormick School of Engineering. Most recently, he was appointed by Illinois Governor Pat Quinn to the newly formed Illinois Innovation Council.
From 1999 to 2010, Dr. Riedel was a member of the board of directors of Oscient Pharmaceuticals Corporation, a biopharmaceutical company, and its predecessor company, Genome Therapeutics Corporation, a genomics company.
Dr. Riedel was a postdoctoral fellow at Harvard University from 1984 to 1987 and an Assistant Professor and Associate Professor of medicine and biochemistry at Boston University School of Medicine from 1987 to 1991, is an adjunct professor at Boston University School of Medicine, and an adjunct professor of Medicine at Northwestern University’s Feinberg School of Medicine and was a visiting professor at Massachusetts Institute of Technology in 1992. In 2009, Dr. Riedel was elected as a member of the Austrian Academy of Sciences.
Dr. Riedel has been a member of our Board since April 2011. He is the Chair of the Compensation Committee and a member of the Science and Medicine Committee. Given his experience as a senior executive in the healthcare field, Dr. Riedel brings to the Board invaluable scientific and commercial expertise, as well as a keen understanding of the biotechnology industry, drug discovery and development, and pharmaceutical management.
Dr. Riedel received his Diploma in biochemistry from the University of Frankfurt in 1981 and his Ph.D. in biochemistry from the University of Frankfurt in 1983.
Robert M. Whelan, Jr. has nearly forty years of investment banking experience working predominantly with high technology and healthcare companies. He has been the President of Whelan & Company, LLC, providing business and financial consulting and strategic services to a broad range of companies, since 2001. From 2001 to 2005, Mr. Whelan served as Managing Director of Valuation Perspectives, Inc., a consulting firm. Prior to that, he held a number of senior-level positions at various investment banking and brokerage firms. Mr. Whelan was Vice Chairman of Prudential Volpe Technology Group, the technology investment banking and research division of Prudential Securities. Prior to Prudential Volpe, he was Chief Operating Officer, Managing Director, board member and Head of Investment Banking of Volpe Brown Whelan & Company, a private technology and healthcare investment banking, brokerage and asset management firm. Volpe Brown Whelan & Company was acquired by Prudential Securities in 1999.
From 2008 to 2009, Mr. Whelan was a Fellow at the Harvard University Advanced Leadership Initiative, an innovative year-long program aimed at providing a rigorous educational curriculum for exceptional leaders who have reached the height of their professions and are seeking to contribute their skills to solve global social problems.
Mr. Whelan currently serves as chairman of the board of directors of Aspen Technology, Inc., a publicly traded provider of software and services for the process industries based in Burlington, Massachusetts.
Mr. Whelan has been a member of our Board since April 2010. He serves on the Audit Committee and Compensation Committee. His extensive investment-banking experience provides him with a wealth of knowledge in dealing with financial, accounting and regulatory matters as he offers the Board insight into the views of shareholders, investors, analysts and others in the financial community.
Mr. Whelan received a B.A. in history from Dartmouth College and a M.B.A. from Stanford University Graduate School of Business.
5
Table of Contents
Class 3 Directors (Term to Expire in 2015)
Harvey J. Berger, M.D. is our principal founder and has served as our Chairman of the Board and Chief Executive Officer since April 1991. He served as our President from April 1991 to September 2003 and from December 2004 to present. From 1986 to 1991, Dr. Berger held a series of executive management positions at Centocor, Inc., a biotechnology company, including Executive Vice President and President, Research and Development Division. He has also held senior academic and administrative appointments at Emory University, Yale University and the University of Pennsylvania and was an Established Investigator of the American Heart Association. Dr. Berger currently serves as a member of the Dean’s Council of Yale School of Medicine.
Dr. Berger, as the principal founder of ARIAD and our Chief Executive Officer for 23 years, brings to the Board a unique combination of strategic vision, leadership skills, and critical knowledge of our operations, research and development programs, commercialization efforts, and the biopharmaceutical industry generally. We benefit from his deep experience in evaluating ARIAD’s opportunities and challenges, his perspectives in shaping the Company’s long-term strategy, and his active leadership in the execution of our operating objectives across the Company’s numerous mission-critical business functions. In addition, Dr. Berger is one of our largest stockholders, beneficially owning approximately 2.4% of our common stock, which directly aligns his interests with those of all of our stockholders. Our Board believes that these attributes, together with Dr. Berger’s demonstrated years of success in building ARIAD into a biopharmaceutical company with its first marketed cancer medicine and an advancing pipeline of highly promising drug candidates, distinctly qualify him for service as a director and as the Chairman of the Board.
Dr. Berger received his A.B. degree in Biology from Colgate University and his M.D. degree from Yale University School of Medicine. He obtained further medical and research training at the Massachusetts General Hospital and Yale-New Haven Hospital.
Sarah J. Schlesinger, M.D. has spent more than 20 years working in the field of cellular immunity, including as clinical director of the laboratory led by the late Ralph M. Steinman, M.D., 2011 Nobel Laureate in Physiology or Medicine. She is currently Senior Attending Physician and Associate Professor of Clinical Investigation at the Laboratory of Cellular Physiology and Immunology at The Rockefeller University. Prior to joining The Rockefeller University in 2003, Dr. Schlesinger was a scientist at the International AIDS Vaccine Initiative in New York City from 2002 to 2003. From 1996 to 2002, Dr. Schlesinger was a Research Physician/Pathologist at the Division of Retrovirology at Walter Reed Army Institute of Research, having previously served, from 1994 to 2002, as Staff Pathologist at the Armed Force Institute of Pathology in Washington, DC. Dr. Schlesinger trained in Surgery at the Albert Einstein College of Medicine and began her career in pathology at Georgetown University in Washington, DC, and hospitals in New York including Buffalo General, Hospital New York and the Manhattan Eye, Ear and Throat Hospital.
Dr. Schlesinger has been a member of our Board since July 2013. She is a member of the Science and Medicine Committee. Dr. Schlesinger leads clinical trials and also chairs the research education and training committee of the Center for Clinical and Translational Science at The Rockefeller University Hospital. She is co-director of Rockefeller’s Clinical Scholars program, the Certificate in Clinical and Translational Sciences program and is the vice-chair of the hospital’s Institutional Review Board. Widely published in her field, Dr. Schlesinger has been recognized with numerous awards for her research and teaching. She also belongs to a number of prominent medical societies including the United States and Canadian Academy of Pathology, the American Association for the Advancement of Science and the College of American Pathologists Dr. Schlesinger brings to the Board expertise in scientific research and clinical trials.
Dr. Schlesinger received her bachelor’s degree from Wellesley College in Wellesley, MA, and her M.D. from Rush Medical College in Chicago.
Wayne Wilson has over thirty years of business, financial, and accounting experience. He has been an independent business advisor since 2002. From 1995 to 2002, he served in various roles, including as President, Chief Operating Officer, and Chief Financial Officer, at PC Connection, Inc., a Fortune 1000 direct marketer of information technology products and services. From 1986 to 1995, Mr. Wilson was a partner in the assurance and advisory services practice of Deloitte & Touche LLP.
6
Table of Contents
Mr. Wilson has been a member of our Board since October 2008 and was appointed as lead director in January 2014. He is chair of the Audit Committee and a member of the Nominating and Corporate Governance Committee. Mr. Wilson is also a member of the boards of directors of FairPoint Communications, Inc., a telecommunications company, Hologic, Inc., a medical diagnostics and device company focusing on women’s health, and Edgewater Technology, Inc., a technology management consulting firm. He previously served as a director of Cytyc Corporation, a medical diagnostics and device company. Mr. Wilson brings substantial general business and financial expertise to our Board, as well as our Audit Committee. His background and extensive experience in financial accounting and reporting make him well versed in accounting principles and financial reporting rules and regulations, and he is well equipped to evaluate financial results and generally oversee the financial reporting process of a publicly traded corporation, making him highly qualified to be the Board’s lead director.
Mr. Wilson received an A.B. degree in political science from Duke University and an M.B.A. from the University of North Carolina at Chapel Hill.
Agreements with Dr. Denner and Sarissa Capital
On February 20, 2014, we entered into a Nomination and Standstill Agreement with Dr. Denner and Sarissa Capital Management LP and certain of its affiliated funds and entities, referred to hereinafter collectively as the Sarissa Group.
Pursuant to this agreement, the Board increased the size of the Board from eight to nine members and appointed Dr. Denner to the Board as a Class 1 director to serve until the 2016 annual meeting of stockholders. Dr. Denner was also appointed as a member of the Nominating and Corporate Governance Committee of the Board. We also agreed to appoint an additional director, referred to as the Additional Designee, selected by the Board and approved by Dr. Denner, as a Class 2 director with a term expiring at the 2017 annual meeting of stockholders. We expect to make this appointment in 2014, after the annual meeting of stockholders.
In addition, under the terms of the agreement, during the Standstill Period, as defined below, the Sarissa Group has agreed not to solicit proxies regarding any matter to come before a meeting of our stockholders, including for the election of directors. Among other provisions, the Sarissa Group has also agreed that, subject to certain exceptions, during the Standstill Period the Sarissa Group will not acquire beneficial ownership of additional shares of our voting stock. The agreement generally defines the “Standstill Period” as the period beginning February 20, 2014 and ending on the earlier of the date, if any, that the Sarissa Group gives notice of the nomination of two or more directors at the 2015 annual meeting of stockholders and the date on which Dr. Denner resigns from the Board.
The agreement also provides that, for so long as Dr. Denner is a member of the Board, we will give prior notice to the Sarissa Group before the advance notice deadline in our bylaws if Dr. Denner or the Additional Designee will not be nominated for election at any future annual meeting of stockholders when their current terms expire. Following the appointment of the Additional Designee and for so long as Dr. Denner is a member of the Board, we have agreed not to increase the size of the Board above ten members. Pursuant to the agreement, Dr. Denner will automatically resign if the Sarissa Group no longer beneficially owns at least 6 million shares of our common stock.
In conjunction with the agreement, we and the Sarissa Group also entered into a Confidentiality Agreement governing the provision of confidential information obtained by Dr. Denner during his service on the Board to the Sarissa Group.
The foregoing is not a complete description of the terms of our agreements with Dr. Denner and the Sarissa Group. For a further description of the terms of the agreements, including copies thereof, please see our Current Report on Form 8-K that we filed with the SEC on February 21, 2014.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and officers, and persons who own more than 10% of our common stock, to file reports of securities ownership and changes in that ownership with the SEC. Officers, directors, and greater than 10% beneficial owners are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file. To our knowledge, based solely upon a review of the copies of the forms furnished to us and written representations that no other forms were required, we believe that all Section 16(a) filing requirements were timely met during the fiscal year ended December 31, 2013, except that one report covering one stock option grant to Martin J. Duvall on December 9, 2013 was filed late.
7
Table of Contents
Corporate Code of Conduct and Ethics
Our Corporate Code of Conduct and Ethics applies to all of our employees and directors. Any changes in or waivers from our Corporate Code of Conduct and Ethics will be included in a Current Report on Form 8-K within four business days following the date of the change or waiver, unless website posting of the amendments or waivers is then permitted by NASDAQ rules. Our Corporate Code of Conduct and Ethics is publicly available on the Investors section of our website at http://investor.ariad.com under the heading “Corporate Governance.”
Corporate Governance Guidelines
Our Corporate Governance Guidelines, which were developed and are overseen by the Nominating and Corporate Governance Committee, establish basic principles of corporate governance by which the Board operates. These guidelines address selection, composition and independence of the Board, director compensation, majority voting in director elections and director resignation in the event of a failure to receive the required vote, evaluation of the performance of the Board and its committees, the structure and operations of the committees of the Board, the establishment and implementation of corporate governance guidelines, principles and practices, leadership development, and succession planning.
Under our Corporate Governance Guidelines, so long as the Chief Executive Officer is also Chairman of the Board, the Board shall appoint one of the independent directors to serve in the role of lead director. His or her role is to support the independent directors in meeting their responsibilities as independent directors. As such, he or she is responsible for oversight of those processes of the Board that independent directors are required to perform. In addition, he or she presides at meetings of the non-management directors.
The Nominating and Corporate Governance Committee is responsible for the establishment, implementation, and oversight of our Corporate Governance Guidelines, Conflict of Interest Policy for Board of Directors, and other corporate governance guidelines, policies, and practices. Our Corporate Governance Guidelines and Conflict of Interest Policy are publicly available on the Investors section of our website at http://investor.ariad.com under the heading “Corporate Governance.”
Majority Voting in Director Elections
On April 28, 2014, we amended our Amended and Restated By-laws to provide that our directors must be elected by a majority of votes cast in uncontested elections and by a plurality of votes cast in contested elections. This change will be in effect for the election of directors starting with this year’s annual meeting of stockholders. In connection with this change, we have also adopted a director resignation policy as part of our Corporate Governance Guidelines. Under this policy, the Board will only nominate directors for election or re-election who have submitted an irrevocable letter of resignation that will be effective upon (1) their failure to receive the required number of votes for reelection at the next annual meeting of stockholders at which they face reelection and (2) acceptance of such resignation by the Board. If an incumbent director fails to receive the number of votes required for reelection, the Nominating and Corporate Governance Committee will act on an expedited basis to determine whether to accept the director’s resignation and will submit its recommendation for prompt consideration to the Board, who, with the director in question abstaining, will decide whether to accept the director’s resignation, taking into account such factors as it deems relevant. Such factors may include the stated reasons why stockholders voted against such director’s reelection, the qualifications of the director and whether accepting the resignation would cause us to fail to meet any applicable listing standards or would violate state law.
Board Committees
The Board currently has four standing committees: the Audit Committee, the Compensation Committee, the Nominating and Corporate Governance Committee and the Science and Medicine Committee. The Science and Medicine Committee was created in October 2013 to assist management in promoting, maintaining and continually enhancing ARIAD’s scientific excellence and clinical scholarship as fundamental corporate values and drivers of corporate success. During 2013, we also had an Executive Committee, but the committee did not meet in 2013 and we decided to eliminate the committee in February 2014. Each committee meets periodically throughout the year, reports its actions and recommendations to the Board, receives reports from senior management, annually evaluates its performance and has the authority to retain outside advisors in its discretion. The primary responsibilities of each committee are summarized below and set forth in more detail in each committee’s written charter, which can be found on the Investors section of our website at http://investor.ariad.com under the heading “Corporate Governance.”
8
Table of Contents
The information under the caption “Director Independence and Committee Qualifications” in Item 13 of this Form 10-K/A is incorporated herein by reference.
Audit Committee
| • | Oversee management’s maintenance of the reliability and integrity of our accounting policies and financial reporting and disclosure practices; | CURRENT COMMITTEE MEMBERS:
Wayne Wilson, Chair Jay R. LaMarche Massimo Radaelli, Ph.D. Robert M. Whelan, Jr.
OTHER COMMITTEE MEMBERS DURING 2013: None | ||
|
• |
Oversee management’s establishment and maintenance of processes to ensure that we have an adequate system of internal control; |
|||
|
• |
Oversee management’s establishment and maintenance of processes to ensure our compliance with legal and regulatory requirements that may impact our financial reporting and disclosure obligations; |
|||
|
• |
Review our independent registered public accounting firm’s qualifications and independence; |
|||
|
• |
Appoint, compensate, and oversee the work of our independent registered public accounting firm; |
|||
|
• |
Pre-approve all audit and non-audit services performed by our independent registered public accounting firm; |
|||
|
• |
Review, in consultation with our management and independent registered public accounting firm, the scope and results of reviews of our quarterly financial statements, audits of our annual financial statements, and audits of our system of internal control over financial reporting; |
|||
|
• |
Perform other additional duties and responsibilities, including reviewing, evaluating, and approving related person or similar transactions or relationships and recommending approval of such transactions to the disinterested and independent members of the Board, if necessary; and |
|||
|
• |
Oversee our compliance with applicable laws, regulations and corporate policies, including our Code of Conduct and Ethics. |
9
Table of Contents
Compensation Committee
| • | Assess the performance of and approve, or recommend for approval by the Board, the compensation of our executive officers; | CURRENT COMMITTEE MEMBERS:
Norbert G. Riedel, Ph.D., Chair Athanase Lavidas, Ph.D. Robert M. Whelan, Jr.
OTHER COMMITTEE MEMBERS DURING 2013: Massimo Radaelli, Ph.D., Chair | ||
|
• |
Analyze our officer and director compensation plans, policies, and programs; |
|||
|
• |
Administer our stock-based compensation and executive compensation plans; and |
|||
|
• |
Review and approve all proposed compensation disclosures, including the Compensation Discussion and Analysis (“CD&A”), for inclusion in our proxy statement and review all recommendations by stockholders of the compensation of our named executive officers and the frequency of voting by stockholders on the compensation of our named executive officers. |
Nominating and Corporate Governance Committee
| • | Identify and evaluate individuals to become directors; | CURRENT COMMITTEE MEMBERS:
Athanase Lavidas, Ph.D., Chair Jay R. LaMarche Wayne Wilson Alexander J. Denner, Ph.D.
OTHER COMMITTEE MEMBERS DURING 2013: Massimo Radaelli, Ph.D. | ||
|
• |
Make recommendations to the Board concerning the size, structure, and composition of the Board and its committees; |
|||
|
• |
Monitor the process to assess the Board’s effectiveness; |
|||
|
• |
Review and assess the adequacy of our corporate governance, including our Corporate Governance Guidelines and our Board Conflict of Interest Policy; and |
|||
|
• |
Oversee matters relating to the independence (including potential conflicts of interest), education, operation, and effectiveness of the Board and its committees. |
Science and Medicine Committee
| • | Consult with and advise management regarding the strategy, focus and direction of our research and development, clinical programs and initiatives, as well as competitive and other factors that may affect those programs and initiatives; | CURRENT COMMITTEE MEMBERS:
Sarah Schlesinger, M.D., Chair Norbert G. Riedel, Ph.D. Massimo Radaelli, Ph.D.
