Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Nuo Therapeutics, Inc. | v364962_8k.htm |

Corporate Presentation January 2014 OTCQX: CMXI 1

Forward - Looking Statements Statements contained in this presentation not relating to historical facts are forward - looking statements that are intended to fall within the safe harbor rule under the Private Securities Litigation Reform Act of 1995. The information contained in the forward looking statements is inherently uncertain, and Cytomedix's actual results may differ materially due to a number of factors, many of which are beyond Cytomedix's ability to predict or control, including many, among many others , risks and uncertainties related to the Company’s ability to successfully execute its AutoloGel™ and Angel ® sales strategies, to successfully launch its efforts in outpatient and other clinics and to achieve AutoloGel ™ expected reimbursement rates in 2014 and thereafter, to successfully negotiate with hospitals and physician offices as anticipated and to realize the anticipated sales growth from such treatments; to meet its stroke trial enrollment rates, to successfully realize sales of the Angel Technology under the Arthrex licensing arrangement resulting in the royalty stream to the Company, the Company’s ability to successfully integrate the Aldagen acquisition, to successfully manage contemplated clinical trials, to manage and address the capital needs, human resource, management, compliance and other challenges of a larger, more complex and integrated business enterprise, viability and effectiveness of the Company’s sales approach and overall marketing strategies, commercial success or acceptance by the medical community, competitive responses, the Company's ability to raise additional capital and to continue as a going concern. These forward - looking statements are subject to known and unknown risks and uncertainties that could cause actual events to differ materially from the forward - looking statements. More information about some of these risks and uncertainties may be found in the reports filed with the Securities and Exchange Commission by Cytomedix at http://www.sec.gov. 2

Cytomedix “Commercial, Growth - Stage Company Specializing in the Development of Innovative, Platelet Based Wound Care Technologies” 3

Cytomedix Highlights • Favorable product reimbursement for 2014 • Broad Medicare patient access via CMS Coverage with Evidence Development (CED) determination • Significant near - term market opportunity in $2.2 billion U.S. chronic wound care market • Definable and scalable commercial path • On - going royalty stream from the licensing of non - core asset 4

5

Uniquely Positioned • Addresses a clear unmet need in the $2.2 billion advanced wound care market • Best - in - class point of care product with strong clinical data • Only Platelet Rich Plasma (PRP) system cleared by FDA for wound care • Unique reimbursement position with CMS • Financial advantage in changing reimbursement environment • Scaled commercial launch to address a segmented market • Opportunity for strategic partnerships as a means to further accelerate sales growth 6

Safe & Rapid Point of Care Product S mall volume of blood is drawn B lood is placed into centrifuge to separate the platelets and PRP PRP is activated via introduction of reagents causing: • R elease of growth factors • F ormation of a fibrin matrix scaffold • Transformation of the liquid to a gel The bioactive gel is applied topically to the wound Average time for procedure: 5 - 8 minutes 7

Positioned to Address Unmet Market Needs Unique Effective • AutoloGel changes the healing trajectory in chronic wounds • Effective across a broad range of wound etiologies – Robust body of clinical data – Complete healing in Randomized Controlled DFU trial (81.3%) – Complete healing in Wagners III/IV Case Series trial (84%) – Large observational case series with 9 6.5% response rate in 2.2 weeks Reimbursed • National Coverage Determination (NCD ) Effective August 2, 2012 • AutoloGel specific evidence collection under CED determination – Diabetic foot ulcers, Pressure ulcers, Venous ulcers – Hospital outpatient payment effective January 1, 2014 – Protocols approved and claims guidance issued • AutoloGel is the only Platelet Rich Plasma System that is FDA cleared for wound care – FDA cleared September 2007 – IP protected device and gel formulation – Very broad label - Indicated for all exuding wounds 8
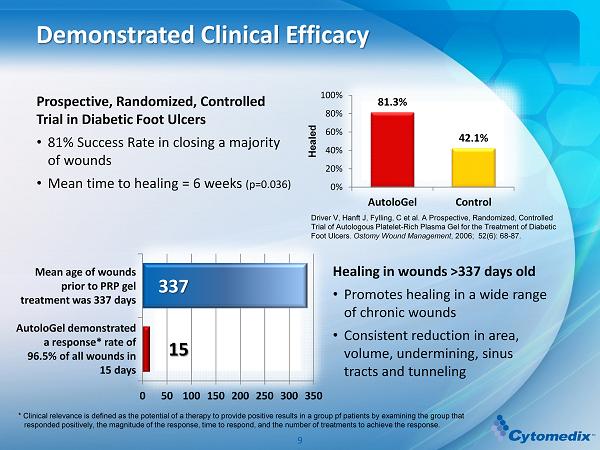
Prospective, Randomized, Controlled Trial in Diabetic Foot Ulcers • 81 % Success Rate in closing a m ajority of w ounds • Mean time to healing = 6 weeks (p=0.036 ) Demonstrated Clinical Efficacy 81.3% 42.1% 0% 20% 40% 60% 80% 100% AutoloGel Control Healed Driver V, Hanft J, Fylling, C et al. A Prospective, Randomized, Controlled Trial of Autologous Platelet - Rich Plasma Gel for the Treatment of Diabetic Foot Ulcers. Ostomy Wound Management , 2006; 52(6): 68 - 87. Healing in wounds >337 days old • Promotes healing in a wide range of chronic wounds • Consistent reduction in area, volume, undermining, sinus tracts and tunneling * Clinical relevance is defined as the potential of a therapy to provide positive results in a group pf patients by examining th e group that responded positively, the magnitude of the response, time to respond, and the number of treatments to achieve the response. 0 50 100 150 200 250 300 350 Mean age of wounds prior to PRP gel treatment was 337 days AutoloGel demonstrated a response* rate of 96.5% of all wounds in 15 days 337 15 9

