Attached files
| file | filename |
|---|---|
| EX-31.1 - EXHIBIT 31.1 - AMERICAN CRYOSTEM Corp | ex31_1.htm |
| EX-32.1 - EXHIBIT 32.1 - AMERICAN CRYOSTEM Corp | ex32_1.htm |
| EX-31.2 - EXHIBIT 31.2 - AMERICAN CRYOSTEM Corp | ex31_2.htm |
| EXCEL - IDEA: XBRL DOCUMENT - AMERICAN CRYOSTEM Corp | Financial_Report.xls |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the fiscal year ended: September 30, 2013 | |
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| Commission file number: 000-54672 | |
American CryoStem Corporation
(Exact name of registrant as specified in its charter)
| Nevada | 26-4574088 | |
| (State or other jurisdiction
of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
1 Meridian Road, Suite 5 Eatontown, NJ 07724 |
||
| (Address of principal executive offices) | ||
| (732) 747-1007 | ||
| (Registrant’s telephone number, including area code) | ||
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, par value $0.001
Indicate by checkmark if registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by checkmark if registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by checkmark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a small reporting company. See definition of “large accelerated filer”, “accelerated filer”, and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer | o | Accelerated Filer | o |
| Non-Accelerated Filer | o | Smaller reporting company | x |
Indicate by checkmark whether the registrant is a shell company (as defined in Rule 12b-2 of the Securities Act). Yes o No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant based on a closing price of $0.33 on March 29, 2013 (the last business day of the registrants most recently completed second fiscal quarter) was approximately $2,065,919.
As of January 7, 2013, the registrant had 32,574,221 shares of its common stock, par value $0.001, outstanding.
Documents incorporated by reference: none.
TABLE OF CONTENTS
| 2 |
FORWARD LOOKING STATEMENTS
Included in this Form 10-K are “forward-looking” statements, as well as historical information. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that the expectations reflected in these forward-looking statements will prove to be correct. Our actual results could differ materially from those anticipated in forward-looking statements as a result of certain factors, including matters described in the section titled “Risk Factors.” Forward-looking statements include those that use forward-looking terminology, such as the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “project,” “plan,” “will,” “shall,” “should,” and similar expressions, including when used in the negative. Although we believe that the expectations reflected in these forward-looking statements are reasonable and achievable, these statements involve risks and uncertainties and we cannot assure you that actual results will be consistent with these forward-looking statements. We undertake no obligation to update or revise these forward-looking statements, whether to reflect events or circumstances after the date initially filed or published, to reflect the occurrence of unanticipated events or otherwise.
| Item 1. | Business |
Company Overview
History
American CryoStem Corporation, which we refer to as “American CryoStem,” “we,” “us,” “our” and “our Company” was incorporated in the state of Nevada on March 13, 2009. On April 20, 2011, we acquired, through our wholly owned subsidiary American CryoStem Acquisition Corporation, substantially all of the assets from, and assumed substantially all of the liabilities of, ACS Global, Inc. (“ACS”) in exchange for our issuance of 21,000,000 shares of our common stock, par value $0.001 per share, to ACS (the “Asset Purchase”). We filed a Current Report on Form 8-K with the Securities and Exchange Commission (SEC) on April 27, 2011 disclosing the Asset Purchase and certain related matters including, but not limited to, the appointment of our present officers and directors as well as the resignation by the former chief executive officer and sole director. Our fiscal year ends September 30 of each calendar year.
Upon the closing of the Asset Purchase: (i) ACS Global became our majority shareholder, (ii) John Arnone was appointed as our chief executive officer and president and Anthony Dudzinski was appointed as our chief operating officer, treasurer and secretary, and (iii) John Arnone and Anthony Dudzinski were appointed to our board of directors, with Mr. Arnone being appointed as Chairman of the Board. Mr. Dudzinski is also a director and the president and treasurer of ACS Global and Mr. Arnone is a director and secretary of ACS Global. Contemporaneously with the Asset Purchase Closing, we sold 1,860,000 shares of Common Stock to accredited investors in a private placement at a purchase price of $0.50 per share for aggregate gross proceeds of $930,000.
Our Business
American CryoStem is a developer, marketer and global licensor of patented adipose tissue-based cellular technologies, bio-materials and related proprietary services with a focus on clinical processing, commercial bio-banking and application development for adipose (fat) tissue and adipose-derived stem cells (ADSCs). We maintain a strategic portfolio of intellectual property and patent applications that form our Adipose Tissue Processing Platform, which supports and promotes a growing pipeline of biologic products and processes, clinical services and international licensing opportunities. Through our ACS Laboratories division, we operate an FDA registered, cGMP compliant human tissue processing, cryo-storage cell culture and differentiation media development facility in Mount Laurel, New Jersey at the Burlington County College Science Incubator.
Our growth strategy is centered on expanding our research and development through scientific collaborations and developing revenue through the sale and licensing of our patented products and services to fully capitalize on: (1) adipose tissue and adipose derived stem cell (ADSC) technologies (2) scientific breakthroughs incorporating ADSCs that have been rapidly shaping the fast growing Regenerative and Personalized Medicine industries; (3) providing these growth industries with a standardized cell processing platform and, (4) enhancing the delivery of healthcare through cellular-based therapies and applications which address disease treatment, wound and burn healing, joint repair and management, and personalized health and beauty care, and (5) building a network of physicians for the delivery of our products and services.
| 3 |
We market a proprietary, patented clinical processing methodology for the collection, preparation and cryopreservation of adipose tissue in its raw form without manipulation, bio-generation or the addition of animal-derived products or other chemical materials requiring removal upon retrieval. We believe that this core process makes each sample suitable for use in cosmetic tissue grafting procedures or for further processing to adult stem cells for other types of cellular therapies. Currently, there are over 100 therapeutic and orthopedic applications for adipose tissue and adult stem cell treatments identified or in use globally, and more discoveries are being made each day.
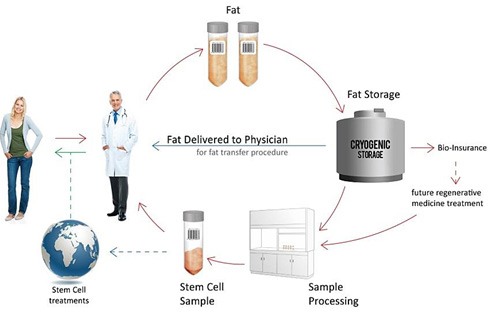
Products and Services
American CryoStem is focused on developing multiple high margin business lines capable of generating sustainable, recurring revenue streams from each of our developed products and services. Our products and services are the result of more than four years of scientific development and investment of approximately $5 million dollars. The Company also incorporates all of its proprietary and patented or patent pending laboratory products, such as our ACSelerate™ cell culture media, into all of our processing product production and contract manufacturing services.
To date, we have generated minimal revenue; however, subject to, among other factors, obtaining the requisite financing, management believes that we are well positioned to leverage our developed products and services as the basis for a host of Regenerative Medicine uses and future applications.
The following products and services are designed to become the basis of, or an integral part of, numerous planned revenue generating and cellular therapy development activities: Our products and services are:
| ● | CELLECT® | ● | Tissue Collection methodology designed for physicians to facilitate the collection and overnight shipping of an individual’s adipose tissue to our FDA registered laboratory; |
| ● | ATGRAFT™ | ● | Tissue processing at our Laboratory of a customer’s adipose tissue and its preparation for long term storage in different configuration sizes allowing future retrieval for tissue grafting procedures or Regenerative Medicine applications |
| 4 |
| ● | ATCELL™ | ● | Clinical Processing to separate the component parts (cells) of an individual’s adipose tissue removing the adipocytes and red blood cells thereby creating the ATCELL™ stem cell lines for storage, expansion, or differentiation |
| ● | Clinical and Research grade donor ATCELL™ lines for use with collaborative partners in cellular therapy research and application development and optimization, cell morphology and characterization assays, and growth analysis. | ||
| ● | ACS Laboratories™ | ● | Manufacturing and sale of our patented ACSelerate-SFM™ and ACSelerate-LSM™ cell culture media products |
| ● | Creation and sale of research grade ATCELL™ | ||
| ● | Participation and support of all collaborative research projects | ||
| ● | Contract manufacturing, including Autokine-CM® | ||
|
● |
Provide testing services for physicians performing in-office procedures and tissue processing | |
| ● | International Licensing™ | ● | Standard Operating Procedures (SOPs) and all associated components and products |
| ● | Consulting and Marketing Review and Assessment | ||
| ● | CELLECT® | ||
| ● | ATGRAFT™ | ||
| ● | ATCELL™ | ||
| ● | Adipose tissue processing, cellular expansion and product manufacture |
CELLECT® Validated Collection, Transportation, and Storage System – An unbreakable “chain of custody” clinical solution for physicians to collect and deliver adipose tissue samples utilizing proprietary and patent pending methods and materials. The CELLECT® service is monitored in real-time and assures the highest tissue and cell viability upon laboratory receipt.
We believe American CryoStem is the first tissue bank to globally incorporate through its CELLECT® service the International Blood Banking identification and labeling and product identification coding system. The coding was developed in conjunction with the American Association of Blood Banks (AABB), the American Red Cross and the International Society of Blood Transfusion (ISBT). These groups formed the International Council for Commonality in Blood Banking Automation (ICCBBA) and developed the ISBT 128 Standard for machine readable labeling. This labeling system is an acceptable machine readable labeling standard, product description, and bar coding system for FDA Center for Biologics Evaluation and Research under 21 CFR 606.12(c) 13. American CryoStem conforms to this standard in its Mount Laurel facility and all cellular and tissue products produced at the facility carry our W3750 ICCBBA facility identifier allowing any physician, hospital, clinic, laboratory and regulator worldwide to identify the origin and obtain additional information of any sample produced at an American CryoStem facility. The Company will promote this standard in all laboratories that license or utilize our technology. The CELLECT® service is included in our pending patent application PCT/US2011/39260.
ATGRAFT™ Adipose Tissue Storage Service – A clinical fat storage solution allowing physicians to provide their patients with multiple tissue/stem cell storage options. The ATGRAFT™ Service, through one liposuction procedure allows individuals the benefit of multiple cosmetic or regenerative procedures by using their own stored adipose tissue as a natural biocompatible filler or cellular therapy application without the trauma of further liposuctions. Potential ATGRAFT™ uses and procedures include breast reconstruction, layered augmentation, buttocks enhancement or volume corrections of the hands, feet, face and neck areas that experience significant adipose tissue (fat) volume reduction as we age. ATGRAFT™ is processed and stored utilizing our cGMP standards so that any stored fat tissue sample may be retrieved in the future and re-processed to create ATCELL™ our clinical grade stem cell product for use in Regenerative Medicine applications. The AGRAFT™ products and services are incorporated into our pending patent application PCT/US13/44621.
| 5 |
The standard fees for ATGRAFT™ tissue processing and initial storage ranges from $750 to $2,500, depending on the volume of tissue processed. The annual storage fee is $200 for up to 100ml of tissue. Storage of tissue over 100ml is billed an additional $1 per 1ml annually. These fees may be paid by the collecting/treating physician or the consumer. The Company earns additional fees ranging from $100 to $500 plus shipping costs, paid by the physician upon retrieval, for the thawing, packaging and shipment of the stored samples to the physician for immediate use upon receipt.
ATGRAFT™ Storage Retrieval fees are determined by the storage configuration as follows:
| ● | Small Sample package – for storages of 100ml of adipose tissue or less. Storages sizes are 4ml vials and 25ml cryo storage bags or a single 100ml cryo-storage bag. The small storage package is ideally suited for the physician to market additional procedures to the client for hands, feet, face and neck and for the correction of small surgical defects. | |
| ● | Medium Sample package – for storage of 100ml to 300ml of adipose tissue. Storage sizes are 25ml and 100ml cryo storage bags. The medium storage package is ideally suited for the physician to use in follow up corrections to same day tissue transfers and minor surgical defect corrections as well as larger procedures to the hands, feet, face and neck and breasts. | |
| ● | Large Storage package – for storage of over 300ml of adipose tissue. Storage sizes are available in 25ml and 100ml cryo storage bags. The large storage package is ideally suited for secondary large volume procedures such as layered breast augmentation and buttock lifts and corrections following large surgical procedures. | |
| ● | Custom Package – storage configuration for pre planned procedures. The company adjusts the fees based upon the final storage configuration. This package permits a physician to pre-plan reconstructive procedures prior to any initial planned surgical procedure such as post mastectomy or lumpectomy reconstructions and corrections. |
The ATGRAFT™ service creates patient retention and a significant revenue opportunity for the participating physician to promote additional procedures and generate additional fees from waste material collected during liposuction procedures. These additional fees can be generated with significantly lower physician costs by eliminating the overhead associated with performing a liposuction for each procedure. Physician cost savings may include: materials, supplies, equipment, and the expenses of utilizing a surgical center, hospital operating room or an in-office aseptic procedure room. The ATGRAFT™ service is designed to operate under the minimally manipulated regulations contained in both 21 CFR 1271.10 and PHS 361.
ATCELL™ Adipose Derived Stem Cells (ADSCs) – Multiple lines of clinically processed and characterized ADSCs created using the Company’s propitiatory SOPs and patented cell culture media. ATCELL™ is the Company’s trademarked name for its ADSC and differentiated cell products. The Company has created multiple master and differentiated lines and labels them according to their characterization. (i.e. ATCELL-SVF™ (stromal vascular fraction), ATCELL–CH™ (differentiated chondrocytes) etc.) The ATCELL™ lines are custom created for patients desiring to store their cells for future or current use. Donated research samples are prepared for sale to researchers for use in application development collaborations. The Company charges fees ranging from $750 to process a previously stored ATGRAFT™ sample and a minimum of $1,500 for newly collected tissue samples seeking further component processing. All customer and donated research samples submitted for processing must utilize the CELLECT® collection system to conform to our internal cGMP Standard Operating Procedures (SOPs).
The Company earns additional fees based upon the storage configuration of the final ATCELL™ samples, and for additional culturing in the ACSelerate™ cell culture and differentiation media. Cell culturing and differentiation can be performed upon receipt of the sample or at any time following the initial processing and cryopreservation of the ATGRAFT™, SVF or ATCELL™ samples. ATCELL™ is ideally suited for expansion and differentiation into additional cell types utilizing the ACSelerate-SFM™ (serum free media) or ACSelerate-LSM™ (low serum media) differentiation media. The ATCELL products and services are incorporated into our pending patent filing US Serial No. 13/646,647.
ACSelerate™ Cell Culture Media Products – Patented cell culture media products for growing human stromal cells (including all cells found in human skin, fat and other connective tissue). ACSelerate™ cell culture media is available animal free (serum free), which is suitable for human clinical and therapeutic uses; and a low serum version for application development and research purposes.
| 6 |
On August 2, 2011, the Company was issued United States patent number 7,989,205 for “Cell Culture Media, Kits and Methods of Use.” The granted claims include media variations for cellular differentiation of ADSCs into osteoblasts (bone), chondrocytes (cartilage), adipocytes (fat), neural cells, and smooth muscles cells in both HSA (clinical) grade and FBS (research) grade. This patent covers both non-GMP research grades and GMP clinical grades suitable for cell culture of adipose-derived stem cells intended for use in humans.
The most widely used cell culture medium today for growing and differentiating stem cell cultures for in vitro diagnostics and research contains 10% or more fetal bovine serum (FBS). The use of FBS and other animal products in clinical cellular therapy application development and manufacture raises concerns and generates debates within the scientific and regulatory community relating to potential human/animal cross-contamination. These same concerns may also need to be addressed through additional expensive and expansive testing and documentation with the FDA during the application and approval process for new cellular therapies. FDA concerns are evidenced in their Guidance’s and Guidelines regarding cellular therapy involving human cells, tissues and products (HCT/Ps) published and maintained by the FDA such as: Guidance for Industry: Source Animal, Product, Preclinical and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans, FDA Final Guidance, April 2003. It is our belief that eliminating or greatly reducing FBS in cellular manufacturing, applications and products can eliminate or ease these scientific and regulatory concerns and may prove to be a winning strategy for cellular therapy application developers seeking FDA approval. Similar concerns exist worldwide in markets that are governed by agencies similar to the FDA, such as the EMA in the European Union and the controlling regulatory bodies in Japan, Korea and China, among others.
The patented ACSelerate™ cell culture media line was specifically developed to address increasing industry demand for animal serum-free cell culture products and for the acceleration of products from the laboratory to the patient.
Currently, our media products are being utilized by our research partners engaged in developing novel new cellular applications and treatments. The Company supports these efforts by also making non-client donated ATCELL™ samples available for research purposes and for internal product development through our research programs. These cell lines are highly sought after by private researchers and universities for use in pre-clinical trial studies and in-vitro research. The ability of the Company to provide clinical grade materials for these research and development collaborators, partners and other third parties further extends the Company’s ability to become a primary source of clinical grade materials and services necessary to support approved applications and treatments.