OTHER COMMITTEE MEMBERS DURING 2013: None | ||
|
• |
Identify and discuss significant emerging science and technology trends and issues, including their potential impact on our research and development and clinical programs, plans or policies; |
|||
|
• |
Help lead periodic updates and discussions with the Board regarding our progress in achieving our strategic research and development and clinical goals and objectives; and |
|||
|
• |
Consult with and advise management, as appropriate, regarding our internal and external investments in science and technology and for any material external investments in research and development that require approval by the Board and assist the Board in evaluating such opportunities. |
10
Table of Contents
Executive Officers
The following table sets forth certain information regarding our executive officers, including their ages as of June 25, 2014, which is the date of our 2014 annual meeting of stockholders.
| Name |
Age |
Position | ||
| Harvey J. Berger, M.D. | 64 | Chairman of the Board of Directors, Chief Executive Officer and President | ||
| Timothy P. Clackson, Ph.D. | 49 | President of Research and Development and Chief Scientific Officer | ||
| Edward M. Fitzgerald | 59 | Executive Vice President, Chief Financial Officer and Treasurer | ||
| Martin J. Duvall | 52 | Executive Vice President and Chief Commercial Officer | ||
| David L. Berstein, Esq. | 62 | Senior Vice President, General Counsel and Chief Intellectual Property Counsel, and Secretary | ||
| Daniel M. Bollag, Ph.D | 53 | Senior Vice President, Regulatory Affairs and Quality | ||
| Maria E. Cantor | 46 | Senior Vice President, Corporate Affairs and Human Resources | ||
| Hugh M. Cole | 49 | Senior Vice President, Chief Business Officer | ||
| Frank G. Haluska, M.D., Ph.D. | 55 | Senior Vice President, Clinical Research and Development and Chief Medical Officer | ||
Biographical information for Dr. Berger is set forth above under the caption “Board of Directors.”
Timothy P. Clackson, Ph.D. has served as our President of Research and Development and Chief Scientific Officer since June 2010. Previously, he served as our Senior Vice President and Chief Scientific Officer from September 2003 to June 2010.
Edward M. Fitzgerald has served as our Executive Vice President, Chief Financial Officer and Treasurer since June 2010. Previously, he served as our Senior Vice President, Chief Financial Officer and Treasurer from May 2002 to June 2010.
Martin J. Duvall has served as our Executive Vice President, Chief Commercial Officer since December 2013, having served as our Senior Vice President, Commercial Operations since September 2011. Previously, from 2010 to 2011, he served as Senior Vice President and General Manager of Merck and Company’s global oncology franchise. From 2009 to 2010, Mr. Duvall led global marketing and commercial operations at Abraxis Bioscience, Inc. From 2004 to 2009, Mr. Duvall held roles leading commercial operations, commercial development and oncology strategy for MGI Pharma, Inc. and its acquirer, Eisai Pharmaceuticals.
David L. Berstein, Esq. has served as our Senior Vice President, General Counsel and Chief Intellectual Property Officer and Secretary since November 2013, having served as our Senior Vice President and Chief Intellectual Property Officer since May 2008. Previously, he served as our Senior Vice President and Chief Patent Counsel from June 2003 to June 2007.
Daniel M. Bollag, Ph.D. has served as our Senior Vice President, Regulatory Affairs and Quality since January 2009. He previously was Vice President, Regulatory Affairs for Genzyme Corporation, a biotechnology company, from 2006 to 2008.
Maria E. Cantor has served as our Senior Vice President, Corporate Affairs and Human Resources since November 2013, having served as our Senior Vice President, Corporate Affairs since January 2012. Previously, she served as our Vice President, Corporate Communications and Investor Relations since July 2008. Ms. Cantor held several positions of increasing responsibility at Genzyme Corporation from 2001 to 2008, most recently serving as Senior Director, Corporate Communications.
11
Table of Contents
Hugh M. Cole has served as our Senior Vice President and Chief Business Officer since March 2014. Previously, from 2007 to 2014, Mr. Cole held management positions at Shire Pharmaceuticals, most recently as Senior Vice President, Strategic Planning and Program Management and, previously, as a global franchise head, and before that, as Vice President, Business Development. Previously he held senior positions in business and corporate development at Oscient Pharmaceuticals (formerly, Genome Therapeutics) and at Millennium Pharmaceuticals and its affiliates.
Frank G. Haluska, M.D., Ph.D. has served as our Senior Vice President, Clinical Research and Development and Chief Medical Officer since January 2012, having held the position of Vice President and Chief Medical Officer since June 2010. Previously, he served as our Vice President, Clinical Affairs from May 2009 to June 2010, Vice President, Clinical Research from July 2008 to May 2009, and Senior Medical Director from October 2007 to July 2008.
12
Table of Contents
| ITEM 11: | EXECUTIVE COMPENSATION |
Compensation Discussion and Analysis
Executive Summary
OVERVIEW
Our success in 2013 was made possible in large measure by our ability to attract, retain and motivate talented and experienced individuals across all areas of our business, including our named executive officers (“NEOs”):
| Harvey J. Berger, M.D. | Chairman of the Board of Directors, Chief Executive Officer and President | |
| Timothy P. Clackson, Ph.D. | President of Research and Development and Chief Scientific Officer | |
| Edward M. Fitzgerald | Executive Vice President, Chief Financial Officer and Treasurer | |
| Martin J. Duvall | Executive Vice President and Chief Commercial Officer | |
| Daniel M. Bollag, Ph.D. | Senior Vice President, Regulatory Affairs and Quality | |
We believe that our compensation program has been and will continue to be effective in attracting, retaining and motivating the right executive team during this critical phase of ARIAD’s evolution from a research and development-stage enterprise to a fully integrated, commercial-stage global oncology company. Our compensation program for our NEOs was supported by 82.7% of the “Say-on-Pay” advisory votes cast by shareholders at our 2013 annual meeting of shareholders. Based on the effectiveness of our compensation program and after consideration of last year’s “Say-on-Pay” advisory vote, the Compensation Committee determined to continue the same fundamental structure for our executive compensation program this year, with certain refinements discussed below.
PERFORMANCE SUMMARY AND RELATED COMPENSATION DECISIONS
2013 has been a challenging year for ARIAD. Although we achieved our key strategic objectives in the first three quarters of the year, including the initial commercial launch in the United States of our first new cancer medicine, Iclusig® (ponatinib), the achievement of marketing authorization for Iclusig by the European Commission as an orphan medicinal product for two indications and the commencement of sales in Europe in the second half of the year, we experienced a significant setback in the fourth quarter, when the U.S. Food and Drug Administration, or FDA, placed a partial clinical hold on all additional patient enrollment in clinical trials of Iclusig and issued several Drug Safety Communications. On October 31, 2013, we temporarily suspended marketing and commercial distribution of Iclusig in the United States, in response to a request by the FDA, while we negotiated an update to the prescribing information and a risk mitigation strategy.
As a result of the challenges that have arisen with respect to Iclusig, our Compensation Committee held a number of meetings in the last quarter of 2013 to evaluate the evolving situation and consider appropriate actions with respect to our executive compensation programs. Among key factors discussed and considered by the Compensation Committee were the following:
| • | The continued appropriateness of our peer group given the decrease in ARIAD’s market value immediately after the FDA action; |
| • | Our executive compensation philosophy of generally targeting executives’ cash and equity compensation at the 65th percentile of the market; |
| • | The appropriateness of paying cash bonuses to the executive team for 2013, even though most of the metrics set out at the beginning of the year had been achieved, given the FDA action relating to Iclusig and its effect on our stockholders; |
13
Table of Contents
| • | The decrease in our stock value and the reduced incentive value of previously granted stock options and other equity awards; and |
| • | To assess the degree of alignment between our Chief Executive Officer’s pay and performance, the Compensation Committee also considered “realizable” pay relative to our peer group. The Compensation Committee noted that the compensation awarded to our Chief Executive Officer over the last three years has been closely aligned with the performance of our stock (TSR) over the same period, both on an absolute basis and relative to the companies in our peer group. See “Pay for Performance” for further discussion of and charts relating to the alignment of Chief Executive Officer pay and our stock performance. |
Following the suspension of commercial distribution of Iclusig in the United States, ARIAD management continued to work with the FDA to gain re-approval of Iclusig and resume commercial distribution of the drug. On December 20, 2013, we obtained FDA approval to resume marketing and commercial distribution of Iclusig under a revised U.S. Prescribing Information, or USPI, and a Risk Evaluation and Mitigation Strategy, or REMS. As a result, we announced that we had resumed marketing and commercial distribution of Iclusig through an exclusive specialty pharmacy. This continued progress towards resuming normal commercial distribution of Iclusig in the United States has been recognized by the market, as reflected in the increase in our stock price since we announced the FDA’s approval to resume marketing and commercial distribution.
In the context of these ongoing developments, our Compensation Committee continued to evaluate and consider the status of Iclusig in the first quarter of 2014, as it made final decisions regarding 2013 and 2014 executive compensation. Key decisions made by the Committee in late 2013 and early 2014 included:
| • | No cash bonuses to executives under the 2013 annual performance award program. Although we had achieved most of our strategic objectives during the first three quarters of 2013, the Compensation Committee determined that no cash bonus should be delivered given the adverse FDA action on Iclusig during the fourth quarter of 2013. As a result, each of our NEOs was positioned below the market 25th percentile in terms of his total cash compensation for 2013. |
| • | No base salary merit or market-based adjustment increases for 2014. Notwithstanding the progress we have made in resuming commercial distribution of Iclusig in the United States, the Compensation Committee determined that base salaries for our NEOs should be maintained at 2013 levels. |
| • | 2014 executive equity grants are even more heavily focused on performance. Although we have historically utilized performance-based equity grants tied to key milestones as an important part of executives’ annual equity awards, the Compensation Committee approved increasing the proportion of executives’ equity awards tied to key performance milestones from 33% of the total award in 2013 to 50% of the total award in 2014. This very-heavy focus on performance-based equity places us at the forefront of the market, and ensures that half of each executive’s award will be earned only if ARIAD is able to successfully execute on its strategy. The remaining half of each NEO’s 2014 equity award is being delivered in time-based restricted stock units vesting in equal installments over the next three years. |
In making these compensation decisions our Compensation Committee also considered the results of our annual Say on Pay vote held in 2013. At our 2013 annual meeting, our shareholders approved the compensation program for our NEOs by a vote of 82.7% in favor. Notwithstanding the continued support of our investors for the structure of our compensation programs, the Compensation Committee believed that the changes described above were warranted and appropriate to maintain strong alignment between our executives and our shareholders and to continue to emphasize our historically strong linkage between executive pay and our Company’s performance.
14
Table of Contents
KEY COMPONENTS OF COMPENSATION AND RELATED PERFORMANCE PERIODS
We provide three basic forms of direct compensation to our executive officers: base salary, bonuses (which we refer to as “annual performance awards”), and long-term equity incentive awards. Our Compensation Committee, with the assistance of Radford, our compensation consultant, and Dr. Berger, our Chief Executive Officer, reviews each of these compensation components annually for each of the nine executive officers on our executive leadership team, including our NEOs, and adjusts each component based primarily on corporate and individual performance.
In order to understand the compensation reported in this Form 10-K/A, it is important to be aware of the fact that two separate fiscal years play a role in our compensation decisions. This is because the time periods over which performance is assessed vary depending on the particular component of compensation, and SEC disclosure rules require us to discuss awards determined based on 2013 performance in different fiscal years. Specifically, the performance review periods are as follows:
| • | Base Salary – The Compensation Committee sets executive salaries in the first quarter of each fiscal year, effective as of January 1st of that year. Typically, a target percentage level of salary increase is established for the executive team, and each executive’s salary is then determined based on an assessment of the corporate and individual performance objectives applicable to that executive during the preceding year. Thus, the salary amounts reported in the Summary Compensation Table later in this Form 10-K/A for fiscal 2013 were established based on 2012 corporate and individual performance. Salary amounts for fiscal 2014, which as discussed above are unchanged from 2013 (with the exception of Mr. Duvall, who was promoted last year), are briefly described below and will be set forth in the Summary Compensation Table next year. |
| • | Annual Performance Awards – These awards are determined after the completion of the fiscal year to which they apply, based on an evaluation of each executive’s performance in relation to corporate and individual objectives for that year. Therefore, unlike the 2013 salary amounts, which were based on 2012 performance, our executives’ annual performance awards for 2013, as reported in the Summary Compensation Table for fiscal 2013, were based on 2013 performance. |
| • | Long-term Equity Incentive Awards – These forward-looking awards are determined in the first quarter of each fiscal year, based on performance during the previous year. Consequently, the 2013 long-term equity incentive awards reported in the Summary Compensation Table and Grant of Plan-Based Awards Table for fiscal 2013 were established based on 2012 performance. The long-term equity incentive awards granted in early 2014 based on 2013 performance are briefly described below and will be set forth in the Summary Compensation Table and Grant of Plan-Based Awards Table next year. |
PAY MIX
Our Compensation Committee considers the mix of the elements of compensation discussed above to be critical in driving our “pay-for-performance” philosophy. The vast majority of our executives’ annual compensation is delivered in short- or long-term incentive compensation whose value is contingent upon the achievement of specific performance targets, appreciation of our stock value, or both. In 2013, as in the past, our Chief Executive Officer’s pay mix was more than 90% comprised of performance-based compensation via our annual performance awards (referred to as STI in the table below) and grants of stock options, restricted stock units and performance shares (referred to as LTI in the table below), and only 9% was delivered via “guaranteed” compensation in the form of his base salary. By comparison, among our peer group listed below in the section entitled “Use of Competitive Market Compensation Data and Compensation Benchmark”, the median pay mix included twice the level of guaranteed base pay, at 20% of the total. Percentages are based on target values for each component of Chief Executive Officer pay, including the fair value of equity awards, including performance shares, on the date of grant:
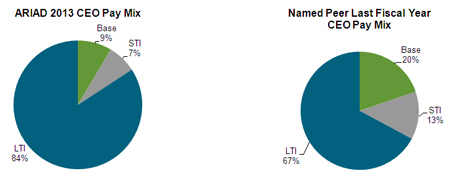
15
Table of Contents
PAY FOR PERFORMANCE
Our Compensation Committee is strongly committed to the principle that compensation delivered to our NEOs should be strongly aligned with value creation for our shareholders. This principle is reflected both in the pay mix referenced above and in our significant historical and continuing use of performance-based equity to deliver executives’ annual long-term incentive awards. Over the past five years, annual pay levels have closely tracked the change in our stock value year-over-year, highlighting the effectiveness of our programs in advancing this goal. Our use of long-term incentives and focus on performance-based vehicles, whether via stock options, whose value is wholly contingent upon stock price performance, or performance shares that vest only upon the achievement of designated milestones/performance targets, ensures that our executives are aligned with our shareholders. We also issue time-based restricted stock units, which further align executives with our shareholders by helping executives build a meaningful, direct ownership stake in our stock.
The following chart compares our total shareholder return, or TSR, over the last five years with the total reported compensation of our Chief Executive Officer (as disclosed in the Summary Compensation Table) over the same period. For 2013, when our stock price declined in the last quarter following the announcement of the partial clinical hold on new patient enrollment in clinical trials of Iclusig and the temporary suspension of marketing and commercial distribution of Iclusig in the United States, the table reflects not only the Chief Executive Officer’s reported pay, which includes the value of his long-term incentive awards at the beginning of the year, but also his 2013 “realizable” pay, which reflects cash plus the year-end value of his 2013 equity grants.
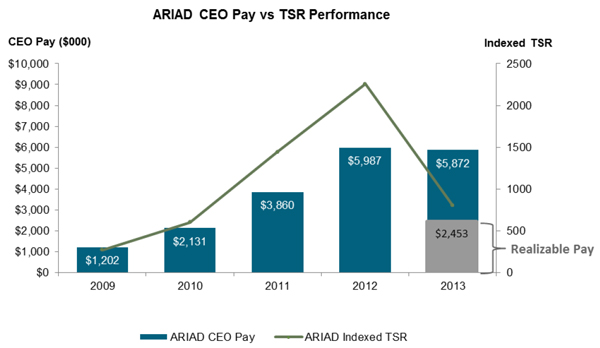
As shown in the table above, the compensation awarded to our Chief Executive Officer over the last five years closely tracked our TSR over the same period. Consistent with our objective to align the interests of our executives
16
Table of Contents
with the interests of our shareholders, as the price of our common stock appreciated over the last five years, the value of stock option awards, the most significant component of the compensation of our Chief Executive Officer (as well as the other NEOs), increased as well. With the decline in ARIAD’s market value by the end of 2013, the actual, realizable value of Dr. Berger’s 2013 compensation decreased as well.
To assess the degree of alignment between our Chief Executive Officer’s pay and performance, the Compensation Committee also considers “realizable” pay relative to our peer group. We believe that the compensation awarded to our Chief Executive Officer in the last three years displayed a strong connection to our TSR performance over the same period both on an absolute basis and relative to the companies in our peer group, as shown in the following chart.
