Attached files
| file | filename |
|---|---|
| 8-K - REXAHN PHARMACEUTICALS, INC. 8-K 1-14-2014 - Ocuphire Pharma, Inc. | form8k.htm |
Exhibit 99.1

January 2014
Revolutionizing the Treatment of Cancer

2
Safe Harbor Statement
The statements that follow (including projections and business trends) are forward-looking
statements. Rexahn's actual results may differ materially from anticipated results, and
expectations expressed in these forward-looking statements, as a result of certain risks and
uncertainties, including Rexahn's lack of profitability, the need for additional capital to
operate its business to develop its product candidates; the risk that Rexahn's development
efforts relating to its product candidates may not be successful; the possibility of being unable
to obtain regulatory approval of Rexahn's product candidates; the risk that the results of
clinical trials may not be completed on time or support Rexahn's claims; demand for and
market acceptance of Rexahn's drug candidates; Rexahn's reliance on third party researchers
and manufacturers to develop its product candidates; Rexahn's ability to develop and obtain
protection of its intellectual property; and other risk factors set forth from time to time in our
filings with the Securities and Exchange Commission. Rexahn assumes no obligation to update
these forward-looking statements.
statements. Rexahn's actual results may differ materially from anticipated results, and
expectations expressed in these forward-looking statements, as a result of certain risks and
uncertainties, including Rexahn's lack of profitability, the need for additional capital to
operate its business to develop its product candidates; the risk that Rexahn's development
efforts relating to its product candidates may not be successful; the possibility of being unable
to obtain regulatory approval of Rexahn's product candidates; the risk that the results of
clinical trials may not be completed on time or support Rexahn's claims; demand for and
market acceptance of Rexahn's drug candidates; Rexahn's reliance on third party researchers
and manufacturers to develop its product candidates; Rexahn's ability to develop and obtain
protection of its intellectual property; and other risk factors set forth from time to time in our
filings with the Securities and Exchange Commission. Rexahn assumes no obligation to update
these forward-looking statements.

3
Rexahn: Revolutionizing the Treatment of Cancer
Identify novel drug targets which are specific to cancer cells:
● Increased efficacy, reduced toxicity
● Efficacy against multiple drug resistant cancer cells
● Synergism with existing cytotoxic compounds
Develop in-licensed targeted drug delivery platforms:
● Nano-Polymer-Drug Conjugate System (NPDCS) combines existing
anticancer agents with a polymer/signaling moiety which directs the
drug directly to the tumor
anticancer agents with a polymer/signaling moiety which directs the
drug directly to the tumor
● Lipid-Coated Albumin Nanoparticle (LCAN) to enhance delivery of
oligonucleotides
oligonucleotides
5
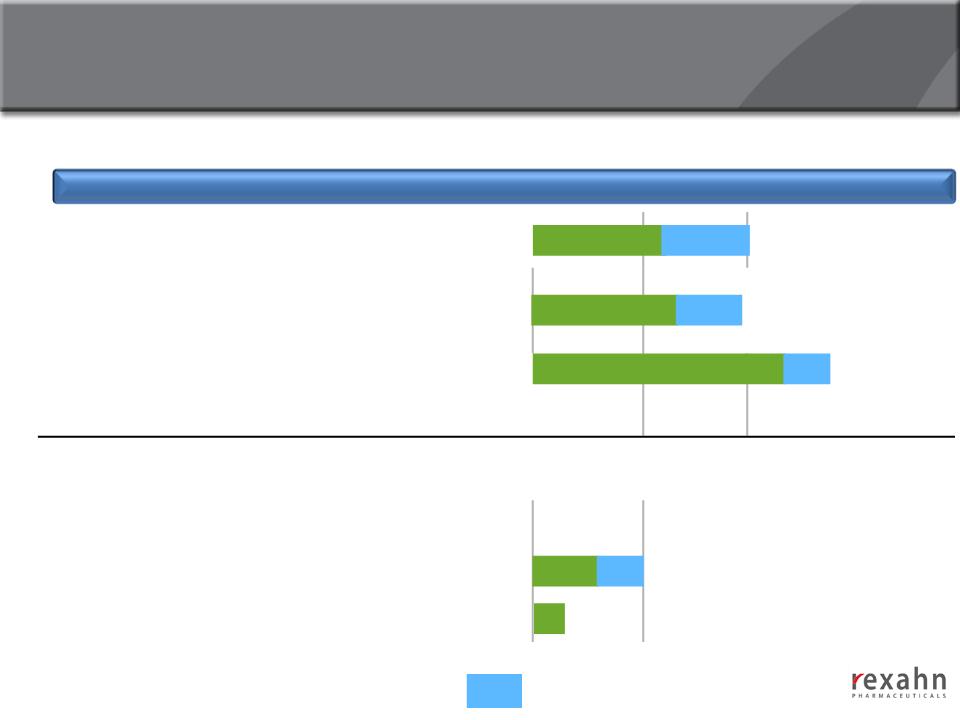
Deep Oncology Pipeline
Drug Candidate
Mechanism of Action
Preclinical
Phase I
Phase II
Phase III
|
SupinoxinTM (RX-5902)
|
p68 RNA Helicase
Inhibitor |
|
|
||
|
RX-3117
|
Cancer Cell Specific
nucleoside analog
|
|
|
||
|
Archexin®
|
Akt1 Inhibitor
|
|
|||
|
|
|
|
|
|
|
|
RX-21101
RX-0201-nano
|
Docetaxel Conjugate
Akt1 inhibitor
|
|
|
|
Targeted Drug Delivery Platform
2014
2014
2014
2014
- Anticipated progress during 2014

