Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Sagent Pharmaceuticals, Inc. | d658421d8k.htm |
 Sagent
Pharmaceuticals 32
nd
Annual J.P. Morgan
Healthcare Conference
January 15, 2014
A Global Pharmaceutical
Company
Exhibit 99.1 |
 2
Disclaimer
The
following
information
contains,
or
may
be
deemed
to
contain,
“forward-looking
statements”
(as
defined
in
and
made
pursuant
to
the
safe
harbor
provisions
of
the
U.S.
Private
Securities
Litigation
Reform
Act
of
1995).
Most
forward-looking
statements
contain
words
that
identify
them
as
forward-looking,
such
as
“may”,
“plan”,
“seek”,
“will”,
“expect”,
“intend”,
“estimate”,
“anticipate”,
“believe”,
“project”,
“opportunity”,
“target”,
and
“continue”
or
other
words
that
relate
to
future
events,
as
opposed
to
past
or
current
events.
By
their
nature,
forward-looking
statements
are
not
statements
of
historical
facts
and
involve
risks
and
uncertainties
because
they
relate
to
events
and
depend
on
circumstances
that
may
or
may
not
occur
in
the
future.
These
statements
give
our
current
expectation
of
future
events
or
our
future
performance
and
do
not
relate
directly
to
historical
or
current
events
or
our
historical
performance.
As
such,
our
future
results
may
vary
from
any
expectations
or
targets
expressed
in,
or
implied
by,
the
forward
looking
statements
included
in
this
presentation,
possibly
to
a
material
degree.
We
cannot
assure
you
that
the
assumptions
made
in
preparing
any
of
the
forward-looking
statements
will
prove
accurate
or
that
any
performance
targets
will
be
realized.
We
expect
that
there
will
be
differences,
which
could
be
significant,
between
performance
targets
and
actual
results.
All
forward-looking
statements
included
in
this
presentation
speak
only
as
of
the
date
made,
and
we
undertake
no
obligation
to
update
or
revise
publicly
any
such
forward-looking
statements,
whether
as
a
result
of
new
information,
future
events,
or
otherwise.
In
particular,
we
caution
investors
not
to
place
undue
weight
on
certain
forward-looking
statements
pertaining
to
potential
growth
opportunities
or
performance
targets
set
forth
herein.
Actual
results
may
vary
significantly
from
these
statements.
For
a
discussion
of
some
of
the
important
factors
that
could
cause
our
results
to
differ
materially
from
those
expressed
in,
or
implied
by,
the
forward-looking
statements
included
in
this
presentation,
investors
should
refer
to
our
risk
factors,
as
they
may
be
amended
from
time
to
time,
set
forth
in
our
Quarterly
Report
on
Form
10-Q
filed
with
the
SEC
on
August
6,
2013.
This
presentation
includes
a
discussion
of
certain
non-GAAP
financial
measures.
Please
refer
to
the
appendix
to
this
presentation
for
further
definition
of
these
measures
and
a
reconciliation
of
such
non-GAAP
measures
to
the
most
closely
comparable
GAAP
measures. |
 3
Investment Highlights
•
Fastest growing Generic Injectable company in U.S. Market , a consolidating industry
with high barriers to entry
•
Extensive global partner network with state-of-the-art facilities enabling
accelerated growth relative to conventional start-ups
•
Solid relationships at all levels of the supply chain enhance responsiveness to
industry demands
•
Vertical integration through acquisition of SCP complements partnering model, as
well as provides additional growth opportunities and a footprint
in China
•
Exceptional quality and safety track record; global excellence in manufacturing
•
Strong sequential margin and revenue growth driven by diverse and deep product
portfolio
•
Experienced management team with great track record, has delivered on product
approvals and financial performance |
 4
Track Record of Success
•
Jeffrey Yordon, Founder, CEO and Chairman
–
Formerly COO and President of APP; held senior
positions with LyphoMed, YorPharm, Gensia and
Faulding
–
Expert in China and India pharmaceutical
industries
–
43 years experience in injectable
pharmaceuticals; including leading four
successful injectable start-ups
Unique Business Model with Strong Track Record
Sagent Pharmaceuticals has revolutionized the way injectable generic drugs are produced and
