Attached files
| file | filename |
|---|---|
| EX-31.2 - CERTIFICATION - Rafina Innovations Inc. | ex312.htm |
| EX-31.1 - CERTIFICATION - Rafina Innovations Inc. | ex311.htm |
| EX-32.1 - CERTIFICATION - Rafina Innovations Inc. | ex321.htm |
| EXCEL - IDEA: XBRL DOCUMENT - Rafina Innovations Inc. | Financial_Report.xls |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C.20549
Form 10-K
|
(Mark One)
|
|
|
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the fiscal year ended December 31, 2013
|
|
|
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the transition period from __________ to __________
|
|
|
000-53089
|
|
|
Commission File Number
|
|
|
HCi VioCare
|
|
|
(Exact name of registrant as specified in its charter)
|
|
|
Nevada
|
30-0428006
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
Centrum Offices, 38 Queen Street, Glasgow, U.K.
|
G1 3DX
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
(0808) 178 4373
|
|
|
(Registrant’s telephone number, including area code)
|
|
|
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
|
|
Title of each class
|
Name of each exchange on which registered
|
|
n/a
|
n/a
|
|
Securities registered pursuant to Section 12(g) of the Exchange Act:
|
|
Common Stock
|
|
Title of class
|
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
|
Yes
|
[ ]
|
No
|
[X]
|
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act.
|
Yes
|
[ ]
|
No
|
[X]
|
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
|
Yes
|
[X]
|
No
|
[ ]
|
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
|
Yes
|
[X]
|
No
|
[ ]
|
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.
|
Yes
|
[ ]
|
No
|
[X]
|
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
[ ]
|
Accelerated filer
|
[ ]
|
|
Non-accelerated filer
|
[ ]
|
Smaller reporting company
|
[X]
|
|
(Do not check if a smaller reporting company)
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
|
Yes
|
[ ]
|
No
|
[X]
|
|
The aggregate market value of voting and non-voting common stock held by non-affiliates of the registrant as at June 30, 2013(the last business day of the registrant’s most recently completed second quarter),was approximately $110,000 based on the $0.20 per share which was the last selling price of the Company’s common stock, assuming solely for the purpose of this calculation that all directors, officers and greater than 10% stockholders of the registrant are affiliates. The determination of affiliate status for this purpose is not necessarily conclusive for any other purpose.
|
APPLICABLE ONLY TO REGISTRANTS INVOLVED IN BANKRUPTCY
PROCEEDINGS DURING THE PAST 5 YEARS:
Indicate by check mark whether the issuer has filed all documents and reports required to be filed by Section 12, 13, or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court.
|
Yes
|
[ ]
|
No
|
[ ]
|
APPLICABLE ONLY TO CORPORATE REGISTRANTS
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date.
|
5,003,650 shares of common stock issued and outstanding as of March 24, 2014
|
DOCUMENTS INCORPORATED BY REFERENCE
List hereunder the following documents if incorporated by reference and the Part of the Form 10-K (e.g. Part I, Part II, etc.) into which the document is incorporated: (1) Any annual report to security holders; (2) Any proxy or information statement; and (3) Any prospectus filed pursuant to Rule 424(b) or (c) of the Securities Act of 1933. The listed documents should be clearly described for identification purposes.
|
None
|
|
Page
|
||
|
PART I
|
||
|
Business
|
2 | |
|
Risk Factors
|
19 | |
|
Unresolved Staff Comments
|
29 | |
|
Properties
|
29 | |
|
Legal Proceedings
|
29 | |
|
Mine Safety Disclosures
|
29 | |
|
PART II
|
||
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
31 | |
|
Selected Financial Data
|
32 | |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
33 | |
|
Quantitative and Qualitative Disclosures About Market Risk
|
34 | |
|
Financial Statements and Supplementary Data
|
34 | |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
35 | |
|
Controls and Procedures
|
35 | |
|
Other Information
|
36 | |
|
PART III
|
||
|
Directors, Executive Officers and Corporate Governance
|
37 | |
|
Executive Compensation
|
39 | |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
41 | |
|
Certain Relationships and Related Transactions, and Director Independence
|
42 | |
|
Principal Accounting Fees and Services
|
43 | |
|
PART IV
|
||
|
Exhibits, Financial Statement Schedules
|
44 | |
| 45 |
Forward Looking Statements
This Annual Report on Form 10-K (“Annual Report”) contains forward-looking statements. These statements relate to future events or our future financial performance. A number of important factors could cause our actual results to differ materially from those expressed in any forward-looking statements made by us in this Form 10-K. In some cases, you can identify forward-looking statements by terminology such as "may," "should," "expects," "plans," "anticipates," "believes," "estimates," "predicts," "potential," or "continue" or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks in the section entitled "Risk Factors" and the risks set out below, any of which may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the "Risk factors" section of this annual report. These risks include, by way of example and not in limitation:
|
·
|
we have a limited operating history, and have never generated any revenue, and we currently have no commercial products;
|
|
·
|
we currently have one technology which requires further development to commercialize its applications. Our ability to generate product revenues, which may not occur for several years, if ever, will depend on the successful development and commercialization of our SocketFit technology;
|
|
·
|
our current and any future collaborations with third parties for the development and commercialization of our current product or any newly acquired products or businesses may not be successful;
|
|
·
|
we intend to acquire and operate clinics related to our business, however, there can be no assurance that we will successful in doing so or if that if we do acquire such clinics that they will be profitable;
|
|
·
|
risks related to the failure to successfully manage or achieve growth of our business if we are successful in development of our technology or the acquisition of clinics; and
|
|
·
|
other risks and uncertainties related to our business strategy.
|
This list is not an exhaustive list of the factors that may affect any of our forward-looking statements. These and other factors should be considered carefully and readers should not place undue reliance on our forward-looking statements.
Forward looking statements are made based on management’s beliefs, estimates and opinions on the date the statements are made and we undertake no obligation to update forward-looking statements if these beliefs, estimates and opinions or other circumstances should change. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
The safe harbors of forward-looking statements provided by Section 21E of the Exchange Act are unavailable to issuers of penny stock. As we issued securities at a price below $5.00 per share, our shares are considered penny stock and such safe harbors set forth under the Private Securities Litigation Reform Act of 1995 are unavailable to us.
Our financial statements are stated in United States dollars and are prepared in accordance with United States generally accepted accounting principles.
In this annual report, unless otherwise specified, all dollar amounts are expressed in United States dollars and all references to "common stock" refer to the common shares in our capital stock.
As used in this Annual Report, the terms "we," "us," "Company," "our" and "VioCare" mean HCi VioCare, unless otherwise indicated.
Background
The address of our principal executive office is Centrum Office, 38 Queen Street, Glasgow, UK G1 3DX. Our telephone number is (0808) 178 4373. The Company’s web-site is currently under development and in beta testing.
Our common stock is quoted on the Over the Counter Bulletin Board (“OTCBB”) and the OTC Markets Inc. owned and operated Inter-dealer Quotation System (“OTCQB”) under the symbol "VICA".
We were incorporated in the State of Nevada on March 26, 2007 as a company intending to sell medical devices in the northern regions of China. Our intent was to seek strategic relationships with medical device manufacturers both in China and North America with the aim to be their sales and distribution agent in northern China. We also intended to assist Chinese medical device manufacturers on the development of the North American market. The Company did not find suitable relationships with which to progress its business until it undertook a change in management in September 2013. With the change in management, the Company determined that it would initially concentrate on the development and marketing of medical devices in Europe, more particularly initially in Scotland, where management had identified several opportunities for entry into the markets for prosthetics and orthotics. We are currently engaged in healthcare innovation in the fields of prosthetics and orthotics (P&O), and we intend to be engaged in the operation of P&O total rehabilitation clinics. We have two recently incorporated wholly-owned Scottish subsidiaries: HCi VioCare Technologies Limited and HCi VioCare Clinics UK Limited, which were incorporated on January 15, 2014.
On December 27, 2013, pursuant to approval of the board of directors and the majority stock holder on November 28, 2013 and November 29, 2013 respectively, the Company filed a Certificate of Amendment to the Articles of Incorporation of the Company for the purpose of clearly providing the directors of the Company with the authority to issue both common and preferred stock without approval by the stockholders and to grant the authority of the directors of the Company to issue such shares of common and/or preferred stock in one or more series, with such voting power, designation, preferences and rights or qualifications, limitations or restriction as they may determine by resolution of the Board of Directors.
On January 13, 2014, the Company filed a Certificate of Designation with the Secretary of State of the State of Nevada. The Certificate of Designation sets forth the rights, preferences and privileges of a class of the Company’s preferred stock. Such class shall be designated as the “Series A Preferred Stock” and the number of shares constituting such series shall be 5,000,000 shares. The holders of Series A Preferred Stock will be entitled to a preference over all of the shares of the Company’s common stock. The holders of common stock and the holders of Series A preferred stock vote together as a single class with the holders of the Series A preferred stock having 50 votes per share of Series A preferred stock and the holders of common stock having 1 vote per share of common stock. Holders of Series A Preferred Stock are entitled to notice of any stockholders’ meeting. No shares of Series A preferred stock have been issued as of the filing date of this report.
On February 12, 2014, we acquired the intellectual property rights over what we believe to be an innovative medical technology which we refer to as the ‘SocketFit’ technology.
On March 21, 2014, the Company’s name changed from China Northern Medical Device, Inc. to HCi VioCare in regard to actions taken on November 28, 2013, when the Board of Directors of the Company (the “Board”) approved, and recommended to the Majority Stockholder that they approve the Name Change. On November 29, 2013, the Majority Stockholder approved the Name Change by written consent in lieu of a meeting, in accordance with Nevada law. On February 21, 2014, the Company submitted the Name Change to FINRA for their review and approval. On February 26, 2014, the Company received notification from FINRA that on March 20, 2014, FINRA would announce the Name Change on the FINRA 03/20/2014 Daily List and that the Name Change would take effect at the open of business on March 21, 2014. At that time the Company’s new symbol became VICA. The Company filed an amendment to our Articles of Incorporation with the Secretary of State of Nevada changing our name to HCi VioCare to be effective on March 21, 2014.
Other than as set out herein, we have not been involved in any bankruptcy, receivership or similar proceedings, nor have we been a party to any material reclassification, merger, consolidation or purchase or sale of a significant amount of assets not in the ordinary course of our business.
Our Current Business – Research and Development and Marketing of devices for healthcare innovation and operation of Prosthetics and Orthotics clinics
We are a development stage company, and are primarily engaged in the research and development and marketing of innovative medical technologies, and intend to be engaged in the operation of prosthetics and orthotics (“P&O”) and diabetic foot total rehabilitation clinics.
On the 15th of January, 2014, we formed our wholly-owned subsidiary, HCi VioCare Technologies Limited (“VioCare Technologies”), a corporation incorporated pursuant to the laws of Scotland, under registration number SC467480. VioCare Technologies intends to research, develop and commercialize state of the art, new, innovative medical devices, methods and products in the fields of prosthetics, orthotics, rehabilitation, bioengineering, mobility, diabetes, diabetic foot, tissue mechanics, ultrasonics, medical signal processing and analysis, medical technology, orthopedics and robotic surgery. VioCare Technologies also plans to expand its research and development reach, to create a stable pipeline of new products and upgrades in the fields of prosthetics, orthotics, and diabetes. VioCare Technologies is intended to be staffed by a world class scientific team, and its current research and development (“R&D”) takes place in Scotland, UK. Dr. Christos Kapatos, the developer of our recently acquired SocketFit technology detailed below will head the R&D and expects to work with a Scientific Advisory Board which we are currently forming in order to ensure that we have the best possible advice and guidance on our developing technologies.
On the 12th of February, 2014 we acquired intellectual property rights ("IPR”) over an innovative medical technology entitled ‘SocketFit’, as well as over any additional technologies under development or that may be developed in the future. SocketFit is a system that will help overcome technical and resource hurdles endemic to the prosthetic sector. The system has been designed with the aim of offering optimally fitted prosthetic sockets that will reduce the number of prostheses made for, resulting in a reduced number of visits by the patient to the prosthetic, and also assisting in the rehabilitation of amputees. VioCare Technologies intends to fund any further development on the aforementioned innovative technology, as well as to complete the development of five additional technologies in P&O and wearable devices which it is currently in negotiation to acquire from Dr. Kapatos. More specifically, it intends to launch three products which it hopes to acquire from Dr. Kapatos during the 2nd and 3d quarter of the year 2014 (Light-IS, W-Mode, and H-Cast). These products require less research and development to get to market. Remaining products that it hopes to acquire it will launch in 2015 and 2016 (SocketFit, A-Cone, D-Ring). As at the date of this filing, the only technology which has been acquired by VioCare Technologies is SocketFit which will take a minimum of two years to get to market from the commencement of the research and development of the product.
SocketFit:
Details of the Project
The number of amputees world-wide is estimated to be 20 million, and for most amputees finding a well-fitted prosthesis is far from easy. Traditional methods of design, manufacture and fitting of a prosthetic socket are typically carried out by “artisan” techniques but unfortunately often result in ill-fitting devices that make wearing a prosthesis almost intolerable for a large number of amputees.
A prosthetic socket is a custom-made “cone” that connects the rest of the prosthesis (foot, shank and knee) to an amputee’s residual limb. Sockets generally need to be replaced once or twice a year. While many significant technological advances have been made with the design and manufacture of prosthetic components, such as electronic knee assemblies and feet, socket design has not kept up. Over the past 20 years a great deal of research has been undertaken to automate the process of socket design and manufacturing, but it has met with limited success. Most sockets continue to be created with hand-sculpted plaster moulds made by the Prosthetist or a technician hours or days after examining the amputee. The result is that, typically, one in four sockets are discarded because of their poor fit.
Many prosthetists, (if not all), are frustrated by the lack of an objective method that would ensure an optimum fit of the socket. This ‘need’ for assistance instigated the advent of industrial sector technologies to be taken up in the field, even though these systems, in reality, lack the necessary ‘ingredient’ to allow the optimum socket to be produced. They merely attempt to replicate current practices in a digital manner (CAD-CAM systems), without taking into account the biomechanical or anatomical characteristics of the residual limb.
The key element of the project is work done by Dr. Christos Kapatos to improve the nature of the data used in socket modeling software. Finite element analysis has been used widely in a variety of applications, including prosthetics. But its prosthetics applications have suffered from the fact that only external boundary data and limited information on the nature of the internal tissue was provided. The results were not promising. By including far more data on the nature of the internal anatomy as well as, for the first time ever (to our knowledge) data on the bio-mechanical properties of the tissue to the FEA, a system can be created that enables Prosthetists to build a socket that evenly distributes weight, provides enhanced comfort, and raises the bar across the industry on socket creation.
SocketFit is a digital system for assessing an amputee’s residual limb and for the production of truly functional and comfortable prosthetic sockets. It takes account of the external and internal geometry of the amputee’s stump, the biomechanical properties of the each individual soft tissue layer i.e. skin, fat, muscle and bone, and the boundary and loading conditions of a complete prosthesis to generate a virtual 3D model of the residual limb. It is then possible to produce an accurate, functional and comfortable prosthetic socket.
Socket Fit Background IPR
Existing techniques of socket production can neither provide information relating to the internal geometry of the residual limb nor offer information on the biomechanical properties of living tissue. The introduction of a system that is capable of providing the Prosthetist with on-line information concerning a detailed mapping of the residual limb and the pressure distribution at the stump/socket interface, as well as the maximum stresses in the tissue, is an innovation in this field and offers the following key advantages over existing techniques:
|
·
|
Provides optimally fitted prosthesis.
|
|
·
|
Provides the Prosthetist with on-line information concerning the detailed mapping of the residual limb (external and internal geometry and structure).
|
|
·
|
Real diagnosis of residual limb problems.
|
|
·
|
Accelerates the rehabilitation of the patient.
|
|
·
|
Improves the quality of prosthetic socket design.
|
|
·
|
Reduces costs by reducing the number of visits to the Prosthetist by the patient.
|
|
·
|
Reduces costs by reducing materials and time wastage due to poor fitted prostheses.
|
|
·
|
Improves the physical and mental well-being of the patients and therefore increase the usage of their prosthesis as they will be more satisfied and feel more comfortable wearing them.
|
|
·
|
The system requires minimum training and familiarisation by the Prosthetists.
|
|
·
|
Compact, mobile, and cost-effective.
|
Dr Christos Kapatos has carried out extensive research in designing a new system to aid the creation of sockets for prosthetic limbs. This research differed from other research as it aimed to acquire and utilise not only the external shape of a residual limb but also:
|
·
|
The internal anatomy of the limb.
|
|
·
|
Bio-mechanical properties of each individual tissue layer of the residual limb.
|
|
·
|
Known boundary conditions (i.e. socket template information and the effects of internal and external forces on the residual limb).
|
By minimising the time and cost of socket production and by reducing the number of faulty sockets (it has been reported that a quarter of all prostheses are currently rejected due to poor fit), there will be a reduction in costs incurred by health services and insurance companies worldwide as well as great benefits to the amputee.
Socket-Fit consists out of three modules:
|
1.
|
The Ring - a device to scan the residual limb and export data.
|
|
2.
|
Data Tools – software to analyse and collate the data into intelligible information.
|
|
3.
|
Socket Modelling – FEA simulation procedures that combine scanned information with known boundary conditions to create a model of a socket for the specific amputee.
|
The system consists of a “ring” attached on a vertical axis, capable of moving vertically along this axis as well as spinning, with the use of a stepper motor and a liner stepper slider. The “ring” is equipped with ultrasound transducers and load transducers as well as with rotary position sensors. An ultrasound transducer and a load transducer are placed on top of each other at the tip of a protruding (spring-supported) arm at the internal side of the ring. A rotary position sensor is also attached on the base of the arm. Two such arms are present on the device.
The external geometry of the medium under examination, is acquired by the position data from the stepper instruments and the use of the rotary position sensors; as the ring moves vertically, spinning at the same time, the spring-supported arms comes in contact with the entire residual limb, mapping every detail and transmitting it to a PC.
The internal geometry is acquired with the use of the on-board ultrasound transducers. Information on the mechanical properties of the tissues are also been captured.
All the data are telemeter to the control box for onward transmission to the PC. Software generates a 3D image of the stump’s external and internal geometry. FEA and optimisation software generate the optimum design for the socket in order to obtain the best pressure distribution, and therefore the most comfortable prosthesis for the amputee. (Figures 1 and 2).
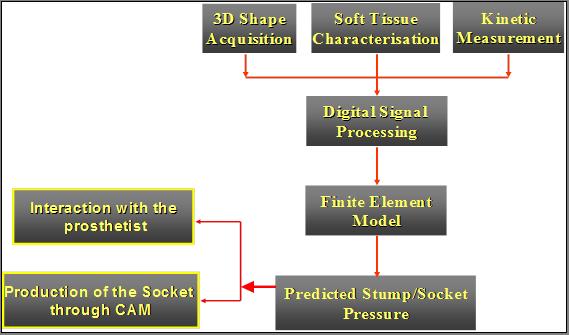
Figure 1, Basic elements of the SocketFit System.
