Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - MEDICAN ENTERPRISES, INC. | Financial_Report.xls |
| EX-10.1 - EXHIBIT 10.1 - MEDICAN ENTERPRISES, INC. | ex101.htm |
| EX-31.2 - EXHIBIT 31.2 - MEDICAN ENTERPRISES, INC. | ex312.htm |
| EX-21.1 - EXHIBIT 21.1 - MEDICAN ENTERPRISES, INC. | ex211.htm |
| EX-32 - EXHIBIT 32 - MEDICAN ENTERPRISES, INC. | ex32.htm |
| EX-3.3 - EXHIBIT 3.3 - MEDICAN ENTERPRISES, INC. | ex33.htm |
| EX-31.1 - EXHIBIT 31.1 - MEDICAN ENTERPRISES, INC. | ex311.htm |
| EX-10.2 - EXHIBIT 10.2 - MEDICAN ENTERPRISES, INC. | ex102.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
|
[X]
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the fiscal year ended December 31, 2013
|
[ ]
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from to
MEDICAN ENTERPRISES, INC.
(Exact name of registrant as specified in charter)
Nevada
(State or other jurisdiction of incorporation)
|
000-53408
|
87-0474017
|
|
|
(Commission File Number)
|
(IRS Employer Identification No.)
|
|
5955 Edmond Street, Suite 102
Las Vegas, NV 89118
|
||
|
(Address of Principal Executive Offices)
|
||
|
(800) 416-8802
(Registrant’s Telephone Number, Including Area Code)
|
||
Securities Registered Pursuant to Section 12(b) of the Act: None
Securities Registered Pursuant to Section 12(g) of the Act: Common Stock, par value $0.001
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [X]
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes [ ] No [X]
Indicate by check mark if the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
(1) Yes [X] No [ ] (2) Yes [X] No [ ]
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). Yes [X] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company:
|
Large accelerated filer
|
[ ]
|
Accelerated filer
|
[ ]
|
|
Non-accelerated filer
|
[ ]
|
Smaller reporting company
|
[X]
|
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes [ ] No [X]
The aggregate estimated market value was determined by multiplying the approximate number of shares of common stock held by non-affiliates by the average bid price of such stock $0.0105 on the last day of the Company’s second fiscal quarter, as quoted on the Over-the-Counter Bulletin Board (the “OTCBB”) of the Financial Industry Regulatory Authority (“FINRA”). There were 1,325,062 (pre-forward split) shares of common voting stock held by non-affiliates, valued in the aggregate at $13,913.
Outstanding Shares
As of April 8, 2014, the Registrant had 40,321,664 shares of common stock outstanding.
Documents Incorporated by Reference
See Part IV, Item 15.
TABLE OF CONTENTS
|
Page
|
|
|
CAUTIONARY STATEMENT REGARDING FORWARD LOOKING STATEMENTS
|
ii |
|
PART I
|
1 |
|
ITEM 1. BUSINESS
|
1 |
|
ITEM 1A. RISK FACTORS
|
18
|
|
ITEM 1B. UNRESOLVED STAFF COMMENTS
|
27
|
|
ITEM 2: PROPERTIES
|
27
|
|
ITEM 3: LEGAL PROCEEDINGS
|
27
|
|
ITEM 4: MINE SAFETY DISCLOSURES
|
27 |
|
PART II
|
|
|
ITEM 5: MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
28
|
|
ITEM 6: SELECTED FINANCIAL DATA
|
30
|
|
ITEM 7: MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
30
|
|
ITEM 7A: QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
31
|
|
ITEM 8: FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
32
|
|
ITEM 9A: CONTROLS AND PROCEDURES
|
45
|
|
ITEM 9B: OTHER INFORMATION
|
45
|
|
PART III
|
46 |
|
ITEM 10: DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
46
|
|
ITEM 11: EXECUTIVE COMPENSATION
|
49 |
|
ITEM 12: SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
49 |
|
ITEM 13: CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
51 |
|
ITEM 14: PRINCIPAL ACCOUNTING FEES AND SERVICES
|
52 |
|
PART IV
|
52 |
|
ITEM 15: EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
52 |
i
CAUTIONARY STATEMENT REGARDING FORWARD LOOKING STATEMENTS
This Annual Report and the documents we have filed with the United States Securities and Exchange Commission (the “SEC”) that are incorporated by reference herein contain forward-looking statements, within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that involve significant risks and uncertainties. Any statements contained, or incorporated by reference, in this Annual Report that are not statements of historical fact may be forward-looking statements. When we use the words “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “will” and other similar terms and phrases, including references to assumptions, we are identifying forward-looking statements. Forward-looking statements involve significant risks and uncertainties which may cause our actual results, performance or achievements to be materially different from those expressed or implied by forward-looking statements.
A variety of factors, some of which are outside our control, may cause our operating results to fluctuate significantly. They include, but are not limited to:
|
|
•
|
the new and evolving regulatory requirements in Canada regarding the production and distribution of medical marijuana;
|
|
|
•
|
our ability to secure and maintain our relationships with our key commercial partners;;
|
|
|
•
|
our expectations regarding the commercial market for our products;
|
|
|
•
|
the effect of competition; and
|
|
|
•
|
the availability of required additional financing.
|
As more fully described in this Annual Report under the heading “Risk Factors,” many important factors may affect our ability to achieve our stated objectives and to manufacture and commercialize our products, including, among other things, our ability to:
|
|
•
|
obtain substantial additional funds to commence and expand our planned operations;
|
|
|
•
|
obtain and maintain all necessary regulatory approvals and licenses and to operate our business in compliance with the same;
|
|
|
•
|
meet obligations and required milestones under agreements;
|
|
|
•
|
retain key executives, and attract, retain and motivate qualified personnel;
|
|
|
•
|
be capable of manufacturing and distributing our product in commercial quantities at reasonable costs; and
|
|
|
•
|
compete against others in a profitable manner.
|
Moreover, new risks regularly emerge and it is not possible for our management to predict all risks, nor can we assess the impact of all risks on our business or the extent to which any risk, or combination of risks, may cause actual results to differ from those contained in any forward-looking statements. All forward-looking statements included in this Annual Report are based on information available to us on the date hereof. Except to the extent required by applicable laws or rules, we undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained throughout this Annual Report and the documents we have filed with the SEC.
ii
PART I
ITEM 1. BUSINESS
Medican Enterprises, Inc. was organized under the laws of the State of Nevada on October 27, 1988, under the name “Extant Investments, Inc.”
As described below, we are a pharmaceutical company developing a business of producing and distributing medical marijuana in Canada as authorized by Health Canada. Other businesses have been operated through our company in the past. The following are the material organizational business developments related to our company since inception:
Corporate History (1990-2012)
Commencing on or about December 5, 1990, pursuant to a Registration Statement on Form S-18 filed with the SEC and Exchange Commission and a prospectus dated as of such date, our company offered and sold units consisting of common stock and warrants that was closed on January 31, 1991.
Effective May 17, 1991, we acquired all of the issued and outstanding shares of common stock of Sentinel Diagnostics, Inc., an Arizona corporation, pursuant to an Agreement and Plan of Reorganization, and changed our name to “Sentinel Scientific, Inc.” The Sentinel Diagnostics business operations were discontinued in late 1992 for lack of funding.
Effective August 10, 1993, and pursuant to a Reorganization Agreement, we acquired all of the outstanding shares of common stock of A.F.C. Entertainment, Inc., a corporation organized under The Companies Act of Barbados, and changed our name to “TC X Calibur, Inc.”
Effective December 31, 1993, we acquired all of the outstanding shares of common stock of Film Opticals Investments, Limited, a corporation organized under the laws of the Province of Ontario, Canada (“Film Opticals”).
In 1993, Film Opticals of Canada Limited, a wholly-owned subsidiary of Film Opticals (“Film Opticals of Canada”), sought court protection by filing a Notice of Intention to Make a Proposal pursuant to Subsection 50.4(1) of the Bankruptcy and Insolvency Act of Canada, because of a dispute with a creditor pursuant to a secured promissory note, and a trustee (the “Trustee”) was appointed to oversee Film Opticals of Canada’s financial management in the Ontario Justice Court, General Division, Case No. B163/94. Operation of the Film Opticals business continued pending a resolution of the dispute with this creditor.
On or about December 28, 2004, pursuant to resolutions adopted by our Board of Directors and approved by the holders of a majority our outstanding shares of common stock, we sold substantially all of our assets by the conveyance of our wholly-owned subsidiary, Film Opticals (and its subsidiary, Film Opticals of Canada), and our film library (“Film Library”), to Film Opticals of Canada 2004 Limited, a newly formed corporation organized under the Province of Ontario, Canada (“New Film Opticals”), and a wholly-owned subsidiary of Berliner Holdings, Inc. (“Berliner”). Berliner was wholly-owned by our then President, Claus Voellmecke. As consideration of the purchase and sale of these assets, Berliner agreed to cancel 500,000 shares of our common stock that it owned and agreed, together with New Film Opticals, to assume, pay and/or compromise all of our outstanding claims or liabilities related to Film Opticals and our Film Library and indemnify and hold us harmless from them. For additional information, please see our 8-K Current Report, our 8-K/A-1 Current Report and our 8-K/A-2 Current Report that have been previously filed with the Securities and Exchange Commission on or about December 8, 2004, December 9, 2004 and January 4, 2005, respectively, and which include a copy of the Definitive Proxy Statement that was mailed to our stockholders on or about December 8, 2004.
In addition to the above referenced sale of substantially all of our assets on or about December 28, 2004, pursuant to resolutions adopted by our Board of Directors and approved by the holders of a majority of our outstanding shares of common stock, we amended our Articles of Incorporation to change our capitalization to add a class of preferred stock, and gave our Board of Directors authority to effect recapitalizations and/or name changes without further stockholder approval.
1
On or about September 15, 2005, certain shares of our outstanding common stock were acquired pursuant to a private transactions which shares, when accumulated with shares of our common stock that were already owned by these persons, represented a controlling interest in us.
On November 2, 2007, we announced the execution of a Letter of Intent to acquire EV Rental, LLC, a California limited liability company. On March 3, 2008, we terminated our obligations under the Letter of Intent.
On December 28, 2007, in the OTCBB trading market on that date, we effected a forward split of our outstanding common stock on a basis of 2.3943 for one, while retaining the current par value of $0.001 per share, with all fractional shares rounded up to the nearest whole share, and with appropriate adjustments in our capital accounts. All share references and computations herein take into account this recapitalization.
On February 28, 2008, we filed Restated Articles of Incorporation with the State of Nevada, a copy of which is incorporated herein by reference. See Part IV, Item 15.
On September 11, 2008, we filed a registration statement on Form 10 of the SEC registering our common stock under Section 12(g) of the Exchange Act. This registration statement became effective on or about November 9, 2008.
There were no material business developments in 2009.
On November 29, 2010, in the OTCBB trading market on that date, we effected a reverse split of our outstanding common stock on a basis of one for four, while retaining the current par value of $0.001 per share, with all fractional shares rounded up to the nearest whole share, and with appropriate adjustments in our capital accounts. All share references and computations herein take into account this recapitalization.
There were no material business developments in 2011 or 2012.
Recent Developments
On June 25, 2013, Kenneth Williams, currently our Chief Executive Officer and a director of our company, entered into a series of Stock Purchase Agreements (the “SPAs”) with Jenson Services and several other shareholders through which Mr. Williams collectively purchased 858,946 shares of our company, representing approximately 65% of our outstanding common stock for total consideration of $96,501. At the closing of the SPAs, Mr. Williams became the holder of an aggregate of approximately 65% of our outstanding shares of common stock, and a change of control of our company occurred.
On that same date, Travis T. Jenson resigned as the Principal Executive Officer and as a director of our company and Jason Jenson resigned as the Treasurer and Secretary of the Company. Neither individual has any dispute against our company, and their resignations were a part of the transaction by which Mr. Williams acquired control of our company. At this time, we also increased the number of members of the Board of Directors from three to four, which was within the range of directors allowed by its Bylaws. Michael Thompson was appointed as a director of our company and Mr. Williams was appointed as a director and the Chief Executive Officer and Chief Financial Officer of our company. On July 19, 2013, Jason Jenson resigned as a director of our company. On July 22, 2013, Harold Jensen resigned as a director of our company. These resignations were not as a result of any dispute with our company and as also a part of the transaction by which Mr. Williams acquired control of our company.
As approved by our Board of Directors and majority shareholder on July 2, 2013, and subsequently announced to the shareholders via mailing, we filed Articles of Amendment to our Articles of Incorporation on August 6, 2013 changing our name to MEDICAN ENTERPRISES, INC. This name change became effective with FINRA, September 18, 2013, and our stock symbol was changed to “MDCN”.
2
On August 16, 2013, our board of directors approved a stock dividend, on a basis of 20:1 (the “Stock Dividend”), pursuant to which, our shareholders as at September 24, 2013 received twenty (20) shares of our common stock for each one (1) share of common stock held. The pay-out date as approved by our board of directors and FINRA was September 25, 2013.
Upon completion of the Stock Dividend, our issued and outstanding shares increased from 1,325,062 to 26,501,240 shares of common stock.
In the fourth quarter of 2013, we formed two subsidiaries in anticipation of our emerging Canadian medical marijuana business: Medican Systems, Inc., a Yukon corporation (“Medican Systems”), and Medican (Delta) Systems, Inc., a British Columbia corporation (“Medican Delta”). At that time, we were contemplating a business model based on providing information about and within the medical cannabis industry through publications and through forging strategic relationships with individuals and related businesses within the industry. We have since modified our business plan to be a holding company for a business engaged in the licensed production and distribution of medical marijuana in Canada. We formed a third subsidiary, CanaLeaf Systems, Inc., a corporation incorporated under laws of Canada under the Canada Business Corporation Act (“CanaLeaf”) on March 25, 2014. through which we will pursue our current business plan.
On November 15, 2013, Medican Delta entered into a management services agreement (the “Management Services Agreement”) with IHL Medical Marijuana Ltd., an affiliate of International Herbs Ltd. (“IHL”) and LFG Advisory Ltd. and LFG Advisory & Accounting Ltd. (LFG Advisory Ltd. and LFG Advisory & Accounting Ltd., collectively, “LFG”). Under the terms of the Management Services Agreement, IHL and LFG agree to promote and develop the business of Medican Delta and assist Medican Delta with obtaining licensed medical marijuana producer status from Health Canada, the department of the federal Canadian government which oversees public health matters. IHL is a world leader in the fresh herbs and specialty produce industry, and has assisted numerous organizations in meeting their strategic goals with the Canadian government. LFG is an independent corporate finance and accounting advisory firm, based in Vancouver, British Columbia.
On January 21, 2014, we appointed a new director, Gary Johnson.
On February 18, 2014, we appointed a new Chief Financial Officer, Wayne Hansen.
On March 13, 2014, we and our subsidiaries Medican Systems and CanaLeaf (referred to collectively herein as “Medican CanaLeaf”) entered into an Amended and Restated Management Services Agreement (the “Amended Management Services Agreement”) with International Herbs (BC) LTD., an affiliate company of IHL (“IHLBC”) and LFG. Under the terms of the Amended Management Services Agreement, we modified, clarified and restated the terms of the original Management Services Agreement entered into on November 15, 2013 among the parties to provide for updated timelines and payment schedules. According to the Amended Management Services Agreement, the parties agree to cooperate and work together to promote and develop the business of Medican CanaLeaf and to assist Medican CanaLeaf in partnering with International Herbs Medical Marijuana Ltd. (“IHMML”), also an affiliate of IHL and a company that is applying to obtain licensed producer status from Health Canada. The Amended Management Services Agreement amends the dates of the three phases of management services provided or to be provided by IHL and LFG. Phase I was deemed completed on February 24, 2014 and consisted of consulting services and the formulation of a business strategy. All fees due under Phase I have been paid in full to IHL and LFG. Phase II of the Amended Management Services Agreement is defined as the period between February 25, 2014 and March 28, 2014 (or such other date as the parties may mutually agree). During Phase II, LFG agreed to provide Medican CanaLeaf with consulting services in order to successfully acquire 50% ownership in IHMML and develop its business infrastructure. IHL agreed to cooperate with Medican CanaLeaf to design, build and develop a facility in Canada in which it will lawfully produce and from which it will lawfully distribute marijuana (which we anticipate will be our Atholville facility as described below, the “Facility”), to hire employees as deemed necessary for the operation of the Facility and all business conducted at and in relation to the Facility, to develop and implement systems and processes to optimize the production and profitability of all business conducted at and in relation to the Facility, to ensure compliance with and adherence to all applicable laws and regulations, and to prepare the Facility for a final site visit and inspection by the government and regulatory personnel. Upon completion of Phase II, Medican CanaLeaf will pay to IHL a fee of CAD$500,000 and will pay LFG a fee in the amount of CAD$100,000. The third phase of the Amended Management Services Agreement commences after the completion of Phase II and runs until a date of completion to be agreed upon by the parties (“Phase III”). During Phase III, LFG will provide Medican CanaLeaf with consulting services in relation to corporate finance work required by CanaLeaf, internal accounting services and assistance with the development of business infrastructure. IHL agrees to assist Medican CanaLeaf with the development of a strategic plan on an as-needed basis. Medican CanaLeaf agrees to pay LFG CAD$17,500 per month plus applicable taxes for the duration of Phase III. In addition to the payments mentioned above, during Phase One and Phase Two, Medican CanaLeaf agrees to pay LFG an amount equal to two percent (2%) of all money procured from investors, and the Company shall issue shares of its common stock as equity compensation to IHL and LFG or its nominees totaling 5,000,000 shares of issued and outstanding common stock of our company.
