Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AERIE PHARMACEUTICALS INC | d683122d8k.htm |
 Leading Innovation in Glaucoma
The Next Generation
Company Overview
February 26, 2014
Exhibit 99.1 |
 Any
discussion of the potential use or expected success of our product candidates is subject to our
product
candidates
being
approved
by
regulatory
authorities.
In
addition,
any
discussion
of
clinical
data
results for our AR-13324 product candidate relate to the results in our Phase 2
clinical trials. Our product candidate PG324 has only been tested in
preclinical animal models. The information in this presentation is current
only as of its date and may have changed or may change in the future. We
undertake no obligation to update this information in light of new information, future
events or otherwise. We are not making any representation or warranty that the
information in this presentation is accurate or complete.
2
Important Information
Certain
statements
in
this
presentation
are
“forward-looking statements”
within
the
meaning
of
the
federal securities laws, including beliefs, expectations, estimates, projections
and statements relating to our business plans, prospects and objectives, and
the assumptions upon which those statements are based.
Words
such
as
“may,”
“will,”
“should,”
“would,”
“could,”
“believe,”
“expects,”
“anticipates,”
“plans,”
“intends,”
“estimates,”
“targets,”
“projects”
or similar expressions are intended to identify these forward-
looking statements.
These statements are based on the Company’s current plans and
expectations.