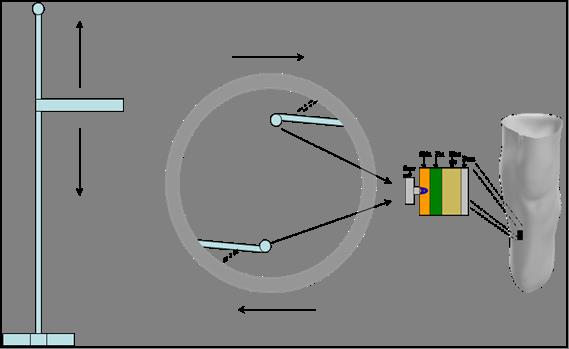
Figure 2, Schematic diagram of the system.
The software
Automation software that controls the hardware, and collects and pre-processes the data.
The automation software calibrates all the sensors and initialises them, controls the motion of both the stepper motor and the slider and acquires the scanning data. These are then saved in a series of ASCI format files and are passed to the appropriate analysis software.
The 1D ultrasonic data are processed by dedicated signal analysis software, which by incorporating a number of advanced analysis methods, such as de-convolution, spectral analysis and speckle registration, produces 2D images/maps of the medium under investigation. It also calculates the displacement caused to the different tissue layers inside the medium by the load applied by the contact “arms”.
The displacement data supplied by the ultrasonic analysis software, together with the pressure data collected by the pressure sensor, are fed in custom made bio-mechanical properties calculation software. The data, in the form of stress vs. strain are processed and the bio-mechanical properties of the tissue layers in the medium under examination calculated.
The final step of the analysis is the Finite Elements Analysis software, or FEA.
A 3D geometry is generated by the 2D images supplied by the ultrasonic analysis software and the appropriate bio-mechanical properties are input into the FE model. The FEA software then generates the shape a prosthetic socket that applies even pressure at the entire surface of the specific medium, residual limb, apart of course from specific points that cannot take any pressure.
The final data from the FEA analysis will be transferred, by means of email or uploaded on data cloud to a 3D carver or a 3D printer where the prosthetic socket, or in the case of a carver the negative mould, will be created. The amputee will then wear the prosthesis and continue with his/her way of living.
1. Control and Acquisition Software
The vertical and rotational movement of the Ring is controlled by the control software. Based upon thorough testing of the device the optimum resolution for rotational movement was calculated to be 1 degree and for vertical movement 1 mm.
The software then acquires and saves the scanning data for later use in the Ultrasonic Analysis and Bio-mechanical Properties Software. Data is saved in a series of ASCI format files and then passed to the appropriate analysis software. Stored data includes:
|
·
|
pressure data
|
|
·
|
displacement data
|
|
·
|
positional data
|
|
·
|
coordinates data
|
|
·
|
ultrasound data
|
2. Ultrasonic Analysis Software
The Ultrasonic Analysis Software is a set of signal and image processing algorithms that have been combined to analyse and process the scanned data.
The algorithms were initially developed and used very successfully in the seismic exploration industry. The algorithms are been adapted and optimised to work with Ultrasound for the first time.
The 1D ultrasonic data derived from the scan is processed by dedicated signal analysis software, which by incorporating a number of advanced analysis methods, such as de-convolution, spectral analysis and speckle registration, produces 2D images/maps of the medium under investigation.
Additionally it also calculates the displacement caused to the different tissue layers inside the medium by the load applied by the contact “arms”.
2D Images will only include the following anatomical information:
|
·
|
Bones (including exact positional information)
|
|
·
|
Fat (note we are only interested in the fat surrounding the muscle and not any fat in between muscles in the centre of the leg)
|
|
·
|
Other Materials (all other materials will be grouped together as one, i.e. different muscle groups, etc.)
|
The displacement data supplied by the ultrasonic analysis software, together with the pressure data collected by the pressure sensor, are fed to a custom-made bio-mechanical properties calculation software.
3. Volume Rendering
This module will take each of the 2D slices and generate a 3D representation of the limb. This representation will be fed into the Socket Modeling module.
There is still some debate around the best tools to use for this module. Options include:
|
·
|
developing a bespoke application
|
|
·
|
use an existing application
|
|
·
|
use existing facilities within FEA
|
4. Bio-Mechanical Tools
The Bio-Mechanical Tools are a set of algorithms that use Bio-Mechanical data derived from the scans to generate stress-strain curves for the tissue within the limb.
This software can predict the stress distribution at any point inside the residual limb, by just knowing the surface pressure applied. The calculated stress, together with the known strain that is calculated by the ultrasonic analysis software from tissue displacement data, produce actual stress vs. strain curves which characterise the tissue layers derived from.
This information will also be fed into the Socket Modelling module.
Socket Modelling
The final step of the analysis is the Finite Elements Analysis software, or FEA. The FEA module incorporates all the information output from the Data Tools (external and internal geometry and bio-mechanical properties) against known Boundary Information to create a model of a customised “uniform pressure” socket for the amputee.
Boundary Information will include:
|
1.
|
Standard Socket Information – template sockets exist which are modified to suit individuals.
|
|
2.
|
Information regarding the impact of external and internal forces (e.g. weight of the amputee).
|
The FEA software then generates the shape a prosthetic socket that applies even pressure at the entire surface of the specific medium, residual limb, apart of course from specific points that cannot take any pressure.
The final data from the FEA analysis will be transferred, by means of email, CDs or any other media, to a 3D carver or a 3D printer where the prosthetic socket, or in the case of a carver the negative mould, will be created. The amputee will then wear the prosthesis and continue with his/her way of living.
Current Status of SocketFit
We have just acquired the rights to the Background IPR and are putting together our development plans for ongoing research and development which we hope will result in a marketable product. We plan to finalize research and development and to launch the product within two years of commencement of the research and development.
Clinics:
On January 15, 2014,we formed our other wholly-owned Scottish subsidiary, HCi VioCare Clinics UK Limited with registration number SC467486 (“VioCare Clinics”), with the intent to create the first state-of-the-art chain of Prosthetic and Orthotic (P&O) and diabetic foot clinics with global reach, while capitalizing on the aforementioned internally developed technologies. VioCare Clinics will establish and/or acquire movement rehabilitation clinics, limb fitting centers and diabetic foot centers, in the UK, Europe and the Middle East, initially. These centers will offer standardized and specialized rehabilitation and consultation services for the physically impaired, as well as for their families, friends, their workplaces and companies that employ or work with them. Some of the sections that will be covered by the centers are:
|
·
|
Prosthetic rehabilitation;
|
|
·
|
Orthotic rehabilitation;
|
|
·
|
Diabetic foot;
|
|
·
|
Foot congenital disorder and musculoskeletal defects;
|
|
·
|
Movement assisting devices;
|
|
·
|
Gait analysis;
|
|
·
|
Pediatric movement analysis and consultation;
|
|
·
|
Living and working spaces design and consultation for people with disabilities;
|
|
·
|
Walking training and body strengthening following amputation, stroke or accident;
|
|
·
|
«Phantomlimb» syndrome consultation;
|
|
·
|
Workshops and educational and training programs; and
|
|
·
|
Expert opinion mediation with insurance companies and organizations.
|
The primary customers will be the physically impaired:
|
·
|
Amputees;
|
|
·
|
People with spasticity;
|
|
·
|
People with walking difficulties;
|
|
·
|
People with genetic abnormalities;
|
|
·
|
People with deformities;
|
|
·
|
People with musculoskeletal pain;
|
|
·
|
People with diabetes;
|
|
·
|
People with flat foot problems or just pain caused by long working hours or hard labor; and
|
|
·
|
Companies or organizations that employ or work with people with disabilities.
|
However the market is not limited to these people; our customers will be families with children with disabilities and businesses that employ disabled persons.
VioCare Clinics will operate under British standards, and will be certified by the International Society of Prosthetics and Orthotics. We have commenced negotiations to acquire our initial clinic in Scotland and hope to have the acquisition completed before the end of our second report quarter, June 30, 2014.
Market and Industry
It is estimated that the global health market is growing by 6-8% per annum, which suggests it will double in the next 10 years. The global Orthopedic Prosthetics market seems to be following the same pattern, as it is growing rapidly, and is projected to exceed US$ 4.5 billion by 2017, according to the 2011 Global Data’s report “Orthotics and Prosthetics - Global Pipeline Analysis, Competitive Landscape and Market Forecasts to 2017”.
Demand for orthopedic prosthetics products is driven by a multitude of factors, including:
|
·
|
growing elderly population in the West: The percentage of people aged 65 and older will increase at a rate three times that of younger generations. Subsequently, the frequency of vascular diseases and diabetes – diseases that often lead to amputation – will increase considerably;
|
|
·
|
causes of amputation: the most common causes of amputation are prosperity diseases related to diseases, such as diabetes and vascular diseases. Accidents account for only 26% of all amputations in developed countries. Indicatively, it is estimated that the number of people with diabetes worldwide (382 million as of November 2013) is expected to reach 592 million by 2035. As a result of the general increase in diabetes and vascular diseases, the number of amputations in the western world is also likely to continue to increase;
|
|
·
|
rising incidence of degenerative joint diseases, such as osteoporosis and arthritis, and increasing numbers of sports injuries;
|
|
·
|
social demands: the growing desire of more and more disabled individuals – especially young people – to become independent, lead active lifestyles, and go about their daily routines without outside help;
|
|
·
|
renewal: the lifetime of traditional prosthetic devices used by previous generations is three to five years. Also prosthetic sockets require replacement after three to six months;
|
|
·
|
market niche: reorganization of healthcare services has led to increased pressure to lower costs within the health sector, which in turn increases the demand for the efficiency of the solutions chosen; and
|
|
·
|
the growing demand for orthopedic prosthetics products is also driving the development of innovative medical devices forward. Advancements in biological science and stem cells technology, as well as rapid technological advances in medical science and development of durable and improved materials and implants have led to large number of people requiring physical rehabilitation, thus contributing to the market’s growth.
|
Overall, the market is set to grow significantly as the incidence of amputation increases. The number of amputees living on developing countries is estimated by the World Health Organization to have reached 300 million with up to 95% lacking access to prosthetic devices, thereby creating an increasing consumer base in need for affordable and reliable orthopedic prosthetics products and services that will improve the amputees’ long-term health and well-being.
Current solutions are inadequate, however, as state-of-the-art solutions from the US and Europe are cost-prohibitive, while low-cost devices run the risk of poor quality and/or unreliable performance. Especially in low-income areas and in developing countries, where the P&O industry is highly fragmented and poorly organized, with significant disparities in terms of clinical infrastructure, provision of medical rehabilitation and assistive devices, demographics (such as gender and/or socioeconomic inequalities), and funding.
Competition
The global Orthopedic Prosthetics industry is highly competitive. The major companies in the sector with whom we compete, through our subsidiary VioCare Clinics, are: Otto Bock with a market share of circa 20%, Ossur with a turnover of around $74 million, USMC who is mostly active in the USA, Blatchford that dominates the domestic market in the UK, and Hanger. The ten largest firms in the field have a combined market share of approximately 40%, and the remaining 60% is divided amongst over 90 smaller companies. In addition, we compete, through our second subsidiary VioCare Technologies, with companies such as General Electric, Siemens, and Google.
Although we are a development stage company with limited operating history, it is intended that our scientific team will lead the technological diversification of the clinics, thereby setting our Company apart from the competition. We will capitalize on our Scientific Board’s vast network of connections to attract customers from neighboring nations and will seek cooperation with global Medical Tourism operators. Moreover, our internally developed technologies are innovative and address very important problems. SocketFit, for example, we believe is unlike any known product currently being sold in the prosthetics sector, and, as such, it does not currently have any direct competitors. Although its target market is niche, given its innovative character and provided that it addresses a significant issue in the sector, it could potentially achieve high penetration levels.
Furthermore, we hope to compete successfully in the prosthetics orthotics industry also by keeping our costs low, relying on the strength of our management’s contacts, and using our size and experience to our advantage by adapting quickly to changing market conditions or responding swiftly to potential opportunities.
Research and Development
As at December 31, 2013, the date of this Annual Report, we had expended a total of $2,739 on research and development.
Intellectual Property
We have acquired exclusive ownership of the innovative medical device ‘SocketFit’. Dr. Kapatos in conjunction with another development Company had prior filed a patent application which has been abandoned. Significant development of the technology has been undertaken since the filing of the abandoned patent and, after corresponding with our patent agents, it was deemed in the best interests of the Company not to revive the old patent, but to apply for new – multiple patents on the technology, and use the old patent as background technology, as new innovations have sprang out of the initial patent and additional fields of application have been identified.
Government Regulations
SocketFit
Our initial market for our products is intended to be the EU, particularly Scotland and Greece. However, we may also market our products in the USA once we have established our European market.
EU Directives
We will be required to comply with the EU Directives regarding medical devices, which apply to all EU member states, including Greece and the UK. The basic relevant EU Directive which applies to our case is Directive 93/42/EEC (as amended by 2007/47/EC) regulating medical devices, as well as Directive 98/68/EEC that requires the manufacturers to place CE marking on their products to demonstrate compliance with the above regulations.
Overview of Directive 93/42/EEC concerning medical devices
|
·
|
Definition and scope of medical devices
|
According to Article 1, par. 2(a), ‘medical device’ means any instrument, apparatus, appliance, material or other article, whether used alone or in combination, together with any accessories, including software intended by the manufacturer to be used specifically for diagnosis or therapeutic purposes or both and necessary for its proper application which:
(a) intended by the manufacturer to be used for human beings for the purpose of:
(i) diagnosis, prevention, monitoring, treatment or alleviation of disease,
(ii) diagnosis, monitoring, treatment, alleviation, or compensation for an injury or handicap,
(iii) investigation, replacement or modification of the anatomy or of a physiological process,
(iv) control of conception; and
(b) which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means.
Having considered the nature of the imaging system and the intended uses, it is our view that the SocketFit system does falls under the above Directive, since, as previously described, the device is intended at least to investigate the anatomy of the amputee’s residual limb and probably to monitor the amputation. The Directive includes in the definition of “medical device” products which are intended for the purpose of investigation of the anatomy (Article 1, par. 2(a) third indent) as well as those for the purpose of diagnosis or monitoring an injury or handicap (article 2(a) second indent). Therefore even very simple claims relating to presenting an image of the anatomy in a clinical context would in our view result in its being classified as a device.
|
·
|
Classification
|
The Directive also lays down rules in Annex IX for the classification of devices based essentially on the potential risks involved, with class III devices having the highest potential risk and class I device the lowest.
Although the SocketFit technology contacts the patient, the imager does not itself enter the stump and is only used once the stump surface has healed. It can therefore be considered to be surgically non invasive and in contact with intact skin only. As it functions by delivering energy in the form of ultrasound to the patient, the imager would be considered as an active device.
Rules 9-12 apply to active devices, however rules 9 and 11 can be eliminated as the system is not therapeutic and does not administer or remove medicines or other substances to or from the patient.
Rule 10 applies to active devices for diagnosis including diagnostic ultrasound. Although the title of the rule is active devices for diagnosis, it should be noted that it also covers devices which are intended for monitoring and it includes all MRI equipment. It can therefore be argued that this rule would apply to the system even if very basic claims are made. As it is not intended to image radiopharmaceuticals, monitor vital signs, or emit ionizing radiation, the system would be a class IIa device under rule 10.
In the event that Rule 10 is considered not to apply, then rule 12 “All other active devices” would apply which would result in the system being a class I device. In this case it is important to consider whether the system has a measuring function and here three criteria are applied:
|
-
|
Is the device intended to measure quantitatively an anatomic parameter?
|
|
-
|
Is it displayed in legal units or compared to at least one point of reference in legal units?
|
|
-
|
Is accuracy implied by implicit or explicit claims with a significant adverse effect in the patient’s health and safety if not achieved?
|
The first parameter would certainly apply regardless of the claims. In the event that the system is intended to be used to compare images over time in order to assess changes in the stump anatomy or/and that it is used to optimise socket geometry then the second and third parameters would also apply and the device would be considered to have a measuring function.
In our view, based on the information provided so far, it is most likely that the system will be a class IIa. If it is considered a class I device it is likely that it will be deemed to have a measuring function. These differences in classification are important as they will affect the conformity assessment routes which can be applied under the directive and the actions which must be taken in order to place the CE mark on the product, pursuant to the aforementioned EU Directive 98/68/EEC.
|
·
|
Conformity Assessment Route
|
The conformity assessment process: In general terms, a manufacturer wishing to place their products on the market under this Directive must:
|
-
|
assign his devices to one of the relevant risk categories defined in the Directive;
|
|
-
|
ensure that the device meets the ‘essential requirements’ specified in Annex I of the Directive;
|
|
-
|
follow the appropriate conformity assessment procedure; and
|
|
-
|
if appropriate (depending on the risk category of the device), ensure that an independent certification body (called a ‘notified body’) is involved in the conformity assessment procedure.
|
Manufacturers are free to apply to any notified body in the EU designated to carry out the desired conformity assessment procedure, regardless of which Member State that notified body is designated in.
As indicated previously, there are three possible classifications: Class IIa, Class I and class I with a measuring function (class Im) which are considered here in turn:
|
i)
|
Class I: This is the simplest scenario as it does not require certification from a Notified Body. In order to place the CE mark on the system, we would simply follow the conformity assessment route in Annex VII of the Directive which is commonly known as self-certification. This involves:
|
|
-
|
Preparation of a technical file on the system (this should include all information required to demonstrate conformity with the essential requirements in Annex I of the Directive);
|
|
-
|
Preparation and implementation of procedure for reviewing experience once on the market, including complaints and positive feedback (known as Post market Surveillance) and for applying any corrective actions;
|
|
-
|
Preparation and implementation of procedure for notifying the relevant competent authorities of any reportable incidents or near incident;
|
|
-
|
Signing a declaration of conformity that the products meet the provisions of the directive which apply to them; and
|
|
-
|
Registering the device under article 14 of the Directive with the relevant competent authority. As we are located in the UK, this would be the Medicines and Healthcare Regulatory Authority, MHRA.
|
Although no Notified Body input is required in this case, it is important to note that the MHRA operates active surveillance of class I devices and may well request the technical file. There is no requirement to have a certified quality system, however we would recommend that ISO 13485 is applied and indeed some customers may require this.
|
ii)
|
Class I Measuring function: In this case Annex VII also applies as above but in addition the procedure in annex IV, V or VI must be applied for those aspects relating to the measuring function. This means that a Notified Body certification is required. Annex VI is arguably the simplest of the annexes as the Notified Body certification is focused on final inspection and testing, however this will depend on the nature of the manufacturing processes and the type of testing which can be conducted routinely. Annex V covers the whole of manufacture and would be the simplest route for a company who already has a quality system such as ISO 13485 in place. Annex IV requires the Notified Body to test every product or to sample batches of every product before they are placed on the market. This is generally not recommended due to cost and inconvenience, unless it is planned only to manufacture small numbers of product infrequently.
|
|
iii)
|
Class IIa: The choices are the same as for a class I measuring device i.e. Annex VII plus either Annex Iv, V or VI. However the Notified Body surveillance concerns the whole of the device not just the measuring function. There is an additional choice – Annex II in which the quality system and Notified Body assessment has to cover both manufacture and design. However, this is not recommended as it involves additional unnecessary work in this case.
|
Once the necessary steps have been successfully completed, the CE marking must be affixed to the medical device. The CE marking has to be placed visibly and legibly on the product or, if not possible due to the nature of the product, be affixed to the packaging and the accompanying document. The CE marking shall consist of the initials 'CE' taking the following form:

The various components of the CE marking must have the same vertical dimension and may not be smaller than 5 mm. If the CE marking is reduced or enlarged, the proportions given in the graduated drawing above must be respected.
