Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TETRALOGIC PHARMACEUTICALS Corp | a14-10136_18k.htm |
| EX-99.1 - EX-99.1 - TETRALOGIC PHARMACEUTICALS Corp | a14-10136_1ex99d1.htm |
Exhibit 99.2
|
|
2.60 1.80 2.15 3.30 3.75 5.10 0.15 5.10 4.50 - logo Corporate Presentation April 2014 0.15 |
|
|
Forward-Looking Statements This presentation contains forward-looking statements. These statements are not guarantees of future performance and involve a number of unknown risks, assumptions, trends, uncertainties and factors that are beyond our control. Given these risks, assumptions and uncertainties, you should not place undue reliance on these forward-looking statements. Forward-looking statements in this presentation include statements regarding development plans, potential therapeutic indications and the potential of any of our product candidates to address unmet medical needs. The clinical data included in this presentation is preliminary and the studies and trials are ongoing. There can be no assurance that the data generated at the end of the studies and trials will be consistent with the preliminary results described in this presentation. In addition, future data generated in the studies and trials may demonstrate trends not apparent at this time. There can be no assurance that future studies and trials will generate positive results or that any of our product candidates will receive regulatory approvals. All statements contained in this presentation are made only as of the date of this presentation and are subject to uncertainty and changes. Except as required by law, we expressly disclaim any responsibility to update our forward-looking statements, whether as a result of new information, future events or otherwise. