Attached files
| file | filename |
|---|---|
| 8-K - 8-K - INNOVUS PHARMACEUTICALS, INC. | v375371_8k.htm |

Corporate Presentation April 21, 2014

Statements under the Private Securities Litigation Reform Act, as amended : With the exception of the historical information contained in this presentation, the matters described herein contain forward - looking statements that involve risks and uncertainties that may individually, mutually, or materially impact the matters herein described, including, but not limited to, Innovus Pharmaceuticals, Inc . ’s (the “Company”) ability to execute its business plan, obtain regulatory approval for products under development, enter into partnering agreements, realize revenue and pursue growth opportunities, some of which are outside the control of the Company . Readers and attendees are cautioned not to place undue reliance on these forward - looking statements as actual results could differ materially from the forward - looking statements contained herein . Attendees are urged to read the risk factors set forth in the Company ’ s most recent annual report on Form 10 - K, subsequent quarterly reports filed on Form 10 - Q and its most recent SEC filings . Company disclaims any intention to update this presentation . Safe - Harbor Statement 2

To be the leader in providing safe and effective non - prescription medicine and consumer care products to improve men and women’s sexual health and vitality. Innovus Pharma’s Vision 3

Innovus Pharma Unique Operating Strategy • Clinically tested with proven product efficacy • Rapid delivery to market through our current sales channels – minimal capital and R&D needed Optimize and expand underserved geographies • Commercial partnerships with established sales force targeting medical professionals • Benefit from limited product availability/competition in underserved geographies One Goal Matters • To be the leader in OTC and consumer care products to improve men and women’s sexual health and vitality • Our scoreboard is SHAREHOLDER VALUE 4 Acquire market ready proven OTC products Low cost operating structure • 2013 cash burn <700K US • Highly efficient flat management structure, with experience and proven track record

About Us • Commercial - stage, OTC pharmaceutical company based in San Diego • Emerging leader in non - prescription medicine and consumer care products for male and female sexual medicine, health and vitality • Management with proven track record in commercialization, partnering, M&A and execution • Strong commercial pipeline generating revenue from multiple regions across the world . • Four commercial products and multiple SKUs in multiple markets • Currently anticipate acquiring or licensing 3 new products in 2014 • 2014 Current Guidance : $ 1 . 5 M - 2 . 0 M in revenues 5

x Secured ~$1.7M in financing x Removed the “going concern” from our audit x Expanded management team x Added three commercial products (CIRCUMSerum ™ , Zestra® and Zestra® Glide) x Two commercial partnerships signed for a potential of up to ~$30M for CIRCUMserum™ and EjectDelay™ with Ovation Pharma x Filed for CIRCUMSerum ™ approval as an NHP in Canada x Approval of EjectDelay ™ in Canada as OTC for premature ejaculation x Acquired Semprae Labs with two revenue generating products (Zestra® and Zestra ® Glide with past ~1M US in revenue) x Over 7 0% increase in market cap from the end of 2102 to the end of 2013 Achieved Key 2013 Milestones 6

Product Route Indication Territory Status Zestra® Topical Female sexual dysfunction W orldwide Marketed US/Canada Zestra®Glide Topical Female Lubricant Worldwide Marketed US/Canada CIRCUMserum™ Topical Reduced Penile Sensitivity Ex - US Marketed EjectDelay™ Topical Premature Ejaculation Worldwide Marketed US Commercial Pipeline 7