delivered. Our ground-breaking business model is changing the industry dynamic and
providing access to a wide range of critical drugs when and how our customers need it
most. As a result,
we can satisfy the demands of today and anticipate the needs of tomorrow. Key Highlights
•
Track record of strong performance on product
launches, ANDA approvals and ANDA submissions
•
Extensive pipeline with 69 ANDAs pending approval
or launch
(1)
•
53 marketed products, 144 presentations
(1)
•
Indications: anti-infective, oncolytic and critical care
•
Presentations: single and multi-dose vials, pre-filled
ready-to-use syringes and premix bags
(1) As of December 31, 2013 |
 5
Corporate Evolution
Company founded in 2006
•
Built upon long-standing expertise and experience
in Generic Injectables
•
Virtual model based upon global network of
strong partner relationships
•
Product development focused on high volume,
low margin opportunities to build scale
2006
2010
Sales of $74 million in 2010,
including addressing the market
shortage in Heparin
2011
2012
Launched 16 new products,
driving nearly break-even
operating performance in the
fourth quarter
2013
Completed a successful IPO
and listed on NASDAQ in
April 2011
2014
Formal guidance to be provided Mid February, 2014
•
Estimated
launch
of
10
-
15
products
•
Continued investment in SCP to support product
development initiatives and capacity expansion
•
Market consolidation driving increased partnering
opportunities and accelerated investment in
product development
•
FDA timelines impact approval timelines
Revised revenue guidance of $230MM -
$250MM
•
YTD reported adjusted margins exceed long-term
targets, validating P&L leverage
•
Vertical Integration capabilities supplementing
virtual base model via consolidation of SCP facility
•
Launched 12 new products, including Zoledronic
Acid at market formation
•
Completed a successful secondary offering in
September 2013 to support SCP investment, pipeline
development and business development activity |
 6
Extensive Global Partner Network
Sagent’s Fundamental Initiative with Partners
•
Quality
–
Facility Support, cGMP training, audit
assistance and integration of Sagent System
•
Regulatory
–
Submission and Compliance Expertise and
become Regulatory Agent for partner
•
Sales/Marketing/Distribution
–
Ability to navigate the complex US distribution
network and in the future others
Industry Consolidation has accelerated the rate of partnering opportunities to a level never
experienced in our history |
 Sagent
Pharmaceuticals
Strategic Growth
Drivers
A Global Pharmaceutical
Company |
 8
Drivers of Long-Term Growth
Strong
Industry
Dynamics
Deep
Development
Pipeline
Investing For
the Future
Growing
Product
Portfolio
Industry
Leading
Sales
Organization |
 9
New Significant Initiative
Strong Industry Dynamics
Several significant macro economic and industry factors are driving demand and shaping
opportunity for Sagent Pharmaceuticals
Drug Shortage Crisis
•
U.S has had as many as 300 drug shortages annually
–
82% of hospitals have delayed patient treatment as a
result of a shortage
–
63% of hospitals were unable to provide recommended
treatment
•
The vast majority of these shortages are injectable products
•
Reasons contributing to shortages
–
2003 Medicare Act
–
Limited global capacity, with a dated domestic platform
–
Industry consolidation
–
Hoarding
–
API
•
Sagent’s Commitment to shortage reduction
–
Product selection
–
SCP objectives
–
Broad global network, providing supply alternatives:
Sodium Bicarbonate
Generic Market Growth
•
Generic market to increase by $100 Billion in 5 years
–
Global market to hit $231 billion by 2017
–
Largest opportunity in injectable drugs with annual
growth rate 10%
Not Price
•
Growth Statistics per Frost & Sullivan
GPOs
Hospitals
Wholesalers
Distributors
•
Industry consolidation creating vast opportunities
•
Huge potential in China, India, Russia, Brazil, Turkey and
S. Korea –
current footprint and business partners |
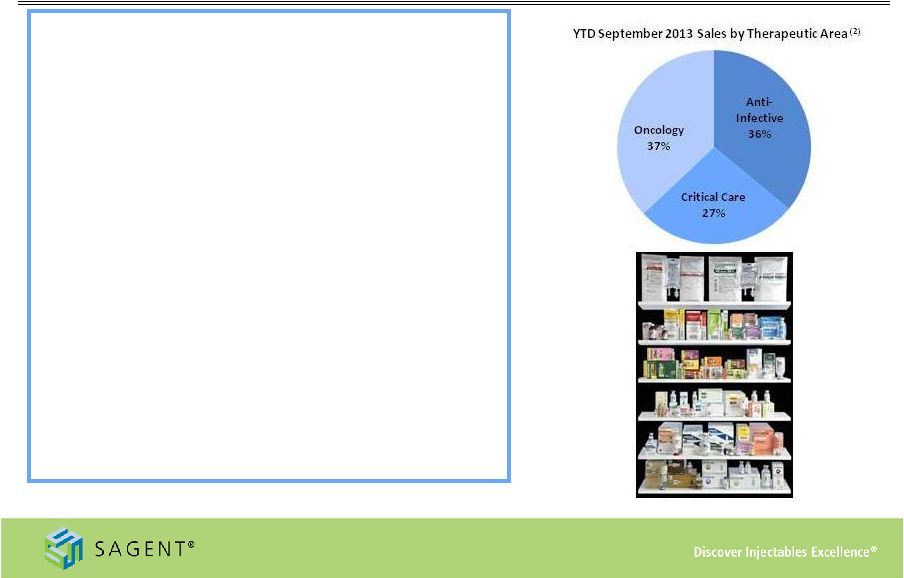 10
Growing Product Portfolio
•
Diverse marketed products -
53 products/144
SKUs
(1)
•
