Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Oncotelic Therapeutics, Inc. | d665783d8k.htm |
 Novel
Vascular Disrupting Agents For Orphan Oncology Indications
February 2014
Exhibit 99.1 |
 2
2
This presentation contains forward-looking statements under the meaning of the Private
Securities Litigation Reform Act of 1995.
These
statements
give
our
current
expectations
or
forecasts
and
use
words
such
as
“anticipate,”
“estimate,”
"expect,"
“believe,”
and other words of similar meaning. Any or all of the forward-looking statements in
this presentation may turn out to be wrong. They can be affected by inaccurate
assumptions we might make or by known or unknown risks and uncertainties
including
but
not
limited
to,
the
efficacy
of
our
product
candidates,
their
efficacy
at
acceptable
dosage
levels,
the
ability
to
raise
capital when needed and on reasonable terms, projections of potential commercial sales of
company products, the results and progress of clinical trials, developing the
necessary manufacturing processes and gaining all necessary regulatory approvals,
both in the United States and internationally. Consequently, no forward-looking
statement can be guaranteed and actual results
may
differ
materially.
Additional
information
concerning
factors
that
could
cause
actual
results
to
materially
differ
from
those in the forward-looking statements are contained in our most recent reports to the
Securities and Exchange Commission including our Form 10-Q, 8-K and 10-K
reports. However, we undertake no obligation to publicly update forward-looking
statements, whether as a result of new information, future events or otherwise. We note
these factors for investors as permitted by the Private Securities Litigation Reform
Act of 1995. The information in this document has been prepared solely for
informational purposes and does not constitute an offer to sell or the solicitation
of an offer to purchase any securities from any entities described herein. Any such offer will be made solely
by means of the prospectus contained in the registration statement (the "Registration
Statement") filed by OXiGENE, Inc. (the "Company") with the Securities
and Exchange Commission (the "SEC"). The information contained herein may not be used in
connection with an offer or solicitation by anyone in any jurisdiction in which such offer
or solicitation is not permitted by law or in which the person making the offer or
solicitation is not qualified to do so or to any person to whom it is unlawful to much
such offer or solicitation.
INVESTING IS SPECULATIVE AND INVOLVES RISK OF LOSS. YOU SHOULD REVIEW CAREFULLY THE
REGISTRATION STATEMENT, INCLUDING THE DESCRIPTION OF THE RISKS AND OTHER TERMS BEFORE
MAKING A DECISION TO INVEST. The Company has filed a Registration Statement
(including a prospectus) with the SEC for the offering
to
which
this
presentation
relates.
Before
you
invest,
you
should
read
the
prospectus
contained
in
the
Registration
Statement, the information incorporated by reference into the Registration Statement, and
other documents the Company has filed with the SEC for more complete information
about the Company and the offering. You may get these documents for free by
visiting EDGAR on the SEC website at http://www.sec.gov
Alternatively, the Company or the placement agent participating
in the offering will arrange to send you the prospectus contained in the Registration
Statement if you request it by calling H.C. Wainwright & Co., LLC at (212)
356-0500. Safe Harbor And Free Writing Prospectus Statement
|
 3
OXiGENE, Inc. (OXGN) Corporate Snapshot
Development-stage biotechnology company
Lead product candidate: ZYBRESTAT
®
A vascular disrupting agent (VDA) being developed
for ovarian cancer and other solid tumors
primarily in combination with other anti-cancer
agents which has shown utility and tolerability to
date in combination
with
Avastin
®
and other
cancer drugs
Follow-on product: OXi4503
A
2
nd
–
generation, dual-mechanism VDA being
developed for acute myeloid leukemia (AML)
Robust intellectual property (IP) portfolio
consists of more than 120 patents worldwide
Balance Sheet (as of YE 2013)
No debt: cash $7.0M, projected
to last into mid 3Q14
Equity Metrics (January 2014)
Mkt Cap: $14.6M
Shr Out: 5.6M (11.0M fully diluted) |
 OXiGENE (OXGN)
Investment Thesis Compelling Valuation
Currently trading at a market cap of approximately $14 million
Leveraging $200+ million previously invested primarily in VDA development
Cost-Efficient and Risk-Mitigated Development Strategy
Ongoing and planned Ph 2 trials supported by foundations, non-profit
research institutions and larger pharmaceutical companies
Significant Potential Value-Creating Events on the Horizon
Readout of Avastin
®
+/-
ZYBRESTAT
®
Ph 2 in ovarian cancer
–
primary
endpoint results expected 1H14
Potential initiation of FDA interaction and planning of pivotal NDA program for
Avastin
®
+ ZYBRESTAT
®
combination
Initiation of the planned Phase 2 trial in neuroendocrine tumors
(NETs)
Demonstrate clinical activity for OXi4503 Phase 1 trial in AML
Actively pursuing development and commercialization agreements with
established industry leaders
4 |
 OXiGENE
Leadership Team TEAM MEMBER
EXPERIENCE
Peter Langecker
MD PhD
Chief Executive Officer
CIBA GEIGY (Novartis), Schering-Plough
(Merck), Coulter (GSK), SUGEN (Pfizer),
Intarcia, DURECT
Barbara Riching
CPA
Chief Financial Officer
Abgenix (Amgen), ALZA (J&J), Natural
Wonders, Ernst & Young
Alice Varga
MS/MA
VP Regulatory Affairs and Quality Assurance
Syntex (Roche), SUGEN (Pfizer), PDL,
Genentech/Roche, Cell Genesys (Bio
Sante), Geron
Kathleen Lee PhD MBA
VP Chemistry, Manufacturing and Controls (CMC)
Syntex (Roche), Scios (J&J), InteKrin
Jai Balkissoon MD, FACS
Clinical Development Consultant
NCI Bethesda, Chiron (Novartis),
Genentech/Roche, PPD
Dai Chaplin PhD
Scientific Advisor and Board Member
University College, London, Cancer
Research Campaign UK, RPR, Aventis
5 |
 ZYBRESTAT
®
:
Reversible
Tubulin
Depolymerizing
Agent
Current chemotherapy is
notorious for killing healthy cells along with cancerous ones –
ZYBRESTAT®
is
designed
to
change
that
What is ZYBRESTAT?
