Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a14-11055_18k.htm |
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a14-11055_1ex99d1.htm |
Exhibit 99.2
|
|
AMAG Pharmaceuticals First Quarter 2014 Financial Results April 24, 2014 AMAG Pharmaceuticals, Inc. 1100 Winter Street Waltham, MA 02451 617.498.3300 www.amagpharma.com A SPECIALTY PHARMACEUTICAL COMPANY DEDICATED TO BRINGING TO MARKET THERAPIES THAT IMPROVE PATIENTS’ LIVES. Copyright 2014 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
Forward Looking Statements 1 This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein which do not describe historical facts, including but not limited to, statements regarding: (i) our mission to build a profitable, multi-product specialty pharmaceutical company; (ii) Feraheme demand growth; (iii) plans to increase share of the current chronic kidney disease (CKD) intravenous (IV) iron market and the growth opportunity for Feraheme in the current indication; (iv) the market opportunity for MuGard and key levers for MuGard’s success in 2014; (v) expansion opportunities, including plans for our potential label expansion for the broader iron deficiency anemia (IDA) indication for Feraheme and the related call audience; (vi) expectations, including timing and next steps, of regulatory matters related to the potential Feraheme and Rienso label expansion opportunities; (vii) the expected timing and magnitude of milestone payments; (viii) our business development goals and opportunities; (ix) the impact of business development transactions on EBITDA and our ability to optimize after-tax cash flows with business development transactions; (x) key financial metrics for 2014; (xi) our 2014 goals, including plans to maximize Feraheme opportunities, to drive MuGard use though increased patient referrals and expanded payer coverage, to execute additional, quality