Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - Vericel Corp | a14-5343_1ex23d1.htm |
| EX-5.1 - EX-5.1 - Vericel Corp | a14-5343_1ex5d1.htm |
|
As filed with the Securities and Exchange Commission on . |
|
Registration No. 333- |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
AASTROM BIOSCIENCES, INC.
(Exact name of registrant as specified in its charter)
|
Michigan |
|
94-3096597 |
24 Frank Lloyd Wright Drive
Lobby K
Ann Arbor, Michigan 48105
(734) 418-4400
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Dominick C. Colangelo
President and Chief Executive Officer
Aastrom Biosciences, Inc.
Lobby K
Ann Arbor, Michigan 48105
(734) 418-4400
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Mitchell S. Bloom
Danielle M. Lauzon
Goodwin Procter LLP
Exchange Place
Boston, Massachusetts 02109
Telephone: (617) 570-1000
Facsimile: (617) 523-1231
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement, as determined by the selling shareholder.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated |
|
Accelerated |
|
Non-accelerated filer o |
|
Smaller reporting |
CALCULATION OF REGISTRATION FEE
|
|
|
|
|
Proposed |
|
Proposed |
|
|
| |||
|
|
|
|
|
Maximum |
|
Maximum |
|
|
| |||
|
Title of Each Class of |
|
Amount to be |
|
Offering Price |
|
Aggregate |
|
Amount of |
| |||
|
Securities to be Registered(1) |
|
Registered (2) (3) |
|
Per Share (4) |
|
Offering Price (4) |
|
Registration Fee |
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock (no par value) |
|
1,748,063 |
|
$ |
3.47 |
|
$ |
6,065,779 |
|
$ |
781 |
|
(1) This Registration Statement also relates to the rights to purchase shares of Series A Junior Participating Cumulative Preferred Stock of the Registrant which are attached to all shares of Common Stock outstanding as of, and issued subsequent to, August 15, 2011, pursuant to the terms of the Registrant’s Shareholder Rights Agreement, dated August 11, 2011, as amended on March 9, 2012. Until the occurrence of certain prescribed events, the Rights are not exercisable, are evidenced by the certificates for the Common Stock and will be transferred with and only with such stock.
(2) The registrant is registering for resale, from time to time, up to 1,700,000 shares of its common stock that the registrant may sell and issue to Lincoln Park Capital Fund, LLC (or Lincoln Park) pursuant to a Purchase Agreement (or the Purchase Agreement), dated January 21, 2014, by and between Lincoln Park and the registrant relating to the sale of up to $15,000,000 in shares of common stock of the registrant. Pursuant to Rule 416(a) under the Securities Act of 1933, the registrant is also registering hereunder an indeterminate number of shares that may be issued and resold resulting from stock splits, stock dividends or similar transactions.
(3) Represents (i) up to 1,700,000 shares that may be sold to Lincoln Park pursuant to the Purchase Agreement and (ii) up to 48,063 shares that will be issued to Lincoln Park as commitment shares.
(4) Estimated pursuant to Rule 457(c) solely for the purpose of calculating the registration fee, based upon the average of the high and low prices for the registrant’s common stock as reported on The NASDAQ Capital Market on February 4, 2014, a date within five business days of the filing of this registration statement, of $3.47.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
|
|
|
|
|
| ||
|
PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION |
DATED FEBRUARY 10, 2014 | ||||
|
|
|
|
|
| ||
1,748,063 Shares
of Common Stock

|
|
|
|
This prospectus relates to the resale, from time to time, of up to 1,748,063 shares of our common stock by Lincoln Park Capital Fund, LLC. Lincoln Park Capital Fund, LLC is sometimes referred to in this prospectus as “selling shareholder” or “Lincoln Park”. The shares of common stock being offered by Lincoln Park are issuable pursuant to a Purchase Agreement we entered into with Lincoln Park on January 21, 2014, which we refer to in this prospectus as the Purchase Agreement. See the section of this prospectus entitled “The Lincoln Park Transaction” for a description of the Purchase Agreement and the section entitled “Selling Shareholder” for additional information about Lincoln Park. Such registration does not mean that Lincoln Park will actually offer or sell any of these shares. We will not receive any proceeds from the sales of shares of our common stock by Lincoln Park; however, we may receive proceeds of up to $15,000,000 under the Purchase Agreement.
Lincoln Park is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act of 1933, as amended. Lincoln Park may offer the shares pursuant to this prospectus for resale in a number of different ways through public or private placement transactions and at varying prices. The prices at which Lincoln Park may sell the shares will be determined by the prevailing market price for the shares or in privately negotiated transactions. See “Plan of Distribution” for additional information.
Our common stock is traded on the NASDAQ Capital Market under the symbol “ASTM”. On February 4, 2014, the last reported sales price of our common stock was $3.56 per share.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 10 of this prospectus for a discussion of information that should be considered in connection with an investment in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
|
|
The date of this Prospectus is , 2014.
|
|
Page |
|
|
|
|
ii | |
|
|
|
|
1 | |
|
|
|
|
7 | |
|
|
|
|
9 | |
|
|
|
|
10 | |
|
|
|
|
23 | |
|
|
|
|
24 | |
|
|
|
|
24 | |
|
|
|
|
25 | |
|
|
|
|
26 | |
|
|
|
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
27 |
|
|
|
|
29 | |
|
|
|
|
33 | |
|
|
|
|
34 | |
|
|
|
|
35 | |
|
|
|
|
CERTAIN PROVISIONS OF MICHIGAN LAW AND OF OUR CHARTER AND BYLAWS |
38 |
|
|
|
|
39 | |
|
|
|
|
41 | |
|
|
|
|
41 | |
|
|
|
|
41 | |
|
|
|
|
42 | |
|
|
|
|
44 |
You may rely only on the information provided or incorporated by reference in this prospectus and the documents incorporated herein and therein by reference, or in a prospectus supplement or amendment thereto. We have not and Lincoln Park has not authorized anyone to provide you with information different from that contained in or incorporated by reference into this prospectus. We and the underwriters are offering to sell, and seeking offers to buy, common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus, any free writing prospectus, or document incorporated by reference is accurate only as of its date, regardless of the time of delivery of this prospectus or of any sale of our common stock.
Information contained on our website is not part of this prospectus. You should read this prospectus together with additional information described under the heading “Where You Can Find More Information” below. In various places in this prospectus, we refer you to sections for additional information by indicating the caption heading of the other sections. All cross-references in this prospectus are to captions contained in this prospectus, unless otherwise indicated.
For investors outside the United States: We have not and Lincoln Park has not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside the United States.
PRESENTATION NOTE: We implemented a twenty-to-one reverse stock split on October 16, 2013. All share numbers and prices in this prospectus have been adjusted to reflect the reverse stock split, unless indicated otherwise.
This summary highlights information contained elsewhere in this prospectus or incorporated by reference in this prospectus. This summary does not contain all of the information that you should consider in making your investment decision. You should read the entire prospectus carefully, especially the discussion regarding the risks of investing in our securities under the heading “Risk Factors” beginning on page of this prospectus and our financial statements and related notes incorporated by reference in this prospectus, before investing in our securities. In this prospectus, “Aastrom,” the “Company,” “we,” “us,” and “our” refer to Aastrom Biosciences, Inc. Please refer to our Glossary at the end of this Prospectus for certain industry-specific and technical definitions.
Aastrom Biosciences, Inc.
Business Overview
We are a clinical-stage biotechnology company focused on developing innovative cell therapies that repair and regenerate damaged tissue for use in the treatment of severe, chronic ischemic cardiovascular diseases. We are developing patient-specific (autologous) multicellular therapies utilizing our proprietary, highly automated and scalable manufacturing system. Our manufacturing technology platform, the Aastrom Replicell System (ARS), enables the expansion of a variety of cell types, including the production of multicellular therapies expanded from an adult patient’s own bone marrow, which can be delivered directly to damaged tissues using conventional syringes and cell injection catheter systems.
Our lead product, ixmyelocel-T, has demonstrated multiple biological activities that promote tissue repair and regeneration by reducing inflammation, promoting angiogenesis and remodeling ischemic tissue. Preclinical and clinical data suggest that ixmyelocel-T is safe and effective in treating patients with severe, chronic ischemic cardiovascular diseases such as advanced heart failure due to dilated cardiomyopathy (DCM), the third leading cause of heart failure, and critical limb ischemia (CLI), the most severe form of peripheral arterial disease (PAD).
Our lead ixmyelocel-T clinical development program is for the treatment of advanced heart failure due to ischemic DCM. Ixmyelocel-T has been granted a U.S. Orphan Drug designation by the U.S. Food and Drug Administration (FDA) for the treatment of DCM, which we believe provides an efficient and cost-effective path to approval for ixmyelocel-T in this heart failure indication. We are currently enrolling our phase 2b ixCELL-DCM study, which is a randomized, double-blind, placebo-controlled clinical trial for patients with advanced heart failure due to ischemic DCM. The study is designed to enroll 108 patients at approximately 35 sites across the United States and Canada. We also have ongoing ixmyelocel-T clinical programs for the treatment of CLI and craniofacial reconstruction, as well as preclinical research and development programs for the treatment of cardiovascular diseases.
Our Therapy
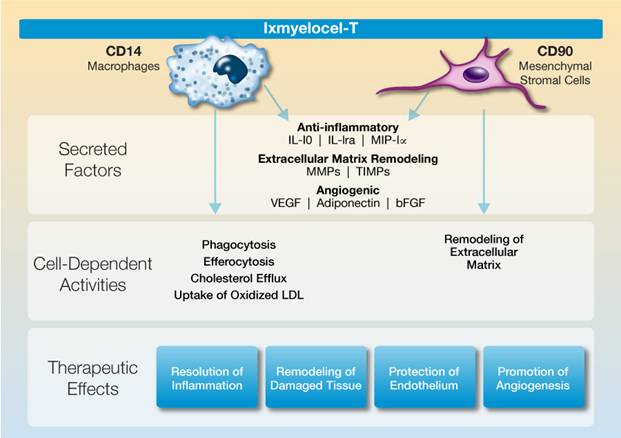
Ixmyelocel-T is a unique multicellular product derived from an adult patient’s own bone marrow. Our proprietary cell manufacturing process significantly expands the mesenchymal stromal cells (MSCs) and M2-like anti-inflammatory macrophages in the patient’s bone marrow mononuclear cells while retaining many of the hematopoietic cells. These cell types are known to regulate the immune response and play a key role in tissue repair and regeneration by resolving pathologic inflammation, promoting angiogenesis, and remodeling ischemic tissue. Ixmyelocel-T is the only multicellular product known to have expanded cell populations of both MSCs and M-2 like anti-inflammatory macrophages.
MSCs and M2-like macrophages have a wide range of biological activities that promote repair and regeneration of damaged tissues through the paracrine effects of their secreted factors, as well as their direct cell activities. These cells produce high levels of potent anti-inflammatory and angiogenic factors, as well as factors involved in extracellular matrix remodeling. These cells also have direct activities such as phagocytosis of cellular debris and apoptotic cells, which control the inflammatory response, uptake of LDL and removal of cholesterol, and
remodeling of extracellular matrix. We believe that, together, these paracrine effects and direct cell activities are responsible for ixmyelocel-T’s demonstrated therapeutic effects of resolving inflammation, promoting angiogenesis, and remodeling and repairing damaged tissue.
The following illustration summarizes the multiple biological activities of ixmyelocel-T that promote repair and regeneration of ischemic tissue:
Ixmyelocel-T has several features that we believe are primarily responsible for success in treating adult patients with severe ischemic cardiovascular diseases such as DCM and critical limb ischemia:
Patient-specific (autologous) — we start with the patient’s own cells, which are accepted by the patient’s immune system, allowing the cells to integrate into existing functional tissues. We believe that this characteristic of our therapy eliminates both the risk of rejection and the need to use immunosuppressive therapy pre- or post-therapy. Our data also suggests that ixmyelocel-T provides the potential for long-term engraftment and tissue repair.
Expanded — we begin with a small amount of bone marrow from the patient (up to 60 ml) and significantly expand the number of certain cell types, primarily MSCs and M2-like anti-inflammatory macrophages, to a substantially greater number than are present in the patient’s own bone marrow (up to 200 times the number of certain cell types compared with the starting bone marrow).
Multicellular — we believe the multiple cell types in ixmyelocel-T, which are normally found in bone marrow but in smaller quantities, possess the key functions required for reducing chronic inflammation and promoting angiogenesis and tissue repair. By reducing inflammation, we believe that ixmyelocel-T provides the ideal conditions to allow for the growth of new tissue and blood vessels.
Minimally invasive — our procedure for collecting bone marrow can be performed in an out-patient setting and takes approximately 15 minutes. Administration of ixmyelocel-T for the treatment of DCM is performed in the cardiac catheterization laboratory using a cell injection catheter system in a one-time procedure. For diseases such as CLI, administration of ixmyelocel-T is performed with a syringe in an outpatient setting in a one-time, approximately 20 minute procedure.
Safe — bone marrow and bone marrow-derived therapies have been used safely and efficaciously in medicine for over three decades. Ixmyelocel-T leverages this body of scientific study and medical experience, and appears well tolerated in over 200 patients treated to date.
Our Technology Platform
Our patient-specific multicellular therapies are manufactured using the Company’s proprietary Aastrom Repicell System (ARS) cell manufacturing system. Our manufacturing process is conducted in a highly-automated, fully-closed and rigorously controlled system. Our system is highly scalable and reproducible and located in a 5,000-square-foot centralized manufacturing facility in Ann Arbor, Michigan. Production is conducted under current Good Manufacturing Practices (cGMP) guidelines required by the FDA with current annual capacity to treat up to 3,000 patients.

Our Strategy
Our objective is to become the leading global biotechnology company in the development, manufacture, and commercialization of autologous multicellular therapies for the treatment of severe ischemic cardiovascular diseases. To achieve this objective, we intend to:
· Complete our phase 2b ixCELL-DCM clinical study for the treatment of advanced heart failure due to ischemic DCM and, if successful, progress ixmyelocel-T into pivotal phase 3 clinical studies for this orphan indication.
· Complete patient follow-up in the REVIVE-CLI study to evaluate safety and efficacy endpoints, and pursue opportunities through investigator-sponsored studies and strategic relationships to continue to develop ixmyelocel-T as a stand-alone and/or adjunct therapy for the treatment of critical limb ischemia.
· Conduct additional preclinical and clinical studies of ixmyelocel-T to pursue additional high-value indications for the treatment of severe ischemic cardiovascular diseases.
· Utilize our proprietary ARS cell-expansion manufacturing platform to expand our product portfolio of cell therapies for the treatment of immune/inflammatory, cardiovascular and fibrovascular diseases.
· Leverage our leading proprietary cell manufacturing platform and expertise to provide manufacturing services and capabilities to other development and commercial-stage biopharmaceutical companies.
· Prepare to commercialize ixmyelocel-T through continued development of our internal commercialization capabilities and/or strategic partnerships for North America, Europe and Asia.
Our Clinical Development Programs
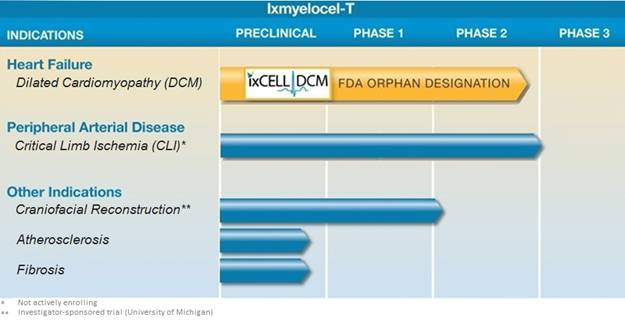
Our clinical development programs are focused on addressing areas of high unmet medical need in severe, chronic ischemic cardiovascular diseases. We have completed our Phase 1/2 clinical trials in DCM and we are currently enrolling our phase 2b ixCELL-DCM study, which is a randomized, double-blind, placebo-controlled clinical trial for patients with advanced heart failure due to ischemic DCM. Ixmyelocel-T has been granted a U.S. Orphan Drug designation by the FDA for the treatment of DCM. We also have ongoing ixmyelocel-T clinical programs for the treatment of CLI and craniofacial reconstruction.
The following summarizes the status of our clinical programs:
Heart Failure Due to Dilated Cardiomyopathy
Heart failure represents a significant unmet medical need and a growing public health problem. The American Heart Association reports that there are approximately 6 million patients currently suffering from heart failure in the United States and an estimated 650,000 new cases in the U.S. each year. Current medical costs to treat these patients exceed $25 billion and this is expected to more than triple to nearly $80 billion by 2030 as a result of a growing patient population and the high cost of the limited treatment alternatives for advanced heart failure patients, as described below.
DCM is the third leading cause of heart failure and the leading cause of heart transplantation in the United States. DCM is a disease characterized by weakening of the heart muscle, thinning of the heart walls, enlargement of the heart chambers, and the inability to sufficiently pump blood throughout the body. Patients with DCM typically present with symptoms of congestive heart failure, including limitations in physical activity and shortness of breath. Ischemic DCM is associated with atherosclerotic cardiovascular disease and prior heart attacks and is the most common form of dilated cardiomyopathy, representing an estimated 60% of all DCM patients. Patient prognosis depends on the stage and cause of the disease, but is typically characterized by a very poor quality of life and a high mortality rate.
Current treatments for ischemic DCM patients that are refractory to further medical therapy such as prescription drugs, devices, and/or further revascularization procedures including bypass surgery and angioplasty, are limited to
heart transplantation and placement of left ventricular assist devices (LVADs). There are less than 2,500 heart transplantations in the United States each year. Many refractory DCM patients are not eligible for heart transplantation and transplants are extremely expensive at an estimated cost of over $1 million. LVADs are also expensive at an estimated cost of over $175,000 and have a mortality rate of 50% at two years.
A majority of advanced heart failure patients that are refractory to medical therapy have DCM, and we believe that the refractory ischemic DCM market represents a substantial market opportunity for ixmyelocel-T. These refractory ischemic DCM patients are currently the target patient population for our clinical development of ixmyelocel-T, with approximately 175,000 patients in the United States alone. Ixmyelocel-T has been granted a U.S. Orphan Drug designation by the FDA for the treatment of DCM, which we believe provides an efficient and cost-effective path to approval for ixmyelocel-T in this heart failure indication.