For Female Sexual Desire/Arousal 8 • Zestra is a topical treatment for Female Sexual Interest/Arousal Disorder (Desire & Arousal) • Zestra® is the only clinically proven consumer care product with statistically significant clinical efficacy in 276 women with FSAD • Zestra® is the only clinically proven product that sold over 11 million doses so far and is currently being sold in retailer such as Walmart, and Target and through distributors such as McKesson, and Cardinal Health. • Zestra franchise is patent protected (US & Europe) until 2021 • The OTC market for Female Sexual Arousal Disorder is estimated at $400 million world wide and $200 million domestic. [1] [ 1 ] Mass Market Retailer, 8 / 08
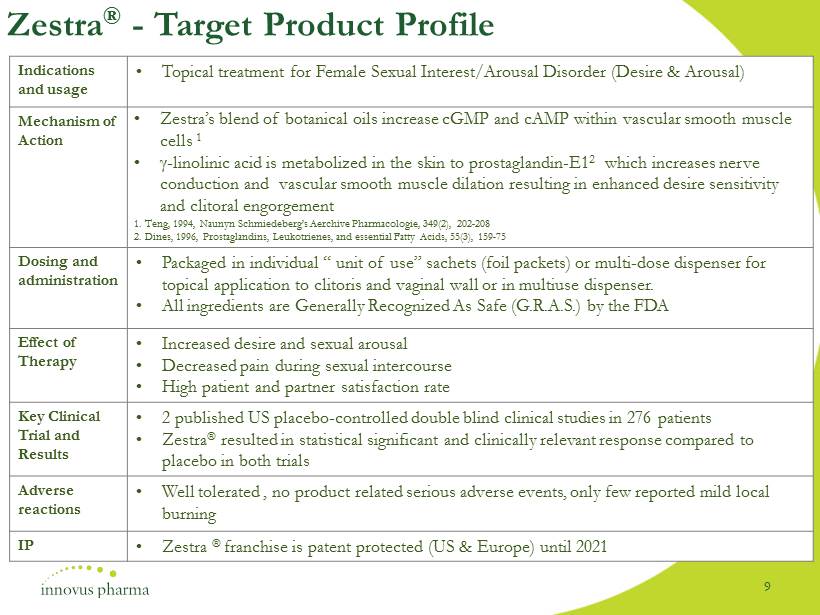
Zestra ® - Target Product Profile Indications and usage • Topical treatment for Female Sexual Interest/Arousal Disorder (Desire & Arousal) Mechanism of Action • Zestra’s blend of botanical oils increase cGMP and cAMP within vascular smooth muscle cells 1 • - linolinic acid is metabolized in the skin to prostaglandin - E1 2 which increases nerve conduction and vascular smooth muscle dilation resulting in enhanced desire sensitivity and clitoral engorgement 1. Teng, 1994, Naunyn Schmiedeberg’s Aerchive Pharmacologie, 349(2), 202 - 208 2. Dines, 1996, Prostaglandins, Leukotrienes, and essential Fatty Acids, 55(3), 159 - 75 Dosing and administration • Packaged in individual “ unit of use” sachets (foil packets) or multi - dose dispenser for topical application to clitoris and vaginal wall or in multiuse dispenser. • All ingredients are Generally Recognized As Safe (G.R.A.S.) by the FDA Effect of Therapy • Increased desire and sexual arousal • Decreased pain during sexual intercourse • High patient and partner satisfaction rate Key Clinical Trial and Results • 2 published US placebo - controlled double blind clinical studies in 276 patients • Zestra resulted in statistical significant and clinically relevant response compared to placebo in both trials Adverse reactions • Well tolerated , no product related serious adverse events, only few reported mild local burning IP • Zestra franchise is patent protected (US & Europe) until 2021 9
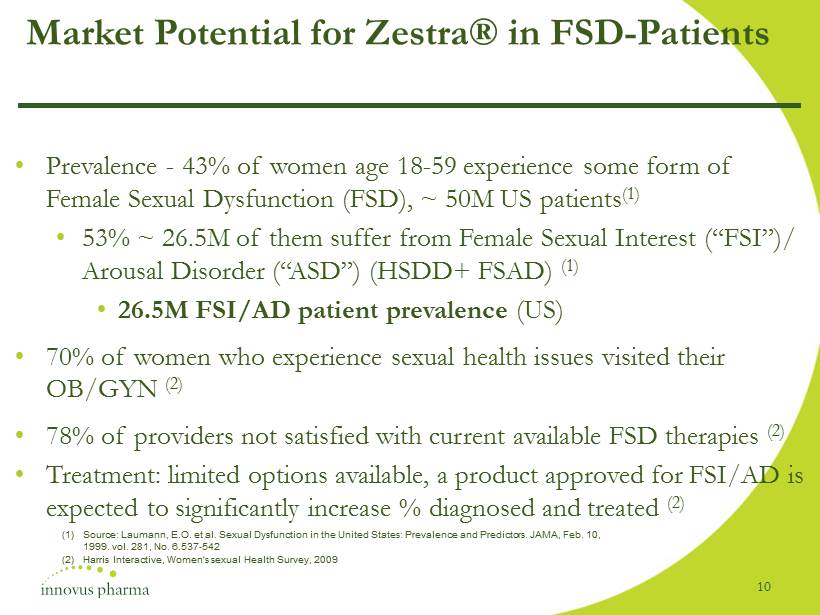
Market Potential for Zestra® in FSD - Patients • Prevalence - 43% of women age 18 - 59 experience some form of Female Sexual Dysfunction (FSD), ~ 50M US patients (1) • 53% ~ 26.5M of them suffer from Female Sexual Interest (“FSI”)/ Arousal Disorder (“ASD”) (HSDD+ FSAD) (1) • 26.5M FSI/AD patient prevalence (US) • 70% of women who experience sexual health issues visited their OB/GYN (2) • 78% of providers not satisfied with current available FSD therapies (2) • Treatment: limited options available, a product approved for FSI/AD is expected to significantly increase % diagnosed and treated (2) (1) Source: Laumann, E.O. et al. Sexual Dysfunction in the United States: Prevalence and Predictors. JAMA, Feb. 10, 1999. vol. 281, No. 6.537 - 542 (2) Harris Interactive, Women's sexual Health Survey, 2009 10