When the product is subject to other Directives covering other aspects and which also provide for the ‘CE’ marking, the accompanying documents must indicate that the product also conforms to those other Directives.
If a Notified Body has been involved in the conformity assessment procedure, its identification number must also be displayed.
Scottish Regulations
The following UK regulation implements the above EU medical devices Directives and amendments to date, and are enforced by the MHRA (The UK Medicines and Healthcare Products Regulatory Authority):
|
·
|
Statutory Instruments 2002 No. 618 (Consolidated legislation) that regulate, among others, general medical devices in part II, thus implementing Directive 93/42/EEC (as amended by the Medical Devices Regulations 2008 No. 2936 which transpose Directive 2007/47/EC into UK law).
|
Pursuant to Regulation 44, a manufacturer with a registered place of business in the UK, who places a relevant device on the market (remember that for these purposes, market means the EU market), or who makes available a device for performance evaluation under his own name must register with the MHRA. In addition, a person with a registered places of business in the UK who (a) places a relevant device on the UK market, or (b) who makes a device available for performance evaluation, on behalf of a manufacturer who does not have a registered place of business in the Community or in a state which is party to an Association Agreement, must register with the MHRA. The process is set out on their website.
Greek Regulations
|
1.
|
Presidential Decree ΔΥ8δ/οικ.130648/2009 for the harmonization of Greek law with the Directive 93/42/EEC on medical devices in conjunction with Circular 62701/27-11-2003 of the National Medicines Agency:
|
|
·
|
According to Article 1 par. 9 and Article 2 par. 2, “the relevant regulatory authority is the National Medicines Agency” that takes all necessary measure for the marketing and/or use of the relevant product, and ensures the product’s security and quality compliance;
|
|
·
|
Article 14 states that manufacturers of and/or anyone who markets medical devices in the Greek territory, must be registered with the National Medicines Agency. The registration process is detailed on the website of the National Medicines Agency but essentially requires the same actions as laid down in the description of the EU Directive 93/42 above.
|
|
2.
|
Presidential Decree ΔΥ8δ/Γ.Π.οικ.1348/2004 on the principles and guidelines of good distribution practice of medical devices, compliance with which is ensured by the National Medicines Agency. These principles are defined in Clause 3, some of which are the following:
|
|
-
|
special transportation. storage, preservation, and handling conditions, for public health protection reasons, according to international and European quality standards;
|
|
-
|
the staff responsible for implementing the principles in question must be trained according to its duties;
|
|
-
|
detailed record keeping of all purchase and/or sale of products; and
|
|
-
|
appropriate facilities and equipment so that proper product storage and distribution is ensured.
|
USA Regulations
Essentially, medical devices are subject to the general controls of the Federal Food Drug & Cosmetic (FD&C) Act which are contained in the final procedural regulations in Title 21 Code of Federal Regulations Part 800-1200 (21 CFR Parts 800 - 1299). These controls are the baseline requirements that apply to all medical devices necessary for marketing, proper labeling and monitoring its performance once the device is on the market.
The Company will be required to determine how FDA may classify our device - which one of the three classes the device may fall into. Unless exempt, FDA will classify our device. Classification identifies the level of regulatory control that is necessary to assure the safety and effectiveness of a medical device. Most importantly, the classification of the device will identify, unless exempt, the marketing process (either premarket notification [510(k)] or premarket approval (PMA)) the manufacturer must complete in order to obtain FDA clearance/approval for marketing.
The Company will need to review the development of data and/or information necessary to submit a marketing application, and to obtain FDA clearance to market. For some [510(k)] submissions and most PMA applications, clinical performance data is required to obtain clearance to market. In these cases, conduct of the trial must be done in accord with FDA's Investigational Device Exemption (IDE) regulation, in addition to marketing clearance.
We do not believe that we will be required to submit for a PMA but believe that SocketFit will comply by the submission of a 510(k) to the FDA.
Each person who wants to market in the U.S., a Class I, II, and III device intended for human use, for which a Premarket Approval (PMA) is not required, must submit a 510(k)to FDA unless the device is exempt from 510(k) requirements of the Federal Food, Drug, and Cosmetic Act (the Act) and does not exceed the limitations of exemptions in .9 of the device classification regulation chapters (e.g., 21 CFR 862.9, 21 CFR 864.9). Before marketing a device, each submitter must receive an order, in the form of a letter, from FDA which finds the device to be substantially equivalent (SE) and states that the device can be marketed in the U.S. This order "clears" the device for commercial distribution.
A 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is at least as safe and effective, that is, substantially equivalent, to a legally marketed device (21 CFR 807.92(a)(3)) that is not subject to PMA. Submitters must compare their device to one or more similar legally marketed devices and make and support their substantial equivalency claims. A legally marketed device, as described in 21 CFR 807.92(a)(3), is a device that was legally marketed prior to May 28, 1976 (pre-amendments device), for which a PMA is not required, or a device which has been reclassified from Class III to Class II or I, or a device which has been found SE through the 510(k) process. The legally marketed device(s) to which equivalence is drawn is commonly known as the "predicate." Although devices recently cleared under 510(k) are often selected as the predicate to which equivalence is claimed, any legally marketed device may be used as a predicate. Legally marketed also means that the predicate cannot be one that is in violation of the Act.
Until the submitter receives an order declaring a device SE, the submitter may not proceed to market the device. Once the device is determined to be SE, it can then be marketed in the U.S. The SE determination is usually made within 90 days and is made based on the information submitted by the submitter.
Please note that FDA does not perform 510(k) pre-clearance facility inspections. The submitter may market the device immediately after 510(k) clearance is granted. The manufacturer should be prepared for an FDA quality system (21 CFR 820) inspection at any time after 510(k) clearance.
What is Substantial Equivalence
A 510(k) requires demonstration of substantial equivalence to another legally U.S. marketed device. Substantial equivalence means that the new device is at least as safe and effective as the predicate.
A device is substantially equivalent if, in comparison to a predicate it:
|
·
|
has the same intended use as the predicate; and
|
|
·
|
has the same technological characteristics as the predicate;
|
|
|
or
|
|
·
|
has the same intended use as the predicate; and
|
|
·
|
has different technological characteristics and the information submitted to FDA;
|
|
-
|
does not raise new questions of safety and effectiveness; and
|
|
-
|
demonstrates that the device is at least as safe and effective as the legally marketed device.
|
A claim of substantial equivalence does not mean the new and predicate devices must be identical. Substantial equivalence is established with respect to intended use, design, energy used or delivered, materials, chemical composition, manufacturing process, performance, safety, effectiveness, labeling, biocompatibility, standards, and other characteristics, as applicable.
A device may not be marketed in the U.S. until the submitter receives a letter declaring the device substantially equivalent. If FDA determines that a device is not substantially equivalent, the applicant may:
|
-
|
resubmit another 510(k) with new data,
|
|
-
|
request a Class I or II designation through the de novo process,
|
|
-
|
file a reclassification petition, or
|
|
-
|
submit a premarket approval application (PMA).
|
Who is Required to Submit a 510(k)
The Act and the 510(k) regulation (21 CFR 807) do not specify who must apply for a 510(k). Instead, they specify which actions, such as introducing a device to the U.S. market, require a 510(k) submission.
The following four categories of parties must submit a 510(k) to the FDA:
|
1.
|
Domestic manufacturers introducing a device to the U.S. market;
|
|
|
Finished device manufacturers must submit a 510(k) if they manufacture a device according to their own specifications and market it in the U.S. Accessories to finished devices that are sold to the end user are also considered finished devices. However, manufacturers of device components are not required to submit a 510(k) unless such components are promoted for sale to an end user as replacement parts. Contract manufacturers, those firms that manufacture devices under contract according to someone else’s specifications, are not required to submit a 510(k).
|
|
2.
|
Specification developers introducing a device to the U.S. market;
|
|
|
A specification developer develops the specifications for a finished device, but has the device manufactured under contract by another firm or entity. The specification developer submits the 510(k), not the contract manufacturer.
|
|
3.
|
Re-packers or re-labelers who make labeling changes or whose operations significantly affect the device.
|
|
|
Re-packagers or re-labelers may be required to submit a 510(k) if they significantly change the labeling or otherwise affect any condition of the device. Significant labeling changes may include modification of manuals, such as adding a new intended use, deleting or adding warnings, contraindications, etc. Operations, such as sterilization, could alter the condition of the device. However, most re-packagers or re-labelers are not required to submit a 510(k).
|
|
4.
|
Foreign manufacturers/exporters or U.S. representatives of foreign manufacturers/exporters introducing a device to the U.S. market.
|
Please note that all manufacturers (including specification developers) of Class II and III devices and select Class I devices are required to follow design controls (21 CFR 820.30) during the development of their device. The holder of a 510(k) must have design control documentation available for FDA review during a site inspection. In addition, any changes to the device specifications or manufacturing processes must be made in accordance with the Quality System regulation (21 CFR 820) and may be subject to a new 510(k).
When a 510(k) is Required
A 510(k) is required when:
|
1.
|
Introducing a device into commercial distribution (marketing) for the first time. After May 28, 1976 (effective date of the Medical Device Amendments to the Act), anyone who wants to sell a device in the U.S. is required to make a 510(k) submission at least 90 days prior to offering the device for sale, even though it may have been under development or clinical investigation before that date. If your device was not marketed by your firm before May 28, 1976, a 510(k) is required.
|
|
2.
|
You propose a different intended use for a device which you already have in commercial distribution. The 510(k) regulation (21 CFR 807) specifically requires a 510(k) submission for a major change or modification in intended use. Intended use is indicated by claims made for a device in labeling or advertising. Most, if not all changes in intended use will require a 510(k). Please note that prescription use to over the counter use is a major change in intended use and requires the submission of a new 510(k).
|
There is a change or modification of a legally marketed device and that change could significantly affect its safety or effectiveness. The burden is on the 510(k) holder to decide whether or not a modification could significantly affect safety or effectiveness of the device. Any modifications must be made in accordance with the Quality System regulation, 21 CFR 820, and recorded in the device master record and change control records. It is recommended that the justification for submitting or not submitting a new 510(k) be recorded in the change control records.
A new 510(k) submission is required for changes or modifications to an existing device, where the modifications could significantly affect the safety or effectiveness of the device or the device is to be marketed for a new or different indication for use.
Other Requirements Besides Marketing Clearance
Premarket Requirements: Labeling, Registration, Listing
Before marketing clearance is obtained the manufacturer must assure that the device is properly labeled in accordance with FDA's labeling regulations. Once clearance for marketing is obtained, the manufacturer must register their establishment and list the type of device they plan to market with the FDA. All registration and listing information (Annual, Initial or Updates) are to be submitted electronically unless FDA grants you a waiver.
Post-market Requirements: Quality System, Medical Device Reporting
Once on the market, there are post-market surveillance controls with which a manufacturer must comply. These requirements include the Quality Systems (QS) (also known as Good Manufacturing Practices, GMPs) and Medical Device Reporting (MDR) regulations. The QS regulation is a quality assurance requirement that covers the design, packaging, labeling and manufacturing of a medical device. The MDR regulation is an adverse event reporting program.
Regulations for P&O Clinics
The operation of our clinics will be subject to, and must comply with, the respective country’s government regulations. Given our planned acquisition or establishment of clinics in Scotland, UK and Athens, Greece, we are currently most interested in the respective applicable regulations. As we determine to establish clinics in other jurisdictions we will be required to understand and be compliant with the laws in those respective jurisdictions in which we intend to operate.
Scotland
|
1.
|
The Health and Social Work Professions Order 2001, made under section 60 of the Health Act 1999, established the UK Health & Care Professions Council (HCPC) that regulates, among others, prosthetists/orthotists in the UK:
|
|
·
|
according to the interpretation set out in Schedule 3, “relevant professions means arts therapists; chiropodists; clinical scientists; dietitians; medical laboratory technicians; occupational therapists; orthoptists; paramedics; physiotherapists; prosthetists and orthotists; radiographers; and speech and language therapists”;
|
|
·
|
according to article 3, par. 1, the Council is responsible for setting out “the standards of education, training, conduct and performance for members of the relevant professions and to ensure the maintenance of those standards”;
|
|
·
|
according to article 5, “the Council shall establish and maintain a register of members of the relevant professions”;
|
|
·
|
the HCPC sets out for all registrants standards of conduct, performance and ethics, and standards for CPD, as well as standards of proficiency for prosthetists/orthotists specifically.
|
|
2.
|
The Health and Care Professions Council (Constitution) Order 2009 which sets out the composition of the Council (as amended by the Health and Care Professions Council (Constitution) Order 2014);
|
|
3.
|
The Regulation of Care (Scotland) Act of 2001 established the Scottish Commission for the Regulation of Care (‘the Care Commission’), and set out the care services that it will regulate, i.e. all adult, child and independent healthcare services in Scotland, one of which independent healthcare services is ‘independent clinics’. As of April 1st 2011, the work of the Care Commission passed to a new body, the Care Inspectorate. Regulation of independent healthcare, including regulation of independent clinics, has passed to Healthcare Improvement Scotland. Healthcare Improvement Scotland is currently responsible for regulating independent hospitals, voluntary hospices, and private psychiatric hospitals. Regulation of independent clinics, independent medical agencies and independent ambulance services has not yet commenced. The Scottish Government consulted on the future arrangements for regulation of independent healthcare services, including the definition and scope of the services that should be regulated last year and are currently considering the way forward.
|
Greece:
We will be governed in Greece by Law 2072/1992 on Regulations for the profession of the technical specialist of prosthetic and orthotic products and other rehabilitation products, and other provisions. More specifically the law states that companies that operate laboratories of prosthetic, orthotic and other rehabilitation products can be formed by any person or legal entity, provided that they have as their chief technical officer an individual licensed as a technical specialist of prosthetic and orthotic products and other rehabilitation products. There is also a clause in the laws of Greece that refer to the necessary mechanical equipment, the specifications of the laboratory space, the required technical staff, and the committee in charge for granting the relevant permit, all of which are determined by a decision of the Minister of Health. In a review of the laws and conversations with the Ministry of Health directly this clause has not yet been enacted and there is no supporting documentation in regard to the requirements. We will be required to monitor the laws closely to ensure that if and when further regulations are enacted we are compliant.
We will be required to obtain those licenses, permits or other authorizations currently required to launch our products and operate our P&O clinics conduct exploration and other programs. It is possible that the costs and delays associated with such compliance could become so prohibitive that we may decide to not proceed with our business plans.
Employees
The Company does not have any direct employees, We presently have a consulting agreement with our Chief Executive Officer, Sotirios Leontaritis which was entered into on January 15, 2014, whereby Mr. Leontaritis shall provide services to the Company as the Company’s President and Chief Executive Officer in regards to the Company’s management and operations for the period from January 1, 2014 to December 31, 2017. Under the terms of the agreement, the Company agreed to pay to Mr. Leontaritis US$60,000 per annum payable in monthly payments of US$5,000 a month for the term of the contract. Further, under the terms of the contract, Mr. Leontaritis will receive cash compensation of US$5,000 and a stock award of 1,000,000 shares of common stock of the Company in consideration of his services as an officer and director for the period from September 10, 2013 to December 31, 2013. On January 22, 2014, the Company issued the 1,000,000 shares to Mr. Leontaritis pursuant to the agreement.
On December 1, 2013, we entered into a verbal compensation agreement with our current Chief Financial Officer, Grigorios Tsourtos whereby Mr. Tsourtos will receive €1,000 per month for his services as Chief Financial Officer. Mr. Tsourtos will be paid as a consultant and will not have an employment agreement with the Company.
We presently do not have pension, health, annuity, insurance, stock options, profit sharing or similar benefit plans; however, we may adopt such plans in the future.
Available Information
Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports that we file with the Securities and Exchange Commission, or SEC, are available at the SEC's public reference room at 100 F Street, N.E., Washington, D.C. 20549. The public may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a website at www.sec.gov that contains reports, proxy and information statements and other information regarding reporting companies.
An investment in our securities should be considered highly speculative due to various factors, including the nature of our business and the present stage of our development. An investment in our securities should only be undertaken by persons who have sufficient financial resources to afford the total loss of their investment. In addition to the usual risks associated with investment in a business, the following is a general description of significant risk factors which should be considered. You should carefully consider the following material risk factors and all other information contained in this Annual Report before deciding to invest in our common stock. If any of the following risks occur, our business, financial condition and results of operations could be materially and adversely affected. Additional risks and uncertainties we do not presently know or that we currently deem immaterial may also impair our business, financial condition or operating results.
Risks Associated with our Business
Implications of being an emerging growth company
As a company with less than $1 billion in revenue during our last fiscal year, we qualify as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and we may remain an emerging growth company until December 31, 2018, subject to satisfaction of certain conditions. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure and other requirements that are applicable to other public companies that are not emerging growth companies. In particular, in this annual report, we have provided only two years of audited financial statements and have not included all of the executive compensation related information that would be required if we were not an emerging growth company. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
We are a development stage company with a limited business history and have losses that we expect to continue into the future. If the losses continue we will have to suspend operations or cease functioning.
We were incorporated on March 26, 2007, and have only started our proposed business but have not realized any revenues. Our net loss since inception is $1,329,651. We have never had significant operations and have no significant assets. We have yet to generate positive earnings and there can be no assurance that we will ever operate profitably. We are in the development stage, and have a limited operating history, upon which an evaluation of our future success or failure can be made. Prior to completion of the development stage, we anticipate that we will incur increased operating expenses without realizing any revenues. We therefore expect to incur significant losses into the foreseeable future. If our business plan is not successful and we are not able to operate profitably, then our stock may become worthless and investors may lose all of their investment in our company.
Our ability to achieve and maintain profitability and positive cash flow is dependent upon:
|
•
|
our ability to find profitable markets in which to operate our P&O total rehabilitation clinics and launch our products;
|
|
•
|
our ability to generate revenues; and
|
|
•
|
our ability to manage research, development and costs of operations.
|
While we believe that our business plan has merit, there can be no assurance that we will be able to achieve any of the above and be successful in our future planned operations, and if our losses continue we will have to suspend operations or cease functioning.
Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
Our operations to date have been limited to organizing and staffing our company, developing and securing our technology, and raising capital. We have not yet demonstrated our ability to successfully complete development of any product candidates, obtain marketing approvals, manufacture a commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history.
In addition, as a new business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition from a company with a research and development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We currently have no saleable products and may never have saleable products
We have only recently acquired our first technology and in order to have a marketable product which is saleable we must undertake additional research and development and, if successful, develop a market for our products. There can be no assurance that we will successfully complete our research and development and ultimately have a marketable product or that, if we do finalize the development of our technology, that a market will develop.
