Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Fortress Biotech, Inc. | d649047d8k.htm |
   NASDAQ: CNDO
December 2013
Lindsay A. Rosenwald, MD
Chairman, President and CEO
Exhibit 99.1 |
   Statements in this presentation that are not descriptions of historical facts are
forward-looking statements within the meaning of the “safe
harbor” provisions of the Private Securities Litigation
Reform Act of 1995. We have attempted to identify forward-looking statements by
terminology including “anticipates,”
“believes,”
“can,”
“continue,”
“could,”
“estimates,”
“expects,”
“intends,”
“may,”
“plans,”
“potential,”
“predicts,”
“should,”
or “will”
or the negative of these terms or other
comparable terminology. Forward-looking statements are based on
management’s current expectations and are subject to risks and
uncertainties that could negatively affect our business, operating results,
financial condition and stock price. Factors that could cause actual results to
differ materially from those currently anticipated risks include
those set forth in our SEC filings
including, in particular, risks relating to: our ability to attract, integrate and
retain key personnel; the results of research and development activities;
uncertainties relating to preclinical and clinical testing, financing and
strategic agreements and relationships; the early stage of products under
development; our need for substantial additional funds; government regulation; patent
and intellectual property matters; our ability to successfully manufacture TSO in
the US; dependence on third party manufacturers; and competition. We
expressly disclaim any obligation or undertaking to update or revise any
statements contained herein to reflect any change in our expectations or any
changes in events, conditions or circumstances after the date of this
presentation. Forward-Looking Statements
Forward-Looking Statements
2 |
   »
Experienced management team and board of directors
-
In both drug development and business development
-
Dedicated to maximizing shareholder
value
»
Business development expertise
-
Products and technologies acquisitions and licensing
-
Investment and spin-out opportunities
-
Creative financing solutions
»
Two biologic product candidates in clinical
stage development -
Focused on autoimmune diseases and cancer immunotherapy
-
Strong proprietary position
Value Proposition
Value Proposition
3 |
   Lindsay
A. Rosenwald, MD Chairman, President and CEO
»
Prolific and successful investor in the life sciences industry
for over 20 years as Chairman of Paramount BioCapital
»
Co-Portfolio Manager and Partner of Opus Point Partners
Lucy Lu, MD
EVP & Chief Financial Officer
»
Senior Analyst at Citi Investment Research
»
Over 10 years of biotech equity research experience
George C. Avgerinos, PhD
Sr. VP, Biologics Operations
»
Divisional VP, Global Process and Manufacturing Sciences,
Abbvie
»
Over 30 years experience in biopharmaceutical process
development including Humira™
process and manufacturing
Key Management and Board Members
Key Management and Board Members
4
Kevin Horgan, MD
Chief Medical Officer
»
more than 25 years of academic and pharmaceutical experience
»
SVP and Chief Medical officer at Soligenix
»
Vice President of Clinical Immunology at Janssen Biotech
Michael S. Weiss
Co-Vice Chairman
»
Co-Portfolio Manager and Partner of Opus Point Partners, LLC
(since 2009)
»
Executive Chairman, Interim CEO and President of TG Therapeutics
»
Chairman and CEO of Keryx Biopharmaceuticals
Eric K. Rowinsky, MD
Co-Vice Chairman
»
World renown oncologist, former CMO at ImClone, board of
Biogen/Idec
»
Chief Medical Officer and Head of Research and Development;
Executive Vice President
at
Stemline Therapeutics |
   »
Products and technologies
-
We intend to pursue product and technology acquisitions and
licenses.
»
Investment opportunities
-
We may make controlling and non-controlling investments in
companies with proprietary products and/or technologies where we
believe shareholder value will be enhanced, while preserving capital
and strength of our balance sheet.
»
Spin-out opportunities
-
From time to time we may spin out new companies based on current
and new technologies and products that we develop, license or
acquire.