Competitive Landscape – Zestra ® vs. OTC Products • No Rx products approved in the US for FASD • Rx in development are in Phase II/III and need years of additional development if they ever succeed. • Focus on lubrication; no clinical claims for FSD - Lubricants address vaginal dryness only - No efficacy for arousal, desire or orgasm disorder • Options available in retail or online stores include: - KY Jelly® (55 - 60% of OTC market) ~$160M in sales worldwide - Astroglide® - Durex® - Lyriana® - Arginine arousal gel - Fragmented market - HerGel® - Vigel® 1 Mass Market Retailer, 8/08 OTC market Worldwide: ~$400 million US: $200 million 1 OTC Options 11
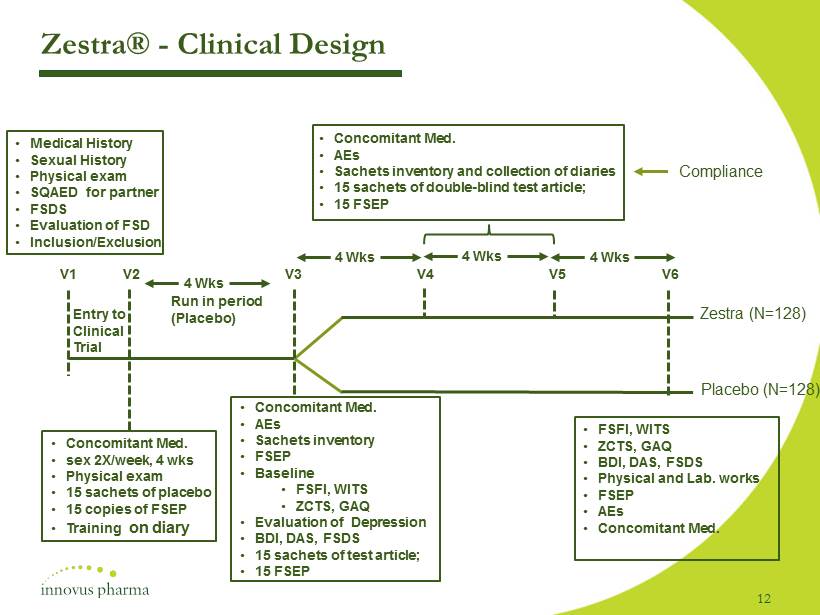
Zestra® - Clinical D esign 12 • Medical History • Sexual History • Physical exam • SQAED for partner • FSDS • Evaluation of FSD • Inclusion/Exclusion V1 V2 V3 V4 V5 V6 • Concomitant Med. • sex 2X/week, 4 wks • Physical exam • 15 sachets of placebo • 15 copies of FSEP • Training on diary Run in period (Placebo) Entry to Clinical Trial • Concomitant Med. • AEs • Sachets inventory • FSEP • Baseline • FSFI, WITS • ZCTS, GAQ • Evaluation of Depression • BDI, DAS, FSDS • 15 sachets of test article; • 15 FSEP 4 Wks 4 Wks 4 Wks 4 Wks • Concomitant Med. • AEs • Sachets inventory and collection of diaries • 15 sachets of double - blind test article; • 15 FSEP Compliance • FSFI, WITS • ZCTS, GAQ • BDI, DAS, FSDS • Physical and Lab. works • FSEP • AEs • Concomitant Med. Zestra (N=128) Placebo (N=128)

