Attached files
| file | filename |
|---|---|
| EX-10.9 - EXHIBIT 10.9 - NanoVibronix, Inc. | v367378_ex10-9.htm |
| EX-23.1 - EXHIBIT 23.1 - NanoVibronix, Inc. | v367378_ex23-1.htm |
| EX-10.12 - EXHIBIT 10.12 - NanoVibronix, Inc. | v367378_ex10-12.htm |
| EX-10.10 - EXHIBIT 10.10 - NanoVibronix, Inc. | v367378_ex10-10.htm |
| EX-10.11 - EXHIBIT 10.11 - NanoVibronix, Inc. | v367378_ex10-11.htm |
As filed with the Securities and Exchange Commission on February 6, 2014.
SEC File No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
NANO VIBRONIX, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 3842 | 01-0801232 |
|
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
105 Maxess Road, Suite S124
Melville, NY 11747
(631) 574-4410
(Address, including zip code, and telephone
number,
including area code, of registrant’s principal executive offices)
Harold Jacob, M.D.
Chief Executive Officer
Nano Vibronix, Inc.
105 Maxess Road, Suite S124
Melville, NY 11747
(631) 574-4410
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
Copies of all communications, including communications sent to agent for service, should be sent to:
|
Rick A. Werner, Esq. Haynes and Boone, LLP 30 Rockefeller Plaza, 26th Floor New York, New York 10112 Tel. (212) 659-7300 Fax (212) 884-8234 |
Mitchell S. Nussbaum, Esq. Angela M. Dowd, Esq. Loeb & Loeb LLP 345 Park Avenue, 18th Floor New York, NY 10154 Tel. (212) 407-4000 Fax (212) 504-3013 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ |
| Non-accelerated filer | ¨ | Smaller reporting company | x |
| (Do not check if a smaller reporting company) |
CALCULATION OF REGISTRATION FEE
| TITLE OF EACH CLASS OF SECURITIES TO BE REGISTERED |
PROPOSED MAXIMUM AGGREGATE OFFERING PRICE(1)(2) |
AMOUNT OF REGISTRATION FEE |
|||||||||
| Common stock, par value $0.001 per share (3) | $ | $ | |||||||||
| Common stock purchase warrants (4) | $ | $ | - | ||||||||
| Common stock underlying the common stock purchase warrants (3)(5) | $ | $ | |||||||||
| Underwriter’s common stock purchase warrants (4)(6) | $ | $ | - | ||||||||
| Common stock included in underwriter’s common stock purchase warrants (3) | $ | $ | |||||||||
| Total | $ | 6,000,000 | $ | 772.80 | |||||||
| (1) | Includes shares and warrants that the underwriter has the option to purchase to cover over-allotments, if any. |
| (2) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
| (3) | Pursuant to Rule 416 under the Securities Act of 1933, as amended, the securities being registered hereunder include such indeterminate number of additional shares of common stock as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. |
| (4) | No fee required pursuant to Rule 457(g) under the Securities Act of 1933, as amended. |
| (5) | There will be issued a warrant to purchase one share of common stock for every one share of common stock offered. The warrants are exercisable at a per share price equal to 125% of the common stock public offering price. |
| (6) | Represents 5% of the shares to be sold in this offering including shares that may be sold upon exercise of the underwriter’s over-allotment option. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission acting pursuant to said section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED FEBRUARY 6, 2014
PRELIMINARY PROSPECTUS

Nano Vibronix, Inc.
Shares of Common Stock
Warrants to Purchase Shares of Common Stock
_________________
This is the initial public offering of securities of Nano Vibronix, Inc. We are offering to sell shares of our common stock together with warrants to purchase shares of our common stock. One share of common stock is being sold together with a warrant. Each warrant entitles the holder to purchase one share of our common stock at an exercise price equal to 125% of the offering price of the common stock, subject to adjustment as described herein. Each warrant will be exercisable immediately and will expire on , 2019.
Prior to this offering, there has been no public market for our securities. The initial public offering price is expected to be between $ and $ per share of common stock and $ per warrant. We have applied to list our shares of common stock for quotation on the Nasdaq Capital Market under the symbol “NVBX.” No assurance can be given that our application will be approved. If the application is not approved, we will not complete this offering. We do not intend to apply to list the warrants on any securities exchange.
Investing in our securities is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page 7 of this prospectus before making a decision to purchase our securities.
We qualify as an “emerging growth company” as defined in the Jumpstart our Business Startups Act of 2012, or JOBS Act and, as such, may elect to comply with certain reduced public company reporting requirements for future filings. Please read the related disclosure contained on pages 17 and 28 of this prospectus.
| Per Share | Per Warrant | Total | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting discounts and commissions(1) | $ | $ | $ | |||||||||
| Proceeds, before expenses, to Nano Vibronix, Inc. | $ | $ | $ | |||||||||
(1) The underwriter will receive compensation in addition to the underwriting discount, as set forth in the section entitled “Underwriting” beginning on page 69 upon the closing of this offering, which consists of three-year compensation warrants entitling the underwriter to purchase 5.0% of the aggregate number of shares of common stock issued in this offering, including shares issued pursuant to the exercise of the over-allotment option, with an exercise price equal to 125% of the price per share of the common stock sold in this offering. We have also agreed to reimburse the underwriter for certain expenses incurred by the underwriter up to an amount not to exceed $25,000 for all expenses other than legal fees plus $125,000 for legal fees upon completion of this offering. See the heading entitled “Underwriting” on page 69 of this prospectus for additional disclosure regarding compensation to the underwriter payable by us.
We have granted the underwriter an option, exercisable one or more times in whole or in part, to purchase up to additional shares of common stock and/or up to an additional warrants from us at the public offering price, less the underwriting discount, within 30 days from the date of this prospectus to cover over-allotments, if any. If the underwriter exercises the option in full, the total underwriting discounts and commissions payable will be $ , and the total proceeds to us, before expenses, will be $ .
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriter expects to deliver the securities against payment in New York, New York on , 2014.
The date of this prospectus is , 2014
______________________________________
Chardan Capital Markets, LLC
______________________________________
TABLE OF CONTENTS
| PROSPECTUS SUMMARY | 1 |
| RISK FACTORS | 7 |
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS | 21 |
| USE OF PROCEEDS | 23 |
| CAPITALIZATION | 24 |
| DILUTION | 25 |
| DIVIDEND POLICY | 26 |
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 26 |
| BUSINESS | 33 |
| MANAGEMENT | 55 |
| EXECUTIVE COMPENSATION | 58 |
| CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS | 59 |
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 61 |
| DESCRIPTION OF SECURITIES | 63 |
| SHARES ELIGIBLE FOR FUTURE ISSUANCE | 67 |
| UNDERWRITING | 69 |
| LEGAL MATTERS | 72 |
| EXPERTS | 72 |
| WHERE YOU CAN FIND ADDITIONAL INFORMATION | 72 |
| INDEX TO FINANCIAL STATEMENTS | F-1 |
You should rely only on the information contained in this prospectus. We have not authorized any other person to provide you with information different from or in addition to that contained in this prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any jurisdiction where an offer or sale is not permitted.
Until , 2014 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Industry and Market Data
In this prospectus, we rely on and refer to information and statistics regarding our industry. We obtained this statistical, market and other industry data and forecasts from publicly available information. While we believe that the statistical data, market data and other industry data and forecasts are reliable, we have not independently verified the data.
PROSPECTUS SUMMARY
This summary highlights information contained in other parts of this prospectus. Because it is a summary, it does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should read the entire prospectus carefully, including our consolidated financial statements and the related notes included in this prospectus and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
When used herein, unless the context requires otherwise, references to the “Company,” “Nano,” “we,” “our” and “us” refer to Nano Vibronix, Inc., a Delaware corporation, and, where appropriate, its consolidated subsidiaries.
The Company
We are a medical device company focusing on noninvasive biological response-activating devices that target wound healing and pain therapy and can be administered at home, without the assistance of medical professionals. Our products currently consist of:
| · | WoundShieldTM, a patch-based therapeutic ultrasound device that facilitates tissue regeneration and wound healing by using ultrasound to increase local capillary perfusion, which is the flow of blood to beds of extremely small blood vessels, and tissue oxygenation, which is the increase in the concentrations of oxygen within the tissue; |
| · | NanoVibronix NPWT (Negative Pressure Wound Therapy), a small, lightweight pump with features that allow contamination-free handling of infected wound exudate (fluid) and enhanced patient mobility; |
| · | PainShieldTM, a disposable patch-based therapeutic ultrasound technology to treat pain, muscle spasm and joint contractures (permanent shortening of a joint) by delivering a localized ultrasound effect to treat pain and induce soft tissue healing in a targeted area; and |
| · | UroShieldTM, an ultrasound-based product that is designed to prevent biofilm (a matrix of microorganisms required for bacteria to grow and cause infections) in urinary catheters, increase antibiotic efficacy and decrease pain and discomfort associated with urinary catheter use. |
Each of our WoundShield, PainShield and UroShield products employs a small, disposable transducer (a device that converts a signal in one form of energy to another form of energy) that transmits low frequency, low intensity ultrasound acoustic waves that seek to repair and regenerate tissue, musculoskeletal and vascular structures and increase antibiotic efficacy. Through their size, effectiveness and ease of use, these products are intended to eliminate the need for technicians and medical personnel to manually administer ultrasound treatment through large transducers, thereby promoting patient independence and enabling more cost-effective home-based care. Our NanoVibronix NPWT is based on an existing standard of care for wound therapy treatment and employs a technology that drains open cavity wounds and seeks to accelerate wound healing.
PainShield and NanoVibronix NPWT are currently approved for marketing in the U.S. by the U.S. Food and Drug Administration and all of our products, except for NanoVibronix NPWT, have CE Mark approval in the European Union. We anticipate that we will apply for CE Mark approval for NanoVibronix NPWT within six months after the closing of the offering to which this prospectus relates. We have a Canadian medical device license for PainShield and UroShield, a certificate allowing us to sell PainShield, WoundShield and UroShield in Israel, a certificate allowing us to sell PainShield in Australia, and we are able to sell PainShield, WoundShield and UroShield in India and Ecuador based on our CE Mark. In addition, our distributor in Korea has applied for approval to sell PainShield and UroShield, and our distributor in Chile has applied for approval to sell PainShield. We generally apply, through our distributor, for approval in a particular country for a particular product only when we have a distributor in place with respect to such product.
In addition to the need to obtain regulatory approvals, as described above, we anticipate that sales volumes and prices of our WoundShield and PainShield products will depend in large part on the availability of coverage and reimbursement for self-administered use from third party payers. Third party payers include governmental programs such as Medicare and Medicaid in the U.S., private insurance plans and workers’ compensation plans. We do not currently have reimbursement codes for self-administered use or clinical use of WoundShield in any of the markets in which we have regulatory authority to sell WoundShield. Of the markets in which we have regulatory authority to sell PainShield, we have reimbursement codes in the United States (i.e., Current Procedural Terminology codes or “CPT codes”) for clinical use only, but do not have such reimbursement codes for self-administered use of the product. NanoVibronix NPWT is generally reimbursed by governmental and other third-party payers in the U.S. With respect to UroShield, which will be used primarily in a clinical setting, we do not currently have reimbursement codes in any of the markets in which we have regulatory authority to sell UroShield. We anticipate that we will begin to seek reimbursement codes for self-administered and clinical use of our products in the markets in which we have regulatory authority to sell such products after the closing of this offering, however, there is no guarantee that we will be successful in obtaining such codes quickly, or at all.
| 1 |
Assuming we are able to obtain adequate financing, including through this offering, we plan to continue to work on the further commercialization of WoundShield and further marketing of NanoVibronix NPWT. We intend to integrate our WoundShield ultrasound technology into NanoVibronix NPWT, which we believe would make NanoVibronix NPWT a superior product within the negative pressure wound therapy market. We also intend to conduct ongoing clinical trials of our PainShield product, with the aim of obtaining a favorable reimbursement code. With respect to our UroShield product, we are currently seeking a strategic partner that is active in the urology market and would be interested in integrating UroShield into its range of products. If we locate such a partner, we anticipate that we would continue to pursue U.S. Food and Drug Administration approval of UroShield.
Since our formation, we have had recurring losses and negative cash flows from operating activities. For the year ended December 31, 2012, we had a net loss of $1,275,000, with revenues of $166,000. For the nine months ended September 30, 2013, we had a net loss of $1,412,000, with revenues of $203,000. As of September 30, 2013, we had an accumulated deficit of $13,626,000 and a total stockholders’ deficit of $2,768,000. Because we have had recurring losses and negative cash flows from operating activities, substantial doubt exists regarding our ability to remain in operation at the same level we are currently performing. Further, the report of Kost Forer Gabbay & Kasierer, a member firm of Ernst & Young Global, our independent registered public accounting firm, with respect to our financial statements at December 31, 2012 and 2011 and the years ended December 31, 2012 and 2011, includes an explanatory paragraph as to our potential inability to continue as a going concern.
Ultrasound Technology and Our Products
As noted above, each of our products, other than NanoVibronix NPWT, is based on the use of low frequency ultrasound, which delivers energy through mechanical vibrations in the form of sound waves. Ultrasound has long been used in physical therapy, physical medicine, rehabilitation and sports medicine. Moreover, there is a growing body of research that supports the positive biological effects of ultrasound.
Our proprietary technology consists of a small, thin (1 millimeter) transducer that is capable of transmitting ultrasonic acoustic waves onto treatment surfaces with a radius of up to 10 centimeters. This technology allows us to treat wounds by implanting our transducers into a small, portable self-adhering acoustic patch, thereby eliminating the need for technicians and medical personnel to manually administer ultrasound therapy, which should reduce the cost of therapy. Moreover, we believe that the delivery of ultrasound through our portable devices is more effective than existing products, as our technology is better positioned to target the affected areas of the body.
While there are currently a number of products on the market that treat pain through ultrasound therapy, we believe that our products differentiate themselves because they are portable, without the requirement to be plugged into an outlet and they have a frequency of 100kHz (in contrast to other devices, which have a frequency of 1MHz), which means they do not produce heat that can damage tissue. They can therefore be self-administered by the patient without the need to be moved about the treated area by the patient or a clinician, they can be applied for a significantly longer period without the risk of tissue damage and they do not require the use of gel. We are aware of one product under U.S. Food and Drug Administration review and with CE Mark approval that we understand does not need to be plugged in and operates at a frequency of 3 MHz, which its manufacturer claims overcomes the need for movement around the treated area and allows for a longer treatment period. We understand that this product does not generate surface acoustic waves as our products do, which means that the treatment area is generally limited to that of the transducer’s diameter, that the use of transmission gel is still required and that the transducer thickness is significantly greater than ours (approximately 1.5cm).
Markets for Our Products
We believe our products compete and/or will compete in the markets described below:
| · | Wound-Healing Devices Market. Our WoundShield, NanoVibronix NPWT and integrated product are aimed at the market for wound-healing devices. The global wound care device market is continuously growing and expected to reach $20.3 billion by 2015 (“Anticipated market in 2015, Wound Care Products: A Global Strategic Business Report,” September 2011). The negative pressure wound therapy market is expanding, in light of recent approvals in Japan and a growing diabetes patient pool and currently is estimated at approximately $2 billion (“Negative Pressure Wound Therapy Market to 2017,” GBI Research, June 2012). |
| · | Pain Market. Our PainShield product is aimed at the pain treatment market. Pain is one of the most common conditions that hinder quality of life of vast populations of patients on a regular basis. According to Bonica’s Management of Pain (2001), a work considered current in the industry based on available industry data, and Landro L, “New Ways to Treat Pain: Tricking the Brain, Blocking the Nerves in Patients When all Else Has Failed,” Wall Street Journal, May 11, 2010, approximately 25% of the U.S. population, 75 million people, suffer from chronic pain. We estimate that approximately 150 million individuals globally suffer from chronic pain. |
| 2 |
| · | Catheter Market. Our UroShield product is complementary to products in the catheter market. According to Urological Catheters – A Global Strategic Business Report, Global Industry Analysis Inc. 2003, over 55 million indwelling urinary catheters are sold annually worldwide. |
Risks Associated with Our Business
Our ability to operate our business and achieve our goals and strategies is subject to numerous risks as discussed more fully in the section titled “Risk Factors,” including, without limitation:
| · | The timing of clinical studies and eventual U.S. Food and Drug Administration approval of WoundShield and our other product candidates. |
| · | Regulatory actions that could adversely affect the price of or demand for our approved products. |
| · | Market acceptance of existing and new products. |
| · | Favorable or unfavorable decisions about our products from government regulators, insurance companies or other third-party payers. |
| · | Our intellectual property portfolio. |
| · | We are not currently meeting the minimum sales requirements necessary to prevent our license agreement related to NanoVibronix NPWT from terminating in August 2014, and even if we meet such sales requirements, the license will no longer be exclusive after such date. |
| · | Our ability to recruit and retain qualified regulatory and research and development personnel. |
| · | Unforeseen changes in healthcare reimbursement for any of our approved products. |
| · | Lack of financial resources to adequately support our operations. |
| · | Difficulties in maintaining commercial scale manufacturing capacity and capability. |
| · | Our ability to generate internal growth. |
| · | Changes in our relationship with key collaborators. |
| · | Our failure to comply with regulatory guidelines. |
| · | Uncertainty in industry demand and patient wellness behavior. |
| · | General economic conditions and market conditions in the medical device industry. |
Corporate and Other Information
We were organized as a Delaware corporation on October 20, 2003. Our principal executive offices are located at 105 Maxess Road, Suite S124, Melville, NY 11747. Our telephone number is (631) 574-4410. Our website address is www.nanovibronix.com. Information accessed through our website is not incorporated into this prospectus and is not a part of this prospectus.
| 3 |
The Offering
| Securities offered by us: | shares of common stock and warrants to purchase up to shares of common stock. |
| Common stock outstanding prior to the offering: | shares |
| Common stock outstanding after this offering: | shares ( shares if the warrants are exercised in full). |
| Terms of warrants: |
Exercise price: $ , which is equal to 125% of the offering price of the common stock in this offering.
Exercisability: Each warrant is exercisable for one share of common stock, subject to adjustment as described herein.
Exercise period: Each warrant will be exercisable immediately and will expire on , 2019.
See “Description of Securities” for more information. |
| Over-allotment option to be offered by us: | We have granted the underwriter the right to purchase up to additional shares of common stock and/or up to additional warrants from us at the public offering price less the underwriting discount within 30 days from the date of this prospectus to cover over-allotments. |
| Use of proceeds: | We estimate that our net proceeds from this offering, without exercise of the over-allotment option, will be approximately $ . We intend to use the proceeds of this offering for marketing activities, clinical and regulatory activities, research and development, intellectual property protection and operations and general working capital. See “Use of Proceeds” beginning on page 23 of this prospectus. |
Underwriter compensation warrants:
|
We will issue to the underwriter, upon closing of this offering, compensation warrants entitling the underwriter to purchase 5.0% of the aggregate number of shares of common stock issued in this offering, including shares issued pursuant to the exercise of the over-allotment option. The underwriter warrants will have a term of three years and may be exercised commencing 12 months after the date of effectiveness of the Registration Statement on Form S-1 of which this prospectus forms a part. The underwriter warrants may be exercised on a cashless basis if not registered. |
| Market for our securities: | We have applied for listing of our common stock on the Nasdaq Capital Market under the symbol “NVBX.” |
| Risk factors: | Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 7 of this prospectus. |
| 4 |
The number of shares of common stock outstanding after this offering is based on shares outstanding on , after giving effect to a one-for- reverse stock split of our common stock effected on and excludes:
| · | 1,842,106 shares of common stock issuable upon the exercise of warrants with an exercise price of $0.38 per share; |
| · | 2,319,062 shares of common stock issuable upon the exercise of warrants with an exercise price of $0.199 per share; |
| · | 2,525,704 shares of common stock issuable upon the exercise of currently outstanding options with a weighted average exercise price of $1.23; |
| · | 274,296 shares of common stock available for future issuance under our 2004 Global Share Option Plan; | |
| · | the shares of common stock issuable upon the exercise of the warrants offered hereby; and | |
| · | the shares of common stock issuable upon the exercise of the underwriter’s compensation warrants. |
Except as otherwise indicated, information in this prospectus reflects or assumes:
| · | a one-for- reverse split of our common stock, which will occur prior to the pricing of this offering; |
| · | the conversion of all outstanding shares of our convertible preferred stock into an aggregate of shares of common stock, which will occur automatically upon the closing of this offering; |
| · | the conversion of all outstanding convertible indebtedness into an aggregate of shares of common stock, which will occur automatically upon the closing of this offering, and assuming a conversion date; |
| · | the exchange of all outstanding warrants to purchase preferred stock into warrants to purchase an aggregate of shares of common stock with an exercise price of $ per share, which will occur automatically upon the closing of this offering; |
| · | the filing of our amended and restated certificate of incorporation and the effectiveness of our restated bylaws, which will occur immediately prior to the completion of this offering; and |
| · | that the underwriter does not exercise its over-allotment option. |
| 5 |
Summary Consolidated Financial Information
(in thousands, except per share data)
The following summary consolidated financial data should be read in conjunction with the consolidated financial statements and the related notes thereto and the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. We derived the statement of operations data for the years ended December 31, 2011 and 2012 and balance sheet data as of December 31, 2011 and 2012 from the audited consolidated financial statements in this prospectus. Those consolidated financial statements were audited by Kost Forer Gabbay & Kasierer, a member firm of Ernst & Young Global, our independent registered public accounting firm. We derived the statement of operations data for the nine months ended September 30, 2013 and 2012 and the balance sheet data as of September 30, 2013 and 2012 from the unaudited condensed consolidated financial statements in this prospectus. We have prepared the unaudited financial information on a basis consistent with our audited consolidated financial statements and have included, in our opinion, all adjustments, consisting only of normal recurring adjustments, that we consider necessary for a fair presentation of the financial information set forth in those statements. Our historical results are not necessarily indicative of the results that may be expected in any future period, and our interim results are not necessarily indicative of the results to be expected for the full fiscal year.
| Years Ended | Nine Months Ended | |||||||||||||||
| December 31, | September 30, | |||||||||||||||
| 2012 | 2011 | 2013 | 2012 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Statement of Operations Data: | ||||||||||||||||
| Revenue | $ | 166 | $ | 94 | $ | 203 | $ | 109 | ||||||||
| Cost of revenues | 50 | 29 | 81 | 33 | ||||||||||||
| Gross profit | 116 | 65 | 122 | 76 | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development, net | 572 | 510 | 498 | 519 | ||||||||||||
| Selling and marketing | 190 | 150 | 183 | 132 | ||||||||||||
| General and administrative | 128 | 87 | 362 | 98 | ||||||||||||
| Total operating expenses | 890 | 747 | 1,043 | 749 | ||||||||||||
| Operating loss | 774 | 682 | 921 | 673 | ||||||||||||
| Financial expense, net | 501 | 41 | 491 | 370 | ||||||||||||
| Net loss | $ | 1,275 | 723 | 1,412 | 1,043 | |||||||||||
| Total comprehensive loss | $ | 1,275 | 723 | 1,412 | 1,043 | |||||||||||
| Deemed dividend | - | 873 | - | - | ||||||||||||
| Net loss attributable to holders of common stock | $ | 1,275 | $ | 1,596 | $ | 1,412 | $ | 1,043 | ||||||||
| Net basic and diluted loss per share | $ | (1.18 | ) | $ | (1.47 | ) | $ | (1.3 | ) | $ | (0.96 | ) | ||||
| Weighted average number of common stock used in computing basic and diluted net loss per share | 1,085,060 | 1,085,060 | 1,085,060 | 1,085,060 | ||||||||||||
| Balance Sheet Data: | ||||||||||||||||
| Cash and cash equivalents | $ | 101 | $ | 893 | $ | 51 | $ | 249 | ||||||||
| Working capital(1) | 90 | 839 | (271 | ) | 182 | |||||||||||
| Total assets | 349 | 1,085 | 435 | 437 | ||||||||||||
| Total long-term liabilities | 2,086 | 1,566 | 2,684 | 1,941 | ||||||||||||
| Total stockholders’ deficiency | (1,832 | ) | (585 | ) | (2,768 | ) | (1,604 | ) | ||||||||
| (1) | Working capital is equal to the difference between total current assets and total current liabilities. |
| 6 |
RISK FACTORS
Investing in our securities involves a high degree of risk. You should carefully consider the following risk factors and all other information contained in this prospectus, including the consolidated financial statements and the related notes appearing at the end of this prospectus, before purchasing our securities. If any of the following risks actually occur, they may materially harm our business and our financial condition and results of operations. In any such event, the market price of our securities could decline and you could lose all or part of your investment.
Risks Related to our Business
The report of our independent registered public accounting firm contains an explanatory paragraph as to our ability to continue as a going concern, which could prevent us from obtaining new financing on reasonable terms or at all.
Because we have had recurring losses and negative cash flows from operating activities, substantial doubt exists regarding our ability to remain in operation at the same level we are currently performing. Further, the report of Kost Forer Gabbay & Kasierer, a member firm of Ernst & Young Global, our independent registered public accounting firm, with respect to our financial statements at December 31, 2012 and 2011 and the years ended December 31, 2012 and 2011, includes an explanatory paragraph as to our potential inability to continue as a going concern. This may adversely affect our ability to obtain new financing on reasonable terms or at all.
We have a history of losses and we expect to continue to incur losses and may not achieve or maintain profitability.
For the year ended December 31, 2012, we had a net loss of $1,275,000, with revenues of $166,000. For the nine months ended September 30, 2013, we had a net loss of $1,412,000, with revenues of $203,000. As of September 30, 2013, we had an accumulated deficit of $13,626,000 and a total stockholders’ deficit of $2,768,000. We expect to incur losses for at least the next year, as we continue to incur expenses related to seeking U.S. Food and Drug Administration approval for our WoundShield product and seek market acceptance of our PainShield and NanoVibronix NPWT products, which will require costly clinical trials and research, further product development and professional fees associated with regulatory compliance. Even if we succeed in commercializing our new products, we may not be able to generate sufficient revenues to cover our expenses and achieve sustained profitability or be able to maintain profitability.
If we are unable to raise additional capital, our clinical trials and product development will be limited and our long-term viability will be threatened; however, if we do raise additional capital, your percentage ownership as a stockholder could decrease and constraints could be placed on the operations of our business.
We have experienced negative operating cash flows since our inception and have funded our operations primarily from proceeds of the sale of our securities, with only limited revenue being generated from our product sales. We will seek to obtain additional funds in the future through equity or debt financings, or strategic alliances with third parties, either alone or in combination with equity financings. These financings could result in substantial dilution to the holders of our common stock, or require contractual or other restrictions on our operations or on alternatives that may be available to us. If we raise additional funds by issuing debt securities, these debt securities could impose significant restrictions on our operations through the imposition of restrictive covenants and requiring us to pledge assets in order to secure repayment. In addition, if we raise funds through the sale of equity, we may issue equity securities with rights superior to our common stock, including voting rights, rights to proceeds upon our liquidation or sale, rights to dividends and rights to appoint board members. Any such required financing may not be available in amounts or on terms acceptable to us, and the failure to procure such required financing could have a material adverse effect on our business, financial condition and results of operations, or threaten our ability to continue as a going concern.
| 7 |
A variety of factors could impact the timing and amount of any required financings, including, without limitation:
| · | unforeseen developments during our clinical trials; |
| · | delays in our receipt of required regulatory approvals; |
| · | delayed market acceptance of our products; |
| · | unanticipated expenditures in our acquisition and defense of intellectual property rights, and/or the loss of those rights; |
| · | the failure to develop strategic alliances for the marketing of some of our product candidates; |
| · | unforeseen changes in healthcare reimbursement for any of our approved products; |
| · | lack of financial resources to adequately support our operations; |
| · | difficulties in maintaining commercial scale manufacturing capacity and capability; |
| · | unanticipated difficulties in operating in international markets; |
| · | unanticipated financial resources needed to respond to technological changes and increased competition; |
| · | unforeseen problems in attracting and retaining qualified personnel; |
| · | enactment of new legislation or administrative regulations; |
| · | the application to our business of new regulatory interpretations; |
| · | claims that might be brought in excess of our insurance coverage; |
| · | the failure to comply with regulatory guidelines; and |
| · | the uncertainty in industry demand. |
In addition, although we have no present commitments or understandings to do so, we may seek to expand our operations and product line through acquisitions or joint ventures. Any acquisition or joint venture would likely increase our capital requirements.
If we fail to obtain an adequate level of reimbursement for our approved products by third party payers, there may be no commercially viable markets for our approved products or the markets may be much smaller than expected.
The availability and levels of reimbursement by governmental and other third party payers affect the market for our approved products. The efficacy, safety, performance and cost-effectiveness of our product and product candidates, and of any competing products, will determine the availability and level of reimbursement. Reimbursement and healthcare payment systems vary significantly by country, and include both government sponsored healthcare and private insurance. To obtain reimbursement or pricing approval in some countries, we may be required to produce clinical data, which may involve one or more clinical trials, that compares the cost-effectiveness of our approved products to other available therapies. We may not obtain reimbursement or pricing approvals in markets we seek to enter in a timely manner, if at all. Our failure to receive reimbursement or pricing approvals in target markets would negatively impact market acceptance of our products in these jurisdictions, placing us at a material cost disadvantage to our competitors.
| 8 |
Even if we obtain reimbursement approvals for our products, we believe that, in the future, reimbursement for any of our products or product candidates may be subject to increased restrictions both in the U.S. and in international markets. Future legislation, regulation or policies of third party payers that limit reimbursement may adversely affect the demand for our products currently under development and our ability to sell our products on a profitable basis. In addition, third party payers continually attempt to contain or reduce the costs of healthcare by challenging the prices charged for healthcare products and services.
In the U.S., specifically, health care providers, such as hospitals and clinics, generally rely on third-party payers. Third-party reimbursement is dependent upon decisions by the Centers for Medicare and Medicaid Services, contracted Medicare carriers or intermediaries, individual managed care organizations, private insurers, foreign governmental health programs and other payers of health care costs. Failure to receive or maintain favorable coding, coverage and reimbursement determinations for our products by these organizations could discourage medical practitioners from using our products due to their costs. In addition, with recent federal and state government initiatives directed at lowering the total cost of health care, the U.S. Congress and state legislatures will likely continue to focus on health care reform including the reform of the Medicare and Medicaid entitlement programs, and on the cost of medical products and services, which could limit reimbursement. Additionally, third-party payers are increasingly challenging the prices charged for medical products and services. We may be unable to sell our products on a profitable basis if third-party payers deny coverage, provide low reimbursement rates or reduce their current levels of reimbursement.
The medical device and therapeutic product industries are highly competitive and subject to rapid technological change. If our competitors are better able to develop and market products that are safer and more effective than any products we may develop, our commercial opportunities will be reduced or eliminated.
Our success depends, in part, upon our ability to maintain a competitive position in the development of technologies and products. We face competition from established medical device companies, such as Misonix Inc., Celleration Inc., Kinetic Concepts, Inc. and Smith & Nephew plc, manufacturers of certain portable ultrasound devices capable of self-administered use, as well as from academic institutions, government agencies, and private and public research institutions in the U.S. and abroad. Most, if not all, of our principal competitors have significantly greater financial resources and expertise than we do in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals, marketing approved products, protecting and defending their intellectual property rights and designing around the intellectual property rights of others. Other small or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements, or mergers with, or acquisitions by, large and established companies, or through the development of novel products and technologies.
The industry in which we operate has undergone, and we expect it to continue to undergo, rapid and significant technological change, and we expect competition to intensify as technological advances are made. Our competitors may be able to respond to changes in technology or the marketplace faster than us. Our competitors may develop and commercialize medical devices that are safer or more effective or are less expensive than any products that we may develop. We also compete with our competitors in recruiting and retaining qualified scientific and management personnel, in establishing clinical trial sites and patient registration for clinical trials, and in acquiring technologies complementary to our programs or advantageous to our business. Given our small size and lack of resources, we are often at a disadvantage with our competitors in all of these areas, which could limit or eliminate our commercial opportunities.
We face the risk of product liability claims and may not be able to obtain insurance.
Our business exposes us to the risk of product liability claims that are inherent in the development of medical devices and products. If the use of one or more of our products harms people, we may be subject to costly and damaging product liability claims brought against us by clinical trial participants, consumers, health care providers, pharmaceutical companies or others selling our products. We currently carry clinical trial and product liability insurance for the products we sell. However, we cannot predict all of the possible harms or side effects that may result and, therefore, the amount of insurance coverage we hold may not be adequate to cover all liabilities we might incur. We intend to expand our insurance coverage to include the sale of additional commercial products as we obtain marketing approval for our product candidates in development and as our sales expand, but we may be unable to obtain commercially reasonable product liability insurance for such products. If we are unable to obtain insurance at an acceptable cost or otherwise protect against potential product liability claims and we continue to make sales, or if our coverages turns out to be insufficient, we may be exposed to significant liabilities, which may materially and adversely affect our business and financial position. If we are sued for any injury allegedly caused by our or our collaborators’ products and do not have sufficient insurance coverage, our liability could exceed our total assets and our ability to pay the liability. A product liability claim or series of claims brought against us would decrease our cash and could reduce our value or marketability.
Our product candidates may not be developed or commercialized successfully.
Our product candidates are based on a technology that has not been used previously in the manner we propose and must compete with more established treatments currently accepted as the standards of care. Market acceptance of our products will largely depend on our ability to demonstrate their relative safety, efficacy, cost-effectiveness and ease of use.
| 9 |
We are subject to the risks that:
| · | the U.S. Food and Drug Administration or a foreign regulatory authority finds our product candidates ineffective or unsafe; |
| · | we do not receive necessary regulatory approvals; |
| · | the regulatory review and approval process may take much longer than anticipated, requiring additional time, effort and expense to respond to regulatory comments and/or directives; |
| · | we are unable to get our product candidates in commercial quantities at reasonable costs; and |
| · | the patient and physician community does not accept our product candidates. |
In addition, our product development program may be curtailed, redirected, eliminated or delayed at any time for many reasons, including:
| · | adverse or ambiguous results; |
| · | undesirable side effects that delay or extend the trials; |
| · | the inability to locate, recruit, qualify and retain a sufficient number of clinical investigators or patients for our trials; and |
| · | regulatory delays or other regulatory actions. |
Additionally, we currently have limited experience in marketing or selling our products, and we have a limited marketing and sales staff and distribution capabilities. Developing a marketing and sales force is time-consuming and will involve the investment of significant amounts of financial and management resources, and could delay the launch of new products or expansion of existing product sales. In addition, we compete with many companies that currently have extensive and well-funded marketing and sales operations. If we fail to establish successful marketing and sales capabilities or fail to enter into successful marketing arrangements with third parties, our ability to generate revenues will suffer.
Furthermore, even if we enter into marketing and distributing arrangements with third parties, we may have limited or no control over the sales, marketing and distribution activities of these third parties, and these third parties may not be successful or effective in selling and marketing our products. If we fail to create successful and effective marketing and distribution channels, our ability to generate revenue and achieve our anticipated growth could be adversely affected. If these distributors experience financial or other difficulties, sales of our products could be reduced, and our business, financial condition and results of operations could be harmed.
We cannot predict whether we will successfully develop and commercialize our product candidates. If we fail to do so, we will not be able to generate substantial revenues, if any.
The loss of our key management would likely hinder our ability to execute our business plan.
As a small company with eight full-time employees, our success depends on the continuing contributions of our management team and qualified personnel and on our ability to attract and retain highly qualified personnel. We face intense competition in our hiring efforts from other medical device companies, as well as from universities and nonprofit research organizations, and we may have to pay higher salaries to attract and retain qualified personnel. We are also at a disadvantage in recruiting and retaining key personnel as our small size and limited resources may be viewed as providing a less stable environment, with fewer opportunities than would be the case at one of our larger competitors. The loss of one or more of these individuals, or our inability to attract additional qualified personnel, could substantially impair our ability to implement our business plan.
Our failure to protect our intellectual property rights could diminish the value of our solutions, weaken our competitive position and reduce our revenue.
We regard the protection of our intellectual property, which includes patents and patent applications, trade secrets, trademarks and domain names, as critical to our success. We strive to protect our intellectual property rights by relying on federal, state and common law rights, as well as contractual restrictions. We enter into confidentiality and invention assignment agreements with our employees, consultants and contractors, and confidentiality agreements with parties with whom we conduct business in order to limit access to, and disclosure and use of, our proprietary information. However, these contractual arrangements and the other steps we have taken to protect our intellectual property may not prevent the misappropriation of our proprietary information or deter independent development of similar technologies by others.
| 10 |
We have obtained patents and we have patent applications pending in both the U.S. and foreign jurisdictions. There can be no assurance that our patent applications will be approved, that any patents issued will adequately protect our intellectual property, or that these patents will not be challenged by third parties or found to be invalid or unenforceable. We have also obtained trademark registration in the U.S. and in foreign jurisdictions. Effective trade secret, trademark and patent protection is expensive to develop and maintain, both in terms of initial and ongoing registration requirements and the costs of defending our rights. We may be required to protect our intellectual property in an increasing number of jurisdictions, a process that is expensive and may not be successful or which we may not pursue in every location. We may, over time, increase our investment in protecting our intellectual property through additional patent filings that could be expensive and time-consuming.
Monitoring unauthorized use of our intellectual property is difficult and costly. Our efforts to protect our proprietary rights may not be adequate to prevent misappropriation of our intellectual property. We may not be able to detect unauthorized use of, or take appropriate steps to enforce, our intellectual property rights. Further, our competitors may independently develop technologies that are similar to ours but which avoid the scope of our intellectual property rights. Further, the laws in the U.S. and elsewhere change rapidly, and any future changes could adversely affect us and our intellectual property. Our failure to meaningfully protect our intellectual property could result in competitors offering solutions that incorporate our most technologically advanced features, which could seriously reduce demand for our products. In addition, we may in the future need to initiate infringement claims or litigation. Litigation, whether we are a plaintiff or a defendant, can be expensive, time-consuming and may divert the efforts of our technical staff and managerial personnel, which could harm our business, whether or not the litigation results in a determination that is unfavorable to us. In addition, litigation is inherently uncertain, and thus we may not be able to stop our competitors from infringing our intellectual property rights.
We could incur substantial costs and disruption to our business as a result of any claim of infringement of another party’s intellectual property rights, which could harm our business and operating results.
In recent years, there has been significant litigation in the U.S. over patents and other intellectual property rights. From time to time, we may face allegations that we or customers who use our products have infringed the trademarks, copyrights, patents and other intellectual property rights of third parties, including allegations made by our competitors or by non-practicing entities. We cannot predict whether assertions of third party intellectual property rights or claims arising from these assertions will substantially harm our business and operating results. If we are forced to defend any infringement claims, whether they are with or without merit or are ultimately determined in our favor, we may face costly litigation and diversion of technical and management personnel. Most of our competitors have substantially greater resources than we do and are able to sustain the cost of complex intellectual property litigation to a greater extent and for longer periods of time than we could. Furthermore, an adverse outcome of a dispute may require us, among other things: to pay damages, potentially including treble damages and attorneys’ fees, if we are found to have willfully infringed a party’s patent or other intellectual property rights; to cease making, licensing or using products that are alleged to incorporate or make use of the intellectual property of others; to expend additional development resources to redesign our products; and to enter into potentially unfavorable royalty or license agreements in order to obtain the rights to use necessary technologies. Royalty or licensing agreements, if required, may be unavailable on terms acceptable to us, or at all. In any event, we may need to license intellectual property which would require us to pay royalties or make one-time payments. Even if these matters do not result in litigation or are resolved in our favor or without significant cash settlements, the time and resources necessary to resolve them could harm our business, operating results, financial condition and reputation.
Our failure to meet certain minimum sales requirements under our license agreement could result in the termination of our exclusive license with respect to our NanoVibronix NPWT product and/or require us to make certain cash payments.