Key indication areas and products include:
–
Oncology:
Calcium Leucovorin, Docetaxel,
Fludarabine, Gemcitabine, Oxaliplatin, Topotecan,
Zoledronic Acid
–
Anti-Infective:
Azithromycin, Cefepime, Fluconazole,
Levofloxacin, Nafcillin, Oxacillin
–
Critical Care:
Adenosine, Heparin, Propofol,
Rocuronium
–
Filed an additional 12 products in 2013
(2) As of September 30, 2013
(1) As of December 31, 2013
More than 25% of our product portfolio holds the
#1 or #2 market position |
 11
Growing Product Portfolio
Approximately $6 billion of potential IMS market value in planned launches from 2013 through
2016 |
 12
Deep Development Pipeline
•
New product pipeline includes 39
products, represented by 69 ANDAs
(1)
•
Sagent currently has two approved
products represented by seven ANDAs
pending commercial launch
(1)
•
10 to 15 product launches expected in
FY’14
(1) As of December 31, 2013
Sagent’s ANDA Submission Rate
An additional 24 products represented by
41 ANDAs under initial development
(1) |
 13
Industry Leading Sales Organization
•
34 person sales and marketing team
–
Average tenure of sales
representatives is about 25 years in
same geographic location
–
Contracting professionals have an
average of approximately 30 years
experience
•
Customer relationships are a key strength
–
Management and key executives hold
long-standing relationships with key
GPO, distributor and wholesaler
decision-makers |
 14
Investing for the Future
Portfolio Breadth |
 Sagent
Pharmaceuticals
Recent
Developments
A Global Pharmaceutical
Company |
 16
Sagent China Pharmaceuticals (SCP)
Vertical Integration
•
Completed the acquisition of our partner’s 50% interest
in KSCP for $25 million, in June of 2013. Payable in
installments through September 2015
–
State-of-the-art isolation technology for aseptic filing
–
FDA and current Good Manufacturing Practices
compliant facility
–
Facility received EIR in the first half of 2013; first
product launched in November
–
Multiple products developed and filed from SCP in 2013
•
Provides for increased responsiveness to drug shortages:
SGNT controls its own products
•
Pursuing additional investment in product development
and capacity expansion
–
$12 -
$16 million annual operating costs
–
$30 -
$35 million capital expansion in final stages of
development, high speed isolator technology |
 Sagent China
Pharmaceuticals (SCP) Key Initiatives
•
Develop injectable formulations for
the US and domestic Chinese markets
•
Focus on developing and formulating
niche products and complex
molecules
•
Housed in a leading Science and
Technology Center in Chengdu, China
•
Positions SCP as a local thought leader
and long-term positioning to enter
domestic market
•
Expected to be 2
largest
pharmaceutical market by 2015
•
Market growth driven by increasing
demand in cities and counties and
growth of the middle class
•
Demand for high quality drugs
growing and price is aligning with
developed markets
•
Regulatory pathway evolving
Global Development Center
China Pharma Market
nd |
 18
Key Products Driving Growth
Recent launches
•
Propofol
–
Launched three single-dose, single-patient vial presentations in 2013 in 20mL,
50 mL and 100mL sizes
–
Market is approximately $230 million, based on IMS data, with limited
number of competitors
–
Product is highly valued by our customers and has experienced intermittent
shortages over the last few years
•
Docetaxel
–
Launched in August 2013
–
Market is approximately $330 million, based upon November 2013 IMS data, with
limited number of competitors
–
Received early FDA approval in anticipation of potential market shortages driven
by API supply issues
•
Zoledronic Acid
–
Launched vials in March 2013 at market formation, followed by
4mg and 5mg pre-mixed bags in second half 2013
–
Market is approximately $600 million, based upon November 2013 IMS data with
continued price erosion
–
Multiple presentation strategy demonstrates the strength of Sagent’s model
|
 19
Key Products Driving Growth
Future launches
•
Iron Sucrose
–
Generic form of Venofer®
–
High barrier to entry for generic manufacturing
–
Received
FDA
Complete
Response
Letter,
December
2013.