We have conducted two phase 2a multicenter, randomized, open-label clinical studies in patients with ischemic DCM and nonischemic DCM investigating surgical (IMPACT-DCM) and catheter-based (Catheter-DCM) delivery of ixmyelocel-T. We reported 12-month data for the surgical IMPACT-DCM study at the Heart Failure Society of America meeting in September 2011 and final 12-month results from the Catheter-DCM study at the Society for Cardiovascular Angiography and Interventions (SCAI) 2012 Scientific Sessions. Results from these studies demonstrated that ixmyelocel-T was well-tolerated in patients with DCM. In the Catheter-DCM study and post-surgery in the IMPACT-DCM study, the incidence of adverse events was comparable between the ixmyelocel-T groups and the control groups. Cardiac failure was reported more frequently in the control group relative to ixmyelocel-T in both studies.
While these exploratory phase 2a studies were not powered for determining differences in efficacy between treatment groups, there were consistent trends of clinically meaningful improvement in clinical endpoints observed in the ischemic DCM groups in both studies. In the combined ischemic DCM groups across both studies, major adverse cardiovascular events (MACE) were experienced by a lower percentage of ixmyelocel T-treated patients compared to control patients, representing a 45% reduction in the number of patients having a MACE event. Likewise, patients in the combined ischemic DCM groups that were treated with ixmyelocel-T had a lower average number of MACE events at 12 months compared to those in the control group, representing a 61% reduction in the average number of MACE events per patient. MACE is the recommended endpoint (mortality and cardiovascular hospitalizations) in Phase 3 heart failure studies as stated in the FDA 2009 Somatic Cell Therapy for Cardiac Diseases Draft Guidance. Consistent positive trends also were observed in several secondary efficacy measures in the ischemic DCM groups. The majority of ixmyelocel T-treated patients, but not placebo-treated patients (both IDCM and NIDCM), had improvement in NYHA Class over the 12 months following treatment. Improvement in NYHA Class is considered clinically meaningful. There was also a trend toward improved function, with a higher percentage of ixmyelocel T-treated IDCM patients showing an improvement in ejection fraction and increased 6 minute walk performance compared to the IDCM control patients.
We are currently enrolling patients in the Phase 2b ixCELL-DCM clinical study, which is a multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of ixmyelocel-T in patients with advanced heart failure due to ischemic DCM. The study is designed to enroll 108 patients at approximately 30 sites in the U.S. and Canada. Patients will be followed for 12 months for the primary efficacy endpoint of MACE events, defined as all-cause deaths, all-cause hospitalizations, and unplanned outpatient or emergency department visits for IV treatment of acute worsening heart failure. Secondary endpoints include clinical, functional, structural, symptomatic, quality of life, and biomarker measures at 3, 6 and 9 months. Patients will be followed for an additional 12 months for safety. We expect to complete enrollment of the ixCELL-DCM study by the end of the first quarter of 2014, and have top-line efficacy results in the second quarter of 2015.
Critical Limb Ischemia
CLI is the most serious and advanced stage of PAD resulting from chronic inflammation and lipid accumulation. PAD is a chronic atherosclerotic disease that progressively restricts blood flow in the limbs and can lead to serious medical complications. This disease is often associated with other serious clinical conditions including hypertension, cardiovascular disease, dyslipidemia, diabetes, obesity and stroke. CLI is used to describe
patients with chronic ischemia-induced pain (even at rest) or tissue loss (ulcers or gangrene) in the limbs, often leading to amputation and death. Many CLI patients are considered unsuitable for revascularization as they have exhausted all other reasonable treatment options and will likely require amputation. The one-year and four-year mortality rates for CLI patients that are unsuitable for revascularization that progress to amputation are approximately 25% and 70%, respectively. Currently, there are an estimated 250,000 CLI patients that are unsuitable for revascularization in the United States.
Ixmyelocel-T has shown significant promise in the treatment of CLI patients with existing tissue loss that are unsuitable for revascularization. Our U.S. Phase 2b RESTORE-CLI program was a multi-center, randomized, double-blind, placebo-controlled clinical trial designed to evaluate the safety and efficacy of ixmyelocel-T in the treatment of patients with CLI that are unsuitable for revascularization. It was the largest multi-center, randomized, double-blind, placebo-controlled cellular therapy study ever conducted in CLI patients. We completed enrollment of this trial in February 2010 with a total of 86 patients at 18 sites across the United States.
Final results of the Phase 2b RESTORE-CLI clinical trial were presented at the American Heart Association Scientific Sessions in November 2011 and published in the peer-reviewed journal Molecular Therapy in April 2012. Patients in the treatment arm showed a 62% reduction in risk relative to placebo in the primary efficacy endpoint of time to first occurrence of treatment failure (p=0.0032). While the study was not powered to show statistical significance in the secondary endpoint of amputation free survival, results from a subgroup of 45 patients with wounds at baseline (the approximate profile of the Phase 3 patient population) showed a 61% reduction in risk (21% ixmyelocel-T treated versus 44% control event rate; p=0.0802). The study also met the primary safety endpoint with no meaningful differences between the treated and control groups.
We initiated the Phase 3 REVIVE-CLI clinical study, a multicenter, randomized, double-blind, placebo controlled study to evaluate the efficacy and safety of ixmyelocel-T in patients with CLI, in 2012. We had previously received Fast Track Designation from the FDA for use of ixmyelocel-T for the treatment of CLI and reached agreement with the FDA on a Special Protocol Assessment. Patients were randomized 1:1 and were to be followed for 12 months for the primary efficacy endpoint of amputation-free survival. On March 27, 2013 we announced that we were stopping enrollment in the study for strategic business reasons. This study has been amended and is ongoing for the 41 patients that are enrolled in the study, and we plan to continue following these patients for 12 months to evaluate safety and certain efficacy measures. We expect to have results from this study in the second quarter of 2014.
Risks Associated with Our Business
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. The risks are discussed more fully in the “Risk Factors” section of this prospectus beginning on page 10 of this prospectus. These risks include, but are not limited to, the following:
· We currently depend heavily on the success of ixmyelocel-T, our sole product candidate. Any failure to commercialize ixmyelocel-T, or significant delays in doing so, will have a material adverse effect on our business, operating results and financial condition.
· Our product development programs are based on novel technologies and are inherently risky.
· We may not be able to raise the required capital to conduct our operations and develop and commercialize our products.
· If we do not keep pace with our competitors and with technological and market changes, our products will become obsolete and our business may suffer.
· If our patents and proprietary rights do not provide substantial protection, then our business and competitive position will suffer.
· Our past losses and expected future losses cast doubt on our ability to continue as a going concern and operate profitably.
Company Information
We were incorporated under the laws of the State of Michigan on March 24, 1989. Our principal executive offices are located at 24 Frank Lloyd Wright Drive, Lobby K, Ann Arbor, Michigan 48105 and our telephone number is (734) 418-4400. Our website address is www.aastrom.com. The reference to our website is intended to be an inactive textual reference and, except for the documents incorporated by reference as noted above, the information on, or accessible through, our website is not part of this prospectus.
Agreement with Lincoln Park
On January 21, 2014, we entered into a Purchase Agreement and a Registration Rights Agreement (or the Registration Rights Agreement) with Lincoln Park, pursuant to which Lincoln Park has agreed to purchase from us up to $15,00,000 in shares of our common stock, subject to certain limitations from time to time over a 30-month period commencing on the date of effectiveness of the registration statement, of which this prospectus is a part, which provides for the resale of such shares pursuant to the Registration Agreement. The shares issuable to Lincoln Park under the Purchase Agreement are being offered pursuant to this prospectus.
Upon the effectiveness of the registration statement, and subject to the satisfaction of the other conditions of the Purchase Agreement, we may direct Lincoln Park from time to time and at our sole discretion to purchase shares of our common stock up to an aggregate amount of $15,000,000. By means of a Regular Purchase, so long as at least one business day has passed since the most recent purchase, we may direct Lincoln Park to purchase up to 50,000 shares of our common stock at the Regular Purchase Price, increasing to amounts up to 100,000 shares of our common stock depending upon the closing sale price of our common stock. Additionally, we may direct Lincoln Park to purchase additional amounts as Accelerated Purchases if on the date of a Regular Purchase the closing sale price of our common stock equals or exceeds $3.00.
There is no upper limit on the price per share that Lincoln Park must pay for our common stock under the Purchase Agreement, but in no event will shares be sold to Lincoln Park under a Regular Purchase on a day our closing price is less than the minimum floor price of $2.50 per share.
As consideration for Lincoln Park’s commitment to purchase our common stock pursuant to the Purchase Agreement, we issued to Lincoln Park 48,063 shares of our common stock (or the Initial Commitment Shares) on January 21, 2014 as consideration for its commitment to purchase shares pursuant to the Purchase Agreement. In the event the initial registration statement is insufficient to cover all of the shares issuable under the Purchase Agreement and we file a new registration statement to cover any remaining shares not covered by the initial registration statement, we will issue to Lincoln Park an additional 48,063 shares (or the Additional Commitment Shares).
The proceeds received by us under the Purchase Agreement are expected to be used for working capital and general corporate purposes as described further in this prospectus.
The Purchase Agreement limits our sales of shares of common stock to Lincoln Park to the maximum number of shares of our common stock that we may issue without breaching our obligations under applicable rules of the NASDAQ Capital Market (approximately 1,148,843 shares, or 19.99% of our total outstanding common stock as of the date of the Purchase Agreement, which we refer to as the 19.99% shareholder approval limitation) or obtaining shareholder approval under such rules, unless the average price of all applicable sales of common stock exceed a “Base Price” (or $4.13, representing our closing consolidated bid price on January 17, 2014, plus an incremental amount to account for the issuance of commitment shares) such that the sales to Lincoln Park are considered to be at least “at market” under applicable NASDAQ rules.
As a result, although the Purchase Agreement provides that we may sell up to $15,000,000 in shares of our common stock to Lincoln Park, only 1,748,063 shares are being offered under this prospectus, which represents (i) up to 1,748,063 shares that we may sell and issue to Lincoln Park from time to time in the future pursuant to the Purchase Agreement after the registration statement of which this prospectus forms a part is declared effective, and (ii) up to 48,063 shares as Initial Commitment Shares that we have previously issued to Lincoln Park under the Purchase Agreement. This aggregate number of shares may or may not cover all of such shares to be purchased by and issued to Lincoln Park under the Purchase Agreement, depending on the purchase price per share. In the event the initial registration statement is insufficient to cover all of the shares issuable under the Purchase Agreement, we may elect to file a new registration statement so as to cover all of the shares potentially issuable.
As of January 21, there were 5,747,087 shares of our common stock issued and outstanding, of which 5,318,519 shares were held by non-affiliates. If all of the 1,748,063 shares offered by Lincoln Park under this prospectus were issued and outstanding as of the date hereof, such shares would represent approximately 30% of the total common stock outstanding and approximately 33% of the total number of outstanding shares held by non-affiliates.
The actual number of shares to be purchased by Lincoln Park under the Purchase Agreement is variable, depending on the market price of our common stock at the time of each sale. Accordingly, we cannot predict the actual total number of shares to be issued to Lincoln Park. This prospectus covers 1,748,063 shares of common stock. As of the date hereof, we do not currently have any plans or intent to issue to Lincoln Park any shares pursuant to the Purchase Agreement beyond the 1,748,063 shares offered hereby. However, if we elect to issue and sell to Lincoln Park pursuant to the Purchase Agreement more than the 1,748,063 shares offered under this prospectus, which we have the right but not the obligation to do, up to the $15,000,000 maximum in shares of our common stock, we would first be required to register for resale under the Securities Act any additional shares we may elect to sell to Lincoln Park before we can sell such additional shares, which could cause additional substantial dilution to our shareholders. The number of shares issued pursuant to the Purchase Agreement and ultimately offered for resale by Lincoln Park depends on the number of shares purchased by Lincoln Park under the Purchase Agreement.
There are substantial risks to our shareholders as a result of the sale and issuance of common stock to Lincoln Park under the Purchase Agreement. These risks include substantial dilution, significant declines in our stock price and our inability to draw sufficient funds when needed. See “Risk Factors.” Issuance of our common stock to Lincoln Park under the Purchase Agreement will not affect the rights or privileges of our existing shareholders, except that the economic and voting interests of our existing shareholders will be diluted as a result of any such issuance. Although the number of shares of common stock that our existing shareholders own will not decrease, the shares owned by our existing shareholders will represent a smaller percentage of our total outstanding shares after any such issuance to Lincoln Park.
|
Issuer |
Aastrom Biosciences, Inc. |
|
|
|
|
Common stock offered by selling shareholder |
Up to 1,748,063 shares of common stock consisting of :
- 48,063 commitment shares issued to Lincoln Park and - 1,700,000 shares we may sell to Lincoln Park under the Purchase Agreement. |
|
|
|
|
|
|
|
Common stock outstanding before this offering |
5,890,980 shares of common stock. |
|
|
|
|
Common stock to be outstanding after this offering |
7,639,043 shares of common stock. |
|
|
|
|
Use of Proceeds |
We will not receive any proceeds from the sales of shares of our common stock by Lincoln Park; however, we may receive proceeds of up to $15,000,000 under the Purchase Agreement for the sale of such shares to Lincoln Park. See “Use of Proceeds” for a more complete description of our intended use of the net proceeds from this offering. |
|
|
|
|
Risk Factors |
You should carefully read “Risk Factors” in this prospectus for a discussion of factors that you should consider before deciding to invest in our common stock. |
|
|
|
|
NASDAQ Capital Market symbol |
ASTM |
The number of shares of our common stock that will be outstanding immediately after this offering is based on 5,890,980 shares of common stock outstanding as of January 31, 2014 and excludes common stock issuable upon the exercise of stock options, warrants, common stock issuable upon the conversion of preferred stock outstanding , and 48,063 shares of our common stock issued to Lincoln Park on January 21, 2014 as a commitment fee.
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below and in the documents incorporated by reference in this prospectus and any prospectus supplement, as well as other information we include or incorporate by reference into this prospectus and any applicable prospectus supplement, before making an investment decision. Our business, financial condition or results of operations could be materially adversely affected by the materialization of any of these risks. The trading price of our securities could decline due to the materialization of any of these risks, and you may lose all or part of your investment. This prospectus and the documents incorporated herein by reference also contain forward-looking statements that involve risks and uncertainties. Actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks described below and in the documents incorporated herein by reference, including (i) our Annual Report on Form 10-K for the year ended December 31, 2012 and (ii) other documents we file with the SEC that are deemed incorporated by reference into this prospectus.
Risks Related to Our Business
Our past losses and expected future losses cast doubt on our ability to continue as a going concern and operate profitably.
As of September 30, 2013, we had $10,816,000 of cash and cash equivalents. This is not sufficient to sustain our operations for one year. In light of our financial position, we are evaluating strategic and financial opportunities in the short-term in order to maintain adequate liquidity through December 31, 2014 and beyond. Other than the Purchase Agreement with Lincoln Park, which is subject to certain limitations and conditions (see “Recent Developments – Agreement with Lincoln Park”), we could continue to sell shares through an At-the-Market Sales Agreement (ATM) in order to raise additional capital, though there are certain factors, such as volume of trading in our common stock, our stock price and the ability to terminate the agreement with notice, which could limit the amount we could raise in a short period of time. On a longer term basis, we will need to raise additional funds in order to complete product development programs and complete clinical trials needed to market and commercialize our products. We cannot be certain that such funding will be available on favorable terms, if at all. Some of the factors that will impact our ability to raise additional capital and our overall success include: the rate and degree of progress for our product development, the rate of regulatory approval to proceed with clinical trial programs, the level of success achieved in clinical trials, the requirements for marketing authorization from regulatory bodies in the United States and other countries, the liquidity and market volatility of our equity securities, regulatory and manufacturing requirements and uncertainties, technological developments by competitors, and other factors. If we cannot raise such funds, we will not be able to develop or enhance products, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements, which would have a material adverse impact on our business, financial condition and results of operations. As a result of the need to raise additional capital and a net capital deficiency, there is uncertainty regarding our ability to maintain liquidity sufficient to operate our business effectively over at least the next twelve months, which raises substantial doubt as to our ability to continue as a going concern. The consolidated financial statements incorporated by reference in this prospectus do not include any adjustments that might result from the outcome of this uncertainty.
We were incorporated in 1989 and have experienced substantial operating losses since inception. As of September 30, 2013, we had accumulated a deficit of approximately $284,797,000 and we have continued to incur losses since that date. These losses have resulted principally from costs incurred in the research and development (including clinical trials) of our cell culture technologies and our cell manufacturing system, general and administrative expenses, and the prosecution of patent applications. We expect to continue to incur significant operating losses over the next several years and at least until, and probably after, product sales increase, primarily owing to our research and development programs, including preclinical studies and clinical trials, and the establishment of marketing and distribution capabilities necessary to support commercialization efforts for our products. We cannot predict with any certainty the amount of future losses. Our ability to achieve profitability will depend, among other things, on successfully completing the development of our product candidates, timely initiation and completion of clinical trials, obtaining regulatory approvals, establishing manufacturing, sales and marketing arrangements with third parties, maintaining supplies of key manufacturing components, acquisition and development of complementary activities and raising sufficient cash to fund our operating activities. Therefore, we may not be able to achieve or sustain profitability.
We may not be able to raise the required capital to conduct our operations and develop and commercialize our products.
We will require substantial additional capital resources in order to conduct our operations, complete our product development programs, complete our clinical trials needed to market our products (including a Phase 2b clinical trial for DCM), and commercialize these products and cell manufacturing facilities. In order to grow and expand our business, to introduce our new product candidates into the marketplace and to acquire or develop complementary business activities, we will need to raise a significant amount of additional funds. We will also need significant additional funds or a collaborative partner, or both, to finance the research and development activities of our cell product candidates for additional indications. Accordingly, we are continuing to pursue additional sources of financing.
Our future capital requirements will depend upon many factors, including:
· continued scientific progress in our research, clinical and development programs;
· costs and timing of conducting clinical trials and seeking regulatory approvals;
· competing technological and market developments;
· avoiding infringement and misappropriation of third-party intellectual property;
· obtaining valid and enforceable patents that give us a competitive advantage;
· our ability to establish additional collaborative relationships;
· our ability to effectively launch a commercial product;
· the effect of commercialization activities and facility expansions, if and as required; and
· complementary business acquisition or development opportunities.