If healthcare professionals do not adopt our products in sufficient numbers or as rapidly as we anticipate, our operating results will be harmed.
The success of any of our products which we may develop will depend heavily on acceptance by healthcare professionals who will prescribe and recommend our products, and our failure to maintain a high level of confidence by key healthcare professionals in our products could adversely affect our business.
Consumers may not use our services, and thus we may never become profitable.
Our products represent a significant change from traditional diabetes treatment, and patients may be reluctant to accept them, or may not find them preferable to conventional treatment. In addition, patients may not comply with recommended treatment guidelines which could compromise the effectiveness of their treatment. Our success will depend upon the rapid acceptance of our products by a large number of potential patients to whom we intend to actively market. Market acceptance will depend in part upon the recommendations of prosthetists and orthotists, as well as other factors including effectiveness, safety, reliability, improved treatment aesthetics and greater comfort and hygiene compared to conventional prosthetic-orthotic products. Furthermore, consumers may not respond to our direct marketing campaigns or we may be unsuccessful in reaching our target audience. If consumers prove unwilling to adopt our products as rapidly or in the numbers that we anticipate, our operating results will be harmed.
If the manufacturers of our products encounter problems or delays in the manufacturing process, our ability to generate revenue will be limited.
We intend to rely on third parties for our manufacturing process if we determine to manufacture and market our products directly. Our rapid growth may exceed the capacity of these manufacturers to produce our products in sufficient quantities to support our growth. In the event of delivery delays or shortages of our products, our business and growth prospects may be harmed.
The manufacturers of our products may encounter difficulties in scaling up production to meet demand, including:
|
·
|
problems involving production yields;
|
|
·
|
shortages of key manufacturing equipment;
|
|
·
|
shortages of qualified personnel; and
|
|
·
|
failure to develop new software processes.
|
If demand for our products exceeds manufacturing capacity, we could develop a substantial backlog of customer orders. If our manufacturers are unable to establish and maintain larger-scale manufacturing capabilities, our ability to generate revenue will be limited and our reputation in the marketplace would be damaged.
If we do not enhance our product offerings, either through the acquisition of or investment in new and complementary technologies, products or businesses, or our research and development efforts, we may be unable to compete effectively.
Our success depends in part on our ability to continually enhance and broaden our product offerings in response to changing customer demands, competitive pressures and technologies, and our ability to increase our market share. Accordingly, we expect to consider opportunities to acquire or make investments in other technologies, products and businesses that could enhance our capabilities, complement our current technology, or expand the breadth of our markets or customer base. Acquisitions and strategic investments involve numerous risks, including:
|
·
|
problems assimilating the purchased technologies, products or business operations;
|
|
·
|
issues maintaining uniform standards, procedures, controls and policies;
|
|
·
|
unanticipated costs associated with the acquisition or strategic investment;
|
|
·
|
diversion of management's attention from our core business;
|
|
·
|
adverse effects on existing business relationships with third-party distributers, suppliers and customers;
|
|
·
|
risks associated with entering new markets in which we have limited experience;
|
|
·
|
our ability to obtain regulatory approvals to market new products; and
|
|
·
|
potential loss of key employees of acquired businesses.
|
While we are in negotiations for further acquisitions, currently our sole commitment is for the development of our recently acquired SocketFit technology. We do not know if we will be able to successfully complete any further acquisitions, or whether we will be able to successfully integrate any acquired business, product or technology into our business or retain any key personnel, suppliers or distributors. Our ability to successfully grow through acquisitions depends upon our ability to identify, negotiate, complete and integrate suitable acquisitions and to obtain any necessary financing. If we are unable to integrate any acquired technology, products or business effectively, our business, financial condition and results of operations could be materially adversely affected.
Furthermore, research and development efforts may require a substantial investment of time and resources before we are adequately able to determine the commercial viability of a new product, technology, material or other innovation. In addition, even if we are able to successfully develop enhancements or new generations of our products, these enhancements or new generations of products may not produce net sales in excess of the costs of development and they may be quickly rendered obsolete by changing customer preferences or the introduction by our competitors of products embodying newer technologies or features.
Issues related to the quality and safety of our products, packaging or sterilization could cause a product recall resulting in harm to our reputation and negatively impact our operating results.
Issues related to quality and safety of products, packaging or sterilization could jeopardize our image and reputation. Negative publicity related to these types of concerns, whether valid or not, might negatively impact demand for our products, or cause production and delivery disruptions. We may need to recall products if they become unfit for use.
Our business exposes us to risks of product liability claims, and we may incur substantial expenses if we are sued for product liability.
Medical devices involve an inherent risk of product liability claims. We may be held liable if any product we develop or any product that uses or incorporates any of our technologies causes injury or is otherwise found unsuitable. Although we intend to continue to maintain product liability insurance, adequate insurance may not be available on acceptable terms and may not provide adequate coverage against potential liabilities. A product liability claim, regardless of its merit or eventual outcome, could result in significant legal defense costs. Costs associated with these potential claims would increase our expenses, and could have a material adverse effect on our business, financial condition and results of operations.
Software defects may be discovered in our products which would damage our ability to sell our products, our results of operations, financial condition and cash flows.
Our systems will incorporate sophisticated computer software. Complex software frequently contains errors, especially when first introduced. Because our products are designed to be used to perform complex interventional procedures, we expect that physicians and hospitals will have an increased sensitivity to the potential for software and other defects. We cannot assure you that our software will not experience errors or performance problems in the future. If we experience software errors or performance problems, we would likely also experience:
|
•
|
loss of revenue;
|
||
|
•
|
increase in reportable adverse events to applicable authorities;
|
||
|
•
|
delay in market acceptance of our products;
|
||
|
•
|
damage to our reputation;
|
||
|
•
|
additional regulatory filings;
|
||
|
•
|
product recalls;
|
||
|
•
|
increased service or warranty costs; and/or
|
||
|
•
|
product liability claims relating to the software defects.
|
We may fail to receive positive clinical results for our products in development that require clinical trials, and even if we receive positive clinical results, we may still fail to receive the necessary clearance or approvals to market our products.
In the development of new products or new indications for, or modifications to, existing products, we may conduct or sponsor clinical trials. Clinical trials are expensive and require significant investment of time and resources. Clinical trials are subject to regulations for clinical studies in each respective country and, failure to comply with such regulations, including, but not limited to, failure to obtain adequate consent of subjects, failure to adequately disclose financial conflicts, or failure to report data or adverse events accurately, could result in fines, penalties, and/or suspension of trials.
Our business may be harmed by technological and therapeutic changes, or the demand for our services could be diminished by the development of alternative treatments. Future innovations could make our inventions obsolete.
Our success will depend, in part, on continued demand for products that will incorporate our inventions. Changes in technology or customer requirements could render these inventions obsolete or unmarketable.
We will rely on information technology systems for accounting and finance, inventory management, engineering, distribution and other functions, and to maintain our research and development data. If such adopted information technology systems fail to adequately perform these functions, or if we experience an interruption in their operation, our business, financial condition and results of operations could be adversely affected.
The efficient operation of our business will be dependent on our information technology systems which we intend to develop. We will rely on our information technology systems to effectively manage:
|
·
|
sales and marketing, accounting and financial functions;
|
|
·
|
order entry, order fulfillment and inventory replenishment processes;
|
|
·
|
engineering tasks; and
|
|
·
|
our research and development data.
|
The failure of our intended information technology systems to perform as we anticipate, or our failure to effectively implement new systems, could disrupt our business and product development and could result in decreased sales, increased overhead costs, excess inventory and product shortages, all of which could have a material adverse effect on our business, financial condition and results of operations. In addition, our information technology systems are vulnerable to damage or interruption from:
|
·
|
tornado, earthquake, fire, flood and other natural disasters;
|
|
·
|
terrorist attacks and attacks by computer viruses or hackers;
|
|
·
|
power loss; and
|
|
·
|
network failure of computer systems, Internet, telecommunications or data.
|
Any such interruption could have material adverse effect on our business, financial condition and results of operations.
Our success depends in part on our proprietary technology and if we are unable to successfully enforce our intellectual property rights, our competitive position may be harmed.
Our success will depend in part on our ability to maintain existing intellectual property and to obtain and maintain further intellectual property protection for our products, in the U.K., Europe, the U.S.A., and in other countries. Our inability to do so could harm our competitive position. We do not currently have any patents related to our technology and our current technology could be at risk if we do not file for patent protection. We intend to apply for patents on all of our intellectual property as we finalize the development of the technology. We will rely on our IP portfolio of future patent applications to protect a large part of our intellectual property and our competitive position. However, our future patent filings may not issue as patents. Additionally, any patents issued to us may be challenged, invalidated, held unenforceable, circumvented, or may not be sufficiently broad to prevent third parties from producing competing products similar in design to our products. In addition, protection afforded by foreign patents may be more limited than that provided under U.S. patents and intellectual property laws.
We rely on confidentiality agreements that could be breached and may be difficult to enforce which could have a material adverse effect on our business and competitive position.
Our policy is to enter into agreements relating to the non-disclosure of confidential information with third parties, including our contractors, consultants, advisors and research collaborators, as well as into agreements that purport to require the disclosure and assignment to us of the rights to the ideas, developments, discoveries and inventions of our employees and consultants while we employ them. However, these agreements can be difficult and costly to enforce. Moreover, to the extent that our contractors, consultants, advisors and research collaborators apply or independently develop intellectual property in connection with any of our projects, disputes may arise as to the proprietary rights to this type of information. If a dispute arises, a court may determine that the right belongs to a third party, and enforcement of our rights can be costly and unpredictable. In addition, we rely on trade secrets and proprietary know-how that we will seek to protect in part by confidentiality agreements with our employees, contractors, consultants, advisors or others. Despite the protective measures we employ, we still face the risk that:
|
•
|
these agreements may be breached;
|
|
|
•
|
these agreements may not provide adequate remedies for the applicable type of breach; or
|
|
|
•
|
our trade secrets or proprietary know-how will otherwise become known.
|
Any breach of our confidentiality agreements or our failure to effectively enforce such agreements would have a material adverse effect on our business and competitive position.
The medical device industry is characterized by extensive litigation over patents and other intellectual property rights and we could become subject to litigation that could be costly, result in the diversion of management's time and efforts, require us to pay damages, and/or prevent us from marketing our existing or future products.
We may be the subject of patent or other litigation in the future. An adverse determination in a patent suit in any litigation or interference proceeding to which we may become a party could subject us to significant liabilities. An adverse determination of this nature could also put our patents, when granted, at risk of being invalidated or interpreted narrowly or require us to seek licenses from third parties. Licenses may not be available on commercially reasonable terms or at all, in which event our business would be materially adversely affected. We may also receive letters from third parties drawing our attention to their patent rights. While we do not believe that we infringe any valid and enforceable rights which have been brought to our attention, there may be other more pertinent rights of which we are presently unaware. If we infringe the patents or proprietary rights of other parties, our ability to grow our business will be severely limited. The defense and prosecution of intellectual property suits, interference proceedings and related legal and administrative proceedings could result in substantial expense to us and significant diversion of effort by our technical and management personnel.
Extensive and changing government regulation of the healthcare industry may be expensive to comply with and exposes us to the risk of substantial government penalties.
We face risks related to our international operations, including the need to obtain necessary foreign regulatory clearance or approvals. If we fail to obtain regulatory clearances in countries in which we intend to operate for products under development, we will not be able to commercialize these products in those countries. Sales of our products will be subject to regulatory requirements that vary widely from country to country. We may be unable to obtain regulatory approvals in the countries in which we intend to operate or to market our products. We may also incur significant costs in attempting to obtain and in maintaining regulatory approvals. If we experience delays in receipt of approvals to market our products or if we fail to receive these approvals, we may be unable to market our products or enhancements in our intended markets in a timely manner, if at all.
|
·
|
Noncompliance with applicable regulatory requirements can result in enforcement action which may include recalling products, ceasing product marketing, and paying significant fines and penalties, which could limit product sales, delay product shipment and adversely affect our profitability.
|
Our results of operations will suffer if we are unable to manage our operations effectively.
The sale and shipment of our products across international borders will subject us to extensive foreign governmental trade, import and export, and custom regulations and laws. Compliance with these regulations is costly and exposes us to penalties for non-compliance. Other laws and regulations that can significantly impact us include various anti-bribery laws and anti-boycott laws. Any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments, restrictions on certain business activities and exclusion or debarment from government contracting. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our distribution and sales activities. In addition, some of the countries in which we may sell our products may be subject to political, economic or social instability. Our intended international operations expose us and our distributors to risks inherent in operating in foreign jurisdictions. These risks include:
|
·
|
adverse changes in tariffs and trade restrictions;
|
|
·
|
political, social and economic instability and increased security concerns;
|
|
·
|
longer collection periods and difficulties in collecting receivables from foreign entities;
|
|
·
|
exposure to different legal standards;
|
|
·
|
transportation delays and difficulties of managing international distribution channels;
|
|
·
|
lack of stringent protection of intellectual property;
|
|
·
|
potentially adverse tax consequences and the complexities of foreign value-added tax systems;
|
|
·
|
difficulties in staffing and managing foreign operations;
|
|
·
|
obstacles to obtaining domestic and foreign export, import and other governmental approvals, permits and licenses and compliance with foreign laws;
|
|
·
|
limitations on the repatriation of earnings; and
|
|
·
|
increased financing costs.
|
These risks may limit or disrupt our operations, restrict the movement of funds or result in the deprivation of contractual rights or the taking of property by nationalization or expropriation without fair compensation. Our continued success as a global company depends, in part, on our ability to develop and implement policies and strategies that are effective in anticipating and managing these and other risks in the countries in which we do business. Failure to manage these and other risks may have a material adverse effect on our operations in any particular country and on our business as a whole.
If we cannot successfully implement our business strategy, our business, results of operations and potential for growth will be adversely affected.
Our business strategy includes the development and marketing of our products and the obtaining of regulatory approval to sell our products in certain key markets and the acquisition or direct development of P&O clinics in various jurisdictions. Our ability to achieve our business strategy is subject to a variety of factors, many of which are beyond our control. In addition, the implementation of our business strategy may not improve our operating results. We may decide to alter or discontinue aspects of our business strategy and may adopt alternative or additional strategies due to business or competitive factors not currently foreseen, such as the introduction of new products or technologies by our competitors or the lack of clients for our clinics. Any failure to successfully implement our business strategy would have a material adverse effect on our business, financial condition and results of operations.
If we lose our key personnel or are unable to attract and retain key personnel, we may be unable to pursue business opportunities or develop our products.
We are highly dependent upon the experience and ability of certain key employees in our management and clinical bioengineering teams, including Sotirios Leontaritis, Director, President, Treasurer, and Chief Executive Officer, Nikolaos Kardaras, Director and Secretary, Grigorios Tsourtos, our Chief Financial Officer and Christos Kapatos, Director, who heads the development of our technology. The loss of the services of any one of those individuals could have an adverse effect on our business, and may significantly delay or prevent the achievement of our product development and other business objectives. Our future success will also depend on our ability to identify, recruit, train and retain additional qualified personnel. There is currently a shortage of skilled clinical, bioengineering and management personnel and intense competition for these personnel. Thus, we may not be successful in retaining our key personnel or their services.
Risks Associated with our Common Stock
We are an "emerging growth company," and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and may remain an emerging growth company until December 31, 2018, provided that, if the market value of our common stock that is held by non-affiliates exceeds $700 million as of any June 30 before that time or if we have annual gross revenues of $1 billion or more in any fiscal year, we would cease to be an emerging growth company as of December 31 of the applicable year. We also would cease to be an emerging growth company if we issue more than $1 billion of non-convertible debt over a three-year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
|
·
|
providing only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced "Management's discussion and analysis of financial condition and results of operations" disclosure;
|
|
·
|
not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting;
|
|
·
|
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements;
|
|
·
|
reduced disclosure obligations regarding executive compensation; and
|
|
·
|
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
|
We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of reduced reporting burdens in this prospectus. In particular, in this prospectus, we have provided only two years of audited financial statements and have not included all of the executive compensation related information that would be required if we were not an emerging growth company. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
In addition, the JOBS Act also provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to delay such adoption of new or revised accounting standards, and as a result, we may not comply with new or revised accounting standards on the relevant dates, on which adoption of such standards is required for public companies that are not emerging growth companies. As a result of such election, our financial statements may not be comparable to the financial statements of other public companies.
We do not intend to pay dividends on any investment in the shares of our stock.
We have never paid any cash dividends and currently do not intend to pay any dividends for the foreseeable future. To the extent that we require additional funding currently not provided for in our financing plan, our funding sources may prohibit the payment of a dividend. Because we do not intend to declare dividends, any gain on an investment in our company will need to come through an increase in the stock’s price. This may never happen and investors may lose all of their investment in us.
Because we can issue additional shares of common stock, purchasers of our common stock may incur immediate dilution and may experience further dilution.
We are authorized to issue up to 100,000,000 shares of common stock, of which 5,003,650 shares are issued and outstanding. Our board of directors has the authority to cause us to issue additional shares of common stock, and to determine the rights, preferences and privileges of such shares, without consent of any of our stockholders. Consequently, the stockholders may experience more dilution in their ownership of our stock in the future.
Because we can issue shares of preferred stock which will have certain voting rights, which, if voted collectively, could result in a control of any matters put before the Company’s stockholders, the shareholders of our common stock will suffer substantial dilution if issued or converted.
We have the right to issue a total of 5,000,000 shares of Series A Preferred Stock. Except with respect to matters which adversely affect the holders of Series A Preferred Stock, as required by law, or as required by the Articles of Incorporation, the holders of Series A Preferred and the holders of Common Stock shall be entitled to notice of any stockholders' meeting and to vote as a single class upon any matter submitted to the stockholders for a vote, on the following basis:
|
(a)
|
holders of Common Stock shall have one vote per share of Common Stock held by them; and
|
|
(b)
|
holders of Series A Preferred Stock shall have 50 votes per share of Series A Preferred Stock held by them.
|
Holders of the Series A Preferred Stock have voting rights with respect to matters that generally require the approval of voting stockholders, in addition to voting rights as specifically required by Nevada law . In the event the holders of Series A Preferred Stock vote collectively as to any matter put before a vote, such holders of Series A Preferred Stock could control the vote. While the holders of Series A Preferred Stock have not advised that they intend to vote collectively on any matter, nor are we aware of any voting agreements currently in place, we cannot know with certainty that such holders of Series A Preferred Stock will not vote collectively in the future or enter into any voting agreements.
Further, the holders of any Series A Preferred Stock will have the right to convert to common stock at their election on the basis of 20 shares of common stock for each one share of Series A Preferred Stock issued, thus the holders of our common stock would suffer substantial dilution upon conversion.
A decline in the price of our common stock could affect our ability to raise further working capital, it may adversely impact our ability to continue operations, and we may go out of business.