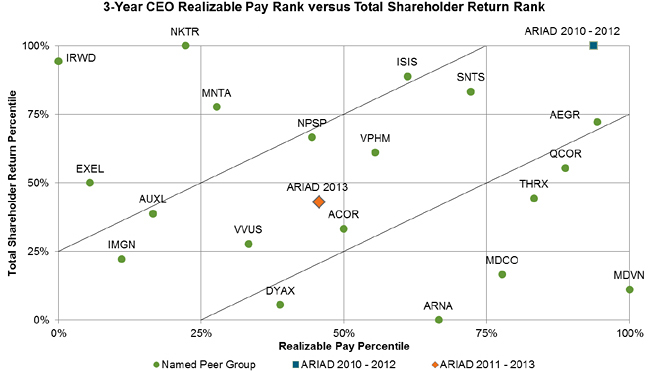
For both the three-year period from 2011-2013 and the preceding three years from 2010-2012, ARIAD’s relative TSR ranking is closely tied to our Chief Executive Officer pay ranking, falling squarely within the “zone of alignment” indicated in the chart. The Compensation Committee believes these analyses confirm the continued effectiveness of our executive compensation programs.
BUSINESS HIGHLIGHTS
We present below business highlights for our 2013 fiscal year to assist our shareholders in understanding the compensation decisions made with respect to reported 2013 annual performance awards, and business highlights for 2012 to facilitate an understanding of our reported 2013 salary determinations and long-term equity incentive awards.
Fiscal 2013
2013 was both a very successful and a challenging year for ARIAD. Since the beginning of 2013, we:
| • | Commenced sales and marketing of Iclusig in the United States in the first quarter of 2013 and in certain European countries in the second half of 2013, recognizing $45.2 million in product revenue during 2013, compared to no product revenue in the prior year. |
| • | Obtained marketing authorization for Iclusig from the European Commission in July 2013, commenced sales of Iclusig in Germany, the United Kingdom, France, Austria and the Netherlands, and laid the groundwork to expand commercialization of Iclusig to all of the major markets in Europe during 2014, subject to obtaining pricing and reimbursement approvals. |
17
Table of Contents
| • | Successfully worked with the FDA to obtain revised prescribing information and resume marketing and commercial distribution of Iclusig in the United States in December 2013, following the temporary suspension of Iclusig in October 2013 in the United States due to safety concerns raised by the FDA. |
| • | Continued development of Iclusig in patients with gastrointestinal stromal tumors, or GIST, in a Phase 2 clinical trial, which is almost fully enrolled, with the goal of completing patient enrollment upon lifting of the partial clinical hold expected in the second quarter of 2014. |
| • | Announced the initiation of a pivotal global Phase 2 trial of our next drug candidate, AP26113, in patients with locally advanced or metastatic non-small cell lung cancer, or NSCLC, who were previously treated with crizotinib, the current standard of care. The ALTA (ALK in Lung Cancer Trial of AP26113) trial is designed to determine the safety and efficacy of AP26113 in refractory NSCLC patients who test positive for anaplastic lymphoma kinase, or ALK, oncogene. We expect this trial to be the basis for our initial filing for regulatory approval of AP26113. |
| • | Continued our internal drug discovery efforts, as a result of which we expect to nominate a potential best-in-class development candidate in the second half of 2014. |
| • | Strengthened our balance sheet through a common stock offering of $310.0 million in net proceeds at the beginning of 2013, ending the year with cash, cash equivalents and marketable securities of $237.2 million, compared to $164.4 million at the end of 2012. |
Fiscal 2012
Our 2012 fiscal year was one of great success and progress at ARIAD as we advanced Iclusig towards commercialization:
| • | We received accelerated approval in the United States of Iclusig for the treatment of adult patients with chronic, accelerated or blast phase chronic myeloid leukemia (CML) that is resistant or intolerant to prior tyrosine kinase inhibitor (TKI) therapy or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) that is resistant or intolerant to prior TKI therapy. This approval followed completion of our NDA submission ahead of schedule. |
| • | We recruited, hired and trained a complete commercial organization in the U.S. and were fully prepared for the U.S. commercial launch of Iclusig upon receipt of marketing approval from the FDA. All of the key functions in our U.S. commercial organization, including account specialists, market access, and marketing, were put in place and we began implementing our commercial plans for Iclusig. |
| • | In Europe, we submitted a Marketing Authorization Application (MAA) for Iclusig to the European Medicines Agency seeking marketing approval in the EU of Iclusig in adult patients with resistant or intolerant CML or Ph+ ALL. The Committee for Medicinal Products for Human Use granted ARIAD’s request for accelerated assessment of the MAA. |
| • | In preparation for our planned commercial launch of Iclusig in Europe, we established our European headquarters in Lausanne, Switzerland, and recruited and hired our General Manager of European operations, along with other key members of our European leadership team. We began recruiting medical science liaisons and sales representatives in each of other major markets in Europe. We also established early-access programs for Iclusig, established the supply chain in key markets and implemented initial pricing and reimbursement activities. |
| • | We advanced the development of AP26113, our investigational ALK inhibitor, and presented positive clinical proof-of-concept data showing the promise of AP26113 as our next internally discovered cancer medicine that overcomes drug resistance. Compelling anti-tumor activity of AP26113 in patients with ALK-positive non-small cell lung cancer (NSCLC) and initial anti-tumor activity in patients with EGFR-mutant NSCLC were presented at the European Society of Medical Oncology meeting. Importantly, AP26113 showed clinical activity in ALK-positive NSCLC patients with brain metastases. |
18
Table of Contents
| • | We initiated the global, Phase 3 EPIC trial of Iclusig in patients with newly diagnosed CML. This trial compares Iclusig to imatinib and has a primary endpoint of major molecular response at 12 months of treatment. |
| • | We initiated a Phase 1/2 clinical trial of Iclusig in resistant or intolerant CML and Ph+ ALL patients in Japan in the second half of 2012. The trial is designed to establish the recommended dose of Iclusig in Japanese patients, confirm its anti-leukemic activity in this patient population, and provide the necessary data required for regulatory approval of Iclusig in Japan. |
| • | Our 2012 achievements positioned us to further strengthen our balance sheet at the beginning of 2013 through an underwritten public offering yielding net proceeds of $310 million. |
THE PHILOSOPHY AND IMPORTANT FEATURES OF OUR COMPENSATION PROGRAM
The philosophy underlying our compensation program has three foundational objectives:
| • | First, we endeavor to attract and retain the best available executive talent to lead our Company, recognizing that we do so in a highly competitive environment. Thus, our Compensation Committee works closely with Radford to identify our “peer group” and other companies within our competitive environment and then to align the components of our compensation program at levels that will reasonably position us to successfully compete. We discuss this below under the heading “Use of Competitive Market Compensation Data and Compensation Benchmark.” |
| • | Second, we seek to motivate our executives to achieve ambitious corporate goals by placing a substantial portion of our executives’ compensation at risk and then rewarding high levels of performance in the pursuit of those goals. We consider this “pay-for-performance” philosophy to be central to our success to date and pivotal to our success in the future. Moreover, as described below under the heading “Targets and Performance Multipliers,” we believe that a system which provides substantially greater rewards for individual performance that “exceeds requirements” or is “outstanding” in comparison to performance that merely “meets requirements” creates a powerful incentive for executives to perform at extraordinary levels. |
| • | Third, we strive to align the interests of our executive officers with those of our shareholders. We do this not only by paying for performance aimed at enhancing shareholder value, but also by structuring a substantial portion of our executives’ compensation as long-term equity compensation. In 2013, approximately 84% of the value of our Chief Executive Officer’s compensation consisted of long-term equity, including the value of performance shares at the target level. |
Use of Competitive Market Compensation Data and Compensation Benchmark
COMPETITIVE MARKET COMPENSATION DATA
As noted above, we draw upon a pool of executive talent that is highly sought after by similarly situated biotechnology companies, as well as larger pharmaceutical and biotechnology companies from which we frequently recruit, both within and outside our geographic area. We believe that the compensation practices of our peer group in particular, as well as of these larger companies and our industry in general, provide useful information to help us compete in this arena. Therefore, our Compensation Committee works closely with Radford and our management each year to review and update a comparator group of companies considered to be our peer group to ensure its continued relevance as ARIAD evolves as a company. The Compensation Committee also reviews broader life science industry data to further inform its decisions. With Radford’s assistance, our Compensation Committee uses two primary market frames of reference (which we collectively refer to as the “market”) against which to compare our executive compensation practices, as follows:
| • | Select Peer Group – A select group of national biotechnology companies at a similar stage of development as our Company, with similar headcount, market capitalization, short- and long-term growth objectives, and similar therapeutic targets. |
| • | Radford Global Life Sciences Survey – A national survey of executive compensation levels and practices that covers approximately sixty executive positions in over 600 multinational life sciences organizations. |
19
Table of Contents
We do not apply a specific weighting to either data source when making compensation comparisons. Instead, our Compensation Committee reviews composite market data synthesized by Radford from these two groups showing levels of cash, equity, and total compensation for all comparable officers relative to the elements of compensation paid to our officers.
In October and November 2013, our Compensation Committee met with Radford and discussed various factors relating to the selection of a peer group, including the continued reasonableness of the companies comprising the prior year’s peer group and ARIAD’s near- and mid-term profile in the context of the FDA action on Iclusig. Based on this review, the Compensation Committee selected a peer group of companies for compensation comparisons for 2013. This peer group consisted of public companies in the biopharmaceutical industry with a commercial branded drug, with annual revenues generally less than $1 billion, and market capitalization and headcount targeted from one-third to three times the value and size, respectively, of ARIAD at the time of the review.
The 2013 peer group reflects substantial changes from the 2012 group, primarily based on the Compensation Committee’s decision that it was appropriate to target smaller market capitalization and revenue companies than in the past. Ten companies from last year’s peer group were removed from the list for 2013: Alkermes plc, BioMarin Pharmaceuticals Inc., Cubist Pharmaceuticals, Inc., Incyte Corporation, Jazz Pharmaceuticals plc, Regeneron Pharmaceuticals, Inc., Salix Pharmaceuticals, Ltd., Seattle Genetics, Inc. and United Therapeutics, Inc. were all removed because they were above the updated market value criterion and Onyx Pharmaceuticals, Inc. was acquired by Amgen. To replace the peers that were removed, 11 new companies were added, with each company falling within the 1/3 to 3x target range of ARIAD’s revenue and market value. The new additions were: Aegerion Pharmaceuticals, Inc., Arena Pharmaceuticals, Inc., Dyax Corp., Exelixis, Inc., ImmunoGen, Inc., Isis Pharmaceuticals, Inc., Momenta Pharmaceuticals, Inc., Nektar Therapeutics, NPS Pharmaceuticals, Inc., Santarus, Inc. and Vivus, Inc.
As a result of these changes, the 2013 peer group consists of the following 19 companies:
| Acorda Therapeutics, Inc. | Ironwood Pharmaceuticals, Inc. | Questcor Pharmaceuticals, Inc. | ||
| Aegerion Pharmaceuticals, Inc. | Isis Pharmaceuticals, Inc. | Santarus, Inc. | ||
| Arena Pharmaceuticals, Inc. | The Medicines Company | Theravance, Inc. | ||
| Auxilium Pharmaceuticals, Inc. | Medivation, Inc. | ViroPharma Incorporated | ||
| Dyax Corp. | Momenta Pharmaceuticals, Inc. | Vivus, Inc. | ||
| Exelixis, Inc. | Nektar Therapeutics | |||
| ImmunoGen, Inc. | NPS Pharmaceuticals, Inc. | |||
COMPENSATION BENCHMARK
In connection with our efforts to maintain a competitive compensation program, our Compensation Committee annually establishes a benchmark for our executive compensation packages relative to other companies in our market. This benchmark is expressed as a “percentile of the market.” Beginning in 2012, recognizing that ARIAD was transitioning from a clinical-stage biotechnology company into a commercial-stage enterprise and that our future success would depend even more on competitive compensation positioning, our Compensation Committee adopted a benchmark generally targeting each element of executive compensation, as well as total compensation at the 65th percentile of the market, and the Compensation Committee maintained this target in 2013 in recognition of the very competitive market for executive talent in which we continued to operate and the critical importance of attracting and retaining such talent as we continue our evolution into a fully integrated, global oncology company.
The compensation benchmark is not intended to set a ceiling or a floor on any executive’s compensation. Instead, the actual value received by an executive in a given year may fluctuate above or below this level based on the actual level of payout or value creation to the executive under our performance-based short- and long-term incentive programs. As discussed below under “Targets and Performance Multipliers,” if an executive substantially exceeds the corporate and individual performance objectives applicable to him or her, the executive may receive compensation above the 65th percentile of market. Alternatively, an executive may receive less than 100% of the compensation target depending on his or her performance.
20
Table of Contents
Targets and Performance Multipliers
As noted above, each year our Compensation Committee analyzes the competitive market compensation data prepared by our compensation consultant to establish the following compensation parameters for each of our three levels of NEOs:
| • | A target percentage increase in base salary, |
| • | A target annual cash performance award based on a percentage of base salary, and |
| • | A target amount of long-term equity incentive award. |
With respect to our NEOs, the three officer tiers are as follows:
| • | Tier I – our Chief Executive Officer, Dr. Berger |
| • | Tier II – our Executive Vice Presidents, Dr. Clackson, Mr. Duvall and Mr. Fitzgerald |
| • | Tier III – our Senior Vice President, Dr. Bollag |
PERFORMANCE MULTIPLIERS
We believe that one of the most important motivators for our executives is the opportunity to earn compensation greater than the established targets by exceeding applicable performance requirements. We accomplish this through a system of “performance multipliers,” which reward exceptional performance at substantially higher levels than performance that merely meets requirements of the position. Under our performance multiplier approach, the level of performance of each executive directly influences his or her increase in base salary, annual performance award and long-term equity incentive award relative to target awards, and such amounts may be greater or less than the target levels based on the assessment and rating of each officer’s performance. Our Compensation Committee establishes the performance multiplier scale, which ranged from 0% – 160% for salary and long-term equity awards for fiscal 2012, and 0% – 200% for annual cash performance awards for fiscal 2013. Each executive officer’s performance was rated on a scale from “unsatisfactory” to “outstanding.” As an example of the impact of our “performance multipliers” on 2013 annual performance awards, an executive whose performance “meets requirements” would receive only 50% of his or her target bonus, whereas an executive whose performance “exceeds requirements” could earn up to 150% of his or her target bonus, and an executive whose performance is assessed as “outstanding” would receive up to 200% of his or her target bonus.
Process for Determining Executive Compensation
Our Compensation Committee is responsible for, among other duties, reviewing the performance of our Chief Executive Officer and reviewing and recommending his compensation for approval by the Board. The Compensation Committee is also responsible for reviewing the assessment of performance of our other executive officers conducted by our Chief Executive Officer and reviewing and approving their compensation in consultation with our Chief Executive Officer. While our Compensation Committee has ultimate authority and responsibility for approving all executive officer compensation, our Chief Executive Officer plays an active role in such decisions, except with respect to his own compensation where he participates in neither the deliberations nor the decision.
At the beginning of each year, the executive leadership team of the Company establishes annual corporate objectives, which are reviewed and discussed with the Compensation Committee and the Board and form the basis for our annual operating plan. The status of our corporate objectives, as well as our performance relative to our operating plan, are reviewed and discussed with the Board regularly during the year. Based on the annual corporate objectives and the associated operating plan, each of the executive officers is responsible for developing plans and managing the key initiatives and activities designed to achieve our objectives.
21
Table of Contents
Generally, at the end of each year, each of the executive officers provides a detailed self-assessment of his or her performance relative to the established corporate and individual objectives, as well as to key leadership and management measures described below. In addition, each executive officer is evaluated on a confidential basis by several of his or her peers, subordinates, and in some instances, external colleagues, selected by our Chief Executive Officer. The Chief Executive Officer reviews and evaluates all of these assessments and completes an overall evaluation of performance for each officer for the year, taking into account his judgment regarding the Company’s overall progress, each officer’s contribution to the achievement of corporate objectives, his or her achievement of individual objectives, and his or her performance in relation to leadership and management measures. Specific numerical weightings or ratings are not applied to individual corporate or personal objectives; rather, the performance of the executive officer is evaluated as a whole in the context of his or her peers at the same level of management within the Company. Our assessments in 2013 concluded that we had achieved most of our corporate objectives through the first three quarters of the year. With the challenges regarding Iclusig that we faced in the fourth quarter, the unified focus of the executive leadership team turned to obtaining FDA approval to resume marketing and distribution of Iclusig in the United States, which was achieved before year-end.
The level of performance is then used by the Chief Executive Officer to guide his recommendations to the Compensation Committee regarding each executive officer’s performance rating. As part of this process, the Compensation Committee, with input from the Chief Executive Officer, reviews the overall performance of the Company relative to the key corporate objectives established at the beginning of the year. This assessment, along with the assessments made by the Chief Executive Officer regarding the individual officers, forms the basis for the decisions regarding the individual’s performance rating. Performance ratings can range from “unsatisfactory” to “meets requirements” to “exceeds requirements” to “outstanding”. The performance rating is then used to determine the performance multiplier applicable to the executive, which forms the basis for increases in salary, annual performance awards and long-term incentive awards.
The Compensation Committee also undertakes a comprehensive review of the Chief Executive Officer’s performance based on its evaluation of our Company’s overall performance, the Chief Executive Officer’s individual contributions to achievement of key objectives, his strategic leadership of the Company and his demonstration of our vision and corporate values, including a commitment to building shareholder value. His overall performance is rated, and his compensation is adjusted using the same performance scale as the other executive officers. The Compensation Committee makes these determinations in executive session and then makes recommendations to the Board for subsequent approval.