Currently
developing
comprehensive response, which should be filed first half 2014, 2014 approval unlikely.
–
Market is approximately $325 million based on November 2013 IMS data
•
Pentobarbital
–
Generic form of Nembutal®
–
High barrier to entry for generic manufacturing, Sagent will be first generic
–
Anticipate approval in first half of 2014
–
Market estimated to be approximately $30 million
•
Adenosine Injection
–
Generic form of Adenoscan®
–
Approved ANDA, launch pending first to file 6 month exclusivity to expire March 2014
–
Market is approximately $55 million based on November 2013 IMS data
–
Sagent sales organization has extensive historical experience with the product
|
 20
Strong & Experienced Management Team
Excel Rx, Premier, VA
Albert Patterson
EVP, Nat’l Accts & Corp Dev
Michael Logerfo
EVP Legal
APP, Fujisawa, LyphoMed
APP, Fujisawa
Landauer, Cardinal Health, KPMG
Nanolnk, Ovation Pharmaceuticals, NeoPharm, Physican Quality
Care, Bristol Myers Squibb
APP, LyphoMed, YorPharm, Gensia, Faulding
Jeffrey Yordon
CEO and Chairman
James Hussey
President
Jonathon Singer
EVP and CFO
Lorin Drake
Corp VP Sales
Ravi Malhotra
CSO
Flavine Holding Co, Attorney |
 Sagent
Pharmaceuticals
Financial Highlights
A Global Pharmaceutical
Company |
 22
Sequential Growth
Heparin
Topotecan
Levofloxacin
Gemcitabine
Cefepime
Azithromycin
Oxaliplatin
Oxacillin
Leucovorin
Key
Launches
•Indicates
mid-point
of
2013
revenue
guidance
range;
2013
Adjusted
Gross
Margin
is
the
midpoint
of
the
guidance
range,
both
as
reported
in
the
third
quarter
earnings
release,
dated
November
5,
2013
•Adjusted
Gross
Profit
is
a
non-GAAP
measure.
Please
refer
to
slide
#26:
Appendix
–
Non
GAAP
Reconciliation
for
further
detail.
Zoledronic Acid
Docetaxel |
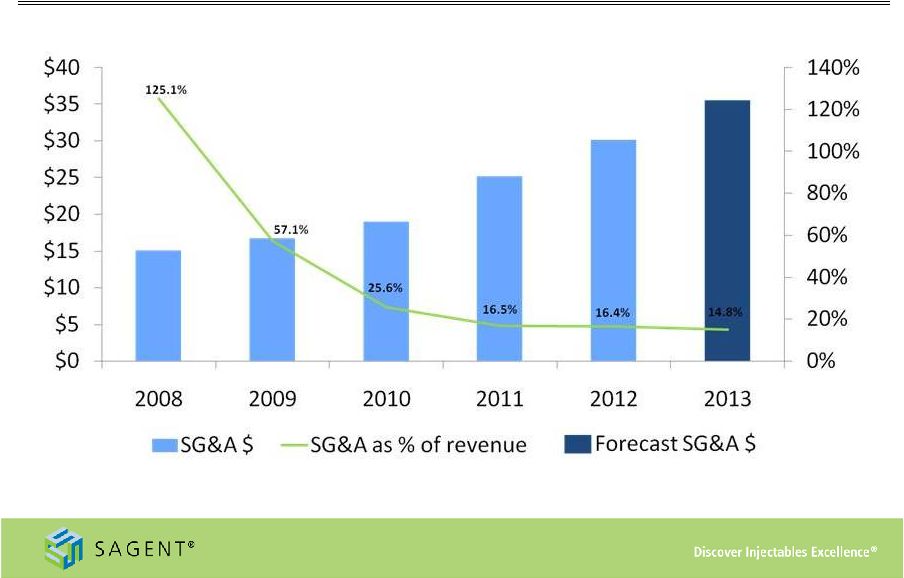 23
SG&A Spending
*
Midpoint
of
expected
2013
SG&A
Expense
and
Revenue
as
reported
in
the
third
quarter
earnings
release,
dated
November
5,
2013 |
 24
YTD 2013 Financial Performance
New product launches drive increases in revenue and record earnings
Key Developments in 2013
•
Successful secondary stock offering
•
Acquired remaining 50% interest in SCP
•
Launch of Zoledronic acid at market formation
•
James Hussey joined as President
•
Amended supply agreement with Actavis
•
Approval and launch of first product from SCP facility
Operating Performance
•
Net revenue of $180.6 million, 38% increase from first
nine months of 2012
•
Adjusted gross profit
(2)
of $62.2 million, or 34% of net revenue
–
Increase from $23.6 million, or 18% of net revenue in 2012
•
Net income of $26.0 million, compared to net loss of $16.8
million in first nine months of 2012
–
Diluted EPS of $0.90 per share in first nine months of 2013
(1)
As of September 30, 2013
(2)
Adjusted
Gross
Profit
is
a
non-GAAP
measure.