ZYBRESTAT
®
, (fosbretabulin, CA4P), kills tumor
cells by selectively blocking those blood vessels that
carry vital oxygen and nutrients to tumors
What does that do?
ZYBRESTAT disrupts the scaffolding, and within
hours the normally flat cells become round, blocking
the blood flow to the tumor and causing massive
tumor cell death
How does it work?
ZYBRESTAT preferentially binds to a protein called
“Tubulin”
within the cells that line the interior of
tumor blood vessels
6 |
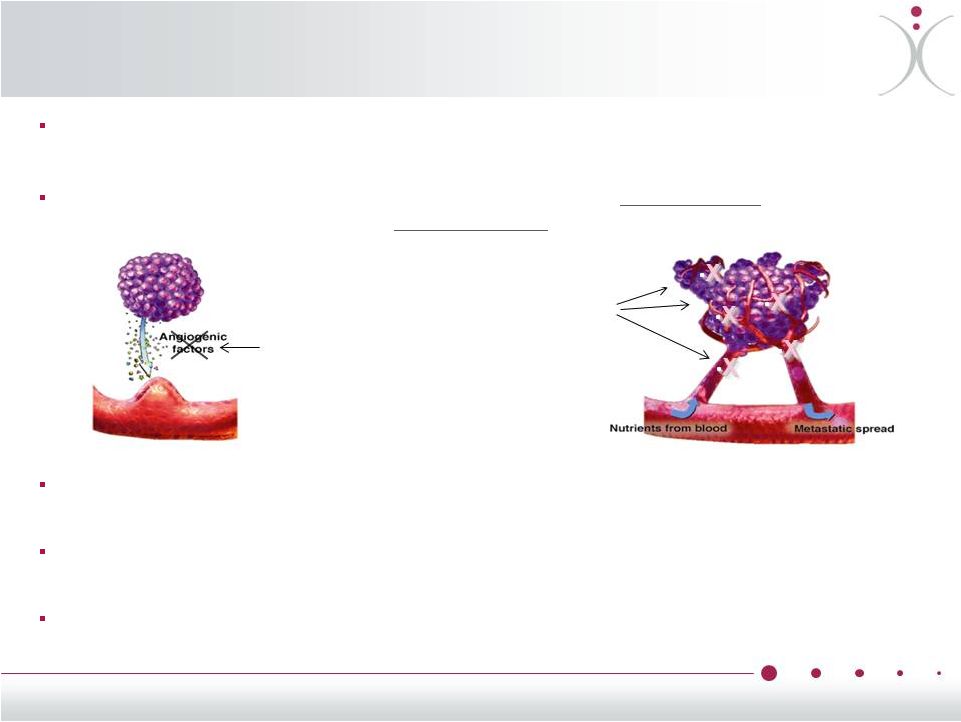 VDAs: A
Targeted Approach to Tumor Necrosis Reducing tumor access to blood, oxygen and
nutrients is very effective anti-cancer therapy as
demonstrated
by
anti-VEGF
therapy
like
bevacizumab
(Avastin
®
)
VDAs reduced blood flow to the center of the tumors by > 70% in 7/7 clinical trials as
shown using 4 different imaging modalities
Blood
flow
reduction
leads
to
tumor
necrosis
of
those
regions
of
tumors
believed
to
be
associated with worse prognosis, often resistant to therapy
Combining VDAs and Anti-VEGFs as well as other anti-cancer drugs have demonstrated