In addition to an evaluation of the level of achievement of our corporate and certain individual objectives, each executive officer is evaluated as to key leadership and management measures, including each individual’s:
| • | Contribution to the management team and development and application of leadership skills reflective and supportive of our corporate values, vision and mission, |
| • | Ability to attract, hire, manage, retain, and motivate talent in support of the achievement of our objectives, |
| • | Management of his or her functions and responsibilities within established financial budgets and forecasts, and |
| • | Management of regulatory compliance requirements related to his or her responsibilities. |
There are no thresholds or maximum levels of achievement applicable to these individual objectives or the corporate objectives. In addition, no specific weight is given to any of these factors in determining base salary increases, annual performance awards or long-term equity incentive awards for any of our executive officers. Instead, as noted above, each executive officer’s performance is evaluated as a whole, taking into account all of these objectives and measures, encouraging collaboration among senior executives in achieving inter-dependent corporate objectives.
22
Table of Contents
Elements of Total Compensation
As briefly described above, we provide three basic forms of direct compensation to our executive officers: base salary, annual performance awards, and long-term equity incentive awards.
BASE SALARY
Base salary is intended to provide all of our employees with a fair and competitive base level of compensation that reflects their job function, organizational level, experience and tenure, and sustained performance over time. Executive officer base salary levels are set using these criteria.
On an annual basis, our executives are eligible for a salary increase. The amount of this increase, if any, is determined by our Compensation Committee, which establishes a target salary increase based on analysis of market compensation data and the recommendation of our compensation consultant.
Each executive’s actual salary increase, if any, is determined based on the executive’s performance in achieving key corporate and individual objectives established at the beginning of each year, internal pay equity, and demonstrated levels of core job competency and effective leadership. As discussed above under “Targets and Performance Multipliers,” the executive’s performance rating leads to the application of a performance multiplier, which results in the actual salary adjustment. Adjustments to base salary levels typically are made in the first quarter of each year and are paid retroactively to January 1 of that year.
ANNUAL PERFORMANCE AWARDS
Annual performance awards are intended to reward our executive officers for achievement of corporate, individual, and key leadership and management objectives on an annual basis. Prior to payment of our 2012 annual performance awards to our Chief Executive Officer, his payments had been made, in whole or in part, in the form of shares of common stock, in order to conserve cash to fund our priority research and development programs and commercialization efforts. Also prior to payment of our 2012 annual performance awards, our other executive officers received payment in cash, but were required to defer such payments under our 2005 Executive Compensation Plan, as amended (the “2005 Executive Compensation Plan”), which also allowed us to conserve cash for operations. For performance in 2012, annual performance awards to our Chief Executive Officer and other NEOs were paid in cash in recognition of the transition of the Company to a commercial organization, which is also consistent with the practices of our peers and the broader life sciences marketplace. For performance in 2013, no annual performance awards were paid to our Chief Executive Officer or any of our NEOs for the reasons described above.
As discussed above under “Targets and Performance Multipliers,” our Compensation Committee annually establishes target annual performance awards for the different tiers of executives, which are expressed as a percentage of base salary. These target awards are then adjusted for each executive through the application of a performance multiplier based on the individual executive’s performance rating for the year.
LONG-TERM EQUITY INCENTIVE AWARDS
Long-term equity incentive awards are also intended to reward our executive officers for achievement of corporate, individual, and key leadership and management objectives. In addition, such awards are intended to align the interests of all of our executive officers with those of our stockholders, promote progress toward achieving our long-term strategy and assist in long-term retention of our executive officers. As such, long-term equity incentive awards for our executive officers are made in the form of stock options, restricted stock units and/or performance shares. Stock options and restricted stock units generally vest annually over three years. Performance shares vest upon the achievement of one or more key corporate objectives or metrics and, once achieved, are subject in certain cases to further time-based vesting to provide further retention. The mix of types of equity awards provides various long-term incentives. Stock options align the interests of our executives with those of our stockholders in that value will only be realized from these awards if our stock price increases over time; restricted stock units provide some certainty of receiving some amount of compensation during periods of market volatility and provide the opportunity for our executives to maintain holdings of our common stock; and performance shares provide a direct link between achievement of key corporate objectives that we expect will drive long-term value for our stockholders. Long-term equity incentive awards are granted under our 2006 Long-Term Incentive Plan. The target long-term equity incentive awards for each officer are based on recommendations by Radford, taking into consideration the value of equity-based awards and total compensation of executives of companies in the composite market compiled by Radford, as described under “Competitive Market Compensation Data” above.
23
Table of Contents
For performance shares granted in March 2012, the performance objective was regulatory approval by the European Medicines Agency of a Marketing Authorization Application (MAA) for Iclusig prior to the end of 2016, and if not obtained by then, the award would terminate and have no value. This objective was achieved in July 2013 and pursuant to the terms of the performance share awards, 160% of the target award was earned with immediate vesting of 50% of the total award and 25% vesting on each of the first and second anniversaries of the achievement of the objective. For performance shares granted in March 2013, the performance objective is tied to continued success in certain research and development initiatives through the end of 2016, with the number of shares to be issued ranging from 50% to 160% of target, and the vesting schedule varying, depending on the year in which such performance objective is achieved. If the program objective is not obtained prior to the end of 2016, the performance shares will terminate and have no value.
MIX OF DIRECT COMPENSATION COMPONENTS
As our executive officers have increasing responsibility for and impact on our results, we place greater emphasis on variable, performance-based compensation and longer term compensation vehicles in the form of equity awards and deferred performance awards.
We do not have specific policies nor do we use formulas to determine a mix of total compensation. We intend for total compensation to vary based on our progress towards achievement of corporate, departmental and team objectives, as well as individual performance goals. For fiscal 2013, our annual performance awards and long-term equity incentive awards, including the value of performance shares awarded at target level, represent over 75% of the total direct compensation (excluding benefits) on average for our NEOs.
BENEFITS AND PERQUISITES
In addition to general benefits offered to all other salaried employees, we provide our executive officers with supplemental long-term disability insurance and long-term care insurance, tax return preparation services and an auto allowance in accordance with their employment agreements. These are the only perquisites we provided to our executive officers during 2013. Perquisites represent less than 2% of each NEO’s total compensation as set forth in the Summary Compensation Table below. We believe that our benefits and perquisites represent competitive market practices for executives at companies within our peer group. They are offered as a means to attract and retain our executive officers.
EMPLOYMENT AGREEMENTS
We have entered into employment agreements with our NEOs. Each of these agreements provides for certain payments and other benefits if the executive’s employment terminates under certain circumstances other than for “cause,” including in connection with a “change in control.” See the subsection “Narrative to Summary Compensation Table and Grants of Plan-Based Awards Table” for a description of the agreement terms impacting current compensation and “Potential Payments upon Termination or Change in Control” for a description of applicable severance and change in control benefits.
Our Compensation Committee believes that change-in-control and severance arrangements are important parts of the overall compensation program for our NEOs. Change-in-control provisions help to secure the continued employment and dedication of our executive officers, to reduce any concern that they might have regarding their own continued employment prior to or following a change in control, and to promote a continuity of management during a corporate transaction. Severance arrangements are used primarily to attract, retain and motivate individuals with the requisite experience and ability to drive our success. Severance arrangements also serve, in part, as consideration to secure commitments from our executive officers not to compete with us after termination of their employment.
COMPENSATION COMMITTEE POLICY REGARDING CHANGE IN CONTROL SEVERANCE PAYMENTS
Effective April 2010, our Compensation Committee adopted a policy that restricts our Company from entering into any future agreement that provides an executive officer with a severance payment following a change in control of our Company, except in the case of a termination event (i.e., a “double-trigger”). In addition, the policy also restricts our Company from entering into any future agreement that provides an executive officer with the right to receive excise tax gross-ups following a change in control, except in certain unusual circumstances. Effective April
24
Table of Contents
2013, our Compensation Committee revised this policy eliminating the exception to allow for an excise tax-gross in certain unusual circumstances. We have not entered into any agreements with any of our executive officers that provide for an excise tax gross-up, other than Dr. Berger’s employment agreement, which pre-dated the April 2010 policy.
Factors Considered in the Determination of Executive Compensation
2012 PERFORMANCE CONSIDERED FOR 2013 SALARY AND LONG-TERM EQUITY INCENTIVE AWARDS
As described above under “Key Components of Compensation and Related Performance Periods,” in early 2013, our Compensation Committee made decisions regarding 2013 salary increases and 2013 grants of long-term equity incentive awards, in each case based on 2012 performance. The Compensation Committee determined the performance rating of each executive officer based on his or her contribution to the achievement of our key corporate objectives for 2012, as well as each officer’s performance in relation to individual goals and leadership and management measures. Our key corporate objectives applicable to our executive officers for 2012 corresponded to ARIAD’s 2012 accomplishments described in the “Executive Summary” section above under “Business Highlights – Fiscal 2012.” Briefly summarized:
| • | We completed the regulatory submission of and obtained U.S. marketing approval for Iclusig. |
| • | We established European operations in preparation for the European commercial approval and launch of Iclusig in 2013. |
| • | We continued to advance the clinical development of AP26113. |
| • | We enhanced our discovery efforts with the goal of designating our next development candidate. |
| • | We managed substantial growth in personnel and internal systems. |
| • | We positioned the Company to further strengthen our balance sheet through an underwritten public offering in early 2013. |
Dr. Berger’s 2013 Salary and Equity Awards
Our Chief Executive Officer’s salary increase and long-term equity incentive awards were based predominantly on our Company’s level of achievement of above-described key corporate accomplishments, as well as successfully demonstrating the following:
| • | Building long-term shareholder value, |
| • | Building and retaining our senior management team, |
| • | Exemplifying our corporate values, mission and vision, |
| • | Developing and executing on our long-term strategy, and |
| • | Providing long-term leadership for our company. |
Based on the above, Dr. Berger’s 2012 performance was rated outstanding. His 2013 salary increase to $751,000 reflects a merit increase of 3%, plus an adjustment of approximately 4.7% based on an assessment of market data and Dr. Berger’s performance. His long-term equity incentive awards delivered in March 2013 based on 2012 performance consisted of 95,000 performance shares, 108,000 restricted stock units and 216,000 stock options, delivering roughly 60% of his target value via performance-based awards (including stock options and performance shares at the target level).
Dr. Clackson’s 2013 Salary and Equity Awards
Dr. Clackson, as President of Research and Development and Chief Scientific Officer, continued to have broad responsibilities for the management of our business, including leading our discovery research, preclinical
25
Table of Contents
development, clinical development, medical affairs, manufacturing and program and alliance management. Based on his contributions to the achievement of our corporate objectives for 2012, as well as his performance in relation to individual objectives and leadership and management standards, Dr. Clackson’s performance was rated as exceeding requirements.
His 2013 salary increase to $493,000 reflects a merit increase of 3%, plus an adjustment of approximately 2.3% based on an assessment of market data and Dr. Clackson’s performance. His long-term equity incentive awards delivered in March 2013 based on 2012 performance consisted of 35,000 performance shares, 32,500 restricted stock units and 65,000 stock options, delivering roughly 60% of his target value via performance-based awards (including stock options and performance shares at the target level).
Mr. Fitzgerald’s 2013 Salary and Equity Awards
Mr. Fitzgerald, as Executive Vice President and Chief Financial Officer, continued to have broad responsibilities for many aspects of our operations and business. These included planning and overseeing the growth and international expansion of our business, leading the planning and implementation of key systems necessary to support the needs of our business, managing significant initiatives that provided additional funding for our programs, and effectively managing our spending in support of our key corporate objectives. Based on his contributions to the achievement of our corporate objectives for 2012, as well as his performance in relation to individual objectives and leadership and management standards, Mr. Fitzgerald’s performance was rated as exceeding requirements.
His 2013 salary increase to $466,000 reflects a merit increase of 3%, plus an adjustment of approximately 3.4% based on an assessment of market data and Mr. Fitzgerald’s performance. His long-term equity incentive awards delivered in March 2013 based on 2012 performance consisted of 35,000 performance shares, 32,500 restricted stock units and 65,000 stock options, delivering roughly 60% of his target value via performance-based awards (including stock options and performance shares at the target level).
Mr. Duvall’s 2013 Salary and Equity Awards
Mr. Duvall, as Senior Vice President, Commercial Operations in 2012, had responsibilities for the management of our commercial business on a global basis, including the development and implementation of our commercial strategy related to the commercial launch of Iclusig in the United States and Europe and the building of our commercial teams, systems and processes necessary to support the launch. Based on his contributions to the achievement of our corporate objectives for 2012, as well as his performance in relation to individual objectives and leadership and management standards, Mr. Duvall’s performance was rated as exceeding requirements.
His 2013 salary increased to $420,000, from $388,000 in 2012, reflecting a merit increase of 3% plus an adjustment of approximately 5.2% based on an assessment of market data and Mr. Duvall’s performance. His long-term equity incentive awards delivered in March 2013 based on 2012 performance consisted of 20,000 performance shares, 24,000 restricted stock units and 48,000 stock options, delivering roughly 60% of his target value via performance-based awards (including stock options and performance shares at the target level). In December 2013, in connection with his promotion to Executive Vice President, Chief Commercial Officer, Mr. Duvall’s annual salary was increased to $455,000 and he was granted an additional 35,000 stock options.
Dr. Bollag’s 2013 Salary and Equity Awards
Dr. Bollag, as Senior Vice President, Regulatory Affairs and Quality, has responsibilities for the management of our regulatory affairs and quality operations on a global basis, including the development and implementation of regulatory strategy related to our products and product candidates. Based on his contributions to the achievement of our corporate objectives for 2012, as well as his performance in relation to individual objectives and leadership and management standards, Dr. Bollag’s performance was rated as exceeding requirements.
His 2013 salary increased to $387,000, from $362,000 in 2012, reflecting a merit increase of 3% plus an adjustment of approximately 3.9% based on an assessment of market data and Dr. Bollag’s performance. His long-term equity incentive awards delivered in March 2013 based on 2012 performance consisted of 20,000 performance shares, 24,000 restricted stock units and 48,000 stock options, delivering roughly 60% of his target value via performance-based awards (including stock options and performance shares at the target level).
26
Table of Contents
2013 PERFORMANCE CONSIDERED FOR 2013 ANNUAL PERFORMANCE AWARDS AND 2014 SALARY AND LONG-TERM EQUITY INCENTIVE AWARDS
2013 Annual Performance Awards
Target annual performance awards for 2013 for all members of our executive leadership team except Dr. Berger were targeted at the 65th percentile of the market. For Dr. Berger, the Compensation Committee set the target annual performance award above the 75th percentile in order to increase the proportion of Dr. Berger’s compensation that is variable based on company performance. Although we did achieve most of our corporate objectives during 2013, the Compensation Committee decided not to pay any cash bonuses to our NEOs because of the FDA action on Iclusig.
2013 Annual Performance Awards
| Name | Target Percentage |
Performance Multiplier |
Actual Award Percentage |
Actual Award |
||||||||||||
| H. Berger |
85 | % | — | — | $ | 0 | ||||||||||
| T. Clackson |
50 | % | — | — | $ | 0 | ||||||||||
| E. Fitzgerald |
50 | % | — | — | $ | 0 | ||||||||||
| M. Duvall |
45 | %(1) | — | — | $ | 0 | ||||||||||
| D. Bollag |
45 | % | — | — | $ | 0 | ||||||||||
| (1) | Mr. Duvall’s target bonus percentage increased from 45% to 50% upon his promotion to Executive Vice President, Chief Commercial Officer in December 2013. |
2014 Salary and Long-term Equity Incentive Awards
The Compensation Committee also determined that no increase in base salaries should be provided to Dr. Berger or the other NEOs. However, in January 2014, the Compensation Committee did approve a 2014 salary increase of $35,000 for Mr. Duvall retroactive to January 1, 2014 in connection with Mr. Duvall’s promotion in December 2013 to Executive Vice President, Chief Commercial Officer.
In January 2014, the Compensation Committee approved long-term incentive awards to our NEOs. These awards were made based on consideration of the challenges presented by the FDA action on Iclusig, the considerable diminution in the value of previously granted equity awards, and the need to restore meaningful go-forward incentives to the executive leadership team with respect to our efforts to resume regular marketing of Iclusig and continue advancement on our other clinical goals. The Compensation Committee initially determined a target number of stock options to be delivered to each member of the executive leadership team and then determined the appropriate equity mix among long-term incentive vehicles (e.g., stock options, performance shares and restricted stock unit awards). For 2013, the Compensation Committee determined not to issue stock options and converted the number of stock options that would have been granted into performance shares at a ratio of 1.6 to 1 and into restricted stock units at a ratio of 2 to 1 based on equivalent accounting values of the various types of awards. With respect to the award of restricted stock units, the Committee used a premium conversion ratio to account for the downside protection in the award and the absence of performance-orientation inherent in performance shares and stock options. Based on these calculations, equity grants were made as to 50% of the value of such awards in the form of performance shares, on a 1.6 to 1 basis, and as to the remaining 50% of the value of such awards in the form of time-based restricted stock units that vest ratably over three years, on a 2 to1 basis. Regarding the performance shares, 50% of each executive’s award will be earned based on the achievement of specific research and development objectives with the remaining 50% based on the achievement of specific commercial objectives.
| Name | 2014 Salary |
Performance Shares at Target(#) |
Restricted Stock Units(#) |
Grant Date Fair Value of Equity |
||||||||||||
| H. Berger |
$ | 751,000 | 239,000 | (1) | 261,000 | (2) | $ | 3,695,000 | ||||||||
| T. Clackson |
$ | 493,000 | 77,000 | 88,000 | $ | 1,219,350 | ||||||||||
| E. Fitzgerald |
$ | 466,000 | 77,000 | 88,000 | $ | 1,219,350 | ||||||||||
| M. Duval |
$ | 455,000 | 77,000 | 88,000 | $ | 1,219,350 | ||||||||||
| D. Bollag |
$ | 387,000 | 77,000 | 88,000 | $ | 1,219,350 | ||||||||||
27
Table of Contents
| (1) | Dr. Berger will not earn more than the target number of shares in order to comply with the 500,000 per employee share award limit set forth in the 2006 Long-Term Incentive Plan. |
| (2) |
Dr. Berger’s award was calculated at 272,000 shares but was reduced by 11,000 shares in order to comply with the 500,000 per employee share award limit set forth in the 2006 Long-Term Incentive Plan. |
For the performance shares granted in January 2014, the number of shares earned will range from 160% to 50% of target, with the actual number of shares earned and the vesting schedule varying depending on the year in which such performance objectives are obtained. If the performance objectives are not obtained prior to the dates set forth in the award, which includes an outside date of 2017, the award will terminate and have no value.
