Attached files
| file | filename |
|---|---|
| 8-K - 8-K - NephroGenex, Inc. | a14-11428_18k.htm |
Exhibit 99.1
|
|
Company Overview April 2014 April 2012 |
|
|
Thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting kidney diseases, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our 10-K Statement filed with the Securities and Exchange Commission during March 2014. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law. - Forward Looking Statements 2 You should read carefully our “Cautionary Note Regarding Forward-looking Statements and Industry Data” and the factors described in the “Risk Factors” sections of our Registration Statement to better understand the risks and uncertainties inherent in our business. |
|
|
Corporate Overview Late-stage company targeting pathogenic oxidative chemistries to treat kidney disease Oral Pyridorin (Phase 3) to slow progression of diabetic nephropathy (DN) IV Pyridorin (Preclinical) to treat acute kidney injury (AKI) Oral Pyridorin for DN is in Phase 3 of Development. It demonstrated >50% treatment effect in patient population accepted by FDA in previous Phase 2 trials FDA Fast Track Designation Proof of concept established in two large Phase 2a studies Preferred patient population identified in large Phase 2b study (307 patients) Benign safety profile Expect first patient in trial during Q2 2014 SPA (Special Protocol Assessment) with FDA New fully approvable endpoint that reduces trial follow-up time & cost by ~50% compared to other DN Phase 3 programs 3 |
|
|
The NephroGenex Pipeline Clinical Program / Indication Preclinical Phase I Phase II Phase III Worldwide Commercial Rights ORAL PYRIDORIN ® Diabetic Nephropathy IV PYRIDORIN ® Acute Kidney Injury Clinical Program Oral BID 300 mg tablet targets pathogenic oxidative chemistries 2015 IV formulation for hospital patients - Pathologic oxidative chemistries 4 |
|
|
The NephroGenex Team Board of Directors Member Affiliation, Position Pierre Legault NephroGenex, Director J. Wesley Fox, Ph.D. NephroGenex, Director Robert Seltzer Care Capital, Director Eugen Steiner, M.D. BioStratum, Director Martin Vogelbaum Rho Ventures, Director Jim Mitchum Director & Chairman of Audit Committee Chairman of the Board Richard Markham Care Capital, Partner Aventis, COO & Vice Chairman Aventis Pharma, CEO Member Affiliation, Position Pierre Legault, CEO Sanofi-Aventis, President Worldwide Dermatology Eckerd, President Rite Aid, CAO OSI Pharmaceuticals, CFO Prosidion Ltd, CEO Board Member, Forest Labs & Regado Biosciences J. Wesley Fox, Ph.D., President & CSO BioStratum, Co-Founder & CSO EnzyMed, Co-Founder and President Abbott Laboratories, Scientist Mark Klausner, M.D., CMO CorMedix, CMO Johnson & Johnson, VP Harvard Medical School, M.D., Clinical Fellow John Hamill, CFO Savient Pharmaceuticals, CFO PharmaNet, CFO Management Team Experienced management team focused on clinical success 5 |
|
|
Oral Pyridorin for Diabetic Nephropathy Overview 6 |
|
|