The Company manufactures several versions of its ACSelerate™ cell culture media including:
| ● | ACSelerate-SFM™ - our flagship, clinical grade, cGMP manufactured animal product and serum free cell culture media, which is ideally suited for the rapid expansion of adipose-derived cell samples for direct use or further culturing into other cell types; | |
| ● | ACSelerate-CY™ - for differentiation of ATCELL™ into chondrocytes (ATCELL-CY™), which are suitable for use in cartilage repair applications in knees and other joints for patients suffering from joint injury, osteoarthritis and other diseases that cause degeneration of joint cartilage; | |
| ● | ACSelerate-OB™ - for differentiation of ATCELL™ into osteoblasts (ATCELL-OB™) for the repair of bone injuries resulting from traumatic injury and musculoskeletal diseases; | |
| ● | ACSelerate-AD™ - for differentiation of ATCELL™ into adipocytes (ATCELL-AD™) for the repair of adipose tissue defects resulting from injury or surgical procedures and is designed for those patients without an appropriate amount of body fat for corrective tissue transfer procedures; | |
| ● | ACSelerate-MY™ - for differentiation of ATCELL ™ into myocytes (ATCELL-MY™) for the repair of muscle defects and loss as the result of traumatic injury, surgery or systemic disease; | |
| ● | ACSelerate-GY™ - a clinical grade, non-DMSO (Dimethyl Sulfoxide) cellular cryopreservation media designed to conform to certain FDA and PHS 361 exemptions available for marketing our ATGRAFT™ service. |
The Company is optimizing additional versions of the ACSelerate™ media product line to address differentiation of ATCELL™, ADSCs into neural, lung and other specific cell types that may be necessary for use in future clinical applications. Many of these applications are not currently approved by the US Food and Drug Administration.
| 7 |
ACS Laboratories™: Laboratory Product Sales, Contract Manufacturing and Professional Services–ACS Laboratories™ is an unincorporated subsidiary of American CryoStem Corporation, responsible for the sale and licensing of all the Company’s patented and patent pending cellular, cell culture, processing and testing products to professional, institutional and commercial clients. The Company operates a separate website (acslaboratories.com) to distinguish the sale of commercial and research products from its consumer products and services, which are marketed on its main website (americancryostem.com). ACS Laboratories™ manufactures the full line of ACSelerate™ cell culture media and ATCELL™ products; and provides these products to our collaborative partners as further discussed below.
ACS Laboratories™ also provides services to physicians and other medical professionals that perform tissue transfer and other cellular therapy services in same day procedures in their own office. Physicians can arrange to submit samples to the laboratory for sterility, viability, cellular density and growth assay analysis. Many physicians that provide tissue transfer services do not have the facilities and equipment necessary to perform this type of testing. Large diagnostic and testing laboratories do not currently offer these services. The Company’s fees range from $200 for sterility testing up to $1000 for comprehensive testing and analysis.
Contract Manufacturing, Autokine-CM® Anti-Aging, Autologous Skin Care Product Line
Under agreement with Personal Cell Sciences (PCS), we manufacture the key ingredient Autokine-CM® (autologous adipose derived stem cell conditioned medium) for PCS’ U-Autologous™ anti-aging topical formulation. Each product is genetically unique to the patient and custom blended, deriving its key ingredients from the individual client’s own stem cells. The Company provides its CELLECT® Tissue Collection service to collect the required tissue to manufacture the U-Autologous™ product and processes it under the same cGMP standard operating procedures that it developed for the ATGRAFT™ and ATCELL™ cell processing services utilizing ACSelerate™ cell culture media. The Company receives collection, processing and long term storage fees and earns a royalty on all U-Autologous™ product sales. The utilization of the Company’s core services in its contract manufacturing relationship provides opportunities for the Company to promote its ATGRAFT™ and ATCELL™ products for an individual’s cosmetic purposes.
Our Company’s contract manufacturing services can be extended to develop custom and/or white label products and services for both local and global cosmetic and regenerative medicine companies, physicians, wellness clinics and med spas. The Company intends to expand its relationships and contract manufacturing regionally through its physician networks and globally through its International Licensing Program.
Product Development
Our strategic approach is to design, develop and launch new products and services that utilize our existing products and services to provide the Company with opportunities to produce near term cash flow, strong recurring revenue streams, strong international licensing partners and complementary scientific data. We focus on products, services and applications that require tissue collection and processing as the initial requirement to produce cellular therapies and products. These products and services can include adipose tissue and stem cell sample processing and storage as a form of personal “bio-insurance,” adipose tissue (fat) storage for cosmetic fat engraftment procedures, and the creation and production of topical applications and ingredients used by other companies in the wound care and cosmetic industries.
We are focusing our efforts on the expansion of our products and services pipelines based upon our intellectual property portfolio, collaborative development relationships, product sales and distribution, and international licensing and partnering opportunities. Our current activities include supporting our university and industry collaborations by providing our products and services with the expectation that our products and services become the basis for new adipose tissue and stem cell based Regenerative Medicine and cellular therapy applications. This strategy allows for our research partners and their application development to begin with clinically harvested and processed adipose tissue and ADSCs (ATCELL™), which we believe can be a significant step toward accelerating the development of new treatments.
Collaboration and Partnering Opportunities
Protein Genomics and Formation of Autogenesis Corporation
In 2012, American CryoStem entered into a Memorandum of Understanding (MOU) outlining our initial collaborative efforts with Protein Genomics, Inc. (PGEN) to test and develop new products by combining certain components of our respective intellectual property and patented products. We have provided PGEN and its research partner, Development Engineering Sciences (DES), with Adipose Derived Stem Cells (ATCELL™) and our patented cell culture mediums (ACSelerate™) for testing with PGEN’s patented products designed for the wound healing market. Research and development has been ongoing since late 2012 and notable progress has been achieved. In October of this year, the early results of this initiative was the subject of local media coverage in Arizona showcasing the groundwork laid by PGEN, DES and American CryoStem in providing assistance in what we believe is a quicker way to heal skin injuries using a patient’s stem cells.
| 8 |
As a result of the success realized in the early stage of this research collaboration, we recently entered into a formal joint venture with Protein Genomics through the incorporation of Autogenesis, Corp. as required in the 2012 MOU. Each company (CRYO and PGen) initially has an equal 50% ownership interest. All products capable of being commercialized, as well as any new intellectual property, resulting from the ongoing scientific collaboration will be wholly-owned by Autogenesis. This is a very exciting turn of events for us and is representative of how we believe additional research collaborations with our Company’s technology may evolve in the future.
Rutgers University
In May of this year, American CryoStem entered into Material Transfer Agreements with three research scientists at Rutgers University allowing them to utilize the Company’s autologous Adipose-Derived Stem Cells (ATCELL™) and patented, serum free, GMP grade cell culture and differentiation mediums (ACSelerate™) for evaluation with the anticipation to implement additional agreements to research, develop and commercialize innovative new cellular therapies targeting incurable diseases, neurological disorders and the $5 billion global wound care market.
In December of this year, American CryoStem and Rutgers University executed a Collaboration and Research Agreement involving stem cell differentiation molecules and molecular biological reagents under the direction and supervision of Dr. KiBum Lee, the PRINCIPAL INVESTIGATOR (PI) for the research. Our collaborative efforts have advanced rapidly and new intellectual property is anticipated to result from this work. Based on the collaborative efforts under the Collaboration and Research Agreement, our Company’s patent counsel is preparing two patent applications based upon earlier developments which are now optioned to American CryoStem. In addition, American CryoStem’s agreement with Rutgers University allows us the use of intellectual property and biomaterials developed by Dr. Lee and his team in combination with our ATCELL™ and ACSelerate™ products for the development of new cellular therapies and regenerative medicine applications. To support the new discoveries, Dr. Lee and our professionals will develop, file and publish patent applications, research papers, government and private grant funding applications to support future clinical studies as appropriate.
Further collaboration and research agreements are currently in negotiation with Rutgers researchers focusing on wound healing and topical delivery of our innovative products.
Institutional Review Board Approval of Protocols
In an effort to make it easier for other physicians and researchers to study the safety of SVF and ADSCs (ATCELL™), we sought approval from the Institutional Review Board (IRB) of the International Cell Surgical Society (ICSS) of our protocols for the processing of ATCELL-SVF™ and culturing of stem cells from adipose tissue ATCELL™. The two protocols, titled: Autologous Adipose Tissue-Derived Stromal Vascular Fraction (SVF) Containing Adult Stem Cells with Isolation of SVF, and Culturing of Adipose Derived Stem Cells (ADSCs) For Use in Institutional Review Board Studies, provide appropriate processing, storage and testing methods necessary to move the clinical investigative process towards uniform treatments. The collection of cGMP processing and outcome data from IRB approved protocols is required by prevailing FDA regulations and guidance for approval of regenerative cellular therapies including, at a minimum, potency (cell count), contamination testing and cell viability.
The ICSS IRB thoroughly evaluated every step of our standardized processing protocols (SOPs), which serve to isolate the SVF or ADSCs from a patient’s adipose tissue. The objective of the IRB is to assess these protocols to ensure the highest patient safety possible and to minimize the risks for those participating in innovative research and investigational studies. Shortly following the end of the third fiscal quarter, ended June 30, 2013, the ICSS IRB approved the protocols.
| 9 |
The Company is making available its processing services utilizing the IRB-approved protocols to physicians and clinical researchers for inclusion in their studies. By adopting these standardized and repeatable protocols (SOPs) and utilizing our laboratory services, researchers can focus their resources on application development rather than creating, validating and managing a clinical laboratory for the preliminary processing of tissue and cellular samples. These studies do not currently involve actual human clinical trials, but affords the IRB the opportunity to endorse our repeatable, standardized and validated processing methodologies for the isolation of SVF and for tissue culture expansion of ADSCs obtained from SVF as the basis for future human clinical study.
Management intends to pursue additional collaborative and partnering opportunities as a strategic method to enhance awareness of and expand the distribution of our patented products, services, technologies and expertise in the IRB-approved clinical processing of adult adipose tissue for autologous (self) use and ADSCs. We believe that as the pace of clinical trials and result reporting increase and scientific and peer reviewed papers are published, new opportunities to market our existing products, services and Intellectual Property portfolio may also emerge.
Additionally, on November 21, 2013 the Company was approved by ICSS for an IRB titled, “Comparative Viability Assessment of Human Adipose Tissue Before and After Cryopreservation” to support a pending clinical study of our ATGRAFT™ products and services and the development of publications in support of our patented technologies.
Moreover, we believe that the combination of our validated cellular processing capabilities and patented products give us an economical platform to develop and produce cellular therapy applications for injection or intravenous therapy, topical applications, burn and wound healing, joint repair, disease treatments and Cosmeceuticals. The clinical methods and products we have developed are designed to permit a variety of treatments for any patient with their own genetically matched raw materials ATCELL™ and ATGRAFT™. Autologous cellular therapies have shown promising results for safety and efficacy in a variety of applications in published early stage clinical trial results and application studies.
Our Company has filed multiple patent applications for our products and methods to be used in the IRB studies, which include:
| ● | ACSelerate-SF™ (animal serum free) and ACSelerate-LS™ (low dose bovine serum) adipose stromal cell culture and differentiation medium in clinical and research grades; | |
| ● | The CELLECT® collection and tracking system for collecting tissue and cellular samples; | |
| ● | Adipose tissue, stromal vascular fraction (SVF) and adipose-derived mesenchymal cell processing, expansion and differentiation; | |
| ● | Storage preparation methods for adipose tissue, stromal vascular fraction (SVF) and adipose derived cellular samples; | |
| ● | Testing and quality management methods, systems, data collection and maintenance; | |
| ● | Cryoprotectant for the storage of adipose tissue samples; and | |
| ● | The ATGRAFT™ service for the collection, preparation, storage and retrieval of adipose tissue as biocompatible fillers for cosmetic and plastic surgery. |
Regulatory Information
The Company has spent years developing processing methodologies and the testing laboratory facilities which are designed to be in compliance with all current Good Manufacturing Practices (cGMP) and current Good Tissue Practices (cGTP) as defined by the United States Public Health Service Act (“PHS” or the “PHS Act”) and the Food and Drug Administration (FDA) regulations as they relate to the operation of a tissue processing and storage facility.
The Company’s Mount Laurel facility has been registered with the FDA (FEI 3008307548) as a processing and storage facility for Human Cells, Tissues and Cellular and Tissue Based Products (HCT/Ps) since 2010. In 2013, we further registered the facility with the State of New York (CP169TP136) and the State of California (CNC80948). These state registrations required the submission of our SOPs for review by the respective State Health Departments, and annual updates to maintain the registrations are required. In addition, we have discussed our operations with the State of New Jersey Health Department and Department of Environmental Protection (DEP) to ascertain any special regulations to which we may be subject. Based upon these discussions, and our use of a registered medical waste disposal company, we do not believe at this time we have any special registrations or regulations for compliance with the State of New Jersey. Our New Jersey Medical Waste Generator registration number is 036439.
| 10 |
The Company is also subject to complying with a significant body of FDA and PHS regulation; the regulations governing our business are mainly contained within 21 CFR 1271.10, 800, 600, 200, 210 and 211. The forgoing regulations govern all aspects of the Company’s SOPs, which we have reviewed with our FDA consultants.
Our SOPs are the key to properly operating a clinical tissue processing facility. To ensure delivery of the highest quality services, we have also designed them to provide a basis for accreditation by the American Association of Blood Banks (AABB), the American Association of Tissue Banks (AATB) and the Foundation for the Accreditation of Cellular Therapy (FACT-JACIE). We have consistently endeavored to ensure that our processes, methodologies and procedures are and remain among the highest standards in the global tissue collection, processing and storage market. To this end, we have equipped ourselves with state-of-the-art quality processing and testing equipment, which help to ensure that every sample that is collected and processed is sterile (free from bacterial contamination and adventitious agents), viable and capable of significant growth and expansion. While published studies generally report total viable cells, our assessment testing also reports each sample’s sterility and growth capabilities.
Quality Management
The Company’s quality management program ensures that during processing and testing of each adipose tissue or SVF sample, the appropriate quality management tests and processing methodologies are performed and the data is collected, recorded and reviewed by the laboratory management team.
Chain of Custody Control
Central to the individual sample testing is an unbroken chain of custody and tracking. Sample tracking begins with the creation of each collection box. All samples, processing, quality management, batch, and storage documents and records, are coded with a unique number. All records and testing samples are cross referenced and verified as required by the standard operating procedures.
Testing Design and Standard Operating Procedures
Testing methods are standardized and operate under a complete set of validated SOPs and Quality Management (QM) processes. All SOPs are designed to be in compliance with the US Food and Drug Administration’s cGMP/cGTP regulations and guidance for aseptic processing. Strict QM is enforced to avoid and/or record any process deviations.
Intellectual Property
From our very early stages, our strategy has been to invest in intellectual property protection. This strategy is intended to strengthen our Company’s foundation in any defensive or offensive legal challenge. In addition, we are developing our IP portfolio to ensure and enhance our business flexibility and allow us to gain favorable terms in potential future collaborative partnerships with third parties. Our intellectual property portfolio currently includes one issued U.S. patent (No. 7989205, Cell Culture Media Kits and Methods of Use); and five pending patent applications which are detailed in the following chart:
| PATENT TITLE | USE OF PATENT | APPLICATION # |
| A Business Method for “Collection, Cryogenic Storage and Distribution of a Biological Sample Material" | Company Core Tissue Collection Processing and Storage Methodology | (PCT/US2011/39260) filed June 6, 2011, and claiming a priority date of June 7, 2010 from provisional application 61/352,217 |
| Systems and Methods for “The Digestion of Adipose Tissue Samples Obtained From a Client for Cryopreservation" | Adipose Tissue Digestion Laboratory Processing Methods | U.S. Serial No. 13/646,647 filed October 5, 2012, and claiming a priority date of October 6, 2011 from provisional application 61/544,103 |
| Compositions and Methods for “Collecting, Washing, Cyroprocessing, Recovering and Return of Lipoaspirate to Physicians for Autologous Adipose Transfer Procedures” | Company Adipose Tissue Storage Platform for Cosmetic Procedures | PCT/US13/44621 Filed June 6, 2013 and claiming a priority date of June 7, 2012
|
| Stem Cell-Based Therapeutic Devices and Methods | Combining ADSCs with Biomaterials for healing and tissue growth | U. S. Serial No. 61/773,112 filed March 10, 2013 |
| Autologous Serum for Transport of Isolated Stromal Vascular Fraction or Adipose Derived Stem Cells | Utilization of Autologous Blood Components for the Transport of Adipose Derived Cells to a Patient | U.S. Serial No. 61/810,970 filed April 11, 2013 |
| 11 |
Trademarks
In addition to patents, the Company has registered or filed application for the following trademarks with the U.S. Patent and Trademark Office: American CryoStem®, CELLECT® and ATGRAFT™. We plan to file for registration additional trademarks for our current and future products, slogans and themes used in our branding and marketing initiatives, including, for example, ACSelerate-SFM™, ACSelerate- LSM™ and ATCELL™.
The Company has also secured a number of online domain names relevant to its business, including www.americancryostem.com and www.acslaboratories.com.
Market Size and Opportunities
By leveraging and capitalizing on our proprietary Adipose Tissue Processing Platform, our Company is working to address multiple high growth, multi-billion dollar market opportunities, including those prevailing within the Regenerative Medicine, Cosmeceuticals and Cell Culture Media and Medical Tourism markets.
Regenerative Medicine Market
According to a leading research firm focused on the biotechnology, healthcare and life sciences industries, TriMark Publications categorizes the Regenerative Medicine market into three main categories:
| ● | Tissue Engineering; | |
| ● | Biomaterials; and | |
| ● | Biomolecules (scaffolds, growth factors and stem cell therapy). |
TriMarkPublications.com cites in its “Regenerative Medicine Markets” report (March 2013) “…the Regenerative Medicine market continues to witness significant advances in clinical efficacy, regulatory approval and product commercialization of cell based therapies which will catapult to over $35 billion by 2019. Affirmative results produced from the application of adult stem cells have resulted in greater government and private sector investment in research and development of new cell therapies. Investment made into the regenerative medicine market include firms that harvest, process, purify, expand, cryopreserve, store or administer stem cells.”1
Medical Tourism
1 http://www.trimarkpublications.com/regenerative-medicine-markets/
| 12 |
KPMG International’s 2011 report on medical tourism credits the industry’s success to the ease of global transportation which is attracting an increasing number of people taking advantage of cost-effective, quality medical care at low cost destinations with world-class medical capabilities.