3
In early March 2014, our Board of Directors and majority stockholder approved an increase of our authorized shares of common stock from 50,000,000 to 100,000,000 shares.
On March 24, 2014, Medican Systems entered into a nonbinding Letter Agreement with IHMML that described the terms through which Medican would, subject to entering into definitive documentation and other conditions, become a 50% holder of the issued and outstanding common shares of IHMML by way of subscription for 41,600,000 common shares of IHMML (the “Investment Shares”) for an aggregate subscription price of CAD$52,000,000 (the “Investment Proceeds”). IHMML will then use the Investment Proceeds to acquire certain lands, retrofit and/or build a marijuana growing operation and attend to the growing, marketing, research and development, training, distribution and retail sale of medical marijuana in Canada as regulated by Health Canada.
On April 8, 2014 CanaLeaf entered into a Subscription Agreement with IHMML through which CanaLeaf has agreed to purchase from IMML, by way of subscription, 41,600,000 common shares of IHMML (the “Investment Shares”) for an aggregate subscription price of CAD$52,000,000 (the “Investment Proceeds”). IHMML will then use the Investment Proceeds to acquire certain lands, retrofit and/or build a marijuana growing operation Facility and attend to the growing, marketing, research and development, training, distribution and retail sale of medical marijuana in Canada as regulated by Health Canada.
Pursuant to the Subscription Agreement, we (as CanaLeaf’s parent company) shall issue 6,000,000 common share purchase warrants to IHMML’s shareholders or shareholders’ designee exercisable within two years of the date of the Subscription Agreement at an exercise price of CAD$2.86 per share.
In the Subscription Agreement, CanaLeaf subscribes for the Investment Shares at a subscription price of CAD$1.25 per share, for an aggregate subscription price equal to the Investment Proceeds. The subscription for the initial Investment Shares shall be completed on or about April 30, 2014, provided that the Investment Proceeds shall be advanced pursuant to the following investment schedule:
|
(a)
|
4,000,000 Shares will be purchased on April 30, 2014 in consideration for payment of CAD$5,000,000;
|
|
(b)
|
An additional 8,000,000 Shares will be purchased on May 31, 2014 in consideration for payment of CAD$10,000,000;
|
|
(c)
|
An additional 4,800,000 Shares will be purchased on June 30, 2014 in consideration for payment of CAD$6,000,000;
|
|
(d)
|
An additional 8,800,000 Shares will be purchased on July 31, 2014 in consideration for payment of CAD$11,000,000;
|
|
(e)
|
An additional 2,400,000 Shares will be purchased on August 31, 2014 in consideration for payment of CAD$3,000,000;
|
4
|
(f)
|
An additional 8,000,000 Shares will be purchased on September 30, 2014 in consideration for payment of CAD$10,000,000; and
|
|
(g)
|
An additional 5,600,000 Shares will be purchased on October 31, 2014 in consideration for payment of CAD$7,000,000.
|
IHMML is expected to use the Investment Proceeds to acquire certain lands, retrofit a marijuana growing operation and support the business plans of IHMML, namely, the licensed growing, marketing, research and development, training distribution and retail sale of medical marijuana in Canada as regulated by Health Canada. IHMML will enter, or will cause LandCo (as defined below) to enter, into contracts of purchase and sale for the purchase of the following buildings and adjoining lands: (a) a building in Atholville, New Brunswick with 300,000 square foot of grow space within a 393,000 square foot facility (the “Atholville Facility”); (b) a 273,000 square foot facility, in Poekmouche, New Brunswick (the “Poekmouche Facility”), and (c) a property with a 25,000 square foot facility in Delta, British Columbia (the “Delta Facility”). Subject to CanaLeaf completing its purchase of all of the Shares that it has agreed to purchase pursuant to the Subscription Agreement, IHMML agrees to use all reasonable commercial efforts to complete or cause LandCo to complete the purchase of the Atholville Facility, the Poekmouche Facility and the Delta Facility (collectively, the “Investment Property”). It is contemplated that the Investment Property will be acquired in a separate company or companies from IHMML (collectively, “LandCo”). IHMML agrees to structure the purchase of the Investment Property such that either: (a) IHMML will have a direct or indirect 100% interest in the Investment Property; or (b) the Shareholders will have a direct or indirect 100% interest in the Investment Property, in the same proportions as their Share ownership interest in IHMML, assuming the full subscription for Shares by CanaLeaf. To the extent required, the parties will enter into a shareholders’ agreement governing the terms of ownership of the shares of LandCo, the terms of which will be substantially the same as the terms of the Shareholders’ Agreement negotiated by the parties and to be signed on or before April 30, 2013. If LandCo acquires the Investment Property, then LandCo will lease such lands to IHMML at reasonable rental rate agreed between LandCo and IHMML.
In addition to the foregoing terms, the parties also agree in the Subscription Agreement to:
|
(a)
|
ensure that the IHMML’s current shareholders (who consist of Rick Brar, Monty Sikka, Mark Catroppa, Kevin Coft, Mansimrat Kaur Brar) have the ability to nominate two individuals to the board of directors of the our company (of which all nominations are subject to the approval of our shareholders);
|
|
(b)
|
ensure that the shareholders of IHMML execute consulting agreements and non-competition agreements that limit the shareholders’ involvement with any companies that compete with IHMML as long as the shareholders’ are involved with IHMML and for two years after their
involvement ceases; and
|
|
(c)
|
ensure that the Company remains compliant in its reporting obligations as a public company.
|
In conjunction with the Subscription Agreement, the parties have negotiated a Shareholders’ Agreement with CanaLeaf, Medican Systems., Zenabis Limited Partnership (the shareholder of 41,600,000 common shares of IHMML), and IHMML to be signed on or before April 30, 2014 upon delivery of the first $5,000,000 payment. The Shareholders’ Agreement sets forth certain material terms of the shareholders of our company following CanaLeaf’s purchase of shares in the Subscription Agreement. The Shareholders’ Agreement sets for the terms of corporate governance of IHMML, our company, and CanaLeaf, rights of the parties thereto to purchase and sell interests of IHMML, and other similar provisions standard for this type of agreement.
The Shareholders’ Agreement also sets forth what will happen if CanaLeaf fails to deliver all the funds required under the Subscription Agreement. If CanaLeaf has not on or before December 31, 2014 purchased all the Investment Shares that it has agreed to purchase pursuant to the Subscription Agreement, then the following will occur:
5
|
1.
|
if the total amount paid by CanaLeaf to IHMML for Investment Shares issued pursuant to the Subscription Agreement is CAD$10,000,000 or less, CanaLeaf will forfeit all Investment Shares purchased by and issued to CanaLeaf, all of which will be cancelled without any payment or other consideration, liability or obligation of any party hereto to CanaLeaf;
|
|
2.
|
if the total amount paid by CanaLeaf to IHMML for Shares issued pursuant to the Subscription Agreement is more than CAD$10,000,000 but not more than CAD$25,000,000, IHMML shall have the option, exercisable on notice to CanaLeaf on or before December 31, 2014, to purchase all Shares held by CanaLeaf at a price of CAD$1.00 per Share;
|
|
3.
|
if the total amount paid by CanaLeaf to IHMML for Shares issued pursuant to the Subscription Agreement is more than CAD$25,000,000, or if IHMML did not exercise its option pursuant to the Shareholders’ Agreement, CanaLeaf will be entitled to retain the Shares purchased pursuant to the Subscription Agreement, but they will not have any of the sell interests or rights defined in the Shareholders’ Agreement.
|
Overview of Current Business
We are a pharmaceutical business currently focused on developing, distributing and marketing medical marijuana in Canada. We have established joint-venture partnerships with experienced agriculture and pharmaceutical distribution companies in Canada to facilitate this business. We also are actively searching to acquire smaller operations in the medical marijuana industry in Canada with the intention of increasing their profits through economies of scale.
We currently have three subsidiaries through which we operate our business.
6
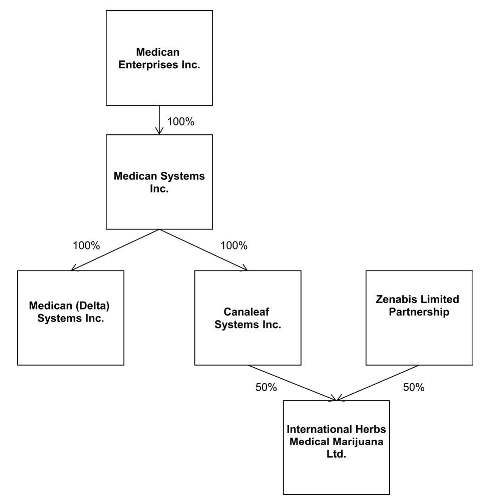
Medican Systems is a corporation incorporated under the laws of the Territory of the Yukon under incorporation number 535642 on December 30, 2013. The authorized share structure is an unlimited number of common shares without par value, with 100 common shares issued to our company, making Medican Systems our direct wholly-owned subsidiary. The primary focus of Medican Systems is to function as a holding company for Canadian based investments, joint ventures and opportunities.
Medican (Delta). is a corporation incorporated under the laws of the Province of British Columbia under incorporation number BC0989867 on December 31, 2013. The authorized share structure is an unlimited number of common shares without par value, with 100 common shares issued to Medican Systems. The primary focus of Medican Delta is to pursue opportunities in the medical marijuana industry in and around the city of Delta in British Columbia, Canada.
CanaLeaf is a corporation incorporated under laws of Canada under the Canada Business Corporation Act under incorporation number 883348-6 on March 25, 2014. The authorized share structure is an unlimited number of common shares. CanaLeaf is our principal operating subsidiary.
CanaLeaf is the entity that is a party to the Amended Management Services Agreement that creates a business partnership with IHL, LFG and our company.
IHL is Western Canada’s largest fresh herb grower and importer. Founded in 1990, IHL is the leading specialty herbal produce grower in North American, carrying products such as fresh herbs, baby vegetables, lettuces and gourmet salads. IHL and Canaleaf are members of the Canadian National Medical Marijuana Association (the “CNMMA”). The CNMMA was created to ensure all Canadian patients who benefit from medical marijuana have access to the highest quality of products and services to meet their health care needs in a well regulated environment. CanaLeaf plans to create opportunities for political lobbying and exposure through the CNMMA.
7
IHMML will be assisting with the marketing of our Company. IHMML is a full-service provider of marketing, information technology, and call center services dedicated to the pharmaceutical sector. They have 12 years of experience providing superior service and success in the pharmaceutical industry with a novel, automated prescription ordering/refill system, delivery system, and customer contact process.
CanaLeaf leverages the respective expertise of IHL and IHMML and is being led by the management team of IHL and IHMML. CanaLeaf is the consumer-facing brand name for our business, and we believe the partnership between IHL and IHMML, with our company providing the capital markets financing opportunities for the business, will allow for CanaLeaf to emerge as a leading Canadian company in medical marijuana, as we will be able to fulfill stringent requirements, understand the vision and can meet the needs of patients, can expand quickly with research and development, and horticulture advancements. With this combination of expertise and abilities, we believe CanaLeaf is poised to become an industry leader within the medical marijuana community. IHMML also owns the rights for Zenabis, another consumer facing brand that will be developed in conjunction with CanaLeaf.
Health Canada’s “Marihuana for Medical Purposes Regulations” (the “MMPR”) treats dried marijuana as much as possible like a medication by creating a licensing scheme for the commercial production and distribution of the dried marijuana for medical purposes. The introduction of the MMPR in December of 2012 allows for regulated commercial-scale production beginning in 2014. The reformed legislation now prohibits patients from growing their own medical marijuana and only licensed commercial producers will be able to control production and distribution. Health Canada has also stated that their own commercial facility will cease operations, thus opening up the market to new commercial licenses. Commercial producers licensed under the new MMPR regulations will be permitted to grow strains of their choosing based on market demand and Health Canada will allow producers to set pricing for their various strains. Portions of the MMPR were enacted in July 2013 and April 1, 2014. On March 21, 2013 a Canadian federal court judge issued an injunction that exempts currently licensed marijuana users and producers from the MMPR, until their case can be heard. This means that people with existing authorization to possess, designated-person production, or personal-use production licenses issued under the old rules are currently still be permitted to possess and/or grow medical marijuana past April 1, 2014. All new patients will be required to obtain a prescription under the new programs.
We believe that IHL is an ideal partner with the Canadian federal government on this initiative. As an agricultural grower and distributor IHL has the growing, testing and security expertise to be a licensed producer of medical marijuana and implement this program to its full extent.
The Amended Management Services Agreement with IHL and the Subscription Agreement are key steps in establishing our company as a leader in the medical marijuana industry in Canada. Currently, Health Canada has begun a major shift from designated growers to licensed commercial growers. Under the designated grower program, production was limited to two patients per grower and four patients per residential address. Under the new system, there will be no limit to the number of patients for each licensed commercial grower. To capitalize on this new development, we are working with IHL to implement our business model by becoming a licensed commercial grower and receive a commercial growing license (the “License”).
Up until April 1, 2014 in Canada, medical uses of marijuana were governed by the Marijuana Medical Access Regulations (the “MMAR”). The MMAR granted the Canadian government the sole authority to produce and sell medical marijuana to patients. As a result, only one company was contracted with the Canadian government to produce and sell to patients. The MMAR’s proposed changes will have effectively privatized the medical marijuana industry and thus, the government will no longer have contracts to grow marijuana but will open that option to free enterprise. This means that there is no subsidy for the costs of goods and therefore, growers will be allowed to set their prices. This change is unprecedented and because it is a federal initiative growers from any province or region can operate throughout Canada. This is the first development of its kind and the first time Canadian companies can produce medical marijuana. Therefore, growers that receive a License will effectively control the medical marijuana business.
8
On April 8, 2014, CanaLeaf entered into an Subscription Agreement through which CanaLeaf will became a 50% holder of the issued and outstanding common shares of IHMML by way of subscription for 41,600,000 common shares of IHMML for an aggregate subscription price of CAD$52,000,000. IHMML will use these investment proceeds to acquire certain lands, retrofit and/or build a marijuana growing operation and attend to the growing, marketing, research and development, training, distribution and retail sale of medical marijuana in Canada as regulated by Health Canada.
Specifically, with the funds delivered by CanaLeaf, IHMML will commence operations in an initial 393,000 square foot facility located in Atholville, New Brunswick. CanaLeaf has received notice from Health Notice that its application is completed and has undergone a preliminary site assessment from Health Canada. IHMML will commence retrofitting the facility with capital upgrades and will begin immediately building the growing rooms for 300,000 square feet in production area.
While we produce at Atholville, a second 273,000 square foot facility in Poekmouche, New Brunswick and a third 25,000 square foot facility in Delta, British Columbia will be retrofitted and secured for production. The Delta facility will serve as a growing distribution and R&D center for the Company in Western Canada.
Our Products and Their Markets
We are actively meeting with other companies and individuals in the medical marijuana industry to establish relationships and partnerships that we believe will benefit us going forward.
Through our CanaLeaf subsidiary, we are working with IHMMLL to obtain a License through Health Canada to become a licensed commercial grower of medical marijuana. CanaLeaf,, under Health Canada’s new regulations, intends and to begin operations in a commercial property in Atholville, New Brunswick. CanaLeaf will produce medical marijuana, conduct research and development, and coordinate its distribution efforts at the Atholville facility. We also plan to retrofit and secure a second location in Poekmouche, New Brunswick and a third facility in Delta, British Columbia.
CanaLeaf has a four-pronged approach to furthering its products offered and services provided: research and development, horticultural supremacy, distribution and market penetration, and asset security. These four principles will be the focus of our growth and potential partnerships in the future.