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, those that are set forth under the heading “Risk Factors” in TetraLogic Pharmaceuticals Corporation’s (the “Company”) Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on March 19, 2014. You can review the Company’s filings and other documents for free by visiting EDGAR on the SEC website: www.sec.gov. “TetraLogic Pharmaceuticals” is a registered trademark and “TetraLogic” and the “TetraLogic Pharmaceuticals” logo are unregistered trademarks of the Company. Other trade names, trademarks and service marks appearing in this presentation are the property of their respective owners. Solely for convenience, the trademarks, service marks and trade names in this presentation are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. |
|
|
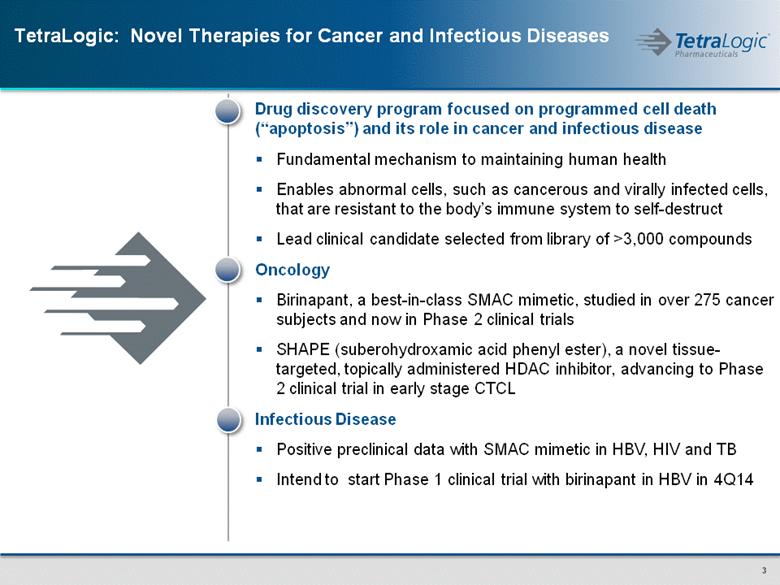
TetraLogic: Novel Therapies for Cancer and Infectious Diseases Drug discovery program focused on programmed cell death (“apoptosis”) and its role in cancer and infectious disease Fundamental mechanism to maintaining human health Enables abnormal cells, such as cancerous and virally infected cells, that are resistant to the body’s immune system to self-destruct Lead clinical candidate selected from library of >3,000 compounds Oncology Birinapant, a best-in-class SMAC mimetic, studied in over 275 cancer subjects and now in Phase 2 clinical trials SHAPE (suberohydroxamic acid phenyl ester), a novel tissue-targeted, topically administered HDAC inhibitor, advancing to Phase 2 clinical trial in early stage CTCL Infectious Disease Positive preclinical data with SMAC mimetic in HBV, HIV and TB Intend to start Phase 1 clinical trial with birinapant in HBV in 4Q14 |
|
|