KPMG reported, “This increase in demand for global medical tourism has grown the industry at a rate of 20%-30% annually with a market size of $100 billion in 2012 up from $78.5 billion in 2010. Patients from developed countries are traveling abroad for medical care due to rising healthcare costs, demographic changes and delays in obtaining access to care at home. The rapidly aging population and a growing shortage of healthcare providers is creating a demand gap likely to widen with the baby boomer population coming to senior age. This need for healthcare will continue to grow and individuals will seek out novel opportunities obtain care further fueling the medical tourism industry.”2
Cell Culture Market
The Cell Culture market is $2.3 billion according to a report in Genetic Engineering & Biotechnology News (January 12, 2012 Vol. 32 No.2) “…and is expected to grow to $3.9 billion by 2015; this equates to 70% growth over a six-year period.”3 “This expected growth may be further enhanced by an expected shortage of bovine serum, a major component in research and the manufacture of certain cellular therapy products.” according to “Peak Serum: Implications of serum supply for cell therapy manufacturing,” a commentary by David A. Brindley published in RegenMed (2012, January 7(1), 7-13), which further states: “Without a sustainable supply or viable alternatives to these components, the commercial-scale production of cell therapies will be impossible, halting the momentum of the industry.”4
Cosmeceutical Market
Many industry experts agree that Cosmeceuticals have become the fastest growing segment of the Cosmetics and Personal Care industry. Cosmeceutical products have a big emphasis on scientifically advanced formulations and often contain active ingredients that can also be found in pharmaceutical products. This continued emergence of increasingly sophisticated active ingredients is said to be the main driving force behind the growth of this segment, which is rapidly evolving into a significant category of the personal care industry.
US retail sales of Cosmeceuticals in 2011 totaled $9.7 billion with ongoing annual sales gains expected to boost the market to $11.7 billion by 2016, according to a Packaged Facts report released in July 2012.
In a report titled Global Cosmeceuticals Market Outlook 2016, published February 2013, RNCOS reports that the worldwide market is estimated to be valued at $30.5 billion and is likely to grow at a consistent CAGR of 7.7% during the period 2012 through 2016.5
Marketing and Distribution
A key objective of our marketing strategy is to position American CryoStem in the market as the “Gold Standard” for adipose tissue processing, cellular processing and cell storage, cellular expansion, therapeutic applications, and, research and commercial uses of adipose tissue within the current regulatory framework. The combination of a traditional sales approach supported by continuous internal and external marketing programs will be closely coordinated with the expansion of our laboratory processing capabilities. Our initial marketing efforts are intended to disseminate current and future uses of adipose tissue and adult stem cells which support our business model, products and services. We intend to employ both print advertising and social media sales campaigns. In addition, we plan to utilize key leaders, and early adopters in the medical community as a marketing resource to enhance awareness of our proprietary, patented products and services and to increase the number of surgeons who join our network and collaborate with us.
2 http://www.kpmg.com/CH/en/Library/Articles-Publications/Documents/Sectors/pub-20120207-issues-monitor-healthcare-medical-tourism-en.pdf
3 http://www.genengnews.com/gen-articles/cell-culture-media-market-maturing/3981/
4 http://www.futuremedicine.com/doi/abs/10.2217/rme.11.112?journalCode=rme
5 http://www.researchandmarkets.com/research/mbmvbh/global
| 13 |
We have also initiated a direct marketing program focused on reaching plastic and cosmetic surgeons and have an initial group of providers that have begun to offer our services to their patients. This marketing initiative has been implemented using a traditional sales approach common to the pharmaceutical and biotechnology industries. This fundamental sales approach at the core of our marketing activities is being strategically and tactically expanded using a combination of in-house sales personnel and outside independent channels.
Our plan provides for a comprehensive integrated marketing approach using various traditional and new media, such as the Internet, social media/blogging, video, print, TV, radio and trade shows to reach targeted potential consumers and promote awareness of our Company and our branded products and services. The essence of this targeted strategy is to reach the end-users as quickly as possible and to accelerate the adoption curve of our products and services. We also plan to utilize outside marketing resources and trade groups to increase the number of surgeons willing to offer our products and services to their patients.
Development of Regional U.S. Markets
Physician Network
The Company continues to develop regional relationships to leverage its new products and services through existing cosmetic surgery and regenerative medicine practices. American CryoStem continues to develop and expand its network of physicians seeking to adopt its products and services initially focusing on surgeons performing liposuction, tissue transfer and regenerative procedures involving the use of adipose tissue. The Company intends to expand its efforts to medical professionals interested in Regenerative Medicine applications utilizing ADSCs and establish itself as a primary source of collection processing and preparation of cellular therapies as they are developed and approved for patient use by the FDA.
The Stern Center
During our first fiscal quarter ended December 31, 2012, we announced the initiation of adult stem cell and adipose tissue collection at the Stern Center for Aesthetic Surgery in Bellevue Washington. Dr. Frederick Stern, a member of the Company’s Scientific and Medical Advisory Board, founded the Stem Center in 1997. The Stern Center offers state-of-the-art laser and cosmetic surgical techniques to patients throughout the western U.S., and is one of the premier laser-assisted liposuction centers in the Pacific Northwest.
Dr. Park Avenue
In September 2013, we announced the opening of three new adipose tissue collection centers at Dr. Park Avenue’s New Jersey locations. Dr. Park Avenue is a leading provider of aesthetic and cosmetic services in the Tri-State area with locations in Brick Township, Franklin Lakes and Hoboken, New Jersey. Dr. Park Avenue’s newest center, located in Hoboken, held its grand opening in late September; in conjunction with the opening, Dr. Park Avenue formally introduced our ATGRAFT™ service for patients interested in fat grafting as an alternative to artificial fillers by using their own stored fat tissue to undergo transfer procedures to the face, hands, breast and buttocks.
Development of International Markets
International Licensing Program
Globally, many jurisdictions outside the US currently permit use of cellular therapies and regenerative medicine applications. The Company has received numerous inquiries concerning the sale or licensing of our products and services in foreign jurisdictions. The Company believes that the inquiries to date are a result of the global boom in Medical Tourism and the slow pace of approval of cellular therapies and regenerative medicine applications in the U.S. To address these inquiries and to expand the Company’s sales, marketing and branding opportunities the Company has designed and is offering an International Licensing Program.
| 14 |
The program is designed to permit the licensing of the company’s products and services to organizations that meet the Company’s financial and technical criteria. The licensing program allows for a variety of business relationship including franchising, partnering and joint venturing. Marketing efforts to date have been to clinics, physician and hospitals in foreign jurisdictions capable of rapidly building or committing the appropriate facilities and personnel to create the required laboratory facilities to operate the CELLECT®, ATGRAFT™ and ATCELL™ services in their local market. Strategically, the Company’s international licensees will maintain the branding of the Company’s services along the lines of the highly publicized “Intel Inside” branding program.
Qualified Licensees can quickly take advantage of the rapidly expanding opportunity to collect, process, store and culture individual stem cell samples for their clients with the comfort and confidence that they are providing services that have been developed to U.S. FDA Standards. Core to the relationship is the developed proprietary and patent pending processing and laboratory operational methodologies contained in our Standard Operating Procedures (SOPs), Training Programs, Continuous Quality Management (QM) and Product Testing Programs, and Laboratory Operation manuals.
The licensing program may be initiated through a letter of intent (LOI) agreement between the Company and the prospective licensee. This agreement is designed for due diligence and facility qualifications purposes. The Company may receive an initial fee under the agreement which is credited toward future royalty payments.
Our licensing program can be broken down into four operating levels, CELLECT®, ATGRAFT™, ATCELL™ and Contract Manufacturing:
| ● | Level One is the opportunity for a licensee or partner to secure a territory and implement the CELLECT® program for the collection, transportation, tracking and monitoring of tissue samples which are delivered to a laboratory for processing and storage until needed. A Level One licensee can quickly offer our branded services without, equipping and staffing their own local facility. Level One licensees are generally treatment facilities operated by physicians with limited laboratory facilities. |
| ● | Level Two is the opportunity for an existing laboratory facility to expand its services to include a CELLECT® and ATGRAFT™ services and to perform these services in a defined geographic location. A level two licensee must be capable of maintaining the services and provide local storage services in line with ACS requirements, guidelines and SOPs. |
| ● | Level Three is the opportunity for an existing stem cell laboratory with basic experience in the processing and storage of stem cells, umbilical cords or other tissues to quickly adopt complete turnkey adipose derived stem cell processing capabilities by incorporating the Company’s CELLECT®, ATGRAFT™ and ATCELL™ services and ACSelerate™ products. A Level Three licensee will also be able to participate in clinical application production and research in conjunction with the ACS Mount Laurel laboratory facility. |
| ● | Level Four is the opportunity for an existing hospital, biotechnology manufacturer, research university or large laboratory facility to implement all of the Company’s services in a defined geographic territory. A Level Four licensee may become a regional processing and manufacturing hub with the ability to manufacture and distribute the Company’s patented products, initiate and develop new treatments and applications and participate in local and global research and development of cellular therapies and regenerative medicine applications. |
Significant to our international development activities is our continuous reinforcement of American CryoStem’s branded services and patented products, services, technologies and SOPs as the highest quality core platform for standardized, repeatable and verifiable method to implement cellular therapies and regenerative medicine utilizing ADSCs.
BALS Institute
On April 23, 2013, we announced receipt of our first commercial international shipment of adipose tissue for processing and long term cryostorage. The master sample was shipped to the Company by BALS (Biomedical and Life Sciences) Institute (BALS), a Hong Kong-based regenerative medicine company and client of Personal Cell Sciences Corp. (PCS), the developer of U-Autologous skin care products and formulations. The product uses an individual’s own adult stem cells to create and supply that individual with his or her own personalized anti-aging skin care line.
| 15 |
As part of the contract manufacturing arrangement between American CryoStem and PCS, we are responsible for clinically testing, processing, culturing and storing samples shipped from PCS clients to create Autokine-CM, the key ingredient in the U-Autologous formulation. BALS Institute has teamed with PCS to ensure the people in Greater China gain access to safe, quality and effective life science technologies through partnerships with leading international corporations.
We have committed extensive resources to establishing and perfecting our international shipping methodologies and protocols, ensuring that our processes meet the highest possible standards of regulatory compliance for shipment of biologic materials. As a result, our FDA registered laboratory and cryostorage facilities in New Jersey are now able to send and receive viable tissue samples to and from clients globally.
Scientific and Medical Advisory Board
As part of our marketing campaign to reach and educate physicians, we are actively seeking to bring highly qualified peer leaders onto our Scientific and Medical Advisory Board to assist us in our industry speaking and education platform. This physician education platform is designed to focus on industry needs and demands as they relate to current and future treatments using our adipose tissue and adult stem cell technologies. Additionally certain members of our advisory board provide assistance and input to management on the oversight of our research relationships, laboratory development and quality management systems. To date, we have succeeded in winning the following members to our Scientific and Medical Advisory Board:
| ● | David K. Moscatello, Ph.D. − Chief Scientist | |
| Dr. Moscatello earned his Bachelor of Science degree in Microbiology from Pennsylvania State University in 1975, and a PhD in Biology with a focus on cancer cell biology from Purdue University in 1984. He has held teaching positions at Purdue University and Richard Stockton College of New Jersey. He returned to research as an NIH Post-Doctoral fellow at the Kimmel Cancer Institute at Thomas Jefferson University working in the laboratory of Dr. Albert Wong. In October 1999, Dr. Moscatello accepted a position at Coriell Institute to pursue his own research interests. He is a member of the American Association for the Advancement of Science, the American Association for Cancer Research and the American Society for Cell Biology. Dr. Moscatello’s primary research interests involve the isolation, culture and characterization of adult tissue-derived stem cells, i.e. stem and multi-potent progenitor cells other than embryonic stem cells. He has had articles published in a variety of media, including Nature, Journal of Biological Chemistry, International Journal of Cancer, British Journal of Cancer and Cancer Research. Dr. Moscatello advises American CryoStem regarding its laboratory operations and processing. |
| ● | Fredric A. Stern, MD, FACS | |
| Dr. Stern is the founder and Medical Director of the Stern Center for Aesthetic Surgery in Bellevue, Washington. Following his education at Columbia University Medical School, Dr. Stern earned his Board Certification in Ophthalmology at the University of Washington, and underwent extensive additional training in oculofacial plastic and laser surgery. In 1987, he joined Virginia Mason Medical Center in Seattle, serving as Director of the Oculoplastic Surgery Division for ten years. While at Virginia Mason, Dr. Stern performed an extensive number of cosmetic laser procedures. He is honored to have been chosen as one of a select group of instructors of the Botox Cosmetic® National Education Faculty, as well as the Radiesse™ Medical Education Faculty. Dr. Stern is also an instructor for the Sciton™ Laser. In 2011, he was voted the Best Plastic Surgeon in Western Washington by KING 5 (NBC affiliate) TV’s viewing audience. Dr. Stern is a Fellow of the American College of Surgeons, the American Academy of Facial Plastic and Reconstructive Surgeons, the American Academy of Cosmetic Surgery, and the American Society of Liposuction Surgery, as well as a member of the International Society of Hair Restoration Surgery. In addition, over the past several years, he has appeared on Northwest Afternoon, Evening Magazine, as well as KOMO, KIRO and Q13 news, discussing and demonstrating the latest techniques in facial and eyelid laser cosmetic surgery, Botox® and laser-assisted liposuction. He is also an accomplished winemaker & published novelist. Dr. Stern’s latest novel is a medical thriller titled, The Sigma Project. |
| ● | Richard Jacoby, MD – Laboratory Director | |
| Dr. Jacoby is a board certified dermatologist, pathologist and dermatopathologist. Dr. Jacoby’s extensive career includes a significant body of peer reviewed publications, numerous medical journal editorial positions and professional and academic lecture invitations. Dr. Jacoby is or has been a member of numerous professional and scientific societies including the American Academy of Dermatology, the American Medical Association and the International Society of Dermatopathology. In addition to his role with American CryoStem, he is also part of the academic faculty, specializing in Health Policy, at a major Philadelphia, Pa. university medical center. Dr. Jacoby received his BA in psychology from New York University and his MD from Jefferson Medical College of Thomas Jefferson University. He has practiced clinical dermatology and dermatopathology in academic, private and corporate settings. His past corporate positions include Chief Medical Officer of two pathology laboratory companies and Regional Managing Director of a publicly traded laboratory company. |
| 16 |
| ● | Mel Bircoll, MD | |
| Dr. Bircoll was the first plastic surgeon to perform liposuction in North America. He pioneered that operation and saw it from its early beginnings to become what is now the most frequently performed cosmetic procedure worldwide. Dr. Bircoll is also the originator of Fat Transfer (Autologous Fat Transplantation, AFT). His landmark presentation of Fat Transfer Using Liposuction Techniques (1984) established this procedure for breast augmentation, facial rejuvenation, hand rejuvenation and a host of reconstructive procedures. He is board certified by the American Board of Plastic Surgery and the American Board of Cosmetic Surgery. He is a member of the American Society of Plastic Surgery and the American Academy of Cosmetic Surgery. Dr. Bircoll is retired from 25 years of private practice in Beverly Hills, California. He is currently actively lecturing and teaching the techniques of Fat Transfer and Fat Storage for stem cell extraction, as well as cosmetic and reconstructive applications. Dr. Bircoll recently presented the latest application of his Fat Transfer/Storage/Serial Injection concepts for breast cancer prevention surgery. |
| ● | Burt D. Ensley, Ph.D. | |
| Dr. Ensley is the Chief Executive Officer and Chairman of Protein Genomics, Inc. He previously served as Chief Executive Officer of Phytotech, Inc. and President of NuCycle Therapy, Inc. prior to their sale. In addition, Dr. Ensley headed the Specialty Chemicals Group at Amgen, Inc. for nearly a decade. He holds a PhD in Microbiology from University of Georgia; is a Fellow of the American Academy of Microbiology; served on the BIO Directorate Board of the National Science Foundation; and is the Board Co-Chair of the University of Arizona’s BIO5 Institute. Dr. Ensley holds 19 issued U.S. patents. |
| ● | Dayong Gao, Ph.D. | |
| Dr. Gao is a world-renowned Professor of Mechanical Engineering and Biomedical Engineering at the University of Washington in Seattle. He has been actively engaged in cryopreservation research for more than 20 years, with specific emphasis on fundamental and applied cryobiology, which is the investigation of mechanisms in cryo-injury and cryo-protection with respect to living biological systems at low temperatures; with the development of optimal methods and technologies for the cryopreservation; and with the banking of living cells and tissues for biomedical applications. Dr. Gao has published 175 research papers in prestigious scientific/biomedical journals, with over 250 papers/abstracts in conference proceedings. He has obtained 16 patents, and authored two scientific books and numerous chapters in 17 scientific books. He currently serves on the Editorial Board, as Editor-in-Chief, of six scientific journals, and is the Editor of the Cryopreservation Engineering section of Biopreservation and Biobanking. His research in cryobiology and cryopreservation has been funded by the National Institutes of Health, the American Cancer Society, the Bill and Melinda Gates Foundation, the American Heart Association, the Whitaker Foundation, the Washington Research Foundation and the Kentucky Science Foundation, among others. Dr. Gao graduated with B.Sc. degree from the University of Science and Technology in China, and received a Ph.D. in Mechanical Engineering from Concordia University, Montreal, Canada. |
| ● | Richard Goldfarb, MD, FACS | |
| Dr. Goldfarb established the Center for SmartLipo with the vision of providing advanced treatments and techniques to help patients restore and maintain a more youthful appearance. He has formed a team of specialists, each with a unique strength in treating the various parts of the face and body. Included are Aesthetic Laser and Liposuction Specialists, Facial Plastic Surgeons, a Plastic and Reconstructive Surgeon and a Medical Weight Loss team. As a group, they are unequaled in their ability to provide comprehensive consultative and treatment options to achieve an individual’s aesthetic goals. Doctors visit the Center for SmartLipo from all over the world on a regular basis to learn state-of-the-art cosmetic treatments and techniques from Dr. Goldfarb and his team. In view of his unrivaled expertise and skills, Dr. Goldfarb is highly sought after to lecture and train physicians internationally on numerous cosmetic laser and surgery topics. He is board certified and a Fellow of the American College of Surgeons, in addition to the American Society of Laser Medicine and Surgery. Dr. Goldfarb graduated from the University of Health Sciences / Finch University, The Chicago Medical School with top honors in Surgery. He completed his surgical training at Northeastern Ohio College of Medicine. He completed additional training in Cosmetic Surgery at the University of Pennsylvania, Department of Plastic Surgery and Yale University. He has over 30 years of General and Vascular Surgery experience, and is a Cosmetic Surgery Specialist. |
| 17 |
| ● | Roy D. Mittman, MD, PA | |
| Dr. Mittman currently serves as a senior partner of Seaview Orthopaedic and Medical Associates (SOMA) located in Ocean, New Jersey. He has assembled a team of highly qualified board certified, fellowship trainedphysicians to practice at SOMA specializing in general orthopaedics, as well as surgery of the Spine, Hand/Wrist, Knee/Shoulder, Total Joints, Foot and Ankle, Sports Medicine, Pain Management and Osteoporosis. SOMA currently operates six locations committed to providing quality care in Monmouth and Ocean Counties. After earning a Bachelor of Arts degree at John Hopkins University, Dr. Mittman earned his Medical Degree at the Albert Einstein College of Medicine in New York and completed orthopaedic training in 1978 at Montefiore Hospital in New York. He is a member of the New Jersey Orthopaedic Society, Orthopaedic Surgeons of New Jersey, Monmouth County Medical Society and the American College of Sports Medicine. |
| ● | Peter Levitch, BA, MA | |
| Mr. Levitch is President of his own consulting firm, Peter Levitch and Associates, located in New Jersey. He provides clients guidance in the development of pharmaceuticals, medical devices, biologics and diagnostics. Mr. Levitch also possesses a background in the clinical evaluation and FDA regulatory approval phases of product development in the biotechnology industry. He has consulted for more than 200 pharmaceutical and biotechnology companies including Amgen, Inc.; Genentech, Inc.; DuPont; Monsanto Company; Johnson & Johnson Family of Companies, Beckman Coulter, Inc.; Chiron Corp.; Eastman Kodak Company and EMD Serono, Inc. He has authored several papers for publication and has lectured all over the country on various topics including Good Manufacturing Practices (GMP), the FDA approval process, and preparing INDs and NDAs. Peter has also served as an FDA liaison for FDA/company meetings. He is listed in Who’s Who in Finance and Industry, is the co-founder of the Regulatory Affairs Professional Society, and a member of the Drug Information Association and the New York Academy of Sciences. |
| ● | Alan H. Davis | |
| Mr. Davis is currently a partner in and the Chief Operating Officer of Novare, LLC. NovareBiologistics was created to meet the need of transporting and storing laboratory materials, including biological samples at required temperature anywhere within the U.S. Over the past 20 years, Mr. Davis has concentrated on business development and sales in biotechnology, manufacturing and software technology. Previously, he was primarily involved in retailing. |
Corporate Information
Our principal executive offices are located at 1 Meridian Road, Eatontown, New Jersey 07724 and our telephone number is (732) 747-1007. Our website is www.americancryostem.com. We also lease and operate a tissue processing laboratory in Mount Laurel, New Jersey at the Burlington County College Science Incubator located on the Burlington County College campus. Our laboratory website address is www.acslaboratories.com.
