Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - ThermoGenesis Holdings, Inc. | ex23_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): February 18, 2014
CESCA THERAPEUTICS INC.
(Formerly Known As ThermoGenesis Corp.)
(Exact name of registrant as specified in its charter)
|
Delaware
|
000-16375
|
94-3018487
|
|
(State or other jurisdiction of
incorporation or organization)
|
(Commission File Number)
|
(I.R.S. Employer Identification No.)
|
2711 Citrus Road
Rancho Cordova, California 95742
(Address and telephone number of principal executive offices) (Zip Code)
(916) 858-5100
(Registrant's telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| o | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| o | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| o | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| o | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
INTRODUCTORY NOTE
On February 18, 2014, Cesca Therapeutics Inc. (formerly known as ThermoGenesis Corp.) (the “Company”) completed the acquisition of TotipotentRX Corporation (“TotipotentRX”) pursuant to the terms of the previously announced Agreement and Plan of Merger, dated as of July 15, 2013 (the “Merger Agreement”), by and among the Company, TotipotentRX, Mitchel Sivilotti, and Kenneth Harris.
The following is certain historical information regarding TotipotentRX’s business, its financial information and pro forma information regarding the Company and TotipotentRX.
Item 8. Other Matters.
TotipotentRX’s Business
Company Overview
TotipotentRX Corporation or TotipotentRX, is focused in the research, development, and commercialization of autologous cell-based therapeutics for use in regenerative medicine.
TotipotentRX Corporation (formerly MK Alliance) is the surviving corporation of a merger whereby TotipotentRX merged with and into MK Alliance, Inc. and MK Alliance changed its name to TotipotentRX. Through this merger, in addition to its U.S. operation, TotipotentRX has two wholly-owned subsidiaries in Gurgaon, a suburb of New Dehli, India. Unless otherwise indicated, reference to TotipotentRX includes its predecessors and its subsidiaries. (TotipotentRX Cell Therapy Pvt. Ltd. and TotipotentSC Scientific Product Pvt. Ltd.).
TotipotentRX Strategy
Regenerative Medicine is the future of healthcare and TotipotentRX is focused on commercializing innovative and cost effective ways of treating our aging population. As we live longer, our dependency on chronic health supportive therapies grows and the resulting long-term economic impact is not sustainable. Clearly, the ideal treatment options for medicine are curative in nature, and similarly to surgical procedures, only cellular therapy can provide sustainable physiological improvements. According to the Alliance for Regenerative Medicine, Annual Report 2013, by reducing hospital care, physician and professional services, and nursing/home healthcare through sustainable “curative therapies,” the U.S. could reduce its healthcare costs by up to $250 billion/year. Allogeneic cell developments introduce unknown levels of safety risk, which may or may not be offset by the potential benefit. In contrast, autologous therapies bridge this safety gap, though the absolute prerequisite to fulfill treatment efficacy (cell potency), reproducibility, and eventual commercialization requirements have remained challenging until now. TotipotentRX’s strategy has been to address these gaps through process control-optimization and controlling all high impact variables so the autologous cells (active material) can achieve their endpoint(s).
Today, over $200 million of autologous cell therapy type products are sold around the globe. TotipotentRX believes that the reason the market isn’t larger isn’t due to a lack of physician and patient interest – it’s the result of an oversimplification and general misunderstanding of the process steps surrounding both scientific (the cell handling and treatment methods) as well as market commercialization (scale up) needs.
2
TotipotentRX’s strategy is to maximize the cellular treatment benefit by addressing the critical steps necessary for introducing viable functional cells required for each clinical indication. TotipotentRX manages this process through absolute control of all parameters involved in the cell production and delivery process:
Parameters with major impact on clinical outcomes in cellular therapies:
| · | Environmental & chemical exposure (Safety & Efficacy) |
| · | Equipment & disposable impact (Safety & Efficacy) |
| · | Cell source and patient specific variabilities (Efficacy) |
| · | Treatment protocol (critical analysis of each step from collection through delivery) (Safety & Efficacy) |
| · | Cell Dosage and Purity (Safety & Efficacy) |
| · | Clinical Trial Design (Safety, Efficacy & Commercialization) |
In order to achieve competitive advantages in these parameters, TotipotentRX has created a framework that surrounds and controls the cell therapeutic research, development, and commercialization process through “in-house” clinical laboratory and cell production capabilities, “in-house” clinical trials through our clinical research organization, “in-house” customized medical device development as well as unparalleled access to patients and leading surgeons through long-term partnerships with leading healthcare providers, such as Fortis Healthcare.
TotipotentRX’s Current Business
TotipotentRX is focused on four companion core competencies to serve patients, physicians and partners in the regenerative medicine market space:
| · | Therapeutic |
| · | Contract Clinical Trial Services |
| · | Cell Manufacturing and Banking |
| · | Medical Device Development and Commercialization |
Therapeutic
TotipotentRX views the combination product as the key to the autologous market. Through control of a mobile manufacturing process with a disposable single use “pharma manufacturing in a box,” every parameter of the cellular treatment is controlled in the operation theater and/or catheterization lab. We believe the direct result of a rapid intra-operative process is a marked improvement in cellular safety and a reduction in the loss of potency and thus speaks to the fundamental premise that using our technology with autologous cells equals clinical efficacy.
The chart below (Autologous Clinical Technology) summarizes the salient features of the proprietary bedside manufacturing process (in a kit) in clinical trials by TotipotentRX. In brief, each specific clinical indication is independently reviewed, studied, engineered, and tested with three main goals: (i) to ensure cell safety, viability, potency for each appropriate application by our clinical research and development team; (ii) to ensure rapid, reproducible (user independent) cell delivery addressing the constraints faced by surgeons conducting surgical treatments on a day to day basis; and (iii) to ensure patient safety. TotipotentRX believes it is essential to provide assurance of potent cellular product and equally essential to provide a process which can be conducted by surgeons and healthcare professionals with ease, without the risk of operator error, and in a rapid cost effective manner. Through many years of direct physician and patient exposure we have distilled the elements of our mobile bedside manufacturing technology to the precise therapy specific needs in a easy to use disposable “kit”, ancillary equipment system, and indication specific protocol; each configured and customized to one of our therapeutic candidates. It is expected this technology system and therapeutic biological will follow the U.S. FDA PMA “combination product” process in the U.S., and be available for use in any operating room (O.R.) for on-label treatments with minimal user training.
3
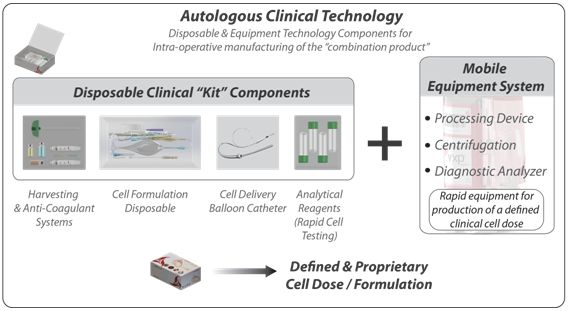
The disposable clinical kit, specific to each clinical indication, is designed to provide a customized pre-market approved solution for each of our clinical applications. The purpose of the kit is to provide a highly controlled, regulated and fully integrated solution to surgeons eliminating any perceived need for additional uncontrolled products/processes during the treatment protocol. TotipotentRX believes that this complete end-to-end solution is a necessity for both regulatory approval and surgeon adoption. Not only does this approach address quality control deviations and directly address the FDA’s requirement for combination product quality, but it also provides an ease of use to the physician user by eliminating the need for pre-surgery preparations such as disposable supply chain and/or cold chain cell management. Each disposable kit contains the four major component categories, which work in concert with the mobile equipment system and in conjunction with the proprietary protocol:
Autologous Clinical Technology Kit Component Categories:
1. Cell Harvest and Anti-coagulation System
2. Cell Processing and Formulation System
3. Cell Diagnostic System
4. Cell Delivery System
TotipotentRX Proprietary Solution – The Combination Product
Component 1: In brief, depending on the source and quantity of the cellular material (i.e. bone marrow, peripheral blood, and cord blood), an integrated cell collection system is provided, which is fully validated to the precise cell collection scenario experienced by the physician at the point-of-care. Using the example of bone marrow source material, TotipotentRX has optimized a proprietary formulation of cell and patient-tested anti-coagulant (an FDA approved polypeptide) designed to minimize adverse effects on the stem cells. To provide further detail on the unique specification of this, TotipotentRX can provide an overview of the features critical to its anti-coagulant system (a chemical added to bone marrow aspirate to prevent the formation of microthrombae) without inhibiting the chemokine receptor 4 (CXCR4)/stromal cell-derived factor-1 (SDF-1) axis, which plays a crucial role in homing to and engraftment of progenitor cells (1, 2). The importance of the CXCR4/SDF-1 axis is unambiguous in the arena of regenerative medicine, and chemicals that interfere with this mechanism impact the overall efficacy of the infused cellular product. Heparins are the most commonly employed anticoagulants for bone marrow aspiration, and are reported to disrupt the pivotal CXCR4/SDF-1 axis (3). The heparin-treated bone marrow cells become unresponsive due to inhibition of the CXCR4 receptor internalization that further blocks CXCR4 downstream signaling. In response to this data, TotipotentRX has created a proprietary formulation designed to avoid this cell inactivation mechanism; a major differentiator to our platform.
4
Component 2: TotipotentRX’s cell processing and formulation system is designed in exclusive collaboration with ThermoGenesis to integrate “smart” and time-tested medical technology into one of the most critical steps of TotipotentRX’s bedside cell manufacturing system. This automated computer-aided technology enables a “hands-free” user-independent infrared cellular selection system that defines a unique cell dose in less than 30 minutes. TotipotentRX refers to this platform as the VXP™ technology as is based on an evolution of the ThermoGenesis AXP™ System, a technology which has been used in processing over 600,000 cellular samples and several thousand human transplants over the past 10 years. The AXP’s quality record ensures that one of the cornerstones of our process will operate to a clinical commercial standard and is scalable.
Component 3: In advance of the cell delivery, the importance of cellular diagnostics has, until now, not been adequately addressed at the point-of-care in both the autologous and allogeneic cell therapy arena. The FDA has underlined this gap in various communications where “No lot of any licensed product shall be released by the manufacturer prior to the completion of tests for conformity with standards applicable to such product.” (21 CFR 610.1), which include tests for potency, sterility, purity, and identity (21 CFR Part 610 B). These requirements apply to all biological products, including autologous and single patient allogeneic products, where a lot may be defined as a single dose (U.S. FDA Guidance for Industry. Potency Tests for Cellular and Gene Therapy Product CBER, Jan. 2011). TotipotentRX addresses this requirement through a mobile cell diagnostic platform designed to specifically and rapidly address pre-transplant cell diagnostics. TotipotentRX’s system, which we anticipate to be part of the combination product, a rapid feedback system provides a clear differentiator that ensures a traceable record of defined cellular doses specific to the clinical application, and in the event of cellular insufficiency, and immediate notification to the surgical team to harvest additional cells thus ensuring each patient is treated with an effective dose.
Component 4: The cell delivery system is a cell friendly system designed to ensure cells being injected/transplanted into the patient are unchanged by the physics and chemistry of and throughout the delivery device’s lumen and surface coating, including control of the cell product physical inputs, and the location of the target delivery is a highly refined, controlled and measurable process. Each step is created to minimize potential harm to the cellular product during delivery to the patient’s target organ of interest and can survive to initiate therapeutic benefit. Though in retrospect TotipotentRX considers this principle of cell-based treatments obvious, many examples of competitive poor delivery dynamics have likely been the cause of poor study results and as a result, TotipotentRX has engaged leading medical technology partners to collaborate with TotipotentRX’s scientific and clinical team on the customized design and integration of cell delivery devices into the TotipotentRX cell manufacturing kit. Taken together, the Cell Therapy Combination Product Kit (Disposable, Equipment, Diagnostics and Protocol) is a controlled cellular production process under the control of the physician as represented by an example from the TotipotentRX AMIRST study procedure below.
5

References in this section:
| (1) | Takahashi M. (2011) Role of the SDF-1/CXCR4 system in myocardial infarction. Circ. J., 74, 418 |
| (2) | Prokoph S, Chavakis E, Levental KR et al. (2012) Sus- tained delivery of SDF-1a from heparin-based hydrogels to attract circulating pro-angiogenic cells, Biomaterials, 33, 4792 |
| (3) | Seeger FH, Rasper T, Fischer A, Reinholz MM, Hergenrei- der, E, Dimmeler S et al. (2012) Heparin disrupts the CXCR4/SDF-1 axis and impairs the functional capacity of bone marrow-derived mononuclear cells used for cardio- vascular repair, Circ Res., 111, 854 |
Therapy Candidates:
TotipotentRX's lead therapeutic technology core combination product platform just outlined is TotiCell™ an intraoperative rapid system for harvesting, preparing, testing, and delivering a therapeutic dose of autologous bone marrow derived or peripheral blood derived cells and proteins. TotipotentRX’s integrated treatment kits (unique to each indication) are designed to comply with the FDA's Combination Product definition and as a result combine a proprietary, effective and safe cell formulation specific to the disease indication with all the required medical devices to harvest, process, quality control and deliver the therapeutic cell dose at the patient's bedside in 60-90 minutes.
The TotiCell platform is currently in various stages of Pilot and Phase 1b trials as a potential treatment for vascular and orthopedic indications.
| · | To date TotipotentRX has completed 10 pilot or phase 1b clinical trials, having a net non-dilutive market “cost equivalent” value exceeding $17M. |
| · | Approximately 600 patients have been treated to date using the TotiCell approach. |
The therapeutic candidates of primary focus by TotipotentRX include advancing the development in acute myocardial infarction, critical limb ischemia and orthopedic regeneration spinal fusion and avascular necrosis. TotipotentRX anticipates receiving regulatory clinical trial approval for advancing to the next stage of clinical trials in each. Initial safety pilot or Phase 1b (safety & efficacy) studies have been executed in several additional indications (see Therapy Pipeline Chart below), and TotipotentRX anticipates pursuing partnerships in the other indications prior to advancing into Phase II.
6
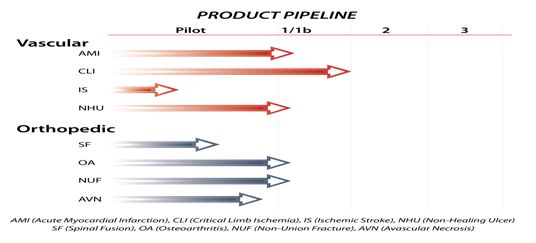
Key Target Indications
The company will initially focus on the following three indications:
| 1) | Acute Myocardial Infarction (AMI): |
AMI is a rapid development of myocardial necrosis due to a critical imbalance between oxygen supply and demand to the myocardium (heart muscle) often caused by the occlusion of a coronary artery – commonly known as a heart attack. Certain patients who suffer an AMI may experience a Left Ventricular Ejection Fraction below 40.0%, and therefore often proceed to suffer from near and medium term ischemic cardiomyopathy, a process leading to chronic heart failure and a high morbidity and mortality rate. This progression of disease and the management of it is extremely expensive to society, health care payers, and government. Our treatment intends to limit the deterioration of the patient towards heart failure, and thus having a significant positive benefit to the patient (quality of life and economic contribution) and healthcare payer system (economic contribution).
Cardiovascular disease (CVD) is the number one cause of morbidity and mortality worldwide. An estimated 17.3 million people died from CVDs in 2008, representing 30.0% of all global deaths. Of these deaths, an estimated 7.3 million were due to coronary heart disease. TotipotentRX’s treatment focuses on patients who have low ejection fractions (<40.0%) three to ten days post AMI, and have a predictably high mortality rate one to five years post infarct. TotipotentRX estimates the resulting addressable market to be a more than 700,000 patients per annum worldwide with 90,000 to 100,000 patients in the U.S. alone.
Over the past decade, the use of regenerative medicine methodologies for cardiovascular disease, and specifically bone marrow derived progenitor cell therapy for AMI has been tested in more than 35 Phase I and Phase II clinical studies demonstrating overall safety and measurable clinical benefit 12-61 months post treatment as evaluated by improvement of the Left Ventricular Ejection Fraction (LVEF) post AMI. Delewi, et al, European Heart Journal, Sept 2013, reported in a meta-analysis study the impact of intracoronary bone marrow cells showed a positive 2.55% LVEF improvement in six months or less with a 95.0% confidence interval in 1641 patients comprising 16 randomized controlled trials.
In 2010, TotipotentRX began developing the TotiCell program with the intent of targeting the low LVEF post AMI indication. This program was named AMIRST (Acute Myocardial Infarction Rapid-Delivery of Stem cell Therapy) and the estimated time to market is 7 years from the commencement of the Phase 1 double blinded randomized controlled clinical study.
7
In September 2013, TotipotentRX completed a 24 month case study follow-up on a 43 year old, non-diabetic, non-obese, smoker male who presented with symptoms of AMI. On admission, the AMI was confirmed with electrocardiogram ST elevation and biochemical tests. The patient was normo-tensive with no family history of ischemic heart disease. The patient presented with two hours of chest pain with a 2 mm ST segment elevation in the anterior leads. The patient’s Left Ventricular Ejection Fraction, LVEF, was estimated to be around 35.0 % by bedside 2D ECHO. Primary PCA was performed using a routine technique, and a single drug-eluting stent was deployed in the proximal LAD with TIMI-3 grade flow results. Post- procedure, the patient’s LVEF remained < 40.0% at the 120 hour point as measured by MuGA and ECHO, which met our inclusion criteria and is predictive of a higher than acceptable one year mortality rate. The patient was advised that he met the inclusion criteria for a clinical trial program using his own (autologous) bone marrow concentrated progenitor stem cells. The clinical trial is registered with clinicaltrials.gov (NCT01536106) and is approved by the Institutional Ethics Committee (IEC) (IEC Approval # TIEC/2011/32/02). The patient, Primary Investigator and Clinical Investigator concurred, and consent was obtained. On the 6th day post PTCA/stent implant, the patient was transferred to the heart catheterization laboratory, and the AMIRST protocol was completed. The entire procedure was completed within 90 minutes. As a safety study, the patient was followed up for 24 months and evaluated with standard diagnostic metrics. No serious adverse event or re-hospitalization event was reported, demonstrating the safety of this adjuvant treatment. The patient’s LVEF improved from 35.0% at the time of the AMIRST treatment to 60.3% on the 24 month final exam.
