Attached files
| file | filename |
|---|---|
| 8-K - MAINBODY - Xenetic Biosciences, Inc. | mainbody.htm |
| EX-9.2 - EX9_2 - Xenetic Biosciences, Inc. | ex9_2.htm |
THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION. PART 2 (EXPLANATORY STATEMENT) OF THIS DOCUMENT COMPRISES AN EXPLANATORY STATEMENT IN COMPLIANCE WITH SECTION 897 OF THE COMPANIES ACT 2006. THIS DOCUMENT CONTAINS DETAILS OF A PROPOSED ACQUISITION WHICH, IF IMPLEMENTED, WILL RESULT IN THE CANCELLATION OF THE XENETIC SHARES FROM ADMISSION TO TRADING ON AIM, A MARKET OPERATED BY THE LONDON STOCK EXCHANGE.
If you are in any doubt as to the action you should take, you are recommended to seek your own professional advice immediately from your stockbroker, bank manager, solicitor, accountant, tax adviser or other independent financial adviser who, if you are in the United Kingdom, should be authorised under the Financial Services and Markets Act 2000 (as amended), or from another appropriately authorised independent financial adviser if you are in a territory outside the United Kingdom.
If you have sold or otherwise transferred all your Shares, please forward this document (together with the related Forms of Proxy) at once to the purchaser or transferee, or to the stockbroker, bank or other agent through whom the sale or transfer was effected, for delivery to the purchaser or transferee. However, these documents should not be forwarded or transmitted in or into any jurisdiction in which such act would constitute a violation of the relevant laws in such jurisdiction. If you have sold or transferred only part of your holding of Shares, please retain these documents and consult the bank, stockbroker or other agent through whom the sale or transfer was effected.
The distribution of this document and the accompanying documents in jurisdictions other than the United Kingdom may be restricted by law and therefore persons into whose possession this document and the accompanying documents come should inform themselves about and observe any such restrictions. Any failure to comply with any such restrictions may constitute a violation of the securities laws of any such jurisdiction.
GSL’s common stock is quoted on OTCBB operated by the Financial Industry Regulatory Authority, Inc. (“FINRA”) and the OTCQB operated by OTC Markets Group, Inc. It is expected that quotation of the GSL Consideration Shares will become effective subject to the satisfaction of certain conditions, including the sanction of the Scheme by the Court, on 27 January 2014.
Shareholders should carefully read the whole of this document. In addition this document should be read in conjunction with the relevant sections of the documents listed as incorporated by reference on page 52, and the accompanying blue and white Forms of Proxy.
RECOMMENDED ACQUISITION
of
XENETIC BIOSCIENCES PLC
(Incorporated in England and Wales under the Companies Act 1985 with registered number 03213174)
by
GENERAL SALES & LEASING, INC.
(to be effected by a scheme of arrangement under Part 26 of the Companies Act 2006)
This document should be read as a whole. Your attention is drawn to the letter from the Chairman of Xenetic set out in Part 1 of this document, which contains the recommendation of the Xenetic Directors (save for Roman Knyazev) that you vote in favour of the Scheme at the Court Meeting and in favour of the Special Resolution to be proposed at the General Meeting. A letter from London Bridge Capital explaining the Acquisition appears in Part 2 of this document and constitutes an explanatory statement in accordance with section 897 of the Companies Act.
Notices of the Meetings, each of which will be held at the offices of Pinsent Masons LLP, 30 Crown Place, London EC2A 4ES, United Kingdom, on 17 December 2013, are set out at the end of this document. The Court Meeting will start at 10.00 a.m. GMT and the General Meeting will start at 10.30 a.m.GMT (or as soon thereafter as the Court Meeting shall have been concluded or adjourned).
Capitalised words and phrases used in this document have the meanings given to them in Part 9 of this document.
IMPORTANT NOTICE
The distribution of this document and/or the accompanying documents in jurisdictions other than the United Kingdom may be restricted by law and therefore persons into whose possession this document and the accompanying documents come should inform themselves about, and observe, such restrictions. Any failure to comply with the restrictions may constitute a violation of the securities laws of any such jurisdiction. Neither this document nor the accompanying documents constitute an offer or an invitation to purchase any securities or a solicitation of an offer to sell any securities pursuant to these documents or otherwise in any jurisdiction in which such offer or solicitation is unlawful. This document and the accompanying documents have been prepared in connection with a proposal in relation to a scheme of arrangement pursuant to and for the purpose of complying with English law and the Code and information disclosed may not be the same as that which would have been prepared in accordance with laws of jurisdictions outside England and Wales. Nothing in this document or the accompanying documents should be relied on for any other purpose.
The statements contained herein are made as at the date of this document, unless some other time is specified in relation to them, and service of this document will not give rise to any implication that there has been no change in the facts set forth herein since such date. Nothing contained herein will be deemed to be a forecast, projection or estimate of the future financial performance of the Company, the Xenetic Group, GSL or the GSL Group.
No person has been authorised to make representations on behalf of the Company or GSL concerning the Acquisition or the Scheme which are inconsistent with the statements contained in this document and any such representations, if made, may not be relied upon as having been so authorised. The summaries of the principal provisions of the Scheme contained in this document are qualified in their entirety by reference to the Scheme itself, the full text of which is set out in Part 4 of this document. Each Shareholder is advised to read and consider carefully the text of the Scheme itself.
No person should construe the contents of this document as legal, financial or tax advice but should consult their own advisers in connection with the matters contained herein, including without limitation in relation to holding US securities.
London Bridge Capital Limited, which is authorised and regulated in the United Kingdom by the Financial Conduct Authority, is acting exclusively for the Company as its financial adviser and no one else in connection with the Acquisition and will not be responsible to anyone other than the Company for providing the protections afforded to clients of London Bridge Capital Limited nor for providing advice in connection with the Acquisition or the content of, or any other matter or arrangement described or referred to in, this document. Neither London Bridge Capital Limited nor any of its directors, officers, subsidiaries or affiliates owes or accepts any duty, liability or responsibility whatsoever (whether direct or indirect, whether in contract, in tort, under statute or otherwise) to any person who is not a client of London Bridge Capital Limited in connection with the Acquisition or any other matter referred to in this document, any statement contained herein or otherwise.
N+1 Singer, which is authorised and regulated in the United Kingdom by the Financial Conduct Authority, is acting exclusively for the Company as its nominated adviser and broker only and no one else in connection with the Acquisition and will not be responsible to anyone other than the Company for providing the protections afforded to clients of N+1 Singer nor for providing advice in connection with the Acquisition or the content of, or any other matter or arrangement described or referred to in, this document. Neither N+1 Singer nor any of their respective directors, officers, subsidiaries or affiliates owes or accepts any duty, liability or responsibility whatsoever (whether direct or indirect, whether in contract, in tort, under statute or otherwise) to any person who is not a client of N+1 Singer in connection with the Acquisition or any other matter referred to in this document, any statement contained herein or otherwise.
Please be aware that addresses, electronic addresses and certain other information provided by Xenetic Shareholders, persons with information rights and other relevant persons in connection with the receipt of communications from Xenetic may be provided to GSL during the Offer Period as required under Section 4 of Appendix 4 to the City Code.
| 2 |
This document has been prepared for the purposes of complying with English law and the information disclosed may be different from that which would have been disclosed if this document had been prepared in accordance with the laws of jurisdictions outside England and Wales. Overseas Shareholders should consult their own legal and tax advisers with regard to the legal and tax consequences of the Scheme and the Acquisition on their particular circumstances.
The release, publication or distribution of this document in jurisdictions other than the United Kingdom and the availability of any offer to shareholders in Xenetic who are not resident in the United Kingdom may be affected by the laws or regulations of any such jurisdictions. Accordingly, any persons who are subject to the laws or regulations of any jurisdiction other than the United Kingdom should inform themselves of, and observe, any applicable requirements.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This document contains statements about Xenetic and GSL that are or may be forward-looking statements. All statements other than statements of historical facts included in this document may be forward-looking statements. Without limitation, any statements preceded or followed by or that include the words “targets”, “plans”, “believes”, “expects”, “aims”, “intends”, “will”, “may”, “anticipates”, “estimates”, “projects” or words or terms of similar substance or the negative thereof, are forward-looking statements. Forward-looking statements include statements relating to the following: (i) future capital expenditures, expenses, revenues, earnings, synergies, economic performance, indebtedness, financial condition, dividend policy, losses and future prospects; (ii) business and management strategies and the expansion and growth of Xenetic’s or GSL operations; and (iii) the effects of government or stock exchange regulation on Xenetic’s or GSL’s business or securities.
Such forward-looking statements involve risks and uncertainties that could significantly affect expected results and are based on certain key assumptions. Many factors could cause actual results to differ materially from those projected or implied in any forward-looking statements. Due to such uncertainties and risks, readers are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date hereof. Each of Xenetic and GSL disclaims any obligation to update any forward-looking or other statements contained in this document, except as required by applicable law.
DISCLOSURE REQUIREMENTS OF THE CITY CODE
Under Rule 8.3(a) of the Code, any person who is interested in 1 per cent. or more of any class of relevant securities of an offeree company or of any securities exchange offeror (being any offeror other than an offeror in respect of which it has been announced that its offer is, or is likely to be, solely in cash) must make an Opening Position Disclosure following the commencement of the offer period and, if later, following the announcement in which any securities exchange offeror is first identified.
An Opening Position Disclosure must contain details of the person’s interests and short positions in, and rights to subscribe for, any relevant securities of each of (i) the offeree company and (ii) any securities exchange offeror(s). An Opening Position Disclosure by a person to whom Rule 8.3(a) applies must be made by no later than 3.30 pm (London time) on the 10th business day following the commencement of the offer period and, if appropriate, by no later than 3.30 p.m. (London time) on the 10th business day following the announcement in which any securities exchange offeror is first identified. Relevant persons who deal in the relevant securities of the offeree company or of a securities exchange offeror prior to the deadline for making an Opening Position Disclosure must instead make a Dealing Disclosure.
Under Rule 8.3(b) of the Code, any person who is, or becomes, interested in 1 per cent. or more of any class of relevant securities of the offeree company or of any securities exchange offeror must make a Dealing Disclosure if the person deals in any relevant securities of the offeree company or of any securities exchange offeror. A Dealing Disclosure must contain details of the dealing concerned and of the person’s interests and short positions in, and rights to subscribe for, any relevant securities of each of (i) the offeree company and (ii) any securities exchange offeror, save to the extent that these details have previously been disclosed under Rule 8. A Dealing Disclosure by a person to whom Rule 8.3(b) applies must be made by no later than 3.30 p.m. (London time) on the business day following the date of the relevant dealing.
| 3 |
If two or more persons act together pursuant to an agreement or understanding, whether formal or informal, to acquire or control an interest in relevant securities of an offeree company or a securities exchange offeror, they will be deemed to be a single person for the purpose of Rule 8.3.
Opening Position Disclosures must also be made by the offeree company and by any offeror and Dealing Disclosures must also be made by the offeree company, by any offeror and by any persons acting in concert with any of them (see Rules 8.1, 8.2 and 8.4). Opening Position Disclosures have already been made both by GSL and by Xenetic.
Details of the offeree and offeror companies in respect of whose relevant securities Opening Position Disclosures and Dealing Disclosures must be made can be found in the Disclosure Table on the Takeover Panel’s website at www.thetakeoverpanel.org.uk, including details of the number of relevant securities in issue, when the offer period commenced and when any offeror was first identified. You should contact the Panel’s Market Surveillance Unit on +44 (0)20 7638 0129 if you are in any doubt as to whether you are required to make an Opening Position Disclosure or a Dealing Disclosure.
NOTICE TO OVERSEAS SHAREHOLDERS
The implications of the Scheme and the Acquisition for Overseas Shareholders may be affected by the laws of the relevant jurisdictions. Overseas Shareholders should inform themselves about and observe any applicable legal requirements. It is the responsibility of each Overseas Shareholder to satisfy himself as to the full observance of the laws of the relevant jurisdiction in connection therewith, including the obtaining of any governmental, exchange control or other consents which may be required, or the compliance with other necessary formalities which are required to be observed and the payment of any issue, transfer or other taxes due in such jurisdiction.
The Acquisition relates to the acquisition of shares in a UK public company and is proposed to be made by means of a scheme of arrangement under Part 26 of the Companies Act. In particular, with respect to investors in the United States, a transaction effected by means of a scheme of arrangement is not subject to the tender offer rules under the US Exchange Act. Accordingly, the Scheme is subject to the disclosure requirements, rules and practices applicable in the United Kingdom to schemes of arrangement, which differ from the requirements of US tender offer rules. Financial information on the Xenetic Group included in the relevant documentation has been prepared in accordance with accounting standards applicable to listed companies in the UK, being IFRS as adopted by the European Union. These may not be comparable to the financial statements of US companies.
Overseas Shareholders in the United States should note the matters set forth in paragraph 17 of Part 2 of this document.
The terms of the Scheme are set out in full in Part 4 of this document. The purpose of the Scheme is to enable GSL to become the owner of the entire issued and to be issued share capital of the Company. This is to be achieved by the cancellation of the Scheme Shares held by Scheme Shareholders and the application of the reserve arising from such cancellation in paying up in full New Shares which have an aggregate nominal value equal to the aggregate nominal value of the Scheme Shares cancelled and issuing the same to GSL. Scheme Shareholders will then receive 56 GSL Consideration Shares for every 175 Scheme Shares held by them on the basis of the Acquisition (as is more fully described herein).
As a result of the Scheme, Scheme Shareholders will be acquiring GSL Consideration Shares from General Sales & Leasing, Inc., a Nevada corporation, in accordance with United States securities laws and the Equivalent Document.
The GSL Consideration Shares to be issued under the Scheme have not been, and will not be, registered under the US Securities Act of 1933, as amended (the “Securities Act”), or under the securities laws of any state, district or other jurisdiction of the United States, the Republic of South Africa, Canada or Japan.
It is expected that the GSL Consideration Shares will be issued in reliance upon the exemption from the registration requirements of the Securities Act provided by Section 3(a)(10) thereof on the basis of the Court Meeting, which will consider, among other things, the fairness of the terms of the Scheme. Under applicable US securities laws, shareholders who are or will be deemed to be affiliates of Xenetic or GSL, prior to or after the Effective Date, will be subject to certain transaction restrictions relating to the GSL Consideration Shares received in connection with the Scheme.
| 4 |
PUBLICATION ON WEBSITE
A copy of this document is, and will be available, free of charge for inspection on the Company’s website at www.xeneticbio.com/investorrelations during the course of the Acquisition but should not be forwarded or transmitted in or into or from any Overseas Jurisdiction.
For the avoidance of doubt, the content of the website referred to above is not incorporated into and does not form part of this document.
COPIES OF THIS DOCUMENT
If you have received this document in electronic form or by it being published on Xenetic’s website: www.xeneticbio.com/investorrelations, you can obtain a hard copy of the document by contacting Share Registrars Limited on +44 (0) 1252 821390. Lines are open 9.00 a.m. to 5.00 p.m. (London time) Monday to Friday (except UK public holidays). The helpline cannot provide advice on the merits of the Acquisition or the Scheme nor give any financial, legal or tax advice. You will not receive a hard copy of this document unless you so request. You may also inform Share Registrars Limited that you wish all future documents, announcements and information in relation to the Acquisition or the Scheme to be sent to you in hard copy.
| 5 |
ACTION TO BE TAKEN
VOTING AT THE COURT MEETING AND THE GENERAL MEETING
There will be two separate meetings of Shareholders: the Court Meeting and the General Meeting. Scheme Shareholders will be entitled to vote at the Court Meeting and all Shareholders will be entitled to vote at the General Meeting. The Court Meeting and the General Meeting will be held at the offices of Pinsent Masons LLP, 30 Crown Place, London EC2A 4ES, United Kingdom, on 17 December 2013 at 10.00 GMT and 10.30 a.m. GMT respectively (or, in the case of the General Meeting, if later, as soon as the Court Meeting has been concluded or adjourned). The Scheme requires approval of the Scheme Resolutions to be tabled at both of these Meetings.
Please check that you have received the following with this document:
● a blue Form of Proxy for use in respect of the Court Meeting;
● a white Form of Proxy for use in respect of the General Meeting; and
● a reply-paid envelope for use in the UK for the return of the Forms of Proxy.
If you have not received all of these documents, please contact the Company’s Registrars, Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL, or call on +44 (0) 1252 821390 between 9.00 a.m. and 5.00 p.m. GMT Monday to Friday (except UK bank holidays).
To vote on the Scheme:
It is important that, for the Court Meeting in particular, as many votes as possible are cast so that the Court may be satisfied that there is a fair representation of Scheme Shareholder opinion. Whether or not you plan to attend the Meetings, each eligible Shareholder is requested to complete and sign both the blue and white Forms of Proxy and return them, in accordance with the instructions printed thereon, by post or, during normal business hours only, by hand to the Company’s Registrars, Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL or by fax to Share Registrars Limited on +44 (0) 1252 719232 or by scan and email to Share Registrars Limited at proxies@shareregistrars.uk.com, as soon as possible, but in any event so as to be received by no later than:
| ● in respect of the blue Form of Proxy for the Court Meeting: | 10.00 a.m. GMT on 15 December 2013 |
| ● in respect of the white Form of Proxy for the General Meeting: | 10.30 a.m. GMT on 15 December 2013 |
(or in the case of any adjournment, not later than 48 hours before the time fixed for the holding of the adjourned Meeting). If you wish to appoint more than one proxy you should request additional Forms of Proxy from Share Registrars Limited and submit them in accordance with the instructions set out in this document.
Returning the Forms of Proxy will enable your votes to be counted at the Meetings in the event of your absence. If the blue Form of Proxy for use at the Court Meeting is not returned by 10.00 a.m. GMT on 15 December 2013, it may be handed to the Chairman of the Court Meeting at the Court Meeting before the start of the Court Meeting. However, in the case of the General Meeting, unless the white Form of Proxy is returned by 10.30 a.m. GMT on 15 December 2013, it will be invalid. The completion and return of a Form of Proxy will not prevent you from attending and voting at the relevant Meeting, or any adjournment thereof, in person should you wish to do so and are so entitled.
Shareholders are encouraged to return their Forms of Proxy as soon as possible, to ensure they arrive before the relevant deadline.
To vote at the Meetings using a proxy appointment through CREST
CREST members who wish to appoint a proxy or proxies through the CREST electronic proxy appointment service may do so using the procedures described in the CREST Manual. CREST personal members or other CREST sponsored members, and those CREST members who have appointed a service provider(s), should refer to their CREST sponsor or voting service provider(s), who will be able to take the appropriate action on their behalf.
| 6 |
In order for a proxy appointment or instruction made using the CREST service to be valid, the appropriate CREST message (a “CREST Proxy Voting Instruction”) must be properly authenticated in accordance with Euroclear’s specifications, and must contain the information required for such instruction, as described in the CREST Manual (available via www.euroclear.com/CREST). The message, regardless of whether it constitutes the appointment of a proxy or is an amendment to the instruction given to a previously appointed proxy, must, in order to be valid, be transmitted so as to be received by the issuer’s agent (ID 7RA36 by 6.00 p.m. on 13 December 2013 in the case of the Court Meeting and by 6.00 p.m. on 13 December 2013 in the case of the General Meeting (or, in the case of any adjournment, no later than 6.00 p.m. on the day two days before the day of the adjourned meeting (excluding any day which is not a working day) in respect of the Court Meeting or by no later than 6.00 p.m. on the day two days before the adjourned meeting (excluding any day which is not a working day) in respect of the General Meeting)). For this purpose, the time of receipt will be taken to be the time (as determined by the time stamp applied to the message by the CREST Application Host) from which the issuer’s agent is able to retrieve the message by enquiry to CREST in the manner prescribed by CREST. After this time any change of instructions to proxies appointed through CREST should be communicated to the appointee through other means.
CREST members and, where applicable, their CREST sponsors or voting service providers should note that Euroclear does not make available special procedures in CREST for any particular message. Normal system timings and limitations will, therefore, apply in relation to the input of CREST Proxy Voting Instructions. It is the responsibility of the CREST member concerned to take (or if the CREST member is a CREST personal member, or sponsored member, or has appointed a voting service provider, to procure that his or her CREST sponsor or voting service provider(s) take(s)) such action as shall be necessary to ensure that a message is transmitted by means of the CREST system by any particular time. In connection with this, CREST members and, where applicable, their CREST sponsors or voting system providers are referred, in particular, to those sections of the CREST Manual concerning the practical limitations of the CREST system and timings.
Xenetic may treat as invalid a CREST Proxy Voting Instruction in the circumstances set out in Regulation 35(5)(a) of the Uncertificated Securities Regulations.
Appointment of multiple proxies and multiple proxy voting instructions
Shareholders are entitled to appoint a proxy in respect of some or all of their Shares. Shareholders are also entitled to appoint more than one proxy. A space has been included in the Forms of Proxy to allow you to specify the number of Shares in respect of which that proxy is appointed. If you return the blue Form of Proxy duly executed but leave this space blank, your blue Form of Proxy will be invalid. If you return the white Form of Proxy duly executed but leave this space blank, you will be deemed to have appointed the proxy in respect of all of your Shares. If you wish to appoint more than one proxy in respect of your shareholding you should contact the Company’s Registrars, Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL or call Share Registrars Limited on +44 (0) 1252 821390 between 9.00 a.m. and 5.00 p.m. GMT Monday to Friday (except UK bank holidays) for further Forms of Proxy or photocopy the Form of Proxy as required.
You may appoint more than one proxy in relation to a Meeting, provided that each proxy is appointed to exercise the rights attached to different Shares held by you. The following principles shall apply in relation to the appointment of multiple proxies:
| (a) | The Company will give effect to the intentions of Shareholders and include votes wherever and to the fullest extent possible. |
| (b) | Where a Form of Proxy does not state the number of Shares to which it applies (a “blank proxy”) then, subject to the following principles where more than one proxy is appointed, that proxy is deemed to have been appointed in relation to the total number of Shares registered in the name of the appointing member (the “member’s entire holding”). In the event of a conflict between a blank proxy and a proxy which does state the number of Shares to which it applies (a “specific proxy”), the specific proxy shall be counted first, regardless of the time it was delivered or received (on the basis that each white Form of Proxy was received prior to the deadline for receipt of white Forms of Proxy and, as far as possible, the conflicting white Form of Proxy should be judged to be in respect of different Shares) and remaining Shares will be apportioned to the blank proxy (pro rata if there is more than one). |
| 7 |
| (c) | Where there is more than one proxy appointed and the total number of Shares in respect of which proxies are appointed is no greater than the Shareholder’s entire holding, it is assumed that proxies are appointed in relation to different Shares, rather than that conflicting appointments have been made in relation to the same Shares. That is, there is only assumed to be a conflict where the aggregate number of Shares in respect of which proxies have been appointed exceeds the Shareholder’s entire holding. |
| (d) | When considering conflicting proxies, later proxies will prevail over earlier proxies, and which proxy is later will be determined on the basis of which proxy is last delivered (or received). Proxies in the same envelope will be treated as having been sent and received at the same time, to minimise the number of conflicting proxies. |
| (e) | If conflicting proxies are sent or received at the same time in respect of (or deemed to be in respect of) an entire holding, and if the Company is unable to determine which was delivered or received last, none of them will be treated as valid. |
| (f) | Where the aggregate number of Shares in respect of which proxies are appointed exceeds a Shareholder’s entire holding and it is not possible to determine the order in which they were delivered or received (or they were all delivered or received at the same time), the number of votes attributed to each proxy will be reduced pro rata. |
| (g) | Where the application of paragraph (f) above gives rise to fractions of Shares, such fractions will be rounded down. |
| (h) | If a Shareholder appoints a proxy or proxies and then decides to attend the Court Meeting or General Meeting in person and vote using his poll card, then the vote in person will override the proxy vote(s). If the vote in person is in respect of the Shareholder’s entire holding then all proxy votes will be disregarded. If, however, the Shareholder votes at the Meetings in respect of less than the Shareholder’s entire holding, then if the Shareholder indicates on his polling card that all proxies are to be disregarded, that shall be the case; but if the Shareholder does not specifically revoke proxies, then the vote in person will be treated in the same way as if it were the last received proxy and earlier proxies will only be disregarded to the extent that to count them would result in the number of votes being cast exceeding the Shareholder’s entire holding. |
| (i) | In
relation
to
paragraph
(h)
above,
in
the
event
that
a
Shareholder
does
not
specifically
revoke
proxies, it will not be possible for the Company to determine the intentions of the Shareholder in this regard. However, in light of the aim to include votes wherever and to the fullest extent possible, it will be assumed that earlier proxies should continue to apply to the fullest extent possible. |
Assistance
If you have any questions relating to the Meetings, this document or the completion and return of the Forms of Proxy, please address your questions in writing to the Company’s Registrars, Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL, or call Share Registrars Limited on +44 (0) 1252 821390 between 9.00 a.m. and 5.00 p.m. GMT Monday to Friday (except UK bank holidays). Please note that no advice on the merits of the matters described in this document or legal, tax or financial advice can or will be given by Share Registrars.
IT IS IMPORTANT THAT, FOR THE COURT MEETING, AS MANY VOTES AS POSSIBLE ARE CAST SO THAT THE COURT MAY BE SATISFIED THAT THERE IS A FAIR REPRESENTATION OF SCHEME SHAREHOLDER OPINION. SCHEME SHAREHOLDERS ARE THEREFORE STRONGLY URGED TO COMPLETE, SIGN AND RETURN THEIR FORMS OF PROXY AS SOON AS POSSIBLE.
Date
This document is dated 21 November 2013.
| 8 |
TABLE OF CONTENTS
| Page | ||
| EXPECTED TIMETABLE OF PRINCIPAL EVENTS | 10 | |
| PART 1 | LETTER FROM THE CHAIRMAN OF XENETIC BIOSCIENCES PLC | 11 |
| PART 2 | EXPLANATORY STATEMENT | 23 |
| PART 3 | CONDITIONS AND FURTHER TERMS OF THE ACQUISITION | 37 |
| PART 4 | THE SCHEME OF ARRANGEMENT | 48 |
| PART 5 | FINANCIAL INFORMATION RELATING TO THE XENETIC GROUP | 52 |
| PART 6 | UK TAXATION | 54 |
| PART 7 | INFORMATION RELATING TO GSL | 55 |
| PART 8 | ADDITIONAL INFORMATION | 79 |
| PART 9 | DEFINITIONS | 96 |
| PART 10 | NOTICE OF COURT MEETING | 101 |
| PART 11 | NOTICE OF GENERAL MEETING | 104 |
| 9 |
EXPECTED TIMETABLE OF PRINCIPAL EVENTS
| Event | Time and/or date |
| Latest time for lodging blue Forms of Proxy | 10.00 a.m. on 15 December 2013(1) |
| for the Court Meeting | |
| Latest time for lodging white Forms of Proxy | 10.30 a.m. on 15 December 2013(1) |
| for the General Meeting | |
| Voting Record Time for Court Meeting and | 6.00 p.m. on 13 December 2013(2) |
| General Meeting | |
| Court Meeting | 17 December 2013 |
| General Meeting | 17 December 2013(3) |
| The folowing dates are subject to change, please see Note (4) below: | |
| Last day of dealings in, and for registration of | 22 January 2014 |
| transfers of, Xenetic Shares | |
| Scheme Record Time | 6.00 p.m. on 22 January 2014 |
| Suspension of Xenetic Shares from trading on AIM | 7.30 a.m. on 23 January 2014 |
| Court Hearing (Sanction of Scheme and | 23 January 2014 |
| Reduction of Capital) | |
| Effective Date of the Scheme | 23 January 2014 |
| Cancellation of admission of Xenetic Shares to | 7.00 a.m. on 24 January 2014 |
| trading on AIM | |
| Quotation of GSL Consideration Shares on OTCBB | 27 January 2014 |
| and OTCQB becomes effective | |
| Latest date for despatch of GSL Consideration Share | 7 February 2014 |
| certificates under the Scheme |
The Court Meeting and the General Meeting will each be held at the offices of Pinsent Masons LLP, 30 Crown Place, London EC2A 4ES, United Kingdom.
Notes:
| (1) | If the blue Form of Proxy for the Court Meeting is not returned by the above time, it may be handed to the Chairman of the Court Meeting before the start of that Meeting. However, the white Form of Proxy for the General Meeting must be lodged by 10.30 a.m. GMT on 15 December 2013 in order to be valid. Please see ‘Action To Be Taken’ in paragraph 15 of Part 1 of this document. |
| (2) | If either the Court Meeting or the General Meeting is adjourned, the Voting Record Time for the adjourned meeting will be 6.00 p.m. GMT on the date two working days before the date set for the adjourned Meeting. |
| (3) | The General Meeting will commence at 10.30 a.m. GMT or, if later, immediately after the conclusion of the Court Meeting or any adjournment thereof. |
| (4) | These times and dates are indicative only and will depend, among other things, on the date upon which the Court sanctions the Scheme and confirms the associated Reduction of Capital. |
Unless otherwise stated, all references in this document to times are to times in London, England.
The dates given are based on current expectations and may be subject to change. If any of the expected dates change, the Company will give adequate notice of the change by issuing an announcement through a Regulatory Information Service.
| 10 |
PART 1
LETTER FROM THE CHAIRMAN OF XENETIC BIOSCIENCES PLC
XENETIC BIOSCIENCES PLC
(Incorporated in England and Wales under the Companies Act 1985 with registered number 03213174)
| Directors: | Registered Office: |
| Sir Brian Richards (Non-executive Chairman) | London Bioscience Innovation Centre |
| M Scott Maguire (Chief Executive Officer) | 2 Royal College Street |
| Colin William Hill (Chief Financial Officer) | London |
| Professor Gregory Gregoriadis (Chief Scientific Officer) | NW1 0NH |
| Dr Dmitry D Genkin (Non-executive director) Firdaus Jal Dastoor (Non-executive director) Artur Isaev (Non-executive director) | |
| Roman Knyazev (Non-executive director) | |
| 21 November 2013 |
To: Shareholders and, for information only, to holders of options and warrants Dear Shareholder
RECOMMENDED
OFFER
BY
GENERAL SALES & LEASING, INC
FOR
XENETIC BIOSCIENCES PLC
(to
be effected by means of a Scheme of Arrangement
under Part 26 of the Companies Act 2006)
1. Introduction
On 12 November 2013, Xenetic announced that the boards of GSL and Xenetic had reached agreement on the terms of a recommended proposal for the Acquisition under which GSL will acquire the entire issued and to be issued share capital of Xenetic.
Xenetic has been implementing its longer term strategy to migrate its corporate seat to, and expand in, the US, noting the significant value gap that exists between the biotechnology sector in the UK and in the US, where Xenetic believes the sector is better understood and more widely followed. Completion of the Acquisition will be the first step in the intended process of achieving a subsequent listing on an Exchange in a manner which the Board believes can be achieved more easily, more quickly and in a more cost-effective manner than would be possible were Xenetic to apply for such a quotation itself.
I am writing to you to explain:
| (a) | the background to, and terms of, the Acquisition; |
| (b) | why the Xenetic Directors (except for Roman Knyazev) consider the Acquisition to be fair and reasonable to Shareholders; and |
| (c) | why the Xenetic Directors (except for Roman Knyazev) are recommending that Shareholders vote in favour of the Scheme at the Court Meeting and the Special Resolution at the General Meeting as such Xenetic Directors have irrevocably agreed to do (or procure to be done) in respect of the Xenetic Shares held beneficially by them. |
| 11 |
Roman Knyazev is an employee and investment manager of Rusnano (a significant shareholder in Synbio, the 45 per cent. shareholder of Xenetic). He was appointed to the Board as a nominated director by Synbio pursuant to the relationship agreement between Synbio and Xenetic dated 28 November 2011 (the “Relationship Agreement”). As a result of his employment by Rusnano, a Russian state-owned entity, Mr Knyazev is unable to express a view on any transaction involving a board recommendation for shareholders to vote either in favour or against any particular proposal.
Since his appointment, the Xenetic Board has been aware of this situation and is wholly supportive of it and such a situation has not arisen to date. This does not affect Mr Knyazev’s ability to participate as a director of Xenetic as regards day-to-day and operational matters. Dr Dmitry Genkin and Dr Artur Isaev are directors of both Xenetic and SynBio (but not of Rusnano) and, as stated below, have voted in favour of the Scheme and recommend it to Xenetic Shareholders along with the remainder of the Xenetic Board.
The Scheme is conditional, amongst other things, on the approval by Shareholders of the Scheme Resolutions, proposed at the General Meeting.
The purpose of this document is therefore to provide details of the Scheme and to convene the General Meeting at which the Scheme Resolutions required to effect the Scheme will be proposed. The Notice of General Meeting is set out on page 104 of this document.
Shareholders are strongly advised to read this document carefully and seek their own independent financial, tax and legal advice and to consider, amongst other matters, paragraph 5 below headed ‘Background to and reasons for the Xenetic Directors’ recommendation of the Acquisition’, before casting their votes.
2. Summary of the terms of the Acquisition
The Acquisition is to be implemented by means of a scheme of arrangement between Xenetic and Scheme Shareholders under Part 26 of the Companies Act and involves a reduction of capital under section 648 of the Companies Act. Details of the Acquisition (including the Conditions to which it is subject) are set out in Part 2, Part 3 and Part 4 of this document. The Scheme requires the requisite approval of the Scheme Shareholders at a meeting convened by the Court and the subsequent sanction of the Court. The Reduction of Capital requires the approval of Shareholders at the General Meeting and the subsequent confirmation of the Court. Once the Scheme becomes Effective, the terms will be binding on all Scheme Shareholders irrespective of whether or not they attend or vote at the Meetings (and if they attend and vote, whether or not they vote in favour).
The purpose of the Scheme is to enable GSL to become the holder of the entire issued and to be issued share capital of Xenetic. This is to be achieved by the cancellation of the Scheme Shares and the application of the reserve resulting from such cancellation in paying up in full such number of New Shares as is equal to the number of Scheme Shares cancelled, and issuing the same to GSL, in circumstances where the Scheme Shareholders on the register of members at the Scheme Record Time will receive:
for every 175 Scheme Shares: 56 GSL Consideration Shares
Xenetic Shareholders holding 174 Scheme Shares or less will be entitled to vote at the Meetings. They will not however receive any GSL Consideration Shares. The GSL Consideration Shares attributable to their Scheme Shares will be aggregated and sold for cash on behalf of such Xenetic Shareholders as soon as reasonably practicable following completion of the Acquisition at the then prevailing market price on the OTCBB or OTCQB. The proceeds will then be converted into sterling at the exchange rate prevailing at that time and Xenetic Shareholders with entitlements to proceeds of such sale of less than £1 will have their proceeds aggregated and donated to charity. Those with entitlements to proceeds of £1 or more will receive sterling cheques for their entitlements as soon as reasonably practicable after such sale. Xenetic Shareholders with entitlements to proceeds of £1 or more will be able to opt to have such proceeds donated to charity. The white Form of Proxy contains a box to tick should such a Scheme Shareholder wish to elect to donate his or her proceeds to charity.
Any Xenetic Shareholders to the extent holding a number of Scheme Shares not divisible by 175, will also have such Scheme Shares aggregated together with similar Scheme Shares of other Xenetic Shareholders to result in GSL Consideration Shares to be sold on behalf of such Xenetic Shareholders in the same manner as explained above. Again, Xenetic Shareholders with entitlements to proceeds of such sale of less than £1 will have their proceeds aggregated and donated to charity. Those with entitlements to proceeds of £1 or more will receive sterling cheques for their entitlements as soon as reasonably practicable after such sale. Xenetic Shareholders with entitlements to proceeds of £1 or more will also be able to opt to have such proceeds donated to charity on the same basis as above.
| 12 |
On the basis of the value of a GSL Consideration Share of 18.59 pence as at 11 November 2013 set out at Part 12 of this document, the Acquisition values the entire existing issued share capital of the Company at approximately £24.3 million and each Scheme Share at approximately 5.95 pence. This represents a premium or discount of approximately:
| ● | a premium of 8.18 per cent. to the Closing Price per Scheme Share of 5.5 pence on 20 November 2013 (being the latest practicable date prior to the posting of this document); |
| ● | a discount of 8.49 per cent. to the Closing Price per Scheme Share of 6.5 pence on 11 November 2013 (being the latest practicable date prior to the issue of the Announcement); |
| ● | a premium of 5.75 per cent. to the Closing Price per Scheme Share of 5.625 pence on 20 October 2013 (being the Business Day prior to the announcement that Xenetic was in discussions with GSL); and |
| ● | a premium of 5.75 per cent. to the Closing Price per Scheme Share of 5.625 pence on 15 July 2013 (being the Business Day prior to the commencement of the Offer Period). |
The expected transaction timetable is set out on page 10 of this document. It is expected that the Acquisition and the resolutions required to implement the Scheme will be put to Xenetic Shareholders at the Court Meeting and the General Meeting which are expected to be held on 17 December 2013. It is expected that, subject to satisfaction or waiver of the Conditions, the Effective Date will be 23 January 2014. If the Scheme becomes Effective, it will be binding on all Scheme Shareholders, irrespective of whether or not they attended or voted at the Court Meeting or the General Meeting. Further details of the Scheme, including the arrangements for settlement of the consideration payable to Scheme Shareholders, are set out in the Explanatory Statement in Part 2 of this document.
Application has been made in accordance with AIM Rule 41 to the London Stock Exchange to cancel the admission to trading on AIM of Xenetic Shares. Cancellation is conditional upon the Scheme (and therefore the Acquisition) having become Effective. The announcement to be issued by Xenetic on 22 November 2013 provides Xenetic Shareholders with due notice of the intended suspension from trading on AIM which is expected to take place at 7.30 a.m. on 23 January 2014, and of the intended cancellation of trading on AIM which is expected to take place at 7.00 a.m. on 24 January 2014.
Accordingly the last day of dealings in, and for registration of transfers of, Xenetic Shares is presently anticipated to be 22 January 2014. The result of the Court Hearing will be announced on 23 January 2014. No transfers of Xenetic Shares will be registered after market close on 22 January 2014.
3. GSL Consideration Shares
The GSL Consideration Shares to be issued as consideration for the Acquisition will be issued in certificated form only. There is expected to be issued 130,520,137 shares of US$0.01 par value each in the capital of GSL assuming no further shares are issued by Xenetic up to the Effective Date. Fractions of GSL Consideration Shares will not be allotted or issued pursuant to the Acquisition.
It is the intention of the Proposed Directors and Xenetic that GSL, following the completion of the Acquisition, will seek a listing of its shares on an Exchange at the earliest practicable opportunity.
The GSL Consideration Shares will, in the meantime be available for quotation on the OTCBB and the OTCQB and will be issued free from all liens, charges, encumbrances and other third party rights and/or interests of any nature whatsoever. It is expected that the GSL Consideration Shares to be issued will be entitled to be deposited, in the normal course, into an individual shareholder’s trading account and then available for immediate trading.
| 13 |
Xenetic Shareholders should, however, be aware that the deposit of their GSL Consideration Shares into their existing trading accounts within the UK or outside may be limited due to the internal rules of operations of the shareholder’s chosen broker-dealer or its clearing firm. It is not uncommon for some broker-dealers and/or their clearing firms to require, among other things, the following items to effectuate the deposit of newly issued OTCBB and OTCQB shares: (1) substantial information on the transaction in which the shareholder received the shares; and/or (2) a legal opinion related to the shares. Moreover, some firms may even outright refuse to accept the deposit of newly issued OTCBB and OTCQB shares, among other limitations, due to such internal rules. As a result, neither GSL, nor Xenetic, can assure any shareholder that they will be able to deposit their GSL Consideration Shares in their existing brokerage accounts or that they will not be required to provide additional information or documentation, not otherwise provided by either company with stock certificates. Consequently, shareholders may be required to set up a new trading account with a different broker-dealer or otherwise find themselves subject to delays and other issues in the deposit of their GSL Consideration Shares. The deposit of the GSL Consideration Shares will be the responsibility of the recipient shareholder.