We license the technology that is the basis of our NanoVibronix NPWT product. Under the license agreement, we have the exclusive license to manufacture, market, sell, lease and distribute the technology within the U.S. until August 2014. Thereafter, the term of the license agreement will be extended automatically on a non-exclusive basis for an additional one- or three-year term if we meet certain minimum sales requirements. We are not currently meeting these requirements. If we do not meet these requirements by August 2014, we may be forced to renegotiate our license agreement on less favorable terms or lose our ability to sell our NanoVibronix NPWT product. In addition, we are obligated to pay a royalty payment of 5% of gross revenues from the sale of our pumps and $0.70 per canister. If we have not paid aggregate royalty payments of at least $150,000 by August 2014, we will have to pay the difference. For a description of this license agreement, see “Business—Intellectual Property—License Agreements” below.
| 11 |
Risks Related to the Regulation of Our Products
We are subject to extensive governmental regulation, including the requirement of U.S. Food and Drug Administration approval or clearance, before our product candidates may be marketed.
The process of obtaining U.S. Food and Drug Administration approval is lengthy, expensive and uncertain, and we cannot be sure that our product candidates will be approved in a timely fashion, or at all. If the U.S. Food and Drug Administration does not approve or clear our product candidates in a timely fashion, or at all, our business and financial condition would likely be adversely affected.
Both before and after approval or clearance of our product candidates, we, our product candidates, our suppliers and our contract manufacturers are subject to extensive regulation by governmental authorities in the U.S. and other countries. Failure to comply with applicable requirements could result in, among other things, any of the following actions:
| · | warning letters; |
| · | fines and other monetary penalties; |
| · | unanticipated expenditures; |
| · | delays in U.S. Food and Drug Administration approval and clearance, or U.S. Food and Drug Administration refusal to approve or clear a product candidate; |
| · | product recall or seizure; |
| · | interruption of manufacturing or clinical trials; |
| · | operating restrictions; |
| · | injunctions; and |
| · | criminal prosecutions. |
In addition to the approval and clearance requirements, numerous other regulatory requirements apply, both before and after approval or clearance, to us, our products and product candidates, and our suppliers and contract manufacturers. These include requirements related to the following:
| · | testing; |
| · | manufacturing; |
| · | quality control; |
| · | labeling; |
| · | advertising; |
| · | promotion; |
| · | distribution; |
| · | export; |
| · | reporting to the U.S. Food and Drug Administration certain adverse experiences associated with the use of the products; and |
| · | obtaining additional approvals or clearances for certain modifications to the products or their labeling or claims. |
We are also subject to inspection by the U.S. Food and Drug Administration to determine our compliance with regulatory requirements, as are our suppliers and contract manufacturers, and we cannot be sure that the U.S. Food and Drug Administration will not identify compliance issues that may disrupt production or distribution, or require substantial resources to correct.
| 12 |
The U.S. Food and Drug Administration’s requirements may change and additional government regulations may be promulgated that could affect us, our product candidates, and our suppliers and contract manufacturers. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action. There can be no assurance that we will not be required to incur significant costs to comply with such laws and regulations in the future, or that such laws or regulations will not have a material adverse effect upon our business.
Failure to obtain regulatory approval in foreign jurisdictions will prevent us from marketing our products abroad.
International sales of our products and any of our product candidates that we commercialize are subject to the regulatory requirements of each country in which the products are sold. Accordingly, the introduction of our product candidates in markets outside the U.S. where we do not already possess regulatory approval will be subject to regulatory approvals in those jurisdictions. The regulatory review process varies from country to country. Many countries impose product standards, packaging and labeling requirements, and import restrictions on medical devices. In addition, each country has its own tariff regulations, duties and tax requirements, as well as reimbursement and healthcare payment systems. The approval by foreign government authorities is unpredictable and uncertain, and can be expensive. We may be required to perform additional pre-clinical, clinical or post-approval studies even if U.S. Food and Drug Administration approval has been obtained. Our ability to market our approved products could be substantially limited due to delays in receipt of, or failure to receive, the necessary approvals or clearances.
We are uncertain regarding the success of our clinical trials for our products in development.
We believe that all of our products in development will require clinical trials to determine their safety and efficacy by regulatory bodies in their target markets, including the U.S. Food and Drug Administration and various foreign regulators. There can be no assurance that we will be able to successfully complete the U.S. and foreign regulatory approval processes for products in development. In addition, there can be no assurance that we will not encounter additional problems that will cause us to delay, suspend or terminate our clinical trials. In addition, we cannot make any assurance that clinical trials will be deemed sufficient in size and scope to satisfy regulatory approval requirements, or, if completed, will ultimately demonstrate our products to be safe and efficacious.
The adoption of healthcare reform in the U.S. may adversely affect our business and financial results.
On March 23, 2010, President Obama signed into law major healthcare reform legislation under the Patient Protection and Affordable Care Act of 2010, or the PPACA, which was modified on March 30, 2010 by the enactment of the Health Care and Education Reconciliation Act of 2010. Under the PPACA, it is expected that expanded healthcare coverage will be made available to an additional 30 million Americans. The increased costs to the U.S. government from the PPACA are expected to be funded through a combination of payment reductions for providers over time and several new taxes. The PPACA imposes, among other things, an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the U.S. beginning in 2013, resulting in an anticipated cost to the medical device industry of up to $20 billion over the next decade. We believe that we will be exempt from this excise tax with respect to PainShield under the exemption for devices of a “type which is generally purchased by the general public at retail for individual use,” but based on our initial assessment, we believe we are subject to it with respect to NanoVibronix NPWT and we will also need to assess whether we are subject to it with respect to other products when they are approved for sale in the U.S. The PPACA also provides for the establishment of an Independent Medicare Advisory Board that could recommend changes in payment for physicians under certain circumstances beginning in 2014. In addition, the PPACA authorizes certain voluntary demonstration projects beginning no later than 2013 around development of bundling payments for acute, inpatient hospital services, physician services, and post acute services for episodes of hospital care. The PPACA increases fraud and abuse penalties and expands the scope and reach of the Federal Civil False Claims Act and government enforcement tools, which may adversely impact healthcare companies.
The U.S. Supreme Court heard a constitutional challenge to the PPACA and in June 2012 held that the PPACA is constitutional. However, states are allowed to opt out of the expansion of eligibility criteria for Medicaid under the PPACA. In addition to the PPACA, the effect of which cannot presently be quantified given its recent enactment, various healthcare reform proposals have also emerged at the state level. We cannot predict whether future healthcare initiatives will be implemented at the federal or state level or the effect any future legislation or regulation will have on us. However, we anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for any approved product, and could adversely affect our business. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. Insurers may also refuse to provide any coverage of uses of approved products for medical indications other than those for which the U.S. Food and Drug Administration has granted market approvals, all of which may adversely affect our business, financial condition and results of operations, possibly materially.
| 13 |
If we fail to comply with the U.S. federal Anti-Kickback Statute and similar state laws, we could be subject to criminal and civil penalties and exclusion from the Medicare and Medicaid programs, which would have a material adverse effect on our business and results of operations.
A provision of the Social Security Act, commonly referred to as the federal Anti-Kickback Statute, prohibits the offer, payment, solicitation or receipt of any form of remuneration in return for referring, ordering, leasing, purchasing or arranging for, or recommending the ordering, purchasing or leasing of, items or services payable by Medicare, Medicaid or any other federal healthcare program. The federal Anti-Kickback Statute is very broad in scope and many of its provisions have not been uniformly or definitively interpreted by existing case law or regulations. In addition, most of the states have adopted laws similar to the federal Anti-Kickback Statute, and some of these laws are even broader than the federal Anti-Kickback Statute in that their prohibitions are not limited to items or services paid for by federal healthcare programs, but instead apply regardless of the source of payment. Violations of the federal Anti-Kickback Statute may result in substantial civil or criminal penalties and exclusion from participation in federal healthcare programs.
All of our financial relationships with healthcare providers and others who provide products or services to federal healthcare program beneficiaries are potentially governed by the federal Anti-Kickback Statute and similar state laws. We believe our operations are in compliance with the federal Anti-Kickback Statute and similar state laws. However, we cannot be certain that we will not be subject to investigations or litigation alleging violations of these laws, which could be time-consuming and costly to us and could divert management’s attention from operating our business, which in turn could have a material adverse effect on our business. In addition, if our arrangements were found to violate the federal Anti-Kickback Statute or similar state laws, the consequences of such violations would likely have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Our Organization and Our Securities
We are currently controlled by our executive officers, directors and principal stockholders, and after this offering, our executive officers, directors and principal stockholders will have significant influence regarding all matters submitted to our stockholders for approval.
As of February 6, 2014, our directors, executive officers and 5% or greater stockholders beneficially owned approximately 65.5% of our voting capital stock. When this offering is completed, our directors, executive officers and 5% or greater stockholders will, in the aggregate, beneficially own shares representing % of our voting capital stock, assuming such persons do not purchase any shares of common stock in this offering. As a result, if these stockholders were to choose to act together, they would be able to exercise significant influence with respect to all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, will exercise significant influence with respect to the election of directors and approval of any merger, consolidation, sale of all or substantially all of our assets or other business combination or reorganization. This concentration of voting power could delay or prevent an acquisition of us on terms that other stockholders may desire. The interests of this group of stockholders may not always coincide with your interests or the interests of other stockholders, and they may act in a manner that advances their best interests and not necessarily those of other stockholders, and might affect the prevailing market price for our securities.
The price of our securities may be volatile, and the market price of our securities after this offering may drop below the price you pay.
The initial public offering price per share may vary from the market prices of our common stock that prevail after the offering. If an active market for our stock develops and continues, the price of such stock nevertheless may be volatile. Market prices for securities of early-stage medical device companies have historically been particularly volatile. As a result of this volatility, you may not be able to sell your common stock at or above the initial public offering price paid per share. The factors that may cause the market price of our securities to fluctuate include, but are not limited to:
| · | progress, or lack of progress, in developing and commercializing our products; |
| · | favorable or unfavorable decisions about our products or intellectual property from government regulators, insurance companies or other third-party payers; |
| · | our ability to recruit and retain qualified regulatory and research and development personnel; |
| 14 |
| · | changes in investors’ and securities analysts’ perception of the business risks and conditions of our business; |
| · | changes in our relationship with key collaborators; |
| · | changes in the market valuation or earnings of our competitors or companies viewed as similar to us; |
| · | changes in key personnel; |
| · | depth of the trading market in our common stock; |
| · | termination of the lock-up agreement or other restrictions on the ability of us or any of our existing stockholders to sell shares after this offering; |
| · | changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; |
| · | the granting or exercise of employee stock options or other equity awards; |
| · | realization of any of the risks described under this section entitled “Risk Factors”; and |
| · | general market and economic conditions. |
In addition, the equity markets have experienced significant price and volume fluctuations that have affected the market prices for the securities of newly public companies for a number of reasons, including reasons that may be unrelated to our business or operating performance. These broad market fluctuations may result in a material decline in the market price of our common stock and you may not be able to sell your common stock at prices you deem acceptable. In the past, following periods of volatility in the equity markets, securities class action lawsuits have been instituted against public companies. Such litigation, if instituted against us, could result in substantial cost and the diversion of management attention.
Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment.
If you purchase securities in this offering, the public offering price that you pay per share of common stock will be substantially higher than the net tangible book value per share of our common stock immediately after this offering. Therefore, you will incur an immediate dilution of $ (or %) in net tangible book value per share of common stock from the price you paid, based on the public offering price of $ per share. The exercise of outstanding warrants and options may result in further dilution of your investment, but only if the public offering price is greater than $ per share. In addition, if we raise funds by issuing additional shares or convertible securities in the future, the newly issued shares may further dilute your ownership interest.
Future sales of our common stock, or the perception that future sales may occur, may cause the market price of our common stock to decline, even if our business is doing well.
Sales of substantial amounts of our common stock in the public market after this offering, or the perception that these sales may occur, could materially and adversely affect the price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. The shares of common stock sold in this offering will be freely tradable, without restriction, in the public market, except for any shares sold to our affiliates.
| 15 |
In connection with this offering, we and our officers and directors have agreed prior to the commencement of this offering, subject to limited exceptions, not to sell or transfer any shares of common stock for 180 days after the date of this prospectus without the consent of Chardan Capital Markets LLC. However, Chardan Capital Markets LLC may release these shares from any restrictions at any time. We cannot predict what effect, if any, market sales of shares held by any stockholder or the availability of shares for future sale will have on the market price of our common stock.
Approximately 18,868,218 shares of common stock may be sold in the public market by existing stockholders on or about 181 days after the date of this prospectus, subject to volume and other limitations imposed under the federal securities laws. Sales of substantial amounts of our common stock in the public market after the completion of this offering, or the perception that such sales could occur, could adversely affect the market price of our common stock and could materially impair our ability to raise capital through offerings of our common stock. See the section entitled “Shares Eligible for Future Sale” for a more detailed description of the restrictions on selling shares of our common stock after this offering.
We are issuing warrants to purchase shares of common stock in this offering. We will also issue a warrant to the underwriter in this offering to purchase an additional shares of common stock. In addition, as of February 6, 2014, we had outstanding options to purchase 2,525,704 shares of our common stock and outstanding warrants to purchase an aggregate of 4,161,168 shares of our common stock. We plan to register for offer and sale the shares of common stock that are reserved for issuance pursuant to outstanding options. Shares covered by such registration statements upon the exercise of stock options generally will be eligible for sale in the public market, except that affiliates will continue to be subject to volume limitations and other requirements of Rule 144 under the Securities Act of 1933, as amended. The issuance or sale of such shares could depress the market price of our common stock.
An active trading market may not develop for our securities, and you may not be able to sell your common stock at or above the initial public offering price or warrant exercise price per share.
There is no established trading market for our common stock, and the market for our common stock may be highly volatile or may decline regardless of our operating performance. Prior to this offering, you could not buy or sell our equity publicly. An active public market for our common stock may not develop or be sustained after this offering. We cannot predict the extent to which investor interest in our company will lead to the development of an active trading market in our common stock or how liquid that market might become. If a market does not develop or is not sustained, it may be difficult for you to sell your common stock at the time you wish to sell it, at a price that is attractive to you, or at all.
The initial public offering price per share has been determined through negotiation between us and representatives of the underwriter, and may not be indicative of the market prices that prevail after this offering. You may not be able to sell your common stock at or above the initial public offering price or warrant exercise price per share.
Due to the speculative nature of warrants, there is no guarantee that it will ever be profitable for holders of the warrants to exercise the warrants.
The warrants being offered do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the warrants may exercise their right to acquire the common stock and pay an exercise price of $[ ] per share (which is equal to 125% of the public offering price of the common stock), prior to the expiration of the five-years term on , 2019, after which date any unexercised warrants will expire and have no further value. Moreover, following this offering, the market value of the warrants is uncertain and there can be no assurance that the market value of the warrants will equal or exceed their public offering price. There can be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the warrants, and, consequently, whether it will ever be profitable for holders of the warrants to exercise the warrants.
It is unlikely that an active trading market for the warrants will develop.
The warrants will not be liquid investments because no public trading market currently exists for the warrants and it is unlikely that a market will develop. Potential purchasers of the warrants should consider carefully the limited liquidity of such investment. We are not obligated, and do not intend, to apply for the listing of the warrants on any securities exchange. Even if a trading market for the warrants were to develop, it may not continue, and a purchaser of the warrants may not be able to sell such warrants at or above the price at which they were purchased.
Holders may be unable to exercise the warrants if we do not maintain a current prospectus and comply with applicable securities laws.
No warrants will be exercisable unless at the time of exercise a prospectus relating to the common stock issuable upon exercise of the warrants is current and the common stock has been registered or qualified or is deemed to be exempt under the securities laws of the state of residence of the holder of the warrants. Under the terms of the warrant agreement, we have agreed to meet these conditions and use our best efforts to maintain a current prospectus relating to the common stock issuable upon exercise of the warrants until the expiration of the warrants. However, we cannot assure you that we will be able to do so, and if we do not maintain a current prospectus related to the common stock issuable upon exercise of the warrants, holders will be unable to exercise their warrants and we will not be required to settle any such warrant exercise. If the prospectus relating to the common stock issuable upon the exercise of the warrants is not current or if the common stock is not qualified or exempt from qualification in the jurisdictions in which the holders of the warrants reside, we will not be required to net cash settle or cash settle the warrant exercise, the warrants may have no value, the market for the warrants may be limited and the warrants may expire worthless.
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the Securities and Exchange Commission and The Nasdaq Stock Market, have imposed various requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these new compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time consuming and costly. We expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage.
| 16 |
We are obligated to develop and maintain proper and effective internal controls over financial reporting. We may not complete our analysis of our internal controls over financial reporting in a timely manner, or these internal controls may have one or more material weaknesses, which may adversely affect investor confidence in our company and, as a result, the value of our common stock.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be evaluated frequently. Section 404 of the Sarbanes-Oxley Act requires public companies to conduct an annual review and evaluation of their internal controls and attestations of the effectiveness of internal controls by independent auditors. We would be required to perform the annual review and evaluation of our internal controls no later than in connection with the second annual report on Form 10-K filed after the offering to which this prospectus relates. However, we initially expect to qualify as a smaller reporting company and as an emerging growth company, and thus, we would be exempt from the auditors’ attestation requirement until such time as we no longer qualify as a smaller reporting company and an emerging growth company. We would no longer qualify as a smaller reporting company if the market value of our public float exceeded $75 million as of the last day of our second fiscal quarter in any fiscal year following this offering. We would no longer qualify as an emerging growth company at such time as described in the risk factor immediately below.
We are in the early stages of the costly and challenging process of compiling the system and processing documentation necessary to evaluate and correct a material weakness in internal controls needed to comply with Section 404. The material weakness relates to our being a small company with a limited number of employees which limits our ability to assert the controls related to the segregation of duties. During the evaluation and testing process, if we identify one or more additional material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are effective. If we are unable to assert that our internal control over financial reporting is effective, we could lose investor confidence in the accuracy and completeness of our financial reports, which would cause the price of our common stock to decline.
While we currently qualify as an “emerging growth company” under the Jumpstart of Business Startups Act of 2012, or the JOBS Act, we will lose that status at the latest by the end of 2018, which will increase the costs and demands placed upon our management.
We will continue to be deemed an emerging growth company until the earliest of (i) the last day of the fiscal year during which we had total annual gross revenues of $1 billion (as indexed for inflation); (ii) the last day of the fiscal year following the fifth anniversary of the date of the first sale of common stock under this registration statement; (iii) the date on which we have, during the previous 3-year period, issued more than $1 billion in non-convertible debt; or (iv) the date on which we are deemed to be a ‘large accelerated filer,’ as defined by the Securities and Exchange Commission, which would generally occur upon our attaining a public float of at least $700 million. Once we lose emerging growth company status, we expect the costs and demands placed upon our management to increase, as we would have to comply with additional disclosure and accounting requirements, particularly if we would also no longer qualify as a smaller reporting company.
We are an “emerging growth company” and we cannot be certain that the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
The JOBS Act permits “emerging growth companies” like us to rely on some of the reduced disclosure requirements that are already available to smaller reporting companies. As long as we qualify as an emerging growth company or a smaller reporting company, we would be permitted to omit the auditor’s attestation on internal control over financial reporting that would otherwise be required by the Sarbanes-Oxley Act, as described above and are also exempt from the requirement to submit “say-on-pay”, “say-on-pay frequency” and “say-on-parachute” votes to our stockholders and may avail ourselves of reduced executive compensation disclosure that is already available to smaller reporting companies.
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the exemption from complying with new or revised accounting standards provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, as long as we are an emerging growth company. An emerging growth company can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the benefits of this until we are no longer an emerging growth company or until we affirmatively and irrevocably opt out of this exemption. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
| 17 |
We will cease to be an emerging growth company at such time as described in the risk factor immediately above. Until such time, however, we cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile and could cause our stock price to decline.
Our management will have broad discretion over the use of the proceeds we receive in this offering, and may not apply the proceeds in ways that increase the value of your investment.
We estimate that net proceeds of the sale of the securities that we are offering will be approximately $ million, or $ million if the underwriter exercises its option to purchase additional securities in this offering in full, based on an assumed initial public offering price of $ per share of common stock, the midpoint of the price range set forth on the cover page of this prospectus, and $ per warrant. We currently intend to use the net proceeds of the offering for marketing activities, clinical and regulatory activities, research and development and intellectual property protection. Depending on the outcome of these activities, our plans and priorities may change and we may apply the net proceeds of this offering differently than we currently anticipate. Moreover, you will not have the opportunity to influence our decision on how to use the proceeds from this offering. We may use the proceeds for corporate purposes that do not immediately enhance our prospects for the future or increase the value of your investment. See “Use of Proceeds.”
Anti-takeover provisions of our certificate of incorporation, our bylaws and Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove the current members of our board and management.
Certain provisions of our amended and restated certificate of incorporation and bylaws that will be in effect upon the completion of this offering could discourage, delay or prevent a merger, acquisition or other change of control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove members of our board of directors. These provisions also could limit the price that investors might be willing to pay in the future for our securities, thereby depressing the market price of our securities. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions, among other things:
| · | allow the authorized number of directors to be changed only by resolution of our board of directors; |
| · | authorize our board of directors to issue, without stockholder approval, preferred stock, the rights of which will be determined at the discretion of the board of directors and that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that our board of directors does not approve; |
| · | establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings; and |
| · | limit who may call a stockholder meeting. |
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law that may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of the voting rights on our common stock, from merging or combining with us for a prescribed period of time.
| 18 |
If securities or industry analysts do not publish research or reports or publish unfavorable research about our business, the price of our securities and their trading volume could decline.
The trading market for our securities will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of us the trading price for our securities would be negatively affected. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our securities, the price of our securities would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, interest in the purchase of our securities could decrease, which could cause the price of our securities and their trading volume to decline.
We may be subject to ongoing restrictions related to grants from the Israeli Office of the Chief Scientist.
Through our Israeli subsidiary, we received grants of $436,815 from the Office of the Chief Scientist of the Israeli Ministry of Industry, Trade and Labor, or the Office of the Chief Scientist, for research and development programs related to products that we are not currently commercializing or marketing. Because we are no longer developing the product to which the grants relate, we do not believe that we are subject to any material conditions with respect to the grants, except for the restrictions on our ability to make certain transfers of the technology or intellectual property related to these grants described below. We could in the future determine to apply for further grants. If we receive any such grants, we would have to comply with specified conditions, including paying royalties with respect to grants received. If we fail to comply with these conditions in the future, sanctions might be imposed on us, such as grants could be cancelled and we could be required to refund any payments previously received under these programs.
Pursuant to the Israeli Encouragement of Industrial Research and Development Law, any products developed with grants from the Office of the Chief Scientist are required to be manufactured in Israel and certain payments may be required in connection with the change of control of the grant recipient and the financing, mortgaging, production, exportation, licensing and transfer or sale of its technology and intellectual property to third parties, which will require the Office of the Chief Scientist’s prior consent and, in case such a third party is outside of Israel, extended royalties and/or other fees. This could have a material adverse effect on and significant cash flow consequences to us if, and when, any technologies, intellectual property or manufacturing rights are exported, transferred or licensed to third parties outside Israel. If the Office of the Chief Scientist does not wish to give its consent in any required situation or transaction, we would need to negotiate a resolution with the Office of the Chief Scientist. In any event, such a transaction, assuming it was approved by the Office of the Chief Scientist, would involve monetary payments, such as royalties or fees, of not less than the applicable funding received from the Office of the Chief Scientist plus interest, not to exceed, in aggregate, six times the applicable funding received from the Office of the Chief Scientist.
Because we do not expect to pay cash dividends for the foreseeable future, you must rely on appreciation of our common stock price for any return on your investment. Even if we change that policy, we may be restricted from paying dividends on our common stock.
We do not intend to pay cash dividends on shares of our common stock for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend upon results of operations, financial performance, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant. Accordingly, you will have to rely on capital appreciation, if any, to earn a return on your investment in our common stock. Investors seeking cash dividends in the foreseeable future should not purchase our common stock.
Our ability to use our net operating loss carry forwards and certain other tax attributes may be limited.
Our ability to utilize our federal net operating loss, carryforwards and federal tax credit may be limited under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended. The limitations apply if an “ownership change,” as defined by Section 382, occurs. Generally, an ownership change occurs if the percentage of the value of the stock that is owned by one or more direct or indirect “five percent shareholders” increases by more than 50% over their lowest ownership percentage at any time during the applicable testing period (typically three years). If we have experienced an “ownership change” at any time since our formation, we may already be subject to limitations on our ability to utilize our existing net operating losses and other tax attributes to offset taxable income. In addition, future changes in our stock ownership, which may be outside of our control, may trigger an “ownership change” and, consequently, Section 382 and 383 limitations. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carryforwards and other tax attributes to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us.
| 19 |
Risks Related to our Operations in Israel
We conduct our operations in Israel and therefore our results may be adversely affected by political, economic and military instability in Israel and its region.
Our principal offices are located in Israel and most of our officers, employees and directors are residents of Israel. Accordingly, political, economic and military conditions in Israel and the surrounding region may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors. Any hostilities involving Israel or the interruption or curtailment of trade within Israel or between Israel and its trading partners could adversely affect our operations and results of operations and could make it more difficult for us to raise capital. During the winter of 2012, Israel was engaged in an armed conflict with Hamas, a militia group and political party operating in the Gaza Strip. This conflict involved missile strikes against civilian targets in various parts of Israel and negatively affected business conditions in Israel. Recent political uprisings and civil resistance demonstrations in various countries in the Middle East, including Egypt and Syria, are affecting the political stability of those countries. It is not clear how this instability, or the Arab Spring in general, will develop and how it will affect the political and security situation in the Middle East. This instability may lead to deterioration of the political relationships that exist between Israel and these countries, and have raised concerns regarding security in the region and the potential for armed conflict. In addition, it is widely believed that Iran, which has previously threatened to attack Israel, has been stepping up its efforts to achieve nuclear capability. Iran is also believed to have a strong influence among extremist groups in the region, such as Hamas in Gaza and Hezbollah in Lebanon. The tension between Israel and Iran and/or these groups may escalate in the future and turn violent, which could affect the Israeli economy generally and us in particular. Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions and could harm our results of operations. For example, any major escalation in hostilities in the region could result in a portion of our employees being called up to perform military duty for an extended period of time. Parties with whom we do business have sometimes declined to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary.
Our commercial insurance does not cover losses that may occur as a result of events associated with the security situation in the Middle East. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability in the region would likely negatively affect business conditions and could harm our results of operations.
Further, in the past, the State of Israel and Israeli companies have been subjected to an economic boycott. Several countries still restrict business and trade activity with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial condition or the expansion of our business.
Our operations may be disrupted as a result of the obligation of management or personnel to perform military service.
Many of our male employees in Israel, including members of our senior management, perform up to one month, and in some cases more, of annual military reserve duty until they reach the age of 45 or older and, in the event of a military conflict, may be called to active duty. There have also been periods of significant call-ups of military reservists, and it is possible that there will be military reserve duty call-ups in the future. Our operations could be disrupted by the absence of a significant number of our employees. Such disruption could materially adversely affect our business, financial condition and results of operations.
| 20 |
Because a certain portion of our expenses is incurred in currencies other than the U.S. dollar, our results of operations may be harmed by currency fluctuations and inflation.
Our reporting and functional currency is the U.S. dollar. Most of the royalty payments from our agreements with our development and/or commercialization partners are payable in U.S. dollars, and we expect our revenues from future licensing agreements to be denominated mainly in U.S. dollars or in Euros. We pay a substantial portion of our expenses in U.S. dollars; however, a portion of our expenses, related to salaries of the employees in Israel and payment to part of the service providers in Israel and other territories, are paid in New Israeli Shekels, or NIS, and in other currencies. In addition, a portion of our financial assets is held in NIS and in other currencies. As a result, we are exposed to the currency fluctuation risks. For example, if the NIS strengthens against the U.S. dollar, our reported expenses in U.S. dollars may be higher than anticipated. In addition, if the NIS weakens against the U.S. dollar, the U.S. dollar value of our financial assets held in NIS will decline.
It may be difficult for investors in the U.S. to enforce any judgments obtained against us or any of our directors or officers.
Almost all of our assets are located outside the U.S., although we do maintain a permanent place of business within the U.S. In addition, all of our officers and some of our directors are nationals and/or residents of countries other than the U.S., and all or a substantial portion of such persons’ assets are located outside the U.S. As a result, it may be difficult for investors to enforce within the U.S. any judgments obtained against us or any of our non-U.S. directors or officers, including judgments predicated upon the civil liability provisions of the securities laws of the U.S. or any state thereof. Additionally, it may be difficult to assert U.S. securities law claims in actions originally instituted outside of the U.S. Israeli courts may refuse to hear a U.S. securities law claim because Israeli courts may not be the most appropriate forums in which to bring such a claim. Even if an Israeli court agrees to hear a claim, it may determine that the Israeli law, and not U.S. law, is applicable to the claim. Further, if U.S. law is found to be applicable, certain content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process, and certain matters of procedure would still be governed by the Israeli law. Consequently, you may be effectively prevented from pursuing remedies under U.S. federal and state securities laws against us or any of our non-U.S. directors or officers.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains “forward-looking statements,” which include information relating to future events, future financial performance, financial projections, strategies, expectations, competitive environment and regulation. Words such as “may,” “should,” “could,” “would,” “predicts,” “potential,” “continue,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” and similar expressions, as well as statements in future tense, identify forward-looking statements. Forward-looking statements should not be read as a guarantee of future performance or results and may not be accurate indications of when such performance or results will be achieved. Forward-looking statements are based on information we have when those statements are made or management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
| · | The timing of clinical studies and eventual U.S. Food and Drug Administration approval of WoundShield and our other product candidates. |
| · | Regulatory actions that could adversely affect the price of or demand for our approved products. |
| · | Market acceptance of existing and new products. |
| · | Favorable or unfavorable decisions about our products from government regulators, insurance companies or other third-party payers. |
| · | Our intellectual property portfolio. |
| · | We are not currently meeting the minimum sales requirements necessary to prevent our license agreement related to NanoVibronix NPWT from terminating in August 2014, and even if we meet such sales requirements, the license will no longer be exclusive after such date. |
| 21 |
| · | Our ability to recruit and retain qualified regulatory and research and development personnel. |
| · | Unforeseen changes in healthcare reimbursement for any of our approved products. |
| · | Lack of financial resources to adequately support our operations. |
| · | Difficulties in maintaining commercial scale manufacturing capacity and capability. |
| · | Our ability to generate internal growth. |
| · | Changes in our relationship with key collaborators. |
| · | Changes in the market valuation or earnings of our competitors or companies viewed as similar to us. |
| · | Our failure to comply with regulatory guidelines. |
| · | Uncertainty in industry demand and patient wellness behavior. |
| · | General economic conditions and market conditions in the medical device industry. |
| · | Future sales of large blocks of our common stock, which may adversely impact our stock price. |
| · | Depth of the trading market in our common stock. |
| · | Termination of the lock-up agreement or other restrictions on the ability of us or any of our existing stockholders to sell shares after this offering; |
The foregoing does not represent an exhaustive list of matters that may be covered by the forward-looking statements contained herein or risk factors that we are faced with that may cause our actual results to differ from those anticipated in our forward-looking statements. Please see “Risk Factors” for additional risks which could adversely impact our business and financial performance. Moreover, new risks regularly emerge and it is not possible for us to predict or articulate all risks we face, nor can we assess the impact of all risks on our business or the extent to which any risk, or combination of risks, may cause actual results to differ from those contained in any forward-looking statements. All forward-looking statements included in this prospectus are based on information available to us on the date of this prospectus. Except to the extent required by applicable laws or rules, we undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained above and throughout this prospectus.
| 22 |
USE OF PROCEEDS
We estimate that the net proceeds from the sale of the securities we are offering will be approximately $ . If the underwriter fully exercises the over-allotment option, the net proceeds of the securities we sell will be approximately $ . “Net proceeds” is what we expect to receive after paying the underwriting discount and other expenses of the offering. For the purpose of estimating net proceeds, we are assuming that the public offering price will be $ per share of common stock, the midpoint of the price range set forth on the cover page of this prospectus, and $ per warrant.
We intend to use the net proceeds as follows:
| · | We expect to use approximately $ for marketing and sales activities, which may include investment in branding our products, producing marketing materials, targeting certain publications, attending exhibitions and medical shows and visiting customers and potential customers. |
| · | We expect to use approximately $ for clinical and regulatory activities, specifically to complete the following clinical trials: (i) Woundshield ultrasound patch enhances perfusion of blood, 30 patient trial; (ii) Woundshield ultrasound patch enhances wound healing, 200 patient trial; and (iii) PainShield for Trigeminal Neuralgia 80 patient trial. See “Business – Our Products – WoundShield and NanoVibronix NPWT – Clinical Trials” and “Business – Our Products – PainShield – Clinical Trials” for more information on these anticipated clinical trials. |
| · | We expect to use approximately $ for research and development, including the integration of WoundShield and NanoVibronix NPWT and other product innovations, improvements and integration. |
| · | We expect to use approximately $ for intellectual property protection, to support filed patent applications and obtain additional intellectual property protection if needed. |
| · | We expect to use the balance of the net proceeds for operations and general working capital requirements. |
We will also receive proceeds upon any cash exercise of the warrants sold in this offering. If all of these warrants were to be exercised at the exercise price of $ per share, then we would receive net proceeds of approximately $ . We expect to use these proceeds, if any, for operations and general working capital requirements at the time of such exercise.
Investors are cautioned, however, that expenditures may vary substantially from these estimates. Investors will be relying on the judgment of our management, who will have broad discretion regarding the application of the proceeds of this offering. The amounts and timing of our actual expenditures will depend upon numerous factors, including our potential investments in new businesses, the amount of cash generated by our operations, the amount of competition and other operational factors. We may find it necessary or advisable to use portions of the proceeds from this offering for other purposes.
From time to time, we evaluate these and other factors and we anticipate continuing to make such evaluations to determine if the existing allocation of resources, including the proceeds of this offering, is being optimized.
Circumstances that may give rise to a change in the use of proceeds include:
| · | the timing of clinical studies and eventual U.S. Food and Drug Administration approval of WoundShield and our other product candidates; |
| · | the need or desire on our part to accelerate, increase or eliminate existing initiatives due to, among other things, changing market conditions and competitive developments; and |
| · | the availability of other sources of cash including cash flow from operations and new bank debt financing arrangements, if any. |
Until we use the net proceeds of this offering, we will invest the funds in short-term, investment grade, interest-bearing securities.
A $1.00 increase or decrease in the assumed initial public offering price of $ per share of common stock (the midpoint of the price range set forth on the front cover of this prospectus) would increase or decrease the net proceeds to us from this offering by $ , assuming the number of shares offered by us remains the same and after deducting estimated underwriting discounts and commission and estimated offering expenses payable by us. An increase or decrease of shares in the number of shares offered by us would increase or decrease the total consideration paid to us by new investors by $ , assuming the initial public offering price of $ per share (the midpoint of the price range set forth on the front cover of this prospectus) remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. The information discussed above is illustrative only and will adjust based on the actual public offering price and other terms of this offering determined at pricing.
| 23 |
CAPITALIZATION
The following table summarizes our capitalization as of September 30, 2013:
| · | on an actual basis; |
| · | on a pro forma basis, giving effect to |
| o | a one-for- reverse split of our common stock, which will occur prior to the pricing of this offering; |
| o | the conversion of all outstanding shares of our convertible preferred stock into an aggregate of shares of common stock; we believe we will be able to obtain the necessary consents to effect the conversion of all of our outstanding preferred stock automatically upon the closing of the offering; |
| o | the conversion of all outstanding convertible indebtedness into an aggregate of shares of common stock; we believe we will be able to obtain the necessary consents to effect the conversion of all of our outstanding convertible debt automatically upon the closing of this offering, regardless of the proceeds to be received in the offering, and assuming a conversion date; and |
| o | the filing of our amended and restated certificate of incorporation and the effectiveness of our restated bylaws, which will occur immediately prior to the completion of this offering. |
| · | on a pro forma, as adjusted basis, giving effect to (1) all of the above, (2) our receipt of the net proceeds from the sale by us in this offering of shares of common stock at an assumed public offering price of $ per share, the midpoint of the range set forth on the cover page of this prospectus, and warrants at an assumed public offering price of $ per warrant, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us and (3) the application of the net proceeds we will receive from this offering in the manner described in “Use of Proceeds.” |
The pro forma information below is illustrative only and our capitalization following the completion of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this table together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our audited and unaudited consolidated financial statements and the related notes appearing elsewhere in this prospectus.
| September 30, 2013 | ||||||||||||
| Actual | Pro Forma | As Adjusted | ||||||||||
| (in thousands) (unaudited) | ||||||||||||
| Convertible promissory notes to common stock | $ | 185 | $ | $ | ||||||||
| Embedded feature of convertible promissory notes | 121 | |||||||||||
| Stockholders’ deficiency: | ||||||||||||
| Common stock of $ 0.001 par value - 24,000,000 shares authorized and 1,085,060 shares issued and outstanding actual; shares authorized and shares issued and outstanding pro forma; shares authorized and shares issued and outstanding pro forma as adjusted | 1 | |||||||||||
| Preferred stock, par value $0.001; 18,000,000 shares authorized actual; shares authorized pro forma and pro forma as adjusted | ||||||||||||
| Series A preferred stock of $ 0.001 par value - 700,000 shares authorized and 394,232 shares issued and outstanding actual; no shares authorized and no shares issued and outstanding pro forma and pro forma as adjusted | * | |||||||||||
| Additional paid-in capital | 10,857 | |||||||||||
| Accumulated deficit | (13,626 | ) | ||||||||||
| Total stockholders’ deficiency | (2,768 | ) | ||||||||||
| Total liabilities and stockholders' deficiency | $ | 435 | ||||||||||
* Represents an amount lower than $1.
| 24 |
DILUTION
Our net tangible book value on September 30, 2013 was approximately $(2,768,000), or $(2.55) per share. “Net tangible book value” is total assets minus the sum of liabilities and intangible assets. “Net tangible book value per share” is net tangible book value divided by the total number of shares outstanding.
After giving effect to adjustments relating to the offering, our pro forma net tangible book value on September 30, 2013, would have been $ , or $ per share and $ per warrant. The adjustments made to determine pro forma net tangible book value per share are the following:
| · | The pro forma adjustments referenced under “Capitalization.” |
| · | An increase in total assets to reflect the net proceeds of the offering as described under “Use of Proceeds” (assuming that the public offering price will be $ per share of common stock, the midpoint of the range set forth on the cover page of this prospectus, and $ per warrant). |
| · | The addition of the number of shares offered by this prospectus to the number of shares outstanding. |
The following table illustrates the pro forma increase in net tangible book value of $ per share and the dilution (the difference between the offering price per share and net tangible book value per share) to new investors:
| Assumed public offering price per share of common stock | $ | |||
| Net tangible book value per share as of September 30, 2013 | $ | (2.55 | ) | |
| Pro forma net tangible book value per share of September 30, 2013 | $ | |||
| Increase in pro forma net tangible book value per share attributable to the offering | $ | |||
| Pro forma as adjusted net tangible book value per share as of September 30, 2013 after giving effect to the offering | $ | |||
| Dilution in pro forma net tangible book value per share to new investors in the offering | $ |
The following table shows, on a pro forma basis as described above, the difference between existing stockholders and new investors with respect to the number of shares purchased from us, the total consideration paid and the average price paid per share. The table assumes that the public offering price will be $ per share of common stock, the midpoint of the range set forth on the cover page of this prospectus, and $ per warrant.
| Shares Purchased | Total Consideration | Average Price | ||||||||||||||||||
| Number | Percent | Amount | Percent | Per Share | ||||||||||||||||
| Existing stockholders | % | $ | % | $ | ||||||||||||||||
| New investors | % | $ | % | $ | ||||||||||||||||
| Total | % | $ | % | |||||||||||||||||
The foregoing tables and calculations are based on the number of shares of our common stock outstanding as of September 30, 2013 and exclude:
| · | 1,315,790 shares of common stock issuable upon the exercise of warrants with an exercise price of $0.38 per share; |
| · | 2,319,062 shares of common stock issuable upon the exercise of warrants with an exercise price of $0.199 per share; |
| · | 2,513,704 shares of common stock issuable upon the exercise of currently outstanding options with a weighted average exercise price of $1.23; and |
| · | 286,296 shares of common stock available for future issuance under our 2004 Global Share Option Plan. |
| 25 |
To the extent any of these outstanding options or warrants is exercised, the dilution to new investors would be reduced. To the extent all of such outstanding options and warrants had been exercised as of September 30, 2013, the pro forma as adjusted net tangible book value per share after this offering would be $ , and total dilution per share to new investors would be $ .