Employees
Currently, we have six employees and continue to use consultants on an as needed basis. As we grow, we will need to attract an unknown number of additional qualified employees, however we could be unsuccessful in attracting and retaining the persons needed.
Available information
We file electronically with the U.S. Securities and Exchange Commission (SEC) our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. The public can obtain materials that we file with the SEC through the SEC’s website at http://www.sec.gov or at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. Information on the operation of the Public Reference Room is available by calling the SEC at 800-SEC-0330.
| 18 |
| Item 1A. | Risk Factors |
You should carefully consider the risks described below, together with all of the other information included in this report, in considering our business and prospects. The risks and uncertainties described below are not the only ones facing the Company. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations. The occurrence of any of the following risks could harm our business, financial condition or results of operations.
Recurring losses and negative cash flows from operations raise substantial doubt about our ability to continue as a going concern and we may not be able to continue as a going concern.
Our recurring losses from operations and negative cash flows from operations raise substantial doubt about our ability to continue as a going concern and as a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements for the fiscal year ended September 30, 2013 with respect to this uncertainty. Substantial doubt about our ability to continue as a going concern may create negative reactions to the price of our Common Stock and we may have a more difficult time obtaining financing.
We expect to incur increased operating expenses for the foreseeable future. The amount of net losses and the time required for us to reach and sustain profitability are uncertain. The likelihood of our success must be considered in light of the problems, expenses, difficulties, and delays frequently encountered in connection with a development stage business, including, but not limited to, uncertainty as to development and the time required for our planned services to become available in the marketplace. There can be no assurance that we will ever generate revenues or achieve profitability at all or on any substantial basis. These matters raise substantial doubt about our ability to continue as a going concern. If we cease or curtail our development activities, it is highly likely that you would lose your entire investment in our Company.
We will require substantial additional capital to pursue our business plan.
We have incurred negative cash flows since inception from our developmental activities, and at this time as well as for the foreseeable future will finance (until we can generate sufficient revenues, if ever, to cover expenses) our activities and overhead expenses through the issue and sale of debt or equity securities. The recoverability of the costs incurred by us to date is highly uncertain and is dependent upon achieving commercial production and sales of our services, of which no assurances can be given. Our prospects must be considered in light of the risks, expenses and difficulties which are frequently encountered by companies in the development stage in the emerging industry that we hope to commence operations in.
We have financed our development activities since inception through the sale of securities. Our capital requirements will depend on many factors, including, among other things, the cost of developing our business and marketing activities, the efficacy and effectiveness of our proposed services, costs (whether or not foreseen), the length of time required to collect accounts receivable we may in the future generate, competing technological and market developments and acceptance. Changes in our proposed business or business plan could materially increase our capital requirements. We cannot assure you that our proposed plans will not change or that changed circumstances will not result in the depletion of our capital resources more rapidly than currently anticipated.
We will need to obtain substantial additional financing to, among other things, fund the future development of any services we attempt to undertake and for general working capital purposes. Any additional equity financing, if available, may be dilutive to stockholders and any such additional equity securities may have rights, preferences or privileges that are senior to those of the holders of shares of our Common Stock. Debt financing, if available, will require payment of interest and may involve our granting security interests on our assets and restrictive covenants that could impose limitations on our operating flexibility.
Our ability to obtain needed financing may be impaired by such factors as the capital markets, our capital structure, our development stage, the lack of an active market for shares of our Common Stock, and our lack of profitability, all of which would impact the availability or cost of future financings. We cannot assure prospective investors that we will be able to obtain requisite financing in a timely fashion or at all and, if obtained, on acceptable terms. Our inability to obtain needed financing on acceptable terms would have a material adverse effect on the implementation of our proposed business plan.
| 19 |
Our future plans and operations are dependent on our ability to secure adequate funding and the absence of unexpected delays or adverse developments. We may not be able to secure required funding.
The statements in this Form 10-K concerning future events or developments or our future activities, such as concerning current or planned research and development activities, anticipated products and services, anticipated commercial introduction of products and services, and other statements concerning our future operations and activities, are forward-looking statements that in each instance assume that we are able to obtain sufficient funding in the near term and thereafter to support such activities and continue our operations and planned activities in a timely manner. There can be no assurance that this will be the case. Also, such statements assume that there are no significant unexpected developments or events that delay or prevent such activities from occurring. Failure to timely obtain sufficient funding, or unexpected development or events, could delay the occurrence of such events or prevent the events described in any such statements from occurring which could adversely affect our business, financial condition and results of operations.
Our limited operating history may make it difficult to evaluate our business and our future viability.
We are in the relatively early stage of operations and have only a limited operating history on which to base an evaluation of our business and prospects. Even if we successfully obtain additional funding, we are subject to the risks associated with companies with a limited operating history, including: the need for additional financing; the uncertainty of research and development efforts; successful commercialization of our products and services; market and customer acceptance of our products and services; unexpected issues with federal or state regulatory authorities; competition from larger organizations; dependence on key personnel; uncertain patent or other intellectual property protection; fluctuations in expenses; and dependence on corporate partners and collaborators. Any failure to successfully address these risks and uncertainties could seriously harm our business and prospects. We may not succeed given the technological, marketing, strategic and competitive challenges we will face. The likelihood of our success must be considered in light of the expenses, difficulties, complications, problems and delays frequently encountered in connection with the growth of a new business, the continuing development of new drug technology, and the competitive and regulatory environment in which we operate or may choose to operate in the future.
Many of our products, services and technologies are in early stages of development.
Processing and cryogenic storage of adipose tissue and stem cells is in the early stages of development, and there can be no assurance that our business will be successful. Further, potential products based upon individuals’ stem cells will require extensive additional research and development before any commercial introduction. There can be no assurance that any future research and development will result in viable products or meet efficacy standards.
Cell therapy is a developing field and a significant market for our services has yet to emerge.
Cell therapy and regenerative medicine is a developing field, with few cell therapy products or services approved for clinical and/or commercial use. We are wholly dependent on the acceptance of cell therapy (and specifically stem cells) to develop into a large and profitable industry. We hope to develop services related to the collection, processing and storage of stem cells. We believe the market for cell and tissue-based therapies is in its infancy, substantially research oriented and financially speculative and has yet to achieve substantial commercial success. Stem cell products and services may in general be susceptible to various risks, including undesirable and unintended side effects, unintended immune system responses, inadequate therapeutic efficacy, lack of acceptance by physicians, hospital and consumers, or other characteristics that may prevent or limit their approval or commercial use. Management believes that the demand for stem cell processing and the number of people who may use cell or tissue-based therapies is difficult, if not impossible, to forecast. Our success is dependent on the establishment of a market for our proposed services and our ability to capture a share of this market.
Our proposed services may not attain commercial acceptance absent endorsement by physicians.
Our proposed services will compete against individual cellular samples derived from alternate sources, such as bone marrow, umbilical cord blood and perhaps embryos. We believe that physicians and hospitals are historically slow to adopt new technologies like ours, whatever the merits, when older technologies continue to be supported by established providers. Overcoming such inertia often requires very significant marketing expenditures or definitive product performance and/or pricing superiority. Management currently believes physicians’ and hospitals’ inertia and skepticism to be a significant barrier as we attempt to gain market penetration with our proposed services. Failure to achieve market acceptance of our proposed services could have a material adverse effect on our future prospects.
| 20 |
If we should in the future become required to obtain regulatory approval to market and sell our proposed services we will not be able to generate any revenues until such approval is received.
The medical industry is subject to stringent regulation by a wide range of authorities. While we believe that, given our proposed business, we are not presently required to obtain regulatory approval to market our services we cannot predict whether regulatory clearance will be required in the future and, if so, whether such clearance will at such time be obtained, whether for the stem cells and/or any other services that we are developing or may attempt to develop. Should such regulatory approval in the future be required, our services may be suspended or may not be able to be marketed and sold in the United States until we have completed the regulatory clearance process as and if implemented by the FDA. Satisfaction of regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product or service and would require the expenditure of substantial resources.
If regulatory clearance of a service we propose to provide is granted, this clearance may be limited to those particular states and conditions for which the service is demonstrated to be safe and effective, which would limit our ability to generate revenue. We cannot ensure that any service developed by us will meet all of the applicable regulatory requirements needed to receive marketing clearance. Failure to obtain regulatory approval will prevent commercialization of our services where such clearance is necessary. There can be no assurance we will obtain regulatory approval of our proposed services that may require it.
As we evolve from a company primarily involved in the research and development of our technology into one that is also involved in the commercialization of our technology, we may have difficulty managing our growth and expanding our operations.
As our business grows, we may need to add employees and enhance our management, systems and procedures. We may need to successfully integrate our internal operations with the operations of various third party services providers to produce and market commercially viable products. We may also need to manage additional relationships with various collaborative partners, suppliers and other organizations. Expanding our business may place a significant burden on our management and operations. We may not be able to implement improvements to our management information and control systems in an efficient and timely manner and we may discover deficiencies in our existing systems and controls. Our failure to effectively respond to such changes may make it difficult for us to manage our growth and expand our operations.
We are wholly dependent on John Arnone and Anthony Dudzinski.
We are wholly dependent on John Arnone and Anthony Dudzinski, our only executive officers and directors. Our future performance will depend on the continued services of such persons and our ability to retain such persons and to hire additional qualified persons. The loss of either of Mr. Arnone or Mr. Dudzinski, or both, would materially and adversely affect our proposed business. We have entered into employment agreements with Mr. Arnone or Mr. Dudzinski who have waived portions of their compensation for fiscal 2013 and 2014. There are no assurances they will continue to do so. The employment agreements among other terms, permit each of Mr. Arnone and Mr. Dudzinski to conduct other business activities outside of their employment with us.
We have not obtained any “key-man” life insurance policies nor do we presently plan to obtain or maintain any such policies on Mr. Arnone, Mr. Dudzinski or any other of our employees.
We may be unable to protect our intellectual property from infringement by third parties, and third parties may claim that we are infringing on their intellectual property, either of which could materially and adversely affect us.
We intend to rely on patent protection, trade secrets, technical know-how and continuing technological innovation to protect our intellectual property, and we expect to require any employees, consultants and advisors that we may hire or engage in the future to execute confidentiality and assignment of inventions agreements in connection with their employment, consulting or advisory relationships. There can be no assurance, however, that these agreements will not be breached or that we will have adequate remedies for any such breach.
Despite our efforts to protect our intellectual property, third parties may infringe or misappropriate our intellectual property or may develop intellectual property competitive with ours. Our competitors may independently develop similar technology or otherwise duplicate our proposed processes or services. As a result, we may have to litigate to enforce and protect our intellectual property rights to determine their scope, validity or enforceability. Intellectual property litigation is particularly expensive, time-consuming, diverts the attention of management and technical personnel and could result in substantial cost and uncertainty regarding our future viability. The loss of intellectual property protection or the inability to secure or enforce intellectual property protection would limit our ability to produce and/or market our services in the future and would likely have an adverse effect on any revenues we may in the future be able to generate by the sale or license of such intellectual property.
| 21 |
We may be subject to costly litigation in the event our future services or technology infringe upon another party’s proprietary rights. Third parties may have, or may eventually be issued, patents that would be infringed by our technology. Any of these third parties could make a claim of infringement against us with respect to our technology. We may also be subject to claims by third parties for breach of copyright, trademark or license usage rights. Any such claims and any resulting litigation could subject us to significant liability for damages or injunctions precluding us from utilizing our technology or services or marketing or selling any products or services under the same. An adverse determination in any litigation of this type could require us to design around a third party’s patent, license alternative technology from another party or otherwise result in limitations in our ability to use the intellectual property subject to such claims.
Risks Related to Our Common Stock
We are authorized to issue 300,000,000 shares of Common Stock and 50,000,000 shares of “blank check” preferred stock, the issuance of which could, among other things, reduce the proportionate ownership interests of current shareholders.
We are authorized to issue 300,000,000 shares of Common Stock and 50,000,000 shares of “blank check” preferred stock. As of September 30, 2013, there were 32,285,721 shares of Common Stock and no shares of preferred stock issued and outstanding. Our Board of Directors has the ability, without seeking shareholder approval, to issue additional shares of Common Stock and/or to designate, establish the terms and conditions of, and issue shares of preferred stock for such consideration, if any, as the board of directors may determine. Any such shares of preferred stock could have dividend, liquidation, conversion, voting or other rights, which could adversely affect the voting power or other rights of the holders of shares of Common Stock. In the event of such issuance, the preferred stock could, among other items, be used as a method of discouraging, delaying or preventing a change in control of our Company, which could have the effect of discouraging bids for our Company and thereby prevent security-holders from receiving the maximum value for their shares of our Common Stock.
Our Common Stock is currently traded on the OTCQB and is subject to additional trading restrictions as a “penny stock,” which could adversely affect the liquidity and price of such stock. If our Common Stock remains subject to the SEC’s penny stock rules, broker-dealers may experience difficulty in completing customer transactions and trading activity in our securities may be adversely affected.
Our Common Stock currently trades on the OTCQB. The OTCQB may be viewed by investors as a less desirable, and less liquid, marketplace. As a result, an investor may find it more difficult to purchase, dispose of or obtain accurate quotations as to the value of our Common Stock.
Because our Common Stock is not listed on any national securities exchange, such shares will also be subject to the regulations regarding trading in “penny stocks,” which are those securities trading for less than $5.00 per share, and that are not otherwise exempted from the definition of a penny stock under other exemptions provided for in the applicable regulations. The following is a list of the general restrictions on the sale of penny stocks:
| ● | Before the sale of penny stock by a broker-dealer to a new purchaser, the broker-dealer must determine whether the purchaser is suitable to invest in penny stocks. To make that determination, a broker-dealer must obtain, from a prospective investor, information regarding the purchaser’s financial condition and investment experience and objectives. Subsequently, the broker-dealer must deliver to the purchaser a written statement setting forth the basis of the suitability finding and obtain the purchaser’s signature on such statement. |
| ● | A broker-dealer must obtain from the purchaser an agreement to purchase the securities. This agreement must be obtained for every purchase until the purchaser becomes an “established customer.” The Securities Exchange Act of 1934, or the Exchange Act, requires that before effecting any transaction in any penny stock, a broker-dealer must provide the purchaser with a “risk disclosure document” that contains, among other things, a description of the penny stock market and how it functions and the risks associated with such investment. These disclosure rules are applicable to both purchases and sales by investors. | |
| ● | A dealer that sells penny stock must send to the purchaser, within 10 days after the end of each calendar month, a written account statement including prescribed information relating to the security. |
| 22 |
These requirements can severely limit the liquidity of securities in the secondary market because fewer brokers or dealers are likely to be willing to undertake these compliance activities. As a result of our Common Stock not being listed on a national securities exchange and the rules and restrictions regarding penny stock transactions, an investor’s ability to sell to a third party and our ability to raise additional capital may be limited. We make no guarantee that market-makers will make a market in our Common Stock, or that any market for our Common Stock will continue.
Our principal stockholders have significant influence over us, they may have significant influence over actions requiring stockholder approval, and your interests as a stockholder may conflict with the interests of those persons.