TotipotentRX has designed a 30 patient Phase Ib (Safety and Preliminary Efficacy), Randomized Double-Blind, Placebo-Controlled, multi-center study to be conducted at Fortis Escorts Hospital (New Delhi), Fortis Flt. Lt. Rajan Dhall Hospital (New Delhi), and Care Hospital (Hyderabad). This study is scheduled to commence in the fourth quarter of calendar 2014 and TotipotentRX anticipates completion within 18 months.
The protocol in brief:
| · | Bone marrow is aspirated in all the patients and they may or may not receive the bone marrow for their treatment depending on a blinded random selection process. |
| · | The aspirated bone marrow enters the TotiCell process – controlled collection, processing, and delivery at a recorded dosage and within 60-90 minutes to each patient selected for the treatment arm. Patients not selected for immediate treatment (placebo arm) will have their cell dose cryopreserved for a potential cross-over study. |
| · | The manufactured cells are infused through a specialized catheter into the infarct-related artery in the same operative procedure less than 10 days following an AMI), which TotipotentRX believes is the optimum time for cellular intervention immediately following the pro-inflammatory reaction of the body to the ischemic injury. |
| · | Infusate cells migrate / home to areas of cardiac need in response to controlled ischemic events. |
The objective of this Phase 1b is to:
| · | Evaluate the safety of intracoronary infusion of our unique composition of autologous bone marrow mononuclear cells utilizing the TotiCell proprietary process for the treatment of patients with acute myocardial infarction (AMI). |
| · | To measure changes in ventricular hemodynamic, infarct size, viable myocardium and cardiac remodeling following intracoronary of the TotiCell Cellular product. |
| · | Measure the rehospitalization rate specific to understanding the anticipated positive economics for supporting reimbursement. |
8
| 2) | Critical Limb Ischemia (CLI): |
Inadequate blood flow to the limbs leading to chronic ischemic rest pain, ulcers, or gangrene of the feet, legs or both. CLI is a serious form of peripheral artery disease (PAD) also often referred to as peripheral vascular disease (PVD), which is caused by atherosclerosis, the hardening and narrowing of the arteries over time due to the buildup of fatty deposits called plaque.
Prevalence of CLI has been increasing in recent years, affecting several million people across the globe. PAD has been estimated to occur in 10.0-25.0% percent of adults older than 55. The standard of therapy for severe limb-threatening ischemia is either surgical or endovascular revascularization that aims to improve the blood flow to the affected extremity. In the absence of revascularization options (up to 40.0% of CLI patients are not candidates for revascularization) or the subsequent failure, of such surgical inventions, such patients with CLI will require amputation within 6 months. Patients requiring major amputation face a diminished quality of life, an unfavorable life expectancy and extensive resources for their post-amputation rehabilitation and course. The 1-year amputation-free survival rate for patients diagnosed with CLI is 45.0%; the mortality rate is approximately 25.0% and may be as high as 45.0% in those who have undergone amputation. Management of this end-stage disease process consumes a significant amount of healthcare resources.
TotipotentRX estimates the resulting number of “no-option” CLI patients to exceed 250,000 in the U.S., a large addressable market.
Bone marrow (BM)-derived stem and progenitor cells have been identified as a potential new therapeutic option to induce therapeutic angiogenesis, and have demonstrated positive outcomes in both pre-clinical and early clinical models. This approach aims at improving the vascularization of the ischemic leg so that perfusion improves sufficiently for wound healing to occur, and at resolving resting pain ultimately allowing limb salvage.
In 2011, in collaboration with ThermoGenesis, TotipotentRX began a 17 patient Phase Ib (Safety and Preliminary Efficacy), single-center open label study at Fortis Escorts Hospital (New Delhi). The purpose was to demonstrate the safety and efficacy of TotiCell manufactured bone marrow mononuclear cells injected into ischemic tissue of patients with non-reconstructable critical limb ischemia. The one-year follow-up of this study was concluded in August of 2013 and the results were published in January 2014. In advance of publishing the final results TotipotentRX provided the market with interim updates which showed that TotipotentRX achieved the primary endpoint of safety after 12 months and the patients had retained their leg and showed measurable improvement in blood flow and pain scores. The final published results of the feasibility/Phase Ib were:
| • | Major limb Amputation free survival rates - 82.4% |
| • | Pain reduction - mean VAS score pre-therapy 7.8 ± 0.97 and 12 month follow-up 0.2 ± 0.58 on a scale of 0-10, p=0.0005 |
| • | 6-minute walking distance - mean distance pre-therapy of 14.5 meters ± 37.57 and 12 month follow-up of 157 meters ± 100.92, p=0.0039 |
| • | Open wound healing - 11 patients had gangrene with or without ulceration pre-treatment and all patients had neither gangrene nor ulceration at 12 month follow-up |
| • | Vasculogenesis in the treated leg - both collateral vessel numbers improved, p=0.0156 in distal thigh, p=0.0313 in proximal leg, and vessel size improvements in the distal thigh, p=0.0156 and proximal leg, p=0.0625, and TcPO2 levels (mean pre-therapy of 14.66 ± 6.93 improved to 35.75 ± 17.04, p=0.0032) |
9
This study also served as the proof of principle for our acute myocardial infarction therapy, demonstrating that vasculogenesis was possible in the human subject using TotipotentRX’s combination product.
This program name has been updated to CLIRST (Critical Limb Ischemia Rapid-delivery of Stem cell Therapy) and we will initiate our application of the pivotal study to commence the next phase of TotipotentRX’s clinical data collection in the U.S., European Union and India.
The Protocol in brief:
| · | Bone marrow is aspirated in all the patients (all subjects treated) in the heart catheterization lab. TotipotentRX only considered “no option” patients for inclusion in response to the requirements of the institutional ethics committee overseeing this study. |
| · | The aspirated bone marrow enters the TotiCell process – controlled collection, processing, and delivery at a recorded dosage and within 60-90 minutes to each subject selected for the treatment arm. |
| · | The bedside manufactured cells are injected intra-muscularly into the affected limb in accordance with a grid-based strategy to ensure appropriate cell distribution. |
| · | Cells migrate / home to areas of vascular need in response to ongoing ischemia in the affected limb. |
| 3) | Orthopedic Repair: |
TotipotentRX has two lead candidates in the orthopedics space: (1) Degenerative Disc Disease and (2) Avascular Necrosis.
Degenerative disc disease refers to a condition in which pain is caused from a damaged spine disc. A wide range of symptoms and severity is associated with this condition, and in more than 400,000 patients per year in the U.S. leads to surgical removal of the diseased disc. Once the disc (vertebral cushion) is removed between two discs, the effected vertebrae are “fused” both mechanically and biologically. The biological fusion is typically completed by inserting a bone scaffold between the vertebrae, and seeding the scaffold with biologicals (proteins, growth factors and/or stem cells) to induce growth of a solid supportive boney fusion. Over the past decade the use of recombinant human bone morphogenic protein (BMP) has been used to supercharge the biological growth of the bone implant. However, recent studies have raised significant safety concerns about the use of this growth factor, and most recently Lad et al (Neurology 2013) reported that the use of rhBMP increases the risk of non-malignant tumors by more than 30.0%. The current rhBMP U.S. market is believed to exceed $700 million. In a recent survey of orthopedic physicians asked what they would be replacing BMP should the U.S. FDA take steps to restrict the on-label allowances of rhBMP, more than 31.0% said they would use autologous bone graft with stem cells.
TotipotentRX believes the addressable U.S. market is large, with more than 120,000 procedures per year.
This procedure involves the use of autologous bone marrow stem cells, and as it is a minimally manipulated homologous use of the patients own cells TotipotentRX, upon consultation with the U.S. FDA, anticipates in will seek CBER approval to complete a Phase II/III Investigational Device Exemption (IDE) clinical trial. If this path is approved, the potential time to market will be considerably shorter than our other product indications.
10
This program is called the Vertebrae Fusion Rapid Implant Stem cell Therapy (VFIRST).
Avascular necrosis (AVN) is a disease resulting from the temporary or permanent loss of blood supply to the bones. Without blood, the bone tissue dies, and ultimately the bone may collapse. If the process involves the bones near a joint (typically the hip), it often leads to collapse of the joint surface. AVN is also known as osteonecrosis, aseptic necrosis, and ischemic necrosis.
| · | The incidence rate of AVN is 1 in 27,200 U.S. adults (approx. 10,000 – 20,000 new cases per year) |
| · | There are five (5) grades of disease development: |
Grade I (least severe): painful but difficult to diagnose on MRI
Grade II: still asymptomatic but recognizable in MRI
Grade III: painful and flattening of femoral head
Grade IV: increased pain and collapse of necrotic segment
Grade V: cystic changes are seen
We have completed a pilot study (investigator initiated) of 15 subjects having Grade II and II/III AVN of the femoral head. Our procedure involves the use of autologous bone marrow derived enriched progenitor cells, a allogeneic bone graft in combination with autologous (patient’s own) biologics to enhance cell survival. The company has filed a patent on both the methods and on a device for calculating the allograft to cell volume ratio. The company is considering options for seeking fast track status of its AVN therapy in pediatric patients.
In addition to our initial pursuit of the above four indications, we have the following in-human clinical initiatives also underway:
|
Other Target Diseases and Treatments
|
||
|
Indication
|
|
Status
|
|
Osteoarthritis
|
|
Pilot Phase
|
|
Non-Union Fracture
|
|
Pilot Complete, Under Review
|
|
Chronic Dermal Wounds
|
|
Pilot Phase
|
|
Ischemic Brain Injury
|
|
Under Review
|
Contract Clinical Trial Services
TotipotentRX has a leading cell therapy CRO team of clinical scientists, physicians, and graduate level scientific associates. This program emerged as a necessity for conducting cell-based clinical trials as few CRO’s understand the complexities of conducting autologous cellular therapy programs. TotipotentRX combines its expertise in intra-operative medical management, cellular manufacturing, device validation and regulatory affairs to provide complete and seamless cellular drug and device clinical services. This service is now being marketed to regenerative medicine biotech and academic centers seeking high quality, rapid enrollment, and lower cost clinical trial studies.
TotipotentRX’s full service cellular biological and medical device services include clinical study design, cost effective contract Phase I-II clinical trials, regulatory consultation, physician/surgeon training and support for cellular product potency validation, handling and delivery of the cellular therapy, and fully validated laboratory services for cellular bioanalytics. The services offered ensure patient safety under Good Clinical Practices (GCP), quality laboratory documentation under Good Laboratory Practices (GLP), and quality cell processing and handling under both Good Manufacturing Practices (GMP) and Good Tissue Practices (GTP).
11
TotipotentRX's scientific and clinical team's comprehension of the complex issues of cellular therapy trials translates into earlier identification of potential problems, hands on decision making, patient and care giver support, physician training and support all leading to superior results. We believe TotipotentRX’s experience in cellular therapy allows us to work smarter, minimize cost, and maximize time efficiencies for TotipotentRX's client trials.
Employee Expertise includes:
| · | Project Management |
| · | Clinical Research Associates for Clinical Support & Monitoring |
| · | Medical Monitors and Physician Oversight |
| · | Quality Assurance teams |
| · | Regulatory Specialists |
TotipotentRX’s exclusive partnership with Fortis Healthcare has also lead to unique marketing opportunities where the CRO offering has been strategically planned in cooperation and co-marketing programs are underway. The first such co-marketing program was launched at the 2012 International Stem Cell Society Meeting in Seattle Washington. Leveraging Fortis’ experience and traditional clinical trial infrastructure, which includes more than 100 trials underway presently, allowing TotipotentRX to focus on the cell therapy unique requirements without additional overhead.
Cell Manufacturing and Banking
Over the past decade, the use of adult cell tissues such as bone marrow cells, cord blood cells and adipose cells have held great promise for treatments of debilitating diseases such as genetic abnormalities, cancer, cardiovascular disease, neurological injury, and secondary effects of adult onset diabetes. TotipotentRX operates advanced clinical cell manufacturing, processing, testing, and storage facilities compliant with GMP, GTP, and GLP and registered with the U.S. FDA and Indian Drug Control (DCGI). With our current infrastructure including the GLP laboratory, GMP development and GCP compliant clinical research teams we house an extremely broad set of capabilities available for internal development needs as well as to outside clients. In exploiting our world-class infrastructure within the Fortis Healthcare network, we provide cell banking (cryopreservation) and cell manufacturing services to both clinical trial clients and patient clients.
Cell Banking
TotipotentRX operates a premium private cord blood and tissue banking service within the Fortis Healthcare system. As a contractual component of TotipotentRX’s services to Fortis, the NovaCord™ brand was created and deployed at five Fortis Hospitals across New Delhi as well as other Fortis locations in the surrounding regions. The GMP facility is marketed through traditional and telesales teams to pregnant mothers planning to deliver their babies at Fortis Hospitals pan-India.
To support the cryogenic storage requirements of the cord blood industry as well as clinical trial clients, TotipotentRX operates a state-of-the-art cryopreservation facility fully equipped with semi-automated controlled rate freezing and stem cell sample long term storage containment dewars as well as a patient sample bank for quality control purposes. TotipotentRX has implemented robust standard operating procedures (SOPs), quality processes, and in-house diagnostic testing to ensure every sample has full traceability, and the facility is licensed by the state and national drug control departments and certified by the British Standards Institute for ISO 9001, GMP, GCP, and GLP.
12
Cell Manufacturing
TotipotentRX offers cGMP-compliant cell therapy manufacturing through its ISO Class 7 (Class 10,000) clean room infrastructure meeting U.S. and international requirements. We have experience with multiple platforms of closed-system processing and can adapt to the specific cell culturing strategy of our client. At request, we can also employ our medical device assets to provide customized disposable systems specific to the unique needs of an individual project.
A testament to TotipotentRX cell therapy service capabilities, in August 2013, in collaboration with the Fortis Memorial Research Institute, TotipotentRX announced the successful launch of its pediatric bone marrow transplant program at the Fortis-TotiRX Centre for Cellular Medicine in New Delhi, India. The new program achieved its first 100-day survival milestone following an allogeneic bone marrow engraftment in a pediatric patient with aplastic anemia. The successful transplant was performed through the use of our cell manufacturing and cryopreservation facilities and has paved the way for the expansion of the bone marrow transplant program at Fortis, a much needed service in the region.
Medical Device Development and Commercialization
TotipotentRX currently operates a medical device assembly and supply business at its Gurgaon (New Delhi N.C.R.) facility in India. This facility was designed to house the organizations sales and operations departments, which cater specifically to the design, assembly and supply of medical devices and kits to the regenerative medicine market, primarily private cord blood banks. This business segment can be divided into two distinct groups:
1. Device Manufacturing
TotipotentRX’s device assembly facility is ISO 13485 certified (British Standards Institute) and has manufacturing clean room infrastructure validated to ISO Class 7 to meet the requirements of European CE marking and U.S. FDA 510(k) for sterile disposable assembly and sterile liquid filling applications. This facility has the capability to assemble regulated product disposables and medical convenience kits. This flexibility allows a unique position to provide rapid, low cost, customized products to the regenerative medicine disposables market.
To support the sales and distribution department the operations group is comprised of the following specialized departments to ensure our customer base is supplied with high quality products:
| · | Finance & Accounts |
| · | Regulatory (product registrations, dossier creation and updates, facility registrations) |
| · | Quality Assurance (quality management, product occurrence tracking and vigilance systems) |
| · | Logistics & Supply Chain |
| · | Engineering (Product Design and advanced engineering support) |
| · | Production |
| · | Warehouse (controlled raw material and finished product storage) |
2. Medical Device Sales and Distribution
TotipotentRX markets a growing product line of consumables under the “TotipotentSC” brand. These products are currently sold to the South Asian and European cord blood bank markets for use in umbilical cord blood collection, processing, and cryopreservation as well as transport of umbilical cord tissue.
13
Cord Blood Banking disposables are either:
| · | Manufactured / assembled in-house |
| · | Sourced through long-term strategic private label agreements |
| · | Distributed in collaboration with specialized suppliers |
TotipotentRX primary customers are private (or public) cord blood banks, which provide an offering to pregnant parents to collect and store their cord blood. These products are sold for single use applications in the GMP laboratory of cord blood banks. Product sales are generally smooth as a business-to-business sale based on annual or multi-year purchase contracts with defined pricing and delivery periods.
Major Products
|
Description
|
Market
|
Distribution / TotiSC Brand
|
Regulatory Approval
|
Sales Territory
|
|
Collection Bag
|
Cord Blood
|
Distribution
|
CE, DCGI
|
Global (outside U.S.)
|
|
Manual Processing Set
|
Cord Blood
|
TotiSC Brand
|
CE, DCGI
|
Global (outside U.S. & EU)
|
|
Manual Processing Set
|
Cord Blood
|
Distribution
|
CE, U.S. FDA, DCGI
|
India and South Asia
|
|
Cord Blood Stem Cell Freezing Bags
|
Cord Blood
|
TotiSC Brand
|
CE, DCGI
|
Global (outside U.S. & EU)
|
|
Stem Cell Freezing Bags
|
Regen. Med.
|
TotiSC Brand
|
CE, DCGI
|
Global (outside U.S.)
|
|
Cryo Overwraps Bags
|
Cord Blood
|
TotiSC Brand
|
N/A
|
Global
|
|
Bone Marrow Processing
|
Regen Med.
|
Distribution
|
CE, U.S. FDA
|
India and South Asia
|
|
Cord Blood Collection Kits
|
Cord Blood
|
TotiSC Brand
|
Convenience Kit (not required)
|
Global
|
|
GMP Cell Expansion Reagents
|
Regen. Med.
|
Distribution
|
N/A
|
India
|
Government Regulations
The development, clinical trials, and marketing of our cell therapy products are subject to the laws and regulations of the U.S. (FDA), European Union (EMEA) and other countries including India as projected in TotipotentRX’s commercial sales strategy. TotipotentRX’s belief is that its clinical approach represents a moderately low regulatory and patient risk due to the following characteristics of its products:
| 1. | Autologous cell source |
| 2. | Cells are non-manipulated (or minimally manipulated) |
| 3. | Homologous (orthopedic indications) and Non-Homologous (vascular indications) |
14
In accordance with historical regulatory publications, rules, and decisions, we assess our regulatory risk to be lower than our competitors. TotipotentRX ranks the risk profile of its clinical candidates in order of highest to lowest risk as AMI, CLI and Spinal Fusion or Avascular Necrosis, respectively. Moreover, relative to TotipotentRX’s competition, it believes that it has a meaningful reduction in time to market where in spite of positive trial outcomes, these competitors’ allogeneic risks remain unknown and may require additional years (and comparatively larger patient populations) in the pivotal studies to conclusively demonstrate the absence of adverse or unpredicted results.