enhanced preclinical and clinical activity and are subject of ongoing clinical
research •
Anti-VEGF
•
VDA
Tubulin-targeted
ascular
isrupting
gents
(VDAs)
work
from
the
inside
of
the
tumor
while
anti-VEGF
compounds
work
from
the
outside
of
the
tumor
V
D
A
7 |
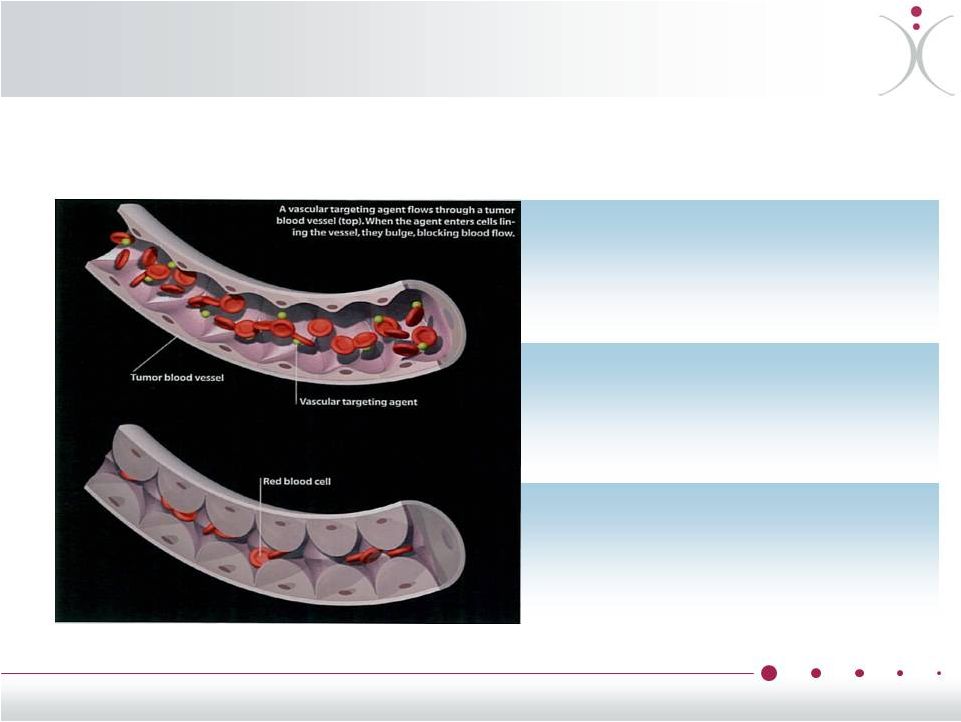
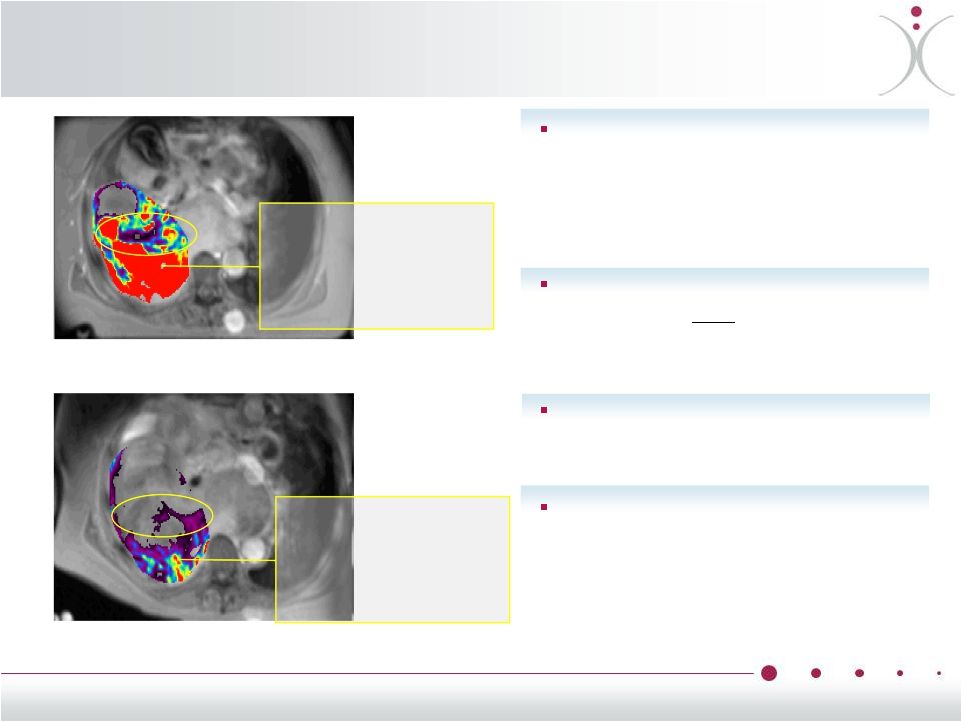 About Vascular
Disrupting Agents (VDAs) Tumor flood flow before VDA
Tumor blood flow 1 hour after VDA
8
Reducing tumor blood supply is a
proven mechanism used by
commercialized anti-VEGF drugs
such as Avastin
®
(bevacizumab)
VDAs disrupt blood flow and
vasculature only
within tumors,
sparing normal cells and organs
VDAs are potentially useful in a
wide range of tumors
VDAs potentially improve clinical
outcomes when used in
combination with other types of
anti-cancer therapies
Red areas
have high
blood flow
Blood flow
significantly
reduced |
 After many
years of research, we believe ZYBRESTAT
®
may
become the first
commercialized VDA…
9 |
 …
attracting the support of
numerous prestigious
foundations, non-profit
research institutions and “big
pharma”
companies.