Diversified Oncology Therapeutic Opportunities (a) Acute Myeloid Leukemia / Myelodysplastic Syndromes / Acute Lymphoblastic Leukemia Pre-clinical Phase 1 Phase 2 Status Oncology AML / MDS / ALL(a) Birinapant alone Investigator-initiated Phase 1/2 clinical trial in progress Myelodysplastic Syndromes (MDS) Birinapant plus azacitidine Concluding Phase 1 clinical trial. Target start of randomized Phase 2 trial is 1H14 Colorectal Cancer (CRC) Birinapant plus irinotecan-containing regimen Phase 1/2 clinical trial completed. Intend to start a randomized Phase 2 clinical trial upon additional financing Ovarian Cancer (Amgen Collaboration) Birinapant plus conatumumab Phase 1/2 clinical trial commenced 4Q13 Cutaneous T-Cell Lymphoma (CTCL) SHAPE Target start of Phase 2 clinical trial is 4Q14 Infectious Disease Hepatitis B, HIV, Tuberculosis Birinapant Target start of Phase 1 clinical trial in Hepatitis B is 4Q14 |
|
|
Oncology - Clinical Development Programs - Birinapant - SHAPE |
|
|
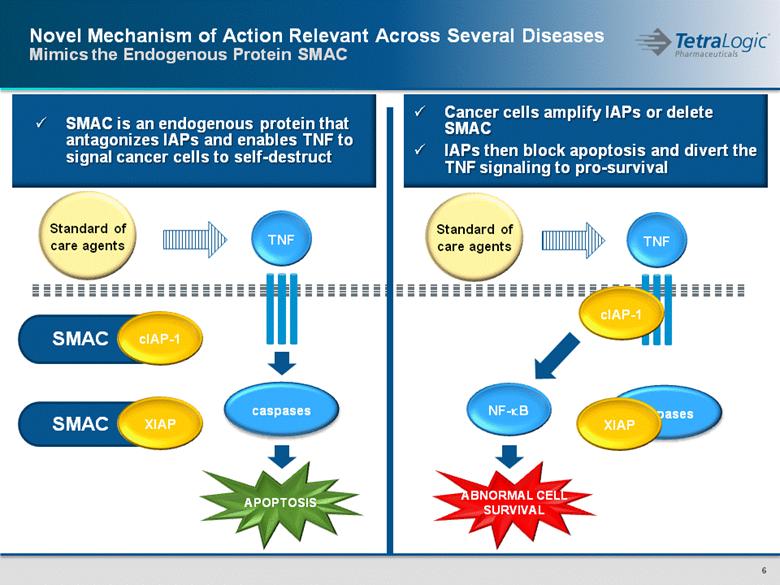
SMAC SMAC XIAP Novel Mechanism of Action Relevant Across Several Diseases Mimics the Endogenous Protein SMAC cIAP-1 Standard of care agents SMAC is an endogenous protein that antagonizes IAPs and enables TNF to signal cancer cells to self-destruct ABNORMAL CELL SURVIVAL TNF Standard of care agents caspases cIAP-1 NF-kB Cancer cells amplify IAPs or delete SMAC IAPs then block apoptosis and divert the TNF signaling to pro-survival XIAP TNF caspases APOPTOSIS |
|
|
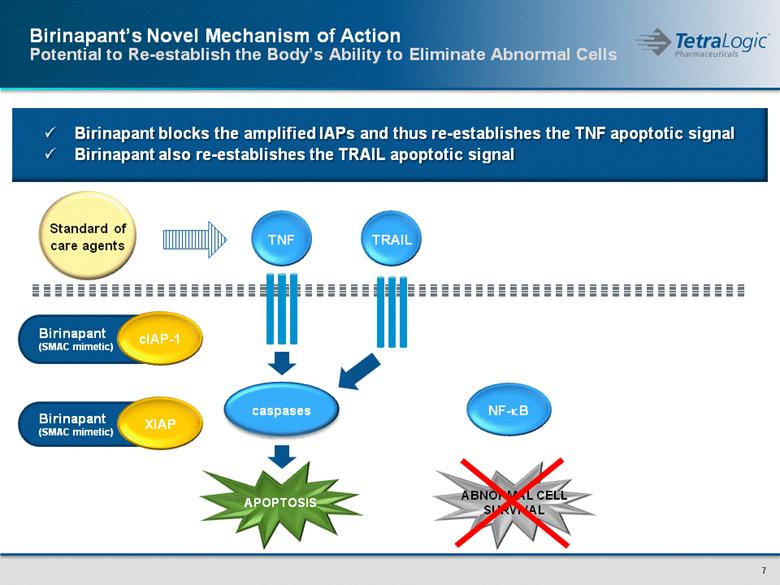
Birinapant (SMAC mimetic) Birinapant (SMAC mimetic) ABNORMAL CELL SURVIVAL Birinapant’s Novel Mechanism of Action Potential to Re-establish the Body’s Ability to Eliminate Abnormal Cells TNF cIAP-1 XIAP Standard of care agents Birinapant blocks the amplified IAPs and thus re-establishes the TNF apoptotic signal Birinapant also re-establishes the TRAIL apoptotic signal TRAIL NF-kB caspases APOPTOSIS |
