Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TESARO, Inc. | a14-8047_18k.htm |
| EX-99.1 - EX-99.1 - TESARO, Inc. | a14-8047_1ex99d1.htm |
Exhibit 99.2
|
|
Immuno-Oncology Collaboration and License Agreement for TIM-3, LAG-3 and PD-1 Antibody Programs March 13, 2014 |
|
|
Safe Harbor Statement Statements made in this presentation about TESARO, Inc. that are not descriptions of historical facts are forward-looking statements reflecting the current beliefs and expectations of management. Forward-looking statements are sometimes identified by words such as “plan,” “may,” “will,” “expect,” and similar expressions referencing future events, conditions or circumstances. These forward-looking statements involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Actual results could differ materially from those expressed or implied by these forward-looking statements as a result of various factors, including, without limitation, the various risks described in our Annual Report on Form 10-K for the year ended December 31, 2012 and our subsequent filings with the SEC. TESARO, Inc. undertakes no obligation to update or revise any forward-looking statement for any reason. Rolapitant, niraparib, TSR-011 and our immuno-oncology candidates are investigational products that have not been approved by any regulatory agency. The most frequently observed adverse events in the two completed rolapitant Phase 3 studies were balanced across treatment arms and included fatigue, alopecia and loss of appetite. Phase 1 data indicate that the most frequently observed adverse events for niraparib at a 300 milligram dose included grade 1/2 anemia, fatigue and nausea. Phase 1 data indicate that TSR-011 was well tolerated at therapeutic dose levels, and the most frequently occurring dose limiting toxicities included ECG changes and dysaethesia, both of which were reversible. |
|
|
Lonnie Moulder Chief Executive Officer |
|
|
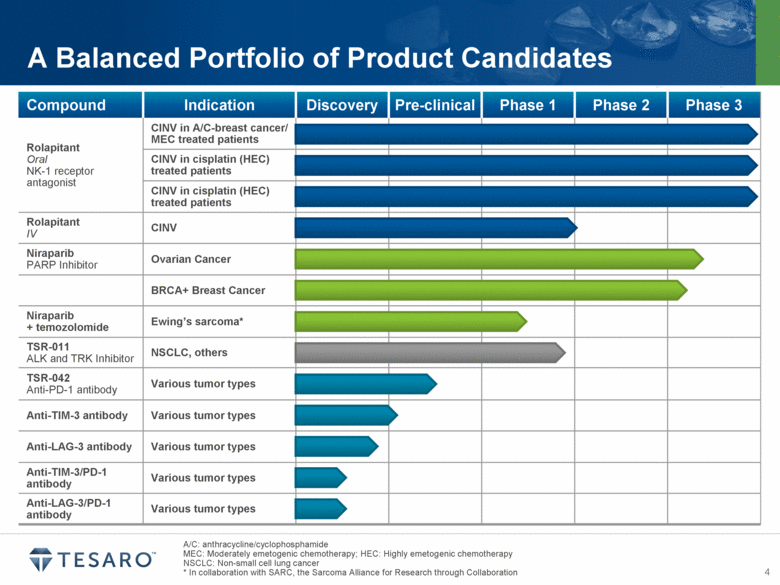
A/C: anthracycline/cyclophosphamide MEC: Moderately emetogenic chemotherapy; HEC: Highly emetogenic chemotherapy NSCLC: Non-small cell lung cancer * In collaboration with SARC, the Sarcoma Alliance for Research through Collaboration A Balanced Portfolio of Product Candidates Compound Indication Discovery Pre-clinical Phase 1 Phase 2 Phase 3 Rolapitant Oral NK-1 receptor antagonist CINV in A/C-breast cancer/ MEC treated patients CINV in cisplatin (HEC) treated patients CINV in cisplatin (HEC) treated patients Rolapitant IV CINV Niraparib PARP Inhibitor Ovarian Cancer BRCA+ Breast Cancer Niraparib + temozolomide Ewing’s sarcoma* TSR-011 ALK and TRK Inhibitor NSCLC, others TSR-042 Anti-PD-1 antibody Various tumor types Anti-TIM-3 antibody Various tumor types Anti-LAG-3 antibody Various tumor types Anti-TIM-3/PD-1 antibody Various tumor types Anti-LAG-3/PD-1 antibody Various tumor types |
|
|
Lonnie Moulder Chief Executive Officer |
|
|