10 |
 ZYBRESTAT
®
:
Dual
Track
to
Potential
Commercialization
ZYBRESTAT
®
–
Clinically validated VDA
Clinical activity in ATC, advanced ovarian cancer and other indications
Good
tolerability
observed
to
date
as
mono-
and
combination
therapy
in
400+
patients
Orphan Drug status for ATC and ovarian cancer in US and EU
US/WW -
Ovarian cancer clinical lead indication
Activity and tolerability observed to date in combination with taxane-based
chemotherapy or anti-VEGF therapy for ovarian cancer in Phase 1 and 2 clinical
studies Combination
with
anti-VEGF
(Avastin
®
)
–
randomized
phase
2
data
expected
1H2014
If Phase 2 is positive potential for subsequent partnered pivotal FDA NDA program
Neuroendocrine tumors –
potential novel indication for VDAs
Positive preclinical results presented at AACR
Potentially rapid development pathway, robust intellectual property
Phase 2 study in patients with carcinoid tumors in planning
EU approval path targeted for anaplastic thyroid cancer (ATC)
Potential
for
2016
commercialization
of
ZYBRESTAT
®
Approval possible based on current data under “Exceptional Circumstances”
Ongoing compassionate use program in EU and other countries
11 |
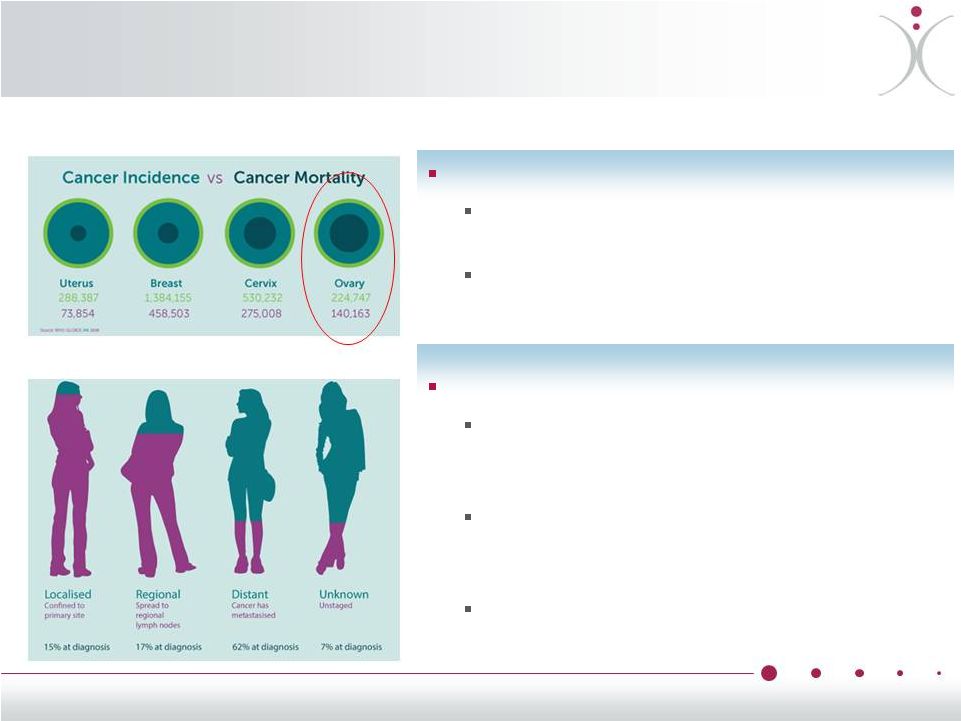 Why Ovarian
Cancer? Aggressive, stealthy cancer
~22,000 new cases in the U.S. annually,
~220,000 new cases worldwide
Over 60% of patients have distant
spread at diagnosis resulting in poor
prognosis
Dire need for better therapies
Highly lethal cancer that is responsible
for the deaths of approximately 14,000
women in the U.S. each year
Rapid resistance to existing FDA-
approved treatments ultimately limits
their effectiveness
5-year survival rates of only 47%,
largely unchanged since the 1990s
•
Annual Incidence And Mortality Rates
of Female Cancers Worldwide
•
Ovarian Cancer Spread at Diagnosis
12 |
 Collaborative
effort between OXGN, Gynecologic Oncology Group (GOG), NCI (CTEP), Genentech /
Roche Ongoing Phase 2 trial (GOG0186I) in ovarian cancer
sponsored by GOG/CTEP
Based on compelling preclinical data of bevacizumab/ZYBRESTAT
combination
Positive results from earlier Phase 1 trial
9/14 treated patients (60%) with ovarian cancer and other solid
tumors had disease stabilization (m = 55 days)
2/4 patients with ovarian cancer had stable disease including a
CA125 response lasting > 1 year
Longer blood-flow shutdown shown via functional imaging with
ZYBRESTAT
Avastin
®
+/-
ZYBRESTAT
®
in Solid Tumors
13 |
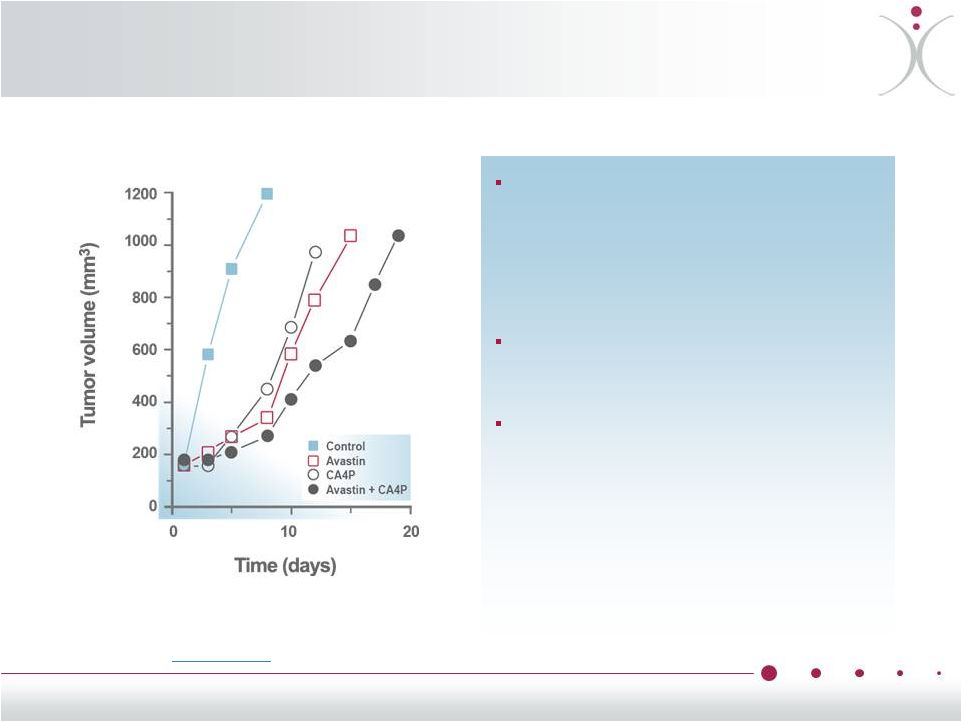 Preclinical
Data Support Effects of ZYBRESTAT (CA4P) + Bevacizumab
Combination Response of Caki-1 (renal cell) tumors
to bevacizumab (2 mg/kg, twice a
week for 2 weeks) and ZYBRESTAT
(CA4P) 100 mg/kg, 3 times a week
for 2 weeks or the combination of
the ZYBRESTAT + bevacizumab
Data shown represent the median
tumor responses of groups of
8-10 mice
The combination of ZYBRESTAT +
bevacizumab resulted in greater
reduction of tumor growth rates than
in either compound alone
Siemann
et
al.,
Anticancer
Res.