After receipt of the certificates representing their GSL Consideration Shares, shareholders may deposit their GSL Consideration Shares, subject to the limitations discussed above regarding deposits, either by providing their certificate to the broker-dealer where their trading account is set up, or by arranging for their GSL Consideration Shares to be electronically transferred into the account. If delivered in electronic form at this later date, the shareholder will need to confirm that their broker-dealer allows for such transfers of OTCBB shares, and then provide the transfer agent with the necessary information regarding such Shares and their trading account to allow the electronic transfer to occur. Shares transferred in this manner will be tradable by the shareholder upon acceptance of the deposit into the trading account consistent with the rules of the broker-dealer with which such Shares are deposited. While Xenetic will assist where it can, because the deposit of the GSL Consideration Shares is dependent on the individual processing rules of the shareholder’s choice of broker-dealer, it will be the responsibility of the individual shareholder receiving their GSL Consideration Share certificate to process and facilitate the deposit of such Shares. Xenetic will take no responsibility or liability whatsoever for ensuring the deposit of the GSL Consideration Shares into a specific shareholder trading account or with a specific broker-dealer.
The GSL Consideration Shares will be capable of being held in both certificated and uncertificated form, and will be issued credited as fully paid and will rank pani passu in all respects with the New GSL Shares, including as to voting rights and the right to receive and retain all dividends and other distributions declared, paid or made after the Effective Date. The New GSL Shares and GSL Consideration Shares will be denominated in US Dollars.
The GSL Consideration Shares will be issued on implementation of the Scheme to Scheme Shareholders on the register immediately following the Scheme Record Time. Details of the rights attaching to the GSL Consideration Shares are set out in paragraph 4 of Part VIII of the Equivalent Document, the text of which is set out for convenience at Part 7 of this document, and in paragraph D of Part 3 of this document.
Upon the Scheme becoming Effective and the receipt by Xenetic Shareholders of their entitlements to GSL Consideration Shares, the protections contained within the City Code will not apply to such holdings. Please refer to paragraph C of Part 3 of this document.
4. Conditions to the Acquisition
The implementation of the Acquisition is subject to the Conditions set out in section A of Part 3 of this document. To become Effective, the Acquisition requires, amongst other things:
| (a) | the approval of the Scheme at the Court Meeting by the necessary majority of the Scheme Shareholders present and voting, either in person or by proxy; |
| (b) | the passing of the Special Resolution at the General Meeting; |
| (c) | the Court sanctioning the Scheme and confirming the Reduction of Capital at the Court Hearing; |
| (d) | the satisfaction or waiver of the other Condition; and |
| (e) | the delivery to the Registrar of Companies of copies of the Court Order(s) sanctioning the Scheme and confirming the Reduction of Capital together with a Statement of Capital. |
Shareholders are referred to Part 3 of this document for the full details of the Conditions.
| 14 |
5. Background to and reasons for the Xenetic Directors’ recommendation of the Acquisition
The Board believes that at the present time smaller quoted bioscience companies, in general, have a higher profile and a better investor following in the US than is found in European markets in general and in the UK market, in particular. The Xenetic Directors believe that by achieving a listing for Xenetic on an Exchange, significant benefits will accrue to Xenetic and its shareholders including, but not exclusively:
| (a) | the ability to attract and incentivise qualified US personnel with orphan drug development and launch experience; |
| (b) | easier access to finance on reasonable terms; |
| (c) | a better rating of Xenetic’s shares; and |
| (d) | higher levels of share trading liquidity. |
The Board has considered a number of options to achieve the goal of migration to the US, including seeking a quotation of Xenetic’s shares directly on an Exchange and seeking a merger with an existing US quoted biotechnology company with complementary activities. Following careful consideration of the issues, the Board has determined that the most appropriate way of achieving this outcome is to be acquired by an SEC-reporting company and then seek to achieve a quotation on an Exchange within a reasonable period; and to effect such an acquisition by the Scheme.
Xenetic’s plan is for it to be acquired on a share-for-share basis by GSL, where the level of effective dilution to Xenetic’s Shareholders does not exceed 3 per cent. as at the Effective Date. This means that, other than for such dilution and the fees and costs of the Acquisition (which are deemed to be the “cost” of achieving Xenetic’s aims), the group formed by the completion of the Acquisition will be effectively the same group as at present, with the same assets and liabilities, save that it will have a US holding company (GSL) with an enhanced ability to achieve a listing on an Exchange. Whilst Xenetic and GSL have commenced developing a plan regarding such a listing, there is no guarantee that such a listing can be achieved.
In particular, GSL is subject to various regulatory requirements, including those of the SEC and the various trading exchanges where its equity shares may trade. Any securities exchange (for example, NASDAQ) can and will apply its own rules and/or analysis to GSL’s eligibility for listing which may adversely impact the Enlarged Group’s attempt to be listed on a securities exchange or the tradability of its shares.
6. Irrevocable Undertakings
Xenetic has sought and received irrevocable undertakings from those Xenetic Directors who are beneficial owners of Xenetic Shares to vote in favour of the Scheme Resolutions in respect of their entire beneficial holdings of Xenetic Shares, a total of 11,271,086 Xenetic Shares, representing approximately 2.76 per cent. of the existing issued ordinary share capital of Xenetic, as at 20 November 2013 (being the latest practicable date prior to the publication of this document).
Xenetic has sought and received irrevocable undertakings from certain other Xenetic Shareholders who beneficially own or otherwise control Xenetic Shares to vote in favour of the Scheme Resolutions in relation to a total of 96,668,741 Xenetic Shares, representing approximately 23.7 per cent. of the existing issued ordinary share capital of Xenetic as at 20 November 2013 (being the latest practicable date prior to the publication of this document).
In relation to the Scheme Resolutions, Xenetic has therefore received total irrevocable undertakings in respect of 107,939,827 Xenetic Shares representing, in aggregate, 26.46 per cent. of the existing issued ordinary share capital of Xenetic as at 20 November 2013 (being the latest practicable date prior to the publication of this document).
Further details of the above irrevocable undertakings are set out in paragraph 7 of Part 8 of this document.
7. Information on Xenetic and current trading and prospects
Xenetic is committed to becoming a US-based and Exchange-listed speciality drug developer focused on achieving full value recognition of the Xenetic patent-protected portfolio with its core technologies and targeted therapeutic offerings.
| 15 |
Xenetic is currently a UK based biopharmaceutical company with core technology capabilities, intended to facilitate the development of a new generation of biological drugs, vaccines and oncology therapies.
Together with its collaborative partners SynBio, Pharmasynthez, SIIL and Baxter, the Group is developing a pipeline of next generation biotherapeutics based on its proprietary PolyXen, Oncohist and ImuXen platform technologies. The Group is pursuing a development strategy of clinical advancement through its own efforts and those of its collaborative partners (such as SynBio, Pharmsynthez, SIIL and Baxter) as further described below.
As announced on 31 July 2013, the Group has received regulatory approval in Australia and New Zealand to commence its Phase II FDA/ICH-compliant clinical trial for ErepoXen®, the Group’s PolyXen®-based biologic candidate for anaemia in pre-dialysis patients with Chronic Kidney Disease (“CKD”). Since that date, the drug has been released for export from India (where it is produced by SIIL). It is now available at the Group’s 10 clinical sites in Australia and New Zealand ready for first patient dosing in the trial being managed by Novotech, the clinical research organisation chosen to conduct the trial. The Company expects that it will be able to provide guidance on its first interim trial results in H2-2014. The trial is expected to finally report completion of Phase II studies by H1-2015.
SynBio:
Phase III trials for ErepoXen® in Russia have progressed rather more slowly than originally anticipated due to patient recruitment limitations. SynBio has now both increased the number of clinical trial sites and made some changes to the trial protocol in order to increase the rate of recruitment. Accordingly, the Group retains a high degree of confidence in the ultimate clinical outcome and the resultant marketing of the product in Russia, the former CIS and elsewhere.
Clinical trials on OncoHistTM candidate for Acute Myeloid Leukaemia are ongoing. The Phase I/II(a) dose-ranging phase of the trial for Non-Hodgkin’s Lymphoma has been completed. In neither case have there been any reported Serious Adverse Events (“SAEs”).
In December 2011, Dmitry Genkin and Peter Kruglyakov undertook to use best endeavours to procure by 16 January 2012 the novation, licence and/or re-transfer back to the Group of certain Cryonix/Onchoist intellectual property previously transferred and/or licensed out of SymbioTech to SynBio or its connected persons. While progress has been made, the relevant documentation has not yet been signed.
Pharmsynthez:
The ImuXen®-based Multiple Sclerosis candidate, MyeloXenTM has completed Stage I trials in healthy volunteers with no SAEs. The trial has now entered the Stage II stage in patients. The dose-ranging phase has been completed with no SAEs reported. The dose confirmation studies in patients are expected to commence within 3 months.
Pre-clinical work on PulmoXenTM has been completed. Pharmsynthez imminently expects to receive Russian regulatory approval to move this PolyXen®-based biologic proprietary candidate for Cystic Fibrosis into the clinic.
SIIL:
The most recently announced advance in Indian clinical work on the Group’s ErepoXen® product candidate relates to the screening of patients for a Phase I/II trial, treating CKD patients on dialysis.
Dana-Farber:
Work on this previously announced collaborative agreement with Dana-Farber Cancer Institute in Boston, examining the novel mechanism of action of OncoHistTM, is still in progress. The Group is awaiting interim data from the SynBio Phase I/II(a) trial prior to submitting an Investigational New Drug (“IND”) filing with the US FDA.
Baxter:
The US$1m license extension fee receivable in June 2013 was duly settled by Baxter, thereby granting that company a 12-month extension to file an IND for their PSA-Factor VIII candidate for haemophilia.
| 16 |
Summary:
The primary objective of the Group is monetising the Group’s IP by transitioning out of the research phase and into product- and indication-specific product development. In summary, the Group’s product development pipeline is as follows:
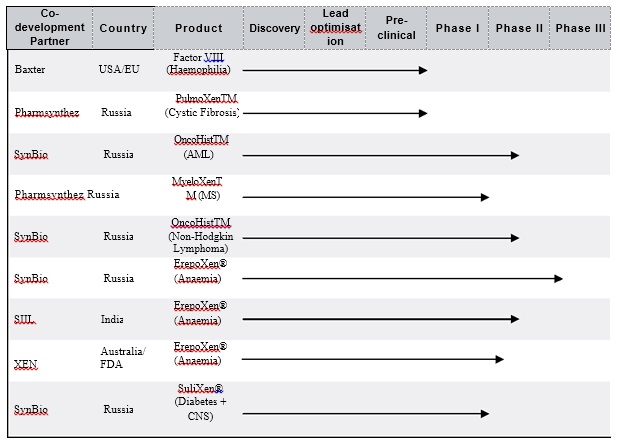
The above table provides an aggregate overview of the drug development pipeline for the Group. To achieve the most rapid and effective development of the candidates most likely to make rapid progress into and through the clinic, it is Xenetic’s intention that, from completion of Xenetic’s move of all drug development activities to the US, there will be a distillation and concentration of its internal development programmes to three candidates, being:
(1) PSA-EPO (Anaemia)
(2) OncoHist (Leukaemia)
(3) PSA-Dnase (Cystic Fibrosis).
8. Funding Outlook
In the announcement of its interim report and unaudited condensed financial statements for the six months ended 30 June 2013 released on 30 September 2013, the Company stated:
“... new capital is planned to be raised in the last quarter of 2013 (whether or not the US listing process has been concluded by that time) and in the second or third quarter of 2014, to fund planned activities through September 2014.”
As at 20 November 2013, being the nearest practicable date prior to the publication of this document, the Group had cash reserves of approximately £3.3 million. As indicated in the interim report, due to the increase in operating costs arising from expenditures such as the stepping-up of the US transition process and the Australian PSA-EPO clinical trials, the monthly capital expenditure rate has risen from an historical rate of circa £250,000 to circa £450,000 per calendar month over the quarter ended June 2013; further increases are expected in the upcoming two quarters partly as a consequence of planned completion of the US transition but also to the expected roll-out of the PSA-EPO clinical trial.
| 17 |
This position is in line with the long-term strategy of the Group. It has not proven practicable to raise capital in the last quarter of 2013 given the Company’s preparation for the Scheme. New capital is planned to be raised in the first half of 2014, to fund planned activities through September 2014. Further announcements will be made by Xenetic (or GSL as the case may be) in due course.
9. Risk Factors
The business of the Group is subject to various risk factors, the principal ones being set out in Part 13 of this document. These risks will continue to apply after the Scheme becomes Effective, in relation to the Enlarged Group and the GSL Consideration Shares.
10. Information on GSL
GSL Shares are currently available for quotation on the OTCBB and OTCQB. There has been only limited trading recently in such shares and therefore they are not considered to have a public valuation or market.
GSL is not currently identified as a ‘shell company’ under relevant SEC rules and regulations, which means that its stock is not subject to certain limitations contained in these rules. There can be no assurance, however, that the SEC and/or various regulatory bodies in the US will not reject this classification and effectively apply the shell company rules to GSL in the future. Additionally, any Exchange may apply its own rules and/or analysis and reach the conclusion that GSL was or is a shell company.
Any such determination, either by the SEC, one of the regulatory bodies in the US or an Exchange could adversely impact the Enlarged Group’s attempt to be listed on an Exchange or the tradability of its shares, and/or the cost and time involved in doing so. This may include being subject to the “seasoning rules” of an Exchange prior to being quoted, in effect requiring GSL to wait a year or more before being allowed to obtain a listing on such Exchange.
GSL was incorporated in August 2011 for the purpose of owning and operating helicopters for use in sightseeing tours and as pilot training aircraft. Whilst there is demand for hourly helicopter rental in Las Vegas, GSL believes that it is in its best interests to enter into the Acquisition of Xenetic, and dispose of its current business operations.
Accordingly, on 12 November 2013, GSL entered into an agreement with its largest shareholder, Oxbridge Technology Partners, SA (“Oxbridge”), a Belize Corporation, pursuant to which Oxbridge will acquire the entirety of GSL’s existing business, which acquisition is conditional upon and will complete immediately after the Scheme becomes Effective and the New Shares in Xenetic have been issued to GSL. Under this disposition agreement Oxbridge will exchange its 100,000,000 GSL Shares for: (a) the shares of GSL’s two subsidiaries, Shift It Media and General Aircraft, Inc., and (b) the payment of US$430,000 in cash, subject to the Scheme becoming Effective. The assets and liabilities associated with such businesses will thereby pass to Oxbridge. Oxbridge’s 100,000,000 GSL Shares will be retired to treasury. Further details of this agreement are set out in paragraph 9(c) of Part VIII of the Equivalent Document, the text of which is set out in Part 7 of this document.
GSL will also undertake a share consolidation in advance of the Scheme becoming Effective whereby every holder of GSL Shares will receive 1 New GSL Share of US$0.01 par value for every 10 GSL Shares of US$0.001 par value held on the consolidation date.
Subject to fulfilment (or as the case may be waiver) of the Conditions, GSL will issue the GSL Consideration Shares to the Scheme Shareholders pursuant to the Scheme. In connection with this, GSL has on or about the date hereof published information on itself and its securities in a document equivalent to a prospectus for the purposes of the Prospectus Rules, for which GSL is responsible. The text of this Equivalent Document is reproduced for convenience in Part 7 of this document.
The GSL Director will resign from the board of GSL when the Scheme becomes Effective and will confirm that he has no outstanding claims against GSL. The Proposed Directors will thereupon be appointed as directors of GSL. After the Effective Date, the board of GSL will review the needs of the business regarding whether to appoint additional directors. Such directors, if appointed, may come from the existing group of Xenetic Directors, be appointed from within the Enlarged Group or be recruited externally, in each case on terms of be agreed at the time.
Pending its conversion to a private limited company, the board of Xenetic will be reduced in number to Mr Maguire and Mr Hill only. It is also the intention to change the name of GSL to Xenetic Biosciences, Inc. on or before the Effective Date.
| 18 |
11. GSL’s strategic plans for Xenetic’s management, employees and places of business
Immediately after the Effective Date, the GSL Director will resign and the Proposed Directors will join the board of GSL as directors with the intention that their service agreements and/or terms of appointment will be equivalent or substantially equivalent to their current terms, and, where relevant, subject to applicable US laws and regulations.
No material changes to the number of Xenetic Group employees (or their current terms of employment) are expected as a result of the conclusion of the transaction contemplated by the Scheme, or from the Enlarged Group’s ongoing business strategy- which will be maintained on the same business model as has been executed by the Company for some years.
The principal objectives of the project are to re-domicile Xenetic’s activities to the US in order for the Company more effectively to develop its proprietary product pipeline with greater access to both a productdevelopment-focused labour pool, and development capital, these being key factors in the successful monetisation of the Company’s intellectual property portfolio.
For those in the UK and European small-cap biotech sector, the United States is recognised globally as the sector leader, and, in the case of Xenetic, especially so in terms of the range of skills located within Massachusetts where the Company is currently establishing its new drug development centre. Also, in terms of access to the US capital markets, where the magnitude of development capital available to emerging companies is appreciably greater than that available in European markets.
Xenetic does not have any employee pension plans to which it contributes. Employees will remain employed by their current Xenetic employer, and their employment rights will be unaffected. However, independently of the Scheme, and in accordance with its stated strategy, in 2012 Xenetic initiated some operational restructuring (which is ongoing) in anticipation of the re-location of operations to the US. This is exemplified by the hiring of senior US-based staff in May 2012, followed by the identification of new office and laboratory facilities in Lexington, Massachusetts. These facilities, where possible utilising fixed assets transferred from the UK, will become fully operational in December 2013. Consequently, the UK laboratories will be closed as there is neither the need for (nor the capital available) to sustain two scientific sites. The corporate office of Xenetic is in London and the new Lexington facility will, in due course, become the corporate hub of the business.
It is important to recognise that these operational issues have been and will continue to be conducted wholly independently of the Scheme. The purpose of the Scheme is a technical mechanism to relocate the domicile of the holding company of the Xenetic Group from the UK to the US, by installing a US company as the new holding company for the Xenetic Group. GSL will not have any other business or operations to be affected by the Scheme after it has acquired Xenetic. There is expected to be no change to the business or strategy of Xenetic following completion of the Scheme.
The current sole director of GSL has played no part in developing the US strategy of Xenetic and will resign on completion of the Scheme. The Proposed Directors (and such other directors as join the board of GSL going forward) will then lead the business, of which GSL will become the holding company. The business will be conducted in substantially the same manner as hitherto.
It is GSL’s intention, if practicable, to establish a mechanism in due course whereby smaller UK-based shareholders will be able to trade out of their positions after the Scheme has become Effective. However, should such holders wish to retain their holdings in the longer term, they will be encouraged to do so notwithstanding that they will need to utilise the services of a UK broker with facilities for trading US shares.
The Scheme will apply to all Scheme Shares, which will be cancelled and the resulting New Shares issued to GSL. As Xenetic will be 100 per cent. owned by GSL, there will therefore not be any trading facilities maintained for securities in Xenetic after the Effective Date. Please refer to paragraph 14 of Part 2 of this document (Explanatory Statement) in relation to the proposed cancellation of trading of Xenetic Shares on AIM.
| 19 |
12. Xenetic Share Schemes, options and warrants
The effect of the Scheme on outstanding options held by directors, employees and others under the Xenetic Share Option Plans and on other options and warrants can be found at paragraphs 6 and 7 of Part 2 of this document. Xenetic also proposes to enter into certain new arrangements with related parties, also as described in paragraph 7 of Part 2 of this document.
13. Suspension and cancellation of admission of Xenetic Shares to trading on AIM
Application has been made in accordance with AIM Rule 41 to the London Stock Exchange to cancel the admission to trading on AIM of Xenetic Shares. Cancellation is conditional upon the Scheme (and therefore the Acquisition) having become Effective. The announcement to be issued by Xenetic on 22 November 2013 provides Xenetic Shareholders with due notice of the intended suspension from trading on AIM which is expected to take place at 7.30 a.m. on 23 January 2014, and of the intended cancellation of trading on AIM which is expected to take place at 7.00 a.m. on 24 January 2014.
Accordingly the last day of dealings in, and for registration of transfers of, Xenetic Shares is presently anticipated to be 22 January 2014. The result of the Court Hearing will be announced on 23 January 2014. No transfers of Xenetic Shares will be registered after market close on 22 January 2014.
Your attention is drawn to paragraph 14 of Part 2 (Explanatory Statement) of this document in relation to cancellation of Xenetic Shares from admission to trading on AIM.
14. Re-registration of Xenetic
It is intended that, as soon as reasonably practicable after the Scheme becomes Effective, the Company will be re-registered as a private limited company in accordance with the Companies Act.
15. Action to be Taken
The Scheme and the Acquisition are subject to the satisfaction (or as the case may be waiver) of the Conditions set out in section A of Part 3 of this document. In order to become Effective, the Scheme must be approved by a majority in number of those Scheme Shareholders who are present and vote either in person or by proxy at the Court Meeting and who represent 75 per cent. or more in value of all Scheme Shares held by such Shareholders. In addition, the Special Resolution to give effect to the Scheme must be passed at the General Meeting. Under the Companies Act, the Scheme is also subject to the approval of the Court. Once the Scheme becomes Effective, the terms will be binding on all Scheme Shareholders irrespective of whether or not they attend or vote at the Meetings (and if they attend and vote, whether or not they vote in favour).
You will find enclosed with this document:
(a) a blue Form of Proxy for use at the Court Meeting;
(b) a white Form of Proxy for use at the General Meeting; and
(c) a reply-paid envelope for use in the UK for the return of the Forms of Proxy.
To vote at the Meetings
Whether or not you intend to attend the Court Meeting and/or the General Meeting, you are requested to complete and sign the enclosed blue and white Forms of Proxy and return them in accordance with the instructions printed on them. Completed Forms of Proxy should be returned, in accordance with the instructions printed thereon, by post or, during normal business hours only, by hand to the Company’s Registrars, Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL or by fax to Share Registrars Limited on +44 (0) 1252 719232 or by scan and email to Share Registrars Limited at proxies@shareregistrars.uk.com, as soon as possible and, in any event, so as to be received by the times set out below:
(a) blue Forms of Proxy for the Court Meeting 10.00 a.m. GMT on 15 December 2013
(b) white Forms of Proxy for the General Meeting 10.30 a.m. GMT on 15 December 2013
| 20 |
(or in the case of any adjournment, not later than 48 hours before the time fixed for the holding of the adjourned Meeting).
If you wish to appoint more than one proxy you should request additional proxy forms from Share Registrars and submit them in accordance with the instructions set out in this document.
If you hold your Shares in uncertificated form, you may vote using the CREST Proxy Voting Service in accordance with the procedures set out in the CREST Manual (please also refer to the notes to the notices of the Court Meeting and the General Meeting set out in Parts 10 and 11 respectively of this document).
If the blue Form of Proxy for use at the Court Meeting is not lodged by the time specified above, it may be handed to the chairman of the Court Meeting at the start of the Court Meeting and will still be valid. However, in the case of the white Form of Proxy for the General Meeting, it will be invalid unless it is lodged by the time specified above with the Company’s Registrars, Share Registrars Limited, Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL. The completion and return of the relevant Form of Proxy will not prevent you from attending and voting in person at the relevant Meeting, or at any adjournment thereof, if you so wish and are so entitled.
It is important that, for the Court Meeting, as many votes as possible are cast so that the Court may be satisfied that there is a fair and reasonable representation of the opinions of the Scheme Shareholders. Therefore, whether or not you intend to attend the Meetings, you are strongly urged to sign and return your Forms of Proxy for both the Court Meeting and the General Meeting as soon as possible.
Notices convening the Court Meeting and the General Meeting are set out in Parts 10 and 11 of this document respectively.
If you have any questions relating to the Meetings, this document or the completion and return of the Forms of Proxy, please address your questions in writing to the Company’s Registrars, Share Registrars Limited, Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL, or call Share Registrars Limited on +44 (0) 1252 821390 between 9.00 a.m. and 5.00 p.m. GMT Monday to Friday (except U.K. bank holidays). Please note that no advice on the merits of the matters described in this document or legal, tax or financial advice can or will be given.
16. Overseas Shareholders
Overseas Shareholders should refer to paragraph 17 of Part 2 (Explanatory Statement) of this document.
17. Further information
The terms of the Scheme are set out in full in Part 4 of this document. Please read carefully the remainder of this document, including the letter from London Bridge Capital, financial adviser to the Company, set out in Part 2 of this document. Please note that the information contained in this letter is not a substitute for reading the remainder of this document.
Your attention is also drawn to the further information contained in this document and, in particular, to the Conditions and Further Terms of the Acquisition in Part 3 of this document, the financial information on Xenetic referred to in Part 5 of this document, UK taxation issues described in Part 6, information on GSL in Part 7 and the additional information in Part 8 of this document. You should read the whole of this document.
18. Recommendation
The Xenetic Directors (other than Roman Knyazev for the reasons stated above), who have been so advised by London Bridge Capital, as the independent financial adviser to Xenetic for the purposes of Rule 3 of the City Code, consider the terms of the Scheme to be fair and reasonable insofar as Xenetic Shareholders are concerned. In providing its advice, London Bridge Capital has taken into account the Xenetic Directors’ commercial assessments. In addition, the Xenetic
| 21 |
Directors (other than Roman Knyazev) consider the terms of the Acquisition to be in the best interests of Xenetic Shareholders as a whole.
Accordingly, the Xenetic Directors (other than Roman Knyazev), unanimously recommend that Xenetic Shareholders vote in favour of the Acquisition and the Scheme Resolutions to be proposed at the Court Meeting and the General Meeting, as they have irrevocably undertaken to do in respect of their entire beneficial shareholdings in the Company, amounting to, in aggregate, 11,271,086 Shares, representing approximately 2.76 per cent. per cent. of the existing issued ordinary share capital of the Company as at 20 November 2013 (being the latest practicable date prior to the publication of this document).
Yours faithfully
/s/ Brian Richards
Sir Brian Richards Chairman
| 22 |
PART 2
EXPLANATORY STATEMENT
(in compliance with section 897 of the Companies Act 2006)
London Bridge Capital Limited 30 Percy Street
London
W1T 5DB
21 November 2013
To Shareholders and, for information only, to holders of options and warrants Dear Shareholder,
RECOMMENDED
ACQUISITION OF XENETIC BIOSCIENCES PLC
BY GENERAL SALES & LEASING, INC.
1. Introduction
On 12 November 2013, the Company announced that the boards of Xenetic and GSL had reached agreement on the terms of a recommended proposal for the acquisition of Xenetic by GSL. GSL is a US operating company whose main business involves the owning and operation of helicopters for use in sightseeing tours and as pilot training aircraft. Immediately following completion of the Acquisition, all of the current operating businesses of GSL will be disposed of and the only ongoing operating activities will be those of the Group.
On the basis of the value as at 11 November 2013 of a GSL Share set out in Part 12 of 5.95 pence per Scheme Share, the Acquisition values the entire existing issued share capital of the Company at approximately £24.3 million and each Xenetic Share at approximately 5.95 pence. This represents a discount of approximately 8.49 per cent. to 6.5 pence, being the Closing Price per Scheme Share on 11 November 2013 (being the Business Day prior to the date of the Announcement).
The Acquisition is to be implemented by means of a Court-sanctioned scheme of arrangement between the Company and the Scheme Shareholders under Part 26 of the Companies Act and involves a reduction of capital under section 648 of the Companies Act. The Scheme requires the approval of the Scheme Shareholders at a meeting convened by the Court and the subsequent sanction of the Court. The Reduction of Capital requires the approval of Shareholders at a General Meeting and the subsequent confirmation of the Court. Once the Scheme becomes Effective, the terms will be binding on all Scheme Shareholders whether or not they voted in favour of the Scheme.
The Xenetic Directors have been advised by London Bridge Capital in connection with the Acquisition. London Bridge Capital has been authorised by the Xenetic Directors to write to you to explain the terms of the Acquisition and the Scheme and to provide you with other relevant information.
Your attention is drawn to the letter from Sir Brian Richards, the Chairman of Xenetic, set out in Part 1 of this document, which forms part of this Explanatory Statement. That letter contains, amongst other matters, a summary of the Acquisition and the background to and the reasons for the recommendation of the Xenetic Directors (save for Roman Knyazev) to the Shareholders to vote in favour of the Scheme Resolutions.
The conditions and further terms of the Acquisition are set out in full in Part 3 of this document. Your attention is also drawn to the information in the other Parts of this document, which form part of the Explanatory Statement pursuant to section 897 of the Companies Act.
Statements made or referred to in this letter regarding GSL’s reasons for making the Acquisition, and/or intentions or expectations of or concerning GSL, reflect the views of GSL. Statements made or referred to in this letter regarding the background to and reasons for the recommendation of the Xenetic Directors, the recommendation of the Xenetic Directors, information concerning the business of Xenetic, and/or intentions or expectations of or concerning Xenetic, reflect the views of the Xenetic Directors (other than Roman Knyazev).
| 23 |
Scheme Shareholders should read the whole of this document and take independent advice before deciding whether or not to vote in favour of the Scheme and the Scheme Resolutions.
2. Summary of the terms of the Acquisition
Under the terms of the Scheme, the Scheme Shares will be cancelled and in exchange, upon the Scheme becoming Effective, Scheme Shareholders on the Company’s register of members at the Scheme Record Time will receive:
for every 175 Scheme Shares: 56 GSL Consideration Shares
Upon the Scheme becoming Effective, Scheme Shareholders will hold 97.39 per cent. of the issued and to be issued shares of GSL, the significant majority of the then shares then in issue.
In accordance with Rule 24.11 of the Code, London Bride Capital has provided an Estimate of Value of the GSL Consideration Shares as at 11 November 2013, and has advised that each GSL Consideration Share should be valued at 18.59p. Further details of the bases and assumptions underlying this valuation are set out in Part 12 of this document.
3. Structure of the Acquisition
(a) Introduction
The Acquisition will be effected by means of a scheme of arrangement between the Company and Scheme Shareholders under Part 26 of the Companies Act involving a reduction of capital under section 648 of the Companies Act. The terms of the Scheme are set out in full in Part 4 of this document. The purpose of the Scheme is to enable GSL to become the owner of the entire issued and to be issued share capital of the Company. This is to be achieved by the cancellation of the Scheme Shares held by Scheme Shareholders and the application of the reserve arising from such cancellation in paying up in full New Shares which have an aggregate nominal value equal to the aggregate nominal value of the Scheme Shares cancelled and issuing the same to GSL. Scheme Shareholders will then receive 56 GSL Consideration Shares for every 175 Scheme Shares held by them on the basis of the Acquisition (as is more fully described in paragraph 2 above) and the Equivalent Document.
Prior to the Announcement, GSL did not hold any Scheme Shares. GSL has not acquired any Scheme Shares since the date of the Announcement. GSL has agreed to subscribe, following the General Meeting but prior to the Scheme Record Time, and to hold until after the Effective Date, one fully paid Xenetic Share. This will mean that GSL will be a member of Xenetic on the Effective Date and accordingly there will be no requirement under section 593 of the Companies Act for an independent valuation of the New Shares. The additional Xenetic Share to be subscribed for or acquired by GSL in advance of the Scheme Record Time will not be a Scheme Share and will not be subject to the Scheme. Save as set out in the preceding sentence, the Scheme Shares amount to all Shares and, upon the Scheme becoming Effective, GSL will own the entire issued ordinary share capital of the Company.
The implementation of the Acquisition and the Scheme is subject to the Conditions, which are set out in Part 3 of this document, and which include the approval of the Acquisition by Scheme Shareholders and the sanction of the Scheme and the Reduction of Capital by the Court.
For the Scheme (including the Reduction of Capital) to become Effective, the Scheme Resolutions implementing the Scheme must be passed by Shareholders at the Meetings. The Scheme must be approved by a majority in number of those Scheme Shareholders present and voting either in person or by proxy at the Court Meeting representing 75 per cent. or more in value of all Scheme Shares held by such Scheme Shareholders. Scheme Shareholders are entitled to attend the Court Hearing in person or to be represented by counsel.
| 24 |
The Reduction of Capital involved in the Scheme requires the approval of the Special Resolution at the General Meeting and the subsequent confirmation of the Court at the Court Hearing.
The Scheme is not capable of becoming Effective unless each Condition to the Acquisition has been satisfied or, where relevant, waived. The Scheme and Reduction of Capital will become Effective on the filing of office copies of the Court Order and a statement of capital with the Registrar of Companies.
Once the Scheme becomes Effective, the terms will be binding on all Scheme Shareholders irrespective of whether or not they attend or vote at the Meetings (and if they attend and vote, whether or not they vote in favour).
Application has been made in accordance with AIM Rule 41 to the London Stock Exchange to cancel the admission to trading on AIM of Xenetic Shares. Cancellation is conditional upon the Scheme (and therefore the Acquisition) having become Effective. The announcement to be issued by Xenetic on 22 November 2013 provides Xenetic Shareholders with due notice of the intended suspension from trading on AIM which is expected to take place at 7.30 a.m. on 23 January 2014, and of the intended cancellation of trading on AIM which is expected to take place at 7.00 a.m. on 24 January 2014.
Accordingly the last day of dealings in, and for registration of transfers of, Xenetic Shares is presently anticipated to be 22 January 2014. The result of the Court Hearing will be announced on 23 January 2014. No transfers of Xenetic Shares will be registered after market close on 22 January 2014.
If the Scheme becomes Effective, the New Shares will be issued to GSL fully paid and free from all liens, equitable interests, charges, encumbrances and other third party rights of any nature whatsoever and together with all rights attaching to them, including the right to receive and retain all dividends and distributions (if any) declared, made or payable after the Effective Date. The Company will not declare, make or pay any dividends or distributions prior to the Effective Date.
On the Effective Date, share certificates in respect of all Scheme Shares will cease to be valid and should be destroyed.
If for any reason the Scheme does not become Effective, the transactions described above will not take effect and Scheme Shareholders will retain their existing holdings of Scheme Shares and admission of the Shares to trading on AIM will not be cancelled.
(b) The Meetings
Before the Court’s approval can be sought to sanction the Scheme, the Scheme will require approval by the holders of Scheme Shares at the Court Meeting and the passing of the Special Resolution by Shareholders at the General Meeting to approve the Reduction of Capital and other related issues.
Notices of the Court Meeting and the General Meeting are set out in Parts 10 and 11 of this document respectively. All holders of Scheme Shares whose names appear on the register of members of the Company at the Voting Record Time will be entitled to attend and vote at the relevant Meeting in respect of the number of Scheme Shares respectively registered in their names at the relevant time, as further described below.
The Court Meeting
The Court Meeting, which has been convened for 10.00 a.m. on 17 December 2013, is being held at the direction of the Court to seek the approval of Scheme Shareholders for the Scheme (with or without modification).
At the Court Meeting, voting will be by way of a poll and each Scheme Shareholder present in person or by proxy will be entitled to one vote for each Scheme Share held. The approval required at the Court Meeting is a majority in number of those Scheme Shareholders present and voting, either in person or by proxy, representing 75 per cent. or more in value of all Scheme Shares held by such Scheme Shareholders.
| 25 |
For the Court Meeting it is important that as many votes as possible are cast so that the Court may be satisfied that there is a fair representation of Scheme Shareholder opinion. Therefore, whether or not you intend to attend the Meetings, you are strongly urged to sign and return your Forms of Proxy for both the Court Meeting and General Meeting as soon as possible.
The General Meeting
The General Meeting has been convened for 10.30 a.m. on 17 December 2013 (or as soon thereafter as the Court Meeting has been concluded or adjourned), to consider and, if thought fit, pass the Special Resolution (which requires votes in favour representing at least 75 per cent., of the votes cast) to:
| (i) | authorise the Xenetic Directors to take all such action as they may consider necessary or appropriate for carrying the Scheme into effect; |
| (ii) | approve the cancellation of the share capital of the Company in accordance with the Scheme, the capitalisation of the reserve arising upon the cancellation of the Scheme Shares and the subsequent issue of New Shares to GSL (or its nominee(s)) in accordance with the Scheme; |
| (iii) | authorise the Xenetic Directors pursuant to section 551 of the Companies Act to allot securities in the Company; and |
| (iv) | approve certain amendments to the Articles (including as referred to below). |
Amendments to the Articles
It is proposed that prior to the Court Hearing the Articles be amended so as to ensure that any Xenetic Shares which are issued after the General Meeting but prior to the Court Hearing will be subject to and bound by the Scheme. Any Xenetic Shares issued on the exercise of options or warrants granted under the Xenetic Share Schemes or otherwise after the Court Hearing will not be subject to the Scheme. Accordingly, it is also proposed that the Articles be amended so that any Xenetic Shares issued to any person other than GSL on or after the Scheme Record Time will automatically be acquired by GSL in consideration for the issue by GSL to such person of such number of GSL Consideration Shares which would have been issued had such Xenetic Shares been Scheme Shares.
The proposed amendments to the Articles are set out in full in the notice of the General Meeting in Part 11 of this document.
Registered Shareholders
Each holder of Scheme Shares who is entered in the Company’s register of members at 6.00 p.m. GMT on the Voting Record Time will be entitled to attend and vote at the Court Meeting. Each holder of Shares entered in the Company’s register of members at 6.00 p.m. GMT on the Voting Record Time will be entitled to attend and vote at the General Meeting.
If either Meeting is adjourned, only those relevant Shareholders on the register of members at 6.00 p.m. GMT on the date which is two days before the date of the adjourned meeting will be entitled to attend and vote. Such a Shareholder is entitled to appoint a proxy or proxies to attend and, on a poll, to vote instead of him or her. A proxy need not be a Shareholder. A blue Form of Proxy for the Court Meeting and a white Form of Proxy for the General Meeting are enclosed. To be valid, the blue Form of Proxy (in respect of the Court Meeting) must reach the Company’s Registrars, Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL (or by fax to Share Registrars Limited on +44 (0) 1252 719232 or by scan and email to Share Registrars Limited at proxies@shareregistrars.uk.com) by 10.00 a.m. on 15 December 2013, or it may be handed to the Chairman of the Court Meeting before the start of the Meeting. To be valid, the white Form of Proxy (in respect of the General Meeting) must reach Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL (including by fax or email as aforesaid) by 10.30 a.m. GMT on 15 December 2013.
Whether or not you intend to attend the Court Meeting and/or the General Meeting, you are requested to complete and sign the enclosed blue and white Forms of Proxy and return them in accordance with the instructions printed on them.
| 26 |
If you hold your Xenetic Shares in uncertificated form, you may vote using the CREST Proxy Voting Service in accordance with the procedures set out in the CREST Manual (please also refer to the notes to the notices of the Court Meeting and the General Meeting set out in Parts 10 and 11 of this document respectively). Proxies submitted through CREST (under CREST Participant ID 7RA36) must be received by Share Registrars Limited by no later than 6.00 p.m. on 13 December 2013 in the case of the Court Meeting and by 6.00 p.m. on 13 December 2013 in the case of the General Meeting (or, in the case of an adjourned meeting, no later than 6.00 p.m. on the day two days before the date of the adjourned meeting (excluding any day which is not a working day) in respect of the Court Meeting or by no later than 6.00 p.m. on the day two days before the adjourned meeting (excluding any day which is not a working day) in respect of the General Meeting).
Shareholders who return completed Forms of Proxy may still attend the Meetings instead of their proxies and vote in person if they wish and are entitled to do so. In the event of a poll on which a Shareholder votes in person, his/her proxy votes lodged with the Company will be excluded.
Shareholders are entitled to appoint a proxy in respect of some or all of their Shares and are also entitled to appoint more than one proxy. A space has been included on the Forms of Proxy to allow Shareholders to specify the number of Shares in respect of which that proxy is appointed. Shareholders who return a white Form of Proxy duly executed but leave this space blank will be deemed to have appointed the proxy in respect of all of their Shares.
Shareholders who wish to appoint more than one proxy in respect of their shareholding should contact the Company’s Registrars, Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL, or call Share Registrars on +44 (0)1252 821390 between 9.00 a.m. and 5.00 p.m. GMT Monday to Friday (except UK bank holidays) for further Forms of Proxy or photocopy the Forms of Proxy as required. Such Shareholders should also read the notes on the Forms of Proxy and note the principles that will be applied in relation to multiple proxies. Shareholders should note that if they wish to appoint more than one proxy they should request additional proxy forms from Share Registrars and submit them in accordance with the instructions set out above.