business development transactions and to continue to operate the business with financial discipline; (xii) our 2014 financial outlook, including projected sales, cost of goods sold, operating expenses, cash and investments balance and adjusted EBITDA; and (xiii) our statement that AMAG is well positioned for success in 2014 and beyond are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others: (1) uncertainties regarding the likelihood and timing of potential approval of Feraheme in the U.S. in the broader IDA indication in light of the complete response letter we received from the FDA informing us that our supplemental new drug application (sNDA) for the broader indication could not be approved in its present form and stating that we had not provided sufficient information to permit labeling of Feraheme for safe and effective use for the proposed broader indication, (2) the possibility that following the U.S. Food and Drug Administration’s (FDA’s) review of post-marketing safety data, including reports of serious anaphylaxis, cardiovascular events, and death, the FDA will request additional technical or scientific information, new studies or reanalysis of existing data, on-label warnings, post-marketing requirements/commitments or risk evaluation and mitigation strategies (REMS) in the current CKD indication for Feraheme, (3) uncertainties regarding our and Takeda Pharmaceutical’s ability to successfully compete in the IV iron replacement market both in the U.S. and outside the U.S., including the EU, including as a result of limitations, restrictions or warnings in Feraheme’s/Rienso’s current or future label that put Feraheme/Rienso at a competitive disadvantage, (4) uncertainties regarding Takeda’s ability to obtain regulatory approval for Feraheme in Canada, and Rienso in the EU, in the broader IDA patient population, (5) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso and in turn affect sales, or our ability to market the product both in the U.S. and outside of the U.S., including the EU, (6) uncertainties regarding the manufacture of Feraheme/Rienso or MuGard, (7) uncertainties relating to our patents and proprietary rights both in the U.S. and outside the U.S., (8) the risk of an Abbreviated New Drug Application (ANDA) filing following the FDA’s recently published draft bioequivalence recommendation for ferumoxytol, (9) uncertainties regarding our ability to compete in the oral mucositis market in the U.S. and (10) other risks identified in our filings with the U.S. Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K for the year ended December 31, 2013 and subsequent filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. |
|
|
Agenda 2 Topic Speaker Opening Remarks Bill Heiden, CEO Commercial Performance Ed Jordan, SVP Sales & Marketing Expansion Opportunities Bill Heiden, CEO Financial Highlights & Outlook Frank Thomas, COO Closing Remarks Bill Heiden, CEO |
|
|
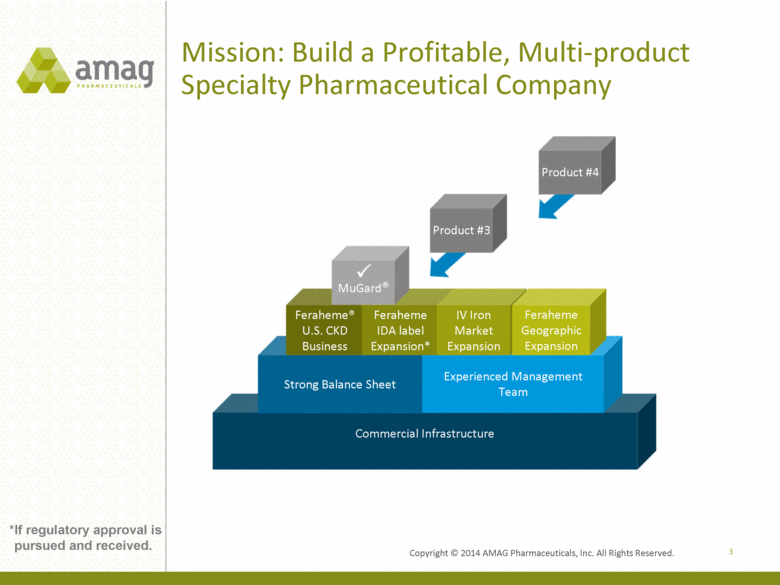
Mission: Build a Profitable, Multi-product Specialty Pharmaceutical Company Commercial Infrastructure Focus on Hematology/Oncology, Hospital and Nephrology Strong Balance Sheet Experienced Management Team Feraheme® U.S. CKD Business Feraheme IDA label Expansion* IV Iron Market Expansion Feraheme Geographic Expansion Product #3 3 MuGard® Product #4 *If regulatory approval is pursued and received. |
|
|
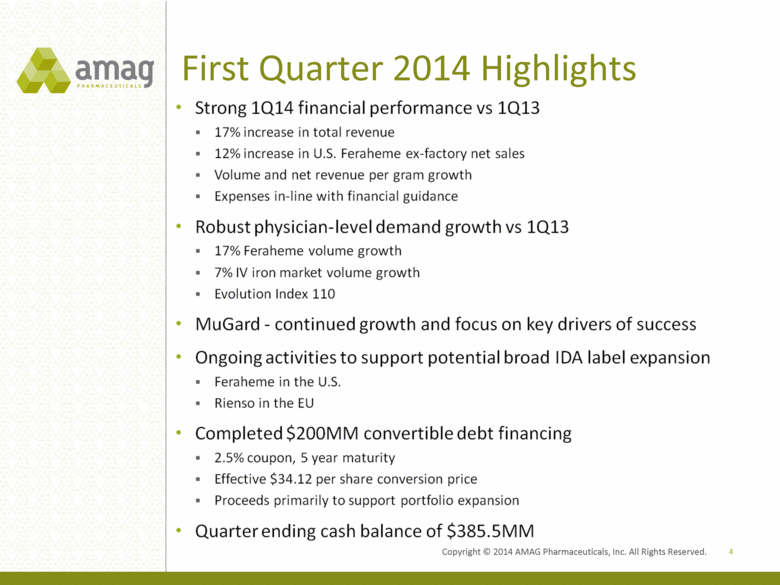
First Quarter 2014 Highlights 4 Strong 1Q14 financial performance vs 1Q13 17% increase in total revenue 12% increase in U.S. Feraheme ex-factory net sales Voulme and net revenue per gram growth Expenses in-line with financial guidance Robust physician-level demand growth vs 1Q13 17% Feraheme volume growth 7% IV iron market volume growth