Based on the number of outstanding shares of our Common Stock held by our stockholders as of September 30, 2013, our directors, executive officers and their respective affiliates owned approximately 65% of our outstanding shares of Common Stock. As a result, those stockholders have the ability to exert a significant degree of influence with respect to the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. The interests of these persons may not always coincide with our interests or the interests of our other stockholders. This concentration of ownership could harm the market price of our Common Stock by (i) delaying, deferring or preventing a change in corporate control, (ii) impeding a merger, consolidation, takeover or other business combination involving us, or (iii) discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. The significant concentration of stock ownership may adversely affect the trading price of our Common Stock due to investors’ perception that conflicts of interest may exist or arise.
Mr. Arnone is presently involved with another entity that operates in an industry similar to ours but that management does not believe to be in competition with us. We may in the future seek to initiate a business relationship with, and/or acquisition of, this other entity. Management cannot assure you that any such business relationship or acquisition, if consummated, would be on terms favorable to us.
Our stockholders may experience significant dilution as a result of any additional financing using our securities.
We will need to raise significant additional capital in order to maintain and continue our operations. To the extent that we raise additional funds by issuing equity securities or securities convertible into or exercisable for equity securities, our stockholders may experience significant dilution.
We have not paid dividends on our Common Stock in the past and do not expect to pay dividends on our Common Stock for the foreseeable future. Any return on investment may be limited to the value of our Common Stock.
No cash dividends have been paid on our Common Stock, and we do not expect to pay cash dividends on our Common Stock in the foreseeable future. Payment of dividends would depend upon our profitability at the time, cash available for those dividends, and other factors as our board of directors may consider relevant. If we do not pay dividends, our Common Stock may be less valuable because a return on a stockholder’s investment will only occur if our stock price appreciates.
A sale of a substantial number of shares of our Common Stock may cause the price of our Common Stock to decline and may impair our ability to raise capital in the future.
Our Common Stock is currently traded on the OTCQB, and there have been and may continue to be periods when it could be considered “thinly-traded,” meaning that the number of persons interested in purchasing our Common Stock at or near bid prices at any given time may be relatively small or non-existent. Finance transactions resulting in a large amount of newly issued shares that become readily tradable or other events that cause stockholders to sell shares, could place downward pressure on the trading price of our stock. In addition, the lack of a robust resale market may require a stockholder who desires to sell a large number of shares of Common Stock to sell the shares in increments over time to mitigate any adverse impact of the sales on the market price of our stock. If our stockholders sell, or the market perceives that our stockholders intend to sell for various reasons, substantial amounts of our Common Stock in the public market, the market price of our Common Stock could decline. Sales of a substantial number of shares of our Common Stock may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
| 23 |
As a “thinly-traded” stock, large sales can place downward pressure on our stock price.
Our stock experiences periods when it could be considered “thinly traded.” Financing transactions resulting in a large number of newly issued shares that become readily tradable, or other events that cause current shareholders to sell shares, could place further downward pressure on the trading price of our stock. In addition, the lack of a robust resale market may require a shareholder who desires to sell a large number of shares to sell the shares in increments over time to mitigate any adverse impact of the sales on the market price of our stock.
Shares eligible for future sale may adversely affect the market for our common stock.
We presently have 6,600,000 options and no warrants to purchase shares of our common stock outstanding. If and when these securities are exercised into shares of our common stock, the number of our shares of common stock outstanding will increase. Such increase in our outstanding share, and any sales of such shares, could have a material adverse effect on the market for our common stock and the market price of our common stock.
In addition, from time to time, certain of our shareholders may be eligible to sell all or some of their shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, promulgated under the Securities Act of 1933, which we refer to in this prospectus as the Securities Act, subject to certain limitations. In general, pursuant to Rule 144, after satisfying a six month holding period: (i) affiliated shareholders (or shareholders whose shares are aggregated) may, under certain circumstances, sell within any three month period a number of securities which does not exceed the greater of 1% of the then outstanding shares of common stock and (ii) non-affiliated shareholders may sell without such limitations, provided we are current in our public reporting obligations. Rule 144 also permits the sale of securities by non-affiliates that have satisfied a one year holding period without any limitation or restriction. Any substantial sale of our common stock pursuant to Rule 144 or pursuant to any resale prospectus may have a material adverse effect on the market price of our securities.
| Item 1B. | Unresolved Staff Comments |
Not applicable.
| Item 2. | Description of Property |
We currently rent office space at 1 Meridian Road, Eatontown, NJ 07724 for our corporate offices. Additionally we rent laboratory space at the Burlington County College Science Incubator 100 Technology Way, Mount Laurel NJ 08054. Our laboratory facility consists of approximately 1300 square feet of leased space located in the science incubator on the campus of the Burlington Community College located in Mount Laurel, New Jersey.
| Item 3. | Legal Proceedings |
We are currently not involved in any litigation that we believe could have a material adverse effect on our financial condition or results of operations. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of the executive officers of our Company or any of our subsidiaries, threatened against or affecting our company, our common stock, any of our subsidiaries or of our companies or our subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect upon the Company.
| Item 4. | Mine Safety Disclosures |
Not applicable.
| 24 |
| Item 5. | Market for Common Equity and Related Stockholder Matters |
(a) Market Information
Our common stock was listed on the OTCQB under the symbol “RAPP” on November 12, 2010. In June 2011, we changed our name to American CryoStem Corporation and since June 15, 2011, our common stock has traded under the stock symbol “CRYO” on the OTCQB. The following table shows the reported high and low closing prices per share for our common stock for each quarterly period as noted. The over-the-counter market quotations set forth for our common stock reflect interdealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| 2012 Fiscal Year | ||||||||
| Quarter ended | High | Low | ||||||
| December 31, 2011 | $ | 1.75 | $ | 0.50 | ||||
| March 31, 2012 | $ | 1.35 | $ | 0.20 | ||||
| June 30, 2012 | $ | 0.95 | $ | 0.15 | ||||
| September 30, 2012 | $ | 0.58 | $ | 0.12 | ||||
| 2013 Fiscal Year | ||||||||
| Quarter ended | High | Low | ||||||
| December 31, 2012 | $ | 0.65 | $ | 0.13 | ||||
| March 31, 2013 | $ | 0.55 | $ | 0.25 | ||||
| June 30, 2013 | $ | 0.51 | $ | 0.30 | ||||
| September 30, 2013 | $ | 0.46 | $ | 0.25 | ||||
(b) Holders of Common Equity
As of September 30, 2013, there were approximately 128 holders of record of our common stock. This figure does not take into account those shareholders whose certificates are held in the name of broker-dealers or other nominees.
(c) Dividend Information
We have never paid any cash dividends on our common shares, and we do not anticipate that we will pay any dividends with respect to those securities in the foreseeable future. Our current business plan is to retain any future earnings to finance the expansion development of our business.
(d) Sales of Unregistered Securities
During the fiscal year ended September 30, 2013, we issued 3,417,359 common shares in connection with the conversion by the holders of $1,196,075 principal amount of 8% unsecured Convertible Notes.
(e) Securities Authorized For Issuance Under Equity Compensation Plans
During the fiscal year ended September 30, 2013, we issued 50,000 common shares to an advisory board member in connection with the exercise of 50,000 options issued at an exercise price of $0.15 under the 2011 American CryoStem Corporation Stock Option Plan. The Company accepted a $7,500 balance due to the option holder in payment for the Option exercise.
| 25 |
The Company applies ASC 718, “Accounting for Stock-Based Compensation” to account for its option issues. Accordingly, all options granted are recorded at fair value using a generally accepted option pricing model at the date of the grant. For purposes of determining the option value at issuance, the fair value of each option granted is measured at the date of the grant by the option pricing model with the following assumptions:
| FY 2013 | FY 2012 | |||||||
| Dividend yield | 0.00 | % | 0.00 | % | ||||
| Risk free interest rate | 0.25 | % | 0.50 | % | ||||
| Volatility | 16.60 | % | 68.04 | % | ||||
The fair values generated by option pricing model may not be indicative of the future values, if any, that may be received by the option holder.
Transfer Agent
Our transfer agent is Olde Monmouth Stock Transfer Co., Inc. located at 200 Memorial Parkway, Atlantic Highlands, New Jersey 07716. Its contact phone is 732-872-2727.
| Item 6. | Selected Financial Data |
Not applicable.
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Forward Looking Statements
This annual report on Form 10-K and other reports filed by American CryoStem Corporation (the “Company”) from time to time with the U.S. Securities and Exchange Commission (the “SEC”) contain or may contain forward-looking statements and information that are based upon beliefs of, and information currently available to, the Company’s management as well as estimates and assumptions made by Company’s management. We and our representatives may from time to time make written or oral statements that are “forward-looking,” including statements contained in this annual report and other filings with the Securities and Exchange Commission (the “SEC”), reports to our stockholders and news releases. All statements that express expectations, estimates, forecasts or projections are forward-looking statements. In addition, other written or oral statements which constitute forward-looking statements may be made by us or on our behalf. Words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “seek,” “estimate,” “project,” “forecast,” “may,” “should,” variations of such words and similar expressions are intended to identify such forward-looking statements. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions which are difficult to predict. Therefore, actual outcomes and results may differ materially from what is expressed or forecasted in or suggested by such forward-looking statements. We undertake no obligation to update or revise any of the forward-looking statements after the date of this annual report to conform forward-looking statements to actual results. Important factors on which such statements are based are assumptions concerning uncertainties, including but not limited to, uncertainties associated with the following:
| ● | Inadequate capital and barriers to raising the additional capital or to obtaining the financing needed to implement our business plan; | |
| ● | Our failure to earn revenues or profits; | |
| ● | Inadequate capital to continue business; | |
| ● | Volatility or decline of our stock price; | |
| ● | Potential fluctuation in quarterly results; | |
| ● | Rapid and significant changes in markets; | |
| ● | Litigation with or legal claims and allegations by outside parties; and | |
| ● | Insufficient revenues to cover operating costs. |
The following discussion should be read in conjunction with the financial statements and the notes thereto which are included in this annual report. This discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ substantially from those anticipated in any forward-looking statements included in this discussion as a result of various factors. Readers are cautioned not to place undue reliance on these forward-looking statements, which are only predictions and speak only as of the date hereof. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee future results, levels of activity, performance, or achievements.
| 26 |
Background
We were incorporated in the State of Nevada on March 13, 2009. On April 20, 2011, we acquired, through our wholly owned subsidiary American CryoStem Acquisition Corporation, substantially all of the assets from, and assumed substantially all of the liabilities of, ACS Global, Inc. (“ACS”) in exchange for our issuance of 21,000,000 shares of our common stock, par value $0.001 per share, to ACS (the “Asset Purchase”). We filed a Current Report on Form 8-K with the Securities and Exchange Commission on April 27, 2011 disclosing the Asset Purchase and certain related matters including, but not limited to, the appointment of our present officers and directors as well as the resignation by the former chief executive officer and sole director. Our fiscal year ends September 30 of each calendar year.
Overview
American CryoStem Corporation, which we refer to as “we,” “us,” “our” and “our Company,” is a developer, marketer and global licensor of patented adipose tissue-based cellular technologies and related proprietary services with a focus on clinical processing, commercial bio-banking and application development for adipose (fat) tissue and autologous adipose-derived regenerative cells (ADRCs). We maintain a strategic portfolio of intellectual property and patent applications that form our Adipose Tissue Processing Platform, which supports and promotes a growing pipeline of biologic products and processes, clinical services and international licensing opportunities. Through our ACS Laboratories division, we operate an FDA registered, cGMP compliant human tissue processing, cryostorage and cell culture and differentiation media development facility in Mount Laurel, New Jersey at the Burlington County College Science Incubator.
Our growth strategy is centered on expanding our research and development through scientific collaborations and developing revenue through the sale and licensing of our patented products and services to fully capitalize on: (1) adipose tissue and adipose derived stem cell (ADSC) technologies (2) scientific breakthroughs incorporating ADSCs that have been rapidly shaping the fast growing Regenerative and Personalized Medicine industries; (3) providing these growth industries with a standardized cell processing platform and, (4) enhancing the delivery of healthcare through cellular-based therapies and applications which address disease treatment, wound and burn healing, joint repair and management, and personalized health and beauty care, and (5) building a network of physicians for the delivery of our products and services.
We intend to pursue opportunities to generate revenue from the development of our intellectual property. This intellectual property includes processing and testing methods developed in our laboratories that may be licensed to researchers and other companies currently researching and developing cellular therapies and regenerative medicine products. Management believes that as the adipose derived stem cell business continues to grow there is an opportunity to assist in the development of our products and services through sale of our developed products, licensing of our processing and testing methods and potential collaborative development opportunities. Although our management intends to pursue these new lines of business, there can be no assurance that we will be able to generate revenue from these sources.
Through our ACS Laboratories division, our Company operates its flagship FDA registered, cGMP human tissue processing, cryostorage and cell culture and differentiation media development facility in Mount Laurel, New Jersey. On a mission to fulfill the pressing need to set a global gold standard for end-to-end clinical collection, processing, tracking and storage, American CryoStem has spent nearly six years designing and constructing the necessary clinical framework capable of replicating its protocols in markets around the world.
The Company has developed a number of products and services for the adipose tissue and adipose derived stem cell market. These products and services currently include:
| ● | CELLECT® | ● | Tissue Collection methodology designed for physicians to facilitate the collection and overnight shipping of an individual’s adipose tissue to our FDA registered laboratory; |
| 27 |
| ● | ATGRAFT™ | ● | Tissue processing at our Laboratory of a customer’s adipose tissue and its preparation for long term storage in different configuration sizes allowing future retrieval for tissue grafting procedures or Regenerative Medicine applications |
| ● | ATCELL™ | ● | Clinical Processing to separate the component parts (cells) of an individual’s adipose tissue removing the adipocytes and red blood cells thereby creating the ATCELL™ stem cell lines for storage, expansion, or differentiation |
| ● | Clinical and Research grade donor ATCELL™ lines for use with collaborative partners in cellular therapy research and application development and optimization, cell morphology and characterization assays, and growth analysis. | ||
| ● | ACS Laboratories™ | ● | Manufacturing and sale of our patented ACSelerate-SFM™ and ACSelerate-LSM™ cell culture media products |
| ● | Creation and sale of research grade ATCELL™ | ||
| ● | Participation and support of all collaborative research projects | ||
| ● | Contract manufacturing, including Autokine-CM® | ||
| ● | Provide testing services for physicians performing in-office procedures and tissue processing |
| ● | International Licensing™ | ● | Standard Operating Procedures (SOPs) and all associated components and products |
| ● | Consulting and Marketing Review and Assessment | ||
| ● | CELLECT® | ||
| ● | ATGRAFT™ | ||
| ● | ATCELL™ | ||
| ● | Adipose tissue processing, cellular expansion and product manufacture |
On December 1, 2013, the Company executed two additional agreements (1) the Cooperative Research Agreement and (2) the Research Evaluation and License Option Agreement, with Rutgers University for further collaboration and intellectual property development with Dr. Kibum Lee. The Cooperative Research Agreement calls for the Company to provide to Dr. Lee’s laboratory and staff with additional materials to continue their research utilizing the Company’s ATCELL™ and ACSelerate™ products. The Agreement also provides for the Company to have exclusive access to certain identified Rutgers intellectual property and for the joint ownership of any additional intellectual property developed. The Research Evaluation and License Option Agreement provides a platform for the Company to be the exclusive developer and licensor for the commercial development of any new intellectual property and patent rights. The Company will also be managing all patent application and prosecution for any technologies developed under the Agreements.
On October 18, 2013, the Company formed Autogenesis Corporation (“Autogenesis”) as part of its collaborative agreement to develop wound healing products and other cellular therapies with privately-held Protein Genomics (PGen). The Company is jointly owned by American CryoStem and Protein Genomics. Autogenesis will be separately funded and will serve as the dedicated business unit focused on continuing and accelerating the research and development of innovative new products and biotechnologies that combine American CryoStem’s ATCELL™ (adipose derived regenerative cells) and ACSelerate™ cell media culture products with PGen’s Elastatropin® human-based protein materials.
In July 2013, our Company received approvals from the Institutional Review Board (IRB) of the International Cell Surgical Society for the Company’s processing protocols for isolating Stromal Vascular Fraction (SVF) or ADSCs from a patient’s adipose tissue; and for culturing stem cells from adipose tissue (ATCELL™). These protocols provide validated testing methods necessary to move the clinical investigative process towards uniform disease treatments’ and provide the collection of cGMP processing and outcome data required by prevailing FDA regulations and guidance for approval of regenerative cellular therapies.
| 28 |
On April 5, 2012 the Company entered into a Collaboration Agreement with Protein Genomics, Inc (PGen) to test and develop new products combining certain intellectual property and patented products. Initially the Company provided PGEN with research materials and its patented cell culture media for testing with PGen’s proprietary patented products designed for the wound healing market. Initial testing has been completed and on September 1, 2012 the Company and PGen entered into a Memorandum of Understanding (MOU) to further develop products based upon the results of the initial collaboration. The terms of the MOU call for the creation of a new entity to be jointly owned by the Company and PGen for the joint development and ownership of any jointly developed intellectual property and to provide a separate vehicle to fund the additional scientific work. Protein Genomics, Inc is a private Company under the control of Burt Ensley, PhD, a member of the Company’s Medical and Scientific Advisory Board.
In September of 2011 we completed the required testing, validation and verification of our core processing methodology and laboratory equipment. The laboratory is located in the science incubator on the Campus of the Burlington Community College located in Mount Laurel, NJ.
On August 2, 2011, we were awarded US Patent No. US 7,989,205 B2, titled “Cell Culture Media, Kits, and Methods of Use”. This Patent was assigned to us by our Chief Scientist, Dr. David Moscatello in 2010 and was originally filed on October 4, 2006. We have continued to develop tools and cellular processing methods that have the potential to result in new products and services being offered for commercial sale and licensing at a future date. Our management cannot predict if a market for these products will develop and therefore cannot predict the potential impact these products will have upon our revenue.
American CryoStem is also focused on securing licensing arrangements with qualified partners around the world to institute and operate its turnkey clinical laboratories that are properly equipped for processing and storing adipose tissue and ADSCs for use in Regenerative and Personalized Medicine applications.