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share of common stock, the midpoint of the range set forth on the cover page of this prospectus, would increase (decrease) our pro forma as adjusted net tangible book value by approximately $ , the pro forma as adjusted net tangible book value per share by approximately $ per share and the dilution to investors in this offering by approximately $ per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The information above assumes that the underwriter does not exercise its over-allotment option. If the underwriter exercises its over-allotment option in full, the pro forma as adjusted net tangible book value will increase to $ per share, representing an immediate increase to existing stockholders of $ per share and an immediate dilution of $ per share to new investors.
DIVIDEND POLICY
In the past, we have not declared or paid cash dividends on our common stock, and we do not intend to pay any cash dividends on our common stock. Rather, we intend to retain future earnings (if any) to fund the operation and expansion of our business and for general corporate purposes. Subject to legal and contractual limits, our board of directors will make any decision as to whether to pay dividends in the future.
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of financial condition and results of operations in conjunction with our consolidated financial statements and the related notes thereto included elsewhere in this prospectus. In addition to historical information, the following discussion and analysis includes forward-looking information that involves risks, uncertainties and assumptions. Our actual results and the timing of events could differ materially from those anticipated by these forward-looking statements as a result of many factors, including those discussed under “Risk Factors” and elsewhere in this prospectus. See “Cautionary Note Regarding Forward-Looking Statements” included elsewhere in this prospectus.
Overview
We are a medical device company focusing on noninvasive biological response-activating devices that target wound healing and pain therapy and can be administered at home, without the assistance of medical professionals. Our WoundShield, PainShield and UroShield products are backed by novel technology which relates to ultrasound delivery through surface acoustic waves. Our NanoVibronix NPWT employs a technology that drains open cavity wounds and seeks to accelerate wound healing.
Recent Events
On February 5, 2013, we issued secured convertible promissory notes to certain investors. The convertible promissory notes were initially issued in the original aggregate principal amount of $100,000. On each of March 28, 2013, June 3, 2013, August 5, 2013, October 7, 2013, December 9, 2013 and February 6, 2014, such principal amount was increased by $100,000, so that the total current principal amount outstanding is $700,000. The convertible promissory notes mature on the earlier of April 30, 2014, the closing date of a financing in which we sell an aggregate of at least $250,000 of our debt or equity securities or on an accelerated date if there is an event of default, upon which date the entire outstanding principal balance and any outstanding fees or interest will be due and payable in full. The convertible promissory notes bear interest at the rate of 6% per annum, which rate is increased to 10% upon and during the occurrence of an event of default. In addition, the convertible promissory notes are convertible either at the holders’ option or upon maturity into shares of our common stock at an initial conversion price of $0.38 per share, subject to adjustment for stock splits, fundamental transactions or similar events. The holders of the convertible promissory notes have a security interest in all of our assets and those of our subsidiaries. To date, no principal or interest has been paid on these notes. See “Liquidity and Capital Resources—Nine Months Ended September 30, 2013 Compared to Nine Months Ended September 30, 2012—Convertible Promissory Notes” below for more information on the terms of these notes.
| 26 |
In connection with the issuance of the convertible promissory notes described above, on each of February 5, 2013, March 28, 2013, June 3, 2013 August 5, 2013, October 7, 2013, December 9, 2013 and February 6, 2014, we issued warrants to purchase 263,158 shares of common stock (in aggregate warrants to purchase 1, 842,106 shares), with an exercise price of $0.38 per share (subject to adjustment), to the participating investors. See “Description of Securities—Warrants—February 2013 Warrants” below for more information on the terms of these warrants.
Critical Accounting Policies
Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
As applicable to these consolidated financial statements, the most significant estimates and assumptions relate to estimation of fair value of stock based compensation and the estimation of the fair value of warrants.
Functional currency
The accompanying consolidated financial statements have been prepared in U.S. dollars.
We believe that the currency of the primary economic environment in which our operations are conducted is the U.S. dollar; thus the dollar is our functional currency. The majority of the proceeds from our financing activities are received in U.S. dollars. Although a portion of our subsidiary’s expenses are dominated in NIS (mostly salary and rent), a substantial portion of our expenses are denominated in U.S. dollars. In addition, most of our assets and liabilities are in U.S. dollars and we expect that most of our revenues will be generated in U.S. dollars.
Transactions and balances originally denominated in U.S. dollars are presented at their original amounts. Transactions and balances in other currencies have been remeasured into U.S. dollars in accordance with Financial Accounting Standards Board Accounting Standards Codification (“ASC”) 830, “Foreign Currency Matters.”
All transaction gains and losses from the remeasurement of monetary balance sheet items denominated in non- U.S. dollar currencies are reflected in the statement of operations in financial expenses, net, as appropriate.
Revenue recognition
We generate revenues from the sale of our products to end users. Revenues from those products are recognized in accordance with Staff Accounting Bulletin No. 104, “Revenue Recognition,” when delivery has occurred, persuasive evidence of an agreement exists, the vendor’s fee is fixed or determinable, no further obligation exists and collectability is probable.
The Company’s agreements with its distributors do not contain any price protection guarantees, rights of return or other post-shipment obligations.
Stock-based compensation
We account for stock-based compensation in accordance with ASC 718, “Compensation-Stock Compensation” (“ASC 718”). ASC 718 requires companies to estimate the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service period in our consolidated statements of operations.
We recognize compensation expense for the value of our awards granted based on the straight line method over the requisite service period of each of the awards, net of estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term “forfeitures” is distinct from “cancellations” or “expirations” and represents only the invested portion of the surrendered option. Ultimately, the actual expenses recognized over the vesting period will only be for those shares that vest.
| 27 |
We selected the Black-Scholes-Merton option pricing model as the most appropriate fair value method for our stock option awards. This option-pricing model requires a number of assumptions, of which the most significant are the expected stock price volatility and the expected option term. Expected volatility was calculated based upon similar traded companies’ historical share price movements. The expected option term represents the period that our stock options are expected to be outstanding. We currently use a simplified method to estimate the period that our stock options are expected to be outstanding, based on the terms of the awards. We will continue to use this method until sufficient historical exercise data supports using expected life assumptions. The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with an equivalent term. We use an expected dividend rate of zero, as we have historically not paid dividends and have no foreseeable plans to pay dividends.
Because there has been no public market for our common stock, we have determined the fair value of the common stock underlying all of our options and warrants at the time of grant by considering a number of objective and subjective factors. We have obtained the assistance of an independent valuation firm and applied a market approach using recent third-party transactions in our equity. The fair value of the underlying shares of common stock will continue to be determined by our management until such time as the common stock is listed or quoted on an established stock exchange, national market system or other quotation system.
Income taxes
We account for income taxes in accordance with ASC 740, “Income Taxes” (“ASC 740”). ASC 740 prescribes the use of the liability method, whereby deferred tax asset and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. We provide a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value. As of September 30, 2013 and September 30, 2012, we provided a full valuation allowance.
We implement a two-step approach to recognize and measure uncertain tax positions. The first step is to evaluate the tax position taken or expected to be taken in a tax return by determining if the weight of available evidence indicates that it is more likely than not that, on an evaluation of the technical merits, the tax position will be sustained on audit, including resolution of any related appeals or litigation processes. The second step is to measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement. We believe that our tax positions are all highly certain of being upheld upon examination. As such, as of September 30, 2013, December 31, 2012 and September 30, 2012, we had not recorded a liability for uncertain tax positions.
Convertible promissory notes
We account for our outstanding convertible promissory notes in accordance with ASC 470-20, “Debt with Conversion and Other Options” (“ASC 470-20”) and ASC 815 “Derivatives and Hedging” (“ASC 815”). In accordance with ASC 815, we bifurcate all embedded derivatives that require bifurcation and account for them separately from the convertible debt. Based upon a third party valuation, we allocated the proceeds from each issuance between the freestanding liability (convertible debt) component, which is accounted for at cost, and the embedded derivative component, which is remeasured on each reporting date.
In addition, under the guidelines of ASC 470-20, we measure an embedded beneficial conversion feature on the date of issuance, by allocating a portion of the proceeds equal to the intrinsic value of the feature to additional paid in capital. The intrinsic value of the feature is calculated on the date of issuance using the effective conversion price which results from the allocation of the proceeds between the convertible debt and the embedded derivative component. The intrinsic value is limited to the portion of the proceeds allocated to the convertible debt. The beneficial conversion feature is amortized to our consolidated statements of comprehensive loss over the term of the liability. We recognize an embedded beneficial conversion feature related to our convertible series B-2 promissory notes.
Warrant liability
The fair value of the liability for our warrants issued to investors in 2013 was calculated using the Binomial model. We accounted for these warrants according to the provisions of ASC 815 and, based on the anti-dilution protections contained in the warrants, we classified them as liabilities, measured at fair value for each reporting period until they are exercised or expire, with changes in fair value recognized in our statement of operations as financial income or expense.
Extended Transition Period for “Emerging Growth Companies”
We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of the Jumpstart Our Business Act of 2012 (known as the JOBS Act). This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result of this election, our consolidated financial statements may not be comparable to companies that comply with public company effective dates. Because our consolidated financial statements may not be comparable to companies that comply with public company effective dates, investors may have difficulty evaluating or comparing our business, performance or prospects in comparison to other public companies, which may have a negative impact on the value and liquidity of our common stock.
| 28 |
Results of Operations
Nine Months Ended September 30, 2013 Compared to Nine Months Ended September 30, 2012
Revenues. Our revenues for the nine months ended September 30, 2013 and 2012 were approximately $203,000 and $109,000, respectively, an increase of approximately $94,000, or 86.2%, between the periods. The increase was attributable primarily to an increase in volume of sales, mainly contributed by sales to new distributors that came onboard after the nine months ended September 30, 2012.
For the nine months ended September 30, 2013, the percentage of revenues attributable to our products was: PainShield – 79.9%; UroShield – 8.6%; and NanoVibronix NWPT – 11.5%. For the nine months ended September 30, 2012, the percentage of revenues attributable to our products was: PainShield - 94.4%; UroShield – 5.6%; and NanoVibronix NWPT - 0%. For the nine months ended September 30, 2013 and 2012, the percentage of revenues attributable to our disposable products was 37.7% and 40.7%, respectively. For the nine months ended September 30, 2013 and 2012, the portion of our revenues that was derived from distributors was 43.8% and 51.0%, respectively.
Gross Profit. For the nine months ended September 30, 2013, gross profit (revenues less cost of revenues) increased by approximately 60.5%, or $46,000, to approximately $122,000 from approximately $76,000 during the same period in 2012. The key driver of the increase in gross profit was our increase in revenues, described above.
Gross profit as a percentage of revenues was approximately 60.1% for the nine months ended September 30, 2013 and approximately 69.7% for the nine months ended September 30, 2012. The decrease was primarily due to a write-off of inventory in the amount of approximately $19,000 during the nine months ended September 30, 2013.
Research and Development Expenses. For the nine months ended September 30, 2013 and 2012, research and development expenses were approximately $498,000 and $519,000, respectively, a decrease of approximately 4.1%, or $21,000, between the periods. The decrease resulted primarily from a decrease in patent and clinical research expenses of approximately $128,000, a decrease in materials and subcontractors expenses of approximately $60,000 and a decrease in facilities expenses associated with and allocated to research and development activities of approximately $45,000, offset by an increase in stock-based compensation expenses of approximately $199,000, to approximately $212,000 from approximately $13,000.
Our research and development expenses as a percentage of total revenues were approximately 245.3% and 476.2% for the nine months ended September 30, 2013 and 2012, respectively. The decrease was due primarily to our increase in revenues, described above.
Research and development expenses consist mainly of payroll expenses to employees involved in research and development activities, stock based compensation expenses, expenses related to subcontracting, patents, clinical trial and facilities expenses associated with and allocated to research and development activities
Selling and Marketing Expenses. For the nine months ended September 30, 2013 and 2012, selling and marketing expenses were approximately $183,000 and $132,000, respectively, an increase of approximately 38.6%, or $51,000, between the periods. The increase was mainly due to an increase in stock-based compensation expenses of approximately $40,000, to approximately $48,000 from approximately $8,000.
Our selling and marketing expenses as a percentage of total revenues were approximately 90.2% and 121.1% for the nine months ended September 2013 and 2012, respectively. The decrease was due to our increase in revenues, described above.
Sales and marketing expenses consist mainly of payroll expenses to direct sales and marketing employees, stock-based compensation expenses, travel expenses, trade show expenses, and facilities expenses associated with and allocated to sales and marketing activities.
General and Administrative Expenses. For the nine months ended September 30, 2013 and 2012, general and administrative expenses were approximately $362,000 and $98,000, respectively, an increase of approximately 269.4%, or $264,000, between the periods. The increase was mainly due to an increase in stock-based compensation expenses of approximately $213,000, to approximately $216,000 from approximately $3,000 and increased accounting and audit expenses of approximately $44,000.
General and administrative expenses as a percentage of total revenues were approximately 178.3% and 89.9% for the first nine months of 2013 and 2012, respectively. The increase was due to the increase in general and administrative expenses, described above.
| 29 |
Our general and administrative expenses consist mainly of payroll expenses for management and administrative employees, share-based compensation expenses, accounting and facilities expenses associated with general and administrative activities.
Financial Expenses, net. For the nine months ended September 30, 2013 and 2012, financial expenses, net were approximately $491,000 and $370,000, respectively, an increase of approximately 32.7%, or $121,000, between the periods. The increase resulted primarily from the amortization of the beneficial conversion feature of our convertible series B-2 promissory notes.
Net Loss. Our net loss increased by approximately $369,000, or 35.4%, to approximately $1,412,000 for the nine months ended September 30, 2013 from approximately $1,043,000 during the same period in 2012. The increase in net loss resulted primarily from the factors described above.
Twelve Months Ended December 2012 Compared to Twelve Months Ended December 31, 2011
Revenues. For the twelve months ended December 31, 2012 and 2011, our revenues were approximately $166,000 and $94,000, respectively, an increase of approximately 76.6%, or $72,000, between the periods. The increase was attributable primarily to an increase in volume of sales, mainly contributed by sales to new distributors that came onboard during the twelve months ended December 31, 2012.
For the twelve months ended December 31, 2012, the percentage of revenues attributable to our products was: PainShield - 93.5%; UroShield - 4.5%; and NanoVibronix NWPT - 2.0%. For the twelve months ended December 31, 2011, the percentage of revenues attributable to our products was: PainShield - 93.5%; UroShield - 6.5%; and NanoVibronix NWPT - 0%. For the twelve months ended December 31, 2012 and 2011, the percentage of revenues attributable to our disposable products was 39.7% and 46.7%, respectively. For the twelve months ended December 31, 2012 and 2011, the portion of our revenues that was derived from distributors was 58.5% and 56.9%, respectively.
Gross Profit. For the twelve months ended December 31, 2012, gross profit increased by approximately 78.5%, or $51,000, to approximately $116,000 from approximately $65,000 during the same period in 2011. The key driver of the increase in gross profit was our increase in revenues, described above.
Gross profit as a percentage of revenues was 69.9% for the twelve months ended December 31, 2012 and 69.1% for the same period in 2011. The increase was primarily due to our increase in revenues, described above.
Research and Development Expenses. For the twelve months ended December 31, 2012 and 2011, research and development expenses were approximately $572,000 and $510,000, respectively, an increase of approximately 12.2%, or $62,000, between the periods. The increase is primarily comprised of a decrease in government grants of approximately $113,000, due to receipt of $113,000 from the Office of the Chief Scientist during 2011 and no such grant during 2012, and an increase in royalty payment of approximately $75,000 due to a payment in 2012 we were required to make under a licensing agreement, partially offset by decreases in salary and subcontractors expenses of approximately $46,000, stock-based compensation expenses of approximately $30,000, facilities expenses associated with and allocated to research and development activities of approximately $27,000 and patent and clinical research expenses of approximately $22,000.
Our research and development expense as a percentage of total revenues was approximately 344.6% and 542.6% in 2012 and 2011, respectively. The decrease was due to our increase in revenues, described above.
Selling and Marketing Expenses. For the twelve months ended December 31, 2012 and 2011, selling and marketing expenses were approximately $190,000 and $150,000, respectively, an increase of approximately 26.7%, or $40,000, between the periods. The increase resulted primarily from increased salary expenses.
Selling and marketing expenses as a percentage of revenue decreased to approximately 114.5% in 2012 from approximately 159.6% in 2011. The decrease was due to our increase in revenues, described above.
General and Administrative Expenses. For the twelve months ended December 31, 2012 and 2011, general and administrative expenses were approximately $128,000 and $87,000, respectively, an increase of approximately 47.1%, or $41,000, between the periods. The increase resulted primarily from an increase of approximately $27,000 in salary expenses and approximately $16,000 in legal fees.
General and administrative expenses as a percentage of revenue decreased to approximately 77.1% in 2012 from approximately 92.6% in 2011. The decrease was due to the increase in our revenues, described above.
| 30 |
Financial Expenses, net. For the twelve months ended December 31, 2012 and 2011, financial expenses, net were $501,000 and $41,000, respectively, an increase of approximately 1,122.0%, or $460,000, between the periods. The increase resulted primarily from approximately $222,000 due to the amortization of the beneficial conversion feature of our convertible series B-2 promissory notes, which were issued in November 2011, and interest expense of approximately $235,000 related to our convertible series B-1 promissory notes and convertible series B-2 promissory notes.
Net Loss. Our net loss increased by approximately $552,000, or 76.3%, to approximately $1,275,000 for the twelve months ended December 31, 2012 from approximately $723,000 during the same period in 2011. The increase in net loss resulted primarily from the factors described above.
Liquidity and Capital Resources
We continue to incur losses and negative cash flows from operating activities. For the nine months ended September 30, 2013, we had losses of approximately $1,412,000 and negative cash flows from operating activities of approximately $447,000. These conditions raise substantial doubts about our ability to continue as a going concern. Our ability to continue to operate is dependent upon raising additional funds to finance our activities. We aim to have our securities listed on the NASDAQ Stock Market, for the purpose of raising capital to finance our operations. There are no assurances, however, that we will be successful in obtaining an adequate level of financing to qualify for a NASDAQ listing, or necessary for the long-term development and commercialization of our products.
We are currently meeting our short-term liquidity requirements with the proceeds of our secured convertible promissory notes, which are borrowings from a related party (see “Certain Relationships and Related Transactions”). We intend to use the proceeds of the offering to which this prospectus relates to meet such short-terms requirements as well as to advance our long-term plans. It is our current belief that the proceeds of this offering will provide sufficient funding to meet our liquidity needs for more than a year.
Our future capital requirements and the adequacy of our available funds will depend on many factors, including our ability to successfully commercialize our products, our development of future products and competing technological and market developments. However, we may be unable to raise sufficient additional capital when we require it or upon terms favorable to us. In addition, the terms of any securities we issue in future financings may be more favorable to new investors and may include preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect on the holders of any of our securities then outstanding. If we are unable to obtain adequate funds on reasonable terms, we will need to curtail operations significantly, including possibly postponing anticipated clinical trials or entering into financing agreements with unattractive terms.
Nine Months Ended September 30, 2013 Compared to Nine Months Ended September 30, 2012
General. As of September 30, 2013, we had cash and cash equivalents of approximately $51,000, compared to approximately $249,000 as of September 30, 2012. The decrease is attributable primarily to our net loss. We have historically met our cash needs through a combination of issuance of equity, borrowing activities and sales. Our cash requirements are generally for product development, research and development cost, marketing and sales activities, finance and administrative cost, capital expenditures and general working capital.
Cash used in our operating activities was approximately $447,000 for the nine months ended September 30, 2013 and approximately $640,000 for the same period in 2012. The most significant usage of cash in our operating activities for the nine months ended September 30, 2013 and 2012 was a net loss of approximately $1,412,000 and $1,043,000, respectively, offset during the nine months ended September 30, 2013 by approximately $476,000 in non-cash stock-based compensation.
Cash used in our investing activities was approximately $3,000 during the nine months ended September 30, 2013, compared to approximately $4,000 during the same period in 2012.
Cash provided by financing activities was approximately $400,000 for the nine months ended September 2013, due to the issuance of convertible promissory notes, compared to no cash used in or provided by financing activities for the same period in 2012.
Convertible Promissory Notes. As of September 30, 2013, we had convertible series B-1 promissory notes and convertible series B-2 promissory notes with an aggregate principal amount outstanding of approximately $2,536,765, with aggregate accrued interest of $501,099, and secured convertible promissory notes with an aggregate principal amount of $400,000, with aggregate accrued interest of $10,866. As of September 30, 2013, no principal or interest had been paid on these notes.
| 31 |
The convertible series B-1 promissory notes mature on the earlier of November 15, 2014 or on an accelerated date if there is an event of default, upon which date the entire outstanding principal balance and any outstanding fees or interest will be due and payable in full. The convertible series B-1 promissory notes bear interest at the rate of 10% per annum, compounded annually. In addition, the convertible series B-1 promissory notes are convertible at any time at the holder’s option into shares of our series B-1 participating convertible preferred stock at an initial conversion price of $0.284 per share, subject to adjustment for stock dividends, stock splits or combinations. The convertible series B-1 promissory notes will automatically convert into series B-1 participating convertible preferred stock upon the occurrence of (i) an aggregate investment in us of $3 million or more in a transaction or series of transactions or (ii) a fundamental transaction. These notes will convert into common stock automatically upon the closing of this offering.
The terms of the convertible series B-2 promissory notes are the same as those of the convertible series B-1 promissory notes, except that the initial conversion price is $0.199. These notes will convert into common stock automatically upon the closing of this offering.
As of September 30, 2013, the secured convertible promissory notes were scheduled to mature on the earlier of December 31, 2013 (which date was subsequently extended to February 28, 2014), the closing date of a financing in which we sell an aggregate of at least $250,000 of our debt or equity securities or on an accelerated upon an event of default, upon which date the entire outstanding principal balance and any outstanding fees or interest would be due and payable in full. The secured convertible promissory notes bear interest at the rate of 6% per annum, which rate is increased to 10% upon and during the occurrence of an event of default. Events of default are comprised of: (i) failure to pay indebtedness under the notes when due; (ii) a default in a covenant, obligation or agreement under the notes or related documents; (iii) any representation, warranty or certification made by us under the notes is false or incorrect in any material respect on the date made; (iv) the occurrence of a liquidation, insolvency or bankruptcy event; (v) the entry of certain final judgments against us; (vi) our failure to make required payments under other debt, the acceleration of the maturity date on other debt, or a demand or requirement that we redeem, repurchase or retire other debt prior to its maturity; (vii) a material adverse effect, as defined in the notes; (viii) any material impairment in the value of the collateral or the priority of the lenders’ liens; (ix) any levy upon, seizure or attachment of a material portion of the collateral; (x) our assertion that any transaction document related to the notes is invalid or unenforceable; and (xi) the lenders cease to have a perfected lien in any of the collateral, subject to certain exceptions. The secured convertible promissory notes are convertible either at the holders’ option or upon maturity into shares of our common stock at an initial conversion price of $0.38 per share, subject to adjustment for stock splits, fundamental transactions or similar events. The holders of the secured convertible promissory notes have a security interest in all of our assets and those of our subsidiaries. These notes will convert into common stock automatically upon the closing of this offering.
Twelve Months Ended December 31, 2012 Compared to Twelve Months Ended December 31, 2011
General. As of December 31, 2012, we had cash and cash equivalents of approximately $101,000, as compared to approximately $893,000 as of December 31, 2011. The decrease is attributable primarily to our net loss.
Cash used in our operating activities was approximately $787,000 for the twelve months ended December 31, 2012, and approximately $547,000 for the same period in 2011. The most significant usage of cash in our operating activities for the twelve months ended December 31, 2012 and 2011 was a net loss of approximately $1,275,000 and $723,000, respectively, which during the twelve months ended December 31, 2012 included the amortization of the beneficial conversion feature of our convertible series B-2 promissory notes in the amount of approximately $238,000, which is a non-cash item.
Cash used in our investing activities was approximately $5,000 during the twelve months ended December 31, 2012, compared to approximately $2,000 during the same period in 2011.
| 32 |
Cash flow provided by financing activities was approximately $1,325,000 for the twelve months ended December 31, 2011, compared to no cash flow used in or provided by financing activities for the same period in 2012. The principal reason for the cash flow from financing activities during 2011 was approximately $1,000,000 from the issuance of convertible series B-1 promissory notes and approximately $325,000 from the issuance of series B participating convertible preferred stock.
Convertible Promissory Notes. As of December 31, 2012, we had convertible series B-1 promissory notes and convertible series B-2 promissory notes with an aggregate principal amount outstanding of approximately $2,536,765, with aggregate accrued interest of $290,044. As of December 31, 2012, no principal or interest had been paid on these notes.
Material Commitments
Under the terms of the license agreement for NanoVibronix NWPT, if we have not paid aggregate royalty payments of at least $150,000 by August 2014, we are required to pay the difference. We made an advance payment of $75,000 on account of future royalties during 2012 and remain obligated to pay $75,000 by August 2014.
Off Balance Sheet Arrangements
As of September 30, 2013, we have no off-balance sheet transactions, arrangements, obligations (including contingent obligations), or other relationships with unconsolidated entities or other persons that have, or may have, a material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Factors That May Affect Future Operations
We believe that our future operating results will continue to be subject to quarterly variations based upon a wide variety of factors, including the ordering patterns of our distributors, timing of regulatory approvals, the implementation of various phases of our clinical trials and manufacturing efficiencies due to the learning curve of utilizing new materials and equipment. Our operating results could also be impacted by a weakening of the Euro and strengthening of the New Israeli Shekel, or NIS, both against the U.S. dollar. Lastly, other economic conditions we cannot foresee may affect customer demand, such as individual country reimbursement policies pertaining to our products.
BUSINESS
Overview
We were organized as a Delaware corporation in October 2003. Through our wholly-owned subsidiary, NanoVibronix Ltd., a private company incorporated under the laws of the State of Israel, we focus on noninvasive biological response-activating devices that target wound healing and pain therapy and can be administered at home, without the assistance of medical professionals. Our products currently consist of:
| · | WoundShield, a patch-based therapeutic ultrasound device that facilitates tissue regeneration and wound healing by using ultrasound to increase local capillary perfusion and tissue oxygenation; |
| · | NanoVibronix NPWT, a small, lightweight pump with features that allow contamination-free handling of infected wound exudate and enhanced patient mobility; |
| 33 |
| · | PainShield, a disposable patch-based therapeutic ultrasound technology to treat pain, muscle spasm and joint contractures by delivering a localized ultrasound effect to treat pain and induce soft tissue healing in a targeted area; and |
| · | UroShield, an ultrasound-based product that is designed to prevent biofilm in urinary catheters, increase antibiotic efficacy and decrease pain and discomfort associated with urinary catheter use. |
Each of our WoundShield, PainShield and UroShield products employs a small, disposable transducer that transmits low frequency, low intensity ultrasound acoustic waves that seek to repair and regenerate tissue, musculoskeletal and vascular structures and increase antibiotic efficacy. Through their size, effectiveness and ease of use, these products are intended to eliminate the need for technicians and medical personnel to manually administer ultrasound treatment through large transducers, thereby promoting patient independence and enabling more cost-effective home-based care. Our NanoVibronix NPWT is based on an existing standard of care for wound therapy treatment and employs a technology that drains open cavity wounds and seeks to accelerate wound healing.
PainShield and NanoVibronix NPWT are currently approved for marketing in the U.S. by the U.S. Food and Drug Administration and all of our products, except for NanoVibronix NPWT, have CE Mark approval in the European Union. We anticipate that we will apply for CE Mark approval for NanoVibronix NPWT within six months after the closing of the offering to which this prospectus relates. We have a Canadian medical device license for PainShield and UroShield, a certificate allowing us to sell PainShield, WoundShield and UroShield in Israel, a certificate allowing us to sell PainShield in Australia, and we are able to sell PainShield, WoundShield and UroShield in India and Ecuador based on our CE Mark. In addition, our distributor in Korea has applied for approval to sell PainShield and UroShield, and our distributor in Chile has applied for approval to sell PainShield. We generally apply, through our distributor, for approval in a particular country for a particular product only when we have a distributor in place with respect to UroShield.
In addition to the need to obtain regulatory approvals, as described above, we anticipate that sales volumes and prices of our WoundShield and PainShield products will depend in large part on the availability of coverage and reimbursement for self-administered use from third party payers. Third party payers include governmental programs such as Medicare and Medicaid in the U.S., private insurance plans and workers’ compensation plans. We do not currently have reimbursement codes for self-administered use or clinical use of WoundShield in any of the markets in which we have regulatory authority to sell WoundShield. Of the markets in which we have regulatory authority to sell PainShield, we have reimbursement codes in the United States (i.e., Current Procedural Terminology codes or “CPT codes”) for clinical use only, but do not have such reimbursement codes for self-administered use of the product. NanoVibronix NPWT is generally reimbursed by governmental and other third-party payers in the U.S. With respect to UroShield, which will be used primarily in a clinical setting, we do not currently have reimbursement codes in any of the markets in which we have regulatory authority to sell UroShield. We anticipate that we will begin to seek reimbursement codes for self-administered and clinical use of our products in the markets in which we have regulatory authority to sell such products after the closing of this offering, however, there is no guarantee that we will be successful in obtaining such codes quickly, or at all.
Assuming we are able to obtain adequate financing, including through this offering, we plan to continue to work on the further commercialization of WoundShield and further marketing of NanoVibronix NPWT. We intend to integrate our WoundShield ultrasound technology into NanoVibronix NPWT, which we believe would make NanoVibronix NPWT a superior product within the negative pressure wound therapy market. We also intend to conduct ongoing clinical trials of our PainShield product, with the aim of obtaining a favorable reimbursement code. With respect to our UroShield product, we are currently seeking a strategic partner that is active in the urology market and would be interested in integrating UroShield into its range of products. If we locate such a partner, we anticipate that we would continue to pursue U.S. Food and Drug Administration approval of the product.
| 34 |
Ultrasound Technology and Our Products
As noted above, each of our products, other than NanoVibronix NPWT, is based on the use of low frequency ultrasound, which delivers energy through mechanical vibrations in the form of sound waves. Ultrasound has long been used in physical therapy, physical medicine, rehabilitation and sports medicine. Moreover, there is a growing body of research that supports the positive biological effects of ultrasound. A recent study indicates that low frequency ultrasound increases nerve regeneration (Crisci AR, Ferreira AL, “Low-intensity pulsed ultrasound accelerates the regeneration of the sciatic nerve after neurotomy in rats”, Ultrasound Med. Biol. 2002 October; 28(10):1335-41). According to Atland, et. al., low frequency ultrasound also has important therapeutic metabolic effects (Altland OD, Dalecki D, Suchkova VN, Francis CW, “Low-intensity ultrasound increases endothelial cell nitric oxide synthase activity and nitric oxide synthesis”, J. Thromb. Haemost. 2004 April; 2(4):637-43). In addition, there is evidence that ultrasound increases the healing of fractures (Warden SJ, Favaloro JM, Bennell KL, McMeeken JM, Ng KW, Zajac JD, Wark JD, “Low-intensity pulsed ultrasound stimulates the bone-forming response in UMR-106 cells”, Biochem. Biophys. Res. Commun. 2001 August 24; 286(3):443-50 and Warden SJ, Bennell KL, McMeeken JM, Wark JD, “Acceleration of fresh fracture repair using the sonic accelerated fracture healing system (SAFHS)”, Calcif. Tissue Int. 2000 February; 66(2):157-63).
Research has further shown that ultrasound therapy has resulted in increased collagen repair (Da Cunha A, Parizotto NA, Vidal BC, “The effect of therapeutic ultrasound on repair of the achilles tendon (tendo calcaneus) of the rat”, Ultrasound Med. Biol. 2001 December; 27(12):1691-6), improved resolution of inflammation (Young SR, Dyson M, “Macrophage responsiveness to therapeutic ultrasound”, Ultrasound Med. Biol. 1990; 16(8):809-16) and increased tissue healing (Young SR, Dyson M, “Effect of therapeutic ultrasound on the healing of full-thickness excised skin lesions”, Ultrasonics. 1990 May; 28(3):175-80), which are all important factors in the wound healing process. Furthermore, research has shown that ultrasound therapy can contribute to increased membrane permeability (Sundaram J, Mellein BR, Mitragotri S, “An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes,” Biophys. J. 2003 May; 84(5):3087-101) and accelerated fibrinolysis, a process that prevents blood clots from growing and becoming problematic (Harpaz D, “Ultrasound enhancement of thrombolytic therapy: observations and mechanisms”, Int. J. Cardiovasc Intervent. 2000 June; 3(2):81-89), which collectively improve the tissue regeneration process and healing of wounds. Sonophoresis, a process that increases the absorption of semisolid topical compounds, including medications, into the skin, is an additional significant effect of ultrasound therapy (Tezel A, Paliwal S, Shen Z, Mitragotri S, “Low-frequency ultrasound as a transcutaneous immunization adjuvant”, Vaccine 2005 May 31; 23(29):3800-7).
In general, ultrasound causes the benefits cited above by increasing local blood circulation, increasing vascular wall permeability, promoting protein secretion, promoting enzymatic reactions, accelerating nitric oxide production, promoting angiogenesis (the formation of new blood vessels from pre-existing vessels) and promoting fibroblast proliferation (fibroblasts are a type of cell that play a critical role in wound healing).
Our proprietary technology consists of a small, thin (1 millimeter) transducer that is capable of transmitting ultrasonic acoustic waves onto treatment surfaces with a radius of up to 10 centimeters. This technology allows us to treat wounds by implanting our transducers into a small, portable self-adhering acoustic patch, thereby eliminating the need for technicians and medical personnel to manually administer ultrasound therapy, which should reduce the cost of therapy. Moreover, we believe that the delivery of ultrasound through our portable devices is more effective than existing products, as our technology is better positioned to target the affected areas of the body.
While there are currently a number of products on the market that treat pain through ultrasound therapy, we believe that our products differentiate themselves because they are portable, without the requirement to be plugged into an outlet and they have a frequency of 100kHz (in contrast to other devices, which have a frequency of 1MHz), which means they do not produce heat that can damage tissue. They can therefore be self-administered by the patient without the need to be moved about the treated area by the patient or a clinician, they can be applied for a significantly longer period without the risk of tissue damage and they do not require the use of gel. We are aware of one product under U.S. Food and Drug Administration review and with CE Mark approval that we understand does not need to be plugged in and operates at a frequency of 3 MHz, which its manufacturer claims overcomes the need for movement around the treated area and allows for a longer treatment period. We understand that this product does not generate surface acoustic waves as our products do, which means that the treatment area is generally limited to that of the transducer’s diameter (see the diagram below), that the use of transmission gel is still required and that the transducer thickness is significantly greater than ours (approximately 1.5cm).
| 35 |
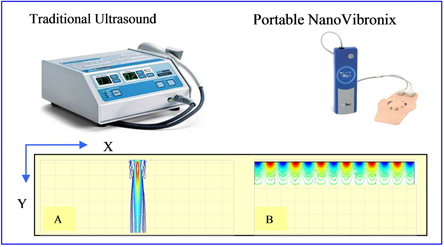
Traditional ultrasound device and our portable ultrasound patch-based device and a comparison of their energy distribution, where the X-axis represents treatment surface and the Y-axis represents ultrasound energy penetration depth within tissue.
In a comparison of a traditional ultrasound device and our portable ultrasound patch-based device, the bulk wave conventional ultrasound machines with handheld transducers distribute the energy deeply into the body, as shown above in diagram (A) on the left. In comparison, our device distributes the energy on the surface, as shown in diagram (B), thereby greatly increasing the treatment area. Our transducers may also be incorporated into treatment patches, including patches that are designed to deliver medicine and other compounds through the skin. The generation and delivery of low frequency ultrasound over a period of time to a specific area has been termed “targeted slow-release ultrasound”. We believe that this delivery method of ultrasound may be comparable to that of slow release medication in the pharmaceutical industry. This “targeted slow-release” capability is intended to allow for more frequent targeting of the intended treatment area and thus may result in a more effective therapeutic response.
Our Products
WoundShield and NanoVibronix NPWT
WoundShield
Our WoundShield product is intended to treat acute and chronic wounds with a disposable treatment patch that delivers localized therapeutic low frequency ultrasound. The WoundShield patch has two configurations: one that is placed adjacent to the wound and another, called the instillation patch, that is placed on the wound to enable instillation through sonophoresis, a process that increases the absorption of semisolid topical compounds, including medications, into the skin. Based on studies conducted by BIO-EC Microbiology Laboratory and Rosenblum, we believe that our WoundShield product possesses significant potential for the treatment of, among other things, diabetic foot ulcers and burns (Gasser P, Study Report delivered by BIO-EC Microbiology Laboratory, Dec 2007, which we ordered, paid for, and provided devices for; Rosenblum J, “Surface Acoustic Wave Patch Diathermy Generates Healing In Hard To Heal Wounds,” European Wound Management Association 2011, for which we supplied devices but had no further involvement).
Picture of WoundShield Driver and Instillation Patch
| 36 |
WoundShield delivers surface acoustic waves to the location of the wound. Surface acoustic waves move laterally across the surface of the wound, which enables the transfer of the acoustic energy of the waves along the entire wound surface in a continuous and consistent mode, providing access to the waves’ benefits for a longer treatment period than conventional ultrasound without the need for supervision or a treatment session by a clinician.
WoundShield has been found to have a positive effect on the epithelialization (healing by the growth of epithelial cells) of diabetic wounds, as well as on the stimulation of the precursors of dermal and epidermal (skin) growth. As such, it is a useful adjunct to wound care by increasing dermal and epidermal growth, including glycosaminoglycans, or GAGs (which bind to extracellular proteins like collagen, fibronectin, laminin, etc. and retain considerable amounts of water, thus preserving the skin structure) as well as the amount of collagen (a protein that helps skin heal) and decreasing the number of cells in mitosis (a type of cell division) (Gasser P, Study Report delivered by BIO-EC Microbiology Laboratory, Dec 2007, which we ordered, paid for, and provided devices for; Rosenblum J, “Surface Acoustic Wave Patch Diathermy Generates Healing In Hard To Heal Wounds,” European Wound Management Association 2011, for which we supplied devices but had no further involvement). In addition, the WoundShield instillation patch allows for administration of therapeutic agents into the wound area through a sonophoresis effect.