Known and unknown risks, uncertainties and other factors could cause actual
results to differ
materially
from
those
contemplated
by
the
statements.
In
evaluating
these
statements,
you
should
specifically consider various factors that may cause our actual results to differ
materially from any forward-looking statements. These risks and
uncertainties are described more fully in our prospectus filed with the SEC
on October 28, 2013 and in the quarterly and annual reports that we file with the SEC,
particularly
in
the
sections
titled
“Risk
Factors”
and
“Management’s
Discussion
and
Analysis
of
Financial
Condition and Results of Operation.”
Such forward-looking statements only speak as of the date they
are made.
We undertake no obligation to publicly update or revise any forward-looking
statements, whether because of new information, future events or otherwise,
except as otherwise required by law. |
 3
Aerie –
Next Generation in Glaucoma Therapies
AR-13324
Novel Dual Action
All Products Fully
Owned by Aerie
PG324 Breakthrough
Triple Action
•
Fixed combination of AR-13324 and latanoprost
•
Potential for maximal IOP lowering
•
Expect P2b results mid-2014, P3
readiness mid-2015
•
Inhibits ROCK and NET, targets diseased tissue
•
Consistent IOP lowering,
may lower EVP
•
Expect P3 efficacy data mid-2015, NDA filing mid-2016
Large Market
Opportunity
•
$4.5B US/EU/JP Market with significant unmet needs
•
Multiple MOAs, once daily, high efficacy and safety
•
Late-stage/potential blockbuster revenue opportunity
•
Full patent protection through at least 2030
•
Will retain US rights and plan to partner in