Oral Pyridorin Market Potential DN patients progress from high risk, to early stage (microalbuminuria), to overt nephropathy (macroalbuminuria), to end-stage-renal-disease (ESRD) and dialysis 19 M diagnosed diabetics in the US, of which 33% (6.3 M) exhibit kidney disease 2.8 M macroalbuminuria (overt nephropathy) 3.5 M microalbuminuria and 3.6 M additional patients are at high risk of progressing to DN Diabetic nephropathy is a significant healthcare financial burden Current approved therapies are marginally effective Only ACEIs and ARBs are approved for DN, which do not treat the underlying cause of the disease A significant unmet medical need remains for treatments that can slow or halt DN Current treatments do not address underlying causes of DN, and patients progressively deteriorate Potential for significant worldwide revenues Could potentially treat 10 M patients in U.S. 7 |
|
|
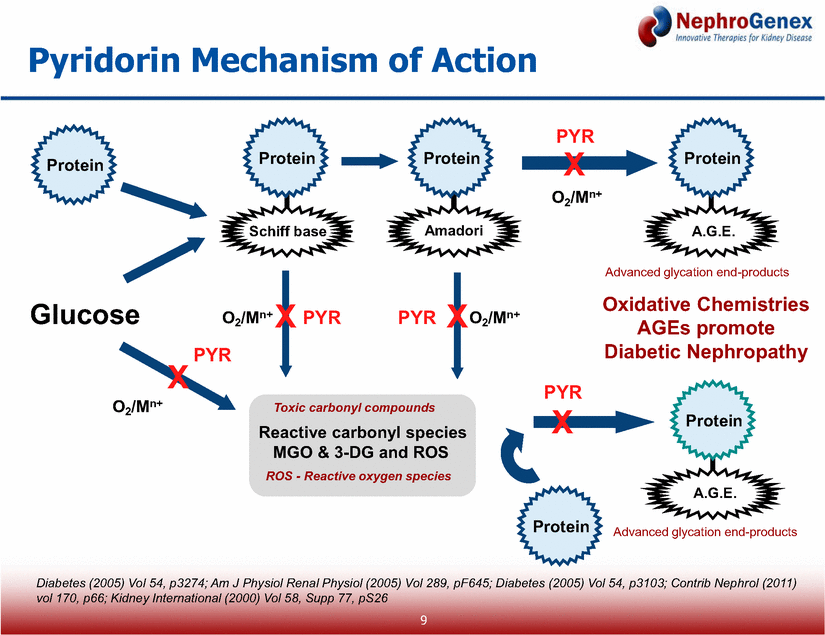
Hyperglycemia increases pathogenic oxidative chemistries* Elevated levels of oxidative chemistries promote the development and progression of DN* Pyridorin targets oxidative chemistries, a key underlying cause of DN DN is the leading cause of dialysis and chronic kidney failure Pyridorin is an effective drug candidate for treating DN Pyridorin is the only advanced drug candidate for DN targeting an underlying cause of the disease Validated in numerous preclinical studies and three Phase 2 studies * Diabetes (2005) Vol 54, p3274; Am J Physiol Renal Physiol (2005) Vol 289, pF645; Diabetes (2005) Vol 54, p3103; Contrib Nephrol (2011) vol 170, p66; Kidney International (2000) Vol 58, Supp 77, pS26 N H 2 O H C H 3 C H O H N H 2 2 C Pyridorin Pathophysiology of DN 8 |
|
|
Pyridorin Mechanism of Action O2/Mn+ Protein Protein Protein Schiff base O2/Mn+ O2/Mn+ Reactive carbonyl species MGO & 3-DG and ROS O2/Mn+ Protein Protein Amadori A.G.E. Protein A.G.E. Glucose Toxic carbonyl compounds Advanced glycation end-products ROS - Reactive oxygen species Advanced glycation end-products PYR PYR PYR PYR X X X X X PYR Oxidative Chemistries AGEs promote Diabetic Nephropathy Diabetes (2005) Vol 54, p3274; Am J Physiol Renal Physiol (2005) Vol 289, pF645; Diabetes (2005) Vol 54, p3103; Contrib Nephrol (2011) vol 170, p66; Kidney International (2000) Vol 58, Supp 77, pS26 9 |
|
|
Patient Population Number of Subjects PYR, Placebo Baseline SCr PYR Placebo SCr Change from Baseline PYR Placebo Pyridorin Treatment Effect P value All Patients 57, 27 1.75 0.64 1.96 0.86 0.11 0.26 0.34 0.92 68% 0.0322 Pyridorin at a 250 mg bid dose is well tolerated and slows the rate of SCr increase in moderate to advanced disease patients Two multi-center, randomized, double-blind, placebo-controlled trials Number of centers: 20 (U.S., Belgium, United Kingdom, Canada, South Africa) Dose: 50mg BID x 2 weeks; 100mg BID x 2 weeks; 250mg BID x 20 weeks Study objectives: assess safety, tolerability and biological activity. All analyses done on combined studies Diabetic patients with DN due to type 1 or type 2 diabetes N=84 (57 treated, 27 placebo) Patients had macroalbuminuria and a bSCr < 3.5 mg/dL Nephrogenex Phase 2a PYR-205-207 Note: Virtually all patients on standard of care at screening –ACEI/ARB therapy and blood pressure control 10 |
|
|
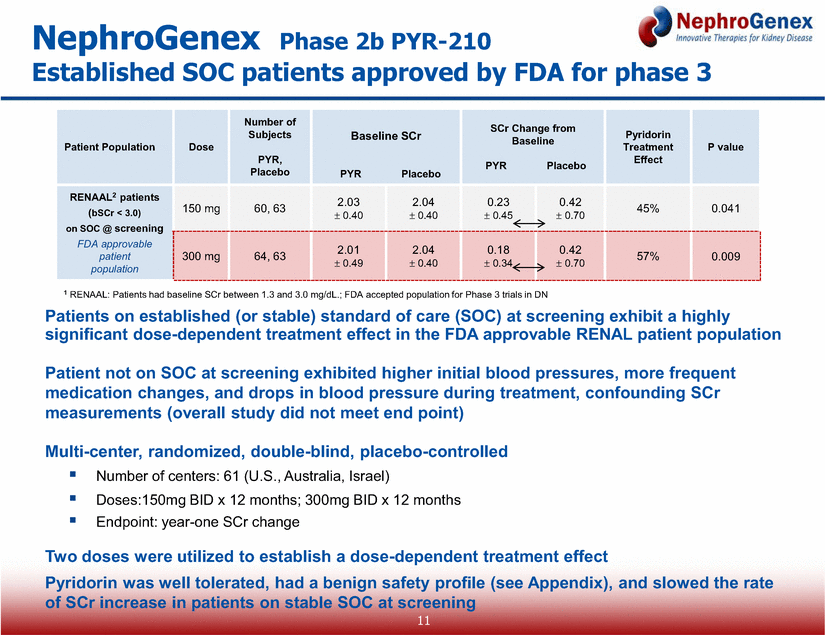