A prolonged decline in the price of our common stock could result in a reduction in the liquidity of our common stock and a reduction in our ability to raise capital. Because we may attempt to acquire a significant portion of the funds we need in order to conduct our planned operations through the sale of equity securities, a decline in the price of our common stock could be detrimental to our liquidity and our operations because the decline may cause investors to not choose to invest in our stock. If we are unable to raise the funds we require for all our planned operations, we may be forced to reallocate funds from other planned uses and may suffer a significant negative effect on our business plan and operations, including our ability to develop new products and continue our current operations. As a result, our business may suffer, and not be successful and we may go out of business. We also might not be able to meet our financial obligations if we cannot raise enough funds through the sale of our common stock and we may be forced to go out of business.
Our stock is a penny stock. Trading of our stock may be restricted by the Securities and Exchange Commission’s penny stock regulations which may limit a stockholder’s ability to buy and sell our stock.
Our stock is a penny stock. The Securities and Exchange Commission has adopted Rule 15g-9 which generally defines “penny stock” to be any equity security that has a market price (as defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. Our securities are covered by the penny stock rules, which impose additional sales practice requirements on broker-dealers who sell to persons other than established customers and “accredited investors”. The term “accredited investor” refers generally to institutions with assets in excess of $5,000,000 or individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouse. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document in a form prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer’s confirmation. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for the stock that is subject to these penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny stock rules discourage investor interest in and limit the marketability of our common stock.
FINRA sales practice requirements may also limit a stockholder's ability to buy and sell our stock.
In addition to the “penny stock” rules promulgated by the Securities and Exchange Commission, the Financial Industry Regulatory Authority (FINRA) has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, the FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock.
Technology company stock prices are especially volatile, and this volatility may depress the price of our common stock.
The stock market has experienced significant price and volume fluctuations, and the market prices of technology companies have been highly volatile. We believe that various factors may cause the market price of our common stock to fluctuate, perhaps substantially, including, among others, the following:
|
•
|
announcements of developments in our patent enforcement actions, should patents be granted to us;
|
|
•
|
developments or disputes concerning our inventions or patents, if granted;
|
|
•
|
our competitors' technological innovations;
|
|
•
|
developments in relationships with licensees;
|
|
•
|
variations in our quarterly operating results;
|
|
•
|
our failure to meet or exceed securities analysts' expectations of our financial results;
|
|
•
|
a change in financial estimates or securities analysts' recommendations;
|
|
•
|
changes in management's or securities analysts' estimates of our financial performance;
|
|
•
|
changes in market valuations of similar companies;
|
|
•
|
the current sovereign debt crises affecting several countries in the European Union and concerns about sovereign debt of the United States;
|
|
•
|
announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures, capital commitments, new technologies, or patents, if granted; and
|
|
•
|
failure to complete significant transactions.
|
The financial crisis affecting the banking system and financial markets and the uncertainty in global economic conditions, which began in late 2007 and has continued up until today, have resulted in a tightening in the credit markets, a low level of liquidity in many financial markets, and extreme volatility in the credit, equity and fixed income markets. If investors have concerns that our business, operating results and financial condition will be negatively impacted by global economic conditions, our stock price could fluctuate significantly in future periods.
In addition, we believe that fluctuations in our stock price during applicable periods can also be impacted by court rulings and/or other developments in our patent licensing and enforcement actions. Court rulings in patent enforcement actions are often difficult to understand, even when favorable or neutral to the value of the patents we intend to apply for and our overall business, and we believe that investors in the market may overreact, causing fluctuations in our stock prices that may not accurately reflect the impact of court rulings on our business operations and assets.
In the past, companies that have experienced volatility in the market price of their stock have been the objects of securities class action litigation. If our common stock was the object of securities class action litigation, it could result in substantial costs and a diversion of management's attention and resources, which could materially harm our business and financial results.
Risks Related to our Financial Results and Need for Additional Financing
We may be unable to raise additional capital if it should be necessary, which could harm our ability to compete.
We expect to incur significant expenditures in order to establish an international brand, to develop both product and process technology, and to operate P&O clinics around the world. We do not currently have the funds available to effectuate our business plan. We have to date relied on loans from our chief executive officer, however, we cannot be assured that he will continue to fund operations and we may require substantial funding that may not be available through loans. We will be required to seek substantial funding in the equity markets or by loans for operations. We may not be able to raise funds when needed, or on acceptable terms.
We may not generate sufficient cash flow to service our debt, pay our contractual obligations and operate our business.
Our ability to make payments on our indebtedness and contractual obligations, and to fund our operations depends on our future performance and financial results, which, to a certain extent, are subject to general economic, financial, competitive, regulatory and other factors, including the interest rate environment, that are beyond our control. Until such time as we can establish operations to generate sufficient liquidity to meet our obligations as they come due you are at risk of our business failing and the loss of your investment. There can be no assurance that our business will generate sufficient cash flow, if any, from operations in the future to service our debt, pay our contractual obligations and operate our business as currently planned.
Our financial results may vary significantly from quarter-to-quarter due to a number of factors, which may lead to volatility in our stock price.
Our quarterly revenue and results of operations may vary significantly from quarter-to-quarter if, and when, we commence revenues from operations. This variability may lead to volatility in our stock price as research analysts and investors respond to these quarterly fluctuations. These fluctuations are due to numerous factors, including: fluctuations in consumer demand for our products; our ability to design and deliver products to our consumers in a timely and cost-effective manner; quality control problems in the manufacturing operations; our ability to timely obtain adequate quantities of the components used in our products; new product introductions and enhancements by us and our competitors; unanticipated increases in costs or expenses; and fluctuations in foreign currency exchange rates. The foregoing factors are difficult to forecast, and these, as well as other factors, could materially and adversely affect our quarterly and annual results of operations.
Prolonged negative economic conditions in domestic and global markets may adversely affect our suppliers and consumers, which could harm our financial position.
Global credit and financial markets have experienced extreme disruptions over the past few years, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates, and uncertainty about economic stability. Although these markets have recently shown signs of rebounding, there can be no assurance that further deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be adversely affected by the recent economic downturn, volatile business environments and continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate further, or do not improve, it may make debt or equity financings, if needed, more difficult, more costly and more dilutive. Failure to secure financing, if needed, in a timely manner, on favorable terms or at all could have a material adverse effect on our growth strategy, financial performance and stock price, and could require us to delay or abandon certain aspects of our business strategy. These negative changes in domestic and global economic conditions or additional disruptions of either or both of the financial and credit markets may have a material adverse effect on our business, financial condition and results of operations.
None.
We currently have offices Glasgow, Scotland which we use for our Glasgow operations while we seek other office space in Glasgow as we commence the development of our technology. Our executive officer, Sotirios Leontaritis has provided office space free of charge in Athens, Greece currently. Since March 10th, 2014 the Company also operates from a branch office at 2A Kolokotroni Street, 17563 Paleo Faliro, Greece.The address of our current office in Glasgow is Centrum Office, 38 Queen Street, Glasgow, UK G1 3DX Our telephone number is (0808) 178 4373.
We have also rented temporary space in Glasgow which we use for our research and development activities. We are seeking a permanent space in Glasgow where we can undertake these activities.
We know of no material, active or pending legal proceedings against our Company, nor of any proceedings that a governmental authority is contemplating against us.
Not Applicable
PART II
Market Price of and Dividends on the Registrant’s Common Equity and Related Stockholder Matters
Market Information
The Company's common stock is currently quoted on the Over-the-Counter-Bulletin Board (“OTCBB”) and the OTC Markets QB(OTCQB) under the trading symbol “VICA.” The Company had no trading in its securities until October 21.2013, therefore the information provided herein is solely for the quarter ended December 31, 2013.
|
Quarter
|
High ($)
|
Low ($)
|
||||||
|
4th Quarter ended 12/31/2013
|
4.00 | 0.70 | ||||||
|
3rd Quarter ended 9/30/2013
|
-0- | -0- | ||||||
|
2nd Quarter ended 6/30/2013
|
-0- | -0- | ||||||
|
1st Quarter ended 3/31/2013
|
-0- | -0- | ||||||
|
4th Quarter ended 12/31/2012
|
-0- | -0- | ||||||
|
3rd Quarter ended 9/30/2012
|
-0- | -0- | ||||||
|
2nd Quarter ended 6/302012
|
-0- | -0- | ||||||
|
1st Quarter ended 3/31/2012
|
-0- | -0- | ||||||
The above information was taken from information on the OTC Markets website at www.otcmarkets.com. The quotations provided may reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
Holders
As of March 21, 2014, there were 21record holders of the Company’s common stock (which number does not include the number of stockholders whose shares are held by a brokerage house or clearing agency, but does include such brokerage houses or clearing agencies as one record holder
Dividends
We have never declared or paid dividends on our common stock. We intend to retain earnings, if any, to support the development of our business and therefore do not anticipate paying cash dividends for the foreseeable future. Payment of future dividends, if any, will be at the discretion of our board of directors after taking into account various factors, including current financial condition, operating results and current and anticipated cash needs.
Recent Sales of Unregistered Securities; Use of Proceeds from Registered Securities
Recent Sales of Unregistered Securities
During the three months ended December 31, 2013 and the subsequent period to the date of the filing of this annual report, the Company issued the following shares:
On January 15, 2014, the Board of Directors of the Company approved the execution of a consulting agreement between the Company and Sotirios Leontaritis (“Leontaritis”), whereby Leontaritis shall provide services to the Company as the Company’s President and Chief Executive Officer in regards to the Company’s management and operations for the period from January 1, 2014 to December 31, 2017. Under the terms of the agreement, the Company agreed to pay to Leontaritis US$60,000 per annum payable in monthly payments of US$5,000 a month for the term of the contract. Further, under the terms of the contract, Leontaritis will receive cash compensation of US$5,000 and a stock award of 1,000,000 shares of common stock of the Company in consideration of his services as an officer and director for the period from September 10, 2013 to December 31, 2013. On January 22, 2014, the Company issued the 1,000,000 shares to Leontaritis pursuant to the agreement.
On February 13, 2014, the Company issued the required 500,000 shares of common stock required as share consideration under an acquisition agreement between the Company’s newly incorporated wholly-owned subsidiary, HCi VioCare Technologies Limited and Christos Kapatos for the acquisition of certain intellectual property rights.
The shares of Common Stock referenced herein were issued in reliance upon the exemption from securities registration afforded by the provisions of Regulation S of the Securities Act of 1933, as amended, (“Securities Act”), as promulgated by the U.S. Securities and Exchange Commission under the Securities Act. Our reliance upon the exemption under Rule 903 of Regulation S of the Securities Act was based on the fact that the sales of the securities were completed in an "offshore transaction", as defined in Rule 902(h) of Regulation S. We did not engage in any directed selling efforts, as defined in Regulation S, in the United States in connection with the sale of the securities. The investor was not a US person, as defined in Regulation S, and was not acquiring the securities for the account or benefit of a US person.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
On December 6, 2013, 46,350 shares of common stock were returned to the Company by two stockholders of the Company and were cancelled and returned to treasury without consideration.
Securities Authorized for Issuance Under Equity Compensation Plans
The Company does not currently have any equity compensation plans and thus no securities have been issued.
As a “smaller reporting company”, we are not required to provide the information required by this Item.
The following discussion should be read in conjunction with our audited financial statements and the related notes that appear elsewhere in this Annual Report. The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in such forward looking statements. Factors that could cause or contribute to such differences include those discussed below and elsewhere in this Annual Report.
Our financial statements are stated in United States dollars and are prepared in accordance with United States generally accepted accounting principles.
This Form 10-K contains certain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. For this purpose any statements contained in this Form 10-K that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, words such as “may,” “will,” “expect,” “believe,” “anticipate,” “estimate” or “continue” or comparable terminology are intended to identify forward-looking statements. These statements by their nature involve substantial risks and uncertainties, and actual results may differ materially depending on a variety of factors, many of which are not within our control. These factors include but are not limited to economic conditions generally and in the industries in which we may participate; competition within our chosen industry, including competition from much larger competitors; technological advances and failure to successfully develop business relationships.
Business Overview
We were incorporated on March 26, 2007 under the laws of the State of Nevada.
The Company has not yet generated revenues from planned principal operations and is considered a development stage company. The Company was formed to sell medical devices with an emphasis on portable medical devices designed for home treatments with the initial focus in the northern regions of China. The Company’s intent was to seek strategic relationships with medical device manufacturers both in China and North America with the aim to be their sales and distribution agent in Northern China and to assist Chinese medical device manufacturers on the development of the North American market.
On September 10, 2013, the controlling shareholder of the Company sold his controlling interest in the shares of the Company and there was a change in the Board of Directors of the Company, effecting a change in control of the Company. The business of the Company remains in the field of medical devices and other opportunities related to their uses. We are currently engaged in healthcare innovation by the technology development and marketing of prosthetics and orthotics (P&O), and we intend to be engaged in the operation of P&O total rehabilitation clinics.
Results of Operations for the Year ended December 31, 2013 Compared to the Year ended December 31, 2012
We have experienced losses since inception. We generated $0 in revenues from operations during the year ended December 31, 2013 and December 31, 2012. Expenses during the year ended December 31, 2013 were $1,338,538 which consisted of office rent of $3,596, office expenses of $41,882, consultancy fees of $13,197, share based compensation totaling $1,250,000 which amount was based on the valuation of a stock award granted to our Chief Executive Officer on grant date, professional fees of $17,862, research and development expenses of $2,739 and travel and entertainment expenses of $9,262 giving us a net loss from operations of $1,338,538. For the same period in 2012, our expenses were $13,627, consisting of office rent of $4,800, office expenses of $2,107, and professional fees of $6,720 resulting in a net operating loss of $13,627. Our expenses during the year ended December 31, 2013 increased substantially over the expenses of the year ended December 31, 2013 as we had increased operations due to the change in control of management of the Company and the expenses related to negotiating and acquiring assets for the proposed ongoing business of the Company. The decrease in our office rent from $4,800 for the year ended December 31, 2012 to $3,596 for the year ended December 31, 2013 is attributable to the discontinuance of the office lease in China. Office expenses increased to $42,278 (2013) from $2,107 due to the
Company purchasing office supplies, payments for administration and secretarial services and generally ramping up operations. Our professional fees increased from $6,720 for the year ended December 31, 2012 to $17,862 for the year ended December 31, 2013, as we required additional professional services and increased filing obligations for actions being undertaken to commence full operations of the Company. As well, we incurred research and development expenses of $2,739, consultancy fees of $13,197, share based compensation of $1,250,000 which amount was based on the valuation of a stock award granted to our Chief Executive Officer on grant date and travel and entertainment expenses of $9,262 related to increased operations with no comparable expenses for December 31, 2012. For the period of March 26, 2007 (inception) through the period ended December 31, 2013, our expenses from operations were $1,694,448 and consisted of office rent of $30,396, office expenses of $56,125, consultancy fees of$13,197, share based compensation of $1,250,000 which amount was based on the valuation of a stock award granted to our Chief Executive Officer on grant date, professional fees of $307,729, research and development expenses of $2,739 and travel and entertainment expenses of $9,262 for a total loss of $1,338,921 which included an interest income of $219 and a loss on foreign exchange of $383.
Capital Resources and Liquidity
At December 31, 2013, we had no available cash on hand and no assets. We had current liabilities of $1,322,152, with $39,900 in accounts payable and accrued expenses of which $30,959 was due to related parties, related party advances of $32,252, and $1, 250,000 in liabilities to be settled with common stock with respect to a common stock award granted to the Company’s Chief Executive Officer.
Advances from related parties totaled $32,252 due to the provision of funding from the Company’s Chief Executive Officer, Mr. Sotirios Leontaritis to finance the Company’s operation due to lack of cash resources and fees due to related parties for consulting. During fiscal 2013, Mr. Wu, the prior sole director and officer of the Company provided a total of $22,460 in funding to the Company, which amount was contributed to additional paid in capital by Mr. Wu by way of a debt conversion agreement.
The Company has funded its operations to the date of this report by the sale of equity and by advances from related parties. There can be no assurance that this funding will continue or that funding will be available when required.
The Company will need to raise a total of $5,000,000 to fund its proposed operations for the next twelve months. It intends to fund operations by a combination of related party loans and equity placements. The Company intends to undertake a registration statement to raise a total of $15,000,000 which it hopes to file prior to the end of the second quarter. In the interim, the Company is seeking equity private placements with which to fund operations until such time as it can file and clear the registration statements. To date the Company has been funded by loans from our President, Sotirios Leontaritis.
There can be no assurance that any funding will be available on satisfactory terms and that Mr. Leontaritis will continue to fund operations during this phase of development of the Company.
Going Concern
The Company incurred net losses of $1,338,921 and $13,627 for the fiscal years ended December 31, 2013 and 2012, respectively and $1,694,612 for the period March 26, 2007 (inception) through December 31, 2013. In addition, the Company had a stockholders' deficiency of $1,322,152 at December 31, 2013. While a total of $1,250,000 of this amount relates to the fair market value of share based compensation, these factors raise substantial doubt about the Company's ability to continue as a going concern.
There can be no assurance that sufficient funds required during the next year or thereafter will be generated from operations or that funds will be available from external sources such as debt or equity financings or other potential sources. The lack of additional capital resulting from the inability to generate cash flow from operations or to raise capital from external sources would force the Company to substantially curtail or cease operations and would, therefore, have a material adverse effect on its business. Furthermore, there can be no assurance that any such required funds, if available, will be available on attractive terms or that they will not have a significant dilutive effect on the Company's existing stockholders.
The accompanying financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
Need for Additional Financing
|
|
Costs associated with being a public company are much higher than those of a private company. There are present legal and accounting expenses, reporting requirements to the SEC, and investor relation costs that must be borne by a public company but not by a private company. These costs can be a burdensome expense which could adversely affect our financial survival. The ongoing regulatory costs, reporting requirements, and management details, which must be met when maintaining a public company, may make the economic viability of our Company very doubtful. In addition the Company has projected it will need to raise $5,000,000 to meet its planned business objectives and there can be no assurance those funds will be available if and when needed.
In the past we have relied on advances from our president to cover our operating costs. Subsequent to the period covered by this report, the Company has acquired its first technology which requires further research and development in order to take the products to market. As well, as of the date of filing of this report, the Company is negotiating to acquire an operating business which is currently in positive cash flow. There can be no assurance that funds for the development of the Company’s newly acquired technology will be available if and when needed, or if funding is acquired that the technology development and marketing will be successful. Further, there can be no assurance that the Company will close on the proposed acquisition of its first clinic or that the funding planned to grow this project will be available if and when needed.
We do not have any off-balance sheet arrangements, financings, or other relationships with unconsolidated entities or other persons, also known as “special purpose entities” (SPEs).
As a “smaller reporting company”, we are not required to provide the information required by this Item.