SupinoxinTM(RX-5902)

7
Supinoxin: Best-in-Class p68 Helicase Inhibitor
n Inhibition of phosphorylated p68 RNA helicase
n Blocks upregulation of cancer related genes
n Solid tumors: pancreas, NSCLC, colon, renal and other solid
tumors
tumors
n Anti-proliferative effects
n Synergistic with cytotoxic agents
n Efficacy against drug resistant cancer cells
n Orally bioavailable
n New chemical entity with a strong patent position
n Phase I clinical trial in cancer patients initiated August 2013
n Initial data expected in Q1 2014
Mechanism
Current and Future
Indications
Indications
Advantages
Patent
Clinical Development
8
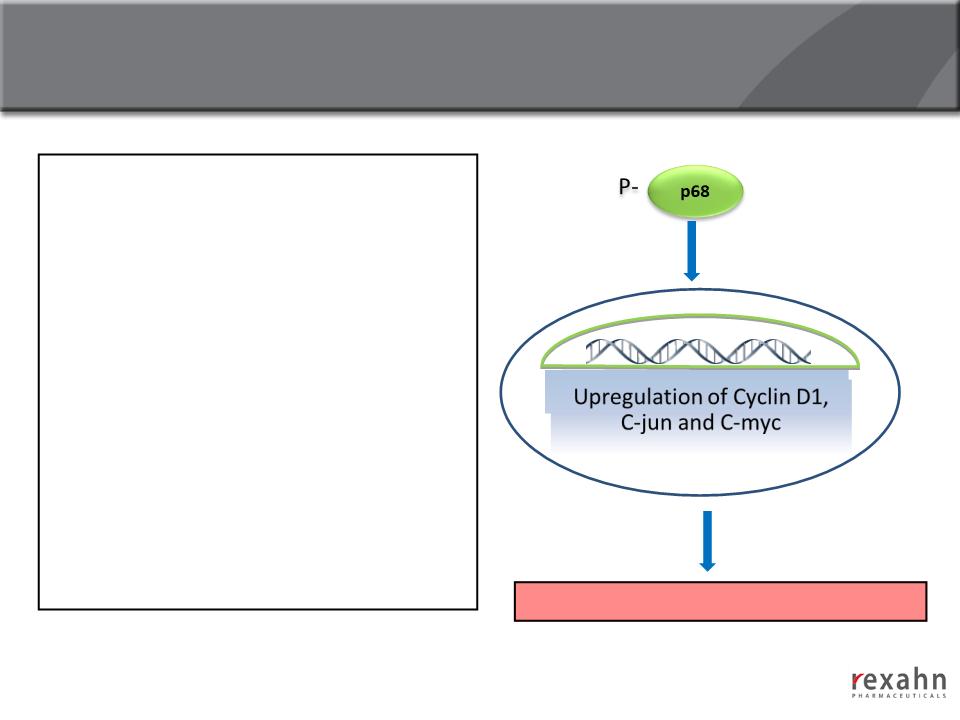
Supinoxin: Mechanism of action
n Phosphorylated p68 is highly
expressed in cancer cells but not in
normal cells, and upregulates
cancer-related genes
expressed in cancer cells but not in
normal cells, and upregulates
cancer-related genes
n Supinoxin selectively inhibits
phosphorylated p68 RNA Helicase
phosphorylated p68 RNA Helicase
● Decreased proliferation/growth
of cancer cells
of cancer cells
● Synergism with cytotoxic agents
● Activity against drug resistant
cancer cells
cancer cells
Cancer cell Proliferation/Tumor growth