JP/EU |
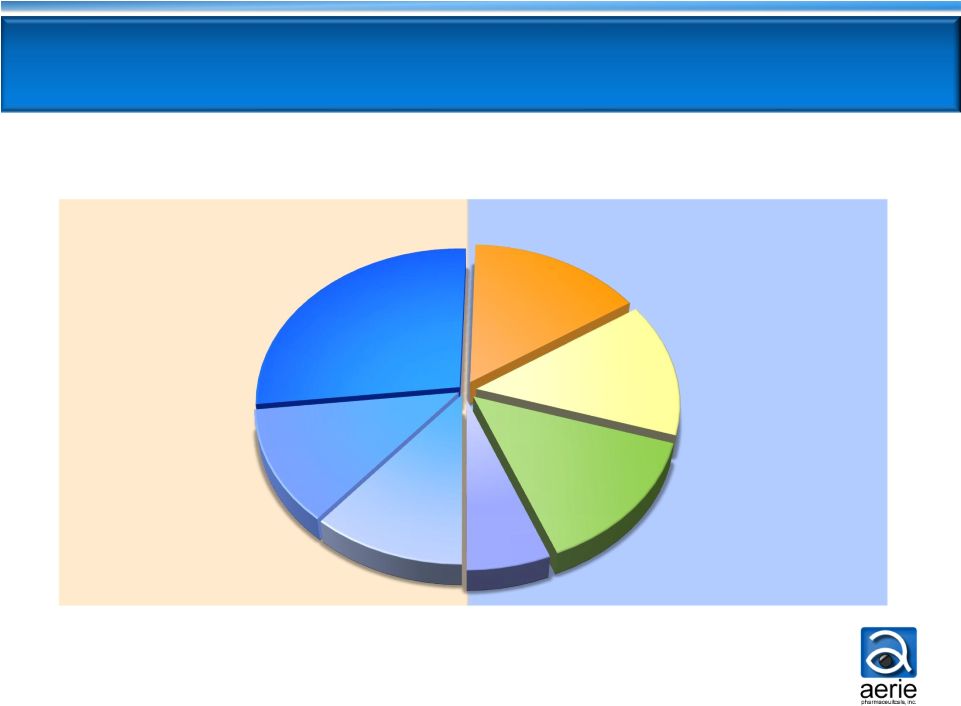 4
Currently Prescribed Glaucoma Therapies
Once Daily
2-3 Times
Daily
Non-PGA
Market
PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic
Anhydrase Inhibitor US Glaucoma Market
IMS TRx data, FY 2012
PGA
Market
Bimatoprost,
11%
Travoprost,
12%
Latanoprost,
27%
BB, 15%
BB Fixed
Combo, 14%
AA, 14%
CAI, 6% |
 Largest Rx market in ophthalmology
-
US 28.5M Rx; annual US sales $1.9B ($4.5B US/EU/JP)
2.2M glaucoma patients in the US expected to grow to
over 3M by 2020
~50% of patients use multiple medications to control
disease
No drugs with new MOA launched in 20 years
None of the existing drugs target the diseased tissue, or
Trabecular Meshwork (primary drain), or lower EVP
None of the existing drugs have shown consistent
efficacy across a broad range of pressures
Significant Glaucoma Market Opportunities
1
National Eye Institute
1
5 |
 Inflow (fluid production)
AA, BB, CAI
PGAs
decrease
increase
increase
Aerie’s Novel Products Address All IOP
Control Mechanisms and May Lower EVP
Outflow
Secondary Drain
(Uveoscleral)
Outflow
Primary Drain
(Trabecular Meshwork)
6
Triple Action
PG324
Dual Action
AR-13324 |
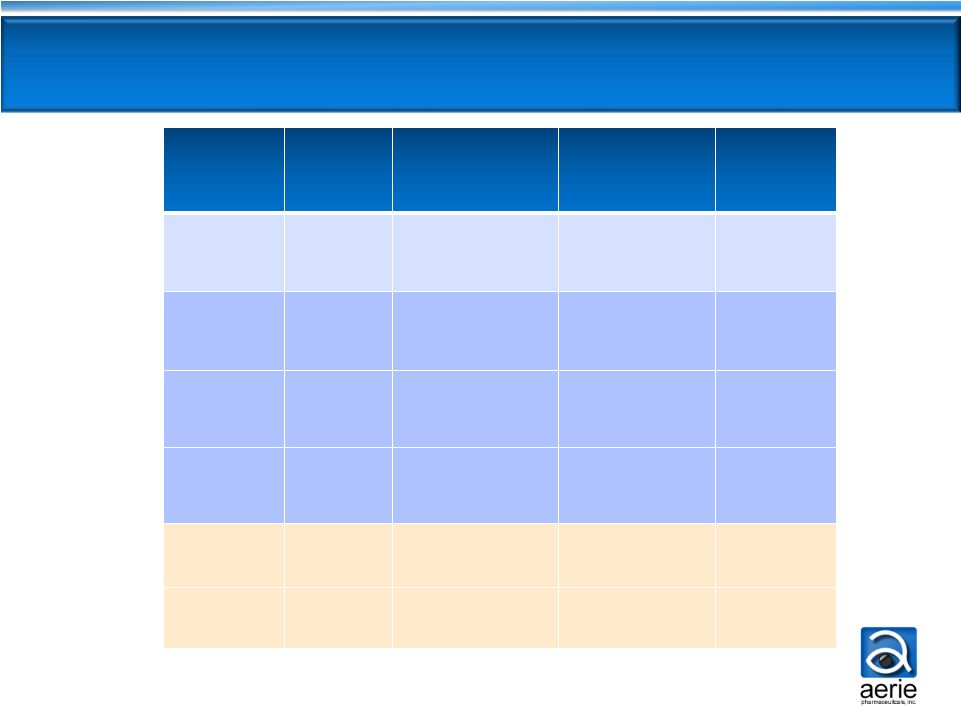 7
The Competitive Landscape
Clinical
Efficacy
Dosing/
Day
Tolerability
Peak Product
Sales*
(US/EU)
Targeting
Diseased
Tissue
PGA
High
1x
Hyperemia
Iris Color Change
Other Cosmetic
$1.7B
No
Beta
Blocker
Moderate
2x
Cardiopulmonary
Contraindications
>$500M
No
CAI
Low
2 -
3x
Sulfonamide
Contraindication
Bitter Taste
>$500M
No
Alpha
Agonist
Low
2 -
3x
Allergy
Drowsiness
>$400M