Evolution Index 110 MuGard - continued growth and focus on key drivers of success Ongoing activities to support potential broad IDA label expansion Feraheme in the U.S. Rienso in the EU Completed $200MM convertible debt financing 2.5% coupon, 5 year maturity Effective $34.12 per share conversion price Proceeds primarily to support portfolio expansion Quarter ending cash balance of $385.5MM Copyright 2014 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
FERAHEME PERFORMANCE Copyright © 2014 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
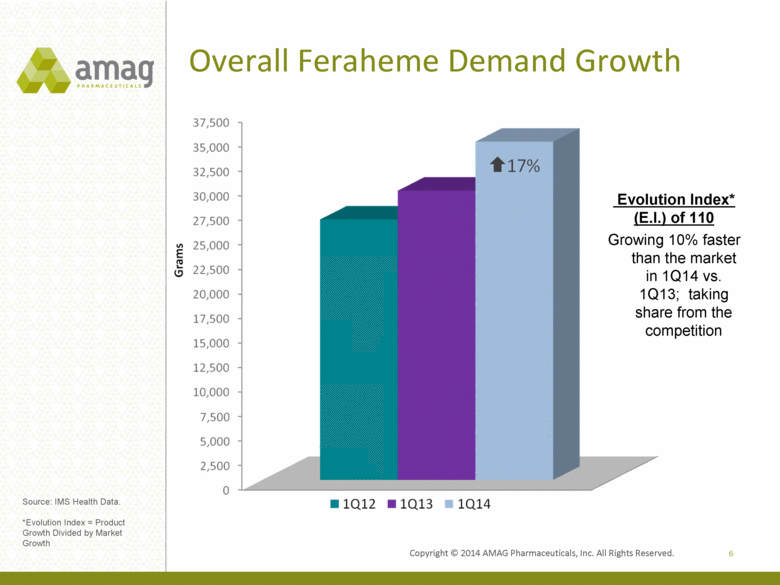
Overall Feraheme Demand Growth 6 Source: IMS Health Data. *Evolution Index = Product Growth Divided by Market Growth Evolution Index* (E.I.) of 110 Growing 10% faster than the market in 1Q14 vs. 1Q13; taking share from the competition |
|
|
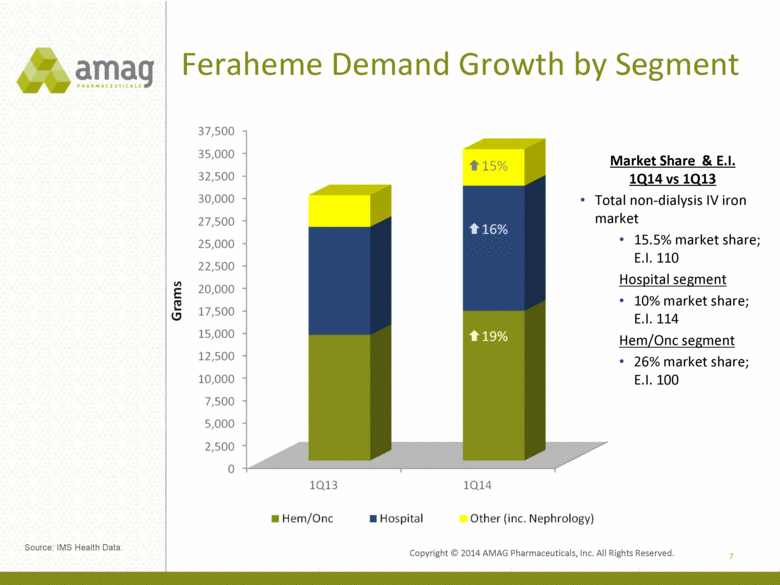
Feraheme Demand Growth by Segment 7 Market Share & E.I. 1Q14 vs 1Q13 Total non-dialysis IV iron market 15.5% market share; E.I. 110 Hospital segment 10% market share; E.I. 114 Hem/Onc segment 26% market share; E.I. 100 Source: IMS Health Data. |
|
|
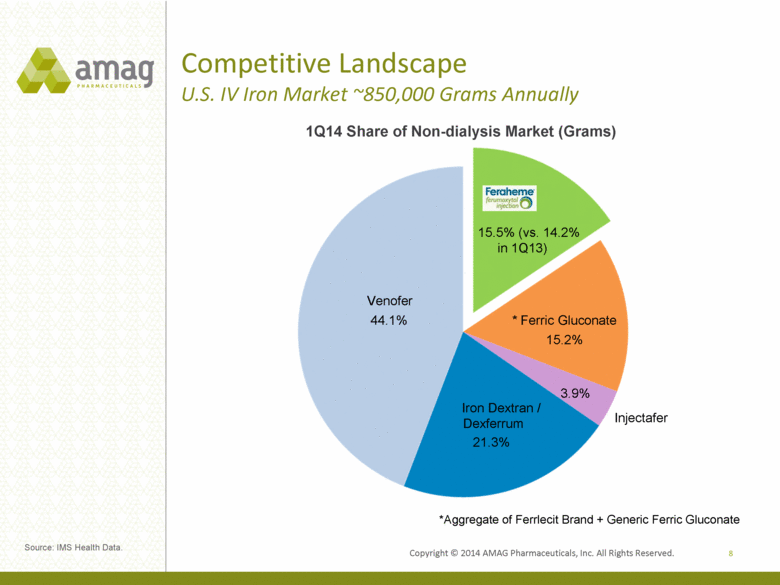
Competitive Landscape U.S. IV Iron Market ~850,000 Grams Annually 8 *Aggregate of Ferrlecit Brand + Generic Ferric Gluconate 1Q14 Share of Non-dialysis Market (Grams) Venofer Iron Dextran / Dexferrum Injectafer * Ferric Gluconate 15.5% (vs. 14.2% in 1Q13) Source: IMS Health Data. 15.2% 44.1% 3.9% 21.3% |
|
|
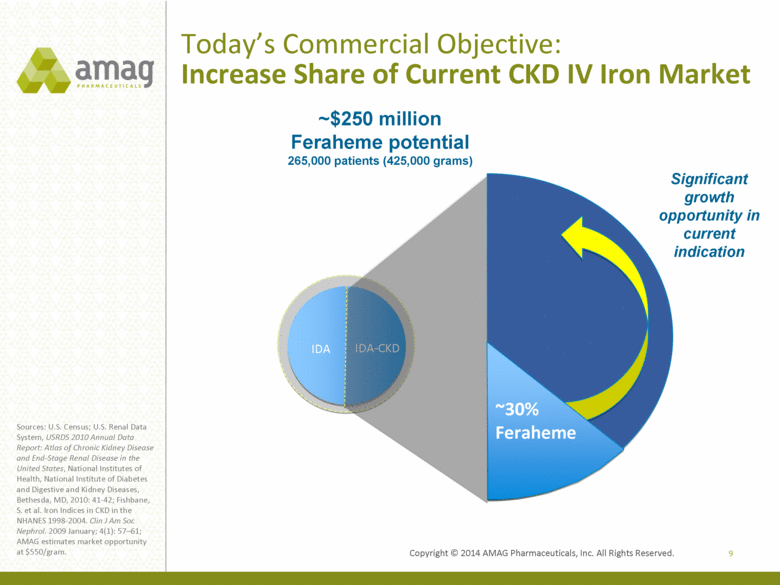
Today’s Commercial Objective: Increase Share of Current CKD IV Iron Market 9 Sources: U.S. Census; U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010: 41-42; Fishbane, S. et al. Iron Indices in CKD in the NHANES 1998-2004. Clin J Am Soc Nephrol. 2009 January; 4(1): 57–61; AMAG estimates market opportunity at $550/gram. IDA IDA-CKD ~30% Feraheme Significant growth opportunity in current indication ~$250 million Feraheme potential 265,000 patients (425,000 grams) |