Evaluation Methods • Primary endpoints: • FSFI scores • FSEP scores as recorded in a diary • Secondary endpoints: • T reatment satisfaction questionnaire (WITS ) • C onsumer testing survey (ZCTS) • T wo GAQs • Q1: If Sexual Satisfaction improved • Q2: If Number of successful encounters increased • FSDS, BDI and DAS • S exual encounter frequency • D ropout rates • Safety : Monitoring adverse events (AEs) 13
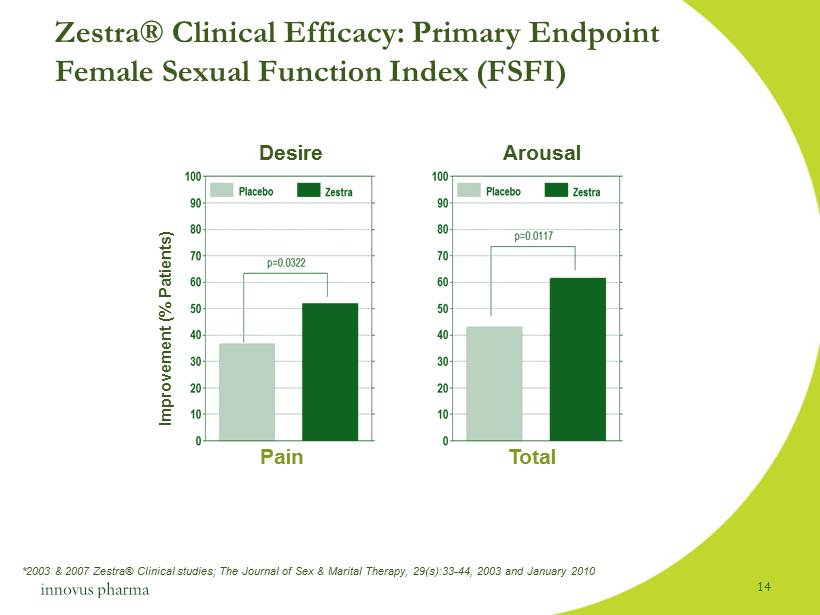
Zestra® Clinical Efficacy : Primary Endpoint Female Sexual Function Index (FSFI) Improvement (% Patients) Desire Arousal Pain Total *2003 & 2007 Zestra® Clinical studies; The Journal of Sex & Marital Therapy, 29(s):33 - 44, 2003 and January 2010 14
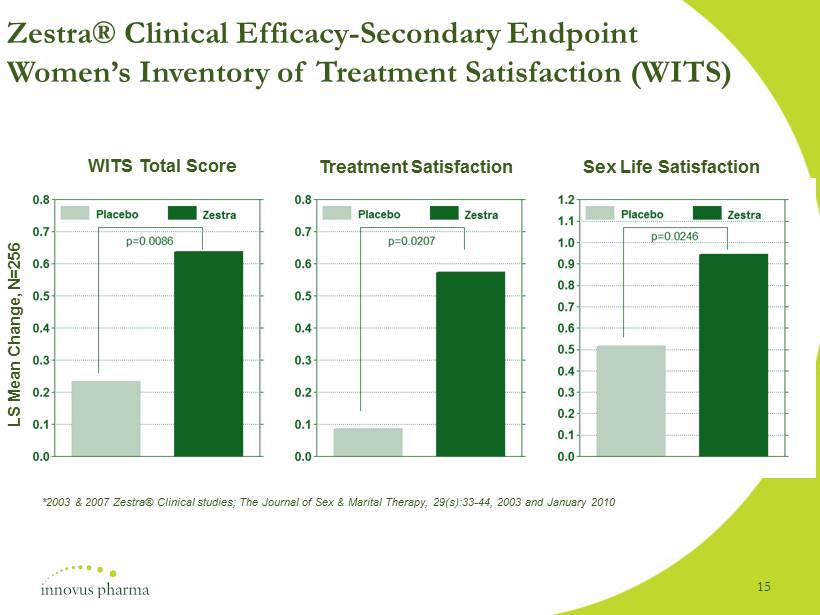
Zestra ® Clinical Efficacy - Secondary Endpoint Women’s Inventory of Treatment Satisfaction (WITS) Treatment Satisfaction WITS Total Score Sex Life Satisfaction LS Mean Change, N=256 *2003 & 2007 Zestra® Clinical studies; The Journal of Sex & Marital Therapy, 29(s):33 - 44, 2003 and January 2010 15

