Attached files
| file | filename |
|---|---|
| 8-K - 8-K - INTERMUNE INC | d682081d8k.htm |
 ASCEND Top-Line Results
February 25, 2014
Exhibit 99.1 |
 Forward-looking Statements
This
presentation
contains
forward-looking
statements
made
pursuant
to
the
"safe
harbor"
provisions
of
the
Private
Securities Litigation Reform Act of 1995. Investors are cautioned that, without
limitation, statements in this presentation regarding InterMune's plans and
expectations; the estimated patient populations suffering from IPF and market potential
for pirfenidone in the United States, including potential pricing estimates;
InterMune's expectations regarding the timing for resubmission of its new
drug application with the FDA for pirfenidone; the potential to make pirfenidone available as a
medicine to IPF patients in the United States; anticipated timing of initiating a
commercial launch for pirfenidone and the size of a commercial salesforce to
market pirfenidone in the Unites States; InterMune’s intention to present additional data
on the ASCEND trial at the American Thoracic Society meeting in May 2014; and
expectations regarding the regulatory approval process and prospects for
success thereof are forward-looking statements. All forward-looking statements
included
in
this
presentation
are
based
on
information
available
to
InterMune
as
of
the
date
hereof,
and
InterMune
assumes no obligation to update any such forward-looking statements. Actual
results could differ materially from those described in the
forward-looking statements. Factors that could cause or contribute to such differences include, but are
not
limited
to,
those
discussed
in
detail
under
the
heading
“Risk
Factors”
in
InterMune’s
periodic
reports
filed
with
the
SEC, including but not limited to the following: (i) the risks related to the
uncertain, lengthy and expensive clinical development process for the
company’s product candidates; (ii) risks related to the regulatory process for pirfenidone,
including that the results of the ASCEND trial may not be satisfactory to the FDA
to receive regulatory approval; (iii) risks related
to
unexpected
regulatory
actions
or
delays
or
government
regulation
generally;
(iv)
risks
related
to
the
company’s
manufacturing strategy; (v) government, industry and general public pricing
pressures; (vi) risks related to the company’s ability to successfully
launch and commercialize pirfenidone; and (vii) the company’s ability to maintain intellectual
property
protection.
The
risks
and
other
factors
discussed
above
should
be
considered
only
in
connection
with
the
risks
and other factors discussed in detail in InterMune’s Form 10-K and its
other periodic reports filed with the SEC, which are also available at
www.intermune.com. 1 |
 Topics
of Today’s Call IPF Disease Overview
2
ASCEND Top-Line Results
NDA Resubmission Timeline
Questions and Answers
Assessing Clinically Meaningful Changes
in Forced Vital Capacity (FVC) |
 Dan
Welch Chairman, CEO and President |
 Our
Franchise: Idiopathic Pulmonary Fibrosis (IPF) A Large, Lethal Orphan Disease
Progressive scarring of the
lungs with no known cause
–
Median survival: 2-5
years*
Large market in North
America + European Top-15:
–
118K to 158K prevalence
–
28K to 37K incidence
Esbriet
®
is the only approved
IPF medicine in Europe or
Canada
% Patients Surviving at 5 Years
100
0
80
60
40
20
Lung
Cancer
IPF
Ovarian
Cancer
PAH
Colo-
rectal
Cancer
Breast
Cancer
*
Bjoraker JA, Am J Respir Crit Care Med. 1998 Jan; 157(1):199-203.
4 |
 IPF in
the United States Large Orphan Disease
–
Incidence ~ 15-20K/year
–
Prevalence ~ 30-50K mild/moderate
Desperate Unmet Need
–
No FDA-approved IPF medicines
Attractive Business Model
–
Value based, orphan disease pricing
–
Small molecule margins
–
No third-party royalties
–
Specialty target audience: 3,000 MDs
–
Targeted
salesforce
of
~
75
-
100
reps
U.S. IPF Prevalence
Mild to Moderate
(30,000-50,000)
Severe
(~20,000)
5 |
 Jonathan Leff, M.D.