|
|
MUGARD PERFORMANCE |
|
|
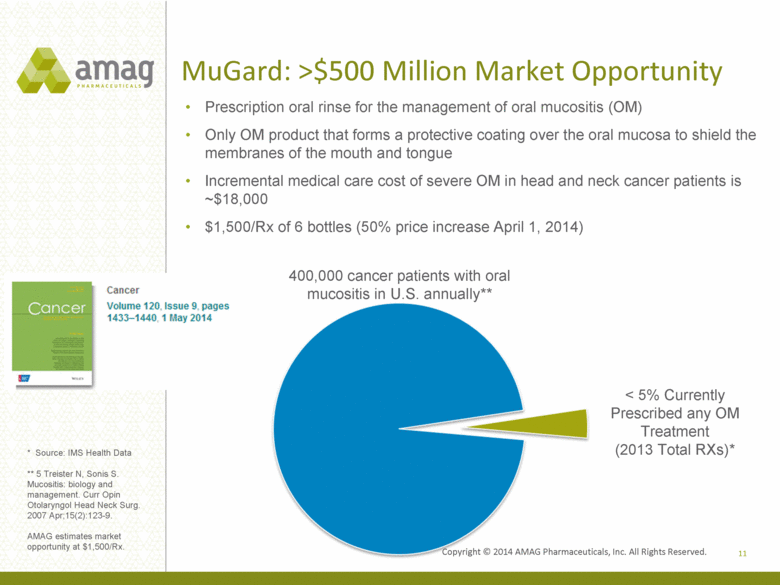
MuGard: >$500 Million Market Opportunity Prescription oral rinse for the management of oral mucositis (OM) Only OM product that forms a protective coating over the oral mucosa to shield the membranes of the mouth and tongue Incremental medical care cost of severe OM in head and neck cancer patients is ~$18,000 $1,500/Rx of 6 bottles (50% price increase April 1, 2014) < 5% Currently Prescribed any OM Treatment (2013 Total RXs)* 400,000 cancer patients with oral mucositis in U.S. annually** * Source: IMS Health Data ** 5 Treister N, Sonis S. Mucositis: biology and management. Curr Opin Otolaryngol Head Neck Surg. 2007 Apr;15(2):123-9. AMAG estimates market opportunity at $1,500/Rx. 11 |
|
|
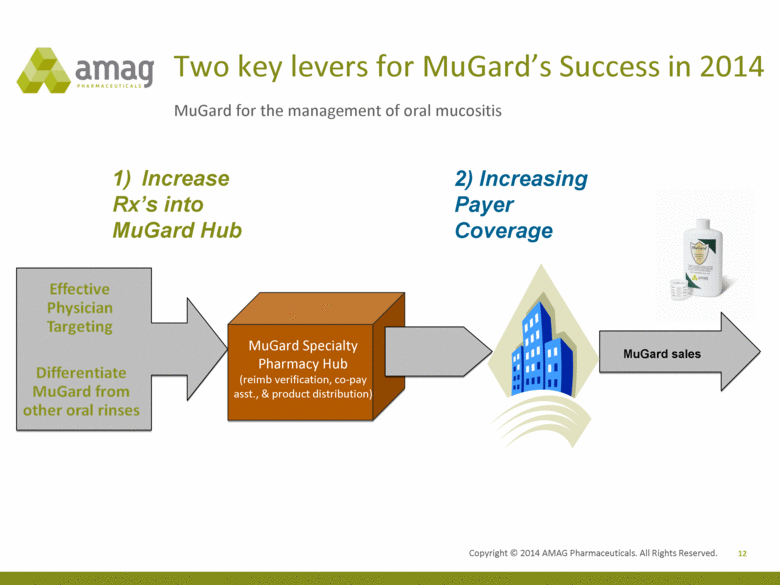
12 MuGard for the management of oral mucositis Two key levers for MuGard’s Success in 2014 Increase Rx’s into MuGard Hub 2) Increasing Payer Coverage MuGard Specialty Pharmacy Hub (reimb verification, co-pay asst., & product distribution) MuGard sales Effective physician Targeting Differentiate MuGard from other oral rinses |
|
|
EXPANSION OPPORTUNITIES Copyright © 2014 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
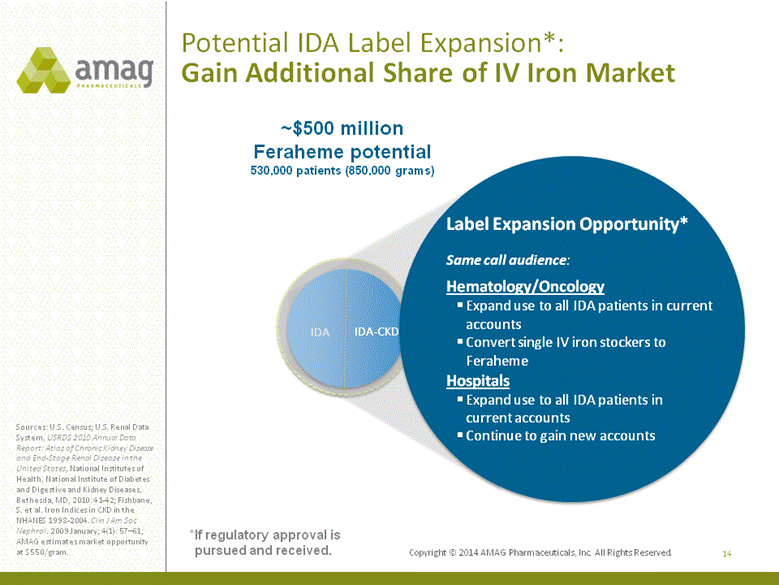
14 Sources: U.S. Census; U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010: 41-42; Fishbane, S. et al. Iron Indices in CKD in the NHANES 1998-2004. Clin J Am Soc Nephrol. 2009 January; 4(1): 57–61; AMAG estimates market opportunity at $550/gram. ~$500 million Feraheme potential 530,000 patients (850,000 grams) IDA IDA-CKD Label Expansion Opportunity* Same call audience: Hematology/Oncology Expand use to all IDA patients in current accounts Convert single IV iron stockers to Feraheme Hospitals Expand use to all IDA patients in current accounts Continue to gain new accounts Potential IDA Label Expansion*: Gain Additional Share of IV Iron Market *If regulatory approval is pursued and received. |