10
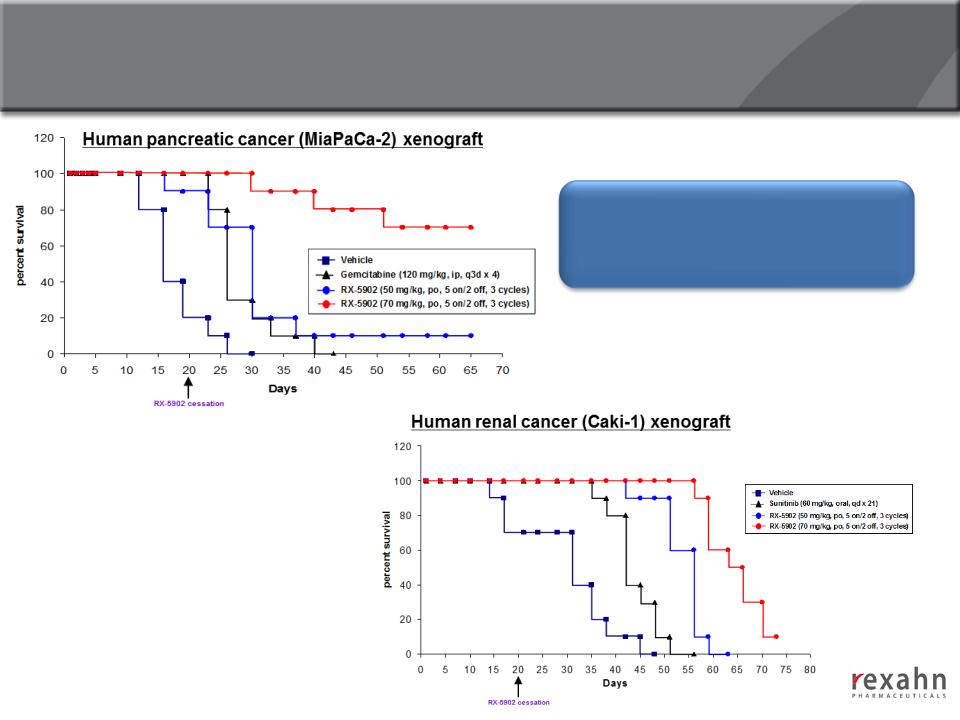
Supinoxin: Increased Survival in Human Renal Cell
Carcinoma and Pancreatic Cancer Xenograft Models
Carcinoma and Pancreatic Cancer Xenograft Models
Treatment with Supinoxin on days 1
to 20 produced a survival benefit
beyond 65 days
to 20 produced a survival benefit
beyond 65 days

