Attached files
| file | filename |
|---|---|
| 8-K - 8-K - QUESTCOR PHARMACEUTICALS INC | d622123d8k.htm |
| EX-99.2 - EX-99.2 - QUESTCOR PHARMACEUTICALS INC | d622123dex992.htm |
| EX-99.1 - EX-99.1 - QUESTCOR PHARMACEUTICALS INC | d622123dex991.htm |
 1
1
Third Quarter 2013
Conference Call
Third Quarter 2013
Conference Call
NASDAQ:
QCOR
NASDAQ:
QCOR
Exhibit 99.3 |
 2
•
Today’s webcast, accompanying slide presentation
and archived replay is available online at
http://ir.questcor.com/events.cfm
•
Telephone replay is available by dialing:
–
U.S.: (855) 859-2056.
–
International: (404) 537-3406.
–
Passcode: 76004546
•
Today’s webcast, accompanying slide presentation
and archived replay is available online at
http://ir.questcor.com/events.cfm
•
Telephone replay is available by dialing:
–
U.S.: (855) 859-2056.
–
International: (404) 537-3406.
–
Passcode: 76004546
Conference Call Logistics
2 |
 3
Safe Harbor Statement
Note: Except for the historical information contained herein, this press release contains
forward-looking statements that have been made pursuant to the Private Securities
Litigation Reform Act of 1995. These statements relate to future events or our future financial
performance. These statements are only predictions. Actual events or results may differ
materially. Factors that could cause or contribute to such differences include, but are
not limited to, the following: Our reliance on Acthar for substantially all of our net sales and profits;
Reductions in vials used per prescription resulting from changes
in treatment regimens by physicians or patient compliance with physician
recommendations; Our ability to receive high reimbursement levels from third party payers; The
complex nature of
our
manufacturing
process
and
the
potential
for
supply
disruptions
or
other
business
disruptions;
The
lack
of
patent protection
for
Acthar
and
the
possible
FDA
approval
and
market
introduction
of
competitive
products;
Our
ability
to
continue
to generate revenue from sales of Acthar
to treat on-label indications associated with NS, MS, IS or rheumatology-related
conditions, and our ability to develop other therapeutic uses for Acthar; Research and
development risks, including risks associated with Questcor's work in the area of NS and
Lupus, our efforts to develop and obtain FDA approval of Synacthen, our reliance on
third-parties to conduct research and development and the ability of
research and development to generate successful results; The results of any pending or future
litigation, investigations or claims, including government investigations and private
securities litigation; Our ability
to
comply
with
federal
and
state
regulations,
including
regulations
relating
to
pharmaceutical
sales
and
marketing
practices; Regulatory
changes
or
other
policy
actions
by
governmental
authorities
and
other
third
parties
in
connection
with
U.S.
health
care reform
or
efforts
to
reduce
federal
and
state
government
deficits;
An
increase
in
the
proportion
of
our
Acthar
unit
sales
comprised
of Medicaid-eligible patients and government entities; Our ability to estimate
reserves required for Acthar used by government entities and Medicaid-eligible patients and
the impact that unforeseen invoicing of historical Medicaid prescriptions may have upon
our results; Our ability to effectively manage our growth, including the expansion of our
sales forces, planned international expansion, and
our
reliance
on
key
personnel;
Our
ability
to
integrate
the
BioVectra
business
with
our
business
and
to
manage,
and
grow,
a contract manufacturing business; Our ability to comply with foreign regulations related to
the operating of BioVectra's business and the
international
sales
of
Synacthen;
The
impact
to
our
business
caused
by
economic
conditions;
Our
ability
to
protect
our proprietary rights; The risk of product liability lawsuits; Our ability to successfully
enter into, and operate in, international markets; The risk of unfavorable changes in
currency exchange rates; Unforeseen business interruptions and
security breaches; Volatility in Questcor's Acthar shipments, estimated channel inventory, and
end-user demand, as well as volatility in our stock price; Our ability and
willingness
to
continue
to
pay
our
quarterly
dividend
or
make
future
increases
in
our
quarterly
dividend;
and
Other
risks discussed in Questcor's annual report on Form 10-K for the year ended December 31,
2012 as filed with the Securities and Exchange Commission, or SEC, on February 27, 2013,
and other documents filed with the SEC. The risk factors and other information contained
in these documents should be considered in evaluating Questcor's prospects and future
financial performance.