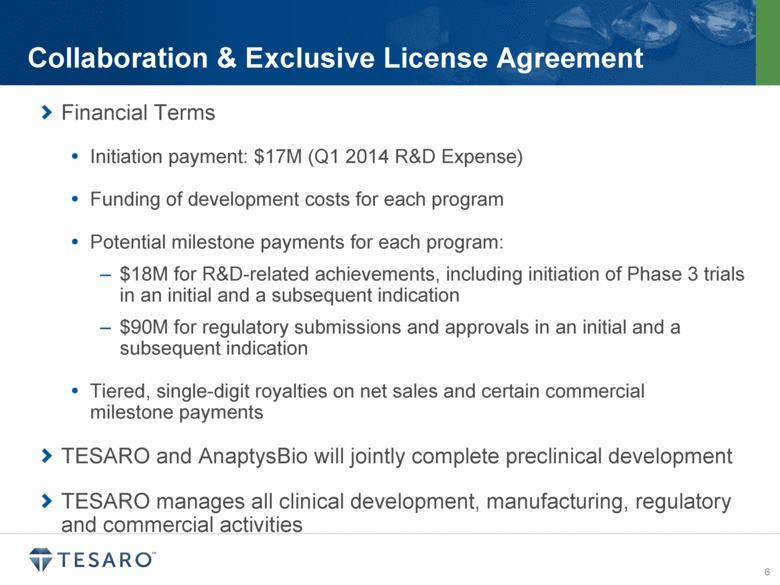
Financial Terms Initiation payment: $17M (Q1 2014 R&D Expense) Funding of development costs for each program Potential milestone payments for each program: $18M for R&D-related achievements, including initiation of Phase 3 trials in an initial and a subsequent indication $90M for regulatory submissions and approvals in an initial and a subsequent indication Tiered, single-digit royalties on net sales and certain commercial milestone payments TESARO and AnaptysBio will jointly complete preclinical development TESARO manages all clinical development, manufacturing, regulatory and commercial activities Collaboration & Exclusive License Agreement |
|
|
Mary Lynne Hedley, Ph.D. President |
|
|
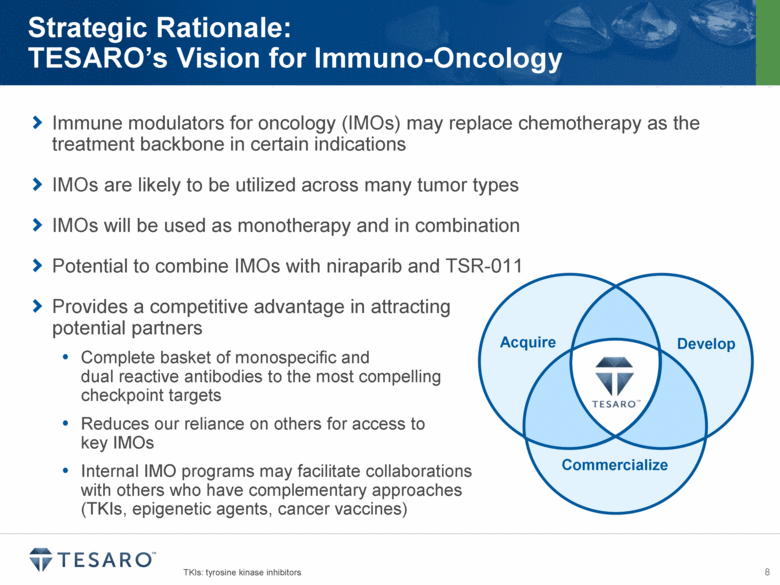
Immune modulators for oncology (IMOs) may replace chemotherapy as the treatment backbone in certain indications IMOs are likely to be utilized across many tumor types IMOs will be used as monotherapy and in combination Potential to combine IMOs with niraparib and TSR-011 Provides a competitive advantage in attracting potential partners Complete basket of monospecific and dual reactive antibodies to the most compelling checkpoint targets Reduces our reliance on others for access to key IMOs Internal IMO programs may facilitate collaborations with others who have complementary approaches (TKIs, epigenetic agents, cancer vaccines) Strategic Rationale: TESARO’s Vision for Immuno-Oncology Develop Acquire Commercialize TKIs: tyrosine kinase inhibitors |
