Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TESARO, Inc. | a13-23786_18k.htm |
| EX-99.1 - EX-99.1 - TESARO, Inc. | a13-23786_1ex99d1.htm |
Exhibit 99.2
|
|
Third-Quarter Operating Results November 7, 2013 |
|
|
Safe Harbor Statement Statements made in this presentation about TESARO, Inc. that are not descriptions of historical facts are forward-looking statements reflecting the current beliefs and expectations of management. Forward-looking statements are sometimes identified by words such as “plan,” “may,” “will,” “expect,” and similar expressions referencing future events, conditions or circumstances. These forward-looking statements involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Actual results could differ materially from those expressed or implied by these forward-looking statements as a result of various factors, including, without limitation, the various risks described in our Annual Report on Form 10-K for the year ended December 31, 2012 and our subsequent filings with the SEC. TESARO, Inc. undertakes no obligation to update or revise any forward-looking statement for any reason. |
|
|
Lonnie Moulder Chief Executive Officer |
|
|
Ted English Vice President, Finance & Administration |
|
|
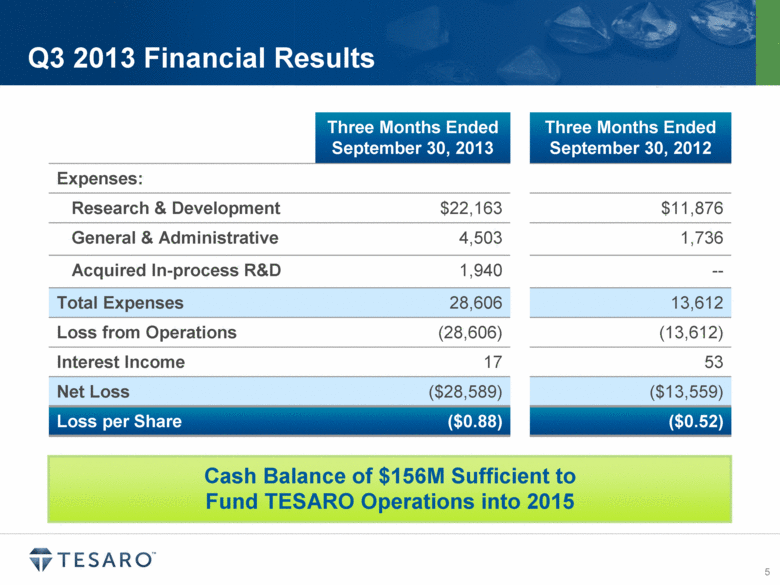
Q3 2013 Financial Results Three Months Ended September 30, 2013 Three Months Ended September 30, 2012 Expenses: Research & Development $22,163 $11,876 General & Administrative 4,503 1,736 Acquired In-process R&D 1,940 -- Total Expenses 28,606 13,612 Loss from Operations (28,606) (13,612) Interest Income 17 53 Net Loss ($28,589) ($13,559) Loss per Share ($0.88) ($0.52) Cash Balance of $156M Sufficient to Fund TESARO Operations into 2015 |