We entered into an ATM on June 16, 2011 (as amended to date, the “ATM”), which allows us to raise approximately $20,000,000 through sales of our common stock from time to time. However, there are certain factors, such as volume of trading in our common stock, our stock price and the ability to terminate the agreement with notice, which limit the amount that can be raised in a short period of time through the ATM. Regardless of the usage of the ATM, we will need to raise additional capital in order to fund the clinical trials of ixmyelocel-T for DCM, complete our product development programs, complete clinical trials needed to market our products and commercialize these products.
Additionally, we may direct Lincoln Park to purchase up to $15,000,000 worth of shares of our common stock under the Purchase Agreement over a 30-month period generally in amounts up to 50,000 shares of our common stock on any such business day. However, there can be no assurance that we will be able to receive any or all of the additional funds from Lincoln Park because the Purchase Agreement contain limitations, restrictions, events of default and other provisions that could limit our ability to cause Lincoln Park to buy common stock from us, including that: (i) Lincoln Park shall not purchase any shares of our common stock on any business day that the closing sale price of our common stock is less than $2.50 per share, subject to adjustment as set forth in the Purchase Agreement, and (ii) Lincoln Park shall not own more than 9.99% of our common stock under the Purchase Agreement. Assuming a purchase price of $4.13 per share (the closing sale price of the common stock on January 17, 2014, plus an incremental amount to account for the issuance of commitment shares) and the purchase by Lincoln Park of the full 1,700,000 shares registered hereunder, proceeds to us would only be $7,021,000. In addition, under the applicable rules of the NASDAQ Capital Market, if we seek to issue shares which may be aggregated with shares sold to Lincoln Park under the Purchase Agreement in excess of 1,148,843 or 19.99% of the total common stock outstanding as of the date of the Purchase Agreement, we may be required to seek shareholder approval in order to be in compliance with the NASDAQ Capital Market rules.
Our reliance on Lincoln Park as a source of funding will depend on a number of factors, including the prevailing market price of our common stock and the extent to which we are able to secure working capital from other sources. If obtaining sufficient funding from Lincoln Park were to prove unavailable or prohibitively dilutive, we will need to raise additional funds in order to complete our product development programs, complete clinical trials needed to market our products (including clinical trials for our DCM program), and commercialize these products. Even if we sell all $15,000,000 under the Purchase Agreement to Lincoln Park, we may still need additional capital to fully implement our business, operating and development plans. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the consequences could be a material adverse effect on our business, operating results, financial condition and prospects
Because of our long-term funding requirements, we may try to access the public or private equity markets if conditions are favorable to complete a financing, even if we do not have an immediate need for additional capital at that time, or whenever we require additional operating capital. In addition, we may seek collaborative relationships, incur debt and access other available funding sources. This additional funding may not be available to us on reasonable terms, or at all. Some of the factors that will impact our ability to raise additional capital and our overall success include:
· the rate and degree of progress for our product development;
· the rate of regulatory approval to proceed with clinical trial programs;
· the level of success achieved in clinical trials;
· the requirements for marketing authorization from regulatory bodies in the United States and other countries;
· the liquidity and market volatility of our equity securities; and
· regulatory and manufacturing requirements and uncertainties, and technological developments by competitors.
If adequate funds are not available in the future, we may not be able to develop or enhance our products, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements and we may be required to delay or terminate research and development programs, curtail capital expenditures, and reduce business development and other operating activities. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the consequences could have a material adverse effect on our business, operating results, financial condition and prospects.
Failure to obtain and maintain required regulatory approvals would severely limit our ability to sell our products.
We must obtain the approval of the FDA before commercial sales of our cell product candidates may commence in the United States, which we believe will ultimately be the largest market for our products. We will also be required to obtain additional approvals from various foreign regulatory authorities to initiate sales activities of cell products in those jurisdictions. If we cannot demonstrate the safety, purity and potency of our product candidates, including our cell product candidates, produced in our production system, the FDA or other regulatory authorities could delay or withhold regulatory approval of our product candidates.
Finally, even if we obtain regulatory approval of a product, that approval may be subject to limitations on the indicated uses for which it may be marketed. Even after granting regulatory approval, the FDA and regulatory agencies in other countries continue to review and inspect marketed products, manufacturers and manufacturing facilities, which may create additional regulatory burdens. Later discovery of previously unknown problems with a product, manufacturer or facility may result in restrictions on the product or manufacturer, including a withdrawal of the product from the market. Further, regulatory agencies may establish additional regulations that could prevent or delay regulatory approval of our products.
We currently depend heavily on the success of ixmyelocel-T, our sole product candidate. Any failure to commercialize ixmyelocel-T, or significant delays in doing so, will have a material adverse effect on our business, operating results and financial condition.
We have invested a significant portion of our efforts and financial resources in the development of ixmyelocel-T. Our ability to generate future product revenue depends heavily on the successful development and commercialization of ixmyelocel-T. The successful commercialization of ixmyelocel-T will depend on several factors, including the following:
· obtaining marketing approvals from the FDA and other foreign regulatory authorities;
· successful enrollment of patients in our ongoing clinical studies of ixmyelocel-T;
· successful completion of our ongoing clinical studies of ixmyelocel-T;
· the successful audit of our facilities by additional regulatory authorities;
· maintaining the cGMP and cGTP compliance of our manufacturing facility;
· maintaining current manufacturing arrangements with third parties and establishing new manufacturing arrangements;
· our development of a successful sales and marketing organization for ixmyelocel-T;
· an acceptable safety and efficacy profile of our product candidates following approval;
· the availability of reimbursement to patients from healthcare payers for our drug products, if approved; and
· other risks described in this “Risk Factors” section.
Any failure to commercialize ixmyelocel-T or significant delays in doing so will have a material adverse effect on our business, results of operations and financial condition.
Our sole product candidate, ixmyelocel-T, is still in clinical development. If we do not successfully continue or complete the clinical development of ixmyelocel-T, our likelihood of success as a company and our ability to finance our operations will be substantially harmed.
Our near-term prospects substantially depend upon our ability to successfully continue and complete clinical trials of our lead product candidate, ixmyelocel-T, and to demonstrate its safety and efficacy, as well as its superiority over existing therapies and standards of care, if any. We are currently enrolling patients with ischemic DCM for the ixCELL-DCM trial, a Phase 2b clinical trial, and have recently treated the first patients in the trial. All of our other potential product candidates are in preclinical research or early clinical development. Our ability to finance our company and to generate revenues will depend heavily on our ability to obtain favorable results in the ongoing and planned clinical trials of ixmyelocel-T, including the ongoing ixCELL-DCM Phase 2b clinical trial, and to successfully develop and commercialize ixmyelocel-T. Ixmyelocel-T could be unsuccessful if it:
· does not demonstrate acceptable safety and efficacy in clinical trials, or otherwise does not meet applicable regulatory standards for approval;
· does not offer sufficient, clinically meaningful therapeutic or other improvements over existing or future drugs used to treat the DCM indications for which it is being tested;
· is not capable of being produced in commercial quantities at acceptable costs; or
· is not accepted as safe, efficacious, cost-effective, less costly and preferable to current therapies in the medical
community and by third-party payers.
If we are not successful in developing and commercializing ixmyelocel-T or are significantly delayed in doing so, our financial condition and future prospects may be adversely affected and we may experience difficulties in raising the substantial additional capital required to fund our business.
Our product development programs are based on novel technologies and are inherently risky.
We are subject to the risks of failure inherent in the development of products based on new technologies. The novel nature of our therapeutics creates significant challenges in regard to product development and optimization, manufacturing, government regulation, third-party reimbursement and market acceptance. For example, if regulatory agencies have limited experience in approving cellular therapies for commercialization, the development and commercialization pathway for our therapies may be subject to increased uncertainty, as compared to the pathway for new conventional drugs.
Any changes in the governmental regulatory classifications of our products could prevent, limit or delay our ability to market or develop our products.
The FDA establishes regulatory requirements based on the classification of a product. Because our product development programs are designed to satisfy the standards applicable to biological licensure for our cellular products, any change in the regulatory classification or designation would affect our ability to obtain FDA approval of our products. Each of these cell products is, under current regulations, regulated as a biologic, which requires a BLA.
Our inability to complete our product development activities successfully would severely limit our ability to operate or finance operations.
In order to commercialize our cell product candidates in the United States, we must complete substantial clinical trials and obtain sufficient safety, purity and potency results to support required registration approval and market acceptance of our cell product candidates. We may not be able to successfully complete the development of our product candidates, or successfully market our technologies or product candidates. We, and any of our potential collaborators, may encounter problems and delays relating to research and development, regulatory approval and intellectual property rights of our technologies and product candidates. Our research and development programs may not be successful, and our cell culture technologies and product candidates may not facilitate the production of cells outside the human body with the expected results. Our technologies and cell product candidates may not prove to be safe and efficacious in clinical trials, and we may not obtain the requisite regulatory approvals for our technologies or product candidates and the cells produced in such products. If any of these events occur, we may not have adequate resources to continue operations for the period required to resolve any issues delaying commercialization and we may not be able to raise capital to finance our continued operation during the period required for resolution of any such issues.
We must successfully complete our clinical trials to be able to market certain of our products.
To be able to market therapeutic cell products in the United States, we must demonstrate, through extensive preclinical studies and clinical trials, the safety and efficacy of our processes and product candidates. If our clinical trials are not successful, our products may not be marketable. The results of early stage clinical trials do not ensure success in later clinical trials, and interim results are not necessarily predictive of final results.
Our ability to complete our clinical trials in a timely manner depends on many factors, including the rate of patient enrollment. Patient enrollment can vary with the size of the patient population, the proximity of suitable patients to clinical sites, perceptions of the utility of cell therapy for the treatment of certain diseases, and the eligibility criteria for the study. For example, patients enrolling in our studies need to provide an adequate amount of bone marrow to process and expand for injection and some patients may not be able to provide sufficient starting material despite our study inclusion and exclusion criteria designed to prevent this. Bone marrow is an inherently variable starting material. We have experienced delays in patient accrual in our previous clinical trials. On March 27, 2013, we announced that we were stopping enrollment in the Phase 3 REVIVE clinical trial due to the slow patient accrual rate for the study and to optimize the use of our financial resources. If we experience similar delays in patient enrollment
for other clinical trials, we could experience increased costs and delays associated with these trials, which would impair our product development programs and our ability to market our products.
Furthermore, the FDA monitors the progress of clinical trials and it may suspend or terminate clinical trials at any time due to patient safety or other considerations.
Our research programs are currently directed at improving product functionality for certain clinical indications, improving product shelf life, and decreasing the cost of manufacturing our products. These production process changes may alter the functionality of our cells and require various additional levels of experimental and clinical testing and evaluation. Any such testing could lengthen the time before these products would be commercially available.
Even if successful clinical results are reported for a product from a completed clinical trial, this does not mean that the results will be sustained over time, or will be sufficient for a marketable or regulatory approvable product.
We may rely on third parties to conduct some of our clinical trials, and their failure to perform their obligations in a timely or competent manner may delay development and commercialization of our product candidates.
We may use clinical research organizations (CROs) to assist in conduct of our clinical trials. There are numerous alternative sources to provide these services. However, we may face delays outside of our control if these parties do not perform their obligations in a timely or competent fashion, or if we are forced to change service providers. Any third party that we hire to conduct clinical trials may also provide services to our competitors, which could compromise the performance of their obligations to us. If we experience significant delays in the progress of our clinical trials, the commercial prospects for product candidates could be harmed and our ability to generate product revenue would be delayed or prevented. In addition, we and any provider that we retain will be subject to Good Clinical Practice, (GCP) requirements. If GCP and other regulatory requirements are not adhered to by us or our third-party providers, the development and commercialization of our product candidates could be delayed.
Any failure of such CRO to successfully accomplish clinical trial monitoring, data collection, safety monitoring and data management and the other services it provides for us in a timely manner and in compliance with regulatory requirements could have a material adverse effect on our ability to complete clinical development of our products and obtain regulatory approval. Problems with the timeliness or quality of the work of a CRO may lead us to seek to terminate the relationship and use an alternate service provider. However, making such changes may be costly and may delay our trials, and contractual restrictions may make such a change difficult or impossible. Additionally, it may be difficult to find a replacement organization that can conduct our trials in an acceptable manner and at an acceptable cost.
Failure of third parties, including Vention Medical, to manufacture or supply certain components, equipment, disposable devices and other materials used in our cell manufacturing process would impair our cell product development.
We rely on third parties, including Vention Medical (Vention), to manufacture and/or supply certain of our devices/manufacturing equipment and to manufacture and/or supply certain components, equipment, disposable devices and other materials used in our cell manufacturing process to develop our cell products. Vention is our sole supplier of cell cassettes for which it would be difficult to obtain alternate sources of supply on a short-term basis. If any of our manufacturers or suppliers fails to perform its respective obligations, or if our supply of certain components, equipment, disposable devices and other materials is limited or interrupted, it could impair our ability to manufacture our products, which would delay our ability to conduct our clinical trials or market our product candidates on a timely and cost-competitive basis, if at all.
In addition, we may not be able to continue our present arrangements with our suppliers, supplement existing relationships, establish new relationships or be able to identify and obtain the ancillary materials that are necessary to develop our product candidates in the future. Our dependence upon third parties for the supply and manufacture of these items could adversely affect our ability to develop and deliver commercially feasible products on a timely and competitive basis.
Manufacturing of our cell products in centralized facilities may increase the risk that we will not have adequate quantities of our cell products for clinical programs.
We are subject to regulatory compliance and quality assurance requirements at our production site in Ann Arbor, Michigan. This site could be subject to ongoing, periodic, unannounced inspection by regulatory agencies to ensure strict compliance with GMP regulations and other governmental regulations. We do not have redundant cell manufacturing sites. In the event our cell production facility is damaged or destroyed or is subject to regulatory restrictions, our clinical trial programs and other business prospects would be adversely affected.
Even if we obtain regulatory approvals to sell our products, lack of commercial acceptance could impair our business.
We will be seeking to obtain regulatory approvals to market our cell products for tissue repair treatments. Even if we obtain all required regulatory approvals, we cannot be certain that our products and processes will be accepted in the marketplace at a level that would allow us to operate profitably. Our products may be unable to achieve commercial acceptance for a number of reasons, such as the availability of alternatives that are less expensive, more effective, or easier to use; the perception of a low cost-benefit ratio for the product amongst physicians and hospitals; or an inadequate level of product support from ourselves or a commercial partner. Our technologies or product candidates may not be employed in all potential applications being investigated, and any reduction in applications would limit the market acceptance of our technologies and product candidates, and our potential revenues.
The market for our products will be heavily dependent on third-party reimbursement policies.
Our ability to successfully commercialize our product candidates will depend on the extent to which government healthcare programs, such as Medicare and Medicaid, as well as private health insurers, health maintenance organizations and other third-party payers will pay for our products and related treatments.
Reimbursement by third-party payers depends on a number of factors, including the payer’s determination that use of the product is safe and effective, not experimental or investigational, medically necessary, appropriate for the specific patient and cost-effective. Reimbursement in the United States or foreign countries may not be available or maintained for any of our product candidates. If we do not obtain approvals for adequate third-party reimbursements, we may not be able to establish or maintain price levels sufficient to realize an appropriate return on our investment in product development. Any limits on reimbursement from third-party payers may reduce the demand for, or negatively affect the price of, our products. For example, in the past, published studies suggested that stem cell transplantation for breast cancer, which constituted a significant portion of the overall stem cell therapy market at the time, may have limited clinical benefit. The lack of reimbursement for these procedures by insurance payers has negatively affected the market for our products in this indication in the past.
Managing and reducing health care costs has been a general concern of federal and state governments in the United States and of foreign governments. In addition, third-party payers are increasingly challenging the price and cost-effectiveness of medical products and services, and many limit reimbursement for newly approved health care products. In particular, third-party payers may limit the indications for which they will reimburse patients who use any products that we may develop. Cost control initiatives could decrease the price for products that we may develop, which would result in lower product revenues to us.
Use of animal-derived materials could harm our product development and commercialization efforts.
Some of the manufacturing materials and/or components that we use in, and which are critical to, implementation of our technology involve the use of animal-derived products, including fetal bovine serum. Suppliers or regulatory changes may limit or restrict the availability of such materials for clinical and commercial use. We currently purchase all of our fetal bovine sera from protected herds in Australia and New Zealand. These sources are considered to be the safest and raise the least amount of concern from the global regulatory agencies. If, for example, the so-called “mad cow disease” occurs in New Zealand or in Australia, it may lead to a restricted supply of the serum currently required for our product manufacturing processes. Any restrictions on these materials would impose a potential competitive disadvantage for our products or prevent our ability to manufacture our cell products. The FDA has issued draft regulations for controls over bovine materials. These proposed regulations do not appear to
affect our ability to purchase the manufacturing materials we currently use. However, the FDA may issue final regulations that could affect our operations. Our inability to develop or obtain alternative compounds would harm our product development and commercialization efforts. There are certain limitations in the supply of certain animal-derived materials, which may lead to delays in our ability to complete clinical trials or eventually to meet the anticipated market demand for our cell products.
Given our limited internal manufacturing, sales, marketing and distribution capabilities, we need to develop increased internal capability or collaborative relationships to manufacture, sell, market and distribute our products.
We have only limited internal manufacturing, sales, marketing and distribution capabilities. As market needs develop, we intend to establish and operate commercial-scale manufacturing facilities, which will need to comply with all applicable regulatory requirements. We will also need to develop new configurations of our cell manufacturing system for these facilities to enable processes and cost efficiencies associated with large-scale manufacturing. Establishing these facilities will require significant capital and expertise. We may need to make such expenditures when there are significant uncertainties as to the market opportunity. Any delay in establishing, or difficulties in operating, these facilities will limit our ability to meet the anticipated market demand for our cell products. We intend to get assistance to market some of our future cell products through collaborative relationships with companies with established sales, marketing and distribution capabilities. Our inability to develop and maintain those relationships would limit our ability to market, sell and distribute our products. Our inability to enter into successful, long-term relationships could require us to develop alternate arrangements at a time when we need sales, marketing or distribution capabilities to meet existing demand. We may market one or more of our products through our own sales force. Our inability to develop and retain a qualified sales force could limit our ability to market, sell and distribute our cell products.