Business Development Strategies
Business Development Strategies
5 |
   »
Porcine whipworm ova
-
Represents
a
novel
approach
to
treating
autoimmune
diseases
–
the
“Hygiene Hypothesis”
-
Natural immunomodulator -
regulates T-Reg cells and inflammatory
cytokines
»
Planned and ongoing studies in multiple autoimmune indications
including psoriasis, autism, multiple sclerosis, and ulcerative colitis
»
Natural properties suggest strong potential for a safe profile
»
North and South America and Japanese rights for all indications
TSO: Trichuris suis ova (CNDO-201)
TSO: Trichuris suis ova (CNDO-201)
6 |
   »
Does not multiply in human host
»
Colonization is self-limited in
humans
»
No systemic phase
»
No direct transmission
»
Ova stable
»
Oral dosing; 1 tbsp solution
taken once every 2 weeks
-
Clear, odorless, tasteless
-
Ova are Microscopic
Benefits of Trichuris suis ova (TSO)
Benefits of Trichuris suis ova (TSO)
7 |
   »
12 week, dose ranging study
-
TSO 250, 2500, 7500 or placebo
-
N=240
-
Crohn’s patients
•
CDAI = 220-350
»
Outcome –
Discontinued post 2
nd
Interim analysis
TSO Phase 2 Crohn’s Disease Studies
TSO Phase 2 Crohn’s Disease Studies
8
TRUST
-
I
TRUST
-
II
»
12 week study
-
TSO 7500 or placebo
-
N=250
-
Crohn’s patients
•
CDAI = 220-450
•
The randomization was stratified by
disease activity as measured by
CDAI
»
Outcome –
Did NOT meet overall
primary endpoint of response |
   9
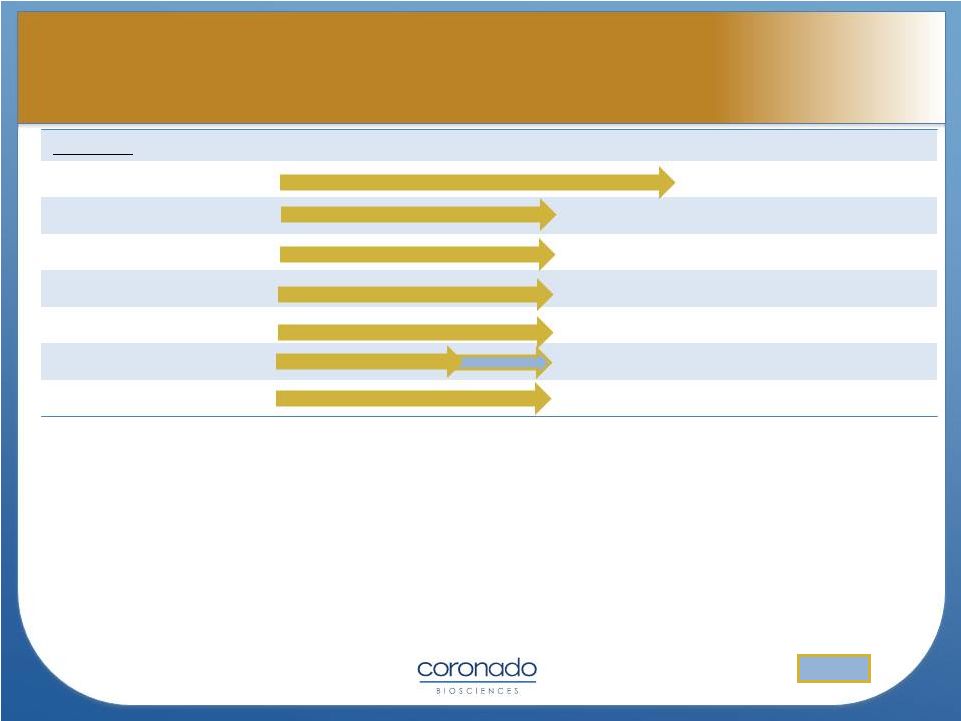
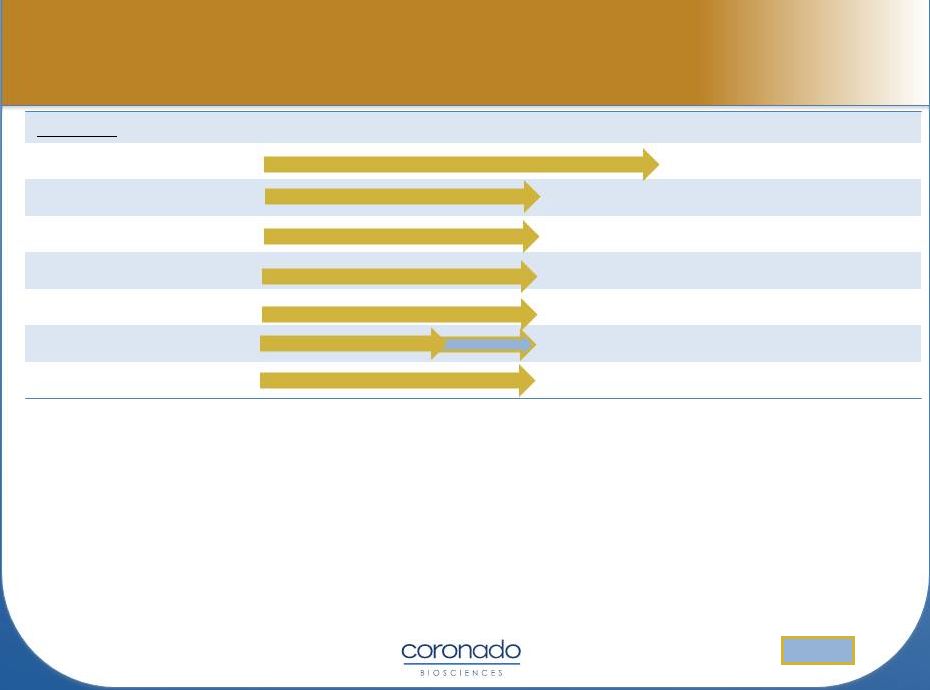
Select TSO Trials
Select TSO Trials
* TSO Phase 2a studies being conducted
as Investigator-Initiated Studies
Planned
OL = Open-Label DB = Double-Blind
SB= Single-Blind PC = Placebo-Controlled
*expected
Indication
Pre-Clinical
Phase 1
Phase 2a*
Phase 2
Phase 3
Design
Ulcerative Colitis
120 pt, DB, PC
Ulcerative Colitis (MOA)
18 pt, DB, PC
Multiple Sclerosis (US)
15 pt, SB
Multiple Sclerosis (EU)
50 pt, DB, PC
Autism
10 pt, DB, Cross
Autism
60 pt, DB, PC
Psoriasis
20 pt, OL
1H2014 (*) |
   »
Numerous threads of evidence suggest that Autism is an