|
|
Mary Lynne Hedley, Ph.D. President |
|
|
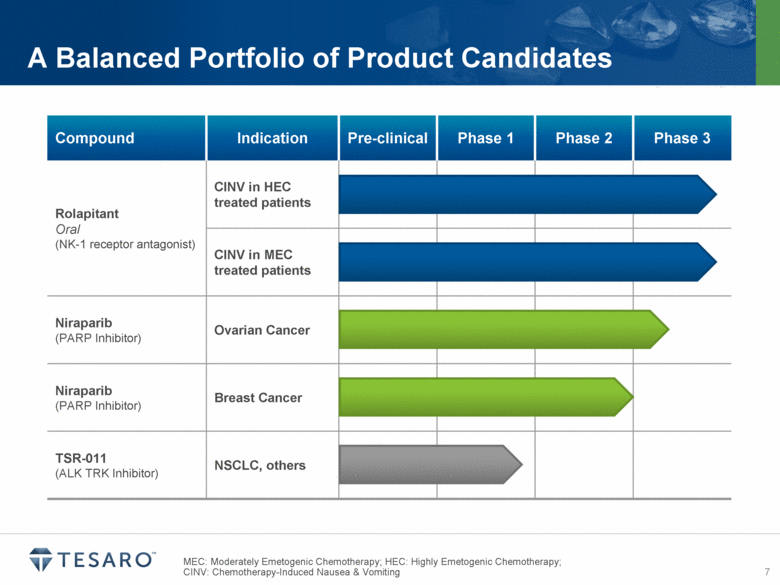
MEC: Moderately Emetogenic Chemotherapy; HEC: Highly Emetogenic Chemotherapy; CINV: Chemotherapy-Induced Nausea & Vomiting A Balanced Portfolio of Product Candidates Compound Indication Pre-clinical Phase 1 Phase 2 Phase 3 Rolapitant Oral (NK-1 receptor antagonist) CINV in HEC treated patients CINV in MEC treated patients Niraparib (PARP Inhibitor) Ovarian Cancer Niraparib (PARP Inhibitor) Breast Cancer TSR-011 (ALK TRK Inhibitor) NSCLC, others |
|
|
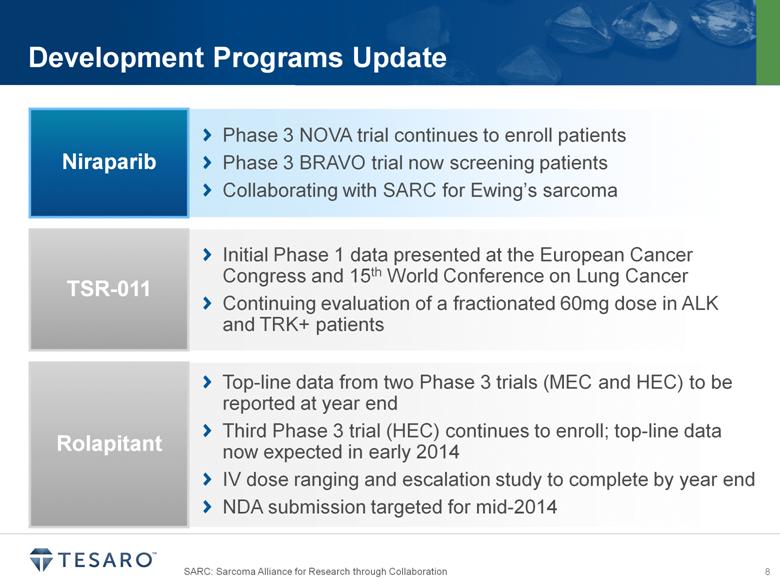
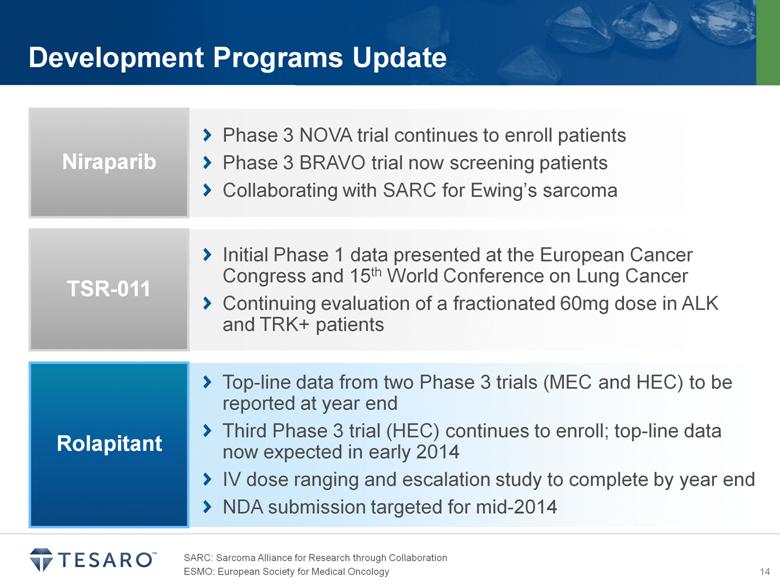
SARC: Sarcoma Alliance for Research through Collaboration Development Programs Update Phase 3 NOVA trial continues to enroll patients Phase 3 BRAVO trial now screening patients Collaborating with SARC for Ewing’s sarcoma Niraparib Initial Phase 1 data presented at the European Cancer Congress and 15th World Conference on Lung Cancer Continuing evaluation of a fractionated 60mg dose in ALK and TRK+ patients TSR-011 Top line data from two Phase 3 trials (MEC and HEC) to be reported at year end Third Phase 3 trial (HEC) continues to enroll; top line data now expected in early 2014 IV dose ranging and escalation study to complete by year end NDA submission targeted for mid-2014 Rolapitant |
|
|
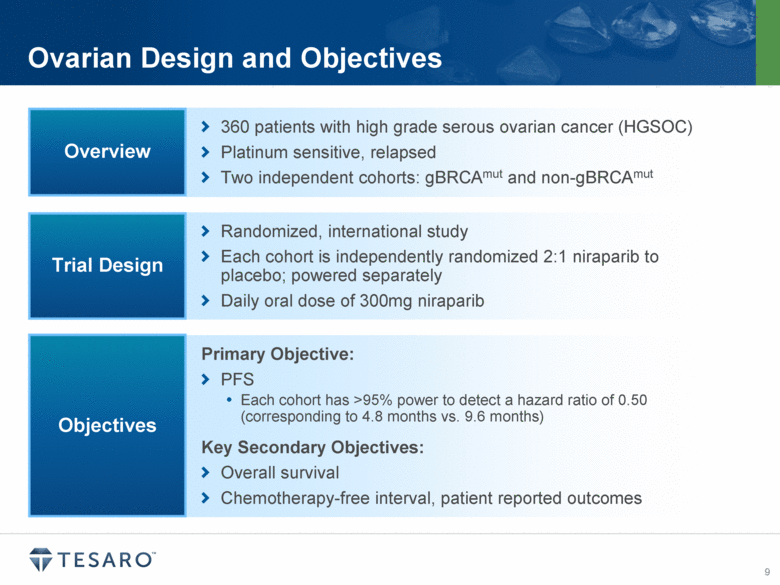