|
|
Birinapant is Generally Well-Tolerated Well-tolerated at pharmacologically active doses Tested in >275 subjects with solid and hematological cancers Several subjects have continued >12 months Dose-limiting toxicities: Elevated serum amylase & lipase, Bell’s Palsy Occurred above Recommended Phase 2 Dose Safely combined with multiple chemotherapeutic regimens Target suppression (cIAP1) and favorable PK in tumors Greater than 50 hour half-life in tumors Selective and prolonged target suppression in tumors NF-ҡB suppression in AML blasts |
|
|
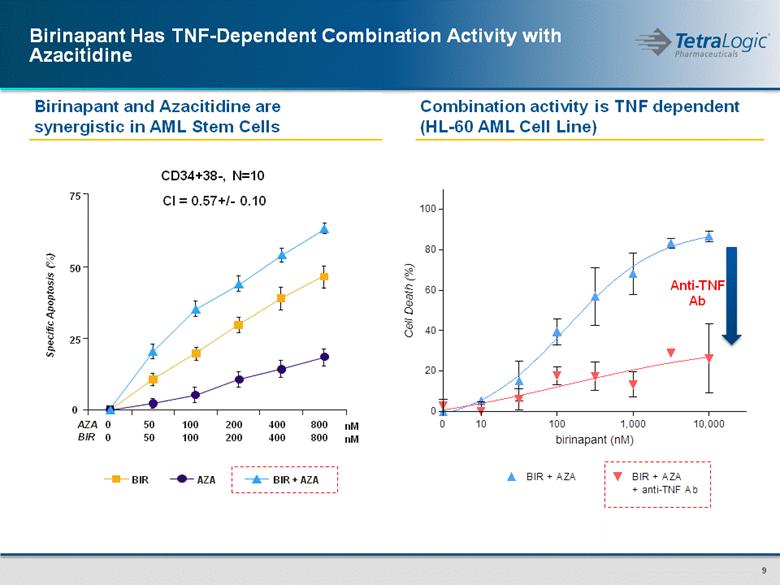
Combination activity is TNF dependent (HL-60 AML Cell Line) Birinapant and Azacitidine are synergistic in AML Stem Cells Birinapant Has TNF-Dependent Combination Activity with Azacitidine Cl = 0.57+/- 0.10 CD34+38-, N=10 BIR AZA BIR + AZA 0 50 100 200 400 800 0 50 100 200 400 800 AZA BIR 0 25 50 75 nM nM Specific Apoptosis (%) Anti-TNF Ab 0 10 100 1,000 10,000 0 20 40 60 80 100 BIR + AZA BIR + AZA + anti-TNF Ab birinapant (nM) Cell Death (%) |
|
|
AML / MDS: Phase 1 Dose Escalation Studies Phase 1 study of single agent birinapant in elderly relapsed / refractory AML patients Evidence of inhibition of cIAP1 and NF-ҡB in circulating AML blasts Evidence of hematological activity in some subjects Decreases in peripheral blast counts In one subject decrease in bone marrow blast count from 60% to 10% Phase 1 study of birinapant plus azacitidine in higher risk MDS Localized pharmacodynamic reaction seen in skin at site of subcutaneous azacitidine injections Phase 2 dose to be 13 mg/m2 twice weekly 3 out of 4 weeks with IV azacitidine |