Patients on established (or stable) standard of care (SOC) at screening exhibit a highly significant dose-dependent treatment effect in the FDA approvable RENAL patient population Patient not on SOC at screening exhibited higher initial blood pressures, more frequent medication changes, and drops in blood pressure during treatment, confounding SCr measurements (overall study did not meet end point) Multi-center, randomized, double-blind, placebo-controlled Number of centers: 61 (U.S., Australia, Israel) Doses:150mg BID x 12 months; 300mg BID x 12 months Endpoint: year-one SCr change Two doses were utilized to establish a dose-dependent treatment effect Pyridorin was well tolerated, had a benign safety profile (see Appendix), and slowed the rate of SCr increase in patients on stable SOC at screening NephroGenex Phase 2b PYR-210 Established SOC patients approved by FDA for phase 3 1 RENAAL: Patients had baseline SCr between 1.3 and 3.0 mg/dL.; FDA accepted population for Phase 3 trials in DN Patient Population Dose Number of Subjects PYR, Placebo Baseline SCr PYR Placebo SCr Change from Baseline PYR Placebo Pyridorin Treatment Effect P value RENAAL2 patients (bSCr < 3.0) on SOC @ screening FDA approvable patient population 150 mg 60, 63 2.03 0.40 2.04 0.40 0.23 0.45 0.42 0.70 45% 0.041 300 mg 64, 63 2.01 0.49 2.04 0.40 0.18 0.34 0.42 0.70 57% 0.009 11 |
|
|
Objective: Evaluate safety efficacy of Pyridorin at a 300 mg twice daily dose compared to placebo to reduce the rate of renal disease progression due to type 2 diabetes mellitus Patient Population Type 2 diabetic nephropathy patients Baseline SCr from 1.3 to 3.0 mg/dL Patients must be on an established and stable regimen of SOC for 6 months Appropriate and stable dose of ACEi/ARB and BP medications Overall trial design Two identical, double-blind Phase 3 studies of 600 patients each Dose 300 mg bid 100 centers world-wide (approximately: 65% U.S. and 35% rest of world) Duration estimated at 3.5 years Powering 247 events 90% powering to detect a 28% treatment effect (>50% treatment effect observed in Phase 2b study in relevant patient population) Endpoint: Time to a 50% SCr increase or ESRD Interim analysis: 300 patients, DSMB review, 12 month increase of SCr level Financial resources: Sufficient resources to reach interim analysis in early 2016 PIONEER Pyridorin Phase 3 Trials Protocol 12 |
|
|
PIONEER Trials Timeline Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 2014 2015 2016 Secured CRO (Collaborative Study Group & Medpace) Expect first patient to enter PIONEER pivotal Phase 3 trial June 2014 Publication on new end point Expect completion TQT cardiac safety study Dec 2014 Anticipate EMA acceptance of new SCr increase end point 2014 Anticipate presentations at American Society of Nephrology (Nov. 2014) Expect completion PIONEER first 300 patient recruitment (Phase 3 PYR-311) Q1 2015 Expect completion 600 patient recruitment PIONEER Summer 2015 Expect completion IV PYR AKI preclinical results Anticipate IND filing for IV PYR AKI Anticipate initiation of IV PYR AKI Phase 1/2 studies Expect PIONEER 300 patients interim analysis Q4 2015/ Q1 2016 Partnership / Collaboration (Initiation of second trial) 13 |