Ovarian Design and Objectives 360 patients with high grade serous ovarian cancer (HGSOC) Platinum sensitive, relapsed Two independent cohorts: gBRCAmut and non-gBRCAmut Overview Randomized, international study Each cohort is independently randomized 2:1 niraparib to placebo; powered separately Daily oral dose of 300mg niraparib Trial Design Primary Objective: PFS Each cohort has >95% power to detect a hazard ratio of 0.50 (corresponding to 4.8 months vs. 9.6 months) Key Secondary Objectives: Overall survival Chemotherapy-free interval, patient reported outcomes Objectives |
|
|
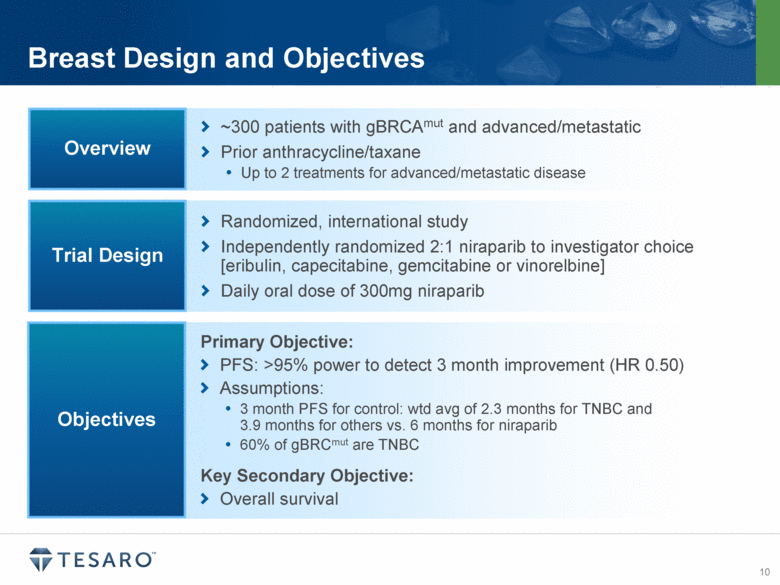
Breast Design and Objectives ~300 patients with gBRCAmut and advanced/metastatic Prior anthracycline/taxane Up to 2 treatments for advanced/metastatic disease Overview Randomized, international study Independently randomized 2:1 niraparib to investigator choice [eribulin, capecitabine, gemcitabine or vinorelbine] Daily oral dose of 300mg niraparib Trial Design Primary Objective: PFS: >95% power to detect 3 month improvement (HR 0.50) Assumptions: 3 month PFS for control: wtd avg of 2.3 months for TNBC and 3.9 months for others vs. 6 months for niraparib 60% of gBRCmut are TNBC Key Secondary Objective: Overall survival Objectives |
|
|
Development Programs Update Phase 3 NOVA trial continues to enroll patients Phase 3 BRAVO trial now screening patients Collaborating with SARC for Ewing’s sarcoma Niraparib Initial Phase 1 data presented at the European Cancer Congress and 15th World Conference on Lung Cancer Continuing evaluation of a fractionated 60mg dose in ALK and TRK+ patients