No
AR-13324
High;
Consistent
1x
Hyperemia
Yes
PG324
Potentially
Highest
1x
TBD
Yes
* Peak sales for best-in-class franchise |
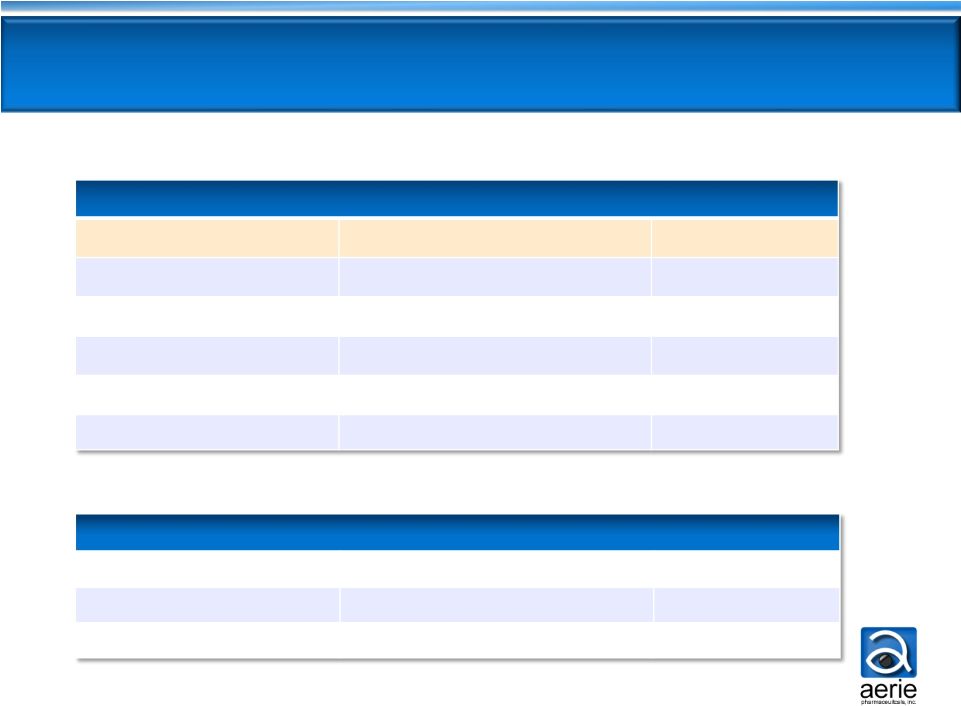 8
New PGAs -
not usable as add-on to current PGAs
Glaucoma Competitors in Pipeline
AR-13324 is the only new MOA drug dosed once-daily
AR
-13324 (Aerie)
ROCK/NET inhibitor (qd)
Phase 2b
K-115 (Kowa)
ROCK inhibitor (bid)
Phase 3
AMA0076
(Amakem)
ROCK inhibitor (bid)
Phase 2a
INO-8875 (Inotek)
Adenosine-A1 agonist (bid)
Phase 2
OPA-6566
(Acucela)
Adenosine-A2a receptor
Phase 1/2
SYL040012 (Sylentis)
beta
Phase 2
New MOAs
RNAi
blocker (bid)
(Japan)
New PGAs
BOL-303259 (B+L)
NO donating
latanoprost
(qd)
Phase 3
DE-117 (Santen)
EP2
agonist (qd)
Phase 2a
ONO-9054 (Ono)
FP/EP3 agonist
(qd)
Phase 1
(bid) |
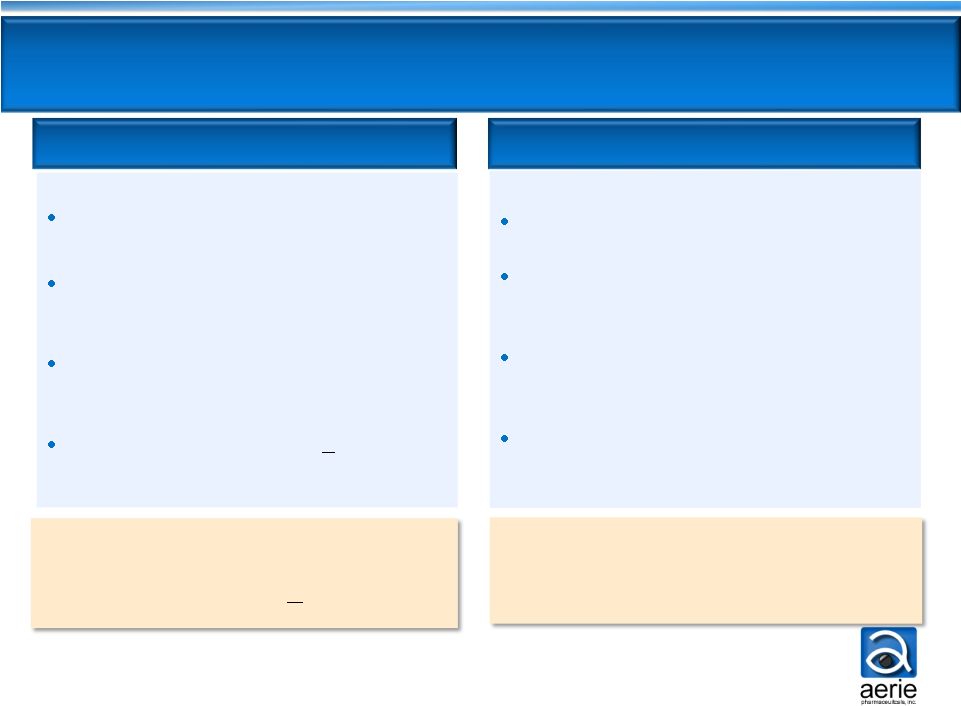 9
Once-daily, consistent IOP lowering
1 drug -
2 MOAs, targets diseased
tissue, and may lower EVP
Well tolerated; no systemic
drug-related AEs
Efficacy expected to be >
current
drugs for majority of patients
Once-daily
Combination of 2 drugs -
3 MOAs,
and may lower EVP
Well tolerated; no systemic
drug-related AEs
Efficacy expected to be superior to all
current drugs
AR-13324 Dual-Action
PG324 Triple-Action
Future drug of choice for 80%
of patients -
IOP <26mmHg
Future drug of choice for
patients requiring maximal
IOP lowering
Aerie Franchise: Highly
Differentiated
Product Profiles |
 10
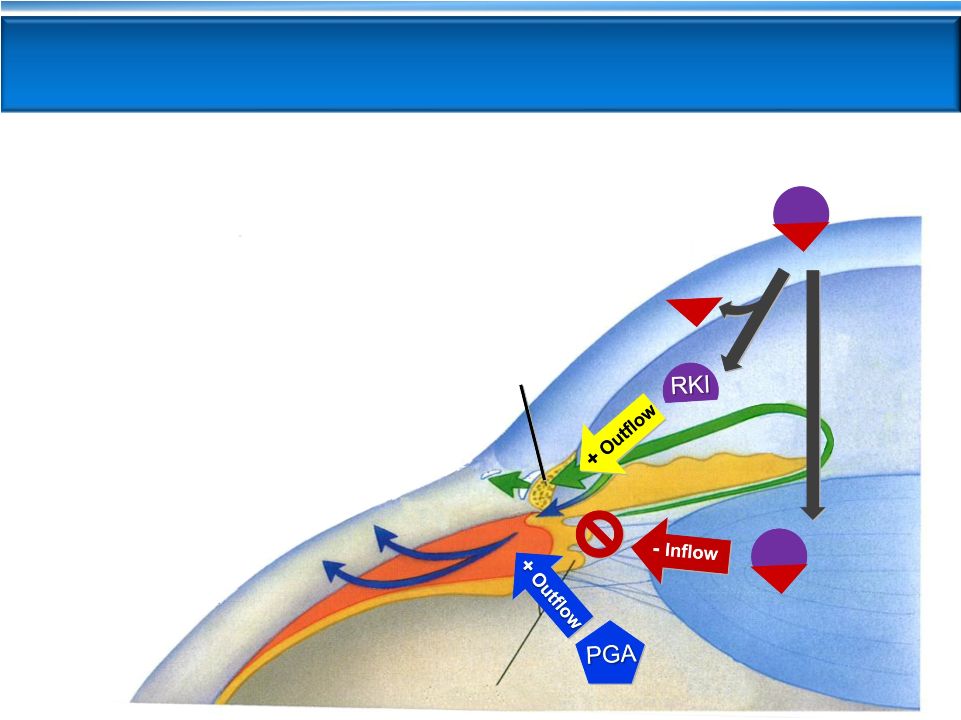
AR-13324
AR-13324
NET
RKI
Trabecular
Meshwork
1.
ROCK inhibition relaxes TM, increases outflow
2.
NET inhibition reduces fluid production
3.
