Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Advaxis, Inc. | v365476_8k.htm |

2013 Advaxis, Inc. | NASDAQ:ADXS Ticker: ADXS Corporate Presentation Empowering the immune system from within™

2013 Advaxis, Inc. | NASDAQ:ADXS Forward Looking Statements This presentation contains forward - looking statements, including, but not limited to: statements as to the anticipated timing of clinical studies and other business developments, statements as to the development of new constructs, expectations as to the adequacy of our cash balances to support our operations for specified periods of time and as to the nature and level of cash expenditures, expectations as to market opportunities, our ability to take advantage of those opportunities, and the risk factors set forth from time to time in Advaxis' SEC filings, including but not limited to its report on Form 10 - K for the fiscal year ended October 31, 2012, available at http://www.sec.gov. The Company undertakes no obligation to publicly release the result of any revision to these forward - looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law. You are cautioned not to place undue reliance on any forward - looking statements. 2

2013 Advaxis, Inc. | NASDAQ:ADXS Why Invest in Advaxis? Proprietary cancer immunotherapy platform technology -- hottest area of cancer research • Live attenuated bacteria stimulate the immune system to view the tumor as a bacterial infection for elimination -- No other technology has the same capability Lead immunotherapy, ADXS - HPV, plans to initiate registrational Phase 3 trials in 20 14 • Improved survival and objective tumor responses in 110 patients with recurrent cervical cancer (Phase 2 clinical study) Plans to file INDs and initiate Phase 1 trials in 2014 for prostate and breast cancers Multiple licensing opportunities • Recently licensed in Asia for ADXS - HPV -- other ADXS - HPV regional deals to follow • Promising opportunity in animal - health based on encouraging survival data in canine osteosarcoma – second immunotherapy, validating platform Low cost manufacturing Orphan Drug Designations Granted Strong balance sheet -- no debt 3

2013 Advaxis, Inc. | NASDAQ:ADXS 1H 2014 • Execute second ex - US HPV deal to market dominate player • Partner ADXS - cHER2 for animal - health indications • Conduct FDA EOP2 meeting for ADXS - HPV in recurrent cervical cancer • Initiate Phase 1/2 high - dose study in recurrent cervical cancer • Dose first patient in Phase 1/2 head & neck cancer trial (Mount Sinai) • Submit IND for ADXS - PSA in prostate cancer • Initiate Phase 1 in prostate cancer with ADXS - PSA • Secure CMO with scale - up and commercial capabilities Anticipated Milestones 4 2H 2014 • Submit IND for ADXS - cHER2 in breast cancer • Initiate global Phase 3 study in recurrent cervical cancer with ADXS - HPV • Initiate Phase 1 study in HPV - associated lung cancer (GBP -- partner in Asia) • Report data (Mount Sinai study)