(c) Conditions to the Acquisition
The Conditions to the Acquisition are set out in full in section A of Part 3 of this document. Inter alia, the implementation of the Acquisition is conditional upon:
| (i) | approval of the Scheme by a majority in number representing not less than 75 per cent. in value of the Scheme Shareholders (or the relevant class or classes thereof, if applicable) who are on the register of members of the Company at the Voting Record Time, present and voting, whether in person or by proxy, at the Court Meeting and any separate class meeting which may be required by the Court or any adjournment thereof; |
| (ii) | the Special Resolution being duly passed at the General Meeting (or at any adjournment thereof); and |
| (iii) | the sanction of the Scheme and the confirmation of the Reduction of Capital (in either case with or without modification or with modification (but subject to such modification being acceptable to GSL and the Company)) and office copies of the Court Order and of a statement of capital being delivered to the Registrar of Companies. |
The Conditions set out above are not capable of being waived in whole or in part.
The Acquisition is also conditional upon the other Conditions set out in section A of Part 3 to this document, which are not otherwise summarised in paragraphs (i) to (iii) inclusive above, being satisfied or as the case may be waived.
(d) Sanction of the Scheme and confirmation of the Reduction of Capital by the Court
Under the Companies Act, the Scheme requires the sanction of the Court and the Reduction of Capital requires the confirmation of the Court. The Court Hearing is expected to be held on 23 January 2014. GSL has confirmed that it has consented to being represented by Xenetic’s counsel at the Court Hearing so as to consent to the Scheme and to undertake to the Court to be bound thereby.
The Scheme (and associated Reduction of Capital) will become Effective in accordance with its terms on delivery of office copies of the Court Order and of a statement of capital to the Registrar of Companies.
| 27 |
If the Scheme becomes Effective, it will be binding on all Shareholders irrespective of whether or not, being entitled to do so, they attended or voted in favour of the Scheme at the Court Meeting or in favour of the Special Resolution at the General Meeting.
If the Scheme does not become Effective on or before 30 June 2014 (or such later date and time (if any) as the Company and GSL may agree and, if appropriate, the Court may approve) the Scheme will not become Effective and the Scheme will not proceed.
(e) Modification of the Scheme
The Company and GSL may jointly consent, on behalf of all persons affected, to any modification of, or addition to, this Scheme or to any condition approved or imposed by the Court. The Court would be unlikely to approve any modification of, or additions to, or to impose a condition to, the Scheme which might be material to the interests of the Scheme Shareholders unless Scheme Shareholders were informed of such modification, addition or condition. It would be a matter for the Court to decide, in its discretion, whether or not a further meeting of Scheme Shareholders should be held in these circumstances. Similarly, if a modification, addition or condition is put forward which, in the opinion of the Xenetic Directors, is of such a nature or importance that it requires the consent of Scheme Shareholders, the Xenetic Directors will not take the necessary steps to enable the Scheme to become Effective unless and until such consent is obtained.
| 4. | Irrevocable Undertakings |
Xenetic has sought and received irrevocable undertakings from those Xenetic Directors who are beneficial owners of Xenetic Shares to vote in favour of the Scheme Resolutions in respect of their entire beneficial holdings of Xenetic Shares, a total of 11,271,086 Xenetic Shares, representing approximately 2.76 per cent. of the existing issued ordinary share capital of Xenetic, as at 20 November 2013 (being the latest practicable date prior to the publication of this document).
Xenetic has sought and received irrevocable undertakings from certain other Xenetic Shareholders who beneficially own or otherwise control Xenetic Shares to vote in favour of the Scheme Resolutions in relation to a total of 96,668,741 Xenetic Shares, representing approximately 23.7 per cent. of the existing issued ordinary share capital of Xenetic as at 20 November 2013 (being the latest practicable date prior to the publication of this document).
In relation to the Scheme Resolutions, Xenetic has therefore received total irrevocable undertakings in respect of 107,939,827 Xenetic Shares representing, in aggregate, 26.46 per cent. of the existing issued ordinary share capital of Xenetic as at 20 November 2013 (being the latest practicable date prior to the publication of this document).
Further details of the above irrevocable undertakings are set out in paragraph 7 of Part 8 of this document.
| 5. | Background to, and reasons for, the Acquisition |
The Xenetic Directors believe that at the present time smaller quoted bioscience companies, in general, have a higher profile and a better investor following in the US than is found in European markets in general and in the UK market, in particular. It believes that by achieving a listing for Xenetic on an Exchange, significant benefits will accrue to Xenetic and its shareholders including, but not exclusively:
| (a) | the ability to attract and incentivise qualified personnel with orphan drug development and launch experience; |
| (b) | easier access to finance on reasonable terms; |
| (c) | a better rating of Xenetic’s shares; and |
| (d) | higher levels of share trading liquidity. |
The Xenetic Directors have considered a number of options to achieve the goal of migration to the US, including seeking a quotation of Xenetic’s shares directly on a US stock market and seeking a merger with an existing US quoted biotechnology company with complementary activities. Following careful consideration of the issues, the Xenetic Directors have determined that the best way is to be acquired by an SEC –reporting, unlisted company, which can seek to achieve a quotation on an Exchange within a reasonable period; and to effect such an acquisition by the Scheme.
| 28 |
Xenetic’s plan is for it to be acquired on a share-for-share basis by GSL, where the level of effective dilution to Xenetic’s Shareholders does not exceed 3 per cent. as at the Effective Date. This means that, other than for such dilution and the fees and costs of the Acquisition (which are deemed to be the “cost” of achieving Xenetic’s aims), the group formed by the completion of the Acquisition will be effectively the same group as at present, with the same assets and liabilities, save that it will have a US holding company (GSL) with an enhanced ability to achieve a listing on an Exchange. Whilst Xenetic and GSL have commenced developing a plan regarding such a listing, there is no guarantee that such a listing can be achieved.
In particular, GSL is subject to various regulatory requirements, including those of the SEC and the various trading exchanges where its equity shares may trade. Any securities exchange (for example, NASDAQ) can and will apply its own rules and/or analysis to GSL’s eligibility for listing which may adversely impact the Enlarged Group’s attempt to be listed on a securities exchange or the tradability of its shares.
6. Xenetic Employee Share Schemes
In accordance with Rule 15 of the Code, the following proposals for replacing the current Xenetic Share Schemes with new schemes in relation to New GSL Shares under US laws, conferring no additional benefits will be put to relevant holders.
(a) Outstanding rights to subscribe for Xenetic Shares granted under the Xenetic employee Share Option Plans
Rights to subscribe for a total of 16,154,007 Xenetic Shares are outstanding as at 20 November 2013 (the nearest practicable date to the publication of this document) at exercise prices of between one penny and 46.6p per Share.
Options to subscribe for 7,669,815 Xenetic Shares are outstanding at exercise prices of between one penny and 37.75p per Share under the terms of the Xenetic 2000 Share Option Plan. Such share options will, if they are neither exercised nor exchanged for the grant of replacement share options as mentioned below within the period of six months after the Effective Date, lapse and cease to be exercisable at the end of that period.
If the Scheme becomes Effective, the holders of such options to subscribe for Xenetic Shares will be invited by GSL to exchange their existing share options for corresponding rights (without additional benefit) to acquire GSL Consideration Shares, such ‘exchange of options’ being conditional upon the Scheme becoming Effective. Such new rights will be in the form of options to acquire New GSL Shares exercisable only in accordance with the terms of replacement option grants as described at paragraph 9.3 of Part 8 of this document.
Options to subscribe for 8,484,192 Xenetic Shares are outstanding under the terms of the Plan (the “Xenetic 2007 Share Option Plan”) at exercise prices of between 6.75p and 46.6p per share. Such share options will, if they are not already, become immediately exercisable (regardless of whether any performance targets or vesting conditions have then been met or satisfied) upon the Effective Date, and will remain exercisable for a period of 40 days thereafter, at the end of which they will, if not exercised, lapse and cease to be exercisable.
It is intended that the holders of share options granted under the Xenetic 2007 Share Option Plan will each be invited by GSL to release their rights under such share options in consideration of the grant by GSL of rights which are equivalent (without additional benefit) but relate to New GSL Shares. Such invitations will be conditional upon the Scheme becoming Effective and must be accepted, if at all, so that such exchange will take effect as soon as practicable after the Scheme has effect. Such replacement options will be granted on terms such that, in each case, the total market value of New GSL Shares that are subject to the replacement option is equal to the total market value of the Xenetic Shares that were subject to the old option immediately before the release of rights under that option, and the total amount payable for the acquisition of shares under the replacement option is equal to the total amount that would have been payable for the acquisition of shares under the old option.
| 29 |
For these purposes, the exercise prices payable for the subscription of shares in GSL will be expressed in US$ and the market value of shares in GSL will be determined by reference to the rate of exchange from sterling into US$ quoted by Barclays Bank plc on the Business Day last preceding the date on which such grant of replacement share options becomes effective. Insofar as existing share options granted under the Xenetic 2007 Share Option Plan are exercisable only subject to the attainment of target levels of share price, the replacement options will be subject to correspondingly adjusted share price targets.
The grant by GSL of replacement share options as described above will not confer any additional benefits on the holders of existing rights to subscribe for Xenetic Shares.
(b) Joint ownership of Xenetic Shares by Directors under Xenetic JSOP
Certain of the Directors of Xenetic hold, jointly with the JSOP Plan Trustee (the “Co-Owner”), the beneficial interest in an aggregate of 33,400,606 Xenetic Shares upon and subject to the terms of Joint Ownership Agreements (“JOAs”) entered into pursuant to the terms of the Xenetic JSOP in June 2010 and March 2012. Under a JOA, and subject to the vesting of the participant’s interest, a participant will, when the jointly-owned shares are sold, be entitled to a share of the proceeds of sale equal to the growth in market value of the jointly-owned shares above their market value at the time of the JOA (being 7.75p in the case of those JOAs entered into in June 2010 and 10.625p in the case of those entered into in March 2012) plus a rate of simple interest on that amount (which, in the case of JOAs entered into in June 2010, is 8.387 per cent. accruing over a maximum period of 5 years and, in the case of JOAs entered into in March 2012, is 7.65 per cent. accruing over a maximum period of 3 years). Awards in the form of JOAs have been made to each of Scott Maguire and Colin Hill (“the Participating Directors”) in 2010 and 2012. Of the jointly-owned shares, the 5,318,479 awarded in 2010 have become fully vested.
Awards of jointly-owned Xenetic Shares made in 2012 vest on the attainment of share price targets (15p, 40 per cent.; 25p, 65 per cent.; 35p, 90 per cent.; and 50p, 100 per cent.) or upon a “Change of Control” (which includes if a 30 per cent.+ shareholder increases their stake by any amount). If, after 2 March 2017, the share price has not attained 35p, the Co-Owner may call upon the Participating Director to give up his interest in the unvested jointly owned shares for a nominal sum. If it has attained 35p, but the 50p target has not then been achieved, the Co-owner must wait until after 2 March 2020 before it can require the Participating Director to give up his interest in the unvested shares.
If the Participating Director ceases employment within the group at any time by reason of either (a) misconduct or (b) voluntary resignation without notice (otherwise than in circumstances amounting to constructive dismissal), the Co-owner has the right to call upon the Participating Director to dispose of his interest in unvested shares for a nominal sum.
A Participating Director has the right, in respect of the awards of jointly-owned shares made in 2012, exercisable at any time after 2 March 2015, or, if earlier, on a “Change of Control,” to require the Co-owner to join in selling vested jointly-owned shares, the proceeds being divided in accordance with the JOA. The Participating Director would receive the growth in value above 13.058p (this being the initial market value of a Xenetic Share at the time of award of 10.625p + the Carrying Cost at 7.65 per cent. which accrues over only a 3-year period). The proposed Acquisition of the Company by GSL will not be a “Change of Control” for these purposes and will not trigger an early vesting of the interests of Participating Directors in the jointly-owned Xenetic Shares awarded in 2012.
Title to the jointly-owned shares is held by Roy Nominees Limited as nominee for the joint owners.
Under the terms of the Scheme, joint owners of Xenetic Shares will be treated in the same manner as all other members of Xenetic. Under the terms of the JOAs, once the Scheme has effect, the joint owners will thereafter jointly hold the beneficial interest in GSL Shares acquired pursuant to the Scheme upon and subject to the terms of the relevant JOA. The share price targets mentioned above will be adjusted by applying the ratio of 56/175 and converting the resulting target prices into US$ at the rate quoted by Barclays Bank PLC on the Business Day last preceding the date on which the Scheme becomes Effective.
Jointly-owned shares were issued at a subscription price equal to their market value at the time of issue. Of that value the amounts shown in the table at paragraph 4.2 in Part 8 of this document were paid by the directors and the balance was funded by loans made by Xenetic to the Co-Owner. When the joint ownership arrangements terminate (which will be when either both directors have ceased employment or Co-Owner has exercised call options either to require the jointly-owned shares to be sold or to buy back from the directors their interests in the jointly-owned shares, or when the jointly-owned shares have been sold) any amounts held by the Co-Owner will be returned to Xenetic, either by way of repayment of loans outstanding or upon a winding-up of the Company’s Guernsey Purpose Trust.
| 30 |
7. Holders of other options and warrants
General
In accordance with Rule 15 of the Code, the following proposals for extending the offer constituted by the Scheme to other outstanding options and warrants will be effected, conferring no additional benefits for relevant holders.
It is proposed that the Articles be amended so as to ensure that any Xenetic Shares which are issued, inter alia following the exercise, in accordance with their terms, of options or warrants after the General Meeting but prior to the Scheme Record Time, will be subject to and bound by the Scheme. Any Xenetic Shares issued inter alia following the exercise, in accordance with their terms, of options or warrants after the Scheme Record Time will not be subject to the Scheme. Accordingly, it is also proposed that the Articles be amended so that any Xenetic Shares issued to any person (other than GSL) on or after the Scheme Record Time will automatically be acquired by GSL in consideration for the issue by GSL to such person of such number of GSL Consideration Shares which would have been issued had such Xenetic Shares been Scheme Shares included in the Scheme.
The Company considers that this mechanism achieves the same offer for option and warrant holders as if they had been Scheme Shareholders (while preserving the terms and conditions of existing options and warrants) and accordingly that this mechanism provides an appropriate offer in relation to such matters for the purposes of Rule 15 of the Code.
Proposed arrangements with related parties
Alongside equity subscriptions previously made by them, Xenetic has previously negotiated warrant arrangements with some of its collaborative partners. These take the form of investment incentives designed to allow those parties to benefit from growth in shareholder value anticipated to arise as a result of the collective efforts of Xenetic and its collaborators in the positive clinical development of Xenetic’s drug candidate pipeline.
All such arrangements have been based upon aspirational share price enhancement resulting from pre-clinical and clinical development milestones being achieved; such as, for example, the filing of Innovative New Drug (“IND”) applications or progression through regulated clinical trials whether in western Europe or elsewhere. This has customarily been the case given that Xenetic’s three principal co-development agreements are with two Russian entities (SynBio and Pharmsynthez) and with Serum Institute of India. Xenetic has also previously granted warrant rights to individuals within certain of these collaborative partnerships, designed to align the interests of operational field management with those of Xenetic’s shareholders and directors.
At the time of the making any such grants/awards, it is clearly difficult to account for future influences which can conspire against planned developments by way both as to absolute negative influences coming to bear, and in relation to the timing of events. Such has been the case with both SynBio and SIIL, where the warrant entitlements granted in 2011 have been rendered either effectively worthless (SIIL) or are now recognised as being relatively very much less attainable (SynBio) than was first considered reasonable by the parties at the date of grant.
Accordingly, Xenetic has been in discussions with SIIL and SynBio to revisit the original terms of grant, with a view to their warrants being renewed and/or re-priced with the benefit of current levels of understanding and development plans resulting from Xenetic’s migration to the United States. Details of the existing warrants of SynBio and SIIL to be replaced and/or re-priced are set out in paragraph 10.1 of Part 8 of this document. Additionally, now that product development lies at the heart of Xenetic’s activities, the Directors believe that it is again appropriate that further operational management awards should be considered.
| 31 |
Additionally, in 2011 SynBio provided Xenetic with loan facilities to fund its operations. Details of these loan facilities are set out at paragraph 10.1 of Part 8 of this document. Xenetic is now in discussions with SynBio as regards the repayment of the loan by way of a further issue of shares to them after the Scheme becomes Effective.
Arrangements in relation to the matters above have not yet been agreed. To the extent the Scheme becomes Effective and such arrangements (or other arrangements with related parties or their connected persons or concert parties, including any revision to the agreement with FDS Pharma as summarised at paragraph 10 of Part 8) are concluded within six months of the Effective Date, GSL will put appropriate resolutions to independent shareholders of GSL to approve the agreed arrangements, whether or not such resolutions are required to be considered by such shareholders under applicable laws or regulations (including the laws of Nevada). The relevant related party (and its connected persons and concert parties) would not be permitted to vote on any resolution in relation to any matter proposed in relation to itself.
8. GSL Consideration Shares
Subject to fulfilment (or as the case may be waiver) of the conditions, GSL will issue the GSL Consideration Shares to the Scheme Shareholders pursuant to the Scheme. In connection with this, GSL has on or about the date hereof published information on itself and its securities in a document equivalent to a prospectus for the purposes of the Prospectus Rules, for which GSL is responsible. The text of this Equivalent Document is reproduced for convenience in Part 7 of this document.
9. Financial effects of the Acquisition for Xenetic Shareholders
This paragraph 9 sets out, for illustrative purposes only, and on the bases and assumptions set out in the notes below, the financial effects on the value for a holder of 1,750 Xenetic Shares, assuming the Scheme becomes effective.
| Effect on the value of 1,750 Xenetic Shares | Pence | Note |
| Value of 560 GSL Consideration Shares | 10,410.4 | (i) |
| Market value of 1,750 Xenetic Shares | 9,625 | (ii) |
| Increase/(decrease) in capital value | 785.4 | (iii) |
| This represents an increase/(decrease) of | 8.16% | (iv) |
Notes:
| (i) | The value of 5.95 pence per Xenetic Share implied by the terms of the Scheme is calculated based on the Merger Ratio multiplied by the valuation as at 11 November 2013 of a GSL Consideration Share of 18.59 pence, as estimated by London Bridge Capital, set out in Part 12. |
| (ii) | Xenetic’s closing share price of 5.5 pence on 20 November 2013 (being the last practicable date prior to the posting of this document). |
| (iii) | In assessing the financial effects of receiving GSL Consideration Shares, no account of any potential tax liability of any Xenetic Shareholder has been taken. |
| (iv) | (iii) as a proportion of (ii) in per cent. terms. |
In assessing the financial effects of the Scheme, the table shown in paragraph 5 of Part 8 should also be referred to as it gives an indication of the recent historical range of the Xenetic share price.
In assessing the financial effect on the income for a holder of 1,750 Xenetic Shares assuming the Scheme becomes Effective, it should be noted that it is not the intention of the Enlarged Company to make distributions by way of dividend payments for the foreseeable future following the Scheme becoming Effective. The Boards of Xenetic and GSL consider that it will be in the Enlarged Group’s shareholders’ best interests to reinvest the profits of the Enlarged Group in business growth opportunities. The Proposed Directors of GSL will regularly review the dividend policy as the Enlarged Group’s asset portfolio and financial position develop over the forthcoming years.
10. Information on Xenetic
Information on Xenetic and its current trading and prospects is set out in paragraphs 7 and 8 in Part 1 (Letter from the Chairman of Xenetic) of this document.
| 32 |
11. Information on GSL
Information on GSL and its current trading and prospects is set out in paragraphs 10 and 11 in Part 1 (Letter from the Chairman of Xenetic), and in its Equivalent Document the text of which is appears for convenience in Part 7 of this document. The GSL Director has taken responsibility for such information.
12. Directors, Management and Employees
Information regarding the intentions of GSL for the management and employees of Xenetic is set out in paragraph 11 of Part 1 (Letter from the Chairman of Xenetic) of this document.
The GSL Director will resign from the board of GSL when the Scheme becomes Effective and will confirm that he has no outstanding claims against GSL. The Proposed Directors will thereupon be appointed as directors of GSL.
After the Effective Date, the board of GSL will review the needs of the business regarding whether to appoint additional directors. Such directors, if appointed, may come from the existing group of Xenetic Directors, be appointed from within the Enlarged Group or be recruited externally, in each case on terms of be agreed at the time.
Pending its conversion to a private limited company, the board of Xenetic will with effect from the Effective Date be reduced in number to Mr Maguire and Mr Hill only. It is also the intention to change the name of GSL to Xenetic Biosciences, Inc. on or before the Effective Date.
13. The Directors and the effect of the Scheme on their interests
The Shares held by the Directors will be subject to the Scheme. Information on the Shares and the options in respect of Shares held by the Directors, their immediate families and any related trusts is provided in paragraph 4 of Part 8 of this document. Details of the irrevocable undertakings provided by the Directors in respect of their legal and/or beneficial interests in Shares are set out in paragraph 7 of Part 8 of this document.
Particulars of the service agreements and letters of appointment of the Xenetic Directors are set out in paragraph 9 of Part 8 of this document. As regards the Proposed Directors it is intended to replicate these on the same commercial terms without conferring additional benefits as near as possible (subject to applicable laws and regulations) in relation to GSL. The new agreements and letters of appointment will be governed by New York law.
Save as set out above, the effect of the Scheme on the interests of the Directors does not differ from its effect on the like interest of any other Shareholder.
14. Suspension and cancellation of admission of Shares to trading on AIM
Application has been made in accordance with AIM Rule 41 to the London Stock Exchange to cancel the admission to trading on AIM of Xenetic Shares. Cancellation is conditional upon the Scheme (and therefore the Acquisition) having become Effective. The announcement to be issued by Xenetic on 22 November 2013 provides Xenetic Shareholders with due notice of the intended suspension from trading on AIM which is expected to take place at 7.30 a.m. on 23 January 2014, and of the intended cancellation of trading on AIM which is expected to take place at 7.00 a.m. on 24 January 2014.
Accordingly the last day of dealings in, and for registration of transfers of, Xenetic Shares is presently anticipated to be 22 January 2014. The result of the Court Hearing will be announced on 23 January 2014. No transfers of Xenetic Shares will be registered after market close on 22 January 2014.
Upon the Scheme becoming Effective, Xenetic will become a wholly owned subsidiary of GSL and each existing certificate representing a holding of Shares shall cease to be valid for any purpose and each holder of certificates representing Shares shall be bound at the request of the Company to deliver up the same to the Company or to any person nominated by Company for cancellation. In addition, on the Effective Date, entitlements to Xenetic Shares held within the CREST System will be cancelled.
| 33 |
15. Settlement
Xenetic Shareholders holding 174 Scheme Shares or less will be entitled to vote at the Meetings. They will not however receive any GSL Consideration Shares. The GSL Consideration Shares attributable to their Scheme Shares will be aggregated and sold for cash on behalf of such Xenetic Shareholders as soon as reasonably practicable following completion of the Acquisition at the then prevailing market price on the OTCBB or OTCQB. The proceeds will then be converted into sterling at the exchange rate prevailing at that time and Xenetic Shareholders with entitlements to proceeds of such sale of less than £1 will have their proceeds aggregated and donated to charity. Those with entitlements to proceeds of £1 or more will receive sterling cheques for their entitlements as soon as reasonably practicable after such sale. Xenetic Shareholders with entitlements to proceeds of £1 or more will be able to opt to have such proceeds donated to charity. The white Form of Proxy contains a box to tick should such a Scheme Shareholder wish to elect to donate his or her proceeds to charity.
Any Xenetic Shareholders to the extent holding a number of Scheme Shares not divisible by 175, will also have such Scheme Shares aggregated together with similar Scheme Shares of other Xenetic Shareholders to result in GSL Consideration Shares to be sold on behalf of such Xenetic Shareholders in the same manner as explained above. Again, Xenetic Shareholders with entitlements to proceeds of such sale of less than £1 will have their proceeds aggregated and donated to charity. Those with entitlements to proceeds of £1 or more will receive sterling cheques for their entitlements as soon as reasonably practicable after such sale. Xenetic Shareholders with entitlements to proceeds of £1 or more will also be able to opt to have such proceeds donated to charity on the same basis as above.
Subject to the Scheme becoming Effective, save with the consent of the Panel, settlement of the consideration to which any Scheme Shareholder is entitled under the Scheme will be implemented in full, in accordance with the terms set out below, free of any liens, rights of set-off, counterclaims or other analogous rights to which GSL may otherwise be, or claim to be, entitled against such Scheme Shareholder.
It should be noted that all documents and remittances sent through the post will be sent at the risk of the person(s) entitled thereto.
(a) Scheme Shares held in certificated form
Any GSL Consideration Shares to which Shareholders are entitled pursuant to the Scheme will be issued on the Effective Date. Certificates for the GSL Consideration Shares will be despatched by post no later than 14 days after the Effective Date in prepaid envelopes addressed to the persons thereto entitled at their respective addresses, as appearing in the register of members of Xenetic at the Scheme Record Time (or, in the case of joint holders, to the address of that joint holder whose name stands first in the said register in respect of such joint holding).
Pending the despatch of certificates for GSL Consideration Shares, temporary documents of title will not be issued and transfers of GSL Consideration Shares in certificated form will be certified against the register of GSL.
Every holder of Xenetic Shares will be bound at the request of Xenetic to deliver up to Xenetic the existing certificate(s) for cancellation or to destroy the certificate(s). On the Effective Date, the certificates for the Scheme Shares will cease to be valid and should be destroyed on receipt of the consideration to which the holder is entitled.
(b) Scheme Shares held in uncertificated form
Where, at the Scheme Record Time, a Scheme Shareholder holds Scheme Shares in uncertificated form, he or she will be issued with any GSL Consideration Shares to which he or she is entitled in certificated form, in the same way as set out above.
After receipt of the certificates representing their GSL Consideration Shares, shareholders may deposit their GSL Consideration Shares, subject to the limitations discussed above regarding deposits, either by providing their certificate to the broker-dealer where their trading account is set up, or by arranging for their GSL Consideration Shares to be electronically transferred into the account. If delivered in electronic form at this later date, the shareholder will need to confirm that their broker-dealer allows for such transfers of OTCBB shares, and then provide the transfer agent with the necessary information regarding such Shares and their trading account to allow the electronic transfer to occur. Shares transferred in this manner will be tradable by the shareholder upon acceptance of the deposit into the trading account consistent with the rules of the broker-dealer with which such Shares are deposited. While Xenetic will assist where it can, because the deposit of the GSL Consideration Shares is dependent on the individual processing rules of the shareholder’s choice of broker-dealer, it will be the responsibility of the individual shareholder receiving their GSL Consideration Share certificate to process and facilitate the deposit of such Shares. Xenetic will take no responsibility or liability whatsoever for ensuring the deposit of the GSL Consideration Shares into a specific shareholder trading account or with a specific broker-dealer.
| 34 |
As from the Scheme Record Time, each holding of Scheme Shares credited to any stock account in CREST will be disabled and all Scheme Shares will be removed from CREST in due course thereafter.
16. Taxation
Your attention is drawn to Part 6 of this document which provides information in respect of UK taxation.
Shareholders who are in any doubt about their tax position, or who are subject to taxation in a jurisdiction outside the UK are strongly advised to contact an appropriate professional independent tax adviser immediately.
17. Overseas shareholders
The availability of the Scheme to persons resident in, or citizens of, jurisdictions outside the United Kingdom may be affected by the laws of such relevant jurisdictions. Persons who are not resident in the United Kingdom should inform themselves about and observe any applicable requirements. It is the responsibility of each Overseas Shareholder to satisfy themselves as to the full observance of the laws of the relevant jurisdiction in connection therewith, including the obtaining of any governmental exchange control or other consents which may be required or compliance with other necessary formalities which are required to be observed and the payment of any issue, transfer or other taxes due in such jurisdiction.
This document has been prepared for the purposes of complying with English law and the information disclosed may be different from that which would have been disclosed if this document had been prepared in accordance with the laws of jurisdictions outside England and Wales.
The Acquisition relates to the acquisition of shares in a UK company and is proposed to be made by means of a scheme of arrangement under English law. A transaction effected by means of a scheme of arrangement is not subject to the tender offer rules under the US Exchange Act. Accordingly, the Scheme is subject to the disclosure requirements, rules and practices applicable in the United Kingdom to schemes of arrangement, which differ from the requirements of US tender offer rules.
Financial information on Xenetic included in the relevant documentation has been prepared in accordance with accounting standards applicable to listed companies in the UK, being IFRS. These may not be comparable to the financial statements of US companies.
This document does not constitute an offer to sell or issue or the solicitation of an offer to buy or subscribe for securities in any jurisdiction in which such offer or solicitation is unlawful.
The terms of the Scheme are set out in full in Part 4 of this document. The purpose of the Scheme is to enable GSL to become the owner of the entire issued and to be issued share capital of the Company. This is to be achieved by the cancellation of the Scheme Shares held by Scheme Shareholders and the application of the reserve arising from such cancellation in paying up in full New Shares which have an aggregate nominal value equal to the aggregate nominal value of the Scheme Shares cancelled and issuing the same to GSL. Scheme Shareholders will then receive 56 GSL Consideration Shares for every 175 Scheme Shares held by them on the basis of the Acquisition and the Equivalent Document (as is more fully described herein).
As a result of the Scheme, Scheme Shareholders will be acquiring GSL Consideration Shares from General Sales & Leasing, Inc., a Nevada corporation, in accordance with United States securities laws.
| 35 |
The GSL Consideration Shares to be issued under the Scheme have not been, and will not be, registered under the US Securities Act of 1933, as amended (the “Securities Act”), or under the securities laws of any state, district or other jurisdiction of the United States, the Republic of South Africa, Canada or Japan.
It is expected that the GSL Consideration Shares will be issued in reliance upon the exemption from the registration requirements of the Securities Act provided by Section 3(a)(10) thereof on the basis of the Court Meeting, which will consider, among other things, the fairness of the terms of the Scheme. Under applicable US securities laws, shareholders who are or will be deemed to be affiliates of Xenetic or GSL, prior to or after the Effective Date, will be subject to certain transaction restrictions relating to the GSL Consideration Shares received in connection with the Scheme.
18. Action to be taken
It is important that, for the Court Meeting in particular, as many votes as possible are cast so that the Court may be satisfied that there is a fair representation of Shareholder opinion. Your attention is drawn to paragraph 15 of the letter from the Chairman of Xenetic set out in Part 1 of this document, which explains the action you should take in relation to the Acquisition and the Scheme.
19. Further information
The terms of the Scheme are set out in full in Part 4 of this document. Your attention is also drawn to the further information contained in this document which forms part of the explanatory statement pursuant to section 897 of the Companies Act. In particular, your attention is drawn to the Conditions and Further Terms of the Acquisition in Part 3, the financial information on the Company in Part 5, the information on GSL in Part 7 and the Additional Information set out in Part 8 of this document.
Yours faithfully
/s/ Adam D. Hart
Adam D. Hart Director
For and on behalf of
London Bridge Capital Limited
| 36 |
PART 3
CONDITIONS AND FURTHER TERMS OF THE ACQUISITION
A. Conditions of the Acquisition
1. The Acquisition will be conditional upon the Scheme becoming unconditional and becoming effective by no later than 5.00 p.m. London time on 30 June 2014, or such later date (if any) as GSL and Xenetic may, with the consent of the Panel, agree and, if required, the Court may allow.
2. The Scheme will be conditional upon:
| (1) | its approval by a majority in number representing not less than 75 per cent. in value of the Scheme Shareholders (or the relevant class or classes thereof, if applicable) present and voting, either in person or by proxy, at the Court Meeting and at any separate class meeting which may be required by the Court or at any adjournment of any such meeting; |
| (2) | all resolutions necessary to approve and implement the Scheme being duly passed by the requisite majority or majorities at the General Meeting of Xenetic or at any adjournment of that meeting; and |
| (3) | the sanction of the Scheme with or without modification (but subject to any such modification being acceptable to GSL and Xenetic) and the confirmation of the Reduction of Capital by the Court and: |
| (i) | the
delivery
of
a
copy
of
the
necessary
Court
Order(s)
and
the
requisite
statement
of
capital to the Registrar of Companies; and |
| (ii) | if the Court so orders for it to become effective, the registration of the Court Order(s) and the statement of capital by the Registrar of Companies. |
| (iii) |
3. Subject as stated in Part B below and to the requirements of the Panel, the Acquisition will also be conditional on the following Condition and, accordingly, the necessary actions to make the Scheme effective will not be taken unless the following Condition (as amended if appropriate) has been satisfied or, where relevant, waived:
(1) all necessary filings or applications having been made in connection with the Acquisition and all statutory or regulatory obligations in any jurisdiction having been complied with in connection with the Acquisition and all authorisations, orders, recognitions, grants, consents, licences, confirmations, clearances, permissions and approvals (collectively “Consents”) reasonably deemed necessary or appropriate by GSL and Xenetic for or in respect of the Acquisition having been obtained in terms and in a form satisfactory to GSL and Xenetic and all necessary statutory or regulatory obligations in connection with the Acquisition in any jurisdiction having been complied with in all material respects. Such filings include, but are not limited to, filings with the Secretary of State of Nevada effectuating the Acquisition and filings with the SEC, all as required by law.
B. Certain further terms of the Acquisition
To the extent permitted by law and subject to the requirements of the Panel, GSL and Xenetic reserve the right to waive, in whole or in part, Condition 3(1) above. Conditions 1 and 2 cannot be waived.
Condition 3(1) must be fulfilled or waived by no later than 11.59pm on the date immediately preceding the date of the Court hearing to sanction the Scheme, failing which the Scheme will lapse. GSL shall be under no obligation to waive or treat as satisfied Condition 3(1) by a date earlier than the latest date specified above for the fulfilment or waiver thereof, notwithstanding that the other Conditions of the offer may at such earlier date have been waived or fulfilled and that there are at such earlier date no circumstances indicating that any of such Conditions may not be capable of fulfilment.
If GSL is required by the Panel to make an offer for Xenetic Shares under the provisions of Rule 9 of the Code, GSL may make such alterations to any of the above Conditions as are necessary to comply with the provisions of that Rule.
| 37 |
The availability of the Acquisition to persons not resident in the United Kingdom may be affected by the laws of the relevant jurisdictions. Persons who are not resident in the United Kingdom should inform themselves about and observe any applicable requirements.
The Acquisition will be governed by English law and will be subject to the jurisdiction of the English courts. The Acquisition will comply with the applicable rules and regulations of the Financial Services Authority and the London Stock Exchange and the Code.
Each of the Conditions shall be regarded as a separate Condition and shall not be limited by reference to any other Condition.
Under Rule 13.5 of the City Code, GSL may not invoke a condition to the Scheme so as to cause the Scheme not to proceed, to lapse or to be withdrawn unless the circumstances which give rise to the right to invoke the condition are of material significance to GSL in the context of the Scheme. The determination of the whether or not such a condition can be invoked would be made by the Panel. Conditions 1 and 2 are not subject to this provision of the Code.
The GSL Consideration Shares will be issued credited as fully paid and will rank pani passu in all respects with the New GSL Shares.
| C. | City Code on Takeovers and Mergers |
The City Code on Takeovers and Mergers applies, inter alia, to offers for all public companies (other than open ended investment companies) which have their registered office in the United Kingdom and the securities of which are admitted to trading on AIM. As a company incorporated in England with Xenetic Shares admitted to trading on AIM, Xenetic is subject to the provisions of the City Code.
The Takeover Panel is an independent body, whose main functions are to issue and administer the City Code and to supervise and regulate takeovers and other matters to which the City Code applies in accordance with the general principles and rules set out in the City Code. The City Code applies to the Xenetic and certain dealings in its shares. The City Code also applies to a range of persons who participate in, or are connected with, or who in any way seek to influence, intervene in, or benefit from takeovers and other matters to which the City Code applies. A copy of the City Code can be obtained at and further guidance on the City Code can be sought, if required, by telephoning the Panel on +44 (0) 20 7382 9026.
Xenetic Shareholders should note that, with effect from the Effective Date, the protections afforded by the City Code will cease to apply in relation to Xenetic and its securities. The City Code will not apply in relation to GSL and its securities, including the GSL Consideration Shares.
| D. | Comparison of material differences between the rights of Shareholders of Xenetic Shares and holders of GSL Consideration Shares |
Xenetic Shareholders’ rights are governed by English law, including the Companies Act. Once the Scheme becomes Effective, all Scheme Shares will be cancelled and Scheme Shareholders will receive GSL Consideration Shares. Because GSL is a corporation incorporated under Nevada law, the rights of the holders of GSL Consideration Shares will be governed by the Nevada Revised Statutes along with GSL’s Articles of Incorporation and Bylaws.
Certain of the principal attributes of the Xenetic Shares and the GSL Consideration Shares are similar. However, there are differences between the rights of Shareholders of Xenetic under English law and the rights of shareholders of GSL Consideration Shares under Nevada law, following the Scheme becoming Effective. In addition, there are differences between GSL’s Articles of Incorporation and Bylaws and Xenetic’s articles of association.
The following is a summary comparison of the material differences between the rights of Shareholders of Xenetic Shares under English law (and its articles of association) and the rights they will have as shareholders of GSL Consideration Shares under Nevada law and GSL’s Articles of incorporation and Bylaws.
| 38 |
The discussion in this section does not include a description of rights or obligations under the U.S. federal securities laws, relevant NASDAQ listing requirements or standards or Xenetic’s or GSL’s corporate governance or other policies.
Provisions currently applicable to Xenetic Shareholders |
Provisions applicable to holders of GSL Consideration Shares |
| Authorised Capital and allotment of new shares | |
There is no concept of authorised share capital under English law.
However, a public company is required to have an allotted share capital of a nominal value that is not less than the amount of the authorised minimum in order to obtain a trading certificate or re-register as a public company which is currently prescribed as £50,000 or €57,100 (section 763, Companies Act and Regulation 2 of the Companies (Authorised Minimum) Regulations 2009 (SI 2009/2425).
Directors may only allot shares or grant rights to subscribe for shares or to convert any security into shares if they have been given prior authorisation for the proposed allotment in accordance with section 551 of the Companies Act by an ordinary resolution.
According to Xenetic’s Articles of Association, the special rights conferred upon the holders of any shares or class of shares shall, unless otherwise provided by the Articles of Association or the terms of issue of the shares concerned, be deemed to be varied by a reduction of capital paid up on those shares but shall be deemed not to be varied by the creation or issue of further shares ranking pani passu with them does not make specific provision, as the Board may determine.
However, no member shall attend or vote unless all moneys presently payable by him in respect of that share have been paid.
According to Xenetic’s Articles of Association, whenever the share capital of the Company is divided into different classes of shares, the rights attached to any class of shares in issue may (unless otherwise provided by the terms of issue of the shares of that class) from time to time be varied or abrogated, whether or not the Company is being wound up, either with the consent in writing of the holders of three-fourths in nominal value of the issued shares of the class or with the sanction of a special resolution passed at a separate meeting of such holders (but not otherwise). |
The authorised share capital of GSL will as at the Effective Date consist of (i) 10,000,000 shares of preferred stock, par value $0.001 per share (of which none have been issued to date) and (ii) 300,000,000 shares of common stock, par value $0.01 (of which the equivalent of 13,500,000 will have been issued).
The shares of preferred stock may be issued in series, and shall have such voting powers, full or limited, or no voting powers, and such designations, preferences and relative participating, optional or other special rights, and qualifications, limitations or restrictions thereof, as shall be stated and expressed in the resolution or resolutions providing for the issuance of such stock adopted from time to time by GSL’s board of directors. The board of directors is expressly vested with the authority, without shareholder approval, to determine and fix in the resolution or resolutions providing for the issuances of preferred stock the voting powers, designations, preferences and rights, and the qualifications, limitations or restrictions thereof, of each such series to the full extent now or hereafter permitted by the laws of the State of Nevada.
|
| Voting Rights | |
Under Xenetic’s Articles of Association, each Xenetic Share has one vote on all matters submitted to a vote of shareholders at any general meeting or at any separate meeting of the holders of any class of shares in Xenetic.