|
|
Opportunity: Grow U.S. IV Iron Market 15 IV Iron IDA* IV Iron IDA-CKD 4,000,000 patients diagnosed with IDA placed on oral iron therapy IV Iron IDA-CKD IV Iron IDA* Oral Iron IDA* Oral Iron IDA-CKD *If regulatory approval is pursued and received. 4,000,000 patients diagnosed with IDA placed on oral iron therapy Current CKD call points Potential future call points* Oncology Nephrology Gastroenterology Rheumatology Women’s Health |
|
|
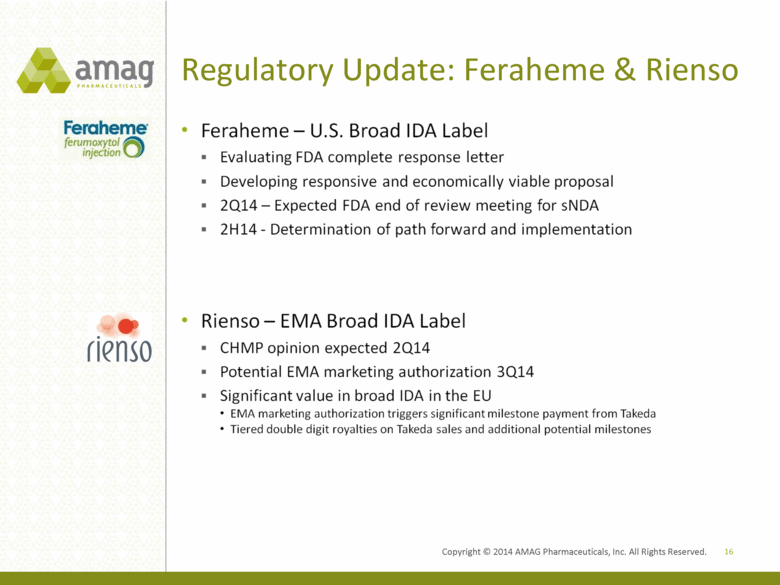
Regulatory Update: Feraheme & Rienso 16 Feraheme U.S. Broad IDA Label Evaluating FDA complete response letter Developing responsive and economically viable proposal 2Q14 Expected FDA end of review meeting for sNDA 2H14 - Determination of path forward and impelmentation Rienso EMA Broad IDA Label CHMP opinion expected 2Q14 Potential EMA marketing authorization 3Q14 Significant value in broad IDA in the EU EMA marketing authorization triggers significant milestone payment for Takeda Tiered double digit royalties on Takeda sales and additional potential milestones |
|
|
Targeted Business Development 17 Financial Similarly sized specialty company Eliminate overlapping infrastructure and increase EBITDA Examine transactions to optimize after-tax cash flows ~ 75 small private companies with U.S. marketed specialty products Strategic Opportunities aligned with Feraheme growth strategy Gastroenterology, rheumatology, women’s health Bulls-Eye Hem/onc or hospital company or product $10 MM–$60 MM/yr. revenue potential IP runway Immediately accretive ~90 branded specialty products with revenues between $51-$100MM ~130 branded specialty products with revenues between $10-$50MM |
|
|