ROCK inhibition may lower Episcleral
Venous Pressure (EVP)
Episcleral
Veins
Schlemm’s
Canal
Mechanisms of Action:
NET
RKI
Cornea
Ciliary Processes
Uveoscleral
Outflow |
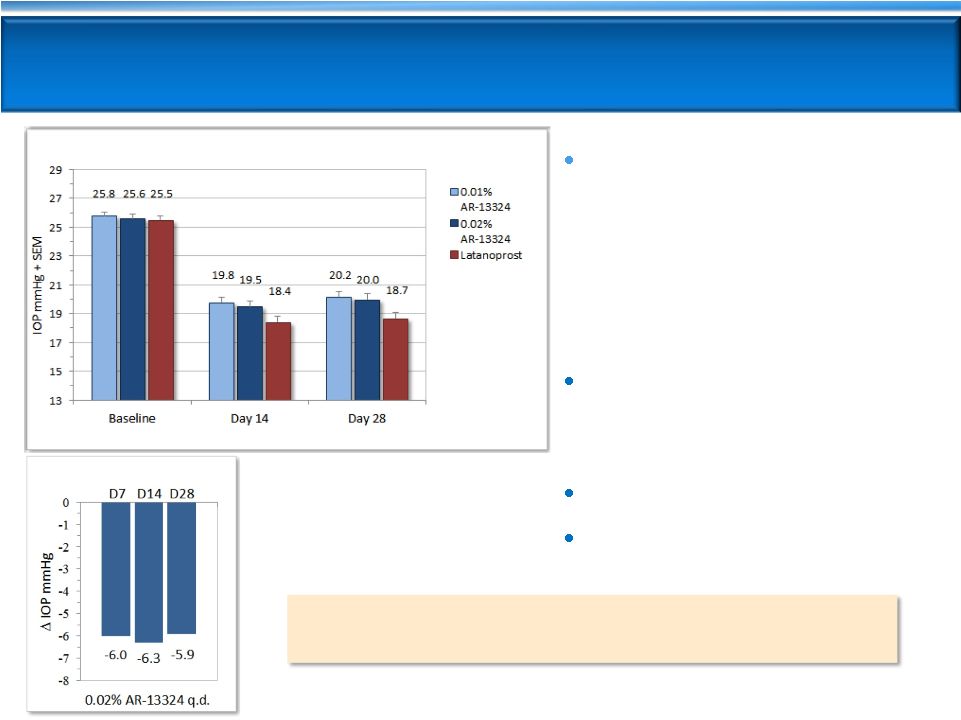 11
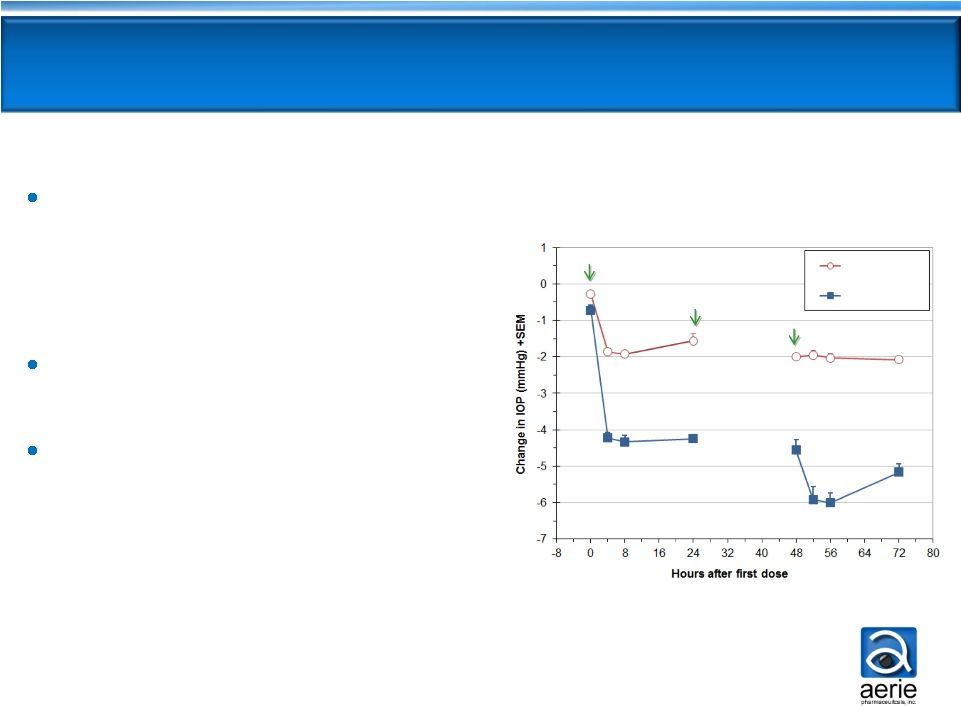
AR-13324 Demonstrated Strong IOP Lowering
in Phase 2b
13 glaucoma NCEs advanced from Phase 2 to Phase 3
since 1970s -
All approved
Once-daily PM dosing of
0.02% AR-13324 is highly
effective
-
IOP -5.7 and -6.2 mmHg on
D28 and D14
-
Consistent efficacy through D28
AR-13324 efficacy results
within ~1 mmHg of
latanoprost
Favorable tolerability profile
No systemic side effects
AR-13324 Efficacy at 8 AM
Diurnal Average IOP -
Entry IOP 22-36 mmHg (n=221) |
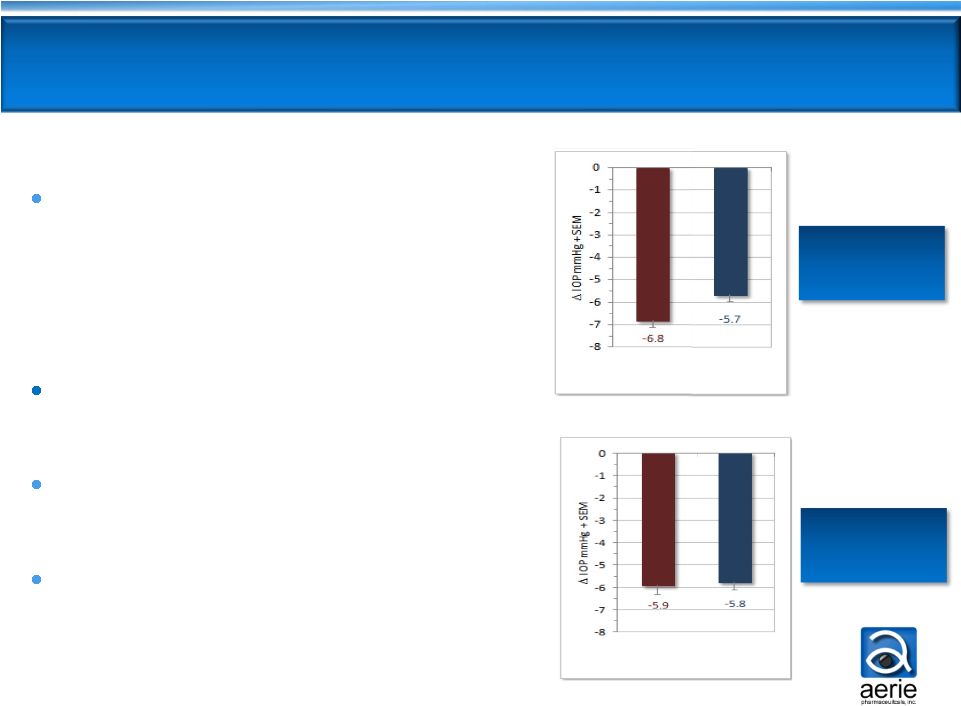 AR-13324 and latanoprost
clinically and statistically
equivalent in patients with
moderately elevated
IOPs of 22
- 26 mmHg
Latanoprost loses ~1 mmHg
efficacy
AR-13324 maintains consistent
efficacy
Latanoprost does not target the
diseased tissue –
AR-13324 does
Latanoprost
0.02%
AR-13324
Latanoprost
0.02%
AR-13324
Phase
2b
baseline
IOP
entry
requirements:
24,
22,
22
mmHg
(8am,
10am,
4pm)
Full Patient Population
Moderately Elevated IOP
12
Differentiated AR-13324 Efficacy
Profile Informs
Phase 3 Study Design Baseline
22 –
36 mmHg
(n=221)
Baseline
22 –
26 mmHg
(n=106) |
 13
New MOA
May Represent Breakthrough
Recently reported study confirms AR-13324 lowered EVP by
35%
in
a
preclinical
in
vivo
model
2
1
Aerie Phase 1 PK Study Press Release dated January 9, 2014
2
Aerie Press Release dated February 25, 2014
AR-13324
lowered
average
IOP
by
over
30%
-
from
16
to
11
mmHg
1
Currently available drugs are typically less effective at lower IOPs
Consistent efficacy of AR-13324 across baseline IOPs may be
explained by lowering of EVP
High efficacy of AR-13324 in normotensive volunteers with