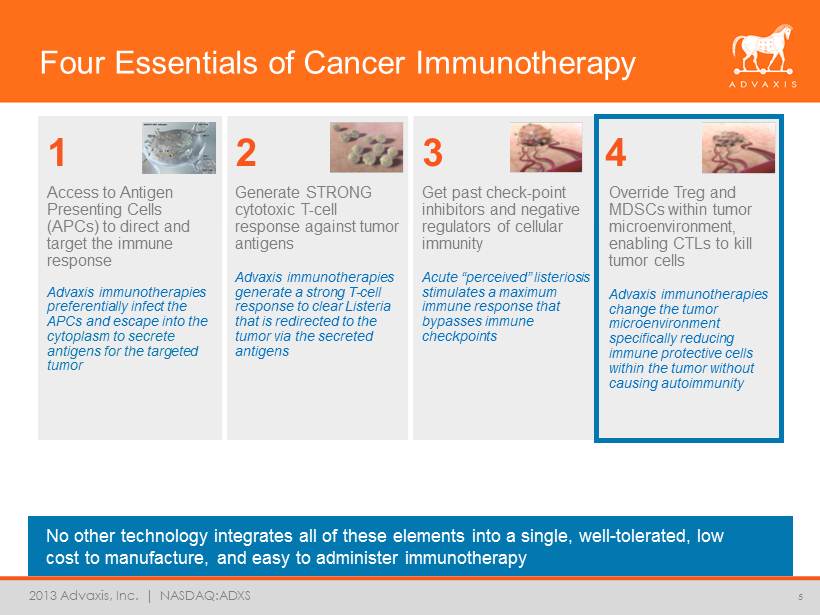
2013 Advaxis, Inc. | NASDAQ:ADXS Four Essentials of Cancer Immunotherapy No other technology integrates all of these elements into a single, well - tolerated, low cost to manufacture, and easy to administer immunotherapy 5 Access to Antigen Presenting Cells (APCs) to direct and target the immune response Advaxis immunotherapies preferentially infect the APCs and escape into the cytoplasm to secrete antigens for the targeted tumor 1 2 3 4 Generate STRONG cytotoxic T - cell response against tumor antigens Advaxis immunotherapies generate a strong T - cell response to clear Listeria that is redirected to the tumor via the secreted antigens Get past check - point inhibitors and negative regulators of cellular immunity Acute “ perceived ” listeriosis stimulates a maximum immune response that bypasses immune checkpoints Override Treg and MDSCs within tumor microenvironment, enabling CTLs to kill tumor cells Advaxis immunotherapies change the tumor microenvironment specifically reducing immune protective cells within the tumor without causing autoimmunity
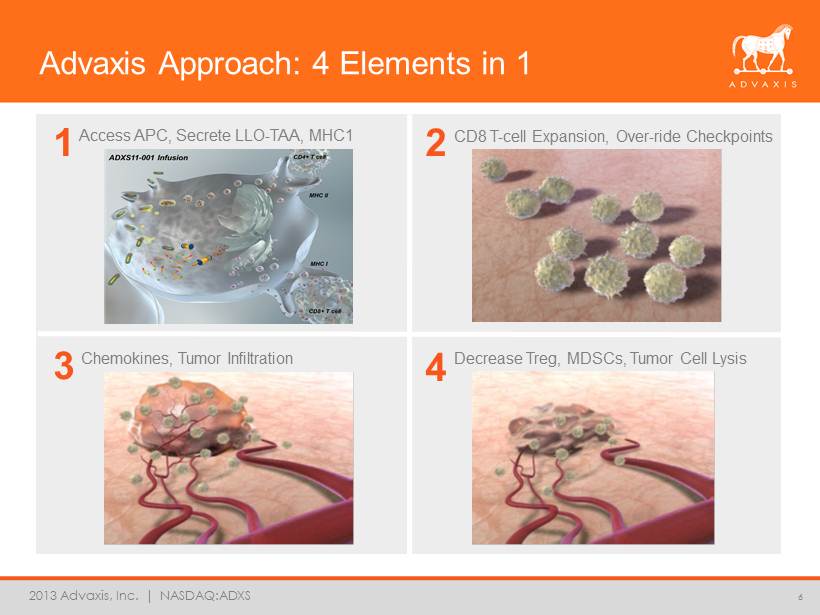
2013 Advaxis, Inc. | NASDAQ:ADXS Advaxis Approach: 4 Elements in 1 6 Access APC, Secrete LLO - TAA, MHC1 CD8 T - cell Expansion, Over - ride Checkpoints Chemokines, Tumor Infiltration Decrease Treg, MDSCs, Tumor Cell Lysis 1 2 3 4
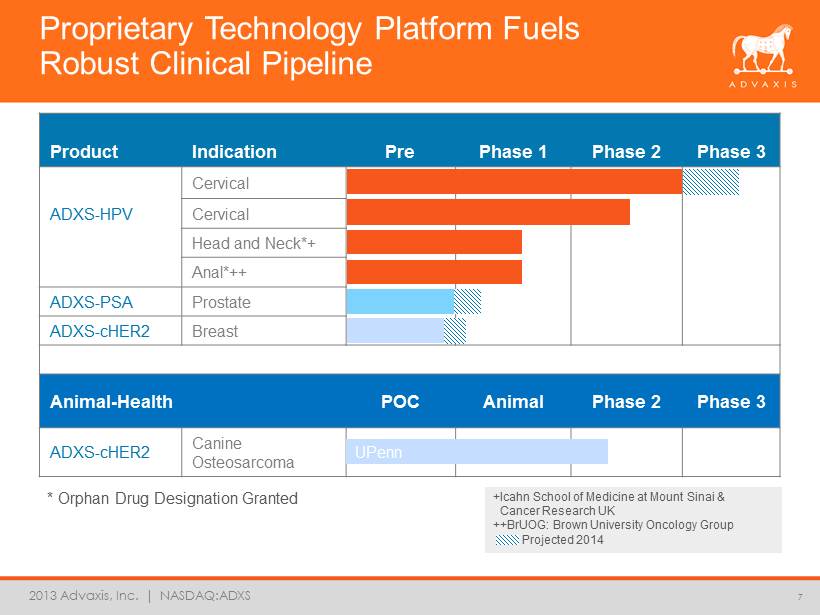
2013 Advaxis, Inc. | NASDAQ:ADXS +Icahn School of Medicine at Mount Sinai & Cancer Research UK ++BrUOG: Brown University Oncology Group Projected 2014 Product Indication Pre Phase 1 Phase 2 Phase 3 Cervical ADXS - HPV Cervical Head and Neck*+ Anal*++ ADXS - PSA Prostate ADXS - cHER2 Breast Animal - Health POC Animal Phase 2 Phase 3 ADXS - cHER2 Canine Osteosarcoma Proprietary Technology Platform Fuels Robust Clinical Pipeline 7 UPenn * Orphan Drug Designation Granted
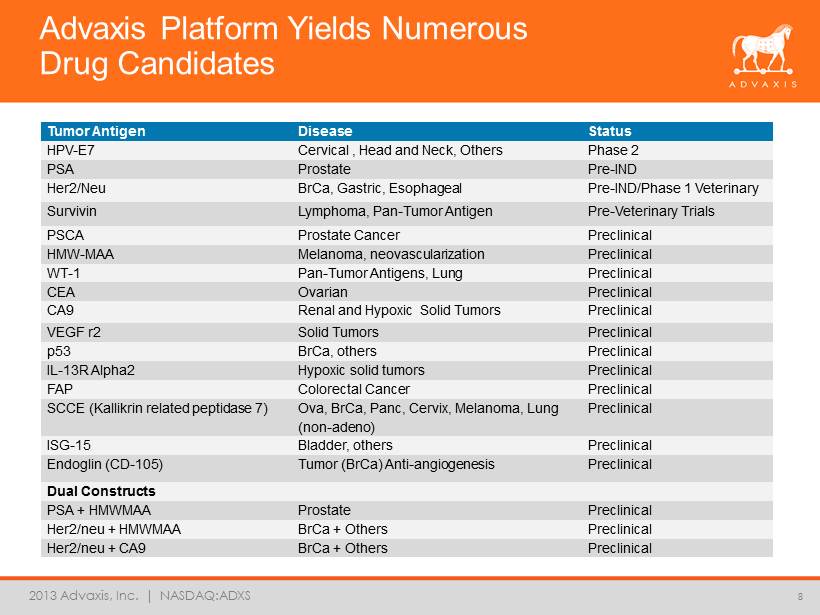
2013 Advaxis, Inc. | NASDAQ:ADXS Advaxis Platform Yields Numerous Drug Candidates 8 Tumor Antigen Disease Status HPV - E7 Cervical , Head and Neck, Others Phase 2 PSA Prostate Pre - IND Her2/Neu BrCa, Gastric, Esophageal Pre - IND/Phase 1 Veterinary Survivin Lymphoma, Pan - Tumor Antigen Pre - Veterinary Trials PSCA Prostate Cancer Preclinical HMW - MAA Melanoma, neovascularization Preclinical WT - 1 Pan - Tumor Antigens, Lung Preclinical CEA Ovarian Preclinical CA9 Renal and Hypoxic Solid Tumors Preclinical VEGF r2 Solid Tumors Preclinical p53 BrCa, others