RX-3117

12
RX-3117: Novel DNA synthesis inhibitor
n Cancer cell specific nucleoside compound that inhibits DNA
synthesis
synthesis
n Activated by UCK1 & UCK2
n Solid tumors: pancreas, NSCLC, colon, renal and other solid
tumors
tumors
n Effective against gemcitabine-resistant human cancer cell
lines
lines
n Orally administered
n Specifically targeted against cancer cells; reduced adverse
events
events
n New chemical entity with a strong patent position
n Completed exploratory Phase I clinical trial in cancer patients
• Confirmed oral bioavailability and safety
n Phase Ib clinical trial in cancer patients initiated Dec 2013
Mechanism of Action
Current and Future
Indications
Indications
Advantages
Patent
Clinical Development
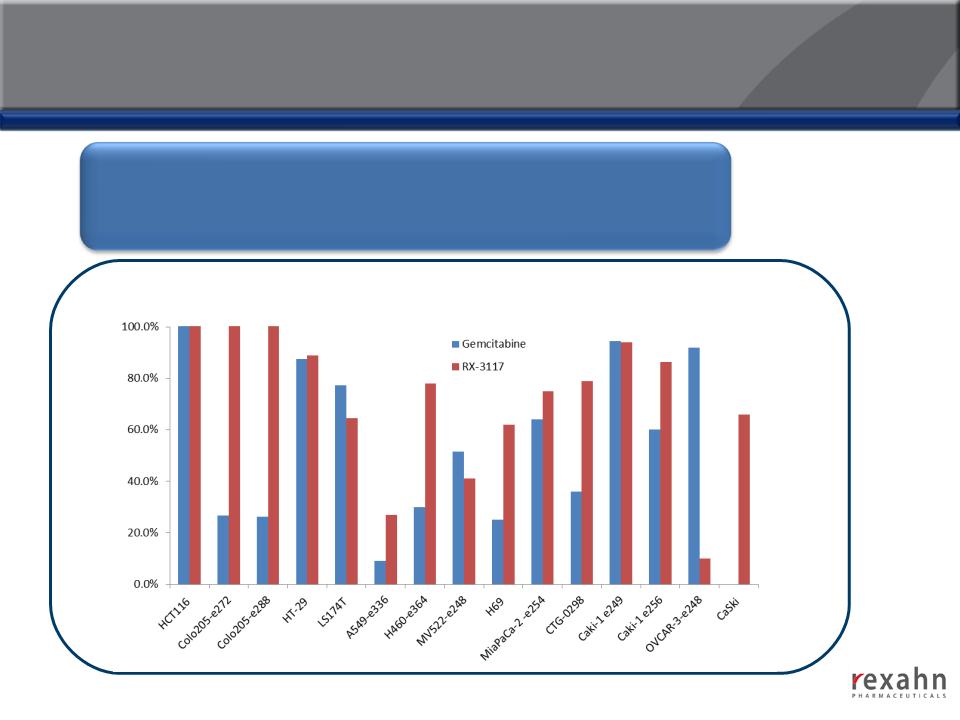
RX-3117: Compelling Efficacy in Animal Models
13
RX-3117 has shown robust anti-tumor effects across a broad variety
of tumor types in animal models (Colon, Non-Small Cell Lung, Small
Cell Lung, Pancreatic, Renal, Ovarian and Cervical)
of tumor types in animal models (Colon, Non-Small Cell Lung, Small
Cell Lung, Pancreatic, Renal, Ovarian and Cervical)
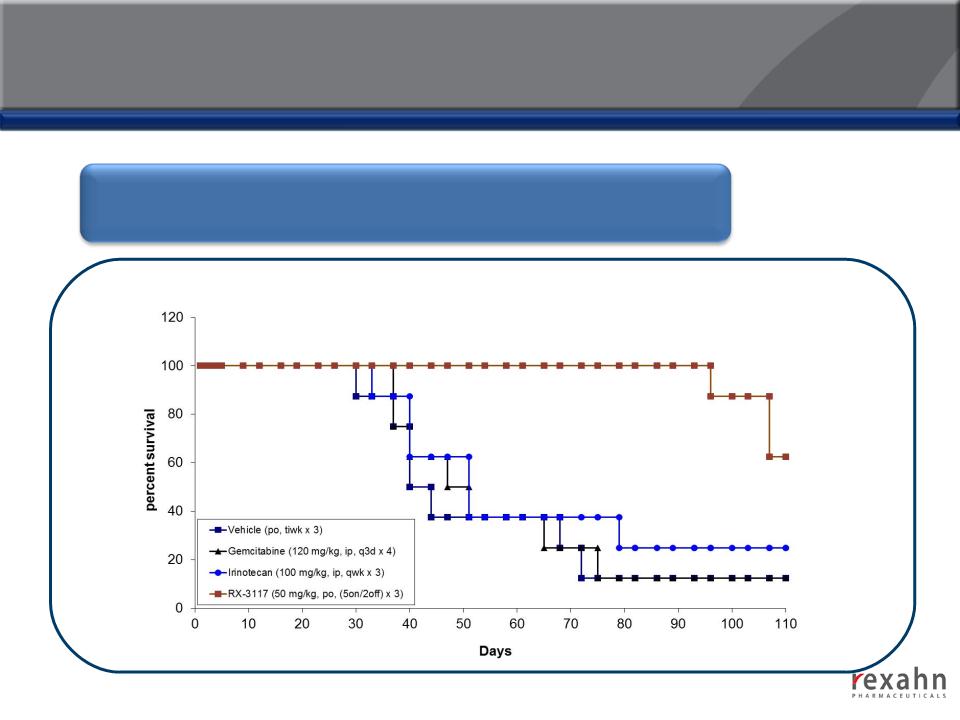
RX-3117: Compelling Efficacy in Animal Models
14
RX-3117 offers significant benefits based on overall-survival via oral
administration in nude mice
administration in nude mice
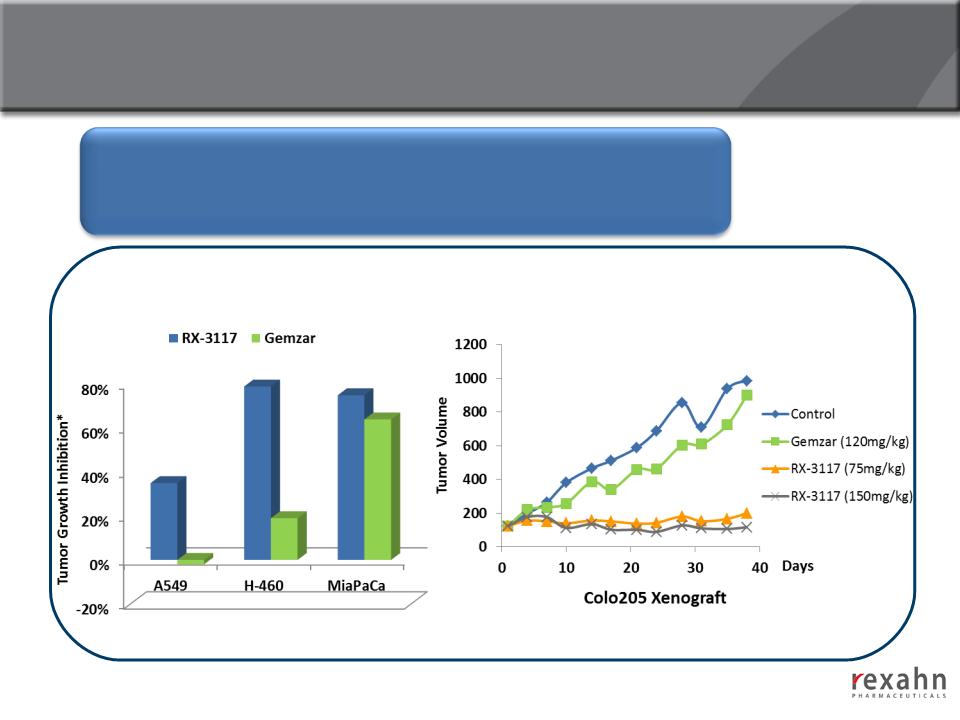
15
RX-3117: Efficacy in Gemcitabine-Resistant Cell Lines
The efficacy of RX-3117 was examined in 12 different human tumor
(Colon, Non-Small Cell Lung, Small Cell Lung, Pancreatic, Renal,
Ovarian and Cervical)
(Colon, Non-Small Cell Lung, Small Cell Lung, Pancreatic, Renal,
Ovarian and Cervical)