Zestra® Safety Profile 16

Zestra® - Commercial Zestra® and Zestra® Glide® are available in the US and Canada including: US Retailers such as: Canadian Retailers such as: • Walmart Loblaws • Target Shoppers Drug Mart • K - Mart Rexall • Drugstore.com Distributors: • Cardinal Health • McKesson Drug Company • HD Smith • Drug Emporium 17

EjectDelay ™ For Premature Ejaculation • EjectDelay™ is an OTC gel (Benzocaine 7 . 5 % ) indicated for the treatment of premature ejaculation . • EjectDelay ™ is supported by a clinical study which improved the intra - vaginal ejaculation latency time by 4 . 6 minutes when compared to placebo treatment . • Products with Benzocaine 7 . 5 % for the treatment of premature ejaculation have superior clinical efficacy than other over the counter products, including Lidocaine, for the treatment of (Premature Ejaculation) PE . • PE is the most common form of male sexual dysfunction, affecting 30 % of men . [ 1 ] 18 [1] J Urol, suppl.,2008;179:340, abstract 988
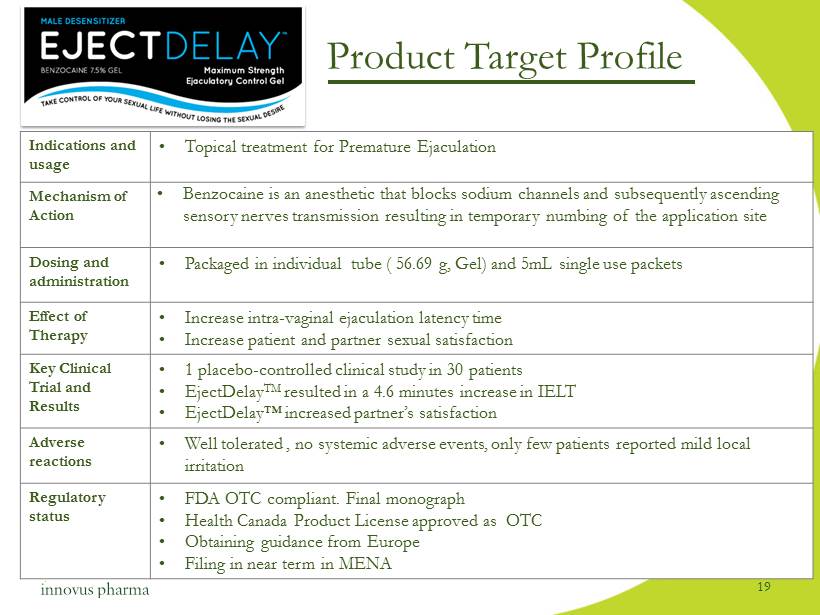
Product Target Profile Indications and usage • Topical treatment for Premature Ejaculation Mechanism of Action • Benzocaine is an anesthetic that blocks sodium channels and subsequently ascending sensory nerves transmission resulting in temporary numbing of the application site Dosing and administration • Packaged in individual tube ( 56.69 g, Gel) and 5mL single use packets Effect of Therapy • Increase intra - vaginal ejaculation latency time • Increase patient and partner sexual satisfaction Key Clinical Trial and Results • 1 placebo - controlled clinical study in 30 patients • EjectDelay TM resulted in a 4.6 minutes increase in IELT • EjectDelay™ increased partner’s satisfaction Adverse reactions • Well tolerated , no systemic adverse events, only few patients reported mild local irritation Regulatory status • FDA OTC compliant. Final monograph • Health Canada Product License approved as OTC • Obtaining guidance from Europe • Filing in near term in MENA 19