Many key processes in wound healing are dependent upon an adequate supply of oxygen. Diabetic foot ulcers are particularly in need of an adequate oxygen supply because the disease often results from poor perfusion (blood flow) and decreased oxygen tension. Oxygen is also important for the immune system to ensure bacterial killing, synthesis of collagen, fibroblast proliferation (fibroblasts are a type of cell that play a critical role in wound healing), oxidative (taking place in the presence of oxygen) pathways for adenosine triphosphate, or ATP, formation (ATP transports chemical energy within cells for metabolism) and the nitric oxide dependent signaling pathways. It is generally believed that a lack of available oxygen is a basic contributing factor in the perpetuation of these wounds. Recently, wound healing experts have developed a technique of perfusing ischemic wounds (which occur when blood flow is blocked) with hyper-oxygenated saline, while the wound is being treated with ultrasound, also known as sonication. This localized oxygenation therapy has many advantages over the use of hyperbaric chambers (large chambers in which the oxygen pressure is above normal), a common method for delivering oxygen to wounds, as it is more cost-effective, can be done at the patient’s bedside and can be administered more frequently. The WoundShield instillation patch was tested as a potential ultrasound technology for this localized oxygen therapy and its performance exceeded the performance of the other ultrasound technologies tested. In one study (Morykwas M, “Oxygen Therapy with Surface Acoustic Waveform Sonication,” European Wound Management Association 2011; we supplied devices for this study, but had no further involvement with it), oxygen sensors were placed in the wound bed to directly measure partial pressure of oxygen in an ischemic wound bed on a pig. The wound was perfused with hyperbaric oxygen and sonicated using the WoundShield instillation patch. With surface acoustic wave ultrasound technology, tissue oxygen levels (partial pressure of oxygen in the blood, or PaO2) were raised from a range of 20 mmHg (millimeters of mercury) to 60 mmHg in peripheral (periwound) areas, a 3 centimeter distance away from the transducer, and from 40 mmHg to greater than 100 mmHg in the central wound bed lying below the WoundShield instillation patch (see table below). The results of this study illustrated that the WoundShield instillation patch allowed oxygen to directly enter into the wound. The direct entry of the oxygen increased the amount of oxygen reaching the wound, which has been shown to advance the healing process. In addition, we believe that WoundShield’s small size, lower cost and ease of use makes localized oxygen treatment commercially viable.

In 2012, results were published of a human feasibility trial for the WoundShield instillation patch that was performed at Duke University in North Carolina. Seven patients were treated with the WoundShield instillation patch for their wounds and average tissue oxygen levels (PaO2) increased by an average of 58% over baseline (Covington S, “Ultrasound-Mediated Oxygen Delivery to Lower Extremity Wounds,” Wounds 2012; 24(8)). We supplied devices for this trial, but had no further involvement with it. Based upon the results of this trial, we are planning a series of clinical trials with an end point claim that our WoundShield product enhances perfusion in chronic wounds.
| 37 |
NanoVibronix NPWT
NanoVibronix NPWT is a small, lightweight pump that provides for enhanced patient mobility through negative pressure wound therapy. Negative pressure wound therapy is a topical treatment intended to promote healing in acute and chronic wounds affected by conditions including diabetes, arterial insufficiency (a lack of enough blood flow through the arteries) and venous insufficiency (impaired blood flow through the veins). Negative pressure wound therapy promotes wound healing by delivering controlled and regulated negative pressure (a vacuum) to the wound bed. This distributed negative pressure helps draw wound edges together, removes infectious materials and actively promotes granulation at the cellular level. The vacuum system of NanoVibronix NPWT provides drainage for open cavity wounds, which promotes healing in acute or chronic wounds and severe burns, and allows for infected wound exudate to be disposed of safely without cross contamination. Negative pressure wound therapy is an accepted standard of care for wound treatment and carries favorable reimbursement coding.

Picture of NanoVibronix NPWT
Integrated WoundShield and NanoVibronix NPWT Product
While we are currently developing and marketing WoundShield and NanoVibronix NPWT as separate products, we intend to develop and market a new product that integrates the two. We anticipate that the integration of the WoundShield ultrasound technology and the NanoVibronix NPWT platform will create a product that provides the benefits of both negative pressure wound therapy and ultrasound therapy. We believe that this new device will be very attractive due its ability to deliver multiple methods of wound therapy in one device. This product, however, will require separate approval by the U.S. Food and Drug Administration. To secure early approval for this product, we intend to design a clinical trial that is similar to the clinical trials being designed for WoundShield that focus on enhancing perfusion in chronic wounds. We anticipate that the integrated WoundShield and NanoVibronix NPWT product could be developed and submitted for U.S. Food and Drug Administration approval in the fourth quarter of 2014.
Market for Wound-Healing Devices
The global wound care device market is continuously growing and expected to reach $20.3 billion by 2015 (“Anticipated market in 2015, Wound Care Products: A Global Strategic Business Report,” September 2011). In addition, the negative pressure wound therapy market is expanding, in light of recent approvals in Japan and a growing diabetes patient pool and currently is estimated at approximately $2 billion (“Negative Pressure Wound Therapy Market to 2017,” GBI Research, June 2012).
According to a report entitled “Advances in Wound Closure Technology” by Frost and Sullivan (2005), approximately 25% of all patients with diabetes develop a foot or leg ulceration at some time during the course of their disease. Some 3.5 million individuals globally suffer from diabetes related foot or leg ulcerations each year. In addition, according to the National Hospital Ambulatory Medical Survey (2000-2004), approximately 500,000 patients receive medical treatment annually for burn injuries in the U.S., with the global number estimated at 1 million. There are also policy-based factors that may increase the size of the wound care market. For example, the Commonwealth of Massachusetts announced a policy not to pay for patients who develop Grade 3 or 4 pressure ulcers acquired in a healthcare facility. We anticipate that these types of decisions will be made on a more widespread basis, which may create a large market opportunity for wound care products, including WoundShield. Furthermore, in 2009, the Centers for Medicare and Medicaid Services announced that they would stop reimbursements for treatment of certain complications that they believed were preventable with proper care. One such complication was surgical site infections after certain elective procedures, including some orthopedic surgeries and bariatric surgery. We believe that such developments incentivize medical care providers to invest in reducing the risk of infection through the use of wound care products, including WoundShield and our planned NanoVibronix NPWT product with WoundShield technology.
| 38 |
Competition for WoundShield and NanoVibronix NPWT
The market for advanced wound care includes a large number of competitors, such as Kinetic Concepts, Inc., or KCI, Smith and Nephew plc and Convatec Inc., all of whom market wound-healing medical devices. Due to their size, in general these companies may have significant advantages over us. These competitors have their own distribution networks for their products, which gives them an advantage over us in reaching potential customers. In addition, they are vertically-integrated, which may allow them to maximize efficiencies that we cannot achieve with our third-party suppliers and distributors. Finally, because of their significantly greater resources, they could potentially choose to focus on research and development of technology similar to ours, more than we are able to. In general, we believe that these competitors have, and will continue to have, substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and sales, distribution and personnel resources than we do. However, we believe that our products differentiate us from these competitors, and we will be competitive on the basis of our advantageous technology.
At present, ultrasound treatment for wounds is limited only to wound debridement (removal of damaged tissue or foreign objects from a wound) and such products are marketed by Misonix Inc., which produces SonicOne products, and Celleration Inc., which produces the MIST Therapy System. Due to their size, in general these companies may have the same advantages over us discussed with respect to our competitors in the paragpraph above. However, both of these ultrasound devices are indicated for use only in medical clinics and require an operator to deliver their treatment, thus limiting their use and application. The MIST Therapy System is a non-contact ultrasound device that delivers ultrasound through a mist that is applied directly on the wound.
We believe that these therapies are less advantageous than WoundShield because they require an operator to deliver the treatment and the removal of bandages to target the wound bed. In contrast, the WoundShield patch sits on normal skin bordering the open wound and no manipulation of the wound bandage is required. Moreover, WoundShield can be self-administered, without an operator, in both clinics and home settings. We also believe that WoundShield will be able to provide superior wound care therapy at a lower price than the existing products being used by medical practitioners. As such, we believe that facilities that are reimbursed based upon diagnosis-related groups will be more inclined to adopt WoundShield because it will provide the same therapeutic results at a significantly lower cost than traditional ultrasound therapies.
The most common method of oxygen administration for wound healing is hyperbaric oxygen therapy, especially to treat specific ulcerations in diabetic patients. Hyperbaric oxygen therapy has been shown to increase vascular endothelial growth factor expression, which measures the creation of new blood vessels (Fok TC, at el, "Hyperbaric oxygen results in increased vascular endothelial growth factor (VEGF) protein expression in rabbit calvarial critical-sized defects", Schulich School of Medicine and Dentistry, University of Western Ontario, Canada). The activation of endothelial cells by VEGF sets in motion a series of steps toward the creation of new blood vessels (J Lewis et al, National Cancer Institute, Understanding Cancer and Related Topics, Understanding Angiogenesis). We believe that the WoundShield instillation patch, which can be used as an oxygen instillation system, will be complementary to, or in some cases an alternative, to the use of hyperbaric chamber therapy. This complementary treatment option will allow the treating physician greater therapeutic versatility in treating wounds. For a certain populace of patients, we believe that the WoundShield instillation patch could provide physicians with an alternative to hyperbaric oxygen therapy because it provides the same benefits as hyperbaric oxygen therapy at a lower cost to the patient. There are a number of competitors in the hyperbaric chamber therapy market, including over twelve companies in the U.S. Due to their size, in general these companies may have the same advantages over us discussed with respect to our competitors in the first paragpraph of this section. However, we believe that the WoundShield instillation patch possesses certain advantages over the existing hyperbaric chamber therapy, including lower cost and greater ease of use. In addition, we do not believe that the WoundShield instillation patch will necessarily compete with hyperbaric chamber therapy, but rather will often complement such therapy.
While we believe that WoundShield is well positioned to capture a share of the wound care market, WoundShield may be unable to achieve its anticipated place in the wound care market due to a number of factors, including, but not limited to, an inability to obtain the approval of the U.S. Food and Drug Administration, its failure to treat wounds for which it is indicated and its failure to be adopted by health care practitioners and facilities or patients because of its status as a new product in a market that relies on patient-focused initiative to treat wounds.
In the negative pressure wound therapy market, in 2010, Kinetic Concepts, Inc. possessed a 74% global market share. Smith & Nephew plc was the only major company at that time to compete with Kinetic Concepts, Inc. However, due to the expiration of Kinetic Concepts, Inc.’s patents for negative pressure technology, additional companies such as Medela, Talley Group Limited and Mölnlycke Health Care have entered this market and now offer competing products in the U.S. and abroad. It is predicted that Kinetic Concepts, Inc.’s global market share will decline as more companies enter the negative pressure market (“Negative Pressure Wound Therapy Market to 2017,” GBI Research, June 2012). While the current negative pressure wound therapy market contains both established companies and a growing number of smaller competitors, we believe NanoVibronix NPWT will be able to establish itself in the market due to its competitive pricing and the fact that all of the product’s parts that come in contact with wound exudates are disposable. However, as more negative pressure wound therapy pumps are marketed, it is possible that NanoVibronix NPWT will be unable to compete against more competitively priced alternatives.
| 39 |
In addition, we believe that there currently is no product in the negative pressure wound therapy market similar to the anticipated integrated WoundShield and NanoVibronix NPWT product, which we believe should be able to provide both negative pressure therapy and the benefits of ultrasound therapy. However, we note that because this integrated product is still in its early stages, its anticipated success is dependent upon a number of factors, including WoundShield obtaining U.S. Food and Drug Administration approval and the ability for the integrated product to be commercialized prior to the commercialization of any similar products by our competitors.
Regulatory Strategy
For a general discussion of the U.S. Food and Drug Administration approval process with respect to our products, and regulation of our products in general, see “–Government Regulation” below.
Our general regulatory strategy for our WoundShield products is focused on seeking U.S. Food and Drug Administration approval for our products for a variety of indications. We received 510(k) clearance from the U.S. Food and Drug Administration to market NanoVibronix NPWT in the U.S. in August 2012. Such clearance is for patients who would benefit from a suction device (negative pressure to help promote wound healing by removing fluids including irrigation and body fluids, wound exudates and infectious materials). Examples of appropriate wound types include diabetic/neuropathic ulcers, pressure ulcers, chronic wounds, acute wounds, dehisced wounds, partial-burns, and flaps and grafts.
WoundShield obtained CE Mark approval in November 2012 for use in wound healing. Following preliminary clinical studies that demonstrated WoundShield’s ability to enhance blood perfusion, we plan to design a clinical trial for WoundShield with an end point of enhanced perfusion in chronic wounds. We believe that this trial will take approximately six months. We are finalizing the protocol for this study, which we anticipate will commence before the end of the year. We intend to coordinate this trial with the Centers for Medicare and Medicaid Services, private insurers and the U.S. Food and Drug Administration to insure that the data generated will be adequate to obtain U.S. Food and Drug Administration approval and reimbursement when used in the outpatient setting. We believe that seeking U.S. Food and Drug Administration approval for an indication limited to enhancing perfusion in chronic wounds will require less complicated clinical trials than if we were to seek approval for other indications, such as wound healing, which in turn will shorten the time necessary to commercialize WoundShield.
Following U.S. Food and Drug Administration approval for WoundShield, and once the product is developed, we intend to submit a second U.S. Food and Drug Administration application for the integrated WoundShield and NanoVibronix NPWT product. Similar to our clinical trials for WoundShield, we intend to structure these trials with an end point claim of enhanced perfusion in chronic wounds. We believe that the approval process for the integrated product should be the shorter 510(k) clearance, because both components will already have U.S. Food and Drug Administration approval.
We also intend to begin clinical studies that compare localized surface acoustic wave-enhanced oxygen therapy for wounds versus either the current standard of care for such wounds or treatment with the hyperbaric chamber for ischemic wounds. For these studies, we intend to coordinate with the U.S. Food and Drug Administration to insure that the data generated will be adequate to obtain U.S. Food and Drug Administration approval for the WoundShield instillation patch and lead to reimbursement of this product. Once we have obtained U.S. Food and Drug Administration approval for certain limited indications for our WoundShield products, we intend to seek approval for a wider range of indications, including wound healing.
Sales and Marketing
In January 2013, we entered into an exclusive distribution agreement for the distribution of NanoVibronix NPWT in the U.S. The distributor has begun marketing NanoVibronix NPWT through participating in product presentations at conferences related to wound therapy and targeting distributors who are selling or renting negative pressure wound therapy equipment to clinics and hospitals.
We have sold limited numbers of our WoundShield products through our website and our distributor in Italy. Following completion of this offering, we intend to aggressively market WoundShield in Europe and pursue the necessary approvals to commence marketing in the U.S.
Clinical Trials
Negative pressure wound treatment is not a novel technology and already has a reimbursement code. Therefore, clinical trials with respect to NanoVibronix NPWT are not needed.
| 40 |
With respect to WoundShield, to date, we have conducted the following evaluation studies:
| Purpose | Doctor/Location | Time, subjects |
Objectives | Results | ||||
|
Clinical evaluation
Physician initiated |
Dr. J. Rosenblum, Shaare Zedek Medical Center |
2008
8 patients |
To evaluate novel technology on wound healing in diabetic foot ulcers.
|
Therapy showed significant changes in wound, wound size was reduced, patients felt less pain, necrotic tissue was less adhesive, necrotic tissue decreased in size. | ||||
|
Clinical evaluation
Physician initiated |
Dr. J. Rosenblum, Shaare Zedek Medical Center |
2010
8 patients |
To evaluate novel technology on wound healing in diabetic foot ulcers.
|
The device had a positive effect on both epitheliazation of diabetic wounds as and stimulating the precursors of dermal and epidermal growth. | ||||
|
Clinical evaluation
Physician initiated |
Dr. S. Covington |
2010
7 patients |
The study aimed to determine if hyper oxygenated saline delivered by surface acoustic waves improves tissue oxygenation in lower extremity wounds.
|
Surface acoustic wave technology in conjunction with oxygenated saline can increase interstitial oxygen in wound bed. This trial to validate proof of concept was put on hold due to financial constraints. |
If we are able to obtain sufficient funding, we anticipate conducting the following clinical trials:
| Trial | Place | Start Date/Timing | Objectives | |||
Woundshield ultrasound patch enhances perfusion of blood 30 patient trial |
University of North Carolina (Rex Hospital) | February 2014
6-month duration |
Safety and efficacy of Woundshield in enhancing blood flow and oxygenation of wounds. | |||
Woundshield ultrasound patch enhances wound healing 200 patient trial |
University of North Carolina (Rex Hospital) | August 2014
12-month duration |
Safety and efficacy of WoundShield in wound healing. | |||
| Woundshield ultrasound patch combined with NanoVibronix NPWT 200 patient trial | University of North Carolina (Rex Hospital) | February 2015
12-month duration |
Safety and efficacy of combination product in enhancing instillation therapy in NanoVibronix NPWT. |
PainShield
PainShield is an ultrasound diathermy device (diathermy is the production of heat in a part of the body by high-frequency electric currents), consisting of a driver unit and a disposable patch, which contains our proprietary therapeutic transducer. It delivers a localized ultrasound effect to treat pain and induce soft tissue healing in a targeted area, while keeping the level of ultrasound energy at a safe and consistent level of 0.4 watts. We believe that PainShield is the smallest and most portable therapeutic ultrasound device on the market and the only product in which the ultrasound transducer is integrated in a therapeutic disposable application patch.
The existing ultrasound therapy devices being used for pain reduction are primarily large devices used exclusively by clinicians in medical settings. PainShield is able to deliver ultrasound therapy without being located in a health care facility or clinic because it is portable, due to it being lightweight and battery operated. Because it is patch based and easy to apply, PainShield does not require medical personnel to apply ultrasound therapy to the patient. The patient benefits include its ease of application and use, faster recovery time, high compliance, safety and efficacy (Adahan M, et al, “A Sound Solution to Tendonitis: Healing Tendon Tears With a Novel Low-Intensity, Low-Frequency Surface Acoustic Ultrasound Patch,” American Academy of Physical Medicine and Rehabilitation Vol. 2, 685-687, July 2010). PainShield can be used by patients at home or work or in clinical setting and can be used even while the patient is sleeping. Its range of applications includes acute and chronic pain reduction and anti-inflammatory treatment.

Picture of PainShield with Patch
PainShield is used to treat tendon disease and trigeminal neuralgia (a chronic pain condition that affects the trigeminal or 5th cranial nerve, one of the most widely distributed nerves in the head); previously, the therapeutic options for these disorders have been very limited. PainShield has also been used to treat pelvic and abdominal pain. To date, the only treatment options for these conditions are pain medication and surgery.
| 41 |
Market for PainShield
Pain is one of the most common conditions that hinder quality of life of vast populations of patients on a regular basis. Pain-related complaints are the most common reason patients seek treatment from physicians (Prince V, “Pain Management in Patients with Substance-Use Disorders,” Pain Management, PSAP-VII, Chronic Illnesses). According to Bonica’s Management of Pain (2001), a work considered current in the industry based on available industry data, and Landro L, “New Ways to Treat Pain: Tricking the Brain, Blocking the Nerves in Patients When all Else Has Failed,” Wall Street Journal, May 11, 2010, approximately 25% of the U.S. population, 75 million people, suffer from chronic pain. We estimate that approximately 150 million individuals globally suffer from chronic pain. Studies have shown that low-frequency ultrasound treatment has yielded positive results for a variety of indications, including tendon injuries and short-term pain relief (Warden SJ, “A new direction for ultrasound therapy in sports medicine,” Sports Med. 2003; 33 (2):95-107), chronic low back pain (Ansari NN, Ebadi S, Talebian S, Naghdi S, Mazaheri H, Olyaei G, Jalaie SA, “Randomized, single blind placebo controlled clinical trial on the effect of continuous ultrasound on low back pain,” Electromyogr Clin Neurophysiol. 2006 Nov; 46(6):329-36) and sinusitis (Ansari NN, Naghdi S, Farhadi M, Jalaie S, “A preliminary study into the effect of low-intensity pulsed ultrasound on chronic maxillary and frontal sinusitis,” Physiother Theory Pract. 2007 Jul-Aug; 23(4):211-8). We believe that PainShield’s technology, portability and ease of use may result in it becoming an attractive product in the pain management and therapy field.
Competition
There are numerous products and approaches currently utilized to treat chronic pain. The pharmacological approach, which may be the most common, focuses on drug-related treatments. Alternatively, there are a large number of non-pharmacological pain treatment modalities available, such as ultrasound, transcutaneous electrical nerve stimulation, or TENS, laser therapy and pulsed electromagnetic treatment. In addition, there are some technologies and devices in the market that utilize low frequency ultrasound or patch technology. Many patients are initially prescribed anti-pain medication; however, ongoing use of drugs may cause substantial side effects and lead to addiction. Therefore, patients and clinicians have shown great interest in alternative pain therapy using medical devices that do not carry these side effects.
The currently available ultrasound treatments for chronic pain have generally been accepted by the medical community as standard treatment for pain management. However, the traditional ultrasound treatments, such as those manufactured or distributed by Mettler Electronics Corp, Metron USA and Zimmer MedizinSysteme, are stationary devices found only in clinics and other health care facilities that need to be administered to patients by health care professionals. We are aware of three companies that market smaller ultrasound devices capable of certain self-administered use for the treatment of pain: Koalaty Products, Inc., Sun-Rain System Corp. and PhysioTEC. These devices generally function in the same manner, at the same frequency and with the same administration and safety requirements and limitations as traditional, larger ultrasound devices. We are also aware of one product under U.S. Food and Drug Administration review and with CE Mark approval, marketed by ZetrOZ, Inc., that we understand may eliminate certain of these requirements and limitations, namely the requirement to be plugged in, the need for movement around the treated area and the relatively short safe treatment period. However, like traditional ultrasound, we understand that this product does not generate surface acoustic waves as our products do, which means that the treatment area is generally limited to that of the transducer’s diameter, that the use of transmission gel is still required and that the transducer thickness is significantly greater than ours (approximately 1.5cm). In addition, there are other patch-based methods of pain treatment, such as TENS therapy. TENS therapy is generally not supported by widespread clinical evidence of its efficacy. In addition, TENS therapy may be painful and irritating for the patient due to the muscle contractions resulting from the electrical pulses. PainShield combines the efficacy of ultrasound treatment for pain with the ease of use and portability of a patch-based system. PainShield also may be self-administered by the patient, including while the patient is sleeping. However, if we are unable to obtain widespread insurance coverage and reimbursement for PainShield, its acceptance as a pain management treatment would likely be hindered, as patients may be reluctant to pay for the product out-of-pocket.
Regulatory Strategy
PainShield received 510(k) clearance from the U.S. Food and Drug Administration in October 2008 for treatment of selected medical conditions such as relief of pain, muscle spasms and joint contraction. PainShield received CE Mark approval in July 2008 and was also approved for sale by the Israeli Ministry of Health in 2010. We have a Canadian medical device license for PainShield, a certificate allowing us to sell PainShield in Australia, and we are able to sell PainShield in India and Ecuador based on our CE Mark. In addition, our distributors in Korea and Chile have applied for approval to sell PainShield, which approvals we believe we will receive within a few months.
In the U.S., PainShield falls under the diathermy classification for the treatment of pain for initial reimbursement purposes. The permitted reimbursement codes can be used in the outpatient supervised medical setting. We intend to coordinate with the Centers for Medicare and Medicaid Services and private insurers so that reimbursement can be extended to cover the administration of PainShield outside of health care facilities and clinics. In addition, we intend to conduct clinical trials in order to effectively market PainShield for a larger range of indications.
| 42 |
Sales and Marketing
PainShield was introduced in 2009 as a treatment for pain and other clinical problems, such as tendonitis, sports injuries, pelvic pain and neurologic pain and we have sold approximately 1,000 units and 6,000 treatment patches since its introduction. We have entered into distribution agreements in North America, Europe, Asia, the Middle East and Australia and New Zealand for the distribution of PainShield. We intend to seek additional distribution opportunities in Europe, East Asia and South America. In addition, we sell PainShield directly to patients through our website.
Clinical Trials
To date, we have conducted the clinical trials set forth below:
| Purpose | Doctor/Location |
Time, subjects |
Objectives | Results | ||||
|
A sound solution for Trigerminal Neuralgia
Physician initiated |
Dr. Ch. Adahan Shiba medical Center |
2009
15 patients |
· Reduction in pain · Reduction in disability · Improvement of function and quality of life · Accelerating of healing |
73% of the subjects experienced complete or near complete relief. | ||||
|
Randomized control trial examining the efficacy of low intensity low frequency Surface Acoustic wave ultrasound in trigerminal neuralgia pain
For Ph.D., Funded by Israeli Ministry of Health |
Dr. M. Zwecker
Chaim Sheba Medical Center, Tel Hashomer, Israel |
2012-2012
19 patients |
· Reduction in pain · Reduction in disability · Improvement of function and quality of life · Accelerating of healing |
In conclusion this study supports the hypothesis that the application of Low Intensity Low Frequency Surface Acoustic Wave Ultrasound (LILF/SAW) may be associated with a clinically significant reduction of pain severity among patients suffering from trigerminal neuralgia disease. | ||||
|
Treating Rutgers university athletic injuries with bandaid sized ultrasound unit Painshield
|
R. Monaco, G. Sherman, Rutgers University Athletic, Rutgers, New Jersey |
2011
40 patients |
· To assess the pain, functional capacity and discomfort of the subject · To assess the subject’s quality of life · To assess the injury status · To assess the efficacy of the treatment · To assess compliance factors
|
Preliminary results: Active group: 70% had improvement, 30% no change Sham group: 70% no change, 30% had improvement This is a really good indication of the effectiveness of the device.
Lack of funding for statistical analysis has stopped this trial prior to fulfillment. | ||||
| Reduction of chronic abdominal and pelvic pain, urological and GI symptoms using wearable device delivering low frequency ultrasound |
D. Wiseman, Synechion Institute for Pelvic Pain |
2011
19 patients |
· To assess the efficacy of Painshield for pelvic and related pain | Improvement in pain related symptoms noted for all symptoms. |
| 43 |
If we are able to obtain sufficient funding, we anticipate conducting the following clinical trials:
| Trial | Place | Start Date/Timing | Objectives | |||
| PainShield for Trigeminal Neuralgia 80 patient trial | To be determined | To be determined | Safety and Efficacy of PainShield in Trigeminal Neuralgia | |||
|
PainShield for Pelvic Pain 200 patient trial |
To be determined | To be determined | Safety and Efficiacy of PainShield in Chronic Pelvic Pain |
UroShield
UroShield is intended to prevent biofilm, increase antibiotic efficacy in the catheter lumen and decrease pain and discomfort associated with urinary catheter use. It is designed to be used with any type of indwelling urinary catheter regardless of the material or coating. We believe UroShield is the first medical device on the market that attempts to simultaneously address all of the aforementioned catheter-related issues. UroShield is similar in design to WoundShield and PainShield, in that it uses a driver unit that produces low frequency, low intensity ultrasound. The driver unit connects to a disposable transducer that is clipped onto the external portion of the catheter to deliver ultrasound therapy to all catheter surfaces as well as the tissue surrounding the catheter.

Picture of UroShield with actuator
The UroShield system has the following advantageous effects:
| · | Prevention or Reduction of Biofilm. The low frequency ultrasound generated by UroShield has been shown to decrease adherence of bacteria to catheter surfaces, thereby reducing biofilm. Biofilm is the complex matrix required for bacteria to grow and cause infection. See the discussion of our Heidelberg 1 trial below. |
| · | Decreased Catheter Associated Pain and Discomfort. We believe that UroShield creates an acoustic envelope on the surfaces of the catheter, which decreases friction and tissue trauma, pain and discomfort caused by the catheter. In addition, the tissue in contact with the catheter remains healthier and less traumatized as a result of the application of low frequency and low intensity ultrasound (Tenke P, “The effectiveness of acoustic energy induced by UroShield in the prevention of bacteriuria and the reduction of patients’ complaints related to long-term indwelling urinary catheters,” 26th Annual Congress of the European Association of Urology (EAU) Congress, Vienna, March 2011; we supplied devices for this study and paid for electron microscopy analysis, but had no further involvement with it). |
| · | Acoustically Augmented Antibiotic Therapy. Antibiotic resistance in biofilm bacteria is a well-known phenomenon. Although it has been known that ultrasound can increase antibiotic efficacy in in-vitro models, we do not believe that there has been a practical ultrasound-based medical device that was able to augment antibiotic efficacy in the clinical setting. UroShield technology has been shown to eradicate biofilm-residing bacteria by greater than 85% when applied simultaneously with an antibiotic in three clinically relevant species, escherichia coli, staphylococcus epidermidis and pseudomonas aeruginosa (Banin E, et al., “Surface acoustic waves increase the susceptibility of Pseudomonas aeruginosa biofilms to antibiotic treatment,” Biofouling, August 2011; we supplied devices for this study, but had no further involvement with it). |
| · | Preservation of the Patency of Catheters. We believe that low frequency ultrasound applied to catheters will add an anti-clogging effect and will preserve patency of catheters. This effect is achieved by ultrasound waves creating an acoustic layer on the inner lumen of the urinary catheter, thereby preventing adherence of biological material and bilofilm formation. We believe that this anti-clogging benefit will help prevent local infection and sepsis secondary to catheter obstruction. |
| 44 |
UroShield has undergone a number of clinical trials. The Heidelberg 1 trial, which we sponsored, was a 22 patient randomized, double blind, sham-controlled, independent trial that tested UroShield’s safety and ability to prevent biofilm in patients with an indwelling Foley catheter. The trial demonstrated that UroShield prevented biofilm in all patients with the active device as compared to biofilm being found in seven of eleven of the control patients. In addition, there was a marked decrease in pain, discomfort and spasm in the active UroShield patients, as evidenced by a statistically significant decrease in the requirement for the medications required to treat urinary catheter associated pain and discomfort (Ikinger U, “Biofilm Prevention by Surface Acoustic Nanowaves: A New Approach to Urinary Tract Infections?,” 25th World Congress of Endourology and SWL, Cancun, Mexico, October 2007).
In a subsequent physician-sponsored trial known as Heidelberg 2, 40 patients who underwent radical prostatectomies were divided into two groups, with the active group receiving one intra-operative dose of antibiotics and UroShield and the control group receiving one intra-operative dose of antibiotics and then five subsequent doses over three days. At the end of the trial, the control group had four cases of bacteruria, as compared to one in the active group. In a third trial, a physician-sponsored open label trial, ten patients who received emergency placement of a urinary catheter due to acute obstruction were given a UroShield device and followed with regard to their pain, discomfort, spasm and overall well-being. Within 24 hours, all patients showed improvement and increased toleration of the catheter (Zillich S., Ikinger U, “Biofilmprävention durch akustische Nanowellen: Ein neuer Aspekt bei katheterassoziierten Harnwegsinfektionen?,” Gesellschaft für Urologie, Heilbronn, Germany, May 2008). We supplied devices for this trial, but had no further involvement with it.
Market for UroShield
According to Urological Catheters – A Global Strategic Business Report, Global Industry Analysis Inc. 2003, over 55 million indwelling urinary catheters are sold annually worldwide. In addition, as of October 1, 2008, Medicare stopped authorizing its payment to hospitals in which patients have developed a catheter-associated urinary tract infection that was not present on admission. This provides hospitals in the U.S. with a substantial financial incentive to reduce the occurrence of such infections through the use of products such as UroShield, which help prevent infections hospitals would otherwise have to treat without reimbursement. In addition, it has been noted that the Centers for Medicare & Medicaid Services may fine hospitals in the future when their patients develop catheter acquired urinary tract infection, which will likely increase the incentive of hospitals to invest in technologies that may prevent this complication (Brown J, et al. “Never Events: Not Every Hospital-Acquired Infection Is Preventable, Clinical Infectious Diseases, 2009, 49 (5)).
Competition
Several types of products have been introduced to address the growing problem of catheter-acquired infection and biofilm formation on catheter surfaces. Manufacturers offer antibiotic-coated and antiseptic-impregnated catheters. In addition, manufacturers have produced silver-coated catheters, which have been shown in small studies to delay bacteruria for about two to four days. However, larger studies did not corroborate this result; on the contrary, silver hydrogel was associated with overgrowth of gram positive bacteria in the urine (Riley DK, Classen DC, “A large randomized clinical trial of a silver-impregnated urinary catheter: lack of efficacy and staphylococcal superinfection,” Am. J. Med. 1995 April; 98(4):349-56).
UroShield has been designed to be added to any type of catheter, including Foley catheters and silver-coated catheters, to improve a catheter’s infection prevention performance. UroShield is not intended to replace any existing products or technologies, but instead is intended to assist these existing products or technologies in preventing catheter-acquired urinary injury and catheter associated complications. UroShield may be unable to achieve its anticipated catheter market share due to a number of factors, including, but not limited to, an inability to obtain approval of the U.S. Food and Drug Administration and failure to be adopted by health care practitioners and facilities because of its status as a new product in the market, without an established niche.
Regulatory Strategy
UroShield received CE Mark approval in September 2007 and was also approved for sale by the Israeli Ministry of Health in 2008. We have a Canadian medical device license for UroShield and we are able to sell UroShield in India and Ecuador based on our CE Mark. In addition, our distributors in Korea and Chile have applied for approval to sell UroShield, which approvals we believe we will receive within a few months.
In the European Union, UroShield has been marketed for the prevention of biofilm, decreased pain and discomfort associated with urinary catheters and increased antibiotic efficacy. In the U.S., we intend to seek clearance from the U.S. Food and Drug Administration through the de novo classification process for UroShield. We submitted our application for 510(k) approval on January 3, 2011. On May 21, 2012, we received a response from the U.S. Food and Drug Administration proposing that the approval go through the de novo route, which will require clinical trials with proposed study protocols to be pre-cleared by the U.S. Food and Drug Administration. We are currently seeking a strategic partner that is active in the urology market to help fund the clinical trials for UroShield to support our U.S. Food and Drug Administration application. We have not made any further submissions to the U.S. Food and Drug Administration related to UroShield.
Sales and Marketing
We are currently seeking a strategic partner that is active in the urology market and would be interested in integrating UroShield into its range of products. We have sold limited numbers of our UroShield products through our website and our distributor in Italy.
| 45 |
Clinical Trials
To date, we have conducted the clinical trials set forth below:
| Purpose | Doctor/Location |
Time, subjects |
Objectives | Results | ||||
|
To assess the safety of the UroShield
Double Blind, Comparative, Randomized Study for the Safety Evaluation of the UroShield System (HD1) |
Dr. U. Ikinger, Salem Academic Hospital, University of Heidelberg, Germany |
2005-2006
40 patients |
To demonstrate that the use of the UroShield is safe and that the device is well tolerated by the patients and user friendly to the medical staff. Efficacy objectives were to demonstrate that the UroShield helps in prevention of biofilm formation in comparison with the urinary catheter alone, as well as bacteriuria. |
UroShield was both safe and well tolerated. UroShield proved markedly efficacious in prevention of biofilm. Subjects required significantly less medications than the control group for catheter related pain and discomfort. | ||||
|
Double Blind, Comparative, Randomized Study for the Safety Evaluation of the UroShield System (HD2)
Physician initiated |
Dr. U. Ikinger, Salem Academic Hospital, University of Heidelberg, Germany |
2007
40 patients |
To demonstrate that the use of the UroShield is safe and helps in prevention of biofilm formation and UTI in comparison with the urinary catheter alone, as well as decrease antibiotic use. | In this trial, only 1/20 patients in UroShield device (no antibiotics) group developed urinary tract infection compared to 4/20 patients within control group treated with the antibiotic prophylaxis alone. | ||||
|
The Effect of UroShield on Pain and Discomfort in Patients Released from the Emergency Room with Urinary Catheter Due to Urine Incontinence
Physician initiated |
Shaare Zedek Medical Center Jerusalem, Israel. |
2007
10 patients |
The study aimed to assess the effectiveness of the UroShield in reducing pain and discomfort levels and improve the well-being of the subjects. Efficacy objectives included reduction of pain, spasm, burning and itching sensation levels of the subjects. |
The results demonstrated a reduction in pain, itching, burning and spasm levels. Additionally, the well-being of the subjects showed a significant increase.
| ||||
|
The Use of the UroShield Device in Patients with Indwelling Urinary Catheters
Open labeled, comparative, randomized study |
Dr. Shenfeld Shaare Zedek Medical Center Jerusalem, Israel. |
2007-2009
40 patients |
Patient complaints related to catheter regarding pain according to VAS scale and discomfort according to 0-10 scale Presence of Clinically Significant UTI Presence of Bacteriuria Presence of Biofilm Use of medication
|
UroShield device was effective in reducing postoperative catheter related pain discomfort and bladder spasms. There was also a notable trend towards reduction of bacteriuria. | ||||
|
Evaluation of the UroShield in urinary and nephrostomies to reduce bacteruria
Physician initiated |
Prof. P.Tenke, Hungary |
2010-2011
26 patients
|
· Pain, disability and QOL · Catheter patency · Bacteriuria / UTI · Hospitalization period · Analgesics and Antibiotics intake |
Showed reduction in pain and significant decrease in bacteriuria rate. |
If we are able to locate a strategic partner or otherwise obtain sufficient funding, we anticipate conducting the following clinical trial:
| Trial | Place | Start Date/Timing | Objectives | |||
| UroShield FDA trial 80 patient trial | To be determined | To be determined | Safety and efficacy of UroShield in urinary catheter related pain and infection |
| 46 |
Third Party Reimbursement
We anticipate that sales volumes and prices of the products we commercialize will depend in large part on the availability of coverage and reimbursement from third party payers. Third party payers include governmental programs such as Medicare and Medicaid, private insurance plans and workers’ compensation plans. These third party payers may deny coverage and reimbursement for a product or therapy, in whole or in part, if they determine that the product or therapy was not medically appropriate or necessary. The third party payers also may place limitations on the types of physicians or clinicians that can perform specific types of procedures. In addition, third party payers are increasingly challenging the prices charged for medical products and services. Some third party payers must also pre-approve coverage for new or innovative devices or therapies before they will reimburse healthcare providers who use the products or therapies. Even though a new product may have been approved or cleared by the U.S. Food and Drug Administration for commercial distribution, we may find limited demand for the device until adequate reimbursement has been obtained from governmental and private third party payers.
In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific product lines and procedures. There can be no assurance that procedures using our products will be considered medically reasonable and necessary for a specific indication, that our products will be considered cost-effective by third party payers, that an adequate level of reimbursement will be available or that the third party payers’ reimbursement policies will not adversely affect our ability to sell our products profitably.
In the U.S., some insured individuals are receiving their medical care through managed care programs, which monitor and often require pre-approval of the services that a member will receive. Some managed care programs are paying their providers on a per capita basis, which puts the providers at financial risk for the services provided to their patients by paying these providers a predetermined payment per member per month, and consequently, may limit the willingness of these providers to use products, including ours.
One of the components in the reimbursement decision by most private insurers and governmental payers, including the Centers for Medicare & Medicaid Services, which administers Medicare, is the assignment of a billing code. Billing codes are used to identify the procedures performed when providers submit claims to third party payers for reimbursement for medical services. They also generally form the basis for payment amounts. We anticipate that our distributors will be responsible for the process for obtaining billing codes for our products.
The initial phase of establishing a professional billing code for a medical service typically includes applying for a Current Procedural Terminology, or CPT, Category III code. This is a tracking code without relative value assigned that allows third party payers to identify and monitor the service as well as establish value if deemed medically necessary. The process includes CPT application submission, clinical discussion with Medical Professional Society CPT advisors as well as American Medical Association CPT Editorial Panel review. A new CPT Category III code will be assigned if the American Medical Association CPT Editorial Panel committee deems it meets the applicable criteria and is appropriate.
| 47 |
The secondary phase in the CPT billing code process includes the establishment of a permanent CPT Category I code in which relative value is analyzed and established by the American Medical Association. The approval of this code is based on, among other criteria, widespread usage and established clinical efficacy of the medical service.