The trials TotipotentRX has conducted in India were all compliant with the applicable Indian Council for Medical Research, and Ministry of Health Order No. V.25011/375/2010-HR rules specific to oversight and rulemaking related to stem cell research and therapy in addition to requisite institutional ethics board and institutional stem cell committee approvals, and all future studies will be compliant with the newest guidelines issued by the Drugs Controller General (India) in the second half of 2013. Both the U.S. and E.U. regulatory agencies are experienced with accepting Indian clinical trial data(1)(2). The U.S. Food and Drug Administration issued a Final Rule in October 2008 revising §21 CFR 312.120(a) and further clarifying their position in a Guidance Document in March 2012, where they will accept as support for a U.S. Investigational New Drug (IND) or application for marketing approval a well-designed and well-conducted foreign clinical study not conducted under a U.S. IND if the study is conducted in accordance with Good Clinical Practices (GCP) and where the sponsor is able to validate the data from the study through an onsite inspection by FDA if necessary. GCP includes review and approval by an IEC before initiating a study, continuing review of an ongoing study by an IEC, and obtaining and documenting the freely given informed consent of the subject before initiating a study.
India has become a major region for conducting clinical studies of equivalent quality and regulatory stringency to the U.S.(3), with the advantage of recognizing a significant savings in clinical costs, accessing many more patients, and enrolling patients faster. Nevertheless, we understand the necessity to conduct multi-centered trials and aim, in all cases, to select sites in countries, which are on TotipotentRX’s commercialization roadmap. TotipotentRX anticipates doing all pilot and Phase I trials in our economically advantageous CRO in India, and then moving onto market/region specific multi-center trials in Phases 2 & 3.
| (1) | Fortis Escorts has completed two U.S. FDA approved cellular therapy CLI studies for competitors prior to initiating the TotipotentRX-ThermoGenesis study. |
| (2) | Foreign clinical trials conducted in India exceeded $2.5 billion. |
| (3) | Fortis Healthcare currently serves as a clinical trial facility for more than 100 U.S. or EMEA IND/IDE multi-site clinical trials per annum, demonstrating their proficiency and acceptable Good Clinical Practices as required by the U.S. FDA. |
TotipotentRX’s regulatory plan focuses on obtaining pre-market approval (PMA) from the FDA or the equivalent via the EMEA and national authorities in Europe as well as other national territories. Therefore, TotipotentRX has designed and anticipates that our studies comply with the guidelines of these regulatory authorities per the Combination Products as defined by the U.S. FDA.
Competition
The field of cell therapy development is competitive. There are a number of companies that are developing stem cell-based therapies for cardiovascular and/or orthopedic indications, including, but not limited to MesoBlast, Ltd., Osiris Therapeutics, Inc., Baxter International, Inc., Athersys, Ltd., Neostem, Inc., Aastrom Biosciences, Inc., Cytori Therapeutics, Inc., Cytomedix, Inc., Pluristem Therapeutics Inc., and Bioheart, Inc. These companies are utilizing a number of different cellular approaches in pursuit of commercially successful therapeutics. TotipotentRX’s competitors employ either autologous or allogeneic-based development programs with cells from three main sources: bone marrow derived cells, fat derived cells, and peripheral blood or cord blood/tissue cells.
15
To our knowledge, TotipotentRX is the only company providing point-of-care fully integrated autologous combination products.
TotipotentRX’s sees the following main advantages to its approach:
Autologous vs. Allogeneic. TotipotentRX employs an autologous cell source (donor and recipient are the same person) to all of its therapeutic candidates) therefore having an inherently lower safety risk profile with respect to transplantation related toxicity, cell durability, and the potential need for immunosuppressive drugs. Allogeneic sources (that is where the donor and recipient are different persons) face a series of technical limitations that we believe will likely impact their clinical value through both an increased risk of treatment success (therapeutic efficacy from a single cell source), potential immunogenicity and the risk of extensive (and expensive) reviews and potential delays by regulatory authorities for safety concerns.
TotiCell is Minimally Manipulated. The majority of TotipotentRX’s competitors employ one or more cell manipulation techniques in order to manufacture their final cell product. The listing of these techniques would be exhaustive, however, we can attempt to summarize these to the following: (i) exposure to chemical agents (i.e. for cell isolation/purification), (ii) cell expansion (ex vivo laboratory-based culturing to achieve necessary cell quantities), (iii) cryopreservation (maintenance of cell stock for “off the shelf” supply for patient treatment) and (iv) transport (between multiple laboratory-based devices and through the hands of many different human technicians at collection sites, within laboratory environments and when transporting between geographical locations). Of note, the U.S. National Institutes of Health “NIH” released a critical study in May 2011 showing expanded mesenchymal stem cells (MSCs) experience unacceptable genetic changes after 2 expansion cycles (Campasi et al, ISCT Annual Meeting, Rotterdam, 2011). To achieve a therapeutic dose, one typically needs to carryout 4-6 expansion cycles. Therefore, this “cell-in-a-bottle” concept appears to have considerably more risk than originally believed. Additionally, Galipeau et al published a landmark paper in 2012 demonstrating that cryopreserved MSCs display a loss of immunosuppression (their main benefit) compared to fresh MSC cells - thus, cryopreserved “cells in a bottle” may have challenges meeting their primary efficacy endpoints without some additional process steps immediately prior to injection to overcome the negative impact of the heat shock protein up regulation due to cryopreservation. These two stability concerns demonstrate challenges that allogeneic cells face in gaining regulatory approval in any conditions which are non-life threatening (no-option). TotipotentRX believes even under the most stringent controls these manipulation steps may have a functional impact on cells, a manifestation that could present itself early in the development path or later after extensive clinical trial investment. Our approach has been to eliminate as many variables and handling steps as possible in producing the cellular product through rapid (60min) intraoperative automation assisted bedside protocols. By providing fresh cells that have only been subjected to short-term ex vivo exposure TotipotentRX believes it maximizes the potency while minimizing the complexity (risk) of the cell treatment process.
Bone Marrow As A Cell Source. Bone marrow has long been studied and has repeatedly demonstrated its direct involvement in the regenerative process in humans and animals. Though initially associated only with hematopoiesis (creation and maintenance of the blood system), bone marrow has been shown not only to contain and produce vascular and bone progenitor cells (endothelial, osteoblasts, mesenchymal cells) but also actively release these cells from the bone marrow interior into the blood stream in response to acute injuries such as a cardiovascular ischemic event. This natural healing process provides a highly logical approach to exploit and to build a therapeutic platform as designed by TotipotentRX in the TotiCell technology.
16
Experienced Cell Therapy Development Company. TotipotentRX has been developing point of care cell therapies for over six years and as a result we believe our experience in testing and commercializing combination products (devices/biologic therapeutics) and translating protocols into a point of care environment is unmatched in this space. TotipotentRX believes it is likely the only company providing a front to back systemized process from collection of the cells to controlled delivery with time tested medical device technologies - all with extreme attention to the surgeon’s requirements and today’s evolving surgical environment.
TotipotentRX’s approach maximizes simplicity to avoid the inherent pitfalls of complex product systems; use the body’s own cells and cellular factors in a therapeutically viable dose without significant manipulation and delivers them to the required target in minutes versus days or weeks. With this approach TotipotentRX believes it is optimally positioned in the market to provide large patient populations access to safe, effective and economically curative treatments.
Collaborations
TotipotentRX applies great value to effective partnerships; both for clinical development and corporate commercial goals, often these are combined for the collective benefit of all stakeholders. A testament to this are ongoing collaborations with many orthopedic and vascular surgeons, and interventional cardiologists through TotipotentRX’s global reach. Though TotipotentRX cannot provide a complete list of its dedicated collaborators TotipotentRX can name those involved in projects that have graduated to the next level of clinical development:
(1) Prof. (Dr.) Sheila Kar, M.D. Dr. Kar is the past Chief of Cardiology at Cedars Sinai Medical Center in Los Angeles, and Assistant Clinical Professor of Medicine at the University of California, Los Angeles. Project Involvement: AMI Study.
(2) Dr. Ashok Seth, M.D. Dr. Seth is currently the Chairman and Head of Cardiology at Fortis Escorts in New Delhi. Dr. Seth has contributed extensively to the growth, development and scientific progress of Cardiology especially Interventional Cardiology in India and across the world. Project Involvement: AMI / CLI Studies.
(3) Dr. Harsha Hegde, M.B.B.S., M.S., Dr. Hegde, previously the Head of Orthopedics for the Fortis Hospital Group in New Delhi, and presently Director of Orthopedics at Nova Healthcare (a Goldman Sachs backed Indian hospital chain). Dr. Hedge is ranked as one of the top five spinal surgeons in Asia, and currently leads the orthopedic stem cell program with TotipotentRX. Project involvement: Spinal Fusion.
In response to TotipotentRX’s clinical translation and commercialization interests, in 2010, TotipotentRX signed a five (5) plus a one (1) year extension exclusive partnership with Fortis Healthcare Limited, the largest hospital and clinical conglomerate in Asia. In essence, TotipotentRX’s corporate partnership with Fortis provides it with access to the following unparalleled market advantages:
| 1. | World Class Facilities and Equipment |
| · | Daily exposure to the administrative and working structure within the healthcare network provides meaningful intelligence on product and service design in order to exceed the expectation of the clinical customers, patients, regulators and payer companies by providing solutions which speak to a completely optimized economically delivered cell treatment approach. |
17
| · | Fortis Healthcare also prides itself on staying at the forefront of medical technologies, where TotipotentRX is given access to the latest diagnostic and imaging systems, a necessity for high quality clinical data collection. |
| · | Fortis Healthcare is an accredited clinical research organization, with its centralized ethics oversight committee approved by the Indian Drugs Controller General. Additionally, its Escorts operations which houses the central ethics oversight committee holds accreditation from the Joint Commission International, the accrediting body for hospitals in the U.S. and abroad. Currently, Fortis participates in more than 100 international clinical trials. |
| 2. | Access to Patients and Internationally Trained Physicians |
| · | 72 Hospitals (6 countries), 10,000 inpatient beds comprising a hospital group twice the size as Kaiser Permanente, with access to economic, clinical, and epidemiological statistics from a database built on 15,000 patients entering Fortis’ facilities every day. |
| · | Diverse and numerous patient populations meeting our study inclusion requirements thus reducing trial related risks particularly with patient selection and adequate enrollment within aggressive timelines. |
| · | The Fortis Hospital brand attracts world class internationally trained and recognized physicians, a critical component of our clinical strategy. |
| 3. | Hospital Embedded Contract Research Organization. |
| · | TotipotentRX’s in-house CRO, benefits from the facilities, technology, patient and physician access in this exclusive relationship with Fortis Healthcare. The combined benefit of this unique advantage is highly controlled studies produced more quickly and economically than TotipotentRX’s competitors. |
In addition to physician and hospital-focused partnerships, TotipotentRX has also invested heavily into the essential devices integral to our combination products. Though several of these partnerships remain confidential they are built upon gaining the unique positioning for the following:
| 1. | The requirement for unique, proprietary and portable device technology critical to the production of rapid cellular transplants. Our mobile manufacturing requires unique instruments, disposable devices and reagents from collection through patient delivery. |
| 2. | The requirement for rapid commercialization (therapeutic market access). TotipotentRX recognizes the regulatory, market penetration and competitive challenges in the industry and has taken definitive steps which mitigate our risks by utilizing partnerships having particular expertise in their technology area. |
Research and Development
TotipotentRX’s current technology has been in development since 2009 through low cost investigator-initiated, or TotipotentRX sponsored, or co-sponsored studies with corporate partners such as ThermoGenesis. By leveraging these development models we have achieved our clinical data generation goals by conducting trials in lower cost world class facilities with a direct investment exceeding $2 million. We estimated this approach has saved our company and shareholders an estimated $12 million to date (due to our in-house CRO and the intelligent use of our foreign facilities and partnerships) as compared to a similar clinical investment funded in the U.S. During this period, having completed ten pilot/phase 1 studies, TotipotentRX has selected 3 indications for immediate investment including AMI, CLI, and Bone Regeneration in either spinal fusion or avascular necrosis as previously detailed in the therapeutic candidates section above. Upon commencement of the next round of clinical studies, TotipotentRX will be assessing its capacity to expand beyond its current targets by considering Phase 1b trials in ischemic stroke, non-healing dermal ulcers and osteoarthritis.
18
Infrastructure, Licenses & Certifications
Collectively, TotipotentRX currently operates four global locations, three in India and one in Los Angeles, California. In India, TotipotentRX has designed and built two specialized facilities specifically catering to the needs of the cell therapy market and a third facility housing administrative activities for the Asia region. In total, the facilities in India cover approximately 12,000 square feet of space.
TotipotentRX Centre for Cellular Medicine (Fortis Collaboration) is located within the newly opened Fortis Memorial Research Institute (FMRI), which is the flagship hospital of Fortis Healthcare, a group comprised of more than 72 primary and tertiary care facilities. TotipotentRX is located within the Oncology wing of FMRI and enjoys exclusive rights in providing all cellular therapies/services through this collaboration. TotipotentRX’s unique facility (details below) was designed to maximize our ability to advance our stem cell clinical development goals as well as provide immediate commercial services to the Fortis Healthcare network.
Cellular Medicine Facility
Location: Gurgaon (New Delhi N.C.R.), India inside FMRI
Major Certifications/Registrations: U.S. FDA Registered; GLP/GMP/GCP and ISO 9001 Certified from the British Standards Institute (BSI); Cord Blood Banking License from the Drug Controller of India
| 1. | Class 10,000 Cell Manufacturing & Cell Expansion Suites |
| 2. | Dedicated Cord Blood & Tissue Banking Suites |
| 3. | Clinical Diagnostic, Research Analytical Instrumentation, and Cryopreservation Facilities |
| 4. | Contract Research Organization |
| 5. | Research & Development Laboratory |
Located within 5 miles of our Cellular Medicine Facility TotipotentRX leases a property custom designed by TotipotentRX to house capabilities for proprietary medical device design and GMP production. Understanding the unique needs of the regenerative medicine market, we believe that the ability to create customized kits and device solutions “in-house” is a necessity to support our internal clinical goals as well as support a growing market.
Device Manufacturing Facility
Location: Gurgaon (New Delhi N.C.R.), India
Major Certifications: ISO 13485 (BSI)
| 1. | Class 10,000 Clean Rooms and requisite functional facilities for quality, regulatory, manufacturing, logistics etc. |
| 2. | Sterile Product Capabilities: Non-Drug Liquid Filling and Medical Device Assembly/Tailing |
| 3. | Non-Sterile Capabilities: Medical Convenience Kit production |
Intellectual Property
TotipotentRX believes that patent protection is important for products and methods to maintain a proprietary position. In the U.S., TotipotentRX currently has four provisional patents pending (submitted to the USPTO) and two additional methods (in patent drafting mode) to protect the methods and or designs of products, which TotipotentRX intends to market. In addition, TotipotentRX has service marks for certain products and services.
19
Intellectual Property Summary (Patent and Publication List)
| 1. | Sanghi, V, et al. (2013) Clinical Case Report: Safety Study of Autologous Bone Marrow Concentrate Enriched in Progenitor Cells (BMCEPC) as an Adjuvant in the Treatment of Acute Myocardial Infarction (Manuscript under preparation) |
| 2. | Bukhari S, et al. (2013) Safety and Efficacy of Autologous Bone Marrow Mononuclear Cells in Patients With Severe Critical Limb Ischemia (Manuscript under preparation) |
| 3. | Ponemone V, et al. (2012) Intrathecal administration of autologous bone marrow cells with 10.0% hematocrit – RBCs are clinically safe, Annual ISCT Meeting, Poster 116, Seattle, WA |
| 4. | Ponemone V, et al. (2012) Autologous bone marrow derived stem cell graft facilitates remodeling of non union fractures, Annual ISCT Meeting, Poster 191, Seattle, W A |
Pending Patent Applications
| 1. | PCT/US2014/010745 – Rapid Infusion of Autologous Bone Marrow Derived Stem Cells |
Employees
As of November 30, 2013, TotipotentRX had 46 employees, 14 of whom were engaged in manufacturing, providing cell-based services, procurement and quality control, 9 in research and new product development, and 23 in sales, marketing and administration. None of its employees are represented by a collective bargaining agreement, nor has it experienced any work stoppage.
Facilities
Collectively, TotipotentRX currently operates four global locations, three in India and one in Los Angeles, California. In India, TotipotentRX has designed and built two specialized facilities specifically catering to the needs of the cell therapy market and a third facility housing administrative activities for the Asia region. In total, the facilities in India cover approximately 12,000 square feet of space.
Legal Proceedings
TotipotentRX is not a party to nor is any of its property subject to any pending legal proceedings. In the normal course of operations, TotipotentRX may have disagreements or disputes with employees, vendors or customers. These disputes are seen by TotipotentRX’s management as a normal part of business, and there are no currently pending actions or threatened actions that management believes would have a significant material impact on TotipotentRX’s financial position.
Market for TotipotentRX’s Common Stock and Dividend Matters
TotipotentRX’s common stock is not traded on any exchange or quoted on any automated quotation system. As of February 18, 2014, there were approximately 13 stockholders of record. TotipotentRX has not paid cash dividends on its common stock.
20
TotipotentRX Management’s Discussion And Analysis Of Financial Condition And Results Of Operations
CERTAIN STATEMENTS CONTAINED IN THIS SECTION AND OTHER PARTS OF THIS DOCUMENT WHICH ARE NOT HISTORICAL FACTS ARE FORWARD-LOOKING STATEMENTS AND ARE SUBJECT TO CERTAIN RISKS AND UNCERTAINTIES. TOTIPOTENTRX’S ACTUAL RESULTS MAY DIFFER SIGNIFICANTLY FROM THOSE RESULTS.
The following discussion should be read in conjunction with TotipotentRX’s consolidated financial statements.
Overview
TotipotentRX Corporation is engaged in the research, development, and commercialization of cell-based services, therapeutics and devices for use in regenerative medicine. TotipotentRX is focused on four companion competencies to serve patients, physicians and partners: Therapeutic, Contract Clinical Trial Services, Cell Manufacturing and Banking and Medical Device Development and Commercialization. TotipotentRX sells medical devices and equipment for collection, transportation, and processing of cord blood, cord tissue, bone marrow, and peripheral blood; reagents for culturing and assaying stem cells; and services to hospital and surgeons for processing autologous cellular therapies at the point of care.