Commercial Landscape 10

Significant Change in 2014 • Skin substitutes unconditionally packaged by CMS • AutoloGel granted favorable Medicare reimbursement for hospital outpatient & physician office • NPWT and hyperbaric therapy continue to see pricing pressure • Expected changes in promotional effort across market players 11

Change Creates Opportunity • Significant revenue potential – 5% market penetration > $300M • Multiple wound types in various segments – DFU, VLU, PU – Hospital outpatient, LTAC, Physician office • A sizable shift in reimbursement landscape – Improved AutoloGel outpatient reimbursement – Packaging of segment - leading skin substitutes 12

Competitive Technologies Brands FDA Clearance CMS Coverage Source AutoloGel (Cytomedix) 510k All exuding wounds DFU, PU, VU DFU, PU, VU Autologous Apligraf (Organogenesis) PMA DFU, VU DFU, VU Expanded Allogeneic Dermagraft (Shire) PMA DFU DFU Expanded Allogeneic EpiFIX ( MiMedx ) NA* DFU, VU Allogeneic Grafix (Osiris) NA* Pass - through only Allogeneic V.A.C. Negative Pressure ( Kinetic Concepts) 510k All wound types All wounds N/A * NA - No approved/cleared indication as FDA regulated by Human Cells, Tissue and Cellular, and Tissue - based Products under Sec tion 361. Legend: DFU : Diabetic Foot Ulcer PU : Pressure Ulcer VU : Venous Ulcer 13
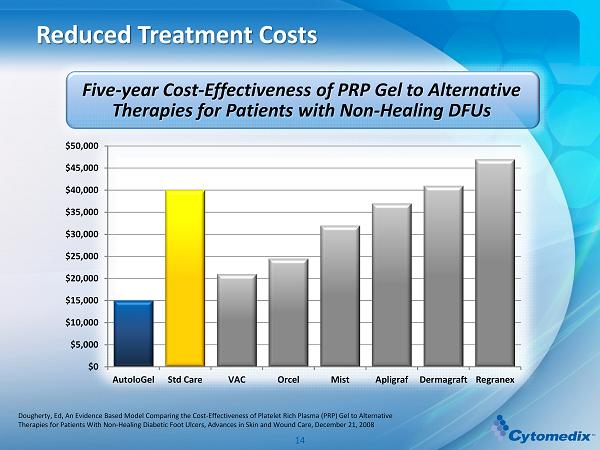
Reduced Treatment Costs Dougherty, Ed, An Evidence Based Model Comparing the Cost - Effectiveness of Platelet Rich Plasma (PRP) Gel to Alternative Therapies for Patients With Non - Healing Diabetic Foot Ulcers, Advances in Skin and Wound Care, December 21, 2008 Five - year Cost - Effectiveness of PRP Gel to Alternative Therapies for Patients with Non - Healing DFUs $0 $5,000 $10,000 $15,000 $20,000 $25,000 $30,000 $35,000 $40,000 $45,000 $50,000 AutoloGel Std Care VAC Orcel Mist Apligraf Dermagraft Regranex 14
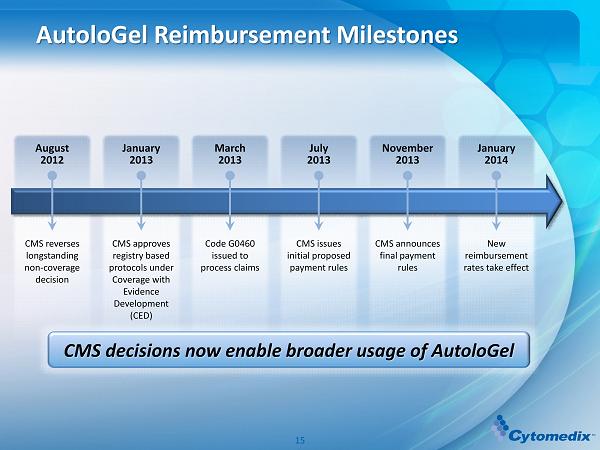
AutoloGel Reimbursement Milestones CMS reverses longstanding non - coverage decision August 2012 CMS approves registry based protocols under Coverage with Evidence Development (CED) January 2013 Code G0460 issued to process claims March 2013 CMS issues initial proposed payment rules July 2013 CMS announces final payment rules November 2013 New reimbursement rates take effect January 2014 CMS decisions now enable broader usage of AutoloGel 15
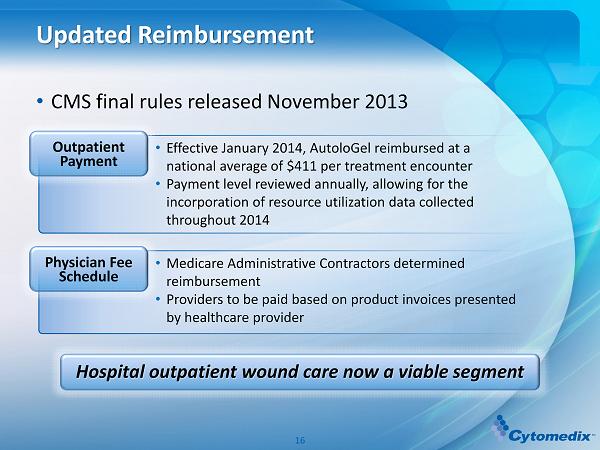
Updated Reimbursement • CMS final rules released November 2013 Hospital outpatient wound care now a viable segment • Effective January 2014, AutoloGel reimbursed at a national average of $411 per treatment encounter • Payment level reviewed annually, allowing for the incorporation of resource utilization data collected throughout 2014 Outpatient Payment • Medicare Administrative Contractors determined reimbursement • Providers to be paid based on product invoices presented by healthcare provider Physician Fee Schedule 16