We believe that the overall escalating costs of medical products and services has led to, and will continue to lead to, increased pressures on the healthcare industry to reduce the costs of products and services. In addition, recent healthcare reform measures, as well as legislative and regulatory initiatives at the federal and state levels, create significant additional uncertainties. There can be no assurance that third party coverage and reimbursement will be available or adequate, or that future legislation, regulation, or reimbursement policies of third party payers will not adversely affect the demand for our products or our ability to sell these products on a profitable basis. The unavailability or inadequacy of third party payer coverage or reimbursement would have a material adverse effect on our business, operating results and financial condition.
The Diagnosis Related Group System, or DRG, is the system of reimbursement that is used in the United States for hospitalized patients as well as patients who are cared for in skilled nursing facilities and long term care facilities. These facilities are not subject to the same reimbursement codes as described above. In the DRG system, each patient admitted to the hospital or facility is assigned a code based on his or her diagnosis. That code is known to be associated with an average hospital stay and the health care facility is reimbursed for the amount of days as defined by the DRG code, regardless of how many days the patient is in the facility. This system gives a strong incentive for these health care facilities to deliver efficient care and to complete the needed treatment as quickly as possible. For example, if the patient has a wound that requires healing before discharge and they succeed in treating the wound in less hospital days than allowed by the DRG code for this diagnosis, the facility will be rewarded by being paid more for more days than the patient was actually in the hospital for. Conversely, if the treatment takes longer, the facility would actually lose income, as they will be paid for the DRG code only. This system serves as a stimulus for these facilities to purchase and utilize devices and technologies that allow more efficient therapy.
PainShield. PainShield is presently reimbursed in the U.S. by many private insurers for use of the ultrasound device in a supervised medical setting and is reimbursed in units of 15 minutes up to an hour a day, 5 hours a week and 20 hours a month. If the device is efficacious in the treatment of the patient’s condition, the treatment period can be extended in some cases for months. Presently, PainShield is typically purchased by a clinic that bills the existing reimbursement codes. PainShield is not reimbursed for therapy in the home setting. Following completion of the offering to which this prospectus relates, we intend to work to obtain reimbursement in the home setting as well as codes that would allow for reimbursement for use of the non-disposable and disposable components of the PainShield device. Our anticipated clinical trials for PainShield would support this effort.
NanoVibronix NPWT. Negative pressure wound therapy is an accepted advanced wound care therapeutic modality and is reimbursable under accepted codes. NanoVibronix NPWT is reimbursable under a code that describes a stationary or portable negative pressure wound therapy electrical pump that provides controlled subatmospheric pressure designed for use with negative pressure wound therapy dressings and canisters to promote wound healing.
WoundShield. We believe that the initial usage of the WoundShield patch will be in the hospital setting. Reimbursement in the hospital setting is governed by the diagnosis-related group system, which does not require specific reimbursement codes. In parallel to introducing the WoundShield device to hospitals, we intend to apply for reimbursement codes for outpatient use. Although obtaining these codes can take two to five years and may require extensive clinical data, we believe that the desirable characteristics of the WoundShield may serve as an incentive to insurance companies to grant these codes more quickly.
| 48 |
UroShield. We expect UroShield to be used in hospital settings and therefore reimbursed under the diagnosis-related group reimbursement system. In addition, we anticipate that UroShield will initially be purchased privately until a reimbursement code is obtained. However, we believe that if we can empirically demonstrate UroShield’s efficacy in preventing recurrent hospital admission in chronic Foley catheter patients and reducing overall per-patient cost, third party payers may accelerate the reimbursement approval process since the device could reduce their overall per-patient cost.
Research and Development Expenses
During the years ended December 31, 2012 and 2011, we spent approximately $572,000 and $623,000 on research and development activities, respectively.
Intellectual Property
Patents
We believe that our patent portfolio provides us with sufficient protection of our patentable intellectual property. We have six patents registered in the U.S. and three filed applications. Granted U.S. Patent No. 7,393,501 (having the following foreign counter-parts: China ZL03818327.7; Israel 165422; Japan 4504183; India 246351; Australia 2003231892; European Union 1511414 B), “Method, apparatus and system for treating biofilms associated with catheters” and granted U.S. Patent No. 7,829,029 (having the following foreign counter-parts: China ZL200780019732.3 and European Union 1998834), “Acoustic add-on device for biofilm prevention in urinary catheter,” both relate to the use of surface acoustic waves to prevent biofilm formation on indwelling catheters. These granted U.S. patents expire on December 19, 2023 and August 28, 2029, respectively. Granted U.S. Patent No. 7,892,191 (having the following foreign counter-parts: Russia 2419395 and Australia 2005331251), “Nanovibration coating process for medical devices using multi vibration modes of thin piezo element” and U.S Patent Application No. 11/710,616, “System and method for SAW treatment of medical devices,” relate to methods of generating surface acoustic waves on medical device surfaces on both indwelling medical devices and implants to prevent biofilm formation. U.S. Patent No. 7,892,191 will expire on December 19, 2023. U.S. Patent Application No. 11/710,615 (having the following foreign counter-parts: China ZL200780014875.5; applications in India, European Union, Canada and Israel), “System and method for surface acoustic waves treatment of skin,” relates to methods of using surface acoustic waves for treatment of skin for the purpose of wound-healing, reducing infection, pain reduction and cosmetic enhancements. U.S Patent Application No. 13/521,060, “Method for friction reduction in medical tubing and applications using this method,” relates to the use of acoustic lubrication (complex vibrations) to reduce friction between indwelling medical devices and vital tissue.
We also license three patents pursuant to a license agreement with Piezo-Top Ltd and PMG Medica Ltd., U.S. Patent No. 6,454,716 B1, “A system and method for detection of fetal heartbeat,” and U.S. Patent No. 6,964,640 B2, “A system and method for detection of motion,” relate to certain technology related to biofilm prevention for medical purposes, including biofilm prevention in indwelling catheters, biofilm prevention in dialysis and respiratory assist devices and control of bacteria in hospital and outpatient environments by biofilm prevention and the killing of bacteriato. These patents expire on May 23, 2020 and January 22, 2023, respectively. U.S. Patent No. 7,431,892 B2, “Apparatus for sterilizing a liquid with focused acoustic standing waves,” relates to our original work introducing multiple modes of power into an ultrasonic transducer for purpose of sterilizing liquids. This patent has been the genesis of the more practical patents described above. This patent expires on July 29, 2024. See “—License Agreements” below.
We believe the granted patents, patent applications and license agreements (described below) collectively cover our existing products to the extent necessary, and may be useful for protecting our future technology developments. With respect to our NanoVibronix NPWT product, negative wound pressure therapy is an established technology and we believe that our ability to use such technology is not dependent upon or limited by the terms or existence of a particular patent or patents. Nonetheless, we are party to a license agreement related to our negative pressure wound therapy technology, described below. We intend to continue patenting new technology as it is developed, and to actively pursue any infringement of any of our patents.
To date, we are not aware of other companies that have patent rights to a system and method for surface acoustic wave treatment.
| 49 |
Trademarks
We believe that our product brand names are an important factor in establishing and maintaining brand recognition. We have the following trademark registrations in the U.S.: NanoVibronix®, WoundShield®, PainShield®, UroShield® and “Curing though prevention”®. Generally, the protection afforded for trademarks is perpetual, if they are renewed on a timely basis, if registered, and continue to be used properly as trademarks.
License Agreements
In October 2003, we entered into a license agreement with Piezo-Top Ltd and PMG Medica Ltd, pursuant to which we were granted an exclusive, worldwide license for the duration of the patent life of U.S. Patent No. 6,454,716 B1, U.S. Patent No. 6,964,640 B2 and U.S. Patent No. 7,431,892 B2 (see “—Patents” above). In exchange for the license, we paid Piezo-Top Ltd and PMG Medica Ltd payments of (i) $5,000 each after the first round of investment in us, (ii) $7,500 each after the second round of investment in us, and (iii) $25,000 each after either the third round of investment, the purchase of at least 40% of our stock or our initial public offering. We have made all three of the required payments under this agreement.
In December 2011, we entered into a license agreement with AC Engineering Ltd. for the exclusive license to manufacture, market, sell, lease and distribute AC Engineering Ltd.’s negative pressure wound therapy technology within the U.S. for a term of two years, commencing from the date of the U.S. Food and Drug Administration approval of the negative pressure wound therapy product, which occurred in August 2012. This technology is the basis of our NanoVibronix NPWT product. The license agreement also granted us a non-exclusive license to use AC Engineering Ltd.’s know-how related to the product and to develop modifications and improvements to the product and to integrate the product into our existing products. The term of the license agreement will be extended automatically for an additional three year non-exclusive term if we sell at least 3,500 units of pumps during the first two years of the license agreement, and the term of the license agreement will be extended automatically thereafter for an additional one year non-exclusive term if we sell at least 1,000 units of pumps during the preceding year. We are obligated to pay AC Engineering Ltd. a royalty payment of 5% of gross revenues from the sale of the pumps and $0.70 per canister. If we have not paid AC Engineering Ltd. aggregate royalty payments of at least $150,000 by the end of the initial two year term, we will have to pay the difference.
Government Regulation
U.S. Food and Drug Administration Regulation
Each of our products must be approved or cleared by the U.S. Food and Drug Administration before it is marketed in the U.S. Before and after approval or clearance in the U.S., our product candidates are subject to extensive regulation by the U.S. Food and Drug Administration under the Federal Food, Drug, and Cosmetic Act and/or the Public Health Service Act, as well as by other regulatory bodies. The U.S. Food and Drug Administration regulations govern, among other things, the development, testing, manufacturing, labeling, safety, storage, record-keeping, market clearance or approval, advertising and promotion, import and export, marketing and sales, and distribution of medical devices and pharmaceutical products. Two of our products, PainShield and NanoVibronix NPWT, have already obtained 510(k) marketing approval by the U.S. Food and Drug Administration.
U.S. Food and Drug Administration Approval or Clearance of Medical Devices
In the U.S., medical devices are subject to varying degrees of regulatory control and are classified in one of three classes depending on the extent of controls the U.S. Food and Drug Administration determines are necessary to reasonably ensure their safety and efficacy:
| · | Class I: general controls, such as labeling and adherence to quality system regulations; |
| · | Class II: special controls, pre-market notification (510(k)), specific controls such as performance standards, patient registries and post-market surveillance and additional controls such as labeling and adherence to quality system regulations; and |
| · | Class III: special controls and approval of a pre-market approval, or PMA, application. |
All our products are classified as Class II medical devices and require U.S. Food and Drug Administration authorization prior to marketing, by means of 510(k) clearance, except for our UroShield product, which we intend to seek clearance from the U.S. Food and Drug Administration through the de novo classification process, described below.
| 50 |
To request marketing authorization by means of a 510(k) clearance, we must submit a pre-market notification demonstrating that the proposed device is substantially equivalent to another legally marketed medical device, has the same intended use, and is as safe and effective as a legally marketed device and does not raise different questions of safety and effectiveness than a legally marketed device. 510(k) submissions generally include, among other things, a description of the device and its manufacturing, device labeling, medical devices to which the device is substantially equivalent, safety and biocompatibility information and the results of performance testing. In some cases, a 510(k) submission must include data from human clinical studies. Marketing may commence only when the U.S. Food and Drug Administration issues a clearance letter finding substantial equivalence. The typical duration to receive 510(k) approval is approximately nine months from the date of the initial 510(k) submission, although there is no guaranty that the timing will not be longer.
In the past, the 510(k) pathway for product marketing required only the proof of significant equivalence in technology for a given indication with a previously cleared device. Currently, there has been a trend of the U.S. Food and Drug Administration requiring additional clinical work to prove efficacy in addition to technological equivalence. Thus, no matter which regulatory pathway we may take in the future towards marketing products in the U.S., we believe we will be required to provide clinical proof of device effectiveness.
After a device receives 510(k) clearance, any product modification that could significantly affect the safety or effectiveness of the product, or that would constitute a significant change in intended use, requires a new 510(k) clearance or, if the device would no longer be substantially equivalent, would require a PMA. If the U.S. Food and Drug Administration determines that the product does not qualify for 510(k) clearance, then a company must submit and the U.S. Food and Drug Administration must approve a PMA before marketing can begin.
A PMA application must provide a demonstration of safety and effectiveness, which generally requires extensive pre-clinical and clinical trial data. Information about the device and its components, device design, manufacturing and labeling, among other information, must also be included in the PMA. As part of the PMA review, the U.S. Food and Drug Administration will inspect the manufacturer’s facilities for compliance with quality system regulation requirements, which govern testing, control, documentation and other aspects of quality assurance with respect to manufacturing. If the U.S. Food and Drug Administration determines the application or manufacturing facilities are not acceptable, the U.S. Food and Drug Administration may outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the U.S. Food and Drug Administration ultimately may decide that the application does not satisfy the regulatory criteria for approval. During the review period, a U.S. Food and Drug Administration advisory committee, typically a panel of clinicians and statisticians, is likely to be convened to review the application and recommend to the U.S. Food and Drug Administration whether, or upon what conditions, the device should be approved. The U.S. Food and Drug Administration is not bound by the advisory panel decision. While the U.S. Food and Drug Administration often follows the panel’s recommendation, there have been instances where the U.S. Food and Drug Administration has not. If the U.S. Food and Drug Administration finds the information satisfactory, it will approve the PMA. The PMA approval can include post-approval conditions, including, among other things, restrictions on labeling, promotion, sale and distribution, or requirements to do additional clinical studies post-approval. Even after approval of a PMA, a new PMA or PMA supplement is required to authorize certain modifications to the device, its labeling or its manufacturing process. Supplements to a PMA often require the submission of the same type of information required for an original PMA, except that the supplement is generally limited to that information needed to support the proposed change from the product covered by the original PMA. The typical duration to receive PMA approval is approximately two years from the date of submission of the initial PMA application, although there is no guaranty that the timing will not be longer.
As describe above, we anticipate that our UroShield product will receive, a de novo review from the U.S. Food and Drug Administration. De novo is a two-step process that requires a company to submit a 510(k) and complete a standard review, including an analysis of the risk to the patient and operator associated with the use of the device and the substantial equivalence rationale. Once that has been accomplished, and the medical device in question has been determined to be not substantially equivalent to another approved device, the product is automatically classified as a Class III device. The manufacturer can then submit a request for an evaluation to have the product reclassified from Class III into Class I or Class II. The U.S. Food and Drug Administration will review the device classification proposal and either recommend special controls to create a new Class I or II device classification or determine that the product is a Class III device. If the U.S. Food and Drug Administration determines that the level of risk associated with the use of the device is appropriate for a Class II or Class I designation, then the product can be cleared as a 510(k) and the U.S. Food and Drug Administration will issue a new classification regulation and product code. If the device is not approved through de novo review, then it must go through the standard PMA process for Class III devices.
| 51 |
Clinical Trials of Medical Devices
One or more clinical trials are generally required to support a PMA application and more recently are becoming necessary to support a 510(k) submission. Clinical studies of unapproved or uncleared medical devices or devices being studied for uses for which they are not approved or cleared (investigational devices) must be conducted in compliance with U.S. Food and Drug Administration requirements. If an investigational device could pose a significant risk to patients, the sponsor company must submit an investigational device exemption application to the U.S. Food and Drug Administration prior to initiation of the clinical study. An investigational device exemption application must be supported by appropriate data, such as animal and laboratory test results, showing that it is safe to test the device on humans and that the testing protocol is scientifically sound. The investigational device exemption will automatically become effective 30 days after receipt by the U.S. Food and Drug Administration unless the U.S. Food and Drug Administration notifies the company that the investigation may not begin. Clinical studies of investigational devices may not begin until an institutional review board has approved the study.
During the study, the sponsor must comply with the U.S. Food and Drug Administration’s investigational device exemption requirements. These requirements include investigator selection, trial monitoring, adverse event reporting, and record keeping. The investigators must obtain patient informed consent, rigorously follow the investigational plan and study protocol, control the disposition of investigational devices, and comply with reporting and record keeping requirements. The sponsor, the U.S. Food and Drug Administration, or the institutional review board at each institution at which a clinical trial is being conducted may suspend a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable risk. During the approval or clearance process, the U.S. Food and Drug Administration typically inspects the records relating to the conduct of one or more investigational sites participating in the study supporting the application.
Post-Approval Regulation of Medical Devices
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
| · | the U.S. Food and Drug Administration quality systems regulation, which governs, among other things, how manufacturers design, test, manufacture, exercise quality control over, and document manufacturing of their products; |
| · | labeling and claims regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and |
| · | the Medical Device Reporting regulation, which requires reporting to the U.S. Food and Drug Administration of certain adverse experiences associated with use of the product. |
Good Manufacturing Practices Requirements
Manufacturers of medical devices are required to comply with the good manufacturing practices set forth in the quality system regulations promulgated under section 520 of the Food, Drug and Cosmetic Act. Current good manufacturing practices regulations require, among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation. The manufacturing facility for an approved product must meet current good manufacturing practices requirements to the satisfaction of the U.S. Food and Drug Administration pursuant to a pre-PMA approval inspection before the facility can be used. Manufacturers, including third party contract manufacturers, are also subject to periodic inspections by the U.S. Food and Drug Administration and other authorities to assess compliance with applicable regulations. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations, and civil and criminal penalties. Adverse experiences with the product must be reported to the U.S. Food and Drug Administration and could result in the imposition of marketing restrictions through labeling changes or in product withdrawal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following the approval.
| 52 |
International Regulation
We are subject to regulations and product registration requirements in many foreign countries in which we may sell our products, including in the areas of product standards, packaging requirements, labeling requirements, import and export restrictions and tariff regulations, duties and tax requirements. The time required to obtain clearance required by foreign countries may be longer or shorter than that required for U.S. Food and Drug Administration clearance, and requirements for licensing a product in a foreign country may differ significantly from U.S. Food and Drug Administration requirements.
The primary regulatory environment in Europe is the European Union, which consists of 25 member states and 42 competent authorities encompassing most of the major countries in Europe. In the European Union, the European Medicines Agency and the European Union Commission determined that WoundShield, PainShield and UroShield are to be regulated as medical device products. These products are classified as Class II devices. These devices are CE Marked and as such can be marketed and distributed within the European Economic Area. We are required to be recertified each year for CE by Intertek, which conducts an annual audit. The audit procedure, which includes on-site visits at our facility, requires us to provide Intertek with information and documentation concerning our management system and all applicable documents, policies, procedures, manuals, and other information.
The primary regulatory bodies and paths in Asia, Australia, Canada and Latin America are determined by the requisite country authority. In most cases, establishment registration and device licensing are applied for at the applicable Ministry of Health through a local intermediary. The requirements placed on the manufacturer are typically the same as those contained in ISO 9001 or ISO 13485, requirements for quality management systems published by the International Organization of Standardization. In some countries outside Europe, we are or will be able to sell on the basis of our CE Mark. We have a Canadian medical device license for PainShield and UroShield, a certificate allowing us to sell PainShield, WoundShield and UroShield in Israel, a certificate allowing us to sell PainShield in Australia, and we are able to sell PainShield, WoundShield and UroShield in India and Ecuador based on our CE Mark. In addition, our distributor in Korea has applied for approval to sell PainShield and UroShield, and our distributor in Chile has applied for approval to sell PainShield. We generally apply, through our distributor, for approval in a particular country for a particular product only when we have a distributor in place with respect to such product.
European Good Manufacturing Practices
In the European Union, the manufacture of medical devices is subject to good manufacturing practice, as set forth in the relevant laws and guidelines of the European Union and its member states. Compliance with good manufacturing practice is generally assessed by the competent regulatory authorities. Typically, quality system evaluation is performed by a notified body, which also recommends to the relevant competent authority for the European Community CE Marking of a device. The competent authority may conduct inspections of relevant facilities, and review manufacturing procedures, operating systems and personnel qualifications. In addition to obtaining approval for each product, in many cases each device manufacturing facility must be audited on a periodic basis by the notified body. Further inspections may occur over the life of the product.
U.S. Anti-Kickback and False Claims Laws
In the U.S., there are federal and state anti-kickback laws that prohibit the payment or receipt of kickbacks, bribes or other remuneration intended to induce the purchase or recommendation of healthcare products and services. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation in federal healthcare programs. These laws are potentially applicable to manufacturers of products regulated by the U.S. Food and Drug Administration as medical devices, such as us, and hospitals, physicians and other potential purchasers of such products. Other provisions of federal and state laws provide civil and criminal penalties for presenting, or causing to be presented, to third-party payers for reimbursement, claims that are false or fraudulent, or which are for items or services that were not provided as claimed. In addition, certain states have implemented regulations requiring medical device and pharmaceutical companies to report all gifts and payments over $50 to medical practitioners. This requirement does not apply to instances involving clinical trials.
| 53 |
Customers
We initially sell our products both through our website and distribution agreements, though a majority of our sales are currently through distributors. We currently have exclusive distribution agreements for our products with medical product distributors based in the U.S. (for PainShield since 2012, for NanoVibronix NPWT since 2013), Italy (since 2013), India (since 2012), Australia (since 2012), New Zealand (since 2012), Ecuador (since 2012), United Kingdom (since 2010) and Israel (since 2012). In May 2013, we entered into a non-exclusive distribution agreement for the distribution of PainShield for all indications other than abdominal and pelvic pain in the U.S. and Canada.
We are currently in discussions with multiple distribution companies in Europe, Asia, and Latin America. Current and future agreements with distributors stipulate that, while we are responsible for training, providing marketing guidance, marketing materials, and technical guidance, distributors will be responsible for carrying out local marketing activities and sales. In addition, in most cases, all sales costs, including sales representatives, incentive programs, and marketing trials, will be borne by the distributor. Under current agreements, distributors purchase our products from us at a fixed price. Our current agreements with distributors are generally for a term of approximately two to three years and automatically renew for an additional annual terms unless modified by either party.
Manufacturing and Suppliers
We assemble our own products at our facilities in Nesher, Israel. All of the component parts of our products are readily available from a number of manufacturers and suppliers. We order component parts on an as-needed basis, generally from the manufacturer that provides us with the most competitive pricing. Our most significant suppliers are APC International, Ltd., Rotel Product Engineering Ltd. and Amit Industries L.T.D (AmiCell). We do not have written agreements with any of these suppliers, but we believe any one could be easily replaced if necessary.
Employees
As of February 6, 2014, we had eight full-time employees and one part-time employee. Our employees are not party to any collective bargaining agreements. We consider our relations with our employees to be good. We believe that our future success will depend, in part, on our continued ability to attract, hire and retain qualified personnel.
Properties
We lease an office and manufacturing facility in Nesher, Israel and an office in Melville, New York. Our lease for the facility in Nesher expires December 31, 2015, with an option to renew annually. The space is approximately 230 square meters. We pay approximately $2,700 per month under our lease. Our lease for the facility in Melville expires May 24, 2014, with an option to renew annually. The space is approximately 15 square meters. We pay $915 per month under our lease. We believe that our facilities are adequate to meet our current and proposed needs.
Legal Proceedings
From time to time, we may be involved in litigation that arises through the normal course of business. As of the date of this filing, we are not a party to any material litigation nor are we aware of any such threatened or pending litigation.
There are no material proceedings in which any of our directors, officers or affiliates or any registered or beneficial shareholder of more than 5% of our common stock, or any associate of any of the foregoing is an adverse party or has a material interest adverse to our interest.
| 54 |
MANAGEMENT
The following table sets forth information regarding our executive officers and the members of our board of directors. All directors hold office for one-year terms until the election and qualification of their successors. Officers are elected by the board of directors and serve at the discretion of the board.
| Name | Age | Position | ||
| Harold Jacob, M.D. | 60 | Chief Executive Officer, Chairman of the Board of Directors and Acting Principal Financial Officer | ||
| Jona Zumeris, Ph.D. | 63 | Vice President of Technology and Director | ||
| Ira Greenstein | 52 | Director |
Harold Jacob, M.D., Chief Executive Officer and Chairman of the Board of Directors. Dr. Jacob has served as our chief executive officer and chairman of the board of directors since September 2003. Dr. Jacob is also currently performing the functions of a principal financial officer. Dr. Jacob is our co-founder and has worked extensively in medical device development. Dr. Jacob also served part-time as an attending gastroenterologist at Shaare Zedek Medical Center in Jerusalem, Israel from 2004 to March 2011. Since April 2011, he has been an attending physician in Gastroenterology at Hadassah University Hospital in Jerusalem, Israel. From 1999 to the present, Dr. Jacob has served as the president of Medical Instrument Development Inc., which provides consulting services to start-up and early stage companies and patents its own proprietary medical devices. From 1997 to 2003, Dr. Jacob served as director of medical affairs at Given Imaging Ltd., a company that developed the first swallowable wireless pill camera for inspection of the intestines. Dr. Jacob also currently serves as a director for Oramed Pharmaceuticals Inc., a pharmaceutical company focused on the development of innovative orally ingestible capsule medication. We believe that Dr. Jacob’s qualifications to serve on our board include his years of experience in the biomedical industry and his experience serving in management roles of various companies. In addition, as chief executive officer, Dr. Jacob’s position on the board ensures a unity of vision between the broader goals our company and our day-to-day operations.
Jona Zumeris, Ph.D., Vice President of Technology and Director. Dr. Zumeris is our co-founder and has served as our vice president of technology since September 2003. From 1999 to 2003, Professor Zumeris served as director of research and development for PMG Medica Ltd., a medical device company focused on ultrasound and piezomechanics technology. Dr. Zumeris was a founder, president and director of research and development of Nanomotion Ltd., a company that designs and manufactures motion solutions using ceramic servo motors, drivers and controllers, from 1993 to 1996. Dr. Zumeris’s extensive experience in the nano-technology and medical fields, especially in leadership and research roles, provide him the appropriate experience to serve on our board.
Ira Greenstein, Director. Mr. Greenstein has served as our director since August 2009. Mr. Greenstein has served as president of Genie Energy Ltd., an energy and gas holding company, since December 2011. Mr. Greenstein currently also serves as counsel to the chairman of IDT Corporation, a multinational holding company with operations primarily in the telecommunications industry, and served as the president of IDT Corporation from 2001 through 2011 and counsel to the chairman of IDT Corporation in 2000 and 2001. Mr. Greenstein serves on the boards of directors of Document Security Systems, Inc. and Ohr Pharmaceuticals, Inc. Mr. Greenstein’s experience serving in corporate roles in publicly-traded companies provides him with knowledge that assists in directing our long-term strategy.
| 55 |
There are no family relationships among any of our directors and executive officers. Dr. Jacob is party to certain agreements related to his service as chief executive officer described in the “Executive Compensation” section of this prospectus.
Director Independence
Our board of directors has determined that Ira Greenstein satisfies the requirements for independence set out in Section 5605(a)(2) of the Nasdaq Stock Market Rules and that he has no material relationship with us (other than being a director and/or a stockholder). In making its independence determinations, the board of directors sought to identify and analyze all of the facts and circumstances relating to any relationship between a director, his immediate family or affiliates and our company and our affiliates and did not rely on categorical standards other than those contained in the Nasdaq rule referenced above.
Board Committees
Upon the consummation of this offering, our board of directors will establish an audit committee, a nominating and corporate governance committee and a compensation committee, each of which will have the composition and responsibilities described below.
Audit Committee. The audit committee will consist of , each of whom our board has determined to be financially literate and qualify as an independent director under Section 5605(a)(2) of the rules of the Nasdaq Stock Market. In addition, qualifies as a financial expert, as defined in Item 407(d)(5)(ii) of Regulation S-K. The function of the audit committee will be to assist the board of directors in its oversight of (1) the integrity of our financial statements, (2) our compliance with legal and regulatory requirements and (3) the qualifications, independence and performance of our independent auditors.
Nominating and Corporate Governance Committee. The nominating and corporate governance committee will consist of , each of whom our board has determined qualifies as an independent director under Section 5605(a)(2) of the rules of the Nasdaq Stock Market. The primary function of the nominating and corporate governance committee will be to identify individuals qualified to become board members, consistent with criteria approved by the board, and select the director nominees for election at each annual meeting of stockholders.
Compensation Committee. The compensation committee will consist of , each of whom our board has determined qualifies as an independent director under Section 5605(a)(2) of the rules of the Nasdaq Stock Market, as an “outside director” for purposes of Section 162(m) of the Internal Revenue Code and as a “non-employee director” for purposes of Section 16b-3 under the Securities Exchange Act of 1934, as amended. The function of the compensation committee will be to discharge the board of directors’ responsibilities relating to compensation of our directors and executives and our overall compensation programs. The primary objective of the compensation committee will be to develop and implement compensation policies and plans that are appropriate for us in light of all relevant circumstances and which provide incentives that further our long-term strategic plan and are consistent with our culture and the overall goal of enhancing enduring stockholder value.
Board Leadership Structure
The board of directors is committed to promoting our effective, independent governance. Our board believes it is in our best interests and the best interests of our stockholders for the board to have the flexibility to select the best director to serve as chairman at any given time, regardless of whether that director is an independent director or the chief executive officer. Consequently, we do not have a policy governing whether the roles of chairman of the board and chief executive officer should be separate or combined. This decision is made by our board of directors, based on our best interests considering the circumstances at the time.
| 56 |
We do not currently separate the roles of chief executive officer and chairman of the board. Our board of directors has determined, in connection with our adoption of certain corporate governance principles in connection with this offering, that one of our independent directors should serve as a lead director at any time when the title of chairman is held by an employee director or there is no current chairman. The lead director’s responsibilities will include, among other things, presiding over periodic meetings of our independent directors and overseeing the function of our board of directors and committees. Our board of directors intends to appoint as our lead independent director effective upon the closing of this offering.
Role in Risk Oversight
Our board of directors oversees an enterprise-wide approach to risk management, designed to support the achievement of business objectives, including organizational and strategic objectives, to improve long-term organizational performance and enhance stockholder value. The involvement of our board of directors in setting our business strategy is a key part of its assessment of management’s plans for risk management and its determination of what constitutes an appropriate level of risk for the company. The participation of our board of directors in our risk oversight process includes receiving regular reports from members of senior management on areas of material risk to our company, including operational, financial, legal and regulatory, and strategic and reputational risks.
While our board of directors has the ultimate responsibility for the risk management process, senior management and various committees of our board of directors will also have responsibility for certain areas of risk management.
Our senior management team is responsible for day-to-day risk management and regularly reports on risks to our full board of directors or a relevant committee. Our finance and regulatory personnel serve as the primary monitoring and evaluation function for company-wide policies and procedures, and manage the day-to-day oversight of the risk management strategy for our ongoing business. This oversight includes identifying, evaluating, and addressing potential risks that may exist at the enterprise, strategic, financial, operational, compliance and reporting levels.
The audit committee will focus on monitoring and discussing our major financial risk exposures and the steps management has taken to monitor and control such exposures, including our risk assessment and risk management policies. As appropriate, the audit committee will provide reports to and receive direction from the full board of directors regarding our risk management policies and guidelines, as well as the audit committee’s risk oversight activities.
In addition, the compensation committee will assess our compensation policies to confirm that the compensation policies and practices do not encourage unnecessary risk taking. The compensation committee will review and discuss the relationship between risk management policies and practices, corporate strategy and senior executive compensation and, when appropriate, report on the findings from the discussions to our board of directors. Our compensation committee intends to set performance metrics that will create incentives for our senior executives that encourage an appropriate level of risk-taking that is commensurate with our short-term and long-term strategies.
Code of Ethics
In connection with this offering, our board of directors will adopt a code of business conduct and ethics that will apply to all of our employees, officers, and directors. Upon completion of this offering, the full text of our code of business conduct and ethics will be available on our website. Information on, or accessible through, our website is not part of this prospectus. We expect that any amendments to the code, or any waivers of its requirements, that apply to directors or executive officers, will be disclosed on our website.
| 57 |
EXECUTIVE COMPENSATION
2013, 2012 and 2011 Summary Compensation Table
The table below sets forth, for our last three fiscal years, the compensation earned by our named executive officers, Harold Jacob, M.D., our chief executive officer and chairman of the board of directors, and Jona Zumeris, our vice president of technology and a member of our board of directors. No other executive officer had compensation of greater than $100,000 for the last three fiscal years.
| Name and Principal Position | Year | Salary ($)(1) | Bonus ($) | Option Awards ($) | All Other Compensation ($)(1) | Total ($)(1) | ||||||||||||||||||
| Harold Jacob, M.D. | 2013 | - | - | 182,728 | (2) | 15,709 | (3) | 198,473 | ||||||||||||||||
| Chief Executive Officer and | 2012 | - | - | - | 16,442 | (3) | 16,442 | |||||||||||||||||
| Chairman of the Board of Directors | 2011 | 24,769 | (4) | - | - | 46,764 | (5) | 71,533 | ||||||||||||||||
| Jona Zumeris, Ph.D. | 2013 | 52,584 | - | 187,536 | (2) | 33,669 | (6) | 273,789 | ||||||||||||||||
| Vice President of Technology | 2012 | 58,739 | 36,153 | (6) | 94,892 | |||||||||||||||||||
| and Director | 2011 | 60,346 | 35,510 | (6) | 95,856 | |||||||||||||||||||
| (1) | Compensation amounts received in non-U.S. currency have been converted into U.S. dollars using the average exchange rate for the applicable year. The average exchange rate for each of 2013, 2012 and 2011 was 3.765 NIS per dollar, 4.014 NIS per dollar and 3.724 NIS per dollar, respectively. |
| (2) | The amounts in this column reflect the dollar amounts to be recognized for financial statement reporting purposes with respect to the twelve month period ended December 31, 2013 in accordance with FASB ASC Topic 718. Fair value is based on the Black-Scholes option pricing model using the fair value of the underlying shares at the grant date. For additional discussion of the valuation assumptions used in determining stock-based compensation and the grant date fair value for stock options, see “Management’s Discussion and Analysis of Financial Condition and Results of Operation - Critical Accounting Policies - Stock-based compensation” and Note 2—“Significant Accounting Policies” and Note 9—“Stockholders’ Equity (Deficiency)” of the Notes to Consolidated Financial Statements as of December 31, 2012 included in this prospectus.
| |
| (3) | Represents car-related benefits for Dr. Jacob. |
| (4) | Represents salary for January through May of 2011, after which time Dr. Jacob voluntarily served without cash compensation. |
| (5) | As compensation for his service from June through December of 2011, Dr. Jacob received series B-1 promissory notes in the original aggregate principal amount of $25,000. The terms of the notes are described under “Certain Relationships and Related Transactions” below. This amount also includes $15,470 of car-related benefits for Dr. Jacob and $6,294 of other benefits, comprised of contributions towards a pension fund, disability insurance, severance pay, an advanced study fund and recreation pay. |
| (6) | Comprised of car-related benefits for Dr. Zumeris of $18,715 in 2013, $19,665 in 2012 and $18,717 in 2011 and other benefits, comprised of contributions towards a pension fund, disability insurance, severance pay, an advanced study fund and recreation pay, of $14,954 in 2013, $16,488 in 2012 and $16,793 in 2011. |
Agreement with Harold Jacob, M.D.
Prior to 2011, we and NanoVibronix Ltd., our wholly-owned Israeli subsidiary, were party to an employment agreement with Dr. Jacob, pursuant to which Dr. Jacob served as chief executive officer of us and NanoVibronix Ltd. Dr. Jacob’s salary was set by oral agreement, but he was entitled to a car, which we lease on his behalf, and contributions towards a pension fund, disability insurance, severance pay, an advanced study fund and recreation pay, which are customary or statutorily prescribed in Israel. Dr. Jacob was also entitled to 15 vacation days. Dr. Jacob’s employment agreement contained confidentiality restrictions and other terms and provisions that are customary in Israel.
Beginning in June 2011, Dr. Jacob voluntarily waived his cash salary. As compensation for his service from May through December of 2011, Dr. Jacob received series B-1 promissory notes in the original aggregate principal amount of $25,000 on November 22, 2011. We and Dr. Jacob orally agreed to terminate his employment agreement beginning in 2012, and Dr. Jacob continues to serve without cash compensation or other benefits, except the use of a car.
Agreement with Jona Zumeris, Ph.D.
NanoVibronix Ltd., our wholly-owned Israeli subsidiary, is party to an employment agreement with Dr. Zumeris, pursuant to which Dr. Zumeris serves as its vice president of technology. Dr. Zumeris’s salary pursuant to the agreement is 19,500 NIS per month, which was increased to 20,000 NIS per month by oral agreement commencing in December 2012, and he is entitled to a car, which we lease on his behalf, and contributions towards a pension fund, disability insurance, severance pay and an advanced study fund and recreation pay, which are customary or statutorily prescribed in Israel. Dr. Zumeris is also entitled to 15 vacation days. Dr. Zumeris’s employment agreement contains confidentiality restrictions and other terms and provisions that are customary in Israel.
Outstanding Equity Awards at Fiscal Year End
The following table provides information on the holdings of stock options of the named executive officer at December 31, 2013. This table includes unexercised and unvested options awards. Each outstanding award is shown separately.
| 58 |
| Option Awards | ||||||||||||||||
| Number of | Number of | |||||||||||||||
| Securities | Securities | |||||||||||||||
| Underlying | Underlying | Option | ||||||||||||||
| Unexercised | Unexercised | Exercise | Option | |||||||||||||
| Date of | Options (#) | Options (#) | Price | Expiration | ||||||||||||
| Name | Grant | Exercisable | Unexercisable | ($) | Date | |||||||||||
| Harold Jacob, M.D. | December 31, 2004 | 60,000 | - | 0.01 | December 31, 2014 | |||||||||||
| December 13, 2007 | 30,000 | - | 10.35 | December 13, 2017 | ||||||||||||
| December 9, 2010 | 75,000 | (1) | - | 0.17 | December 9, 2020 | |||||||||||
March 28, 2013 |
760,000 |
- | 0.01 | March 28, 2023 | ||||||||||||
Jona Zumeris, Ph.D. |
March 28, 2013 |
780,000 |
- | 0.01 | March 28, 2023 | |||||||||||
| (1) | Granted as compensation for services as an executive officer in light of his below market salary. |
2004 Global Share Option Plan
In November 2004, our board of directors adopted the 2004 Global Share Option Plan, pursuant to which 2,800,000 shares of our common stock are reserved for issuance as awards to employees, directors, consultants and other service providers. The purpose of the 2004 Global Share Option Plan is to provide an incentive to attract and retain directors, officers, consultants, advisors and employees, to encourage a sense of proprietorship and stimulate an active interest of such persons in our development and financial success. The 2004 Global Share Option Plan is administered by our board of directors.
On March 28, 2013, we granted options under the 2004 Global Share Option Plan to the following executive officers and directors. The options all vested immediately.
| Number of Securities | Option | Option | ||||||||
| Underlying Unexercised Options (#) | Exercise | Expiration | ||||||||
| Name | Exercisable | Price ($) | Date | |||||||
| Harold Jacob, M.D. | 760,000 | 0.01 | March 28, 2023 | |||||||
| Jona Zumeris, Ph.D | 780,000 | 0.01 | March 28, 2023 | |||||||
| Paul Packer(1) | 210,000 | 0.28 | March 28, 2023 | |||||||
| (1) Mr. Packer resigned as a member of our board of directors, effective as of January 15, 2014. | ||||||||||
Director Compensation
We paid no compensation to our non-employee directors for the one year periods ended December 31, 2013 and 2012 and have paid no compensation during 2014 to date, other than the awards described above. The following table shows information concerning the compensation of our directors other than Dr. Jacob and Dr. Zumeris, during the twelve months ended December 31, 2013.
| Name | Fees Earned or Paid in Cash ($) | Option Awards ($)(1) | All Other Compensation ($) | Total ($)(1) | ||||||||||||
| Sim Fass(2) | - | - | - | - | ||||||||||||
| Ira Greenstein | - | - | - | - | ||||||||||||
| Paul Packer(3) | - | 22,761 | (4) | - | 22,761 | |||||||||||
| (1) | The amounts in this column reflect the dollar amounts to be recognized for financial statement reporting purposes with respect to the twelve month period ended December 31, 2013 in accordance with FASB ASC Topic 718. Fair value is based on the Black-Scholes option pricing model using the fair value of the underlying shares at the grant date. For additional discussion of the valuation assumptions used in determining stock-based compensation and the grant date fair value for stock options, see “Management’s Discussion and Analysis of Financial Condition and Results of Operation - Critical Accounting Policies - Stock-based compensation” and Note 2—“Significant Accounting Policies” and Note 9—“ Stockholders’ Equity (Deficiency)” of the Notes to Consolidated Financial Statements as of December 31, 2012 included in this prospectus. |
| (2) | Mr. Fass resigned as a member of our board of directors, effective as of January 29, 2014. |
| (3) | Mr. Packer resigned as a member of our board of directors, effective as of January 15, 2014. |
| (4) | See “—2004 Global Share Option Plan” above for more information on this award. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Generally, we do not to enter into related party transactions unless the members of the board who do not have an interest in the potential transaction have reviewed the transaction and determined that (i) we would not be able to obtain better terms by engaging in a transaction with a non-related party and (ii) the transaction is in our best interest. This policy applies generally to any transaction in which we are to be a participant and the amount involved exceeds the lesser of $120,000 or one percent of the average of our total assets at year end for the previous two completed fiscal years, and in which any related person had or will have a direct or indirect material interest. This policy is not currently in writing. In addition, our audit committee will be required to pre-approve any related party transactions pursuant to its charter.
