Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ignyta, Inc. | d679231d8k.htm |
| EX-99.1 - EX-99.1 - Ignyta, Inc. | d679231dex991.htm |
| Exhibit 99.2
|
Exhibit 99.2
RXDX-101
&
RXDX-102
Justin Gainor, MD
February 20th, 2014
|
|
Background
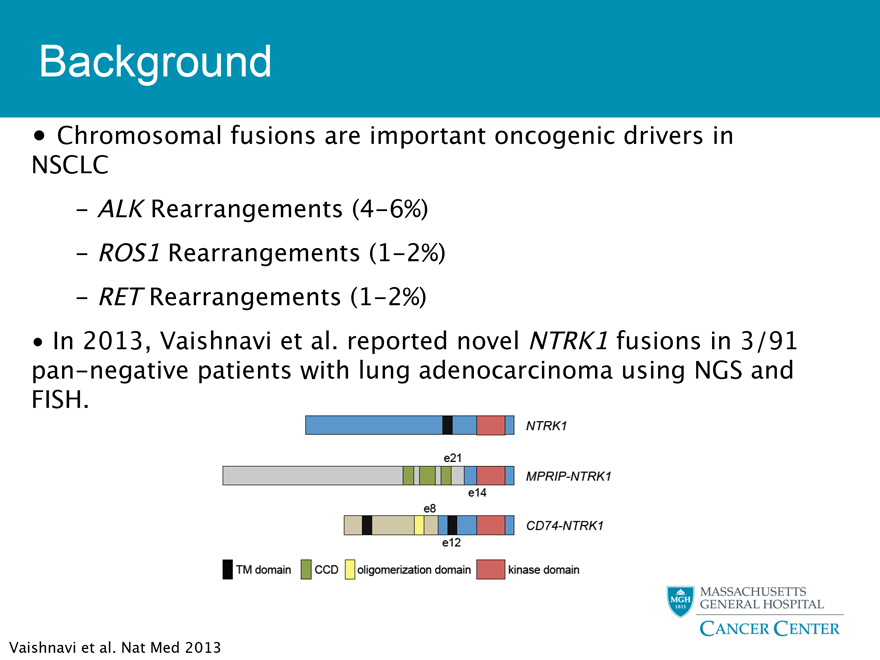
Chromosomal fusions are important oncogenic drivers in NSCLC
- ALK Rearrangements (4-6%)
- ROS1 Rearrangements (1-2%)
- RET Rearrangements (1-2%)
In 2013, Vaishnavi et al. reported novel NTRK1 fusions in 3/91 pan-negative patients with lung adenocarcinoma using NGS and FISH.
Vaishnavi et al. Nat Med 2013
|
|
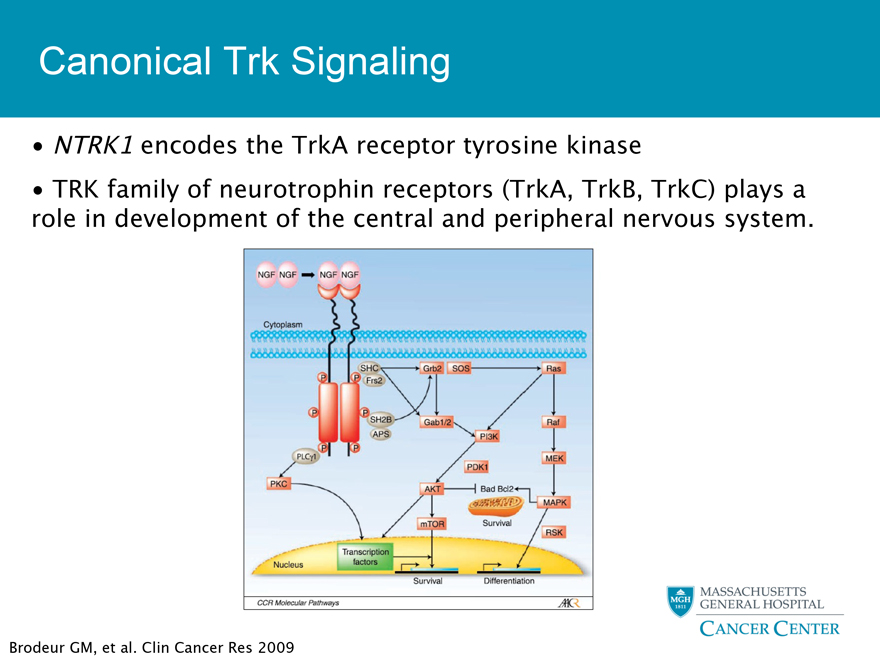
Canonical Trk Signaling
NTRK1 encodes the TrkA receptor tyrosine kinase
TRK family of neurotrophin receptors (TrkA, TrkB, TrkC) plays a role in development of the central and peripheral nervous system.