Preclinical IL - 13R Alpha2 Hypoxic solid tumors Preclinical FAP Colorectal Cancer Preclinical SCCE (Kallikrin related peptidase 7) Ova, BrCa, Panc, Cervix, Melanoma, Lung (non - adeno) Preclinical ISG - 15 Bladder, others Preclinical Endoglin (CD - 105) Tumor (BrCa) Anti - angiogenesis Preclinical Dual Constructs PSA + HMWMAA Prostate Preclinical Her2/neu + HMWMAA BrCa + Others Preclinical Her2/neu + CA9 BrCa + Others Preclinical
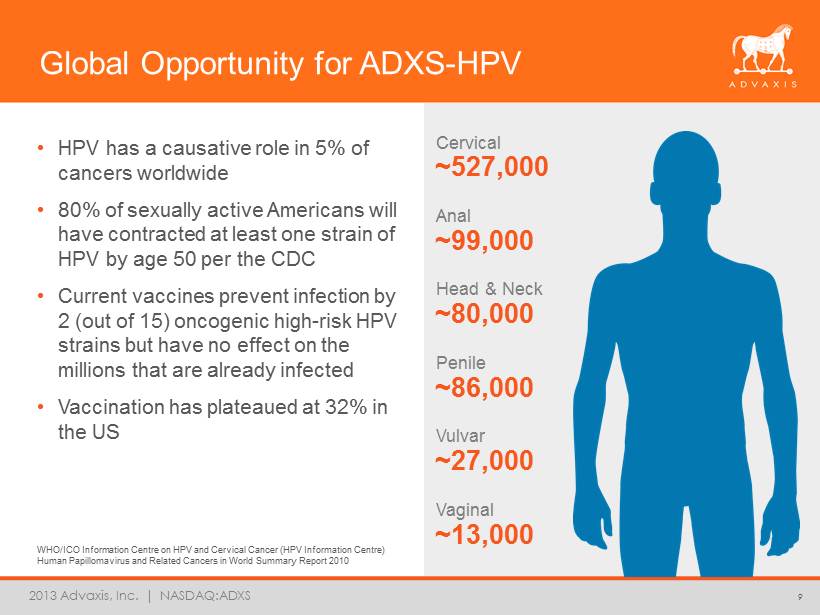
2013 Advaxis, Inc. | NASDAQ:ADXS Global Opportunity for ADXS - HPV • HPV has a causative role in 5% of cancers worldwide • 80% of sexually active Americans will have contracted at least one strain of HPV by age 50 per the CDC • Current vaccines prevent infection by 2 (out of 15) oncogenic high - risk HPV strains but have no effect on the millions that are already infected • Vaccination has plateaued at 32% in the US 9 ~80,000 Head & Neck ~527,000 Cervical ~99,000 Anal ~27,000 Vulvar ~86,000 Penile ~13,000 Vaginal WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre) Human Papillomavirus and Related Cancers in World Summary Report 2010
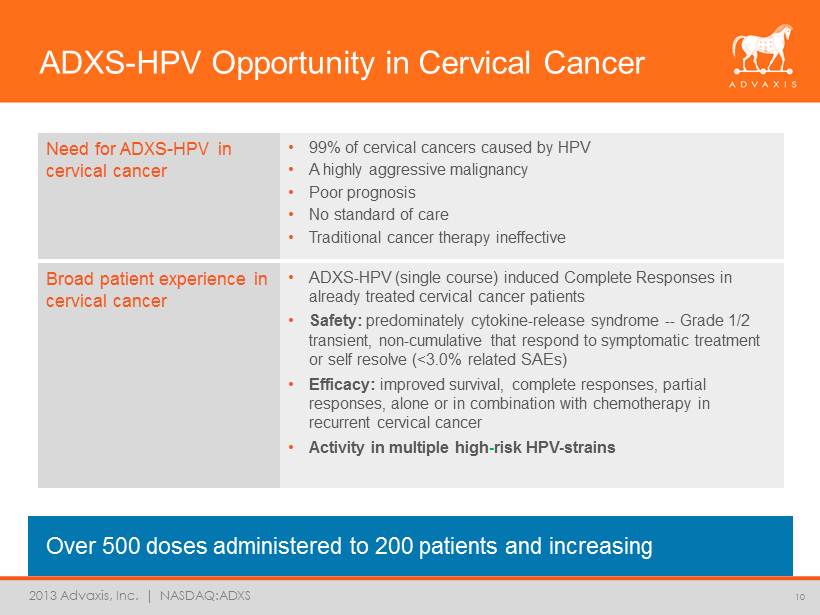
2013 Advaxis, Inc. | NASDAQ:ADXS Need for ADXS - HPV in cervical cancer • 99% of cervical cancers caused by HPV • A highly aggressive malignancy • Poor prognosis • No standard of care • Traditional cancer therapy ineffective ADXS - HPV Opportunity in Cervical Cancer Over 500 doses administered to 200 patients and increasing 10 Broad patient experience in cervical cancer • ADXS - HPV (single course) induced Complete Response s in already treated cervical cancer patients • Safety: predominately cytokine - release syndrome -- Grade 1/2 transient, non - cumulative that respond to symptomatic treatment or self resolve (<3.0% related SAEs) • Efficacy: improved survival, complete responses, partial responses, alone or in combination with chemotherapy in recurrent cervical cancer • Activity in multiple high - risk HPV - strains
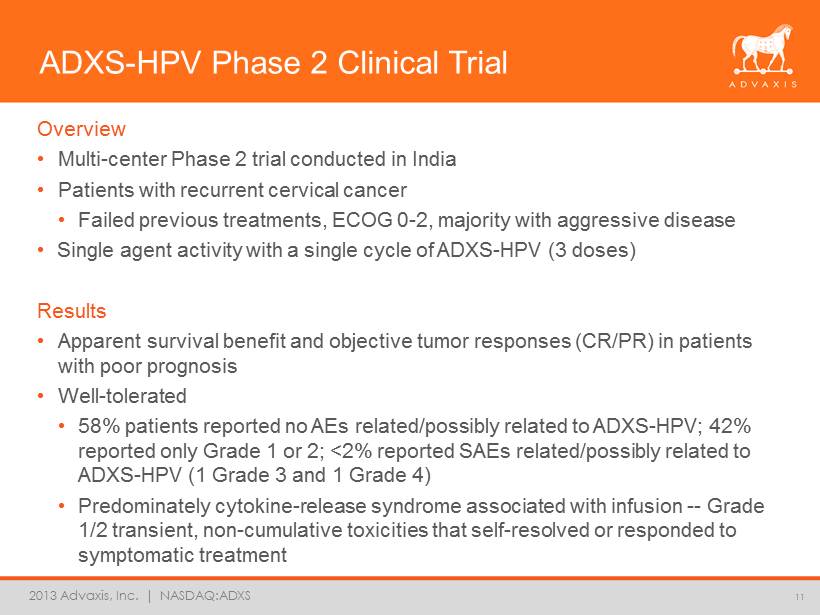
2013 Advaxis, Inc. | NASDAQ:ADXS ADXS - HPV Phase 2 Clinical Trial Overview • Multi - center Phase 2 trial conducted in India • Patients with recurrent cervical cancer • Failed previous treatments, ECOG 0 - 2, majority with aggressive disease • Single agent activity with a single cycle of ADXS - HPV (3 doses) Results • Apparent survival benefit and objective tumor responses (CR/PR) in patients with poor prognosis • Well - tolerated • 58% patients reported no AEs related/possibly related to ADXS - HPV; 42% reported only Grade 1 or 2; <2% reported SAEs related/possibly related to ADXS - HPV (1 Grade 3 and 1 Grade 4) • Predominately cytokine - release syndrome associated with infusion -- Grade 1/2 transient, non - cumulative toxicities that self - resolved or responded to symptomatic treatment 11