There are several regulatory requirements related to the entrance into the medical marijuana industry in Canada. Under the new MMPR, interested parties must apply to Health Canada to become a licensed producer. Licensed producers can be authorized to possess, sell, provide, ship, deliver, transport, destroy, and produce marijuana for medical purposes under the MMPR. CanaLeaf, through its partnerships with IHL and IHMML, has already taken steps to meet all requirements of the new MMPR, including:
|
·
|
Designating a senior person in charge, a responsible person in charge, and an alternate responsible person in charge who meet all eligibility requirements;
|
|
·
|
Completion of a site application;
|
|
·
|
Obtaining the proper personal security clearances;
|
|
·
|
Meeting the physical requirements for the cultivation and storage areas at a facility;
|
|
·
|
Submitting a completed licensed producer application.
|
9
Sales and Marketing
The sales and marketing for CanaLeaf will be led by IHMML. As a first move into the commercial production of medicinal marijuana based on MMPR regulations, the establishment of key distribution channels will be critical to CanaLeaf’s success. CanaLeaf will focus on developing two primary distribution strategies as sanctioned by MMPR regulations.
Direct Sales to Consumers via Online or Telephone Channels
|
·
|
Upon registration, consumers in Canada will be able to directly and securely purchase or re-order CanaLeaf products via the company's website which will be further enhanced through search engine optimization (“SEO”) strategies. According to the regulations of Health Canada, only patients within Canada will be able to purchase these products through these channels.
|
|
·
|
In order for customers to register with CanaLeaf and order its products they must provide the following documents:
|
|
o
|
A medical document - A medical document must be completed in full and signed by a health care practitioner.
|
|
o
|
An Authorization to Possess - This document is issued by Health Canada and can only be used until the end of its validity period, or the expiry date if no separate validity date is indicated.
|
|
o
|
A Form B "Medical Practitioner's Form" - While this form is primarily for use under the Marihuana Medical Access Regulations, if the customer’s health care practitioner has already completed it, it may be used during the transition period so long as it is within one year of the date it was signed by the health care practitioner.
|
|
·
|
CanaLeaf will ensure the highest level of security of its systems to ensure the confidentiality and security of customer and transactional information; system will be integrated with, a provider of technology solutions and support for commercial cannabis growing operations, for patient profiles, document storage and e-commerce functionalities.
|
|
·
|
CanaLeaf’s website will provide the relevant and necessary information in order to educate and advise customers to make informed purchasing decisions.
|
|
·
|
Online channels can further be expanded into mobile channels to provide greater ease of access by customers to CanaLeaf products.
|
|
·
|
Shipping will be completed by Canada Post Secured Shipping (the only approved carrier for Controlled Substances) requiring customer signature upon receipt of the product.
|
|
·
|
Per MMPR Section 5 “Packaging, Labelling and Shipping”, shipments will include the following features.
|
|
o
|
Product will only be sent to the customer’s home, shelter or his/her primary care physician.
|
|
o
|
Container for shipping will have no smell or visible indicators for the product, tamper evident and child resistant.
|
|
o
|
Shipment will contain standard information about the product and client specific label.
|
|
o
|
Maximum package size will be 30g per immediate container (150g max per shipment) measured by the percentage weight of delta-9-tetrahydrocannabinol (THC) and of cannabidiol.
|
10
|
o
|
Package will be clearly labelled “Keep Out of Reach of Children” as well as list the expiration date based on stability testing prior to shipping.
|
Sales via Intermediaries (Medical Professionals)
Critical to not only the sales of CanaLeaf medicinal marijuana products, but also the growth of the overall industry will be the ability to gain support and promotion of medicinal marijuana by health care professionals. CanaLeaf will work closely with medical professionals to provide educational and awareness training to ensure they understand the benefits of its products and incentivize professionals to recommend CanaLeaf products to patients that may benefit from its use, CanaLeaf will also verify the physician is able to practice and is not prohibited from prescribing controlled substances and/or narcotics. Further verification will be made with the physician’s office to ensure that the medical documentation and quantity is complete and correct. Medical professionals will be able to scan bar codes to place direct orders online or via the phone through a dedicated line.
Once CanaLeaf has been able to establish a stronger brand and gain recognition of its product quality, medical professionals can be strategically approached to expand this channel. However, it should be noted that intermediary channels are strongly dependent on brand and quality development and thus must be developed concurrently to optimize CanaLeaf's strategy.
Distribution Approach
Distribution strategy will be overseen by the senior management team of IHMML. Their experience has seen over $500 million in revenues and they have mastered the demanding complexity of software development in the pharmacy space. Integrating the merchant processing systems with proprietary software programs, they have fine-tuned the process that is vital in supporting fraud prevention tactics. As well, their group of over 100 trained customer support representatives with experience in the health care industry leads to thorough experience in pharmaceutical processing.
We will be negotiating with large pharmaceutical and medical sales companies within Canada to market our medical marijuana to physicians throughout Canada. Typically such firms charge 2% of all sales and that rate has been factored into our model.
11
Market
On a global scale, the environment pertaining to medical marijuana usage is rapidly changing across the globe. In the Czech Republic, the health ministry awarded its first license to import marijuana, months after the European Union member legalized marijuana for medical use. Uruguay made history by becoming the first country in the world to legalize the sale and production of cannabis. In the United States, the states of Colorado and Washington both voted to make the sale and use of marijuana legal. Eighteen other American states allow the use of medical marijuana. However, the legal environment in the United States does not currently allow for us to directly be involved in any cultivation or distribution or dispensing of medical marijuana.
Canada is also effecting major changes in the regulation of medical marijuana through the rollout of the recently-enacted MMPR. Health Canada provides two reasons for the regulatory changes: first, to address concerns about the safety of home grow-ops; and secondly, to reduce the cost of administering a program that has proven more popular than anticipated. There is a tremendous business opportunity for companies that obtain their commercial growers license as the Canadian government project revenues for the new medical marijuana industry to reach $1.3 billion by 2024.
Competition
Although Health Canada’s regulations regarding the production and distribution of medical marijuana have only changed this year, we have several competitors in this business area, and expect there to be a great deal more competition in this area as marijuana use becomes more mainstream and more Licenses are granted by Health Canada.
With the recent rollout of the new MMPR, production and sale of medical marijuana will be limited to commercial producers who are able to meet strict government and health regulations. This will effectively eliminate the 3,405 individuals licensed to grow for others and 18,063 individuals allowed to grow marijuana for personal use. As well, it will eliminate the monopoly currently enjoyed by the Saskatchewan medical marijuana producer Prairie Plant Systems. This will likely result in market consolidation with players building up scale to meet current demand. However, on March 21, 2014, a Canadian federal court judge issued an injunction that exempts currently licensed marijuana users and producers from the MMPR. Accordingly, people with existing authorization to possess, designated-person production, or personal-use production licenses issued under the old rules will still be permitted to possess and/or grow medical marijuana past April 1, 2014.
CanaLeaf has received notice from Health Canada that its application to Health Canada is complete. The market to obtain a licence from Health Canada is competitive as there were over 400 medical marijuana licence applications made over the past year, with only a few official licences granted thus far.
We believe that competition will likely result from two key sources:
|
1.
|
Incumbent producers/retailers with established customer base, market access and production facilities. These players would likely to have already established scale beyond household production however lack operational and management expertise to expand their market base. These operators will likely require substantial investments in order to upgrade its facilities to meet new regulatory requirements. Smaller incumbents would either be too small or lack the necessary expertise to compete against commercial enterprises or do not want to expose their operations due to previous legal infractions.
|
12
|
2.
|
New operators looking to leverage the opportunities provided through market liberalization will generally be enterprises possessing stronger technical and/or financial backing. These operators will likely have sound understanding of the industry and regulatory requirements, technical expertise in R&D of strains suitable and desired by the market as well as financing to establish sufficient scale to capture a substantial amount of market share from the onset. These new entrants may also purchase smaller incumbents in order to gain quicker access to key consumer markets.
|
Although competition from both sources will be likely, the second source of competition will likely pose a greater threat to the commercial viability of IMMHL. The following is an analysis of the existing competitive landscape, their key individual company characteristics and how CanaLeaf competes.
Key competitors expected in the market upon formal establishment of the MMPR regulations as of April 1, 2014 will include Canna Farms Limited, CanniMedicare Inc., Peace Naturals Projects Inc., Tweed Marijuana Inc., GreenLeaf Medicinals Ltd., In The Zone Produce Ltd., ThunderBird Biomedical Inc., and Whistler Medical Marijuana Corporation. These companies are currently registered commercial producers with Health Canada as of April 8th, 2014. CanaLeaf has identified key threats posed by these companies, their key value proposition within the market and has developed differentiating strategies against each company.
In response to these competitors, CanaLeaf has established key areas of differentiation and a strong set of value propositions which we believe we allow us to emerge as a leader within the industry. We aim to provide a pharmaceutical grade medical marijuana product at $7.50/gram, which is on par with several of the top players. CanaLeaf is an integrated distribution and growing facility. Given our plans for 600,000 square feet of production, our economies of scale will continue to make us price competitive particularly against smaller players such as Cannimed, Cannafarms, Tweed and Mettrum. In addition to CanaLeaf’s larger production capacity and advanced facilities, CanaLeaf also boasts a strong team of experienced technical experts with deep industry expertise and research and development capabilities in effectively responding to market needs.
A summary of what we believe are our key competitive advantages include:
|
|
·
|
Industry Experience: We believe CanaLeaf’s management team forms a key component of our competitive advantage. The founders of CanaLeaf currently own and operate Western Canada’s largest herb grower and importer at IHL. The management team at IHL will bring its experience and expertise into the commercial medical marijuana market.
|
|
|
·
|
Research and Development: Through our partnership with RPC Science & Engineering (“RPC”), a New Brunswick firm specializing in research and development for medical marijuana companies, and IHMML, we will be able to focus on research and development to increase production efficiencies which will allow us to further advance new medicinal marijuana strains for sale. Our partnership with RPC will also allow us to improve on our quality assurance procedures, ensuring we sell only the highest-quality medicinal marijuana to the Canadian market.
|
|
|
·
|
Government Relationships: From years of experience in agriculture, CanaLeaf’s management team has formed key relationships with officials at Health Canada that will lobby the interests of IHLMM. We believe these relationships will assist us in understanding the regulatory environment, expedite administration, as well as allow us to be privy to potential changes in regulations. We further believe these key relationships will allow us to adapt to the changing legal and market conditions.
|
|
|
·
|
Scale: As an entrant into a highly fragmented industry, it will be a critical imperative to establish operations at a scale that will capture as much market share as possible from the onset in order to establish a stronger market position going forward. CanaLeaf expects to acquire the New Brunswick property with sufficient size to be able to support growing space of 500,000 square feet with a production capacity of up to 450 kilograms per week. As per financial estimates on market potential and usage, our business plan calls for CanaLeaf to capture initially a 2% market share and continue to grow that until maximum capacity is reached, at which time additional growing space (within the financial projections) will be established to meet greater demand.
|
|
|
·
|
Quality Control: CanaLeaf will partner with RPC to conduct analytical services to ensure that Division 4 of the MMPR requirements on quality for the production and sale of dried marijuana in Canada are effectively met. RPC is licensed by Health Canada to perform analysis of cannabis for levels of cannabinol, delta-9-tetrahydrocannabinol and cannabidiol. Test methods are performed using validated, documented procedures which comply with regulatory requirements.
|
13
Intellectual Property
None.
Reports to Security Holders
We are subject to the reporting and other requirements of the Exchange Act and we intend to furnish our shareholders annual reports containing financial statements audited by our independent registered public accounting firm and to make available quarterly reports containing unaudited financial statements for each of the first three quarters of each year. We file Quarterly Reports on Form 10-Q, Annual Reports on Form 10-K and Current Reports on Form 8-K with the SEC in order to meet our timely and continuous disclosure requirements. We may also file additional documents with the SEC if they become necessary in the course of our Company’s operations.
The public may read and copy any materials that we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is www.sec.gov.
Effect of Existing or Probable Governmental Regulations on the Business
United States
While we intend to focus our business efforts entirely in Canada at least initially, we may, if regulatory and business conditions allow, operate in the United States. Cannabis is not legal to grow in the U.S. under U.S. federal law, although it may be imported and sold in the U.S. Importation is subject to a “zero tolerance” policy as a controlled substance under the U.S. Controlled Substances Act. In certain states, the growth and cultivation of cannabis is legal (California, Colorado, Hawaii, Kentucky, Maine, Maryland, Montano, North Dakota, Oregon, Vermont and West Virginia), although states are resistant to allow the cultivation of cannabis due to resistance from the U.S. Department of Drug Enforcement Agency (the “DEA”) and prohibitions of federal law.
Upon the production and sale of marijuana-based products to consumers, our products will be required to comply with various regulations, including approvals of the federal, state and local governments. We have no current plans to participate in any of these activities in the United States until such time as the legal environment has stabilized and there can be legal certainty surrounding any activities in the medical marijuana industry in the United States.
Canada
The legislation pertaining to medical marijuana has undergone significant recent changes in Canada. The new MMPR was announced on December 2012 as a Health Canada initiative by the Government of Canada. The purpose of this legislation was to allow for the regulation of commercial-scale production of medical marijuana. The new MMPR supersedes all other Medical Marijuana laws in Canada. In 2001 there were around 100 authorized users and this number grew to 40,000 by 2013. The rapid growth in the user base required more oversight from a health and safety standpoint as the old system was simply not economical in the long-run. This change in effect causes growers under the previous program to cease and desist on April 1, 2014. Only corporations or entities who meet the stringent requirements of the new Licensed Producer status (“LP”), under the new MMPR will be allowed to produce medical marijuana in Canada. Municipal governments across Canada, along with local and federal law enforcement are anticipating the effective closure of all current licensed growers (under the old program).
14
Health Canada’s role under the old program was to authorize and license individuals with medical support and administer production contracts. Under the new program, Health Canada will regulate the Licensed Producers, including licensing audit and inspection. Support for access to medical marijuana will be through physicians or nurse practitioners. Doctors are able to recommend medical marijuana to treat chronic diseases and conditions. Medical marijuana is used for AIDS, cancer, ADHD, multiple sclerosis, nausea resulting from chemotherapy, Crohn's disease, glaucoma, epilepsy, insomnia, migraines, arthritis and lack of appetite. Key differences between the old programs and new program are as follows:
|
Old Program
|
New Program
|
|
|
Support for Access
|
Physician
|
Physician or nurse practitioner
|
|
Authorization
|
Application to Health Canada
|
Registration with Licensed Producer
|
|
Production
|
1) Purchase from Health Canada
2) Personal use production
3) Designated-person production
|
Licensed Producer only
|
|
Distribution
|
Health Canada supply sent through secure courier; designated producer can distribute in person or by mail
|
Licensed Producer (secure direct delivery)
|
|
Health Canada Role
|
Authorize and license individuals with medical support; administer production contract
|
Regulate Licence Producers, including licensing audit and inspection
|
Commencing April 2014, under the new MMPR Program commercial growers are able to grow strains based on market demand and Health Canada will allow producers to set prices based on the different strains. CanaLeaf has unique competitive advantages that allow us to leverage the expertise of RPC Science & Engineering for the testing and establishing protocols for pharmaceutical grade medical marijuana. In addition to lowering long-term development costs, our industry partnership with RPC gives CanaLeaf credibility via an independent certification body.
It is anticipated that this major change will produce a vacuum in the supply of high grade medical marijuana. A new market is opening up and the economics are attractive: there is an existing client base, supply is currently limited (and will continue to be so), and there is significant demand for high-quality, laboratory tested and Health Canada standardized medical marijuana.
The MMPR authorizes three key activities: the possession of dried marijuana for medical purposes by individuals who have the support of an authorized health care practitioner; the production of dried marijuana by licensed producers; and the sale and distribution of dried marijuana by specific regulated parties to individuals who can possess it. Licensed producers would be subject to regulatory requirements related to security; good production practices; packaging, labeling and shipping; record keeping and reporting; and distribution. They would also be subject to Health Canada inspections.
15
Commercial producers licensed under the new MMPR regulations are permitted to grow strains of their choosing based on market demand, and Health Canada will allow producers to set pricing for their various strains.
Key Dates in the Evolution of Canadian Attitudes and Laws Regarding Marijuana
In 1999, the first two Canadian patients received federal permission to use marijuana. In 2001, the Canadian Medical Marihuana Access Regulations grant legal access to cannabis for individuals with HIV/AIDS and other illnesses. Authorized patients can grow their own pot or obtain it from authorized producers or Health Canada.
In 2013, new regulations change the Canadian Medical Marijuana Access rules, shifting to licensed commercial growers for supply of medical marijuana and away from homegrown MM. Approximately 37,800 people are authorized to possess medical marijuana under the federal program, increasing from less than 100 in 2001.