Premature Ejaculation ( PE ) DEFINITION: 1. The absence of voluntary control over ejaculation resulting in ejaculation either preceding vaginal entry or occurring immediately upon vaginal entry . 2. Intra - Vaginal Ejaculation Latency Time < 1 minute as compared to ~4 - 5min for non PE population [1] MARKET SIZE: 1. Premature ejaculation is the most common form of male sexual dysfunction, affecting 30% of men [2] 1. J Sex Med, 2005;2:358 - 367 2. J Urol, suppl., 2008; 179: 340, abstract 988 20
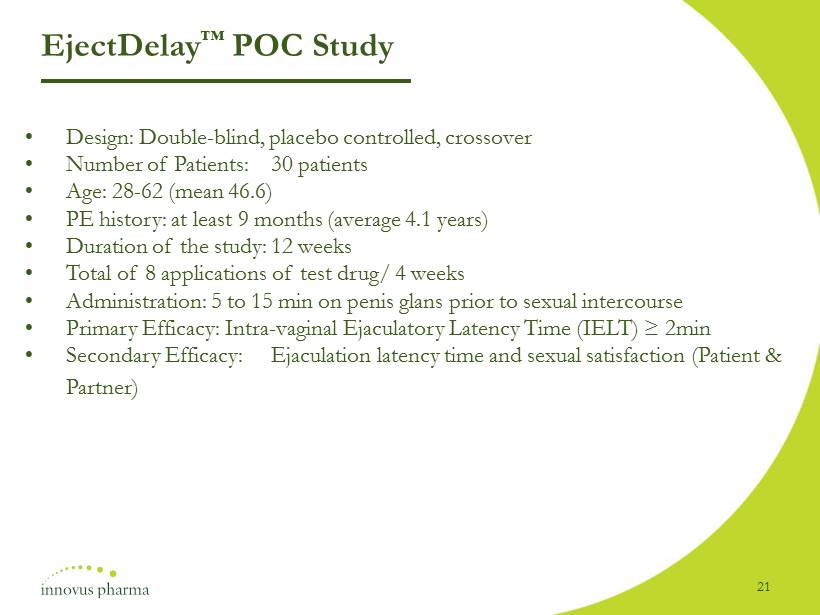
EjectDelay ™ POC Study • Design: Double - blind , placebo controlled, crossover • Number of Patients: 30 patients • Age: 28 - 62 (mean 46.6) • PE history: at least 9 months (average 4.1 years) • Duration of the study: 12 weeks • Total of 8 applications of test drug/ 4 weeks • Administration: 5 to 15 min on penis glans prior to sexual intercourse • Primary Efficacy: Intra - vaginal Ejaculatory Latency Time (IELT) ≥ 2min • Secondary Efficacy: Ejaculation latency time and sexual satisfaction (Patient & Partner) 21

EjectDelay ™ - Clinical Design • Medical History • Sexual History • Physical exam • Inclusion/Exclusion • Training on application of test drug • Training on Patient diaries • Sex 1X/week • Hand remaining test tubes • Physical exam • Collection of Patient Diary questionnaire • Collection of Patient’s adverse events questionnaire Treatment period Follow up period (Week 2 & Week 4) EjectDelay™ 8 applications Lidocaine 8% 8 applications Placebo 8 applications (N=30) (4 Weeks) (4 Weeks) (4 Weeks) 22

EjectDelay™: Clinical Efficacy - I - ELT Benzocaine 7.5% achieved the highest primary and secondary endpoint efficacy 23