|
|
Exclusivity Patents and New Chemical Entity (NCE) Japan Post marketing surveillance system provides exclusivity for up to 8 years (NCE 8 years exclusivity) Method of use (patient population), manufacturing and new chemical entity (NCE) status: U.S. September 2028 including patent extension dosage & patient population IP protection (NCE 5 years exclusivity) Europe June 2024 and extension by country dosage & patient population IP protection and NCE 10 years exclusivity) Canada June 2024 dosage & patient population IP protection and NCE 8 years exclusivity 14 Pyridorin for DN is unencumbered: No future royalties and one minimal $200K milestone at approval |
|
|
Corporate Summary Experienced management team focused on clinical success Diabetic nephropathy represents a large and growing unmet need Oral Pyridorin could potentially treat as many as 10 M patients, more than half of the diabetic population in the US No FDA approved alternative treatments for DN Pyridorin could significantly decrease the rate of progression of diabetic nephropathy, the onset of ESRD and improve patient quality of life Oral Pyridorin Pivotal Phase 3 program in DN initiated Demonstrated benign safety profile in Phase 1 and phase 2 studies >50% treatment effect in patient population from Phase 2b study selected for the Phase 3 program First patient in Phase 3 trial expected in June 2014 FDA Fast Track, SPA agreement with endpoint and patient population that reduces the time, cost and risk of Phase 3 development 15 |
|
|
Appendix |
|
|
Multi-center, randomized, double-blind, placebo-controlled trial Number of centers: 46 Location of centers: U.S. Dose: 50mg BID x 24 weeks Study objectives: assess safety, tolerability and biological activity Diabetic patients with DN due to type 1 or type 2 diabetes N = 128 (65 treated, 63 placebo) Patients had macroalbuminuria and a baseline SCr (bSCrdL A dose of 50 mg bid was examined in mild to moderate disease patients Pyridorin was well tolerated and slowed the rate of SCr increase SCr levels are used as diagnostic indicators of kidney function Pyridorin Phase 2a Program PYR-206 17 |
|
|
Mild to Moderate Diabetic Nephropathy Dose 50 mg BID Patient Population Number of Subjects PYR, Placebo Baseline SCr PYR Placebo SCr Change from Baseline PYR Placebo Pyridorin Treatment Effect P value All Patients 65, 63 1.27 0.34 1.33 0.38 0.12 0.40 0.16 0.28 27% 0.2426 Type 2 Diabetes 40, 40 1.28 0.34 1.30 0.36 0.08 0.29 0.17 0.30 53% 0.0573 34, 30 1.54 0.21 1.65 0.28 0.13 0.53 0.26 0.33 50% 0.0691 Type 2 Diabetes, 22, 19 1.53 0.20 1.59 0.73 0.06 0.37 0.29 0.35 79% 0.0074 Note: Virtually all patients on standard of care at screening ACEI/ARB therapy and blood pressure control Pyridorin at a 50 mg bid dose is well tolerated and slows the rate of SCr increase in mild to moderate disease patients Clinical Results PYR-206 18 Baseline SCr > 1.3 mg/dL |
|
|