Cash Requirements
We will require additional capital to fund marketing, operational expansion, processing staff training, as well as for working capital. We are attempting to raise sufficient funds than would enable us to satisfy our cash requirements for a period of the next 12 to 24 months. We have minimal long term debt and have been able to meet our past financial obligations. In order to finance further market development with the associated expansion of operational capabilities for the time period discussed above, we will need to raise additional working capital. However, we cannot assure you we can attract sufficient capital to enable us to fully fund our anticipated cash requirements during this period. In addition, we cannot assure you that the requisite financing, whether over the short or long term, will be raised within the necessary time frame or on terms acceptable to us, if at all. Should we be unable to raise sufficient funds we may be required to curtail our operating plans if not cease them entirely. As a result, we cannot assure you that we will be able to operate profitably on a consistent basis, or at all, in the future.
In order to move our Company through its next critical growth phase of development and commercialization and to ensure we are in position to support our research collaborations and market penetration strategies, in September 2013 we engaged a new investment banker and financial advisor. With a goal of raising capital in early to mid-2014, we have been working in close concert with the corporate finance team to determine our tactical approach to the equity markets, with particular emphasis on identifying the best deal structure to attract and retain meaningful capital sponsorship from both the retail and institutional investing communities, while limiting dilution to our current shareholders.
We expended $392,420 during the fiscal year ended September 30, 2013 in professional fees (principally legal, consulting and accounting). In addition, we expended $257,905 for Research and Development during the 12-month period ended September 30, 2013 primarily on the continuing development and optimization of the Company’s patented cell culture medium products and the development of the new intellectual property, adipose tissue processing and storage services and materials.
Going Concern
As of the date of this annual report, there is substantial doubt regarding our ability to continue as a going concern as we have not generated sufficient cash flow to fund our proposed stem cell business.
We have suffered recurring losses from operations since our inception. In addition, we have yet to generate an internal cash flow from our business operations or successfully raised the financing required to expand our business. As a result of these and other factors, our independent auditor has expressed substantial doubt about our ability to continue as a going concern. Our future success and viability, therefore, are dependent upon our ability to generate capital financing. The failure to generate sufficient revenues or raise additional capital may have a material and adverse effect upon us and our shareholders.
| 29 |
Our plans with regard to these matters encompass the following actions: (i) obtaining funding from new investors to alleviate our working capital deficiency, and (ii) implementing a plan to generate sales of our proposed products. Our continued existence is dependent upon our ability to resolve our liquidity problems and achieve profitability in our current business operations. However, the outcome of management’s plans cannot be ascertained with any degree of certainty. Our financial statements do not include any adjustments that might result from the outcome of these risks and uncertainties.
Liquidity and Capital Resources
As of the fiscal year ended September 30, 2013, the Company had a cash balance of $115,932 and accounts receivable of $2,003. Our principal source of funds has been sales of our securities. Should we be unable to raise sufficient funds, we will be required to curtail our operating plans if not cease them entirely. We cannot assure you that we will generate the necessary funding to operate or develop our business. Please see “Cash Requirements” above for our existing plans with respect to raising the capital we believe will be required. In the event that we are able to obtain the necessary financing to move forward with our business plan, we expect that our expenses will increase significantly as we attempt to grow our business. Accordingly, estimates for future financing required may not be accurate and must be considered in light these circumstances.
Commitments
As of the date of this annual report, our material capital commitments were (I) the continued funding of the expansion of our marketing efforts and laboratory processing capabilities, (ii) an equipment lease in the amount of $31,140 for laboratory equipment with monthly payments of $1,869.74 and the final payment due March 2015, and (iii) the current lease for the laboratory spaces at the Burlington County College Science Incubator, Laboratory 110 and 108.
In connection with the closing of the April 2011, Asset Purchase we assumed (i) an unsecured note payable in the face amount of $65,000 with interest payable upon maturity of 6%, the note was exchanged at maturity with the Company’s 8% Convertible note. (ii) unsecured liabilities without interest of $133,812 due to ACS Global, the majority shareholder of the Company, for certain prepaid expenses made by ACS Global prior to the closing of the transaction. there is no due date associated with this liability. During fiscal 2013 the Company has paid certain expenses on behalf of ACS Global, Inc and the balance owed to the Company by ACS Global is $139,447 as of the date of this report.
During fiscal year 2013, the Company issued a principal amount of $1,379,875 of 8% Convertible Notes due September 30, 2014 and received proceeds of $1,379,875. The notes are convertible into restricted shares of the Company’s common stock at any time until maturity by the holder at $0.35 per share. The Company may also prepay the notes at any time upon at least 30 days written notice to the holder(s) either in whole or in part. Upon any prepayment by the Company of the convertible notes the Company shall issue to the holder a warrant to purchase 250 shares of common stock for each $1,000 of principal prepaid. Each warrant issued upon prepayment shall have an exercise price of $0.35 per share of common stock and shall be exercisable for a period of two years from the date of the prepayment. Certain purchasers of the convertible notes elected to convert a principal amount of $1,200,043 resulting in the issuance of 3,467,359 restricted shares of the Company’s common stock. The convertible notes have an interest rate of 8%, are convertible in to the Company’s common stock at a conversion price of $0.35 per share and mature in September 2014. Of the convertible notes issued, $1,196,075 of the notes was converted. The Company recorded $259,225 in interest expense as a beneficial conversion feature of the convertible notes in its statement of operations in fiscal year 2013. The Company had $190,800 in principal amount of the notes outstanding as of September 30, 2013.
We anticipate that any further capital commitments that may be incurred will be financed principally through the issuance of our securities. However, we cannot assure you that additional financing will be available to us on a timely basis, on acceptable terms, or at all.
| 30 |
Off Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Results of Operations
Comparison of the Twelve Months Ended September 30, 2013 to the Year Ended September 30, 2013:
Revenue. Our total revenue was $11,529 for the 12 months ended September 30, 2013, compared to $21,364 for the same period ended September 30, 2012.
Selling, General and Administrative Expenses. Selling, general and administrative expenses (“SG&A”) for the 12 months ended September 30, 2013 were $1,723,494, as compared to SG&A of $1,014,202 for the 12 months ended September 30, 2012.
Net Income (Loss). Our net loss for the 12 months ended September 30, 2013 was $(2,399,744), which includes a non-cash loss of $676,250 recorded for the issuance of options during the period, compared to a net Loss of $(2,364,259) for the year ended September 30, 2012.
Liquidity and Capital Resources
Liquidity and Financial Position
As shown in the accompanying financial statements, we incurred a net loss of $2,399,744 for the year ended September 30, 2013 which includes a non-cash loss of $676,250 recorded for the issuance of options during the period as compared to net loss of $2,374,410 for the previously fiscal period ended September 30, 2012. At the year ended September 30, 2013 our current assets were $117,935 and our total assets were $569,032 and our total liabilities were $621,516. Our liabilities exceeded its assets by $52,484.
Outlook To date we have worked with minimal capital and we remain in the early stages of marketing our services. We intend to accelerate our marketing strategies and expand our marketing efforts subject to available capital and the success of its sales strategies. We have also identified several additional products and services that are complimentary to the current services associated with collecting, processing and storage of adipose tissue and adipose derived stem cells, and cell culture media and cellular products.
We are also aggressively pursuing additional marketing opportunities in our target markets to further expand the delivery of our services to clients. These additional marketing programs are designed to provide clients interested in taking advantage of our services and the burgeoning cellular therapy and regenerative medicine market.
We had a cash balance of $115,932 as of the date of this annual report. Our principal source of funds has been sales of our securities.
Should we be unable to raise sufficient funds, we will be required to curtail our operating plans if not cease them entirely. We cannot assure you that we will generate the necessary funding to operate or develop our business. Please see “Cash Requirements” above for our existing plans with respect to raising the capital we believe will be required.
In the event that we are able to obtain the necessary financing to move forward with our business plan, we expect that our expenses will increase significantly as we attempt to grow our business. Accordingly, estimates for future financing required may not be accurate and must be considered in light these circumstances.
Application of Critical Accounting Policies
Our financial statements and accompanying notes are prepared in accordance with generally accepted accounting principles in the United States. Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. These estimates and assumptions are affected by management’s application of accounting policies. Critical accounting policies include revenue recognition, impairment of marketing rights and accounting for legal contingencies.
| 31 |
We recognize revenue in accordance with Staff Accounting Bulletin No.101, “Revenue Recognition in Financial Statements.” Sales are recorded when products are shipped to customers. Provisions for discounts and rebates to customers, estimated returns and allowances and other adjustments are provided for in the same period the related sales are recorded.
We evaluate our long-lived assets for financial impairment on a regular basis in accordance with Statement of Financial Accounting Standards No. 121, “Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of” evaluates the recoverability of long-lived assets not held for sale by measuring the carrying amount of the assets against the estimated discounted future cash flows associated with them. At the time such evaluations indicate that the future discounted cash flows of certain long-lived assets are not sufficient to recover the carrying value of such assets, the assets are adjusted to their fair values.
New Accounting Pronouncements
In July 2012, the FASB issued changes to the testing of indefinite-lived intangible assets for impairment, similar to the goodwill changes issued in September 2011. These changes provide an entity the option to first assess qualitative factors to determine whether the existence of events or circumstances leads to a determination that it is more likely than not (more than 50%) that the fair value of an indefinite-lived intangible asset is less than its carrying amount. Such qualitative factors may include the following: macroeconomic conditions; industry and market considerations; cost factors; overall financial performance; and other relevant entity-specific events. If an entity elects to perform a qualitative assessment and determines that an impairment is more likely than not, the entity is then required to perform the existing two-step quantitative impairment test, otherwise no further analysis is required. An entity also may elect not to perform the qualitative assessment and, instead, proceed directly to the two-step quantitative impairment test. These changes become effective for any indefinite-lived intangible asset impairment test performed on January 1, 2013 or later, although early adoption is permitted. Upon adoption of these changes, management plans to proceed directly to the two-step quantitative test for indefinite-lived intangible assets. As these changes should not affect the outcome of the impairment analysis of an indefinite-lived intangible asset, management has determined these changes will not have a material impact on the financial statements.
Critical Accounting Policies
We prepare financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”), which requires us to make estimates and assumptions that affect the amounts reported in our combined and consolidated financial statements and related notes. We periodically evaluate these estimates and assumptions based on the most recently available information, our own historical experience and various other assumptions that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Since the use of estimates is an integral component of the financial reporting process, actual results could differ from those estimates. Some of our accounting policies require higher degrees of judgment than others in their application. We believe the following accounting policies involve the most significant judgments and estimates used in the preparation of our financial statements.
Basis of Presentation
Our financial statements are presented on the accrual basis of accounting in accordance with generally accepted accounting principles in the United State of America, whereby revenues are recognized in the period earned and expenses when incurred.
Management’s Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Long-Lived Assets
We review and evaluate our long-lived assets for impairment whenever events or changes in circumstances indicate that their net book value may not be recoverable. When such factors and circumstances exist, we compare the assets’ carrying amounts against the estimated undiscounted cash flows to be generated by those assets over their estimated useful lives. If the carrying amounts are greater than the undiscounted cash flows, the fair values of those assets are estimated by discounting the projected cash flows. Any excess of the carrying amounts over the fair values are recorded as impairments in that fiscal period.
| 32 |
Statement of Cash Flows
For purposes of the statement of cash flows, we consider all highly liquid investments (i.e., investments which, when purchased, have original maturities of three months or less) to be cash equivalents.
Fair Value of Financial Instruments
Our financial instruments consist of cash and cash equivalents. The fair value of cash and cash equivalents approximates the recorded amounts because of the liquidity and short-term nature of these items.
Recent Accounting Pronouncements
We have reviewed all recently issued, but not yet effective, accounting pronouncements and do not believe that any future adoption of such pronouncements will have a material impact on our financial condition or the results of our operations.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk |
This item is not applicable because we are a smaller reporting company.
| 33 |
| Item 8. | Financial Statements and Supplementary Data |
Audited Financial Statements and Schedules
September 30, 2012 and September 30, 2013
American CryoStem Corporation
Index to Financial Statements
| F-1 |
Report of Independent Registered Public Accounting Firm
The Shareholders,
American CryoStem Corporation
We have audited the accompanying balance sheets of American CryoStem Corporation as of September 30, 2013 and 2012, and the related statements of operations, stockholders’ equity, and cash flows for each of the two years in the period ended September 30, 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of American CryoStem Corporation as of September 30, 2013 and 2012, and the results of its operations and its cash flows for each of the two years in the period ended September 30, 2013 in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses and negative cash flows from operations that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also discussed in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Donahue Associates LLC
Monmouth Beach, New Jersey
December 10, 2013
| F-2 |
American CryoStem Corporation
(Audited)
| For the Fiscal Year Ended September 30, | ||||||||
| 2013 | 2012 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 115,932 | $ | 4,039 | ||||
| Accounts receivable | 2,003 | 0 | ||||||
| Total current assets | 117,935 | 4,039 | ||||||
| Other assets: | ||||||||
| Other deposit | 0 | 5,000 | ||||||
| Security deposit | 5,800 | 5,800 | ||||||
| Patent | 163,935 | 126,273 | ||||||
| Equipment- net | 281,362 | 318,587 | ||||||
| Total assets | $ | 569,032 | $ | 459,699 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable & accrued expenses | $ | 260,129 | $ | 222,209 | ||||
| Capital lease payable | 20,239 | 18,321 | ||||||
| Total current liabilities | $ | 280,368 | $ | 240,530 | ||||
| Note payable to shareholder | 0 | 72,475 | ||||||
| Convertible notes payable | 190,800 | 0 | ||||||
| Capital lease payable | 10,901 | 31,140 | ||||||
| Payable to shareholder | 139,447 | 139,812 | ||||||
| Shareholders’ equity: | ||||||||
| Common stock- $.001 par value, authorized 300,000,000 shares authorized, issued and outstanding, 28,158,362 shares at September 30, 2012 and 32,285,721 at September 30, 2013 | $ | 32,286 | $ | 28,159 | ||||
| Additional paid in capital | 5,990,623 | 3,623,232 | ||||||
| Accumulated deficit | -6,075,393 | -3,675,649 | ||||||
| Total shareholders’ deficit | -52,484 | -24,258 | ||||||
| Total Liabilities & Shareholders’ Deficit | $ | 569,032 | $ | 459,699 | ||||
See the accompanying notes to the financial statements.
| F-3 |
American CryoStem Corporation
CONSOLIDATED statements of operations
(Audited)
| For the Fiscal Year Ended September 30, | ||||||||
| 2013 | 2012 | |||||||
| Net sales revenue | $ | 11,529 | $ | 21,364 | ||||
| Cost of sales | (5,118.00 | ) | (658.00 | ) | ||||
| Gross margin on sales | $ | 6,411 | $ | 20,706 | ||||
| General and administrative expenses: | ||||||||
| Professional fees | 392,420.00 | 326,188.00 | ||||||
| Research & development | 257,905.00 | 304,273.00 | ||||||
| Administration | 1,477,608.00 | 1,743,949.00 | ||||||
| Total general & administrative expenses | 2,127,933.00 | 2,374,410.00 | ||||||
| Net loss from operations | $ | (2,121,522 | ) | $ | (2,353,704 | ) | ||
| Other income (expenses): | ||||||||
| Interest income | 0.00 | 2.00 | ||||||
| Interest expense | (278,222.00 | ) | (10,557.00 | ) | ||||
| Net loss before provision for income taxes | (2,399,744.00 | ) | (2,364,259.00 | ) | ||||
| Provision for income taxes | 0.00 | 0.00 | ||||||
| Net loss | $ | (2,399,744 | ) | $ | (2,364,259 | ) | ||
| Basic & fully diluted net loss per common share: | ||||||||
| Net loss | $ | (0.08 | ) | $ | (0.09 | ) | ||
| Weighted average of common shares outstanding: | ||||||||
| Basic & fully diluted | 29,472,803.00 | 27,383,587.00 | ||||||
See the accompanying notes to financial statements.
| F-4 |
American CryoStem Corporation
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Audited)
| For the Fiscal Year Ended September 30, | ||||||||
| 2013 | 2012 | |||||||
| Operating Activities: | ||||||||
| Net loss | $ | (2,399,744 | ) | $ | (2,364,259 | ) | ||
| Adjustments to reconcile net income items not requiring the use of cash: | ||||||||
| Labor & salaries | 912,250 | 1,397,735 | ||||||
| Interest expense | 259,225 | 3,900 | ||||||
| Depreciation & amortization expense | 42,539 | 35,271 | ||||||
| Changes in other operating assets and liabilities: | ||||||||
| Accounts receivable | (2,003 | ) | 18,062 | |||||
| Other deposit | 5,000 | (5,000 | ) | |||||
| Accounts payable and accrued expenses | 37,920 | 111,944 | ||||||
| Net cash used by operations | $ | (1,144,813 | ) | $ | (802,347 | ) | ||
| Investing activities: | ||||||||
| Patents & trademarks | $ | (41,977 | ) | $ | (27,360 | ) | ||
| Security deposits | — | (2,500 | ) | |||||
| Purchase of equipment | (999 | ) | (20,180 | ) | ||||
| Net cash used by investing activities | (42,976 | ) | (50,040 | ) | ||||
| Financing activities: | ||||||||
| Convertible notes converted to common stock | $ | 1,200,043 | $ | 779,000 | ||||
| Payment of capital lease | (18,321 | ) | (34,904 | ) | ||||
| Issuance of convertible notes | 118,325 | — | ||||||
| Payable to shareholder | (365 | ) | 5,000 | |||||
| Net cash provided by financing activities | 1,299,682 | 749,096 | ||||||
| Net increase (decrease) in cash | $ | 111,893 | $ | (103,291 | ) | |||
| Cash balance at September 30 | $ | 115,932 | $ | 4,039 | ||||
| Supplemental disclosures of cash flow information: | ||||||||
| Interest paid during the fiscal year | $ | 4,119 | $ | 6,657 | ||||
| Income taxes paid during the fiscal year | $ | — | $ | — | ||||
See the accompanying notes to financial statements.
| F-5 |
American CryoStem Corporation
Statements of Changes in Shareholders’ Equity
For the Years Ended September 30, 2013 and 2012
| Common | Par | Paid in | Retained | Total | ||||||||||||||||
| Shares | Value | Capital | Deficit | Deficit | ||||||||||||||||
| Balance at September 30, 2011 | 26,475,362 | $ | 26,476 | $ | 1,448,180 | $ | (1,311,390 | ) | $ | 163,266 | ||||||||||
| Issuance of common stock | 1,658,000 | 1,658 | 777,442 | 779,100 | ||||||||||||||||
| Shares issued to pay invoice | 25,000 | 25 | 12,475 | 12,500 | ||||||||||||||||
| Issuance of options | 1,385,135 | 1,385,135 | ||||||||||||||||||
| Net loss | (2,364,259 | ) | (2,364,259 | ) | ||||||||||||||||
| Balance at September 30, 2012 | 28,158,362 | $ | 28,159 | $ | 3,623,232 | $ | (3,675,649 | ) | $ | (24,258 | ) | |||||||||
| Convertible notes converted to common stock | 3,467,359 | 3,467 | 1,196,576 | 1,200,043 | ||||||||||||||||
| Shares issued to consultants | 660,000 | 660 | 235,340 | 236,000 | ||||||||||||||||
| Issuance of options | 676,250 | 676,250 | ||||||||||||||||||
| Issuance of convertible notes | 259,225 | 259,225 | ||||||||||||||||||
| Net loss | (2,399,744 | ) | (2,399,744 | ) | ||||||||||||||||
| Balance at September 30, 2013 | 32,285,721 | $ | 32,286 | $ | 5,990,623 | $ | (6,075,393 | ) | $ | (52,484 | ) | |||||||||
See the accompanying notes to financial statements.
| F-6 |
Note 1. Organization of the Company and Significant Accounting Policies
American CryoStem Corporation (the “Company”) is a publicly held corporation formed on March 13, 2009 in the state of Nevada as R&A Productions Inc. (R&A).