TSR-011 Top line data from two Phase 3 trials (MEC and HEC) to be reported at year end Third Phase 3 trial (HEC) continues to enroll; top line data now expected in early 2014 IV dose ranging and escalation study to complete by year end NDA submission targeted for mid-2014 Rolapitant SARC: Sarcoma Alliance for Research through Collaboration |
|
|
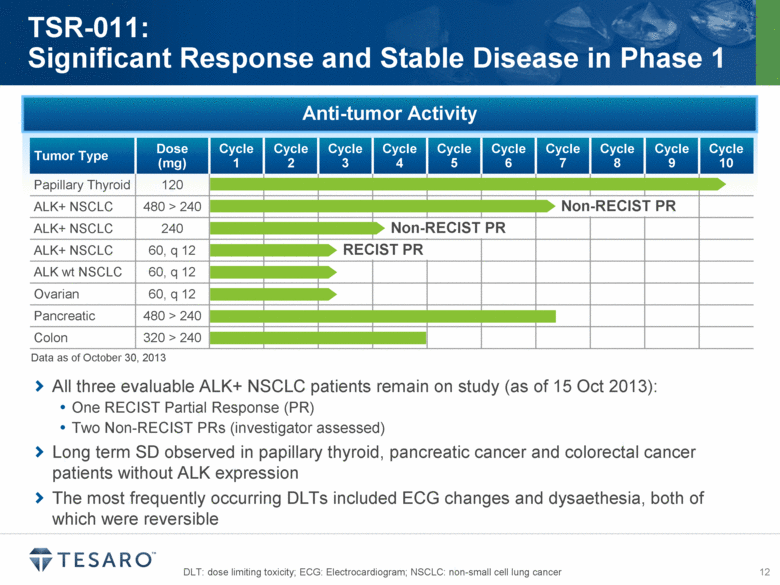
All three evaluable ALK+ NSCLC patients remain on study (as of 15 Oct 2013): One RECIST Partial Response (PR) Two Non-RECIST PRs (investigator assessed) Long term SD observed in papillary thyroid, pancreatic cancer and colorectal cancer patients without ALK expression The most frequently occurring DLTs included ECG changes and dysaethesia, both of which were reversible DLT: dose limiting toxicity; ECG: Electrocardiogram; NSCLC: non-small cell lung cancer TSR-011: Significant Response and Stable Disease in Phase 1 Anti-tumor Activity Tumor Type Dose (mg) Cycle 1 Cycle 2 Cycle 3 Cycle 4 Cycle 5 Cycle 6 Cycle 7 Cycle 8 Cycle 9 Cycle 10 Papillary Thyroid 120 ALK+ NSCLC 480 > 240 ALK+ NSCLC 240 ALK+ NSCLC 60, q 12 ALK wt NSCLC 60, q 12 Ovarian 60, q 12 Pancreatic 480 > 240 Colon 320 > 240 Non-RECIST PR RECIST PR Non-RECIST PR Data as of October 30, 2013 |
|
|
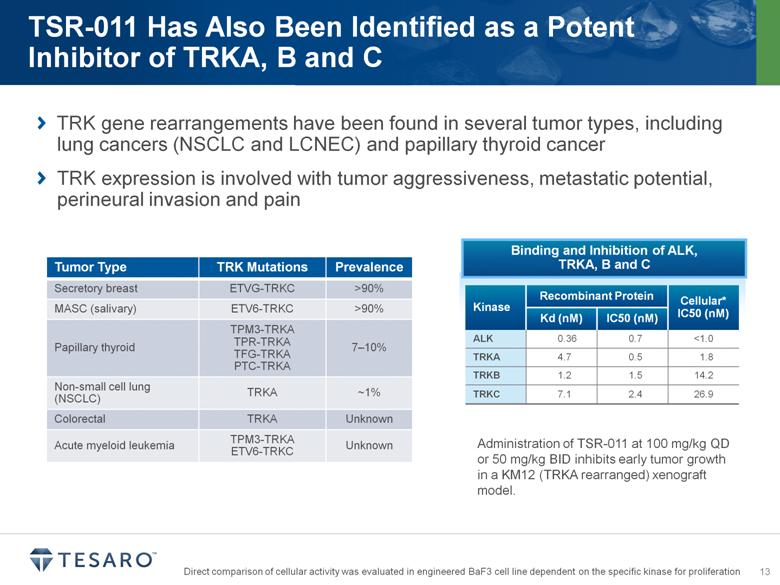
TRK gene rearrangements have been found in several tumor types, including lung cancers (NSCLC and LCNEC) and papillary thyroid cancer TRK expression is involved with tumor aggressiveness, metastatic potential, perineural invasion and pain Tumor Type TRK Mutations