2014 Target Annual Performance Awards
In March 2014, the Compensation Committee approved 2014 target annual performance awards as a percentage of 2014 salaries which annual performance awards will be paid in 2015 based on 2014 performance. The targets were maintained at 2013 levels except for Mr. Duvall, whose target bonus increased in connection with his promotion to Executive Vice President, Chief Commercial Officer in December 2013, and are as follows:
| Name | 2014 Target Payout as a % of 2014 Base Salary |
|||
| H. Berger |
85 | % | ||
| T. Clackson |
50 | % | ||
| E. Fitzgerald |
50 | % | ||
| M. Duvall |
50 | % | ||
| D. Bollag |
45 | % | ||
The actual payout of the 2014 annual performance awards may be above or below target based on performance.
Hedging Transactions, Pledges of Stock, and Insider Trading Policy
Our insider trading policy expressly bars our executive officers, directors and employees from engaging in hedging transactions such as buying or selling puts and calls on ARIAD stock. We also prohibit our executive officers, directors and employees from purchasing ARIAD stock on margin and pledging our stock as collateral. In addition, we prohibit our officers, directors and employees from purchasing or selling ARIAD securities while in possession of material, non-public information, or otherwise using such information for their personal benefit. We also permit and encourage our executives and directors to enter into trading plans that are intended to comply with the requirements of Rule 10b5-1 promulgated under the Exchange Act when they desire to prudently diversify their asset portfolios and exercise their stock options before their scheduled expiration dates.
Tax Deductibility of Compensation
Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”) limits the deduction a public company is permitted for compensation paid to the chief executive officer and to the three most highly compensated executive officers (other than the chief executive officer or chief financial officer). Generally, amounts paid in excess of $1,000,000 to a covered executive cannot be deducted, unless the compensation is paid pursuant to a plan that is performance related, non-discretionary and has been approved by shareholders. Our 2006 Long-Term Incentive Plan permits the issuance of performance-based stock awards that would be compliant with Section 162(m). In 2013, we granted performance shares that are intended to comply with Section 162(m). We have not adopted a policy that all executive compensation be fully deductible.
28
Table of Contents
Summary Compensation Table
The following table sets forth the compensation paid to or accrued on behalf of our Chief Executive Officer, our Chief Financial Officer and our three other most highly compensated executive officers, whom we refer to collectively as our named executive officers, during the fiscal years ended December 31, 2011, 2012 and 2013.
| Name and Principal Position | Year | Salary | Bonus | Stock Awards (1) |
Option Awards (1) |
All
Other Compensation (2) |
Total | |||||||||||||||||||||
| Harvey J. Berger, M.D. Chairman, Chief Executive Officer, and President |
|
2013 2012 2011 |
|
$ |
749,754 695,777 663,923 |
|
$ |
— 976,000 372,000 |
|
$ |
2,256,120 1,911,350 1,530,765 |
|
$ |
2,831,090 2,368,901 1,264,736 |
|
$ |
35,473 34,810 28,234 |
|
$ |
5,872,437 5,986,838 3,859,658 |
| |||||||
| Timothy P. Clackson, Ph.D. President of Research and Development, Chief Scientific Officer |
|
2013 2012 2011 |
|
|
492,423 466,077 417,000 |
|
|
— 365,000 300,000 |
|
|
678,925 602,000 304,980 |
|
|
851,949 911,971 390,918 |
|
|
31,979 31,030 25,378 |
|
|
2,055,275 2,376,078 1,438,276 |
| |||||||
| Edward M. Fitzgerald Executive Vice President, Chief Financial Officer and Treasurer |
|
2013 2012 2011 |
|
|
465,354 436,461 397,308 |
|
|
— 329,000 179,000 |
|
|
678,925 376,250 304,980 |
|
|
851,949 775,272 390,918 |
|
|
30,306 29,636 23,163 |
|
|
2,026,533 1,946,619 1,295,369 |
| |||||||
| Martin J. Duvall Executive Vice President and Chief Commercial Officer |
2013 | 427,262 | — | 501,360 | 748,558 | 23,457 | 1,700,637 | |||||||||||||||||||||
| Daniel M. Bollag, Ph.D. Senior Vice President, Regulatory Affairs and Quality |
2013 | 386,423 | — | 501,360 | 629,131 | 30,772 | 1,547,687 | |||||||||||||||||||||
| (1) | The amounts included under “Stock Awards” and “Option Awards” represent the aggregate grant date fair value for stock awards and option awards granted during the year, computed in accordance with FASB ASC Topic 718. The assumptions used in the calculation of the grant date fair value of the stock awards and option awards granted in 2013 are set forth in note 13 to our audited consolidated financial statements titled “Stock-Based Compensation” included in the Original Filing. The grant date fair value of performance shares awarded in 2013 was determined to be $0 and therefore is not included in the table. The grant date fair value of performance shares awarded in 2013, assuming the maximum potential value is achieved, is $3,108,400 for Dr. Berger; $1,145,200 for Dr. Clackson; $1,145,200 for Mr. Fitzgerald; $654,400 for Mr. Duvall and $654,400 for Dr. Bollag. |
| (2) | Amounts included under “All Other Compensation” for 2013 consist of: (i) matching contributions to our defined contribution retirement savings plan ($7,500 for each of Dr. Berger, Dr. Clackson, Mr. Fitzgerald, Mr. Duvall and Dr. Bollag); and (ii) other compensation ($27,973 for Dr. Berger, $24,479 for Dr. Clackson, $22,806 for Mr. Fitzgerald, $15,957 for Mr. Duvall and $23,272 for Dr. Bollag) consisting of the cost of supplemental long-term disability and long-term care insurances, an annual auto allowance, and tax preparation services for each of the named executive officers. |
29
Table of Contents
Grants of Plan-Based Awards in 2013
The following table shows information regarding grants of equity awards that were made during the year ended December 31, 2013 to each of our named executive officers. All awards were made under our 2006 Long-Term Incentive Plan. There were no grants of non-equity incentive plan awards to our named executive officers during 2013.
| Estimated Future Payouts Under Equity Incentive Plan Awards: (1) |
All Other Stock Awards: Number of Shares of Stock or Units (#) |
All Other Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/sh) |
Grant Date Fair Value of Stock and Option Awards (2) |
||||||||||||||||||||||||||||
| Grant Date |
Threshold (#) |
Target (#) | Maximum (#) |
|||||||||||||||||||||||||||||
| Harvey J. Berger |
03/19/13 | 216,000 | (4) | $ | 20.89 | $ | 2,831,090 | |||||||||||||||||||||||||
| 03/19/13 | 108,000 | (3) | $ | 2,256,120 | ||||||||||||||||||||||||||||
| 03/19/13 | 47,500 | 95,000 | 152,000 | — | ||||||||||||||||||||||||||||
| Timothy P. Clackson |
03/19/13 | 65,000 | (4) | $ | 20,89 | $ | 851,949 | |||||||||||||||||||||||||
| 03/19/13 | 32,500 | (3) | $ | 678,925 | ||||||||||||||||||||||||||||
| 03/19/13 | 17,500 | 35,000 | 56,000 | — | ||||||||||||||||||||||||||||
| Edward M. Fitzgerald |
||||||||||||||||||||||||||||||||
| 03/19/13 | 65,000 | (4) | $ | 20.89 | $ | 851,949 | ||||||||||||||||||||||||||
| 03/19/13 | 32,500 | (3) | $ | 678,925 | ||||||||||||||||||||||||||||
| 03/19/13 | 17,500 | 35,000 | 56,000 | — | ||||||||||||||||||||||||||||
| Martin J. Duvall |
03/19/13 | 48,000 | (4) | $ | 20.89 | $ | 629,131 | |||||||||||||||||||||||||
| 03/19/13 | 24,000 | (3) | $ | 501,360 | ||||||||||||||||||||||||||||
| 03/19/13 | 10,000 | 20,000 | 32,000 | — | ||||||||||||||||||||||||||||
| 12/09/13 | 35,000 | (5) | $ | 4.29 | $ | 119,427 | ||||||||||||||||||||||||||
| Daniel M. Bollag |
03/19/13 | 48,000 | (4) | $ | 20,89 | $ | 629,131 | |||||||||||||||||||||||||
| 03/19/13 | 24,000 | (3) | $ | 501,360 | ||||||||||||||||||||||||||||
| 03/19/13 | 10,000 | 20,000 | 32,000 | — | ||||||||||||||||||||||||||||
| (1) | These awards are performance shares that will vest based on the achievement, and timing of the achievement, of certain research and development initiatives prior to the end of 2016 provided that the executive is then employed with us. |
| (2) | The grant date fair values of the awards have been determined in accordance with FASB ASC Topic 718. The assumptions used in the calculation of the grant date fair values of these awards are set forth in Note 13 to our audited financial statements for the year ended December 31, 2013 included in the Original Filing. |
| (3) | These awards are in the form of restricted stock units that vest as to 33 1/3% of the awards on each of March 19, 2014, 2015 and 2016 provided that the executive is then employed with us, at which times the underlying shares of common stock will be delivered to the recipient, subject to the terms of the recipient’s executive employment agreement. |
| (4) | The stock options vest as to 33 1/3% of the award on each of March 19, 2014, 2015 and 2016 provided that the executive is then employed with us, subject to the terms of the recipient’s executive employment agreement. |
| (5) | The stock option vests as to 25% of the award on each of December 9, 2014, 2015, 2016 and 2017. |
30
Table of Contents
Narrative to Summary Compensation Table and Grants of Plan-Based Awards Table
Employment agreements with our named executive officers provide for base salary as may be adjusted annually, annual bonus opportunities, participation in our benefit plans, the opportunity to receive equity awards, and post-termination benefits and obligations.
Dr. Berger’s employment agreement has been in effect since April 2010. It was automatically renewed in December 2013 and will expire on December 31, 2016, subject to automatic renewal for successive three-year terms absent notice to the contrary by either party, and following a change in control (as defined in the agreement), until the later of the expiration of the then current term or the second anniversary of the date on which the change in control occurs. The employment agreements with our other named executive officers provided for initial terms, which automatically renew for successive one-year terms absent notice to the contrary by either party. In April 2014, the terms of employment of each of our named executive officers, other than Dr. Berger, were extended to December 31, 2016. Each employment agreement specifies a minimum level of base salary for the executive, but gives our Compensation Committee authority to increase the executive’s base salary from time to time.
Dr. Berger’s employment agreement, as currently in effect, provides that we shall pay him a discretionary cash bonus based on a target of not less than fifty percent of his then current salary, with the actual amount determined by our Board. In 2012, we paid half of the value of Dr. Berger’s 2011 bonus in shares of common stock and half in cash. The employment agreements for the other named executive officers provide for discretionary bonuses based on a target of thirty percent of their then current salaries, payable in the form of stock options, stock awards, restricted stock units, deferred compensation or cash, as determined by our Board. The annual targets are reviewed and established each year by the Compensation Committee. The target awards for 2013 are set forth in the Compensation Discussion and Analysis under the heading “Process for Determining Executive Compensation”.
In 2011, we awarded performance shares to each of the named executive officers who were employed at that time that would vest upon obtaining regulatory approval from the FDA to market Iclusig prior to the end of 2016. This objective was achieved in December 2012 and, in accordance with the terms of the grant, 50% of the award vested in December 2012 and the remaining 50% vested in December 2013. In 2012, we awarded performance shares to each of the named executive officers that would vest upon obtaining regulatory approval from the European Medicines Agency to market Iclusig in the European Union prior to the end of 2016. The objective was achieved in July 2013 and, in accordance with the terms of the grant, 50% of the award vested in July 2013, 25% will vest in July 2014 and the remaining 25% will vest in July 2015. In 2013, we awarded performance shares to each of the named executive officers that will vest upon the achievement, and timing of the achievement, of certain research and development initiatives prior to the end of 2016. The number of shares that will vest and the vesting schedule are dependent on when prior to the end of 2016 the performance milestone is achieved. If the performance milestone is not achieved prior to December 31, 2016, these performance shares will terminate and have no value.
The employment agreements also provide that each executive is entitled to, among other things, participation in any incentive, stock award or bonus plan, pension, group insurance, and fringe benefits on the same basis as executives at a comparable level; group health, disability and life insurance; paid vacation; an auto allowance of $750 per month which was increased to $1,000 per month in 2012; standard tax preparation and planning services; reimbursement of business expenses; indemnification and directors’ and officers’ insurance coverage; and for executives who had received credit under our sabbatical policy prior to its termination in 2008, a lump sum payment upon termination of employment in good standing equal to three months of their base salary for each sabbatical that was fully earned under the policy. In addition, Dr. Berger’s employment agreement provides him with medical malpractice insurance with coverage reasonably satisfactory to Dr. Berger and legal costs to enforce the employment agreement on an as-incurred basis subject to repayment if we prevail.
The employment agreements with our named executive officers also provide for severance payments upon termination of employment by us without cause, termination by the executive for material breach of the agreement by us, non-renewal (for Dr. Berger only) or termination in connection with a change in control. Dr. Berger’s agreement also provides for a tax gross-up in the event that any amounts payable by us or our successor are subject to excise taxes under Sections 280G and 4999 of the Code. See “Potential Payments upon Termination or Change in Control” for a description of these provisions in the employment agreements.