EjectDelay™: Clinical Efficacy - I - ELT 1 . McMahon CG. Dapoxetine : a new option in the medical management of premature ejaculation. Ther Adv Urol. 2012 October; 4(5): 233 - 251 2. Culley C. PSD 502: A second Phase III, randomized, double - blind, placebo - controlled study in premature ejaculation (PE) patients in the US and Europe , AUA 2010, Abstract# 1493 24
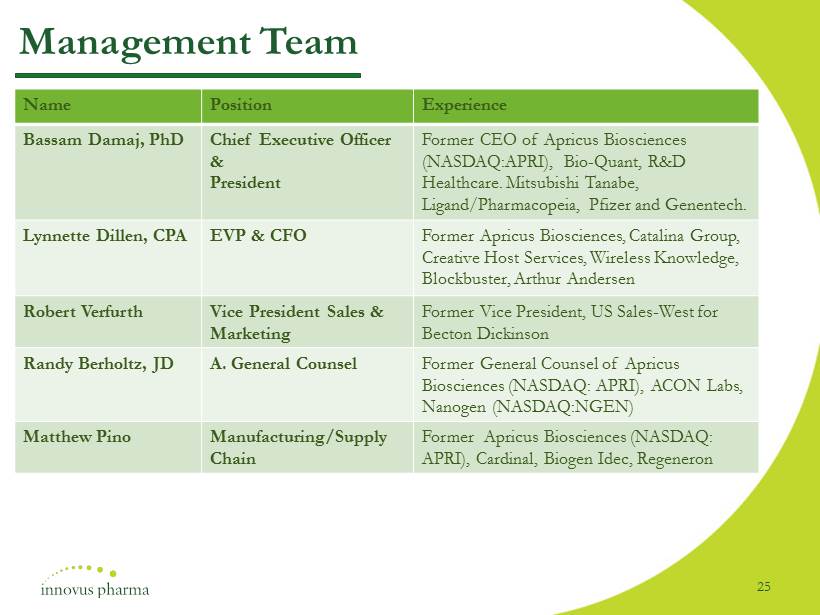
Management Team Name Position Experience Bassam Damaj, PhD Chief Executive Officer & President Former CEO of Apricus Biosciences (NASDAQ:APRI), Bio - Quant, R&D Healthcare. Mitsubishi Tanabe, Ligand/Pharmacopeia, Pfizer and Genentech. Lynnette Dillen, CPA EVP & CFO Former Apricus Biosciences, Catalina Group, Creative Host Services, Wireless Knowledge, Blockbuster, Arthur Andersen Robert Verfurth Vice President Sales & Marketing Former Vice President, US Sales - West for Becton Dickinson Randy Berholtz, JD A. General Counsel Former General Counsel of Apricus Biosciences (NASDAQ: APRI), ACON Labs, Nanogen (NASDAQ:NGEN) Matthew Pino Manufacturing/Supply Chain Former Apricus Biosciences (NASDAQ: APRI), Cardinal, Biogen Idec, Regeneron 25
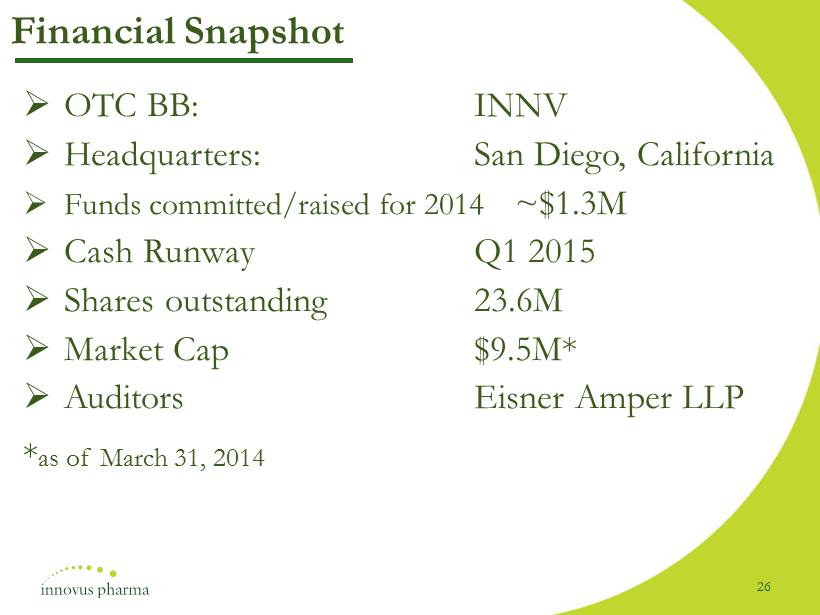
» OTC BB : INNV » Headquarters : San Diego, California » Funds committed/raised for 2014 ~ $ 1 . 3 M » Cash Runway Q 1 2015 » Shares outstanding 23 . 6 M » Market Cap $ 9 . 5 M* » Auditors Eisner Amper LLP * as of March 31 , 2014 Financial Snapshot 26

Why Invest in INNV ? • Multiple Near - Term Milestones » Four commercial products (Zestra®, Zestra® Glide, EjectDelay™ & CIRCUMSerum™) » Introduction of additional products through acquisition » Announcing multiple commercial partnerships in 2014 worldwide » Launch of EjectDelay™ in Canada in 2014 » Expansion of Zestra® US Retailers throughout 2014 » Zestra® expansion of market share Canada via P harma partners. » Exiting 2014 operationally cash flow positive • Unique Business Strategy and Niche Market • E xperienced and proven management 27

Contact Bassam Damaj, Ph.D. President & CEO or Lynnette Dillen, CPA EVP & CFO +1 858 964 - 5123 bdamaj@innovuspharma.com ldillen@innovuspharma.com 28