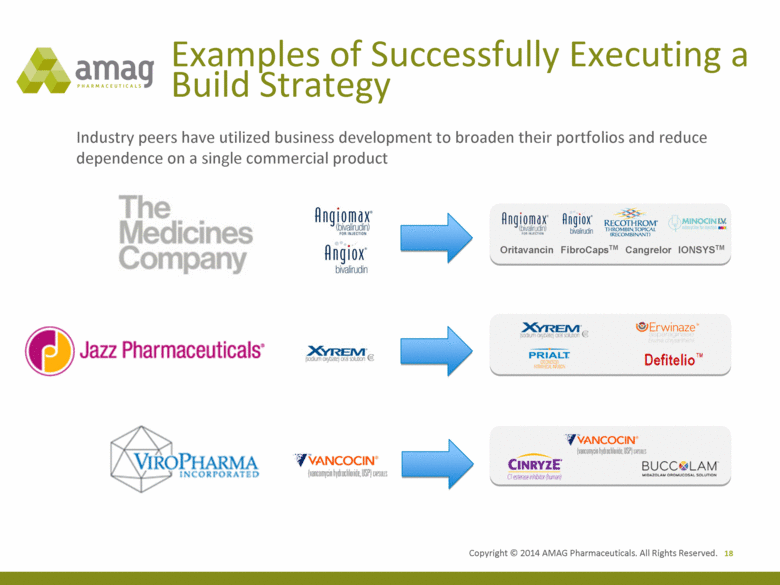
Examples of Successfully Executing a Build Strategy 18 Industry peers have utilized business development to broaden their portfolios and reduce dependence on a single commercial product Oritavancin FibroCapsTM IONSYSTM Cangrelor |
|
|
FINANCIAL HIGHLIGHTS & OUTLOOK Copyright © 2014 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
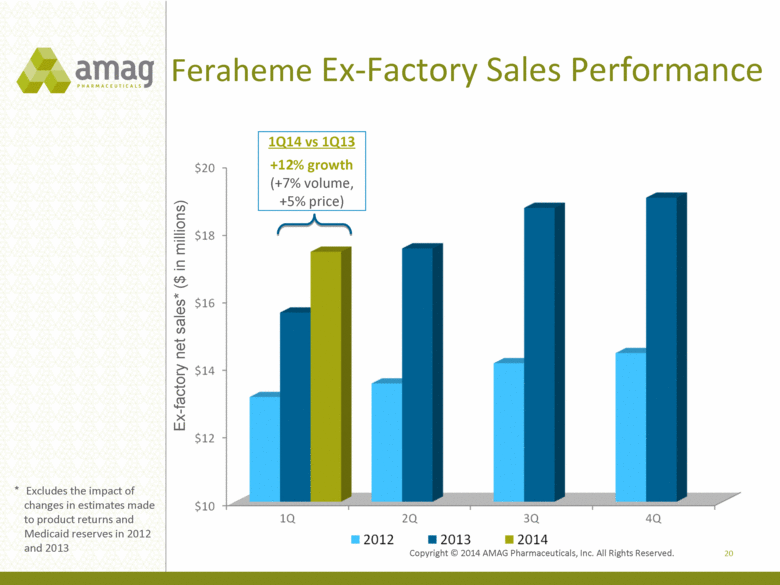
Feraheme Ex-Factory Sales Performance 20 Ex-factory net sales* ($ in millions) * Excludes the impact of changes in estimates made to product returns and Medicaid reserves in 2012 and 2013 1Q14 vs 1Q13 +12% growth (+7% volume, +5% price) |
|
|
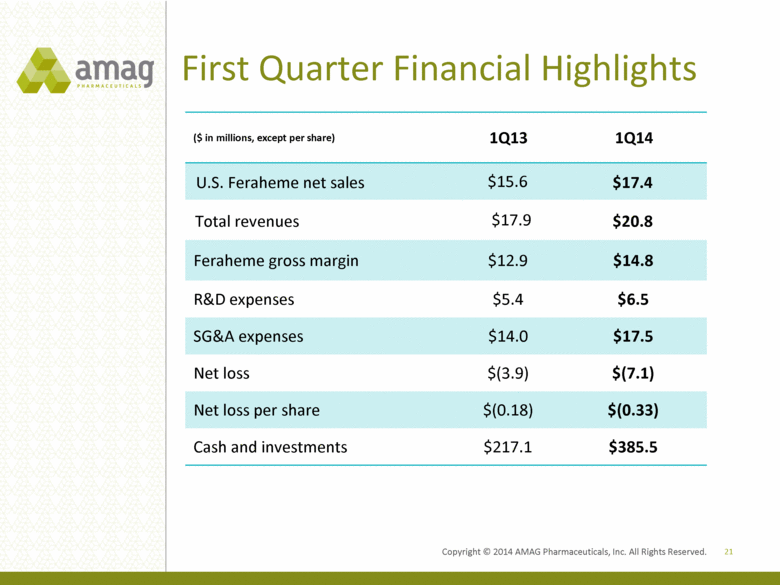
First Quarter Financial Highlights ($ in millions, except per share) 1Q13 1Q14 U.S. Feraheme net sales $15.6 $17.4 Total revenues $17.9 $20.8 Feraheme gross margin $12.9 $14.8 R&D expenses $5.4 $6.5 SG&A expenses $14.0 $17.5 Net loss $(3.9) $(7.1) Net loss per share $(0.18) $(0.33) Cash and investments $217.1 $385.5 21 Copyright 2014 AMAG Pharmaceuticals, Inc. All Right Reserved. |
|
|
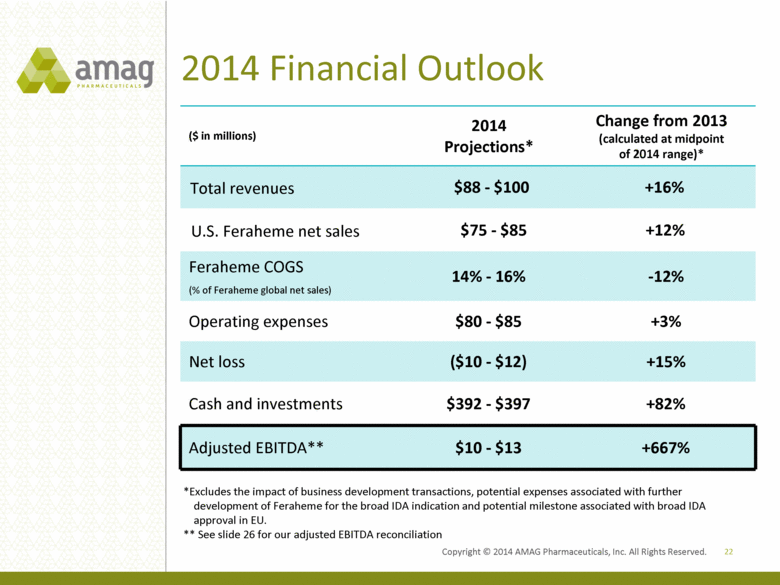
2014 Financial Outlook ($ in millions) 2014 Projections* Change from 2013 (calculated at midpoint of 2014 range)* Total revenues $88 - $100 +16% U.S. Feraheme net sales $75 - $85 +12% Feraheme COGS (% of Feraheme global net sales) 14% - 16% -12% Operating expenses $80 - $85 +3% Net loss ($10 - $12) +15% Cash and investments $392 - $397 +82% Adjusted EBITDA** $10 - $13 +667% 22 *Excludes the impact of business development transactions, potential expenses associated with further development of Feraheme for the broad IDA indication and potential milestone associated with broad IDA approval in EU. ** See slide 26 for our adjusted EBITDA reconciliation Copyright 2014 AMAG Pharmaceuticals, Inc. All Right Reserved. |
|
|
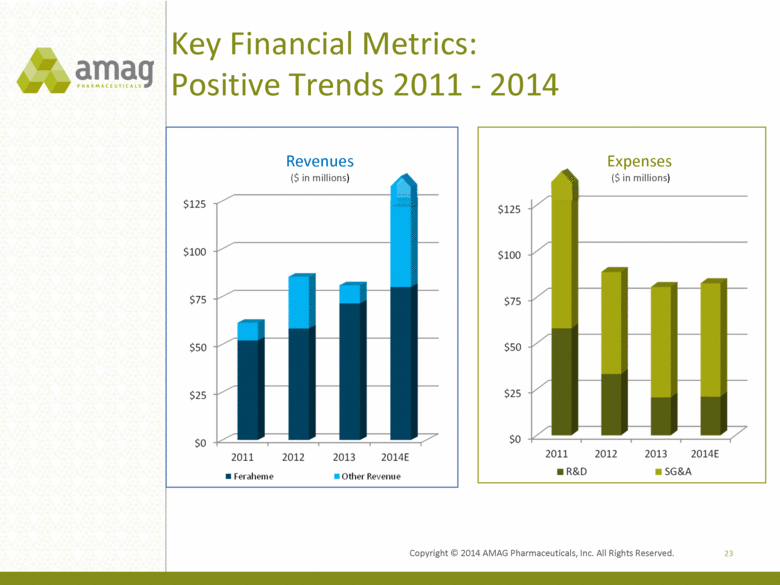