Subject to the provisions of the Companies Act and without prejudice to any rights attached to any existing shares, any share may be issued with such rights or restrictions as the Company may by ordinary resolution determine, or in the absence of such determination, or so far as any such resolution does not make specific provision, as the Board may determine.
However, no member shall attend or vote unless all moneys presently payable by him in respect of that share have been paid.
According to Xenetic’s Articles of Association, whenever the share capital of the Company is divided into different classes of shares, the rights attached to any class of shares in issue may (unless otherwise provided by the terms of issue of the shares of that class) from time to time be varied or abrogated, whether or not the Company is being wound up, either with the consent in writing of the holders of three-fourths in nominal value of the issued shares of the class or with the sanction of a special resolution passed at a separate meeting of such holders (but not otherwise). |
Under GSL’s Articles of Incorporation, holders of GSL common stock are entitled to one vote for each share of such stock. Voting rights of preferred stock are determined by the board of GSL and may vary from time to time. No preferred shares have been defined or issued at this time.
Subject to the rights of the holders of any series of preferred stock, if issued, and except as otherwise required by applicable law, GSL’s Articles of Incorporation or, for the election of directors, the affirmative vote of a majority of the combined voting power of the outstanding shares present in person or represented by proxy at the meeting and entitled to vote on the subject matter shall be the act of the shareholders.
Subject to the rights of the holders of any series of preferred stock, if issued, directors of GSL shall be elected by a plurality of the combined voting power of the outstanding shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors.
|
| 39 |
| Quroum | |
Xenetic’s Articles of Association provide that no business shall be transacted at any general meeting unless a quorum is present when the meeting proceeds to business. The absence of a quorum shall not preclude the appointment of a chairman in accordance with the provisions of the Articles, which shall not be treated as part of the business of the meeting. Two members present in person or by proxy and entitled to vote upon the business to be transacted at the meeting shall be a quorum. For class meetings of the holders of any class of shares of Xenetic, the same provision apply, mutatis mutandis, except that in the case of a meeting held in connection with the variation or abrogation of the rights attached to the shares of the class the necessary quorum shall be two persons at least holding or representing by proxy at least one-third in nominal amount of the issued shares of the class. |
GSL’s Bylaws provide that, unless otherwise provided by law, holders of a majority of the capital stock of GSL issued and outstanding and entitled to vote present in person or by proxy at the meeting constitutes a quorum for the transaction of business at the meeting. |
| Annual General Meetings of Shareholders | |
The Companies Act requires Xenetic to hold annual general meetings and that the election and reelection of a relevant class of directors shall take place thereat. Meetings will take place at such date, time and place, as may be specified by the Xenetic Board in the notice of meeting.
English law requires that each annual general meeting must take place within the six month period beginning with the day following the end of the company’s fiscal year, but does not specify what business must be transacted at the annual general meeting, nor are there any restrictions on business. However, the annual general meeting is usually the meeting that is used for matters which must be dealt with each financial year, such as the re-election of directors, fixing the remuneration of auditors and consideration of the annual accounts, directors’ report and auditors’ report. |
GSL’ Bylaws provide that an annual meeting of shareholders for the purpose of electing directors and of transacting any other business shall be held each year at such date, time and place, as may be specified by the GSL board of directors in the notice of the meeting.
Under Nevada law, one or more shareholders, who have the right to exercise at least 15 per cent. of the voting power, may petition the district court of Nevada to order a meeting to elect directors if the annual meeting has not been held within 18 months following the date of the last election of directors. |
| 40 |
Provisions currently applicable to Xenetic Shareholders |
Provisions applicable to holders of GSL Consideration Shares |
| Special General Meetings of Shareholders | |
The Xenetic Board can call general meetings at such date, time and place, as they may specify in the notice of meeting.
Under English law, one or more shareholders holding voting shares representing at least five per cent. of such of the paid-up capital as carries the right of voting at general meetings of Xenetic can require (or requisition) Xenetic to call and hold a general meeting.
The only business that may be conducted at a general meeting is the business that is referred to in the relevant notice of such general meeting. |
Subject to the rights of the holders of preferred stock, GSL’s Bylaws provide that special meetings of shareholders may only be called by (i) the Chairman of the Board of Directors, (ii) GSL’s Chief Executive Officer or (iii) the Board of Directors by a vote of the majority of total number of authorized directors. GSL’s Bylaws provide that only such business shall be conducted at a special meeting of shareholders as shall have been brought before the meeting pursuant to the notice of that special meeting.
|
| Notice of Annual General Meetings | |
The Xenetic Board can call general meetings at such date, time and place, as they may specify in the notice of meeting.
The notice of the general meeting must state the date, time and place of the meeting and the general nature of the business to be dealt with, and where it is proposed to pass a particular resolution as a special resolution the notice should state as such (as well as include the text of the proposed special resolution).
The notice of any annual general meeting should also identify the meeting as being an annual general meeting. Business transacted at a general meeting of shareholders shall be limited to the purposes stated in the notice.
Xenetic’s Articles of Association provide that an annual general meeting must be called by at least 21 clear days’ notice and by not less than 14 clear days’ notice for a general meeting other than the annual general meeting. |
Generally, the Nevada Revised Statutes requires that notice is given to shareholders of the place (if any), date, and hour, and means of remote communication, if any, of each annual and special shareholders’ meeting, at least 10 days, but no more than 60 days, before the meeting date. The notice must also state the purpose or purposes for which the meeting is called.
GSL’s Bylaws provide that all meetings of shareholders are to be held at such time and place as may be stated in the notice of the meeting and that such notice must state: (i) the place, if any, date and hour thereof; the means of remote communication, if any, by which shareholders and proxy holders may be deemed to be present in person and vote at such meeting, and (ii) the purpose or purposes for which the meeting is called.
GSL’s Bylaws provide that notice of an annual or special meeting will be given to each shareholder entitled to vote thereat at least 10 days but not more than 60 days before the date of the meeting.
|
| 41 |
Provisions currently applicable to Xenetic Shareholders |
Provisions applicable to holders of GSL Consideration Shares |
| Shareholder Nominations of Directors | |
Shareholders, whether individually or collectively, who meet the relevant thresholds can requisition general meetings and require certain resolutions to be proposed at general meetings, and these resolutions may include nominations of persons for election to the Xenetic board of directors (see “Special Meetings of Shareholders” and “Shareholder Proposals” above).
The shareholders of Xenetic also have the right in Xenetic’s Articles of Association to elect directors by ordinary resolution. The Xenetic Board also has the right in the Xenetic articles to elect directors.
|
GSL’s Bylaws provide that, subject to the rights of the holders of any preferred stock, nominations of persons for election to the GSL board of directors at any meeting of shareholders at which directors will be elected may be made (a) by or at the direction of the board of directors (or any duly authorised committee thereof) or (b) by any shareholder of GSL that: complies with the notice procedures described below.
For a nomination to be made by a shareholder, such shareholder must give timely notice thereof in proper written form to the Secretary of GSL. To be timely, a shareholder’s written notice to the Secretary of GSL must be delivered to or mailed and received at the principal executive offices of GSL, in the case of: (x) an annual meeting, not less than 60 days nor more than 90 days prior to the first anniversary of the date of the immediately preceding year’s annual meeting of shareholders; provided, however, that if the date of the annual meeting is advanced more than 30 days prior to or delayed by more than 30 days after the anniversary of the preceding year’s annual meeting, to be timely, notice by the shareholder must be so received not less than 60 days nor more than 90 days prior to the date of such annual meeting of shareholders, or, in the event public announcement of the date of such annual meeting is first made by GSL fewer than seventy (70) days prior to the date of such annual meeting, not later than the close of business on the tenth day following the day on which public disclosure of the date of the annual meeting is first given or made. To be in proper written form, a shareholder’s notice to the Secretary of GSL must include all the information set forth in the Bylaws. |
| Standard Conduct for Directors; Composition of the Board | |
English law imposes certain specific obligations on the directors of Xenetic. In addition to certain common law and equitable principles, there are statutory director duties, including seven codified duties as follows:
(1) to act in a way he or she considers, in good faith, would be most likely to promote the success of the company for the benefit of its shareholders as a whole; (2) to act in accordance with the company’s constitution and exercise powers only for the purposes for which they are conferred; (3) to exercise independent judgment; (4) to exercise reasonable care, skill and diligence; (5) to avoid conflicts of interest; (6) not to accept benefits from third parties; and (7) to declare an interest in a proposed transaction with the company.
Xenetic’s articles provide that the number of directors shall not be less than two, unless otherwise determined by an ordinary resolution of Xenetic’s shareholders. |
In general, the Nevada Revised Statutes does not contain any specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of the board of directors is thus determined by decisions of the courts of the State of Nevada. In general, directors have a duty to act in good faith, on an informed basis and in a manner they reasonably believe to be in the best interests of the shareholders.
GSL’s Articles of Incorporation and Bylaws provide that the number of directors of the corporation shall be not less than one and not more than 13, and the exact number of directors shall be fixed by the board of directors.
|
| 42 |
| Removal of Directors | |
| Under English law, shareholders in Xenetic may remove a director of Xenetic without cause by ordinary resolution, irrespective of any provisions in the Xenetic Articles of Association. | Nevada law provides that any director or the entire board of directors may be removed, with or without cause, by the holders of two-thirds of the shares then entitled to vote at an election of directors, except directors may not be removed in certain situations in the case of a corporation having cumulative voting. It should be noted that GSL’s Bylaws contain a provision that allows directors to be removed by the affirmative vote of the holders of a majority of the shares outstanding. As this provision is in conflict with the Nevada Revised Statutes, the statute is governing, not the bylaws. |
| Declaration of Dividends | |
The shareholders may declare dividends by ordinary resolution, except that no dividend may exceed the amount recommended by the Xenetic board of directors. Furthermore, the Xenetic Articles of Association also provide that a general meeting declaring a dividend may, upon recommendation by the Xenetic Board, direct by ordinary resolution that the dividend shall be satisfied wholly or partly by the distribution of assets, including (without limitation) paid up shares or debentures of another corporation. Xenetic’s Articles of Association authorise the Board to declare interim dividends (including wholly or partly by the distribution of assets) if the Xenetic Board considers that they are justified by the profits of Xenetic available for distribution. |
The Nevada Revised Statutes prohibits distributions to shareholders when the distributions would (i) render the corporation unable to pay its debts as they become due in the usual course of business; or (ii) render the corporation’s total assets less than the sum of its total liabilities plus the amount that would be needed to satisfy the preferential rights upon dissolution of shareholders whose preferential rights are superior to those receiving the distribution. |
| 43 |
Provisions currently applicable to Xenetic Shareholders |
Provisions applicable to holders of GSL Consideration Shares |
| Preemptive Rights | |
Under English law, the issuance for cash of: (1) equity securities (i.e., shares other than shares which, with respect to dividends or capital, carry a right to participate only up to a specified amount in a distribution); or (2) rights to subscribe for, or convert securities into, such equity securities, must be offered first to the existing ordinary shareholders inproportion to their respective nominal amounts (i.e., par values) of their holdings. English law permits a company’s shareholders by special resolution or a provision in a company’s articles of association to exclude pre-emptive rights for a period of up to five years. |
Under Nevada law, a shareholder is not entitled to preemptive rights to subscribe
for additional
GSL’s Articles of Incorporation do not provide such rights to the holders of GSL’s common stock. However, such rights could later be part of the rights in a newly created preferred class of stock. |
| Takeover Matters | |
Xenetic is currently subject to the UK Takeover Code and thus the ability of its directors to engage in defensive measures to seek to frustrate bids is restricted. Xenetic and its Shareholders currently have the benefit of the protections and regulation prescribed by the Takeover Code.
However, as described in section C of this Part 3, Xenetic will cease to have the benefit of the Takeover Code when it has been acquired by GSL. |
GSL will not be subject to the Takeover Code as described in Section C of this Part 3. Pursuant to the Nevada Revised Statutes, unless a corporation’s articles of incorporation provides otherwise, a merger or consolidation of the corporation or the sale, lease or exchange of all or substantially all of a corporation’s assets generally requires the approval of the board of directors and (except in limited circumstances) the affirmative vote of a majority of the voting power of the outstanding stock entitled to vote on the transaction. Pursuant to the Nevada Revised Statutes, a Corporation or other entity owning at least 90 per cent. of the outstanding shares of a subsidiary corporation may effect a merger with or into such subsidiary by resolution of the board of directors of the parent and without any action on the part of the board of directors or of the other shareholders of the subsidiary. A Nevada court will generally uphold board of director decisions to adopt anti-takeover measures in the face of a potential takeover where the directors are able to show that: (1) the directors had reasonable grounds for believing that there was a danger to corporate policy and effectiveness from an acquisition proposal; and the board action taken was reasonable in relation to the threat posed.
In its articles of incorporation, GSL has elected not to be governed by the provisions of Sections 78.411 to 78.444 of the Nevada Revised Statutes, which generally prohibits “business combinations”, including mergers, sales and leases of assets, issuances of securities and similar transactions by a corporation or a subsidiary with an interested shareholder who beneficially owns 10 per cent. or more of a corporation’s voting stock within three years after the person or entity becomes an interested shareholder, unless: (i) the board of directors of the corporation has approved, before the time that the shareholder became an interested shareholder, either the business combination or the transaction that resulted in the person becoming an interested shareholder; (ii) upon consummation of the transaction that resulted in the person becoming an interested shareholder, the person owns at least 85 per cent. of the corporation’s voting stock (excluding shares owned by directors who are officers and shares owned by employee stock plans in which participants do not have the right to determine confidentially whether shares will be tendered in a tender or exchange offer); or (iii) at or after the person or entity becomes an interested shareholder, the business combination is approved by the board of directors and authorized by the vote of at least 662/3 per cent. of the outstanding voting stock not owned by the interested shareholder at an annual or special meeting. |
| 44 |
Provisions currently applicable to Xenetic Shareholders |
Provisions applicable to holders of GSL Consideration Shares |
| Appraisal/Dissenters Rights | |
| Under English law, unless otherwise agreed by contract, a shareholder is generally entitled to bring actions for breach of directors’ duties on behalf of the Company or may inter alia apply to the court by petition for an order on the ground (a) that the company’s affairs are being or have been conducted in a manner that is unfairly prejudicial to the interests of members generally or of some part of its members (including at least himself), or (b) that an actual or proposed act or omission of the company (including an act or omission on its behalf) is or would be so prejudicial. | Pursuant to the Nevada Revised Statutes, a shareholder of a Nevada corporation is generally entitled, subject to certain specified circumstances, to dissent from a vote on approving certain merger or consolidation transactions and demand appraisal of the fair value of such shareholder’s shares in the event the corporation is a party to a merger or consolidation, subject to specified exceptions. |
| Amendment to the Articles of Incorporation/Bylaws | |
The Companies Act provides that the articles of association of a company may be amended by special resolution. The Companies Act retains the principle that a member is not bound by any alterations made to the articles subsequent to him becoming a member, if the alteration has the effect of increasing his liability to the company or requires him to take more shares. A member may give his written consent to the alteration and is then bound by it.
|
Pursuant to the Nevada Revised Statutes, unless the articles of incorporation requires a greater vote, an amendment to the articles of incorporation requires: (1) recommendation of the board of directors; (2) the affirmative vote of a majority of the voting power of the outstanding stock entitled to vote; and (3) subject to certain class rights, the affirmative vote of a majority of the voting power of the outstanding stock of each class entitled to vote.
Pursuant to the Bylaws, only the Board of Directors has the power to adopt, amend or repeal bylaws. |
| 45 |
Provisions currently applicable to Xenetic Shareholders |
Provisions applicable to holders of GSL Consideration Shares |
| Related Party Transactions | |
Pursuant to the Companies Act, specific transactions with directors may require approval of members of the Company. Xenetic’s Articles of association also contains specific provisions regarding director’s interests and sets out when disclosure of the directors’ interests and conflicts need to be made and in which circumstances the director’s right to vote at meeting of the Board or a committee of the Board may be curtailed. |
Pursuant to the Nevada Revised Statutes any contract or transaction between the corporation and one or more of its directors or officers is neither void nor voidable solely because the interested director or officer was present, participates or votes at the board or board committee meeting that authorizes the contract or transaction, if either: (i) the director’s or officer’s interest is made known to the disinterested directors or the shareholders of the corporation, who thereafter approve the transaction in good faith; (ii) (i) the director’s or officer’s interest is not known to the interested director or officer at the time of the transaction or (iii) the contract or transaction is fair to the corporation as of the time it is approved or ratified by either the board of directors, a committee thereof, or the shareholders. |
| Indemnification of Officers and Directors | |
Under English law, the fiduciary and general duties of directors are owed to the company, so shareholders or third parties will normally only have a cause of action against the company, not against individual directors. However, directors may also incur liabilities to shareholders and third parties where they act in a way which creates a personal obligation. This is not lightly implied, but could be assumed, for example, by an express representation by a director accepting a personal obligation to the shareholder or third party. Under the Companies Act a company may not generally exempt a director from, or indemnify him against, liability in connection with any negligence, default, breach of duty or breach of trust by him in relation to the company. This means that a company cannot exempt a director from liability for breach of one or more of his duties to the company or limit his liability for such a breach. Pursuant to Xenetic’s Articles of Association, subject to the provisions of the Companies Acts but without prejudice to any indemnity to which he may otherwise be entitled, every Director, Alternate Director, Secretary or other officer (other than the Auditors) of Xenetic or of any associated company shall be indemnified out of the assets of Xenetic against all costs, charges, expenses, losses, damages and liabilities (“Liabilities”) incurred by him in or about the execution of his duties or the exercise of his powers or otherwise in relation thereto and, where Xenetic is a trustee of an occupational pension scheme, against all Liabilities incurred in connection with Xenetic’s activities as a trustee of the pension scheme, including (without prejudice to the generality of the foregoing) any liability incurred by him in defending any proceedings, whether civil, criminal or regulatory which relate to anything done or omitted or alleged to have been done or omitted by him as an officer or employee of Xenetic or of any associated company in which judgment is given in his favour or in which he is acquitted, or which are otherwise disposed of without any finding or admission of material breach of duty on his part or in connection with any application in which relief is granted to him by the court from liability for negligence, default, breach of duty or breach of trust in relation to the affairs of Xenetic. |
The Nevada Revised Statutes permits GSL to indemnify any persons who are, or are threatened to be made, parties to any threatened, pending or completed legal action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was a director, officer, employee or agent of GSL or is or was serving at the request of GSL as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of GSL and, with respect to any criminal action or proceeding, had no reasonable cause to believe that the person’s conduct was unlawful. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, GSL must indemnify him against the expenses that such officer or director actually and reasonably incurred. GSL may indemnify the same category of persons in an action by or in the right of GSL under the same conditions, but only for expenses (including attorneys’ fees), provided that no indemnification is permitted without judicial approval if such person is adjudged to be liable to the corporation. GSL’s articles of incorporation and Bylaws provide that GSL shall indemnify and hold harmless, to the fullest extent permitted by applicable law as it presently exists or may hereafter be amended, any person who was or is made or is threatened to be made a party or is otherwise involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative by reason of the fact that he, or a person for whom he is the legal representative, is or was a director or officer of GSL or while a director or officer is or was serving at the request of GSL as a director, officer, employee or agent of another corporation or of a partnership, joint venture, limited liability company, trust, enterprise or nonprofit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses (including attorneys’ fees) incurred by such person. GSL shall be required to indemnify or make advances to a person in connection with a proceeding (or part thereof) initiated by such person only if the proceeding (or part thereof) was authorized by GSL’s board of directors. GSL shall pay the expenses (including attorneys’ fees) incurred by a director, officer or any other person entitled to indemnification in defending any proceeding in advance of its final disposition, provided, however, that the payment of expenses incurred by a director or officer in advance of the final disposition of the proceeding shall be made only upon receipt of an undertaking by the director or officer to repay all amounts advanced if it should be ultimately determined that the director or officer is not entitled to be indemnified. GSL’s board of directors may, to the fullest extent permitted by applicable law as it presently exists, or may hereafter be amended from time to time, authorize an appropriate officer or officers to purchase and maintain at GSL’s expense insurance: (i) to indemnify GSL for any obligation which it incurs as a result of the indemnification of directors and officers; and (ii) to indemnify or insure directors and officers against liability in instances in which they may not otherwise be indemnified by GSL. |
| 46 |
PART 4
THE SCHEME OF ARRANGEMENT
IN THE HIGH COURT OF JUSTICE CHANCERY DIVISION COMPANIES COURT |
No 7321 of 2013 |
IN THE MATTER OF XENETIC BIOSCIENCES PLC
and
IN THE MATTER OF THE COMPANIES ACT 2006
SCHEME OF ARRANGEMENT
(under Part 26 of the Companies Act 2006) between
XENETIC BIOSCIENCES PLC
and
THE
HOLDERS OF SCHEME SHARES
(as hereinafter defined)
PRELIMINARY
(A) In this Scheme, the following expressions have the meanings stated, unless they are inconsistent with the subject or context:
| “business day” | a day on which London Stock Exchange plc is open for the transaction of business; |
| “Code” | the City Code on Takeovers and Mergers of the United Kingdom; |
| “Companies Act” | the Companies Act 2006, as amended; |
| “Company” or “Xenetic” | Xenetic Biosciences plc, incorporated in England and Wales with company registration number 03213174; |
| “Court” | the High Court of Justice in England and Wales; |
| “Court Hearing” | the hearing by the Court at which the Court order is made; |
| “Court Meeting” | the meeting of the Scheme Shareholders (and any adjournment thereof) convened pursuant to an order of the Court under Part 26 of the Companies Act for the purpose of considering and, if thought fit, approving this Scheme; |
| “Court Order” | the order of Court sanctioning this Scheme under Part 26 of the Companies Act and confirming the reduction of the Company’s capital by the cancellation of the Scheme Shares under section 648 of the Companies Act; |
| “Effective Date” | the date on which this Scheme becomes effective in accordance with clause 5 of this Scheme; |
| “Group” | Xenetic and its subsidiaries; |
| “GSL” | General Sales & Leasing, Inc. a company incorporated in the state of Nevada, US |
| 47 |
“GSL Consideration Shares” |
the New GSL Shares to be issued credited as fully paid to Scheme Shareholders pursuant to the Scheme and the Equivalent Document (expected to be up to a maximum of 130,520,137 New GSL shares assuming Xenetic does not issue further shares); |
| “GSL Group” | GSL and its subsidiaries; |
| “GSL Shares” | Common Stock of GSL with a par value of US$0.001 per share; |
| “GMT” | Greenwich Mean Time; |
| “Holder” | a registered holder of Shares including a person entitled by transmission; |
| “New GSL Shares” | new GSL Shares of Common Stock with a par value of US$0.01 proposed to be issued and credited as fully paid pursuant to the share consolidation of GSL (before the Scheme becomes Effective); |
| “New Shares” | the new ordinary shares of 0.5 pence each in the capital of the Company to be issued in accordance with clause 1.2 of this Scheme; |
| “Reduction of Capital” | the reduction of the share capital of the Company by the cancellation and extinguishing of the Scheme Shares, provided for in clause 1.1 of this Scheme; |
| “Scheme” | this scheme of arrangement under Part 26 of the Companies Act between the Company and the Scheme Shareholders in its present form or with or subject to any modification, addition or condition agreed between the Company and GSL and approved or imposed by the Court; |
| “Scheme Record Time” | 6.00 p.m. GMT on the business day before the Court Hearing; |
| “Scheme Shareholders” | holders of Scheme Shares; |
| “Scheme Shares” | the aggregate of: (i) the Shares in issue at the date of this Scheme; (ii) the Shares (if any) issued after the date of this Scheme and prior to the Voting Record Time; and (iii) the Shares (if any) issued on or after the Voting Record Time and prior to the Scheme Record Time either on terms that the original Holder or any subsequent Holder thereof shall be bound by this Scheme or in respect of which the Holder thereof shall have agreed in writing to be bound by this Scheme, (iv) in each case other than any Shares which are registered in the name of or beneficially owned by GSL or any member of the GSL Group; |
| “Shares” | ordinary shares of 0.5 pence each in the capital of the Company; |
| “Statement of Capital” | the statement of capital (approved by the Court) showing as altered by the Court Order in so far as it confirms the Reduction of Capital, the information required by section 649 of the Companies Act with respect to Xenetic’s share capital; |
| “subsidiary” | has the meaning ascribed to it in the Companies Act; and |
| “Voting Record Time” | 6.00 p.m. GMT on the day which is two working days before the date of the Court Meeting or, if the Court Meeting is adjourned, 6.00 p.m. GMT on the day which is two working days before the date of such adjourned meeting. |
| 48 |
| (B) | References to “clauses” are to clauses of this Scheme and references to time are to London time. Any reference to any provision of any legislation shall include any amendment, modification, re-enactment or extension thereof. |
| (C) | GSL has agreed to be represented by Xenetic’s counsel at the Court Hearing, to consent to the Scheme and to undertake to the Court to be bound thereby and to execute and do, or procure to be executed and done, all such documents, acts and things as may be necessary or desirable to be executed or done by it or on its behalf for the purpose of giving effect to this Scheme. |
| (D) | As at the date of this document GSL does not hold, and no member of the GSL Group holds, any Shares. |
| (E) | The provisions of this Scheme are subject to the Court confirming the Reduction of Capital and, accordingly, they may not be implemented until a certified copy of the Court Order (together with a statement of capital) has been delivered to, and registered by, the Registrar of Companies. |
THE SCHEME
| 1. | Cancellation of the Scheme Shares |
1.1 The share capital of the Company shall be reduced by cancelling and extinguishing all of the Scheme Shares.
1.2 Subject to and forthwith upon the said Reduction of Capital taking effect, and notwithstanding anything to the contrary in the Company’s articles of association, the reserve arising in the books of account of the Company as a result of the said Reduction of Capital shall be capitalised and applied in paying up in full at par such number of New Shares as shall be equal to the number of Scheme Shares cancelled pursuant to clause 1.1 of this Scheme, which shall be allotted and issued credited as fully paid (free from all liens, charges, equitable interests and encumbrances) to GSL and/or its nominee(s).
| 2. | Consideration for cancellation of the Scheme Shares |
2.1 In consideration of the cancellation of the Scheme Shares pursuant to clause 1.1 of this Scheme and the allotment and issue to GSL and/or its nominee(s) of the New Shares, GSL shall, contingently upon the Reduction of Capital provided for in clause 1.1 of this Scheme taking effect (and subject as hereinafter provided) in accordance with clause 5 of this Scheme, issue to each holder of Scheme Shares (as appearing in the register of members of the Company at the Scheme Record Time):
for every 175 Scheme Shares: 56 GSL Consideration Shares
2.2 The provisions of this clause 2 of the Scheme shall be subject to any prohibition or condition imposed by law.
| 3. | Certificates and cancellation |
With effect from and including the Effective Date, all certificates representing Scheme Shares shall cease to have effect as documents of title or to be valid for any purpose and each holder of Scheme Shares shall be bound at the request of the Company to deliver up the same to the Company or to any person nominated by the Company for cancellation or to destroy the same.
| 49 |
4. Settlement
4.1 In the case of Scheme Shares which at the Scheme Record Time are held in certificated form, any GSL Consideration Shares to which Shareholders are entitled pursuant to the Scheme will be issued on the Effective Date. Certificates for the GSL Consideration Shares will be despatched by post no later than 14 days after the Effective Date in prepaid envelopes addressed to the persons thereto entitled at their respective addresses, as appearing in the register of members of Xenetic at the Scheme Record Time (or, in the case of joint holders, to the address of that joint holder whose name stands first in the said register in respect of such joint holding).
Pending the despatch of certificates for GSL Consideration Shares, temporary documents of title will not be issued and transfers of GSL Consideration Shares in certificated form will be certified against the register of GSL. Every holder of Xenetic Shares will be bound at the request of Xenetic to deliver up to Xenetic the existing certificate(s) for cancellation or to destroy the certificate(s). Euroclear will also be instructed to cancel the entitlements to Xenetic Shares cancelled as part of the Scheme.
4.2 In the case of Scheme Shares which at the Scheme Record Time are held in uncertificated form, GSL will procure that Scheme Shareholders will be issued any GSL Consideration Shares to which he or she is entitled in certificated form in the same way as set out in clause 4.1 above.
4.3 The provisions of this clause 4 of this Scheme shall take effect subject to any prohibition or condition imposed by law or regulation.
5. The Effective Date
5.1 This Scheme and Reduction of Capital, which it includes, shall become effective in accordance with its terms as soon as the following shall have occurred, in the following order, that:
| (a) | a copy of the Court Order sanctioning this Scheme shall have been delivered to the Registrar of Companies; and |
| (b) | a copy of the Court Order (insofar as it relates to the Capital Reduction) and the Statement of Capital shall have been delivered to the Registrar of Companies. |
5.2 Unless this Scheme shall become effective on or before 5.00 p.m. London time on 30 June 2014 (or such later date, if any as GSL and the Company may agree and the Court may allow), this Scheme shall never become effective.
6. Modification
GSL and the Company may jointly consent, on behalf of all persons affected, to any modification of, or addition to, this Scheme or to any condition approved or imposed by the Court.
Dated:
21 November 2013
| 50 |
PART 5
FINANCIAL INFORMATION RELATING TO THE XENETIC GROUP
Basis of financial information
The following table sets out the financial information in respect of Xenetic as required by Rule 24.3(e) of the Code. Reference in the first column is to Rule 24.3(a) of the Code, referring to information to be set out in accordance with Rule 24.3(e). The documents referred to in the table, the contents of which have been announced through a Regulatory Information Service, are incorporated by reference into this document and are available in ‘read only’ format for viewing or downloading, free of charge, on the Company’s website at http://www.xeneticbio.com/financial-reports.
Incorporation of information by reference
| No Information | Source of Information
|
1. Turnover, net profit or loss before and after taxation, the charge for tax, extraordinary items, minority interests, the amount absorbed by dividends and earnings and dividends per share for Xenetic for the two years ended 31 December 2012.
|
Xenetic Annual Report 31 December 2012. If you are reading this document in hard copy, please enter the web address below in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address below to be brought to the page containing the relevant document. http://www.xeneticbio.com/financial-reports
|
Xenetic Annual Report 31 December 2011. If you are reading this document in hard copy, please enter the web address below in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address below to be brought to the page containing the relevant document. http://www.xeneticbio.com/financial-reports | |
2. A statement of the assets and liabilities shown in the audited accounts for Xenetic for the year ended 31 December 2012, being the last published audited accounts.
|
Xenetic Annual Report 31 December 2012 and Balance Sheet. If you are reading this document in hard copy, please enter the web address below in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address below to be brought to the page containing the relevant document. http://www.xeneticbio.com/financial-reports
|
| 3. A cash flow statement provided in the audited accounts for Xenetic for the year ended 31 December 2012, being the last published audited accounts. | Xenetic Annual Report 31 December 2012 and Consolidated Statement of Cash Flows. If you are reading this document in hard copy, please enter the web address below in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address below to be brought to the page containing the relevant document. http://www.xeneticbio.com/financial-reports
|
| 51 |
| 4. Turnover, net profit or loss before and after taxation, the charge for tax, extraordinary items, minority interests, the amount absorbed by dividends and earnings and dividends per share for Xenetic in respect of any interim statement or preliminary announcement made since the last published audited accounts. | Xenetic 2013 interim results ended 30 June 2013.
If you are reading this document in hard copy, please enter the web address below in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address below to be brought to the page containing the relevant document.
http://www.xeneticbio.com/financial-reports
|
| 5. Significant accounting policies, together with any points from the notes to the accounts which are of major relevance to an appreciation of the figures. | Xenetic Annual Report 31 December 2012 and the Notes to the Consolidated Financial Statements. If you are reading this document in hard copy, please enter the web address below in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address below to be brought to the page containing the relevant document. http://www.xeneticbio.com/financial-reports Xenetic Annual Report 31 December 2011 and the Notes to the Financial Statements. |
If you are reading this document in hard copy, please enter the web address below in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address below to be brought to the page containing the relevant document. http://www.xeneticbio.com/financial-reports
|
The Company will send within two Business Days, without charge, to each person to whom a copy of this document has been sent, on their request, a copy of any documents incorporated by reference in this document. Requests should be addressed to the Directors at Xenetic Biosciences plc, Greener House, 66-68 Haymarket, London SW1Y 4RF.
| 52 |
PART 6
UK TAXATION
The following statements are intended only as a general guide to certain UK tax considerations and do not purport to be a complete analysis of all potential UK tax consequences of the Scheme. They are based on current UK legislation and what is understood to be the current published practice of HM Revenue & Customs as at the date of this document, both of which may change, possibly with retroactive effect. They apply only to Scheme Shareholders who are resident (and, in the case of individuals domiciled) for tax purposes in (and only in) the UK (except in so far as express reference is made to the treatment of non-UK residents) and who hold their Scheme Shares as an investment (other than under an individual savings account), and who are the absolute beneficial owners of both their Scheme Shares and any dividends paid on them. The tax position of certain categories of Scheme Shareholders who are subject to special rules (such as persons acquiring their Scheme Shares by exercising share options under the Xenetic Share Schemes or in connection with employment, dealers in securities, insurance companies and collective investment schemes) is not considered.
Scheme Shareholders who are in any doubt about their tax position or who may be subject to tax in a jurisdiction other than the UK are strongly recommended to consult their own professional tax advisers.
A. UK taxation of chargeable gains
Liability to UK taxation of chargeable gains will depend on the individual circumstances of Scheme Shareholders. Acquisition of GSL Consideration Shares
A Scheme Shareholder who, together with persons connected with him, does not hold more than five per cent. of, or of any class of, shares in or debentures of, the Company should not be treated as having made a disposal of his Scheme Shares for the purposes of UK taxation of chargeable gains to the extent that he receives GSL Consideration Shares in exchange for his Scheme Shares under the Scheme. Instead the GSL Consideration Shares will be treated as the same asset as his Scheme Shares, acquired at the same time as his Scheme Shares.
Any Scheme Shareholder who, either alone or together with persons connected with him, holds more than five per cent. of, or of any class of, shares in or debentures of the Company, is advised that an application for clearance will be made to HM Revenue & Customs under section 138 of the Taxation of Chargeable Gains Act 1992 in respect of the Scheme. If such clearance is given, any shareholder will be treated in the manner described in the preceding paragraph.
Disposal of GSL Consideration Shares
A subsequent disposal of the GSL Consideration Shares may, depending on individual circumstances, give rise to a liability to United Kingdom taxation of chargeable gains and, accordingly, any gain deferred on approval of the Scheme may, on such disposal, become chargeable to taxation.
Indexation allowance may be available to reduce any chargeable gain arising (but not to create or increase any allowable loss) to Scheme Shareholders within the charge to corporation tax.
B. Tax on dividends on GSL Consideration Shares
GSL will not be required to withhold tax at source when paying a dividend to UK Scheme Shareholders.
C. Stamp duty and stamp duty reserve tax (“SDRT”)
No stamp duty or SDRT will be payable by Scheme Shareholders as a result of the Scheme.
Part 6 of this document is not intended to be, and should not be construed to be, legal or taxation advice to any particular Scheme Shareholder. Any Scheme Shareholder who has any doubt about his own taxation position, or who is subject to taxation in any jurisdiction other than the UK should consult his professional taxation advisor immediately.
| 53 |
PART 7
INFORMATION
RELATING TO GSL
TEXT OF EQUIVALENT DOCUMENT
THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION. If you are in any doubt as to the action you should take, you are recommended to seek your own independent financial advice immediately from your stockbroker, bank manager, solicitor, accountant or other independent financial adviser duly authorised under the Financial Services and Markets Act 2000, if you are in the United Kingdom, or, if not, from another appropriately authorised independent financial adviser.
This document is being sent to the shareholders of Xenetic Biosciences plc (“Xenetic”). If you have sold or otherwise transferred all of your shares in Xenetic, please forward this document, together with the accompanying documents, at once, to the purchaser or transferee or to the bank, stockbroker, or other agent through whom the sale or transfer was effected for transmission to the purchaser or transferee. However, such documents should not be forwarded or transmitted in or into a Restricted Jurisdiction (as defined herein) or any other jurisdiction if to do so would constitute a violation of the relevant laws of such jurisdiction. The distribution of this document in jurisdictions other than the UK may be restricted by law and therefore persons into whose possession this document comes should inform themselves about and observe any such restrictions. Any failure to comply with those restrictions may constitute a violation of the securities laws of any such jurisdiction.
This document does not comprise a prospectus prepared in accordance with the Prospectus Rules under Section 73A of FSMA and has not been approved by the Financial Conduct Authority in accordance with Section 85 of FSMA.
You should read the whole of this document prior to making any decision. Your attention is drawn to the section of this document entitled “Risk Factors” for details of certain factors that should be considered.
Equivalent Document
published
in connection with the issue of shares of Common Stock with par value of $0.001
each in the capital of General Sales and Leasing, Inc. (“GSL”) pursuant to the acquisition by
General Sales and Leasing, Inc.
of the entire issued share capital of
Xenetic Biosciences plc
to be effected by a scheme of arrangement under Part 26 of the Companies Act 2006
GSL’s Shares are quoted on the OTCBB operated by the Financial Industry Regulatory Authority, Inc. and the OTCQB operated by OTC Markets Group, Inc. It is expected that the GSL Consideration Shares to be issued pursuant to the Acquisition will be available to shareholders subject to the satisfaction of certain conditions, including the sanction of the Scheme by the Court, on 27 January 2014.
This document does not constitute an offer to sell, or a solicitation of an offer to acquire GSL Consideration Shares in any jurisdiction in which such offer or solicitation is unlawful. In particular, this document is not for distribution in or into the United States of America, Canada, Australia, the Republic of South Africa or Japan. The GSL Consideration Shares have not been and will not be registered under the United States Securities Act 1933 (as amended) or under the securities legislation of a Restricted Jurisdiction or in any country, territory or possession where to do so may contravene local securities laws or regulations. Accordingly, the GSL Consideration Shares may not, subject to certain exceptions, be offered or sold, directly or indirectly in or into a Restricted Jurisdiction or to any national, citizen or resident of a Restricted Jurisdiction.
| 54 |
CONTENTS
| PART I SUMMARY | 57
|
| PART II RISK FACTORS | 59
|
| PART III DEFINITIONS | 63
|
| PART IV EXPECTED TIMETABLE AND STATISTICS | 65
|
| PART V DIRECTOR, REGISTERED OFFICE AND ADVISERS | 66
|
| PART VI INFORMATION ON THE ACQUISITION | 67
|
| 1. Introduction | 67
|
| 2. Background to and Reasons for the Acquisition | 67
|
| 3. The Acquisition | 68
|
| 4. Directors | 69
|
| 5. Information on Xenetic | 69
|
| 6. US Federal Securities Law Restrictions on Transfer | 71
|
| 7. Corporate Governance | 71
|
| 8. Incentive Plans | 71
|
| 9. Dividend Policy | 72
|
| 10. Current Trading and Prospects | 72
|
| 11. Taxation | 72
|
| 12. Further Information | 72
|
| PART VII FINANCIAL INFORMATION ON GSL | 73
|
| PART VIII ADDITIONAL INFORMATION | 74
|
| 1. Responsibility Statements | 74
|
| 2. Incorporation, History and Conduct of Business | 74
|
| 3. Share Capital | 74
|
| 4. Certificate of Incorporation, Bylaws and Governing Law | 74
|
| 5. Director’s Interests | 76
|
| 6. Substantial Shareholdings | 76
|
| 7. Director’s Service Contracts | 77
|
| 8. Details of Subsidiaries | 77
|
| 9. Material Contracts | 77
|
| 10. Taxation | 77
|
| 11. Related Party Transactions | 78
|
| 12. Litigation | 78
|
| 13. Working Capital | 78
|
| 14. Source of Information | 78
|
| 15. No Significant Change | 78
|
| 16. General | 78
|
| 17. Documents Available for Inspection | 78
|
| 55 |
PART I
SUMMARY
This summary should be read as an introduction to the full text of this document and any decision to accept under the Scheme should be based on consideration of the full text of this document as a whole. Where a claim relating to the information contained in this document is brought before a court, a plaintiff investor may, under the national legislation of a member state of the European Economic Area, have to bear the costs of translating this document before the legal proceedings are initiated. Civil liability attaches to those persons who are responsible for this summary, including any translation of this summary, but only if this summary is misleading, inaccurate or inconsistent when read together with the other parts of this document.