Moderate to Advanced Diabetic Nephropathy Dose 250 mg bid Patient Population Number of Subjects PYR, Placebo Baseline SCr PYR Placebo SCr Change from Baseline PYR Placebo Pyridorin Treatment Effect P value All Patients 57, 27 1.75 0.64 1.96 0.86 0.11 0.26 0.34 0.92 68% 0.0322 Type 2 Diabetes 45, 22 1.74 0.67 1.94 0.92 0.12 0.27 0.38 1.02 68% 0.0498 42, 19 2.00 0.55 2.37 0.67 0.12 0.30 0.47 1.09 74% 0.0454 Type 2 Diabetes, 33, 15 2.00 0.58 2.40 0.73 0.14 0.31 0.55 1.22 75% 0.058 Note: Virtually all patients on standard of care at screening ACEI/ARB therapy and blood pressure control Clinical Results PYR-205/207 Pyridorin at a 250 mg bid dose is well tolerated and slows the rate of SCr increase in moderate to advanced disease patients 19 |
|
|
Clinical Results PYR-210 Patient Population Dose Number of Subjects PYR, Placebo Baseline SCr PYR Placebo SCr Change from Baseline PYR Placebo Pyridorin Treatment Effect P value Patients NOT on SOC at screening3 Pre-specified analysis 300 mg 36, 34 2.32 0.50 2.34 0.67 0.62 0.75 0.31 0.68 n/a n/a 150 mg 30, 34 2.33 0.56 2.34 0.67 0.73 0.90 0.31 0.68 n/a n/a RENAAL2 patients (bSCr < 3.0) on SOC @ screening FDA approvable patient population 150 mg 60, 63 2.03 0.40 2.04 0.40 0.23 0.45 0.42 0.70 45% 0.041 300 mg 64, 63 2.01 0.49 2.04 0.40 0.18 0.34 0.42 0.70 57% 0.009 1 SOC: ACEI/ARB therapy and blood pressure control. Patients not on SOC at screening entered an 8 week run-in period 2 RENAAL: Patients had baseline SCr between 1.3 and 3.0 mg/dL.; FDA accepted population for Phase 3 trials in DN 3 Pre-specified analyses to evaluate patients not on SOC at screening and requiring a run-in period versus patients on established SOC at screening Type 2 Diabetic Nephropathy Patients With and Without SOC 1 at screening 300 & 150 mg bid Patients on SOC at screening exhibit a highly significant dose-dependent treatment effect in the FDA approvable RENAAL patient population Patients not on SOC at screening exhibit higher blood pressures and more frequent medication changes that can confound SCr values Phase 3 Dose & Patient Population 20 |
|
|
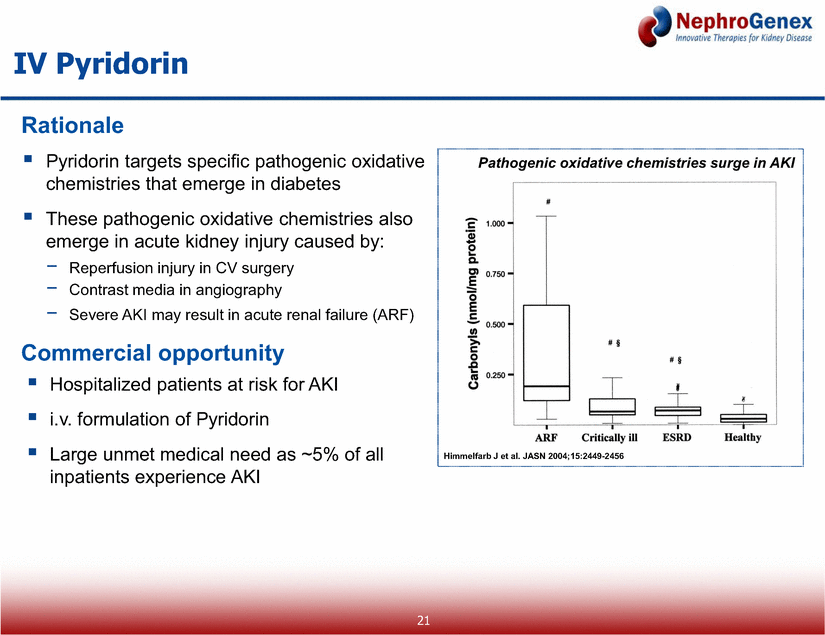
Pyridorin targets specific pathogenic oxidative chemistries that emerge in diabetes These pathogenic oxidative chemistries also emerge in acute kidney injury caused by: Reperfusion injury in CV surgery Contrast media in angiography Severe AKI may result in acute renal failure (ARF) IV Pyridorin Rationale Hospitalized patients at risk for AKI i.v. formulation of Pyridorin Large unmet medical need as ~5% of all inpatients experience AKI Commercial opportunity Himmelfarb J et al. JASN 2004;15:2449-2456 Pathogenic oxidative chemistries surge in AKI 21 |
|
|
Safety Conclusions There were no meaningful differences between groups in AEs There was possibly an increase in diarrhea & constipation with the higher Pyridorin 300 mg bid dose Diarrhea: Placebo = 6%, 150 mg = 3%, 300 mg = 11 % Constipation: Placebo = 4%, 150 mg = 4%, 300 mg = 10 % There were no differences between groups in SAEs There were no differences between groups in mortality or ESRD There were no differences between groups for laboratory parameters (hematology, chemistry, HbA1c) There was no effect of Pyridorin on the QTc interval CONCLUSION: Pyridorin has a benign safety profile with the possible exception of a small increase in diarrhea and constipation with the higher dose of 300 mg bid Confidential 22 |