|
|
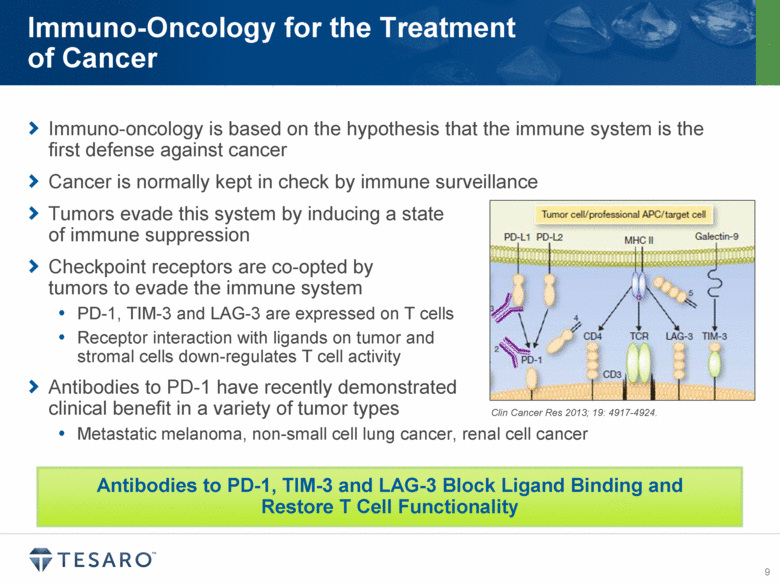
Immuno-oncology is based on the hypothesis that the immune system is the first defense against cancer Cancer is normally kept in check by immune surveillance Tumors evade this system by inducing a state of immune suppression Checkpoint receptors are co-opted by tumors to evade the immune system PD-1, TIM-3 and LAG-3 are expressed on T cells Receptor interaction with ligands on tumor and stromal cells down-regulates T cell activity Antibodies to PD-1 have recently demonstrated clinical benefit in a variety of tumor types Metastatic melanoma, non-small cell lung cancer, renal cell cancer Immuno-Oncology for the Treatment of Cancer Antibodies to PD-1, TIM-3 and LAG-3 Block Ligand Binding and Restore T Cell Functionality Clin Cancer Res 2013; 19: 4917-4924. |
|
|
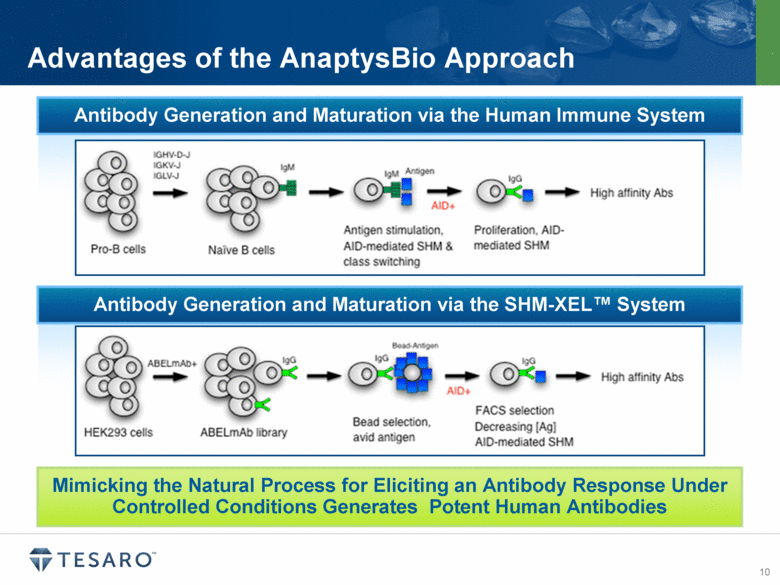
Advantages of the AnaptysBio Approach Mimicking the Natural Process for Eliciting an Antibody Response Under Controlled Conditions Generates Potent Human Antibodies Antibody Generation and Maturation via the Human Immune System Antibody Generation and Maturation via the SHM-XEL™ System |
|
|
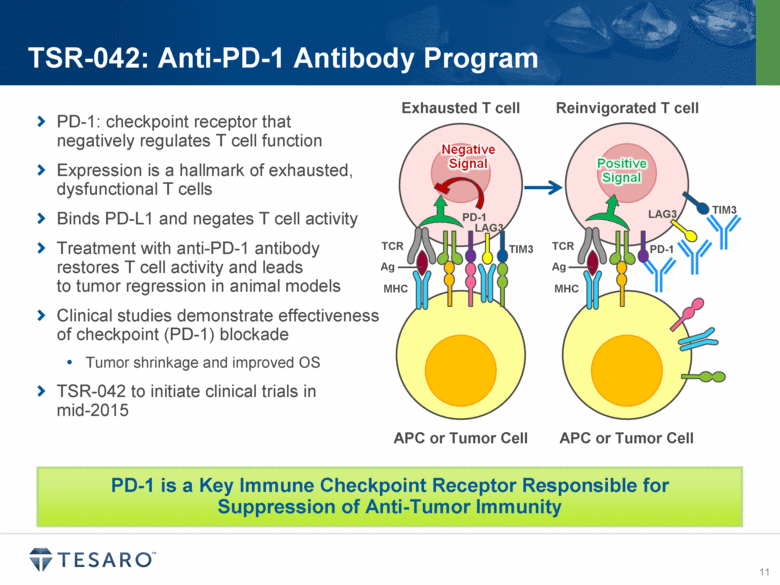