Note: Except for the historical information contained herein, this press release contains
forward-looking statements that have been made pursuant to the Private Securities
Litigation Reform Act of 1995. These statements relate to future events or our future financial
performance. These statements are only predictions. Actual events or results may differ
materially. Factors that could cause or contribute to such differences include, but are
not limited to, the following: Our reliance on Acthar for substantially all of our net sales and profits;
Reductions in vials used per prescription resulting from changes
in treatment regimens by physicians or patient compliance with physician
recommendations; Our ability to receive high reimbursement levels from third party payers; The
complex nature of
our
manufacturing
process
and
the
potential
for
supply
disruptions
or
other
business
disruptions;
The
lack
of
patent protection
for
Acthar
and
the
possible
FDA
approval
and
market
introduction
of
competitive
products;
Our
ability
to
continue
to generate revenue from sales of Acthar
to treat on-label indications associated with NS, MS, IS or rheumatology-related
conditions, and our ability to develop other therapeutic uses for Acthar; Research and
development risks, including risks associated with Questcor's work in the area of NS and
Lupus, our efforts to develop and obtain FDA approval of Synacthen, our reliance on
third-parties to conduct research and development and the ability of
research and development to generate successful results; The results of any pending or future
litigation, investigations or claims, including government investigations and private
securities litigation; Our ability
to
comply
with
federal
and
state
regulations,
including
regulations
relating
to
pharmaceutical
sales
and
marketing
practices; Regulatory
changes
or
other
policy
actions
by
governmental
authorities
and
other
third
parties
in
connection
with
U.S.
health
care reform
or
efforts
to
reduce
federal
and
state
government
deficits;
An
increase
in
the
proportion
of
our
Acthar
unit
sales
comprised
of Medicaid-eligible patients and government entities; Our ability to estimate
reserves required for Acthar used by government entities and Medicaid-eligible patients and
the impact that unforeseen invoicing of historical Medicaid prescriptions may have upon
our results; Our ability to effectively manage our growth, including the expansion of our
sales forces, planned international expansion, and
our
reliance
on
key
personnel;
Our
ability
to
integrate
the
BioVectra
business
with
our
business
and
to
manage,
and
grow,
a contract manufacturing business; Our ability to comply with foreign regulations related to
the operating of BioVectra's business and the
international
sales
of
Synacthen;
The
impact
to
our
business
caused
by
economic
conditions;
Our
ability
to
protect
our proprietary rights; The risk of product liability lawsuits; Our ability to successfully
enter into, and operate in, international markets; The risk of unfavorable changes in
currency exchange rates; Unforeseen business interruptions and
security breaches; Volatility in Questcor's Acthar shipments, estimated channel inventory, and
end-user demand, as well as volatility in our stock price; Our ability and
willingness
to
continue
to
pay
our
quarterly
dividend
or
make
future
increases
in
our
quarterly
dividend;
and
Other
risks discussed in Questcor's annual report on Form 10-K for the year ended December 31,
2012 as filed with the Securities and Exchange Commission, or SEC, on February 27, 2013,
and other documents filed with the SEC. The risk factors and other information contained
in these documents should be considered in evaluating Questcor's prospects and future
financial performance. |
 4
•
8,132 vials shipped, up 45% YOY
•
$236.3M net sales, up 68% YOY
•
$1.52 GAAP diluted EPS, up 67% YOY
•
$1.68 Non-GAAP diluted EPS, up 73% YOY
•
$17.1M R&D investment, up 114% YOY
•
8,132 vials shipped, up 45% YOY
•
$236.3M net sales, up 68% YOY
•
$1.52 GAAP diluted EPS, up 67% YOY
•
$1.68 Non-GAAP diluted EPS, up 73% YOY
•
$17.1M R&D investment, up 114% YOY
Q3-2013 Financial Highlights
Note: See Reconciliation of Non-GAAP Adjusted Financial Disclosure slide
12. |
 5
Paid Rx
Comparison
Q3 –
2013
Q3 –
2012
Q2 –
2013
NS
370 -
380
7%
7%
MS
1,370 -
1,400
4%
8%
IS
225 -
230
33%
7%
Rheumatology
450 -
460
N/M
43%
Total
2,450 -
2,500 **
30%
10%
New Paid Acthar Prescriptions by
Therapeutic Area*
* Includes prescriptions covered by commercial carriers, Medicare, Medicaid and
Tricare in all periods regardless of the rebate percentage applicable in those
periods. ** Total number of prescriptions includes all paid prescriptions.