HCi VioCare
(Formerly China Northern Medical Device, Inc. )
A Development Stage Company
FINANCIAL STATEMENTS
At December 31, 2013 and 2012 and
for the years ended December 31, 2013 and 2012
TABLE OF CONTENTS
|
PAGE
|
|||
|
F-2
|
|||
|
F-3
|
|||
|
F-4
|
|||
|
F-5
|
|||
|
F-6
|
|||
|
F-7 to F-14
|
|
KEITH K. ZHEN, CPA
|
|
CERTIFIED PUBLIC ACCOUNTANT
|
|
2070 WEST 6TH STREET - BROOKLYN, NY 11223 - TEL (347) 408-0693 - FAX (347) 602-4868 - EMAIL :KEITHZHEN@GMAIL.COM
|
|
To the Board of Directors and Stockholders of
|
|
HCi VioCare
|
|
( A development stage company)
|
|
We have audited the accompanying balance sheet of HCi VioCare (Formerly China Northern Medical Device, Inc.) ( a development stage company) as of December 31, 2013 and 2012, and the related statements of income, stockholders' equity and comprehensive income, and cash flows for each of the years in the two-year period ended December 31, 2013, and the period March 26, 2007 (inception) through December 31, 2013. China Northern Medical Device, Inc’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
|
|
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
|
|
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of China Northern Medical Device, Inc. ( a development stage company) as of December 31, 2013 and 2012, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2013, and the period March 26, 2007 (inception) through December 31, 2013 in conformity with accounting principles generally accepted in the United States of America.
|
|
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred an operating loss for each of the years in the two-year period ended December 31, 2013, and as of December 31, 2013, has a shareholders' deficiency. These factors raise substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also described in Note 2. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
|
|
/s/Keith K. Zhen, CPA
|
|
Keith K. Zhen, CPA
|
|
Brooklyn, New York
|
|
April 14, 2014
|
| HCi VioCare |
|
(Formerly China Northern Medical Device, Inc.)
|
|
(A Development Stage Company)
|
|
December 31,
|
December 31,
|
|||||||
|
2013
|
2012
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets:
|
||||||||
|
Cash and cash equivalents
|
$ | - | $ | 113 | ||||
|
Prepaid office rent
|
- | 800 | ||||||
|
Total Current Assets
|
- | 913 | ||||||
|
Total Assets
|
$ | - | $ | 913 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current Liabilities:
|
||||||||
|
Accounts payable and accrued expenses (Note 5)
|
$ | 8,941 | $ | 6,604 | ||||
|
Accounts payable and accrued expenses - related party (Note 6)
|
30,959 | - | ||||||
|
Advances from related party (Note 6)
|
32,252 | - | ||||||
| Liabilities to be settled with common stock | 1,250,000 | - | ||||||
|
Total Current Liabilities
|
1,322,152 | 6,604 | ||||||
|
Stockholders' Equity:
|
||||||||
|
Preferred stock, par value $0.0001, 5,000,000 shares authorized; none issued and outstanding as of December 31, 2013 and 2012
|
- | - | ||||||
|
Common stock, par value $0.0001, 100,000,000 shares authorized; 3,503,650 shares issued and outstanding as of December 31, 2013;
|
||||||||
|
3,550,000 shares issued and oustanding as of December 31, 2012
|
350 | 355 | ||||||
|
Additional paid-in capital
|
372,110 | 349,645 | ||||||
|
Deficit accumulated during the development stage
|
(1,694,612 | ) | (355,691 | ) | ||||
|
Stockholders' deficiency
|
(1,322,152 | ) | (5,691 | ) | ||||
|
Total Liabilities and Stockholders' Deficiency
|
$ | - | $ | 913 | ||||
See Notes to Financial Statements
|
|
| HCi VioCare |
|
(Formerly China Northern Medical Device, Inc.)
|
|
(A Development Stage Company)
|
|
For the Period
|
||||||||||||
|
March 26, 2007
|
||||||||||||
|
For the Year Ended
|
(inception) through
|
|||||||||||
|
December 31,
|
December 31,
|
|||||||||||
|
2013
|
2012
|
2013
|
||||||||||
|
Revenues
|
||||||||||||
|
Sales
|
$ | - | $ | - | $ | - | ||||||
|
Costs of Sales
|
- | - | - | |||||||||
|
Gross Profit
|
- | - | - | |||||||||
|
Operating Expenses
|
||||||||||||
|
Office rent
|
3,596 | 4,800 | 30,396 | |||||||||
|
Office expenses
|
41,882 | 2,107 | 56,125 | |||||||||
|
Consultancy Fees
|
13,197 | - | 38,197 | |||||||||
| Share based compensation | 1,250,000 | - | 1,250,000 | |||||||||
|
Professional fees
|
17,862 | 6,720 | 307,729 | |||||||||
|
Research and development
|
2,739 | - | 2,739 | |||||||||
|
Travel and entertainment
|
9,262 | - | 9,262 | |||||||||
|
Total Operating Expenses
|
1,338,538 | 13,627 | 1,694,448 | |||||||||
|
Income (Loss) from Operations
|
(1,338,538 | ) | (13,627 | ) | (1,694,448 | ) | ||||||
|
Other Income (Expenses)
|
||||||||||||
|
Foreign exchange gain (loss)
|
(383 | ) | - | (383 | ) | |||||||
|
Interest Income
|
- | - | 219 | |||||||||
|
Total Other Income (Expenses)
|
(383 | ) | - | (164 | ) | |||||||
|
Income (Loss) before Provision for Income Tax
|
(1,338,921 | ) | (13,627 | ) | (1,694,612 | ) | ||||||
|
Provision for Income Tax
|
- | - | - | |||||||||
|
Net Income (Loss)
|
$ | (1,338,921 | ) | $ | (13,627 | ) | $ | (1,694,612 | ) | |||
|
Basic and fully diluted earnings (loss) per share
|
$ | (0.38 | ) | $ | (0.00 | ) | ||||||
|
Weighted average shares outstanding
|
3,546,138 | 3,550,000 | ||||||||||
See Notes to Financial Statements
| HCi VioCare |
|
(Formerly China Northern Medical Device, Inc.)
|
|
(A Development Stage Company)
|
| FOR PERIOD MARCH 26, 2007 (INCEPTION) THROUGH DECEMBER 31, 2013 |
|
Common Stock
|
Additional
|
Retained Earnings | ||||||||||||||||||
|
No Par Value
|
Paid-in
|
Accumulated
|
||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
(Deficit)
|
Totals
|
||||||||||||||||
|
Balances at the date of inception on March 26, 2007
|
- | $ | - | $ | - | $ | - | $ | - | |||||||||||
|
Proceeds from issuance of common stock
|
3,000,000 | 300 | 39,700 | - | 40,000 | |||||||||||||||
|
Net income (loss)
|
- | - | - | (99,151 | ) | (99,151 | ) | |||||||||||||
|
Balances at December 31, 2007
|
3,000,000 | 300 | 39,700 | (99,151 | ) | (59,151 | ) | |||||||||||||
|
Proceeds from issuance of common stock
|
550,000 | 55 | 109,945 | - | 110,000 | |||||||||||||||
|
Net income (loss)
|
- | - | - | (151,799 | ) | (151,799 | ) | |||||||||||||
|
Balances at December 31, 2008
|
3,550,000 | 355 | 149,645 | (250,950 | ) | (100,950 | ) | |||||||||||||
|
Net income (loss)
|
- | - | - | (32,340 | ) | (32,340 | ) | |||||||||||||
|
Balances at December 31, 2009
|
3,550,000 | 355 | 149,645 | (283,290 | ) | (133,290 | ) | |||||||||||||
|
Net income (loss)
|
- | - | - | (35,376 | ) | (35,376 | ) | |||||||||||||
|
Balances at December 31, 2010
|
3,550,000 | 355 | 149,645 | (318,666 | ) | (168,666 | ) | |||||||||||||
|
Net income (loss)
|
- | - | - | (23,398 | ) | (23,398 | ) | |||||||||||||
|
Balances at December 31, 2011
|
3,550,000 | 355 | 149,645 | (342,064 | ) | (192,064 | ) | |||||||||||||
|
Debt conversion
|
- | - | 200,000 | - | 200,000 | |||||||||||||||
|
Net income (loss)
|
- | - | - | (13,627 | ) | (13,627 | ) | |||||||||||||
|
Balances at December 31, 2012
|
3,550,000 | 355 | 349,645 | (355,691 | ) | (5,691 | ) | |||||||||||||
|
Debt conversion
|
- | - | 22,460 | - | 22,460 | |||||||||||||||
|
Retierment of 46,350 shares of common stock
|
(46,350 | ) | (5 | ) | 5 | - | - | |||||||||||||
|
Net income (loss)
|
- | - | - | (1,338,921 | ) | (1,338,921 | ) | |||||||||||||
|
Balances at December 31, 2013
|
3,503,650 | $ | 350 | $ | 372,110 | $ | (1,694,612 | ) | $ | (1,322,152 | ) | |||||||||
See Notes to Financial Statements
| HCi VioCare |
|
(Formerly China Northern Medical Device, Inc.)
|
|
(A Development Stage Company)
|
|
For the Period
|
||||||||||||
|
March 26, 2007
|
||||||||||||
|
For the Year Ended
|
(inception) through
|
|||||||||||
|
December 31,
|
December 31,
|
|||||||||||
|
2013
|
2012
|
2013
|
||||||||||
|
Operating Activities
|
||||||||||||
|
Net income (loss)
|
$ | (1,338,921 | ) | $ | (13,627 | ) | $ | (1,694,612 | ) | |||
|
Adjustments to reconcile net income (loss) to Net cash provided (used) by operating activities:
|
||||||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Decrease (Increase) in prepaid office rent
|
800 | (800 | ) | - | ||||||||
|
Increase (decrease) in accounts payable and accrued expenses
|
2,337 | (10,495 | ) | 8,941 | ||||||||
|
Increase (decrease) in accounts payable and accrued expenses - related party
|
30,959 | - | 30,959 | |||||||||
| Increase (decrease) in liabilities to be settled with common stock | 1,250,000 | - | 1,250,000 | |||||||||
|
Net cash provided (used) by operating activities
|
(54,825 | ) | (24,922 | ) | (404,712 | ) | ||||||
|
Investing Activities
|
||||||||||||
|
Net cash (used) by investing activities
|
- | - | - | |||||||||
|
Financing Activities
|
||||||||||||
|
Proceeds from issuance of common stock
|
- | - | 150,000 | |||||||||
|
Loans from a shareholder
|
54,712 | 25,000 | 254,712 | |||||||||
|
Net cash provided (used) by financing activities
|
54,712 | 25,000 | 404,712 | |||||||||
|
Increase (decrease) in cash
|
(113 | ) | 78 | - | ||||||||
|
Cash at beginning of period
|
113 | 35 | - | |||||||||
|
Effects of exchange rates on cash
|
- | - | - | |||||||||
|
Cash at end of period
|
$ | - | $ | 113 | $ | - | ||||||
|
Supplemental Disclosures of Cash Flow Information:
|
||||||||||||
|
Cash paid (received) during year for:
|
||||||||||||
|
Interest
|
$ | - | $ | - | $ | - | ||||||
|
Income taxes
|
$ | - | $ | - | $ | - | ||||||
|
Non-cash financing activities:
|
||||||||||||
|
Shares cancelled without consideration
|
$ | 5 | $ | - | $ | 5 | ||||||
|
Contribution of loans from former director to additional paid-in capital
|
22,460 | - | 222,460 | |||||||||
| $ | 22,465 | $ | - | $ | 222,465 | |||||||
See Notes to Financial Statements
F-6
HCi VioCare
(Formerly China Northern Medical Device, Inc.)
(A Development Stage Company)
Note 1 - ORGANIZATION AND BUSINESS BACKGROUND
HCi VioCare (formerly China Northern Medical Device, Inc.) ("VICA" or the "Company") was incorporated on March 26, 2007 under the laws of the State of Nevada. The Company has selected December 31 as its fiscal year end.
The Company has not yet generated revenues from planned principal operations and is considered a development stage company as defined in the Accounting Standards Codification ("ASC") 915, "Development Stage Entities", issued by the Financial Accounting Standards Board ("FASB"). The Company was formed to sell medical devices with an emphasis on portable medical devices designed for home treatments with the initial focus in the northern regions of China. The Company’s intent was to seek strategic relationships with medical device manufacturers both in China and North America with the aim to be their sales and distribution agent in Northern China and to assist Chinese medical device manufacturers on the development of the North American market.
On September 10, 2013, the controlling shareholder of the Company sold his controlling interest in the shares of the Company and there was a change in the Board of Directors of the Company, effecting a change in control of the Company. The business of the Company remains in the field of medical devices and other opportunities related to their uses. We are currently engaged in healthcare innovation by the technology development and marketing of prosthetics and orthotics (P&O), and we intend to be engaged in the operation of P&O total rehabilitation clinics.
Note 2 - GOING CONCERN
The Company incurred net losses of $1,338,921 and $13,627 for the years ended December 31, 2013 and 2012, respectively and $1,694,612 for the period March 26, 2007 (inception) through December 31, 2013. In addition, the Company had a working capital deficiency of $1,322,152 and a stockholders' deficiency of $1,322,152 at December 31, 2013. These factors raise substantial doubt about the Company's ability to continue as a going concern.
There can be no assurance that sufficient funds required during the next year or thereafter will be generated from operations or that funds will be available from external sources such as debt or equity financings or other potential sources. The lack of additional capital resulting from the inability to generate cash flow from operations or to raise capital from external sources would force the Company to substantially curtail or cease operations and would, therefore, have a material adverse effect on its business. Furthermore, there can be no assurance that any such required funds, if available, will be available on attractive terms or that they will not have a significant dilutive effect on the Company's existing stockholders.
The accompanying financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
During the period March 26, 2007 (inception) through December 31, 2013, the Company relied heavily for its financing needs on its Chief Executive Officer and President as more fully disclosed in Note 6.
Note 3 - CONTROL BY PRINCIPAL STOCKHOLDER/OFFICER
Our Chief Executive Officer owns beneficially and in the aggregate, the majority of the voting power of the Company. Accordingly, the Chief Executive Officer has the ability to control the approval of most corporate actions, including approving significant expenses, increasing the authorized capital stock and the dissolution, merger or sale of the Company's assets.
F-7
HCi VioCare
(Formerly China Northern Medical Device, Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
Note 4 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America ("US GAAP") and are presented in U.S. dollars.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results when ultimately realized could differ from these estimates.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand, deposits in banks with maturities of three months or less, and all highly liquid investments which are unrestricted as to withdrawal or use, and which have original maturities of three months or less.
Concentrations of Credit Risk
Financial instruments that subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents. The Company maintains its cash and cash equivalents with high-quality institutions.
Fair Value of Financial Instruments
The carrying value of financial instruments including cash and cash equivalents, receivables, prepaid expenses, accounts payable and accrued expenses, approximates their fair value due to the relatively short-term nature of these instruments.
Impairment of Long-life Assets
Long-lived assets and certain identifiable intangibles are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
Foreign Currencies
The Company’s functional currency is USD and the reporting currency is USD. The effect of exchange rate changes on transactions denominated in currencies other than the functional currency are recorded as a gain/loss on foreign transactions, which is included into the profit and loss for the period.
F-8
HCi VioCare
(Formerly China Northern Medical Device, Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
Note 4 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Revenue Recognition
The Company recognizes revenue when the earnings process is complete and persuasive evidence of an arrangement exists. This generally occurs when products are shipped to unaffiliated customers, both title and the risks and rewards of ownership are transferred or services have been rendered and accepted, the selling price is fixed or determinable, and collectability is reasonably assured.
Advertising Costs
The Company expenses advertising costs as incurred or the first time the advertising takes place, whichever is earlier, in accordance with the FASB ASC 720-35. Advertising costs were immaterial for the fiscal years ended December 31, 2013 and 2012, respectively.
Research and Development Costs
The Company charges research and development costs to expense when incurred in accordance with the FASB ASC 730, “Research and Development”. Research and development costs were $2,739 and $0 for the years ended December 31, 2013 and 2012, respectively.
Related parties
For the purposes of these financial statements, parties are considered to be related if one party has the ability, directly or indirectly, to control the party or exercise significant influence over the party in making financial and operating decisions, or vice versa, or where the Company and the party are subject to common control or common significant influence. Related parties may be individuals or other entities.
Stock-based compensation
Stock-based compensation follows the guidance codified in the Compensation – Stock Compensation Topic of FASB ASC (“ASC 718”). The Company determines the value of stock issued at the date of grant. It also determines at the date of grant, the value of stock at fair market value or the value of services rendered (based on contract or otherwise) whichever is more readily determinable.
Income Taxes
The Company accounts for income tax in accordance with FASB ASC 740, "Income Taxes", which requires the asset and liability approach for financial accounting and reporting for income taxes. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance related to deferred tax assets is recorded when it is more likely than not that some portion or all of the deferred tax assets will not be realized.
The Company has an accumulated deficient from operations. Because there is no certainty that we will realize taxable income in the future, we did not record any deferred tax benefit as a result of these losses.
F-9
HCi VioCare
(Formerly China Northern Medical Device, Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
Note 4 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Earnings (Loss) Per Share
The Company reports earnings per share in accordance with FASB ASC 260, “Earnings Per Share.” FASB ASC 260 requires presentation of basic and diluted earnings per share in conjunction with the disclosure of the methodology used in computing such earnings per share. Basic earnings (loss) per share is computed by dividing income (loss) available to common shareholders by the weighted-average number of common shares outstanding during the period. Diluted earnings per share is computed similar to basic earnings per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. There are no potentially dilutive securities outstanding (options and warrants) for the year ended December 31, 2013 and 2012, respectively.
Comprehensive Income
FASB ASC 220, “Comprehensive Income", establishes standards for reporting and display of comprehensive income, its components and accumulated balances. Comprehensive income as defined includes all changes in equity during a period from non-owner sources.
Segment Reporting
FASB ASC 820 “Segments Reporting” establishes standards for reporting information about operating segments on a basis consistent with the Company’s internal organization structure as well as information about geographical areas, business segments and major customers in financial statements. Our proposed business segments are expected to span more than one geographical area. Specifically the Company intends to operate prosthetic and orthotic rehabilitation clinics with various European based locations, as well as a corporate development and technology center which will undertake ongoing research and marketing activities.
Fair Value of Measurements
Accounting principles generally accepted in the United States define fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. Additionally, the inputs used to measure fair value are prioritized based on a three-level hierarchy. This hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
Level 1: Unadjusted quoted prices in active markets for identical assets or liabilities
Level 2: Input other than quoted market prices that are observable, either directly or indirectly, and reasonably available. Observable inputs reflect the assumptions market participants would use in pricing the asset or liability and are developed based on market data obtained from sources independent of the Company.
Level 3: Unobservable inputs. Unobservable inputs reflect the assumptions that the Company develops based on available information about what market participants would use in valuing the asset or liability.
An asset or liability’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Availability of observable inputs can vary and is affected by a variety of factors. The Company uses judgment in determining fair value of assets and liabilities and Level 3 assets and liabilities involve greater judgment than Level 1 and Level 2 assets or liabilities.
F-10
HCi VioCare
(Formerly China Northern Medical Device, Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
Note 4 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Recent Accounting Pronouncements
In March 2013, the FASB issued guidance on when foreign currency translation adjustments should be released to net income. When a parent entity ceases to have a controlling financial interest in a subsidiary or group of assets that is a business within a foreign entity, the parent is required to release any related cumulative translation adjustment into net income. Accordingly, the cumulative translation adjustment should be released into net income only if the sale or transfer results in the complete or substantially complete liquidation of the foreign entity in which the subsidiary or group of assets had resided. The guidance is effective prospectively beginning January 1, 2014. It is not expected to have a material impact in the financial statements.