EVP, Research and Development |
 Clinically Meaningful Changes in FVC in IPF Studies (1)
How to Determine a Clinically Relevant Change
The Clinical Reality:
–
Disease heterogeneity –
individuals progress at different rates
–
A population mean may underrepresent the true clinical impact to
an individual
–
Therefore group MEAN decline in FVC is not the most informative measure of
treatment effect for individual patients
What is the minimal clinically important difference (MCID) in FVC in IPF patients?
–
MCID is the difference to an individual patient
that is meaningful
–
MCID for percent predicted FVC (%FVC) in an IPF patient is 2-6%*
–
%FVC declines of 10% in an IPF patient translate to ~4-8X increased risk of
death
+
Categorical Change in %FVC is the clinically meaningful metric:
Proportion of patients with a %FVC decline 10%
7
* du Bois RM Am J Respir Crit Care Med 184: 1382-9, 2011 +
du Bois RM Am J Respir Crit Care Med 184: 459-66, 2011
|
 8
Why is Categorical Change in FVC clinically meaningful?
–
Measures changes in an individual patient
–
Addresses the statistical challenge of the population mean
–
Can be easily tied to the MCID (a population mean cannot)
–
Relevant to clinicians and regulators
–
Esbriet label in Europe and Canada only reference Categorical FVC
Change
Categorical FVC Change: Measure of Clinical Meaningfulness in ASCEND
displayed two ways:
•
Proportion of patients with a %FVC decline 10%
•
Proportion of patients with no decline in %FVC
Clinically Meaningful Changes in FVC in IPF Studies (2) |
 The
ASCEND Study: A Randomized, Double-blind, Placebo
Controlled Trial of Pirfenidone in Patients
with Idiopathic Pulmonary Fibrosis |
 ASCEND Study Design: Overview
Randomized, double-blind, placebo controlled trial
Eligible patients randomized (1:1) to treatment with