Please
refer
to
slide
#26:
Appendix
–
Non
GAAP
Reconciliation
for
further
detail |
 25
Investment Highlights
•
Fastest growing Generic Injectable company in U.S. Market , a consolidating industry
with high barriers to entry
•
Extensive global partner network with state-of-the-art facilities enabling
accelerated growth relative to conventional start-ups
•
Solid relationships at all levels of the supply chain enhance responsiveness to
industry demands
•
Vertical integration through acquisition of SCP complements partnering model, as
well as provides additional growth opportunities and a footprint
in China
•
Exceptional quality and safety track record; global excellence in manufacturing
•
Strong sequential margin and revenue growth driven by diverse and deep product
portfolio
•
Experienced management team with great track record, has delivered on product
approvals and financial performance |
 Appendix
– Non-GAAP Reconciliation
Nine months ended
Nine months ended
September 30,
September 30,
2008
2009
2010
2011
2012
2013
2008
2009
2010
2011
2012
2013
Adjusted Gross Profit
73
$
435
$
9,460
$
20,833
$
36,746
$
62,172
$
0.6%
1.5%
12.8%
13.7%
20.0%
34.4%
Sagent portion of gross
profit earned by Sagent
Agila joint venture
-
(2)
417
2,064
5,639
1,909
0.0%
0.0%
0.6%
1.4%
3.1%
1.1%
Gross Profit
73
$
437
$
9,043
$
18,769
$
31,107
$
60,263
$
0.6%
1.5%
12.2%
12.3%
16.9%
33.4%
Reconciliation of GAAP to non-GAAP Information (in thousands)
Sagent Pharmaceuticals, Inc.
Year ended December 31,
Year ended December 31,
Percentage of net revenues
We use the non-GAAP financial measure “Adjusted Gross Profit” and corresponding
ratios. We define Adjusted Gross Profit as gross profit plus our share of the gross
profit earned through our Sagent Agila joint venture which is included in the Equity in net (income) loss of joint ventures line on
the Consolidated Statements of Operations. We believe that Adjusted Gross Profit is
relevant and useful supplemental information for our investors. Our management believes
that the presentation of this non-GAAP financial measure, when considered together with our GAAP financial measures
and the reconciliation to the most directly comparable GAAP financial measure, provides a more
complete understanding of the factors and trends affecting Sagent than could be
obtained absent these disclosures. Management uses Adjusted Gross Profit and corresponding ratios to make
operating and strategic decisions and evaluate our performance. We have disclosed this
non-GAAP financial measure so that our investors have the same financial data that
management uses with the intention of assisting you in making comparisons to our historical operating results and analyzing
our underlying performance. Our management believes that Adjusted Gross Profit provides
a useful supplemental tool to consistently evaluate the profitability of our products
that have profit sharing arrangements. The limitation of this measure is that it includes an item that does not have an
impact on gross profit reported in accordance with GAAP. The best way that this
limitation can be addressed is by using Adjusted Gross Profit in combination with our
GAAP reported gross profit. Because Adjusted Gross Profit calculations may vary among other companies, the Adjusted Gross
Profit figures presented below may not be comparable to similarly titled measures used by
other companies. Our use of Adjusted Gross Profit is not meant to and should not be
considered in isolation or as a substitute for, or superior to, any GAAP financial measure. You should carefully evaluate
the following table reconciling Adjusted Gross Profit to our GAAP reported gross profit for
the period presented (dollars in thousands). Adjusted Gross
Profit 26 |
 |