On July 2, 2013, TotipotentRX merged with and into MK Alliance which existed as the surviving company and subsequently changed its name to TotipotentRX. Prior to the merger, TotipotentRX was a 77.0% owned subsidiary of MK Alliance.
Research and Development Activities
Over the last five years TotipotentRX has invested approximately $2,000,000 in research and development activities associated with conducting clinical trials and introducing new products in the regenerative medicine market. In addition to research and development costs of $197,000 disclosed in Footnote 1 of the Financial Statements of TotipotentRX, TotipotentRX also incurred other costs associated with conducting clinical trials including capital expenditures and executive time to design and manage clinical trials.
Critical Accounting Policies
TotipotentRX’s discussion and analysis of its financial condition and results of operations are based upon TotipotentRX’s consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these consolidated financial statements requires TotipotentRX to make estimates and judgments that affect reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. TotipotentRX bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
TotipotentRX believes the following critical accounting policies affect its more significant judgments and estimates used in the preparation of its consolidated financial statements.
Revenue Recognition
Revenues from the sale of TotipotentRX’s products and services are recognized when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. Amounts billed in excess of revenue recognized are recorded as deferred revenue on the balance sheet.
21
Revenue arrangements with multiple delivered are divided into units of accounting if certain criteria are met, including whether the delivered item(s) has value to the customer on a stand-alone basis. Revenue for each unit of accounting is recognized as the unit of accounting is delivered. Arrangement consideration is allocated to each unit of accounting based upon the relative fair value determined from third-party evidence of the separate units of accounting. TotipotentRX accounts for collection, processing and testing of the umbilical cord blood and the storage as separate units of accounting. Revenue generated from storage contracts is deferred and recorded ratably over the life of the agreement, up to 21 years.
Inventory Reserves
TotipotentRX states inventories at lower of cost or market value determined on a first-in, first-out basis. TotipotentRX provides inventory allowances when conditions indicate that the selling price could be less than cost due to physical deterioration, obsolescence, changes in price levels, or other causes, which it includes as a component of cost of revenues. Additionally, TotipotentRX provides reserves for excess and slow-moving inventory on hand that are not expected to be sold to reduce the carrying amount of slow-moving inventory to its estimated net realizable value. The reserves are based upon estimates about future demand from our customers and distributors and market conditions. Because some of TotipotentRX products are highly dependent on current customer use and validation, and completion of regulatory and field trials, there is a risk that we will forecast incorrectly and purchase or produce excess inventories. As a result, actual demand may differ from forecasts and TotipotentRX may be required to record additional inventory reserves that could adversely impact the gross margins. Conversely, favorable changes in demand could result in higher gross margins when products previously reserved are sold.
Shipping and handling fees billed to customers are included in net revenues, while the related costs are included in cost of goods sold.
Allowance for Doubtful Accounts
TotipotentRX evaluates the collectability of accounts receivable and determines the appropriate reserve for doubtful accounts based on a combination of factors. TotipotentRX writes off uncollectible accounts receivable based upon specific criteria, including when internal and/or external efforts to collect the amounts are unsuccessful, whether a customer has accounts past due over a specified period of time, and whether a customer has filed for or has been placed in bankruptcy. Allowances are recorded for all other receivables based on analysis of historical trends of write-offs and recoveries. In addition, in circumstances where we are aware of a specific customer’s inability to meet its financial obligation, a specific allowance for doubtful accounts is recorded to reduce the receivable to the net amount reasonably expected to be collected. Our judgment is required as to the impact of certain of these items and other factors as to ultimate realization of our accounts receivable. If the financial condition of our customers were to deteriorate, additional allowances may be required.
Stock-Based Compensation
TotipotentRX measures compensation expense for all share-based awards at fair value on the date of grant and recognizes compensation expense over the service period for awards expected to vest. TotipotentRX uses the Black-Scholes option-pricing model to determine the fair-value for awards and recognizes compensation expense on a straight-line basis over the awards’ vesting periods. Management has determined the Black-Scholes fair value of stock option awards and related share-based compensation expense with the assistance of third-party valuation specialists. Determining the fair value of stock options at the grant date requires the use of highly subjective assumptions, including the expected term of the option and the expected volatility of TotipotentRX’s stock price. The determination of the grant date fair value of options using an option-pricing model is affected by TotipotentRX’s estimated common stock fair value as well as assumptions regarding a number of other complex and subjective variables. In valuing the options, management makes assumptions about risk-free interest rates, dividend yields, volatility, and weighted-average expected lives, of the options.
22
Risk-free rate. Risk-free interest rates are derived from U.S. Treasury securities as of the option grant date.
Expected dividend yields. Expected dividend yields are based on TotipotentRX’s historical dividend payments, which have been zero to date.
Volatility. Absent a public market for its shares, TotipotentRX estimated volatility of its share price based on the volatilities of industry peers in the regenerative medicine space in which
TotipotentRX operates.
Expected term. TotipotentRX estimates the weighted-average expected life of options as the average of the vesting option schedule and the term of the award, which is known as the simplified method, since, due to the limited period of time share-based awards have been exercisable, TotipotentRX does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term.
Forfeiture rate. As the majority of the stock options granted were immediately vested, a 0% forfeiture rate was used.
Income Taxes
We account for income taxes using the asset and liability method, under which we recognize deferred tax assets and liabilities for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and for net operating loss and tax credit carryforwards. Tax positions that meet a more-likely-than-not recognition threshold are recognized in the first reporting period that it becomes more-likely-than-not such tax position will be sustained upon examination. We record potential accrued interest and penalties related to unrecognized tax benefits in income tax expense.
As a multinational corporation, we are subject to complex tax laws and regulations in various jurisdictions. The application of tax laws and regulations is subject to legal and factual interpretation, judgment, and uncertainty. Tax laws themselves are subject to change as a result of changes in fiscal policy, changes in legislation, evolution of regulations and court rulings. Therefore, the actual liability for U.S. or foreign taxes may be materially different from our estimates, which could result in the need to record additional liabilities or potentially to reverse previously recorded tax liabilities.
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date. A valuation allowance is recorded against any deferred tax assets when, in the judgment of management, it is more likely than not that all of or part of a deferred tax asset will not be realized. In assessing the need for a valuation allowance, we consider all positive and negative evidence, including recent financial performance, scheduled reversals of temporary differences, projected future taxable income, availability of taxable income in carryback periods and tax planning strategies. Based on a review of such information, we believe that insufficient positive evidence exists to support that we will more likely than not be able to realize the majority of our foreign and U.S. federal and state deferred tax assets. Therefore, we have recorded a valuation allowance against our deferred tax assets to the extent that they are not expected to be recoverable against taxes previously paid in available carryback periods.
23
Results of Operations
The following is management’s discussion and analysis of certain significant factors which have affected TotipotentRX’s financial condition and results of operations during the periods included in the accompanying consolidated financial statements.
Results of Operations for the Nine Months ended September 30, 2013 as Compared to the Nine Months ended September 30, 2012
Net Revenues
Net revenues for the nine months ended September 30, 2013 were $1,130,000 compared to $791,000 for the nine months year ended September 30, 2012, an increase of $339,000 or 43%. The increase is primarily due a large cord blood banking customer that resumed placing regular orders in late calendar 2012, new cord blood banking customers and growth in the Company’s own cord blood banking program of $333,000.
Gross Profit
Gross profit was $301,000 or 27% of revenues for the nine months ended September 30, 2013, as compared to $275,000 or 35% of revenues for the nine months ended September 30, 2012. The increase in gross profit of $26,000 or 9% was primarily the result of increased revenue, partially offset by a change in the mix of products and services sold and an increase in contractual expenses associated with the Company’s own cord blood banking program. The decrease in the gross profit margin is due to an increase in cord blood banking revenues which have a lower gross profit margin than point of care revenues.
Selling, General and Administrative
Selling, general and administrative expenses were $1,146,000 for the nine months ended September 30, 2013 compared to $859,000 for the nine months ended September 30, 2012, an increase of $287,000 or 33%. The increase is primarily the result of costs associated with the pending merger of ThermoGenesis and TotipotentRX of $247,000 and an increase in selling expenses of $47,000 relating to the Company’s own cord blood banking program.
Depreciation
Depreciation expense was $34,000 for the nine months ended September 30, 2013, which is comparable the nine months ended September 30, 2012.
Interest and Other Income (Expense)
Interest and other income (expense), net, was ($12,000) for the nine months ended September 30, 2013, which is comparable to the nine months ended September 30, 2012.
Adjusted EBITDA
Adjusted EBITDA was $806,000 loss for the nine months ended September 30, 2013, as compared to $551,000 loss for the nine months ended September 30, 2012, an increase in the loss of $255,000 or 46%. The increase is primarily the result of an increase in the operating loss of $264,000, partially offset by an increase in depreciation of $9,000.
24
Provision for Income Taxes
Provisions for income taxes were zero for the nine months ended September 30, 2013, as compared to $37,000 for the nine months ended September 30, 2012. The decrease is the result of having no taxable income for the nine months ended September 30, 2013.
Liquidity and Capital Resources
At September 30, 2013, the company’s cash and cash equivalents balance was $509,000 and working capital of $44,000. This compares to cash and cash equivalents balance of $1,035,000 and working capital of $809,000 at December 31, 2012.
Net cash used in operating activities for the nine months ended September 30, 2013 was $491,000 primarily due to the net loss of $891,000, offset by depreciation of $72,000, an increase in accounts payable of $348,000 and an increase in deferred revenue of $106,000.
Cash flows from investing activities include capital expenditures of $12,000.
Cash flows from investing activities include a new note payable of $34,000, partially offset by payments on the note payable of $4,000.
TotipotentRX believes that it has sufficient cash and working capital for its operations for at least the next 12 months.
Results of Operations for the Year Ended December 31, 2012 as Compared to the Year Ended December 31, 2011
Net Revenues
Net revenues for the year ended December 31, 2012 were $1,177,000 compared to $1,839,000 for the year ended December 31, 2011, a decrease of $662,000, or 36%. Revenues decreased by $800,000 primarily due a large cord blood banking customer placing a stocking order in December 2011 and an overall decrease in other cord blood banking customers’ orders and point of care procedures. This decrease was partially offset by increases of $220,000 relating to growth in the Company’s own cord blood banking program, which was launched in the fourth quarter of 2011.
Gross Profit
Gross profit was $401,000 or 34% of revenues for the year ended December 31, 2012, compared to $830,000 or 45% of revenues for the year ended December 31, 2011. The decrease in gross profit for the year ended December 31, 2012 was primarily the result of lower revenues, change in the mix of products and service sold and an increase in labor costs associated with the addition of personnel to support the launch of manufacturing of cord blood banking products. The decrease in gross profit margin was due to the increase in cord blood banking revenues which have a lower gross profit margin than point of care.
25
Selling, General and Administrative
Selling, general and administrative expenses were $1,473,000 for the year ended December 31, 2012 compared to $1,029,000 for the year ended December 31, 2011, an increase of $444,000 or 43%. The increase in operating costs is primarily due to a non-cash charge of $250,000 relating to the impairment of a non-equity investment, labor and selling expenses of $145,000 associated with growth in our cord blood banking program which was launched in the fourth quarter of 2011, bad debt expense of $46,000 and costs of $45,000 associated with initiating a cardiac clinical study in 2012. These expenses were partially offset by a decrease in stock compensation expense of $104,000 resulting from stock options that were granted in 2011.
Depreciation
Depreciation expense was $45,000 for the year ended December 31, 2012, an increase of $16,000 or 55%, as compared to $29,000 for the year ended December 31, 2011. The increase is primarily the result of having a full year of operations of the Company’s own cord blood banking program which was launched in the fourth quarter of 2011.
Interest and Other Income (Expense), Net
Interest and other expense was $3,000 for the year ended December 31, 2012, which is consistent with the year ended December 31, 2011.
Provisions for Income Taxes
Provisions for income taxes was $37,000 for the year end December 31, 2012, compared to $91,000 for the year ended December 31, 2011, a decrease of $54,000 or 59%. The decrease was primarily the result of a foreign subsidiary having foreign taxable income for the year ended December 31, 2011. The same foreign subsidiary had a taxable loss for the year ended December 31, 2012; however, the tax year end for the foreign subsidiary is March 31, so a portion of the income tax expense for the year ended March 31, 2012 was recognized in the calendar year ended December 31, 2012.
Adjusted EBITDA
Adjusted EBITDA was $777,000 loss for the year ended December 31, 2012, as compared to $63,000 loss for the year ended December 31, 2011, an increase in the loss of $714,000. The increase is primarily the result of an increase in the operating loss of $889,000 and a decrease in stock compensation expense of $104,000, partially offset by an impairment of investment in private company of $250,000 and an increase in depreciation of $29,000.
Liquidity and Capital Resources
At December 31, 2012, the company’s cash and cash equivalents balance was $1,035,000 and working capital of $809,000. This compares to cash and cash equivalents balance of $1,174,000 and working capital of $713,000 at December 31, 2011.
Net cash used in operating activities for the year ended December 31, 2012 was $780,000 primarily due to the net loss of $1,157,000 offset by a loss on impairment of a non- equity investment in a private corporation of $250,000, and an increase in accounts receivable of $226,000
26
Cash flows from investing activities include capital expenditures of $155,000 and proceeds from the sale or maturities of short-term investments of ($185,000). Cash flows from financing activities include the proceeds from sale of subsidiary shares of $630,000 and proceeds from note payable to related party of $60,000.
The outflow of cash due to net cash used in operating activities and from investing activities was offset primarily by the proceeds from the sale of common stock in the amount of $630,000.
TotipotentRX believes that it has sufficient cash and working capital for its operations for the remainder of the calendar year.
Quantitative and Qualitative Disclosure about Market Risk.
Because TotipotentRX was a private company, it is not required to provide information regarding quantitative and qualitative disclosure about market risk.
27
Index to Financial Statements of TotipotentRX (MK Alliance, Inc.)
|
F-2
|
|
|
F-3
|
|
|
F-4
|
|
|
F-5
|
|
|
F-6
|
|
|
|
|
|
F-9
|
|
|
F-10
|
|
|
F-11
|
|
|
F-12
|
|
|
F-13
|
|
|
F-14
|
Index to Unaudited Pro Forma Financial Statements
|
F-26
|
|
|
F-27
|
|
|
F-28
|
|
|
F-29
|
F-1
TotipotentRX Corporation
|
|
September 30,
2013
|
December 31,
2012
|
||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
509,000
|
$
|
1,035,000
|
||||
|
Accounts receivable
|
207,000
|
222,000
|
||||||
|
Inventories
|
91,000
|
73,000
|
||||||
|
Other current assets
|
74,000
|
28,000
|
||||||
|
Total current assets
|
881,000
|
1,358,000
|
||||||
|
Long-term receivables
|
48,000
|
39,000
|
||||||
|
Equipment, net
|
374,000
|
415,000
|
||||||
|
Other assets
|
20,000
|
30,000
|
||||||
|
Total assets
|
$
|
1,323,000
|
$
|
1,842,000
|
||||
|
|
||||||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
589,000
|
$
|
324,000
|
||||
|
Deferred revenue
|
45,000
|
43,000
|
||||||
|
Note payable
|
30,000
|
--
|
||||||
|
Accrued liabilities
|
173,000
|
182,000
|
||||||
|
Total current liabilities
|
837,000
|
549,000
|
||||||
|
Notes payable to related parties
|
328,000
|
312,000
|
||||||
|
Deferred revenue
|
236,000
|
167,000
|
||||||
|
Capital lease payable
|
44,000
|
--
|
||||||
|
Total liabilities
|
1,445,000
|
1,028,000
|
||||||
|
Commitments and contingencies (Note 3)
|
||||||||
|
Stockholders’ Equity:
|
||||||||
|
Common stock, $0.01 par value; 1,000,000 shares authorized; 401,563 issued and outstanding (333,000 at December 31, 2012)
|
4,000
|
3,000
|
||||||
|
Paid-in capital
|
2,493,000
|
2,632,000
|
||||||
|
Accumulated deficit
|
(2,404,000
|
)
|
(1,620,000
|
)
|
||||
|
Accumulated other comprehensive loss
|
(215,000
|
)
|
(155,000
|
)
|
||||
|
Total TotipotentRX Corporation stockholders’ equity
|
(122,000
|
)
|
860,000
|
|||||
|
Noncontrolling interest
|
--
|
(46,000
|
)
|
|||||
|
Total stockholders’ equity (deficit)
|
(122,000
|
)
|
814,000
|
|||||
|
Total liabilities and stockholders’ equity
|
$
|
1,323,000
|
$
|
1,842,000
|
||||
The accompanying notes are an integral part of the condensed consolidated financial statements.
F-2
TotipotentRX Corporation
|
|
Nine Months Ended September 30,
|
|||||||
|
|
2013
|
2012
|
||||||
|
|
||||||||
|
Net revenues
|
$
|
1,130,000
|
$
|
791,000
|
||||
|
Cost of goods sold
|
829,000
|
516,000
|
||||||
|
Gross profit
|
301,000
|
275,000
|
||||||
|
|
||||||||
|
Operating expenses:
|
||||||||
|
Selling, general and administrative expenses
|
1,146,000
|
859,000
|
||||||
|
Depreciation
|
34,000
|
31,000
|
||||||
|
Total operating expenses
|
1,180,000
|
890,000
|
||||||
|
Loss from operations
|
(879,000
|
)
|
(615,000
|
)
|
||||
|
|
||||||||
|
Interest and other income (expense), net
|
(12,000
|
)
|
(1,000
|
)
|
||||
|
|
||||||||
|
Loss before taxes
|
(891,000
|
)
|
(616,000
|
)
|
||||
|
Provision for income taxes
|
--
|
37,000
|
||||||
|
Net Loss
|
(891,000
|
)
|
(653,000
|
)
|
||||
|
Less: Net loss attributable to noncontrolling interests
|
(107,000
|
)
|
(114,000
|
)
|
||||
|
Net loss attributable to TotipotentRX Corporation
|
$
|
(784,000
|
)
|
$
|
(539,000
|
)
|
||
|
|
||||||||
|
Net loss
|
$
|
(891,000
|
)
|
$
|
(653,000
|
)
|
||
|
Other comprehensive (loss):
|
||||||||
|
Foreign currency translation adjustments
|
(46,000
|
)
|
--
|
|||||
|
Comprehensive loss
|
(937,000
|
)
|
(653,000
|
)
|
||||
|
Comprehensive loss attributable to noncontrolling interests
|
(107,000
|
)
|
(114,000
|
)
|
||||
|
Comprehensive loss attributable to TotipotentRX Corporation
|
$
|
(830,000
|
)
|
$
|
(539,000
|
)
|
||
The accompanying notes are an integral part of the condensed consolidated financial statements.