31
Table of Contents
Outstanding Equity Awards at December 31, 2013
The following table lists the outstanding equity awards at December 31, 2013 for each of the named executive officers:
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options: Exercisable (1) |
Number of Securities Underlying Unexercised Options: Unexercisable (1) |
Option Exercise Price |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested |
Market Value of Shares or Units of Stock That Have Not Vested (2) |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested |
Equity Incentive Plan Awards: Market or Payout Values of Unearned Shares, Units or Other Rights that Have Not Vested (2) |
||||||||||||||||||||||||
| Harvey J. Berger |
150,000 | $ | 5.23 | 09/09/14 | ||||||||||||||||||||||||||||
| 150,000 | $ | 7.56 | 10/04/15 | |||||||||||||||||||||||||||||
| 240,000 | $ | 4.64 | 03/06/17 | |||||||||||||||||||||||||||||
| 25,000 | (3) | $ | 4.49 | 04/16/17 | ||||||||||||||||||||||||||||
| 146,667 | 73,333 | (4) | $ | 7.82 | 04/01/21 | |||||||||||||||||||||||||||
| 71,000 | 142,000 | (5) | $ | 15.05 | 03/20/22 | |||||||||||||||||||||||||||
| 216,000 | (6) | $ | 20.89 | 03/19/23 | ||||||||||||||||||||||||||||
| 41,667 | (10) | $ | 284,169 | |||||||||||||||||||||||||||||
| 68,000 | (11) | $ | 463,760 | |||||||||||||||||||||||||||||
| 73,600 | (12) | $ | 501,952 | |||||||||||||||||||||||||||||
| 108,000 | (13) | $ | 736,560 | |||||||||||||||||||||||||||||
| 95,000 | (14) | $ | 647,900 | |||||||||||||||||||||||||||||
| Timothy P. Clackson |
20,657 | $ | 7.56 | 10/04/15 | ||||||||||||||||||||||||||||
| 55,458 | $ | 4.49 | 04/16/17 | |||||||||||||||||||||||||||||
| 24,999 | (3) | $ | 2.62 | 12/08/19 | ||||||||||||||||||||||||||||
| 20,000 | 10,000 | (7) | $ | 3.25 | 06/24/20 | |||||||||||||||||||||||||||
| 36,702 | 22,667 | (4) | $ | 7.82 | 04/01/21 | |||||||||||||||||||||||||||
| 27,334 | 54,666 | (5) | $ | 15.05 | 03/20/22 | |||||||||||||||||||||||||||
| 65,000 | (6) | $ | 20.89 | 03/19/23 | ||||||||||||||||||||||||||||
| 13,000 | (10) | $ | 88,660 | |||||||||||||||||||||||||||||
| 26,666 | (11) | $ | 181,862 | |||||||||||||||||||||||||||||
| 28,000 | (12) | $ | 190,960 | |||||||||||||||||||||||||||||
| 32,500 | (13) | $ | 221,650 | |||||||||||||||||||||||||||||
| 35,000 | (14) | $ | 238,700 | |||||||||||||||||||||||||||||
32
Table of Contents
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options: Exercisable (1) |
Number of Securities Underlying Unexercised Options: Unexercisable (1) |
Option Exercise Price |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested |
Market Value of Shares or Units of Stock That Have Not Vested (2) |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested |
Equity Incentive Plan Awards: Market or Payout Values of Unearned Shares, Units or Other Rights that Have Not Vested (2) |
||||||||||||||||||||||||
| Edward M. Fitzgerald |
50,000 | $ | 5.23 | 09/09/14 | ||||||||||||||||||||||||||||
| 60,000 | $ | 7.56 | 10/04/15 | |||||||||||||||||||||||||||||
| 100,000 | $ | 4.49 | 04/16/17 | |||||||||||||||||||||||||||||
| 30,000 | 10,000 | (7) | $ | 3.25 | 06/24/20 | |||||||||||||||||||||||||||
| 45,333 | 22,667 | (4) | $ | 7.82 | 04/01/21 | |||||||||||||||||||||||||||
| 20,000 | (3) | $ | 14.50 | 01/17/22 | ||||||||||||||||||||||||||||
| 17,000 | 34,000 | (5) | $ | 15.05 | 03/20/22 | |||||||||||||||||||||||||||
| 65,000 | (6) | $ | 20.89 | 03/19/23 | ||||||||||||||||||||||||||||
| 13,000 | (10) | $ | 88,660 | |||||||||||||||||||||||||||||
| 16,666 | (11) | $ | 113,662 | |||||||||||||||||||||||||||||
| 28,000 | (12) | $ | 190,960 | |||||||||||||||||||||||||||||
| 32,500 | (13) | $ | 221,650 | |||||||||||||||||||||||||||||
| 35,000 | (14) | 238,700 | ||||||||||||||||||||||||||||||
| Martin J. Duvall |
50,000 | 50,000 | (8) | $ | 10.28 | 09/19/21 | ||||||||||||||||||||||||||
| 3,334 | 6,666 | (5) | $ | 15.05 | 03/20/22 | |||||||||||||||||||||||||||
| 48,000 | (6) | $ | 20.89 | 03/19/23 | ||||||||||||||||||||||||||||
| 35,000 | (9) | $ | 4.29 | 12/09/23 | ||||||||||||||||||||||||||||
| 3,333 | (11) | $ | 22,731 | |||||||||||||||||||||||||||||
| 5,600 | (12) | $ | 38,192 | |||||||||||||||||||||||||||||
| 24,000 | (13) | $ | 163,680 | |||||||||||||||||||||||||||||
| 20,000 | (14) | 136,400 | ||||||||||||||||||||||||||||||
| Daniel M. Bollag |
37,500 | $ | 1.30 | 03/10/19 | ||||||||||||||||||||||||||||
| 21,333 | 10,667 | (4) | $ | 7.82 | 04/01/21 | |||||||||||||||||||||||||||
| 12,667 | 25,333 | (5) | $ | 15.05 | 03/20/22 | |||||||||||||||||||||||||||
| 48,000 | (6) | $ | 20.89 | 03/19/23 | ||||||||||||||||||||||||||||
| 6,000 | (10) | $ | 40,920 | |||||||||||||||||||||||||||||
| 12,000 | (11) | $ | 81,840 | |||||||||||||||||||||||||||||
| 16,000 | (12) | $ | 109,120 | |||||||||||||||||||||||||||||
| 24,000 | (13) | $ | 163,680 | |||||||||||||||||||||||||||||
| 20,000 | (14) | 136,400 | ||||||||||||||||||||||||||||||
| (1) | Options have ten-year terms. Unless otherwise indicated, the options reported in this table vest 25% per year over the four-year period following the date of grant, provided that the option holder is still an employee of ARIAD. |
| (2) | The market value of the stock awards is determined by multiplying the number of shares by $6.82, the closing price of our common stock on the NASDAQ Global Select Market on December 31, 2013, the last business day of our most recently completed fiscal year. |
| (3) | These options were granted pursuant to our program to grant options to employees and directors upon reaching ten, fifteen or twenty years of service with ARIAD. These options are fully vested upon grant and have a term of ten years. |
| (4) | These options vest on April 1, 2014. |
| (5) | These options vest as to 50% of the balance of the award on March 20, 2014 and March 20, 2015. |
| (6) | These options vest as to 33% of the award on March 19, 2014, 2015 and 2016. |
| (7) | These options vest on June 24, 2014. |
| (8) | These option vests as to 50% of the balance of the award on September 19, 2014 and on September 19, 2015. |
| (9) | This option vests as to 25% of the award on December 9, 2014, 2015, 2016 and 2017. |
| (10) | These restricted stock units vest on April 1, 2014. |
| (11) | These restricted stock units vest as to 50% of the balance of the award on each of March 20, 2014 and 2015. |
| (12) | These performance shares relate to the approval by the European Medicines Agency to market Iclusig in the European Union, at which time 50% of the award vested of this remaining balance, 50% will vest on July 15, 2014 and 50% on July 15, 2015. |
33
Table of Contents
| (13) | These restricted stock units vest as to 33 1/3% of the balance on each of March 19, 2014, 2015 and 2016. |
| (14) | These performance shares vest only if we achieve a certain research and development initiative prior to the end of 2016. These amounts represent target awards with the final number of shares to be issued being dependent upon when, prior to the end of 2016, such performance milestone is achieved. |
Option Exercises and Stock Vested in 2013
The following table contains information regarding option exercises and stock awards that vested during the year ended December 31, 2013 held by our named executive officers.
| Option Awards | Stock Awards | |||||||||||||||
| Shares Acquired on Exercise |
Value Realized on Exercise (1) |
Shares Acquired on Vesting |
Value Realized on Vesting |
|||||||||||||
| Harvey J. Berger |
97,667 | (2) | $ | 1,892,786 | ||||||||||||
| 41,666 | (3) | $ | 762,488 | |||||||||||||
| 62,500 | (4) | $ | 311,250 | |||||||||||||
| 34,000 | (5) | $ | 695,300 | |||||||||||||
| 73,600 | (6) | $ | 1,544,128 | |||||||||||||
| Timothy P. Clackson |
4,766 | $ | 71,632.98 | |||||||||||||
| 44,542 | $ | 702,427.34 | ||||||||||||||
| 38,333 | (2) | $ | 742,894 | |||||||||||||
| 8,631 | $ | 107,369.64 | ||||||||||||||
| 13,000 | (3) | $ | 237,900 | |||||||||||||
| 19,500 | (4) | $ | 97,110 | |||||||||||||
| 13,334 | (5) | $ | 272,680 | |||||||||||||
| 28,000 | (6) | $ | 587,440 | |||||||||||||
| Edward M. Fitzgerald |
33,000 | $ | 468,930.00 | |||||||||||||
| 32,333 | (2) | $ | 626,614 | |||||||||||||
| 13,000 | (3) | $ | 237,900 | |||||||||||||
| 19,500 | (4) | $ | 97,110 | |||||||||||||
| 8,334 | (5) | $ | 170,430 | |||||||||||||
| 28,000 | (6) | $ | 587,440 | |||||||||||||
| Martin J. Duvall |
1,667 | (5) | $ | 34,090 | ||||||||||||
| 5,600 | (6) | $ | 117,488 | |||||||||||||
| 25,000 | (7) | $ | 537,250 | |||||||||||||
| Daniel M. Bollag |
32,333 | (2) | $ | 626,619 | ||||||||||||
| 6,000 | (3) | $ | 109,800 | |||||||||||||
| 9,000 | (4) | $ | 44,820 | |||||||||||||
| 6,000 | (5) | $ | 122,700 | |||||||||||||
| 16,000 | (6) | $ | 335,680 | |||||||||||||
| (1) | Value represents the market value of a share of our common stock at the time of exercise minus the exercise price per share of the option, multiplied by the number of shares acquired upon exercise. |
| (2) | This represents the vesting of restricted stock units granted on March 22, 2010. The value realized is calculated by multiplying the number of shares issued times $19.38, the closing price of our common stock on March 22, 2013. |
34
Table of Contents
| (3) | This represents the vesting of restricted stock units granted on April 1, 2011. The value realized is calculated by multiplying the number of shares issued times $18.30, the closing price of our common stock on April 1, 2013. |
| (4) | This represents the vesting of performance shares granted on April 1, 2011 which became fully vested on December 18, 2013. The value realized is calculated by multiplying the number of shares issued times $4.98, the closing price of our common stock on December 18, 2013. |
| (5) | This represents the vesting of restricted stock units granted on March 20, 2012. The value realized is calculated by multiplying the number of shares issued times $20.45, the closing price of our common stock on March 20, 2013. |
| (6) | This represents the vesting of performance shares granted on April 1, 2011 of which 50% vested on July 15, 2013. The value realized is calculated by multiplying the number of shares issued times $20.98, the closing price of our common stock on July 15, 2013. |
| (7) | This represents the vesting of restricted stock units granted on September 19, 2011, which vested on September 19, 2013. The value realized is calculated by multiplying the number of shares issued times $21.49, the closing price of our common stock on September 19, 2013. |
Non-Qualified Deferred Compensation in 2013
The following table contains information about the participation of our named executive officers in our 2005 Executive Compensation Plan during and as of the year ended December 31, 2013. Dr. Berger does not participate in this plan.
| Name | Executive Contributions ($) |
Registrant Contributions ($) |
Aggregate Earnings ($) (1) |
Aggregate Withdrawals/ Distributions ($) |
Aggregate Balance at Year End ($) |
|||||||||||||||
| Timothy P. Clackson |
— | — | $ | 49,344 | $ | (558,816 | ) | $ | 340,651 | |||||||||||
| Edward M. Fitzgerald |
— | — | 41,707 | (452,951 | ) | 298,816 | ||||||||||||||
| Martin J. Duvall |
— | — | 986 | (27,588 | ) | — | ||||||||||||||
| Daniel M. Bollag |
— | — | 19,507 | (238,140 | ) | 147,979 | ||||||||||||||
| (1) | No portion of the amounts listed in the “Aggregate Earnings” column was reported as compensation in 2013 in the Summary Compensation Table. |
The 2005 Executive Compensation Plan is an unfunded, non-qualified, deferred compensation plan and is intended to comply with the requirements of Section 409A of the Code. The Compensation Committee may from time to time in its sole discretion allow participants to defer payment of part of their compensation under the 2005 Executive Compensation Plan on a pre-tax basis. In addition, the Compensation Committee may elect to issue awards under the 2005 Executive Compensation Plan to participants either as performance-based awards or on an ad hoc basis. Deferred amounts are subject to certain vesting requirements (other than for salary deferrals) and payment provisions as specified at the time of the deferral. Our Compensation Committee, in its sole discretion, determines the terms of awards, including the acceleration or modification of vesting terms. Amounts that are not vested as of the time of a participant’s separation from service are forfeited, subject to the terms of the participant’s executive employment agreement. Vesting of unvested amounts may be accelerated in connection with a change in control at the Compensation Committee’s discretion. The value of amounts deferred under the 2005 Executive Compensation Plan is increased or decreased over time based on the actual total return of specified mutual funds. Participants may choose to receive payment of their vested benefits in either a lump sum or annual installments (but not to exceed twenty years) at either a specified date, upon the first anniversary of their separation from service, or the earlier of these two dates. A participant may subsequently change the form of payment or elect to defer the timing of payment, within certain limits, provided the change is elected at least twelve months before the previously scheduled date for commencement of payment. Any changes to the timing of payment must be deferred for at least five additional years.
35
Table of Contents
Potential Payments upon Termination or Change in Control
Chief Executive Officer
The following is a summary of the severance and change in control benefits provided to Dr. Berger, our Chief Executive Officer, under the terms of his employment agreement in effect since April 2010.
EMPLOYMENT TERMINATION WITHOUT CAUSE
If we terminate Dr. Berger’s employment without “cause” or Dr. Berger terminates his employment for “good reason,” each as defined below, we are obligated to:
| • | Accelerate the vesting of all stock options, stock grants, and similar equity rights and provide for continued exercisability of all awards through their original terms with all rights, and |
| • | Make a cash lump sum payment equal to the sum of three times the executive’s then current annual salary and three times the bonus amount paid for the preceding year, provided that such bonus amount shall not be less than 50% of the executive’s salary. |
“Cause” for purposes of Dr. Berger’s employment agreement consists of:
| • | Willful neglect of duties following written notice and a 30-day opportunity to correct, |
| • | Conviction of a felony involving moral turpitude, or |
| • | Any act of fraud or embezzlement involving us or any of our affiliates. |
Any determination of cause requires at least a two-thirds vote of the entire Board.
Termination for “good reason” for purposes of Dr. Berger’s employment agreement consists of:
| • | A material reduction of duties or authority that continues for 30 days, |
| • | An uncured material breach of the employment agreement or any other material agreement with the Company that continues for 30 days, |
| • | A “Change in Control” (as defined below) occurs and Dr. Berger is not the chief executive officer of the surviving corporation, |
| • | Dr. Berger is no longer elected to our Board, named as Chairman and designated as our chief executive officer, |
| • | Dr. Berger ceases to be our highest ranking executive with the power to appoint and remove all other ARIAD employees, |
| • | A person other than Dr. Berger or a person designated by Dr. Berger is elected ARIAD’s president, |
| • | The retention of any senior executive officer by ARIAD, or an offer to pay compensation to any senior executive officer that in either case is unacceptable to Dr. Berger, in his reasonable judgment, or |
| • | We file for bankruptcy or similar proceedings or an involuntary petition is commenced against us. |
NON-RENEWAL
If we do not renew Dr. Berger’s employment agreement at the end of its term, we are obligated to make a lump sum cash payment equal to two times his then current annual salary and accelerate the vesting of all stock options, stock grants and similar equity rights and provide for continued exercisability of all awards through their original terms with all rights. Any determination not to renew Dr. Berger’s employment requires at least a two-thirds vote of the entire Board.
36
Table of Contents
MEDICAL COVERAGE
Dr. Berger’s employment agreement requires us to continue providing medical coverage in all group health plans for the maximum COBRA continuation period at our expense following any termination of employment other than for death.
CHANGE IN CONTROL
Dr. Berger’s employment agreement provides that in the event of a “Change in Control” (as defined in this subsection), all stock, stock option, stock award and similar equity rights granted to him shall immediately vest and remain fully exercisable through their original term with all rights.
In addition, Dr. Berger has the right to terminate his employment agreement for “good reason” if a Change in Control occurs and he is not the chief executive officer of the surviving corporation, in which case we will pay Dr. Berger the same benefits as if we had terminated his employment without cause. Dr. Berger’s agreement also provides that, following a Change in Control, his term of employment will continue until the later of the existing term or two years and one day following the Change in Control.
A tax gross-up will be paid on Dr. Berger’s behalf if any amounts payable by us (or our successor) become subject to excise taxes under Sections 280G and 4999 of the Code.
Generally, pursuant to Dr. Berger’s employment agreement, a Change in Control occurs if:
| • | Any person makes a tender or exchange offer for our common stock pursuant to which such person acquires 40% or more of our issued and outstanding common stock, |
| • | A merger or consolidation of ARIAD, other than a merger or consolidation of ARIAD in which our voting securities prior to the transaction continue to represent more than 50% of the total voting power of the surviving corporation, or the sale or disposition of all or substantially all of our assets, or |
| • | Any person acquires more than 40% of our issued and outstanding voting securities. |
The following table sets out the estimated potential payments upon termination or a Change in Control for Dr. Berger, based on the assumptions discussed above and assuming such event occurred on December 31, 2013.
| Dr. Berger Payments and Benefits |
Voluntary termination or termination for Cause |
Non- Renewal |
Termination by ARIAD Without Cause or by the Executive with Good Reason Absent a Change-in- Control |
Death, Disability or Acceleration of Vesting upon Change- in-Control without Termination |
Termination by ARIAD without Cause or by the Executive with Good Reason Following a Change-in- Control |
|||||||||||||||
| Severance benefits: |
||||||||||||||||||||
| Lump sum payment |
— | $ | 1,502,000 | $ | 3,379,500 | n/a | $ | 3,379,500 | ||||||||||||
| Healthcare benefits |
$ | 36,982 | 36,982 | 36,982 | n/a | 36,982 | ||||||||||||||
| Acceleration of equity awards: |
||||||||||||||||||||
| Market value of equity vesting on termination (1) |
— | 2,634,339 | 2,634,339 | 3,023,079 | 3,023,079 | |||||||||||||||
| 280G Tax Gross-Up (2) |
n/a | n/a | n/a | n/a | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total Payment |
$ | 36,982 | $ | 4,193,320 | $ | 6,050,820 | $ | 3,023,079 | $ | 6,439,560 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Amounts do not include the value associated with vested stock options. Information about all stock options and other unvested equity awards held by Dr. Berger as of December 31, 2013 is included in the “Outstanding Equity Awards at December 31, 2013” table. |
| (2) | Based on the assumptions set forth above, Dr. Berger’s payments will not result in a tax gross-up payment to him based on Dr. Berger’s taxable wages per Form W-2 for the years 2008 through 2012. The amount of the tax gross-up, if any, that would arise would depend upon the facts and circumstances at the time of a change-in-control and any related employment termination. |
37
Table of Contents
The acceleration of equity awards in the table above includes the value of unvested stock options and restricted stock units, the value of performance shares granted in 2012 the vesting of which is now time based as the performance objective has been achieved, and the value of performance shares granted in 2013 for which the performance objective had not been achieved as of December 31, 2013. The 2013 performance shares vest in connection with a change in control at the maximum award level, or 160% of the target. The values of equity awards included in the table above are based on the intrinsic value of such awards on December 31, 2013 (i.e. the difference between the market value of the Company’s common stock on that date and the exercise or purchase price, if any).