16
RX-3117: Exploratory Phase I clinical Trial (Completed)
n Exploratory Phase I clinical trial in cancer patients was conducted in Europe in 2012
n Objectives:
● Evaluate oral bioavailability and pharmacokinetics
● Assess safety and tolerability
n Drug administration cohorts:
● 20 mg IV (n=3)
● 50 mg oral (n=3)
● 100 mg oral (n=3)
n Results:
● Nine subjects, ages 47 to 67 years, were enrolled
● RX-3117 was orally bioavailable with Tmax of 2-3 hours, T1/2 of 14-21 hours, and
oral bioavailability of 33 to 56%
oral bioavailability of 33 to 56%
● RX-3117 was well tolerated with no post-dose adverse events, laboratory
abnormalities, or ECG changes emerging through 7 days of follow-up
abnormalities, or ECG changes emerging through 7 days of follow-up

17
RX-3117: Phase Ib Study Design (ongoing)
n Initiated December 2013
n Cancer patients with solid tumors
n Up to 30 patients and three clinical sites
n Treatment cycle is 28 days
● Dosing 3 times a week for 3 weeks followed by 1 week off
n Dose Finding Study Design
● Escalation decisions based on safety, dosing, PK, laboratory, etc
● Patients may receive up to 8 cycles of treatment
● Anti-tumor activity secondary endpoint
● Patients will be scanned (CT or MRI) prior to initiating treatment and after every 2
cycles
cycles