|
|
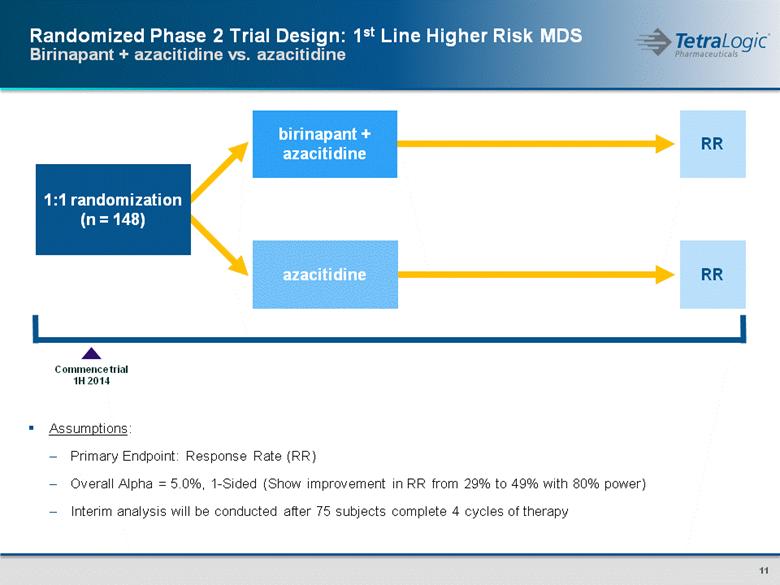
1:1 randomization (n = 148) RR RR azacitidine birinapant + azacitidine Commence trial 1H 2014 Assumptions: Primary Endpoint: Response Rate (RR) Overall Alpha = 5.0%, 1-Sided (Show improvement in RR from 29% to 49% with 80% power) Interim analysis will be conducted after 75 subjects complete 4 cycles of therapy Randomized Phase 2 Trial Design: 1st Line Higher Risk MDS Birinapant + azacitidine vs. azacitidine |
|
|
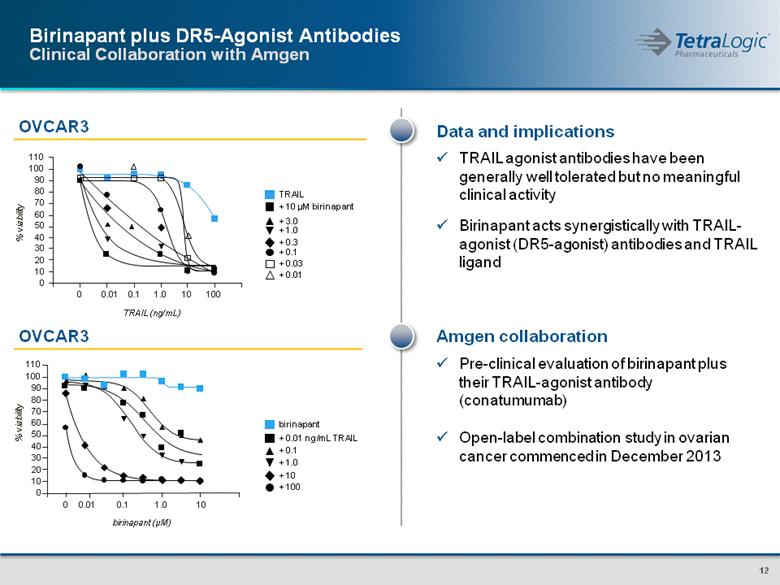
Amgen collaboration Pre-clinical evaluation of birinapant plus their TRAIL-agonist antibody (conatumumab) Open-label combination study in ovarian cancer commenced in December 2013 OVCAR3 Birinapant plus DR5-Agonist Antibodies Clinical Collaboration with Amgen Data and implications TRAIL agonist antibodies have been generally well tolerated but no meaningful clinical activity Birinapant acts synergistically with TRAIL-agonist (DR5-agonist) antibodies and TRAIL ligand OVCAR3 % viability TRAIL (ng/mL) 110 100 90 80 70 60 50 40 30 20 10 0 0 0.01 0.1 1.0 10 100 TRAIL + 10 µM birinapant + 3.0 + 1.0 + 0.3 + 0.1 + 0.03 + 0.01 110 100 90 80 70 60 50 40 30 20 10 0 % viability birinapant (µM) 0 0.01 0.1 1.0 10 birinapant + 0.01 ng/mL TRAIL + 0.1 + 1.0 + 10 + 100 |
|
|
Study Population All Subjects N = 71 Subjects who Failed Immediately Prior Irino Therapy N = 22 KRAS Mutant N = 37 4 month PFS 34% 32% 38% 6 month PFS 21% 18% 24% Median PFS (mo) 2.2 3.0 3.0 Response Rate 8% 14% 8% Disease Control Rate [PR + SD] 63% 59% 68% Historically irinotecan re-treatment has been ineffective in CRC Adding birinapant to irinotecan resulted in some PRs and prolonged PFS of irinotecan-relapsed and irinotecan-refractory