In 2014, patients and producers authorized under the previous regulations are required to destroy their medical marijuana and cannabis seeds, although a Canadian Federal Court injunction has granted a temporary injunction allowing continued use of home-grown medical marijuana until legal arguments can be heard. However, the federal government is arguing against this temporary injunction and stated its goal “to treat dried marijuana as much as possible like other narcotic drugs used for medical purposes." However, the injunction is only for existing users and will not apply for new prescriptions. According to the government, a 2010 RCMP report on medical marijuana grow operations across the country found fires up to 24 times more likely in homes with grow operations than with those without. Growing operations are being set up with hydroponic equipment and being installed without the proper permits or inspections, usually within residential areas.
New Canadian Government Steps to Help Medical Community with Medical Marijuana
On March 31, 2014, the Canadian Federal Government announced that marijuana is not an approved drug or medicine in Canada and has not gone through the rigorous scientific trials for efficacy or safety; however the courts have required reasonable access to a legal source of marijuana for medical purposes. As a result, the Government believes that this must be done in a controlled and regulated fashion to protect public health and safety. Minister Rona Ambrose, Minister of Health has directed Health Canada to work with secure commercial operators licensed to produce marijuana to enhance information sharing with regulatory oversight bodies on how doctors and nurse practitioners are authorizing the use of marijuana. The new measures, including information on dosage, educational material and increased oversight, will decrease the potential for over-prescriptions and negative health impacts. It is assumed that active engagement with the medical community is the first step towards approving the use of marijuana within Canada.
Smaller Reporting Company Status
We are subject to the reporting requirements of Section 13 of the Exchange Act, and we are subject to the disclosure requirements of Regulation S-K of the SEC, as a “smaller reporting company.” That designation will relieve us of some of the informational requirements of Regulation S-K, including comprehensive information in the Summary Compensation Tables, a reduction of information included in the Management’s Discussion and Analysis section, and decreased reporting obligations in Item 404 Transactions with Related Persons.
Sarbanes-Oxley Act
We are also subject to the Sarbanes-Oxley Act of 2002 and related rules and regulations. The Sarbanes-Oxley Act created a strong and independent accounting oversight board to oversee the conduct of auditors of public companies and strengthens auditor independence. It also requires steps to enhance the direct responsibility of senior members of management for financial reporting and for the quality of financial disclosures made by public companies; establishes clear statutory rules to limit, and to expose to public view, possible conflicts of interest affecting securities analysts; creates guidelines for audit committee members’ appointment, compensation and oversight of the work of public companies’ auditors; management assessment of our internal controls; auditor attestation to management’s conclusions about internal controls; prohibits certain insider trading during pension fund blackout periods; requires companies and auditors to evaluate internal controls and procedures; and establishes a federal crime of securities fraud, among other provisions. Compliance with the requirements of the Sarbanes-Oxley Act has the potential to substantially increase our legal and accounting costs.
16
Exchange Act Reporting Requirements
Section 14(a) of the Exchange Act requires all companies with securities registered pursuant to Section 12(g) of the Exchange Act to comply with the rules and regulations of the SEC regarding proxy solicitations, as outlined in Regulation 14A. Matters submitted to our stockholders at a special or annual meeting thereof or pursuant to a written consent will require us to provide our stockholders with the information outlined in Schedules 14A or 14C of Regulation 14; preliminary copies of this information must be submitted to the SEC at least 10 days prior to the date that definitive copies of this information are forwarded to our stockholders.
On September 11, 2008, we filed a registration statement on Form 10 of the SEC registering our $0.001 par value common stock under Section 12(g) of the Exchange Act. This registration statement became effective on or about November 9, 2008.
We are required to file Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q with the SEC on a regular basis, and are required to timely disclose certain material events (e.g., changes in corporate control; acquisitions or dispositions of a significant amount of assets other than in the ordinary course of business; and bankruptcy) in a Current Report on Form 8-K. We are not considered an emerging growth company under the JOBS Act because the Company sold securities pursuant to an effective registration statement prior to December 8, 2011.
Research and Development Costs During the Last Two Fiscal Years
None; not applicable. Once we receive our commercial growers license and commence operations in our two facilities, we expect that there will be significant research and development expenses incurred related to our products and production.
Cost and Effects of Compliance with Environmental Laws
We do not believe that our current or intended business operations are subject to any material environmental laws, rules or regulations that would have an adverse material effect on our business operations or financial condition or result in a material compliance cost; however, we will become subject to all such governmental requirements to which the reorganized, merged or acquired entity is subject or may become subject.
Employees
As of March 31, 2014, we had two employees, our CEO, Ken Williams, and our CFO, Wayne Hansen.
Additional Information
You may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You may also find all of the reports or registration statements that we have previously filed electronically with the SEC at its Internet site at www.sec.gov. Please call the SEC at 1-202-551-8090 for further information on this or other Public Reference Rooms.
17
ITEM 1A. RISK FACTORS
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information included in this Annual Report, before making an investment decision. If any of the following or similar risks actually occurs, our business, financial condition or results of operations could suffer and our business might fail. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment. You should read the section entitled “Cautionary Note Regarding Forward Looking Statements” above for a discussion of what types of statements are forward-looking statements, as well as the significance of such statements in the context of this Annual Report.
Risks Related to Our Business
We have only recently commenced operations and are thus subject to the risks associated with new businesses.
We have only recently commenced our business plan of manufacturing and distributing medical marijuana in Canada. As of the date of this Annual Report, we have no cash resources, products, manufacturing facilities or sales and marketing operations. As such, we are a “start-up” company with no history of revenue-generating operations. We are, and expect for the foreseeable future to be, subject to all the risks and uncertainties, inherent in a new business. As a result, we still must establish many functions necessary to operate a business, including finalizing our managerial and administrative structure, acquiring our manufacturing facilities, continuing product development, assessing and commencing our marketing activities, implementing financial systems and controls, and recruiting personnel.
Accordingly, you should consider our prospects in light of the costs, uncertainties, delays, and difficulties frequently encountered by companies in their pre-revenue generating stages, particularly those in heavily regulated industries like the medical marijuana industry. Potential investors should carefully consider the risks and uncertainties that a company with no operating history will face. In particular, potential investors should consider that there is a significant risk that we will not be able to:
|
|
·
|
implement or execute our current business plan, or that our business plan is sound;
|
|
|
·
|
raise sufficient funds in the capital markets or otherwise to effectuate our business plan;
|
|
|
·
|
maintain and expand our management team and Board of Directors;
|
|
|
·
|
determine that the processes that we are developing are commercially viable; and/or
|
|
|
·
|
attract, enter into, or maintain contracts with, and retain customers.
|
If we cannot execute any one of the foregoing, our business may fail, in which case you would lose the entire amount of your investment in our company.
We must raise at least $52 million Canadian Dollars over the next 12 months in order to commence our business, and we will continue to need additional financing to carry out our business plan.
We need to obtain significant additional funding to successfully launch and continue our business. Such additional funds may not be readily available or may not be available on terms acceptable to us. Specifically, our Subscription Agreement entered into with IHMML on April 8, 2014 requires us to raise CAD$52,000,000 over the next 12 months, and at least CAD$5,000,000 by April 30, 2014. Any failure to acquire these funds will have adverse effects on our operations. Moreover, CanaLeaf will lose all or virtually all of its shares in IHMML under the Subscription Agreement if it does not fund IHMML certain amounts by December 31, 2014. As such, our failure to raise funding in the near future would have a significantly adverse impact on our business viability and could cause our business to fail. We do not currently have any arrangements or credit facilities in place as a source of funds, and there can be no assurance that we will be able to raise sufficient additional capital on acceptable terms, or at all.
18
If we raise additional capital by issuing equity securities, the percentage ownership of our existing stockholders may be reduced, and accordingly these stockholders may experience substantial dilution. We may also issue equity securities that provide for rights, preferences and privileges senior to those of our common stock. Given our need for cash and that equity raising is the most common type of fundraising for companies like ours, the risk of dilution is particularly significant for stockholders of our company.
Debt financing, if obtained, may involve agreements that include liens on our assets, covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, could increase our expenses and require that our assets be provided as a security for such debt. Debt financing would also be required to be repaid regardless of our operating results.
If we raise additional funds through collaborations and licensing arrangements, we may be required to relinquish some rights to our technologies or candidate products, or to grant licenses on terms that are not favorable to us.
We cannot accurately predict the volume or timing of any future sales, making the timing of any revenues difficult to predict.
We may be faced with regulatory or operational challenges that could impede our ability to commence sales of our medical marijuana products. Consequently, we may incur substantial expenses and devote significant management effort and expense in launching sales efforts, which may not result in revenue generation. We must also obtain regulatory approvals to engage in our business, which is subject to risk and potential delays, and which may not actually occur. As such, we cannot accurately predict the volume or timing of any future sales of our products.
We may not be able to effectively manage our growth.
If we are able to launch our business, any growth in or expansion of our business is likely to place a strain on our management and administrative resources, infrastructure, and systems. As with other growing businesses, we expect that we will need to further refine and expand our business development capabilities, our systems and processes, and our access to financing sources. We also will need to hire, train, supervise, and manage new employees. These processes are time consuming and expensive, will increase management responsibilities, and will divert management attention. We cannot assure you that we will be able to:
|
·
|
expand our products effectively or efficiently or in a timely manner;
|
|
·
|
allocate our human resources optimally;
|
|
·
|
meet our capital needs;
|
|
·
|
identify and hire qualified employees or retain valued employees; or
|
|
·
|
incorporate effectively the components of any business or product line that we may acquire in our effort to achieve growth.
|
Our inability or failure to manage our growth and expansion effectively could harm our business and materially and adversely affect our operating results and financial condition.
19
If we incur substantial liability from litigation, complaints, or enforcement actions our financial condition could suffer.
Litigation, complaints, and enforcement actions involving us could consume considerable amounts of financial and other corporate resources, which could have a negative impact on our sales, revenue, profitability and growth prospects. We have not been, and are not currently, subject to any material litigation, complaint or enforcement action regarding our publications by any federal, state or foreign regulatory authority.
We are subject to significant regulation by the Canadian Federal Government
The regulations regarding medical marijuana in Canada have changed significantly since 2013 with the implementation of the Marijuana for Medical Purposes Regulation (MMPR). Our business operations are closely tied to the MMRP in that the receipt of a commercial growers license will be critical to our operational success in the future. Should we or our affiliates be unable to obtain all required licenses, or if the regulations in Canada continue to change, our business would not be able to operate or there could be significant cost to change our operations to remain compliant with the laws and regulations.
We will be required to attract and retain top quality talent to compete in the marketplace.
We believe our future growth and success will depend in part on our ability to attract and retain highly skilled managerial, product development, sales and marketing, and finance personnel. There can be no assurance of success in attracting and retaining such personnel. Shortages in qualified personnel could limit our ability to increase sales of existing products and services and launch new product and service offerings. The population of the area and preference for youth to move from the North to the South of Canada is a risk factor that will require considerable planning and recruitment efforts.
Our forecasts are highly speculative in nature and we cannot predict results in a development stage company with a high degree of accuracy.
Any financial projections, especially those based on ventures with minimal operating history, are inherently subject to a high degree of uncertainty, and their ultimate achievement depends on the timing and occurrence of a complex series of future events, both internal and external to the enterprise. There can be no assurance that potential revenues or expenses we project will, in fact, be received or incurred.
We will be subject to evolving and expensive corporate governance regulations and requirements. Our failure to adequately adhere to these requirements or the failure or circumvention of our controls and procedures could seriously harm our business.
As a publicly traded company, we are subject to various federal, state, and other rules and regulations, including applicable requirements of the Sarbanes-Oxley Act of 2002. Compliance with these evolving regulations is costly and requires a significant diversion of management time and attention, particularly with regard to our disclosure controls and procedures and our internal controls over financial reporting. Our internal controls and procedures may not be able to prevent errors or fraud in the future. Faulty judgments, simple errors or mistakes, or the failure of our personnel to adhere to established controls and procedures, may make it difficult for us to ensure that the objectives of the control system are met. A failure of our controls and procedures to detect other than inconsequential errors or fraud could seriously harm our business and results of operations.
The limited size of our senior management team may hamper our ability to effectively manage a publicly traded company while developing our products, which may harm our business.
Our management team has experience in the management of publicly traded companies and complying with federal securities laws, including compliance with recently adopted disclosure requirements on a timely basis. They realize it will take significant resources to meet these requirements while simultaneously working on developing, marketing, distributing, and protecting our products. Our management will be required to design and implement appropriate programs and policies in responding to increased legal, regulatory compliance and reporting requirements, and any failure to do so could lead to the imposition of fines and penalties and harm our business. The limited size of our management team may hinder our ability to effectively expand our R&D, conduct clinical trials and horticulture advancements, meet regulatory demands, or otherwise develop, market, or sell our products.
20
Our independent auditor's reports on our financial statements for the years ended December 31, 2013 and 2012 includes a "going concern" explanatory paragraph.
Our independent auditor has presented our financial statements on the basis that we are a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business over a reasonable length of time. This means that we may not generate enough funds to pay our general operating expenses and bills from professionals and other advisors that we are obligated to pay.
We may not acquire the facilities as planned and will therefore not be able to commence operations on the timetable or the scale that we have planned.
A key component to our growth and future operations are the retrofitting of two potential facilities in New Brunswick to house our growing and business operations. If these facilities are not obtained or if something happens to these facilities, the company may experience significant delays and expenses as it finds new facilities.
Risks Related to Our Industry
There are many regulations and governmental agencies that regulate the medical marijuana industry, and there will likely be increased and/or changing regulations as the industry becomes more mainstream with more participants.
As the viability and success of this venture is highly dependent on regulatory reform of the medical marijuana industry, particularly in Canada, any changes due to public opinion, government leadership, or lobbying may affect the business environment under which CanaLeaf will be operating. Although reforms and regulatory changes have been widely publicized it is still possible that such regulations may change. Any changes in laws, rules, regulations, public opinion or political support for our business plan could have a material adverse impact on our business prospects.
We are exposed to risks related to fluctuations in the Canadian economy.
Several factors may affect the financial stability of companies operating in Canada, including:
|
·
|
expand our products effectively or efficiently or in a timely manner;
|
|
·
|
allocate our human resources optimally;
|
|
·
|
meet our capital needs;
|
|
·
|
identify and hire qualified employees or retain valued employees; or
|
|
·
|
incorporate effectively the components of any business or product line that we may acquire in our effort to achieve growth.
|
Any of the foregoing events may negatively impact the overall profitability and financial stability of the Company.
There are sales risks associated with the cannabis and medical marijuana industry because cannabis is a controlled substance.
As cannabis is a controlled substance, direct consumer marketing is not allowed. All products can only be prescribed by a physician and, to be successful, companies will have to develop programs targeted to this group. Traditionally in this sector, growers have targeted users as opposed to the doctors. The new regulations will change this traditional approach and will require growers to target doctors. If we are unable to properly conduct sales in a regulated environment or target the appropriate audiences for our medical marijuana products, our results of operations and business prospects could be substantially impaired.
21
We are operating in a highly competitive industry and may not be able to compete successfully.
We are involved in a highly competitive industry where we may compete with numerous other companies in the medical marijuana industry, who may have far greater resources, more experience, and personnel perhaps more qualified than we do. There can be no assurance that we will be able to successfully compete against these other entities, particularly since we are just a start-up enterprise.
Our reputation in the industry will be very important as we grow the business, and any negative impact on our reputation could be damaging to our business.
As a producer and a retailer of a controlled substance in Canada that has been commonly associated with various other narcotics, violence, and criminal activities, it is expected that such a venture will likely result in widespread negative publicity and public opinion. Lack of understanding and awareness of the medical benefits associated with cannabis is poorly understood across the mainstream public despite various efforts to build such awareness.
There are safety operational risks related to the cultivation and storage of our products at the facilities.
Given the nature of the product and its lack of legal availability outside of the therapeutic channels, as well as the concentration of abundant stock within one facility, will likely result in risks due to shrinkage as well as theft. Also, as an agricultural product, there will be vulnerabilities due to various diseases and pests impacting not only yield and revenue but overall product quality to consumers.
There are risks related to the quality and quality control of our products.
We may be subject to liability of our products and must ensure quality control of the product at every stage. If a patient has an adverse reaction to the products, we may be subject to product liability related to the products and their quality in relation to the intended use of the products..
Increased popularity with the legalization of medical cannabis in Canada and the United States could result in unfavorable media coverage, which could ultimately negatively affect our business.
Our business industry receives a high degree of media coverage in the United States. The legalization of cannabis, both for medical uses and recreational uses, is becoming popular in the United States and Canada. This will increase the amount of media coverage our business industry receives. This media coverage could be negative coverage that may affect how others view our business. If this occurs, we may have difficulty attracting new customers.
Cannabis is not legal to grow in the U.S. under federal law, although it may be imported and sold in the U.S. Importation is subject to a “zero tolerance” policy as a controlled substance under the U.S. Controlled Substances Act.