average IOP of 16 mmHg also supports lowering of EVP
No currently marketed products are known to lower EVP,
which contributes up to half of IOP in normotensive subjects
|
 10,444 individuals were
screened for the prevalence of Primary Open-Angle Glaucoma (POAG)
Baltimore Eye Survey
* Sommer A, Tielsch JM, Katz J et al. Relationship between intraocular pressure and
primary open angle glaucoma among white and black Americans: The Baltimore
eye survey. Arch Ophthalmol 1991;109:1090-1095 14
Baseline IOP
(mmHg)
Percentage of POAG
Patients Identified
Cumulative Percentage
<
15
13%
13%
16-18
24%
37%
19-21
22%
59%
22-24
19%
78%
25-29
10%
88%
30-34
9%
97%
>
35
3%
100%
~80% of Glaucoma IOPs Are <
26 mmHg
at Time of Diagnosis |
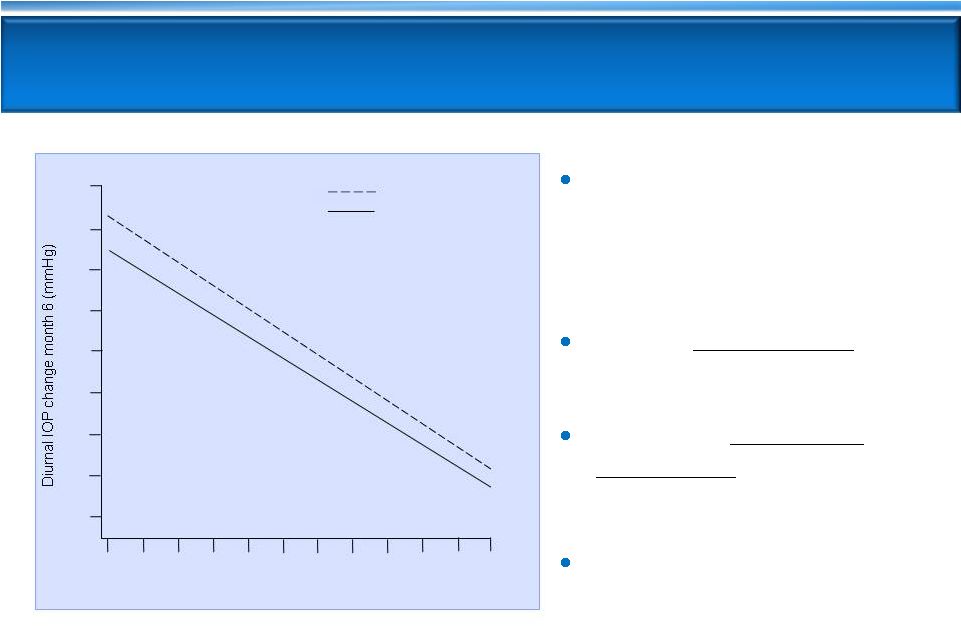 Latanoprost and timolol
lose 0.5 mmHg efficacy for
every 1 mmHg drop in
baseline IOP
Timolol less effective
than
latanoprost at all baselines
AR-13324 equivalent/
non-inferior
to latanoprost
at baselines 22 –
26 mmHg
Timolol is the standard
comparator for glaucoma
Phase 3 trials
Pooled data from three latanoprost registration studies.
Hedman and Alm; European Journal Ophthalmology;2000
0
-2
-4
-6
-8
-10
-12
-14
-16
Timolol (n = 369)
Latanoprost (n = 460)
16
18
20
22
24
26
28
30
32
34
36
38
Untreated diurnal IOP (mmHg)
Latanoprost and Timolol Show Reduced Efficacy
at Lower Baseline IOPs
15 |
 AR-13324 Registration Trial Design
Primary efficacy endpoint: IOP at all
time points through Day 90
Non-inferiority design vs. timolol
-
95% CI within 1.5 mmHg at all time points,
within 1.0 mmHg at a majority of time points
Planned entry IOP:
Minimum
21 mmHg, maximum 26 mmHg
-
FDA has agreed to Aerie proposal for entry IOPs
with no expected impact on label
-
AR-13324 non-inferior to latanoprost at entry