On March 20, 2009, we issued 8,696 shares of series B participating convertible preferred stock and warrants to purchase 8,696 shares of series B participating convertible preferred stock to Paul Packer, who was then a member of our board of directors and who remained a director until January 15, 2014, in exchange for consideration of $150,000. On January 1, 2010, we issued 2,899 shares of series B participating convertible preferred stock and warrants to purchase 2,899 shares of series B participating convertible preferred stock to a fund controlled by Mr. Packer, in exchange for consideration of $50,000. On July 12, 2011, we issued 5,797 shares of series B participating convertible preferred stock and warrants to purchase 5,797 shares of series B participating convertible preferred stock to a fund controlled by Mr. Packer, in exchange for consideration of $100,000. The warrants had an exercise price of $17.25 per share and a five-year term. The series B participating convertible preferred stock was convertible into shares of our common stock at a rate of one common share per series B participating convertible preferred share.
| 59 |
On November 22, 2011, we issued convertible series B-1 promissory notes to certain investors. These investors include three funds controlled by Mr. Packer. The notes purchased by these funds were in the original aggregate principal amount of $180,000. Dr. Jacob, our Chief executive officer and chairman, also participated in the offering. As compensation for his service from May through December of 2011, Dr. Jacob received notes in the original aggregate principal amount of $25,000. The convertible series B-1 promissory notes mature on the earlier of November 15, 2014 or on an accelerated date if there is an event of default, upon which date the entire outstanding principal balance and any outstanding fees or interest will be due and payable in full. The convertible series B-1 promissory notes bear interest at the rate of 10% per annum, compounded annually. In addition, the convertible series B-1 promissory notes are convertible at any time at the holder’s option into shares of our series B-1 participating convertible preferred stock at an initial conversion price of $0.284 per share, subject to adjustment for stock dividends, stock splits or combinations. Our series B-1 participating convertible preferred stock is convertible into shares of our common stock at a rate of one common share per series B-1 participating convertible preferred share. The convertible series B-1 promissory notes will automatically convert into series B-1 participating convertible preferred stock upon the occurrence of (i) an aggregate investment in us of $3 million or more in a transaction or series of transactions or (ii) a fundamental transaction. To date, no principal or interest has been paid on these notes. As of February 6, 2014, an aggregate of $42,166 in interest has accrued on the notes. These notes will convert into common stock automatically upon the closing of this offering.
On November 22, 2011, we issued convertible series B-2 promissory notes in the original aggregate principal amount of $340,329 and warrants to purchase 513,575 shares of series B-2 participating convertible preferred stock to Mr. Packer and the two funds described above in exchange for the cancellation of the preferred stock and warrants described above. The principal amount of the notes was equal to the original investment in the series B participating convertible preferred stock plus simple interest at 8% from the date of the original investment. The number of shares underlying the warrants was equal to Mr. Packer’s and the two funds’ proportionate share of 30 percent of the number of shares into which the convertible series B-1 promissory notes were convertible. The terms of the convertible series B-2 promissory notes are the same as those of the convertible series B-1 promissory notes, except that the initial conversion price is $0.199 per share of series B-2 participating convertible preferred stock. Our series B-2 participating convertible preferred stock is convertible into shares of our common stock at a rate of one common share per series B-2 participating convertible preferred share. To date, no principal or interest has been paid on these notes. As of February 6, 2014, an aggregate of $82,143 in interest has accrued on the notes. These notes will convert into common stock automatically upon the closing of this offering. The warrants had an exercise price of $0.199 per share and a seven-year term. On , the warrants were exchanged for warrants to purchase shares of common stock at an exercise price of $ per share. For a description of these new warrants, see “Description of Securities—Warrants— 2013 Warrants.”
On February 5, 2013, March 28, 2013, June 3, 2013, August 5, 2013, October 7, 2013, December 9, 2013 and February 6, 2014, we issued secured convertible promissory notes to two funds controlled by Mr. Packer. The notes were initially issued in the original aggregate principal amount of $100,000. On each date listed above, such principal amount was increased by $100,000. The sixth amended and restated secured convertible promissory notes issued on and February 6, 2014 have an original aggregate principal amount of $700,000. The convertible promissory notes mature on the earlier of April 30, 2014, the closing date of a financing in which we sell an aggregate of at least $250,000 of our debt or equity securities or on an accelerated date if there is an event of default, upon which date the entire outstanding principal balance and any outstanding fees or interest will be due and payable in full. The convertible promissory notes bear interest at the rate of 6% per annum, which rate is increased to 10% upon and during the occurrence of an event of default. In addition, the convertible promissory notes are convertible either at the holders’ option or upon maturity into shares of our common stock at an initial conversion price of $0.38 per share, subject to adjustment for stock splits, fundamental transactions or similar events. The holders of the convertible promissory notes have a security interest in all of our assets and those of our subsidiaries. To date, no principal or interest has been paid on these notes. As of February 6, 2014, an aggregate of $21,288 in interest has accrued on the notes. These notes will convert into common stock automatically upon the closing of this offering.
In connection with the issuance of the notes described above, on each of February 5, 2013, March 28, 2013, June 3, 2013, August 5, 2013, October 7, 2013, December 9, 2013 and February 6, 2014, we issued warrants to purchase up to an aggregate of 263,158 shares of common stock, with an exercise price of $0.38 per share, subject to adjustment, to the two funds controlled by Mr. Packer.
Following the completion of this offering, the Company intends, if and to the extent requested by or on behalf of Mr. Packer, to exchange the shares of common stock he beneficially owns for shares of our series C preferred stock. The series C preferred stock would be established by our board of directors prior to such exchange. We anticipate that each share of our series C preferred stock would be convertible into shares of our common stock (subject to adjustment as provided in the related certificate of designation of preferences) at any time at the option of the holder, provided that the holder would be prohibited from converting series C preferred stock into shares of our common stock if, as a result of such conversion, the holder, together with its affiliates, would own more than 4.99% of the total number of shares of our common stock then issued and outstanding. For more information regarding the series C preferred stock, see “Description of Securities – Preferred Stock – Series C Convertible Preferred Stock.”
| 60 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information with respect to the beneficial ownership of our common stock as of February 6, 2014 by:
| · | each person known by us to beneficially own more than 5.0% of our common stock; |
| · | each of our directors; |
| · | each of the named executive officers; and |
| · | all of our directors and executive officers as a group |
The percentages of common stock beneficially owned are reported on the basis of regulations of the Securities and Exchange Commission governing the determination of beneficial ownership of securities. Under the rules of the Securities and Exchange Commission, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or to direct the voting of the security, or investment power, which includes the power to dispose of or to direct the disposition of the security. Except as indicated in the footnotes to this table, each beneficial owner named in the table below has sole voting and sole investment power with respect to all shares beneficially owned and each person’s address is c/o Nano Vibronix, Inc., 105 Maxess Road, Suite S124, Melville, NY 11747. As of February 6, 2014, we had 19,690,608 shares outstanding.
| Name of Beneficial Owner | Number of Shares Beneficially Owned (1) |
Percentage Beneficially Owned Prior to Offering (1) (2) |
Percentage Beneficially Owned After the Offering (1) (2) |
|||||||||
| 5% Owners | ||||||||||||
| CollabRx, Inc.(2) | 1,303,788 | (3) | 6.6 | % | % | |||||||
| IDT Corporation(4) | 1,230,318 | (5) | 6.2 | % | % | |||||||
| Paul Packer (6) | 8,674,050 | (7) | 39.0 | % | % | |||||||
| Miriam Winder-Kelly(8) | 1,699,453 | (9) | 8.5 | % | % | |||||||
| Officers and Directors | ||||||||||||
| Harold Jacob, M.D. | 1,537,255 | (10) | 7.4 | % | % | |||||||
| Jona Zumeris, Ph.D. | 1,264,824 | (11) | 6.2 | % | % | |||||||
| Ira Greenstein | 471,319 | (12) | 2.3 | % | % | |||||||
| All directors and executive officers as a group (3 persons) | 3,273,398 | 15.2 | % | % | ||||||||
* Less than one percent (1%).
| (1) | Shares of common stock beneficially owned and the respective percentages of beneficial ownership of common stock assume the exercise of all options, warrants and other securities convertible into common stock beneficially owned by such person or entity currently exercisable or exercisable within 60 days of February 6, 2014. Shares issuable pursuant to the exercise of stock options and warrants exercisable within 60 days are deemed outstanding and held by the holder of such options or warrants for computing the percentage of outstanding common stock beneficially owned by such person, but are not deemed outstanding for computing the percentage of outstanding common stock beneficially owned by any other person. In addition, shares of common stock beneficially owned and the respective percentages of beneficial ownership of common stock assume all of the adjustments described on page 4 under “Prospectus Summary – The Offering.” All conversions are deemed to have occurred on February 6, 2014. |
| (2) | CollabRx’s address is 44 Montgomery Street, Suite 800, San Francisco, California 94104. |
| 61 |
| (3) | Comprised of shares of common stock to be issued upon the conversion of preferred stock. |
| (4) | IDT Corporation’s address is 520 Broad Street, Newark, New Jersey 07102. |
| (5) | Comprised of (i) 990,664 shares of common stock to be issued upon the conversion of convertible series B-2 promissory notes and (ii) 239,654 shares of common stock that may be purchased upon the exercise of warrants. |
| (6) | Mr. Packer’s address is 805 Third Avenue, 15th Floor, New York, NY 10022. |
| (7) | Comprised of (i) 17,253 shares of common stock held by Globis Capital Partners, L.P., (ii) 807,105 shares of common stock to be issued upon the conversion of preferred stock held by Globis Capital Partners, L.P., (iii) 347,677 shares of common stock to be issued upon the conversion of convertible series B-1 promissory notes held by Globis Capital Partners, L.P., (iv) 638,704 shares of common stock to be issued upon the conversion of convertible series B-2 promissory notes held by Globis Capital Partners, L.P., (v) 1,628,193 shares of common stock that may be purchased by Globis Capital Partners, L.P. upon the exercise of warrants, (vi) 1,518,500 shares of common stock to be issued upon the conversion of secured convertible promissory notes held by Globis Capital Partners, L.P., (vii) 6,678 shares of common stock held by Globis Overseas Fund, Ltd., (viii) 312,415 shares of common stock to be issued upon the conversion of preferred stock held by Globis Overseas Fund, Ltd., (ix) 108,649 shares of common stock to be issued upon the conversion of convertible series B-1 promissory notes held by Globis Overseas Fund, Ltd., (x) 355,584 shares of common stock to be issued upon the conversion of convertible series B-2 promissory notes held by Globis Overseas Fund, Ltd., (xi) 454,444 shares of common stock that may be purchased by Globis Overseas Fund, Ltd. upon the exercise of warrants, (xii) 379,625 shares to be issued upon the conversion of secured convertible promissory notes held by Globis Overseas Fund, Ltd., (xiii) 325,947 shares to be issued upon the conversion of convertible series B-1 promissory notes held by Globis International Investments L.L.C., (xiv) 3,339 shares of common stock held by Mr. Packer, (xv) 156,207 shares to be issued upon the conversion of preferred stock held by Mr. Packer, (xvi) 1,128,686 shares to be issued upon the conversion of convertible series B-2 promissory notes held by Mr. Packer, which will occur automatically upon the closing of this offering, (xvii) 273,044 shares of common stock that may be purchased by Mr. Packer upon the exercise of warrants, and (xviii) 212,000 shares of common stock that may be purchased upon the exercise of stock options held by Mr. Packer.
Mr. Packer is the managing member of Globis Capital Advisors, L.L.C., which is the general partner of Globis Capital Partners, L.P. Mr. Packer is the managing member of Globis Capital, L.L.C., which is the general partner of Globis Capital Management, L.P., which is the investment manager of Globis Overseas Fund, Ltd. Mr. Packer is also the managing member of Globis International Investments L.L.C. Mr. Packer is deemed to have beneficial ownership of the shares held by Globis Capital Partners, L.P., Globis Overseas Fund, Ltd. and Globis International Investments L.L.C.
Following the completion of this offering, the Company intends, if and to the extent requested by or on behalf of Mr. Packer, to exchange the shares of common stock he beneficially owns for shares of our series C preferred stock. The series C preferred stock would be established by our board of directors prior to such exchange. We anticipate that each share of our series C preferred stock would be convertible into shares of our common stock (subject to adjustment as provided in the related certificate of designation of preferences) at any time at the option of the holder, provided that the holder would be prohibited from converting series C preferred stock into shares of our common stock if, as a result of such conversion, the holder, together with its affiliates, would own more than 4.99% of the total number of shares of our common stock then issued and outstanding. Therefore, we anticipate that Mr. Packer will cease to beneficially own more than 5% of our common stock. For more information regarding the series C preferred stock, see “Description of Securities – Preferred Stock – Series C Convertible Preferred Stock.” |
| (8) | Ms. Winder-Kelly’s address is 900 Abel Wolman Municipal Bldg. 200N. Holliday St. Baltimore, MD 21202. |
| (9) | Comprised of (i) 5,797 shares of common stock to be issued upon the conversion of preferred stock, (ii) 1,363,748 shares of common stock to be issued upon the conversion of convertible series B-2 promissory notes and (iii) 329,908 shares of common stock that may be purchased upon the exercise of warrants. |
| (10) | Comprised of (i) 59,370 shares of common stock held by Medical Instrument Development Inc., an entity controlled by Dr. Jacob, (ii) 357,703 shares of common stock to be issued upon the conversion of convertible series B-2 promissory notes held by Medical Instrument Development Inc., (iii) 86,533 shares of common stock that may be purchased by Medical Instrument Development Inc. upon the exercise of warrants, (iv) 108,649 shares of common stock to be issued upon the conversion of convertible series B-1 promissory notes held by Dr. Jacob and (v) 925,000 shares of common stock that may be purchased by Dr. Jacob upon the exercise of stock options. |
| (11) | Comprised of (i) 484,824 shares of common stock held by Piezo Top Ltd, an entity controlled by Dr. Zumeris, and (ii) options to purchase 780,000 shares of common stock held by Dr. Zumeris. |
| (12) | Comprised of (i) 375,887 shares of common stock to be issued upon the conversion of convertible series B-2 promissory notes, (ii) 90,932 shares of common stock that may be purchased upon the exercise of warrants and (iii) 4,500 shares of common stock that may be purchased upon the exercise of stock options. |
| 62 |
DESCRIPTION OF SECURITIES
Upon the completion of this offering, our restated certificate of incorporation will authorize us to issue up to shares of common stock, $0.001 par value per share, and shares of preferred stock, $0.001 par value per share, all of which shares of preferred stock will be undesignated. Our board of directors may establish the rights and preferences of the preferred stock from time to time. Following the completion of this offering, the Company intends, if and to the extent requested by or on behalf of Mr. Packer, to exchange the shares of common stock he beneficially owns for shares of series C preferred stock. The series C preferred stock would be established by our board of directors prior to such exchange. The anticipated terms of the series C preferred stock are described below. As of February 6, 2014, there were 19,690,608 shares of common stock outstanding, held of record by approximately 130 stockholders.
Common Stock
The holders of common stock are entitled to one vote per share. Our certificate of incorporation does not provide for cumulative voting. The holders of our common stock are entitled to receive ratably such dividends, if any, as may be declared by the board of directors out of legally available funds. Upon liquidation, dissolution or winding-up, the holders of our common stock are entitled to share ratably in all assets that are legally available for distribution. The holders of our common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of any series of preferred stock, which may be designated solely by action of the board of directors and issued in the future.
Warrants
Each warrant entitles the holder to purchase one share of our common stock at an initial exercise price of $ (which is equal to 125% of the offering price of the common stock). Each warrant will be exercisable immediately upon issuance and will expire on , 2019.
The warrants will be represented issued in registered form, in each case pursuant to a warrant agreement between VCorp Services, LLC, as warrant agent, and us.
The exercise price and number of shares issuable upon exercise of the warrants may be adjusted upon the occurrence of certain events, including but not limited to any stock split, stock dividend, recapitalization, reorganization, merger or consolidation. However, warrants will not be adjusted for issuances of common stock or securities convertible or exercisable into common stock at a price below the then current exercise price of the warrants.
The warrants may be exercised, at the option of each holder, in whole or in part, upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price for the number of shares of our common stock purchased upon such exercise, by certified check payable to us or by wire transfer of immediately available funds to an account designated by us. Subject to applicable laws, the warrants may be transferred at the option of the holders upon surrender of the warrants to us together with the appropriate instruments of transfer.
The warrant holders do not have the rights or privileges of holders of common stock and any voting rights until they exercise their warrants and receive shares of common stock. After the issuance of shares of common stock upon exercise of the warrants, each holder will be entitled to one vote for each share of common stock held of record on all matters to be voted on by stockholders.
No warrants will be exercisable unless at the time of exercise a prospectus relating to common stock issuable upon exercise of the warrants is current and the common stock has been registered or qualified or deemed to be exempt under the securities laws of the state of residence of the holder of the warrants. Under the terms of the warrant agreement, we have agreed to meet these conditions and use our best efforts to maintain a current prospectus relating to common stock issuable upon exercise of the warrants until the expiration of the warrants. However, we cannot assure you that we will be able to do so, and if we do not maintain a current prospectus related to the common stock issuable upon exercise of the warrants, holders will be unable to exercise their warrants and we will not be required to settle any such warrant exercise. If the prospectus relating to the common stock issuable upon the exercise of the warrants is not current or if the common stock is not qualified or exempt from qualification in the jurisdictions in which the holders of the warrants reside, we will not be required to net cash settle or cash settle the warrant exercise, the warrants may have no value, the market for the warrants may be limited and the warrants may expire worthless.
| 63 |
Preferred Stock
All 222,620 currently outstanding shares of series A-1 preferred stock will be converted automatically to common stock at a conversion rate of 11.695 shares of common stock per share of series A-1 preferred stock upon the completion of this offering. This conversion rate reflects an antidilution adjustment made in connection with our November 2011 promissory note issuances. All 171,612 currently outstanding shares of series A-2 preferred stock will be converted automatically to common stock at a conversion rate of one share of common stock per share of series A-2 preferred stock upon the completion of this offering. The owners of a majority of the outstanding shares of series A-1 and series A-2 preferred stock voting as a single class may cause all shares of series A preferred stock to be converted into shares of common stock. Following the completion of this offering, our board of directors will have the authority, without further action by our stockholders, to issue up to shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The purpose of authorizing our board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of us and may adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of common stock until the board of directors determines the specific rights attached to that preferred stock.
Series C Convertible Preferred Stock
We anticipate that, if and when established, the series C preferred stock would rank as follows:
• senior to all of our common stock;
• senior to any class or series of our capital stock hereafter created specifically ranking by its terms junior to the preferred stock;
• on parity with any class or series of our capital stock hereafter created specifically ranking by its terms on parity with the preferred stock; and
• junior to any class or series of our capital stock hereafter created specifically ranking by its terms senior to the series C preferred stock;
in each case, as to distributions of assets upon our liquidation, dissolution or winding up whether voluntarily or involuntarily.
Each share of our series C preferred stock would be convertible into one share of our common stock (subject to adjustment as provided in the related certificate of designation of preferences) at any time at the option of the holders, provided that each holder would be prohibited from converting series C preferred stock into shares of our common stock if, as a result of such conversion, any such holder, together with its affiliates, would own more than 4.99% of the total number of shares of our common stock then issued and outstanding (subject to an increase, upon at least 61 days’ notice by the holders of the series C preferred stock, to up to 9.99%).
In the event of our liquidation, dissolution, or winding up, each holder of our series C preferred stock could elect to receive either (i) in preference to any payments made to the holders of our common stock and any other junior securities, a payment for each share of series C preferred stock then held equal $0.001, plus an additional amount equal to any dividends declared but unpaid on such shares, and any other fees or liquidated damages then due and owing thereon or (ii) the amount of cash, securities or other property to which such holder would be entitled to receive with respect to each share of series C preferred stock if such share of series C preferred stock had been converted to common stock immediately prior to such liquidation, dissolution, or winding up (without giving effect to any conversion limitations).
Shares of series C preferred stock would not be entitled to receive any dividends, unless and until specifically declared by our board of directors. However, holders of our series C preferred stock would be entitled to receive dividends on shares of series C preferred stock equal (on an as-if-converted-to-common-stock basis) to and in the same form as dividends actually paid on shares of the common stock when such dividends are specifically declared by our board of directors. We would not be obligated to redeem or repurchase any shares of preferred stock. Shares of series C preferred stock would not otherwise be entitled to any redemption rights, or mandatory sinking fund or analogous fund provisions.
Each holder of series C preferred stock would be entitled to the number of votes equal to the number of whole shares of common stock into which the shares of series C preferred stock held by such holder is then convertible (subject to the beneficial ownership limitations set forth in the related certificate of designation of preferences) with respect to any and all matters presented to the stockholders for their action or consideration. Holders of series C preferred stock would vote together with the holders of common stock as a single class, except as provided by law and except that the consent of holders of a majority of the outstanding series C preferred stock would be required to amend the terms of the series C preferred stock.
The series C preferred stock would not limit or qualify the rights of the holders to be sold in this offering except for potential liquidation preference described above.
| 64 |
Other Warrants
February 2013 Warrants
On February 5, 2013, in connection with the issuance of our initial secured convertible promissory notes, we issued to the same accredited investors warrants to purchase up to an aggregate of 263,158 shares of common stock at an exercise price of $0.38 per share. The warrants contain full ratchet anti-dilution price protection upon the issuance of equity or equity-linked securities at an effective common stock purchase price of less than $0.38 per share as well as other customary anti-dilution protection. The holders of such warrants have the right to exercise the warrants by means of a cashless exercise. Upon the occurrence of certain change of control transactions, then any holder of the warrants shall, upon exercise, have the right to acquire the same securities as if it had exercised the warrants immediately before the date on which a record is taken for such transaction, or, if no such record is taken, the date as of which the record holders of shares of common stock are to be determined for the participation in such transaction. The warrants expire on February 5, 2018.
March 2013 Warrants
On March 28, 2013, in connection with the issuance of our amended and restated secured convertible promissory notes, we issued to the same accredited investors warrants to purchase up to an aggregate of 263,158 shares of common stock at an exercise price of $0.38 per share. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on March 28, 2018.
June 2013 Warrants
On June 3, 2013, in connection with the issuance of our second amended and restated secured convertible promissory notes, we issued to the same accredited investors warrants to purchase up to an aggregate of 263,158 shares of common stock at an exercise price of $0.38 per share. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on June 3, 2018.
August 2013 Warrants
On August 5, 2013, in connection with the issuance of our third amended and restated secured convertible promissory notes, we issued to the same accredited investors warrants to purchase up to an aggregate of 263,158 shares of common stock at an exercise price of $0.38 per share. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on August 5, 2018.
October 2013 Warrants
On October 7, 2013, in connection with the issuance of our fourth amended and restated secured convertible promissory notes, we issued to the same accredited investors warrants to purchase up to an aggregate of 263,158 shares of common stock at an exercise price of $0.38 per share. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on October 7, 2018.
December 2013 Warrants
On December 9, 2013, in connection with the issuance of our fifth amended and restated secured convertible promissory notes, we issued to the same accredited investors warrants to purchase up to an aggregate of 263,158 shares of common stock at an exercise price of $0.38 per share. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on December 9, 2018.
February 2014 Warrants
On February 6, 2014, in connection with the issuance of our sixth amended and restated secured convertible promissory notes, we issued to the same accredited investors warrants to purchase up to an aggregate of 263,158 shares of common stock at an exercise price of $0.38 per share. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on February 6, 2019.
2014 Warrants
Automatically upon the closing of this offering, we will issue warrants to purchase up to an aggregate of shares of common stock at an exercise price of $0.199 per share in exchange for warrants to purchase an equal number of shares of series B-2 preferred stock, at the same exercise price. The warrants will otherwise be identical to the warrants for which they are exchanged. The warrants will contain customary anti-dilution protection. The holders of such warrants will have the right to exercise the warrants by means of a cashless exercise. Upon the occurrence of certain change of control transactions, then any holder of the warrants shall, upon exercise, have the right to acquire the same securities as if it had exercised the warrants immediately before the consummation of such transaction. The warrants will expire on November 15, 2018.
Lock-up Agreements
In connection with this offering, we, our directors and executive officers have agreed not to offer, sell, contract to sell, pledge, grant options to purchase, or otherwise dispose of any shares of our common stock or securities exchangeable for or convertible into our common stock for a period of 180 days after the date of this prospectus supplement without the prior written consent of Chardan Capital Markets LLC. This agreement does not apply to the issuance of shares upon the exercise of rights to acquire shares of common stock pursuant to any existing stock option or similar equity incentive or compensation plan. Our directors and executive officers have agreed, subject to certain exceptions, not to, directly or indirectly, sell, hedge, or otherwise dispose of any shares of common stock, options to acquire shares of common stock or securities exchangeable for or convertible into shares of common stock, for a period of 180 days after the date of this prospectus supplement without the prior written consent of Chardan Capital Markets LLC.
| 65 |
However, in the event that either (i) during the last 17 days of the “lock-up” period, we release earnings results or material news or a material event relating to us occurs or (ii) prior to the expiration of the “lock-up” period, we announce that we will release earnings results during the 16-day period beginning on the last day of the “lock-up” period, the expiration of the “lock-up” will be extended until the expiration of the 18-day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless Chardan Capital Markets LLC waives, in writing, such an extension.
Anti-Takeover Effect of Delaware Law, Certain Charter and Bylaw Provisions
Our certificate of incorporation and bylaws contain provisions that could have the effect of discouraging potential acquisition proposals or tender offers or delaying or preventing a change of control of our company. These provisions are as follows:
| · | they provide that special meetings of stockholders may be called only by our chairman, our president or by a resolution adopted by a majority of our board of directors; |
| · | they do not include a provision for cumulative voting in the election of directors. Under cumulative voting, a minority stockholder holding a sufficient number of shares may be able to ensure the election of one or more directors. The absence of cumulative voting may have the effect of limiting the ability of minority stockholders to effect changes in our board of directors; and |
| · | they allow us to issue, without stockholder approval, up to shares of preferred stock that could adversely affect the rights and powers of the holders of our common stock. |
We are subject to the provisions of Section 203 of the General Corporation Law of the State of Delaware, an anti-takeover law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. For purposes of Section 203, a “business combination” includes a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder, and an “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years prior did own, 15% or more of the voting stock of a corporation.
Indemnification of Directors and Officers
Section 145 of the General Corporation Law of the State of Delaware provides, in general, that a corporation incorporated under the laws of the State of Delaware, as we are, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Our certificate of incorporation and bylaws provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the provisions of the General Corporation Law of the State of Delaware, as amended from time to time, subject to any permissible expansion or limitation of such indemnification, as may be set forth in any stockholders’ or directors’ resolution or by contract. Any repeal or modification of these provisions approved by our stockholders will be prospective only and will not adversely affect any limitation on the liability of any of our directors or officers existing as of the time of such repeal or modification.
We are also permitted to apply for insurance on behalf of any director, officer, employee or other agent for liability arising out of his actions, whether or not the General Corporation Law of the State of Delaware would permit indemnification.
Disclosure of Commission Position on Indemnification for Securities Act Liabilities
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to our directors, officers and persons controlling us, we have been advised that it is the Securities and Exchange Commission’s opinion that such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable.
| 66 |
SHARES ELIGIBLE FOR FUTURE ISSUANCE
Prior to this offering, there has been no public market for our securities, and we cannot predict the effect, if any, that market sales of our securities or the availability of our securities for sale will have on the market price of our securities prevailing from time to time. Nevertheless, sales of substantial amounts of our common stock, including shares issued upon exercise of outstanding options, in the public market following this offering could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through the sale of our equity securities.
Upon the closing of this offering, we will have a total of shares of our common stock outstanding, based on the shares of our common stock outstanding as of February 6, 2014, assuming (i) the conversion of all outstanding shares of our convertible preferred stock into an aggregate of shares of common stock, which will occur automatically upon the closing of this offering, (ii) the conversion of all outstanding convertible indebtedness into an aggregate of shares of common stock, which will occur automatically upon the closing of this offering and (iii) a one-for- reverse split of our common stock, which will occur immediately prior to the closing of this offering. Of these outstanding shares, all of the shares of common stock sold in this offering will be freely tradable, except that any shares purchased in this offering by our affiliates, as that term is defined in Rule 144 under the Securities Act of 1933, as amended, would only be able to be sold in compliance with the Rule 144 limitations described below.
The remaining outstanding shares of our common stock will be deemed “restricted securities” as defined in Rule 144. Restricted securities may be sold in the public market only if they are registered under the Securities Act or if they qualify for an exemption from registration under Rule 144 or Rule 701 promulgated under the Securities Act of 1933, as amended, which rules are summarized below. In addition, our officers and directors have entered into lock-up agreements with the underwriter under which they have agreed, subject to specific exceptions, not to sell any of our stock for at least 180 days following the date of this prospectus, as described below. As a result of these agreements, subject to the provisions of Rule 144 or Rule 701, based on an assumed offering date of , 2014, shares will be available for sale in the public market as follows:
| · | Beginning on the date of this prospectus, all of the shares of common stock sold in this offering will be immediately available for sale in the public market; |
| · | Beginning 181 days after the date of this prospectus, subject to possible extension as described in “Underwriting” below, additional shares of common stock will become eligible for sale in the public market, of which shares will be held by affiliates and subject to the volume and other restrictions of Rule 144, as described below; and |
| · | The remainder of the shares will be eligible for sale in the public market from time to time thereafter, subject in some cases to the volume and other restrictions of Rule 144, as described below. |
| 67 |
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person would be entitled to sell those shares upon expiration of the lock-up agreements described below, without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell upon expiration of the lock-up agreements described above, within any three-month period, a number of shares that does not exceed the greater of:
| · | 1% of the number of shares of common stock then outstanding, which will equal approximately shares immediately after this offering; or |
| · | the average weekly trading volume of common stock on the Nasdaq Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale. |
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required by that rule to wait until 90 days after the date of this prospectus before selling those shares pursuant to Rule 701, subject to the market standoff agreements and lock-up agreements described above.
Stock Options
As soon as practicable after the closing of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act of 1933, as amended, covering all of the shares of our common stock subject to outstanding options and the shares of our common stock reserved for issuance under our stock plans. In addition, we intend to file a registration statement on Form S-8 or such other form as may be required under the Securities Act of 1933, as amended, for the resale of shares of our common stock issued upon the exercise of options that were not granted under Rule 701. We expect to file this registration statement as soon as permitted under the Securities Act of 1933, as amended. However, the shares registered on Form S-8 may be subject to the volume limitations and the manner of sale, notice and public information requirements of Rule 144 and will not be eligible for resale until expiration of the lock-up and market standoff agreements to which they are subject.
Lock-up Agreements
For a description of the lock-up agreements with the underwriter that restrict sales of shares by us and our executive officers and directors, see the information under the heading “Underwriting.”
| 68 |
UNDERWRITING
Under the terms and subject to the conditions contained in an underwriting agreement, we have agreed to sell to the underwriter named below, and the underwriter has agreed to purchase from us, the number of shares of common stock and warrants set forth opposite its name below:
| Underwriter | Number of
Shares | Number of Warrants | ||||||
| Chardan Capital Markets LLC | ||||||||
| Total | ||||||||
The underwriting agreement provides that the obligation of the underwriter to purchase the securities offered hereby is subject to certain conditions and that the underwriter is obligated to purchase all of the securities offered hereby if any of the securities are purchased.
If the underwriter sells more shares of common stock and warrants than the above number, the underwriter has an option for 30 days to buy up to an aggregate of additional shares of common stock and/or up to additional warrants from us at the public offering price less the underwriting commissions and discounts to cover these sales.
The underwriter proposes to offer to the public the securities purchased pursuant to the underwriting agreement at the public offering price on the cover page of this prospectus. After completion of this offering, the underwriter may change the offering price and other selling terms at various times. In connection with the sale of the securities to be purchased by the underwriter, the underwriter will be deemed to have received compensation in the form of underwriting commissions and discounts.
Commission and Expenses
The underwriter has advised us that it proposes to offer the securities to the public at the public offering prices set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share of common stock and $ per warrant. After this offering, the public offering price and concession may be changed by the underwriter. No such change shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus. The securities are offered by the underwriter as stated herein, subject to receipt and acceptance by the underwriter and subject to their right to reject any order in whole or in part. The underwriter has informed us that it does not intend to confirm sales to any accounts over which it exercises discretionary authority.
We have agreed to pay to the underwriter a fee equal to 9% of the aggregate sales price of the securities sold in this offering, which fee is to be paid by means of a discount from the offering price to purchasers in the offering. In addition, we have agreed to reimburse the underwriter for all of its agreed-upon, actual and out-of-pocket expenses, including but not limited to reasonable and documented travel, legal fees and other expenses, incurred in connection with the offering, whether or not the offering is completed, subject to presentation of appropriate documentation evidencing such out-of-pocket expenses up to a maximum of $25,000 for all expenses other than legal fees plus $125,000 for legal fees upon completion of this offering. In the event that this offering is not consummated, we will not be required to reimburse the underwriter for more than $25,000 for legal fees and we will not be required to reimburse the underwriter for any other out of pocket expenses. Notwithstanding the foregoing, if the underwriter terminates the underwriting agreement prior to closing under certain circumstances, including if we breach the terms of the underwriting agreement or there is a material adverse change impacting the markets generally or the Company specifically, then the foregoing limitations on reimbursement of expenses shall not apply. We estimate that expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $ .
| 69 |
The following table summarizes the public offering price, underwriting discounts and commissions and proceeds before expenses to us assuming both no exercise and full exercise of the underwriter’s option to purchase additional securities:
| Total | ||||||||||||||
| Per Share | Per Warrant | Without Over- |
With Over- Allotment |
|||||||||||
| Public offering price | $ | $ | $ | $ | ||||||||||
| Underwriting discounts and commissions | ||||||||||||||
| Proceeds, before expenses, to us | ||||||||||||||
Underwriter Compensation Warrants
The Company shall issue to the underwriter, upon the closing of the offering, and, if applicable, upon the closing of the over-allotment option, underwriter warrants equal in number to 5.0% of the aggregate number of shares of common stock issued under the offering and, in the event that the over-allotment option is exercised, including 5% of the shares of common stock issued pursuant to the exercise of the over-allotment option. Each underwriter warrant will have a term of three years commencing one year from the date on which the Registration Statement on Form S-1 of which this prospectus forms a part is declared effective by the Securities and Exchange Commission and may be exercised on a cashless basis if not registered. The underwriter warrants will have an exercise price equal to 125% of the per share price of the common stock sold in this offering. The underwriter warrants are exercisable commencing twelve months after the date on which the Registration Statement on Form S-1 of which this prospectus forms a part is declared effective by the Securities and Exchange Commission. The underwriter warrants are not redeemable by us.
The underwriter warrants and the shares of our common stock underlying such warrants are deemed to be underwriting compensation by the FINRA and are therefore subject to a 180-day lock-up pursuant to FINRA Rule 5110(g)(1). The underwriter (or permitted assignee under the rule) may not sell, transfer, assign, pledge or hypothecate the underwriter warrants or the securities underlying these warrants, nor will it engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the underwriter warrants or the underlying securities for a period of 180 days from the date on which the Registration Statement on Form S-1 of which this prospectus forms a part is declared effective by the Securities and Exchange Commission, except to any FINRA member participating in the offering and their bona fide officers or partners.
Lock Up Agreements
We have agreed not to offer, sell, contract to sell, pledge, grant options to purchase, or otherwise dispose of any shares of our common stock or securities exchangeable for or convertible into our common stock for a period of 180 days after the date of this prospectus supplement without the prior written consent of Chardan Capital Markets LLC. This agreement does not apply to the issuance of shares upon the exercise of rights to acquire shares of common stock pursuant to any existing stock option or similar equity incentive or compensation plan. Our directors and executive officers have agreed, subject to certain exceptions, not to, directly or indirectly, sell, hedge, or otherwise dispose of any shares of common stock, options to acquire shares of common stock or securities exchangeable for or convertible into shares of common stock, for a period of 180 days from the date on which the Registration Statement on Form S-1 of which this prospectus forms a part is declared effective by the Securities and Exchange Commission without the prior written consent of Chardan Capital Markets LLC.
However, in the event that either (i) during the last 17 days of the “lock-up” period, we release earnings results or material news or a material event relating to us occurs or (ii) prior to the expiration of the “lock-up” period, we announce that we will release earnings results during the 16-day period beginning on the last day of the “lock-up” period, the expiration of the “lock-up” will be extended until the expiration of the 18-day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless Chardan Capital Markets LLC waives, in writing, such an extension.
Indemnification
The underwriting agreement provides that we will indemnify the underwriter against certain liabilities that may be incurred in connection with this offering, including liabilities under the Securities Act, or to contribute payments that the underwriter may be required to make in respect thereof.
Listing
We have applied to list our shares of common stock on the Nasdaq Capital Market, subject to notice of issuance, under the symbol “ NVBX.”
| 70 |
Electronic Distribution
A prospectus in electronic format may be made available on websites or through other online services maintained by the underwriter of this offering, or by its affiliates. Other than the prospectus in electronic format, the information on the underwriter’s website and any information contained in any other website maintained by an underwriter is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the underwriter in its capacity as underwriter, and should not be relied upon by investors.
Price Stabilization, Short Positions and Penalty Bids
In connection with the offering the underwriter may engage in stabilizing transactions, syndicate covering transactions and penalty bids with respect to the common stock only, and in over-allotment transactions with respect to the common stock and the warrants, in each case in accordance with Regulation M under the Exchange Act:
| · | Stabilizing transactions permit bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum. |
| · | Over-allotment involves sales by the underwriter of securities in excess of the number of securities the underwriter is obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by the underwriter is not greater than the number of securities that it may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. The underwriter may close out any covered short position by either exercising its over-allotment option and/or purchasing securities in the open market. |
| · | Syndicate covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriter will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which it may purchase shares through the over-allotment option. If the underwriter sells more shares than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriter is concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. |
| · | Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriter makes any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriter makes any representations that the underwriter will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
No Public Market
Prior to this offering, there has not been a public market for our securities in the U.S. and the public offering price for our securities will be determined through negotiations between us and the underwriter. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the underwriter believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant.
| 71 |
We offer no assurances that the initial public offering price will correspond to the price at which our common stock will trade in the public market subsequent to this offering or that an active trading market for our common stock will develop and continue after this offering.