F-3
TotipotentRX Corporation
|
|
Common Stock
|
Paid-in
|
Accumulated
|
Accumulated
Other
Comprehensive
|
Total
TotipotentRX
Corporation
Stockholder’s
|
Non-
Controlling
|
Total
|
|||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Capital
|
Deficit
|
Loss
|
Equity
|
Interests
|
Equity
|
||||||||||||||||||||||||
|
Balance, December 31, 2011
|
333,000
|
$
|
3,000
|
$
|
1,799,000
|
$
|
(682,000
|
)
|
$
|
(136,000
|
)
|
$
|
984,000
|
$
|
126,000
|
$
|
1,110,000
|
|||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Net (loss)
|
--
|
--
|
--
|
(539,000
|
)
|
--
|
(539,000
|
)
|
(114,000
|
)
|
(653,000
|
)
|
||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Sale of subsidiary shares to noncontrolling interests
|
--
|
--
|
832,000
|
--
|
--
|
832,000
|
48,000
|
880,000
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Stock-based compensation
|
--
|
--
|
1,000
|
--
|
--
|
1,000
|
--
|
1,000
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Balance, September 30, 2012
|
333,000
|
$
|
3,000
|
$
|
2,632,000
|
$
|
(1,221,000
|
)
|
$
|
(136,000
|
)
|
$
|
1,278,000
|
$
|
60,000
|
$
|
1,338,000
|
|||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Balance, December 31, 2012
|
333,000
|
$
|
3,000
|
$
|
2,632,000
|
$
|
(1,620,000
|
)
|
$
|
(155,000
|
)
|
$
|
860,000
|
$
|
(46,000
|
)
|
$
|
814,000
|
||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Net (loss)
|
--
|
--
|
--
|
(784,000
|
)
|
--
|
(784,000
|
)
|
(107,000
|
)
|
(891,000
|
)
|
||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Acquisition of noncontrolling interest
|
68,563
|
1,000
|
(140,000
|
)
|
--
|
(14,000
|
)
|
(153,000
|
)
|
153,000
|
--
|
|||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Stock-based compensation
|
--
|
--
|
1,000
|
--
|
--
|
1,000
|
--
|
1,000
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Foreign currency translation
|
--
|
--
|
--
|
--
|
(46,000
|
)
|
(46,000
|
)
|
--
|
(46,000
|
)
|
|||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Balance, September 30, 2013
|
401,563
|
$
|
4,000
|
$
|
2,493,000
|
$
|
(2,404,000
|
)
|
$
|
(215,000
|
)
|
$
|
(122,000
|
)
|
--
|
$
|
(122,000
|
)
|
||||||||||||||
The accompanying notes are an integral part of the condensed consolidated financial statements.
F-4
TotipotentRX Corporation
|
|
Nine Months Ended September 30,
|
|||||||
|
|
2013
|
2012
|
||||||
|
Cash flows from operating activities:
|
||||||||
|
Net loss
|
$
|
(891,000
|
)
|
$
|
(653,000
|
)
|
||
|
Adjustments to reconcile net loss to net cash (used in) provided by operating activities:
|
||||||||
|
Stock-based compensation expense
|
1,000
|
1,000
|
||||||
|
Depreciation and amortization
|
72,000
|
63,000
|
||||||
|
Interest accrued on notes payable to related parties
|
16,000
|
12,000
|
||||||
|
Net changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable, net
|
(43,000
|
)
|
(143,000
|
)
|
||||
|
Inventories
|
(48,000
|
)
|
26,000
|
|||||
|
Other current assets
|
(49,000
|
)
|
(11,000
|
)
|
||||
|
Other assets
|
7,000
|
(2,000
|
)
|
|||||
|
Accounts payable
|
348,000
|
(34,000
|
)
|
|||||
|
Accrued liabilities
|
(10,000
|
)
|
(19,000
|
)
|
||||
|
Deferred revenue
|
106,000
|
100,000
|
||||||
|
|
||||||||
|
Net cash used in operating activities
|
(491,000
|
)
|
(660,000
|
)
|
||||
|
Cash flows from investing activities:
|
||||||||
|
Capital expenditures
|
(12,000
|
)
|
(149,000
|
)
|
||||
|
Purchase of short-term investments
|
--
|
(65,000
|
)
|
|||||
|
Proceeds from sales or maturities of short-term investments
|
--
|
189,000
|
||||||
|
|
||||||||
|
Net cash used in investing activities
|
(12,000
|
)
|
(25,000
|
)
|
||||
|
Cash flows from financing activities:
|
||||||||
|
Proceeds from note payable
|
34,000
|
--
|
||||||
|
Payment on note payable
|
(4,000
|
)
|
--
|
|||||
|
Proceeds from sale of subsidiary shares
|
--
|
630,000
|
||||||
|
|
||||||||
|
Net cash provided by financing activities
|
30,000
|
630,000
|
||||||
|
Effects of foreign currency rate changes on cash
|
(53,000
|
)
|
(9,000
|
)
|
||||
|
Net decrease in cash and equivalents
|
(526,000
|
)
|
(64,000
|
)
|
||||
|
Cash and cash equivalents at beginning of period
|
1,035,000
|
1,174,000
|
||||||
|
Cash and cash equivalents at end of period
|
$
|
509,000
|
$
|
1,110,000
|
||||
|
|
||||||||
|
Supplemental non-cash financing information:
|
||||||||
|
Equipment acquired with capital lease
|
$
|
55,000
|
--
|
|||||
The accompanying notes are an integral part of the condensed consolidated financial statements.
F-5
TotipotentRX Corporation
(Unaudited)
| 1. | Summary of Significant Accounting Policies |
Nature of Operations
TotipotentRX (the “Company”, “TotiRX”) is focused in the research, development and commercialization of autologous cell-based therapeutics for use in regenerative medicine. TotiRX is headquartered in Los Angeles, California with two subsidiaries in New Delhi/Gurgaon, India. The subsidiaries jointly operate sales and services branch offices in Chennai and Mumbai, India, and TotiRX operates a branch office in Bangalore, India.
On July 2, 2013, TotiRX, a California corporation, and MK Alliance, Inc., incorporated in California in 2007, entered into an Agreement and Plan of Merger whereby TotiRX merged with and into MK Alliance, Inc., with MK Alliance, Inc. as the survivor company and subsequently changed its name to TotiRX. Immediately prior to the merger, MK Alliance owned approximately 77% of the outstanding shares of TotiRX. Each outstanding share of TotiRX not owned by the surviving corporation was converted into 0.351657 of the surviving corporation. In addition, options to purchase 31,000 shares (10,901 after conversion) of TotiRX were assumed by the surviving corporation.
Interim Reporting
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (U.S. GAAP) for interim financial information. Accordingly, certain information and footnote disclosures normally included in annual financial statements prepared in accordance with U.S. GAAP have been condensed or omitted pursuant to accounting principles applicable for interim periods. In the opinion of management, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included. Operating results for the nine month period ended September 30, 2013, are not necessarily indicative of the results that may be expected for the year ending December 31, 2013. These unaudited condensed consolidated financial statements should be read in conjunction with the consolidated financial statements and the notes thereto included in this Form S-4/A-2.
Recently Adopted Accounting Pronouncements
In February 2013, the FASB issued ASC 2013-02, which is an update to improve the reporting of reclassifications out of accumulated other comprehensive income (“AOCI”). Companies are also required to present reclassifications by component when reporting changes in AOCI balances. We adopted ASC 2013-02 effective January 1, 2013. The adoption of ASC 2013-02 did not have a material impact on our consolidated results of operations or financial condition.
Subsequent Events
The Company evaluated events occurring between September 30, 2013 and December 11, 2013, the date the financial statements were available to be issued.
| 2. | Commitments and Contingent Liabilities |
In the normal course of operations, the Company may have disagreements or disputes with customers, employees or vendors. Such potential disputes are seen by management as a normal part of business. As of September 30, 2013, management believes any liability that may ultimately result from the resolution of these matters will not have a material adverse effect on our consolidated financial position, operating results or cash flows.
F-6
| 3. | Stock-Based Compensation |
The shares and price per share information reflected in this footnote 3 have been presented to give effect to the TotiRX/MK Alliance merger. The following is a summary of option activity for our stock option plan:
|
|
Number of
Shares
|
Weighted-
Average
Exercise Price
|
Weighted- Average
Remaining
Contractual Life
(Years)
|
|||||||||
|
Outstanding at December 31, 2012
|
10,901
|
$
|
17.28
|
|||||||||
|
|
||||||||||||
|
Granted
|
--
|
--
|
||||||||||
|
Forfeited/cancelled
|
--
|
--
|
||||||||||
|
Expired
|
--
|
--
|
||||||||||
|
Exercised
|
--
|
--
|
||||||||||
|
Outstanding at September 30, 2013
|
10,901
|
$
|
17.28
|
2.3
|
||||||||
|
Exercisable at September 30, 2013
|
10,726
|
$
|
17.28
|
2.3
|
||||||||
|
Vested and expected to vest at September 30, 2013
|
10,901
|
$
|
17.28
|
2.3
|
||||||||
Non-vested stock option activity for the nine months ended September 30, 2013, is as follows:
|
|
Non-Vested Stock
Options
|
Weighted-Average
Grant Date Fair Value
|
||||||
|
Outstanding at December 31, 2012
|
264
|
$
|
12.82
|
|||||
|
Granted
|
--
|
--
|
||||||
|
Vested
|
88
|
$
|
12.82
|
|||||
|
Forfeited
|
--
|
--
|
||||||
|
Outstanding at September 30, 2013
|
176
|
$
|
12.82
|
|||||
| 4. | Note Payable |
In August 2013, the Company entered into a note payable for $34,000 to be paid in ten monthly installments beginning in September 2013 and ending in June 2014. The note payable has an annual interest rate of 5.24%.
| 5. | Proposed Merger with ThermoGenesis Corp |
On July 15, 2013, TotiRX entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), with ThermoGenesis Corp. (“ThermoGenesis”) providing for the merger of TotiRX into ThermoGenesis, with ThermoGenesis surviving. ThermoGenesis is a public company and a leading designer and supplier of clinical technologies for processing, storage and administration of stem cells used in the practice of regenerative medicine.
F-7
Assuming the merger is consummated, a TotiRX stockholder will receive, in exchange for each share of TotiRX common stock held by such stockholder immediately before the closing of the Merger, approximately 30.284 shares of ThermoGenesis common stock. After the merger, the former stockholders of TotiRX will own approximately 12,490,841 shares of ThermoGenesis common stock, in the aggregate representing approximately 43% ThermoGenesis shares of common stock outstanding, excluding shares of common stock subject to options and warrants.
The Merger Agreement was unanimously approved by the boards of directors of both companies. The contemplated merger is subject to the approval of TotiRX and ThermoGenesis respective stockholders at stockholders meetings and satisfaction of other closing conditions, including the filing of a registration statement with the SEC. Further, the combined company will be named Cesca Therapeutics to better reflect the combined products and services of the two companies. It is anticipated that the merger will close during the first quarter of calendar 2014.
The Merger Agreement contains certain termination rights for both TotiRX, on the one hand, and ThermoGenesis, on the other, and further provides that, upon termination of the Merger Agreement under specified circumstances, including, but not limited to, termination due to a failure by one party to recommend approval of the Merger, a party soliciting an acquisition proposal in breach of the Merger Agreement, or a party entering into an agreement with a third-party related to an acquisition proposal, that breaching party may be required to pay to the other party a termination fee of $500,000.
F-8
The Board of Directors
MK Alliance, Inc.
We have audited the accompanying consolidated financial statements of MK Alliance, Inc. (the “Company”) which comprise the consolidated balance sheets as of December 31, 2012 and 2011, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity and cash flows for the years then ended, and the related notes to the consolidated financial statements.
Management’s Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of these financial statements in conformity with U.S. generally accepted accounting principles; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free of material misstatement, whether due to fraud or error.
Auditor’s Responsibility
Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of MK Alliance, Inc. at December 31, 2012 and 2011, and the consolidated results of its operations and its cash flows for the years then ended in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young, LLP
Sacramento, CA
November 8, 2013
F-9
MK Alliance, Inc.
|
|
December 31,
|
|||||||
|
|
2012
|
2011
|
||||||
|
ASSETS
|
||||||||
|
Currents Assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
1,035,000
|
$
|
1,174,000
|
||||
|
Short-term investments
|
--
|
123,000
|
||||||
|
Accounts receivable, net of allowance for doubtful accounts of $17,000 ($6,000 at December 31, 2011)
|
222,000
|
82,000
|
||||||
|
Inventories
|
73,000
|
49,000
|
||||||
|
Other current assets
|
28,000
|
23,000
|
||||||
|
Total current assets
|
1,358,000
|
1,451,000
|
||||||
|
Long-term receivables, net of allowance of $41,000 ($0 at December 31, 2011)
|
39,000
|
4,000
|
||||||
|
Equipment, net
|
415,000
|
363,000
|
||||||
|
Investment in private corporation
|
--
|
250,000
|
||||||
|
Other assets
|
30,000
|
29,000
|
||||||
|
Total assets
|
$
|
1,842,000
|
$
|
2,097,000
|
||||
|
|
||||||||
|
Liabilities and Stockholders’ Equity
|
||||||||
|
Current Liabilities:
|
||||||||
|
Accounts payable
|
$
|
324,000
|
$
|
292,000
|
||||
|
Deferred revenue
|
43,000
|
52,000
|
||||||
|
Stock subscription payable
|
--
|
250,000
|
||||||
|
Accrued liabilities
|
182,000
|
144,000
|
||||||
|
Total current liabilities
|
549,000
|
738,000
|
||||||
|
Notes payable to related parties
|
312,000
|
236,000
|
||||||
|
Deferred revenue
|
167,000
|
13,000
|
||||||
|
Total liabilities
|
1,028,000
|
987,000
|
||||||
|
Commitments and contingencies (Note 6)
|
||||||||
|
Stockholders’ Equity:
|
||||||||
|
Common stock, $0.01 par value; 1,000,000 shares authorized; 333,000 issued and outstanding at December 31, 2012 and 2011
|
3,000
|
3,000
|
||||||
|
Paid-in capital
|
2,632,000
|
1,799,000
|
||||||
|
Accumulated deficit
|
(1,620,000
|
)
|
(682,000
|
)
|
||||
|
Accumulated other comprehensive loss
|
(155,000
|
)
|
(136,000
|
)
|
||||
|
Total MK Alliance, Inc. stockholders’ equity
|
860,000
|
984,000
|
||||||
|
Non controlling interest
|
(46,000
|
)
|
126,000
|
|||||
|
Total stockholders’ equity
|
814,000
|
1,110,000
|
||||||
|
Total liabilities and stockholders’ equity
|
$
|
1,842,000
|
$
|
2,097,000
|
||||
The accompanying notes are an integral part of the consolidated financial statements.
F-10
MK Alliance, Inc.
|
|
Year Ended December 31,
|
|||||||
|
|
2012
|
2011
|
||||||
|
|
||||||||
|
Net revenues
|
$
|
1,177,000
|
$
|
1,839,000
|
||||
|
Cost of goods sold
|
776,000
|
1,009,000
|
||||||
|
Gross profit
|
401,000
|
830,000
|
||||||
|
|
||||||||
|
Operating expenses:
|
||||||||
|
Selling, general and administrative expenses
|
1,473,000
|
1,029,000
|
||||||
|
Depreciation
|
45,000
|
29,000
|
||||||
|
Total operating expenses
|
1,518,000
|
1,058,000
|
||||||
|
Loss from operations
|
(1,117,000
|
)
|
(228,000
|
)
|
||||
|
|
||||||||
|
Interest and other income (expense), net
|
(3,000
|
)
|
(3,000
|
)
|
||||
|
|
||||||||
|
Loss before taxes
|
(1,120,000
|
)
|
(231,000
|
)
|
||||
|
Provision for income taxes
|
37,000
|
91,000
|
||||||
|
Net Loss
|
(1,157,000
|
)
|
(322,000
|
)
|
||||
|
Less: Net loss attributable to noncontrolling interests
|
(219,000
|
)
|
(94,000
|
)
|
||||
|
Net loss attributable to MK Alliance, Inc.
|
$
|
(938,000
|
)
|
$
|
(228,000
|
)
|
||
|
|
||||||||
|
Net loss
|
$
|
(1,157,000
|
)
|
$
|
(322,000
|
)
|
||
|
Other comprehensive (loss):
|
||||||||
|
Foreign currency translation adjustments
|
(20,000
|
)
|
(144,000
|
)
|
||||
|
Comprehensive loss
|
(1,177,000
|
)
|
(466,000
|
)
|
||||
|
Comprehensive loss attributable to noncontrolling interests
|
(220,000
|
)
|
(107,000
|
)
|
||||
|
Comprehensive loss attributable to MK Alliance, Inc.
|
$
|
(957,000
|
)
|
$
|
(359,000
|
)
|
||
The accompanying notes are an integral part of the consolidated financial statements.