If we do not keep pace with our competitors and with technological and market changes, our products will become obsolete and our business may suffer.
The markets for our products are very competitive, subject to rapid technological changes, and vary for different candidates and processes that directly compete with our products. Our competitors may have developed, or could in the future develop, new technologies that compete with our products or even render our products obsolete. As an example, in the past, published studies have suggested that hematopoietic stem cell therapy use for bone marrow transplantation, following marrow ablation due to chemotherapy, may have limited clinical benefit in the treatment of breast cancer, which was a significant portion of the overall hematopoietic stem cell transplant market. This resulted in the practical elimination of this market for our cell-based product for this application.
Our cell manufacturing system is designed to improve and automate the processes for producing cells used in therapeutic procedures. Even if we are able to demonstrate improved or equivalent results, the cost or process of treatment and other factors may cause researchers and practitioners to not use our products and we could suffer a competitive disadvantage. Finally, to the extent that others develop new technologies that address the targeted application for our products, our business will suffer.
The current credit and financial market conditions may exacerbate certain risks affecting our business.
We rely upon third parties for certain aspects of our business, including collaboration partners, wholesale distributors, contract clinical trial providers, contract manufacturers and third-party suppliers. Because of the recent tightening of global credit and the volatility in the financial markets, there may be a delay or disruption in the performance or satisfaction of commitments to us by these third parties, which could adversely affect our business.
If we cannot attract and retain key personnel, our business may suffer.
Our success depends in large part upon our ability to attract and retain highly qualified scientific and management personnel. We face competition for such personnel from other companies, research and academic institutions and other entities. Further, in an effort to conserve financial resources, we have implemented reductions in our work force on four previous occasions, most recently in the first quarter of 2013. As a result of these and other factors, we may not be successful in hiring or retaining key personnel. Our inability to replace any key employee could harm our operations.
Risks Related to Intellectual Property
If our patents and proprietary rights do not provide substantial protection, then our business and competitive position will suffer.
Our success depends in large part on our ability to develop or license intellectual property rights to protect our proprietary products and technologies. This involves complex legal, scientific, and factual questions and uncertainties. We rely upon patent, trade secret, copyright and contract laws to protect proprietary technology and trademark law to protect brand identities. However, we cannot assure you that any patent applications filed by, assigned to, or licensed to us will be granted, and that the scope of any of our issued or licensed patents will be sufficiently broad to offer meaningful protection. In addition, our issued patents or patents licensed to us could be successfully challenged, invalidated, held to be unenforceable, or circumvented so that our patent rights would not create an effective competitive barrier. We also cannot assure you that the inventors of the patents and applications that we own or license were the first to invent or the first to file on the inventions, or that a third party will not claim ownership in one of our patents or patent applications. We cannot assure you that a third party does not have or will not obtain patents that dominate the patents we own or license now or in the future. Furthermore, we rely on exclusive, world-wide licenses relating to the production of human cells granted to us by the University of Michigan. If we materially breach such agreements or otherwise fail to materially comply with such agreements, or if such agreements expire or are otherwise terminated by us, we may lose our rights under the patents held by the University of Michigan. At the latest, each of these licenses will terminate when the patent underlying the license expires, with the last to expire during the third quarter of 2014. Once the patents expire, third parties may be able to practice the inventions covered by those patents and thus compete with us.
Patent law relating to the scope of claims in the biotechnology field is evolving and our patent rights in this country and abroad are subject to this uncertainty.
We also rely on trade secrets and un-patentable know-how that we seek to protect, in part, by confidentiality agreements with our employees, consultants, suppliers and licensees. These agreements may be breached, and we might not have adequate remedies for any breach. Our competitors may also independently develop technologies substantially equivalent or superior to ours. If this were to occur, our business and competitive position would suffer.
Intellectual property litigation could harm our business.
Our success will also depend in part on our ability to develop commercially viable products without infringing the proprietary rights of others. Our cell processing system and cell compositions utilize a wide variety of technologies and we can give no assurance that we have identified or can identify all inventions and patents that may be infringed by development and manufacture of our cell compositions. Although we have not been subject to any filed infringement claims, patents could exist or could be filed which would prohibit or limit our ability to market our products or maintain our competitive position. In the event of an intellectual property dispute, we may be forced to litigate. Such litigation is typically protracted and the results are unpredictable. Intellectual property litigation would divert management’s attention from developing our products and would force us to incur substantial costs regardless of whether we are successful. An adverse outcome could subject us to significant liabilities to third parties including treble damages and the opposing party’s attorney fees, and force us to pay significant license fees and royalties or cease the development and sale of our products and processes.
We have hired and will continue to hire individuals who have experience in cell culture and cell based therapeutics and may have confidential trade secret or proprietary information of third parties. We caution these individuals not to use or reveal this third-party information, but we cannot assure you that these individuals will not use or reveal this third-party information. Thus, we could be sued for misappropriation of proprietary information and trade secrets. Such claims are expensive to defend and could divert our attention and could result in substantial damage awards and injunctions that could have a material adverse effect on our business, financial condition or results of operations.
We may need to initiate lawsuits to protect or enforce our patents or other proprietary rights, which would be expensive and, if unsuccessful, may cause us to lose some of our intellectual property rights.
To protect or enforce our patent rights, it may be necessary for us to initiate patent litigation proceedings against third parties, such as infringement suits or interference proceedings. These lawsuits would be expensive, take significant time and would divert management’s attention from other business concerns. These lawsuits could put our patents at risk of being invalidated, held unenforceable, or interpreted narrowly, and our patent applications at risk of not being issued. Further, these lawsuits may provoke the defendants to assert claims against us. The patent position of biotechnology firms is highly uncertain, involves complex legal and factual questions and recently has been the subject of much litigation. We cannot assure you that we will prevail in any of such suits or proceedings or that the damages or other remedies awarded to us, if any, will be commercially valuable.
The government maintains certain rights in technology that we develop using government grant money and we may lose the revenues from such technology if we do not commercialize and utilize the technology pursuant to established government guidelines.
Certain of our and our licensors’ research have been or are being funded in part by government grants. As a result of such funding, the United States government has established guidelines and has certain rights in the technology developed with the grant. These rights include a non-exclusive, fully paid-up, worldwide license under such inventions for any governmental purpose. In addition, the United States government has the right to require us to grant an exclusive license under any of such inventions to a third party if the United States government determines that: (i) adequate steps have not been taken to commercialize such inventions; (ii) such action is necessary to meet public health or safety needs; or (iii) such action is necessary to meet requirements for public use under federal regulations. Additionally, under the federal Bayh Dole Act, a party which acquires an exclusive license for an invention that was partially funded by a federal research grant is subject to the following government rights: (x) products using the invention which are sold in the United States are to be manufactured substantially in the United States, unless a waiver is obtained; (y) the government may force the granting of a license to a third party who will make and sell the needed product if the licensee does not pursue reasonable commercialization of a needed product using the invention; and (z) the United States government may use the invention for its own needs. If we fail to meet these guidelines, we would lose our exclusive rights to these products, and we would lose potential revenue derived from the sale of these products.
Potential product liability claims could affect our earnings and financial condition.
We face an inherent business risk of exposure to product liability claims in the event that the manufacture and/or use of our products during clinical trials, or after commercialization, results in adverse events. As a result, we may incur significant product liability exposure, which could exceed existing insurance coverage. We may not be able to maintain adequate levels of insurance at reasonable cost and/or on reasonable terms. Excessive insurance costs or uninsured claims would increase our operating loss and adversely affect our financial condition.
Risks Related to Our Common Stock
We may be unable to continue as a going concern in which case our securities will have little or no value.
We have incurred substantial losses since inception and have a net capital deficiency. This raises substantial doubt about our ability to continue as a going concern. In the event we are not able to continue operations you will likely suffer a complete loss of your investment in our securities.
Our common stock price has been volatile and future sales of shares of common stock could have an adverse effect on the market price of such shares.
The market price of shares of our common stock has been volatile, ranging in closing price between $3.21 and $28.20 during the twelve months ended December 31, 2013, which has been retroactively adjusted for our twenty-to-one reverse stock split on October 16, 2013. The price of our common stock may continue to fluctuate in response to a number of events and factors, such as:
· clinical trial results;
· the amount of our cash resources and our ability to obtain additional funding;
· announcements of research activities, business developments, technological innovations or new products by us or our competitors;
· entering into or terminating strategic relationships;
· regulatory developments in both the United States and abroad;
· disputes concerning patents or proprietary rights;
· changes in our revenues or expense levels;
· public concern regarding the safety, efficacy or other aspects of the products or methodologies we are developing;
· news or reports from other stem cell, cell therapy or regenerative medicine companies;
· reports by securities analysts;
· status of the investment markets;
· concerns related to management transitions; and
· delisting from The NASDAQ Capital Market.
Any of these events may cause the price of our shares to fall, which may adversely affect our business and financing opportunities. In addition, the stock market in general and the market prices for biotechnology companies in particular have experienced significant volatility recently that often has been unrelated to the operating performance or financial conditions of such companies. These broad market and industry fluctuations may adversely affect the trading price of our common stock, regardless of our operating performance or prospects.
You will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase in this offering.
Because the public offering price per share of our common stock is higher than the net tangible book value per share of our common stock, you will suffer dilution in the net tangible book value of the common stock you purchase in this offering. Based on an assumed public offering price of $3.56 per share, if you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of approximately $1.76 per share in the net tangible book value of the common stock. See the section entitled “Dilution” in this prospectus for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
The sale of our common stock through future equity offerings may cause dilution and could cause the price of our common stock to decline.
During 2013, we sold (i) an aggregate of 278,294 shares of common stock pursuant to our ATM through September 30, 2013, and (ii) in August 2013, we offered an aggregate of 1,500,000 shares of our common stock, and warrants to acquire another 1,500,000 shares under a Form S-1 registration statement, which became effective on August 14, 2013. The ATM, which as of September 30, 2013 had remaining capacity of approximately $16,500,000, permits us to sell our common stock from time to time under a registration statement on Form S-3 filed in November 2010, pursuant to which we registered $75,00,000 of our securities for public sale. However, there are certain factors, such as volume of trading in our common stock, our stock price and the ability to terminate the agreement with notice, which limit the amount that can be raised in a short period of time through the ATM.
Sales of our common stock offered through future equity offerings may result in substantial dilution to the interests of other holders of our common stock. The sale of a substantial number of shares of our common stock to investors, or anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect sales.
The sale or issuance of our common stock to Lincoln Park may cause dilution and the sale of the shares of common stock acquired by Lincoln Park, or the perception that such sales may occur, could cause the price of our common stock to fall.
Under the Purchase Agreement with Lincoln Park, upon effectiveness of the registration statement for resale of the shares offered by this prospectus, and subject to other conditions, we may direct Lincoln Park to purchase up to $15,000,000 of our shares of common stock over a 30-month period. We have issued to Lincoln Park 48,063 shares of common stock as Initial Commitment Shares as of the date of this prospectus, and may in the future issue an additional 48,063 shares of common stock as Additional Commitment Shares as a fee for its commitment to purchase the shares, if any, registered for resale under any additional registration statement. The number of shares ultimately offered for sale by Lincoln Park under this prospectus is dependent upon the number of shares purchased by Lincoln Park under the Purchase Agreement. Depending on market liquidity at the time, sales of shares we issue to Lincoln Park may cause the trading price of our common stock to decline.
Subject to certain conditions, we generally have the right to control the timing and amount of any sales of our shares to Lincoln Park, except that, pursuant to the terms of our agreements with Lincoln Park, we would be unable to sell shares to Lincoln Park if and when the market price of our common stock is below $2.50 per share or if Lincoln Park would own more than 9.99% of our common stock for stock sold to it under the Purchase Agreement. The purchase price for the shares that we may sell to Lincoln Park will fluctuate based on the price of our common stock and other factors determined by us. As such, Lincoln Park may ultimately purchase all, some or none of the shares of our common stock offered pursuant to this prospectus and, after it has acquired shares, Lincoln Park may resell all, some or none of those shares. Therefore, sales to Lincoln Park by us pursuant to either or both of the purchase agreements could result in substantial dilution to the interests of other holders of our common stock. Additionally, the sale of a substantial number of shares of our common stock to Lincoln Park, or the anticipation of such sales, could cause the trading price of our common stock to decline and could make it more difficult for us to sell equity or equity-related securities in the future.
Some of our outstanding warrants include anti-dilution protection for any issuance of securities lower than the exercise price of such warrants such as is contemplated by this offering if such lower issuance occurs prior to the exercise or during the exercise period of the warrants. This anti-dilution protection could result in dilution to the shareholders and may contribute to downward pressure on the trading price of our common stock.
As of September 30, 2013 we have outstanding Class A warrants to purchase 226,229 shares of common stock issued in January 2010 and warrants to purchase 15,405 shares of common stock issued December 2010, with current exercise prices of $25.40 per common share and $5.60 per common share before any adjustment related to this offering, respectively. These warrants contain anti-dilution provisions that reduce the exercise price of the warrants if we issue or sell, or are deemed to have issued or sold, any shares of its common stock or securities exercisable or convertible into shares of common stock for no consideration or for a consideration per share less than the applicable exercise price in effect immediately prior to the time of such issue or sale, as is contemplated by this offering. The exercise of the warrants at prices below the market price of our common stock could adversely affect the price of shares of our common stock. In addition, sales of the shares of our common stock issuable upon exercise of the warrants could have a depressive effect on the price of our common stock, particularly if there is not a coinciding increase in demand by purchasers of our common stock.
Eastern Capital Limited holds a large percentage of our outstanding capital stock and has significant influence over the outcome of corporate actions requiring shareholder approval; and such shareholder’s priorities for our business may be different from other shareholders’.
Eastern Capital Limited (Eastern Capital) has not entered into a lock-up agreement in connection with this offering. All of the accumulated dividends in Series B-1 non-voting preferred stock and outstanding Series B-2 voting preferred stock, representing a significant amount of our outstanding capital stock on a fully-converted basis, are held by Eastern Capital. The accumulated dividends in our Series B-1 non-voting preferred stock are exchangeable for shares of Series B-2 voting preferred stock and, in March 2017, are convertible into shares of our common
stock. Based solely on the number of shares of Series B-2 preferred stock that Eastern Capital held as of September 30, 2013, Eastern Capital has beneficial ownership of approximately nine percent (9%) (calculated on an as converted to common stock basis and excluding any shares that will accrue as a dividend on the shares of Series B-2 preferred) of our voting securities based on the approximately 6,554,000 shares of common stock and Series B-2 preferred stock outstanding as of January 31, 2014. Furthermore, in connection with the March 2012 financing, we amended our Shareholder Rights Plan described below under “Description of Capital Stock” to allow Eastern Capital to acquire beneficial ownership of up to 49.9% of the Company’s outstanding securities without being deemed an “Acquiring Person” for purposes of our Shareholder Rights Plan. As a result of their current ownership and their ability to acquire more of our securities, they will be able to significantly influence the outcome of any financing transaction or other matter submitted to our shareholders for approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transaction. The interests of Eastern Capital may differ from the interests of our other shareholders. For example, Eastern Capital could delay or prevent a change of control of the Company even if such a change of control would benefit our other shareholders. The significant concentration of stock ownership may adversely affect the trading price of our common stock due to our investors’ perception that conflicts of interest may exist or arise.
In addition, the shares of Series B-1 preferred stock and the shares of Series B-2 preferred stock which may be issued upon exchange of the shares of Series B-1 preferred stock have certain rights, preferences and privileges that rank senior to the shares of our common stock. For example, the shares of Series B-1 preferred stock and Series B-2 preferred stock are entitled to receive a liquidation preference prior to any payment being made to holders of common stock upon a voluntary or involuntary liquidation, dissolution or winding up of the Company, or, in certain cases, if we experience a change of control. Furthermore, if the shares of Series B-1 preferred stock are never exchanged for shares of Series B-2 preferred stock and/or converted into shares of our common stock, at any time after March 2017, we may be required to redeem the then outstanding shares of Series B-1 preferred stock and any dividend shares accrued thereon at a price equal to the greater of (A) $3.25 (subject to adjustments for stock splits and similar events) plus all accrued dividends and (B) the then fair market value of a share of common stock multiplied by the number of shares of common stock into which such share of Series B-1 preferred stock is then convertible. Such redemption would be completed in three annual installments beginning not more than 120 days after we receive a request for redemption. The requirement for us to redeem Eastern Capital’s shares of Series B-1 preferred stock in cash could diminish our working capital, the consequences of which could have a material adverse effect on our business, operating results, financial condition and prospects.
Our corporate documents and Michigan law contain provisions that may make it more difficult for us to be acquired.
Our Board of Directors (Board) has the authority, without shareholder approval, to issue additional shares of preferred stock and to fix the rights, preferences, privileges and restrictions of these shares without any further vote or action by our shareholders. Michigan law contains a provision that makes it more difficult for a 10% shareholder, or its officers, to acquire a company. This authority, together with certain provisions of our charter documents, may have the effect of making it more difficult for a third party to acquire, or of discouraging a third-party from attempting to acquire, control of our company. This effect could occur even if our shareholders consider the change in control to be in their best interest. We have adopted a shareholder rights plan, the purpose of which is, among other things, to enhance our Board’s ability to protect shareholder interests and to ensure that shareholders receive fair treatment in the event any coercive takeover attempt of our company is made in the future. The shareholder rights plan could make it more difficult for a third party to acquire, or could discourage a third party from acquiring, our company or a large block of our company’s common stock.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the documents that we incorporate by reference, contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (Securities Act) and Section 21E of the Securities Exchange Act of 1934, as amended (Exchange Act). Any statements about our expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and may be forward-looking. These statements are often, but are not always, made through the use of words or phrases such as “anticipates,” “estimates,” “plans,” “projects,” “trends,” “opportunity,” “comfortable,” “current,” “intention,” “position,” “assume,” “potential,” “outlook,” “remain,” “continue,” “maintain,” “sustain,” “seek,” “achieve,” “continuing,” “ongoing,” “expects,” “believe,” “intend” and similar words or phrases, or future or conditional verbs such as “will,” “would,” “should,” “could,” “may,” or similar expressions. Accordingly, these statements involve estimates, assumptions and uncertainties which could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified in their entirety by reference to the factors discussed throughout this report, and in particular those factors referenced in the section “Risk Factors.”