Brodeur GM, et al. Clin Cancer Res 2009
|
|
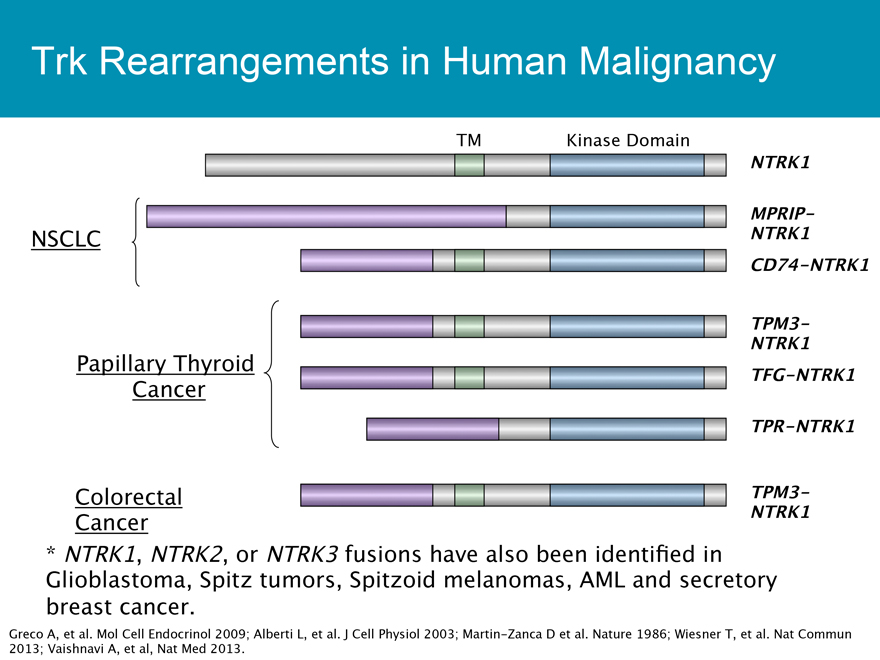
Trk Rearrangements in Human Malignancy
TM Kinase Domain
NTRK1
MPRIP-
NSCLC NTRK1
CD74-NTRK1
TPM3-
NTRK1
Papillary Thyroid
TFG-NTRK1
Cancer
TPR-NTRK1
ColorectalTPM3-
Cancer NTRK1
* NTRK1, NTRK2, or NTRK3 fusions have also been identified in
Glioblastoma, Spitz tumors, Spitzoid melanomas, AML and secretory breast cancer.