PD-1: checkpoint receptor that negatively regulates T cell function Expression is a hallmark of exhausted, dysfunctional T cells Binds PD-L1 and negates T cell activity Treatment with anti-PD-1 antibody restores T cell activity and leads to tumor regression in animal models Clinical studies demonstrate effectiveness of checkpoint (PD-1) blockade Tumor shrinkage and improved OS TSR-042 to initiate clinical trials in mid-2015 TSR-042: Anti-PD-1 Antibody Program PD-1 is a Key Immune Checkpoint Receptor Responsible for Suppression of Anti-Tumor Immunity APC or Tumor Cell APC or Tumor Cell Exhausted T cell Reinvigorated T cell TCR Ag MHC TCR Ag MHC PD-1 PD-1 TIM3 TIM3 LAG3 LAG3 |
|
|
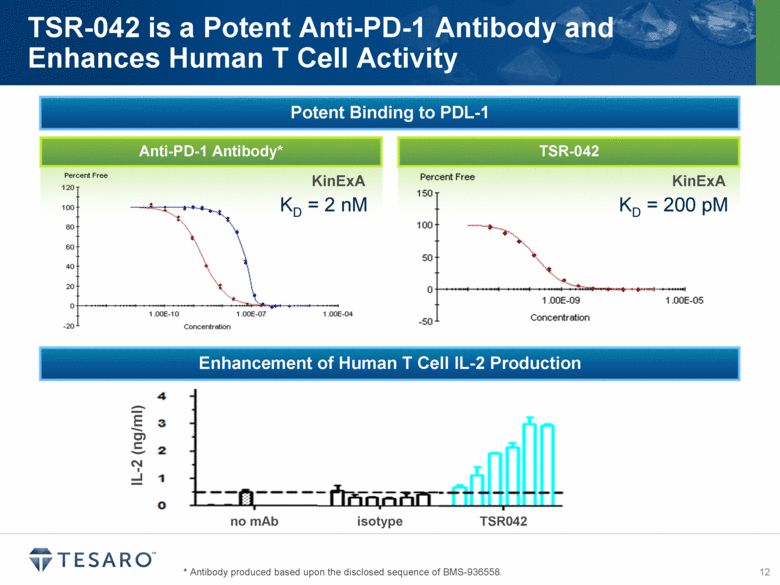
TSR-042 Anti-PD-1 Antibody* * Antibody produced based upon the disclosed sequence of BMS-936558. TSR-042 is a Potent Anti-PD-1 Antibody and Enhances Human T Cell Activity KinExA KD = 2 nM KinExA KD = 200 pM IL-2 (ng/ml) isotype TSR042 no mAb Potent Binding to PDL-1 Enhancement of Human T Cell IL-2 Production |
|
|
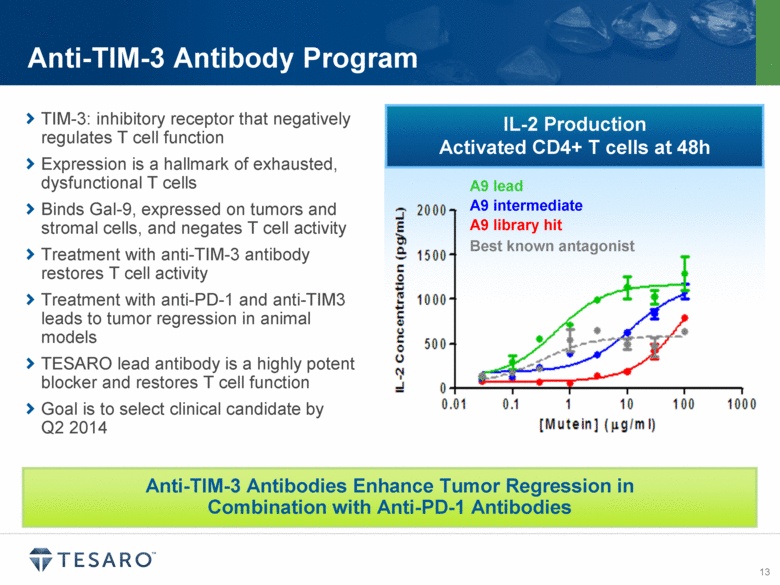
IL-2 Production Activated CD4+ T cells at 48h Anti-TIM-3 Antibody Program Anti-TIM-3 Antibodies Enhance Tumor Regression in Combination with Anti-PD-1 Antibodies TIM-3: inhibitory receptor that negatively regulates T cell function Expression is a hallmark of exhausted, dysfunctional T cells Binds Gal-9, expressed on tumors and stromal cells, and negates T cell activity Treatment with anti-TIM-3 antibody restores T cell activity Treatment with anti-PD-1 and anti-TIM3 leads to tumor regression in animal models TESARO lead antibody is a highly potent blocker and restores T cell function Goal is to select clinical candidate by Q2 2014 Best known antagonist A9 library hit A9 lead A9 intermediate |