IOPs of 22-26 mmHg in Phase 2b
Phase 3 Protocol
AR-13324 0.02%
dosed QD PM
vs.
Timolol
dosed BID
~1200 patients
90 days efficacy
100+
patients for
1 year safety
16 |
 17
AR
-
13324 Summary
Potential drug of choice for 80% of patients –
those
with
IOPs <
26mmHg
Once daily, high efficacy, consistent IOP lowering, good
tolerability and safety profile through Phase 2
The only drug to target the diseased tissue, with added MOAs
of lowering fluid production and potentially lowering EVP
Next Steps:
Phase 3 expected to commence in mid-2014
Phase 3 90-day efficacy and short-term safety results expected
mid-2015 NDA filing expected by
mid-2016
|
 AR-13324 FIXED COMBINATION WITH
LATANOPROST
AR-13324
MOAs:
NET
RKI
18
TM
Outflow
Ciliary Processes
Uveoscleral
Outflow
Latanoprost
Cornea
1.
ROCK inhibition restores TM outflow
2.
NET inhibition reduces fluid production
3.
ROCK inhibition may lower
Episcleral Venous Pressure
4.
PGA receptor
activation
increases uveoscleral
outflow
Triple-Action PG324
NET
RKI |
 First product to lower IOP through
all three known mechanisms, and
also potentially lower EVP
-
Once-daily: 1 drop, 2 drugs, 3 MOAs
High efficacy in predictive
primate model
Human proof of concept
established in prior ROCKi/PGA
combination trials
-
Demonstrated significant IOP
lowering beyond PGA alone
Dose
Dose
Dose
PG324 vs. Latanoprost, Primates (n=6/group)
Latanoprost
0.02% PG324
19
Day 2 -
No IOP
measured
PG324: Triple-Action Fixed Combination |
 Primary efficacy endpoint: Mean
diurnal IOP on Day 28
Two concentrations of PG324 vs.
AR-13324 0.02% and latanoprost
Trial design similar to registration
trial for fixed-dose combination
-
1-3 mmHg superiority vs. components
previously accepted by FDA
20
PG324 Phase 2b Clinical Trial Design
Phase 2b Protocol
PG324 0.01%
vs.
PG324 0.02%
vs.
AR-13324 0.02%
vs.