Based on internal company estimates. |
 6
Acthar Business Diversification Continues
Based on Acthar Net Sales
Based on internal company estimates. |
 7
•
Understanding the biological properties of
melanocortin peptides such as Acthar and Synacthen
–
Specific biochemical pathways, cells, and tissues
–
Immunomodulation and anti-inflammatory effects
•
Understanding the benefit of Acthar in devastating
medical conditions
•
Building the body of evidence surrounding the efficacy
and safety of Acthar on-label indications
•
Demonstrating the clinical benefit of Acthar and
Synacthen in new indications
•
Understanding the biological properties of
melanocortin peptides such as Acthar and Synacthen
–
Specific biochemical pathways, cells, and tissues
–
Immunomodulation and anti-inflammatory effects
•
Understanding the benefit of Acthar in devastating
medical conditions
•
Building the body of evidence surrounding the efficacy
and safety of Acthar on-label indications
•
Demonstrating the clinical benefit of Acthar and
Synacthen in new indications
R&D Objective: Addressing Unmet Medical
Needs with Acthar and Synacthen |
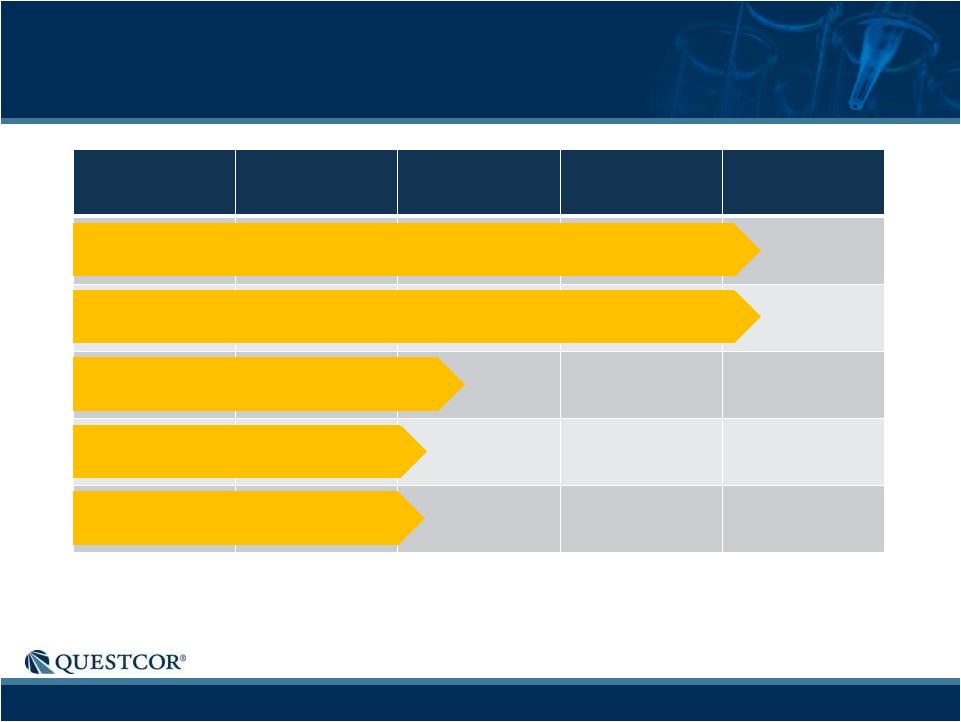 8
Pre-Clinical
Phase 1
Phase 2
Phase 3
Phase 4
Company-Sponsored Acthar Clinical Trials
IDIOPATHIC MEMBRANOUS NEPHROPATHY
SYSTEMIC LUPUS ERYTHEMATOSUS
DIABETIC NEPHROPATHY
AMYOTROPHIC LATERAL SCLEROSIS
ACUTE RESPIRATORY DISTRESS SYNDROME |
 9
ALS Phase 2 Open-Label Safety Study for Acthar
•
Goals
–
Assess short-term safety and tolerability of Acthar in ALS
–
Inform dosage selection for future studies
•
Study Design
–
Enroll up to 40 patients at multiple sites in U.S.