In April 2011, R&A purchased substantially all the assets and liabilities of American CryoStem Corporation (ACS) for 21 million shares of common stock. ACS was deemed to be the accounting acquirer. At that time, the former operations of R&A were discontinued and the name of the Company was changed to American CryoStem Corporation.
The Company is in the business of collecting adipose tissue, processing it to separate the adult stem cells, and preparing such stem cells for long-term storage. The process allows individuals to preserve their stem cells for future personal use in cellular therapy. The adipose derived stem cells are prepared and stored in their raw form without manipulation, bio-generation or the addition of biomarkers or other materials, making them suitable for use in cellular treatments and therapies offered by existing and planned treatment centers worldwide. Individualized collection and storage of adult stem cells provides personalized medicine solutions by making the patient’s own preserved stem cells available for future cellular therapies.
Use of Estimates - The preparation of the financial statements in conformity with United States generally accepted accounting principles (“GAAP”) uniformly applied requires management to make reasonable estimates and assumptions that affect the reported amounts of the assets and liabilities and disclosure of contingent assets and liabilities and the reported amounts of revenues and expenses at the date of the financial statements and for the period they include. Actual results may differ from these estimates.
Cash and interest bearing deposits - For the purpose of calculating changes in cash flows, cash includes all cash balances and highly liquid short-term investments with an original maturity of three months or less.
Revenue Recognition – The Company recognizes revenue from the processing of adipose tissue into usable stem cells once all the procedures have been performed and the client sample has been stored in the Company’ cryogenic storage tank. Storage revenues for stored client samples are recognized on an annual basis on the anniversary date of the storage.
Long Lived Assets - The Company reviews for the impairment of long-lived assets whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. An impairment loss would be recognized when estimated future cash flows expected to result from the use of the asset and its eventual disposition is less than its carrying amount.
Fixed Assets – Fixed assets are stated at cost. Depreciation expense is computed using the straight-line method over the estimated useful life of the assets, which is estimated as follows:
| Office equipment | 5 Years | |
| Lab equipment & furniture | 7 Years | |
| Lab software | 5 Years |
Income taxes - The Company accounts for income taxes in accordance with generally accepted accounting principles which require an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually for differences between financial statement and income tax bases of assets and liabilities that will result in taxable income or deductible expenses in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets and liabilities to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period adjusted for the change during the period in deferred tax assets and liabilities.
The Company follows the accounting requirements associated with uncertainty in income taxes using the provisions of Financial Accounting Standards Board (FASB) ASC 740, Income Taxes. Using that guidance, tax positions initially need to be recognized in the financial statements when it is more likely than not the positions will be sustained upon examination by the tax authorities. It also provides guidance for derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. As of September 30, 2013 and September 30, 2012, the Company has no uncertain tax positions that qualify for either recognition or disclosure in the financial statements. All tax returns from fiscal years 2009 to 2012 are subject to IRS audit.
| F-7 |
Recently Issued Accounting Pronouncements
There are no recently issued accounting pronouncements that have a material impact on the Company’s financial statements.
Note 2. Going Concern
The accompanying financial statements have been presented in accordance with GAAP, which assumes the continuity of the Company as a going concern. However, the Company has incurred significant losses since its inception and has no material revenues to date and continues to rely on financing and the issuance of shares to raise capital to fund its business operations. Management’s plans with regard to this matter are as follows:
On August 26, 2013, the Company entered into an Agreement with an investment banker as the exclusive financial advisor and placement agent in connection with a private offering of the Company’s securities. The Company is completing the due diligence and expects to begin the offering in the first quarter of fiscal 2014.
The Company plans to continue to fund its operations through capital fundraising activities in 2014 until the new commercial facilities generate sufficient revenue to support its operations.
Note 3. Loss Per Share
The Company applies ASC 260, “Earnings per Share” to calculate loss per share. In accordance with ASC 260, basic net loss per share has been computed based on the weighted average of common shares outstanding during the years, adjusted for the financial instruments outstanding that are convertible into common stock during the years. The Company has 2,850,000 and 6,600,000 options outstanding for fiscal years 2012 and 2013, the effects of the options are not included in the calculation of loss per share since their inclusion would be anti-dilutive.
Net loss per share is computed as follows:
| September 30, 2013 | September 30, 2012 | |||||||
| Net loss | (2,399,744.00 | ) | (2,364,259.00 | ) | ||||
| Weighted average shares outstanding | 29,472,803.00 | 27,383,587.00 | ||||||
| Basic & fully diluted net loss per common share: | ||||||||
| Net gain (loss) | (0.08 | ) | (0.09 | ) | ||||
Note 4. Equipment
The equipment owned by the Company is comprised as follows:
| September 30, 2013 | September 30, 2012 | |||||||
| Office equipment | $ | 26,637 | $ | 26,638 | ||||
| Lab furniture | 642 | 642 | ||||||
| Office furniture | 999 | 0 | ||||||
| Lab equipment | 246,407 | 246,407 | ||||||
| Lab software | 123,000 | 123,000 | ||||||
| Accumulated depreciation | -116,323 | -78,100 | ||||||
| Equipment- net | $ | 281,362 | $ | 318,587 | ||||
Lab equipment includes $88,000 of leased equipment. Depreciation expense on this leased asset for 2012 was $12,571 and $12,521 for 2013.
| F-8 |
Note 5. Patents
On August 2, 2011, the Company was awarded U.S. Patent No. US 7,989,205 B2, titled Cell Culture Media, Kits, and Methods of Use. The Patent is for cell culture media kits for the support of primary culture of normal non-hematopoietic cells of mesodermal origin suitable for both research and clinical applications. The Company filed and maintains a continuation (U.S. Serial No. 13/194,900) with additional claims pending.
The Company has filed the following additional patents to extend its intellectual property to encompass additional aspects of the Company’s platform processing technologies:
| ● | A Business Method for Collection Cryogenic Storage and Distribution of a Biologic Sample Material PCT/US2011/39260 |
| ● | Systems and Methods for the Digestion of Adipose Tissue Samples Obtained from a Client for Cryopreservation U.S. Serial No. 13/646,647 filed October 5, 2012 |
| ● | Compositions and Methods for Collecting, Washing, Cryopreserving, Recovering and Return of Lipaspirates to Physician for Autologous Adipose Transfer Procedures PCT/US13/44621 filed June 6, 2013 |
| ● | Stem Cell-Based Therapeutic Devices and Methods U.S. Serial No. 61/773,112 Filed March 10, 2013 |
| ● | Autologous Serum for Transport of Isolated Stromal Vascular Fraction or Adipose Derived Stem Cells 61/810,970 Filed April 11, 2013 |
Note 6. Debt
During fiscal year 2013, the Company issued $1,379,875 of convertible notes. The convertible notes have an exercise price of $0.35 and mature in September 2014. Of the convertible notes issued, $1,196,076 of the notes was exercised. The Company recorded $259,225 in interest expense as a beneficial conversion feature of the convertible notes in its statement of operations in fiscal year 2013.
The following table describes the Company’s debt outstanding at September 30, 2013:
| Debt | Carrying Value | Maturity | Rate | |||||||
| Capital lease | $ | 31,140 | March 31, 2015 | 10.00 | % | |||||
| Convertible notes | $ | 190,800 | September 30, 2014 | 8.00 | % | |||||
| Due to shareholder | $ | 139,447 | Demand | 0.00 | % | |||||
Note 7. Administration Expense
Detail of administrative expenses in the statements of operations is as follows:
| F-9 |
| September 30, 2013 | September 30, 2012 | |||||||
| Advertising & promotion | $ | 20,026 | $ | 17,536 | ||||
| Automobile | 7,529 | 5,019 | ||||||
| Bank fees | 624 | 1,016 | ||||||
| Business meetings | 11,036 | 4,772 | ||||||
| Consulting | 199,402 | 113,473 | ||||||
| Depreciation & amortization | 42,539 | 35,271 | ||||||
| Dues & subscriptions | 5,112 | 1,663 | ||||||
| Insurance | 2,303 | 27,420 | ||||||
| Travel & meals | 48,744 | 21,608 | ||||||
| Administration | 51,568 | 49,126 | ||||||
| Labor & salaries | 1,031,879 | 1,412,635 | ||||||
| Rent | 30,000 | 40,686 | ||||||
| Postage | 15,384 | 4,960 | ||||||
| Telecommunications | 8,123 | 5,419 | ||||||
| Website maintenance | 3,339 | 3,345 | ||||||
| Total | $ | 1,477,608 | $ | 1,743,949 | ||||
Note 8. Common Stock Issuances
During fiscal year ended September 30, 2012, the Company issued 1,558,000 shares of common stock and received proceeds of $779,000. In addition, an option holder exercised 100,000 options and the Company received proceeds of $100.
During fiscal year ended September 30, 2012, the Company issued 25,000 shares of common stock to pay an invoice totaling $12,500.
During fiscal year ended September 30, 2013, the Company issued 3,467,359 shares of common stock as a result of the convertible notes exercised as discussed in Note 6.
During fiscal year ended September 30, 2013, an option holder exercised 50,000 options for common stock in exchange for the retirement of a payable to the option holder of $7,500.
During fiscal year ended September 30, 2013, the Company issued 660,000 shares of common stock to consultants for services rendered valued at $236,000.
Note 9. Option Issuances
During fiscal year ended September 30, 2012, the Company issued 3,000,000 options with an average exercise price of $0.14. The Company recorded an expense of $1,385,135 as a result of the issue.
During fiscal year ended September 30, 2013, the Company issued 3,750,000 options with an average exercise price of $0.18. The Company recorded an expense of $676,250 as a result of the issue.
The Company applies ASC 718, “Accounting for Stock-Based Compensation” to account for its option issues. Accordingly, all options granted are recorded at fair value using a generally accepted option pricing model at the date of the grant. For purposes of determining the option value at issuance, the fair value of each option granted is measured at the date of the grant by the option pricing model with the following assumptions:
| FY 2013 | FY 2012 | |||||||
| Dividend yield | 0.00 | % | 0.00 | % | ||||
| Risk free interest rate | 0.25 | % | 0.50 | % | ||||
| Volatility | 16.60 | % | 68.04 | % | ||||
The fair values generated by option pricing model may not be indicative of the future values, if any, that may be received by the option holder.
| F-10 |
The following is a summary of common stock options outstanding at September 30, 2012 and September 30, 2013:
| Wgtd Avg | Wgtd Years | |||||||||||
| Amount | Exercise Price | to Maturity | ||||||||||
| Outstanding at September 30, 2011 | 0 | |||||||||||
| Issues | 3,000,000 | |||||||||||
| Exercises | -100,000 | |||||||||||
| Expires | 0 | |||||||||||
| Outstanding at September 30, 2012 | 2,900,000 | $ | 0.14 | 4.58 | ||||||||
| Issues | 3,750,000 | $ | 0.18 | 4.16 | ||||||||
| Exercises | -50,000 | |||||||||||
| Expires | 0 | |||||||||||
| Outstanding at September 30, 2013 | 6,600,000 | $ | 0.18 | 4.16 | ||||||||
Note 10. Fair Values of Financial Instruments
Fair Value Measurements under generally accepted accounting principles clarifies the principle that fair value should be based on the assumptions market participants would use when pricing an asset or liability and establishes a fair value hierarchy that prioritizes the information used to develop those assumptions. Under the standard, fair value measurements are separately disclosed by level within the fair value hierarchy as follows:
| ● | Level 1 - Quoted prices in active markets for identical assets or liabilities. |
| ● | Level 2 - Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which all significant inputs are observable or can be derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities. |
| ● | Level 3 - Unobservable inputs to the valuation methodology that are significant to the measurement of fair value of assets or liabilities. |
To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, for disclosure purposes, the level in the fair value hierarchy within which the fair value measurement is disclosed and is determined based on the lowest level input that is significant to the fair value measurement.
Cash, accounts receivable, other deposit, security deposit, accounts payable and accrued expenses, capital lease payable, payable to shareholder, convertible notes payable, and note payable to shareholder in the balance sheet are estimated to approximate fair market value at September 30, 2013 and September 30, 2012 because of their short term nature.
Note 11. Commitments & Contingencies
Operating Leases – The Company has two operating leases for its laboratory facilities at the Burlington County College Science Incubator in Burlington, New Jersey. Each lease is for a term of one year with a monthly rent of $1,650 per laboratory.
Capital Lease – The Company has a capital lease for laboratory equipment. The minimum lease payments due on the capital lease are as follows:
| F-11 |
| 2014 | $ | 22,440 | ||
| 2015 | $ | 11,220 | ||
| Total minimum lease payments | $ | 33,660 | ||
| Less amounts representing interest | -2,520 | |||
| Present value of net minimum lease payments | $ | 31,140 |
Note 12. Reliance on Key Personnel
The Company largely relies on the efforts of its Chief Operating Officer and its Chief Executive Officer and Chairman of its Board of Directors. A withdrawal of the efforts of the Chief Operating Officer or the Chief Executive Officer and Chairman would have a material adverse effect on the Company’s ability to continue as a going concern.
Note 13. Litigation
The Company is not party to any pending litigation against it and is not aware of any litigation contemplated against it as of September 30, 2013 that may have a material effect upon the Company.
Note 14. Subsequent Events
On October 18, 2013, the Company formed Autogenesis Corporation (“Autogenesis”) as part of its collaborative agreement to develop wound healing products and other cellular therapies with privately-held Protein Genomics (PGen). The Company is jointly owned by American CryoStem and Protein Genomics. Autogenesis will be separately funded and will serve as the dedicated business unit focused on continuing and accelerating the research and development of innovative new products and biotechnologies that combine American CryoStem’s ATCELL™ (adipose derived regenerative cells), and ACSelerate™ cell media culture products with PGen’s Elastatropin® human-based protein materials.
On December 1, 2013, the Company executed two additional agreements (1) the Cooperative Research Agreement and (2) the Research Evaluation and License Option Agreement, with Rutgers University for further collaboration and intellectual property development with Dr. Kibum Lee. The Cooperative Research Agreement calls for the Company to provide to Dr. Lee’s laboratory and staff with additional materials to continue their research utilizing the Company’s ATCELL™ and ACSelerate™ products. The Agreement also provides for the Company to have exclusive access to certain identified Rutgers intellectual property and for the joint ownership of any additional intellectual property developed. The Research Evaluation and License Option Agreement provides a platform for the Company to be the exclusive developer and licensor for the commercial development of any new intellectual property and patent rights. The Company will also be managing all patent application and prosecution for any technologies developed under the Agreements.
| F-12 |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
(a) Evaluation of Disclosure and Control Procedures
The Company’s disclosure controls and procedures are designed to ensure (i) that information required to be disclosed by the Company in the reports the Company files or submits under the Exchange Act are recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms; and (ii) that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including its principal executive officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Our principal executive officer and principal financial officer evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of September 30, 2013, and concluded that the disclosure controls and procedures were effective as a whole.
(b) Management’s Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining an adequate system of internal control over financial reporting, as defined in Rule 13a-15(f) under the Exchange Act. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with Generally Accepted Accounting Principles (“GAAP”).
Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable assurance of such reliability and may not prevent or detect misstatements. Also, projection of any evaluation of effectiveness to future periods is subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management has conducted, with the participation of our Chief Executive Officer and our Principal Accounting Officer, an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2013. Management’s assessment of internal control over financial reporting used the criteria set forth in SEC Release 33-8810 based on the framework established by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control over Financial Reporting – Guidance for Smaller Public Companies. Based on this evaluation, Management concluded that our system of internal control over financial reporting was effective as of December 31, 2013, based on these criteria.
