Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a13-24764_28k.htm |
| EX-10.2 - EX-10.2 - AMICUS THERAPEUTICS, INC. | a13-24764_2ex10d2.htm |
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a13-24764_2ex99d1.htm |
| EX-10.1 - EX-10.1 - AMICUS THERAPEUTICS, INC. | a13-24764_2ex10d1.htm |
Exhibit 99.2
|
|
Broad Strategic Update Conference Call November 20, 2013 |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to the ongoing collaboration with GSK, preclinical and clinical development of Amicus’ candidate drug products, financing plans, and the timing and reporting of results from clinical trials evaluating Amicus’ candidate drug products. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2012. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. 2 |
|
|
Strategic Overview |
|
|
Four Pillars of Amicus’ Broad Strategic Repositioning Callidus Biopharma, Inc. Acquisition Organizational Restructuring and Realignment New Fabry Agreement with GSK Rare Diseases $40M Equity and Expected Debt Financing |
|
|
Strengthening Biologics Business Strategy Acquisition of Callidus Biopharma strengthens Amicus’ Pompe program and expected to advance time to clinic by >1 year Amended GSK collaboration provides global rights to Fabry next-generation ERT 3 next-generation ERTs for Fabry, Pompe and MPS I expected to enter clinic in next 3 years Addition of ex-US rights clinical and commercial responsibilities for Fabry monotherapy program maximize chances for success $15M in equity and $25M in expected debt financing; and organizational restructuring to extend cash runway through late 2015 Amicus Now Strongly Positioned and Well-Capitalized to Advance Next-Generation Enzyme Replacement Therapies |
|
|
Pompe Program Update & Callidus Biopharma Acquisition |
|
|
Pompe Program Strategy Significant unmet medical need in Pompe Human proof-of-concept for CHART™ platform already established in Pompe Amicus has been developing proprietary next-generation ERT utilizing CHART platform Callidus’ Pompe ERT and platform technology complimentary to Amicus’ CHART™ platform Program Goal to Develop Better rhGAA Combined With CHART™ Technology Elevated Glycogen in Muscle |