patients We intend to start a randomized clinical trial if we obtain additional financing Birinapant in 3rd Line CRC (a) Grothey et al., “Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, Ph 3 trial,” Lancet, 2013, 381, 303-12 CRC (≥ 3rd Line) Phase 1/2 TetraLogic-sponsored trial (irinotecan plus birinapant) Phase 3 CORRECT Trial(a) Placebo N = 255 7% 2% 1.7 0% 15% |
|
|
Oncology - Clinical Development Programs - Birinapant - SHAPE |
|
|
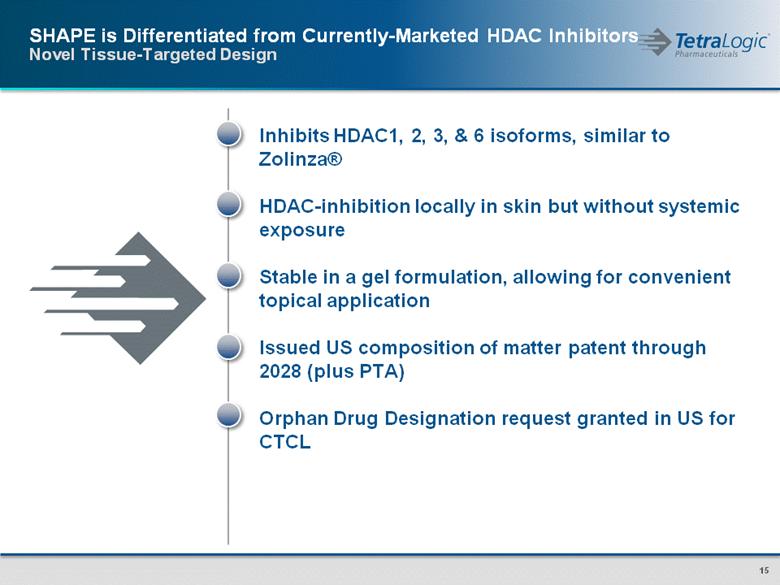
SHAPE is Differentiated from Currently-Marketed HDAC Inhibitors Novel Tissue-Targeted Design Inhibits HDAC1, 2, 3, & 6 isoforms, similar to Zolinza® HDAC-inhibition locally in skin but without systemic exposure Stable in a gel formulation, allowing for convenient topical application Issued US composition of matter patent through 2028 (plus PTA) Orphan Drug Designation request granted in US for CTCL |
|
|
PD demonstrates localized dermal acetylation Ethanol-based Vehicle 0.5% SHAPE (3.5 mg/mL) PK demonstrates tissue-targeting with lack of systemic exposure SHAPE Shows Desired Pharmacokinetic and Pharmacodynamic Profile Human Blood vs. Human Skin Homogenate Time (min.) Fraction Remaining (%) Acetylation in keratinocytes indicated by brown nuclear stain |
|
|
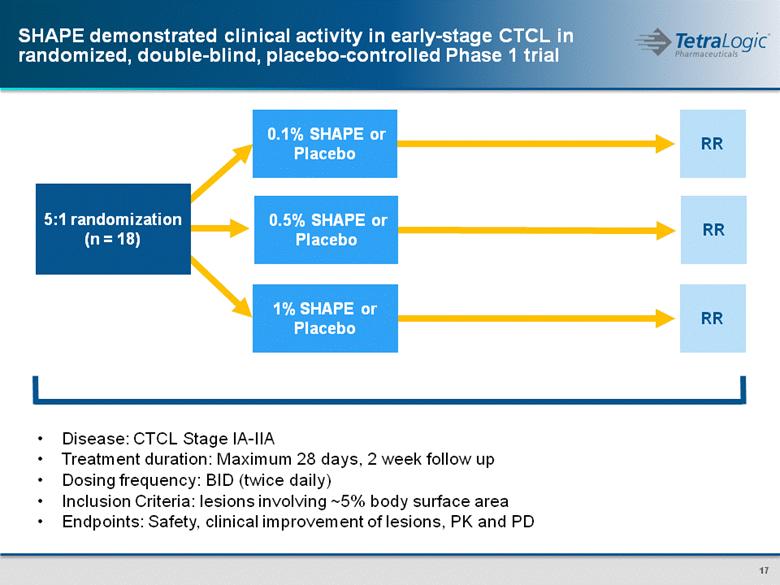
SHAPE demonstrated clinical activity in early-stage CTCL in randomized, double-blind, placebo-controlled Phase 1 trial 5:1 randomization (n = 18) RR RR 1% SHAPE or Placebo 0.1% SHAPE or Placebo Disease: CTCL Stage IA-IIA Treatment duration: Maximum 28 days, 2 week follow up Dosing frequency: BID (twice daily) Inclusion Criteria: lesions involving ~5% body surface area Endpoints: Safety, clinical improvement of lesions, PK and PD RR 0.5% SHAPE or Placebo |