Prevalence Secretory breast ETVG-TRKC >90% MASC (salivary) ETV6-TRKC >90% Papillary thyroid TPM3-TRKA TPR-TRKA TFG-TRKA PTC-TRKA 7–10% Non-small cell lung (NSCLC) TRKA ~1% Colorectal TRKA Unknown Acute myeloid leukemia TPM3-TRKA ETV6-TRKC Unknown Binding and Inhibition of ALK, TRKA, B and C Kinase Recombinant Protein Cellular* IC50 (nM) Kd (nM) IC50 (nM) ALK 0.36 0.7 <1.0 TRKA 4.7 0.5 1.8 TRKB 1.2 1.5 14.2 TRKC 7.1 2.4 26.9 Administration of TSR-011 at 100 mg/kg QD or 50 mg/kg BID inhibits early tumor growth in a KM12 (TRKA rearranged) xenograft model. Direct comparison of cellular activity was evaluated in engineered BaF3 cell line dependent on the specific kinase for proliferation |
|
|
Development Programs Update Phase 3 NOVA trial continues to enroll patients Phase 3 BRAVO trial now screening patients Collaborating with SARC for Ewing’s sarcoma Niraparib Initial Phase 1 data presented at the European Cancer Congress and 15th World Conference on Lung Cancer Continuing evaluation of a fractionated 60mg dose in ALK and TRK+ patients TSR-011 Top line data from two Phase 3 trials (MEC and HEC) to be reported at year end Third Phase 3 trial (HEC) continues to enroll; top line data now expected in early 2014 IV dose ranging and escalation study to complete by year end NDA submission targeted for mid-2014 Rolapitant SARC: Sarcoma Alliance for Research through Collaboration ESMO: European Society for Medical Oncology |
|
|
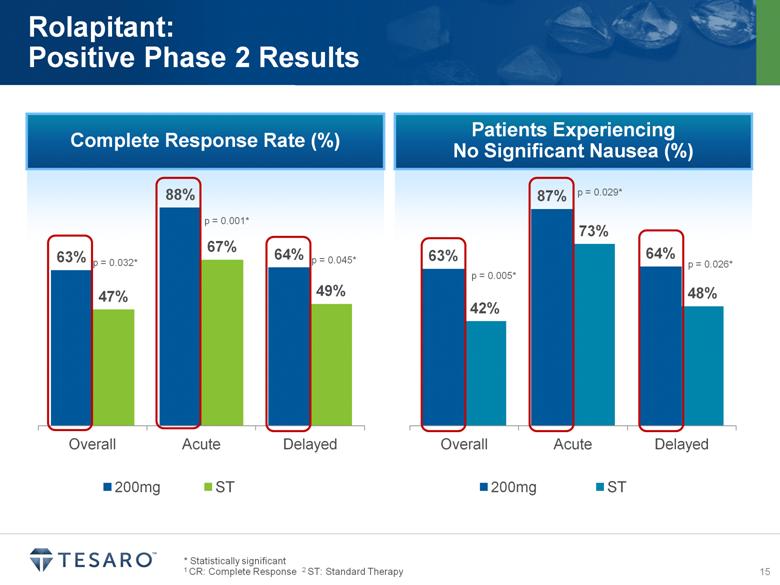