Introduction
On 12 November 2013, GSL announced the terms of a proposed acquisition of the entire issued share capital of Xenetic by GSL by way of a Scheme and a Reduction of Capital.
Information on the Acquisition
The Acquisition is to be implemented by means of a Scheme between Xenetic and its shareholders and also involves a reduction of Xenetic’s capital. Under the terms of the Scheme, the outstanding Xenetic shares shall be cancelled and, upon the Scheme becoming Effective, the Xenetic Shareholders shall receive in consideration for the cancellation of their shares:
for every 175 Xenetic Shares : 56 GSL Consideration Shares
The capital reserve created by the cancellation in the reserves of Xenetic shall be capitalised and applied in paying up new Xenetic Shares which shall then be issued by Xenetic to GSL. The full details of the Scheme are set out in the Scheme Document.
Information on GSL
GSL was incorporated in August 2011 for the purpose of owning and operating helicopters for use in sightseeing tours and as pilot training aircraft. On 11 February 2013, with the appointment of its new sole officer and Director, Ari L. Nagler, GSL began to operate an online advertising business through its newly-formed subsidiary, Shift It Media Co. The helicopter rental business was subsequently moved into another wholly-owned subsidiary, General Aircraft, Inc.
While GSL has experienced a reasonable demand and for its helicopter rental and online advertising businesses, because of limited growth results over the past year and increasing expenses in relation to maintaining the public entity and its SEC filing requirements, the Director considers it in the best interests of GSL to enter into the Acquisition of Xenetic. GSL intends to dispose of the helicopter rental and online advertising businesses through the transfer of its two subsidiaries to its largest shareholder immediately following the Acquisition so that its sole focus can be on the business of Xenetic.
For a more complete discussion of GSL, its financials, risk factors and history, it is recommended that you review GSL’s disclosure filings made on the US SEC’s online EDGAR system. Indeed, this document is supplemented by and reference is made to the information contained in GSL’s filings which can be found at: www.sec.gov.
Specific attention is made to the list of all GSL’s:
● filings at: http://www.sec.gov/cgi-bin/browse-
edgar?company=general+sales&owner=exclude&action=getcompany
● final amended form S-1/A filing at:
http://www.sec.gov/Archives/edgar/data/1534525/000125529412000158/0001255294-12- 000158-index.htm
| 56 |
● most recent form10-K filing at:
http://www.sec.gov/Archives/edgar/data/1534525/000125529412000898/0001255294-12- 000898-index.htm
● its most recent form 10-Q filing at:
http://www.sec.gov/Archives/edgar/data/1534525/000125529413000560/0001255294-13- 000560-index.htm
Information on Xenetic
Xenetic Biosciences was founded in as Lipoxen Technologies Limited in 1997 by the liposomologist Professor Gregory Gregoriadis, PhD DSc, as a spin-out from The School of Pharmacy at the University of London. Xenetic listed on the AIM market operated by the London Stock Exchange on 17 January 2006. Since flotation, Xenetic has steadily progressed the development of all of:
● PolyXen, its natural polymer-based enabling technology for next generation biologic drugs;
● ImuXen, its natural liposome-based enabling technology platform for next generation vaccines; and
● OncoHist, its natural technology for the delivery of oncology therapies.
Risk Factors
The material risk factors relating to GSL fall into a number of areas and are set out more fully in Part II entitled “Risk Factors”.
| 57 |
PART II
RISK FACTORS
The Director makes no warranty or guarantee of performance of GSL. Past performance is not necessarily a guide to future performance, especially in view of the proposed change in business plan as discussed throughout this document. Post events, experience derived from these, or assumptions derived from any of these, do not predetermine the future.
The Director believes the following risks to be the most significant. Additional risks and uncertainties not presently known to the Director, or which the Director currently deems immaterial, may also have an adverse effect upon GSL.
The information set out below does not purport to be an exhaustive summary of the risks affecting GSL nor are they set out in any particular order of priority. If any of the following risks were to materialise, GSL’s business, financial condition, results or future operations could be materially and adversely affected with consequent impact on the financial position of GSL.
For a more complete list of risk factors, please review GSL’s filing on form S-1/A online at the US SEC’s EDGAR site at:
http://www.sec.gov/Archives/edgar/data/1534525/000125529412000158/mainbody.htm.
General Economic Risk
Both US and world economic conditions may affect the performance of GSL. Factors such as inflation, currency fluctuations, interest rates, recession, supply and demand of capital and industrial disruption or unemployment have an impact on business costs, future operations, earnings and stock market prices. GSL’s operations, business and profitability could be affected by these and other economic and political factors, which are beyond the control of GSL.
Business, strategy, growth and competition
The ability of GSL to implement its strategy in a competitive market requires effective planning and management control systems. GSL’s future growth will depend on its ability to expand and improve operational, financial and management information and control systems in line with GSL’s growth. This growth and expansion could place a significant strain on GSL’s financial, management and other resources, particularly if such growth and/or expansion occurs rapidly. Failure to do so could have an adverse effect on GSL’s business, financial condition and results of operations.
Revenue and profit growth
The results of GSL’s operations may fluctuate. GSL may not be able to achieve sustained revenue growth and profitability in the future as its results are influenced by a number of factors, many of which are beyond GSL’s control. Ultimately, if GSL does not realize sufficient revenue levels to sustain profitability, it may be required to seek financing and/or borrowing in the longer term, which may not be available or which may be on adverse terms.
Dependence on key personnel
GSL’s success depends to a significant extent upon the retention of the services of members of the senior management or other key personnel. Any loss (whether temporary or permanent) could have a material adverse effect on the business, financial condition or results of operations of GSL.
Ability to recruit and retain staff
The future success of GSL depends to a significant extent on its ability to hire and retain key development and operational personnel. There can be no assurance that GSL will be able to continue to retain and attract qualified personnel for the development of GSL’s products and business.
| 58 |
Permits
There can be no guarantee that any permits, consents or approvals required from third parties in connection with existing or new projects will be issued or granted to GSL. A failure by GSL to obtain such permits, consents or approvals may affect GSL’s ability to execute or complete existing and/or new projects.
Capital expenditure and development risk
Projects in which GSL is involved often require significant capital expenditure during the implementation stage, and often may only generate a return at a later date. Furthermore, changing conditions, unforeseen circumstances and financing costs during development periods may result in losses or diminished profits from such projects.
Market for common stock
The common stock is eligible for quotation on the OTC Bulletin Board. If for any reason, however, our securities later becomes ineligible for continued quotation on the OTC Bulletin Board, or a public trading market does not develop, purchasers of the common stock may have difficulty selling their securities should they desire to do so and purchasers of our common stock may lose their entire investment if they are unable to sell our securities.
FINRA sales practice requirements
In addition to the “penny stock” rules described below, FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may have the effect of reducing the level of trading activity in our common stock. As a result, fewer broker-dealers may be willing to make a market in our common stock, reducing a stockholder’s ability to resell shares of our common stock.
Limit on secondary trading
Common stock holders may not be able to resell the shares in any state in the United States unless and until the shares of our common stock are qualified for secondary trading under the applicable securities laws of such state or there is confirmation that an exemption, such as listing in certain recognized securities manuals, is available for secondary trading in such state. There can be no assurance that we will be successful in registering or qualifying our common stock for secondary trading, or identifying an available exemption for secondary trading in our common stock in every state. If we fail to register or qualify, or to obtain or verify an exemption for the secondary trading of, our common stock in any particular state, the shares of common stock could not be offered or sold to, or purchased by, a resident of that state. In the event that a significant number of states refuse to permit secondary trading in our common stock, the market for the common stock will be limited which could drive down the market price of our common stock and reduce the liquidity of the shares of our common stock and a stockholder’s ability to resell shares of our common stock at all or at current market prices, which could increase a stockholder’s risk of losing some or all of his investment.
Dividend policy
GSL has never declared or paid any cash dividends on our common stock. GSL currently intends to retain future earnings, if any, to finance the expansion of its business. As a result, the Director does not anticipate paying any cash dividends in the foreseeable future. The payment of any future dividends will be at the discretion of the Director after taking into account various factors, including but not limited to GSL’s financial condition, operating results, cash needs, growth plans and the terms of any credit agreements that GSL may be a party to at the time. Accordingly, investors must rely on sales of their own common stock after price appreciation, which may never occur, as the only way to realize their investment.
“Penny Stock” rules
Broker-dealer practices in connection with transactions in “penny stocks” are regulated by penny stock rules adopted by the SEC. Penny stocks generally are equity securities with a price of less than $5.00 (other than securities registered on some national securities exchanges or quoted on NASDAQ). The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and, if the broker-dealer is the sole market maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market, and monthly account statements showing the market value of each penny stock held in the customer’s account. In addition, broker-dealers who sell these securities to persons other than established customers and “accredited investors” must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. Consequently, these requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security subject to the penny stock rules, and investors in our common stock may find it difficult to sell their shares.
| 59 |
Dilution
Generally, existing shareholders will experience dilution of their ownership percentage in GSL if and when additional shares of common stock are offered and sold. In the future, GSL may be required to seek additional equity funding in the form of private or public offerings of its common stock. In the event that GSL undertakes subsequent offerings of common stock, shareholders’ ownership percentage, voting power as a common shareholder, and earnings per share, if any, will be proportionately diluted. This may, in turn, result in a substantial decrease in the per-share value of the common stock.
Government policies and legislation
The introduction of new policies or legislation or amendments to existing policies or legislation by governments or the interpretation of those laws in the State of Nevada or other jurisdictions under which GSL operates could impact adversely on the assets, operations and ultimately the financial performance of GSL.
Regulatory risk
GSL is not currently identified by its management as a Shell Company as defined under SEC rules and regulations, which means that its stock is not subject to certain limitations contained in these rules. There can be no assurance, however, that the SEC and/or various regulatory bodies in the US will not reject this classification and effectively apply the Shell Company rules to GSL in the future. Additionally, any Exchange may apply its own rules and/or analysis to the classification of GSL and reach a different conclusion than GSL’s management. Any such determination, either by the SEC, one of the other regulatory bodies in the US or an Exchange, could adversely impact GSL’s attempt to be listed on an Exchange or the tradability of its shares in its current markets, and/or the cost and time involved in doing so.
Taxation
Any change in GSL’s tax status or in taxation legislation in any of the countries in which GSL operates could affect GSL’s ability to provide returns to shareholders.
No guarantee of listing
Xenetic’s plan is for it to be acquired on a share-for-share basis by GSL, where the level of effective dilution to Xenetic’s shareholders is modest. This means that, other than for an acceptable level of dilution (which is deemed to be the “cost” of achieving GSL’s aims) and the fees and costs of the Acquisition including the costs involved in the GSL Hive Out Agreement described below, the group formed by the completion of the Acquisition will be as near as possible the same as Xenetic’s is currently constituted, with the same assets and liabilities, save that it would have a US holding company with an enhanced ability to achieve a listing on a major United States securities exchange. While Xenetic and GSL have already had general discussions regarding such a listing, the process of getting listed on a major securities exchange in the US is complex and subject to the independent rules of the Exchange. Consequently, there can be no assurance or guarantee given that such a listing can or will be achieved by GSL.
| 60 |
Forward-Looking Statements
This Document includes statements that are (or may be deemed to be) “forward-looking statements.” These forward-looking statements can be identified by the use of forward-looking terminology including the terms “believes,” “continues,” “expects,” “intends,” “may,” “would” or “should” or, in each case, their negative or other variations or comparable terminology. These forward-looking statements include all matters that are not historical facts. Prospective Investors should not place undue reliance on forward-looking statements, the timeframe of which starts with the date of this Document.
Forward-looking statements involve risk and uncertainty because they relate to future events and circumstances. Forward-looking statements contained in this Document based on past trends or activities should not be taken as a representation that such trends or activities will continue in the future.
Subject to any requirement under applicable laws and regulations, neither GSL nor its Director undertakes to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
| 61 |
PART III
DEFINITIONS
The following definitions apply throughout this Document unless the context otherwise requires:
| “Acquisition” | the proposed acquisition of the Scheme Shares by GSL to be implemented by way of the Scheme on the terms and subject to the Conditions set out in the Scheme Document and any subsequent revision, variation, extension or renewal thereof; |
| “Act” | the UK Companies Act 2006 (as amended);
|
| “Board” | means the board of directors of GSL from time to time;
|
| “Business Day” | a day (other than a Saturday or a Sunday) on which banks are generally open for business in the City of London;
|
| “Court” | the High Court of Justice in England and Wales;
|
| “Director” | the sole director of GSL at the date of this document;
|
| “document” | this document;
|
| “Effective” | the Scheme becoming effective pursuant to its terms;
|
| “Effective Date” | the day on which the Scheme becomes Effective;
|
| “EDGAR” | the SEC’s Electronic Data-Gathering, Analysis, and Retrieval system;
|
| “Exchange” | any recognised US stock exchange, including (but not limited to) NASDAQ;
|
| “Exchange Act” | the United States Securities Exchange Act of 1934 (as amended);
|
| “FINRA” | the Financial Industry Regulatory Authority, Inc.; |
| “FSMA” | the Financial Services and Markets Act 2000 of the United Kingdom;
|
| “General Meeting” | the General Meeting of the Xenetic Shareholders; |
| “GMT” | Greenwich Mean Time; |
| “GSL” | General Sales and Leasing, Inc.; |
| “GSL Consideration Shares” | expected to be 130,520,137 New Shares to be issued credited as fully paid to Scheme Shareholders pursuant to the Scheme and the Equivalent Document;
|
| “GSL Hive Out Agreement” | the contingent agreement by GSL to transfer its two existing subsidiaries to its largest shareholder, as described in paragraph 9(c) of Part VIII of this document;
|
| “NASDAQ” | the NASDAQ Stock Market; |
| “Nevada Revised Statutes” | the current codified laws of the State of Nevada; |
| “New Shares” | new shares of Common Stock with a par value of $0.01 proposed to be issued and credited as fully paid pursuant to the Share Consolidation;
|
| 62 |
| “Oxbridge” | Oxbridge Technology Partners, SA, a Belize Corporation;
|
| “Reduction of Capital” | the reduction of the share capital of Xenetic associated with the cancellation and extinguishing of the Scheme Shares under section 641 of the Act;
|
| “Restricted Jurisdiction” | the United States, Canada, Australia, the Republic of South Africa or Japan;
|
| “Scheme” | the scheme of arrangement proposed to be made under Part 26 of the Act between Xenetic and the holders of Scheme Shares with or subject to any modification, addition or condition approved or imposed by the Court and agreed to by Xenetic and GSL and incorporating a Reduction of Capital;
|
| “Scheme Document” | the formal document to be presented to the Xenetic Shareholders in connection with the Scheme and the Reduction of Capital, a copy of which is available in ‘read only’ format for viewing or downloading, free of charge, on GSL’s website at http://www.xeneticbio.com/investorrelations.aspx. If you are reading this document in hard copy, please enter the web address provided in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address provided to be brought to the page containing the relevant document;
|
| “Scheme Shares” | the Xenetic Shares in issue at the Effective Date other than any shares which are registered in the name of or beneficially owned by GSL;
|
| “SEC” | the United States Securities and Exchange Commission; |
| “Securities Act” | the United States Securities Act of 1933 (as amended); |
| “Share Consolidation” | the share consolidation described in paragraph 3(c) of Part VIII of this document;
|
| “Shares” | shares of Common Stock of par value $0.001 each in the capital of GSL;
|
| “SOX” | the United States Sarbanes-Oxley Act of 2002; |
| “UK” or “United Kingdom” | the United Kingdom of Great Britain and Northern Ireland;
|
| “US” or “United States” | the United States of America; |
| “Xenetic” | Xenetic Biosciences plc, incorporated in England and Wales (company number 3213174);
|
| “Xenetic Shareholder” | a holder of Xenetic Shares; |
| “Xenetic Shares” | ordinary shares of £0.005 each in the capital of Xenetic; |
| “£” | UK pounds sterling, the lawful currency of the United Kingdom; and |
| “$” or “US Dollars” | United States dollars, the lawful currency of the United States. |
All references to statute or other forms of UK legislation in this document shall, unless otherwise stated, be references to statutes or forms of legislation of the UK and any references to provisions of any legislation shall include any amendment, modification, re-enactment or extension thereof.
| 63 |
PART IV
EXPECTED TIMETABLE AND STATISTICS
| Event | Time and/or date |
| General Meeting | 17 December 2013 |
| Court Meeting | 23 January 2014 |
| Effective Date of the Scheme | 23 January 2014 |
General notes:
| 1. | Reference to times in this document are to London time unless otherwise stated. |
| 2. | The times and dates set out in the expected timetable above and mentioned throughout this document may be adjusted by Xenetic and GSL. |
| 3. | Different deadlines and procedures for return of forms may apply in certain cases. |
STATISTICS
| Number of existing Shares in issue as at the date of this document | 135,000,000 |
| Number of New Shares in issue following the Share Consolidation | 13,500,000 |
| Number of New Shares in issue following closing of the GSL Hive Out Agreement | 3,500,000 |
| Number of GSL Consideration Shares expected to be issued pursuant to the Acquisition | 130,520,137 |
| Aggregate number of shares of Common Stock of GSL anticipated to be in issue following 134,020,137 the Acquisition | |
| GSL Consideration Shares as a percentage of the enlarged issued share capital of GSL | 97.39% |
| following the Acquisition |
| 64 |
PART V
DIRECTOR, REGISTERED OFFICE AND ADVISERS
| Company | General Sales and Leasing, Inc.
|
| Director | Ari L. Nagler (President, CEO and CFO)
|
| Principal Executive Office | 16445 North 91st St. Suite 103 Scottsdale Arizona 85260 USA
|
| Legal Adviser to GSL as to English law | DWF LLP Capital House 85 King William Street London EC4N 7BL
|
| Legal Adviser to GSL as to Nevada law | Cane Clark LLP 3273Warm Springs Rd. Las Vegas Nevada 89120 USA |
| 65 |
PART VI
INFORMATION ON THE ACQUISITION
1. Introduction
On 12 November 2013, GSL and Xenetic announced the terms of a proposed acquisition of the entire issued share capital of Xenetic by GSL by way of a Scheme and a Reduction of Capital.
| 2. | Background to and Reasons for the Acquisition |
GSL was incorporated in August 2011 for the purpose of owning and operating helicopters for use in sightseeing tours and as pilot training aircraft. GSL purchased a helicopter, a Robinson R44 Raven II, which seats three passengers in addition to the pilot. The helicopter is hangered at North Las Vegas Airport, an executive and general aviation airport in North Las Vegas, Nevada. The helicopter is currently being completely overhauled as required by the manufacturer and the FAA, based on it exceeding 2200 hours of operation. When the overhaul is complete (expected sometime in November), the helicopter will be rented through a third-party manager, Elite Aviation VGT, LLC (“Elite”), on an hourly basis to tour operators for use in sightseeing tours of the Las Vegas strip, as well as for helicopter pilot training flights. GSL is in the process of negotiating a new agreement with Elite and expects to get a net rental rate of $185-$200 per hour for the use of the helicopter.
On 11 February 2013, with the appointment of its new sole officer and Director, Ari L. Nagler, GSL began to operate an online advertising business through its newly-formed subsidiary, Shift It Media Co. The helicopter rental business was subsequently moved into another wholly-owned subsidiary, General Aircraft, Inc.
While GSL has experienced a reasonable demand and for its helicopter rental and online advertising businesses, because of limited growth results over the past year and increasing expenses in relation to maintaining the public entity and its SEC filing requirements, the Director considers it in the best interests of GSL to enter into the proposed Acquisition. GSL intends to dispose of the helicopter rental and online advertising businesses through the transfer of its two subsidiaries to its largest shareholder immediately following the Acquisition so that its sole focus can be on the business of Xenetic.
For a more complete discussion of GSL, its financials, risk factors and history, it is recommended that you review GSL’s disclosure filings made on the US SEC’s online EDGAR system. Information contained in GSL’s filings on the EDGAR system, which can be found at www.sec.gov, are incorporated herein by this reference.
Specific attention is directed to the following:
| ● | the list of all of GSL’s filings at: http://www.sec.gov/cgi-bin/browseedgar?company=general+sales&owner=exclude&action=getcompany |
| ● | GSL’s final amended form S-1/A filing at: |
http://www.sec.gov/Archives/edgar/data/1534525/000125529412000158/0001255294-12- 000158-index.htm
| ● | GSL’s most recent form 10-K filing at: |
http://www.sec.gov/Archives/edgar/data/1534525/000125529412000898/0001255294-12- 000898-index.htm
| ● | GSL’s most recent form 10-Q filing at: |
http://www.sec.gov/Archives/edgar/data/1534525/000125529413000560/0001255294-13- 000560-index.htm
Financial Information
Current financial information in respect of GSL is available for viewing or downloading, free of charge, on the SEC’s EDGAR website at:
http://www.sec.gov/Archives/edgar/data/1534525/000125529413000560/0001255294-13-000560- index.htm
| 66 |
If you are reading this document in hard copy, please enter the web address provided in your web browser to be brought to the page containing the relevant document. If you are reading this document in electronic form, please click on the web address provided to be brought to the page containing the relevant document.
3. The Acquisition
The Acquisition is to be implemented by means of a Scheme between Xenetic and the Xenetic Shareholders and also involves a reduction of Xenetic’s capital. The Scheme requires the requisite approval of the Xenetic Shareholders at a meeting convened by the Court and the subsequent sanction of the Court. In addition, a Reduction of Capital shall take place which requires the approval of the Xenetic Shareholders at a General Meeting and the subsequent confirmation of the Court. Once the Scheme becomes Effective, the terms will be binding on all Xenetic Shareholders whether or not they voted in favour of the Scheme.
The purpose of the Scheme is to enable GSL to acquire the all the outstanding Xenetic Shares, following which GSL would own the entire issued ordinary share capital of Xenetic. Under the terms of the Scheme, which is subject to the satisfaction (or, where applicable, waiver) of the conditions and further terms set out in the Scheme Document, the outstanding Xenetic Shares shall be cancelled and, upon the Scheme becoming Effective, the existing Xenetic Shareholders shall receive in consideration for the cancellation of their shares:
for every 175 Xenetic Shares : 56 GSL Consideration Shares
The capital reserve created by the cancellation in the reserves of Xenetic shall be capitalised and applied in paying up new Xenetic Shares in the capital of Xenetic which shall then be issued by Xenetic to GSL.
Prior to the Announcement, GSL held no Xenetic Shares. GSL has not acquired any Xenetic Shares since the date of the Announcement.
The expected transaction timetable is set out on page [65] of this document. It is expected that the Acquisition and the resolutions required to implement the Scheme will be put to the Xenetic Shareholders at the Court meeting and the General Meeting which are expected to be held on 17 December 2013. It is expected that, subject to satisfaction or waiver of the conditions of the Scheme, the Effective Date shall be 23 January 2014. If the Scheme becomes Effective, it will be binding on all Xenetic Shareholders, irrespective of whether or not they attended or voted at the Court meeting or the General Meeting.
The full details of the Scheme are set out in the Scheme Document.
It is expected that the GSL Consideration Shares issued to Xenetic Shareholders will be entitled to deposit, in the normal course, into an individual shareholder’s trading account and then be available for trading on the OTCBB operated by FINRA and the OTCQB operated by OTC Markets Group, Inc. The GSL Consideration Shares will be issued free from all liens, charges, encumbrances and other third party rights and/or interests of any nature whatsoever. Such GSL Consideration Shares will be issued in either electronic or certificated form, will be issued as fully paid and will rank pani passu in all respects with the existing outstanding shares of GSL, including as to voting rights and the right to receive and retain all dividends and other distributions declared, paid or made after the Effective Date. The GSL Consideration Shares will be denominated in US Dollars.
Xenetic Shareholders should, however, be aware that the deposit of their GSL Consideration Shares into their existing trading accounts may be restricted or delayed based on the rules of their individual stockbroker or its designated clearing house. It is not uncommon for many stockbrokers to require a substantial amount of additional documentation to effectuate the deposit and subsequent trading of newly issued OTCBB and OTCQB shares. Consequently, shareholders may be required to set up a new trading account with a different stockbroker or otherwise find themselves subject to delays and other problems in the deposit of their GSL Consideration Shares. While GSL will assist where it can, because the deposit of the GSL Consideration Shares is dependent on the individual processing rules of the shareholder’s choice of stockbroker, it will be the responsibility of the individual shareholder receiving the GSL Consideration Shares to process and facilitate the deposit of the shares. GSL will take no responsibility or liability whatsoever for ensuring the deposit of the GSL Consideration Shares into a specific shareholder trading account or with a specific stockbroker.
| 67 |
4. Directors
GSL has established a different governance and executive management structure than that of a typical company incorporated under English law. The control and management of GSL is divided between stockholders, a board of directors and officers of GSL. The Board is elected by the stockholders at a meeting called for that purpose. The Board is entitled to exercise its powers through committees and the appointment of officers. Officers have general powers and duties of day-to-day supervision and management of GSL, and can be granted specific authority under an agreement or by resolution of the Board. For example, the functions of “Managing Director” and “Finance Director” in English companies are typically undertaken in a Nevada corporation by the chief executive officer (“CEO”) and chief financial officer (“CFO”), respectively (who, in these roles, are officers and not directors, of GSL).
GSL currently has only one director whose biographical details are set out in brief below:
Ari L. Nagler (President, CEO and CFO): Mr Nagler is GSL’s President, Chief Executive Officer, Chief Financial Officer, Secretary, Treasurer, and Director. Since 2010, he has been the Chief Executive Officer and cofounder of Nuad Networks, an online advertising business which is currently delivering over 500 million monthly online ad impressions. From 2009 until 2010, Mr. Nagler was Vice President of Ace Air, where he managed a 130 employee HVAC contracting business. From 2004 until 2009, he was the principal of World Class Investments / Ari Nagler, PLLC, where he negotiated, managed, and closed M&A transactions. From 2004 until 2008, Mr. Nagler was also owner and President of Candleloft/The Candle Maker, a manufacturing, retail, and wholesale business. Mr. Nagler graduated from Cornell University in 1996 with a Bachelor of Science: Organizational Development and Management Consulting Concentration.
Immediately following the Acquisition, it is intended that the Director shall resign and the following persons, who are currently directors of Xenetic, shall be appointed to the Board:
● M Scott Maguire (Chief Executive Officer);
● Colin William Hill (Chief Financial Officer);
● Firdaus Jal Dastoor (Non-executive director); and
● Artur Isaev (Non-executive director).
5. Information on Xenetic History and Background
Xenetic was incorporated on 12 June 1996 under the name Greenchip Investments plc. Xenetic was admitted to AIM on 20 October 2000 with its principal goal and investment strategy being to bring into European markets, technology-driven business ventures, with a particular focus on life science, environmental technology and internet related technologies.
On 23 December 2005, Xenetic acquired Lipoxen Technologies Limited to fulfil its investment strategy and to provide significant prospects to grow shareholder value. Concurrently, Xenetic raised approximately £3.5 million through a placing of ordinary shares, changed its name to Lipoxen plc and the enlarged group was re-admitted to AIM.
Pursuant to an agreement executed on 4 August 2011, Xenetic raised approximately £12 million through a further placing of ordinary shares and changed its name to Xenetic Biosciences plc.
Business
Xenetic is a UK-based biopharmaceutical company providing expertise in the development of a whole new generation of drugs, cancer therapies and vaccines.
Working with some of the largest pharmaceutical organisations in the world, Xenetic provides specialist delivery solutions to improve the efficacy and performance of drugs and vaccines in a number of key medical areas including oncology.
Xenetic is also developing a pipeline of next generation biotherapeutics based on its proprietary PolyXen, Oncohist and ImuXen platform technologies.
| 68 |
Directors
Sir Brian Richards, CBE, BSc, PhD, DSc (Non-executive Chairman): Sir Brian Richards was appointed non-executive Chairman of Xenetic in June 2005. He has extensive experience of chairing boards of public companies and is currently the non-executive Chairman of Alizyme plc, Cozart plc, VASTox plc and MAN Mali (Guernsey) Limited. Sir Brian previously served as executive Chairman of British Biotechnology Limited, a company he co-founded, and has had non-executive chairmanships or directorships of several biopharmaceutical companies including Peptide Therapeutics (later Acambis plc), Oxford Biomedica plc and CeNeS Pharmaceuticals plc.
M Scott Maguire, MBA (Chief Executive Officer): Mr Maguire joined Xenetic as Chief Executive Officer in April 2004. His background is in life science and healthcare investment banking and he has advised many US and European companies on capital raisings and commercial development over his 22 year career. Mr Maguire began his banking career with Merrill Lynch in 1987 in New York and after receiving his MBA in 1993, he joined the healthcare division of W.R. Grace National Medical Care where he helped create the international healthcare division. In 1996 he co-founded the Arthur Andersen global healthcare corporate finance practice based in London. Mr. Maguire is currently director of Healthcare Capital Partners Limited, a healthcare corporate finance and proprietary investment boutique he co-founded in 2002 and a non-executive director of Renal Services Ltd, a company focused on dialysis service provision in the UK.
Colin William Hill, ACMA (Chief Financial Officer): Mr Hill was appointed Chief Financial Officer at Xenetic in June 2007. Prior to joining Xenetic he was Finance Director (2001-2003) and non-executive Chairman (2003) at Greenchip Investments plc. Mr Hill has been a member of the Chartered Institute of Management Accountants since 1968 and spent 15 years in industry specialising in corporate turnaround and development work before becoming a freelance consultant in 1981. Since that time, he has focused on due diligence relating to corporate finance assignments in small and medium enterprises and public companies with small market capitalisations in the UK, USA, and overseas. Between 1998 and 2008 Mr Hill was Group Finance Director of Arlington Group, a company listed on AIM.
Professor Gregory Gregoriadis, PhD, DSc (Chief Scientific Officer): Professor Gregory Gregoriadis is Xenetic’s Chief Scientific Officer. In 1997 he founded Xenetic (then known as Lipoxen) as a spin out from The School of Pharmacy, University of London, where he was Head of the Centre for Drug Delivery Research. Prior to this, Professor Gregoriadis was Head of the Liposomal Therapeutics Group at the Medical Research Council. He is an internationally acknowledged expert in drug and vaccine delivery. In 1971, he was the first to introduce liposomes as vehicles for drug and vaccine delivery and in 1991 he introduced the use of polysialic acid as a means to improve the pharmacological action of peptide and protein drugs. He has published nearly 400 research papers, reviews and articles, as well as 27 volumes on drug delivery and targeting. Professor Gregoriadis’ achievements have been honoured with the Controlled Release Society Founders Award (1994), the A.D. Bangham FRS Life Achievement Award (1995), election as a Fellow of the American Association of Pharmaceutical Scientists (1998), a DSc from the University of London (2001) and The Journal of Drug Targeting Life Achievement Award (2008) all for exceptional contributions to the field of drug and vaccine delivery. Professor Gregoriadis’ seminal contributions to the field of drug and vaccine delivery are also reflected in his founding in 1978 of the on-going Gordon Research Conference series ‘Drug Carriers in Medicine and Biology’, his directorships of the NATO Advanced Studies Institute’s ‘Targeting of Drugs and Vaccines’ from 1981 until 1999, his Chairmanship of the ‘Liposome Advances’ conference series from 1990 until 2006 and his position as Emeritus Professor at the University of London. Since 2003 Professor Gregoriadis has been President of the International Liposome Society.
Dr Dmitry D Genkin (Non-executive Director): Dr Genkin joined Xenetic as a non-executive Director in 2000. He has the Russian equivalent of an MD in Internal Therapy and studied drug delivery under Professor Gregoriadis at The School of Pharmacy, University of London in 1992 and the Department of Clinical Pharmacology at Karolinska Hospital, Stockholm from 1992 until 1993. Since 1993 Dr Genkin has headed a number of Russia’s largest pharmaceutical companies including Pharmavit which had 27 per cent. of the Russian pharmaceutical market. In 1998 he was awarded the silver medal by the Russian Natural Science Academy. Dr Genkin is currently a partner and director of FDS Pharma and Chairman of Pharmsynthez which is listed on the Moscow stock exchange
Firdaus Jal Dastoor, FCS (Non-executive Director): Mr Dastoor was appointed non-executive Director at Xenetic in July 2007. He is a Fellow Member of The Institute of Company Secretaries of India and began his career as a company secretary. He was Company Secretary of the Poonawalla Group until 1994. He then took on assignments involved in business development strategies and operations. Mr Dastoor is on the board of several companies operating in the field of engineering products, life sciences and biotech, international trade, financial services and quality standards certifications. Currently, he is a Group Director of the Poonawalla Group of Companies in charge of Finance and Corporate Affairs.
| 69 |
Artur Isaev (Non-executive Director): Mr. Artur Isaev, born in 1970, is a General Director and a majority shareholder of Human Stem Cells Institute OJSC, Russia’s public biotech company, headquartered in Moscow. Mr. Isaev has a degree in Medicine and MBA. He started his business career as a top manager in a brokerage, investment and auditing companies. In 2003 he founded Human Stem Cells Institute and from the very beginning has occupied the post of its General Director. Mr. Isaev is a vice-president of a nongovernmental Organization of Experts in Cell Technologies and Regenerative Medicine.
Roman Knyazev (Non-Executive Director): Mr Knyazev joined Xenetic as a non-executive Director in 2012. He has economic background from Moscow State University. Since 2009 Roman Knyazev has been a member of Investment department specializing in healthcare of RUSNANO, Russia’s biggest nanotechnological fund. Before that Roman has worked for PWC, advisory department. Roman is also a member of the board of Synbio – the biggest Xenetic’s shareholder.
Financial Information
Financial information in respect of Xenetic is available in ‘read only’ format for viewing or downloading, free of charge, on Xenetic’s website at http://www.xeneticbio.com/financial-reports. If you are reading this document in hard copy, please enter the web address provided in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address provided to be brought to the page containing the relevant document.
6. US Federal Securities Law Restrictions on Transfer
The GSL Consideration Shares shall not be registered under the Securities Act or qualified for resale under the applicable securities laws of any of the States of the US. The GSL Consideration Shares are only being offered and sold pursuant to an exemption from registration afforded by Section 3(a)(10) of the Securities Act on the basis of the Court Meeting, which will consider, among other things, the fairness of the terms of the Scheme.
7. Corporate Governance
GSL is governed by the Nevada Revised Statutes, its Certificate of Incorporation and its Bylaws. The Board is responsible for the proper management of GSL. The Director intends that GSL will comply with all corporate governance regimes required under the laws of the State of Nevada in the USA applicable to a company of the size and type of GSL, as well as the reporting obligations it is required to meet under US federal law.
GSL is currently considered a voluntary filer under the US securities laws, and, thus, not subject to various more stringent filing requirements set forth under the Exchange Act, and the more recent rules set forth under SOX. It is anticipated, however, that following completion of the Acquisition, GSL will become subject to these more stringent reporting requirements of the Exchange Act as well as SOX. Compliance with the Exchange Act and SOX will entail significant additional general and administrative expenses and management attention, along with additional burdens and costs on individual large shareholders, the directors and officers.
8. Incentive Plans
The Director recognises the importance of GSL’s employees to the performance of GSL’s business and believe that performance based share incentives are an effective way to motivate and retain high calibre employees and to align the interests of the management and key employees. While GSL has no such incentive plan at this time, it intends to put an incentive arrangement in place that mirrors the current plans offered by Xenetic to its directors and employees following the closing of the Acquisition.
| 70 |
9. Dividend Policy
GSL does not anticipate paying dividends to its shareholders in the near future, and will instead use available earnings to grow and improve GSL’s business. The declaration and payment by GSL of any future dividends will depend on the results of GSL’s operations, its financial condition, cash requirements and prospects, the distributable reserves of GSL and other factors deemed by the Board to be relevant at the time.
10. Current Trading and Prospects
GSL has incurred losses since incorporation in August 2011. The Director intends to build near term revenue streams through the sale of its two subsidiaries and their businesses, and through the operation of the business of Xenetic following the closing of the Acquisition.
11. Taxation
You are strongly advised to consult your own independent professional tax advisers regarding the tax consequences of owning the GSL Consideration Shares.
12. Further Information
Your attention is drawn to the remainder of this document and you are urged not to rely on summaries or individual parts only and to carefully review Part II of this document, which contains certain risk factors relating to any investment in GSL, to Part VIII of this document, which contains further additional information on GSL, and to GSL’s reports provided through links contained in this document to the SEC EDGAR system website.
| 71 |
PART VII
FINANCIAL INFORMATION ON GSL
Financial information in respect of GSL, which has been filed with the SEC, is incorporated by reference into this document and is available in ‘read only’ format for viewing or downloading, free of charge, on the EDGAR website. If you are reading this document in hard copy, please enter the web address below in your web browser to be brought to the page containing the relevant document. If you are reading this document in soft copy, please click on the web address below to be brought to the page containing the relevant document.
http://www.sec.gov/cgi-bin/browse-
edgar?company=general+sales&match=&CIK=&filenum=&State=&Country=&SIC=&owner=exclude&Find= Find+Companies&action=getcompany
| 72 |
PART VIII
ADDITIONAL INFORMATION
1. Responsibility Statements
GSL and its Director, to the best of their knowledge after taking all reasonable care to ensure that such is the case, confirm that: (a) the information contained in this document is in accordance with the facts as they are known; (b) the information contained in this document does not omit anything likely to affect the import of such information; and (c) as a result and consistent with this understanding, GSL and Director accept responsibility for the information contained herein.
2. Incorporation, History and Conduct of Business
| (a) | GSL was incorporated as General Aircraft, Inc. on 9 August 2011 in the State of Nevada with its principal place of business located at 5389 Golden Barrel Ave., Las Vegas, NV 89141. On 11 February 2013, GSL’s principal place of business changed to 16445 North 91st St., Suite 103, Scottsdale, Arizona 85260. GSL’s name was changed to General Sales and Leasing, Inc. on 13 February 2013. |
| (b) | It is the intention of the Director to continue to conduct the affairs of GSL through the closing of the Acquisition so that it remains an operating company. |
3. Share Capital
(a) Set out below are details of GSL’s issued share capital as at the date of this document:
| $ Number | |
| Common Stock with par value of $0.001 each 135,000 | 135,000,000 |
(b) GSL’s authorised share capital consists of:
| (i) | 10,000,000
shares
of
Preferred
Stock,
with
$0.001
par
value,
of
which
none
have
been
issued. The voting rights, rate of dividends preference in relation to other classes or series, and rights in the event of liquidation related to shares of Preferred Stock of any series are determined by the Board and may vary from time to time. |
| (ii) | 300,000,000 shares of Common Stock, with $0.001 par value, of which 135,000,000 have been issued. Holders of Common Stock have voting rights equal to one vote for each share of Common Stock held and are entitled to receive dividends when, and if declared by the Board subject to the rights of any Preferred Stock having preference as to dividends. In the event of liquidation or dissolution, subject to the rights of any Preferred Stock and creditors, Holders’ are entitled to share rateably in any distributions made on behalf of GSL. Holders of Common Stock do not have conversion, redemption or pre-emptive rights. |
(c) In advance of the Scheme becoming Effective, GSL intends to undertake a share consolidation whereby every current holder of Shares shall receive 1 New GSL Share for every 10 Shares held on the date of consolidation. Therefore, the issued share capital following such share consolidation is expected to be 13,500,000 shares of Common Stock with par value of $0.01 each.
(d) Following the implementation of the Share Consolidation, it is expected that Oxbridge shall own 10,000,000 New Shares prior to the closing of the GSL Hive Out Agreement.