immune mediated disorder linked to the hygiene hypothesis (HH)
-
Gene –
environment mismatch is likely responsible for the dramatic
increase in incidence
-
Increase parallels that of asthma and other HH conditions
»
Strong evidence for GI issues and immune dysregulation in
Autism patients
-
Up to 70% of the patients have current or past GI issues, and
endoscopy often shows low level inflammation in these patients
-
Abnormal cytokines, B cell and T cell subsets, and low levels of
regulatory cytokines
-
Evidence of activation of the innate immune system in the CNS
-
Maternal autoimmune diagnosis is a recognized risk factor
»
Anecdotal evidence and the interim data from Dr. Hollander’s
study point to potential efficacy
Scientific Rationale for TSO for Autism
Scientific Rationale for TSO for Autism
10 |
   »
Ongoing double-blind, placebo-controlled, randomized cross-
over study in 10 adult, high functioning autism patients at
Montefiore Medical Center
»
In the first 5 patients that completed the study, there was a
statistically significant separation from placebo in favor of TSO
on three measures of disease that reflect a core feature of
autism (the restrictive interests and repetitive behavior domain)
-
Montefiore-Einstein Rigidity Scale (MERS)
-
Repetitive Behavior Scale-Revised (RBS-R) Sameness Scale
-
Social Responsiveness Scale (SRS)-Repetitive Behaviors Scale
»
TSO is well tolerated
»
Final results are expected in mid 2014
Pilot Study of TSO in Autism
Pilot Study of TSO in Autism
11 |
   »
Three issued US patents entitled ‘Use of Parasitic Biological Agents for
Prevention and Control of Autoimmune Disease’
directed to compositions,
methods of producing compositions, and methods of autoimmune disease with
helminths –
exp. 12/2018
»
Five additional pending patents
* Expiration dates do not include any patent term extension
TSO Intellectual Property
TSO Intellectual Property
12
-
‘Use of Parasitic Biological Agents for Disease Prevention and Control’ - directed to
the treatment of animals/man with a Th1 or Th2 mediated autoimmune disease – exp.