In certain states, the growth and cultivation of cannabis is legal (California, Colorado, Hawaii, Kentucky, Maine, Maryland, Montano, North Dakota, Oregon, Vermont and West Virginia), although states are resistant to allow the cultivation of cannabis due to resistance from the U.S. Department of Drug Enforcement Agency (“DEA”) and prohibitions of federal law. Upon the production and sale of marijuana-based products to consumers, our products will be required to comply with various regulations at the province and Canadian levels. At this time, our product will only be sold in Canada under the MMPR.
22
Risks Relating to Ownership of Our Securities
Our stock price may be volatile, which may result in losses to our shareholders.
The stock markets have experienced significant price and trading volume fluctuations, and the market prices of companies listed on the Over-the-Counter Bulletin Board quotation system in which shares of our common stock are listed, have been volatile in the past and have experienced sharp share price and trading volume changes. The trading price of our common stock is likely to be volatile and could fluctuate widely in response to many factors, including the following, some of which are beyond our control:
|
·
|
variations in our operating results;
|
|
·
|
changes in expectations of our future financial performance, including financial estimates by securities analysts and investors;
|
|
·
|
changes in operating and stock price performance of other companies in our industry;
|
|
·
|
additions or departures of key personnel; and
|
|
·
|
future sales of our common stock.
|
Domestic and international stock markets often experience significant price and volume fluctuations. These fluctuations, as well as general economic and political conditions unrelated to our performance, may adversely affect the price of our common stock.
Our common shares are thinly traded and you may be unable to sell at or near ask prices, or at all.
We do not have a liquid market for our common stock, and we cannot predict the extent to which an active public market for trading our common stock will be achieved or sustained.
This situation is attributable to a number of factors, including the fact that we are a small company that is relatively unknown to stock analysts, stockbrokers, institutional investors and others in the investment community who generate or influence sales volume. Even if we came to the attention of such persons, those persons tend to be risk-averse and may be reluctant to follow, purchase, or recommend the purchase of shares of an unproven company such as ours until such time as we become more seasoned and viable. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that current trading levels will be sustained.
The market price for our common stock is particularly volatile given our status as a relatively small company, which could lead to wide fluctuations in our share price. You may be unable to sell your common stock at or above your purchase price if at all, which may result in substantial losses to you.
Shareholders should be aware that, according to SEC Release No. 34-29093, the market for penny stocks has suffered in recent years from patterns of fraud and abuse. Such patterns include (1) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (2) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (3) boiler room practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (4) excessive and undisclosed bid-ask differential and markups by selling broker-dealers; and (5) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines of practical limitations to prevent the described patterns from being established with respect to our securities. The occurrence of these patterns or practices could increase the volatility of our share price.
23
An active trading market for our common stock may not develop or be sustained.
As we are in our early stages, an investment in our company will likely require a long-term commitment, with no certainty of return. Although our common stock is listed for quotation on the OTCBB and OTCQB markets under the symbol of MDCN, we cannot predict whether an active market for our common stock will ever develop in the future. In the absence of an active trading market:
|
|
·
|
investors may have difficulty buying and selling or obtaining market quotations;
|
|
|
·
|
market visibility for shares of our common stock may be limited; and
|
|
|
·
|
a lack of visibility for shares of our common stock may have a depressive effect on the market price for shares of our common stock.
|
The OTCBB and OTCQB markets are relatively unorganized, inter-dealer, over-the-counter markets that provide significantly less liquidity than NASDAQ or the NYSE MKT (formerly known as the NYSE AMEX). In this event, there would be a highly illiquid market for our common stock and you may be unable to dispose of your common stock at desirable prices, or at all. Moreover, there is a risk that our common stock could be delisted from the OTCBB and OTCQB, in which case it might be listed on the so called “Pink Sheets”, which is even more illiquid than the OTCQB.
The lack of an active market impairs your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your shares. An inactive market may also impair our ability to raise capital to continue to fund operations by selling shares and may impair our ability to acquire additional intellectual property assets by using our shares as consideration.
We may not maintain qualification for OTC Bulletin Board or OTCQB inclusion, and therefore you may be unable to sell your shares.
Trading of our common stock could be suspended. If for any reason our common stock does not become eligible or maintain eligibility for quotation on the OTCBB or OTCQB or a public trading market does not develop, purchasers of shares of our common stock may have difficulty selling their shares should they desire to do so. If we are unable to satisfy the requirements for quotation on the OTCBB and OTCQB, any quotation of in our common stock could be conducted in the “pink” sheets market. As a result, a purchaser of our common stock may find it more difficult to dispose of, or to obtain accurate quotations as to the price of their shares. This would materially and adversely affect the liquidity of our securities.
Our majority stockholders will control our company for the foreseeable future, including the outcome of matters requiring stockholder approval.
Our officers and directors collectively have approximately 43.05% beneficial ownership of our company. As a result, such individuals will have the ability, acting together, to control the election of our directors and the outcome of corporate actions requiring stockholder approval, such as: (i) a merger or a sale of our company, (ii) a sale of all or substantially all of our assets, and (iii) amendments to our articles of incorporation and bylaws. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to our other stockholders and be disadvantageous to our stockholders with interests different from those individuals. Certain of these individuals also have significant control over our business, policies, and affairs as officers or directors of our company. Therefore, you should not invest in reliance on your ability to have any control over our company.
There may be limitations on the effectiveness of our internal controls, and a failure of our control systems to prevent error or fraud may materially harm our company.
24
Proper systems of internal controls over financial accounting and disclosure are critical to the operation of a public company. As we are a start-up company, we are at the very early stages of establishing, and we may be unable to effectively establish such systems, especially in light of the fact that we expect to operate as a publicly reporting company. This would leave us without the ability to reliably assimilate and compile financial information about our company and significantly impair our ability to prevent error and detect fraud, all of which would have a negative impact on our company from many perspectives. Moreover, we do not expect that disclosure controls or internal controls over financial reporting, even if established, will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Failure of our control systems to prevent error or fraud could materially adversely impact us.
We do not anticipate paying any cash dividends to our common shareholders.
We presently do not anticipate that we will pay dividends on any of our common stock in the foreseeable future. If payment of dividends does occur at some point in the future, it would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any common stock dividends will be within the discretion of our Board of Directors. We presently intend to deploy available capital to execute our business plan; accordingly, we do not anticipate the declaration of any dividends for common stock in the foreseeable future.
Because the SEC imposes additional sales practice requirements on brokers who deal in shares of penny stocks, some brokers may be unwilling to trade our securities. This means that you may have difficulty reselling your shares, which may cause the value of your investment to decline.
Our shares are classified as penny stocks and are covered by Section 15(g) of the Exchange Act which imposes additional sales practice requirements on brokers-dealers who sell our securities in this offering or in the aftermarket. For sales of our securities, broker-dealers must make a special suitability determination and receive a written agreement prior from you to making a sale on your behalf. Because of the imposition of the foregoing additional sales practices, it is possible that broker-dealers will not want to make a market in our common stock. This could prevent you from reselling your shares and may cause the value of your investment to decline.
Financial Industry Regulatory Authority (“FINRA”) sales practice requirements may limit your ability to buy and sell our common stock, which could depress the price of our shares.
FINRA rules require broker-dealers to have reasonable grounds for believing that an investment is suitable for a customer before recommending that investment to the customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status and investment objectives, among other things. Under interpretations of these rules, FINRA believes that there is a high probability such speculative low-priced securities will not be suitable for at least some customers. Thus, FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our shares, have an adverse effect on the market for our shares, and thereby depress our share price.
Volatility in our common share price may subject us to securities litigation.
The market for our common stock is characterized by significant price volatility as compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. Although we are not currently the subject of any pending litigation, we may, in the future, be the target of such litigation. Securities litigation could result in substantial costs and liabilities and could divert management's attention and resources.
25
The elimination of monetary liability against our director, officer and employees under Nevada law and the existence of indemnification rights of our director, officer and employees may result in substantial expenditures by our Company and may discourage lawsuits against our director, officer and employees.
Our articles of incorporation provide that directors and officers of the Company will not be personally liable to the Company or any of its stockholders for damages for breach of fiduciary duty as a director or officer except for acts or omissions which involve intentional misconduct, fraud or knowing violation of law, or the payment of dividends. In addition, our bylaws implement indemnification provisions requiring the Company to indemnify our directors to the fullest extent permitted by state law, and permit our board of directors to indemnify our officers. We may also have contractual indemnification obligations under our employment agreements with our officers. The foregoing indemnification obligations could result in our company incurring substantial expenditures to cover the cost of settlement or damage awards against directors and officers, which we may be unable to recoup. These provisions and resultant costs may also discourage our company from bringing a lawsuit against directors and officers for breaches of their fiduciary duties, and may similarly discourage the filing of derivative litigation by our shareholders against our directors and officers even though such actions, if successful, might otherwise benefit our company and shareholders.
As a public company, our business is subject to regulations related to corporate governance and public disclosure that have increased both our costs and the risk of noncompliance.
Because our common stock is publicly traded, we are subject to certain rules and regulations of federal, state and financial market exchange entities charged with the protection of investors and the oversight of companies whose securities are publicly traded. These entities, including the Public Company Accounting Oversight Board, the SEC and FINRA, have issued requirements and regulations and continue to develop additional regulations and requirements in response to corporate scandals and laws enacted by Congress, most notably the Sarbanes-Oxley Act of 2002. Our efforts to comply with these regulations have resulted in, and are likely to continue resulting in, increased general and administrative expenses and diversion of management time and attention from revenue-generating activities to compliance activities. Because new and modified laws, regulations and standards are subject to varying interpretations in many cases due to their lack of specificity, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This evolution may result in continuing uncertainty regarding compliance matters and additional costs necessitated by ongoing revisions to our disclosure and governance practices.
Sales of our currently issued and outstanding stock may become freely tradable pursuant to Rule 144 and may dilute the market for your shares and have a depressive effect on the price of the shares of our common stock.
A majority of the outstanding shares of our common stock are “restricted securities” within the meaning of Rule 144 under the Securities Act (“Rule 144”). As restricted shares, these shares may be resold only pursuant to an effective registration statement or under the requirements of Rule 144 or other applicable exemptions from registration under the Securities Act and as required under applicable state securities laws. Rule 144 provides in essence that one year following a company ceasing to be a “shell company” and filing Form 10 information with the SEC to that effect, a non-affiliate who has held restricted securities for a period of at least six months may sell their shares of common stock. Under Rule 144, affiliates who have held restricted securities for a period of at least six months may, under certain conditions, sell every three months, in brokerage transactions, a number of shares that does not exceed the greater of 1% of a company’s outstanding shares of common stock or the average weekly trading volume during the four calendar weeks prior to the sale (the four calendar week rule does not apply to companies quoted on the OTCQB). A sale under Rule 144 or under any other exemption from the Securities Act, if available, or pursuant to subsequent registrations of our shares of common stock, may have a depressive effect upon the price of our shares of common stock in any active market that may develop.
Because we were once a “shell company,” if we ever become delinquent with the filing of our reports, Rule 144 will no longer be available until and unless we become current.
Rule 144 provides, as indicated above, that sales of securities of a former shell company may only be made once the applicable waiting period has terminated and only if appropriate current information is available by the company, and that it has filed all relevant periodic reports that it is required to file. If we become delinquent with our SEC reports, any holders of restricted securities will no longer be able to sell until, if ever, the company becomes current. No assurance can be made that we will be able to remain current with its reports given the costs of an international audit and difficulty in raising capital.
26
ITEM 1B. UNRESOLVED STAFF COMMENTS
None; not applicable.
ITEM 2: PROPERTIES
Our principal offices are located at 5955 Edmond Street, Suite 102, Las Vegas, Nevada 89118. Our telephone number is 800-416-8802. Our office is approximately 185 square feet in size. The lease is month-to-month and the rate is $1,500 per month.
The Company, Through CanaLeaf’s Subscription Agreement with IHMML, we expect to commence operations in an initial leased 393,000 square foot facility located in Atholville, New Brunswick and in two other potential facilities (a 293,000 square foot facility in Poekmouche, New Brunswick and a 25,000 square foot facility in Delta, British Columbia.)
ITEM 3: LEGAL PROCEEDINGS
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties and an adverse result in these or other matters may arise from time to time that may harm our business. Except as set forth below, we are currently not aware of any such legal proceedings or claims that we believe will have a material adverse effect on our business, financial condition or operating results.
ITEM 4: MINE SAFETY DISCLOSURES
None; not applicable.
27
PART II
ITEM 5: MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is not traded on any exchange. Our common stock is quoted on OTC Bulletin Board and OTCQB Market under the trading symbol “MDCN”. We cannot assure you that there will be a market in the future for our common stock.
OTC Bulletin Board and OTCQB securities are not listed and traded on the floor of an organized national or regional stock exchange. Instead, securities transactions are conducted through a telephone and computer network connecting dealers. Issuers on these listing services are, like us, traditionally smaller companies that do not meet the financial and other listing requirements of a national or regional stock exchange.
The following table sets forth, for the periods indicated over the last two years, the high and low closing bid quotations, as reported by the OTC Bulletin Board, and represents prices between dealers, does not include retail markups, markdowns or commissions, and may not represent actual transactions:
|
2013
|
Closing Bid High
|
Closing Bid Low
|
||||||
|
January 1 – March 31
|
.0105
|
.008
|
||||||
|
April 1 – June 30
|
.0105
|
.0105
|
||||||
|
July 1 – September 30
|
.5
|
.0105
|
||||||
|
October 3 – December 30
|
.42
|
.31
|
||||||
|
2012
|
||||||||
|
January 1 – March 31
|
.0255
|
.0255
|
||||||
|
April 2 – June 29
|
.0255
|
.0255
|
||||||
|
July 2 – September 28
|
.0255
|
.008
|
||||||
|
October 1 – December 31
|
.008
|
.008
|
||||||
|
2014
|
||||||||
|
January 1- March 31
|
4.10
|
.31
|
||||||
These prices were obtained from OTC Markets and do not necessarily reflect actual transactions, retail markups, mark downs or commissions.
Holders
As of April 8, 2014, there were 182 stockholders of record of our common stock. This number does not include shares held by brokerage clearing houses, depositories or others in unregistered form.
Dividends
Any decisions regarding dividends will be made by our board of directors. We currently intend to retain and use any future earnings for the development and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Our board of directors has complete discretion on whether to pay dividends, subject to the approval of our stockholders. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant.
Securities Authorized for Issuance under Equity Compensation Plans
We do not have in effect any compensation plans under which our equity securities are authorized for issuance and we do not have any outstanding stock options.
28
Recent Sales of Unregistered Securities
We effected a forward split paid as a dividend on September 19, 2013.
On October 2, 2013, we issued 10,000 shares of common stock to Kenneth Williams, our Chief Executive Officer, and 10,000 shares of common stock to Michael Thompson in consideration of their service as a board member of the Company. The issuance of such securities was exempt from registration pursuant to Section 4(a)(2) of the Securities Act.
On November 25, 2013, we issued 500,000 shares of common stock to Cecil Sinow for cash consideration of $125,000, or $0.25 per share. The issuance of such securities was exempt from registration pursuant to Section 4(a)(2) of the Securities Act and Regulation D, Rule 506(b), promulgated thereunder. There was no general solicitation, and the investor is financially sophisticated.
On November 25, 2013, we issued 100,000 shares of common stock to our legal counsel as payment for its legal and general advisory and consulting services. The issuance of such securities was exempt from registration pursuant to Section 4(a)(2) of the Securities Act.
On December 6, 2013, we issued 20,000 shares of common stock to a business consultant for consulting and general business advisory services. The issuance of such securities was exempt from registration pursuant to Section 4(a)(2) of the Securities Act.
On December 6, 2013, we issued 10,000 shares of common stock to a creditor as consideration for an extension for repayment of an outstanding debt. The issuance of such securities was exempt from registration pursuant to Section 4(a)(2) of the Securities Act.
On January 29, 2014, we issued 20,000 shares of common stock to Kenneth Williams, 20,000 shares of common stock to Michael Thompson, and 100,000 shares of common stock to Gary Johnson in consideration for their service as board members of the Company. The issuance of such securities was exempt from registration pursuant to Section 4(a)(2) of the Securities Act.
On March 11, 2014, we issued an aggregate of 6,324,800 shares of common stock of the Company to eighteen individual sophisticated investors. The issuance of such securities was exempt from registration pursuant to Section 4(a) (2) of the Securities Act and Regulation D, Rule 506(b), promulgated thereunder. There was no general solicitation, and the investor is financially sophisticated.
On March 25, 2014, the Company issued 220,000 shares of common stock to four investors in a private placement offering. The issuance of such securities was exempt from registration pursuant to Section 4(a) (2) of the Securities Act and Regulation D, Rule 506(b), promulgated thereunder. There was no general solicitation, and the investor is financially sophisticated.