|
|
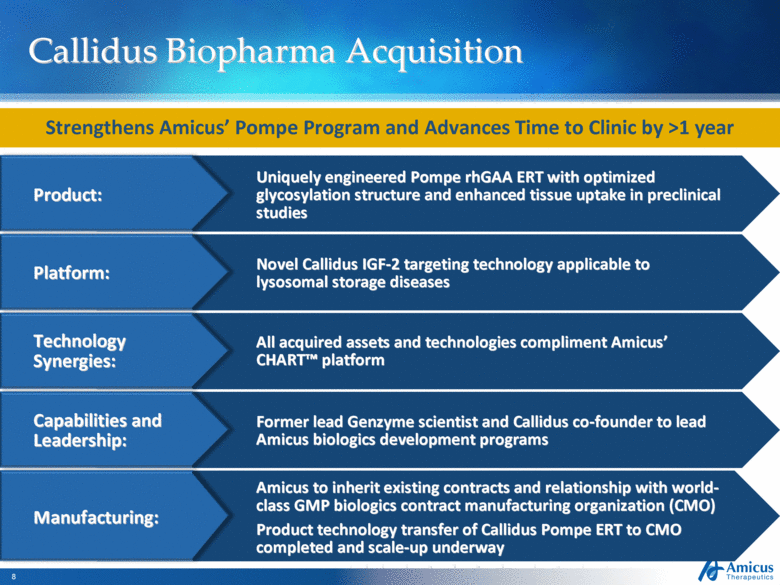
Callidus Biopharma Acquisition Strengthens Amicus’ Pompe Program and Advances Time to Clinic by >1 year Product: Uniquely engineered Pompe rhGAA ERT with optimized glycosylation structure and enhanced tissue uptake in preclinical studies Platform: Novel Callidus IGF-2 targeting technology applicable to lysosomal storage diseases Technology Synergies: All acquired assets and technologies compliment Amicus’ CHART™ platform Capabilities and Leadership: Former lead Genzyme scientist and Callidus co-founder to lead Amicus biologics development programs Manufacturing: Amicus to inherit existing contracts and relationship with world-class GMP biologics contract manufacturing organization (CMO) Product technology transfer of Callidus Pompe ERT to CMO completed and scale-up underway |
|
|
Next-Generation Pompe ERT Callidus’ ERT Has Demonstrated Significant Advantages in Preclinical Studies that May Be Further Improved By Co-Formulating with AT2220 L6 Myoblast Uptake (Preliminary Results) 10000 8000 6000 4000 0 0 100 200 300 400 500 2000 Endogenous GAA levels Internalized GAA Activity (F460) GAA (nM) Lumizyme rhGAA Callidus rhGAA vIGF2-Tagged rhGAA ~10x > Lumizyme >50x > Lumizyme |
|
|
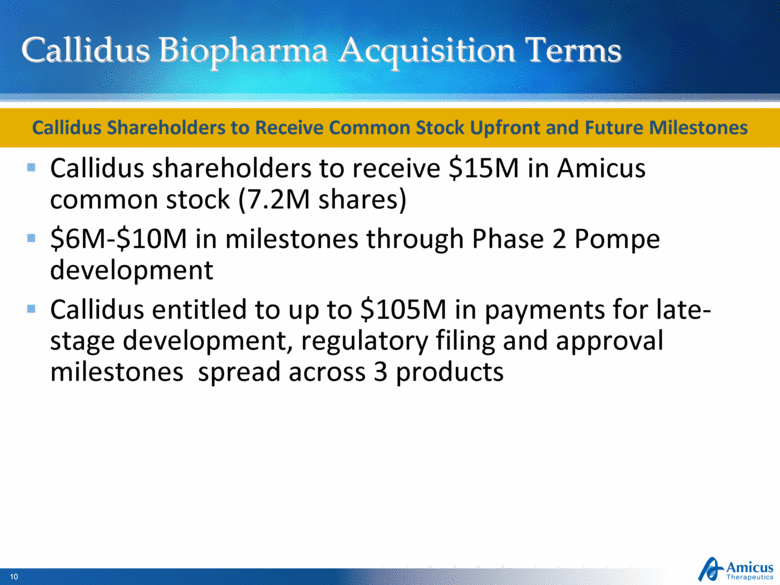
Callidus Biopharma Acquisition Terms Callidus Shareholders to Receive Common Stock Upfront and Future Milestones Callidus shareholders to receive $15M in Amicus common stock (7.2M shares) $6M-$10M in milestones through Phase 2 Pompe development Callidus entitled to up to $105M in payments for late-stage development, regulatory filing and approval milestones spread across 3 products |
|
|
Fabry Program & GSK Collaboration Update |
|
|