2008
Jul-Aug;28(4B):2027-31
14 |
 GOG 0186I:
Ongoing Phase 2 Study in Ovarian Cancer
First randomized study to test anti-VEGF + VDA in advanced
ovarian
cancer
–
no
cytotoxic
chemotherapy
involved
2nd-line, 3rd-line platinum-sensitive and platinum-resistant ovarian
cancer at 67 clinical sites, 107 patients, controlled, 1:1 randomization
Dosing: Bevacizumab +/-
ZYBRESTAT, q 3 weeks
Primary endpoint: demonstrate median progression free survival
(PFS) increase from 50% to 65%
Enrollment complete, two successful interim safety analyses and
successful futility analysis for primary endpoint reported
Primary endpoint readout expected 1H2014
Event-driven based on PFS
Go/no go for potential pivotal study and potential end-of-Phase-2
meeting with FDA 2H2014
Potential path to NDA/ EU MAA filings in ovarian cancer
15 |
 Attractive
Potential Commercial Opportunity Target patient population:
Recurrent
ovarian
cancer
–
80%
of
all
ovarian
cancer
patients
ZYBRESTAT in Ovarian Cancer:
Use of anti-VEGF agents has been shown to be effective across
different
tumor
types
–
potential
for
greater
activity
in
combination
with VDA like
ZYBRESTAT®
AVASTIN®
already approved as single agent for ovarian cancer in
EU and other countries
Absence of chemotherapy and good tolerability would make the
combination regimen with anti-VEGF agents attractive to patients
Orphan drug status for ovarian cancer in US and EU for
ZYBRESTAT®
Potential for pivotal program exploring novel
approach targeting tumor blood supply
®
16 |
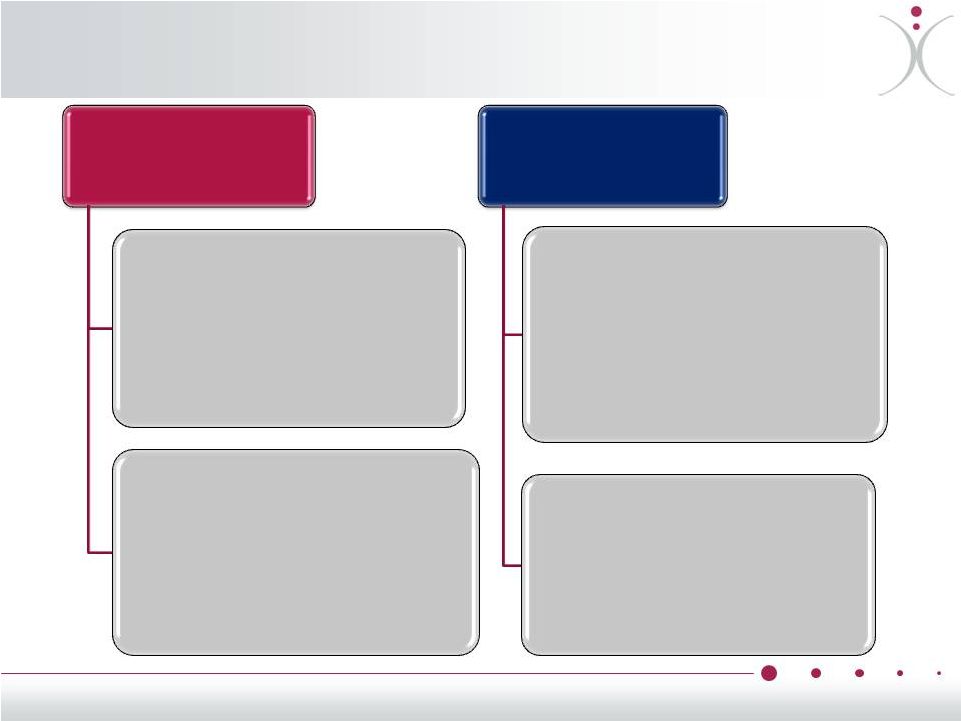 Risk-Mitigated, Cost Effective Development
Pivotal Anti-VEGF
Combination
Program
Potential Future
Exploratory
Combinations
Ongoing Phase 2 with
Avastin
®
(bevacizumab)
With Votrient
®
(pazopanib)
Potential Future Study with
Avastin
®
(bevacizumab)
With weekly Taxol
®
(paclitaxel)
ZYBRESTAT in Ovarian Cancer – ®
•
Daily Votrient
®
p.o. +/-
ZYBRESTAT
®
i.v., weekly x 3, q 4 weeks
•
Potentially Sponsored by UK Non-Profit
Cancer Research Organization and Involved
Drug Manufacturers and OXiGENE
•
Multicenter study, up to 120 patients
•
Primary endpoint: PFS
•
Initiation dependent on future funding and
external partner
•
Potential Pivotal Phase 3 study
•
Avastin
®
q 3 weeks +/-
ZYBRESTAT
®
i.v., q 3
weeks
•
Sample size, Primary Endpoint: based on FDA
feedback if Phase 2 study is positive
•
Potential for CTEP/GOG support of follow-on
study
•
Weekly paclitaxel +/-
weekly ZYBRESTAT
®
i.v., q 4 weeks
•
Multicenter study, up to 120 patients
•
Primary endpoint: PFS
•
Initiation dependent on future funding and
external partner
Avastin
®
q
3
weeks
+/-
ZYBRESTAT
®
i.v., q 3
weeks
Sponsored by Genentech / Roche, GOG, NCI
107 patients enrolled at 67 clinical sites
Primary Endpoint: PFS
Primary endpoint data expected in 1H2014
•
•
•
•
•
17 |
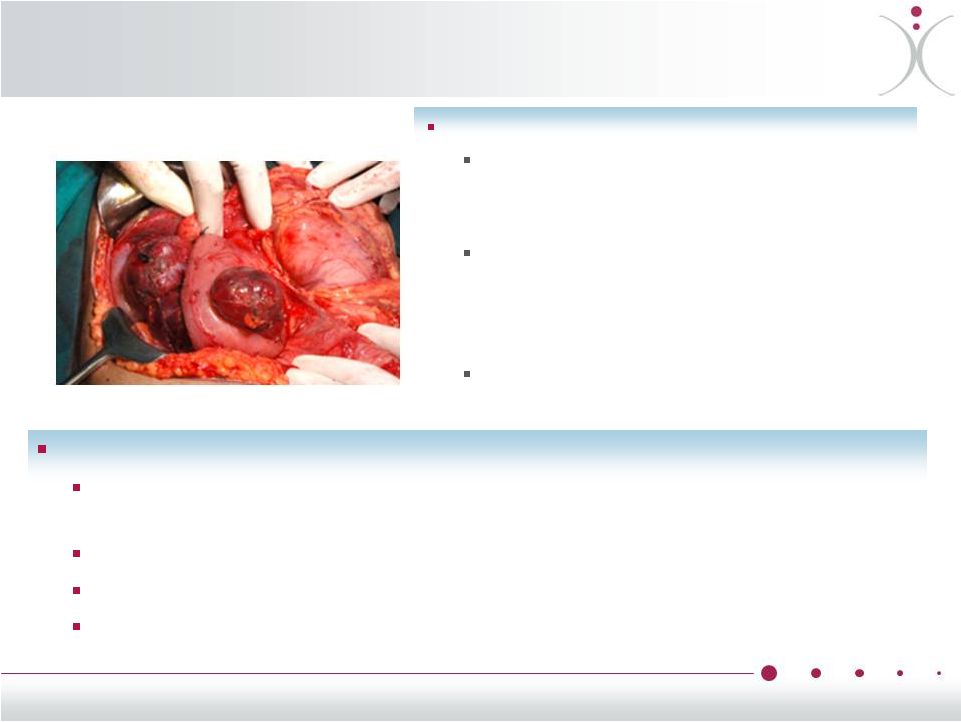 Neuroendocrine
Tumors Including Carcinoid Slow-Growing, Biologically Active
Tumors Neuroendocrine tumors (NETs)
overproduce biologically active substances
such as serotonin or insulin
These can cause debilitating symptoms,
including episodic flushing, diarrhea,