On March 25, 2014, the Company issued 175,624 shares of common stock to a creditor in exchange for forgiveness of two shareholders’ loans. The issuance of such securities was exempt from registration pursuant to Section 4(a) (2) of the Securities Act.
On March 25, 2014, the Company issued 500,000 shares of common stock to Perera Holdings Ltd. as consideration for services rendered under the Master Services Agreement. The issuance of such securities was exempt from registration pursuant to Section 4(a) (2) of the Securities Act.
On March 25, 2014, the Company issued 500,000 shares of common stock to Fong Capital Holdings Ltd. as consideration for services rendered under the Master Services Agreement. The issuance of such securities was exempt from registration pursuant to Section 4(a) (2) of the Securities Act.
On March 25, 2014, the Company issued 4,000,000 shares of common stock to Canada Bioceutical Corp. as consideration for services rendered under the Master Services Agreement. The issuance of such securities was exempt from registration pursuant to Section 4(a) (2) of the Securities Act.
29
On March 28, 2014, the Company issued 190,000 shares of common stock to an individual investor in a private placement offering. The issuance of such securities was exempt from registrations pursuant to Section 4(a) (2) of the Securities Act and Regulation D, Rule 506(b), promulgated thereunder. There was no general solicitation, and the investor is financially sophisticated.
Use of Proceeds of Registered Securities
There were no proceeds received during the three years ended December 31, 2013, from the sale of registered securities.
Purchases of Equity Securities by Us and Affiliated Purchasers
None; not applicable.
ITEM 6: SELECTED FINANCIAL DATA
See Item 8.
ITEM 7: MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Prospective investors should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included elsewhere in this Annual Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. See “Cautionary Note Regarding Forward-Looking Statements”. You should review the “Risk Factors” section of this Annual Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Plan of Operation
Our plan of operation for the next 12 months from the date of this filing is to continue to establish joint ventures with key individuals in the industry, and (through our subsidiary CanaLeaf and its partners)obtain approval from Health Canada as a licensed commercial grower and open and begin operations in the facility in the Atholville facility.
We expect that we will have significant foreseeable cash requirements related to the Management Services Agreement with IHL and the subscription agreement with IHMMA.
Under the Subscription Agreement, the Company has agreed to subscribe to shares in IHMMA according to the following schedule and in the following amounts:
|
(a)
|
4,000,000 Shares will be purchased on April 30, 2014 in consideration for payment of CAD$5,000,000;
|
|
(b)
|
a further 8,000,000 Shares will be purchased on May 31, 2014 in consideration for payment of CAD$10,000,000;
|
|
(c)
|
a further 4,800,000 Shares will be purchased on June 30, 2014 in consideration for payment of CAD$6,000,000;
|
30
|
(d)
|
a further 8,800,000 Shares will be purchased on July 31, 2014 in consideration for payment of CAD$11,000,000;
|
|
(e)
|
a further 2,400,000 Shares will be purchased on August 31, 2014 in consideration for payment of CAD$3,000,000;
|
|
(f)
|
a further 8,000,000 Shares will be purchased on September 30, 2014 in consideration for payment of CAD$10,000,000; and
|
|
(g)
|
a further 5,600,000 Shares will be purchased on October 31, 2014 in consideration for payment of CAD$7,000,000.
|
Additionally we will have expenses that will relate to maintaining our Company’s good standing or the payment of expenses associated with legal fees, accounting fees, and other general operating expenses.
Results of Operations
Year Ended December 31, 2013 Compared to the Year Ended December 31, 2012
Our current operations began on October 1, 2013. General and administrative expenses were $367,042 for the year ended December 31, 2013, compared to $9,467 for the period ended December 31, 2012. General and administrative expenses were comprised mainly of accounting, legal, consulting, and compliance fees. We had a net loss of $376,449 for the period ended December 31, 2013 compared to a net loss of $18,904 for the period ended December 31, 2012. The increase in net loss for the period ended December 31, 2013, is due to the increase in general and administrative expenses resulting from consulting fees and other services arising from the change in control of the company.
Liquidity and Capital Resources
We had no cash or cash equivalents on hand. If additional funds are required, such funds may be advanced by management or shareholders. Compensation to these individuals has been rendered in the form of stock payments and warrants as described in Note 7 to the financial statements. Various loans for advances made to the company were also incurred as described in note 3 to the financial statements.
Off-Balance Sheet Arrangements
We had no Off-Balance Sheet arrangements during the fiscal year ended December 31, 2013.
ITEM 7A: QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
31
ITEM 8: FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
MEDICAN ENTERPRISES, INC.
CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2013
TABLE OF CONTENTS
|
Report of Independent Registered Public Accounting Firms
|
33
|
|
Consolidated Balance Sheets
|
35
|
|
Consolidated Statements of Operations
|
36
|
|
Consolidated Statements of Stockholders’ Deficit
|
37
|
|
Consolidated Statements of Cash Flows
|
38
|
|
Notes to Consolidated Financial Statements
|
39
|
32

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Medican Enterprises, Inc.
5955 Edmond Street Suite 102
Las Vegas,NV 89118
We have audited the accompanying consolidated balance sheet of Medican Enterprises, Inc. (a development stage company) as of December 31, 2013 and the related statements of operations, changes in stockholders’ deficit, and cash flows for the year ended December 31, 2013 and the period from reactivation January 1, 2005 to December 31, 2013. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. The consolidated financial statements of the Company as of December 31, 2012, and for the period from reactivation January 1, 2005 through December 31, 2012, were audited by another auditor; whose report dated March 12, 2013; expressed an unqualified opinion on the financial statements and also included an explanatory paragraph that raised substantial doubt about the Company’s ability to continue as a going concern.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Medican Enterprise, Inc. as of December 31, 2013 and the results of its operations and its cash flows for the year ended December 31, 2013, and the period from reactivation January 1, 2005 to December 31, 2013 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern as indicated in Note 2. The Company has had no revenues and an accumulated deficit of $1,094,467. These conditions, among others, raise substantial doubt about the Company’s ability to continue as a going concern. Management's plans concerning these matters are also described in the Note 2, which includes the raising of additional equity financing. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Anton & Chia LLP
Newport Beach, California
April 15, 2014
33
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
Medican Enterprises, Inc. (formerly known as TC X Calibur, Inc.) [a development stage company]
We have audited the accompanying consolidated balance sheet of Medican Enterprises, Inc. (formerly known as TC X Calibur, Inc.) [a development stage company] as of December 31, 2012, and the related consolidated statement of operations, stockholders’ deficit, and cash flows for the year ended December 31, 2012 and for the period from reactivation [January 2005] through December 31, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform an audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company has determined that it is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purposes of expressing an opinion on the effectiveness of the Company’s internal controls over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Medican Enterprises, Inc. [a development stage company] as of December 31, 2012, and the consolidated results of its operations and cash flows for the year ended December 31, 2012, and for the period from reactivation through December 31, 2012, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has incurred a loss from operations and negative operating cash flows during the period from reactivation through December 31, 2012. These issues raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustment that might result from the outcome of this uncertainty.
/s/ Mantyla McReynolds, LLC
Mantyla McReynolds, LLC
Salt Lake City, Utah
March 12, 2013
34
|
MEDICAN ENTERPRISES, INC.
(A DEVELOPMENT STAGE COMPANY)
|
||||||||
|
CONSOLIDATED BALANCE SHEETS
|
||||||||
|
DECEMBER 31, 2013 and 2012
|
||||||||
|
December 31, 2013
|
December 31, 2012
|
|||||||
|
ASSETS
|
||||||||
|
Current asset:
|
||||||||
| $ | - | $ | - | |||||
|
Total assets
|
$ | - | $ | - | ||||
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$ | 176,187 | $ | - | ||||
|
Payable to related parties
|
- | 93,581 | ||||||
|
Accrued Interest - related parties
|
4,172 | 9,437 | ||||||
|
Notes Payable - related parties
|
116,529 | - | ||||||
|
Total current liabilities
|
296,888 | 103,018 | ||||||
|
Total liabilities
|
296,888 | 103,018 | ||||||
|
Stockholders’ equity (deficit):
|
||||||||
|
Preferred Stock -- 5,000,000 shares authorized, $.001 par value; 0 shares issued and outstanding
|
- | - | ||||||
|
Common stock - 50,000,000 shares authorized, $.001 par value;
|
||||||||
|
27,151,240 shares issued and outstanding as of December 31, 2013
|
||||||||
|
and 26,501,240 as of December 31, 2012
|
27,151 | 26,501 | ||||||
|
Additional paid-in capital
|
770,428 | 588,499 | ||||||
|
Accumulated deficit
|
(613,885 | ) | (613,885 | ) | ||||
|
Accumulated development stage equity
|
(480,582 | ) | (104,133 | ) | ||||
|
Total stockholders' deficit
|
(296,888 | ) | (103,018 | ) | ||||
|
Total liabilities and stockholders' deficit
|
$ | - | $ | - | ||||
|
The accompanying notes are an integral part of these consolidated financial statements.
|
||||||||
35
|
MEDICAN ENTERPRISES, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF OPERATIONS
|
||||||||||||
|
From
|
||||||||||||
|
For the
|
For the
|
reactivation
|
||||||||||
|
Year
|
Year
|
(01/01/05)
|
||||||||||
|
Ended
|
Ended
|
through
|
||||||||||
|
December 31,
|
December 31,
|
December 31,
|
||||||||||
|
2013
|
2012
|
2013
|
||||||||||
|
INCOME
|
$ | - | $ | - | $ | - | ||||||
|
OPERATING EXPENSES
|
||||||||||||
|
Selling, General, and Administrative Expense
|
367,042 | 9,467 | 461,738 | |||||||||
|
Total Operating Expenses
|
367,042 | 9,467 | 461,738 | |||||||||
|
Loss from operations
|
(367,042 | ) | (9,467 | ) | (461,738 | ) | ||||||
|
Other Income (Expense):
|
||||||||||||
|
Related Party Interest Expense
|
(9,407 | ) | (9,437 | ) | (18,844 | ) | ||||||
|
Net Other Expense
|
(9,407 | ) | (9,437 | ) | (18,844 | ) | ||||||
|
NET LOSS APPLICABLE TO COMMON SHARES
|
$ | (376,449 | ) | $ | (18,904 | ) | $ | (480,582 | ) | |||
|
NET LOSS PER BASIC AND DILUTED SHARES
|
$ | (0.01 | ) | $ | (0.01 | ) | $ | (0.02 | ) | |||
|
WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING
|
26,569,076 | 26,501,240 | 26,501,240 | |||||||||
|
The accompanying notes are an integral part of these consolidated financial statements.
|
||||||||||||
36
|
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' DEFICIT
From Reactivation at January 1, 2005 Through the Year Ended December 31, 2013
|
||||||||||||||||||||||||
|
Number of
Shares
|
Common
Stock
|
Additional
Paid-in
Capital
|
Accumulated
Deficit
|
Accumulated
Deficit During
Development
Stage
|
Net
Stockholders'
Equity Deficit
|
|||||||||||||||||||
|
Balance, January 1, 2005
|
26,501,240 | 26,501 | 588,499 | (613,885 | ) | 1,115 | ||||||||||||||||||
|
Net loss for the year ended December 31, 2005
|
(32,564 | ) | (32,564 | ) | ||||||||||||||||||||
|
Balance, December 31, 2005
|
26,501,240 | 26,501 | 588,499 | (613,885 | ) | (32,564 | ) | (31,449 | ) | |||||||||||||||
|
Net loss for the year ended December 31, 2006
|
(6,488 | ) | (6,488 | ) | ||||||||||||||||||||
|
Balance, December 31, 2006
|
26,501,240 | 26,501 | 588,499 | (613,885 | ) | (39,052 | ) | (37,937 | ) | |||||||||||||||
|
Net loss for the year ended December 31, 2007
|
(7,905 | ) | (7,905 | ) | ||||||||||||||||||||
|
Balance, December 31, 2007
|
26,501,240 | 26,501 | 588,499 | (613,885 | ) | (46,957 | ) | (45,842 | ) | |||||||||||||||
|
Net loss for the year ended December 31, 2008
|
(14,696 | ) | (14,696 | ) | ||||||||||||||||||||
|
Balance, December 31, 2008
|
26,501,240 | 26,501 | 588,499 | (613,885 | ) | (61,653 | ) | (60,538 | ) | |||||||||||||||
|
Net loss for the year ended December 31, 2009
|
(6,905 | ) | (6,905 | ) | ||||||||||||||||||||
|
Balance, December 31, 2009
|
26,501,240 | 26,501 | 588,499 | (613,885 | ) | (68,558 | ) | (67,443 | ) | |||||||||||||||
|
Net loss for the year ended December 31, 2010
|
(8,636 | ) | (8,636 | ) | ||||||||||||||||||||
|
Balance, December 31, 2010
|
26,501,240 | 26,501 | 588,499 | (613,885 | ) | (77,194 | ) | (76,079 | ) | |||||||||||||||
|
Net loss for the year ended December 31, 2011
|
(8,035 | ) | (8,035 | ) | ||||||||||||||||||||
|
Balance, December 31, 2011
|
26,501,240 | 26,501 | 588,499 | (613,885 | ) | (85,229 | ) | (84,114 | ) | |||||||||||||||
|
Net loss for the year ended December 31, 2012
|
(18,904 | ) | (18,904 | ) | ||||||||||||||||||||
|
Balance, December 31, 2012
|
26,501,240 | 26,501 | 588,499 | (613,885 | ) | (104,133 | ) | (103,018 | ) | |||||||||||||||
|
Common stock issued for services ($0.25/share)
|
650,000 | 650 | 161,850 | - | - | 162,500 | ||||||||||||||||||
|
Related party debt forgiveness
|
- | - | 9,603 | - | - | 9,603 | ||||||||||||||||||
|
Contributions from directors
|
- | - | 10,476 | - | - | 10,476 | ||||||||||||||||||
|
Net loss for the year ended December 31, 2013
|
- | - | - | - | (376,449 | ) | (376,449 | ) | ||||||||||||||||
|
Balances at December 31, 2013
|
$ | 27,151,240 | $ | 27,151 | $ | 770,428 | $ | (613,885 | ) | $ | (480,582 | ) | $ | (296,888 | ) | |||||||||
|
The accompanying notes are an integral part of these financial statements.
|
||||||||||||||||||||||||
37
|
MEDICAN ENTERPRISES, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENT OF CASH FLOWS
FROM REACTIVATION AT JANUARY 1, 2013 TO DECEMBER 31, 2013
|
||||||||||||
|
For the
|
For the
|
From
reactivation
(01/01/05)
|
||||||||||
|
Year Ended
|
Year Ended
|
through
|
||||||||||
|
December 31,
|
December 31,
|
December31,
|
||||||||||
|
2013
|
2012
|
2013
|
||||||||||
|
Operating Activities:
|
||||||||||||
|
Net loss
|
$ | (376,449 | ) | $ | (18,904 | ) | $ | (480,582 | ) | |||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Common stock issued for services
|
162,500 | - | 162,500 | |||||||||
|
Change in operating assets and liabilities:
|
||||||||||||
|
Prepaid Expense
|
- | - | 18,750 | |||||||||
|
Accounts Payable
|
176,187 | - | 175,760 | |||||||||
|
Payables to Related Parties
|
22,948 | 9,467 | 99,321 | |||||||||
|
Accrued interest - related party
|
(5,265 | ) | 9,437 | 4,172 | ||||||||
|
Net Cash Used in Operating Activities
|
(20,079 | ) | - | (20,079 | ) | |||||||
|
Investing Activities:
|
$ | - | $ | - | $ | - | ||||||
|
Financing Activities:
|
||||||||||||
|
Related party debt forgiveness
|
9,603 | - | 9,603 | |||||||||
|
Contributions from directors
|
10,476 | - | 10,476 | |||||||||
|
Net Cash provided by financing activities
|
20,079 | - | 20,079 | |||||||||
|
Net Increase in Cash
|
- | - | - | |||||||||
|
Cash, Beginning of Year
|
- | - | - | |||||||||
|
Cash, End of Year
|
$ | - | $ | - | $ | - | ||||||
|
Supplemental Disclosures of Cash flow information:
|
||||||||||||
|
Cash paid for interest
|
$ | - | $ | - | $ | - | ||||||
|
Cash paid for taxes
|
$ | - | $ | - | $ | - | ||||||
|
The accompanying notes are an integral part of these consolidated financial statements.
|
||||||||||||
38
Medican Enterprises, Inc.