Amicus and GSK Amend Fabry Collaboration “With internal expertise and established relationships within the rare disease community, we believe Amicus is well positioned to maintain momentum of the programs, maximizing their potential for success, which we hope will provide benefits to patients living with Fabry disease. GSK will continue to support Amicus through our equity investment and share in the future value of migalastat HCl as the Fabry program meets certain regulatory and sales milestones.” -Moncef Slaoui, Chairman of GSK R&D |
|
|
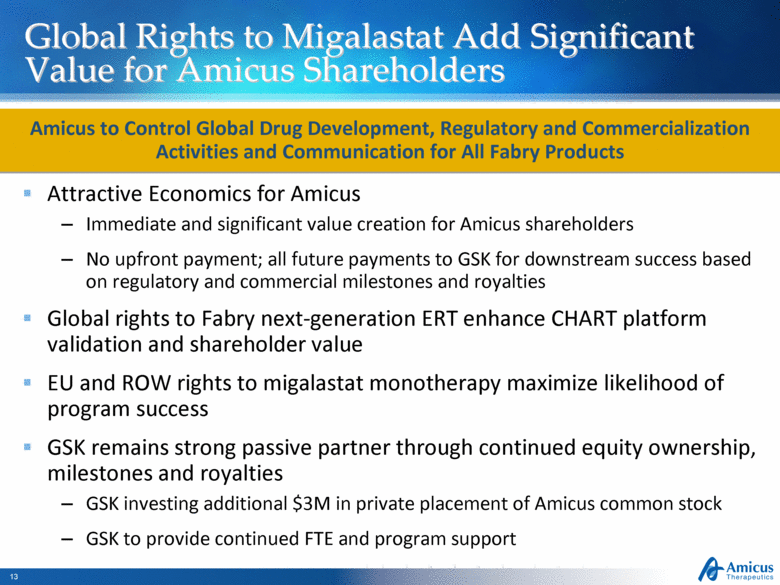
Global Rights to Migalastat Add Significant Value for Amicus Shareholders Attractive Economics for Amicus Immediate and significant value creation for Amicus shareholders No upfront payment; all future payments to GSK for downstream success based on regulatory and commercial milestones and royalties Global rights to Fabry next-generation ERT enhance CHART platform validation and shareholder value EU and ROW rights to migalastat monotherapy maximize likelihood of program success GSK remains strong passive partner through continued equity ownership, milestones and royalties GSK investing additional $3M in private placement of Amicus common stock GSK to provide continued FTE and program support Amicus to Control Global Drug Development, Regulatory and Commercialization Activities and Communication for All Fabry Products |
|
|
Financing & Organizational Restructuring/Realignment |
|
|
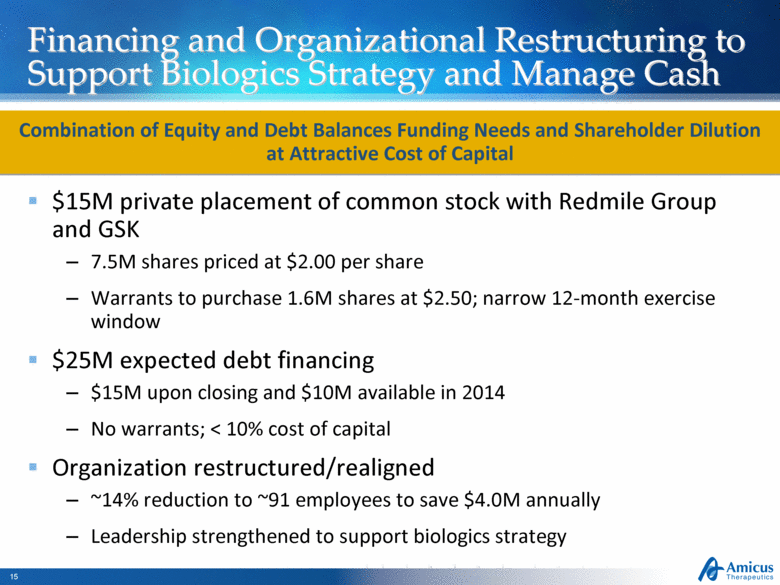
Financing and Organizational Restructuring to Support Biologics Strategy and Manage Cash $15M private placement of common stock with Redmile Group and GSK 7.5M shares priced at $2.00 per share Warrants to purchase 1.6M shares at $2.50; narrow 12-month exercise window $25M expected debt financing $15M upon closing and $10M available in 2014 No warrants; < 10% cost of capital Organization restructured/realigned ~14% reduction to ~91 employees to save $4.0M annually Leadership strengthened to support biologics strategy Combination of Equity and Debt Balances Funding Needs and Shareholder Dilution at Attractive Cost of Capital |