wheezing, potentially carcinoid heart
disease or hypoglycemia
Low mitotic index often limits
effectiveness of chemotherapy
Limited Therapeutic Options
Somatostatin analogues such as Sandostatin
®
(octreotide) help to control
symptoms, but the effect is often only temporary
Serotonin inhibitors in development (only effective in serotonin-producing
tumors). Non-responders
are
limited
to
surgical
resection
-
if
possible
Chemotherapy only effective if mitotic index is high
18 |
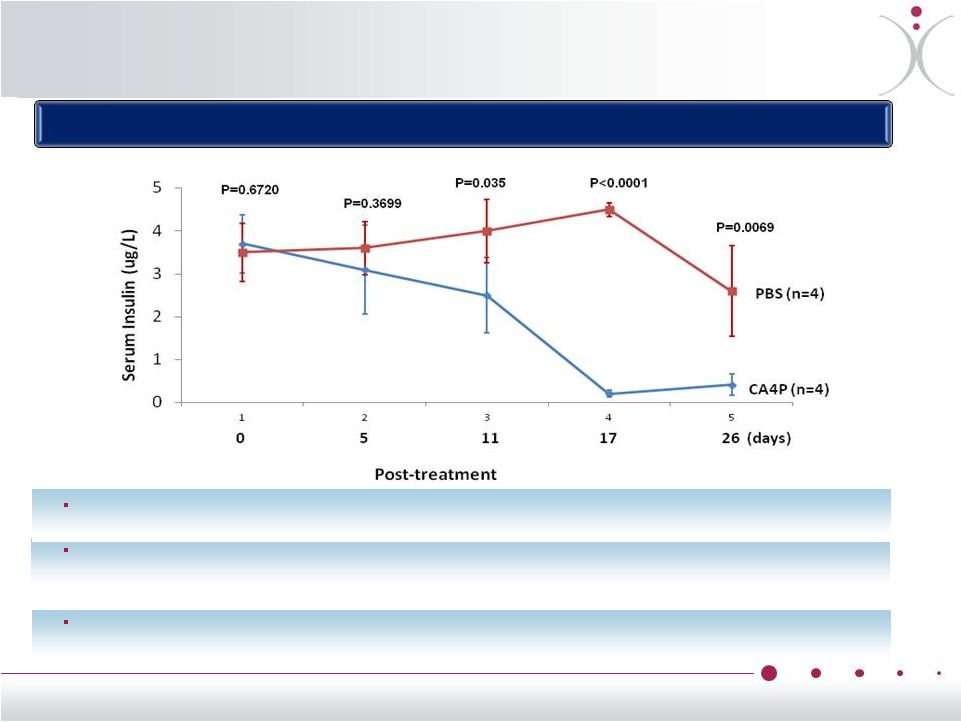 ZYBRESTAT
in
Pancreatic
NET
•* ZiQiang
Yuan et al., Albert Einstein College of Medicine, Bronx, NY, 2013 AACR AACR-NCI-EORTC
International Conference on Molecular Targets and Cancer Therapeutics
Effect of CA4P on serum insulin level in Men1 KO mice with IP injection of CA4P
or PBS for four weeks
ZYBRESTAT
®
decreased
insulin
levels
in
functional
transgenic
mouse
model *
®
Treatment with ZYBRESTAT
®
resulted in a significant and sustained decrease in circulating insulin, with maximum effect seen by day 17
(CA4P group 0.213 ± 0.075 µg/L, versus PBS
group 4.578 ± 0.161 µg/L, p<0.0001)
The reduction in insulin was accompanied by a significantly (p=0.0128) reduced tumor size in the CA4P group (1.83 ± 1.27 mm
2
) compared to the PBS group (9.88 ±
2.99 mm
2
).
19 |
 Sandostatin
®
(octreotide) +/-
ZYBRESTAT
®
Planned Phase 2 Self-Controlled Study
20 Sandostatin®-refractory
GI- NET patients with increased
biomarker levels (5-HIAA and/or chromogranin A) or clinical
symptoms
Primary endpoints: Biomarker (5-HIAA and/or chromogranin A
levels)
Secondary endpoints: Symptom control, Quality of Life
Rationale: Positive Preclinical Results, Potentially Rapid
Development Pathway, Robust Intellectual Property
Positive preclinical data in prolactinoma and insulinoma models
Potentially rapid readout and development based on biomarkers
Exclusive, worldwide licensing agreement with Angiogene for the
use of VDAs in the treatment of carcinoid syndrome and other
neuroendocrine tumors (NETs)
Additional IP filed
20 |
 ZYBRESTAT
®
in Anaplastic Thyroid Cancer
Completed Randomized Ph. 2/3 (FACT) Trial Results
80 patients with biopsy-proven ATC at 40 clinical sites
Paraplatin®
(carboplatin) / Taxol®
(paclitaxel) +/-
ZYBRESTAT®
Primary endpoint: Median survival: 5.2 vs. 4.0 months (NS), HR 0.72
Secondary endpoints: 1-year survival 26 vs. 9%; OR rate 20 vs. 16%
Target 2016 EMA marketing authorization in EU
Potential
for
approval
via
“Exceptional
Circumstances”
pathway
May not require additional clinical data
Anaplastic Thyroid Cancer (ATC)
Highly aggressive and lethal cancer with limited
treatment options
~2,000 total patients annually in the US and EU
Orphan drug status in both the US and EU
21 |
 ZYBRESTAT
®
in
ATC:
Data
Suggest
Clinical
Benefit
•* Remick S. et al; Thyroid. 2009 March;
19(3): 233–240; **Sosa et al; Thyroid 2013 May 30. [Epub ahead of print]
Phase 1 study in solid tumors –
single agent therapy
Including 7 ATC patients: 1 SD, 1 PR, and one long-lasting CR (14 years)
Open label Phase 2 study in ATC patients –
single agent therapy