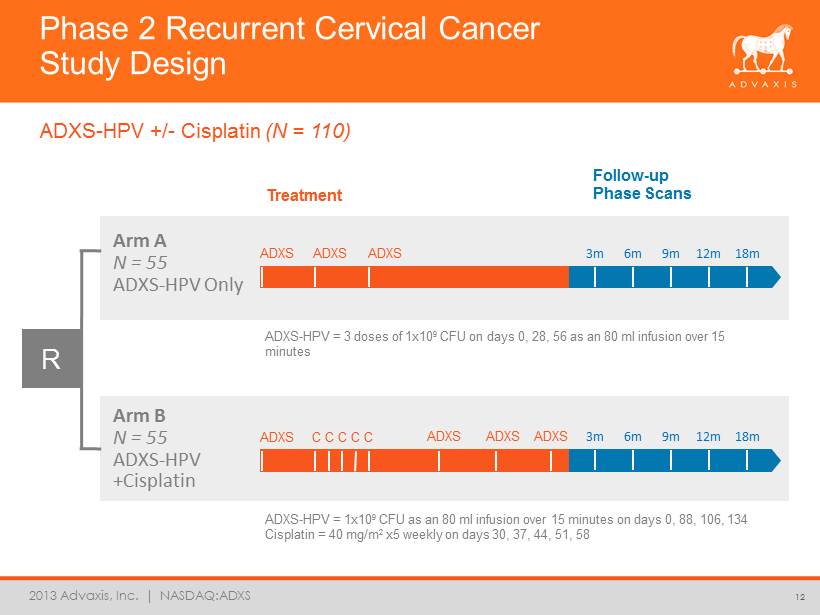
2013 Advaxis, Inc. | NASDAQ:ADXS 3m 6m 9m 12m 18m Phase 2 Recurrent Cervical Cancer Study Design 12 ADXS - HPV +/ - Cisplatin (N = 110) Arm A N = 55 ADXS - HPV Only Arm B N = 55 ADXS - HPV +Cisplatin Treatment Follow - up Phase Scans ADXS ADXS ADXS ADXS ADXS ADXS 3m 6m 9m 12m 18m R ADXS C C C C C ADXS - HPV = 3 doses of 1x10 9 CFU on days 0, 28, 56 as an 80 ml infusion over 15 minutes ADXS - HPV = 1x10 9 CFU as an 80 ml infusion over 15 minutes on days 0, 88, 106, 134 Cisplatin = 40 mg/m 2 x5 weekly on days 30, 37, 44, 51, 58
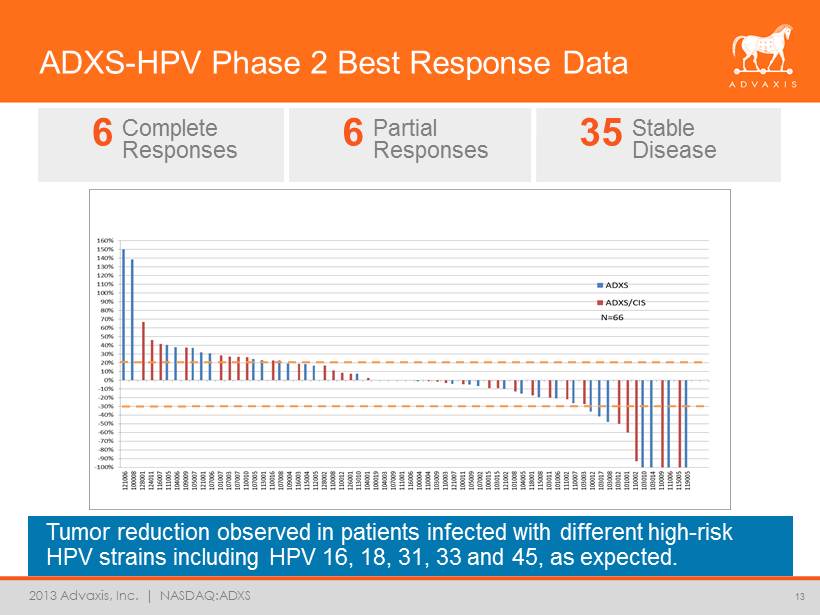
2013 Advaxis, Inc. | NASDAQ:ADXS ADXS - HPV Phase 2 Best Response Data Tumor reduction observed in patients infected with different high - risk HPV strains including HPV 16, 18, 31, 33 and 45, as expected. 13 6 Complete Responses 6 Partial Responses 35 Stable Disease
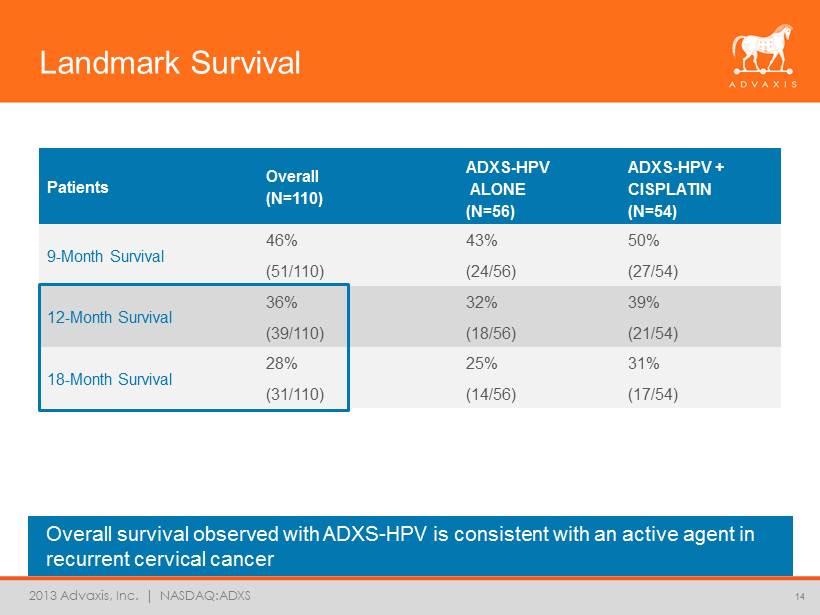
2013 Advaxis, Inc. | NASDAQ:ADXS Landmark Survival Overall survival observed with ADXS - HPV is consistent with an active agent in recurrent cervical cancer 14 Patients Overall (N=110) ADXS - HPV ALONE (N=56) ADXS - HPV + CISPLATIN (N=54) 9 - Month Survival 46% (51/110) 43% (24/56) 50% (27/54) 12 - Month Survival 36% (39/110) 32% (18/56) 39% (21/54) 18 - Month Survival 28% (31/110) 25% (14/56) 31% (17/54)
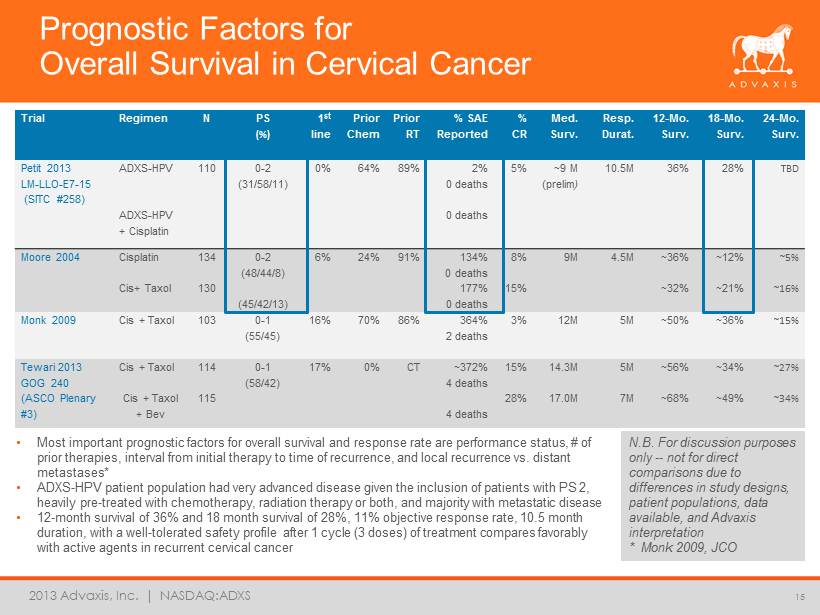
2013 Advaxis, Inc. | NASDAQ:ADXS Prognostic Factors for Overall Survival in Cervical Cancer Trial Regimen N PS (%) 1 st line Prior Chem Prior RT % SAE Reported % CR Med. Surv. Resp. Durat. 12 - Mo. Surv. 18 - Mo. Surv. 24 - Mo. Surv. Petit 2013 LM - LLO - E7 - 15 (SITC #258) ADXS - HPV ADXS - HPV + Cisplatin 110 0 - 2 (31/58/11) 0% 64% 89% 2% 0 deaths 0 deaths 5% ~9 M (prelim ) 10.5M 36% 28% TBD Moore 2004 Cisplatin Cis+ Taxol 134 130 0 - 2 (48/44/8) (45/42/13) 6% 24% 91% 134% 0 deaths 177% 0 deaths 8% 15% 9M 4.5M ~36% ~32% ~12% ~21% ~5% ~16% Monk 2009 Cis + Taxol 103 0 - 1 (55/45) 16% 70% 86% 364% 2 deaths 3% 12M 5M ~50% ~36% ~15% Tewari 2013 GOG 240 (ASCO Plenary #3) Cis + Taxol Cis + Taxol + Bev 114 115 0 - 1 (58/42) 17% 0% CT ~372% 4 deaths 4 deaths 15% 28% 14.3M 17.0M 5M 7M ~56% ~68% ~34% ~49% ~27% ~34% • Most important prognostic factors for overall survival and response rate are performance status, # of prior therapies, interval from initial therapy to time of recurrence, and local recurrence vs. distant metastases* • ADXS - HPV patient population had very advanced disease given the inclusion of patients with PS 2, heavily pre - treated with chemotherapy, radiation therapy or both, and majority with metastatic