In February 2013, the FASB issued guidance for the recognition, measurement and disclosure of obligations resulting from joint and several liability arrangements for which the total amount of the obligation within the scope of the guidance is fixed at the reporting date. Examples include debt arrangements, other contractual obligations and settled litigation. The guidance requires an entity to measure such obligations as the sum of the amount that the reporting entity agreed to pay on the basis of its arrangement among its co-obligors plus additional amounts the reporting entity expects to pay on behalf of its co-obligors. The guidance is effective January 1, 2014 and is not expected to have a material impact in the financial statements.
Note 5 - ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses consist of the following:
|
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
Accounts payable
|
$
|
941
|
$
|
-
|
||||
|
Accrued professional fees
|
8,000
|
6,604
|
||||||
|
Total accounts payable and accrued expenses
|
$
|
8,941
|
$
|
6,604
|
||||
Note 6 - RELATED PARTY TRANSACTIONS
The Company received loans from its former CEO and controlling shareholder, Mr. Jinzhao Wu to finance the Company’s operations due to a lack of cash resources. These loans were unsecured, non-interest bearing and had no fixed terms of repayment, therefore, and they were deemed payable on demand. Cash flow from this activity is classified as cash flows from financing activity. The total borrowing from Mr. Wu was $200,000 for the period March 26, 2007 (inception) through December 31, 2012. On December 31, 2012, the Company entered into a debt conversion agreement with Mr. Wu, pursuant to which $200,000 of debt owed to Mr. Wu by the Company was contributed to additional paid-in capital of the Company. The borrowing from Mr. Wu for the period January 1, 2013 through September 9, 2013 was $22,460. On September 9, 2013, the Board of Directors approved that Mr. Wu contribute his loans to the Company in the total amount of $22,460, as additional paid-in capital of the Company. On September 9, 2013 Mr. Wu sold his controlling interest in the Company to the Company’s current Chief Executive Officer, President, Treasurer and Director, Mr. Sotirios Leontaritis in a private transaction. On September 10, 2013 Mr. Wu resigned all positions as an officer and director.
On September 9, 2013 Mr. Sotirios Leontaritis acquired 3,000,000 shares of the Company’s common stock, the controlling interest, from the Company’s former sole officer and director in a private transaction. On September 10, 2013 Mr. Leontaritis was appointed President, Treasurer and Director of the Company.
F-11
HCi VioCare
(Formerly China Northern Medical Device, Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
Note 6 - RELATED PARTY TRANSACTIONS (continued)
For the period from September 10, 2013 through December 31, 2013, Mr. Sotirios Leontaritis advanced funds to the Company to settle certain operating expenses incurred in the normal course, and incurred expense items for reimbursement. A total of $24,590 has been recorded as accounts payable, related party for reimbursable expenses, and a further $32,252 has been recorded on the balance sheets as advances from related party as of December 31, 2013, which amount bears no interest, unsecured, and is due on demand, without a formal loan agreement.
On January 15, 2014, the Board of Directors of the Company approved the execution of a consulting agreement between the Company and Sotirios Leontaritis (“Leontaritis”), whereby Leonataritis shall provide services to the Company as the Company’s President and Chief Executive Officer in regards to the Company’s management and operations for the period from January 1, 2014 to December 31, 2017. Under the terms of the agreement, the Company agreed to pay to Leontaritis US$60,000 per annum payable in monthly payments of US$5,000 a month for the term of the contract. Further, under the terms of the contract, Leontaritis will receive cash compensation of US$5,000 and a stock award of 1,000,000 shares of common stock of the Company in consideration of his services as an officer and director for the period from September 10, 2013 to December 31, 2013. On January 22, 2014, the Company issued the 1,000,000 shares to Leontaritis pursuant to the agreement. As of December 31, 2013, the Company expensed $1,255,000in consulting fees and share based compensation, of which $5,000 will be paid in cash and an amount of $1,250,000 was the deemed the value of the stock award which has been expensed as share based compensation and is recorded on the Company’s balance sheets as “Liabilities to be settled with common stock” at December 31, 2013.
During the year ended December 31, 2013, Mr. Nikolaos Kardaras, the Secretary and a director of the Company invoiced the Company in the amount of $2,727 (GBP 1,403) for services rendered which amount was recorded as attorney fees and was fully paid by December 31, 2013.
On November 27, 2013, the Board of Directors of the Company appointed Mr. Grigorios Tsourtos as Chief Financial Officer of the Company. During the fiscal year ended December 31, 2013, the Company accrued $1,369 (EUR 1,000) as consulting fees for his services rendered under accounts payable and accrued expenses – related party.
Note 7 - CAPITAL STOCK
The Articles of Incorporation authorize the Company to issue 5,000,000 shares of preferred stock with a par value of $0.0001, and 100,000,000 shares of common stock with a par value of $0.0001. No shares of preferred stock have been issued. Upon formation of the Company, 3,000,000 shares of common stock were issued for $40,000.
The Company completed a public offering on March 14, 2008. The Company issued 550,000 shares of common stock to 40 PRC citizen shareholders for $110,000. The common stock issued and outstanding immediately after the offering was 3,550,000.
On December 31, 2012, the Company entered into debt conversion agreement with Mr. Wu, formerly the Company's sole officer and director, pursuant to which $200,000 of debt owed to Mr. Wu by the Company was contributed to additional paid-in capital of the Company.
On September 9, 2013, the Board of Directors approved the further contribution by Mr. Wu of outstanding loans to the Company in the amount of $22,460, which amount has been allocated to additional paid-in capital of the Company.
On December 6, 2013, 46,350 shares of common stock were cancelled and returned to treasury without consideration.
F-12
HCi VioCare
(Formerly China Northern Medical Device, Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
Note 7 - CAPITAL STOCK (continued)
On December 27, 2013, pursuant to approval of the board of directors and the majority stock holder on November 28, 2013 and November 29, 2013 respectively, the Company filed a Certificate of Amendment to the Articles of Incorporation of the Company for the purpose of clearly providing the directors of the Company with the authority to issue both common and preferred stock without approval by the stockholders and to grant the authority of the directors of the Company to issue such shares of common and/or preferred stock in one or more series, with such voting power, designation, preferences and rights or qualifications, limitations or restriction as they may determine by resolution of the Board of Directors.
As at December 31, 2013 the Company has a total of 3,503,650 shares issued and outstanding.
The Company issued a stock award of 1,000,000 shares of common stock of the Company to an officer and director of the Company pursuant to a consulting agreement (ref Note 6 – Related party transactions). The shares issued have been valued at $1.25 per share, the fair market value of the shares on the grant date as share based compensation which totals $1,250,000. The 1,000,000 shares of common stock were not issued at December 31, 2013, and therefore are recorded as “Liabilities to be settled with common stock” on the Company’s balance sheets. On January 22, 2014, the Company issued the 1,000,000 shares to Mr. Leontaritis pursuant to the agreement.
Note 8 - OFFICE LEASE
During the period up to September 10, 2013, the Company rented an office premise and attached facilities for its headquarters. The rental expense amounted to $3,596 and $4,800 for the year ended December 31, 2013 and 2012, respectively.
Note 9 - SUBSEQUENT EVENTS
Designation of Series A Preferred Stock
On January 13, 2014, the Company filed a Certificate of Designation with the Secretary of State of the State of Nevada. The Certificate of Designation sets forth the rights, preferences and privileges of a class of the Company’s preferred stock. Such class shall be designated as the “Series A Preferred Stock” and the number of shares constituting such series shall be 5,000,000 shares. The holders of Series A Preferred Stock will be entitled to a preference over all of the shares of the Company’s common stock. Holders of Series A Preferred Stock shall have 50 votes per share of Series A Preferred Stock held by them and shall be entitled to notice of any stockholders’ meeting and to vote as a single class upon any matter submitted to the stockholders for a vote. Each share of Series A Preferred Stock is convertible into 20 shares of our common stock at any time at the holder’s option. Shares of Series A Preferred Stock shall not be entitled to any dividends.
Consulting Agreement with Mr. Sotirios Leontaritis, President and CEO of the Company
On January 15, 2014, the Board of Directors of the Company approved the execution of a consulting agreement between the Company Sotirios Leontaritis (“Leontaritis”), whereby Leonataritis shall provide services to the Company as the Company’s President and Chief Executive Officer in regards to the Company’s management and operations for the period from January 1, 2014 to December 31, 2017. Under the terms of the agreement, the Company agreed to pay to Leontaritis US$60,000 per annum payable in monthly payments of US$5,000 a month for the term of the contract. Further, under the terms of the contract, Leontaritis will receive cash compensation of US$5,000 and a stock award of 1,000,000 shares of common stock of the Company in consideration of his services as an officer and director for the period from September 10, 2013 to December 31, 2013. On January 22, 2014, the Company issued the 1,000,000 shares to Leontaritis pursuant to the agreement.
F-13
HCi VioCare
(Formerly China Northern Medical Device, Inc.)
(A Development Stage Company)
NOTES TO FINANCIAL STATEMENTS
Note 9 - SUBSEQUENT EVENTS (continued)
Incorporation of Two New Subsidiaries
On January 15, 2014, the Company incorporated two wholly-owned subsidiaries in Scotland, U.K., HCi VioCare Technologies Limited and HCi VioCare Clinics UK Limited. The Company intends to operate in Scotland under these two subsidiaries, one of which will undertake the development and marketing of technologies and the other which is intended to acquire or establish operating clinics related to the field of prosthetics and orthotics.
Acquisition of a Patented Technology
On February 12, 2014, the Company, through our newly incorporated Scottish subsidiary, HCi VioCare Technologies Limited (“Viocare”), entered into an acquisition agreement with Christos Kapatos, a director of both the Company and Viocare, to acquire all rights and interest in and to a patented technology known as “Socket-Fit”. Socket-Fit is a digital system for assessing an amputee’s residual limb and for the production of truly functional and comfortable prosthetic sockets. The technology takes account of the external and internal geometry of the amputee’s stump, the biomechanical properties of each individual soft tissue layer and the boundary and loading conditions of a complete prosthesis to generate a virtual 3D model of the residual limb making it possible to product any accurate, functional and comfortable prosthetic socket. By minimizing the time and cost of socket production and reducing the number of faulty sockets there will be a reduction in costs incurred by health services and insurance companies worldwide as well as benefits to the amputee. The Company intends through the acquisition of the background intellectual property rights (“IPR”) to undertake and fund, through its U.K. subsidiary, a project as defined in the acquisition agreement to improve the nature of the data used in socket modeling software with a view to creating a system that will enable prosthetists to build a socket that evenly distributes weight, provides enhanced comfort, and can be marketed and used across the industry for improved socket creation.
Consideration for the acquisition by Viocare of the exclusive, perpetual, revocable, transferable, royalty free worldwide ownership of the IPR and knowhow to develop and exploit the IPR was the issuance of 500,000 shares of the common stock of the Company to Kapatos. It is the intent of the Company to enter into a consulting agreement with Kapatos which will provide for services for the further development and commercialization of the IPR.
On February 13, 2014, the Company issued the required 500,000 shares of common stock required as share consideration under the acquisition agreement to Kapatos thus completing the acquisition of the IPR.
Change of the Company’s Name
On February 19, 2014, the Company filed a Certificate of Amendment with the Secretary of State of Nevada to change the name of the Company to HCi VioCare effective March 21, 2014. The name change has been submitted to FINRA for their approval. On the effective date, March 21, 2014 or such other later date upon which the Company receives approval from FINRA, the Company’s name will change to HCi VioCare and its CUSIP number will change to “40416H105”, and the Company’s new trading symbol will be “VICA”.
In the fiscal years ended December 31, 2013 and 2012, there have been no changes in the Company’s auditors or the Company’s accounting policies, nor have there been any disagreements with our accountants.
Evaluation of Disclosure Controls and Procedures
Our management, under supervision and with the participation of the Company’s Principal Executive Officer and Principal Financial Officer, evaluated the effectiveness of our disclosure controls and procedures, as defined under Exchange Act Rule 13a-15(e). Based upon this evaluation, the Principal Executive Officer and Principal Financial Officer concluded that, as of December 31, 2013, because of the material weakness in our internal control over financial reporting (“ICFR”) described below, our disclosure controls and procedures were not effective.
Disclosure controls and procedures are controls and other procedures that are designed to ensure that required information to be disclosed in our reports filed or submitted under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that required information to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and our principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined under Exchange Act Rules 13a-15(f) and 14d-14(f). Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
All internal control systems, no matter how well designed, have inherent limitations and may not prevent or detect misstatements. Therefore, even those systems determined to be effective can only provide reasonable assurance with respect to financial reporting reliability and financial statement preparation and presentation. In addition, projections of any evaluation of effectiveness to future periods are subject to risk that controls become inadequate because of changes in conditions and that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2013. In making the assessment, management used the criteria issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on its assessment, management concluded that, as of December 31, 2013, our internal control over financial reporting was not effective and that material weaknesses in ICFR existed as more fully described below.
As defined by Auditing Standard No. 5, “An Audit of Internal Control Over Financial Reporting that is Integrated with an Audit of Financial Statements” established by the Public Company Accounting Oversight Board (“PCAOB”), a material weakness is a deficiency or combination of deficiencies that results in more than a remote likelihood that a material misstatement of annual or interim financial statements will not be prevented or detected. In connection with the assessment described above, management identified the following control deficiencies that represent material weaknesses as of December 31, 2013:
|
1)
|
Lack of an independent audit committee or audit committee financial expert, and no independent directors. We do not have any members of the Board who are independent directors and we do not have an audit committee. These factors may be counter to corporate governance practices as defined by the various stock exchanges and may lead to less supervision over management;
|
|
|
2)
|
The relatively small number of people who are responsible for bookkeeping functions prevents us from segregating duties within our internal control system. The inadequate segregation of duties is a weakness because it could lead to the untimely identification and resolution of accounting and disclosure matters or could lead to a failure to perform timely and effective reviews. This may result in a failure to detect errors in spreadsheets, calculations or assumptions used to compile the financial statements and related disclosures as filed with the SEC;
|
|
3)
|
Insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of US GAAP and SEC disclosure requirements;
|
Management's Remediation Initiatives
As of December 31, 2013, management assessed the effectiveness of our internal control over financial reporting. Based on that evaluation, it was concluded that during the period covered by this report, the internal controls and procedures were not effective due to deficiencies that existed in the design or operation of our internal controls over financial reporting. However, management believes these weaknesses did not have an effect on our financial results. During the course of our evaluation, we did not discover any fraud involving management or any other personnel who play a significant role in our disclosure controls and procedures or internal controls over financial reporting.
Due to a lack of financial and personnel resources, we are not able to, and do not intend to, immediately take any action to remediate these material weaknesses. We will not be able to do so until, if ever, we acquire sufficient financing and staff to do so. We will implement further controls as circumstances, cash flow, and working capital permits. Notwithstanding the assessment that our ICFR was not effective and that there were material weaknesses as identified in this report, we believe that our financial statements contained in our Annual Report on Form 10-K for the period ended December 31, 2013, fairly presents our financial position, results of operations, and cash flows for the periods covered, as identified, in all material respects.
Management believes that the material weaknesses set forth above were the result of the scale of our operations and intrinsic to our small size. Management also believes that these weaknesses did not have an effect on our financial results.
This Annual Report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to the rules of the Securities and Exchange Commission that permit the Company to provide only management’s report in this annual report.
Changes in Internal Control over Financial Reporting
During the period covered by this report, there were no changes (including corrective actions with regard to significant deficiencies or material weaknesses) in our internal controls over financial reporting that occurred that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
PART III
Executive Officer and Directors
The following individuals serve as the directors and executive officers of our Company as of the date of this annual report.
|
Name
|
Position Held with Our Company
|
Age
|
Date First Elected or
Appointed
|
|
Sotirios Leontaritis
|
Chief Executive Officer, President, Treasurer, and Director
|
53
|
September 10, 2013
|
|
Nikolaos Kardaras
|
Secretary and Director
|
63
|
September 10, 2013
|
|
Christos Kapatos
|
Director
|
36
|
September 30, 2013
|
|
Grigorios Tsourtos
|
Chief Financial Officer
|
35
|
November 27, 2013
|
Our Board of Directors consists of only one class. All of the directors will serve until the next annual meeting of stockholders; until their successors are elected and qualified; or until their earlier death, retirement, resignation or removal. There are no family relationships among directors and executive officers. There are no arrangements or understandings between any director or executive officer and any other person(s) pursuant to which a director or executive officer was selected. Our executive officers are elected by our Board of Directors and hold office until their death, resignation or removal from office.
Our Board of Directors believes that the Board and our executive officer possesses a range of talent, skill, and experience sufficient to provide sound and prudent guidance with respect to our operations and interests. The information below with respect to our sole director and officer includes the individual’s experience, qualifications, attributes, and skills that led our Board of Directors to the conclusion that he or she should serve as a director and/or executive officer.
We know of no pending proceedings to which any director, member of senior management, or affiliate is either a party adverse to us, or our subsidiaries, or has a material interest adverse to us or our subsidiaries.
None of our executive officers or directors have been involved in any bankruptcy proceedings within the last five years, been convicted in or has pending any criminal proceedings, been subject to any order, judgment or decree enjoining, barring, suspending or otherwise limiting involvement in any type of business, securities or banking activity or been found to have violated any federal or state securities or commodities law.
Business Experience
The following is a brief account of the education and business experience during at least the past five years of each director, executive officer and key employee of our company, indicating the person’s principal occupation during that period, and the name and principal business of the organization in which such occupation and employment were carried out.
Mr. Leontaritis has been a self-employed private businessman residing in Athens, Greece and has been for the past five years. He has been involved in the public markets for over 25 years in numerous capacities. In 2004, he was the co-founder of a public bio-tech startup company in Athens, Greece. In 2007 he co-founded a solar energy company also in Greece. For the past two years, Mr. Leontaritis has been working on a project for rehabilitation centers, diabetic foot care and research and development of health care technologies with a Cyprus corporation, H.C.I. VioCare Ltd., of which he is currently the sole shareholder.
He is not an officer and director of any other reporting issuers.
Grigorios Tsourtos – Chief Financial Officer
Mr. Tsourtos, holds a degree as an associate CPA from the Certified Practicing Accountants of Australia which he received in November, 2012. He also has a Bachelor in Business Administration, with a major in Accounting and Finance that he received from the Athens University of Economics in 2003.
Mr. Tsourtos is a finance professional with multi-industry experience. From July 2013 to present he is employed with Huawei Technologies as the Chief Accountant for the Greek rep office which covers five countries, Greece, Cyprus, Albania, FYROM, and Bulgaria with a combined turnover of €200M per annum. His duties include contract negotiation, project management, cash flow planning, monthly and annual closing of financial reports, annual external audit coordination and tax and statutory compliance. From July 2012 to July 2013, he was employed with Terna Energy as their controller of U.S. Operations where he was responsible for financial control for the operations in the U.S market, reporting to the Group Controller and the Deputy CFO. From July 2008 to July 2012 he was the Controller and accounting manager for Upstream Mobile Marketing, reporting directly to the CFO and supervising the day to day operations of accounting and finance functions, which comprised headquarters and ten regional offices in Europe, the Americas, the Middle East and Africa. From February 2004 to July 2008 he was an associate auditor with Crowe Horwath.