Because the factors referred to in the preceding paragraph could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements we make, you should not place undue reliance on any such forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made, and we undertake no obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding:
· potential strategic collaborations with others;
· future capital needs;
· adequacy of existing capital to support operations for a specified time;
· product development and marketing plans;
· features and successes of our cellular therapies;
· manufacturing and facility capabilities;
· clinical trial plans and anticipated results, including the publication thereof;
· anticipation of future losses;
· replacement of manufacturing sources;
· commercialization plans; or
· revenue expectations and operating results.
We have never declared dividends on our common stock, and currently do not plan to declare dividends on shares of our common stock in the foreseeable future. We expect to retain our future earnings, if any, for use in the operation and expansion of our business. Subject to the foregoing, the payment of cash dividends in the future, if any, will be at the discretion of our Board and will depend upon such factors as earnings levels, capital requirements, our overall financial condition and any other factors deemed relevant by our Board.
We will not receive any proceeds from the sales of shares of our common stock by Lincoln Park; however, we may receive proceeds of up to $15,000,000 under the Purchase Agreement over the 30-month period following the effective date of the registration statement of which this prospectus is a part assuming that we sell all of the shares available thereunder and excluding the cost of the shares issued to Lincoln Park for its commitment. However, there can be no assurance we will sell any or all of the shares to Lincoln Park or that they will resell such shares offered hereby.
We estimate that we will use the proceeds under the Purchase Agreement as follows:
· to help fund the internal and external clinical development costs associated with the ixCELL-DCM Phase 2b clinical trial, the REVIVE-CLI study, and investigator-sponsored trials;
· to help fund development costs associated with preclinical studies and to further develop our manufacturing platform; and
· to help fund general corporate purposes, including internal development costs, working capital, general administrative costs and the prosecution and maintenance of our intellectual property.
We will be required to raise substantial additional capital to continue to fund the clinical development of our cell therapy applications. We may raise additional capital through additional public or private financings, as well as collaborative relationships, incurring debt and other available sources. Please see the discussion of the risks associated with our liquidity in the section “Risk Factors.”
MARKET PRICE OF OUR COMMON STOCK
Our common stock is traded on The NASDAQ Capital Market under the symbol “ASTM.” Our common stock has, from time to time, traded on a limited, sporadic and volatile basis. The table below shows the high and low closing prices for our common stock for the periods indicated, as reported by NASDAQ. Prices per share of our common stock have been adjusted for the twenty-for-one reverse stock split on October 16, 2013 on a retroactive basis.
|
|
|
Price Ranges |
| ||||
|
|
|
High |
|
Low |
| ||
|
Fiscal Year Ending December 31, 2013 |
|
|
|
|
| ||
|
First Quarter |
|
$ |
28.20 |
|
$ |
14.00 |
|
|
Second Quarter |
|
16.00 |
|
8.02 |
| ||
|
Third Quarter |
|
15.48 |
|
5.40 |
| ||
|
Fourth Quarter |
|
5.70 |
|
3.21 |
| ||
|
|
|
|
|
|
| ||
|
Fiscal Year Ended December 31, 2012 |
|
|
|
|
| ||
|
First Quarter |
|
$ |
44.00 |
|
$ |
35.60 |
|
|
Second Quarter |
|
52.80 |
|
38.80 |
| ||
|
Third Quarter |
|
43.60 |
|
31.40 |
| ||
|
Fourth Quarter |
|
32.60 |
|
23.00 |
| ||
The following table summarizes all compensation earned by or paid to Dominick C. Colangelo, our chief executive officer effective March 1, 2013, Daniel R. Orlando, our chief operating officer (served as interim president and chief executive officer until March 1, 2013), Ronnda L. Bartel, our chief scientific officer, Sharon M. Watling, our vice president of clinical development until April 26, 2013, Brian D. Gibson, our vice president of finance, chief accounting officer and treasurer until August 23, 2013 and Michael W. Elliston, our controller, chief accounting officer and treasurer since August 23, 2013, (the “named executive officers”) during the fiscal years ended December 31, 2011, 2012 and 2013, respectively.
2013 SUMMARY COMPENSATION TABLE
|
|
|
|
|
|
|
|
|
Option |
|
Non-Equity Incentive |
|
All Other |
|
|
| ||||||
|
Name and Principal |
|
|
|
Salary |
|
Bonus |
|
Awards |
|
Plan |
|
Compensation |
|
Total |
| ||||||
|
Position |
|
Year |
|
($) |
|
($) |
|
($)(1) |
|
Compensation($)(2) |
|
($)(3) |
|
($) |
| ||||||
|
Dominick C. Colangelo |
|
Dec. 2013 |
|
$ |
358,333 |
(4) |
$ |
— |
|
$ |
937,884 |
|
$ |
89,583 |
|
$ |
59,264 |
(5) |
$ |
1,445,064 |
|
|
President and CEO |
|
Dec. 2012 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
|
|
Dec. 2011 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Daniel R. Orlando, |
|
Dec. 2013 |
|
$ |
285,000 |
|
$ |
35,000 |
(6) |
$ |
— |
|
$ |
45,600 |
|
$ |
46,597 |
(7) |
$ |
412,197 |
|
|
Chief Operating Officer |
|
Dec. 2012 |
|
$ |
99,948 |
(8) |
$ |
— |
|
$ |
485,466 |
|
$ |
— |
|
$ |
443 |
|
$ |
585,857 |
|
|
|
|
Dec. 2011 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Ronnda L. Bartel, |
|
Dec. 2013 |
|
$ |
260,000 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
28,859 |
(9) |
$ |
288,859 |
|
|
Chief Scientific Officer |
|
Dec. 2012 |
|
$ |
253,079 |
|
$ |
— |
|
$ |
209,174 |
|
$ |
— |
|
$ |
9,619 |
|
$ |
471,872 |
|
|
|
|
Dec. 2011 |
|
$ |
243,389 |
|
$ |
14,814 |
(10) |
$ |
235,939 |
|
$ |
85,186 |
|
$ |
7,145 |
|
$ |
586,473 |
|
|
Sharon M. Watling, |
|
Dec. 2013 |
|
$ |
79,811 |
(11) |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
185,299 |
(13) |
$ |
265,110 |
|
|
Vice President Clinical Development |
|
Dec. 2012 |
|
$ |
245,000 |
|
$ |
— |
|
$ |
199,062 |
|
$ |
— |
|
$ |
10,429 |
(13) |
$ |
454,491 |
|
|
|
|
Dec. 2011 |
|
$ |
210,625 |
(12) |
$ |
— |
|
$ |
333,179 |
|
$ |
70,000 |
|
$ |
9,714 |
(13) |
$ |
623,518 |
|
|
Brian D. Gibson, |
|
Dec. 2013 |
|
$ |
110,000 |
(14) |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
32,435 |
(16) |
$ |
142,435 |
|
|
Vice President of Finance, Chief Accounting |
|
Dec. 2012 |
|
$ |
165,000 |
|
$ |
— |
|
$ |
319,367 |
|
$ |
— |
|
$ |
21,145 |
(16) |
$ |
505,512 |
|
|
Officer and Treasurer |
|
Dec. 2011 |
|
$ |
123,333 |
(15) |
$ |
— |
|
$ |
105,532 |
|
$ |
40,000 |
|
$ |
6,468 |
(16) |
$ |
275,333 |
|
|
Michael W. Elliston, |
|
Dec. 2013 |
|
$ |
113,455 |
(17) |
$ |
— |
|
$ |
— |
|
$ |
17,500 |
|
$ |
4,811 |
(18) |
$ |
135,766 |
|
|
Controller, Chief Accounting Officer and |
|
Dec. 2012 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Treasurer |
|
Dec. 2011 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
|
|
|
|
(1) |
|
Amount reflects the grant date fair value of the named executive officer’s stock options, calculated in accordance with FASB ASC Topic 718. For purposes of this calculation, we have disregarded forfeiture assumptions. For a discussion of the assumptions used in calculating these values, see Note 3 to our consolidated financial statements in our annual report on Form 10-K for the fiscal year ended December 31, 2012 filed with the SEC on March 18, 2013. |
|
|
|
|
|
(2) |
|
Amounts reflected in this column were awarded pursuant to individual’s employment agreement, as applicable, as described in more detail below. |
|
|
|
|
|
(3) |
|
The all other compensation column includes Aastrom contributions to 401(k) Supplemental Retirement Plans (401(k) Plan), as detailed in footnotes 5, 7, 13, 16 and 18. None of the named executive officers received perquisites having an aggregate value of $10,000 or more in the fiscal years ended December 31, 2013, 2012 or 2011, respectively. All other compensation also includes the portion of medical, dental, vision and long term disability premiums by Aastrom on behalf of the named executive officers. These benefits are offered to all full-time Aastrom employees. |
|
|
|
|
|
(4) |
|
Mr. Colangelo became our President and Chief Executive Officer effective as of March 1, 2013. This amount represents the salary earned by Mr. Colangelo subsequent to his employment. |
|
|
|
|
|
(5) |
|
This amount includes Aastrom contributions made to Mr. Colangelo’s 401(k) Plan of $6,898 and $35,124 of reimbursable commuting expenses in the fiscal year ended December 31, 2013. |
|
|
|
|
|
(6) |
|
This amount represents a bonus paid to Mr. Orlando for his service as Aastrom’s interim President and Chief Executive Officer. |
|
|
|
|
|
(7) |
|
This amount includes Aastrom contributions made to Mr. Orlando’s 401(k) Plan of $10,747 and $18,326 of reimbursable commuting expenses in the fiscal year ended December 31, 2013. |
|
|
|
|
|
(8) |
|
This amount represents the salary earned by Mr. Orlando during fiscal 2012 after his employment commenced in August 2012. |
|
|
|
|
|
(9) |
|
This amount includes vacation payout of $20,000 that was paid in January 2014. |
|
|
|
|
|
(10) |
|
Represents the cash performance bonus awarded to Dr. Bartel on May 2, 2012 and January 18, 2011 based on the achievement of goals for Aastrom and Dr. Bartel set by the Compensation Committee of the Board of Directors. |
|
|
|
|
|
(11) |
|
Represents the salary earned by Dr. Watling prior to April 26, 2013. |
|
|
|
|
|
(12) |
|
Effective March 22, 2011, Dr. Watling was made an executive officer of Aastrom. This amount represents the salary earned by Dr. Watling during the fiscal year ended December 31, 2011. |
|
|
|
|
|
(13) |
|
These amounts include Aastrom contributions made to Dr. Watling’s 401(k) Plan of $2,501, $8,575 and $8,084 in the fiscal years ended December 31, 2013, 2012 and 2011, respectively. The amount in 2013 also includes $163,333 in severance and $18,846 in vacation payout. |
|
|
|
|
|
(14) |
|
Represents the salary earned by Mr. Gibson prior to August 31, 2013. |
|
|
|
|
|
(15) |
|
Effective October 13, 2011, Mr. Gibson was promoted from Controller to Vice President of Finance, Chief Accounting Officer and Treasurer and executive officer of Aastrom. This amount represents the salary earned by Mr. Gibson during the fiscal year ended December 31, 2011. |
|
|
|
|
|
(16) |
|
These amounts include Aastrom contributions made to Mr. Gibson’s 401(k) Plan of $4,331, $5,775 and $4,748 in the fiscal years ended December 31, 2013, 2012 and 2011, respectively. The amount in 2013 also includes $12,693 in vacation payout. |
|
|
|
|
|
(17) |
|
Effective August 23, 2013, Mr. Elliston was appointed Treasurer, Secretary and Principle Finance and Accounting Officer and executive officer of Aastrom. This amount represents the salary earned by Mr. Elliston during the fiscal year ended December 31, 2013. |
|
|
|
|
|
(18) |
|
This amount includes Aastrom contributions made to Mr. Elliston’s 401(k) Plan of $3,945 during the fiscal year ended December 31, 2013. |
STOCK OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information, as of January 31, 2014, or as otherwise set forth below, with respect to the beneficial ownership of Aastrom’s common stock by (i) all persons known by Aastrom to be the beneficial owners of more than 5% of the outstanding common stock of Aastrom; (ii) each director and director nominee of Aastrom, (iii) each executive officer of Aastrom named in the Summary Compensation Table, and (iv) all executive officers and directors of Aastrom as a group.
|
|
|
Shares Owned(1) |
| ||
|
Name and Address of Beneficial Owner(2) |
|
Number of |
|
Percentage of |
|
|
Robert L. Zerbe(4) |
|
15,611 |
|
* |
|
|
Ronald M. Cresswell(5) |
|
16,763 |
|
* |
|
|
Alan L. Rubino(6) |
|
15,758 |
|
* |
|
|
Nelson M. Sims(7) |
|
16,967 |
|
* |
|
|
Dominick C. Colangelo(8) |
|
17,189 |
|
* |
|
|
Daniel R. Orlando(9) |
|
9,876 |
|
* |
|
|
Ronnda L. Bartel(10) |
|
23,756 |
|
* |
|
|
Sharon M. Watling(11) |
|
— |
|
* |
|
|
Brian D. Gibson(12) |
|
— |
|
* |
|
|
Michael W. Elliston(13) |
|
3,995 |
|
* |
|
|
Eastern Capital Limited(14) |
|
1,402,889 |
|
21.4 |
% |
|
All officers and directors as a group (10 persons and 1 company)(15) |
|
1,522,804 |
|
23.2 |
% |
|
* |
|
Represents less than 1% of the outstanding shares of Aastrom’s common stock equivalents. |
|
|
|
|
|
(1) |
|
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting and investment power with respect to shares. Except as indicated in the footnotes to this table, to the knowledge of Aastrom, the persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to community property laws, where applicable. The number of shares owned and percentage ownership amounts include shares of restricted stock granted under Aastrom’s Amended and Restated 2004 Equity Incentive Plan (the “2004 Plan”) and the Aastrom 2009 Omnibus Incentive Plan (the “Incentive Plan”). Pursuant to the rules of the SEC, the number of shares of Aastrom’s common stock deemed outstanding includes shares issuable pursuant to options held by the respective person or group that are currently exercisable or may be exercised within 60 days of January 31, 2014. |
|
|
|
|
|
(2) |
|
The address for each beneficial owner is 24 Frank Lloyd Wright Drive, Lobby K, Ann Arbor, MI 48105, except Eastern Capital Limited which is P.O. Box 31300, Grand Cayman, KY1-1206 Cayman Islands. |
|
|
|
|
|
(3) |
|
Calculated on the basis of 5,939,043 shares of common stock plus 12,308 shares of Series B-2 preferred stock on an as converted basis for a total of 6,554,443 common stock equivalents outstanding as of January 31, 2014. |
|
|
|
|
|
(4) |
|
Includes 15,611 shares issuable upon exercise of options held by Dr. Zerbe that are exercisable within the 60-day period following January 31, 2014. |
|
|
|
|
|
(5) |
|
Includes 16,763 shares issuable upon exercise of options held by Dr. Cresswell that are exercisable within the 60-day period following January 31, 2014. |
|
|
|
|
|
(6) |
|
Includes 15,758 shares issuable upon exercise of options held by Mr. Rubino that are exercisable within the 60-day period following January 31, 2014. |
|
|
|
|
|
(7) |
|
Includes 16,967 shares issuable upon exercise of options held by Mr. Sims that are exercisable within the 60-day period following January 31, 2014. |
|
(8) |
|
Includes 17,189 shares issuable upon exercise of options held by Mr. Colangelo that are exercisable within the 60-day period following January 31, 2014. |
|
|
|
|
|
(9) |
|
Includes 9,876 shares issuable upon exercise of options held by Mr. Orlando that are exercisable within the 60-day period following January 31, 2014. |
|
|
|
|
|
(10) |
|
Includes 23,756 shares issuable upon exercise of options held by Dr. Bartel that are exercisable within the 60-day period following January 31, 2014. |
|
|
|
|
|
(11) |
|
Dr. Watling does not have any shares issuable upon exercise of options that are exercisable within the 60-day period following January 31, 2014. |
|
|
|
|
|
(12) |
|
Mr. Gibson does not have any shares issuable upon exercise of options that are exercisable within the 60-day period following January 31, 2014. |
|
|
|
|
|
(13) |
|
Includes 3,995 shares issuable upon exercise of options held by Mr. Elliston that are exercisable within the 60-day period following January 31, 2014. |
|
|
|
|
|
(14) |
|
Includes the common share equivalent for 12,308 shares of Series B-2 preferred stock that are convertible into 50 common shares for each share of Series B-2 preferred stock and vote on an as converted basis and 362,500 of common stock issuable upon exercise of warrants. |
|
|
|
|
|
(15) |
|
Includes 478,857 shares issuable upon exercise of options/warrants that are exercisable within the 60-day period following January 31, 2014. |
General
On January 21, 2014, we entered into the Purchase Agreement and the Registration Rights Agreement with Lincoln Park. Pursuant to the terms of the Purchase Agreement, Lincoln Park has agreed to purchase from us up to $15,000,000 in shares of our common stock, subject to certain limitations. Under the terms of the Purchase Agreement, we may, from time to time and at our sole discretion during a 30-month period commencing on the date of the effectiveness of the registration statement of which this prospectus is a part, subject to the conditions of the Purchase Agreement, direct Lincoln Park to purchase shares of our common stock up to an aggregate amount of $15,000,000. The amount we receive and the per share purchase price depends on whether the purchase is a Regular Purchase or an Accelerated Purchase. An aggregate of 1,748,063 shares issuable to Lincoln Park under the Purchase Agreement are being offered pursuant to this prospectus. The proceeds received by us under the Purchase Agreement are expected to be used for clinical development costs and general corporate purposes as described further in this prospectus.
Pursuant to the terms of the Registration Rights Agreement, we have filed with the SEC the registration statement that includes this prospectus to register for resale under the Securities Act shares of common stock that may be issued and sold to Lincoln Park under the Purchase Agreement. Although the Purchase Agreement provides that we may sell up to $15,000,000 in shares of our common stock to Lincoln Park, only 1,748,063 shares are being offered under this prospectus, which represents (i) up to 1,700,000 shares that we may sell and issue to Lincoln Park from time to time in the future pursuant to the Purchase Agreement after the registration statement of which this prospectus forms a part is declared effective, and (ii) up to 48,063 shares as Commitment Shares we are obligated to issue to Lincoln Park.