F-11
MK Alliance, Inc.
|
Common Stock
|
Accumulated
Other
|
Total MK
Alliance, Inc.
|
Non-
|
|||||||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Paid-in
Capital
|
Accumulated
Deficit
|
Comprehensive
Loss
|
Stockholder’s
Equity
|
Controlling
Interests
|
Total
Equity
|
||||||||||||||||||||||||
|
Balance, December 31, 2010
|
333,000
|
$
|
3,000
|
$
|
827,000
|
$
|
(454,000
|
)
|
$
|
(5,000
|
)
|
$
|
371,000
|
$
|
--
|
$
|
371,000
|
|||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Net (loss)
|
--
|
--
|
--
|
(228,000
|
)
|
--
|
(228,000
|
)
|
(94,000
|
)
|
(322,000
|
)
|
||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Sale of subsidiary shares to noncontrolling interests
|
--
|
--
|
867,000
|
--
|
--
|
867,000
|
233,000
|
1,100,000
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Stock-based compensation
|
--
|
--
|
105,000
|
--
|
--
|
105,000
|
--
|
105,000
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Foreign currency translation
|
--
|
--
|
--
|
--
|
(131,000
|
)
|
(131,000
|
)
|
(13,000
|
)
|
(144,000
|
)
|
||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Balance, December 31, 2011
|
333,000
|
3,000
|
1,799,000
|
(682,000
|
)
|
(136,000
|
)
|
984,000
|
126,000
|
1,110,000
|
||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Net (loss)
|
--
|
--
|
--
|
(938,000
|
)
|
--
|
(938,000
|
)
|
(219,000
|
)
|
(1,157,000
|
)
|
||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Sale of subsidiary shares to noncontrolling interests
|
--
|
--
|
832,000
|
--
|
--
|
832,000
|
48,000
|
880,000
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Stock-based compensation
|
--
|
--
|
1,000
|
--
|
--
|
1,000
|
--
|
1,000
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Foreign currency translation
|
--
|
--
|
--
|
--
|
(19,000
|
)
|
(19,000
|
)
|
(1,000
|
)
|
(20,000
|
)
|
||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||
|
Balance, December 31, 2012
|
333,000
|
$
|
3,000
|
$
|
2,632,000
|
$
|
(1,620,000
|
)
|
$
|
(155,000
|
)
|
$
|
860,000
|
$
|
(46,000
|
)
|
$
|
814,000
|
||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-12
MK Alliance, Inc.
|
|
Year Ended December 31,
|
|||||||
|
|
2012
|
2011
|
||||||
|
Cash flows from operating activities:
|
||||||||
|
Net loss
|
$
|
(1,157,000
|
)
|
$
|
(322,000
|
)
|
||
|
Adjustments to reconcile net loss to net cash (used in) provided by operating activities:
|
||||||||
|
Stock-based compensation expense
|
1,000
|
105,000
|
||||||
|
Depreciation and amortization
|
89,000
|
60,000
|
||||||
|
Interest accrued on notes payable to related parties
|
16,000
|
16,000
|
||||||
|
Loss on impairment of investment in private corporation
|
250,000
|
--
|
||||||
|
Net changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable, net
|
(226,000
|
)
|
(38,000
|
)
|
||||
|
Inventories
|
(13,000
|
)
|
(49,000
|
)
|
||||
|
Other current assets
|
(6,000
|
)
|
(15,000
|
)
|
||||
|
Other assets
|
(2,000
|
)
|
(10,000
|
)
|
||||
|
Accounts payable
|
74,000
|
225,000
|
||||||
|
Accrued liabilities
|
43,000
|
110,000
|
||||||
|
Deferred revenue
|
151,000
|
74,000
|
||||||
|
Net cash (used in) provided by operating activities
|
(780,000
|
)
|
156,000
|
|||||
|
Cash Flows from investing activities:
|
||||||||
|
Capital expenditures
|
(155,000
|
)
|
(276,000
|
)
|
||||
|
Purchase of short-term investments
|
(63,000
|
)
|
(277,000
|
)
|
||||
|
Proceeds from sales or maturities of short-term investments
|
185,000
|
138,000
|
||||||
|
Investment in private corporation
|
--
|
(250,000
|
)
|
|||||
|
Net cash used in investing activities
|
(33,000
|
)
|
(665,000
|
)
|
||||
|
Cash flows from financing activities:
|
||||||||
|
Proceeds from sale of subsidiary shares
|
630,000
|
1,100,000
|
||||||
|
Stock subscription payable
|
--
|
250,000
|
||||||
|
Proceeds from note payable to related party
|
60,000
|
--
|
||||||
|
Net cash provided by financing activities
|
690,000
|
1,350,000
|
||||||
|
Effects of foreign currency rate changes on cash
|
(16,000
|
)
|
(107,000
|
)
|
||||
|
Net (decrease) increase in cash and equivalents
|
(139,000
|
)
|
734,000
|
|||||
|
Cash and cash equivalents at beginning of year
|
1,174,000
|
440,000
|
||||||
|
Cash and cash equivalents at end of year
|
$
|
1,035,000
|
$
|
1,174,000
|
||||
|
Supplemental disclosures of cash flow information:
|
||||||||
|
Income taxes paid
|
$
|
56,000
|
$
|
54,000
|
||||
The accompanying notes are an integral part of the consolidated financial statements.
F-13
MK Alliance, Inc.
| 1. | Summary of Significant Accounting Policies |
Nature of Operations
MK Alliance, Inc. (“the Company”) is focused in the research, development and commercialization of autologous cell-based therapeutics for use in regenerative medicine. MK Alliance, Inc., and its majority held subsidiary, TotipotentRX Corporation (“TotiRX”), are headquartered in Los Angeles, California with two subsidiaries, TotipotentRX Cell Therapy Pvt. Ltd. (“TotiRX India”) and TotipotentSC Product Pvt. Ltd. (“TotiSC India”) in Gurgaon, a suburb of New Delhi, India. The subsidiaries jointly operate sales and services branch offices in Chennai and Mumbai, India, and TotiRX operates a branch office in Bangalore, India.
The Company sells the following technologies: (1) medical devices and equipment for collection, transportation, and processing of cord blood, cord tissue, bone marrow, and peripheral blood; (2) reagents for culturing and assaying stem cells; (3) services to hospitals and surgeons for processing autologous cellular therapies at the point of care. The Company has a GMP ISO 13485 Medical device manufacturing facility in Gurgaon, India.
TotiRX and TotiRX India are primarily a cellular therapy research and development company with human cell therapy development initiatives in cardiac disease, vascular disease, and orthopedic regeneration. TotiRX’s commercial efforts are focused on providing human clinical trial contract services, and providing cellular therapy services to its partner Fortis Healthcare Ltd. (“FHL”). Inclusive in the sales and service offering are cord blood banking, and point-of-care devices, kits and services to the FHL physicians and surgeons. The subsidiary has a GMP ISO 9001 (U.S. FDA registered) cellular processing and banking facility in Gurgaon, India. TotiSC India is primarily a cellular therapy medical device company providing devices and kits to both the cord blood banking and point-of-care hospital markets. TotiSC’s commercial efforts are primarily focused in Europe and Southeast Asia. The subsidiary has a GMP ISO 13485 medical device manufacturing facility in Gurgaon, India.
The Company sells most of its products and services on a direct basis. The Company’s main sales geographies are India and Europe.
The Company’s subsidiaries, TotiRX and TotiRX India, sell their services globally. The sales and service program with FHL is primarily focused in India.
The Company’s subsidiary, TotiSC India, sells its products and services where it has government registration (licenses), and this currently includes all of the European Union, India, Singapore and territories which accept European Union CE medical device approval.
Accounting Principles
The financial statements and accompanying notes are prepared in accordance with accounting principles generally accepted in the United States of America.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the parent company, MK Alliance Inc., its wholly-owned subsidiary, Totipotent SC Corporation, majority-owned subsidiary, TotiRX and its wholly-owned subsidiary, TotiRX India. All significant intercompany balances and transactions have been eliminated in consolidation.
F-14
Cash, Cash Equivalents and Short-term Investments
Holdings of highly liquid investments with maturities of three months or less when purchased are considered to be cash equivalents. Short-term investments are comprised of certificates of deposits which are classified as available for sale and have maturities greater than three months, but not exceeding one year.
Fair Value of Financial Instruments
The Company establishes the fair value of its assets and liabilities using the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date (exit price) and under a fair value hierarchy based on the inputs used to measure fair value. The three levels of the fair value hierarchy are as follows:
Level 1—Quoted prices in active markets for identical assets and liabilities;
Level 2—Observable inputs, other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; and
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to fair value of the assets or liabilities.
The carrying values of cash and cash equivalents, short-term investments, accounts receivable and accounts payable, approximate fair value due to their short duration. As of December 31, 2012, the Company had no Level 1, 2 or 3 financial instruments. As of December 31, 2011, the Company had certificates of deposits of $123,000 in cash equivalents and short-term investments, each, classified as Level 1 assets. As of December 31, 2011, the Company did not have any Level 2 or 3 financial instruments.
Foreign Currencies
Assets and liabilities recorded in foreign currencies are translated at the exchange rate on the balance sheet. The functional currency in India is the Indian rupee (INR). Revenue and expenses are translated at average rates of exchange prevailing during the year. Cash flows were also translated at average exchange rates for the period, therefore, amounts reported on the consolidated statement of cash flows did not necessarily agree with changes in the corresponding balances on the consolidated balance sheet. Equity accounts other than retained earnings are translated at the historic exchange rate on the date of investment. Translation adjustments of $20,000 and $144,000 for the years ended December 31, 2012 and 2011 resulting from this process are recorded to other comprehensive loss.
Accounts Receivable
Accounts receivable are stated net of an allowance for doubtful accounts. The Company estimates an allowance based on its historical collection trends, age of outstanding receivables and existing economic conditions. A customer’s receivable balance is considered past-due based on its contractual terms. Past-due receivable balances are written-off when the Company’s internal collection efforts have been unsuccessful in collecting the amount due.
Allowance for Doubtful Accounts
The Company evaluates the collectability of accounts receivable and determines the appropriate reserve for doubtful accounts based on a combination of factors. The Company writes off uncollectible accounts receivable based upon specific criteria, including when internal and/or external efforts to collect the amounts are unsuccessful, whether a customer has accounts past due over a specified period of time, and whether a customer has filed for or has been placed in bankruptcy. Allowances are recorded for all other receivables based on analysis of historical trends of write-offs and recoveries. In addition, in circumstances where we are aware of a specific customer’s inability to meet its financial obligation, a specific allowance for doubtful accounts is recorded to reduce the receivable to the net amount reasonably expected to be collected. Our judgment is required as to the impact of certain of these items and other factors as to ultimate realization of our accounts receivable. If the financial condition of our customers were to deteriorate, additional allowances may be required.
F-15
Inventories
Inventories are stated at the lower of cost or market. Cost is determined using the first-in, first-out method.
Inventory Reserves
The Company states inventories at lower of cost or market value determined on a first-in, first-out basis. The Company provides inventory allowances when conditions indicate that the selling price could be less than cost due to physical deterioration, obsolescence, changes in price levels, or other causes, which it includes as a component of cost of revenues. Additionally, the Company provides reserves for excess and slow-moving inventory on hand that are not expected to be sold to reduce the carrying amount of slow-moving inventory to its estimated net realizable value. The reserves are based upon estimates about future demand from our customers and distributors and market conditions. Because some of the Company’s products are highly dependent on current customer use and validation, and completion of regulatory and field trials, there is a risk that we will forecast incorrectly and purchase or produce excess inventories. As a result, actual demand may differ from forecasts and the Company may be required to record additional inventory reserves that could adversely impact the gross margins. Conversely, favorable changes in demand could result in higher gross margins when products previously reserved are sold.
Equipment
Equipment is recorded at cost and presented net of depreciation and amortization. Depreciation is primarily accounted for on the straight-line method based on estimated useful lives. The amortization of leasehold improvements is based on the shorter of the lease term of the life of the improvement. Betterments and large renewals which extend the life of the asset are capitalized whereas maintenance and repairs and small renewals are expensed as incurred.
Revenue Recognition
Revenues from the sale of the Company’s products and services are recognized when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. Amounts billed in excess of revenue recognized are recorded as deferred revenue on the balance sheet.
Revenue arrangements with multiple deliverables are divided into units of accounting if certain criteria are met, including whether the delivered item(s) has value to the customer on a stand-alone basis. Revenue for each unit of accounting is recognized as the unit of accounting is delivered. Arrangement consideration is allocated to each unit of accounting based upon the relative fair value determined from third-party evidence of the separate units of accounting. The Company accounts for collection, processing and testing of the umbilical cord blood and the storage as separate units of accounting. Revenue generated from storage contracts is deferred and recorded ratably over the life of the agreement, up to 21 years.
Shipping and handling fees billed to customers are included in net revenues, while the related costs are included in cost of goods sold.
F-16
Stock-Based Compensation
The Company measures compensation expense for all share-based awards at fair value on the date of grant and recognizes compensation expense over the service period for awards expected to vest. The Company uses the Black-Scholes option-pricing model to determine the fair-value for awards and recognizes compensation expense on a straight-line basis over the awards’ vesting periods. Management has determined the Black-Scholes fair value of stock option awards and related share-based compensation expense with the assistance of third-party valuation specialists. Determining the fair value of stock options at the grant date requires the use of highly subjective assumptions, including the expected term of the option and the expected volatility of the Company’s stock price. The determination of the grant date fair value of options using an option-pricing model is affected by the Company’s estimated common stock fair value as well as assumptions regarding a number of other complex and subjective variables. In valuing the options, management makes assumptions about risk-free interest rates, dividend yields, volatility, and weighted-average expected lives, of the options.
Risk-free rate. Risk-free interest rates are derived from U.S. Treasury securities as of the option grant date.
Expected dividend yields. Expected dividend yields are based on the Company’s historical dividend payments, which have been zero to date.
Volatility. Absent a public market for its shares, the Company estimated volatility of its share price based on the volatilities of industry peers in the regenerative medicine space in which the Company operates.
Expected term. The Company estimates the weighted-average expected life of options as the average of the vesting option schedule and the term of the award, which is known as the simplified method, since, due to the limited period of time share-based awards have been exercisable, the Company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term.
Forfeiture rate. As the majority of the stock options granted were immediately vested, a 0% forfeiture rate was used.
Income Taxes
The tax years 2007-2012 remain open to examination by the major taxing jurisdictions to which we are subject; however, there is no current investigation. The Company’s policy is to recognize interest and penalties related to the underpayment of income taxes as a component of income tax expense.
We account for income taxes using the liability method. Under this method, deferred tax assets are based on differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes and are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. These deferred tax assets include net operating loss carryforwards, fixed assets and reserves and accruals. The net deferred tax asset has been fully offset by a valuation allowance because of our history of losses. Utilization of operating losses may be subject to annual limitation due to ownership change provisions of the Internal Revenue Code of 1986 and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization.
Research and Development Expenses
For the years ended December 31, 2012 and 2011 the Company had $139,000 and $58,000, respectively of research and development expenses included in selling, general and administrative expenses in the statement of operations.
F-17
Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Recently Adopted Accounting Pronouncements
In July 2012, the FASB issued ASU 2012-02, which is an update to Topic 350, “Intangibles – Goodwill and Other”. This update provides additional guidance in performing impairment tests for indefinite-lived intangible assets by simplifying how an entity tests those assets for impairment. The update allows an entity to make a qualitative assessment about the likelihood that an indefinite-lived intangible asset is impaired to determine whether it should perform a qualitative impairment test. The Company adopted ASU 2012-02 effective December 31, 2012. The adoption of ASU 2012-02 did not have a material impact on our consolidated results of operations or financial condition.
In June 2011, the FASB issued ASU No. 2011-05, “Presentation of Comprehensive Income.” The guidance improves the comparability of financial reporting and facilitates the convergence of U.S. GAAP and IFRS by amending the guidance in ASC 220, “Comprehensive Income”. Under the amended guidance, an entity has the option to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In both choices, the entity is required to present on the face of the financial statements reclassification adjustments for items that are reclassified from other comprehensive income to net income in the statement(s) where the components of net income and the components of other comprehensive income are presented. The Company adopted this guidance retrospectively for the year ending December 31, 2011. The adoption of the guidance did not have a material impact on our financial condition or results of operations.
In May 2011, the Financial Accounting Standards Board (“FASB”) issued an Accounting Standards Updates (“ASU”) to the Fair Value Measurement Topic of the FASB ASC. This update was issued in order to achieve common fair value measurement and disclosure requirements in U.S. GAAP and International Financial Reporting Standards (“IFRS”). The update clarifies that (i) the highest and best use concept applies only to the fair value measurement of nonfinancial assets, (ii) specific requirements pertain to measuring the fair value of instruments classified in a reporting entity’s shareholders’ equity and, (iii) a reporting entity should disclose quantitative information about unobservable inputs used in a fair value measurement that is categorized within Level 3 of the fair value hierarchy. The update changes requirements with regard to the fair value of financial instruments that are managed within a portfolio and with regard to the application of premiums or discounts in a fair value measurement. In addition, the update increased disclosure requirements regarding Level 3 fair value measurements to include the valuation processes used by the reporting entity and the sensitivity of the fair value measurement to changes in unobservable inputs and the interrelationships between the unobservable inputs, if any. The Company adopted ASU 2011-04 effective January 1, 2012. The adoption of ASU 2011-04 did not have a material impact on our consolidated results of operations or financial condition.
Recently Issued Accounting Pronouncements
In February 2013, the FASB issued ASC 2013-02, which is an update to improve the reporting of reclassifications out of accumulated other comprehensive income (“AOCI”). Companies are also required to present reclassifications by component when reporting changes in AOCI balances. The updated accounting guidance is effective for fiscal years, and interim periods within those years, beginning after December 15, 2012 on a prospective basis. This guidance is not expected to have a material impact on our financial condition or results of operations.
F-18
In March 2013, the FASB issued ASU 2013-05, “Foreign Currency Matters” (Topic 830) which provides guidance on a parent’s accounting for the cumulative translation adjustment upon derecognition of a subsidiary or group of assets within a foreign entity. This new guidance requires that the parent release any related cumulative translation adjustment into net income only if the sale or transfer results in the complete or substantially complete liquidation of the foreign entity in which the subsidiary or group of assets had resided. The new guidance will be effective for us beginning July 1, 2014. The Company is currently assessing the potential impact, if any, the adoption of ASU 2013-05 may have on its consolidated financial statements.
In July 2013, the FASB issued ASU 2013-11, “Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists”. This amendment requires entities to present an unrecognized tax benefit, or a portion of an unrecognized tax benefit, as a reduction to a deferred tax asset for a net operating loss carryforward or a similar tax loss or a tax credit carryforward, unless certain conditions exist. This guidance is effective prospectively for annual reporting periods (and the interim periods within) beginning after December 15, 2013. Early adoption and retrospective application are permitted. The Company expects to adopt this guidance effective July, 2014. The Company is currently assessing the potential impact, if any, the adoption of ASU 2013-11 may have on its consolidated financial statements.