Greco A, et al. Mol Cell Endocrinol 2009 ; Alberti L, et al. J Cell Physiol 2003 ; Martin-Zanca D et al. Nature 1986 ; Wiesner T, et al. Nat Commun 2013 ; Vaishnavi A, et al, Nat Med 2013.
|
|
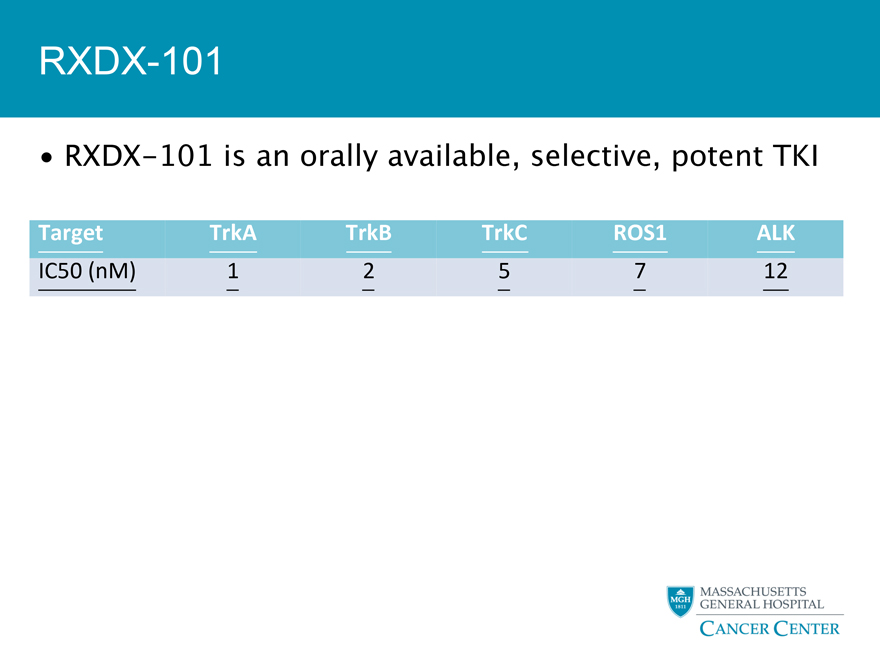
RXDX-101
RXDX-101 is an orally available, selective, potent TKI
Target TrkA TrkB TrkC ROS1 ALK
IC50
(nM) 1 2 5 7 12
|
|
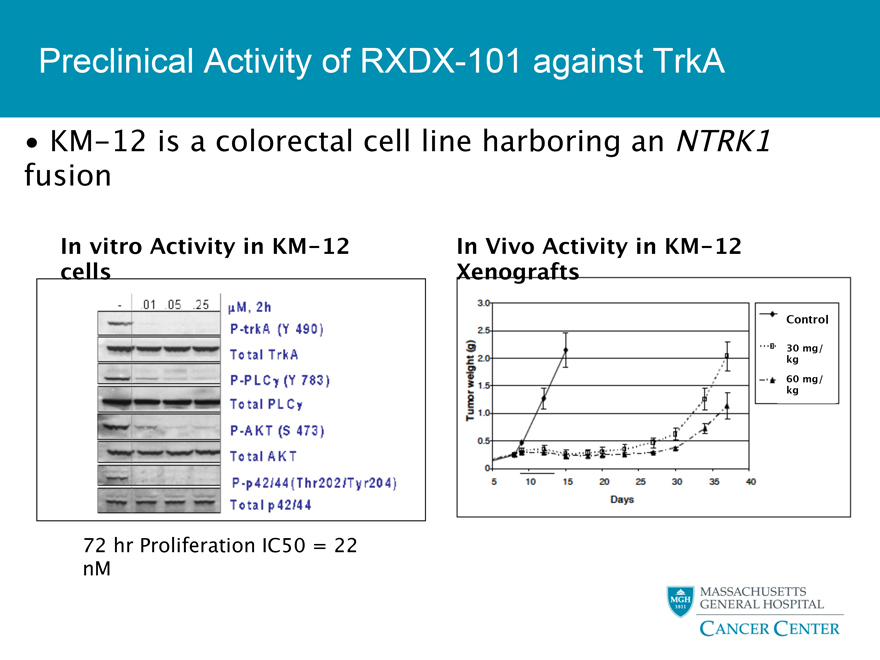
Preclinical Activity of RXDX-101 against TrkA
KM-12 is a colorectal cell line harboring an NTRK1 fusion
In vitro Activity in KM-12In Vivo Activity in KM-12 cells Xenografts
Control