11/2023
-
‘Production of a Viable, Storable Worm Egg Suspension’ - directed to a process
for preparation of TSO using an acid wash - exp. 3/2028 - ‘Method for Characterizing the Biological Activity of
Helminth Eggs, in particular Trichuris Eggs’ – exp. 5/2029
-
‘Treatment with Helminths’ directed to methods of treating obesity and IBS - exp.
10/2029
-
‘Compositions and Methods for Treating IBD’ - directed to a method of treating IBD by
contacting an isolated dendritic/macrophage cell with a helminth – exp. ~9/2032 |
   »
NK cells represent the key component of the body’s innate immune
surveillance system
»
Proof of principle established in patients with high-risk refractory or
relapsed acute myeloid leukemia (AML)
»
Activation with CNDO-109 does not require toxic cytokines or long-
term culture/expansion, and does not change NK cell phenotypes
»
Preclinical activity demonstrated in multiple myeloma, breast
cancer, prostate cancer and ovarian cancer
CNDO-109: Activated Natural Killer Cells
CNDO-109: Activated Natural Killer Cells
13 |
 »
Activated ex vivo by tumor cell
lysate (CNDO-109)
»
Effective from autologous or
allogeneic NK cell source
»
Uniquely positioned in patients
with “minimal residual disease”
»
Remains active after
freeze/thaw
CNDO-109 Mechanism of Action
CNDO-109 Mechanism of Action
14
Trigger
receptor
Trigger
receptor
Priming
receptor
Priming
receptor
Trigger
receptor
Priming
receptor
Resting
Donor NK
Priming
signal
Trigger
ligand
Priming –
Signal 1
Triggering –
Signal 2
“Serial Killer”
Tumor lysis
Dead
Tumor
cell
Primed
Donor NK
Patient’s
tumor
Primed
Donor NK
CNDO-109 |
   15
CNDO-109 Phase 1 Study in AML
CNDO-109 Phase 1 Study in AML
»
Phase 1 investigator sponsored open-label trial
»
To determine the safety of infusion of allogeneic Tumor-activated
NK (TaNK) cells after low dose radiotherapy plus chemotherapy in
high-risk relapse or refractory AML patients
»
Enrolled 8 AML patients
-
5 in Complete Remission at time of study entry
-
1 patient in partial relapse (PR) at time of study entry
»
3/5 experienced a longer CR than their previous CR, in addition
PR patient achieved CR
Kottaridis, et al., ASH 2011 |
   16
CNDO-109 Clinical Development
CNDO-109 Clinical Development
»
Initiated Phase 1/2 allogeneic clinical trial for the treatment of
relapsed AML
»
Future autologous studies in other tumor types are possible (e.g.
multiple myeloma, breast, ovarian and prostate) |
   »
Core patent -
“Method for activating natural killer cells by tumor
cell preparation in vitro”
-
Issued in U.S. -
exp. 1/2029
-
Issued in Australia –
exp. 3/2026
-
Pending in Europe, Canada, Japan and India
»
Patent pending -
“Preserved Compositions of Activated NK Cells
and Methods of Using the Same”
–
exp. 7/2030
»
Provisional
application
pending
in
the
U.S.
-
“Compositions
and
Methods for Treating Viral Infections”
* Expiration dates do not include any patent term extension
CNDO-109 Intellectual Property
CNDO-109 Intellectual Property
17 |
   Financials
Financials
18
Listed on NASDAQ: CNDO
Market Cap as of 12/20/2013
~$75M
Shares Outstanding
35.5M
-
Additional 4.0 M options and warrants
-
Additional 3.9 M restricted shares
Cash Position as of 9/30/2013
$106.3M
Total Debt as of 9/30/2013
$15.0M
Accounts Payable as of 9/30/2013
$1.1M
Working Capital as of 9/30/2013
$96.9M |