4. Certificate of Incorporation, Bylaws and Governing Law
GSL is governed by its constitutional documents, which consist of the Certificate of Incorporation and Bylaws, and is regulated by the laws of the State of Nevada in the US, provided, in large, by the Nevada Revised Statutes. The following is a summary of certain provisions of its Certificate of Incorporation and Bylaws:
| 73 |
(a) Certificate of Incorporation
The Certificate of Incorporation was first filed in Nevada on 9 August 2011 with an amendment filed on 12 February 2013. They contain provisions, inter alia, to the following effect:
(i) Purpose
The purpose of GSL is stated to be any legal purpose. (ii) Authorised capital stock
GSL is authorised to issue 310,000,000 shares, divided into “Common Stock” and “Preferred Stock”, each having a par value of $0.001. Of the 310,000,000 shares, GSL may issue 300,000,000 shares of Common Stock and 10,000,000 shares of Preferred Stock. Increasing or decreasing the authorised capital of GSL requires an amendment to GSL’s Certificate of Incorporation. Amending the Certificate of Incorporation requires the approval of the holders of a majority of the issued shares entitled to vote.
(iii) Voting rights attaching to Common Shares
Holders of Common Shares are entitled to one vote per share. (iv) Directors liability and indemnification
The liability of GSL’s directors is eliminated to the maximum extent allowed under Nevada Revised Statutes.
(b) Bylaws
GSL’s Bylaws, dated 3 August 2011, contain provisions, inter alia, to the following effect: (i) Board of Directors
The Bylaws state that the business and affairs of GSL shall be managed by its Board. The number of directors shall be fixed from time to time by resolution of the Board, with the number of directors being no less than 1 nor more than 13.
Each director shall hold office until their successors are appointed. A Chairman of the Board shall be elected from among the directors, and, if present, shall preside at all meetings of the Board and of the shareholders. The Chairman has such powers and duties as the Board prescribes.
The Board shall hold a meeting immediately after the annual meeting of the shareholders. Regular meetings of the Board can be held with the consent of the directors. Special meetings of the Board may be called by or at the request of the Chairman, the President or any two directors. Written notice of such special meetings must be given to each of the directors.
A majority of the number of directors shall constitute a quorum for the transaction of business at any meetings of the Board. The passing of resolutions of the Board require the approval of a majority of the directors present at a quorate meeting of the Board.
(ii) Officers
The Bylaws provide for the following officers of GSL: Chairman, Chief Executive Officer, President, one or more Vice Presidents, Chief Financial Officer, Treasurer and Controller. The officers of GSL serve at the pleasure of the Board and may be removed at any time by the Board.
(iii) Meetings of the shareholders
The annual meeting of the shareholders shall be held on such day as shall be fixed by the Board. Special meetings of the shareholders may be called by the Chairman, Chief Executive Officer or by the Board.
| 74 |
The Board may designate any place as the place for the annual meeting of the shareholders or for any special meeting called by the Board. Written notice of a shareholders’ meeting shall, unless otherwise prescribed by Nevada law, be delivered not less than 10 nor more than 60 days before the meeting.
The presence in person or by proxy of holders of not less than 50 per cent. of outstanding shares entitled to vote on the business to be transacted shall constitute a quorum at any shareholders’ meeting. The approval of the majority of the Common shares represented at a quorate meeting shall be required to pass a shareholders’ resolution. At all meetings of the shareholders, a shareholder may vote in person or by proxy.
(iv) Dividends
The Board may from time to time declare, and GSL may pay, dividends on GSL’s issued share capital.
5. Director’s Interests
| (a) | As at 20 November 2013 (the latest practicable date prior to the publication of this document), the Director holds no Shares in GSL. |
| (b) | In addition to his directorship in GSL and/or its subsidiaries, the Director currently holds or has within the 5 years preceding the date of this document held the following directorships and is or has within such period been a partner in the following firms: |
| Name | Present Directorship | Past Directorship
|
| Ari L. Nagler | Develas, LLC | Ari Nagler, PLLC DC Retail, LLC, dba Candleloft/ The Candle Maker Nuad Networks LLC |
| (c) | At the date of this document, the Director: |
| (i) | does not have any convictions in relation to fraudulent offences for at least the previous 5 years; |
| (ii) | was not a director of a company, a member of an administrative, management or supervisory body or a senior manager of a company within the previous 5 years which has entered into any bankruptcy, receivership or liquidation proceedings; and |
| (iii) | has not been subject to any official public incrimination and/or sanctions by statutory or regulatory authorities (including designated professional bodies) or has not been disqualified by a court from acting as a member of the administrative, management or supervisory bodies of an issuer or from acting in the management or conduct of the affairs of any issuer for at least the previous 5 years. |
6. Substantial Shareholdings
(a) In addition to the holdings disclosed in paragraph 5 above, GSL is aware of the following persons who may, directly or indirectly, be interested in 3 per cent. or more of the issued share capital of GSL:
| At the date of this document | Folowing the Share Consolidation, Acquisition and closing of the GSL Hive Out Agreement | |||
| Name | Number of Shares | Percentage of issued Shares | Number Percentage of Shares issued Shares | |
| Oxbridge | 100,000,000 | 74.07% | Nil | Nil |
| 75 |
7. Director’s Service Contracts
There is presently no written service contract between GSL and its sole Director, Ari L. Nagler. Presently, Mr Nagler is not paid a regular salary by GSL but has a variable salary arrangement based on his performance. Since becoming GSL’s CEO earlier this year, Mr Nagler has been paid $15,000 by GSL pursuant to this arrangement.
8. Details of Subsidiaries
GSL has the following directly held wholly-owned subsidiary undertakings:
| Country of | Proportion of shares | |||
| registration or | Nature of | Type of | and voting | |
| Name | incorporation | business | shares held | rights held |
| Shift It Media Co | Nevada, USA | Advertising | Common Stock | 100% |
| General Aircraft, Inc. | Nevada, USA | Aircraft rentals | Common Stock | 100% |
9. Material Contracts
Save as disclosed in this paragraph, there are no contracts (other than contracts entered into in the ordinary course of business) which (i) have been entered into by GSL within the 2 years immediately preceding the date of this document and which are, or may be, material to GSL, or (ii) contain any provision under which GSL have any obligation or entitlement which at the date of this document is material to GSL.
(a) Purchase Money Promissory Note and Security Agreement
Pursuant to an agreement dated 11 August 2011, in connection with the acquisition of a Robinson R44 helicopter, GSL made a promise to pay Western Intermountain Holdings Trust the principal sum of $212,812.50 at a rate of 6 per cent. per annum. All principal and accrued interest then outstanding is repayable by GSL on or prior to 11 August 2016. The liabilities under this agreement are being assumed by Oxbridge under the GSL Hive Out Agreement.
(b) Financing Agreement and Security Agreement
Pursuant to an agreement dated 2 August 2013, North Star Capital Group, Inc. provided GSL with a line of credit financing of up to $220,000 in connection with the overhaul of the Robinson R44 helicopter. Interest is being charged at 9.5 per cent. on the outstanding principal at any time, and all principal and accrued interest is repayable by 2 August 2015. At the present time, North Star has advanced $170,000 for the overhaul process, which is in process. The liabilities under this agreement are being assumed by Oxbridge under the GSL Hive Out Agreement.
(c) GSL Hive Out Agreement
Pursuant to an agreement dated 12 November 2013 between GSL, Oxbridge, Shift It Media Co and General Aircraft, Inc., GSL has agreed to transfer the entire issued share capital of each of Shift It Media Co and General Aircraft, Inc. to Oxbridge along with any assets relating to the business conducted by Shift It Media Co and General Aircraft, Inc. Oxbridge shall assume any liabilities connected with the business being transferred and has indemnified GSL for any losses arising out of such liabilities. In addition, the 100,000,000 Shares held (or such other number of shares held following any corporate reorganization, split or otherwise) by Oxbridge in GSL shall be retired back into treasury upon the closing of the Acquisition. Furthermore, GSL shall make a payment of $430,000 to Oxbridge following such closing. The GSL Hive Out Agreement is unconditional in all respects save for the Scheme becoming Effective. Following the disposal contemplated by the GSL Hive Out Agreement, GSL’s assets and liabilities will exclusively be the assets and liabilities of Xenetic.
10. Taxation
Prospective investors should consult their tax advisors regarding the US federal, state, local, and non-US income and other tax considerations that may affect them as a result of the Acquisition.
| 76 |
11. Related Party Transactions
GSL has not entered into any related party transactions during the period from incorporation of GSL until the date of this document.
12. Litigation
There are no governmental, legal or arbitration proceedings (including any such proceedings which are pending or threatened of which GSL is aware), during the previous 12 months which may have, or have had in recent past, significant effects on GSL and/or GSL’s financial position or profitability.
13. Working Capital
The Director is of the opinion, having made due and careful enquiry, that the working capital available to GSL is sufficient working capital for its present requirements, that is for at least 12 months from the date of this document.
14. Source of Information
In relation to information provided by third parties, GSL confirms that that information has been accurately reproduced and as far as GSL is aware and able to ascertain from information published by that third party, no facts have been omitted which would render the reproduced information inaccurate or misleading.
15. No Significant Change
Other than the financing agreement with North Star Capital Group described in paragraph 9(b) above, there has been no significant change in the trading or financial position of GSL since 31 May 2013 (being the date to which the last financial information of GSL was prepared).
16. General
| (a) | There are no specific dates on which entitlement to dividends or interest thereon on Shares arises and there are no arrangements in force for the waiver of future dividends. |
| (b) | Other than described in paragraph 9(c) above, no agreement, arrangement or understanding (including any compensation arrangement) exists between GSL or any person acting in concert with GSL for the purposes of the Acquisition and any of the Directors, or recent directors, shareholders or recent shareholders of GSL or any person interested or recently interested in Common Stock having any connection with or dependence upon or which is conditional on the outcome of, the Acquisition. |
| (c) | No proposal exists in connection with the Acquisition for any payment or other benefit to be made or given by GSL or any person acting in concert with GSL for the purposes of the Acquisition to the Director as compensation for loss of office or as consideration for, or in connection with, his retirement from office. |
| (d) | The aggregate fees and expenses which are expected to be incurred by GSL in connection with the Acquisition are estimated to amount to $52,500 plus applicable VAT. |
17. Documents Available for Inspection
Copies of this document are available for inspection during normal business hours on any weekday (Saturdays, Sundays and public holidays excepted) at DWF LLP, Capital House, 85 King William Street, London EC4N 7BL and at GSL’s principal place of business until the date of the Scheme.
Dated: 21 November 2013
| 77 |
PART 8
ADDITIONAL INFORMATION
1. Responsibility Statements
| (a) | The Directors, whose names are set out in paragraph 2(a) below, accept responsibility for all the information contained in this document, other than information for which GSL accepts responsibility as stated in sub-paragraph (b) of this paragraph 1, and the Directors (except for Roman Knyazev) accept responsibility for the recommendation of the Acquisition and any opinion attributable to the Directors relating to such recommendation contained in this document. To the best of the knowledge and belief of the Directors (who have taken all reasonable care to ensure that such is the case), the information contained in this document for which they accept responsibility, is in accordance with the facts and does not omit anything likely to affect the import of such information. |
| (b) | The GSL Director accepts responsibility for the information contained in this document and the Equivalent Document relating to GSL and statements of intention by GSL in each case only in relation to the period up to the Effective Date. To the best of the knowledge and belief of the GSL Director (who has taken all reasonable care to ensure that such is the case), the information contained in this document and the Equivalent Document for which he accepts responsibility, is in accordance with the facts and does not omit anything likely to affect the import of such information. |
2. The Directors and GSL Directors
| (a) | The Directors of Xenetic and their respective functions, are as follows: |
– Sir Brian Richards (Non-executive Chairman)
– M Scott Maguire (Chief Executive Officer)
– Colin William Hill (Chief Financial Officer)
– Professor Gregory Gregoriadis (Chief Scientific Officer)
– Dr Dmitry D Genkin (Non-executive director)
– Firdaus Jal Dastoor (Non-executive director)
– Artur Isaev (Non-executive director)
– Roman Knyazev (Non-executive director)
The Company, whose registered number is 03213174, has its registered office at London Bioscience Innovation Centre, 2 Royal College Street, London NW1 0NH. The business address of each Director is that of the Company’s registered office.
| (b) | The GSL Director and his functions are as set out in paragraph 4 of Part VI of the Equivalent Document, the text of which is set out in Part 7 of this document. GSL is a company as described in paragraph 2 of Part VIII of the Equivalent Document. |
3. Concert Parties
3.1 In addition to the GSL Directors, companies in the GSL Group, Oxbridge and their respective directors and (if relevant) related pension funds, there are no other persons who are (or are deemed to be) acting in concert with GSL for the purposes of the Acquisition.
3.2 In addition to the Xenetic Directors, Xenetic’s related companies, their directors and related nominees and pension funds, the only other persons who are (or are deemed to be) acting in concert with Xenetic for the purposes of the Acquisition are: Igor Nikolaev, a previous director appointed to the Board by SynBio and currently an observer to the Board; London Bridge Capital, Xenetic’s Rule 3 adviser in connection with the Acquisition; and SynBio and the following additional persons:
| (i) | OAO Human Stem Cell Institute (HSCI); |
| (ii) | OAO Russian Corporation of Nanotechnologies (Rusnano); |
| (iii) | FDS Pharma Associates LP (UK), jointly controlled by OOO Baltic Finance (represented by Igor Nikolaev) and Angport Limited; |
| 78 |
| (iv) | Pharmsynthez; |
| (v) | Cryonix Joint Stock Company (50 per cent. owned by HSCI); |
| (vi) | Angport Limited (BVI) (controlled by Dmitry Genkin); |
| (vii) | Pharma Research Limited (Limited Partner of FDS Pharma); |
| (viii) | Pharma Industries Limited (General Partner of FDS Pharma); |
| (ix) | OOO Baltic Finance; |
| (x) | Black Island Capital Limited (controlled by Igor Nikolaev); |
| (xi) | Rock Nominees Limited (controlled by Igor Nikolaev); and |
| (xii) | the directors of each of the corporate entities listed above, including without limitation Artur Isaev and Dmitry Genkin. |
4. Interests and dealings
4.1 Definitions
For the purposes of this Part 8 of this document:
(a) “acting in concert” has the meaning set out in the Code;
(b) “arrangement” includes any indemnity or option arrangements and any agreement or understanding, formal or informal, of whatever nature, relating to relevant securities which may be an inducement to deal or refrain from dealing;
(c) “dealing” or “dealt” includes the following:
| (i) | the acquisition or disposal of relevant securities, of the right (whether conditional or absolute) to exercise or direct the exercise of the voting rights attaching to relevant securities, or of general control of relevant securities; |
| (ii) | the taking, granting, acquisition, disposal, entering into, closing out, termination, exercise (by either party) or variation of an option (including a traded option contract) in respect of any relevant securities; |
| (iii) | subscribing or agreeing to subscribe for relevant securities; |
| (iv) | the exercise or conversion, whether in respect of new or existing securities, of any relevant securities carrying conversion or subscription rights; |
| (v) | the offer of, disposal of, entering into, closing out, exercise (by either party) of any rights under, or variation of, a derivative referenced directly or indirectly, to relevant securities; |
| (vi) | entering into, terminating or varying the terms of any agreement to purchase or sell relevant securities; and |
| (vii) | any other action resulting, or which may result, in an increase or decrease in the number of relevant securities in which a person is interested or in respect of which he has a short position; |
(d) “derivative” includes any financial product whose value, in whole or in part, is determined directly or indirectly by reference to the price of an underlying security;
(e) “disclosure date” means 20 November 2013 (being the latest practicable date prior to the publication of this document);
(f) “disclosure period” means the period commencing on 16 July 2012 (being the date 12 months prior to the commencement of the Offer Period) and ending on the disclosure date;
(g) a person is treated as having an “interest in securities” if he has long economic exposure, whether absolute or conditional, to changes in the price of those securities (and a person who only has a short position in securities is not treated as interested in those securities). In particular, a person is treated as “interested” in securities if:
(i) he owns them;
| 79 |
(ii) he has the right (whether conditional or absolute) to exercise or direct the exercise of the
voting rights attaching to them or has general control of them;
(iii) by virtue of any agreement to purchase, option or derivative, he;
(A) has the right or option to acquire them or call for their delivery; or
(B) is under an obligation to take delivery of them,
whether the right, option or obligation is conditional or absolute and whether it is in the money or otherwise; or
(iv) he is a party to any derivative:
(A) whose value is determined by reference to their price; and (B) which results, or may result, in his having a long position in them.
(h) “relevant securities” means:
| (i) | Xenetic Shares and any other securities of Xenetic conferring voting rights; |
| (ii) | equity share capital of Xenetic or, as the context requires, GSL; |
| (iii) | securities of Xenetic which carry substantially the same rights as the GSL Consideration Shares; or |
| (iv) | securities of Xenetic or, as the context requires, GSL carrying conversion or subscription rights into any of the foregoing; |
(i) “short position” means any short position (whether conditional or absolute and whether in the
money or otherwise) including any short position under a derivative, any agreement to sell or any delivery obligation or right to require another person to purchase or take delivery.
4.2 Interests in relevant securities of Xenetic
(a) As at the disclosure date, the beneficial interests of the Xenetic Directors in the share capital of Xenetic were as follows. Roman Knyazev and Artur Isaev were not interested in any Xenetic Shares other than indirectly by virtue of the above concert parties being interested by virtue of the interest of SynBio referred to in paragraph 4.2(d) below.
| Number of Xenetic Shares beneficially held | Percentage of aggregate Xenetic Shares in issue | |
| Name of Director | ||
| Sir Brian Richards | 2,262,606 | 0.55% |
| Scott Maguire (held by Canaccord Nominees Ltd) | 1,401,361 | 0.34% |
| Colin Hill (held by Pershing Nominees) | 1,825,420 | 0.45% |
| Professor Gregory Gregoriadis | 4,650,208 | 1.14% |
| Firdaus Dastoor | 1,125,000 | 0.28% |
| Dr Dmitry Genkin (held by FDS Pharma) | 64,560 | 0.02% |
| 80 |
(b) As at the disclosure date, the following Xenetic Directors had the following outstanding options and awards under the Xenetic Share Schemes: Vesting Date/ | ||||||
| Name of | Date of | No. of | Exercise | Performance | ||
| Director | grant | shares | price (p) | Plan | Target | Lapse Date |
| Scott Maguire | 16.01.2006 | 6,200,250 | 1.00 | Xenetic 2000 | fully vested | 15.01.2016 |
| Share Option Plan | ||||||
| 10.06.2010 | 1,547,096 | 11.00 | Xenetic 2007 | fully vested | 09.06.2020 | |
| Share Option Plan | ||||||
| Colin Hill | 10.06.2010 | 1,323,396 | 11.00 | Xenetic 2007 | fully vested | 09.06.2020 |
| Share Option Plan | ||||||
| 10.06.2010 | 74,566 | 11.00 | Xenetic 2007 | Share price of 20p | 09.06.2020 | |
| Share Option Plan | ||||||
| 10.06.2010 | 74,566 | 11.00 | Xenetic 2007 | Share price of 40p | 09.06.2020 | |
| Share Option Plan | ||||||
| 10.06.2010 | 74,568 | 11.00 | Xenetic 2007 | Share price of 100p | 09.06.2020 | |
| Share Option Plan | ||||||
(c)
As at the disclosure date, the following Xenetic Directors held interests as beneficial co-owners Amount Subscription paid by Rate of Price of the director to | |||||||
| Name of | Date of | No. of | Vesting | Threshold | Carrying | jointly-owned | acquire their |
| Director | JOA | shares | hurdles | Amount | Cost | shares | interest |
| Scott Maguire | 10 June 2010 |
3,857,084 | None | 7.75p per share | 8.387% p.a. over up to | 7.75p | £4,705.64 |
| (plus the carrying cost) | 5 years | ||||||
02 March 2012 |
23,380,673 | Share price targets | 10.625p per share | 7.65 % p.a. over up to | 10.625p | £32,265.33 | |
| (plus the carrying cost) | 3 years | ||||||
| Colin Hill | 10 June 2010 |
1,461,395 | None | 7.75p per share | 8.387% p.a. over up to | 7.75p | £1,782.90 |
| (plus the carrying cost) | 5 years | ||||||
02 March 2012 |
4,701,454 | Share price targets | 10.625p per share | 7.65% p.a. over up to |
10.625p | £6,488.01 | |
| (plus the carrying cost) | 3 years | ||||||
(References to the carrying cost are to the amount by which the threshold amount is increased over a 5 year period calculated by reference to a rate of simple interest applied to the original threshold amount over that period.)
| (d) | As at the disclosure date, other than Dmitry Genkin as set out at paragraph 4.2(a) above the interests of persons acting in concert with Xenetic and their connected persons in relevant securities in Xenetic were as follows: |
| (i) | by virtue of the agreement between Xenetic and FDS Pharma dated 10 October 2005 summarised at paragraph 10.1 of this Part 8, FDS Pharma is interested in up to 10,174,340 Xenetic Shares; |
| (ii) | by virtue of SynBio’s holding of such Xenetic Shares, SynBio and its concert parties listed in paragraph 3.2 above are interested in 184,755,238 Xenetic Shares, approximately 45.9 per cent. of the issued share capital of Xenetic; |
| (iii) | by virtue of the warrants held by SynBio described at paragraph 10.1 of Part 8 of this document, SynBio is interested in 11,080,000 further Xenetic Shares; |
| (iv) | Igor Nikolaev is interested in 4,127,743 Xenetic Shares including the 1,950,000 Xenetic Shares held by/for Black Island Capital Limited; and |
| (v) | Black Island Capital Limited is interested in 1,950,000 Xenetic Shares. 82 |
| 81 |
(e) As at the disclosure date, the persons acting in concert with GSL and their connected persons had no interests in relevant securities in Xenetic.
4.3 Dealings in relevant securities of Xenetic
| (a) | Save as set out below, during the Offer Period there have been no dealings in Xenetic Shares by the Xenetic Directors, their immediate families, related trusts or any connected persons or by any of Xenetic’s concert parties: |
On the following dates Igor Nikolaev, an observer to the Board of Xenetic, sold the following Xenetic Shares at the prices stated per Xenetic Share. Mr Nikolaev retains an interest of 4,127,743 Xenetic Shares following such sales.
| Date | Number of Xenetic Shares | Price |
| 18.07.13 | 100,000 | 7p |
| 02.08.13 | 400,000 | 6.25p |
| 28.08.13 | 500,000 | 6.5p |
| 04.10.13 | 500,000 | 5.5p |
| 07.10.13 | 200,000 | 5.5p |
| 18.10.13 | 800,000 | 5.5p |
| 21.10.13 | 633,274 | 5.5p |
| (b) | During the disclosure period (as defined above), there have been no dealings in Xenetic Shares by the GSL Director, his immediate family, related trusts or any connected person or any dealings by any of GSL’s concert parties. |
4.4 Interests in relevant securities of GSL
| (a) | As at the disclosure date, the persons acting in concert with Xenetic (and their connected persons) had no interests in relevant securities in GSL. |
| (b) | As at the disclosure date, the GSL Director, members of his immediate family, related trusts and connected persons had no interests in relevant securities of GSL. |
| (c) | As at the disclosure date, Oxbridge held 100,000,000 GSL Shares. Save for this holding, persons acting in concert with GSL and their connected persons had no interests in relevant securities in GSL. |
| (d) | As at the disclosure date, the GSL Director had no outstanding options or awards in relation to GSL. |
4.5 Dealings in relevant securities of GSL
| (a) | During the disclosure period, there have been no dealings in GSL Shares by the Xenetic Directors, their immediate families, related trusts or any commented persons or by any concert parties of Xenetic or such persons. |
| (b) | During the disclosure period (as defined above), there have been no dealings in GSL Shares by the GSL Directors, their immediate families, related trusts or any connected persons or by any concert parties of GSL or such persons. |
4.6 General
Save as disclosed in this paragraph 4 of this Part 8, as at the disclosure date:
| (a) | neither GSL, nor the GSL Director nor (in the case of the GSL Director) any member of his family or related trusts or companies or (so far as the GSL Director is aware having made due and careful enquiry) other connected persons, nor any person acting in concert with GSL, nor any person with whom GSL or any person acting in concert with GSL had a dealing arrangement (save for the irrevocable undertakings described in this Part 8), was interested in, directly or indirectly, nor had any right to subscribe for, or had any short position in relation to, any relevant securities of GSL nor had any such person dealt in any relevant securities of GSL during the disclosure period; |
| 82 |
| (b) | neither GSL, nor the GSL Director nor (in the case of the GSL Director) any member of his family or related trusts or companies or (so far as the GSL Director is aware having made due and careful enquiry) other connected persons, nor any person acting in concert with GSL, nor any person with whom GSL or any person acting in concert with GSL had a dealing arrangement (save for the irrevocable undertakings described in this Part 8), was interested in, directly or indirectly, nor had any right to subscribe for, or had any short position in relation to, any relevant securities of Xenetic nor had any such person dealt in any relevant securities of Xenetic during the disclosure period; |
| (c) | neither Xenetic, nor any of the Xenetic Directors nor (in the case of Xenetic Directors) any member of their respective families or related trusts or companies or (so far as the Xenetic Directors are aware having made due and careful enquiry) other connected persons, nor any person acting in concert with Xenetic, nor any person with whom Xenetic or any person acting in concert with Xenetic had a dealing arrangement (save for the irrevocable undertakings described in this Part 8), was interested in, directly or indirectly, nor had any right to subscribe for, or had any short position in relation to, any relevant securities of Xenetic nor had any such person dealt in any relevant securities of Xenetic since the beginning of the Offer Period; |
| (d) | neither Xenetic, nor any of the Xenetic Directors nor (in the case of Xenetic Directors) any member of their respective families or related trusts or companies or (so far as the Xenetic Directors are aware having made due and careful enquiry) other connected persons, nor any person acting in concert with Xenetic, nor any person with whom Xenetic or any person acting in concert with Xenetic had a dealing arrangement, was interested in, directly or indirectly, nor had any right to subscribe for, or had any short position in relation to, any relevant securities of GSL nor had any such person dealt in any relevant securities of GSL since the beginning of the Offer Period; |
| (e) | neither Xenetic, GSL, nor any person acting in concert with Xenetic or GSL has borrowed or lent (including for these purposes any financial collateral arrangements) any relevant securities in Xenetic or GSL (save for any borrowed shares which have either been on-lent or sold); |
| (f) | save
for
the
irrevocable
undertakings
given
by
Xenetic
Directors
as
described
in
this
Part
8,
there is no arrangement of the kind referred to in Note 11 on the definition of “acting in concert” set out in the City Code relating to relevant securities in Xenetic which exists between GSL or any person acting in concert with GSL and any other person, nor between Xenetic or any person acting in concert with Xenetic and any other person; |
| (g) | Xenetic has not redeemed or purchased any relevant securities of Xenetic during the disclosure period; |
| (h) | GSL has not redeemed or purchased any relevant securities of GSL or agreed to redeem or purchase during the disclosure period, save pursuant to the GSL Hive Out Agreement. |
5. Market quotations
The following table shows the Closing Prices of Xenetic Shares and GSL Shares for the first business day in each of the six months immediately prior to the date of this document, for 15 July 2013 (the Business Day prior to the commencement of the Offer Period) and for 20 November 2013 (being the latest practicable business day prior to the publication of this document):
| Share price Share price | Xenetic | GSL |
| Date (2013) | (pence) | (US$) |
| 3 June | 6.75 | 0.15 |
| 1 July | 6.125 | 0.15 |
| 15 July | 5.625 | 0.15 |
| 1 August | 6.375 | 0.15 |
| 2 September | 6.625 | 0.15 |
| 1 October | 5.75 | 0.15 |
| 1 November | 6.125 | 0.02 |
| 20 November | 5.50 | 0.02 |
| 83 |
6. Dealings
Dealings in relevant securities of Xenetic
There have been no dealings in Xenetic Shares between 16 July 2013 (being the start of the Offer Period) and 20 November 2013 (being the latest practicable date prior to the publication of this document) by the Xenetic Directors, their immediate families, related trusts and (so far as the Xenetic Directors are aware having made due and careful enquiry) any other person whose interests in Xenetic Shares a director is taken to be interested in pursuant to Part 22 of the Companies Act.
There have been no dealings in Xenetic Shares between 16 July 2013 (being the start of the Offer Period) and 20 November 2013 (being the latest practicable date prior to the publication of this document) by the GSL Director, his immediate family, related trusts and (so far as the GSL Director is aware having made due and careful enquiry) any other person whose interests in Xenetic Shares such person is taken to be interested in pursuant to Part 22 of the Companies Act.
7. Irrevocable undertakings Xenetic Directors
The following Xenetic Directors who beneficially own or otherwise control Xenetic Shares have given irrevocable undertakings to vote or procure the vote in favour of the Scheme at the Court Meeting (or otherwise to be bound by the Scheme) and the Special Resolution to be proposed at the General Meeting in relation to the following Xenetic Shares:
| Percentage of issued | ||
| Number of share capital | ||
| Name of Director | Xenetic Shares | of Xenetic |
| Sir Brian Richards | 2,262,606 | 0.55% |
| Scott Maguire (held by Canaccord Nominees Ltd) | 1,401,361 | 0.34% |
| Colin Hill (held by Pershing Nominees) | 1,825,420 | 0.45% |
| Professor Gregory Gregoriadis | 4,650,208 | 1.14% |
| Firdaus Dastoor | 1,125,000 | 0.28% |
| Dr Dmitry Genkin (held by FDS Pharma) | 6,491 | 0.002% |
| Total | 11,271,086 | 2.76% |
Xenetic Shareholders
Xenetic has also sought and received irrevocable undertakings from those other Xenetic Shareholders set out below to vote in favour of the Scheme Resolutions (and if the Acquisition is subsequently structured as a Takeover Offer, to accept any such offer made by GSL) in respect of their entire holdings in Xenetic Shares, representing approximately 23.7 per cent. of the existing issued ordinary share capital of Xenetic as at 20 November 2013 (being the last practicable date prior to the publication of this document).
| Percentage of issued | ||
| Number of share capital | ||
| Name | Xenetic Shares | of Xenetic |
| Serum Institute of India Limited (including | 42,137,665 | 10.33% |
| Poonwalla Investments & Industries Pvt Ltd and | ||
| Chanda Investment and Trading Company Pvt Limited) | ||
| RBC Trustees (Guernsey) Limited (Roy Nominees, for the | 33,400,606 | 8.19% |
| Xenetic JSOP trustee, Scott Maguire and Colin Hill) (“RBC”) | ||
| Baxter Healthcare SA | 7,325,470 | 1.80% |
| Securities Services Nominees Limited | 13,805,000 | 3.38% |
| Total | 96,668,741 | 23.7% |
| 84 |
All the irrevocable undertakings given by the Xenetic Directors and Xenetic Shareholders above remain binding in the event of higher competing offer for Xenetic. The irrevocable undertakings however become binding on such directors and shareholders (and will apply to their Xenetic Shares) only upon publication of a firm intention to make the Offer pursuant to Rule 2.7 of the City Code (namely the issue of the Announcement) where such Offer is publicly recommended by the board of Xenetic and, in the case of Serum Institute of India Limited and its connected persons, to any required Reserve Bank of India approvals. All such irrevocable undertakings will lapse and have no further effect if:
3.1 the Scheme lapses or is withdrawn;
3.2 GSL announces, with the consent of the Panel, that it does not intend to make or proceed with the Scheme; or
3.3 the Scheme has not become effective by 5.00 p.m. London time on 30 June 2014. Escrow Arrangements
Pursuant to an acquisition agreement dated 4 August 2011 made between (1) Xenetic and (2) the sellers in respect of the acquisition by Xenetic of the entire issued share capital of SymbioTech, 15,000,000 Xenetic Shares held by Synbio are subject to escrow arrangements, as contained in such acquisition agreement and in an escrow agreement between (1) SynBio, (2) Xenetic and (3) Lea Yeat Limited. Further details of this escrow arrangement are set out in paragraph 10.1 of this Part 8 (SymbioTech Acquisition Agreement).
Xenetic intends that these escrow arrangements in respect of such Xenetic Shares shall terminate with effect from completion of the Scheme and be replaced with equivalent escrow arrangements in respect of the GSL Consideration Shares attributable to them, for the remainder of the Escrow Period (as defined in such acquisition agreement), ending on 16 January 2015.
8. Lock ins and orderly market undertakings
Subject to the Scheme completing, the Xenetic Shareholder irrevocable undertakings set out in paragraph 7 above (save for RBC) also contain customary 12 month lock-in plus 12 month orderly marketing undertakings in relation to any GSL Consideration Shares received by such Xenetic Shareholders pursuant to the Scheme. The equivalent provisions for the Xenetic Directors set out in paragraph 7 above and RBC are a lock in for 6 months (or until an earlier admission to an Exchange), plus an orderly marketing undertaking for 6 months.
9. Directors’ service contracts or arrangements
9.1 Xenetic Directors’ service contracts or arrangements
Set out below are details of the service agreements or letters of appointment of each of the Xenetic Directors:
Sir Brian Richards has entered into a letter of appointment with the Company dated 22 December 2005, pursuant to which he was appointed as a non-executive Chairman of the Company until such time as his appointment is terminated by either party on three months’ notice or for cause inter alia if Sir Brian is in material breach of his obligations under the letter or any of his fiduciary duties owed to the Company. Sir Brian is entitled to remuneration of £3,000 per annum, which is payable monthly in arrears, and reimbursement of all out of pocket expenses incurred in the performance of his duties. Sir Brian has also performed consultancy services to Xenetic under a separate contract, for which he was paid £72,000 for the period 1 January 2013 to date. Sir Brian has agreed not to be directly or indirectly employed, engaged, concerned or interested in any business or undertaking which competes with any business of the Company or any of its subsidiaries, save with the prior sanction of the Board.
Scott Maguire has entered into a service agreement with the Company dated 3 November 2009, pursuant to which he was appointed as a chief executive officer of the Company until such time as his appointment is terminated by either party on 12 months’ notice or for cause inter alia if Mr Maguire is in material breach of his obligations under the letter or any of his fiduciary duties owed to the Company. Mr Maguire is entitled to remuneration of £330,000 per annum, which is payable monthly in arrears, and reimbursement of all out of pocket expenses incurred in the performance of his duties. Mr Maguire has agreed not to be directly or indirectly employed, engaged, concerned or interested in any business or undertaking which competes with any business of the Company or any of its subsidiaries, save with the prior sanction of the Board.
| 85 |
Colin Hill has entered into a service agreement with the Company dated 2 July 2007, pursuant to which he was appointed as chief financial officer of the Company until such time as his appointment is terminated by either party on 12 months’ notice or for cause inter alia if that Mr Hill is in material breach of his obligations under the letter or any of his fiduciary duties owed to the Company. Mr Hill is entitled to remuneration of £154,000 per annum, which is payable monthly in arrears, and reimbursement of all out of pocket expenses incurred in the performance of his duties. Mr Hill has agreed not to be directly or indirectly employed, engaged, concerned or interested in any business or undertaking which competes with any business of the Company or any of its subsidiaries, save with the prior sanction of the Board.
Professor Gregory Gregoriadis has entered into a letter of appointment with the Company dated 23 December 2005, pursuant to which he was appointed as chief scientific officer of the Company until such time as his appointment is terminated by either party on three months’ notice or for cause. The appointment may be immediately terminated for cause inter alia if Professor Gregoriadis is in material breach of his obligations under the letter or any of his fiduciary duties owed to the Company. Professor Gregoriadis is entitled to remuneration of £3,000 per annum, which is payable monthly in arrears, and reimbursement of all out of pocket expenses incurred in the performance of his duties. Professor Gregoriadis has also performed consultancy services to Xenetic under a separate contract, for which he was paid £87,000 for the period 1 January 2013 to date. Professor Gregoriadis has agreed not to be directly or indirectly employed, engaged, concerned or interested in any business or undertaking which competes with any business of the Company or any of its subsidiaries, save with the prior sanction of the Board.
Dr Dmitry Genkin has entered into a letter of appointment with the Company dated 22 December 2005, pursuant to which he was appointed as a non-executive Director of the Company until such time as his appointment is terminated by either party on three months’ notice or for cause. The appointment may be immediately terminated for cause inter alia if Dr Genkin is in material breach of his obligations under the letter or any of his fiduciary duties owed to the Company. Dr Genkin is entitled to remuneration of £3,000 per annum, which is payable monthly in arrears, and reimbursement of all out of pocket expenses incurred in the performance of his duties. Dr Genkin has also performed consultancy services to Xenetic under a separate contract, for which he was paid £48,000 for the period 1 January 2013 to date. Dr Genkin has agreed not to be directly or indirectly employed, engaged, concerned or interested in any business or undertaking which competes with any business of the Company or any of its subsidiaries, save with the prior sanction of the Board.
Mr Firdaus Dastoor has been appointed as a non-executive Director of the Company until such time as his appointment is terminated by either party on three months’ notice or for cause. Mr Dastoor is entitled to remuneration of £3,000 per annum, which is payable monthly in arrears, and reimbursement of all out of pocket expenses incurred in the performance of his duties.
Mr Artur Isaev has been appointed as a non-executive Director of the Company until such time as his appointment is terminated by either party on three months’ notice or for cause. Mr Isaev is entitled to remuneration of £3,000 per annum, which is payable monthly in arrears, and reimbursement of all out of pocket expenses incurred in the performance of his duties.
Mr Roman Knyazev appointed as a non-executive Director of the Company pursuant to the Relationship Agreement. Mr Knyazev is entitled to remuneration of £3,000 per annum, which is payable monthly in arrears, and reimbursement of all out of pocket expenses incurred in the performance of his duties.
9.2 GSL Director
On the Effective Date the sole director of GSL will resign and will waive all claims against GSL with effect from completion of the Scheme. The Proposed Directors will be appointed to the board of GSL on the Scheme becoming Effective.
| 86 |
9.3 Summary of the principal terms of replacement share options to be granted by GSL in exchange for options granted under the Xenetic Share Option Plans
The replacement options to be granted over GSL Shares are intended to be on terms equivalent to those of the existing rights held by employees to subscribe for Xenetic Shares. Any replacement option granted in consideration for the release of an option to subscribe for Xenetic Shares which has not yet vested will vest and become exercisable only after such date, or upon the achievement of equivalent performance targets, as are specified in the terms of the existing option.
All replacement options will lapse and cease to be exercisable at the end of the tenth anniversary of the grant of the existing option over Xenetic Shares, or at such other time as was notified to the optionholder when the existing option was granted or the earlier time when the existing option would, by its terms (or as has been otherwise agreed between Xenetic and the optionholder) lapse and cease to be exercisable.
Any employee optionholders who choose not to release their existing options in consideration for the grant of an option in GSL will have the right to subscribe for Xenetic shares for a period (i) of 40 days in the case of options granted under the Xenetic 2007 Share Option Plan; or (ii) of 6 months in the case of options granted under the Xenetic 2000 Share Option Plan, beginning in each case with the date on which the Scheme becomes Effective.
GSL Shares over which replacement options are to be granted
The number of GSL Shares over which a replacement option will be granted in exchange for the release of existing rights to subscribe for Xenetic Shares will be determined in the same manner as that in which shareholders in Xenetic will, under the terms of the Scheme, acquire a number of shares of common stock in GSL in substitution for their Xenetic Shares.
Price per GSL Share payable upon the exercise of a replacement option
The aggregate exercise price payable for the acquisition of shares under a replacement option to acquire GSL Shares will (except as described below) be determined by dividing the aggregate price payable on exercise in full of the original option to subscribe for Xenetic Shares by the number of GSL Shares over which the replacement option is granted and converting that price into US dollars after conversion at the rate quoted by Barclays Bank PLC on the Business Day last preceding the date on which the grant of replacement share options becomes effective.
To ensure compliance with the UK statutory requirements relating to tax-favoured options originally granted by Xenetic as “Enterprise Management Incentives” (“EMI Options”), it may be necessary to adjust the number of GSL Shares and the exercise price per GSL Share over which a replacement option is granted, to take account of any changes in the respective market values of Xenetic Shares and GSL Shares at the time of grant of the replacement options. Any such adjustment will be subject to the agreement of Her Majesty’s Revenue & Customs and the Proposed Directors.