The amounts in the table do not include the value of the lump sum payment ($563,250) we will make to Dr. Berger upon termination of employment in good standing for amounts previously earned under our now terminated sabbatical policy, as described in subsection “Narrative to Summary Compensation Table and Grants of Plan-Based Awards Table.”
Other Named Executive Officers
The following is a description of the potential payments upon termination or change in control with respect to the named executive officers other than Dr. Berger:
EMPLOYMENT TERMINATION WITHOUT CAUSE
If we terminate the employment of our other named executive officers without cause, we are obligated to continue payment of the executive’s then current salary for a period of twelve months following the effective date of termination; accelerate vesting of all stock, stock options, stock awards, and similar equity awards and deferred compensation awards granted to the executive that would have otherwise vested during the remaining term of that executive’s contract, subject to the normal post termination exercise period; and continue payment of all benefits covered under COBRA for up to one year.
“Cause” for purposes of the other named executive officers’ employment agreements consists of:
| • | The officer’s failure to perform any of his material duties, |
| • | The conviction of the officer of any felony, |
| • | Commission of any crime related to the officer’s employment with the Company, |
| • | Violation of any law or regulation related to our business, |
| • | Conduct that could result in unfavorable publicity for us in a material way, |
| • | Unprofessional conduct inconsistent with the officer’s position with the Company, |
| • | Failure to comply with our written policies, or |
| • | Breach of the terms of the employment agreement. |
The executive officer has a right to cure any conduct that constitutes cause, if curable, within 30 days after receiving written notice.
For purposes of determining payments upon termination, a termination of the employment agreement by the named executive officer due to an uncured breach by us of the employment agreement is treated as a termination of the executive’s employment without cause.
NON-RENEWAL
If we do not renew the employment agreement of our other named executive officers, no severance benefit is payable. In addition, any unvested stock awards, stock options, restricted stock or restricted stock units, performance shares or unvested portions of past bonuses in the form of deferred compensation shall be forfeited to the Company.
38
Table of Contents
EMPLOYMENT TERMINATION WITH CAUSE
Any unvested stock awards, stock options, restricted stock or restricted stock units, performance shares or unvested portions of past bonuses in the form of deferred compensation shall be forfeited to the Company in the event the employment is terminated by the Company for Cause.
CHANGE IN CONTROL
In the event of a consummation of a “Change in Control”, if within one year following such occurrence the Company terminates the executive officer’s employment without cause or the executive officer resigns for good reason, we are obligated to accelerate the vesting of all stock options, stock grants and similar equity rights and deferred compensation awards and provide for continued exercisability of all awards through their original terms with all rights. We are also obligated to continue to pay the named executive officer their then current salary for a period of 24 months from the effective date of termination. Good reason means the involuntary relocation of more than 25 miles, the material breach of the employment agreement by the Company, or the material diminution of the officer’s responsibilities, which situation remains uncured within a specified timeframe after notice.
The Change in Control definition with respect to the employment agreements for the other named executive officers is similar to the employment agreement for Dr. Berger except that the threshold for acquisition of shares of our common stock is 50% for the other named executive officers.
The following tables set out the estimated potential payments upon termination or a change in control for Dr. Clackson, Mr. Fitzgerald, Mr. Duvall and Dr. Bollag, based on the assumptions following the table and assuming such event occurred on December 31, 2013. The total of continued payments in the case of termination by ARIAD without cause in the tables below reflect the remaining terms of the employment agreements with each of these executives. The footnotes to all of the tables follow the last table.
| Dr. Clackson Payments and benefits |
Voluntary Termination or Termination for Cause |
Death or Disability | Termination by ARIAD Without Cause or by the Executive for ARIAD’s Material Breach |
Termination by ARIAD without Cause or by the Executive for Good Reason within One Year after a Change in Control |
||||||||||||
| Severance benefits: |
||||||||||||||||
| Total of continued payments |
— | — | $ | 493,000 | $ | 986,000 | ||||||||||
| Healthcare benefits |
— | $ | 24,992 | 24,992 | 37,488 | |||||||||||
| Non-qualified benefits (1) |
— | — | 340,651 | 340,651 | ||||||||||||
| Acceleration of equity awards: |
||||||||||||||||
| Market value of equity vesting on termination (2) |
— | 258,952 | 384,652 | 1,100,752 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Payment |
— | $ | 283,945 | $ | 1,243,295 | $ | 2,464,892 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
39
Table of Contents
| Mr. Fitzgerald Payments and benefits |
Voluntary Termination or Termination for Cause |
Death or Disability | Termination by ARIAD Without Cause or by the Executive for ARIAD’s Material Breach |
Termination by ARIAD without Cause or by the Executive for Good Reason within One Year after a Change in Control |
||||||||||||
| Severance benefits: |
||||||||||||||||
| Total of continued payments |
— | — | $ | 466,000 | $ | 932,000 | ||||||||||
| Healthcare benefits |
— | $ | 24,654 | 24,654 | 36,982 | |||||||||||
| Non-qualified benefits (1) |
— | — | 298,816 | 298,816 | ||||||||||||
| Acceleration of equity awards: |
||||||||||||||||
| Market value of equity vesting on termination (2) |
— | 232,233 | 350,552 | 1,032,552 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Payment |
— | $ | 256,887 | $ | 1,140,023 | $ | 2,300,350 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Mr. Duvall Payments and benefits |
Voluntary Termination or Termination for Cause |
Death or Disability | Termination by ARIAD Without Cause or by the Executive for ARIAD’s Material Breach |
Termination by ARIAD without Cause or by the Executive for Good Reason within One Year after a Change in Control |
||||||||||||
| Severance benefits: |
||||||||||||||||
| Total of continued payments |
— | — | $ | 420,000 | $ | 840,000 | ||||||||||
| Healthcare benefits |
— | $ | 24,654 | 24,654 | 36,982 | |||||||||||
| Non-qualified benefits (1) |
— | — | — | — | ||||||||||||
| Acceleration of equity awards: |
||||||||||||||||
| Market value of equity vesting on termination (2) |
— | 62,037 | 107,162 | 531,393 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Payment |
— | $ | 86,692 | $ | 551,817 | $ | 1,408,375 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Dr. Bollag Payments and benefits |
Voluntary Termination or Termination for Cause |
Death or Disability | Termination by ARIAD Without Cause or by the Executive for ARIAD’s Material Breach |
Termination by ARIAD without Cause or by the Executive for Good Reason within One Year after a Change in Control |
||||||||||||
| Severance benefits: |
||||||||||||||||
| Total of continued payments |
— | — | $ | 387,000 | $ | 774,000 | ||||||||||
| Healthcare benefits |
— | $ | 24,992 | 24,992 | 37,488 | |||||||||||
| Non-qualified benefits (1) |
— | — | 147,979 | 147,979 | ||||||||||||
| Acceleration of equity awards: |
||||||||||||||||
| Market value of equity vesting on termination (2) |
— | 131,093 | 190,960 | 613,800 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Payment |
— | $ | 156,086 | $ | 750,931 | $ | 1,573,267 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Amounts are based on the value and vesting schedule of unvested deferred compensation awards under our 2005 Executive Compensation Plan at December 31, 2013; no such awards were made and deferred in 2013. |
| (2) | Amounts do not include the value associated with vested stock options. Information about all stock options and other unvested equity awards held by the named executive officers as of December 31, 2013 is included in the “Outstanding Equity Awards at December 31, 2013” table. |
40
Table of Contents
The acceleration of equity awards in the tables above includes the value of unvested stock options and restricted stock units, as well as the value of performance shares granted in 2012 the vesting of which is now time based as the performance objective has been achieved. In the case of termination by the Company without Cause or by the officer for the Company’s material breach, awards that would have vested during the remaining term of the officer’s employment agreement accelerate. In the case of termination by the Company without Cause or by the officer for good reason within one year of a Change in Control, all such outstanding awards immediately vest and remain exercisable according to their terms. In addition, performance shares granted in 2013, for which the performance objective has not yet been achieved, will immediately vest upon termination in connection with a change in control at the maximum level, or 160% of the award, and are included in the above tables.
The amounts in the above tables do not include the value of the lump sum payments payable upon termination in good standing to Dr. Clackson ($123,250) and Mr. Fitzgerald ($116,500) for amounts previously earned under our now terminated sabbatical policy, as described in the “Narrative to Summary Compensation Table and Grants of Plan-Based Awards Table” above. The amounts in the above tables also do not reflect any reduction in payments related to the modified economic cutback provisions in the executive officers’ employment agreements that may apply on or after a change-in-control.
Assumptions Regarding Post Termination Payment Tables
Except as otherwise specifically noted above, the tables presented on the preceding pages were prepared as though each named executive officer’s employment was terminated on December 31, 2013, using the closing price of our common stock on that date ($6.82). We are required by the SEC to use these assumptions. With those assumptions taken as a given, we believe that the remaining assumptions listed above, which are necessary to produce these estimates and reflect solely our interpretation of our contractual obligations, are reasonable in the aggregate. However, the executives’ employment was not terminated on December 31, 2013, and a change in control did not occur on that date. There can be no assurance that a termination of employment, a change in control or both would produce the same or similar results as those described if either or both of them occur on any other date or at any other price of our common stock, or if any assumption is not correct in fact.
Director Compensation
Director Compensation Policy in Effect During 2013
Effective January 2, 2012, the Board of Directors adopted the following policy for compensation of the non-management members of our Board:
| • | A one-time grant upon initial appointment or election to the Board of 40,000 stock options, which vest over three years in equal amounts on the first, second and third anniversaries of the date of grant; |
| • | Annual cash compensation of $50,000, paid in equal quarterly amounts on or about the last day of each calendar quarter. In lieu of cash, a director may elect to receive $50,000 worth of restricted shares of our common stock on January 31, subject to a lapsing repurchase right as described below. The number of shares to be issued will be determined based on the volume weighted average price (“VWAP”) of our common stock for the month of December of the prior year. A director electing to receive his or her retainer in shares of our common stock must make such election by January 15 of each calendar year; and |
| • | An annual equity grant of 14,000 restricted shares of our common stock, made on or about January 31 of each calendar year, subject to a lapsing repurchase right as described below. The number of restricted shares to be awarded will be evaluated annually to take into account the underlying value of the shares. The value of restricted share awards made on January 31, 2013 to each director was $278,320. |
For stock option awards, the exercise price of each award is the closing price of our common stock as quoted on the NASDAQ Global Select Market on the date of grant. These awards have terms of ten years, subject to earlier termination. For restricted stock awards, we have the right to repurchase the restricted shares for a nominal amount if the director ceases to be a member of the Board or ceases to provide us with other services following their service as a director. Our right to repurchase the restricted shares lapses as to 25% of the shares on each of March 31, June 30, September 30 and December 31 of the year of the award. All awards are granted under and subject to the terms and conditions of our 2006 Long-Term Incentive Plan, as amended (the “2006 Long-Term Incentive Plan”).
41
Table of Contents
No other compensation, in the form of cash or otherwise, was paid to our non-management directors other than reimbursement of their reasonable expenses incurred in attending Board and committee meetings. Management directors do not receive any compensation for their service as directors.
Set forth below is information concerning the compensation paid to or earned by our non-management directors during 2013.
2013 Director Compensation Table
| Name | Fees Earned or Paid in Cash |
Stock Awards (1) |
Option Awards (1)(2) |
Total | ||||||||||||
| Jay R. LaMarche |
$ | 50,000 | $ | 278,320 | — | $ | 328,320 | |||||||||
| Athanase Lavidas, Ph.D. |
$ | 50,000 | $ | 278,320 | — | $ | 328,320 | |||||||||
| Massimo Radaelli, Ph.D. |
$ | 50,000 | $ | 278,320 | — | $ | 328,320 | |||||||||
| Norbert G. Riedel, Ph.D. |
$ | 50,000 | $ | 278,320 | — | $ | 328,320 | |||||||||
| Sarah J. Schlesinger, M.D. (3) |
$ | 25,000 | — | $ | 533,536 | $ | 558,536 | |||||||||
| Robert M. Whelan, Jr. |
$ | 50,000 | $ | 278,320 | — | $ | 328,320 | |||||||||
| Wayne Wilson |
$ | 50,000 | $ | 278,320 | — | $ | 328,320 | |||||||||
| (1) | As of December 31, 2013, no director held any restricted stock. |
| (2) | The amounts included under “Stock Awards” and “Option Awards” represent the aggregate grant date fair value for stock awards and option awards granted during the year, computed in accordance with FASB ASC Topic 718. The assumptions used in the calculation of the grant date fair value of the stock awards and option awards are set forth in note 13 to our audited consolidated financial statements, entitled “Stock-Based Compensation,” included in the Original Filing. As of December 31, 2013, each non-management director had the following aggregate number of stock options outstanding: Mr. LaMarche – 70,000; Dr. Lavidas – 20,000; Dr. Radaelli – 52,319; Dr. Riedel – 25,000; Dr. Schlesinger– 40,000; Mr. Whelan – 25,000; and Mr. Wilson – 25,000. |
| (3) | Dr. Schlesinger was appointed to the Board on July 15, 2013. |
Director Compensation Policy in Effect as of January 1, 2014
With the help of our compensation consultant, based on a review of our 2014 peer group of companies listed below in the section entitled “Use of Competitive Market Compensation Data and Compensation Benchmark” and in order to more closely align our director compensation with those companies, the Compensation Committee approved an increase in the compensation to be paid to our non-management directors effective January 1, 2014. We believe that our revised compensation policy recognizes the increased time and responsibilities required of our directors for their service on the Board and the Board committees. The revised policy is as follows:
| • | A one-time grant upon initial appointment or election to the Board of 75,000 stock options, which vest over three years in equal amounts on the first, second and third anniversaries of the date of grant; |
| • | Annual cash compensation of $70,000, paid in equal quarterly amounts on or about the last day of each calendar quarter. In lieu of cash, a director may elect to receive the equivalent value in restricted shares of our common stock on January 31, subject to a lapsing repurchase right as to 25% of the shares on March 31, June 30, September 30 and December 31 of the year of the award. The number of shares to be issued will be determined based on the volume weighted average price (VWAP) of our common stock for the month of December of the prior year. Such election to be paid in shares in lieu of cash must be made by January 15 of each calendar year; and |
| • | An annual equity grant of 25,000 stock options and 12,500 restricted stock units which will vest as to 25% of the shares on March 31, June 30, September 30 and December 31 of the year of the award. |
For stock option awards, the exercise price of each award is the closing price of our common stock as quoted on the NASDAQ Global Select Market on the date of grant. These awards have terms of ten years, subject to earlier
42
Table of Contents
termination. The annual cash component will be prorated for any director who joins the Board during the year beginning on the first day of the fiscal quarter in which he or she was initially appointed or elected and the individual may elect to be paid in shares in lieu of cash. If a director dies, resigns or is removed during any quarter, he or she shall be entitled to a cash payment (or shares in lieu thereof) on a pro-rated basis through his or her last day of service.
Director Stock Ownership Guidelines
In 2012, we adopted stock ownership guidelines, to be phased in over five years, for the non-employee directors. The guideline for the non-employee directors is ownership of our common stock with a value of at least three times the annual cash compensation. Newly elected directors will have five years from when they are first elected or appointed to the Board to comply with these guidelines. All of our non-employee directors with the exception of Sarah Schlesinger and Alexander Denner, who each recently joined the Board, are in early compliance with our stock ownership guidelines.
Compensation Practices and Policies Relating to Risk Management
Consistent with SEC disclosure requirements, we have assessed the Company’s compensation policies, practices and awards, including the use of performance shares, and have concluded that our compensation policies, practices and awards do not create risks that are reasonably likely to have a material adverse effect on the Company. Our management assessed the Company’s compensation and benefits programs to determine if the programs’ provisions and operations create undesired or unintentional risk of a material nature. We do not have any programs where a participant may be able to directly affect variability or timing of payout. Rather, our compensation programs include a combination of fixed base salaries, cash bonuses, long-term incentive awards, including performance-based compensation, and employee retirement plans that are generally uniform in design and operation throughout the Company and with all levels of employees.
In 2012, we implemented a minimum stock ownership guideline for our Chief Executive Officer equal to six times his base salary, to be phased in over five years, as well as minimum stock ownership guidelines for the non-employee members of our Board of Directors equal to three times their annual cash compensation, to be phased in over five years. Our Chief Executive Officer already meets this minimum stock ownership guideline and our non-employee directors, with the exception of Drs. Denner and Schlesinger, who recently joined the Board, are in early compliance with this guideline. We believe the adoption of such guidelines further aligns the interests of our Chief Executive Officer and our Board of Directors with those of our shareholders.
In April 2014, we adopted the Recoupment Principles which we will use, together with the compensation clawback requirements set forth in the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, to create a formal clawback and incentive recoupment policy before the end of 2014. The policy will provide the Compensation Committee with broad discretion to recoup certain incentive awards in instances of material violations of company policy that cause significant harm to us and in certain instances of a failure to manage or monitor conduct or risks appropriately. This policy will also provide for the recovery of incentive based compensation in the event of a significant restatement of financial results of the Company caused by executive fraud or willful misconduct.
Based on the foregoing, we believe that our compensation policies, practices and awards do not create risks that are likely to have a material adverse effect on the Company as a whole. We also believe that our incentive compensation arrangements provide incentives that do not encourage risk-taking beyond the organization’s ability to effectively identify and manage significant risks, are compatible with our effective internal controls and our risk management practices, and are supported by the oversight and administration of the Compensation Committee with regard to executive compensation programs.