Affiliations
The underwriter and its affiliates may in the future provide, various investment banking and other financial services for us for which services they may in the future receive, customary fees. Except for services provided in connection with this offering, the underwriter has not provided any investment banking or other financial services to us and we do not expect to retain the underwriter to perform any investment banking or other financial services to us for at least 90 days after the date of this prospectus.
LEGAL MATTERS
The validity of the securities offered hereby will be passed upon for us by Haynes and Boone, LLP, New York, New York. The underwriter is being represented by Loeb & Loeb, LLP, New York, New York, in connection with the offering.
EXPERTS
Our consolidated financial statements as of December 31, 2011 and 2012 and for the years ended December 31, 2011 and 2012 included in this prospectus have been audited by Kost Forer Gabbay & Kasierer, a member firm of Ernst & Young Global, an independent registered public accounting firm, as stated in their report appearing herein (which contains an explanatory paragraph describing conditions that raise a substantial doubt about our ability to continue as a going concern as described in Note 1b to the consolidated financial statements), and are included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed a registration statement on Form S-1 with the Securities and Exchange Commission in connection with this offering. In addition, as a result of this offering, we will become subject to the full informational requirements of the Securities Exchange Act of 1934, as amended, and, accordingly, will file annual, quarterly and current reports and other information with the Securities and Exchange Commission. You may read and copy the registration statement and any other documents we have filed at the Securities and Exchange Commission’s Public Reference Room at 100 F Street, N.W., Washington, D.C. 20549, on official business days during the hours of 10:00 am to 3:00 pm.. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the Public Reference Room. Our Securities and Exchange Commission filings are also available to the public at the Securities and Exchange Commission’s Internet site at “http://www.sec.gov”. Upon completion of this offering, you will also be able to access, free of charge, our reports filed with the Securities and Exchange Commission through our website (www.nanovibronix.com). Reports filed with or furnished to the Securities and Exchange Commission will be available as soon as reasonably practicable after they are filed with or furnished to the Securities and Exchange Commission. None of the information contained on, or that may be accessed through our websites or any other website identified herein is part of, or incorporated into, this prospectus. All website addresses in this prospectus are intended to be inactive textual references only.
This prospectus is part of the registration statement and does not contain all of the information included in the registration statement. Whenever a reference is made in this prospectus to any of our contracts or other documents included as exhibits to this registration statement, the reference may not be complete and, for a copy of the contract or document included as exhibits to this registration statement, you should refer to the exhibits that are a part of the registration statement.
| 72 |
NANO VIBRONIX INC.AND ITS SUBSIDIARY
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. DOLLARS IN THOUSANDS
| Page | |
| CONSOLIDATED FINANCIAL STATEMENTS AS OF DECEMBER 31, 2012 |
|
| Report of Independent Registered Public Accounting Firm | F-2 |
| Consolidated Balance Sheets | F-3 |
| Consolidated Statements of Comprehensive Loss | F-5 |
| Consolidated Statements of Changes in Stockholders' Equity (Deficiency) | F-6 |
| Consolidated Statements of Cash Flows | F-7 |
| Notes to Consolidated Financial Statements | F-8 |
|
CONDENSED CONSOLIDATED FINANCIAL STATEMENTS AS OF SEPTEMBER 30, 2013 UNAUDITED |
|
| Condensed Consolidated Balance Sheets | F-28 |
| Condensed Consolidated Statements of Comprehensive Loss | F-30 |
| Condensed Consolidated Statements of Changes in Stockholders' Deficiency | F-31 |
| Condensed Consolidated Statements of Cash Flows | F-33 |
| Notes to Condensed Consolidated Financial Statements | F-34 |
- - - - - - - - - - - - - -
| F-1 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of
NANO VIBRONIX INC.
We have audited the accompanying consolidated balance sheets of Nano Vibronix Inc. ("the Company") and its subsidiary as of December 31, 2012 and 2011, and the related consolidated statements of comprehensive loss, changes in stockholders' equity (deficiency) and cash flows for each of the two years ended December 31, 2012. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financing reporting. Our audit includes consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, based on our audits, the consolidated financial statements referred to above, present fairly, in all material respects, the consolidated financial position of the Company and its subsidiary as of December 31, 2012 and 2011, and the consolidated results of their operations, changes in stockholders' equity (deficiency) and cash flows for each of the two years ended December 31, 2012, in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 1b, the Company has incurred recurring losses and negative cash flows from operating activities during the year ended December 31, 2012. Its ability to continue to operate is dependent upon obtaining additional financial support. These conditions as described in Note 1b, raise substantial doubt about the Company's ability to continue as a going concern. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
| /s/ Kost Forer Gabbay & Kasierer | ||
| Tel-Aviv, Israel | KOST FORER GABBAY & KASIERER | |
| November 12, 2013 | A Member of Ernst & Young Global |
| F-2 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 101 | $ | 893 | ||||
| Trade receivables | 7 | 6 | ||||||
| Prepaid expenses and other accounts receivable (Note 3) | 17 | 44 | ||||||
| Inventories | 60 | - | ||||||
| Total current assets | 185 | 943 | ||||||
| PROPERTY AND EQUIPMENT, NET (Note 4) | 28 | 32 | ||||||
| SEVERANCE PAY FUND | 136 | 110 | ||||||
| Total assets | $ | 349 | $ | 1,085 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-3 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands (except share data)
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| LIABILITIES AND STOCKHOLDERS' DEFICIENCY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 20 | $ | 19 | ||||
| Other accounts payables (Note 5) | 75 | 85 | ||||||
| Total current liabilities | 95 | 104 | ||||||
| LONG-TERM LIABILITIES: | ||||||||
| Convertible Promissory notes (Note 7) | 1,946 | 1,452 | ||||||
| Accrued severance pay | 140 | 114 | ||||||
| Total long-term liabilities | 2,086 | 1,566 | ||||||
| COMMITMENTS AND CONTINGENT LIABILITIES (Note 6) | ||||||||
| STOCKHOLDERS' DEFICIENCY (Note 9): | ||||||||
| Stock capital - | ||||||||
| Common stock of $ 0.001 par value - Authorized: 24,000,000 shares at December 31, 2012 and 2011; Issued and outstanding: 1,085,060 shares at December 31, 2012 and 2011 |
1 | 1 | ||||||
| Series A Preferred stock of $ 0.001 par value - Authorized: 700,000 shares at December 31, 2012 and 2011; Issued and outstanding: 394,232 shares at December 31, 2012 and 2011 |
- | *) | - | *) | ||||
| Additional paid-in capital | 10,381 | 10,353 | ||||||
| Accumulated deficit | (12,214 | ) | (10,939 | ) | ||||
| Total stockholders' deficiency | (1,832 | ) | (585 | ) | ||||
| Total liabilities and stockholders' deficiency | $ | 349 | $ | 1,085 | ||||
| *) | Represents an amount lower than $ 1. |
The accompanying notes are an integral part of the consolidated financial statements.
| F-4 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
U.S. dollars in thousands
| Year ended December 31, |
||||||||
| 2012 | 2011 | |||||||
| Revenues | $ | 166 | $ | 94 | ||||
| Cost of revenues | 50 | 29 | ||||||
| Gross profit | 116 | 65 | ||||||
| Operating expenses: | ||||||||
| Research and development, net | 572 | 510 | ||||||
| Selling and marketing | 190 | 150 | ||||||
| General and administrative | 128 | 87 | ||||||
| Total operating expenses | 890 | 747 | ||||||
| Operating loss | 774 | 682 | ||||||
| Financial expense, net (Note 10) | 501 | 41 | ||||||
| Net loss | $ | 1,275 | $ | 723 | ||||
| Total comprehensive loss | $ | 1,275 | $ | 723 | ||||
| Deemed dividend | - | 873 | ||||||
| Net loss attributable to holders of common stock | $ | 1,275 | $ | 1,596 | ||||
| Net basic and diluted loss per share (Note 12) | $ | (1.18 | ) | $ | (1.47 | ) | ||
| Weighted average number of common stock used in computing basic and diluted net loss per share | 1,085,060 | 1,085,060 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-5 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS' EQUITY (DEFICIENCY)
U.S. dollars in thousands (except share data)
| Total | ||||||||||||||||||||||||||||
| Preferred stock | Common stock | Additional paid- in |
Accumulated | stockholders' equity |
||||||||||||||||||||||||
| Number | Amount | Number | Amount | capital | Deficit | (deficiency) | ||||||||||||||||||||||
| Balance as of January 1, 2011 | 453,959 | $ | - | *) | 1,085,060 | $ | 1 | $ | 9,507 | $ | (9,343 | ) | $ | 165 | ||||||||||||||
| Issuance of Series B Preferred stock | 18,840 | - | *) | - | - | 325 | - | 325 | ||||||||||||||||||||
| Deemed dividend in respect of equity restructuring | - | - | - | - | 873 | (873 | ) | - | ||||||||||||||||||||
| Cancellation of Series B Preferred stock upon issuance of B-2 Promissory notes | (78,567 | ) | - | *) | - | - | (1,557 | ) | - | (1,557 | ) | |||||||||||||||||
| Benefit component of convertible notes and warrants | - | - | - | - | 1,142 | - | 1,142 | |||||||||||||||||||||
| Stock-based compensation related to options granted to consultants and employees | - | - | - | - | 63 | - | 63 | |||||||||||||||||||||
| Total comprehensive loss | - | - | - | - | - | (723 | ) | (723 | ) | |||||||||||||||||||
| Balance as of December 31, 2011 | 394,232 | - | *) | 1,085,060 | 1 | 10,353 | (10,939 | ) | (585 | ) | ||||||||||||||||||
| Stock-based compensation related to options granted to consultants and employees | - | - | - | - | 28 | - | 28 | |||||||||||||||||||||
| Total comprehensive loss | - | - | - | - | - | (1,275 | ) | (1,275 | ) | |||||||||||||||||||
| Balance as of December 31, 2012 | 394,232 | $ | - | *) | 1,085,060 | $ | 1 | $ | 10,381 | $ | (12,214 | ) | $ | (1,832 | ) | |||||||||||||
| *) | Represents an amount lower than $ 1. |
The accompanying notes are an integral part of the consolidated financial statements.
| F-6 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands
| Year ended December 31, |
||||||||
| 2012 | 2011 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (1,275 | ) | $ | (723 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 9 | 10 | ||||||
| Stock based compensation | 28 | 63 | ||||||
| Benefit component of B-2 promissory notes | 238 | 16 | ||||||
| Decrease (increase) in trade receivables | (1 | ) | 22 | |||||
| Decrease in prepaid expenses and other accounts receivable | 27 | 89 | ||||||
| Increase in inventories | (60 | ) | - | |||||
| Increase (decrease) in accounts payable | 1 | (2 | ) | |||||
| Decrease in accrued liabilities and expenses | (10 | ) | (38 | ) | ||||
| Decrease in accrued severance pay, net | - | (5 | ) | |||||
| Accrued interest on promissory notes | 256 | 21 | ||||||
| Net cash used in operating activities | (787 | ) | (547 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchase of property and equipment | (5 | ) | (2 | ) | ||||
| Net cash used in investing activities | (5 | ) | (2 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from issuance of promissory notes | - | 1,000 | ||||||
| Proceeds from issuance of Series B Preferred stock, net | - | 325 | ||||||
| Net cash provided by financing activities | - | 1,325 | ||||||
| Increase (decrease) in cash and cash equivalents | (792 | ) | 776 | |||||
| Cash and cash equivalents at the beginning of the period | 893 | 117 | ||||||
| Cash and cash equivalents at the end of the period | $ | 101 | $ | 893 | ||||
| Supplemental information and disclosure of non-cash financing transactions: | ||||||||
| Benefit component of B-2 Promissory notes and warrants | $ | - | $ | 1,142 | ||||
| Deemed dividend in respect of equity restructuring | $ | - | $ | 873 | ||||
| Issuance of B-2 promissory note in respect of Preferred B stock cancellation | $ | - | $ | 1,557 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-7 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands (except share data and per share data)
| NOTE 1:- | GENERAL |
| a. | Nano Vibronix Inc. (“the Company”), a U.S. (Delaware) corporation, commenced operations on October 20, 2003 and is a medical device company focusing on noninvasive biological response-activating devices that target wound healing and pain therapy and can be administered at home, without the assistance of medical professionals. |
The Company's principal research and development activities are conducted in Israel through its wholly-owned subsidiary, Nano Vibronix (Israel 2003) Ltd., a company registered in Israel which commenced operations in October 2003.
| b. | During the ended year December 31, 2012, the Company continues to incur losses and negative cash flows from operating activities amounting to $ 1,275 and $ 787, respectively. These conditions raise substantial doubts about the Company's ability to continue as a going concern. The Company's ability to continue to operate is dependent upon raising additional funds to finance its activities. The Company plans to raise capital to finance its operations. There are no assurances, however, that the Company will be successful in obtaining an adequate level of financing needed for the long-term development and commercialization of its products. The consolidated financial statements do not include any adjustments with respect to the carrying amounts of assets and liabilities and their classification that might be necessary should the Company be unable to continue as a going concern. |
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES |
The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States ("U.S. GAAP").
| a. | Use of estimates: |
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
| F-8 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| b. | Financial statements in U.S. dollars: |
The accompanying financial statements have been prepared in U.S. dollars.
The majority of the Company's finances are received in U.S. dollars. Although a portion of the Company's expenses are dominated in New Israeli Shekel ("NIS") (mostly salary and rent), a substantial portion of the expenses are denominated in U.S. dollar. In addition, most of the Company's assets and liabilities are in U.S. dollars and management expects that most of its revenues will be generated in US dollars. The Company's management believes that the currency of the primary economic environment in which the operations of the Company and its subsidiary are conducted is the U.S. dollar; thus the dollar is the functional currency of the Company and its subsidiary.
Transactions and balances originally denominated in U.S. dollars are presented at their original amounts. Transactions and balances in other currencies have been remeasured into U.S. dollars in accordance with ASC 830, "Foreign Currency Matters" ("ASC 830").
All transaction gains and losses from the remeasurement of monetary balance sheet items denominated in non-dollar currencies are reflected in the statement of operations in financial expenses, net, as appropriate.
| c. | Principles of consolidation: |
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, Nano Vibronix (Israel 2003) Ltd. All intercompany balances and transactions have been eliminated upon consolidation.
| d. | Cash equivalents: |
Cash equivalents are short-term highly liquid investments that are readily convertible to cash with original maturities of three months or less at the date acquired.
| e. | Inventories: |
Inventories are stated at the lower of cost or market value. Inventory write-offs are provided to cover risks arising from slow-moving items or technological obsolescence. The Company periodically evaluates the quantities on hand relative to current and historical selling prices and historical and projected sales volume. Based on this evaluation, provisions are made when required to write-down inventory to its market value. As of December 31, 2012, no provision was recorded.
Inventories include finished products and raw materials. Cost is determined using the “first-in, first-out” method.
| f. | Property and equipment: |
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated by the straight-line method over the estimated useful lives of the assets, at the following annual rates:
| % | |
| Computers and peripheral equipment | 33 |
| Office furniture and equipment | 10 – 15 (mainly 10) |
| F-9 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| g. | Impairment of long-lived assets: |
The Company's property and equipment are reviewed for impairment in accordance with ASC 360, "Property Plant and Equipment", whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the assets. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less selling costs. During the years ended December 31, 2012 and 2011, no impairment losses have been identified.
| h. | Severance pay: |
The Company's liability for severance pay in respect of its subsidiary is calculated pursuant to Israel's Severance Pay Law, based on the most recent salary of the employees multiplied by the number of years of employment, as of the balance sheet date. Employees are entitled to one month's salary for each year of employment or a portion thereof. The Company's liability for all of its Israeli employees is covered by monthly deposits for insurance policies and/or pension funds and by an accrual. The value of these policies and/or funds is recorded as an asset in the Company's balance sheet. The deposited funds include profits accumulated to the balance sheet date. The deposited amounts may be withdrawn only upon the fulfillment of the obligations pursuant to the Israel's Severance Pay Law or labor agreements. The value of the deposited funds is based on the cash surrendered value of these policies, and includes immaterial profits.
Severance expenses (income) for the years ended December 31, 2012 and 2011, amounted to $ 26 and $ (37), respectively.
| i. | Revenue recognition: |
The Company generates revenues from the sale of its products to distributors and end users, which are usually doctors as well as patients using the product at home. Revenues from those products are recognized in accordance with Staff Accounting Bulletin No. 104, "Revenue Recognition" ("SAB No. 104"), when delivery has occurred, persuasive evidence of an agreement exists, the vendor's fee is fixed or determinable, no further obligation exists and collectability is probable.
The Company’s agreements with its distributors do not contain any price protection guarantees, rights of return or other post-shipment obligations.
Revenues derived from product sales to distributors do not contain any price protection guarantee, rights of return or other post-shipment obligation.
| j. | Research and development costs, net: |
Research and development costs, net of grants received, are charged to the statement of operations, as incurred.
| F-10 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| k. | Government grants: |
Royalty-bearing grants from the Government of Israel for funding approved research and development activities are recognized at the time the Company is entitled to such grants, on the basis of the costs incurred, and included as a reduction from research and development costs. During the years ended December 31, 2012 and 2011, research and development grants amounted to $ 0 and $ 113, respectively.
| l. | Income taxes: |
The Company accounts for income taxes in accordance with ASC 740, "Income Taxes". This Statement prescribes the use of the liability method whereby deferred tax asset and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value. As of December 31, 2012 and 2011, a full valuation allowance was provided by the Company.
The Company implements a two-step approach to recognize and measure uncertain tax positions. The first step is to evaluate the tax position taken or expected to be taken in a tax return by determining if the weight of available evidence indicates that it is more likely than not that, on an evaluation of the technical merits, the tax position will be sustained on audit, including resolution of any related appeals or litigation processes. The second step is to measure the tax benefit as the largest amount that is more than 50% (cumulative basis) likely to be realized upon ultimate settlement. The Company believes that its tax positions are all highly certain of being upheld upon examination. As such, as of December 31, 2012 and 2011 the Company has not recorded a liability for uncertain tax positions.
| m. | Accounting for stock-based compensation: |
The Company accounts for stock-based compensation in accordance with ASC 718, "Compensation-Stock Compensation" which requires companies to estimate the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the Company's consolidated statements of operations.
The Company recognizes compensation expenses for the value of its awards granted based on the straight line method over the requisite service period of each of the awards, net of estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
| F-11 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
The Company selected the Black-Scholes-Merton option pricing model as the most appropriate fair value method for its stock-options awards. The option-pricing model requires a number of assumptions, of which the most significant are the expected stock price volatility and the expected option term. Expected volatility was calculated based upon similar traded companies' historical share price movements. The expected option term represents the period that the Company's stock options are expected to be outstanding. The Company currently uses simplified method until sufficient historical exercise data will support using expected life assumptions .The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with an equivalent term.
The Company has historically not paid dividends and has no foreseeable plans to pay dividends.
The Company applies ASC 505-50, "Equity-Based Payments to Non-Employees" with respect to options and warrants issued to non-employees.
The fair value of the shares of Common Stock underlying the options and warrants had been determined by the Company`s management with assistance of an independent valuation firm by applying of market approach using recent third-party transactions in the equity of the Company. Because there has been no public market for the Common Stock, management has determined fair value of the Common Stock at the time of grant of options by considering a number of objective and subjective factors. The fair value of the underlying shares of Common Stock shall be determined by management until such time as the Common Stock is listed on an established stock exchange, national market system or other quotation system.
| n. | Fair value of financial instruments: |
ASC 820, "Fair Value Measurements and Disclosures", defines fair value as the price that would be received to sell an asset or paid to transfer a liability (i.e., the "exit price") in an orderly transaction between market participants at the measurement date.
In determining fair value, the Company uses various valuation approaches. ASC 820 establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company's assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances.
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
| F-12 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
As a basis for considering such assumptions, ASC 820 establishes a three-tier value hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value:
| Level 1 - | Valuations based on quoted prices in active markets for identical assets that the Company has the ability to access. Valuation adjustments and block discounts are not applied to Level 1 instruments. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these products does not entail a significant degree of judgment. |
| Level 2 - | Valuations based on one or more quoted prices in markets that are not active or for which all significant inputs are observable, either directly or indirectly. |
| Level 3 - | Valuations based on inputs that are unobservable and significant to the overall fair value measurement. |
The carrying amounts of cash and cash equivalents, trade receivables, prepaid expenses and other accounts receivable, accounts payable and other accounts payable approximate their fair value due to the short-term maturities of such instruments.
| o. | Convertible promissory notes: |
The Company applies ASC 470-20, "Debt with Conversion and Other Options" ("ASC 470-20"), when it can’t elect the fair value option under ASC 825, "Financial Instruments". In accordance with ASC 470-20 the Company first allocates the proceeds to freestanding liability instrument that are measured at fair value at each reporting date, based on their fair value. The remaining proceeds are allocated between the convertible debt and all other freestanding instruments based on the relative fair values of the instruments at the time of issuance. In accordance with ASC 815 "Derivatives and Hedging" ("ASC 815"), the Company bifurcates all embedded derivatives that require bifurcation and accounts for them separately from the convertible debt.
Embedded derivatives that are separated from the convertible debt are bifurcated based on their fair value and remeasured on each reporting date.
In addition, under the guidelines of ASC 470-20, the Company measures and recognizes the embedded beneficial conversion feature on the commitment date. The beneficial conversion feature is measured by allocating a portion of the proceeds equal to the intrinsic value of the feature to additional paid-in-capital. The intrinsic value of the feature is calculated on the commitment date using the effective conversion price which had resulted subsequent to the allocation of the proceeds between the convertible debt and all other freestanding instruments. This intrinsic value is limited to the portion of the proceeds allocated to the convertible debt.
The Company applied ASC 470-20 and ASC 815 to the Convertible promissory notes (see Note 7).
| F-13 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| p. | Basic and diluted net loss per share: |
Basic net loss per share is computed based on the weighted average number of Common stock outstanding during each year. Diluted net loss per share is computed based on the weighted average number of Common stock outstanding during each year plus dilutive potential equivalent Common stock considered outstanding during the year, in accordance with ASC 260, "Earnings per Share."
All outstanding stock options and warrants have been excluded from the calculation of the diluted net loss per share because all such securities are anti-dilutive for all periods presented.
| q. | Concentrations of credit risk: |
Financial instruments that potentially subject the Company and its subsidiary to concentrations of credit risk consist principally of cash and cash equivalents and trade receivables.
Cash and cash equivalents are invested in major banks in the United States and Israel. Such deposits in the United States and in Israel may be in excess of insured limits and are not insured in other jurisdictions. Management believes that the financial institutions that hold the Company's investments are financially sound and, accordingly, minimal credit risk exists with respect to these investments.
Trade receivables are mainly derived from sales to customers, located in the United States of America, Israel, Europe and India. The Company performs ongoing credit evaluation of its customers and to date has not experienced any material losses.
The Company and its subsidiary have no off-balance-sheet concentration of credit risk such as foreign exchange contracts, option contracts or other foreign hedging arrangements.
| r. | Impact of recently issued accounting standards: |
In May 2011, the Financial Accounting Standards Board (FASB) issued guidance that changed the requirement for presenting "Comprehensive Income" in the consolidated financial statements. The update requires an entity to present the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. The update is effective for fiscal years, and interim periods within those years, ending after December 15, 2012 and should be applied retrospectively. The adoption of the standard had no impact on the Company's financial position or results of operations, but resulted in a change in the presentation of its basic consolidated financial statements. The Company elected to present comprehensive income in a single continuous statement of comprehensive income.
| F-14 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 3:- | PREPAID EXPENSES AND OTHER ACCOUNTS RECEIVABLE |
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Prepaid expenses | $ | - | $ | 5 | ||||
| Other accounts receivable | 17 | 39 | ||||||
| $ | 17 | $ | 44 | |||||
| NOTE 4:- | PROPERTY AND EQUIPMENT, NET |
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Cost: | ||||||||
| Computers and peripheral equipment | $ | 92 | $ | 87 | ||||
| Office furniture and equipment | 8 | 8 | ||||||
| 100 | 95 | |||||||
| Accumulated depreciation: | ||||||||
| Computers and peripheral equipment | 65 | 57 | ||||||
| Office furniture and equipment | 7 | 6 | ||||||
| 72 | 63 | |||||||
| Depreciated cost | $ | 28 | $ | 32 | ||||
Depreciation expenses for the years ended December 31, 2012 and 2011 were $ 9 and $ 10, respectively.
| NOTE 5:- | OTHER ACCOUNTS PAYABLES |
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Employees and payroll accruals | $ | 51 | $ | 53 | ||||
| Accrued expenses | 24 | 32 | ||||||
| $ | 75 | $ | 85 | |||||
| F-15 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 6:- | COMMITMENTS AND CONTINGENT LIABILITIES |
| a. | The Company leases office facilities and motor vehicles under operating leases, which expire on various dates, the latest of which is 2015. |
Future minimum lease commitments under non-cancelable operating lease agreements as of December 31, 2012 are as follows:
| Year ended December 31, | Operating leases | |||
| 2013 | $ | 47 | ||
| 2014 | 34 | |||
| 2015 | 31 | |||
| Total | $ | 112 | ||
Rent and related expenses were $ 46 and $ 39 for the years ended December 31, 2012 and 2011, respectively.
| b. | Royalties to the Office of the Chief Scientist ("the OCS"): |
Under the Company's subsidiary research and development agreements with the OCS and pursuant to applicable laws, the Company is required to pay royalties at the rate of 3-3.5% of sales of products developed with funds provided by the OCS, up to an amount equal to 100% of the OCS research and development grants received, linked to the dollar including accrued inters at the LIBOR rate. The Company is obligated to repay the Israeli Government for the grants received only to the extent that there are sales of the funded products.
As of December 31, 2012, the Company has a contingent obligation to pay royalties in the principal amount of approximately $ 453. In addition, the OCS may impose certain conditions on any arrangement under which it permits the Company to transfer technology or development out of Israel.
| c. | In December 2011, the Company entered into a license agreement with a third party, to manufacture and sell its products. The Company will market and sell the third party's products and will pay future royalties as a percentage of future revenue. In regard with this agreement, during 2012 the Company paid an advance payment in an amount of $ 75 on account of the future royalties, which was recorded to the consolidated statement of comprehensive loss in the year ended December 31, 2012, due to the uncertainty of the Company`s ability to sell the products. In addition, the Company is obligated to pay additional amount of $ 75, in case the actual paid royalties until 2014 will not exceed the minimum of $ 150, as defined in the agreement. Since the additional future royalties creates an executor contract to the Company, and in accordance with ASC 450-20, the liability was not recorded in the Company's consolidated financial statements as of December 31, 2012 and 2011, and will be recorded only when occurred. |
| F-16 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 7:- | CONVERTIBLE PROMISSORY NOTES |
In November 2011, the Company issued Convertible B-1 Promissory Notes (the "B-1 Promissory Notes") to new and existing stockholders for a consideration of $ 1,000. The B-1 Promissory Notes bears 10% annual interest and will be automatically converted into series B-1 Participating Convertible Preferred stock ("series B-1 Stock"), upon certain events as defined in the agreement, at a fix conversion price of $ 0.284 per share. In case the B-1 Promissory Notes will not be converted, the Company shall pay the unpaid principal amount and interest accrued on the earlier of an "Event of Default" (as defined in the agreement) or November 15, 2014 (the "Maturity Date").
Following the above mentioned, the Company's "old Series B" Participating Convertible Preferred stock and Warrants to Preferred B stock, issued during 2009 through 2011 pursuant to the subscription agreement from 2009 (the "Old Series B Investor") (see also Note 9) were automatically cancelled and its holders received Convertible B-2 Promissory Notes (the "B-2 Promissory Notes"). The B-2 Promissory Notes are in the principal amount equal to the sum of: (1) the purchase price paid by the investor, (2) any purchase price previously paid in connection with any prior exercise of warrants to Preferred B stock, plus (3) simple interest at 8% per annum from the date of payment of such amounts reflecting an aggregate amount of $1,557. The B-2 Promissory Notes terms are identical to the B-1 Promissory Notes terms, except that such B-2 Promissory Notes are convertible into stock of series B-2 Participating Convertible Preferred stock of the Company, par value $ 0.001 per share ("series B-2 stock") and the conversion price set forth in such notes is $ 0.199 per share (reflecting a 30% discount on the B-1 Promissory Notes' conversion price mentioned above). The B-2 Promissory notes are considered to be a liability pursuant to ASC 480 "Distinguishing Liabilities from Equity". The convertible notes are presented at accreted value, which includes the principal amount of the convertible notes less any discount and accumulated interest accrued over the term of the convertible notes, using the interest method.
In addition, the Company issued to the "Old Series B Investors" warrants to purchase 2,319,062 B-2 Preferred stock with a fix exercise price of $ 0.199 (reflecting a 30% discount on the fair value of the Company's Preferred stock on that date). The warrants shall expire on November 15, 2018. The fair value of the warrants on the issuance date was $571 was recorded in equity in accordance with ASC 470.
As a result of issuing the warrants and as a result of the discount on the conversion price of the B-2 Promissory note, the Company recorded in 2011 benefit component in the amount of $ 1,142, to be amortized over the terms of the B-2 Promissory Notes.
| F-17 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 8:- | TAXES ON INCOME |
| a. | As of December 31, 2012, the U.S. Company had federal and state net operating carry forward tax losses of approximately $ 8,930. The federal operating loss can be offset against taxable income for 20 years. Utilization of the U.S. net operating losses may be subject to substantial limitations due to the change of ownership provisions of the Internal Revenue Code of 1986. |
| b. | Foreign tax: |
| 1. | Tax rates: |
The Israeli corporate tax rate was 24% in 2011and 25% in 2012.
On July 30, 2013, the Israeli Parliament (the Knesset) approved the second and third readings of the Economic Plan for 2013-2014 ("Amended Budget Law") which consists, among others, of fiscal changes whose main aim is to enhance long-term collection of taxes.
These changes include, among others, raising the Israeli corporate tax rate from 25% to 26.5%, cancelling the lowering of the tax rates applicable to preferred enterprises (9% in development area A and 16% in other areas), taxing revaluation gains and increasing the tax rates on dividends within the scope of the Law for the Encouragement of Capital Investments to 20% effective from January 1, 2014.
| 2. | Income Tax (Inflationary Adjustments) Law, 1985: |
According to the law, until 2007, the results for tax purposes were adjusted for the changes in the Israeli CPI.
In February 2008, the "Knesset" (Israeli parliament) passed an amendment to the Income Tax (Inflationary Adjustments) Law, 1985, which limits the scope of the law starting 2008 and thereafter. Starting 2008, the results for tax purposes are measured in nominal values, excluding certain adjustments for changes in the Israeli CPI carried out in the period up to December 31, 2007. The amendment to the law includes, inter alia, the elimination of the inflationary additions and deductions and the additional deduction for depreciation starting 2008.
| 3. | The Law for the Encouragement of Capital Investments, 1959: |
According to the law, the Company's subsidiary is entitled to various tax benefits by virtue of the "Beneficiary Enterprise" status granted to the subsidiary, defined by this law.
| F-18 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 8:- | TAXES ON INCOME (Cont.) |
The principal benefits are:
The subsidiary is tax exempt for a benefit period of two years and in the five subsequent years of the benefit period is subject to a reduced tax rate of 10%-25% (based on percentage of foreign ownership).
According to the law, the benefit period commences in the later of the year elected by the subsidiary or the first year in which the subsidiary has taxable income, provided that 12 years have not elapsed from the beginning of the year of election. The subsidiary has elected 2005 as the year of election.
If dividends are distributed out of tax exempt profits, the subsidiary will then become liable for tax at the rate applicable to its profits from the approved enterprise in the year in which the income was earned, as if it had not chosen the alternative track of benefits. The subsidiary's policy is not to distribute dividends out of these profits.
Conditions for the entitlement to the benefits:
The above benefits are conditional upon the fulfillment of the conditions stipulated by the law, regulations published there under and the letters of approval for the specific investments in the Beneficiary Enterprise. In the event of failure to comply with these conditions, the benefits may be canceled and the subsidiary may be required to refund the amount of the benefits, in whole or in part, including interest. The management believes that the subsidiary is meeting the aforementioned conditions.
In December 2010, the "Knesset" (Israeli Parliament) passed the Law for Economic Policy for 2011 and 2012 (Amended Legislation), 2011 ("the Amendment"), which prescribes, among others, amendments in the Law for the Encouragement of Capital Investments, 1959 ("the Law"). The Amendment became effective as of January 1, 2011. According to the Amendment, the benefit tracks in the Law were modified and a flat tax rate applies to the Company's entire preferred income under its status as a preferred company with a preferred enterprise. Commencing from the 2011 tax year, the Company will be able to apply (the waiver is non-recourse) the Amendment and from the elected tax year and onwards, it will be subject to the amended tax rates that are: 2011 and 2012 - 15% (in development area A - 10%), 2013 and 2014 - 12.5% (in development area A - 7%) and in 2015 and thereafter - 12% (in development area A - 6%). Certain "Special Industrial Companies" that meet certain criteria would enjoy further reduced tax rates of 5% in Zone A and 8% elsewhere. The profits of these Industrial Companies would be freely distributable as dividends, subject to a 15% withholding tax (or lower, under an applicable tax treaty).
| F-19 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 8:- | TAXES ON INCOME (Cont.) |
The Company and its subsidiary has tested the impact of the amendment to the Law on its financial statements, and as of the publication of the reports the Company and its subsidiary estimates that it will not move under the initiation of the Law as of the tax year 2012.
This estimation of the Company and its subsidiary might change in the future until the submission of the final decision to the tax authorities, as stated in the amendment to the Law.
| 4. | The Israeli subsidiary has accumulated a net operating tax loss carryforward as of December 31, 2012, amounting to approximately $ 272, which may be carried forward and offset against taxable income in the future for an indefinite period. |
| 5. | The subsidiary has final tax assessments through 2007. |
| c. | Deferred income taxes: |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial purposes and the amounts used for income tax purposes. Significant components of the Company`s deferred tax assets are as follows:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carry forward | $ | 3,615 | $ | 3,628 | ||||
| Temporary differences | 5 | 4 | ||||||
| Deferred tax assets before valuation allowance | 3,620 | 3,632 | ||||||
| Valuation allowance | (3,620 | ) | (3,632 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that all or some portion of the deferred tax assets will not be realized. The ultimate realization of the deferred tax assets is dependent upon the generation of future taxable income during the periods in which temporary differences are deductible and net operating losses are utilized. Based on consideration of these factors, the Company recorded a full valuation allowance at December 31, 2012 and 2011.
| d. | The main reconciling item between the statutory tax rate of the Company and the effective tax rate is the recognition of valuation allowances in respect of deferred taxes relating to accumulated net operating losses carried forward due to the uncertainty of the realization of such deferred taxes. |
| F-20 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 9:- | STOCKHOLDERS' EQUITY (DEFICIENCY) |
| a. | Composition of stock capital: |
| December 31, 2012 and 2011 | ||||||||
| Authorized | Issued and outstanding |
|||||||
| number of shares | ||||||||
| Common stock of $ 0.001 par value | 24,000,000 | 1,085,060 | ||||||
| Series A-1 Preferred stock of $ 0.001 par value | 400,000 | 222,620 | ||||||
| Series A-2 Preferred stock of $ 0.001 par value | 300,000 | 171,612 | ||||||
| Series B-1 Preferred stock of $ 0.001 par value | 4,650,000 | - | ||||||
| Series B-2 Preferred stock of $ 0.001 par value | 12,650,000 | - | ||||||
| b. | Common Stock: |
The Common stock confer upon the holders the right to receive notice to participate and vote in general meetings of the Company, and the right to receive dividends, if declared and to participate in the distribution of the surplus assets and funds of the Company in the event of liquidation, dissolution or winding up of the Company.
| c. | Series A and B Convertible Preferred Stock: |
Liquidation preference - Upon any liquidation, dissolution or winding up of the Company, (i) first, each series B holder will be entitled to be paid, before any distribution or payment is made upon any other securities of the Company, an amount in cash equal to the aggregate Series Issuance Price (subject to adjustments) of all shares of series B Preferred stock held by such holder; (ii) second, each series A holder will be entitled to be paid, before any distribution or payment is made upon any junior securities of the Company, an amount in cash equal to the aggregate Series Issuance Price (subject to adjustments) of all shares of series A Preferred stock held by such holder, (iii) thereafter, each series A holder and series B holder shall participate in any distribution or payment on a pro-rata basis with all junior securities, and such shares shall thereafter confer only the rights of Common stock, as if such holder's Preferred stock had been converted into Common stock.
Voting rights - Each outstanding share of Preferred A and B stock shall have the number of votes equal to the number of whole shares of Common stock, into which such share of
Preferred stock is then convertible.
Conversion - Each share of series A Convertible Preferred stock or series B Convertible Preferred stock shall be convertible, at the option of the holder thereof, at any time and from time to time, and without the payment of additional consideration by the holder thereof, into such number of fully paid and non-assessable shares of Common stock as is determined by dividing the applicable Series Issuance Price by the conversion price in effect at the time of conversion.
| F-21 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 9:- | STOCKHOLDERS' EQUITY (DEFICIENCY) (Cont.) |
In each case, subject to adjustment for any and all recapitalizations, reclassifications, stock splits, reverse stock splits, stock dividends, subdivisions, combinations or similar events.
| d. | On September 9, 2009, the Company signed an investment agreement to raise an amount of up to $ 2,000 for issuance of series B Convertible Preferred stock of $ 0.001 par value each ("series B Preferred stock") and warrants to purchase series B Preferred stock at $ 17.25 per share, exercisable until March 31, 2014 ("series B Warrants"). According to the investment agreement, if the Company shall effect a private placement of at least $ 1,000, including convertible debt, the Securities shall automatically be cancelled and shall represent solely the right to receive such number of securities which would have been issued in consideration for the sum of: (1) the purchase price paid by the Investor, (2) any purchase price previously paid in connection with any prior exercise of the series B Warrants, plus (3) simple interest at 8% per annum from the date of payment of such amounts provided that the purchase price for such securities shall be deemed reduced by thirty percent. |
During 2009 the Company issued 37,639 shares of series B Preferred stock for a consideration of $ 639 (net of $ 10 issuance expenses). In addition, the Company issued 37,639 series B Warrants.
During 2010, the Company issued 22,088 shares of series B Preferred stock for a consideration of $ 381. In addition, the Company issued 22,088 series B Warrants.
During 2011, the Company issued 18,840 shares of series B Convertible Preferred stock for a consideration of $ 325. In addition, the Company issued 18,840 series B Warrants.
| e. | In October 2011, the Company signed an amendment to the investment agreement from 2009, according to which if the Company shall effect a private placement of at least $1,000 in convertible debt, the series B Preferred stock shall automatically be cancelled and shall represent solely the right to receive such number of convertible debt which would have been issued in consideration for the sum of: (1) the purchase price paid by the investor, (2) any purchase price previously paid in connection with any prior exercise of series B Warrants, plus (3) simple interest at 8% per annum from the date of payment of such amounts. The convertible debt exercise price will reflect 30% discount of the private placement price. As a result, the Company recorded deemed dividend in the amount of $ 873 to represent the equity restructuring. |
In November 2011, the Company issued promissory notes to new and existing stockholders for a consideration of $ 1,000 following which the series B Preferred stock and series B Warrants were automatically canceled (see also Note 7).
| F-22 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 9:- | STOCKHOLDERS' EQUITY (DEFICIENCY) (Cont.) |
| f. | Stock option plan: |
In November 2004, the Board of Directors of the Company adopted a stock option plan ("the Plan"), according to which options may be granted to employees, directors and consultants.
Pursuant to the Plan, the Company reserved for issuance 2,800,000 stock of Common stock. Each option entitles the holder to purchase one Ordinary stock of the Company and expires after 10 years from the date of grant. Any options, which are terminated, cancelled, forfeited or not exercised, become available for future grants.