30 mg/ kg
60 mg/ kg
72 hr Proliferation IC50 = 22 nM
|
|
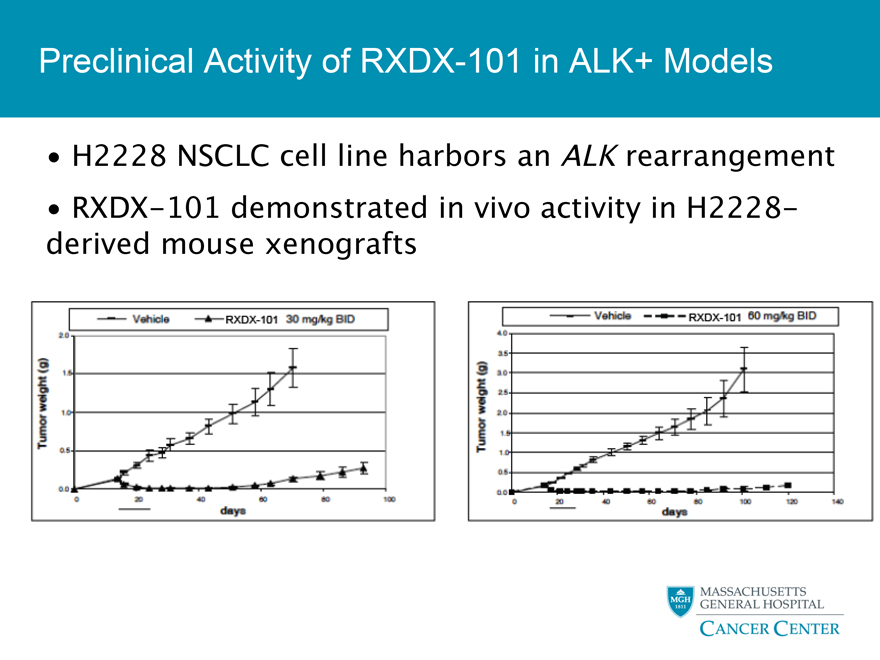
Preclinical Activity of RXDX-101 in ALK+ Models
H2228 NSCLC cell line harbors an ALK rearrangement
RXDX-101 demonstrated in vivo activity in H2228 -derived mouse xenografts
|
|
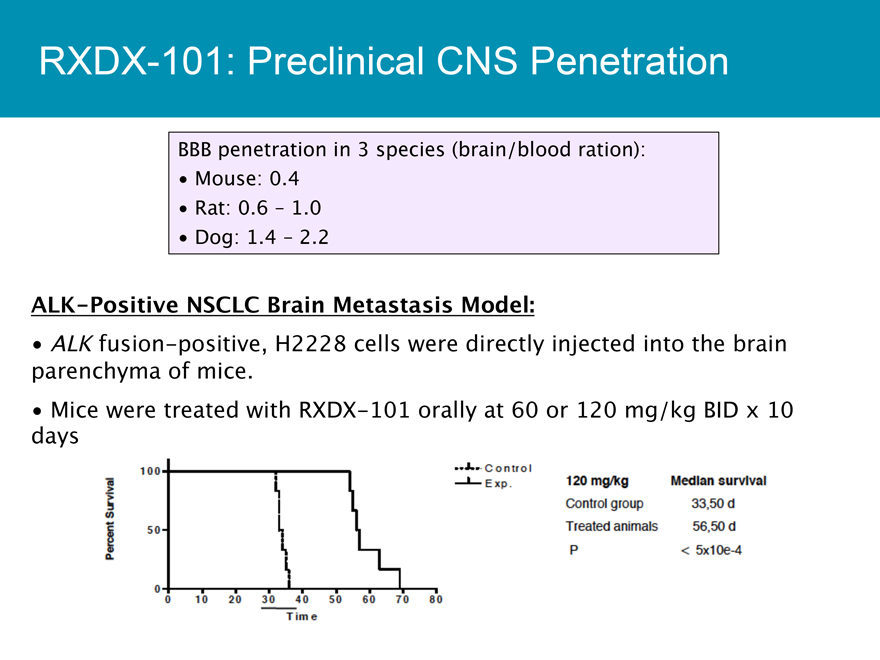
RXDX-101: Preclinical CNS Penetration
BBB penetration in 3 species (brain/blood ration):
Mouse: 0.4
Rat: 0.6 - 1.0
Dog: 1.4 - 2.2
ALK-Positive NSCLC Brain Metastasis Model:
ALK fusion-positive, H2228 cells were directly injected into the brain parenchyma of mice.