–
8-week treatment, plus optional 28-week open label extension
–
Patients randomized to one of four dosing regimens
•
Goals
–
Assess short-term safety and tolerability of Acthar in ALS
–
Inform dosage selection for future studies
•
Study Design
–
Enroll up to 40 patients at multiple sites in U.S.
–
8-week treatment, plus optional 28-week open label extension
–
Patients randomized to one of four dosing regimens
Findings to Drive Design for Pivotal Efficacy Study |
 10
ARDS Phase 2 Safety and Efficacy Study for Acthar
•
Goals
–
Determine if Acthar increases number of ventilator-free days during 28-day
treatment period
–
Assess if Acthar reduces mortality, organ failure, length of hospital or ICU stay
–
Inform dosage selection for future studies
•
Study Design
–
4-week randomized, placebo controlled trial
–
Enroll up to 210 patients at up to 40 sites in U.S.
–
Patients randomized to one of six dosing regimens
•
Goals
–
Determine if Acthar increases number of ventilator-free days during 28-day
treatment period
–
Assess if Acthar reduces mortality, organ failure, length of hospital or ICU stay
–
Inform dosage selection for future studies
•
Study Design
–
4-week randomized, placebo controlled trial
–
Enroll up to 210 patients at up to 40 sites in U.S.
–
Patients randomized to one of six dosing regimens
Findings to Drive Design for Pivotal Efficacy Study |
 11
Q3-2013 Financial Results
Q3 –
2013
Q3 –
2012
Change
Net Sales ($M)
$236.3
$140.3
68%
Fully Diluted, GAAP EPS
$1.52
$0.91
67%
Fully Diluted, Non-GAAP EPS
$1.68
$0.97
73%
Cash and short term investments ($M)
$281.1*
$111.9
Cash flow from operations ($M)
$108.9
$51.2
Diluted shares outstanding
62.1
61.4
* Includes $75 million in restricted cash. |
 12
Reconciliation of Non-GAAP Adjusted
Financial Disclosure
Reconciliation
of
Non-GAAP
Adjusted
Financial
Disclosure
Three Months Ended
Nine Months Ended
September 30,
September 30,
2013
2012
2013
2012
Adjusted net income
$104,368
$59,427
$233,328
$143,943
Share-based compensation expense (1)
(5,269)
(2,855)
(13,807)
(6,908)
Depreciation and amortization expense (2)
(3,127)
(226)
(6,253)
(638)
Interest expense associated with contingent consideration (3)
(188)
0
(572)
0
Interest
expense
associatedwith
R&Dliabilityin
conjunction
with
acquisition
of
Synacthen
(4)
(1,141)
0
(1,140)
0
Compensation expense associated with BV Trust (5)
(202)
0
(534)
0
Foreign currency transaction loss (6)
0
0
(329)
0
Medicaid adjustment for 2002 -2009 (7)
0
0
(7,751)
0
BioVectra purchase price adjustment (8)
0
0
169
0