We cannot predict the actual total number of shares to be issued to Lincoln Park. The aggregate number of shares offered under this prospectus may or may not cover all of such shares to be purchased by and issued to Lincoln Park under the $15,000,000 Purchase Agreement, depending on the purchase price per share. In the event the initial registration statement is insufficient to cover all of the shares issuable under the Purchase Agreement, we may elect to file a new registration statement so as to cover all of the shares potentially issuable. As of the date hereof, we do not currently have any plans or intent to issue to Lincoln Park any shares pursuant to the Purchase Agreement beyond the 1,748,063 shares offered hereby. If we elect to issue and sell to Lincoln Park pursuant to the Purchase Agreement more than the 1,748,063 shares offered under this prospectus, up to the $15,000,000 maximum, we would first be required to register for resale under the Securities Act such additional shares, which could cause substantial additional dilution to our stockholders.
Purchase of Shares under the Purchase Agreement
By means of a Regular Purchase, so long as at least one business day has passed since the most recent purchase, we may direct Lincoln Park to purchase up to 50,000 shares of our common stock at the Regular Purchase Price, increasing to amounts up to 100,000 shares of our common stock depending upon the closing sale price of our common stock. The Regular Purchase Price is the lower of:
· the lowest sale price for our common stock reported on the NASDAQ Capital Market on the purchase date of such shares or
· the arithmetic average of the three lowest closing sale prices for our common stock during the 10 consecutive business days immediately preceding the purchase date of such shares.
Additionally, we may direct Lincoln Park to purchase additional amounts as Accelerated Purchases if on the date of a Regular Purchase the closing sale price of our common stock equals or exceeds $3.00. In such event, we may direct Lincoln Park to purchase on the following day, as an Accelerated Purchase and at the Accelerated Purchase Price, up to the lesser of:
· three times the number of shares for a Regular Purchase or
· up to 30% of the following day’s volume.
The Accelerated Purchase Price is the lower of:
· 97% of the volume weighted average price during (i) the entire trading day on the purchase date, if the volume of shares of our common stock traded on the purchase date has not exceeded a volume maximum calculated in accordance with the Purchase Agreement, or (ii) the portion of the trading day on the purchase date (calculated starting at the beginning of normal trading hours) until such time at which the volume of shares of our common stock traded has exceeded such volume maximum, or
· the closing sale price of our common stock on the purchase date.
There is no upper limit on the price per share that Lincoln Park must pay for our common stock under the Purchase Agreement, but in no event will shares be sold to Lincoln Park under a Regular Purchase on a day our closing price is less than the minimum floor price of $2.50 per share. The Regular Purchase Price and the Accelerated Purchase Price will be equitably adjusted for any reorganization, recapitalization, non-cash dividend, stock split, or other similar transaction occurring during the business days used to compute the Regular Purchase Price or Accelerated Purchase Price.
The Purchase Agreement limits our sales of shares of common stock to Lincoln Park to the maximum number of shares of our common stock that we may issue without breaching our obligations under applicable rules of the NASDAQ Capital Market (approximately 1,148,843 shares, or 19.99% of our total outstanding common stock as of the date of the Purchase Agreement) or obtaining shareholder approval under such rules, unless the average price of all applicable sales of common stock exceed a “Base Price” (or $4.13, representing our closing consolidated bid price on January 17, 2014, plus an incremental amount to account for the issuance of commitment shares) such that the sales to Lincoln Park are considered to be at least “at market” under applicable NASDAQ rules.
Other than as set forth above, there are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing and amount of any sales of our common stock to Lincoln Park. Generally, each time we direct Lincoln Park, subject to the terms of the Purchase Agreement, Lincoln Park will be obligated to purchase such amounts as directed by us. Lincoln Park does not have the right to require us to sell any shares of common stock to them under the Purchase Agreement. We have no obligation to sell any shares under the Purchase Agreement and the actual proceeds that we receive from sales to Lincoln Park could be substantially less than the maximum $15,000,000.
Commitment Shares
As consideration for Lincoln Park’s commitment to purchase our common stock pursuant to the Purchase Agreement, we previously issued to Lincoln Park 48,063 shares as Initial Commitment Shares. Lincoln Park may not assign or transfer its rights and obligations under the Purchase Agreement. In the event the initial registration statement is insufficient to cover all of the shares issuable under the Purchase Agreement and we file a new registration statement to cover any remaining shares not covered by the initial registration statement, we will issue to Lincoln Park 48,063 shares (or the Additional Commitment Shares) as additional consideration.
Effect of Performance of the Purchase Agreement on Our Shareholders
All shares of common stock that are covered by this prospectus are expected to be freely tradable. It is anticipated that shares registered in this offering will be sold over a period of up to 30 months from the date of this prospectus. The sale by Lincoln Park of a significant amount of shares registered in this offering at any given time could cause the market price of our common stock to decline and to be highly volatile. Lincoln Park may ultimately acquire all, some or none of the shares of common stock not yet issued but registered in this offering. After it has acquired such shares, it may sell all, some or none of such shares. Therefore, sales to Lincoln Park by us under the Purchase Agreement may result in substantial dilution to the interests of other holders of our common stock. However, we have the right to control the timing and amount of any sales of our shares to Lincoln Park and the Purchase Agreement may be terminated by us at any time at our discretion without any cost to us.
As of January 21, there were 5,747,087 shares of our common stock issued and outstanding, of which 5,318,519 shares were held by non-affiliates. If all of the 1,748,063 shares offered by Lincoln Park under this prospectus were issued and outstanding as of the date hereof (without taking into account the 19.99% shareholder approval limitation), such shares would represent approximately 30% of the total common stock outstanding and approximately 33% of the total number of outstanding shares held by non-affiliates.
The number of shares ultimately offered for resale by Lincoln Park will be dependent upon the number of shares we sell to Lincoln Park under the Purchase Agreement. The following table shows the amount of proceeds we would receive from Lincoln Park from the sale of shares pursuant to the Purchase Agreement (without accounting for certain fees and expenses), to the extent covered by this prospectus, based on varying assumed average purchase prices:
|
Assumed Average |
|
Number of Registered |
|
Percentage of Outstanding |
|
Proceeds from the Sale of |
| |
|
$2.50 (3) |
|
1,700,000 |
|
22.68 |
% |
$ |
4,250,000 |
|
|
$3.50 |
|
1,700,000 |
|
22.68 |
% |
$ |
5,950,000 |
|
|
$4.50 |
|
1,700,000 |
|
22.68 |
% |
$ |
7,650,000 |
|
|
$5.50 |
|
1,700,000 |
|
22.68 |
% |
$ |
9,350,000 |
|
|
(1) |
Excludes the 48,063 shares to be issued as Commitment Shares. Although the Purchase Agreement provides that we may sell up to $15,000,000 in shares of our common stock to Lincoln Park, we are only registering 1,700,000 shares to be purchased thereunder, which may or may not cover all of such shares purchased by them under the Purchase Agreement, depending on the purchase price per share. As a result, we have included in this column only those shares which are registered in this offering. |
|
|
|
|
(2) |
The denominator is based on 5,795,150 shares outstanding as of January 21, 2014, adjusted to include the 48,063 shares issued as Initial Commitment Shares, and the number of shares set forth in the adjacent column which we would have sold to Lincoln Park. The numerator is based on the number of shares issuable under the Purchase Agreement at the corresponding assumed purchase price set forth in the adjacent column. |
|
|
|
|
(3) |
Under the Purchase Agreement, we may not sell any shares to Lincoln Park in the event the purchase price of such shares is below $2.50. |
|
|
|
|
(4) |
If we seek to issue shares, including shares from other transactions but not included in this offering that may be aggregated with this transaction under the applicable rules of the NASDAQ Capital Market, in excess of 1,748,063 or 19.99% of the total common stock outstanding as of the date of the Purchase Agreement, we may be required to seek shareholder approval in order to be in compliance with the NASDAQ Capital Market rules. |
There are substantial risks to our shareholders as a result of the sale and issuance of common stock to Lincoln Park under the Purchase Agreement. These risks include substantial dilution and declines in our stock price. See “Risk Factors.” Issuances of our common stock to Lincoln Park under the Purchase Agreement will not affect the rights or privileges of our existing shareholders, except that the economic and voting interests of our existing shareholders will be diluted as a result of any such issuance. Although the number of shares of common stock that our existing shareholders own will not decrease, the shares owned by our existing shareholders will represent a smaller percentage of our total outstanding shares after any such issuance to Lincoln Park.
Representations and Warranties; Indemnification
The Purchase Agreement includes customary representations and warranties by us to Lincoln Park. In addition, we have agreed to customary indemnification of Lincoln Park in connection with the Purchase Agreement.
Events of Default
Pursuant to the Purchase Agreement, we cannot sell any shares of common stock to Lincoln Park if an event of default has occurred. Lincoln Park does not have the right to terminate the Purchase Agreement upon any of the events of default set forth below. The following events constitute events of default under the Purchase Agreement, all of which are outside the control of Lincoln Park:
· the effectiveness of the registration statement, of which this prospectus is a part, lapses for any reason (including, without limitation, the issuance of a stop order), or this prospectus is unavailable for sale by us or the resale by Lincoln Park of our common stock offered hereby, and such lapse or unavailability continues for a period of ten consecutive business days or for more than an aggregate of thirty business days in any 365-day period;
· suspension by our principal market of our common stock from trading for a period of one business day;
· the delisting of our common stock from the NASDAQ Capital Market, provided our common stock is not immediately thereafter trading on the New York Stock Exchange, NYSE MKT, the NASDAQ Global Market, the NASDAQ Global Select Market, the NYSE ARCA or the OTC Bulletin Board (or nationally recognized successor thereto);
· the transfer agent’s failure for three business days to issue to Lincoln Park shares of our common stock which Lincoln Park is entitled to receive under the Purchase Agreement;
· any breach of the representations or warranties or covenants contained in the Purchase Agreement or any related agreements which has a material adverse effect on us, only if breach continues for a period of at least five business days;
· any participation in insolvency or bankruptcy proceedings by or against us, which is not discharged within 90 days;
· our common stock is not eligible to be transferred electronically; or
· if we reach the share limit to the extent applicable under the NASDAQ Capital Market rules, and we have not obtained any necessary shareholder approval.
Termination of the Purchase Agreement; No Assignment
We have the unconditional right at any time under the Purchase Agreement for any reason to give notice to Lincoln Park terminating the Purchase Agreement without any cost to us.
No Short-Selling or Hedging by Lincoln Park
Lincoln Park has agreed that any time prior to the termination of the Purchase Agreement neither it nor any of its affiliates shall engage in or enter into, directly or indirectly, any short-sale of our common stock or any hedging transaction that establishes a net short position in our common stock.
This prospectus relates to the possible resale by the selling shareholder, Lincoln Park, of shares of common stock that have been or may be issued to Lincoln Park pursuant to the Purchase Agreement. We are filing the registration statement of which this prospectus forms a part pursuant to the provisions of the Registration Rights Agreement, which we entered into with Lincoln Park on January 21, 2014 concurrently with our execution of the Purchase Agreement, in which we agreed to provide certain registration rights with respect to sales by Lincoln Park of the shares of our common stock that have been or may be issued to Lincoln Park under the Purchase Agreement.
Lincoln Park, as the selling shareholder, may, from time to time, offer and sell pursuant to this prospectus any or all of the shares that we have sold or may sell to Lincoln Park under the Purchase Agreement. The selling shareholder may sell some, all or none of its shares. We do not know how long the selling shareholder will hold the shares before selling them, and we currently have no agreements, arrangements or understandings with the selling shareholder regarding the sale of any of the shares.
The following table presents information regarding the selling shareholder and the shares that it may offer and sell from time to time under this prospectus. The table is prepared based on information supplied to us by the selling shareholder, and reflects its holdings as of January 31, 2014. Neither Lincoln Park nor any of its affiliates has held a position or office, or had any other material relationship, with us or any of our predecessors or affiliates. As used in this prospectus, the term “selling shareholder” means Lincoln Park. Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Exchange Act. The percentage of shares beneficially owned prior to the offering is based on 5,939,043 shares of our common stock actually outstanding as of January 31, 2014.
|
Selling Shareholder |
|
Shares Beneficially |
|
Percentage of |
|
Shares to be Sold in |
|
Percentage of |
|
|
|
|
|
|
|
|
|
|
|
|
|
Lincoln Park Capital Fund, LLC (1) |
|
48,063 (2) |
|
.08% (3) |
|
1,748,063 (4) |
|
.01% |
|
* Less than 1%
(1) Josh Scheinfeld and Jonathan Cope, the Managing Members of Lincoln Park Capital, LLC, are deemed to be beneficial owners of all of the shares of common stock owned by Lincoln Park Capital Fund, LLC. Messrs. Cope and Scheinfeld have shared voting and investment power over the shares being offered under the prospectus filed with the SEC in connection with the transactions contemplated under the Purchase Agreement. Lincoln Park Capital, LLC is not a licensed broker dealer or an affiliate of a licensed broker dealer.
(2) Represents 48,063 shares of our common stock issued to Lincoln Park on January 21, 2014 as a fee for its commitment to purchase additional shares of our common stock under the Purchase Agreement which shares are covered by the registration statement that includes this prospectus. See the description under the heading “The Lincoln Park Transaction” for more information about the Purchase Agreement.
(3) Based on 5,939,043 outstanding shares of our common stock as of January 31, 2014, which includes 48,063 shares of our common stock issued to Lincoln Park on January 21, 2014 as a commitment fee for its commitment to purchase additional shares of our common stock under the Purchase Agreement. Although we may at our discretion elect to issue to Lincoln Park up to an aggregate amount of $15,000,000 of our common stock
under the Purchase Agreement, other than the shares described in the immediately preceding sentence, such shares are not included in determining the percentage of shares beneficially owned before this offering.
(4) Assumes issuance of the maximum 1,748,063 shares being registered hereby, which reflects the 48,063 shares already issued and the issuance of an additional 1,700,000 shares under the Purchase Agreement.
The net tangible book value of our common stock as of September 30, 2013 was $4,950,000, or $1.15 per share. Net tangible book value per share represents our total tangible assets less our total tangible liabilities, divided by the number of shares of common stock before giving effect to the conversion of all outstanding shares of common stock upon the completion of this offering.
Net tangible book value dilution per share to new investors represents the difference between the amount per share paid by purchasers in this offering and the pro forma net tangible book value per share of our common stock immediately after the completion of this offering. After giving effect to our assumed sale of all issuance and sale of 1,700,000 shares of common stock in this offering at an assumed public offering price of $2.50 per share (based on the adjusted minimum floor price at which sales may be made), not including the 48,063 Initial Commitment shares to be issued to Lincoln Park as a fee for its commitment to purchase shares of our common stock, our as adjusted net tangible book value as of September 30, 2013 would have been approximately $8,966,250 or $1.49 per share of common stock. This represents an immediate increase in net tangible book value of $0.34 per share to existing shareholders and an immediate dilution in net tangible book value of $1.01 per share to purchasers of common stock in this offering at the assumed offering price, as illustrated in the following table:
The following briefly summarizes the general terms and provisions of our shares of common preferred stock and the warrants. You should read the provisions of our articles of incorporation, as amended (Charter), our amended and restated bylaws (Bylaws) and other relevant instruments and agreements relating to our securities before you make an investment decision with respect to our shares of common and preferred stock.
The following description of our common and preferred stock and certain provisions of our Charter, and our amended and restated Bylaws, is a summary and is qualified in its entirety by the provisions of our Charter and Bylaws.
Our authorized capital stock consists of 15,000,000 shares of common stock, no par value per share, and 5,000,000 shares of preferred stock, no par value per share. Please see “Certain Provisions of Michigan Law and of Our Charter and Bylaws” for a description of those provisions in our Charter and Bylaws that would have an effect of delaying, deferring or preventing a change in control of the Company and that would operate only with respect to an extraordinary corporate transaction involving us or our subsidiaries.
Common Stock
Holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of shareholders. We do not have a classified Board and shareholders do not have cumulative voting rights. Holders of common stock have no preemptive, redemption or conversion rights and are not subject to future calls or assessments. No sinking fund provisions apply to our common stock. All outstanding shares are fully-paid and non-assessable. In the event of our liquidation, dissolution or winding up, holders of common stock are entitled to share ratably in assets available for distribution, subject to any prior distribution rights of any preferred stock then outstanding. Holders of common stock are entitled to receive proportionately any such dividends declared by our Board, out of legally available funds for dividends, subject to any preferences that may be applicable to any shares of preferred stock that may be outstanding at that time. The rights, preferences and privileges of holders of common stock are set forth in our Charter, which may be amended by the holders of a majority of the outstanding shares of common stock. We have adopted a shareholder rights plan, which could make it more difficult for a third party to acquire, or could discourage a third party from acquiring, our company or a large block of our common stock. Please see the description above in “Certain Provisions of Michigan Law and of our Charter and Bylaws; Transfer Agent and Registrar.”
Preferred Stock
Our Board may issue preferred stock in one or more series without shareholder approval. Our Board may determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock. The issuance of preferred stock, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, could make it more difficult for a third party to acquire, or could discourage a third party from acquiring, a majority of our outstanding voting stock. The rights of holders of our common stock described above, will be subject to, and may be adversely affected by, the rights of any preferred stock that we may designate and issue in the future.
Shareholder Rights Agreement - Series A Junior Participating Cumulative Preferred Stock
On August 11, 2011, our Board adopted a shareholder rights agreement (Rights Agreement), the purpose of which is, among other things, to enhance the Board’s ability to protect shareholder interests and to ensure that shareholders receive fair treatment in the event any coercive takeover attempt of Aastrom is made in the future. The Rights Agreement could make it more difficult for a third-party to acquire, or could discourage a third party from acquiring, us or a large block of our common stock. The following summary description of the Rights Agreement should be read in conjunction with the Rights Agreement, which was filed with the SEC as an exhibit to a Registration Statement on Form 8-A on August 12, 2011 and amended in March 2012 to allow Eastern Capital to acquire beneficial ownership of up to 49.9% of the Company’s outstanding securities without being deemed an “acquiring person” for purposes of our Rights Agreement.