|
|
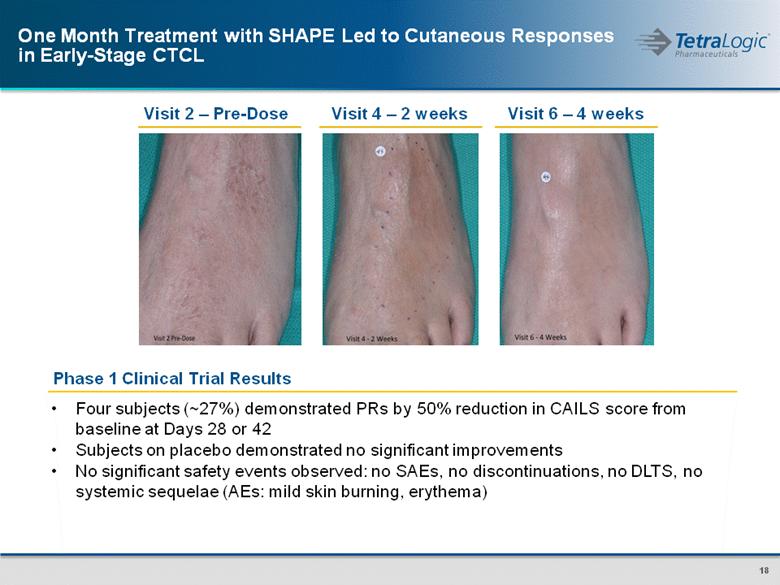
Phase 1 Clinical Trial Results Visit 2 – Pre-Dose Visit 4 – 2 weeks Visit 6 – 4 weeks Four subjects (~27%) demonstrated PRs by 50% reduction in CAILS score from baseline at Days 28 or 42 Subjects on placebo demonstrated no significant improvements No significant safety events observed: no SAEs, no discontinuations, no DLTS, no systemic sequelae (AEs: mild skin burning, erythema) One Month Treatment with SHAPE Led to Cutaneous Responses in Early-Stage CTCL |
|
|
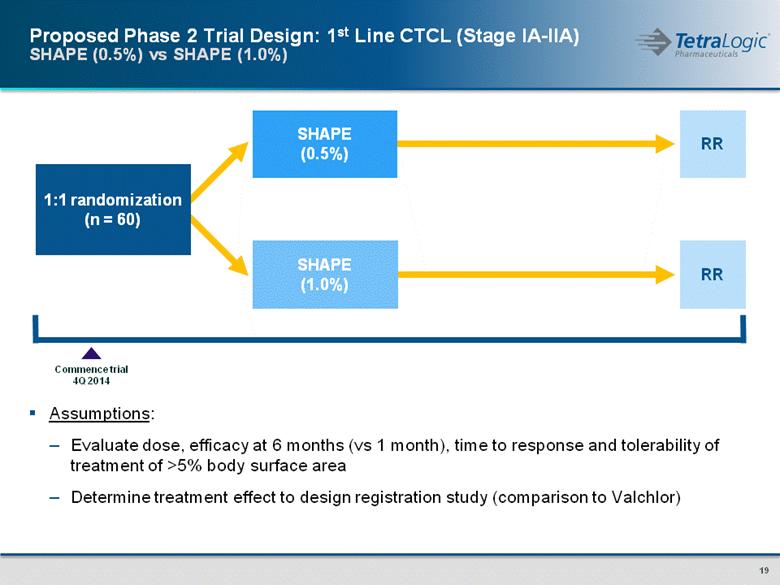
1:1 randomization (n = 60) RR RR SHAPE (1.0%) SHAPE (0.5%) Commence trial 4Q 2014 Assumptions: Evaluate dose, efficacy at 6 months (vs 1 month), time to response and tolerability of treatment of >5% body surface area Determine treatment effect to design registration study (comparison to Valchlor) Proposed Phase 2 Trial Design: 1st Line CTCL (Stage IA-IIA) SHAPE (0.5%) vs SHAPE (1.0%) |
|
|
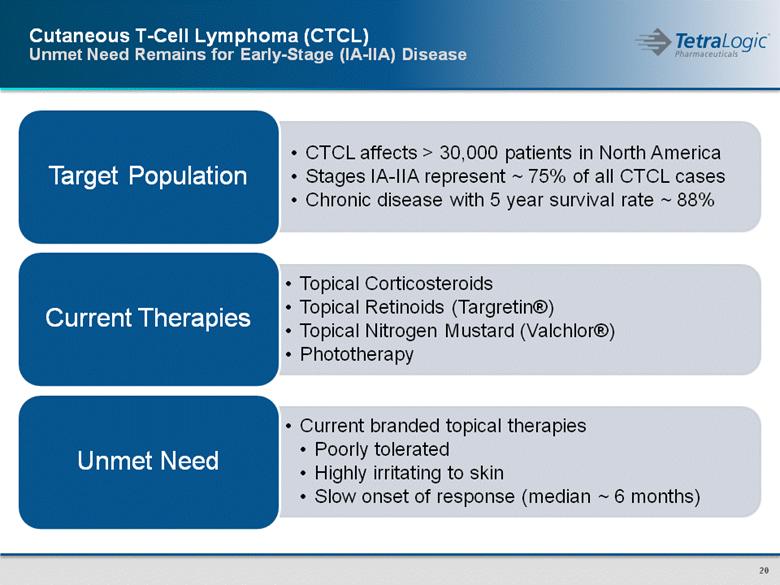
Cutaneous T-Cell Lymphoma (CTCL) Unmet Need Remains for Early-Stage (IA-IIA) Disease |
|
|
Infectious Diseases - Birinapant |
|
|
Birinapant Clears Hepatitis B Virus In Vivo Decrease in Circulating HBV-DNA with Birinapant TNF is Required for Birinapant’s Efficacy TNF is required for birinapant's effect; consistent with the anti-cancer mechanism of action Birinapant collaborates with polymerase inhibitors such as entecavir Note: Studies conducted in lab of Dr. Marc Pellegrini, Walter and Eliza Hall Institute of Medical Research, Australia Control (n=21) +birinapant (n=27) 0 3 6 10 17 24 31 38 45 52 103 104 105 106 107 108 detection limit p=0.79 0.071 0.035 0.116 0.016 0.059 0.018 0.072 ns ns Mean with SEM t-test, Holm-Sidak HBV-DNA (copies/ml) Time after