Exercise of a replacement option
If not already immediately exercisable, a replacement option will normally become exercisable after such date as is specified in the terms or, if later, when it becomes vested in consequence of any performance targets being met. Options granted to employees in the US vest (i.e. become exercisable) in three equal tranches, each tranche having a share price target which will be adjusted as described above.
Replacement options which are EMI Options may, if so permitted by the directors of Xenetic, be exercised within 90 days following the occurrence of a “disqualifying event” (as that term is defined in sections 533 – 536 of the Income Tax (Earnings and Pensions) Act 2003).
Leaving employment
If an Optionholder ceases to hold office or employment with the Xenetic Group (“leaves”) by reason of ill-health, disability, injury, redundancy, transfer out of the Xenetic Group of the business or subsidiary in which he is engaged (or, in the case of options granted in exchange for the release of options originally granted under the Xenetic 2000 Share Option Scheme, retirement) the Optionholder mayretain the Option and exercise it with a limited period of 6 months or one year thereafter. If an Optionholder leaves for any other reason, his option may be exercised only in respect of vested option shares or, in the case of options originally granted under the Xenetic 2007 Share Option Scheme, within one year thereafter but only if the directors of Xenetic so determine in their discretion. If an Optionholder dies, his option may be exercised by his personal representatives within a limited period after the date of death. Options become exercisable in full upon a takeover of GSL. If there occurs a variation in the share capital of GSL by way of capitalisation or rights issue, consolidation or reduction or any other variation of share capital, the Company may make such adjustments to the number of GSL Shares under option and to the exercise price as appropriate to take account of such variation.
| 87 |
Variation of GSL share capital
If there occurs a variation in the share capital of GSL by way of capitalisation or rights issue, consolidation or reduction or any other variation of share capital, the Company may make such adjustments to the number of GSL Shares under option and to the exercise price as appropriate to take account of such variation.
10. Material contracts
10.1 Xenetic Material Contracts
The following contracts, not being contracts entered into in the ordinary course of business, have been entered into by members of the Xenetic Group during the period beginning on 16 July 2011, two years before the commencement of the Offer Period and are or may be material:
FDS Pharma Agreement
On 10 October 2005, the Company and FDS Pharma entered into an agreement for the provision of manufacturing and clinical development services. By such agreement, FDS Pharma will use technology described in the PolyXen patents to manufacture certain product candidates for Lipoxen Technologies Limited (“Lipoxen”) in relation to which FDS Pharma (or sub-contractors appointed with the consent of Lipoxen) will carry out further clinical development work in accordance with an agreed timetable and development programme.
Lipoxen was legally acquired in January 2006 in an all-share transaction by way of a reverse takeover (“RTO”) by the Company (then named Greenchip Investments plc), which company, at completion, changed its name to Lipoxen plc. At the date of the RTO each Lipoxen share was exchanged for ~1.356 new Company shares, issued as fully paid. In 2011, Lipoxen plc changed its name to Xenetic Biosciences plc.
In consideration for FDS Pharma agreeing to conduct initial development and certain manufacturing services, including work to develop certain cell lines exclusive to Lipoxen, Lipoxen previously allotted 15,000,000 ordinary shares in Lipoxen to FDS Pharma (equivalent to approximately 20.35 million Xenetic Shares using the ratio above). FDS Pharma is entitled to receive an additional maximum 7.5 million ordinary shares in Lipoxen (approximately 10,174,340 Xenetic Shares) which will be issued provided that FDS Pharma has achieved certain development milestones.
The Company wishes to modify the product candidates (being the original subject of this agreement) such that both the original product candidates are expected to be substituted. The specific deliverables under the agreement may also change. Xenetic and FDS Pharma are therefore in discussions as to the precise nature and scope of any changes that may be agreed. It therefore remains the intention of the parties to maintain the concept of future performance milestones triggering a future issue to FDS Pharma of up to 10,174,340 Xenetic Shares. By virtue of this agreement, FDS Pharma is therefore interested in up to 10,174,340 Xenetic Shares.
To the extent that changes are made to the agreement with FDS Pharma (being a concert party of Xenetic) and/or relevant shares are required to be issued in due course under such agreement, the principles summarised at paragraph 7 of Part 2 (Explanatory Statement) will apply.
| 88 |
SymbioTec Acquisition Agreement
On 4 August 2011 the Company entered into an acquisition agreement with the sellers including SynBio to acquire the entire issued share capital of SymbioTec.
Under the acquisition agreement the sellers have provided various warranties, indemnities and undertakings to the Company, subject to certain agreed limitations on liability.
The consideration for the acquisition of the entire issued share capital of SymbioTec was the issue to the sellers of 80,000,000 Ordinary Shares. A proportion of such Ordinary Shares held by Synbio (currently 15,000,000 Ordinary Shares) has been retained in an escrow account to be used, amongst other things, for sale into the market to meet any losses occasioned by a breach of the warranties or indemnity claims under the acquisition agreement subject to time limits and other limitations of liability.
Subscription Agreement
The Company and SynBio entered into the subscription agreement, dated 4 August 2011, pursuant to which SynBio conditionally agreed to subscribe for 110,800,000 Ordinary Shares for cash at a price of 11p per Ordinary Share.
SynBio Co-Development Agreement
On 4 August 2011 the Company and SynBio entered into a co-development agreement pursuant to which the Company and SynBio have agreed to collaborate in the development of PolyXen® technology.
Under the Co-Development Agreement the Company has granted an exclusive licence to SynBio to develop pharmaceutical products using certain molecules and PolyXen® technology that prolongs the active life and improves the pharmacokinetics of therapeutic proteins and peptides (as well as conventional drugs) in the Russian market and the commonwealth of independent states (comprising Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyzstan, Republic of Moldova, Tajikistan, Turkmenistan, Ukraine and Uzbekistan. SynBio has granted an exclusive licence to the Company to use the pre-clinical and clinical data generated by SynBio in certain agreed products.
Relationship Agreement
On 28 November 2011 the Company entered into a relationship agreement with SynBio in order to maintain the independence of the Company from SynBio and the Company’s suitability as an AIM company. SynBio has agreed to ensure that the Company is capable of carrying on its business independently. For so long as SynBio holds not less than 40 per cent. of the voting rights of the Company, it shall be entitled to nominate two non-executive directors to be appointed to the Board. Igor Nikolaev has also been appointed by SynBio as an observer to the Xenetic Board but does not have any voting rights.
The above relationship agreement will remain in force until the Scheme becomes Effective. Xenetic’s intention is that Synbio and GSL will, conditional upon the Scheme becoming Effective, replace the above relationship agreement with a new directors’ appointment agreement governed by the laws of the State of Nevada on similar terms, to apply in relation to New GSL Shares held by Synbio and its connected persons from time to time after the Effective Date.
SynBio Warrant Instrument
On 4 August 2011 the Company entered into a warrant instrument pursuant to which SynBio received Warrants granting the right to subscribe for up to 11,080,000 Ordinary Shares at an exercise price of 33 pence per Ordinary Share. The warrants were issued at a nil premium and are exercisable at any time after January 2014 for three years thereafter. SynBio may exercise the warrants only to the extent the total number of Ordinary Shares held by it and any concert party following an exercise of the warrants do not exceed 50 per cent. of the then issued ordinary share capital of the Company. The warrants are not transferable.
| 89 |
As described in paragraph 7 of Part 2 (Explanatory Statement) of this document, the SynBio warrants are intended to be replaced by new and/or re-priced warrants to be issued in due course on terms to be agreed.
Synbio loan facilities
On 10 May 2011 SynBio granted an unsecured loan facility of US$1,100,000 (increased on 23 August 2011 to US$1,500,000 and on 24 October 2011 to US$1,700,000) to Xenetic for working capital purposes. The aggregate amount of principal and accrued interest (at 0.67 per cent. compounded per month) outstanding under such loans as at 20 November 2013 (being the latest practicable date prior to the publication of this document) was approximately US$681,042. As described in paragraph 7 of Part 2 (Explanatory Statement) of this document, the loan facilities is intended to be repaid by way of an issue of New GSL Shares in due course on terms to be agreed.
SIIL Master Agreement
On 4 August 2011 the Company entered into a master agreement with SIIL in order to amend and restate the terms of the arrangement between them. Under the terms of the master agreement SIIL surrendered up to 14 product candidates, the development rights for which were vested in SIIL under the terms of certain historical agreements, back to the Company. The consideration for such changes was the issue and allotment to SIIL of 9,000,000 Ordinary Shares. SIIL also agreed to subscribe for 2,500,000 Ordinary Shares for cash at 11p per Ordinary Share.
SIIL agreed not to offer, dispose of, directly or indirectly, such consideration shares and subscription shares for a period of 24 months from the date of receipt of such shares) and further agreed to prohibit such disposal, except through the Company’s brokers, for a further 24 months following the expiry of such period.
In addition, the master agreement: (a) adjusts the economic interest in sales, royalties and/or licensing income arising from sales of the ErepoXen® product in certain territories from 50:50 to 75:25 between the Company and SIIL and (b) adjusts the share of revenues between the parties in respect of the sales of the product in certain other territories
SIIL Warrant Instrument
On 4 August 2011 the Company entered into a SIIL warrant instrument pursuant to which SIIL received warrants granting the right to subscribe for up to (in aggregate) 7,500,000 Ordinary Shares from time to time during a period of twenty four months from execution of the warrant instrument at an average exercise price of 20 pence per Ordinary Share. The warrants are not transferable.
As described in paragraph 7 of Part 2 (Explanatory Statement) of this document, the SIIL warrants are intended to be replaced by new and/or re-priced warrants to be issued in due course on terms to be agreed.
Novotech Master Agreement
On 6 February 2013 the Company and Novotech (Australia) Pty Limited (“Novotech”) entered into an agreement pursuant to which the Company retained the services of Novotech to perform clinical research and related services in connection with certain clinical research projects of the Company.
Novotech Clinical Trial Agreement
Further to the Novotech Master Agreement, on 18 March 2013 the Company and Novotech entered into an agreement pursuant to which Novotech agreed to provide the Company with clinical services relating to phase II, open-label, multi-centre and sequential dose finding trial to assess the efficacy and safety of multiple doses of polysialylated human erythropoietin (PSA-EPO) in chronic kidney disease (‘CKD’) subjects who are not receiving dialysis nor receiving erythropoiesis-stimulating agents (‘ESA’).
Baxter Warrant Instrument
On 15 September 2010, in relation to an exclusive research, development and licence agreement with Baxter Healthcare SA (“Baxter”), the Company issued a warrant instrument (as subsequently
| 90 |
supplemented) pursuant to which Baxter received warrants granting the right to subscribe up to 14,338,430 Ordinary Shares at £0.0902 per Ordinary Share (namely an aggregate exercise price of US$2,000,000, based on the US$: GBP exchange rate agreed with Baxter of 1.5464). The warrants are exercisable at any time up to 30 June 2015 and are also exercisable within 60 days after a change of control of the Company. To the extent Baxter exercises its warrants in accordance with the warrant instrument, the terms of the Scheme and/or the amended Articles of Association would apply in relation to the exercise of such warrants.
Parcae Capital Agreement
On 14 February 2013, the Company entered into a consultancy agreement with Parcae Capital Corporation (“Parcae”) governed by the laws of the State of Mass., under which Parcae provides for a period of 24 months certain financial consultancy services to the Company in relation to its migration to the US by way of the Scheme, and in relation to US investor relations matters. Parcae is paid a monthly retainer of US$10,000 under the agreement, plus reasonable travel costs and expenses.
Parcae is also incentivised in line with the increase in the market value of GSL following completion of the Scheme. The incentive arrangements provide for fees to be payable up to a maximum of $396,970 (excluding the monthly retainer of US$10,000) should the market capitalisation (based on the value of the issued shares of GSL on Admission) thereafter achieve relevant hurdles between US$101 million and US$400 million within a 12 month period of the Scheme becoming Effective.
Parcae’s liability for non-performance under the agreement is limited to the lower of the cash compensation Parcae receives under the agreement, and the actual damage suffered by the Company as a result of such non-performance.
10.2 GSL Material Contracts
Paragraph 9 of Part VIII of the Equivalent Document the text of which is set out in Part 7 of this document contains details of those contracts, not being contracts entered into in the ordinary course of business, entered into by members of the GSL Group since the period beginning on 16 July 2011 (being two years before the commencement of the Offer Period) and which are or may be material.
11. Material change
Save as disclosed in this document, there has been no significant change in the financial or trading position of Xenetic since 30 September 2013 (the date on which the latest published interim Accounts was published).
Save as disclosed in this document and in the Equivalent Document, there has been no significant change in the financial or trading position of GSL since 31 May 2013 (the date to which the last published 10Q accounts of GSL were prepared).
12. Sources and bases of information
In this document, unless otherwise stated or the context otherwise requires, the following bases and sources have been used.
12.1 References to a percentage of Xenetic Shares are based on 407,875,428 issued Xenetic Shares as set out in the confirmation by Xenetic pursuant to Rule 2.10 of the Code contained in the Announcement.
12.2 References to a percentage of GSL Shares are based on 135,000,000 issued GSL Shares as set out in the confirmation by GSL pursuant to Rule 2.10 of the Code contained in the Announcement. GSL will also undertake a share consolidation in advance of the Scheme becoming Effective whereby every holder of GSL Shares will receive 1 New GSL Share for every 10 GSL Shares held on the consolidation date.
| 91 |
12.3 The Closing Prices of a Xenetic Share as set out in paragraph 5 of this Part 8 are sourced from Bloomberg and (in the case of GSL) from Nasdaq: www.nasdaq.com/symbol/gaif/interactivechart?timeframe=6m&charttype=line).
12.4 Unless otherwise stated, the financial information relating to Xenetic is extracted from the Annual Report and Accounts.
12.5 Unless otherwise stated, the financial information relating to GSL is extracted from GSL’s last published annual report and accounts as at 31 August 2012.
12.6 The terms of the Acquisition value the entire issued share capital of Xenetic at approximately £24.3 million, based on the estimated valuation of a GSL Consideration Share of 18.59 pence and the number of Xenetic Shares in issue of 407,875,428 on 11 November 2013 (being the Business Day prior to the date of the Announcement).
12.7 The maximum number of 130,520,137 GSL Consideration Shares that may be issued, assuming full approval of the Acquisition, is calculated by applying the offer ratio of 56 GSL Consideration Shares for every 175 Scheme Shares to the 407,875,428 Xenetic Shares in issue on 11 November 2013. This calculation takes no account of fractions or of any Xenetic Shares that may be issued after the date of the Announcement as a result of the exercise of options or warrants, but it does reflect the share consolidation in relation to GSL that will be effected in advance of the Scheme becoming Effective whereby every holder of GSL Shares will receive 1 New GSL Share for every 10 GSL Shares held on the consolidation date.
12.8 The percentage that the GSL Consideration Shares will represent as a proportion of the issued share capital of the Enlarged Group, of 97.39 per cent., is calculated by dividing 130,520,137 GSL Consideration Shares to be issued pursuant to the Scheme by the aggregate of: (i) the 135,000,000 GSL Shares in issue on 11 November 2013 (being the Business Day prior to the date of the Announcement) less the 100,000,000 GSL Shares currently held by Oxbridge that are proposed to be retired to treasury (as set out in paragraph 10.1 of this Part 8) both of which, being subject to the share consolidation (described in paragraph 12.7) will convert into 13,500,000 New GSL Shares and 10,000,000 New GSL Shares, respectively and (ii) the 130,520,137 GSL Consideration Shares.
12.9 For purposes of Part 12, closing share prices for Xenetic of 6.5p on 11 November 2013, of 5.625p on 20 October 2013 and of 5.625p on 15 July 2013 have been derived from Factset.
13. General
13.1 London Bridge Capital has given and has not withdrawn its written consent to the issue of this document with the inclusion herein of the references to its name in the form and context in which they appear and to the inclusion in this document of the letter relating to the Estimated Value of a GSL Consideration Share set out in Part 12.
13.2 N+1 Singer has given and has not withdrawn its written consent to the issue of this document with the inclusion herein of the references to its name in the form and context in which they appear.
13.3 GSL has given and has not withdrawn its written consent to the issue of this document with the inclusion herein of the references to its name in the form and context in which they appear.
13.4 All references to time in this document and the Forms of Proxy are to London time unless the context provides otherwise.
13.5 The International Securities Identification Number for Xenetic Shares is GB00B08NWV55. 13.6 The International Securities Identification Number for GSL Shares is US3707291056.
13.7 Settlement of the consideration to which each Scheme Shareholder is entitled under the Scheme will be implemented in full in accordance with the terms of the Scheme without regard to any lien or right of set-off, counterclaim or other analogous right to which GSL may otherwise be or claim to be, entitled against any such Scheme Shareholder.
| 92 |
13.8 The aggregate fees and expenses which are expected to be incurred by GSL in connection with the Acquisition are estimated to amount to US$47,500. This aggregate number consists of the following categories:
| (a) | financial and corporate broking advice: US$zero |
| (b) | legal advice: US$45,000 |
| (c) | accounting advice US$zero |
| (d) | public relations advice US$zero |
| (e) | other professional services US$zero |
| (f) | other costs and expenses: US$2,500 |
13.9 The aggregate fees and expenses expected to be incurred by Xenetic in connection with the Acquisition are estimated to amount to £1,300,000 including non-recoverable applicable VAT. This aggregate number consists of the following categories:
| (a) | financial and corporate broking advice: £224,000 |
| (b) | legal advice: £540,000 |
| (c) | accounting advice £485,000 |
| (d) | public relations advice £5,000 |
| (e) | other professional services £10,000 |
| (f) | other costs and expenses: £6,000 |
| (g) | non-recoverable VAT: £30,000 |
13.10 There is no agreement, arrangement or understanding whereby any of the New Shares to be issued to GSL pursuant to the Scheme will be transferred by GSL to any other person.
13.11 No agreement, arrangement or understanding (including compensation arrangement) exists between GSL or any person acting in concert with it and any of the directors, recent directors, shareholders or recent shareholders of Xenetic, or any person interested or recently interested in shares of Xenetic, having any connection with or dependence upon the Scheme or Acquisition.
14. Documents available for inspection
14.1 A copy of this document and the documents listed in paragraph 14 below are available free of charge on Xenetic’s website, www.xeneticbio.com/investorrelations until the Effective Date.
14.2 Copies of the following documents are available for inspection during normal business hours on any weekday (Saturdays, Sundays and public holidays excepted) at the Company’s corporate office at Greener House, 66-68 Haymarket, London SW1Y 4RF until the Effective Date:
| (a) | the Articles; |
| (b) | a draft of the Articles as proposed to be amended at the General Meeting; |
| (c) | the constitutional documents of GSL; |
| (d) | this document and the Equivalent Document; |
| (e) | the GSL Hive Out Agreement; |
| (d) | the audited consolidated financial statements of Xenetic for the financial years ended 31 December 2011 and 2012 and interim results for the period ended 30 June 2013; |
| (e) | the agreement with Parcae Capital referred to in paragraph 10 of this Part 8; |
| (f) | the letters of consent referred to in sub-paragraphs 13.1 to 13.3 above; |
| (g) | the Announcement; |
| (h) | the irrevocable undertakings referred to in paragraph 7 above of this Part 8; |
| 93 |
| (i) | this document and the Forms of Proxy; |
| (j) | the annual report and accounts for GSL for the financial year ended 31 August 2012; |
| (k) | the letter from London Bridge Capital relating to the Estimated Value of a New GSL Share included in Part 12; |
| (l) | the Xenetic 2000 Share Option Scheme (as amended); |
| (m) | the Xenetic 2007 Share Option Scheme (as amended); |
| (n) | the Joint Ownership Agreements between the JSOP Plan Trustee and each of Scott Maguire and Colin Hill; and |
| (o) | the draft forms of Replacement Share Option Agreement between GSL and holders of Xenetic Share Options relating to the grant of rights to acquire GSL Shares in exchange for rights to subscribe for Xenetic Shares granted under the Xenetic Share Option Plans. |
Dated: 21 November 2013
| 94 |
PART 9
DEFINITIONS
In this document (with the exception of Part 4 (The Scheme of Arrangement), Part 5 (Financial information relating to the Xenetic Group), Part 10 (Notice of Court Meeting) and Part 11 (Notice of General Meeting)), the following words and expressions have the following meanings, unless the context requires otherwise:
| “Acquisition” | the recommended acquisition of the entire issued and to be issued ordinary share capital of Xenetic by GSL to be implemented by way of the Scheme on the terms and subject to the Conditions set out in this document and any subsequent revision, variation, extension or renewal thereof;
|
| “Admission” | the quotation of the GSL Consideration Shares on the OTCBB and OTCQB;
|
| “AIM” | AIM, the market of that name operated by the London Stock Exchange;
|
| “AIM Rules” | the ‘AIM Rules for Companies’ published by the London Stock Exchange, as amended from time to time;
|
| “Announcement” | the announcement made by the Company on 12 November 2013 through a Regulatory Information Service relating to the Acquisition pursuant to and in accordance with Rule 2.7 of the Code;
|
| “Articles” | the articles of association of the Company from time to time;
|
| “Board” | the Directors of the Company as at the date of this document;
|
| “Business Day” | a day, other than a Saturday, Sunday or public holiday, on which commercial banks are open for business in the City of London, United Kingdom, and are not authorised or required by law to remain closed in New York, New York, United States;
|
| “Closing Price” | the middle market closing price of a Xenetic Share as derived from the Daily Official List;
|
| “Code” or “City Code” | the United Kingdom City Code on Takeovers and Mergers;
|
| “Companies Act” | the Companies Act 2006 (as amended);
|
| “Company” or “Xenetic” | Xenetic Biosciences plc, incorporated in England and Wales with company registration number 03213174;
|
| “the Company’s Guernsey Purpose Trust” | the trust established by the Company in 2010 for the purpose of acting as co-owner of jointly-owned Xenetic Shares pursuant to the Xenetic JSOP;
|
| “Conditions” | the conditions of the Acquisition set out in section A of Part 3 of this document;
|
| “Court” | the High Court of Justice in England and Wales;
|
| “Court Hearing” | the hearing of the Court at which the Court Order is made;
|
| “Court Meeting” | the meeting of the Scheme Shareholders convened by order of the Court pursuant to Part 26 of the Companies Act to be held at 10.00 a.m. GMT on 17 December 2013 to consider and, if thought fit, approve the Scheme, notice of which is set out in Part 10 of this document (including any adjournment thereof);
|
| 95 |
| “Court Order” | the order of the Court sanctioning the Scheme under Part 26 of the Companies Act and confirming the reduction of the Company’s capital by the cancellation of the Scheme Shares provided by the Scheme under section 648 of the Companies Act;
|
| “CREST” | the relevant system (as defined in the Uncertificated Securities Regulations) of which Euroclear is the Operator (as defined in the Uncertificated Securities Regulations);
|
| “Daily Official List” | the Daily Official List of the London Stock Exchange;
|
| “Directors” or “Xenetic Directors” | the directors of Xenetic (save as the context may require Roman Knyazev);
|
| “Effective” | the Scheme having become effective pursuant to its terms;
|
| “Effective Date” | the day on which the Scheme becomes effective in accordance with clause 5 of the Scheme;
|
| “Enlarged Group” | the GSL Group as enlarged by the Acquisition;
|
| “Equivalent Document” | a document equivalent to a prospectus published by GSL and for which GSL is responsible, the text of which is provided for convenience in Part 7 of this document (pages 55-78);
|
| “Euroclear” | Euroclear UK & Ireland Limited;
|
| “Exchange” | any recognised US stock exchange, including (but not limited to) NASDAQ, that has registered with the SEC under section 6 of the US Exchange Act;
|
| “Explanatory Statement” | the explanatory statement relating to the Acquisition proposal, asset out in Part 2 of this document, which together with the documents incorporated therein, constitutes the explanatory statement as required by section 897 of the Companies Act;
|
| “FDA” | the Food and Drug Administration, an agency of the Department of Health and Human Services of the US;
|
| “FDS Pharma” | the FDS Pharma Associates Limited Partnership (UK);
|
| “Financial Conduct Authority” | the Financial Conduct Authority of the UK;
|
| “Form of Proxy” | as the context may require, either or both of (i) the blue Form of Proxy for use at the Court Meeting, and (ii) the white Form of Proxy for use at the General Meeting, each of which accompanies this document;
|
| “FSMA” | the Financial Services and Markets Act 2000 (as amended);
|
| “General Meeting” | the general meeting of the Shareholders to be held at 10.30 a.m. GMT on 17 December 2013 (or as soon thereafter as the Court Meeting shall have been concluded), notice of which is set out in Part 11 of this document (including any adjournment thereof);
|
| “GMT” | Greenwich Mean Time;
|
| “Group” or “Xenetic Group” | the Company and its subsidiaries;
|
| “GSL” | General Sales & Leasing Inc., a corporation incorporated under the laws of the State of Nevada with its principal executive office at 16445 North 91st Street, Suite 103, Scottsdale Arizona 85260 and SEC CIK#0001534525;
|
| “GSL Consideration Shares” | up to an expected maximum of 130,520,137 New GSL Shares to be issued credited as fully paid to Scheme Shareholders pursuant to the Scheme and the Equivalent Document;
|
| 96 |
| “GSL Director” | the current director of GSL;
|
| “GSL Group” | GSL and its subsidiaries;
|
| “GSL Hive Out Agreement” | the Agreement of Conveyance, Transfer and Assignment of Subsidiaries and Assumption of Obligations in respect of the existing business of GSL;
|
| “GSL Shares” | shares of common stock in the capital of GSL with a nominal value of US$0.001 per share;
|
| “IFRS” | International Financial Reporting Standards as adopted by the European Union as applied in accordance with the provisions of the Companies Act;
|
| “Joint Ownership Agreement” (or “JOA”) | an agreement relating to the acquisition, holding, and disposal of Xenetic Shares made between the JSOP Plan Trustee and a participant pursuant to and in accordance with the Xenetic JSOP;
|
| “the JSOP Plan Trustee” | RBC cees Trustee Guernsey Limited as trustee of the Company’s Guernsey Purpose Trust;
|
| “Listing Rules” | the rules and regulations made by the Financial Conduct Authority in its capacity as the UK Listing Authority under the financial Services and Markets Act 2000 and contained in the UK Listing Authority’s publication of the same name;
|
| “London Bridge Capital” | London Bridge Capital Limited, financial adviser to Xenetic;
|
| “London Stock Exchange” | London Stock Exchange plc;
|
| “Meetings” | the Court Meeting and the General Meeting (and “Meeting” means either of them);
|
| “Merger Ratio” | for every 175 Scheme Shares, 56 GSL Consideration Shares;
|
| “N+1 Singer” | N+1 Singer Advisory LLP, together with its affiliates, acting as the Company’s nominated adviser and broker;
|
| “NASDAQ” | the NASDAQ Global Select Market, The NASDAQ Global Market or the NASDAQ Capital Market, operated by The NASDAQ Stock Market LLC;
|
| “New GSL Shares” | new GSL shares of common stock with a nominal value of US$0.01 proposed to be issued and credited as fully paid pursuant to the share consolidation of GSL before the Scheme becomes Effective;
|
| “New Shares” | the new ordinary shares of 0.5p each in the capital of Xenetic to be issued and credited as fully paid to GSL pursuant to the Scheme;
|
| “Notice of General Meeting” | the notice of General Meeting set out in Part 11 of this document;
|
| “Offer Period” | the offer period (as defined in the City Code) relating to Xenetic which commenced on 16 July 2013;
|
| “OTCBB” | the OTC Bulletin Board, operated by the Financial Industry Regulatory Authority, Inc.;
|
| “OTCQB” | the OTCQB marketplace, operated by OTC Markets Group, Inc.;
|
| 97 |
| “Overseas Shareholders” | Shareholders whose registered addresses are outside the UK or who are citizens or residents of countries other than the UK and “Overseas Jurisdiction” shall be construed accordingly;
|
| “Pounds” or “£” or “sterling” | UK pounds sterling, the lawful currency of the UK;
|
| “Proposed Directors” | the proposed new directors of GSL (being Scott Maguire, Dmitry Genkin, Artur Isaev and Firdaus Dastoor) who will assume office upon the Scheme becoming Effective;
|
| “Reduction of Capital” | the reduction of the share capital of the Company associated with the cancellation and extinguishing of the Scheme Shares provided for in clause 1.1 of the Scheme under section 648 of the Companies Act;
|
| “Registrar of Companies” | the Registrar of Companies in England and Wales;
|
| “Regulatory Information Service” | any of the services set out in schedule 12 of the Listing Rules; |
| “Scheme” or “Scheme of Arrangement” | the scheme of arrangement proposed to be made under Part 26 of the Companies Act between the Company and the holders of Scheme Shares as set out in Part 4 of this document, with or subject to any modification, addition or condition approved or imposed by the Court and agreed to by the Company and GSL and incorporating a reduction of capital under section 648 of the Companies Act;
|
| “Scheme Record Time” | 6.00 p.m. GMT on the business day prior to the Court Hearing;
|
| “Scheme Resolutions” | the resolution to be proposed at the Court Meeting and the Special Resolution to be proposed at the General Meeting, in both cases to approve and give effect to the Scheme;
|
| “Scheme Shareholders” | the holders of Scheme Shares;
|
| “Scheme Shares” | (i) the Shares in issue at the date of this document;
(ii) any Shares issued after the date of this document and before the Voting Record Time; and
(iii) any Shares issued at or after the Voting Record Time but on or before the Scheme Record Time in respect of which the original or any subsequent holders thereof are, or shall have agreed in writing to be, bound by the Scheme, in each case other than any Shares which are registered in the name of or beneficially owned by GSL or any member of the GSL Group;
|
| “SEC” | the United States Securities and Exchange Commission;
|
| “Shareholders” or “Xenetic Shareholders” | the holders of Shares;
|
| “Shares” or “Xenetic Shares” | ordinary shares of 0.5 pence each in the capital of the Company;
|
| “Share Options” | rights to subscribe for Xenetic Shares granted pursuant to the Xenetic Share Schemes;
|
| “SIIL” or “Serum Institute of India” | the Serum Institute of India Limited, a company incorporated under the laws of India having its principal place of business at S.No. 212/2, Off Sol; Poonawalla Road, Hadapsar, Pune-411 028, Maharashtra, India;
|
| 98 |
| “Special Resolution” | the special resolution set out in the Notice of General Meeting to be proposed at the General Meeting to approve, amongst other things, the Scheme and the Reduction of Capital;
|
“subsidiary” and “subsidiary undertaking”
|
have the meanings given by the Companies Act; |
| “ SymbioTec” | SymbioTec Gesellschaft zur Forschung und Entwicklung auf dem Gebiet der Biotechnologie GmbH; |
| “SynBio” | SynBio LLC, a limited liability company incorporated under the laws of the Russian Federation, Main State Registration Number 1117746126321, having its registered office at building 2, 55/1, Leninsky Prospekt, Moscow, Russian Federation;
|
| “Takeover Offer” | a takeover offer as that term is defined in section 974 of the Companies Act;
|
| “Takeover Panel” or “Panel” | the United Kingdom Panel on Takeovers and Mergers;
|
| “Uncertificated Securities Regulations” | the Uncertificated Securities Regulations 2001 (s1 2001 No. 3755) as (amended);
|
| “United Kingdom” or “UK” | the United Kingdom of Great Britain and Northern Ireland;
|
| “United States” or “US” | the United States of America;
|
| “US Exchange Act” | the United States Securities Exchange Act of 1934, as amended, and rules and regulations thereunder;
|
| “US$” or “$” | United States dollars, the lawful currency of the United States;
|
| “Voting Record Time” | 6.00 p.m. GMT on the day which is two working days before the date of the Court Meeting or, if such Court Meeting is adjourned, 6.00 p.m. GMT on the day which is two working days before the day of such adjourned meeting;
|
| “Xenetic 2000 Share Option Plan” | the Company’s Unapproved Share Option Plan established on 18 July 2000 (as amended);
|
| “Xenetic 2007 Share Option Plan” | the Company’s 2007 Share Option Scheme established on 9 August 2007 (as amended in 2010 and 2012);
|
| “Xenetic Group” | Xenetic and its subsidiaries;
|
| “the Xenetic JSOP” | the Company’s Executive Shared Ownership Plan approved and established by shareholders of the Company on 23 July 2009;
|
| “Xenetic Option Holder” | an individual who holds options granted pursuant to the Xenetic Share Schemes; and
|
| “Xenetic Share Option Plans” | the Xenetic 2000 Share Option Plan and the Xenetic 2007 Share Option Plan.
|
In this document and the Forms of Proxy, references to the singular includes the plural and vice versa, unless the context otherwise requires.
| 99 |
PART 10
NOTICE OF COURT MEETING
IN THE HIGH COURT OF JUSTICE CHANCERY DIVISION COMPANIES COURT REGISTRAR DERRETT |
No. 7321 of 2013 |
IN THE MATTER OF XENETIC BIOSCIENCES PLC
and
IN THE MATTER OF THE COMPANIES ACT 2006
NOTICE IS HEREBY GIVEN that by an order dated 21 November 2013 made in the above matters the Court has given permission for a meeting (the “Court Meeting”) to be convened of the holders of Scheme Shares (as defined in the Scheme of Arrangement referred to below), for the purpose of considering and, if thought fit, approving (with or without modification) a scheme of arrangement (the “Scheme of Arrangement”) proposed to be made between Xenetic Biosciences PLC (the “Company”) and the Scheme Shareholders (as defined in the Scheme of Arrangement) and that such meeting will be held at the offices of Pinsent Masons LLP, 30 Crown Place, London EC2A 4ES United Kingdom, on 17 December 2013 at 10.00 a.m. Greenwich Mean Time (“GMT”), at which place and time all holders of the Scheme Shares (as defined in the Scheme of Arrangement) are requested to attend.
A copy of the Scheme of Arrangement and a copy of the explanatory statement required to be furnished pursuant to section 897 of the Companies Act 2006 are incorporated in the document of which this notice forms part.
At the Court Meeting, the following resolution will be proposed:
“That the scheme of arrangement dated 21 November 2013 (the “Scheme”), between the Company and the Scheme Shareholders (as defined in the Scheme), a print of which has been produced to this meeting and, for the purposes of identification, signed by the chairman hereof in its original form or with or subject to any modification, addition or condition approved or imposed by the Court, be approved and the directors of the Company be authorised to take all such actions as they consider necessary or appropriate for carrying the Scheme into effect.”
Scheme Shareholders entitled to attend and vote at the meeting may vote in person at the Court Meeting or they may appoint another person, whether a member of the Company or not, as their proxy to attend and vote in their stead. A blue Form of Proxy for use at the Court Meeting is enclosed with this notice. Further details are set out below. Completion of the blue Form of Proxy will not prevent a Scheme Shareholder from attending and voting at the said meeting, or any adjournment thereof, in person if he wishes to do so.
Scheme Shareholders who hold Scheme Shares through CREST and who wish to appoint a proxy or proxies through the CREST Electronic Proxy Service may do so for the Court Meeting and any adjournment(s) thereof by using the procedures described in the CREST Manual, which can be viewed at www.euroclear.com/CREST. CREST personal members or other CREST sponsored members, and those CREST members who have appointed a voting service provider, should refer to their CREST sponsor or voting service provider, who will be able to take the appropriate action on their behalf.
In order for a proxy appointment or instruction made using the CREST service to be valid, the appropriate CREST message (a “CREST Proxy Voting Instruction”) must be properly
| 100 |
authenticated in accordance with the specifications of Euroclear UK & Ireland Limited (“Euroclear”) and must contain the information required for such instructions, as described in the CREST Manual. The message, regardless of whether it relates to the appointment of a proxy or to an amendment to the instruction given to a previously appointed proxy, must, in order to be valid, be transmitted so as to be received by Share Registrars Limited (participant ID 7RA36) by no later than 6.00 p.m. on 13 December 2013 (or, in the case of an adjourned meeting, no later than 6.00 p.m. on the day two days before the day of the adjourned meeting (excluding any date which is not a working day)). For this purpose, the time of receipt will be taken as the time (as determined by the time stamp applied to the message by the CREST Applications Host) from which Share Registrars Limited are able to retrieve the message by enquiry to CREST in the manner prescribed by CREST. After this time, any change of instructions to proxies appointed through CREST should be communicated to the appointee through other means.
CREST members and, where applicable, their CREST sponsor or voting service provider, should note that Euroclear does not make available special procedures in CREST for any particular messages. Normal system timings and limitations will therefore apply in relation to the input of CREST Proxy Voting instructions. It is the responsibility of the CREST member concerned to take (or, if the CREST member is a CREST personal member or sponsored member or has appointed a voting service provider, to procure that his or her CREST sponsor or voting service provider takes) such action as shall be necessary to ensure that a message is transmitted by means of the CREST system by any particular time. In connection with this, CREST members and, where applicable, their CREST sponsor or voting service provider are referred, in particular, to those sections of the CREST Manual concerning practical limitations of the CREST system and timings.
The Company may treat as invalid a CREST Proxy Voting Instruction in the circumstances set out in regulation 35(5)(a) of the Uncertificated Securities Regulations 2001.
Scheme Shareholders are entitled to appoint a proxy in respect of some or all of their Scheme Shares. Scheme Shareholders are also entitled to appoint more than one proxy. A space has been included in the blue Form of Proxy to allow Scheme Shareholders to specify the number of Scheme Shares in respect of which that proxy is appointed. Scheme Shareholders who return the blue Form of Proxy duly executed but leave this space blank will be deemed to have appointed the proxy in respect of all their Scheme Shares.
Scheme Shareholders who wish to appoint more than one proxy in respect of their shareholding should contact Share Registrars Limited for further blue Forms of Proxy. Such Scheme Shareholders should also read the notes to the Notice of the General Meeting set out in Part 11 of the document of which this notice forms part and note the principles that will be applied in relation to multiple proxies.
In the case of joint holders of Scheme Shares, the vote of the senior who tenders a vote, whether in person or by proxy, will be accepted to the exclusion of the vote(s) of the other joint holder(s) and for this purpose, seniority will be determined by the order in which the names stand in the register of members of the Company in respect of the joint holding.
Entitlement to attend and vote at the meeting or any adjournment thereof and the number of votes which may be cast thereat will be determined by reference to the register of members of the Company at 6.00 p.m. GMT on the day which is two working days before the date of the meeting or adjourned meeting (as the case may be). In each case, changes to the register of members of the Company after such time shall be disregarded.
It is requested that the blue Form of Proxy (together with any power of attorney or other authority under which it is signed, or a notarially certified copy of such power of authority) be lodged by post with the Company’s registrar, Share Registrars Limited, Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL or by hand (during normal business hours) or by fax to Share Registrars Limited on +44 (0) 1252 719232 or by scan and email to Share Registrars Limited at proxies@shareregistrars.uk.com at the same address not less than 48 hours before the time appointed for the said meeting but if forms are not so lodged, they may be handed to the Chairman before the start of the meeting.
| 101 |
Scheme Shareholders should note that if they wish to appoint more than one proxy they should request additional blue Forms of Proxy from the Company’s registrars, Share Registrars Limited and submit them in accordance with the instructions set out in the preceding paragraphs.
By the said order, the Court has appointed Sir Brian Richards, or, failing him, Colin Hill, or, failing him, Scott Maguire of Xenetic Biosciences plc each of London Bioscience Innovation Centre, 2 Royal College Street, London NW1 0NH, to act as chairman of the Court Meeting and has directed the chairman to report the result thereof to the Court.