In connection with the adoption of the Rights Agreement, the Board declared a dividend distribution of one preferred stock purchase right (Right) for each outstanding share of common stock to shareholders of record as of the close of business on August 15, 2011. In addition, one Right will automatically attach to each share of common stock issued between August 15, 2011 and the distribution date. The Rights currently are not exercisable and are attached to and trade with the outstanding shares of common stock. Under the Rights Agreement, the Rights become exercisable if a person or group becomes an “acquiring person” by acquiring 15% or more of the outstanding shares of common stock or if a person or group commences a tender offer that would result in that person owning
15% or more of the common stock. If a person or group becomes an “acquiring person,” each holder of a Right (other than the acquiring person and its affiliates, associates and transferees) would be entitled to purchase, at the then-current exercise price, such number of shares of our preferred stock which are equivalent to shares of common stock having a value of twice the exercise price of the Right. If we are is acquired in a merger or other business combination transaction after any such event, each holder of a Right would then be entitled to purchase, at the then-current exercise price, shares of the acquiring company’s common stock having a value of twice the exercise price of the Right.
Each share of preferred stock is entitled to payment of a quarterly dividend, an increased vote multiple, and a liquidation preference. In addition, each share of preferred stock is granted the exclusive right to vote for two additional members of the Board whose positions are created upon the vesting of such rights upon holders of preferred stock. Once purchased, said shares are not redeemable by the Company.
The Rights may be redeemed in whole, but not in part, at a price of $0.001 per Right (payable in cash, common stock or other consideration deemed appropriate by the Board) by the Board only until the earlier of (i) the time at which any person becomes an “acquiring person” or (ii) the expiration date of the Rights Agreement. Immediately upon the action of the Board ordering redemption of the Rights, the Right will terminate and thereafter the only right of the holders of Rights will be to receive the redemption price. The Rights will expire at the close of business on August 15, 2021, unless previously redeemed or exchanged by us as described above.
Series B Convertible Preferred Stock
On March 9, 2012, the Company completed the sale of 12,308 shares of Series B-1 Non-Voting Convertible Preferred Stock (Series B-1 preferred stock) at an offering price of $3,250 per share. In addition to the Series B-1 preferred stock, which was issued at the closing, the Company also authorized Series B-2 Voting Convertible preferred Stock (Series B-2 preferred stock). The Series B-1 preferred stock and Series B-2 preferred stock collectively are referred to as the Series B preferred stock. The Series B preferred stock is convertible, at the option of the holder thereof at any time after the five year anniversary of the closing of the offering, into shares of common stock at a conversion price of $3.25 per share of common stock, at a conversion ratio of one share of preferred stock for fifty shares of common stock. At any time after the five year anniversary of issuance, the Company may elect to convert any or all outstanding shares of Series B preferred stock into shares of common stock, subject to certain limitations. Dividends on the Series B preferred stock will be cumulative and compound daily, at a rate of 11.5% per annum, payable upon conversion, liquidation, redemption or other similar events, and payable in cash or Series B-1 preferred stock until the five year anniversary of issuance. As of September 30, 2013, there are approximately 121,050 accumulated but undeclared Series B-1 dividend shares. Unless prohibited by Michigan law governing distributions to shareholders, the Series B-1 preferred stock shall be redeemable at the option of holder of the Series B-1 preferred stock commencing at any time after the five year anniversary of issuance, liquidation, winding up, dissolution or other similar events, subject to certain terms and limitations.
On August 12, 2013, the Company amended the Series B preferred stock agreement to remove the cash redemption provision, modify the liquidation preferences for the Series B-2 preferred stock and to increase the redemption price for the Series B-1 preferred stock. The redemption price, prior to the five year anniversary, is now equal to $7,430 multiplied by the number of Series B-1 preferred shares redeemed minus the Company’s closing stock price multiplied by the number of common shares into which the outstanding Series B-2 preferred stock are convertible. The redemption price, after the five year anniversary, is the amount equal to the greater of the Series B offering price plus accrued dividends or the conversion value in common stock. As a result of the amendment to the agreement, the Series B preferred stock has been reclassified from mezzanine into shareholders’ equity (deficit).
Transfer Agent
The transfer agent of our common stock is Continental Stock Transfer & Trust Company.
CERTAIN PROVISIONS OF MICHIGAN LAW AND OF OUR CHARTER AND
BYLAWS
We are subject to certain anti-takeover provisions of the Michigan Business Corporation Act (MBCA) that could delay or make more difficult a merger or tender offer involving us. Chapter 7A of the MBCA prevents, in general, an “interested shareholder” (defined generally as a person owning 10% or more of a corporation’s outstanding voting shares) from engaging in a “business combination” (as defined therein) with a Michigan corporation unless:
(a) the board of directors issues an advisory statement, holders of 90% of the shares of each class of stock entitled to vote approve the transaction, and holders of two-thirds of the “disinterested” shares of each class of stock approve the transaction; (b) the interested shareholder has been an interested shareholder for at least five years and has not acquired beneficial ownership of any additional shares of the corporation subsequent to the transaction which resulted in such shareholder being classified as an interested shareholder, and meets certain requirements, including provisions relating to the fairness of the price and the form of consideration paid; or (c) the board of directors, by resolution, exempts a particular interested shareholder from these provisions prior to the interested shareholder becoming an interested shareholder. The MBCA also contains certain other provisions that could have anti-takeover effects.
Our Charter does not provide shareholders with the right to act without a meeting and does not provide for cumulative voting in the election of directors. The amendment of any of these provisions would require approval by holders of at least a majority of the shares of our outstanding common stock.
These and other provisions of our Charter or Bylaws, as well as our Rights Agreement described above under “Description of Capital Stock,” could have the effect of deterring certain takeovers or delaying or preventing certain changes in control or changes in our management, including transactions in which shareholders might otherwise receive a premium for their shares over then-current market prices.
The common stock offered by this prospectus is being offered by Lincoln Park, the selling shareholder. The common stock may be sold or distributed from time to time by the selling shareholder directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may be
changed. The sale of the common stock offered by this prospectus may be effected in one or more of the following methods:
· ordinary brokers’ transactions;
· transactions involving cross or block trades;
· through brokers, dealers, or underwriters who may act solely as agents;
· “at the market” into an existing market for the common stock;
· in other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents;
· in privately negotiated transactions; or
· any combination of the foregoing.
In order to comply with the securities laws of certain states, if applicable, the shares may be sold only through registered or licensed brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the registration or qualification requirement is available and complied with.
Brokers, dealers, underwriters, or agents participating in the distribution of the shares as agents may receive compensation in the form of commissions, discounts, or concessions from the selling shareholder and/or purchasers of the common stock for whom the broker-dealers may act as agent. The compensation paid to a particular broker-dealer may be less than or in excess of customary commissions.
Lincoln Park is an “underwriter” within the meaning of the Securities Act.
Neither we nor Lincoln Park can presently estimate the amount of compensation that any agent will receive. We know of no existing arrangements between Lincoln Park, any other shareholder, broker, dealer, underwriter, or agent relating to the sale or distribution of the shares offered by this prospectus. At the time a particular offer of shares is made, a prospectus supplement, if required, will be distributed that will set forth the names of any agents, underwriters, or dealers and any compensation from the selling shareholder, and any other required information.
We will pay all of the expenses incident to the registration, offering, and sale of the shares to the public other than commissions or discounts of underwriters, broker-dealers, or agents. We have also agreed to indemnify Lincoln Park and related persons against specified liabilities, including liabilities under the Securities Act.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and controlling persons, we have been advised that in the opinion of the SEC this indemnification is against public policy as expressed in the Securities Act and is therefore, unenforceable.
Lincoln Park and its affiliates have agreed not to engage in any direct or indirect short selling or hedging of our common stock during the term of the Purchase Agreement.
We have advised Lincoln Park that while it is engaged in a distribution of the shares included in this prospectus it is required to comply with Regulation M promulgated under the Securities Exchange Act of 1934, as amended. With certain exceptions, Regulation M precludes the selling shareholder, any affiliated purchasers, and any broker-dealer or other person who participates in the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or purchases made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability of the shares offered hereby this prospectus.
This offering will terminate on the date that all shares offered by this prospectus have been sold by Lincoln Park.
Our common stock is listed on the NASDAQ Capital Market under the symbol “ASTM.”
Certain legal matters, including the legality of the securities offered, will be passed upon for us by Dykema Gossett PLLC, Ann Arbor, Michigan, acting as special counsel to the Company. In connection with the offering, other legal matters will be passed upon for us by Goodwin Procter LLP, Boston, Massachusetts.
The consolidated financial statements and management’s assessment of the effectiveness of internal control over financial reporting (which is included in Management’s Report on Internal Control over Financial Reporting) incorporated in this Prospectus by reference to the Annual Report on Form 10-K for the year ended December 31, 2012 have been so incorporated in reliance on the report (which contains an explanatory paragraph relating to the Company’s ability to continue as a going concern as described in Note 1 to the consolidated financial statements) of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange Commission (SEC) a registration statement on Form S-1 under the Securities Act with respect to the securities offered by this prospectus. This prospectus, which forms a part of the registration statement, does not contain all the information set forth in the registration statement, as permitted by
the rules and regulations of the SEC. For further information with respect to us and the securities offered by this prospectus, reference is made to the registration statement.
Statements contained in this prospectus as to the contents of any contract or other document that we have filed as an exhibit to the registration statement are qualified in their entirety by reference to the exhibits for a complete statement of their terms and conditions.
We are subject to the information requirements of the Exchange Act and, in accordance therewith, file annual, quarterly and special reports, proxy statements and other information with the SEC. You may read and copy any document we file, including the registration statement, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You may call the SEC at 1-800-SEC-0330 for further information on the operation of the Public Reference Room. These documents also may be accessed through the SEC’s electronic data gathering, analysis and retrieval system, or EDGAR, via electronic means, including the SEC’s home page on the Internet (www.sec.gov). You may also inspect the registration statement on this website.
This prospectus incorporates by reference important business and financial information that we file with the SEC and that we are not including in or delivering with this prospectus. As the SEC allows, incorporated documents are considered part of this prospectus, and we can disclose important information to you by referring you to those documents. We incorporate by reference the documents listed below:
· our annual report on Form 10-K for the period ended December 31, 2012, filed with the SEC on March 18, 2013;
· our quarterly reports on Form 10-Q for the quarters ended March 31, 2013, filed with the SEC on May 8, 2013, June 30, 2013, filed with the SEC on August 7, 2013 and September 30, 2013, filed with the SEC on November 12, 2013;
· our current reports on Form 8-K, filed with the SEC on March 8, 2013, March 29, 2013, April 9, 2013, April 23, 2013 (including exhibit 99.1 thereto), May 3, 2013, May 13, 2013, May 24, 2013, August 12, 2013, August 14, 2013, August 26, 2013, September 27, 2013, October 10 , 2013, November 29, 2013, January 14, 2014 and January 27, 2014, respectively (excluding any information furnished in such reports under Item 2.02, Item 7.01 or Item 9.01);
· our definitive Proxy Statements on Schedule 14A for the Annual Meeting of Shareholders, filed with the SEC on March 22, 2013 and for the Special Meeting of Shareholders, filed with the SEC on September 5, 2013;
· the description of the rights to purchase shares of our Series A Junior Participating Cumulative Preferred Stock contained in the Registration Statement on Form 8-A, filed with the SEC on August 12, 2011, including any amendment or report for the purpose of updating such description; and
· the description of our common stock contained in our registration statements on Form S-1, filed with the SEC on November 1, 1996, filed with the SEC on including any amendment or report filed for the purpose of updating such description.
Pursuant to Rule 412 under the Securities Act, any statement contained in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or any other subsequently filed document that is deemed to be incorporated by reference into this prospectus modifies or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You may request a copy of any or all of these filings, at no cost, by writing to us at: Aastrom Biosciences, Inc., 24 Frank Lloyd Wright Drive, Lobby K, Ann Arbor, Michigan 48105, Attention: Investor Relations, or by telephoning us at (734) 418-4400. These filings may also be obtained through our website located at http://www.aastrom.com. The reference to our website is intended to be an inactive textual reference and, except for the documents incorporated by reference as noted above, the information on, or accessible through, our website is not intended to be part of this prospectus.
You should rely only on the information incorporated by reference or provided in this prospectus or any supplement. We have not authorized anyone else to provide you with different information. You should not assume that information in this prospectus or any supplement is accurate as of any date other than the date on the front of these documents.
We advise that there have been no material changes in our affairs that have occurred since the end of the latest fiscal period for which audited financial statements were included in the latest Form 10-K and that have not been described in a Form 8-K filed under the Exchange Act.
|
TERM |
|
DEFINITION |
|
Adverse Event |
|
Any adverse change in health or “side-effect” that occurs in a person participating in a clinical trial, from the time they consent to joining the trial until a pre-specified period of time after their treatment has been completed. |
|
Autologous (Patient Specific) |
|
Originating from the patient receiving treatment. (Aastrom uses only autologous cells) |
|
BLA — Biologics License Application |
|
An application containing product safety, efficacy and manufacturing information required by the FDA to market biologics products in the United States. |
|
CLI — Critical Limb Ischemia |
|
A vascular disease characterized by insufficient blood flow in the lower extremities that causes severe pain, tissue loss or both. |
|
Chemistry, Manufacturing, and Control |
|
The composition, manufacture, and control of the drug substance and the drug product. It is information on the identification, quality, purity, and strength of the investigational product. |
|
Controlled Clinical Trial |
|
A clinical study that compares patients receiving a specific treatment to patients receiving an alternate treatment for the condition of interest. The alternate treatment may be another active treatment, standard of care for the condition and/or a placebo (inactive) treatment. |
|
DCM — Dilated Cardiomyopathy |
|
A chronic cardiac disease where expansion of the patient’s heart reduces the pumping function to a point that the normal circulation of blood cannot be maintained. |
|
Double-Blind Clinical Trial |
|
Clinical trials in which neither the patient nor the physician know if the patient received the experimental treatment or a control/placebo. |
|
FDA — Food & Drug Administration |
|
The U.S. FDA ensures that medicines, medical devices, and radiation-emitting consumer products are safe and effective. Authorized by Congress to enforce the Federal Food, Drug, and Cosmetic Act and several other public health laws, the agency monitors the manufacture, import, transport, storage, and sale of $1 trillion worth of goods annually. |
|
GMP — Good Manufacturing Practice |
|
GMP regulations require that manufacturers, processors, and packagers of drugs, medical devices, some food, and blood take proactive steps to ensure that their products are safe, pure, and effective. GMP regulations require a quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mix-ups, and errors. |
|
Hematopoietic Stem Cells |
|
Stem cells that give rise to all the blood cell types including myeloid (monocytes and macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes/platelets, dendritic cells), and lymphoid lineages (T-cells, B-cells, NK-cells). |
|
IMPACT-DCM |
|
Our U.S. Phase 2 dilated cardiomyopathy clinical trial. |
|
IND — Investigational New Drug |
|
An application submitted to the FDA for a new drug or biologic that, if allowed, will be used in a clinical trial. |
|
Ischemia |
|
A shortage or inadequate flow of blood to a body part (commonly an organ or tissue) caused by a constriction or obstruction of the blood vessels supplying it. |
|
LVEF — Left Ventricular Ejection Fraction |
|
The fraction of blood pumped out of the left ventricle with each heart beat. |
|
Mesenchymal stromal cells |
|
Connective tissue cells that, in the case of bone marrow derived MSC, function to support blood forming cells and secrete anti-inflammatory factors. |
|
TERM |
|
DEFINITION |
|
M2 anti-inflammatory macrophages |
|
Specialized blood cells that remove damaged tissue and bacteria and secrete anti-inflammatory factors. |
|
Open-label Clinical Trial |
|
A trial in which both the treating physician and the patient know whether |
|
|
|
they are receiving the experimental treatment or control/placebo treatment. |
|
|
|
|
|
Orphan Drug Designation |
|
“Orphan drug” refers to a drug or biologic that is intended for use in the treatment of a rare disease or condition. Orphan drug designation from the U.S. Food and Drug Association (FDA) qualifies the sponsor to receive certain benefits from the Government in exchange for developing the drug for a rare disease or condition. The drug must then go through the FDA marketing approval process like any other drug or biologic which evaluates for safety and efficacy. Usually a sponsor receives a quicker review time and lower application fees for an orphan product. |
|
Phase 1 Clinical Trial |
|
A Phase 1 trial represents an initial study in a small group of patients to test for safety and other relevant factors. |
|
Phase 2 Clinical Trial |
|
A Phase 2 trial represents a study in a moderate number of patients to assess the safety and efficacy of a product. |
|
Phase 2b Clinical Trial |
|
A Phase 2b trial is a moderately-sized Phase 2 trial that is more specifically designed assess the efficacy of a product than a Phase 2a trial. |
|
Phase 3 Clinical Trial |
|
Phase 3 studies are initiated to establish safety and efficacy in an expanded patient population at multiple clinical trial sites and are generally larger than trials in earlier phases of development. |
|
Prospective Clinical Trial |
|
A clinical trial in which participants are identified and then followed throughout the study going forward in time. |
|
Randomized Clinical Trial |
|
A clinical trial in which the participants are assigned randomly to different treatment groups. |
|
Somatic Cell |
|
Any of the cells responsible for forming the body of an organism such as internal organs, bones, skin, connective tissues and blood. |
|
Stem Cell |
|
Unspecialized (undifferentiated) cells that retain the ability to divide throughout a lifetime and give rise to more specialized (differentiated) cells which take the place of cells that die or are lost. In culture, these undifferentiated cells possess the ability to divide for indefinite periods in culture and may give rise to highly specialized cells. |
Part II—INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The expenses payable by Aastrom Biosciences, Inc. (the “Registrant” or the “Company”) in connection with the issuance and distribution of the securities being registered (other than underwriting discounts and commissions, if any) are set forth below. Each item listed is estimated, except for the Securities and Exchange Commission (the “SEC”) registration fee.
|
Securities and Exchange Commission registration fee |
|
$ |
861 |
| |
|
Legal fees and expenses |
|
40,000 |
| ||
|
Accounting fees and expenses |
|
10,000 |
| ||
|
Printing fees and expenses |
|
5,000 |
| ||
|
Transfer agent and registrar fees |
|
5,000 |
| ||
|
Miscellaneous fees and expenses |
|
4,204 |
| ||
|
|
|
|
| ||
|
Total |
|
$ |
65,000 |
| |
Item 14. Indemnification of Directors and Officers
Sections 1561 through 1571 of the Michigan Business Corporation Act (the “MBCA”) authorize a corporation to grant or a court to award, indemnity to directors, officers, employees and agents in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act.