Subsequent Events
The Company evaluated events occurring between the end of the most recent fiscal year and November 8, 2013 the date the financial statements were available to be issued. Refer to Note 12 for further information on subsequent events.
| 2. | Inventories |
Inventories consisted of the following at December 31:
|
|
2012
|
2011
|
||||||
|
Supplies
|
$
|
28,000
|
$
|
39,000
|
||||
|
Raw materials
|
18,000
|
4,000
|
||||||
|
Finished goods
|
27,000
|
6,000
|
||||||
|
|
$
|
73,000
|
$
|
49,000
|
||||
| 3. | Equipment |
Equipment comprised the following at December 31:
|
|
2012
|
2011
|
||||||
|
Machinery and equipment
|
$
|
330,000
|
$
|
205,000
|
||||
|
Computer and software
|
65,000
|
57,000
|
||||||
|
Leasehold improvements
|
80,000
|
93,000
|
||||||
|
Office equipment
|
99,000
|
82,000
|
||||||
|
|
574,000
|
437,000
|
||||||
|
Less accumulated depreciation
|
(159,000
|
)
|
(74,000
|
)
|
||||
|
Net equipment
|
$
|
415,000
|
$
|
363,000
|
||||
F-19
| 4. | Investment in Private Corporation |
In October 2011, TotiRX entered into a Stock Purchase Agreement (“Agreement”) with a private corporation. Under the terms of the Agreement, TotiRX agreed to purchase two $250,000 tranches of common stock comprised of 834 shares at $299.76 per share. The first tranche was purchased concurrent with signing the agreement and is recorded at the historical cost of $250,000 at December 31, 2011.
During the quarter ended December 31, 2012, TotiRX and the other company terminated the stock purchase agreement for breach. Based on management’s belief that the private company’s products still required significant development before they would be ready for commercial sale, the investment was considered fully impaired and the carrying value of $250,000 was written off to selling, general and administrative expenses.
| 5. | Notes Payable to Related Parties |
On May 28, 2008, the Company entered into promissory notes for $75,000 each with two of its officers. The notes bore interest at 8% and had an original maturity date of May 28, 2011. The notes were amended on May 28, 2011, May 28, 2012 and May 28, 2013 to provide for an interest rate of 7% and a maturity date of May 27, 2014.
In August 2008, the Company entered into a $30,000 promissory note with one of the above officers. The note bore interest at 8% and had an original maturity date of August 28, 2011. The note was amended in August 2011, 2012 and 2013 to provide for an interest rate of 7% and a maturity date of August 28, 2014.
In December 2012, the Company entered into a $60,000 promissory note with one of the above officers. The note bears interest of 7% and matures on December 19, 2013.
As the Company has consistently demonstrated its intent to extend the notes for an additional year, the notes payable to related parties balance has been classified as long-term at December 31, 2012 and 2011.
Through December 31, 2012, no interest had been paid on the loans. The accrued interest of $72,000 and $56,000 at December 31, 2012 and 2011, respectively, is included in the notes payable to related parties balance.
| 6. | Commitments and Contingencies |
As part of the agreement with FHL, the Company has guaranteed a minimum monthly revenue to FHL of 375,000 INR (approximately $7,000 as of December 31, 2012) subject to foreign currency fluctuations once all specified government approvals are in place. As the required government approvals were not received until July 2013, there were no payments made under this requirement for the years ended December 31, 2012 and 2011. The agreement ends May 2016.
The Company is subject to legal proceedings and claims which arise in the ordinary course of its business. In the opinion of management, the amount of ultimate liability with respect to these actions will not materially affect the financial position of the Company.
| 7. | Rental and Lease Information |
The Company leases its facilities and certain equipment. Rental expenses for the years ended December 31, 2012 and 2011 amounted to $62,000 and $65,000, respectively. The annual future minimum lease payments for the non-cancelable operating lease are as follows:
|
2013
|
$
|
32,000
|
||
|
2014
|
34,000
|
|||
|
2015
|
6,000
|
|||
|
Total
|
$
|
72,000
|
F-20
| 8. | Stock-Based Compensation |
The Company’s subsidiary, TotiRX has a Stock Option Plan which permits the grant of options to employees, directors, consultants and advisers. There are 50,000 shares authorized to be issued under this Plan. The exercise price of the options shall not be less than 85% of the fair market value of the stock. Generally, the stock options granted through December 31, 2012 have a maximum term of five years and vest immediately. The following is a summary of option activity for our stock option plan:
|
|
Number of
Shares
|
Weighted-
Average
Exercise Price
|
Weighted- Average
Remaining
Contractual Life
(Years)
|
|||||||||
|
Outstanding at December 31, 2011
|
31,000
|
$
|
6.077
|
|||||||||
|
|
||||||||||||
|
Granted
|
--
|
--
|
||||||||||
|
Forfeited/cancelled
|
--
|
--
|
||||||||||
|
Expired
|
--
|
--
|
||||||||||
|
Exercised
|
--
|
--
|
||||||||||
|
Outstanding at December 31, 2012
|
31,000
|
$
|
6.077
|
3
|
||||||||
|
Exercisable at December 31, 2012
|
30,250
|
$
|
6.077
|
3
|
||||||||
|
Vested and expected to vest at December 31, 2012
|
31,000
|
$
|
6.077
|
3
|
||||||||
Non-vested stock option activity for the year ended December 31, 2012, is as follows:
|
|
Non-Vested Stock
Options
|
Weighted-Average
Grant Date Fair Value
|
||||||
|
Outstanding at December 31, 2011
|
1,000
|
$
|
4.51
|
|||||
|
Granted
|
--
|
|||||||
|
Vested
|
250
|
$
|
4.51
|
|||||
|
Forfeited
|
--
|
|||||||
|
Outstanding at December 31, 2012
|
750
|
$
|
4.51
|
|||||
The weighted average grant date fair value of options granted during the year ended December 31, 2011 was $3.52. There were no options granted during the year ended December 31, 2012. The total fair value of options vested during the years ended December 31, 2012 and 2011 was $1,000 and $105,000.
The fair value of stock options granted on January 30, 2011 was estimated using the following weighted average assumptions:
|
Expected life (years)
|
2.5
|
|||
|
Risk-free interest rate
|
0.82
|
%
|
||
|
Expected volatility
|
109
|
%
|
||
|
Dividend yield
|
0
|
%
|
F-21
The estimated per share value of common stock was based on a valuation performed by an independent third-party valuation specialist. The risk-free interest rates are based on U.S. Treasury securities at maturity with an equivalent term. The Company estimates the weighted average expected life of the options, following the permitted simplified method, as the mid-point between the vesting date and the end of the contractual term of the awards, since the Company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term. There has been no trading activity in the stock and as such, volatility represents management’s best estimate of future volatility based on reviewing the average volatility of stock prices for similar publicly traded companies. The Company has not declared or paid dividends in the past and does not currently expect to do so in the foreseeable future.
| 9. | Stockholders’ Equity |
In December 2011, the Company sold 8,696 shares of common stock in TotiRX for $200,000 and in January 2012, the Company sold 38,262 shares of TotiRX common stock for $880,000. The shares of common stock were sold with warrants to purchase 5,700 shares of common stock. Also, in December 2011, $250,000 of cash was received in advance of issuing the stock certificates and was recorded as a stock subscription payable as of December 31, 2011. The shares were sold to noncontrolling interests to increase their percentage ownership to approximately 23%. The warrants may be exercised by the holders at an exercise price of $23.00 per share. The warrants expire upon the earlier of November 30, 2014 or the sale of TotiRX.
In January 2011, the Company sold 148,000 shares of common stock for $900,000 to noncontrolling interests for an approximately 19% ownership.
| 10. | Concentrations |
The Company’s assets and operations are primarily located in India, except for certain overhead and sales functions, which are located in the United States.
Revenues from one customer totaled $363,000 or 31% and $328,000 or 18% of net revenues for the years ended December 31, 2012 and 2011. At December 31, 2012, this customer individually accounted for 10% of total accounts receivable. Revenues from another customer totaled $176,000 or 15% and $911,000 or 50% of net revenues for the years ended December 31, 2012 and 2011. Revenues from another customer totaled $92,000 or 8% and $291,000 or 16% for the years ended December 31, 2012 and 2011.
| 11. | Income Taxes |
The income tax provision for the years ended December 31, 2012 and 2011 varies from amounts computed by applying the federal statutory tax rate to income before income taxes due to state income taxes, penalties and interest, foreign taxes and valuation allowance.
At December 31, 2012, we had net operating loss carryforwards for federal and state income tax purposes of approximately $1,026,000 and $693,000 respectively, that are available to offset future income. The federal and state loss carryforwards expire in various years between 2017 and 2032.
F-22
The provision for (benefit from) income taxes consists of the following:
|
|
December 31,
|
|||||||
|
|
2012
|
2011
|
||||||
|
Current
|
||||||||
|
Federal
|
$
|
23,000
|
$
|
13,000
|
||||
|
State
|
||||||||
|
Foreign
|
$
|
14,000
|
$
|
78,000
|
||||
|
Total current
|
$
|
37,000
|
$
|
91,000
|
||||
|
Deferred:
|
||||||||
|
Federal
|
$
|
(253,000
|
)
|
$
|
(95,000
|
)
|
||
|
State
|
(19,000
|
)
|
(3,000
|
)
|
||||
|
Foreign
|
(101,000
|
)
|
(54,000
|
)
|
||||
|
Total deferred
|
$
|
(373,000
|
)
|
$
|
(152,000
|
)
|
||
|
Valuation allowance
|
$
|
373,000
|
$
|
152,000
|
||||
|
Total income tax expense
|
$
|
37,000
|
$
|
91,000
|
||||
Loss before income taxes include losses relating to non-U.S. operations of approximately $376,000 for 2012 and profits related to non-U.S. operations of approximately $150,000 for 2011.
Significant components of the Company’s deferred tax liabilities and assets are as follows:
|
|
December 31,
|
|||||||
|
|
2012
|
2011
|
||||||
|
Deferred tax assets
|
||||||||
|
Net operating losses
|
$
|
547,000
|
$
|
284,000
|
||||
|
Fixed assets
|
76,000
|
-
|
||||||
|
Reserves & accruals
|
42,000
|
26,000
|
||||||
|
Total deferred tax assets
|
665,000
|
310,000
|
||||||
|
Deferred tax liabilities
|
||||||||
|
Fixed assets
|
(6,000
|
)
|
||||||
|
Total deferred tax liabilities
|
-
|
(6,000
|
)
|
|||||
|
Valuation Allowance
|
(665,000
|
)
|
(304,000
|
)
|
||||
|
Net deferred tax assets
|
$
|
-
|
$
|
-
|
||||
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company established a full valuation allowance against its net deferred tax assets due to uncertainty surrounding realization of such assets. The valuation allowance increased by approximately $361,000 and $70,000 for 2012 and 2011, respectively.
The Company is subject to an IRS pending fine for the late filing of Form 5471 (Information on Foreign Controlled Corporations) for the years of 2010 and 2011. The maximum potential liability to the Company is $30,000 plus interest. The Company is disputing the fine. However, they have assessed that it is more likely than not going to be upheld. The Company has accrued $23,000 for the year ended December 31, 2012 and $13,000 for the year ended December 31, 2011, which includes both the penalties and interest.
F-23
| 12. | Subsequent Events |
Merger with MK Alliance, Inc. and TotiRX
On July 2, 2013, TotiRX, a California corporation, and MK Alliance, Inc., a California corporation entered into an Agreement and Plan of Merger whereby TotiRX merged with and into MK Alliance, Inc., with MK Alliance, Inc. as the survivor company and subsequently changed its name to TotiRX. Immediately prior to the merger, MK Alliance owned approximately 77% of the outstanding shares of TotiRX. Each outstanding share of TotiRX owned by the noncontrolling interest was converted into 0.351657 of the surviving corporation. In addition, options to purchase 31,000 shares of TotiRX were assumed by the surviving corporation subject to adjustment due to conversion.
Proposed Merger with ThermoGenesis Corp.
On July 15, 2013, TotiRX entered into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), with ThermoGenesis Corp. (“ThermoGenesis”) providing for the merger of TotiRX into ThermoGenesis, with ThermoGenesis surviving. ThermoGenesis is a public company and a leading designer and supplier of clinical technologies for processing, storage and administration of stem cells used in the practice of regenerative medicine.
Assuming the merger is consummated, a TotiRX stockholder will receive, in exchange for each share of TotiRX common stock held by such stockholder immediately before the closing of the Merger, approximately 30.284 shares of ThermoGenesis common stock. After the merger, the former stockholders of TotiRX will own approximately 12,490,800 shares of ThermoGenesis common stock, in the aggregate representing approximately 43% ThermoGenesis shares of common stock outstanding, excluding shares of common stock subject to options and warrants.
The Merger Agreement was unanimously approved by the boards of directors of both companies. The contemplated merger is subject to the approval of TotiRX and ThermoGenesis respective stockholders at stockholders meetings and satisfaction of other closing conditions, including the filing of a registration statement with the SEC. Further, the combined company will be named Cesca Therapeutics to better reflect the combined products and services of the two companies. It is anticipated that the merger will close during the first quarter of calendar 2014.
The Merger Agreement contains certain termination rights for both TotiRX and ThermoGenesis and further provides that, upon termination of the Merger Agreement under specified circumstances, including, but not limited to, termination due to a failure by one party to recommend approval of the Merger, a party soliciting an acquisition proposal in breach of the Merger Agreement, or a party entering into an agreement with a third-party related to an acquisition proposal, that breaching party may be required to pay to the other party a termination fee of $500,000.
F-24
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The following unaudited pro forma condensed combined financial information gives effect to the proposed merger of ThermoGenesis Corp. (“ThermoGenesis”) and TotipotentRX Corporation (“TotiRX”). For accounting purposes ThermoGenesis is considered to be acquiring TotiRX because the existing stockholders of ThermoGenesis will have a controlling interest in the combined company.
The Merger will be accounted for as a “purchase,” as that term is used under generally accepted accounting principles, for accounting and financial reporting purposes. Under purchase accounting, the assets (including identifiable intangible assets) and liabilities (including executory contracts and other commitments) of TotipotentRX as of the effective date of the Merger will be recorded at their respective fair values and added to those of ThermoGenesis. Any excess of purchase price over the fair values is recorded as goodwill. Consolidated financial statements of ThermoGenesis issued after the Merger would reflect these fair values and would not be restated retroactively to reflect the historical consolidated financial position or results of operations of TotipotentRX. The purchase method of accounting is based on ASC 805, Business Combinations. The consideration transferred used in the pro forma information below is primarily based on the closing price and number of ThermoGenesis common shares outstanding on November 30, 2013. Significant changes in the fair value of ThermoGenesis common shares between the filing of this document and the acquisition date could have a material impact on the consideration transferred and, as a result, the residual amount recognized as goodwill after recognizing the fair values of identifiable assets acquired and liabilities assumed as of the acquisition date. In addition, transactions by either ThermoGenesis or TotiRX such as borrowings or stock issuances could also have a material impact on the fair values of assets acquired and liabilities assumed at the acquisition date.
The unaudited pro forma condensed combined financial information presented below is based on the historical financial statements of ThermoGenesis and TotiRX, adjusted to give effect to the acquisition of ThermoGenesis and TotiRX for accounting purposes. The pro forma adjustments are described in the accompanying notes presented on the following pages.
The unaudited pro forma condensed combined balance sheet as of September 30, 2013 gives effect to the proposed merger as if it occurred on September 30, 2013 and combines the historical balance sheets of ThermoGenesis and TotiRX as of September 30, 2013. The TotiRX balance sheet information was derived from its unaudited condensed balance sheet as of September 30, 2013 included herein. The ThermoGenesis balance sheet information was derived from its unaudited balance sheet included in its Form 10-Q for the three months ended September 30, 2013 and also included herein. The unaudited pro forma condensed combined statements of operations for the three months ended September 30, 2013 and the year ended June 30, 2013 are presented as if the merger was consummated on July 1, 2012, and combines the historical results of ThermoGenesis and TotiRX for the three months ended September 30, 2013 and the year ended June 30, 2013. The historical results of TotiRX were derived from its unaudited condensed statements of operations for the six months ended June 30, 2013 and 2012 included in the Form S-4 filed November 8, 2013, the nine months ended September 30, 2013 and its audited consolidated statements of operations for the year ended December 31, 2012. The historical results of ThermoGenesis were derived from its unaudited condensed statement of operations for the three months ended September 30, 2013 included in its Form 10-Q for the quarterly period ended September 30, 2013, and its audited consolidated statement of operations for the year ended June 30, 2013 included in its Form 10-K for the year ended June 30, 2013.
The unaudited pro forma condensed combined financial information has been prepared for illustrative purposes and is not intended to represent the condensed combined financial position or results of operations in future periods or what the results actually would have been had ThermoGenesis and TotiRX been a combined company during the specified periods. The pro forma adjustments are based on the preliminary information available at December 20, 2013. The unaudited pro forma condensed combined financial information and accompanying notes are qualified by reference to and should be read in conjunction with (i) the TotiRX historical financial statements and notes thereto as of and for the nine months ended September 30, 2013 and the year ended December 31, 2012 included elsewhere herein, and (ii) the historical financial statements and notes thereto for the year ended June 30, 2013, included in ThermoGenesis’ Annual Report on Form 10-K, and the three months ended September 30, 2013 included in ThermoGenesis Report on Form 10-Q, filed with the Securities and Exchange Commission.