Key Financial Metrics: Positive Trends 2011 - 2014 23 Revenues ($ in millions) Expenses ($ in millions) Copyright 2014 AMAG Pharmaceuticals, Inc. All Right Reserved. |
|
|
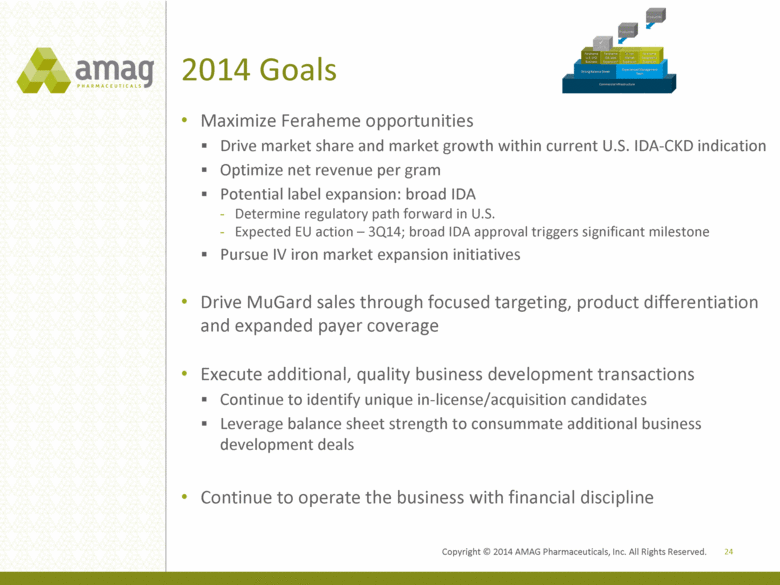
2014 Goals Maximize Feraheme opportunities Drive market share and market growth within current U.S. IDA-CKD indication Optimize net revenue per gram Potential label expansion: broad IDA Determine regulatory path forward in U.S. Expected EU action – 3Q14; broad IDA approval triggers significant milestone Pursue IV iron market expansion initiatives Drive MuGard sales through focused targeting, product differentiation and expanded payer coverage Execute additional, quality business development transactions Continue to identify unique in-license/acquisition candidates Leverage balance sheet strength to consummate additional business development deals Continue to operate the business with financial discipline 24 Copyright 2014 AMAG Pharmaceuticals, Inc. All Right Reserved. |
|
|
Well positioned for success in 2014. . . and beyond. AMAG Pharmaceuticals, Inc. 1100 Winter Street Waltham, MA 02451 617.498.3300 www.amagpharma.com A SPECIALTY PHARMACEUTICAL COMPANY DEDICATED TO BRINGING TO MARKET THERAPIES THAT IMPROVE PATIENTS’ LIVES. Copyright 2014 AMAG Pharmaceuticals, Inc. All Rights Reserved. |
|
|
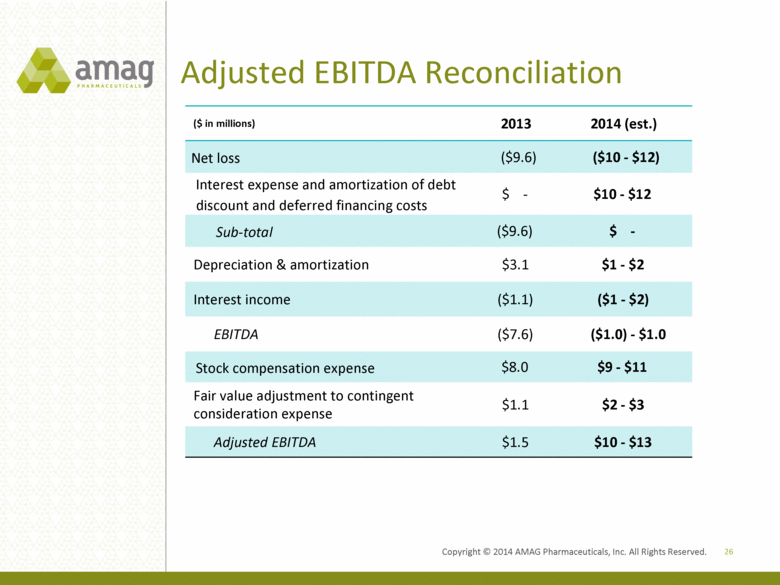
Adjusted EBITDA Reconciliation 26 ($ in millions) 2013 2014 (est.) Net loss ($9.6) ($10 - $12) Interest expense and amortization of debt discount and deferred financing costs $ - $10 - $12 Sub-total ($9.6) $ - Depreciation & amortization $3.1 $1 - $2 Interest income ($1.1) ($1 - $2) EBITDA ($7.6) ($1.0) - $1.0 Stock compensation expense $8.0 $9 - $11 Fair value adjustment to contingent consideration expense $1.1 $2 - $3 Adjusted EBITDA $1.5 $10 - $13 Copyright 2014 AMAG Pharmaceuticals, Inc. All Rights Reserved. |