43
Table of Contents
Compensation Committee Interlocks and Insider Participation
During the fiscal year ended December 31, 2013, Drs. Lavidas, Radaelli and Riedel and Mr. Whelan served as members of our Compensation Committee. In 2013, none of our executive officers served on the board of directors or compensation committee of any entity that had one or more executive officers serving as a member of our Board or Compensation Committee. There are no family relationships between or among the members of our Board or executive officers.
Compensation Committee Report
The Compensation Committee of the Board has reviewed and discussed with members of management the Compensation Discussion and Analysis (“CD&A”) section included in this Form 10-K/A, as required by Item 402(b) of Regulation S-K. Based on this review and discussion, the Compensation Committee recommended to the Board that the CD&A be included in this Form 10-K/A.
Members of the Compensation Committee
Norbert G. Riedel, Ph.D., Chair
Athanase Lavidas, Ph.D.
Robert M. Whelan, Jr.
44
Table of Contents
| ITEM 12: | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The following table sets forth, as of April 28, 2014, certain information with respect to (i) each person (including any “group” as defined in Section 13(d)(3) of Exchange Act known to us to own beneficially more than 5% of our common stock, (ii) each of our directors and director nominees, (iii) each executive officer named in the Summary Compensation Table under “Executive Compensation” (referred to as our named executive officers), and (iv) all of our current directors and executive officers as a group. In accordance with the rules promulgated by the SEC, such ownership includes shares currently owned, as well as shares that the named person has the right to acquire within 60 days of April 28, 2014, including, but not limited to, shares that the named person has the right to acquire through the exercise of any option and restricted stock units that will be vested as of that date. Except as otherwise indicated, the stockholders listed in the table have sole voting and investment powers with respect to the common stock shown as beneficially owned. Percentage ownership is based on 186,798,293 shares of common stock outstanding as of April 28, 2014.
| Name and Address ** | Number and Nature
of Shares Beneficially Owned |
Percent of Class | ||||||
| FMR LLC |
17,076,102 | (1) | 8.89 | % | ||||
| The Vanguard Group, Inc. |
10,335,936 | (2) | 5.38 | % | ||||
| Sarissa Capital Management LP |
12,000,000 | (3) | 6.25 | % | ||||
| Harvey J. Berger, M.D. |
4,554,577 | (4) | 2.37 | % | ||||
| Timothy P. Clackson, Ph.D. |
694,845 | (5) | * | |||||
| Edward M. Fitzgerald |
664,595 | (6) | * | |||||
| Martin J. Duvall |
104,966 | (7) | * | |||||
| Daniel M. Bollag, Ph.D. |
258,896 | (8) | * | |||||
| Alexander J. Denner, Ph.D. |
12,000,000 | (3) | 6.25 | % | ||||
| Jay R. LaMarche |
610,151 | (9) | * | |||||
| Athanase Lavidas, Ph.D. |
132,881 | (10) | * | |||||
| Massimo Radaelli, Ph.D. |
139,069 | (11) | * | |||||
| Robert M. Whelan, Jr. |
76,109 | (12) | * | |||||
| Wayne Wilson |
131,750 | (13) | * | |||||
| Norbert G. Riedel, Ph.D. |
128,109 | (14) | * | |||||
| Sarah Schlesinger |
18,750 | (15) | * | |||||
| All current directors and executive officers as a group (17 persons) |
21,960,909 | (16) | 11.39 | % | ||||
| * | Indicates less than 1% of the outstanding shares of common stock. |
| ** | Addresses are given for beneficial owners of more than 5% of the outstanding common stock only. |
| (1) | This information is based solely on information contained in a Schedule 13G/A filed with the SEC on January 10, 2014 by FMR LLC. The shares are held by various subsidiaries of FMR LLC that have voting and dispositive power over the shares, as detailed in the filing. |
| (2) | This information is based solely on information contained in a Schedule 13G/A filed with the SEC on February 11, 2014 by The Vanguard Group, Inc. The shares are held by various subsidiaries of The Vanguard Group, Inc. that have voting and dispositive power over the shares, as detailed in the filing. |
| (3) | This information is based solely on information contained in a Schedule 13D/A filed with the SEC on February 21, 2014 by Sarissa Capital Management LP. The shares are held by various affiliates of Sarissa |
45
Table of Contents
| Capital Management LP, including Dr. Denner, that have voting and dispositive power over the shares, as detailed in the filing. By virtue of his position as the Chief Investment Officer of Sarissa Capital and as the managing member of Sarissa Capital’s general partner and as controlling the ultimate general partner of each of the Sarissa Funds, Dr. Denner may be deemed to have the shared power to vote or direct the vote of (and the shared power to dispose or direct the disposition of) the 12,000,000 shares held by Sarissa Funds. |
| (4) | Includes 999,000 shares issuable upon exercise of stock options. Includes 1,714,286 shares owned by Ocean Capital Partners, LLC, an investment entity owned by Dr. Berger and his immediate family and for which Dr. Berger has the right to vote and dispose of the shares; and 740,050 shares owned by Dr. Berger’s spouse and daughters. Dr. Berger disclaims beneficial ownership of the shares held by his spouse and daughters |
| (5) | Includes 291,817 shares issuable upon exercise of stock options. |
| (6) | Includes 393,667 shares issuable upon exercise of stock options. |
| (7) | Includes 72,667 shares issuable upon exercise of stock options. |
| (8) | Includes 110,833 shares issuable upon exercise of stock options. |
| (9) | Includes 76,250 shares issuable upon exercise of stock options and 6,696 shares held by Mr. LaMarche’s spouse. |
| (10) | Includes 26,250 shares issuable upon exercise of stock options. |
| (11) | Includes 58,569 shares issuable upon exercise of stock options. |
| (12) | Includes 31,250 shares issuable upon exercise of stock options. |
| (13) | Includes 31,250 shares issuable upon exercise of stock options. |
| (14) | Includes 31,250 shares issuable upon exercise of stock options. |
| (15) | Includes 6,250 shares issuable upon exercise of stock options. |
| (16) | See notes 3 through 15 above. Also includes 423,112 shares of common stock and 571,348 shares issuable upon the exercise of stock options held by executive officers not listed in the table above. |
Equity Compensation Plan Information
The following table provides certain aggregate information with respect to all of our equity compensation plans in effect as of December 31, 2013:
| Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
Weighted Average Exercise Price of Outstanding Options, Warrants and Rights |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in first column) |
|||||||||
| Equity Compensation Plans Approved by Securityholders |
12,627,068 | 9.05 | 8,495,040 | |||||||||
| Equity Compensation Plans not Approved by Securityholders |
N/A | N/A | N/A | |||||||||
|
|
|
|
|
|||||||||
| Total |
12,627,068 | (1) | 8,495,040 | (2) | ||||||||
|
|
|
|
|
|||||||||
| (1) | Consists of options to purchase 864,122 shares of common stock granted under our 2001 Stock Plan, as amended, and options to purchase 9,515,546 shares of common stock, 1,472,200 restricted stock units and performance shares for up to a maximum of 775,200 shares of common stock granted under our 2006 Long-Term Incentive Plan, as amended. |
| (2) | Consists of 8,079,200 shares available for issuance under our 2006 Long-Term Incentive Plan, as amended, and 415,840 shares available for issuance under our 1997 Employee Stock Purchase Plan, as amended. Does not include the additional 8,000,000 shares that would be authorized for issuance under the 2014 Long-Term Incentive Plan or the additional 750,000 shares which would be authorized under the 1997 Employee Stock Purchase Plan, as amended, if approved at our 2014 annual meeting of stockholders. |
46
Table of Contents
| ITEM 13: | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Related Person Transactions Approval Policy
All related person transactions are reviewed and approved in advance by our Audit Committee or other independent body of our Board. In general, a related person transaction is defined as any transaction (other than setting compensation) in which we or any subsidiary or affiliate is a participant and in which any of the following persons has or will have a direct or indirect material interest: our executive officers, our Board members and nominees, beneficial holders of more than 5% of our securities, immediate family members of any of the foregoing persons, and any other persons who the Board determines may be considered to be related persons as defined by the rules and regulations of the SEC.
Our Audit Committee or its chair or other independent body of our Board, as the case may be, will approve only those related person transactions that are determined to be in, or not inconsistent with, the best interests of our company and our stockholders, taking into account all available facts and circumstances as it determines in good faith to be necessary. These facts and circumstances will typically include, but not be limited to, the benefits of the transaction to us and our stockholders, the impact on a director’s independence in the event the related person is a director or nominee, an immediate family member of a director or nominee, or an entity in which a director or nominee is a partner, shareholder, or executive officer, the availability of other sources for comparable products or services, the terms of the transaction, and the terms of comparable transactions that would be available to unrelated third parties or to employees generally. No member of our Audit Committee or our Board will participate in any review, consideration, or approval of any related person transaction with respect to which the member or any of his or her immediate family members or other business affiliates is the related person.
In reviewing and approving such transactions, our Audit Committee or other independent body of our Board will obtain, or will direct management to obtain on its behalf, all information that it believes to be relevant and important to its review of the transaction prior to approval. Following receipt and review of the necessary information, a discussion will be held of the relevant factors deemed to be necessary prior to approval. If a discussion is not deemed to be necessary, approval may be given by unanimous written consent of our Audit Committee or other independent body of our Board. This approval authority may also be delegated to the chair of our Audit Committee in some circumstances. No related person transaction shall be entered into prior to completing these procedures.
Our policies as described above are included in the Audit Committee Charter as approved by our Audit Committee and our Board. As required under SEC rules, transactions that involve an amount in excess of $120,000, in which we are a participant and a related person is determined to have a direct or indirect material interest, are disclosed in our annual report on Form 10-K and/or proxy statement.
Transactions with Related Persons
We have no related person transactions to report.
Indemnification
We indemnify our directors and our executive officers to the fullest extent permitted by law so that they will be free from undue concern about personal liability in connection with their service to the Company. This is required under our Amended and Restated By-laws, and we have also entered into agreements with those individuals contractually obligating us to provide this indemnification to them.
Director Independence and Committee Qualifications
The Board has determined that each of our directors, except Dr. Berger, is an “independent director” as such term is defined by The NASDAQ Stock Market LLC (“NASDAQ”). The Board has also determined that each member of the Compensation Committee, the Audit Committee and the Nominating and Corporate Governance Committee meets the independence requirements applicable to each such committee member prescribed by NASDAQ and the SEC. The Board has further determined that Messrs. LaMarche, Whelan and Wilson are “audit committee financial experts” as defined in the rules of the SEC.
47
Table of Contents
The Nominating and Corporate Governance Committee annually reviews the independence of all directors and reports its findings to the Board. The Nominating and Corporate Governance Committee has reviewed each director’s status by applying the standards for director independence and the criteria to determine “audit committee financial expert” status and by evaluating self-evaluation questionnaires and other information supplied by each director or otherwise independently obtained. On the basis of this review, the Nominating and Corporate Governance Committee delivered a report to our Board upon which our Board made its determinations of each director’s status.
In making these determinations, the Nominating and Corporate Governance Committee considered that in the ordinary course of business, relationships and transactions may occur between the Company and its subsidiaries and entities with which some of our directors are or have been affiliated. In addition, each year the Committee evaluates, based on questionnaires completed by the directors, whether the directors have any conflicts of interest with the Company under our Conflict of Interest Policy, a copy of which is publicly available on the Investors section of our website at http://investor.ariad.com under the heading “Corporate Governance.”
48
Table of Contents
| ITEM 14: | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The Audit Committee of our Board of Directors has selected Deloitte & Touche LLP (“Deloitte”) to be our independent registered public accounting firm for the year ending December 31, 2014. Our Board of Directors has ratified this selection. Deloitte has served as our independent registered public accounting firm since 1991. Deloitte has advised us that it does not have any direct or indirect financial interest in ARIAD.
Before it selected Deloitte as our independent registered public accounting firm, our Audit Committee carefully considered the qualifications of Deloitte, including the firm’s performance in prior years, the competence of personnel assigned to our engagement and its reputation for integrity, quality, and competence in the fields of accounting and auditing. Our Audit Committee also considered whether Deloitte’s provision of non-audit services to ARIAD is compatible with that firm’s independence.
Audit and Non-Audit Fees
For the fiscal years ending December 31, 2013 and 2012, we incurred the following fees for the services of Deloitte and its member firms:
| 2013 | 2012 | |||||||
| Audit Fees |
$ | 2,339,589 | $ | 767,873 | ||||
| Audit-Related Fees |
— | 20,397 | ||||||
| Tax Fees |
375,392 | 1,122,801 | ||||||
| All Other Fees |
2,200 | 6,700 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 2,617,181 | $ | 1,917,771 | ||||
Audit Fees
Audit Fees include fees for the audit of our annual financial statements, the reviews of our quarterly financial statements included in reports on Form 10-Q, and the audit of our system of internal control over financial reporting and fees related to statutory audit requirements of our subsidiaries, as well as fees for work that generally only our independent registered public accounting firm can provide including reviews of registration statements that include the firm’s consent, the provision of comfort letters in connection with stock offerings and statutory audit work. The increase in Audit Fees in 2013 reflects the growth and expansion of the Company, including the commercial launch of Iclusig in the United States and Europe and the geographic expansion of the Company in Europe, necessitating an increase in audit procedures to support Deloitte’s reports on our consolidated financial statements and system of internal control over financial reporting. We expect that Audit Fees may decrease in 2014 due to the reduction of time required on audit procedures connected to revenue, inventory and other matters related to the initial commercial launch of Iclusig in 2013.
Audit-Related Fees
Audit-Related Fees include fees for services that are reasonably related to the audit or reviews of our consolidated financial statements and are not reported under Audit Fees above. These services include consultation on financial accounting and reporting matters.
Tax Fees
Tax Fees include fees for preparation of tax returns as well as general tax planning and advice. Fees for these recurring services totaled approximately $375,000 in 2013 and $72,000 in 2012. These tax fees increased due to the increased tax compliance requirements associated with our international organization as well as supporting planning and advisory services as we grow and expand. We expect that fees for these recurring tax services will increase in 2014 as needed to fulfill our tax compliance requirements.
49
Table of Contents
Tax fees in 2012, in addition to the recurring fees described above, included assistance with the assessment of the tax implications of the design and implementation of our international tax structure. These non-recurring services included:
| • | Transfer pricing analysis for tax purposes related to the transfer of certain intellectual property rights to our wholly-owned subsidiary in Switzerland (approximately $403,000); |
| • | Analysis of the tax impacts in the United States of the transfer of intellectual property rights to our Swiss subsidiary (approximately $143,000); |
| • | Preparation and submission of tax ruling requests with Swiss tax authorities, and analysis of tax regulations applicable to subsidiaries in Europe (approximately $337,000); and |
| • | General advice on tax matters and review of documentation prepared by the Company, legal counsel and other consultants (approximately $167,000). |
These services were substantially completed in 2012. In engaging Deloitte on these matters, management and the Audit Committee considered Deloitte’s significant expertise in corporate taxation, including Swiss taxation, their significant expertise in biotechnology/pharmaceutical business and taxation, and their deep knowledge of our history, business, strategy and operations. As such, we determined that the engagement of Deloitte would ensure high quality advice and input, pertinent to our business and consistent with our overall business strategy, and completion of the work in a timely and efficient manner. The Audit Committee also discussed and determined that the provision of these services was compatible with Deloitte’s independence from the Company in the conduct of its audit services.
All Other Fees
All Other Fees include fees associated with services not captured in the three preceding categories, which includes access to and usage of Deloitte’s accounting research database.
Our Audit Committee pre-approved all of the services set forth above, pursuant to our pre-approval policy described below.
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-audit Services of Independent Auditors
Consistent with SEC policies regarding auditor independence, our Audit Committee has responsibility for appointing, setting compensation and overseeing the work of the independent registered public accounting firm. In recognition of this responsibility, our Audit Committee has established a policy to pre-approve all audit and permissible non-audit services provided by the independent registered public accounting firm.
Prior to engagement of an independent registered public accounting firm for the next year’s audit and also on a quarterly basis, management submits a report to our Audit Committee for their approval, outlining the services planned or anticipated to be rendered by the independent registered public accounting firm, and the estimated fees for such services. The services are outlined according to the four categories of services defined above, Audit, Audit-Related, Tax and All Other. Actual fees incurred relative to estimated fees are reported to our Audit Committee each quarter.
Prior to engagement, the Audit Committee pre-approves these services by category of service. The fees are budgeted and the Audit Committee requires our independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service. During the year, circumstances may arise when it may become necessary to engage our independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the Audit Committee requires specific pre-approval before engaging our independent registered public accounting firm.
To ensure prompt consideration of unexpected services, our Audit Committee has delegated authority to the Chair of our Audit Committee to pre-approve services to be rendered. Any such actions taken by the Chair must be reported to our Audit Committee at its next scheduled meeting.
50
Table of Contents
| ITEM 15: | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
The financial statements, financial statement schedules and exhibits listed in the exhibit index of the Original Filing and the exhibits listed in the exhibit index of this Form 10-K/A are filed with, or incorporated by reference in, this Form 10-K/A.
51
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Act of 1934, the registrant has duly caused this Amendment No. 1 to Annual Report on Form 10-K/A to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Cambridge and Commonwealth of Massachusetts on the 30th day of April, 2014.
| ARIAD PHARMACEUTICALS, INC. | ||
| By: | /s/ Harvey J. Berger, M.D. | |
| Name: | Harvey J. Berger, M.D. | |
| Title: | Chairman, Chief Executive Officer and President | |
52
Table of Contents
EXHIBIT LIST
| Exhibit |
Exhibit Description | |
| 31.1 | Certification of the Chief Executive Officer | |
| 31.2 | Certification of the Chief Financial Officer | |
53