Impairment of purchased technology (9)
0
(659)
(485)
(662)
Net income –
GAAP
$94,441
$55,687
$202,626
$135,735
Adjusted net income per share –
basic
$1.77
$1.01
$4.00
$2.36
Share-based compensation expense (1)
(0.09)
(0.05)
(0.24)
(0.11)
Depreciation and amortization expense (2)
(0.05)
0.00
(0.11)
(0.01)
Interest expense associated with contingent consideration (3)
0.00
—
(0.01)
—
Interest
expense
associatedwith
R&Dliabilityin
conjunction
with
acquisition
of
Synacthen
(4)
(0.02)
(0.02)
Compensation expense associated with BV Trust (5)
0.00
—
(0.01)
—
Foreign currency transaction loss (6)
—
—
(0.01)
—
Medicaid adjustment for 2002 -2009 (7)
—
—
(0.13)
—
BioVectra purchase price adjustment (8)
—
—
0.00
—
Impairment of purchased technology (9)
—
(0.01)
(0.01)
(0.01)
Net income per share –basic
$1.60
$0.95
$3.47
$2.23
Adjusted net income per share -
diluted
$1.68
$0.97
$3.82
$2.25
Share-based compensation expense (1)
(0.08)
(0.05)
(0.23)
(0.11)
Depreciation and amortization expense (2)
(0.05)
0.00
(0.10)
(0.01)
Interest expense associated with contingent consideration (3)
0.00
—
(0.01)
—
Interest
expense
associatedwith
R&Dliabilityin
conjunction
with
acquisition
of
Synacthen
(4)
(0.02)
(0.02)
Compensation expense associated with BV Trust (5)
0.00
—
(0.01)
—
Foreign currency transaction loss (6)
—
—
(0.01)
—
Medicaid adjustment for 2002 -2009 (7)
—
—
(0.13)
—
BioVectra purchase price adjustment (8)
—
—
0.00
—
Impairment of purchased technology (9)
—
(0.01)
(0.01)
(0.01)
Net income per share –diluted
$1.52
$0.91
$3.32
$2.12
Net sales –Questcor
$227,296
$140,339
$531,113
$348,760
Net sales -BioVectra
9,050
0
24,935
0
Consolidated net sales
236,346
140,339
556,048
348,760
Medicaid adjustment
0
0
11,500
0
Adjusted consolidated net sales
$236,346
$140,339
$567,548
$348,760
Notes
to
Reconciliation
of
Non-GAAP
Adjusted
Financial
Disclosure
Netincome
per
share
–
basic
and
diluted
may
not
foot
due
to
rounding.
Use
of
Non-GAAP
Financial
Measures
Our
“non-GAAP adjusted
net
income”
excludes
the
following
items
from GAAP
net income:
1.
Share-based
compensation
expense.
2.
Depreciation
and
amortization
expense,
including
amortization
expense
on
our
purchased
intangibles.
3.
Interest
expense
associated
with
the
net
present
value
adjustment
on
our
contingent
consideration.
4.
Interest
expense
associated
with
the
net
present
value
adjustment
on
the
R&D
liability
in conjunction
with
acquisition
of
Synacthen.
5. Compensation
expense associated
with
the
BV
Trust agreement.
6. Foreign
currency
transaction
loss.
7.