disease • 12 - month survival of 36% and 18 month survival of 28%, 11% objective response rate, 10.5 month duration, with a well - tolerated safety profile after 1 cycle (3 doses) of treatment compares favorably with active agents in recurrent cervical cancer N.B. For discussion purposes only -- not for direct comparisons due to differences in study designs, patient populations, data available, and Advaxis interpretation * Monk 2009, JCO 15

2013 Advaxis, Inc. | NASDAQ:ADXS Next steps towards registration in this highly aggressive malignancy • Conduct EOP2 meeting with FDA, finalize Phase 3 protocols, submit SPA • Conduct 2 pivotal Phase 3 trials (multi - national trials with partner participation) • Conduct Phase 1/2 study with high dose, immunology endpoints and repeat cycles • Complete GOG NCI Phase 2 study of ADXS - HPV in 67 patients with recurrent/refractory cervical cancer Next Steps for ADXS - HPV in Recurrent Cervical Cancer 16
2013 Advaxis, Inc. | NASDAQ:ADXS Opportunity for ADXS - HPV in Head & Neck Cancer 17 Need for ADXS - HPV in head and neck cancer • HPV - associated head & neck cancer is increasing at an epidemic rate due to changing sexual practices • 25%+ of head & neck cancer caused by HPV • Current therapies lead to poor quality of life Clinical development • Discuss development plan with the FDA under ODD • Phase 1/2 “window of opportunity” study in 25 patients with early disease in US Icahn School of Medicine at Mount Sinai • Phase 1 to evaluate the use of ADXS - HPV for the treatment of HPV - associated head and neck cancer in 27 patients (REALISTIC) • Future Phase 2 and 3 studies to be determined Orphan Drug Designation Granted
2013 Advaxis, Inc. | NASDAQ:ADXS Opportunity for ADXS - HPV in Anal Cancer Orphan Drug Designation Granted 18 Need for ADXS - HPV in anal cancer • 80 - 100% caused by HPV • Current therapies are toxic and have long - term side effects • No therapy for recurrent disease Clinical development • Discuss development plan with the FDA under ODD • Phase 1/2 study in 25 patients with HPV - associated anal cancer Brown University Oncology Group (BrUOG) • Future Phase 2 and 3 studies within RTOG to be determined

2013 Advaxis, Inc. | NASDAQ:ADXS Create long - term value for the Company by forming relationships with market dominant biopharmaceutical companies that validate our platform • Seeking partnerships that are aligned with the Company ’ s clinical and commercialization objectives Commercialization and Partnering Strategy 19 Strategy • Retain US rights to ADXS - HPV • License ADXS - HPV on regional basis outside the US • License ADXS - cHER2 for animal - health • Seek worldwide partnerships for larger indications (prostate & breast) Existing Partnerships • Asia: Global BioPharma