Mice were treated with RXDX-101 orally at 60 or 120 mg/kg BID x 10 days
|
|
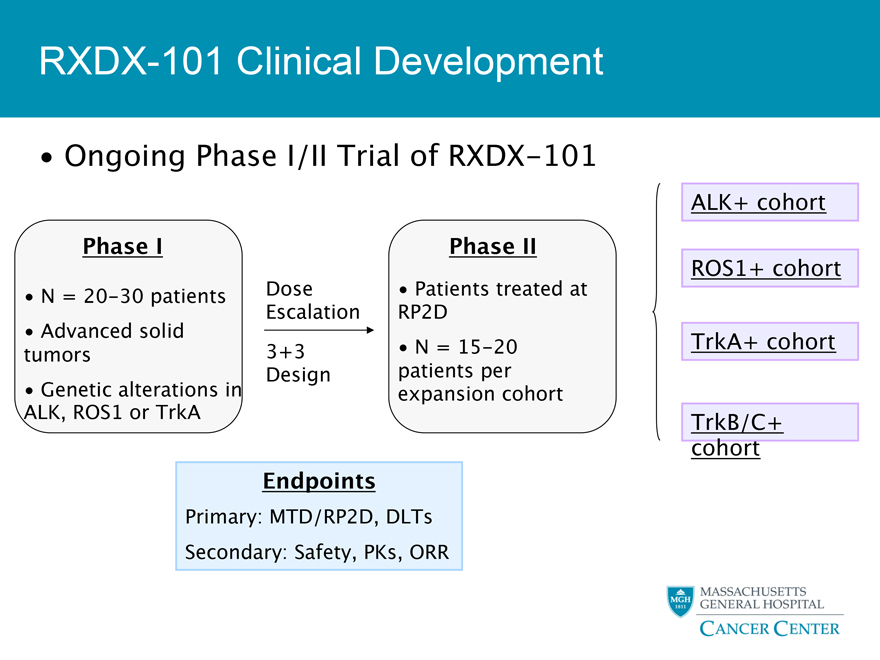
RXDX-101 Clinical Development
Ongoing Phase I/II Trial of RXDX-101
ALK+ cohort
Phase I Phase II
ROS1+ cohort
N = 20-30 patients Dose Patients treated at Escalation RP2D
Advanced solid TrkA+ cohort tumors 3+3 N = 15-20 Design patients per
Genetic alterations in expansion cohort
ALK, ROS1 or TrkA TrkB/C+
cohort
Endpoints
Primary: MTD/RP2D, DLTs
Secondary: Safety, PKs, ORR
|
|
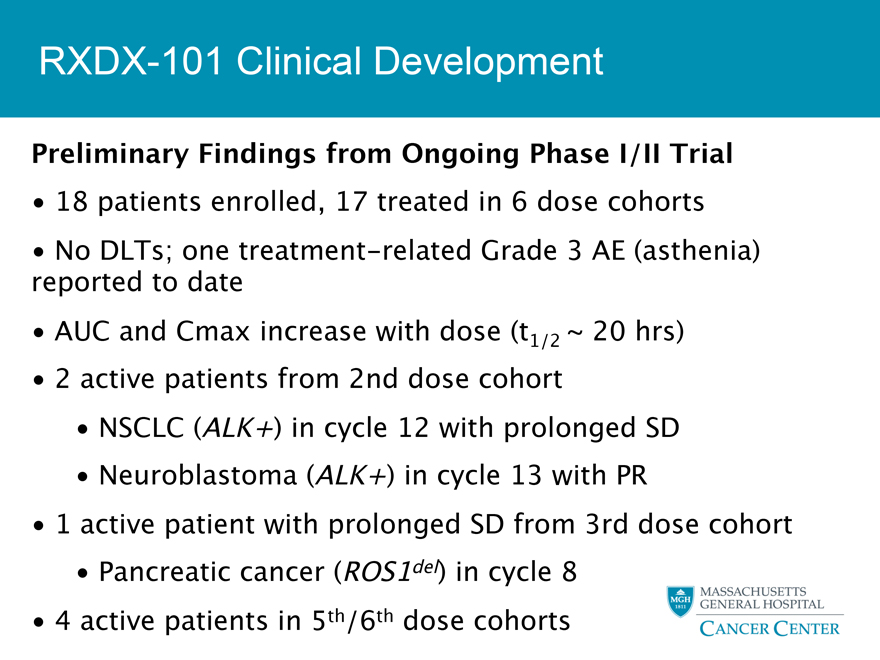
RXDX-101 Clinical Development
Preliminary Findings from Ongoing Phase I/II Trial
18 patients enrolled, 17 treated in 6 dose cohorts
No DLTs; one treatment-related Grade 3 AE (asthenia) reported to date
AUC and Cmax increase with dose (t1/2 ~ 20 hrs)
2 active patients from 2nd dose cohort
NSCLC (ALK+) in cycle 12 with prolonged SD