|
|
Financial Guidance $60.5M cash position at September 30, 2013 Additional $40M in equity and expected debt financing $80-85M in cash expected at year-end 2013 Detailed FY14 financial guidance to be announced early 2014 Projected Year-End Cash, Including Proceeds from Equity and Expected Debt Financings, Expected to Fund Operations into Late 2015 |
|
|
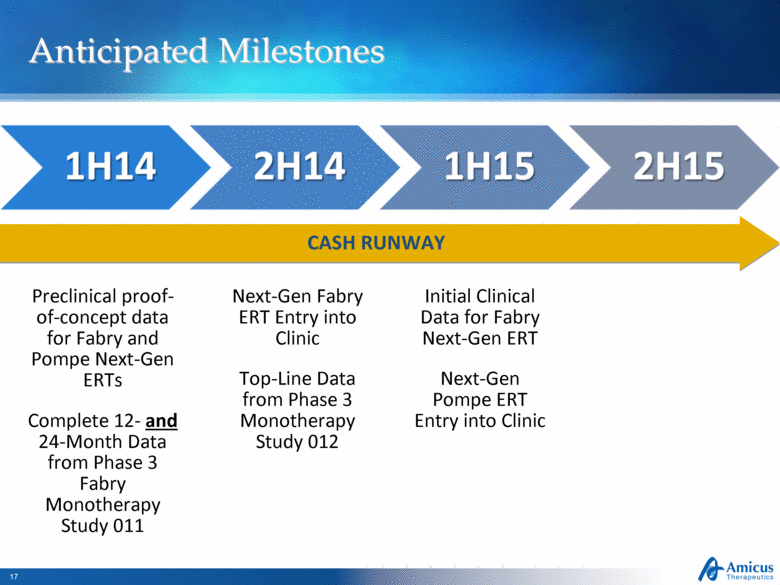
Anticipated Milestones Preclinical proof-of-concept data for Fabry and Pompe Next-Gen ERTs Complete 12- and 24-Month Data from Phase 3 Fabry Monotherapy Study 011 Next-Gen Fabry ERT Entry into Clinic Top-Line Data from Phase 3 Monotherapy Study 012 CASH RUNWAY Initial Clinical Data for Fabry Next-Gen ERT Next-Gen Pompe ERT Entry into Clinic |
|
|
Strength in Business Development Activities $350M+ Secured Since 2007 IPO, Largely Through Business Development Activities $250M+ in Business Development Deals <$100M in Follow-on Equity Offerings |
|
|
Broad Strategic Update Conference Call November 20, 2013 at the forefront of therapies for rare and orphan diseases |
|
|
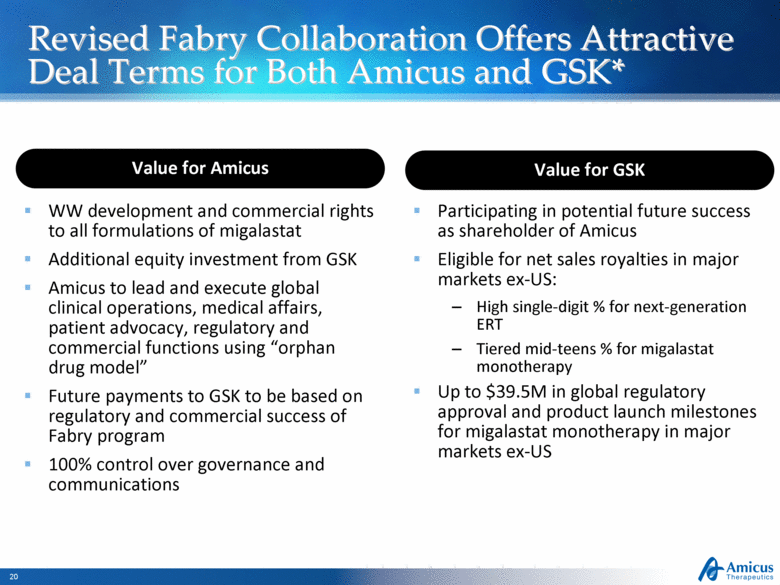
Revised Fabry Collaboration Offers Attractive Deal Terms for Both Amicus and GSK* WW development and commercial rights to all formulations of migalastat Additional equity investment from GSK Amicus to lead and execute global clinical operations, medical affairs, patient advocacy, regulatory and commercial functions using “orphan drug model” Future payments to GSK to be based on regulatory and commercial success of Fabry program 100% control over governance and communications Participating in potential future success as shareholder of Amicus Eligible for net sales royalties in major markets ex-US: High single-digit % for next-generation ERT Tiered mid-teens % for migalastat monotherapy Up to $39.5M in global regulatory approval and product launch milestones for migalastat monotherapy in major markets ex-US Value for Amicus Value for GSK |