Complete Response Rate (%) * Statistically significant 1 CR: Complete Response 2 ST: Standard Therapy Rolapitant: Positive Phase 2 Results p = 0.032* p = 0.001* p = 0.045* Patients Experiencing No Significant Nausea (%) p = 0.029* p = 0.026* p = 0.005* |
|
|
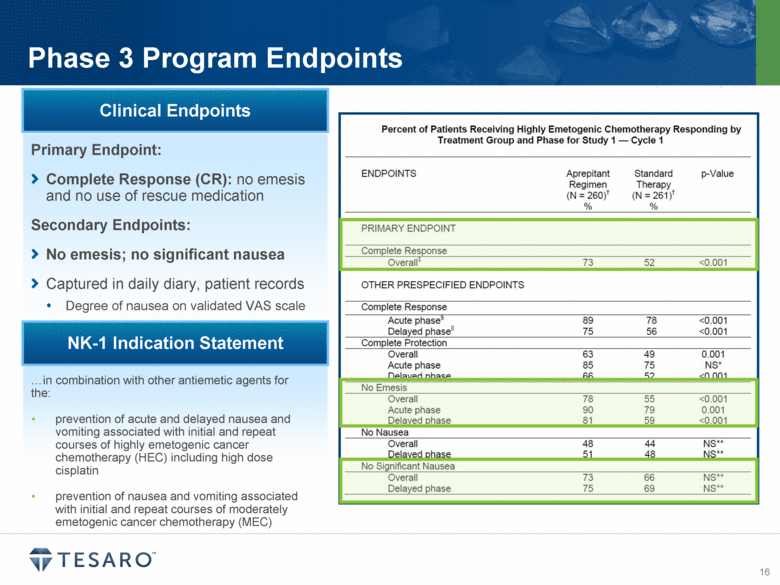
Phase 3 Program Endpoints Primary Endpoint: Complete Response (CR): no emesis and no use of rescue medication Secondary Endpoints: No emesis; no significant nausea Captured in daily diary, patient records Degree of nausea on validated VAS scale Clinical Endpoints in combination with other antiemetic agents for the: prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high dose cisplatin prevention of nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC) NK-1 Indication Statement |
|
|
November 7, 2013 Lonnie Moulder Chief Executive Officer |
|
|
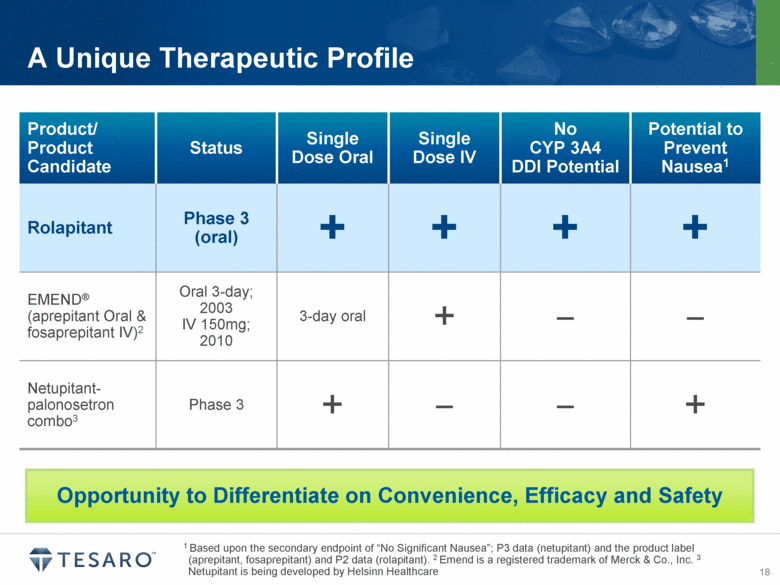
1 Based upon the secondary endpoint of “No Significant Nausea”; P3 data (netupitant) and the product label (aprepitant, fosaprepitant) and P2 data (rolapitant). 2 Emend is a registered trademark of Merck & Co., Inc. 3 Netupitant is being developed by Helsinn Healthcare A Unique Therapeutic Profile Product/ Product Candidate Status Single Dose Oral Single Dose IV No CYP 3A4 DDI Potential Potential to Prevent Nausea1 Rolapitant Phase 3 (oral) + + + + EMEND® (aprepitant Oral & fosaprepitant IV)2 Oral 3-day; 2003 IV 150mg; 2010 3-day oral + – – Netupitant-palonosetron combo3 Phase 3 + – – + Opportunity to Differentiate on Convenience, Efficacy and Safety |
|
|
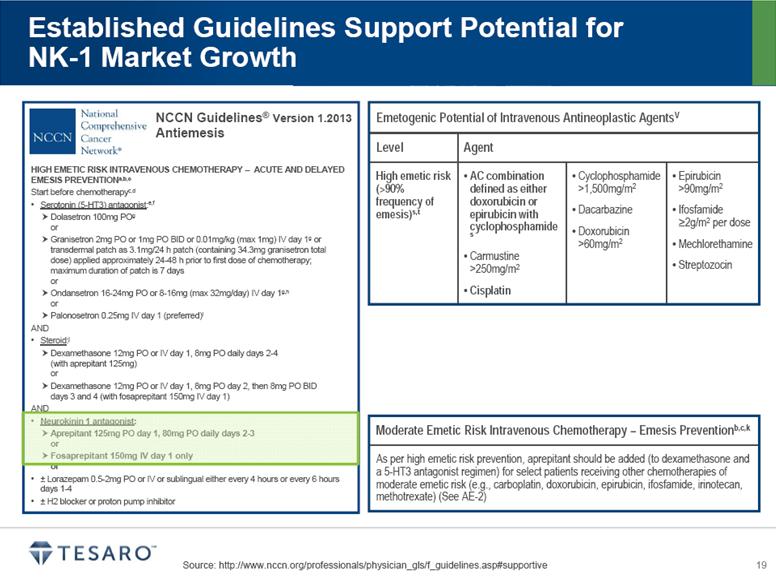