Latanoprost
All dosed QD PM
~300 patients
28 days |
 Potential for use by patients requiring maximal IOP lowering
and/or patients with advanced disease state
Potential to be the most efficacious IOP-lowering drug in
glaucoma with at least three and potentially four MOAs
Expected to be the first PGA fixed combination product in the
US, and dosed as a single drop once daily
Next Steps:
Phase 2b first patient dosed early 1Q 2014
Phase 2b results expected mid-2014
Phase 3 preparatory work commences second half of 2014
Phase 3 readiness expected mid
2015
PG324
Summary
21 |
 H1
2014 H2 2014
H2 2015
H1 2015
H1 2016
22
Key Milestones Financed by IPO Proceeds
Early-2014
:
PG324
Start Phase 2b clinical trial
Mid-2014
:
AR-13324
Start Phase 3
registration trials
Mid-2015
AR-13324
Efficacy results from
Phase 3 expected
Mid-2016
AR-13324
NDA filing expected
Mid-2014: PG324
Results from Phase
2b expected
Mid-2015
:
PG324
Phase 3-prep
:
: |
 Financial Summary
AR-13324
Phase 3 registration trials
Direct clinical -
$25.0M
Non-clinical -
$12.4M
PG324
Phase 2b clinical trial
Direct clinical -
$2.5M
Non-clinical -
$1.0M
Phase 3 enabling activities
Non-clinical -
$6.3M
G&A and working capital -
$20.8M
Use of IPO Proceeds:
$68M through Mid-2016
Additional PG324 Needs:
$40M through Mid-2017
PG324
Phase 3 registration trials
Direct clinical -
$27.5M
12 Months G&A
and
working capital -
$12.5
23
Targeted
Mid-2017:
Expected NDA Approval
for
AR
-
13324
Expected NDA Filing for PG324 |
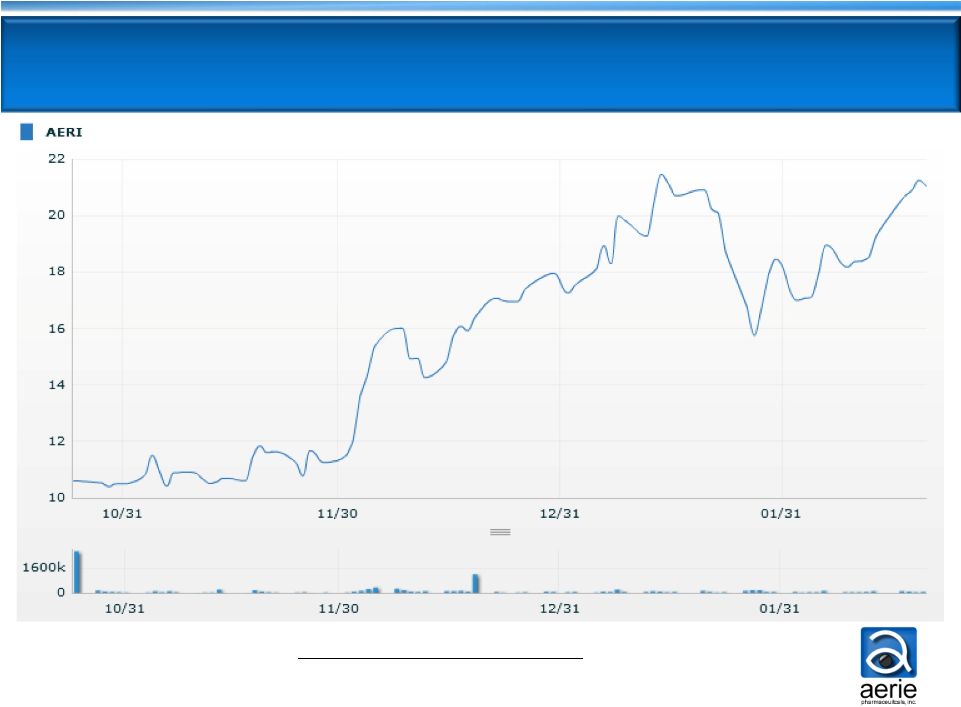 Market
Cap
(dollars
in
millions)
24
Stock & Market Cap Performance
10/31/13
-
$243.7
12/31/13
-
$418.2
2/21/14
-
$490.6 |
 AR-13324
Novel Dual Action
All Products Fully
Owned by Aerie
PG324 Breakthrough
Triple Action
•
Fixed combination of AR-13324 and latanoprost
•
Potential for maximal IOP lowering
•
Expect P2b results mid-2014, P3
readiness
mid-2015
•
Inhibits ROCK and NET, targets diseased tissue
•
Consistent
IOP
lowering,
may
lower
EVP
•
Expect P3 efficacy data mid-2015, NDA filing mid-2016
Large Market
Opportunity
•
$4.5B US/EU/JP Market with significant unmet needs
•
Multiple MOA’s, once daily, high efficacy and safety
•
Late-stage/potential blockbuster revenue opportunity
•
Full patent protection through at least 2030
•
Will retain US rights and plan to partner in
JP/EU
25
Aerie –
Next Generation in Glaucoma Therapies |