Notes to the Consolidated Financial Statements
Years Ended December 31, 2013 and 2012
NOTE 1 ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Organization
Medican Enterprises, Inc. (“the Company”) was incorporated in Nevada in October, 1988, under the name Extant Investments, Inc. In 1991, the Company merged with and changed its name to Sentinel Scientific, Inc. From 1991 to 1993, the Company was involved with research and development of biomedical technologies, but ceased active operations due to lack of operating capital. In August, 1993, the Company merged with A.F.C. Entertainment, Inc. (“A.F.C.”), a Barbados corporation, which was involved with the foreign film industry. In December, 1993, the Company purchased all of the shares of Film Optical Investments Limited, a corporation organized in the Province of Ontario, Canada (“Film Opticals”) in exchange for 120,000 of its common shares. With the acquisition of Film Opticals, the Company had been engaged in the business of providing a full range of motion picture printing services and creative titles, credits and optical effects for features, commercials, theatrical and television programs. The foreign film library, acquired with the merger of A.F.C., remained intact, but funding constraints curtailed the Company’s ability to develop and market this business. Because of these constraints, the board of directors elected on December 8, 2004, to sell Film Opticals. On August 6, 2013, The Company changed its name to Medican Enterprises, Inc.
We are a pharmaceutical business currently focused on developing, distributing and marketing medical marijuana in Canada. We have established joint-venture partnerships with experienced agriculture and pharmaceutical distribution companies in Canada to facilitate this business. We also are actively searching to acquire smaller operations in the medical marijuana industry in Canada with the intention of increasing their profits through economies of scale.
We currently have three subsidiaries through which we operate our business.
Medican Systems. is a corporation incorporated under the laws of the Territory of the Yukon under incorporation number 535642 on December 30, 2013. The authorized share structure is an unlimited number of common shares without par value, with 100 common shares issued to our company, making Medican Systems our direct wholly-owned subsidiary. The primary focus of Medican Systems is to function as a holding company for Canadian based investments, joint ventures and opportunities.
Medican (Delta). is a corporation incorporated under the laws of the Province of British Columbia under incorporation number BC0989867 on December 31, 2013. The authorized share structure is an unlimited number of common shares without par value, with 100 common shares issued to Medican Systems. The primary focus of Medican Delta is to pursue opportunities in the medical marijuana industry in and around the city of Delta in British Columbia, Canada.
CanaLeaf is a corporation incorporated under laws of Canada under the Canada Business Corporation Act under incorporation number 883348-6 on March 25, 2014. The authorized share structure is an unlimited number of common shares. CanaLeaf is our principal operating subsidiary.
The consolidated financial statements of the Company have been prepared in accordance with U. S. generally accepted accounting principles. The consolidated financial statements of the Company include the accounts of Medican Enterprises, Inc. and its wholly-owned subsidiaries. All significant intercompany transactions have been eliminated. The following summarizes the more significant of such policies:
(b) Statement of Cash Flows
For purposes of the statement of cash flows, the Company considers all highly liquid debt instruments purchased with a maturity of three months or less to be cash equivalents. During the period ending December 31, 2013 and 2012 the Company did not have non-cash investing or financing activities.
39
(c) Income Taxes
The Company applies the provisions of Financial Accounting Standards Board Accounting Standard Codification (“ASC”) 740 Income Taxes. The Standard requires an asset and liability approach for financial accounting and reporting for income taxes, and the recognition of deferred tax assets and liabilities for the temporary differences between the financial reporting basis and tax basis of the Company’s assets and liabilities at enacted tax rates expected to be in effect when such amounts are realized or settled. Due to a loss from inception, the Company has no tax liability. At this time the Company has no deferred taxes arising from temporary differences between income for financial reporting and income tax purposes.
The Company classifies tax-related penalties and net interest on income taxes as income tax expense. As of December 31, 2013 and 2012, no income tax expense had been incurred or accrued.
(d) Net Loss Per Common Share
Basic loss per common share is based on the weighted-average number of shares outstanding. Diluted income or loss per share is computed using weighted average number of common shares plus dilutive common share equivalents outstanding during the period using the treasury stock method. There are no common stock equivalents outstanding, thus, basic and diluted income or loss per share calculations are the same. All per share calculations reflect the effects of the forward stock split.
(e) Impairment of Long-Lived Assets
The Company reviews long-lived assets, at least annually, to determine if impairment has occurred and whether the economic benefit of the asset (fair value for assets to be used and fair value less disposal costs for assets to be disposed of) is expected to be less than the carrying value. Triggering events, which signal further analysis, consist of a significant decrease in the asset’s market value, a substantial change in the use of an asset, a significant physical change in the asset, a significant change in the legal or business climate that could affect the asset, an accumulation of costs significantly in excess of the amount originally expected to acquire or construct the asset, or a history of losses that imply continued losses associated with assets used to generate revenue. The Company has no long-lived assets as of December 31, 2013 and 2012.
(f) Use of Estimates in Preparation of Financial Statements
The preparation of financial statements in conformity with U. S. generally accepted accounting principles (“US GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
(g) Revenue Recognition
The Company shall recognize revenues in accordance with the Securities & Exchange Commission Staff Accounting Bulletin (SAB) number 104, “Revenue Recognition.” SAB 104 clarifies application of U.S. generally accepted accounting principles to revenue transactions. Accordingly the Company shall recognize revenues when earned which shall be as products or services are delivered to customers. The Company shall also record accounts receivable for revenue earned but not yet collected. An allowance for bad debts shall be provided based on estimated losses. For revenue received in advance of service the Company shall record a current liability as deferred revenue until the earnings process is complete.
(h) Impact of New Accounting Standards
The Company has reviewed all recently issued, but not yet adopted, accounting standards in order to determine their effects, if any, on its results of operation, financial position or cash flows. Based on that review, the Company believes that none of these pronouncements will have a significant effect on its consolidated financial statements.
40
NOTE 2 LIQUIDITY/GOING CONCERN
The Company has development stage losses of $480,582, and has had negative cash flows from operating activities during the period from reactivation (January 1, 2005) through December 31, 2013. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustment that might result from the outcome of this uncertainty. Management plants to continue the operations that it has started organically within the Company since October 1, 2013. The Company currently operates as a pharmaceutical business currently focused on developing, distributing and marketing medical marijuana in Canada. The Company has established joint-venture partnerships with experienced agriculture and pharmaceutical distribution companies in Canada to facilitate this business. Management of the Company is also actively searching to acquire smaller operations in the medical marijuana industry in Canada with the intention of increasing their profits through economies of scale.
NOTE 3 RELATED PARTY TRANSACTIONS
During the years ended December 31, 2012, the Company borrowed $9,467 from a shareholder to pay operating expenses. The loan is non-interest bearing, unsecured and payable on demand. The balance of the loan as of December 31, 2012 was $93,581. The aggregate amount of related party loans is non-interest bearing, unsecured and payable on demand. However, the Company imputes interest on the loan at 10% per annum. Imputed interest expense on related party loans for the years ended December 31, 2012 totaled $9,437.
The Company offices for 2012 were those of then-President, Travis T. Jenson, and were provided at no cost. The shareholder incurred no incremental costs in providing this office space to the Company.
The Company had expenses and payables paid in its behalf by a shareholder in the amount of $116,529 during the period ended December 31, 2013. The Company imputes interest at 10% per annum. Accrued interest related party payables for the year ended December 31, 2013 totaled $4,172. As of December 31, 2013, the total amount of note payable to related parties was $116,529.
On June 25, 2013 the Company executed two promissory notes with a former shareholder. The promissory notes were for funds already paid on behalf of the Company as a partial settlement of those debts. This creditor forgave a balance due to them of $9,603 and assigned the remaining balances to a third party shareholder.
NOTE 4 EQUITY
As of December 27, 2007, the Company effected a 2.3943-for-1 forward stock split of its issued and outstanding shares of common stock. The split effectively increased the number of shares of common stock by 3,086,541 shares. On November 29, 2010, the Company effected a 1-for-4 reverse split of its issued and outstanding shares of common stock. The reverse split decreased the number of shares of common stock by 3,975,102 shares. All common share and per share amounts have been restated for all periods presented for this stock split.
On August 16, 2013, the Company’s board of directors approved a forward split paid as a stock dividend, on a basis of 20:1 (the “Stock Dividend”), pursuant to which, the Company’s shareholders as at September 24, 2013 received twenty (20) shares of our Company’s common stock for each one (1) share of common stock currently held. The pay-out date as approved by the board of directors and Financial Industry Regulatory Authority is September 25, 2013. All prior years' capital accounts of the Company have been retroactively restated to reflect the equivalent number of common stock based on the forward-split ratio of 20:1.
On October 4, 2013, 20,000 shares were issued to individuals in exchange for services. The shares were valued at $0.25 per share and had a par value of $0.001.
41
On November 25, 2013, 500,000 shares were issued for cash consideration of $125,000. The shares were valued at $0.25 per share and had a par value of $0.001.
On November 25, 2013, 100,000 shares were issued for professional services. The shares were valued at $0.25 per share and had a par value of $0.001.
On December 6, 2013, 30,000 shares were issued in exchange for services. The shares were valued at $0.25 per share and had a par value of $0.001.
NOTE 5 INCOME TAXES
The provision for income taxes consists of the following:
|
2013
|
2012
|
|||||||
|
Current tax
|
$ | - | $ | - | ||||
|
Deferred tax benefit
|
(127,993 | ) | (6,427 | ) | ||||
|
Benefits of operating loss carryforwards
|
127,993 | 6,427 | ||||||
|
Provision for Income Tax
|
$ | - | $ | - | ||||
Below is a summary of deferred tax asset calculations on net operating loss carry forward amounts as of December 31, 2013.
|
Description
|
NOL Balance
|
Tax
|
Rate
|
|||||||||
|
Net Operating Loss
|
$ | 1,073,579 | $ | 365,017 | 34 | % | ||||||
|
Related Party Interest Accrual
|
9,407 | 3,198 | 34 | % | ||||||||
|
Valuation Allowance
|
(368,215 | ) | ||||||||||
|
Deferred Tax Asset - 12/31/2013
|
$ | - | ||||||||||
Below is a summary of deferred tax asset calculations on net operating loss carry forward amounts as of December 31, 2012.
|
Description
|
NOL Balance
|
Tax
|
Rate
|
|||||||||
|
Net Operating Loss
|
$ | 706,537 | $ | 40,222 | 34 | % | ||||||
|
Related Party Interest Accrual
|
9,437 | 3,209 | 34 | % | ||||||||
|
Valuation Allowance
|
(243,431 | ) | ||||||||||
|
Deferred Tax Asset - 12/31/2012
|
$ | - | ||||||||||
A valuation allowance is provided when it is more likely than not that some portion of the deferred tax asset will not be realized. Currently there is no reasonable assurance that the Company will be able to take advantage of a deferred tax asset. Thus, an offsetting allowance has been established for the deferred asset. The valuation allowance has increased by $127,993, from the prior year balance of $243,431, as of December 31, 2012.
42
The Company has the following operating loss carry forwards available at December 31, 2013:
|
Operating Losses
|
||||
|
Expires
|
Amount
|
|||
|
2020
|
$ | 38,250 | ||
|
2021
|
12,382 | |||
|
2022
|
17,151 | |||
|
2023
|
14,274 | |||
|
2024
|
529,784 | |||
|
2025
|
32,564 | |||
|
2026
|
6,488 | |||
|
2027
|
7,905 | |||
|
2028
|
14,696 | |||
|
2029
|
6,905 | |||
|
2030
|
8,636 | |||
|
2031
|
8,035 | |||
|
2032
|
9,467 | |||
|
2033
|
367,042 | |||
|
Total
|
$ | 1,073,579 | ||
Reconciliation between income taxes at the statutory tax rate (34%) and the actual income tax provision for continuing operations follows:
|
2013
|
2012
|
|||||||
|
Expected Tax Provision
|
$ | (127,993 | ) | $ | (6,427 | ) | ||
|
Effect of:
|
||||||||
|
Increase in Valuation Allowance
|
127,993 | 6,427 | ||||||
|
Actual Tax Provision
|
$ | - | $ | - | ||||
Uncertain Tax Positions
The Company has not made any adjustments to deferred tax asset or liabilities. The Company did not identify any material uncertain tax positions of the Company on returns that have been filed or that will be filed. The Company has not had operations and is carrying a Net Operating Loss as disclosed above. Since it is not unlikely that the Net Operating loss will ever produce a tax benefit, even if examined by taxing authorities and disallowed entirely, there would be no effect on the financial statements.
The Company has filed income tax returns in the U.S. All years prior to 2010 are closed by expiration of the statute of limitations. The tax year ended December 31, 2010, will close by expiration of the statute of limitations on April 15, 2014. The years ended December 31, 2011, 2012 and 2013 are open for examination.
43
NOTE 6 SUBSEQUENT EVENTS
Sale of Stock
In March of 2014, stock was sold to various individuals at below market prices in a private placement. A total of 3,544,800 shares with a par value of $.001 were sold to various individuals at for an aggregate purchase price of $1,163,700 CAD. As of the purchase date of these payments, the value in US currency was $1,045,584.
Payment of Stock
In March of 2014, stock was issued to various individuals as payment for services rendered. A total of 9,625,624 shares with a par value of $.001 per share were issued..
Issuance of Stock Warrants
In January of 2014, stock warrants were issued. A total of 500,000 warrants were issued to purchase stock at $0.50. Par value of shares is $0.001 per share.
In March of 2014, stock warrants were issued to various individuals. A total of 2,444,800 warrants were issued to purchase stock at $.25, and a total of 550,000 warrants were issued to purchase stock at an exercise price of $.50. Par value of shares is $.001 per share.
Convertible Promissory Notes
On March 31, 2014, the Company entered into a convertible promissory note agreement for $57,500 with LG Capital Funding, LLC. The note has an interest rate of 8% per annum and is accrued. The note, at the holder’s option, is convertible at a 30% discount to market on average of the lowest 3 average closing prices over the prior 20 trading days. The Company also entered into a Securities Purchase Agreement with LG and may receive funds from a second note in the amount of $57,500 in the future.
On March 31, 2014, the Company entered into a convertible promissory note agreement for $57,500 with Adar Bays, LLC. The note has an interest rate of 8% per annum and is accrued. The note, at the holder’s option, is convertible at a 30% discount to market on average of the lowest 3 average closing prices over the prior 20 trading days. The Company also entered into a Securities Purchase Agreement with LG and may receive funds from a second note in the amount of $57,500 in the future.
Corporate Events
On March 25, 2014, we filed a Definitive Information Statement announcing that on March 6, 2014 the Company’s majority shareholder and Board of Directors authorized an increase in the authorized shares of the Company from 50,000,000 shares of common stock to 100,000,000 shares of common stock. The Information Statement has been mailed to shareholders and the Amendment to our company’s Articles of Incorporation will be filed twenty days after the mailing date.
44
ITEM 9: CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
ITEM 9A: CONTROLS AND PROCEDURES
Our management, with the participation of our principal executive and our principal financial officer, evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures as of the end of the period covered by the Annual Report were effective such that the information required to be disclosed by us in reports filed under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding disclosure. A controls system cannot provide absolute assurance, however, that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes of accounting principles generally accepted in the United States.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance of achieving their control objectives.
Our management, with the participation of the Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our internal control over financial reporting as of December 31, 2013. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control - Integrated Framework (2013). Based on this evaluation, our management, with the participation of the Chief Executive Officer and Chief Financial Officer, concluded that, as of December 31, 2013, our internal control over financial reporting was not effective.
This Annual Report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to rules of the Security and Exchange Commission that permit the Company to provide only management’s report in this Annual Report.
Changes in Internal Control Over Financial Reporting
There have been no changes in internal control over financial reporting.
ITEM 9B: OTHER INFORMATION
On August 16, 2013, our company’s board of directors approved a stock dividend, on a basis of 20:1 (the “Stock Dividend”), pursuant to which, our Company’s shareholders as at September 24, 2013 received twenty (20) shares of our Company’s common stock for each one (1) share of common stock currently held. The pay-out date was approved by our board of directors and Financial Industry Regulatory Authority is September 25, 2013.
On March 25, 2014, we filed a Definitive Information Statement announcing that on March 6, 2014 the Company’s majority shareholder and Board of Directors authorized an increase in the authorized shares of the Company from 50,000,000 shares of common stock to 100,000,000 shares of common stock. The Information Statement has been mailed to shareholders and the Amendment to our company’s Articles of Incorporation will be filed at least twenty days after the mailing date.