The Company’s amended and restated bylaws (the “Bylaws”) provide that the Company shall, to the fullest extent authorized or permitted by the MBCA, or other applicable law, indemnify a director or officer who was or is a party or is threatened to be made a party to any proceeding by or in the right of the Company to procure a judgment in its favor by reason of the fact that such person is or was a director, officer, employee or agent of the Company, against expenses, including actual and reasonable attorneys’ fees, and amounts paid in settlement incurred in connection with the action or suit, if the indemnitee acted in good faith and in a manner the person reasonably believed to be in, or not opposed to, the best interests of the Company or its shareholders. This section also requires the Company to advance expenses incurred by any agent of the Company in defending any proceeding prior to the final disposition of such proceeding upon receipt of an undertaking by or on behalf of the agent to repay such amount unless it shall be determined ultimately that the agent is entitled to be indemnified.
The Bylaws also authorize the Company to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the Company against any liability asserted against or incurred by such person in such capacity or arising out of such person’s status as such, regardless of whether the Company would have the power to indemnify such person against such liability under the provisions of the MBCA.
The Company has entered into indemnification agreements with certain individuals which contain provisions that may in some respects be broader than the specific indemnification provisions contained under applicable law. The indemnification agreement may require the Company, among other things, to indemnify such directors, officers and key personnel against certain liabilities that may arise by reason of their status or service as directors, officers or employees of the Company, to advance the expenses incurred by such parties as a result of any threatened claims or proceedings brought against them as to which they could be indemnified, and to the maximum extent that insurance coverage of such directors, officers and key employees under the Company’s directors’ and officers’ liability insurance policies is maintained.
Section 1209 of the MBCA permits a Michigan corporation to include in its articles of incorporation a provision eliminating or limiting a director’s liability to a corporation or its shareholders for monetary damages for breaches of fiduciary duty. The enabling statute provides, however, that liability for breaches of the duty of loyalty, acts or
omissions not in good faith or involving intentional misconduct or knowing violations of the law, or the receipt of improper personal benefits cannot be eliminated or limited in this manner. The Company’s Restated Articles of Incorporation (as amended, the “Charter”) includes a provision which eliminates, to the fullest extent permitted by the MBCA, director liability for monetary damages for breaches of fiduciary duty.
Item 15. Recent Sales of Unregistered Securities
The following is a summary of all securities that we have sold within the past three years without registration under the Securities Act of 1933, as amended.
On March 9, 2012, the Company entered into a Securities Purchase Agreement with Eastern Capital Limited, a Cayman exempted company (“ECL”), to sell 12,308 shares of Series B-1 Non-Voting Preferred Stock in a private placement to ECL, an “accredited investor” (as defined in Regulation D) under the Securities Act, at a price of $3,250.00 per share. The Series B-1 Shares were exchanged on a one-for-one basis for shares of the Series B-2 Voting Preferred Stock of the Company. The sales of the shares of Series B preferred stock were made only to a select number of accredited investors in reliance upon the exemptions from registration afforded by Rule 506 of Regulation D as promulgated by the SEC under the Securities Act and/or Section 4(2) of the Securities Act.
On June 27, 2012, the Company entered into separate warrant exchange agreements with each of certain holders of the Company’s outstanding warrants to purchase the Company’s common stock, issued in connection with the Company’s December 2010 public offering, with an exercise price of $64.40 and an expiration date of December 15, 2015. Pursuant to such warrant exchange agreements, on June 27, 2012, the Company issued an aggregate of 191,667 shares of Common Stock to Great Point Partners and its affiliated investment funds, Heights Capital Management and its affiliated investment funds, Deerfield Capital and its affiliated investment funds, and Millenium Management and its affiliated investment funds in exchange for the surrender of an aggregate of 383,333 warrants.
On July 30, 2012, the Company announced the results of its previously announced offer to exchange (the “Exchange Offer”) any warrant to purchase shares of common stock, no par value per share, of the Company issued in connection with the Company’s December 2010 public offering, that was tendered and accepted, for shares of the Company’s common stock. Such Exchange Offer was made upon the terms and subject to the conditions set forth in the Company’s offer to exchange, dated June 28, 2012, and in the related Exchange Offer materials filed as exhibits to the Tender Offer Statement on Schedule TO originally filed with the Securities and Exchange Commission on June 28, 2012, as amended. The Exchange Offer expired at 5:00 p.m., Eastern Standard Time, on Friday, July 27, 2012.
The issuance of shares of Common Stock in the warrant exchanges was made pursuant to the exemption from the registration requirements of the Securities Act of 1933, as amended, provided by Section 3(a)(9) of the Securities Act. No proceeds were received and no commissions were paid by the Company in connection with the Exchange Offer.
On January 21, 2014, we completed a private placement to Lincoln Park Capital Fund, LLC pursuant to which we have the right to sell to Lincoln Park up to $15,000,000 in shares of common stock, subject to certain limitations, from time to time over the 30-month period commencing on the date that a registration statement covering the resale of the shares is declared effective by the SEC. The issuance and sale was made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based on the offering of such securities to one investor, the lack of any general solicitation or advertising in connection with such issuance, the representation of such investor that it was an accredited investor and that it was purchasing the shares for its own account and without a view to distribute them.
Item 16. Exhibits and Financial Statements Schedules
(a) Exhibits
A list of exhibits filed with this registration statement on Form S-1 is set forth on the Exhibit Index and is incorporated herein by reference.
(b) Financial Statement Schedules
All schedules have been omitted because either they are not required, are not applicable or the information is otherwise set forth in the financial statements and related notes thereto.
Item 17. Undertakings.
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement;
(2) To include any prospectus required by Section 10(a)(3) of the Securities Act;
(3) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement; and
(4) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(b) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(d) The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, as amended, the information omitted from a form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933, as amended, shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, as amended, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, or the Act, may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Ann Arbor, state of Michigan on February 10, 2014.
|
|
AASTROM BIOSCIENCES, INC. | |
|
|
|
|
|
|
By: |
/s/ Dominick C. Colangelo |
|
|
|
Dominick C. Colangelo |
|
|
|
|
|
|
|
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Dominick C. Colangelo |
|
President, Chief Executive Officer and Director |
|
February 10, 2014 |
|
Dominick C. Colangelo |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/s/ Michael W. Elliston |
|
Controller, Chief Accounting Officer and Treasurer |
|
February 10, 2014 |
|
Michael W. Elliston |
|
(Principal Financial and Accounting Officer) |
|
|
|
|
|
|
|
|
|
* |
|
Chairman of the Board of Directors |
|
February 10, 2014 |
|
Robert L. Zerbe, M.D. |
|
|
|
|
|
|
|
|
|
|
|
* |
|
Director |
|
February 10, 2014 |
|
Ronald M. Creswell, Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
* |
|
Director |
|
February 10, 2014 |
|
Alan L. Rubino |
|
|
|
|
|
|
|
|
|
|
|
* |
|
Director |
|
February 10, 2014 |
|
Nelson M. Sims |
|
|
|
|
|
|
|
|
|
|
|
*/s/ Dominick C. Colangelo |
|
|
|
February 10, 2014 |
|
Dominick C. Colangelo, Attorney-in-fact |
|
|
|
|
EXHIBIT INDEX
|
Exhibit |
|
Description |
|
3.1 |
|
Restated Articles of Incorporation of the Company, filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 17, 2009 and incorporated herein by reference (File No. 000-22025). |
|
|
|
|
|
3.2 |
|
Certificate of Amendment to Restated Articles of Incorporation of the Company dated February 9, 2010, filed as Exhibit 3.2 to the Company’s Post-Effective Amendment No. 1 to Form S-1 filed on March 31, 2010 and incorporated herein by reference (File No. 333-160044). |
|
|
|
|
|
3.3 |
|
Certificate of Amendment to Restated Articles of Incorporation of the Company dated March 22, 2011, filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K, filed on March 25, 2011 and incorporated herein by reference (File No. 000-22025). |
|
|
|
|
|
3.4 |
|
Certificate of Designation, Preferences and Rights, of the Company classifying and designating the Series A Junior Participating Cumulative Preferred Stock, attached as Exhibit 3.1 to the Company’s Current Report on Form 8-A filed on August 12, 2011, incorporated herein by reference. |
|
|
|
|
|
3.5 |
|
Amended and Restated Certificate of Designations, Preferences and Rights, of the Company classifying and designating the Series B-1 Non-Voting Convertible Preferred Stock and the Series B-2 Voting Convertible Preferred Stock, attached as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on August 12, 2013, incorporated herein by reference. |
|
|
|
|
|
3.6 |
|
Amended and Restated Bylaws, filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on November 12, 2010 and incorporated herein by reference. |
|
|
|
|
|
4.1 |
|
Specimen Common Stock Certificate, filed as Exhibit 4.1 to Amendment No. 2 to the Company’s Registration Statement on Form S-1/A filed on December 20, 1996 and incorporated herein by reference. |
|
|
|
|
|
4.2 |
|
Shareholder Rights Agreement, dated as of August 11, 2011, between the Company and Continental Stock Transfer & Trust Company, as Rights Agent, attached as Exhibit 4.1 to the Company’s Current Report on Form 8-A filed on August 12, 2011, incorporated herein by reference. |
|
|
|
|
|
4.3 |
|
Amendment to Shareholder Rights Agreement, dated as of March 9, 2012, between the Company and Continental Stock Transfer & Trust Company, as Rights Agent, attached as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on March 9, 2012, incorporated herein by reference. |
|
|
|
|
|
5.1 # |
|
Opinion of Dykema Gossett PLLC. |
|
|
|
|
|
10.1 |
|
Form of Indemnification Agreement, attached as Exhibit 10.1 to the Company’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference. |
|
|
|
|
|
10.2 |
|
Amended and Restated 1992 Incentive and Non-Qualified Stock Option Plan and forms of agreements thereunder, attached as Exhibit 10.5 to the Company’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference. |
|
10.3 |
|
Form of Employment Agreement, attached as Exhibit 10.8 to the Company’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference. |
|
|
|
|
|
10.4 |
|
License Agreement, dated March 13, 1992, between the Company and the University of Michigan and amendments thereto dated March 13, 1992, October 8, 1993 and June 21, 1995, attached as Exhibit 10.17 to the Company’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference. |
|
|
|
|
|
10.5 |
|
2001 Stock Option Plan, attached as Exhibit 10.72 to the Company’s Annual Report on Form 10-K for the year ended June 30, 2002, incorporated herein by reference. |
|
|
|
|
|
10.6 |
|
2004 Equity Incentive Plan, attached as Exhibit 10.82 to Amendment No. 1 to the Company’s Quarterly Report on Form 10-Q/A for the quarter ended September 30, 2004, incorporated herein by reference. |
|
|
|
|
|
10.7 |
|
Form of Option and Restricted Stock Award Agreements for Grants under 2004 Equity Incentive Plan, attached as Exhibit 10.84 to the Company’s Annual Report on Form 10-K for the year ended June 30, 2005, incorporated herein by reference. |
|
|
|
|
|
10.8 |
|
Amendment dated December 5, 2002 to License Agreement with the University of Michigan, attached as Exhibit 10.87 to the Company’s Annual Report on Form 10-K for the year ended June 30, 2005, incorporated herein by reference. |
|
|
|
|
|
10.9 |
|
2004 Equity Incentive Plan, as amended, attached as Exhibit 99.1 to the Company’s Current Report on Form 8-K filed on November 8, 2006, incorporated herein by reference. |
|
|
|
|
|
10.10 |
|
Forms of Grant Notice and Stock Option Agreement for Grants under 2004 Equity Incentive Plan, as amended, attached as Exhibit 99.2 to the Company’s Current Report on Form 8-K filed on November 8, 2006, incorporated herein by reference. |
|
|
|
|
|
10.11 |
|
Form of Purchase Agreement, attached as Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on October 16, 2007, incorporated herein by reference. |
|
|
|
|
|
10.12 |
|
Form of Warrant, attached as Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on October 16, 2007, incorporated herein by reference. |
|
|
|
|
|
10.13 |
|
Lease agreement between Domino’s Farms Office Park, LLC and the Company, as amended., attached as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 9, 2013, incorporated herein by reference. |
|
|
|
|
|
10.14 |
|
2009 Omnibus Incentive Plan, attached as Appendix II to the Company’s Proxy Statement filed on October 9, 2009, incorporated herein by reference. |
|
|
|
|
|
10.15 |
|
Class A Warrant Agreement, dated as of January 21, 2010, by and between the Company and Continental Stock Transfer & Trust Company (incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 27, 2010). |
|
|
|
|
|
10.16 |
|
Class B Warrant Agreement, dated as of January 21, 2010, by and between the Company and Continental Stock Transfer & Trust Company (incorporated herein by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the SEC on January 27, 2010). |
|
|
|
|
|
10.17 |
|
Underwriting Agreement, dated as of January 15, 2010, and between the Company and Oppenheimer & Co. Inc. (incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed |
|
|
|
on January 15, 2010). |
|
|
|
|
|
10.18 |
|
Form of indemnification agreement entered into between the Company and each of its directors, attached as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on August 31, 2010, incorporated herein by reference. |
|
|
|
|
|
10.19 |
|
Amended Code of Business Conduct and Ethics, attached as Exhibit 14.1 to the Company’s Current Report on Form 8-K filed on August 31, 2010, incorporated herein by reference. |
|
|
|
|
|
10.20 |
|
Contract Manufacturing and Supply Agreement, dated as of November 1, 2010, by and between Vention Medical (formerly ATEK Medical, LLC) and the Company (incorporated herein by reference to Exhibit 10.30 to the Company’s Annual Report on Form 10-KT for the year ended December 31, 2010). |
|
|
|
|
|
10.21 |
|
Warrant Agreement, dated as of December 15, 2010, by and between the Company and Continental Stock Transfer & Trust Company (incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on December 16, 2010). |
|
|
|
|
|
10.22 |
|
Underwriting Agreement, dated as of December 10, 2010, and between the Company and Stifel, Nicolaus & Company, Incorporated, Needham & Company, LLC and Roth Capital Partners (incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on December 10, 2010). |
|
|
|
|
|
10.23 |
|
Amendment to the 2009 Omnibus Incentive Plan, dated March 21, 2011 (incorporated herein by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K, filed on March 25, 2011). |
|
|
|
|
|
10.24 |
|
Employment Agreement with Ronnda L. Bartel, PhD, dated March 22, 2011 (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed on March 25, 2011). |
|
|
|
|
|
10.25 |
|
Senior Executive Incentive Bonus Plan (incorporated herein by reference to Exhibit 10.3 to the Company’s current Report on Form 8-K, filed on March 25, 2011). |
|
|
|
|
|
10.26 |
|
At Market Issuance Sales Agreement, dated June 16, 2011, by and among the Company and McNicoll, Lewis & Vlak LLC, as amended (incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on November 29, 2013). |
|
|
|
|
|
10.27 |
|
Master Services Agreement by and between the Company and PPD, made and entered into as of September 23, 2011 (the “Master Services Agreement”) (incorporated herein by reference to Exhibit 10.28 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012). |
|
|
|
|
|
10.28 |
|
Project Addendum to the Master Services Agreement, dated as of November 16, 2011 (incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed November 22, 2011). |
|
|
|
|
|
10.29 |
|
Registration Rights Agreement, dated March 9, 2012, between the Company and Eastern Capital Limited, attached as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on March 9, 2012, incorporated herein by reference. |
|
|
|
|
|
10.30 |
|
Securities Purchase Agreement, dated as of March 9, 2012, by and between the Company and Eastern Capital Limited (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on March 9, 2012). |
|
|
|
|
|
10.31 |
|
Employment Agreement, dated as of April 3, 2013, by and between the Company and Daniel R. Orlando (incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on April 9, 2013). |
|
10.32 |
|
Employment Agreement, dated as of October 26, 2012, by and between the Company and Brian Gibson (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on October 26, 2012). |
|
|
|
|
|
10.33 |
|
Amendment to the 2009 Omnibus Incentive Plan, dated May 3, 2012 (incorporated herein by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, filed on August 7, 2012). |
|
|
|
|
|
10.34 |
|
Form of Warrant Exchange Agreement, dated June 27, 2012 (incorporated herein by reference to Exhibit 10.1 to the Company’s Report on Form 8-K, filed on June 27, 2012). |
|
|
|
|
|
10.35 |
|
Executive Resignation Agreement, executed on December 12, 2012 and effective December 14, 2012, by and between the Company and Tim M. Mayleben (incorporated herein by reference to Exhibit 10.30 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012). |
|
|
|
|
|
10.36 |
|
Executive Employment Agreement, executed March 4, 2013 and effective March 1, 2013, by and between the Company and Dominick C. Colangelo (incorporated herein by reference to Exhibit 10.1 to the Company’s Report on Form 8-K, filed on March 8, 2013). |
|
|
|
|
|
10.37 |
|
Form of Warrant Exercise Agreement, dated September 24, 2013 (incorporated herein by reference to Exhibit 10 to the Company’s Report on Form 8-K, filed on September 27, 2013). |
|
|
|
|
|
10.38 |
|
Consulting Services Agreement, executed January 9, 2014 and effective January 1, 2014, by and between the Company and Ronnda L. Bartel (incorporated herein by reference to Exhibit 10.1 to the Company’s Report on Form 8-K, filed on January 14, 2014). |
|
|
|
|
|
10.39 |
|
Underwriting Agreement, dated as of August 13, 2013, by and between the Company and Aegis Capital Corp. (incorporated herein by reference to Exhibit 1.1 to the Company’s Registration Statement on Form S-1 (File No. 333-188186) filed on August 13, 2013). |
|
|
|
|
|
10.40 |
|
Purchase Agreement, dated as of January 21, 2014, by and between the Company and Lincoln Park Capital Fund, LLC (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on January 27, 2014). |
|
|
|
|
|
10.41 |
|
Registration Rights Agreement, dated as of January 21, 2014, by and between the Company and Lincoln Park Capital Fund, LLC (incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on January 27, 2014). |
|
|
|
|
|
23.1# |
|
Consent of PricewaterhouseCoopers LLP. |
|
|
|
|
|
23.2# |
|
Consent of Dykema Gossett PLLC (included in Exhibit 5.1 hereto). |
|
|
|
|
|
24.1# |
|
Power of Attorney (included in signature pages to this Registration Statement). |
|
# |
|
Filed herewith. |