2013 Advaxis, Inc. | NASDAQ:ADXS • Exclusive licensing agreement with Global BioPharma (GBP), funded by a group of investors led by Taiwan Biotech Company Ltd. (TBC) • TBC is one of the top 5 biopharmaceutical companies in Taiwan • Formed Global BioPharma (GBP) solely to focus on development and commercialization of ADXS - HPV for the treatment of HPV - associated cancers in Asia (29 countries) • Territory covers >4B people with >200,000 annual diagnoses of cervical cancer (roughly 40% of the world ’ s cases) Licensing Agreement in Asia 20 GBP • Conduct registration trials for cervical cancer and explore development in lung, head and neck, and anal cancers • Responsible for all clinical development and commercialization costs (including 150 patients in US and Asia registrational programs) • Establish manufacturing for its own territories • Purchased common stock from Advaxis at market with option to purchase additional shares at 150% premium Advaxis will receive • Event - based financial milestones • A nnual development fee • A nnual net sales royalty payments in the high single to double digits

2013 Advaxis, Inc. | NASDAQ:ADXS Immunotherapy targeting cancers overexpressing HER2 • Including breast cancer and others ADXS - cHER2 for the Treatment of Canine Osteosarcoma and Human Breast Cancers Animal - Health Program • Phase 1 data in canine osteosarcoma (CO) show encouraging survival in companion dogs treated with ADXS - cHER2 vs. those untreated • Naturally occurring tumors in companion dogs not animals bred for research • Data validate platform technology • Data support further development and basis for regulatory pathway to advance toward conditional approval with USDA 21 Human Program • Preliminary canine osteosarcoma data provide rationale to advance ADXS - cHER2 into a Phase 1 for breast cancer and other HER2 driven cancers going forward
2013 Advaxis, Inc. | NASDAQ:ADXS Animal - Health -- Products in Development Canine Osteosarcoma (OS) • Approximately 8,000 - 20,000 dogs per year in the US • Highly aggressive mesenchymal tumor - - medium to large breeds • Standard of care treatment: amputation and post operative chemotherapy • High rate of recurrence • 9 months -- 1 year median survival, 25% of dogs survive two years 22 Canine Lymphoma (T - cell and B - cell) • 5 million new cases per year in the US (1 in 15 dogs) • 80 - 100% express Survivin • Median Survival T - cell Lymphoma ~55 - 162 days • Median Survival B - cell Lymphoma ~127 - 256 days Current Clinical Program Targeted Indication
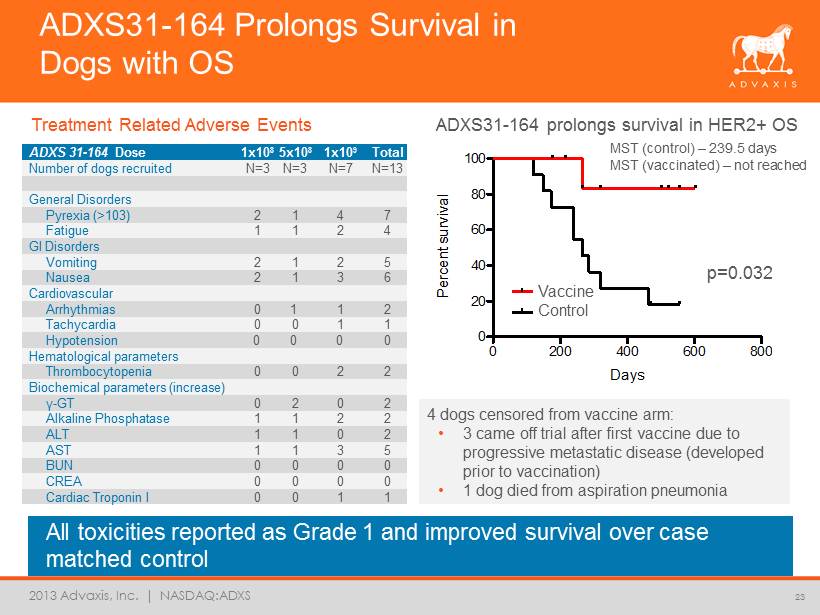