As of December 31, 2012, under the Plan, 2,426,296 options were available for future grants.
| 1. | Option issued to employees |
| A summary of the Company's options activity and related information with respect to options granted to employees for the year ended December 31, 2012 is as follows: |
| Year ended December 31, |
||||||||||||||||
| 2012 | 2011 | |||||||||||||||
| Amount of options |
Weighted average exercise price |
Amount of options |
Weighted average exercise price |
|||||||||||||
| Outstanding - beginning of the year | 327,791 | $ | 7.89 | 330,816 | $ | 4.49 | ||||||||||
| Granted | - | $ | - | - | $ | - | ||||||||||
| Exercised | - | $ | - | - | $ | - | ||||||||||
| Expired or Forfeited | - | $ | - | (3,025 | ) | $ | 4.63 | |||||||||
| Outstanding - end of the year | 327,791 | $ | 7.89 | 327,791 | $ | 7.89 | ||||||||||
| Exercisable at end of year | 324,116 | $ | 3.88 | 312,441 | $ | 3.84 | ||||||||||
The weighted average remaining contractual life as of December 31, 2012 is 4.63 years. The aggregated intrinsic value of outstanding options and exercisable options, as of December 31, 2012 is $ 23 and $ 24, respectively.
As of December 31, 2012 the unrecognized compensation cost is $ 7 and $ 6 to be recognized in 2013 and 2014, respectively.
| F-23 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 9:- | STOCKHOLDERS' EQUITY (DEFICIENCY) (Cont.) |
| 2. | Option issued to non employees |
The Company's outstanding options granted to consultants as of December 31, 2012 are as follows:
| Issuance date | Options for Common stock |
Average exercise price per share |
Options exercisable |
Expiration date | ||||||||||
| December 2004 | 10,225 | $ | 7.38 | 10,225 | December 2014 | |||||||||
| April 2005 | 2,250 | $ | 6.74 | 2,250 | April 2015 | |||||||||
| December 2005 | 1,000 | $ | 6.74 | 1,000 | December 2015 | |||||||||
| September 2006 | 3,500 | $ | 3.37 | 3,500 | September 2016 | |||||||||
| December 2007 | 15,000 | $ | 12.08 | 15,000 | December 2017 | |||||||||
| October 2008 | 938 | $ | 6.74 | 938 | October 2018 | |||||||||
| April 2009 | 7,500 | $ | 10.35 | 6,812 | April 2019 | |||||||||
| December 2010 | 5,500 | $ | 0.284 | 5,500 | December 2020 | |||||||||
| Total | 45,913 | 45,225 | ||||||||||||
The fair value of the Company's stock options granted to non-employees for the year ended December 31, 2011 and 2012 was using the following weighted average assumptions:
| Year ended December 31, |
||||||||
| 2012 | 2011 | |||||||
| Dividend yield | 0 | % | 0 | % | ||||
| Expected volatility | 82 | % | 82 | % | ||||
| Risk-free interest | 0.72% - 1.48 | % | 0.46% - 1.53 | % | ||||
| Expected term (years) | 6 - 8 | 4 - 9 | ||||||
| 3. | Stock-based compensation: |
The stock based expense recognized in the financial statements for services received from employees and non-employees is shown in the following table:
| Year ended December 31, |
||||||||
| 2012 | 2011 | |||||||
| Research and development, net | $ | 17 | $ | 47 | ||||
| Selling and marketing | 7 | 9 | ||||||
| General and administrative | 4 | 7 | ||||||
| $ | 28 | $ | 63 | |||||
| F-24 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 10:- | FINANCIAL EXPENSE, NET |
| Year ended December 31, |
||||||||
| 2012 | 2011 | |||||||
| Income interest | $ | (1 | ) | $ | (1 | ) | ||
| Bank commission expenses | 4 | 2 | ||||||
| Interest on promissory notes | 256 | 21 | ||||||
| Benefit component of promissory notes | 238 | 16 | ||||||
| Other | 4 | 3 | ||||||
| $ | 501 | $ | 41 | |||||
| NOTE 11:- | GEOGRAPHIC INFORMATION AND MAJOR CUSTOMER DATA |
| Summary information about geographic areas: |
| ASC 280, “Segment Reporting,” establishes standards for reporting information about operating segments. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company manages its business on the basis of one reportable segment, and derives revenues from selling its products mainly through distributor agreements. The following is a summary of revenues within geographic areas: |
| Year ended December 31, |
||||||||
| 2012 | 2011 | |||||||
| United States | $ | 67 | $ | 27 | ||||
| Israel | 20 | 14 | ||||||
| Europe | 20 | 21 | ||||||
| India | 16 | 9 | ||||||
| Rest of the world | 43 | 23 | ||||||
| $ | 166 | $ | 94 | |||||
| During the years ended December 31, 2012 and 2011, there were no sales to a single customer exceeding 10% of the Company’s revenues. |
| The Company's long-lived assets are all located in Israel. |
| NOTE 12:- | BASIC AND DILUTED NET LOSS PER SHARE |
The following table sets forth the computation of the Company's basic and diluted net loss per share of Common stock:
| Year ended December 31 |
||||||||
| 2012 | 2011 | |||||||
| Net loss attributable to holders of Common stock as reported | $ | (1,275 | ) | $ | (1,596 | ) | ||
| Weighted average number of Common stock used in computing basic and diluted net loss per share | $ | 1,085,060 | 1,085,060 | |||||
| Net loss per share of Common stock, basic and diluted | $ | (1.18 | ) | $ | (1.47 | ) | ||
For the years ended December 31, 2012 and 2011, all outstanding options and warrants have been excluded from the calculation of the diluted net loss per share since their effect was anti-dilutive.
| F-25 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 13:- | SUBSEQUENT EVENTS |
| a. | On February 5, 2013 the Company signed an agreement with certain investors ("The Agreement"), according to which the Company issued convertible promissory notes (the "Note") in consideration for $100. The convertible promissory notes mature on the earlier of June 30, 2013, the closing date of a financing in which the Company shall sell an aggregate of at least $250 of its debt or equity securities or such accelerated date as a result of an occurrence of an event of default as defined in The Agreement, upon which date the entire outstanding principal balance and any outstanding fees or interest will be due and payable in full. The convertible promissory notes bear interest at the rate of 6% per annum, which rate is increased to 10% upon and during the occurrence of an event of default as defined in The Agreement. In addition, the convertible promissory notes are convertible either at the Company`s option or upon maturity of the promissory notes into Common stock at an initial conversion price of $0.38 per share, subject to adjustment for stock splits, fundamental transactions or similar events. |
In addition, the Company issued to the stockholder 263,158 warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants shall expire on February 5, 2018.
On March 28, 2013 the Company signed an amendment to The Agreement and issued convertible promissory notes to certain investors in the principal amount of $ 200. In addition, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on March 28, 2018.
On June 3, 2013 the Company signed a second amendment to The Agreement and issued to convertible promissory notes to certain investors in the principal amount of $ 300. The Note are convertible to Common stock and bears 6% interest, computed annually and should be fully due and payable at the earliest of September 30, 2013 or the closing date of a financing round as defined in the agreement. In addition, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on June 3, 2018.
On August 5, 2013, the Company signed a third amendment to The Agreement and issued convertible promissory notes to certain investors in the principal amount of $400. The Note are convertible to Common stock and bears 6% interest, computed annually and should be fully due and payable at the earliest of November 30, 2013 or the closing date of a financing round as defined in the agreement. In addition, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on August 5, 2018.
| F-26 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
| NOTE 13:- | SUBSEQUENT EVENTS (Cont.) |
On October 7, 2013, the Company signed a fourth amendment to The Agreement and issued convertible promissory notes to certain investors in the principal amount of $500. The Note are convertible to Common stock and bears 6% interest, computed annually and should be fully due and payable at the earliest of December 31, 2013 or the closing date of a financing round as defined in the agreement. In addition, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on October 7, 2018.
| b. | In March 2013, the Company granted 1,750,000 options fully vested for Common stock to 3 executive officers and directors. The average exercise price of the options is $ 0.04 and the options will expire in March 2023. |
| c. | The Company evaluates events or transactions that occur after the balance sheet date but prior to the issuance of financial statements to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure. For its consolidated financial statements as of December 31, 2012, the Company evaluated subsequent events through November 12, 2013, which is the date the consolidated financial statements were issued. |
- - - - - - - - - - - - - -
| F-27 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONDENSED CONSOLIDATED BALANCE SHEETS
U.S. DOLLARS IN THOUSANDS
| September 30, | December 31, | |||||||
| 2013 | 2012 | |||||||
| Unaudited | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 51 | $ | 101 | ||||
| Trade receivables | 20 | 7 | ||||||
| Prepaid expenses and other accounts receivable | 105 | 17 | ||||||
| Inventories | 72 | 60 | ||||||
| Total current assets | 248 | 185 | ||||||
| PROPERTY AND EQUIPMENT, NET | 24 | 28 | ||||||
| SEVERANCE PAY FUND | 163 | 136 | ||||||
| Total assets | $ | 435 | $ | 349 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-28 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONDENSED CONSOLIDATED BALANCE SHEETS
U.S. DOLLARS IN THOUSANDS (except share data)
| September 30, | December 31, | |||||||
| 2013 | 2012 | |||||||
| Unaudited | ||||||||
| LIABILITIES AND STOCKHOLDERS' DEFICIENCY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 16 | $ | 20 | ||||
| Other accounts payable | 197 | 75 | ||||||
| Convertible Promissory notes to common stock | 185 | - | ||||||
| Embedded feature of Convertible Promissory notes | 121 | - | ||||||
| Total current liabilities | 519 | 95 | ||||||
| LONG-TERM LIABILITIES: | ||||||||
| Convertible Promissory notes to preferred stock | 2,400 | 1,946 | ||||||
| Warrants to purchase Common stock | 116 | - | ||||||
| Accrued severance pay | 168 | 140 | ||||||
| Total long-term liabilities | 2,684 | 2,086 | ||||||
| STOCKHOLDERS' DEFICIENCY: | ||||||||
| Stock capital - | ||||||||
| Common stock of $ 0.001 par value - Authorized: 24,000,000 shares at September 30, 2013 and December 31, 2012; Issued and outstanding: 1,085,060 shares at September 30, 2013 and December 31, 2012 | 1 | 1 | ||||||
| Series A Preferred stock of $ 0.001 par value - Authorized: 700,000 shares at September 30, 2013 and December 31, 2012; Issued and outstanding: 394,232 shares at September 30, 2013 and December 31, 2012 | - | *) | - | *) | ||||
| Additional paid-in capital | 10,857 | 10,381 | ||||||
| Accumulated deficit | (13,626 | ) | (12,214 | ) | ||||
| Total stockholders' deficiency | (2,768 | ) | (1,832 | ) | ||||
| Total liabilities and stockholders' deficiency | $ | 435 | $ | 349 | ||||
| *) | Represents an amount lower than $ 1. |
The accompanying notes are an integral part of the consolidated financial statements.
| F-29 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
U.S. DOLLARS IN THOUSANDS (except share and per share data)
| Nine months ended September 30, |
Year ended December 31, |
|||||||||||
| 2013 | 2012 | 2012 | ||||||||||
| Unaudited | ||||||||||||
| Revenues | $ | 203 | $ | 109 | $ | 166 | ||||||
| Cost of revenues | 81 | 33 | 50 | |||||||||
| Gross profit | 122 | 76 | 116 | |||||||||
| Operating expenses: | ||||||||||||
| Research and development, net | 498 | 519 | 572 | |||||||||
| Selling and marketing | 183 | 132 | 190 | |||||||||
| General and administrative | 362 | 98 | 128 | |||||||||
| Total operating expenses | 1,043 | 749 | 890 | |||||||||
| Operating loss | 921 | 673 | 774 | |||||||||
| Financial expense, net | 491 | 370 | 501 | |||||||||
| Net loss | $ | 1,412 | $ | 1,043 | $ | 1,275 | ||||||
| Total comprehensive loss | $ | 1,412 | $ | 1,043 | $ | 1,275 | ||||||
| Net loss attributable to holders of common stock | $ | 1,412 | $ | 1,043 | $ | 1,275 | ||||||
| Net basic and diluted loss per share | $ | (1.3 | ) | $ | (0.96 | ) | $ | (1.18 | ) | |||
| Weighted average number of common stock used in computing basic and diluted net loss per share | 1,085,060 | 1,085,060 | 1,085,060 | |||||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-30 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS' DEFICIENCY
U.S. DOLLARS IN THOUSANDS (except share data)
| Preferred stock | Common stock | Additional paid-in |
Accumulated | Total stockholders' |
||||||||||||||||||||||||
| Number | Amount | Number | Amount | capital | deficit | deficiency | ||||||||||||||||||||||
| Balance as of January 1, 2012 | 394,232 | $ | - | *) | 1,085,060 | $ | 1 | $ | 10,353 | $ | (10,939 | ) | $ | (585 | ) | |||||||||||||
| Stock based compensation related to options granted to consultants and employees | - | - | - | - | 28 | - | 28 | |||||||||||||||||||||
| Total comprehensive loss | - | - | - | - | - | (1,275 | ) | (1,275 | ) | |||||||||||||||||||
| Balance as of December 31, 2012 | 394,232 | - | *) | 1,085,060 | 1 | 10,381 | (12,214 | ) | (1,832 | ) | ||||||||||||||||||
| Stock based compensation related to options granted to consultants and employees | - | - | - | - | 476 | - | 476 | |||||||||||||||||||||
| Total comprehensive loss | - | - | - | - | - | (1,412 | ) | (1,412 | ) | |||||||||||||||||||
| Balance as of September 30, 2013 (unaudited) | 394,232 | $ | - | *) | 1,085,060 | $ | 1 | $ | 10,857 | $ | (13,626 | ) | $ | (2,768 | ) | |||||||||||||
| *) | Represents an amount lower than $ 1. |
The accompanying notes are an integral part of the consolidated financial statements.
| F-31 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONDENSED STATEMENTS OF CHANGES IN STOCKHOLDERS' DEFICIENCY (CONT.)
U.S. DOLLARS IN THOUSANDS (except share data)
| Preferred stock | Common stock | Additional paid-in |
Accumulated | Total stockholders' |
||||||||||||||||||||||||
| Number | Amount | Number | Amount | capital | Deficit | Deficiency | ||||||||||||||||||||||
| Balance as of January 1, 2012 | 394,232 | $ | - | *) | 1,085,060 | $ | 1 | $ | 10,353 | $ | (10,939 | ) | $ | (585 | ) | |||||||||||||
| Stock based compensation related to options granted to consultants and employees | - | - | - | - | 24 | - | 24 | |||||||||||||||||||||
| Total comprehensive loss | - | - | - | - | - | (1,043 | ) | (1,043 | ) | |||||||||||||||||||
| Balance as of September 30, 2012 (unaudited) | 394,232 | $ | - | *) | 1,085,060 | $ | 1 | $ | 10,377 | $ | (11,982 | ) | $ | (1,604 | ) | |||||||||||||
| *) | Represents an amount lower than $ 1. |
The accompanying notes are an integral part of the consolidated financial statements.
| F-32 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. DOLLARS IN THOUSANDS
| Nine months ended September 30, |
Year ended December 31, |
|||||||||||
| 2013 | 2012 | 2012 | ||||||||||
| Unaudited | ||||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | (1,412 | ) | $ | (1,043 | ) | $ | (1,275 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation | 7 | 6 | 9 | |||||||||
| Stock based compensation | 476 | 24 | 28 | |||||||||
| Benefit component of B-2 promissory notes | 262 | 167 | 238 | |||||||||
| Valuation of embedded feature of Convertible Promissory notes | (12 | ) | - | - | ||||||||
| Valuation of warrants to purchase Common stock | 24 | - | - | |||||||||
| Increase in trade receivables | (13 | ) | - | (1 | ) | |||||||
| Decrease (increase) in prepaid expenses and other accounts receivable | (9 | ) | 23 | 27 | ||||||||
| Increase in inventories | (12 | ) | (5 | ) | (60 | ) | ||||||
| Increase (decrease) in accounts payable | (4 | ) | 12 | 1 | ||||||||
| Increase (decrease) in other accounts payable | 43 | (16 | ) | (10 | ) | |||||||
| Increase in accrued severance pay, net | 1 | - | - | |||||||||
| Accrued interest on promissory notes | 202 | 192 | 256 | |||||||||
| Net cash used in operating activities | (447 | ) | (640 | ) | (787 | ) | ||||||
| Cash flows from investing activities: | ||||||||||||
| Purchase of property and equipment | (3 | ) | (4 | ) | (5 | ) | ||||||
| Net cash used in investing activities | (3 | ) | (4 | ) | (5 | ) | ||||||
| Cash flows from financing activities: | ||||||||||||
| Proceeds from issuance of promissory notes | 400 | - | - | |||||||||
| Net cash provided by financing activities | 400 | - | - | |||||||||
| Decrease in cash and cash equivalents | (50 | ) | (644 | ) | (792 | ) | ||||||
| Cash and cash equivalents at the beginning of the period | 101 | 893 | 893 | |||||||||
| Cash and cash equivalents at the end of the period | $ | 51 | $ | 249 | $ | 101 | ||||||
| Non-cash financing transactions: | ||||||||||||
| Issuance expenses | $ | 79 | $ | - | $ | - | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-33 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
NOTE 1:- GENERAL
| a. | Nano Vibronix Inc. ("the Company"), a U.S. (Delaware) corporation, commenced operations on October 20, 2003 and is a medical device company focusing on noninvasive biological response-activating devices that target wound healing and pain therapy and can be administered at home, without the assistance of medical professionals. |
The Company's principal research and development activities are conducted in Israel through its wholly-owned subsidiary, Nano Vibronix (Israel 2003) Ltd., a company registered in Israel, which commenced operations in October 2003.
| b. | During the period ended September 30, 2013, the Company continues to incur losses and negative cash flows from operating activities amounting to $ 1,412 and $ 447, respectively. These conditions raise substantial doubts about the Company's ability to continue as a going concern. The Company's ability to continue to operate is dependent upon raising additional funds to finance its activities. The Company plans to raise capital to finance its operations. There are no assurances, however, that the Company will be successful in obtaining an adequate level of financing needed for the long-term development and commercialization of its products. The consolidated financial statements do not include any adjustments with respect to the carrying amounts of assets and liabilities and their classification that might be necessary should the Company be unable to continue as a going concern. |
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES
The significant accounting policies applied in the annual consolidated financial statements of the Company as of December 31, 2012 are applied consistently in these financial statements, with the exception of the following:
Warrants:
The Company accounts for certain warrants held by investors which include down round protection as a liability according to provisions of ASC 815-40, "Derivatives and Hedging - Contracts in Entity`s Own Equity", ("ASC 815") which provides new two-step model to be applied in determining whether a financial instrument or an embedded feature is indexed to an issuer`s own sock and thus able to qualify to be a derivative financial instrument. The Company measures the warrants at fair value with the assistance of an independent valuation firm by applying the Bionomical option pricing model in each reporting period until they are exercised or expired, with changes in the fair value being recognized in the Company`s statement of operations as financial income or expense, as appropriate. For more information see Note 4.
| F-34 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
NOTE 3:- UNAUDITED INTERIM FINANCIAL STATEMENTS
The accompanying unaudited condensed consolidated financial statements as of September 30, 2013 have been prepared in accordance with the U.S. generally accepted accounting principles for interim financial information. Accordingly, they do not include all the information and footnotes required by generally accepted accounting principles in the United States for complete financial statements. In the opinion of management, the unaudited interim consolidated financial statements include all adjustments of normal recurring nature necessary for a fair presentation of the Company’s consolidated financial position as of September 30, 2013, the Company’s consolidated results of operation and the consolidated cash flow for the nine months ended September 30, 2013. Results for the nine months ended September 30, 2013 are not necessarily indicative of the results that may be expected for the year ended December 31, 2013.
NOTE 4:- FAIR VALUE MEASURMENTS
ASC 820, "Fair Value Measurements and Disclosures" ("ASC 820"), defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and consider assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions and risk of nonperformance.
ASC 820 also establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument's categorization within the fair value hierarchy is based on the lowest level of input that is significant to the fair value measurement. ASC 820 establishes three levels of inputs that may be used to measure fair value.
| Level 1 - | quoted prices in active markets for identical assets or liabilities; |
| Level 2 - | inputs other then Level 1 that are observable, either directly or indirectly, such as quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; or. |
| Level 3 - | unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets and liabilities. |
During February through September 2013, the Company issued convertible promissory notes for an aggregated amount of $ 400, convertible to Common stock with an exercise price of $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events (see also Note 5). The Company accounts for the conversion feature at fair value, with changes in fair values being recognized in the Company’s statement of comprehensive loss as financial income or expenses.
| F-35 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
NOTE 4:- FAIR VALUE MEASURMENTS (Cont.)
In addition, in February 2013, the Company issued to the stockholder 263,158 warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants shall expire on February 5, 2018.
In March 2013, the Company issued to the stockholder 263,158 warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on March 28, 2018.
In June 2013, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on June 3, 2018.
In August 2013, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on August 5, 2018.
The Company accounts for the warrants to purchase Common stock held by the stockholders (each of which include weighted average anti-dilution protection) as a liability according to the provisions of ASC 815-40, " Derivatives and Hedging – Contracts in Entity`s Own Equity".
The Company measures the warrants and the conversion feature of the convertible promissory notes at fair value with the assistance of an independent valuation firm by applying the Bionomical option pricing model in each reporting period until they are exercised or expired, with changes in fair values being recognized in the Company’s statement of operations as financial income or expenses.
In estimating the warrants' and the conversion feature fair value the Company used the following assumptions:
| Issuance date and September 30, | ||
| 2013 | ||
| Dividend yield (1) | 0% | |
| Expected volatility (2) | 58% - 59% | |
| Risk-free interest (3) | 0.07% - 0.1% | |
| Expected term (years) (4) | 0.75 - 1 |
| F-36 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
NOTE 4:- FAIR VALUE MEASURMENTS (Cont.)
| (1) | Dividend yield - was based in the fact that the Company has not paid dividends to its stockholders in the past and does not expect to pay dividends to its stockholders in the future. |
| (2) | Expected volatility - was calculated based on actual historical stock price movements of companies in the same industry over the term that is equivalent to the expected term of the option. |
| (3) | Risk-free interest - based on yield rate of non-index linked U.S. Federal Reserve treasury stock. |
| (4) | Expected term - the expected term was based on the maturity date of the warrants. |
Fair value measurement using significant unobservable inputs (Level 3):
| Fair value of warrants to Common stock and embedded feature |
||||
| Balance at January 1, 2013 | $ | - | ||
| Fair value of warrants | 92 | |||
| Change in fair value of warrants | 24 | |||
| Fair value of embedded feature | 133 | |||
| Change in fair value of embedded feature of Convertible Promissory notes | (12 | ) | ||
| Balance at September 30, 2013 | $ | 237 | ||
In addition the Company’s financial instruments also includes cash and cash equivalents, trade receivables, prepaid expenses and other accounts receivable, accounts payable and other accounts payable. The fair value of these financial instruments was not materially different from their carrying values as of September 30, 2013 due to the short-term maturities of such instruments.
NOTE 5:- CONVERTIBLE PROMISSORY NOTES
On February 5, 2013 the Company signed an agreement with certain investors ("The Agreement"), according to which the Company issued convertible promissory notes (the "Note") in consideration for $100. The convertible promissory notes mature on the earlier of June 30, 2013, the closing date of a financing in which the Company shall sell an aggregate of at least $ 250 of its debt or equity securities or such accelerated date as a result of an occurrence of an event of default as defined in The Agreement, upon which date the entire outstanding principal balance and any outstanding fees or interest will be due and payable in full. The convertible promissory notes bear interest at the rate of 6% per annum, which rate is increased to 10% upon and during the occurrence of an event of default as defined in The Agreement. In addition, the convertible promissory notes are convertible either at the investor`s option or upon maturity of the promissory notes into Common stock at an initial conversion price of $ 0.38 per share, subject to adjustment for stock splits, fundamental transactions or similar events.
| F-37 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
NOTE 5:- CONVERTIBLE PROMISSORY NOTES (Cont.)
In addition, the Company issued to the stockholder 263,158 warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38. The warrants shall expire on February 5, 2018.
On March 28, 2013 the Company signed an amendment to The Agreement and issued convertible promissory notes to certain investors in the principal amount of $ 200. In addition, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on March 28, 2018.
On June 3, 2013 the Company signed a second amendment to The Agreement and issued to convertible promissory notes to certain investors in the principal amount of $ 300. The Note are convertible to Common stock and bears 6% interest, computed annually and should be fully due and payable at the earliest of September 30, 2013 or the closing date of a financing round as defined in the agreement. In addition, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on June 3, 2018.
On August 5, 2013, the Company signed a third amendment to The Agreement and issued convertible promissory notes to certain investors in the principal amount of $400. The Note are convertible to Common stock and bears 6% interest, computed annually and should be fully due and payable at the earliest of November 30, 2013 (which date was subsequently extended to February 28, 2014. See also Note 8b) or the closing date of a financing round as defined in the agreement. In addition, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants (see Note 4), other than that the warrants expire on August 5, 2018.
NOTE 6:- STOCKHOLDERS' DEFICIENCY
On March 28, 2013, the Company's Board of Directors approved the grant of 1,930,000 and 210,000 options to employees and non-employees, respectively, at an exercise price between $ 0.01 and $ 0.28 per share. Such options to employees and non employees are fully vested on the date of grant. The options shall have ten years terms, unless otherwise approved by the Board of Directors and shell be issued under the 2004 Equity Incentive Plan ("2004 Plan").
Upon such approval, under 2004 Plan, 286,296 options are available for future grants to employees, directors and non employees of the Company and its subsidiary.
| F-38 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
NOTE 6:- STOCKHOLDERS' DEFICIENCY (Cont.)
The total compensation cost related to all of the Company's equity-based awards, recognized during the period of nine months ended September 30, 2013 and 2012 were comprised as follow:
| Nine months period ended September 30, |
||||||||
| 2013 | 2012 | |||||||
| Research and development, net | $ | 212 | $ | 13 | ||||
| Selling and marketing | 48 | 8 | ||||||
| General and administrative expenses | 216 | 3 | ||||||
| Total stock-based compensation expenses | $ | 476 | $ | 24 | ||||
NOTE 7:- GEOGRAPHIC INFORMATION AND MAJOR CUSTOMER DATA
Summary information about geographic areas:
The Company manages its business on the basis of one reportable segment, and derives revenues from selling its products mainly through distributor agreements. The following is a summary of revenues within geographic areas:
| Nine months period ended September 30, |
||||||||
| 2013 | 2012 | |||||||
| United States | $ | 122 | $ | 38 | ||||
| Israel | 10 | 14 | ||||||
| Europe | 15 | 15 | ||||||
| India | 9 | 12 | ||||||
| Rest of the world | 47 | 30 | ||||||
| $ | 203 | $ | 109 | |||||
During the nine months period ended September 30, 2013 and 2012, there were no sales to a single customer exceeding 10% of the Company's revenues.
The Company's long-lived assets are all located in Israel.
NOTE 8:- SUBSEQUENT EVENTS
| a. | On October 7, 2013, the Company signed a fourth amendment to The Agreement (see Note 5) and issued convertible promissory notes to certain investors in the principal amount of $500. The Note are convertible to Common stock and bears 6% interest, computed annually and should be fully due and payable at the earliest of December 31, 2013 or the closing date of a financing round as defined in the agreement. In addition, the |
| F-39 |
NANO VIBRONIX INC. AND ITS SUBSIDIARY
NOTE 8:- SUBSEQUENT EVENTS (Cont.)
| Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on October 7, 2018. |
| b. | On December 9, 2013, the Company signed a fifth amendment to The Agreement (see Note 5) and issued convertible promissory notes to certain investors in the principal amount of $600. The Note are convertible to Common stock and bears 6% interest, computed annually and should be fully due and payable at the earliest of February 28, 2014, or the closing date of a financing round as defined in the agreement. In addition, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on December 9, 2018. |
| c. | On February 6, 2014, the Company signed a sixth amendment to The Agreement (see Note 5) and issued convertible promissory notes to certain investors in the principal amount of $700. The Note are convertible to Common stock and bears 6% interest, computed annually and should be fully due and payable at the earliest of April 30, 2014, or the closing date of a financing round as defined in the agreement. In addition, the Company issued to the stockholder 263,158 additional warrants to purchase Common stock. The exercise price at which the warrant may be exercised shall be $ 0.38 subject to adjustment for stock splits, fundamental transactions or similar events. The warrants contain identical terms to those of the February 2013 Warrants, other than that the warrants expire on February 6, 2019. | |
| d. | The Company evaluates events or transactions that occur after the balance sheet date but prior to the issuance of financial statements to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure. For its interim consolidated financial statements as of September 30, 2013 (unaudited) and for the nine month period then ended (unaudited), the Company evaluated subsequent events through February 6, 2014, the date that the consolidated financial statements were issued. |
- - - - - - - - - - - - - -
| F-40 |
Shares of Common Stock
Warrants to Purchase
Shares of Common Stock

Nano Vibronix, Inc.
PROSPECTUS
, 2014
Chardan Capital Markets, LLC
Until , 2014 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable by us in connection with the sale and distribution of the common stock being registered. All amounts are estimates except for the Securities and Exchange Commission registration fee, the FINRA filing fee, and the Nasdaq listing fee.
| Securities and Exchange Commission Registration Fee | $ | |||
| FINRA Filing Fee | ||||
| Nasdaq Listing Fee | $ | |||
| Accounting Fees and Expenses | $ | |||
| Legal Fees and Expenses | $ | |||
| Transfer Agent Fees | $ | |||
| Printing Expenses | $ | |||
| Miscellaneous Fees and Expenses | $ | |||
| Total | $ |
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law provides, in general, that a corporation incorporated under the laws of the State of Delaware, as we are, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Our certificate of incorporation and bylaws provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the provisions of the Delaware General Corporation Law, as amended from time to time, subject to any permissible expansion or limitation of such indemnification, as may be set forth in any stockholders’ or directors’ resolution or by contract.
Any repeal or modification of these provisions approved by our stockholders will be prospective only and will not adversely affect any limitation on the liability of any of our directors or officers existing as of the time of such repeal or modification.
| II-1 |
We are also permitted to apply for insurance on behalf of any director, officer, employee or other agent for liability arising out of his actions, whether or not the Delaware General Corporation Law would permit indemnification.
Item 15. Recent Sales of Unregistered Securities.
During 2010 and 2011, we issued an aggregate of 40,928 shares of series B preferred stock and warrants to purchase 40,928 shares of series B preferred stock, with an exercise price of $17.25 per share and a five-year term, to certain investors for consideration of $706,010. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by either Regulation S under the Securities Act of 1933, as amended, or Section 4(2) of the Securities Act of 1933, as amended. Each of the investors was either an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) or not a “U.S. person” (as that term is defined in Rule 902 of Regulation S) at the time of the transactions.
On December 9, 2010, we issued options to purchase 5,500 shares of common stock at an exercise price of $0.284 as compensation to certain consultants. The options have a ten-year term. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by either Regulation S under the Securities Act of 1933, as amended, or Section 4(2) of the Securities Act of 1933, as amended. Each of the investors was either an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) or not a “U.S. person” (as that term is defined in Rule 902 of Regulation S) at the time of the transactions.
On November 22, 2011, we issued convertible series B-1 promissory notes to certain investors in consideration for $1,000,000. The notes are convertible to series B-1 preferred stock and bear interest at 10% per annum, compounded annually. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by either Regulation S under the Securities Act of 1933, as amended, or Section 4(2) of the Securities Act of 1933, as amended. Each of the investors was either an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) or not a “U.S. person” (as that term is defined in Rule 902 of Regulation S) at the time of the transactions.
On November 22, 2011, we issued convertible series B-2 promissory notes and warrants to purchase 2,319,062 shares of series B-2 preferred stock to the holders of series B participating convertible preferred stock and warrants in exchange for the cancellation of such previously-held preferred stock and warrants. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were exchanged pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by Section 3(a)(9) of the Securities Act of 1933, as amended.
On February 5, 2013, we signed an agreement with certain investors according to which we issued convertible promissory notes in consideration for $100,000, in such original principal amount. The notes are convertible to common stock and bear interest at 6% annually. In addition on such date, we issued warrants to purchase up to an aggregate of 263,158 shares of common stock with an exercise price of $0.38 per share, subject to adjustments, and a five-year term. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) of the Securities Act of 1933, as amended. Each investor was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the transaction.
On March 28, 2013, we signed an amendment to the agreement described above and issued convertible promissory notes in consideration for an additional $100,000 with the same terms described above, so that the aggregate original principal amount was increased to $200,000. In addition on such date, we issued warrants to purchase up to an aggregate of 263,158 shares of common stock with an exercise price of $0.38 per share, subject to adjustments, and a five-year term. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) of the Securities Act of 1933, as amended. Each investor was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the transaction.
| II-2 |
On June 3, 2013, we signed a second amendment to the agreement described above and issued convertible promissory notes in consideration for $100,000 with the same terms described above, so that the aggregate original principal amount was increased to $300,000. In addition on such date, we issued warrants to purchase up to an aggregate of 263,158 shares of common stock with an exercise price of $0.38 per share, subject to adjustments, and a five-year term. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) of the Securities Act of 1933, as amended. Each investor was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the transaction.
On August 5, 2013, we signed a third amendment to the agreement described above and issued convertible promissory notes in consideration for $100,000 with the same terms described above, so that the aggregate original principal amount was increased to $400,000. In addition on such date, we issued warrants to purchase up to an aggregate of 263,158 shares of common stock with an exercise price of $0.38 per share, subject to adjustments, and a five-year term. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) of the Securities Act of 1933, as amended. Each investor was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the transaction.
On October 7, 2013, we signed a fourth amendment to the agreement described above and issued convertible promissory notes in consideration for $100,000 with the same terms described above, so that the aggregate original principal amount was increased to $500,000. In addition on such date, we issued warrants to purchase up to an aggregate of 263,158 shares of common stock with an exercise price of $0.38 per share, subject to adjustments, and a five-year term. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) of the Securities Act of 1933, as amended. Each investor was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the transaction.
On December 9, 2013, we signed a fifth amendment to the agreement described above and issued convertible promissory notes in consideration for $100,000 with the same terms described above, so that the aggregate original principal amount was increased to $600,000. In addition on such date, we issued warrants to purchase up to an aggregate of 263,158 shares of common stock with an exercise price of $0.38 per share, subject to adjustments, and a five-year term. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) of the Securities Act of 1933, as amended. Each investor was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the transaction.
On February 6, 2014, we signed a sixth amendment to the agreement described above and issued convertible promissory notes in consideration for $100,000 with the same terms described above, so that the aggregate original principal amount was increased to $700,000. In addition on such date, we issued warrants to purchase up to an aggregate of 263,158 shares of common stock with an exercise price of $0.38 per share, subject to adjustments, and a five-year term. The securities issued in the above described transactions were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold pursuant to the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) of the Securities Act of 1933, as amended. Each investor was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the transaction.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
| Exhibit No. | Description |
| 1.1+ | Form of Underwriting Agreement |
| 3.1* | Amended and Restated Certificate of Incorporation, as presently in effect |
| 3.2* | Bylaws, as presently in effect |
| 3.3+ | Form of Amended and Restated Certificate of Incorporation, to be in effect upon completion of this offering |
| 3.4+ | Form of Amended and Restated Bylaws, to be in effect upon completion of this offering |
| 4.1* | Form of Common Stock Certificate |
| 4.2+ | Form of Warrant Agreement by and between the Company and VCorp Services, LLC and Form of Warrant Certificate |
| II-3 |
| 5.1+ | Opinion of Haynes and Boone, LLP |
| 10.1* | License Agreement, dated October 26, 2003, by and among Nano Vibronix, Inc., Piezo-Top Ltd, and PMG Medica Ltd |
| 10.2* | License Agreement, dated December 11, 2011, by and between Nano Vibronix, Inc. and AC Engineering Ltd. |
| 10.3* | Form of Series B-1 Promissory Note |
| 10.4* | Form of Subscription Agreement for Series B-1 Convertible Promissory Notes |
| 10.5* | Form of Series B-2 Promissory Note |
| 10.6* | Form of Series B-2 Participating Convertible Preferred Stock Purchase Warrant |
| 10.7* | Form of Subscription Agreement for Series B Convertible Preferred Stock and Warrants |
| 10.8* | First Amendment to Subscription Agreement for Series B Convertible Preferred Stock and Warrants, dated November 14, 2011, by and between NanoVibronix, Inc. and the investors signatory thereto |
| 10.9 | Sixth Amended and Restated Securities Purchase Agreement, dated February 6, 2014, by and between Nano Vibronix, Inc. and Globis Overseas Fund, Ltd. |
| 10.10 | Sixth Amended and Restated Securities Purchase Agreement, dated February 6, 2014, by and between Nano Vibronix, Inc. and Globis Capital Partners, L.P. |
| 10.11 | Sixth Amended and Restated Secured Convertible Promissory Note, dated February 6, 2014, by Nano Vibronix, Inc. in favor of and Globis Overseas Fund, Ltd. |
| 10.12 | Sixth Amended and Restated Secured Convertible Promissory Note, dated February 6, 2014, by Nano Vibronix, Inc. in favor of and Globis Capital Partners, L.P. |
| 10.13* | Form of 2013 and 2014 Warrant to Purchase Common Stock |
| 10.14* | Nano Vibronix, Inc. 2004 Global Share Option Plan |
| 10.15* | Personal Employment Agreement, dated April 1, 2008, by and among Nano Vibronix, Inc., Nano-Vibronix (Israel 2003) Ltd and Harold Jacob |
| 10.16* | Amendment to Personal Employment Agreement, dated December 1, 2008, by and among Nano Vibronix, Inc., Nano-Vibronix (Israel 2003) Ltd and Harold Jacob |
| 10.17* | Personal Employment Agreement, dated March 1, 2008, by and between Nano-Vibronix (Israel 2003) Ltd and Jona Zumeris |
| 21.1* | List of Subsidiaries |
| 23.1 | Consent of Kost Forer Gabbay & Kasierer, a member firm of Ernst & Young Global |
| 23.2+ | Consent of Haynes and Boone, LLP (included in Exhibit 5.1) |
| 24.1 | Power of Attorney (included on signature page) |
+ To be filed by amendment.
* Previously filed.
| II-4 |
(b) Financial Statement Schedules
No financial statement schedules are provided because the information is not required or is shown either in the financial statements or the notes thereto.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(i) If the registrant is relying on Rule 430B (§230.430B of this chapter):
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; or
(ii) If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
| II-5 |
(5) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-6 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Melville, State of New York on February 6, 2014.
| NANO VIBRONIX, INC. | |||
| By: | /s/ Harold Jacob | ||
| Name: Harold Jacob, M.D. | |||
| Title: Chief Executive Officer | |||
KNOW ALL MEN BY THESE PRESENTS, that we, the undersigned officers and directors of Nano Vibronix, Inc., a Delaware corporation (the “Company”), do hereby constitute and appoint Harold Jacob and Ira Greenstein, and each of them, as his or her true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments, exhibits thereto and other documents in connection therewith) to this Registration Statement and any subsequent registration statement filed by the registrant pursuant to Rule 462(b) of the Securities Act of 1933, as amended, which relates to this Registration Statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
In accordance with the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| Chief Executive Officer and | ||||
| Chairman of the Board of Directors | ||||
| /s/ Harold Jacob | (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) |
February 6, 2014 | ||
| Harold Jacob, M.D. | ||||
| /s/ Jona Zumeris | Vice President of Technology and Director | February 6, 2014 | ||
| Jona Zumeris, Ph.D. | ||||
| /s/ Ira Greenstein | Director | February 6, 2014 | ||
| Ira Greenstein | ||||
| II-7 |