45
PART III
ITEM 10: DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors and Executive Officers
The following sets forth information about our director and executive officer as of the date of this report:
|
Name
|
Age
|
Positions Held
|
|
Kenneth Williams
|
42
|
CEO, Director
|
|
Michael Thompson
|
43
|
Director
|
|
Gary Johnson
|
61
|
Director
|
|
Wayne Hansen
|
71
|
CFO
|
Background and Business Experience
Mr. Kenneth Williams
Mr. Williams, age 42, is a director and the Chief Executive Officer of the Company. He has over eighteen years of work experience in information technology, security and network engineering. Mr. Williams has held various positions as a systems and network engineer and network architect. From 2004-2011, Mr. Williams worked at the University of Michigan-MBNI Research as a research network specialist. In 2011, Mr. Williams was appointed as a chief technology officer in the public sector (at Digagogo Ventures Corp.) and later served as the chief executive officer and director. Since 2008, Mr. Williams has been a state-authorized caregiver in the Michigan Medical Marijuana Program.
Mr. Wayne Hansen
Mr. Hansen, age 71, is the Chief Financial Officer of the Company. Mr. Hansen has an extensive history of corporate financial management. Mr. Hansen is President of Caulfield Capital Management Inc. and has served in that capacity since 1997. He was formerly a Managing Partner of Asia Liaison and Practice Partner of BDO Dunwoody.
The Honorable Gary Johnson
Mr. Johnson, age 61, is the former two-term Republican governor of New Mexico. Raised in North Dakota and then New Mexico, Mr. Johnson graduated from the University of New Mexico in 1975. Mr. Johnson started a construction business in 1974, which grew into a multi-million dollar enterprise in the decades since. Mr. Johnson won the governorship of New Mexico in 1994 as an upstart Republican candidate, making a name for himself over the course of his time in office as a libertarian-minded conservative. Mr. Johnson has been active in libertarian causes, including marijuana legalization, since leaving office. Mr. Johnson was the Libertarian party's nominee for president in 2012. Mr. Johnson is an accomplished athlete competing in cycling and skiing and has climbed the highest mountain on 6 of the 7 continents. In 2003, Mr. Johnson summited Mount Everest.
Mr. Michael Thompson
Mr. Thompson, age 43, received a BS in Psychology and Sociology from the University of Oregon and a Masters in Marriage and Family Therapy. From 2009 - 2013, Mr. Thompson worked in the pharmaceutical industry as a sales representative for Amgen, Inc. He left that position in 2013 to focus his efforts on alternate projects. He has started and operates a retail and wholesale electronic cigarette company. Mr. Thompson has been involved in the medical marijuana industry as a licensed provider since early 2012. He has several years of hands-on cultivation experience, which include indoor, outdoor and advance hydroponic production. He also has in-depth knowledge and experience on the processing of the cannabis flower and by-products into a variety of marketable products. Mr. Thompson has been involved in efforts to legalize cannabis both for medicinal and recreational purposes. As a part of those efforts, he has gained a broad understanding of the changing laws regarding medical marijuana. Currently, he is heading a group designed to capitalize on mainstreaming the industry in areas where recreational use of marijuana has been legalized. The goal of that endeavor is to provide an upscale retail experience for a broad range of customers. Mr. Thompson brings his expertise from both a production and retail perspective to the Board.
46
Significant Employees
Other than the foregoing named officers and directors, we have no full-time employees whose services are materially significant to our business and operations, although we rely heavily on our consultant that helps administer our website.
Family Relationships
None.
Involvement in Other Public Companies
Mr. Williams is the Chief Technology Officer of Digagogo Ventures Corp.
Involvement in Certain Legal Proceedings
During the past 10 years, to our knowledge, none of our present or former directors, executive officers or persons nominated to become directors or executive officers has been the subject of any of the following:
(1) A petition under the federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two (2) years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two (2) years before the time of such filing;
(2) Such person was convicted in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
(3) Such person was the subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him or her from, or otherwise limiting, the following activities:
(i) Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
(ii) Engaging in any type of business practice; or
(iii) Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws;
(4) Such person was the subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than sixty (60) days the right of such person to engage in any activity described in paragraph (f)(3)(i) of this section, or to be associated with persons engaged in any such activity;
47
(5) Such person was found by a court of competent jurisdiction in a civil action or by the SEC to have violated any federal or state securities law, and the judgment in such civil action or finding by the SEC has not been subsequently reversed, suspended, or vacated;
(6) Such person was found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated;
(7) Such person was the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
(i) Any federal or state securities or commodities law or regulation; or
(ii) Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
(iii) Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
(8) Such person was the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
Compliance with Section 16(a) of the Exchange Act
The common stock of the Company is registered under the Exchange Act, and therefore, the officers, directors and holders of more than 10% of our outstanding shares are subject to the provisions of Section 16(a) which requires them to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and our other equity securities. Officers, directors and greater than 10% beneficial owners are required by SEC regulations to furnish us with copies of all Section 16(a) reports they file. We have reviewed the filings and have determined that the Company’s officers and directors are not in compliance with this requirement.
Code of Ethics
We have not adopted a code of ethics that applies to our executive officers and directors. When we do adopt a code of ethics, we will disclose it in a Current Report on Form 8-K.
Nominating Committee
We have not established a Nominating Committee because, due to our lack of material operations and the fact that we presently have only four directors and executive officers. We believe that we are able to effectively manage the issues normally considered by a Nominating Committee. Following the entry into any business or the completion of any acquisition, merger or reorganization, a further review of this issue will no doubt be necessitated.
If we do establish a Nominating Committee, we will disclose this change to our procedures in recommending nominees to our Board of Directors.
Audit Committee
Our board of directors has determined that it does not have a member of its audit committee who qualifies as an "audit committee financial expert" as defined in Item 407(d)(5)(ii) of Regulation S-K, and is "independent" as the term is used in Item 7(d)(3)(iv) of Schedule 14A under the Securities Exchange Act of 1934, as amended.
48
We believe that our board of directors is capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting. We believe that retaining an independent director who would qualify as an "audit committee financial expert" would be overly costly and burdensome and is not warranted in our circumstances given the stage of our development and the fact that we have not generated any significant profitability to date. In addition, we currently do not have a nominating, or a compensation committees or committees performing similar functions nor do we have a written nominating, compensation or audit committee charter. Our director does not believe that it is necessary to have such committees because they believe the functions of such committees can be adequately performed by our board of directors.
ITEM 11: EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to the named persons for services rendered in all capacities during the noted periods. No other executive officer received total annual salary and bonus compensation in excess of $100,000.
|
Stock
|
Option
|
All Other
|
|||||||||||||||||||||||
| Name and |
Salary
|
Bonus
|
Awards
|
Awards
|
Compensation
|
Total
|
|||||||||||||||||||
|
Principal Position
|
Year
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
||||||||||||||||||
|
Kenneth Williams
|
2013
|
$ | 0 | $ | 0 | $ | 4200 | $ | 0 | $ | 0 | $ | 4,200 | ||||||||||||
|
Michael Thompson
|
2013
|
$ | 0 | $ | 0 | $ | 4200 | $ | 0 | $ | 0 | $ | 4,200 | ||||||||||||
|
Wayne Hansen
|
2013
|
$ | 5000 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 5,000 | ||||||||||||
Each of Kenneth Williams and Michael Thompson received 10,000 shares of common stock on October 4, 2013 for their services as directors of the Company.
Summary of Employment Contracts and Material Terms
On January 29, 2014, we entered into an employment contract with Wayne Hansen, our Chief Financial Officer. Under the terms of the contract, Mr. Hansen agreed to serve as Chief Financial Officer of the Company and will be compensated under the following terms: (i) Five Thousand dollars ($5,000) for the month of December 2013; (ii) Five Thousand dollars ($5,000) for the month of January 2014; (iii) Seven Thousand Five Hundred dollars ($7,500) for the month of February 2014; (iv) Seven Thousand Five Hundred dollars ($7,500) for the month of March 2014; (v) Ten Thousand dollars ($10,000) for the month of April 2014; (vi) Following April 2014, Ten Thousand dollars ($10,000) per month until amended by either party; (vii) At the option of either party, the compensation can be converted from cash to shares at a conversion rate of .25 per share. All shares converted will not be free trading until six months from the date of the agreement to be signed upon conversion; (viii) This compensation will be subject to adjustment pursuant to our employee compensation policies applicable to senior executives, as in effect from time to time.
Compensation of Directors
Our board members receive 10,000 shares of our common stock quarterly for their service on our board. Mr. Johnson received 100,000 shares of our common stock when he joined the Board of Directors during the first quarter of 2014.
ITEM 12: SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Security Ownership of Certain Beneficial Owners
The following table sets forth the ownership by any person known to us to be the beneficial owner of more than five percent (5%) of any of our outstanding voting securities as of April 8, 2014. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. The persons named in the table below have sole voting power and investment power with respect to all shares of common stock shown as beneficially owned by them. The percentage of beneficial ownership is based upon 40,531,664 shares of common stock outstanding at that date.
49
Beneficial Owners
|
Title of Class
|
Name and Address of
Beneficial Owners
|
Amount and Nature of Beneficial Ownership
|
Percent of Class
|
||||||
|
Common
|
Canada Bioceutical Corp
|
4,000,000 | 9.84 | % | |||||
|
#2700-700 W Georgia St
PO Box 10057
|
|||||||||
|
Vancouver, BC V7Y 1B8
Canada
|
|||||||||
|
Common
|
Kenneth Williams
|
17,208,920 | 42.35 | % | |||||
|
CEO, Director
|
|||||||||
|
885 Cliffs Dr #304
|
|||||||||
|
Ypsilanti, MI 48198
|
|||||||||
Management
|
Title of Class
|
Name and Address of
Beneficial Owners
|
Amount and Nature of Beneficial Ownership
|
Percent of Class
|
||||||
|
Common
|
Kenneth Williams
|
17,208,920 | 42.35 | % | |||||
|
CEO, Director
|
|||||||||
|
885 Cliffs Dr #304
|
|||||||||
|
Ypsilanti, MI 48198
|
|||||||||
|
Common
|
Wayne Hansen
|
100,000 | 0.3 | % | |||||
|
CFO
|
|||||||||
|
565 Hawthorne Rise
|
|||||||||
|
Parksville, BC V9P 2KE
Canada
|
|||||||||
|
Common
|
Gary Johnson
|
100,000 | 0.3 | % | |||||
|
Director
|
|||||||||
|
PO Box 1858
|
|||||||||
|
El Prado, NM 87529
|
|||||||||
|
Common
|
Michael Thompson
|
30,000 | 0.1 | % | |||||
|
4906 S. Lincoln Way
|
|||||||||
|
Spokane, WA 99224
|
|||||||||
|
Common
|
All Officers and directors as a group (three)
|
17,438,920 | 43.05 | % | |||||
SEC Rule 13d-3 generally provides that beneficial owners of securities include any person who, directly or indirectly, has or shares voting power and/or investment power with respect to such securities, and any person who has the right to acquire beneficial ownership of such security within 60 days. Any securities not outstanding which are subject to such options, warrants or conversion privileges exercisable within 60 days are treated as outstanding for the purpose of computing the percentage of outstanding securities owned by that person. Such securities are not treated as outstanding for the purpose of computing the percentage of the class owned by any other person. At the present time there are no outstanding options or warrants.
50
Changes in Control
There are no additional present arrangements or pledges of our securities which may result in a change in control of the Company. However, there are no provisions in our Articles of Incorporation or Bylaws that would delay, defer or prevent a change in control.
Securities Authorized for Issuance under Equity Compensation Plans
None, not applicable.
ITEM 13: CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with Related Persons
On June 25, 2013, Ken Williams, our Chief Executive Officer, entered into a series of Stock Purchase Agreements through which he acquired a majority of the then-issued and outstanding shares of the Company. Pursuant to that transaction, a change of control occurred.
Promoters and Certain Control Persons
We did not have any promoters at any time during the past five fiscal years.
Parents of the Smaller Reporting Company
We have no parent company.
Director Independence
For purposes of determining director independence, we have applied the definitions set out in NASDAQ Rule 5605(a)(2). The OTCBB on which shares of our Common Stock are quoted does not have any director independence requirements. The NASDAQ definition of “Independent Officer” means a person other than an Executive Officer or employee of the Company or any other individual having a relationship, which in the opinion of the Company's Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Based on this definition, Mr. Johnson and Mr. Thompson would be considered independent directors.
Description of Securities
Common Stock
We are authorized to issue up to 50,000,000 shares of common stock, par value $0.001 per share. Each outstanding share of common stock entitles the holder thereof to one vote per share on all matters. The holders of shares of our common stock are entitled to dividends out of funds legally available when and as declared by our board of directors. All of the issued and outstanding shares of our common stock are duly authorized, validly issued, fully paid and non-assessable. To the extent that additional shares of our common stock are issued, the relative interests of existing stockholders will be diluted.
On March 6, 2014, the Company filed a Definitive 14-C Information Statement to inform its holders of record that the Board approved an increase of the Company’s authorized shares of common stock from 50,000,000 to 100,000,000 shares, par value $0.001 per share. The Information Statement was mailed to shareholders on March 25, 2014 and an Amendment to the Articles of Incorporation will be filed with the Nevada Secretary of State twenty days following that mailing date.
51
Preferred Stock
We are authorized to issue up to 5,000,000 shares of preferred stock, par value $0.001 per share. None of these shares are designated, issued or outstanding.
ITEM 14: PRINCIPAL ACCOUNTING FEES AND SERVICES
The following is a summary of the fees billed to us by our principal accountants during the fiscal years ended December 31, 2013 and 2012:
|
Fee Category
|
2012
|
2013
|
||||||
|
Audit Fees
|
$ | 5,950 | $ | 13,319 | ||||
|
Audit-related Fee
|
0 | $ | 1400 | |||||
|
Tax Fees
|
400 | $ | 0 | |||||
|
All Other Fees
|
0 | $ | 0 | |||||
|
Total Fees
|
$ | 6,350 | $ | 14,719 | ||||
Audit Fees - Consists of fees for professional services rendered by our principal accountants for the audit of our annual financial statements and review of the financial statements included in our Forms 10-Q or services that are normally provided by our principal accountants in connection with statutory and regulatory filings or engagements.
Audit-related Fees - Consists of fees for assurance and related services by our principal accountants that are reasonably related to the performance of the audit or review of our financial statements and are not reported under “Audit fees.”
Tax Fees - Consists of fees for professional services rendered by our principal accountants for tax compliance, tax advice and tax planning.
All Other Fees - Consists of fees for products and services provided by our principal accountants, other than the services reported under “Audit fees,” “Audit-related fees,” and “Tax fees” above.
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Auditors
We have not adopted an Audit Committee; therefore, there is no Audit Committee policy in this regard. However, we do require approval in advance of the performance of professional services to be provided to us by our principal accountant. Additionally, all services rendered by our principal accountant are performed pursuant to a written engagement letter between us and the principal accountant.
PART IV
ITEM 15: EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)(1)(2) Financial Statements. See the audited financial statements for the year ended December 31, 2013 contained in Item 8 above which are incorporated herein by this reference.
52
(a)(3) Exhibits. The following exhibits are filed as part of this Annual Report:
|
Exhibit No.
|
Identification of Exhibit
|
|
3.1
|
Restated Articles of Incorporation(3)
|
|
3.2
|
Bylaws(1)
|
|
3.3
|
Amended Articles of Incorporation*
|
|
10.1
|
Amended Master Services Agreement dated March 13, 2014*
|
|
10.2
|
Subscription Agreement dated April 8, 2014*
|
|
14.1
|
Code of Ethics(2)
|
|
21.1
|
List of Subsidiaries*
|
|
31.1
|
Certification of Principal Executive Officer as adopted pursuant to section 302 of the Sarbanes-Oxley Act of 2002*
|
|
31.2
|
Certification of Principal Financial Officer as adopted pursuant to section 302 of the Sarbanes-Oxley Act of 2002*
|
|
32.2
|
Certification of Principal Executive and Financial Officer pursuant to 18 U.S.C section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*
|
|
101.INS
|
XBRL Instance Document*
|
|
101.SCH
|
XBRL Taxonomy Extension Schema*
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase*
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase*
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase*
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase*
|
(1) Filed with our initial Form 10 and incorporated herein by reference.
(2) Filed with our initial Form 10-KSB for December 31, 2006, and incorporated herein by reference.
(3) Filed with our initial Form 10-KSB for December 31, 2007, and incorporated herein by reference.
(4) Incorporated herein by reference.
*Filed herewith.
53
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
MEDICAN ENTERPRISES, INC
|
Date:
|
April 15, 2014
|
By:
|
/s/Kenneth Williams
|
|
|
Kenneth Williams, CEO, and Director
|
Pursuant to the requirements of the Securities Exchange Act of 1934 this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Date:
|
April 15, 2014
|
By:
|
/s/Kenneth Williams
|
|
|
Kenneth Williams, CEO, and Director
|
||||
|
Date:
|
April 15, 2014
|
By:
|
/s/Wayne Hansen
|
|
|
Wayne Hansen, Chief Financial Officer
|
54