2013 Advaxis, Inc. | NASDAQ:ADXS All toxicities reported as Grade 1 and improved survival over case matched control 23 Survival of Data 4:Survival proportions 0 200 400 600 800 0 20 40 60 80 100 Legend Legend Days P e r c e n t s u r v i v a l ADXS31 - 164 Prolongs Survival in Dogs with OS ADXS 31 - 164 Dose 1x10 8 5x10 8 1x10 9 Total Number of dogs recruited N=3 N=3 N=7 N=13 General Disorders Pyrexia (>103) 2 1 4 7 Fatigue 1 1 2 4 GI Disorders Vomiting 2 1 2 5 Nausea 2 1 3 6 Cardiovascular Arrhythmias 0 1 1 2 Tachycardia 0 0 1 1 Hypotension 0 0 0 0 Hematological parameters Thrombocytopenia 0 0 2 2 Biochemical parameters (increase) γ - GT 0 2 0 2 Alkaline Phosphatase 1 1 2 2 ALT 1 1 0 2 AST 1 1 3 5 BUN 0 0 0 0 CREA 0 0 0 0 Cardiac Troponin I 0 0 1 1 ADXS31 - 164 prolongs survival in HER2+ OS Survival proportions: Survival of Data 1 Time P e r c e n t s u r v i v a l 0 100 200 300 400 0 50 100 Control Vaccine p=0.032 4 dogs censored from vaccine arm: • 3 came off trial after first vaccine due to progressive metastatic disease (developed prior to vaccination) • 1 dog died from aspiration pneumonia MST (control) – 239.5 days MST (vaccinated) – not reached Vaccine Control Treatment Related Adverse Events
2013 Advaxis, Inc. | NASDAQ:ADXS ADXS - PSA for the Treatment of Prostate Cancer 24 Our approach • Target cells expressing PSA with our immunotherapy • Conducted a pre - IND meeting with the FDA to discuss the CMC, pharmacology, toxicology, and clinical plans for ADXS - PSA • Establish proof - of - concept Clinical development x Required toxicology studies completed and GMP drug product manufactured for the Phase 1 clinical study • IND to be filed with the FDA for ADXS - PSA in the treatment of prostate cancer in the first half of 2014 • Phase 1 study to be initiated in first half of 2014 in the US Need for ADXS - PSA in prostate cancer • Large market • Expect ADXS - PSA to have similar safety profile to that of ADXS - HPV • Potential partnering opportunity

2013 Advaxis, Inc. | NASDAQ:ADXS Why Invest in Advaxis? Proprietary cancer immunotherapy platform technology -- hottest area of cancer research • Live attenuated bacteria stimulate the immune system to view the tumor as a bacterial infection for elimination -- No other technology has the same capability Lead immunotherapy, ADXS - HPV, plans to initiate registrational Phase 3 trials in 20 14 • Improved survival and objective tumor responses in 110 patients with recurrent cervical cancer (Phase 2 clinical study) Plans to file INDs and initiate Phase 1 trials in 2014 for prostate and breast cancers Multiple licensing opportunities • Recently licensed in Asia for ADXS - HPV -- other ADXS - HPV regional deals to follow • Promising opportunity in animal - health based on encouraging survival data in canine osteosarcoma – second immunotherapy, validating platform Simple, robust, and compliant manufacturing process Orphan Drug Designations Granted Strong balance sheet -- no debt 25