|
|
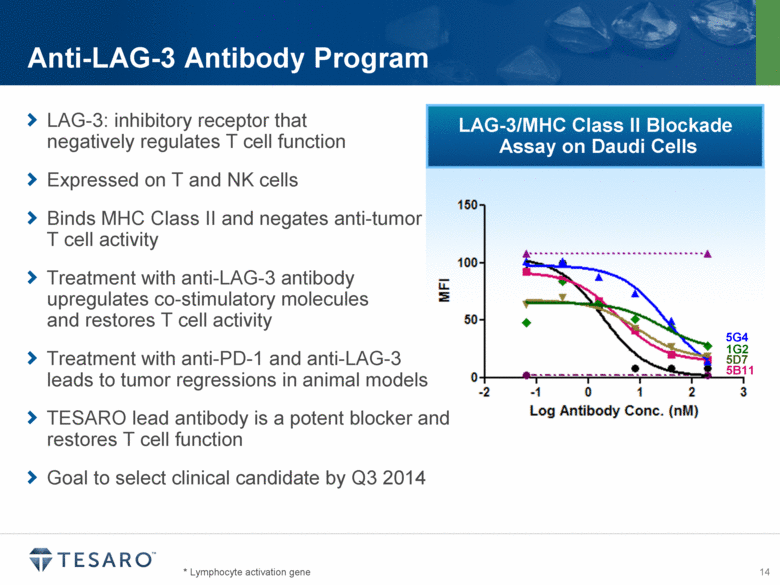
LAG-3/MHC Class II Blockade Assay on Daudi Cells * Lymphocyte activation gene Anti-LAG-3 Antibody Program LAG-3: inhibitory receptor that negatively regulates T cell function Expressed on T and NK cells Binds MHC Class II and negates anti-tumor T cell activity Treatment with anti-LAG-3 antibody upregulates co-stimulatory molecules and restores T cell activity Treatment with anti-PD-1 and anti-LAG-3 leads to tumor regressions in animal models TESARO lead antibody is a potent blocker and restores T cell function Goal to select clinical candidate by Q3 2014 5G4 1G2 5D7 5B11 |
|
|
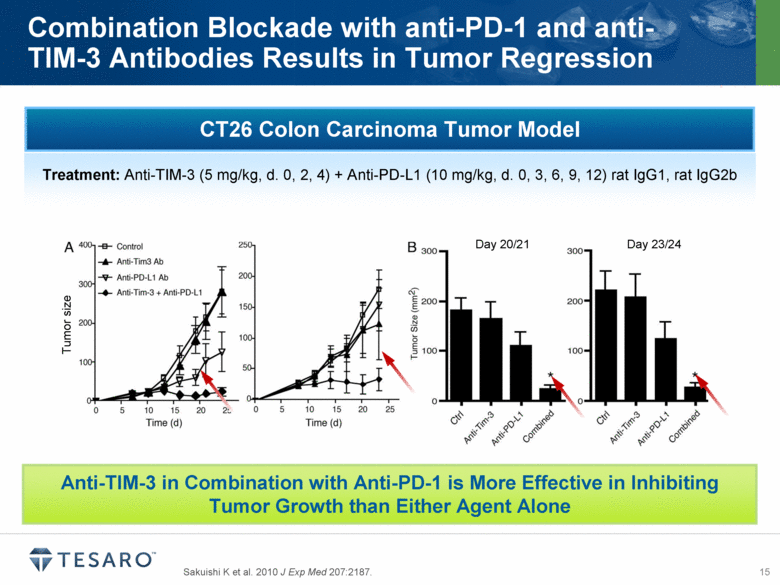
Sakuishi K et al. 2010 J Exp Med 207:2187. Combination Blockade with anti-PD-1 and anti-TIM-3 Antibodies Results in Tumor Regression Tumor size Day 20/21 Day 23/24 CT26 Colon Carcinoma Tumor Model Treatment: Anti-TIM-3 (5 mg/kg, d. 0, 2, 4) + Anti-PD-L1 (10 mg/kg, d. 0, 3, 6, 9, 12) rat IgG1, rat IgG2b Anti-TIM-3 in Combination with Anti-PD-1 is More Effective in Inhibiting Tumor Growth than Either Agent Alone |
|
|
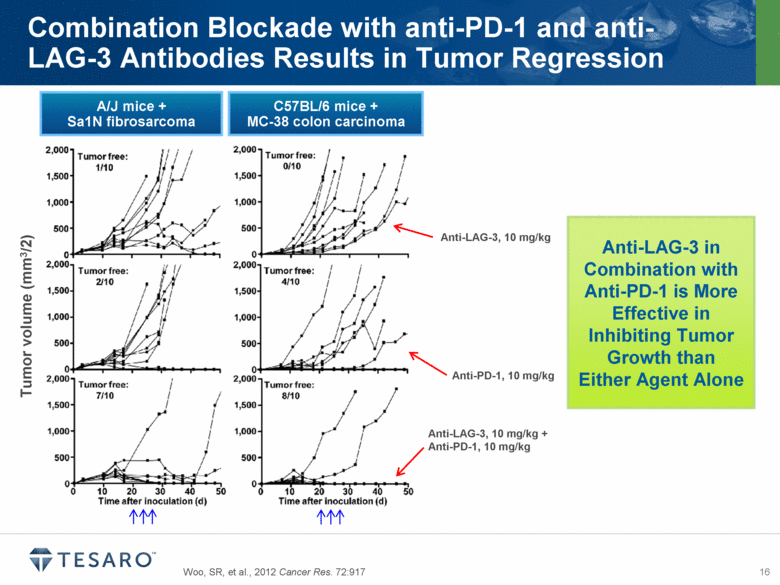
Woo, SR, et al., 2012 Cancer Res. 72:917 Combination Blockade with anti-PD-1 and anti-LAG-3 Antibodies Results in Tumor Regression Anti-PD-1, 10 mg/kg Anti-LAG-3, 10 mg/kg Anti-LAG-3, 10 mg/kg + Anti-PD-1, 10 mg/kg Tumor volume (mm3/2) A/J mice + Sa1N fibrosarcoma C57BL/6 mice + MC-38 colon carcinoma Anti-LAG-3 in Combination with Anti-PD-1 is More Effective in Inhibiting Tumor Growth than Either Agent Alone |