Archexin®
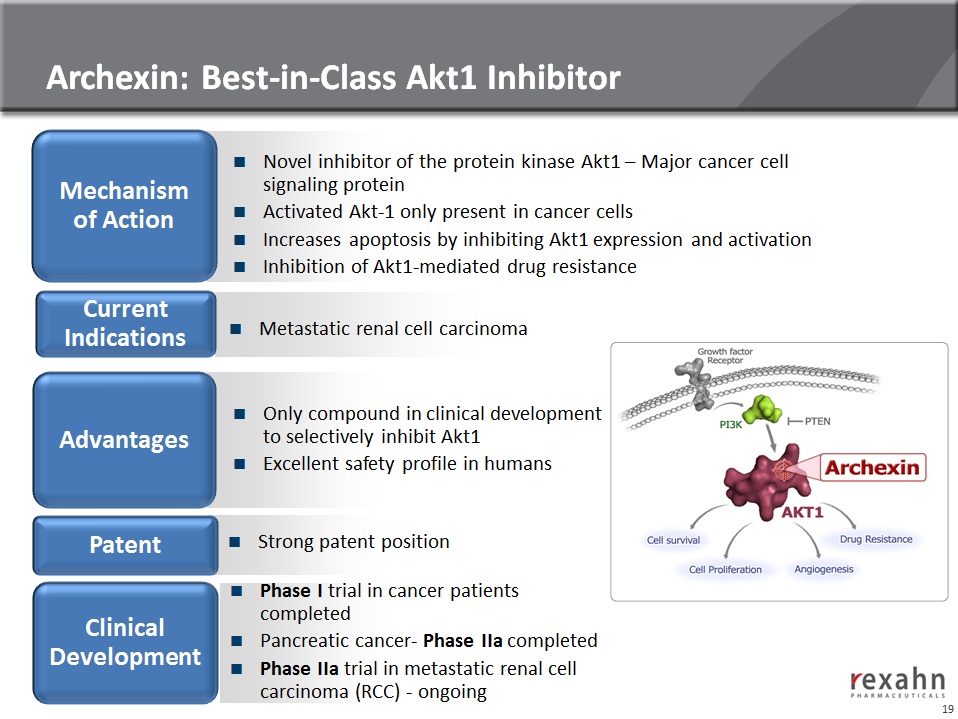
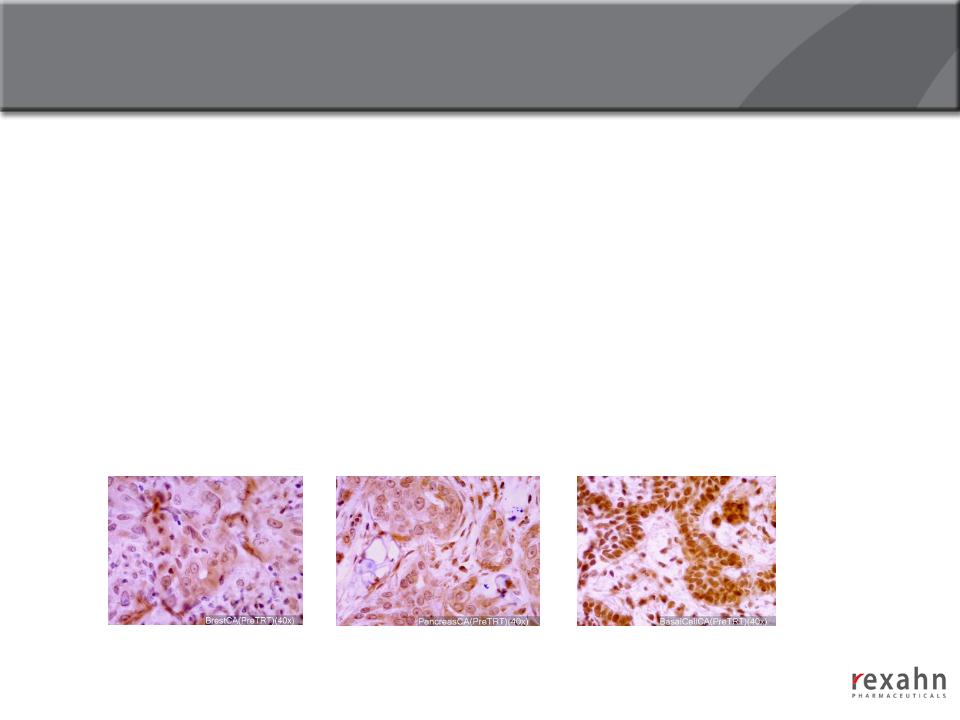
20
Archexin: Phase I Clinical trial (completed)
n Phase I objective
● To determine maximum tolerated dose, safety and pharmacokinetic profiles
n Phase I results:
● MTD was 250 mg/m2/d in Patients with an advanced cancer after up to two cycles
of treatment
of treatment
● The dose limiting toxicity was Grade 3 fatigue; no significant hematological
abnormalities
abnormalities
n Phospho-Akt1 being developed as a clinical biomarker
J Clin Onc, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement), 2007: 3564