(c) Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act, during our most recently completed fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
(d) Attestation Report of the Registered Public Accounting Firm
This annual report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to an exemption for smaller reporting companies.
| Item 9B. | Other Information |
None.
| 34 |
| Item 10. | Directors, Executive Officers, and Corporate Governance |
The following table and biographical summaries set forth information, including principal occupation and business experience, about our directors and executive officers at September 30, 2013:
| Name | Age | Position | Officer and/or Director Since | |||
| John Arnone | 56 | Chairman and Chief Executive Officer | 2011 | |||
| Anthony F. Dudzinski | 51 | Chief Operating Officer and Director | 2011 | |||
| John DiFolco | 29 | Executive Vice President and Director of Marketing | 2013 |
John Arnone – Chairman and Chief Executive Officer
Mr. Arnone has been the Chairman of American CryoStem since 2008 and Chief Executive Officer since 2011. Prior to his involvement in the life sciences/biotechnology industries, he spent 25 years in the investment banking/financial services industry as an investment banker and a proactive investor. Over a 25 year period and holding six NASD licenses, Mr. Arnone founded, managed and operated two general securities broker-dealers based in New York specializing in strategic planning, corporate structure, financial planning and new business development. Over the years, he has provided advisory and business management services as a founder, officer, director and/or shareholder to both mid-level and development stage private and public companies. Mr. Arnone also co-founded and operated a global entertainment distribution corporation with 120 employees, and under his guidance the Company was voted medium wholesaler of the year in the music industry (1997, 1998 and 2000) by the National Association of Recording Merchants. Mr. Arnone holds a degree in Business Administration and a Bachelors of Art in Economics from Kean University in New Jersey.
Anthony F. Dudzinski − Chief Operating Officer and Director
Mr. Dudzinski is a founder of American CryoStem as well as its Chief Operating Officer. He is primarily focused on building and maintaining the Company’s operational and laboratory infrastructure and their compliance with current regulations. Mr. Dudzinski has been in the life sciences and biotechnology sector for more than eight years and has more than 25 years of experience in areas of senior management with a variety of public and private companies. Beginning in the securities industry with a focus on regulatory compliance and operations, he combined this experience with the biotechnology industry while building new investment vehicles focused on life sciences and biotechnology companies in 2004. Mr. Dudzinski’s past positions include Chief Executive Officer, President, Chief Operating Officer and Director of small and medium-sized organizations, including a publicly traded company with approximately 300 employees. He was also the President and Chief Operating Officer of a privately operated, registered broker-dealer with more than 175 sales associates. In addition to this experience, he was a founder and Chief Executive Officer of a number of publicly available exchange traded funds; and the Founder, Chairman and Chief Operating Officer of a target date fund complex and a registered investment company.
John DiFolco – Executive Vice President & Director of Marketing
Mr., DiFolco has served as American CryoStem’s Director of Marketing since 2008 and was named Executive Vice President in 2013. His core focus is to create and implement marketing campaigns targeting prospective customers and business partners. Mr. DiFolco has been has been involved directly and indirectly in creating and implementing marketing campaigns for various organizations over the years. He has introduced new products and services to new markets and as a result increased overall awareness and revenue. Mr. DiFolco is highly skilled in assessing the market landscape and determining and implementing tactical strategies to successfully market a company’s services through multiple channels, including social networking, search engine marketing, local web searching and grass roots print media campaigns. He has a deep understanding of technology, specifically web development and the creation of digital media.
| 35 |
Compliance with Section 16(A) of the Exchange Act
Section 16(a) of the Exchange Act requires the Company’s directors, executive officers and persons who beneficially own 10% or more of a class of securities registered under Section 12 of the Exchange Act to file reports of beneficial ownership and changes in beneficial ownership with the SEC. Directors, executive officers and greater than 10% stockholders are required by the rules and regulations of the SEC to furnish the Company with copies of all reports filed by them in compliance with Section 16(a).
Based solely on our review of certain reports filed with the Securities and Exchange Commission pursuant to Section 16(a) of the Securities Exchange Act of 1934, as amended, the reports required to be filed with respect to transactions in our common stock during the fiscal year ended September 30, 2013, were timely with the exception of:
| John S. Arnone, President and Chief Executive Officer | 4 | Mr. Arnone failed to file a Form 4 covering options to purchase common stock granted to him on September 28, 2013. | ||
| Anthony Dudzinski, Chief Financial Officer | 4 | Mr. Dudzinski failed to file a Form 4 covering options to purchase common stock granted to him on September 28, 2013. |
CORPORATE GOVERNANCE
Board Committees
Our board of directors does not have separate audit, nominating or compensation committees. Our entire board of directors performs the functions of these committees.
Audit Committee Financial Expert
We have not made a determination as to whether any of our directors would qualify as an audit committee financial expert.
Nominating Procedures
We have not adopted any procedures by which our security holders may recommend nominees to our board of directors.
| Item 11. | Executive Compensation |
The following table sets forth compensation information for services rendered by certain of our executive officers in all capacities during the last two completed fiscal years. The following information includes the dollar value of base salaries and certain other compensation, if any, whether paid or deferred.
Summary Compensation Table
| Name and Position(s) | Year | Salary($) | Bonus | Stock Awards | Option Awards1 | Total Compensation | ||||||||||||||
| John S. Arnone | 2013 | $ | 36,826 | $ | 187,528 | |||||||||||||||
| President and CEO | 2012 | $ | 18,000 | N/A | N/A | $ | 408,829 | $ | 426,826 | |||||||||||
| Anthony F. Dudzinski | 2013 | $ | 27,850 | $ | 187,528 | |||||||||||||||
| Chief Operating Officer | 2012 | $ | 28,000 | N/A | N/A | $ | 265,501 | $ | 285,151 | |||||||||||
1 This column represents the aggregate grant-date fair value of the awards computed in accordance with FASB ASC Topic 718. These amounts represent the accounting value for these awards and do not necessarily correspond to the actual value that may be realized by the named executive officer. The assumptions used in the calculation of these amounts for the fiscal year ended September 30, 2013 are described in the Notes to our financial statements included in this Annual Report.
Employment Contracts
The Company has an Employment Agreement with John Arnone, President and Chief Executive Officer of the Company, which began on October 1, 2012 and expires on October 1, 2017. The Board of Directors determines and approves the annual compensation paid to Mr. Arnone, which was set at $150,000 in base compensation for Fiscal 2013. During Fiscal 2013, Mr. Arnone was paid $37,826. Mr. Arnone agreed to waive the balance of his base compensation for the fiscal 2013.
| 36 |
The Company has an Employment Agreement with Anthony Dudzinski, Chief Operating Officer of the Company, which began on October 1, 2012 and expires on October 1, 2017. The Board of Directors determines and approves the annual compensation paid to Mr. Dudzinski, which was set at $150,000 in base compensation for Fiscal 2013. During Fiscal 2013, Mr. Dudzinski was paid $27,856. Mr. Dudzinski has agreed to waive the balance of his base compensation for Fiscal 2013.
Compensation of Directors
We do not compensate any of our directors for their services as directors. However, we do reimburse our directors for expenses incurred in attending board meetings and may issue stock or stock options for their time.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management |
The following table sets forth certain information regarding the beneficial ownership of our Common Stock by: (i) each person who, to our knowledge, beneficially owns 5% or more of our Common Stock and (ii) each of our directors and officers. Unless otherwise indicated, each of the stockholders listed below has sole voting and investment power over its shares of Common Stock beneficially owned.
| Name and Address of Beneficial Owner | Number of Shares | Percent of Class 1 | ||||||
| Directors and Named Executive Officers 2: | ||||||||
| John S. Arnone 3 | 22,880,000 | 70.87 | % | |||||
| Anthony Dudzinski 4 | 22,580,000 | 69.94 | % | |||||
| All directors and named executive officers as a group (2 persons) | ||||||||
| Other 5% or Greater Beneficial Owners | ||||||||
| ACS Global, Inc. | 21,000,000 | 65.04 | % | |||||
1 Beneficial ownership is calculated based on 32,285,721 shares of Common Stock issued and outstanding as of September 30, 2013, together with securities exercisable or convertible into shares of Common Stock within sixty (60) days of the date hereof for each stockholder. Beneficial ownership is determined in accordance with Rule 13d-3 of the Commission. The number of shares of Common Stock beneficially owned by a person includes shares of Common Stock issuable upon conversion of securities and subject to options or warrants held by that person that are currently convertible or exercisable or convertible or exercisable within sixty (60) days of the date hereof. The shares of Common Stock issuable pursuant to those convertible securities, options or warrants are deemed outstanding for computing the percentage ownership of the person holding such convertible securities, options or warrants but are not deemed outstanding for the purposes of computing the percentage ownership of any other person.
2 Unless otherwise specified, the address for the directors and officers is c/o American CryoStem Corporation at 1 Meridian Road, Eatontown, NJ 07724.
3 Mr. Arnone presently owns 14,250,000 shares of Common Stock of ACS Global and has the right to receive an additional 12,000,000 such shares upon the conversion of Series C Preferred Stock of ACS Global owned by him. As a result, he beneficially owns 35.8% percent of the ACS Global Common Stock. Mr. Arnone is also an officer and a director of ACS Global. Consequently, Mr. Arnone is a control person of ACS Global and may as such be deemed to “beneficially own” the 21,000,000 shares of Common Stock owned by ACS Global. Mr. Arnone, however, disclaims beneficial ownership of all such shares. Mr. Arnone also holds 1,880,000 options to purchase the Company’s Common Stockof which 880,000 expire September 4, 2017 and 1,000,000 expire on September 28, 2018.
4 Mr. Dudzinski presently owns 2,020,000 shares of ACS Global Common Stock and has the right to receive an additional 12,000,000 such shares upon the conversion of ACS Global preferred stock owned by him. As a result, he beneficially owns 19.16% percent of the ACS Global Common Stock. Mr. Dudzinski is also an officer and a director of ACS Global. Consequently, Mr. Dudzinski is a control person of ACS Global and may as such be deemed to “beneficially own” the 21,000,000 shares of Common Stock owned by ACS Global. Mr. Dudzinski, however, disclaims beneficial ownership of all such shares. Mr. Dudzinski also holds 1,580,000 options to purchase the Company’s Common Stockof which 580,000 expire September 4, 2017 and 1,000,000 expire on September 28, 2018.
| 37 |
Description of Securities
We are authorized to issue 300,000,000 shares of Common Stock, par value $0.001 per share and 50,000,000 shares of preferred stock, par value $0.0001 per share. As of January 07, 2013, there were 32,574,221 shares of Common Stock and no shares of preferred stock issued and outstanding.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information with respect to the outstanding equity awards to our named executive officers during fiscal 2013:
| Option Awards | |||||||||||||||||||
| Name | Number of securities underlying unexercised options (#) Exercisable | Number of securities underlying unexercised options (#) Unexercisable | Equity incentive plan awards: Number of securities underlying unexercised options (#) | Option exercise price ($) | Option expiration date |
||||||||||||||
| John S. Arnone | 1,000,000 | — | 880,000 | $ | 0.20 | 9/28/2018 | |||||||||||||
| Anthony Dudzinski | 1,000,000 | — | 580,000 | $ | 0.20 | 9/28/2018 | |||||||||||||
Option Plans
On September 18, 2011 our Board of Directors approved the “American CryoStem Corporation Incentive Stock Option Plan” (the “2011 Plan”). Under the Plan, officers, directors, employees and consultants to the Company may be granted options to purchase shares of the Company’s common stock, par value $0.001 per share. There are 3,000,000 shares of common stock reserved for issuance under the Plan. The Plan is administered under the authority of the Stock Option Plan Committee (the “Committee”). The Company issued all of the Options available under the 2011 Plan in Fiscal 2012. To date 150,000 options from the 2011 Plan have been exercised and a total of 2,850,000 remain outstanding at an average weighted exercise price of $0.14.
On May 1, 2013 our Board of Directors approved the 2013 American CryoStem Corporation Incentive Stock Option Plan (the “2013 Plan”). Under the Plan, officers, directors, employees and consultants to the Company may be granted options to purchase shares of the Company’s common stock, par value $0.001 per share. There are 5,000,000 shares of common stock reserved for issuance under the Plan. The Plan is administered under the authority of the Stock Option Plan Committee (the “Committee”). During 2013 the Company granted a total of 3,750,000 at a weighted average price of $0.18 to certain employees, advisory board members and consultants. To date no Options issued under the plan have been exercised.
Our current Board of Directors serves as the Option Plan Committee. The Plan further provides for the Committee to set the terms of any Options granted at the time of the grant and terminates ten years from its effective date and is subject to final shareholder approval.
On September 18, 2011, our Board of Directors approved the Annual Bonus Performance Plan for Executive Officers. To promote the success of our Company by providing to participating executives bonus incentives that qualify as performance-based compensation within the meaning of Section 162(m) of the Internal Revenue Code of 1986 as amended. The plan provides for the granting of up to an aggregate amount of bonuses awarded to all Participants of up to 10% of our income before taxes. The plan shall be administered by a Committee currently consisting of our Board of Directors. No bonuses have been granted under this plan during fiscal 2013.
| 38 |
| Item 13. | Certain Relationships and Related Transactions |
On April 20, 2011, we acquired, through our wholly owned subsidiary American CryoStem Acquisition Corporation, substantially all of the assets from, and assumed substantially all of the liabilities of, ACS Global, Inc. (formerly known as American CryoStem Corporation) a Nevada corporation (“ACS Global”), in exchange for 21,000,000 shares of our Common Stock. At the time of the acquisition, John Arnone, our Chairman of the Board, CEO and President was a director and the secretary of ACS Global and Anthony Dudzinski, one of our directors and our Chief Operating Officer, Treasurer and Secretary was a director, president and secretary of ACS Global. In addition, Mr. Arnone owns 14,250,000 shares of Common Stock of ACS Global and has the right to receive an additional 12,000,000 such shares upon the conversion of Series C Preferred Stock of ACS Global owned by him and Mr. Dudzinski owns 2,020,000 shares of ACS Global Common Stock and has the right to receive an additional 12,000,000 such shares upon the conversion of ACS Global preferred stock owned by him. As a result, assuming the conversion of such preferred stock, Mr. Arnone and Mr. Dudzinski would have been deemed to be the beneficial owners of approximately 35.8% and 19.1% of the Common Stock of ACS Global, respectively. Further, Mr. Arnone and Mr. Dudzinski, as control persons of ACS Global may be deemed to beneficially own the 21,000,000 shares of our Common Stock issued to ACS Global in the acquisition. Each of Mr. Arnone and Mr. Dudzinski disclaim such beneficial ownership.
Mr. Arnone remains a Director and Secretary of ACS Global and Mr. Dudzinski remains as a Director, President and Treasurer of ACS Global.
On October 18, 2013, the Company formed Autogenesis Corporation (“Autogenesis”) as part of its collaborative agreement to develop wound healing products and other cellular therapies with privately-held Protein Genomics (PGen). The Company is jointly owned by American CryoStem and Protein Genomics. Autogenesis will be separately funded and will serve as the dedicated business unit focused on continuing and accelerating the research and development of innovative new products and biotechnologies that combine American CryoStem’s ATCELLs (adipose derived regenerative cells) and ACSelerate cell media culture products with PGen’s Elastatropin® human-based protein materials. Mr. Burt Ensley, a member of our Scientific and Advisory Board, is the CEO of Protein Genomics. Mr. Dudzinski is the CEO and Director of Autogeneis Corporation, Mr. Arnone is Secretary and a Director of Autogenesis corporation and Mr. Ensley is the Chief Scientific Officer and Director of autogenesis.
Under agreement with Personal Cell Sciences (PCS), we manufacture the key ingredient Autokine-CM® (autologous adipose derived stem cell conditioned medium) for PCS’ U-Autologous™ anti-aging topical formulation. Each product is genetically unique to the patient and custom blended, deriving its key ingredients from the individual client’s own stem cells. The Company provides its CELLECT® Tissue Collection service to collect the required tissue to manufacture the U-Autologous product and processes it under the same cGMP standard operating procedures that it developed for the ATGRAFT™ and ATCELL™ cell processing services utilizing ACSelerate™ cell culture media. The Company receives collection, processing and long term storage fees and earns a royalty on all U-Autologous product sales. The utilization of the Company’s core services in its contract manufacturing relationship provides opportunities for the Company to promote its ATGRAFT™ and ATCELL™ products for an individual’s cosmetic purposes. Mr. Arnone is the CEO of Personal Cell Sciences, Corp.
Director Independence
Using the definition of “independent” using the rules of The Nasdaq Stock Market, we have determined that neither John Arnone nor Anthony Dudzinski are independent.
| Item 14. | Principal Accountant Fees and Services |
Audit Fees
The aggregate fees billed by Donahue Associates, for professional services rendered for the audit of our annual financial statements for fiscal year ended September 30, 2013 were $10,000 and $10,000 for the fiscal year ended September 2012.
Audit-Related Fees
There were no other fees billed by Donahue Associates, LLC for professional services rendered, other than as stated under the captions Audit Fees.
Tax Fees
There were no other fees billed Donahue Associates, LLC for professional services rendered, other than as stated under the captions Audit Fees.
All Other Fees
There were no other fees billed by Donahue Associates, LLC for professional services rendered, other than as stated under the captions Audit Fees, Audit-Related Fees, and Tax Fees.
| 39 |
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) (1) Financial Statements.
The financial statements listed in the Index to Consolidated Financial Statements appearing on page F-1 of this Form 10-K are filed as a part of this report.
(2) Financial Statement Schedules
There are no financial statement schedules included in this annual report.
(3) The exhibits listed below are filed as part of this annual report.
| Number | Exhibit | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema Document | |
| 101.CAL | XBRL Taxonomy Calculation Linkbase Document | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB | XBRL Taxonomy Label Linkbase Document | |
| 101.PRE | XBRL Taxonomy Presentation Linkbase Document |
| (1) | Incorporated by reference to the registrant’s current report on Form 8-K filed on June 15, 2011 | |
| (2) | Incorporated by reference to the registrant’s current report on Form 8-K filed on April 27, 2011 | |
| (3) | Incorporated by reference to the registrant’s registration statement on Form S-1 filed on February 16, 2010 |
| 40 |
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| DATE: January 14, 2013 | American CryoStem Corporation. | ||
| (Registrant) | |||
| By: | /s/ John S, Arnone | ||
| John S Arnone | |||
| President, CEO and Director | |||
In accordance with the Securities Exchange Act, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| January 14, 2013 | /s/ John S, Arnone |
| John S. Arnone, President, CEO and
Chairman of the Board (principal executive officer, principal financial and accounting officer) | |
| January 14, 2013 | /s/ Anthony F. Dudzinski |
| Anthony Dudzinski, COO, Treasurer, Secretary and Director |
| 41 |