Neuroblastoma (ALK+) in cycle 13 with PR
1 active patient with prolonged SD from 3rd dose cohort
Pancreatic cancer (ROS1del) in cycle 8
4 active patients in 5th/6th dose cohorts
|
|
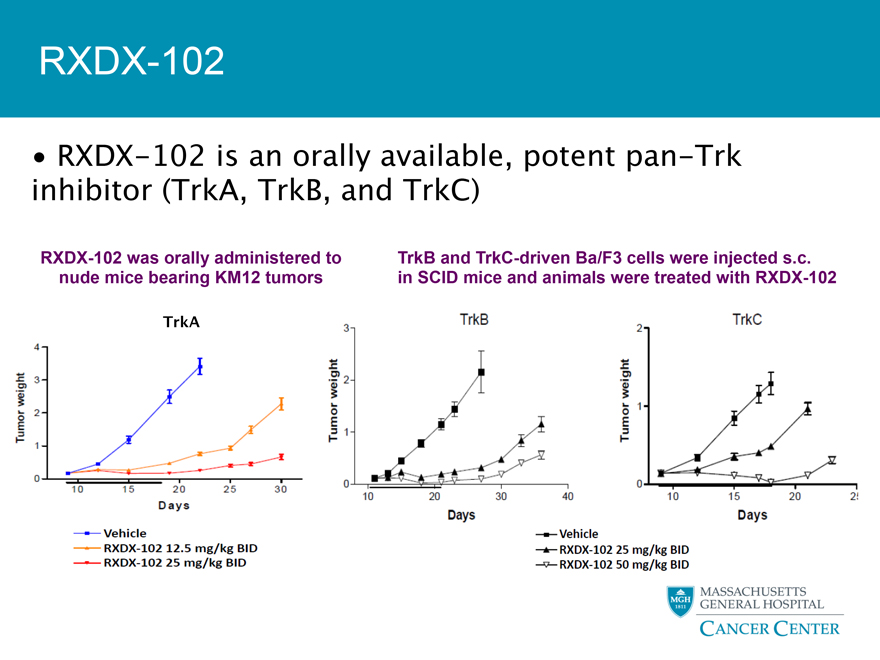
RXDX-102
RXDX-102 is an orally available, potent pan-Trk inhibitor (TrkA, TrkB, and TrkC)
RXDX-102 was orally administered to TrkB and TrkC-driven Ba/F3 cells were injected s.c. nude mice bearing KM12 tumors in SCID mice and animals were treated with RXDX-102
TrkA
|
|
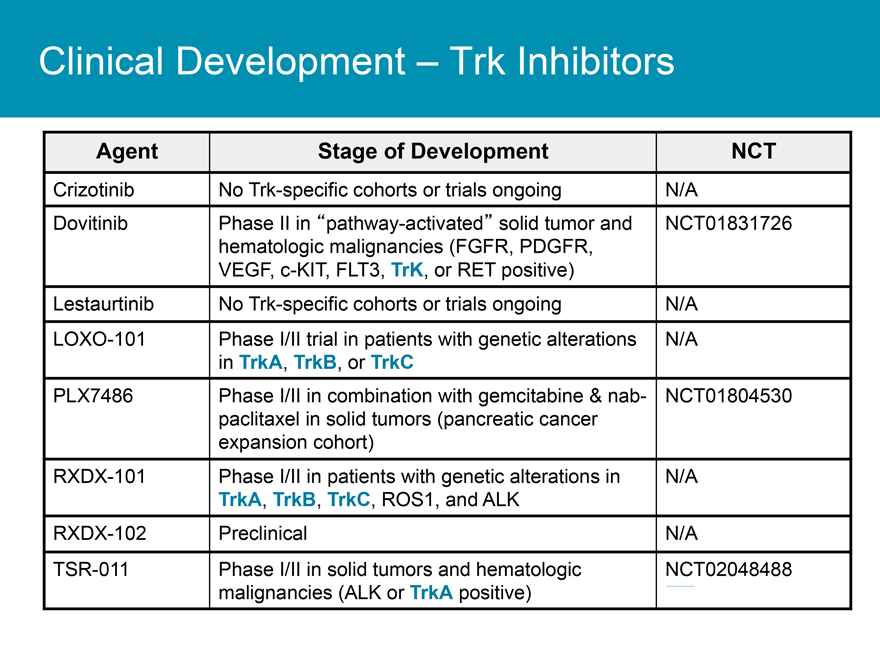
Clinical Development _ Trk Inhibitors
Agent Stage of Development NCT
Crizotinib No Trk-specific cohorts or trials ongoing N/A
Dovitinib Phase II in “pathway-activated” solid tumor and NCT01831726 hematologic malignancies (FGFR, PDGFR, VEGF, c-KIT, FLT3, TrK, or RET positive)
Lestaurtinib No Trk-specific cohorts or trials ongoing N/A LOXO-101 Phase I/II trial in patients with genetic alterations N/A in TrkA, TrkB, or TrkC
PLX7486 Phase I/II in combination with gemcitabine & nab- NCT01804530 paclitaxel in solid tumors (pancreatic cancer expansion cohort) RXDX-101 Phase I/II in patients with genetic alterations in N/A
TrkA, TrkB, TrkC, ROS1, and ALK
RXDX-102 Preclinical N/A
TSR-011 Phase I/II in solid tumors and hematologic NCT02048488 malignancies (ALK or TrkA positive)
|
|
Summary
RXDX-101 is a orally available pan-Trk, ROS1 and ALK inhibitor.
RXDX-101 demonstrates in vitro and in vivo activity in TrkA- and ALK-driven lung cancer models.
A Phase I/II trial of RXDX-101 is currently ongoing. No DLTs have been reported to date.
Additional genotyping studies are needed to determine the frequency of NTRK fusions in other malignancies and in the general NSCLC population, which may inform clinical development of Trk inhibitors.