Commercial Considerations • AutoloGel Positioning – Clinical benefit of product with published evidence – Financial benefit created by CMS reimbursement changes • Market Penetration Objectives – Hospital outpatient programs in specific geographies – Add high - volume Long Term Acute Care hospitals – Selectively target high volume Physician Office practices 17

Commercial Considerations • Near Term – Focused commercial execution top priority – Multiple examples to emulate – Specialized 30 person direct sales team by December 2014 – Add enabling functions to maximize representative efficiency Scalable and flexible model which can be accelerated if proven effective 18

Commercial Considerations • Longer Term – Implement targeted marketing efforts focused on AutoloGel as superior option in outpatient wound care – Continue scaling commercial organization commensurate with market adoption and company financial results – Expand coverage and reimbursement in adjacent segments • Private insurers • Veterans Administration – Create evidence of value proposition for AutoloGel in the US health care market (e.g. cost benefit model) 19

0 1000 2000 3000 4000 5000 6000 2013 2014 2015 2016 2017 2018 2019 2020 2021 Projected US Sales $Millions Advanced Dressings NPWT Biologic Dressings* Projected Market for Advanced Wound Therapies Source: MedMarket Diligence. Wound Management, Worldwide Market & Forecast. March 2013 NPWT = Negative Pressure Wound Therapy * Biologic Dressings = Growth factor products and skin substitutes 20

Management Team • Martin Rosendale – President & Chief Executive Officer – Joined Cytomedix, Inc. in March 2008 as COO, appointed CEO in July 2008 – CEO of Core Dynamics, Inc. – Leadership positions with American Red Cross, SangStat , North American Vaccine and ZLB Bioplasma – Served on the boards of Transplant Recipients International Organization and the American Red Cross Biomedical Services, San Jose Region – 24+ years of specialty medical experience – Bachelor of Science degree (Microbiology) from California State University • Steven A. Shallcross – EVP & Chief Financial Officer – Appointed CFO of Cytomedix in May 2013 – Held CFO and senior financial positions at Empire Petroleum Partners, LLC, Sensors for Medicine and Science, Inc., Innocoll Holdings, Inc., Vanda Pharmaceuticals, Inc., MiddleBrook Pharmaceuticals, Inc. – Certified Public Accountant. Bachelor’s degree in Accounting from the University of Illinois and MBA from the University of Chicago, Booth School of Business • Dean E. Tozer – Corporate Development (Consultant) – Most recently VP of Corporate Development for Shire Regenerative Medicine – Sr. VP of Advanced BioHealing from 2006 until its acquisition by Shire in June 2011 – Industry advisor from 2000 to 2006 – Various senior international and domestic responsibilities at DuPont and Searle – Bachelor’s degree in Commerce from Saint Mary’s University in Halifax Nova Scotia. Certified M anagement Accountant designation in Ontario, Canada 21

22