|
|
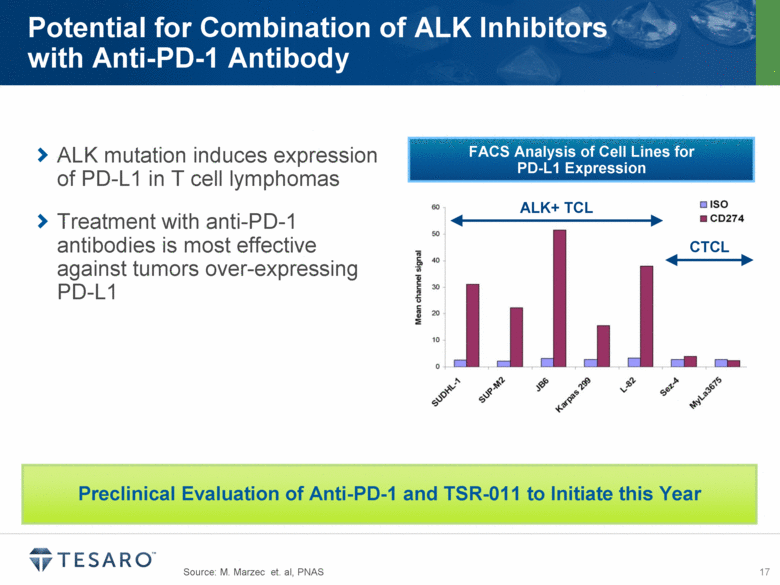
Source: M. Marzec et. al, PNAS Potential for Combination of ALK Inhibitors with Anti-PD-1 Antibody ALK mutation induces expression of PD-L1 in T cell lymphomas Treatment with anti-PD-1 antibodies is most effective against tumors over-expressing PD-L1 FACS Analysis of Cell Lines for PD-L1 Expression ALK+ TCL CTCL Preclinical Evaluation of Anti-PD-1 and TSR-011 to Initiate this Year |
|
|
Immuno-oncology platform provides a portfolio of candidates focused on high value targets and augments the value of TESARO’s other pipeline candidates Enhances prospects for future collaborations Anticipated milestones: Potential first-in-class anti-TIM-3 antibody clinical candidate to be selected in Q2 2014 Anti-LAG-3 antibody candidate to be selected by Q3 2014 Dual reactive antibody clinical candidates targeting PD-1/TIM-3 and PD-1/LAG-3 to be selected by first-quarter 2015 Preclinical investigation of TSR-042 (anti-PD-1 antibody) with each of TSR-011 (ALK/TRK inhibitor) and niraparib (PARP inhibitor) in 2014 First clinical trial of TSR-042 projected to begin in mid-2015 Advancing Our Oncology Strategy Summary |
|
|
Q&A |
|
|
March 13, 2014 Immuno-Oncology Collaboration and License Agreement for TIM-3, LAG-3 and PD-1 Antibody Programs |