2013 Advaxis, Inc. | NASDAQ:ADXS Experienced Management Team & Board of Directors Management Team Daniel J. O ’ Connor, Esq. President and Chief Executive Officer • 15 years of executive, legal, regulatory, compliance, manufacturing and quality experience in the biopharmaceutical industry • Former Senior VP and General Counsel of ImClone Systems Incorporated • Played a key role in development, licensing and commercialization of Erbitux ® and was the executive leader who enabled the company to be sold to Eli Lilly in 2008 for $6.5B Robert Petit, Ph.D. Executive Vice President and Chief Scientific Officer • 25 years experience in oncology drug development • U.S. medical strategy lead for Yervoy ® program at Bristol Myers Squibb (NYSE: BMY) as the Director of Medical Strategy for oncology products and Director of Global Clinical Research • VP of Clinical Development at MGI Pharma and Aesgen, Inc. Gregory Mayes Executive Vice President, Chief Operating Officer • 20 years experience in operations and bio - pharmaceuticals, Executive Committee for Dendreon Corp., President, Unigene Laboratories, VP, GC, Chief Compliance Officer, ImClone Systems Inc., Senior Counsel, AstraZeneca Pharmaceuticals Mark Rosenblum Chief Financial Officer, Secretary, and Senior Vice President • 25 years experience in accounting and financial leadership. VP, Chief Accounting Officer of Wellman, Inc., a $1.2B chemical company; CFO and Secretary, HemoBioTech, Inc. Chris French Vice President, Executive Director of Medical Affairs • 20 years of research and pharmaceutical experience in drug development • Management positions in medical affairs, regulatory affairs, business development and scientific communications • U.S. Director of Oncology Scientific Communications, Bristol Myers Squibb., Senior Director, MGI Pharma and VP Regulatory and Scientific Affairs, Aesgen 26 Board of Directors David Sidransky, MD • Co - Founder & Chairman, Champions Oncology • Professor, Johns Hopkins, Oncology Medicine James Patton, MD, MBA, Chairman • VP, Millennium Oncology Management • Founder & Chairman, VAL Health Roni A. Appel • Managing Director, LibertyView Equity Partners Richard Berman • Former CEO, Easylink Services • Former SVP, Bankers Trust Company • Director, Lustros, Inc., and Neostem, Inc. Thomas McKearn, MD • Founder, Cytogen Corporation Thomas A. Moore • Former VP, Proctor & Gamble Daniel J. O ’ Connor, Esq. • President & CEO, Advaxis

2013 Advaxis, Inc. | NASDAQ:ADXS 27 Advaxis, Inc. 305 College Road East Princeton, NJ 08540 T: 609.452.9813 ir@advaxis.com www.advaxis.com