pirfenidone 2403 mg/d or matched placebo for 52 weeks
Centralized review of HRCT, SLB, spirometry and deaths
instituted to confirm eligibility and ensure high-quality
efficacy assessments
127 sites in 9 countries (U.S., Australia, Brazil, Croatia,
Israel, Mexico, New Zealand, Peru, and Singapore)
10 |
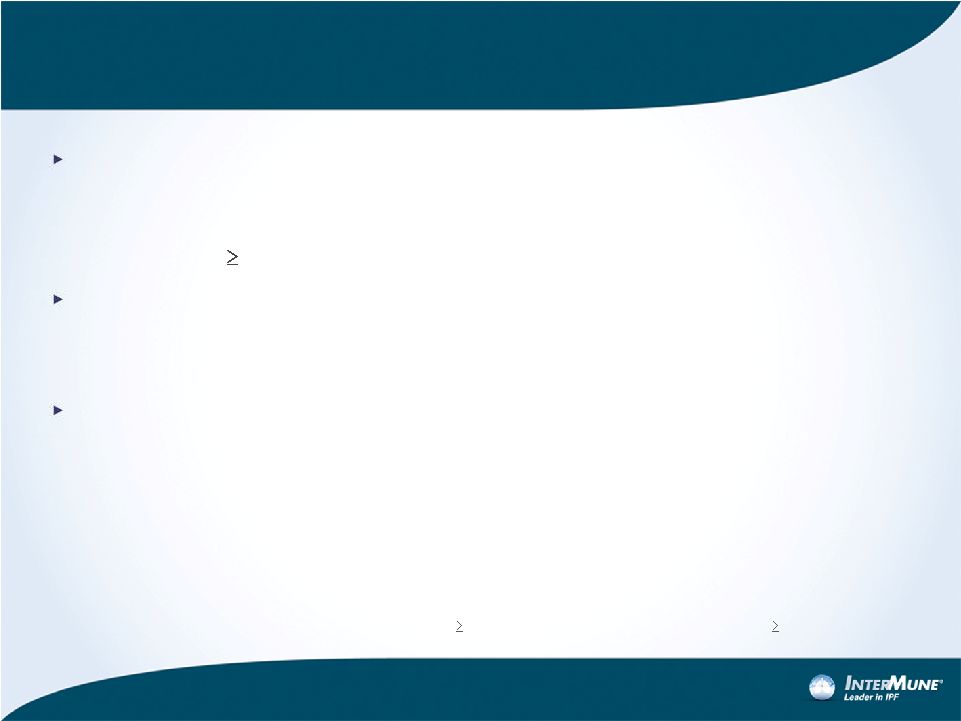 ASCEND Study Design: Pre-Specified Efficacy
Endpoints
Primary Endpoint: %FVC Change from Baseline to Week 52
–
Primary analysis: Rank ANCOVA model
–
Magnitude of effect: categorical analysis of 2 clinically important thresholds
of change (
10% decline or death, no decline)
Key Secondary Endpoints*
–
6MWT distance (6MWD) change in meters from Baseline to Week 52
–
Progression-free survival (PFS)
†
Additional Secondary Endpoints
–
All-cause mortality (ASCEND alone and pooled with CAPACITY)
–
Treatment-emergent IPF-related mortality (ASCEND alone and pooled with
CAPACITY)
–
Dyspnea change from Baseline to Week 52 (UCSD SOBQ score)
11
*
Tested for multiple comparisons using the Hochberg procedure
†
Defined
as
time
to
first
occurrence
of
death,
confirmed
10%
absolute
decline
in
FVC,
or
confirmed
50
m
decline
in
6MWD |
 ASCEND Study: Key Eligibility Criteria
Age 40–80 years
Confident diagnosis of IPF based on central review of
HRCT +/-
SLB
Percent predicted FVC
50% and
90%
Percent predicted DLco
30% and
90%
FEV
1
/FVC ratio
0.80
6MWD
150 m
12 |
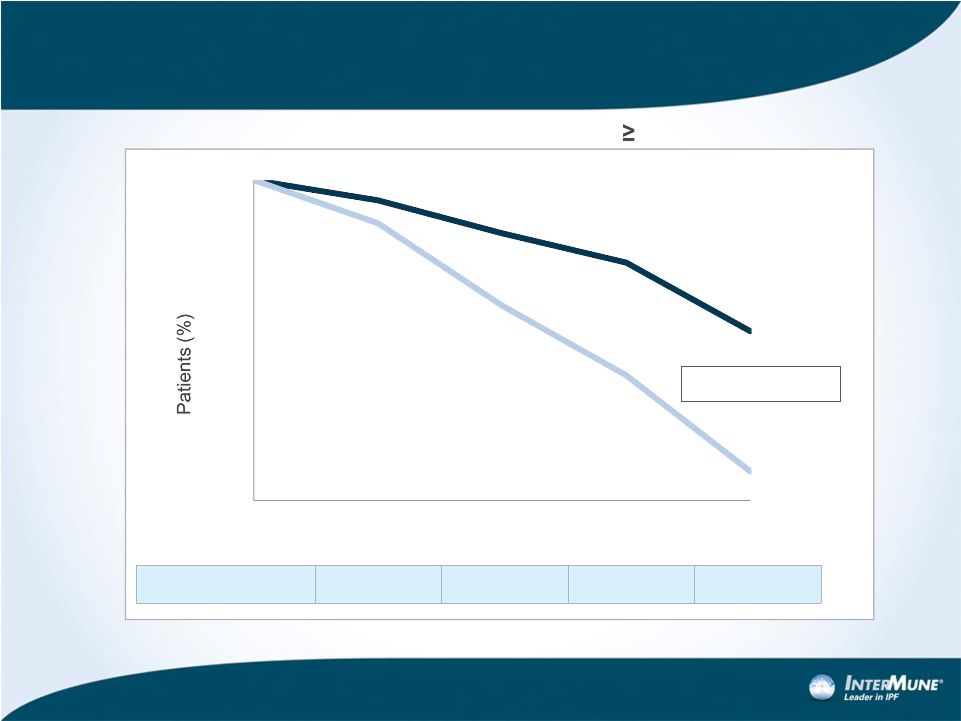 Primary Efficacy Analysis: %FVC Change at Week 52
Relative Difference
54.0%**
58.0%**
57.8%**
47.9%*
13
Pirfenidone (N=278)
Placebo (N=277)
p<0.000001*
Proportion
of
Patients
with
%FVC
Decline
10%
or
Death
*Rank ANCOVA (pirfenidone vs. placebo)
**Rank ANCOVA p<0.0001
0
10
20
30
0
13
26
39
52
Week |
 Primary Efficacy Analysis: %FVC Change at Week 52