F-25
as of September 30, 2013
|
|
ThermoGenesis
Historical
|
TotiRX
Historical
|
Pro Forma
Adjustments
|
|
Pro Forma
Combined
|
||||||||||||
|
ASSETS
|
|
||||||||||||||||
|
Current assets:
|
|
||||||||||||||||
|
Cash and cash equivalents
|
$
|
5,306,000
|
$
|
509,000
|
$
|
1,223,000
|
(a)
|
$
|
7,038,000
|
||||||||
|
Accounts receivable, net
|
4,209,000
|
207,000
|
(108,000
|
)
|
(b)
|
4,308,000
|
|||||||||||
|
Inventories
|
4,413,000
|
91,000
|
(1,000
|
)
|
(b)
|
4,503,000
|
|||||||||||
|
Prepaid expenses and other current assets
|
319,000
|
74,000
|
--
|
|
393,000
|
||||||||||||
|
Total current assets
|
14,247,000
|
881,000
|
1,114,000
|
|
16,242,000
|
||||||||||||
|
|
|
||||||||||||||||
|
Long-term receivables, net
|
--
|
48,000
|
--
|
|
48,000
|
||||||||||||
|
Equipment, net
|
2,189,000
|
374,000
|
--
|
|
2,563,000
|
||||||||||||
|
Other assets
|
48,000
|
20,000
|
--
|
|
68,000
|
||||||||||||
|
Intangibles
|
--
|
--
|
7,667 ,000
|
(c)
|
7,667,000
|
||||||||||||
|
Goodwill
|
--
|
--
|
305,000
|
(d)
|
305,000
|
||||||||||||
|
|
$
|
16,484,000
|
$
|
1,323,000
|
$
|
9,086,000
|
|
$
|
26,893,000
|
||||||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
||||||||||||||||
|
|
|
||||||||||||||||
|
Current liabilities:
|
|
||||||||||||||||
|
Accounts payable
|
$
|
3,218,000
|
$
|
589,000
|
$
|
(108,000
|
)
|
(b)
|
$
|
3,699,000
|
|||||||
|
Deferred revenue
|
549,000
|
45,000
|
(42,000
|
)
|
(e)
|
552,000
|
|||||||||||
|
Other current liabilities
|
1,521,000
|
203,000
|
--
|
|
1,724,000
|
||||||||||||
|
Total current liabilities
|
5,288,000
|
837,000
|
(150,000
|
)
|
|
5,975,000
|
|||||||||||
|
|
|
||||||||||||||||
|
Notes payable to related parties
|
--
|
328,000
|
(328,000
|
)
|
(f)
|
--
|
|||||||||||
|
Deferred revenue
|
76,000
|
236,000
|
(218,000
|
)
|
(e)
|
94,000
|
|||||||||||
|
Capital lease and other non-current liabilities
|
--
|
44,000
|
|
44,000
|
|||||||||||||
|
|
|
||||||||||||||||
|
Stockholders’ equity:
|
|
||||||||||||||||
|
Common stock
|
16,000
|
4,000
|
9,000
|
(f)
|
29,000
|
||||||||||||
|
Paid in capital
|
127,594,000
|
2,493,000
|
6,870,000
|
(f)
|
136,957,000
|
||||||||||||
|
Accumulated deficit
|
(116,490,000
|
)
|
(2,404,000
|
)
|
2,688,000
|
(f),(g)
|
(116,206,000
|
)
|
|||||||||
|
Accumulated other comprehensive loss
|
--
|
(215,000
|
)
|
215,000
|
(f)
|
--
|
|||||||||||
|
Total stockholders’ equity
|
11,120,000
|
(122,000
|
)
|
9,782,000
|
|
20,780,000
|
|||||||||||
|
|
$
|
16,484,000
|
$
|
1,323,000
|
$
|
9,086,000
|
|
$
|
26,893,000
|
||||||||
See accompanying notes.
F-26
for the Three Months Ended September 30, 2013
|
|
ThermoGenesis
Historical
|
TotiRX
Historical
|
Pro Forma
Adjustments
|
|
Pro Forma
Combined
|
||||||||||||
|
|
|
||||||||||||||||
|
Net revenues
|
$
|
3,644,000
|
$
|
363,000
|
$
|
(56,000
|
)
|
(b)
|
$
|
3,951,000
|
|||||||
|
Cost of revenues
|
2,253,000
|
234,000
|
(55,000
|
)
|
(b)
|
2,432,000
|
|||||||||||
|
Gross profit
|
1,391,000
|
129,000
|
(1,000
|
)
|
|
1,519,000
|
|||||||||||
|
|
|
||||||||||||||||
|
Expenses:
|
|
||||||||||||||||
|
Sales and marketing
|
715,000
|
--
|
--
|
|
715,000
|
||||||||||||
|
Research and development
|
833,000
|
--
|
--
|
|
833,000
|
||||||||||||
|
General and administrative
|
2,142,000
|
486,000
|
(571,000
|
)
|
(h)
|
2,057,000
|
|||||||||||
|
Total operating expenses
|
3,690,000
|
486,000
|
(571,000
|
)
|
|
3,605,000
|
|||||||||||
|
|
|
||||||||||||||||
|
Loss from operations
|
(2,299,000
|
)
|
(357,000
|
)
|
570,000
|
|
(2,086,000
|
)
|
|||||||||
|
|
|
||||||||||||||||
|
Interest and other income (expense), net
|
--
|
(6,000
|
)
|
--
|
|
(6,000
|
)
|
||||||||||
|
|
|
||||||||||||||||
|
Net loss
|
$
|
(2,299,000
|
)
|
$
|
(363,000
|
)
|
$
|
570,000
|
|
$
|
(2,092,000
|
)
|
|||||
|
|
|
||||||||||||||||
|
Per share data:
|
|
||||||||||||||||
|
Basic and diluted net loss per common share
|
$
|
(0.14
|
)
|
|
$
|
(0.07
|
)
|
||||||||||
|
|
|
||||||||||||||||
|
Shares used in computing per share data
|
16,662,891
|
|
29,391,445
|
||||||||||||||
See accompanying notes.
F-27
for Year Ended June 30, 2013
|
|
ThermoGenesis
Historical
|
TotiRX
Historical
|
Pro Forma
Adjustments
|
|
Pro Forma Combined
|
||||||||||||
|
|
|
||||||||||||||||
|
Net revenues
|
$
|
17,963,000
|
$
|
1,478,000
|
$
|
(161,000
|
)
|
(b)
|
$
|
19,280,000
|
|||||||
|
Cost of revenues
|
11,598,000
|
1,047,000
|
(156,000
|
)
|
(b)
|
12,489,000
|
|||||||||||
|
Gross profit
|
6,365,000
|
431,000
|
(5,000
|
)
|
|
6,791,000
|
|||||||||||
|
|
|
||||||||||||||||
|
Expenses:
|
|
||||||||||||||||
|
Sales and marketing
|
2,955,000
|
--
|
--
|
|
2,955,000
|
||||||||||||
|
Research and development
|
2,991,000
|
--
|
--
|
|
2,991,000
|
||||||||||||
|
General and administrative
|
5,645,000
|
1,689,000
|
286,000
|
(h)
|
7,620,000
|
||||||||||||
|
Gain on sale of product lines
|
(2,161,000
|
)
|
-
|
-
|
|
(2,161,000
|
)
|
||||||||||
|
Total operating expenses
|
9,430,000
|
1,689,000
|
286,000
|
|
11,405,000
|
||||||||||||
|
Loss from operations
|
(3,065,000
|
)
|
(1,258,000
|
)
|
(291,000
|
)
|
|
(4,614,000
|
)
|
||||||||
|
|
|
||||||||||||||||
|
Interest and other income (expense), net
|
(21,000
|
)
|
(10,000
|
)
|
-
|
|
(31,000
|
)
|
|||||||||
|
|
|
||||||||||||||||
|
Net loss
|
$
|
(3,086,000
|
)
|
$
|
(1,268,000
|
)
|
$
|
(291,000
|
)
|
|
$
|
(4,645,000
|
)
|
||||
|
|
|
||||||||||||||||
|
Per share data:
|
|
||||||||||||||||
|
Basic and diluted net loss per common share
|
$
|
(0.19
|
)
|
|
$
|
(0.16
|
)
|
||||||||||
|
|
|
||||||||||||||||
|
Shares used in computing per share data
|
16,526,578
|
|
29,192,843
|
||||||||||||||
See accompanying notes.
F-28
|
Note 1.
|
Basis of Pro Forma Presentation
|
On July 15, 2013, ThermoGenesis and TotiRX entered into an Agreement and Plan of Merger and Reorganization (the Merger Agreement) in connection with the merger, TotiRX will merge with and into ThermoGenesis (the Merger). The merged company will be renamed Cesca Therapeutics.
At the effective time of the merger, the outstanding shares of TotiRX common stock will be converted into the right to receive 12,490,841 shares of ThermoGenesis common stock representing approximately 43% of the shares of outstanding common stock of the combined company, excluding the issuance of shares pursuant to ThermoGenesis and TotiRX outstanding options and warrants. Additionally, at the effective time of the merger, notes payable to related parties will be repaid through payment of $150,000 cash and issuance of shares of ThermoGenesis common stock equal to the remaining balance divided by the 10 day volume weighted average price. The Merger is intended to qualify as a tax-free reorganization under the provisions of Section 368(a) of the Code and is subject to customary closing conditions, including approval by ThermoGenesis and TotiRX stockholders.
Because ThermoGenesis’ stockholders will own approximately 57% of the common shares of the combined company immediately following consummation of the Merger, ThermoGenesis is deemed to be the acquirer for accounting purposes.
Accordingly, the assets and liabilities of TotiRX will be recognized at fair value upon consummation of the Merger, which is expected to be the acquisition date for accounting purposes, and any direct costs of the Merger will be charged to expense in the period incurred.
|
Note 2.
|
Estimated Consideration Transferred and Purchase Price Allocation
|
As of November 29, 2013, there were approximately 16,677,909 shares of ThermoGenesis common stock outstanding. Based upon a closing price of $0.736 on November 29, 2013, the fair value of such ThermoGenesis common shares was approximately $12,275,000. The estimated consideration transferred consists of the following:
|
|
Shares
|
Value
|
||||||
|
ThermoGenesis common stock at $0.736 per share
|
12,490,841
|
$
|
9,198,000
|
|||||
|
Repayment of related party notes payable
|
||||||||
|
Cash payment
|
150,000
|
|||||||
|
ThermoGenesis common stock at $0.749 per share
|
237,713
|
178,000
|
||||||
|
Total preliminary estimated purchase price
|
12,728,554
|
$
|
9,526,000
|
|||||
F-29
The per share price of ThermoGenesis common stock is subject to change until the acquisition date and, as a result, the estimated consideration transferred and resultant goodwill, if any, will fluctuate. Below are the significant changes to the pro forma condensed combined balance sheet that would occur upon a 20% increase or decrease in the price of ThermoGenesis common stock based on an estimated stock price of $0.736 per share at November 30, 2013:
|
Stock Price
|
% Change in
Stock Price
|
Total Preliminary
Estimated
Purchase Price
|
Goodwill
|
|||||||||||
|
$
|
0.589
|
-20%
|
|
$
|
7,685,000
|
--
|
||||||||
|
$
|
0.736
|
0%
|
|
$
|
9,526,000
|
$
|
305,000
|
|||||||
|
$
|
0.884
|
20%
|
|
$
|
11,370,000
|
$
|
2,149,000
|
|||||||
Under the acquisition method of accounting, identifiable assets acquired and liabilities assumed will be recognized and measured at fair value at the acquisition date, with any residual amount from the consideration transferred being recognized as goodwill. For purposes of this unaudited pro forma condensed combined financial information, ThermoGenesis and TotiRX have made an estimate of the acquisition date fair values of assets acquired and liabilities assumed, with the residual value recognized as goodwill, as follows:
|
Tangible Assets
|
||||
|
Current assets
|
$
|
881,000
|
||
|
Property, plant, and equipment
|
374,000
|
|||
|
Other non-current assets
|
68,000
|
|||
|
Total tangible assets acquired
|
1,323,000
|
|||
|
Intangible Assets
|
||||
|
Clinical protocols
|
6,041,000
|
|||
|
Other intangible assets
|
2,714,000
|
|||
|
Total intangible assets acquired
|
8,755,000
|
|||
|
Total assets acquired
|
10,078,000
|
|||
|
Less: liabilities assumed (excluding notes payable to related parties)
|
(857,000
|
)
|
||
|
Total identifiable net assets
|
9,221,000
|
|||
|
Goodwill
|
305,000
|
|||
|
Total preliminary estimated purchase price
|
$
|
9,526,000
|
||
A final determination of estimated consideration transferred and fair value, which cannot be made prior to the acquisition date, will be based on the actual net assets of TotiRX that exist as of the acquisition date. The assets identified and amounts recorded at the acquisition date may differ materially from the information presented in this pro forma financial information as a result of:
| · | The fair value of ThermoGenesis common stock at the acquisition date; |
| · | The final valuation of the tangible and intangible assets of TotiRX; |
| · | TotiRX’s net debt (as defined in the Merger Agreement) as of the acquisition date; |
| · | TotiRX’s net cash (as defined in the Merger Agreement) as of the acquisition date; |
| · | The timing of the acquisition date; and |
| · | Other changes in TotiRX’s net assets that occur prior to the acquisition date, which could cause material differences in the information presented herein. |
F-30
|
Note 3.
|
Pro Forma Adjustments
|
The following adjustments have been reflected in the unaudited pro forma condensed combined balance sheet as of September 30, 2013 and unaudited pro forma condensed combined statement of operations for the three months ended September 30, 2013 and the year ended June 30, 2013.
| (a) | To record the net effect on cash and cash equivalents as follows: |
|
|
Three months ended
September 30, 2013
|
Twelve months ended
June 30, 2013
|
Total
|
|||||||||
|
Source of Cash to fund the Merger
|
||||||||||||
|
Cash
|
$
|
789,000
|
$
|
434,000
|
$
|
1,223,000
|
||||||
|
|
||||||||||||
|
Uses of Cash to fund the Merger
|
||||||||||||
|
Professional fees to support the merger reflected in the Company’s September 30, 2013 balance sheet
|
865,000
|
873,000
|
1,738,000
|
|||||||||
|
Repayment of notes payable to related parties
|
--
|
(150,000
|
)
|
(150,000
|
)
|
|||||||
|
Incremental payments for new employment contracts
|
(76,000
|
)
|
(289,000
|
)
|
(365,000
|
)
|
||||||
|
|
$
|
789,000
|
$
|
434,000
|
$
|
1,223,000
|
||||||
| (b) | To eliminate intercompany revenue, purchases, inventory, receivable and payable balances as follows: |
|
|
Three months ended
September 30, 2013
|
Twelve months ended
June 30, 2013
|
||||||
|
Receivables/Payables
|
$
|
108,000
|
127,000
|
|||||
|
|
||||||||
|
ThermoGenesis sales to TotiRX
|
$
|
56,000
|
161,000
|
|||||
|
Decrease to cost of goods for inventory sold to third parties
|
(55,000
|
)
|
(156,000
|
)
|
||||
|
Decrease to inventory for items not yet sold to third parties
|
(1,000
|
)
|
(5,000
|
)
|
||||
F-31
|
(c)
|
To record the adjustment to intangible assets as follows:
|
|
Intangibles Asset
|
Fair Value
|
Useful
Life
|
Amortization for
three months ended
September 30, 2013
|
Amortization for
twelve months ended
June 30, 2013
|
Total
Amortization
|
|||||||||||||||
|
Customer Relationships
|
$
|
439,000
|
1
|
$
|
110,000
|
$
|
439,000
|
$
|
549,000
|
|||||||||||
|
Non-Compete Agreements
|
1,456,000
|
5
|
73,000
|
291,000
|
364,000
|
|||||||||||||||
|
Device Registration
|
141,000
|
5
|
7,000
|
28,000
|
35,000
|
|||||||||||||||
|
Licenses
|
354,000
|
5
|
18,000
|
71,000
|
89,000
|
|||||||||||||||
|
Trade Name
|
324,000
|
8
|
10,000
|
41,000
|
51,000
|
|||||||||||||||
|
Clinical Protocols
|
6,041,000
|
10
|
--
|
--
|
--
|
|||||||||||||||
|
Total
|
$
|
8,755,000
|
$
|
218,000
|
$
|
870,000
|
$
|
1,088,000
|
||||||||||||
|
|
||||||||||||||||||||
|
|
$
|
8,755,000
|
||||||||||||||||||
|
|
(1,088,000
|
)
|
||||||||||||||||||
|
|
$
|
7,667,000
|
||||||||||||||||||
Intangible assets are amortized once they start to provide economic benefit, using the straight-line method over their useful life. Clinical protocols are not expected to provide economic benefit until they are introduced to the marketplace. Since it is not certain when each clinical protocol will be introduced into the marketplace, no amortization for the clinical protocols is reflected in the unaudited pro forma condensed information.
| (d) | To record the adjustment for goodwill resulting from the merger (see note 2). |
| (e) | To record the adjustment to deferred revenue to reflect estimated fair value. |
|
Deferred revenue
|
$
|
(42,000
|
)
|
|
|
Long term deferred revenue
|
$
|
(218,000
|
)
|
| (f) | To record adjustment to eliminate TotiRX common stock, paid-in-capital, accumulated deficit, accumulated other comprehensive income, and non-controlling interest and repayment of notes payable to related parties in exchange for ThermoGenesis common stock and cash (see note 2). |
| (g) | To record adjustment to accumulated deficit as follows: |
|
Eliminate TotiRX accumulated deficit
|
$
|
2,404,000
|
||
|
Amortization of acquired intangible assets
|
(1,088,000
|
)
|
||
|
Legal, accounting and financial advisory fees to support the merger.
|
1,738,000
|
|||
|
Incremental costs of employment agreements associated with the merger
|
(365,000
|
)
|
||
|
Eliminate intercompany gross profit
|
(1,000
|
)
|
||
|
|
$
|
2,688,000
|
F-32
| (h) | To record adjustment to general and administrative as follows: |
|
|
Three months ended
Sep 2013
|
Twelve months ended
Jun 2013
|
||||||
|
Amortization of acquired intangible assets
|
$
|
218,000
|
$
|
870,000
|
||||
|
Incremental costs of employment agreements associated with the merger
|
76,000
|
289,000
|
||||||
|
Less legal, accounting and financial advisory fees to support the merger and reflected in the unaudited pro forma condensed combined financial information
|
(865,000
|
)
|
(873,000
|
)
|
||||
|
|
$
|
(571,000
|
)
|
$
|
286,000
|
|||
It is estimated that an additional $300,000 of legal, accounting and financial advisory fees directly attributable to the merger will be incurred within the next 12 months. These fees have not been included in the unaudited pro forma condensed combined financial information.
|
Note 4.
|
Pro Forma Net Loss Per Share of Common Stock
|
The number of shares of common stock used to calculate the basic and diluted earnings per share was calculated as follows:
|
|
Three months ended
Sep 2013
|
Twelve months ended
Jun 2013
|
||||||
|
Shares used for computing ThermoGenesis historical per share data
|
16,662,891
|
16,526,578
|
||||||
|
Shares to be issued for acquisition of net assets
|
12,490,841
|
12,490,841
|
||||||
|
Shares to be issued to repay notes payable to related parties
|
237,713
|
175,424
|
||||||
|
|
29,391,445
|
29,192,843
|
||||||
F-33
EXHIBIT INDEX
|
Exhibit No.
|
Document Description
|
Incorporation by Reference
|
|
|
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm
|
Filed herewith.
|
||
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Cesca Therapeutics Inc.
|
|
|
|
|
a Delaware Corporation
|
|
|
|
|
|
|
|
|
/s/ Dan T. Bessey
|
|
|
|
|
|
Dan T. Bessey, Chief Financial Officer
|
|