21
Archexin: Phase IIa Study in Metastatic
Pancreatic Cancer (completed)
Pancreatic Cancer (completed)
§ Open label 2-stage study to assess the safety and efficacy of Archexin in
combination with gemcitabine
combination with gemcitabine
§ 31 subjects enrolled (10 for safety, 21 for efficacy) with ages ranging 18-65 years
with metastatic pancreatic cancer
with metastatic pancreatic cancer
§ Archexin in combination with gemcitabine provided a median survival of 9.1
months compared to the historical survival data of 5.65 months (Burris et al., 1997,
J. Clin Oncol 15:2403) for standard single agent gemcitabine therapy
months compared to the historical survival data of 5.65 months (Burris et al., 1997,
J. Clin Oncol 15:2403) for standard single agent gemcitabine therapy

Corporate Overview
Milestones | Highlights
Milestones | Highlights

24
Major Milestones for 2014
n Initial data from Supinoxin Phase I Clinical trial (1Q14)
n Complete RX-3117 corporate partnership (mid year)
n Complete Supinoxin Phase I clinical trial (4Q14)
n Complete safety component of Archexin Phase IIa clinical trial (4Q14)
n Complete patient enrollment in RX-3117 Phase I clinical trial (4Q14/1Q15)

25
Financial Highlights
|
Rexahn Financial Highlights
|
|
|
Ticker
|
RNN
|
|
Exchange
|
NYSE MKT
|
|
Market Price (1/10/14)
|
$1.14
|
|
Market Capitalization (1/10/14)
|
$167 MM
|
|
Shares Outstanding (12/31/13)
|
147 MM
|
|
Insider Ownership
|
10%
|
|
Cash Balance (12/31/13)
|
$19 MM
|
|
Monthly Est. Cash Burn
|
$0.9 MM
|

26
Rexahn Investment Highlights
Pipeline
§Supinoxin (RX-5902): Phosphorylated p68 RNA Helicase inhibitor. Phase I clinical trial in
cancer patients with solid tumors; initiated August 2013
cancer patients with solid tumors; initiated August 2013
§RX-3117: Next generation cancer cell specific nucleoside analog. Completed European
exploratory Phase I trial in cancer patients with solid tumors. IND filed and Phase Ib clinical
trial in cancer patients initiated in December 2013
exploratory Phase I trial in cancer patients with solid tumors. IND filed and Phase Ib clinical
trial in cancer patients initiated in December 2013
§Archexin: Akt1 inhibitor completed an exploratory Phase IIa clinical trial in pancreatic
cancer. A Phase IIa clinical trial in cancer patients with metastatic renal cell carcinoma
initiated in December 2013
cancer. A Phase IIa clinical trial in cancer patients with metastatic renal cell carcinoma
initiated in December 2013
§Nano-Polymer-Drug Conjugate System (NPDCS)
§ RX-21101: polymer conjugated form of docetaxel containing a signaling moiety
which directs the drug into the tumor maximizing efficacy and minimizing toxicity
which directs the drug into the tumor maximizing efficacy and minimizing toxicity
§Lipid-Coated Albumin Nanoparticle (LCAN)
§ RX-0201-nano: nanoliposomal Akt1 inhibitor, similar to Archexin®
Rapidly advancing pipeline: Initiated three clinical trials in 2013 with data in 2014
Strong Intellectual Property position

Revolutionizing the Treatment of Cancer
REXAHN PHARMACEUTICALS, INC.
15245 Shady Grove Road, Suite 455
Rockville, MD 20850
Tel. 240-268-5300 | Fax. 240-268-5310
www.rexahn.com
15245 Shady Grove Road, Suite 455
Rockville, MD 20850
Tel. 240-268-5300 | Fax. 240-268-5310
www.rexahn.com