26 patients with biopsy-proven ATC
Median overall survival: 4.7 months
1-year survival: 23%, comparing favorably to historical control (<10%)*
FACT Study –
randomized, controlled Phase 2/3 study in ATC patients
80 patients with biopsy-proven ATC
Carboplatin/paclitaxel +/-
ZYBRESTAT
®
randomized 2:1;
q 3 weeks x 6, followed by ZYBRESTAT
®
maintenance therapy or
observation, until evidence of progression
22 |
 FACT Study
– Summary of Results
FACT Study results
One-year survival rate 25.5% vs. 8.7%
Median Overall Survival 5.2 vs. 4.0 months (HR 0.72)
25% higher Objective Response rate (20% vs. 16%)
Forest Plot of Hazard Ratios for OS in subgroups
Lower
HR
with
use
of
ZYBRESTAT
®
for
patients
60
years
of
age,
prior
thyroid surgery, prior chemotherapy, prior radiation therapy, stage IVC
disease and tumor size >6 cm; suggesting greater antitumor activity in more
advanced tumors
Safety
Addition of ZYBRESTAT
®
to standard chemotherapy was observed to be
well tolerated with AEs primarily related to ATC and disease progression
Treatment-related AEs were easily clinically managed
23 |
 ZYBRESTAT
®
Clinical Safety Profile Observed in Clinical trials To Date
No cumulative toxicities or cytotoxic-like side-effects
Most AEs related to underlying disease or disease
progression
Adverse events are mainly low grade, reversible,
transient, and manageable
Absence
of
significant
cardiac
side
effects
-
transient
mild to moderate hypertension effectively managed
through antihypertensive prophylaxis
Transient myelosuppression in association with
chemotherapy, manageable without stopping therapy
Sequence of administration relevant for
myelosuppression
–
less
if
ZYBRESTAT
given
after
chemotherapy
400+
patient safety
data set
24 |
 Unique, Dual
Mechanism of Action Tubulin-mediated cell shape change of endothelial and
leukemic cells Metabolized by oxidative enzymes (e.g., tyrosinases and peroxidases)
into an orthoquinone species with direct cytotoxic effects on tumor cells
Compelling Preclinical and Early Clinical Data
Recommended dose, objective responses and significant blood flow
reductions shown in Ph 1/2 studies in refractory solid tumors
Demonstrated near-complete elimination of FLT-3 mutated human AML
(leukemic clone) in systemic xenograft in SCID mouse model
Ongoing Dose-Escalating Phase 1 Study in Hematologic Malignancies
Robust Intellectual Property (IP)
Protected by both composition-of-matter and method-of-use patents
Exclusive, worldwide license to the combretastatins discovered and
isolated by researchers at Arizona State University (ASU)
OXiGENE’s OXi4503: A 2nd-Generation VDA
25 |
 Robust
Intellectual Property Portfolio ZYBRESTAT®
United States
Europe
Japan
Composition of Matter: fosbretabulin
tromethamine
September 2021
US 7659261, 7524832 &
7659262
September 2021
EPO 1320534
September 2021
JP 4149804
Method of modulating tumor growth
or metastasis by administration of fosbretabulin
and paclitaxel
December 2021
US 6,538,038
N/A
N/A
Method of treating NET including carcinoid
tumor symptoms by administering fosbretabulin
June 2033
PCT* pending