Mr. Tsourtos is not an officer and director of any other reporting issuers.
Nikolaos Kardaras – Secretary and Director
Mr. Nikolaos Kardaras is member of the Piraeus Bar, having practiced law since 1976. He graduated from the Law School of Athens, Greece in 1974. His current practice encompasses the disciplines of maritime, commercial, civil, initiatives and investments law, acquisition and mergers, and international shipping and commercial transactions.
Mr. Kardaras is not an officer or director of any other reporting issuers.
Dr. Christos Kapatos – Director
Dr. Kapatos operates a bioengineering technology consulting business, HCinnovations, Glasgow, where he has been the consultant and the managing director since 2009. He specializes in the fields of bioengineering, education, medical devices, healthcare automation, advanced prosthetics, orthotics and diabetic technologies, and medical technology startups. He has published a number of papers and has filed patents for prosthetic technologies. From 2008 to 2011 he was the innovation and commercialization director for Diolab Ltd, an international medical equipment company. From 2008 to present, he has worked with Scotsig, a company with small to medium equipment technology in the field of medical signal processing, as a medical devices, innovation and commercialization consultant. Dr. Kapatos received a BEng degree from Northumbria University in 1998, an Msc degree in bioengineering from the University of Strathclyde in 1999, a Msc degree related to new venture creation from Caledonian University Business School in 2007, and a Phd in Bioengineering from the University of Strathclyde in 2006. He has worked with Mr. Leontaritis on projects for H.C.I. VioCare Ltd.
Dr. Kapatos is not an officer and director of any other reporting issuers.
Code of Ethics
The Company has adopted a Code of Ethics applicable to its Chief Executive Officer and Chief Financial Officer. This Code of Ethics was filed as an exhibit to the Form 10K for the year ending December 31, 2008 filed on April 6, 2009.
We will provide a copy of the Code of Ethics to any person without charge, upon request. Requests can be sent to our company at the address on the cover of this annual report.
Nominating Committee
There have been no material changes to the procedures by which security holders may recommend nominees to the Company's board of directors.
Audit Committee
At this time, the Company is not required to have an audit committee. Further, since there no independent members of the Board it is not feasible at this time to have an audit committee. The Board of Directors performs the same functions as an audit committee. The Board of Directors in performing its functions as an audit committee has determined that it does not have an audit committee financial expert.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our directors, executive officers, and stockholders holding more than 10% of our outstanding common stock, to file with the Securities and Exchange Commission initial reports of ownership and reports of changes in beneficial ownership of our common stock. Executive officers, directors and greater-than-10% stockholders are required by SEC regulations to furnish us with copies of all Section 16(a) reports they file.
Based on a review of Forms 3, 4, and 5 and amendments thereto furnished to the registrant during its most recent fiscal year ending December 31, 2013, there were no late filings. We note in a review of the filings with the Securities and Exchange Commission that Mr. Jinzhao Wu has not filed a Form 4 or Form 5 reporting the disposition of his shares of the Company during fiscal 2013.
|
·
|
Our principal executive officer for the fiscal years ended December 31, 2013 and 2012.
|
|
SUMMARY COMPENSATION TABLE
|
||||||||||||||||||
|
Name
and Principal
Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Change in
Pension
Value and
Nonqualified
Deferred
Compensation
Earnings
($)
|
All
Other
Compensati
on
($)
|
Total
($)
|
|||||||||
|
Sotirios Leontaritis(1)
Chief Executive Officer, President, Treasurer, Secretary and director
|
2013
|
5,000 |
Nil
|
1,250,000 | (2) |
Nil
|
Nil
|
Nil
|
Nil
|
1,255,000 | ||||||||
|
Jinzhao Wu(3)
Chief Executive Officer, President, Treasurer, Secretary and director
|
2013
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
|||||||||
|
Jinzhao Wu(3)
Chief Executive Officer, President, Treasurer, Secretary and director
|
2012
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
Nil
|
|||||||||
(1) Mr. Sotirios Leontaritis was appointed principal executive officer on September 9, 2013.
(2) This amount is calculated solely for the purposes of disclosure based on the closing bid price of the stock at the date of issuance at $1.25 per share. The compensation received by Mr. Leontaritis pursuant to a consulting agreement entered into with the Company effective January 1, 2014 which provided for the retroactive payments for 2013 as reflected above.
(3)Mr. Wu was our principal executive officer for the fiscal year ended December 31, 2012 and through to September 9, 2013 when he resigned from the position.
Outstanding Equity Awards at Fiscal Year-End
None.
Option Grants and Exercises
We do not currently have any stock option plans. There were no option grants during fiscal 2013 or 2012.
Employment Agreements
On November 27, 2013, the Board of Directors of the Company appointed Mr. Grigorios Tsourtos as Chief Financial Officer of the Company. During the fiscal year ended December 31, 2013, the Company accrued $1,369 (€1,000) consulting fees for his services rendered. Mr. Tsourtos is not an employee of the Company, but has a verbal agreement to provide his consulting services as Chief Financial Officer for €1,000 per month.
As of the fiscal year ended December 31, 2013, we had not entered into employment agreement with any of our other directors or officers. Subsequent to the fiscal year ended December 31, 2013, on January 15, 2014, the Board of Directors of the Company approved the execution of a consulting agreement between the Company and its President and Chief Executive Officer, Sotirios Leontaritis (“Leontaritis”), whereby Leontaritis will provide services to the Company as the Company’s President and Chief Executive Officer in regards to the Company’s management and operations for the period from January 1, 2014 to December 31, 2017. Under the terms of the agreement, the Company will pay to Leontaritis US$60,000 per annum payable in monthly payments of US$5,000 a month for the term of the contract. Further, under the terms of the contract, Leontaritis received cash compensation of US$5,000 and a stock award of 1,000,000 shares of common stock of the Company in consideration of his services for the period from September 10, 2013 to December 31, 2013.
Compensation of Directors
During the most recent fiscal year, no directors were provided any compensation for their role as directors of the Company.
The Company has made no arrangements for the cash remuneration of its directors, except that they will be entitled to receive reimbursement for actual, demonstrable out-of-pocket expenses, including travel expenses, if any, made on the Company’s behalf.
Compensation Committee
We do not currently have a compensation committee. The Chief Executive Officer’s executive compensation has been approved by our Board of Directors. All of our other compensation contracts have been negotiated by our Chief Executive Officer and approved by our Board of Directors.
Security Ownership of Certain Beneficial Owners
Based on 5,003,650 shares of common stock issued and outstanding as of March 24, 2014, the beneficial owners holding more than 5% of the outstanding common stock, taking into account beneficial ownership of common stock if all outstanding options, warrants, rights and conversion privileges (to which the applicable 5% stockholders have the right to exercise in the next 60 days) are exercised and additional shares of common stock are issued, are disclosed below:
|
Title of Class
|
Name and address of Beneficial Owner
|
Amount and Nature
of Beneficial Ownership
|
Percentage of Class (1)
|
|
Common Stock
|
Sotirios Leontaritis
|
4,000,000 shares held directly
|
79.94%
|
(1)Beneficial ownership is determined in accordance with SEC rules and includes voting or investment power with respect to securities. All shares of common stock subject to options or warrants exercisable within 60 days of March 24, 2014 are deemed to be outstanding and beneficially owned by the persons holding those options or warrants for the purpose of computing the number of shares beneficially owned and the percentage ownership of that person. They are not, however, deemed to be outstanding beneficially owned for the purpose of computing the percentage ownership of any other person. Subject to the paragraph above, the percentage ownership of outstanding shares is based on 5,003,650 shares of common stock outstanding as of March 24, 2014.
Security Ownership of Management
The following table shows, as of March 24, 2014, the shares of the Company’s common stock beneficially owned by each director (including each nominee), by each of the executive officers and by all directors and executive officers as a group. Information is also provided regarding beneficial ownership of common stock if all outstanding options, warrants, rights and conversion privileges (to which the applicable officers and directors have the right to exercise in the next 60 days) are exercised and additional shares of common stock are issued.
|
Title of Class
|
Name of Beneficial Owner
|
Amount and Nature
of Beneficial Ownership
|
Percentage of Class (1)
|
|
Common Stock
|
Sotirios Leontaritis
|
4,000,000 shares held directly
|
79.94%
|
|
Common Stock
|
Christos Kapatos
|
500,000 shares held directly
|
9.99%
|
|
Common Stock
|
Nikolaos Kardaras
|
-0-
|
0%
|
|
Common Stock
|
Grigorios Tsourtos
|
-0-
|
0%
|
|
All Officers and Directors as a Group
|
4,500,000
|
89,93%
|
(1)Beneficial ownership is determined in accordance with SEC rules and includes voting or investment power with respect to securities. All shares of common stock subject to options or warrants exercisable within 60 days of March 24, 2014 are deemed to be outstanding and beneficially owned by the persons holding those options or warrants for the purpose of computing the number of shares beneficially owned and the percentage ownership of that person. They are not, however, deemed to be outstanding beneficially owned for the purpose of computing the percentage ownership of any other person. Subject to the paragraph above, the percentage ownership of outstanding shares is based on 5,003,650 shares of common stock outstanding as of March 24, 2014.
Changes in Control
On September 9, 2013, Jinzhao Wu, the then principal shareholder of the Company entered into a Stock Purchase Agreement which provided for the sale of 3,000,000 shares of common stock of the Company to Sotirios Leontaritis thus effecting a change in control of the Company.
Securities authorized for issuance under equity compensation plans.
None.
Certain Relationships and Related Transactions
The Company received loans from its former CEO and controlling shareholder, Mr. Jinzhao Wu to finance the Company’s operations due to a lack of cash resources. These loans were unsecured, non-interest bearing and had no fixed terms of repayment, therefore, they were deemed payable on demand. Cash flow from this activity is classified as cash flows from financing activity. The total borrowing from Mr. Wu was $200,000 for the period March 26, 2007 (inception) through December 31, 2012. On December 31, 2012, the Company entered into a debt conversion agreement with Mr. Wu, pursuant to which $200,000 of debt owed to Mr. Wu by the Company was contributed to additional paid-in capital of the Company. The borrowing from Mr. Wu for the period January 1, 2013 through September 9, 2013 was $22,460 (2012 - $19,000). On September 9, 2013, the Board of Directors approved that Mr. Wu contribute his loans to the Company in the total amount of $22,460, as additional paid-in capital of the Company. On September 9, 2013 Mr. Wu sold his controlling interest in the Company to the Company’s current Chief Executive Officer, President, Treasurer and Director, Mr. Sotirios Leontaritis in a private transaction. On September 10, 2013 Mr. Wu resigned all positions as an officer and director.
On September 9, 2013 Mr. Sotirios Leontaritis acquired 3,000,000 shares of the Company’s common stock, the controlling interest, from the Company’s former sole officer and director in a private transaction. On September 10, 2013 Mr. Leontaritis was appointed President, Treasurer and Director of the Company.
During the fiscal year ended December 31, 2013, Mr. Sotirios Leontaritis advanced funds to the Company to settle certain operating expenses incurred in the normal course and also incurred expense items for reimbursement. A total of $24,590 has been recorded as accounts payable-related party for reimbursable expenses, and a further $32,252 has been recorded on the balance sheets as advances from related party as of December 31, 2013, which amount bears no interest and is due on demand.
During the year ended December 31, 2013, Mr. Nikolaos Kardaras, the Secretary and a director of the Company invoiced the Company in the amount of $2,727 (£1,403) for services rendered.
On November 27, 2013, the Board of Directors of the Company appointed Mr. Grigorios Tsourtos as Chief Financial Officer of the Company. During the fiscal year ended December 31, 2013, the Company accrued $1,369 (€1,000) consulting fees for his services rendered.
|
Review, Approval or Ratification of Transactions with Related Persons
|
We do not currently have any written policies and procedures for the review, approval or ratification of any transactions with related persons.
|
Promoters and Certain Control Persons
|
None.
|
Parents
|
None.
|
Director Independence
|
As of the date of this Annual Report, we do not have any independent directors.
We have developed the following categorical standards for determining the materiality of relationships that the directors may have with the Company. This information is not available on our Web site because such site is currently under development.
A director shall not be deemed to have a material relationship with the Company that impairs the director's independence as a result of any of the following relationships:
| 1. |
the director is an officer or other person holding a salaried position of an entity (other than a principal, equity partner or member of such entity) that provides professional services to the Company and the amount of all payments from the Company to such entity during the most recently completed fiscal year was less than two percent of such entity’s consolidated gross revenues;
|
||
| 2. |
the director is the beneficial owner of less than five (5%) per cent of the outstanding equity interests of an entity that does business with the Company;
|
||
| 3. |
the director is an executive officer of a civic, charitable or cultural institution that received less than the greater of one million ($1,000,000) dollars or two (2%) per cent of its consolidated gross revenues, as such term is construed by the New York Stock Exchange for purposes of Section 303A.02(b)(v) of the Corporate Governance Standards, from the Company or any of its subsidiaries for each of the last three (3) fiscal years;
|
||
| 4. |
the director is an officer of an entity that is indebted to the Company, or to which the Company is indebted, and the total amount of either the Company's or the business entity's indebtedness is less than three (3%) per cent of the total consolidated assets of such entity as of the end of the previous fiscal year; and
|
||
| 5. |
the director obtained products or services from the Company on terms generally available to customers of the Company for such products or services. The Board retains the sole right to interpret and apply the foregoing standards in determining the materiality of any relationship.
|
The Board shall undertake an annual review of the independence of all non-management directors. To enable the Board to evaluate each non-management director, in advance of the meeting at which the review occurs, each non-management director shall provide the Board with full information regarding the director’s business and other relationships with the Company, its affiliates and senior management.
Directors must inform the Board whenever there are any material changes in their circumstances or relationships that could affect their independence, including all business relationships between a director and the Company, its affiliates, or members of senior management, whether or not such business relationships would be deemed not to be material under any of the categorical standards set forth above. Following the receipt of such information, the Board shall re-evaluate the director's independence.
The aggregate fees billed for the most recently completed fiscal year ended December 31, 2013 and for the fiscal year ended December 31, 2012 for professional services rendered by the principal accountant for the audit of our annual financial statements and review of the financial statements included in our quarterly reports on Form 10-Q and services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for these fiscal periods were as follows:
|
Year Ended
|
||||||||
|
December 31, 2013
$
|
December 31, 2012
$
|
|||||||
|
Audit Fees
|
11,000 | 11.000 | ||||||
|
Audit Related Fees
|
0 | -0- | ||||||
|
Tax Fees
|
0 | -0- | ||||||
|
All Other Fees
|
0 | -0- | ||||||
|
Total
|
11,000 | 11,000 | ||||||
Our board of directors pre-approves all services provided by our independent auditors. All of the above services and fees were reviewed and approved by the board of directors either before or after the respective services were rendered.
Our board of directors has considered the nature and amount of fees billed by our independent auditors and believes that the provision of services for activities unrelated to the audit is compatible with maintaining our independent auditors’ independence.
PART IV
Exhibits:
|
3.1.1
|
Articles of Incorporation
|
Incorporated by reference to our Registration Statement filed with the SEC on Form SB-2 dated June 29, 2007.
|
|
3.1.2
|
Certificate of Amendment to the Articles of Incorporation
|
Incorporated by reference to our Definitive 14C filed with the SEC on December 27, 2013
|
|
3.1.3
|
Certificate of Amendment to the Articles of Incorporation
|
Incorporated by reference to our Definitive 14C filed with the SEC on March 3, 2014
|
|
3.2
|
Bylaws
|
Incorporated by reference to our Registration Statement filed with the SEC on Form SB-2/A dated October 11, 2007.
|
|
4.1
|
Certificate of Designation
|
Incorporated by reference to our Definitive 14C filed with the SEC on December 27, 2013
|
|
10.1
|
Marketing Research Agreement
|
Incorporated by reference to our Registration Statement
|
|
10.2
|
Loan Agreement
|
Incorporated by reference to our Registration Statement filed with the SEC on Form SB-2/A dated January 28,
2008.
|
|
.10.3
|
Subscription Agreement
|
Incorporated by reference to our Registration Statement filed with the SEC on Form SB-2 dated June 29, 2007.
|
|
10.4
|
Contract between the Company and Sotirios Leontaritis dated effective January 1, 2014
|
Incorporated by reference to our Form 8-K filed with the SEC on January 21, 2014
|
|
10.5
|
Acquisition Agreement between HCi VioCare Technologies Limited and Christos Kapatos dated February 2, 2014
|
Incorporated by reference to our Form 8-K filed with the SEC on February 14, 2014.
|
|
14.1
|
Code of Ethics
|
Incorporated by reference to the registration statement filed with the SEC on Form 10-K dated April 6, 2009.
|
|
31.1
|
Certification of Principal Executive Officer filed pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
Filed herewith
|
|
31.2
|
Certification of Principal Financial Officer filed pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
Filed herewith
|
|
32.1
|
Certification of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
Filed herewith
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase **
|
Filed herewith
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase **
|
Filed herewith
|
|
101.INS
|
XBRL Instance Document **
|
Filed herewith
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase **
|
Filed herewith
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase **
|
Filed herewith
|
|
101.SCH
|
XBRL Taxonomy Extension Schema **
|
Filed herewith
|
**Pursuant to Rule 406T of Regulation S-T, these interactive data files are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933 or Section 18 of the Securities Exchange Act of 1934 and otherwise are not subject to liability.
Financial Statements.
The following financial statements are included in this report:
|
Page
|
|
|
Audited Financial Statements
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-2 |
|
Balance Sheets
|
F-3 |
|
Statements of Operations
|
F-4 |
|
Statements of Changes in Stockholders’ Equity
|
F-5 |
|
Statements of Cash Flows
|
F-6 |
|
Notes to Audited Financial Statements
|
F-7 to F-14 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
HCi VioCare
|
|||
|
Date:
|
April 14, 2014
|
By:
|
/s/ Sotirios Leontaritis
|
|
Name:
|
Sotirios Leontaritis
|
||
|
Title:
|
Chief Executive Officer and President (Principal Executive Officer)
|
||
|
Date:
|
April 14, 2014
|
By:
|
/s/ Grigorios Tsourtos
|
|
Name:
|
Grigorios Tsourtos
|
||
|
Title:
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer)
|
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Date:
|
April 14, 2014
|
By:
|
/s/ Sotirios Leontaritis
|
|
Name:
|
Sotirios Leontaritis
|
||
|
Title:
|
President, Chief Executive Officer, Treasurer and Director (Principal Executive Officer)
|
||
|
Date:
|
April 14, 2014
|
By:
|
/s/ Nikolaos Kardaras
|
|
Name:
|
Nikolaos Karadas
|
||
|
Title:
|
Secretary and Director
|
|
Date:
|
April 14, 2014
|
By:
|
/s/ Christos Kapatos
|
|
Name:
|
Christos Kapatos
|
||
|
Title:
|
Director
|