Source: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive Established Guidelines Support Potential for NK-1 Market Growth HIGH EMETIC RISK INTRAVENOUS CHEMOTHERAPY – ACUTE AND DELAYED EMESIS PREVENTIONa,b,c Start before chemotherapyc,d Serotonin (5-HT3) antagonist:e,f Dolasetron 100mg POg or Granisetron 2mg PO or 1mg PO BID or 0.01mg/kg (max 1mg) IV day 1g or transdermal patch as 3.1mg/24 h patch (containing 34.3mg granisetron total dose) applied approximately 24-48 h prior to first dose of chemotherapy; maximum duration of patch is 7 days or Ondansetron 16-24mg PO or 8-16mg (max 32mg/day) IV day 1g,h or Palonosetron 0.25mg IV day 1 (preferred)i AND Steroid:j Dexamethasone 12mg PO or IV day 1, 8mg PO daily days 2-4 (with aprepitant 125mg) or Dexamethasone 12mg PO or IV day 1, 8mg PO day 2, then 8mg PO BID days 3 and 4 (with fosaprepitant 150mg IV day 1) AND Neurokinin 1 antagonist: Aprepitant 125mg PO day 1, 80mg PO daily days 2-3 or Fosaprepitant 150mg IV day 1 only or ± Lorazepam 0.5-2mg PO or IV or sublingual either every 4 hours or every 6 hours days 1-4 ± H2 blocker or proton pump inhibitor NCCN Guidelines® Version 1.2013 Antiemesis ® Emetogenic Potential of Intravenous Antineoplastic AgentsV Level Agent High emetic risk (>90% frequency of emesis)s,t AC combination defined as either doxorubicin or epirubicin with cyclophosphamide s Carmustine >250mg/m2 Cisplatin Cyclophosphamide >1,500mg/m2 Dacarbazine Doxorubicin >60mg/m2 Epirubicin >90mg/m2 Ifosfamide >2g/m2 per dose Mechlorethamine Streptozocin Moderate Emetic Risk Intravenous Chemotherapy – Emesis Preventionb,c,k As per high