June 2033
PCT *pending
June 2033
PCT* pending
OXi4503
Composition
of
Matter:
OXi4503
–
salt
form
including potassium
October 2021
US 7,078,552
April 2021
EPO 1278758
February 2020
pending
Method of treating myeloid neoplasm by
administering OXi4503
November 2028
November 2028
November 2028
JP 5302328
•
*A Patent Cooperation Treaty (PCT) application establishes a filing date in all 148 contracting
states. OXiGENE is the owner or exclusive licensee
of more than 120 patents worldwide
26 |
 OXiGENE
- Where is the Opportunity?
Significant Therapeutic and Commercial Potential
Potential to significantly improve outcomes in solid tumor indications
Example: Antisoma received $75M upfront and $915 M in potential
development-based milestones for ASA404 from Novartis
Challenging Development History Limits Competition
Apart from OXiGENE only 3 other companies are known to be currently
developing VDAs, none of which have progressed to the submission
stage
OXiGENE believes it has determined the optimal way ZYBRESTAT
®
should be developed and brought to the market
ZYBRESTAT®
May Become the First Commercialized VDA
Clinically meaningful activity with no significant safety issues
to date
Previously identified cardiovascular response was found to be easily
managed with no abrogation of the anti-tumor effects
27 |
 OXiGENE
- Financial Overview
Cost-Efficient, “Virtual Company”
Infrastructure (as of YE 2013)
No debt, cash: $7.0M, projected to last into mid 3Q14
–
Raised $9.1M in 2013 via ATM and two PIPEs, including proceeds from exercise of
PIPE warrants, net of redemption and expenses.
Common Stock: 5.6M outstanding (11.0M fully diluted)
Use of Proceeds from Offering
Completion
of
the
ongoing
GOG0186I
trial
in
ovarian
cancer
–
most
of
the costs were borne by NCI/CTEP
If GOG0186I is positive, initiate pursuit of pivotal program with FDA
input and potential partner
If
GOG0186I
is
positive,
partial
funding
of
potential
Phase
1/2
trial
in
ovarian
cancer
with
®
in
combination
with
Votrient®
EU
MAA
filing
for
®
®
combo
in
ATC
including
manufacturing
of
mandatory
registration
lots
of
®
Initiate Phase 2 study in gastrointestinal neuroendocrine tumors
/
ZYBRESTAT
ZYBRESTAT
Paraplatin
Taxol
28 |
 OXiGENE (OXGN)
Investment Thesis Compelling Valuation
Currently trading at a market cap of approximately $14 million
Leveraging $200+ million previously invested primarily in VDA development
Cost-Efficient and Risk-Mitigated Development Strategy
Ongoing and planned Ph 2 trials supported by foundations, non-profit
research institutions and larger pharmaceutical companies
Significant Potential Value-Creating Events on the Horizon
Readout of Avastin
®
+/-
ZYBRESTAT
®
Ph 2 in ovarian cancer
–
primary
endpoint results expected 1H14
Potential initiation of FDA interaction and planning of pivotal NDA program for
Avastin
®
+ ZYBRESTAT
®
combination
Initiation of the planned Phase 2 trial in neuroendocrine tumors
(NETs)
Demonstrate clinical activity for OXi4503 Phase 1 trial in AML
Actively pursuing development and commercialization agreements with
established industry leaders
29 |