first birinapant dose (d) IgG1 Control + vehicle (n=6) IgG1 Control + birinapant (n=6) Anti-TNF + birinapant (n=6) 103 104 105 106 107 108 0 7 14 28 35 21 0 3 7 10 14 day Neutralizing antibody birinapant detection limit HBV-DNA (copies/ml) Time after first dose (d) HBV+ C57BL/6 |
|
|
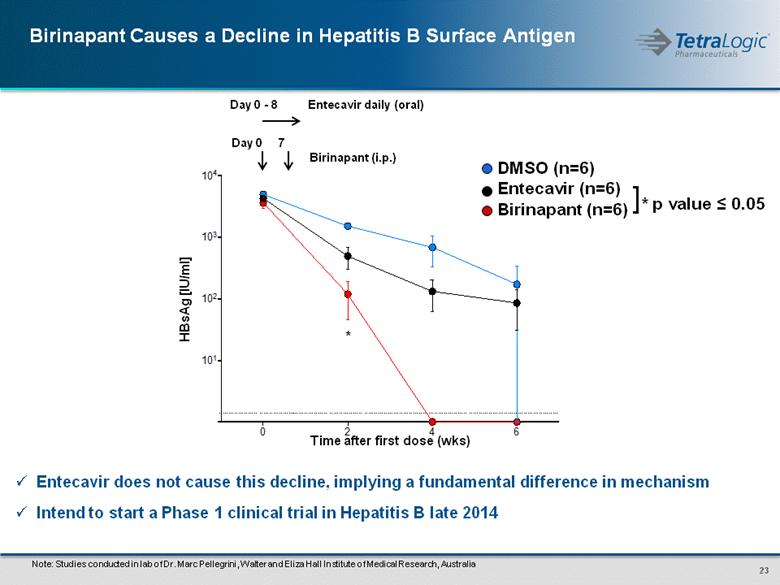
Entecavir does not cause this decline, implying a fundamental difference in mechanism Intend to start a Phase 1 clinical trial in Hepatitis B late 2014 Note: Studies conducted in lab of Dr. Marc Pellegrini, Walter and Eliza Hall Institute of Medical Research, Australia Day 0 - 8 Entecavir daily (oral) Day 0 7 Birinapant (i.p.) HBsAg [IU/ml] Time after first dose (wks) DMSO (n=6) Entecavir (n=6) Birinapant (n=6) * p value ≤ 0.05 ] |
|
|
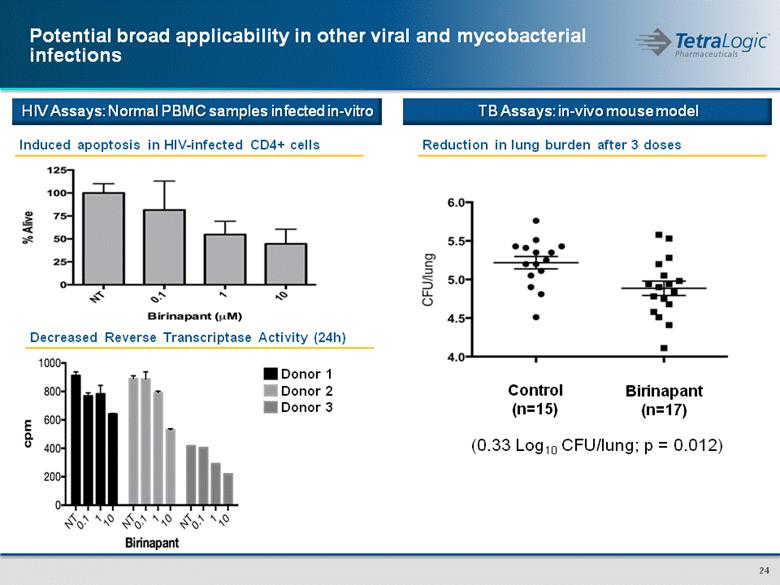
Potential broad applicability in other viral and mycobacterial infections Induced apoptosis in HIV-infected CD4+ cells Decreased Reverse Transcriptase Activity (24h) Donor 1 Donor 2 Donor 3 Reduction in lung burden after 3 doses Control (n=15) Birinapant (n=17) (0.33 Log10 CFU/lung; p = 0.012) HIV Assays: Normal PBMC samples infected in-vitro TB Assays: in-vivo mouse model |
|
|
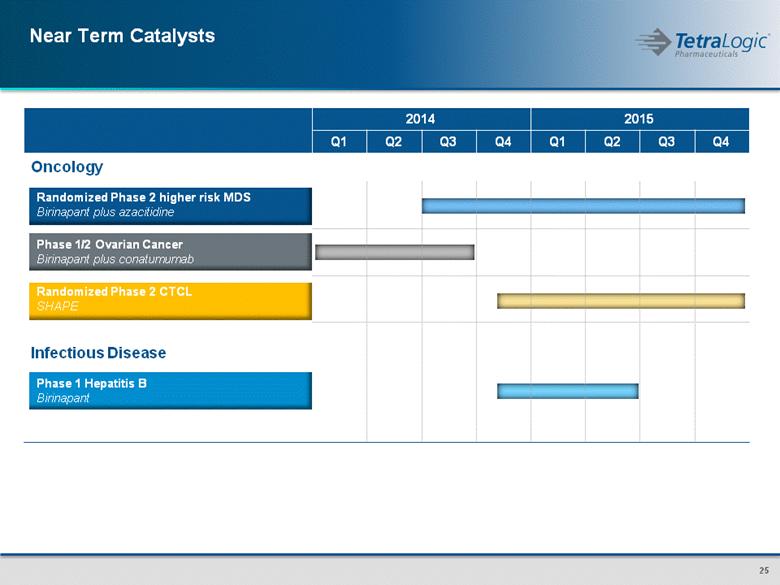
Near Term Catalysts 2014 2015 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Oncology Randomized Phase 2 higher risk MDS Birinapant plus azacitidine Phase 1/2 Ovarian Cancer Birinapant plus conatumumab Randomized Phase 2 CTCL SHAPE Infectious Disease Phase 1 Hepatitis B Birinapant |
|
|
Investment Highlights Novel therapeutic approaches Strong clinical data Expanding into infectious diseases Multiple value-creating milestones Proven management team |
|
|
2.60 1.80 2.15 3.30 3.75 5.10 0.15 5.10 4.50 - logo 0.15 |