*Rank ANCOVA (pirfenidone vs. placebo at week 52)
47.9% Reduction
132.5% Increase
14
Proportion of Patients with Clinically Important Thresholds of %FVC Change
p<0.000001*
0
10
20
30
40
10%
Decline
or
Death
No Decline (Change >0%)
Pirfenidone (N=278)
Placebo (N=277)
> |
 Key
Secondary Endpoints*: 6MWD and PFS 15
Relative
Reduction
P-value
(Rank ANCOVA)
6MWD Results
27.5%
0.0360
Proportion
of
Patients
with
6MWD
Decline
50
meters
or
Death
Hazard Ratio
(95% CI)
P-value
(Log Rank)
PFS Results
0.57
(0.43-0.77)
0.0001
Progression-Free Survival (PFS)**
*Two key secondary endpoints pre-specified with Hochberg procedure for alpha
control **Defined
as
time
to
first
occurrence
of
death,
confirmed
10%
absolute
decline
in
%FVC,
or
confirmed
50
m
decline
in
6MWD |
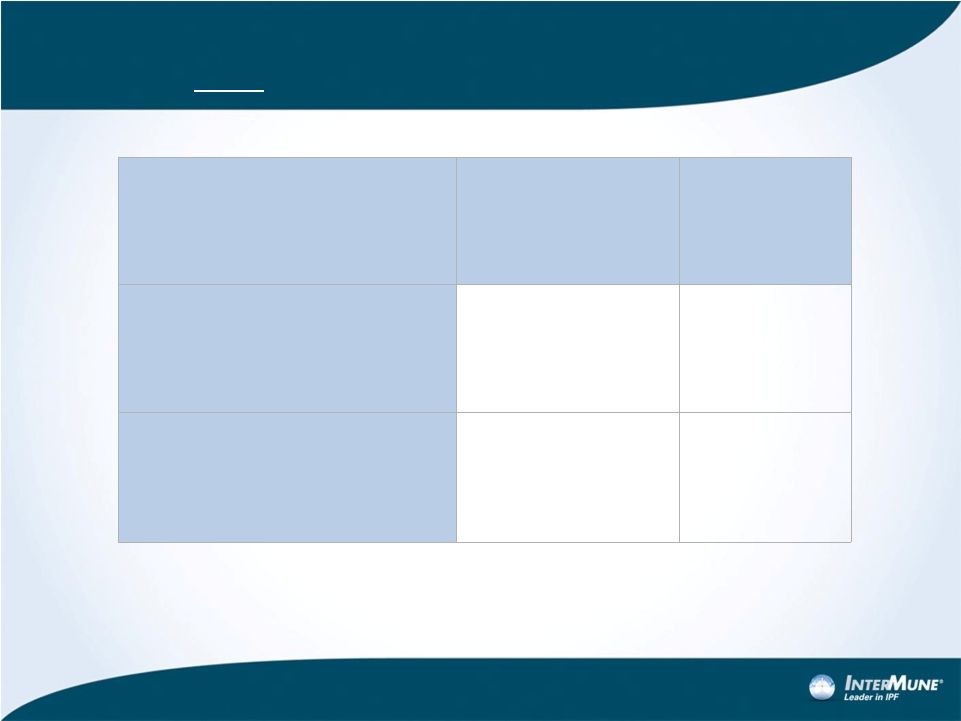 Pre-Specified Mortality Analyses:
ASCEND Only
Patients, n (%)
HR
(95% CI)
P-value
‡
All-cause mortality
0.55 (0.26–1.15)
0.1045
Treatment-emergent
IPF-related mortality*
0.44 (0.11–1.72)
0.2258
*
Deaths
adjudicated
by
mortality
assessment
committee
as
directly
related
to
IPF
†
Occurring
during
treatment
period
(from
first
dose
up
to
28
days
after
last
dose
of
study
drug)
‡
Log-rank test
16
† |
 Pre-Specified Mortality Analyses:
ASCEND
and
CAPACITY
(52-week
data)
Pooled
Patients, n (%)
HR
(95% CI)
P-value
‡
All-cause mortality
0.52 (0.31–0.87)
0.0107
Treatment
emergent
IPF-related
mortality*
0.32 (0.14–0.76)
0.0061
*
Deaths
adjudicated
by
mortality
assessment
committee
(ASCEND)
or
investigator
(CAPACITY)
as
directly
related
to
IPF and does not include deaths in CAPACITY beyond one year
†
Occurring
during
treatment
period
(from
first
dose
up
to
28
days
after
last
dose
of
study
drug)
‡
Log-rank test
17
† |
 Overall Safety Results
18