emetic risk prevention, aprepitant should be added (to dexamethasone and a 5-HT3 antagonist regimen) for select patients receiving other chemotherapies of moderate emetic risk (e.g., carboplatin, doxorubicin, epirubicin, ifosfamide, irinotecan, methotrexate) (See AE-2) |
|
|
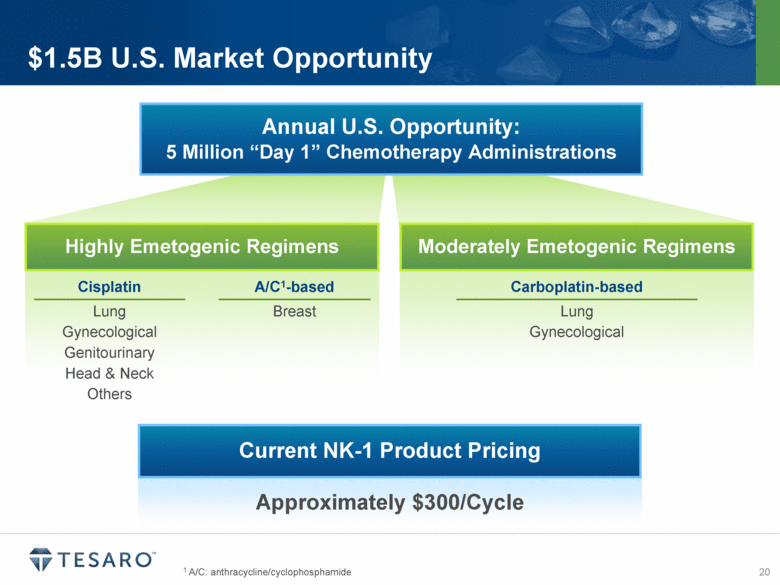
1 A/C: anthracycline/cyclophosphamide $1.5B U.S. Market Opportunity Annual U.S. Opportunity: 5 Million “Day 1” Chemotherapy Administrations Approximately $300/Cycle Highly Emetogenic Regimens Moderately Emetogenic Regimens Cisplatin A/C1-based Lung Gynecological Genitourinary Head & Neck Others Breast Carboplatin-based Lung Gynecological Current NK-1 Product Pricing |
|
|
Commercial Organization Design Approximately 120 Total Headcount Opportunity for Significant Leverage |
|
|
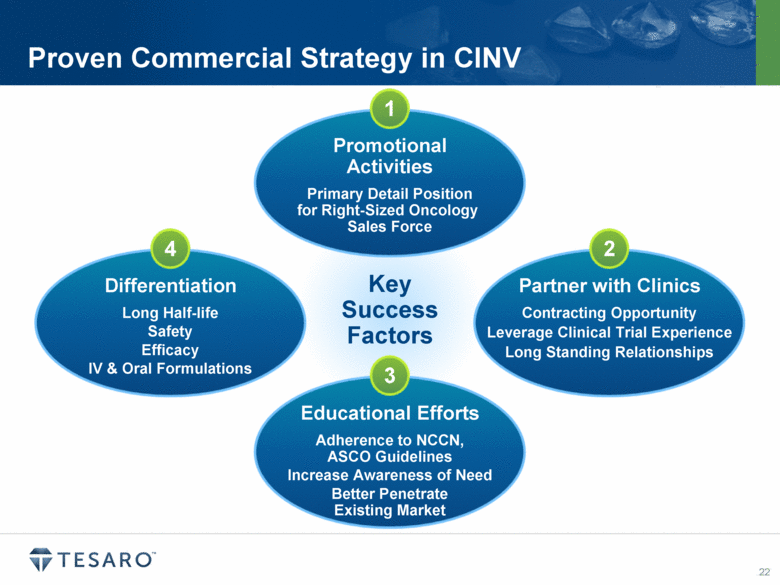
Proven Commercial Strategy in CINV Key Success Factors 1 3 2 4 |
|
|
[LOGO] |
|
|
Corporate Goals Initiated the Phase 3 trial of niraparib in ovarian cancer Presented niraparib Phase 1 data at ASCO Presented rolapitant DDI data at MASCC Begin the rolapitant IV dose finding and escalation study Present initial clinical data for TSR-011 at ECC Initiate patient treatment in the Phase 3 trial of niraparib in breast cancer by year end Announce top-line results of rolapitant oral pivotal program Two Phase 3 trials (MEC and HEC) at year end Third Phase 3 trial (HEC) in early 2014 Continue to evaluate the clinical activity of TSR-011 in ALK- and TRK-positive patients |
|
|
Third-Quarter Operating Results November 7, 2013 |