Medicaid
adjustment
for
prior
period
2002
-
2009
8. BioVectra
purchase
price adjustment related
to
a
labor
rebate
received
in
the second
quarter 2013
9. Impairment
of
purchased
technology
related
to
our
acquisition
of
Doral. |
 13
•
8,132 vials shipped, up 45% YOY
•
$236.3M net sales, up 68% YOY
•
$1.52 GAAP diluted EPS, up 67% YOY
•
$1.68 Non-GAAP diluted EPS, up 73% YOY
•
$17.1M R&D investment, up 114% YOY
•
8,132 vials shipped, up 45% YOY
•
$236.3M net sales, up 68% YOY
•
$1.52 GAAP diluted EPS, up 67% YOY
•
$1.68 Non-GAAP diluted EPS, up 73% YOY
•
$17.1M R&D investment, up 114% YOY
Q3-2013 Financial Highlights
Note: See Reconciliation of Non-GAAP Adjusted Financial Disclosure slide
12. |
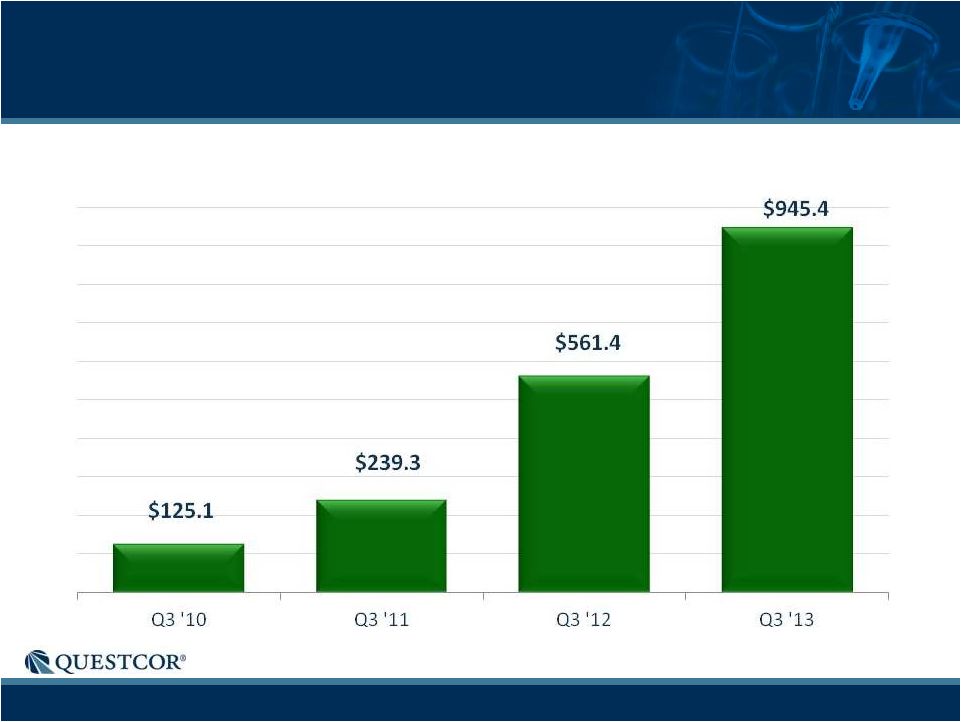 14
3-Year Net Sales Growth
Annualized Quarterly Net Sales ($M)
Each bar represents the applicable third quarter net sales multiplied by 4.
|
 15
3-Year Net Sales Growth
Quarterly Net Sales ($M) |
 16
•
Increased penetration of current Acthar markets
–
Lupus, RA are new Acthar markets in very early development
•
Pilot selling effort beginning soon in pulmonology for on-label
symptomatic sarcoidosis indication
–
Possible Acthar role in current dermatology and ophthalmology
indications also currently being evaluated for commercial potential
•
R&D focused on Acthar label expansion, Synacthen U.S. market
opportunity
–
ALS, ARDS, diabetic nephropathy and others
•
Untapped international market opportunities
•
Strong free cash flow generation enables possible product
acquisitions/partnering
•
Increased penetration of current Acthar markets
–
Lupus, RA are new Acthar markets in very early development
•
Pilot selling effort beginning soon in pulmonology for on-label
symptomatic sarcoidosis indication
–
Possible Acthar role in current dermatology and ophthalmology
indications also currently being evaluated for commercial potential
•
R&D focused on Acthar label expansion, Synacthen U.S. market
opportunity
–
ALS, ARDS, diabetic nephropathy and others
•
Untapped international market opportunities
•
Strong free cash flow generation enables possible product
acquisitions/partnering
A Strong Foundation for Growth |
 17
17
Third Quarter 2013
Conference Call
Third Quarter 2013
Conference Call
NASDAQ:
QCOR
NASDAQ:
QCOR |