Pirfenidone was generally safe and well tolerated with only 4% more
treatment discontinuations due to AEs than placebo
The most common AEs with higher incidence in the pirfenidone group
were
primarily
gastrointestinal
(e.g.,
nausea
and
dyspepsia)
and
skin-
related (e.g.,
rash)
Aminotransferase
elevations
3
times
the
ULN
occurred
in
2.9%
versus
0.7% in placebo, were reversible and were an infrequent cause of
treatment discontinuation
The safety profile of pirfenidone was generally consistent with
observations from our previous Phase 3 CAPACITY studies, open-label
extension studies and post-marketing experience |
 Summary of Treatment Emergent Adverse Events*
Patients (%)
Pirfenidone
(N=278)
Placebo
(N=277)
Any adverse event
277 (99.6%)
272 (98.2%)
Grade 3
58 (20.9%)
61 (22.0%)
Grade 4
8 (2.9%)
14 (5.1%)
Any serious adverse event (SAE)
55 (19.8%)
69 (24.9%)
Hospitalizations due to respiratory,
thoracic, and mediastinal SAEs**
10 (3.6%)
31 (11.2%)
Treatment-emergent death
8 (2.9%)
15 (5.4%)
Any AE leading to treatment D/C
40 (14.4%)
30 (10.8%)
* Occurring during treatment period (from first dose up to 28 days after last dose
of study drug) **SAEs in MedDRA system organ class
19 |
 Summary of Top-Line ASCEND Results
Primary efficacy endpoint of FVC change achieved (p<0.000001) with
clinically meaningful effect size
Both key secondary endpoints of 6MWD and PFS achieved
Effect not demonstrated on dyspnea (UCSD SOBQ)
Additional secondary endpoints showed evidence of an effect on
mortality
Pirfenidone was generally safe and well tolerated with few treatment
discontinuations
20 |
 Dan
Welch Chairman, CEO and President |
 Next
Steps Anticipate NDA resubmission by early Q3 2014
Class 2 NDA resubmission; six-month review
If approved, a U.S. launch could occur in Q2 2015.
22 |
 Questions and Answers |
 |