The Scheme of Arrangement will be subject to the subsequent sanction of the Court. Dated: 21 November 2013
Pinsent Masons LLP 30 Crown Place London EC2A 4ES
Solicitors for the Company
| 102 |
PART 11
NOTICE OF GENERAL MEETING
XENETIC BIOSCIENCES PLC
(the “Company”)
(Registered in England and Wales with company registration number 03213174)
NOTICE IS HEREBY GIVEN that a GENERAL MEETING of the Company will be held at the offices of Pinsent Masons LLP, 30 Crown Place, London EC2A 4ES United Kingdom at 10.30 a.m. Greenwich Mean Time (“GMT”) on 17 December 2013 (or as soon thereafter as the Court Meeting (as defined in the document of which this notice forms part) convened for 10.00 a.m. GMT on the same day and at the same place, by an order of the High Court of Justice in England & Wales (the “Court”), shall have concluded or been adjourned) for the purpose of considering and, if thought fit, passing the following resolution which will be proposed as a special resolution:
SPECIAL RESOLUTIONS
1. THAT for the purpose of giving effect to the scheme of arrangement dated 21 November 2013 (in its original form or with or subject to any modification, addition or condition agreed between the Company and GSL and approved or imposed by the Court (the “Scheme”)) proposed to be made between the Company and the Scheme Shareholders (as defined in the Scheme), a print of which has been produced to the meeting and (for the purpose of identification only) signed by the Chairman thereof:
(a) the Directors of the Company be authorised to take all such actions as they may consider necessary or appropriate for carrying the Scheme into effect;
(b) the share capital of the Company be reduced by cancelling and extinguishing all the Scheme Shares (as defined in the Scheme);
(c) subject to and forthwith upon the reduction of share capital referred to in paragraph (b) of this resolution taking effect and notwithstanding anything to the contrary in the articles of association of the Company:
| (i) | the Company create such number of New Shares (as defined in the Scheme) as shall be equal to the number of Scheme Shares cancelled as aforesaid; |
| (ii) | the reserve arising in the books of account of the Company as a result of such reduction of capital taking effect shall be capitalised and applied in paying up in full at par all of the New Shares so created, which shall be allotted and issued, credited as fully paid, to GSL and/or its nominee(s); |
| (iii) | the Directors of the Company be generally and unconditionally authorised for the purposes of section 551 of the Companies Act 2006 to allot the New Shares referred to in paragraph (c)(i) of this resolution, provided that: (1) the maximum aggregate nominal amount of the shares which may be allotted under this authority shall be the aggregate nominal amount of the New Shares created pursuant to paragraph (c)(i) of this resolution, (2) this authority shall expire on the fifth anniversary of the date of this resolution and (3) this authority shall be in addition to and without prejudice to any other authority under the said section 551 of the Companies Act 2006 previously granted and in force on the date on which this resolution is passed; and |
(d) with effect from the passing of this resolution, the articles of association of the Company be amended by the adoption and inclusion of the following new article 180:
“SCHEME OF ARRANGEMENT
180.1 In this article, the “Scheme” means the scheme of arrangement dated 21 November 2013, between the Company and the holders of its Scheme Shares (as defined in the Scheme) under Part 26 of the Companies Act 2006 in its original form or with or subject to any modification, addition or condition approved or imposed by the Court and/or agreed by the Company and General Sales & Leasing, Inc. (“GSL”) and (save as defined in this article) expressions defined in the Scheme shall have the same meanings in this article.
| 103 |
180.2 Notwithstanding any other provision of these articles, if the Company issues any shares (other than to GSL or its nominee(s)) after the adoption of this article and before the Scheme Record Time, such shares shall be issued subject to the terms of the Scheme and shall be Scheme Shares for the purposes thereof and the holders of such shares, and any subsequent holder of such shares (other than GSL and/or its nominee or nominees) shall be bound by the Scheme accordingly.
180.3 Subject to the Scheme becoming Effective, if any shares are issued to any person (a “New Member”) (or transferred to any subsequent holder or any nominee of such New Member or any subsequent holder) (other than under the Scheme or to GSL or its nominee(s)) after the Scheme Record Time (the “Transfer Shares”), they shall (on the Effective Date or, if later, on issue) be immediately transferred to GSL (or as it may direct) in consideration of the issue by GSL to the New Member (or to any transferee if such shares have been so transferred to any subsequent holder or any nominee of such New Member or any subsequent holder) of such number of shares in GSL for each share equal to the consideration per Scheme Share issuable pursuant to the Scheme as if each such share had been a Scheme Share.
180.4 On any reorganisation of, or material alternation to, the share capital of the Company (including, without limitation, any sub-division and/or consolidation), the value of the consideration per share to be paid under paragraph 180.3 of this article shall be adjusted by the Directors in such manner as the Company’s auditors may determine to be appropriate to reflect such reorganisation or alteration. References in this article to shares shall, following such adjustment, be construed accordingly.
180.5 To give effect to any transfer required by paragraph 180.3 above, the Company may appoint any person as attorney for the New Member (or any subsequent holder or any nominee of such New Member or any such subsequent holder) to transfer the Transfer Shares to GSL and/or its nominee(s) and do all such other things and execute and deliver all such documents as may in the opinion of the attorney be necessary or desirable to vest the Transfer Shares in GSL and/or its nominee(s) and pending such vesting, to exercise all such rights attaching to the Transfer Shares as GSL may direct. If any attorney is so appointed, the New Member (or any subsequent holder or any nominee of such New Member or any such subsequent holder) shall not thereafter (except to the extent that the attorney fails to act in accordance with the directions of GSL) be entitled to exercise any rights attaching to the Transfer Shares unless so agreed by GSL. The attorney shall be empowered to execute and deliver as transferor a form of transfer or instructions of transfer on behalf of the New Member (or any subsequent holder or any nominee of such New Member or any such subsequent holder) in favour of GSL and/or its nominee(s) and the Company may give good receipt for the consideration for the Transfer Shares and may register GSL and/or its nominee(s) as holder thereof and issue to it certificates for the same. The Company shall not be obliged to issue a certificate to the New Member (or any subsequent holder or any nominee of such New Member or any such subsequent holder) for the Transfer Shares. GSL shall issue the relevant shares in GSL by such date as GSL will agree with the Company and in any event within 10 business days of the issue of the Transfer Shares to the New Member.
180.6 If the Scheme shall not have become Effective by the date referred to in clause 5.2 of the Scheme, this article 180 shall not be effective.
Dated: 21 November 2013
| Registered Office | BY ORDER OF THE BOARD |
| London Bioscience Innovation Centre | Colin Hill |
2 Royal College Street London NW1 0NH |
Director |
Corporate Office Greener House 66-68 Haymarket London SW1Y4RF |
| 104 |
NOTES TO NOTICE:
Entitlement to attend and vote
1. Only those members registered on the Company’s register of members at: (a) 6.00 p.m. GMT on 13 December 2013; or,
| (b) | if this General Meeting is adjourned, at 6.00 p.m. GMT on the day two working days prior to the adjourned General Meeting, |
shall be entitled to attend and vote at the General Meeting.
Website giving information regarding the General Meeting
2. Information regarding the General Meeting is available on the Company’s website, www.xeneticbio.com/investorrelations.
Attending in person
3. If you wish to attend the General Meeting in person, you may be asked for your name and address to confirm your identity.
Appointment of proxies
4. If you are a member of the Company at the time set out in Note 1 above, you are entitled to appoint a proxy to exercise all or any of your rights to attend, speak and vote at the General Meeting and you should have received a white Form of Proxy. You can only appoint a proxy using the procedures set out in these Notes and the notes to the white Form of Proxy.
5. A proxy does not need to be a member of the Company but must attend the General Meeting to represent you. Details of how to appoint the Chairman of the General Meeting or another person as your proxy using the white Form of Proxy are set out in the notes to the white Form of Proxy. If you wish your proxy to speak on your behalf at the General Meeting you will need to appoint your own choice of proxy (not the Chairman) and give your instructions directly to them.
6. You may appoint more than one proxy provided each proxy is appointed to exercise rights attached to different shares. You may not appoint more than one proxy to exercise rights attached to any one share. To appoint more than one proxy, one or more additional white Forms of Proxy may be obtained by contacting Share Registrars Limited on +44 (0) 1252 821390 or you may photocopy your white Form of Proxy. Please follow the instructions in the explanatory notes to the Form of Proxy in relation to the appointment of more than one proxy.
7. If no voting indication is given, your proxy will vote or abstain from voting at his or her discretion. Your
proxy will vote (or abstain from voting) as he or she thinks fit in relation to any other matter which is put before the General Meeting.
Appointment of proxy using hard copy white Form of Proxy
8. The notes to the white Form of Proxy explain how to direct your proxy to vote on the Resolution or withhold their vote.
To appoint a proxy using the white Form of Proxy, the form must be:
| ● | completed and signed; |
| ● | sent or delivered to Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL (including by fax to Share Registrars Limited on +44 (0) 1252 719232 or by scan and email to Share Registrars Limited at proxies@shareregistrars.uk.com); and |
| 105 |
| ● | received by Share Registrars Limited no later than 10.30 a.m. GMT on 15 December 2013 (or, if the General Meeting is adjourned, no later than 48 hours before the time of the adjourned General Meeting). |
In the case of a member which is a company, the white Form of Proxy must be executed under its common seal or signed on its behalf by an officer of the company or an attorney for the company. Any power of attorney or any other authority under which the white Form of Proxy is signed (or a duly certified copy of such power or authority) must accompany the white Form of Proxy.
A pre-addressed envelope has been included for use in returning your proxy form. Please note that postage has not been paid for non-UK shareholders.
| 9. | CREST members who wish to appoint a proxy or proxies through the CREST electronic proxy appointment service may do so by using the procedures described in the CREST Manual, which can be viewed electronically at www.euroclear.com/CREST. CREST Personal Members or other CREST sponsored members, and those CREST members who have appointed a service provider(s), should refer to their CREST sponsor or voting service provider(s), who will be able to take the appropriate action on their behalf. |
| 10. | To appoint a proxy or to give or amend an instruction to a previously appointed proxy via the CREST system, the CREST message (a ‘CREST Proxy Voting Instruction’) must be properly authenticated with CRESTCo’s specifications, and must contain the information required for such instruction, as described in the CREST Manual. This message, regardless of whether it constitutes the appointment of a proxy or is an amendment to the instruction given to a previously appointed proxy must, in order to be valid, be transmitted so as to be received by the issuer’s agent (CREST Participant ID 7RA36) by 6.00 p.m. on 13 December 2013. For this purpose the time of receipt will be taken to be the time (as determined by the timestamp applied to the message by the CREST Applications Host) from which the issuer’s agent is able to retrieve the message by enquiry to CREST in the manner prescribed by CREST. After this time any change of instructions to proxies appointed through CREST should be communicated to the appointee through other means. For further information on CREST procedures, limitations and system timings please refer to the CREST Manual. |
CREST members and, where applicable, their CREST sponsor or voting service provider, should note that Euroclear does not make available special procedures in CREST for any particular messages. Normal system timings and limitations will therefore apply in relation to the input of CREST Proxy Voting Instructions. It is the responsibility of the CREST member concerned to take (or, if the CREST member is a CREST personal member or sponsored member or has appointed a voting service provider, to procure that his or her CREST sponsor or voting service provider takes) such action as shall be necessary to ensure that a message is transmitted by means of the CREST system by any particular time. In connection with this, CREST members and, where applicable, their CREST sponsor or voting service provider are referred, in particular, to those sections of the CREST Manual concerning practical limitations of the CREST system and timings.
| 11. | The Company may treat as invalid a CREST Proxy Instruction in the circumstances set out in Regulation 35(5)(a) of the Uncertificated Securities Regulations. In any case a proxy form must be received by the Company’s Registrars no later than 6.00 p.m. on 13 December 2013. |
Joint holders
| 12. | In the case of joint holders, where more than one of the joint holders purports to appoint a proxy, only the appointment submitted by the most senior holder will be accepted. Seniority is determined by the order in which the names of the joint holders appear in the Company’s register of members in respect of the joint holding (the first-named being the most senior). |
Changing proxy instructions
| 13. | To change your proxy instructions simply submit a new proxy appointment using the methods set out above. Note that the cut-off time for receipt of proxy appointments (see above) also applies in relation to amended instructions; any amended proxy appointment received after the relevant cut-off time will be disregarded. Where you have appointed a proxy using the hard-copy white Form of Proxy and |
| 106 |
would like to change the instructions using another hard-copy white Form of Proxy, please contact Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL (including by fax to Share Registrars Limited on +44 (0) 1252 719232 or by scan and email to Share Registrars Limited at proxies@shareregistrars.uk.com). If you submit more than one valid proxy appointment, the appointment received last before the latest time for the receipt of proxies will take precedence.
Termination of proxy appointments
| 14. | In order to revoke a proxy instruction you will need to inform the Company by sending a signed hard copy notice clearly stating your intention to revoke your proxy appointment to Share Registrars Limited of Suite E, First Floor, 9 Lion & Lamb Yard, Farnham, Surrey GU9 7LL. In the case of a member which is a company, the revocation notice must be executed under its common seal or signed on its behalf by an officer of the company or an attorney for the company. Any power of attorney or any other authority under which the revocation notice is signed (or a duly certified copy of such power or authority) must be included with the revocation notice. The revocation notice must be received by Share Registrars Limited no later than 10.30 a.m. on 15 December 2013 (or, if the General Meeting is adjourned, no later than 48 hours before the time of the adjourned General Meeting). If you attempt to revoke your proxy appointment but the revocation is received after the time specified, then, subject to the paragraph directly below, your proxy appointment will remain valid. |
Appointment of a proxy does not preclude you from attending the General Meeting and voting in person. If you have appointed a proxy and attend the General Meeting in person, your proxy appointment will automatically be terminated.
Corporate Representatives
| 15. | A corporation which is a member can appoint one or more corporate representatives who may exercise, on its behalf, all its powers as a member provided that no more than one corporate representative exercises power over the same share. |
Issued ordinary shares and total voting rights
| 16. | As at 20 November 2013 (being the latest practicable date prior to the publication of this document), the Company’s issued ordinary share capital comprised 407,875,428 ordinary shares of 0.5 pence each. Each ordinary share carries the right to one vote at a general meeting of the Company and, therefore, the total number of voting rights in the Company as at 20 November 2013 is 407,875,428. |
| 107 |
PART 12
RULE 24.11 VALUATION LETTER: ESTIMATE OF VALUE OF GSL CONSIDERATION SHARES The following is the full text of a letter from London Bridge Capital Limited to the Xenetic Directors:
The Directors
Xenetic Biosciences PLC
Greener House 66-68 Haymarket
London
SW1Y 4RF
12 November 2013
Recommended Acquisition of Xenetic Biosciences PLC by General Sales & Leasing, Inc. Estimate of Value of GSL Consideration Shares
Dear Sirs,
Pursuant to the requirements of the Code, you have requested our opinion as to the estimated value of the GSL Consideration Shares receivable in respect of Scheme Shares (the “Estimate of Value”). Under the terms of the Scheme, for each 175 Scheme Shares held, Xenetic Shareholders will be entitled to receive 56 GSL Consideration Shares.
Although the GSL Shares are currently tradable on the OTCBB, there has been little trading in such shares and therefore they are not considered to have a public valuation. Whilst it is expected that the GSL Consideration Shares will be tradable on the OTCBB following the completion of the Acquisition, and that the Scheme Shares will cease to be traded on AIM, it is not proposed that the GSL Consideration Shares will be admitted to trading on any recognised stock exchange or other market after the Effective Date for some time, although it is the stated aim of the Proposed Directors and of Xenetic to seek such a listing at the earliest practicable time.
Capitalised terms used in this letter will, unless otherwise stated, have the same meaning given to them in the Scheme Document.
Purpose
This Estimate of Value has been provided to the Xenetic Directors solely for the purposes of Rule 24.11 of the City Code and shall not be used or relied upon for any other purpose whatsoever. It is not addressed to and may not be used or relied upon by any third party for any purpose whatsoever and London Bridge Capital Limited (“London Bridge Capital”) expressly disclaim any duty or liability to any third party with respect to the contents of this letter.
This letter sets out our opinion as to the Estimate of Value on the basis calculated below as at the date of this letter. We have assumed for this purpose that, as at the date of this letter, the Scheme had become effective in accordance with its terms, so that GSL had full control of Xenetic, that the GSL Consideration Shares had been issued, and that the entire existing business of GSL will have been transferred to Oxbridge Technology Partners, SA.
This Estimate of Value does not represent the value that a holder of GSL Consideration Shares may realise on any future sale; such a value may be higher or lower than the figure in this letter. London Bridge Capital assumes no obligation to update or revise this Estimate of Value based upon circumstances or events occurring after the date hereof.
| 108 |
Information
In arriving at the Estimate of Value, we have, amongst other things:
| 1. | considered the value of a Xenetic Share at the close of business on 11 November 2013, being the latest practicable day prior to the issue of this letter; |
| 2. | considered the estimated total costs of the Scheme and Acquisition of £1.33 million; |
| 3. | considered the stated value of net assets that will be held by GSL following the transfer of the existing business of GSL to Oxbridge Technology Partners, SA (“Oxbridge”) of £nil and the associated payment of $430,000 to be made by GSL to Oxbridge; |
| 4. | considered the share consolidation to be undertaken by GSL in advance of the Scheme becoming Effective whereby every holder of GSL Shares will receive 1 New GSL Share for every 10 GSL Shares held on the consolidation date; |
| 5. | considered a maximum number of GSL Consideration Shares to be issued of 130,520,137; |
| 6. | considered the US$:£ exchange rate of 1.598 at the close of business on 11 November 2013, being the latest practicable day prior to the issue of this letter; and |
| 7. | considered such other factors and performed such other analyses as we considered appropriate. |
We have relied on, and assumed, without independent verification, the accuracy and completeness of the information reviewed by us for the purposes of this opinion. We have made no independent valuation or appraisal of the assets and liabilities of Xenetic nor of GSL, nor have we sought or been provided with any such valuation or appraisal. Our opinion is necessarily based on financial, economic, market and other conditions in effect, and the information made available to us, as at 11 November 2013 (being the latest practicable day prior to the issue of this letter).
The valuation of the GSL Consideration Shares is inherently imprecise and is subject to certain uncertainties and contingencies, all of which are difficult to predict and are beyond our control. In performing this analysis, London Bridge Capital has made various assumptions. Consequently, the view expressed in this letter is not necessarily indicative of the amount which might be realised upon a sale of the GSL Consideration Shares to a third party. This Estimate of Value may differ substantially from estimates available from other sources. In addition, our view would be expected to fluctuate with changes in prevailing market and industry conditions, the financial conditions and prospects of GSL and other factors which generally influence the valuation of companies and securities.
Methodology
| 1. | London
Bridge
Capital
has
calculated
the
market
valuation
of
Xenetic
by
multiplying
the
number
of Scheme Shares in issue and admitted to trading on AIM by the value of a Scheme Share at the close of business on 11 November 2013, being the latest practicable day prior to the issue of this letter. This sum amounts to £26.5 million (“Xenetic Market Valuation”). |
| 2. | London Bridge Capital has deducted from the Xenetic Market Valuation both the estimated costs of the Scheme and Acquisition, amounting to £1.33 million, and the $430,000 payment to be made by GSL to Oxbridge to calculate the “Enlarged Group Valuation” of £24.9 million; |
| 3. | London Bridge Capital has divided the Enlarged Group Valuation by the total number of New GSL Shares in issue following the completion of the Acquisition, resulting in an “Estimate of Value” of a GSL Consideration Share of 18.59 pence. |
The taxation position of individual Xenetic Shareholders will vary and so we have not taken account of the effects of any taxation exemptions, allowances or reliefs available for the purposes of income, capital gains, inheritance or any other applicable tax, duty or levy, notwithstanding that these may be significant in the case of some Xenetic Shareholders.
No account has been taken of any potential transaction costs that a holder of GSL Consideration Shares may incur in realising such shares.
| 109 |
Opinion
On the basis of and subject to the foregoing, the Estimate of Value as at 11 November 2013 (being the estimated value of each GSL Consideration Share receivable in respect of Scheme Shares) is 18.59 pence.
General
London Bridge Capital is acting for Xenetic and no one else in connection with the Acquisition. London Bridge Capital will receive fees from Xenetic in respect of these services. London Bridge Capital will not be responsible to anyone other than Xenetic for providing the protections afforded to clients of London Bridge Capital, nor for providing advice in connection with the Acquisition, the content of the Scheme Document or any matter referred to herein.
Xenetic Shareholders are urged to read carefully all the information contained in the Scheme Document including the Risk Factors. In particular, Xenetic Shareholders should note that:
| 1. | the GSL Consideration Shares will not be listed on any recognised stock exchange or other market following the completion of the Acquisition; |
| 2. | whilst Xenetic and GSL have commenced developing a plan regarding a listing of the Enlarged Group on an Exchange, there is no guarantee that such a listing can be achieved; |
| 3. | whilst it is expected that the GSL Consideration Shares will be tradable on the OTCBB following the completion of the Acquisition, the Scheme Shares will cease to be traded on AIM; |
| 4. | whilst
it
is
expected
that
Enlarged
Group
will
continue
not
to
be
classified
as
a
“shell’
company,
there can be no assurance that the SEC or an Exchange may not object to the current classification as made by GSL or in the case of an Exchange apply its own rules and or analysis to GSL’s determination of its shell status, and reclassify the Enlarged Group as a shell where such determination, either by the SEC or an Exchange could adversely impact the Enlarged Group’s attempt to be listed on an exchange or the tradability of its shares and thereby affect its valuation. |
In providing this Estimate of Value, London Bridge Capital expresses no opinion or recommendation to any person as to whether they should vote in favour of the Acquisition. Xenetic Shareholders are recommended to seek their own independent financial advice.
Yours faithfully,
| /s/ Adam Hart |
| Adam Hart |
| Director |
| For and on behalf of |
| London Bridge Capital Limited |
| 110 |
PART 13
RISK FACTORS
The Xenetic Directors consider the following risks to be the most significant for Xenetic Shareholders. In addition to the other relevant information set out in this document, these risks should be considered carefully by Xenetic Shareholders when deciding what action to take at the Meetings and/or in evaluating an investment in the Company (or, after the Scheme becomes Effective, GSL) into which the Group’s operations will merge. Such investment may not be suitable for all of the Company’s existing or prospective investors.
If you are in any doubt about the action you should take, you should consult a person authorised under the Financial Services and Markets Act 2000, as amended, who specialises in advising on the acquisition of shares and other securities. It should be noted that the risks described below are not the only risks faced by the Group. There may be additional risks that the Xenetic Directors currently consider not to be material or of which they are currently unaware. The risks set out below are not presented in any assumed order of priority.
The risks below relate to the Group. They will similarly continue to apply to the Enlarged Group after the Scheme becomes Effective and references to the Company and the Group shall be construed accordingly.
Development of the Company’s products carries certain risks
Development of product candidates is lengthy and expensive with no guarantee that they will reach market. Any product candidate must be put through extensive research and development, including pre-clinical and clinical testing, which will be costly. In addition, there are a significant number of reasons why a product might not be successful including:
– the inability of clinical trials to demonstrate that the product is safe and effective in humans;
– the failure to obtain required regulatory approvals;
– the lack of adequate funding to finance development; or
– the failure to establish and maintain any collaborative third party agreements to support product
development.
The Company has a limited history as a speciality drug developer. The Company is also exposed to the risk that the delay or failure of individual product development programmes will adversely affect the Company’s product development pipeline.
Governments may introduce further legislation which could impact on the Company’s business
The Company is unable to forecast what additional legislation or regulations may be enacted in the future which could have an adverse affect on the Group’s business, financial condition and results of operations as well as prospects.
The Company has a history of losses, and as a result the Group cannot be certain that it will achieve profitability
The Company has a history of operating losses since its inception. In order to support the development of the Group’s product candidates, future expenses may be incurred which are considerably in excess of its revenue. No assurances can be given that the operations of the Group will become profitable. The level of operating expenditures will vary depending upon the stage of development of proprietary proteins and vaccines and the number and nature of collaborations of the Group. The Group may not develop any products and may continue to incur substantial losses even if revenues increase.
| 111 |
The Company has not yet commercialised any products or technologies, and as a result the Group may never become profitable
The Company has not yet commercialised any products or technologies, and may never be able to do so. The Directors do not know when or if the Group will complete any of its product development efforts, obtain regulatory approval for any product candidates incorporating its technologies, or successfully commercialise any approved products.
The Group has limited product development and commercial manufacturing experience
To date, the Company has not manufactured, on a commercial scale, any pharmaceutically active proteins or vaccines. The Company confronts scale-up risks associated with protein and vaccine manufacturing and has sought and continues to have collaborators, licencees or contract manufacturers to manufacture most of the compounds necessary to commercialise its technologies. The Group may become dependent on suppliers that could discontinue its supply arrangements or change supply terms to its disadvantage. The success of the Group depends on its ability to manufacture these compounds on a commercial scale or to obtain commercial quantities, in either case, at reasonable cost. Manufacturing processes also must comply with current Good Manufacturing Practices, or cGMP. The Group may not be able to manufacture or obtain sufficient quantities of the products it develops to meet its needs for pre-clinical or clinical development.
Success is partly dependent on the performance of third party collaborators
The Group will continue to rely on collaborative partners to co-develop and manufacture certain products and to commercialise some of the products made using the Company’s technologies. The Company has entered into several significant collaboration agreements and the success of the product candidates is highly dependent on its collaborators, most notably SynBio, FDS Pharma and the Serum Institute of India. If the relationship with either of these parties is adversely affected, the Company’s development programme may also be adversely impacted. These collaborators also have significant discretion over the allocation of their resources and the Group cannot guarantee that they will devote adequate resources to these collaborations. Failure of collaborators could adversely affect the ability of the Group to develop product candidates and could materially affect operations.
The partnering strategy of the Group entails many risks, including:
–the Group may not be successful in producing its technologies to commercial scale and even if it produces to commercial scale, it may not be able to perform in a manner that meets regulatory approval;
–the Group may not be successful in applying its technologies to the needs of its collaborative partners;
–collaborators of the Group may not be successful in, or may not remain committed to, co-developing products of the Group or commercialising products incorporating technologies of the Group;
–collaborators are not obliged to market or commercialise products of the Group or products incorporating technologies of the Group; and
–the collaboration agreements in existence may be terminated by partners of the Company on short notice.
The Group may be exposed to product liability and related risks
The use in humans of compounds developed by the Group or incorporating its technologies may result in product liability claims. Product liability claims can be expensive to defend, and may result in large settlements of claims or judgments against the Group. The Group may not be able to obtain insurance cover at a reasonable cost or in sufficient amounts to protect it against losses.
Intellectual Property and Patent Protection
The Group’s success depends in part on its ability to establish, protect and enforce proprietary rights,
including patents for its innovations and there can be no assurance that any intended patent applications will mature into patents or that existing patents or future patents will adequately protect the Group’s products and technology.
| 112 |
If the Group fails to obtain adequate protection for its intellectual property, the Group’s competitors may be able to take advantage of the Group’s research and development efforts. In addition, there is no assurance that the Group’s competitors will be unable to develop competing products which do not infringe the rights obtained by the Group.
The laws of some foreign countries may not protect intellectual property rights of the Group to the same extent as European and U.S. laws. The Group may need to participate in proceedings to determine the validity of patents belonging to it, licensed to it or belonging to its competitors, which could result in substantial costs and the diversion of the Group’s efforts. Infringement of intellectual property is often difficult to prove. Legal actions against third party infringers are usually costly and lengthy, and their outcome is uncertain. An adverse outcome could subject the Group to significant liabilities and require the Group either to cease using a technology or to pay licence fees. Finally, some patent protection in Europe is not available to the Group in foreign countries due to the differences in the patent laws of those countries. Patents can also be challenged by competitors. The Group’s patent position is therefore never certain and involves complex legal and factual issues and applications.
There is no assurance that third parties have no granted or pending patent rights which may inhibit the Group’s freedom to exploit its own proprietary rights. Since patent applications are maintained in secrecy for 18 months, the Company cannot be certain that it was the first to make the inventions covered by the pending applications or that it was the first to file patent applications for such inventions. If patents are granted to other parties that contain claims having a scope that is interpreted to cover any of the Group’s products or processes, there can be no assurance that the Company will be able to obtain licences to such patents at reasonable cost, if at all, or be able to develop or obtain alternative technology.
The commercial success of the Group will also depend, amongst other things, on its (and outside parties’) ability to preserve the confidentiality of its own and outside parties’ know how. There can be no assurance that obligations to maintain the Group’s or such outside parties’ trade secrets and/or know how will not be breached or that such trade secrets and/or know-how will not otherwise become known in a manner which provides the Group with no practical recourse. In addition, where copyright, design right and/or know how protect the Group’s products or technology, there can be no assurance that a competitor or potential competitor will not independently develop the same product or technology.
Competitors may develop better or more successful products
Competitors of the Group may succeed in developing products and technologies that are more effective or less costly that would render products or technologies of the Group, or both, obsolete or uncompetitive. The Group’s competitors may also have significantly greater financial, marketing and human resources and may have more experience in research and development. As a result, the Group’s competitors may develop safer or more effective drugs, implement more effective sales and marketing programmes or be able to establish superior proprietary positions which would render the Group’s and/or its partners’ products and/or technologies obsolete or otherwise uncompetitive. In addition, the Group anticipates that it will face increased competition in the future as new companies enter the Group’s markets and alternative drugs and technologies become available. The Group’s products under development are based on a range of different technologies and are expected to address a number of different markets. The Group’s competitive position will be determined in part by the potential indication for which the Group’s products are developed and ultimately approved by regulatory authorities. In addition, the first pharmaceutical product to reach the market may be at a significant competitive advantage relative to later entrants to the market. Accordingly, the relative speed with which the Group or its collaborative partners can develop products, complete the clinical trials and approval processes and supply commercial quantities of the products to the market, are expected to be important competitive factors.
The Group’s competitors are developing products that could compete with the product candidates the Group is developing. The Group and its collaborators will need to persuade patients and physicians to adopt its products over its competitor’s products. If they fail to build a strong position in their markets this would have a material adverse effect on the Group’s profitability.
Necessary regulatory approvals may not be obtained by the Group or its collaborators or long delays and large expenditures may be incurred in obtaining such approvals Pharmaceutical product candidates manufactured using the Company’s technologies must undergo an extensive regulatory approval process before commercialisation. This process is regulated by EMA and the FDA and by comparable agencies in other countries. The regulatory agencies have substantial discretion to terminate clinical trials, require additional testing, delay or withhold registration and marketing approval, and mandate product withdrawals.
| 113 |
The Company has not and its collaborators have not submitted any product candidates incorporating the Company’s technologies for approval to EMA or to the FDA or any other regulatory authority. If any product candidate manufactured using the Company’s technology is submitted for regulatory approval, it may not receive the approvals necessary for commercialisation. Any delay in receiving, or failure to receive, these approvals would adversely affect the ability of the Group to generate product revenues from milestone payments and royalties, and significant sums will already have been spent in pursuing approval.
Collaboration partners’ manufacturing processes would be subject to continued review by regulatory agencies. Any discovery of unknown problems with products of the Group, products incorporating its technologies, or manufacturing processes could result in restrictions on such products or manufacturing processes, including potential withdrawal of the products.
Safety and efficacy standards
As part of the regulatory approval process, the Company must conduct pre-clinical studies and clinical trials for each of its product candidates to demonstrate safety and efficacy. The number of pre-clinical studies and clinical trials that will be required varies depending on the product candidate, the indication being evaluated, the trial results and the regulations applicable to the particular product candidate. The results of pre-clinical studies and initial clinical trials of the Company’s product candidates do not necessarily predict the results of later stage clinical trials. Unapproved product candidates in later stages of clinical trials may fail to show the desired safety and efficacy despite having progressed through initial clinical trials. There can be no assurance that the data collected from the pre-clinical studies and clinical trials of the Company’s product candidates will be sufficient to satisfy the relevant regulatory authorities, or approval from local ethics committees. In addition, the continuation of a particular study after review by an independent data safety monitoring board or review body does not necessarily indicate that all clinical trials will ultimately be successfully completed.
Human Volunteers
Many factors affect enrolment in clinical trials, including the size of the patient or subject population, the novelty and complexity of use of trial material for healthcare professionals, the proximity of patients or subjects to clinical sites, the eligibility criteria for the trial, competing clinical trials, new products approved for the conditions the clinical trial is investigating and adverse publicity surrounding the clinical trials of other product development companies. As a result of all these factors, the Company’s clinical trials may take longer to enrol patients or subjects than anticipated. Delays in patient or subject enrolment in the clinical trials may increase the Company’s costs and slow down its product development and approval process. Clinical trials may also be subject to delays stemming from patient or subject withdrawal or from lower than expected event rates. The clinical trials may also incur increased costs if enrolment is increased in order to achieve the desired number of events. The Company’s development costs will also increase if it needs to perform more, or larger, clinical trials than planned.
Safety concerns and side effects
The Company’s product candidates may produce unexpected side effects or serious adverse events which could interrupt, delay or halt clinical trials of the Company’s product candidates and could result in the relevant regulatory authorities denying approval of its product candidates for any or all targeted indications. An independent data safety monitoring board, the relevant regulatory authorities or the Company itself may suspend or terminate clinical trials at any time. There can be no assurances that any of the Company’s product candidates will ultimately prove to be safe for human use. The Company’s clinical trials could also be delayed or terminated in the event that the product candidate being tested is in the same class of product as a marketed product that is revealed to cause side effects. Any delays in completing clinical trials will delay the Company’s ability to generate revenue from product sales, and the Company may have insufficient capital resources to support its operations in the longer term. Even if the Company does have sufficient capital resources, its ability to generate meaningful revenues or become profitable may be delayed.
| 114 |
Foreign Exchange Risk
As a consequence of the international nature of its business, the Group is exposed to risks associated with changes in foreign currency exchange rates. Most of the agreements the Company has entered into are denominated in US dollars while the Company’s operating costs are in sterling. The Company has not entered into any contracts to reduce its currency risk exposure. Therefore currency fluctuation could have a significant impact on financial results and cash flow of the Group.
Dependence on key employees
The future success of the Group is substantially dependent on the continued services and performance of its senior management and other key personnel in the various areas of its business. Whilst the Group has entered into employment arrangements with each of its key personnel with the aim of securing their services, the retention of their services cannot be guaranteed. The Company is dependent on certain scientific and management personnel. Incentivisation of key employees to remain with the Group remains critical to the Group’s success. The loss of those employees could weaken the Group’s scientific and management capabilities, resulting in delays in the development of its drugs and impacting negatively on the Group’s business. The biotechnology industry has a highly competitive market for qualified scientific and managerial employees. Competitors may try to recruit some of the Group’s important employees. Recruiting and retaining management and scientific personnel as the Group develops will be critical to the Group’s success.
Manufacturing
There can be no assurance that the Group’s or its partners’ product candidates will be capable of being produced in commercial quantities at acceptable cost or that, if introduced, they will achieve market acceptance. Development of product candidates involves a lengthy and complex process. Any product candidate which the Group wishes to offer commercially to the public must be put through extensive research and pre-clinical and clinical development which will be costly to the Group. This development process takes several years. In addition, the Group or its partners will need to obtain regulatory approvals to conduct clinical trials and manufacture drugs before they can be marketed. Results of pre-clinical studies are not necessarily indicative of results that may be obtained in clinical trials and results in early clinical trials may be different from those obtained in long-term testing or in general use. Adverse or inconclusive results from pre-clinical testing or clinical trials may substantially delay, or halt entirely, the development of products. The Group may fail to successfully develop a product candidate for many reasons, including:
–the failure to establish any collaborative third party agreements to support drug development;
–the failure to produce a promising compound in sufficient quantities to conduct clinical trials;
–or to manufacture the compound at commercially acceptable quantities and prices;
–the failure of the drug in pre-clinical studies;
–the inability of clinical trials to demonstrate that the drug is safe and effective in humans; or
–the failure to obtain required regulatory approvals.
The Group’s success also depends on acceptance of the Group’s products by the market, including by physicians and third-party payers, and consequently the Group’s progress may be adversely affected if it is unable to achieve market acceptance of its products. Some factors that may affect the rate and level of market acceptance of any of the Group’s products include:
–the existence or entry onto the market of superior competing products or therapies;
–the price of the Group’s products compared to competing products;
–public perception regarding the safety, efficacy and benefits of the Group’s products compared to competing products or therapies;
–the effectiveness of the sales and marketing efforts of the Group’s marketing partners;
–regulatory developments related to manufacturing or use of the Group’s products;
–the willingness of physicians to adopt a new treatment regimen; and
–publicity concerning the product type in general.
| 115 |
The biotechnology and pharmaceutical industries are subject to rapid technological change which could affect the success of the Group’s drugs or make them obsolete. The field of biotechnology is characterised by significant and rapid technological change. Research and discoveries by others may result in medical insights or breakthroughs which render the Group’s product candidates less competitive or even obsolete before they generate revenue. The Group’s product candidates use specialised manufacturing processes for which there a few suitable manufacturing contractors. There can be no assurance that the contractors who are currently able to manufacture the Group’s product candidates will continue to make capacity available at economic prices, or that suitable new contractors will enter the market.
Manufacturing processes that are effective and practical at the small scale required by the early stages of clinical development may not be appropriate at the higher scale required for later stages of clinical development or for commercial supply. There can be no assurance that the Group will be able to adapt current processes or develop new processes suitable for the scale required by later stages of clinical development or commercial supply in a timely or cost-effective manner, nor that contractors will be able to provide sufficient manufacturing capacity when required.
Adverse actions by animal rights activists could interfere with research activities
Activities of the Group or its collaborators’ activities, operations and research, and services conducted for the Group by third parties, could be adversely affected by animal rights activists. Any such adverse action could delay research projects and decrease the ability to conduct future research and development. Any significant interruption of the ability of the Group to conduct its business operations, research and development activities, or manufacturing operations could reduce revenue and increase expenses.
Adverse public opinion
The pharmaceutical industry is frequently subject to adverse publicity on many topics, including corporate governance, product recalls and research and discovery methods, as well as to political controversy over the impact of novel techniques and therapies on humans, animals and the environment. Adverse publicity about the Group, its collaborators, its products, or any other part of the industry may hurt the Group’s public image, which could harm its operations, cause its share price to decrease or impair its ability to gain market acceptance for its products.
Growth management
The Directors anticipate that further expansion will be required to address the anticipated growth in the markets in which the clients of the Group operate. The future success of the Group will depend, in part, on its ability to manage this anticipated expansion. Such expansion is expected to place significant demands on management, support functions, accounting, sales and marketing and other resources. If the Group is unable to manage its expansion effectively, its business and financial results could suffer.
Investment in AIM securities
Investment in shares traded on AIM (or as the case may be on OTCBB or the OTCQB) are perceived to involve a higher degree of risk and be less liquid than investment in companies whose shares are listed on the Official List and traded on the London Stock Exchange’s market for listed securities or on an Exchange. An investment in shares traded on such markets may be difficult to realise. Shareholders should be aware that the value of shares may go down as well as up and that the market price of the shares may not reflect the underlying value of the Group. Shareholders may therefore realise less than, or lose all of, their investment.
Potentially volatile share price and liquidity
The share price of quoted emerging companies can be highly volatile and shareholdings illiquid. The price at which shares are quoted and the price at which investors may realise their shares may be influenced by a significant number of factors, some specific to the Company and its operations and some which affect quoted companies generally. These factors could include the
performance of the Enlarged Group, large purchases or sales of shares, legislative changes and general, economic, political or regulatory conditions.
| 116 |
Additional risk factors relating to GSL General
The risks set out in Part II of the Equivalent Document (as reproduced at pages 59-62 of this document) apply to GSL and its securities. Accordingly such risks will also apply in relation to the GSL Consideration Shares after the Scheme becomes Effective.
SEC and Exchange classification risk
GSL Shares are currently available for quotation on the OTCBB and OTCQB. There has been only limited trading recently in such shares and therefore they are not considered to have a public valuation or market.
GSL does not currently identify itself as a ‘shell company’ under relevant SEC rules and regulations, which means that its stock is not subject to certain limitations contained in these rules. There can be no assurance, however, that the SEC and/or various regulatory bodies in the US will not reject this identification and effectively apply the shell company rules to GSL in the future. Additionally, any Exchange may apply its own rules and/or analysis and reach the conclusion that GSL was or is a shell company.
Any such determination, either by the SEC, one of the regulatory bodies in the US or an Exchange, could adversely impact the Enlarged Group’s attempt to be listed on an Exchange or the tradability of its shares, and/or the cost and time involved in doing so. This may include being subject to the “seasoning rules” of an Exchange prior to being quoted, in effect requiring GSL to wait a year or more before being allowed to obtain a listing on such Exchange.
| 117 |
